- 1Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
- 2SA-MRC Unit on Child and Adolescent Health, University of Cape Town, Cape Town, South Africa
Introduction: The availability and use of surfactant replacement therapy (SRT) for respiratory distress syndrome (RDS) in low- and middle-income countries (LMICs) is variable with unclear impact on infant outcomes. This review evaluates the published evidence on SRT in the management of preterm neonates with RDS in LMICs, with a focus on SRT availability, administration, timing, type, and cost.
Methods: A systematic scoping review of seven databases was conducted, following the Preferred Items for Systematic Reviews and Meta-Analysis guidelines extension for Scoping Reviews. English language systematic reviews and observational and experimental studies, published between January 2010 and July 2023, were eligible for this review. Case reports, small case series, and qualitative studies were excluded. Titles and abstracts were screened by one reviewer and full text by two independent researchers. Sufficiently homogeneous randomized controlled trials (RCTs) were synthesized using random-effects meta-analyses, while other results were synthesized narratively. Primary outcomes for meta-analyses were (1) need for invasive mechanical ventilation (IMV), (2) development of bronchopulmonary dysplasia (BPD), and (3) in-hospital mortality.
Results: After screening 483 titles/abstracts and 266 full texts, 113 articles were included in the final review (52 RCTs, 50 observational studies, and 11 systematic reviews). Studies reported both INtubation-SURfactant-Extubation (INSURE) and Less/Minimal Invasive Surfactant Administration/Treatment (LISA/MIST) methods of SRT, with different threshold criteria for implementation. There was moderate certainty evidence that using LISA/MIST reduced the need for IMV [risk difference (RD): 0.10 (95% confidence interval, CI: 0.04–0.17); p = 0.001] compared with INSURE, with a borderline effect on BPD [RD: 0.04 (95% CI: 0.00–0.08); p = 0.05] and no significant effect on mortality [RD: 0.01 (95% CI: −0.02 to 0.04; p = 0.5)]. There was low certainty evidence that poractant alfa (200 mg/kg) was associated with a reduced need for mechanical ventilation compared with beractant (100 mg/kg) (RD 0.10 (95% CI: 0.02–0.18); p = 0.01), with a similar reduction in mortality [RD: 0.07 (95% CI: 0.01–0.13); p = 0.02]. No cost-effectiveness studies were identified.
Conclusion: LISA/MIST should be used in preference to INSURE. Poractant alfa (200 mg/kg) is conditionally recommended in preference to beractant (100 mg/kg). Regionally relevant cost-effectiveness studies are needed.
Introduction
A leading cause of neonatal mortality (NNM), preterm birth and associated complications, result in approximately 1 million infant deaths annually, 99% of which occur in low- and middle-income countries (LMICs). NNM, accounting for almost 50% of deaths in children under 5 years (1), accounts for an increasing proportion of deaths, as other causes have greatly reduced in the past few decades. While the contribution of NNM to childhood deaths increases, more neonates are born [and in high-income countries (HICs), surviving] at an even younger gestational age (GA) (2). Achieving the target of fewer than 12 neonatal deaths per 1,000 births, as stipulated in the Sustainable Development Goals (SDGs) of 2030 (3), will require a strengthening of antenatal and perinatal care.
Approximately 45% of prematurity-related deaths in LMICs are attributable to respiratory distress syndrome (RDS) (4) caused by lung immaturity and surfactant deficiency (5). Respiratory interventions such as continuous positive airway pressure (CPAP) and surfactant replacement therapy (SRT) have improved the outcomes of preterm infants with RDS and are part of standard care in HICs (6, 7). However, in 2020, only 33% of African countries had access to SRT and 63% to CPAP (6). Even in settings where these interventions are available, costs may be prohibitive (6, 8–18). Furthermore, most of the evidence supporting their use is from HICs (14, 16, 19, 20), with a lack of data from Africa and other LMICs. Inaccurate gestational age assessments, inadequate antenatal care and underutilization of antenatal steroids, urban–rural and public–private resource discordance, and wide variation in the number of live births occurring outside health facilities further complicate a standardized SRT approach in resource-limited LMIC settings.
In 2016, Sankar et al. (21) published a seminal systematic review on the efficacy, safety, feasibility, and cost-effectiveness of SRT in low-resource settings. The review confirmed a significant reduction in both mortality and risk of air leaks in preterm neonates who received SRT. Suggested areas for future research included defining ideal timing for rescue SRT, its cost- effectiveness, exploration of less invasive modalities of SRT, and the effect of SRT on bronchopulmonary dysplasia (BPD) in resource-restricted settings (RRS) (21).
A growing body of research in LMICs around the threshold criteria, ideal timing, surfactant type and dose, method of administration, and respiratory support pre- and post-SRT has emerged in the last decade. Studies in HICs have demonstrated that Less Invasive Surfactant Administration (LISA) reduces the composite outcome of death or BPD compared with the INtubation-SURfactant-Extubation (INSURE) method (22).
This systematic scoping review aimed to provide an update on the availability and practices of SRT and their effect on infant outcomes in LMICs, and to make recommendations for use, to inform policy and identify priority research areas.
Methods
This study was a systematic scoping review (23) of the published literature on the use of SRT in the treatment of RDS in preterm neonates in LMICs. The objectives were (1) to explore the availability and use of SRT in LMICs and describe the range of current practice and (2) to describe the impact of SRT on infant outcomes, including the need for invasive mechanical ventilation (IMV), on BPD prevalence, and on mortality.
Protocol and registration
A scoping review protocol was created a priori. This paper adheres to Preferred Items for Systematic Reviews and Meta-Analysis guidelines extension for Scoping Reviews (PRISMA-ScR) (24), and as per these guidelines, registration as a systematic review was not required.
Ethics
An ethical waiver was granted on the grounds that “As the systematic review involves published literature available through publicly accessible electronic databases, research ethics review and approval is not required” (HREC REF 301/2021).
Eligibility criteria
Published systematic reviews and observational and experimental studies that focused on SRT, with or without ancillary therapies, in preterm neonates with or at-risk of RDS, conducted in LMICs as defined by World Bank Income (WBI) categories (25), were considered for inclusion. Specifically, studies describing the availability, use (criteria for use, method, and timing of administration), complications, in-hospital outcomes, and mortality associated with SRT in these settings were considered for inclusion. Case reports, case series, and qualitative studies were excluded. Unpublished data or research in progress were not included.
Information sources
Primary systematic searches were conducted in EBSCOhost (Africawide and CINAHL), Web of Science, Scopus, Scielo, and Cochrane, using comprehensive search strategies with controlled vocabulary and Boolean operators, while Google Scholar was used supplementarily to capture literature not indexed in traditional databases and identify additional sources through citation searching, ensuring breadth of coverage appropriate for a scoping review's exploratory aims. Peer-reviewed literature published in English between January 2010 and June 2025 and meeting eligibility criteria was considered for inclusion. The final and most recent search was executed on 9 October 2025.
Search strategy
Search strategies were customized for each database's indexing system while maintaining core medical subject headings (MeSH) terms. The filters used included date and language limits as specified above. The full search strategy for PubMed was
#1 “Pulmonary Surfactants"[Mesh] OR “Surface-Active Agents"[Mesh]
#2 surfactant*[tiab] OR “surface active agent*"[tiab] OR “lung surfactant*"[tiab] OR exogenous surfactant*[tiab] OR beractant[tiab] OR survanta[tiab] OR poractant[tiab] OR curosurf[tiab] OR calfactant[tiab] OR infasurf[tiab] OR bovactant[tiab] OR lucinactant[tiab]
#3 #1 OR #2
#4 “Respiratory Distress Syndrome, Newborn"[Mesh]
#5 “respiratory distress syndrome"[tiab] OR RDS[tiab] OR “hyaline membrane disease"[tiab] OR HMD[tiab] OR “neonatal respiratory distress"[tiab]
#6 #4 OR #5
#7 “Infant, Premature"[Mesh] OR “Infant, Extremely Premature"[Mesh] OR “Infant, Very Low Birth Weight"[Mesh] OR “Infant, Extremely Low Birth Weight"[Mesh] OR “Infant, Low Birth Weight"[Mesh]
#8 preterm*[tiab] OR premature*[tiab] OR “low birth weight"[tiab] OR LBW[tiab] OR VLBW[tiab] OR ELBW[tiab] OR “very low birth weight"[tiab] OR “extremely low birth weight"[tiab]
#9 #7 OR #8
#10 “Developing Countries"[Mesh] OR “Africa"[Mesh] OR “Asia"[Mesh] OR “South America"[Mesh] OR “Central America"[Mesh] OR “Caribbean Region"[Mesh]
#11 LMIC*[tiab] OR “low income countr*"[tiab] OR “middle income countr*"[tiab] OR “low and middle income"[tiab] OR “developing countr*"[tiab] OR “developing nation*"[tiab] OR “developing world"[tiab] OR “less developed countr*"[tiab] OR “under developed countr*"[tiab] OR “underdeveloped countr*"[tiab] OR “third world"[tiab] OR “low resource"[tiab] OR “limited resource"[tiab] OR “resource limited"[tiab] OR “resource poor"[tiab]
#12 [LIST OF INDIVIDUAL LMIC COUNTRIES]
#13 #10 OR #11 OR #12
#14 #3 AND #6 AND #9 AND #13
Filters: Humans
Selection of sources of evidence
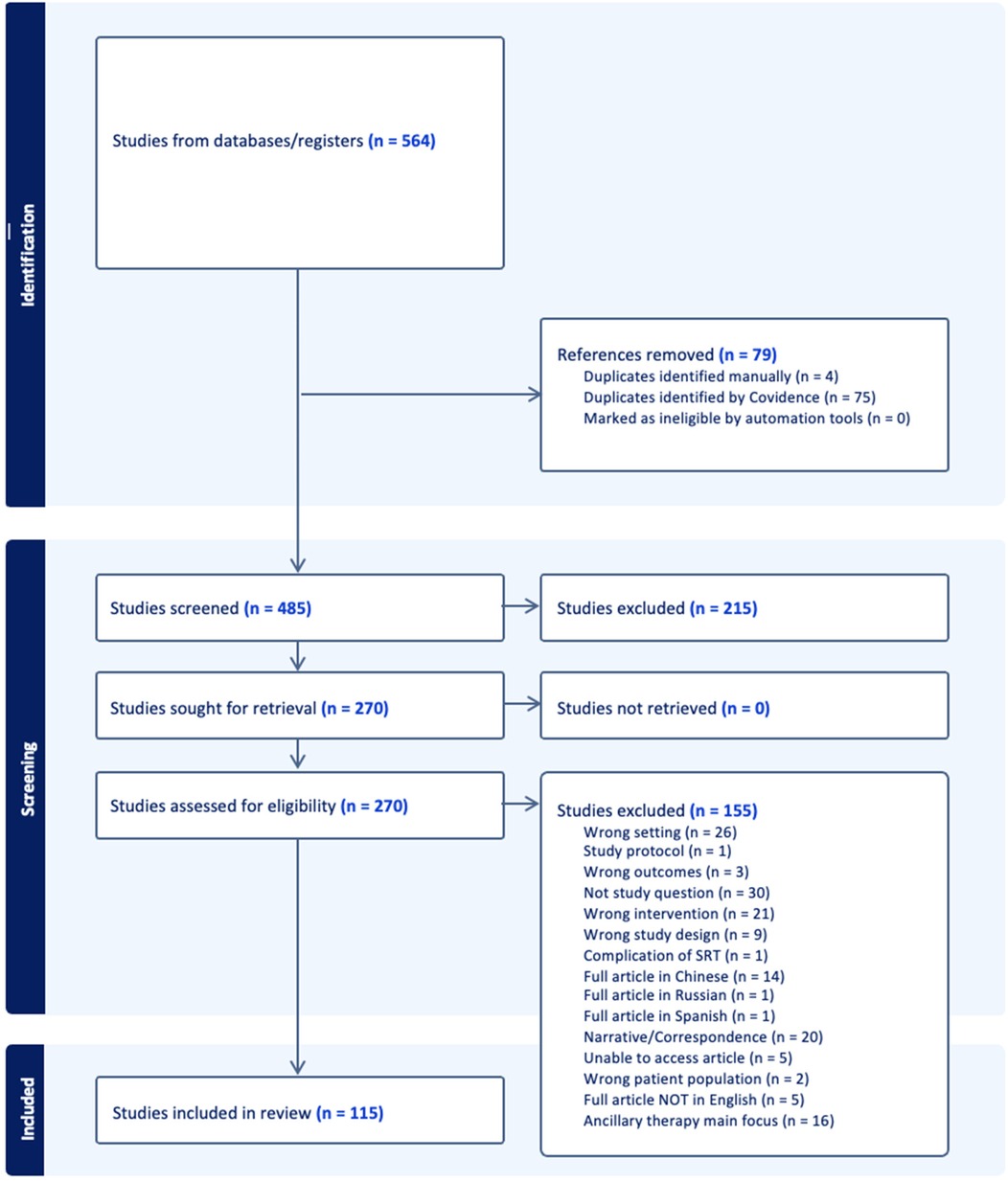
Following the search, identified citations were uploaded into both EndNote (26) and Covidence (https://www.covidence.org/), and duplicates were removed. One reviewer screened titles and abstracts against the inclusion criteria to identify potentially relevant studies. Once retrieved, full texts were reviewed for inclusion by two independent researchers. The online software program, Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia, 2023), was used for the article screening and selection process. Any disagreements arising between reviewers were resolved through discussion and/or with an additional reviewer/s in the case of disagreement. The results of the search and study inclusion process, as well as reasons for exclusion of sources of evidence at full text review, were recorded and reported in PRISMA-ScR flow diagram (Figure 1) (24).
Data charting process
Data from included studies were extracted in a standardized manner, using a self-developed data extraction form and charted in Microsoft Excel (Microsoft Corporation, 2018) for analysis.
Data items
The extracted data included the following: (a) country, (b) World Health Organization (WHO) region, (c) WBI group, (d) setting (public/private, tier of healthcare, etc.), (e) study design and grouping, (f) time frame, (g) ethics approval, (h) baseline characteristics (overall sample, intervention group, control group), (i) inclusion criteria, (j) exclusion criteria, (k) surfactant details [type, dosage, indications, timing, method of administration, use of premedication, rank of healthcare provider performing SRT, number of attempts of SRT, mean time dose(s) given, definition of SRT failure, implementation strategy, barriers and facilitators for use], (l) characteristics of ancillary therapies [including non-invasive ventilation (NIV), IMV, antenatal steroid use, methylxanthine use, postnatal steroid use, oxygen therapy, settings used, etc.], and (m) outcomes, including mortality and other adverse events.
Critical appraisal of individual sources of evidence
In the case of studies being amenable to pooling results with meta-analyses, a combination of the “Grading of Recommendations, Assessment, Development, and Evaluations” (GRADE) criteria (27) and Joanna Briggs Institute (JBI) critical appraisal tool checklists (for observational studies) (23) were applied to inform the certainty of results (see the summary of findings’ tables). The Robvis tool (28) was used to generate critical appraisal infographics for pooled randomized controlled trials (RCTs).
Synthesis of results
While the primary purpose of this study was a scoping review to map the literature on the topic, secondary meta-analyses of homogeneous RCTs were conducted after assessing for clinical and methodological homogeneity. Clinical homogeneity was evaluated by comparing study populations (gestational age, birth weight), interventions (surfactant type, dose, delivery method), comparators, and outcome definitions. Methodological homogeneity was assessed by examining study design quality, risk of bias, and outcome measurement approaches. Sufficiently homogeneous RCTs, considering interventions, outcomes, and study sample, were synthesized using random-effects meta-analyses, while other results were synthesized narratively. Primary outcomes for meta-analyses were (1) progression to IMV, (2) development of BPD, and (3) in-hospital mortality.
Statistical heterogeneity was subsequently assessed using the I2 statistic post hoc, with I2 > 50% considered substantial heterogeneity. When substantial statistical heterogeneity was detected despite clinical homogeneity, we planned to explore potential sources through sensitivity or subgroup analyses. Certainty of evidence was assessed using GRADE methodology, with heterogeneity contributing to downgrading when I2 > 50%. IBM SPSS Statistics (version 28.0.1.1) was used for meta-analysis, and a p-value of <0.05 was considered statistically significant.
Results
Selection of sources of evidence
A total of 975,639 articles were identified on the initial search, the titles of which were screened by the first author. Following title screening, 564 citations were exported to Covidence. After removing duplicates, 485 titles and abstracts were screened with a resultant 265 full texts assessed for eligibility. After excluding 150 studies, 115 articles were included in the final review (Figure 1). The breakup for these articles was 53 RCTs, 11 systematic reviews, and 51 observational studies (Supplementary Appendix 1, Supplementary Tables S1–S3).
Characteristics of the sources of evidence
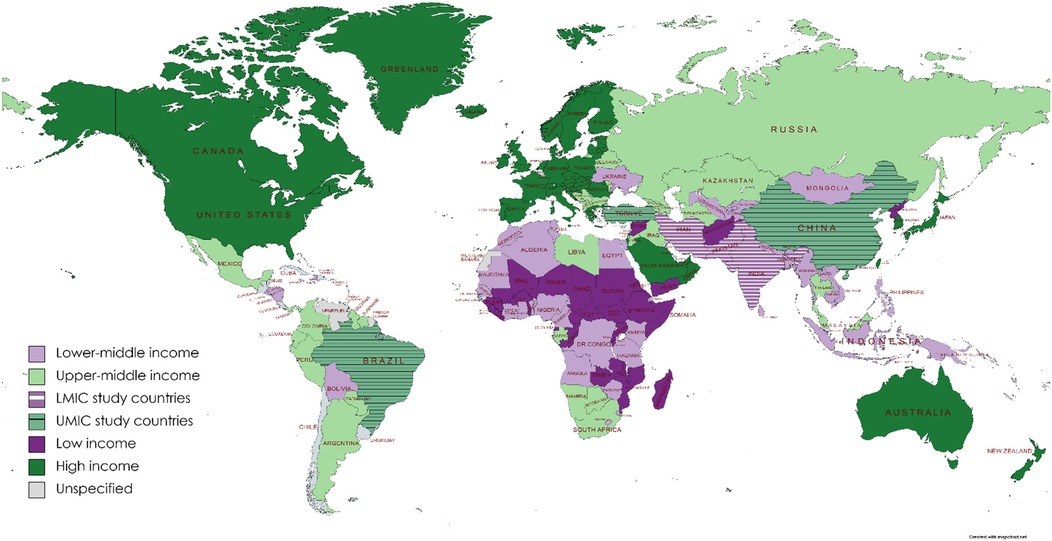
Excluding systematic reviews (n = 11), most (74%) articles were published between 2014 and 2017 (33%) and 2018 and 2021 (41%), with a trend to an increasing number of publications over time. In terms of WBI group classification (25) (Figure 2), 44% (n = 45) of studies originated from low- to middle-income countries and the remainder from high-to middle-income countries. Most publications were from the WHO regions of Eastern Mediterranean (n = 29; 28.4) or Europe (n = 28; 27.5%), with the African region publishing only 6 (5.9%) studies (Figure 3). Study methodology was almost equally split between RCTs (46%) and observational (44%) designs, with only one each of “cost-effectiveness” and “diagnostic accuracy” studies. Studies were conducted in 19 countries, most frequently Turkey (24.5%), followed by Iran (22.5%), with many countries producing only one included study.
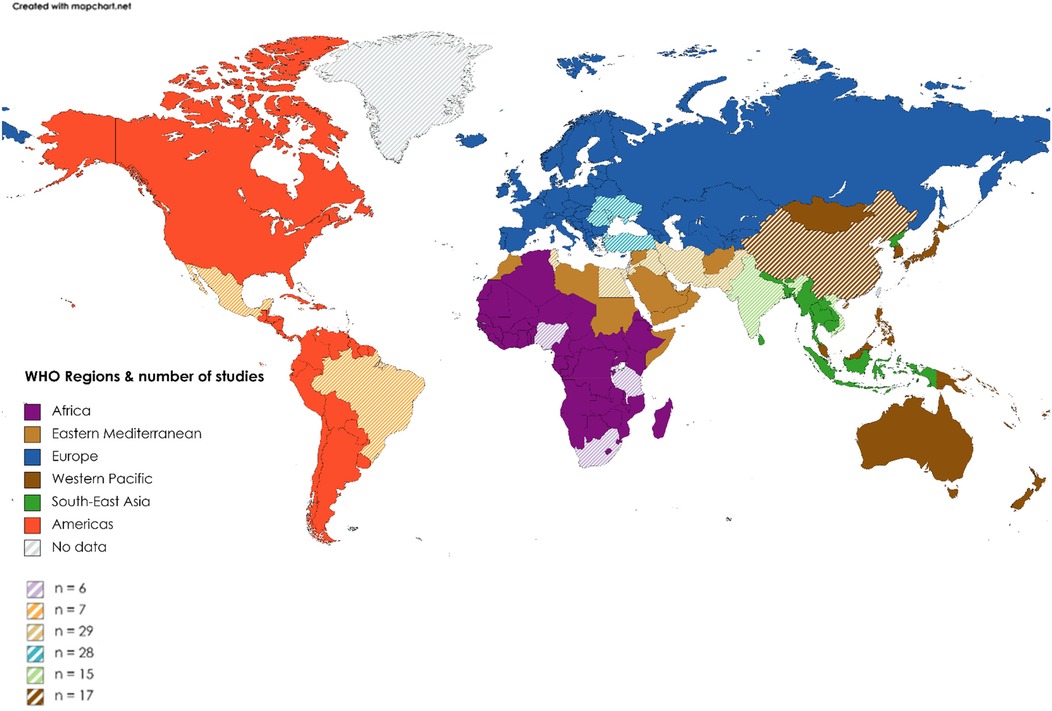
Figure 3. Distribution of publications by WHO region. Color, WHO region; Striped areas, countries from region that contributed to publication count (see map key for the total number of publications per WHO region).
A total sample of 6,524 infants was derived from all included RCTs. Sample sizes varied widely per study, with most RCTs utilizing samples of between 40 and 99 participants and most observational studies including ≥200 infants. Studies were only included if at least a proportion of the population were likely to be premature neonates; however, the range of gestational ages was highly heterogeneous. Many studies also used infant birth weight as an inclusion criterion, with most using 500 g as a lower limit. The male and female genders were mostly equally represented in the samples. Most studies were conducted in level III neonatal intensive care units (NICUs) and typically under the auspices of specialist pediatricians or neonatologists.
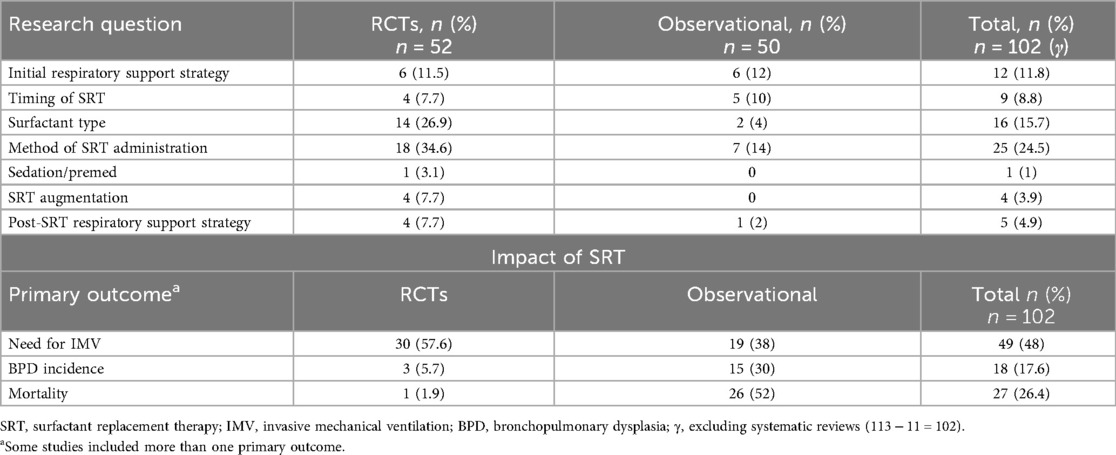
The most frequent research question pertained to the method of administration of SRT (24.5%), with most comparing less invasive methods vs. endotracheal administration. Other questions covered the topics of initial respiratory support strategies prior to and/or mitigating the need for SRT (11.8%) and post-SRT respiratory support strategies (4.9%), timing of SRT (8.8%), surfactant type (15.7%), and SRT augmentation (3.9%), while one study addressed the topic of peri-SRT sedation. With regard the outcomes of interest, the need for IMV was the most commonly reported outcome (48%), followed by mortality (26.4%) and BPD incidence (17.6%) (Table 1).
Critical appraisal within sources of evidence
Studies that were pooled for meta-analysis displayed generally low levels of bias (see Supplementary Appendix 2, Supplementary Figures S1–S3). Limitations may include selection bias given that all non-English publications were excluded, for pragmatic reasons. In addition, despite certain groups of studies being amenable to pooling for meta-analysis, heterogeneity was present and rendered evidence of moderate to low certainty.
Synthesis of findings
Availability and use of SRT in LMICs
The most well-represented countries were Turkey and Iran. No randomized controlled studies were identified from any African country. Several papers reported that surfactant, while available, is often not readily accessible because of its prohibitive cost (6–18). The coverage of surfactant in African countries is estimated to be <40% (29), despite its inclusion in the WHO Essential Drug List. In addition, surfactant is generally used only in centers that are also able to offer mechanical ventilation (13), and the availability (14, 15, 17) thereof is an additional challenge. Even supplemental oxygen and x-ray facility availability (15, 16) were far from ubiquitous. A lack of national health insurance schemes or severe limitations therein mean that in some countries, surfactant and any ancillary therapy are given only where parents can afford it, out of pocket (9, 13). In certain settings, the cost of SRT exceeds the average per capita Gross National Product (GNP) of the country (9), rendering “cost rather than care” (9) the driver of SRT.
Threshold criteria/indications for SRT
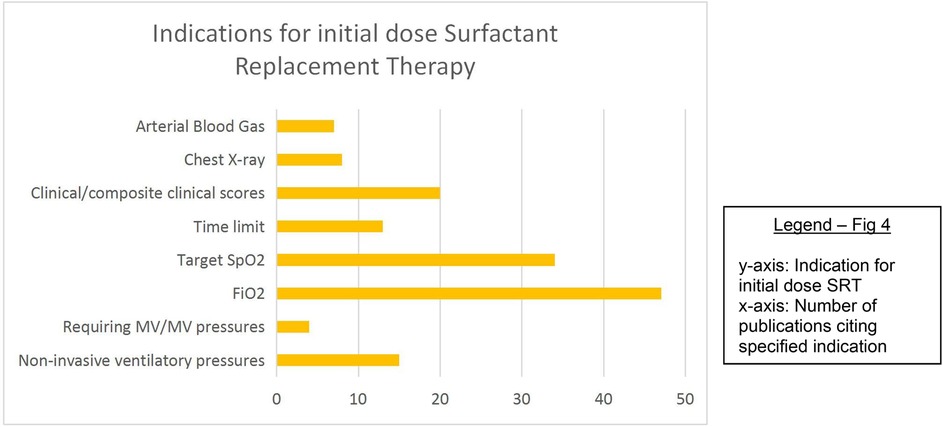
Fifty-six percent of studies described the threshold at which the first dose of SRT was administered. These include, in order of descending frequency: fraction of inspired oxygen (FiO2), target oxygen saturation (SpO2), clinical features or composite clinical score [e.g., Silverman Anderson score (30), Acute Care of At-Risk Newborns (ACoRN) (31)], non-invasive ventilatory pressure cut-off values, time limits, radiographic findings, arterial blood gas analysis, and the requirement for IMV/IMV pressure cut-off values. Most studies used a combination of these factors, especially FiO2, target SpO2, and time limits (Figure 4). Vardar et al. (32) developed a lung ultrasound (LUS) severity score [reference standard called chest radiograph (CXR)], which predicted the need for administering initial and subsequent surfactant doses with promising specificity and sensitivity.
A FiO2 threshold of requiring ≥0.4 to meet target saturation was most frequently employed as the indication to provide SRT, with the commonest target saturation range being 90%–95%. Eight studies (22, 33–39) used a target saturation range with a lower limit of 85%, and four studies (40–43) used a FiO2 threshold of ≥0.50.
Type and dose of surfactant
Sixteen studies (14 RCTs) compared different types of surfactants for several outcomes (8, 14, 33, 41, 44–55). Only one retrospective study (14) compared natural with synthetic surfactant; the remainder compared naturally derived surfactants. Sixty percent of these studies compared beractant (Survanta, AbbVie Laboratories, Chicago, IL, USA) and poractant alfa (Curosurf, Chiesi Farmaceutici, Parma, Italy) (eight RCTs and one observational study).
Excluding cost-effectiveness and diagnostic studies, guidelines, systematic reviews, and studies directly comparing surfactant types not used as standard practice, 73.5% of original studies specified either the type or dose, or both, of the initial bolus of surfactant routinely used in their settings or study. Of those where the type was known, porcine surfactant was used in 60%, bovine in 34.5%, and either bovine or porcine (depending on availability) in 5.5%. Supplementary Tables S4 and S5 display the various types and trade names of surfactants used in the included studies. The most frequent type/dose combination used was poractant alfa (200 mg/kg), followed by beractant (100 mg/kg), according to the manufacturer's recommended dosages.
Kandraju et al. (56) mentioned the lack of availability of Curosurf in the Indian market for a few months during the authors' study period, while an additional four studies (6, 9, 42, 57) indicated that the type of surfactant used was entirely dependent on availability.
Method of administration
Seventeen RCTs (22, 40, 58–73) and six observational studies (37, 66, 74–77) compared various methods of surfactant administration (Supplementary Appendix 1, Supplementary Tables S1, S2) generally, and “less invasive” methods [predominantly LISA but also use of a laryngeal mask airway (LMA) (40)/via thin intratracheal catheter (CATH) (59)/Minimally Invasive Surfactant Administration (MISA) (67)/Minimal Invasive Surfactant Treatment (MIST) (63, 70, 72)/TakeCare (61)] involving either a feeding, vascular, or other catheter as an intratracheal conduit for SRT were compared with intratracheal administration via the endotracheal tube (ETT) for determining several outcomes, including those pertinent to this review.
Excluding those studies that compared two methods of administration, LISA alone was used in 11.7% of studies, INSURE in 51.9,; either/or both these techniques in 3.9%, and the method was not specified in 32.4%.
Reported strengths of the LISA technique include the following: it is relatively easy to learn (22) and the use of an infant feeding tube as a conduit is cost-effective and immediately available (68); while other studies report concerns regarding procedural sedation (or lack thereof) (63, 70, 78) and staff reluctance to transition to this method (76).
As shown in Supplementary Tables S1 and S2, heterogeneity existed between studies in terms of the type and size of the intratracheal conduit used, its depth of insertion, the type of NIV maintained during the less invasive procedure, and the exact methodology used to deliver SRT. The control procedures also differed.
Timing of administration
Ten studies [four RCTs (56, 79–81) and six observational (75, 82–86)] investigated the role of timing of SRT on outcomes, including the need for IMV, mortality, and persistence of patent ductus arteriosus (PDA). SRT timing ranged from “with the first breath” to up to 72 h after birth. No two studies tested the same window of time. Included studies broadly divided the administration into “prophylactic” use (given soon after birth, usually based on GA) or “rescue” use (given once certain clinical parameters met). Most units appear to be using clinical indications, rendering time a secondary consideration. The extreme heterogeneity of the data precluded meta-analysis. Five of the studies (56, 81–83, 86) demonstrated statistically significant findings, with “early” surfactant having more favorable outcomes than “late” SRT.
SRT augmentation
Four RCTs (87–90) described the application of SRT augmentation with 75% of these originating from Iran. Two (88, 90) described intratracheal therapy in addition to intratracheal surfactant—one (88) reported the instillation of budesonide 0.25 mg/kg, while the other (90), salbutamol 0.2 mg/kg. A third study (89) described nebulization of the infant with salbutamol 0.15 mg/kg by a micropump nebulizer 10 min prior to SRT. The fourth study (87) described the addition of salbutamol 0.2 mg/kg, although it was not clear by what route this was administered. In both studies comparing salbutamol (89, 90) with a control, the need for IMV was significantly lower in the intervention group. The incidence of BPD in the SRT + budesonide group was significantly lower than that in the control group (88). However, the samples were small in size, BPD definitions were not standardized (limiting generalizability), and the quality of evidence was poor. Neurodevelopmental (and other long-term) follow-up was lacking, and further research in RRS is required.
Respiratory support strategies—pre- and post-SRT
Fourteen studies—10 RCTs (43, 91–99) and 4 observational studies (34, 57, 100, 101)—examined the mode of respiratory support either/both prior to and following SRT. Most compared the use of NIV other than CPAP [duo positive airway pressure (DUOPAP), bilevel positive airway pressure (BiPAP), and non-invasive intermittent positive pressure ventilation (nIPPV)] or non-invasive high frequency oscillatory ventilation (nHFOV) vs. CPAP, with a small number comparing invasive with non-invasive ventilation or high flow nasal cannula (HFNC) with other respiratory support.
Premedication practices
Excluding systematic reviews, eight studies (19, 40, 50, 59, 71, 88, 102, 103) reported the use of premedication or analgesia during SRT: 22.2% with LISA, 22.2% with INSURE, and 55.6% with both/either method of delivery. Premedication or analgesia was not reported in 75% of included studies. Fourteen studies (34, 37, 56, 61, 64, 65, 67–69, 74, 75, 77, 79, 80) reported the use of no premedication or analgesia, and four studies (68, 69, 72, 102) reported the use of non-pharmacological measures such as nesting and swaddling during SRT delivery. A variety of drugs were employed by those using pharmacological measures, including fentanyl (50, 88, 102), atropine (59), morphine (71), combinations of remifentanil (40) and midazolam (40), or atropine and ketamine (103), phenobarbitone (19), and in the case of the LMA vs. LISA, lidocaine gel (40).
Outcomes
A lack of standardization with regard to terminology, procedures, and outcome measures rendered much of the data inhomogeneous and meaningfully incomparable. However, sufficiently homogeneous studies were pooled in meta-analyses for the primary outcomes of this review. The only factor found to impact the outcome of mortality, with moderate certainty of evidence, was that of poractant alfa over beractant.
Type of surfactant
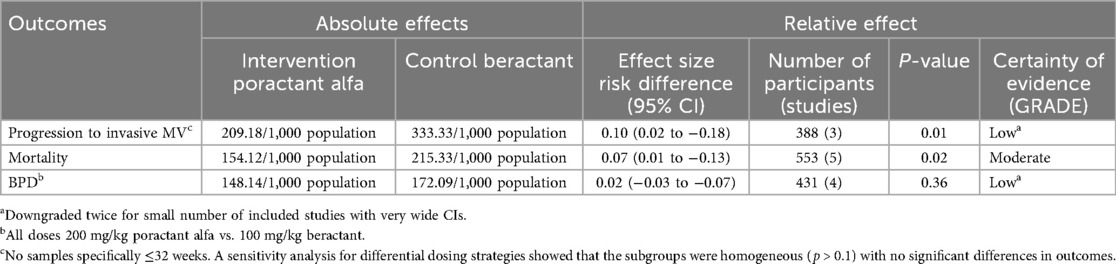
Poractant alfa was found to be superior to beractant for mortality [pooled risk difference (95% confidence interval, CI) 0.07 (0.01–0.12); p = 0.02] and progression to IMV (pooled risk difference 0.10 (0.02–0.28); p = 0.01), with no difference in the proportion developing BPD [pooled risk difference 0.02 (−0.03 to 0.07); p = 0.36] (Table 2; Figure 5). There was no significant difference between dosing schedules or gestational age groups on post hoc sensitivity analysis.
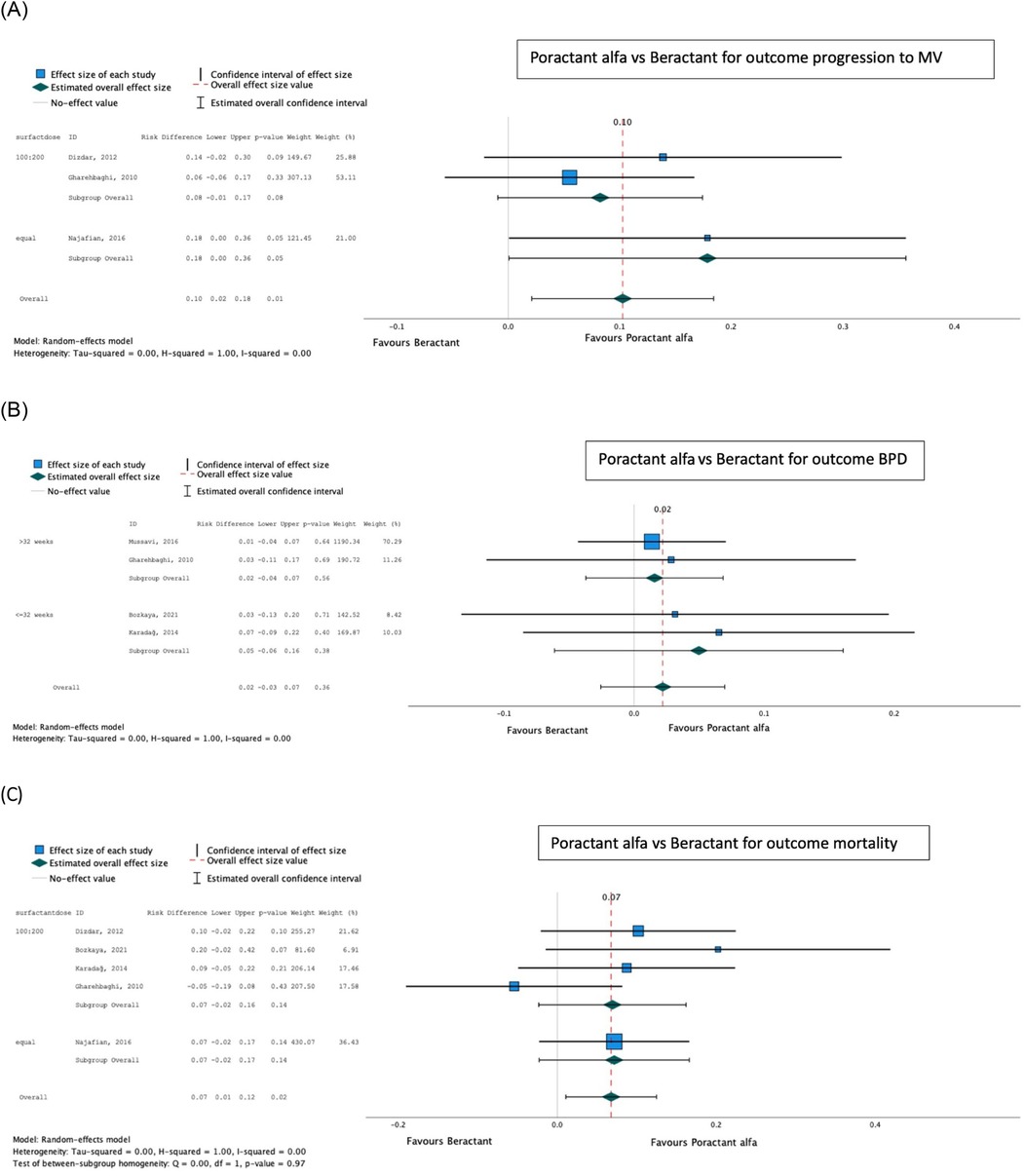
Figure 5. Effect of poractant alfa vs. beractant on progression to invasive mechanical ventilation (A), bronchopulmonary dysplasia (B), and mortality (C).
Method of administration
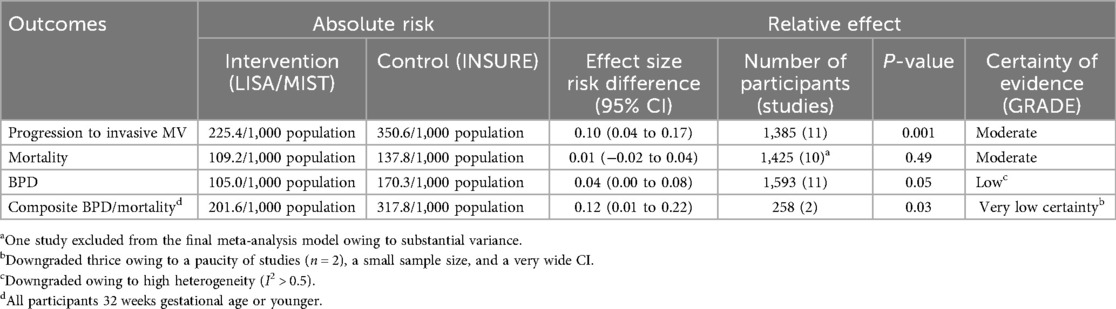
LISA/MIST was favored over INSURE for the outcome progression to IMV [pooled risk difference (95% CI) 0.10 (0.04–0.17); p < 0.001], while no significant difference was found between the techniques for the development of BPD [pooled risk difference 0.03 (−0.01 to 0.08); p = 0.12] or mortality [pooled risk difference 0.02 (−0.02 to 0.06); p = 0.29] (Table 3; Figure 6).
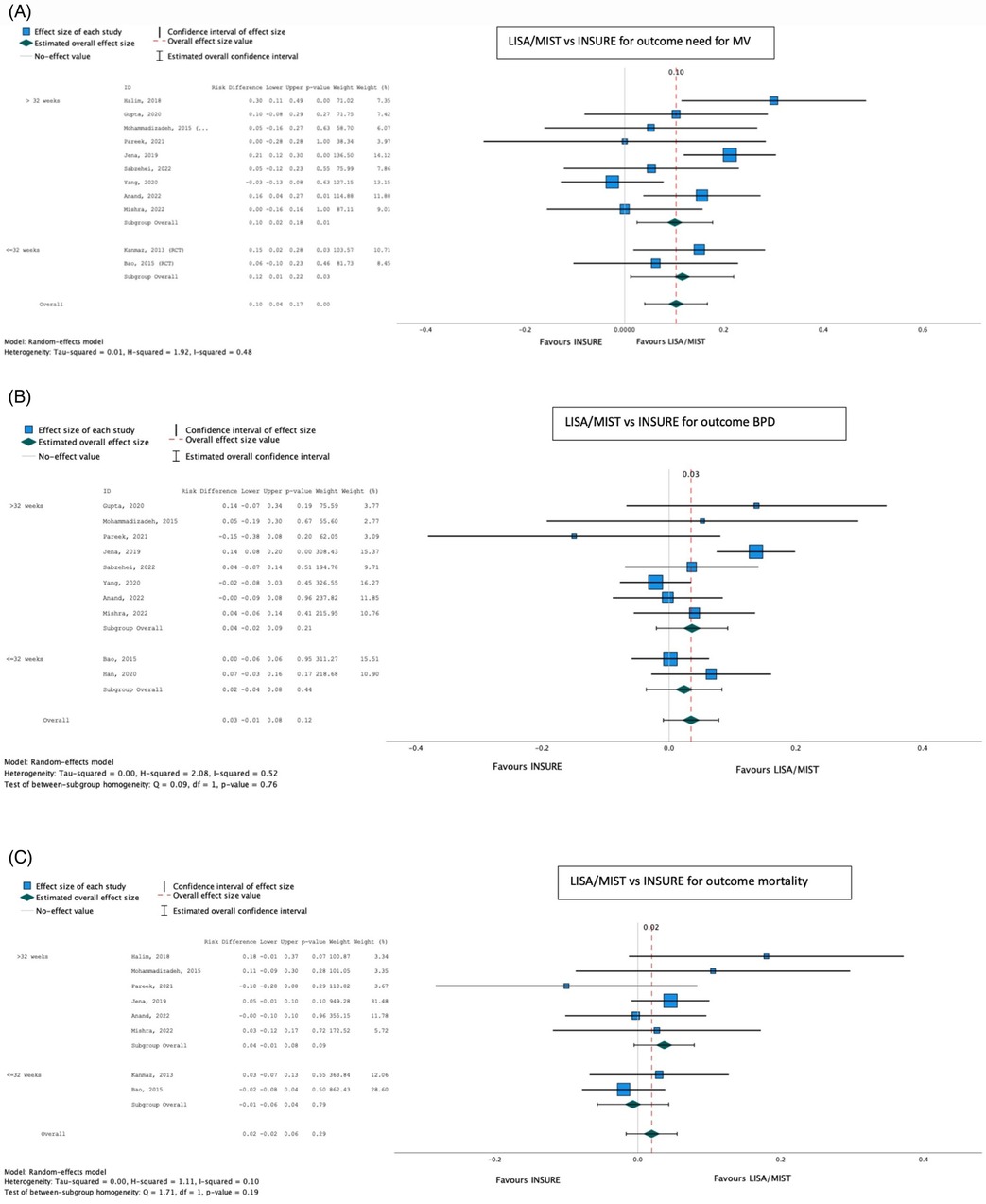
Figure 6. Effect of LISA/MIST vs. INSURE on progression to invasive mechanical ventilation (A), bronchopulmonary dysplasia (B), and mortality (C).
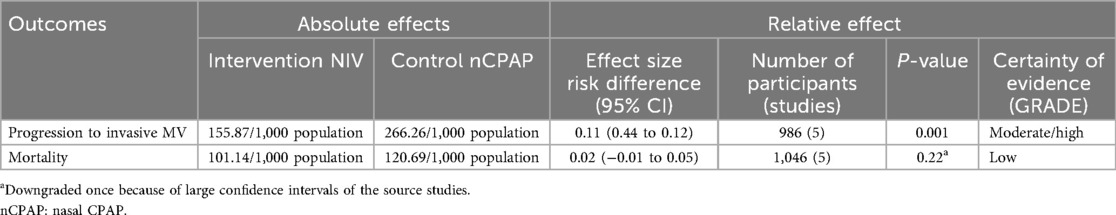
On meta-analysis, the other methods of NIV (BiPAP, nIPPV, and nHFOV) (both pre- and post-SRT), were associated with a reduced need for IMV compared with CPAP [pooled risk difference 0.11 (0.04–0.28); p < 0.001], with no significant effect on mortality [pooled risk difference 0.02 (−0.01 to 0.05); p = 0.22] (Table 4; Figure 7).
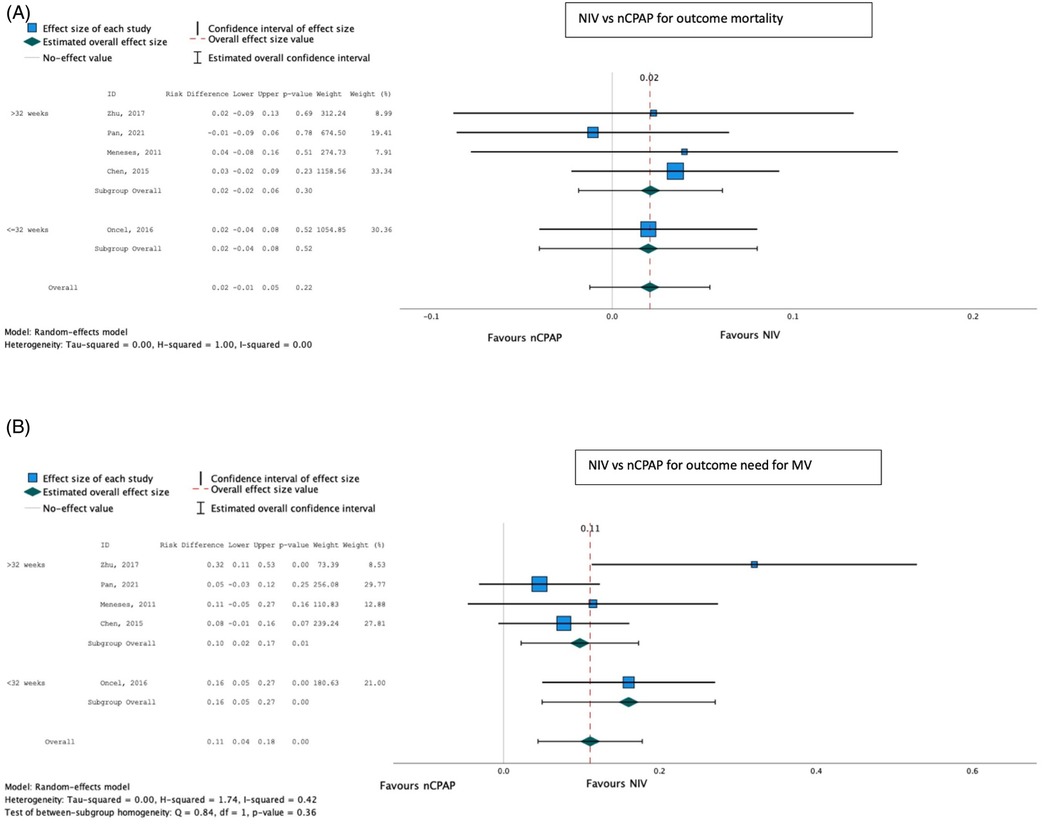
Figure 7. Effect of NIV other than CPAP vs. CPAP on mortality (A) and need for invasive mechanical ventilation (B).
Discussion
In this scoping review, key findings were moderate certainty of evidence to support the use of less invasive methods of SRT to limit the need for IMV. LISA/MIST was preferable to INSURE. Poractant alfa is conditionally recommended in preference to beractant for its superiority in limiting the need for invasive ventilation and reducing mortality. No recommendations could be made about the potential for different interventions to reduce the burden of BPD, nor for the superiority of a particular mode of NIV other than CPAP. While not within the scope of this review, data on medium- and longer-term outcomes, as well as cost-effectiveness, were sparse. This review highlighted large gaps in data from LMIC countries on surfactant practices, the gaps possibly due to unavailability of surfactant, lack of basic support in the management of RDS, or lack of reporting of use and outcomes. Africa, South America, and Indonesia were especially poorly represented (6).
The noted knowledge gaps include very limited reporting of local availability of surfactant in most LMICs; best clinical parameters for reliably indicating surfactant need; most appropriate method and timing of delivery of SRT, along with procedural sedation (taking into consideration local practice and constraints); as well as SRT impact on long-term outcomes.
The number of studies comparing INSURE with less invasive methods of SRT has increased, but most studies suggest that INSURE remains the predominant method of SRT administration, particularly in RRS. This has been attributed partly to the lag period of technology transfer from LMICs to HICs, with the cost of technology, lack of knowledge, and inadequate support systems being contributing factors to this lag period (14).
In keeping with guidelines from HICs (104), less invasive SRT administration demonstrated superiority over INSURE in mitigating the need for ventilation. A Cochrane review (105) published in 2021 concluded that SRT via a thin catheter (vs. via the ETT) has a similar rate of adverse effects and yet is associated with a lower intubation rate in the first 72 h, a reduced incidence of major complications and in-hospital mortality, and a reduced risk of death or BPD. However, some experts (106) in HICs suggest that the clinical potential of LISA techniques is overstated and urge the conduct of further high-quality studies.
Limited published data from LMICs on other “less invasive” methods of SRT administration—such as via laryngeal or supraglottic airways (SALSA), or aerosolization—were noted. Because of the lack of expertise in laryngoscopy in many LMICs, surfactant administration through SALSA is a promising method of SRT (107). It is relatively easy to insert a supraglottic airway device (SAD) into the mouth and advance it until it meets resistance. However, minimal published evidence involving SALSA in LMICs was available for inclusion in this study, and the quality of evidence was low (108). An important recent development has been the manufacture of smaller SADs that can be used in infants weighing less than 1,000 g (109). Aerosolization of surfactant is the least invasive method of delivering it, requiring no airway manipulation. Several studies have shown that although it is considered safe, the efficacy of aerosolization is still inconclusive (110).
Poractant was found to be superior to beractant for reducing mortality and progression to IMV. This is in keeping with a Cochrane review (111), which demonstrated an increased risk of in-hospital mortality, oxygen requirement at 36 weeks' gestational age, and PDA requiring treatment in infants treated with beractant vs. poractant. However, a lack of dose-equivalent comparison groups of appropriate sample size was a potential reason for differences. However, Boshoff Coyles (112) demonstrated no significant differences in outcomes between two groups of infants given these different surfactants at comparable dosages. Further high-quality as well as cost-effectiveness studies are needed.
With increasing survival at younger gestational ages, the importance of subsequent morbidity is paramount. The current review found that neither the technique of administration, the type of surfactant used, nor the type of respiratory support (NIV vs. CPAP) had any significant effect on the outcome of BPD, despite all three being associated with a reduced need for invasive ventilation, which is a proxy for the likelihood of long-term morbidity. The need for invasive ventilation may be an inappropriate proxy for this outcome, as it is only one of many factors contributing to BPD development, which is known to occur in infants who have never been invasively ventilated (106).
The use of NIV other than CPAP was found to be superior to CPAP with regard to the need for IMV, yet no effect on mortality was shown. This contrasts with the results of an RCT published in 2015 (113), which found that non-invasive neurally adjusted ventilatory assist (NIV NAVA) had no significant effect on oxygen requirements or the need for invasive ventilation in preterm newborns compared with CPAP. A RCT (91) that compared HFNC vs. CPAP found that although INSURE failure was higher in the HFNC group, HFNC was easier to use for nurses, better tolerated for infants, and facilitated more attachment between infants and parents—considerations that may justify its use. A three-arm multicenter randomized controlled trial comparing HFNC vs. CPAP vs. NIPPV as primary respiratory support in infants ≥32 weeks GA is currently registered with the Chinese Clinical Trial Registry (114). However, high-tech NIV is unlikely to be available in many RRS, and availing CPAP—even in its most rudimentary forms—remains a priority (6, 9, 13, 15–19, 115–119).
The answer to a “one-size-fits-all-SRT-protocol-for-best-outcomes” remains elusive. This is true even in HICs. A recent publication, The Respiratory Distress Syndrome Neonatal Expert Taskforce (RDS-NExT) (118) initiative, convened a panel of experts from various regions of the United States to establish consensus on best clinical surfactant practices. One finding was the absence of standard practices in respiratory management and surfactant administration. This variability demonstrates a lack of consensus regarding how SRT is used in the neonatal setting even in HICs. Tailored SRT approaches should be based on the best local evidence. For example, in LMICs, a lack of routine early pregnancy ultrasound scans and high rates of intrauterine growth restriction diminish the utility of birthweight and GA estimates in SRT decision-making (119). Clinical parameters, then, should be prioritized, as is the case in most guidelines, including those from high-income settings.
The 2022 Update of the European Consensus Guidelines on the Management of Respiratory Distress Syndrome (104) advise that surfactant be given where there is worsening RDS with FiO2 >0.30 on CPAP pressures ≥6 cmH2O, or where LUS suggests surfactant deficiency, and that even lower FiO2 thresholds be considered for very immature infants. Variability in precise FiO2 or SPO2 cut-offs observed in studies may reflect resource constraints. The use of higher FiO2 or lower saturation target thresholds—as noted in some of the included studies—raises the question of whether “permissive hypoxemia” is clinically consequential, which is another research gap. On the other hand, the financial burden of administering surfactant at lower thresholds of FiO2 is an important consideration in LMICs, where the cost of surfactant is often borne by the families of the infants (29). The RDS-NExT study highlights that individual clinical parameters and available resources must always be considered in deciding when and how SRT is delivered (118).
The factor of timing of SRT may be especially important in RRS where mothers may have received little to no antenatal care or steroids or where infants are often “outborn” and require transport (sometimes over great distances) to health facilities. While a Cochrane (120) review found that early stabilization with CPAP and selective SRT (“rescue”) to infants requiring intubation yielded less risk of chronic lung disease or death, the trials included were mostly from HICs, thus limiting the generalizability of these findings. Prophylactic surfactant may be beneficial dependent on the setting. While the heterogeneity of timing among studies included in this review precluded meta-analysis, those with statistically significant findings tended to favor early over late administration. A lack of equipment and expertise outside of the NICU setting may mean that alternate measures of delivering SRT are needed.
Procedural sedation is an underexplored component of optimal SRT. Laryngoscopy and airway manipulation can precipitate dangerous physiological responses, including apnea, bradycardia, and laryngospasm, as well as discomfort in the absence of appropriate sedation (106). A balance of light sedation/analgesia is needed to mitigate these responses, while not impeding a quick extubation post procedure. Few studies included premedication practices, despite the recommendation by both American and European guidelines (121, 122) that sedation be provided for laryngoscopy. Considering that overly deep sedation creates the need for prolonged ventilation, while insufficient sedation may result in neurosensory impairment associated with early neonatal pain experiences (102), this is another important area of study for future research.
This research project used a mixed review methodology, which was planned a priori to include meta-analyses if sufficiently homogeneous data were available to pool results within specific research subquestions. The exclusion of non-English studies, which was pragmatically necessary for this unfunded study, is a substantial limitation of the review, as relevant studies from LMICs may have been omitted. There is potential bias implicit in the scoping review methodology, and given the limitations of selection bias and variable heterogeneity between studies in this scoping review, a collaborative LMIC multi-institutional research project may be an innovative research project to reduce heterogeneity and address gaps in data. We recommend prospective systematic reviews specific to each population intervention control outcome (PICO)-type subquestion to confirm efficacy and safety.
Conclusion
In LMICs, where invasive mechanical ventilation is a scarce resource, LISA should be recommended in preference to INSURE. Poractant alfa (200 mg/kg) is also conditionally recommended in preference to beractant (100 mg/kg) for SRT, but regionally relevant cost- effectiveness studies are recommended to inform recommendations for implementation in LMICs. Observational studies regarding local practice and equipment and resource availability are needed to better describe current practice, shortfalls, and opportunities for strengthening SRT practices and improving outcomes. While more data have accumulated in the past decade, many gaps remain. In striving to achieve the SDG of fewer than 12 per 1,000 neonatal deaths, bundles of care from antenatal care and steroid coverage through to SRT and IMV must be strengthened.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
CP: Conceptualization, Data curation, Writing – review & editing, Methodology, Investigation, Writing – original draft, Formal analysis, Visualization. LT: Writing – review & editing, Supervision. HZ: Supervision, Writing – review & editing. BM: Methodology, Formal analysis, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors, wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1685625/full#supplementary-material
Abbreviations
BiPAP, bilevel positive airway pressure; BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; CXR, chest radiograph/x-ray; DUOPAP, duo positive airway pressure; ETT, endotracheal tube; FiO2, fraction of inspired oxygen; GA, gestational age; GRADE, grading of recommendations, assessment, development, and evaluations; HFNC, high flow nasal cannula; HIC, high-income country; IMV, invasive mechanical ventilation; INSURE, INtubation-SURfactant-Extubation; JBI, Joanna Briggs Institute; LISA, Less Invasive Surfactant Administration; LMICs, low- and middle-income countries; LUS, lung ultrasound; MeSH, medical subject headings; MIST, Minimal Invasive Surfactant Treatment; nHFOV, non-invasive high frequency oscillatory ventilation; NICU, neonatal intensive care unit; nIPPV, non-invasive intermittent positive pressure ventilation; NNM, neonatal mortality; PDA, patent ductus arteriosus; PRISMA-ScR, preferred items for systematic reviews and meta-analysis guidelines extension for scoping reviews; RCT, randomized controlled trial; RDS, respiratory distress syndrome; RRS, resource-restricted settings; SDGs, Sustainable Development Goals; SpO2, oxygen saturation; SRT, surfactant replacement therapy; WBI, World Bank Income; WHO, World Health Organization.
References
1. World Health Organization. Newsroom: Preterm Birth 2023 (October 14, 2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed October 14, 2023).a
2. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10(1):S2. doi: 10.1186/1742-4755-10-S1-S2
3. United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development [Internet]. Resolution adopted by the General Assembly on 25 September 2015, A/RES/70/1. New York: United Nations (2015) [cited 2025 October 27]. Available online at: https://digitallibrary.un.org/record/3923923?v=pdf
4. Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. (2022) 6(2):106–15. doi: 10.1016/S2352-4642(21)00311-4
5. Ekhaguere OA, Okonkwo IR, Batra M, Hedstrom AB. Respiratory distress syndrome management in resource limited settings—current evidence and opportunities in 2022. Front Pediatr. (2022) 10:961509. doi: 10.3389/fped.2022.961509
6. Lategan I, Price C, Rhoda NR, Zar HJ, Tooke L. Respiratory interventions for preterm infants in LMICs: a prospective study from Cape Town, South Africa. Front Glob Womens Health. (2022) 3:817817. doi: 10.3389/fgwh.2022.817817
7. Ballot DE, Chirwa T, Ramdin T, Chirwa L, Mare I, Davies VA, et al. Comparison of morbidity and mortality of very low birth weight infants in a Central Hospital in Johannesburg between 2006/2007 and 2013. BMC Pediatr. (2015) 15:20. doi: 10.1186/s12887-015-0337-4
8. Rebello CM, Precioso AR, Mascaretti RS. A multicenter, randomized, double-blind trial of a new porcine surfactant in premature infants with respiratory distress syndrome. Einstein (Sao Paulo). (2014) 12(4):397–404. doi: 10.1590/S1679-45082014AO3095
9. Okonkwo IR, Okolo AA. The scope and extent of exogenous surfactant utilization in Nigerian health care facilities: benefits of its regular use to outcome of premature babies. J Matern Fetal Neonatal Med. (2020) 33(8):1276–81. doi: 10.1080/14767058.2018.1517320
10. Singh D, Rana KS, Mathai S. Role of prophylactic surfactant in preterm infants. Med J Armed Forces India. (2011) 67(2):138–41. doi: 10.1016/S0377-1237(11)60012-9
11. Chen C, Tian T, Liu L, Zhang J, Fu H. Gender-related efficacy of pulmonary surfactant in infants with respiratory distress syndrome: a STROBE compliant study. Medicine (Baltimore). (2018) 97(17):e0425. doi: 10.1097/MD.0000000000010425
12. Wang H, Gao X, Liu C, Yan C, Lin X, Yang C, et al. Morbidity and mortality of neonatal respiratory failure in China: surfactant treatment in very immature infants. Pediatrics. (2012) 129(3):e731–40. doi: 10.1542/peds.2011-0725
13. Hamilton N, Trotman H. Challenges faced in translating the benefits of surfactant replacement therapy to a resource-limited setting. Am J Perinatol. (2017) 34(8):742–8. doi: 10.1055/s-0036-1598023
14. Patel DV, Bansal SC, Shah M, Patel CL, Patil K, Nimbalkar SM. Natural versus synthetic surfactant therapy in respiratory distress syndrome of prematurity. Indian J Pediatr. (2022) 89(11):1086–92. doi: 10.1007/s12098-022-04166-4
15. Crivceanscaia L, Avasiloaiei A, Moscalu M, Stamatin M. Short-term predictive factors for the outcome of preterm infants depending on the method of respiratory support. Med Surg J Rev Med Chir. (2017) 121(4):681–8. Available online at: https://www.revmedchir.ro/index.php/revmedchir/article/view/40
16. Khan EA, Hashmey I. Surfactant use in premature neonates <37 weeks gestation: experience and outcome at a tertiary care hospital. J Pak Med Assoc. (2015) 65(5):486–90. PMID: 26028381.26028381
17. Vidyasagar D, Velaphi S, Bhat VB. Surfactant replacement therapy in developing countries. Neonatology. (2011) 99(4):355–66. doi: 10.1159/000326628
18. Xuan NM. Outcomes of preterm infants 26–34 week’ gestation at a Vietnamese children hospital. Med Sci. (2020) 24(104):2119–25. Available online at: https://www.discoveryjournals.org/medicalscience/current_issue/v24/n104/A43.pdf
19. Nakhshab M, Tajbakhsh M, Khani S, Farhadi R. Comparison of the effect of surfactant administration during nasal continuous positive airway pressure with that of nasal continuous positive airway pressure alone on complications of respiratory distress syndrome: a randomized controlled study. Pediatr Neonatol. (2015) 56(2):88–94. doi: 10.1016/j.pedneo.2014.05.006
20. Nanda D, Nangia S, Thukral A, Yadav CP. A new clinical respiratory distress score for surfactant therapy in preterm infants with respiratory distress. Eur J Pediatr. (2020) 179(4):603–10. doi: 10.1007/s00431-019-03530-5
21. Sankar MJ, Gupta N, Jain K, Agarwal R, Paul VK. Efficacy and safety of surfactant replacement therapy for preterm neonates with respiratory distress syndrome in low- and middle-income countries: a systematic review. J Perinatol. (2016) 36:S36–48. doi: 10.1038/jp.2016.31
22. Bao Y, Zhang G, Wu M, Ma L, Zhu J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr. (2015) 15:21. doi: 10.1186/s12887-015-0342-7
23. The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 Edition/Supplement. The Joanna Briggs Institute (2015).
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Hamadeh N, Van Rompaey C, Metreau E, Eapen SG. New World Bank Country Classifications by Income Level: 2022-2023 [Internet]. Washington (DC): World Bank (2022) [cited 2025 Oct 27]. Available online at: https://blogs.worldbank.org/en/opendata/new-world-bank-country-classifications-income-level-2022-2023
27. The GRADE Working Group. GRADE Handbook: For Grading Quality of Evidence and Strength of Recommendations [Internet]. (2013) [cited 2025 Oct 27]. Available online at: https://gdt.gradepro.org/app/handbook/handbook.html
28. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12(1):55–61. doi: 10.1002/jrsm.1411
29. Tooke L, Ehret DEY, Okolo A, Dlamini-Nqeketo S, Joolay Y, Minto'o S, et al. Limited resources restrict the provision of adequate neonatal respiratory care in the countries of Africa. Acta Paediatr. (2022) 111(2):275–83. doi: 10.1111/apa.16050
30. Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics. (1956) 17(1):1–10. Available online at: https://www.scirp.org/reference/referencespapers?referenceid=374103013353856
31. . “Respiratory”. In: Boulton JE, Coughlin K, O'Flaherty D, Solimano A, Boulton JE, Coughlin K, et al., editors. ACoRN: Acute Care of at-Risk Newborns: A Resource and Learning Tool for Health Care Professionals. Oxford University Press (2021). p. 47–96.
32. Vardar G, Karadag N, Karatekin G. The role of lung ultrasound as an early diagnostic tool for need of surfactant therapy in preterm infants with respiratory distress syndrome. Am J Perinatol. (2020). p. 1547–56. doi: 10.1055/s-0040-1714207
33. Najafian B, Karimi-Sari H, Khosravi MH, Nikjoo N, Amin S, Shohrati M. Comparison of efficacy and safety of two available natural surfactants in Iran, Curosurf and Survanta in treatment of neonatal respiratory distress syndrome: a randomized clinical trial. Contemp Clin Trials Commun. (2016) 3:55–9. doi: 10.1016/j.conctc.2016.04.003
34. Mahmoud RA. Effectiveness of nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure in preterm infants after less invasive surfactant administration. Int J Pediatr (Mashhad). (2019) 7(1):8915–24. doi: 10.22038/ijp.2018.34600.3047
35. Danaei N, Seddigh M, Ghorbani R, Nooripour S. Effective factors of INSURE method failure in treatment of respiratory distress syndrome in preterm infants. Int J Pediatr (Mashhad). (2017) 5(11):6069–76. doi: 10.22038/ijp.2017.26022.2221
36. Eras Z, Dizdar EA, Kanmaz G, Guzoglu N, Aksoy HT, Altunkaya GB, et al. Neurodevelopmental outcomes of very low birth weight preterm infants treated with poractant alfa versus beractant for respiratory distress syndrome. Am J Perinatol. (2014) 31(6):463–8. doi: 10.1055/s-0033-1351659
37. Silahli M, Tekin M. The comparison of LISA and INSURE techniques in term of neonatal morbidities and mortality among premature infants. Acta Biomed. (2020) 91(4):e2020189. doi: 10.23750/abm.v91i4.8845
38. Naseh A, Yekta BG. INSURE method (INtubation-SURfactant-Extubation) in early and late premature neonates with respiratory distress: factors affecting the outcome and survival rate. Turk J Pediatr. (2014) 56(3):232–7. PMID: 2534159325341593
39. Hashim QM, Aasam AIA, Alwan HMO, Nasrawi AJ. Effect of surfactant replacement therapy in preterm with respiratory distress syndrome in low sources center Al-Zahraa Teaching Hospital at AlNajaf city-Iraq. Curr Pediatr Res. (2021) 25(8):738–43. Available online at: https://www.currentpediatrics.com/abstract/effect-of-surfactant-replacement-therapy-in-preterm-with-respiratory-distress-syndrome-in-low-sources-center-alzahraa-teaching-hos-18025.html
40. Barbosa RF, Simões ESAC, Silva YP. A randomized controlled trial of the laryngeal mask airway for surfactant administration in neonates. J Pediatr (Rio J). (2017) 93(4):343–50. doi: 10.1016/j.jped.2016.08.007
41. Gharehbaghi MM, Sakha SH, Ghojazadeh M, Firoozi F. Complications among premature neonates treated with beractant and poractant alfa. Indian J Pediatr. (2010) 77(7):751–4. doi: 10.1007/s12098-010-0097-y
42. Abdallah Y, Mkony M, Noorani M, Moshiro R, Bakari M, Manji K. CPAP failure in the management of preterm neonates with respiratory distress syndrome where surfactant is scarce. A prospective observational study. BMC Pediatr. (2023) 23(1):211. doi: 10.1186/s12887-023-04038-6
43. Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: a randomized controlled trial. Pediatrics. (2011) 127(2):300–7. doi: 10.1542/peds.2010-0922
44. Yilmaz FH, Tarakci N, Gultekin ND, Yucel M, Kececi R, Ozturk ENY, et al. Comparison of the efficacy of three natural surfactants in preterm Turkish newborns with respiratory distress syndrome. J Pediatr Res. (2021) 8(2):124–30. doi: 10.4274/jpr.galenos.2020.04864
45. Jain K, Nangia S, Ballambattu VB, Sundaram V, Sankar MJ, Ramji S, et al. Goat lung surfactant for treatment of respiratory distress syndrome among preterm neonates: a multi-site randomized non-inferiority trial. J Perinatol. (2019) 39:3–12. doi: 10.1038/s41372-019-0472-0
46. Dizdar EA, Sari FN, Aydemir C, Oguz SS, Erdeve O, Uras N, et al. A randomized, controlled trial of poractant alfa versus beractant in the treatment of preterm infants with respiratory distress syndrome. Am J Perinatol. (2012) 29(2):95–100. doi: 10.1055/s-0031-1295648
47. Bozdağ Ş, Dilli D, Gökmen T, Dilmen U. Comparison of two natural surfactants for pulmonary hemorrhage in very low-birth-weight infants: a randomized controlled trial. Am J Perinatol. (2015) 32(3):211–8. doi: 10.1055/s-0034-1389090
48. Bozkaya D, Dizdar EA, Korkut S, Ceran B, Alkan M, Oğuz ŞS. Evaluation of different types of natural surfactants by lung ultrasound in respiratory distress syndrome. Am J Perinatol. (2021) 38(6):590–6. doi: 10.1055/s-0039-1700856
49. Mirzarahimi M, Barak M. Comparison efficacy of Curosurf and Survanta in preterm infants with respiratory distress syndrome. Pak J Pharm Sci. (2018) 31(2):469–72. PMID: 29618436.29618436
50. Mussavi M, Mirnia K, Asadollahi K. Comparison of the efficacy of three natural surfactants (Curosurf, Survanta, and Alveofact) in the treatment of respiratory distress syndrome among neonates: a randomized controlled trial. Iran J Pediatr. (2016) 26(5):1–10. doi: 10.5812/ijp.5743
51. Macooie AA, Fakour Z, Roanaghi P. Comparative evaluation of the effects of BLES and Survanta on treatment of respiratory distress syndrome in newborns. J Family Med Prim Care. (2018) 7(5):1063–7. doi: 10.4103/jfmpc.jfmpc_188_17
52. Karadag A, Ozdemir R, Degirmencioglu H, Uras N, Dilmen U, Bilgili G, et al. Comparison of three different administration positions for intratracheal beractant in preterm newborns with respiratory distress syndrome. Pediatr Neonatol. (2016) 57(2):105–12. doi: 10.1016/j.pedneo.2015.04.012
53. Sarokolai ZK, Niknafs P, Azizzadeh F, Bijari BB, Mousavi H. BLES versus Curosurf for treatment of respiratory distress in preterm neonates and their adverse effects. Iran J Pediatr. (2018) 28(4):e11734. doi: 10.5812/ijp.11734
54. Terek D, Gonulal D, Koroglu OA, Yalaz M, Akisu M, Kultursay N. Effects of two different exogenous surfactant preparations on serial peripheral perfusion Index and tissue carbon monoxide measurements in preterm infants with severe respiratory distress syndrome. Pediatr Neonatol. (2015) 56(4):248–55. doi: 10.1016/j.pedneo.2014.11.004
55. Kadıoğlu Şimşek G, Kanmaz Kutman HG, Canpolat FE, Oğuz ŞS. Effect of two different early rescue surfactant treatments on mortality in preterm infants with respiratory distress syndrome. Clin Respir J. (2020) 14(3):285–90. doi: 10.1111/crj.13130
56. Kandraju H, Murki S, Subramanian S, Gaddam P, Deorari A, Kumar P. Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: a randomized controlled trial. Neonatology. (2013) 103(2):148–54. doi: 10.1159/000345198
57. Fallahi M, Taslimi Taleghani N, Afje SA, Shamshiri AR, Esmaili F, Radfar M, et al. Predictors of success rate in different initial respiratory supports in very low birthweight infants with respiratory distress. Arch Iran Med. (2020) 23(11):724–31. doi: 10.34172/aim.2020.96
58. Halim A, Shirazi H, Riaz S, Gul SS, Ali W. Less invasive surfactant administration in preterm infants with respiratory distress syndrome. J Coll Physicians Surg Pak. (2019) 29(3):226–330. doi: 10.29271/jcpsp.2019.03.226
59. Mohammadizadeh M, Ardestani AG, Sadeghnia AR. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. J Res Pharm Pract. (2015) 4(1):31–6. doi: 10.4103/2279-042X.150053
60. Nayeri FS, Esmaeilnia Shirvani T, Aminnezhad M, Amini E, Dalili H, Moghimpour Bijani F. Comparison of INSURE method with conventional mechanical ventilation after surfactant administration in preterm infants with respiratory distress syndrome: therapeutic challenge. Acta Med Iran. (2014) 52(8):596–600. Available online at: https://acta.tums.ac.ir/index.php/acta/article/view/456825149882
61. Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. (2013) 131(2):e502–9. doi: 10.1542/peds.2012-0603
62. Li XF, Cheng TT, Guan RL, Liang H, Lu WN, Zhang JH, et al. Effects of different surfactant administrations on cerebral autoregulation in preterm infants with respiratory distress syndrome. J Huazhong Univ Sci Technolog Med Sci. (2016) 36(6):801–5. doi: 10.1007/s11596-016-1665-9
63. Okur N, Uras N, Buyuktiryaki M, Oncel MY, Saria FN, Yarci E, et al. Neonatal pain and heart rate variability in preterm infants treated with surfactant: a pilot study. Arch Argent Pediatr. (2019) 117(6):397. doi: 10.5546/aap.2019.eng.397
64. Pareek P, Deshpande S, Suryawanshi P, Sah LK, Chetan C, Maheshwari R, et al. Less invasive surfactant administration (LISA) vs. intubation surfactant extubation (InSurE) in preterm infants with respiratory distress syndrome: a pilot randomized controlled trial. J Trop Pediatr. (2021) 67(4):fmab086. doi: 10.1093/tropej/fmab086
65. Jena SR, Bains HS, Pandita A, Verma A, Gupta V, Kallem VR, et al. Surfactant therapy in premature babies: SurE or InSurE. Pediatr Pulmonol. (2019) 54(11):1747–52. doi: 10.1002/ppul.24479
66. Ngo XM, Le GC. Treatment of respiratory distress syndrome in premature infants: a comparison of LISA and INSURE methods in the Vietnamese context. Med Sci. (2020) 24(104):1845–52. Available online at: https://www.discoveryjournals.org/medicalscience/current_issue/v24/n104/A6.pdf
67. Han T, Liu H, Zhang H, Guo M, Zhang X, Duan Y, et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Front Pediatr. (2020) 8:182. doi: 10.3389/fped.2020.00182
68. Anand R, Nangia S, Kumar G, Mohan MV, Dudeja A. Less invasive surfactant administration via infant feeding tube versus InSurE method in preterm infants: a randomized control trial. Sci Rep. (2022) 12(1):21955. doi: 10.1038/s41598-022-23557-3
69. Mishra A, Joshi A, Londhe A, Deshmukh L. Surfactant administration in preterm babies (28–36 weeks) with respiratory distress syndrome: LISA versus InSurE, an open-label randomized controlled trial. Pediatr Pulmonol. (2023) 58(3):738–45. doi: 10.1002/ppul.26246
70. Sabzehei MK, Basiri B, Shokouhi M, Ghahremani S, Moradi A. Comparison of minimally invasive surfactant therapy with intubation surfactant administration and extubation for treating preterm infants with respiratory distress syndrome: a randomized clinical trial. Clin Exp Pediatr. (2022) 65(4):188–93. doi: 10.3345/cep.2021.00297
71. Sadeghnia AR, Mahjoor Z, Barekatain B. Comparison of surfactant administration efficacy in the treatment of respiratory distress syndrome in preterm neonates: aerosolization versus INSURE. Iran J Pediatr. (2022) 32(2):e120633. doi: 10.5812/ijp-120633
72. Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur J Pediatr. (2020) 179(8):1287–93. doi: 10.1007/s00431-020-03682-9
73. Yang G, Hei M, Xue Z, Zhao Y, Zhang X, Wang C. Effects of less invasive surfactant administration (LISA) via a gastric tube on the treatment of respiratory distress syndrome in premature infants aged 32 to 36 weeks. Medicine (Baltimore). (2020) 99(9):e19216. doi: 10.1097/MD.0000000000019216
74. Buyuktiryaki M, Alarcon-Martinez T, Simsek GK, Canpolat FE, Tayman C, Oguz SS, et al. Five-year single center experience on surfactant treatment in preterm infants with respiratory distress syndrome: LISA vs. INSURE. Early Hum Dev. (2019) 135:32–6. doi: 10.1016/j.earlhumdev.2019.06.004
75. Dobryanskyy DO, Menshykova AO, Salabay ZV, Detsyk OY. Neonatal preterm respiratory care in Ukraine: an observational study of outcomes in relation to timing and methods of surfactant treatment. Am J Perinatol. (2020):889–96. doi: 10.1055/s-0040-1719183
76. Xu C-c, Bao Y-y, Zhao J-x, Cheng K, Sun L, Wu J-y, et al. Effects of less invasive surfactant administration versus intubation-surfactant-extubation on bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: a single-center, retrospective study from China. BMC Pulm Med. (2022) 22(1):1–6. doi: 10.1186/s12890-022-02270-x
77. Zhang X, Pan J, Zhu L, Ye Y, Fan Z, Chen X, et al. Less invasive surfactant administration for the treatment of neonatal respiratory distress syndrome combined with noninvasive ventilation in Anhui Province, China: a retrospective cohort study. Clin Pediatr (Phila). (2023) 62(9):1109–17. doi: 10.1177/00099228231152859
78. Panza R, Laforgia N, Bellos I, Pandita A. Systematic review found that using thin catheters to deliver surfactant to preterm neonates was associated with reduced bronchopulmonary dysplasia and mechanical ventilation. Acta Paediatr. (2020) 109(11):2219–25. doi: 10.1111/apa.15374
79. Rong Z, Chang L, Cheng H, Wang H, Zhu X, Peng F, et al. A multicentered randomized study on early versus rescue Calsurf administration for the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. (2019) 36(14):1492–7. doi: 10.1055/s-0039-1678530
80. Okulu E, Arsan S, Mungan Akın İ, Alan S, Kılıç A, Atasay B. Early or later prophylactic INSURE in preterm infants of less than 30 weeks’ gestation. Turk J Pediatr. (2015) 57(1):1–8. PMID: 26613214.26613214
81. Kong X, Cui Q, Hu Y, Huang W, Ju R, Li W, et al. Bovine surfactant replacement therapy in neonates of less than 32 weeks’ gestation: a multicenter controlled trial of prophylaxis versus early treatment in China—a pilot study. Pediatr Neonatol. (2016) 57(1):19–26. doi: 10.1016/j.pedneo.2015.03.007
82. Duman N, Tüzün F, Sever AH, Arslan MK, İşcan B, Dilek M, et al. Nasal intermittent positive pressure ventilation with or without very early surfactant therapy for the primary treatment of respiratory distress syndrome. J Matern Fetal Neonatal Med. (2016) 29(2):252–7. doi: 10.3109/14767058.2014.997203
83. Vamseedhar Y, Raju YP, Mohan CR. Role of surfactant administration in premature infants with acute respiratory distress syndrome. J Evol Med Dent Sci. (2015) 4(59):10257–62. doi: 10.14260/jemds/2015/1478
84. You H, Huang X. Effect of pulmonary surfactant on the prevention of neonatal respiratory distress syndrome in premature infants. Am J Transl Res. (2021) 13(4):3642–9. PMID: 34017546.34017546
85. Liu L, Deng Q. Profound effect of pulmonary surfactant on the treatment of preterm infants with respiratory distress syndrome. Contrast Media Mol Imaging. (2022) 2022:4166994. doi: 10.1155/2022/4166994
86. Canpolat FE, Simsek GK, Webbe J, Buyuktiryaki M, Karacaglar NB, Elbayiyev S, et al. Late administration of surfactant may increase the risk of patent ductus arteriosus. Front Pediatr. (2020) 8:130. doi: 10.3389/fped.2020.00130
87. Babaei H, Talebhagh A, Pirkashani LM. The effect of surfactant accompanied by ventolin on the respiratory distress syndrome in premature newborns: a clinical trial study. Int J Pediatr (Mashhad). (2019) 7(8):9803–15. doi: 10.22038/ijp.2019.39481.3364
88. Gharehbaghi MM, Ganji S, Mahallaei M. The efficacy of intra-tracheal budesonide with surfactant in treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Med J Tabriz Univ Med Sci. (2021) 43(1):48–54.
89. Çelik HT, Yurdakök M, Korkmaz A, Yiğit Ş. Does inhaled salbutamol before surfactant therapy have any beneficial effect? Turk J Pediatr. (2018) 60(6):669–74. doi: 10.24953/turkjped.2018.06.007
90. Dehdashtian M, Malakian A, Aramesh MR, Mazori A, Aletayeb MH, Shirani A, et al. Effectiveness of intratracheal salbutamol in addition to surfactant on the clinical course of newborns with respiratory distress syndrome: a clinical trial. Ital J Pediatr. (2016) 42:1–5. doi: 10.1186/s13052-016-0215-1
91. Kadivar MM, Mosayebi ZM, Razi NM, Nariman SM, Sangsari RM. High flow nasal cannulae versus nasal continuous positive airway pressure in neonates with respiratory distress syndrome managed with INSURE method: a randomized clinical trial. Iran J Med Sci. (2016) 41(6):494–500. PMID: 27853329.27853329
92. Malakian A, Aramesh MR, Agahin M, Dehdashtian M. Non-invasive duo positive airway pressure ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: a randomized controlled trial. BMC Pediatr. (2021) 21(1):301. doi: 10.1186/s12887-021-02741-w
93. Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2016) 101(4):F323–8. doi: 10.1136/archdischild-2015-308204
94. Chen L, Wang L, Li J, Wang N, Shi Y. Noninvasive ventilation for preterm twin neonates with respiratory distress syndrome: a randomized controlled trial. Sci Rep. (2015) 5:14483. doi: 10.1038/srep14483
95. Zhu XW, Zhao JN, Tang SF, Yan J, Shi Y. Noninvasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in preterm infants with moderate-severe respiratory distress syndrome: a preliminary report. Pediatr Pulmonol. (2017) 52(8):1038–42. doi: 10.1002/ppul.23755
96. Shokouhi M, Basiri B, Sabzehei MK, Mandiankhoo M, Pirdehghan A. Efficacy and complications of humidified high-flow nasal cannula versus nasal continuous positive airway pressure in neonates with respiratory distress syndrome after surfactant therapy. Iran Red Crescent Med J. (2024) 21(2):1–7. doi: 10.5812/ircmj.83615
97. Akbarian-Rad Z, Mohammadi A, Khafri S, Ahmadpour-Kacho M, Zahed-Pasha Y, Haghshenas-Mojaveri M. Comparison of heated humidified high flow nasal cannula and nasal continuous positive airway pressure after surfactant administration in preterm neonates with respiratory distress syndrome. Clin Respir J. (2020) 14:740–7. doi: 10.1111/crj.13191
98. Pan R, Chen GY, Wang J, Zhou ZX, Zhang PY, Chang LW, et al. Bi-level nasal positive airway pressure (BiPAP) versus nasal continuous positive airway pressure (CPAP) for preterm infants with birth weight less than 1500 g and respiratory distress syndrome following INSURE treatment: a two-center randomized controlled trial. Curr Med Sci. (2021) 41(3):542–7. doi: 10.1007/s11596-021-2372-8
99. Sabzehei MK, Basiri B, Goli MA, Solgi MS, Eghbalian F, Moradi A. Nasal intermittent positive pressure ventilation (NIPPV) vs. nasal continuous positive airway pressure (NCPAP) after less invasive surfactant administration (LISA) in preterm infants with respiratory distress syndrome. Int J Pediatr (Mashhad). (2022) 10(5):15972–81.
100. Afjeh SA, Sabzehei MK, Khoshnood Shariati M, Shamshiri AR, Esmaili F. Evaluation of initial respiratory support strategies in VLBW neonates with RDS. Arch Iran Med. (2017) 20(3):158–64. PMID: 28287810.28287810
101. Buyuktiryaki M, Okur N, Sari FN, Ozer Bekmez B, Bezirganoglu H, Cakir U, et al. Comparison of three different noninvasive ventilation strategies as initial respiratory support in very low birth weight infants with respiratory distress syndrome: a retrospective study. Arch Pediatr. (2020) 27(6):322–7. doi: 10.1016/j.arcped.2020.06.002
102. Sk H, Saha B, Mukherjee S, Hazra A. Premedication with fentanyl for less invasive surfactant application (LISA): a randomized controlled trial. J Trop Pediatr. (2022) 68(2):fmac019. doi: 10.1093/tropej/fmac019
103. Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A, et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics. (2012) 129(4):e952–9. doi: 10.1542/peds.2011-1365
104. Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. (2023) 120(1):3–23. doi: 10.1159/000528914
105. Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. (2021) 5(5):CD011672. doi: 10.1002/14651858.CD011672
106. De Luca D, Shankar-Aguilera S, Bancalari E. LISA/MIST: complex clinical problems almost never have easy solutions. Semin Fetal Neonatal Med. (2021) 26(2):101230. doi: 10.1016/j.siny.2021.101230
107. Zapata HA, Fort P, Roberts KD, Kaluarachchi DC, Guthrie SO. Surfactant administration through laryngeal or supraglottic airways (SALSA): a viable method for low-income and middle-income countries. Front Pediatr. (2022) 10:853831. doi: 10.3389/fped.2022.853831
108. Calevo MG, Veronese N, Cavallin F, Paola C, Micaglio M, Trevisanuto D. Supraglottic airway devices for surfactant treatment: systematic review and meta-analysis. J Perinatol. (2019) 39(2):173–83. doi: 10.1038/s41372-018-0281-x
109. Kaluarachchi DC, Katheria A, Peebles PJ, Guthrie SO, Kakkilaya V, Dargaville PA. Prophylactic surfactant therapy in the era of less invasive surfactant delivery. J Perinatol. (2025):1–5. doi: 10.1038/s41372-025-02420-z
110. Härtel C, Glaser K, Speer CP. The miracles of surfactant: less invasive surfactant administration, nebulization, and carrier of topical drugs. Neonatology. (2021) 118(2):225–34. doi: 10.1159/000516106
111. Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, Rojas-Reyes MX. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. (2015) 2015(12):CD010249. doi: 10.1002/14651858.CD010249.pub2
112. Boshoff Coyles L, Joolay Y, Tooke L. Bovine or porcine: does the type of surfactant matter? J Trop Pediatr. (2020) 66(5):534–41. doi: 10.1093/tropej/fmaa011
113. Kallio M, Koskela U, Peltoniemi O, Kontiokari T, Pokka T, Suo-Palosaari M, et al. Neurally adjusted ventilatory assist (NAVA) in preterm newborn infants with respiratory distress syndrome—a randomized controlled trial. Eur J Pediatr. (2016) 175(9):1175–83. doi: 10.1007/s00431-016-2758-y
114. Zhou R, Xiong T, Tang J, Huang Y, Liu W, Zhu J, et al. High-flow nasal cannula (HFNC) vs. continuous positive airway pressure (CPAP) vs. nasal intermittent positive pressure ventilation as primary respiratory support in infants of ≥32 weeks gestational age (GA): study protocol for a three-arm multi-center randomized controlled trial. Trials. (2023) 24(1):647. doi: 10.1186/s13063-023-07665-7
115. Gupta N, Saini SS, Murki S, Kumar P, Deorari A. Continuous positive airway pressure in preterm neonates: an update of current evidence and implications for developing countries. Indian Pediatr. (2015) 52(4):319–28. doi: 10.1007/s13312-015-0632-z
116. Van Wyk L, Tooke L, Dippenaar R, Rhoda N, Lloyd L, Holgate S, et al. Optimal ventilation and surfactant therapy in very-low-birth-weight infants in resource-restricted regions. Neonatology. (2020) 117(2):217–24. doi: 10.1159/000506987
117. Murki S, Deorari A, Vidyasagar D. Use of CPAP and surfactant therapy in newborns with respiratory distress syndrome. Indian J Pediatr. (2014) 81(5):481–8. doi: 10.1007/s12098-014-1405-8
118. Bhandari V, Black R, Gandhi B, Hogue S, Kakkilaya V, Mikhael M, et al. RDS-NExT workshop: consensus statements for the use of surfactant in preterm neonates with RDS. J Perinatol. (2023) 43:982–90. doi: 10.1038/s41372-023-01690-9
119. Stevenson A, Joolay Y, Levetan C, Price C, Tooke L. A comparison of the accuracy of various methods of postnatal gestational age estimation; including Ballard score, foot length, vascularity of the anterior lens, last menstrual period and also a clinician’s non-structured assessment. J Trop Pediatr. (2021) 67(1):fmaa113. doi: 10.1093/tropej/fmaa113
120. Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. (2012) 2012(3):CD000510. doi: 10.1002/14651858.CD000510.pub2
121. Kumar P, Denson SE, Mancuso TJ. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. (2010) 125(3):608–15. doi: 10.1542/peds.2009-2863
122. Ancora G, Lago P, Garetti E, Merazzi D, Savant Levet P, Bellieni CV, et al. Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatr. (2019) 108(2):208–17. doi: 10.1111/apa.14606
123. Pal S, Ghosh M. Risk factors and prediction models for less invasive surfactant administration failure in preterm infants: a retrospective cohort study in a low-and-middle income country. Pediatr Pulmonol. (2025) 60(5):e71127. doi: 10.1002/ppul.71127
Keywords: surfactant, preterm, neonate, respiratory distress syndrome, resource-limited settings, low- and middle-income countries
Citation: Price CA, Tooke L, Zar HJ and Morrow BM (2025) A systematic scoping review of the use of surfactant replacement therapy for respiratory distress syndrome in preterm neonates in low- and middle-income countries. Front. Pediatr. 13:1685625. doi: 10.3389/fped.2025.1685625
Received: 14 August 2025; Accepted: 13 October 2025;
Published: 7 November 2025.
Edited by:
David Warburton, Children's Hospital Los Angeles, United StatesReviewed by:
Scott Guthrie, Vanderbilt University Medical Center, United StatesMariam John Amin Ibrahim, Ain Shams University, Egypt
Copyright: © 2025 Price, Tooke, Zar and Morrow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caris A. Price, cHJjY2FyMDA1QG15dWN0LmFjLnph
 Caris A. Price
Caris A. Price Lloyd Tooke
Lloyd Tooke Heather J. Zar
Heather J. Zar Brenda M. Morrow
Brenda M. Morrow