- 1Department of Pediatrics, Penn State College of Medicine, Penn State Health, Hampden Medical Center, Enola, PA, United States
- 2Department of Pediatrics, University of Mississippi Medical Center, Jackson, MS, United States
This discussion explores the complex aspects of managing nutrition for preterm infants during the critical transition from exclusive parenteral nutrition (PN) to full enteral feeding (EN). The primary objectives of nutritional care in this very low birth weight infants (VLBW) population are to promote growth rates comparable to those in utero and enhance key neurodevelopmental milestones. The transition phase is characterized by the gradual increase of enteral feeds concurrently with the reduction and eventual cessation of parenteral nutrition. This period presents several key challenges in clinical practice, marked by notable variability: (A) determining the appropriate timing and criteria for initiating enteral feeds; (B) optimizing the rate at which enteral feed volumes are safely increased; (C) deciding the specific enteral volume threshold when to initiate human milk fortification to meet increasing caloric and protein demands; (D) establishing the optimal timing for discontinuing intravenous lipid emulsions (ILE); and (E) identifying the precise enteral volume threshold that dictates when to cease parenteral amino acid administration. To navigate these complexities and ensure seamless nutrient administration, practical recommendations for effective management are crucial. These include advocating for early fortification of human milk, judicious use of concentrated parenteral nutrition to provide adequate nutrients in lower fluid volumes, and strategically minimizing the overall duration of the transition phase. Implementing these evidence-informed steps aims to ensure smooth nutrition management, optimize nutrient delivery, and significantly reduce the pervasive risk of postnatal growth failure in extremely low birth weight infants.
Introduction
Substantial improvements in the survival rates of preterm infants have highlighted the ongoing complexities of their nutritional management, which is significantly influenced by gestational age, postmenstrual age (PMA), and inherent physiological immaturity. Small preterm infants often face feeding intolerance due to gut immaturity, manifesting as gastric dysmotility and intestinal hypomotility, alongside other complicating conditions. This challenge can slow the progression toward complete enteral nutrition. Consequently, parenteral nutrition is crucial in bridging the nutritional gap while enteral feeds gradually increase. This combined approach ensures adequate nutrient delivery to meet the infant's essential needs, ultimately supporting growth and improving health outcomes (1). Key objectives are to achieve extrauterine growth rates comparable to those in utero, promote gut maturation, and optimize immediate and long-term health outcomes, including neurodevelopment (2–5). Simultaneously, careful nutritional modulation is required to mitigate the risks of excessive postnatal weight gain and potential long-term sequelae such as obesity, diabetes, and cardiovascular disorders (6, 7). This careful balance extends to macronutrient provision; for instance, studies suggest excessive protein administration early in life may be associated with adverse neurodevelopmental outcomes (8), underscoring the critical need to provide sufficient protein for growth while avoiding potential harm from excess.
For many preterm infants, particularly those born at less than 32 weeks' gestation, nutritional support typically commences with exclusive parenteral nutrition (PN). Initial management often targets a fluid intake of approximately 100 ml/kg/day, providing 46–56 kcal/kg/day. Caloric delivery via PN is subsequently advanced over the following days, with a goal of 110–130 kcal/kg/day, to adequately support extrauterine growth and meet the high metabolic demands of this population.
Nutrition guidelines
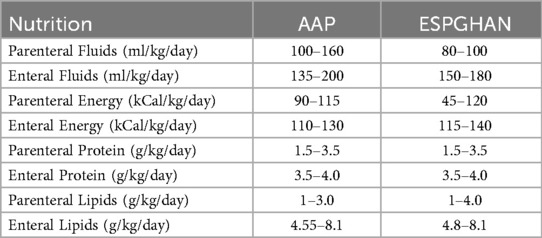
Current nutritional guidelines offer specific recommendations for distinct feeding stages in preterm infants. For instance, the American Academy of Pediatrics (AAP) (Table 1) recommends a minimum parenteral nutrition (PN) intake of 90 kcal/kg/day for extremely low birth weight (ELBW) infants (9). Once full enteral nutrition (EN) is achieved, caloric targets typically increase to 110–130 kcal/kg/day, often corresponding to fluid volumes approaching 150 ml/kg/day.
Despite these established benchmarks for exclusive PN and full EN, specific, evidence-based guidelines for managing nutrition during the transition phase are notably lacking, especially for ELBW infants. This transition phase is generally defined as the period when exclusive PN is slowly reduced and eventually stopped as EN volumes gradually increase.
However, a lack of agreement continues regarding the specific enteral feeding volumes that define the initiation and cessation of this critical phase. While the ultimate goal is often to reach full enteral feeds (e.g., ∼150 ml/kg/day), the transition occurs within intermediate volumes. For instance, Wang et al., in a systematic review supporting this need for definition, proposed a quantitative operational definition, suggesting the transition phase could encompass the period when minimum enteral feeding volumes increase from 30 ml/kg/day up to approximately 120 ml/kg/day (10). Nevertheless, significant variation regarding this upper threshold remains evident in clinical practice and the literature. The phase of nutrition when PN is combined with advancing enteral nutrition from 30 ml/kg/day to 120 ml/kg/day is considered the transitional phase of nutrition.
Practice challenges
Research on the transition from parenteral to enteral nutrition in preterm infants consistently underscores the need for well-defined, evidence-based nutrient targets during this critical period. Managing nutrition in these infants is complicated by considerable variability in clinical practices worldwide. Key areas where these practices often differ include:
1. The timing and criteria for initiating enteral feeds:
Significant variation exists in practices for initiating enteral feeds, including the timing and whether a trophic feeding phase precedes advancement. In many centers, starting enteral feedings very early, often within 12 h. of birth, is standard practice. Administering the mother's own milk in sequence, starting with the initially produced colostrum, offers significant benefits because colostrum is uniquely rich in valuable bioactive compounds (1). Early progressive feeding, started without a trophic phase, was investigated in a single-center randomized controlled trial of 60 extremely preterm infants (<1,000 g). The study found that this approach increased infants' time on full enteral feeds and decreased their need for total parenteral nutrition (PN) without raising the incidence of other health complications (11). However, if a center practices trophic feeding, this initial non-nutritive phase can prolong PN duration and delay the time to reach complete enteral nutrition.
2. The rate at which enteral feed volumes are increased:
Historically, it was believed that slower advancement of smaller enteral volumes could help protect against Necrotizing Enterocolitis (NEC), even though it delayed full feedings (12). However, current evidence does not support this cautious approach (13). A review of 10 randomized controlled trials (RCTs) involving 3,753 infants found no reduction in the risk of NEC or death when comparing slower feeding advancement rates of 15–20 ml/kg/day to faster rates of 30–40 ml/kg/day in preterm very low birth weight (VLBW) infants (13). Similarly, the SIFT RCT, which included 2,793 preterm infants, showed no significant difference in the incidence of late-onset sepsis, NEC, or survival without moderate-to-severe neurodevelopmental disabilities between advancement rates of 30 ml/kg/day and 18 ml/kg/day (14). If the rate of advancement of feeds is slow, it may prolong the PN phase and unfortified breast milk provision, thus compromising nutrient delivery.
3. The specific enteral volume threshold determines when to start human milk fortification:
It is clearly evident that human milk reduces NEC compared to preterm formulas (15). Hence, human milk is preferred as the primary source of enteral feeding in infants with very low birth weights. Since human milk alone does not meet the nutritional requirements of the growing preterm infant, it is recommended to fortify it to a 24-calorie/oz (16). However, the enteral volume at which such fortification occurs varies widely (17). Such variance can result in the suboptimal supply of nutrients. One systematic review compared early (enteral volume of 40 ml/kg/day or less) vs. late fortification (≥75 ml/kg/day) (18). No differences were found between groups for in-hospital growth, risk of NEC, feed intolerance, sepsis, or mortality. The Cochrane review also reached a similar conclusion (19). However, one study noted that cumulative protein intake was higher with early fortification (EF) (20). The cumulative protein intake (g/kg) in the first 4 weeks of life was higher in the EF group [98.6 [93.8, 104] vs. 89.6 [84.2, 96.4], P < 0.001]. Therefore, the timing of human milk fortification is essential to ensure optimal provision of nutrients. Moreover, the fortification, standard, or individualized fortification may also impact nutrient delivery (21). By enhancing protein intake and supporting better somatic and head growth, adjustable fortification represents a practical approach to optimizing the nutritional value of fortified human milk.
4. The timing for discontinuing intravenous lipid emulsions (ILE):
The American Academy of Pediatrics' nutrition recommendation for preterm infants does not provide optimal timing for discontinuing parenteral lipids. The recommended enteral fat provision for ELBW is about 4.5–8 g/kg/day (22). It is suggested that at least 0.5 g/kg/day of lipids is needed to prevent essential fatty acid deficiency (1). Fortified human milk provides approximately 4.8 g/kg/day of fat at a volume of 100 ml/kg (23). Hence, reducing the intravenous lipids to 1 g/kg/day when the fortified human milk volume is at 80 ml/kg/day and discontinuing the intravenous lipids at 100 ml/kg/day of enteral volume of fortified human milk is safe and provides the recommended fat provision. This suggested approach also highlights the importance of earlier human milk fortification.
5. The enteral volume threshold dictates when to cease parenteral amino acid administration:
Studies suggest that the gut and liver consume almost 40 to 50% of the amino acids during the first pass (24, 25). Therefore, premature discontinuation of parenteral amino acids may lead to an inadequate supply of protein in the VLBW infant. It is suggested that parenteral nutrition be weaned when the enteral volume reaches 75 ml/kg/day (25). However, the recommendation is unclear about whether the fortification of human milk is initiated before weaning from parenteral nutrition. The current recommendation from AAP is to discontinue parenteral nutrition once the enteral volume reaches 100–120 ml/kg/day.
Recognizing these varying approaches emphasizes the challenges of optimizing care. However, a thorough examination of the rationale and implications for each specific variation in practice exceeds the scope of this article.
Transition phase studies
As there are specific guidelines for the parenteral and enteral phases of nutrition in infants with very low birth weight (VLBW), the provision of nutrients may remain consistent during these phases (26, 27). However, the absence of such recommendations during the transition phase of nutrition results in an inconsistent and highly variable approach to nutrition by neonatal caregivers. Miller et al. noted that overall growth was adequate during the parenteral and enteral phases of nutrition; however, growth was compromised during the transitional phase (28). Growth velocity of <10 g/kg/day was considered poor growth. The incidence of poor growth during the parenteral, enteral, and transition phases of nutrition was 22.5%, 17.1%, and 46.1%, respectively. The odds of poor growth during the transition phase of nutrition were 5.4 (95% CI: 1.66–17.52). In this study, the authors identified that growth was compromised during the transitional phase primarily due to reduced protein intake as the parenteral nutrition was weaned and enteral nutrition was introduced. The study recommends maintaining protein intake at or above 3 g/kg/day and early human milk fortification with enteral feeds at 80 ml/kg/day.
Brennan et al., in their observational study, demonstrated macronutrient and energy deficits in the transition phase of nutrition in <34 weeks preterm infants (29). The study emphasizes the implementation of a nutrition phase (PN, transition phase, and EN) rather than a chronological age (postmenstrual age) approach to identify nutrient deficits at each phase, particularly during the transition phase, when enteral feeds reach 80 ml/kg/day and early fortification of human milk is initiated.
A subsequent prospective study by the same research group used a nutrient model to identify optimal amino acid provision during the parenteral nutritional transition for preterm infants (BW ≤1,500 g, GA <34 weeks) (30). The model yielded an optimal target of 3.5 g of amino acids per 100 ml. However, the interpretation requires caution, as clarification is needed on whether this represents a target concentration within fluids or how it translates to the standard daily intake goal (g/kg/day). The stated aim of this target was to support adequate growth and prevent nutrient deficits.
In this study (30), the researchers also proposed strategies to boost protein intake during the transition, such as customized PN formulations and appropriate human milk fortification. Their protocol included a specific fluid calculation method: enteral feeds below 40 ml/kg/day did not contribute to the fluid totals used for adjusting PN rates. This was intended to maintain consistent parenteral fluid and nutrient delivery, particularly during the initiation of minimal enteral feeds.
In a separate study, Miller et al. conducted a retrospective comparative analysis (31) that implemented a targeted nutrition protocol. They compared a study group receiving concentrated PN within a restricted total fluid volume of 100 ml/kg/day to a control group with a higher total fluid volume of 140 ml/kg/day. Both groups aimed for similar nutritional goals, targeting an energy intake of 100–120 kcal/kg/day and a protein intake greater than 3 g/kg/day, with intravenous lipids providing less than 50% of the calories derived from PN.
The weight-for-age z scores at birth, one week old, and during the transition phase were similar between groups. The findings revealed that the study group (restricted fluids with concentrated PN) achieved significantly higher weight-for-age z-scores by the end of the transition phase compared to the control group, with this advantage persisting until 35 weeks postmenstrual age. However, the authors noted that the actual delivery of protein and calories often fell short of targets when infants received total enteral feed volumes between approximately 100–130 ml/kg/day. This shortfall was potentially linked to the protocol's threshold for initiating human milk fortification, which was delayed until enteral volumes reached 100–120 ml/kg/day.
A similar correlation of the critical role of protein during the transition phase was highlighted by Liotto and the group in their NICU (32). The authors noted that very low birth weight infants with adequate growth velocity of at least 15 g/kg/day had higher enteral protein intake during the main parenteral period and higher parenteral protein and energy intakes during the main enteral nutritional intake period, suggesting a careful adjustment of protein during the transition phase.
To understand the potential risk of poor growth in the transition nutrition phase in very low birth weight infants, Immeli and group conducted a retrospective cohort study (33). The study highlights that the prolonged transition phase from parenteral to enteral nutrition correlates with lower cumulative intakes of energy, protein, fat, and carbohydrates at 28 days of age. The study specifically demonstrated that a prolonged transition phase of over 12 days in VLBW infants resulted in significantly lower weight and head circumference z-score changes at term-equivalent age compared to those with a shorter transition phase of 7 days. The study also reported more negative associations with the transition duration in boys than in girls. Interestingly, in another retrospective study by Alur et al., female ELBW infants experienced a significant decrease in weight percentiles during the transition phase compared to males with similar calorie and protein intake (34).
Our suggested approach to the transition phase
We designed a new approach to nutrient provision for ELBW infants, carefully considering the challenges previously outlined (25–32). Building on this foundation and the studies discussed above, we suggest a practical strategy for the transition phase (TP), which includes concentrating PN, fortifying human milk earlier, and reducing the overall duration of the TP (35):
(A) Parenteral nutrition is initiated immediately at birth with 3.5 g/kg/day of amino acids in preterm infants with birth weights of 1,000–1,500 grams and 2.5–3 g/kg/day in preterm infants with BW of <1,000 grams (1), and 100 ml/kg/day of PN.
(B) Additional fluids, such as low-concentration dextrose-containing fluids, may be provided to meet the insensible losses.
(C) Lipids are started at 1 g/kg/day and are advanced if the triglyceride concentrations are less than 265 mg/dl (36). The lipid fluid volumes are in addition to the PN fluid.
(D) Dextrose concentrations are modified to keep the blood glucose concentrations below 180 mg/dl.
(E) Trophic enteral feeds are initiated as soon as possible if hemodynamically stable at 20 ml/kg/day. The feeds are advanced at 20 ml/kg/day daily if the infant is stable until a total enteral feed volume of 150–160 ml/kg/day is achieved. The duration of the trophic feeds is based on the infant's clinical stability.
(F) The feeds are either mother's milk or donor breast milk. The feeds are fortified to 24 kcal/oz once an enteral feed volume of 50 ml/kg/day is achieved. AAP recommends as much human milk, either donor or mother's own, as possible before 34 weeks postconceptional age to reduce the risk of necrotizing enterocolitis. However, donor milk may have lower protein, immunoglobulins, and electrolyte content, and up to 30% reduction in fat absorption may account for poor postnatal growth (9)
(G) The choice between fortifying human milk with preterm formula powder vs. a commercial human milk fortifier (HMF) is complex. Although some evidence indicates similar growth outcomes (non-inferiority) between these methods (37), commercial HMFs are specifically formulated to complement the nutrient profile of human milk.
(H) Among individualized fortification strategies, adjustable fortification using a BUN (blood urea nitrogen) level of 10 mg/dl as the cutoff may be more convenient compared to targeted fortification, which requires breast milk analyzers and is labor-intensive. However, neither approach is superior to the other (38). Decisions are therefore typically multifactorial, guided by institutional guidelines, specific nutrient targets, product availability, cost, potential tolerance issues, and the infant's clinical condition. The PN is weaned as enteral feeds are advanced to keep total PN and enteral fluids at 150 ml/kg/day.
Monitoring the growth of preterm infants during their hospital stay is essential for early detection of extrauterine growth restriction (EUGR). Failing to meet expected growth rates can result in adverse health outcomes, including impaired neurodevelopment and increased morbidity (39, 40).
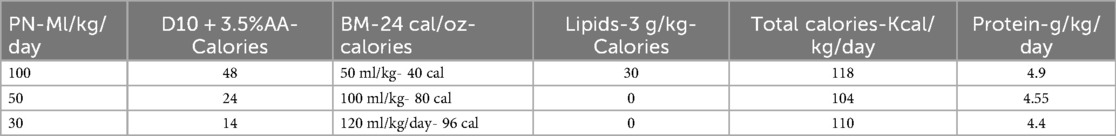
The total calories provided at 100 ml/kg/day of PN [Dextrose10%w and 3.5 g/kg/day of amino acids (AA)] and 50 ml/kg/day of 24 kcal/oz mother's milk (BM-24) or donor breast milk will be 88 kcal/kg/day, and with lipids at 3 g/kg/day will provide 118 kcal/kg/day (Table 2).
When enteral feeds are advanced to 100 ml/kg/day and lipids are discontinued, the PN will be at 50 ml/kg/day to provide a total calories of 104 kcal/kg/day. When the PN is discontinued at 120 ml/kg/day of the enteral volume of BM-24, the total calories provided will be 110 kcal/kg/day as noted (refer to the table with nutritional characteristics). The total combined protein would be between 4.4 and 4.9 g/kg/day. The higher protein provided during the transition phase may help mitigate the first-pass effect (24, 25, 41), and the consensus suggests that increasing enteral intake by up to 50% will have a minimal impact on the systemic availability of amino acids (24). Thus, throughout the transition phase, adequate calories and protein are provided.
We also strongly recommend that a randomized controlled trial of the transition phase of nutrition may help clarify the optimal approach.
Conclusions
In summary, extremely low birth weight infants, in particular, and those who experienced fetal growth restriction, are at risk for poor postnatal growth and require tailored nutritional interventions (38). Hence, neonatal caregivers should pay careful attention to the five challenges outlined: timing of initiating enteral feeds, rate of advancement of enteral feed volumes, enteral volumes at which human milk fortification is introduced, timing of discontinuation of ILE, and enteral volumes at which PN is discontinued for streamlining the nutrient delivery during TP.
Addressing these challenges with diligence is crucial for streamlining nutrient delivery. Our proposed TP phase policy offers a framework for developing robust TP nutrition guidelines.
Every neonatal unit caring for ELBW infants needs to create its specific nutritional guidelines for the transition phase. This ensures a seamless shift from parenteral to enteral nutrition and helps minimize postnatal growth failure. We strongly advocate for multicenter prospective trials to identify the most effective nutritional approaches during this critical period.
Author contributions
PA: Conceptualization, Writing – review & editing, Methodology, Investigation, Writing – original draft, Visualization. SR: Writing – original draft, Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Academy of Pediatrics. In: Greer FR, Abrams SA, editors. Pediatric Nutrition. Elk Grove Village, IL: American Academy of Pediatrics (2025). doi: 10.1542/9781610027700
2. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK, National Institutes of Child Health and Human Development Neonatal Research Network. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. (2006) 117(4):1253–61. doi: 10.1542/peds.2005-1368
3. Ganapathy S. Long chain polyunsaturated fatty acids and immunity in infants. Indian Pediatr. (2009) 46(9):785–90.19812424
4. Roggero P, Giannì ML, Orsi A, Amato O, Piemontese P, Liotto N. Implementation of nutritional strategies decreases postnatal growth restriction in preterm infants. PLoS One. (2012) 7(12):e51166. doi: 10.1371/journal.pone.0051166
5. Skinner AM, Narchi H. Preterm nutrition and neurodevelopmental outcomes. World J Methodol. (2021) 11(6):278–93. doi: 10.5662/wjm.v11.i6.278
6. Bavineni M, Wassenaar TM, Agnihotri K, Ussery DW, Lüscher TF, Mehta JL. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur Heart J. (2019) 40(14):1107–12. doi: 10.1093/eurheartj/ehz025
7. Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. (2014) 15(10):804–11. doi: 10.1111/obr.12214
8. Blanco CL, Gong AK, Schoolfield J, Green BK, Daniels W, Liechty EA. Impact of early and high amino acid supplementation on ELBW infants at 2 years. JPediatr Gastroenterol Nutr. (2012) 54(5):601–7. doi: 10.1097/MPG.0b013e31824887a0
9. Greer F. Pediatric nutrition. In: Greer FR, Abrams SA, editors. Nutritional Needs of the Preterm Infant. Elk Grove Village, IL: American Academy of Pediatrics (2025). doi: 10.1542/9781610027700-ch5
10. Wang N, Zhang J, Wang B, Yu Z, Zhang J, Qu L. Relationship between nutrient intakes in the transition phase and postnatal growth of preterm infants: a systematic review. Ital J Pediatr. (2023) 49(1):13. doi: 10.1186/s13052-022-01406-3
11. Salas AA, Li P, Parks K, Lal CV, Martin CR, Carlo WA. Early progressive feeding in extremely preterm infants: a randomized trial. Am J Clin Nutr. (2018) 107(3):365–70. doi: 10.1093/ajcn/nqy012
12. De Rose DU, Umberto D, Lapillonne A, Iacobelli S, Capolupo I, Dotta A, et al. Nutritional strategies for preterm neonates and preterm neonates undergoing surgery: new insights for practice and wrong beliefs to uproot. Nutrients. (2024) 16(11):1719. doi: 10.3390/nu16111719
13. Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. (2021) 8(8):CD001241.34427330
14. Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E. SIFT Investigators Group. Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med. (2019) 381(15):1434–43. doi: 10.1056/NEJMoa1816654
15. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. (2010) 156(4):562–7.e1. doi: 10.1016/j.jpeds.2009.10.040
16. Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Ann Nutr Metab. (2011) 58(suppl 1):8–18. doi: 10.1159/000323381
17. Beggs MR, Bando N, Unger S, O'Connor DL. State of the evidence from clinical trials on human milk fortification for preterm infants. Acta Paediatr. (2022) 111(6):1115–20. doi: 10.1111/apa.16283
18. Basu S, Upadhyay J, Singh P, Kumar M. Early versus late fortification of breast milk in preterm infants: a systematic review and meta- analysis. Eur J Pediatr. (2020) 179(7):1057–68. doi: 10.1007/s00431-020-03677-6
19. Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database Syst Rev. (2020) 2020(7):CD013392.
20. Shah SD, Dereddy N, Jones TL, Dhanireddy R, Talati AJ. Early versus delayed human milk fortification in very low birth weight infants-a randomized controlled trial. J Pediatr. (2016) 174:126–31.e1. doi: 10.1016/j.jpeds.2016.03.056
21. Arslanoglu S, Boquien C-Y, King C, Lamireau D, Tonetto P, Barnett D, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front Pediatr. (2019) 7:76.30968003
22. Koletzko B, Wieczorek S, Cheah F-C, Domellöf M, van Goudoever JB, Poindexter BB. Recommended nutrient intake levels for preterm infants. World Rev Nutr Diet. (2021) 122:191–7. doi: 10.1159/000514772
23. Enfamil® Human Milk Fortifier Acidified liquid. Mead Johnson Nutrition. Available online at: https://hcp.meadjohnson.com/s/product/a4R4J000000PpQmUAK/enfamil-liquid-human-milk-fortifier-standard-protein (Accessed May 5, 2025).
24. van der Schoor SR, Wattimena DL, Huijmans J, Vermes A, van Goudoever JB. The gut takes nearly all: threonine kinetics in infants. Am J Clin Nutr. (2007) 86:1132–8. doi: 10.1093/ajcn/86.4.1132
25. van den Akker CH, Saenz de Pipaon M, van Goudoever JB. Proteins and amino acids. World Rev Nutr Diet. (2021) 122:75–88. doi: 10.1159/000514748
26. Embleton ND, Jennifer Moltu S, Lapillonne A, Van Den Akker CH, Carnielli V, Fusch C. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. J Pediatr Gastroenterol Nutr. (2023) 76(2):248–68. doi: 10.1097/MPG.0000000000003642
27. Robinson DT, Calkins KL, Chen Y, Cober MP, Falciglia GH, Church DD. Guidelines for parenteral nutrition in preterm infants: the American society for parenteral and enteral nutrition. JPEN J Parenter Enteral Nutr. (2023) 47(7):830–58. doi: 10.1002/jpen.2550
28. Miller M, Vaidya R, Rastogi D, Bhutada A, Rastogi S. From parenteral to enteral nutrition: a nutrition-based approach for evaluating postnatal growth failure in preterm infants. JPEN J Parenter Enteral Nutr. (2014) 38(4):489–97. doi: 10.1177/0148607113487926
29. Brennan AM, Fenton S, Murphy BP, Kiely ME. Transition phase nutrition recommendations: a missing link in the nutrition management of preterm infants. JPEN J Parenter Enteral Nutr. (2018) 42(2):343–51. doi: 10.1177/0148607116686289
30. Brennan AM, Kiely ME, Fenton S, Murphy BP. Standardized parenteral nutrition for the transition phase in preterm infants: a bag that fits. Nutrients. (2018) 10(2):170. doi: 10.3390/nu10020170
31. Miller M, Donda K, Bhutada A, Rastogi D, Rastogi S. Transitioning preterm infants from parenteral nutrition: a comparison of 2 protocols. JPEN J Parenter Enteral Nutr. (2017) 41(8):1371–9. doi: 10.1177/0148607116664560
32. Liotto N, Amato O, Piemontese P, Menis C, Orsi A, Corti MG, et al. Protein intakes during weaning from parenteral nutrition drive growth gain and body composition in very low birth weight preterm infants. Nutrients. (2020) 12(5):1298. doi: 10.3390/nu12051298
33. Immeli L, Sankilampi U, Mäkelä PM, Leskinen M, Sund R, Andersson S, et al. Length of nutritional transition associates negatively with postnatal growth in very low birthweight infants. Nutrients. (2021) 13(11):3961. doi: 10.3390/nu13113961
34. Alur P, et al. Calorie intake is associated with weight gain during transition phase of nutrition in female extremely low birth weight infants. Biol Sex Differ. (2020) 11(1):16. doi: 10.1186/s13293-020-00295-7
35. Alur P, Kalikkot Thekkeveedu R, Meeks M, Hart KC, Desai J, Johnson M, et al. The transition phase of nutrition: a pragmatic approach in elbw infants. J Investig Med. 2023;68(2):435–710. doi: 10.1136/jim-2020-SRM
36. Carnielli VP, Correani A, Giretti I, D'Ascenzo R, Bellagamba MP, Burattini I, et al. Nutritional care of preterm infants: scientific basis and practical guidelines. World Rev Nutr Diet. (2021) 122:198–211. doi: 10.1159/000514751
37. Chinnappan A, Sharma A, Agarwal R, Thukral A, Deorari A, Sankar MJ. Fortification of breast milk with preterm formula powder vs human milk fortifier in preterm neonates: a randomized noninferiority trial. JAMA Pediatr. (2021) 175(8):790–6. doi: 10.1001/jamapediatrics.2021.0678
38. De Rose DU, Umberto D, Maggiora E, Maiocco G, Morniroli D, Vizzari G, et al. Improving growth in preterm infants through nutrition: a practical overview. Front Nutr. (2024) 11:1449022. doi: 10.3389/fnut.2024.1449022
39. Morniroli D, Tiraferri V, Maiocco G, De Rose DU, Cresi F, Coscia A, et al. Beyond survival: the lasting effects of premature birth. Front Pediatr. (2023) 11:1213243. doi: 10.3389/fped.2023.1213243
40. De Rose DU, Umberto D, Cota F, Gallini F, Bottoni A, Fabrizio GC, et al. Extra-uterine growth restriction in preterm infants: neurodevelopmental outcomes according to different definitions. Eur J Paediatr Neurol. (2021) 33:135–45. doi: 10.1016/j.ejpn.2021.06.004
Keywords: nutrition, transition phase, VLBW (very low birth weight), preterm infant, parenteral nutrition
Citation: Alur P and Ramarao S (2025) Transition phase of nutrition—optimizing nutrient administration. Front. Pediatr. 13:1658550. doi: 10.3389/fped.2025.1658550
Received: 2 July 2025; Accepted: 22 August 2025;
Published: 5 September 2025.
Edited by:
Simonetta Costa, Casilino General Hospital, ItalyReviewed by:
Domenico Umberto De Rose, Bambino Gesù Children's Hospital (IRCCS), ItalyAmanda Salley, Greenville Health System, United States
Copyright: © 2025 Alur and Ramarao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep Alur, cHhhOTZAcHN1LmVkdQ==; cGFsdXJAcGVubnN0YXRlaGVhbHRoLnBzdS5lZHU=
 Pradeep Alur
Pradeep Alur Sumana Ramarao
Sumana Ramarao