- 1Neurobiology of Aging and Disease Laboratory, Lee Kong Chian School of Medicine, Nanyang Technological University Singapore, Singapore
- 2Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kota Bharu, Malaysia
- 3Brain & Behaviour Cluster and Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kota Bharu, Malaysia
Natural products remain a crucial source of drug discovery for accessible and affordable solutions for healthy aging. Centella asiatica (L.) Urb. (CA) is an important medicinal plant with a wide range of ethnomedicinal uses. Past in vivo and in vitro studies have shown that the plant extract and its key components, such as asiatic acid, asiaticoside, madecassic acid and madecassoside, exhibit a range of anti-inflammatory, neuroprotective, and cognitive benefits mechanistically linked to mitoprotective and antioxidant properties of the plant. Mitochondrial dysfunction and oxidative stress are key drivers of aging and neurodegenerative disease, including Alzheimer’s disease and Parkinson’s disease. Here we appraise the growing body of evidence that the mitoprotective and antioxidative effects of CA may potentially be harnessed for the treatment of brain aging and neurodegenerative disease.
Introduction
Centella asiatica (L.) Urb. (CA) is a medicinal plant commonly consumed in salads or juices in several countries, including Malaysia, India, Sri Lanka, Indonesia and China (Hashim, 2011; Maulidiani et al., 2012; Bachok et al., 2014; Singh et al., 2014). CA has a wide range of ethnomedical applications, including treatment of gastrointestinal disorders, skin diseases, fever, and cognitive and memory problems (Gohil et al., 2010; Jahan et al., 2012; Sabaragamuwa et al., 2018). Studies of the plant extract and its bioactive compounds have revealed a broad range of pharmacological and therapeutic effects, including anti-ulcer (Zheng et al., 2016), anti-microbial (Idris and Nadzir, 2017), cytoprotective (Choi et al., 2016; Tewari et al., 2016), anti-inflammatory (Choi et al., 2016; Park et al., 2017; Ho et al., 2018), anti-oxidant (Zhao et al., 2014; Dewi and Maryani, 2015; Intararuchikul et al., 2019) and mitoprotective (Gray et al., 2017; Zhang et al., 2017; Gray et al., 2018c) properties. The bioactive components of CA readily cross the blood brain barrier and exert beneficial neuroactive effects in a range of models of aging (Zweig et al., 2021) and neurodegenerative disease including Alzheimer’s disease (AD) (Gray et al., 2018c; Matthews et al., 2019) and Parkinson’s disease (PD) (Gopi and Arambakkam Janardhanam, 2017; Teerapattarakan et al., 2018). Recent studies have associated these neuroprotective and anti-inflammatory effects with increased expression of proteins essential for mitochondrial bioenergetics and antioxidant genes (Gray et al., 2018c; Lu et al., 2021; Zweig et al., 2021). Mitochondria play a pivotal role in aging and neurodegeneration, regulating energy metabolism, immune responses and cell death pathways (Moreira et al., 2010; Rizzuto et al., 2012; Mills and O'Neill, 2016; Sun et al., 2016; Shah et al., 2019). Hence, this review focuses on the potential therapeutic application of CA for the treatment of brain-aging and neurodegenerative disease through restoration of mitochondrial function and inhibition of oxidative damage.
Centella asiatica (L.) Urb. (CA): The Medicinal Plant
Botany and Geographical Distribution of Centella asiatica (L.) Urb.
CA is commonly known by several names, including gotu kola in Sinhala, pegaga in Malay, ‘léi gōng gēn’ in Chinese and Asian or Indian Pennywort in English (Jahan et al., 2012; Orhan, 2012; Singh et al., 2014; Gajbhiye et al., 2016). CA belongs to the Apiaceae family, which is native to Asian countries and parts of China as well as several other parts of the world, such as northern Australia and the Western Pacific. The plant grows horizontally, with long, slender and tender prostrate stolons that can extend up to 2 m and are characterized by long internodes and nodes. Each node of the stem bears one to three leaves that are about 2–6 cm in length and 1.5–5 cm in width with a slightly cupped circular-reniform shape and palmately netted veins. CA is odorless and flowers from April to June with fascicled umbels that consist of three to four sessile flowers. These flowers bear 4-mm-long fruits that range in shape from oval to globular. Found up to 1800 m above sea level, CA grows in a wide range of habitats, such as open sunny areas, swamps, paddy fields as well as along the banks of lakes and ponds and on stone walls and rocks (Roy et al., 2013; Singh et al., 2014; Sirichoat et al., 2015; Gajbhiye et al., 2016).
Centella asiatica (L.) Urb. and its Major Phytochemical Constituents
CA contains amino acids, alkaloids, carbohydrates, vitamins, minerals, terpenes of various categories (such as monoterpenes, sesquiterpenes, diterpenes, triterpenes and tetraterpene) and phenolic compounds (such as the flavonoids, tannins and other constituents). The phytochemistry of CA has previously been comprehensively reviewed by Brinkhaus et al. (2000), Gray et al. (2018a) and Torbati et al. (2021) therefore will only be summarized briefly here. Terpenes are the dominant group of chemical constituents of CA, with triterpenes being the major and most important component of CA, serving as a marker constituent for quality control analyses (Rafi et al., 2018). The triterpenes (Figure 1) found in CA are mostly pentacyclic triterpenic acids (sapogenins), such as the asiatic acid (PubChem CID: 119034, National Center for Biotechnology Information, 2021a) and madecassic acid (PubChem CID: 73412, National Center for Biotechnology Information, 2021b), and their respective triterpenoid glycosides (saponins, with a trisaccharide moiety linked to the aglycones), such as asiaticoside (PubChem CID: 52912190, National Center for Biotechnology Information, 2021c) and madecassoside (PubChem CID: 131801373, National Center for Biotechnology Information, 2021d) (Azerad, 2016; Rafi et al., 2018).
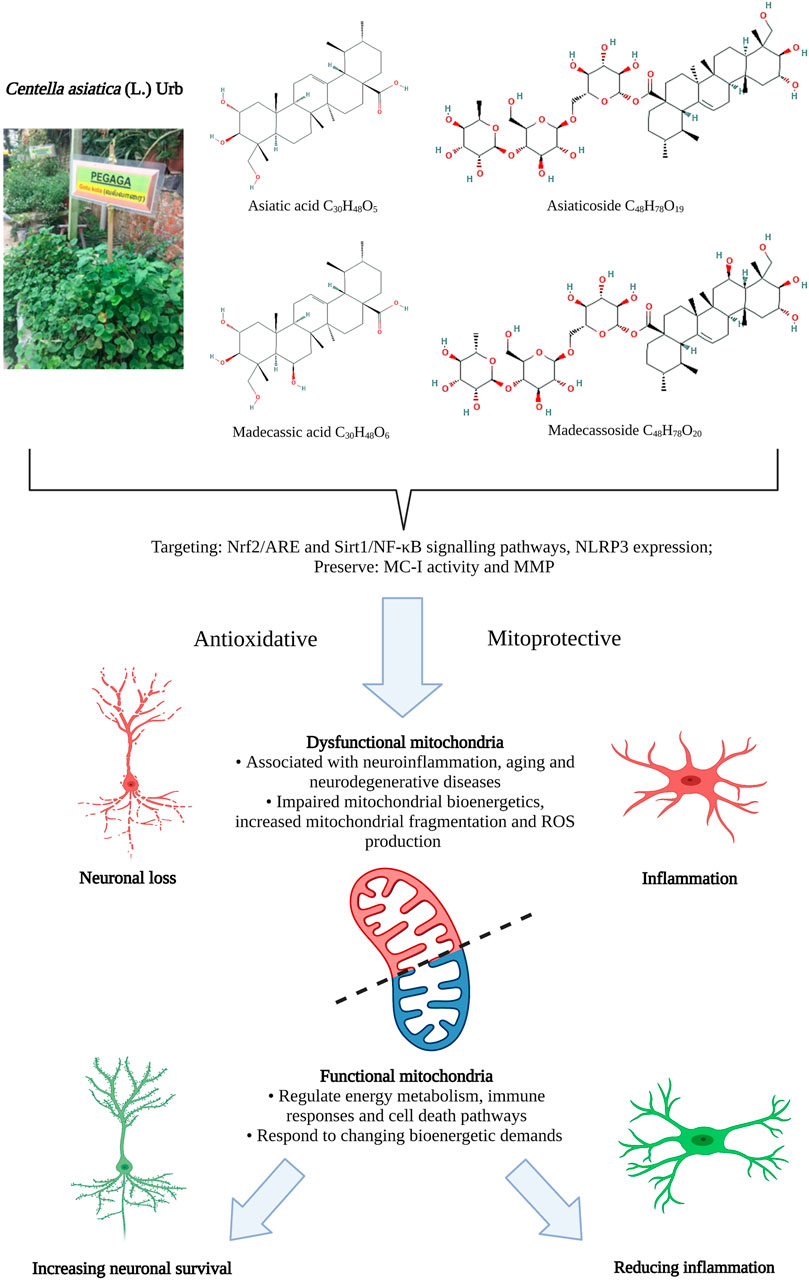
FIGURE 1. Antioxidative and mitoprotective activities of Centella asiatica and its main components. Mitochondrial dysfunction in regulating energy metabolism in response to changing bioenergy demands is closely associated with neuroinflammation in aging and neurodegenerative diseases. The antioxidative and mitoprotective activities of CA targeting mitochondrial and oxidative functions may confer neuroprotective benefits that could potentially be harnessed to treat aging and neurodegenerative diseases and improve functional behavioral outcomes. ARE, antioxidant response element genes; MC-I, mitochondrial complex I; MMP, mitochondrial membrane potential; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing three; Nrf2, NF-E2-p45-related factor 2; Sirt1, Sirtuin 1. Figure created with BioRender.com.
CA extract has been widely studied in the form of ethanolic (Sari et al., 2014; Sari and Rochmah, 2015; Binti Mohd Yusuf Yeo et al., 2018; Suri et al., 2018; Wong et al., 2019; Wong et al., 2020), methanolic (Veerendra Kumar and Gupta, 2003; Arora et al., 2018) and aqueous (Mitha et al., 2016; Gray et al., 2018c; Chintapanti et al., 2018) extract as well as leaf juice (Rao et al., 2007; Thirawarapan et al., 2019). Of these different preparations of CA, it was found that the ethanolic extract retained the highest amount of the triterpenes asiatic acid and asiaticoside compared to other solvents (Puttarak and Panichayupakaranant, 2013; Gajbhiye et al., 2016).
Wide chemotypic variations in triterpenoids were found in CA planted in different growing regions, altitudes and localities (Long et al., 2012; Singh et al., 2014; Srivastava et al., 2014). Genotypic and phenotypic variability have been associated with differences in phytochemicals content of CA including macronutrients, micronutrients, phenolics, flavonoids, tannin, anthocyanin, carotenoids and ascorbic acid (Thomas et al., 2010; Singh et al., 2014; Lal et al., 2017; Chandrasekara et al., 2020). Other than geographical and genotypical influences, the phytochemical compositions of CA also vary due to seasonal variations associated with the cultivation and harvesting procedures, light conditions, as well as the drying conditions post-harvesting (Maulidiani et al., 2012; Rahajanirina et al., 2012; Alqahtani et al., 2015; Plengmuankhae and Tantitadapitak, 2015). This underlines the potential challenges involved in the study of CA plant extract, as differences in specific phytochemical composition may influence the efficacy of the extract.
Neuroactive Effects of Centella asiatica (L.) Urb.: Crossing the Blood Brain Barrier
Several pharmacokinetic studies have confirmed that bioactive components of CA can cross the blood brain barrier (BBB) when administered peripherally, although the transport mechanisms of these phytochemicals remain largely unknown. For example, asiatic acid, asiaticoside and madecassoside were found to accumulate in the brains of animals administered with CA extract or the respective single components (Yin et al., 2012; Anukunwithaya et al., 2017a; Anukunwithaya et al., 2017b). A recent study using primary porcine brain endothelial cells as in vitro BBB model also reported that asiatic acid, asiaticoside and madecassoside exhibit high permeability across the BBB (Hanapi et al., 2021). The bioavailability of these phytochemicals in brain tissue after peripheral administration (Yin et al., 2012; Anukunwithaya et al., 2017a; Anukunwithaya et al., 2017b) indicates they cross the BBB at adequate concentrations to exert neuroactive effects supporting the potential use of these compounds as neurotherapeutics.
Neuroactive Effects of Centella asiatica (L.) Urb.: Cognition
Cognitive-enhancing effects of CA extract have been described in numerous studies, in both normal animals and models of aging and neurodegenerative disease (Doknark et al., 2014; Sari et al., 2014; Sirichoat et al., 2015; Yolanda et al., 2015; Wong et al., 2019; Sbrini et al., 2020). In early studies, CA extract was found to improve memory and ameliorate biochemical and mitochondrial dysfunction in a mouse model of aging (Kumar et al., 2011). In other studies CA was found to confer protection against hippocampal dysfunction, a region of the brain that plays a critical role in learning and memory and is severely affected in AD (Veerendra Kumar and Gupta, 2003; Giribabu et al., 2014). Further, key bioactive components of CA have also been shown to affect learning and memory in models of aging and neurodegenerative disease. For example, asiaticoside has been found to enhance cognitive performance in aged animals (Lin et al., 2013) and a rat model of AD (Zhang et al., 2017). The cognitive effects of CA extract have been linked to changes in synaptic plasticity (Lin et al., 2013) and excitatory neurotransmission (Wanasuntronwong et al., 2018; Wong et al., 2020) as well as improved neuronal health and survival in models of aging and disease (Gray et al., 2018b; Gray et al., 2018c). Here we will examine the evidence that CA and its phytochemicals provide cognitive benefits in aging and neurodegenerative disease via mitoprotective and antioxidant mechanisms (Soumyanath et al., 2012; Chen et al., 2016; Gray et al., 2016; Gray et al., 2017; Matthews et al., 2019).
Targeting Mitochondria in Aging and Neurodegenerative Disease: Role for Centella asiatica (L.) Urb.
Mitochondrial dysfunction is closely associated with aging (López-Otín et al., 2013; Sun et al., 2016), AD (Moreira et al., 2010; Yoo et al., 2020) and PD (Yang et al., 2020). Mitochondria regulate energy metabolism, immune responses and cell-death pathways through their highly flexible and dynamic network. The mitochondrial network responds to changing bioenergetic demands by adjusting the rate of mitochondrial fission and fusion—a function that was found to be affected in most age-associated neurodegenerative conditions (Shah et al., 2019). Studies have shown that age-related toxic protein aggregates, such as Alzheimer’s beta amyloid (Aβ), induce mitochondrial dysregulation by binding to mitochondrial proteins. For example, Aβ has been found to bind to the mitochondrial fission protein (Drp1), and the mitochondrial voltage-dependent anion channel (VDAC) (Manczak et al., 2011; Manczak et al., 2018). These abnormal protein interactions affect mitochondrial biogenesis, increase mitochondrial fragmentation and induce free radical production (John and Reddy, 2020).
Mitochondria are the primary source of free radicals, otherwise known as reactive oxygen species (ROS), and ROS overproduction leads to oxidative damage. Oxidative damage further affects the mitochondrial respiratory chain function in generating energy in the form of adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) (Elfawy and Das, 2019). Perturbations in the electron transport chain function and/or reduction in the mitochondrial membrane potential lead to a vicious cycle of mitochondrial stress, which results in decreased ATP production and increased ROS production (Szalardy et al., 2015; Zorova et al., 2018). The brain is highly susceptible to both bioenergetic dysfunction and oxidative damage due to the high energy demands associated with neurotransmission and a high lipid content, respectively. The use of antioxidant strategies has been reported to provide a protective benefit against aging and neurodegenerative diseases. Further, enhancing mitochondrial biogenesis and quality control may be an efficient strategy for preventing mitochondrial disorders (Smith et al., 2012; Suliman and Piantadosi, 2016; Murphy and Hartley, 2018) and providing neuroprotection in AD and PD mouse models (Johri and Beal, 2012). Several therapeutic approaches that aim to protect against neurodegeneration and inflammation by improving brain bioenergetics, rescuing mitochondrial dysfunction and reducing oxidative damage are being developed (Cunnane et al., 2020; Fairley et al., 2021). In this section, we will focus on the mitoprotective and antioxidative effects of CA and its key phytochemicals as potential therapeutic agents that can 1) promote neuronal health and survival, and 2) reduce neuroinflammation.
Neuroprotective Effects of Centella asiatica (L.) Urb. and its Major Constituents: Antioxidative and Mitoprotective Effects
Neuroprotective effects of CA have been described in several models of neurodegenerative disease and injury, linked to effects on mitochondrial energy production, oxidative stress and mitochondrial-induced apoptosis. For example, the CA extract, asiatic acid has been shown to prevent mitochondrial morphology abnormalities in a rat model of kainic acid-induced seizure, which protected synaptic function and alleviated cognitive deficits (Lu et al., 2021). In a separate study, the CA phytochemical asiaticoside was found to inhibit Aβ-induced neuronal apoptosis by restoring and maintaining mitochondrial membrane potential (Song et al., 2018). Several potential molecular mechanisms mediating the mitoprotective effects of CA have been proposed, including increased conductance and stabilization of VDAC (Tewari et al., 2016). VDAC plays a critical role in cell survival, transport of substrates for energy production and maintenance of mitochondrial membrane potential (Camara et al., 2017), making it a target of interest in regulating mitochondrial function.
Meanwhile, other studies have implicated CA and its bioactive components in the regulation of important antioxidant response signaling pathways. In mouse models of AD, CA extract has been found to promote antioxidative responses, countering Aβ pathology-driven oxidative stress, mitigating neuronal loss around the plaques and improving memory function (Gray et al., 2017; Gray et al., 2018c). CA extract has also been found to protect rotenone-induced parkinsonism rats against lipid peroxidation, dopaminergic neuronal death and locomotor deficit. These protective effects were associated with increased antioxidant enzyme expression and preservation of mitochondrial complex I activity, which is responsible for the rate-limiting step in OXPHOS (Teerapattarakan et al., 2018). Madecassoside was also found to be effective at ameliorating the deficits observed in PD rat models via its antioxidative activities, maintaining the redox balance (Xu et al., 2013). Similarly, asiaticoside has been found to reduce oxidative stress induced by rotenone (Gopi and Arambakkam Janardhanam, 2017; Subaraja and Vanisree, 2019). Likewise, asiatic acid provided antioxidative benefits in a drosophila PD model, protecting mitochondria against rotenone-induced oxidative stress and apoptosis. The antioxidative properties of asiatic acid are also thought to mediate neuroprotection and improve spatial memory function in animals treated with valproic acid (Xu et al., 2012; Umka Welbat et al., 2016). Outside of the brain, antioxidative effects of CA are also observed in other organs and systems. For example, CA extract was found to inhibit lipid peroxidation in rotenone-treated rats (Intararuchikul et al., 2019) and regulate lipid metabolism via antioxidant effect (Zhao et al., 2014). These findings support the notion that the neuroprotective effects of CA and its bioactive components are at least in part mediated through enhanced antioxidative responses.
CA-induced antioxidative responses have been linked to the higher expression of antioxidant response element genes (AREs) activated via Nrf2 (NF-E2-p45-related factor two, encoded by the NFE2L2 gene) (Matthews et al., 2019). The Nrf2/ARE signaling cascade regulates a plethora of cellular activities, including metabolic reprogramming, mitochondrial physiology and biogenesis, antioxidant stress response, drug detoxification, inflammation, autophagy and unfolded protein response and proteostasis (Dinkova-Kostova and Abramov, 2015; He et al., 2020). Altered expression of Nrf2-targeted genes is associated with AD, and previous studies have demonstrated that the activation of Nrf2 ameliorates Aβ pathology and cognitive deficits in AD mouse models (Bahn et al., 2019). Consequently, activation of Nrf2 pathway represents a promising therapeutic direction for enhancing mitochondrial quality control and biogenesis in aging and neurodegenerative diseases (Kerr et al., 2017; Gureev et al., 2019; Gureev and Popov, 2019; Brandes and Gray, 2020; Bento-Pereira and Dinkova-Kostova, 2021). Subsequent studies found that Nrf2 is a crucial component of the mitoprotective effects of CA, whereby long-term CA treatment improved the cognitive performance of wild type but not Nrf2 deficient mice (Nrf2 knockout) (Zweig et al., 2021). Further, these studies associated hippocampal mitochondrial dysfunction with cognitive performance.
In addition to the general ability to induce antioxidant responses, disease-specific mitoprotective effects of CA have also been identified in models of PD. For example, CA components have been shown to block the translocation of α-synuclein to the mitochondria, therefore maintaining mitochondrial membrane integrity and ATP production (Ding et al., 2018). Further, pre-treatment with asiatic acid significantly decreased mitochondrial ROS production in a 1-methyl-4-phenyl-pyridine (MPP+)-induced neuroblastoma model of PD and protected the cells form the loss of mitochondrial membrane potential (Chen et al., 2019). Additionally, CA and its triterpenoids may also reduce ROS production (Gray et al., 2017; Nataraj et al., 2017), thus potentially restoring mitochondrial function in the central nervous system (Onyango et al., 2017). For example, madecassic acid inhibited ROS production in human retinal microvascular endothelial cells (hMRECs) following hypoxia-induced oxidative stress (Yang et al., 2016). The molecular targets mediating these effects are yet to be elucidated and whether they are generalized to other disease models remains to be determined.
Anti-Inflammatory Effects of Centella asiatica (L.) Urb. and its Major Constituents
The mitochondrial and metabolic fitness of the brain’s innate immune system plays an important role in the neuroinflammatory responses involved in neurodegenerative diseases (Paolicelli and Angiari, 2019)—a concept known as “immunometabolism” (O'Neill et al., 2016). Mitochondrial-dependent OXPHOS and fatty acid oxidation (FAO) are associated with anti-inflammatory responses (Mills and O'Neill, 2016) while, on the other hand, inflammatory responses are associated with a shift toward non-mitochondrial erobic glycolysis (Rodríguez-Prados et al., 2010; Galván-Peña and O’Neill, 2014). This switch toward erobic glycolysis causes several functional changes: 1) rapid supply of ATP, 2) proinflammatory cytokine production, 3) rearrangement of the tricarboxylic acid (TCA) cycle and accumulation of intermediate metabolites, such as succinate and citrate, and 4) repurposing of the electron transport chain (ETC) to produce ROS (Lampropoulou et al., 2016; Millet et al., 2016; Mills et al., 2016). Furthermore, microglial activation releases neurotoxic factors, such as mitochondrial-generated ROS, that exacerbate the neuroinflammation, thus resulting in neuronal death and neurodegeneration (González et al., 2014; Simpson and Oliver, 2020). Microglia are metabolically plastic and, hence, are potential therapeutic targets for the treatment of AD using metabolic reprogramming strategies (Fairley et al., 2021).
CA and its derivatives have also been shown to affect inflammatory responses through the regulation of mitochondrial and oxidative functions. Asiatic acid, asiaticoside and madecassoside have been found to demonstrate anti-inflammatory effects through a reduction of cytokine levels and the activation of microglia in stroke models (Krishnamurthy et al., 2009; Chen et al., 2014; Luo et al., 2014). Sirtuin 1 (Sirt 1) protein is an important epigenetic regulator for many physiological processes, modulating downstream pathways by targeting proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and plays a role in alleviating oxidative stress (Elibol and Kilic, 2018). In an immortalized microglial cell line, asiatic acid was found to prevent LPS-induced neuroinflammation by enhancing Sirt1 expression while suppressing NF-κB activation, attenuated the production of nitric oxide and the expression of inducible nitric oxide synthase (iNOS) and reduced the expression and release of inflammatory cytokines in response to LPS-induced inflammation (Qian et al., 2018). Asiatic acid was shown to protect BV2 cells from LPS-induced damage by suppressing NLRP3 (NLR family pyrin domain containing three) expression and decreasing mitochondrial ROS, effectively ameliorating mitochondrial dysfunction (Chen et al., 2019).
Anti-inflammatory effects have also been reported in models of AD. In a study that used the intracerebroventricular infusion of toxic forms of Alzheimer’s Aβ, the neuroprotective effects of asiaticoside in Aβ-infused rats were suggested as being associated with the anti-inflammatory properties of asiaticoside, hence mitigating mitochondrial injuries and regulating the expression of apoptosis markers (Zhang et al., 2017). The mitoprotective effects of asiatic acid have been demonstrated in earlier studies that targeted the regulation of the mitochondrial membrane potential and ROS production (Xiong et al., 2009; Xu et al., 2012). Taken together, these findings demonstrate that CA and its major phytochemicals inhibit ROS production and ameliorate mitochondrial dysfunction, reducing detrimental inflammatory responses.
Conclusion
Plants produce chemically, structurally and molecularly diverse phytochemicals that determine their evolutionary success. These compounds represent biological functions and continue to provide crucial novel pharmacological leads for the treatment of human diseases. CA and its phytochemicals have wide ethnopharmacological applications in various cultures, and its biological effects have been substantiated in numerous studies. These findings suggest that CA confers pleiotropic neuroprotective and anti-inflammatory benefits through its mitoprotective and antioxidative effects, which could potentially be harnessed for the treatment of aging and neurodegenerative diseases. Further research is still needed to determine the synergistic effects, safety, efficacy, bioavailability and metabolism of these components.
Author Contributions
JW, AB, and JA wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
AB acknowledges funding support from the Nanyang Assistant Professorship from Nanyang Technological University Singapore. JW and JA acknowledge funding support from the NKEA Research Grant Scheme (NRGS Grant, NH1014D049) for the research presented in this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alqahtani, A., Tongkao-on, W., Li, K. M., Razmovski-Naumovski, V., Chan, K., and Li, G. Q. (2015). Seasonal Variation of Triterpenes and Phenolic Compounds in australian Centella asiatica (L.) Urb. Phytochem. Anal. 26 (6), 436–443. doi:10.1002/pca.2578
Anukunwithaya, T., Tantisira, M., Shimada, T., Sai, Y., and Khemawoot, P. (2017a). Multiple Oral Dosing Pharmacokinetics of Standardized Extract of Centella asiatica ECa 233 and its Inductive Effect on Efflux Transporters in Rats. PMIO 4 (02), e66–e73. doi:10.1055/S-0043-114669
Anukunwithaya, T., Tantisira, M., Tantisira, B., and Khemawoot, P. (2017b). Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Med. 83 (08), 710–717. doi:10.1055/s-0042-122344
Arora, R., Kumar, R., Agarwal, A., Reeta, K. H., and Gupta, Y. K. (2018). Comparison of Three Different Extracts of Centella asiatica for Anti-amnesic, Antioxidant and Anticholinergic Activities: In Vitro and In Vivo Study. Biomed. Pharmacother. 105, 1344–1352. doi:10.1016/j.biopha.2018.05.156
Azerad, R. (2016). Chemical Structures, Production and Enzymatic Transformations of Sapogenins and Saponins from Centella asiatica (L.) Urban. Fitoterapia 114, 168–187. doi:10.1016/j.fitote.2016.07.011
Bachok, M. F., Mohd Yusof, B.-N., Ismail, A., and Hamid, A. A. (2014). Effectiveness of Traditional Malaysian Vegetables ('ulam') in Modulating Blood Glucose Levels. Asia Pac. J. Clin. Nutr. 23 (3), 369–376. doi:10.6133/apjcn.2014.23.3.01
Bahn, G., Park, J.-S., Yun, U. J., Lee, Y. J., Choi, Y., Park, J. S., et al. (2019). NRF2/ARE Pathway Negatively Regulates BACE1 Expression and Ameliorates Cognitive Deficits in Mouse Alzheimer's Models. Proc. Natl. Acad. Sci. USA 116 (25), 12516–12523. doi:10.1073/pnas.1819541116
Bento-Pereira, C., and Dinkova-Kostova, A. T. (2021). Activation of Transcription Factor Nrf2 to Counteract Mitochondrial Dysfunction in Parkinson's Disease. Med. Res. Rev. 41 (2), 785–802. doi:10.1002/med.21714
Binti Mohd Yusuf Yeo, N. A., Muthuraju, S., Wong, J. H., Mohammed, F. R., Senik, M. H., Zhang, J., et al. (2018). Hippocampal Amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic Acid GluA1 (AMPA GluA1) Receptor Subunit Involves in Learning and Memory Improvement Following Treatment with Centella asiatica Extract in Adolescent Rats. Brain Behav. 8 (9), e01093. doi:10.1002/brb3.1093
Brandes, M. S., and Gray, N. E. (2020). NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 12, 175909141989978. doi:10.1177/1759091419899782
Brinkhaus, B., Lindner, M., Schuppan, D., and Hahn, E. G. (2000). Chemical, Pharmacological and Clinical Profile of the East Asian Medical Plant Centella Aslatica. Phytomedicine 7 (5), 427–448. doi:10.1016/s0944-7113(00)80065-3
Camara, A. K. S., Zhou, Y., Wen, P.-C., Tajkhorshid, E., and Kwok, W.-M. (2017). Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 8, 460. doi:10.3389/fphys.2017.00460
Chandrasekara, C. H. W. M. R. B., Sumanarathne, R. A. P. I., and Bandaranayake, P. C. G. (2020). Centellaasiatica Morphotypes Differ Genetically as Well as Macronutrients Content, Total Phenolic Content and Chemical Fingerprints of Leaves. J. Agric. Sci. 15, 75. doi:10.4038/jas.v15i1.8673
Chen, S., Yin, Z.-J., Jiang, C., Ma, Z.-Q., Fu, Q., Qu, R., et al. (2014). Asiaticoside Attenuates Memory Impairment Induced by Transient Cerebral Ischemia-Reperfusion in Mice through Anti-inflammatory Mechanism. Pharmacol. Biochem. Behav. 122, 7–15. doi:10.1016/j.pbb.2014.03.004
Chen, C.-L., Tsai, W.-H., Chen, C.-J., and Pan, T.-M. (2016). Centella asiatica Extract Protects against Amyloid β1-40-induced Neurotoxicity in Neuronal Cells by Activating the Antioxidative Defence System. J. Traditional Complement. Med. 6 (4), 362–369. doi:10.1016/j.jtcme.2015.07.002
Chen, D., Zhang, X.-Y., Sun, J., Cong, Q.-J., Chen, W.-X., Ahsan, H. M., et al. (2019). Asiatic Acid Protects Dopaminergic Neurons from Neuroinflammation by Suppressing Mitochondrial ROS Production. Biomolecules Ther. 27 (5), 442–449. doi:10.4062/biomolther.2018.188
Chintapanti, S., Pratap Reddy, K., and Sreenivasula Reddy, P. (2018). Behavioral and Neurochemical Consequences of Perinatal Exposure to lead in Adult Male Wistar Rats: Protective Effect by Centella asiatica. Environ. Sci. Pollut. Res. 25 (13), 13173–13185. doi:10.1007/s11356-018-1500-x
Choi, M.-J., Zheng, H.-M., Kim, J. M., Lee, K. W., Park, Y. H., and Lee, D. H. (2016). Protective Effects of Centella asiatica Leaf Extract on Dimethylnitrosamine-Induced Liver Injury in Rats. Mol. Med. Rep. 14 (5), 4521–4528. doi:10.3892/mmr.2016.5809
Cunnane, S. C., Trushina, E., Morland, C., Prigione, A., Casadesus, G., Andrews, Z. B., et al. (2020). Brain Energy rescue: an Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 19 (9), 609–633. doi:10.1038/s41573-020-0072-x
Dewi, R. T., and Maryani, F. (2015). Antioxidant and α-Glucosidase Inhibitory Compounds of Centella Asiatica. Proced. Chem. 17, 147–152. doi:10.1016/j.proche.2015.12.130
Ding, H., Xiong, Y., Sun, J., Chen, C., Gao, J., and Xu, H. (2018). Asiatic Acid Prevents Oxidative Stress and Apoptosis by Inhibiting the Translocation of α-Synuclein into Mitochondria. Front. Neurosci. 12, 431. doi:10.3389/fnins.2018.00431
Dinkova-Kostova, A. T., and Abramov, A. Y. (2015). The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 88 (Pt B), 179–188. doi:10.1016/j.freeradbiomed.2015.04.036
Doknark, S., Mingmalairak, S., Vattanajun, A., Tantisira, B., and Tantisira, M. H. (2014). Study of Ameliorating Effects of Ethanolic Extract of Centella asiatica on Learning and Memory Deficit in Animal Models. J. Med. Assoc. Thai. 97 Suppl 2 (Suppl. 2), S68–S76.
Elfawy, H. A., and Das, B. (2019). Crosstalk between Mitochondrial Dysfunction, Oxidative Stress, and Age Related Neurodegenerative Disease: Etiologies and Therapeutic Strategies. Life Sci. 218, 165–184. doi:10.1016/j.lfs.2018.12.029
Elibol, B., and Kilic, U. (2018). High Levels of SIRT1 Expression as a Protective Mechanism against Disease-Related Conditions. Front. Endocrinol. 9, 614. doi:10.3389/fendo.2018.00614
Fairley, L. H., Wong, J. H., and Barron, A. M. (2021). Mitochondrial Regulation of Microglial Immunometabolism in Alzheimer's Disease. Front. Immunol. 12, 257. doi:10.3389/fimmu.2021.624538
Gajbhiye, N. A., Makasana, J., Saha, A., Patel, I., and Jat, R. S. (2016). LC-ESI-MS/MS Method for Simultaneous Determination of Triterpenoid Glycosides and Aglycones in Centella asiatica L. Chromatographia 79 (11), 727–739. doi:10.1007/s10337-016-3089-x
Galván-Peña, S., and O’Neill, L. A. (2014). Metabolic Reprograming in Macrophage Polarization. Front. Immunol. 5, 420. doi:10.3389/fimmu.2014.00420
Giribabu, N., Srinivasarao, N., Swapna Rekha, S., Muniandy, S., and Salleh, N. (2014). Centella asiatica Attenuates Diabetes Induced Hippocampal Changes in Experimental Diabetic Rats. Evidence-Based Complement. Altern. Med. 2014, 1–10. doi:10.1155/2014/592062
Gohil, K., Patel, J., and Gajjar, A. (2010). Pharmacological Review on Centella asiatica: A Potential Herbal Cure-All. Indian J. Pharm. Sci. 72 (5), 546–556. doi:10.4103/0250-474X.78519
González, H., Elgueta, D., Montoya, A., and Pacheco, R. (2014). Neuroimmune Regulation of Microglial Activity Involved in Neuroinflammation and Neurodegenerative Diseases. J. Neuroimmunol. 274 (1-2), 1–13. doi:10.1016/j.jneuroim.2014.07.012
Gopi, M., and Arambakkam Janardhanam, V. (2017). Asiaticoside: Attenuation of Rotenone Induced Oxidative burden in a Rat Model of Hemiparkinsonism by Maintaining the Phosphoinositide-Mediated Synaptic Integrity. Pharmacol. Biochem. Behav. 155, 1–15. doi:10.1016/j.pbb.2017.02.005
Gray, N. E., Harris, C. J., Quinn, J. F., and Soumyanath, A. (2016). Centella asiatica Modulates Antioxidant and Mitochondrial Pathways and Improves Cognitive Function in Mice. J. Ethnopharmacology 180, 78–86. doi:10.1016/j.jep.2016.01.013
Gray, N. E., Zweig, J. A., Matthews, D. G., Caruso, M., Quinn, J. F., and Soumyanath, A. (2017). Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Aβ-Exposed Hippocampal Neurons. Oxidative Med. Cell Longevity 2017, 1–8. doi:10.1155/2017/70230912017
Gray, N. E., Alcazar Magana, A., Lak, P., Wright, K. M., Quinn, J., Stevens, J. F., et al. (2018a). Centella asiatica: Phytochemistry and Mechanisms of Neuroprotection and Cognitive Enhancement. Phytochem. Rev. 17 (1), 161–194. doi:10.1007/s11101-017-9528-y
Gray, N. E., Zweig, J. A., Caruso, M., Martin, M. D., Zhu, J. Y., Quinn, J. F., et al. (2018b). Centella asiatica Increases Hippocampal Synaptic Density and Improves Memory and Executive Function in Aged Mice. Brain Behav. 8, e01024. doi:10.1002/brb3.1024
Gray, N. E., Zweig, J. A., Caruso, M., Zhu, J. Y., Wright, K. M., Quinn, J. F., et al. (2018c). Centella asiatica Attenuates Hippocampal Mitochondrial Dysfunction and Improves Memory and Executive Function in β-amyloid Overexpressing Mice. Mol. Cell Neurosci. 93, 1–9. doi:10.1016/j.mcn.2018.09.002
Gureev, A. P., and Popov, V. N. (2019). Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 44 (10), 2273–2279. doi:10.1007/s11064-018-02711-2
Gureev, A. P., Shaforostova, E. A., and Popov, V. N. (2019). Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 10, 435. doi:10.3389/fgene.2019.00435435
Hanapi, N. A., Mohamad Arshad, A. S., Abdullah, J. M., Tengku Muhammad, T. S., and Yusof, S. R. (2021). Blood-brain Barrier Permeability of Asiaticoside, Madecassoside and Asiatic Acid in Porcine Brain Endothelial Cell Model. J. Pharm. Sci. 110 (2), 698–706. doi:10.1016/j.xphs.2020.09.015
Hashim, P. (2011). Centella asiatica in Food and Beverage Applications and its Potential Antioxidant and Neuroprotective Effect. Int. Food Res. J. 18 (4), 1215.
He, F., Ru, X., and Wen, T. (2020). NRF2, a Transcription Factor for Stress Response and beyond. Ijms 21 (13), 4777. doi:10.3390/ijms21134777
Ho, P. J., Sung, J. J., Cheon, K. K., and Tae, H. J. (2018). Anti-inflammatory Effect of Centella asiatica Phytosome in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis. Phytomedicine 43, 110–119. doi:10.1016/j.phymed.2018.04.013
Idris, F. N., and Nadzir, M. M. (2017). Antimicrobial Activity of Centella asiatica on Aspergillus niger and Bacillus Subtilis. Chem. Eng. Trans. 56, 1381–1386. doi:10.3303/CET1756231
Intararuchikul, T., Teerapattarakan, N., Rodsiri, R., Tantisira, M., Wohlgemuth, G., Fiehn, O., et al. (2019). Effects of Centella Asiatica extract on Antioxidant Status and Liver Metabolome of Rotenone-Treated Rats Using GC-MS. Biomed. Chromatogr. 33 (2), e4395. doi:10.1002/bmc.4395
Jahan, R., Hossain, S., Seraj, S., Nasrin, D., Khatun, Z., Das, P. R., et al. (2012). Centella asiatica (L.) Urb.: Ethnomedicinal Uses and Their Scientific Validations. Am. -Eurasian J. Sustain. Agric. 6 (4), 261–270.
John, A., and Reddy, P. H. (2021). Synaptic Basis of Alzheimer's Disease: Focus on Synaptic Amyloid Beta, P-Tau and Mitochondria. Ageing Res. Rev. 65, 101208. doi:10.1016/j.arr.2020.101208
Johri, A., and Beal, M. F. (2012). Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 342 (3), 619–630. doi:10.1124/jpet.112.192138
Kerr, F., Sofola-Adesakin, O., Ivanov, D. K., Gatliff, J., Gomez Perez-Nievas, B., Bertrand, H. C., et al. (2017). Direct Keap1-Nrf2 Disruption as a Potential Therapeutic Target for Alzheimer's Disease. Plos Genet. 13 (3), e1006593. doi:10.1371/journal.pgen.1006593
Krishnamurthy, R. G., Senut, M.-C., Zemke, D., Min, J., Frenkel, M. B., Greenberg, E. J., et al. (2009). Asiatic Acid, a Pentacyclic Triterpene from Centella Asiatica, Is Neuroprotective in a Mouse Model of Focal Cerebral Ischemia. J. Neurosci. Res. 87 (11), 2541–2550. doi:10.1002/jnr.22071
Kumar, A., Prakash, A., and Dogra, S. (2011). Centella asiatica Attenuates D-Galactose-Induced Cognitive Impairment, Oxidative and Mitochondrial Dysfunction in Mice. Int. J. Alzheimer's Dis. 2011, 347569. doi:10.4061/2011/347569
Lal, R. K., Gupta, P., and Dubey, B. K. (2017). Genetic Variability and Associations in the Accessions of Manduk Parni {Centella asiatica (L)}. Ind. Crops Prod. 96, 173–177. doi:10.1016/j.indcrop.2016.11.056
Lampropoulou, V., Sergushichev, A., Bambouskova, M., Nair, S., Vincent, E. E., Loginicheva, E., et al. (2016). Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cel Metab. 24 (1), 158–166. doi:10.1016/j.cmet.2016.06.004
Lin, X., Huang, R., Zhang, S., Wei, L., Zhuo, L., Wu, X., et al. (2013). Beneficial Effects of Asiaticoside on Cognitive Deficits in Senescence-Accelerated Mice. Fitoterapia 87, 69–77. doi:10.1016/j.fitote.2013.03.023
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2013). The Hallmarks of Aging. Cell 153 (6), 1194–1217. doi:10.1016/j.cell.2013.05.039
Long, H. S., Stander, M. A., and Van Wyk, B.-E. (2012). Notes on the Occurrence and Significance of Triterpenoids (Asiaticoside and Related Compounds) and Caffeoylquinic Acids in Centella Species. South Afr. J. Bot. 82, 53–59. doi:10.1016/j.sajb.2012.07.017
Lu, C.-W., Lin, T.-Y., Pan, T.-L., Wang, P.-W., Chiu, K.-M., Lee, M.-Y., et al. (2021). Asiatic Acid Prevents Cognitive Deficits by Inhibiting Calpain Activation and Preserving Synaptic and Mitochondrial Function in Rats with Kainic Acid-Induced Seizure. Biomedicines 9, 284. doi:10.3390/biomedicines9030284
Luo, Y., Yang, Y.-P., Liu, J., Li, W.-H., Yang, J., Sui, X., et al. (2014). Neuroprotective Effects of Madecassoside against Focal Cerebral Ischemia Reperfusion Injury in Rats. Brain Res. 1565, 37–47. doi:10.1016/j.brainres.2014.04.008
Manczak, M., Calkins, M. J., and Reddy, P. H. (2011). Impaired Mitochondrial Dynamics and Abnormal Interaction of Amyloid Beta with Mitochondrial Protein Drp1 in Neurons from Patients with Alzheimer's Disease: Implications for Neuronal Damage. Hum. Mol. Genet. 20 (13), 2495–2509. doi:10.1093/hmg/ddr139
Manczak, M., Kandimalla, R., Yin, X., and Reddy, P. H. (2018). Hippocampal Mutant APP and Amyloid Beta-Induced Cognitive Decline, Dendritic Spine Loss, Defective Autophagy, Mitophagy and Mitochondrial Abnormalities in a Mouse Model of Alzheimer's Disease. Hum. Mol. Genet. 27 (8), 1332–1342. doi:10.1093/hmg/ddy042
Matthews, D. G., Caruso, M., Murchison, C. F., Zhu, J. Y., Wright, K. M., Harris, C. J., et al. (2019). Centella asiatica Improves Memory and Promotes Antioxidative Signaling in 5XFAD Mice. Antioxidants 8 (12), 630. doi:10.3390/antiox812063012
Maulidiani, H., Khatib, A., Shaari, K., Abas, F., Shitan, M., Kneer, R., et al. (2012). Discrimination of Three Pegaga (Centella) Varieties and Determination of Growth-Lighting Effects on Metabolites Content Based on the Chemometry of 1H Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 60 (1), 410–417. doi:10.1021/jf200270y
Millet, P., Vachharajani, V., McPhail, L., Yoza, B., and McCall, C. E. (2016). GAPDH Binding to TNF-α mRNA Contributes to Posttranscriptional Repression in Monocytes: A Novel Mechanism of Communication between Inflammation and Metabolism. J.I. 196 (6), 2541–2551. doi:10.4049/jimmunol.1501345
Mills, E. L., and O'Neill, L. A. (2016). Reprogramming Mitochondrial Metabolism in Macrophages as an Anti-inflammatory Signal. Eur. J. Immunol. 46 (1), 13–21. doi:10.1002/eji.201445427
Mills, E. L., Kelly, B., Logan, A., Costa, A. S. H., Varma, M., Bryant, C. E., et al. (2016). Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 167 (2), 457–470.e13. doi:10.1016/j.cell.2016.08.064
Mitha, K. V., Yadav, S., and Ganaraja, B. (2016). Improvement in Cognitive Parameters Among Offsprings Born to Alcohol Fed Female Wistar Rats Following Long Term Treatment with Centella Asiatica. Indian J. Physiol. Pharmacol. 60 (2), 167–173.
Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A., and Perry, G. (2010). Mitochondrial Dysfunction Is a Trigger of Alzheimer's Disease Pathophysiology. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1802, 2–10. doi:10.1016/j.bbadis.2009.10.006
Murphy, M. P., and Hartley, R. C. (2018). Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 17 (12), 865–886. doi:10.1038/nrd.2018.174
Nataraj, J., Manivasagam, T., Justin Thenmozhi, A., and Essa, M. M. (2017). Neuroprotective Effect of Asiatic Acid on Rotenone-Induced Mitochondrial Dysfunction and Oxidative Stress-Mediated Apoptosis in Differentiated SH-SYS5Y Cells. Nutr. Neurosci. 20 (6), 351–359. doi:10.1080/1028415X.2015.1135559
National Center for Biotechnology Information (2021a). PubChem Compound Summary for CID 119034, Asiatic acid. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/Asiatic-acid (Retrieved June 8, 2021)
National Center for Biotechnology Information (2021b). PubChem Compound Summary for CID 73412, Madecassic acid. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/Madecassic-acid (Retrieved June 8, 2021)
National Center for Biotechnology Information (2021c). PubChem Compound Summary for CID 52912190. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/52912190 (Retrieved June 8, 2021)
National Center for Biotechnology Information (2021d). PubChem Compound Summary for CID 131801373. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/131801373 (Retrieved June 8, 2021)
O'Neill, L. A. J., Kishton, R. J., and Rathmell, J. (2016). A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 16 (9), 553–565. doi:10.1038/nri.2016.70
Onyango, I. G., Khan, S. M., and Bennett, J. P. (2017). Mitochondria in the Pathophysiology of Alzheimer S and Parkinson S Diseases. Front. Biosci. 22, 854–872. doi:10.2741/4521
Orhan, I. E. (2012). Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evidence-Based Complement. Altern. Med. 2012, 1–8. doi:10.1155/2012/946259
Paolicelli, R. C., and Angiari, S. (2019). Microglia Immunometabolism: From Metabolic Disorders to Single Cell Metabolism. Semin. Cel Dev. Biol. 94, 129–137. doi:10.1016/j.semcdb.2019.03.012
Park, J., Choi, J., Son, D., Park, E., Song, M., Hellström, M., et al. (2017). Anti-inflammatory Effect of Titrated Extract of Centella asiatica in Phthalic Anhydride-Induced Allergic Dermatitis Animal Model. Ijms 18, 738. doi:10.3390/ijms180407384
Plengmuankhae, W., and Tantitadapitak, C. (2015). Low Temperature and Water Dehydration Increase the Levels of Asiaticoside and Madecassoside in Centella asiatica (L.) Urban. South Afr. J. Bot. 97, 196–203. doi:10.1016/j.sajb.2015.01.013
Puttarak, P., and Panichayupakaranant, P. (2013). A New Method for Preparing Pentacyclic Triterpene Rich Centella asiatica Extracts. Nat. Product. Res. 27 (7), 684–686. doi:10.1080/14786419.2012.686912
Qian, Y., Xin, Z., Lv, Y., Wang, Z., Zuo, L., Huang, X., et al. (2018). Asiatic Acid Suppresses Neuroinflammation in BV2 Microgliaviamodulation of the Sirt1/NF-κB Signaling Pathway. Food Funct. 9 (2), 1048–1057. doi:10.1039/c7fo01442b
Rafi, M., Handayani, F., Darusman, L. K., Rohaeti, E., Wahyu, Y., Sulistiyani, S., et al. (2018). A Combination of Simultaneous Quantification of Four Triterpenes and Fingerprint Analysis Using HPLC for Rapid Identification of Centella asiatica from its Related Plants and Classification Based on Cultivation Ages. Ind. Crops Prod. 122, 93–97. doi:10.1016/j.indcrop.2018.05.062
Rahajanirina, V., Rakotondralambo Raoseta, S. n. O., Roger, E., Razafindrazaka, H., Pirotais, S., Boucher, M., et al. (2012). The Influence of Certain Taxonomic and Environmental Parameters on Biomass Production and Triterpenoid Content in the Leaves of Centella asiatica (L.) Urb. From Madagascar. Chem. Biodiversity 9 (2), 298–308. doi:10.1002/cbdv.201100073
Rao, M. K., Rao, M. S., and Rao, G. S. (2007). Treatment with Centalla Asiatica (Linn) Fresh Leaf Extract Enhances Learning Ability and Memory Retention Power in Rats. Neurosciences (Riyadh) 12 (3), 236–241.
Rizzuto, R., De Stefani, D., Raffaello, A., and Mammucari, C. (2012). Mitochondria as Sensors and Regulators of Calcium Signalling. Nat. Rev. Mol. Cel Biol. 13 (9), 566–578. doi:10.1038/nrm3412
Rodríguez-Prados, J.-C., Través, P. G., Cuenca, J., Rico, D., Aragonés, J., Martín-Sanz, P., et al. (2010). Substrate Fate in Activated Macrophages: a Comparison between Innate, Classic, and Alternative Activation. J.I. 185 (1), 605–614. doi:10.4049/jimmunol.0901698
Roy, D. C., Barman, S. K., and Shaik, M. M. (2013). Current Updates on Centella asiatica: Phytochemistry, Pharmacology and Traditional Uses. Med. Plant Res. 3. doi:10.5376/mpr.2013.03.0004
Sabaragamuwa, R., Perera, C. O., and Fedrizzi, B. (2018). Centella asiatica (Gotu Kola) as a Neuroprotectant and its Potential Role in Healthy Ageing. Trends Food Sci. Technol. 79, 88–97. doi:10.1016/j.tifs.2018.07.024
Sari, D. C. R., and Rochmah, M. A. (2015). The Effects of Ethanol Extracts of Centella asiatica Leaf on Serial Serum Brain Derived Neurotrophin Factor (BDNF) Concentration of Rats (Sprague Dawley) Following Chronic Stress. Kls 2, 159–167. doi:10.18502/kls.v2i1.136
Sari, D. C. R., Aswin, S., Susilowati, R., Ar-Rochmah, M., Prakosa, D., Romi, M., et al. (2014). Ethanol Extracts of Centella asiatica Leaf Improves Memory Performance in Rats after Chronic Stress via Reducing Nitric Oxide and Increasing Brain-Derived Neurotrophic Factor (BDNF) Concentration. GSTF J. Psych 1, 9. doi:10.7603/s40790-014-0009-01
Sbrini, G., Brivio, P., Fumagalli, M., Giavarini, F., Caruso, D., Racagni, G., et al. (2020). Centella asiatica L. Phytosome Improves Cognitive Performance by Promoting BDNF Expression in Rat Prefrontal Cortex. Nutrients 12 (2), 355. doi:10.3390/nu12020355
Shah, S. I., Paine, J. G., Perez, C., and Ullah, G. (2019). Mitochondrial Fragmentation and Network Architecture in Degenerative Diseases. PLoS One 14 (9), e0223014. doi:10.1371/journal.pone.0223014
Simpson, D. S. A., and Oliver, P. L. (2020). ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 9 (8), 743. doi:10.3390/antiox9080743
Singh, S., Singh, D. R., Banu, V. S., and N, A. (2014). Functional Constituents (Micronutrients and Phytochemicals) and Antioxidant Activity of Centella asiatica (L.) Urban Leaves. Ind. Crops Prod. 61, 115–119. doi:10.1016/j.indcrop.2014.06.045
Sirichoat, A., Chaijaroonkhanarak, W., Prachaney, P., Pannangrong, W., Leksomboon, R., Chaichun, A., et al. (2015). Effects of Asiatic Acid on Spatial Working Memory and Cell Proliferation in the Adult Rat hippocampus. Nutrients 7 (10), 8413–8423. doi:10.3390/nu7105401
Smith, R. A. J., Hartley, R. C., Cochemé, H. M., and Murphy, M. P. (2012). Mitochondrial Pharmacology. Trends Pharmacol. Sci. 33 (6), 341–352. doi:10.1016/j.tips.2012.03.010
Song, D., Jiang, X., Liu, Y., Sun, Y., Cao, S., and Zhang, Z. (2018). Asiaticoside Attenuates Cell Growth Inhibition and Apoptosis Induced by Aβ1-42 via Inhibiting the TLR4/NF-κB Signaling Pathway in Human Brain Microvascular Endothelial Cells. Front. Pharmacol. 9, 28. doi:10.3389/fphar.2018.00028
Soumyanath, A., Zhong, Y.-P., Henson, E., Wadsworth, T., Bishop, J., Gold, B. G., et al. (2012). Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer's Disease: Investigation of a Possible Mechanism of Action. Int. J. Alzheimer's Dis. 2012, 1–9. doi:10.1155/2012/381974
Srivastava, S., Verma, S., Gupta, A., Rajan, S., and Rawat, A. (2014). Studies on Chemotypic Variation in Centella asiatica (L.) Urban from Nilgiri Range of India. J. Planar Chromatogr. - Mod. TLC 27 (6), 454–459. doi:10.1556/jpc.27.2014.6.9
Subaraja, M., and Vanisree, A. J. (2019). The Novel Phytocomponent Asiaticoside-D Isolated from Centella asiatica Exhibits Monoamine Oxidase-B Inhibiting Potential in the Rotenone Degenerated Cerebral Ganglions of Lumbricus Terrestris. Phytomedicine 58, 152833. doi:10.1016/j.phymed.2019.152833
Suliman, H. B., and Piantadosi, C. A. (2016). Mitochondrial Quality Control as a Therapeutic Target. Pharmacol. Rev. 68 (1), 20–48. doi:10.1124/pr.115.011502
Sun, N., Youle, R. J., and Finkel, T. (2016). The Mitochondrial Basis of Aging. Mol. Cel 61 (5), 654–666. doi:10.1016/j.molcel.2016.01.028
Suri, A. A., Handayani, A., Ferhad, A., Farida, S., and Redjeki, S. (2018). Effect of Centella asiatica Ethanol Extract in Spatial Working Memory on Adult Male Rats. Adv. Sci. Lett. 24 (8), 6109–6111. doi:10.1166/asl.2018.12641
Szalárdy, L., Zádori, D., Klivényi, P., Toldi, J., and Vécsei, L. (2015). Electron Transport Disturbances and Neurodegeneration: From Albert Szent-Györgyi's Concept (Szeged) till Novel Approaches to Boost Mitochondrial Bioenergetics. Oxidative Med. Cell Longevity 2015, 1–19. doi:10.1155/2015/498401
Teerapattarakan, N., Benya-Aphikul, H., Tansawat, R., Wanakhachornkrai, O., Tantisira, M. H., and Rodsiri, R. (2018). Neuroprotective Effect of a Standardized Extract of Centella asiatica ECa233 in Rotenone-Induced Parkinsonism Rats. Phytomedicine 44, 65–73. doi:10.1016/j.phymed.2018.04.028
Tewari, D., Mukhopadhyay, M., Nekkanti, M. S., Vallabhaneni, S., Sahu, G., Jetti, S. K., et al. (2016). Cytoprotective Effect of Centella asiatica Is Mediated through the Modulation of Mitochondrial Voltage-dependent Anion Channel (VDAC) and Scavenging of Free Radicals. J. Funct. Foods 21, 301–311. doi:10.1016/j.jff.2015.11.047
Thirawarapan, S. S., Jariyapongsakul, A., Suvitayavat, W., Muangnongwa, S., and Sribusarakum, A. (2019). Anti-hypertensive and Cerebral Blood Flow Improving Actions of Centella asiatica (L.) Urban Leaves Juice in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Pharm. Sci. Asia 46 (3), 184–192. doi:10.29090/psa.2019.03.018.0002
Thomas, M. T., Kurup, R., Johnson, A. J., Chandrika, S. P., Mathew, P. J., Dan, M., et al. (2010). Elite Genotypes/chemotypes, with High Contents of Madecassoside and Asiaticoside, from Sixty Accessions of Centella asiatica of South India and the Andaman Islands: For Cultivation and Utility in Cosmetic and Herbal Drug Applications. Ind. Crops Prod. 32 (3), 545–550. doi:10.1016/j.indcrop.2010.07.003
Torbati, F. A., Ramezani, M., Dehghan, R., Amiri, M. S., Moghadam, A. T., Shakour, N., et al. (2021). Ethnobotany, Phytochemistry and Pharmacological Features of Centella asiatica: A Comprehensive Review. Adv. Exp. Med. Biol. 1308, 451–499. doi:10.1007/978-3-030-64872-5_25
Umka Welbat, J., Sirichoat, A., Chaijaroonkhanarak, W., Prachaney, P., Pannangrong, W., Pakdeechote, P., et al. (2016). Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients 8 (5), 303. doi:10.3390/nu8050303
Veerendra Kumar, M., and Gupta, Y. (2003). Effect of Centella asiatica on Cognition and Oxidative Stress in an Intracerebroventricular Streptozotocin Model of Alzheimer's Disease in Rats. Clin. Exp. Pharmacol. Physiol. 30 (5-6), 336–342. doi:10.1046/j.1440-1681.2003.03842.x
Wanasuntronwong, A., Wanakhachornkrai, O., Phongphanphanee, P., Isa, T., Tantisira, B., and Tantisira, M. H. (2018). Modulation of Neuronal Activity on Intercalated Neurons of Amygdala Might Underlie Anxiolytic Activity of a Standardized Extract of Centella asiatica ECa233. Evidence-Based Complement. Altern. Med. 2018, 1–8. doi:10.1155/2018/3853147
Wong, J. H., Muthuraju, S., Reza, F., Senik, M. H., Zhang, J., Mohd Yusuf Yeo, N. A. B., et al. (2019). Differential Expression of Entorhinal Cortex and Hippocampal Subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) and N-Methyl-D-Aspartate (NMDA) Receptors Enhanced Learning and Memory of Rats Following Administration of Centella asiatica. Biomed. Pharmacother. 110, 168–180. doi:10.1016/j.biopha.2018.11.044
Wong, J. H., Reza, F., Muthuraju, S., Chuang, H. G., Zhang, J., Senik, M. H., et al. (2020). Acute Application of Centella asiatica Extract Enhanced AMPAR-Mediated Postsynaptic Currents in Rat Entorhinal Cortex. J. Integr. Neurosci. 19 (2), 217–227. doi:10.31083/j.jin.2020.02.50
Xiong, Y., Ding, H., Xu, M., and Gao, J. (2009). Protective Effects of Asiatic Acid on Rotenone- or H2O2-Induced Injury in SH-SY5Y Cells. Neurochem. Res. 34 (4), 746–754. doi:10.1007/s11064-008-9844-0
Xu, M.-f., Xiong, Y.-y., Liu, J.-k., Qian, J.-j., Zhu, L., and Gao, J. (2012). Asiatic Acid, a Pentacyclic Triterpene in Centella asiatica, Attenuates Glutamate-Induced Cognitive Deficits in Mice and Apoptosis in SH-SY5Y Cells. Acta Pharmacol. Sin. 33 (5), 578–587. doi:10.1038/aps.2012.3
Xu, C.-L., Qu, R., Zhang, J., Li, L.-F., and Ma, S.-P. (2013). Neuroprotective Effects of Madecassoside in Early Stage of Parkinson's Disease Induced by MPTP in Rats. Fitoterapia 90, 112–118. doi:10.1016/j.fitote.2013.07.009
Yang, B., Xu, Y., Hu, Y., Luo, Y., Lu, X., Tsui, C. K., et al. (2016). Madecassic Acid Protects against Hypoxia-Induced Oxidative Stress in Retinal Microvascular Endothelial Cells via ROS-Mediated Endoplasmic Reticulum Stress. Biomed. Pharmacother. 84, 845–852. doi:10.1016/j.biopha.2016.10.015
Yang, L., Mao, K., Yu, H., and Chen, J. (2020). Neuroinflammatory Responses and Parkinson' Disease: Pathogenic Mechanisms and Therapeutic Targets. J. Neuroimmune Pharmacol. 15 (4), 830–837. doi:10.1007/s11481-020-09926-7
Yin, M.-C., Lin, M.-C., Mong, M.-C., and Lin, C.-Y. (2012). Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 60 (31), 7697–7701. doi:10.1021/jf302529x
Yolanda, D. A., Sari, D. C. R., Rochmah, M. A., and Suharmi, S. (2015). The Dose Variations Effect of Centella asiatica Ethanol Extract o Escape Latency's Distance Morris Water Maze After Chronic Electrical Stress. Kls 2, 146–153. doi:10.18502/kls.v2i1.134
Yoo, S.-M., Park, J., Kim, S.-H., and Jung, Y.-K. (2020). Emerging Perspectives on Mitochondrial Dysfunction and Inflammation in Alzheimer's Disease. BMB Rep. 53 (1), 35–46. doi:10.5483/BMBRep.2020.53.1.274
Zhang, Z., Li, X., Li, D., Luo, M., Li, Y., Song, L., et al. (2017). Asiaticoside Ameliorates β-amyloid-induced Learning and Memory Deficits in Rats by Inhibiting Mitochondrial Apoptosis and Reducing Inflammatory Factors. Exp. Ther. Med. 13 (2), 413–420. doi:10.3892/etm.2016.4004
Zhao, Y., Shu, P., Zhang, Y., Lin, L., Zhou, H., Xu, Z., et al. (2014). Effect of Centella Asiaticaon Oxidative Stress and Lipid Metabolism in Hyperlipidemic Animal Models. Oxidative Med. Cell Longevity 2014, 1–7. doi:10.1155/2014/154295
Zheng, H.-M., Choi, M.-J., Kim, J. M., Cha, K. H., Lee, K. W., Park, Y. H., et al. (2016). Centella asiatica Leaf Extract Protects against Indomethacin-Induced Gastric Mucosal Injury in Rats. J. Med. Food 19 (1), 38–46. doi:10.1089/jmf.2015.3464
Zorova, L. D., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., Jankauskas, S. S., et al. (2018). Mitochondrial Membrane Potential. Anal. Biochem. 552, 50–59. doi:10.1016/j.ab.2017.07.009
Zweig, J. A., Brandes, M. S., Brumbach, B. H., Caruso, M., Wright, K. M., Quinn, J. F., et al. (2021). Loss of NRF2 Accelerates Cognitive Decline, Exacerbates Mitochondrial Dysfunction, and Is Required for the Cognitive Enhancing Effects of Centella asiatica during Aging. Neurobiol. Aging 100, 48–58. doi:10.1016/j.neurobiolaging.2020.11.019
Keywords: medicinal plants, neuroprotection, mitochondria, neurodegeneration, centella asiatica (L.) Urb, antioxidative, mitoprotective
Citation: Wong JH, Barron AM and Abdullah JM (2021) Mitoprotective Effects of Centella asiatica (L.) Urb.: Anti-Inflammatory and Neuroprotective Opportunities in Neurodegenerative Disease. Front. Pharmacol. 12:687935. doi: 10.3389/fphar.2021.687935
Received: 30 March 2021; Accepted: 17 June 2021;
Published: 29 June 2021.
Edited by:
Tahir Ali, University of Calgary, CanadaReviewed by:
Atif Ali Khan Khalil, National University of Medical Sciences (NUMS), PakistanKevin Spelman, Consultant, Ashland, OR, United States
Copyright © 2021 Wong, Barron and Abdullah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jafri Malin Abdullah, YnJhaW5zY2llbmNlc0BnbWFpbC5jb20=
 Jia Hui Wong
Jia Hui Wong Anna M. Barron
Anna M. Barron Jafri Malin Abdullah
Jafri Malin Abdullah