- Department of Pediatrics, WuXi No.2 People’s Hospital, Wuxi, Jiangsu, China
Objectives: Attention-Deficit/Hyperactivity Disorder (ADHD) affects approximately 5% of children globally and is characterized by symptoms of inattention, hyperactivity, and impulsivity. This study examines the impact of a multi-micronutrient nutritional formula combined with aerobic exercise on ADHD symptoms, cognitive function, specifically the domain of executive function, and sleep quality.
Methods: A retrospective analysis was conducted on 220 children aged 6–12 years diagnosed with ADHD according to the American Psychiatric Association (APA) guidelines, who had been treated at WuXi No.2 People's Hospital from November 2022 to October 2024. Participants were divided into two groups: the Multi-Micronutrient Nutritional Formula (MNF) group (n = 111) who received a three-month dietary supplementation and a MNF combined with Aerobic Exercise (MNF-AE) group (n = 109) who received both the nutritional formula and daily aerobic exercise. Evaluations included the Conners Parent Symptom Questionnaire (PSQ), Stroop Test, Wisconsin Card Sorting Test (WCST), Alternate Uses Task, and the Children's Sleep Habits Questionnaire (CSHQ).
Results: Post-treatment, the MNF-AE group exhibited significant improvements in ADHD symptoms, demonstrated by reduced PSQ scores (mean 30.75 ± 3.87) compared to the MNF group (32.42 ± 4.17; P = 0.002). The MNF-AE group also showed enhanced cognitive processing speed and selective attention, evidenced by faster Stroop Test times (Stroop Word: 23.03 ± 7.53 s vs. 25.86 ± 5.37 s; P = 0.002). Executive function improved significantly, as evidenced by higher total correct scores in the WCST (76.98 ± 10.74 vs. 73.75 ± 12.44; P = 0.041) and creativity (assessed by the Alternate Uses Task) improved in originality and fluency (all P < 0.05). Moreover, the MNF-AE group had better overall sleep quality as indicated by lower CSHQ scores (72.63 ± 6.87 vs. 74.95 ± 6.22; P = 0.009).
Conclusions: Combining multi-micronutrient supplementation with aerobic exercise may offer potential benefits for managing ADHD symptoms, improving cognitive function, as well as enhancing sleep quality in children with ADHD. These findings suggest potential additive effects of this combined intervention.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a prevalent neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity. Affecting approximately 5% of children worldwide (1), ADHD poses significant challenges not only to affected individuals but also to families, educators, and health care systems (2, 3). The etiology of ADHD is multifaceted, involving a combination of genetic, environmental, and neurobiological factors. This complexity necessitates diverse therapeutic approaches that target various dimensions of the disorder. Traditional management strategies primarily involve pharmacological interventions such as psychostimulants and non-stimulant medications (4–6). However, these treatments may not adequately address all aspects of ADHD, often prompting the exploration of alternative and complementary therapeutic options.
Emerging evidence suggests that nutritional and lifestyle modifications can play a crucial role in ADHD management, potentially addressing underlying physiological and cognitive deficits. However, as highlighted by Lange et al. (7), despite promising preliminary findings on the benefits of minerals and specific diets, there remains insufficient evidence to recommend the use of micronutrients or probiotics, underscoring the need for further comprehensive studies. Nutritional factors, including deficiencies in essential vitamins and minerals, have been implicated in the pathophysiology of ADHD. Micronutrients such as zinc, magnesium, iron, and B-complex vitamins are fundamental to neurotransmitter synthesis and brain function. For instance, zinc and magnesium are integral to dopamine regulation, a neurotransmitter critical for attention and impulse control (8, 9). Iron is essential for dopamine biosynthesis, with deficiencies linked to more severe inattentiveness, restlessness, and hyperactivity (10, 11). B vitamins further contribute to cognitive health by supporting neurotransmitter metabolism and reducing oxidative stress, which may ameliorate ADHD symptoms (12, 13).
Concurrent with nutritional considerations, physical exercise is gaining recognition as a beneficial adjunct therapy for ADHD. Regular physical activity has been associated with enhanced cognitive performance and bolstered brain health (14). Aerobic exercise, in particular, has demonstrated efficacy in improving attention and executive function in children with ADHD (15). These cognitive benefits have been hypothesized to stem from increased cerebral blood flow, upregulation of neurotrophins such as brain-derived neurotrophic factor (BDNF), and modifications in catecholaminergic neurotransmission (16). Collectively, these changes support synaptic plasticity, neurogenesis, and optimal neurotransmitter availability, which are crucial for attenuating ADHD symptoms such as inattention, hyperactivity, and impulsivity (17, 18).
The integration of multi-micronutrient formulations with aerobic exercise presents a promising multimodal approach to ADHD management. By concurrently addressing nutritional deficiencies and promoting physical fitness, this strategy offers the potential to enhance both neurological and physiological aspects of health. Previous research suggests that combined interventions may result in substantial improvements in attention, behavior, and cognitive functions (19). However, comprehensive investigations exploring these combined effects remain sparse, necessitating further research to elucidate the underlying mechanisms and optimize intervention protocols.
To address this gap, the present study evaluated the impact of a multi-micronutrient nutritional formula combined with aerobic exercise on key outcomes in children aged 6–12 years diagnosed with ADHD. This study aimed to comprehensively evaluate the benefits of a combined multi-micronutrient nutritional formula with aerobic exercise on symptom reduction, cognitive enhancement, improved executive function, and better sleep quality in children with ADHD.
Methods
Case selection: participant identification and grouping
Ethics statement: approval and consent waiver
The study received approval from the Institutional Review Board and Ethics Committee of WuXi No.2 People's Hospital. Since the research exclusively utilized de-identified patient data, posing no potential harm or impact on participants, the need for informed consent was waived. This waiver was granted in accordance with the regulatory and ethical standards applicable to retrospective research.
Study design and data collection: retrospective analysis approach
A retrospective analysis was performed on 220 children diagnosed with ADHD at WuXi No.2 People's Hospital between November 2022 and October 2024. The participants were categorized into two groups based on their treatment regimen. The first group, the Multi-Micronutrient Nutritional Formula (MNF) group, included 111 patients who received only the multi-micronutrient nutritional formula. The second group, the Multi-Micronutrient Nutritional Formula combined with Aerobic Exercise (MNF-AE) group, comprised of 109 patients who received both the multi-micronutrient nutritional formula and aerobic exercise therapy. Allocation to the MNF or MNF-AE group was based on the treatment regimen prescribed by the attending physician, ensuring that the allocation process was consistent with clinical practice and minimized potential selection bias. Eligible participants were identified from the hospital's medical records system, which contained comprehensive data on all ADHD patients treated during the specified period, encompassed demographic information, scores from the PSQ, WCST outcomes, performance on the Alternate Uses Task, and scores from the CSHQ.
The sample size for this study was calculated using GraphPad software. The calculation was based on the following assumptions: a medium effect size (Cohen's d = 0.5), a two-tailed significance level of α = 0.05, and a desired statistical power of 95%. It was estimated that each group required a minimum of 105 participants to detect a significant difference in mean scores using a two-sided, two-sample t-test with equal variances. This ensures adequate power to identify meaningful differences between the MNF and MNF-AE groups.
Inclusion and exclusion criteria: defining study eligibility
Participants were children diagnosed with ADHD, aged 6–12 years old. Specific inclusion and exclusion criteria are detailed below:
Inclusion criteria comprised the following: (1) a diagnosis of ADHD as per the American Psychiatric Association (APA) guidelines (20); (2) aged between 6 and 12 years (under age 6 years excluded due to potential challenges in their participation in the intervention; over 12 years excluded to avoid complications arising from complex developmental changes that could influence the study outcomes); and (3) availability of comprehensive medical records for each participant to facilitate thorough review and analysis.
The exclusion criteria were as follows: (1) the presence of exercise contraindications as per the American College of Sports Medicine (ACSM) guidelines (21), or any acute or chronic conditions potentially impacting the effectiveness of aerobic exercise intervention; (2) allergy to the micronutrient supplement administered in the study; (3) a diagnosis of other severe psychiatric or neurological disorders; (4) the presence of a severe physical illness or disability; (5) current use of asthma or allergic rhinitis medications; (6) the use of melatonin or similar substances for sleep enhancement; (7) participation in ongoing behavioral therapy or psychological treatment; and (8) intellectual disability or a history of brain injury.
Intervening method: treatment regimens detailed: MNF and AE regimens
Both regimes were three-months in duration. The MNF group underwent a regimen involving a multi-micronutrient nutritional formula. This formula included essential micronutrients with adjusted dosages: Zinc (8 mg/day), Magnesium (150 mg/day), Iron (8 mg/day), Vitamin B1 (Thiamine, 0.9 mg/day), Vitamin B2 (Riboflavin, 1.1 mg/day), Vitamin B3 (Niacin, 12 mg/day), Vitamin B6 (Pyridoxine, 1.0 mg/day), Vitamin B12 (Cobalamin, 1.8 µg/day), and Folate (200 µg/day) These micronutrients were provided through high-quality supplements sourced from DSM Nutritional Products to ensure purity and potency. Additionally, participants received these nutrients through carefully monitored natural food sources such as red meat, seafood, legumes, whole grains, nuts, seeds, leafy green vegetables, and fortified foods. The supplements were administered in capsule form, ensuring ease of consumption and consistent dosage. The daily dietary intake was supervised by professional medical personnel, ensuring that all children consumed the same foods. No other dietary restrictions were imposed on the participants during the MNF regimen, except for adherence to the standardized multi-micronutrient formula.
The MNF-AE group combined the multi-micronutrient nutritional formula with aerobic exercise. Participants engaged in a daily aerobic exercise regimen consisting of a 5-min warm-up, 20 min of running, and a 5-min cool-down, totaling 30 min of exercise daily. This was conducted through outdoor running accompanied by one of the parents to ensure safety and support, while participants wore chest-belt heart rate monitors (V800, Polar Electro Oy, Finland) to track their heart rates. The exercise intensity was maintained at 50%–70% of each participant's Heart Rate Reserve (HRR), as recommended by the ACSM guidelines (21) for moderate intensity. The HRR was calculated as the difference between each participant's Maximal Heart Rate (MHR) and Resting Heart Rate (RHR).
This study employed a retrospective design, wherein data on both exercise adherence and nutritional supplement intake were collected post-intervention using structured interviews and medical records. All intervention protocols described herein were consistent with standard clinical practices during the original treatment period, ensuring methodological consistency and reliability.
Data collection and outcome measurement: assessment tools utilized
ADHD symptoms
The Conners PSQ was employed to assess ADHD symptoms both before and after the intervention. It comprises a total of 48 items, each rated on a 4-point scale ranging from 0 to 3, resulting in a maximum possible score of 144. A higher score reflects more severe behavioral problems. The PSQ demonstrates a Cronbach's α of 0.86, indicating high reliability (22).
Stroop test
To assess attentional inhibitory control, participants completed the Stroop Color-Word Interference Test. In this test, participants were required to quickly and accurately verbally identify the ink color of displayed words. The Stroop Test included three conditions: Stroop Word, Stroop Color, and Stroop Color-Word. In the Stroop Word condition, participants completed 50 trials with color names printed in black ink. For the Stroop Color condition, there were 50 trials where color names appeared within rectangles matching the corresponding color. In the Stroop Color-Word condition, 50 trials consisted of color names printed in ink of a different color. Each condition's trials were arranged on a sheet of paper, with participants instructed to name the colors from top to bottom across 10 items per row and from left to right across 5 columns. The primary outcome measured in the Stroop Test was the time score, defined as the average time taken (in seconds) to correctly name the ink color for each trial. Lower time scores indicate better performance, reflecting more efficient attentional inhibitory control. The Stroop Color-Word Interference Test was selected because it has been shown to effectively measure executive function, particularly attentional inhibitory control. Additionally, this test was sensitive to acute exercise effects, making it suitable for evaluating changes in cognitive performance following interventions (23).
Wisconsin card sorting test
The WCST was employed to evaluate the executive function of participants. This test includes four stimulus cards and 128 response cards. Participants were instructed to sort the response cards based on one of three attributes—color, shape, or number—of the stimulus cards. After each attempt, the examiner provided feedback, indicating whether the match was “Correct” or “Incorrect”. Once a participant successfully matched 10 consecutive cards according to a specific attribute, termed the “nature of category”, the sorting rule was changed without prior notice. The test concluded either after the participant completed six categories or exhausted all 128 response cards.
For statistical analysis, six raw scores were recorded: Total Correct, Perseverative Responses, Perseverative Errors, Non-perseverative Errors, Conceptual Level Response, and Categories Completed. Perseverative Responses indicate the participant's tendency to continue sorting based on the previous category rule after it has changed. Perseverative Errors occur when the participant persists in following the old rule despite the change. Non-perseverative Errors were mistakes made while trying to adapt to the new rule. The Conceptual Level Response measures the ratio of correct sorts to total sorts within each category phase. Categories Completed refer to the number of category rules successfully mastered and completed by the participant. The WCST was chosen as one of the most widely used tasks for evaluating executive function. It was particularly effective in assessing cognitive flexibility, problem-solving abilities, and set-shifting (23).
Performance on the alternate uses task
To assess participants' cognitive flexibility and creativity, their performance on the “Alternate Uses Task” was evaluated. In this task, participants were required to generate as many alternative and novel uses as possible for a series of common household items. Each participant was given 60 s to provide verbal responses for each stimulus object, which were recorded with a dictation device placed before them. Prior to the task, an investigator trained in cognitive assessments delivered standardized instructions. During each session, participants completed one set of stimulus objects, consisting of three distinct items. Responses for each item were assessed based on four criteria: fluency, flexibility, originality, and elaboration. Elaboration scores ranged from 0 to 2 points, reflecting the depth and complexity of the ideas presented for each object. The Alternate Uses Task was included in our study because it has been found to be a valid and reliable cognitive test for assessing cognitive flexibility and creativity (24).
Sleep quality
The CSHQ was utilized to evaluate the children's sleep behaviors and disturbances. Parents rated their children's sleep conditions using a three-point scale: “frequently” (5–7 times per week), “sometimes” (2–4 times per week), and “rarely or never” (0–1 time per week). The CSHQ comprises 48 items, with 33 of these being scored and divided into 8 subscales. The total score can range from 33 to 99, with higher scores indicating poorer sleep quality. The CSHQ demonstrated a Cronbach's α coefficient of 0.73, indicating acceptable reliability (25).
Statistical analysis: utilizing SPSS for data evaluation
Data analysis was conducted in SPSS 29.0 (SPSS Inc., Chicago, Illinois, USA). Continuous variables were assessed for normal distribution using the Shapiro–Wilk test. In cases where the continuous data followed a normal distribution, differences between groups were evaluated using the T-test. Categorical data were analyzed using the chi-square test. The test statistic is denoted as χ2. Throughout this study, any P-value below 0.05 was set as the threshold for statistical significance. Categorical data is presented as [n (%)], continuous data is presented as mean ± standard deviation (SD).
Results
Basic data
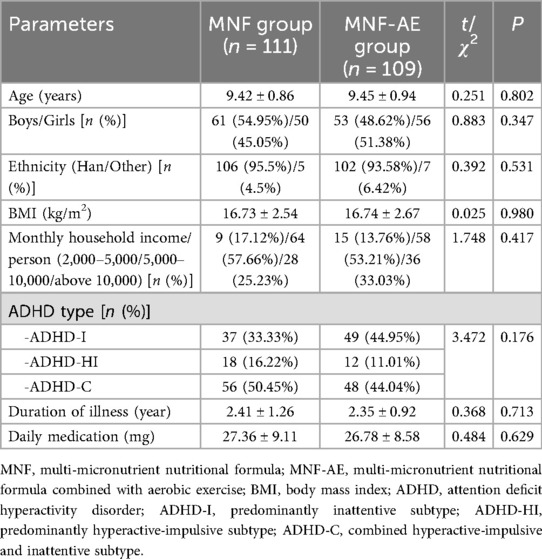
The mean age, gender distribution, ethnic distribution BMI, monthly household income per capita, distribution of ADHD subtypes, illness duration and average daily medication dosage showed no significant variation (P > 0.05). Thus, both groups were comparable (Table 1).
ADHD symptoms
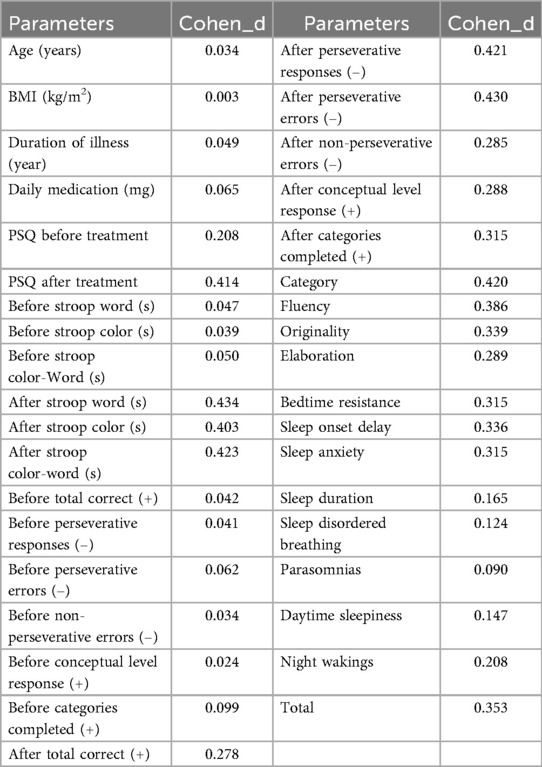
Prior to treatment, there was no difference in mean PSQ scores (P = 0.127) (Figure 1). However, after the intervention, the MNF-AE group exhibited significantly lower PSQ scores compared to the MNF group (P = 0.002).
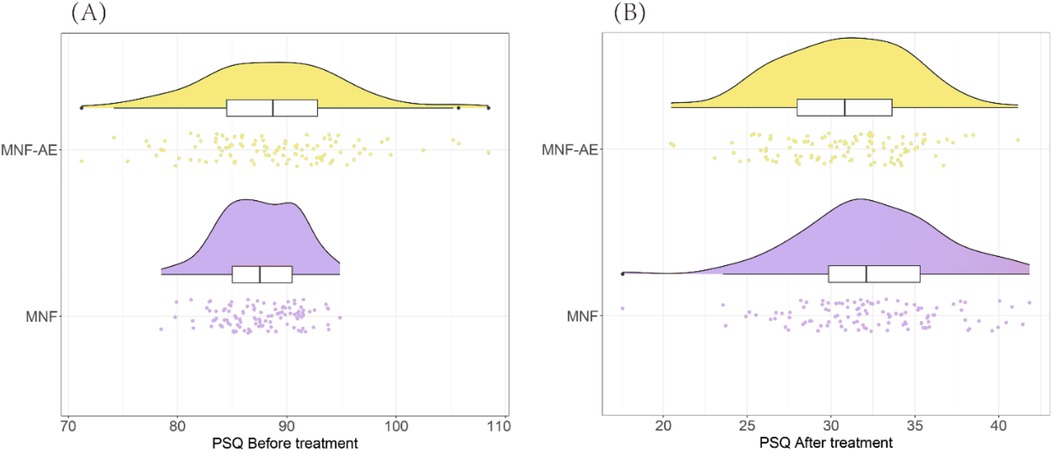
Figure 1. Comparison of PSQ score between two groups. (A) PSQ before treatment; (B) PSQ after treatment. ns: ns: no statistically significant difference; **: P < 0.01. PSQ: Conners Parent Symptom Questionnaire.
Attentional inhibitory control
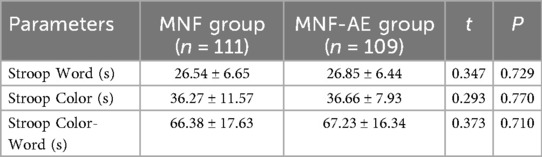
Before treatment there was no difference in mean Stroop Word (P = 0.729), Stroop Color (P = 0.770), or Stroop Color-Word test times (P = 0.710) (Table 2).
After treatment, the MNF-AE group demonstrated significantly faster completion times compared to the MNF group in the Stroop Word (P = 0.002) the Stroop Color (P = 0.003), and Stroop Color-Word test (P = 0.002) (Figure 2).
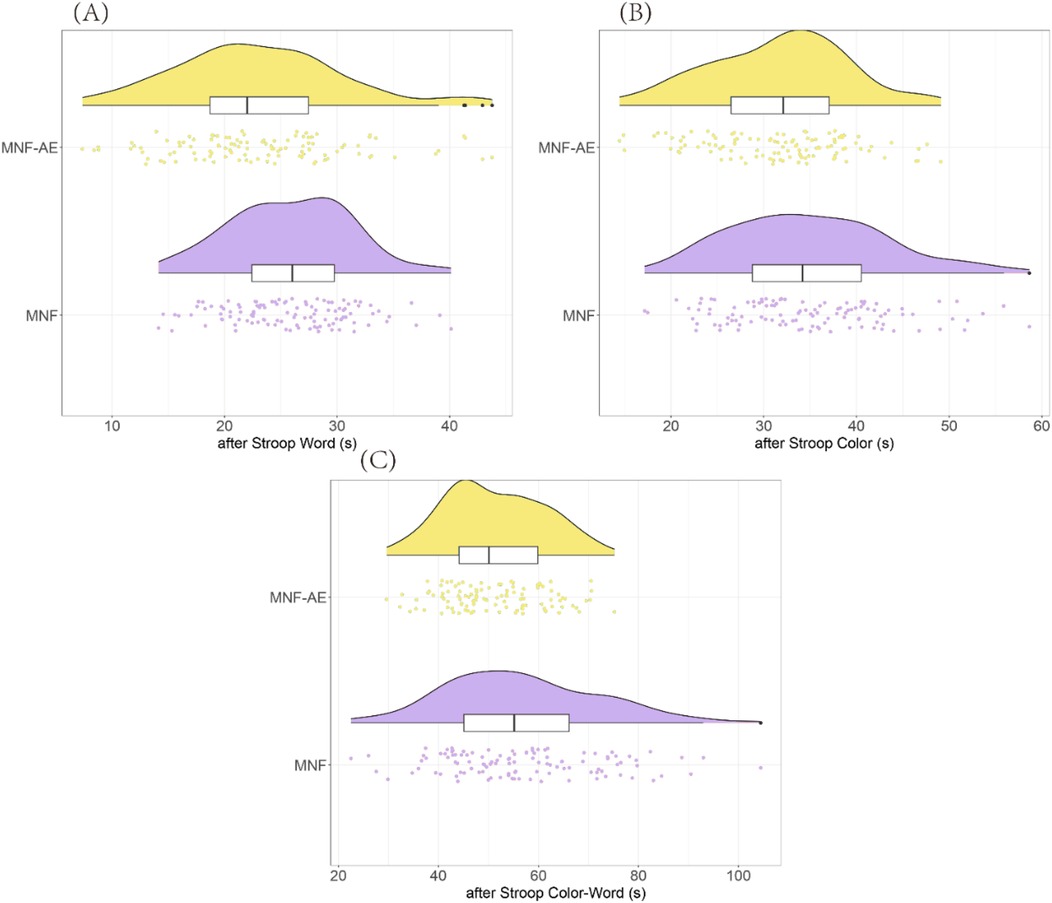
Figure 2. Comparison of stroop test results between two groups (after treatment). (A) Stroop Word; (B) Stroop Color; (C) Stroop Color-Word. **: P < 0.01.
Executive function
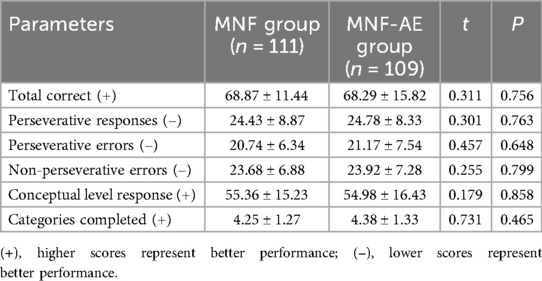
Before treatment there was no difference between groups for total correct scores (P = 0.756), perseverative responses (P = 0.763), perseverative errors (P = 0.648), non-perseverative errors (P = 0.799), conceptual level response (P = 0.858), or categories completed (P = 0.465) (Table 3).
After treatment, the MNF-AE group demonstrated a significantly higher total correct score (P = 0.041), significantly fewer perseverative responses (P = 0.002) and perseverative errors (P = 0.002), significantly higher conceptual level responses (P = 0.034), and completed more Categories (P = 0.020) compared to the MNF group. However non-perseverative errors were lower in the MNF group compared to the MNF-AE group (P = 0.036) (Figure 3).
Figure 3. Comparison of WCST results between two groups (after treatment). (A) Total Correct; (B) Perseverative Responses; (C) Perseverative Errors; (D) Non-perseverative Errors; (E) Conceptual Level Response; (F) Categories Completed. (+): higher scores represent better performance; (–): lower scores represent better performance. *: P < 0.05; **: P < 0.01. WCST: Wisconsin Card Sorting Test.
Cognitive function
The MNF-AE group outperformed the MNF group in scores for Category (P = 0.002), Fluency (P = 0.005), Originality (P = 0.013), and Elaboration (P = 0.033) (Table 4).
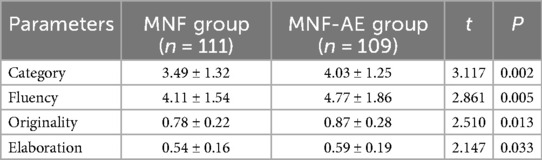
Table 4. Comparison of performance on the alternate uses task following aerobic exercise between two groups.
Sleep parameters
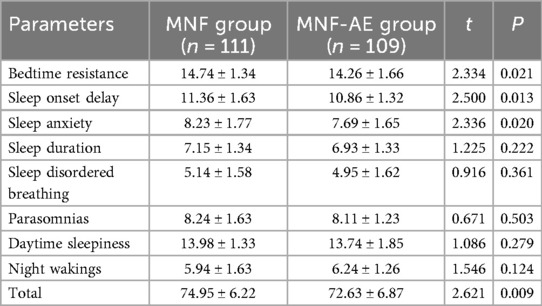
Post-intervention, the MNF-AE group showed better improvement in bedtime resistance (P = 0.021), sleep onset delay (P = 0.013), and sleep anxiety (P = 0.020) compared to the MNF group (Table 5). No significant differences were observed in sleep duration (P = 0.222), sleep-disordered breathing (P = 0.361), parasomnias (P = 0.503), daytime sleepiness (P = 0.279), or night wakings (P = 0.124). Additionally, total CSHQ scores were significantly lower in the MNF-AE group (P = 0.009). The effect sizes for various parameters were calculated and are summarized in Table 6.
Discussion
The major finding of the present study was that the addition of aerobic exercise into a regimen of multi-micronutrient supplementation significantly improved cognition, sleep, and ADHD symptomology in children with ADHD. Our findings indicate that integrating aerobic exercise into a regimen of multi-micronutrient supplementation improved PSQ scores in children with ADHD. This improvement can be attributed to the combined effects of nutrient support, which addresses deficiencies critical for neurotransmitter synthesis and neural function, and exercise-induced neuroplasticity, promoting brain regions involved in executive function and attention (26, 27). Specifically, the MNF-AE group likely benefited from better regulation of dopamine and norepinephrine due to nutrient intake (28), and enhanced synaptic plasticity and neurogenesis from aerobic exercise (29), leading to improved ADHD symptom management as reflected in the PSQ scores. These findings are consistent with Zhu et al.'s (30) systematic review and network meta-analysis, which found that closed-skill activities dominated by aerobic exercises were particularly effective in improving hyperactivity/impulsivity and inattentionin children and adolescents with ADHD.
It appears that the incorporation of aerobic exercise provides a complementary mechanism for improving sleep, cognition, and symptomology in children with ADHD, potentially operating through enhanced cerebral blood flow and the upregulation of neurotrophins like BDNF. Exercise-induced BDNF formation was known to promote synaptic plasticity and neurogenesis, potentially leading to improved cognitive outcomes, such as those demonstrated by Jaberi et al. (31), who found that physical exercise increases BDNF levels, enhancing learning and memory. The present study observed improvements in cognitive performance (better performance on the Stroop Test and WCST) when aerobic exercise was used in addition to a MNF which adds evidence for the use of aerobic exercise to improve attentional control and executive functioning. This aligns with previous research showing that regular physical activity can lead to structural changes in the brain, particularly in areas associated with executive function such as the prefrontal cortex and hippocampus (17, 18, 32, 33). However, it is important to consider other perspectives. For instance, an umbrella review by Dastamooz found strong evidence for the effectiveness of exercise in improving inattention and inhibitory control but noted weaker evidence for emotional and social outcomes (34). Therefore, while our findings support the cognitive benefits of aerobic exercise, future research should further explore its impact on these less robust outcomes.
A proposed mechanism for this improvement in attentional control and executive functioning with the addition of aerobic exercise is the ability of aerobic exercise to modulate catecholaminergic activity. Specifically, dopamine and norepinephrine, which are crucial in regulating attention and impulse control. Exercise-induced increases in these neurotransmitters' availability are suggested to optimize arousal mechanisms and enhance cognitive clarity (35, 36), which may explain the improvements in improved cognitive processing speed and selective attention with the addition of aerobic exercise observed in the present study.
The improvement in executive function indices, such as reduced perseverative errors and increased total correct scores in the WCST observed for the MNF-AE group in the present study, highlights the potential for physical activity to influence cognitive flexibility. Evidence from prior literature suggests that these improvements may be mediated by the exercise-induced enhancement of prefrontal cortex function, which is critical for executive task performance (37). This may be of specific benefit to children with ADHD for whom responses to changing contingencies is a common challenge as improved problem-solving skills facilitate adaptive responses to changing contingencies (24).
The Alternate Uses Task results suggest that the combined intervention positively affects divergent thinking through changes in neural pathways that promote novel idea generation. This process aligns with reports of dynamic changes in functional connectivity post-exercise, fostering a cognitive environment conducive to creative thinking (38, 39).
Additionally, sleep quality, assessed via the CSHQ, showed marked improvement in the MNF-AE group. While significant enhancements were observed in specific aspects of sleep, it is important to acknowledge that not all sleep parameters demonstrated improvements. The combination of aerobic exercise and micronutrient supplementation appears to be particularly effective in enhancing sleep onset, reducing bedtime resistance, and alleviating sleep-related anxiety. A critical factor here was that both exercise and micronutrients may exert beneficial effects on the sleep-wake cycle, more so when systematically combined (40, 41). Exercise generally promotes better sleep through thermoregulatory and circadian rhythm influences, while adequate nutrient intake ensures the presence of precursors required for sleep modulating neurotransmitters such as serotonin and melatonin (42, 43). These effects likely contributed to better sleep onset, reduced anxiety, and overall improved sleep quality in the MNF-AE group, offering the additional benefit of potentially mitigating the negative impact of poor sleep on ADHD symptoms (44, 45).
Interestingly, our study also suggests cultural and behavioral elements associated with dietary habits and physical activity can significantly influence health outcomes in children with ADHD. The structured regimen likely promoted a consistent daily routine, facilitating adherence, which were particularly beneficial in managing ADHD. Furthermore, the consistency in exercise and diet may instill discipline, indirectly benefiting behavioral management (46–48).
The combination of multi-micronutrient supplementation (MNF) and aerobic exercise offers a promising approach to managing ADHD symptoms in children. By addressing nutritional deficiencies, MNF ensures that essential vitamins and minerals are available for optimal neurotransmitter function, which supports better regulation of critical neurotransmitters like dopamine and norepinephrine (7, 49). Aerobic exercise complements this by enhancing cerebral blood flow and promoting neurotrophin upregulation, such as BDNF, which fosters synaptic plasticity and neurogenesis (50). Together, these interventions create an environment conducive to improved ADHD behavioral management (15). In real-world settings, this combined approach can be particularly beneficial for children with ADHD. It not only helps manage core symptoms but also establishes a structured routine that aids in maintaining consistency and discipline. For example, the integration of regular physical activity and balanced nutrition can help children develop healthier habits, leading to long-term benefits in their daily functioning and overall quality of life. These findings suggest that combining nutritional and physical activity interventions could provide a holistic and non-pharmacological strategy for ADHD management, potentially reducing reliance on traditional treatments and improving outcomes for affected children.
While this study provides promising insights into the effects of multi-micronutrient supplementation combined with aerobic exercise in managing ADHD in children, it was not without limitations. The sample size was relatively small, which may limit the generalizability of the findings to broader populations. The reliance on parent-reported measures also introduces potential bias. Objective sleep measurements, such as actigraphy or polysomnography, would provide more reliable data and should be included in future research to better understand the impact of the intervention on sleep. Future research with larger, more diverse populations and a comprehensive assessment of confounding variables was necessary to validate these findings and optimize the intervention protocol. Furthermore, the retrospective nature of this study meant that treatments were not randomized or prospectively assigned. This design limitation might have introduced biases and confounding factors that could affect the validity of our findings. The study lacked both a control group receiving usual treatment and an exercise-only group, making it difficult to attribute observed improvements directly to the intervention. Another limitation is that the caloric value of the food was not adjusted to account for the higher energy expenditure in the MNF-AE group due to daily aerobic exercise. This means that the energy balance may have differed between groups, potentially affecting the comparability of the MNF intervention. Future research should address this limitation by ensuring that caloric intake is adjusted to match energy expenditure across different intervention groups, thereby providing more reliable comparisons. Our statistical analysis did not adjust for potential confounders, such as baseline cognitive function, parental education level, and comorbid conditions, which could have influenced the outcomes. This omission is particularly critical because it may lead to biased estimates of the intervention's effectiveness. Additionally, it did not explore interaction effects between time and intervention. This limitation is especially important when considering outcomes susceptible to learning effects, such as performance on the Stroop test. Recognizing these limitations, we advocate for future studies employing prospective, randomized designs with comprehensive statistical adjustments to better understand the effects of multi-micronutrient supplementation combined with aerobic exercise on ADHD symptoms in children.
Conclusion
In conclusion, while traditional treatments for ADHD focus on symptom management, the addition of a multi-micronutrient nutritional formula combined with aerobic exercise may offer potential benefits as part of a multimodal approach. This strategy not only addresses underlying nutritional deficiencies and enhances physical health but also supports cognitive and neurochemical processes linked to ADHD. These results underscore the potential value of considering holistic interventions that encompass dietary and lifestyle modifications alongside conventional therapies. Future research should explore long-term effects, optimal intervention durations, and potential biochemical markers that can further elucidate the mechanisms driving these benefits, paving the way for more personalized and integrative ADHD management strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Wuxi No.2 Peoples Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants legal guardians/next of kin due to the retrospective nature of the study and the use of de-identified data.
Author contributions
YY: Conceptualization, Writing – original draft, Data curation, Formal analysis. YW: Writing – review & editing, Writing – original draft, Methodology, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Donzelli G, Llopis-Gonzalez A, Llopis-Morales A, Cioni L, Morales-Suárez-Varela M. Particulate matter exposure and attention-deficit/hyperactivity disorder in children: a systematic review of epidemiological studies. Int J Environ Res Public Health. (2019) 17:67. doi: 10.3390/ijerph17010067
2. Zayats T, Neale BM. Recent advances in understanding of attention deficit hyperactivity disorder (ADHD): how genetics are shaping our conceptualization of this disorder. F1000Res. (2019) 8(F1000 Faculty Rev):2060. doi: 10.12688/f1000research.18959.1
3. Chen M, Li H, Wang J, Dillman JR, Parikh NA, He L. A multichannel deep neural network model analyzing multiscale functional brain connectome data for attention deficit hyperactivity disorder detection. Radiol Artif Intell. (2019) 2:e190012. doi: 10.1148/ryai.2019190012
4. Weibel S, Menard O, Ionita A, Boumendjel M, Cabelguen C, Kraemer C, et al. Practical considerations for the evaluation and management of attention deficit hyperactivity disorder (ADHD) in adults. Encephale. (2020) 46:30–40. doi: 10.1016/j.encep.2019.06.005
5. Gilboa Y, Helmer A. Self-Management intervention for attention and executive functions using equine-assisted occupational therapy among children aged 6–14 diagnosed with attention deficit/hyperactivity disorder. J Altern Complement Med. (2020) 26:239–46. doi: 10.1089/acm.2019.0374
6. Eke H, Janssens A, Newlove-Delgado T, Paul M, Price A, Young S, et al. Clinician perspectives on the use of national institute for health and care excellence guidelines for the process of transition in attention deficit hyperactivity disorder. Child Care Health Dev. (2020) 46:111–20. doi: 10.1111/cch.12718
7. Lange KW, Lange KM, Nakamura Y, Reissmann A. Nutrition in the management of ADHD: a review of recent research. Curr Nutr Rep. (2023) 12:383–94. doi: 10.1007/s13668-023-00487-8
8. Skalny AV, Mazaletskaya AL, Ajsuvakova OP, Bjørklund G, Skalnaya MG, Chernova LN, et al. Magnesium status in children with attention-deficit/hyperactivity disorder and/or autism spectrum disorder. Soa Chongsonyon Chongsin Uihak. (2020) 31:41–5. doi: 10.5765/jkacap.190036
9. Noorazar SG, Malek A, Aghaei SM, Yasamineh N, Kalejahi P. The efficacy of zinc augmentation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: a randomized controlled trial. Asian J Psychiatr. (2020) 48:101868. doi: 10.1016/j.ajp.2019.101868
10. Robberecht H, Verlaet AAJ, Breynaert A, De Bruyne T, Hermans N. Magnesium, iron, zinc, copper and selenium Status in attention-deficit/hyperactivity disorder (ADHD). Molecules. (2020) 25:4440. doi: 10.3390/molecules25194440
11. Degremont A, Jain R, Philippou E, Latunde-Dada GO. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: a systematic review. Nutr Rev. (2021) 79:615–26. doi: 10.1093/nutrit/nuaa065
12. Topal Z, Tufan AE, Karadag M, Gokcen C, Akkaya C, Sarp AS, et al. Evaluation of peripheral inflammatory markers, serum B12, folate, ferritin levels and clinical correlations in children with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). Nord J Psychiatry. (2022) 76:150–7. doi: 10.1080/08039488.2021.1946712
13. Sourander A, Silwal S, Upadhyaya S, Surcel HM, Hinkka-Yli-Salomäki S, McKeague IW, et al. Maternal serum vitamin B12 and offspring attention-deficit/hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry. (2021) 30:1449–62. doi: 10.1007/s00787-020-01621-5
14. Chen C, Nakagawa S. Physical activity for cognitive health promotion: an overview of the underlying neurobiological mechanisms. Ageing Res Rev. (2023) 86:101868. doi: 10.1016/j.arr.2023.101868
15. Yang G, Liu Q, Wang W, Liu W, Li J. Effect of aerobic exercise on the improvement of executive function in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. Front Psychol. (2024) 15:1376354. doi: 10.3389/fpsyg.2024.1376354
16. Vancampfort D, Firth J, Stubbs B, Schuch F, Rosenbaum S, Hallgren M, et al. The efficacy, mechanisms and implementation of physical activity as an adjunctive treatment in mental disorders: a meta-review of outcomes, neurobiology and key determinants. World Psychiatry. (2025) 24:227–39. doi: 10.1002/wps.21314
17. Mehren A, Reichert M, Coghill D, Müller HHO, Braun N, Philipsen A. Physical exercise in attention deficit hyperactivity disorder—evidence and implications for the treatment of borderline personality disorder. Borderline Personal Disord Emot Dysregul. (2020) 7:1. doi: 10.1186/s40479-019-0115-2
18. Mehren A, Özyurt J, Thiel CM, Brandes M, Lam AP, Philipsen A. Effects of acute aerobic exercise on response inhibition in adult patients with ADHD. Sci Rep. (2019) 9:19884. doi: 10.1038/s41598-019-56332-y
19. Westwood SJ, Bozhilova N, Criaud M, Lam SL, Lukito S, Wallace-Hanlon S, et al. The effect of transcranial direct current stimulation (tDCS) combined with cognitive training on EEG spectral power in adolescent boys with ADHD: a double-blind, randomized, sham-controlled trial. IBRO Neurosci Rep. (2022) 12:55–64. doi: 10.1016/j.ibneur.2021.12.005
20. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. (2013) 25:191–2. doi: 10.1590/s2317-17822013000200017
21. Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. (2013) 12:215–7. doi: 10.1249/JSR.0b013e31829a68cf
22. Guo J, Li D. Effects of image-sandplay therapy on the mental health and subjective well-being of children with autism. Iran J Public Health. (2021) 50:2046–54. doi: 10.18502/ijph.v50i10.7505
23. Zheng Y, Liang JM, Gao HY, Yang ZW, Jia FJ, Liang YZ, et al. An open-label, self-control, prospective study on cognitive function, academic performance, and tolerability of osmotic-release oral system methylphenidate in children with attention-deficit hyperactivity disorder. Chin Med J (Engl). (2015) 128:2988–97. doi: 10.4103/0366-6999.168948
24. Ludyga S, Gerber M, Mücke M, Brand S, Weber P, Brotzmann M, et al. The acute effects of aerobic exercise on cognitive flexibility and task-related heart rate variability in children with ADHD and healthy controls. J Atten Disord. (2020) 24:693–703. doi: 10.1177/1087054718757647
25. Li SH, Jin XM, Shen XM, Wu SH, Jiang F, Yan CH, et al. Development and psychometric properties of the Chinese version of children’s sleep habits questionnaire. Zhonghua Er Ke Za Zhi. (2007) 45:176–80.17504618
26. Wulaer B, Kunisawa K, Hada K, Jaya Suento W, Kubota H, Iida T, et al. Shati/Nat8l deficiency disrupts adult neurogenesis and causes attentional impairment through dopaminergic neuronal dysfunction in the dentate gyrus. J Neurochem. (2021) 157:642–55. doi: 10.1111/jnc.15022
27. Ishihara Y, Honda T, Ishihara N, Namba K, Taketoshi M, Tominaga Y, et al. A CCR5 antagonist, maraviroc, alleviates neural circuit dysfunction and behavioral disorders induced by prenatal valproate exposure. J Neuroinflammation. (2022) 19:195. doi: 10.1186/s12974-022-02559-y
28. Dalnoki L, Hurks PPM, Gubbels JS, Eussen S, Mommers M, Thijs C. Exploring the relationship of dietary intake with inattention, hyperactivity, and impulsivity, beyond ADHD. J Atten Disord. (2025) 29:70–9. doi: 10.1177/10870547241293946
29. van Riper SM, Tempest GD, Piccirilli A, Ma Q, Reiss AL. Aerobic exercise, cognitive performance, and brain activity in adolescents with attention-deficit/hyperactivity disorder. Med Sci Sports Exerc. (2023) 55:1445–55. doi: 10.1249/MSS.0000000000003159
30. Zhu F, Zhu X, Bi X, Kuang D, Liu B, Zhou J, et al. Comparative effectiveness of various physical exercise interventions on executive functions and related symptoms in children and adolescents with attention deficit hyperactivity disorder: a systematic review and network meta-analysis. Front Public Health. (2023) 11:1133727. doi: 10.3389/fpubh.2023.1133727
31. Jaberi S, Fahnestock M. Mechanisms of the beneficial effects of exercise on brain-derived neurotrophic factor expression in Alzheimer’s disease. Biomolecules. (2023) 13:1577. doi: 10.3390/biom13111577
32. Vysniauske R, Verburgh L, Oosterlaan J, Molendijk ML. The effects of physical exercise on functional outcomes in the treatment of ADHD: a meta-analysis. J Atten Disord. (2020) 24:644–54. doi: 10.1177/1087054715627489
33. Villa-González R, Villalba-Heredia L, Crespo I, Del Valle M, Olmedillas H. A systematic review of acute exercise as a coadjuvant treatment of ADHD in young people. Psicothema. (2020) 32:67–74. doi: 10.7334/psicothema2019.211
34. Dastamooz S, Sadeghi-Bahmani D, Farahani MHD, Wong SHS, Yam JCS, Tham CCY, et al. The efficacy of physical exercise interventions on mental health, cognitive function, and ADHD symptoms in children and adolescents with ADHD: an umbrella review. EClinicalMedicine. (2023) 62:102137. doi: 10.1016/j.eclinm.2023.102137
35. Chan YS, Jang JT, Ho CS. Effects of physical exercise on children with attention deficit hyperactivity disorder. Biomed J. (2022) 45:265–70. doi: 10.1016/j.bj.2021.11.011
36. Kesting S, Weeber P, Schönfelder M, Renz BW, Wackerhage H, von Luettichau I. Exercise as a potential intervention to modulate cancer outcomes in children and adults? Front Oncol. (2020) 10:196. doi: 10.3389/fonc.2020.00196
37. Hosseini L, Farokhi-Sisakht F, Badalzadeh R, Khabbaz A, Mahmoudi J, Sadigh-Eteghad S. Nicotinamide mononucleotide and melatonin alleviate aging-induced cognitive impairment via modulation of mitochondrial function and apoptosis in the prefrontal cortex and hippocampus. Neuroscience. (2019) 423:29–37. doi: 10.1016/j.neuroscience.2019.09.037
38. França AP, Schamne MG, de Souza BS, da Luz Scheffer D, Bernardelli AK, Corrêa T, et al. Caffeine consumption plus physical exercise improves behavioral impairments and stimulates neuroplasticity in spontaneously hypertensive rats (SHR): an animal model of attention deficit hyperactivity disorder. Mol Neurobiol. (2020) 57:3902–19. doi: 10.1007/s12035-020-02002-4
39. Custodio RJP, Kim HJ, Kim J, Ortiz DM, Kim M, Buctot D, et al. Hippocampal dentate gyri proteomics reveals Wnt signaling involvement in the behavioral impairment in the THRSP-overexpressing ADHD mouse model. Commun Biol. (2023) 6:55. doi: 10.1038/s42003-022-04387-5
40. Qiu H, Liang X. Change in sleep latency as a mediator of the effect of physical activity intervention on executive functions among children with ADHD: a secondary analysis from a randomized controlled trial. J Autism Dev Disord. (2024) 54:3069–77. doi: 10.1007/s10803-023-06018-2
41. Trakada G. Dietary nutrient intake and sleep. Nutrients. (2023) 15:2276. doi: 10.3390/nu15102276
42. González-Devesa D, Sanchez-Lastra MA, Outeda-Monteagudo B, Diz-Gómez JC, Ayán-Pérez C. Effectiveness of exercise on sleep quality in attention deficit hyperactivity disorder: a systematic review and meta-analysis. Children (Basel). (2025) 12:119. doi: 10.3390/children12020119
43. Mason L, Connolly J, Devenney LE, Lacey K, O'Donovan J, Doherty R. Sleep, nutrition, and injury risk in adolescent athletes: a narrative review. Nutrients. (2023) 15:5101. doi: 10.3390/nu15245101
44. Zeibig JM, Seiffer B, Sudeck G, Rösel I, Hautzinger M, Wolf S. Transdiagnostic efficacy of a group exercise intervention for outpatients with heterogenous psychiatric disorders: a randomized controlled trial. BMC Psychiatry. (2021) 21:313. doi: 10.1186/s12888-021-03307-x
45. Hong GCC, Conduit R, Wong J, Di Benedetto M, Lee E. Diet, physical activity, and screen time to sleep better: multiple mediation analysis of lifestyle factors in school-aged children with and without attention deficit hyperactivity disorder. J Atten Disord. (2021) 25:1847–58. doi: 10.1177/1087054720940417
46. Huang R, Ridout SJ, Harris B, Ridout KK, Raja K. Pharmacist medication management of adults with attention deficit: an alternative clinical structure. Perm J. (2020) 24:19.122. doi: 10.7812/TPP/19.122
47. Curtis DF, Heath CL, Hogan WJ. Child skills training for attention-deficit/hyperactivity disorder (ADHD): a randomized controlled trial of structured dyadic behavior therapy (SDBT). Psychotherapy (Chic). (2021) 58:68–80. doi: 10.1037/pst0000294
48. Halt AH, Hirvonen TT, Koskela J, Kerkelä M, Hurtig T. Attention-deficit/hyperactivity disorder is not associated with overweight in adolescence but is related to unhealthy eating behavior and limited physical activity. Nord J Psychiatry. (2023) 77:591–9. doi: 10.1080/08039488.2023.2198504
49. Visternicu M, Rarinca V, Burlui V, Halitchi G, Ciobică A, Singeap AM, et al. Investigating the impact of nutrition and oxidative stress on attention deficit hyperactivity disorder. Nutrients. (2024) 16:3113. doi: 10.3390/nu16183113
Keywords: ADHD, nutritional supplementation, aerobic exercise, cognitive function, executive function, sleep quality
Citation: Ye Y and Wang Y (2025) Benefits of multi-micronutrient nutritional formula combined with aerobic exercise on children with attention deficit hyperactivity disorder: improvements in symptoms, cognition, executive function, and sleep. Front. Pediatr. 13:1650588. doi: 10.3389/fped.2025.1650588
Received: 20 June 2025; Accepted: 3 September 2025;
Published: 26 September 2025.
Edited by:
Elizabeth C. Matsui, The University of Texas at Austin, United StatesReviewed by:
Alinda Nur Ramadhani, University of Aisyiyah Surakarta, IndonesiaLamiaa Abd El Hakeem Ali, Cairo University, Egypt
Öznur Adigüzel Akman, Zonguldak Bulent Ecevit University, Türkiye
Copyright: © 2025 Ye and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang, d2FuZ3l1bnl1ZXJAMTI2LmNvbQ==
 Yanjuan Ye
Yanjuan Ye Yun Wang
Yun Wang