- 1Department of Thyroid and Breast Surgery, Chongming Hospital Affiliated to Shanghai University of Medicine and Health Sciences, Shanghai, China
- 2Shanghai University of Medicine and Health Sciences, Shanghai, China
- 3Department of Pathology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Breast Surgery, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
A 12-year-old Chinese adolescent girl presented with a 2-month history of left nipple bleeding. Physical examination revealed bloody and brownish nipple discharge. Ultrasonography identified a 1 mm solid mass within a dilated duct, and MRI showed heterogeneous enhancement. Surgical exploration revealed a well-defined, medium-textured mass within the duct, which was completely excised. Histopathological analysis confirmed an intraductal fibroadenoma with stromal infarction and hemorrhage, explaining the nipple discharge. Postoperatively, the patient recovered well without recurrence during a 12-month follow-up. Fibroadenoma, the most common benign breast lesion in adolescents, arises from estrogen-sensitive intralobular fibroblasts and may involve ductal structures, as in this case. Spontaneous infarction of intraductal fibroadenoma is exceptionally rare and can present with bloody discharge, necessitating differentiation from other intraductal lesions such as ductal adenoma and intraductal papilloma. This case highlights the importance of timely diagnosis and management of breast lesions in adolescents, emphasizing the need to distinguish between physiological changes and pathological conditions. The report also discusses the etiology of spontaneous infarction in intraductal fibroadenoma and its clinical implications, contributing to the understanding of this rare presentation in pediatric patients.
Introduction
During adolescence, the female breast undergoes various changes influenced by genetic, nutritional, endocrine, and other factors (1). These breast changes in adolescent patients can be either physiological or pathological, highlighting the critical importance of timely detection and identification of malignant lesions. The incidence of breast diseases is relatively low in adolescent girls, with fibroadenoma being the most common condition (2). Fibroadenoma is a benign lesion composed of both epithelial and stromal components. It arises from an abnormal increase in the sensitivity of intralobular fibroblasts to estrogen, which may be related to abnormalities in the quantity or quality of estrogen receptors within these fibroblasts (3). When fibroadenoma occurs within the duct, it is referred to as intraductal fibroadenoma. This condition holds significant diagnostic importance regarding symptoms and pathology, necessitating differentiation from other conditions such as ductal adenoma and intraductal papilloma. Spontaneous infarction of intraductal fibroadenoma is an exceptionally rare finding in recent literature.
In this case report, we present a 12-year-old girl who presented with bloody nipple discharge from the left breast. Imaging studies revealed an intraductal mass, which was subsequently managed surgically. Postoperative pathology confirmed the diagnosis of intraductal fibroadenoma with infarction, elucidating the clinical symptom of bloody discharge. Furthermore, this article explores the differential diagnosis of benign intraductal lesions and discusses the potential etiologies and clinical manifestations of spontaneous infarction in intraductal fibroadenoma.
Case presentation
The patient is a 12-year-old Chinese adolescent female who presented with a two-month history of nipple discharge, which had evolved from pale yellow to brown in color, accompanied by intermittent breast distension and tenderness. Physical examination revealed no evidence of erythema, swelling, or ulceration on the skin surface. Upon manual compression of the left nipple, bloody discharge was elicited, and no palpable mass was identified (Figure 1A). The patient experienced menarche at 11 years of age and has maintained regular menstrual cycles. Her personal history is negative for smoking, alcohol use, and substance abuse. There is no family history of genetic or hereditary disorders, and she has no significant history of specific environmental or occupational exposures.
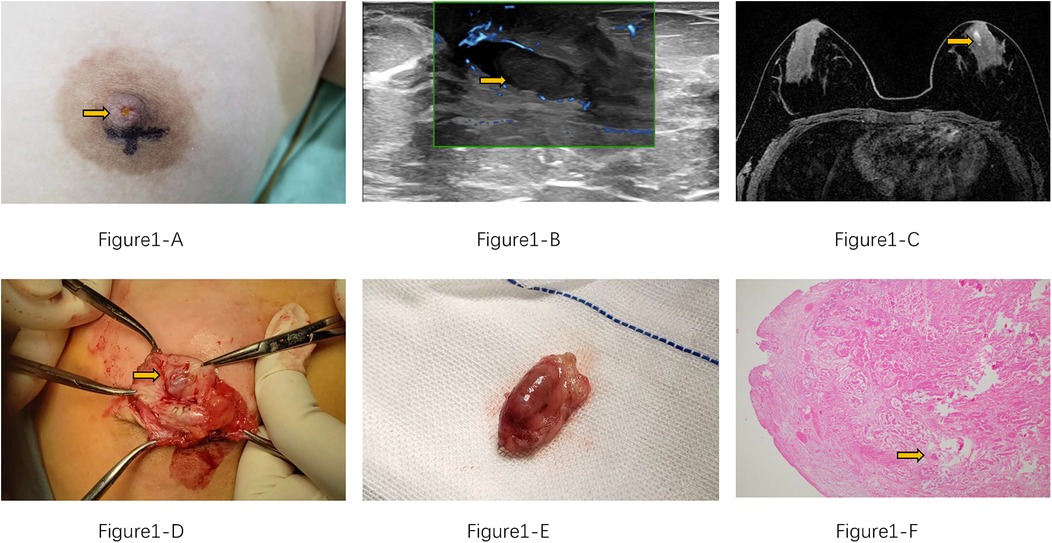
Figure 1. (A) Brownish discharge from the patient's left nipple (yellow arrow). (B) Ultrasound shows a dilated duct with an intraluminal mass (yellow arrow). (C) MRI reveals ductal dilation with a filling defect (yellow arrow). (D) Intraoperative view of the intraluminal mass within the duct. (E) Complete surgical excision of the mass. (F) HE*40 histopathological section shows the tumor infarction is evident, with fibrin and red blood cells alternating faintly visible within the vascular lumen. (yellow arrow).
Post-admission ultrasound revealed localized dilation of the left breast duct posterior to the nipple, with a maximum internal diameter of 5.2 mm. A linear hypoechoic lesion measuring approximately 13 mm × 5 mm was identified within the dilated duct (Figure 1B). The lesion exhibited a regular morphology, parallel orientation, and heterogeneous echogenicity, suggestive of intraductal papilloma, and was classified as BI-RADS Category 3. No significant lymphadenopathy was observed in the bilateral axillary regions. Magnetic resonance imaging (MRI) showed a linear hyperintense signal on T1-weighted imaging (T1WI) medial to the left nipple, with rim enhancement post-contrast. A small, round nodule (0.7 cm in diameter) was noted posteriorly (Figure 1C), displaying well-defined margins, isointensity on T2-weighted imaging (T2WI), and hyperintensity on diffusion-weighted imaging (DWI), with an apparent diffusion coefficient (ADC) value of 1.72 × 10−3 mm2/s. The lesion demonstrated linear enhancement and a moderate-rising time-intensity curve (TIC). It was provisionally classified as BI-RADS Category 3, with intraductal papilloma considered a differential diagnosis. No abnormal laboratory findings were noted preoperatively.
Subsequently, the patient underwent surgical excision of the left breast lesion. Intraoperatively, yellowish discharge was observed from the left nipple, and a dilated duct with a palpable mass measuring 8 mm was noted posterior to the nipple. The mass had a moderate consistency and clear boundaries. The resected tissue was a grayish-white and grayish-red mass, measuring 20 × 15 × 10 mm (Figures 1D,E). Histopathological examination of the paraffin-embedded specimen revealed an intracanalicular fibroadenoma with infarction (Figure 1F). Figure 2 demonstrates the patient's treatment timeline. The patient was followed up at our institution every three months postoperatively. No recurrence of nipple discharge was observed during the one-year follow-up period.
Discussion
Intraductal fibroadenoma is an exceptionally rare condition, with only 14 cases reported in the literature. It predominantly affects adolescent females, particularly those aged 15 years or younger (4, 5), though a small number of cases have been documented in patients aged 30 to 50 years (6, 7). Occurrences in elderly patients are exceedingly rare (8). Postmenarchal adolescent females are consistently identified as the high-risk group for this condition (4, 9–11).
In younger patients, intraductal fibroadenoma often exhibits complex pathological features, overlapping with papilloma, intraductal adenoma, fibroadenoma, and benign phyllodes tumor (6, 12). In contrast, fibroadenomas in older, perimenopausal women are typically solitary, grow more slowly, and carry a marginally increased risk of associated pathology, such as sclerosing adenosis or carcinoma in situ within complex fibroadenomas, often prompting a lower threshold for diagnostic excision to rule out malignancy. The etiology is thought to mirror that of conventional fibroadenoma, potentially linked to hormonal imbalances involving estrogen and progesterone, as well as abnormalities in estrogen receptor functionality in fibroblasts (9, 13). Clinically, adolescent females typically present with a rapidly enlarging, palpable breast mass over several months, sometimes accompanied by bloody nipple discharge and/or breast pain. Imaging studies usually reveal a well-circumscribed, mildly lobulated, heterogeneous mass with uniform density (4, 6). In cases of malignant transformation, imaging may show heterogeneous density, irregular margins, and reduced apparent diffusion coefficient (ADC) values (14).
Breast fibroadenomas can be classified into three distinct types: simple fibroadenoma, juvenile fibroadenoma, and multicentric fibroadenoma. Among these, simple fibroadenoma is the most prevalent, characterized by small tumor size and slow growth. Juvenile fibroadenoma, which has the lowest incidence, typically develops after menarche and exhibits rapid growth. A rare subtype of juvenile fibroadenoma is giant juvenile fibroadenoma (3). Intraductal fibroadenoma may follow a similar classification; however, it is less frequently described in the current literature.
Histologically, intraductal fibroadenoma is characterized by fibroadenomatous polypoid structures growing within ducts or multiple polyps resembling papillary hyperplasia, often filling the ductal lumen (6, 12). It typically presents as solitary or multiple adenomatous nodules, featuring proliferative tubular myoepithelium with luminal and basal cells surrounded by fibrous stroma (15). Differentiation from other intraductal lesions, such as ductal adenoma and intraductal papilloma, is essential. Ductal adenoma, a benign lesion confined to the ductal lumen, is distinguished by well-demarcated margins, duct wall thickening, fibrosis, or plaque-like calcification, often with abundant elastic tissue. Its solid component consists of glandular structures within a hyalinized stroma, composed of biphasic epithelial and myoepithelial cells. Epithelial cells exhibit cuboidal to columnar morphology with intense eosinophilic staining, while myoepithelial cells are smaller and cuboidal to flattened, with clear or faintly eosinophilic staining. A key feature is apical cytoplasmic snouting in epithelial cells at the glandular lumen interface (16, 17). Intraductal papillomas, more common in perimenopausal women, feature ductal dilation and a complex fibrovascular core lined by myoepithelial and luminal epithelial cells. Multicentric peripheral papillomas carry a higher risk of malignant transformation (18, 19). Intraductal fibroadenomas often share overlapping features with papillomas, adenomas, fibroadenomas, and benign phyllodes tumors, complicating diagnosis (6, 12). Table 1 summarizes these differential diagnostic features.
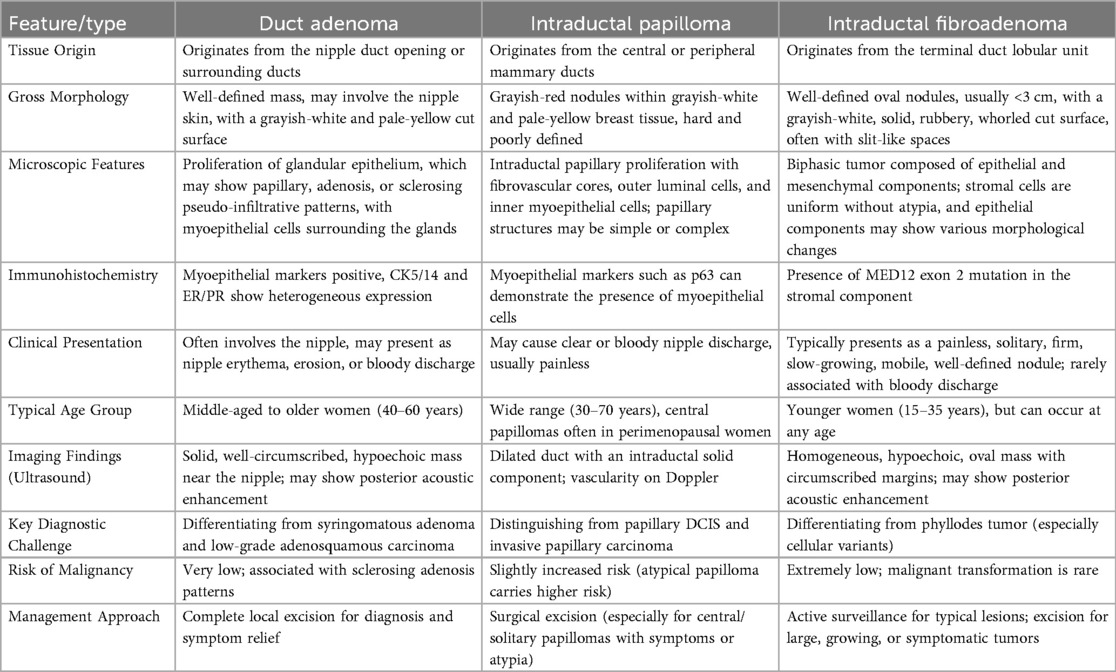
Table 1. A comprehensive comparison of clinical, pathological, and diagnostic features among duct adenoma, intraductal papilloma, and intraductal fibroadenoma, highlighting key distinguishing points for clinical practice.
The specific mechanisms underlying the development of intraductal fibroadenoma have been scarcely explored in the literature, and the precise etiology remains poorly understood. Current hypotheses suggest that the formation of intraductal fibroadenoma is primarily driven by the hyperplasia of stromal and connective tissues. The connective tissue frequently infiltrates the ductal wall, with stromal cells originating from the periductal and perilobular connective tissue. When the base of the protruding stroma is broad or expansive, it exerts compressive forces on the opposing ductal wall, leading to its eventual narrowing and the subsequent formation of a fibroadenoma (12, 20, 21).
Spontaneous infarction of fibroadenomas is a rare phenomenon, occurring in only 0.5%–1.5% of cases (11, 22), with intraductal fibroadenomas exhibiting this feature even more infrequently. Clinically, it often manifests as rapid enlargement of a pre-existing breast mass over a short period, accompanied by severe pain and, in some cases, bloody nipple discharge. Spontaneous infarction can occur in various benign breast lesions, such as fibroadenomas, intraductal papillomas, and phyllodes tumors, particularly during phases of hormonal fluctuation. Bloody nipple discharge may also arise when benign lesions involve the areolar region or ducts (10).
The mechanisms underlying spontaneous infarction in intraductal fibroadenomas are attributed to both physiological and iatrogenic factors. Physiological factors include degenerative changes, such as calcification and hyaline degeneration, and ischemic events within hyperplastic tumor tissues. Iatrogenic factors, such as trauma or fine-needle aspiration, can induce thrombotic vascular changes, while hormonal imbalances from oral medications may also contribute (5). Histologically, infarction in fibroadenomas often presents as a solitary cyst with yellow-red vegetations and central necrosis. For intraductal fibroadenomas, pre-treatment evaluation should include culture of ductal secretions, measurement of estrogen and progesterone levels, and ultrasound imaging (10). Although ultrasound has limited sensitivity for detecting intratumoral infarction, it remains a valuable diagnostic tool and a critical basis for clinical diagnosis.
The treatment of intraductal fibroadenoma in adolescents, as reported in the majority of literature, involves local surgical excision of the tumor (4–6, 11, 12). Follow-up at 6 or 24 months postoperatively has shown no recurrence or related complications in these cases. In the present case, the patient underwent simple mass excision, and no recurrence of nipple discharge was observed during the 12-month follow-up period. These findings demonstrate that intraductal fibroadenoma, as a benign condition, has an excellent prognosis following complete surgical resection of the lesion. Figure 3 provides a clear clinical pathway for differential diagnosis and management of three common breast lesions based on their characteristic presentations, imaging findings, and standardized assessment criteria.
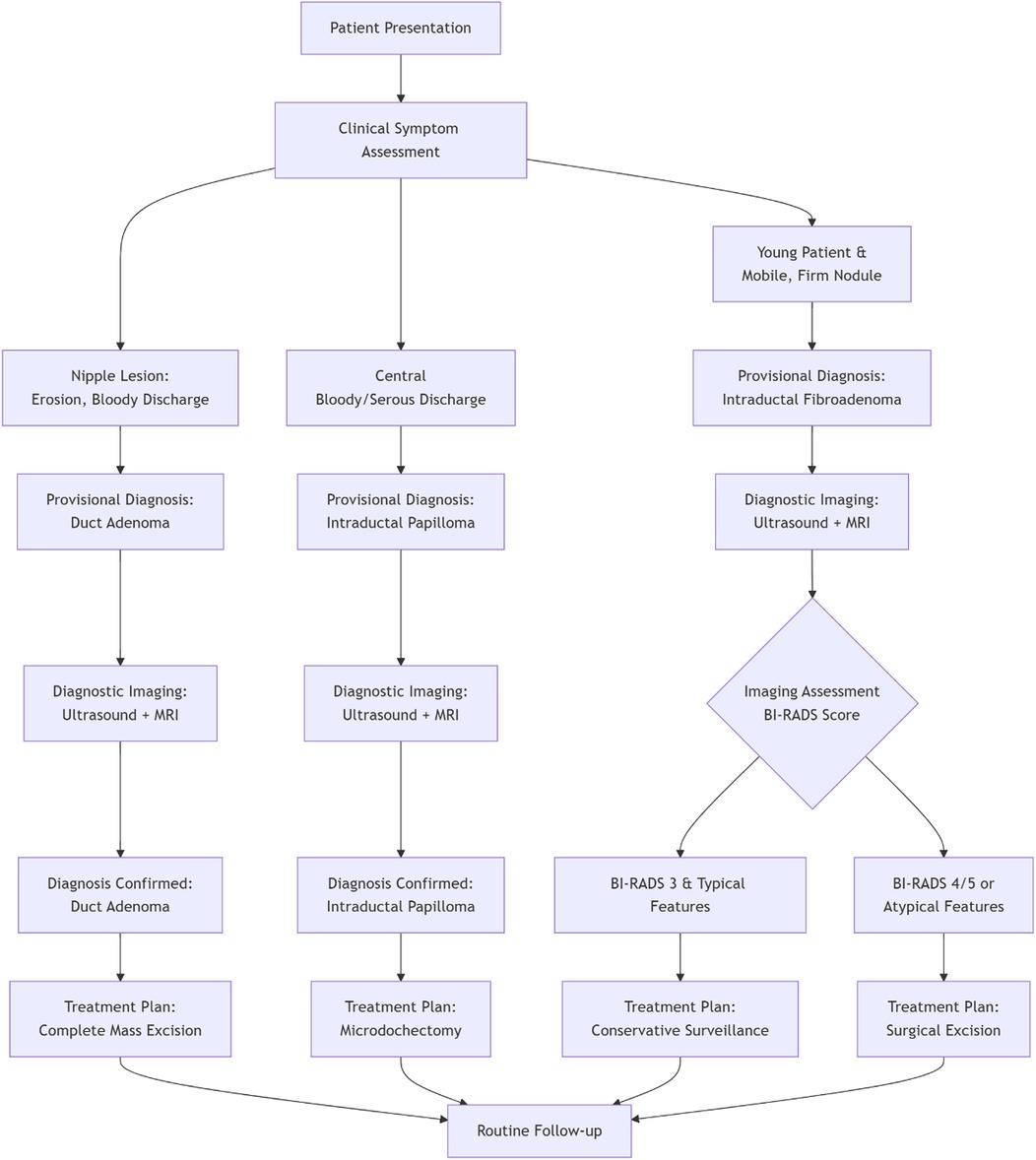
Figure 3. Clinical decision pathway for the diagnosis and management of common intraductal breast lesions.
The patient's postoperative course was uneventful; quarterly follow-up examinations at our institution for one year confirmed no recurrence of nipple discharge. While this affirms the short-term efficacy of the intervention, it underscores the necessity of a structured long-term follow-up strategy to ensure ongoing patient well-being.
In summary, this case highlights a rare instance of intraductal fibroadenoma with spontaneous infarction in an adolescent female, presenting with bloody nipple discharge. The surgical approach should be guided by comprehensive preoperative diagnosis and evaluation. For such lesions, thorough differential diagnosis both preoperatively and intraoperatively is crucial. In adolescent females, to minimize impact on normal breast development and future lactation, simple mass excision is preferred over regional duct excision for confirmed intraductal fibroadenomas. This strategy maximizes preservation of breast function and morphology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PG: Writing – original draft, Investigation. WY: Methodology, Writing – original draft. WG: Writing – original draft, Visualization. PW: Writing – review & editing, Methodology. WL: Writing – review & editing, Conceptualization, Funding acquisition, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by Shanghai Higher Education Society Annual Planning Project (2QYB24057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Silva NK. Breast development and disorders in the adolescent female. Best Pract Res Clin Obstet Gynaecol. (2017) 48:40–50. doi: 10.1016/j.bpobgyn.2017.08.009
2. Adekeye A, Lung KC, Brill KL. Pediatric and adolescent breast conditions: a review. J Pediatr Adolesc Gynecol. (2023) 36:5–13. doi: 10.1016/j.jpag.2022.11.001
3. Salati SA. Breast fibroadenomas: a review in the light of current literature. Pol Przegl Chir. (2020) 93:40–8. doi: 10.5604/01.3001.0014.5676
4. Hayano F, Yamada S, Nakano S, Watanabe T, Sasaguri Y, Koga S. Intraductal fibroadenoma under the nipple in an 11-year-old female. Diagn Pathol. (2014) 9:32. doi: 10.1186/1746-1596-9-32
5. Shrateh ON, Doudin DRA, Torman Y, Abulihya M. Spontaneous infarction of breast fibroadenoma unrelated to any known risk factor: a case report. Int J Surg Case Rep. (2023) 105:108031. doi: 10.1016/j.ijscr.2023.108031
6. Chung A, Scharre K, Wilson M. Intraductal fibroadenomatosis: an unusual variant of fibroadenoma. Breast J. (2008) 14:193–5. doi: 10.1111/j.1524-4741.2007.00553.x
7. Feliciano YZ, Freire R, Net J, Yepes M. Ductal and lobular carcinoma in situ arising within an enlarging biopsy proven fibroadenoma. BMJ Case Rep. (2021) 14:e237017. doi: 10.1136/bcr-2020-237017
8. Marumoto A, Steinemann S. An uncommon pairing of common tumors: case report of ductal carcinoma within fibroadenoma. Hawaii J Med Public Health. (2019) 78:39–43.30766763
9. Alessandrini L, Gobbi D, Zanon GF, Dall’Igna P, Cecchetto G, Alaggio R. Concurrent fibroadenoma and intraductal papilloma—a recurring complex lesion in a premenarcheal girl. Pathol Res Pract. (2012) 209:120–3. doi: 10.1016/j.prp.2012.10.011
10. Liu H, Yeh M-L, Lin K-J, Huang C-K, Hung C-M, Chen Y-S. Bloody nipple discharge in an adolescent girl: unusual presentation of juvenile fibroadenoma. Pediatr Neonatol. (2010) 51:187–9. doi: 10.1016/S1875-9572(10)60036-8
11. Fowler CL. Spontaneous infarction of fibroadenoma in an adolescent girl. Pediatr Radiol. (2004) 34:988–90. doi: 10.1007/s00247-004-1250-4
12. Cummings MC, da Silva L, Papadimos DJ, Lakhani SR. Fibroadenoma and intraduct papilloma—a common pathogenesis? Virchows Arch. (2009) 455:271–5. doi: 10.1007/s00428-009-0817-8
13. Ni YB, Tse GM. Pathological criteria and practical issues in papillary lesions of the breast—a review. Histopathology. (2016) 68:22–32. doi: 10.1111/his.12866
14. Tagliati C, Lanni G, Cerimele F, Di Martino A, Calamita V, Lucidi Pressanti G, et al. Low diffusion level within a fibroadenoma as the sole sign of ductal carcinoma in situ: a case report. Breast Dis. (2021) 40:347–51. doi: 10.3233/BD-201080
15. Bezić J. Intraductal fibroadenoma and intraductal phyllodes tumour—a part of the spectrum of the breast ductal adenoma? Virchows Arch. (2009) 456:105–6. doi: 10.1007/s00428-009-0870-3
16. Azzopardi JG, Salm R. Ductal adenoma of the breast: a lesion which can mimic carcinoma. J Pathol. (1984) 144:15–23. doi: 10.1002/path.1711440103
17. Matsubayashi RN, Momosaki S, Muranaka T. Ductal adenoma of breast: imaging characteristics and radiologic-pathologic correlation of unique findings which reflect “pseudoinvasion”. Breast Cancer. (2016) 23:597–606. doi: 10.1007/s12282-015-0608-9
18. Tay TKY, Tan PH. Papillary neoplasms of the breast—reviewing the spectrum. Mod Pathol. (2021) 34:1044–61. doi: 10.1038/s41379-020-00732-3
19. Kulka J, Madaras L. Papillary lesions of the breast. Virchows Arch. (2021) 480:65–84. doi: 10.1007/s00428-021-03182-7
20. Cheatle GL. Hyperplasia of epithelial and connective tissues in the breast: its relation to fibro-adenoma and other pathological conditions. Br J Surg. (1923) 10:436–55. doi: 10.1002/bjs.1800104003
21. Demetrakopoulos NJ. Three-dimensional reconstruction of a human mammary fibroadenoma. Q Bull Northwest Univ Med Sch. (1958) 32:221–8.13579145
Keywords: intraductal fibroadenoma, adolescent breast lesions, nipple discharge, stromal infarction, benign breast tumors
Citation: Gao P, Yang W, Guan W, Wu P and Lv W (2025) Intraductal fibroadenoma presenting with nipple bleeding in an adolescent: a case report and literature review. Front. Pediatr. 13:1661307. doi: 10.3389/fped.2025.1661307
Received: 26 August 2025; Accepted: 31 October 2025;
Published: 14 November 2025.
Edited by:
Nektarios I. Koufopoulos, University General Hospital Attikon, GreeceReviewed by:
Gloria Pelizzo, University of Milan, ItalyMenelaos Samaras, National and Kapodistrian University of Athens, Greece
Copyright: © 2025 Gao, Yang, Guan, Wu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjie Lv, bHZ3ZW5qaWVfMjAyMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Pingfa Gao1,†
Pingfa Gao1,† Ping Wu
Ping Wu Wenjie Lv
Wenjie Lv