- Neonatal Department, Tianjin First Central Hospital, Tianjin, China
Background: Parenteral nutrition-associated cholestasis (PNAC) is common among very and extremely preterm infants (VPT). This study aims to investigate the relationship between the duration of parenteral nutrition (PN), enteral nutrition (EN), and the PN/EN ratio and the occurrence of PNAC in VPT, with the goal of providing a basis for the early identification of high-risk infants in clinical practice.
Methods: A total of 230 VPT were retrospectively enrolled and divided into two groups based on the occurrence of PNAC. Baseline characteristics such as gestational age, sex, and birth weight, as well as clinical features, were compared between groups. Multivariable logistic regression was used to analyze the association between the duration of enteral nutrition (EN), parenteral nutrition (PN), and the development of PNAC. Interaction effects between PN, EN, the PN/EN ratio, and clinical variables were also explored. Restricted cubic spline (RCS) regression was employed to assess potential nonlinear relationships between PN, EN duration, PN/EN ratio, and PNAC. Predictive performance was evaluated using the area under the receiver operating characteristic curve (AUC).
Results: Infants in the PNAC group had significantly lower gestational age, birth weight, and Apgar scores compared to the non-PNAC group. In contrast, the incidence of premature rupture of membranes and mechanical ventilation was significantly higher. In VPT, longer PN duration, shorter EN duration, and a higher PN/EN ratio were significantly associated with increased risk of PNAC, showing linear or near-linear trends. ROC analysis indicated that the PN/EN ratio had better predictive performance for PNAC than either PN or EN duration alone. Interaction analysis revealed that the association between PN/EN and PNAC risk was stronger in infants with lower birth weight and lower 1-minute Apgar scores.
Conclusions: Longer PN duration, shorter EN duration, and a higher PN/EN ratio are significant risk factors for PNAC in VPT. The PN/EN ratio demonstrated the best predictive accuracy. The association between PN/EN and PNAC was more pronounced in infants with lower birth weight and lower 1-minute Apgar scores.
1 Introduction
In recent years, with advances in perinatal medicine and neonatal intensive care, the survival rate of very and extremely preterm infants (gestational age <32 weeks) (VPT) has significantly improved (1–3). However, due to the immaturity of multiple organ systems, these infants are at high risk for various complications after birth (4, 5). Among them, parenteral nutrition-associated cholestasis (PNAC) is a relatively common yet serious condition (6). Recent studies have reported that the incidence of PNAC among newborns is approximately 5%–30%, depending on gestational age, birth weight, and duration of parenteral nutrition (7, 8). It is primarily characterized by elevated direct bilirubin levels and impaired liver function, which may progress to liver fibrosis, cirrhosis, or even liver failure, severely affecting both short- and long-term outcomes (9).
The exact pathogenesis of PNAC remains unclear. However, accumulating evidence suggests a strong association with prolonged dependence on parenteral nutrition (PN) (10). Due to gastrointestinal immaturity, VPT often cannot tolerate timely or sufficient enteral nutrition (EN), resulting in extended use of PN and imbalanced nutrient intake, which can lead to cholestasis. In addition, other factors such as infection, low birth weight, low gestational age, hypoxic-ischemic injury, medication use, and intestinal diseases (e.g., necrotizing enterocolitis) have also been implicated in the development of PNAC (8, 11). Although the prevention and management of PNAC have received increasing clinical attention, its specific risk factors remain controversial, and findings from different studies are inconsistent.
Some existing studies have shown a positive association between the duration of PN and the risk of PNAC in preterm infants, while early initiation and adequate intake of EN may offer protective effects (12). However, research specifically focusing on the risk of PNAC in VPT remains limited. Moreover, most previous studies have assessed either PN or EN duration independently, which may not fully capture the overall nutritional balance in these infants (13, 14). The PN/EN ratio, as a composite indicator reflecting the nutritional structure, may serve as a more sensitive predictor of PNAC risk. Nevertheless, current research on this ratio is scarce and lacks systematic analysis. This study innovatively uses the PN/EN ratio as an evaluation indicator, systematically analyzes its association with the risk of PNAC, and conducts interaction analyses, aiming to provide a scientific basis for early identification of high-risk infants and optimization of nutritional management strategies.
2 Materials and methods
2.1 Study population
This retrospective study included VPT born at our hospital between JAN 2021 and DEC 2024. Based on the occurrence of parenteral nutrition-associated cholestasis (PNAC), the infants were divided into PNAC and non-PNAC groups. Inclusion criteria were as follows: According to World Health Organization (WHO) standards, VPT with a gestational age of less than 32 weeks were included; received both parenteral nutrition (PN) and enteral nutrition (EN) during hospitalization; no history of surgical intervention. Exclusion criteria were: congenital biliary tract malformations, inherited metabolic diseases, or other known hepatobiliary conditions associated with cholestasis; gestational age ≥32 weeks; major structural anomalies such as congenital heart disease or gastrointestinal malformations; death or transfer before NICU discharge; congenital intrauterine infection; absence of PN or EN; incomplete clinical data.
2.2 Data collection
Clinical data were obtained from the electronic medical record system, including: sex, gestational age, birth weight, mode of delivery, Apgar scores at 1 and 5 min, singleton or multiple birth, gestational hypertension, gestational diabetes, intrauterine infection (chorioamnionitis), premature rupture of membranes (PROM), use of mechanical ventilation postnatally, and small for gestational age (SGA). Whether diagnosed with patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), late-onset sepsis (LOS), extrauterine growth restriction (EUGR), length of hospital stay, duration of parenteral nutrition (PN), duration of enteral nutrition (EN), PN/EN ratio, and time to initiate enteral nutrition. The diagnostic criteria for PNAC were: direct bilirubin (DBIL) ≥ 2 mg/dl or total bilirubin (TBIL) ≥ 5 mg/dl, with DBIL accounting for ≥20% of TBIL (15), after excluding other possible causes of cholestasis.
All VPT followed the standardized feeding guidelines of our Neonatology Department. The formulation and starting doses of PN were applied according to a unified protocol, including total initial energy, amounts of protein, carbohydrates, and fat, as well as the concentrations and infusion rates of amino acids, glucose, and lipid emulsion, to ensure consistency in nutritional management among all infants.
2.3 Statistical analysis
All statistical analyses were performed using R software version 4.4.1. Continuous variables were expressed as median (minimum–maximum) and compared using the Mann–Whitney U test or independent sample t-test, as appropriate. Categorical variables were presented as frequency (percentage) and analyzed using Fisher's exact test or Chi-square test. All patients were divided into four quartile groups according to the values of PN duration, EN duration, and PN/EN ratio, ranked from lowest to highest: Q1 (0%–25%), Q2 (25%–50%), Q3 (50%–75%), and Q4 (75%–100%). The Q1 group was used as the reference group in subsequent analyses. Specifically, for PN duration: Q1 = 8–13 days, Q2 = 13–18 days, Q3 = 18–23 days, and Q4 = 23–28 days; for EN duration: Q1 = 14–21 days, Q2 = 21–27 days, Q3 = 27–34 days, and Q4 = 34–39 days; and for the PN/EN ratio: Q1 = 0.2–0.5, Q2 = 0.5–0.7, Q3 = 0.7–0.9, and Q4 = 0.9–1.6. Multivariate logistic regression was used to construct three models to evaluate the association of PN, EN, and PN/EN with PNAC:
Model 1: unadjusted.
Model 2: adjusted for gestational age, sex, and birth weight.
Model 3: adjusted for gestational age, sex, birth weight, 1-minute Apgar score, 5-minute Apgar score, postnatal mechanical ventilation.
Restricted cubic spline (RCS) regression was used to explore the nonlinear relationships between PN, EN, PN/EN, and PNAC. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to evaluate the predictive performance of each indicator, and the DeLong test was used to compare differences in predictive ability. Additionally, interaction analyses were conducted to examine the relationship between PN/EN and PNAC risk across different strata of gestational age, birth weight, and Apgar scores. A two-sided P-value < 0.05 was considered statistically significant.
3 Results
3.1 Comparison of clinical characteristics between the PNAC and non-PNAC groups
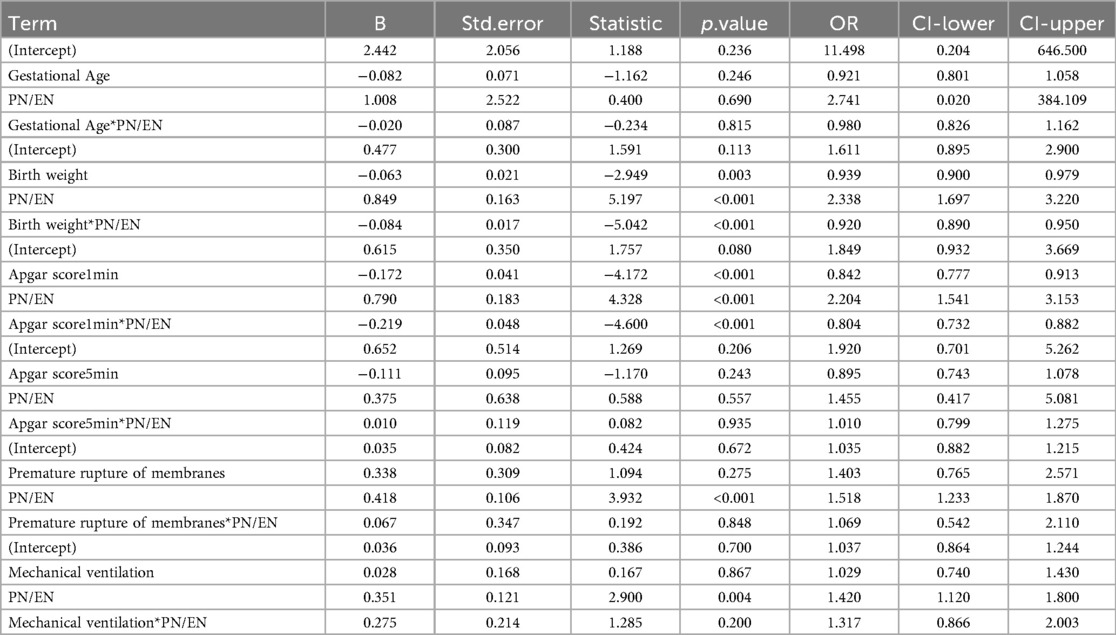
The results showed that among 230 VPT, 82 cases (35.65%) developed PNAC, while 148 cases (64.35%) did not. Compared with the non-PNAC group, the PNAC group had significantly lower gestational age (P = 0.002), lower birth weight (P < 0.001), lower Apgar scores at 1 min (P = 0.004) and 5 min (P = 0.003), a higher incidence of PROM (P = 0.004), a higher proportion requiring mechanical ventilation after birth (P = 0.001), a higher incidence of PDA (P < 0.001), a higher incidence of NEC (P = 0.009), a longer hospital stay (P = 0.007), a longer duration of PN (P < 0.001), a shorter duration of EN (P = 0.042), and a higher PN/EN ratio (P < 0.001) (Table 1).
3.2 Effects of PN, EN, and PN/EN on the occurrence of PNAC
The results showed that the duration of PN was positively associated with the risk of PNAC, with PNQ2–Q4 groups having higher risk compared to the lowest quartile group (PNQ1); however, only the PNQ4 group showed a significant increase in risk across all models (Table 2). The duration of EN was negatively associated with PNAC risk, with higher quartile groups showing markedly reduced risk, which remained significant after adjusting for gestational age, sex, birth weight, and Apgar scores (Table 3). An increased PN/EN ratio was associated with a higher risk of PNAC, with the highest quartile group (PN/ENQ4) showing the most pronounced risk, and the PN/ENQ3 group also showing increased risk in Models 1 and 2 (Table 4). Overall, these trends remained stable after multivariable adjustment, suggesting that the duration and proportion of PN and EN play an important role in the occurrence of PNAC.
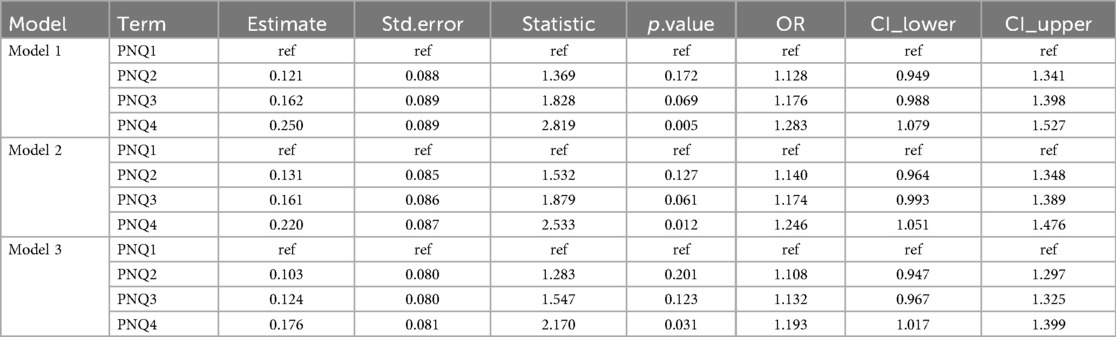
Table 2. The impact of the duration of parenteral nutrition (PN) on the occurrence of parenteral nutrition-associated cholestasis (PNAC).
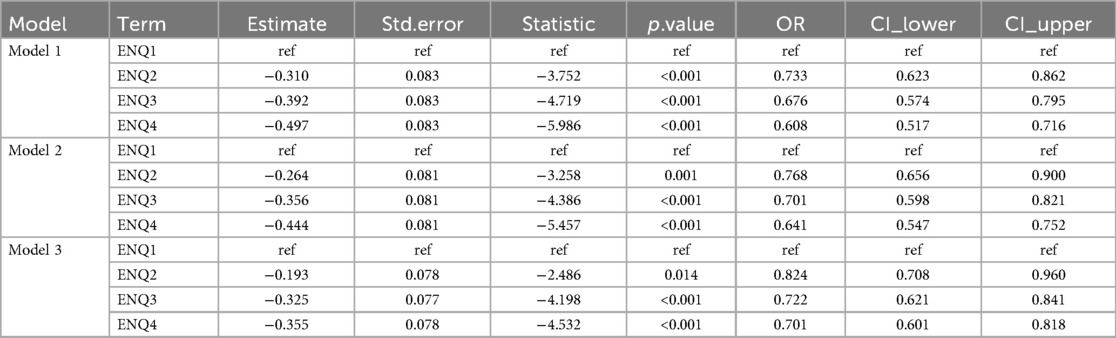
Table 3. The impact of the duration of enteral nutrition (EN) on the occurrence of parenteral nutrition-associated cholestasis (PNAC).
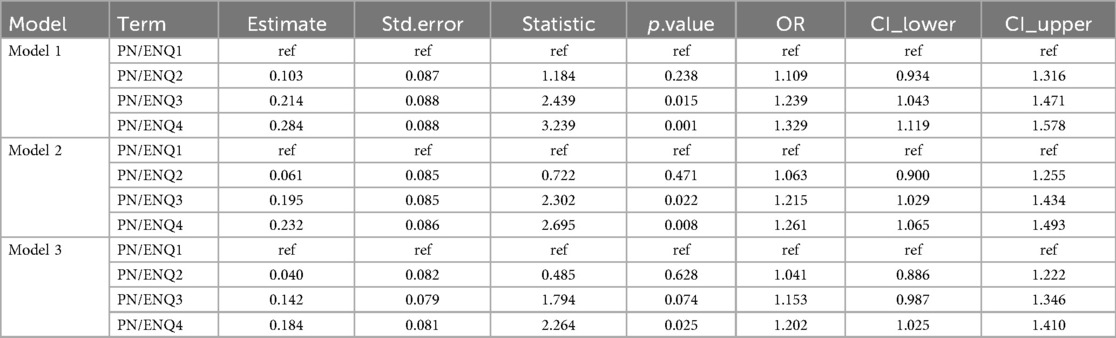
Table 4. The impact of the duration of parenteral nutrition (PN) and enteral nutrition (EN) on the occurrence of parenteral nutrition-associated cholestasis (PNAC).
3.3 Restricted cubic spline (RCS) analysis of the association between PN, EN, PN/EN and PNAC
As PN duration increased, EN duration decreased, and PN/EN ratio increased, the risk of PNAC showed a significant upward trend (P for TOTAL all < 0.05). Nonlinear analysis indicated that the relationships between changes in PN, EN, and PN/EN and PNAC were not significantly nonlinear (P for Nonlinear all > 0.05), meaning that these three variables were linearly or approximately linearly related to PNAC (Figures 1A–C).
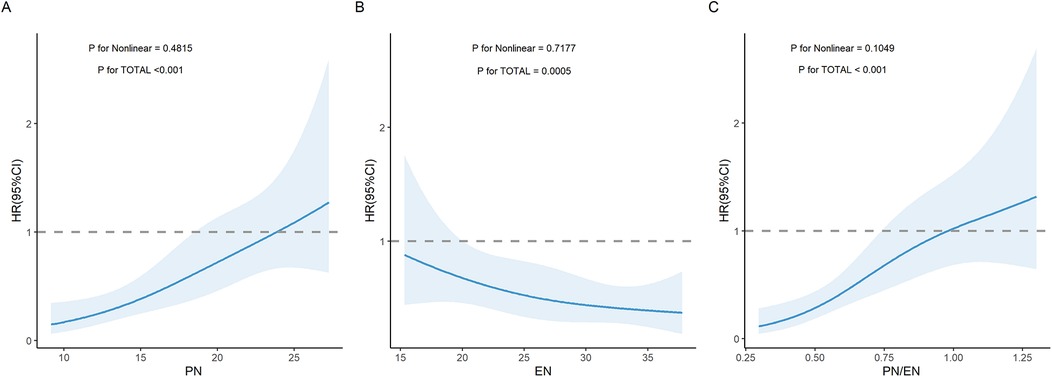
Figure 1. Restricted cubic spline (RCS) curves illustrating the association between nutritional parameters and the risk of PNAC. (A) Association between PN duration and PNAC risk. (B) Association between EN duration and PNAC risk. (C) Association between PN/EN ratio and PNAC risk. PN, parenteral nutrition; EN, enteral nutrition; PNAC, parenteral nutrition-associated cholestasis; HR, hazard ratio.
3.4 ROC curve analysis of the predictive ability of PN, EN, and PN/EN for PNAC occurrence
The results showed that the AUC values for PN, EN, and PN/EN were 0.645, 0.579, and 0.676, respectively, with PN/EN demonstrating the best predictive ability, followed by PN and EN. The optimal thresholds indicated that when PN duration exceeded 16.5 days, EN duration was less than 25.5 days, and the PN/EN ratio was higher than 0.659, the model predicted the occurrence of PNAC (Figures 2A–C). DeLong's test showed that the predictive ability of PN/EN was significantly better than that of EN (P = 0.006), but there was no significant difference compared with PN.
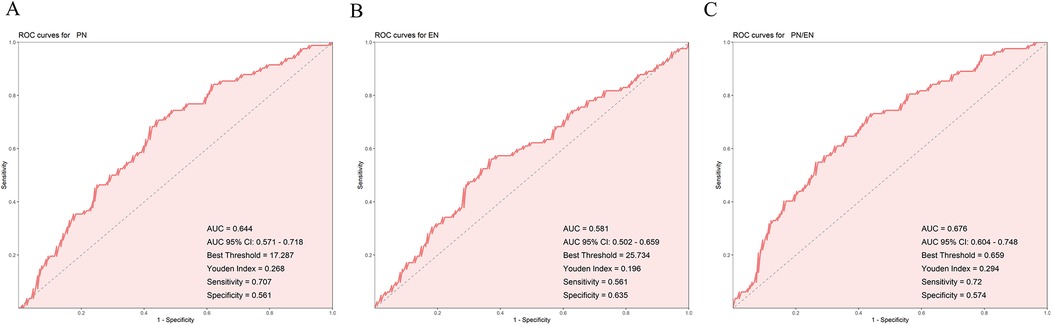
Figure 2. Receiver operating characteristic (ROC) curves for predicting PNAC. (A) ROC curve for PN duration. (B) ROC curve for EN duration. (C) ROC curve for PN/EN ratio. PN, parenteral nutrition; EN, enteral nutrition; PNAC, parenteral nutrition-associated cholestasis; ROC, receiver operating characteristic; AUC, area under the curve.
3.5 Interaction analysis
Based on the above analysis, the PN/EN ratio showed the strongest predictive ability. Gestational age, birth weight, 1-minute and 5-minute Apgar scores, premature rupture of membranes, and mechanical ventilation were covariates included in Model 3 and also variables that showed significant differences in the baseline analysis. These variables, having already demonstrated associations with PNAC risk, were therefore included in the interaction analysis with PN/EN to identify the combinations or conditions under which PN/EN has a stronger or weaker effect on PNAC risk. The results showed a significant interaction between PN/EN and birth weight, with stronger interaction associated with a lower risk of PNAC. This indicates that the higher the birth weight, the weaker the impact of PN/EN on PNAC (enhanced protective effect), meaning PN/EN has a greater effect on low birth weight preterm infants. Similarly, there was a significant interaction between the 1-minute Apgar score and PN/EN; the stronger the interaction, the lower the risk of PNAC. This suggests that the lower the Apgar score, the stronger the influence of PN/EN on the risk of PNAC. In other words, infants in poorer initial condition are more likely to develop PNAC due to a higher PN ratio. Interactions between PN/EN and other factors were not significant (Table 5).
4 Discussion
Our study found that for VPT, the longer the duration of PN, the higher the risk of developing PNAC. This may be because PN bypasses enteral feeding, leading to a lack of food stimulation in the intestines, which reduces the secretion of cholecystokinin, inhibits gallbladder contraction, decreases bile discharge, and causes cholestasis (16). The absence of nutrient intake in the intestines also causes bile acid retention, reducing the reabsorption of bile acids by the intestines (17). To maintain this balance, hepatocytes overproduce bile acids, ultimately leading to bile acid metabolism disorder (18). Excess amino acids and prolonged lipid emulsion infusion in PN can produce hepatotoxic substances and free radicals, damaging hepatocytes (19). Lack of enteral stimulation disrupts the intestinal barrier and flora, allowing endotoxins to trigger hepatic inflammation and worsen cholestasis (20, 21).
In contrast, longer EN duration was associated with a lower risk of PNAC. This could be because EN, especially formula containing fat, stimulates the intestine to secrete cholecystokinin (CCK), promoting gallbladder emptying and reducing bile stasis. EN also promotes the intestinal reabsorption of bile acids, preventing bile acid metabolism disorders (22). Nutrients in EN, such as glutamine (23) and short-chain fatty acids (SCFAs), maintain the normal proliferation of intestinal epithelial cells, protecting the intestinal barrier function and inhibiting the excessive growth of pathogenic bacteria (e.g., Escherichia coli, Klebsiella). Additionally, nutrients in EN, such as taurine and choline, further support liver fat metabolism, reducing the risk of PNAC (24, 25).
This study primarily identified critical thresholds for PN, EN, and PN/EN, which can provide clinicians with precise reference values. When PN duration exceeds 16.5 days, the risk of PNAC in VPT significantly increases. When PN/EN exceeds 0.659, the risk of PNAC significantly increases, suggesting clinicians should pay attention to the balance between PN and EN. When developing nutrition management plans for VPT, if PN/EN is higher than this threshold, re-evaluation may be necessary. The interaction analysis results are also one of the important findings of this study. The higher the birth weight, the weaker the influence of PN/EN on PNAC (enhanced protection), indicating that VPT with higher birth weights have more mature hepatobiliary function and greater tolerance to the toxicity potentially caused by PN. Therefore, even with a relatively high PN/EN ratio (i.e., a higher proportion of parenteral nutrition), these infants are less likely to develop cholestasis. VPT with lower Apgar scores had a higher risk of developing PNAC when the PN/EN ratio was elevated. This also suggests that individualized nutritional interventions should be considered for such high-risk infants, optimizing the timing and proportion of PN and transitioning to EN as early as possible to reduce the risk of PNAC. However, it should be noted that lower 1- and 5-minute Apgar scores are physiologically expected in VPT, which may limit the interpretability of the interaction results.
In this study, the incidence of PNAC among VPT was 36%, which is comparable to previously reported rates (26, 27). VPT have immature hepatic enzyme systems, with reduced bile secretion and excretion capacity, and their intestinal function is underdeveloped, leading to poor tolerance of enteral nutrition (28). Prolonged parenteral nutrition may induce hepatocellular steatosis, cholestasis, and inflammatory responses, significantly increasing the risk of PNAC, highlighting the importance of early optimization of parenteral and enteral nutrition strategies in VPT.
Previous studies have analyzed the risk factors for PNAC in very low birth weight infants and preterm infants (12, 29), but research specifically focusing on VPT remains limited. Prior studies have suggested that prolonged PN duration and delayed initiation of EN are associated with an increased risk of PNAC, which is consistent with the findings of this study (14, 30). Building on this, our study further quantified the risk gradient of the PN/EN ratio, identified specific thresholds, and conducted interaction analyses, providing a basis for individualized nutritional intervention strategies.
This study also has certain limitations. First, it is a retrospective study with potential selection bias. Second, the study scale was relatively small and did not experimentally validate the mechanisms by which PN and EN affect PNAC development in VPT. Future research could conduct larger-scale prospective randomized controlled trials as well as more in-depth physiological and biochemical experiments to validate the findings of this study.
5 Conclusion
This study demonstrates that PN and EN are important influencing factors for the development of PNAC in VPT. Specifically, PN and the PN/EN ratio show a linear or near-linear positive correlation with PNAC occurrence, while EN shows a linear or near-linear negative correlation. Among these, the predictive performance of the PN/EN ratio is the strongest. Interaction analysis indicates that in infants with higher birth weight and higher 1-minute Apgar scores, the impact of PN/EN on the risk of PNAC occurrence is weakened. In summary, we have identified the threshold for PN/EN, clarified the optimal timing for advancing EN in VPT requiring PN, and provided a reference basis for developing individualized nutritional management strategies for these infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Neonatal Department, Tianjin First Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
XM: Writing – review & editing, Writing – original draft. YZ: Writing – review & editing, Methodology. YG: Writing – review & editing, Conceptualization. LY: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheong JLY, Burnett AC, Treyvaud K, Spittle AJ. Early environment and long-term outcomes of preterm infants. J Neural Transm (Vienna). (2020) 127(1):1–8. doi: 10.1007/s00702-019-02121-w
2. Platt MJ. Outcomes in preterm infants. Public Health. (2014) 128(5):399–403. doi: 10.1016/j.puhe.2014.03.010
3. Cheong JLY, Lee KJ, Boland RA, Spittle AJ, Opie GF, Burnett AC, et al. Changes in long-term prognosis with increasing postnatal survival and the occurrence of postnatal morbidities in extremely preterm infants offered intensive care: a prospective observational study. Lancet Child Adolesc Health. (2018) 2(12):872–9. doi: 10.1016/s2352-4642(18)30287-6
4. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol. (2016) 33(3):318–28. doi: 10.1055/s-0035-1571202
5. Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. (2016) 40(8):497–509. doi: 10.1053/j.semperi.2016.09.002
6. Satrom K, Gourley G. Cholestasis in preterm infants. Clin Perinatol. (2016) 43(2):355–73. doi: 10.1016/j.clp.2016.01.012
7. Lauriti G, Zani A, Aufieri R, Cananzi M, Chiesa PL, Eaton S, et al. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr. (2014) 38(1):70–85. doi: 10.1177/0148607113496280
8. Wang N, Yan W, Hong L, Lu L, Feng Y, Wu J, et al. Risk factors of parenteral nutrition-associated cholestasis in very-low-birthweight infants. J Paediatr Child Health. (2020) 56(11):1785–90. doi: 10.1111/jpc.14826
9. Madnawat H, Welu AL, Gilbert EJ, Taylor DB, Jain S, Manithody C, et al. Mechanisms of parenteral nutrition-associated liver and gut injury. Nutr Clin Pract. (2020) 35(1):63–71. doi: 10.1002/ncp.10461
10. Beath SV, Kelly DA. Total parenteral nutrition-induced cholestasis: prevention and management. Clin Liver Dis. (2016) 20(1):159–76. doi: 10.1016/j.cld.2015.08.009
11. Chen CY, Chen HL. The risk factors of parenteral nutrition-associated cholestasis in preterm infants. Pediatr Neonatol. (2009) 50(5):181–3. doi: 10.1016/s1875-9572(09)60060-7
12. Alkharfy TM, Ba-Abbad R, Hadi A, Sobaih BH, AlFaleh KM. Total parenteral nutrition-associated cholestasis and risk factors in preterm infants. Saudi J Gastroenterol. (2014) 20(5):293–6. doi: 10.4103/1319-3767.141688
13. Lee HH, Jung JM, Nam SH, Lim G, Chung ML. Risk factor analysis of parenteral nutrition-associated cholestasis in extremely low birth weight infants. Acta Paediatr. (2016) 105(7):e313–9. doi: 10.1111/apa.13441
14. Veenstra M, Danielson L, Brownie E, Saba M, Natarajan G, Klein M. Enteral nutrition and total parenteral nutrition components in the course of total parenteral nutrition-associated cholestasis in neonatal necrotizing enterocolitis. Surgery. (2014) 156(3):578–83. doi: 10.1016/j.surg.2014.04.031
15. Kim AY, Lim RK, Han YM, Park KH, Byun SY. Parenteral nutrition-associated cholestasis in very low birth weight infants: a single center experience. Pediatr Gastroenterol Hepatol Nutr. (2016) 19(1):61–70. doi: 10.5223/pghn.2016.19.1.61
16. Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. (2007) 14(1):63–7. doi: 10.1097/MED.0b013e3280122850
17. Chaudhari S, Kadam S. Total parenteral nutrition in neonates. Indian Pediatr. (2006) 43(11):953–64.17151398
18. Wang N, Wang J, Zhang T, Huang L, Yan W, Lu L, et al. Alterations of gut microbiota and serum bile acids are associated with parenteral nutrition-associated liver disease. J Pediatr Surg. (2021) 56(4):738–44. doi: 10.1016/j.jpedsurg.2020.06.035
19. Henkel J, Alfine E, Saín J, Jöhrens K, Weber D, Castro JP, et al. Soybean oil-derived poly-unsaturated fatty acids enhance liver damage in NAFLD induced by dietary cholesterol. Nutrients. (2018) 10(9):1326. doi: 10.3390/nu10091326
20. Dembinski J, Behrendt D, Reinsberg J, Bartmann P. Endotoxin-stimulated production of IL-6 and IL-8 is increased in short-term cultures of whole blood from healthy term neonates. Cytokine. (2002) 18(2):116–9. doi: 10.1006/cyto.2002.0880
21. Bertolini A, Fiorotto R, Strazzabosco M. Bile acids and their receptors: modulators and therapeutic targets in liver inflammation. Semin Immunopathol. (2022) 44(4):547–64. doi: 10.1007/s00281-022-00935-7
22. Xiao F, Gao X, Hu H, Le J, Chen Y, Shu X, et al. Exclusive enteral nutrition exerts anti-inflammatory effects through modulating microbiota, bile acid metabolism, and immune activities. Nutrients. (2022) 14(21):4463. doi: 10.3390/nu14214463
23. van Zwol A, Neu J, van Elburg RM. Long-term effects of neonatal glutamine-enriched nutrition in very-low-birth-weight infants. Nutr Rev. (2011) 69(1):2–8. doi: 10.1111/j.1753-4887.2010.00359.x
24. Vermeulen MA, van Stijn MF, Visser M, Lemmens SM, Houdijk AP, van Leeuwen PA, et al. Taurine concentrations decrease in critically ill patients with shock given enteral nutrition. JPEN J Parenter Enteral Nutr. (2016) 40(2):264–72. doi: 10.1177/0148607114567199
25. Verner A, Craig S, McGuire W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev. (2007) 2007(4):Cd006072. doi: 10.1002/14651858.CD006072.pub2
26. Bae HJ, Shin SH, Kim EK, Kim HS, Cho YS, Gwak HS. Effects of cyclic parenteral nutrition on parenteral nutrition-associated cholestasis in newborns. Asia Pac J Clin Nutr. (2019) 28(1):42–8. doi: 10.6133/apjcn.201903_28(1).0007
27. Vongbhavit K, Underwood MA. Predictive value of the aspartate aminotransferase to platelet ratio Index for parenteral nutrition-associated cholestasis in premature infants with intestinal perforation. JPEN J Parenter Enteral Nutr. (2018) 42(4):797–804. doi: 10.1177/0148607117722755
28. Potter CJ. Cholestasis in the premature infant. Clin Perinatol. (2020) 47(2):341–54. doi: 10.1016/j.clp.2020.02.009
29. Gupta K, Wang H, Amin SB. Parenteral nutrition-associated cholestasis in premature infants: role of macronutrients. JPEN J Parenter Enteral Nutr. (2016) 40(3):335–41. doi: 10.1177/0148607114555161
30. Matsukubo M, Sugita K, Muto M, Yano K, Harumatsu T, Kurimoto T, et al. Long-term parenteral nutrition and delayed establishment of enteral nutrition in extremely low birth weight infants with high enterostomy site is associated with prolonged cholestasis. Pediatr Surg Int. (2024) 41(1):37. doi: 10.1007/s00383-024-05946-z
Keywords: very and extremely preterm infants (VPT), parenteral nutrition-associated cholestasis (PNAC), parenteral nutrition (PN), enteral nutrition (EN), interaction
Citation: Ma X, Zhou Y, Guo Y and Ye L (2025) Predictive role of parenteral and enteral nutrition duration in parenteral nutrition-associated cholestasis among very preterm infants. Front. Pediatr. 13:1651046. doi: 10.3389/fped.2025.1651046
Received: 20 June 2025; Accepted: 25 August 2025;
Published: 11 September 2025.
Edited by:
Thomai Karagiozoglou- Lampoudi, International Hellenic University, GreeceReviewed by:
Koshiro Sugita, Kagoshima University Hospital, JapanAikaterini Drougia, University Hospital of Ioannina, Greece
Copyright: © 2025 Ma, Zhou, Guo and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolei Ma, TU5CVkM5MTEyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaolei Ma
Xiaolei Ma Ying Zhou†
Ying Zhou† Yanting Guo
Yanting Guo