- Department of Pediatircs, Tianjin Medical University Baodi Hospital, Tianjin, China
Objective: To retrospectively analyze the predictive value of an early neonatal vitamin D (vit D) level for neonatal respiratory failure in newborns.
Methods: The data of 220 hospitalized neonates, including 114 boys (51.8%) and 106 girls (48.2%), were collected from March 2021 to March 2023. Neonates were divided into respiratory failure (n = 52) and non-respiratory failure groups (n = 168) based on the respiratory status at the time of admission. Serum 25-hydroxy vitamin D [25(OH)D] levels were determined using a chemiluminescence immunoassay. The influence of serum 25(OH)D levels and perinatal factors on neonatal respiratory failure were analyzed. The predictive value of 25(OH)D for respiratory failure was evaluated.
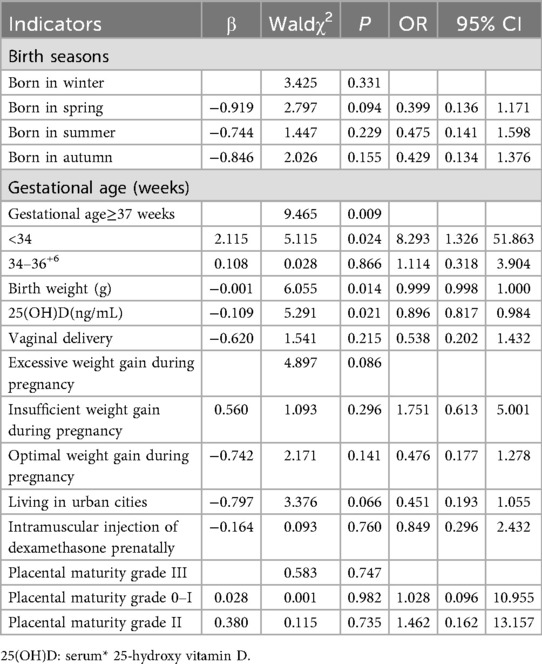
Results: Serum 25(OH)D (OR = 0.896, 95% CI = 0.817–0.984) and birth weight (OR = 0.999, 95% CI = 0.998–1.000) were independent protective factors against neonatal respiratory failure, while gestational age < 34 weeks (OR = 8.293, 95% CI = 1.326–51.863) was an independent risk factor for neonatal respiratory failure (P < 0.05). The area under the receiver operating characteristic curve (AUC) for 25(OH)D alone was 0.620 (95% CI = 0.531-0.708). However, combining the 25(OH)D level with gestational age and birth weight improved the AUC to 0.868 (95% CI = 0.812-0.925) with a sensitivity of 0.750 and 1-specificity of 0.845 (P < 0.001).
Conclusion: 25(OH)D deficiency may increase the risk of neonatal respiratory failure. Combining 25(OH)D with gestational age and birth weight was shown to have better predictive value for respiratory failure than serum 25(OH)D alone.
1 Introduction
Neonatal respiratory failure refers to the inability of newborns to maintain respiratory homeostasis, resulting in ventilation-perfusion mismatch and gas exchange dysfunction. It is a common neonatal emergency worldwide. Over the past seven decades, the fatality rate in high-income countries has decreased significantly; however, due to the lack of neonatal wards and neonatal intensive care units, the mortality rate of neonatal respiratory failure in low - and middle-income countries remains high (1).
Vitamin D (vit D) is a lipid-soluble pre-hormone steroid that has an important role in many physiologic activities. Vit D, upon binding to vit D receptor, interacts with activator and calcium-binding proteins, initiating genomic actions that regulate skeletal and calcium homeostasis, cell proliferation, differentiation, wound healing, repair mechanisms, immune modulation, and inflammatory responses (2). Vit D is expressed in fetal pulmonary cells (3), in which vit D participates in lung development, immune system maturation, and improving pulmonary function (4). Globally, vitamin D deficiency is more common among pregnant women and newborns than in the general population. There are significant differences in vitamin D status among different regions of the World Health Organization, with the highest prevalence of vitamin D deficiency among pregnant women and newborns in Southeast Asia (5). Serum 25-hydroxy vitamin D [25(OH)D] is the major circulating form of vit D and is therefore considered the most important indicator of vit D status worldwide (6).
In addition, vit D may modulate immune functions by enhancing monocyte and macrophage function in innate immunity via induction of cathelicidin, which is an antimicrobial peptide with properties that kill bacteria, viruses, and fungi. Vit D regulates T and B cells in adaptive immunity, especially by aiding the differentiation of T cells into regulatory T cells (Tregs), which are crucial for preventing autoimmune responses (7). Moreover, vit D modulates cytokine production by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines, thereby helping to control inflammatory responses. Many immune cells express vit D receptor and vit D binding to vit D receptor regulates the expression of genes responsible for the immune response. Therefore, vit D has been reported to be an immune modulator (7, 8).
Previous studies have paid more attention to the high correlation between vitamin D in mothers and newborns. The vit D level has been shown to influence postnatal respiratory outcomes. But few studies have focused on neonatal respiratory failure and the connection with vit D. Therefore, this study investigates the predictive value of the serum 25(OH)D level on neonatal respiratory failure was investigated based on the relationship to respiratory illnesses in early infancy.
2 Materials and methods
2.1 Study subjects
The following retrospective study analyzed the clinical data from 220 neonates ≤3 days old, who had been admitted to Tianjin Medical University Baodi Hospital between March 2021 and March 2023. The Ethics Committee of Tianjin Medical University Baodi Hospital (2021-E-0301) provided ethical clearance for this study. The study adhered to the Declaration of Helsinki. Guardians were informed and provided written informed consent for participation in the study.
The inclusion criteria were as follows: neonates ≤3 days old with complete clinical data; respiratory failure occurring within 24 h after production; maternal age between 18 and 45 y. The postnatal C-reactive protein (CRP) level was determined to be normal or abnormal based on neonatal sepsis diagnostic criteria, the postnatal procalcitonin (PCT) level, and the hourly adjusted PCT cut-off value after birth. Respiratory failure was diagnosed according to the 5th edition of the practical neonatology diagnostic criteria, as follows: 1. clinical indicators, including inspiratory depression, groan, central cyanosis, refractory apnea, decreased activity, and respiratory rate >60 beats/min; 2. laboratory indicators, including PACO2 > 60 mmHg, PO2 < 50 mmHg, or oxygen saturation <80% at an FiO2 = 100%; and c. arterial blood gas pH < 7.2. Clinical indicators plus one or more laboratory indicators facilitated the diagnosis of respiratory failure. The causes of respiratory failure include neonatal pneumonia and neonatal respiratory distress syndrome. Anti-infective and respiratory support treatments were administered after admission.
The exclusion criteria were as follows: women with triplets or more; pregnant women who had received vit D or vit A + D supplements for >2 weeks; women with a history of long-term use of drugs affecting vit D metabolism, such as antiepileptics, antineoplastics, antiretrovirals, antituberculosis medications, or glucocorticoids; women who smoked cigarettes or consumed alcohol during pregnancy; neonates >3 d old; and neonates with severe anemia, congenital malformations, severe asphyxia during the perinatal period, or suspected genetic/metabolic disorders.
2.2 Clinical materials
All medical records were collected, systematically re-evaluated, and data extracted based on the diagnostic criteria of the relevant literature. Neonatal baseline data upon admission included gender, gestational age, fetus number (Single or twin pregnancies), birth weight, percentile, and laboratory results [procalcitonin, CRP, and 25(OH)D] from blood samples obtained within 1 h. Birth seasons were included because seasonal factors have a significant impact on Vit D levels (9). Prenatal maternal data included general demographic data, pregnancy complications, and para-clinic test results.
2.3 Sample collection and testing
Because mothers who resided locally did not take a vit D supplement during pregnancy, the possibility of vit D deficiency in newborns was high. 25(OH) levels are routinely checked in newborns to determine the need for vit D supplementation. The Obstetrics Department in our hospital routinely delays umbilical cord ligation for 30 s after delivery to ensure the highest hemoglobin level possible in the newborn. Then, peripheral venous blood samples (3–4 mL) were collected from the femoral vein or radial artery of neonates within 1 h of admission under sterile conditions by specialist nurses with >15 years of experience. The peripheral venous blood samples were allowed to rest for 10–20 min, then centrifuged at 3,000 r/min for 15 min at 4 °C. The serum was separated and stored at −80 °C for subsequent analysis. Serum 25(OH)D was tested by CLIA using the iFlash 3,000G analyzer (YHLO, city, state, country). Tests were carried out using magnetic bead-acridinium ester technology with the 25(OH)D CLIA reagent kit (catalog number, 2021021; http://www.szyhlo.com/products_details/3.html).
2.4 Grouping criteria
The Practical Diagnostic Criteria for Neonatology (5th edition) was used as a reference. Clinical indicators are inspiratory depression, moaning, central cyanosis, refractory apnea, reduced activity, and respiratory rate >60 breaths/min. The laboratory indicators include PaCO2 > 60 mmHg, arterial blood gas (ABG) or FiO2 at 100% with a PO2 < 50 mmHg or SaO2 < 80%, and an ABG pH < 7.2 (10).
Subgroups were classified based on Vit D level: A 25(OH)D level of ≥20 ng/ml(50 nmol/L) was considered sufficient, 12–20 ng/mL(30–50 nmol/L) was insufficient, and <12 ng/ml(30 nmol/L) was deficient (9).
Subgroups were classified based on an abnormal CRP level, as follows (11): CRP ≥ 3 mg/L group within 6 h after birth; CRP ≥ 6 mg/L group from 6 to 24 h after birth; and CRP ≥ 10 mg/L within 24 h after birth.
Subgroups were classified based on abnormal PCT levels as follows (12): PCT level ≥0.5 ug/L group within 6 h after birth; PCT level ≥2 ug/L group between 6 and 24 or between 48 and 60 h after birth; PCT level ≥5 ug/L between 12 and 18 or 36 and 48 h after birth; PCT level ≥10 ug/L between 18 and 36 h; and PCT level ≥1 ug/L between 60 and 72 h.
2.5 Statistical methods
SAS 9.4 was used for data analysis. Categorical data are presented as counts and percentages. The Kolmogorov–Smirnov test was performed to check normality. Data that were normally distributed are expressed as the mean ± standard deviation (mean ± SD). Comparisons were made using an independent samples t-test. Non-normally distributed data are presented as the median and interquartile range [M (IQR)] and the Kruskal–Wallis test, a non-parametric rank-sum test, was used for analysis. ROC curves were used to demonstrate the predictive value of the newborn serum 25(OH)D concentration in conjunction with other markers for risk of respiratory failure. The Youden index, sensitivity, specificity, and AUC were calculated. A P < 0.05 was considered an indicator of statistical significance.
3 Results
3.1 comparison of neonatal baseline characteristics
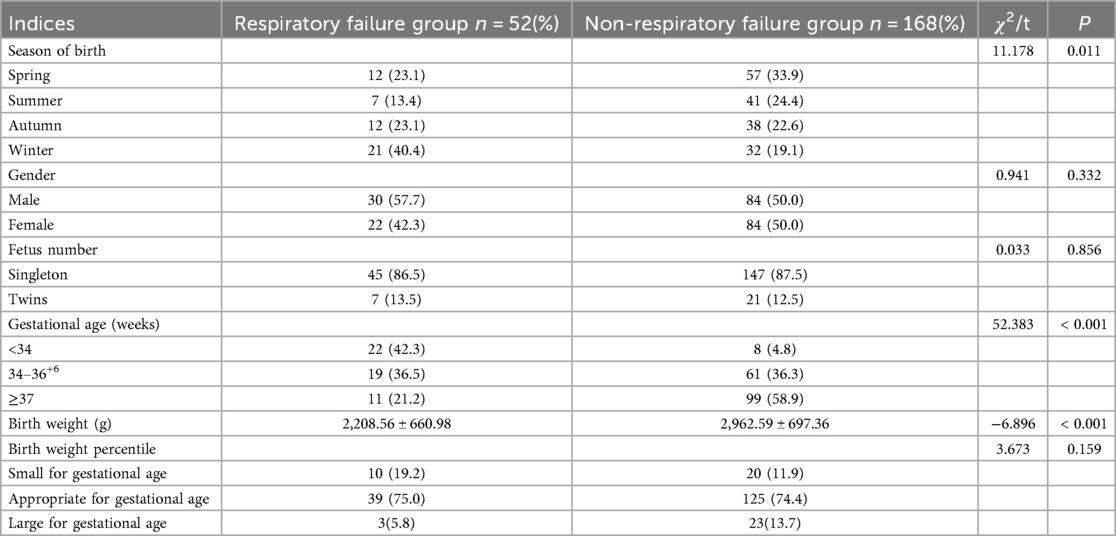
Based on a comparative analysis, statistically significant differences were detected in the incidence of births in the winter (40.4% vs. 19.1%; P = 0.011), preterm births <34 weeks gestations (42.3% vs. 4.8%; P < 0.001), and a decreased mean birth weight (2,208.56 ± 660.98 g vs. 2,962.59 ± 697.36 g; P < 0.001) between the respiratory and non-respiratory failure groups. No statistical difference was detected in the gender distribution (male, 57.7% vs. 50.0%; P = 0.332), number of fetuses (singleton, 86.5% vs. 87.5%; P = 0.856), and birth weight percentile (small-for-gestational-age, 19.2% vs. 11.9%; P = 0.159; Table 1).
3.2 Comparison of neonatal infection indices in different subgroups
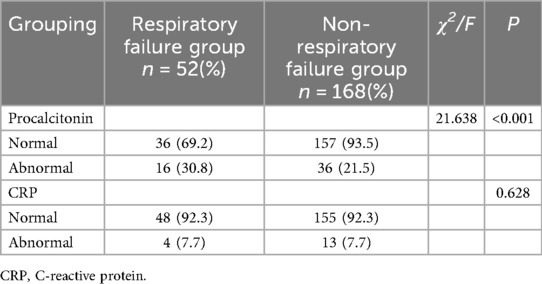
CRP and PCT levels fluctuate greatly in the early stage of neonates. Therefore, in this study a value less than the cut-off value based on age characteristics was selected as normal and a value higher than the cut-off value was selected as the abnormal group. The grouping results are shown in Table 2. The incidence of respiratory failure in neonates with an abnormal PCT level was significantly higher than neonates without respiratory failure (χ2 = 21.638; P < 0.001), while a normal CRP level had no significant effect on the incidence of neonatal respiratory failure (P = 0.628), as shown in Table 2.
3.3 Comparison of maternal factors
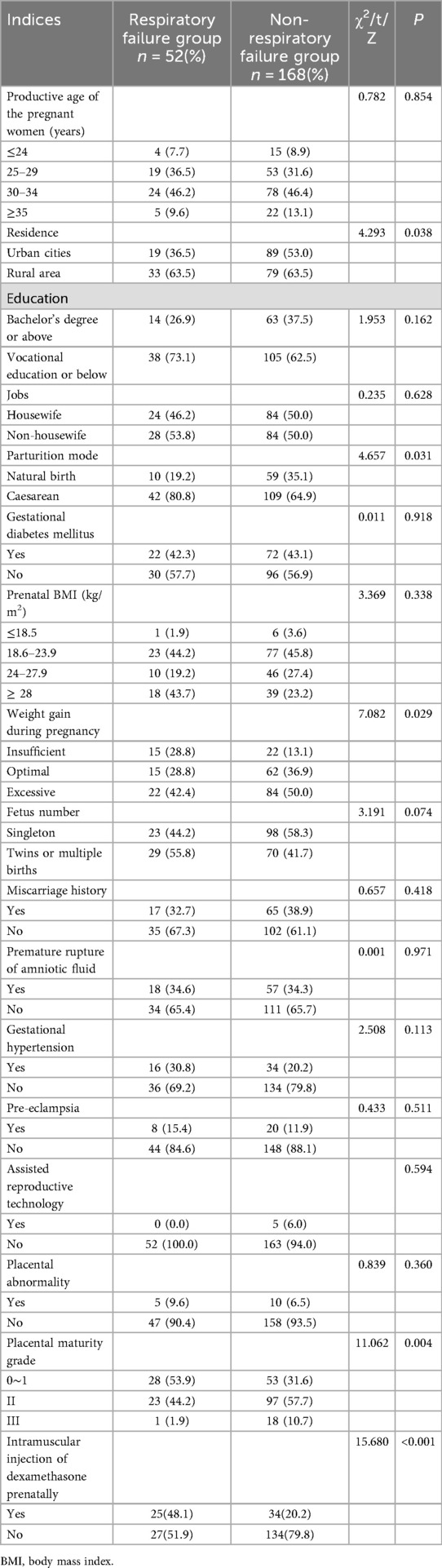
When compared to the control group, more mothers in the respiratory failure group had residences in rural areas than the non-respiratory group (63.5% vs. 47.0%; P = 0.038). The incidence of cesarean delivery was significantly higher in the respiratory failure group than the non-respiratory failure group: (80.8% vs. 64.9%; P = 0.031. The percentage of mothers with insufficient or excessive weight gain was higher in the respiratory failure group than the non-respiratory failure group (28.8% vs. 13.1% and 42.4% vs. 50.0%, respectively; P = 0.029). Moreover, placental immaturity, especially grades 0–1, was noted more frequently in the respiratory failure group (53.9% vs. 31.6%; P = 0.004). Prenatal dexamethasone injection was more frequently performed in the respiratory failure group (48.1% vs. 20.2%; P = 0.000). Other maternal factors, including productive age of the pregnant women, education level, prenatal body mass index, and gestational hypertension, did not differ significantly between the two groups, which may indicate that these factors are less influential on neonatal respiratory failure outcomes (Table 3).
3.4 Comparison of neonatal serum vit D level
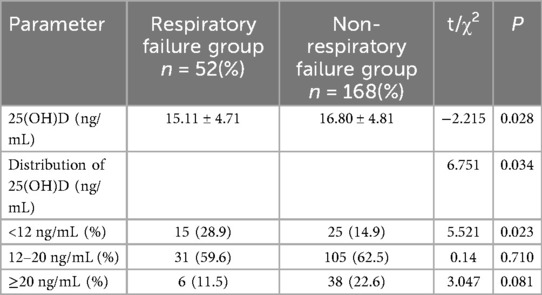
Table 4 compares the serum 25(OH)D levels between the respiratory and non-respiratory failure groups, showing several key statistical findings. The mean 25(OH)D level was significantly lower in the respiratory failure group than the non-respiratory failure group (15.11 ± 4.71 ng/mL vs. 16.80 ± 4.81 ng/mL; t = −2.215, P = 0.028). There was a significantly higher percentage of patients in the respiratory failure group than the non-respiratory failure group with 25(OH)D levels <12 ng/mL (9.45% vs. 30.55%, χ² = 5.521, P = 0.023). There was no significant difference in the 2 groups of patients with 25(OH)D levels ranging from 12–20 ng/mL (32.15% vs. 62.50%; χ² = 0.140, P = 0.710). Compared to the respiratory failure group, A significantly higher percentage of patients in the non-respiratory failure group had a 25(OH)D ≥20 ng/mL (22.60% vs. 10.40%; χ² = 3.047, P = 0.081).
3.5 Binary logistic regression analysis
Table 5 shows the results of binary logistic regression analysis on maternal and neonatal factors associated with neonatal respiratory failure, including those factors with p values < 0.05. Neonates born before 34 completed weeks of gestation had a significantly higher risk of respiratory failure (OR = 8.293, 95% CI = 1.326–51.863; P = 0.024). Therefore, neonates born at <34 weeks gestation had an 8.3-fold increased risk of developing respiratory failure compared to neonates born after 37 completed weeks of gestation. Lower levels of 25(OH)D were associated with an increased probability of neonatal respiratory failure (OR = 0.896, 95% CI = 0.817–0.984; P = 0.021). The probability respiratory failure increased by 10% approximately for each 1 unit incremental decrease in the 25(OH)D concentration. Birth weight was also significantly associated with respiratory failure (OR = 0.999, 95% CI = 0.998–1.000; P = 0.014). Although small, this effect suggested that a lower birth weight was associated with a slightly increased risk for respiratory failure with each 1 g reduction in birth weight associated with a marginal increase in risk. Excessive maternal weight gain during pregnancy was not associated with neonatal respiratory failure (P = 0.086).
3.6 Combination of the serum 25(OH)D level and multiple indicators in diagnosing neonatal respiratory failure
The receiver operating characteristic (ROC) curve for diagnosing neonatal respiratory failure based on the serum 25(OH)D concentration is shown in Figure 1. The area under the ROC curve (AUC) for serum 25(OH)D was 0.620 (95% CI = 0.531–0.708) with the largest Youden index of 0.211 when the cut-off value was 17.6 ng/mL. This finding suggests a sensitivity of 0.423 and a specificity of 0.788 at this cut-off value. The ROC curve remarkably improved in identifying respiratory failure by combining the serum 25(OH)D level with gestational age and birth weight. The AUC for multiple indicators reached 0.868 (95% CI = 0.812–0.925) with a maximum Youden index = 0.595. This combination yielded a sensitivity of 0.750 and specificity of 0.845, reflecting a higher predictive value for neonatal respiratory failure than single indicators (P < 0.001).
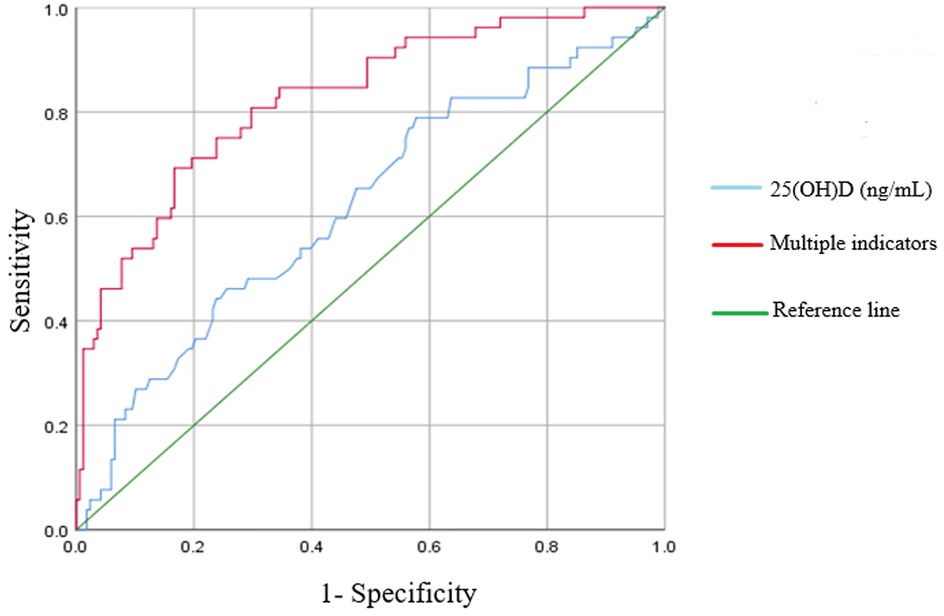
Figure 1. ROC curve comparison of 25(OH)D alone and 25(OH)D combined with multiple indicators in predicting the respiratory failure.
4 Discussion
Our study confirmed that preterm infants at a lower gestational age and birth weight have a higher predisposition to respiratory failure. Among preterm infants <34 weeks gestation, antenatal glucocorticoid exposure was associated with lower rates of acute respiratory distress syndrome but preterm infants still formed most neonatal respiratory failure cases. This finding suggests that close observation of neonatal vit D deficiency is required among neonates with a risk of respiratory complications.
Vit D has been associated with pulmonary function and inflammation. The effects of vit D on inflammatory cells, including dendritic cells, macrophages, T-cells, B-cells, and the structural epithelial cells, are important in mitigating pulmonary diseases (13, 14). Vit D modulates the immune system to enhance lung defense mechanisms against infections and inflammation, which are major contributors to respiratory failure (15). Furthermore, vit D supports pulmonary function by promoting the development of surfactant and improving alveolar fluid clearance, both of which help prevent pulmonary edema (16). Proper maturation of the respiratory system is all the more important in preterm infants because of the risk for respiratory complications due to immature lung development. There is a very close link between vit D levels and respiratory failure in preterm infants (17, 18), which is consistent with the results of the current study.
Other reports have also focused on neonatal and childhood respiratory outcomes as a function of vit D levels (19, 20). In one study, vit D deficiency among preterm neonates was associated with a higher incidence of respiratory distress syndrome, surfactant treatment, and non-invasive ventilation (19). In another study, infants in whom vit D levels were initially low still had a higher rate of respiratory and infectious morbidities, even though neonatal vit D levels improved with supplementation at standard doses, raising the possibility that personalized vit D dosing is required (20).
Severe vit D deficiency in umbilical cord blood has been associated with an increased risk of early birth and respiratory problems (21). Despite standard supplementation, many infants still had inadequate vit D, which is associated with a higher incidence of respiratory and allergic disorders (22). Currently, available studies suggest that vit D deficiency may increase the risk of respiratory failure through several mechanisms. Vit D is essential for fetal lung development and vit D deficiency impairs alveolar formation and surfactant production, leading to respiratory compromise (23). Additionally, vit D regulates immune responses and vit D deficiency can weaken the body's ability to fight infections, making neonates more susceptible to pulmonary infections (e.g., pneumonia), which can progress to respiratory failure (24). Vit D also helps control inflammation and vit D deficiency may exacerbate cytokine release, worsening lung damage in conditions like acute respiratory distress syndrome (ARDS) (25). Moreover, vit D maintains calcium homeostasis. Vit D deficiency leads to hypocalcemia, which eventually impairs respiratory muscle function and may contribute to respiratory failure (26). In conclusion, vitamin D deficiency in newborns increases the risk of neonatal respiratory failure by regulating the immune system and inflammatory responses, affecting the development of lung structure and lung ventilation.
Vit D deficiency in children has also been linked to the risk of developing recurrent respiratory infections (27) and is associated with an increased frequency of hospitalization for respiratory infections (28). Lykkedegn et al. concluded that there is still too little information to confirm or refute vit D in preventing or curing neonatal ARDS and bronchopulmonary dysplasia (BPD) (26) but significant animal and in vitro experiments support such biological roles in pulmonary maturation. Specifically, vit D roles in pulmonary maturation include promotion of alveolarization and maturation of type II alveolar epithelial cells, further adding to the hypothesis that vit D deficiency is a modifiable risk factor for ARDS and BPD (29).
BPD is a chronic respiratory disease that continues into childhood. BPD is defined by hypoxemia, pulmonary hypertension, wheezing, and an increased risk of asthma. The etiology of BPD is not well understood but includes immaturity of pulmonary structure and biochemistry, surfactant deficiency, low antioxidant and immune defenses, and injury from hyperoxia, hypoxia, or barotrauma caused by ventilatory support. These processes disrupt pulmonary epithelial cells, leading to edema, inflammation, and possible exacerbation by infections (30). Confirmation of the hypothesis through further research should include therapeutic studies on the usefulness of vit D in neonatal respiratory disorders.
Vit D-associated regulatory genes are expressed during pulmonary development in mice and humans. Vit D also downregulates disintegrin and metalloproteinase expression (31), which has a role in lung development and function (32). Sakurai et al. emphasized the critical role of vit D in perinatal lung maturation, including alveolar epithelial-mesenchymal interaction, regulation of alveolar fibroblast proliferation, and apoptosis (33). Additionally, vit D exerts beneficial effects on the respiratory system by maintaining integrity of the epithelial barrier and reducing lung injury (34). Vit D facilitates alveolar fluid transport by regulating epithelial sodium channel activity, thereby decreasing pulmonary edema (35). Lung barrier integrity depends on cohesive and tight junctions between epithelial and endothelial cells that form the air-blood barrier. Destruction of this barrier increases vascular permeability, promotes paracellular fluid movement into pulmonary spaces, and exacerbates lung dysfunction (36, 37).
4.1 Limitations of this study
The limitations of this study include the following issues. First, the retrospective design and failure to account for other factors that could influence neonatal respiratory failure, such as maternal nutrition and infection history. Second, the sample size was small from one single center, and the difference in sample size between the two groups was significant. This factor may lead to some bias in the results or statistical analysis. Third, although a correlation between maternal and neonatal vit D levels was observed, supplementation with vit D may have some protective effects. The results should be confirmed in large-scale prospective studies with larger sample sizes and assessment of multiple variable interactions. In fact, studying the interactive role of other micronutrients and prenatal factors associated with vit D may provide a fuller understanding of neonatal health.
5 Conclusion
The combination of neonatal vit D levels, gestational age, and birth weight provides a highly effective predictive model for respiratory failure incidence after birth. Respiratory failure is predicted by vit D deficiency or insufficiency, especially in preterm newborns with a gestational age < 34 weeks and low birth weight. By combining the above indicators, the risk prediction of neonatal respiratory failure may be improved, and effective respiratory support and comprehensive treatment can be actively adopted to reduce the impact of respiratory failure on newborns.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tianjin Medical University Baodi Hospital (2021-E-0301). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contribution
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tochie JN, Sibetcheu AT, Arrey-Ebot PE, Choukem SP. Global, regional and national trends in the burden of neonatal respiratory failure and essentials of its diagnosis and management from 1992 to 2022: a scoping review. Eur J Pediatr. (2024) 183(1):9–50. doi: 10.1007/s00431-023-05238-z
2. Delrue C, Speeckaert MM. Vitamin D and vitamin D-binding protein in health and disease. Int J Mol Sci. (2023) 24(5):4642. doi: 10.3390/ijms24054642
3. Wang P, Tan Z-X, Fu L, Fan Y-J, Luo B, Zhang Z-H, et al. Gestational vitamin D deficiency impairs fetal lung development through suppressing type II pneumocyte differentiation. Reprod Toxicol. (2020) 94:40–7. doi: 10.1016/j.reprotox.2020.03.008
4. Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, et al. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics. (2013) 6:47. doi: 10.1186/1755-8794-6-47
5. Saraf R, Morton SM, Camargo CA Jr, Grant CC. Global summary of maternal and newborn vitamin D status—a systematic review. Matern Child Nutr. (2016) 12(4):647–68. doi: 10.1111/mcn.12210
6. Administration Branch of the Chinese Nutrition Society. Expert consensus on the evaluation and improvement of vitamin D nutritional status. Chin J Health Manag. (2023) 17(04):245–52. doi: 10.3760/cma.j.cn115624-20230105-00009
7. Athanassiou L, Mavragani CP, Koutsilieris M. The immunomodulatory properties of vitamin D. Mediterr J Rheumatol. (2022) 33(1):7–13. doi: 10.31138/mjr.33.1.7
8. Ghaseminejad-Raeini A, Ghaderi A, Sharafi A, Nematollahi-Sani B, Moossavi M, Derakhshani A, et al. Immunomodulatory actions of vitamin D in various immune-related disorders: a comprehensive review. Front Immunol. (2023) 14:950465. doi: 10.3389/fimmu.2023.950465
9. Hansen L, Tjønneland A, Køster B, Brot C, Andersen R, Cohen AS, et al. Vitamin D status and seasonal variation among Danish children and adults: a descriptive study. Nutrients. (2018) 10(11):1801. doi: 10.3390/nu10111801
10. Shao XM, Ye HM, Qiu XS. Practical Neonatology. V5. Beijing: People’s Medical Publishing House Co., Ltd (2019). p. 603.
11. Lu J, Wang DH, Zhong BM. Expert consensus on diagnosis and treatment of neonatal mitochondrial diseases (2024). Chin J Neonatol. (2024) 39(11):647–55. doi: 10.3760/cma.j.issn.2096-2932.2024.11.002
12. Fleiss N, Schwabenbauer K, Randis TM, Polin RA. What’s new in the management of neonatal early-onset sepsis? Arch Dis Child Fetal Neonatal Ed. (2023) 108(1):10–4. doi: 10.1136/archdischild-2021-323532
13. Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet. (2017) 389(10079):1649–59. doi: 10.1016/S0140-6736(17)30312-4
14. Ahmad S, Arora S, Khan S, Mohsin M, Mohan A, Manda K, et al. Vitamin D and its therapeutic relevance in pulmonary diseases. J Nutr Biochem. (2021) 90:108571. doi: 10.1016/j.jnutbio.2020.108571
15. Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. (2011) 86:217–37. doi: 10.1016/B978-0-12-386960-9.00009-5
16. Al-Beltagi M, Rowiesha M, Elmashad A, Elrifaey SM, Elhorany H, Koura HG. Vitamin D status in preterm neonates and the effects of its supplementation on respiratory distress syndrome. Pediatr Pulmonol. (2020) 55(1):108–15. doi: 10.1002/ppul.24552
17. Papalia H, Samonini A, Buffat C, Gras E, des Robert C, Landrier J-F, et al. Low vitamin D levels at birth and early respiratory outcome in infants with gestational age less than 29 weeks. Front Pediatr. (2022) 9:790839. doi: 10.3389/fped.2021.790839
18. Dell'Orto V, Nobile S, Correani A, Marchionni P, Giretti I, Rondina C, et al. Early nasal continuous positive airway pressure failure prediction in preterm infants less than 32weeks gestational age suffering from respiratory distress syndrome. Pediatr Pulmonol. (2021) 56(12):3879–86. doi: 10.1002/ppul.25678
19. Jafari N, Taslimi Taleghani N, Kazemi SA, Abouoasef S, Motamed N, Jalilvand A. Association between vitamin D insufficiency and respiratory problems in premature neonates. Arch Iran Med. (2022) 25(1):32–6. doi: 10.34172/aim.2022.06
20. Yangin Ergon E, Dorum BA, Balki HG, Bako D, Alkan Ozdemir S. A prospective cross-sectional study on the vitamin D status of neonates and the impact of neonates’ standard vitamin D supplementation on neonatal morbidities. Children (Basel). (2024) 11(5):543. doi: 10.3390/children11050543
21. Treiber M, Mujezinović F, Pečovnik Balon B, Gorenjak M, Maver U, Dovnik A. Association between umbilical cord vitamin D levels and adverse neonatal outcomes. J Int Med Res. (2020) 48(10):300060520955001. doi: 10.1177/0300060520955001
22. Dajic K, Bjelakovic B, Kostic A, Vujic A, Jankovic S, Milovanovic J, et al. Hypovitaminosis D in infants: evidence that increased intake of vitamin D reduces the incidence of allergic and respiratory disorders. Int J Clin Pharmacol Ther. (2023) 61(3):96–101. doi: 10.5414/CP204093
23. Binks MJ, Smith-Vaughan HC, Bar-Zeev N, Chang AB, Andrews RM. Vitamin D insufficiency among hospitalised children in the Northern Territory. J Paediatr Child Health. (2014) 50(7):512–8. doi: 10.1111/jpc.12623
24. Dhawan M, Priyanka, Choudhary OP. Immunomodulatory and therapeutic implications of vitamin D in the management of COVID-19. Hum Vaccin Immunother. (2022) 18(1):2025734. doi: 10.1080/21645515.2022.2025734
25. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore). (2019) 98(38):e17252. doi: 10.1097/MD.0000000000017252
26. Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. (2015) 308(7):L587–602. doi: 10.1152/ajplung.00117.2014
27. Jachvadze M, Shanidze L, Gubelidze N, Gogberashvili K. Vitamin D status among Georgian children with high acute respiratory morbidity. Georgian Med News. (2021) 314:95–9. https://pubmed.ncbi.nlm.nih.gov/34248035/
28. Jaybhaye AP, Sangle AL, Ugra D, Chittal RY. A hospital-based study of vitamin D levels in children with recurrent respiratory infections. Cureus. (2022) 14(8):e27864. doi: 10.7759/cureus.27864
29. Hong M, Xiong T, Huang J, Wu Y, Lin L, Zhang Z, et al. Vitamin D supplementation and lower respiratory tract infection in infants: a nested case-control study. Infection. (2023) 51(1):109–18. doi: 10.1007/s15010-022-01845-4
30. Gibson AM, Reddington C, McBride L, Callanan C, Robertson C, Doyle LW. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. (2015) 50(10):987–94. doi: 10.1002/ppul
31. Kovacs CS. Bone metabolism in the fetus and neonate. Pediatr Nephrol. (2014) 29(5):793–803. doi: 10.1007/s00467-013-2461-4
32. Matejek T, Zemankova J, Malakova J, Cermakova E, Skalova S, Palicka V. Severe vitamin D deficiency in preterm infants: possibly no association with clinical outcomes? J Matern Fetal Neonatal Med. (2022) 35(8):1562–70. doi: 10.1080/14767058.2020.1762560
33. Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, et al. 1α, 25 (OH) 2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. (2009) 297(3):L496. doi: 10.1152/ajplung.90539.2008
34. Shi Y-Y, Liu T-J, Fu J-H, Xu W, Wu L-L, Hou A-N, et al. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. (2016) 13(2):1186–94. doi: 10.3892/mmr.2015.4685
35. Nie H, Cui Y, Wu S, Ding Y, Li Y. 1, 25- vitamin D enhances alveolar fluid clearance by upregulating the expression of epithelial sodium channels. J Pharm Sci. (2016) 105(1):333–8. doi: 10.1016/j.xphs.2015.11.022
36. Greiller CL, Suri R, Jolliffe DA, Kebadze T, Hirsman AG, Griffiths CJ, et al. Vitamin D attenuates rhinovirus-induced expression of in-tercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor re-ceptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol. (2019) 187:152–9. doi: 10.1016/j.jsbmb.2018.11.013
Keywords: neonatal, vitamin D deficiency, pregnant woman, perinatal, related factors
Citation: Zhang C (2025) Predictive value of vitamin D levels in neonatal respiratory failure: a retrospective study. Front. Pediatr. 13:1680809. doi: 10.3389/fped.2025.1680809
Received: 6 August 2025; Accepted: 27 October 2025;
Published: 19 November 2025.
Edited by:
Radek Kucera, Charles University, CzechiaReviewed by:
Shuo Wang, Central South University, ChinaRivan Virlando Suryadinata, University of Surabaya, Indonesia
Copyright: © 2025 Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenlu Zhang, emhhbmdjaGVubHUwNDk1QDE2My5jb20=
†ORCID:
Chenlu Zhang
orcid.org/0009-0002-1953-7409
 Chenlu Zhang
Chenlu Zhang