- 1Department of Pediatrics, Ningbo Yinzhou No. 2 Hospital, Ningbo, China
- 2Department of Pediatrics, Ninghai First Hospital, Ningbo, China
Objective: This study aims to compare the systemic inflammatory response index (SIRI) and the systemic immune-inflammatory index (SII) in pediatric community–acquired pneumonia (CAP) caused by the respiratory syncytial virus (RSV) and Mycoplasma pneumoniae (Mp).
Methods: The study included 120 children with single RSV infection (RSV group) and 120 children with single Mp infection (Mp group). The SIRI and SII were calculated by using the formulation neutrophil × monocyte/lymphocyte and platelet × neutrophil/lymphocyte, respectively.
Results: Significant differences were found between RSV and Mp infections in three age-stratified subgroups of <6 months, 3–5 years, and ≥5 years (p < 0.05). The proportions of children presenting with nasal stuffiness and rhinorrhea, shortness of breath, grunting, and gastrointestinal symptoms were markedly higher in the RSV group than those in the Mp group (p < 0.05). The RSV group had lower values of the SIRI and SII than the Mp group (p = 0.010; p = 0.021). The RSV group demonstrated significantly higher values in terms of duration of symptoms before admission, length of hospital stay, proportion of children requiring oxygen supplementation, and proportion of children with severe pneumonia compared with the Mp group (p < 0.05). The incidence of bronchial pneumonia and emphysema was significantly higher in the RSV group than that in the Mp group (p = 0.005; p = 0.001). However, the incidence of patchy shadow was significantly higher in the Mp group than that in the RSV group (p = 0.027).
Conclusion: The SIRI and SII may offer additional value in differentiating CAP associated with RSV and Mp infections in children; both indices may serve as easily accessible blood indicators for clinical decision-making in the management of pediatric CAP.
Introduction
Community-acquired pneumonia (CAP) is the most common cause of both hospitalization and death in children aged under the age of 5 worldwide (1). There were close to 0.22 episodes of CAP per child-year in the year 2010 in low- and middle-income countries, and 10%–17% of these new cases required hospitalization (2). The etiology of childhood CAP is variable and alters according to age and disease severity. Mycoplasma pneumoniae (Mp) is the most common bacterial pathogen detected in children hospitalized with CAP, while respiratory syncytial virus (RSV) is the most commonly detected pathogen (3). With regard to radiological findings to predict the etiology of CAP, lung consolidation is a poor predictor of typical bacterial infection. Pleural effusion was noted to be the best predictor of typical bacterial CAP, but it is far too rare to aid in etiology prediction (4). Prompt and reliable identification of the underlying pathogen is important for reducing diagnostic uncertainty and rationally prescribing antimicrobial treatment for CAP patients requiring hospitalization (5). However, distinguishing between bacterial and viral etiologies of CAP is still challenging in many clinical settings.
In general, microbiological testing is recommended for etiological diagnosis of CAP requiring hospitalization, but gold standard diagnostic tests are often costly and at times unavailable in low-resource settings (6). Alternative reliable and easily accessible laboratory-based predictors are critical to improve discrimination between bacterial and viral etiologies of childhood CAP in low-income and middle-income countries where microbiological testing is unavailable (7). Several serum inflammatory markers, such as C-reactive protein (CRP) and procalcitonin, may be useful for distinguishing between bacterial and viral etiologies of CAP; however, they display a low negative predictive value (8). Therefore, there is an urgent requirement to accelerate the diagnosis and evaluate the prognosis of CAP as well as identify markers with the better potential to differentiate CAP etiology.
Recent evidence has indicated that the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic inflammatory response index (SIRI), and systemic immune-inflammatory index (SII) may provide additional diagnostic and prognostic value for many inflammatory conditions in children, including acute bronchiolitis (9) and sepsis (10). The SIRI, derived from NLR×MLR, and SII, calculated as NLR×PLR, may serve as beneficial biomarkers for predicting the occurrence of necrotizing pneumonia among children with CAP (11). In addition, both the SII and the SIRI were higher in non-survivors of CAP than in survivors, indicating their prognostic value in CAP management (12). A previous study showed that the SII is more sensitive and specific than the NLR, PLR, and SIRI in predicting CAP severity (13). However, limited studies have used these indices to distinguish between bacterial and viral etiologies of childhood CAP. In the present study, we compared SIRI and SII levels in children with CAP associated with RSV and Mp infections.
Methods
Study subjects
The study retrospectively examined 240 children with CAP who were admitted to Ningbo Yinzhou No. 2 Hospital between December 2024 and June 2025. The study was approved by the Ethics Committee of Ningbo Yinzhou No. 2 Hospital. Informed consent from the patients was waived because of the retrospective nature of this study. The inclusion criteria were as follows: (i) a diagnosis of CAP associated with RSV or Mp infection; (ii) duration of illness of 7 days or less; (iii) no prior hospital admission; and (iv) age between 2 months and 14 years. In individuals who were previously well and acquired an infection outside the hospital, a diagnosis of CAP was confirmed based on evidence of acute respiratory tract infection accompanied by radiological findings of lung consolidation, other infiltrate, or pleural effusion by a certified radiologist (14). RSV infection was confirmed based on RSV-positive results of nasopharyngeal swabs using a direct immunofluorescence assay and real-time PCR (Roche, Switzerland). Mp infection was confirmed by serological testing for IgM and IgG antibodies using enzyme-linked immunosorbent assay (ELISA), as well as detection of Mp DNA content by PCR (Roche, Switzerland). The exclusion criteria of this study were as follows: (i) co-infection involving multiple viral pathogen and bacterial pathogen; (ii) radiological findings of lung cavitation by other causes, such as lung abscess, congenital lung abnormalities, or septic pulmonary embolism; (iii) chronic lung disorders, such as cystic fibrosis or bronchiolitis obliterans, malignancy, or primary or severe acquired immunodeficiency; (iv) neurological or neuromuscular illnesses; (v) hospital-acquired pneumonia; or (vi) incomplete medical records.
Calculation of the SIRI and SII
Samples of venous blood were taken from the patients within the first 24 h following admission. The SIRI integrates three types of inflammatory cells—neutrophils, monocytes, and lymphocytes—and is calculated using the formulation neutrophil × monocyte/lymphocyte. The SII integrates platelets, neutrophils, and lymphocytes and is calculated using the formulation platelet × neutrophil/lymphocyte. Platelet, neutrophil, monocyte, and lymphocyte counts were measured using an automated hematology analyzer (Sysmex XN2000; Sysmex, Kobe, Japan) and reported to be 109 cells/L.
Sample size evaluation and statistical analysis
Sample size calculation was computed through a priori power analysis using the G*power software (version 3.1.9.2). To achieve a power of 90% for detecting the difference at a two-sided α level of 0.05, 86 patients were required for each group (172 in total). Accounting for 20% missing data, 120 participants per group were determined to achieve the required power value. Descriptive statistics included medians with an interquartile range (IQR) for non-normally distributed continuous variables and proportions or frequencies for categorical variables. Median data with IQR were analyzed by using non-parametric tests (Mann–Whitney U test). Categorical variables were analyzed using the chi-square test. The obtained data were statistically processed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). A p-value of <0.05 (two tailed) denoted a significant difference.
Results
Comparison of demographic characteristics between RSV and Mp infections
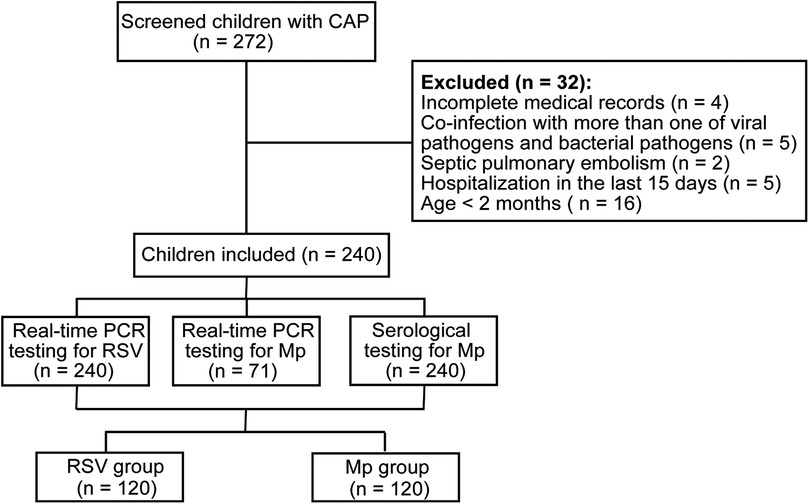
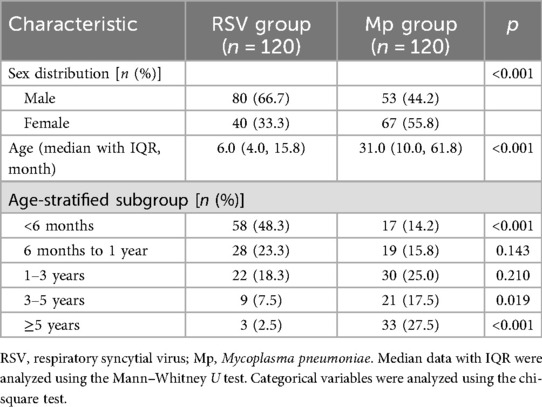
We initially screened 272 children with CAP, of whom 32 were excluded from the final analysis (Figure 1). There were 240 children with CAP in the final analysis, of whom 120 had single RSV infection (RSV group) and 120 had single Mp infection (Mp group). The RSV group consisted of 80 males and 40 females and the Mp group had 53 males and 67 females, suggesting significant differences in sex distribution between RSV and Mp infections (p < 0.05). The median value of age in the RSV group was 6.0 months and the median value of age in the Mp group was 31.0 months, indicating significant differences in age distribution between RSV and Mp infections in children (p < 0.05). In addition, significant differences were found in RSV and Mp infections among the three age-stratified subgroups: <6 months, 3–5 years, and ≥5 years (p < 0.05; Table 1).
Comparison of clinical characteristics between RSV and Mp infections
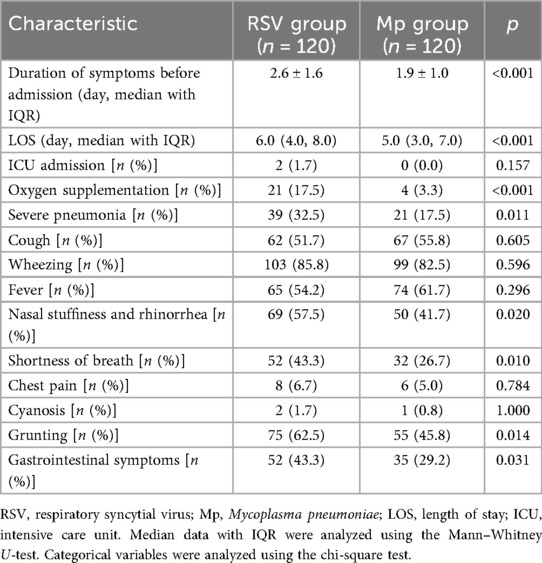
As shown in Table 2, the duration of symptoms before admission, length of hospital stay, and proportion of children requiring oxygen supplementation were significantly higher in the RSV group than in the Mp group (p < 0.001). According to the World Health Organization (WHO) classification of childhood pneumonia, severe pneumonia is defined as the presence of chest indrawing. There were 39 cases of severe pneumonia in the RSV group against 21 cases in the Mp group. There were significantly more cases of severe pneumonia in the RSV group than in the Mp group (p = 0.011, Table 2). The signs or symptoms of acute respiratory tract infections caused by RSV and Mp included cough, wheezing, fever, nasal stuffiness and rhinorrhea, shortness of breath, chest pain, cyanosis, grunting, and gastrointestinal presentations. The proportion of children presenting nasal stuffiness and rhinorrhea, shortness of breath, grunting, and gastrointestinal symptoms was markedly higher in the RSV group than in the Mp group (p < 0.05, Table 2).
Comparison of SIRI and SII levels between RSV and Mp infections
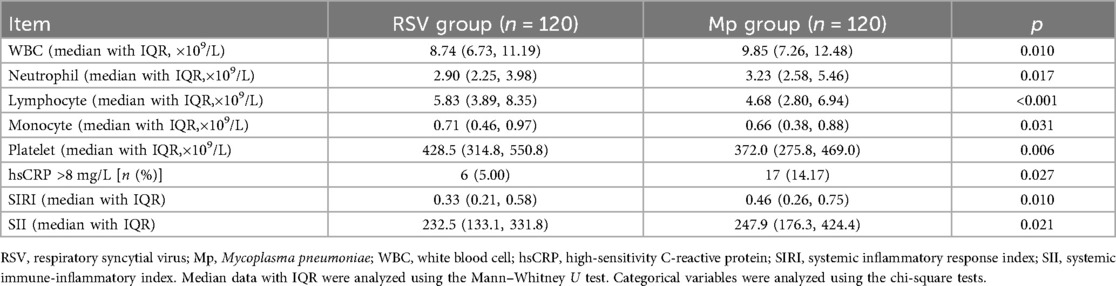
According to the laboratory findings listed in Table 3, the RSV group exhibited fewer counts of white blood cells (WBCs), neutrophils, and lymphocytes than the Mp group (p = 0.010; p = 0.017; p < 0.001). More monocytes and platelets were observed in the RSV group than in the Mp group (p = 0.031; p = 0.006). The proportion of children with high-sensitivity CRP (hsCRC) values >8 mg/L was lower in the RSV group than in the Mp group (p = 0.027). According to the formulations of the SIRI and SII, the RSV group had lower values of the SIRI and SII than the Mp group (p = 0.010; p = 0.021; Table 3). Considering the significant age difference between children with RSV and Mp infections, we performed age-stratified differences of the SIRI and SII. Because of only three children in the age group of ≥5 years presenting with RSV infection, we categorized the age groups as follows in this analysis: <6 months, 6 months to 1 year, and >1 year. As shown in Table 4, the RSV group exhibited lower values of the SIRI and SII than the Mp group in the age stratifications of <6 months and >1 year. No significant difference was observed in these values between RSV and Mp infections with regard to the age group of 6 months to 1 year.
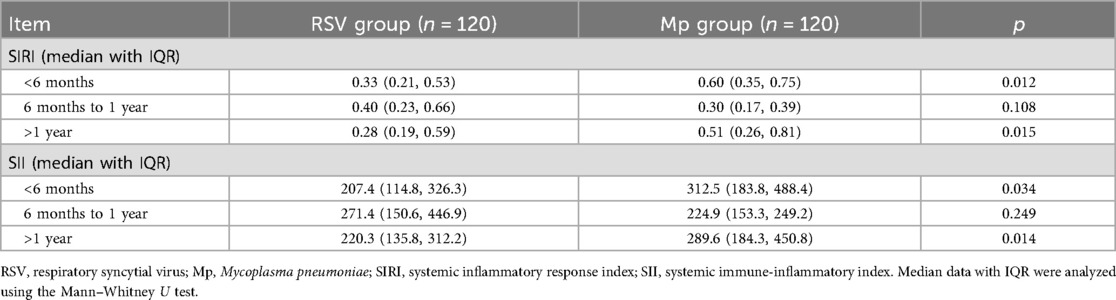
Table 4. Age-stratified differences (<6 months, 6 months to 1 year, and >1 year) of the SIRI and SII between RSV and Mp infections.
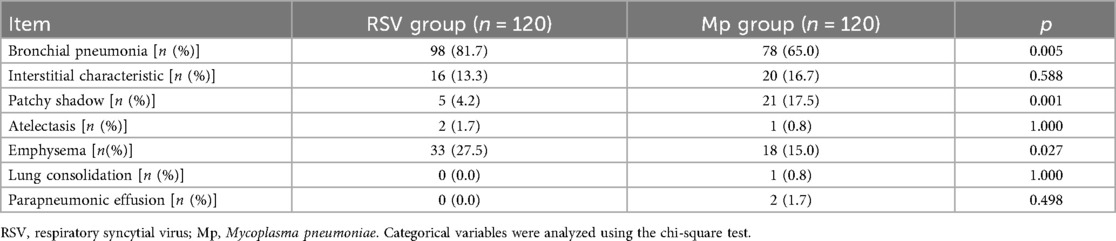
Comparison of radiological findings between RSV and Mp infections
According to the radiological findings of RSV and Mp infections (Table 4), the incidence of bronchial pneumonia and emphysema was significantly higher in the RSV group than in the Mp group (p = 0.005; p = 0.001). The incidence of patchy shadow was significantly higher in the Mp group than in the RSV group (p = 0.027, Table 5). No significant differences were found in the incidence of interstitial characteristics, atelectasis, lung consolidation, and parapneumonic effusion between children with RSV and Mp infections.
Discussion
The present study investigated the roles of SIRI and SII levels in distinguishing between the bacterial and viral etiologies of pediatric CAP. The main finding of the study revealed that children with Mp infection had higher values of the SIRI and SII than those with RSV infection, indicating the potential clinical application of the SIRI and SII as accessible markers in pediatric CAP management.
Earlier work indicated significant differences in bacterial and viral etiologies of CAP according to different demographics and geographic locations (15). Age has been considered one of the most important characteristics to predict RSV and Mp infections (16). RSV (34.0%) was the most common etiological pathogen of CAP in children under the age of 2, accounting for 34% of the total cases. Mp was the most common cause of CAP in children aged 2–18 years, accounting for 45.3% of the total cases. In the present study, children aged <6 months accounted for the highest proportion (48.3%) with RSV infections and children aged >3 years accounted for the highest proportion (45%) with Mp infections. The prevalence and infection patterns of respiratory pathogens in childhood CAP are presented in an age-dependent manner. However, a previous meta-analysis (17) found that respiratory viruses were frequently detected in CAP among children of all ages and geographical regions; this indicates that while age can be included in a predictive model of bacterial and viral etiologies of childhood CAP, it cannot be considered an independent predictor.
The severity of CAP varies with RSV infection. Clinically, symptoms may manifest as mild upper respiratory tract infections or asymptomatic, or even as bronchiolitis or pneumonia. Some severely ill children may present with feeding difficulties, shortness of breath, nasal stuffiness and rhinorrhea, and even respiratory failure, requiring ventilator-assisted treatment (18). In mild cases of Mp infection, patients may exhibit fever and cough. Radiological studies of Mp infection have revealed bronchopneumonia, interstitial pneumonia, increased hilar shadow, and cloudy infiltrative changes. In severe cases of Mp infection, patients may present with persistent high fever, extensive pulmonary consolidation or atelectasis, pleural effusion, and multiple systemic functional impairments outside the lungs (19). In the present study, we also found RSV to be most frequently associated with severe CAP, which concurs with the results of a previous study (14). The main pathological changes of RSV infection include mucosal congestion and edema, necrosis of respiratory epithelium, lymphocyte infiltration, cilia deficiency, neutrophilic inflammation, and fibrin embolism. These pathological changes may be associated with ventilation dysfunction (small airway obstruction) and imbalance in the ventilation-to-perfusion ratio (intrapulmonary shunt) (20). RSV mainly damages bronchioles with a diameter of 75–300 µm and is more likely to cause airway stenosis and ventilation dysfunction, thus leading to severe symptoms such as wheezing and expiratory dyspnea (21). RSV infection, particularly severe manifestations, often occurs in children under 6 months of age who have poor respiratory compensatory capacity due to narrow respiratory tracts and tender mucous membranes (22). Severe pneumonia caused by Mp infection is mainly attributed to a strong immune inflammatory response (23). Severe illness caused by Mp infection mainly occurs in school-aged children, as children at this age have relatively mature immune function development and a strong immune response (24).
Although the NLR, MLR, and PLR were previously used to differentiate between viral and bacterial pneumonia and predict outcomes, no ideal biomarker has yet been reported (25). The novel inflammatory biomarkers, the SIRI and SII, have recently been described as more sensitive in predicting a poor prognosis in patients with colorectal and esophageal cancer compared with the NLR and PLR (26, 27). The SIRI integrates three blood cell subtypes—neutrophils, monocytes, and lymphocytes. The SII utilizes the absolute counts of neutrophils, platelets, and lymphocytes. The SII and SIRI reflect the balance between inflammation and immune response, both of which play important roles in predicting various outcomes across disease conditions in which the inflammatory process is the primary process (28). Mp is regarded as one of the bacterial pathogens—in addition to Streptococcus pneumoniae and Staphylococcus aureus—that is responsible for necrotizing pneumonia (29). Elmeazawy et al. (11) noted that both the SII and the SIRI could predict the occurrence of necrotizing pneumonia in children, upon performing an analysis on admitted patients. The severity of Mp infection is closely correlated with the extent of the host's immune-inflammatory response; therefore, immune-related parameters are more likely to exhibit changes during Mp infection compared with RSV infection. In our study, children with RSV infection exhibited lower values of the SIRI and SII than those with Mp infection. Therefore, Mp infection exhibits a stronger immune inflammatory response compared with RSV infection.
Our study has some limitations. No significant differences were observed in SIRI and SII values between RSV and Mp infections in the age group of 6 months to 1 year, which is possibly the result of the small sample size in this age group or population from a single medical center. The retrospective nature of this study also limits the ability to infer causality between these biomarkers and CAP severity.
In conclusion, the findings of the present study suggest that the SIRI and SII may provide additional value to distinguish between CAP associated with RSV and Mp infections in children. These two inflammatory biomarkers—the SII and SIRI—may serve as easily accessible blood indicators for clinical decision-making in pediatric CAP management. More in-depth and well-designed large-scale studies are required to confirm whether both biomarkers can be integrated into a predictive model of bacterial and viral etiologies of childhood CAP. In addition, the role of the SII and SIRI in monitoring the treatment efficacy of Mp infection and the incidence of antimicrobial resistance can also be investigated in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Ningbo Yinzhou No. 2 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study was a retrospective one.
Author contributions
H-HZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. K-LQ: Resources, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors, wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meyer Sauteur PM. Childhood community-acquired pneumonia. Eur J Pediatr. (2024) 183:1129–36. doi: 10.1007/s00431-023-05366-6
2. Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. (2013) 3:010401. doi: 10.7189/jogh.03.010401
3. Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol. (2018) 90:421–8. doi: 10.1002/jmv.24963
4. Arnold SR, Jain S, Dansie D, Kan H, Williams DJ, Ampofo K, et al. Association of radiology findings with etiology of community acquired pneumonia among children. J Pediatr. (2023) 261:113333. doi: 10.1016/j.jpeds.2023.01.010
5. Mardian Y, Menur Naysilla A, Lokida D, Farida H, Aman AT, Karyana M, et al. Approach to identifying causative pathogens of community-acquired pneumonia in children using culture, molecular, and serology tests. Front Pediatr. (2021) 9:629318. doi: 10.3389/fped.2021.629318
6. Lokida D, Farida H, Triasih R, Mardian Y, Kosasih H, Naysilla AM, et al. Epidemiology of community-acquired pneumonia among hospitalised children in Indonesia: a multicentre, prospective study. BMJ Open. (2022) 12:e057957. doi: 10.1136/bmjopen-2021-057957
7. Farida H, Triasih R, Lokida D, Mardian Y, Salim G, Wulan WN, et al. Epidemiologic, clinical, and serum markers may improve discrimination between bacterial and viral etiologies of childhood pneumonia. Front Med (Lausanne). (2023) 10:1140100. doi: 10.3389/fmed.2023.1140100
8. Wrotek A, Robakiewicz J, Pawlik K, Rudzinski P, Pilarska I, Jaron A, et al. The etiology of community-acquired pneumonia correlates with serum inflammatory markers in children. J Clin Med. (2022) 11:5506. doi: 10.3390/jcm11195506
9. Kizilsoy OF, Korkmaz MF, Senkan GE, Bozdemir SE, Korkmaz M. Relationship between the systemic immune-inflammatory index and the severity of acute bronchiolitis in children. Lab Med. (2024) 55:169–73. doi: 10.1093/labmed/lmad055
10. Aydogan S, Dilli D, Soysal C, Akduman H, Orun UA, Tasar M, et al. Role of systemic immune-inflammatory index in early diagnosis of sepsis in newborns with CHD. Cardiol Young. (2022) 32:1826–32. doi: 10.1017/S1047951122001202
11. Elmeazawy R, Ayoub D, Morad LM, El-Moazen AMF. Role of systemic immune-inflammatory index and systemic inflammatory response index in predicting the diagnosis of necrotizing pneumonia in children. BMC Pediatr. (2024) 24:496. doi: 10.1186/s12887-024-04818-8
12. Baran B, Yetkin NA, Rabahoglu B, Tutar N, Gulmez I. Assessment of mortality risk in patients with community-acquired pneumonia: role of novel inflammatory biomarkers. J Clin Lab Anal. (2025) 39:e70081. doi: 10.1002/jcla.70081
13. Wang S, Wan Y, Zhang W. The clinical value of systemic immune inflammation Index (SII) in predicting the severity of hospitalized children with Mycoplasma pneumoniae pneumonia: a retrospective study. Int J Gen Med. (2024) 17:935–42. doi: 10.2147/IJGM.S451466
14. Liu YN, Zhang YF, Xu Q, Qiu Y, Lu QB, Wang T, et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. (2023) 4:e330–9. doi: 10.1016/S2666-5247(23)00031-9
15. Li ZJ, Zhang HY, Ren LL, Lu QB, Ren X, Zhang CH, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. (2021) 12:5026. doi: 10.1038/s41467-021-25120-6
16. Lee E, Kim CH, Lee YJ, Kim HB, Kim BS, Kim HY, et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis. (2020) 20:132. doi: 10.1186/s12879-020-4810-9
17. Pratt MTG, Abdalla T, Richmond PC, Moore HC, Snelling TL, Blyth CC, et al. Prevalence of respiratory viruses in community-acquired pneumonia in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2022) 6:555–70. doi: 10.1016/S2352-4642(22)00092-X
18. Zheng G, Zhan C, Pan H, Lu Y, Zhang H, Xu X, et al. Genetic diversity of respiratory syncytial virus in children with community-acquired pneumonia in Guangzhou: an epidemiological update. Pediatr Res. (2025). doi: 10.1038/s41390-025-04214-7
19. Yang S, Lu S, Guo Y, Luan W, Liu J, Wang L. A comparative study of general and severe Mycoplasma pneumoniae pneumonia in children. BMC Infect Dis. (2024) 24:449. doi: 10.1186/s12879-024-09340-x
20. Sitthicharoenchai P, Alnajjar S, Ackermann MR. A model of respiratory syncytial virus (RSV) infection of infants in newborn lambs. Cell Tissue Res. (2020) 380:313–24. doi: 10.1007/s00441-020-03213-w
21. Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol. (2015) 235:266–76. doi: 10.1002/path.4462
22. Antillon M, Li X, Willem L, Bilcke J, RESCEU investigators, Jit M, et al. The age profile of respiratory syncytial virus burden in preschool children of low- and middle-income countries: a semi-parametric, meta-regression approach. PLoS Med. (2023) 20:e1004250. doi: 10.1371/journal.pmed.1004250
23. Zhu Y, Luo Y, Li L, Jiang X, Du Y, Wang J, et al. Immune response plays a role in Mycoplasma pneumoniae pneumonia. Front Immunol. (2023) 14:1189647. doi: 10.3389/fimmu.2023.1189647
24. Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol. (2016) 7:800. doi: 10.3389/fmicb.2016.00800
25. Ng WW, Lam SM, Yan WW, Shum HP. NLR, MLR, PLR and RDW to predict outcome and differentiate between viral and bacterial pneumonia in the intensive care unit. Sci Rep. (2022) 12:15974. doi: 10.1038/s41598-022-20385-3
26. Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2018) 65:503–10. doi: 10.1016/j.intimp.2018.10.002
27. Xie QK, Chen P, Hu WM, Sun P, He WZ, Jiang C, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. (2018) 16:273. doi: 10.1186/s12967-018-1638-9
28. Islam MM, Satici MO, Eroglu SE. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: an extensive literature review. Turk J Emerg Med. (2024) 24:8–19. doi: 10.4103/tjem.tjem_198_23
Keywords: community-acquired pneumonia, respiratory syncytial virus, mycoplasma pneumonia, systemic inflammatory response index, systemic immune-inflammatory index
Citation: Zhou H-H and Qian K-L (2025) Comparison of the systemic inflammatory response index and the systemic immune-inflammatory index in pediatric community–acquired pneumonia caused by respiratory syncytial virus and Mycoplasma pneumoniae. Front. Pediatr. 13:1694856. doi: 10.3389/fped.2025.1694856
Received: 29 August 2025; Accepted: 30 October 2025;
Published: 21 November 2025.
Edited by:
Maurizio Aricò, Azienda Sanitaria Locale di Pescara, ItalyReviewed by:
Inke Nadia D. Lubis, University of North Sumatra, IndonesiaRupalakshmi Vijayan, St Elizabeth Hospital, United States
Copyright: © 2025 Zhou and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Hui Zhou, emhoMTk5MjEwMDlAMTYzLmNvbQ==
 Hui-Hui Zhou
Hui-Hui Zhou Kai-Li Qian
Kai-Li Qian