- 1Department of Neonatology, Children's Medical Center, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Perinatology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 3Sichuan Clinical Research Center for Birth Defects, Luzhou, Sichuan, China
Necrotizing enterocolitis (NEC) is an acute, life-threatening intestinal disorder in neonates, associated with notably high mortality. It is characterized by insidious and non-specific early clinical manifestations, a rapid disease progression course, and often results in long-term sequelae in affected infants, such as short bowel syndrome and neurodevelopmental impairments. The pathogenesis of NEC remains complex and not fully elucidated; thus, the screening and validation of biomarkers with high specificity, high sensitivity, and clinical applicability constitutes a core strategy to enhance the efficacy of early diagnosis and accuracy of prognostic assessment for this disease. This article aims to systematically synthesize the current clinical dilemmas in the field of NEC and the update status of relevant clinical guidelines, with a focus on reviewing the research advances of both traditional and emerging biomarkers in the contexts of NEC early diagnosis, disease staging, severity stratification, prediction of surgical intervention requirements, and prognostic evaluation. Additionally, it analyzes the consistencies and discrepancies between cutting-edge research findings and clinical guidelines, and prospects the future development direction of precision diagnosis and treatment for NEC.
1 Introduction
Necrotizing enterocolitis (NEC) is a severe inflammatory intestinal disorder that poses a life threat to preterm infants and accounts for a major cause of death in neonatal intensive care units (NICUs) (1). This disease predominantly affects preterm infants (accounting for 90% of cases), with an incidence rate of 0.5‰–5.0‰ and a domestic mortality rate as high as 10%–50% (2, 3). Despite significant advancements in perinatal medicine and neonatal intensive care technologies, the incidence and mortality rates of NEC remain persistently high, presenting a major clinical challenge. The pathogenesis of NEC is complex and has not been fully elucidated. Currently, its diagnosis and treatment primarily rely on the comprehensive assessment of clinical manifestations, laboratory tests, and imaging examinations (4, 5), which is associated with limitations such as delayed diagnosis, inaccurate assessment, and difficulty in prediction. In recent years, research focus has shifted to the molecular level; a large number of potential biomarkers—ranging from serum proteins and fecal microbiota to urine metabolites—have been successively identified. The integration of multi-omics technologies with machine learning algorithms has driven the advancement of NEC diagnosis and treatment toward precision medicine. This article reviews the research progress of traditional and emerging biomarkers in the early diagnosis, staging and severity assessment, prediction of surgical needs, and prognosis evaluation of NEC.
2 Clinical challenges
The clinical management of NEC is fraught with substantial challenges, with core issues including delayed diagnosis, difficulty in differential diagnosis, and rapid disease progression. First and foremost, the primary clinical challenge manifests as non-specific early manifestations: its symptoms overlap significantly with those of other common neonatal conditions (e.g., sepsis, feeding intolerance) (6), often leading to difficulty in differentiation and subsequent delay in the initiation of optimal treatment. Secondly, NEC is characterized by rapid progression: some infants can deteriorate from Bell Stage I to full-thickness intestinal wall necrosis, perforation, and even sepsis or shock (Bell Stage III) within hours (7), resulting in a sharp increase in mortality. Furthermore, differential diagnosis is exceptionally challenging: NEC shares striking similarities with neonatal sepsis, spontaneous intestinal perforation, and other conditions in terms of clinical manifestations and laboratory findings, yet their treatment strategies differ significantly (8); thus, accurate differentiation is critical for guiding treatment. Additionally, the Bell Staging Criteria—currently the primary tool for assessing disease severity—suffers from strong subjectivity, as its evaluation of abdominal signs relies on clinicians' subjective judgment. Moreover, characteristic imaging findings such as pneumatosis intestinalis and portal venous gas either appear late or present atypically. Finally, clinical practice also faces the dilemma of a lack of reliable prognostic prediction tools, making it difficult to reliably identify which infants will progress to severe stages requiring surgery or develop long-term sequelae such as short bowel syndrome and neurodevelopmental delay (9, 10).
3 Guideline summary
Currently, authoritative guidelines both domestically and internationally have established a standardized framework for the diagnosis and management of NEC, which is based on clinical manifestations and centered on imaging findings. Regarding diagnosis, guidelines generally regard the combination of clinical manifestations (e.g., progressive abdominal distension, bilious vomiting, hematochezia) and characteristic findings on abdominal radiography (plain film) (e.g., pneumatosis intestinalis, portal venous gas) as the “gold standard” for diagnosis and staging. Additionally, dynamic abdominal sign monitoring via ultrasound, intestinal oxygenation assessment using infrared spectroscopy, and surveillance of laboratory parameters (including complete blood count, C-reactive protein, procalcitonin, interleukin-6, and blood gas analysis) are recommended, while routine testing of fecal calprotectin is not advised (11, 12). In terms of treatment, once NEC is suspected or confirmed, the core principles include immediate fasting, gastrointestinal decompression, and administration of broad-spectrum antibiotics and nutritional support via the intravenous route (12). For surgical intervention, radiologically confirmed pneumoperitoneum (indicating intestinal perforation) constitutes an absolute indication, whereas failure of conservative medical treatment or persistent disease deterioration serves as a relative indication (13). Notably, the international expert consensus published in 2025 specifically emphasizes the importance of regular pain assessment [e.g., using the Neonatal Pain, Agitation, and Sedation Scale (N-PASS)] and prophylactic analgesia for infants with NEC (particularly those with Bell stage ≥ II). It recommends a regimen of acetaminophen combined with opioids, which reflects the advancement in humanistic care for infants in the management of NEC (14). In terms of prevention, guidelines strongly recommend breastfeeding and the administration of glucocorticoids to mothers at risk of preterm birth (12).
4 Traditional biomarkers
4.1 Inflammatory markers
4.1.1 Serum Amyloid A
Serum Amyloid A (SAA) is an acute-phase reactant protein whose levels rise rapidly in the early stage of infection, correlate with the severity of inflammation, and participate in the inflammatory process by regulating proinflammatory cytokines and angiogenesis (15–17). This property endows it with potential value in the early diagnosis of NEC, serving as an auxiliary indicator for the early diagnosis of NEC. A study by Qian et al. (18) revealed that the combined detection of SAA, platelet-to-lymphocyte ratio (PLR), and procalcitonin (PCT) exhibited higher diagnostic value for NEC than single SAA detection (AUC = 0.856, sensitivity = 84.3%, specificity = 87.5% vs. AUC = 0.807, sensitivity = 83.1%, specificity = 78.8%). However, a study by Reisinger et al. (19) pointed out that the combined use of SAA and intestinal fatty acid-binding protein (I-FABP) did not significantly improve the diagnostic accuracy of NEC, suggesting insufficient specificity of SAA in this combination mode. This may be attributed to the mismatch in pathophysiological time windows between SAA and I-FABP.
Existing studies also clearly demonstrate that SAA levels are closely associated with the staging and severity of NEC. Cetinkaya et al. (20) found through dynamic monitoring of SAA levels that the SAA levels of NEC infants at the initial onset (0 h) were significantly higher than those at later stages (24 h, 48 h). Moreover, infants with Bell stage II–III showed higher SAA levels at all monitoring time points compared with those with stage I. When the cut-off value was set at 23.3 mg/dl, SAA could distinguish NEC from sepsis. This result suggests that changes in SAA levels not only serve as an important reference indicator for predicting the severity of NEC but also assist in differential diagnosis for early detection of the disease. In addition, a study by Qian et al. (18) further confirmed that SAA levels showed a positive correlation with the severity of NEC—i.e., as the severity of NEC increased, SAA levels increased correspondingly—further verifying the clinical significance of SAA in evaluating the staging and severity of NEC.
Meanwhile, SAA also holds certain research value in predicting the surgical indications of NEC. A study by Coufal et al. (21) found that infants who progressed to stage IIIB had significantly higher SAA levels than those with stage II or IIIA. Furthermore, when SAA was used in combination with indicators such as fatty acid-binding protein (FABP) and trefoil factor 3 (TFF-3), it could predict NEC-related imaging features (e.g., pneumatosis intestinalis or portal venous gas), providing a reference for the assessment of NEC surgical indications. In addition, through ROC curve analysis, Chen et al. (22) found that when the cut-off value of SAA was 19.25 mg/L, the AUC for predicting surgical needs was 0.784; however, when SAA was combined with four indicators [C-reactive protein [CRP], neutrophil-to-lymphocyte ratio [NLR], and platelet distribution width [PDW]], the AUC significantly increased to 0.974. This indicates that combined detection of specific biomarkers including SAA enables more accurate determination of whether NEC infants require surgery and selection of the appropriate surgical timing, providing stronger support for clinical surgical decision-making.
There is also a certain association between SAA and disease prognosis. A nested case-control study followed up 126 NEC infants for 60 days, and the results showed that the serum SAA levels of infants in the death group at the time of diagnosis were significantly higher than those in the survival group. More importantly, Cox proportional hazards regression analysis confirmed that high SAA expression was an independent risk factor for poor prognosis of neonatal NEC, regardless of whether other confounding factors were adjusted (23). This suggests that SAA is not only an inflammatory marker but also directly associated with the risk of death. Although long-term studies that directly track the neurodevelopment or growth of NEC infants in the years following diagnosis are still lacking, SAA has become an important biomarker for evaluating the prognosis of NEC due to its strong association with disease severity, surgical needs, and short-term mortality. In clinical practice, dynamic monitoring of SAA levels combined with other indicators (e.g., CRP, NLR, PCT, PLR, PDW) can improve the accuracy of prognosis prediction.
4.1.2 C-reactive protein
C-reactive protein (CRP) is an acute-phase reactant protein synthesized by the liver under the induction of interleukin-6 (IL-6). It increases 6–8 h after the onset of inflammation, peaks at 48–72 h, and its levels can quantify the degree of inflammation (24, 25). It is widely used in the assessment of diseases such as cancer (26), autoimmune diseases (27, 28), and cardiovascular diseases (29, 30). Currently, as a single indicator, CRP has obvious limitations in the early diagnosis of NEC. On one hand, its elevation is not exclusive to NEC and may be associated with other inflammatory diseases or infections. A controlled study showed that there was no statistically significant difference in high-sensitivity C-reactive protein (hs-CRP) levels between the NEC group and the sepsis group, indicating insufficient specificity (31). On the other hand, its diagnostic efficacy as a single indicator is inadequate. A study demonstrated that the value of CRP in diagnosing NEC in preterm infants is lower than that of intestinal tissue oxygen content (rSO2), and its sensitivity also needs to be improved (32). This may be because preterm infants are prone to various infectious diseases, and CRP alone cannot distinguish the source of inflammation. In contrast, rSO2 focuses on changes in local intestinal oxygenation and is significantly less affected by inflammation in other parts of the body. Meanwhile, numerous studies have confirmed that CRP levels are closely associated with the staging and severity of NEC. A foreign prospective study showed that regardless of whether sepsis was complicated or not, CRP levels were significantly abnormal in infants with Bell stage Ⅱ/Ⅲ NEC (33). Moreover, CRP levels in stage Ⅱ infants without complications mostly returned to normal within 9 days; if CRP levels continued to rise, it indicated a risk of complications. A domestic prospective study involving 142 cases further confirmed that CRP levels in infants with stage Ⅲ NEC were higher than those in infants with stage Ⅰ/Ⅱ NEC before treatment, on the day after treatment, and during the recovery period (34). Additionally, CRP levels showed a further increase on the day after treatment. This trend clearly reflects the association between CRP levels and disease severity, supporting CRP as an important reference indicator for evaluating the staging and severity of NEC. Furthermore, CRP has certain clinical reference value in determining the surgical indications for NEC and selecting the timing of surgery. The results of a study by Duci et al. (35) showed that elevated CRP levels were positively correlated with the surgical needs of NEC infants. Meanwhile, dynamic monitoring of CRP changes can also help assess the progression of intestinal necrosis, providing a basis for clinically evaluating the necessity of surgery. The aforementioned domestic prospective study indicated that CRP levels before treatment and on the day after treatment had predictive value for NEC surgery (with optimal cut-off values of 14.6 mg/L and 42.9 mg/L, respectively) (34). The conclusion from the foreign prospective study—that persistent elevation of CRP indicates a risk of complications—can also indirectly provide a reference for the selection of surgical timing, helping clinicians formulate more reasonable surgical decisions (33). In addition, there is an association between CRP and the prognosis of NEC infants. A study by Lu et al. (36) clearly pointed out that elevated CRP levels are associated with the prognosis of NEC, and the CRP levels of infants with poor prognosis were significantly higher than those with good prognosis. However, current research data on the specific mechanism of association between CRP and the long-term prognosis of NEC as well as more detailed clinical studies remain limited. In the future, more long-term follow-up studies are needed to further clarify the specific value and application mode of CRP in the evaluation of the long-term prognosis of NEC.
4.1.3 Procalcitonin
Procalcitonin (PCT), an infectious biomarker produced by thyroid C cells, holds certain value in the early diagnosis of NEC. It can be detected within 2 h after the onset of severe bacterial infection, rises rapidly at 6 h, and peaks at 8–24 h, exhibiting high diagnostic efficacy for bacterial infections (37, 38). A prospective case-control study by Elfarargy et al. (39) showed that PCT levels in infants with NEC were significantly higher than those in the healthy control group, and this difference could assist in the early identification of NEC. Meanwhile, a study by Turner et al. (40) found that PCT has potential for differential diagnosis: PCT levels in infants with sepsis (up to 4.1 ng/ml) were significantly higher than those in infants with NEC, suggesting that PCT can be used to distinguish NEC from systemic infections, further providing a reference for the early diagnosis of NEC. Existing studies have indicated that PCT levels are also closely associated with the staging and severity of NEC, and the magnitude of its elevation is linked to disease progression. A retrospective cohort study demonstrated that PCT levels in infants with NEC stage Ⅲ were higher than those in infants with stage Ⅰ/Ⅱ; this difference serves as an important basis for evaluating the staging and severity of NEC (41). Additionally, the combined detection of PCT and mean platelet volume (MPV) not only improves diagnostic efficacy (AUC = 0.895 vs. AUC = 0.706 for PCT alone) but also acts as an effective tool for determining the severity of NEC. PCT can also be used for surgical risk stratification. The results of a study by Liebe et al. (42) showed that a PCT level ≥1.4 ng/ml indicates the need for surgical intervention; this threshold provides clinicians with a clear reference indicator for assessing whether an infant requires surgery, facilitating more rational judgment of surgical indications. However, the study also found that single PCT detection cannot fully distinguish NEC from sepsis, and this finding differs from the results of Turner et al. (40). PCT also shows significant value in evaluating the prognosis of neonatal NEC. A retrospective multicenter study involving 188 infants with NEC found that the first PCT level detected at the onset of symptoms was an independent predictor of post-NEC intestinal stenosis (RR = 1.82; 95% CI = 0.98–3.15; P = 0.009) (43). This implies that monitoring PCT levels in the early stage of the disease helps identify infants at higher risk of developing intestinal stenosis in the future, thereby enabling more intensive follow-up.
4.2 Immune markers
4.2.1 Interleukins
Interleukins (ILs) are signal proteins that facilitate cell-cell interactions, exhibiting pro-inflammatory or anti-inflammatory activities and mediating a variety of immune responses. Certain subtypes play key roles in inflammatory regulation and intestinal injury in NEC (44). A study involving animal models and clinical trials showed that IL-6 levels were significantly higher in the NEC group than in the healthy group (P < 0.05), and this abnormal level could assist in the identification of early-stage NEC (45). The diagnostic value of IL-33 is even more prominent: a study by Cakir et al. (46) demonstrated that IL-33 levels in the NEC group were significantly higher than those in the non-NEC group at 1, 3, and 7 days after disease onset. This level difference from non-affected populations can indicate the possibility of NEC in the early stage of the disease, and IL-33 can serve as a potential marker for follow-up monitoring, providing a reference for early disease tracking. Additionally, although IL-1β and IL-17 are not directly used as early diagnostic indicators, their mechanism of promoting inflammation by disrupting the intestinal tight junction (TJ) barrier (IL-1β increases luminal antigen penetration, while IL-17 directly damages intestinal cell junctions) is closely associated with the key pathogenesis of NEC (TJ barrier defects). Their abnormal expression can indirectly reflect the early intestinal injury status, providing potential mechanistic references for early diagnosis (47–49). Meanwhile, changes in the levels of multiple IL subtypes are closely related to the staging and severity of NEC. A retrospective study indicated that IL-6 levels showed a clear positive correlation with the severity of NEC, specifically presenting as a gradient in NEC infants: stage III > stage II > stage I (50). Moreover, abnormal IL-6 levels can also act as a marker for intestinal ischemic injury, further linking to disease severity. IL-33 exhibits a sustained upward trend in NEC stage III, and this dynamic elevation feature can indicate that the disease has progressed to a more severe stage (46). Furthermore, certain IL subtypes have predictive value for determining the surgical indications of NEC, among which the role of IL-8 is particularly clear. A study involving the collection of intestinal samples found that IL-8 has predictive value for the surgical needs of very low birth weight (VLBW) infants; changes in its levels can assist clinicians in judging whether this specific population requires surgical intervention (51). Although IL-10 does not directly indicate surgical needs, it has predictive value for the disease progression of VLBW infants (52). Since the degree of disease progression is one of the important factors determining the need for surgery, IL-10 can indirectly provide references for the assessment of surgical indications by predicting disease progression, helping clinicians make a more comprehensive judgment on the necessity of surgery. Prognostic assessment is crucial for the long-term quality of life of surviving infants, and certain IL subtypes also play important roles in this aspect. Persistently high expression of IL-8 during the post-treatment recovery period may be associated with persistent intestinal inflammation and recurrence risk, which directly affect the long-term recovery quality of infants; changes in IL-8 levels can indirectly indicate the possibility of poor long-term prognosis (51). A study by Jiankang et al. (53) found that by combining serum IL-6 levels (>6.25 ng/ml) with abdominal ultrasound indicators, the 1-year survival rate of infants in the high-risk group (Subgroup A, 55.6%) was significantly lower than that in the low-risk group (Subgroup B, 88.0%). This strongly confirms that IL-6 combined with imaging examinations can effectively identify high-risk infants, exhibiting good predictive value for prognosis, thereby guiding more active intervention and follow-up.
4.2.2 Tumor necrosis factor-alpha
As a key pro-inflammatory mediator, Tumor Necrosis Factor-α (TNF-α) causes intestinal injury by activating inflammatory cells and increasing vascular permeability (54). A study involving 92 infants with NEC revealed that the serum TNF-α levels of NEC infants were significantly higher than those of healthy neonates (55). Moreover, the combination of TNF-α and serum Resistin exhibited higher specificity for NEC diagnosis compared with single-marker detection (AUC = 0.952, specificity = 97.7% vs. AUC = 0.819, specificity = 65.1%). This indicates that serum TNF-α detection can assist in NEC diagnosis, and the combined diagnostic efficacy is superior. The aforementioned study also found that serum TNF-α levels were higher in infants with NEC stage Ⅲ than in those with stage Ⅱ (55). A similar conclusion was drawn from another study on preterm infants, where TNF-α levels showed a positive correlation with NEC staging (r = 0.51, P < 0.01) (56). Furthermore, a study including 124 NEC infants further confirmed that as the disease progressed from stage Ⅰ to stage Ⅲ, the TNF-α concentration in infants increased progressively with each stage (57). These findings collectively indicate that TNF-α levels can serve as an objective indicator for evaluating the severity of NEC. Certainly, TNF-α is also associated with the prognosis of NEC. The aforementioned study showed that the serum TNF-α levels in the poor prognosis group were significantly higher than those in the good prognosis group (P < 0.05) (55). In addition, a study by Gou (58) found that when TNF-α >38 ng/dl was combined with blood lactic acid >9.0 mmol/L, the risk of poor prognosis in infants increased significantly, suggesting that this combination can be used as an auxiliary indicator for prognosis assessment. As a core pro-inflammatory factor, TNF-α plays a key role in the occurrence and development of NEC. Clinically, combining it with other biomarkers may help establish more reliable prediction models.
4.2.3 Immune cells
Regulatory T cells (Treg) exert functions of eliminating autoreactive T cells, inducing self-tolerance, and suppressing inflammation (59); they play a protective role in NEC by suppressing inflammation and maintaining immune homeostasis. A study by Pacella et al. (60) found that a reduced frequency of Tregs at birth is an independent risk factor for NEC development (β = 2.98, P = 0.039), suggesting that Treg levels may be used for early auxiliary diagnosis of NEC. Research has shown that Th17/Treg imbalance is involved in NEC progression: melatonin can reduce Th17 cells and increase Tregs by activating the AMPK/SIRT1 signaling pathway, thereby improving intestinal immune imbalance (61). Clinical evidence indicates that Treg expression in monocytes of NEC infants is decreased, while exogenous TGF-β and IL-10 can upregulate Tregs (62), implying its potential as a therapeutic target. Although Treg-based therapy shows promising prospects for clinical application, future treatment strategies may require combined interventions (e.g., simultaneous blockade of IL-6 signaling) to achieve more precise immune regulation (63).
As key participants in intestinal mucosal immunity, γδ T cells regulate local inflammatory responses by rapidly secreting cytokines (64). A study comparing cytokine expression in γδ T cells isolated from intestinal epithelial lymphocytes (IELs) between necrotic intestinal segments of NEC infants and those with intestinal atresia found that the proportion of γδ T cells in the necrotic intestinal segments of NEC infants was significantly reduced, while the expression of pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-17) mediated by these cells was increased. This suggests that γδ T cell dysfunction may exacerbate intestinal immune imbalance (65). Mechanistically, a study by Weitkamp et al. (66) revealed that the deficiency of intraepithelial γδ T cells (γδ IELs) impairs intestinal barrier function and promotes NEC development. Intervention experiments demonstrated that Bifidobacterium can alleviate NEC-related intestinal injury by increasing the number of intestinal epithelial γδ T cells, confirming its feasibility as a therapeutic target (67). Therefore, maintaining or restoring γδ T cell homeostasis may serve as a novel strategy for NEC prevention and treatment.
4.2.4 Toll-like receptor 4
Toll-like receptor 4 (TLR4) is a core molecule in the pathogenesis of NEC; it drives intestinal inflammation and immune dysregulation by inducing intestinal epithelial cell death, recruiting pro-inflammatory leukocytes, and causing intestinal hypoperfusion (48, 68–70). In recent years, studies on several TLR4-targeted intervention strategies have shown therapeutic potential. A study by Zhang et al. (65) found that β-glucan can improve intestinal barrier function by inhibiting the TLR4/NF-κB pathway, thereby reducing the risk of NEC in newborn mice. A study by Kovler et al. (71) demonstrated that enteric glial cell deficiency may promote NEC through TLR4 activation and intestinal motility disorders, suggesting that the repair of enteric glial cells could be a potential therapeutic approach. Additionally, a study by Liu et al. (72) revealed that the expression levels of both TLR4 and necroptosis-related proteins are upregulated in NEC patients and animal models; moreover, inhibiting necroptosis can significantly alleviate intestinal inflammatory injury, indicating that anti-necroptosis therapy is a potential direction for relieving NEC symptoms.
4.2.5 The complement
Complement 5a (C5a), a complement activation product, is identified as a key pathogenic factor in NEC by mediating mesenteric ischemia/reperfusion injury. A study by Lian et al. (73) showed that urinary C5a is abnormally elevated in the early stage of intestinal injury, suggesting its potential as an early diagnostic biomarker for NEC. Furthermore, a study by Tayman et al. (74) found that serum and urinary C5a levels were significantly elevated in infants with NEC; among these, serum C5a could effectively predict the risk of death and surgical intervention (P < 0.05), indicating that serum C5a can be used to assess disease prognosis. Additionally, complement C3 expression is upregulated in mesenteric ischemia models and shows a positive correlation with the degree of intestinal tissue damage. This further supports that excessive activation of the complement system may collectively drive the occurrence and development of NEC (75).
4.3 Gut-related markers
4.3.1 Intestinal fatty acid-binding protein
Intestinal Fatty Acid-Binding Protein (I-FABP) is specifically expressed in the epithelial cells of small intestinal mucosal villi and is rapidly released into the bloodstream upon intestinal ischemia or inflammatory injury (76, 77); studies have confirmed it as a sensitive biomarker for the early diagnosis of NEC. A mouse model study showed that serum I-FABP levels could be detected to increase as early as 15 min after intestinal ischemia onset, and the levels continued to rise with prolonged ischemia duration (78). This enables the capture of early intestinal injury signals before the appearance of typical NEC symptoms, providing a basis for early disease identification. Meanwhile, urinary I-FABP also exhibits potential for early diagnosis. A study by Coufal et al. (21) found that urinary I-FABP can be used to distinguish NEC from sepsis: urinary I-FABP levels in the NEC group were significantly higher than those in the sepsis group, which can help rule out interference from other infectious diseases and improve the accuracy of early diagnosis. Additionally, a study by Saran et al. (79) pointed out that the urinary I-FABP/creatinine ratio (urinary I-FABP/Cr) further optimizes diagnostic efficacy—when this ratio is 3.6 pg/mmol, the sensitivity and specificity for diagnosing NEC stage Ⅱ/Ⅲ reach 96% and 99.5%, respectively—providing a more reliable quantitative indicator for the early accurate diagnosis of NEC.
Changes in I-FABP levels (in both serum and urine forms) are also closely associated with the staging and severity of NEC, serving as important references for assessing disease conditions. Regarding serum I-FABP, multiple studies have shown that serum I-FABP levels in infants with NEC stage Ⅲ are significantly higher than those in the healthy control group and infants with stage Ⅰ/Ⅱ (P < 0.05) (80–82). This trend has been verified in both animal models of NEC (rat ileal tissue models) and clinical prospective studies involving neonates with a gestational age < 32 weeks (83, 84). In terms of urinary I-FABP, a study by Shaaban et al. (85) found that urinary I-FABP levels were positively correlated with NEC severity, specifically presenting as a gradient in infants: stage Ⅲ > stage Ⅱ > stage Ⅰ (P < 0.05). Furthermore, a study by Evennett et al. (86) noted that elevated urinary I-FABP is associated with the extent of intestinal involvement—higher urinary I-FABP levels are observed when the intestine is extensively involved—further confirming that it can reflect the severity of intestinal injury through level differences. Although a meta-analysis showed that the overall diagnostic value of urinary I-FABP and its ratio (AUC = 0.81) is slightly lower than that of serum I-FABP (AUC = 0.84), both have clear clinical significance in the assessment of NEC staging and severity (87).
Meanwhile, I-FABP (especially in urinary form) holds important predictive value for determining the surgical indications of NEC. The results of a study by El-Abd et al. (88) showed that there is a clear diagnostic threshold for urinary I-FABP (4.13 ng/g). When this threshold is used to assist in predicting the surgical needs of NEC infants, the sensitivity and specificity reach 100% and 76.19%, respectively—providing a clear quantitative reference for clinically judging the need for surgical intervention. Additionally, the aforementioned study by Evennett et al. (86) also found that urinary I-FABP levels are significantly correlated with the length of intestinal resection (RHO = 0.92, P = 0.001), i.e., higher urinary I-FABP levels indicate more severe intestinal injury and potentially longer intestinal segments requiring resection. This further provides a basis for formulating surgical plans (e.g., assessment of intestinal resection range) and helps clinicians grasp surgical indications more accurately.
Although ultra-long-term studies directly demonstrating the relationship between I-FABP and the growth and development of NEC infants in the years following diagnosis are currently lacking, several studies have confirmed that its high levels in the acute phase are associated with more severe disease and a higher risk of complications. A retrospective study involving 105 infants with suspected NEC found that serum I-FABP levels in the survival group were significantly lower than those in the death group (P < 0.05), indicating a direct association with the risk of death (89). High I-FABP levels suggest more severe full-thickness intestinal injury, which is the main pathological basis for intestinal stenosis (90). A study pointed out that high urinary I-FABP levels in NEC infants on the first day of refeeding indicate a higher risk of subsequent intestinal stenosis complications (91).
4.3.2 Liver fatty acid-binding protein
Although Liver Fatty Acid-Binding Protein (L-FABP) is widely expressed in tissues such as the liver, intestine, and kidney, it is also released into the bloodstream upon intestinal injury, thus holding certain value in the early diagnosis of NEC. A prospective study showed that when symptoms of NEC appear during the disease course, L-FABP levels in infants with NEC (at any stage) are significantly higher than those in healthy controls (81). Another prospective cohort study involving preterm infants with a gestational age <32 weeks and/or birth weight <1,500 g found that L-FABP levels are positively correlated with the risk of NEC, supporting its role as an early warning indicator for NEC (92). Meanwhile, similar to Intestinal Fatty Acid-Binding Protein (I-FABP), L-FABP can also assist in distinguishing NEC from sepsis (21), reducing the interference of infectious diseases on the early diagnosis of NEC and further improving the accuracy of early identification. Furthermore, a study by Pelsers et al. (93) revealed that preoperative L-FABP levels are significantly elevated in patients with intestinal injury; notably, L-FABP content is the highest in the ileum (40-fold higher than that of I-FABP in each intestinal segment), exhibiting high sensitivity to intestinal injury. This allows L-FABP to capture injury signals in the early stage of the disease, providing support for the early diagnosis of NEC. Additionally, the study] also indicated that preoperative L-FABP levels are significantly elevated in patients with intestinal injury, while they decrease rapidly after surgery (93). This dynamic change can reflect the repair of intestinal injury and the efficacy of surgical intervention. Based on this, monitoring changes in L-FABP levels can assist in determining the timing of surgery. Currently, research on L-FABP in NEC remains limited, and further studies are needed to verify its clinical efficacy in disease staging and prognostic assessment.
4.3.3 Fecal calprotectin
Fecal Calprotectin (FC), a member of the S100 protein family, is a 36 kDa calcium-binding protein primarily derived from neutrophils (accounting for 60% of cytoplasmic proteins), and its concentration is positively correlated with the degree of inflammation (94). Since FC exhibits high stability in feces (stable for 7 days at room temperature) and its concentration in healthy individuals is approximately 6 times that in plasma, it is widely used in clinical practice for monitoring intestinal inflammation (94, 95). A study on exclusively breastfed infants with suspected NEC found that FC levels in the NEC group were higher than those in the healthy group (96). A meta-analysis by Yanqiu et al. (97) indicated that FC has high value for the early diagnosis of NEC (sensitivity = 0.86, specificity = 0.80, AUC = 0.913).A multicenter prospective study showed that the combined detection of FC and Lipocalin-2 (LCN2) can improve the sensitivity of early prediction for NEC; notably, changes in these indicators can be observed as early as 10 days before symptom onset, providing advance warning for the early identification of NEC (98, 99). Additionally, monitoring FC levels can also assess the severity and staging of NEC. A study by Hu et al. (100) demonstrated that FC levels in the NEC group increased progressively with disease staging (stage III > stage II > stage I) (P < 0.05), suggesting a positive correlation between FC levels and disease severity. Thus, differences in FC levels can assist in evaluating the severity of NEC in infants. However, there is currently controversy regarding the association between FC and NEC staging. The core point of contention lies in the association between FC levels and postnatal days: a study by Yoon et al. (101) suggested that FC levels are affected by gestational age—FC levels increase with postnatal age in infants with a gestational age <26 weeks, while the opposite trend is observed in those with a gestational age ≥26 weeks. In contrast, a study by Farghaly et al. (96) found no correlation between FC and postnatal days. It is hypothesized that the conflicting conclusions may stem from differences in sample size; this controversy requires larger-scale studies to clarify, thereby enabling FC to play a more accurate role in the staging assessment of NEC. FC also holds certain value in the evaluation of long-term prognosis of NEC. A study by Chen et al. (102) found that the median FC level in infants who developed intestinal stenosis (a common long-term complication of NEC) was significantly higher than that in the non-stenosis group (P < 0.001), suggesting that FC levels can serve as a reference indicator for predicting the risk of post-surgical intestinal stenosis in NEC infants.
4.3.4 VOCs
Fecal volatile organic compounds (VOCs) are components of fecal odor and metabolic products of the intestinal microbiota. As the preclinical stage of NEC is associated with alterations in intestinal microbiota composition, VOCs represent potential biomarkers for non-invasive prediction of NEC. A prospective study found that the absence of four specific esters (including 2-ethylhexyl acetate) was detected 4 days before the onset of NEC, which may have marker significance for the early diagnosis of NEC (103).Meanwhile, a study by de et al. (104) revealed that the fecal VOC profiles of infants with NEC could be distinguished from those of the healthy group and the sepsis group 2–3 days before the appearance of clinical symptoms (with a sensitivity of 83.3% and a specificity of 75.0%). This suggests that VOC analysis via eNose may serve as a non-invasive tool for the early prediction of NEC. Using gas chromatography-mass spectrometry (GC-MS), Probert et al. (105) detected a specific set of VOCs in NEC infants before disease onset, and their levels were positively correlated with the development of NEC. This VOC panel includes 3-(methylthio)propionaldehyde, benzaldehyde, 2-phenylacetaldehyde, 2-methylpropanal, 3-methylbutanol, and 2-methylbutanol. The aforementioned studies confirm that changes in VOCs precede the clinical onset of NEC, and this finding holds important significance for the early prediction of NEC.
5 Emerging biomarkers
5.1 Genomics
Genomics, the study of all genes in an organism, has made significant progress in understanding the pathogenesis, risk assessment, and therapeutic strategies of NEC. Genetic polymorphism refers to one or more variations in gene sequences, which may affect an individual's susceptibility to certain diseases. The following studies have identified multiple genetic susceptibility factors associated with NEC, all confirming that genetic polymorphisms involved in immune response, inflammatory regulation, and intestinal development are closely linked to NEC. Through gene resequencing, a study by Zhou et al. (106) found that the rs2075783 polymorphism in exon 1 of the GM2A gene and the rs1048719 polymorphism in the intronic region of this gene are associated with the development of NEC, while the rs11465996 polymorphism in the promoter region of the MD-2 gene is associated with the severity of NEC. A prospective multicenter cohort study showed that variations in NFKB1 (g.-24519delATTG) and NFKBIA (g.-1004A>G) are associated with NEC development (P < 0.05) (107). A study by Zhang et al. (108) indicated that the TC + CC genotypes and C allele of IL-17F rs763780 are associated with both susceptibility to NEC and the severity of NEC. The first domestic study on vitamin D and its receptor demonstrated that the VDR Fok1 C/T genetic polymorphism plays a role in the development of NEC (109). Furthermore, associations between genetic polymorphisms and NEC-related surgery have also been identified. A cohort study of very low birth weight (VLBW) infants found that carriers of ≥2 variant alleles of NOD2 had an increased risk of developing NEC requiring surgery (OR = 3.57; 95% CI: 1.27–10.04; P = 0.03) (110). A study by Yasuhara et al. (111) reported a novel familial pathogenic variant of GATA6 associated with NEC complicated by intestinal perforation.
Additionally, progress has been made in studies on genetic polymorphisms and short-term outcomes of NEC. A study by Ya et al. (112) found that the CXCL5-156 C allele is a risk factor for death in NEC infants (P < 0.05). Notably, some studies have also identified genetic polymorphisms that are not merely associated with increased NEC risk. A study by Strauss et al. (113) found that the HIF1A rs11549465T allele independently reduces the risk of NEC, providing a new research direction for NEC prevention. A study by Cao et al. (114) identified a gene with dual effects: HMGB1 rs1360485 increases susceptibility to NEC but predicts better survival outcomes. Moreover, research on gene epigenetics has enhanced understanding of NEC pathogenesis. Serial studies by Good et al. (115, 116) showed that NEC tissues exhibit genome-wide hypermethylation, which is associated with transcriptional abnormalities and has potential for non-invasive detection—this provides new opportunities for developing novel diagnostic methods for NEC. Currently, a research gap exists: large-scale long-term follow-up studies that directly link these genetic and epigenetic markers to endpoints such as long-term growth and development, neurodevelopment, and long-term intestinal function recovery in NEC infants are lacking. Future research could integrate these markers to identify infants at risk of adverse long-term prognosis in the early stages of the disease, thereby enabling more targeted interventions and follow-up.
5.2 Transcriptomics
Transcriptomics, which investigates gene transcription and its regulatory mechanisms at the global level, studies gene expression from the RNA perspective. As a key subfield of gene expression research, it provides in-depth insights into gene regulation and cellular activities. By exploring signaling pathways critical to the development of NEC, the following studies have identified novel therapeutic approaches. Previous research has confirmed that melatonin treats NEC by correcting Treg/Th17 imbalance (61); similarly, researchers have found via transcriptomic analysis of intestinal tissues that melatonin reduces bile acid toxicity and alleviates intestinal injury through the SIRT1/FXR pathway (117). A study by Gao et al. (118) demonstrated through transcriptomic analysis that butyrate mitigates intestinal inflammation by inhibiting phosphorylation of the PI3K-Akt pathway and enhancing the expression of tight junction (TJ) proteins. A study by Chen et al. (119) showed that Bacillus fragilis regulates the microbiota-bile acid metabolism axis via the FXR-NLRP3 pathway, restores intestinal dysbiosis and abnormal bile acid metabolism, and thereby alleviates intestinal injury. Using methods including immunofluorescence staining, Western blotting, and reverse transcription-quantitative PCR (RT-qPCR), a study by Zhang et al. (120) found that Saccharomyces boulardii (SB) exerts a protective effect against NEC through the SIRT1/NF-κB pathway. Integrated studies of transcriptomics and microbiomics have also revealed microbiota-host interactions, where changes in the abundance of specific microbes may affect intestinal immune responses and barrier function. Hosfield et al. (121) performed fecal microbiome analysis via 16S rRNA sequencing and showed that both microbial diversity and the relative abundance of Lactobacillus were significantly higher in the control group than in the NEC group, while the relative abundance of E. coli was lower in the control group. This indicates that NEC development is associated with intestinal dysbiosis. A study by Zhai et al. (122) found that NEC infants are often accompanied by changes in the intestinal bacterial genome, and variations in microbiota composition are related to the severity of the disease. Another study confirmed intestinal inflammation in the small intestine and colon using quantitative reverse transcription-polymerase chain reaction (qRT-PCR), evaluated the intestinal microbiome via 16S rRNA sequencing, and analyzed the intestinal microbiome of NEC piglets. The results showed that the changes in the intestinal microbiome of NEC piglets were consistent with those of preterm infants with NEC, characterized by reduced microbial diversity and increased abundances of Gammaproteobacteria and Enterobacteriaceae (123). Additionally, one of the aforementioned studies found that the protective effect of SB against NEC is associated with the regulation of the intestinal microbiome: compared with the normal group, the control group showed a significant reduction in the richness of intestinal microbiota composition, and the NEC group exhibited a further decrease in intestinal microbial richness (120). However, intervention with the probiotic Saccharomyces boulardii significantly improved the enrichment of intestinal microbiota in neonatal mice with NEC.
Transcriptomic studies have also identified gene expression signatures in the intestinal tissues of NEC infants, providing potential targets for the development of NEC interventions. Han et al. (124) performed whole-transcriptome RNA sequencing on NEC samples and found that HK2, a pathogenic hypoxia-related gene, was upregulated. Egozi et al. (125) combined single-cell sequencing and bulk transcriptomics and showed that epithelial cells in NEC tissues abnormally activate pro-inflammatory genes. A retrospective study by Pan et al. (126) conducted whole-blood transcriptomic analysis and indicated that colonic differentially expressed genes (e.g., AOAH, STAT3) are associated with the degree of pathological lesions. These gene targets may thus enable early intervention for NEC. Notably, transcriptomic data are large in scale and complex to analyze, and it remains challenging to fully decipher genetic-environmental interactions. Future research should integrate multi-omics technologies, artificial intelligence (AI), and clinical cohorts to overcome these challenges and better leverage transcriptomics to improve neonatal health.
5.3 Proteomics
Proteomics primarily investigates the expression, modification, interaction, and function of proteins in organisms. In the research on neonatal necrotizing NEC, the application of proteomics focuses on the following aspects. First, through proteomic analysis, researchers can identify NEC-associated changes in protein expression and signaling pathways, thereby unraveling the molecular mechanisms of the disease. Zhong et al. (127) performed proteomic and ubiquitin-proteomic analyses on intestinal macrophages and showed that RNF31-mediated ubiquitination and degradation of IKKα activates NF-κB/M1 macrophage polarization; inhibiting this pathway can alleviate intestinal inflammation. A study establishing in vivo and in vitro models found that butyrate upregulates Fut2 expression via the MEK4-JNK pathway, thereby enhancing the intestinal barrier (128). A study by Nguyen et al. (129) revealed that TGF-β2 exerts a protective effect in NEC by regulating oxidative stress and the TLR4 signaling pathway.
Second, proteomic technologies enable comprehensive analysis of protein expression profiles in intestinal tissues and blood samples from NEC patients, uncovering disease-associated biomarkers. A multicenter prospective study reported that liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of samples—using SWATH/DIA acquisition and cross-compatible proteomic software—identified a panel of 36 fecal proteins that can predict the development of NEC one week in advance (130). Wang et al. (131) compared the necrotic segments of intestinal tissue with adjacent normal intestinal segments (in a control setting) and found that TRAF6 and CXCL8/IL-8 were significantly upregulated in both NEC intestinal tissues and serum, suggesting their potential as important predictive factors for the early diagnosis of NEC. Mackay et al. (132) analyzed serum protein levels in neonates with and without NEC and showed that alpha-fetoprotein (AUC = 0.926), glucagon (AUC = 0.860), and IGHA1/IGHA2 (AUC = 0.826) could effectively distinguish NEC cases from non-NEC cases. Additionally, a study evaluating 92 inflammation-related proteins using a high-throughput OLINK proteomic platform found that 11 biomarkers (with upregulated expression, including IL-8, IL-24, CCL20, OPG, TSLP, TRAIL, MMP-10, CXCL1, MCP-4, TNFSF14, and LIF) hold high value in identifying NEC and determining its severity (133). Among these, the combination of IL-8, IL-24, and CCL20 exhibited the optimal predictive value for distinguishing NEC from the healthy group, NEC from sepsis, and different degrees of disease inflammation (AUC = 0.909 vs. 0.782 vs. 0.919). The combination of IL-8, OPG, MCP-4, IL-24, LIF, and CCL20 could distinguish NEC stage Ⅱ from stage Ⅲ (AUC = 0.977) (133). Similarly, several studies have also found abnormally high expression of CCL20, TSLP, and CXCL1 in NEC (134–136). Notably, proteomic technologies are still evolving. Significant differences may exist between laboratories in terms of sample processing, data acquisition, and data analysis, which may lead to reduced comparability of research results.
5.4 Metabolomics
Metabolomics delves into the metabolic profiling of patients with NEC using high-precision technical approaches such as mass spectrometry (MS) and nuclear magnetic resonance (NMR), uncovering the metabolic dysregulation underlying the disease. Patients with NEC exhibit abnormal changes in a variety of metabolites; these changes not only involve basic physiological processes such as energy metabolism and amino acid metabolism but also are closely associated with complex mechanisms including intestinal microecology and oxidative stress. In two previously mentioned studies (118, 119), transcriptomics was integrated with metabolomics. By analyzing the metabolism of hesperidin, bile acids, and intestinal metabolites in NEC, these studies further deepened the understanding of how butyrate and Bacteroides fragilis alleviate intestinal inflammation in NEC. Multiple studies have utilized metabolomics to analyze differences in serum/urine metabolites, and combined with ROC curve analysis, these studies suggest that such metabolites hold diagnostic value for NEC and are promising potential biomarkers. A study focusing on preterm infants with abdominal symptoms and gestational age ≤34 weeks employed MRM-based targeted metabolomics to measure TCA cycle metabolites. It found that reduced levels of certain TCA metabolites (including succinic acid, L-malate, and oxaloacetic acid) as well as decreased species diversity have potential value for the early diagnosis of NEC (137). A prospective case-control study by Thomaidou et al. (138) identified that certain phospholipids and their derivatives (e.g., L-carnitine) could be used as biomarkers for the early detection of late-onset sepsis (LOS) and NEC. Additionally, a multicenter prospective case-control study using targeted high-performance liquid chromatography (HPLC) analysis showed alterations in several specific amino acids in samples collected 1–3 days before NEC onset (139), suggesting that early diagnostic biomarkers for NEC may be identified among these altered amino acids. Several other studies also integrated metabolomics techniques and, through analyzing intestinal microbial diversity, consistently found significant differences in the composition and distribution of microbiota between the NEC group and the control group. At the phylum level, studies have reported contradictory patterns of microbial abundance in the NEC group: some studies showed a decrease in Actinobacteria and Proteobacteria (137) and an increase in Firmicutes (140), while others observed an increase in Proteobacteria (140, 141) and a decrease in Firmicutes (141). At the genus level, the NEC group exhibited a significant reduction in Bifidobacterium and Lactobacillus, an enrichment of Streptococcus, and contradictory results regarding the abundance of Propionibacterium (137, 140, 142–145). At the species level, the direction of changes in Staphylococcus and Enterococcus varied across studies, but Bacillus was consistently enriched before the onset of NEC/sepsis (142, 143, 145).
5.5 Machine learning
Machine Learning (ML) technology is reshaping the clinical research paradigm for NEC, particularly demonstrating significant potential in early diagnosis, severity stratification, and prognostic assessment. Multiple studies have validated the application value of ML in this field. For instance, one study constructed classification models using XGBoost, decision trees, and artificial neural networks (ANNs). Results showed that XGBoost exhibited the optimal performance in the differential diagnosis of NEC, with a sensitivity of 80.48%, a specificity of 100%, and an AUC of 0.902 (146). Another study based on the decision tree algorithm identified nine key diagnostic criteria [including apnea, lethargy, occult blood in stool, abdominal distension, gestational age, postnatal age at onset, feeding volume, disseminated intravascular coagulation (DIC), and occult rectal bleeding]. This model outperformed the traditional modified Bell staging criteria in identifying NEC (147). Furthermore, a multimodal AI system integrated feature engineering, machine learning, and deep learning technologies. By leveraging clinical data from 379 NEC patients in the week prior to surgery, the system achieved effective prediction of surgical needs (148). The study further analyzed 4,535 abdominal x-rays and clinical parameters from 1,823 infants with suspected NEC, highlighting the importance of multi-source information fusion in enhancing model performance. In addition, a single-center retrospective study involving 536 infants demonstrated that the predictive model constructed by combining feature selection algorithms with Support Vector Machines (SVM) could efficiently distinguish between NEC and non-NEC cases (AUROC = 0.932), as well as between medical NEC and surgical NEC (AUC = 0.835) (149). This further validates the clinical application prospects of ML in NEC-assisted diagnosis and risk stratification. However, machine learning (ML) also has limitations. The performance of ML models is highly dependent on the scale, completeness, and representativeness of training data. For necrotizing enterocolitis (NEC), the limited sample size, uneven case distribution, and potential selection bias in retrospective studies pose challenges to model training. Additionally, the “uninterpretability” of ML models leads to low trust among clinicians in their results. Especially in high-stakes decision-making (e.g., determining the timing of surgery), clinicians tend to rely on traditional indicators rather than ML predictions. Moreover, the clinical translation of ML models faces certain difficulties. In the future, prospective, multi-center studies are needed to improve model interpretability and promote the clinical translation of ML in NEC management.
Beyond the traditional and emerging biomarkers mentioned above, several clinical manifestations and comprehensive assessment indicators also hold significant value in evaluating the condition of NEC. Early NEC often presents with abdominal distension, gastric residuals, or hematochezia. While these symptoms serve as warning signs, their diagnostic specificity is limited. Abdominal distension is common in preterm infants and can also be caused by non-NEC factors such as feeding intolerance and constipation; moreover, the incidence of hematochezia in fulminant NEC (fNEC) is significantly lower than that in typical NEC (12.9% vs. 49.0%), indicating that hematochezia is not a reliable single-indicator basis for diagnosis (7). In terms of feeding assessment, gastric residual volume has traditionally been monitored to evaluate NEC risk. However, recent studies have suggested that such monitoring may be more reasonable only when accompanied by other gastrointestinal symptoms—this avoids gastric enzyme loss or mucosal irritation caused by frequent manipulations (150). Based on clinical symptoms, integrating objective indicators of internal environment disturbance can significantly improve the accuracy of assessment. For instance, metabolic acidosis indicated by blood gas analysis is a key marker reflecting the deterioration of systemic conditions. The MD7 scoring system, constructed based on multiple metabolic indicators, quantifies the metabolic status of infants. Studies have shown that an MD7 score ≥3 is significantly associated with an increased risk of requiring surgical intervention (151). A systematic review and meta-analysis further demonstrated that the MD7 score has a sensitivity of 0.77 and a specificity of 0.73 for identifying NEC cases requiring surgery (152). Another study revealed that serum Relmβ can be combined with the MD7 score to further improve the accuracy of predicting surgical timing (153). In recent years, near-infrared spectroscopy (NIRS) technology has provided a new approach for the early warning of NEC. This technology enables non-invasive, continuous monitoring of abdominal regional oxygen saturation (A-rSO2), directly reflecting intestinal microcirculatory perfusion and oxygenation status. A study by Yangbo and Dan (154) showed that a decrease in intestinal rSO2 is significantly associated with an increased risk of NEC development, and the AUC for diagnosing NEC using rSO2 combined with CRP reaches 0.870 (95% CI: 0.791–0.950). Another retrospective study also confirmed that rSO2 combined with PCT and mean platelet volume (MPV) has good predictive value for NEC severity (155). Additionally, intestinal ultrasound can accurately assess intestinal injury and diagnose NEC. Studies have identified several specific ultrasound findings of NEC—such as pneumatosis intestinalis, portal venous gas, changes in intestinal wall morphology and perfusion, intestinal motility status, and peritoneal effusion—and ultrasound has shown superior imaging performance compared to x-ray for detecting these features (156). Therefore, in clinical practice, for infants with high-risk factors (e.g., prematurity), once suspicious symptoms appear, clinicians should promptly integrate imaging findings, laboratory indicators, and novel monitoring technologies for comprehensive judgment, and implement dynamic monitoring. This approach aims to improve the capacity for early diagnosis and intervention of NEC.
6 Discussion
6.1 Comparison of diagnostic efficacy of biomarkers
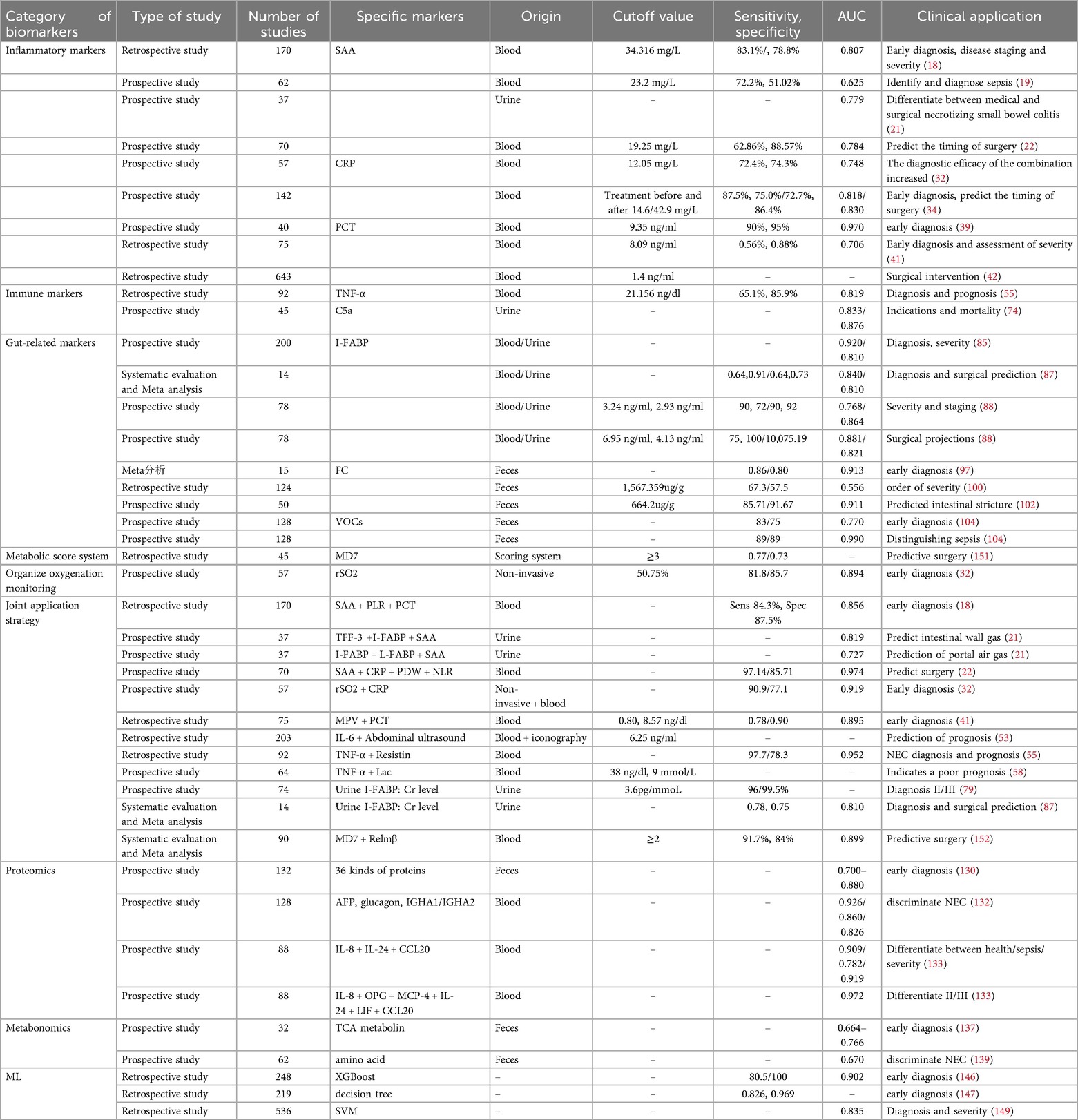
To systematically evaluate the diagnostic value of various biomarkers for NEC, this study summarizes the key indicators of their diagnostic efficacy based on existing literature, including sensitivity (Sens), specificity (Spec), and AUC. Additionally, an integrated perspective has been developed, as shown in Table 1 and Figure 1.
Based on the table above, Intestinal Fatty Acid-Binding Protein (I-FABP) demonstrates promising clinical application prospects due to its relatively high area under the receiver operating characteristic curve (AUROC) values and consistent performance in NEC diagnosis, severity grading, and surgical prediction. This biomarker can be detected in both blood and urine; notably, the urinary I-FABP-to-creatinine ratio (I-FABP:Cr) further simplifies the detection workflow, enhancing its feasibility and applicability in clinical practice. Thus, I-FABP is expected to serve as either a standalone indicator or a core component of combined diagnostic panels, emerging as a preferred tool for NEC screening and disease monitoring. Fecal Calprotectin (FC), a fully non-invasive detection indicator, is particularly suitable for neonates and preterm infants. It exhibits excellent efficacy in early diagnosis and intestinal stenosis prediction (AUROC can reach ≥0.913), making it well-suited for promotion in primary healthcare facilities or long-term follow-up settings. However, FC is associated with inter-individual variability, and fulminant calprotectin levels are relatively high in healthy preterm infants during the first postnatal week (157, 158). For clinical application, unified cut-off values and time windows remain necessary to improve standardization. Procalcitonin (PCT) holds value in distinguishing NEC from sepsis; nevertheless, its diagnostic stability is limited when used alone. It is more appropriately incorporated into combined assessment systems as an auxiliary inflammatory indicator. As a non-invasive, real-time bedside functional monitoring tool, regional tissue oxygen saturation (rSO2) has a standalone AUROC of 0.894, with further improved efficacy when combined with C-reactive protein (CRP). Although equipment costs may limit its popularization, rSO2 is expected to become a routine monitoring modality in neonatal intensive care units (NICUs) with adequate resources. Additionally, several combined strategies exhibit significant advantages. For example, the panel of Serum Amyloid A (SAA) + C-reactive protein (CRP) + Platelet Distribution Width (PDW) + Neutrophil-to-Lymphocyte Ratio (NLR) achieves an AUROC as high as 0.974. This suggests that multi-marker integrated models have substantial potential to improve diagnostic accuracy, making them suitable for early warning in high-risk infants and clinical decision support.
It should be noted that in neonates with congenital heart disease (CHD) complicated by necrotizing enterocolitis (NEC), intestinal hypoperfusion and ischemic injury are caused by insufficient cardiac output (159). For intestinal injury biomarkers such as Intestinal Fatty Acid-Binding Protein (I-FABP) and Fecal Calprotectin (FC), which are less affected by cardiogenic systemic inflammation, attention should be paid to their dynamic change trends rather than single absolute values. This is because CHD neonates may have relatively high baseline levels due to chronic intestinal ischemia, and a significant increase after feeding may be more diagnostically valuable. Similarly, for inflammatory indicators such as C-reactive protein (CRP) and procalcitonin (PCT), which have low specificity, caution is needed to determine whether their elevation is caused by CHD-related chronic systemic inflammation or non-infectious inflammation after cardiac surgery (160). For CHD neonates, dynamic monitoring is also the focus of regional tissue oxygen saturation (rSO2) monitoring. During feeding or hemodynamic fluctuations, a sharp decrease in rSO2 or an extremely high “intestinal-cerebral oxygenation difference” may be more indicative of an intestinal ischemic crisis than absolute values (161).
6.2 Comparison between current guidelines and emerging biomarkers
Currently, there are authoritative guidelines available for the diagnosis and management of neonatal necrotizing enterocolitis (NEC) (12). These guidelines primarily base diagnosis on clinical manifestations and radiological features; however, these indicators typically manifest only after intestinal injury has already occurred, even progressing to the middle or advanced stages. In contrast, the molecular biomarkers discussed in this article—such as Intestinal Fatty Acid-Binding Protein (I-FABP) and Serum Amyloid A (SAA)—are released into the blood or urine at the very early stage of intestinal mucosal injury, providing a critical “time window” for the early identification of NEC. Although the guidelines recommend monitoring inflammatory markers such as C-reactive protein (CRP) and procalcitonin (PCT), which align with the biomarker focus of this article, their insufficient specificity in NEC diagnosis constitutes a significant limitation. This review suggests that the suboptimal diagnostic efficacy or inconsistent results of CRP and PCT observed in many studies may stem from the following factors: First, the early systemic inflammatory response in NEC overlaps considerably with that of infectious diseases such as neonatal sepsis; the confounding effect of infection makes it difficult to accurately distinguish NEC from other infectious conditions using PCT or CRP alone. Second, differences in detection methods, reagent brands, and detection time windows across studies directly compromise the comparability of results. Finally, and most critically, for the special population of preterm infants, there is a lack of unified and reliable cut-off values to define abnormal levels; the use of varying cut-off values in different studies inevitably leads to significant variability in diagnostic sensitivity and specificity. Therefore, while CRP and PCT are excellent inflammatory indicators, their standalone utility is limited. Instead, they need to be combined with other biomarkers with higher intestinal tissue specificity (e.g., I-FABP) or integrated with routine blood parameters such as Platelet-to-Lymphocyte Ratio (PLR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet Distribution Width (PDW) for comprehensive assessment, thereby improving diagnostic accuracy.
Furthermore, the guidelines recommend using near-infrared spectroscopy (NIRS) to monitor local intestinal oxygenation, which is consistent with the findings of this article: whether used alone or in combination with CRP, regional tissue oxygen saturation (rSO2) exhibits favorable diagnostic efficacy. In terms of imaging, the guidelines suggest using ultrasound for dynamic monitoring of changes in abdominal signs; however, this review finds that ultrasound outperforms x-ray in identifying certain NEC-specific findings. Regarding Fecal Calprotectin (FC), the guidelines currently do not recommend it as a routine test; nonetheless, this article proposes that if unified cut-off values and clear detection time windows are established, this indicator still holds potential for clinical translation. In terms of determining surgical timing, the guidelines primarily rely on evidence of deterioration in systemic condition and radiological signs of perforation. In contrast, studies reviewed in this article demonstrate that urinary Complement 5a (C5a), I-FABP, the MD7 scoring system, and multi-marker combined models are significantly more accurate than traditional indicators in predicting the progression of intestinal necrosis, surgical necessity, and infant mortality.
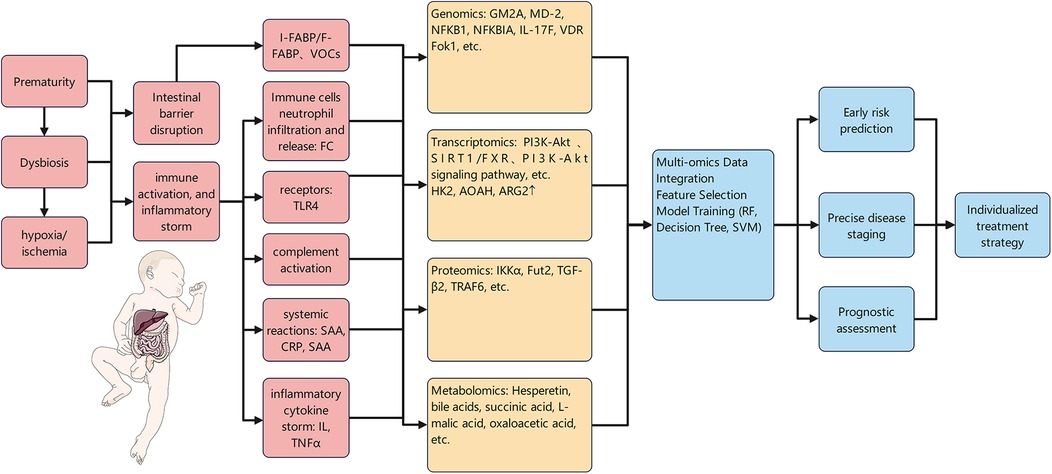
6.3 Advantages and challenges of multi-omics integration
Multi-omics integration provides a powerful tool for deciphering the core driver pathways underlying the development and progression of necrotizing enterocolitis (NEC) by systematically uncovering interaction networks across distinct molecular layers, including the genome, transcriptome, proteome, and metabolome (162). For instance, two studies integrating transcriptomics and metabolomics revealed the synergistic mechanism of butyrate and Bacteroides fragilis in alleviating intestinal inflammation in NEC, laying a theoretical foundation for the development of novel therapeutic strategies (118, 119). Another study, by fusing DNA methylome and transcriptome data, identified widespread hypermethylation in NEC and characterized multiple key genes (e.g., ADAP1, GUCA2A) with suppressed expression due to increased methylation levels. Functionally, these genes are closely associated with intestinal inflammation and barrier integrity. This finding not only deepens the understanding of epigenetic regulatory mechanisms in NEC but also provides potential novel biomarkers for early diagnosis and risk prediction (163). Additionally, machine learning classification models constructed based on single-cell transcriptome and bulk transcriptome data have demonstrated excellent performance in cancer subtyping (164); such approaches are equally applicable to NEC research and are expected to enable precise patient stratification and prognostic assessment by integrating multi-omics information.
Despite the broad prospects of multi-omics integration in NEC research, it still faces numerous challenges in practical application. First, the complexity of data integration cannot be overlooked: the fusion of heterogeneous data (e.g., genome, epigenome, transcriptome, proteome) is itself a major challenge in computational biology (165). Data generated from different laboratories or platforms often exhibit batch effects; effectively eliminating technical variations while retaining meaningful biological signals constitutes a core challenge in data preprocessing. Notably, the “Fountain” deep learning framework based on regularized centroid mapping, proposed by Zhu et al. (166), has achieved significant progress in addressing this issue. Second, the high technical barriers of analytical methods and poor model interpretability limit clinicians' trust in and understanding of model results. Furthermore, the translation of omics signals into clinically applicable biomarkers is a lengthy process, requiring validation in large-scale, multicenter prospective cohorts to confirm that these biomarkers indeed improve clinical outcomes. Finally, simplifying complex multi-omics analysis workflows and integrating them into existing clinical workflows imposes extremely high demands on cost, efficiency, and operability.
7 Conclusion
With the advancement of research and technological innovation, significant progress has been made in studies on biomarkers associated with neonatal necrotizing enterocolitis (NEC). These biomarkers have demonstrated substantial potential in early diagnosis, disease staging, severity assessment, treatment strategy selection, and prognostic evaluation, while also deepening the clinical understanding of the pathophysiological mechanisms underlying NEC. However, an ideal biomarker capable of independently and accurately predicting the occurrence and progression of NEC is yet to be identified. Even though some biomarkers show certain application value, they still have limitations in sensitivity and specificity. Moreover, most studies have small sample sizes, and their results need to be further validated through large-scale clinical research. Based on existing evidence, Intestinal Fatty Acid-Binding Protein (I-FABP) and Fecal Calprotectin (FC) can be regarded as key biomarkers prioritized for clinical application advancement at this stage, owing to their excellent diagnostic accuracy (with AUC values mostly above 0.9) and intestinal specificity. Furthermore, simple combined models constructed by integrating inflammatory indicators such as Serum Amyloid A (SAA), C-reactive protein (CRP), and procalcitonin (PCT) with platelet parameters or tissue oxygenation monitoring (rSO2) have also shown high diagnostic efficacy. These models can be integrated into clinical pathways to assist clinicians in early warning and surgical decision-making. To fundamentally improve the diagnostic capability for NEC, future research should focus on developing predictive and diagnostic models incorporating multi-omics integration and AI-integrated analysis, while promoting the implementation of large-scale, multicenter, prospective cohort studies to facilitate their clinical translation. Through these efforts, it is expected to provide more robust support for the early identification and precise intervention of NEC, thereby making substantial contributions to reducing its morbidity and mortality rates.
Author contributions
AL: Writing – review & editing, Writing – original draft. TL: Writing – review & editing. RZ: Writing – review & editing. SZ: Writing – review & editing. LK: Writing – review & editing. XL: Writing – review & editing. WD: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article was supported by the National Natural Science Foundation of China (82371710), Sichuan Science and Technology Department Major Science and Technology Special Project (2022YFS0062) and Luzhou Science and Technology Programme Project (2022-JYJ-122).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bellodas Sanchez J, Kadrofske M. Necrotizing enterocolitis. Neurogastroenterol Motil. (2019) 31(3):e13569. doi: 10.1111/nmo.13569
2. Shao XM, Ye HM, Qiu XS. Practical Neonatology. 5th ed. Beijing: People’s Medical Publishing House (2019). p. 632.
3. Wang H, Meng X, Xing S, Guo B, Chen Y, Pan YQ, et al. Probiotics to prevent necrotizing enterocolitis and reduce mortality in neonates: a meta-analysis. Medicine (Baltimore). (2023) 102(8):e32932. doi: 10.1097/MD.0000000000032932
4. De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clin Perinatol. (2013) 40(1):109–24. doi: 10.1016/j.clp.2012.12.008
5. D’Angelo G, Impellizzeri P, Marseglia L, Montalto AS, Russo T, Salamone I, et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital J Pediatr. (2018) 44(1):84. doi: 10.1186/s13052-018-0528-3
6. Tao M, Yan L, Lang Y, Shen L, Chen S, Cai N. Development and validation of a nomogram model for predicting the occurrence of necrotizing enterocolitis in premature infants with late-onset sepsis. Eur J Med Res. (2025) 30(1):595. doi: 10.1186/s40001-025-02857-0
7. Chen X, Li Y, Liu Y, Liu T, Shi Y. Fulminant necrotizing enterocolitis: clinical features and a predictive model. BMC Pediatr. (2025) 25(1):546. doi: 10.1186/s12887-025-05902-3
8. Dantes G, Keane OA, Do L, Rumbika S, Ellis NH, Dutreuil VL, et al. Clinical predictors of spontaneous intestinal perforation vs necrotizing enterocolitis in extremely and very low birth weight neonates. J Pediatr Surg. (2024) 59(11):161608. doi: 10.1016/j.jpedsurg.2024.06.017
9. Bethell GS, Hall NJ, Battersby C, Knight M, Darlington AS. Surgeons and neonatologists views about surgical decision-making in necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. (2025) 110:fetalneonatal-2025-328480. doi: 10.1136/archdischild-2025-328480
10. Bazacliu C, Neu J. Necrotizing enterocolitis: long term complications. Curr Pediatr Rev. (2019) 15(2):115–24. doi: 10.2174/1573396315666190312093119
11. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6
12. Evidence-Based Medicine Group. Clinical guidelines for the diagnosis and treatment of neonatal necrotizing enterocolitis (2020). Zhongguo Dang Dai Er Ke Za Zhi. (2021) 23(1):1–11. (Chinese). doi: 10.7499/j.issn.1008-8830.2011145
13. Chinese Medical Association Pediatric Surgery Branch Neonatal Surgery Group. Expert consensus on surgical treatment of necrotizing enterocolitis in newborns. Chin J Pediatr Surg. (2016) 37(10):724–8. doi: 10.3760/cma.j.issn.0253-3006.2016.10.002
14. Ten Barge JA, van den Bosch GE, Allegaert K, Bhatt A, Brindley N, Byrne D, et al. Pain management in preterm infants with necrotizing enterocolitis: an international expert consensus statement. Eur J Pediatr. (2025) 184(6):342. doi: 10.1007/s00431-025-06168-8
15. Expert consensus on clinical application of serum amyloid A in infectious diseases. Chin J Lab Med. (2019) 42(3):186–92. doi: 10.3760/cma.j.issn.1009-9158.2019.03.008
16. Connolly M, Rooney PR, McGarry T, Maratha AX, McCormick J, Miggin SM, et al. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann Rheum Dis. (2016) 75(7):1392–8. doi: 10.1136/annrheumdis-2015-207655
17. Hong C, Shen C, Ding H, Huang S, Mu Y, Su H, et al. An involvement of SR-B1 mediated p38 MAPK signaling pathway in serum amyloid A-induced angiogenesis in rheumatoid arthritis. Mol Immunol. (2015) 66(2):340–5. doi: 10.1016/j.molimm.2015.03.254
18. Qian YP, Liu GH, Zhao YW, Zhang J. Diagnostic value of platelet/lymphocyte ratio combined with serum procalcitonin and amyloid A in neonatal necrotizing enterocolitis. Int J Lab Med. (2022) 43(21):2561–4. 2568. doi: 10.3969/j.issn.1673-4130.2022.21.001
19. Reisinger KW, Van der Zee DC, Brouwers HA, Kramer BW, van Heurn LW, Buurman WA, et al. Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. (2012) 47(9):1640–5. doi: 10.1016/j.jpedsurg.2012.02.027
20. Cetinkaya M, Ozkan H, Köksal N, Akaci O, Ozgür T. The efficacy of serial serum amyloid A measurements for diagnosis and follow-up of necrotizing enterocolitis in premature infants. Pediatr Surg Int. (2010) 26(8):835–41. doi: 10.1007/s00383-010-2635-0
21. Coufal S, Kokesova A, Tlaskalova-Hogenova H, Frybova B, Snajdauf J, Rygl M, et al. Urinary I-FABP, L-FABP, TFF-3, and SAA can diagnose and predict the disease course in necrotizing enterocolitis at the early stage of disease. J Immunol Res. (2020) 2020:3074313. doi: 10.1155/2020/3074313
22. Chen Y, Dai YH, Wu YH, Li JQ, Gao PM. Predictive value of SAA, NLR, CRP and PDW for surgical timing of neonatal necrotizing enterocolitis. Mod Biomed Adv. (2023) 23(6):1066–70. doi: 10.13241/j.cnki.pmb.2023.06.013
23. Jun X, Jinyu Z, Dongli W, Ruoyu D. Serum SAA and IL-8 expression in neonatal necrotizing enterocolitis patients and its correlation with clinical prognosis. Henan Med Res. (2023) 32(9):1640–4. doi: 10.3969/j.issn.1004-437X.2023.09.024
24. Dang XP, Hu XJ, Zhang L, Meng YC, Yang F, Zhang DP, et al. Correlation between intestinal inflammatory infiltration and routine inflammatory markers in blood in neonatal necrotizing enterocolitis. Lab Med Clin. (2021) 18(5):577–81. doi: 10.3969/j.issn.1672-9455.2021.05.001
25. Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Hum Dev. (2017) 105:25–33. doi: 10.1016/j.earlhumdev.2016.12.002
26. Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. (2022) 20(1):301. doi: 10.1186/s12916-022-02506-x
27. Gaitonde S, Samols D, Kushner I. C-reactive protein and systemic lupus erythematosus. Arthritis Rheum. (2008) 59(12):1814–20. doi: 10.1002/art.24316
28. Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. (2021) 51(1):219–29. doi: 10.1016/j.semarthrit.2020.11.005
29. Oikonomou E, Zografos T, Papamikroulis GA, Siasos G, Vogiatzi G, Theofilis P, et al. Biomarkers in atrial fibrillation and heart failure. Curr Med Chem. (2019) 26(5):873–87. doi: 10.2174/0929867324666170830100424
30. Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. (2005) 68(2):766–72. doi: 10.1111/j.1523-1755.2005.00455.x
31. Yang JX, Xie QL, Liang LG. Significance of combined detection of intestinal fatty acid-binding protein and high-sensitivity C-reactive protein in the diagnosis of neonatal necrotizing enterocolitis. Chin Pract Med. (2019) 14(11):6–8. doi: 10.14163/j.cnki.11-5547/r.2019.11.003
32. Jie SS, Dai LY, Zhang J, Zhang YL, Zhang F. Value of intestinal regional tissue oxygen saturation and C-reactive protein in the diagnosis of necrotizing enterocolitis in preterm infants. Chin J Contemp Pediatr. (2022) 24(11):1202–6. doi: 10.7499/j.issn.1008-8830.2204047
33. Pourcyrous M, Korones SB, Yang W, Boulden TF, Bada HS. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics. (2005) 116(5):1064–9. doi: 10.1542/peds.2004-1806
34. Wang L, Ni SW, Zhu KR, Zhou DY, Wang Y. Changes and clinical significance of C-reactive protein and procalcitonin levels in neonates with necrotizing enterocolitis. Chin J Contemp Pediatr. (2018) 20(10):825–30. doi: 10.7499/j.issn.1008-8830.2018.10.008
35. Duci M, Fascetti-Leon F, Erculiani M, Priante E, Cavicchiolo ME, Verlato G, et al. Neonatal independent predictors of severe NEC. Pediatr Surg Int. (2018) 34(6):663–9. doi: 10.1007/s00383-018-4261-1
36. Lu YX, Zhai RQ, Yang H, Wang XH, Li XQ, Yu J. Expression and significance of CRP, I-FABP, and SIRT1 in neonatal necrotizing enterocolitis. J Med Theory Pract. (2023) 36(4):664–6. doi: 10.19381/j.issn.1001-7585.2023.04.049
37. Eleni A, Charalampos A, Helen G, Kosmas S. Emerging biomarkers for prediction and early diagnosis of necrotizing enterocolitis in the era of metabolomics and proteomics. Front Pediatr. (2020) 8:602255. doi: 10.3389/fped.2020.602255
38. Zhu MY, Cao EH. Detection and application of procalcitonin: interpretation of “expert consensus on clinical significance of infection-related biomarkers”. Shanghai Med Pharm J. (2018) 39(1):14–8.
39. Elfarargy MS, El Farargy MS, Atef MM, El-Deeb OS, Elsharaby RM, Elhady HAE. Early biomarkers in neonatal necrotizing enterocolitis: a pilot study. J Popul Ther Clin Pharmacol. (2019) 26(3):e1–8. doi: 10.15586/jptcp.v26i3.602
40. Turner D, Hammerman C, Rudensky B, Schlesinger Y, Wine E, Muise A, et al. Low levels of procalcitonin during episodes of necrotizing enterocolitis. Dig Dis Sci. (2007) 52(11):2972–6. doi: 10.1007/s10620-007-9763-y
41. Cai N, Liao W, Chen Z, Tao M, Chen S. The mean platelet volume combined with procalcitonin as an early accessible marker helps to predict the severity of necrotizing enterocolitis in preterm infants. Int J Gen Med. (2022) 15:3789–95. doi: 10.2147/IJGM.S346665
42. Liebe H, Lewis S, Loerke C, Golubkova A, Leiva T, Stewart K, et al. A retrospective case control study examining procalcitonin as a biomarker for necrotizing enterocolitis. Surg Infect (Larchmt). (2023) 24(5):448–55. doi: 10.1089/sur.2022.366
43. Zhang H, Chen J, Wang Y, Deng C, Li L, Guo C. Predictive factors and clinical practice profile for strictures post-necrotising enterocolitis. Medicine (Baltimore). (2017) 96(10):e6273. doi: 10.1097/MD.0000000000006273
44. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. (2009) 78:539. doi: 10.1016/j.bcp.2009.04.029
45. Chen J, Yan Z, Lin Z, Fan Y, Bao X, Chen X, et al. I-FABP protein/mRNA and IL-6 as biomarkers of intestinal barrier dysfunction in neonates with necrotizing enterocolitis and SPF BALB/c mouse models. J Int Med Res. (2024) 52(6):3000605241254788. doi: 10.1177/03000605241254788
46. Cakir U, Tayman C, Yarci E, Halil H, Buyuktiryaki M, Ulu HO, et al. Novel useful markers for follow-up of necrotizing enterocolitis: endocan and interleukin-33. J Matern Fetal Neonatal Med. (2020) 33(14):2333–41. doi: 10.1080/14767058.2018.1548601
47. Rawat M, Nighot M, Al-Sadi R, Gupta Y, Viszwapriya D, Yochum G, et al. IL1B Increases intestinal tight junction permeability by up-regulation of MIR200C-3p, which degrades occludin mRNA. Gastroenterology. (2020) 159(4):1375–89. doi: 10.1053/j.gastro.2020.06.038
48. Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. (2016) 126(2):495–508. doi: 10.1172/JCI83356
49. König J, Wells J, Cani PD, Garcıa-Rodenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. (2016) 7(10):e196. doi: 10.1038/ctg.2016.54
50. Li Y. Correlation between IL-6, CRP, procalcitonin, platelet count and severity of neonatal necrotizing enterocolitis. Guizhou Med J. (2022) 46(5):747–8. doi: 10.3969/j.issn.1000-744X.2022.05.039
51. Snyder KB, Calkins CL, Golubkova A, Leiva T, Schlegel C, Hunter CJ, et al. Despite recovery from necrotizing enterocolitis infants retain a hyperinflammatory response to injury. J Inflamm Res. (2024) 17:331–41. doi: 10.2147/JIR.S436125
52. Seo YM, Lin YK, Im SA, Sung IK, Youn YA. Interleukin 8 may predict surgical necrotizing enterocolitis in infants born less than 1500 g. Cytokine. (2021) 137:155343. doi: 10.1016/j.cyto.2020.155343
53. Jiankang G, Yanhong B, You L, Guo Y, Zhigang F. Diagnostic and prognostic value of abdominal ultrasound combined with leukocyte interleukin-6 testing for neonatal necrotizing enterocolitis. Chin J Matern Child Clin Med (Electron Ed). (2022) 18(4):433–41. doi: 10.3877/cma.j.issn.1673-5250.2022.04.009
54. Horiuchi T, Mitoma H, Harashima S-i, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). (2010) 49(7):1215–28. doi: 10.1093/rheumatology/keq031
55. Yue H, Tuohui Z, Lei W. Correlation between serum resistin and tumor necrosis factor α expression levels and prognosis of neonatal necrotizing enterocolitis. J Clin Exp Med. (2025) 24(05):527–30. doi: 10.3969/j.issn.1671-4695.2025.05.020
56. Hong W, Ping X, Fengmin W, Yingying J, Chun Z. Clinical significance of measuring blood lactate and tumor necrosis factor-α in necrotizing enterocolitis of preterm infants. Chin J Neonatol. (2013) 28(1):3. doi: 10.3969/j.issn.1673-6710.2013.01.012
57. Lixia H, Hongxia F. The correlation between cytokines and neonatal necrotizing enterocolitis. China Mater Child Health Res. (2019) 30(1):19–22.
58. Gou ZY. Clinical significance of blood lactate and TNF-α levels in premature infants with necrotizing enterocolitis. Chin J Matern Child Health. (2014) 29(35):5841–2.
59. Grover P, Goel PN, Greene MI. Regulatory T cells: regulation of identity and function. Front Immunol. (2021) 12:750542. doi: 10.3389/fimmu.2021.750542
60. Pacella I, Di Chiara M, Prota R, De Luca C, Cardillo A, Potenza E, et al. Reduction in regulatory T cells in preterm newborns is associated with necrotizing enterocolitis. Pediatr Res. (2023) 94(5):1789–96. doi: 10.1038/s41390-023-02658-3
61. Ma F, Hao H, Gao X, Cai Y, Zhou J, Liang P, et al. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics. (2020) 10(17):7730–46. doi: 10.7150/thno.45862
62. Pang Y, Du X, Xu X, Wang M, Li Z. Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int Immunopharmacol. (2018) 59:354–60. doi: 10.1016/j.intimp.2018.04.026
63. Cheng H, Nan F, Ji N, Ma X, Zhang J, Liang H, et al. Regulatory T cell therapy promotes TGF-β and IL-6-dependent pro-inflammatory Th17 cell generation by reducing IL-2. Nat Commun. (2025) 16(1):7644. doi: 10.1038/s41467-025-62628-7
64. Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. (2021) 21(4):221–32. doi: 10.1038/s41577-020-00452-4
65. Zhang X, Zhang Y, He Y, Zhu X, Ai Q, Shi Y. β-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J Transl Med. (2023) 21(1):14. doi: 10.1186/s12967-022-03866-x
66. Weitkamp JH, Rosen MJ, Zhao Z, Koyama T, Geem D, Denning TL, et al. Small intestinal intraepithelial TCRγδ+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS One. (2014) 9(6):e99042. doi: 10.1371/journal.pone.0099042
67. Feng G, He LF, Liu SL, Chen YY, Cui WL. Effect of Bifidobacterium on intestinal γδ T lymphocytes in neonatal rats with necrotizing enterocolitis. Guangdong Med J. (2016) 37(21):3177–80. doi: 10.13820/j.cnki.gdyx.2016.21.005
68. Kovler ML, Sodhi CP, Hackam DJ. Precision-based modeling approaches for necrotizing enterocolitis. Dis Model Mech. (2020) 13:dmm044388. doi: 10.1242/dmm.044388
69. Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. (2014) 289(14):9584–99. doi: 10.1074/jbc.M113.526517
70. Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A. (2013) 110(23):9451–6. doi: 10.1073/pnas.1219997110
71. Kovler ML, Gonzalez Salazar AJ, Fulton WB, Lu P, Yamaguchi Y, Zhou Q, et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med. (2021) 13(612):eabg3459. doi: 10.1126/scitranslmed.abg3459
72. Liu T, Zong H, Chen X, Li S, Liu Z, Cui X, et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr Res. (2022) 91(1):73–82. doi: 10.1038/s41390-021-01457-y
73. Lian CW, Peng B, Guo XY, Fang YL. Predictive value of urinary intestinal fatty acid-binding protein and complement C5a for neonatal necrotizing enterocolitis. Chin Foreign Med Res. (2021) 19(24):52–4. doi: 10.14033/j.cnki.cfmr.2021.24.017
74. Tayman C, Tonbul A, Kahveci H, Uysal S, Koseoğlu B, Tatli MM, et al. C5a, a complement activation product, is a useful marker in predicting the severity of necrotizing enterocolitis. Tohoku J Exp Med. (2011) 224(2):143–50. doi: 10.1620/tjem.224.143
75. Yeramilli VA, Brawner KM, Crossman DK, Barnum SR, Martin CA. RNASeq analysis reveals upregulation of complement C3 in the offspring gut following prenatal stress in mice. Immunobiology. (2020) 225(4):151983. doi: 10.1016/j.imbio.2020.151983
76. Chmurzyńska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. (2006) 47(1):39–48. doi: 10.1007/BF03194597
77. Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. (2005) 352(1–2):15–35. doi: 10.1016/j.cccn.2004.09.001
78. Gollin G, Marks WH. Elevation of circulating intestinal fatty acid binding protein in a luminal contents-initiated model of NEC. J Pediatr Surg. (1993) 28(3):367–70. doi: 10.1016/0022-3468(93)90233-B
79. Saran A, Devegowda D, Doreswamy SM. Urinary intestinal fatty acid binding protein for diagnosis of necrotizing enterocolitis. Indian Pediatr. (2020) 57(9):798–800. doi: 10.1007/s13312-020-1955-y
80. Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. (1999) 34(10):1453–7. doi: 10.1016/S0022-3468(99)90102-1
81. Guthmann F, Börchers T, Wolfrum C, Wustrack T, Bartholomäus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. (2002) 239(1–2):227–34. doi: 10.1023/A:1020508420058
82. Benkoe TM, Mechtler TP, Weninger M, Pones M, Rebhandl W. Serum levels of interleukin-8 and gut-associated biomarkers in diagnosing necrotizing enterocolitis in preterm infants. J Pediatr Surg. (2014) 49(10):1446–51. doi: 10.1016/j.jpedsurg.2014.03.012
83. Mitidiero LF, Simões AL, Gonçalves FL, Figueira RR, Castro e Silva O, Sbragia L. L-FABP and I-FABP expression in newborn rats changes inversely in the model of necrotizing enterocolitis. Acta Cir Bras. (2014) 29(Suppl 2):43–9. doi: 10.1590/S0102-8650201400140009
84. Liu S, Liu Y, Lai S, Xie Y, Xiu W, Yang C. Values of serum intestinal fatty acid-binding protein, fecal calprotectin, and fecal human β-defensin 2 for predicting necrotizing enterocolitis. BMC Pediatr. (2024) 24(1):183. doi: 10.1186/s12887-024-04667-5
85. Shaaban AIE, Alfqy OAE, Shaaban HMK, Al-Maqsoud YH, Assar EH. Potential role of serum intestinal fatty acid-binding protein as a marker for early prediction and diagnosis of necrotizing enterocolitis in preterm neonates. J Indian Assoc Pediatr Surg. (2021) 26(6):393–400. doi: 10.4103/jiaps.JIAPS_218_20
86. Evennett NJ, Hall NJ, Pierro A, Eaton S. Urinary intestinal fatty acid-binding protein concentration predicts extent of disease in necrotizing enterocolitis. J Pediatr Surg. (2010) 45(4):735–40. doi: 10.1016/j.jpedsurg.2009.09.024
87. Yang G, Wang Y, Jiang X. Diagnostic value of intestinal fatty-acid-binding protein in necrotizing enterocolitis: a systematic review and meta-analysis. Indian J Pediatr. (2016) 83(12–13):1410–9. doi: 10.1007/s12098-016-2144-9
88. El-Abd Ahmed A, Hassan MH, Abo-Halawa N, Abdel-Razik GM, Moubarak FA, Sakhr HM. Lactate and intestinal fatty acid binding protein as essential biomarkers in neonates with necrotizing enterocolitis: ultrasonographic and surgical considerations. Pediatr Neonatol. (2020) 61(5):481–9. doi: 10.1016/j.pedneo.2020.03.015
89. Xueli Y, Gang H, Peng T, Juan L. A study on the value of Ⅰ-FABP in early diagnosis and prognostic prediction of necrotizing enterocolitis. Sichuan Med J. (2021) 42(04):404–8. doi: 10.16252/j.cnki.issn1004-0501-2021.04.018
90. Yuanmei L, Chengyan T. Advances in the diagnosis and treatment of post-necrotizing enterocolitis intestinal stricture in newborns. Chin J Pract Pediatr. (2024) 39(05):342–5. doi: 10.19538/j.ek2024050606
91. Kuik SJ, Kalteren WS, Mebius MJ, Bos AF, Hulscher JBF, Kooi EMW. Predicting intestinal recovery after necrotizing enterocolitis in preterm infants. Pediatr Res. (2020) 87(5):903–9. doi: 10.1038/s41390-019-0634-y
92. Ozcan B, Aydemir O, Isik DU, Bas AY, Demirel N. Severe anemia is associated with intestinal injury in preterm neonates. Am J Perinatol. (2020) 37(6):603–6. doi: 10.1055/s-0039-1683982
93. Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. (2003) 36(7):529–35. doi: 10.1016/s0009-9120(03)00096-1
94. Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. (1992) 27(9):793–8. doi: 10.3109/00365529209011186
95. Lee YW, Lee KM, Lee JM, Chung YY, Kim DB, Kim YJ, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. (2019) 34(1):72–80. doi: 10.3904/kjim.2016.324
96. Farghaly MAA, Ali MAM, Ramey S, Said W, Abdelkarem A, Collin M. Characteristics of fecal calprotectin as an early marker for suspected necrotizing enterocolitis in newborns exclusively fed maternal breast milk: a case-control study. Proc Bayl Univ Med Cent. (2023) 37(1):43–7. doi: 10.1080/08998280.2023.2277580
97. Xie Y-Q, Ren C-J, Wang X, Xiang S-W, Wang X-X, Hao L. Role of fecal calprotectin in the diagnosis of neonatal necrotizing enterocolitis: a meta analysis. Zhongguo Dang Dai Er Ke Za Zhi. (2021) 23(4):381–9. doi: 10.7499/j.issn.1008-8830.2010111
98. hibault MP, Tremblay É, Horth C, Fournier-Morin A, Grynspan D, Babakissa C, et al. Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates. Pediatr Res. (2022) 91(1):129–36. doi: 10.1038/s41390-021-01680-7
99. Nakayuenyongsuk W, Christofferson M, Stevenson DK, Sylvester K, Lee HC, Park KT. Point-of-care fecal calprotectin monitoring in preterm infants at risk for necrotizing enterocolitis. J Pediatr. (2018) 196:98–103. doi: 10.1016/j.jpeds.2017.12.069
100. Hu LY, Ban CF. Value of fecal calprotectin combined with intestinal barrier function in grading diagnosis of neonatal necrotizing enterocolitis. Chin J Matern Child Health. (2023) 38(7):1242–5. doi: 10.19829/j.zgfybj.issn.1001-4411.2023.07.022
101. Yoon JM, Park JY, Ko KO, Lim JW, Cheon EJ. Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Korean J Pediatr. (2014) 57(8):351–6. doi: 10.3345/kjp.2014.57.8.351
102. Chen G, Lv X, Tang W. Fecal calprotectin as a non-invasive marker for the prediction of post-necrotizing enterocolitis stricture. Pediatr Surg Int. (2023) 39(1):250. doi: 10.1007/s00383-023-05534-7
103. Garner CE, Ewer AK, Elasouad K, Power F, Greenwood R, Ratcliffe NM, et al. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: a pilot study. J Pediatr Gastroenterol Nutr. (2009) 49(5):559–65. doi: 10.1097/MPG.0b013e3181a3bfbc
104. de Meij TG, van der Schee MP, Berkhout DJ, van de Velde ME, Jansen AE, Kramer BW, et al. Early detection of necrotizing enterocolitis by fecal volatile organic compounds analysis. J Pediatr. (2015) 167(3):562–7. doi: 10.1016/j.jpeds.2015.05.044
105. Probert C, Greenwood R, Mayor A, Hughes D, Aggio R, Jackson RE, et al. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: the DOVE study. Arch Dis Child Fetal Neonatal Ed. (2020) 105(5):474–9. doi: 10.1136/archdischild-2019-318221
106. Zhou W, Yuan W, Huang L, Wang P, Rong X, Tang J. Association of neonatal necrotizing enterocolitis with myeloid differentiation-2 and GM2 activator protein genetic polymorphisms. Mol Med Rep. (2015) 12(1):974–80. doi: 10.3892/mmr.2015.3499
107. Sampath V, Le M, Lane L, Patel AL, Cohen JD, Simpson PM, et al. The NFKB1 (g.-24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res. (2011) 169(1):e51–7. doi: 10.1016/j.jss.2011.03.017
108. Zhang Y, Li K. Association of interleukin-23 receptor and interleukin-17 single nucleotide polymorphisms with necrotizing enterocolitis in Chinese Han preterm infants. Chin J Contemp Pediatr. (2020) 22(2):141–5. doi: 10.7499/j.issn.1008-8830.2020.02.011
109. Barut D, Akisu M, Koroglu OA, Terek D, Ergin F, Onay H, et al. The role of vitamin D receptor gene polymorphism in the development of necrotizing enterocolitis. Pediatr Res. (2023) 94(1):275–9. doi: 10.1038/s41390-022-02426-9
110. Härtel C, Hartz A, Pagel J, Rupp J, Stein A, Kribs A, et al. NOD2 loss-of-function mutations and risks of necrotizing enterocolitis or focal intestinal perforation in very low-birth-weight infants. Inflamm Bowel Dis. (2016) 22(2):249–56. doi: 10.1097/MIB.0000000000000658
111. Yasuhara J, Manivannan SN, Majumdar U, Gordon DM, Lawrence PJ, Aljuhani M, et al. Novel pathogenic GATA6 variant associated with congenital heart disease, diabetes mellitus and necrotizing enterocolitis. Pediatr Res. (2024) 95(1):146–55. doi: 10.1038/s41390-023-02811-y
112. Ya Z, Wenbin D, Shiqiong H, Xi C, Jinyu X. The relationship between CXCR5 Gene polymorphism and cytomegalovirus infection and clinical outcomes of neonatal necrotizing enterocolitis. Chin J Hosp Infect. (2021) 31(12):1761–5. doi: 10.11816/cn.ni.2021-202898
113. Strauss E, Gotz-Więckowska A, Sobaniec A, Chmielarz-Czarnocińska A, Szpecht D, Januszkiewicz-Lewandowska D. Hypoxia-inducible pathway polymorphisms and their role in the complications of prematurity. Genes (Basel). (2023) 14(5):975. doi: 10.3390/genes14050975
114. Cao H, Guo D. Association of high-mobility group box 1 (HMGB1) gene polymorphisms with susceptibility and better survival prognosis in Chinese Han neonatal necrotizing enterocolitis. Med Sci Monit. (2021) 27:e930015. doi: 10.12659/MSM.930015
115. Good M, Chu T, Shaw P, McClain L, Chamberlain A, Castro C, et al. Global hypermethylation of intestinal epithelial cells is a hallmark feature of neonatal surgical necrotizing enterocolitis. Clin Epigenetics. (2020) 12(1):190. doi: 10.1186/s13148-020-00983-6
116. Good M, Chu T, Shaw P, Nolan LS, McClain L, Chamberlain A, et al. Neonatal necrotizing enterocolitis-associated DNA methylation signatures in the colon are evident in stool samples of affected individuals. Epigenomics. (2021) 13(11):829–44. doi: 10.2217/epi-2021-0078
117. Lai J, Li F, Li H, Huang R, Ma F, Gu X, et al. Melatonin alleviates necrotizing enterocolitis by reducing bile acid levels through the SIRT1/FXR signalling axis. Int Immunopharmacol. (2024) 128:111360. doi: 10.1016/j.intimp.2023.111360
118. Gao Y, Yang L, Yao Q, Wang J, Zheng N. Butyrate improves recovery from experimental necrotizing enterocolitis by metabolite hesperetin through potential inhibition the PI3K-Akt pathway. Biomed Pharmacother. (2024) 176:116876. doi: 10.1016/j.biopha.2024.116876
119. Chen Z, Chen H, Huang W, Guo X, Yu L, Shan J, et al. Bacteroides fragilis alleviates necrotizing enterocolitis through restoring bile acid metabolism balance using bile salt hydrolase and inhibiting FXR-NLRP3 signaling pathway. Gut Microbes. (2024) 16(1):2379566. doi: 10.1080/19490976.2024.2379566
120. Zhang K, Zhang X, Lv A, Fan S, Zhang J. Saccharomyces Boulardii modulates necrotizing enterocolitis in neonatal mice by regulating the sirtuin 1/NF-κB pathway and the intestinal microbiota. Mol Med Rep. (2020) 22(2):671–80. doi: 10.3892/mmr.2020.11138
121. Hosfield BD, Pecoraro AR, Baxter NT, Hawkins TB, Markel TA. The assessment of fecal volatile organic compounds in healthy infants: electronic nose device predicts patient demographics and microbial enterotype. J Surg Res. (2020) 254:340–7. doi: 10.1016/j.jss.2020.05.010
122. Zhai G. Study on 16S-rRNA sequencing analysis of intestinal bacterial genomes in preterm infants guiding surgical timing selection for neonatal necrotizing enterocolitis. Chin Med Sci. (2024) 14(11):130–3. doi: 10.20116/j.issn2095-0616.2024.11.30
123. Ragan MV, Wala SJ, Sajankila N, Duff AF, Wang Y, Volpe SG, et al. Development of a novel definitive scoring system for an enteral feed-only model of necrotizing enterocolitis in piglets. Front Pediatr. (2023) 11:1126552. doi: 10.3389/fped.2023.1126552
124. Han Y, Liu X, Kang L, Chen D, Li Y, Zhang H, et al. A potential pathogenic hypoxia-related gene HK2 in necrotizing enterocolitis (NEC) of newborns. BMC Pediatr. (2022) 22(1):617. doi: 10.1186/s12887-022-03664-w
125. Egozi A, Olaloye O, Werner L, Silva T, McCourt B, Pierce RW, et al. Single-cell atlas of the human neonatal small intestine affected by necrotizing enterocolitis. PLoS Biol. (2023) 21(5):e3002124. doi: 10.1371/journal.pbio.3002124
126. Pan X, Muk T, Ren S, Nguyen DN, Shen RL. Blood transcriptomic markers of necrotizing enterocolitis in preterm pigs. Pediatr Res. (2022) 91(5):1113–20. doi: 10.1038/s41390-021-01605-4
127. Zhong X, Zhang R, Yang S, Jin J, Lyu C, Fan J, et al. RNF31-mediated IKKα ubiquitination aggravates inflammation and intestinal injury through regulating NF-κB activation in human and mouse neonates. Life Sci. (2024) 352:122893. doi: 10.1016/j.lfs.2024.122893
128. Zhang DD, Huang ZX, Liu XC, Ding XP, Li L, He Y, et al. Butyrate protects the intestinal barrier by upregulating Fut2 expression via MEK4-JNK signaling pathway activation. Pediatr Res. (2024) 97:128–37. doi: 10.1038/s41390-024-03419-6
129. Nguyen DN, Jiang P, Jacobsen S, Sangild PT, Bendixen E, Chatterton DE. Protective effects of transforming growth factor β2 in intestinal epithelial cells by regulation of proteins associated with stress and endotoxin responses. PLoS One. (2015) 10(2):e0117608. doi: 10.1371/journal.pone.0117608
130. Gagné D, Shajari E, Thibault MP, Noël JF, Boisvert FM, Babakissa C, et al. Proteomics profiling of stool samples from preterm neonates with SWATH/DIA mass spectrometry for predicting necrotizing enterocolitis. Int J Mol Sci. (2022) 23(19):11601. doi: 10.3390/ijms231911601
131. Wang J, Qu M, Qiu A, Yang L, Xu H, Yu S, et al. Quantitative proteomic analysis identifying and evaluating TRAF6 and IL-8 as potential diagnostic biomarkers in neonatal patients with necrotizing enterocolitis. Mol Biotechnol. (2024) 67:1109–21. doi: 10.1007/s12033-024-01111-y
132. Mackay S, Frazer LC, Bailey GK, Miller CM, Gong Q, Dewitt ON, et al. Identification of serum biomarkers for necrotizing enterocolitis using aptamer-based proteomics. Front Pediatr. (2023) 11:1184940. doi: 10.3389/fped.2023.1184940
133. Dong H, Zhang L, Li B, Li J, Chen Y, Richard SA, et al. Screening inflammatory protein biomarkers on premature infants with necrotizing enterocolitis. Inflamm Res. (2023) 72(4):757–68. doi: 10.1007/s00011-023-01702-6
134. Fagbemi AO, Torrente F, Puleston J, Lakhoo K, James S, Murch SH. Enteric neural disruption in necrotizing enterocolitis occurs in association with myenteric glial cell CCL20 expression. J Pediatr Gastroenterol Nutr. (2013) 57(6):788–93. doi: 10.1097/MPG.0b013e3182a86fd4
135. Ying Y, Fang L, Xiaohui Z, Huixian L, Hongyu L, Shuo L, et al. The value of serum Smad7, TSLP, FC, and sTREM-1 in the assessment of necrotizing enterocolitis in newborns. J Clin Exp Med. (2025) 24(14):1544–7.
136. Le H, Wang Y, Zhou J, Li D, Gong Z, Zhu F, et al. Git2 deficiency promotes MDSCs recruitment in intestine via NF-κB-CXCL1/CXCL12 pathway and ameliorates necrotizing enterocolitis. Mucosal Immunol. (2024) 17(5):1060–71. doi: 10.1016/j.mucimm.2024.07.006
137. Du TT, Liu XC, He Y, Gao X, Liu ZZ, Wang ZL, et al. Changes of gut microbiota and tricarboxylic acid metabolites may be helpful in early diagnosis of necrotizing enterocolitis: a pilot study. Front Microbiol. (2023) 14:1119981. doi: 10.3389/fmicb.2023.1119981
138. Thomaidou A, Deda O, Begou O, Lioupi A, Kontou A, Gika H, et al. A prospective, case-control study of serum metabolomics in neonates with late-onset sepsis and necrotizing enterocolitis. J Clin Med. (2022) 11(18):5270. doi: 10.3390/jcm11185270
139. Deianova N, El Manouni El Hassani S, Struijs EA, Jansen EEW, Bakkali A, van de Wiel MA, et al. Fecal amine metabolite analysis before onset of severe necrotizing enterocolitis in preterm infants: a prospective case-control study. Sci Rep. (2022) 12(1):12310. doi: 10.1038/s41598-022-16351-8
140. Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. (2013) 1(1):13. doi: 10.1186/2049-2618-1-13
141. Lindberg TP, Caimano MJ, Hagadorn JI, Bennett EM, Maas K, Brownell EA, et al. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J Matern Fetal Neonatal Med. (2020) 33(3):349–58. doi: 10.1080/14767058.2018.1490719
142. Stewart CJ, Embleton ND, Marrs EC, Smith DP, Nelson A, Abdulkadir B, et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome. (2016) 4(1):67. doi: 10.1186/s40168-016-0216-8
143. Brehin C, Dubois D, Dicky O, Breinig S, Oswald E, Serino M. Evolution of gut microbiome and metabolome in suspected necrotizing enterocolitis: a case-control study. J Clin Med. (2020) 9(7):2278. doi: 10.3390/jcm9072278
144. Feng J, He Y, Liu D, Li L, Chen J, Yu J. The constitution and functional prediction of the microbiota in necrotizing enterocolitis with a gestational age of over 28 weeks. Medicine (Baltimore). (2019) 98(40):e17206. doi: 10.1097/MD.0000000000017206
145. Liu J, Li Y, Feng Y, Pan L, Xie Z, Yan Z, et al. Patterned progression of gut microbiota associated with necrotizing enterocolitis and late onset sepsis in preterm infants: a prospective study in a Chinese neonatal intensive care unit. PeerJ. (2019) 7:e7310. doi: 10.7717/peerj.7310
146. Wenjing G, Huaying L, Wei Z, Junjian L. Machine learning-based differential diagnosis of necrotizing enterocolitis in newborns. Chin Digit Med. (2019) 14(03):50–52 +69. doi: 10.3969/j.issn.1673-7571.2019.03.014
147. Lueschow SR, Boly TJ, Jasper E, Patel RM, McElroy SJ. A critical evaluation of current definitions of necrotizing enterocolitis. Pediatr Res. (2022) 91(3):590–7. doi: 10.1038/s41390-021-01570-y
148. Gao W, Pei Y, Liang H, Lv J, Chen J, Zhong W. Multimodal AI system for the rapid diagnosis and surgical prediction of necrotizing enterocolitis. IEEE Access. (2021) 9:51050–64. doi: 10.1109/ACCESS.2021.3069191
149. Zhenyu L, Ling L, Jiaqi W, Qinlei J, Hui W. Establishment of a machine learning-based prediction model for necrotizing enterocolitis in newborns. Chin J Neonatol (Chin Engl Ed). (2024) 39(3):150–6. doi: 10.3760/cma.j.issn.2096-2932.2024.03.005
150. He LL, Chen WJ, Xu YF, Deng MP, Li YF, Li YY, et al. Research advances in the assessment of gastric residue in extremely low birth weight infants. Chin J Nurs. (2017) 52(S1):78–80. doi: 10.3761/j.issn.0254-1769.2017.z1.037
151. You Z. The application value of DAAS combined with MD7 in the timing selection of neonatal necrotizing enterocolitis surgery. Chin J Mod Surg. (2019) 23(02):102–5. doi: 10.16260/j.cnki.1009-2188.2019.02.006
152. Bethell GS, Jones IH, Battersby C, Knight M, Hall NJ. Methods of identifying surgical necrotizing enterocolitis-a systematic review and meta-analysis. Pediatr Res. (2025) 97(1):45–55. doi: 10.1038/s41390-024-03292-3
153. Lu G. The Value of Serum Biomarkers Combined with MD7 in Predicting Surgical Timing for Necrotizing Enterocolitis in Newborns. Chongqing: Chongqing Medical University (2021). doi: 10.27674/d.cnki.gcyku.2021.001105
154. Yangbo L, Dan Z. Analysis of the diagnostic value of near-infrared spectral detection for red blood cell oxygen saturation (rSO2) in neonatal necrotizing enterocolitis. J Qiqihar Med Coll. (2024) 45(20):1951–4.
155. Meng W, Wang Q, Xu Q, Gao H, Zhou Y, Shao W. Biomarkers in the severity of necrotizing enterocolitis in preterm infants: a pilot study. Int J Gen Med. (2024) 15(17):1017–23. doi: 10.2147/IJGM.S446378
156. Fengqin Y, Yuanyuan H, Xiaowei P, Jun Y. Application progress of ultrasound in neonatal necrotizing enterocolitis. J Gannan Med Univ. (2024) 44(07):698–702. doi: 10.3969/j.issn.1001-5779.2024.07.007
157. van Zoonen AGJF, Hulzebos CV, Muller Kobold AC, Kooi EMW, Bos AF, Hulscher JBF. Serial fecal calprotectin in the prediction of necrotizing enterocolitis in preterm neonates. J Pediatr Surg. (2019) 54(3):455–9. doi: 10.1016/j.jpedsurg.2018.04.034
158. Shores DR, Fundora J, Go M, Shakeel F, Brooks S, Alaish SM, et al. Normative values for circulating intestinal fatty acid binding protein and calprotectin across gestational ages. BMC Pediatr. (2020) 20(1):250. doi: 10.1186/s12887-020-02142-5
159. Burge KY, Gunasekaran A, Makoni MM, Mir AM, Burkhart HM, Chaaban H. Clinical characteristics and potential pathogenesis of cardiac necrotizing enterocolitis in neonates with congenital heart disease. A narrative review. J Clin Med. (2022) 11(14):3987. doi: 10.3390/jcm11143987
160. Xiang LN, Qian C, Rui C, Bei L, Kefeng H, Zhipu L. Changes in serum PCT, IL-6, and CRP during the perioperative period in children with congenital heart disease. J Qingdao Univ (Med Ed). (2020) 56(05):592–6.
161. National Alliance of Cardiovascular Specialty Centers for Children’s Medicine, Chinese Medical Association Pediatric Surgery Branch-Thoracic Surgery Group (CICU Collaboration). Expert consensus on clinical application of near-infrared spectral monitoring technology in pediatric congenital heart disease postoperative care. Chin J Heart Fail Cardiomyop. (2023) 07(1):3–10. doi: 10.3760/cma.j.cn101460-20230129-00004
162. Ivanisevic T, Sewduth RN. Multi-omics integration for the design of novel therapies and the identification of novel biomarkers. Proteomes. (2023) 11(4):34. doi: 10.3390/proteomes11040034
163. Tian B, Xu X, Li L, Tian Y, Liu Y, Mu Y, et al. Epigenetic insights into necrotizing enterocolitis: unraveling methylation-regulated biomarkers. Inflammation. (2025) 48(1):236–53. doi: 10.1007/s10753-024-02054-x
164. Shen J, Lu L, Chen Z, Guo W, Wang S, Liu Z, et al. Multi-omics analysis constructs a novel neuroendocrine prostate cancer classifier and classification system. Sci Rep. (2025) 15(1):13901. doi: 10.1038/s41598-025-96683-3
165. Adam E, Zanoaga MD, Rota R, Cominetti O. A comprehensive protocol and step-by-step guide for multi-omics integration in biological research. J Vis Exp. (2025) (222):e66995. doi: 10.3791/66995
Keywords: neonatal necrotizing enterocolitis, predictive value, biomarkers, neonatal diseases, neonatal, preterm infants
Citation: Liu A, Liang T, Zhang R, Zhao S, Kang L, Lei X and Dong W (2025) Predictive value of biomarkers in neonatal necrotizing enterocolitis. Front. Pediatr. 13:1661371. doi: 10.3389/fped.2025.1661371
Received: 7 July 2025; Accepted: 16 October 2025;
Published: 5 November 2025.
Edited by:
Domenico Umberto De Rose, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Wenqiang Sun, Children's Hospital of Soochow University, ChinaWilliam Carey, Mayo Clinic, United States
Copyright: © 2025 Liu, Liang, Zhang, Zhao, Kang, Lei and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Dong, ZG9uZ3dlbmJpbjIwMDBAMTYzLmNvbQ==
 Anji Liu
Anji Liu Ting Liang1,2,3
Ting Liang1,2,3 Rong Zhang
Rong Zhang Wenbin Dong
Wenbin Dong