- 1School of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, China
- 2Diagnosis and Treatment Center for Children, The Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, China
Background: Autosomal dominant intellectual disability type 39 (MRD39; OMIM # 616521) is caused by heterozygous mutation in the MYT1l gene on chromosome 2p25.3. The MYTL1 encoded protein belongs to a novel class of cystein-cystein-histidine-cystein zinc finger proteins that function in the developing mammalian central nervous system.
Case summary: We report a 1-year-6-month-old girl presenting with global developmental delay (GDD) and autistic behaviors, demonstrating inability to stand independently, crawling mobility, poor response to name calling, and impaired joint attention. Initial developmental assessments yielded a Griffiths Mental Development Scale score of 57 and an ADOS-2 score of 11. Following 20 months of systematic rehabilitative training, the patient achieved independent ambulation, could follow simple commands, and produced phrases under 10 words, though suboptimal response to name calling and joint attention persisted. Re-evaluation showed a Griffiths score of 59 and an ADOS-2 score of 10. Whole-exome sequencing identified a de novo heterozygous missense variant in the MYT1l gene [c.1695G > T; p.(Arg565Ser)]. According to the American College of Medical Genetics and Genomics (ACMG) guidelines, this variant was classified as Likely Pathogenic based on criteria PM6 (de novo status) and PM2 (absence in population databases). Based on the concordant genotype and phenotype, the patient was diagnosed with MYT1l-related neurodevelopmental disorder (MRD39).
Conclusion: We report a case of MYT1l-related disorder presenting with global developmental delay and features of autism spectrum disorder, associated with the previously documented but functionally uncharacterized c.1695G > T (p.Arg565Ser) variant. This case provides valuable clinical evidence supporting the pathogenicity of this variant and contributes to a deeper understanding of the phenotypic spectrum of MYT1l-related conditions.
1 Introduction
Autosomal dominant intellectual disability type 39 (MRD5; OMIM #616521) is caused by heterozygous mutation in the MYT1l gene on chromosome 2p25.3 (1). The MYT1l gene comprises 12 exons and encodes a protein of 1,230 amino acids (1, 2). The core clinical manifestations of MRD39 include intellectual disability, delayed language development, autism spectrum disorder, with partial patients exhibiting distinctive facial features and epileptic seizures (3–5).
In this study, we present a case of a patient diagnosed with MRD39, characterized by significant developmental delay and autistic features, yet notably lacking seizures. Whole-exome sequencing (WES) revealed a de novo heterozygous missense mutation in the MYT1l gene, which aligns with the patient's clinical phenotype. This finding contributes to the expanding knowledge of MYT1l variants and their associated clinical presentations.
2 Case presentation
2.1 Chief complaint
A 1-year-6-month-old girl was referred for evaluation of developmental delay observed over the preceding 8 months.
The patient presented with inability to ambulate independently since birth. This female infant was born at 37 weeks' gestation (G2P2) via spontaneous vaginal delivery, with a birth weight of 3.0 kg. There was no history of intrauterine hypoxia or neonatal resuscitation. She was formula-fed postnatally. Developmental milestones included: head control at 4 months, rolling over at 6 months, independent sitting at 11 months, stable sitting without support at 14 months, crawling at 15 months, and standing independently at 16 months. Clinical observations revealed poor response to name calling, limited eye contact, absence of stranger anxiety, and prominent repetitive head-banging behavior. Initial developmental assessments yielded a Griffiths Mental Development Scale score of 57 and an ADOS-2 score of 11.
2.2 Physical examination
Weight 10 kg, head circumference 46 cm. The child was alert and responsive with regular respirations. No hypopigmented patches or café-au-lait spots were observed. The anterior fontanelle was closed. Ocular motility was normal, and the pupils were isocoric with normal light reflexes. No nuchal rigidity was noted. Cardiopulmonary and abdominal examinations were unremarkable. Cranial nerve assessment showed no abnormalities. Muscle strength and tone were normal in all extremities. Knee jerks and abdominal reflexes were present. Ankle clonus was absent. Kernig's sign, Brudzinski's sign, Babinski sign, and Oppenheim sign were negative bilaterally.
2.3 Family history
The patient's father and mother are healthy, and the patient's 8-year-old brother is healthy.
2.4 Routine clinical examinations
A comprehensive evaluation, including complete blood count, urinalysis, stool analysis, assessments of liver and kidney function, cardiac enzyme levels, electrolyte balance, thyroid function, and both blood and urine genetic metabolic screenings, indicated no abnormalities. Electroencephalography (EEG) revealed no abnormalities, and brain magnetic resonance imaging (MRI) showed no structural lesions.
2.5 Genetic analysis
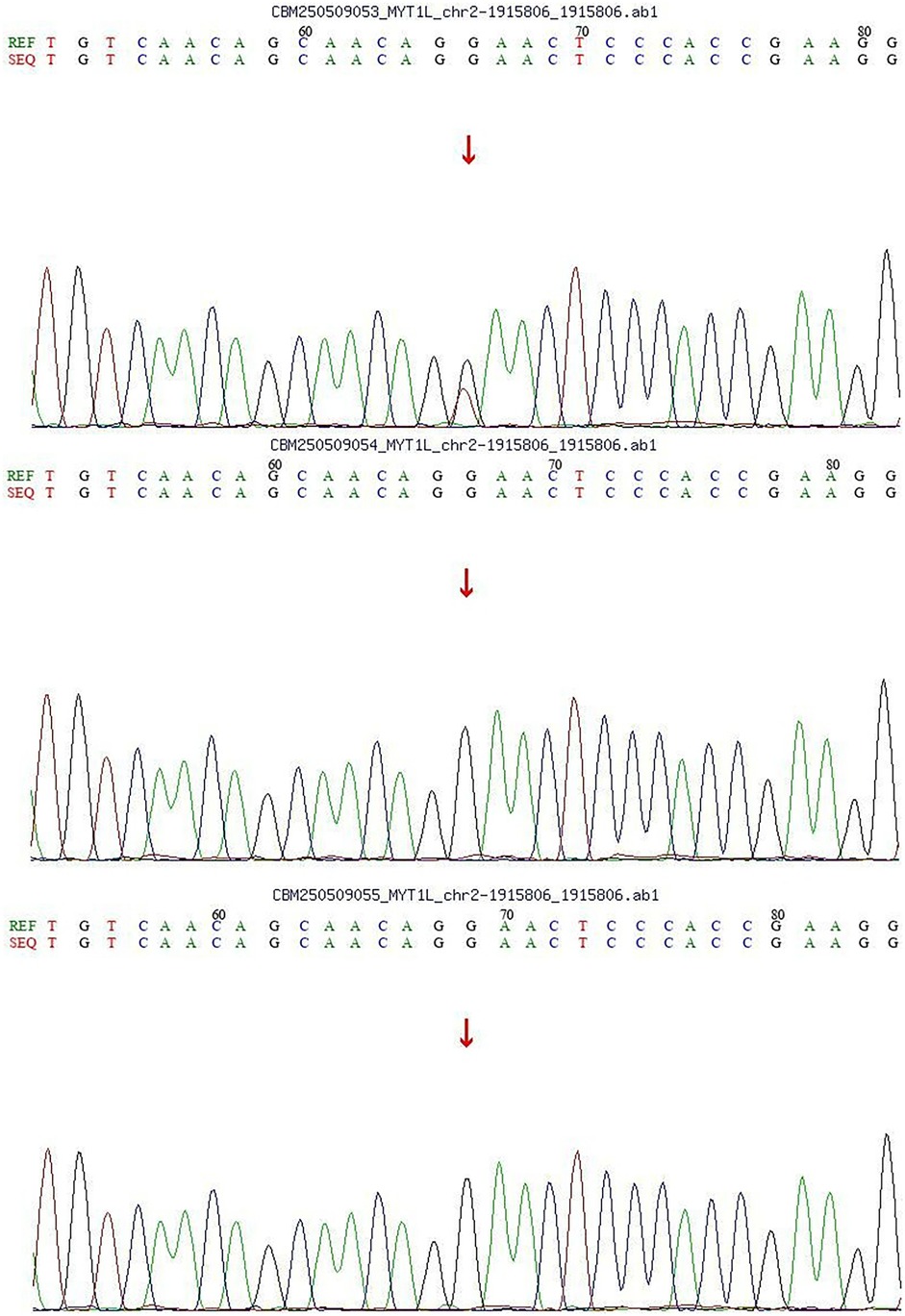
With the consent of the parents, we performed whole exome sequencing (WES) to identify potential pathogenic variants.Following sequencing, a stepwise filtering approach was applied to prioritize candidates: common polymorphisms (allele frequency >0.1% in aggregate population databases including gnomAD, 1,000 Genomes, and ExAC) were excluded, and focus was placed on rare, protein-altering variants. Given the patient's phenotype and negative family history, we prioritized de novo variants. Whole-exome sequencing (WES) revealed a de novo heterozygous missense variant in the MYT1l gene [c.1695G > T; p.(Arg565Ser)]. Parental Sanger sequencing confirmed that both parents carried the wild-type allele for this variant (Figure 1). Based on the ACMG/AMP guidelines, the variant was assessed as Likely Pathogenic, supported by evidence PM6 (confirmed de novo status) and PM2 (absence in population databases).
2.6 Final diagnosis
MRD39, ASD.
2.7 Treatment
A comprehensive rehabilitation program spanning 20 months was implemented for the patients.
2.8 Outcome and follow-up
Following 20 months of systematic rehabilitation training, the patient achieved independent walking and short-distance running, and can speak short phrases of fewer than 10 words. However, persistent deficits include reduced eye contact, poor response to name calling, and stereotypical head-banging behavior. Re-evaluation showed a Griffiths score of 59 and ADOS-2 score of 10.
3 Discussion
The MYT1l gene is integral to neuronal differentiation and neural development. Studies demonstrate that MYT1l sustains neuronal identity by repressing the expression of genes associated with non-neuronal lineages. This repression occurs through its interaction with the Sin3b complex, which is recruited via the N-terminal domain of MYT1l (6). Furthermore, MYT1l exhibits similar genomic binding sites in both neurons and fibroblasts and is typically found in an open chromatin configuration, emphasizing its vital role in maintaining neuronal identity (6).
Beyond its function in neurons, MYT1l also facilitates the differentiation of oligodendrocyte precursor cells (OPCs). Evidence indicates that MYT1l is expressed during myelin formation and remyelination, with its expression being regulated by binding to the Olig1 promoter, thereby promoting OPC differentiation (7). These biological functions position MYT1l as a promising therapeutic target for demyelinating diseases.
In neurodevelopmental disorders, loss-of-function mutations in the MYT1l gene are correlated with intellectual disability, autism spectrum disorder, and obesity (2, 8). Studies suggest that MYT1l deficiency results in the aberrant activation of neuronal developmental programs, consequently impairing neuronal maturation and function[ (9). Moreover, the absence of MYT1l leads to an imbalance in neuronal proportions, predominantly affecting neuronal maturation processes. This developmental imbalance persists throughout the developmental timeline (10).
Mutations in the MYT1l gene are also implicated in schizophrenia and other neuropsychiatric disorders. Research has demonstrated that microdeletions in MYT1l are significantly associated with childhood-onset schizophrenia, indicating that MYT1l may contribute to the pathogenesis of these conditions (11). Additionally, MYT1l expression is linked to memory-related processes in the brain, influencing these processes by regulating the proliferation/differentiation switch of ID-bHLH factors (12).
In this case, both parents were healthy at the child's birth, with no history of intrauterine asphyxia or amniotic fluid contamination, and no family history of genetic disorders. Postnatally, the patient exhibited global developmental delay accompanied by manifestations of autism spectrum disorder. Based on the clinical presentation, whole-exome sequencing (WES) identified a de novo heterozygous missense variant in the MYT1l gene [c.1695G > T; p. (Arg565Ser)], which was confirmed by Sanger sequencing to segregate within the family according to genetic co-segregation principles. According to the ACMG/AMP guidelines, this variant was classified as “Likely Pathogenic” based on criteria PM6 (confirmed de novo status) and PM2 (absence in population databases). Despite the variant's classification as “Likely Pathogenic” and the need for functional validation, the significant overlap between the patient's phenotype and the established MYT1l-associated neurodevelopmental profile, in the absence of other plausible genetic findings, strongly implicates this variant as the most likely underlying cause. Therefore, integrating the compelling clinical and genetic evidence, a definitive diagnosis of Autosomal Dominant Intellectual disability 39 and Autism Spectrum Disorder was established.
The MYT1l variant at position 565 (p.Arg565Ser) (ID: 801647) lacks a definitive pathogenicity assignment in the ClinVar database, necessitating its evaluation using the PM6 and PM2 criteria. While our classification of “Likely Pathogenic” is robustly supported by de novo status and population frequency data, the application of other ACMG criteria warrants further discussion. the PS1 criterion (identical amino acid change as a known Pathogenic variant) could not be applied because this variant lacks independent basis for a “Pathogenic” or “Likely Pathogenic” designation. Similarly, the PP5 criterion (reported as Pathogenic by an authoritative source but with unpublished data) was also not met. Compared to the well-established loss-of-function mechanisms in MYT1l, the pathogenicity of this missense variant remains to be fully elucidated through functional studies. our case, combined with existing ClinVar submissions, adds to the body of evidence linking this specific variant to neurodevelopmental disorders and highlights the need for further evidence to strengthen its classification criteria.
After 20 months of structured rehabilitation training, the patient exhibited notable advancements in motor skills, including the ability to walk independently and run short distances, as well as enhancements in language abilities, demonstrated by the production of short phrases consisting of fewer than 10 words. Nevertheless, the patient continued to experience persistent deficits, such as diminished eye contact, inadequate response to auditory cues such as name calling, and repetitive head-banging behavior. Remarkably, in contrast to previously documented cases, this patient presented with normal findings on brain MRI, EEG results, and no history of seizure episodes.
We report a case of MYT1l-related MRD39, distinguished by pronounced global developmental delay and autism spectrum disorder, and characterized by a novel c.1695G > T missense variant that has not been previously documented. This finding broadens the current understanding of the mutation spectrum and phenotypic variability associated with MYT1l, thereby advancing insights into the genotype-phenotype correlations in conditions related to MYT1l.
Patient perspective
The patient's legal guardian provided written informed consent for the publication of this case report.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Changchun University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. YC: Writing – original draft, Writing – review & editing. YQ: Writing – original draft, Writing – review & editing. XS: Writing – original draft, Writing – review & editing. WW: Writing – original draft, Writing – review & editing. KJ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the child and his family members for their contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. (2012) 367(20):1921–9. doi: 10.1056/NEJMoa1206524
2. Mayo S, Roselló M, Monfort S, Oltra S, Orellana C, Martínez F. Haploinsufficiency of the MYT1l gene causes intellectual disability frequently associated with behavioral disorder. Genet Med. (2015) 17(8):683–4. doi: 10.1038/gim.2015.86
3. Boeri S, Scala M, Madia F, Perucco F, Vozzi D, Capra V, et al. MYT1l Variant inherited by a mosaic father in a case of severe developmental and epileptic encephalopathy. Epileptic Disord. (2023) 25(6):874–9. doi: 10.1002/epd2.20141
4. De Rocker N, Vergult S, Koolen D, Jacobs E, Hoischen A, Zeesman S, et al. Refinement of the critical 2p25.3 deletion region: the role of MYT1l in intellectual disability and obesity. Genet Med. (2015) 17(6):460–6. doi: 10.1038/gim.2014.124
5. Blanchet P, Bebin M, Bruet S, Cooper GM, Thompson ML, Duban-Bedu B, et al. MYT1l Mutations cause intellectual disability and variable obesity by dysregulating gene expression and development of the neuroendocrine hypothalamus. PLoS Genet. (2017) 13(8):e1006957. doi: 10.1371/journal.pgen.1006957
6. Mall M, Kareta MS, Chanda S, Ahlenius H, Perotti N, Zhou B, et al. Myt1l safeguards neuronal identity by actively repressing many non-neuronal fates. Nature. (2017) 544(7649):245–9. doi: 10.1038/nature21722
7. Shi Y, Shao Q, Li Z, Gonzalez GA, Lu F, Wang D, et al. Myt1l promotes differentiation of oligodendrocyte precursor cells and is necessary for remyelination after lysolecithin-induced demyelination. Neurosci Bull. (2018) 34(2):247–60. doi: 10.1007/s12264-018-0207-9
8. Stevens SJ, van Ravenswaaij-Arts CM, Janssen JW, Klein Wassink-Ruiter JS, van Essen AJ, Dijkhuizen T, et al. MYT1l Is a candidate gene for intellectual disability in patients with 2p25.3 (2pter) deletions. Am J Med Genet A. (2011) 155(11):2739–45. doi: 10.1002/ajmg.a.34274
9. Chen J, Fuhler NA, Noguchi KK, Dougherty JD. MYT1l Is required for suppressing earlier neuronal development programs in the adult mouse brain. Genome Res. (2023) 33(4):541–56. doi: 10.1101/gr.277413.122
10. Yen A, Sarafinovska S, Chen X, Skinner DD, Leti F, Crosby M, et al. MYT1l Deficiency impairs excitatory neuron trajectory during cortical development. Nat Commun. (2024) 15(1):10308. doi: 10.1038/s41467-024-54371-2
11. Lee Y, Mattai A, Long R, Rapoport JL, Gogtay N, Addington AM. Microduplications disrupting the MYT1l gene (2p25.3) are associated with schizophrenia. Psychiatr Genet. (2012) 22(4):206–9. doi: 10.1097/YPG.0b013e328353ae3d
12. Kepa A, Martinez Medina L, Erk S, Srivastava DP, Fernandes A, Toro R, et al. Associations of the intellectual disability gene MYT1l with Helix-loop-Helix gene expression, hippocampus volume and hippocampus activation during memory retrieval. Neuropsychopharmacology. (2017) 42(13):2516–26. doi: 10.1038/npp.2017.91
Keywords: autism spectrum disorder, global developmental delay, autosomal dominant intellectual disability type 39, MYT1l, epilepsy
Citation: Wang X, Lin S, Chen Y, Qi Y, Sun X, Wang W and Jiang K (2025) De novo missense mutation in MYT1l leading to autosomal dominant intellectual disability 39 and autism spectrum disorder: a case report. Front. Pediatr. 13:1672911. doi: 10.3389/fped.2025.1672911
Received: 25 July 2025; Accepted: 9 October 2025;
Published: 23 October 2025.
Edited by:
Ammar Husami, Cincinnati Children’s Hospital Medical Center, United StatesReviewed by:
Simon Carlo, Ponce Health Sciences University, Puerto RicoRafiullah Rafiullah, University Hospital Heidelberg, Germany
Copyright: © 2025 Wang, Lin, Chen, Qi, Sun, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangzhu Lin, NjE4NThAMTYzLmNvbQ==
 Xin Wang1,2
Xin Wang1,2 Shuangzhu Lin
Shuangzhu Lin Yangfan Qi
Yangfan Qi Xiaoyu Sun
Xiaoyu Sun Wanqi Wang
Wanqi Wang