- 1Women and Children’s Health Program, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
- 2Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 3Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
- 4Applied Health Research Centre, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
- 5Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Objective: To evaluate the risk of cancer after phototherapy for neonatal hyperbilirubinemia.
Study design: This was a systematic review and meta-analysis. Electronic databases, including PubMed, Embase, and Cochrane Library, were searched. Prospective and retrospective studies, case series, and review studies published between 1970 and 2025 were included. Studies underwent two stages of screening. The first phase was title and abstract screening. The second phase was a full-text review of studies deemed to meet the inclusion criteria. Risk of bias was assessed using ROBINS-E. Inverse-variance weighted multi-level random effects models were used for all analyses.
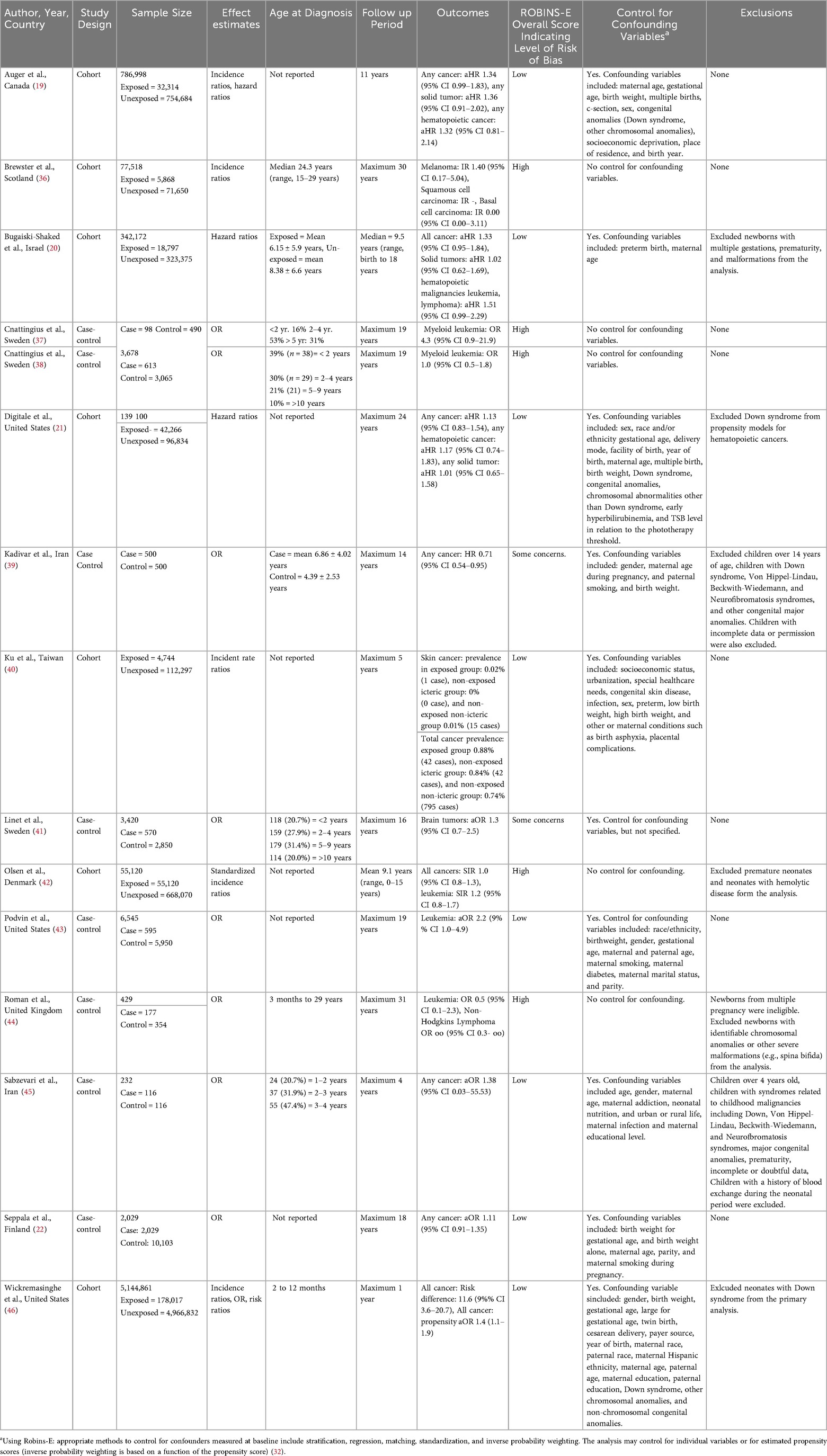
Results: This systematic review and meta-analysis included 15 studies. Risk of bias was low in eight studies, one study was judged to have some concerns, and six studies were determined to have a high risk of bias. A total of 6,675,265 patient data points were included. Studies ranged from 1995–2022, with an age group from 35 weeks to 31 years old. Overall, there was an estimated 24% increased odds of cancer for those who received phototherapy compared to those who did not [OR = 1.24; 95% CI: (1.12, 1.36); p < 0.001].
Conclusions: Phototherapy for neonatal hyperbilirubinemia was associated with a small increased risk of cancer up to age 31 years. This association must be balanced by the well-understood risk of Bilirubin-Induced Neurologic Dysfunction.
Introduction
Phototherapy is the gold standard treatment for neonatal hyperbilirubinemia. In 2022, the American Academy of Pediatrics published revised clinical practice guidelines with increased thresholds for the use of phototherapy for term and near-term infants. Subsequently, a large US hospital network of 22,000 newborns reported a 47% decrease in phototherapy usage since the new guidelines were published, with 2% of newborns receiving phototherapy (1). European studies reported higher rates with close to 4% of neonates receiving phototherapy (2, 3). Phototherapy decreases serum levels of unconjugated bilirubin by changing the conformation of the bilirubin molecule to excretable water-soluble isomers. Without treatment, acute hyperbilirubinemia may lead to Bilirubin-Induced Neurologic Dysfunction (BIND). BIND is the constellation of neurologic signs and symptoms that include acute bilirubin encephalopathy and may result in the sequelae of chronic bilirubin encephalopathy, hearing loss, and visuo-oculomotor disturbances (4–6). Animal and cell culture studies have identified concerns around phototherapy-induced DNA damage, raising questions around a potential cancer risk (7–18). However, its potential as a carcinogen has been investigated in numerous observational studies with mixed results (19–22).
Previous meta-analyses evaluating cancer risk after exposure to phototherapy in the neonatal period have shown a small positive association (23–26), however these meta-analyses analyzed fewer studies than the present one, either because they separated cohort and case control studies (23, 24), or excluded studies included within their systematic reviews from their primary analyses without sufficient explaination (25, 26). The aim of our study was to perform an updated, inclusive, and focused systematic review and meta-analysis evaluating the association between phototherapy in the neonatal period and cancer risk, excluding benign and dysplastic nevi, using weighted analysis, and including only studies with appropriate tests of association or sufficient detail for re-analysis, to increase the reliability of our findings.
Methods
Study design
This is a systematic review and meta-analysis which followed The Cochrane Handbook for Systematic Reviews (27) and the PRISMA 2020 reporting guidelines for reporting Systematic Reviews and Meta-Analyses (28).
Search process
A literature search of electronic databases including: Medline, Embase, Cochrane Central, CINAHL, and Scopus was performed on March 28, 2022 and June 18, 2025. The search strategy underwent a review process in accordance with the Peer Review for Electronic Search Strategies (PRESS) guidelines (29). The search terms and keywords used covered three main concepts, “Phototherapy,” “Neonates,” and “Cancer,” including any of these concepts' synonymous words. The full search strategy can be found in the Supplementary Data.
Inclusion criteria
We included human studies published in English between 1970 and 2025, with neonates of all gestational ages who underwent phototherapy within 28 days of age. Peer-reviewed observational studies, including prospective and retrospective studies, case series studies, and review studies were included.
Exclusion criteria
Studies were excluded if they were theoretical studies based on human cells, in vitro and/or animal studies, studies where phototherapy was the outcome variable, studies where the outcome was not the impact of phototherapy, studies where the outcomes were benign and/or dysplastic nevi, or studies which did not report the side effects of phototherapy. Additionally, one study was excluded because it represented a duplicate sample from another included study.
Outcome
Our primary outcome was overall cancer risk.
Data extraction
Studies underwent two stages of screening. The first phase was title/abstract screening. The second phase was a full-text review of studies deemed to meet the inclusion criteria. Two independent reviewers were assigned to each phase. Each reviewer independently screened studies, determined the eligibility of papers and decided on inclusion status, with two additional independent reviewers resolving any conflicts among the initial reviewers' decisions. Records and data were managed throughout the review using Covidence (30), a systematic review software, and EndNote (31), a bibliographic software.
Risk of bias assessment
Risk of bias was assessed using Risk of Bias in Non-randomized Studies of Exposures (ROBINS-E) (32). Risk of bias was assessed for seven domains, including: confounding, measurement of the exposure, selection of participants into the study (or into the analysis), post-exposure interventions, missing data, measurement of the outcome, and selection of the reported results (32). Two independent reviewers completed the assessment for each study. The overall score was reported as low, some concerns, or high risk of bias.
Statistical analysis
Inverse-variance weighted multi-level random effects models were used for all analyses. Weights were assigned using the inverse of an effect size's variance (i.e., the squared standard error) to determine the strength of evidence within each study. For example, larger studies with less variance in their estimates tend to contribute more weight in the meta-analyses. These models account for heterogeneity between estimates from different study designs, with random effects explicitly modelled for each individual study in the analyses. For the overall cancer risk analyses, an additional random effect was included for heterogeneity from the type of cancers (“skin”, “blood”, “solid organ”, and “any or other”). Results were pooled on the odds ratio (OR) scale. For studies that reported other metrics, such as standardized incidence ratio estimates, the equivalent OR was estimated from the reported data. For studies with zero cancers in one of the cancer adjusted ORs and standard errors were estimated. Egger's tests (33) were performed, and funnel plots were produced for each outcome to assess potential publication bias. All meta-analyses and re-estimation of individual study effects were performed using the metafor package (34) in R version 4.4.2 (35).
Results
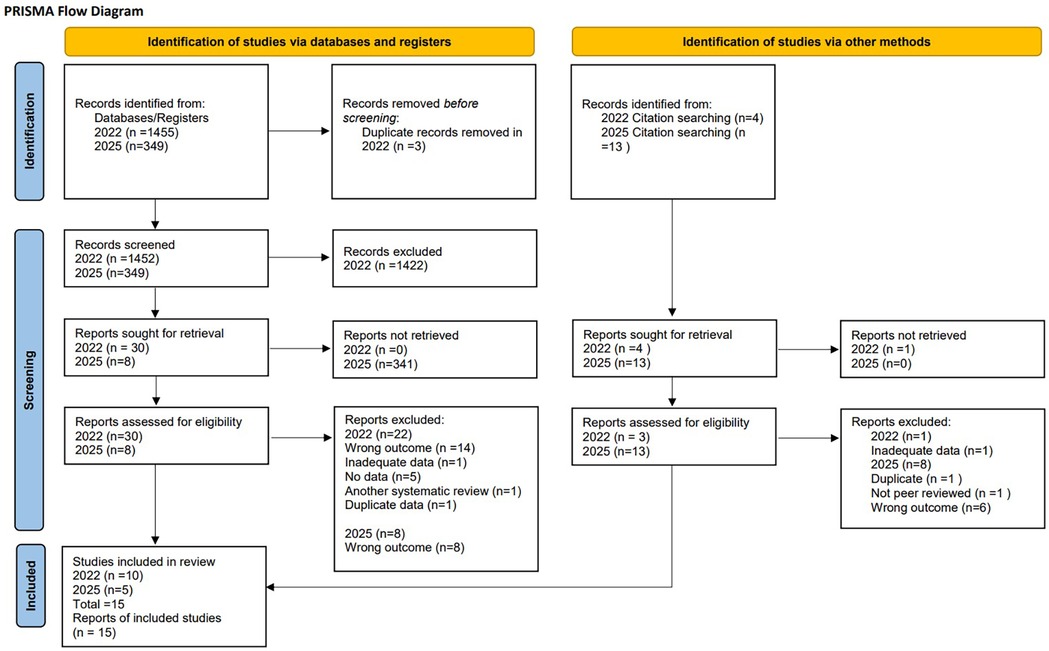
Our systematic review and meta-analysis included 15 studies (19–22, 36–46). See PRISMA flow diagram, Figure 1. A total of 6,675,265 patient data points were included in the analysis through a combination of case-control and retrospective cohort studies. Included studies ranged from 1995–2022, with an age group from 35 weeks to 31 years old, Table 1.
Risk of bias assessment
Using ROBINS-E, eight studies were determined to have a low risk of bias, one study was judged to have some concerns, and six studies were determined to have a high risk of bias based on lack of control for confounding factors (the other domains were assessed as low risk of bias for all studies). Important confounding factors were those where adjustment was expected to lead to an important change in the estimated effect of the exposure (32) (Table 1).
Any cancer risk
Overall, there was an estimated 24% increased odds of cancer for those who received phototherapy compared to those who did not [OR = 1.24; 95% confidence interval (CI): (1.12, 1.36); p < 0.001], Figure 2A. It was estimated that 9.33% of the total variance was due to heterogeneity (I2 = 8.72%). There was insufficient evidence of publication bias in the reporting of any cancer risk (p = 0.45), Figure 3A. Assuming a baseline risk of cancer of (1/463) (47), this estimate would lead to an approximate expected additional 51 cancer diagnoses per 100,000 children treated with phototherapy. This example baseline risk of cancer was taken from Auger et al. (19) and represents the baseline risk over the first 11 years of age.
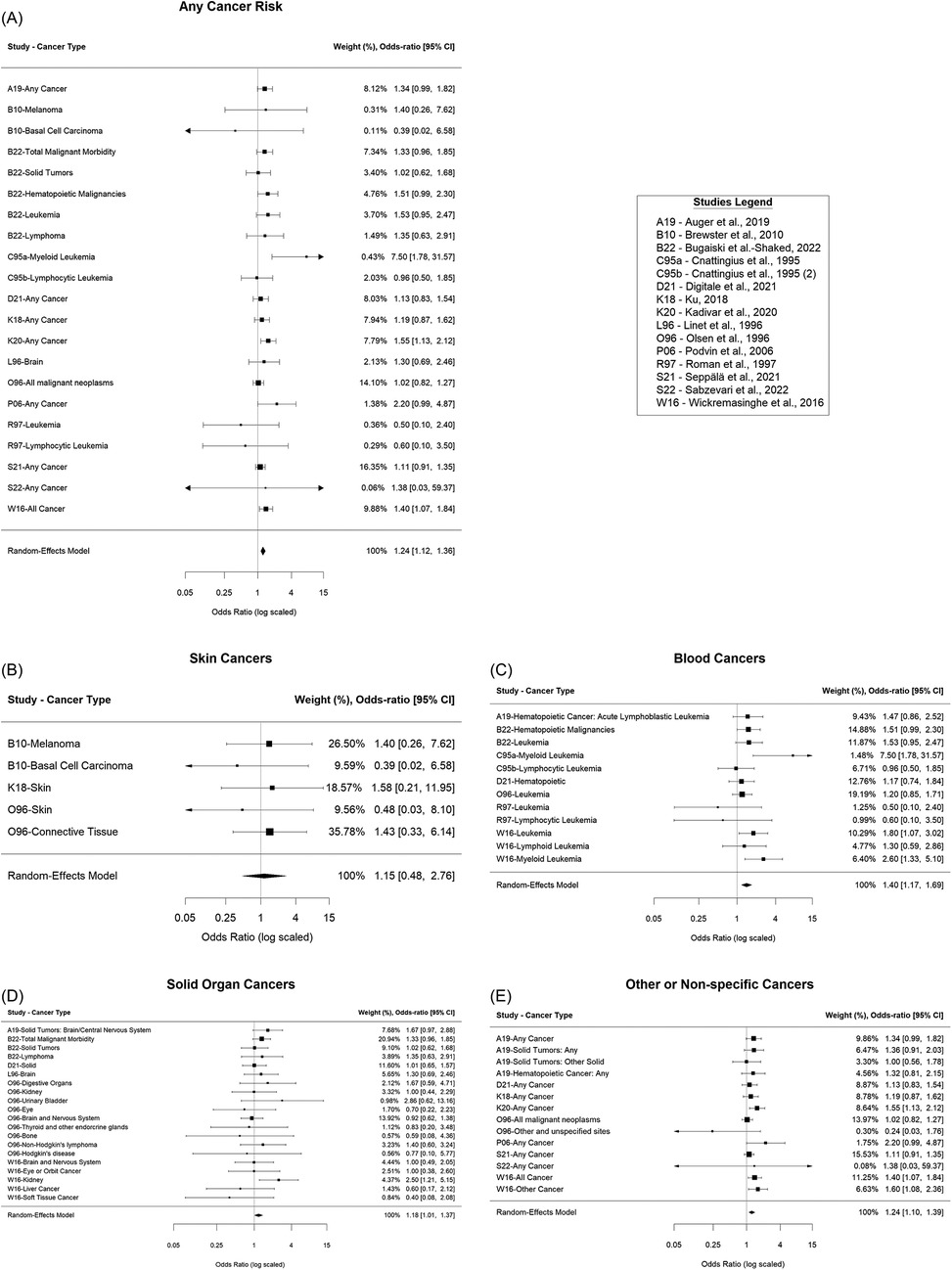
Figure 2. Forest plots. (A) Overall cancer risk, (B) Skin cancer risk, (C) Blood cancer risk, (D) Solid organ cancer risk, (E) Other or non-specific cancer risk.
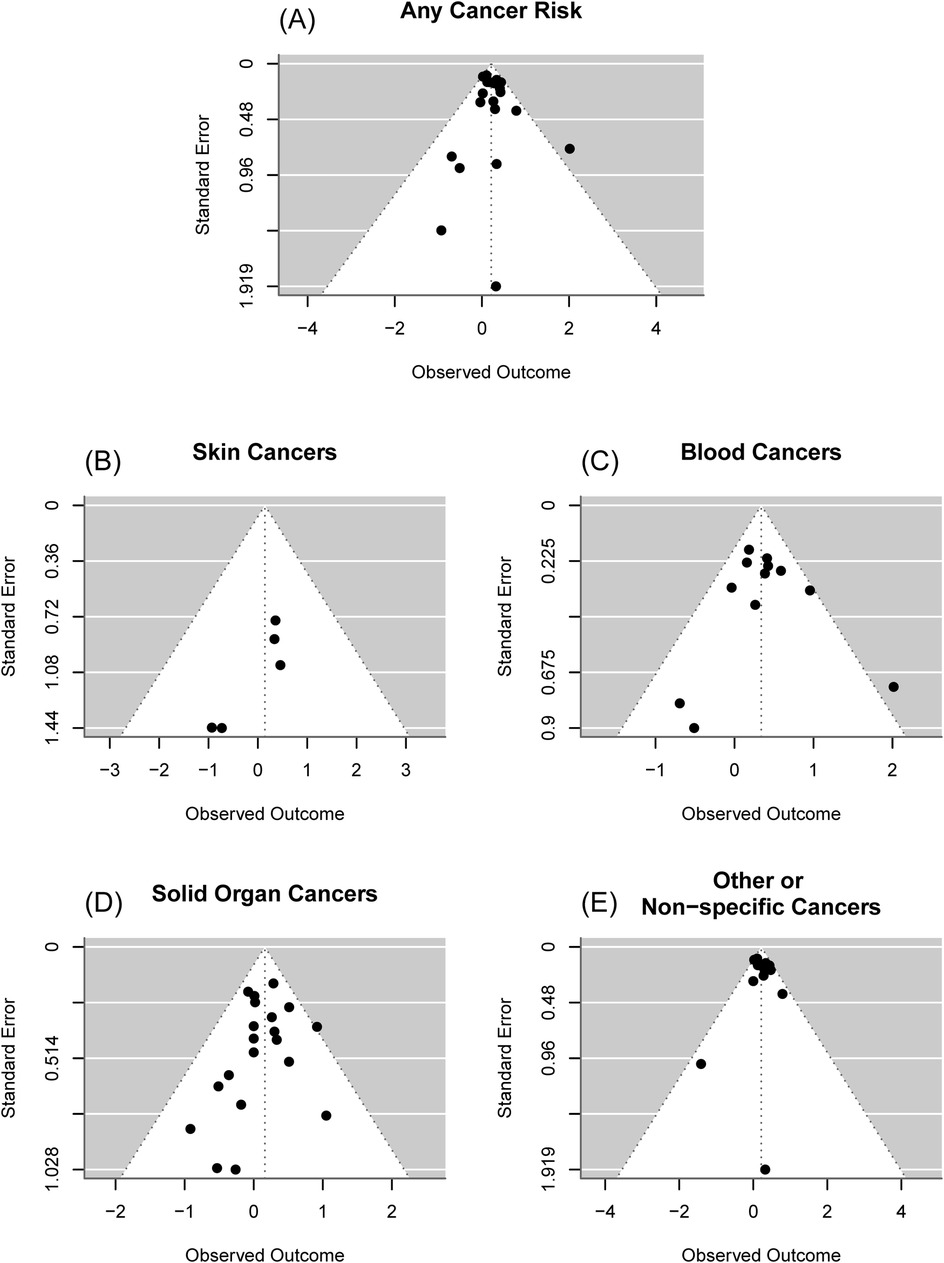
Figure 3. Funnel plots. (A) Overall cancer risk. From an Egger's test, there was insufficient evidence of publication bias in reporting of overall cancer risk (p = 0.98), (B) Skin cancer risk. From an Egger's test, there was insufficient evidence of publication bias in reporting of skin cancer risk (p = 0.34), (C) Blood cancer risk, From an Egger's test, there was insufficient evidence of publication bias in reporting of blood cancer risk (p = 0.73), (D) Solid organ cancer risk. From an Egger's test, there was insufficient evidence of publication bias in reporting of solid organ cancer risk (p = 0.49), (E) Other or non-specific cancer risk. From an Egger's test, there was insufficient evidence of publication bias in reporting of other or non-specific cancer risk (p = 0.73).
Skin cancer risk
For skin cancers, there was insufficient evidence of a difference in the odds of cancer for those who received phototherapy compared to those who did not [OR = 1.15; 95% CI: (0.48, 2.76); p = 0.75], Figure 2B. It was estimated that 0.00% of the total variance was due to heterogeneity (I2 = 0.00%). There was insufficient evidence of publication bias in the reporting of skin cancer risk (p = 0.34), Figure 3B.
Blood cancer risk
For cancers of the blood, there was an estimated 40% increased odds of cancer for those who received phototherapy compared to those who did not [OR = 1.40; 95% CI: (1.17, 1.69); p < 0.001], Figure 2C. It was estimated that 10.36% of the total variance was due to heterogeneity (I2 = 10.36%). There was insufficient evidence of publication bias in the reporting of blood cancer risk (p = 0.73), Figure 3C.
Solid organ cancer risk
For solid organ cancers, there was an estimated 18% increased odds of cancer for those who received phototherapy compared to those who did not [OR = 1.18; 95% CI: (1.01, 1.37); p = 0.04], Figure 2D. It was estimated that 0.00% of the total variance was due to heterogeneity (I2 = 0.00%). There was insufficient evidence of publication bias in the reporting of solid organ cancer risk (p = 0.49), Figure 3D.
Other or non-specific cancer risk
For other or non-specific cancers, there was an estimated 24% increased odds of cancer for those who received phototherapy compared to those who did not [OR = 1.24; 95% CI: (1.10, 1.39); p < 0.001], Figure 2E. It was estimated that 23.44% of the total variance was due to heterogeneity (I2 = 23.44%). There was insufficient evidence of publication bias in reporting of any or other cancer risk (p = 0.73), Figure 3E.
In addition to I2, the measure of variance due to overall heterogeneity, we also estimated τ2, the estimate of between-study variance from the random effects (34). For all reported meta-analyses, the τ2 estimate was 0.00.
Discussion
In this systematic review and meta-analysis of 15 studies and 6,675,265 children, phototherapy for neonatal hyperbilirubinemia was associated with a small increased risk of cancer up to age 31 years (24% increased odds). Our findings are similar to four previous systematic reviews and meta-analyses (23–26); however, there were differences in our methodological approach. The previous systematic reviews and meta-analyses either separated their analyses based on study design (case-control or cohort) (23, 24), or excluded studies from their primary analyses for other reasons (25, 26). We adopted an inclusive approach, pooling all 15 studies into one meta-analysis. Since cancer is a rare outcome, and because relative risks, odds ratios, and other multiplicative effect estimates approximate each other for rare outcomes, we combined estimates from various study designs (case-control or cohort) and analyzed them together. However, we included modelling to account for variance from each study given the different study types and multiple estimates coming from individual studies. Another distinction from three of the four previous meta-analyses evaluating childhood cancer risk (23, 24, 26), is that our study evaluated cancer risk into adulthood. Other differences were that we included only peer-reviewed studies, excluded studies which did not report findings with sufficient detail to obtain or perform appropriate tests of association (48), and excluded studies with duplicate cases [two studies found in our systematic review, Newman et al. (49) and Digitale et al. (21, 49), reported on the same cohort, therefore we only included the later study by Digitale et al.].
In terms of specific cancer types, blood cancers and solid organ cancers were found to have the greatest association with cancer risk, with an estimated 40% and 18% increased odds of cancer, respectively, for those who received phototherapy compared to those who did not. This is consistent with previous meta-analyses, which showed that blood cancers had the greatest association with cancer risk (23–26).
There are several plausible mechanisms for the carcinogenic effects of phototherapy. Phototherapy generates oxygen radicals, which can lead to DNA damage (14). Studies have demonstrated DNA damage and cytokine changes among term neonates, and evidence of oxidative stress among premature neonates after phototherapy (11, 13, 16). Phototherapy may also induce apoptosis and DNA damage specifically in lymphocytes, potentially affecting hematopoiesis and contributing to blood cancer risk (50). Studies have also shown a positive association between the duration of phototherapy and markers of DNA damage (14). DNA damage over time can potentially lead to carcinogenic gene modifications. Similarly, studies have shown that higher intensity light causes more DNA damage than conventional light therapy (51). However, a recent study demonstrated that the negative effect of phototherapy on sister chromatid exchange frequency (an index of genomic stability in response to environmental or genetic mutagens) was temporary. After 3.5 years of follow-up, differences in mean sister chromatid exchange values had disappeared between the majority of children who received phototherapy in the neonatal period and healthy children who had not received phototherapy (52).
It should be noted that several studies included in our meta-analysis did not adjust for confounding variables such as hyperbilirubinemia, prematurity, intrauterine growth restriction, congenital anomalies, as well as maternal factors such as gestational diabetes, all of which may increase cancer risk, as well as the need for phototherapy, and must be considered when interpreting our findings. Adjustment for hyperbilirubinemia may have been avoided in some studies, as it too was collinear with the phototherapy exposure; however, this remains a limitation as there is evidence that hyperbilirubinemia is a confounder that is independently associated with cancer risk (19), along with its more obvious connection to the decision to use phototherapy. The association between hyperbilirubinemia and cancer risk deserves additional attention. A large cohort study and a case-control study included in our meta-analysis evaluated hyperbilirubinemia and cancer risk and demonstrated a small increased risk of cancer (19, 43). Studies have also shown a positive association between hyperbilirubinemia and DNA damage, as well as enhanced apoptosis among circulating lymphocytes of term infants (53).
Other factors that were not addressed in our meta-analysis were the duration and method of phototherapy delivery. It may be postulated that longer durations and greater intensity of light could influence the risk of malignancy (14, 51). These factors were not reported in the majority of studies included in this meta-analysis or previous meta-analyses and therefore could not be assessed.
While our results show an association between phototherapy and cancer, the clinical significance remains unclear. An OR of 1.24 indicates an added 24% increase in the odds of developing cancer after phototherapy exposure; however, since the baseline risk of childhood cancer is very low, phototherapy would lead to a small number of additional cancer diagnoses (e.g., 51/100,000 children treated with phototherapy), some of which may be due to increased risk due to hyperbilirubinemia. This low risk must be balanced with the risk of untreated hyperbilirubinemia. Untreated hyperbilirubinemia in the neonatal period can lead to BIND with devastating neurodevelopmental consequences (4–6). Current phototherapy guidelines (1, 57) are associated with a very low incidence of neurodevelopmental complications from hyperbilirubinemia, with approximately 1 in every 50,000–100,000 children developing chronic bilirubin encephalopathy (58). Historic data demonstrate that higher thresholds for initiating phototherapy and a lack of routine screening for hyperbilirubinemia in the 1990s (59) led to an increased risk of both acute and chronic bilirubin encephalopathy in Canada (58, 60). However, administrative data from the California Department of Developmental Services did not support a resurgence in kernicterus. Trends in acute bilirubin encephalopathy were not reported (61). There is clear evidence that prior to the use of phototherapy for hyperbilirubinemia, neurodevelopmental consequences were much more common, and are still seen in developing countries that have reduced access to bilirubin monitoring and phototherapy (62, 63). While recent meta-analyses including this one show a very small but reproducible risk of cancer after phototherapy, this association must be balanced by the well-understood risk of BIND. This study highlights the importance of adhering to phototherapy guidelines, including close serum bilirubin monitoring of children at risk of severe hyperbilirubinemia, and the appropriate use of phototherapy.
Limitations
This study had some limitations. First, several studies included in our meta-analysis did not control for premature births and other confounding factors. Furthermore, premature neonates may be affected differently by phototherapy as they have distinct physiology from term infants, as well as thinner skin, which may be more vulnerable to the potential mutagenic effects of phototherapy (54, 55). Premature infants are also more likely to require a longer duration of phototherapy compared to term infants with hyperbilirubinemia, which may increase the risk of cancer. Second, only observational studies (case-control and cohort studies) were included in our meta-analysis, and therefore, we cannot make any definitive conclusions about a causal relationship between phototherapy and cancer. Third, the high degree of heterogeneity across individual study designs included in our meta-analysis made interpretation more challenging. Furthermore, the variability in follow-up periods among studies limited our ability to analyze the age at cancer diagnosis. However, the heterogeneity among included studies improved the generalizability of our findings (56). Finally, two studies included in our meta-analysis (Wickramasinghe et al. and Digitale et al.) (21, 46) were conducted in the same state with overlapping study periods. While data was extracted from different administrative databases, there may have been an overlap between their samples.
Conclusions
We found a small increased risk of cancer up to age 31 years (24% increased odds) after neonatal phototherapy. Further research is needed to explore the risk of cancer after phototherapy, adjusting for confounding factors, as well as the potential impacts of phototherapy duration and intensity. Additionally, more research is needed to evaluate the effects of phototherapy in the very premature infant.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SF: Data curation, Methodology, Supervision, Writing – original draft, Conceptualization, Writing – review & editing. CK-S: Writing – review & editing, Formal analysis, Writing – original draft, Supervision, Methodology. MG: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. TJ: Writing – original draft, Supervision, Writing – review & editing. MS: Conceptualization, Methodology, Writing – review & editing, Data curation, Writing – original draft, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1667636/full#supplementary-material
Abbreviations
BIND, bilirubin-induced neurologic dysfunction; OR, odds ratio; CI, confidence intervals.
References
1. Sarathy L, Chou JH, Romano-Clarke G, Darci KA, Lerou PH. Bilirubin measurement and phototherapy use after the AAP 2022 newborn hyperbilirubinemia guideline. Pediatrics. (2024) 153:e2023063323. doi: 10.1542/peds.2023-063323
2. van der Geest BAM, de Mol MJS, Barendse ISA, de Graaf JP, Bertens LCM, Poley MJ, et al. Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study. Sci Rep. (2022) 12:14385. doi: 10.1038/s41598-022-17933-2
3. Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med (Lond). (2017) 78:699–704. doi: 10.12968/hmed.2017.78.12.699
4. Good WV, Wong RJ, Norcia AM, Hou C, Cellucci J, McGovern MQ, et al. Effect of bilirubin on visuocortical development in preterm infants. J Perinatol. (2025) 45:1289–96. doi: 10.1038/s41372-025-02213-4
5. Pranty AI, Shumka S, Adjaye J. Bilirubin-induced neurological damage: current and emerging iPSC-derived brain organoid models. Cells. (2022) 11(17):2647. doi: 10.3390/cells11172647
6. Shapiro SM, Popelka GR. Auditory impairment in infants at risk for bilirubin-induced neurologic dysfunction. Semin Perinatol. (2011) 35:162–70. doi: 10.1053/j.semperi.2011.02.011
7. Aycicek A, Kocyigit A, Erel O, Senturk H. Phototherapy causes DNA damage in peripheral mononuclear leukocytes in term infants. J Pediatr (Rio J). (2008) 84:141–6. doi: 10.2223/JPED.1765
8. Aycicek A, Erel O. Total oxidant/antioxidant status in jaundiced newborns before and after phototherapy. J Pediatr (Rio J). (2007) 83:319–22. doi: 10.2223/JPED.1645
9. Galla A, Kitsiou-Tzeli S, Gourgiotis D, Hadjigeorgiou E, Yousef-Ayash H, Kanarios J, et al. Sister chromatid exchanges in peripheral lymphocytes in newborns treated with phototherapy and vitamin E. Acta Paediatr. (1992) 81:820–3. doi: 10.1111/j.1651-2227.1992.tb12110.x
10. Gathwala G, Sharma S. Oxidative stress, phototherapy and the neonate. Indian J Pediatr. (2000) 67:805–8. doi: 10.1007/BF02726223
11. Gathwala G, Sharma S. Phototherapy induces oxidative stress in premature neonates. Indian J Gastroenterol. (2002) 21:153–4.12385544
12. Karadag A, Yesilyurt A, Unal S, Keskin I, Demirin H, Uras N, et al. A chromosomal-effect study of intensive phototherapy versus conventional phototherapy in newborns with jaundice. Mutat Res. (2009) 676:17–20. doi: 10.1016/j.mrgentox.2009.03.008
13. Kurt A, Aygun AD, Kurt AN, Godekmerdan A, Akarsu S, Yilmaz E. Use of phototherapy for neonatal hyperbilirubinemia affects cytokine production and lymphocyte subsets. Neonatology. (2009) 95:262–6. doi: 10.1159/000171216
14. Ramy N, Ghany EA, Alsharany W, Nada A, Darwish RK, Rabie WA, et al. Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatol. (2016) 36:132–6. doi: 10.1038/jp.2015.166
15. Tsai FJ, Tsai CH, Peng CT, Wang TR. Sister chromatid exchange in Chinese newborn infants treated with phototherapy for more than five days. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. (1998) 39:327–9.9823679
16. Tatli MM, Minnet C, Kocyigit A, Karadag A. Phototherapy increases DNA damage in lymphocytes of hyperbilirubinemic neonates. Mutat Res. (2008) 654:93–5. doi: 10.1016/j.mrgentox.2007.06.013
17. Sirota L, Straussberg R, Gurary N, Aloni D, Bessler H. Phototherapy for neonatal hyperbilirubinemia affects cytokine production by peripheral blood mononuclear cells. Eur J Pediatr. (1999) 158:910–3. doi: 10.1007/s004310051240
18. Speck WT, Rosenkranz HS. Phototherapy for neonatal hyperbilirubinemia–a potential environmental health hazard to newborn infants: a review. Environ Mutagen. (1979) 1:321–36. doi: 10.1002/em.2860010404
19. Auger N, Laverdière C, Ayoub A, Lo E, Luu TM. Neonatal phototherapy and future risk of childhood cancer. Int J Cancer. (2019) 145:2061–9. doi: 10.1002/ijc.32158
20. Bugaiski-Shaked A, Shany E, Mesner O, Sergienko R, Wainstock T. Association between neonatal phototherapy exposure and childhood neoplasm. J Pediatr. (2022) 245:111–6. doi: 10.1016/j.jpeds.2022.01.046
21. Digitale JC, Kim M-O, Kuzniewicz MW, Newman TB. Update on phototherapy and childhood cancer in a northern California cohort. Pediatrics. (2021) 148:e2021051033. doi: 10.1542/peds.2021-051033
22. Seppälä LK, Vettenranta K, Leinonen MK, Tommiska V, Madanat-Harjuoja LM. Preterm birth, neonatal therapies and the risk of childhood cancer. Int J Cancer. (2021) 148:2139–47. doi: 10.1002/ijc.33376
23. Galdos-Bejar M, Belanovic-Ramirez I, Baquedano-Rojas C, Alarcón-Espinosa J, Joya-Arista V, Arrarte-Perez A, et al. Impact of phototherapy on childhood cancer: a comprehensive systematic review and meta-analysis. J Neonatol. (2024) 39:361–74. doi: 10.1177/09732179241304925
24. Kuitunen I, Nikkila A, Kiviranta P, Jaaskelainen J, Auvinen A. Risk of childhood neoplasms related to neonatal phototherapy- a systematic review and meta-analysis. Pediatr Res. (2024) 96:1131–40. doi: 10.1038/s41390-024-03191-7
25. Abdellatif M, Tawfik GM, Makram AM, Abdelsattar MK, Dobs M, Papadopoulos DN, et al. Association between neonatal phototherapy and future cancer: an updated systematic review and meta-analysis. Eur J Pediatr. (2023) 182:329–41. doi: 10.1007/s00431-022-04675-6
26. Hemati Z, Keikha M, Khoshhali M, Kelishadi R. Phototherapy and risk of childhood cancer: a systematic review and meta-analysis. J Neonatal Nurs. (2022) 28:219–28. doi: 10.1016/j.jnn.2022.01.007
27. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (updated August 2024). Cochrane (2024). Available online at: www.cochrane.org/handbook (Accessed June 30, 2025).
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
29. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. (2016) 75:40–6. doi: 10.1016/j.jclinepi.2016.01.021
30. Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia. (2022). Available online at: https://www.covidence.org (Accessed June 30, 2025).
31. Clarivate. EndNote Version X9. Philadelphia, PA. (2018). Available online at: https://endnote.com/ (Accessed June 30, 2025).
32. Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. (2024) 186:108602. doi: 10.1016/j.envint.2024.108602
33. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
34. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
35. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2024).
36. Brewster DH, Tucker JS, Fleming M, Morris C, Stockton DL, Lloyd DJ, et al. Risk of skin cancer after neonatal phototherapy: retrospective cohort study. Arch Dis Child. (2010) 95:826–31. doi: 10.1136/adc.2009.179275
37. Cnattingius S, Zack M, Ekbom A, Gunnarskog J, Linet M, Adami HO. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. (1995) 4:441–5.7549797
38. Cnattingius S, Zack MM, Ekbom A, Gunnarskog J, Kreuger A, Linet M, et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst. (1995) 87:908–14. doi: 10.1093/jnci/87.12.908
39. Kadivar M, Sangsari R, Saeedi M, Ghasemi Tehrani S. Association between neonatal phototherapy and cancer during childhood. Iran J Neonatol. (2020) 11:104–8. doi: 10.22038/ijn.2020.42623.1709
40. Ku MS. Neonatal phototherapy: a novel therapy to prevent allergic skin disease for at least 5 years. Neonatology. (2018) 114:235–41. doi: 10.1159/000489389
41. Linet MS, Gridley G, Cnattingius S, Nicholson HS, Martinsson U, Glimelius B, et al. Maternal and perinatal risk factors for childhood brain tumors (Sweden). Cancer Causes Control. (1996) 7:437–48. doi: 10.1007/BF00052670
42. Olsen JH, Hertz H, Kjaer SK, Bautz A, Mellemkjaer L, Boice JD Jr. Childhood leukemia following phototherapy for neonatal hyperbilirubinemia (Denmark). Cancer Causes Control. (1996) 7:411–4. doi: 10.1007/BF00052666
43. Podvin D, Kuehn CM, Mueller BA, Williams M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. (2006) 20:312–22. doi: 10.1111/j.1365-3016.2006.00731.x
44. Roman E, Ansell P, Bull D. Leukaemia and non-Hodgkin’s lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer. (1997) 76:406–15. doi: 10.1038/bjc.1997.399
45. Sabzevari F, Sinaei R, Bahmanbijari B, Dehghan Krooki S, Dehghani A. Is neonatal phototherapy associated with a greater risk of childhood cancers? BMC Pediatr. (2022) 22:356. doi: 10.1186/s12887-022-03412-0
46. Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal phototherapy and infantile cancer. Pediatrics. (2016) 137:e20151353. doi: 10.1542/peds.2015-1353
47. Spector LG, Pankratz N, Marcotte EL. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. (2015) 62:11–25. doi: 10.1016/j.pcl.2014.09.013
48. Kraemer HC, Gardner C, Brooks Iii JO, Yesavage JA. Advantages of excluding underpowered studies in meta-analysis: inclusionist versus exclusionist viewpoints. Psychol Methods. (1998) 3:23–31. doi: 10.1037/1082-989X.3.1.23
49. Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective cohort study of phototherapy and childhood cancer in Northern California. Pediatrics. (2016) 137:e20151354. doi: 10.1542/peds.2015-1354
50. Yahia S, Shabaan AE, Gouida M, El-Ghanam D, Eldegla H, El-Bakary A, et al. Influence of hyperbilirubinemia and phototherapy on markers of genotoxicity and apoptosis in full-term infants. Eur J Pediatr. (2015) 174:459–64. doi: 10.1007/s00431-014-2418-z
51. Mohamed WW, Niazy WH. Genotoxic effect of phototherapy in term newborn infants with hyperbilirubinemia. J Neonatal Perinatal Med. (2012) 5:381–7. doi: 10.3233/NPM-1261912
52. Kahveci H, Dogan H, Karaman A, Caner I, Tastekin A, Ikbal M. Phototherapy causes a transient DNA damage in jaundiced newborns. Drug Chem Toxicol. (2013) 36:88–92. doi: 10.3109/01480545.2011.653491
53. El-Abdin MYZ, El-Salam MA, Ibrhim MY, Koraa SSM, Mahmoud E. Phototherapy and DNA changes in full term neonates with hyperbilirubinemia. Egypt J Med Human Genet. (2012) 13:29–35. doi: 10.1016/j.ejmhg.2011.11.003
54. Oranges T, Dini V, Romanelli M. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care (New Rochelle). (2015) 4:587–95. doi: 10.1089/wound.2015.0642
55. Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. (2010) 27:125–31. doi: 10.1111/j.1525-1470.2009.00973.x
56. Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med. (1997) 16:753–68. doi: 10.1002/(SICI)1097-0258(19970415)16:7%3C753::AID-SIM494%3E3.0.CO;2-G
57. Ng E, Altit G, Joynt C, Radziminski N, Narvey M. Guidelines for detection and management of hyperbilirubinemia in term and late preterm newborns (≥35 weeks gestational age). Can Paediatr Soc. (2025). Available online at: https://cps.ca/documents/position/hyperbilirubinemia-newborns
58. Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics. (2012) 130:e886–90. doi: 10.1542/peds.2012-0253
59. Newman TB, Maisels MJ. Evaluation and treatment of jaundice in the term newborn: a kinder, gentler approach. Pediatrics. (1992) 89:809–18. doi: 10.1542/peds.89.5.809
60. Sgro M, Kandasamy S, Shah V, Ofner M, Campbell D. Severe neonatal hyperbilirubinemia decreased after the 2007 Canadian guidelines. J Pediatr. (2016) 171:43–7. doi: 10.1016/j.jpeds.2015.12.067
61. Brooks JC, Fisher-Owens SA, Wu YW, Strauss DJ, Newman TB. Evidence suggests there was not a “resurgence” of kernicterus in the 1990s. Pediatrics. (2011) 127:672–9. doi: 10.1542/peds.2010-2476
62. Diala UM, Wennberg RP, Abdulkadir I, Farouk ZL, Zabetta CDC, Omoyibo E, et al. Patterns of acute bilirubin encephalopathy in Nigeria: a multicenter pre-intervention study. J Perinatol. (2018) 38:873–80. doi: 10.1038/s41372-018-0094-y
Keywords: phototherapy, hyperbilirubinemia, cancer, neonatology, systematic review, meta-analysis
Citation: Freeman SJ, Keown-Stoneman CDG, Ghobrial M, Jegathesan T and Sgro MD (2025) Neonatal phototherapy and cancer risk: a systematic review and meta-analysis. Front. Pediatr. 13:1667636. doi: 10.3389/fped.2025.1667636
Received: 16 July 2025; Accepted: 24 October 2025;
Published: 24 November 2025.
Edited by:
Ming-Chou Chiang, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Defne Engür, University of Health Sciences, TürkiyeRen-Huei Fu, Linkou Chang Gung Memorial Hospital, Taiwan
Marjaneh Zarkesh, Gilan University of Medical Sciences, Iran
Copyright: © 2025 Freeman, Keown-Stoneman, Ghobrial, Jegathesan and Sgro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sloane J. Freeman, c2xvYW5lLmZyZWVtYW5AdW5pdHloZWFsdGgudG8=
 Sloane J. Freeman
Sloane J. Freeman Charles D. G. Keown-Stoneman3,4,5
Charles D. G. Keown-Stoneman3,4,5