- Department of Paediatrics,The First Affiliated Hospital of Jinan University, Guangzhou, China
Introduction: Mycoplasma pneumoniae pneumonia (MPP) is a common cause of pediatric community-acquired pneumonia, and coinfections with Haemophilus influenzae (Hi) or influenza virus may alter disease severity. Identifying distinct laboratory patterns may help clinicians recognise coinfections early.
Methods: A retrospective analysis was conducted on 140 hospitalized children with confirmed MPP (2014–2024). Patients were grouped as MPP alone (n = 64), MPP+Hi (n = 36), and MPP+influenza (n = 40). Clinical characteristics, complete blood count (CBC), hypersensitive C-reactive protein (hs-CRP), and biochemical indicators (ALT, AST, CK, CK-MB, urea, creatinine) were compared among groups. Correlation analyses were performed for biochemical markers.
Results: Children with MPP+Hi showed the highest hs-CRP levels (23.93 ± 21.26 mg/L), longest fever duration, and longest hospital stay. The MPP+influenza group had significantly lower WBC (7.25 ± 3.50 × 109/L) and platelet counts (266.00 ± 97.46 × 109/L), and a higher monocyte percentage (10.18 ± 3.29%). Simple MPP cases had the highest lymphocyte percentage. No group differences were found in ALT, AST, CK, CK-MB, urea, or creatinine, although CK-MB was elevated across all groups. Correlation analysis showed weak but significant associations among AST, ALT, CK, and CK-MB.
Conclusion: Coinfection type influenced inflammatory and haematological patterns in children with MPP: Hi coinfection produced stronger inflammatory responses, while influenza coinfection showed viral-associated lymphopenia and thrombocytopenia. Routine laboratory parameters may support earlier recognition of coinfections and guide more targeted clinical management.
1 Introduction
Pneumonia remains one of the leading causes of childhood morbidity and mortality worldwide, with Mycoplasma pneumoniae (MP) recognised as a major contributor to community-acquired pneumonia (CAP) in preschool and school-aged children, accounting for 10%–40% of cases (1, 2). Although M. pneumoniae pneumonia (MPP) is often self-limiting, some children develop severe or refractory forms characterised by prolonged fever, worsening radiographic changes, and, in rare cases, life-threatening complications (3).
In recent years, the incidence of mixed respiratory infections has risen substantially, with coinfections involving MP and other bacterial or viral pathogens increasingly reported in pediatric CAP (4, 5). Among these, Haemophilus influenzae (Hi) and influenza viruses are particularly common and clinically significant (5, 6). These pathogens, independently capable of causing significant respiratory disease, often exacerbate the clinical course when present alongside MP, leading to increased disease severity, prolonged recovery times, and greater healthcare utilization (5, 7). For instance, children with MPP and viral coinfections have been observed to experience longer fevers, more severe pneumonia, and a poorer response to standard treatments (5, 7–9).
Laboratory investigations, especially complete blood counts, hypersensitive C-reactive protein (hs-CRP), and selected biochemical markers, play a pivotal role in differentiating bacterial and viral infections (10). Elevated CRP levels often reflect bacterial involvement, while variations in white blood cell subsets and platelet counts may help to distinguish viral contributions (11, 12). Several studies have emphasized the utility of these parameters for the early recognition of mixed infections, which may allow clinicians to tailor therapy more effectively (13–15).
Despite the clinical significance, there is a paucity of studies focusing on the comparative analysis of clinical features and laboratory findings in children with MPP alone vs. those with coinfections (8). Current literature is often fragmented, focusing on individual pathogens rather than evaluating the combined impact of coinfections on disease presentation, laboratory parameters, and outcomes (16). Addressing this gap, the present study retrospectively analyzes pediatric cases of MPP, MPP + Hi, and MPP + influenza virus coinfection to identify distinctive patterns in clinical features, inflammatory markers, and biochemical indicators. By clarifying these differences, our aim is to provide clinicians with practical insights that may support earlier recognition of coinfections and more informed treatment decisions in children with pneumonia. This study adds novelty by analysing a decade-long, consistently tested paediatric cohort (2014–2024) to directly compare bacterial (H. influenzae) and viral (influenza) coinfections in MPP, using uniform diagnostic methods and integrated correlation analysis to reveal distinct inflammatory profiles.
2 Materials and methods
2.1 Study design and participants
This retrospective study analyzed 140 pediatric patients diagnosed with pneumonia and were admitted to the Department of Pediatrics at the First Affiliated Hospital of Jinan University between January 2014 and January 2024. The inclusion criteria were: (1) diagnosis of MPP based on the “Consensus on the Diagnosis and Treatment of Mycoplasma Pneumoniae Pneumonia (2015 Edition)"issued by the Respiratory Group of the Chinese Pediatric Society, Chinese Medical Association (2015); (2) no prior antibiotic treatment or ineffective antibiotic treatment before hospitalization; (3) completion of sputum culture, MP antibody detection, respiratory virus antigen testing, complete blood count (CBC), hypersensitive C-reactive protein (hs-CRP), and biochemical tests within 48 h of admission; and (4) confirmed detection of MP, Hi, or influenza virus antigens or nucleic acids.
Exclusion criteria included: positive identification of other respiratory viruses or bacteria (besides Hi), positive sputum culture for bacteria other than Hi, history of chronic underlying diseases (e.g., congenital heart disease, immunodeficiencies, bronchopulmonary dysplasia), and inclusion of neonatal patients. We focused specifically on H. influenzae and influenza virus because these were the most frequent coinfecting agents detected in our cohort over the study period. Other bacterial and viral pathogens were excluded due to their low prevalence, which would have yielded very small subgroups and limited statistical comparability. Restricting the analysis to Hi and influenza virus enabled clearer identification of clinically relevant differences. The patients were divided into three groups based on their infection status: Group A (MPP alone, n = 64), Group B (MPP with Hi coinfection, n = 36), and Group C (MPP with influenza virus coinfection, n = 40).
2.2 Microbiological and virological testing
Deep respiratory tract secretions were obtained using sterile suction catheters. Sample quality was assessed via Gram stain microscopy, requiring >25 white blood cells and <10 epithelial cells per high-power field for specimen acceptance. Qualified sputum samples were inoculated onto chocolate agar supplemented with bacitracin and incubated at 35–37°C in a 5% CO₂-enriched atmosphere for 24–48 h. Haemophilus influenzae colonies were identified based on characteristic morphology, positive oxidase test, and growth dependence on both X (hemin) and V (nicotinamide adenine dinucleotide) factors. Confirmatory biochemical identification was performed using standard techniques.
Mycoplasma pneumoniae infection was confirmed by two methods: (1) detection of MP-IgM antibodies via the SERODIA-MYCO II particle agglutination test, with a positive result defined as a titer ≥1:160; and (2) detection of MP RNA from throat swabs using RNA isothermal amplification combined with a gold nanoparticle-based lateral flow assay using commercially available kit (Wuhan Zhongzhi Biotechnology Co., Ltd.). RNA extraction was performed according to the kit instructions. Internal positive and negative controls were included in each run.
For respiratory virus detection, nasopharyngeal swabs were analyzed for six common viruses: respiratory syncytial virus, adenovirus, human metapneumovirus, and parainfluenza virus types 1–3. Detection was performed via one-step reverse transcription polymerase chain reaction (RT-PCR) using commercial kits (Shanghai Bojie Medical Science & Technology Co., Ltd.), following the manufacturer's protocols. Only patients with positive detection of influenza virus antigens or nucleic acids were included.
All laboratory tests and diagnostic workflows were performed using the same core platforms and quality-control procedures throughout 2014–2024. CBC analyses were conducted on the Sysmex XN-Series, hs-CRP on the BN ProSpec® (Siemens Healthineers), and biochemical assays on the OLYMPUS AU640 analyzer. The same Mycoplasma pneumoniae antibody (SERODIA-MYCO II) and RNA detection kits (Wuhan Zhongzhi Biotechnology Co., Ltd.) were used across the study period. Routine internal calibration and participation in external quality-assurance programs ensured analytical consistency despite the long retrospective timeframe.
2.3 Laboratory investigations
2.3.1 Complete blood count (CBC)
CBC parameters, including white blood cell count (WBC), neutrophil percentage (N%), lymphocyte percentage (L%), monocyte percentage (M%), eosinophil percentage (EO%), basophil percentage (BASO%), absolute counts for each cell type (N#, L#, M#, EO#, BASO#), platelet count (PLT), mean platelet volume (MPV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width-coefficient of variation (RDW-CV), and red cell distribution width-standard deviation (RDW-SD), were measured using an automated hematology analyzer (Sysmex XN-Series, Sysmex Corporation, Kobe, Japan). Routine internal and external quality control procedures were followed in accordance with hospital and national laboratory standards.
2.3.2 Hypersensitive C-reactive protein (hs-CRP)
Hs-CRP levels were measured using an immunoturbidimetric assay on an automatic specific protein analyzer (BN ProSpec®, Siemens Healthineers, Germany). A positive threshold was defined as ≥0.50 mg/L. Calibration and quality controls were performed daily according to the manufacturer's guidelines.
2.3.3 Biochemical tests
Fasting venous blood samples were analyzed using an OLYMPUS AU640 automatic biochemical analyzer (Olympus Corporation, Tokyo, Japan). Parameters included alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measured by the International Federation of Clinical Chemistry recommended enzymatic rate method without pyridoxal phosphate activation; creatine kinase (CK) and creatine kinase-MB (CK-MB) measured by the enzymatic method; urea measured by the urease–glutamate dehydrogenase method; and creatinine (CREA) measured by the enzymatic method. Quality control materials (two levels) were run daily to ensure accuracy and precision.
All patients underwent these laboratory tests within 48 h of admission.
2.4 Statistical analysis
Data were analysed using SPSS Statistics version 29.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to assess the normality of data. Normally distributed continuous variables were expressed as mean ± standard deviation and compared between groups using independent-sample t-tests or one-way analysis of variance (ANOVA), followed by Tukey's HSD test. Non-normally distributed data were presented as medians (P25, P75) and compared using the Kruskal–Wallis test, followed by Dunn's multiple range test. Categorical variables were expressed as percentages and compared between groups using the Chi-square (χ2) test. No formal correction for multiple comparisons was applied, as this was an exploratory study. Over-correction could increase Type II error and obscure potential biological patterns; therefore, p-values are presented descriptively. Correlation analyses were conducted using Pearson's or Spearman's methods, depending on data distribution. P-value < 0.05 was considered statistically significant. Effect sizes for significant ANOVA results were small (partial η2 < 0.1), indicating modest group differences.
3 Results
3.1 General clinical characteristics
Significant differences were observed in age, cough duration, fever duration before admission, and length of hospital stay among the three groups (P < 0.05) (Table 1). Children in Group B (MPP with Hi) were the youngest (4.08 ± 2.66 years), followed by Group A (4.91 ± 1.61 years), while Group C (MPP with influenza virus) included older children (5.15 ± 2.95 years). The duration of coughing before admission was longest in Group A (15.26 ± 12.71 days), significantly longer than in Groups B (8.45 ± 9.07 days) and C (7.46 ± 6.72 days). Fever duration was longest in Group B (5.09 ± 4.85 days), compared to Group C (3.58 ± 3.25 days) and Group A (2.50 ± 2.61 days). Hospital stays were also significantly longer in Group B (10.11 ± 4.81 days) compared to Groups A (8.11 ± 2.44 days) and C (8.05 ± 2.04 days).
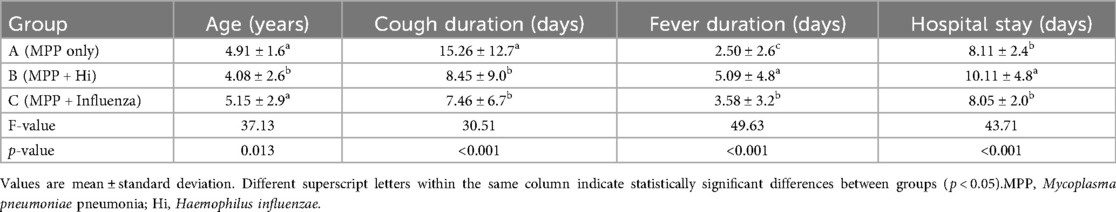
Table 1. Comparison of age, symptom duration, and hospital stay among children with Mycoplasma pneumoniae pneumonia, with and without coinfections (children with MPP + H. influenzae were youngest with longest fever and hospital stay; MPP-only had longest cough).
3.2 Complete blood count and hs-CRP
Significant differences were observed among the three groups in hs-CRP levels, WBC count, neutrophil count (N#), lymphocyte count (L#), monocyte count (M#), platelet count (PLT), lymphocyte percentage (L%), and monocyte percentage (M%). Group B exhibited the highest hs-CRP levels (23.93 ± 21.26 mg/L a), significantly higher than Groups A (12.80 ± 24.01 mg/L ab) and C (9.31 ± 11.87 mg/L b) (P = 0.006). WBC counts were significantly lower in Group C (7.25 ± 3.50 × 10⁹/L b) compared to Groups A (10.79 ± 5.10 × 10⁹/L a) and B (12.04 ± 5.85 × 10⁹/L a) (P < 0.001). Similarly, neutrophil counts (N#) and lymphocyte counts (L#) were lowest in Group C (P = 0.003 and P = 0.001, respectively). Monocyte counts (M#) were highest in Group B and lowest in Group C (P = 0.006). Platelet counts were significantly lower in Group C (P = 0.014). Lymphocyte percentage (L%) was highest in Group A and markedly lower in Groups B and C (P = 0.045). Monocyte percentage (M%) was highest in Group C compared to Groups A and B (P = 0.010). No statistically significant differences were found among groups in neutrophil percentage (N%), eosinophil counts (EO#), or basophil counts (BASO#) (P > 0.05) (Table 2). Similarly, no statistically significant differences were found among the three groups in terms of red blood cell indices and platelet parameters (Table 3).
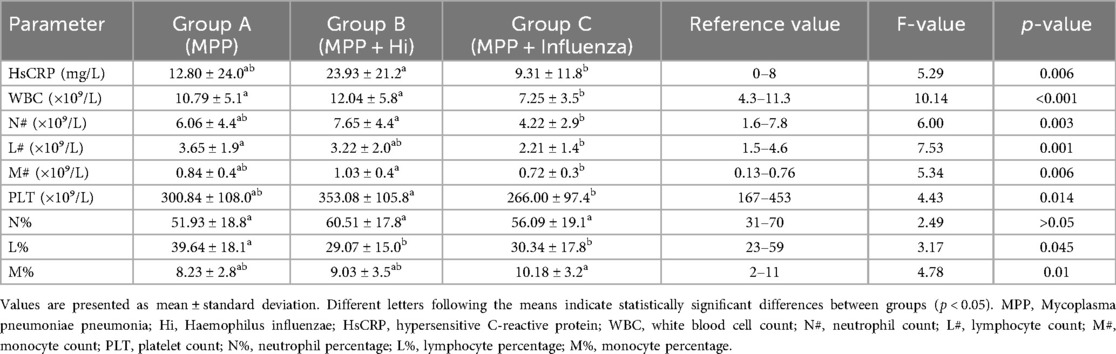
Table 2. Comparison of inflammatory markers and white blood cell counts among children with Mycoplasma pneumoniae pneumonia, with and without coinfections (hs-CRP highest in MPP + H. influenzae; WBC and platelets lowest in MPP + influenza).
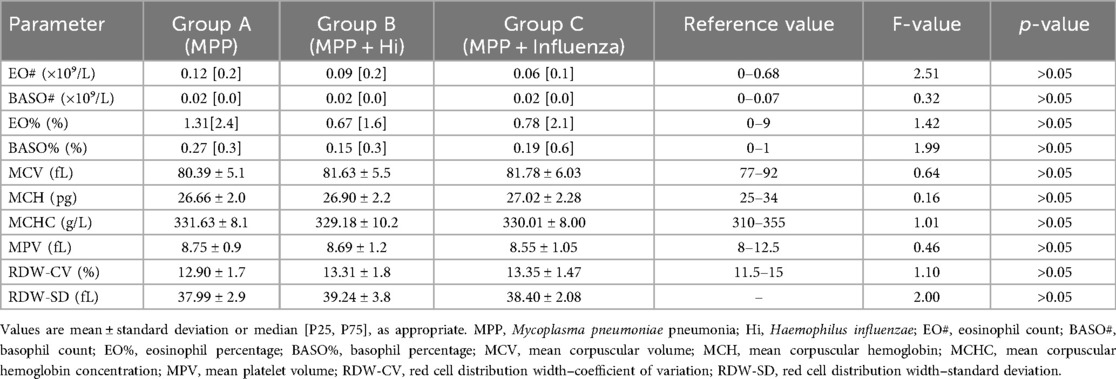
Table 3. Comparison of red blood cell indices and platelet parameters among children with Mycoplasma pneumoniae pneumonia, with and without coinfections (No significant differences in red-cell or platelet indices among groups).
3.3 Complete blood count and hs-CRP
No significant differences were found among the groups for ALT, AST, CK, CK-MB, UREA, and CREA levels (P > 0.05) (Table 3). However, CK-MB values in all groups exceeded the normal reference range, suggesting myocardial involvement despite the absence of statistically significant intergroup differences (Table 4).
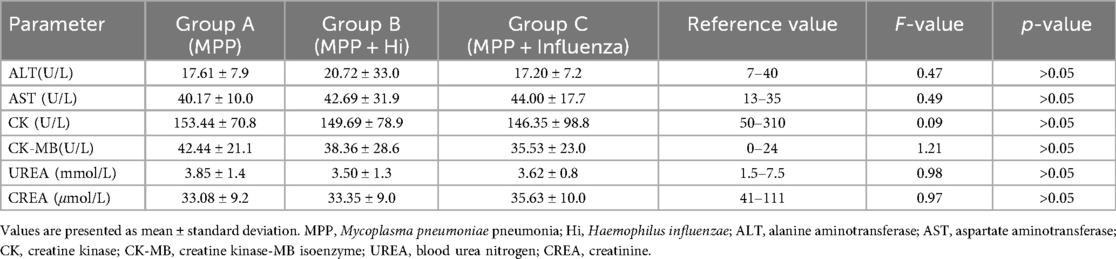
Table 4. Comparison of biochemical indicators among children with Mycoplasma pneumoniae pneumonia, with and without coinfections (No group differences in biochemistry; CK-MB elevated across all groups).
Correlation analysis among biochemical markers showed that AST was positively correlated with ALT (r = 0.402, P < 0.001), CK (r = 0.308, P < 0.001), and CK-MB (r = 0.387, P < 0.001), and negatively correlated with UREA (r = −0.206, P = 0.015) and hs-CRP (r = −0.169, P = 0.046). CK-MB was positively correlated with CK (r = 0.302, P < 0.001) and AST (r = 0.387, P < 0.001), and negatively correlated with UREA (r = −0.210, P = 0.013). Both types of correlation was very weak. No significant correlations were found between CK-MB and ALT, CREA, or hs-CRP (Figure 1).
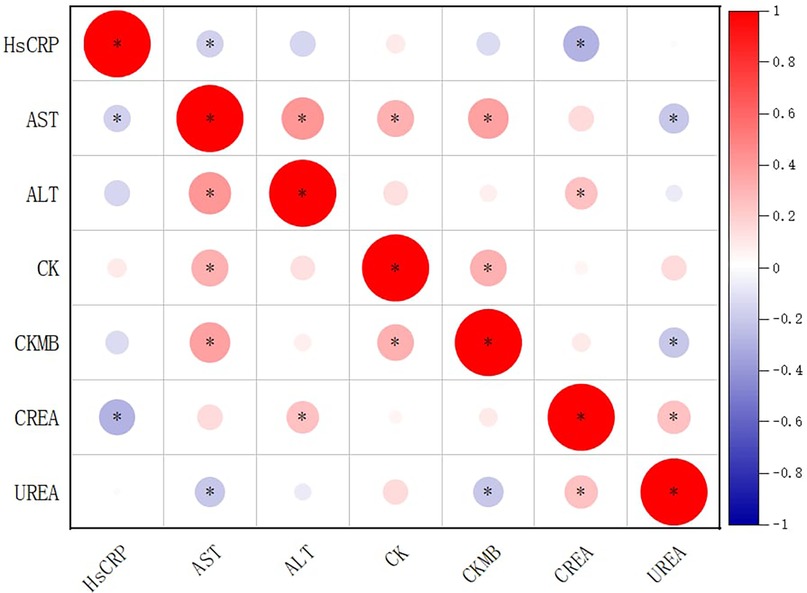
Figure 1. Correlation matrix of biochemical indicators in children with Mycoplasma pneumoniae pneumonia and coinfections. Circle size and color represent correlation strength and direction (red = positive; blue = negative). Asterisks (*) denote correlations significant at p < 0.05. Based on reported p-values, correlations between AST and ALT, AST and CK, AST and CK-MB, CK-MB and CK, and CK-MB and AST would remain significant after multiple-testing correction. Diagonal cells represent self-correlations (r = 1) and are retained for completeness, although they are mathematically trivial.
4 Discussion
Mycoplasma pneumoniae pneumonia is increasingly reported alongside coinfections with Hi or influenza virus, which can worsen clinical outcomes in children due to their immature immunity and physiological vulnerability (3, 17). In this study, we compared the clinical and laboratory characteristics of children with MPP alone and those with coinfections involving Hi or influenza virus. We found that Hi coinfection was associated with elevated inflammatory markers, prolonged fever, and longer hospital stays, whereas influenza virus coinfection was characterized by lymphopenia and thrombocytopenia, reflecting differing host immune responses to bacterial vs. viral pathogens (18–20). These patterns are consistent with prior reports showing that Hi coinfection amplifies inflammatory responses through innate immune activation, resulting in higher hs-CRP and neutrophil counts (21–23), while influenza virus coinfection impairs host defences by inducing lymphocyte apoptosis and immune suppression (24). Clinically, the observed patterns suggest therapeutic implications. Children coinfected with Hi showed the highest hs-CRP, prolonged fever, and hospital stays, indicating a stronger inflammatory response that may warrant early antibacterial treatment. In contrast, MPP + influenza virus cases showed lymphopenia and thrombocytopenia, supporting timely antiviral use. Elevated CK-MB across all groups highlights the need for cardiac monitoring in pediatric MPP regardless of coinfection.
Consistent with previous studies, age and symptom duration also varied across groups: Hi coinfections were more common in younger children, whereas influenza coinfections occurred more often in preschool and school-aged children, reflecting age-related susceptibility patterns (12, 25–28). These trends are consistent with epidemiological data and reinforce the need for age-tailored clinical vigilance. Previous research has reported that coinfections can worsen MPP severity (8, 24, 29). Choo et al. reported that respiratory viral coinfections correlated with longer fever duration and increased risk of severe pneumonia (30). These findings align with our study and extend prior work by directly comparing both bacterial (Hi) and viral (influenza virus) coinfections in MPP.
In addition, platelet counts were significantly lower in the MPP + influenza group, supporting the immunosuppressive effect of viral coinfection. Reduced platelet levels are increasingly recognized as a marker of severity in respiratory infections (24, 31). Although total WBC counts did not differ significantly between MPP and MPP + Hi groups, this may reflect sampling limitations or undetected co-pathogens (32). We also observed weak correlations among biochemical markers such as AST, ALT, CK, and CK-MB. Although these relationships were statistically significant, the effect sizes were small (r ≈ 0.3–0.4) and most likely reflect shared metabolic responses, such as concurrent hepatocellular and myocellular enzyme release, rather than clinically meaningful associations. While this overlap is expected given the enzymes’ related tissue origins, the consistent elevation of CK-MB across all groups remains noteworthy and may indicate mild, nonspecific myocardial or skeletal muscle involvement, warranting cautious clinical observation rather than a presumption of cardiac injury. Such CK-MB elevation in pneumonia may also arise from systemic inflammation, hypoxia, or skeletal muscle strain rather than direct myocardial damage, and therefore should be interpreted with caution (33–35).
From a practical perspective, these observations suggest that routine laboratory data may provide early, actionable clues while awaiting pathogen confirmation. For example, elevated hs-CRP with prolonged fever may alert clinicians to possible Hi coinfection and support timely antibacterial escalation, whereas lymphopenia and thrombocytopenia should raise suspicion of influenza virus coinfection and prompt early antiviral consideration or closer monitoring. Elevated CK-MB, seen across all groups, underscores the importance of cardiac monitoring in children with MPP. Importantly, we are not proposing a diagnostic score. Rather, our findings indicate that widely available laboratory parameters can serve as complementary, rapid indicators, particularly valuable in settings where multiplex diagnostic panels are unavailable, delayed, or cost-prohibitive.
This study has several limitations. It was retrospective and conducted in a single center, limiting generalizability. Subgroup sizes were modest, preventing the use of robust multivariate analyses and restricting our findings to exploratory associations. We also focused only on Hi and influenza virus, as these were the predominant coinfections in our cohort; results may not apply to less common pathogens. In addition, potential seasonal variation in infection rates was not analyzed due to lack of detailed temporal data. Future multicenter studies with larger sample sizes are needed to validate these findings and to assess whether laboratory parameters could be integrated into predictive algorithms alongside molecular diagnostics.
In summary, our results highlight distinct clinical and laboratory profiles of MPP coinfections. Hi coinfection was linked to heightened inflammatory responses and longer illness, while influenza virus coinfection was associated with immune suppression and thrombocytopenia. These patterns may support earlier recognition of coinfections and more tailored treatment, serving as an adjunct to, but not a replacement for, pathogen-directed diagnostic testing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for this study as it involved a retrospective analysis of anonymized clinical data routinely collected during standard medical care. No interventions or identifiable patient information were used, and all procedures complied with institutional and national ethical guidelines for research involving human participants. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors gratefully acknowledge the Department of Pediatrics, The First Affiliated Hospital, Jinan University, Guangzhou, China, for providing the necessary facilities and access to conduct this study. We also extend our sincere thanks to the laboratory staff for their valuable support in sample collection and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ebeledike C, Ahmad T, Martin SD. Pediatric pneumonia (nursing). In: MCM, editor. StatPearls. Florida: StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK536940/
2. Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, et al. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. (2016) 170(3):267–87. doi: doi: 10.1001/jamapediatrics.2015.4276
3. Butpech T, Tovichien P. Mycoplasma pneumoniae pneumonia in children. World J Clin Cases. (2025) 13(5):99149. doi: doi: 10.12998/wjcc.v13.i5.99149
4. Woods CR, Bryant KA. Viral infections in children with community-acquired pneumonia. Curr Infect Dis Rep. (2013) 15:177–83. doi: doi: 10.1007/s11908-013-0324-6
5. Yu A, Ran L, Sun X, Feng T. Significance of respiratory virus coinfection in children with Mycoplasma pneumoniae pneumonia. BMC Pulm Med. (2024) 24(1):585. doi: doi: 10.1186/s12890-024-03380-4
6. Levine OS, Liu G, Garman RL, Dowell SF, Yu S, Yang Y-H. Haemophilus influenzae type B and Streptococcus pneumoniae as causes of pneumonia among children in Beijing, China. Emerg Infect Dis. (2000) 6(2):165–70. doi: doi: 10.3201/eid0602.000209
7. Zhang X, Chen Z, Gu W, Ji W, Wang Y, Hao C, et al. Viral and bacterial co-infection in hospitalised children with refractory Mycoplasma pneumoniae pneumonia. Epidemiology & Infection. (2018) 146(11):1384–8. doi: doi: 10.1017/s0950268818000778
8. Chen Q, Lin L, Zhang N, Yang Y. Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front Pediatr. (2024) 12:1337786. doi: doi: 10.3389/fped.2024.1337786
9. Song Q, Xu B-P, Shen K-L. Effects of bacterial and viral co-infections of Mycoplasma pneumoniae pneumonia in children: analysis report from Beijing children’s hospital between 2010 and 2014. Int J Clin Exp Med. (2015) 8(9):15666–74.26629061
10. Largman-Chalamish M, Wasserman A, Silberman A, Levinson T, Ritter O, Berliner S, et al. Differentiating between bacterial and viral infections by estimated CRP velocity. PLoS One. (2022) 17(12):e0277401. doi: doi: 10.1371/journal.pone.0277401
11. Mouliou DS. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Diseases. (2023) 11(4):132. doi: doi: 10.3390/diseases11040132
12. Vos Q, Lees A, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. (2000) 176(1):154–70. doi: doi: 10.1034/j.1600-065x.2000.00607.x
13. Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. (2013) 57(suppl_3):S139–70. doi: doi: 10.1093/cid/cit578
14. de Nooijer AH, Pickkers P, Netea MG, Kox M. Inflammatory biomarkers to predict the prognosis of acute bacterial and viral infections. J Crit Care. (2023) 78:154360. doi: doi: 10.1016/j.jcrc.2023.154360
15. Endimiani A, Ramette A, Rhoads DD, Jacobs MR. The evolving role of the clinical microbiology laboratory in identifying resistance in gram-negative bacteria: an update. Infect Dis Clin North Am. (2020) 34(4):659–76. doi: doi: 10.1016/j.idc.2020.08.001
16. Qiu-Ju C, Ling-Yu G, Ting-Dong Z, Yang T, Ning H, Ai-Hua W, et al. Routine blood parameters as auxiliary diagnostic tools for Mycoplasma pneumoniae infection in children. J Med Microbiol. (2024) 73(9):001885. doi: doi: 10.1099/jmm.0.001885
17. Fu S, Jia W, Li P, Cui J, Wang Y, Song C. Risk factors for pneumonia among children with coinfection of influenza A virus and Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis. (2024) 43(7):1437–44. doi: doi: 10.1007/s10096-024-04854-3
18. King PT, Sharma R. The lung immune response to nontypeable Haemophilus influenzae (lung immunity to NTHi). J Immunol Res. (2015) 2015(1):706376. doi: doi: 10.1155/2015/706376
19. Kumar N, Sharma S, Barua S, Tripathi BN, Rouse BT. Virological and immunological outcomes of coinfections. Clin Microbiol Rev. (2018) 31(4):e00111–00117. doi: doi: 10.1128/cmr.00111-17
20. Xu J-Q, Zhang W-Y, Fu J-J, Fang X-Z, Gao C-G, Li C, et al. Viral sepsis: diagnosis, clinical features, pathogenesis, and clinical considerations. Mil Med Res. (2024) 11(1):78. doi: doi: 10.1186/s40779-024-00581-0
21. Ding L, Jiang Y. Biomarkers associated with the diagnosis and prognosis of Mycoplasma pneumoniae pneumonia in children: a review. Front Cell Infect Microbiol. (2025) 15:1552144. doi: doi: 10.3389/fcimb.2025.1552144
22. Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory Mycoplasma pneumoniae pneumonia in children. Respir Care. (2015) 60(10):1469–75. doi: 10.4187/respcare.03920
23. Wen J, Yu Y, Feng D, Sun T, Xia P, Li D, et al. Diagnostic Biomarkers for Mycoplasma Pneumoniae Pneumonia: Serum Hs-Crp, Saa, and Il-6. Available at SSRN (2024). Available online at: https://ssrn.com/abstract=4983210 or 10.2139/ssrn.4983210. doi: 10.2139/ssrn.4983210
24. Zhou M, Qi J, Li X, Zhang Z, Yao Y, Wu D, et al. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: a systematic review and meta-analysis. Br J Haematol. (2020) 189(3):438–41. doi: doi: 10.1111/bjh.16655
25. Lee K-L, Lee C-M, Yang T-L, Yen T-Y, Chang L-Y, Chen J-M, et al. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010–2019. J Formos Med Assoc. (2021) 120(1):281–91. doi: doi: 10.1016/j.jfma.2020.08.018
26. Tsang R, Bruce M, Lem M, Barreto L, Ulanova M. A review of invasive Haemophilus influenzae disease in the indigenous populations of North America. Epidemiol Infect. (2014) 142(7):1344–54. doi: doi: 10.1017/s0950268814000405
27. Yang B, Zhang W, Gu W, Zhang X, Wang M, Huang L, et al. Differences of clinical features and prognosis between Mycoplasma pneumoniae necrotizing pneumonia and non-Mycoplasma pneumoniae necrotizing pneumonia in children. BMC Infect Dis. (2021) 21(1):797. doi: doi: 10.1186/s12879-021-06469-x
28. Youn Y-S, Lee K-Y, Hwang J-Y, Rhim J-W, Kang J-H, Lee J-S, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. (2010) 10(48):1–7. doi: doi: 10.1186/1471-2431-10-48
29. Gao J, Xu L, Xu B, Xie Z, Shen K. Human adenovirus coinfection aggravates the severity of Mycoplasma pneumoniae pneumonia in children. BMC Infect Dis. (2020) 20(1):420. doi: doi: 10.1186/s12879-020-05152-x
30. Choo S, Kim S-H, Lee E. Clinical significance of Mycoplasma pneumoniae specific IgM titer in children hospitalized with Mycoplasma pneumoniae pneumonia. BMC Infect Dis. (2022) 22(1):470. doi: doi: 10.1186/s12879-022-07456-6
31. Assinger A. Platelets and infection–an emerging role of platelets in viral infection. Front Immunol. (2014) 5:649. doi: doi: 10.3389/fimmu.2014.00649
32. Trunfio M, Savoldi A, Viganò O, d’Arminio Monforte A. Bacterial coinfections in dengue virus disease: what we know and what is still obscure about an emerging concern. Infection. (2017) 45:1–10. doi: doi: 10.1007/s15010-016-0927-6
33. Qi X, Sun X, Li X, Kong D, Zhao L. Significance changes in the levels of myocardial enzyme in the child patients with Mycoplasma Pneumoniae pneumonia. Cell Mol Biol. (2020) 66(6):41–5. doi: doi: 10.14715/cmb/2020.66.6.8
34. Shen T, Li Y, Liu T, Lian Y, Kong L. Association between Mycoplasma pneumoniae infection, high-density lipoprotein metabolism and cardiovascular health. Biomed Rep. (2024) 20(3):39. doi: doi: 10.3892/br.2024.1729
Keywords: Mycoplasma pneumoniae, Haemophilus influenzae, influenza virus, blood routine, pediatric pneumonia
Citation: Tang X and Chen C (2025) Comparative analysis of blood routine, C-reactive protein, and biochemical markers in children with Mycoplasma pneumoniae pneumonia and its coinfections. Front. Pediatr. 13:1661684. doi: 10.3389/fped.2025.1661684
Received: 8 July 2025; Accepted: 24 October 2025;
Published: 24 November 2025.
Edited by:
Maurizio Aricò, Department of Pediatrics, ItalyReviewed by:
Enrico Valletta, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyMahinder Paul, Albert Einstein College of Medicine, United States
Copyright: © 2025 Tang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chongfeng Chen, c2l6eDg2MEAxNjMuY29t
 Xingjia Tang
Xingjia Tang Chongfeng Chen
Chongfeng Chen