- 1Department of Infectious Diseases, Children’s Hospital of Soochow University, Suzhou, China
- 2Department of Pediatrics, Qingdao Hospital, University of Health and Rehabilitation Sciences, Qingdao, China
Background: Liver injury is a extrapulmonary complication of community-acquired pneumonia (CAP). However, limited data exist on the pathogen distribution and severity of liver injury in children with CAP-associated liver injury. This study aimed to investigate the characteristics of pathogen distribution and the severity of liver injury in children with CAP complicated by liver injury in the Suzhou area.
Methods: A retrospective study was conducted on children with CAP hospitalized at the Children's Hospital of Soochow University between January 2018 and December 2022. The study included children aged over 28 days to under 18 years, categorized into the following age groups: >28 days to 1 year, >1–3 years, >3–5 years, and >5 years. Laboratory examination results, pathogens, and characteristics of liver injury were analyzed.
Results: Among the 1525 children with CAP complicated by liver injury, the male-to-female ratio was 1.4:1. Mild elevation of transaminases were observed in 1,403 cases. In the same age group, there were differences in the proportion of cases with varying degrees of liver injury (p < 0.05). Among the four age groups, both the number of cases and the incidence of liver injury were highest in the >28 days to 1-year-old group. The incidence of liver injury was higher in children with severe community-acquired pneumonia (SCAP). Additionally, the distribution of pathogens varied significantly among age groups (p < 0.001). Children with severe liver injury were mostly accompanied by Mycoplasma pneumoniae (M. pneumoniae) infection (88.89%). Alanine aminotransferase (ALT) levels also varied significantly based on age group, pathogen type, and pneumonia severity (p < 0.05). Importantly, none of the children progressed to liver failure.
Conclusions: In the Suzhou area, children with CAP aged >28 days to 1 year were the most susceptible to liver injury, with mild elevation of transaminases being the most common presentation. Special attention was required for children with CAP complicated by M. pneumoniae infection, as they carried a higher risk of severe liver injury. Children with SCAP were more prone to liver injury. Additionally, pathogen distribution varied across different age groups in children with CAP complicated by liver injury.
Background
CAP is one of the most prevalent childhood diseases, with approximately 120 million new cases reported annually. It remains the leading cause of death in children under 5 years old, posing a significant threat to pediatric health (1, 2). CAP can be caused by various pathogens, including viruses, bacteria, and M. pneumoniae (3). The most common symptoms include fever, and cough. However, some children may also exhibit extrapulmonary manifestations in other systems, with liver injury as a well-recognized complication (4).
As one of the most important organs in the human body, the liver performs vital physiological functions in aspects such as synthesis, metabolism, detoxification, and immunity. There are many causes of liver injury in children, including infections, drugs, poisoning, autoimmune diseases, and inherited metabolic diseases. Clinically, symptoms such as anorexia, vomiting, and abdominal distension may appear, or they may be accompanied by signs such as jaundice of the skin and sclera, and hepatomegaly (5). Infection is the most common cause of liver injury, and the severity and clinical characteristics of liver injury caused by infections with different pathogens vary. Studies have found that after COVID-19 infection, the incidence and severity of liver injury differ among patients of different ages. Neonates have the mildest liver injury, and the incidence of liver injury in children is much lower than that in adults (6).
At present, the pathogenesis of CAP complicated by liver injury has not been fully clarified. The possible mechanisms are considered as follows. First, CAP-associated liver injury may be directly caused by pathogen infection. Viral infection of hepatocytes and bile duct epithelial cells is the cause of liver injury. Pathology of liver tissue shows hepatocyte degeneration, focal necrosis with neutrophil infiltration, and congestion of hepatic sinusoids (7). Secondly, liver tissue contains a large number of cells involved in immune responses and possesses important immune defense and immune regulation functions. Pathogen infection can trigger a systemic inflammatory response. The increased secretion of inflammatory factors such as interleukin-1, interleukin-6, and tumor necrosis factor-α leads to a cytokine storm, which further results in immune-mediated liver injury (8). In addition, children with pneumonia are often accompanied by wheezing and shortness of breath, and may have hypoxemia. The liver has a high demand for oxygen and is easily affected by hypoxia. A reduction in oxygen supply and lipid accumulation in hepatocytes can lead to hepatocyte death (9).
Currently, limited research exists on the severity of liver injury in children with CAP and the distribution of causative pathogens, with no relevant studies reported in Suzhou. This study examines the etiological findings and liver injury severity of 1,525 children with CAP complicated by liver injury who were hospitalized at the Children's Hospital of Soochow University between January 2018 and December 2022. The study aims to provide clinicians with a clearer understanding of the severity of liver injury in children with CAP and the pathogen distribution patterns, facilitating improved diagnosis and management of affected patients.
Materials and methods
Study subjects
This retrospective study was conducted on children with CAP who received treatment at the Children's Hospital of Soochow University from January 2018 to December 2022. Children were included in the study upon meeting the following criteria: age between >28 days and <18 years; fulfilled the diagnostic criteria for CAP; pathogens were identified in the CAP children; and complete clinical data were available. Children with immunodeficiency, inherited metabolic diseases, other infections including human immunodeficiency virus, hepatotropic viruses, Epstein–Barr virus, and cytomegalovirus were excluded. Based on the presence or absence of liver injury, these CAP children were divided into two groups: CAP without liver injury group and CAP complicated by liver injury group (Figure 1). The study received ethical approval from the Ethics Committee of the Children's Hospital of Soochow University, China (approval number: 2023CS171).
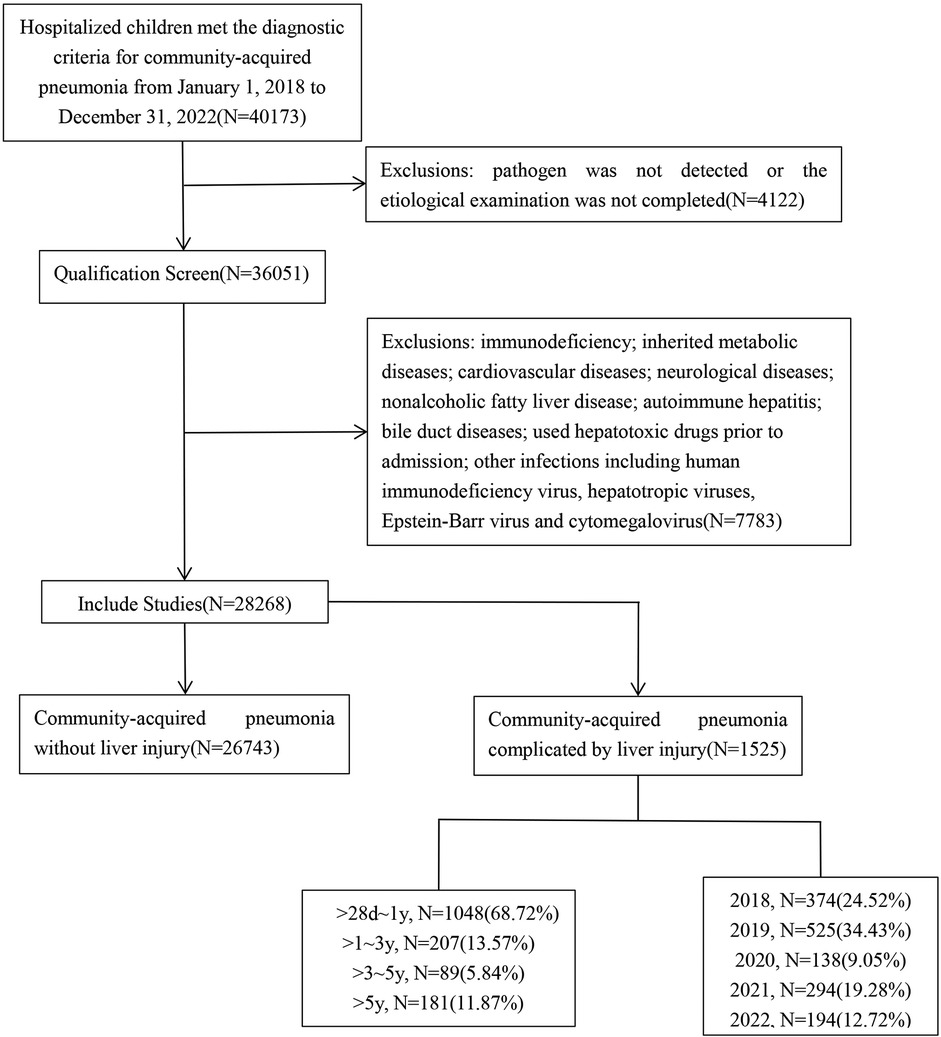
Figure 1. Flowchart of this study. A retrospective study was conducted on children with community-acquired pneumonia (CAP) hospitalized at the Children's Hospital of Soochow University between January 2018 and December 2022, with a total of 40,173 CAP children. According to the inclusion and exclusion criteria, a total of 28,268 children with CAP were enrolled. Based on the presence or absence of liver injury, these 28,268 CAP children were divided into two groups: CAP without liver injury group and CAP complicated by liver injury group.
Diagnostic criteria
Diagnostic criteria for community-acquired pneumonia
The patient presents with respiratory symptoms. Fever, cough, and wheezing are the most common symptoms of CAP. Fixed moist rales can be heard on lung auscultation, and chest imaging suggests pneumonia. Etiological detection can identify the infectious pathogen (10).
Diagnostic criteria for severe pneumonia
Children with CAP are diagnosed with severe pneumonia if they present with one or more of the following conditions: (1) Poor general physical condition; (2) Consciousness disorder; (3) Oxygen saturation <92%, cyanosis or tachypnea, with significantly increased respiratory rate (infants >70 breaths/min, children over 1 year old >50 breaths/min); (4) Ultra-high fever (temperature >41℃) or persistent moderate-high fever for more than 5 days (temperature 38℃–41℃); (5) With dehydration or symptoms of anorexia; (6) Chest radiograph or chest CT shows ≥2/3 infiltrate in one lung, multilobar infiltration, pleural effusion, pneumothorax, atelectasis, pulmonary necrosis, or lung abscess; (7) Occurrence of extrapulmonary complications such as septic shock, and acute renal failure (10).
Diagnostic criteria for liver injury and the degree of severity
Liver injury was defined as an elevation in liver enzyme levels of at least twice the upper limit of normal. An ALT level twice the upper limit of normal and ≤199 U/L indicates mild injury; 200–600 U/L indicates moderate injury; and >600 U/L indicates severe injury (11).
Data collection
The demographic and laboratory characteristics were collected. Demographic characteristics included age, gender. The laboratory values included respiratory etiological detection, MP antibody and liver function tests.
Etiological detection
Nasopharyngeal aspirates collected after admission were analyzed for the presence of respiratory syncytial virus (RSV), human parainfluenza virus (HPIV), and adenovirus (ADV) using direct immunofluorescence. Reverse transcriptase polymerase chain reaction (RT-PCR) was performed to detect influenza A virus (FluA), influenza B virus (FluB), M. pneumoniae, human bocavirus (hBoV), human metapneumovirus (hMPV), and human rhinovirus (HRV). Bacterial detection in nasopharyngeal aspirates was conducted via culture-based methods. Samples were cultured on blood agar plates following clinical standard culture procedures, and bacterial species were identified by quantitative analysis.
Statistical analysis
Statistical analyses were conducted using SPSS 25.0 software (IBM, Armonk, NY, USA). Data were presented as the number [n (%)], with categorical data analyzed using the chi-square, continuity correction or Fisher's exact test, as appropriate. Non-normally distributed data were expressed as medians (interquartile ranges). Comparisons of quantitative variables across different groups were performed using the Mann–Whitney U-test and Kruskal–Wallis H-test. A p-value <0.05 was considered statistically significant.
Results
Children characteristics
A total of 28,268 children with CAP were included in the study. In these CAP children, 1,525 had extrapulmonary complications with liver injury. Among them, 896 were males (58.75%), and 629 were females (41.25%), resulting in a male-to-female ratio of 1.4:1. The youngest child was 1 month old, while the oldest was 204 months old. The median age was 6 months (interquartile range: 3–12 months). Age distribution analysis showed that 1,048 children (68.72%) were aged >28 days to 1 year, 207 (13.57%) were aged >1–3 years, 89 (5.84%) were aged >3–5 years, and 181 (11.87%) were aged >5 years (Figure 1).
Pathogenic characteristics in children with CAP complicated by liver injury across age groups
The >28 days to 1-year age group had the highest number of cases, while the >3–5-year age group had the least. In the >28 days to 1-year group, viral infections were the most prevalent, and M. pneumoniae infections were the least common. As age increased, the proportions of viral and bacterial infections gradually declined, while the proportion of M. pneumoniae infections increased. There were differences in pathogen distribution among different age groups (p < 0.001) (Table 1). Within each age group, the predominant bacterial pathogen varied. In the >28 days to 1-year group, Staphylococcus aureus (S. aureus) was the most frequently detected pathogen. In the other three age groups, Streptococcus pneumoniae (S. pneumoniae) was the most frequently detected pathogen. RSV was the most prevalent viral infection in the >28 days to 1-year and >1–3-year groups. ADV, FluA, and FluB had the highest detection rates in the >3–5-year group, while FluB was the most frequently detected in the >5-year group. The constituent ratio of the number of cases of S. aureus, Hemophilus influenzae (H. influenzae), RSV, HPIV, and M. pneumoniae varied significantly across the different age groups (p < 0.05) for the four CAP age groups with liver injury (Figure 2).
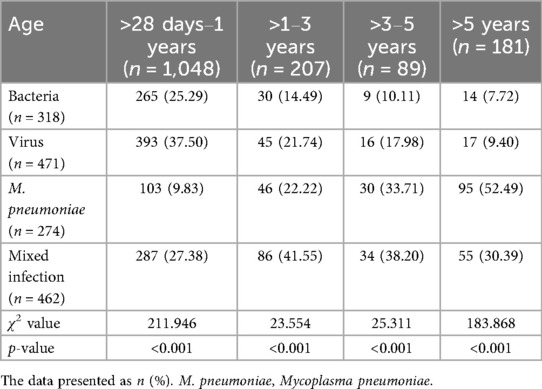
Table 1. Pathogen detection in children with community-acquired pneumonia complicated by liver injury across various age groups.

Figure 2. Pathogen detection in children with community-acquired pneumonia complicated by liver injury. (A–D) Constituent ratio of pathogens, (E–H) Number of pathogens. *p < 0.05, **p < 0.01, ***p < 0.001, ns, no significant difference. S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; P. aeruginosa, Pseudomonas aeruginosa; RSV, respiratory syncytial virus; hBoV, human bocavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; ADV, adenovirus; HPIV, human parainfluenza virus; FluA, influenza A virus; FluB, influenza B virus; M. pneumoniae, Mycoplasma pneumoniae.
Characteristics of mixed pathogen infections
Children with CAP complicated by liver injury in each age group exhibited mixed infections, with a total of 462 cases (30.30%). The highest proportion of mixed infections was observed in children aged >1–3 years (Table 1). The distribution of mixed infections varied significantly among different age groups (p < 0.001) (Figure 2). There were differences in the distribution of different types of mixed infections in the age groups of >28 days to 1 year and >5 years (p < 0.001) (Table 2). In the >28 days to 1-year, >1–3-year, and >3–5-year groups, bacteria-virus mixed infections were the most common. However, in the >5-year group, virus-M. pneumoniae mixed infections were the most prevalent (Table 2). Among children with bacteria-virus mixed infections in the >28 days to 1-year group, the most frequently observed combinations included RSV mixed with S. aureus or S. pneumoniae, or H. influenzae. Among children with virus-M. pneumoniae mixed infections in the >5-year group, the most common combinations involved M. pneumoniae mixed with HRV or HPIV.
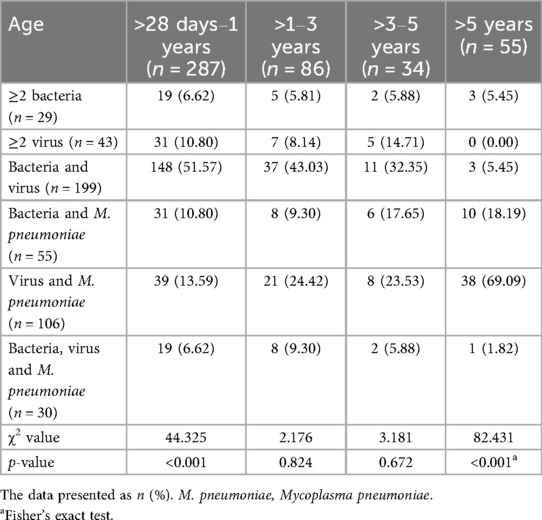
Table 2. Mixed infections in children with community-acquired pneumonia complicated by liver injury across various age groups.
Pathogen distribution characteristics in children with CAP and those with liver injury
There were differences in the constituent ratios of pathogen infections among children with CAP across different age groups (p < 0.05) (Figure 3). The pathogen distribution varied between children with CAP and those with CAP complicated by liver injury. In the group aged >28 days to 1 year, there were differences in the constituent ratios of bacteria, M. pneumoniae, mixed infections, S. aureus, Moraxella catarrhalis (M. catarrhalis), ≥2 bacterial infections, ≥2 viral infections, as well as bacteria-virus, bacteria-M. pneumoniae, and bacteria-virus-M. pneumoniae mixed infections between children with CAP and those with CAP complicated by liver injury. In the >1–3-year group, there were differences in bacteria-virus-M. pneumoniae mixed infections between the children with CAP and those with CAP complicated by liver injury. In the >5-year group, there were differences in the constituent ratios of bacteria, M. pneumoniae, H. influenzae, FluB, ≥2 viruses, and bacteria-virus mixed infections between the children with CAP and those with CAP complicated by liver injury (p < 0.05). For other pathogen infections, no differences were observed between children with CAP and those with CAP complicated by liver injury in any age group (Figure 4).
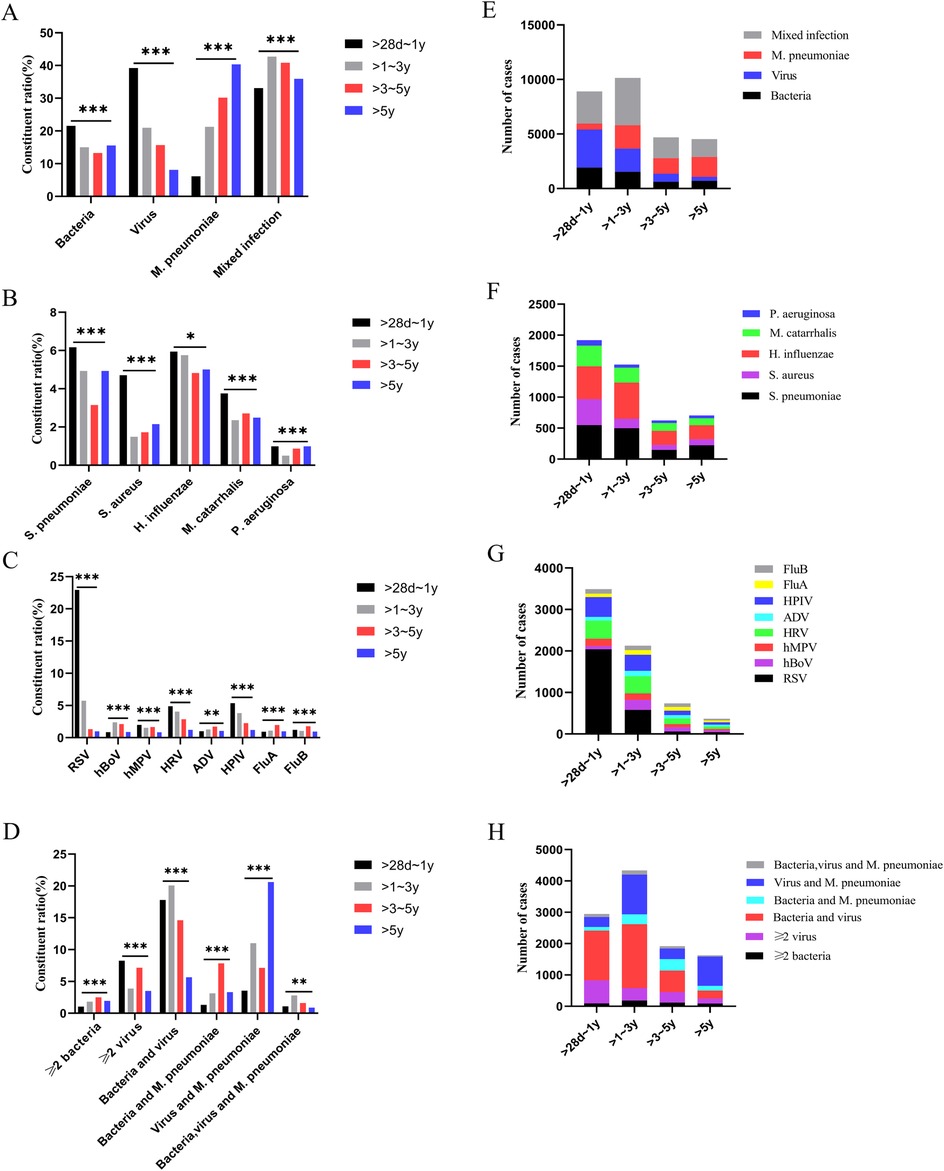
Figure 3. Pathogen detection in children with community-acquired pneumonia. (A–D) Constituent ratio of pathogens, (E–H) Number of pathogens. *p < 0.05, **p < 0.01***, p < 0.001. S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; P. aeruginosa, Pseudomonas aeruginosa; RSV, respiratory syncytial virus; hBoV, human bocavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; ADV, adenovirus; HPIV, human parainfluenza virus; FluA, influenza A virus; FluB, influenza B virus; M. pneumoniae, Mycoplasma pneumoniae.
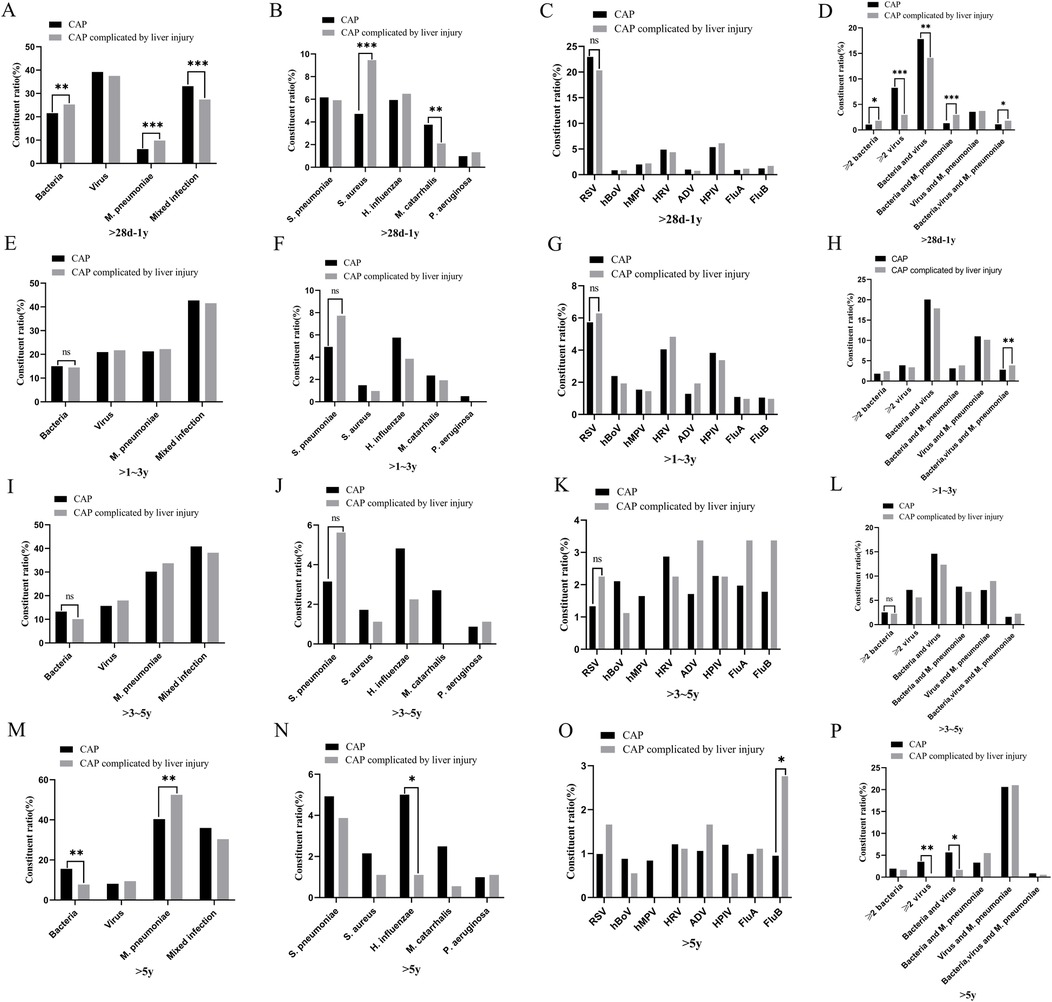
Figure 4. Distribution of pathogens in children with community-acquired pneumonia and those with liver injury. (A–D) >28 days–1-year group, (E–H) >1–3-year group, (I–L) >3–5-year group, (M–P) >5-year group. *p < 0.05, **p < 0.01***, p < 0.001, ns, no significant difference. S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; P. aeruginosa, Pseudomonas aeruginosa; RSV, respiratory syncytial virus; hBoV, human bocavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; ADV, adenovirus; HPIV, human parainfluenza virus; FluA, influenza A virus; FluB, influenza B virus; M. pneumoniae, Mycoplasma pneumoniae.
Liver injury incidence in children with CAP
The incidence of liver injury was 11.77% in the >28 days to 1-year age group, which was significantly higher than that in the other three age groups (p < 0.001) (Figure 5). In this group (>28 days to 1 year), the highest incidence of liver injury (26.27%) was observed in children with mixed bacterial, and M. pneumoniae infection. In the >1–3-year group, the highest incidence of liver injury (6.06%) occurred in children with mixed bacterial, viral, and M. pneumoniae infections. In the >5-year group, children with FluB had the highest incidence of liver injury (11.9%) (p < 0.05) (Figure 5).

Figure 5. Incidence of liver injury in children with community-acquired pneumonia across different age groups and pathogen types. (A) Incidence of liver injury in children with community-acquired pneumonia across different age groups ***p < 0.001; (B) Incidence of liver injury in community-acquired pneumonia caused by different pathogens in the >28 days to 1-year group *p < 0.05; (C) Incidence of liver injury in community-acquired pneumonia caused by different pathogens in the >1–3-year group *p < 0.05; (D) Incidence of liver injury in community-acquired pneumonia caused by different pathogens in the >3–5-year group, ns, no significant difference; (E) Incidence of liver injury in community-acquired pneumonia caused by different pathogens in the >5-year group *p < 0.05. S. pneumoniae, Streptococcus pneumoniae; S. aureus, Staphylococcus aureus; H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; P. aeruginosa, Pseudomonas aeruginosa; RSV, respiratory syncytial virus; hBoV, human bocavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; ADV, adenovirus; HPIV, human parainfluenza virus; FluA, influenza A virus; FluB, influenza B virus; M. pneumoniae, Mycoplasma pneumoniae.
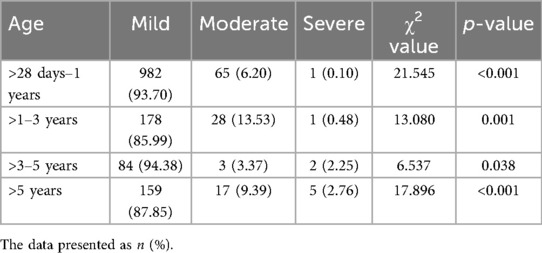
Liver function in children with CAP complicated by liver injury
Among the 1,525 children with CAP complicated by liver injury, 1,403 (92%) had mild elevation of transaminases, 113 (7.41%) had moderate elevation of transaminases, and 9 (0.59%) had severe elevation of transaminases. There were differences in the distribution of liver injury severity within the same age group (p < 0.05) (Table 3). The ALT values varied across different age groups. Children in the >1–3-year, >3–5-year, and >5-year groups had higher ALT levels than those in the >28 days to 1-year group [100.5 (78.5, 138) U/L vs. 92 (79.5, 138) U/L vs. 98.5 (81, 139) U/L vs. 86 (76, 109) U/L]. However, there were no significant differences in ALT values among the >1–3-year, >3–5-year, and >5-year groups (Figure 6A). The children were categorized into four groups based on infectious pathogens, with their ALT values showing significant differences. The M. pneumoniae-infected group had significantly higher ALT levels than the other three groups. In comparison, the mixed infection group had higher ALT levels than both the bacterial infection and viral infection groups [99.5 (81, 140) U/L vs. 92.5 (78, 127) U/L vs. 84 (74, 103.5) U/L vs. 87 (76.5, 107) U/L] (Figure 6D). No differences were observed in the aspartate transaminase (AST) and total bilirubin (TBIL) values among children with CAP complicated by liver injury across different age groups or pathogen infection types (Figure 6).
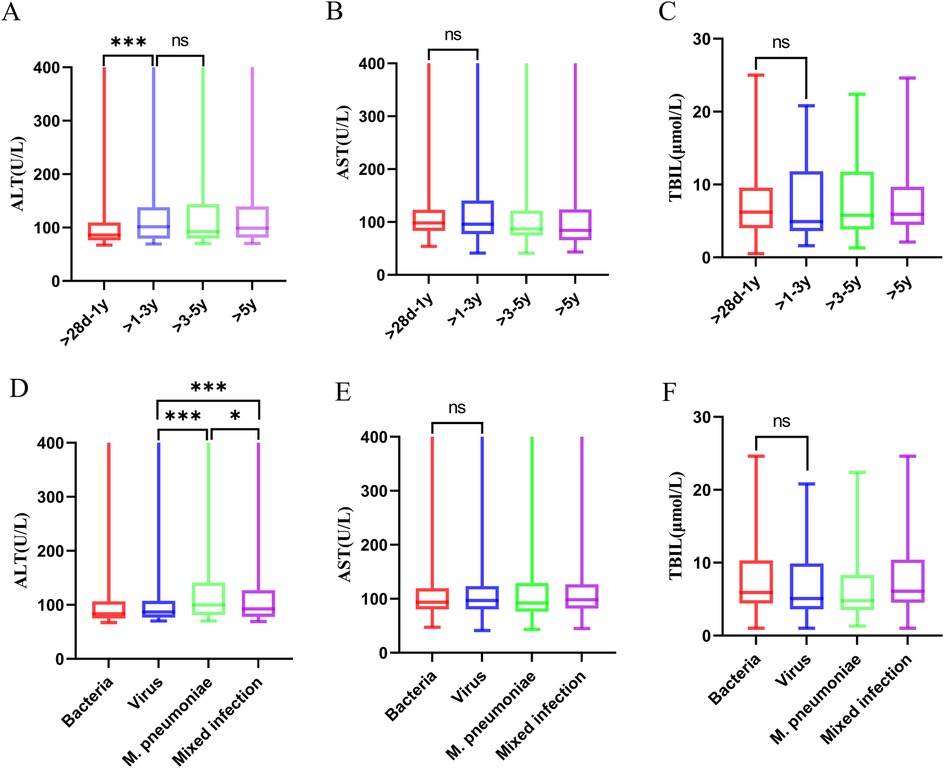
Figure 6. ALT, AST, and TBIL levels across different groups of children with community-acquired pneumonia complicated by liver injury. (A–C) ALT, AST, and TBIL levels across different age groups of children with community-acquired pneumonia complicated by liver injury ***p < 0.001, ns, no significant difference. (D–F) ALT, AST, and TBIL levels in different pathogen infection groups of children with community-acquired pneumonia complicated by liver injury. *p < 0.05, ***p < 0.001, ns, no significant difference. ALT, alanine aminotransferase; AST, aspartate transaminase; TBIL, total bilirubin; MP, Mycoplasma pneumoniae.
Characteristics of patients with non-severe or severe community-acquired pneumonia complicated by liver injury
As shown in Table 4, the levels of ALT was higher in children with SCAP compared to those with non-severe community-acquired pneumonia (NSCAP) (p = 0.005). The incidence of liver injury is higher in SCAP children (p < 0.001). Other clinical and laboratory tests displayed no significant difference.
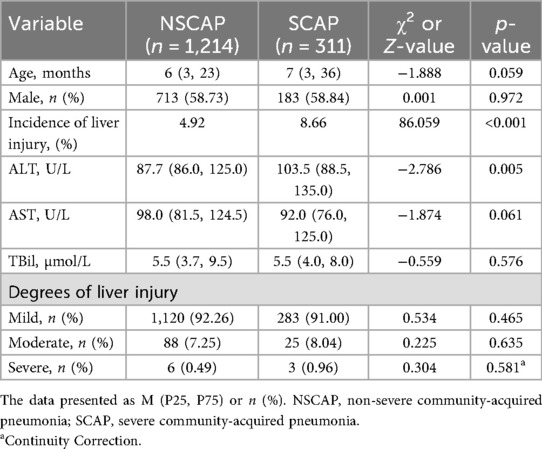
Table 4. Clinical and laboratory characteristics of non-severe and severe community-acquired pneumonia children.
Discussion
CAP is an infection affecting the lung parenchyma and/or interstitium and typically presents with fever, cough, and polypnea. Diagnosis is confirmed through pulmonary imageological examination (12). In addition to respiratory manifestations, extrapulmonary complications are not uncommon in children with CAP, with liver injury being one of the recognized complications (6). Liver injury in CAP occurs due to hepatocyte damage and increased membrane permeability, leading to the release of intracellular enzymes into the bloodstream and a subsequent elevation of liver enzyme levels. ALT is predominantly found in hepatocytes and has the highest intracellular concentration among liver enzymes. When hepatocytes undergo degeneration and necrosis, increased membrane permeability results in ALT release into the bloodstream, making it the most direct and sensitive indicator of liver dysfunction (13). Therefore, this study utilizes serum ALT levels as an indicator to assess the severity of liver injury in children with CAP.
In recent years, several studies have explored the etiological and epidemiological characteristics of CAP (14, 15). However, there is limited information regarding the severity of CAP-associated liver injury and pathogen distribution. In this study, we conducted a retrospective analysis of 1,525 children with CAP complicated by liver injury who were hospitalized at the Children's Hospital of Soochow University between January 2018 and December 2022. This study found that the number of CAP children complicated by liver injury decreased from 2020 to 2022 compared with the two prior years (Figure 1). Notably, the number of affected children in 2020 was significantly lower than in the other four years. This may be related to the varying annual incidence rates of children with CAP complicated by liver injury in the Suzhou region. Following the implementation of prevention and control measures in January 2020 due to the COVID-19 pandemic, there was a significant decline in the mobility and gatherings of people in Suzhou. Additionally, the use of masks in public areas by both travelers and children greatly diminished the risk of cross-infection, contributing to a decline in CAP prevalence. Following the reduction in the prevention and control response level, there was a rise in public gatherings, which led to a corresponding increase in children with CAP. Consequently, the number of children diagnosed with CAP complicated by liver injury increased in 2021 and 2022 (16, 17).
The exact mechanism of CAP-associated liver injury remains unclear. However, it is believed to result from direct pathogen invasion, indirectly by systemic inflammatory responses, and hepatocyte hypoxia, all of which contribute to liver injury (18–20). Among the 1,525 children with CAP complicated by liver injury, the >28 days to 1-year age group had the highest number of cases. Additionally, the incidence of liver injury in this age group was significantly higher than that in the other three age groups. This may be attributed to the relatively low immune function and immature liver development in this age group, making them more susceptible to pathogen-induced liver injury. Therefore, the first year of life represents a critical period for liver function monitoring in children with CAP. Early detection of liver dysfunction allows for timely intervention and symptomatic treatment to prevent further complications.
This study found that the infectious pathogens in children with CAP vary across different age groups. In the >28 days to 1-year group, viral infections were the most prevalent, whereas M. pneumoniae infections were the least common. As age increased, the proportion of viral, and bacterial infections gradually decreased while the proportion of M. pneumoniae infections increased. These findings align with previous studies on pathogen distribution in children with CAP (21, 22). For CAP caused by bacterial infections, H. influenzae, and S. pneumoniae were the two most common pathogens, while RSV, HRV, and HPIV were the most frequently detected viruses in viral pneumonia (23, 24). However, the characteristics of pathogen distribution in children with CAP complicated by liver injury in the Suzhou area differed from those of general children with CAP. In the >28 days to 1-year group, there were differences in the constituent ratios of S. aureus, M. catarrhalis, M. pneumoniae, and various mixed infections between the children with CAP and those with CAP complicated by liver injury. In children aged >1–3 years, a significant difference was observed in the constituent ratio of bacterial, viral, and M. pneumoniae mixed infections between children with CAP alone and those with CAP complicated by liver injury. In children aged >5 years, differences were noted in the constituent ratios of H. influenzae, M. pneumoniae, FluB, ≥2 viral infections, and bacteria-virus mixed infections between both groups. These findings indicate that the incidence of liver injury varies by pathogen type and age group. In the >28 days to 1-year group, bacterial, and M. pneumoniae mixed infections had the highest incidence of liver injury. In the >1–3-year group, bacterial, viral, and M. pneumoniae mixed infections were associated with the highest incidence of liver injury. In the >5-year group, FluB infection had the highest incidence of liver injury. Therefore, special attention should be paid to liver function in children with CAP who are infected with the above-mentioned pathogens in each age group.
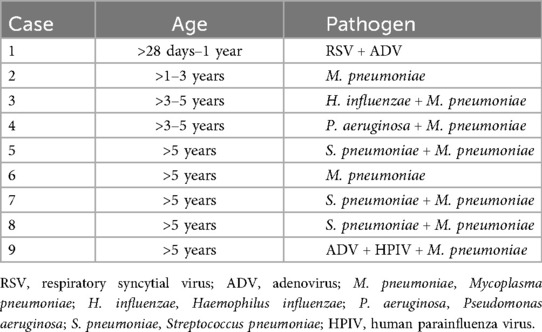
Among the 1,525 children with CAP complicated by liver injury, 1,403 had mild elevation of transaminases, while only a small number exhibited severe liver injury. The liver injury observed in these children was of the hepatocellular type, with bilirubin levels remaining within the normal range, indicating no impairment in bile excretion. Importantly, none of the children progressed to liver failure. These findings suggest that, in clinical practice, most children with CAP children experience only a mild liver injury. After treatment targeting the primary disease, the children recovered well.
This study demonstrated that ALT values among children with CAP complicated by liver injury varied across different age groups. Children in the >1–3-year, >3–5-year, and >5-year groups had higher ALT levels than those in the >28 days to 1-year group. This study also revealed that ALT values vary depending on the infectious pathogens in children with CAP. Children infected with M. pneumoniae had significantly higher ALT levels than those in the other three groups. Additionally, the ALT levels in the mixed infection group were higher than in the bacterial and viral infection groups. In the >28 days to 1-year group, most children were infected with viruses or bacteria, which correlated with milder liver injury compared to older children. Among the 1,525 children in this study, mild elevation of transaminases were the most common, with only nine cases of severe liver injury. An analysis of the pathogen characteristics in these nine children indicated that seven cases involved mixed infections, and eight were accompanied by M. pneumoniae infection (Table 5). Therefore, greater clinical vigilance is required for CAP accompanied by M. pneumoniae infection, as there is a possibility of severe liver injury in these children. Routine liver function tests should be performed to ensure early detection and timely intervention. This study found that children with SCAP are more likely to have elevated transaminases, and the ALT levels in the SCAP group are higher than those in the NSCAP group. Therefore, it is speculated that liver injury may be related to the severity of CAP.
This study has some limitations. First, this is a single-center retrospective study, with a relatively small number of children with CAP complicated by liver injury due to certain pathogens. Second, pathogen detection specimens were collected from nasopharyngeal aspirates, which may not fully represent the causative pathogens of pulmonary infections. Third, this study focused on liver injury in children with CAP infected with common pathogens. Future research should involve multi-center, large-sample prospective clinical studies to validate these findings. Additionally, conducting more extensive pathogen screening and monitoring the outcome of liver injury in children with CAP complicated by liver injury can provide more comprehensive clinical guidance.
Conclusion
Liver injury is a recognized extrapulmonary complication in children with CAP, with mild elevation of transaminases being the most common presentation. The main manifestation was hepatocellular injury rather than cholestatic injury. It occurred most frequently in children with CAP aged >28 days to 1 year. All children basically healed well, none of them progressed to liver failure. Pathogen distribution in CAP complicated by liver injury varied across different age groups. Clinically, greater vigilance is required in M. pneumoniae infection, as children with CAP infected by M. pneumoniae may be at a higher risk of severe liver injury. Children with SCAP were more prone to liver injury. Although liver injury associated with CAP is often transient and reversible, healthcare workers need to monitor liver function and strengthen supportive treatment. Therefore, in clinical practice, active liver function monitoring of children with CAP should be an essential component of clinical management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Children's Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
KW: Writing – original draft. ZL: Writing – original draft. FC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Medical Research Project of Jiangsu Provincial Health Commission (Z2023060), “Scientific and Educational Prosperity” Youth Science and Technology Project of Suzhou (KJXW2022020) and Suzhou Applied Basic Research (Medical and Health) Science and Technology Innovation Project (SYWD2024119).
Acknowledgments
The authors wish to thank the laboratory of the Children's Hospital of Soochow University that provided the data for liver function tests, MP antibody detection, and etiological detection of nasopharyngeal aspirates.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shan W, Shi T, Chen K, Xue J, Wang Y, Yu J, et al. Risk factors for severe community-aquired pneumonia among children hospitalized with CAP younger than 5 years of age. Pediatr Infect Dis J. (2019) 38(3):224–9. doi: 10.1097/INF.0000000000002098
2. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388(10063):3027–35. doi: 10.1016/S0140-6736(16)31593-8
3. Meyer Sauteur PM. Childhood community-acquired pneumonia. Eur J Pediatr. (2024) 183(3):1129–36. doi: 10.1007/s00431-023-05366-6
4. Chee E, Huang K, Haggie S, Britton PN. Systematic review of clinical practice guidelines on the management of community acquired pneumonia in children. Paediatr Respir Rev. (2022) 42:59–68. doi: 10.1016/j.prrv.2022.01.006
5. Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. (2017) 27(21):R1147–51. doi: 10.1016/j.cub.2017.09.019
6. Sadeghi Dousari A, Hosseininasab SS, Sadeghi Dousari F, Fuladvandi M, Satarzadeh N. The impact of COVID-19 on liver injury in various age. World J Virol. (2023) 12(2):91–9. doi: 10.5501/wjv.v12.i2.91
7. Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. (2020) 72(5):1864–72. doi: 10.1002/hep.31480
8. Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. (2018) 36:247–77. doi: 10.1146/annurev-immunol-051116-052415
9. Guo X, Zhu D, Chen H. Clinical features and risk factors of liver injury in patients with Chlamydia psittaci pneumonia-a retrospective analysis. Front Cell Infect Microbiol. (2024) 13:1320758. doi: 10.3389/fcimb.2023.1320758
10. National Health Commission of the People’s Republic of China, State Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of community-acquired pneumonia in children (2019 version). Chin J Clin Infect Dis. (2019) 12(1):6–13. Chinese. doi: 10.3760/cma.j.issn.1674-2397.2019.01.002
11. Green RM, Flamm S. AGA Technical review on the evaluation of liver chemistry tests. Gastroenterology. (2002) 123(4):1367–84. doi: 10.1053/gast.2002.36061
12. Liu YN, Zhang YF, Xu Q, Qiu Y, Lu QB, Wang T, et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. (2023) 4(5):e330–9. doi: 10.1016/S2666-5247(23)00031-9
13. Zhang L, Zhao S. Severe liver injury affects the outcomes and length of hospital stay in children with community-acquired pneumonia. Afr Health Sci. (2022) 22(3):578–89. doi: 10.4314/ahs.v22i3.62
14. Yun KW, Wallihan R, Desai A, Alter S, Ambroggio L, Cohen DM, et al. Clinical characteristics and etiology of community-acquired pneumonia in US children, 2015–2018. Pediatr Infect Dis J. (2022) 41(5):381–87. doi: 10.1097/INF.0000000000003475
15. Roh EJ, Lee MH, Lee JY, Kim HB, Ahn YM, Kim JK, et al. Analysis of national surveillance of respiratory pathogens for community-acquired pneumonia in children and adolescents. BMC Infect Dis. (2022) 22(1):330. doi: 10.1186/s12879-022-07263-z
16. Tang X, Dai G, Jiang X, Wang T, Sun H, Chen Z, et al. Clinical characteristics of pediatric respiratory tract infection and respiratory pathogen isolation during the coronavirus disease 2019 pandemic. Front Pediatr. (2022) 9:759213. doi: 10.3389/fped.2021.759213
17. Qian C, Chen Q, Lin W, Li Z, Zhu J, Zhang J, et al. Incidence of community-acquired pneumonia among children under 5 years in Suzhou, China: a hospital-based cohort study. BMJ Open. (2024) 14(1):e078489. doi: 10.1136/bmjopen-2023-078489
18. Wang Y, Jian S, Li W, Zhao L, Ye G, Shi F, et al. Epigallocatechin-3-gallate ameliorates liver injury secondary to Pseudomonas aeruginosa pneumonia. Int Immunopharmacol. (2022) 112:109239. doi: 10.1016/j.intimp.2022.109239
19. Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. (2021) 41(1):20–32. doi: 10.1111/liv.14730
20. Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. (2018) 30(4):380–7. doi: 10.1097/BOR.0000000000000494
21. Su DQ, Huang HL, Zhuo ZQ. Pathogen distribution and bacterial resistance in children with severe pneumonia: a single-center retrospective study. Medicine (Baltimore). (2021) 100(35):e27128. doi: 10.1097/MD.0000000000027128
22. Suh JH, Ahn B, Song SH, Choi S, Choi SH, Lee H, et al. Etiology and clinical characteristics of community-acquired pneumonia in Korean children during the Pre-COVID-19 period, 2015–2020. J Korean Med Sci. (2023) 38(43):e339. doi: 10.3346/jkms.2023.38.e339
23. Rueda ZV, Aguilar Y, Maya MA, López L, Restrepo A, Garcés C, et al. Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. (2022) 22(1):169. doi: 10.1186/s12887-022-03235-z
Keywords: children, community-acquired pneumonia, liver injury, pathogen distribution, severity
Citation: Wang K, Li Z and Cheng F (2025) Pathogen distribution and liver injury severity in children with community-acquired pneumonia complicated by liver injury in Suzhou, China. Front. Pediatr. 13:1665002. doi: 10.3389/fped.2025.1665002
Received: 13 July 2025; Accepted: 2 September 2025;
Published: 15 September 2025.
Edited by:
Jiehao Cai, Fudan University, ChinaReviewed by:
Naghmeh Satarzadeh, Stem cells and Regenerative Medicine Innovation Center Kerman University of Medical Science, IranAmin Sadeghi Dousari, Kerman University of Medical Sciences, Iran
Copyright: © 2025 Wang, Li and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangfang Cheng, ZmFuZ2ZhbmdfY2hlbmdAMTI2LmNvbQ==
 Kun Wang
Kun Wang ZhengJiayi Li2
ZhengJiayi Li2 Fangfang Cheng
Fangfang Cheng