- 1Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 2Department of Clinical Science, Intervention, and Technology, Karolinska Institutet, Stockholm, Sweden
The global rise in pediatric obesity has paralleled an alarming increase in metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD). MASLD in children represents a significant public health concern due to its potential progression to advanced liver disease and its association with a myriad of cardiometabolic comorbidities. This review elucidates the risk and consequences of pediatric MASLD in the population of children with obesity. It also identifies critical research gaps and outlines future directions for the prevention, diagnosis, and treatment of pediatric MASLD.
Introduction
Pediatric obesity is a serious and evolving public health problem as the rates continue to rise (1), the age of onset decreases (2), and higher degree of obesity has become more prevalent (3). The earlier onset and the greater obesity severity likely contribute to a growing number of children living with obesity-related comorbidities. By 2035, at least 36 million children are projected to have comorbidities attributable to obesity (1). This will dramatically affect pediatric and adult healthcare.
One of the most common comorbidities in pediatric obesity is metabolic dysfunction associated steatotic liver disease (MASLD), which previously was known as non-alcoholic fatty liver disease (NAFLD). Approximately half of the pediatric obesity population is estimated to have MASLD (4). Despite its high prevalence, pediatric MASLD is often undiagnosed (5, 6), and even patients with positive initial screening results frequently lack further evaluation (5).
Given the vast number of children living with obesity, identifying subgroups at the highest risk of developing MASLD is crucial. MASLD can be reversed if detected and managed in the early stage (7). Adult studies have indicated the interaction between sex and age modulates the risk of MASLD (8), yet such an interaction has not been explored in children. Several studies in children have attempted to identify factors associated with the risk of MASLD, including metabolic parameters (9–13) and perinatal factors (14, 15). However, the findings were inconclusive. Moreover, the cross-sectional design of most studies (9, 10, 12, 13) limits the inference of the disease course. Besides, results from single-center studies (9, 11, 12, 14) required cautious interpretation.
Beside risk stratification, understanding the long-term consequences of pediatric MASLD is essential to determine the clinical course of the disease and the care pathway. Yet, current knowledge of pediatric MASLD outcomes remains limited. In adult MASLD, while only a minority develop severe form of MASLD, these patients have high risk of liver-related mortality (16). Conversely, among adults with the milder form of MASLD, cardiometabolic disease is the leading cause of death (17). Whether the findings in adults can be extrapolated to pediatrics is uncertain. Children with MASLD have been suggested to have worse metabolic and liver outcomes than adults (18–22) given that they have early adiposity exposure and long time to develop complications. Yet, because previous studies in children were from selected populations of patients undergoing liver biopsy (18–21), had small sample size (21), or had only few individuals with the event outcomes (22), the precision of the estimates and generalizability are limited. The purpose of this review was to elucidate the risk and consequences of pediatric MASLD in the population of children with obesity.
Epidemiology of pediatric obesity: growing in number in severity
Between 1990 and 2022 the global age-standardized prevalence of obesity in girls increased from 1.7% to 6.9%, while in boys it increased from 2.1% to 9.3% (23). In nearly 90% of countries worldwide, the prevalence of obesity among school-aged children and adolescents has doubled during this period (23). Moreover, obesity has also become more prevalent among preschool-aged children (24). The severity of obesity is also a public health concern. In the pediatric population, high degree of obesity (i.e., class II-III obesity) has increased 1.7-fold globally in the period 2007—2017 compared to 1967—2007 (3). In many European countries, 1 in 4 children with obesity had high degree of obesity (25).
Definition of pediatric obesity: a chronic relapsing disease, not only a condition
The World Health Organization defines obesity as “a chronic complex disease defined by excessive fat deposits that can impair health” (26). Further, obesity has a relapsing nature in a similar sense as, for example, hypertension (27, 28). Hence, long-term care is necessary. Yet, whether obesity is a disease is not universally accepted. It has been argued that a high body mass index (BMI) without any signs or symptoms is not enough to establish obesity diagnosis. Clinical obesity which is denoted as “a condition in which the risk to health associated with excess adiposity has already materialized and can be objectively documented by specific signs and symptoms reflecting biological alterations of tissues and organs, which are consistent with extant illness” was recently proposed (29). However, the specific signs and symptoms that define and measure this condition require further clarification. Also, it is uncertain if the term clinical obesity is appropriate for pediatric population considering that their symptoms are often subtle and cardiometabolic derangements often manifest in later childhood (30).
Causes, risk factors, pathogenesis of obesity: not as simple as eating too much
Obesity occurs as a long-term consequence of positive energy balance (i.e., when energy intake is more than energy expenditure). Nevertheless, the long-term positive energy balance is not caused by personal choice, but rather by a complex interaction between biological, individual, environmental, and societal factors (31–34), see Figure 1. In the molecular level, adipose tissue dysfunction, representing by adipocyte hypertrophy, adipose tissue expandability, hypoxia, and inflammation, does not only affect the development of obesity but also obesity-related comorbidities (35). Additionally, while causality remains unclear, gut dysbiosis has been shown to be associated with obesity development, both in animal models and human studies. For instance, Firmicutes/Bacteroidetes ratio seems to be higher in individuals with obesity than those with normal weight (36). Moreover, from environmental perspective, family socioeconomic position, local communities and culture, public policies on food and agriculture are indicated to influence the development of childhood obesity (31).
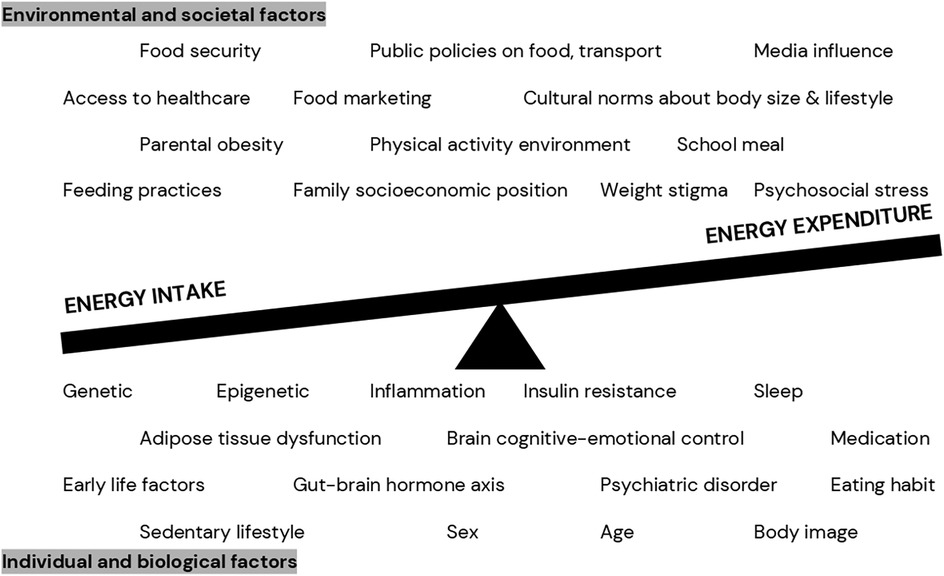
Figure 1. Complex relationship between various factors contributing to positive energy balance in pediatric obesity (31–34).
Measurement and diagnosis of obesity: do we actually measure adiposity?
There is no perfect tool to measure excess adiposity in daily practice. Multicomponent models using densitometry are often considered the gold standard of fat mass measurement. However, they are unsuitable for pediatric population, impractical for routine practice, and not error-free (e.g., hydration status may bias the result) (37).
To date, BMI is still widely utilized as the primary indirect measure of fatness because it is simple, and it identifies correctly most children with excess adiposity (38). Yet, given some substantial limitations of BMI (e.g., affected by muscle mass and stature), the combination of medical history, physical examination, and anthropometric measurement is essential in pediatric obesity work-up (33).
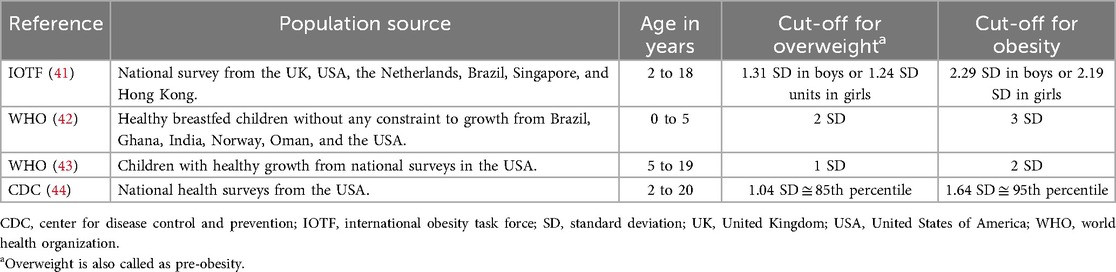
Because children grow in height and weight, BMI in children should be compared with a growth reference adjusted for sex and age and can be measured in a standard deviation score (SDS). A comparison of some well-known growth references is presented in Table 1. Despite the differences in population source and obesity cut-off, these references have high agreement in classifying weight status (39). Overall, the International Obesity Task Force (IOTF) reference yields the lowest prevalence of obesity, while the WHO reference generates the highest (40). For epidemiological studies, the IOTF reference, which is based on pooled international data and linked to the adult obesity cut-off, is widely used (31, 41).
While BMI SDS is generally appropriate to define obesity in children, the use of BMI SDS to monitor weight status over time should be considered carefully, especially in children with high degree of obesity, because it may overestimate or underestimate improvement of weight status depending on the age of the children (45). Non-SDS BMI metrics (e.g., BMI percent of the 95th percentile) have been suggested as the preferrable anthropometric measure in monitoring treatment response (46).
Comorbidities in pediatric obesity: almost all organs are affected
Childhood obesity is associated with increased risk of various conditions or diseases in almost all organs (34). The pathophysiology of obesity-related comorbidities is complex, with low-grade chronic inflammation caused by excess adiposity playing an important role (34). Figure 2 shows some diseases or conditions more prevalent in children with obesity than in their peers with normal weight (47), with MASLD as one of the most common comorbidities in children with obesity.
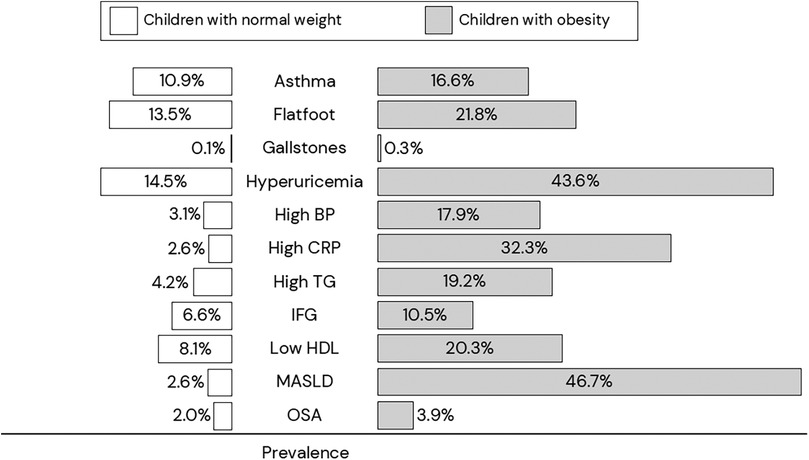
Figure 2. Prevalence of comorbidities in children with normal weight (white rectangle) vs children with obesity (gray rectangle) (47). BP, blood pressure; CRP, C-reactive protein; HDL, high-density lipoprotein cholesterol; IFG, impaired fasting hyperglycemia; MASLD, metabolic-associated steatotic liver disease; OSA, obstructive sleep apnea; TG, triglycerides.
Pediatric obesity management: no shortcuts
Similar to other chronic diseases, childhood obesity requires comprehensive and long-term care (48–51). Moreover, weight regain or rebound is not uncommon (52). Nevertheless, the aim of obesity treatment is not only adiposity reduction and maintenance, but also includes early detection and management of comorbidities, and improvement of quality of life, self-image, and health behavior (33, 48, 49).
Until now, the primary treatment of childhood obesity has been behavioral intervention addressing dietary, physical activity, sleep, and sedentary behavior (31, 48, 49, 51). A reduction of at least 0.25 BMI SDS units during lifestyle intervention has been shown to improve cardiometabolic markers (53, 54). However, obtaining such a BMI SDS reduction is rather challenging. In Sweden, the average BMI SDS reduction after a year of treatment is −0.14 units, with approximately 30% of the patients obtaining a reduction of at least 0.25 SDS units within the first year of obesity treatment (55). A meta-analysis has also shown that the effect of behavioral intervention given in 6–12 months for school-aged children with obesity is generally modest (56) and indicated that longer continued care is required. Additionally, BMI SDS has methodological limitations, especially when utilized in subgroups with high degree of obesity (e.g., in adolescents with class III obesity, a great difference in BMI corresponds to a small difference in SDS) (45).
Other treatment options for pediatric obesity include anti-obesity medications and bariatric surgery. Liraglutide and Semaglutide, belonging to the group of glucagon-like peptide-1 (GLP-1) receptor agonists, have been approved in Europe as pharmacotherapy in adolescents from age 12 years (57, 58). Liraglutide, administered subcutaneously once daily, in combination with lifestyle interventions, demonstrates a mean reduction in BMI SDS of 0.23 units after 56 weeks of treatment among adolescents with obesity and previous poor response to lifestyle alone (59). Semaglutide, administered subcutaneously once weekly, in combination with lifestyle intervention, results in a mean reduction in BMI SDS of 1.1 units after 68 weeks of treatment among adolescents with obesity and overweight who had associated morbidity (60). Setmelanotide, a melanocortin-4 receptor agonist, has been approved for children with obesity due to rare genetic variants that disrupt the melanocortin pathway (61). Additionally, in adult obesity treatment, Tirzepatide, a glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist, has shown good efficacy. Its efficacy and safety in pediatrics are still investigated in an ongoing phase-3 trial (62). Other than medications, bariatric surgery has also been introduced as a treatment option for adolescents with severe obesity (63). Among 81 adolescents with severe obesity treated in the specialized pediatric treatment centers in Sweden, the mean BMI reduction was 13.1 kg/m2 over 5 years (64).
A silent epidemic of MASLD
The estimated prevalence of pediatric MASLD varies depending on the diagnostic tools and the study population. A meta-analysis estimated an overall global prevalence of pediatric MASLD of 7.4% (95% CI: 4.2%–12.8%) in the general population and 52.5% (95% CI: 46.2%–58.7%) in the pediatric obesity population. Despite its high estimated prevalence, MASLD is often underscreened and underdiagnosed in the pediatric obesity population (5).
Among children with MASLD, the prevalence of MASH and fibrosis is challenging to establish given that liver biopsy is usually required to confirm MASH and fibrosis. Among children with biopsy-proven MASLD treated in tertiary care, approximately 20%–50% of them had MASH and 10%–20% had advanced fibrosis at the time of diagnosis (65). However, given the selected population, the prevalence is likely to overestimate the real-world prevalence.
Definition of MASLD: one name with a large spectrum of disease phenotypes
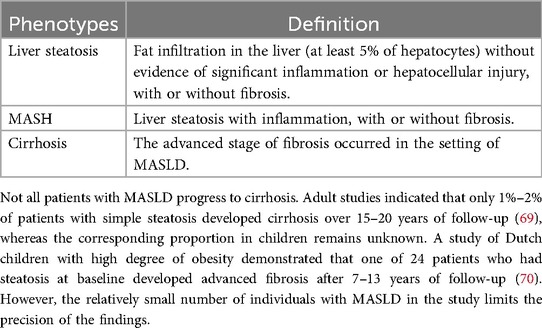
According to a recent international consensus, MASLD is defined as “the presence of hepatic steatosis in conjunction with one cardiometabolic risk factor and no other discernible cause” (66). Despite being a single disease, MASLD encompasses a broad spectrum of phenotypes differentiated by liver histology (7, 66, 67), see Table 2. Worsening histology appears to be associated with higher mortality (68).
NAFLD, MAFLD, MASLD: are they interchangeable?
The term for steatotic liver disease in children has changed over time. After the first case report in the 1980s (71), the disease was called non-alcoholic fatty liver disease (NAFLD). This term has been long criticized for its ambiguity (i.e., “non-alcoholic” does not explain the etiology) and inaccuracy (i.e., alcohol disorder is not a major concern in children). In 2021, a new term called metabolic dysfunction associated fatty liver disease (MAFLD) and a change in diagnostic criteria was proposed (72). As the word “fatty” is considered stigmatizing, among other considerations, in 2023 a new term called metabolic dysfunction associated steatotic liver disease (MASLD) and its detailed diagnosis criteria for adults and pediatrics was established (66). Later in 2024, pediatric societies supported the new term MASLD yet highlighted some essential considerations in diagnosis that are unique in children (73).
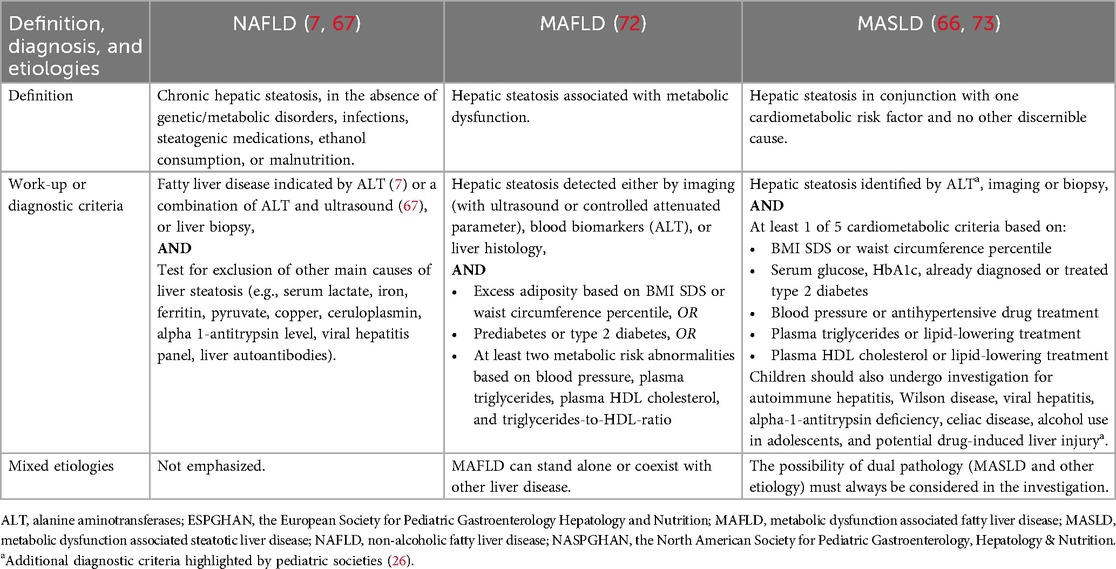
NAFLD, MAFLD, and MASLD are not only about changes in nomenclature but also changes in the definition and diagnostic criteria. A key difference is that, unlike NAFLD, both MAFLD and MASLD require evidence of metabolic dysfunction. Table 3 shows the comparison of definition and diagnostic criteria for NAFLD, MAFLD, and MASLD.
An important question is whether the previous findings on NAFLD or MAFLD studies can be extrapolated to MASLD. The short answer is yes, especially in obesity population. In general pediatric population in the United States, 80% of patients with NAFLD met the criteria for MAFLD, whereas all children with NAFLD fulfilled the MAFLD criteria in a Chinese pediatric obesity population (74). In a Taiwanese cohort of school-aged children, the prevalence of NAFLD and MASLD was equal within the study population (75). In general adult population in Sweden, 99.5% of patients with NAFLD met the MASLD criteria (76).
Causes, risk factors, pathogenesis of MASLD: multiorgan crosstalk
Although not clearly understood yet, the most widely accepted pathogenesis of pediatric MASLD is called the “multiple-hit theory”, whereby MASLD occurs due to crosstalk between multiple organs, including the liver, adipose tissue, pancreas, and gut (77). Briefly, the ‘first hit’ is marked by free fatty acid accumulation in the liver (77). Sources of free fatty acids are dietary intake, lipolysis in adipocytes, and hepatic lipogenesis, which are associated with obesity and insulin resistance (78). The “second hit” is marked by inflammation and cell death due to lipotoxicity. The mechanism of how steatosis environment triggers inflammation remains unclear, but oxidative stress, mitochondrial dysfunction, proinflammatory cytokines imbalance, and dysbiosis of gut microbiota play roles (77, 79). The ‘third hit’ is the sequences of wound healing after inflammation and cell death. Hepatic stellate cells are activated, differentiate to myofibroblast, and subsequently, promote the regeneration of hepatocytes. However, when liver injury occurs repetitively, the regeneration process is impaired, leading to liver fibrosis (78).
Obesity is known as the greatest risk factor for MASLD (73, 80). The interplay between obesity and insulin resistance plays a major role in liver fat accumulation (77). Excessive fructose intake has also been suggested to promote liver steatosis and inflammation by inducing hepatic lipogenesis, increasing hepatic insulin resistance, and affecting the gut-liver axis (81). A large meta-analysis showed that added fructose intake from various food sources such as biscuits, cake, or sugar-sweetened beverages was associated increased risk of MASLD (82). Additionally, while findings on the association between different types of fat intake and MASLD were inconclusive (83), low-fat diet has been reported to be associated with regression of MASLD (84). This suggests the role of fat intake in MASLD pathogenesis. Moreover, the effect of maternal adiposity during pregnancy on the increased liver fat content in the offspring has also been indicated (85).
There are also some non-modifiable factors associated with pediatric MASLD, such as genetic variants, birth weight, and sex. Among all studied genetic variants, the patatin-like phospholipase domain containing 3 (PNPLA3) gene is the most established variant associated with an increased likelihood of liver steatosis and liver injury in children and adolescents (86). Birth weight (both low and high birth weight) and its association with MASLD occurrence and MASLD severity have also been reported (15, 87). However, whether the association between birth weight and MASLD is mediated by childhood obesity is unclear. In addition, boys have a higher likelihood of MASLD compared to girls (12, 13).
In the absence of a good simple test, how to establish MASLD?
Until now, the gold standard to define the presence and severity of pediatric MASLD is liver biopsy. However, biopsy should not be performed in all children with suspected MASLD considering its invasiveness and that general anesthesia is commonly required in children (88, 89). Liver biopsy is recommended in particular cases, for instance to exclude other liver diseases (that cannot be excluded using non-invasive tests), to ascertain advanced disease or possibility of multiple liver diagnoses, before surgical treatment or potentially hepatotoxic medications, and in clinical trials (88, 89). Biopsy also has some drawbacks, such as sampling error (7) and relatively poor inter-rater reliability among hepatopathologists in assessing steatohepatitis (90).
Alanine aminotransferase (ALT), an enzyme found predominantly in hepatocytes, has been recommended by pediatric hepatology and obesity guidelines as a biomarker for MASLD screening (7, 48, 67, 73, 89, 91, 92). Despite its limitations (e.g., within-individual variability, mediocre sensitivity, not a direct marker of steatosis), ALT remains the primary biomarker for pediatric MASLD due to its availability, practicality, low cost, and accuracy compared to other existing non-invasive tests. Moreover, elevated ALT in children with excess adiposity is very likely due to MASLD (7, 73, 93). Nevertheless, ALT threshold for positive MASLD screening is various [e.g., a threshold of 22 U/L for girls and 25 U/L for boys was recommended by the Endocrine Society (91), 35 U/L by ESPGHAN (67), 44 U/L for girls and 52 U/L for boys by NASPGHAN and AAP (7, 48), 48 U/L by the Swedish pediatric guidelines (49)] or not mentioned (94). Also, guidelines’ recommendations vary in what to do when positive screening is found.
Unlike in adult MASLD, the use of prediction scores and imaging to detect steatosis or fibrosis is not as established in children. Some existing biomarkers or prediction scores to detect adult steatosis (fatty liver index score, NAFLD liver fat score) or fibrosis (e.g., aspartate aminotransferase-to-platelet ratio, Fibrosis-4 Index) have low accuracy or need further validations in children (92, 95). To detect steatosis in children, ultrasound has low accuracy in detecting mild steatosis (i.e., liver steatosis <30%) and longitudinal changes of steatosis (7, 48, 92). Better accuracy in quantifying steatosis in children is shown by controlled attenuation parameter (CAP) and magnetic resonance imaging-proton density fat fraction (MRI-PDFF) (96, 97). Yet, further studies to determine the CAP threshold are warranted, and MRI utilization is limited given its high cost and sedation-required for young children. To detect fibrosis, the performance of currently available imaging (e.g., transient elastography and magnetic resonance elastography) in pediatric populations needs to be investigated further (98). Non-invasive test with good accuracy in detecting MASLD in children is urgently needed.
A diagnostic pathway for MASLD has recently been clarified by pediatric societies (Figure 3). In the diagnostic pathway (Figure 3), it is important to note that other causes of liver steatosis in children, including Wilson disease, autoimmune hepatitis, viral hepatitis, alpha 1-antitypsin deficiency, lysosomal acid lipase deficiency, should not be overlooked (99–101).
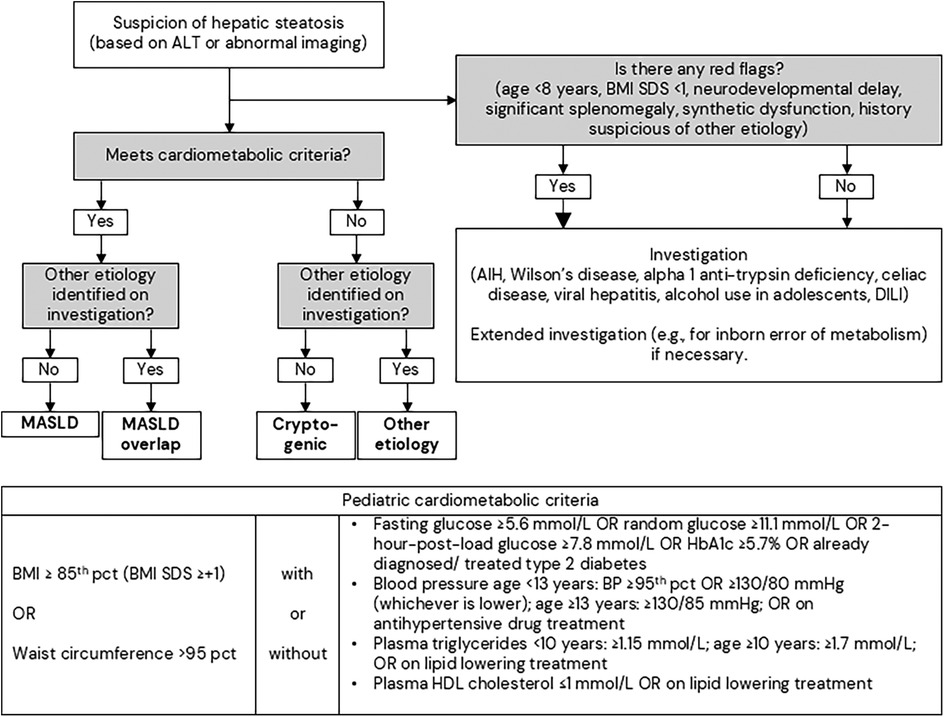
Figure 3. Diagnostic pathway for steatotic liver disease in children. The bigger arrow represents that immediate investigation is required. The figure is modified from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN); European Association for the Study of the Liver (EASL); North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN); Pediatric steatotic liver disease has unique characteristics: A multisociety statement endorsing the new nomenclature. J Pediatr Gastroenterol Nutr. 2024 (73), used under a Creative Commons Attribution 4.0 International License. AIH, autoimmune hepatitis; ALT, alanine aminotransferases; BMI, body mass index; DILI, drug-induced liver injury; MASLD, metabolic dysfunction associated steatotic liver disease; pct, percentile; SDS, standard deviation score.
Comorbidities of MASLD: liver and beyond
A vast literature on adult MASLD showed that MASLD is not only associated with the incidence of cirrhosis and hepatocellular carcinoma (102) but also associated with increased risk of other diseases, including type 2 diabetes (103), cardiovascular disease (104), chronic kidney disease (105), hypertension (106). Whether such comorbidities also occur in pediatric MASLD to a similar extent is uncertain.
With regards to end-stage liver disease, pediatric MASLD has been assumed to be more harmful than the adult type as the disease course starts much earlier (107). However, previous findings were conflicting (21, 70) and large longitudinal studies are lacking.
The association between pediatric MASLD and type 2 diabetes has been shown in a population of 892 children with biopsy-proven MASLD (108) and a national population of Israeli adolescents before military service (22). However, the selected population in the studies (22, 108) limits the generalizability and the small number of patients developing type 2 diabetes (22) makes the estimates uncertain. Limited data an association between MASLD in youth and conditions such as atherosclerosis, decreased bone mineral density, chronic kidney disease, and obstructive sleep apnea (109). Population-based longitudinal studies are required to confirm the findings.
MASLD management: hits two targets with one arrow
Not only for obesity, lifestyle intervention also remains the cornerstone for pediatric MASLD management (7, 94). Reduction in BMI SDS through lifestyle intervention is associated with reduction of liver fat and transaminases (110, 111). In adults, Resmetirom, a thyroid hormone receptor beta-selective agonist, has shown good efficacy in MASH resolution and fibrosis improvement (112). Additionally, Semaglutide and Liraglutide seem to have promising effects on adult MASH resolution (113, 114). However, to date, there is no approved pharmacotherapy for pediatric MASLD. For adolescents with severe obesity and non-cirrhotic MASH, gastric bypass surgery followed by long-term follow-up can be considered (63).
MASLD in pediatric obesity: risk factors and consequences
Male and older age are independently associated with increased risk for MASLD (12, 13). Furthermore, interaction between age and sex on the risk of MASLD was indicated (115); the risk seems to be increasing in boys with increasing age, while the risk tends to be constant in girls. While birthweight is known to be associated with MASLD in general pediatric population (15), a large multinational cohort of children showed that small for gestational age was associated with increased risk of MASLD and other cardiometabolic factors in children with obesity (116).
It has been suggested that early exposure to adiposity and MASLD may lead to worse liver outcomes (21, 107). A recent Swedish nationwide study found that children with obesity had an increased risk for major adverse liver outcomes compared to their peers in the general population (117). Moreover, as adult MASLD is known to be positively associated with type 2 diabetes (103, 118), we also showed that pediatric MASLD increases the risk and accelerates the onset of type 2 diabetes in children with obesity.
While relative weight loss in pediatric obesity has indeed beneficial effects, weight loss in children is complex given their weight and height are naturally growing over time. Several pediatric trials have shown that relative weight loss improves metabolic biomarkers (119) and that reduction of at least 0.25 BMI SDS units is clinically beneficial (53, 54). This beneficial effect of obesity treatment was confirmed in a real-world study showing that reduction of at least 0.25 BMI SDS units in a long-term pediatric obesity treatment reduced the risk of MASLD (120).
Discussion
This review highlights the rapid evolution in our understanding of pediatric obesity and MASLD, from its escalating prevalence to the intricate molecular pathways that drive its progression. The reclassification of NAFLD to MASLD epitomizes a fundamental shift in scientific thought, emphasizing the metabolic underpinnings of the disease and setting the stage for more targeted research and clinical approaches.
When resources in management of pediatric obesity and MASLD are limited, risk-stratified care can be useful. MASLD screening in children with obesity should be prioritized in children with higher degree of obesity, impaired fasting glycemia, or elevated triglycerides. Moreover, perinatal factors, especially birth weight for gestational age, are important to assess in managing pediatric obesity because it is associated with increased risk of developing cardiometabolic diseases. MASLD in pediatric obesity should not be overlooked because it increases the risk of youth-onset type 2 diabetes considerably. Moreover, MASLD in pediatric obesity may contribute to an increased risk of major adverse liver outcomes.
The close link between degree of obesity, cardiometabolic derangement, pediatric MASLD, future risk of youth-onset type 2 diabetes, and severe liver disease underscores the need for collaborative multidisciplinary care. Current screening and treatment guidelines for pediatric MASLD vary widely and are rather inconsistent, contributing to many MASLD cases being undiagnosed or neglected.
In conclusion, pediatric MASLD represents a critical and growing health challenge with far-reaching consequences. While significant progress has been made in understanding its epidemiology and pathogenesis, substantial research gaps persist, particularly regarding long-term outcomes and effective, targeted therapies. Future research direction must prioritize finding accurate non-invasive tests to diagnose and monitor pediatric MASLD. Without reliable non-invasive tests, studies in pediatric MASLD would consistently be hampered by misclassification or selection bias, weakening the internal validity. Developing such a reliable non-invasive test would remarkably accelerate our understanding of pediatric MASLD. Furthermore, a unified international guideline from pediatric hepatology, endocrinology, and obesity experts is of importance to improve the holistic and comprehensive care for children with MASLD. Raising pediatric MASLD awareness and knowledge among healthcare providers (both liver and non-liver specialists) and the population at the greatest risk is important to halt disease progression.
Author contributions
RP: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support was provided by Freemason Foundation for Children's Welfare in Stockholm, the Foundation of Sällskapet Barnavård, and the HRH Crown Princess Lovisa Society for Child Care. The funding sources had no involvement in manuscript writing or the decision to submit the article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lobstein T, Powis J, Jackson-Leach R. World Obesity Atlas 2024. (2024). Available online at: https://data.worldobesity.org/publications/?cat=22 (Accessed June 1, 2025).
2. Cunningham SA, Hardy ST, Jones R, Ng C, Kramer MR, Narayan KMV. Changes in the incidence of childhood obesity. Pediatrics. (2022) 150(2):e2021053708. doi: 10.1542/peds.2021-053708
3. Pinhas-Hamiel O, Hamiel U, Bendor CD, Bardugo A, Twig G, Cukierman-Yaffe T. The global spread of severe obesity in toddlers, children, and adolescents: a systematic review and meta-analysis. Obes Facts. (2022) 15(2):118–34. doi: 10.1159/000521913
4. Li J, Ha A, Rui F, Zou B, Yang H, Xue Q, et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000–2021. Aliment Pharmacol Ther. (2022) 56(3):396–406. doi: 10.1111/apt.17096
5. Sahota AK, Shapiro WL, Newton KP, Kim ST, Chung J, Schwimmer JB. Incidence of nonalcoholic fatty liver disease in children: 2009–2018. Pediatrics. (2020) 146(6):1–4. doi: 10.1542/peds.2020-0771
6. Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. (2005) 147(6):839–42. doi: 10.1016/j.jpeds.2005.07.020
7. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the North American society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. (2017) 64(2):319–34. doi: 10.1097/MPG.0000000000001482
8. Lonardo A, Nascimbeni F, Ballestri S, Fairweather DL, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. (2019) 70(4):1457–69. doi: 10.1002/hep.30626
9. Bedogni G, Gastaldelli A, Manco M, De Col A, Agosti F., Tiribelli C., et al. Relationship between fatty liver and glucose metabolism: a cross-sectional study in 571 obese children. Nutr, Metab Cardiovasc Dis. (2012) 22(2):120–6. doi: 10.1016/j.numecd.2010.05.003
10. Koutny F, Weghuber D, Bollow E, Greber-Platzer S, Hartmann K, Körner A, et al. Prevalence of prediabetes and type 2 diabetes in children with obesity and increased transaminases in European German-speaking countries. analysis of the APV initiative. Pediatr Obes. (2020) 15(4):1–8. doi: 10.1111/ijpo.12601
11. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. (2008) 118(3):277–83. doi: 10.1161/CIRCULATIONAHA.107.739920
12. Jimenez-Rivera C, Hadjiyannakis S, Davila J, Hurteau J, Aglipay M, Barrowman N, et al. Prevalence and risk factors for non-alcoholic fatty liver in children and youth with obesity. BMC Pediatr. (2017) 17(1):113. doi: 10.1186/s12887-017-0867-z
13. Greber-Platzer S, Thajer A, Bohn S, Brunert A, Boerner F, Siegfried W, et al. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr. (2019) 19(1):332. doi: 10.1186/s12887-019-1711-4
14. Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. (2007) 30(10):2638–40. doi: 10.2337/dc07-0281
15. Newton KP, Feldman HS, Chambers CD, Wilson L, Behling C, Clark JM, et al. Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr. (2017) 187:141–146.e1. doi: 10.1016/j.jpeds.2017.03.007
16. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl AM, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. (2021) 385(17):1559–69. doi: 10.1056/nejmoa2029349
17. Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol-related fatty liver disease. Clin Gastroenterol Hepatol. (2019) 17(8):1625–1633.e1. doi: 10.1016/j.cgh.2018.11.033
18. Nobili V, Mantovani A, Cianfarani S, Alisi A, Mosca A, Sartorelli MR, et al. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J Hepatol. (2019) 71(4):802–10. doi: 10.1016/j.jhep.2019.06.023
19. Harlow KE, Africa JA, Wells A, Belt PH, Behling CA, Jain AK, et al. Clinically actionable hypercholesterolemia and hypertriglyceridemia in children with nonalcoholic fatty liver disease. J Pediatr. (2018) 198:76–83.e2. doi: 10.1016/j.jpeds.2018.02.038
20. Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. (2014) 164(4):707–713.e3. doi: 10.1016/j.jpeds.2013.10.071
21. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. (2009) 58(11):1538–44. doi: 10.1136/gut.2008.171280
22. Bardugo A, Bendor CD, Zucker I, Lutski M, Cukierman-Yaffe T, Derazne E, et al. Adolescent nonalcoholic fatty liver disease and type 2 diabetes in young adulthood. J Clin Endocrinol Metab. (2021) 106(1):e34–44. doi: 10.1210/clinem/dgaa753
23. Phelps NH, Singleton RK, Zhou B, Heap RA, Mishra A, Bennett JE, et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. (2024) 403(10431):1027–50. doi: 10.1016/S0140-6736(23)02750-2
24. Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. (2019) 17(1):212. doi: 10.1186/s12916-019-1449-8
25. Spinelli A, Buoncristiano M, Kovacs VA, Yngve A, Spiroski I, Obreja G, et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes Facts. (2019) 12(2):244–58. doi: 10.1159/000500436
26. World Health Organization. Obesity and overweight. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed March 23, 2023).
27. Burki T. European Commission classifies obesity as a chronic disease. Lancet Diabetes Endocrinol. (2021) 9(7):418. doi: 10.1016/S2213-8587(21)00145-5
28. Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes Rev. (2017) 18(7):715–23. doi: 10.1111/obr.12551
29. Rubino F, Batterham RL, Koch M, Mingrone G, le Roux CW, Farooqi IS, et al. Lancet diabetes & endocrinology commission on the definition and diagnosis of clinical obesity. Lancet Diabetes Endocrinol. (2023) 11(4):226–8. doi: 10.1016/S2213-8587(23)00058-X
30. Lycett K, Juonala M, Magnussen CG, Norrish D, Mensah FK, Liu R, et al. Body mass index from early to late childhood and cardiometabolic measurements at 11 to 12 years. Pediatrics. (2020) 146(2):e20193666. doi: 10.1542/peds.2019-3666
31. Jebeile H, Kelly AS, O'Malley G, Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. (2022) 10(5):351–65. doi: 10.1016/S2213-8587(22)00047-X
32. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15(5):288–98. doi: 10.1038/s41574-019-0176-8
33. Lister NB, Baur LA, Felix JF, Hill AJ, Marcus C, Reinehr T, et al. Child and adolescent obesity. Nat Rev Dis Prim. (2023) 9(1):2–10. doi: 10.1038/s41572-023-00435-4
34. Marcus C, Danielsson P, Hagman E. Pediatric obesity—long-term consequences and effect of weight loss. J Intern Med. (2022) 292(6):870–91. doi: 10.1111/joim.13547
35. Blüher M. Understanding adipose tissue dysfunction. J Obes Metab Syndr. (2024) 33(4):275–88. doi: 10.7570/jomes24013
36. Zhuang Z, Zhou P, Wang J, Lu X, Chen Y. The characteristics, mechanisms and therapeutics: exploring the role of gut microbiota in obesity. Diabetes Metab Syndr Obes. (2023) 16:3691–705. doi: 10.2147/DMSO.S432344
37. Norgan N. Laboratory and field measurements of body composition. Public Health Nutr. (2005) 8(7a):1108–22. doi: 10.1079/phn2005799
38. Simmonds M, Llewellyn A, Owen CG, Woolacott N. Simple tests for the diagnosis of childhood obesity: a systematic review and meta-analysis. Obes Rev. (2016) 17(12):1301–15. doi: 10.1111/obr.12462
39. Li K, Haynie D, Palla H, Lipsky L, Iannotti RJ, Simons-Morton B. Assessment of adolescent weight status: similarities and differences between CDC, IOTF, and WHO references. Prev Med. (2016) 87:151–4. doi: 10.1016/j.ypmed.2016.02.035
40. Llorca-Colomer F, Murillo-Llorente MT, Legidos-García ME, Palau-Ferré A, Pérez-Bermejo M. Differences in classification standards for the prevalence of overweight and obesity in children. A systematic review and meta-analysis. Clin Epidemiol. (2022) 14(null):1031–52. doi: 10.2147/CLEP.S375981
41. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. (2012) 7(4):284–94. doi: 10.1111/j.2047-6310.2012.00064.x
42. WHO Multicentre Growth Reference Study Group. WHO Child growth standards based on length/height, weight and age. Acta Paediatr Suppl. (2006) 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x
43. De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. (2007) 85(9):660–7. doi: 10.2471/BLT.07.043497
44. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth charts for the United States: methods and development. Vital Health Stat. (2002) 11(246):1–190.
45. Júlíusson PB, Roelants M, Benestad B, Lekhal S, Danielsen Y, Hjelmesæth J, et al. Severe obesity is a limitation for the use of body mass index standard deviation scores in children and adolescents. Acta Paediatr. (2018) 107(2):307–14. doi: 10.1111/apa.14113
46. Kelly AS, Bahlke M, Baker JL, de Beaufort C, Belin RM, Fonseca H, et al. Considerations for the design and conduct of pediatric obesity pharmacotherapy clinical trials: proceedings of expert roundtable meetings. Pediatr Obes. (2024) 19:1–9. doi: 10.1111/ijpo.13161
47. Sharma V, Coleman S, Nixon J, Sharples L, Hamilton-Shield J, Rutter H, et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes Rev. (2019) 20(10):1341–9. doi: 10.1111/obr.12904
48. Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Executive summary: clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. (2023) 151(2):11–47. doi: 10.1542/peds.2022-060641
49. Nationellt programområde för barns och ungdomars hälsa. Nationellt Vårdprogram För Behandling Av Obesitas Hos Barn Och Ungdomar. (2023).
50. Socialstyrelsen. Nationella Riktlinjer För Vård Och Stöd Vid Adhd Och Autism—Prioriteringsstöd till Beslutsfattare Och Chefer 2022. (2022).
51. UK National Institute for Health and Care Excellence (NICE). Obesity : identification, assessment and management. NICE Clin Guidel No 189. 2022. (2014). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK588750/ (Accessed June 1, 2025).
52. Wiegand S, Keller KM, Lob-Corzilius T, Pott W, Reinehr T, Röbl M, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. (2014) 82(6):380–7. doi: 10.1159/000368963
53. Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. (2016) 101(8):3171–9. doi: 10.1210/jc.2016-1885
54. Ford AL, Hunt LP, Cooper A, Shield JPH. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health?. Arch Dis Child. (2010) 95(4):256–61. doi: 10.1136/adc.2009.165340
55. The Swedish Childhood Obesity Treatment Register. BORIS Annual Report. (2022). Available online at: https://www.e-boris.se/for-vardgivare/arsrapporter/ (Accessed June 1, 2025).
56. Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. (2017) 2017(6):CD012651. doi: 10.1002/14651858.CD012651
57. European Medicines Agency. Wegovy (semaglutide). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy (Accessed September 6, 2024).
58. European Medicines Agency. Saxenda (liraglutide). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (Accessed November 6, 2024).
59. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM., Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. (2020) 382(22):2117–28. doi: 10.1056/nejmoa1916038
60. Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. (2022) 387(24):2245–57. doi: 10.1056/nejmoa2208601
61. Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. (2020) 8(12):960–70. doi: 10.1016/S2213-8587(20)30364-8
62. A Study of Tirzepatide (LY3298176) Once Weekly in Adolescent Participants Who Have Obesity, or Are Overweight With Weight-Related Comorbidities. (2023). Available online at: https://clinicaltrials.gov/ct2/show/NCT06075667. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02603102/full (Accessed September 9, 2024).
63. Nobili V, Vajro P, Dezsofi A, Fischler B, Hadzic N, Jahnel J, et al. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN hepatology committee position statement. J Pediatr Gastroenterol Nutr. (2015) 60(4):550–61. doi: 10.1097/MPG.0000000000000715
64. Olbers T, Beamish AJ, Gronowitz E, Flodmark CE, Dahlgren J, Bruze G, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. (2017) 5(3):174–83. doi: 10.1016/S2213-8587(16)30424-7
65. Yu EL, Schwimmer JB. Epidemiology of pediatric nonalcoholic fatty liver disease. Clin Liver Dis. (2021) 17(3):196–9. doi: 10.1002/cld.1027
66. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79(6):1542–56. doi: 10.1016/j.jhep.2023.06.003
67. Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr. (2012) 54(5):700–13. doi: 10.1097/MPG.0b013e318252a13f
68. Simon TG, Roelstraete B, Hartjes K, Shah U, Khalili H, Arnell H, et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J Hepatol. (2021) 75(5):1034–41. doi: 10.1016/j.jhep.2021.06.034
69. Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. (2005) 129(1):375–8. doi: 10.1053/j.gastro.2005.05.041
70. Draijer L, Voorhoeve M, Troelstra M, Holleboom A, Beuers U, Kusters M, et al. A natural history study of paediatric non-alcoholic fatty liver disease over 10 years. JHEP Rep. (2023) 5(5):100685. doi: 10.1016/j.jhepr.2023.100685
71. Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. (1983) 78(6):374–7. doi: 10.1111/j.1572-0241.1983.tb01898.x
72. Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. (2021) 6(10):864–73. doi: 10.1016/S2468-1253(21)00183-7
73. Baumann U, Koot BGP, Fitzpatrick E, Mann J, Vajro P, Krag A, et al. Paediatric steatotic liver disease has unique characteristics: a multisociety statement endorsing the new nomenclature. J Pediatr Gastroenterol Nutr. (2024) 78(5):1190–6. doi: 10.1002/jpn3.12156
74. Xing Y, Fan J, Wang HJ, Wang H. Comparison of MAFLD and NAFLD characteristics in children. Children. (2023) 10(3):1–13. doi: 10.3390/children10030560
75. Wang CW, Huang PC, Dai CY, Huang JF, Yu ML. Comparative evaluation of pediatric fatty liver disease criteria: MASLD, ESPGHAN, NASPGHAN, and NAFLD—identifying the optimal pediatric label. J Hepatol. (2024) 80(4):e157–9. doi: 10.1016/j.jhep.2024.01.004
76. Hagström H, Vessby J, Ekstedt M, Shang Y. 99% Of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol. (2024) 80(2):e76–7. doi: 10.1016/j.jhep.2023.08.026
77. Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J Gastroenterol. (2018) 24(27):2974–83. doi: 10.3748/wjg.v24.i27.2974
78. Draijer L, Benninga M, Koot B. Pediatric NAFLD: an overview and recent developments in diagnostics and treatment. Expert Rev Gastroenterol Hepatol. (2019) 13(5):447–61. doi: 10.1080/17474124.2019.1595589
79. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. (2018) 68(2):280–95. doi: 10.1016/j.jhep.2017.11.014
80. Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. (2003) 143(4):500–5. doi: 10.1067/S0022-3476(03)00325-1
81. Porto A, Pan Z, Zhou W, Sokol RJ, Klaczkiewicz K, Sundaram SS. Macronutrient and micronutrient intake in adolescents with non-alcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. (2022) 75(5):666–74. doi: 10.1097/MPG.0000000000003578
82. Liu W, Zhai D, Zhang T, Mudoti NG, Chang Q, Liu Y, et al. Meta-analysis of the association between major foods with added fructose and non-alcoholic fatty liver disease. Food Funct. (2023) 14(12):5551–61. doi: 10.1039/d3fo00882g
83. Tsompanaki E, Thanapirom K, Papatheodoridi M, Parikh P, Chotai de Lima Y, Tsochatzis EA. Systematic review and meta-analysis: the role of diet in the development of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2023) 21(6):1462–1474.e24. doi: 10.1016/j.cgh.2021.11.026
84. Alferink LJM, Erler NS, de Knegt RJ, Janssen HLA, Metselaar HJ, Darwish Murad S, et al. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur J Epidemiol. (2020) 35(11):1069–85. doi: 10.1007/s10654-020-00627-2
85. Cantoral A, Montoya A, Luna-Villa L, Roldán-Valadez EA, Hernández-Ávila M, Kershenobich D, et al. Overweight and obesity status from the prenatal period to adolescence and its association with non-alcoholic fatty liver disease in young adults: cohort study. BJOG An Int J Obstet Gynaecol. (2020) 127(10):1200–9. doi: 10.1111/1471-0528.16199
86. Li J, Hua W, Ji C, Rui J, Zhao Y, Xie C, et al. Effect of the patatin-like phospholipase domain containing 3 gene (PNPLA3) I148M polymorphism on the risk and severity of nonalcoholic fatty liver disease and metabolic syndromes: a meta-analysis of paediatric and adolescent individuals. Pediatr Obes. (2020) 15(6):1–11. doi: 10.1111/ijpo.12615
87. Bugianesi E, Bizzarri C, Rosso C, Mosca A, Panera N, Veraldi S, et al. Low birthweight increases the likelihood of severe steatosis in pediatric non-alcoholic fatty liver disease. Am J Gastroenterol. (2017) 112(8):1277–86. doi: 10.1038/ajg.2017.140
88. Dezsőfi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzic N, et al. Liver biopsy in children: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr. (2015) 60(3):408–20. doi: 10.1097/MPG.0000000000000632
89. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. (2018) 67(1):328–57. doi: 10.1002/hep.29367
90. Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. (2020) 73(6):1322–32. doi: 10.1016/j.jhep.2020.06.025
91. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity—assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102(3):709–57. doi: 10.1210/jc.2016-2573
92. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American association for the study of liver diseases (AASLD). Endocr Pract. (2022) 28(5):528–62. doi: 10.1016/j.eprac.2022.03.010
93. Vajro P, Maddaluno S, Veropalumbo C. Persistent hypertransaminasemia in asymptomatic children: a stepwise approach. World J Gastroenterol. (2013) 19(18):2740–51. doi: 10.3748/wjg.v19.i18.2740
94. Arbetsgruppen för fettleversjukdom hos barn och ungdomar Svenska Barnläkarförenings delförening för gastroenterologi hepatologi och nutrition. Utredning Och Handläggning Av Fettleversjukdom Hos Barn Och Ungdomar Med Övervikt Och Fetma. (2021).
95. Koot BGP, Van Der Baan-Slootweg OH, Bohte AE, Nederveen AJ, van Werven JR, Tamminga-Smeulders CLJ, et al. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity. (2013) 21(3):583–90. doi: 10.1002/oby.20173
96. Desai NK, Harney S, Raza R, Al-Ibraheemi A, Shillingford N, Mitchell PD, et al. Comparison of controlled attenuation parameter and liver biopsy to assess hepatic steatosis in pediatric patients. J Pediatr. (2016) 173:160–164.e1. doi: 10.1016/j.jpeds.2016.03.021
97. Jia S, Zhao Y, Liu J, Guo X, Chen M, Zhou S, et al. Magnetic resonance imaging-proton density fat fraction vs. Transient elastography-controlled attenuation parameter in diagnosing non-alcoholic fatty liver disease in children and adolescents: a meta-analysis of diagnostic accuracy. Front Pediatr. (2022) 9:1–11. doi: 10.3389/fped.2021.784221
98. Mosca A, Panera N, Crudele A, Alisi A. Noninvasive diagnostic tools for pediatric NAFLD: where are we now? Expert Rev Gastroenterol Hepatol. (2020) 14(11):1035–46. doi: 10.1080/17474124.2020.1801413
99. Pokorska-Śpiewak M, Kowalik-Mikołajewska B, Aniszewska M, Pluta M, Walewska-Zielecka B, Marczyńska M. Liver steatosis in children with chronic hepatitis B and C: prevalence, predictors, and impact on disease progression. Medicine. (2017) 96(3):e5832. doi: 10.1097/MD.0000000000005832
100. Di Giorgio A, D’Adda A, Marseglia A, Sonzogni A, Licini L, Nicastro E, et al. Biliary features in liver histology of children with autoimmune liver disease. Hepatol Int. (2019) 13(4):510–8. doi: 10.1007/s12072-019-09948-1
101. Besler KJ, Blanchard V, Francis GA. Lysosomal acid lipase deficiency: a rare inherited dyslipidemia but potential ubiquitous factor in the development of atherosclerosis and fatty liver disease. Front Genet. (2022) 13:1013266. doi: 10.3389/fgene.2022.1013266
102. Akbari C, Dodd M, Stål P, Nasr P, Ekstedt M, Kechagias S, et al. Long-term major adverse liver outcomes in 1,260 patients with non-cirrhotic NAFLD. JHEP Rep. (2024) 6(2):100915. doi: 10.1016/j.jhepr.2023.100915
103. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. (2021) 70(5):962–9. doi: 10.1136/gutjnl-2020-322572
104. Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6(11):903–13. doi: 10.1016/S2468-1253(21)00308-3
105. Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. (2014) 11(7):e1001680. doi: 10.1371/journal.pmed.1001680
106. Ciardullo S, Grassi G, Mancia G, Perseghin G. Nonalcoholic fatty liver disease and risk of incident hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2022) 34(4):365–71. doi: 10.1097/MEG.0000000000002299
107. Wong VWS, Ekstedt M, Wong GLH, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. (2023) 79(3):842–52. doi: 10.1016/j.jhep.2023.04.036
108. Newton KP, Wilson LA, Crimmins NA, Fishbein MH, Molleston JP, Xanthakos SA, et al. Incidence of type 2 diabetes in children with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2023) 21(5):1261–70. doi: 10.1016/j.cgh.2022.05.028
109. Pacifico L, Perla FM, Roggini M, Andreoli G, D’avanzo M, Chiesa C. A systematic review of NAFLD-associated extrahepatic disorders in youths. J Clin Med. (2019) 8(6):868. doi: 10.3390/jcm8060868
110. Nobili V, Marcellini M, Devito R, Ciampalini Paolo, Piemonte Fiorella, Comparcola Donatella, et al. NAFLD In children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. (2006) 44(2):458–65. doi: 10.1002/hep.21262
111. Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with nonalcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. (2009) 94(6):437–42. doi: 10.1136/adc.2008.143594
112. Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. (2024) 390(6):497–509. doi: 10.1056/nejmoa2309000
113. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. (2021) 384(12):1113–24. doi: 10.1056/nejmoa2028395
114. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. (2016) 387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X
115. Putri RR, Casswall T, Hagman E. Prevalence of increased transaminases and its association with sex, age, and metabolic parameters in children and adolescents with obesity—a nationwide cross-sectional cohort study. BMC Pediatr. (2021) 21(1):271. doi: 10.1186/s12887-021-02747-4
116. Prinz N, Putri RR, Reinehr T, Danielsson P, Weghuber D, Norman M, et al. The association between perinatal factors and cardiometabolic risk factors in children and adolescents with overweight or obesity: a retrospective two-cohort study. PLoS Med. (2023) 20(1):1–17. doi: 10.1371/journal.pmed.1004165
117. Putri RR, Casswall T, Danielsson P, Marcus C, Hagman E. The association between childhood obesity and major adverse liver outcomes in adolescence and young adulthood. JHEP Rep. (2025) 7(7):101425. doi: 10.1016/j.jhepr.2025.101425
118. Castera L, Laouenan C, Vallet-Pichard A, Vidal-Trécan T, Manchon P, Paradis V, et al. High prevalence of NASH and advanced fibrosis in type 2 diabetes: a prospective study of 330 outpatients undergoing liver biopsies for elevated alt, using a low threshold. Diabetes Care. (2023) 46(7):1354–62. doi: 10.2337/dc22-2048
119. Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. (2012) 130(6):e1647–71. doi: 10.1542/peds.2012-1176
Keywords: obesity, childhood obesity, MASLD, NAFLD, fatty liver, steatosis, pediatric, pediatric obesity
Citation: Putri RR (2025) The evolving landscape of pediatric obesity and metabolic dysfunction-associated steatotic liver disease. Front. Pediatr. 13:1675713. doi: 10.3389/fped.2025.1675713
Received: 29 July 2025; Accepted: 13 October 2025;
Published: 28 October 2025.
Edited by:
Luis Peña-Quintana, University of Las Palmas de Gran Canaria, SpainReviewed by:
Angelo Di Giorgio, Papa Giovanni XXIII Hospital, ItalyAntonella Mosca, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright: © 2025 Putri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Resthie R. Putri, cmVzdGhpZS5wdXRyaUBraS5zZQ==
 Resthie R. Putri
Resthie R. Putri