- 1Department of Pediatrics, Division of Pediatric Cardiology, Izmir Democracy University Buca Seyfi Demirsoy Teaching and Research Hospital, Izmir, Türkiye
- 2Department of Pediatrics, Division of Neonatology, Izmir City Hospital, Izmir, Türkiye
Background: Hemodynamically significant patent ductus arteriosus (hPDA) in premature infants is a common congenital cardiac anomaly associated with substantial morbidity and mortality. Traditional diagnostic methods like echocardiography face challenges such as expertise requirement and inconsistent accessibility. This study investigates the efficacy of the Random Forest machine learning model in predicting hPDA in premature infants, aiming to provide a non-invasive, objective, and reliable alternative.
Methods: This retrospective study analyzed data from 657 premature infants hospitalized between 2014 and 2019. Patients were categorized into hPDA and asymptomatic PDA (aPDA) groups. The Random Forest classification model, implemented in JASP software, utilized prenatal, natal, and postnatal clinical data, including gestational week, birth weight, and the need for resuscitation at birth. Model performance was assessed using metrics such as accuracy, Area Under the Curve, F1 score, Matthews Correlation Coefficient, recall, precision, and feature importance.
Results: The Random Forest model demonstrated strong predictive performance, achieving a test accuracy of 91.7%, an AUC of 0.950, an F1 score of 0.923, and an MCC of 0.775. Notably, the recall for the hPDA group was 100%. Gestational week, birth weight, and the need for resuscitation at birth were identified as the most significant predictors. The model also revealed complex relationships, showing variables deemed statistically insignificant by classical methods (e.g., gender, 5th-minute APGAR score, oligohydramnios) to be significant within the Random Forest framework.
Conclusions: The Random Forest model effectively predicts hPDA risk in premature infants, offering superior predictive power compared to classical statistical analyses. This approach has the potential to enhance early detection, facilitate timely interventions, and support personalized treatment strategies, thereby improving patient outcomes. Further validation through large-scale, multi-center prospective studies is essential for its integration into clinical practice.
1 Introduction
Patent ductus arteriosus(PDA) in premature infants constitutes a prevalent congenital cardiac anomaly linked to substantial morbidity and mortality (1). More precisely, hemodynamically significant PDA commonly affects premature neonates during the initial days of life and has the potential to induce severe systemic consequences, particularly in very low birth weight and highly premature infants (2, 3). As a result, this condition is associated with a range of comorbidities, including chronic lung disease, bronchopulmonary dysplasia, necrotizing enterocolitis, impaired renal function, intraventricular hemorrhage, and elevated mortality rates (4). Consequently, the timely and precise detection of patent ductus arteriosus in premature infants is paramount for mitigating potential complications and enhancing clinical management strategies (5).
Among the traditional diagnostic methods used for premature PDA, echocardiography is considered the gold standard (6). However, this method also presents certain diagnostic challenges and limitations (5). The most significant of these challenges include the requirement for high expertise and its inconsistent accessibility (1). These limitations particularly hinder the continuous monitoring of PDA in premature infants within intensive care units, where hemodynamic status is dynamically changing (3). Consequently, there is a growing need for non-invasive, objective, and reliable alternative methods that can support clinical decision-making processes and enhance diagnostic accuracy (7). In this context, machine learning algorithms offer the potential to analyze extensive clinical data, uncover complex relationships, and develop predictive models (8).
Despite the availability of diverse therapeutic modalities, including pharmacological interventions and surgical procedures and transcatheter closure the ongoing debate and lack of a definitive consensus on the most appropriate management approach for premature PDA continue to persist in current neonatological practice (3, 9–11). This absence of a standardized strategy contributes to divergent treatment paradigms and variable clinical outcomes, further exacerbated by insufficient investigation into the utility of echocardiographic variables for predicting clinical outcomes, especially in the early stages (3).
There is no international consensus on the diagnosis and management of hemodynamically significant patent ductus arteriosus (12–14). This situation primarily stems from the insufficient investigation into the utility of echocardiographic variables for predicting clinical outcomes, especially in the early stages (4, 15). This ambiguity leads to divergent treatment approaches and outcomes, underscoring the necessity for a standardized approach to PDA in premature infants (3, 16). In this context, machine learning algorithms, particularly the Random Forest model, may offer a potential solution for the early prediction of hemodynamically significant PDA, independently of PDA treatments.
This study rigorously investigates the efficacy of the Random Forest model for accurately predicting hemodynamically significant PDA in premature infants. By leveraging a comprehensive dataset comprising pre-delivery, peri-delivery, and post-delivery clinical data alongside physical examination findings from neonates monitored in the neonatal intensive care unit, this research seeks to overcome current diagnostic limitations and facilitate earlier, more targeted interventions.
2 Materials and methods
2.1 Data collection
This study was constructed from retrospective data with the objective of predicting hemodynamically significant patent ductus arteriosus in premature infants. The model encompasses historical data from the prenatal, natal, and postnatal periods, alongside clinical metrics and physical examination results. Data for this study were sourced from 657 patients, hospitalized with a diagnosis of prematurity in the Neonatal Intensive Care Unit of İzmir Medical Point hospital from 2014 to 2019. Patients in the neonatal intensive care unit were monitored by a neonatology specialist, and the diagnosis of PDA was confirmed by a pediatric cardiology specialist. Adhering to Turkish Neonatology Guidelines, the approach to hemodynamically significant PDA (hPDA) in these patients was established through a consensus involving neonatology and pediatric cardiology specialists (5). According to the guideline, treatment for hPDA was initiated in patients presenting with specific clinical and echocardiographic findings. Clinically, these findings included systemic hypotension, pulmonary hyperperfusion, systemic hypoperfusion, dependence on respiratory support, and the development of metabolic acidosis. Echocardiographically, the criteria comprised an LA/Ao ratio >1.5, a PDA diameter >2 mm, abnormal diastolic flow patterns, retrograde flow, ductal steal, and left atrial enlargement (5). The detailed data acquisition process involved gathering each patient's demographic profile, prenatal narrative, birth records, postnatal clinical trajectory, and laboratory analyses.
2.2 Patients groups
PDA was present in all patients' initial echocardiograms. Patients were divided into two groups according to their follow-ups based on echocardiographic and clinical findings. The first group consisted of premature infants diagnosed with hPDA, while the second group included patients in whom hemodynamic PDA was not detected by echocardiography or clinical findings, cases of spontaneous closure during hospitalization, or those not requiring medical and surgical treatment, categorized as the asymptomatic PDA (aPDA) group.
This distinction was made to optimally test both clinically and the machine learning RF model's ability to differentiate hemodynamically significant PDA from aPDA. This grouping is a critical step for evaluating the predictive power of the Random Forest model within the JASP application in distinguishing hemodynamically significant hPDA from non-hemodynamic aPDA.
2.3 Data selection
To rigorously identify the most impactful predictors and optimize the model's performance, the authors subjected the data illustrated in Figure 1 to comprehensive analysis within the Random Forest model, systematically exploring various manual data combinations via the JASP program. From the perspective of the hPDA prediction model, we prioritized prenatal and natal values from these parameters. The variables found to most effectively predict hemodynamically significant PDA(hPDA), necessitating medical or surgical ligation, and distinguishing it from the hemodynamically insignificant aPDA group, included: gender, low birth weight, reduced gestational age, requirement for CPR at birth, Apgar score at five minutes, presence of oligohydramnios, chorioamnionitis, multiple gestations, maternal smoking habits, and the manifestation of bronchopulmonary dysplasia (BPD).
2.4 Statistical analysis and machine learning
Statistical analyses and machine learning methodologies were employed to assess the data using the open-source JASP 0.95.2 software (17). Continuous variables were reported as mean ± standard deviation for parametric distributions and as medians for non-parametric distributions; categorical variables were presented as counts and percentages. The Kolmogorov–Smirnov test was performed to ascertain the normality of data distribution. Bivariate relationships between variables were assessed using a simple correlation test. Differences among categorical variables were investigated through chi-square analysis. For quantitative comparisons, Student's t-test and ANOVA were utilized for normally distributed parameters, while the Mann Whitney U and Kruskal Wallis tests were applied for parameters demonstrating non-normal distribution. A p-value of <0.05 was designated as statistically significant for all analyses.
The Random Forest algorithm, located under the classification section of the machine learning tab in the JASP program, will be employed for machine learning. The dataset will be allocated as follows: 65% for the training set, 20% for the validation set, and 15% for the test set. Key metrics to be recorded include Support, Accuracy, Precision, Recall, False Positive Rate, False Discovery Rate, F1 Score, Matthews Correlation Coefficient, Area Under Curve, Negative Predictive Value, True Negative Rate, and False Negative Rate. Furthermore, for the machine learning model, Mean Decrease in Accuracy, Total increase in Node Purity, and Mean dropout loss values of the features will be documented. This study designates the hemodynamically significant hPDA group (comprising patients treated with medical closure and surgical ligation) and the hemodynamically insignificant, spontaneously resolving aPDA group as the target variables.
3 Results
3.1 Distribution of demographic and clinical variables according to PDA presence
When a total of 657 preterm cases under 37 weeks were examined, hemodynamically PDA was not detected in 487 cases, while it was present in 170 cases.
A comparative analysis of fundamental clinical and demographic variables between infants with hPDA and aPDA revealed notable disparities. Gestational age was significantly reduced in the hPDA cohort, averaging 27(25–28) weeks, in contrast to 29(27–31) weeks in the aPDA cohort (p < 0.001). Correspondingly, birth weight was markedly lower in the hPDA group 895(705–1189 gr) vs. an average of 1,120(873–1,357) g in the aPDA group (p < 0.001). Assessment of APGAR scores indicated comparable 5th-minute scores across both groups [aPDA: 7(7–8), hPDA: 7(7–8); p:0.177], yet 1st-minute scores were marginally depressed in the hPDA group [aPDA: 6(5–7), hPDA: 6(4–7); p:0.007]. Clinical course evaluation demonstrated prolonged hospitalization for hPDA cases [aPDA: 49(30–72) days, hPDA: 61(27–92) days; p:0.005]. Furthermore, the duration of mechanical ventilation was substantially elevated in the hPDA group [aPDA: 0(0–2) days, hPDA: 2(0–7) days; p < 0.001]. (Table 1)
Regarding the gender distribution in Table 1, the aPDA group comprised 246 males and 241 females, while the hPDA group included 82 males and 88 females, with no significant difference observed between the two groups in terms of gender (p = 0.609). There were 92 cases requiring CPR at birth, and 45 of these had hPDA, which yielded a statistically significant result in the hemodynamically significant hPDA group (p < 0.001). Oligohydramnios was present in 30 cases in the aPDA group and 14 cases in the hPDA group (p:0.351); conversely, cases without oligohydramnios were dominant in both groups. Similarly, the presence of chorioamnionitis was detected in a limited number of cases in both groups, and mostly this condition was not observed (p:0.206). No significant difference was found when examining the multiple pregnancy variable (p:0.051). Concerning maternal smoking, a history of smoking was found in 36 cases in the aPDA group and 23 cases in the hPDA group, which was statistically significant (p:0.016). When evaluating the distribution of bronchopulmonary dysplasia, among a total of 287 patients diagnosed with BPD, 106 had hPDA, and this also resulted in a higher prevalence of BPD in all patients with hPDA, which was statistically significant (p:<0.001; Table 1).
3.2 Machine learning results
In the analysis conducted using the Random Forest classification model, generated by running the JASP program on the dataset comprising 657 registered patients, the JASP program automatically evaluated a total of 370 cases. The data were automatically split within the JASP program into a training set (63.7%), a validation set (21%), and a test set (14.8%). The model's accuracy rate was found to be 91.7% on the test set (Table 2, Figure 2).
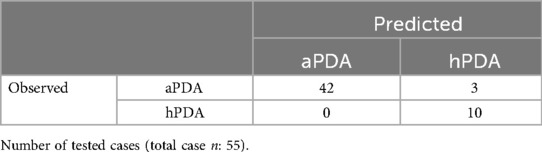
Upon examining the confusion matrix, 42 of the 45 cases aPDA were correctly classified, while 3 cases were incorrectly classified as hPDA. All 10 cases with existing hPDA were correctly predicted. This result indicates that the model has high sensitivity, especially in distinguishing hPDA cases (Table 3).
In the model's performance evaluation, accuracy was found to be 91.7%. The high Area Under the ROC curve (AUC = 0.950) indicates significant discriminative power between the two classes. The Recall value was found to be 100% for the hPDA group (no false negatives) and 93.3% for the aPDA group. Precision values were 100% for hPDA negative and 76.9% for hPDA positive, suggesting a limited number of false positives in the positive class. F1 scores were 0.947 (negative), 0.800 (positive), and an average of 0.923, respectively, revealing that the model exhibits a balanced and strong performance in terms of both sensitivity and precision, especially with a very low risk of missing clinically critical positive cases. These results indicate that the model is particularly strong in detecting hPDA positive cases, but its precision value is somewhat limited due to the relatively low number of positive class instances. A negative predictive value of 66.7% suggests that the model's assessment of aPDA group should be evaluated more carefully (Table 4).
When feature importance metrics were analyzed, birth weight and gestational week emerged as the most influential factors within the model. These variables demonstrated the highest contributions to both node purity increase and mean decrease in accuracy criteria. Furthermore, while the impact of birth resuscitation need, oligohydramnios, chorioamnionitis, bronchopulmonary dysplasia, multiple pregnancy, maternal smoking, gender, and the 5th-minute APGAR score was comparatively lower, it is notable that the mean dropout loss value for the APGAR score was relatively high. In this context, variables such as oligohydramnios, chorioamnionitis, BPD, multiple pregnancy, gender, and 5th-minute Apgar score, which had zero Mean decrease in accuracy and Total increase in Node Puirty values, exhibited high Mean dropout loss scores. Although these data were less influential in the Random Forest tree, they were retained in the final model due to their elevated Mean dropout loss values. These findings underscore that birth weight and gestational week are the most robust predictors for assessing PDA risk in neonates, concurrently indicating that other factors provide modest yet significant contributions to the model's overall predictive capability (Table 5).
4 Discussion
Our study utilized Random Forest assessment within the machine learning framework. Prior to Random Forest modeling, an evaluation of these data using classical statistical methods indicated that p-values were not consistently significant across all variables. Specifically, while gestational week, birth weight, maternal smoking, CPR requirement, 1st-minute APGAR score, and BPD demonstrated statistical significance, factors such as gender, 5th-minute APGAR score, oligohydramnios, chorioamnionitis, and multiple pregnancy were deemed insignificant (Table 1). The Random Forest model effectively identified a range of significant variables, including those like gender, 5th-minute APGAR score, oligohydramnios, chorioamnionitis, and multiple pregnancy, which traditional statistical analyses had previously dismissed as insignificant. This striking divergence underscores the Random Forest model's superior capacity to discern intricate, complex relationships that are often overlooked by conventional approaches, thereby establishing a critical and previously unappreciated role for these factors in hPDA prediction. Our research illustrates how machine learning can convert statistically non-significant data into valuable insights. This underscores the capacity of machine learning models to uncover latent patterns and multi-variable interactions within biomedical datasets, particularly where conventional statistical analyses prove inadequate (8). Consequently, this phenomenon in machine learning warrants further in-depth investigation through new studies, with a call for more comprehensive analysis of data across medical and other domains. These findings robustly affirm that the Random Forest model can offer predictive capabilities extending beyond classical statistical analyses, making substantial contributions to clinical decision-making, particularly in pediatric populations and intricate pathophysiological contexts (18).
In the evaluation of outcomes derived from the Random Forest model, the F1 score and Matthews Correlation Coefficient scores are highlighted as prominent metrics. Research in the existing literature underscores the crucial role of F1 score and MCC values in assessing model efficacy (19, 20). F1 score and MCC values approaching 1 indicate a higher predictive probability of the model. Beyond solely considering MCC and F1 scores, the inclusion of AUC results in the evaluation of machine learning models contributes to improved accuracy. The AUC, defined as the area beneath the Receiver Operating Characteristic curve, encapsulates a model's performance across its full range of classification thresholds and demonstrates greater resilience to class imbalances (21). The text points to existing literature that underscores the criticality of these metrics, citing an example from a heart failure classification study where a Random Forest method achieved high accuracy with an AUC of 0.97 and an MCC of 0.83 (22). Regarding the current study's findings, the Random Forest model demonstrated strong performance with an F1 score of 92.3%, an MCC of 77.5%, and an AUC of 95%, indicating high sensitivity and precision. The text also notes that similar studies in the literature show high success rates for Random Forest models in complex medical diagnostic problems (23). A comparison is drawn to another Random Forest model for PDA risk prediction in preterm infants, which reported an acceptable performance with 76.3% accuracy and 90% specificity (8). The author suggests that their Random Forest model exhibits superior performance in both sensitivity and specificity compared to similar studies, implying it could be a more reliable tool in clinical practice.
In our study, among the data used in the Random Forest model, gestational week, birth weight, and the need for resuscitation at birth were identified as significant predictor variables (Table 5). These findings play a critical role in developing strategies for early diagnosis and treatment, and similarly, the literature confirms that low birth weight and low gestational age are important predictors in identifying neonates at risk for patent ductus arteriosus (8). Additionally, the study by Park et al. yielded results similar to ours. This study showed that infants in the symptomatic PDA group had statistically significantly lower gestational age, lower birth weight, and lower 1st-minute and 5th-minute Apgar scores (1). Indeed, previous different studies have also demonstrated that these parameters are strong predictors for the risk of hPDA development, especially in very low birth weight infants (24). Furthermore, some studies have indicated that each one-week increase in gestational age reduces the probability of PDA, and low birth weight is associated with a high risk of PDA (8). These results are further strengthened by artificial intelligence, highlighting the potential of machine learning models like Random Forest for PDA risk assessment in premature infants. As a result of these studies, more stable artificial intelligence models can be developed to monitor patients more precisely, and potential complications can be predicted early for timely intervention. Specifically, by preventing hemodynamically insignificant PDA with potential for early spontaneous closure from undergoing unnecessary invasive treatment, significant contributions can be made to clinical decision-making processes. Moreover, AI-supported models will enable more personalized and effective treatment plans by creating customized risk profiles based on each patient's individual characteristics (25).
The assessment of feature importance within Random Forest modeling is significantly informed by metrics such as Mean Decrease in Accuracy, Total Increase in Node Purity, and Mean Dropout Loss, which offer critical insights into the model's operational mechanisms (26, 27). These metrics are instrumental in discerning the features that maximally contribute to the model's predictive efficacy, thus articulating the differential relevance of various variables (26). Our investigation identified gestational week, birth weight, and the requirement for resuscitation at birth as paramount determinants within the model, exhibiting substantial contributions to both the augmentation of node purity and the mitigation of Mean Decrease in Accuracy. This observation robustly substantiates the pivotal role of these three core parameters in prognosticating the risk of hemodynamically significant patent ductus arteriosus in preterm neonates. Conversely, the comparatively elevated Mean Dropout Loss for the APGAR score, despite a lesser contribution when juxtaposed with other importance metrics, signifies its non-negligible influence on the model's aggregate performance. Furthermore, variables including oligohydramnios, chorioamnionitis, bronchopulmonary dysplasia, and multiple pregnancy, while displaying a Mean Decrease in Accuracy of 0 and possibly limited effectiveness within the RF model, remain integral foundational data. This caliber of quantitative and qualitative feature importance analysis diminishes the inherent opacity of the model, enabling clinicians to comprehend the most impactful predictive factors and thereby augmenting the model's trustworthiness in clinical decision-making. Consequently, the model's demonstrated high performance, coupled with its robust interpretability and transparency derived from detailed feature importance analysis, not only reinforces its reliability but also significantly enhances its trustworthiness and potential for practical application within the critical medical domain.
Future studies of this nature require support through multi-center, large-cohort studies to facilitate their integration into prospective clinical practice and enhance their generalizability (4). This study, which can be considered a precursor to prospective research, clearly demonstrates the potential of machine learning models to contribute to diagnostic and risk assessment processes in pediatric cardiology (28–31). Future investigations are imperative for further advancing the predictive capacity of this model by integrating richer datasets, such as additional biomarkers or genomic data, which will substantially enhance its prognostic accuracy. These integrations will enable the development of more personalized and accurate approaches for the detection and management of hemodynamically significant PDA in preterm infants (30). Consequently, continued investigation into comparative analyses of diverse algorithmic frameworks and the interpretability of predictive models is imperative. Such efforts are critical for expanding the clinical applicability of machine learning in pediatric cardiology and optimizing its therapeutic advantages (8).
5 Conclusion
This study's findings underscore the Random Forest model's efficacy in evaluating the risk of hemodynamically significant patent ductus arteriosus in premature infants, thereby presenting substantial contributions to clinical utility. The conducted analyses identified factors such as low birth weight, reduced gestational age, and the necessity for CPR at birth as pivotal predictors for PDA development risk. These insights are paramount for the timely identification of neonates at risk and the formulation of suitable therapeutic strategies, allowing for early risk assessment of PDA in neonatal intensive care units, which in turn facilitates the creation of targeted interventions and enhances patient prognosis. Nevertheless, the full integration of these models into prospective clinical applications mandates further validation and reinforcement through extensive, multi-center prospective investigations. As the domains of artificial intelligence and machine learning progressively evolve, the incorporation of such prognostic models into clinical decision support systems is poised to significantly amplify the role of AI in both research endeavors and clinical applications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Izmir Democracy University Buca Seyfi Demirsoy Training and Research Hospital Non-Interventional Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because of accordance with national legislation and institutional requirements, this is permissible for retrospective studies.
Author contributions
OA: Conceptualization, Formal analysis, Methodology, Writing – original draft, Investigation, Software, Supervision. SG: Data curation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors confirm the utilization of Generative AI for linguistic refinement in the preparation of this manuscript. We, the authors, assume full accountability for this usage and attest that the final version has been thoroughly reviewed and verified by us.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aPDA, asymptomatic PDA; hPDA, hemodynamically significant patent ductus arteriosus; CPR, cardiopulmonary resuscitation; BPD, bronchopulmonary dysplasia; RF, random forest; AUC, area under the curve; MCC, Matthews correlation coefficient.
References
1. Park SH, Moon J, Eun HS, Hong J-H, Lee K. Artificial intelligence-based diagnostic support system for patent ductus arteriosus in premature infants. J Clin Med. (2024) 13:2089. doi: 10.3390/jcm13072089
2. Babla K, Shetty S, Kulkarni A. A clinical and echocardiographic approach to evaluation of patent ductus arteriosus in preterm infants. Paediatr Child Health (Oxford). (2020) 30:129. doi: 10.1016/j.paed.2020.01.003
3. Köksal N, Aygün C, Uras N. Turkish Neonatal Society guideline on the management of patent ductus arteriosus in preterm infants. Turk Pediatri Ars. (2018) 53(Suppl 1):S76–87. doi: 10.5152/TurkPediatriArs.2018.01808
4. Umapathi KK, Muller B, Sosnowski C, Thavamani A, Murphy J, Awad S, et al. A novel patent ductus arteriosus severity score to predict clinical outcomes in premature neonates. J Cardiovasc Dev Dis. (2022) 9:114. doi: 10.3390/jcdd9040114
5. Lei H, Ashrafi AH, Chang P, Chang A, Lai WW. Patent ductus arteriosus (PDA) detection in echocardiograms using deep learning. Intell Based Med. (2022) 6:100054. doi: 10.1016/j.ibmed.2022.100054
6. Dasraf D, Djer MM, Advani N. The role of n terminal - probrain natriuretic peptide in the diagnosis of hemodynamic persistent asrteriosus ductus in premature neonates patient. J Phys Conf Ser. (2017) 884:12147. doi: 10.1088/1742-6596/884/1/012147
7. Mohammadi I, Firouzabadi SR, Hosseinpour M, Akhlaghpasand M, Hajikarimloo B, Zeraatian-Nejad S, et al. Using artificial intelligence to predict post-operative outcomes in congenital heart surgeries: a systematic review. BMC Cardiovasc Disord. (2024) 24(1):768. doi: 10.1186/s12872-024-04336-6
8. Jura AMC, Popescu D-E, Cîtu C, Biriş M, Pienar C, Paul C, et al. Predicting risk for patent ductus arteriosus in the neonate: a machine learning analysis. Medicina (B Aires). (2025) 61:603. doi: 10.3390/medicina61040603
9. Méot M, Haddad RN, Patkai J, Abu Zahira I, Di Marzio A, Szezepanski I, et al. Spontaneous closure of the arterial duct after transcatheter closure attempt in preterm infants. Children. (2021) 8(12):1138. doi: 10.3390/children8121138
10. Sathanandam SK, Gutfinger D, O'Brien L, Forbes TJ, Gillespie MJ, Berman DP, et al. Amplatzer piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients≥ 700 grams. Catheter Cardiovasc Interv. (2020) 96(6):1266–76. doi: 10.1002/ccd.28973
11. Haddad RN, Bonnet D, Malekzadeh-Milani S. Embolization of vascular abnormalities in children with congenital heart diseases using medtronic micro vascular plugs. Heart Vessels. (2022) 37(7):1271–82. doi: 10.1007/s00380-021-02007-6
12. Kindler A, Seipolt B, Heilmann A, Range U, Rüdiger M, Hofmann SR. Development of a diagnostic clinical score for hemodynamically significant patent ductus arteriosus. Front Pediatr. (2017) 5:280. doi: 10.3389/fped.2017.00280
13. Wu JJY, Niduvaje K, Lee LY, Amin Z. Retrospective comparison of death or neurodevelopmental outcomes in extremely low birth weight preterm infants following different management options of haemodynamically significant patent ductus arteriosus. BMC Pediatr. (2021) 21(1):457. doi: 10.1186/s12887-021-02920-9
14. Hundscheid T, El-Khuffash A, McNamara PJ, de Boode WP. Survey highlighting the lack of consensus on diagnosis and treatment of patent ductus arteriosus in prematurity. Eur J Pediatr. (2022) 181:2459. doi: 10.1007/s00431-022-04441-8
15. Trahan KF, Shelton EL, Gillam-Krakauer M. Patent ductus arteriosus in extremely preterm infants: update on current diagnostic and treatment options. Curr Treat Options Cardiovasc Med. (2025) 27(1):1–9. doi: 10.1007/s11936-025-01101-6
16. Parkerson S, Philip R, Talati AJ, Sathanandam S. Management of patent ductus arteriosus in premature infants in 2020. Front Pediatr. (2021) 8:590578. doi: 10.3389/fped.2020.590578
18. Hsu J, Yang C, Lin C, Chu S, Huang H, Chiang M, et al. Machine learning algorithms to predict mortality of neonates on mechanical intubation for respiratory failure. Biomedicines. (2021) 9:1377. doi: 10.3390/biomedicines9101377
19. Aggrawal SPR. Elimination and backward selection of features (P-value technique) in prediction of heart disease by using machine learning algorithms. Türk Bilgisayar Mat Eğitim Derg. (2021) 12:2650. doi: 10.17762/turcomat.v12i6.5765
20. Chicco D, Jurman G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics. (2020) 21(1):6. doi: 10.1186/s12864-019-6413-7
21. Singh VK, Pencina M, Einstein AJ, Liang JX, Berman DS, Slomka PJ. Impact of train/test sample regimen on performance estimate stability of machine learning in cardiovascular imaging. Sci Rep. (2021) 11(1):14490. doi: 10.1038/s41598-021-93651-5
22. Chulde-Fernández B, Enríquez-Ortega D, Guevara C, Navas P, Tirado-Espín A, Vizcaíno-Imacaña P, et al. Classification of heart failure using machine learning: a comparative study. Life. (2025) 15:496. doi: 10.3390/life15030496
23. Huertas-García Á, Martí-González C, Maezo RG, Rey AE. A comparative study of machine learning algorithms for anomaly detection in industrial environments: performance and environmental impact. Algor Intell Syst. (2023):373–89. doi: 10.1007/978-981-99-9436-6_26
24. Sun X, Chen L, Gao J. Predictive value of a nomogram model for adverse outcomes in very low birth weight infants with patent ductus arteriosus: a prospective study. Front Pediatr. (2023) 11:1131129. doi: 10.3389/fped.2023.1131129
25. Na JY, Kim D, Kwon AM, Jeon JY, Kim H, Kim C, et al. Artificial intelligence model comparison for risk factor analysis of patent ductus arteriosus in nationwide very low birth weight infants cohort. Sci Rep. (2021) 1(1):22353. doi: 10.1038/s41598-021-01640-5
26. Doubleday K, Zhou J, Zhou H, Fu H. Doubleday - risk controlled decision trees and random forests for precision medicine (2022). Stat Med. (2022) 41(4):719–35. doi: 10.1002/sim.9253
27. Ha HT, Nguyen DTB, Stoeckel T. What is the best predictor of word difficulty? A case of data mining using random forest. Language Testing. (2024) 41:828. doi: 10.1177/02655322241263628
28. Ejaz H, Thyyib T, Ibrahim AK, Nishat A, Jhancy M. Role of artificial intelligence in early detection of congenital heart diseases in neonates. Front Digit Health. (2024) 5:1345814. doi: 10.3389/fdgth.2023.1345814
29. Jone P, Gearhart A, Lei H, Xing F, Nahar J, Lopez-Jimenez F, et al. Artificial intelligence in congenital heart disease. JACC Adv. (2022) 1:100153. doi: 10.1016/j.jacadv.2022.100153
30. Sethi Y, Patel N, Kaka N, Desai A, Kaiwan O, Sheth M, et al. Artificial intelligence in pediatric cardiology: a scoping review. J Clin Med. (2022) 11:7072. doi: 10.3390/jcm11237072
Keywords: patent ductus arteriosus, premature infants, Random Forest model, machine learning, gestational week, birth weight, F1 score
Citation: Ay O and Gunes S (2025) Machine learning-based prediction of hemodynamically significant patent ductus arteriosus in preterm neonates: a pioneering insight. Front. Pediatr. 13:1727418. doi: 10.3389/fped.2025.1727418
Received: 17 October 2025; Revised: 13 November 2025;
Accepted: 13 November 2025;
Published: 2 December 2025.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Raymond N. Haddad, INSERM Biologie Cellulaire, Développement et Évolution, FranceAbdullah Ozyurt, Toros University, Türkiye
Copyright: © 2025 Ay and Gunes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oguzhan Ay, b2d1emhhbmF5MUBnbWFpbC5jb20=
 Oguzhan Ay
Oguzhan Ay Sezgin Gunes
Sezgin Gunes