- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Department of Orthopedics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Purpose: Adolescent Idiopathic Scoliosis (AIS) is a complex three-dimensional spinal deformity, and its etiology and progression mechanisms have not been fully elucidated. This review aims to comprehensively explain the pathogenesis, clinical manifestations, and treatment strategies of AIS from a biomechanical perspective, providing a new theoretical framework for clinical diagnosis and treatment.
Methods: This review strictly follows the PRISMA guidelines for systematic literature search and selection. The search databases include PubMed, Embase, Web of Science, and Cochrane Library, with the cutoff date being June 2025. The search strategy involves a combination of keywords related to AIS, biomechanics, pathogenesis, clinical manifestations, and treatment. By progressively screening titles, abstracts, and full-text articles, relevant high-quality studies were ultimately included for comprehensive analysis.
Results: The pathogenesis of AIS can be conceptualized as a “vicious cycle” driven by the interactional imbalance between passive subsystems (skeletal-ligament), active subsystems (muscles), and neurocontrolled subsystems (central and peripheral nerves). Biomechanical factors play a key role in driving the progression from initial minor imbalances to significant three-dimensional deformities. Clinically, symptoms such as body deformity, back pain, and reduced cardiopulmonary function can all be directly interpreted from a biomechanical perspective. In terms of treatment, all mainstream interventions (including observation, specific exercise rehabilitation, bracing, and surgery) fundamentally rely on biomechanical correction.
Conclusion: The biomechanical perspective provides an indispensable integrative framework for understanding AIS. It unifies the process from molecular abnormalities to macro deformities, linking the diverse clinical manifestations and treatment approaches. Further exploration of biomechanical mechanisms is of significant importance for optimizing treatment timing and improving long-term patient outcomes.
1 Introduction
Scoliosis is a spinal deformity characterized by lateral curvature or vertebral rotation of one or more spinal segments. Idiopathic scoliosis, the most prevalent form, can manifest at any age. However, due to the rapid skeletal growth during puberty, it is most commonly diagnosed during adolescence. AIS has an unclear etiology, which poses significant challenges for clinical diagnosis and treatment. Traditional studies have focused on factors such as genetic predisposition, hormonal secretion abnormalities, and neurological control; however, the critical role of biomechanics in driving the occurrence and progression of AIS has not been systematically elucidated. In this review, we summarize and analyze the pathogenesis, clinical manifestations and treatment strategies of AIS from a biomechanical perspective. By examining AIS through a biomechanical lens, we can understand the fundamental mechanical principles underlying the condition and establish a theoretical basis for evaluating existing methodologies, developing novel intelligent braces, and creating mechanobiological intervention strategies. This article aims to provide clinicians with an integrated biomechanical framework to advance the standards of AIS diagnosis and treatment.
2 Materials and methods
This review strictly follows the guidelines of systematic reviews and PRISMA. Literature searches were conducted in the PubMed, Embase, Web of Science, and Cochrane Library databases, covering the period from the inception of the databases to December 2024. The search strategy combined both medical subject headings (MeSH) and free-text terms, including the following keywords: “adolescent idiopathic scoliosis,” “biomechanics,” “pathogenesis,” “clinical manifestations,” “treatment strategies,” and their combinations.
Study selection was carried out independently by two researchers. Duplicate articles were first excluded, and then titles and abstracts were screened according to predefined inclusion and exclusion criteria, followed by a full-text assessment of potentially eligible studies. Inclusion criteria included: (1) studies addressing the biomechanics of AIS; (2) studies involving pathogenesis, clinical manifestations, or treatment methods; (3) studies containing biomechanical parameters or analysis. Exclusion criteria were: (1) studies not focused on idiopathic scoliosis; (2) conference abstracts or case reports.
Data extraction was performed using a standardized form, which included the following information: first author, publication year, study type, sample characteristics, biomechanics assessment methods, main findings, and conclusions. Two reviewers independently extracted data, and any disagreements were resolved through discussion or consultation with a third researcher. Due to substantial heterogeneity among studies, a meta-analysis was not conducted, and a narrative synthesis method was employed to systematically summarize the results.
3 Results
3.1 Pathogenesis
3.1.1 Fully upright walking
In terms of anatomical structure, the human spine is similar to the spines of many vertebrates, such as chimpanzees, cattle, rats, and mice (1–3). However, among all vertebrates, only humans develop AIS. Some studies suggest that bipedalism and the upright posture are closely related to AIS. Bipedalism and the upright posture lead to a series of changes in the human spine and trunk in three-dimensional space, affecting sagittal shape, axial pelvic-spinal rotation, and transverse plane reverse rotation (4, 5). Under normal physiological conditions, the arrangement of sagittal spinal tissues is strictly regulated, with children and adolescents undergoing changes in spinal alignment during growth and development while maintaining alignment between the spinal and lower limb joint centers (6). Under abnormal physiological conditions, abnormal spinal alignment and reduced rotational stability can lead to spinal deformities such as scoliosis (5, 7). Studies have shown that rats, which walk bipedally and must remain upright, develop scoliosis after pinealectomy, whereas quadrupedal rats with similar pinealectomy do not (8). We performed a force analysis on the spines of dogs (fully quadrupedal), apes (brief bipedal), and humans (fully bipedal), finding that the spines of quadrupedal animals and apes are more horizontally positioned, with the center of gravity located in front of the pelvis, while the human spine is closer to the vertical direction, with the center of gravity above the pelvis (Figure 1). Among all vertebrates, including humans, the spine primarily supports axial compression, which is mainly borne by the anterior column (intervertebral discs, endplates, and vertebral bodies) (9). Due to fully upright walking, the human spine bears a greater gravitational load, which affects the vertical growth of the spine. During growth and development in children and adolescents, incorrect upright and seated postures may form, leading to asymmetric distribution of gravitational load on the spinal epiphyseal plates, ultimately causing asymmetric vertebral growth and wedging, inducing scoliosis (10–12). In this review, we performed a force analysis on individual vertebrae of dogs, apes, and humans (Figure 1), finding that shear loads in the vertebrae of dogs and apes mainly direct toward the abdominal side, which can be countered by the pulling forces of small joints, muscles, and spinal ligaments, keeping the spine in a very stable state (7). In human vertebrae, some vertebrae experience shear loads directed toward the abdominal side, while others experience loads directed toward the back. The human spine is unable to effectively counteract the backward shear loads, leading to asymmetric loading, asymmetric growth, and axial rotational instability, which may result in the occurrence and development of scoliosis (7, 13–15).
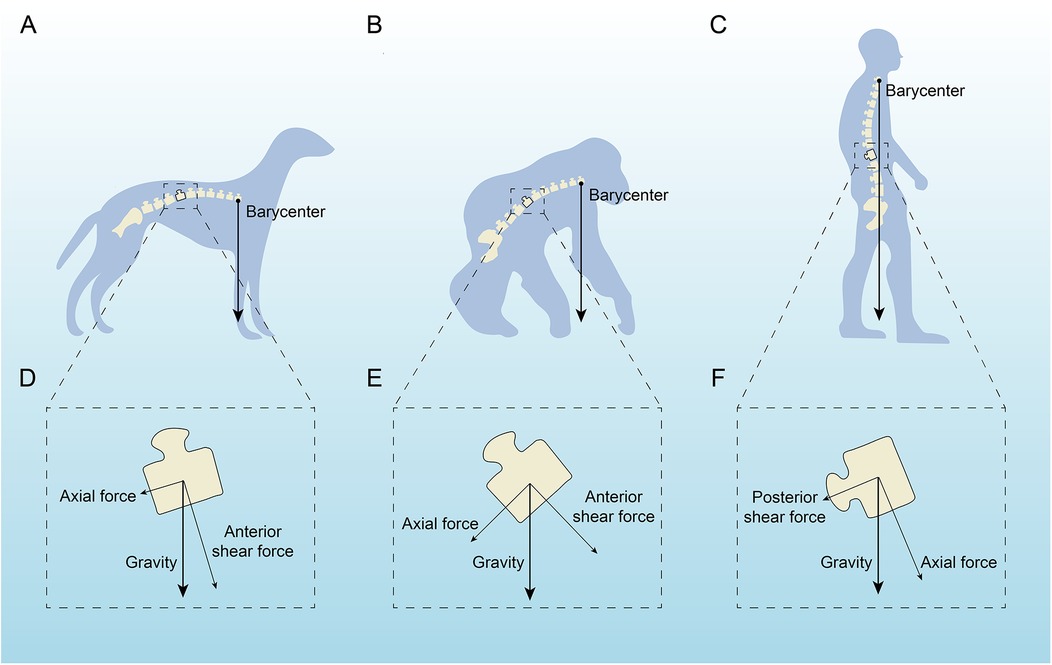
Figure 1. Analysis of the force acting on a single vertebra in dogs, apes and humans. (A,D) A quadruped with the barycenter near the hind legs, highlighting axial and anterior shear forces. (C,F) An intermediate stanced primate with similar force distribution but a slightly shifted barycenter. (C,F) An upright human, showing a central barycenter with axial and posterior shear forces affecting the spine. Arrows indicate force directions relative to gravity.
3.1.2 Regulatory factors
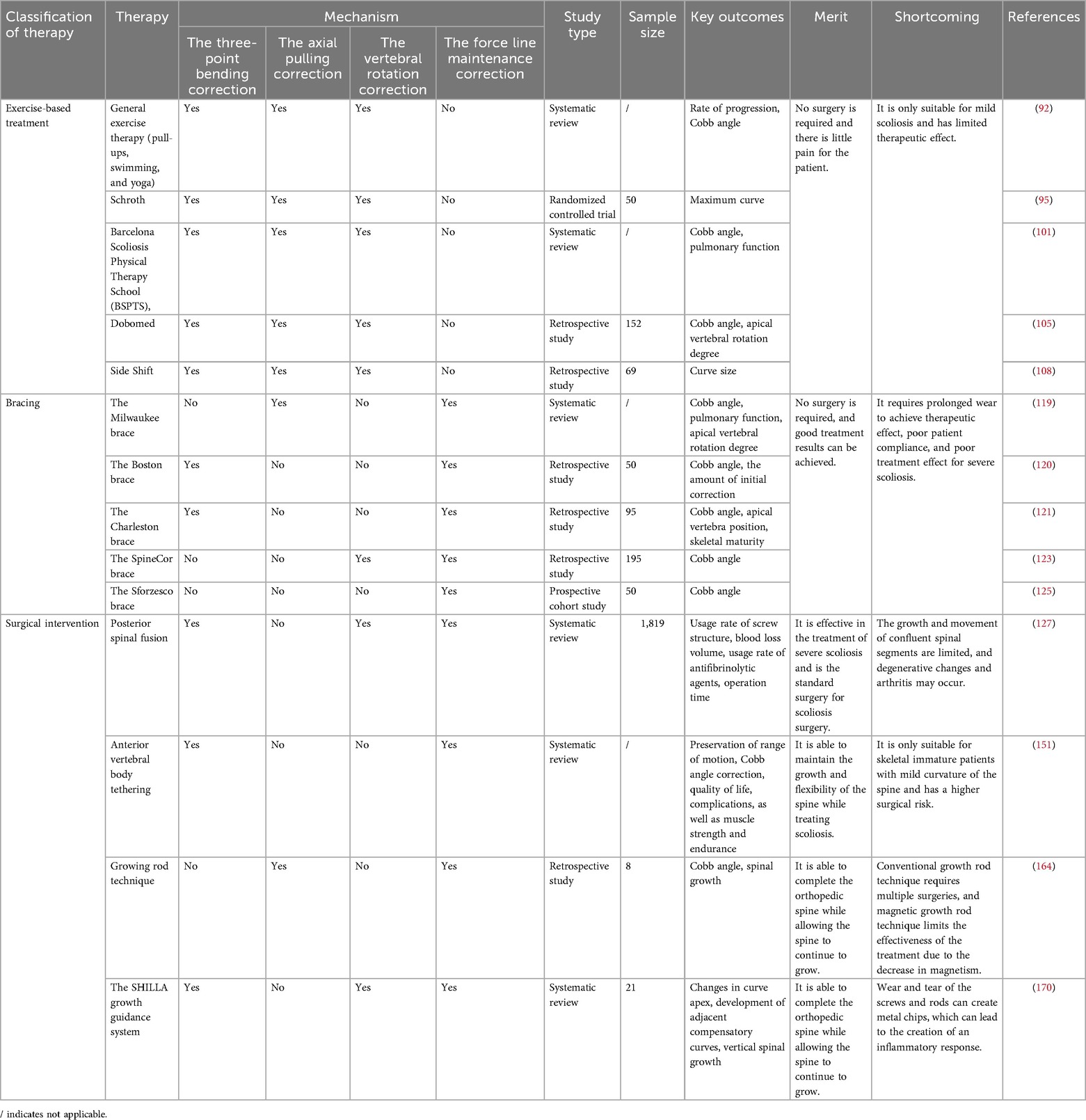
Panjabi proposed the concept of spinal stability through the “Three-Segment Model,” which delineates the spinal stability system into three components: a passive subsystem made up of ligaments and bones, an active subsystem comprised of spinal-associated muscles, and a neural control subsystem (16, 17). Abnormalities in any of these subsystems can lead to biomechanical alterations in the spine, thereby undermining its stability and potentially contributing to the development of AIS (Table 1). The proper growth, development, and functioning of bones, ligaments, muscles, and nerves within the spinal stability system depend on the expression of specific proteins. Disruptions in the expression of these proteins may result in the onset of AIS.
Abnormal skeletal growth and development are crucial mechanisms underlying AIS. The human spine normally maintains a specific mechanical balance to support essential life functions. Abnormalities in vertebral growth, such as accelerated or slowed bone development, excessive or insufficient bone volume, and variations in bone density, can disrupt the bone's mechanical properties, including strength, toughness, and stiffness, potentially leading to AIS. Compared to healthy individuals, patients with AIS exhibit accelerated skeletal growth (18–20) and reduced bone density (21, 22). Additionally, AIS patients experience asymmetrical load distribution at the vertebral epiphyseal plates, which causes uneven vertebral growth and wedging (23, 24). Research indicates that in AIS patients, there is a disturbance in the growth balance between the anterior column (including intervertebral discs, endplates, and vertebrae) and the posterior column (including the vertebral arch and facet joints), with a tendency for overgrowth in the anterior column, resulting in asymmetric spinal development (25–28). Recent studies have shown that not only the abnormal development of vertebral bodies can lead to the occurrence of AIS, but also the deformities of the ribs and thoracic cage ultimately contribute to AIS (29). Abnormal growth and development of the ribs and thoracic cage will subsequently affect the intervertebral discs, and ultimately, the vertebral bodies will deform due to asymmetric loads, muscle forces, growth, and gravity. The growth and development of spinal bones are regulated by various growth-related proteins, which influence bone development and, consequently, the mechanical properties such as strength, toughness, and stiffness. The process begins with bone marrow mesenchymal stem cells, which have high proliferative potential and can differentiate into osteoblasts, chondrocytes, or adipocytes (30, 31). In proteomic studies of bone marrow mesenchymal stem cells (BM-MSCs) from AIS patients, a significant upregulation of pyruvate kinase M2 (PKM2) has been observed. PKM2 negatively correlates with cell differentiation, resulting in weakened osteogenic differentiation of BM-MSCs, inhibited skeletal growth and development, decreased bone mass, and reduced bone strength (32). Additionally, annexin A2 is found to be significantly downregulated in mesenchymal stem cells (MSCs) from AIS patients. Annexin A2 promotes osteogenic differentiation of BM-MSCs, enhances alkaline phosphatase (ALP) activity, and supports mineralization of osteoblasts. Its downregulation is associated with lower bone density and compromised bone strength (32, 33). Moreover, a marked reduction in phosphorylated heat shock protein 27 (HSP27) levels has been reported in osteoblasts and BM-MSCs of AIS patients. Upregulation of phosphorylated HSP27 in these cells promotes the expression of bone formation markers (34). Melatonin deficiency has been shown to induce scoliosis in pinealectomized chickens and mice (35, 36). AIS patients also exhibit dysfunction in melatonin signaling (37). Melatonin, primarily produced by the pineal gland, enhances osteogenic and chondrogenic differentiation of BM-MSCs (38, 39), stimulates osteoblast differentiation, and promotes osteogenesis (40). Furthermore, melatonin inhibits the binding of osteoclast differentiation factors to the receptor activator of nuclear factor-κB (RANK), reducing osteoclast differentiation (41) and thereby increasing bone density and strength (42). In AIS patients, a downregulation of leptin receptors in BM-MSCs has been observed, potentially leading to decreased sensitivity to circulating leptin (43). Leptin directs BM-MSCs towards osteogenic pathways rather than adipogenic ones, enhancing bone density and strength (44, 45). Estrogen also impacts the development of scoliosis. Its primary role in skeletal development is mediated through estrogen receptor alpha. Estrogen's most significant effects on bone metabolism likely involve promoting osteoblast differentiation, inhibiting osteoblast apoptosis, and facilitating osteoclast apoptosis, thus enhancing skeletal growth and bone strength (46, 47).
Research into the paravertebral muscles reveals that changes and asymmetries in these muscles impair spinal posture and movement control, contributing to the progression of AIS. Studies have identified variations in the biomechanical properties of these muscles in AIS, including differences in muscle tension, stiffness, relaxation time, and the Deborah number (indicative of creep). These biomechanical characteristics are linked to the severity of scoliosis (48, 49). The alterations and asymmetries in the paravertebral muscles are associated with the expression of specific genes. Tent5a, an atypical poly(A) polymerase, has been identified as a susceptibility gene for AIS (50). It shows differential expression in the bilateral paravertebral muscles of AIS patients. Inhibiting Tent5a expression can reduce myoblast proliferation, migration, and differentiation, and also inhibit the maturation of type I muscle fibers, leading to decreased muscle asymmetry, improved spinal stress balance, and reduced scoliosis progression (51). Furthermore, PAX3 expression in the paravertebral muscles of AIS patients varies between sides, with a positive correlation between PAX3 levels and muscle volume. The convex side exhibits higher PAX3 expression and muscle volume compared to the concave side, causing an imbalance in the forces exerted on the spine (52). ADGRG6, a member of the adhesion G protein-coupled receptor family, shows a strong association with AIS (53). Inhibition of ADGRG6 leads to decreased tendon fiber density and uneven elastic fiber distribution, lowering elastic modulus and failure stress, thus disrupting spinal stress balance (54). Calmodulin, a crucial calcium-binding protein, regulates calcium signaling and skeletal muscle contraction. In AIS patients, calmodulin is unevenly distributed in the paravertebral muscles, with higher levels on the convex side and lower on the concave side (55). This disparity in contraction strength between the bilateral paravertebral muscles disrupts spinal stress balance. Estrogen influences skeletal muscle activity and regulates vascular endothelial growth factor production, which supports muscle cell differentiation and anti-apoptotic processes (56). In female AIS patients, estrogen receptor type 1 (ESR1) and type 2 (ESR2) show asymmetrical expression in deep paravertebral muscles, with the extent of this asymmetry positively correlated with Cobb angle and progression risk factors (57).
Abnormalities in the nervous system are crucial to the pathogenesis of AIS. The pathogenesis of AIS primarily involves the extrapyramidal system. Numerous studies have demonstrated that dysfunction in afferent nerves, the regulatory center, and efferent nerves can all contribute to the development of AIS (58–60). Regarding afferent pathways, research has shown that AIS patients experience a significant decline in balance while standing with their eyes closed. Additionally, their somatosensory evoked potentials exhibit abnormal latencies and amplitudes, suggesting impaired signal processing in the spinal cord's dorsal columns or the somatosensory cortex (58). Additionally, some AIS patients present with cerebellar tonsil ectopia, which is associated with more severe spinal curvature (61). The biomechanical impact of gene expression in human neural tissue on AIS remains unexplored. However, studies in zebrafish have demonstrated that modulating the expression of certain genes within neural tissue can influence AIS development. For example, overexpression of UNCX in zebrafish larvae results in abnormal branching of primary motor neurons in the tail, which leads to defects in trunk nerve development and disrupts the spinal alignment normally regulated by the nervous system, causing spinal curvature (62). Moreover, irregular deposition of zebrafish scospondin (sspo) within the brain ventricles has been linked to idiopathic-like scoliosis. sspo influences the function of Reissner's fiber, which regulates cerebrospinal fluid homeostasis, neurogenesis, and embryonic development. Inhibiting the irregular deposition of sspo helps maintain spinal balance and alignment (63). In terms of efferent pathways, dysfunction is primarily observed as an imbalance in the excitability of spinal α-motor neurons and associated neuromuscular control deficits. Electromyography studies have revealed that AIS patients' paraspinal muscles display asymmetric electrical activity both at rest and during movement, with abnormal muscle excitability patterns on the convex and concave sides (60). This abnormality may stem from changes in the recruitment sequence and rate of motor neuron pools, leading to an imbalance in muscle strength required to maintain spinal symmetry. During the growth period, this imbalance applies asymmetric stress to the spine, which exacerbates its three-dimensional deformity.
AIS is likely not initiated by a single subunit, but rather a minor defect in one subunit that triggers a positive feedback loop. A slight abnormality in neural control (such as a minor proprioceptive defect) causes the patient to unconsciously adopt a slightly asymmetric posture when standing or moving. Alternatively, a subtle ligamentous laxity in the passive subunit allows the spine to undergo slight deformation more easily under external forces. This initial asymmetric posture leads to biomechanical changes in the spine. In order to maintain this unstable curved posture, the muscles are compelled to compensate. The concave side muscles are forced to contract continuously, resulting in fatigue, spasms, and functional degradation. The convex side muscles are overstretched, further exacerbating the imbalance in muscle strength and coordination. The deformed spine and abnormally functioning muscles send erroneous feedback signals to the neural control system, prompting it to issue increasingly inaccurate commands for muscle control, thereby reinforcing and worsening the faulty posture, eventually leading to progressive, structural three-dimensional spinal deformity. Panjabi's “Three-Segment Model” provides an excellent biomechanical integrative framework for understanding AIS. It reveals that AIS is not a static deformity but rather a dynamically progressing process driven by the imbalance of interactions within the neuromuscular-skeletal system. This calls for a clinical practice that transcends mere orthotics, advocating for a comprehensive treatment strategy aimed at restoring the functionality of the entire spinal system.
3.2 Clinical feature
3.2.1 Somatic deformity
AIS primarily involves one or more vertebral segments bending laterally with associated vertebral rotation, typically developing in adolescents during or around puberty. Mild scoliosis often does not cause significant discomfort or noticeable somatic deformity (64). In contrast, more severe scoliosis can impact adolescent growth and development, leading to noticeable physical deformities and structural abnormalities (65) such as uneven shoulders, asymmetrical scapulae, spinal deviation from the midline, and pelvic tilt (Figure 2A). From a static biomechanical perspective, the rotating vertebrae induce structural deformation in the attached ribs, leading to the clinically visible “scapular prominence” deformity and thoracic asymmetry (66). This disrupts the alignment between the two mechanical bases: the thoracic cage and the pelvis. In order to maintain a centered head position and horizontal eye alignment as much as possible in the sagittal and coronal planes, the body compensates by modifying spinal curvature and pelvic tilt (67). However, this compensatory mechanism is often imperfect, resulting in external signs such as uneven shoulders and scapular asymmetry. Additionally, the normal physiological curvature of the spine is frequently disturbed, further reducing the spine's efficiency in absorbing and dispersing stress, thereby making it more vulnerable to axial loading. These structural changes result in altered biomechanical performance in patients with AIS compared to healthy individuals. Research indicates that AIS patients exhibit reduced hip and pelvic movement during walking, excessive energy expenditure, asymmetric gait patterns, and uneven ground reaction forces (68). During physical activity, a deformed spine, as a dysfunctional movement chain, exhibits significant reductions in range of motion, flexibility, and energy transmission efficiency. Research has confirmed that patients with AIS show distinct biomechanical patterns during walking: the kinematic parameters of the hip joint and pelvis in the horizontal and coronal planes are reduced, which is a compensatory strategy aimed at reducing spinal movement and maintaining trunk stability (69). However, this strategy comes at the cost of reduced movement economy, leading to increased energy expenditure and greater susceptibility to fatigue (69). Additionally, due to changes in the biomechanical advantages of the trunk muscles (such as abnormal length-tension relationships between the convex and concave side muscles) and disturbances in proprioceptive input, patients exhibit step asymmetry and uneven ground reaction force distribution throughout the gait cycle (70).
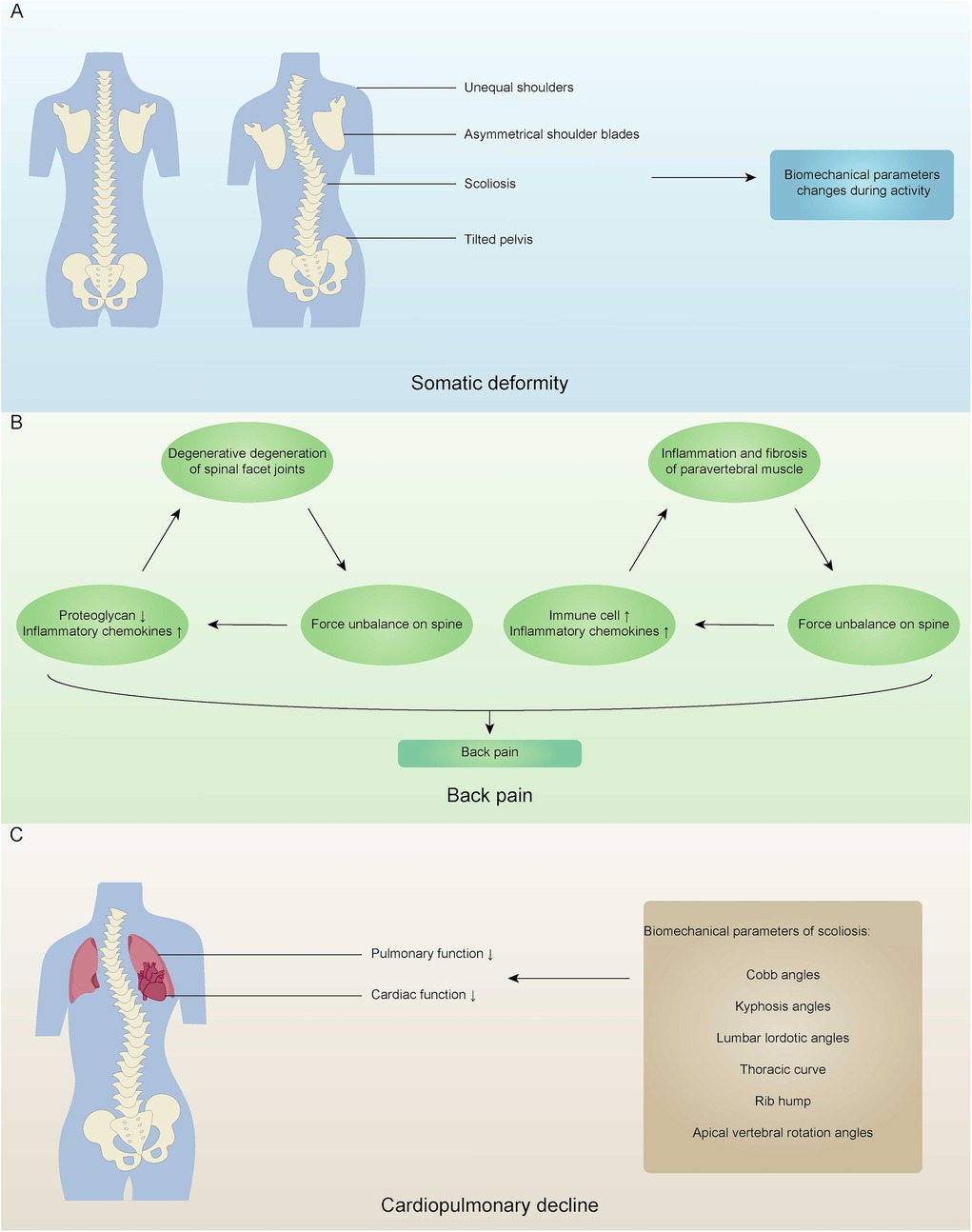
Figure 2. Clinical manifestations of adolescent idiopathic scoliosis. (A) A normal spine and a spine with somatic deformity, highlighting unequal shoulders, asymmetrical shoulder blades, scoliosis, and tilted pelvis, leading to changes in biomechanical parameters during activity. (B) The cycle resulting in back pain, involving degeneration of spinal facet joints, force imbalance on the spine, inflammation of paravertebral muscle, and changes in proteoglycans and inflammatory chemokines. (C) Cardiopulmonary decline due to decreased pulmonary and cardiac function, with biomechanical parameters of scoliosis such as Cobb angles, kyphosis angles, and others.
3.2.2 Back pain
Back pain is a significant clinical feature of AIS. Compared to the control group, patients with AIS experience a higher prevalence of back pain, which is not only more severe but also lasts longer and recurs more frequently (64, 71–73). The etiology of back pain in these patients is multifactorial, involving both biochemical and biomechanical mechanisms (74). Research indicates that AIS patients exhibit notable degenerative changes in the spinal facet joints, including considerable loss of proteoglycans, increased cell density, elevated levels of pro-inflammatory markers, and the presence of prominent small leucine-rich proteoglycan (SLRP) fragments, such as chondroadherin and decorin (75). Facet joints are crucial for maintaining spinal stability, and their degenerative changes are widely recognized as a source of pain in chronic back pain cases (76, 77). When facet joints undergo degeneration, the spine loses its force balance and develops rotational instability, which exacerbates the degeneration of the facet joints and results in significant back pain in AIS patients (78). In a separate prospective study, Samaan et al. found that pain is associated with inflammation of the paravertebral muscles (79). In AIS, muscle inflammation is a process that involves both acute and chronic stages. The repeated cycles of muscle tissue damage associated with the progression of scoliosis may lead to inflammation and fibrosis. Furthermore, paraspinal muscles undergo structural remodeling in response to abnormal mechanical environments. The muscles on the concave side of the spine demonstrate more pronounced fibrosis and fat degeneration, while those on the convex side experience micro-damage and inflammatory responses due to compensatory overuse (80). Consequently, the force exerted by the paravertebral muscles becomes unbalanced, leading to rotational instability in the spine and further increasing inflammatory chemokines and immune cells in the muscle tissue, thus creating a vicious cycle and resulting in significant back pain (79).
3.2.3 Cardiopulmonary decline
As AIS progresses, it adversely affects both pulmonary and cardiac functions. Numerous studies have documented a decline in pulmonary function in patients with AIS (81–83). This decline is often attributed to factors such as distortion of the spine and thoracic cavity, spinal compression on the lungs, secondary rib deformities, and decreased mobility of the chest wall (84–86). From a biomechanical standpoint, the primary cause of reduced pulmonary function in patients with AIS is the compression and restriction imposed by the deformed spine and thoracic cavity. The combination of vertebral rotation and rib hump associated with scoliosis leads to asymmetric collapse of the thoracic cage, significantly reducing the thoracic volume, particularly on the convex side. This structural change impairs pulmonary function through two main mechanisms. Firstly, restrictive ventilatory dysfunction, which results from a decrease in chest wall compliance and limited diaphragm movement, jointly hampers lung expansion. Secondly, local compression of lung tissue can lead to a mismatch between ventilation and perfusion, affecting the efficiency of gas exchange. Researchers have extensively investigated the relationship between spinal deformity parameters and pulmonary function in AIS patients. Recent studies have found that the pulmonary function of patients with AIS is negatively correlated with the Cobb angle. A significant inverse relationship exists between the Cobb angle and both the absolute and predicted values of forced expiratory volume in 1 s, forced vital capacity, vital capacity, and total lung capacity. The results indicate that for every 2.6–4.5 degrees of spinal curvature, a 1% decline in predicted lung function is observed (87). However, because AIS is a three-dimensional spinal deformity, evaluating pulmonary function based solely on the Cobb angle is insufficient. Research has shown that worse pulmonary function is associated with larger proximal and main thoracic Cobb angles, decreased kyphosis angles, increased lumbar lordosis angles, longer thoracic curvature, larger rib humps, greater apical vertebral rotation, and a smaller thoracic cage (88). As AIS advances, severe impairment of pulmonary function can lead to reduced lung volumes, resulting in pulmonary arterial hypertension and impaired cardiac function (89). Furthermore, a correlation has been observed between right ventricular function and the Cobb angle, with severe cases of AIS showing limited right ventricular systolic function (90).
3.3 Therapy
The treatment methods for AIS primarily consist of non-surgical and surgical approaches. Non-surgical methods include exercise-based orthotic treatment and brace orthotic treatment, whereas surgical approaches involve traditional spinal fusion using rods and screws and growing rod surgery. We performed a biomechanical analysis of these treatment methods, categorizing them into four biomechanical correction models: the three-point bending correction model, the axial pulling correction model, the vertebral rotation correction model, and the force line maintenance correction model (Figure 3). The corrective treatment approaches for AIS incorporate one or more of these biomechanical correction models (Table 2).
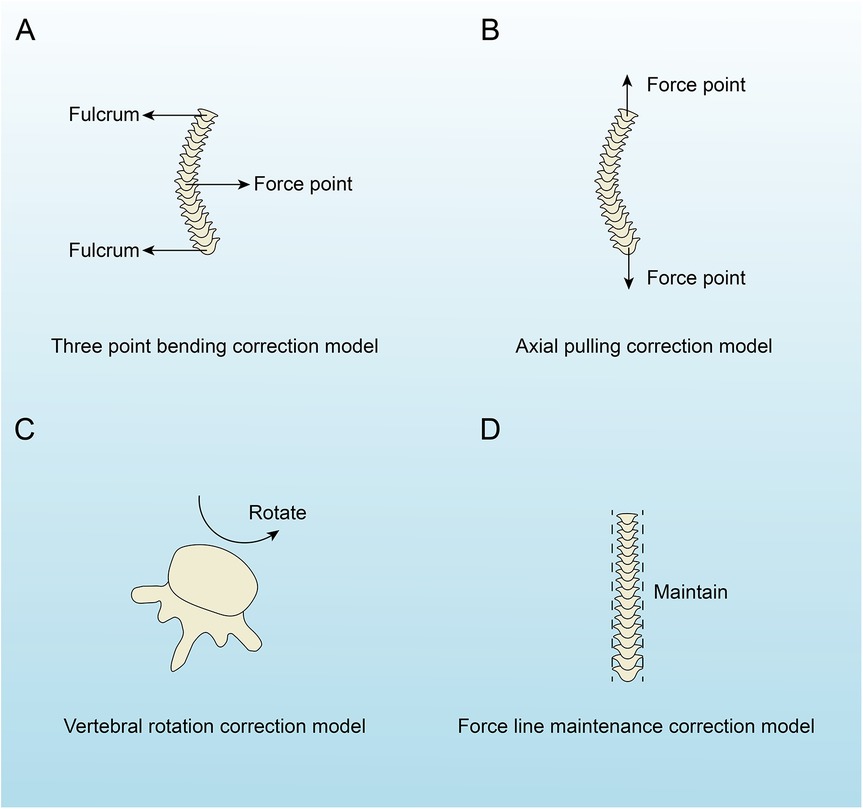
Figure 3. The four biomechanical correction models of adolescent idiopathic scoliosis. (A) Three-point bending model with labeled fulcrum and force point. (B) Axial pulling model with two force points. (C) Vertebral rotation model indicating rotation. (D) Force line maintenance model with vertical alignment labeled “Maintain”.
3.3.1 Exercise-based treatment
Exercise-based treatments encompass general exercise and physiotherapeutic scoliosis-specific exercises (PSSE). General exercise therapy involves low-intensity stretching and strength training activities such as pull-ups, swimming, and yoga, which can help stabilize the spine and alleviate symptoms (91, 92). It is widely accepted that PSSE offers superior therapeutic benefits for AIS compared to general exercise. PSSE is a tailored exercise program designed according to the patient's spinal curvature, degree, and clinical characteristics, with an emphasis on preventing curvature progression (93). Various PSSE methods exist, all based on similar biomechanical principles. Here, we focus on four commonly utilized techniques: Schroth, Barcelona Scoliosis Physical Therapy School (BSPTS), Dobomed, and Side Shift. The method of Schroth involves specific postural correction, correction of breathing patterns, and correction of postural perception. This method strengthens the paravertebral muscles through targeted exercises, aiming for asymmetric sagittal plane alignment, axial elongation, and rotational breathing. These techniques are designed to halt curve progression, correct abnormal spinal curves, reduce pain, increase lung capacity, and improve overall posture and appearance (94, 95). The physical therapy strategies for the thoracic spine (particularly in the T2 to T10 segments) make full use of the biomechanical coupling effect between the ribs and thoracic vertebrae. The “rotational breathing” technique in the Schroth method consciously guides the expansion of the rib space on the convex side, which applies a persistent, lateral de-rotational torque to the connected vertebrae. This mechanically induced force transmitted through the ribs helps correct vertebral rotational deformities and, to some extent, improves the symmetry and mobility of the thoracic cage (96). Schroth, as one of the most representative forms of PSSE, has been strongly supported by extensive evidence from clinical research. In recent years, several high-quality systematic reviews and meta-analyses have consistently demonstrated significant benefits of this therapy in improving Cobb angle, trunk rotation, and quality of life. A meta-analysis by Ceballos-Laita et al. clearly indicated that, compared to conventional treatments, Schroth therapy significantly reduces the Cobb angle in patients with AIS (96). Further analysis by Baumann et al., through a systematic review and meta-analysis of randomized controlled trials, strengthened this conclusion by incorporating observational studies for subgroup analysis, confirming the positive role of Schroth therapy in controlling angle progression (97). In addition to structural parameters, Schroth therapy has also shown positive effects on improving patients' functional outcomes and health-related quality of life. The study by Ceballos-Laita et al. additionally reported significant advantages of this therapy in reducing trunk rotation angles and improving scores on quality of life questionnaires (96). A network meta-analysis by Wang et al. further confirmed these findings, showing long-term benefits of Schroth therapy in improving spinal deformities and quality of life among six specific exercise interventions compared (98). This suggests that its effectiveness extends beyond radiological indicators to include aesthetic improvements and psychosocial well-being, embodying the modern concept of comprehensive treatment. It is noteworthy that the effectiveness of Schroth therapy has been contextualized within the broader framework of exercise interventions. A network meta-analysis by Jiang et al. compared and ranked various PSSEs. Although all exercise regimens demonstrated some degree of effectiveness, the combination of Schroth therapy with core stabilization training was considered the optimal strategy for addressing AIS (99). Chen et al.'s study suggested that exercise interventions can significantly improve AIS, but the differences in improvement between different exercise forms (core strength training, PSSE, yoga, Schroth, and sling) were not statistically significant (100). Current evidence from clinical research consistently supports Schroth therapy as an effective, non-invasive treatment for improving the Cobb angle, trunk rotation, and quality of life in patients with AIS. BSPTS, an evolution of the method of Schroth, uses translation, rotation, and axial elongation exercises to prevent curve progression, correct scoliosis posture, stabilize the spine, and enhance respiratory function (101). BSPTS is typically applicable to patients with mild to moderate scoliosis or as an adjunct to brace treatment to enhance muscle strength and improve posture symmetry. Currently, high-level evidence from evidence-based medicine specifically targeting BSPTS remains relatively limited. Seleviciene et al. explored various physical therapy methods and their efficacy currently used for conservative treatment of AIS in a literature review. The review indicated that BSPTS, as one of the main approaches within PSSE, is clinically applied based on its systematic theoretical framework and clinical observations. However, the review also highlighted that despite the widespread use of PSSE (including BSPTS), research in this field still faces challenges, and many treatments require more high-quality studies to provide strong evidence for their efficacy (102). Future research should focus on identifying the optimal indications for BSPTS, treatment dosages (frequency and duration), and its unique advantages or specific therapeutic effects, thus providing guidance for its precise application in clinical practice. The method of Dobomed, also referred to as the Dobosiewicz's method, is a 3D biomechanical correction technique based on AIS pathomechanics, aiming to correct spinal curvature through 3D vertebral displacement (101). Evidence shows that the method of Dobomed can reduce Cobb angles and vertebral rotation while preventing further curvature progression (103–105). Currently, high-quality randomized controlled trials and traditional meta-analyses specifically targeting Dobomed therapy are relatively limited, which somewhat restricts the independent and precise evaluation of its efficacy. However, the study by Wang et al. systematically compared the efficacy of six different scoliosis-specific exercises for AIS (98). Since no available Dobomed clinical trials met the inclusion criteria, the efficacy of Dobomed therapy for AIS was not compared. Although the study may indicate that certain methods (such as Schroth) show relative advantages in statistical rankings, Dobomed therapy, as part of a comprehensive physical therapy system, still holds clinical value in improving posture and function in AIS patients, particularly for those with specific types of scoliosis, such as reduced thoracic kyphosis. Side Shift therapy includes active 3D automatic correction (in the transverse, coronal, and sagittal planes), excessive corrective movements beyond the midline, trunk shifting opposite the main curve, and repeated correction exercises during growth to influence spinal development (101, 106). Studies suggest that the Side Shift therapy effectively halts curvature progression and is a viable treatment for AIS (107, 108). Currently, evidence from evidence-based medicine specifically targeting the side-shifting method remains limited. A network meta-analysis conducted by Wang et al. compared the efficacy of six different scoliosis-specific exercises on AIS (98), but we cannot directly obtain quantitative comparison data or ranking results regarding the efficacy of the side-shifting method from this study. This situation itself indicates that the side-shifting method is usually incorporated into broader physical therapy programs for research, rather than being assessed as an independent intervention. Furthermore, core stability training may help suppress the progression of scoliosis by optimizing the dynamic stability of the spine and reducing asymmetrical loads applied to the spine during daily activities. Studies show that a scientifically designed core stability training program can be an effective component of PSSE, providing intrinsic biomechanical support for the spine (101). Liu et al. confirmed that core stability training could benefit mild to moderate AIS, but due to heterogeneity, small sample size, and multiple comparisons, the evidence for the efficacy of core stability training is limited (109). The network meta-analyses by Chen et al. and Jiang et al. both included core muscle training as an important control intervention when comparing various exercise interventions. Although these analyses may indicate that some more comprehensive PSSE methods (such as Schroth) are statistically superior, core training, as a foundational intervention, is often integrated into more comprehensive rehabilitation programs to enhance treatment effects (99, 100). The core of combined exercise programs is to maximize treatment effects through synergy. These programs may integrate various elements such as scoliosis-specific exercises (e.g., Schroth), core stability training, symmetry-strengthening exercises, and posture education, aiming to address the three-dimensional deformity and functional impacts of AIS from multiple dimensions. Currently, randomized controlled trials focusing on “combined exercise programs” as an independent intervention are relatively limited. A network meta-analysis by Li et al. compared the short-term effects of different strategies in conservative treatment for AIS, and the results reinforced the short-term efficacy of bracing combined with scoliosis-specific exercises. This study provides indirect but important support for the concept of “combined treatment,” suggesting that combining treatment methods with different mechanisms may be a more promising strategy to address the complexity of AIS through multi-target effects, thereby achieving better overall outcomes (110). In addition to its traditional roles in muscle strengthening and posture correction, physical therapy can also modulate the extrapyramidal system. Targeted proprioceptive training and balance exercises can enhance the functional activity of this system. Through repetitive and patterned motor training, the regulation of spinal motor neurons by structures such as the basal ganglia, cerebellum, and brainstem can be optimized. This process improves the balance and coordination of the paravertebral muscles, achieving greater precision and automaticity without the need for conscious control (111).
3.3.2 Bracing
In the 16th century, the French surgeon Ambroise Pare was the first to employ metal orthotic devices for spinal deformities. Following this, numerous braces and their derivatives, such as the Milwaukee brace and the Lyon brace, were developed for scoliosis treatment (112–114). Brace orthopedic treatment is indicated for AIS with a Cobb angle ranging from 20°–40° (115, 116). Currently, there is a wide variety of braces used for AIS treatment. Negrini et al. proposed a classification system for spinal braces based on criteria including building, rigidity, anatomical classification, aonstruction of the envelope, mechanism of action, and plane of action (117). Although many braces share similar biomechanical mechanisms, we will focus on a few representative examples. The Milwaukee brace, the first widely used brace for scoliosis treatment globally, consists of a rigid orthotic frame designed to correct the curved spine through axial extension. However, due to the substantial discomfort it causes, patient compliance tends to be lower, making it advisable to use the Milwaukee brace only intermittently (118, 119). In 1972, the Boston brace was introduced, primarily constructed from metal, leather, and canvas. This brace features a symmetrical design with a posterior opening and is characterized as a rigid orthotic. Its biomechanical function is based on a three-point bending system, which has been shown to produce favorable therapeutic outcomes (115, 119). A controlled matched study evaluating the effectiveness of the Boston brace for managing greater curves in 50 skeletally immature female patients found that the Boston brace was effective in preventing progression of greater curves when used ≥18 h per day (120). The Charleston brace is a nocturnal scoliosis corrective brace made from a plaster mold, employing a three-point bending mechanism to correct spinal curvature. Studies indicate that the Charleston brace is highly effective in managing AIS, and its nocturnal use has been shown to improve patient compliance and reduce psychological stress to some extent (121). Asymmetric nocturnal braces (such as the Providence brace) facilitate the posterior migration of the nucleus pulposus towards the midline in the apex of the scoliosis curve. The brace exerts a continuous, localized lateral pressure on the convex side of the apex through precisely molded pressure pads, while providing corresponding release space on the concave side. This pressure induces gradual displacement of the nucleus pulposus under sustained low load, progressively correcting the asymmetric deformation of the intervertebral disc (122). The SpineCor brace is a flexible spinal orthosis that includes a rotational strap connecting the pelvis to the shoulder straps. This brace allows for dynamic adjustment of the rotational strap's tension, which is tailored according to the specific curvature patterns of the spine. Research suggests that the SpineCor brace can partially correct scoliosis (123). The Sforzesco brace is a custom-made thoracolumbar orthosis designed according to the SPoRT concept (Symmetric, Patient-oriented, Rigid, Three-dimensional, and Active). It utilizes thrust-based correction techniques for scoliosis and has been shown to have favorable treatment outcomes (124, 125). In the thoracic segments from T2 to T10, the pressure applied by the brace to the ribs is effectively transmitted to the corresponding vertebral bodies via the costovertebral and costotransverse joints, generating a powerful moment for de-rotation and lateral translation, which allows for three-dimensional correction of the thoracic deformity. The brace design often includes a frontal plane flexion mechanism with an opening on the anterior axillary line of the concave side. This design not only prevents excessive pressure on the concave side ribs but, more importantly, increases the volume of the concave thoracic cavity, thus creating physical space for the expansion of restricted lung tissue and the correction of the deformity. For scoliosis of the lumbar or thoracolumbar region below T10, the brace is commonly designed using a constant-volume translation principle. The brace shell forms an inclined surface above the iliac crest on the convex side, angling downward and inward. When the patient lies down, the gravitational force of the trunk, combined with the counteracting force of the brace, pushes the convex side of the lumbar spine upwards toward the midline and neutral position, effectively reducing lateral displacement and rotation (126).
3.3.3 Surgical intervention
AIS is a complex three-dimensional spinal deformity. When AIS continues to deteriorate and non-surgical treatment methods fail to achieve the desired therapeutic outcomes, surgical intervention should be considered. We have summarized the current common surgical treatments for AIS, including vertebral fusion surgery, anterior vertebral body tethering (AVBT), growing rod technique, and the SHILLA growth guidance system.
3.3.3.1 Vertebral fusion surgery
Posterior spinal fusion (PSF) is widely recognized as the standard treatment for AIS, particularly for patients with a Cobb angle greater than 40°–50° (127–129). The primary goals of this surgery are to correct spinal deformities, restore balance, and achieve joint fusion, all while minimizing complications (130). Extensive research has demonstrated that PSF can effectively correct spinal deformities and produce favorable outcomes (129, 131, 132). One of the primary costs of surgery is the inevitable restriction of motion at the fused segments. This sacrifice in mobility may increase the risk of degeneration at adjacent segments, but the stability gained from the procedure is crucial for controlling the progression of deformity. At the same time, a key biomechanical benefit of PSF is the complete elimination of the “accordion phenomenon” within the intervertebral discs at the fused levels. This phenomenon is a descriptive term for the dynamic changes of the intervertebral discs in AIS patients under daily, periodic axial loading. The repetitive collapse and re-expansion of the discs, akin to the opening and closing of an accordion, is believed to potentially exacerbate vertebral wedging and the progression of scoliosis through the Hueter-Volkmann mechanism (133). Alongside the development of PSF, anterior spinal fusion (ASF) has emerged. In the 1970s, Allen Dwyer introduced anterior correction techniques for AIS (134). ASF offers benefits such as fewer segments fused and higher fusion rates, but it has limited potential for correcting spinal curvature and may sometimes result in kyphosis (135, 136). Both anterior and posterior spinal fusion surgeries are invasive procedures that can lead to substantial blood loss, extended hospital stays, postoperative pain, and risk of infection (137). To address these issues, minimally invasive spinal fusion techniques have been developed. These techniques minimize trauma and shorten hospitalization times. Researchers such as Sarwahi et al. and de Bodman et al. have successfully implemented minimally invasive spinal fusion for AIS treatment (138, 139). Nevertheless, minimally invasive spinal fusion surgery has limitations: 1. Its corrective potential is limited, requiring careful patient selection based on curve severity (140). 2. There are numerous constraints in performing osteotomies and spinal fusion with minimally invasive techniques (140, 141). 3. Multiple fluoroscopic images are necessary to accurately position rotated vertebrae (142).
3.3.3.2 Anterior vertebral body tethering
Due to the fusion of vertebrae with screws and rods, vertebral fusion surgery may result in restricted growth and movement of the fused spinal segments, as well as degenerative changes and arthritis in the adjacent segments (143–146). AVBT has been developed as a surgical technique. This method is based on the Hueter-Volkmann principle, which states that applying compressive forces to the growth plates slows down skeletal growth, while tensile forces stimulate it, thereby altering vertebral shape (147, 148). In AVBT, tethers and screws are introduced on the convex side of the curved spine to restrict its growth, while allowing the concave side to grow, thus correcting scoliosis. In animal models, Newton et al. found that using anterior-lateral flexible tethers in calf models can regulate spinal growth while maintaining spinal flexibility (149, 150). Multiple studies have shown that AVBT can progressively correct scoliosis in skeletally immature patients with AIS, demonstrating significant therapeutic effects. Additionally, since it does not involve vertebral fusion, it largely preserves joint mobility and muscle strength (151–153). However, AVBT has limitations, being suitable only for skeletally immature patients with mild spinal curvature, and it requires an anterior approach that necessitates opening the thoracic and abdominal cavities, thereby increasing surgical risks (154). Due to its novelty, the indications for AVBT remain controversial, and further research is needed to define precise indications and the specific structures required for patients (155).
3.3.3.3 Growing rod technique
Growing rod technique is indicated for patients with scoliosis who have not responded to conservative treatment, specifically those with a Cobb angle exceeding 50° and aged under 10 years. This technique employs axial traction on the spine, allowing for spinal correction while facilitating continued growth (156–158). However, the requirement for open surgery every six months to extend the growth rod introduces multiple risks associated with anesthesia and surgical incision complications (159–161). The advent of magnetic growth rod technology offers a promising solution to the challenges posed by traditional growth rods, significantly decreasing the frequency of required surgeries. This technology allows for incremental extensions that closely mimic the physiological growth patterns of the spine (162, 163). Magnetic control technology has considerable advantages in clinical applications, as it enables non-invasive manipulation of magnetic devices within the body through magnetic fields to achieve therapeutic objectives. Current research indicates that magnetic growth rod technology can provide adequate traction for scoliotic spines, effectively manage spinal curvature, and reduce both the number of surgical interventions and associated pain, thereby significantly improving patients' quality of life (162, 164, 165). Nonetheless, the weakening and loss of magnetic force present critical limitations for this surgical approach. An increase in body mass index can cause subcutaneous fat to thicken, which increases the distance between the external control device and the magnetic rod. Specifically, for every 1 mm increase in distance, the driving force diminishes by 2.1%, ultimately compromising the traction efficacy of the magnetic rod on the spine (166).
3.3.3.4 The SHILLA growth guidance system
Similar to growing rod technique, the SHILLA growth guidance system can achieve spinal deformity correction while allowing the spine to continue growing. In the SHILLA growth guidance system, all vertebrae are aligned as much as possible in the coronal, sagittal, and axial planes. Then, only the apex vertebrae of the spinal curvature are fused, and non-locking pedicle screws are placed in the remaining vertebrae. These non-locking pedicle screws can slide along the rod, utilizing the growth potential of the patient's vertebrae to guide the vertical growth of the spine while maintaining the normal sequence of the non-fused vertebrae (167, 168). The SHILLA growth guidance system is intended for pediatric patients under the age of 10 who have severe, progressive, and life-threatening scoliosis. It allows for spinal growth while controlling the deformity, eliminating the need for repeated surgical procedures (169–171). However, the sliding of non-locking pedicle screws along the rod can produce metal debris due to wear, which may lead to inflammatory responses (172).
4 Discussion
AIS is a three-dimensional spinal deformity located in the coronal, sagittal, and horizontal planes, characterized by vertebral rotation and lateral curvature of the spine, making it the most common type of scoliosis (173–175). In orthopedic-related diseases, biomechanical analysis is an important method that helps us gain a comprehensive understanding of the condition and develop better treatment options (176–178). Therefore, studying AIS from a biomechanical perspective is crucial, as it may assist in developing more effective treatment methods, ultimately improving the quality of life for patients with AIS.
This study systematically reviews the role of biomechanics in the pathogenesis, clinical manifestations, and treatment of AIS, revealing that biomechanical factors are present throughout the entire process of AIS development. These factors serve as the core link connecting abnormalities in the skeletal, muscular, and neural control systems. In terms of pathogenesis, the perspective proposed in this review—that the unique biomechanical challenges posed by fully upright walking represent an evolutionary background for the onset of AIS—aligns with Kouwenhoven et al.'s view that “the uniquely human fully upright posture seems to be a prerequisite for AIS development” (12). However, unlike most reviews that focus on a single factor, such as genetics, molecular pathways, or hormonal imbalances (179, 180), this study innovatively connects molecular biology and biomechanics. It highlights that regulatory molecules such as PKM2 and PAX3 may exert their effects by influencing the biomechanical parameters of the bones (e.g., density, toughness) and the force balance in the spine. This provides a mechanobiological framework for understanding how genetic mutations ultimately translate into macroscopic deformities, bridging the gap between mechanistic studies and clinical manifestations found in traditional reviews. This approach complements the “mechanical load-growth feedback” theory emphasized by Stokes et al (122). In terms of clinical presentation and evaluation, the guidelines of the International Scientific Society on Scoliosis Orthopaedic and Rehabilitation Treatment (SOSORT) and classic literature by Weinstein et al. comprehensively describe the body deformities and functional impairments associated with AIS (64, 93). This review further emphasizes the biomechanical nature of these symptoms, linking abstract symptoms to specific mechanical principles. This perspective strengthens the necessity for biomechanical interventions (e.g., specific exercise rehabilitation), aligning with the core recommendations for exercise therapy in the SOSORT guidelines (93). In the analysis of treatment methods, the conclusions of this study share broad consensus with the North American Spine Society (NASS) guidelines and reviews by Negrini et al., particularly in terms of the core concept of bracing treatment—applying biomechanical forces to alter spinal growth patterns, while surgery serves as the ultimate mechanical reconstruction approach (181, 182). The unique contribution of this study lies in the establishment of an evaluation system based on biomechanics as a unified standard to analyze the mechanical principles and pros and cons of different treatment strategies. For example, with regard to novel non-fusion techniques (such as vertebral tethering), the design concept is entirely based on the mechanical inhibition of the growth plate on the convex side of the deformed vertebra. The biomechanical analysis framework provided by this study offers a solid theoretical foundation for understanding and optimizing such technologies, an area that many traditional treatment strategies have yet to explore in depth (183).
Based on the previous discussions, this review does not repeat the conclusions of existing guidelines. Rather, it organically links the etiology, clinical manifestations, and treatment of AIS through an enhanced biomechanical integrative perspective. It not only supports the core treatment principles based on biomechanics found in existing guidelines, but also introduces biomechanical concepts and systematic mechanical analyses, providing new insights into understanding the complex nature of AIS and offering direction for future development of more targeted biomechanical intervention strategies.
Author contributions
DL: Data curation, Writing – original draft, Writing – review & editing. HM: Writing – original draft. SY: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIS, adolescent idiopathic scoliosis; BM-MSCs, bone marrow mesenchymal stem cells; PKM2, pyruvate kinase M2; PKM2, mesenchymal stem cells; ALP, alkaline phosphatase; HSP27, heat shock protein 27; RANK, receptor activator of nuclear factor-κB; ESR1, estrogen receptor type 1; ESR2, estrogen receptor type 2; sspo, scospondin; SLRP, small leucine-rich proteoglycan; PSSE, physiotherapeutic scoliosis-specific exercises; BSPTS, barcelona scoliosis physical therapy school; AVBT, anterior vertebral body tethering; PSF, posterior spinal fusion; ASF, anterior spinal fusion.
References
1. Cotterill PC, Kostuik JP, D'Angelo G, Fernie GR, Maki BE. An anatomical comparison of the human and bovine thoracolumbar spine. J Orthop Res. (1986) 4(3):298–303. doi: 10.1002/jor.1100040306
2. Schlösser TP, Janssen MM, Hogervorst T, Vrtovec T, de Vos J, Öner FC, et al. The odyssey of sagittal pelvic morphology during human evolution: a perspective on different hominoidae. Spine J. (2017) 17(8):1202–6. doi: 10.1016/j.spinee.2017.03.016
3. Janssen MM, de Wilde RF, Kouwenhoven JW, Castelein RM. Experimental animal models in scoliosis research: a review of the literature. Spine J. (2011) 11(4):347–58. doi: 10.1016/j.spinee.2011.03.010
4. Burwell RG, Aujla RK, Kirby AS, Dangerfield PH, Moulton A, Freeman BJ, et al. Leg-arm length ratios correlate with severity of apical vertebral rotation in girls after school screening for adolescent idiopathic scoliosis (AIS): a dynamic pathomechanism in the initiation of the deformity? Stud Health Technol Inform. (2008) 140:189–93. doi: 10.3233/978-1-58603-888-5-189
5. Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, et al. Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop. (2011) 31(1 Suppl):S14–27. doi: 10.1097/BPO.0b013e3181f73c12
6. Abelin-Genevois K. Sagittal balance of the spine. Orthop Traumatol Surg Res. (2021) 107(1S):102769. doi: 10.1016/j.otsr.2020.102769
7. Kouwenhoven JW, Smit TH, van der Veen AJ, Kingma I, van Dieen JH, Castelein RM. Effects of dorsal versus ventral shear loads on the rotational stability of the thoracic spine: a biomechanical porcine and human cadaveric study. Spine (Phila Pa 1976). (2007) 32(23):2545–50. doi: 10.1097/BRS.0b013e318158cd86
8. Machida M, Saito M, Dubousset J, Yamada T, Kimura J, Shibasaki K. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. (2005) 14(9):843–8. doi: 10.1007/s00586-004-0806-1
9. Smit TH. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J. (2002) 11(2):137–44. doi: 10.1007/s005860100346
10. Samadi B, Raison M, Achiche S, Fortin C. Identification of the most relevant intervertebral effort indicators during gait of adolescents with idiopathic scoliosis. Comput Methods Biomech Biomed Engin. (2020) 23(10):664–74. doi: 10.1080/10255842.2020.1758075
11. Drevelle X, Lafon Y, Ebermeyer E, Courtois I, Dubousset J, Skalli W. Analysis of idiopathic scoliosis progression by using numerical simulation. Spine (Phila Pa 1976). (2010) 35(10):E407–12. doi: 10.1097/BRS.0b013e3181cb46d6
12. Kouwenhoven JW, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine (Phila Pa 1976). (2008) 33(26):2898–908. doi: 10.1097/BRS.0b013e3181891751
13. Castelein RM, van Dieen JH, Smit TH. The role of dorsal shear forces in the pathogenesis of adolescent idiopathic scoliosis–a hypothesis. Med Hypotheses. (2005) 65(3):501–8. doi: 10.1016/j.mehy.2005.03.025
14. Kouwenhoven JW, Vincken KL, Bartels LW, Castelein RM. Analysis of preexistent vertebral rotation in the normal spine. Spine (Phila Pa 1976). (2006) 31(13):1467–72. doi: 10.1097/01.brs.0000219938.14686.b3
15. Kouwenhoven JW, Bartels LW, Vincken KL, Viergever MA, Verbout AJ, Delhaas T, et al. The relation between organ anatomy and pre-existent vertebral rotation in the normal spine: magnetic resonance imaging study in humans with situs inversus totalis. Spine (Phila Pa 1976). (2007) 32(10):1123–8. doi: 10.1097/01.brs.0000261563.75469.b0
16. Pope MH, Panjabi M. Biomechanical definitions of spinal instability. Spine (Phila Pa 1976). (1985) 10(3):255–6. doi: 10.1097/00007632-198504000-00013
17. Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. (1992) 5(4):383–9. doi: 10.1097/00002517-199212000-00001
18. Archer IA, Dickson RA. Stature and idiopathic scoliosis. A prospective study. J Bone Joint Surg Br. (1985) 67(2):185–8. doi: 10.1302/0301-620X.67B2.3980522
19. Cheung CS, Lee WT, Tse YK, Tang SP, Lee KM, Guo X, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine (Phila Pa 1976). (2003) 28(18):2152–7. doi: 10.1097/01.BRS.0000084265.15201.D5
20. Wang WJ, Hung VW, Lam TP, Ng BK, Qin L, Lee KM, et al. The association of disproportionate skeletal growth and abnormal radius dimension ratio with curve severity in adolescent idiopathic scoliosis. Eur Spine J. (2010) 19(5):726–31. doi: 10.1007/s00586-009-1247-7
21. Tanabe H, Aota Y, Nakamura N, Saito T. A histomorphometric study of the cancellous spinal process bone in adolescent idiopathic scoliosis. Eur Spine J. (2017) 26(6):1600–9. doi: 10.1007/s00586-017-4974-1
22. Wang ZW, Lee WY, Lam TP, Yip BH, Yu FW, Yu WS, et al. Defining the bone morphometry, micro-architecture and volumetric density profile in osteopenic vs. non-osteopenic adolescent idiopathic scoliosis. Eur Spine J. (2017) 26(6):1586–94. doi: 10.1007/s00586-016-4422-7
23. Pope MH, Stokes IA, Moreland M. The biomechanics of scoliosis. Crit Rev Biomed Eng. (1984) 11(3):157–88. 6391812
24. Stokes IA, Burwell RG, Dangerfield PH. Biomechanical spinal growth modulation and progressive adolescent scoliosis–a test of the “vicious cycle” pathogenetic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis. (2006) 1:16. doi: 10.1186/1748-7161-1-16
25. Shi L, Wang D, Driscoll M, Villemure I, Chu WC, Cheng JC, et al. Biomechanical analysis and modeling of different vertebral growth patterns in adolescent idiopathic scoliosis and healthy subjects. Scoliosis. (2011) 6:11. doi: 10.1186/1748-7161-6-11
26. Crijns TJ, Stadhouder A, Smit TH. Restrained differential growth: the initiating event of adolescent idiopathic scoliosis? Spine (Phila Pa 1976). (2017) 42(12):E726–E32. doi: 10.1097/BRS.0000000000001946
27. Guo X, Chau WW, Chan YL, Cheng JC, Burwell RG, Dangerfield PH. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis–result of disproportionate endochondral-membranous bone growth? Summary of an electronic focus group debate of the IBSE. Eur Spine J. (2005) 14(9):862–73. doi: 10.1007/s00586-005-1002-7
28. Dickson RA. The aetiology of spinal deformities. Lancet. (1988) 1(8595):1151–5. doi: 10.1016/S0140-6736(88)91963-0
29. Jelke G, Moller R, Schmidt K. Obstructive diseases of the respiratory tract. 1. Examination methods and diagnostic criteria of various forms of asthma. Med Klin. (1975) 70(45):1839–44. 1105117
30. Hoang DM, Pham PT, Bach TQ, Ngo AT, Nguyen QT, Phan TT, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. (2022) 7(1):272. doi: 10.1038/s41392-022-01134-4
31. Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. (2019) 37(7):855–64. doi: 10.1002/stem.3016
32. Zhuang Q, Li J, Wu Z, Zhang J, Sun W, Li T, et al. Differential proteome analysis of bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. PLoS One. (2011) 6(4):e18834. doi: 10.1371/journal.pone.0018834
33. Gillette JM, Nielsen-Preiss SM. The role of annexin 2 in osteoblastic mineralization. J Cell Sci. (2004) 117(Pt 3):441–9. doi: 10.1242/jcs.00909
34. He S, Li J, Wang Y, Xiang G, Yang G, Xiao L, et al. Phosphorylated heat shock protein 27 improves the bone formation ability of osteoblasts and bone marrow stem cells from patients with adolescent idiopathic scoliosis. JOR Spine. (2023) 6(3):e1256. doi: 10.1002/jsp2.1256
35. Turgut M, Basaloglu HK, Yenisey C, Ozsunar Y. Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur Spine J. (2006) 15(5):605–12. doi: 10.1007/s00586-005-0972-9
36. Oyama J, Murai I, Kanazawa K, Machida M. Bipedal ambulation induces experimental scoliosis in C57BL/6J mice with reduced plasma and pineal melatonin levels. J Pineal Res. (2006) 40(3):219–24. doi: 10.1111/j.1600-079X.2005.00302.x
37. Azeddine B, Letellier K, Wang da S, Moldovan F, Moreau A. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. (2007) 462:45–52. doi: 10.1097/BLO.0b013e31811f39fa
38. Gao W, Lin M, Liang A, Zhang L, Chen C, Liang G, et al. Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells. J Pineal Res. (2014) 56(1):62–70. doi: 10.1111/jpi.12098
39. Zhang L, Su P, Xu C, Chen C, Liang A, Du K, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARgamma expression and enhancing Runx2 expression. J Pineal Res. (2010) 49(4):364–72. doi: 10.1111/j.1600-079X.2010.00803.x
40. Schulman S, Stigendal L, Jansson JH, Brohult J. Haemorrhagic and thromboembolic complications versus intensity of treatment of venous thromboembolism with oral anticoagulants. Acta Med Scand. (1988) 224(5):425–30. doi: 10.1111/j.0954-6820.1988.tb19606.x
41. Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ. Scientific basis for the potential use of melatonin in bone diseases: osteoporosis and adolescent idiopathic scoliosis. J Osteoporos. (2010) 2010:830231. doi: 10.4061/2010/830231
42. Tresguerres IF, Tamimi F, Eimar H, Barralet JE, Prieto S, Torres J, et al. Melatonin dietary supplement as an anti-aging therapy for age-related bone loss. Rejuvenation Res. (2014) 17(4):341–6. doi: 10.1089/rej.2013.1542
43. Liang G, Gao W, Liang A, Ye W, Peng Y, Zhang L, et al. Normal leptin expression, lower adipogenic ability, decreased leptin receptor and hyposensitivity to leptin in adolescent idiopathic scoliosis. PLoS One. (2012) 7(5):e36648. doi: 10.1371/journal.pone.0036648
44. Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. (2005) 20(6):994–1001. doi: 10.1359/JBMR.050103
45. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. (1999) 140(4):1630–8. doi: 10.1210/endo.140.4.6637
46. Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. (2007) 130(5):811–23. doi: 10.1016/j.cell.2007.07.025
47. Börjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Movérare-Skrtic S, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci U S A. (2011) 108(15):6288–93. doi: 10.1073/pnas.1100454108
48. Liu Y, Pan A, Hai Y, Li W, Yin L, Guo R. Asymmetric biomechanical characteristics of the paravertebral muscle in adolescent idiopathic scoliosis. Clin Biomech (Bristol). (2019) 65:81–6. doi: 10.1016/j.clinbiomech.2019.03.013
49. Pan AX, Hai Y, Liu YZ, Zhang YP, Zhang LM, Li WJ, et al. Assessment of biomechanical properties of paraspinal muscles in adolescent idiopathic scoliosis. Zhonghua Yi Xue Za Zhi. (2018) 98(43):3485–9. doi: 10.3760/cma.j.issn.0376-2491.2018.43.005
50. Kou I, Otomo N, Takeda K, Momozawa Y, Lu HF, Kubo M, et al. Genome-wide association study identifies 14 previously unreported susceptibility loci for adolescent idiopathic scoliosis in Japanese. Nat Commun. (2019) 10(1):3685. doi: 10.1038/s41467-019-11596-w
51. Luo M, Yang H, Wu D, You X, Huang S, Song Y. Tent5a modulates muscle fiber formation in adolescent idiopathic scoliosis via maintenance of myogenin expression. Cell Prolif. (2022) 55(3):e13183. doi: 10.1111/cpr.13183
52. Qin X, He Z, Yin R, Qiu Y, Zhu Z. Abnormal paravertebral muscles development is associated with abnormal expression of PAX3 in adolescent idiopathic scoliosis. Eur Spine J. (2020) 29(4):737–43. doi: 10.1007/s00586-019-06217-5
53. Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. (2013) 45(6):676–9. doi: 10.1038/ng.2639
54. Liu Z, Hussien AA, Wang Y, Heckmann T, Gonzalez R, Karner CM, et al. An adhesion G protein-coupled receptor is required in cartilaginous and dense connective tissues to maintain spine alignment. Elife. (2021) 10:e67781. doi: 10.7554/eLife.67781
55. Acaroglu E, Akel I, Alanay A, Yazici M, Marcucio R. Comparison of the melatonin and calmodulin in paravertebral muscle and platelets of patients with or without adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (2009) 34(18):E659–63. doi: 10.1097/BRS.0b013e3181a3c7a2
56. Leboeuf D, Letellier K, Alos N, Edery P, Moldovan F. Do estrogens impact adolescent idiopathic scoliosis? Trends Endocrinol Metab. (2009) 20(4):147–52. doi: 10.1016/j.tem.2008.12.004
57. Kotwicki T, Tomaszewski M, Andrusiewicz M, Sliwa A, Rusin B, Kotwicka M. Estrogen receptor type 1 and type 2 presence in paravertebral skeletal muscles: expression level and relation to phenotype in children with idiopathic scoliosis. Genes (Basel). (2022) 13(5):739. doi: 10.3390/genes13050739
58. Lao ML, Chow DH, Guo X, Cheng JC, Holmes AD. Impaired dynamic balance control in adolescents with idiopathic scoliosis and abnormal somatosensory evoked potentials. J Pediatr Orthop. (2008) 28(8):846–9. doi: 10.1097/BPO.0b013e31818e1bc9
59. Geissele AE, Kransdorf MJ, Geyer CA, Jelinek JS, Van Dam BE. Magnetic resonance imaging of the brain stem in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (1991) 16(7):761–3. doi: 10.1097/00007632-199107000-00013
60. Chwala W, Koziana A, Kasperczyk T, Walaszek R, Plaszewski M. Electromyographic assessment of functional symmetry of paraspinal muscles during static exercises in adolescents with idiopathic scoliosis. Biomed Res Int. (2014) 2014:573276. doi: 10.1155/2014/573276
61. Cheng JC, Guo X, Sher AH, Chan YL, Metreweli C. Correlation between curve severity, somatosensory evoked potentials, and magnetic resonance imaging in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (. 1999) 24(16):1679–84. doi: 10.1097/00007632-199908150-00009
62. Yonezawa Y, Guo L, Kakinuma H, Otomo N, Yoshino S, Takeda K, et al. Identification of a functional susceptibility variant for adolescent idiopathic scoliosis that upregulates early growth response 1 (EGR1)-mediated UNCX expression. J Bone Miner Res. (2023) 38(1):144–53. doi: 10.1002/jbmr.4738
63. Rose CD, Pompili D, Henke K, Van Gennip JL, Meyer-Miner A, Rana R, et al. SCO-spondin defects and neuroinflammation are conserved mechanisms driving spinal deformity across genetic models of idiopathic scoliosis. Curr Biol. (2020) 30(12):2363–73 e6. doi: 10.1016/j.cub.2020.04.020
64. Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. (2003) 289(5):559–67. doi: 10.1001/jama.289.5.559
65. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. (2008) 371(9623):1527–37. doi: 10.1016/S0140-6736(08)60658-3
66. Behrbalk E, Uri O, Clamp JA, Rickert M, Boszczyk BM. Bilateral reconstructive costoplasty for razorback deformity correction in adolescent idiopathic scoliosis. Eur Spine J. (2015) 24(2):234–41. doi: 10.1007/s00586-014-3619-x
67. Cho JH, Lee CS, Joo YS, Park J, Hwang CJ, Lee DH. Association between sacral slanting and adjacent structures in patients with adolescent idiopathic scoliosis. Clin Orthop Surg. (2017) 9(1):57–62. doi: 10.4055/cios.2017.9.1.57
68. Daryabor A, Arazpour M, Sharifi G, Bani MA, Aboutorabi A, Golchin N. Gait and energy consumption in adolescent idiopathic scoliosis: a literature review. Ann Phys Rehabil Med. (2017) 60(2):107–16. doi: 10.1016/j.rehab.2016.10.008
69. Mahaudens P, Banse X, Mousny M, Detrembleur C. Gait in adolescent idiopathic scoliosis: kinematics and electromyographic analysis. Eur Spine J. (2009) 18(4):512–21. doi: 10.1007/s00586-009-0899-7
70. Yang JH, Suh SW, Sung PS, Park WH. Asymmetrical gait in adolescents with idiopathic scoliosis. Eur Spine J. (2013) 22(11):2407–13. doi: 10.1007/s00586-013-2845-y
71. Sato T, Hirano T, Ito T, Morita O, Kikuchi R, Endo N, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. (2011) 20(2):274–9. doi: 10.1007/s00586-010-1657-6
72. Mayo NE, Goldberg MS, Poitras B, Scott S, Hanley J. The Ste-Justine adolescent idiopathic scoliosis cohort study. Part III: back pain. Spine (Phila Pa 1976). (1994) 19(14):1573–81. doi: 10.1097/00007632-199407001-00005
73. Weinstein SL. The natural history of adolescent idiopathic scoliosis. J Pediatr Orthop. (2019) 39(6):S44–S6. doi: 10.1097/BPO.0000000000001350
74. An JK, Berman D, Schulz J. Back pain in adolescent idiopathic scoliosis: a comprehensive review. J Child Orthop. (2023) 17(2):126–40. doi: 10.1177/18632521221149058
75. Bisson DG, Lama P, Abduljabbar F, Rosenzweig DH, Saran N, Ouellet JA, et al. Facet joint degeneration in adolescent idiopathic scoliosis. JOR Spine. (2018) 1(2):e1016. doi: 10.1002/jsp2.1016
76. Jaumard NV, Welch WC, Winkelstein BA. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J Biomech Eng. (2011) 133(7):071010. doi: 10.1115/1.4004493
77. Manchikanti L, Singh V, Pampati V, Damron KS, Beyer CD, Barnhill RC. Is there correlation of facet joint pain in lumbar and cervical spine? An evaluation of prevalence in combined chronic low back and neck pain. Pain Physician. (2002) 5(4):365–71. doi: 10.36076/ppj.2002/5/365
78. Bisson DG, Sheng K, Kocabas S, Ocay DD, Ferland CE, Saran N, et al. Axial rotation and pain are associated with facet joint osteoarthritis in adolescent idiopathic scoliosis. Osteoarthritis Cartilage. (2023) 31(8):1101–10. doi: 10.1016/j.joca.2023.03.007
79. Samaan MC, Missiuna P, Peterson D, Thabane L. Understanding the role of the immune system in adolescent idiopathic scoliosis: Immunometabolic CONnections to Scoliosis (ICONS) study protocol. BMJ Open. (2016) 6(7):e011812. doi: 10.1136/bmjopen-2016-011812
80. Wajchenberg M, Martins DE, de Paiva Luciano R, Puertas EB, Del Curto D, Schmidt B, et al. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: a cross-sectional study. Medicine (Baltimore). (2015) 94(8):e598. doi: 10.1097/MD.0000000000000598
81. Abdelaal AAM, Abd El Kafy E, Elayat M, Sabbahi M, Badghish MSS. Changes in pulmonary function and functional capacity in adolescents with mild idiopathic scoliosis: observational cohort study. J Int Med Res. (2018) 46(1):381–91. doi: 10.1177/0300060517715375
82. Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine (Phila Pa 1976). (2007) 32(24):2685–93. doi: 10.1097/BRS.0b013e31815a7b17
83. Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty-one patients. J Bone Joint Surg Am. (2005) 87(9):1937–46. doi: 10.2106/JBJS.D.02209
84. Leong JC, Lu WW, Luk KD, Karlberg EM. Kinematics of the chest cage and spine during breathing in healthy individuals and in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (1999) 24(13):1310–5. doi: 10.1097/00007632-199907010-00007
85. Wozniczka JK, Ledonio CGT, Polly DW Jr., Rosenstein BE, Nuckley DJ. Adolescent idiopathic scoliosis thoracic volume modeling: the effect of surgical correction. J Pediatr Orthop. (2017) 37(8):e512–e8. doi: 10.1097/BPO.0000000000000728
86. Johnston CE, Richards BS, Sucato DJ, Bridwell KH, Lenke LG, Erickson M, et al. Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (2011) 36(14):1096–102. doi: 10.1097/BRS.0b013e3181f8c931
87. Kempen DH, Heemskerk JL, Kaçmaz G, Altena MC, Reesink HJ, Vanhommerig JW, et al. Pulmonary function in children and adolescents with untreated idiopathic scoliosis: a systematic review with meta-regression analysis. Spine J. (2022) 22(7):1178–90. doi: 10.1016/j.spinee.2021.12.011
88. Kan MM, Negrini S, Di Felice F, Cheung JP, Donzelli S, Zaina F, et al. Is impaired lung function related to spinal deformities in patients with adolescent idiopathic scoliosis? A systematic review and meta-analysis-SOSORT 2019 award paper. Eur Spine J. (2023) 32(1):118–39. doi: 10.1007/s00586-022-07371-z
89. Tsiligiannis T, Grivas T. Pulmonary function in children with idiopathic scoliosis. Scoliosis. (2012) 7(1):7. doi: 10.1186/1748-7161-7-7
90. Li S, Yang J, Li Y, Zhu L, Lin Y, Li X, et al. Right ventricular function impaired in children and adolescents with severe idiopathic scoliosis. Scoliosis. (2013) 8(1):1. doi: 10.1186/1748-7161-8-1
91. Bettany-Saltikov J, Parent E, Romano M, Villagrasa M, Negrini S. Physiotherapeutic scoliosis-specific exercises for adolescents with idiopathic scoliosis. Eur J Phys Rehabil Med. (2014) 50(1):111–21. 24525556
92. Negrini S, Antonini G, Carabalona R, Minozzi S. Physical exercises as a treatment for adolescent idiopathic scoliosis. A systematic review. Pediatr Rehabil. (2003) 6(3-4):227–35. doi: 10.1080/13638490310001636781
93. Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC, et al. 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. (2018) 13:3. doi: 10.1186/s13013-017-0145-8
94. Weiss HR. The method of Katharina Schroth—history, principles and current development. Scoliosis. (2011) 6:17. doi: 10.1186/1748-7161-6-17
95. Schreiber S, Parent EC, Khodayari Moez E, Hedden DM, Hill DL, Moreau M, et al. Schroth physiotherapeutic scoliosis-specific exercises added to the standard of care lead to better cobb angle outcomes in adolescents with idiopathic scoliosis—an assessor and statistician blinded randomized controlled trial. PLoS One. (2016) 11(12):e0168746. doi: 10.1371/journal.pone.0168746
96. Ceballos-Laita L, Carrasco-Uribarren A, Cabanillas-Barea S, Perez-Guillen S, Pardos-Aguilella P, Jimenez Del Barrio S. The effectiveness of Schroth method in Cobb angle, quality of life and trunk rotation angle in adolescent idiopathic scoliosis: a systematic review and meta-analysis. Eur J Phys Rehabil Med. (2023) 59(2):228–36. doi: 10.23736/S1973-9087.23.07654-2
97. Baumann AN, Orellana K, Oleson CJ, Curtis DP, Cahill P, Flynn J, et al. The impact of patient scoliosis-specific exercises for adolescent idiopathic scoliosis: a systematic review and meta-analysis of randomized controlled trials with subgroup analysis using observational studies. Spine Deform. (2024) 12(3):545–59. doi: 10.1007/s43390-023-00810-x
98. Wang Z, Zhu W, Li G, Guo X. Comparative efficacy of six types of scoliosis-specific exercises on adolescent idiopathic scoliosis: a systematic review and network meta-analysis. BMC Musculoskelet Disord. (2024) 25(1):1070. doi: 10.1186/s12891-024-08223-1
99. Jiang Y, Peng H, Song Y, Huang L, Chen H, Li P, et al. Evaluating exercise therapies in adolescent idiopathic scoliosis: a systematic review with Bayesian network meta-analysis. PeerJ. (2025) 13:e19175. doi: 10.7717/peerj.19175
100. Chen Y, Zhang Z, Zhu Q. The effect of an exercise intervention on adolescent idiopathic scoliosis: a network meta-analysis. J Orthop Surg Res. (2023) 18(1):655. doi: 10.1186/s13018-023-04137-1
101. Berdishevsky H, Lebel VA, Bettany-Saltikov J, Rigo M, Lebel A, Hennes A, et al. Physiotherapy scoliosis-specific exercises—a comprehensive review of seven major schools. Scoliosis Spinal Disord. (2016) 11:20. doi: 10.1186/s13013-016-0076-9
102. Seleviciene V, Cesnaviciute A, Strukcinskiene B, Marcinowicz L, Strazdiene N, Genowska A. Physiotherapeutic scoliosis-specific exercise methodologies used for conservative treatment of adolescent idiopathic scoliosis, and their effectiveness: an extended literature review of current research and practice. Int J Environ Res Public Health. (2022) 19(15):9240. doi: 10.3390/ijerph19159240
103. Durmala J, Dobosiewicz K, Kotwicki T, Jendrzejek H. Influence of asymmetric mobilisation of the trunk on the Cobb angle and rotation in idiopathic scoliosisin children and adolescents. Ortop Traumatol Rehabil. (2003) 5(1):80–5. 17679865
104. Dobosiewicz K, Durmala J, Jendrzejek H, Czernicki K. Influence of method of asymmetric trunk mobilization on shaping of a physiological thoracic kyphosis in children and youth suffering from progressive idiopathic scoliosis. Stud Health Technol Inform. (2002) 91:348–51. 15457753
105. Dobosiewicz K, Durmala J, Czernicki K, Piotrowski J. Radiological results of Dobosiewicz method of three-dimensional treatment of progressive idiopathic scoliosis. Stud Health Technol Inform. (2006) 123:267–72. 17108438
106. Day JM, Fletcher J, Coghlan M, Ravine T. Review of scoliosis-specific exercise methods used to correct adolescent idiopathic scoliosis. Arch Physiother. (2019) 9:8. doi: 10.1186/s40945-019-0060-9
107. Maruyama T, Kitagawa T, Takeshita K, Mochizuki K, Nakamura K. Conservative treatment for adolescent idiopathic scoliosis: can it reduce the incidence of surgical treatment? Pediatr Rehabil. (2003) 6(3-4):215–9. doi: 10.1080/13638490310001642748
108. Mamyama T, Kitagawal T, Takeshita K, Nakainura K. Side shift exercise for idiopathic scoliosis after skeletal maturity. Stud Health Technol Inform. (2002) 91:361–4. 15457756
109. Liu X, Wang Y, Liu M, Zhang Y, Wu Q, Wang Q. The efficacy of core stabilization exercise in mild and moderate adolescent idiopathic scoliosis: a systematic review and meta-analysis. J Orthop Surg Res. (2025) 20(1):214. doi: 10.1186/s13018-025-05612-7
110. Li K, Miao J, Zhang J. Network meta-analysis of short-term effects of different strategies in the conservative treatment of AIS. Eur J Med Res. (2021) 26(1):54. doi: 10.1186/s40001-021-00526-6
111. Kastrinis A, Koumantakis G, Tsekoura M, Nomikou E, Katsoulaki M, Theodosopoulos E, et al. The effect of schroth method on postural control and balance in patients with adolescent idiopathic scoliosis: a literature review. Adv Exp Med Biol. (2023) 1425:469–76. doi: 10.1007/978-3-031-31986-0_45
112. Blount WP, Schmidt AC, Keever ED, Leonard ET. The Milwaukee brace in the operative treatment of scoliosis. J Bone Joint Surg Am. (1958) 40-A(3):511–25. doi: 10.2106/00004623-195840030-00003
113. Zaina F, De Mauroy JC, Grivas T, Hresko MT, Kotwizki T, Maruyama T, et al. Bracing for scoliosis in 2014: state of the art. Eur J Phys Rehabil Med. (2014) 50(1):93–110. 24622051
114. Grivas TB, Kaspiris A. European braces widely used for conservative scoliosis treatment. Stud Health Technol Inform. (2010) 158:157–66. 20543417
115. Lee YJ, Wang WJ, Mohamad SM, Chandren JR, Gani SMA, Chung WH, et al. A comparison between Boston brace and European braces in the treatment of adolescent idiopathic scoliosis (AIS) patients: a systematic review based on the standardised Scoliosis Research Society (SRS) inclusion criteria for brace treatment. Eur Spine J. (2024) 33(2):630–45. doi: 10.1007/s00586-023-08007-6
116. Negrini S, Aulisa AG, Aulisa L, Circo AB, De Mauroy JC, Durmala J, et al. 2011 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis. (2012) 7(1):3. doi: 10.1186/1748-7161-7-3
117. Negrini S, Zaina F, Atanasio S. BRACE MAP, a proposal for a new classification of braces. Stud Health Technol Inform. (2008) 140:299–302. doi: 10.3233/978-1-58603-888-5-299
118. Maruyama T, Takesita K, Kitagawa T, Nakao Y. Milwaukee brace. Physiother Theory Pract. (2011) 27(1):43–6. doi: 10.3109/09593985.2010.503992
119. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. (2016) 24(8):555–64. doi: 10.5435/JAAOS-D-14-00416
120. Wiley JW, Thomson JD, Mitchell TM, Smith BG, Banta JV. Effectiveness of the Boston brace in treatment of large curves in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (2000) 25(18):2326–32. doi: 10.1097/00007632-200009150-00010
121. Lee CS, Hwang CJ, Kim DJ, Kim JH, Kim YT, Lee MY, et al. Effectiveness of the Charleston night-time bending brace in the treatment of adolescent idiopathic scoliosis. J Pediatr Orthop. (2012) 32(4):368–72. doi: 10.1097/BPO.0b013e3182561193
122. Stokes IA. Mechanical modulation of spinal growth and progression of adolescent scoliosis. Stud Health Technol Inform. (2008) 135:75–83. 18401082
123. Coillard C, Leroux MA, Zabjek KF, Rivard CH. Spinecor–a non-rigid brace for the treatment of idiopathic scoliosis: post-treatment results. Eur Spine J. (2003) 12(2):141–8. doi: 10.1007/s00586-002-0467-x
124. Atanasio S, Zaina F, Negrini S. The Sforzesco brace and SPoRT concept: a brace to replace cast in worst curves. Disabil Rehabil Assist Technol. (2008) 3(3):154–60. doi: 10.1080/17483100801905843
125. Negrini S, Atanasio S, Negrini F, Zaina F, Marchini G. The Sforzesco brace can replace cast in the correction of adolescent idiopathic scoliosis: a controlled prospective cohort study. Scoliosis. (2008) 3:15. doi: 10.1186/1748-7161-3-15
126. Landauer F, Trieb KP. Scoliosis: brace treatment—from the past 50 years to the future. Medicine (Baltimore). (2022) 101(37):e30556. doi: 10.1097/MD.0000000000030556
127. Lonner BS, Ren Y, Yaszay B, Cahill PJ, Shah SA, Betz RR, et al. Evolution of surgery for adolescent idiopathic scoliosis over 20 years: have outcomes improved? Spine (Phila Pa 1976). (2018) 43(6):402–10. doi: 10.1097/BRS.0000000000002332
128. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. (2006) 1(1):2. doi: 10.1186/1748-7161-1-2
129. Burton DC, Sama AA, Asher MA, Burke SW, Boachie-Adjei O, Huang RC, et al. The treatment of large (>70 degrees) thoracic idiopathic scoliosis curves with posterior instrumentation and arthrodesis: when is anterior release indicated? Spine (Phila Pa 1976). (2005) 30(17):1979–84. doi: 10.1097/01.brs.0000176196.94565.d6
130. Addai D, Zarkos J, Bowey AJ. Current concepts in the diagnosis and management of adolescent idiopathic scoliosis. Childs Nerv Syst. (2020) 36(6):1111–9. doi: 10.1007/s00381-020-04608-4
131. Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am. (1962) 44-A:591–610. doi: 10.2106/00004623-196244040-00001
132. King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. (1983) 65(9):1302–13. doi: 10.2106/00004623-198365090-00012
133. Bazin L, Ansorge A, Vendeuvre T, Cochard B, Tabard-Fougère A, Vazquez O, et al. Minimally invasive surgery for posterior spinal instrumentation and fusion in adolescent idiopathic scoliosis: current Status and future application. Children (Basel). (2023) 10(12):1882.38136084
134. Dwyer AF, Schafer MF. Anterior approach to scoliosis. Results of treatment in fifty-one cases. J Bone Joint Surg Br. (1974) 56(2):218–24. doi: 10.1302/0301-620X.56B2.218
135. Kohler R, Galland O, Mechin H, Michel CR, Onimus M. The Dwyer procedure in the treatment of idiopathic scoliosis. A 10-year follow-up review of 21 patients. Spine (Phila Pa 1976). (1990) 15(2):75–80. doi: 10.1097/00007632-199002000-00005
136. Hsu LC, Zucherman J, Tang SC, Leong JC. Dwyer instrumentation in the treatment of adolescent idiopathic scoliosis. J Bone Joint Surg Br. (1982) 64(5):536–41. doi: 10.1302/0301-620X.64B5.7142261
137. Lonner BS, Kondrashov D, Siddiqi F, Hayes V, Scharf C. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. (2006) 88(5):1022–34. doi: 10.2106/JBJS.E.00001
138. Sarwahi V, Wollowick AL, Sugarman EP, Horn JJ, Gambassi M, Amaral TD. Minimally invasive scoliosis surgery: an innovative technique in patients with adolescent idiopathic scoliosis. Scoliosis. (2011) 6:16. doi: 10.1186/1748-7161-6-16
139. de Bodman C, Miyanji F, Borner B, Zambelli PY, Racloz G, Dayer R. Minimally invasive surgery for adolescent idiopathic scoliosis: correction of deformity and peri-operative morbidity in 70 consecutive patients. Bone Joint J. (2017) 99-B(12):1651–7. doi: 10.1302/0301-620X.99B12.BJJ-2017-0022.R2
140. Fiore M, Ruffilli A, Viroli G, Barile F, Manzetti M, Faldini C. Minimally invasive surgery using posterior-only pedicle screw fixation in treatment of adolescent idiopathic scoliosis: a systematic review and meta-analysis. J Clin Neurosci. (2022) 99:317–26. doi: 10.1016/j.jocn.2022.03.019
141. Bazin L, Ansorge A, Vendeuvre T, Cochard B, Tabard-Fougère A, Vazquez O, et al. Minimally invasive surgery in patients with adolescent idiopathic scoliosis: is it better than the standard approach? A 2-year follow-up study. Clin Spine Surg. (2016) 29(8):331–40. doi: 10.1097/BSD.0000000000000106
142. Harrison Farber S, Nayar G, Desai R, Reiser EW, Byrd SA, Chi D, et al. Radiation exposure to the surgeon during minimally invasive spine procedures is directly estimated by patient dose. Eur Spine J. (2018) 27(8):1911–7. doi: 10.1007/s00586-018-5653-6
143. Green DW, Lawhorne TW, Widmann RF 3rd, Kepler CK, Ahern C, Mintz DN, et al. Long-term magnetic resonance imaging follow-up demonstrates minimal transitional level lumbar disc degeneration after posterior spine fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976). (2011) 36(23):1948–54. doi: 10.1097/BRS.0b013e3181ff1ea9
144. Kepler CK, Meredith DS, Green DW, Widmann RF. Long-term outcomes after posterior spine fusion for adolescent idiopathic scoliosis. Curr Opin Pediatr. (2012) 24(1):68–75. doi: 10.1097/MOP.0b013e32834ec982
145. Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case-control study. Spine (Phila Pa 1976). (2006) 31(3):275–83. doi: 10.1097/01.brs.0000197652.52890.71
146. Lonner BS, Ren Y, Upasani VV, Marks MM, Newton PO, Samdani AF, et al. Disc degeneration in unfused caudal motion segments ten years following surgery for adolescent idiopathic scoliosis. Spine Deform. (2018) 6(6):684–90. doi: 10.1016/j.jspd.2018.03.013
147. Stokes IA, Spence H, Aronsson DD, Kilmer N. Mechanical modulation of vertebral body growth. Implications for scoliosis progression. Spine (Phila Pa 1976). (1996) 21(10):1162–7. doi: 10.1097/00007632-199605150-00007
148. Mehlman CT, Araghi A, Roy DR. Hyphenated history: the Hueter-Volkmann law. Am J Orthop (Belle Mead NJ). (1997) 26(11):798–800. 9402217
149. Newton PO, Fricka KB, Lee SS, Farnsworth CL, Cox TG, Mahar AT. Asymmetrical flexible tethering of spine growth in an immature bovine model. Spine (Phila Pa 1976). (2002) 27(7):689–93. doi: 10.1097/00007632-200204010-00004
150. Newton PO, Farnsworth CL, Faro FD, Mahar AT, Odell TR, Mohamad F, et al. Spinal growth modulation with an anterolateral flexible tether in an immature bovine model: disc health and motion preservation. Spine (Phila Pa 1976). (2008) 33(7):724–33. doi: 10.1097/BRS.0b013e31816950a0
151. Wong DL, Mong PT, Ng CY, Ong CK, Qian Z, Shao MH, et al. Can anterior vertebral body tethering provide superior range of motion outcomes compared to posterior spinal fusion in adolescent idiopathic scoliosis? A systematic review. Eur Spine J. (2023) 32(9):3058–71. doi: 10.1007/s00586-023-07787-1
152. Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, et al. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976). (2014) 39(20):1688–93. doi: 10.1097/BRS.0000000000000472
153. Mariscal G, Morales J, Pérez S, Rubio-Belmar PA, Bovea-Marco M, Bas JL, et al. Meta-analysis on the efficacy and safety of anterior vertebral body tethering in adolescent idiopathic scoliosis. Eur Spine J. (2023) 32(1):140–8. doi: 10.1007/s00586-022-07448-9
154. Parent S, Shen J. Anterior vertebral body growth-modulation tethering in idiopathic scoliosis: surgical technique. J Am Acad Orthop Surg. (2020) 28(17):693–9. doi: 10.5435/JAAOS-D-19-00849
155. Newton PO. Spinal growth tethering: indications and limits. Ann Transl Med. (2020) 8(2):27. doi: 10.21037/atm.2019.12.159
156. Akbarnia BA, Breakwell LM, Marks DS, McCarthy RE, Thompson AG, Canale SK, et al. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine (Phila Pa 1976). (2008) 33(9):984–90. doi: 10.1097/BRS.0b013e31816c8b4e
157. Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976). (2005) 30(17 Suppl):S46–57. doi: 10.1097/01.brs.0000175190.08134.73
158. Thompson GH, Akbarnia BA, Campbell RM Jr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. (2007) 27(3):354–61. doi: 10.1097/BPO.0b013e3180333eea
159. Elsebai HB, Yazici M, Thompson GH, Emans JB, Skaggs DL, Crawford AH, et al. Safety and efficacy of growing rod technique for pediatric congenital spinal deformities. J Pediatr Orthop. (2011) 31(1):1–5. doi: 10.1097/BPO.0b013e318202c1f0
160. Thompson GH, Akbarnia BA, Kostial P, Poe-Kochert C, Armstrong DG, Roh J, et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976). (2005) 30(18):2039–44. doi: 10.1097/01.brs.0000179082.92712.89
161. Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. (2010) 92(15):2533–43. doi: 10.2106/JBJS.I.01471
162. Cheung KM, Cheung JP, Samartzis D, Mak KC, Wong YW, Cheung WY, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. (2012) 379(9830):1967–74. doi: 10.1016/S0140-6736(12)60112-3
163. Wick JM, Konze J. A magnetic approach to treating progressive early-onset scoliosis. AORN J. (2012) 96(2):163–73. doi: 10.1016/j.aorn.2012.05.008
164. Hickey BA, Towriss C, Baxter G, Yasso S, James S, Jones A, et al. Early experience of MAGEC magnetic growing rods in the treatment of early onset scoliosis. Eur Spine J. (2014) 23(Suppl 1):S61–5. doi: 10.1007/s00586-013-3163-0
165. Akbarnia BA, Cheung K, Noordeen H, Elsebaie H, Yazici M, Dannawi Z, et al. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976). (2013) 38(8):665–70. doi: 10.1097/BRS.0b013e3182773560
166. Gilday SE, Schwartz MS, Bylski-Austrow DI, Glos DL, Schultz L, O’Hara S, et al. Observed length increases of magnetically controlled growing rods are lower than programmed. J Pediatr Orthop. (2018) 38(3):e133–e7. doi: 10.1097/BPO.0000000000001119
167. Morell SM, McCarthy RE. New developments in the treatment of early-onset spinal deformity: role of the Shilla growth guidance system. Med Devices (Auckl). (2016) 9:241–6. doi: 10.2147/MDER.S77657
168. McCarthy RE, Sucato D, Turner JL, Zhang H, Henson MA, McCarthy K. Shilla growing rods in a caprine animal model: a pilot study. Clin Orthop Relat Res. (2010) 468(3):705–10. doi: 10.1007/s11999-009-1028-y
169. McCarthy RE, McCullough FL. Shilla growth guidance for early-onset scoliosis: results after a minimum of five years of follow-up. J Bone Joint Surg Am. (2015) 97(19):1578–84. doi: 10.2106/JBJS.N.01083
170. Wilkinson JT, Songy CE, Bumpass DB, McCullough FL, McCarthy RE. Curve modulation and apex migration using shilla growth guidance rods for early-onset scoliosis at 5-year follow-up. J Pediatr Orthop. (2019) 39(8):400–5. doi: 10.1097/BPO.0000000000000983
171. McCarthy RE, Luhmann S, Lenke L, McCullough FL. The Shilla growth guidance technique for early-onset spinal deformities at 2-year follow-up: a preliminary report. J Pediatr Orthop. (2014) 34(1):1–7. doi: 10.1097/BPO.0b013e31829f92dc
172. Toth JM, Ankomah F, Kawakami N, Uno K. A comparison of the inflammatory host response to particulate debris adjacent to unlocked and locked screws of a growth guidance system for early onset scoliosis. Eur Spine J. (2022) 31(9):2301–10. doi: 10.1007/s00586-022-07271-2
173. Ru L, Zheng H, Lian W, Zhao S, Fan Q. Knowledge mapping of idiopathic scoliosis genes and research hotspots (2002–2022): a bibliometric analysis. Front Pediatr. (2023) 11:1177983. doi: 10.3389/fped.2023.1177983
174. Scaturro D, Balbo A, Vitagliani F, Stramazzo L, Camarda L, Letizia Mauro G. Is there a relationship between idiopathic scoliosis and body mass? A scoping review. Nutrients. (2022) 14(19):4011. doi: 10.3390/nu14194011
175. Trobisch P, Suess O, Schwab F. Idiopathic scoliosis. Dtsch Arztebl Int. (2010) 107(49):875–83. doi: 10.3238/arztebl.2010.0875
176. Iyer P, Hwang M, Ridley L, Weisman MM. Biomechanics in the onset and severity of spondyloarthritis: a force to be reckoned with. RMD Open. (2023) 9(4):e003372. doi: 10.1136/rmdopen-2023-003372
177. Roe S. Biomechanics of fracture fixation. Vet Clin North Am Small Anim Pract. (2020) 50(1):1–15. doi: 10.1016/j.cvsm.2019.08.009
178. Dowdell J, Kim J, Overley S, Hecht A. Biomechanics and common mechanisms of injury of the cervical spine. Handb Clin Neurol. (2018) 158:337–44. doi: 10.1016/B978-0-444-63954-7.00031-8
179. Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J (Engl). (2020) 133(4):483–93. doi: 10.1097/CM9.0000000000000652
180. Gargano G, Oliva F, Migliorini F, Maffulli N. Melatonin and adolescent idiopathic scoliosis: the present evidence. Surgeon. (2022) 20(6):e315–e21. doi: 10.1016/j.surge.2021.07.008
181. Richards BS, Bernstein RM, D'Amato CR, Thompson GH. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS committee on bracing and nonoperative management. Spine (Phila Pa 1976). (2005) 30(18):2068–75. doi: 10.1097/01.brs.0000178819.90239.d0
182. Negrini S, Minozzi S, Bettany-Saltikov J, Chockalingam N, Grivas TB, Kotwicki T, et al. Braces for idiopathic scoliosis in adolescents. Cochrane Database Syst Rev. (2015) 2015(6):CD006850. doi: 10.1002/14651858.CD006850.pub3
Keywords: adolescent idiopathic scoliosis, biomechanics, pathogenesis, clinical manifestations, treatment strategies
Citation: Li D, Mo H and Yang S (2025) From a biomechanical perspective: pathogenesis, clinical manifestations and treatment strategies of adolescent idiopathic scoliosis. Front. Pediatr. 13:1649483. doi: 10.3389/fped.2025.1649483
Received: 19 June 2025; Accepted: 22 September 2025;
Published: 16 October 2025.
Edited by:
Davide Bizzoca, University of Bari Aldo Moro, ItalyReviewed by:
Jean Claude De Mauroy, Independent Researcher, Lyon, FranceVanja Dimitrijević, University of Novi Sad, Serbia
Copyright: © 2025 Li, Mo and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siying Yang, WXN5aW5nNDFAMTYzLmNvbQ==
 Dongmei Li1,2
Dongmei Li1,2 Haokun Mo
Haokun Mo Siying Yang
Siying Yang