- 1The Second Clinical Medical School, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, China
- 2Department of Pediatric Surgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an Shaanxi, China
- 3Department of Pediatric Surgery, Guilin Maternal and Child Health Hospital, Guangxi, Zhuang Autonomous Region, China
- 4Department of Gastrointestinal Surgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
Background: Heterotopic pancreas (HP) is a congenital anomaly characterized by pancreatic tissue entirely separate from the orthotopic pancreas, lacking direct ductal communication and vascular continuity. HP is most frequently found in the upper gastrointestinal tract, particularly the stomach, duodenum, and proximal jejunum, while involvement of the mesentery is relatively rare
Case presentation: We report a case of congenital duodenal atresia with concomitant HP in a 3-day-old neonate. Emergency laparotomy was performed at 72 h of life for membranous duodenal atresia; web excision and duodenoduodenostomy were completed. Preoperative evaluation showed no other associated malformations (e.g., congenital heart disease or Down syndrome). Intraoperatively, a 10.0 × 6.0 mm focus of HP tissue was unexpectedly found at the jejunal mesenteric boundary, approximately 5–7 cm distal to the duodenal obstruction, necessitating en bloc resection with a segment of adjacent bowel. Histopathology confirmed HP. During 12-month postoperative follow-up, the neonate remained clinically stable, with physical development consistent with age-matched normal children, and no disease recurrence.
Conclusions: Given the potential association between HP and duodenal atresia in asymptomatic cases, we propose systematic screening for HP in all neonates with this condition. If identified, concurrent prophylactic resection of the HP lesion during the repair of duodenal atresia is recommended to mitigate the risk of long-term complications, including pancreatitis and gastrointestinal bleeding.
1 Introduction
Congenital duodenal obstruction (CDO), occurring in approximately 1.5 per 10,000 live births, requires early surgical intervention. The co-occurrence of CDO with heterotopic pancreas (HP) represents an exceptionally uncommon clinical presentation. HP refers to pancreatic tissue located outside the native gland, lacking vascular or ductal continuity with the orthotopic pancreas (1). Most individuals with HP remain asymptomatic during life, making its true incidence difficult to determine. Autopsy studies report a prevalence of 0.55% to 15% (1–3), while intraoperative and endoscopic detection rates are approximately 0.18% to 0.2% (4). Due to its frequent subclinical course, HP is often identified incidentally during histologic examination. Reported HP distribution varies across studies due to the absence of systematic evaluation; however, available literature indicates that HP primarily occurs in the stomach, duodenum, jejunum, and Meckel's diverticulum (1, 4). Extraintestinal occurrences (e.g., mesentery, greater omentum, spleen) are significantly rarer, with only isolated cases reported (3, 4).
Herein, we detail a rare case of congenital duodenal atresia associated with jejunal mesenteric heterotopic pancreas (MHP). The neonate required emergency laparotomy 72 h post-birth for CDO. Intraoperatively, HP tissue was incidentally identified at the boundary between the jejunum and its mesentery.
2 Case description
A female 35 + 5-week preterm infant was delivered vaginally following premature rupture of membranes. Antenatal ultrasonography revealed gastric and duodenal dilation with the characteristic “double-bubble sign” and polyhydramnios, prompting admission for suspected CDO.
The mother attended regular prenatal visits. At 32 weeks' gestation, ultrasound demonstrated polyhydramnios and a double-bubble sign, suggesting possible CDO. With no other anomalies detected, she continued routine monitoring. At 35 + 5 weeks, premature membrane rupture resulted in vaginal delivery of a 2,300 g female infant. Apgar scores were 9, 10, and 10 at (1/5/10 min). After birth, the newborn was assessed, with unremarkable findings on evaluation of vital signs, mental status, and physical abdominal examination. At 12 h after delivery, a trial feeding under close monitoring was initiated, beginning with clear water followed by breastfeeding. After increasing the volume of breast milk, the infant developed mild milk vomiting approximately 2 h later. The frequency of emesis increased progressively, and the vomitus transitioned to bilious yellow-green fluid without a feculent odor (5–10 ml/non-projectile episode). Given the prenatal ultrasound findings, radiography was promptly performed. The infant did not pass meconium within the first 24 h and had only two urinations (∼5 ml each), which were without hematuria or mucus. Clinical assessment revealed afebrile status, alertness, and no lethargy/irritability.
Admission laboratory investigations demonstrated the following hematologic and biochemical profiles: white blood cell count (WBC) 16.5 × 10⁹/L (55% neutrophils, 30% lymphocytes); red blood cell count (RBC) 5.2 × 1012/L; hemoglobin (Hb) 170.0 g/L; and platelets (PLT) 250.0 × 10⁹/L. Serum electrolytes were sodium (Na+) 138.0 mmol/L, potassium (K+) 4.0 mmol/L, and chloride (Cl−) 98.0 mmol/L. Preoperative serum amylase was 78.0 U/L (reference range: 25–125 U/L) and lipase was 55.0 U/L (reference range: 0–190 U/L). Hepatic and renal function panels were within normal limits. Abdominal radiography revealed marked gastric and duodenal dilation with the classic “double-bubble” sign (Figure 1). These radiographic findings, in conjunction with the clinical presentation, were highly suggestive of congenital duodenal atresia. Preoperative examinations revealed no other associated malformations (e.g., congenital heart disease or Down syndrome) in the neonate.
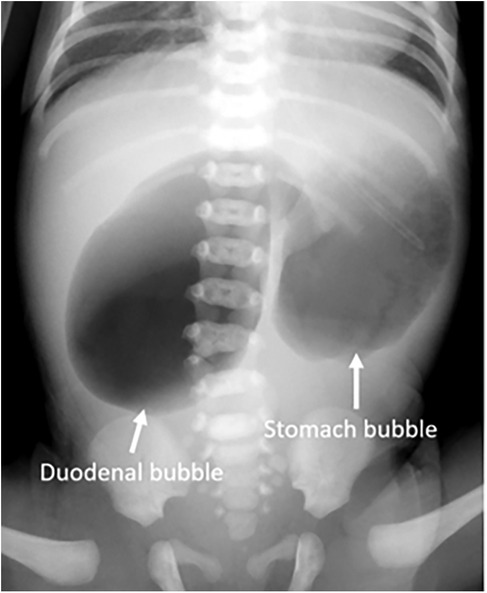
Figure 1. Preoperative x-ray shows the “double bubble” sign with no gas in the distal bowel (arrow).
Based on the “double-bubble” sign and CDO diagnosis, emergency laparotomy was performed 72 h post-birth. Preoperative nasogastric decompression yielded 50 ml of bilious fluid, substantially reducing abdominal distension. Intraoperative exploration confirmed membranous duodenal atresia (Figure 2A), and a duodenoduodenostomy was performed. During the procedure, a systematic exploration of the entire gastrointestinal tract (from the stomach to the rectum) was conducted, and no other obstructive lesions were identified. Incidentally, a 10.0 × 6.0 mm mass adherent to the boundary between the jejunum and its mesentery was identified (Figure 2B). Given potential complication risks, the mass was surgically resected. To ensure complete excision of the lesion and prevent residual pancreatic tissue, approximately 2 cm of the adjacent normal jejunal wall was resected along with the mass. Subsequently, primary jejunojejunostomy was performed using an end-to-end anastomosis technique to restore intestinal continuity. Histopathological examination of the resected specimen confirmed the diagnosis of MHP. Hematoxylin-eosin (H&E) staining of the HP tissue revealed typical acinar, ductal, and islet structures, consistent with Heinrich Type I (Figure 3).
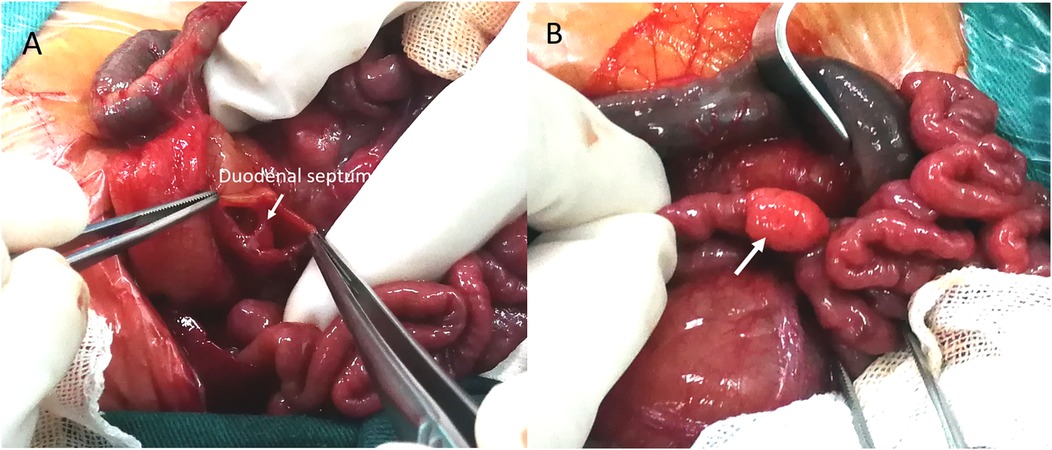
Figure 2. Intraoperative findings. (A) Surgery confirmed duodenal obstruction caused by a membrane (arrow). (B) A photograph of the HP mass in situ shows a 10.0 mm × 6.0 mm yellowish soft tissue mass located at the boundary between the jejunum and mesentery, adhering to the serosal surface of the jejunum (arrow).
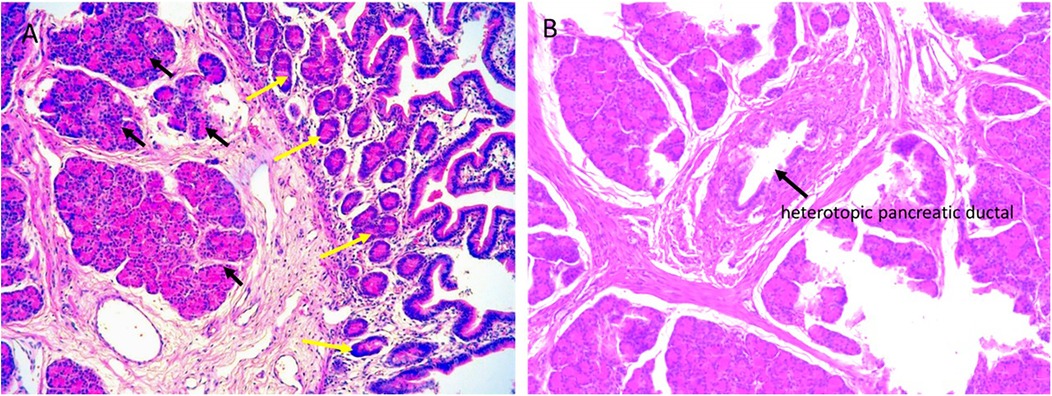
Figure 3. Histopathological analysis of the resected specimen. (A) Microscopically, the lesion consists of HP tissue (black arrow), including acini, islet cells, and HP tissue extending to the jejunal serosa (yellow arrow) (magnification × 200). (B) HP tissue and pancreatic ducts (black arrow) (magnification × 100). The histopathological features of the lesion are consistent with Heinrich Type I.
Postoperative laboratory investigations on day 3 revealed the following results: WBC 13.8 × 10⁹/L (45% neutrophils, 40% lymphocytes); RBC 4.9 × 1012/L; Hb 160.0 g/L; PLT 220.0× 10⁹/L; Na+ 136.0 mmol/L, K+ 3.9 mmol/L, Cl− 96.0 mmol/L. Serum amylase was 65.0 U/L and lipase was 48.0 U/L. Hepatic and renal function panels were within normal limits. The postoperative recovery was uneventful. Prophylactic antibiotics were administered preoperatively and for the first three days after surgery. Minimal enteral feeding was initiated on postoperative day 3 and advanced to full feeding by day 7. The patient was discharged on postoperative day 8. Twelve-month follow-up ultrasound showed no evidence of recurrent HP. Written informed consent for the publication of this case report and any accompanying images was obtained from the neonate's parents prior to manuscript submission.
3 Discussion
HP refers to pancreatic parenchyma anatomically separate from the orthotopic pancreas, lacking continuity with the normal pancreas. First described by Jean Schultz in 1727, HP was not histologically confirmed until Klob's detailed characterization in 1859 (5). HP is typically discovered incidentally during autopsy, surgery, or endoscopy (1, 4). Current literature—primarily case reports and small series—lacks large clinicopathologic studies (6). This gap in the literature, combined with its frequent subclinical presentation, makes it difficult to determine the true incidence.
HP is more common in males than females, with peak onset typically occurring between ages 40 and 60 (3, 4). The most frequent sites for HP anomalies are the duodenum (30%), stomach (25%), and jejunum (15%), followed by Meckel's diverticulum (6%). Ileal involvement is rare (3%) (1). Dolan et al. (3) reported that among 212 patients with HP, 136 were male and 76 were female. The heterotopic tissue was located in the stomach (81 cases), duodenum (77 cases), or upper jejunum (33 cases). The remaining cases involved Meckel's diverticulum (6%), ileum (3%), and various other sites in small proportions. In contrast, Zhang et al. (4) observed a slight female predominance in a cohort of 184 patients with HP (male-to-female ratio: 0.94). This study found the stomach to be the most common location (97 cases, 52.7%), followed by the small intestine (48 cases, 26%), lesser omentum (18 cases, 10%), spleen/portal vein region (5 cases, 2.7%), mesentery (7 cases, 3.8%), distal esophagus (4 cases, 2.2%), hilar region (2 cases, 1%), and gallbladder (1 case, 0.5%). CDO-HP co-occurrence remains exceptionally rare, with pediatric MHP being particularly scarce—rarely reported in children <10 years and exceedingly rare in neonates (7).
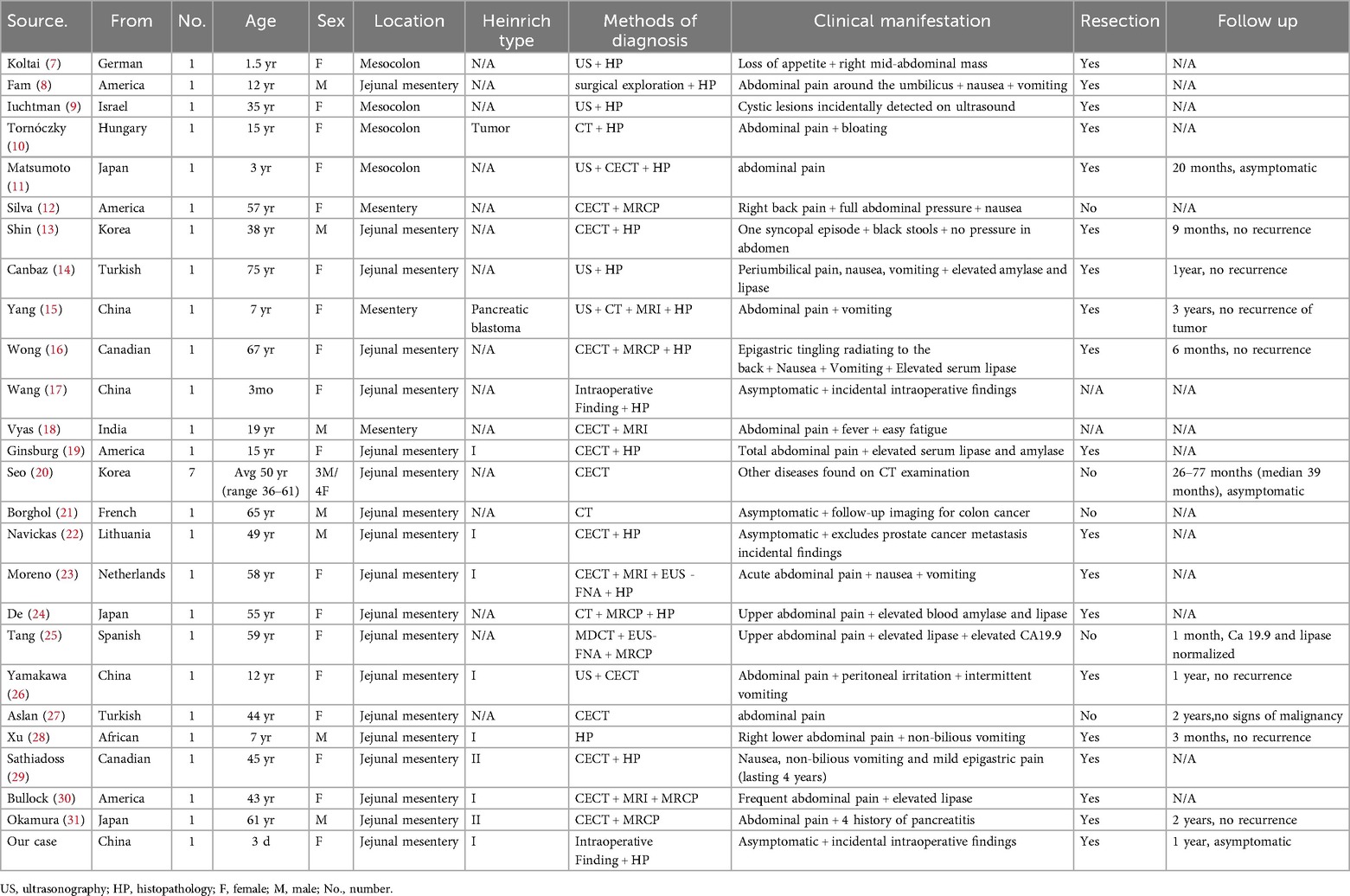
Since most occurrences of HP are in the upper gastrointestinal tract, extraintestinal incidence is substantially lower. We searched PubMed from January 1980 to August 2025 using [(“heterotopic pancrea*” OR “ectopic pancrea*”) AND mesent*]. Reference lists of eligible articles were screened. We included English-language case reports/series describing mesenteric HP with clinical details; our case was also included. A total of 32 reports were identified (7–31) (Table 1). From the table, MHP demonstrated a striking female predominance (68.75% [22/32] female vs. 31.25% [10/32] male), contrasting sharply with the established male predominance in general HP. Anatomically, 78.125% (25/32) involved jejunal mesentery, while 12.5% (4/32) affected colonic mesentery. Three additional cases described MHP in small intestinal mesentery without precise localization (12, 15, 18). Pathologically, most were Heinrich type I, comprising acini, ducts, and islet cells.
CT enhancement patterns reflect HP's microscopic composition: acinar-dominant lesions show homogeneous enhancement similar to or exceeding normal pancreas, while ductal-dominant lesions exhibit heterogeneous, reduced enhancement (32, 33). MHP demonstrates imaging characteristics analogous to orthotopic pancreas. Jejunal MHP typically appears as an elongated, lobulated lesion with a broad jejunal base (34). Mesenteric lesions show higher long-to-short diameter ratios (3.0) than gastric/proximal small bowel lesions (1.4–1.5) (20). MHP maximum diameters exceed gastric HP (4.4 cm vs. 1.8–2.7 cm), with longitudinal jejunal alignment more frequent than at other HP sites (20). Histopathologically, microscopic HP infiltrates jejunal walls into muscularis propria (13). In our case, HP extended to the serosa (Figure 3), suggesting MHP may exhibit extra-tubular growth.
In our review of the literature on MHP, CECT and MRCP proved most diagnostically valuable. Most patients exhibited normal orthotopic pancreas, while the HP typically manifested acute pancreatitis symptoms (12, 14, 23, 27, 30). Additionally, two cases of HP-related malignant transformation have also been reported (10, 15). Nevertheless, even in these presentations, differentiation from alternative conditions remains essential. Key differential diagnoses include acute appendicitis, perforated appendicitis, Meckel's diverticulitis (8, 28, 31), and para-duodenal hernia (19). When evaluating suspected gastrointestinal mesenchymal tumors, carcinoid tumors, lymphomas, and metastases—which may similarly present as homogeneous, well-enhanced soft tissue masses within the mesentery—CECT demonstrates limited reliability for distinguishing MHP (29). Although this patient's preoperative serum amylase and lipase levels were within normal limits and the mesenteric heterotopic pancreas (MHP) showed no secondary organic lesions, the future risk of severe complications—such as pancreatitis or malignant transformation—cannot be excluded. Given this potential for serious disease and malignant progression, local resection of the MHP represents the optimal clinical strategy for long-term prognosis (9, 13, 15, 29).
It is noteworthy that congenital duodenal atresia is frequently associated with other congenital anomalies, with congenital heart disease and Down syndrome being the most common (35). Other associated malformations include renal abnormalities and biliary atresia (36, 37). Therefore, conducting relevant baseline screening is crucial for patients with congenital duodenal atresia, both preoperatively and postoperatively (37). We also noted that Song et al. (38) previously reported a case of congenital duodenal atresia with coexisting heterotopic pancreas (HP), mirroring the dual pathological findings observed in our patient. This similarity raises a fundamental embryological question: Could aberrant migration or differentiation of the pancreatic bud disrupt duodenal recanalization during embryonic development? Importantly, this hypothesis does not amount to an inference of a causal relationship; it merely represents a potential embryological mechanism (38).
Bilious vomiting accompanied by delayed meconium passage in a neonate represents a clinical emergency, necessitating immediate referral to a specialized neonatal surgical center to prevent life-threatening complications such as intestinal ischemia, perforation, or septic shock. In the evaluation of a neonatal mesenteric mass or cyst, key differential diagnoses should include congenital enteric cyst, mesenteric lymphangioma, simple cyst, neoplastic cyst, and infectious cyst. In the present case, the mass was histopathologically confirmed to be HP without signs of metaplasia. An open surgical approach was adopted for this case, rather than a laparoscopic procedure. This decision was made after thorough team discussion, considering our limited experience with neonatal laparoscopic surgery. The primary goals were to ensure the safety of the anastomosis, minimize the risk of intraoperative complications, and optimize postoperative outcomes. The procedure was performed after obtaining informed consent from the child's guardians.
In conclusion, given the potential association between HP and duodenal atresia in asymptomatic cases, we propose systematic screening for HP in all neonates with this condition. If identified, concurrent prophylactic resection of the HP lesion during the repair of duodenal atresia is recommended to mitigate the risk of long-term complications, including pancreatitis and gastrointestinal bleeding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Xi'an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Data curation, Writing – original draft, Writing – review & editing. SiL: Data curation, Writing – original draft, Writing – review & editing. ShL: Formal analysis, Funding acquisition, Resources, Writing – review & editing. YL: Conceptualization, Writing – review & editing. DQ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. National Natural Science Foundation of China, No. 82170676; Natural Science Foundation of Shaanxi Provincial Key Industries Innovation Chain (Cluster)-Social Development Project, No. 2020ZDLSF02-03; Xi'an Talents Plan Project: Clinical Application of Minimally Invasive Treatment of Alimentary Tract Malformation in Children by Combining Medical and Industrial Innovative Technology of Magnetic Surgery, No. XAYC210064; Key Project of Shaanxi Province's 14th Five-Year Education Science Planning for 2024: Research on the Training Model for Top-notch Innovative Talents in Higher Education Institutions of Shaanxi Province, No. SGH24Z16.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pearson S. Aberrant pancreas. Review of the literature and report of three cases, one of which produced common and pancreatic duct obstruction. Arch Surg. (1951) 63(2):168–86. doi: 10.1001/ARCHSURG.1951.01250040172006
2. Alqahtani A, Aljohani E, Almadi F, Billa S, Alqahtani M, Alkhaldi H. Heterotopic pancreatic tissue in the gastric antrum an incidental finding during bariatric surgery: a case report and literature review. Int J Surg Case Rep. (2020) 67:39–41. doi: 10.1016/j.ijscr.2019.12.040
3. Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. (1974) 109(6):762–5. doi: 10.1001/archsurg.1974.01360060032010
4. Zhang Y, Sun X, Gold JS, Sun Q, Lv Y, Li Q, et al. Heterotopic pancreas: a clinicopathological study of 184 cases from a single high-volume medical center in China. Hum Pathol. (2016) 55:135–42. doi: 10.1016/j.humpath.2016.05.004
5. LeCompte MT, Mason B, Robbins KJ, Yano M, Chatterjee D, Fields RC, et al. Clinical classification of symptomatic heterotopic pancreas of the stomach and duodenum: a case series and systematic literature review. World J Gastroenterol. (2022) 28(14):1455–78. doi: 10.3748/wjg.v28.i14.1455
6. Zhang X, Peng L, Wang Z, Pan F, Ren R, Li Y, et al. Extensive heterotopic pancreas in a rare site: a case report and a review of literature. Medicine (Baltimore). (2023) 102(9):e32241. doi: 10.1097/MD.0000000000032241
7. Koltai IL, Hoffmann-von Kap-herr S, Weitzel D. [Accessory pancreatic cyst located in the mesocolon in a child (author’s transl)]. Z Kinderchir. (1981) 32(3):277–82. doi: 10.1055/s-2008-1063270
8. Fam S, O’Briain DS, Borger JA. Ectopic pancreas with acute inflammation. J Pediatr Surg. (1982) 17(1):86–7. doi: 10.1016/s0022-3468(82)80338-2
9. Iuchtman M, Auslander L. Ectopic pancreas cyst in the mesocolon. J Clin Gastroenterol. (1991) 13(6):716–7. doi: 10.1097/00004836-199112000-00022
10. Tornóczky T, Kálmán E, Jáksó P, Méhes G, Pajor L, Kajtár GG, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol. (2001) 54(3):241–5. doi: 10.1136/jcp.54.3.241
11. Matsumoto F, Tohda A, Shimada K, Kubota A. Pancreatic pseudocyst arising from ectopic pancreas and isolated intestinal duplication in mesocolon caused hydronephrosis in a girl with horseshoe kidney. J Pediatr Surg. (2005) 40(7):e5–7. doi: 10.1016/j.jpedsurg.2005.03.069
12. Silva AC, Charles JC, Kimery BD, Wood JP, Liu PT. MR Cholangiopancreatography in the detection of symptomatic ectopic pancreatitis in the small-bowel mesentery. AJR Am J Roentgenol. (2006) 187(2):W195–7. doi: 10.2214/AJR.04.1756
13. Shin SS, Jeong YY, Kang HK. Giant heterotopic pancreas in the jejunal mesentery. AJR Am J Roentgenol. (2007) 189(5):W262–3. doi: 10.2214/AJR.05.1142
14. Canbaz H, Colak T, Düşmez Apa D, Sezgin O, Aydin S. An unusual cause of acute abdomen: mesenteric heterotopic pancreatitis causing confusion in clinical diagnosis. Turk J Gastroenterol. (2009) 20(2):142–5. 19530049
15. Yang X, Wang X. Imaging findings of pancreatoblastoma in 4 children including a case of ectopic pancreatoblastoma. Pediatr Radiol. (2010) 40(10):1609–14. doi: 10.1007/s00247-010-1776-6
16. Wong JC, Robinson C, Jones EC, Harris A, Zwirewich C, Wakefield R, et al. Recurrent ectopic pancreatitis of the jejunum and mesentery over a 30-year period. Hepatobiliary Pancreat Dis Int. (2011) 10(2):218–20. doi: 10.1016/s1499-3872(11)60036-2
17. Wang D, Wei XE, Yan L, Zhang YZ, Li WB. Enhanced CT and CT virtual endoscopy in diagnosis of heterotopic pancreas. World J Gastroenterol. (2011) 17(33):3850–5. doi: 10.3748/wjg.v17.i33.3850
18. Vyas S, Khandelwal A, Sandhu MS, Verma S, Khandelwal N. Asymptomatic heterotopic pancreas in the mesentery. Trop Gastroenterol. (2012) 33(4):289–92. doi: 10.7869/tg.2012.75
19. Ginsburg M, Ahmed O, Rana KA, Boumendjel R, Dachman AH, Zaritzky M. Ectopic pancreas presenting with pancreatitis and a mesenteric mass. J Pediatr Surg. (2013) 48(1):e29–32. doi: 10.1016/j.jpedsurg.2012.10.062
20. Seo N, Kim JH. Characteristic CT features of heterotopic pancreas of the mesentery: “another pancreas” in the mesentery. Clin Imaging. (2014) 38(1):27–30. doi: 10.1016/j.clinimag.2013.09.008
21. Borghol S, Diris B, Alberti N, Crombe A, Laurent F. Ectopic pancreatic tissue in the jejunal mesentery. Diagn Interv Imaging. (2015) 96(11):1233–6. doi: 10.1016/j.diii.2015.01.009
22. Navickas G, Valančienė D. Ektopinė (heterotopinė) kasa plonosios žarnos pasaite: klinikinio atvejo pristatymas ir literatūros apžvalga. Acta Med Litu. (2016) 23(2):147–51. doi: 10.6001/actamedica.v23i2.3332
23. Moreno Pastor A, Olalla Muñoz JR, Esteban Delgado P, Soria Aledo V. Diagnosis of mesenteric ectopic pancreas by secretin-enhanced magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. (2018) 42(2):236–9. doi: 10.1097/RCT.0000000000000665
24. de Kok BM, de Korte FI, Perk LE, Terpstra V, Mieog JSD, Zijta FM. Acute clinical manifestation of mesenteric heterotopic pancreatitis: a pre- and postoperative confirmed case. Case Rep Gastrointest Med. (2018) 2018:5640379. doi: 10.1155/2018/5640379
25. Tang XB, Liao MY, Wang WL, Bai YZ. Mesenteric heterotopic pancreas in a pediatric patient: a case report and review of literature. World J Clin Cases. (2018) 6(14):847–53. doi: 10.12998/wjcc.v6.i14.847
26. Yamakawa K, Kurita A, Terajima H, Yazumi S. Ectopic pancreatitis of the jejunal mesentery. Intern Med. (2018) 57(6):903–4. doi: 10.2169/internalmedicine.9548-17
27. Aslan S, Nural MS. CT Features of asymptomatic heterotopic pancreas in jejunal mesentery. Turk J Gastroenterol. (2019) 30(2):208–10. doi: 10.5152/tjg.2018.18064
28. Xu YE, Hendahewa R. A rare case of mesenteric heterotopic pancreas presenting as an inflammatory mass. J Surg Case Rep. (2020) 2020(2):rjz395. doi: 10.1093/jscr/rjz395
29. Sathiadoss P, Fasih N. Case 307: heterotopic pancreas in jejunal mesentery. Radiology. (2022) 305(2):490–4. doi: 10.1148/radiol.210251
30. Bullock C, Amiraian D, Almerey T, Stauffer J, Komforti MK, LeGout JD. The tale of 2 pancreases: jejunal mesenteric ectopic pancreas causing recurrent ectopic pancreatitis. Radiol Case Rep. (2023) 19(1):464–7. doi: 10.1016/j.radcr.2023.10.049
31. Okamura K, Ishibashi E, Araki K, Kanasaki Y, Kodama K, Kanazawa A, et al. Acute on chronic pancreatitis affecting the ectopic pancreas located in the jejunal mesentery: a case report. Acta Radiol Open. (2024) 13(8):20584601241269617. doi: 10.1177/20584601241269617
32. Yang CW, Liu XJ, Wei Y, Wan S, Ye Z, Yao S, et al. Use of computed tomography for distinguishing heterotopic pancreas from gastrointestinal stromal tumor and leiomyoma. Abdom Radiol (NY). (2021) 46(1):168–78. doi: 10.1007/s00261-020-02631-2
33. Kim DW, Kim JH, Park SH, Lee JS, Hong SM, Kim M, et al. Heterotopic pancreas of the jejunum: associations between CT and pathology features. Abdom Imaging. (2015) 40(1):38–45. doi: 10.1007/s00261-014-0177-y
34. Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic pancreas: histopathologic features, imaging findings, and complications. Radiographics. (2017) 37(2):484–99. doi: 10.1148/rg.2017160091
35. Pijpers AGH, Eeftinck Schattenkerk LD, de Vries R, Broers CJM, Straver B, van Heurn ELW, et al. Cardiac anomalies in children with congenital duodenal obstruction: a systematic review with meta-analysis. Pediatr Surg Int. (2023) 39(1):160. doi: 10.1007/s00383-023-05449-3
36. Hess A, Zino C, Camps J. A rare dual diagnosis of duodenal and biliary atresia in a premature infant. Cureus. (2021) 13(9):e18218. doi: 10.7759/cureus.18218
37. Lum Min SA, Imam M, Zrinyi A, Shawyer AC, Keijzer R. Post-discharge follow-up of congenital duodenal obstruction patients: a systematic review. Pediatr Surg Int. (2023) 39(1):239. doi: 10.1007/s00383-023-05515-w
Keywords: congenital duodenal obstruction, heterotopic pancreas, pediatric surgery, neonate, children
Citation: Sun Y, Li S, Liu S, Li Y and Quan D (2025) Neonatal duodenal atresia with heterotopic pancreas: a case report and literature review. Front. Pediatr. 13:1655746. doi: 10.3389/fped.2025.1655746
Received: 28 June 2025; Accepted: 16 October 2025;
Published: 3 November 2025.
Edited by:
Magd Kotb, Cairo University, EgyptReviewed by:
Juan Carlos Núñez-Enríquez, Mexican Social Security Institute, MexicoCosimo Bleve, San Bortolo Hospital, Italy
Copyright: © 2025 Sun, Li, Liu, Li and Quan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufeng Li, bHlmNzkxMDAxQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Yinmin Sun
Yinmin Sun Siqi Li1,†
Siqi Li1,† Yufeng Li
Yufeng Li