- 1Department of Pediatric Nephrology and Rheumatism and Immunology, Children’s Hospital Affiliated to Shandong University, Jinan, Shandong, China
- 2Department of Pediatric Nephrology and Rheumatism and Immunology, Jinan Children’s Hospital, Jinan, Shandong, China
Hydroxychloroquine (HCQ) therapy is the main treatment for systemic lupus erythematosus (SLE); however, rare adverse effects, including generalized pustular psoriasis (GPP), have been predominantly reported in adults. We herein report the first case of GPP caused by HCQ in a pediatric SLE patient. A 7-year-old girl with SLE developed fever, hepatic dysfunction, and disseminated pustules 3 weeks after starting HCQ. Histopathological examination revealed the characteristic features of GPP, including epidermal hyperkeratosis with parakeratosis, pustule formation above the stratum spinosum and acanthosis. Neutrophils and lymphocytes were observed in the superficial and mid-dermal vascular plexuses. HCQ therapy was discontinued, and the patient received methylprednisolone, intravenous immunoglobulin, meropenem, and hepatoprotective therapy. After 9 days of treatment, the pustules had largely resolved and inflammatory markers had returned to normal. This unprecedented pediatric observation underscores HCQ as a potential trigger for GPP in pediatric patients with SLE and highlights the importance of immediate HCQ discontinuation for optimal outcome. Interleukin-17 and interleukin-36 cytokine pathways may synergistically contribute to pathogenesis, suggesting a role for targeted therapies.
Introduction
Psoriasis is a chronic inflammatory disease which can be divided into non-pustular and pustular forms. Among non-pustular psoriases, plaque psoriasis (also known as psoriasis vulgaris) is the most common subtype, accounting for approximately 90% of cases (1, 2). The chronic inflammatory response characteristic of this condition is primarily driven by the presence of pathogenic T cells in the skin. The T helper 17 (Th17)-interleukin17 (IL-17)-interleukin23 (IL-23) axis constitutes the central mechanism underlying disease pathogenesis (1). Psoriasis cum pustulatione denotes the fleeting, scattered, sterile micropustules that arise within or at the margins of pre-existing psoriatic plaques and patients are usually afebrile with no systemic inflammatory signs. Pustular psoriasis (PP) comprises generalized pustular psoriasis (GPP), impetigo herpetiformis and localized pustular psoriasis. Whether palmoplantar pustulosis should be classified as localized pustular psoriasis or regarded as a distinct entity remains controversial (1, 2).
GPP is a rare, yet potentially life-threatening autoinflammatory dermatosis. GPP presents with erythema, pain, fever, and systemic manifestations; within hours, pinpoint pustules emerge, coalesce, and form lakes of pus (1, 2). IL-17 and interleukin-36(IL-36) are key drivers in the pathogenesis of GPP. The pro-inflammatory activity of IL-36 is further amplified by feedback regulation within the IL-17/IL-36 axis (3). It exhibits heterogeneous progression patterns, ranging from self-limiting to chronic refractory presentations. GPP most commonly is idiopathic and not triggered by drugs. Hydroxychloroquine (HCQ) therapy, the main treatment for systemic lupus erythematosus (SLE), is generally well-tolerated but has been increasingly recognized as a potential trigger for psoriatic flares, especially pustular variants. However, reported cases of HCQ-associated GPP have only been in adults.
Here, we present the first case of HCQ-induced GPP in a pediatric patient with SLE, expanding the spectrum of HCQ-related adverse effects and linking the immunopathogenesis of these conditions. This case underscores the need for careful monitoring to detect early this rare but serious adverse drug reaction in pediatric patients. It also highlights the need for further research to optimize treatments for this population with dual autoinflammatory pathologies.
Case report
A 7-year-old girl presented with a history of a recurrent malar erythema for 3 months, bilateral thigh myalgia for 3 weeks, and febrile illness for 2 days. Physical examination revealed classic cutaneous manifestations, including a butterfly-shaped facial erythema and palmar erythema, accompanied by arthritis. Laboratory investigations demonstrated hematologic (leukocytopenia: 3.62 × 109/L) and immunologic abnormalities [hypocomplementemia: C3 0.223 g/L, C4 <0.0575 g/L; positive ANA (1:1000) and anti-dsDNA antibodies]. These findings confirmed a diagnosis of SLE. Initial management comprised high-dose methylprednisolone pulse therapy (500 mg/day for 3 days) followed by oral prednisone (55 mg/day), supplemented with mycophenolate mofetil (0.25 g BID), belimumab (240 mg/dose), and HCQ (75 mg BID).
Approximately 3 weeks after HCQ therapy, the patient developed a progressively worsening pruritic pustules, which progressed from the lumbar area to the facial, truncal, and extremity regions (Figure 1). A skin biopsy of the right upper limb pustular eruptions showed epidermal hyperkeratosis with parakeratosis, pustule formation above the stratum spinosum and acanthosis. Neutrophils and lymphocytes were observed in the superficial and mid-dermal vascular plexuses (Figure 2).
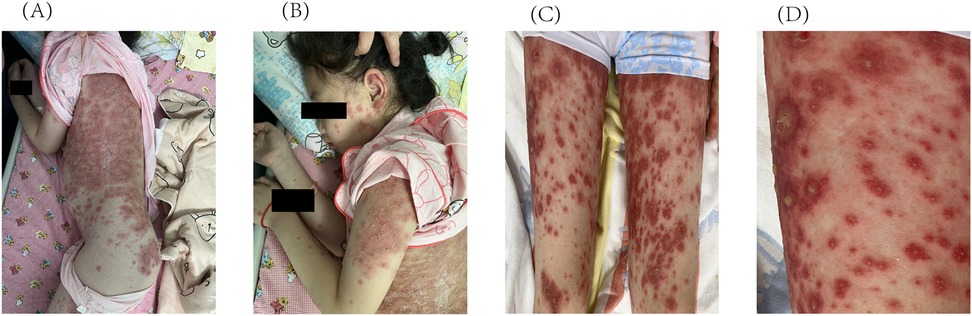
Figure 1. Initial cutaneous manifestations of pustular psoriasis. Multiple pustules on the intense erythema are observed on (A) the trunk, (B) the face and upper limbs, (C) the lower limbs. (D) Enlarged image of the lower limbs.
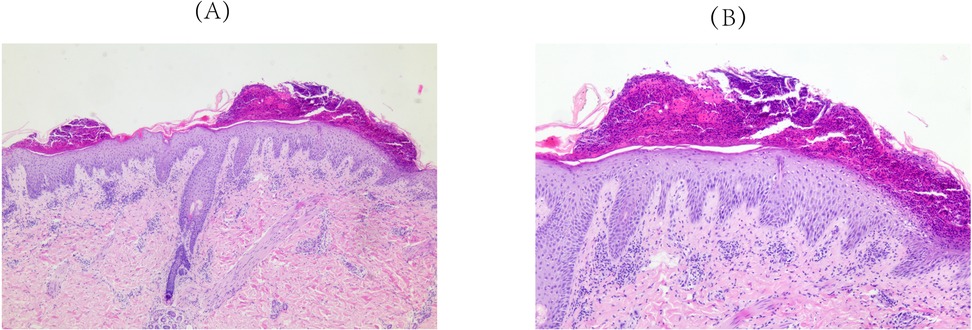
Figure 2. Pathological findings of skin biopsy (right upper limb). Epidermal hyperkeratosis with parakeratosis, pustule formation above the stratum spinosum and acanthosis. Neutrophils and lymphocytes localizes to the superficial and mid-dermal vascular plexuses were observed. (A) Original magnification ×50, (B) Original magnification ×100; hematoxylineosin staining.
The clinical course was complicated by pyrexia with substantially elevated acute phase reactants (C-reactive protein: 93.59 mg/L; procalcitonin: 0.62 ng/ml), necessitating sequential antimicrobial therapy, initially with azithromycin followed by meropenem, and adjunctive intravenous immunoglobulin administration. Significant clinical improvement was observed with therapeutic intervention comprising intravenous administration of methylprednisolone (22 mg BID), immunoglobulin, ammonium glycyrrhizinate-cysteine complex, and vitamin C; oral medications (mycophenolate mofetil 0.25 g BID); immediate cessation of HCQ; and localized topical therapy (including silicone oil cream and compound miconazole nitrate cream). After 9 days of therapy, the pustules had largely resolved with desquamation, demonstrating marked clinical improvement (Figure 3). Although secukinumab biologic therapy was recommended during treatment, it was postponed based on parental preference. At the 12-month follow-up, the patient maintained complete remission without skin lesion recurrence. The prednisone dose was successfully tapered to a maintenance dose of 7.5 mg/day with sustained disease control.
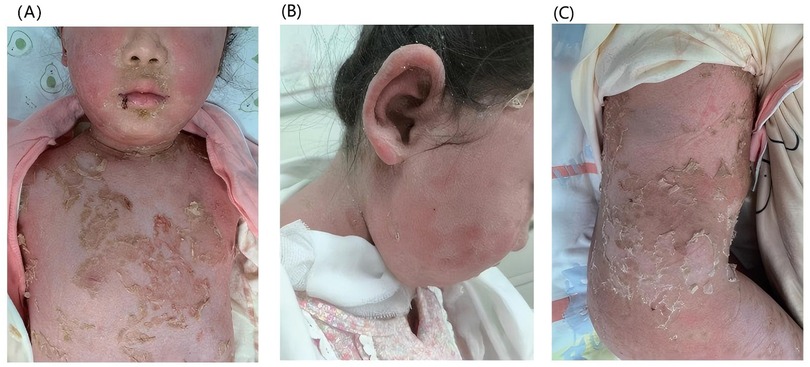
Figure 3. Cutaneous manifestations after nine days of treatment. After treatment, the pustules gradually subsided with subsequent desquamation: (A) the trunk, (B) the face, (C) the trunk and the lower limbs.
Discussion
GPP is a rare variant of psoriasis, accounting for approximately 1% of cases with varying global incidence (4). Some adult-onset GPP typically occurs in patients with prior psoriasis history, while pediatric-onset GPP frequently develops in the absence of prior psoriasis history. It differs between adults and children, with pediatric cases often being less severe. However, severe cases can be life-threatening and often exhibit recurrent episodes (5).
GPP demonstrates histopathological hallmarks of psoriasiform hyperplasia and spongiform pustules of Kogoj, with minimal or absent eosinophils. In contrast, acute generalized exanthematous pustulosis (AGEP), histopathology reveals sub corneal/intraepidermal pustules amid spongiosis, a neutrophil-rich infiltrate with eosinophils. Focal keratinocyte necrosis and dermal edema may be present (6, 7). Unlike our pediatric SLE patient, Liccioli et al. (8) reported the first pediatric case of AGEP induced by HCQ treated for Sjögren Syndrome, posing such a drug as a possible trigger also in children. Additionally, an Iranian study (9) analyzing 20 female patients with HCQ-induced pustular eruptions—whose clinical and histopathologic features did not fully meet criteria for AGEP or PP—identified overlapping characteristics of both conditions. The authors first proposed that this HCQ-triggered overlap presentation may represent a new entity, while acknowledging that further studies are required for validation. Pustular drug reaction with eosinophilia and systemic symptoms (pustular DRESS) evolves from maculopapular eruptions to folliculocentric pustules, accompanied by facial edema, lymphadenopathy, eosinophilia, and multiorgan involvement (notably hepatotoxicity). Histopathology shows dense superficial and deep perivascular infiltrates rich in lymphocytes, eosinophils, and atypical lymphocytes, often with interface dermatitis (10, 11). In this pediatric case, with pustules appearing approximately three weeks after medication use. The histopathology demonstrated classic features of pustular psoriasis. No eosinophils, necrotic keratinocytes were observed. These findings support a diagnosis of GPP and are inconsistent with AGEP. Furthermore, the absence of facial edema, eosinophilia, or multi-organ involvement—along with the histopathological features—rules out pustular DRESS.
Triggers of GPP include abrupt medication withdrawal including corticosteroids or other anti-psoriatic medications, bacterial/viral infections, taking antimalarial agents, immunization, pregnancy, psychological stress, and hypothyroidism (12). Several studies have demonstrated that the most common trigger of GPP is the withdrawal of systemic glucocorticoids (13–15). Conversely, GPP flares have also been reported in patients with non-pustular or localized pustular psoriasis after glucocorticoid exposure (16). The most commonly reported case is the development of GPP in patients with plaque psoriasis treated with corticosteroids (17). In the present case, the child had no prior history of psoriasis and was still in the initial, full-dose induction phase of glucocorticoid therapy. The GPP improved without discontinuing the glucocorticoids, thereby excluding glucocorticoid as the trigger of GPP in this patient. Additionally, the onset of GPP was not preceded by infection at any site, indicating the absence of an infectious trigger. The elevation of acute-phase reactants on day 5 after admission is considered to reflect a systemic inflammatory response. Nonetheless, secondary bacterial infection cannot be completely excluded.
HCQ has an excellent safety profile, dermatological manifestations, particularly maculopapule, erythema, urticaria and pruritus, represent the predominant skin-related adverse reactions, affecting up to 10% of treated patients. Long-term administration may lead to pigmentation changes in skin and mucous membranes. Notably, severe cutaneous adverse reactions including GPP, DRESS, and AGEP remain exceedingly uncommon (18, 19). Recent studies show it may worsen psoriasis and induce GPP, GPP of pregnancy, and palmoplantar pustulosis (20–22). Ten cases of HCQ-induced GPP in adult patients have been documented (Table 1) (20, 21, 23–29). Notably, five of them had SLE as the underlying condition. Currently, although psoriasis and SLE share common pathogenic mechanisms, their coexistence remains relatively rare. Tselios et al. reported 63 cases of psoriasis among 1,823 SLE patients in Canada (incidence rate 3%) (30). According to a report by Ali et al., the incidence of GPP is low, with an estimated prevalence of concurrent GPP and SLE at 0.69% (31). Additionally, Yu et al. reported a case of GPP coexisting with subacute cutaneous lupus erythematosus (32).We herein present the first documented case of HCQ-induced GPP in a pediatric patient with SLE. The causality assessment was carried out using Naranjo scale (Table 2), yielding a total score of 8. This score indicates a “probable” causal relationship between HCQ and the adverse reaction. Despite the potential for rechallenge to raise the score by 2 points (to a “definite” level), ethical and clinical concerns rendered it inadvisable. No other potential GPP triggers, as previously enumerated, were identified in this child. Importantly, upon further inquiry regarding the medication history, the parents explicitly reported that the child developed dry skin and pruritus following the initiation of oral HCQ. With cumulative exposure to the drug, pustular eruptions emerged. After discontinuing HCQ, the patient continued the original treatment for the underlying disease and received ongoing symptomatic supportive care. The condition was relieved, further confirming the role of HCQ in causing GPP.
Emerging evidence has implicated HCQ as a precipitating factor for GPP through the inhibition of epidermal transglutaminase, leading to keratinocyte hyperproliferation via IL-17 overproduction (33). Elevated IL-17 levels in both GPP and SLE suggest a shared pathogenesis (34), with IL-17 inhibitors demonstrating promise for SLE treatment (35). Secukinumab has shown efficacy in adult refractory cases, including lupus nephritis with comorbid psoriasis (36) and HCQ-induced GPP with concurrent SLE, demonstrating rapid improvement and sustained remission (27, 28). Pediatric GPP respond similarly to secukinumab (37). Moreover, IL-36 can bind to the IL-36 receptor, initiating an inflammatory cascade that promotes the expression of various cytokines and neutrophil-attracting chemokines. The expression of IL-36 can be enhanced by inflammatory cytokines derived from other immune cells (such as CD4+ T cells), including IL-17A and IL-23. Increased levels of IL-36 may further drive the differentiation of IL-17-producing CD4+ T cells. These cytokines could establish a positive-feedback loop through autocrine and paracrine mechanisms (38, 39). Interleukin 36RN may be the most common causative gene for GPP. Genetic testing was indeed performed for this child. The results identified a heterozygous missense variant in the IL36RN gene: NM_012275.3: c.129C>A (p.Ser43Arg). But this variant was interpreted as one of “Uncertain Significance” (VUS) according to the ACMG guidelines. The IL-36 receptor inhibitor, spesolimab, achieves remission in adults with refractory GPP/SLE cases after conventional therapy failure (29), with preliminary pediatric data on GPP showing favorable outcomes (40). These findings suggest that inhibitors of the IL-17 and IL-36 pathways are promising therapeutic options for this complex clinical scenario.
GPP is a rare yet severe dermatological condition that can be life-threatening. Although HCQ has been implicated as a potential trigger of GPP, its use requires careful risk-benefit evaluation. For HCQ-induced GPP in pediatric patients with SLE, the prognosis largely depends on timely diagnosis and appropriate therapeutic intervention. While biological agents have been well-documented in the literature as effective treatment options for idiopathic GPP, our case report suggests that prompt drug discontinuation may obviate the need for biologic therapy in cases of drug-induced GPP. Nevertheless, owing to its rare incidence, existing evidence remains limited. Hence, We still need further validation of clinical data.
Patient perspective
The parents of this patient expressed satisfaction with both the treatment process and the clinical outcome; however, they declined the proposed therapy with secukinumab.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Jinan Children's Hospital (Children's Hospital Affiliated to Shandong University). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft, Writing – review & editing. LD: Writing – original draft. HQ: Writing – original draft. YX: Writing – original draft. LK: Writing – original draft. WZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Armstrong AW, Blauvelt A, Callis Duffin K, Huang YH, Savage LJ, Guo L, et al. Psoriasis. Nat Rev Dis Primers. (2025) 11(1):45. doi: 10.1038/s41572-025-00630-5
2. Kshirsagar SJ, Adhav PS, Laddha UD, Ganore JS, Pagar CS, Bambal VR. Navigating psoriasis: from immune mechanisms to natural healing approaches. Int Immunopharmacol. (2025) 144:113626. doi: 10.1016/j.intimp.2024.113626
3. Puig L, Fujita H, Thaçi D, Zheng M, Daly ACH, Leonardi C, et al. Current treatments for generalized pustular psoriasis: a narrative summary of a systematic literature search. Dermatol Ther (Heidelb). (2024) 14(9):2331–78. doi: 10.1007/s13555-024-01230-z
4. Prinz JC, Choon SE, Griffiths CEM, Merola JF, Morita A, Ashcroft DM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. (2023) 37(2):256–73. doi: 10.1111/jdv.18720
5. Puig L, Choon SE, Gottlieb AB, Marrakchi S, Prinz JC, Romiti R, et al. Generalized pustular psoriasis: a global Delphi consensus on clinical course, diagnosis, treatment goals and disease management. J Eur Acad Dermatol Venereol. (2023) 37(4):737–52. doi: 10.1111/jdv.18851
6. Stadler PC, Oschmann A, Kerl-French K, Maul JT, Oppel EM, Meier-Schiesser B, et al. Generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. (2023) 239(3):328–33. doi: 10.1159/000529218
7. Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasis. J Cutan Pathol. (2010) 37(12):1220–9. doi: 10.1111/j.1600-0560.2010.01612.x
8. Liccioli G, Marrani E, Giani T, Simonini G, Barni S, Mori F. The first pediatric case of acute generalized exanthematous pustulosis caused by hydroxychloroquine. Pharmacology. (2019) 104(1–2):57–9. doi: 10.1159/000500406
9. Behrangi E, Hallaji Z, Ghiasi M, Abedini R, Ghanadan A, Khodashenas Z, et al. Hydroxychloroquine-induced unusual generalized pustular cutaneous reaction as a new clinical entity: a case series. Immunoregulation. (2020) 3(1):67–72. doi: 10.32598/IMMUNOREGULATION.3.1.8
10. Gössinger E, Dodiuk-Gad R, Mühleisen B, Oon HH, Oh CC, Maul JT, et al. Generalized pustular psoriasis, acute generalized exanthematous pustulosis, and other pustular reactions: a clinical review. Dermatol Clin. (2024) 42(2):317–28. doi: 10.1016/j.det.2024.01.001
11. Girijala RL, Siddiqi I, Kwak Y, Wright D, Patel DB, Goldberg LH. Pustular DRESS syndrome secondary to hydroxychloroquine with EBV reactivation. J Drugs Dermatol. (2019) 18(2):207–9.30811149
12. Kodali N, Kwatra SG. Current management of generalized pustular psoriasis. Exp Dermatol. (2023) 32(8):1204–18. doi: 10.1111/exd.14765
13. Brenner M, Molin S, Ruebsam K, Weisenseel P, Ruzicka T, Prinz JC. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. (2009) 161(4):964–6. doi: 10.1111/j.1365-2133.2009.09348.x
14. Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. (2022) 23(Suppl 1):21–9. doi: 10.1007/s40257-021-00654-z
15. Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. (2018) 32(10):1645–51. doi: 10.1111/jdv.14949
16. Baker H. Corticosteroids and pustular psoriasis. Br J Dermatol. (1976) 94(suppl 12):83–8. doi: 10.1111/j.1365-2133.1976.tb02274.x
17. Torres T, Antunes J, Tavares Bello R, Varela P, Henrique M, Marques Pinto G, et al. Update on generalized pustular psoriasis. Acta Med Port. (2025) 38(5):321–30. doi: 10.20344/amp.22672
18. Huo R, Wei C, Yang Y, Lin J, Huang X. Hydroxychloroquine: a double-edged sword (review). Mol Med Rep. (2025) 31(4):102. doi: 10.3892/mmr.2025.13467
19. Rapparini L, Cedirian S, Placa ML, Piraccini BM, Raschi E, Starace M. Safety of hydroxychloroquine: what a dermatologist should know. Am J Clin Dermatol. (2025) 26(2):251–64. doi: 10.1007/s40257-025-00919-x
20. Shindo E, Shikano K, Kawazoe M, Yamamoto T, Kusunoki N, Hashimoto Y, et al. A case of generalized pustular psoriasis caused by hydroxychloroquine in a patient with systemic lupus erythematosus. Lupus. (2019) 28(8):1017–20. doi: 10.1177/0961203319854139
21. Ryoo YW, Yun JM, Kim HW, Kim SA. Early-onset generalized pustular psoriasis of pregnancy following hydroxychloroquine use. Ann Dermatol. (2023) 35(Suppl 1):S43–7. doi: 10.5021/ad.21.062
22. Karaalioğlu B, Yıldırım F, Mutlu MY, Akkuzu G, Özgür DS, Bes C. A case of palmoplantar pustular psoriasis induced by hydroxychloroquine in a patient with systemic lupus erythematosus. Int J Rheum Dis. (2022) 25(3):1–3. doi: 10.1111/1756-185X.14392
23. Friedman SJ. Pustular psoriasis associated with hydroxychloroquine. J Am Acad Dermatol. (1987) 16(6):1256–7. doi: 10.1016/s0190-9622(87)80021-x
24. Vine JE, Hymes SR, Warner NB, Cohen PR. Pustular psoriasis induced by hydroxychloroquine: a case report and review of the literature. J Dermatol. (1996) 23(5):357–61. doi: 10.1111/j.1346-8138.1996.tb04031.x
25. Gravani A, Gaitanis G, Zioga A, Bassukas ID. Synthetic antimalarial drugs and the triggering of psoriasis—do we need disease-specific guidelines for the management of patients with psoriasis at risk of malaria? Int J Dermatol. (2014) 53(3):327–30. doi: 10.1111/ijd.12231
26. Maglie R, Dini V, Romanelli M. Pustular psoriasis induced by hydroxychloroquine: a case report to confirm a rare association. J Am Acad Dermatol. (2016) 74(5 Suppl):AB265. doi: 10.1016/j.jaad.2016.02.1027
27. Kishibe M, Takeda K, Honma M, Makino Y, Yamamoto AI. Effectiveness of anti-interleukin-17-receptor antibody for hydroxychloroquine-induced generalized pustular psoriasis in a patient with systemic lupus erythematosus. J Dermatol. (2022) 49(4):e428–9. doi: 10.1111/1346-8138.16514
28. Akaji K, Nakagawa Y, Kakuda K, Takafuji M, Kiyohara E, Murase C. Generalized pustular psoriasis associated with systemic lupus erythematosus successfully treated with secukinumab. J Dermatol. (2021) 48(1):e43–4. doi: 10.1111/1346-8138.15645
29. Minami Y, Shinkuma S, Yokoyama T, Shinoda S, Hamada K, Arima A, et al. A case of generalized pustular psoriasis complicated with SLE successfully treated with spesolimab. J Dermatol. (2024) 51(10):e335–6. doi: 10.1111/1346-8138.17243
30. Tselios K, Yap KS, Pakchotanon R, Polachek A, Su J, Urowitz MB, et al. Psoriasis in systemic lupus erythematosus: a single-center experience. Clin Rheumatol. (2017) 36(4):879–84. doi: 10.1007/s10067-017-3566-0
31. Ali M, Abdullah S, Abdulhameed Y, Elbadawi F. Systemic lupus erythematosus and psoriasis overlap: a case series. Cureus. (2025) 17(5):e84760. doi: 10.7759/cureus.84760
32. Yu X, Jin H, Fang K. A case of generalized pustular psoriasis complicated with subacute cutaneous lupus erythematosus. Med J PUMCH. (2017) 8(2–3):187–9. doi: 10.3969/j.issn.1674-9081.2017.03.020
33. Öncü INS, Güler D, Gürel G. Exacerbation of psoriasis following hydroxychloroquine in a patient with suspected COVID-19. Dermatol Ther. (2021) 34(2):e14806. doi: 10.1111/dth.14806
34. Mohammadi S, Sedighi S, Memarian A. IL-17 is aberrantly overexpressed among under-treatment systemic lupus erythematosus patients. Iran J Pathol. (2019) 14(3):236–42. doi: 10.30699/ijp.2019.94878.1934
35. Ramaswamy M, Tummala R, Streicher K, Nogueira da Costa A, Brohawn PZ. The pathogenesis, molecular mechanisms, and therapeutic potential of the interferon pathway in systemic lupus erythematosus and other autoimmune diseases. Int J Mol Sci. (2021) 22(20):11286. doi: 10.3390/ijms222011286
36. Satoh Y, Nakano K, Yoshinari H, Nakayamada S, Iwata S, Kubo S, et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus. (2018) 27(7):1202–6. doi: 10.1177/0961203318762598
37. Chen Y, Wang Z, Xiang X. Two cases of childhood generalized pustular psoriasis treated with secukinumab. Chin J Dermatol. (2023) 56(9):874–6. doi: 10.35541/cjd.20210214
38. Lee CC, Huang YH, Chi CC, Chung WH, Chen CB. Generalized pustular psoriasis: immunological mechanisms, genetics, and emerging therapeutics. Trends Immunol. (2025) 46(1):74–89. doi: 10.1016/j.it.2024.12.001
39. Bachelez H, Barker J, Burden AD, Navarini AA, Krueger JG. Generalized pustular psoriasis is a disease distinct from psoriasis vulgaris: evidence and expert opinion. Expert Rev Clin Immunol. (2022) 18(10):1033–47. doi: 10.1080/1744666X.2022.2116003
Keywords: generalized pustular psoriasis, hydroxychloroquine, systemic lupus erythematosus, pediatric, adverse drug reaction
Citation: Wang X, Dong L, Qiu H, Xu Y, Kong L and Zhou W (2025) Case Report: Hydroxychloroquine-induced generalized pustular psoriasis in a pediatric patient with systemic lupus erythematosus. Front. Pediatr. 13:1652376. doi: 10.3389/fped.2025.1652376
Received: 23 June 2025; Accepted: 29 September 2025;
Published: 13 October 2025.
Edited by:
Dennis Niebel, University Medical Center Regensburg, GermanyReviewed by:
Ivan Arni Caballero Preclaro, Tondo Medical Center, PhilippinesLukas Sollfrank, University Hospital Erlangen, Germany
Sebaranjan Biswal, Kalinga Institute of Medical Sciences, India
Copyright: © 2025 Wang, Dong, Qiu, Xu, Kong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiran Zhou, d2VpcmFueHVlMjAxNEAxNjMuY29t
 Xue Wang
Xue Wang Linlin Dong
Linlin Dong Hui Qiu
Hui Qiu Yihuai Xu
Yihuai Xu Linxiaoyu Kong
Linxiaoyu Kong Weiran Zhou
Weiran Zhou