- 1Department of PharmacoTherapy, - Epidemiology & -Economics, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, Netherlands
- 2Mental Health Research Institute, Tomsk National Research Medical Center, Russian Academy of Sciences, Tomsk, Russia
- 3Department of Cytology and Genetics, National Research Tomsk State University, Tomsk, Russia
- 4GGZ Westelijk Noord-Brabant, Policy Office for Quality and Innovation of Care (BZI), Halsteren, Netherlands
- 5Department of Psychotherapy and Psychological Counseling, National Research Tomsk State University, Tomsk, Russia
- 6Department of Psychiatry, Addictology and Psychotherapy, Siberian State Medical University, Tomsk, Russia
- 7School of Non-Destructive Testing and Security, Division for Control and Diagnostics, National Research Tomsk Polytechnic University, Tomsk, Russia
- 8Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
Major depressive disorder has become a prominent cause of disability, as lifetime prevalence has increased to ~15% in the Western world. Pharmacological effects of serotonin (5-hydroxytryptamine, 5-HT) are mediated through 5-hydroxytryptamine receptor (5-HTR) binding. Serotonin regulation of amygdala activity is attained through activation of three 5-HT2 family receptor subtypes, 5-HT2A, 5-HT2B, and 5-HT2C. Specifically, HT2A and the HT2C receptors have similar gross cerebral distribution and function, with higher constitutive activity found in HT2C than in HT2A. We investigated the possible association of 5-HTR gene polymorphisms to specific and non-specific antidepressant treatment responses in treatment-free patients in Siberia. 156 patients, aged between 18–70 years and clinically diagnosed with depressive disorders, were treated with antidepressants for 4 weeks. Patients were genotyped for a subset of 29 SNPs from the following 5-HT Receptor genes: HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B and HTR6. Primary outcome was measured by differences in Hamilton Depression Rating Scale (ΔHAM‐D 17) scores between baseline/week two, week two/week four and baseline/week four. Univariate linear regression was initially conducted to determine the 5-HTR SNPs to be studied within the multiple linear regression. Multiple linear regression analyses over the three time periods were conducted for ΔHAM‐D 17 with independent factors including: age, gender, depression diagnosis, antidepressant treatment and selected 5-HTR SNPs. We found improved ∆HAM-D 17 in patients taking tricyclic antidepressants (0–4 weeks: B = 4.85, p = 0.0002; 0–2 weeks: B = 3.58, p = 0.002) compared to patients taking SSRIs. Over the course of study, significant associations between 5-HT receptors SNPs and antidepressant response were not identified.
Introduction
Major depressive disorder (MDD) is a complex, polygenic disease and a leading cause of disability affecting individuals across the globe (Kessler and Bromet, 2013). Although there are multiple antidepressant treatments, nearly a third of patients do not respond positively to the initial prescription (Bauer et al., 2013; Bauer et al., 2015). Efforts to personalize treatment by identifying genetic biomarkers associated with antidepressant response have been inconclusive. This may be due to the heterogeneous character of mood disorders but also to the large number of antidepressant drugs being investigated within the studies, leading to varied mechanisms of action and differing targets.
Currently, patients are prescribed selective serotonin reuptake inhibitors (SSRIs) as first line treatment of depression, targeting serotonin (5-HT) reuptake and increasing the level of 5-HT (Loonen and Ivanova, 2016). 5-HT receptors play a role in the mechanism of action of antidepressants, with the different receptor subfamilies affecting serotonergic activity (Fink and Göthert, 2007; Barnes and Neumaier, 2011). 5-HT receptors are categorized into seven families, 5-HT1 to 5-HT7, each mediating different physiological effects of 5-HT. 5-HT receptors are part of the G-protein coupled receptor (GPCR) superfamily, with the exception of 5-HT3 receptor, a ligand-gated ion channel (Barnes and Neumaier, 2011). Activation of 5-HT2 receptors regulates activity of projection neurons within the amygdala, where high activity is known to be associated with MDD. Within 5-HT2 receptors three receptor subtypes are subcategorized (5-HT2A, 5-HT2B, and 5-HT2C), playing an important role in the regulation of amygdala activity and amygdala-mediated effects, both behavioral and psychological (Bombardi, 2014). 5-HT2A and 5-HT2C receptors modulate dopamine output within the central nervous system and are targets for various mood disorder medications (Van Oekelen et al., 2003; Bubar and Cunningham, 2006). Curiously, 5-HT2C is the only receptor known to undergo RNA editing, achieving greater protein diversity than the other 5-HT receptors (Burns et al., 1997). 5-HT2B receptors, on the other hand, were found to have a transient action in embryogenesis, which suppression was found to lead to morphological defects, and peripheral tissues but its role in the adult brain was undetermined (Nebigil et al., 2001; Leysen, 2004).
In this study, we selected 5-HT receptor SNPs which were previously associated with mood disorders and known to affect 5-HT mechanism of action. Studies have linked 5-HT2C (759-C/T polymorphism) with weight gain (Pooley et al., 2004; Del Castillo et al., 2013; Higgins et al., 2013; Reynolds et al., 2014). Polymorphisms within the HTR2C gene have been previously associated with criminal behavior, antipsychotic drug-induced hyperprolactinemia and tardive dyskinesia (Toshchakova et al., 2018; Ivanova et al., 2017; Loonen et al., 2019; Pozhidaev et al., submitted). Further investigation on 5-HT receptor polymorphisms may build on previous findings to develop a better understanding of mental health disease and treatment side-effects.
Our current research builds on our previous investigations on the Russian depression cohort, expanding the genotype targets to encompass 5-HT receptor single nucleotide polymorphisms (SNPs). The prior studies investigated the possible relationship between PRL and BDNF genotypes and serum protein levels on our depression cohort. BDNF rs6265 and severity of depression were found to be associated in the initial observations (Losenkov et al., submitted). To follow-up the findings, patients were treated with antidepressants for 4 weeks to investigate the potential pharmacogenetic PRL and BDNF markers in antidepressant treatment response (Ochi et al., 2019).
Many patients within our cohort were prescribed SSRIs and SNRIs throughout the study, therefore in this investigation, additional genotyping was conducted to investigate 5-HT receptor SNPs’ roles in treatment response in the cohort. As with the previous study, we investigated the ‘true’ antidepressant response to determine whether associations between 5-HT receptor polymorphisms and antidepressant response are found. This stems from our hypothesis where antidepressant response is delayed roughly 2 weeks and specific response to treatment given should be noticeable in after the 2 weeks. We aim to identify the effect of 5-HT receptor SNPs on antidepressant efficacy within the total and the latter 2 weeks of the study.
Methods
Study Design and Patient Characteristics
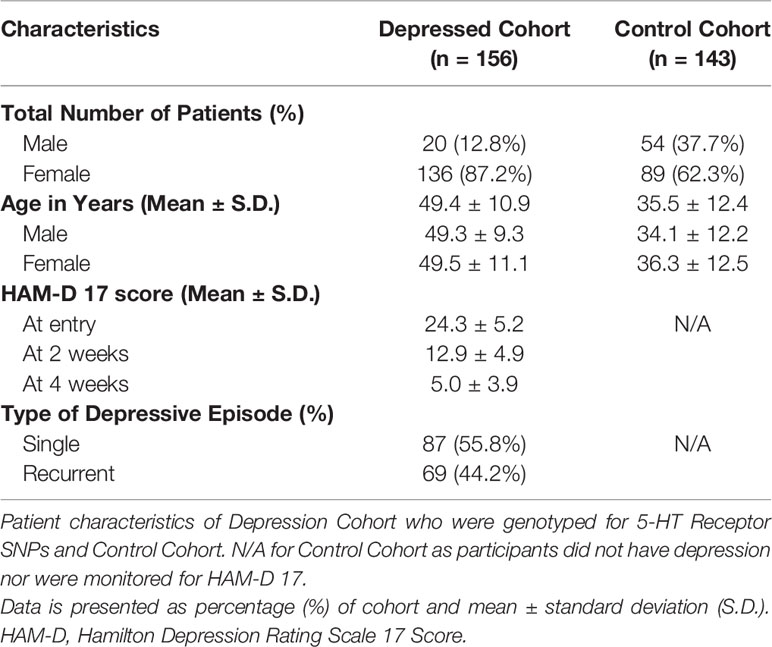
Following the investigation of PRL and BDNF to antidepressant treatment, further genotyping was conducted on 5-HT receptor SNPs. 156 participants from the initial 186, aged 18-70 years old, were genotyped and included based on the clinical diagnosis of a depressive episode (single or recurrent, ICD: F32 or F33). Depressed patients were assessed with the 17-item Hamilton Depression Rating Scale (HAM-D 17) (Hamilton, 1960). The increase in the number of patients compared to the previous study (151 patients genotyped) stems from increased funds available to expand the scope of our study.
The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki 1975, revised in Fortaleza, Brazil, 2013), and approved by the Institutional Medical Review Board (protocol 49 from 23.04.12). Participants were recruited from psychiatric departments of the Mental Health Research Institute, Tomsk National Research Medical Center and provided written informed consent. Patients were excluded from the study considering the following conditions: non-Caucasian ethnicity, schizophrenia, decompensated personality disorders, pregnancy, or any relevant gynecological or endocrine (thyroid) disorder, relevant pharmacological withdrawal symptoms, organic brain disorders (e.g., epilepsy, Parkinson’s disease), or treatment with antidopaminergic drugs (antipsychotic or antiemetic drugs).
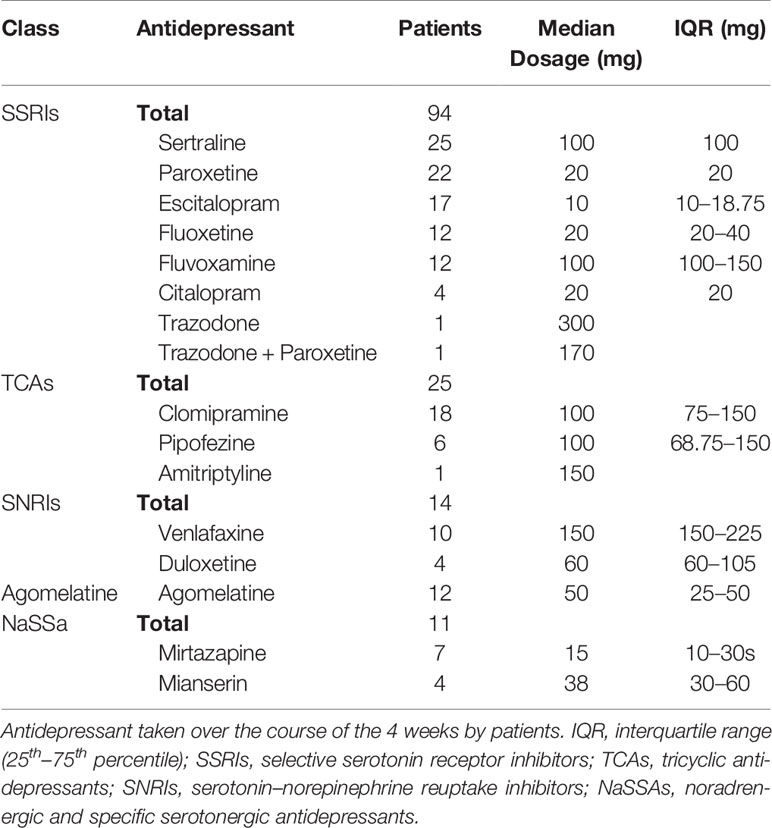
After diagnosing, assessing depressive status and obtaining informed consent, antidepressant treatments were initiated to all patients within our cohort. Out of the studied 156 patients, 133 patients were treated with serotonin reuptake inhibitors [selective serotonin reuptake inhibitors (SSRIs) – 91, tricyclic antidepressants (TCAs) – 23, serotonin and noradrenaline reuptake inhibitors (SNRIs) – 14], 23 patients were treated with serotonin type 2 receptor antagonists (Table 1). Depressed patients were assessed with the 17-item Hamilton Depression Rating Scale (HAM-D 17) (Hamilton, 1960).
A control cohort consisting of 143 healthy participants of both genders, aged from 18 to 60 years old, were enrolled as a reference for the association study. Written informed consent was obtained from the participants and was genotyped to determine the association of 5-HT receptor polymorphisms to depression. The control participants were excluded if they presented the following conditions: chronic physical pathology in exacerbation, pregnancy, or any relevant gynecological or endocrine (thyroid) disorder; relevant pharmacological withdrawal symptoms; or organic brain disorders (e.g., epilepsy, Parkinson’s disease) or mental disorders.
Genotyping
DNA was isolated from the leukocytes in whole peripheral blood from patients using the standard phenol-chloroform micro method and mandatory condition of pre-freezing the blood. The DNA was genotyped for a subset of 29 SNPs from the following 5-HT receptor genes: HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B, and HTR6 in the Laboratory of Genetics of the University of Groningen with the MassARRAY® System (Agena Bioscience™) and in the Laboratory of Molecular Genetics and Biochemistry of the Mental Health Research Institute with “StepOnePlus” (Applied Biosystems) (Supplementary Table 1). Blood sampling method can be found in the previous study (Ochi et al., 2019).
Statistical Analysis
To determine the effect of 5-HT Receptor gene SNPs to antidepressant response, the outcome was measured by the difference in HAM-D 17 score between entry and 2 weeks of treatment (ΔHAM-D 17 0–2 weeks), after two and 4 weeks of treatment (ΔHAM-D 17 2–4 weeks) and entry and 4 weeks of treatment (ΔHAM-D 17 0–4 weeks), with a greater ΔHAM-D 17 score denoting better clinical outcome. Normal distribution was tested utilizing the P-P plot. Due to the small number of homozygous patients for some SNP, patients were combined with heterozygous patients in the analysis to determine the effect of the allele and compared against the major allele.
Patient characteristics (i.e. age, sex, ΔHAM-D 17) were determined using descriptive statistics (Table 2). Due to the larger number of variables to our cohort size, univariate linear regression was conducted initially to determine the variables to be included in the multiple linear regression. As the patient cohort, consisted of 156 patients, we proceeded with 16 variables in the multiple linear regression, utilizing the one in ten rule (Harrell et al., 1984). Multiple linear regression was conducted to identify the independent factors associated with ΔHAM-D 17 between the three time periods, including age, sex, depression diagnosis, type of antidepressant taken and 5-HT receptor gene SNPs. To study specific antidepressants and SNP genotypes, dummy variables were created to determine the effect within each variable. Without the creation of the dummy variables, differentiation between the antidepressant medication and alleles would not have been investigated. Odds ratio (OR) was calculated for HTR2C SNPs, due to the hemizygous nature of the gene in males (as the gene is X-linked). Statistical analysis was conducted with SPSS software (release 25.0). The significance level for descriptive statistics and univariate statistical tests were p < 0.05 and for the multiple linear regression was p < 0.0031, after Bonferroni correction. Power analysis was conducted post-hoc utilizing G*Power (Faul et al., 2007; Faul et al., 2009).
Results
From the initial cohort, 156 patients consisting of 20 men and 136 women, aged 49.4 ± 10.9 years (mean ± standard deviation), were genotyped for the 5-HT receptor SNPs. Over the course of 4 weeks, HAM-D 17 was taken at entry (HAM-D 17 score: 24.3 ± 5.2), at 2 weeks (HAM-D 17 score: 12.9 ± 4.9) and at 4 weeks (HAM-D 17 score: 5.0 ± 3.9) (Table 1). The control cohort included 143 patients, comprising of 54 male and 89 women and aged 35.5 ± 12.4 years. The control cohort was genotyped for 5-HT2C receptor SNPs to determine the Odds Ratio for depression, when compared to the depression cohort.
HTR1A rs1800042 was not included in the analysis as it violated the Hardy-Weinburg Equilibrium distribution. Of the remaining 28 SNPs investigated, 13 of the SNPs had the minor allele combined with the heterozygous allele for analyses (Supplementary Table 1). As we were interested in determining which SNPs influenced antidepressant treatment response, the SNPs genotypes were not categorized into dummy variables for the univariate analyses.
Linear Regression to Determine Covariate Associations With ∆HAM-D 17
Univariate linear regression was conducted for the 5-HTR SNPs to determine which SNPs were to be included in the multiple linear regression analyses of the three time periods for ∆HAM-D 17 (Supplementary Table 2). SNPs found to be significant in the univariate analyses, had a p-value lower than 0.3 or were found to be associated in the multiple linear regression where all 5-HTR SNPs were included into the multiple linear regression analysis.
For the multiple linear regression, the following covariates were included: gender, age, type of diagnosis, antidepressant treatment, HTR1A rs6295, rs749099, HTR1B rs6296, HTR2A rs7997012, rs1928040, rs6312, HTR2C rs5946189, rs6318, rs17326429, HTR3A rs33940208, HTR3B rs1176744, and HTR6 rs1805054. To determine the effect of a SNP’s genotype on antidepressant response and type of antidepressant treatment taken, dummy variables were created for the SNPs and antidepressants treatments. Dummy variables provide an opportunity to determine the effect of the genotypes within SNPs and see which allele affected antidepressant response. Similarly, dummy variable for antidepressant treatments determine the effect treatments have on ∆HAM-D 17, when referenced to SSRIs. Bonferroni correction was imputed by taking the standard alpha (0.05), dividing by the number of covariates (16), leaving 0.0031 as the new alpha to determine significance for the analyses. Post-hoc power analyses from the imputed multiple linear regression R2 are found within Supplementary Table 4.
Throughout the study periods, gender, age and type of diagnosis were not found to be significantly associated with ∆HAM-D. Improved ∆HAM-D 17 was found in patients taking tricyclic antidepressants (0–4 weeks: B = 4.85, p = 0.0002; 0–2 weeks: B = 3.58, p = 0.002) compared to patients taking SSRIs. Further significant associations were not found within the other antidepressants studied in the cohort.
5-HT Receptor Associations
No significant associations for 5-HTRs were identified in the cohort within all three time periods (Table 3, Supplementary Table 3). Of the SNPs identified to be significant in the univariate analyses, HTR2C rs5946189, HTR3A rs33940208 and HTR3B rs1176744 did not result in significance in the multiple linear regression.
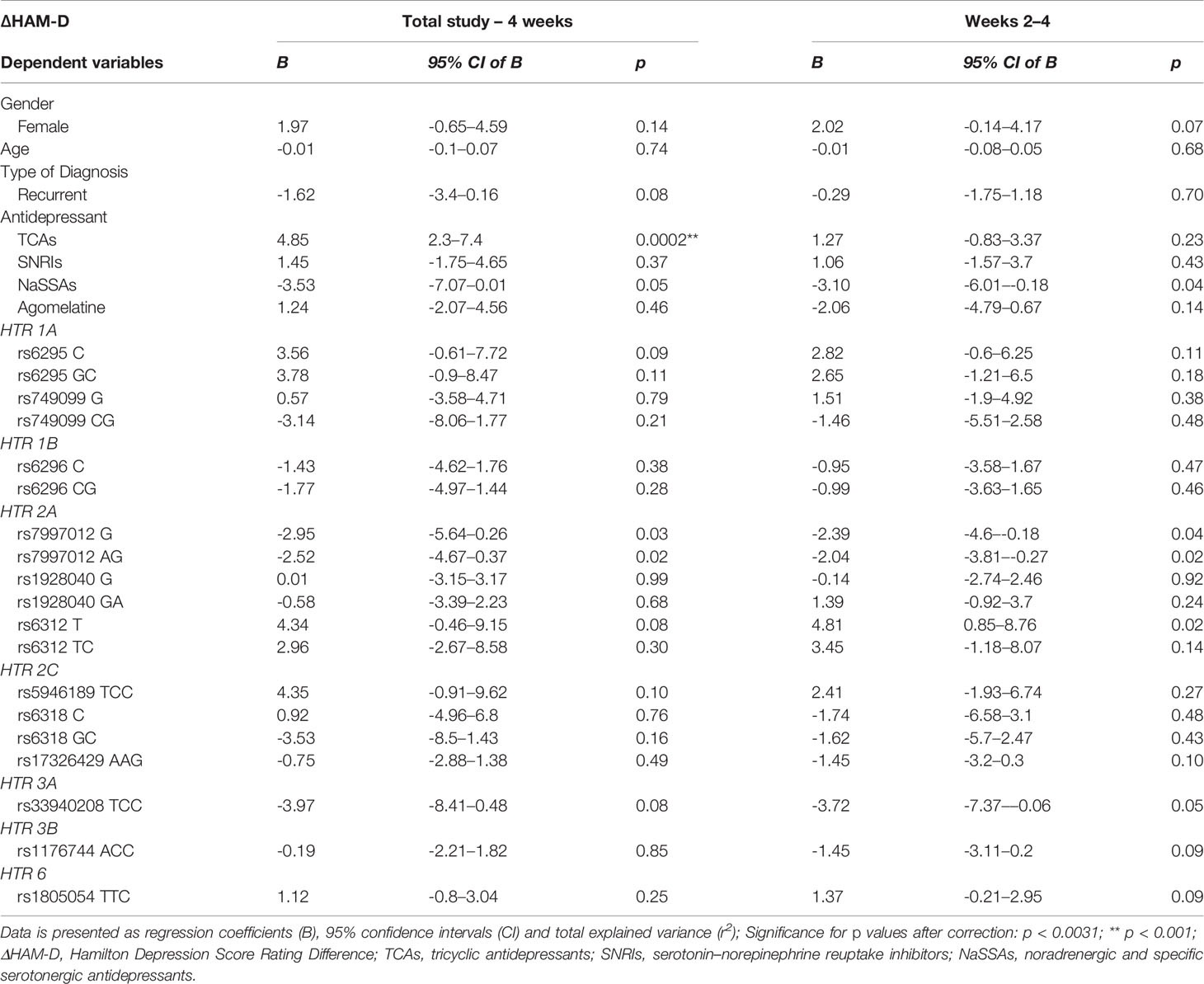
Table 3 Multiple linear regression of total depression cohort covariates (age, gender, diagnosis, type of antidepressant, selected 5-HT receptor gene genotypes) between the four week and week two to four study period.
Within the studied 5-HTR SNPs, suggestive associations were found within the total study and in the last 2 weeks, albeit not significant to the Bonferroni correction. Of note, HTR1A rs6295 homozygous C and heterozygous GC patients were found to indicate increased ∆HAM-D 17 compared to homozygous G patients. HTR2A rs6312 homozygous T and heterozygous TC patients illustrated a positive ∆HAM-D compared to homozygous C patients. HTR2C rs5946189 combined homozygous C and heterozygous TC patients suggest a positive ∆HAM-D compared to homozygous T patients. On the other hand, HTR2A rs7997012 homozygous C and heterozygous CG patients were indicative of decreased ∆HAM-D 17 when compared to homozygous G. HTR3A rs33940208 combined homozygous C and heterozygous TC patients point to a decreased ∆HAM-D 17 when compared to homozygous T. The remaining studied 5-HTR SNPs (HTR1A rs749099, HTR1B rs6296, HTR2A rs1928040, HTR2C rs6318, HTR2C rs17326429, HTR3B rs1176744, HTR6 rs1805054) did not suggest a direction of ∆HAM-D inclination in the total study, nor in the last 2 weeks.
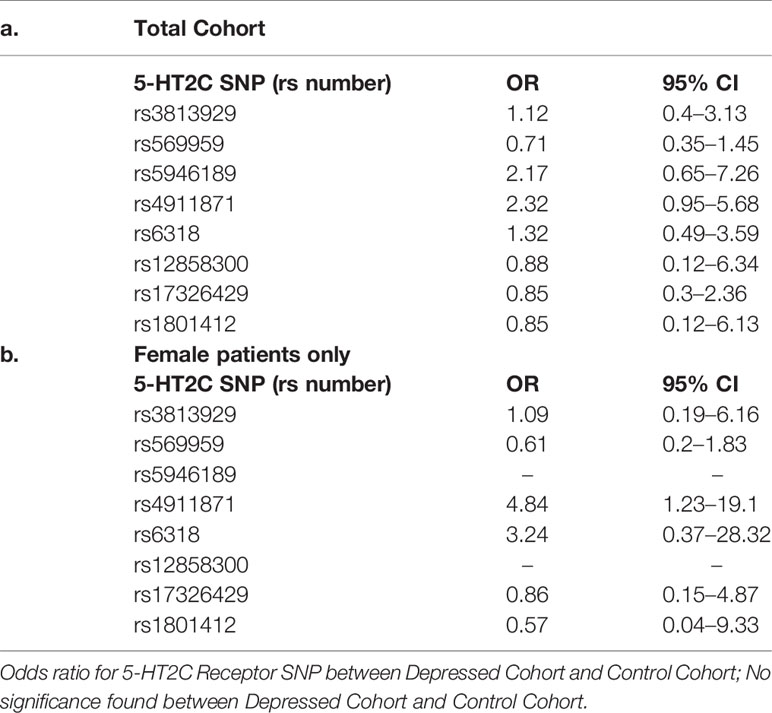
Odds Ratio of Hemizygous 5-HT2C
Odds ratio (OR) was calculated for 5-HT2C genotypes to explore its hemizygous nature and to determine prevalence of depression. Depressed patients were compared against a control group of depression free individuals. In both total cohort and female-only OR comparisons, no significance was found for 5-HT2C receptor SNPs (Table 4). Male-only OR comparison was unable to be conducted due to the small sample size within the depression cohort.
Discussion
Due to the complex nature of depression, identifying a single etiology or pharmacological target for antidepressant treatment has been unconvincing. The monoamine theory stipulates neurotransmission dysfunction of the adrenergic/serotonergic pathways causes a depressive episode, providing a target for antidepressant treatment (Loonen and Ivanova, 2016). 5-HT receptor signaling may provide insight into its role in mediating antidepressant response, such as via the neural circuitry of the hippocampus (Yohn et al., 2017). However, no significant associations were found in our study within the selected 5-HT receptor polymorphisms on antidepressant response. This may stem from the different classes of antidepressants prescribed but as the majority of treatment prescribed are serotonin reuptake inhibitors, the lack of any associations in our cohort does not lend credence to the monoamine theory. On the other hand, the indications found within HTR1A rs6295, HTR2A rs6312, HTR2A rs7997012, HTR2C rs5946189 and HTR3A rs33940208 provide a potential direction for future studies to focus on targeting a specified 5-HT receptor and antidepressants whose mechanism of action target it.
Our results fall in line with previous literature and pharmacogenetic studies targeting 5-HT receptors, as results were inconclusive with indications toward antidepressant response and other mental health disorders (Serretti et al., 2009; Bondy, 2011; Reynolds et al., 2014). While there are some indicative associations within the selected 5-HT receptor polymorphisms, until further investigation with antidepressants targeting the specific 5-HT receptor are conducted, whether there are significant associations will not be known. We decided not to include any HTR2B SNPs in our investigation as its role as classical neurotransmitter receptor has not been clear. Until demonstrated recently, studies failed to demonstrate the presence of 5-HT2B receptors in the brain, whereas now several regions have been identified within the central nervous system (Devroye et al., 2018). 5-HT2B receptors appear to regulate the activity of serotonin transporters, by phosphorylation of the transporters, and may regulate the function of neuroglia, i.e. astrocytes, microglia, etc. (Devroye et al., 2018). This suggestive involvement of HTR2B in the neuroplastic process provides an interesting opportunity to study the antidepressant effects of SSRIs related to HTR2B-associated neuroplastic modulation, in combination with other neuroplastic factors (BDNF), to determine whether a potential relationship to antidepressant treatment response can be identified.
Curious Nature of HTR2C and Depression in Males
The hemizygous nature of HTR2C in males presents an opportunity to investigate whether a genotype affects 5-HT2C receptor activity. Males are unable to be heterozygous for 5-HT2C receptor genotypes since the gene is X-linked, therefore, 5-HT2C genotypes affect males differently than in females. We looked to explore this within our male depressive cohort and determine whether an effect on antidepressant response could be found. However, the small sample size of male depressed patients in our study limited the possibility to investigate the potential pharmacogenetic effect of 5-HT2C receptor genotypes on antidepressant response.
The small number of male depressive patients in our study falls in line with the current prevalence of depression found in males, in comparison to females (Keuhner, 2017). Depression in males is often not investigated which may stem from the underreporting of depressive symptoms, due to a lack of understanding of their symptoms and perceived emasculation of reporting depression (Sigmon et al., 2005). As we look to develop an understanding on personalizing treatment for major depressive disorder, consideration on the effect of gender on the etiology of depression, and subsequent treatment, is required.
Strengths and Limitations
Due to the recurrent nature of depression, exposure from previous treatments may hinder the effectiveness of following treatments. Our cohort consisted of treatment-free patients, thus the potential limiting effect of previous antidepressant treatment exposures (notably SSRI) is limited. Majority of our patients were prescribed serotonin reuptake inhibitors, providing the opportunity to study the effect of 5-HT receptor SNPs on treatment response.
The small number of patients studied in our cohort limited the number of covariates included in our final multiple regression analysis. In addition, the number of antidepressants prescribed to patients varied, limiting the possibility of stratifying the analysis per treatment. Therefore, investigating the mechanism of action of selected antidepressants targeting specific 5-HT receptors was not possible, limiting the scope of the study.
Conclusion and Future Research
Over the course of study, significant associations between 5-HT receptors SNPs and antidepressant response were not identified. Our hypothesis of targeting the ‘true’ antidepressant effect within the last 2 weeks of the study similarly did not result in associations between the SNPs and treatment response. Currently, the first-line of antidepressant treatment for major depressive disorders are SSRIs but our investigation suggests tricyclic antidepressants are more effective as patients were found to respond with greater improvement in HAM-D 17 score over the course of the study.
Sub-analyses of treatment options were limited as the study size was not large enough. Therefore, future studies will need to consider narrowing treatments given, focusing on a specific mechanism of action and whether polymorphisms within the targeted 5-HT receptors will affect antidepressant response. Specifically, HTR2C provides a unique opportunity to explore tailored treatment for male patients due to the hemizygous nature of the gene.
Author’s Note
This study was a collaboration between the Mental Health Research Institute (Tomsk National Research Medical Center of the Russian Academy of Sciences) in Tomsk and the Groningen Research Institute of Pharmacy (GRIP) of the University of Groningen. The Russian part is carried out within the framework of Tomsk Polytechnic University Competitiveness Enhancement Program.
Data Availability Statement
The datasets generated for this study are available on every reasonable request to Prof. Dr. Svetlana A. Ivanova (aXZhbm92YW5paXB6QGdtYWlsLmNvbQ==), following approval of the Board of Directors of the MHRI, in line with local guidelines and regulations.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Medical Review Board of the Mental Health Research Institute (protocol 49 from 23.04.12). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TO and NV share first authorship. AL and SI instigated and designed the study. AL, BW and SI coordinated and supervised the study. TO, NV and IL designed and performed the statistical analysis and contributed to writing the paper. SI wrote the study protocol and selected the SNPs. IL monitored the study. GS collected clinical data. IL, NV, DP and IP isolated DNA, genotyped the samples and recorded all data in an Excel database. NB supervised the clinical work. SI, AL and BW supervised the technical work. TO and AL wrote the manuscript. GS, BW and SI adapted the manuscript. All authors read the paper and agree with its content. Their role justifies their (co)-authorship to this paper.
Funding
Obtaining reagents for genotyping of 5-HT receptor genes was financed by the Russian Foundation for Basic Research (RFBR) grant №17-29-02205 «Development of a molecular genetic panel of depressive disorders based on polymorphisms of the genes of neuronal kinases, neurotrophic proteins and genes of the serotonergic system» and was also used for the salary payments of the Russian authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01462/full#supplementary-material
References
Barnes, N. M., Neumaier, J. F. (2011). Neuronal 5-HT receptors and SERT. Tocris Biosci. Sci. Rev. Ser. 34, 1–15.
Bauer, M., Pfennig, A., Severus, E., Whybrow, P., Angst, J., Möller, H. (2013). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, Part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14 (5), 334–385. doi: 10.3109/15622975.2013.804195
Bauer, M., Severus, E., Köhler, S., Whybrow, P., Angst, J., Möller, H. (2015). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: maintenance treatment of major depressive disorder-update 2015. World J. Biol. Psychiatry 16 (2), 76–95. doi: 10.3109/15622975.2014.1001786
Bombardi, C. (2014). Neuronallocalization of the 5-HT2 receptor family in the amygdaloid complex. Front. Pharmacol. 5, 68. doi: 10.3389/fphar.2014.00068
Bondy, B. (2011). Genetics in psychiatry: are the promises met? World J. Biol. Psychiatry. 12 (2) 81–88. doi: 10.3109/15622975.2010.546428
Bubar, M., Cunningham, K. (2006). Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr. Topics Med. Chem. 6 (18), 40. 1971–1985. doi: 10.2174/156802606778522131
Burns, C. M., Chu, H., Rueter, S. M., Hutehinson, L. K., Canton, H., Sanders-bush, E., et al. (1997). Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387, 303–308. doi: 10.1038/387303a0
Chagraoui, A., Thibaut, F., Skiba, M., Thuillez, C., Bourin, M. (2016). 5-HT2C receptors in psychiatric disorders: a review. Prog. Neuropsychopharmacol. Biol. Psychiatry 66, 120–135. doi: 10.1016/j.pnpbp.2015.12.006
Del Castillo, N., Zimmerman M, B., Tyler, B., Ellingrod, V. L., Calarge, C. (2013). 759C/T Variants of the serotonin (5-HT2C) receptor gene and weight gain in children and adolescents in long-term risperidone treatment. Clin. Pharmacol. Biopharm. 2 (2), 110. doi: 10.4172/2167-065x.1000110
Devroye, C., Cathala, A., Piazza, P., Spampinato, U. (2018). The central serotonin 2B receptor as a new pharmacological target for the treatment of dopamine-related neuropsychiatric disorders: rationale and current status of research. Pharmacol. Ther. 181, 143–155. doi: 10.1016/j.pharmthera.2017.07.014
Di Giovanni, G., Esposito, E., Di Matteo, V. (2011). 5HT2C Receptors in the Pathophysiology of CNS Disease (New York: Humana Press), 557 pages.
Faul, F., Erdfelder, E., Lang, A.-G., Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/bf03193146
Faul, F., Erdfelder, E., Buchner, A., Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. doi: 10.3758/brm.41.4.1149
Feighner, J. P. (1999). Mechanism of action of antidepressant medications. J. Clin. Psychiatry 60 (4), 4–11.
Fink, K. B., Göthert, M. (2007). 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 59 (4), 360–417. doi: 10.1124/pr.107.07103
Guardiola-Lemaitre, B., De Bodinat, C., Delagrange, P., Millan, M. J., Munoz, C., Mocaër, E. (2014). Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br. J. Pharmacol. 171 (15), 3604–3619. doi: 10.1111/bph.12720
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 (1), 56–62. doi: 10.1136/jnnp.23.1.56
Harrell, F. E., Jr., Lee, K. L., Califf, R. M., Pryor, D. B., Rosati, R. A. (1984). “Regression modelling strategies for improved prognostic prediction”. Stat. Med. 3 (2), 143–152. doi: 10.1002/sim.4780030207
Higgins, G. A., Sellers, E. M., Fletcher, P. J. (2013). From obesity to substance abuse: therapeutic opportunities for 5-HT 2C receptor agonists. Trends Pharmacol. Sci. 34 (10), 560–570. doi: 10.1016/j.tips.2013.08.001
Ivanova, S. A., Osmanova, D. Z, Freidin, M. B., Fedorenko, O. Y., Boiko, A. S., Pozhidaev, I. V., et al (2017). Identification of 5-hydroxytryptamine receptor gene polymorphisms modulating hyperprolactinaemia in antipsychotic drug-treated patients with schizophrenia. World J. Biol. Psychiatry 18 (3), 239–246. doi: 10.1080/15622975.2016.1224926
Kessler, R., Bromet, E. (2013). The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 34 (1), 119–138. doi: 10.1146/annurev-publhealth-031912-114409
Kuehner, C. (2017). Why is depression more common among women than among men? Lancet Psychiatry 4 (2), 146–158. doi: 10.1016/S2215-0366(16)30263-2
Leysen, J. (2004). 5-HT2 Receptors. Curr. Drug Target -CNS Neurol. Disord. 3 (1), 11–26. doi: 10.2174/1568007043482598
Loonen, A. J. M., Ivanova, S. A. (2016). Circuits regulating pleasure and happiness—mechanisms of depression. Front. Hum. Neurosci. 10, 1–25. doi: 10.3389/fnhum.2016.00571
Loonen, A. J. M., Wilffert, B., Ivanova, S. A. (2019). Putative role of pharmacogenetics to elucidate the mechanism of tardive dyskinesia in schizophrenia. Pharmacogenomics. doi: 10.2217/pgs-2019-0100
Losenkov, I. S., Mulder, N. J. V., Levchuk, L. A., Vyalova, N. M., Loonen, A. J. M., Bosker, F. J., et al. (submitted). Validation of biomarkers in depression: association between BDNF gene variant rs6265 and the severity of depression in antidepressant drug-naïve depressed patients. Submitted for publication.
Nebigil, C., Etienne, N., Schaerlinger, B., Hickel, P., Launay, J., Maroteaux, L. (2001). Developmentally regulated serotonin 5-HT2B receptors. Int. J. Dev. Neurosci. 19 (4), 365–372. doi: 10.1016/s0736-5748(01)00022-3
Ochi, T., Vyalova, N., Losenkov, I., Levchuk, L., Osmanova, D., Mikhalitskaya, E., et al. (2019). Investigating the potential role of BDNF and PRL genotypes on antidepressant response in depression patients: a prospective inception cohort study in treatment-free patients. J. Affect. Disord. 259, 432–439. doi: 10.1016/j.jad.2019.08.058
Pooley, E. C., Fairburn, C. G., Cooper, Z., Sodhi, M. S., Cowen, P. J., Harrison, P. J. (2004). A 5-HT2C receptor promoter polymorphism (HTR2C-759C/T) is associated with obesity in women, and with resistance to weight loss in heterozygotes. Am. J. Med. Genet. 126B (1), 124–127. doi: 10.1002/ajmg.b.20143
Pozhidaev, I. V., Paderina, D. Z., Fedorenko, O. Y., Kornetova, E. G., Semke, A. V., Loonen, A. J. M., et al. (submitted). 5-Hydroxytryptamine receptors and tardive dyskinesia in schizophrenia. Submitted for publication.
Reynolds, G. P., McGowan, O. O., Dalton, C. F. (2014). Pharmacogenomics in psychiatry: the relevance of receptor and transporter polymorphisms. Br. J. Clin. Pharmacol. 77 (4), 654–672. doi: 10.1111/bcp.12312
Serretti, A., Drago, A., Liebman, M. N. (2009). Pharmacogenetics of antidepressant response. Biomarkers Psychiatr. Disord. 3 (3), 315–353. doi: 10.1007/978-0-387-79251-4_14
Sigmon, S. T., Pells, J. J., Boulard, N. E., Whitcomb-Smith, S., Edenfield, T. M., Hermann, B. A., et al. (2005). Gender differences in self-reports of depression: the response bias hypothesis revisited. Sex Roles 53 (5–6), 401–411. doi: 10.1007/s11199-005-6762-3
Toshchakova, V. A., Bakhtiari, Y., Kulikov, A. V., Gusev, S. I., Trofimova, M. V., Fedorenko, O. Y., et al. (2018). Association of polymorphisms of serotonin transporter (5HTTLPR) and 5-HT2C receptor genes with criminal behavior in Russian criminal offenders. Neuropsychobiology 75 (4), 200–210. doi: 10.1159/000487484
Van Oekelen, D., Luyten, W., Leysen, J. (2003). 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 72 (22), 2429–2449. doi: 10.1016/s0024-3205(03)00141-3
Keywords: 5-HT receptor genes, treatment response, polymorphism, antidepressant, treatment-free depression
Citation: Ochi T, Vyalova NM, Losenkov IS, Paderina DZ, Pozhidaev IV, Loonen AJM, Simutkin GG, Bokhan NA, Ivanova SA and Wilffert B (2019) Limited Associations Between 5-HT Receptor Gene Polymorphisms and Treatment Response in Antidepressant Treatment-Free Patients With Depression. Front. Pharmacol. 10:1462. doi: 10.3389/fphar.2019.01462
Received: 10 September 2019; Accepted: 13 November 2019;
Published: 19 December 2019.
Edited by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceReviewed by:
Dubravka Svob Strac, Rudjer Boskovic Institute, CroatiaLuc Maroteaux, INSERM U839 Institut du Fer à Moulin, France
Copyright © 2019 Ochi, Vyalova, Losenkov, Paderina, Pozhidaev, Loonen, Simutkin, Bokhan, Ivanova and Wilffert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton J. M. Loonen, YS5qLm0ubG9vbmVuQHJ1Zy5ubA==
 Taichi Ochi
Taichi Ochi Natalya M. Vyalova
Natalya M. Vyalova Innokentiy S. Losenkov
Innokentiy S. Losenkov Diana Z. Paderina
Diana Z. Paderina Ivan V. Pozhidaev
Ivan V. Pozhidaev Anton J. M. Loonen
Anton J. M. Loonen German G. Simutkin
German G. Simutkin Nikolay A. Bokhan
Nikolay A. Bokhan Svetlana A. Ivanova
Svetlana A. Ivanova Bob Wilffert1,8
Bob Wilffert1,8