- Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Digestive endoscopy in children is increasingly used for the diagnosis and treatment of a broad range of diseases affecting the stomach, intestines, biliary tract, and pancreas, with the advantages of being minimally invasive and efficient. Endoscopic procedures in children differ from those in adults in terms of both indications and primary objectives. Furthermore, ensuring the safety and comfort of children during the examination necessitates additional considerations, such as the use of appropriately sized endoscopes, carefully tailored sedation protocols, and bowel preparation regimens. This article provides an overview of the diagnostic value of endoscopy in common digestive tract diseases and challenging conditions in children, and it details the clinical applications of various endoscopic therapeutic techniques. Furthermore, the review focuses on several core aspects of endoscopy in children, including age-stratified selection strategies for endoscopic instruments, safety evaluations of sedation and anesthesia protocols, indications and contraindications for various endoscopic techniques, potential procedure-related adverse events, as well as current disparities in the development of endoscopy in children across different regions. Despite substantial progress in the field, challenges remain, including the lack of specialized devices, technical complexity, and gaps in operator training and quality control. Future efforts should emphasize multicenter studies, the development of standardized operating guidelines, and the integration of artificial intelligence and novel imaging technologies to optimize the endoscopy diagnostic and therapeutic system, thereby advancing digestive endoscopy in children toward greater precision, safety, and efficiency.
1 Introduction
Endoscopic technology has become an important tool for diagnosing and treating digestive diseases in children, combining the advantages of being minimally invasive and highly efficient (1). Technically speaking, patient safety and comfort must be considered during the procedure, and therefore certain additional conditions must be met (2). Since the application of endoscopic technology in children began in the 1970s, endoscopic techniques have continued to evolve. In recent years, complex endoscopic procedures such as endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS), and peroral endoscopic myotomy (POEM) have been progressively applied to children and has demonstrated favorable safety and efficacy (3–6).
The field of endoscopy in children continues to face significant challenges, including imperfect implementation of quality standards, a paucity of specialized and standardized assessment tools, and inadequate device compatibility, despite technical advancements that offer minimally invasive options for children (7). This review aims to summarize the clinical applications of existing children's endoscopic techniques, explore future development directions, and promote the precision and individualization of children's endoscopic diagnosis and treatment (Figure 1).
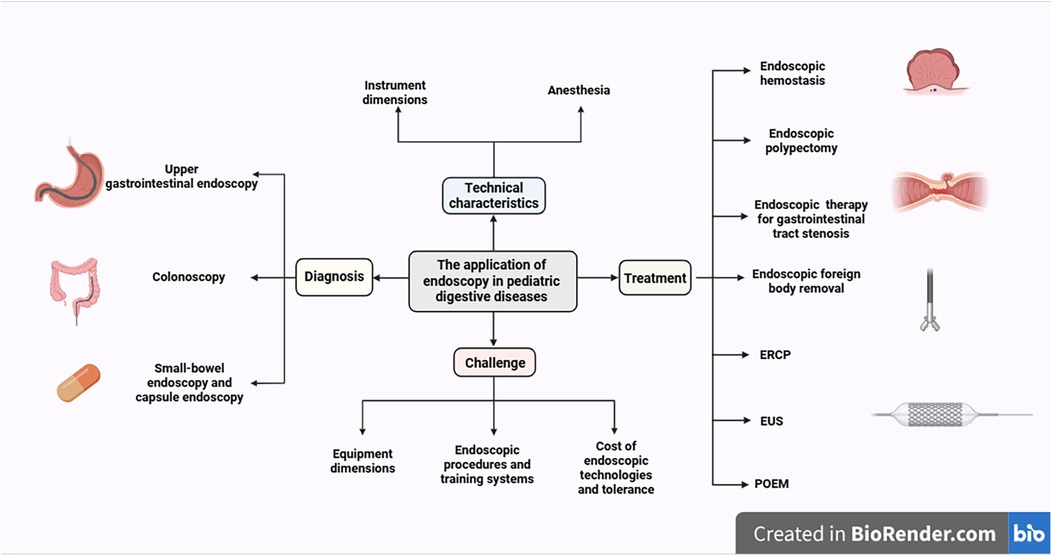
Figure 1. Current applications of gastrointestinal endoscopy in children. (created with BioRender.com).
2 Methods
2.1 Literature search strategy
A systematic literature search was performed using the PubMed database.
2.2 Search terms
The search strategy incorporated a combination of Medical Subject Headings (MeSH) and free-text terms to maximize retrieval. Key search concepts and terms include:
Core Topics: endoscopy in children, gastrointestinal endoscopy, children, endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS), dimensions.
Diagnosis: diagnosis, diagnostic yield, esophagogastroduodenoscopy (EGD), colonoscopy, small-bowel endoscopy, capsule endoscopy.
Therapeutics: therapeutic endoscopy, foreign body removal, dilation, hemostasis, polypectomy.
Specific Conditions: inflammatory bowel disease (IBD), eosinophilic esophagitis (EoE), polyposis syndrome.
2.3 Inclusion criteria
Study Types: Randomized controlled trials, prospective cohort studies, large-scale retrospective studies, systematic reviews, and meta-analyses. Clinical practice guidelines from leading societies (e.g., NASPGHAN, ESPGHAN, ESGE, ASGE) were prioritized.
Population: Studies had to involve a patient population aged ≤18 years.
Publication Date: The search primarily included literature published between January 2008 and August 2025, to cover evidence and technological advances from the past 15 years.
3 Technical characteristics of endoscopy in children
The technical execution of endoscopy in children necessitates careful attention to instrument dimensions; in contrast to adults, the selection of endoscopic devices is tailored to the child's somatotype (1). In infants and young children with a body weight below 10 kg, the use of an ultra-thin gastroscope (outer diameter 5–6 mm) is recommended to minimize airway compression and mucosal injury (2); for weight between 10 and 20 kg, an 8 mm small-diameter gastroscope may be selected (8). In ERCP procedures, children weighing more than 10 kg may use standard adult duodenoscopes with an outer diameter of 11.3 mm (9); those weighing less than 10 kg should use specific duodenoscopes for children with an outer diameter of 7.5 mm (10). EUS is generally indicated only for children exceeding 15 kg; smaller children may be considered to substitute with a bronchial ultrasound probe (10). In addition, endoscopic therapy accessories (e.g., dilating balloons and hemostatic clips) should be matched to the luminal dimensions of children to prevent iatrogenic injury (3).
Endoscopy anesthesia is a critical component to ensure smooth procedures and patient safety. In recent years, multiple sedation regimens and techniques have been applied in clinical practice to optimize sedation efficacy and reduce adverse events. Studies indicate that endoscopy in children often requires deep sedation or general anesthesia, especially for younger or high-risk children and for advanced endoscopic procedures (10, 11). Common sedatives include propofol, midazolam, ketamine, and dexmedetomidine; among these, propofol is widely used due to its rapid onset and quick recovery, but its potential to cause respiratory depression should be noted (12). Dexmedetomidine, when delivered via inhalation, can significantly suppress gag and cough reflexes, reduce intraoperative anesthetic requirements, and improve endoscopist satisfaction with favorable safety (13). However, Johnson et al. found that low-dose dexmedetomidine combined with propofol did not reduce total propofol consumption and was associated with increased postoperative hypotension and prolonged recovery (14). Shafa et al. reported that ketamine combined with lidocaine did not significantly improve hemodynamics but could reduce the dose requirements of a single agent (12). Emerging agents such as remimazolam have shown rapid metabolism and good safety in adults, but pediatric data are still limited (15). Overall, sedation management for endoscopy in children should be individualized, balancing age, weight, and comorbidities to select the best plan, with emphasis on teamwork and postoperative follow-up (1).
4 Gastrointestinal endoscopy in the diagnosis of children
4.1 Esophagogastroduodenoscopy (EGD)
For children, EGD serves as a primary tool for investigating organic diseases. It is crucial for identifying the underlying causes of non-specific symptoms—such as failure to thrive, chronic abdominal pain, recurrent vomiting, and unexplained anemia—and for diagnosing specific conditions, notably gastroesophageal reflux disease (GERD) and eosinophilic esophagitis (EoE) (16, 17). Endoscopic examination allows direct visualization of esophageal mucosal lesions, including erosions, ulcers, or strictures, and can be combined with biopsies for histologic assessment, thereby enhancing diagnostic accuracy (18). For children with GERD, endoscopy can reveal varying degrees of esophagitis, and the strong correlation between pH monitoring and endoscopic findings (Boix-Ochoa score) further validates the diagnostic value of endoscopy (18). Additionally, endoscopy is particularly critical in differentiating EoE from GERD; the diagnosis of EoE can be confirmed by identifying eosinophilic infiltration in the esophageal mucosa (≥15 eosinophils per high-power field) on biopsy (19). In refractory cases, endoscopy can guide therapeutic decisions; for example, Rizvi et al. observed that IgG positivity in esophageal biopsy specimens may aid in detecting response to proton pump inhibitor (PPI) therapy or monitoring disease progression in children with EoE (20).
EGD in children differs from adult practice in several key aspects. A primary distinction lies in biopsy practices: children routinely undergo multi-site biopsies, even in the absence of macroscopic mucosal abnormalities, to avoid missing clinically subtle mucosal diseases. In contrast, biopsies in adults are typically targeted and obtained only from suspicious lesions (9, 10). Secondly, there are divergent focuses in diagnostic indications. In children, EGD is primarily employed to diagnose conditions such as failure to thrive, eosinophilic esophagitis, and inflammatory bowel disease. In adults, the procedure is more often oriented toward screening for malignancies and evaluating conditions like esophageal varices (9, 10).
Transnasal endoscopy (TNE) represents a novel, sedative-free modality for upper GI assessment, particularly advantageous for longitudinal surveillance in children with EoE, GERD, and postoperative esophageal procedures (21, 22). In cohorts of children, TNE demonstrates satisfactory tolerability, shortened procedure times, and biopsy adequacy comparable to conventional endoscopy, enabling accurate quantification of esophageal inflammatory activity (23, 24). Additional strategies, such as video goggle or VR-based unsedated TNE, may further reduce anxiety and improve diagnostic yield (25–27).
4.2 Colonoscopy
Studies have shown that the positive diagnostic yield of colonoscopy in children can exceed 70%, with inflammatory bowel disease (including ulcerative colitis and Crohn's disease) representing the most common diagnosis, accounting for over 40% (28). In addition, colonoscopy can effectively identify conditions such as polyposis and vascular malformations in children, providing important guidance for clinical management (29). For children who have undergone solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT), colonoscopy can reliably detect post-transplant lymphoproliferative disorders (PTLD), infectious colitis, and graft-vs. -host disease (GVHD) (30, 31).
Colon capsule endoscopy (CCE) is a wireless, ingestible mini-endoscope used for the non-invasive examination of the colon. In conditions involving children such as ulcerative colitis, CCE demonstrates high accuracy for assessing disease activity and extent, with reported sensitivity of 95% and specificity of 100%, outperforming modalities like intestinal ultrasound and fecal calprotectin (32, 33). Its advantages include the avoidance of anesthesia, excellent patient tolerance, and its utility as a complementary tool to colonoscopy, particularly for long-term monitoring in children, which can reduce the frequency and risks associated with invasive procedures. However, limitations exist, such as the inability to obtain biopsies or perform therapeutic interventions, a high dependency on adequate bowel preparation, the potential for capsule retention, and substantial cost (32, 33).
In terms of technical performance, colonoscopy in children demonstrates a high success rate, typically exceeding 90%, with relatively low complication rates (34). While adult colonoscopy is primarily utilized for colorectal cancer screening and surveillance, colonoscopy in children is chiefly indicated for diagnosing conditions such as inflammatory bowel disease (IBD) and polyposis syndromes. Consequently, a critical procedural step in children is intubation of the ileocecal valve, which allows for endoscopic examination and biopsy of the terminal ileum (9, 10, 28). This maneuver is critical for the differential diagnosis of IBD, as pathologies like Crohn's disease frequently involve the terminal ileum, making examination of the colon alone insufficient for a comprehensive assessment. Successful ileal intubation thereby enables a definitive diagnosis and accurate disease mapping. Studies confirm that high rates of both cecal and ileal intubation (exceeding 90% for each) are achievable in colonoscopy for children (35), Ileocecal intubation rate should be regarded as a key quality indicator for colonoscopy in children. To enhance safety and diagnostic accuracy, the choice of colonoscope should be tailored to the child's age and weight (8, 28). Through colonoscopy, clinicians can directly visualize mucosal abnormalities and, when combined with histopathology, establish a definitive diagnosis to guide individualized treatment (9, 29).
Adequate bowel preparation is a critical step to ensure the smooth performance of colonoscopy (36). Current commonly used bowel cleansing agents include polyethylene glycol (PEG) and sodium picosulfate magnesium citrate (SPMC); there is no significant difference in cleansing efficacy between them (37, 38). PEG requires high-dose administration and may be poorly tolerated, whereas SPMC has a smaller volume and better palatability, making it more acceptable to children (39).Moreover, the use of SPMC can reduce the need for nasogastric tube insertion, thereby alleviating discomfort for children (37). Split-dose regimens are associated with markedly improved bowel cleanliness compared with day-before dosing, and patient education along with adjunctive tools (e.g., smartphone applications) can further enhance bowel preparation quality (37, 39).
4.3 Small-bowel endoscopy and capsule endoscopy
Small-bowel endoscopy, including push enteroscopy (PE) and device-assisted enteroscopy (primarily balloon-assisted enteroscopy, BAE) are pivotal minimally invasive techniques for small bowel disorders in children (40). PE employs a push-and-pull method with 150–250 cm working-length endoscopes, targeting proximal small bowel lesions such as polyps and Crohn's disease (CD) in children ≥2 years and ≥10 kg (40). BAE enhances access via balloon-anchored pleating: single-balloon enteroscopy (SBE) uses a single overtube balloon (≥3 years, ≥13.5 kg), while double-balloon enteroscopy (DBE) employs dual balloons (≥2 years, ≥12 kg), reaching mid-to-distal segments (40). Both techniques address core indications: obscure gastrointestinal bleeding (OGIB), CD, and polyposis syndromes (e.g., Peutz-Jeghers syndrome, PJS), with BAE enabling therapeutic interventions like polypectomy and stricture dilation (41, 42). PE offers cost-effectiveness and wide availability, while BAE provides superior diagnostic yields (58.8%–78.6% for DBE) and therapeutic versatility, reducing surgical reliance (40, 41). Safety profiles are favorable, with minor complications (abdominal discomfort) predominating, though younger children (<10 years) have slightly higher risks (40, 41).
Capsule endoscopy (CE) play an important role in children for diagnosis, with particularly high sensitivity for the diagnosis and assessment of CD (33, 41), and enable detection of very early-onset inflammatory bowel disease (VEO-IBD). Studies show that 42% of VEO-IBD patients have small-bowel abnormalities detected by CE, with aphthous ulcers being predominant (43). Moreover, the newly proposed Crohn's disease activity index for CE (CE-CD) demonstrates good reliability and predictive value in children, effectively assessing inflammation and predicting clinical outcomes such as hospitalization and relapse (44). For OGIB, CE can identify etiologies including vascular malformations, ulcers, polyps, and Meckel's diverticulum (29, 45, 46). CE also show high accuracy in screening and monitoring polyposis syndromes such as PJS (41, 42, 47). Some reports indicate that CE can clearly visualize characteristic white villi changes in the small intestine, enabling precise diagnosis of intestinal lymphangiectasia (48, 49). Pan-enteric capsule endoscopy (PCE) is a non-invasive endoscopic technique that allows for the simultaneous examination of both the small bowel and colon. It holds significant value in Crohn's disease in children by providing a complete assessment of mucosal inflammation, thereby guiding treat-to-target strategies and improving rates of mucosal healing (50). Its key advantage over traditional capsule endoscopy lies in its ability to provide a comprehensive, one-stop evaluation of the entire bowel, thereby eliminating the need for repeated procedures (50).
CE use in children carries a risk of capsule retention, with reported rates ranging from 0.18% to 2%, and the risk may be higher in CD patients due to intestinal strictures (51–53). Clinically, when intestinal stricture is suspected, a patency capsule (the Agile Patency System) is recommended; this dissolvable capsule of the same size as an endoscopic capsule, equipped with an internal localization marker, can precisely identify the location of strictures and prevent retention (54). To reduce blind spots and missed lesions during CE, magnetically controlled capsule endoscopy (MCE) can achieve precise repositioning via external magnetic guidance, thereby improving safety and sensitivity (55–58). Difficulty swallowing the CE is not uncommon in children due to age, developmental stage, or psychological factors. To address this challenge, endoscopic-assisted delivery has become a well-established solution, primarily utilizing a dedicated delivery device, a transparent cap, or a retrieval basket (59). Evidence confirms that this technique ensures precise capsule placement in the descending duodenum, effectively prevents gastric retention, and significantly increases the completion rate of full small-bowel examination to over 94% (53). Regarding safety, multiple large-scale studies have reported a capsule retention rate of less than 3% with no serious adverse events, demonstrating a favorable risk-benefit profile, particularly for young or swallowing-impaired children (55, 60).
5 Gastrointestinal endoscopy in the treatment of children
5.1 Endoscopic hemostasis
Endoscopic therapy plays a key role in the management of gastrointestinal bleeding in children, with the goals of hemostasis and prevention of rebleeding. For variceal hemorrhage, endoscopic variceal ligation (EVL) is the treatment of choice, providing effective hemostasis with relatively low complication rates (61, 62). In children with esophageal varices, endoscopic sclerotherapy may be used to eradicate varices and achieve hemostasis (63). For nonvariceal bleeding, endoscopic injection of epinephrine combined with mechanical or thermal coagulation (e.g., argon plasma coagulation or metallic clips) can achieve hemostasis (61). Titanium clips are currently the preferred mechanical modality for nonvariceal gastrointestinal bleeding (61). In the very rare Dieulafoy lesions in children, endoscopic therapy is first-line and may include clipping, thermal coagulation, sclerosants, epinephrine injection, or laser therapy (64–66). The advantages of endoscopic treatment include rapid localization of the bleeding source and precise intervention, which significantly reduces the need for surgery and length of hospitalization. Treatment should be individualized according to the child's age and clinical status (61).
Compared with adult endoscopic treatment for gastrointestinal bleeding, etiologically, children predominantly present with juvenile polyps, allergic colitis, or vascular anomalies, whereas adults more commonly have diverticulitis or colorectal cancer (29, 61). Children's cases require a greater reliance on deep sedation or general anesthesia due to children's limited cooperation (67). In addition, we must consider the following special safety aspects for children: meticulous hemodynamic monitoring given children's more fragile physiology, tailored bowel preparation to avoid adverse effects, and family-centered communication to support informed consent (61, 67). Furthermore, endoscopists should prioritize minimizing tissue trauma and ensuring procedural efficiency to reduce complications, reflecting the distinct clinical needs of the population of children (67, 68).
5.2 Endoscopic polypectomy
Endoscopy is also employed for polypectomy, especially for symptomatic polyps and polyposis syndromes (69). There is currently no unified guideline for polypectomy techniques in children. For small polyps (≤5 mm), cold forceps polypectomy (CFP) or cold snare polypectomy (CSP) are commonly employed, with CSP preferred due to higher complete resection rates and lower complication risk (70, 71). For largerpolyps or pedunculated polyps, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) can improve safety, though the choice should be tailored to the individual child (72, 73). In children with PJS, BAE enables safe and effective small-bowel polypectomy, with high symptom relief, reduced risk of intussusception and surgical intervention (74, 75). Some studies suggest that endoscopic ischemic polypectomy (EIP) is associated with fewer complications than conventional polypectomy, making it particularly suitable for children with PJS (76).
Children and adults differ substantially in polyp characteristics, indications, and endoscopic polypectomy techniques. Polyps in children are mostly benign pedunculated juvenile polyps (highly vascularized), while adults have more sessile adenomas with malignant potential (69). Procedures for children are symptom-driven (e.g., rectal bleeding), unlike adult screening-focused practices (69). Technically, children often use cold/hot snare polypectomy, with hot snare preferred for larger polyps, whereas adults prioritize cold snare for <10 mm lesions (69, 72). Critical safety concerns of children include thinner bowel walls, increased bleeding risk from vascular polyps, limited exposure to advanced techniques, and the need to minimize tissue injury—requiring tailored approaches to avoid thermal damage and ensure complete resection without compromising safety (69, 76).
5.3 Endoscopic therapy for gastrointestinal tract stenosis
Endoscopic therapy can also be used for children with gastrointestinal strictures, especially esophageal anastomotic stricture and EoE-related stricture (77). For esophageal stricture, endoscopic balloon dilation (EBD) is the first-line treatment, with a low complication rate (e.g., perforation) but requiring multiple sessions to maintain long-term esophageal patency (78). For refractory stenosis, adjunctive local steroid injections (intralesional steroid injection, ISI) can reduce inflammation and scar formation (79). Additionally, stent implantation provides sustained dilation and is suitable for long-segment strictures or recurrent cases, though risks such as stent migration and mucosal injury should be noted (80, 81). For severe fibrotic strictures, endoscopic electrocautery incisional therapy (EIT) can incise scar tissue to improve dilation outcomes (82). For complex strictures, endoscopic magnetic compression anastomosis (MCA), a minimally invasive option, has been demonstrated as effective, offering a minimally invasive solution for complete occlusion or extreme narrowing of the esophagus by endoscopically guiding placement of magnets to progressively reconstruct the lumen (83). Overall, endoscopy can improve prognosis through multiple therapeutic approaches and reduce the need for open surgery.
Compared with adult endoscopic management of gastrointestinal strictures—often associated with neoplasia or chronic conditions—practice in children emphasizes etiologies such as EoE or congenital anomalies (77). Children-specific safety considerations include: heightened anesthesia-related risks, with potential impacts on neurodevelopment from repeated exposure; reliance on combined barium esophagrams to minimize missed strictures; and trauma prevention in smaller luminal diameters—unlike adults, where perforation is the principal concern (77, 84). Additionally, Strictures in children frequently necessitate more frequent dilations, requiring closer long-term monitoring of outcomes.
5.4 Endoscopic foreign body removal
Endoscopy demonstrates high efficiency and safety in gastrointestinal foreign body retrieval for children, with a low complication rate (85). Endoscopy enables precise localization of the foreign body and, depending on its characteristics, safe extraction using various accessories (e.g., graspers, retrieval nets). This is especially advantageous for high-risk objects such as sharp foreign bodies or batteries, where it can help prevent mucosal injury or perforation (86, 87). According to Oliva and colleagues, the urgency of endoscopy is categorized into four levels: emergency (<4 h), urgent (<24 h), early elective (<48 h), and elective (>48 h), depending on the type and location of the foreign body and the child's clinical symptoms (88). For example, button batteries in the esophagus require emergent removal within 2 h to avoid serious complications (88, 89). Although endoscopic techniques are well established, children with repeated or deliberate ingestion of foreign bodies often have underlying psychiatric disorders, necessitating multidisciplinary collaboration, including psychological interventions (90).
5.5 Application of ERCP in children
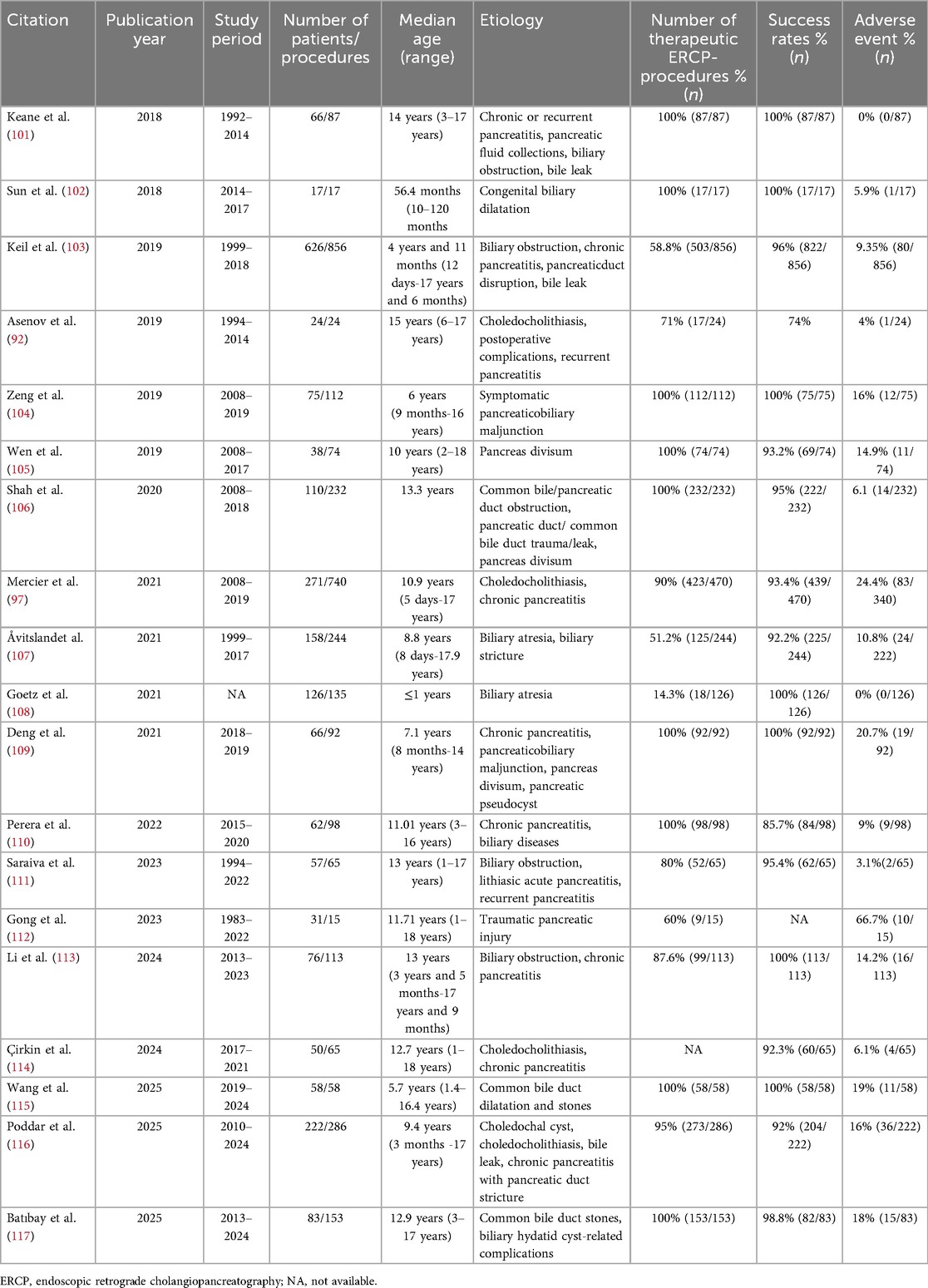
Although many noninvasive diagnostic modalities have supplanted ERCP for diagnosis in children, ERCP demonstrates relatively high safety and efficacy in the treatment of biliary and pancreatic disorders in children (91). Indications for ERCP in children mainly include choledocholithiasis, congenital biliary dilatation (CBD), postoperative complications (e.g., biliary leak, pancreaticopleural fistula), and recurrent pancreatitis, among others (92–94). Several meta-analyses indicate that the overall treatment success rate of ERCP in children ranges from approximately 74% to 95%, with stent placement being the most common therapeutic modality; other approaches include sphincterotomy, stone extraction, dilatation procedures (sphincterotomy and balloon dilation), and balloon dilatation, etc.
The postoperative adverse event rate is about 7%–8%, with postoperative pancreatitis being the most frequent complication (95, 96). Other rare complications include biliary infection, and bleeding (97, 98). Although intestinal complications are not the most common, they are critically important in ERCP for children, these primarily include intestinal strictures, adhesions, and postsurgical anatomical alterations (98, 99). Such conditions-particularly stenosis or adhesions-can prevent the passage of a standard duodenoscope to the duodenal papilla, increasing the risk of cannulation failure and intestinal perforation. To address these challenges, a comprehensive clinical approach is essential. Preoperative imaging should be used to delineate anatomical details. During the procedure, the selection of specialized equipment—such as smaller-caliber duodenoscopes—should be tailored to the child's weight and the degree of stenosis. In cases of severe stricture, balloon dilation may be considered. Crucially, these procedures should be performed in specialized centers equipped with anesthesiology support for children, onsite surgical backup, and endoscopists experienced in managing complex cases in children to maximize safety and manage potential complications effectively (10). The following summarizes recent results on ERCP in children (Table 1). It should be noted that ERCP in children require general anesthesia (10).
Because children are more susceptible to malignant tumors after exposure to ionizing radiation, particular attention must be paid to the risks of radiation exposure. During the procedure, radiation dose should be monitored and radioprotective shielding should be appropriately used to minimize the child's radiation exposure (100).
5.6 Application of EUS in children
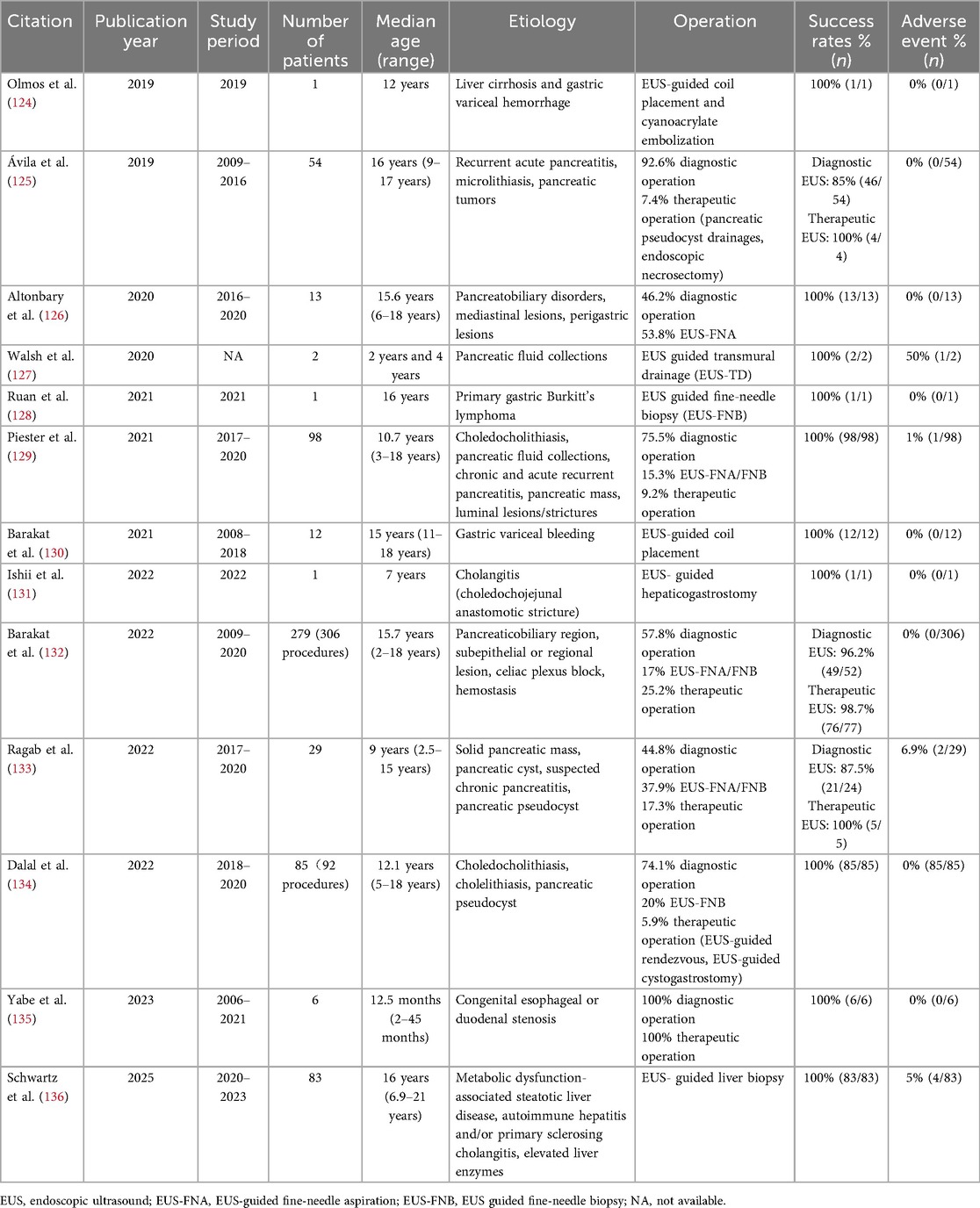
Endoscopic ultrasound (EUS) can clearly delineate pancreaticobiliary structures, with diagnostic performance superior to conventional imaging for chronic pancreatitis, microlithiasis in the biliary tract, and pancreatic pseudocysts, among other conditions (118). Additionally, EUS can be used to guide fine-needle aspiration biopsy, providing histopathological diagnostic confirmation for pancreatic masses and autoimmune pancreatitis (119). In terms of treatment, EUS-guided drainage can be safely performed for children with symptomatic pancreatic pseudocysts, thereby avoiding surgical intervention (120). For children with biliary obstruction, EUS-guided biliary drainage represents an effective alternative, particularly after ERCP failure (119). In children weighing more than 25 kg, adult-endoscopic ultrasound can be safely utilized (121). In smaller children, ultrasound endoscopic probes through standard children endoscope channels may be employed (9, 122). For children weighing more than 15 kg, EUS can be performed safely under intravenous sedation (e.g., propofol) without general anesthesia (119). Infants and young children, due to smaller anatomical structures, may pose greater technical and anesthesia-related challenges (123). The following summarizes recent studies on EUS in children (Table 2). Overall, EUS demonstrates a high technical success rate (118). The complication rate is low, with most adverse events being mild pancreatitis, bleeding, and infection (100, 118).
5.7 POEM for the treatment of achalasia
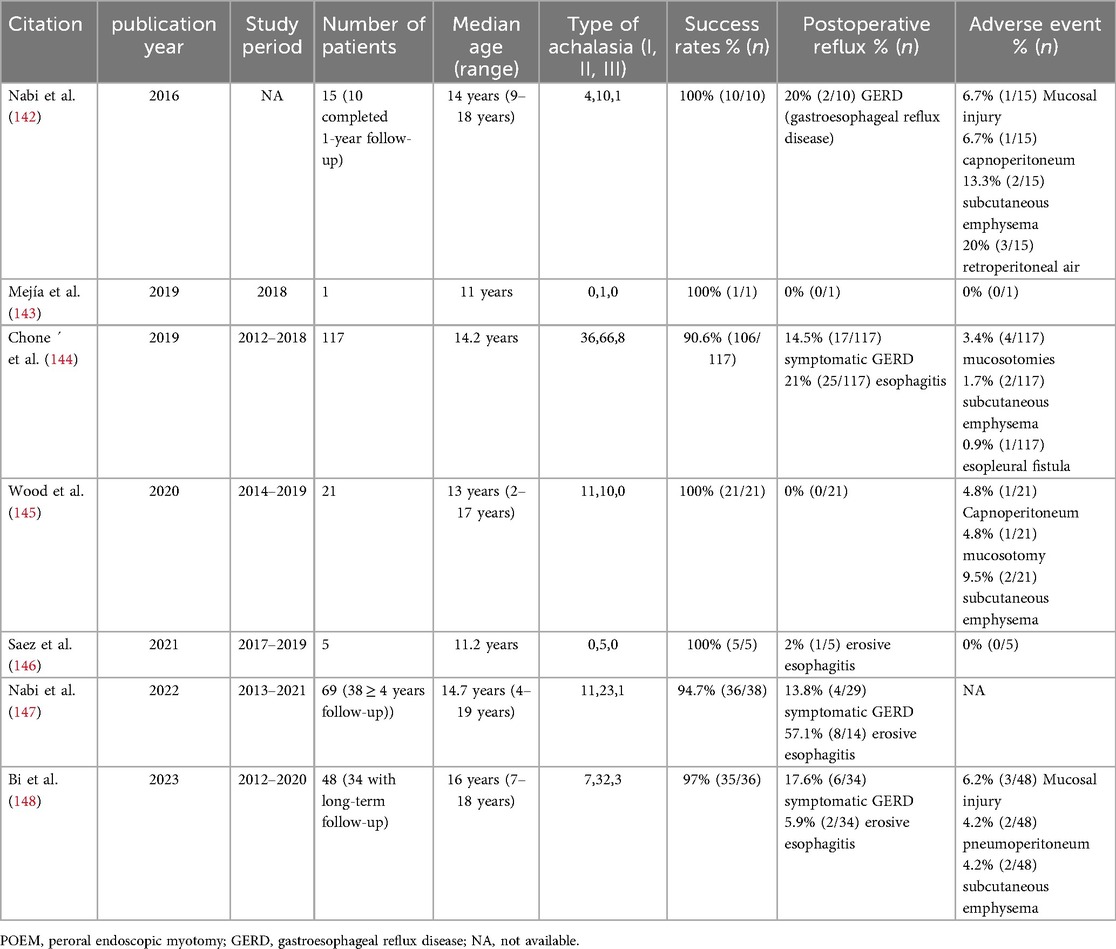
POEM is principally used to treat children with achalasia. A meta-analysis shows technical success and clinical success rates of 97.1% and 88–94.4%, respectively, in children, with postoperative Eckardt scores significantly reduced (137). The following summarize recent results on POEM in children with achalasia (Table 3). The procedure is applicable to all types of achalasia, and remains effective even in previously treated or complex cases (e.g., sigmoid-type achalasia) (138, 139). The advantages of POEM include its minimally invasive nature, rapid postoperative recovery, and shorter hospital stay (140, 141). However, the incidence of postoperative gastroesophageal reflux (GER) is relatively high, with about 26.3% of children developing erosive esophagitis, necessitating long-term follow-up and proton pump inhibitor therapy (137). Overall, POEM provides a safe and effective treatment option for children with achalasia, but its technical complexity and postoperative reflux issues warrant careful consideration in clinical practice.
6 Indications, contraindications, and adverse events of endoscopy in children
Gastrointestinal endoscopy has become an indispensable tool for diagnosing and treating digestive tract and pancreatobiliary diseases in children. Its application emphasizes the principles of being child-centered, safety-first, and purpose-driven. Gastroscopy and colonoscopy serve as the primary modalities for evaluating mucosal lesions in the upper and lower gastrointestinal tract, providing both diagnostic (e.g., for IBD and EoE) and therapeutic (e.g., polypectomy, hemostasis) functions. For the small intestine, which is beyond the reach of traditional endoscopy, capsule endoscopy offers a non-invasive screening method capable of effectively detecting abnormalities. On this basis, device-assisted enteroscopy allows for further examination and treatment of suspicious lesions. In the field of pancreatobiliary diseases, the role of ERCP has shifted entirely from diagnosis to advanced therapeutic interventions such as stone extraction, stent placement, and drainage. Meanwhile, EUS, with its superior imaging and guided puncture capabilities, plays a crucial role in tumor evaluation, cyst drainage, and other areas. Below is a summary of the diagnostic and therapeutic indications for various types of endoscopy (Table 4) (10, 101, 149).
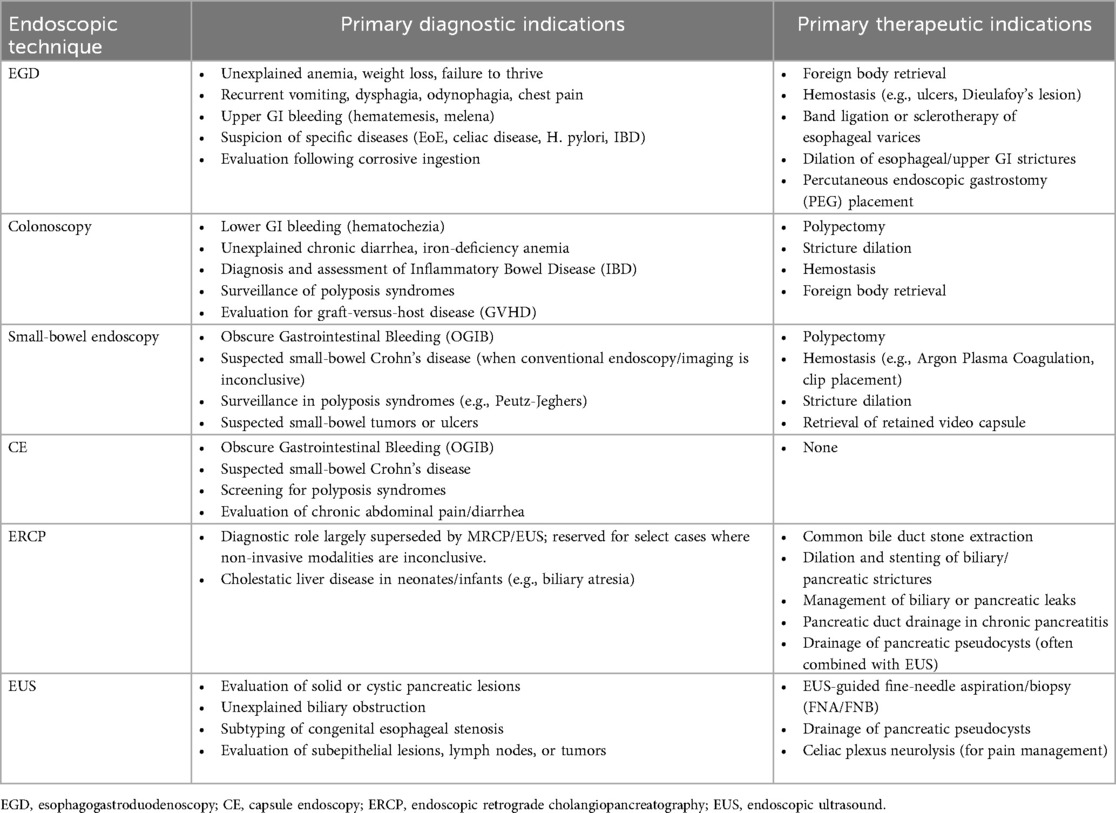
Table 4. Indications for various endoscopic techniques in the diagnosis and treatment of digestive diseases in children.
The safety of gastrointestinal endoscopy in children hinges on strict adherence to contraindications and a comprehensive understanding of potential adverse events (Table 5) (10, 101, 149). While diagnostic procedures such as gastroscopy, colonoscopy, and diagnostic EUS are generally safe with rare serious complications, the risk escalates significantly with the invasiveness and complexity of the intervention. High-risk procedures include therapeutic polypectomy via enteroscopy, post-ERCP pancreatitis, and EUS-guided drainage, whereas the foremost risk of capsule endoscopy is retention at an asymptomatic stricture. To maximize patient safety, all endoscopic procedures must be conducted by an experienced endoscopy in children team in a setting equipped with child-specific devices and under appropriate anesthesia and monitoring. Ultimately, rigorous pre-procedural evaluation (including imaging to exclude contraindications), refined technical skill, and diligent post-procedural observation are paramount to preventing and managing adverse events, thereby ensuring that the benefits of gastrointestinal endoscopy outweigh its risks for children.
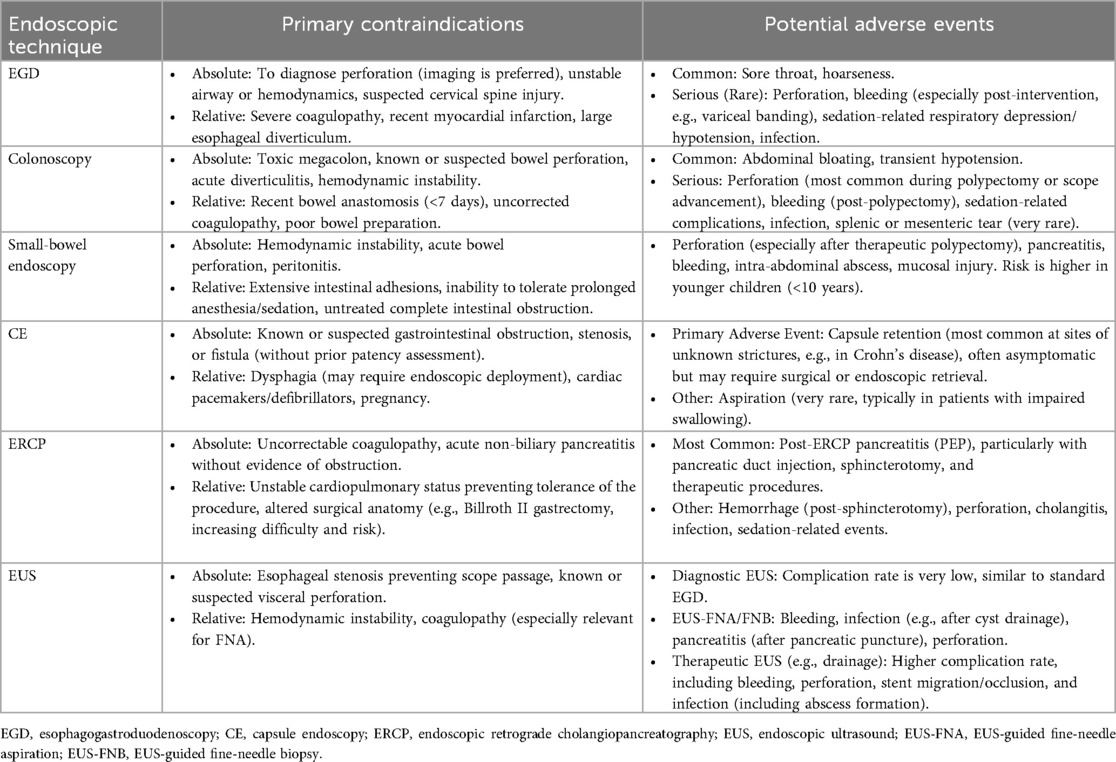
Table 5. Contraindications and postprocedural adverse events of various endoscopic techniques in children.
7 Geographical inequalities in development
In the field of endoscopy in children, there remains a gap of several decades in overall capabilities between low- and middle-income countries (LMICs) and high-income nations, with development being highly uneven across regions. In Europe, under the coordinated leadership of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society of Gastrointestinal Endoscopy (ESGE), a high-quality diagnosis and treatment system has been systematically established. This includes a competency-based, progressive training pathway supported by simulation training, e-learning platforms, and “train-the-trainer” programs. The region has also published the first comprehensive endoscopy in children guideline covering both diagnostic and therapeutic procedures (10, 150). These efforts have significantly enhanced the standardization, safety, and professionalism of endoscopy in children in Europe, providing an important model for global practice.
Asia has made remarkable progress in gastrointestinal endoscopy of children, particularly in the application of techniques and data accumulation. However, the absence of regional guidelines, unsystematic training, lack of unified quality control, and uneven distribution of resources remain key constraints. Future progress depends on regional collaboration, standardized training, guideline development, and optimized resource allocation to improve overall standards (151).
LMICs face substantial challenges in endoscopy in children, with development severely constrained by shortages of funding, equipment, specialized personnel, and infrastructure. There is a lack of systematic quality frameworks and underdeveloped data registry systems. Despite these obstacles, progress has been achieved in some regions through international organizational support, local pioneering efforts, and technological innovation, demonstrating commendable advances under difficult conditions (152).
8 Discussion
A critical challenge in gastrointestinal endoscopy in children lies in the lack of specialized equipment tailored to children's age, size, and anatomical characteristics. Despite strong recommendations for age/weight-appropriate endoscopic tools and children-specific monitoring devices, clinical practice often relies on adult-adapted equipment, compromising procedural safety and efficacy (67). Concurrently, the paucity of children-specific clinical trial data persists–most evidence is extrapolated from adult studies, leading to “very low” quality of evidence for key practices as highlighted by GRADE assessments (67, 68). Limited volumes of children with digestive diseases, even in tertiary centers, further restrict sample sizes, hindering robust validation of procedures and quality metrics (68, 84). Additionally, substantial inter-center heterogeneity is evident globally: training programs vary widely in duration, procedural thresholds, and assessment methods; clinical practices differ markedly in documentation completeness (e.g., bowel preparation quality), technical outcomes (e.g., ileal intubation rates), and adherence to quality standards (67, 68, 84).
High-quality endoscopy in children should embody the following characteristics: it must be safe and effective; it should focus on the experience and understanding of the child and their parents; it must adhere to standardized procedures based on consensus guidelines; and it should pursue continuous quality improvement through data feedback, peer comparison, and technological updates. In 2022, the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and ESPGHAN jointly established the international Endoscopy in children Quality Improvement Network (PEnQuIN). This initiative aims to promote continuous quality improvement in endoscopy in children services worldwide by developing and implementing children-specific quality standards and metrics. Through a multi-level implementation framework involving endoscopists, endoscopic facilities (with standardized operating procedures, electronic reporting systems, and a culture of patient safety), and endoscopic procedures (including the establishment of multicenter collaborative networks and quality control registry systems), and leveraging technologies such as electronic medical records, artificial intelligence, and video recording, the automated collection, analysis, and feedback of quality indicators can be achieved (153). The PEnQuIN initiative represents a comprehensive, sustainable, and child-centered ecosystem for enhancing the quality of endoscopy in children globally, serving as a milestone in ushering the field into an era of high-quality practice.
Nevertheless, the implementation of high-quality endoscopy in children standards and indicators in clinical practice faces multiple challenges: difficulties in translating guidelines into practice; a widespread lack of automated data systems, hindering effective data collection and integration; limited application of emerging technologies such as AI and video assessment in endoscopy in children, coupled with a shortage of validated children-specific algorithms; and resistance among some endoscopists to performance feedback and quality improvement initiatives (154).
Therefore, we call for the establishment of a national endoscopy in children quality registry and collaborative network to facilitate multi-center data sharing and benchmarking; promote the adoption of digital and intelligent tools, such as AI-assisted diagnosis and evaluation systems; enhance endoscopist training and continuous education through modern teaching methods including video assessment and simulation training; and advocate for policy and financial support to integrate endoscopy in children quality improvement into hospital accreditation and health insurance evaluation systems. Guided by the PEnQuIN standards and adapted to local contexts, we should develop tailored endoscopy in children quality guidelines, working collectively to usher in a new era of evidence-based, child-centered, data-driven, and continuously improving endoscopy in children practice.
Author contributions
GuoZ: Writing – original draft. GuiZ: Writing – review & editing. PL: Resources, Visualization, Supervision, Conceptualization, Writing – review & editing. SZ: Supervision, Conceptualization, Writing – review & editing, Visualization, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. National Natural Science Foundation of China (No. 82027801).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nabi Z, Reddy DN. Advanced therapeutic gastrointestinal endoscopy in children—today and tomorrow. Clin Endosc. (2018) 51:142–9. doi: 10.5946/ce.2017.102
2. Lang T. Interfaces in pediatric gastrointestinal endoscopy: who should do it? Visc Med. (2016) 32:7–11. doi: 10.1159/000444116
3. Krasaelap A, Lerner DG. Advances in endoscopic procedures in pediatric patients. Pediatr Clin N Am. (2021) 68:1221–35. doi: 10.1016/j.pcl.2021.07.005
4. Chen W-F, Li Q-L, Zhou P-H, Yao L-Q, Xu M-D, Zhang Y-Q, et al. Long-term outcomes of peroral endoscopic myotomy for achalasia in pediatric patients: a prospective, single-center study. Gastrointest Endosc. (2015) 81:91–100. doi: 10.1016/j.gie.2014.06.035
5. Rollo G, De Angelis P, Torroni F, Balassone V, Iolanda Contini AC, Faraci S, et al. Replogle modified endoscopic vacuum-assisted closure (EVAC) therapy: a new strategy to treat anastomotic leakage and esophageal perforation. J Pediatr Surg. (2024) 59:432–6. doi: 10.1016/j.jpedsurg.2023.09.043
6. Manfredi MA, Clark SJ, Staffa SJ, Ngo PD, Smithers CJ, Hamilton TE, et al. Endoscopic esophageal vacuum therapy: a novel therapy for esophageal perforations in pediatric patients. J Pediatr Gastroenterol Nutr. (2018) 67:706–12. doi: 10.1097/mpg.0000000000002073
7. Barakat MT, Triadafilopoulos G, Berquist WE. Pediatric endoscopy practice patterns in the United States, Canada, and Mexico. J Pediatr Gastroenterol Nutr. (2019) 69:24–31. doi: 10.1097/mpg.0000000000002310
8. Barth BA, Banerjee S, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, et al. Equipment for pediatric endoscopy. Gastrointest Endosc. (2012) 76:8–17. doi: 10.1016/j.gie.2012.02.023
9. Lightdale JR, Acosta R, Shergill AK, Chandrasekhara V, Chathadi K, Early D, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. (2014) 79:699–710. doi: 10.1016/j.gie.2013.08.014
10. Thomson M, Tringali A, Dumonceau J, Tavares M, Tabbers MM, Furlano R, et al. Paediatric gastrointestinal endoscopy: european society for paediatric gastroenterology hepatology and nutrition and European society of gastrointestinal endoscopy guidelines. J Pediatr Gastroenterol Nutr. (2017) 64:133–53. doi: 10.1097/mpg.0000000000001408
11. Cox CB, Laborda T, Kynes JM, Hiremath G. Evolution in the practice of pediatric endoscopy and sedation. Front Pediatr. (2021) 9:687635. doi: 10.3389/fped.2021.687635
12. Shafa A, Abediny R, Shetabi H, Shahhosseini S. The effect of preoperative combined with intravenous lidocaine and ketamine vs. Intravenous ketamine on pediatric patients undergoing upper gastrointestinal endoscopy. Anesth Pain Med. (2023) 13:e130991. doi: 10.5812/aapm-130991
13. Turunc E, Ustun YB, Bilgin S, Kaya C, Koksal E, Dost B. Effect of nebulized dexmedetomidine on gag reflex suppression and sedation quality in pediatric patients undergoing gastrointestinal endoscopy: a randomized controlled trial. BMC Anesthesiol. (2025) 25:227. doi: 10.1186/s12871-025-03106-x
14. Johnson EG, Weaver SG, Batt KL, Weaver RH, Schadler A, Hall SJ. Low-dose adjuvant dexmedetomidine did not decrease propofol sedation requirements in children undergoing gastrointestinal endoscopy. Pharmacotherapy. (2022) 42:792–7. doi: 10.1002/phar.2729
15. Garrett A, Flowers J, Ng V, Tobias JD. Remimazolam for sedation during upper gastrointestinal endoscopy in an adolescent. J Med Cases. (2022) 13:495–8. doi: 10.14740/jmc4013
16. Lightdale JR, Gremse DA, Section on Gastroenterology, Hepatology, and Nutrition, Heitlinger LA, Cabana M, Gilger MA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. (2013) 131:e1684–95. doi: 10.1542/peds.2013-0421
17. Adamiak T, Plati KF. Pediatric esophageal disorders: diagnosis and treatment of reflux and eosinophilic esophagitis. Pediatr Rev. (2018) 39:392–402. doi: 10.1542/pir.2017-0266
18. Lupu VV, Burlea M, Nistor N, Streanga V, Starcea MI, Paduraru G, et al. Correlation between esophageal pH-metry and esophagitis in gastroesophageal reflux disease in children. Medicine. (2018) 97:e12042. doi: 10.1097/MD.0000000000012042
19. Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. (2018) 155:1022–1033.e10. doi: 10.1053/j.gastro.2018.07.009
20. Rizvi SA, Oriala C, Irastorza LE, Bornstein J, Li S, Smadi Y. Esophageal epithelial immunoglobulin G is an important marker for the diagnosis and management of pediatric eosinophilic esophagitis. JGH Open. (2022) 6:402–7. doi: 10.1002/jgh3.12752
21. Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc. (2016) 83:299–306.e1. doi: 10.1016/j.gie.2015.05.044
22. Bose P, Pitman R. Pediatric unsedated transnasal endoscopy: applications, equipment, and future directions. Front Pediatr. (2025) 13:1585705. doi: 10.3389/fped.2025.1585705
23. Shaul E, Kennedy KV, Spergel ZC, Daneshdoost S, Mahon M, Thanawala S, et al. Endoscopic and histologic utility of transnasal endoscopy in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2024) 78:1155–60. doi: 10.1002/jpn3.12170
24. Friedlander JA, Leinwand K, Bhardwaj V, Nguyen N. A guide on transnasal endoscopy: setting up a pediatric unsedated endoscopy program. Front Pediatr. (2024) 11:1267148. doi: 10.3389/fped.2023.1267148
25. Lee J-W, Tai C-S, Chang K-C, Chiu Y-C, Ni Y-H, Wu J-F. The benefits of transnasal endoscopy compared to conventional endoscopy in adolescents. J Formos Med Assoc. (2024):S0929664624005424. doi: 10.1016/j.jfma.2024.11.007
26. Sabe RMM, Elzayat A, Buckley A, Shah JR, Khalili AS, Sferra TJ. Transnasal endoscopy for children and adolescents with eosinophilic esophagitis: a single-center experience. Gastroenterol Res. (2022) 15:155–61. doi: 10.14740/gr1535
27. Nguyen N, Pan Z, Smith C, Friedlander JA. Transnasal endoscopy ease score “TNEase score” to evaluate patient tolerance of unsedated transnasal endoscopy. J Pediatr Gastroenterol Nutr. (2024) 78:381–5. doi: 10.1002/jpn3.12102
28. Yoshioka S, Takedatsu H, Fukunaga S, Kuwaki K, Yamasaki H, Yamauchi R, et al. Study to determine guidelines for pediatric colonoscopy. World J Gastroenterol. (2017) 23:5773. doi: 10.3748/wjg.v23.i31.5773
29. Sahn B, Bitton S. Lower gastrointestinal bleeding in children. Gastrointest Endosc Clin N Am. (2016) 26:75–98. doi: 10.1016/j.giec.2015.08.007
30. Slae M, Pinhasov D, Averbuch D, Davidovics Z, Or EE, Reif S, et al. Evaluation of gastrointestinal symptoms in pediatric patients post hematopoietic stem cell transplantation: ileo-colonoscopy versus sigmoidoscopy. A single-center experience and review of literature. Pediatr Blood Cancer. (2021) 68:e29235. doi: 10.1002/pbc.29235
31. Ylinen E, Merras-Salmio L, Jahnukainen T. Gastrointestinal symptoms and endoscopy findings after pediatric solid organ transplantation: a case series. Pediatr Transplant. (2022) 26:e14374. doi: 10.1111/petr.14374
32. Spyropoulou V, Russo G, Rossi ED, Ruggiero C, Volpe D, D’Arcangelo G, et al. Diagnostic accuracy of multimodal noninvasive follow-up for pediatric ulcerative colitis: a single-center prospective study. J Pediatr Gastroenterol Nutr. (2024) 78:280–8. doi: 10.1002/jpn3.12098
33. Argüelles-Arias F, Donat E, Fernández-Urien I, Alberca F, Argüelles-Martín F, Martínez MJ, et al. Guideline for wireless capsule endoscopy in children and adolescents: a consensus document by the SEGHNP (Spanish society for pediatric gastroenterology, hepatology, and nutrition) and the SEPD (Spanish society for digestive diseases). Rev Esp Enferm Dig. (2015) 107(12):714–31. doi: 10.17235/reed.2015.3921/2015
34. Kudo T, Abukawa D, Nakayama Y, Segawa O, Uchida K, Jimbo K, et al. Nationwide survey of pediatric gastrointestinal endoscopy in Japan. J Gastroenterol Hepatol. (2021) 36:1545–9. doi: 10.1111/jgh.15297
35. Singh HK, Withers GD, Ee LC. Quality indicators in pediatric colonoscopy: an Australian tertiary center experience. Scand J Gastroenterol. (2017) 52:1453–6. doi: 10.1080/00365521.2017.1380224
36. Piccirillo M, Pucinischi V, Mennini M, Strisciuglio C, Iannicelli E, Giallorenzi MA, et al. Gastrointestinal bleeding in children: diagnostic approach. Ital J Pediatr. (2024) 50:13. doi: 10.1186/s13052-024-01592-2
37. Furio S, Lucarini A, Mennini M, Strisciuglio C, Felici E, Ferretti A, et al. Effectiveness and safety of polyethylene-glycol-4000 versus sodium picosulphate plus magnesium oxide and citric acid for bowel cleansing before colonoscopy in children: a systematic review with meta-analysis. J Pediatr Gastroenterol Nutr. (2025) 80:25–33. doi: 10.1002/jpn3.12388
38. Di Nardo G, Barbara G, Borrelli O, Cremon C, Giorgio V, Greco L, et al. Italian Guidelines for the management of irritable bowel syndrome in children and adolescents: joint consensus from the Italian societies of: gastroenterology, hepatology and pediatric nutrition (SIGENP), pediatrics (SIP), gastroenterology and endoscopy (SIGE) and neurogastroenterology and motility (SINGEM). Ital J Pediatr. (2024) 50:51. doi: 10.1186/s13052-024-01607-y
39. Mamula P, Nema N. Bowel preparation for pediatric colonoscopy. Front Pediatr. (2021) 9:705624. doi: 10.3389/fped.2021.705624
40. Hoskins BJ. Deep enteroscopy in children: techniques, applications, and future directions. Front Pediatr. (2025) 13:1562075. doi: 10.3389/fped.2025.1562075
41. Iwama I, Nambu R, Nakayama Y. Small bowel endoscopy for children: collaboration of capsule endoscopy and device-assisted enteroscopy. Dig Endosc. (2023) 35:562–73. doi: 10.1111/den.14511
42. Yamamoto H, Sakamoto H, Kumagai H, Abe T, Ishiguro S, Uchida K, et al. Clinical guidelines for diagnosis and management of Peutz-Jeghers syndrome in children and adults. Digestion. (2023) 104:335–47. doi: 10.1159/000529799
43. Hagiwara S, Shimizu H, Nambu R, Jimbo K, Kaji E, Nishizawa T, et al. Feasibility and safety of small bowel capsule endoscopy in very early-onset inflammatory bowel disease: a multi-institutional study. Inflamm Bowel Dis. (2025):izaf144. doi: 10.1093/ibd/izaf144
44. Oliva S, Veraldi S, Cucchiara S, Russo G, Spagnoli A, Cohen SA. Assessment of a new score for capsule endoscopy in pediatric crohn's disease (CE-CD). Endosc Int Open. (2021) 09:E1480–90. doi: 10.1055/a-1522-8723
45. Cohen SA, Klevens AI. Use of capsule endoscopy in diagnosis and management of pediatric patients, based on meta-analysis. Clin Gastroenterol Hepatol. (2011) 9:490–6. doi: 10.1016/j.cgh.2011.03.025
46. Hansen LØ, Thorndal C, Agache A, Koulaouzidis A. Meckel’s diverticulum discovered by capsule endoscopy: a systematic review of case reports. Scand J Gastroenterol. (2025) 60:414–20. doi: 10.1080/00365521.2025.2487536
47. Phen C, Attard TM. The role of capsule endoscopy in the management of pediatric hereditary polyposis syndromes. J Pediatr Gastroenterol Nutr. (2023) 77:442–4. doi: 10.1097/MPG.0000000000003902
48. Wu J, Huang Z, Ji M, Jiang Z, Wang Y, Tang Z, et al. The diagnostic value of capsule endoscopy in children with intestinal lymphangiectasia. Rev Esp Enferm Dig. (2021) 113:765–9. doi: 10.17235/reed.2021.7682/2020
49. Van Der Reijden SM, Van Wijk MP, Jacobs MAJM, De Meij TGJ. Video capsule endoscopy to diagnose primary intestinal lymphangiectasia in a 14-month-old child. J Pediatr Gastroenterol Nutr. (2017) 64:e161. doi: 10.1097/MPG.0000000000001586
50. Oliva S, Aloi M, Viola F, Mallardo S, Civitelli F, Maccioni F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol. (2019) 17(10):2060–2067.e1. doi: 10.1016/j.cgh.2018.10.015
51. Odeyinka O, Alhashimi R, Thoota S, Ashok T, Palyam V, Azam AT, et al. The role of capsule endoscopy in Crohn’s disease: a review. Cureus. (2022) 14(7):e27242. doi: 10.7759/cureus.27242
52. Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, De Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. (2005) 62:712–6. doi: 10.1016/j.gie.2005.05.002
53. Li L, Zhan X, Li J, Li S, Chen Y, Yang L, et al. Clinical assessment of small bowel capsule endoscopy in pediatric patients. Front Med. (2024) 11:1455894. doi: 10.3389/fmed.2024.1455894
54. Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. (2008) 67:902–9. doi: 10.1016/j.gie.2007.10.063
55. Cheng W, Lin K, Wang L, Wang X, Feng Y, Gu Z, et al. Clinical features of magnetically controlled capsule endoscopy in children: a large, retrospective cohort study. J Pediatr Gastroenterol Nutr. (2025) 80:733–41. doi: 10.1002/jpn3.12472
56. Li J, Zhao X, Su W, Shen R, Xiao Y, Wang X, et al. Magnetically guided capsule endoscopy and magnetic resonance enterography in children with Crohn’s disease: manifestations and the value of assessing disease activity. Front Pharmacol. (2022) 13:894808. doi: 10.3389/fphar.2022.894808
57. Di Nardo G, Micheli F, Cozzi DA, Mercantini P, Parisi P, Baccini F, et al. Magnetic-assisted capsule endoscopy in children with crohn disease: feasibility and impact on gastric transit time. J Pediatr Gastroenterol Nutr. (2023) 76:646–51. doi: 10.1097/MPG.0000000000003733
58. Wang L, Lin K, Cheng W, Wang X, Zhang Y, Feng Y, et al. Innovative nomogram approach to enhance the prediction of Helicobacter pylori infection in children via magnetic-controlled capsule endoscopy. Eur J Pediatr. (2025) 184:139. doi: 10.1007/s00431-024-05962-0
59. Barth BA, Donovan K, Fox VL. Endoscopic placement of the capsule endoscope in children. Gastrointest Endosc. (2004) 60:818–21. doi: 10.1016/S0016-5107(04)02052-8
60. Cohen SA, Oliva S. Capsule endoscopy in children. Front Pediatr. (2021) 9:664722. doi: 10.3389/fped.2021.664722
61. Romano C, Oliva S, Martellossi S, Miele E, Arrigo S, Graziani MG, et al. Pediatric gastrointestinal bleeding: perspectives from the Italian society of pediatric gastroenterology. World J Gastroenterol. (2017) 23:1328. doi: 10.3748/wjg.v23.i8.1328
62. Dai C. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World J Gastroenterol. (2015) 21:2534. doi: 10.3748/wjg.v21.i8.2534
63. Sarma MS, Seetharaman J. Pediatric non-cirrhotic portal hypertension: endoscopic outcome and perspectives from developing nations. World J Hepatol. (2021) 13:1269–88. doi: 10.4254/wjh.v13.i10.1269
64. Chen Y, Sun M, Teng X. Therapeutic endoscopy of a Dieulafoy lesion in a 10-year-old girl: a case report. World J Clin Cases. (2022) 10:1966–72. doi: 10.12998/wjcc.v10.i6.1966
65. Emura T, Hosoda K, Harai S, Oyachi N, Suzuki T, Takada K, et al. Dieulafoy lesion in a two-year-old boy: a case report. J Med Case Reports. (2016) 10:293. doi: 10.1186/s13256-016-1083-4
66. Jeon HK, Kim GH. Endoscopic management of Dieulafoy’s lesion. Clin Endosc. (2015) 48:112. doi: 10.5946/ce.2015.48.2.112
67. Walsh CM, Lightdale JR, Mack DR, Amil-Dias J, Bontems P, Brill H, et al. Overview of the pediatric endoscopy quality improvement network quality standards and indicators for pediatric endoscopy: a joint NASPGHAN/ESPGHAN guideline. J Pediatr Gastroenterol Nutr. (2022) 74:S3–S15. doi: 10.1097/MPG.0000000000003262
68. Scarallo L, Russo G, Renzo S, Lionetti P, Oliva S. A journey towards pediatric gastrointestinal endoscopy and its training: a narrative review. Front Pediatr. (2023) 11:1201593. doi: 10.3389/fped.2023.1201593
69. Kay M, Wyllie R. Pediatric colonoscopic polypectomy technique. J Pediatr Gastroenterol Nutr. (2020) 70:280–4. doi: 10.1097/MPG.0000000000002591
70. Friesen HJ, Attard TM, Liman AYJ, Yasui OW, Walsh CM, Gugig R, et al. Cold snare polypectomy in pediatric polyposis: a multicenter experience. Children. (2025) 12:291. doi: 10.3390/children12030291
71. Ferlitsch M, Hassan C, Bisschops R, Bhandari P, Dinis-Ribeiro M, Risio M, et al. Colorectal polypectomy and endoscopic mucosal resection: european society of gastrointestinal endoscopy (ESGE) guideline—update 2024. Endoscopy. (2024) 56:516–45. doi: 10.1055/a-2304-3219
72. Hoskins BJ, Ng K, Rex DK. Is it time to revisit the need for pediatric polypectomy guidelines? J Pediatr Gastroenterol Nutr. (2025) 81:162–6. doi: 10.1002/jpn3.70113
73. Ye B, Wu Y, Tang X. Risk factors of post-polypectomy bleeding and recurrence in children with colorectal polyps after endoscopic mucosal resection: a retrospective cohort study. Transl Pediatr. (2022) 11:1823–30. doi: 10.21037/tp-22-518
74. Blanco-Velasco G, Hernández-Mondragón OV, Blancas-Valencia JM, Paz-Flores V, Fuentes-Hernández D, Rodríguez-González P, et al. Seguridad y eficacia de la polipectomía en intestino delgado utilizando enteroscopio asistido por balones en pacientes pediátricos con síndrome de Peutz-Jeghers. Rev Gastroenterol Méx. (2018) 83:234–7. doi: 10.1016/j.rgmx.2017.07.003
75. Li B-R, Sun T, Li J, Zhang Y-S, Ning S-B, Jin X-W, et al. Primary experience of small bowel polypectomy with balloon-assisted enteroscopy in young pediatric Peutz–Jeghers syndrome patients. Eur J Pediatr. (2020) 179:611–7. doi: 10.1007/s00431-019-03534-1
76. Dofuku M, Yano T, Yokoyama K, Okada Y, Kumagai H, Tajima T, et al. Management of pediatric Peutz–Jeghers syndrome: highlighting the efficacy and safety of endoscopic ischemic polypectomy. J Pediatr Gastroenterol Nutr. (2025) 80:408–16. doi: 10.1002/jpn3.12458
77. Nguyen N, Kramer RE, Menard-Katcher C. Endoscopy in pediatric eosinophilic esophagitis. Front Pediatr. (2021) 9:713027. doi: 10.3389/fped.2021.713027
78. Yasuda JL, Taslitsky GN, Staffa SJ, Clark SJ, Ngo PD, Hamilton TE, et al. Utility of repeated therapeutic endoscopies for pediatric esophageal anastomotic strictures. Dis Esophagus. (2020) 33:doaa031. doi: 10.1093/dote/doaa031
79. Ngo PD, Kamran A, Clark SJ, Jennings RW, Hamilton TE, Smithers CJ, et al. Intralesional steroid injection therapy for esophageal anastomotic stricture following esophageal atresia repair. J Pediatr Gastroenterol Nutr. (2020) 70:462–7. doi: 10.1097/MPG.0000000000002562
80. Baghdadi O, Yasuda J, Staffa S, Ngo P, Zendejas B, Hamilton T, et al. Predictors and outcomes of fully covered stent treatment for anastomotic esophageal strictures in esophageal atresia. J Pediatr Gastroenterol Nutr. (2022) 74:221–6. doi: 10.1097/MPG.0000000000003330
81. Lange B, Sold M, Kähler G, Wessel LM, Kubiak R. Experience with fully covered self-expandable metal stents for anastomotic stricture following esophageal atresia repair. Dis Esophagus. (2018) 31:221–6. doi: 10.1093/dote/doy061
82. Manfredi MA, Clark SJ, Medford S, Staffa SJ, Ngo PD, Hamilton TE, et al. Endoscopic electrocautery incisional therapy as a treatment for refractory benign pediatric esophageal strictures. J Pediatr Gastroenterol Nutr. (2018) 67:464–8. doi: 10.1097/MPG.0000000000002008
83. Woo R, Wong CM, Trimble Z, Puapong D, Koehler S, Miller S, et al. Magnetic compression stricturoplasty for treatment of refractory esophageal strictures in children: technique and lessons learned. Surg Innov. (2017) 24:432–9. doi: 10.1177/1553350617720994
84. Thakkar K, Holub JL, Gilger MA, Shub MD, McOmber M, Tsou M, et al. Quality indicators for pediatric colonoscopy: results from a multicenter consortium. Gastrointest Endosc. (2016) 83:533–41. doi: 10.1016/j.gie.2015.06.028
85. Khorana J, Tantivit Y, Phiuphong C, Pattapong S, Siripan S. Foreign body ingestion in pediatrics: distribution, management and complications. Medicina. (2019) 55:686. doi: 10.3390/medicina55100686
86. Fung BM, Sweetser S, Song LMWK, Tabibian JH. Foreign object ingestion and esophageal food impaction: an update and review on endoscopic management. World J Gastrointest Endosc. (2019) 11:174–92. doi: 10.4253/wjge.v11.i3.174
87. Kurowski JA, Kay M. Caustic ingestions and foreign bodies ingestions in pediatric patients. Pediatr Clin N Am. (2017) 64:507–24. doi: 10.1016/j.pcl.2017.01.004
88. Oliva S, Romano C, De Angelis P, Isoldi S, Mantegazza C, Felici E, et al. Foreign body and caustic ingestions in children: a clinical practice guideline. Dig Liver Dis. (2020) 52:1266–81. doi: 10.1016/j.dld.2020.07.016
89. Choe JY, Choe B-H. Foreign body removal in children using foley catheter or magnet tube from gastrointestinal tract. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:132. doi: 10.5223/pghn.2019.22.2.132
90. Low Kapalu CM, Uraizee O, Lerner DG, Thomson M, Attard T. Endoscopist experience with pediatric recurrent and intentional foreign body ingestion (RIFBI): management considerations and future directions. J Pediatr Gastroenterol Nutr. (2024) 78:711–9. doi: 10.1002/jpn3.12114
91. Saito T, Terui K, Mitsunaga T, Nakata M, Kuriyama Y, Higashimoto Y, et al. Role of pediatric endoscopic retrograde cholangiopancreatography in an era stressing less-invasive imaging modalities. J Pediatr Gastroenterol Nutr. (2014) 59:204–9. doi: 10.1097/MPG.0000000000000399
92. Asenov Y, Akın M, Cantez S, Gün Soysal F, Tekant Y. Endoscopic retrograde cholangiopancreatography in children: retrospective series with a long-term follow-up and literature review. Turk J Gastroenterol. (2019) 30(2):192–7. doi: 10.5152/tjg.2018.18165
93. Yang J, Lu L, Jin H, Yang J, Zhang X. Endoscopic management of pancreaticopleural fistula in a pediatric patient: a case report and literature review. Medicine. (2020) 99:e20657. doi: 10.1097/MD.0000000000020657
94. Lin TK, Fishman DS, Giefer MJ, Liu QY, Troendle D, Werlin S, et al. Functional pancreatic sphincter dysfunction in children: recommendations for diagnosis and management. J Pediatr Gastroenterol Nutr. (2019) 69:704–9. doi: 10.1097/MPG.0000000000002515
95. Sun R, Xu X, Zheng Q, Zhan J. Therapeutic endoscopic retrograde cholangiopancreatography for pediatric hepato-pancreato-biliary diseases: a systematic review and meta-analysis. Front Pediatr. (2022) 10:915085. doi: 10.3389/fped.2022.915085
96. Hosseini A, Sohouli MH, Sharifi E, Sayyari A, Sridharan K, Tajalli S, et al. Indications, success, and adverse event rates of pediatric endoscopic retrograde cholangiopancreatography (ERCP): a systematic review and meta-analysis. BMC Pediatr. (2023) 23:596. doi: 10.1186/s12887-023-04392-5
97. Mercier C, Pioche M, Albuisson E, Ponchon T, Gonzalez J-M, Barthet M, et al. Safety of endoscopic retrograde cholangiopancreatography in the pediatric population: a multicenter study. Endoscopy. (2021) 53:586–94. doi: 10.1055/a-1209-0155
98. Usatin D, Fernandes M, Allen IE, Perito ER, Ostroff J, Heyman MB. Complications of endoscopic retrograde cholangiopancreatography in pediatric patients; a systematic literature review and meta-analysis. J Pediatr. (2016) 179:160–165.e3. doi: 10.1016/j.jpeds.2016.08.046
99. Wang X-Q, Kong C-H, Ye M, Diao M. Analysis of the efficacy and safety of endoscopic retrograde cholangiopancreatography for the treatment of pediatric pancreatobiliary diseases. World J Gastrointest Surg. (2024) 16:3754–63. doi: 10.4240/wjgs.v16.i12.3754
100. Tagawa M, Morita A, Imagawa K, Mizokami Y. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound in children. Dig Endosc. (2021) 33:1045–58. doi: 10.1111/den.13928
101. Keane MG, Kumar M, Cieplik N, Thorburn D, Johnson GJ, Webster GJ, et al. Paediatric pancreaticobiliary endoscopy: a 21-year experience from a tertiary hepatobiliary centre and systematic literature review. BMC Pediatr. (2018) 18:42. doi: 10.1186/s12887-017-0959-9
102. Sun B, Yu D, Chen J, Tang Y, Wu H. Endoscopic biliary drainage management for children with serious cholangitis caused by congenital biliary dilatation. Pediatr Surg Int. (2018) 34:897–901. doi: 10.1007/s00383-018-4296-3
103. Keil R, Drábek J, Lochmannová J, Šťovíček J, Koptová P, Wasserbauer M, et al. ERCP In infants, children, and adolescents—different roles of the methods in different age groups. PLoS One. (2019) 14:e0210805. doi: 10.1371/journal.pone.0210805
104. Zeng J-Q, Deng Z-H, Yang K-H, Zhang T-A, Wang W-Y, Ji J-M, et al. Endoscopic retrograde cholangiopancreatography in children with symptomatic pancreaticobiliary maljunction: a retrospective multicenter study. World J Gastroenterol. (2019) 25:6107–15. doi: 10.3748/wjg.v25.i40.6107
105. Wen J, Li T, Liu L, Bie L-K, Gong B. Long-term outcomes of therapeutic ERCP in pediatric patients with pancreas divisum presenting with acute recurrent or chronic pancreatitis. Pancreatology. (2019) 19:834–41. doi: 10.1016/j.pan.2019.08.004
106. Shah R, Cohen RZ, Mekaroonkamol P, Taylor A, Freeman AJ, Fritzen C, et al. Retrospective multicenter matched controlled comparison of endoscopic retrograde cholangiopancreatography in pediatric patients: a 10-year experience. J Pediatr Gastroenterol Nutr. (2020) 70:568–73. doi: 10.1097/MPG.0000000000002632
107. Åvitsland TL, Aabakken L. Endoscopic retrograde cholangiopancreatography in infants and children. Endosc Int Open. (2021) 09:E292–6. doi: 10.1055/a-1337-2212
108. Goetz M, Andersen P, Bergman J, Frei N, Schmidt A, Kähler G, et al. ERCP in babies: low risk of post-ERCP pancreatitis—results from a multicentre survey. United European Gastroenterol J. (2020) 8:77–80. doi: 10.1177/2050640619874187
109. Deng Z, Zeng J, Lv C, Jiang L, Ji J, Li X, et al. Prevalence and factors associated with post-endoscopic retrograde cholangiopancreatography pancreatitis in children. Dig Dis Sci. (2021) 66:224–30. doi: 10.1007/s10620-020-06179-5
110. Perera KDR, Nawarathne NMM, Samarawickrama VT, Deraniyagala MP, Luxman WGE, Fernandopulle ANR. Endoscopic retrograde cholangiopancreatography in children: feasibility, success, and safety with standard adult endoscopes and accessories. Pediatr Gastroenterol Hepatol Nutr. (2022) 25:406. doi: 10.5223/pghn.2022.25.5.406
111. Saraiva RO, Borges VP, Silva MJ, Loureiro R, Capela T, Ramos G, et al. Endoscopic retrograde cholangiopancreatography on pediatric patients: experience of a Portuguese adult gastroenterology department. GE Port J Gastroenterol. (2024) 31:110–5. doi: 10.1159/000529090
112. Gong SC, An S, Shin IS, Jung PY. Usefulness of endoscopic retrograde cholangiopancreatography in the diagnosis and treatment of traumatic pancreatic injury in children. Diagnostics. (2023) 13:2044. doi: 10.3390/diagnostics13122044
113. Li Q, Li S, Hou S, Zhang L, Chen S, Wang J, et al. ERCP-Related adverse events in pediatric patients: a 10-years single-site review. Pediatr Surg Int. (2024) 40:199. doi: 10.1007/s00383-024-05784-z
114. Çirkin G, Akarsu M, Öztürk Y, İyilikçi L, Güler Y, Gülpinar Aydin Ö. Evaluation of endoscopic retrograde cholangiopancreatography in Turkish children. Medicine. (2024) 103:e41045. doi: 10.1097/MD.0000000000041045
115. Wang X, Liu H, Li W, Yang H, Yang J, Bian H, et al. Endoscopic retrograde cholangiopancreatography for the treatment of common bile duct dilatation with choledocholithiasis in children: a single-center retrospective cohort study of 58 cases. BMC Pediatr. (2025) 25:535. doi: 10.1186/s12887-025-05888-y
116. Poddar U, Samanta A, Mohindra S, Upadhyaya VD, Kumar B, Srivastava A, et al. Endoscopic retrograde cholangiopancreatography and endoscopic cystogastrostomy in very young children (aged <5 years): feasibility, success, and safety. DEN Open. (2025) 5:e70085. doi: 10.1002/deo2.70085
117. Batıbay E, Yüksekyayla O, Polat M, Bayhan İ, Sevinç M, Dağ A, et al. Endoscopic retrograde cholangiopancreatography in pediatric population: a decade-long experience from 2 tertiary centers. Turk J Gastroenterol. (2025) 36(5):321–7. doi: 10.5152/tjg.2025.24462
118. Patel S, Marshak J, Daum F, Iqbal S. The emerging role of endoscopic ultrasound for pancreaticobiliary diseases in the pediatric population. World J Pediatr. (2017) 13:300–6. doi: 10.1007/s12519-017-0020-y
119. Scheers I, Ergun M, Aouattah T, Piessevaux H, Borbath I, Stephenne X, et al. Diagnostic and therapeutic roles of endoscopic ultrasound in pediatric pancreaticobiliary disorders. J Pediatr Gastroenterol Nutr. (2015) 61:238–47. doi: 10.1097/MPG.0000000000000692
120. Jazrawi SF, Barth BA, Sreenarasimhaiah J. Efficacy of endoscopic ultrasound-guided drainage of pancreatic pseudocysts in a pediatric population. Dig Dis Sci. (2011) 56:902–8. doi: 10.1007/s10620-010-1350-y
121. Lin TK, Troendle DM, Wallihan DB, Barth B, Fox VL, Fishman DS, et al. Specialized imaging and procedures in pediatric pancreatology: a North American society for pediatric gastroenterology, hepatology, and nutrition clinical report. J Pediatr Gastroenterol Nutr. (2017) 64:472–84. doi: 10.1097/MPG.0000000000001371
122. Cohen S, Kalinin M, Yaron A, Givony S, Reif S, Santo E. Endoscopic ultrasonography in pediatric patients with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. (2008) 46:551–4. doi: 10.1097/MPG.0b013e31815ce571
123. Norris N, Vitale DS. Endoscopic treatments for complicated biliary disease in children. Semin Pediatr Surg. (2025) 34:151500. doi: 10.1016/j.sempedsurg.2025.151500
124. Olmos JI, Oleas R, Alcívar JA, Baquerizo-Burgos J, Robles-Medranda C. Endoscopic ultrasound-guided placement of coils and cyanoacrylate embolization in refractory gastric variceal bleeding: a pediatric case report. Endosc Int Open. (2019) 07:E1061–3. doi: 10.1055/a-0915-9532
125. Téllez-Ávila FI, Duarte-Medrano G, Herrera-Mora D, Lopez-Arce G, Leal-García M, Ramírez-Martínez M, et al. Endoscopic ultrasound in pediatric patients with pancreatobiliary disease. Surg Laparosc Endosc Percutan Tech. (2019) 29:271–4. doi: 10.1097/SLE.0000000000000673
126. Altonbary AY, Hakim H, Elkashef W. Role of endoscopic ultrasound in pediatric patients: a single tertiary center experience and review of the literature. World J Gastrointest Endosc. (2020) 12:355–64. doi: 10.4253/wjge.v12.i10.355
127. Walsh LT, Groff A, Mathew A, Moyer MT. Endoscopic management of large peripancreatic fluid collections in two pediatric patients by endoscopic ultrasound-guided transmural drainage. Pediatr Gastroenterol Hepatol Nutr. (2020) 23:105. doi: 10.5223/pghn.2020.23.1.105
128. Ruan W, Febo-Rodriguez L, Daignault C, Gulati N, Narine K, Sher AC, et al. Endoscopic ultrasound-guided diagnosis of Helicobacter pylori-associated gastric Burkitt’s lymphoma in an adolescent patient: a rare case. Clin J Gastroenterol. (2021) 14:88–91. doi: 10.1007/s12328-020-01278-2
129. Piester TL, Liu QY. EUS in pediatrics: a multicenter experience and review. Front Pediatr. (2021) 9:709461. doi: 10.3389/fped.2021.709461
130. Barakat MT, Foley MA, Gugig R. Initial experience with endoscopic ultrasound-guided coil placement for pediatric gastric variceal hemostasis. J Pediatr Gastroenterol Nutr. (2021) 72:532–7. doi: 10.1097/MPG.0000000000003028
131. Ishii S, Koga H, Saito H, Seo S, Ushio M, Takahashi S, et al. Endoscopic ultrasound-guided hepaticogastrostomy in a seven-year-old girl. Intern Med. (2022) 61:3521–4. doi: 10.2169/internalmedicine.9355-22
132. Barakat MT, Cagil Y, Gugig R. Landscape of pediatric endoscopic ultrasound in a United States tertiary care medical center. J Pediatr Gastroenterol Nutr. (2022) 74:657–61. doi: 10.1097/MPG.0000000000003403
133. Ragab KM, El-Kassas M, Madkour A, Okasha HH, Agwa RH, Ghoneem EA. Safety and efficacy of endoscopic ultrasound as a diagnostic and therapeutic tool in pediatric patients: a multicenter study. Ther Adv Gastrointest Endosc. (2022) 15:26317745221136767. doi: 10.1177/26317745221136767
134. Dalal A, Kamat N, Patil G, Daftary R, Maydeo A. Usefulness of endoscopic ultrasound in children with pancreatobiliary and gastrointestinal symptoms. Endosc Int Open. (2022) 10:E192–9. doi: 10.1055/a-1675-2291
135. Yabe K, Matsuoka A, Nakata C, Hasegawa A, Nakazawa T, Horiuchi A, et al. Mini-probe endoscopic ultrasound for the diagnosis of congenital esophageal or duodenal stenosis. J Med Ultrasonics. (2023) 50:177–85. doi: 10.1007/s10396-023-01281-3
136. Schwartz TS, Mouzaki M, Berklite L, Lopez-Nunez OF, Miethke A, Xanthakos SA, et al. Pediatric endoscopic ultrasound-guided liver biopsy: 3-year experience. J Pediatr Gastroenterol Nutr. (2025) 80:920–5. doi: 10.1002/jpn3.70001
137. Nabi Z, Talukdar R, Chavan R, Basha J, Reddy DN. Outcomes of per-oral endoscopic myotomy in children: a systematic review and meta-analysis. Dysphagia. (2022) 37:1468–81. doi: 10.1007/s00455-022-10409-5
138. Hu J-W, Li Q-L, Zhou P-H, Yao L-Q, Xu M-D, Zhang Y-Q, et al. Peroral endoscopic myotomy for advanced achalasia with sigmoid-shaped esophagus: long-term outcomes from a prospective, single-center study. Surg Endosc. (2015) 29:2841–50. doi: 10.1007/s00464-014-4013-9
139. Miranda García P, Casals Seoane F, Gonzalez J-M, Barthet M, Santander Vaquero C. Per-oral endoscopic myotomy (POEM): a new endoscopic treatment for achalasia. Rev Esp Enferm Dig. (2017) 109(10):719–26. doi: 10.17235/reed.2017.4732/2016
140. Evensen H, Kristensen V, Larssen L, Sandstad O, Hauge T, Medhus AW. Outcome of peroral endoscopic myotomy (POEM) in treatment-naive patients. A systematic review. Scand J Gastroenterol. (2019) 54:1–7. doi: 10.1080/00365521.2018.1549271
141. Tashiro J, Petrosyan M, Kane TD. Current management of pediatric achalasia. Transl Gastroenterol Hepatol. (2021) 6:33. doi: 10.21037/tgh-20-215
142. Nabi Z, Ramchandani M, Reddy DN, Darisetty S, Kotla R, Kalapala R, et al. Per oral endoscopic myotomy in children with achalasia cardia. J Neurogastroenterol Motil. (2016) 22:613–9. doi: 10.5056/jnm15172
143. Mejía R, Sáez J, Aranda F, Pattillo JC, Vuletin JF, Gattini D, et al. Miotomía endoscópica por vía oral (POEM) en un paciente pediátrico para tratamiento de la acalasia esofágica. Rev Chil Ped. (2019) 90:88. doi: 10.32641/rchped.v90i1.884
144. Choné A, Familiari P, Von Rahden B, Desai P, Inoue H, Shimamura Y, et al. Multicenter evaluation of clinical efficacy and safety of per-oral endoscopic myotomy in children. J Pediatr Gastroenterol Nutr. (2019) 69:523–7. doi: 10.1097/MPG.0000000000002432
145. Wood LS, Chandler JM, Portelli KE, Taylor JS, Kethman WC, Wall JK. Treating children with achalasia using per-oral endoscopic myotomy (POEM): twenty-one cases in review. J Pediatr Surg. (2020) 55:1006–12. doi: 10.1016/j.jpedsurg.2020.02.028
146. Saez J, Mejia R, Pattillo JC, Vuletin F, Monrroy H, Jaime F, et al. Per oral endoscopic myotomy (POEM) in pediatric patients with esophageal achalasia: first Latin-American experience. J Pediatr Surg. (2021) 56:706–10. doi: 10.1016/j.jpedsurg.2020.06.007
147. Nabi Z, Ramchandani M, Basha J, Goud R, Darisetty S, Reddy DN. POEM Is a durable treatment in children and adolescents with achalasia cardia. Front Pediatr. (2022) 10:812201. doi: 10.3389/fped.2022.812201
148. Bi Y-W, Lei X, Ru N, Li L-S, Wang N-J, Zhang B, et al. Per-oral endoscopic myotomy is safe and effective for pediatric patients with achalasia: a long-term follow-up study. World J Gastroenterol. (2023) 29:3497–507. doi: 10.3748/wjg.v29.i22.3497
149. Di Nardo G, Calabrese C, Conti Nibali R, De Matteis A, Casciani E, Martemucci L, et al. Enteroscopy in children. United European Gastroenterol J. (2018) 6:961–9. doi: 10.1177/2050640618789853
150. Broekaert I, Tzivinikos C, Narula P, Antunes H, Dias JA, Van Der Doef H, et al. European society for paediatric gastroenterology, hepatology and nutrition position paper on training in paediatric endoscopy. J Pediatr Gastroenterol Nutr. (2020) 70:127–40. doi: 10.1097/MPG.0000000000002496
151. Huang JG, Tanpowpong P. Paediatric gastrointestinal endoscopy in the Asian-Pacific region: recent advances in diagnostic and therapeutic techniques. World J Gastroenterol. (2023) 29:2717–32. doi: 10.3748/wjg.v29.i18.2717
152. Rao MS, Gaur A, Bharadwaj HR, Imran S, Tan JK, Abbas S, et al. The current state of pediatric gastroenterology in under-resourced nations. Ann Med Surg. (2025) 87:2218–28. doi: 10.1097/MS9.0000000000003141
153. Walsh CM, Lightdale JR. Pediatric endoscopy quality improvement network (PEnQuIN) quality standards and indicators for pediatric endoscopy: an ASGE-endorsed guideline. Gastrointest Endosc. (2022) 96:593–602. doi: 10.1016/j.gie.2022.06.016
Keywords: endoscopy in children, gastrointestinal, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, endoscope dimensions, anesthesia
Citation: Zhang G, Zhao G, Li P and Zhang S (2025) The application of gastrointestinal endoscopy in children: a narrative review. Front. Pediatr. 13:1691692. doi: 10.3389/fped.2025.1691692
Received: 24 August 2025; Accepted: 10 November 2025;
Published: 24 November 2025.
Edited by:
Fei Liu, Guangzhou Medical University, ChinaReviewed by:
Brett J. Hoskins, Indiana University School of Medicine, United StatesJenifer R. Lightdale, Boston Children’s Hospital, United States
Copyright: © 2025 Zhang, Zhao, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Li, bGlwZW5nQGNjbXUuZWR1LmNu; Shutian Zhang, emhhbmdzaHV0aWFuQGNjbXUuZWR1LmNu
 Guo Zhang
Guo Zhang Guiping Zhao
Guiping Zhao Peng Li
Peng Li Shutian Zhang
Shutian Zhang