- Department of Pediatrics, Fuyang Women and Children's Hospital, Fuyang, China
Background: This study aimed to optimize risk prediction of severe community-acquired pneumonia (CAP) in children through age-stratified selection of albumin-based inflammatory ratios.
Methods: This retrospective study analyzed 1,071 pediatric CAP patients (aged 1–12 years). Propensity score matching (1:2 severe-to-mild ratio) within three age strata (1–3 years, 4–6 years, 7–12 years) generated balanced cohorts (N = 360). Neutrophil percentage-to-albumin ratio (NPAR), C-reactive protein-to-albumin ratio (CAR), and CRP × lymphocyte-to-albumin ratio (CALLY) were evaluated using multivariate logistic regression and ROC analysis.
Results: Severe CAP incidence increased with age (1–3 years: 7.37%; 4–6 years: 12.80%; 7–12 years: 18.04%). Multivariate analysis identified NPAR as the sole independent predictor for younger children (1–3 years: OR = 10.289; 4–6 years: OR = 35.117), while CAR predicted severe CAP in older children (7–12 years: OR = 3.342). ROC analysis demonstrated robust performance for NPAR in 1–6 years (AUC: 0.748–0.807, NPV: 85.9–88.0%) and CAR in 7–12 years (AUC: 0.734, NPV: 83.0%).
Conclusions: NPAR (for ages 1–6 years) and CAR (for ages 7–12 years) serve as effective, age-specific biomarkers for severe CAP, facilitating precise risk stratification with high negative predictive value.
Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality among children under 5 years old globally (1). Data from 2016 showed that lower respiratory tract infections caused a total of 2,377,697 deaths worldwide, including 652,572 deaths among children under 5 years old. Sub-Saharan Africa had the highest proportion (27.4%), followed by South Asia (24.8%); the proportion in Latin America and the Caribbean was 6.8% (2–4). In Colombia, the mortality rate of children under 5 years old due to acute respiratory infections reached 14.89 per 100,000 in 2018 (5). Approximately 155 million new cases of CAP occur globally each year (with an incidence of about 0.22 episodes per child per year), of which as many as 10%–17% require hospitalization (4). Patients with CAP present with varying severity levels. Most have mild CAP and can be treated as outpatients with low mortality rates. Moderate CAP patients require hospitalization but generally survive despite higher mortality risks. Severe CAP cases necessitate ICU management and have extremely high short- and long-term mortality rates (6).
Accurate risk stratification of pediatric CAP severity remains challenging due to heterogeneous clinical presentations and the lack of universally validated biomarkers. Current prediction tools [e.g., Pneumonia Severity Index (PSI), CURB-65] (7) exhibit limited generalizability in children owing to developmental variations in immune responses and pathophysiology. These scores were primarily derived and validated in adult populations, incorporating parameters that are developmentally inappropriate or less relevant in pediatric patients (8). Serum albumin (Alb), a negative acute-phase protein reflecting systemic inflammation and nutritional status, has emerged as a promising biomarker substrate (9). However, isolated albumin measurements lack age-specific discriminatory power. Combining albumin with dynamic inflammatory markers [e.g., C-reactive protein (CRP), neutrophils] into ratios such as Neutrophil-Percentage-to-Albumin Ratio (NPAR), CRP to Alb ratio (CAR), and C-reactive protein-albumin-lymphocyte (CALLY) may amplify prognostic precision by integrating dual pathways of inflammation and metabolic stress. Crucially, pediatric immunity evolves across developmental stages: toddlers (1–3 years) exhibit innate immunity dominance with neutrophil-predominant responses, whereas school-aged children (7–12 years) demonstrate adaptive immune maturation with lymphocyte/CRP interplay. This immunophysiological transition likely modulates biomarker performance but is rarely addressed in existing models. We hypothesize that age-stratified selection of albumin-based ratios would optimize severe CAP prediction by aligning biomarker dynamics with host biology. Validate NPAR, CAR, and CALLY as age-specific predictors of severe CAP in three pediatric age strata (1–3 years, 4–6 years, 7–12 years), thereby enabling precision risk stratification for timely intervention.
Materials and methods
Study design and participants
This retrospective cohort study analyzed 1,071 pediatric patients (aged 1–12 years) with CAP admitted to Fuyang Women and Children's Hospital between January 2022 to December 2024. Patients were stratified into three developmental stages: 1–3 years (toddlers), 4–6 years (preschoolers), and 7–12 years (school-aged children). Severe CAP was defined as requiring intensive respiratory support (mechanical ventilation/high-flow oxygen) or exhibiting septic shock, consistent with IDSA/ATS minor/major criteria adaptations for pediatrics. Djudication of Severe CAP: The diagnosis of severe CAP was initially established in real-time by the attending pediatric intensivist or emergency physician based on standard clinical criteria. For the purpose of this study, all cases meeting the severe CAP definition were independently reviewed and confirmed by two senior pediatricians who were blinded to the biomarker data. Any discrepancies between the reviewers were resolved through discussion and consensus. Inclusion criteria: Radiologically confirmed CAP (chest x-ray); Availability of complete albumin-based inflammatory ratios (NPAR/CAR/CALLY) within 24 h of admission; Age 1–12 years. Exclusion criteria: Immunosuppression (chemotherapy/HIV); Hospital-acquired pneumonia; Chronic organ failure (renal/liver). Rationale for Age Stratification: Patients were stratified into three age groups (1–3 years, 4–6 years, 7–12 years) based on distinct developmental stages in immunology and respiratory physiology. This approach addresses: (1) Differential immune maturation: Transition from innate immunity dominance (1–3 years) to adaptive immunity competence (7–12 years) (reflected in inflammatory biomarker profiles); (2) Anatomic vulnerability: Smaller airway caliber and weaker cough reflex in toddlers vs. school-aged children.
Data collection and Variable definitions
Baseline variables were systematically extracted from electronic medical records:
① Demographics: Age, sex; ② Clinical parameters: APACHE-II score, renal dysfunction (eGFR <90 ml/min/1.73m2), prior pneumonia history; ③ Pathogen data: Viral/bacterial/mixed/other (based on PCR/culture); ④ Inflammatory ratios: NPAR = Neutrophil percentage (%)/Serum albumin (g/dL); CAR = C-reactive protein (mg/L)/Serum albumin (g/dL); CALLY = [CRP (mg/L) × Lymphocyte count (×109/L)]/Serum albumin (g/dL). Lymphocyte and Neutrophil percentage (%) were determined using an XE-2100 hematology analyzer (Sysmex, Kobe, Japan). Serum biochemical parameters, including Albumin (reference range in children: 3.8–5.4 g/dL) and CRP (reference range in children: <5 mg/L), were measured with a HITACHI 7600 automated biochemistry analyzer.
Statistical analysis
Propensity Score Matching (PSM) was performed separately within each age stratum (1–3 years, 4–6 years, 7–12 years) using the “MatchIt” package (version 4.5.0) in R software. Severe-to-mild matching ratio: 1:2 using nearest-neighbor algorithm (caliper = 0.2 SD); Covariates balanced: Age, sex, APACHE-II, renal dysfunction, pathogen type, pneumonia history (SMD < 0.1); Predictive Modeling: Univariate analysis: Mann–Whitney U test (CAR/CALLY)/t-test (NPAR) for severe vs. mild CAP; Multivariate logistic regression: Evaluated independent predictors of severe CAP per age stratum; Final model optimization: Retained only age-stratified significant biomarkers (NPAR for 1–6 years, CAR for 7–12 years). Validation: Discriminative power: ROC curves with AUC/95% CI calculation; Hosmer–Lemeshow test; Clinical utility: Sensitivity, specificity, NPV/PPV at optimal cutoffs.
Results
Severe community-acquired pneumonia incidence and propensity score matching in pediatric patients: overall and age-stratified analysis
A total of 1,071 pediatric community-acquired pneumonia (CAP) patients aged 1–12 years (951 mild, 120 severe) demonstrated an overall severe CAP incidence of 11.20%, with age-stratified rates progressively increasing from 7.37% (1–3 years) to 12.80% (4–6 years) and 18.04% (7–12 years). Following propensity score matching at a 1:2 severe-to-mild ratio within each age stratum, balanced cohorts were established: 111 cases (74 mild/37 severe) for 1–3 years, 144 cases (96 mild/48 severe) for 4–6 years, and 105 cases (70 mild/35 severe) for 7–12 years. All baseline covariates—including age, sex, renal dysfunction, pathogen type, pneumonia history, and APACHE-II score—achieved inter-group balance (standardized mean difference <0.1; p > 0.05) (Table 1).
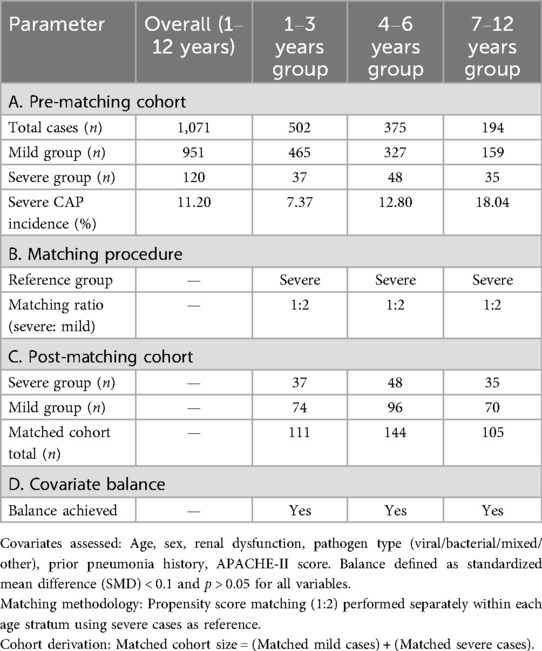
Table 1. Construction of matched cohorts for mild vs. severe pediatric community-acquired pneumonia (CAP).
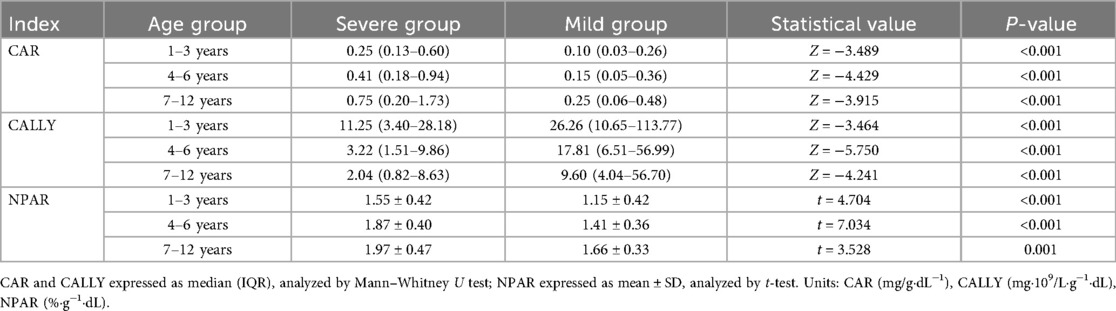
Univariate analysis of inflammatory ratios
All inflammatory ratios exhibited statistically significant differences between severe and mild CAP across age strata (p < 0.001). Specifically, CAR demonstrated consistently higher values in severe cases: median values of 0.25 vs. 0.10 in 1–3 years, 0.41 vs. 0.15 in 4–6 years, and 0.75 vs. 0.25 in 7–12 years patients. In contrast, CALLY showed significantly lower median values in severe groups: 11.25 vs. 26.26 (1–3 years), 3.22 vs. 17.81 (4–6 years), and 2.04 vs. 9.60 (7–12 years). Similarly, NPAR was significantly elevated in severe CAP cases across all age groups: mean values of 1.55 ± 0.42 vs. 1.15 ± 0.42 (1–3 years), 1.87 ± 0.40 vs. 1.41 ± 0.36 (4–6 years), and 1.97 ± 0.47 vs. 1.66 ± 0.33 (7–12 years) (Table 2).
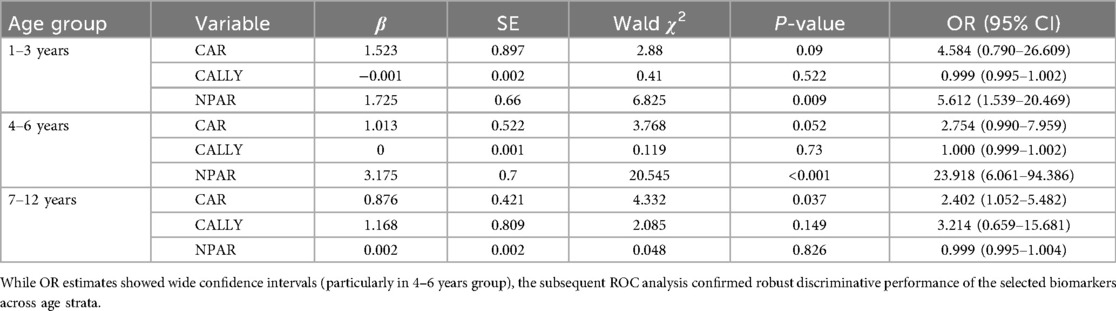
Multivariate logistic regression analysis
Multivariate logistic regression identified distinct age-stratified predictors of severe CAP: In the 1–3 years group, only NPAR demonstrated statistical significance (OR = 5.612, 95% CI: 1.539–20.469; p = 0.009). For 4–6 years patients, NPAR showed dominant predictive value (OR = 23.918, 95% CI: 6.061–94.386; p < 0.001), while CAR failed to reach significance (p = 0.052). Conversely, in the 7–12 years cohort, CAR emerged as the sole significant predictor (OR = 2.402, 95% CI: 1.052–5.482; p = 0.037), with neither CALLY nor NPAR showing significant associations (both p > 0.05) (Table 3).
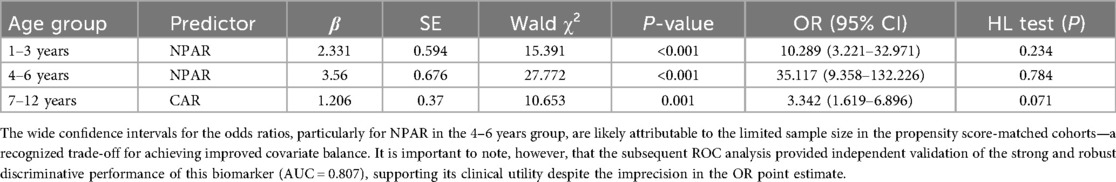
Final predictive models with validation
Final age-optimized models demonstrated strong predictive performance: the NPAR model for 1–3 years (OR = 10.289, 95% CI: 3.221–32.971, p < 0.001), NPAR model for 4–6 years (OR = 35.117, 95% CI: 9.358–132.226, p < 0.001), and CAR model for 7–12 years (OR = 3.342, 95% CI: 1.619–6.896, p = 0.001), all with adequate calibration (Hosmer–Lemeshow p > 0.05). The incidence of severe CAP progressively increased with age, and optimal biomarkers shifted from NPAR in younger children (1–6 years) to CAR in older children (7–12 years) (Table 4). NPAR-based models (1–6 years) achieved NPV > 83%, while the CAR model (7–12 years) maintained NPV > 83% (see Table 5).
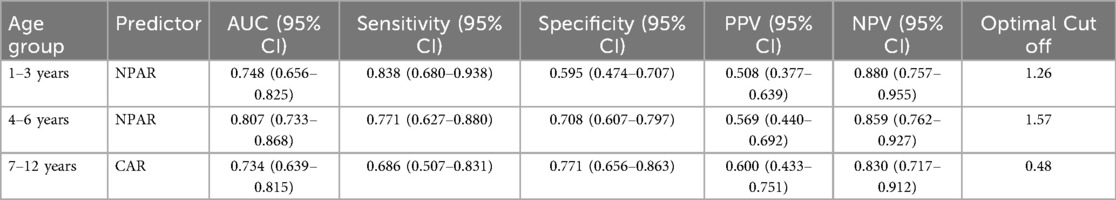
ROC curve analysis
The ROC curve analysis confirmed the robust diagnostic efficacy of our age-stratified models, with all AUCs >0.73 demonstrating clinical utility. Critically, each model achieved high negative predictive value (NPV: 0.83–0.88) for safely excluding severe CAP—particularly vital for 1–3 years toddlers where 88% NPV (0.880, 95% CI: 0.757–0.955) mitigates missed diagnoses. The biologically anchored cutoffs aligned with disease pathophysiology: 1–3 years NPAR > 1.26 (between mild mean 1.15 ± 0.42 and severe mean 1.55 ± 0.42), 4–6 years NPAR > 1.57 (discriminating severe IQR from mild 1.41 ± 0.36), and 7–12 years CAR > 0.48 (2× mild median 0.25). Notably, the 4–6 years NPAR model showed optimal balance (sensitivity 0.771/specificity 0.708, AUC = 0.807), while younger children prioritized sensitivity (0.838) to avert under-triage and adolescents emphasized specificity (0.771) to prevent unnecessary ICU transfers—collectively enabling precision risk-stratification across pediatric developmental stages (Table 5 and Figures 1–3).
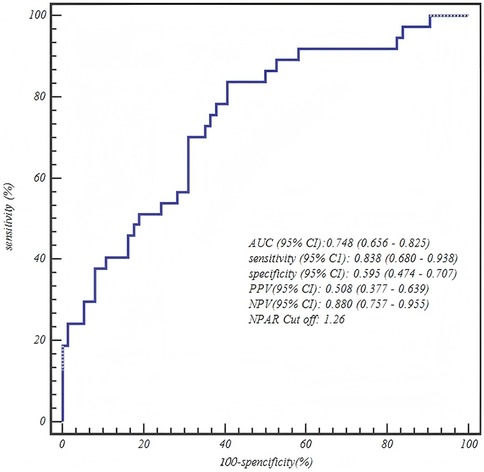
Figure 1. ROC curve of the neutrophil percentage-to-albumin ratio (NPAR) for predicting severe CAP in toddlers (1–3 years).
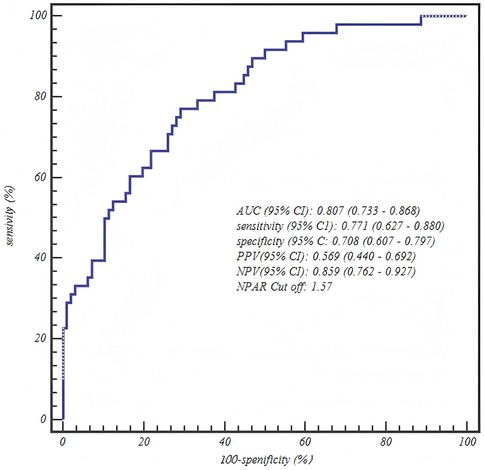
Figure 2. Age-stratified model performance: ROC curve for the neutrophil percentage-to-albumin ratio (NPAR) in predicting severe CAP among children aged 4–6 years.
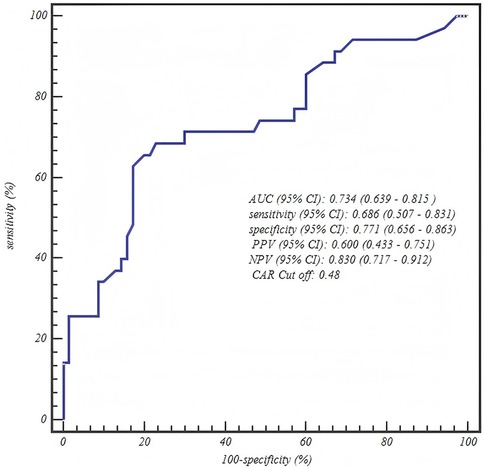
Figure 3. ROC curve of the C-reactive protein-to-albumin ratio (CAR) for predicting severe CAP in school-aged children (7–12 years).
Discussion
CAP is a common and frequently occurring disease of the respiratory system in children. Mild CAP primarily manifests clinically with respiratory symptoms such as fever, cough, and sputum production. Due to fewer alveoli, more developed interstitial tissue, lower lung air volume, and rich pulmonary vasculature in children, inflammation tends to persist and become difficult to resolve. Furthermore, an excessive inflammatory response can cause severe lung damage, making progression to severe CAP more likely (10). When children develop severe CAP, severe hypoxia and toxemia can occur. Beyond symptoms like fever and cough, this can lead to respiratory failure manifestations such as dyspnea and impaired ventilation/perfusion function. It can also cause severe dysfunction in multiple organ systems, including the cardiovascular, nervous, and digestive systems. Severe CAP is a major cause of morbidity and mortality in children worldwide (11). Notably, from a pathophysiological perspective, the systemic inflammatory response and organ dysfunction characterizing severe CAP align with the definition of sepsis, i.e., life-threatening organ dysfunction caused by a dysregulated host response to infection (12). Therefore, it is very important to find early warning indicators and prediction models for severe pneumonia as early as possible.
This study employed rigorous age stratification and propensity score matching (PSM) analysis. Our results revealed a significant age-dependent trend in the incidence of severe CAP among children, which increased from 7.37% in the 1–3 years group to 18.04% in the 7–12 years group. Importantly, baseline characteristics were well-balanced across all age strata after matching. This observed trend is likely explained by age-specific patterns in pathogen distribution and differences in immune system development. In younger children (1–3 years), the risk of severe CAP was relatively low (7.37%). This may be attributed to a virus-dominated infection profile. Supporting this, national surveillance data from Liu et al. indicated a viral detection rate of 50.82% (predominantly RSV and influenza virus) among CAP patients aged ≤5 years, compared to a bacterial positivity rate of only 34.37% (13). Since viral pneumonia is often self-limiting, this pattern aligns with the lower incidence of severe disease we observed in this age group. Conversely, older children (7–12 years) faced a substantially higher risk of severe CAP (18.04%). Two key factors may underlie this increase. First, the pathogen spectrum shifts with age. Liu et al. reported that in children over 6 years, bacterial detection rates (32.53%) become comparable to viral rates (34.37%). Furthermore, viral-bacterial co-infections—such as RSV-Haemophilus influenzae co-infection (OR = 2.62)—were significantly more prevalent in severe CAP cases. Second, immune responses differ in older children. They appear more susceptible to excessive inflammatory reactions. As noted by Principi et al., traditional biomarkers like PCT and CRP can be ambiguous for distinguishing bacterial pneumonia and assessing severity (11). Additionally, the maturation of the immune system with age may sometimes lead to dysregulated inflammatory responses, thereby increasing the risk of tissue damage.
Our findings underscore that the predictive efficacy of albumin-based inflammatory ratios is intrinsically linked to pediatric immune maturation. For toddlers (1–3 years), NPAR emerged as the dominant predictor (OR = 10.289, AUC = 0.748), aligning with their neutrophil-predominant innate immunity. Neutrophils are frontline responders in early childhood infections, and their activity combined with hypoalbuminemia (reflecting acute-phase inflammation) amplifies the signal for severe disease (14). Conversely, in school-aged children (7–12 years), CAR superseded other ratios (OR = 3.342, AUC = 0.734), coinciding with the maturation of adaptive immunity and CRP's role in bacterial challenge amplification (15). This transition mirrors immunological shifts (16, 17): CRP production is more robust in older children due to enhanced hepatocyte response to IL-6, while lymphopenia (integrated in CALLY) may be less specific in bacterial/viral co-infections prevalent in this age group.
Our models' high NPV (83% across all strata) holds transformative potential for clinical practice. In our study on age-stratified albumin-based inflammatory ratios for severe pneumonia risk prediction in children, models like NPAR (1–6 years) and CAR (7–12 years) achieved NPVs of 88.0%, 85.9%, and 83.0% across different age groups. These high NPVs allow safe outpatient management of low-risk cases, reducing unnecessary ICU referrals and optimizing medical resource allocation. This potential is further supported by other clinical studies. In health information exchange (HIE), AI models have demonstrated NPVs of 94.10% to 99.10% in predicting clinical outcomes, as shown in a systematic review (18). Additionally, the ITBvsCD-CEP model achieved an NPV of 83% in differentiating ITB from CD in a multicenter study (19). These high NPVs enhance clinical decision-making, reduce unnecessary interventions, and improve patient care across various medical fields by reliably ruling out conditions. In low-resource settings, NPAR (cutoff >1.26 for 1–3 years; >1.57 for 4–6 years) and CAR (cutoff >0.48 for 7–12 years) can reliably exclude severe CAP, reducing unnecessary ICU referrals. For example, NPV = 88% in toddlers minimizes missed severe cases in a high-volume emergency department. Preschoolers (4–6 years) showed optimal NPAR performance (AUC = 0.807), supporting its use as a first-line tool for this high-risk group.
Our results contrast with adult CAP biomarkers (e.g., PSI/CURB-65), which overlook developmental immunity. While CURB-65 is a validated predictor of mortality from community-acquired pneumonia in adults, it was never intended for use in children (20). Similarly, the PSI and CURB-65 scores have been widely used to predict mortality risk in adult CAP patients, but they may not be as effective in pediatric populations (21). While CAR predicts mortality in adult pneumonia, its age-dependent performance in children—superior only in school-aged cohorts—highlights pediatrics as a distinct biological niche. Similarly, CALLY, validated in chronic inflammation, lacked significance here, possibly due to lymphocyte count variability during acute pediatric infections (22, 23). This reinforces that pediatric biomarker discovery must account for age stratification.
Our definition of severe CAP, based on the requirement for intensive respiratory support or the presence of septic shock, aligns with the modern conceptualization of sepsis (Sepsis-3) (12). Community-acquired pneumonia is one of the most common primary causes of sepsis. Consequently, the most severe form of CAP inherently represents sepsis originating from a pulmonary source. The objective clinical endpoints chosen in this study (e.g., mechanical ventilation and septic shock) accurately capture this critical transition from a localized infection to systemic organ failure. Therefore, the predictive power of NPAR and CAR for severe CAP not only underscores their value in pneumonia severity stratification but also suggests their potential role as early warning biomarkers for identifying children at high risk of progressing from pneumonia to sepsis, providing a stronger conceptual framework for their clinical utility. Weiss et al. validated a multimarker sepsis risk stratification tool in critically ill children, supporting our approach of using inflammatory ratios for early identification of severe CAP progressing to sepsis (24).
The heterogeneity of pediatric CAP, caused by diverse pathogens and host responses, must be considered when interpreting our findings. Although our age-stratified design addressed key immunological differences, the performance of NPAR and CAR within specific subgroups (e.g., viral vs. bacterial infections) requires future investigation. As seen in sepsis research (25), data-driven subphenotyping (e.g., using clustering methods) can reveal distinct subgroups for tailored therapy. Future studies should thus explore how pathogen type and host factors influence these biomarkers to enable truly personalized clinical application. While our study identifies strong associations between NPAR/CAR and severe CAP outcomes, establishing a causal relationship requires further investigation. The observed associations may be influenced by unmeasured confounders or may simply reflect the severity of the underlying inflammatory state. To truly determine if modulating these ratios (e.g., through albumin supplementation or anti-inflammatory therapies) can improve outcomes, future research must move beyond prediction towards causal inference. The framework of target trial emulation (26) provides a rigorous methodology for this purpose. Future prospective studies should be designed to emulate a trial where patients are virtually “randomized” to different management strategies based on their early NPAR/CAR values. This approach would allow us to estimate the causal effect of biomarker-guided therapy on clinical outcomes, ultimately translating these predictive biomarkers into actionable therapeutic targets.
Our study has several limitations. First, as a single-center retrospective analysis, our findings require external validation in diverse populations and healthcare settings to confirm their generalizability, as regional variations in pathogen prevalence and clinical practices may influence biomarker performance. Second, while propensity score matching successfully balanced known confounders, unmeasured covariates (e.g., vaccination status, socioeconomic factors) may still affect the generalizability of our findings. Furthermore, the matching process itself led to a substantial reduction in the analyzable sample size, particularly evident in the 7–12 years cohort (which decreased from 194 to 105 patients). This reduction inevitably diminished the statistical power of our analysis and is a key reason for the wide confidence intervals observed in our multivariate regression models, as noted in the results. Future studies with larger cohorts are needed to obtain more precise estimates. Third, as a retrospective study, we lacked complete data for key variables required to calculate established pneumonia severity scores (e.g., PSI, CURB-65). Consequently, we were unable to directly benchmark the predictive performance of NPAR and CAR against these clinical standards. This important comparison remains a critical objective for future prospective studies that are specifically designed to collect all necessary data for a comprehensive head-to-head evaluation. Fourth, our analysis utilized a single, early measurement of NPAR/CAR (within 24 h of admission) for prediction. While this is clinically relevant for initial triage, it does not capture the temporal dynamics of these biomarkers. As raised The trajectory of NPAR (e.g., its rate of change or persistence at high levels) throughout hospitalization may provide additional prognostic value and reflect response to therapy. Investigating these time-varying patterns represents an important avenue for future research. Finally, the unexpected non-significance of CALLY may reflect lymphocyte count volatility during acute pediatric infections, limiting its utility in CAP risk stratification compared to chronic inflammatory conditions.
For successful translation into clinical practice, the NPAR and CAR ratios can be seamlessly integrated into existing diagnostic workflows. As these ratios are derived from routine laboratory parameters—complete blood count with differentials, CRP, and albumin—they can be automatically calculated and reported alongside standard results by the hospital's laboratory information system or electronic health record. This requires no additional blood draws or costs, presenting a significant advantage for rapid adoption. We propose a straightforward clinical decision pathway: for a child aged 1–6 years with CAP, an NPAR value below the established age-specific cutoff (e.g., >1.26 for 1–3 years; >1.57 for 4–6 years) would support the safety of outpatient management or standard ward care, while a value above the cutoff would flag the need for intensified monitoring and consideration of higher-level care. Similarly, for children aged 7–12 years, the CAR cutoff of >0.48 would guide this triage decision. To definitively establish the value of this biomarker-guided approach, a prospective, multicenter, pragmatic trial is warranted. The critical next step is a head-to-head comparison against current standards of care. This prospective study should be designed to rigorously compare the performance of these age-stratified ratios against both clinical judgment and adapted versions of established adult scores (where feasible in pediatrics) in predicting severe outcomes. The primary goal would be to determine whether the integration of NPAR and CAR into clinical decision rules improves patient outcomes and optimizes resource allocation compared to conventional practices.
Conclusion
In summary, NPAR and CAR serve as developmentally tuned biomarkers for severe CAP risk stratification. Their high NPV enables safe outpatient management of low-risk cases, while directing intensive resources to high-risk children. Future work should focus on translating these ratios into clinical decision-support tools and exploring their role in guiding immunomodulatory therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Conducted in accordance with the Declaration of Helsinki; approved by the Institutional Review Board of Fuyang Women and Children's Hospital (202508010014). Informed consent was waived due to retrospective anonymized data analysis.
Author contributions
YB: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CH: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 2024 Scientific Research Project of the Fuyang Municipal Health Commission (Grant No. FYZC2024-088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. (2018) 18:1191–210. doi: 10.1016/S1473-3099(18)30310-4
2. WHO. Levels and trends in child mortality. WHO (2015). Available online at: http://www.whoint/maternal_child_adolescent/documents/levels_trends_child_mortality_2015/en/ (Accessed August 14, 2020).
3. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2015) 385:430–40. doi: 10.1016/S0140-6736(14)61698-6
4. Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. (2013) 3:010401. doi: 10.7189/jogh.03.010401
5. Tasa de Mortalidad por Infección Respiratoria Aguda (IRA) en Menores de 5 años Georeferenciado. Así Vamos en Salud—indicadores en salud normatividad derechos (2016). Available online at: https://www.asivamosensalud.org/indic adores/enfermedades-transmisibles/tasa-de-mortalidad-por-infeccion respiratoria-aguda-ira-en (Accessed August 14, 2020).
6. Cavallazzi R, Ramirez JA. Definition, epidemiology, and pathogenesis of severe community-acquired pneumonia. Semin Respir Crit Care Med. (2024) 45:143–57. doi: 10.1055/s-0044-1779016
7. Anurag A, Preetam M. Validation of PSI/PORT, CURB-65 and SCAP scoring systems in COVID-19 pneumonia for prediction of disease severity and 14-day mortality. Clin Respir J. (2021) 15:467–71. doi: 10.1111/crj.13326
8. Long X, Li W, Tang Y, Luo Z, Luo J, Fu Z, et al. Online tool for predicting severe cases of childhood community-acquired pneumonia based on the PIRO concept. Pediatr Pulmonol. (2025) 60(7):e71199. doi: 10.1002/ppul.71199
9. Geng M, Zhang K. CRP-albumin-lymphocyte index (CALLYI) as a risk-predicting biomarker in association with osteoarthritis. Arthritis Res Ther. (2025) 27:57. doi: 10.1186/s13075-025-03530-x
10. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the Infectious Diseases Society of America. Clin Infect Dis. (2011) 53:e25–76. doi: 10.1093/cid/cir531
11. Liu YN, Zhang YF, Xu Q, Qiu Y, Lu QB, Wang T, et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. (2023) 4:e330–9. doi: 10.1016/S2666-5247(23)00031-9
12. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
13. Principi N, Esposito S. Biomarkers in pediatric community-acquired pneumonia. Int J Mol Sci. (2017) 18(2):447. doi: 10.3390/ijms18020447
14. Bi L, Liang J, Hu K. Neutrophil percentage-to-albumin ratio (NPAR) as a biomarker for asthma: a cross-sectional analysis of NHANES data. BMC Pulm Med. (2025) 25:269. doi: 10.1186/s12890-025-03701-1
15. Ji W, Li H, Qi Y, Zhou W, Chang Y, Xu D, et al. Association between neutrophil-percentage-to-albumin ratio (NPAR) and metabolic syndrome risk: insights from a large US population-based study. Sci Rep. (2024) 14:26646. doi: 10.1038/s41598-024-77802-y
16. Chen J, Zhang Z, Teng Z, Zeng Q. Association of neutrophil-percentage-to-albumin ratio with all-cause and cardiovascular mortality in patients with diabetes and prediabetes from the NHANES 1999–2018. Sci Rep. (2025) 15:15630. doi: 10.1038/s41598-025-98818-y
17. Seckin B, Ates MC, Kirbas A, Yesilyurt H. Usefulness of hematological parameters for differential diagnosis of endometriomas in adolescents/young adults and older women. Int J Adolesc Med Health. (2018) 32(2):20180078. doi: 10.1515/ijamh-2018-0078
18. Borna S, Maniaci MJ, Haider CR, Maita KC, Torres-Guzman RA, Avila FR, et al. Artificial intelligence models in health information exchange: a systematic review of clinical implications. Healthcare (Basel). (2023) 11:2584. doi: 10.3390/healthcare11182584
19. Limsrivilai J, Lee CK, Prueksapanich P, Harinwan K, Sudcharoen A, Cheewasereechon N, et al. Validation of models using basic parameters to differentiate intestinal tuberculosis from Crohn’s disease: a multicenter study from Asia. PLoS One. (2020) 15:e0242879. doi: 10.1371/journal.pone.0242879
20. Venkatesan S, Myles PR, McCann G, Kousoulis AA, Hashmi M, Belatri R, et al. Development of processes allowing near real-time refinement and validation of triage tools during the early stage of an outbreak in readiness for surge: the FLU-CATs study. Health Technol Assess. (2015) 19:1–132. doi: 10.3310/hta19890
21. Lee MS, Oh JY, Kang CI, Kim ES, Park S, Rhee CK, et al. Guideline for antibiotic use in adults with community-acquired pneumonia. Infect Chemother. (2018) 50:160–98. doi: 10.3947/ic.2018.50.2.160
22. Karakioulaki M, Stolz D. Biomarkers and clinical scoring systems in community-acquired pneumonia. Ann Thorac Med. (2019) 14:165–72. doi: 10.4103/atm.ATM_305_18
23. Deng Y, Huang J, Deng L, Zhou Y, Pan L, Wang J, et al. C-reactive protein-albumin-lymphocyte (CALLY) index as an independent risk factor for postoperative atrial fibrillation recurrence. Clin Cardiol. (2025) 48:e70157. doi: 10.1002/clc.70157
24. Weiss SL, Fitzgerald JC. Pediatric sepsis diagnosis, management, and sub-phenotypes. Pediatrics. (2024) 153(1):e2023062967. doi: 10.1542/peds.2023-062967
25. Yang J, Zhang B, Hu C, Jiang X, Shui P, Huang J, et al. Identification of clinical subphenotypes of sepsis after laparoscopic surgery. Laparosc Endosc Robot Surg. (2024) 7:16–26. doi: 10.1016/j.lers.2024.02.001
Keywords: community-acquired pneumonia, albumin ratios, age stratification, NPAR, CAR, negative predictive value
Citation: Bai Y, Zhang J and Hou C (2025) Age-stratified selection of albumin-based inflammatory ratios: a novel strategy for optimizing risk prediction of severe pneumonia in children. Front. Pediatr. 13:1679957. doi: 10.3389/fped.2025.1679957
Received: 5 August 2025; Accepted: 29 October 2025;
Published: 14 November 2025.
Edited by:
Albert Martin Li, The Chinese University of Hong Kong, ChinaReviewed by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaNeel Patel, Staten Island University Hospital, United States
Copyright: © 2025 Bai, Zhang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxu Hou, ZnlseWhjeDEyM0BzaW5hLmNvbQ==
 Yunbao Bai
Yunbao Bai Jianming Zhang
Jianming Zhang