- 1Department of Neonate, The Third Affiliated Hospital of Zunyi Medical University (The First People's Hospital of Zunyi), Zunyi, Guizhou, China
- 2Department of Pediatric, The Third Affiliated Hospital Zunyi Medical University (The First People's Hospital of Zunyi), Zunyi, Guizhou, China
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by severe intestinal barrier dysfunction and immune dysregulation in patients, with limited clinical treatment options. Recent research highlights the important role of the gut bacterium Akkermansia muciniphila (AKK) in both the development and management of UC. AKK supports the integrity of the intestinal barrier by metabolizing mucins, enhances the production of tight junction proteins (Occludin-1/ZO-1), and influences immune responses by shifting macrophage polarization from M1 to M2, suppressing pro-inflammatory cytokines (IL-1β、IL-6、MCP-1), and activating anti-inflammatory pathways (SCFAs-SLC52A2/FFAR2、AhR). Clinical data indicate that the abundance of AKK in the intestines of patients with UC is significantly reduced, and this decrease is positively correlated with disease activity and relapse rates. Animal studies have demonstrated that adding AKK can restore the thickness of the mucus layer, lower inflammation scores, and improve the composition of gut microbiota. Importantly, the probiotic effects of AKK vary by strain; for instance, strain FSDLZ36M5 notably reduces colitis symptoms, while FSDLZ20M4 may worsen inflammation. These findings suggest that AKK or its metabolites, such as short-chain fatty acid(SCFAs), hold promise as therapeutic targets for the microbiota in patients with UC.Nonetheless, additional research on strain selection and clinical application is essential to refine treatment strategies. This article will review the correlation between the pathogenesis of AKK and UC, and explore the potential application value of AKK as a probiotic in children with UC, providing new insights for the prevention and treatment in patients with UC.
Introduction
Ulcerative colitis (UC) is a gastrointestinal disease characterized by non-specific chronic inflammation of the colonic mucosa and submucosa, with an unclear etiology, and is classified as a type of inflammatory bowel disease (IBD). The clinical symptoms of UC include persistent diarrhea, abdominal pain, and rectal bleeding, with chronic inflammation typically confined to the colonic mucosal layer (1). During acute exacerbations, systemic toxic symptoms may occur, leading to severe complications, high mortality rates, and poor prognosis. Due to the long course of the disease, frequent relapses, and the lack of effective treatment methods, complete recovery is difficult. In children, the disease can not only cause abdominal pain, diarrhea, hematochezia, and extraintestinal symptoms, but may also lead to growth and developmental disorders as well as changes in mental status, with its incidence rising globally (2, 3). However, to date, there are no definitive effective preventive or therapeutic measures for this disease in either adults or children.
The exact mechanisms behind UC are not fully understood, but growing evidence suggests that gut microbiota and their byproducts significantly contribute to the disease's development (4). Additionally, both the initial appearance and subsequent flare-ups of UC are closely linked to the gut microbiome (5). Healthy gut bacteria support normal physiological processes in children by regulating immune responses, aiding metabolism, enhancing digestion and nutrient absorption, and counteracting harmful pathogens (5). Consequently, research into native human gut probiotics has opened new avenues for disease treatment. Notably, Bifidobacterium and Lactobacillus have emerged as well-established therapeutic probiotics available today (6). Research has also highlighted Akkermansia muciniphila (AKK), a Gram-negative, oval bacterium isolated from human feces, which uses mucin for its carbon, nitrogen, and energy needs (7). Since the discovery of this unique bacterium in 2004, AKK has been extensively studied. It is found in breast milk and various parts of the digestive system, with the highest abundance in the colonic mucosal layer (8). It is considered a promising “next-generation beneficial microbe” (9).
The biological characteristics of AKK
AKK is an anaerobic bacterium that thrives on mucus and is integral to the gastrointestinal microbiome, especially in mammalian intestines, being the sole representative of the Verrucomicrobia phylum. It relies exclusively on mucin for its carbon and nitrogen needs, allowing it to flourish within the intestinal mucosal layer. This distinctive metabolic trait positions AKK as vital for supporting intestinal health and balance. It is essential for preserving the function of the intestinal barrier, modulating immune responses, and ensuring metabolic stability (10). A key feature of AKK is its reliance on mucin for growth, which aids in the regeneration of the intestinal mucus layer. In healthy adults, it comprises about 1% to 4% of the total bacterial community in fecal samples, and its levels are inversely related to various inflammatory conditions, indicating a potential protective effect on gut health and overall physiological function by reinforcing the intestinal barrier (11, 12). Furthermore, AKK is known to generate short-chain fatty acids (SCFAs), which are vital for host metabolism and inflammatory processes (13). Research has also demonstrated that this bacterium bolsters the integrity of the intestinal epithelial barrier, solidifying its role as a beneficial component of the gut microbiota (10). It is important to note that the specific characteristics of different AKK strains may lead to varying health outcomes, suggesting that not all strains will provide the same probiotic benefits. This highlights the importance of careful strain selection and assessment for therapeutic uses, indicating that the probiotic effects of AKK are likely dependent on the specific strain (14). As investigations into AKK progress, it remains a significant contributor to gut microbiota composition and a promising candidate for future probiotic treatments aimed at enhancing metabolic health and maintaining gut ecosystem balance (15).
Changes of AKK in UC
AKK is crucial to the gut microbiome and has attracted considerable interest in the study of UC. Research conducted by Qian K. et al. revealed a notable decrease in AKK levels among individuals with UC (16). This decline is especially pronounced in those experiencing active UC, where AKK levels in the colon, cecum, transverse colon, left colon, and rectum are significantly lower (17). Such a reduction may contribute to the impairment of the intestinal mucosal barrier and the intensification of inflammatory reactions (18). Located within the intestinal mucus layer, AKK is vital for preserving gut homeostasis and structural integrity (Figure 1). Studies indicate that AKK, recognized as a mucin-degrading bacterium, may be instrumental in fostering the development of epithelial cells mediated by intestinal stem cells and in sustaining intestinal balance (19). Although AKK degrades mucin, animal experiments have shown that it can increase the production of mucin by enhancing the number and density of goblet cells in mice induced by a high-fat diet, thereby restoring the thickness of the mucus layer and strengthening the intestinal barrier (20). According to Wade H. et al., AKK and its membrane proteins mitigate intestinal inflammatory stress and enhance the healing of intestinal epithelial wounds via CREBH and miR-143/145 (21). In patients with UC, the decline in AKK correlates with intestinal barrier damage, evidenced by reduced mucin expression (notably MUC2), which is essential for the protective function of the epithelial barrier in the colon (22). This change in function may result in heightened intestinal permeability and inflammatory responses, characteristic of UC (16, 17).
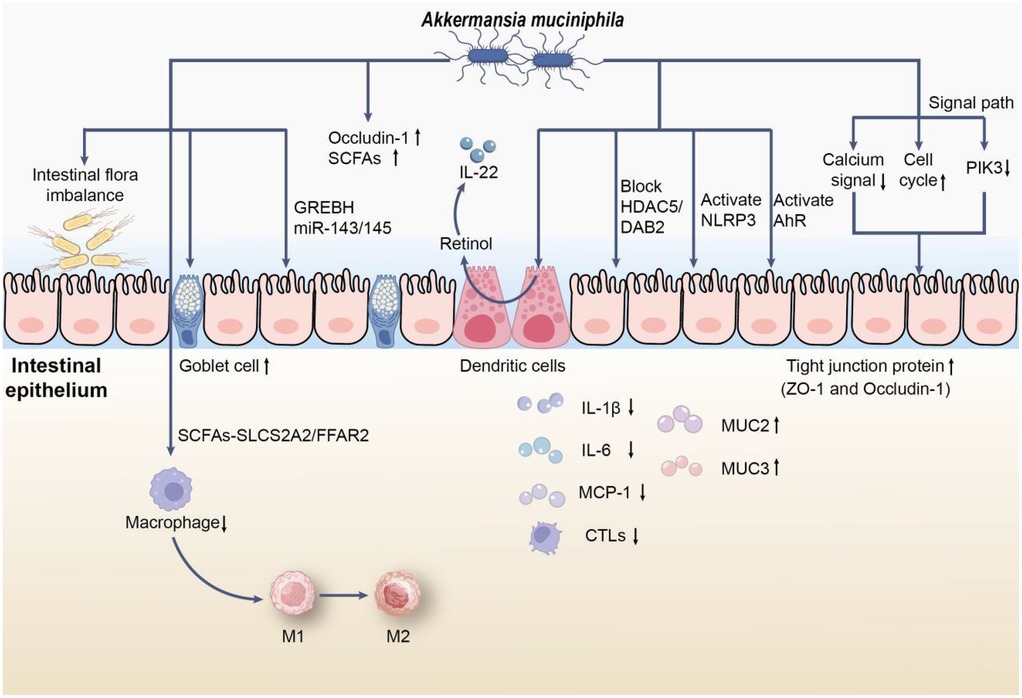
Figure 1. The role of AKK in UC. First, AKK may reduce colonic infiltrating macrophages through the SCFAs-SLC52A2/FFAR2 pathway and stimulate macrophage polarization from M1 to M2 in vivo, thereby alleviating the inflammatory response in mice with UC. In addition, AKK promotes mucin production by inducing an increase in the number and density of goblet cells, restoring the thickness of the mucus layer; simultaneously, its membrane proteins alleviate intestinal inflammatory stress via CREBH and miR-143/145. Moreover, AKK upregulates the expression of Occludin-1 and short-chain fatty acids(SCFAs) receptors, which can also promote the synthesis of retinoic acid in dendritic cells to regulate IL-22 activity and improve colonic damage in mice with UC by blocking the macrophage pro-inflammatory phenotype transition mediated by the HDAC5/DAB2 axis. On the other hand, AKK may reduce apoptosis by activating NLRP3 or AhR signaling pathways, or by downregulating the expression of calcium signaling pathway genes or upregulating cell cycle signaling pathway genes, or by alleviating intestinal epithelial cell inflammation through inhibiting the expression of upstream receptor genes of PI3K. Notably, the supplementation of AKK promotes the expression of tight junction proteins (such as ZO-1 and Occludin-1) and reduces colonic infiltrating cytotoxic T lymphocytes (CTLs), lowering the levels of pro-inflammatory cytokines (such as IL-1β, IL-6, and MCP-1), while promoting the expression of mucins (such as MUC2 and MUC3), further alleviating the inflammatory response.
Zhang L et al. demonstrated that AKK mitigated pathological damage in the colonic tissues of mice, reduced oxidative stress and inflammation, increased the levels of Occludin-1 and short-chain fatty acids(SCFAs) receptors, and promoted the transition of macrophages from the M1 to M2 phenotype in vivo. Following the silencing of FFAR2, the beneficial effects of AKK on cell viability and the M1 to M2 macrophage shift, along with its suppression of oxidative stress, inflammation, apoptosis, metabolic disorders, and necroptosis, were reinstated. This suggests that AKK may influence macrophage polarization via the SCFAs-SLC52A2/FFAR2 pathway, thus alleviating the inflammatory response in mice with dextran sulfate sodium (DSS)-induced UC (23). Additional research indicates that AKK improves colonic damage in DSS-induced UC by inhibiting the pro-inflammatory phenotypic shift in macrophages mediated by the HDAC5/DAB2 axis (24). Furthermore, AKK enhances retinoic acid production in dendritic cells, which helps regulate IL-22 activity and reduce colitis symptoms in mice (25). Moreover, Qu S et al. found that AKK can ease DSS-induced acute colitis through the activation of NLRP3 (26). Additionally, AKK has the ability to activate AhR signaling by influencing tryptophan metabolism, leading to a decrease in colonic inflammation (27).
Research indicates that a decrease in AKK correlates with elevated levels of pro-inflammatory cytokines, suggesting its significant role in regulating immune responses within the gut (13, 28). Dietary inclusion of AKK has been found to strengthen the intestinal barrier by enhancing the production of tight junction proteins like ZO-1 and Occludin-1, which are essential for preserving epithelial cell integrity (29). Luo Y and colleagues discovered that AKK might mitigate cell apoptosis by downregulating critical genes in the calcium signaling pathway or upregulating those in the cell cycle signaling pathway. Additionally, it may reduce inflammation in intestinal epithelial cells by inhibiting the expression of upstream receptor genes in the PI3K pathway (30). Moreover, AKK supplementation has been shown to lower inflammatory markers, offering protection against colitis induced by dextran sulfate sodium (DSS) (18). Importantly, the health advantages of AKK may also stem from its metabolites, particularly short-chain fatty acids (SCFAs), which could significantly influence the host's metabolic health and inflammatory conditions (31). AKK appears to be instrumental in modulating immune responses and enhancing gut health, thereby contributing to reduced inflammation in UC (32). Furthermore, evidence suggests that AKK can restore gut microbiota diversity, increasing the prevalence of beneficial bacteria like Bacteroides and Lactobacillus, which may further bolster its protective effects against colonic inflammation (33). Another significant discovery relates to the strain-specific effects of AKK, revealing that different strains can have distinct impacts on gut health. Liu Q and associates found that among three isolated human AKK strains (FSDLZ39M14, FSDLZ36M5, and FSDLZ20M4) and the AKK type strain ATCC BAA-835, only FSDLZ36M5 exhibited notable protective effects against UC by increasing colon length, decreasing intestinal permeability, and enhancing the expression of anti-inflammatory cytokines. The other strains (FSDLZ39M14, ATCC BAA-835, and FSDLZ20M4) did not demonstrate these benefits, with FSDLZ20M4 showing a tendency to worsen inflammation according to various indicators (34). This highlights the necessity of identifying specific strains with therapeutic potential for UC management and offers genetic targets for the efficient and rapid screening of AKK strains that may alleviate UC symptoms.
The potential therapeutic role of AKK in UC
AKK has become a key candidate for treating UC due to its vital function in supporting gut health and regulating inflammation. Research indicates that adding AKK can lead to notable improvements in the Disease Activity Index (DAI), colon length, and organ index (35). Furthermore, AKK has been shown to decrease the presence of macrophages and cytotoxic T lymphocytes (CTLs) in the colon, which helps to mitigate inflammatory reactions (36). A study by Liu F et al. revealed that AKK supplementation resulted in lower levels of pro-inflammatory cytokines, including IL-1β, IL-6, and MCP-1. This effect may be linked to AKK's involvement in host immune signaling pathways, particularly through members of the nucleotide-binding oligomerization domain-like receptor family, such as NLRP3, which is known to play a role in inflammation regulation (32). In a model of UC induced by dextran sulfate sodium (DSS), AKK supplementation improved intestinal barrier function, decreased the infiltration of inflammatory cells, and increased the expression of mucins like MUC2 and MUC3, which are essential for maintaining intestinal integrity (37). This suggests that enhancing AKK levels through diet or probiotics could be a viable approach for UC treatment. Additionally, the damage to the mucus layer that AKK relies on is thought to significantly contribute to UC's development; thus, its involvement in mucin degradation and metabolic functions may be critical for restoring gut balance (38). While the therapeutic potential of AKK is encouraging, further clinical trials are needed to assess its effectiveness and safety in patients with UC. More in-depth studies on specific strains and their mechanisms are crucial for fully understanding how to incorporate AKK into treatment plans for UC and other gastrointestinal issues (39).
The potential application value of AKK as a probiotic in children with UC
Current research on the role of AKK in IBD primarily focuses on adult and animal models. In pediatric populations, only two studies explicitly mention the association between this bacterium and IBD. Therefore, this section will explore the potential application value of AKK as a probiotic in children with UC.
In patients with UC, the reduction of AKK may be closely related to disease relapse and progression (40). This reduction could lead to a higher likelihood of disease recurrence, prompting studies to suggest that changes in the abundance of AKK may serve as a biomarker for predicting disease relapse (41). In pediatric populations, the gut microbiota is still developing, making it more susceptible to dysbiosis. The ability of AKK to modulate microbial composition and enhance mucosal immunity may be particularly beneficial, leading to the inference that this pathogenic mechanism is also applicable to pediatric patients. Furthermore, AKK has garnered attention for its potential protective role in gut health, especially among children with UC. It is believed that AKK can renew the mucus layer in the gut and enhance barrier integrity, which is often compromised in patients with UC (41). Therefore, enhancing mucosal barrier function is crucial, particularly for children, as further exacerbation of UC may have lasting impacts on their growth and development.Experimental studies have indicated that AKK supplementation can lead to positive outcomes, such as decreased inflammatory cell infiltration and enhanced mucin production (notably MUC2 and MUC3) in the intestinal lining (26). The administration of AKK seems to alleviate colitis symptoms by bolstering intestinal barrier function and mitigating inflammation (11), underscoring its potential as a treatment option for pediatric UC. Importantly, the characteristics of intestinal inflammation include immune cell infiltration and damage to goblet cells (41). The presence of sufficient AKK in the gut may counteract these issues, providing a supplementary strategy for managing pediatric UC. Therefore, AKK and other dietary or therapeutic interventions, such as prebiotics or fecal microbiota transplantation (FMT), are worth exploring to optimize treatment outcomes (42). Furthermore, understanding the phylogenetic variation and phenotypic diversity of AKK may offer further insights into personalized treatment approaches for pediatric UC. Different strains of AKK exhibit unique characteristics that may influence their effectiveness in modulating the gut environment and alleviating UC symptoms (43). Despite these promising findings, further research is needed to elucidate the protective role of AKK in pediatric UC and to establish standardized protocols for its clinical application. Large-scale, randomized controlled trials are essential to validate its efficacy, safety, and long-term benefits in pediatric populations.
In conclusion, AKK is a key element in understanding the microbiota's role in pediatric UC, with its capacity to influence gut health and immune responses presenting a promising path for therapeutic development. Ongoing studies are needed to clarify the specific mechanisms and best practices for employing this bacterium in treating pediatric UC, emphasizing the importance of further investigation into the therapeutic possibilities of probiotics like AKK, Especially in the group of children with UC.
Conclusion
While current research has suggested that AKK may play a significant role in UC, the exact mechanisms involved remain to be explored. Future studies might concentrate on: 1. how AKK interacts with the intestinal immune system; 2. the particular impact of AKK in ulcerative colitis among children; 3. the creation of innovative therapeutic strategies based on AKK, including probiotic products or their metabolites (44, 45).
To conclude, AKK is a crucial component of gut microbiota that significantly influences the development of UC. A decrease in its levels correlates strongly with the severity and relapse of the condition, suggesting that introducing AKK could be a promising treatment approach. Ongoing studies are expected to clarify how it functions, offering fresh perspectives for preventing and managing UC (Figure 2).
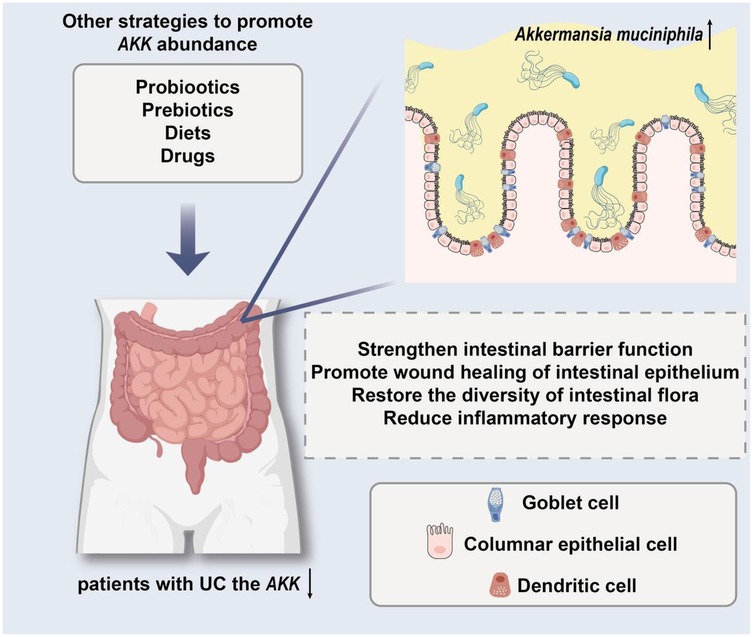
Figure 2. The model demonstrates the protective effect of targeting AKK in the treatment of ulcerative colitis (UC).
Author contributions
NX: Writing – original draft. XX: Writing – review & editing. YM: Writing – review & editing. JJ: Writing – review & editing. SJ: Writing – review & editing. KL: Writing – review & editing. YL: Writing – review & editing. MD: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Grant 1: Mechanism Study on the Involvement of Dysregulated Gut Microbiota in Regulating Breast Milk Jaundice. Grant Number: Qiankehe Foundation - ZK[2021]General371. Grant 2: Exploring the Interactions between Danshensu IIA and the Gut-Brain Axis in Neuroprotection Following Hypoxic-Ischemic Brain Injury in Neonatal Rats. Grant Number: gzwkj2022-399. Grant 3: Role and Mechanism of Dysregulated Gut Microbiota via the SCFAs-GPR41/43 Pathway in the Pathogenesis of Breast Milk Jaundice. Grant Number: 82260317. Source: National Natural Science Foundation of China. Grant 4: Study on the Value of Combined Detection of Umbilical Cord Blood and Urinary Cystatin C in the Early Diagnosis of Neonatal Ischemic Hypoxic Renal Injury Document. Grant Number: Zunshi Kehe HZ Zi (2024) No. 51.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tao Q, Liang Q, Fu Y, Qian J, Xu J, Zhu Y, et al. Puerarin ameliorates colitis by direct suppression of macrophage M1 polarization in DSS mice. Phytomedicine. (2024) 135:156048. doi: 10.1016/j.phymed.2024.156048
2. Kim YE, Kim SH, Kim SP, Park Y, Lee SH, Choi HJ, et al. Epidemiology of pediatric inflammatory bowel disease categorized by age subgroups in Korea. Pediatr Int. (2024) 66(1):e15786. doi: 10.1111/ped.15786
3. LeBlanc JF, Segal JP, de Campos Braz LM, Hart AL. The microbiome as a therapy in pouchitis and ulcerative colitis. Nutrients. (2021) 13(6):1780. doi: 10.3390/nu13061780
4. Seishima J, Iida N, Kitamura K, Yutani M, Wang Z, Seki A, et al. Gut-derived Enterococcus faecium from ulcerative colitis patients promotes colitis in a genetically susceptible mouse host. Genome Biol. (2019) 20(1):252. doi: 10.1186/s13059-019-1879-9
5. Dixit K, Chaudhari D, Dhotre D, Shouche Y, Saroj S. Restoration of dysbiotic human gut microbiome for homeostasis. Life Sci. (2021) 278:119622. doi: 10.1016/j.lfs.2021.119622
6. Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kanarek E, et al. The effectiveness and safety of multi-strain probiotic preparation in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled study. Nutrients. (2021) 13(3):756. doi: 10.3390/nu13030756
7. Ma WY, Feng SX, Chen NH. Advances of Akkermansia muciniphila in regulating host functions. Zhongguo Zhong Yao Za Zhi. (2021) 46(11):2760–5. doi: 10.19540/j.cnki.cjcmm.20210119.60134296573
8. Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. (2019) 12(6):1109–25. doi: 10.1111/1751-7915.13410
9. Li Z, Ke H, Wang Y, Chen S, Liu X, Lin Q, et al. Global trends in Akkermansia muciniphila research: a bibliometric visualization. Front Microbiol. (2022) 13:1037708. doi: 10.3389/fmicb.2022.1037708
10. Sundberg LR, Rantanen N, de Freitas Almeida GM. Mucosal environment induces phage susceptibility in Streptococcus mutans. Phage (New Rochelle). (2022) 3(3):128–35. doi: 10.1089/phage.2022.0021
11. Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, et al. Genotypic and phenotypic diversity among human isolates of Akkermansia muciniphila. mBio. (2021) 12(3):e00478-21. doi: 10.1128/mBio.00478-21
12. Li W, Sun J, Jing Y, Zhao J, Wu Q, Liu J, et al. Comparative genomics revealed wide intra-Species genetic heterogeneity and lineage-specific genes of Akkermansia muciniphila. Microbiol Spectr. (2022) :10(3):e0243921. doi: 10.1128/spectrum.02439-2135536024
13. Xue L, Zhao Y, Wang H, Li Z, Wu T, Liu R, et al. The effects of live and pasteurized Akkermansia muciniphila on DSS-induced ulcerative colitis, gut microbiota, and metabolomics in mice. Food Funct. (2023) 14(10):4632–46. doi: 10.1039/D2FO03493J
14. Ndongo S, Armstrong N, Raoult D, Fournier PE. Reclassification of eight Akkermansia muciniphila strains and description of Akkermansia massiliensis sp. nov. and Candidatus Akkermansia timonensis, isolated from human feces. Sci Rep. (2022) 12(1):21747. doi: 10.1038/s41598-022-25873-0
15. Hou F, Tang J, Liu Y, Tan Y, Wang Y, Zheng L, et al. Safety evaluation and probiotic potency screening of Akkermansia muciniphila strains isolated from human feces and breast milk. Microbiol Spectr. (2023) 11(2):e0336122. doi: 10.1128/spectrum.03361-22
16. Qian K, Chen S, Wang J, Sheng K, Wang Y, Zhang M. A β-N-acetylhexosaminidase amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. (2022) 13(4):2216–27. doi: 10.1039/D1FO04094D
17. Huang Z, Yang W, Wang X, Guo F, Cheng Y, Cao L, et al. Industrially produced rice protein ameliorates dextran sulfate sodium-induced colitis via protecting the intestinal barrier, mitigating oxidative stress, and regulating gut Microbiota. J Agric Food Chem. (2022) 70(16):4952–65. doi: 10.1021/acs.jafc.2c00585
18. Wang T, Shi C, Wang S, Zhang Y, Wang S, Ismael M, et al. Protective effects of companilactobacillus crustorum MN047 against dextran sulfate sodium-induced ulcerative colitis: a fecal Microbiota transplantation study. J Agric Food Chem. (2022) 70(5):1547–61. doi: 10.1021/acs.jafc.1c07316
19. Kim S, Shin YC, Kim TY, Kim Y, Lee YS, Lee SH, et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. (2021) 13(1):1–20. doi: 10.1080/19490976.2021.1892441
20. Trastoy B, Naegeli A, Anso I, Sjögren J, Guerin ME. Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from Akkermansia muciniphila. Nat Commun. (2020) 11(1):4844. doi: 10.1038/s41467-020-18696-y
21. Wade H, Pan K, Duan Q, Kaluzny S, Pandey E, Fatumoju L, et al. Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145. J Biomed Sci. (2023) 30(1):38. doi: 10.1186/s12929-023-00935-1
22. Renaud V, Houde VP, Pilon G, Varin TV, Roblet C, Marette A, et al. The concentration of organic acids in cranberry juice modulates the gut Microbiota in mice. Int J Mol Sci. (2021) 22(21):11537. doi: 10.3390/ijms222111537
23. Zhang L, Wang J, Xu Y, Wei K, Lin W, Hu H, et al. Akkermansia muciniphila relieves inflammatory response in DSS-induced ulcerative colitis in mice through regulating macrophage polarization via SCFAs-SLC52A2/FFAR2 pathway. Naunyn Schmiedebergs Arch Pharmacol. (2025) 398(7):8695–711. doi: 10.1007/s00210-025-03787-8
24. Miao Y, Wang M, Sun H, Zhang Y, Zhou W, Yang W, et al. Akkermansia muciniphila ameliorates colonic injury in mice with DSS-induced acute colitis by blocking macrophage pro-inflammatory phenotype switching via the HDAC5/DAB2 axis. Biochim Biophys Acta Mol Cell Res. (2024) 1871(7):119751. doi: 10.1016/j.bbamcr.2024.119751
25. Liu H, Huang R, Shen B, Huang C, Zhou Q, Xu J, et al. Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice. Microbiome. (2024) 12(1):275. doi: 10.1186/s40168-024-01995-7
26. Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (DSS)-induced acute colitis by NLRP3 activation. Microbiol Spectr. (2021) 9(2):e0073021. doi: 10.1128/Spectrum.00730-21
27. Gu Z, Pei W, Shen Y, Wang L, Zhu J, Zhang Y, et al. Akkermansia muciniphila and its outer protein amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. (2021) 12(20):10184–95. doi: 10.1039/D1FO02172A
28. Liu Y, Yin F, Huang L, Teng H, Shen T, Qin H. Long-term and continuous administration of Bacillus subtilis during remission effectively maintains the remission of inflammatory bowel disease by protecting intestinal integrity, regulating epithelial proliferation, and reshaping microbial structure and function. Food Funct. (2021) 12(5):2201–10. doi: 10.1039/d0fo02786c
29. Zong M, Chang C, Anjum R, Xu H, Guo Y, Pan D, et al. Multifunctional LPxTG-motif surface protein derived from limosilactobacillus reuteri SH 23 in DSS-induced ulcerative colitis of mice. Faseb J. (2023) 37(5):e22895. doi: 10.1096/fj.2022-0252-RR
30. Luo Y, Lan C, Xie K, Li H, Devillard E, He J, et al. Active or autoclaved Akkermansia muciniphila relieves TNF-α-induced inflammation in intestinal epithelial cells through distinct pathways. Front Immunol. (2021) 12:788638. doi: 10.3389/fimmu.2021.788638
31. Kim JS, Kang SW, Lee JH, Park SH, Lee JS. The evolution and competitive strategies of Akkermansia muciniphila in gut. Gut Microbes. (2022) 14(1):2025017. doi: 10.1080/19490976.2021.2025017
32. Liu F, Wang X, Li D, Cui Y, Li X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother Res. (2021) 35(3):1468–85. doi: 10.1002/ptr.6910
33. Dorofeyev A, Dorofeyeva A, Borysov A, Tolstanova G, Borisova T. Gastrointestinal health: changes of intestinal mucosa and microbiota in patients with ulcerative colitis and irritable bowel syndrome from PM2.5-polluted regions of Ukraine. Environ Sci Pollut Res Int. (2023) 30(3):7312–24. doi: 10.1007/s11356-022-22710-9
34. Liu Q, Lu W, Tian F, Zhao J, Zhang H, Hong K, et al. Akkermansia muciniphila exerts strain-specific effects on DSS-induced ulcerative colitis in mice. Front Cell Infect Microbiol. (2021) 11:698914. doi: 10.3389/fcimb.2021.698914
35. Zhang H, Pan Y, Jiang Y, Chen M, Ma X, Yu X, et al. Akkermansia muciniphila ONE effectively ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. NPJ Sci Food. (2024) 8(1):97. doi: 10.1038/s41538-024-00339-x
36. Zheng M, Han R, Yuan Y, Xing Y, Zhang W, Sun Z, et al. The role of Akkermansia muciniphila in inflammatory bowel disease: current knowledge and perspectives. Front Immunol. (2023) 13:1089600. doi: 10.3389/fimmu.2022.1089600
37. Leoncini G, Cari L, Ronchetti S, Donato F, Caruso L, Calafà C, et al. Mucin expression profiles in ulcerative colitis: new insights on the histological mucosal healing. Int J Mol Sci. (2024) 25(3):1858. doi: 10.3390/ijms25031858
38. Xue C, Li G, Gu X, Su Y, Zheng Q, Yuan X, et al. Health and disease: Akkermansia muciniphila, the shining star of the gut Flora. Research (Wash D C). (2023) 6:0107. doi: 10.34133/research.0107
39. Wu Z, Xu Q, Gu S, Chen Y, Lv L, Zheng B, et al. Akkermansia muciniphila ameliorates clostridioides difficile infection in mice by modulating the intestinal microbiome and metabolites. Front Microbiol. (2022) 13:841920. doi: 10.3389/fmicb.2022.841920
40. Mendes-Frias A, Moreira M, Vieira MC, Gaifem J, Costa P, Lopes L, et al. Akkermansia muciniphila and Parabacteroides distasonis as prognostic markers for relapse in ulcerative colitis patients. Front Cell Infect Microbiol. (2024) 14:1367998. doi: 10.3389/fcimb.2024.1367998
41. Singh V, Johnson K, Yin J, Lee S, Lin R, Yu H, et al. Chronic inflammation in ulcerative colitis causes long-term changes in goblet cell function. Cell Mol Gastroenterol Hepatol. (2022) 13(1):219–32. doi: 10.1016/j.jcmgh.2021.08.010
42. Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, et al. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. (2023) 72(7):1308–18. doi: 10.1136/gutjnl-2022-327853
43. Akiyama S, Onoda T, Moue S, Sakamoto N, Sakamoto T, Suzuki H, et al. Association of colonic metaplasia of goblet cells and endoscopic phenotypes of the J pouch in patients with ulcerative colitis: a retrospective pilot study. Intest Res. (2024) 22(1):92–103. doi: 10.5217/ir.2023.00105
44. Han W, Zhuang X. Research progress on the next-generation probiotic Akkermansia muciniphila in the intestine. Food Front. (2021) 2(4):443–8. doi: 10.1002/fft2.87
Keywords: Akkermansia muciniphila, ulcerative colitis, intestinal barrie, immune regulation, strain specificity, microecological therapy
Citation: Xu N, Xiao X, Ma Y, Jing J, Jiang S, Luo K, Li Y and Duan M (2025) Advancements in understanding the relationship between Akkermansia muciniphila and the development of ulcerative colitis. Front. Pediatr. 13:1673156. doi: 10.3389/fped.2025.1673156
Received: 25 July 2025; Accepted: 2 September 2025;
Published: 26 September 2025.
Edited by:
Almuthe Christina Hauer, Medical University Graz, AustriaReviewed by:
Michael J. Lentze, University Hospital Bonn, GermanyCopyright: © 2025 Xu, Xiao, Ma, Jing, Jiang, Luo, Li and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Duan, ZHVhbm1pYW9Aem11LmVkdS5jbg==
†Present Address: Nie Xu, Department of Pediatric, The Third People's Hospital of Bijie, Bijie, Guizhou, China
 Nie Xu
Nie Xu Xiaoping Xiao1
Xiaoping Xiao1 Ying Li
Ying Li