- 1ENT Department, Military Hospital of Tunis, Tunis, Tunisia
- 2Radiology Department, Military Hospital of Tunis, Tunis, Tunisia
- 3Pathology Department, Military hospital of Tunis, Tunis, Tunisia
- 4Pediatry Department, Military Hospital of Tunis, Tunis, Tunisia
Background: Cavernous sinus tuberculosis is an extremely rare manifestation of central nervous system tuberculosis in children, with only two cases reported worldwide. It can mimic malignancy or other inflammatory conditions. Its occurrence in children with primary immunodeficiency, particularly major histocompatibility complex (MHC) class II deficiency, has not yet been described.
Case report: We report an 11-year-old girl with a history of recurrent infections and chronic otitis media. She presented with right orbital swelling, severe headaches, and exophthalmos. Imaging revealed an extensive mass in the sinonasal and orbital regions, extending to the skull base and cavernous sinus. A computed tomography-guided biopsy and histopathology, supported by PCR testing for Mycobacterium tuberculosis, confirmed extensive orbital and cervicofacial tuberculosis. An immunological evaluation and genetic analysis revealed familial MHC class II deficiency. The patient received anti-tuberculosis therapy [isoniazid, rifampin, pyrazinamide, and ethambutol (HRZE) followed by isoniazid and rifampin (HR)], leading to clinical and radiological improvement. She continues with intravenous immunoglobulin replacement therapy every 21 days while awaiting a bone marrow transplantation.
Conclusions: This case highlights the importance of considering tuberculosis in atypical cavernous sinus lesions in children, especially in endemic regions. Severe or unusual infections should prompt evaluation for underlying immunodeficiency.
Introduction
Tuberculosis remains a major public health challenge worldwide, particularly in low- and middle-income countries. In these regions, both the pulmonary and extrapulmonary forms continue to affect vulnerable pediatric populations. While most pediatric tuberculosis cases affect the lungs, extrapulmonary involvement is rare and presents significant diagnostic and therapeutic challenges. Among these rare forms, cervicofacial tuberculosis involving the cavernous sinus and orbit is exceedingly uncommon and may mimic malignancy (1).
In immunocompetent children, Mycobacterium tuberculosis is usually contained within granulomas, which limit its spread. In contrast, children with compromised immunity may present with atypical disease forms, including disseminated or fulminant tuberculosis. Primary immunodeficiencies impair T-cell function and major histocompatibility complex (MHC) class II expression, increasing the risk of severe and recurrent infections caused by intracellular pathogens such as mycobacteria (2).
We report a unique case of extensive, atypical tuberculosis in an 11-year-old girl with familial MHC class II deficiency. Her initial presentation involved isolated orbital symptoms. The diagnosis was confirmed using histopathological and molecular investigations. This case emphasizes the importance of considering both tuberculosis and underlying primary immunodeficiencies in the differential diagnosis of atypical orbital masses in children. We aim to illustrate the diagnostic approach, radiological findings, therapeutic management, and the role of immunological evaluation in managing such complex cases.
Case report
We present the case of an 11-year-old girl who had viral meningoencephalitis at age 5, resulting in unilateral sensorineural hearing loss. Since that episode, she had been followed for bilateral chronic otitis media with recurrent upper respiratory tract infections that were treated as needed with antibiotics, antipyretics, nasal lavage, and supportive care. Her immunizations were complete and age-appropriate, including the Bacillus Calmette–Guérin (BCG) vaccine.
The current episode began approximately 1 month prior to hospital admission, initially presenting as isolated right eyelid edema that was overlooked. Subsequently, she developed severe, persistent bifrontal and occipital headaches, prompting urgent evaluation.
On clinical examination, she had a right eyelid edema and grade III axial right-sided exophthalmos without limitation of ocular movements. Nasal endoscopy revealed inflamed mucosa with purulent discharge from the right superior meatus, consistent with active sinonasal inflammation.
The treating pediatrician prescribed oral amoxicillin-clavulanate (2 g/day for 8 days) and corticosteroids, nasal decongestants, nasal lavage, and oral mucolytics, suspecting acute ethmoiditis. As the symptoms did not show signs of improvement, a referral to our specialized hospital service was deemed necessary for further evaluation.
On admission, left jugulocarotid lymphadenopathy was noted, while the neurological examination was unremarkable. Her height was 137 cm (height-for-age z-score: −1.0) and her weight was 28 kg (weight-for-age z-score: −2.0), indicating she was underweight. Her BMI was 14.7 kg/m2 (BMI-for-age z-score: −2.2), consistent with moderate thinness. Historical records showed persistently low weight, supporting a diagnosis of chronic undernutrition.
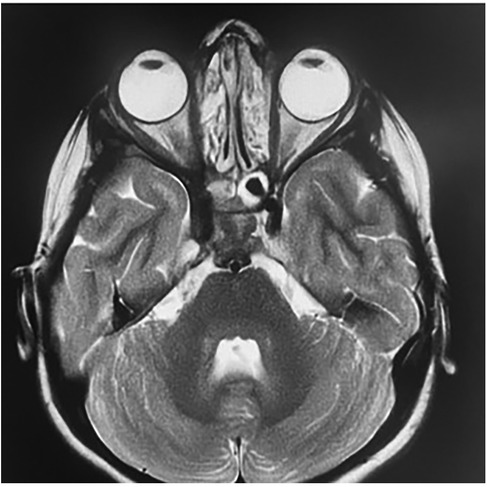
Contrast-enhanced CT of the brain and orbits demonstrated a tissue process involving both nasal cavities and maxillary sinuses, extending into the right extraconal orbital space and ipsilateral cavernous sinus (Figure 1). Orbital and cerebro-cervical MRI demonstrated an expansive tissue signal process affecting the right orbital extraconal space, nasal cavities, right cavernous sinus, pterygopalatine fossa, infratemporal fossa, and foramina of the skull base. Cervical lymphadenopathy was also present (Figure 2).
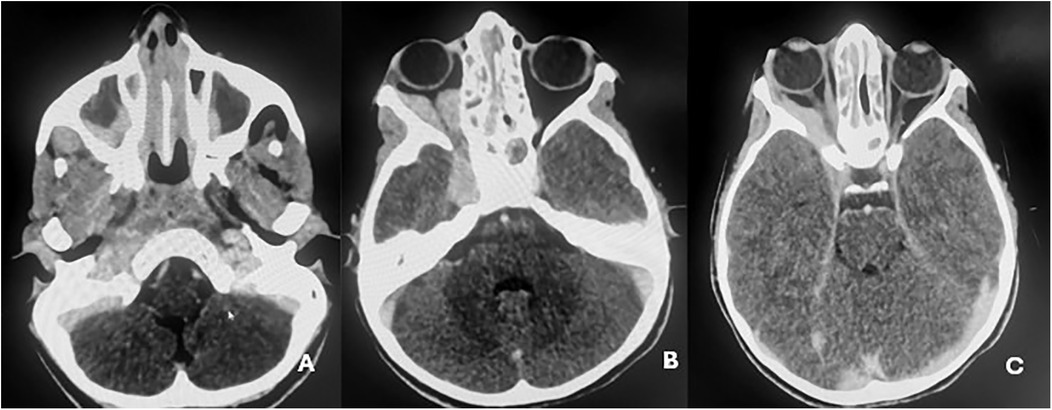
Figure 1. Contrast-enhanced cerebro-orbital scans demonstrating tissue expansion affecting both nasal cavities, extending into the maxillary sinuses (A), and the right extraconal orbital space, extending to the ipsilateral cavernous sinus (B,C).
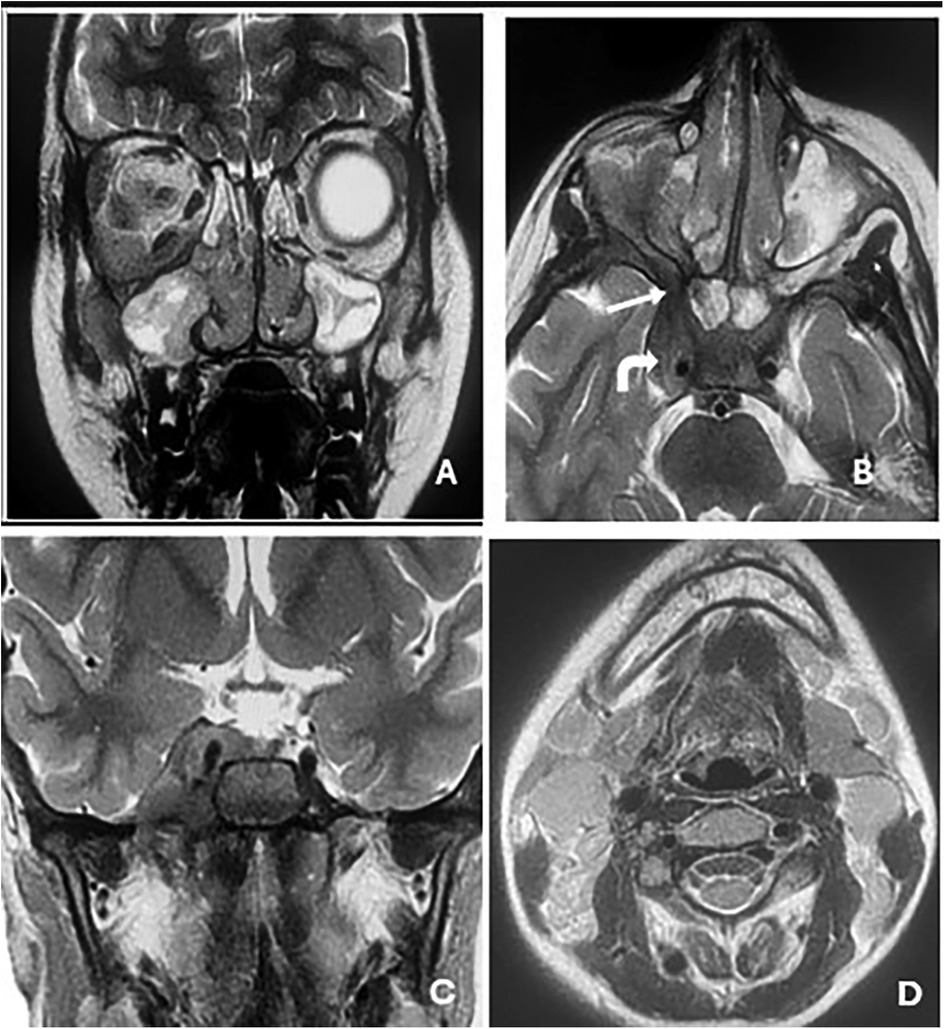
Figure 2. Orbital and cerebro-cervical MRI in T2-weighted sequences in the coronal (A and C) and axial (B) planes showing an expansive tissue signal process invading the right orbital extraconal space and nasal cavities (A); the right cavernous sinus (solid arrow in B); and the pterygopalatine fossa, infratemporal fossa, and the foramina of the skull base, with (B) showing the right round and (C) the right oval. Scan showing non-necrotic bilateral jugulocarotid and spinal lymphadenopathy (D).
To exclude lymphoma, a thoraco-abdomino-pelvic CT scan was performed and revealed no distant lesions. A CT-guided biopsy of the infratemporal mass, along with lymph node excision, was undertaken. The histopathological examination demonstrated an epithelioid granulomatous reaction with multinucleated giant cells and central caseating necrosis, highly suggestive of tuberculosis (Figure 3). PCR testing confirmed the presence of M. tuberculosis, whereas the sputum analysis for acid-fast bacilli (AFB) was negative.
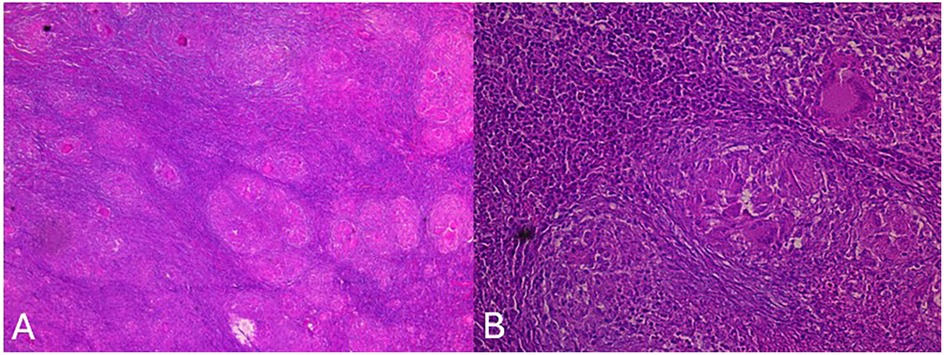
Figure 3. Anatomopathological study showing (HEx4) lymph node parenchyma altered by a specific inflammatory reaction with multiple tuberculous granulomas (A) and (HEx20) an epithelioid granuloma with focal central caseous necrosis and Langhans-type giant cells (B).
Pre-treatment evaluations included an assessment of acetylator status, complete blood count, renal and liver function, and serum electrolytes. The patient was initiated on standard anti-tuberculosis chemotherapy.
In view of the patient's atypically extensive tuberculosis presentation and a history of recurrent pulmonary and gastrointestinal infections in her older sister, an immunodeficiency workup was undertaken.
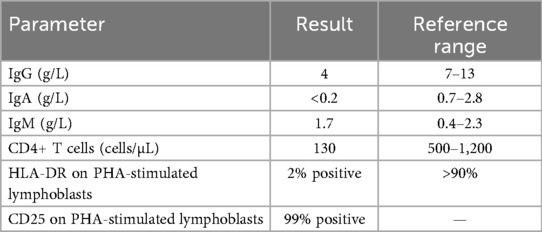
The quantitative analysis demonstrated profound CD4+ T-cell lymphopenia with IgA and IgG hypogammaglobulinemia, while her IgM levels remained within the normal range. Flow cytometry revealed a markedly reduced CD4/CD8 ratio (<0.3).
Peripheral blood mononuclear cells were isolated via Ficoll density gradient, stimulated with phytohemagglutinin (PHA), and labeled with monoclonal antibodies at the specialized immunology laboratory of the Pasteur Institute of Tunis. The analysis demonstrated the near-complete absence of human leukocyte antigen – DR isotype (HLA-DR) expression on activated lymphoblasts (2% positive), whereas the activation marker CD25 was expressed in 99% of the cells, indicating adequate lymphocyte activation. These findings confirmed MHC class II deficiency, consistent with a combined immunodeficiency due to defective HLA class II expression (Table 1).
Similar immunological findings were observed in the patient's older sister, confirming familial primary immunodeficiency due to MHC class II deficiency (bare lymphocyte syndrome type II).
The patient is currently receiving intravenous immunoglobulin replacement therapy every 21 days while awaiting allogeneic hematopoietic stem cell transplantation (HSCT). After 6 months of anti-tuberculosis therapy, comprising 3 months of quadruple therapy (isoniazid, rifampicin, pyrazinamide, and ethambutol) followed by 3 months of dual therapy (isoniazid and rifampicin), clinical follow-up demonstrated complete resolution of her exophthalmos. Subsequent MRI confirmed marked regression of the lesions (Figure 4). At the time of reporting, she was in her ninth month of treatment, exhibiting no evidence of drug resistance and showing progressive weight gain.
Discussion
Cavernous sinus tuberculosis (CST) remains an exceptionally rare condition in children, with only two pediatric cases reported to date. Familial MHC class II deficiency, also referred to as bare lymphocyte syndrome type II, is a rare autosomal recessive primary immunodeficiency disorder, with roughly 150 cases documented worldwide (3). The simultaneous occurrence of these two uncommon conditions in an 11-year-old patient represents an extraordinarily rare clinical scenario.
Tuberculosis affecting the central nervous system (CNS) represents one of the most severe forms of extrapulmonary tuberculosis in children, with a clinical spectrum ranging from tuberculous meningitis (TBM) to compressive myelopathy secondary to vertebral involvement, as well as the development of tuberculomas (4).
The cavernous sinus is an irregularly shaped, endothelium-lined venous structure positioned on either side of the sella turcica. It is located laterally and superiorly to the sphenoid sinus and immediately posterior to the optic chiasm (5). Venous drainage occurs from the central face and frontal sinuses. A notable anatomical characteristic is the absence of valves in its connecting veins, permitting bidirectional blood flow and thereby increasing the risk of infectious spread and thrombosis (5).
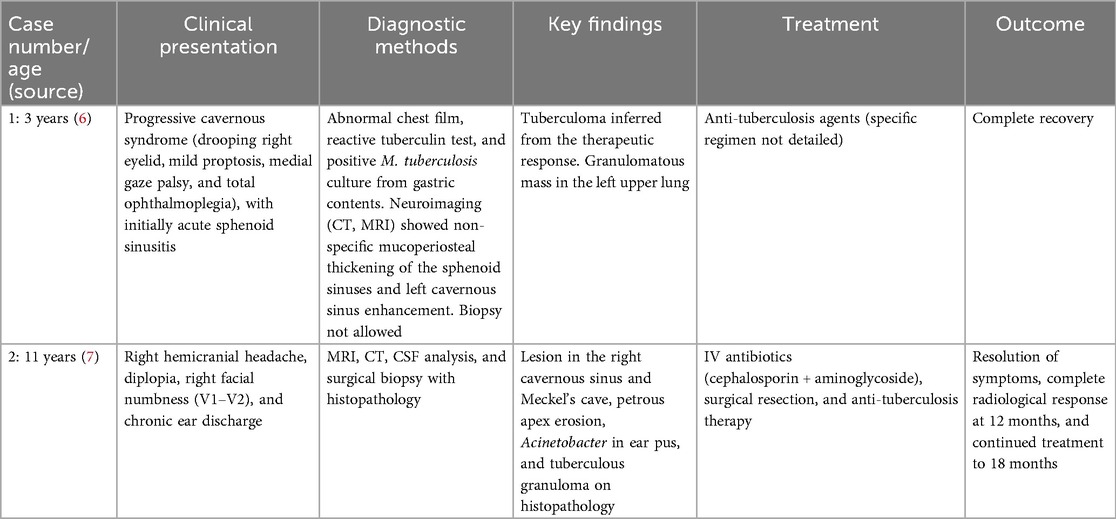
To date, only two pediatric cases of cavernous sinus tuberculoma have been documented. The first, reported in 2007, involved a 3-year-old Thai patient who presented with progressive cavernous syndrome, including right eyelid ptosis, mild proptosis, medial gaze palsy, and eventual total ophthalmoplegia. The initial clinical diagnosis was acute sphenoid sinusitis. The tuberculoma diagnosis was inferred from a reactive tuberculin test, an abnormal chest radiograph showing bilateral reticular infiltration and a calcified nodule, and a positive M. tuberculosis culture from gastric contents. No direct biopsy of the cavernous sinus was performed. The patient achieved complete recovery following anti-tuberculosis therapy (6) (Table 2).
The second case, reported in 2014, involved an 11-year-old patient with a 2-year history of untreated right ear discharge and hearing loss. She presented with right hemicranial headache, diplopia, and facial numbness. The clinical examination revealed right VI cranial nerve palsy and V1–V2 hypoesthesia, accompanied by purulent otorrhea. MRI identified a lesion in the right cavernous sinus and Meckel's cave with erosion of the petrous apex. The lesion was initially suspected to be an inflammatory granuloma secondary to chronic otitis media. A definitive diagnosis was established postoperatively when histopathology demonstrated caseating granulomas consistent with tuberculosis. The patient received anti-tuberculosis therapy following a near-total surgical resection. MRI performed after 12 months confirmed complete resolution of the lesion, and the therapy was continued for a total of 18 months (7) (Table 2).
A cavernous sinus tuberculoma poses a considerable diagnostic challenge, as it may mimic more common conditions, including meningioma, lymphoma, sarcoidosis, or bacterial and fungal infections (3). This similarity frequently leads to diagnostic delays, with empirical therapies sometimes initiated before the commencement of anti-tuberculosis treatment, potentially worsening patient outcomes—particularly in anatomically critical sites such as the CNS (4).
The diagnostic evaluation of CST relies on a combination of imaging and microbiological confirmation. Contrast-enhanced CT and MRI are essential for identifying lesions within the cavernous sinus (3), and MRI can detect early infarcts, basal ganglia enhancement, and basal cistern exudates (8). Nevertheless, imaging findings are often non-specific and may resemble other intracranial masses (3). Direct microbiological confirmation from the affected site remains the gold standard. Due to the paucibacillary nature of pediatric tuberculosis and the challenges in obtaining adequate CNS samples, a definitive confirmation can be difficult (4). The recovery of M. tuberculosis from cerebrospinal fluid (CSF) is typically low, and culture growth is slow (4). Molecular assays, such as the Xpert MTB/RIF test, are recommended for rapid detection and drug-resistance evaluation, although their sensitivity can vary (8). When feasible, tissue biopsy provides definitive confirmation, demonstrating caseating granulomas and allowing subsequent culture and molecular analyses (3). In adult patients, some CST cases were only conclusively diagnosed after surgical resection (9).
Children affected by paucibacillary tuberculosis, which is characterized by a low bacterial load, can nevertheless mount a robust immunological response, resulting in granuloma formation. This contrast creates a diagnostic paradox: although the host immune system attempts to contain the infection through granuloma formation, the low bacterial burden complicates direct detection of M. tuberculosis (8).
In individuals with MHC class II deficiency, the immunological hallmark is the absent or markedly reduced expression of MHC class II molecules (HLA-DR, HLA-DQ, and HLA-DP) on antigen-presenting cells (APCs), including B cells, monocytes, and dendritic cells (3). This defect impairs both cellular and humoral immunity, as these molecules are critical for the development, maturation, and functional activation of CD4+ T helper (Th) cells (3). Patients typically present with severe CD4+ T-lymphocytopenia, an inverted CD4:CD8 ratio, and hypogammaglobulinemia, reflecting impaired Th cell-dependent antibody production by B cells (3). Altered thymic selection of CD4+ T cells further exacerbates this immune defect (10).
CD4+ T cells, activated via MHC class II molecules on APCs, are central to the host's resistance against M. tuberculosis. They orchestrate granuloma formation and maintenance, which contain mycobacteria. In MHC class II deficiency, impaired antigen presentation leads to weak CD4+ T-cell activation and function, explaining the persistent infection and atypical disease dissemination (11, 12). Consequently, granulomas may be present but functionally inadequate, emphasizing the importance of histopathology not only for diagnosis but also for evaluating granulomatous responses in immunodeficient patients.
Typically, an 11-year-old child would have a lower risk of progression to active tuberculosis and would present with mild clinical manifestations. In this patient, the severe CST presentation reflects her underlying primary immunodeficiency. The “Th1 skew” and lymphocyte predominance generally observed in immunocompetent children are markedly compromised in MHC class II deficiency (13).
The management of patients with such conditions requires prolonged and carefully monitored therapy. In young children, higher drug dosages may be necessary to achieve effective bactericidal activity (14). In this patient, the presence of atypical CNS involvement, including the skull base and cavernous sinus, combined with MHC class II deficiency, warranted a 12-month anti-tuberculosis regimen, in line with international guidelines for CNS and osteoarticular tuberculosis (15).
Corticosteroids are frequently administered in children with CNS tuberculosis, particularly those with tuberculous meningitis, to reduce inflammation, cerebral edema, and intracranial pressure (15, 16). In patients living with primary immunodeficiency, corticosteroid use presents a therapeutic challenge, as these agents may suppress immune function while simultaneously mitigating severe inflammatory complications or immune reconstitution inflammatory syndrome (IRIS) (17, 18).
Additional clinical considerations include drug interactions, particularly with rifamycins, which induce cytochrome P450 enzymes and drug transporters, potentially reducing serum concentrations of co-administered immunosuppressants or other therapies required to manage complications associated with MHC class II deficiency (19, 20).
HSCT remains the only curative therapy for individuals with MHC class II deficiency (3). However, active CNS tuberculosis can complicate transplant eligibility. Achieving effective infection control prior to HSCT is essential for optimizing transplant outcomes and minimizing post-transplant complications (10).
Conclusion
This case highlights cavernous sinus tuberculosis in a child with MHC class II deficiency. Tuberculosis should be considered in atypical intracranial lesions, and severe or unusual infections should prompt evaluation for underlying immunodeficiency.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for this study involving human samples in accordance with the local legislation and institutional requirements because our institution does not require it for non experimental studies. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM: Conceptualization, Supervision, Validation, Writing – review & editing. AA: Writing – original draft. SE: Validation, Writing – review & editing. KT: Data curation, Writing – review & editing. MR: Data curation, Writing – review & editing. MM: Conceptualization, Data curation, Writing – review & editing. HB: Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dian S, Ganiem AR, van Laarhoven A. Central nervous system tuberculosis. Curr Opin Neurol. (2021) 34:396–402. doi: 10.1097/WCO.0000000000000920
2. Simone LC, Tuli A, Simone PD, Wang X, Solheim JC. Analysis of major histocompatibility complex class I folding: novel insights into intermediate forms. Tissue Antigens. (2012) 79:249–62. doi: 10.1111/j.1399-0039.2012.01849.x
3. Aluri J, Gupta M, Dalvi A, Mhatre S, Kulkarni M, Hule G, et al. Clinical, immunological, and molecular findings in five patients with major histocompatibility complex class II deficiency from India. Front Immunol. (2018) 9:188. doi: 10.3389/fimmu.2018.00188
4. Kunju P, James J. Central nervous system tuberculosis in children. Pediatr Infect Dis. (2019) 1:23–9. doi: 10.5005/jp-journals-10081-1106
5. Toma N. [Anatomy of the cavernous sinus]. No Shinkei Geka. (2024) 52:560–9 (in Japanese). doi: 10.11477/mf.1436204949
6. Intusoma U, Anuntaseree W, Pruekprasert P, Janjindamai S, Vasiknanonte P. First case report of cavernous sinus tuberculoma in a child. J Pediatr Neurol. (2007) 5:157–60. doi: 10.1055/s-0035-1557372
7. Kumar VRR, Madhugiri VS, Verma SK, Barathi SD, Yadav AK, Bidkar P. Tuberculoma of the cavernous sinus and Meckel’s cave in a child. Pediatr Neurosurg. (2013) 49:369–73. doi: 10.1159/000369030
8. Tristram D, Tobin EH. “Tuberculosis in children”. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2025). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK610681/ (Accessed June 24, 2025).
9. Kapadia S, Patrawalla A. Extrapulmonary tuberculosis presenting as a cavernous sinus syndrome: case report with review of existing literature. IDCases. (2014) 1:97–100. doi: 10.1016/j.idcr.2014.10.010
10. Lum SH, Neven B, Slatter MA, Gennery AR. Hematopoietic cell transplantation for MHC class II deficiency. Front Pediatr. (2019) 7:516. doi: 10.3389/fped.2019.00516
11. Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for toll-like receptors. Nat Rev Microbiol. (2010) 8:296–307. doi: 10.1038/nrmicro2321
12. Petersen HJ, Smith AM. The role of the innate immune system in granulomatous disorders. Front Immunol. (2013) 4:120. doi: 10.3389/fimmu.2013.00120
13. Seddon JA, Chiang SS, Esmail H, Coussens AK. The wonder years: what can primary school children teach us about immunity to Mycobacterium tuberculosis? Front Immunol. (2018) 9:2946. doi: 10.3389/fimmu.2018.02946
14. World Health Organization. “Treatment of TB in children”. In: Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children (2014). 2nd ed. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK214449/ (Accessed June 24, 2025).
15. Nardell EA. Extrapulmonary Tuberculosis (TB)—Infectious Diseases. Merck Manual Professional Edition (2023). Available online at: https://www.merckmanuals.com/professional/infectious-diseases/mycobacteria/extrapulmonary-tuberculosis-tb (Accessed June 24, 2025).
16. Canadian Tuberculosis Standards. Chapter 9: Pediatric Tuberculosis—Management of TB Disease (2023). Available online at: https://manuals.cts-sct.ca/documentation/chapter-9-pediatric-tuberculosis/5-management-of-tb-disease/5-6-adjunctive-therapy/
17. Schutz C, Davis AG, Sossen B, Lai RPJ, Ntsekhe M, Harley YX, et al. Corticosteroids as an adjunct to tuberculosis therapy. Expert Rev Respir Med. (2018) 12:881–91. doi: 10.1080/17476348.2018.1515628
18. National Institutes of Health. Tuberculosis: Pediatric OIs (2023). Available online at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/mycobacterium-tuberculosis (Accessed June 24, 2025).
19. Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. (2003) 42:819–50. doi: 10.2165/00003088-200342090-00003
Keywords: cavernous sinus, MHC class II deficiency, pediatric case report, orbital tuberculosis, paranasal sinus tuberculosis
Citation: Mezri S, Amri A, Essghaier S, Tlili K, Rabhi M, Marmouri M and Barakizou H (2025) Case Report: Atypical extensive orbitofacial tuberculosis extending to the skull base and cavernous sinus revealed major histocompatibility complex class II deficiency in an 11-year-old girl. Front. Pediatr. 13:1663784. doi: 10.3389/fped.2025.1663784
Received: 10 July 2025; Accepted: 9 October 2025;
Published: 4 November 2025.
Edited by:
Sudeep Kumar Maurya, University of Pittsburgh Medical Center, United StatesReviewed by:
Andrew Kobets, Montefiore Medical Center, United StatesLily Gloria Tagoe, Korle Bu Teaching Hospital, Ghana
Copyright: © 2025 Mezri, Amri, Essghaier, Tlili, Rabhi, Marmouri and Barakizou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sameh Mezri, c2FtZWhtZXpyaUB5YWhvby5mcg==
 Sameh Mezri
Sameh Mezri Ameni Amri1
Ameni Amri1 Hager Barakizou
Hager Barakizou