- 1Department of Laboratory Medicine, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
Atypical Hand-foot-mouth disease (HFMD), which is caused predominantly by enteroviruses (EVs) shares overlapping cutaneous manifestations with varicella, a condition triggered by varicella-zoster virus (VZV) and further complicates diagnosis. This study systematically characterized the clinical features, hematological profiles, and infection patterns (single, mixed and sequential infection) of pediatric patients with poorly distinguishable EV and VZV infection to improve diagnostic accuracy and optimize clinical management strategies. A retrospective analysis was conducted on the cases that were difficult to distinguish and were confirmed by polymerase chain reaction (PCR) from 2021 to 2025, including demographics, symptoms, hematological profiles, and infection patterns. Among the 85 patients, 33 had a single EV infection and 46 had a single VZV infection, with 4 mixed infections and 2 sequential infections. Compared with patients in the VZV group, those in the EV group were more often associated with local rashes on the limbs and around the mouth, often accompanied by oral vesicles. In contrast, patients with VZV infection usually exhibited pruritic, widespread skin rashes across the body. Virological testing identified CV-A6 as the dominant EV serotype (51.52%). Hematological profiles revealed increased atypical lymphocytes in patients with VZV infection compared with those with EV infection. Patients with mixed infection maybe correlated with more extensive skin lesions. The clinical differentiation of poorly distinguishable EV and VZV infection presents significant challenges, especially in children with mixed infection or atypical features. In the absence of early virological testing, it is hoped that this study can provide some guidance for the diagnosis of HFMD and chickenpox that are difficult to distinguish or have mixed infection.
Introduction
Enterovirus (EV) and varicella-zoster virus (VZV) are two prominent viral pathogens that commonly affect children worldwide. Hand‒foot‒mouth disease (HFMD), which is classically associated with EV-A71 and Coxsackievirus A16 (CV-A16), manifests as febrile illness accompanied by nonpruritic maculopapular or vesicular eruptions localized on the palmar, plantar, and oral regions (1). However, recent epidemiological studies have highlighted the rise of atypical HFMD variants driven by emerging serotypes such as Coxsackievirus A6 (CV-A6). These strains exhibit a broader anatomical distribution, involving the trunk and limbs, and produce vesiculobullous lesions resembling those of varicella (2–5), thereby complicating clinical differentiation (6).
In contrast, the hallmark centrifugal exanthem progression of chickenpox under VZV infection follows a distinct temporal trajectory: successive waves of pruritic macules, papules, vesicles, and crusted lesions coexist simultaneously within the same anatomical field (7). While virologically divergent, the increasing convergence of cutaneous manifestations in patients with atypical EV infection and modified varicella presentations creates diagnostic ambiguities (8), particularly in endemic regions where both viruses exhibit seasonal cocirculation. These clinical parallels necessitate a critical reevaluation of traditional syndromic classification frameworks.
Conventional laboratory diagnostic methods, such as complete blood count (CBC), often fail to adequately distinguish between EV and VZV infection because of significant overlap in hematological profiles. For example, despite a higher incidence of atypical lymphocytes in VZV patients, this parameter lacks specificity for definitive diagnosis. This diagnostic insufficiency underscores the imperative for molecular confirmation via polymerase chain reaction (PCR) and serotype-specific assays. Notably, CV-A6 has emerged as the predominant EV serotype in severe HFMD cases, underscoring its clinical significance in pediatric populations.
The diagnostic complexity increases further in cases of viral coexistence, which is a relatively rare report but is associated with severe clinical manifestations, including extensive dermatological involvement, prolonged illness duration, and heightened risks of complications such as encephalitis (9, 10). Sequential infection, where one virus precedes the other, present temporal patterns that may reflect potential virus‒virus interactions (11). These interactions, although not fully understood, likely modulate disease severity and immune responses, warranting further investigation.
This investigation systematically compares the clinical and laboratory characteristics of pediatric EV and VZV infection, with a focus on distinguishing atypical HFMD presentations and understanding the impact of mixed infection and sequential infection. By identifying distinguishing markers and analyzing infection patterns, this research seeks to improve diagnostic strategies, inform clinical management and contribute to a broader understanding of virus‒virus interactions.
Materials and methods
Study design
Children with HFMD or chickenpox who could not be distinguished at the first visit to West China Second Hospital from January 2021 to July 2025 were retrospectively analyzed. This was subsequently confirmed by RT-PCR testing as HFMD, chickenpox or both. Patients with chronic conditions or immunodeficiency that could affect clinical outcomes were excluded. Patient records were reviewed for clinical presentation, laboratory findings, and virological tests (RT-PCR for EV and VZV) at the first visit. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of West China Second University Hospital, Sichuan University [Medical Research 2024 Ethics Approval (279)]. Owing to the nature of retrospective studies, informed consent from individual patients was waived. Patient data confidentiality was maintained, and all analyses were performed on de-identified data to ensure privacy.
For the purpose of this study, sequential infection was defined as two separate, laboratory-confirmed (by PCR) symptomatic infection with EV and VZV occurring in the same individual, separated by a time interval of at least 14 days. This criterion was established based on the typical incubation and illness periods of both viruses to ensure that the second infection represented a new, independent event after the complete resolution of symptoms (including skin lesions) from the first infection. Mixed infection was defined as the simultaneous detection of both EV and VZV nucleic acids in a sample collected during a single disease episode.
Data collection
Data collected from medical records included demographic information (age, sex), clinical symptoms (fever, rash characteristics such as location, type, pruritus, oral or pharyngeal lesions, and other associated symptoms), laboratory tests, and virological testing. Laboratory tests focused on complete blood count (CBC), including leukocyte counts and percentages of neutrophils, lymphocytes, and monocytes, with specific attention to the presence of atypical lymphocytes.
Virological testing
Nucleic acid isolation followed pathogen-specific protocols: EV RNA was extracted via the DAAN GENE Viral RNA Kit (Guangzhou, China), whereas VZV DNA was performed via the Sansure Nucleic Acid Extraction System (Changsha, China). Real-time PCR amplification and quantification were conducted on an ABI 7500 Fast System (Thermo Fisher Scientific, USA) via commercial multiplex assays for detecting and analyzing EVs (universal EVs, EV-A71, CV-A16, CV-A6 and CV-A10) (DAAN GENE, China) and VZV (Sansure, China). The sample results were determined to be positive or negative following the instructions of the respective kits.
Statistical analysis
The differences in clinical features and laboratory results between the EV group and VZV group were assessed using appropriate statistical tests. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. Continuous variables, which were not normally distributed and the relatively small sample size of the cohort, were compared using the nonparametric Mann–Whitney U test. Given the exploratory nature of this study aimed at characterizing clinical features, and considering the modest sample size, no adjustments for multiple comparisons were made. The reported P values should therefore be interpreted descriptively and with caution, as they are intended to highlight potential differences rather than to confirm definitive associations. To address this deficiency, we no longer solely rely on the P value. Instead, for categorical variables comparisons, we calculated and reported the effect size, such as odds ratio and its 95% confidence interval. Statistical analyses were conducted via GraphPad Prism 8.0 and SPSS software 19.0, and graphical representations were generated via GraphPad Prism 8.0. A value of P < 0.05 was considered significant.
Results
Overview of the hardly distinguishable cases between HFMD and chickenpox
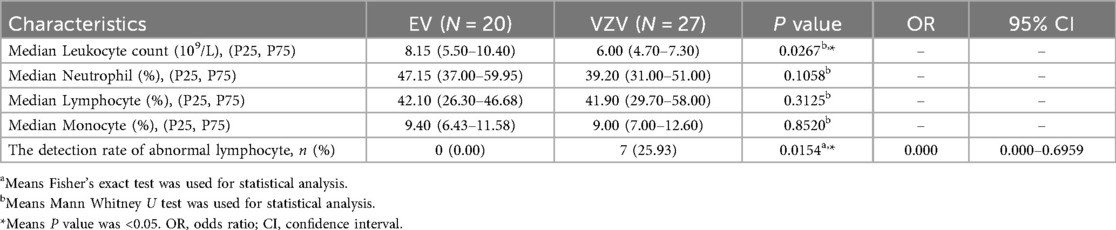
To analyze the clinical characteristics of pediatric patients with hardly distinguishable HFMD-infected EV and chickenpox-infected VZV, we categorized the patients into four groups: EV infection, VZV infection, mixed infection and sequential infection with both viruses. A total of 85 patients were included: 33 with EV infection, 46 with VZV infection, 4 with mixed infection and 2 with a sequential infection involving both viruses (Figure 1A). Among the EV cases, CV-A6 was the predominant serotype, identified in 17 of the 33 patients (Figure 1B).
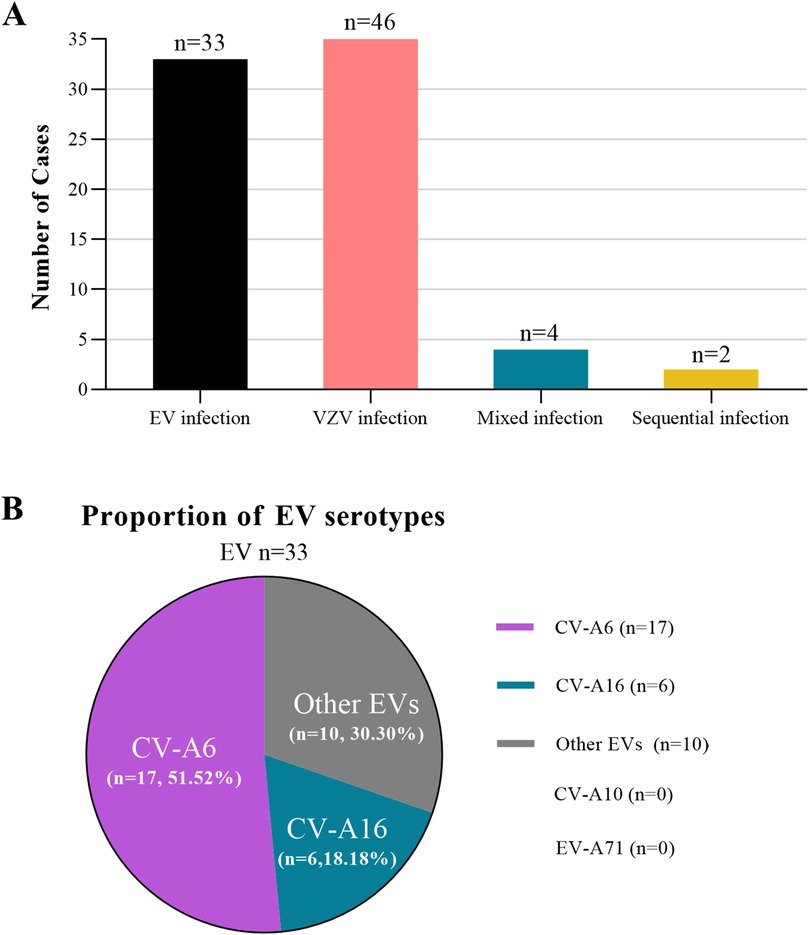
Figure 1. Overview of the hardly distinguishable cases between HFMD and chickenpox. (A) The four hardly distinguishable HFMD and chickenpox groups including laboratory-confirmed EV infection, laboratory-confirmed VZV infection, mixed infection and sequential infection with both viruses. (B) Proportion of EV serotypes in the EV group.
In our cohort, RT-PCR testing for SARS-CoV-2 (COVID-19) was performed in eight participants at the time of enrolment and all results were negative. No systematic screening for other viral or bacterial co-infection was undertaken. We recognise this as a limitation in interpreting co-infection status.
Demographics and clinical presentations of the patients whose cases were difficult to distinguish between HFMD and chickenpox
Table 1 provides a comparative overview of the demographic data and clinical manifestations between the EV- and VZV-infected groups (Table 1). There was a greater percentage of males in both groups, with 63.64% in the EV group and 69.57% in the VZV group. The median age of patients in the EV group (31 months, IQR: 14–42) was lower than that of patients in the VZV group (72 months, IQR: 12–96), although this age difference was not statistically significant.
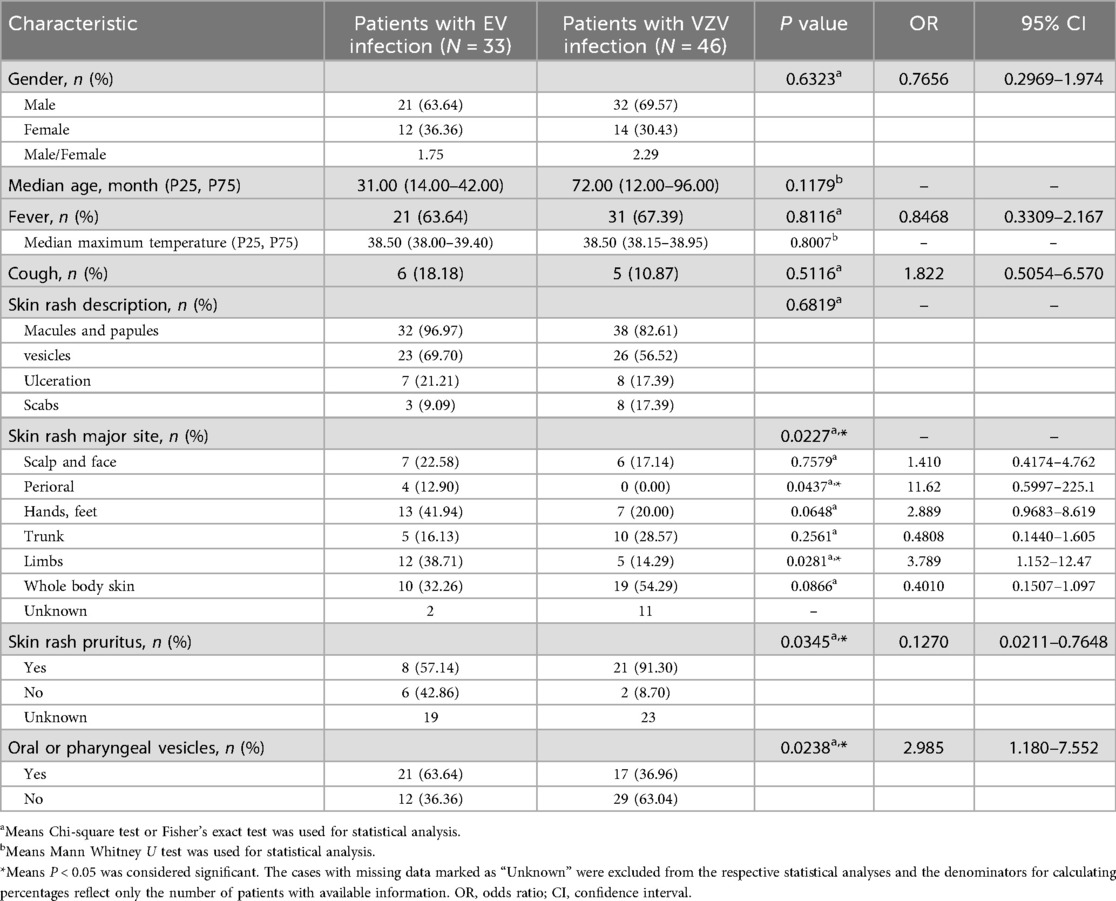
Table 1. Demographic characteristics and clinical manifestations at first visit of patients infected with EV or VZV virus.
Fever was observed in 63.64% of patients with EV infection and 67.39% of patients with VZV infection, with median maximum temperatures of 38.50°C (IQR: 38.00–39.40) and 38.5°C (IQR: 38.15–38.95), respectively. Additionally, 18.18% of EV cases and 10.87% of VZV cases presented with cough. Skin rashes were not significantly different between the two groups. The skin rash types included macules and papules (96.97% in EV, 82.61% in VZV), vesicles (69.70% in EV, 56.52% in VZV), ulceration (21.21% in EV, 17.39% in VZV) and scabs (9.09% in EV, 17.39% in VZV). Pruritic rashes were reported in 57.14% of EV cases and 91.30% of VZV cases, a significant difference (P = 0.0345). Oral or pharyngeal vesicles were more common among EV patients (63.64%) than among VZV patients (36.96%, P = 0.0238). Notably, skin rash involvement was more widespread in VZV infection, affecting the whole body (54.29%), whereas skin lesions in patients with EV infection were more common in the limbs (38.71%, P = 0.0281) and perioral regions (12.90%, P = 0.0437).
Hematological profiles of the hardly distinguishable cases between HFMD and chickenpox
As shown in Table 2, the hematological profiles overlapped significantly between the groups. It should be noted that laboratory data were available for a subset of patients (20/33 in the EV group and 27/46 in the VZV group) as complete blood count testing was not performed on all patients at the initial visit. Among the available data, neutrophil percentages, lymphocyte percentages and monocyte percentages did not differ significantly. Leukocyte count in the EV group was higher than that in the VZV group. However, atypical lymphocytes were detected in 25.93% of VZV cases but were absent in EV infection (P = 0.0154), suggesting a potentially significant marker for VZV.
Sequential and mixed infection patterns
Table 3 highlights infection patterns in patients with mixed and sequential infection. Among the six patients, two presented with anterior‒posterior sequential infection. A 4-year-old female first developed EV-associated symptoms (fever, rash on hands and feet, oral lesions). After the complete resolution of the initial EV infection, she subsequently developed VZV symptoms (pruritic trunk and facial rash) 19 days later. Similarly, a 14-month-old male initially presented with VZV. Following the full resolution of the varicella rash, he developed EV symptoms after 16 days.
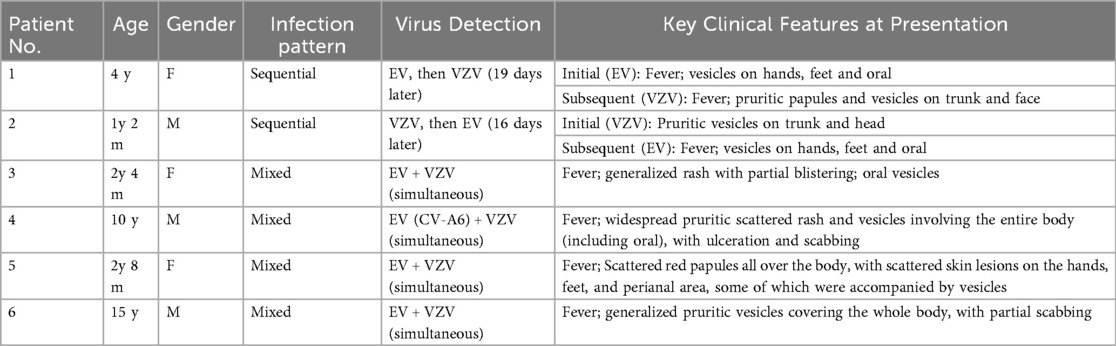
Table 3. The clinical presentation at first visit of individual patients with sequential or mixed EV and VZV infection.
Both the 28-month-old female and the 15-year-old male who were infected with both EV and VZV presented with generalized rashes or vesicles on their bodies. A 10-year-old male with a CV-A6-positive EV presented with extensive rashes with ulceration and scabbing across the body. Another patient, a 32-month-old female, presented with rashes involving the entire body, including the perioral, hands, feet, and perianal regions, accompanied by vesicles and pruritus. These findings suggest that mixed infection lead to more widespread dermatological involvement.
Discussion
Typical HFMD and varicella can often be distinguished by the distribution and characteristics of the rash, whereas atypical HFMD and chickenpox with similar clinical characteristics are often difficult to distinguish. Our findings analyzed the clinical characteristics, hematological profiles, and infection patterns of pediatric patients with cases of HFMD and chickenpox that were difficult to distinguish, providing valuable insights into their differentiation. Specifically, oral mucosal lesions and rashes localized to the limbs and perioral regions were more characteristic of EV infection at the patient's initial visit, whereas pruritic or widespread skin rashes were more indicative of VZV infection. Despite these distinctions, the overlap in clinical presentations, particularly in atypical cases, underscores the diagnostic challenges of relying solely on clinical symptoms for accurate diagnosis.
The laboratory results further highlighted the challenges in differentiating these clinically ambiguous HFMD and chickenpox cases. While neutrophil and lymphocyte percentages showed no significant difference between the EV and VZV groups, two distinct hematological patterns emerged. Patients with EV infection tended to present with a higher leukocyte count. This could reflect a stronger initial inflammatory response, commonly triggered by enterovirus. In contrast, Patients with VZV infection showed a key differentiator: atypical lymphocytes were detected in 25.93% of VZV cases but were completely absent in the EV group (P = 0.015). This finding aligns well with the known lymphotropism of VZV and the specific immune response it provokes, often leading to the appearance of these activated lymphocytes in the blood. Therefore, while total leukocyte count itself offers limited help in distinguishing these infections, the presence of atypical lymphocytes appears to be a more specific indicator pointing towards VZV in diagnostically uncertain scenarios.
Reports of patients co-infected with VZV and enterovirus were limited. Na's case mentioned that a 11-year-old girl who was infected with both VZV and enterovirus developed polymorphic rashes on her face and trunk, single-shaped painful pustules on her palms and soles, and painful ulcers in her mouth (9). Even in another case report, it was discovered that a 14-year-old male youth was simultaneously infected with the varicella-zoster virus and enterovirus, which caused meningitis (10). In our study, four cases of co-infection were identified. All these cases presented with extensive dermatological involvement, including vesicular rashes, ulceration, or scabbing across the body. These findings suggest that mixed infection may lead to more widespread dermatological involvement. It is important to note that the number of cases with mixed infection in our study was very small, which limits the statistical power and the generalizability of our conclusions. The small sample size increases the susceptibility to selection bias and makes it challenging to draw definitive conclusions about the prevalence or distinct clinical course of co-infection compared to single infection. Future studies with larger cohorts are necessary to validate these preliminary observations and to better understand the epidemiology and clinical impact of EV and VZV co-infection. Reports of sequential infection patterns were rare. Whether EV infected first or VZV infected first, the clinical symptoms presented were all related to the corresponding pathogen in our cases. It should be noted that patients with virus-virus co-infection or sequential infection may not be uncommon, as there were not sufficient widespread diagnostic tools available in the past for a complete diagnosis.
It is worth noting that although only 8 participants underwent RT-PCR testing for SARS-CoV-2 and all were negative, we cannot rule out subclinical or other viral/bacterial co-infection, which may modulate host-pathogen interactions. Prior vaccination against SARS-CoV-2 may shift baseline immunity and affect susceptibility or response to other infection (12), even if vaccination status was not uniformly recorded. Moreover, the rapid evolution of SARS-CoV-2 lineages across continents (13) underscores that variant-specific transmissibility or immune evasion could influence co-infection risk or immune-state dynamics. Persistent or long-term SARS-CoV-2 infection (14, 15) may leave residual immune dysfunction or viral reservoirs, thereby altering host responses to additional pathogens. Finally, diagnostic limitations, including low viral load infection, variant escape from standard assays, and incomplete screening of other pathogens (16), mean that undetected co-infections or persistent states might bias the interpretation of our findings. Together, these factors recommend cautious interpretation of causality in our results and support further studies with comprehensive pathogen screening, vaccination history, viral genome analysis, and longitudinal follow-up.
Notably, Coxsackievirus A6 (CV-A6) was predominant in the EV infection group and was detected in 51.52% of the patients. CV-A6 has been identified as a leading serotype in severe and atypical cases of HFMD (17, 18), often mimicking varicella-like presentations with extensive rashes involving the trunk and limbs (3). This overlap complicates the differentiation of HFMD from chickenpox, while atypical chickenpox presentations mimicking HFMD have also been reported (8). The emergence of CV-A6 as a predominant serotype in our study is consistent with recent studies showing that CV-A6 is more likely to cause severe dermatological manifestations and can overlap with symptoms observed in VZV infection (17, 18). This highlights the importance of including CV-A6-specific testing in the diagnostic workup for pediatric patients presenting with atypical HFMD, as it may lead to better differentiation from VZV-related conditions.
The findings of our study have significant implications for improving the diagnosis and management of pediatric EV and VZV infection, especially in regions where both viruses cocirculate. Advanced virological diagnostic methods, such as RT‒PCR and serotype-specific testing, are essential for accurate identification and differentiation. By providing a clearer understanding of the clinical and laboratory features distinguishing EV and VZV infection, as well as highlighting the potential severity of mixed and sequential infection, this research offers essential insights that could improve patient outcomes and treatment strategies.
However, our study has several limitations. Its retrospective design and relatively small sample size may have limited the detection of statistically significant differences between the two groups, particularly in terms of subtle clinical markers. Additionally, the study's focus on a pediatric cohort restricts the generalizability of the findings to adult populations or immunocompromised patients, who may exhibit different clinical or immune responses to EV and VZV infection. As a single-center study, our findings may be subject to selection bias and may not be fully generalizable to other populations or healthcare settings. The absence of follow-up data limits our ability to comment on the long-term outcomes and potential sequelae of these infection, particularly in cases of mixed or sequential infection. Furthermore, while we explored common clinical and laboratory markers, more in-depth investigations into inflammatory biomarkers or cytokine profiles could provide a deeper understanding of the pathophysiology of these infections.
Future studies should aim to include larger, multicenter cohorts with diverse age groups and immune statuses to increase the generalizability of the findings. Advanced diagnostic tools, such as next-generation sequencing, and a deeper exploration of immunological responses in coinfected and sequentially infected patients could significantly refine our understanding of these infections. Investigating the immunopathological mechanisms of EV and VZV interactions will be crucial for improving therapeutic strategies and managing severe cases more effectively.
In conclusion, this study highlights the challenges of distinguishing between EV and VZV infection on the basis of clinical presentation alone, particularly in cases of atypical HFDM, chickenpox or mixed infection. These findings emphasize the importance of early virological testing and serotype-specific diagnostics in improving diagnostic accuracy and informing treatment strategies. The identification of CV-A6 as a predominant serotype in atypical HFMD adds complexity to the clinical landscape, suggesting the need for further research into the full spectrum of EV serotypes and their clinical implications. On the other hand, it is hoped that this study can provide some guidance for the diagnosis of HFMD and chickenpox that are difficult to distinguish or have mixed infection in the absence of early virological testing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Clinical Research Ethics Committee of West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Owing to the nature of retrospective studies, informed consent from patients was waived. Patient data confidentiality was maintained, and all analyses were performed on deidentified data to ensure privacy.
Author contributions
JD: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. YZ: Methodology, Software, Visualization, Writing – original draft. QY: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Xinya Research Foundation from West China Second University Hospital, Sichuan University (Grant No. KX200 to QY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li XW, Ni X, Qian SY, Wang Q, Jiang RM, Xu WB, et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr. (2018) 14(5):437–47. doi: 10.1007/s12519-018-0189-8
2. Yasui Y, Makino T, Hanaoka N, Owa K, Horikoshi A, Tanaka A, et al. A case of atypical hand-foot-and-mouth disease caused by coxsackievirus A6: differential diagnosis from varicella in a pediatric intensive care unit. Jpn J Infect Dis. (2013) 66(6):564–6. doi: 10.7883/yoken.66.564
3. Chen Y, Dai B, Han S, Duan G, Yang H, Chen S, et al. Arising concerns of atypical manifestations in patients with hand, foot, and mouth disease. Vaccines (Basel). (2023) 11(2):405. doi: 10.3390/vaccines11020405
4. Sapia EY, Maroni C, Groisman C, Kromer H, Lihue Rojo G, Dastugue M, et al. Atypical hand-foot-mouth disease virus genotyping in a pediatric hospital in Buenos Aires city, Argentina. Arch Argent Pediatr. (2020) 118(2):e199–203. doi: 10.5546/aap.2020.e199
5. Yan X, Zhang ZZ, Yang ZH, Zhu CM, Hu YG, Liu QB. Clinical and etiological characteristics of atypical hand-foot-and-mouth disease in children from Chongqing, China: a retrospective study. BioMed Res Int. (2015) 2015:802046. doi: 10.1155/2015/802046
6. Buttery VW, Kenyon C, Grunewald S, Oberste MS, Nix WA. Atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6–Minnesota, 2014. MMWR Morb Mortal Wkly Rep. (2015) 64(29):805. doi: 10.15585/mmwr.mm6429a8
7. Kennedy PGE, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. (2018) 10(11):609. doi: 10.3390/v10110609
8. Osawa M, Umemoto N, Tajima N, Sugawara H, Nishida J, Kakurai M, et al. Atypical varicella mimicking hand-foot-mouth disease in an adult patient with malignant lymphoma during chemotherapy. Br J Dermatol. (2004) 151(1):254–6. doi: 10.1111/j.1365-2133.2004.06077.x
9. Na SY, Son YM, Lee HY, Baek JO, Roh JY, Lee JR. A case of varicella combined with hand-foot-mouth disease in a healthy child. Ann Dermatol. (2009) 21(1):98–101. doi: 10.5021/ad.2009.21.1.98
10. Drăgoi AL, Nemeș RM. A unique case of double meningitis with enterovirus and reactivated varicella-zoster virus in a male teenager. Diagn Microbiol Infect Dis. (2024) 110(1):116409. doi: 10.1016/j.diagmicrobio.2024.116409
11. Chia JK, Chia AA. Varicella-zoster virus reactivation during acute enterovirus infection is associated with CD8 lymphocytopenia. BMJ Case Rep. (2009) 2009:bcr08.2008.0803. doi: 10.1136/bcr.08.2008.0803
12. Xie J, Ye F, Deng X, Tang Y, Liang JY, Huang X, et al. Circular RNA: a promising new star of vaccine. J Transl Intern Med. (2023) 11(4):372–81. doi: 10.2478/jtim-2023-0122
13. Hyug Choi J, Sook Jun M, Yong Jeon J, Kim HS, Kyung Kim Y, Ho Jeon C, et al. Global lineage evolution pattern of sars-cov-2 in Africa, America, Europe, and Asia: a comparative analysis of variant clusters and their relevance across continents. J Transl Intern Med. (2023) 11(4):410–22. doi: 10.2478/jtim-2023-0118
14. Ewing AG, Salamon S, Pretorius E, Joffe D, Fox G, Bilodeau S, et al. Review of organ damage from COVID and long COVID: a disease with a spectrum of pathology. Med Rev (2021). (2025) 5(1):66–75. doi: 10.1515/mr-2024-0030
15. Guo M, Shang S, Li M, Cai G, Li P, Chen X, et al. Understanding autoimmune response after SARS-CoV-2 infection and the pathogenesis/mechanisms of long COVID. Med Rev (2021). (2024) 4(5):367–83. doi: 10.1515/mr-2024-0013
16. Kang S, Zheng R. Distribution of the causes of fever of unknown origin in China, 2013–2022. J Transl Intern Med. (2024) 12(3):299–307. doi: 10.2478/jtim-2024-0008
17. Yang X, Li Y, Zhang C, Zhan W, Xie J, Hu S, et al. Clinical features and phylogenetic analysis of severe hand-foot-and-mouth disease caused by coxsackievirus A6. Infect Genet Evol. (2020) 77:104054. doi: 10.1016/j.meegid.2019.104054
Keywords: hand-foot-mouth disease, chickenpox, clinical diagnosis, children, coinfection
Citation: Yang Q, Zhang Y and Duan J (2025) Clinical features of poorly distinguishable HFMD and chickenpox in children: a retrospective analysis. Front. Pediatr. 13:1706776. doi: 10.3389/fped.2025.1706776
Received: 16 September 2025; Revised: 19 November 2025;
Accepted: 25 November 2025;
Published: 10 December 2025.
Edited by:
Zhongjie Shi, Wayne State University, United StatesReviewed by:
Heloisa Helena De Sousa Marques, University of São Paulo, BrazilMd Rifat Al Mazid Bhuiyan, Rajshahi Medical College, Bangladesh
Copyright: © 2025 Yang, Zhang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaxin Duan, amlheGluX2R1YW5Ac2luYS5jbg==; Yiteng Zhang, OTc0NzcyNjk1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Qiuxia Yang
Qiuxia Yang Yiteng Zhang1,2*†
Yiteng Zhang1,2*† Jiaxin Duan
Jiaxin Duan