- 1Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh
- 2Department of Pharmacy, State University of Bangladesh, Dhaka, Bangladesh
- 3Centre for Applied Physics and Radiation Technologies, School of Engineering and Technology, Sunway University, Bandar Sunway, Malaysia
- 4Space Science Centre, Universiti Kebangsaan Malaysia, Bangi, Malaysia
- 5Department of Radiological Sciences, College of Applied Medical Sciences, Taif University, Taif, Saudi Arabia
Nowadays, nitrogenous heterocyclic molecules have attracted a great deal of interest among medicinal chemists. Among these potential heterocyclic drugs, benzimidazole scaffolds are considerably prevalent. Due to their isostructural pharmacophore of naturally occurring active biomolecules, benzimidazole derivatives have significant importance as chemotherapeutic agents in diverse clinical conditions. Researchers have synthesized plenty of benzimidazole derivatives in the last decades, amidst a large share of these compounds exerted excellent bioactivity against many ailments with outstanding bioavailability, safety, and stability profiles. In this comprehensive review, we have summarized the bioactivity of the benzimidazole derivatives reported in recent literature (2012–2021) with their available structure-activity relationship. Compounds bearing benzimidazole nucleus possess broad-spectrum pharmacological properties ranging from common antibacterial effects to the world’s most virulent diseases. Several promising therapeutic candidates are undergoing human trials, and some of these are going to be approved for clinical use. However, notable challenges, such as drug resistance, costly and tedious synthetic methods, little structural information of receptors, lack of advanced software, and so on, are still viable to be overcome for further research.
Introduction
Benzimidazole, alternatively known as 1H-benzimidazole and 1,3-benzodiazole, consists of benzene ring fused with a five-membered imidazole ring, and is an important heterocyclic pharmacophore. Benzimidazole is regarded as a “privileged structure” in heterocyclic chemistry due to its association with a wide range of biological activities (Barot et al., 2013; Alaqeel, 2017).
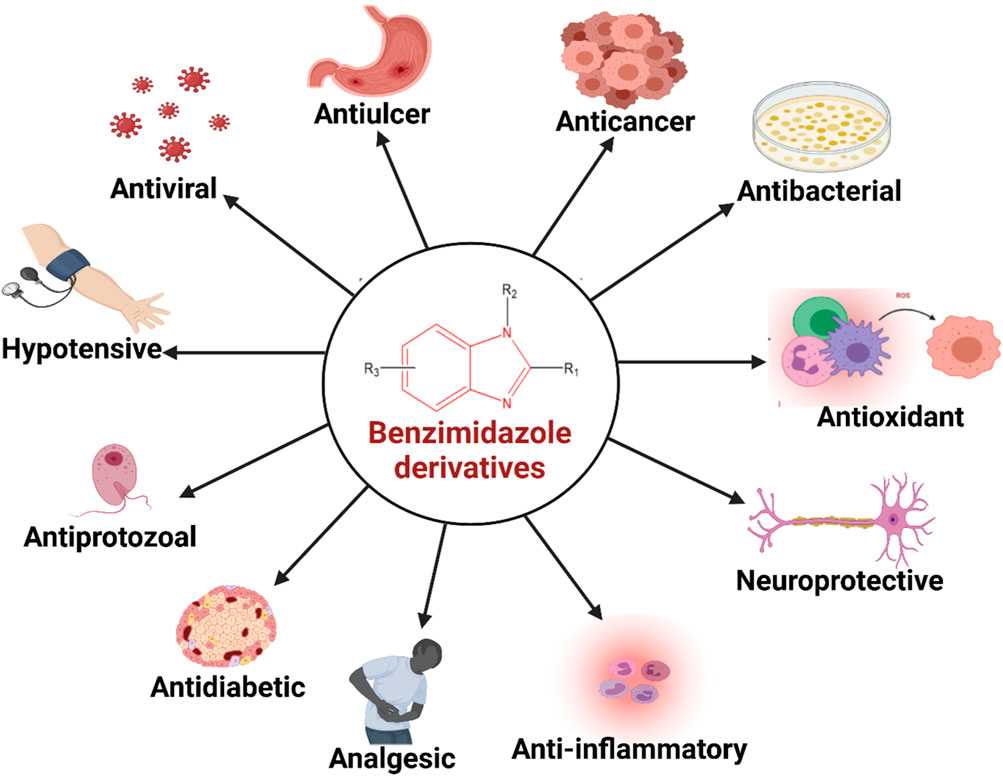
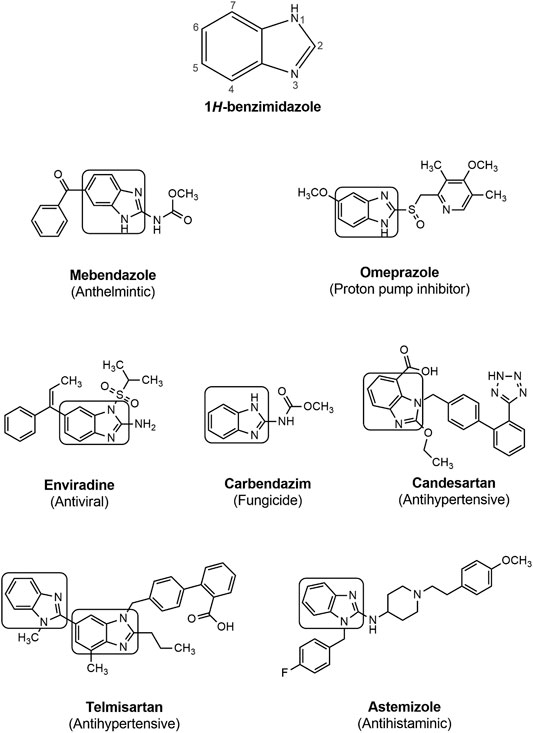
Back in 1940s, benzimidazole was speculated to act similarly as purines to provide biological responses and the first investigation on biological activity of benzimidazole nucleus was reported in 1944 (Woolley, 1944). Interest among the researchers about the synthetic procedure of benzimidazole and its derivatives escalated when Brink et al. (Brink and Folkers, 1949; Emerson et al., 1950) found that 5,6-dimethylbenzimidaozle was a degradation product of vitamin B12 and some of its derivatives also possessed vitamin B12 like activity. These early reports led researchers to the exploration of benzimidazole nucleus for numerous activities. Through the course of many years of research, benzimidazole has emerged as an important heterocyclic system because of its existence in diverse biologically active compounds, such as antiparasitics, antimicrobials, antivirals, antifungals, anticonvulsants, antihypertensives, antihistaminics, analgesics, anti-inflammatory agents, anticancers, anticoagulants and proton pump inhibitors (Figure 1) (Fei and Zhou, 2013; Wang et al., 2015). As a result of changing substituents around the core structure, many drugs of a wide variety of therapeutic lines have been developed such as albendazole, mebendazole, thiabendazole as antihelmintics; enviradine as antiviral; carbendazim as fungicidal; omeprazole, lansoprazole, pantoprazole as proton pump inhibitors; candesartan cilexitil and telmisartan as antihypertensives, and astemizole as antihistaminic agent (Figure 2) (Bansal and Silakari, 2012; Alaqeel, 2017). The high therapeutic potential of benzimidazole related drugs has inspired the medicinal chemists to carry out the synthesis of several novel chemotherapeutic agents containing benzimidazole moiety (Morais et al., 2017).
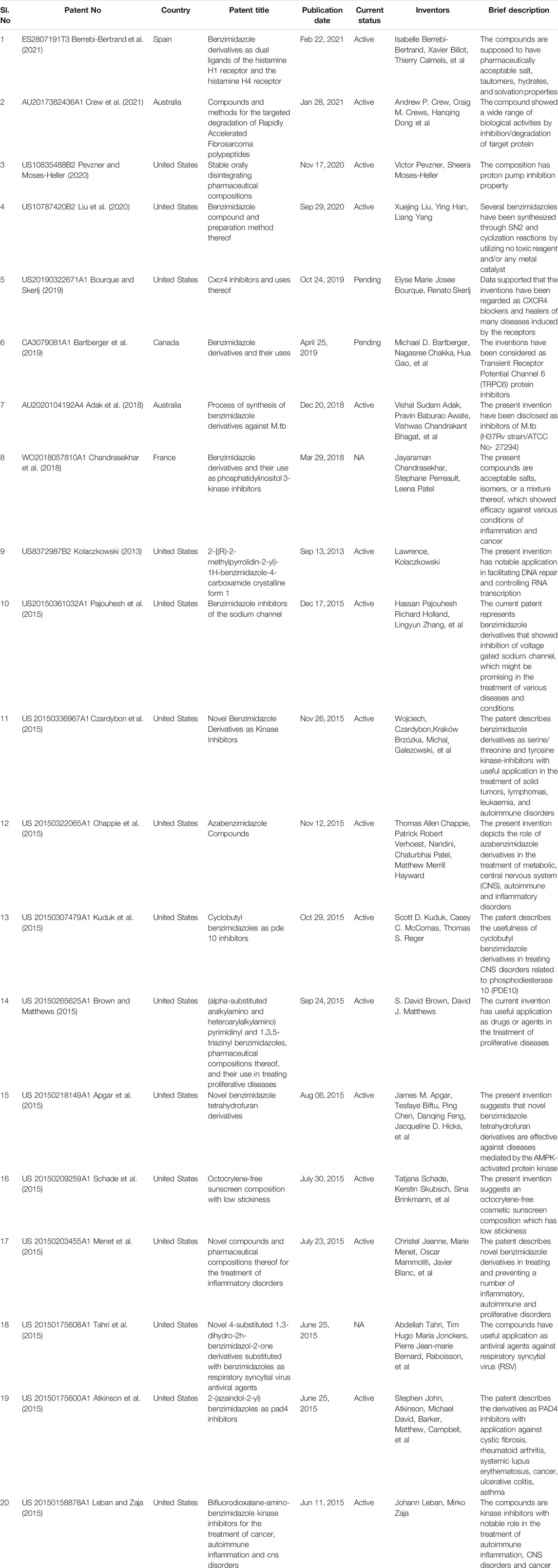
Numerous researches have been accomplished in the past couple of years which produced very intriguing results concerning the chemistry, structure-activity relationship and biological activities of different benzimidazole based compounds. The diverse biological activities displayed by compounds bearing benzimidazole moiety have prompted researchers all around the globe to design and synthesize various benzimidazole analogues. A number of recently published patents on the benzimidazole moiety are listed in Table 1. Several review articles have been published emphasizing on the contribution of benzimidazole nucleus in particular biological activity, e.g. anticancer, analgesic, anti-inflammatory, antimicrobial, antiviral, antitubercular, antiulcer, antihypertensive and antidiabetic property (Bansal and Silakari, 2012; Barot et al., 2013; Keri et al., 2015; Wang et al., 2015; Akhtar et al., 2017). To the best of our knowledge, there is no review article available in the literature which has focused on the most updated information of the diverse biological and therapeutic applications of benzimidazole derivatives. The present review gives a comprehensive account of all biological aspects of benzimidazole derivatives and has included information from the recent studies reported up to 2021. Apart from literature study, this review also provides a thoughtful insight into the latest ongoing research on benzimidazole derivatives in a variety of therapeutic fields.
Biological Activities
The wide variety of benzimidazole derivatives synthesized during the last few years and their diverse biological applications are discussed in the following sections.
Antimicrobial Activity
Antimicrobial and Antifungal Activity
The antimicrobial potential of benzimidazole moiety has been explored notably since late 1990s and early 2000s (Özkay et al., 2011). Considering the huge dimension of research conducted on antimicrobial property of benzimidazole derivatives after 2012, the following section focuses on the up-to-date information on antibacterial and antifungal activities, while antiviral, antiulcer, antiprotozoal and antitubercular properties are discussed in separate sections. Different benzimidazole based compounds with antibacterial and antifungal activities are shown in Figure 3.
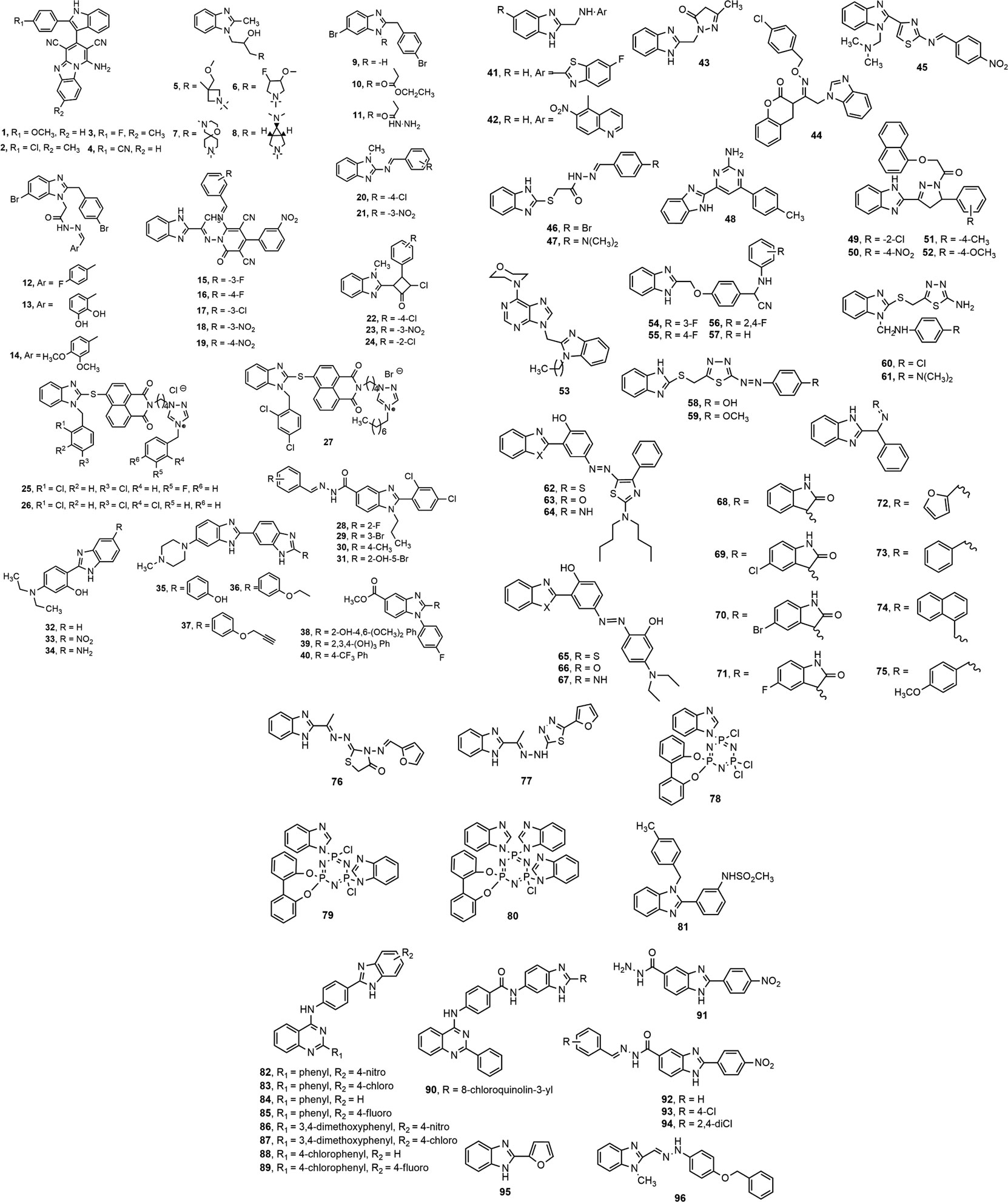
Kathrotiya and Patel (Kathrotiya and Patel, 2013) synthesized a series of indole-based pyrido [1,2-a] benzimidazole derivatives (1–4) and evaluated in vitro antimicrobial activity against some Gram-positive and Gram-negative bacteria and fungi using broth microdilution minimum inhibitory concentration (MIC) method. Compounds 1, 3 and 4 (MIC = 50, 62.5 and 12.5 μg/ml, respectively) displayed prominent antibacterial activity against S. typhi compared to standards ampicillin, chloramphenicol and ciprofloxacin (MIC = 100, 50 and 25 μg/ml, respectively). Compounds 2 and 3 exhibited notable antifungal activity against C. albicans (MIC = 250 μg/ml) in comparison with standard griseofulvin (MIC = 500 μg/ml). The derivatives with 4-methoxy (1) and cyano (4) group at 2-position of indole nucleus were found to possess excellent inhibitory activity against most of the tested organisms. Birajdar et al. (Birajdar et al., 2013) synthesized some amino alcohol derivatives of 2-methyl benzimidazole (5–8) by epoxide ring opening of 2-methyl benzimidazole with different substituted cyclic amines. Compounds 5–8 demonstrated moderate to good activity against Gram-positive (S. aureus) and Gram-negative (E. coli) pathogens in comparison with reference drugs ciprofloxacin and norfloxacin.
Several benzimidazole derivatives with imine functionality (9–14) were prepared by Kahveci et al. (Kahveci et al., 2014) using microwave irradiation as well as conventional method. Compounds 9–14 displayed notable antimicrobial property against the tested microorganisms. Desai et al. (Desai et al., 2014) synthesized a series of 2-pyridone based benzimidazole derivatives (15–19) and investigated in vitro antimicrobial potential against a number of bacterial and fungal strains using conventional broth dilution method. Compounds 15 and 18 (MIC = 12.5–25 µg/ml) showed better antibacterial activity, and 16 and 19 (MIC = 25–100 µg/ml) displayed comparable activity to standard chloramphenicol (MIC = 50 µg/ml). The presence of electron withdrawing groups, e.g. fluoro (15, 16) and nitro (18, 19) at the meta or para position might have contributed for their antimicrobial property. Compound 17 containing chloro group displayed the most remarkable antifungal activity, with MIC values in the range of 25–62.5 µg/ml against three fungal strains compared to standard ketoconazole (MIC = 50 µg/ml). A library of 1-methyl-N-[(substituted-phenylmethylidene)-1H-benzimidazol-2-amines (20–24) were synthesized and reported for notable antimicrobial activity against Gram-positive S. aureus (ATCC 6538), B. pumilus (ATCC 14884) and Gram-negative E. coli (NCTC 10418), P. aeruginosa (ATCC 25619) bacteria compared to reference drug ampicillin (Noolvi et al., 2014).
Luo et al. (Luo et al., 2015) synthesized a series of benzimidazole-based naphthalimide triazoles and triazolium compounds (25–27). The derivatives were assessed for in vitro antibacterial activity against Gram-positive S. aureus, Methicillin-resistant S. aureus, M. luteus and B. subtilis and Gram-negative B. proteus, E. coli, B. typhi, and P. aeruginosa as well as for antifungal activity against A. fumigatus, C. albicans, C. utilis, A. flavus, and S. cerevisiae. The 2-chlorobenzyl triazolium compound 26 and octyl group-containing compound 27 showed the most potent antibacterial activity against S. aureus with an MIC of 2 μg/ml to standard norfloxacin (MIC = 2 μg/ml) and better than standard chloromycin (MIC = 7 μg/ml). Compound 26 and 3-fluorobenzyl moiety bearing compound 25 appeared to be the most prominent antifungal agents, with MIC value in the range of 2–19 μg/ml against the tested fungal strains. Vasantha et al. (Vasantha et al., 2015) synthesized a series of N-arylidene-2-(2,4-dichlorophenyl)-1-propyl-1H-benzo[d]imidazole-5-carbohydrazides derivatives among which compounds 28–31 displayed notable inhibitory effect against A. niger with MIC value of 3.12 μg/ml. Compound 28 demonstrated a MIC value of 3.12 μg/ml against most bacterial and fungal strains and appeared to be a potent antibacterial and antifungal agent.
A series of benzimidazole derivatives were synthesized and evaluated by Padalkar et al. (Padalkar et al., 2016) (32–34) and Chandrika et al. (Chandrika et al., 2016) (35–37), where the compounds 32–34 showed prominent antibacterial activity against S. aureus strain and compounds 35–37 were found to be the most potent antifungal agents against azole-resistant fungal strain C. albicans ATCC 64124 (strain B). Another study reported that among the total 22 synthesized novel 2-substituted fluorinated benzimidazoles, compounds 38–40 showed antimicrobial activity. In contrast, compound 40 containing a trifluoromethyl substituent showed the highest antifungal activity against the fungus C. albicans (Shintre et al., 2017). Similarly, El-Gohary and Shaaban (El-Gohary and Shaaban, 2017) synthesized a series of benzimidazole derivatives (41–43), where compounds 41 and 43 showed notable activity against S. aureus, and compound 42 was found to be the most effective against B. cereus. Compound 41 displayed the highest antifungal potential against C. albicans, whereas 43 exhibited prominent activity against A. fumigatus.
Singh et al. (Singh LR. et al., 2017) prepared a library of coumarin-benzimidazole hybrids and screened them for antimicrobial activity. Compound 44 was found to be the promising broad-spectrum antibacterial agent against P. aeruginosa, S. aureus, B. subtilis and P. vulgaris. A series of N-(substitutedbenzylidene)-4-(1-((dimethylamino)methyl)-1H-benzimidazol-2-yl)thiazol-2-amine derivatives were investigated for antimicrobial activity using agar streak dilution test. Compound 45 appeared to be the most potent among the series. Notably, the presence of an electron-withdrawing group might have contributed to the improved antimicrobial property of the compound (Prasad and Sundararajan, 2017).
Recently, Yadav et al. (Yadav et al., 2018) synthesized 2-substituted benzimidazole derivatives (46–47), where compound 46 emerged as the most potent antibacterial agent against both Gram-positive and Gram-negative bacteria compared to standard cefadroxil. All derivatives exhibited better antifungal activity than the standard fluconazole, and compound 47 showed maximum activity against A. niger (MIC = 0.018 mM). Liu et al. (Liu et al., 2018) designed a series of novel aminopyrimidinyl benzimidazoles as potential antimicrobial agents. Among them, compound 48 showed effective growth inhibition of MRSA, E. coli, and fungus A. flavus, compared to standard drugs chloromycin, norfloxacin, and fluconazole.
Another study reported the evaluation of a series of 1-(3-(1H-benzoimidazol-2-yl)-5-aryl-4-5dihydro-1H-pyrazol-1-yl)-2-(napthalene-1-yloxy) ethanones (49–52), where the electron-withdrawing groups (compound 49 containing chloro group at ortho position and 50 with a nitro group at the para position) were the most effective against bacterial strains. On the contrary, electron releasing groups at the para position (compounds 51 and 52 carrying methyl and methoxy group, respectively) contributed to the most promising antifungal activity against the tested organisms (Desai et al., 2018). Wang et al. (Wang et al., 2018) synthesized a library of purine benzimidazole hybrids and assessed them for antimicrobial potency. Compound 53 exhibited prominent activity against most tested bacterial and fungal strains and multidrug-resistant strain S. aureus, 16 times more potent than the standard norfloxacin (MIC = 4 µg/ml vs. 64 µg/ml). Amongst a series of α-aminonitrile based benzimidazole derivatives (54–57), the compounds 55 and 56 were found to be the most potent antibacterial agents having MIC values ranging between 3.9 and 7.8 μg/ml against different bacterial species. All the compounds exerted illustrious antifungal activity against C. albicans (MIC = 3.9–7.8 µg/ml) compared to the reference drug fluconazole (MIC value <3.9 µg/ml) (Shaikh et al., 2018).
Similarly, some recent publications also reported several potential antibacterial benzimidazole derivatives like compounds 58–61 containing the 1,3,4-thiadiazole ring and azo moiety showed excellent activity against S. aureus, B. subtilis, E. coli, and p. aeruginosa compared to amoxicillin and ciprofloxacin (Mahmoud et al., 2020). Also, the azo linked compounds 62–67 and novel Schiff bases of 2-(1-amino benzyl)-benzimidazole compounds 68–75 indicated moderate to high in vitro inhibition of both gram-positive (S. aureus) and gram-negative (E. coli) bacteria (Mishra et al., 2019; Singhal et al., 2019). Abdel-Motaal et al. (Abdel-Motaal et al., 2020) synthesized some substituted benzimidazole-2yl derivatives. Compounds 76 and 77 containing thiadiazole and thiazolone moieties, respectively, displayed antibacterial potency against S. aureus, E. coli, and B. pumilus comparable to standard gentamicin. Among the compounds 78–80, the compound 80 highly inhibited the B. subtilis and S. aureus bacterial growth compared to the reference drug chloramphenicol (zone of inhibition, mm: 23 and 14 vs. 32 and 30) (İbişoğlu et al., 2020). Besides, co-treatment of compound 81 with colistin exhibited a promising synergistic effect against wild strains E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa with MIC range = 8–16 μg/ml (Dokla et al., 2020). Malasala et al. (Malasala et al., 2021) reported nine more potent antibacterial agents 82–90 with MIC range 4–64 μg/ml against several resistant organisms, including methicillin and vancomycin-resistant S. aureus. The derivatives with 4-nitro, 4-chloro, 4-fluoro, 4-bromo, and unsubstituted exerted good to moderate inhibitory actions against S. aureus and M. tuberculosis H37Rv. Similarly, the analogues with phenyl, 3,4-dimethoxy, and 4-chloro exhibited moderate to good inhibitory property S. aureus and M. tuberculosis H37Rv (Malasala et al., 2021). Furthermore, compounds 91–94 inhibited C. albicans and C. neoformans var. grubii fungal growth with MIC values 4–16 μg/ml, and likewise, compounds 95 and 96 also exerted remarkable antifungal activity (Dhanamjayulu et al., 2019; Amine Khodja et al., 2020; Morcoss et al., 2020).
Antiviral Activity
The antiviral properties of benzimidazole derivatives have been tested against different viral strains; human immunodeficiency virus (HIV), hepatitis B and C virus (HBV and HCV), enteroviruses, respiratory syncytial virus (RSV), human cytomegalovirus (HCMV), bovine viral diarrhea virus (BVDV) and herpes simplex virus-1 (HSV-1) are some to mention (Abu-Bakr et al., 2012). This section focuses on the recent studies involving varied antiviral properties of different benzimidazole derivatives, and their structures are shown in Figure 4.
Benzimidazole Against HIV
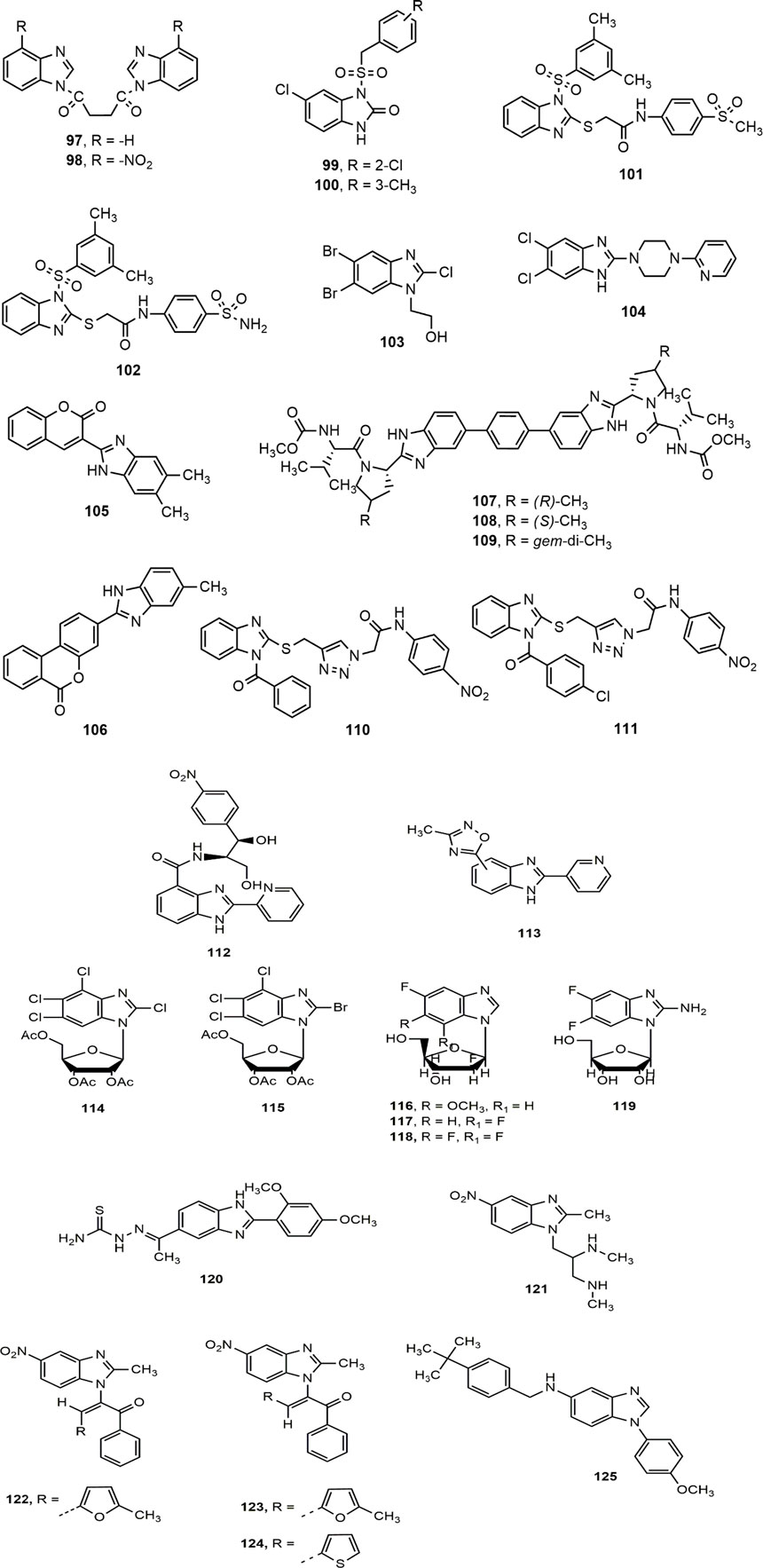
A number of substituted benzimidazole derivatives were synthesized as reverse transcriptase inhibitors (RTIs) against HIV-1 replication, among them compounds 97–98 showed notable antiviral activity against laboratory-adapted strains HIV-1IIIB and HIV-1Ada5 (EC50 = 15.4–40 µM) and primary isolates of HIV-1UG07O and HIV-1VB59 strains (EC50 = 5.28–31.86 µM) (Singh et al., 2015). Besides, Ferro et al. (Ferro et al., 2017) synthesized two series of N1-aryl-benzimidazol-2-one derivatives as non-nucleoside reverse transcriptase inhibitors (NNRTIs) against HIV-1, where the compounds 99–100 were more potent than the standard drug nevirapine (IC50: 1.3 and 0.79 vs. 1.55 µM). The sulfone derivatives 101–102, synthesized by the same research group were also found to be potent HIV-1 NNRTIs with IC50 values of 47 and 50 nM, respectively. The substitution at C-4 position of the arylacetamide portion of the compounds might have contributed for their notable activity against HIV-1IIIB strain in cell-based assays (Monforte et al., 2018). Finally, Srivastava et al. (Srivastava et al., 2020) has recently reported a promising anti-HIV benzimidazole derivative 103 with a low IC50 value of 0.386 × 10–5 μM.
Benzimidazole Against Hepatitis B and C Viruses (HBV and HCV)
The hepatitis B surface antigen (HBsAg), an HBV surface protein is an important mediator of HBV life cycle (Wang et al., 2016). Xu et al. (Xu et al., 2014) carried out a high-throughput screening (HTS), and concluded that the compound 104 inhibited the secretion of HBsAg and HBV virions indicated by EC50 values of 1.5 and 0.6 μM, respectively, along with half cytotoxicity concentration (CC50) value of 24.5 μM.
Moreover, Tsay et al. (Tsay et al., 2013) synthesized a library of hinged benzimidazole-coumarin hybrids and reported the potentiality of compounds 105–106 with EC50 values 3.0 and 5.5 nM, respectively, against hepatitis C virus (HCV), a prime cause of liver cirrhosis and hepatocellular carcinoma. Besides, Henderson et al. (Henderson et al., 2015) synthesized a series of 4-substituted pyrrolidine containing bis-benzimidazole analogues (107–109) and evaluated for their HCV non-structural 5A (NS5A) inhibitory effect with balanced Genotype 1a (G1a) and Genotype 1b (G1b) potency. Compounds 107 (G1a EC50 = 0.028 nM, G1b EC50 = 0.007 nM), 108 (G1a EC50 = 0.026 nM, G1b EC50 = 0.037 nM) and 109 (G1a EC50 = 0.03 nM, G1b EC50 = 0.011 nM) appeared to be most active compounds from the series. Substitution of methyl group (107–108) and geminal dimethyl group (109) at 4-position of pyrrolidine nucleus was likely to contribute for G1a and G1b potency of the compounds. In another study, a set of 2-thiobenzimidazole analogues (110–111) containing triazole moiety were reported as promising HCV inhibitor, where the substituent at position-2 of benzimidazole nucleus played the crucial role to enhance the antiviral potency (Youssif et al., 2016).
Benzimidazole Against Enteroviruses, Cytomegalovirus and Herpes Simplex Virus (HSV)
Two distinct series of benzimidazole derivatives were synthesized, where the compounds 112–113 indicated potent enterovirus (Coxsackie) inhibition with the IC50 values of 1.76 and 1.08 μg/ml, respectively (Xue et al., 2011; Wubulikasimu et al., 2013). The benzimidazole D-ribonucleosides derivatives 114–115 exerted activity in rat cytomegalovirus infected cells, and prevented cleavage of concatemeric viral DNA and nuclear egress of mature viral capsids (Dittmer et al., 2017). In a pair consecutive studies (Kharitonova et al., 2016, 2017), Kharitonova et al. reported that several 2′-deoxy-2′-fluoro-β-D-arabinofuranosyl benzimidazole derivatives (116–118) and 2-amino-5,6-difluorobenzimidazole nucleosides (119) inhibited Herpes Simplex Virus-induced cytopathic effect (CPE). Impressively, the IC50 value of compound 119 was 104 μM, four times lower than that of ribavirin and eight times lower than that of maribavir.
Benzimidazole Against Bovine Viral Diarrhea Virus (BVDV), Rotavirus and Arenaviruses
Among a library of 5-acetyl-2-arylbenzimidazoles analogues, compound 120 appeared to be the most effective antiviral agent against Bovine Viral Diarrhea virus (BVDV, EC50 = 1.11 mM) due to the presence of 2,4-dimethoxy group in the phenyl moiety (Vitale et al., 2012). Shaker et al. (Shaker et al., 2015) synthesized a series of 5-nitro-1H-benzimidazole derivatives (121–124) bearing substitution of heterocyclic rings at position 1. Compounds 121 and 122 exhibited equal potency as standard doxorubicin against A-549, HCT-116, MCF-7 and human liver carcinoma HepG2 cell lines. Besides, compounds 122–124 showed great potential to be used as potent antiviral agents due to their inhibitory effect against rotavirus Wa strain. Finally, compound 125, identified by Dai and co-workers, was found to be potent antiviral agent against Lassa virus envelope glycoprotein (LASV GP) pseudotypes with EC50 value of 1.1 nM (Dai et al., 2013).
Anti-inflammatory and Analgesic Activity
Benzimidazole based compounds are of great importance as anti-inflammatory and analgesic agents because of their property to inhibit cyclooxygenases (COXs), enzymes involved in biosynthesis of important inflammatory mediators called prostaglandins (Akhtar et al., 2017). Apart from the cyclooxygenases (COX), the benzimidazole derivatives interact with transient receptor potential vanilloid-1, cannabinoid receptors, bradykinin receptors, specific cytokines, and 5-lipoxygenase (5-LOX) activating protein. Thus, the compounds derived from benzimidazole moiety show the anti-inflammatory property (Veerasamy et al., 2021). Different benzimidazole derivatives with analgesic and anti-inflammatory properties are shown in Figure 5.
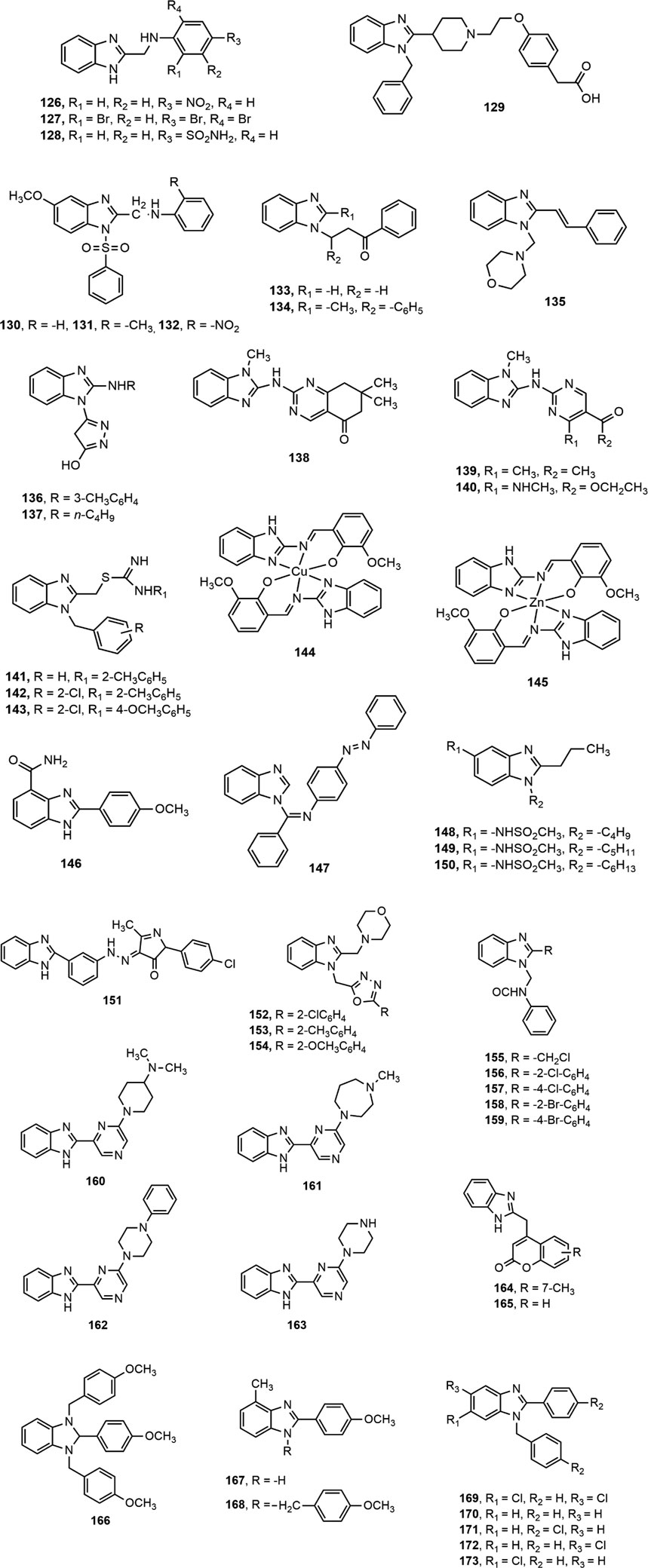
Mariappan et al. (Mariappan et al., 2015) synthesized a set of 2-substituted benzimidazole derivatives and reported that compounds 126–128 were found to be the most promising agents among the series displaying significant (p < 0.01) analgesic and anti-inflammatory effect at a dose level of 100 mg/kg p.o. Li et al. (Li et al., 2015) assessed the synthesized two series of 2-(piperidin-4-yl)-1H-benzo[d]imidazole derivatives for anti-inflammatory activity and found that the compound 129 exhibited the most potent inhibitory activity on nitric oxide and TNF-α production (IC50 = 0.86 and 1.87 µM, respectively). Interestingly, the compound 129 also prevented 33.30 and 50.60% ear oedema in xylene-treated mice at doses of 4 and 12 mg/kg, respectively, compared to standard ibuprofen (26.77 and 39.34% inhibition at 4 and 12 mg/kg dose levels, respectively). Besides, compounds 130–132 showed the potentiality as gastroprotective lead compounds which can be developed into orally active analgesic and anti-inflammatory agents (Gaba and Mohan, 2015). Compounds 133–134, synthesized by Kumar et al. displayed significant analgesic and anti-inflammatory properties, (Kumar et al., 2015) and compound 135 showed better inhibition of acetic acid induced writhing at 20 mg/kg dose than the standard diclofenac (78.12 vs. 75%) (Datar and Limaye, 2015).
Moneer et al. (Moneer et al., 2016) synthesized a series of 5-[2-(substituted amino)-1H-benzimidazol-1-yl]-4H-pyrazol-3-ol derivatives (136–137) and investigated for in vitro cyclooxygenase inhibitory effect using Cayman’s colorimetric COX (ovine) assay. Compounds 136–137 showed remarkable in vitro cyclooxygenase inhibition (IC50 on COX-1: 0.1664 and 0.2272 nM, respectively, and IC50 on COX-2: 0.0370 and 0.0469 nM, respectively) and significant (p < 0.05) reduction of edema volume compared to that of standard diclofenac at all time intervals. Prajapat and Talesara (Prajapat and Talesara, 2016) synthesized several alkoxyphthalimide based benzimidazole derivatives (138–140) and Siddiqui et al. (Siddiqui et al., 2016) prepared a series of 1-{(1-(2-substituted benzyl)-1H-benzo[d]imidazol-2-yl)methyl}-3-arylthioureas (141–143). All the compounds (138–143) exerted notable anti-inflammatory effect compared to standard diclofenac.
A series of Cu(II) and Zn(II) complexes of a 2-[(1H-benzoimidazol-2-ylimino)-methyl]-6-methoxy-phenol Schiff base ligands (144–145) were synthesized from condensation of 2-aminobenzimidazole and o-vanillin (AlAjmi et al., 2016). The both compounds (144–145) at 100 mg/kg b.w. showed around 55% inhibition of inflammation compared to standard diclofenac (65.4% inhibition). Furthermore, Bukhari et al. (Bukhari et al., 2016) reported about anti-inflammatory activity of a series of benzimidazole derivatives where compound 146 was found to be a potent inhibitor of 5-LOX, COX, TNF-α, IL-6 and cytokines among the series. Figure 6 depicts a clear diagram for understanding the association of structural modifications with bioactivities of benzimidazole derivatives against inflammation.
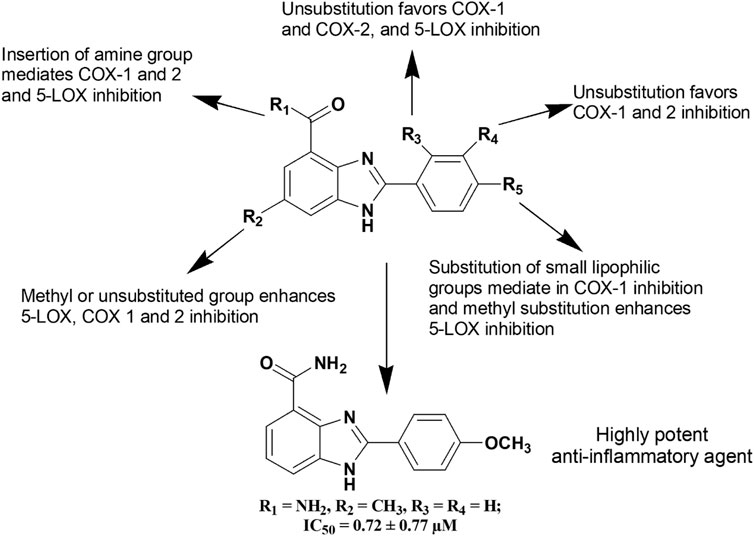
FIGURE 6. Structure activity relationship (SAR) of benzimidazole derivatives having anti-inflammatory activity. The figure represents the SAR studies accomplished by Bukhari et al. (2016).
Eswayah et al. (Eswayah et al., 2017) synthesized several N-substituted benzimidazole derivatives and concluded that compound 147 exhibited prominent analgesic activity indicated by decrease in number of writhing at 50 mg/kg dose compared to standard aspirin (17 vs. 12%). Besides, Sharma et al. (Sharma R. et al., 2017) stated that compounds 148–150 demonstrated notable reduction in edema ranging from 92.7 to 97.6% compared to the standard drugs rofecoxib and indomethacin (78.95 and 75%, respectively). Moreover, compound 151 displayed 72% analgesic activity and 67% protection of inflammation at 20 mg/kg dose in second hour in comparison with standard diclofenac (69% analgesic activity and 65% protection of inflammation) (Chikkula and Sundararajan, 2017). Similarly, Rathore et al. (Rathore et al., 2017) synthesized a series of 1-{(5-substituted-1,3,4-oxadiazol-2-yl)methyl}-2-(morpholinomethyl)-1H-benzimidazole derivatives (152–154) and reported that the compound 152 having chloro group at the ortho position of phenyl ring showed promising anti-inflammatory effect compared to standard indomethacin (74.17 ± 1.28% vs. 57.79 ± 1.71%). Besides, compounds 152–154 also produced remarkable COX-2 inhibition (IC50 range = 8–13.7 µM).
By applying Mannich reaction, Sethi et al. (Sethi et al., 2017) prepared a series of N-benzimidazol-1-yl methyl-benzamide derivatives (155–159), having electron-withdrawing groups chloro and bromo at ortho position of phenyl ring (156–158) and chloromethyl group at 2-position of benzimidazole nucleus (155), asserted significant analgesic and anti-inflammatory activities compared to vehicle control group (10% DMSO, p < 0.05). Moreover, Shankar et al. (Shankar et al., 2017) synthesized a series of 2-(6-alkyl-pyrazin-2-yl)-1H-benzo[d]imidazole derivatives (160–163), where the compound 162 showed maximum selectivity towards COX-2 enzyme among the series (% inhibition 78.68 ± 0.46 and selectivity ratio 3.71) and it might be due to the presence of N-phenyl piperzine moiety in the benzimidazole nucleus. Besides, compounds 160, 161 and 163 demonstrated notable activity against COX-2 enzyme (% inhibition 71.45 ± 0.65, 76.93 ± 0.84 and 58.27 ± 0.25, respectively).
Recently, Sethi et al. (Sethi et al., 2018) synthesized two series of benzimidazole based compounds from the coupling of coumarin and benzimidazole nuclei and narrated the anti-inflammatory activity of compounds 164–165 compared to the standard indomethacin (45 vs. 48%). Brishty et al. (Brishty et al., 2020) synthesized a group of substituted benzimidazole derivatives amongst which compounds 166, 167 and 168 exhibited remarkable analgesic activity by inhibition of acetic acid induced writhing of mice compared to standard diclofenac (88.24, 84.03 and 85.71%, respectively, vs. 90.76%; p < 0.001). In continuation of the research by the group, Saha et al. (Saha et al., 2020) prepared a number of disubstituted benzimidazole derivatives. Compounds 169, 170 and 171 displayed notable analgesic property at a dose of 25 mg/kg by 88.81, 69.40 and 64.93% writhing inhibition, respectively (p < 0.05) in comparison with standard aceclofenac (88.81%). Very recently, the group reported the pharmacological investigation of some of their previously synthesized benzimidazoles (Saha et al., 2021). Compounds 170, 172 and 173 exhibited promising central analgesic potential in radiant heat tail flick method compared to standard morphine (% of elongation 58.07, 51.59, and 76.65, respectively vs. 87.17). Besides, 171, 172 and 173 displayed notable reduction in paw edema (81.75, 79.09 and 86.69%, respectively) comparable to standard aceclofenac (87.83) (Saha et al., 2021).
Antiulcer Activity
Many benzimidazole derivatives are known to possess potent antiulcer activity and H+/K+-ATPase inhibitory properties. During recent times, several new synthetic benzimidazole-based compounds were developed which exhibited similar or better antiulcerogenic potentials compared to the established market preparations. The benzimidazole derivatives with antiulcer activity are shown in Figure 7.
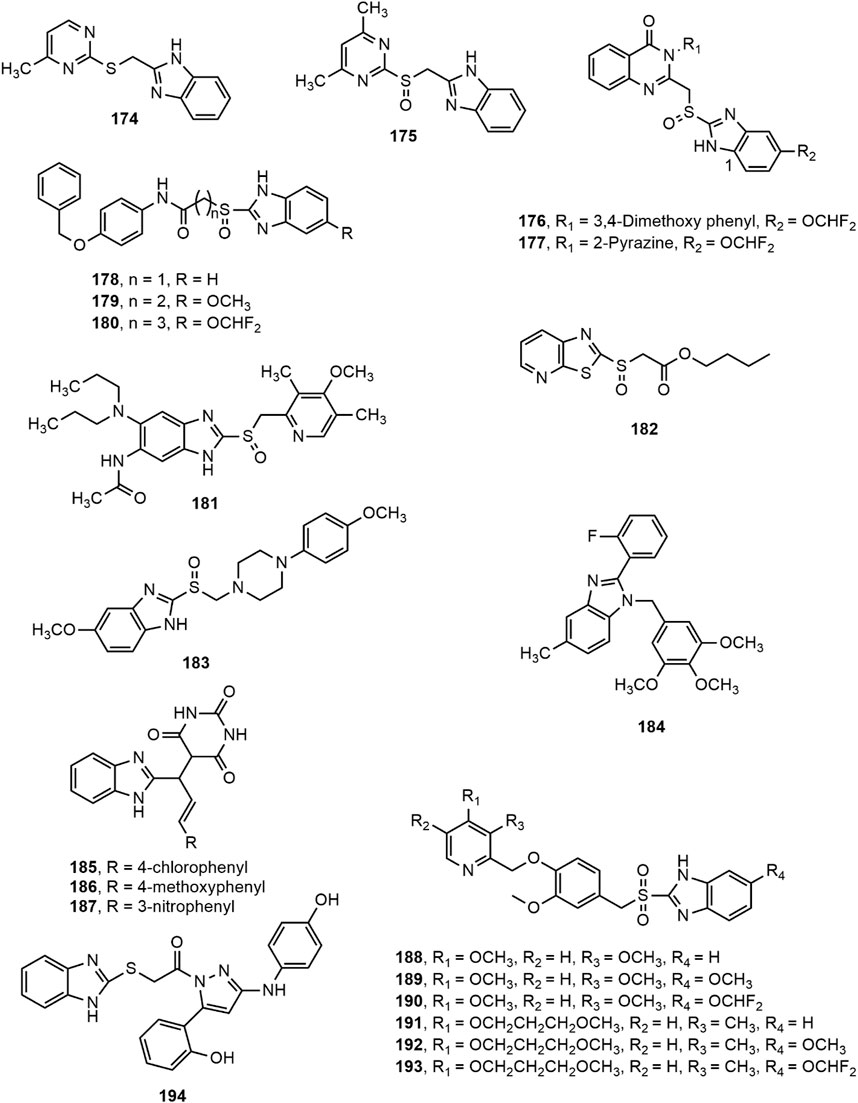
A series of pyrimidylthiomethyl benzimidazoles (e.g. 174) and pyrimidylsulfinylmethyl benzimidazole derivatives (e.g. 175) were developed and screened for antiulcer activity. Compounds 174–175 significantly reduced gastric acid secretion, free acidity and gastric ulcers in the pylorus-ligated rats at 10 and 30 mg/kg doses, where the sulfinyl derivative (175) was found to be more effective than thio derivative (174) (Bariwal et al., 2008). In another study, compounds 176–177 exhibited most prominent antiulcer activity against pylorus ligation-induced, aspirin induced, and ethanol induced ulcer in rat model at a dose level of 10 and 20 mg/kg compared to omeprazole (Patil et al., 2010). Besides, Reddy et al. (Reddy et al., 2011) prepared a series of 2-substituted mercaptobenzimidazole derivatives and reported that compounds 178–180 produced notable antiulcer potentiality at a dose level of 10 mg/kg comparable to omeprazole. Furthermore, compound 181 prevented H+/K+-ATPase enzymatic activity with an IC50 value of 1.6 × 10–5 M and compound 182 displayed prominent effects on inhibition of gastric lesions and gastric acid secretion in a dose dependant manner (0.3–30 mg/kg) (Tanaka et al., 2011; Yan et al., 2011).
Moreover, some benzimidazole-piperazine conjugated analogues were assessed for their in vivo antiulcer property. The 4-methoxy phenyl piperazine substituted benzimidazole derivative (183) appeared to be the most effective agent (Patil et al., 2012). Chang et al. (Chang et al., 2012) developed a series of 3,4,5-trimethoxybenzylbenzimidazole derivatives among which compound 184 (2-fluorophenyl-5-methyl-1-(3,4,5-trimethoxybenzyl) benzimidazole) emerged as the most potent inhibitor of Helicobacter pylori growth and pathogenesis of host cells. The compound specifically inhibited H. pylori adhesion and invasion of gastric epithelial cells, as revealed by in vitro H. pylori infection model. Mathew et al. (Mathew et al., 2013) synthesized a series of substituted benzimidazole derivatives (185–187) and reported that derivatives 185–187 exerted remarkable protection of ulcer (69.58, 69.56 and 67.17%, respectively) at a dose of 50 mg/kg b.w compared to omeprazole (77.37%, 2 mg/kg b.w.). Amongst a series of substituted methoxybenzyl-sulfonyl-1H-benzo[d]imidazole derivatives, compounds 188–193 appeared to be the most potent H+/K+-ATPase inhibitors compared to omeprazole (Rajesh et al., 2017). Finally, some new benzimidazole-pyrazole hybrids were evaluated for in vivo anti ulcerogenic activity using ethanol-induced gastric ulcer model in Albino rats. Compound 194 was found to be the most potent among the series with 83.1% ulcer inhibition at a dose level of 500 μg/kg (Noor et al., 2017).
Antioxidant Activity
Several benzimidazole derivatives have been explored through years for their capacity to act as antioxidants. Different benzimidazole derivatives with antioxidant activity are shown in Figure 8.
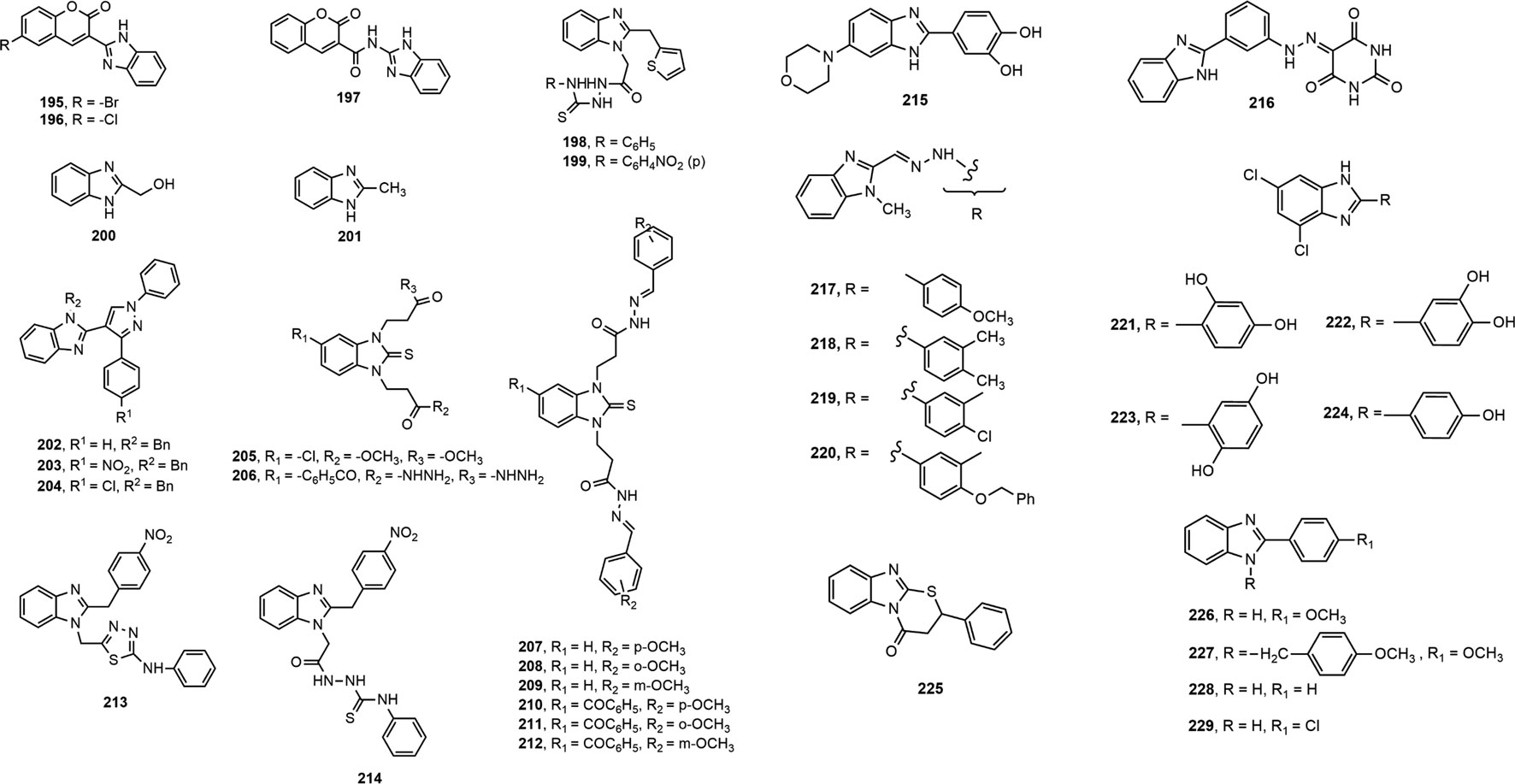
Two series of benzimidazole compounds made through coupling of coumarin derivatives with benzimidazole nucleus either directly or through amide linkage at 2-position were evaluated for antioxidant property (Arora et al., 2014). Compounds 195–197 demonstrated excellent antioxidant activity (IC50 values 19.7, 13.9 and 1.2 µmol/L, respectively) compared to standard butylated hydroxytoluene (BHT, IC50 = 23.4 µmol/L). Among the different benzimidazole derivatives with heterocyclic moieties developed by Mentese et al. (Menteşe et al., 2015), compound 198–199 with a thiophene ring exhibited remarkable antioxidant activity. Poddar et al. synthesized and evaluated the antioxidant property of substituted benzimidazoles by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging method (Poddar et al., 2016). Compound 200 and 201 exhibited mild to moderate antioxidant potential (IC50 = 400.42 and 144.84 µg/ml, respectively) in comparison with standard BHT (IC50 = 51.56 μg/ml).
Among a library of N-substituted pyrazole-containing benzimidazoles, compounds 202–204 attributed prominent antioxidant activity in both DPPH and hydrogen peroxide assay supposed to the presence of benzyl substituent on imidazole nitrogen (Bellam et al., 2017). Besides, Anastassova et al. (Anastassova et al., 2018a) evaluated antioxidant property of compounds 205–206 using tert-butyl hydroperoxide (tert-BOOH) induced oxidative stress on rat hepatocytes, and reported that both compounds showed significant effect comparable to standard quercetin. Similarly, compounds 207–212 displayed notable cytoprotective effect on rat hepatocytes (Anastassova et al., 2018b).
Moreover, a library of 2-(4-nitrobenzyl)-1H-benzimidazole derivatives showed good antioxidant property where compounds 213 and 214 demonstrated prominent inhibitory effect against xanthine oxidase (IC50 = 12.30 ± 0.33 μg/ml) and urease (IC50 = 13.04 ± 0.89 μg/ml), respectively (Karaali et al., 2018). Furthermore, compound 215 had the most potency in the series of 2-(aryl)-6-morpholin-4-yl(or 4-methylpiperazin-1-yl)-1H-benzimidazoles (Özil et al., 2018).
Recently, Baldisserotto et al. (Baldisserotto et al., 2020) synthesized total 39 arylbenzimidazole derivatives and reported their remarkable potency against various free radicals. Figure 9 represents a general structure for describing SAR of benzimidazoles possessing antioxidant activity. The addition of cyano or carboxyl group at 5-position (R1) of the benzimidazole nucleus was responsible for exhibiting medium to high potency against several free radicals. In contrast, the derivatives containing the 5-sulfonic acid group showed poor or no antioxidant properties. Unsubstituted 2-aromatic ring or OH, Cl, Br, 2-OH-napthyl or 4-OH-steryl substitutions enhanced activity. In another study, compound 216 showed 40–80% antioxidant potential at different concentrations (10–100 µM) (Abdelgawad et al., 2019). Amine Khodja et al. reported that compounds 217–220 exerted promising inhibition capacity against various free radicals compared to BHT (IC50 (mean ± SD, µM) for DPPH assay: 40.4 ± 0.9 to 60.4 ± 1.9 vs. 70.8 ± 6.6) (Amine Khodja et al., 2020). Taha et al. (Taha et al., 2020) synthesized 20 benzimidazole derivates and found four potent antioxidant compounds 221–224 with IC50 values (mean ± SEM: 22.42 ± 0.26 to 40.60 ± 0.80) comparable to standard propyl gallate (29.20 ± 1.25). Interestingly, compound 225 exhibited more inhibition (%) of DPPH-free radical than the standard antioxidant Trolox (73% ± 2.42 vs. 70% ± 0.35) (Ramos Rodríguez et al., 2020). Furthermore, compounds 166, 168, 226 and 227 displayed prominent antioxidant property with lower IC50 values than the standard BHT (8.834, 7.519, 0.038 and 0.959 μg/ml, respectively vs. 14.44 μg/ml) (Brishty et al., 2020). Finally, compounds 228 and 229 demonstrated mild antioxidant potential in comparison with standard ascorbic acid (IC50 = 12.25 × 103 and 87.326 × 103 μg/ml, respectively vs. 2.19 μg/ml) (Saha et al., 2021).
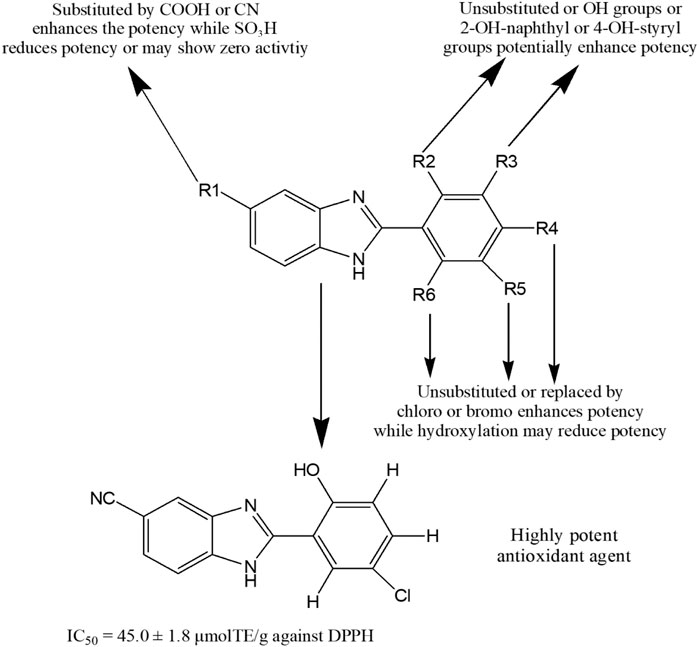
FIGURE 9. Structure activity relationship (SAR) of benzimidazole derivatives having antioxidant potentiality. The figure represents the SAR studies accomplished by Baldisserotto et al. (2020).
Anticancer Activity
Among the anticancer drugs discovered in the recent years, different benzimidazole derivatives occupy an important place. The current review accounts the anticancer activity of benzimidazoles reported after 2013. The benzimidazole derivatives with anticancer activity are shown in Figure 10.
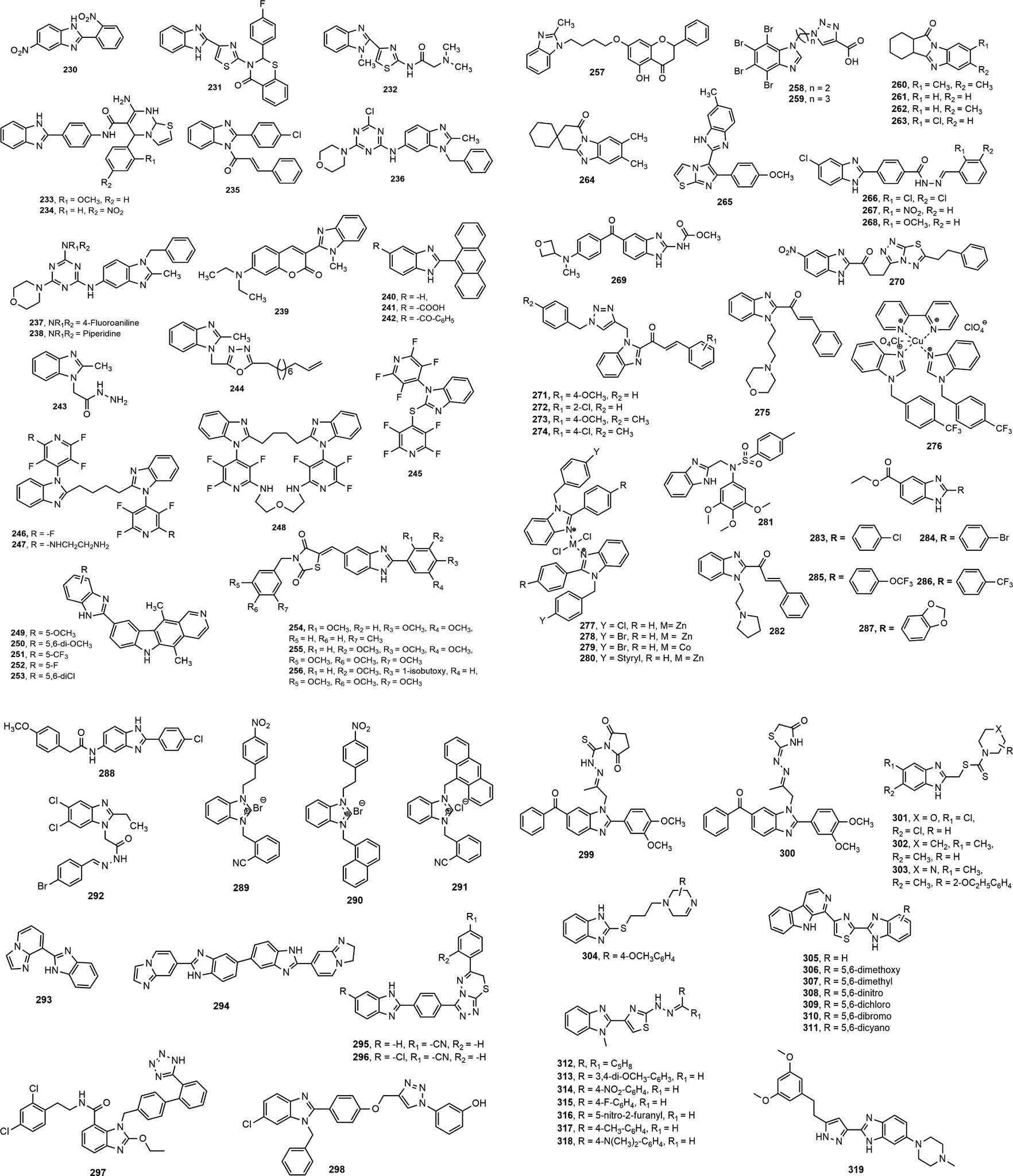
A series of substituted benzimidazole derivatives were evaluated for in vitro anticancer activity in human lung adenocarcinoma A549 cell line at normoxic and hypoxic conditions. Compound 230 was found to be the most cytotoxic agent with hypoxia/normoxia cytotoxic coefficient of 4.75, compared to standard tirapazamine (5.59) (Błaszczak-Świątkiewicz et al., 2014). The benzimidazole-thiazole derivatives 231–232 showed notable anticancer effect against human liver carcinoma cell line (HepG2: IC50 = 0.518 and 0.578 mM) and pheochromocytoma of the rat adrenal medulla cell line (PC12: IC50 = 0.309 and 0.298 mM) (Nofal et al., 2014). Compounds 233–234 with dual inhibition of Aurora A kinase and kinesin spindle protein were found to be the most prominent antitumor agents against various tested cell lines in comparison with standard drug CK0106023 (Abd El-All et al., 2015). Kalalbandi and Seetharamappa synthesized a series of 1-[(2E)-3-phenylprop-2-enoyl]-1H-benzimidazole derivatives among which compound 235 displayed notable antiproliferative activity against nine tumor subpanels, indicated by its selectivity ratios within the range of 0.79–1.53 and 0.47 to 1.69 at the GI50 (growth inhibitory 50%) and TGI level, respectively (Kalalbandi and Seetharamappa, 2015). Similarly, compounds 236–238 with GI50 values of 9.79, 2.58 and 3.81 µM, respectively exhibited broad spectrum antitumor activities, while the compound 236 was found to be the most potent DHFR inhibitor with IC50 value of 1.05 µM (Singla et al., 2015).
Among the derivatives identified by Liu et al. (Liu et al., 2015), compound 239 showed notable inhibition of PI3K-AKT-mTOR pathway having GI50 values in the range of 0.07–0.41 μmol/L against most of the tested cancer cell lines, signifying its potential to be used as anticancer agent. Sontakke and co-workers (Sontakke et al., 2015) synthesized 2-anthryl benzimidazole derivatives (240–242) bearing hydrogen, benzoyl and carboxyl substituents, respectively at 5th position. Compounds 241 and 242 showed anticancer potency against MCF-7 cell lines (IC50: 16.18 and 19.21 µM, respectively) and HL-60 cell lines (IC50: 15.15 and 18.29 µM, respectively) followed by compound 240 (MCF-7 IC50 = 20.48 µM and HL-60 IC50 = 23.23 µM). In another study, compound 243 displayed most notable activity against HeLa cell lines (IC50 = 05.34 ± 1.2 µM) compared to standards doxorubicin and 5-flurouracil (IC50 = 03.56 ± 2.7 and 02.78 ± 2.6 µM, respectively). On the other hand, compound 244 showed marked activity against Hep3B cell line with an IC50 value of 11.10 ± 1.1 µM (Varshney et al., 2015).
Bhambra et al. (Bhambra et al., 2016) synthesized a library of fluoroaryl benzimidazole derivatives (245–248) among which compounds 246–248 demonstrated inhibition against K-562 and MCF-7 cell lines in micromolar range. Compounds 246 and 248 were reported as activators of caspases, which play important role in apoptosis of cancerous cells. Bramhananda Reddy et al. designed and synthesized a library of benzimidazole fused ellipticine derivatives (249–253) and delineated antiproliferative potential against human cancer cell lines Zr-75–1, HeLa, MCF-7 and A-549 with GI50 values of <0.1–34.6 µM, compared to standard etopoxide (GI50 = 0.2–3.08 µM) (Bramhananda Reddy et al., 2016). Sharma et al. (Sharma P. et al., 2017) designed and synthesized a series of benzimidazole bearing thiazolidinedione derivatives, and proved remarkable cytotoxicity of these compounds (254–256) towards PC-3, HeLa, A549 and HT1080 cancer cell lines with IC50 values of 0.096–0.63 µM. In a different study, Compound 257 exhibited potent antiproliferative effect against MFC cells IC50 (mean ± SD) value of 25.72 ± 3.95 μM (Wang et al., 2017). Triazole containing 4,5,6,7-tetrabromo-1H-benzimidazole derivatives 258–259 bearing carboxyl substituent manifested the most prominent inhibitory effect against protein kinase 2 (CK2) with binding affinity value in the range of 1.96–0.91 µM (Chojnacki et al., 2017). Compounds 260–264 exerted antiproliferative property in MTT assay against five human cancer cell lines, breast (T47D), lung (NCl H-522), liver (HepG2), colon (HCT-15) and ovary (PA-1) with IC50 (mean ± SD) value of 7.5 ± 0.3 to 14.6 ± 0.4 μM (Kumar et al., 2018). Upon assessment of antiproliferative property against four cancer cell lines (HeLa, MCF-7, A549 and DU-145 alongside normal HEK-293 cell line), Baig et al. (Baig et al., 2018) enumerated noteworthy IC50 (1.08 µM) of derivative 265 against A549 cell line. Compounds 266–268 displayed prominent cytotoxic activity against A549 and MCF-7 cancer cell lines in comparison with standard drug cisplatin with IC50 values of 0.03–0.06 μM. The presence of 2,4-dichlorobenzylidene (266), 2-nitrobenzylidene (267) and 2-methoxybenzylidene moiety (268) contributed for notable cytotoxic activity of these compounds (Acar Çevik et al., 2018). The oxetanyl substituted compound 269 exhibited cytotoxicity towards a wide range of cancer cell types, e. g. lung, prostate and ovarian cancers with prominent activity against highly aggressive cancer lines (IC50 = 0.9–3.8 µM) (Cheong et al., 2018). Besides, Ibrahim et al. synthesized 2-substituted-5-nitro-benzimidazole derivative 270 as dual inhibitors of c-Met and VEGFR-2 kinases which is important therapeutic target in the treatment of lung (IC50 2.19 ± 0.09 against A549) and colorectal (IC50 10.97 ± 0.09 µM against HCT116) cancers (Ibrahim et al., 2018).
Recently, Djemoui et al. (Djemoui et al., 2020) synthesized several triazole-benzimidazole-chalcone hybrid compounds 271–274 and narrated potential anti-proliferative property of these derivatives against two breast cancer (T47-D and MDA-MB-231) and one prostate cancer cell line (PC3) compared to standard Doxorubicin. It is mentionable here that the chloro substituent 274 at the chalcone ring proliferated the anticancer effects. In a distinct research, a total of 24 new molecules containing benzimidazole group, arene, and alkyl chain-bearing cyclic moieties were synthesized, where the compound 275 impeded the growth of MCF-7 and human ovarian carcinoma (OVCAR-3) cell lines manifesting superior effects to standard cisplatin (IC50 (mean ± SD, µM): 8.91 ± 0.07 vs. 11.7 ± 0.12 and 10.76 ± 0.12 vs. 16.04 ± 0.74, respectively) (Hsieh et al., 2019). A copper (II) complex (276) of benzimidazole derivatives showed excellent potency at 72 h post treatment against prostate cancer cell line (DU145) with IC50 10 µM (Kacar et al., 2020). Besides, two zinc (II) complexes with 2-[2-(benzimidazol-2-yl)-phenyl]-1-methyl-benzimidazole and 1,2-bis(1-methyl-benzimidazol-2-yl)-benzene exerted both dose and time dependent cytotoxicity against breast cancer cell lines (MB-MDA-231) (Su et al., 2019). In another study (Yılmaz et al., 2019), 18 complexes of zinc (II) and cobalt (II) containing 1-benzyl and 2-phenyl moieties showed in vitro potency against human prostate (DU-145) and human ovarian (A-2780) cancer cell lines. Notably, compounds 277–280 at a concentration of 0.1 µM exhibited superior activity against A-2780 cell line in comparison with standard docetaxel. Jian-Song et al. (Jian-Song et al., 2019) synthesized a spectrum of unconventional BZD derivatives and reported their in vitro anticancer property, particularly against three genre of cell lines (MGC-803, PC-3, MCF-7). Notably, compound 281 inhibited predominately of all the three cancer cell lines compared to 5 Fluorouracil (IC50 (mean ± SD, µM): 1.02 ± 0.03 vs. 6.82 ± 1.17, 3.34 ± 0.09 vs. 18.42 ± 1.73, and 5.40 ± 0.51 vs. 17.11 ± 2.94, respectively). The structural modifications of the compound 281 might significantly influence its anti-proliferative property that has been illustrated in Figure 11. In another study (Suk et al., 2019), a benzimidazole derivative carrying a pyrrolidine side chain (282) significantly suppressed sorafenib-resistant cell lines growth in xenograft model by inhibiting the phosphorylation of AKT, p70S6 and the downstream molecule RPS6, has unlocked another milestone in the treatment of hepatocellular carcinoma. Yeong et al. (Yeong et al., 2019) synthesized several new benzimidazole derivatives 283–287 and screened them against sirtuin cancer lines (SIRT1, SIRT2, and SIRT3). Among them, compound 284 elicited significant inhibition of SIRT1-3 compared to tenovin-6 (IC50 (mean ± SD, µM): 7.7 ± 1.4 vs. 42.10, 5.6 ± 1.3 vs. 25.6, and 9.8 ± 2.0 vs. 82.65). Moreover, among 37 synthesized molecules, compound 288 exerted the most inhibition of angiogenesis (79%), and HUVEC and HepG2 cell lines (IC50: 1.47 and 2.57 mM, respectively), and VEGFR-2 kinase inhibition (Yuan et al., 2019).
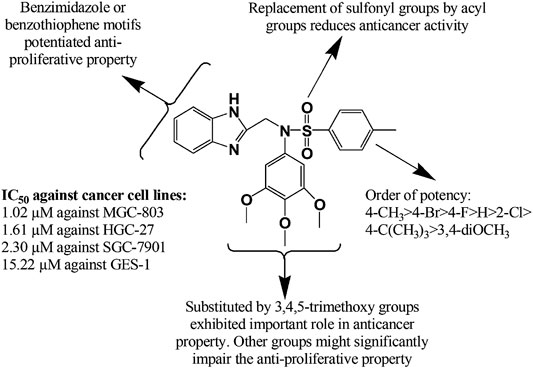
FIGURE 11. Structure activity relationship (SAR) of benzimidazole derivatives having anticancer potentiality. The figure represents the SAR studies accomplished by Song, J. et al. (2019).
Akkoc et al. (Akkoç et al., 2020) synthesized three benzimidazole derivatives 289–291 and reported the most promising anti-breast cancer feature of compound 291 compared to standard cisplatin (IC50 (mean ± SD, µM): 1.26 ± 0.85 vs. 5.77 ± 0.40). In another study, Atmaca et al. (Atmaca et al., 2020) disclosed significant cytotoxicity of compound 292 against breast cancer (MCF-7), prostate cancer (DU-145), and lung cancer (H69AR) with IC50 values of 17.8 ± 0.24, 10.2 ± 1.4 and 49.9 ± 0.22 μg/ml, respectively, compared to 5-Fluorouracil. Compounds 293–294 showed stronger anticancer property against HepG2 (IC50: 26.62 and 20.29, respectively) and DLD-1 cells (IC50: 21.29 and 19.23 µM, respectively) than cisplatin (IC50: 30.38 and 60.79 µM) (Caymaz et al., 2020). Particularly, compound 295–296 demonstrated potent anti-breast cancer effect by obstructing MCF-7 cell growth compared to standard cisplatin (IC50: 0.016 ± 0.001 and 0.018 ± 0.001 vs. 0.020 ± 0.009 µM). Besides, compound 295 displayed efficiency for impeding estrogen-dependent breast cancer by inhibiting aromatase enzyme with IC50 0.032 ± 0.042 µM, compared to IC50 0.024 ± 0.001 µM for letrozole (Acar Çevik et al., 2020). Furthermore, compound 297 blocked neddylation process with superior anticancer property compared to candesartan cilexetil (IC50 5.51 vs 16.43 mM) (Chen et al., 2021). Notably, compound 298 displayed excellent effect in the treatment of lung cancer by inhibiting A-549 and NCI-H460 cell lines growth with IC50 level of 0.63 ± 0.21 μM and 0.99 ± 0.01 μM, respectively, compared to 5-Fluorouracil (IC50 (μM): 1.69 ± 0.90 and 3.20 ± 0.50, respectively). Apart from, the compound 298 significantly suppressed the breast cancer cell lines MCF-7 and MDA-MB-23 with IC50 (μM) values, comparable to 5-Fluorouracil (1.3 ± 0.18 vs. 2.80 ± 0.12, and 0.94 ± 0.02 vs. 0.79 ± 0.09, respectively) (Sridhar Goud et al., 2020).
Meguid et al. (El-Meguid et al., 2020) synthesized an array of novel 6-benzoyl benzimidazole derivatives where most of the compounds exhibited promising anticancer activity with safety profile. Remarkably, compounds 299–300 exhibited superior inhibition of EGFR, HER2, PDGFR-β and VEGFR2, in comparison to erlotinib that opened several promising fighting tools against cervical cancer. In a distinct study, compounds 301–304 strongly prevented breast cancer cell lines manifesting IC50 values of 5.70 9.55, 5.58 and 6.84 μg/ml, respectively compared to standard doxurubucin (IC50 at 4.17 μg/ml) (Nashaat et al., 2020). Furthermore, Sireesha et al. (Sireesha et al., 2021) synthesized a library of hybrid β-carbolines 305–311 that were found to be effective against various cancer cell lines where compounds 306–307 exerted higher in vitro efficacy than the reference etoposide to prevent breast (IC50 against MCF-7: 0.092 ± 0.001 and 0.81 ± 0.062, vs. 2.11 ± 0.024), lung (IC50 against A549: 0.72 ± 0.042 and 1.90 ± 0.88, vs. 3.08 ± 0.135), colon (IC50 against Colo-205: 0.34 ± 0.071 and 0.41 ± 0.12, vs. 0.13 ± 0.017), and ovarian (IC50 against A2780: 1.23 ± 0.55 and 1.80 ± 0.59, vs. 1.31 ± 0.27) cancers. Similarly, Srour et al. (Srour et al., 2020) synthesized a series of benzimidazole derivatives 312–318 and reported promising anticancer potential of 312–316 against breast cancer (IC50 against MCF-7: 5.96–11.91 μM, vs. IC50 of erlotinib; 4.15 μM). Besides, compounds 312, 314, 316, 317 and 318 showed significant cytotoxicity against epidermal growth factor receptor tyrosine kinase with IC50 values of 71.67–152.59 nM compared to IC50 of standard erlotinib 152.59 nM. Finally, Yamani et al. (Yamani et al., 2021) applied scaffolds hybridization technique to formulate a total of 24 pyrazole-benzimidazole derivatives for blocking fibroblast growth factor receptors (FGFRs). Amongst the derivatives, compound 319 selectively inhibited FGFR (1–4) with IC50 values of 0.75, 0.50, 3.05, and 87.90 nM, respectively. Due to acceptable safety and pharmacokinetic profiles along with in vivo anti-tumor potency, the compound 319 is now undergoing with an open-label, multicenter, dose-escalation phase I clinical trial for assessing the safety and tolerability against the adults patients with bladder, gastric, and squamous cell lung cancers (NCT04149691) (Yamani et al., 2021).
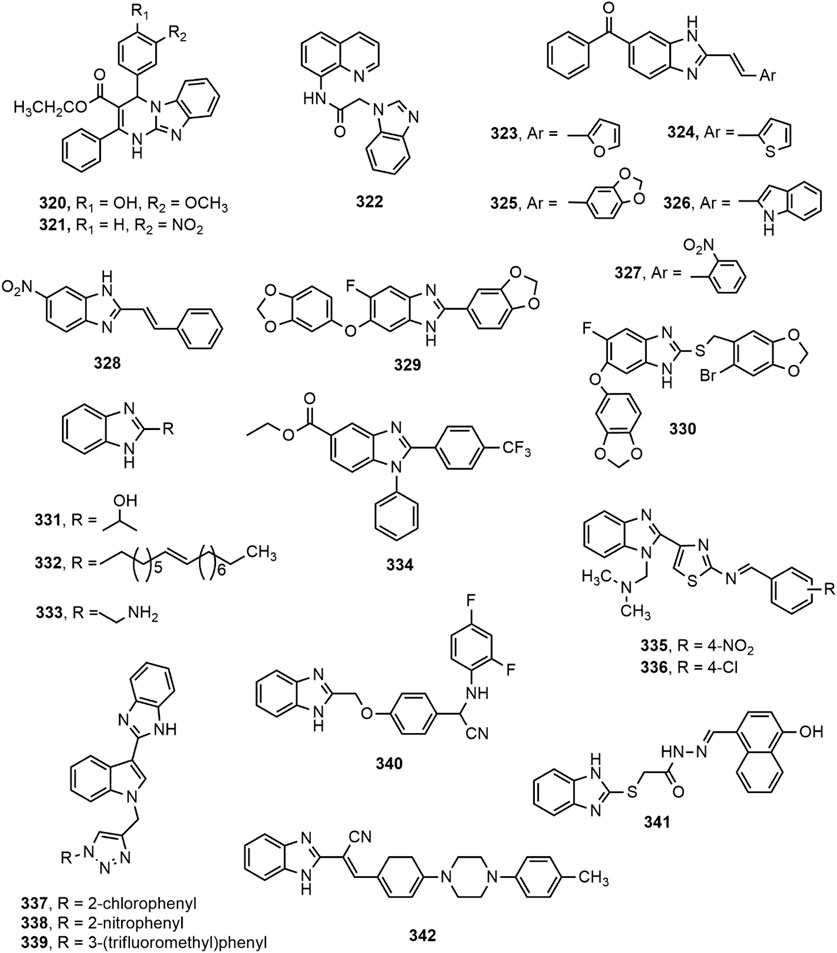
Antitubercular Activity
Compounds containing heterocyclic moieties, such as pyrrole, imidazole and benzimidazole have been reported to demonstrate excellent antitubercular properties (Wang et al., 2015). Benzimidazole scaffold has been on target of the scientists for producing novel antitubercular agents. Different benzimidazole derivatives with antitubercular property are shown in Figure 12.
Warekar et al. (Warekar et al., 2016) synthesized a series of 4-(4-nitro-phenyl)-2-phenyl-1,4-dihydro-benzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylic acid ethyl ester derivatives and reported that compounds 320–321 appeared to be the most promising antitubercular agents among the series with minimum inhibitory concentration (MIC) value of 25 μg/ml against Mycobacterium tuberculosis H37Rv strain. Compound 322 also displayed good activity against same strain under aerobic conditions, as indicated by its IC90 and MIC values (77 µg/ml and >100 μM, respectively) (Mantu et al., 2016). In a different study (Anguru et al., 2017), compounds 323–328 exhibited good activity against M. tuberculosis H37Rv strain while compound 325 emerged as the most promising agent with MIC value of 16 μg/ml.
A number of substituted fluorobenzimidazole derivatives (329–330) were synthesized and evaluated for in vitro antimycobacterial property against pathogenic M. tuberculosis H37Rv strain (ATCC 27294) using MABA method. Compounds 329–330 exhibited notable antitubercular activity against H37Rv strain and their activity was contributed by the incorporation of methylenedioxyphenyl moiety at 2- and 6-position of benzimidazole ring (Nandha et al., 2017). Harika et al. (Harika et al., 2017) synthesized a series of 2-substituted benzimidazole derivatives (331–333) using condensation of o-phenylenediamine with different aliphatic, aromatic, fatty acids, and amino acids, and depicted remarkable antitubercular property against M. tuberculosis H37Rv strain compared to reference drugs pyrazinamide, streptomycin and ciprofloxacin. In addition, Yeong et al. (Yeong et al., 2017) designed two series of benzimidazole derivatives among which compound 334 having trifluoromethyl group displayed antimycobacterial effect against both M. tuberculosis H37Rv strain and the drug-resistant-tuberculosis strain. Compounds 335–336, synthesized by Prasad and Sundararajan, exhibited notable antitubercular activity against M. tuberculosis H37Rv strain with MIC value of 3.9 µg/ml compared to the standard isoniazid (Prasad and Sundararajan, 2017).
Recently, Ashok et al. (Ashok et al., 2018) synthesized a series of indole-benzimidazole-based 1,2,3-triazole hybrids (337–339) by conventional and microwave-assisted methods and evaluated for in vitro antitubercular activity against M. tuberculosis H37Rv strain. The derivatives 337–339 showed prominent antitubercular activity with MIC values in the range of 3.125–6.25 μg/ml. Compound 338 was the most potent among all (MIC = 3.125 μg/ml) which was likely due to the presence of nitro group at ortho position of phenyl ring. Compound 340 displayed notable activity (MIC = 0.05 μg/ml) and emerged as a promising antitubercular agent among the reported series (Shaikh et al., 2018). Compound 341 showing 67.56, 53.45, and 47.56% inhibition against mycobacterial enzymes isocitrate lyase, pantothenate synthetase and chorismate mutase, respectively appeared to be the most potent antitubercular agent among the series (Yadav et al., 2018). Very recently, compound 342 exerted excellent potency against M. tuberculosis H37Rv with IC50 value of 0.78 mg/ml compared to standard ethambutol (IC50 1.56 mg/ml) (Sirim et al., 2020). Gobis et al. (Gobis et al., 2015) have prepared a series of compounds containing novel 2-(2-phenalkyl)-1H-benzo[d]imidazole where the compound bearing methyl groups at the benzimidazole system and phenethyl substituent at the C-2 position with electronegative chlorine atom at the phenyl ring exhibited prominent tuberculostatic property against Mycobacterium tuberculosis strains with MIC values ranging from 0.8 to 1.6 μg/ml (Figure 13).
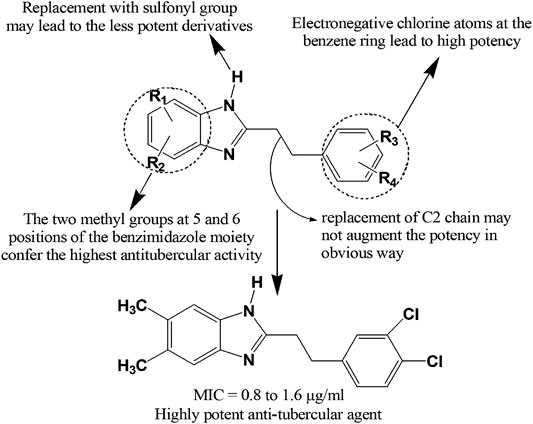
FIGURE 13. Structure activity relationship (SAR) of benzimidazole derivatives having anti-tubercular effects. The figure represents the SAR studies accomplished by Gobis et al. (2015).
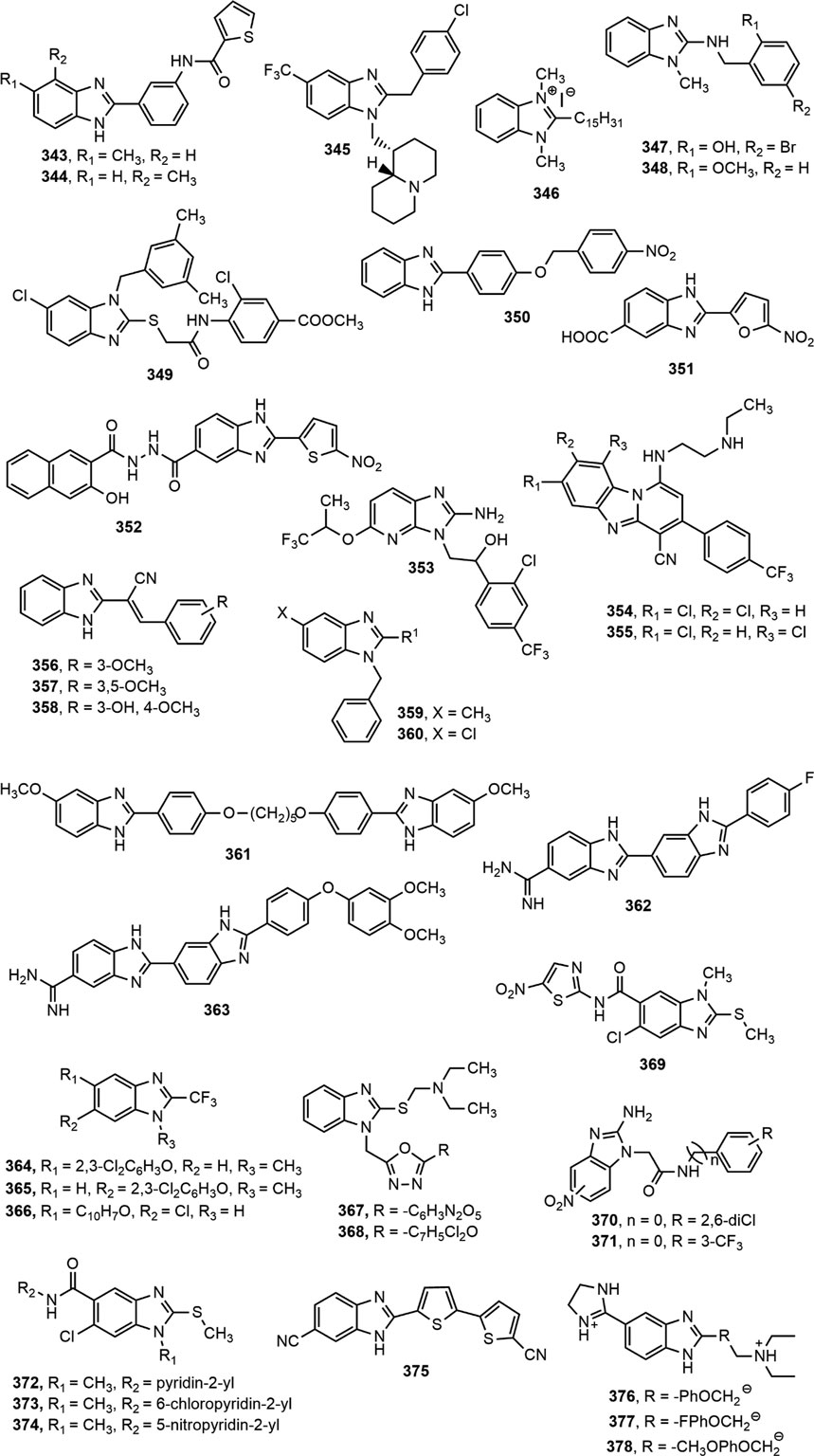
Antiprotozoal Activity
The exploration of benzimidazole nucleus to discover new structural features required for the optimization of novel antiprotozoal agents is of utmost importance. Benzimidazole derivatives with antileishmanial, antimalarial, and antiprotozoal activities against different species are shown in Figure 14.
Benzimidazole Against Leishmania Species
Keurulainen et al. (Keurulainen et al., 2015) synthesized several 2-arylbenzimidazole derivatives and proclaimed extensive inhibitory property of compounds 343–344 against axenic amastigotes of Leishmania donovani. Tonelli and co-workers (Tonelli et al., 2018) reported in vitro antileishmanial activity of derivatives 345–346 with IC50 values of 3.70 and 0.19 µM, respectively against L. tropica, and 4.76 and 0.64 µM, respectively against L. infantum. In another research, a total of 28 N-benzyl-1H-benzimidazol-2-amine derivatives, where compounds 347–348 showed significant (p < 0.05) antileishmanial activity against the amastigote of L. mexicana and L. braziliensis with IC50 values of 2.62 and 3.21 µM, respectively, and their activity was 5.8 and 4.8 times better than standard miltefosine (IC50 = 15.34 µM) (Nieto-Meneses et al., 2018). Upon evaluation of in vitro antileishmanial activity against intracellular amastigotes of L. infantum, compound 349 displayed promising result with IC50 value of 6.8 μM. However, the compound possessed some degree of cytotoxicity with CC50 = 8.0 and 32.0 μM against primary peritoneal mouse macrophages PMM and human fetal lung fibroblasts MCR-5, respectively (De Luca et al., 2018). Recently, compounds 350 exerted moderate antileishmanial effect by inhibiting L. donovani with IC50 value of 68 ± 2.8 μM compared to standard miltefosine (IC50 = 12 ± 0 μM) (Kapil et al., 2019).
Benzimidazole Against Malaria
Among the four Plasmodium species responsible for human malaria, P. falciparum has already started to show resistance to available antimalarial drugs, thus causing the urgency to develop drugs with novel drug targets and new mechanism of action (Singh et al., 2012). In search for compounds with comparable activity to chloroquine, Camacho et al. (Camacho et al., 2011) developed a series of benzimidazole-5-carbohydrazide derivatives and reported that the compounds 351–352 showed prominent in vivo antimalarial potential against rodent P. berghei and appeared to be as effective as chloroquine. Based on structure-activity relationship studies and pharmacokinetics optimization, compound 353 was found to display noticeable efficacy in the humanized P. falciparum mouse model of malaria (Pf/SCID model) with ED90 value of 28.6 mg/kg. The attachment of the neutral hydrophobic group at position 6 of the benzimidazole moiety enhanced this excellent anti-malarial property (Hameed et al., 2014). Singh et al. (Singh K. et al., 2017) reported a series of pyrido[1,2-a]benzimidazole (PBI) antimalarial agents having in vitro anti-plasmodial activity with IC50 values of 0.02–0.95 μM against Pf NF54 strain, and 0.02–1.07 μM against multidrug-resistant Pf K1 strain of P. falciparum. Among which, compounds 354–355 exhibited most prominent in vivo efficacy in mouse P. berghei model due to the presence of chlorine group at C-7, C-8 and C-9 position of benzimidazole moiety. In another study, compounds 356–358 displayed excellent activity with IC50 values of 0.69, 1.60 and 1.61 μM, respectively against chloroquine-sensitive 3D7 strain compared to standard chloroquine (IC50 = 1.53 µM) (Sharma et al., 2018). Recently, compounds 359–360 were synthesized as highly potent antimalarial agents having IC50 values of 0.098 and 0.062 μM, respectively against NF54 strain of P. falciparum (Mueller et al., 2020).
Benzimidazole Against Different Protozoa
Torres-Gómez et al. (Torres-Gómez et al., 2008) synthesized a library of benzimidazole-pentamidine hybrids and evaluated them for antiprotozoal activity against T. vaginalis, E. histolytica, G. lamblia, L. Mexicana and P. berghei. Compound 361 was found to be the most potent from the series, showing 3- and 9- times more activity than standards metronidazole and pentamidine, respectively. Alp et al. (Alp et al., 2009) synthesized several 2’-arylsubstituted-1H,1’H-[2,5’]-bisbenzimidazolyl-5-carboxamidine derivatives and found promising antiparasitic activity of compounds 362 and 363 against P. falciparum, L. donovani, T. brucei rhodesiense and Trypanosoma cruzi. The presence of 4-fluorophenyl (362) and 4-(3,4-dimethoxyphenoxy)phenyl groups (363) at the C-2’ position of amidinobisbenzimidazole moiety contributed for the antiparasitic activity. Among a library of 2-(trifluoromethyl)-1H-benzimidazoles, compounds 364–366 displayed the most prominent in vitro antiparasitic activity against E. histolytica, G. intestinalis, T. vaginalis and T. spiralis (Hernández-Luis et al., 2010). Compounds 367–368 manifested notable activity against Paramecium caudatum and Vorticella campanula compared to standard metronidazole (Maske et al., 2012).
Matadamas-Martínez et al. (Matadamas-Martínez et al., 2016) designed and synthesized a novel nitazoxanide and N-methyl-1H-benzimidazole hybrid molecule 369 which displayed better activity with IC50 value of 0.010 μM than that of standards nitazoxanide, albendazole and metronidazole against G. intestinalis (IC50 = 0.015, 0.037 and 1.224 μM, respectively). Hernández-Núñez et al. (Hernández-Núñez et al., 2017) prepared a series of 2-(2-amino-5(6)-nitro-1H-benzimidazol-1-yl)-N-arylacetamide analogues and illustrated 7-fold more potency of compound 370 than the standard benznidazole against G. intestinalis with an IC50 of 3.95 µM, and 4-fold more activity of compounds 370–371 against T. vaginalis in comparison with benznidazole. Flores-Carrillo and co-workers (Flores-Carrillo et al., 2017) prepared a library of twelve 2-(methylthio)-1H-benzimidazole-5-carboxamide derivatives and investigated their in vitro antiparasitic activity against G. intestinalis, E. histolytica and T. vaginalis. Compounds 372 and 373 showed notable effect against T. vaginalis and G. intestinalis, respectively in comparison with standards albendazole and metronidazole, and compound 374 emerged as a broad-spectrum antiprotozoal agent with activity against all three tested protozoans. Farahat et al. (Farahat et al., 2018) synthesized a series of benzimidazole bichalcophene diamidine derivatives. Compound 375 showed prominent antiparasitic activity towards mice model infected with T. brucei rhodesiense at a dose of 4 × 5 mg/kg i.p. and was found to be more potent than pentamidine, the usual drug of choice to treat African sleeping sickness. Similarly, compounds 376–378 exerted superior efficacy against T. brucei in the treatment of human African trypanosomiasis with IC50 (mean ± SEM) values of 0.47 ± 0.02, 3.67 ± 0.30, and 0.71 ± 0.22, respectively compared to standard nifurtimox (IC50 = 2.0 ± 0.2). The presence of diethylaminoethyl group substantially augmented the antitrypanosomal property of the compounds (376–378). Unsubstituted or methyl substituted aromatic rings or inclusion of an imidazole ring at C-5 also potentiated the activity (Figure 15) (Popov et al., 2020).
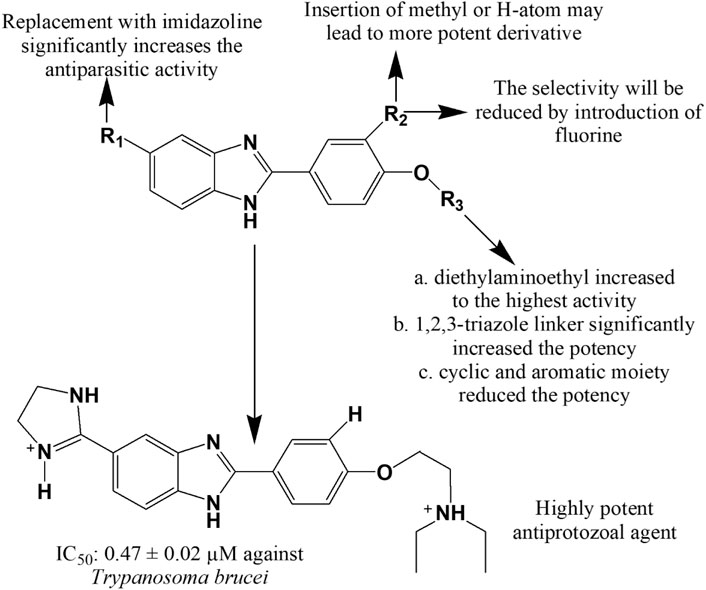
FIGURE 15. Structure activity relationship (SAR) of benzimidazole derivatives effective against Trypanosoma brucei. The figure represents the SAR studies accomplished by Popov et al. (2020).
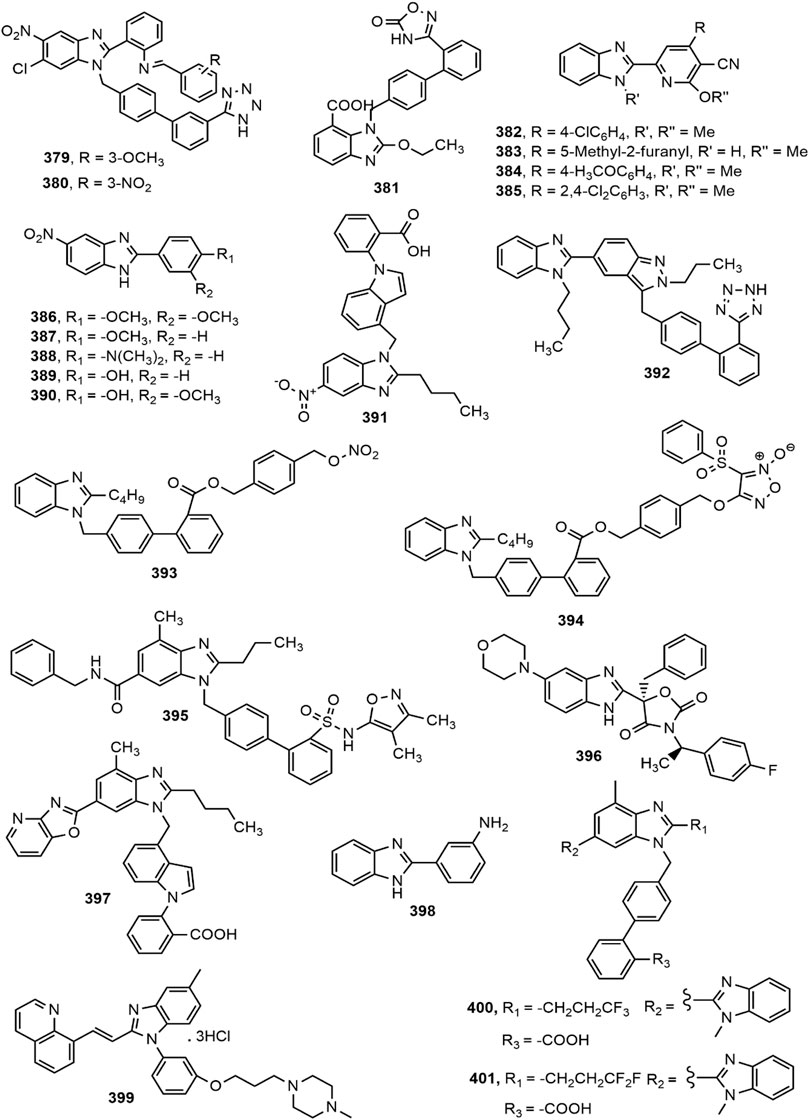
Antihypertensive Activity
A number of marketed antihypertensive drugs comprise benzimidazole moiety, Candesartan cilexetil and Telmisartan are two major examples (Figure 2). Categorically they are the antagonists of angiotensin II receptor playing important role in managing hypertension (Keri et al., 2015). In recent years, a number of scientists have conducted research to prepare benzimidazole based novel antihypertensive agents which provided similar or even better efficacy than the conventional types of antihypertensive drugs. Different benzimidazole derivatives with antihypertensive activity are shown in Figure 16.
Sharma et al. (Sharma et al., 2010) synthesized a series of substituted benzimidazole derivatives and evaluated for their property as angiotensin II receptor antagonists or sartans using invasive (direct) method in Wister rats. Compounds 379 and 380 appeared to be the most prominent antihypertensive agents from the series compared to standard losartan. Kusumoto et al. (Kusumoto et al., 2011) reported about a novel, long-lasting and potent AT1 blocker azilsartan medoxomil and its active metabolite azilsartan (381) and investigated its pharmacological profile in rat and dog models. Oral administration of 0.1–1 mg/kg azilsartan medoxomil in spontaneously hypertensive rats (SHRs) and renal hypertensive dogs demonstrated better effect in reduction of blood pressure at all doses compared to standard drug olmesartan medoxomil. Abou-Seri et al. (Abou-Seri et al., 2011) synthesized a series of 2-alkoxy-4-aryl-6-(1H-benzimidazol-2-yl)-3-pyridinecarbonitrile derivatives. All compounds in the series displayed significant vasodilation properties. Compounds 382–385 showed most prominent activity (IC50 = 0.145, 0.202, 0.210, and 0.214 mM, respectively) compared to standard prazosin hydrochloride (IC50 = 0.487 mM).
Datani et al. (Datani et al., 2012) synthesized a series of twenty novel 5-nitro benzimidazole analogues and screened them for ex vivo vasorelaxant property in rat aorta rings pre-contracted with phenylephrine. Compounds 386–390 exhibited prominent vasorelaxant activity with EC50 value less than 30 μM. Among a new set of 5-nitro benzimidazole derivatives, compound 391 emerged as the most active agent against AT1 with IC50 value of 1.03 ± 0.26 nM (Zhu et al., 2014). The presence of butyl chain on 2-position of benzimidazole moiety (391) helped the derivative to interact and bind tightly with lipophilic pocket of the receptor. Several benzimidazole derivatives containing indazole moiety were synthesized by Lamotte et al. (Lamotte et al., 2014) among which compound 392 displayed potent AT1 receptor antagonism as indicated by IC50 value (0.006 mM).
Two series of nitric oxide (NO) releasing benzimidazole derivatives were synthesized by coupling benzimidazole biphenyl skeleton with nitro ester and furoxan NO-donor moieties, where compounds 393–394 were reported to possess comparable activity to positive control losartan (Zhang et al., 2015). Hao et al. (Hao et al., 2015) designed and synthesized a series of 4′-[(benzimidazol-1-yl)methyl]biphenyl-2-sulphonamide derivatives and reported that compound 395 was found to be the most potent AT1 and Endothelin ETA receptor antagonist with IC50 values 28 and 10 nM, respectively. Upon the identification of a new series of benzimidazole oxazolidinediones as mineralocorticoid receptor (MR) antagonists by high-throughput screening, compound 396 showed similar efficacy as standard drug spironolactone at a dose of 100 mg/kg (p.o.) in rat natriuresis model (Yang et al., 2015).
Bao et al. (Bao et al., 2017) synthesized a series of benzimidazole derivatives which reduced blood pressure in dose-dependent manner in spontaneously hypertensive rats. Among the series, compound 397 exhibited long-lasting efficiency in decreasing blood pressure, with a maximal lowered response of 35.82 ± 6.20 mmHg at 5 mg/kg and 55.98 ± 4.74 mmHg at 10 mg/kg. The compound also showed potent affinity towards AT1 receptor compared to standard telmisartan with IC50 value of 1.13 ± 1.68 nM. In a separate study, Khan et al. (Khan et al., 2018) synthesized a series of 2-phenyl substituted benzimidazoles and assessed antihypertensive activity of these derivatives by using tail cuff method and confirmed excellent antihypertensive property of compound 398 in spontaneously hypertensive rats compared to standard losartan. A very recent report (Yang et al., 2020) ascertained the promising pulmonary hypotensive effect of compound 399 with excellent pharmacokinetic profile in comparison with tadalafil. Finally, compounds 400–401 showed superior inhibition of AT1 receptor (IC50 (mean ± SEM): 0.8 ± 0.1 and 2.3 ± 0.7, respectively) than the both standard losartan and telmisartan (Wu et al., 2020).
Antidiabetic Activity
Several benzimidazole based compounds have displayed promising antidiabetic activity by acting as targets of varied stages of carbohydrate metabolism and some of them have been marketed for the treatment of type 2 diabetes. The structure of benzimidazole derivatives with antidiabetic activity reported within recent years are shown in Figure 17.
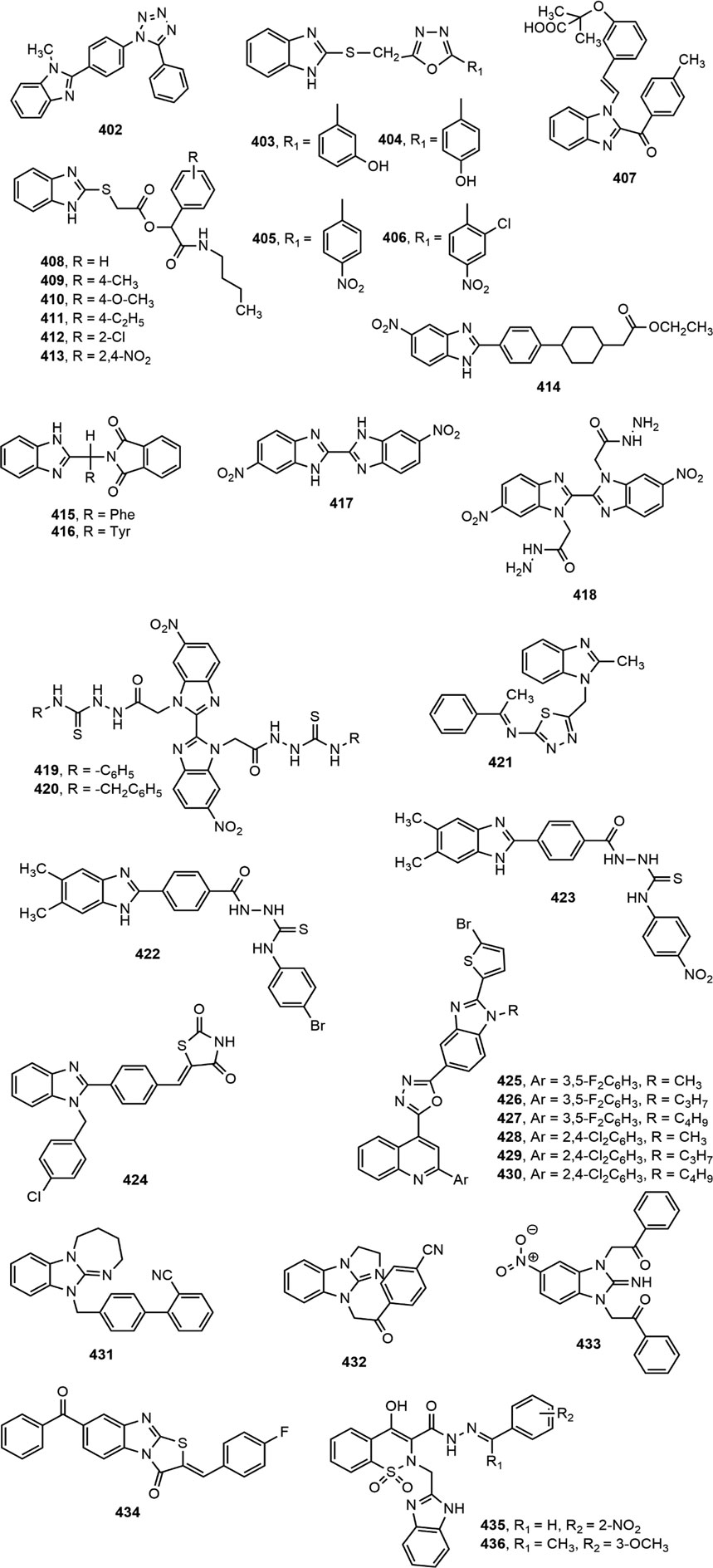
Several 2-(pyridine-2-yl)-1H-benzimidazole derivatives were synthesized by Ishikawa et al. (Ishikawa et al., 2009) among which compound 402 emerged as the most potent and metabolically stable glucokinase activator. Besides, the compound proved its oral glucose lowering efficacy in rat oral glucose tolerance test (OGTT) model. A new series of 4-thiazolidinones and 1,3,4-oxadiazoles bearing 2-mercapto benzimidazole moiety were prepared by Shingalapur et al. (Shingalapur et al., 2010) among which compounds 403–406 produced notable results in OGTT model. All the potent derivatives contained hydroxyl group which might have contributed for their antidiabetic property. Some 2-benzoyl benzimidazole derivatives were assessed for antidiabetic and lipid-lowering effects (Ushiroda et al., 2011). Compound 407 appeared to be an effective peroxisome proliferator-activated receptor (PPARγ) partial agonist. Shaikh et al. (Shaikh et al., 2012) reported one-pot synthesis of carbonyl-amide linkage based benzimidazole derivatives (408–413) via Passerini reaction and demonstrated their antidiabetic potential using rat OGTT model compared to the standard drug glibenclamide.
Diacylglycerol O-acyltransferase-1 (DGAT-1) is an enzyme which catalyzes the formation of triglycerides from diacylglycerol and Acyl-CoA and thereby plays an important role in intestinal fat absorption. DGAT-1 has emerged as an important therapeutic target for the management of different metabolic disorders, such as obesity and diabetes (Birch et al., 2010). Kwak et al. (Kwak et al., 2013) prepared some benzimidazole derivatives as inhibitors of DGAT-1 which contained a phenylcyclohexyl acetic acid group in benzimidazole moiety. Among the derivatives, compound 414 displayed potent in vivo antidiabetic potential in a 4-week study with diet-induced obesity (DIO) mouse model.
The α-glucosidase inhibitors are an important class of antidiabetic agents because of their property to reduce the postprandial glucose level in type-2 diabetic patients (Özil et al., 2016). Mobinikhaledi et al. (Mobinikhaledi et al., 2015) synthesized several new benzimidazole derivatives from amino acids in the presence of phosphorus oxychloride (POCl3). Compounds 415 and 416, upon evaluation of yeast and rat intestinal α-glucosidases inhibitory effect produced most notable results. The IC50 values for compound 416 against yeast and rat intestinal α-glucosidases were reported as 9.1 and 36.7 μM, respectively and thus it appeared to be the most potent benzimidazole among the series. Özil and co-workers (Özil et al., 2016) prepared a series of bis-benzimidazole derivatives by the reaction of o-phenylenediamine and 4-nitro-o-phenylenediamine with oxalic acid using both conventional and microwave techniques. Compounds 417–420 demonstrated prominent α-glucosidase inhibition with IC50 values of 0.54 ± 0.01, 0.44 ± 0.04, 1.24 ± 0.05 and 0.49 ± 0.01 µM, respectively compared to standard acarbose (IC50 13.34 ± 1.26 µM).
Some novel 1,3,4-thiadiazole substituted 2-methyl benzimidazole derivatives were synthesized by conventional methods among which compound 421 exhibited significant in vitro antidiabetic property (Nair et al., 2016). A series of hybrid benzimidazole-thiourea derivatives were synthesized by Zawawi et al. (Zawawi et al., 2017) and evaluated for α-glucosidases inhibitory potential. Compounds 422–423 displayed significant inhibitory properties with IC50 values of 50.57 ± 0.81 and 35.83 ± 0.66 µM, respectively. Besides, Singh et al. (Singh et al., 2018) synthesized a library of N-substituted-benzimidazolyl linked para substituted benzylidene derivatives. Compound 424 bearing 2,4-thiazolidinedione group at 4-position of phenyl ring exhibited pronounced in vitro α-amylase and α-glucosidase inhibitory properties (IC50 = 0.54 ± 0.01 µM) and appeared as a promising antidiabetic agent from the series.
Recently, Bharadwaj et al. (Bharadwaj et al., 2018) have developed several novel benzimidazole derivatives (425–430) and reported excellent antidiabetic activity by applying α-glucosidase inhibitory method with a range of IC50 = 0.66 ± 0.05 to 3.79 ± 0.46 μg/ml compared to standard IC50 value of acarbose (1460.28 ± 244.365). Besides, two potent AMP-activated protein kinase (AMPK) activators (431–432) with multi-target antidiabetic property were reported against LPS-activated murine peritoneal macrophages (Babkov et al., 2019). In another study, two molecules, 1,3-disubstituted-benzimidazole-2-imine (433) and 1,3-thiazolo[3,2-a]benzimidazolone derivative (434), exerted dual effects against dipeptidyl peptidase-IV (DPP-IV) and xanthine oxidase (XO) enzymes with IC50 values less than 200 μM (Tomovic et al., 2020). Finally, Kanwal et al. (Kanwal et al., 2020) prepared a library of benzimidazole-benzothiazine hybrid molecules by Gabriel–Colman rearrangement of methyl 2-(1,1-dioxido-3-oxobenzo[d]isothiazol-2(3H)-yl) acetate, and reported that compounds 435–436 exhibited potential antidiabetic property by inhibiting Ecto-nucleotide pyrophosphatases/phosphodiesterases 1 (ENPP1) 1 and -3.
Anticoagulant Activity
Different substituted benzimidazole derivatives have been explored for several years for their anticoagulant activity and potential use in clinical practice. Benzimidazole derivatives acting as anticoagulants are shown in Figure 18.
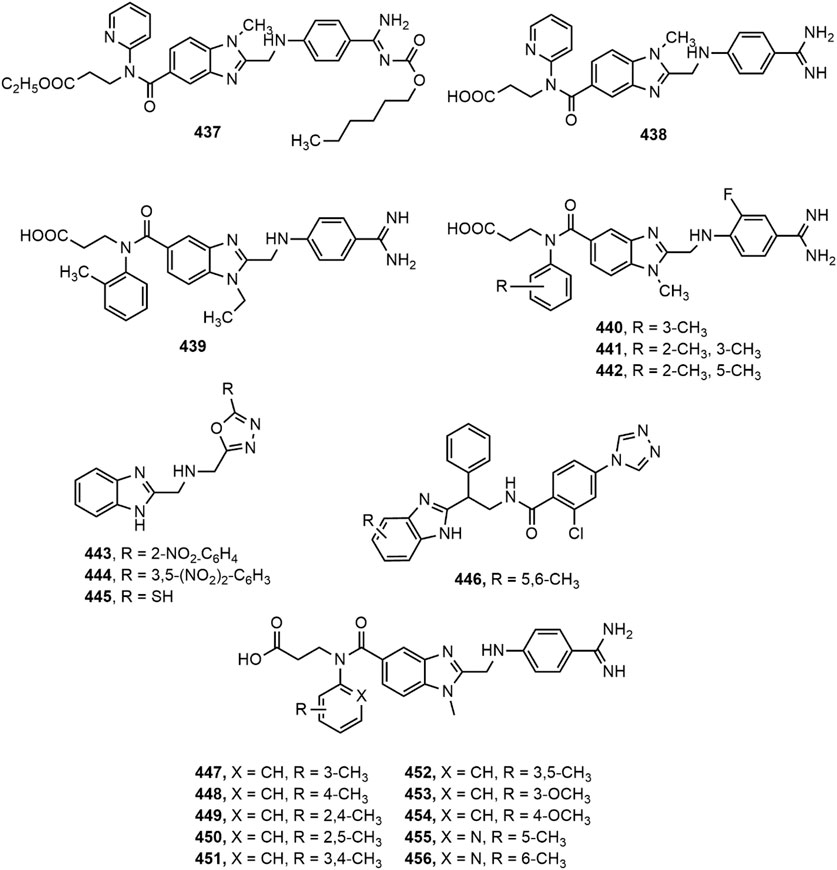
Thrombin (fIIa) is a multifunctional serine protease responsible for the proteolytic cleavage of fibrinogen. Inhibition of thrombin is a crucial mechanism for inhibition of coagulation. Benzimidazole moiety serves as a suitable template for placing a wide range of substituents required for interaction with thrombin (Hauel et al., 2002). Hauel et al. (Hauel et al., 2002) designed and synthesized a series of new benzimidazole derivatives bearing structural similarity to α-NAPAP (N-alpha-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidide), a benzamidine-based powerful inhibitor of thrombin, trypsin and other serine proteases. With the addition of ethyl ester and hexyloxycarbonyl carbamide hydrophobic side chains, improved pharmacokinetic profile was obtained leading to the invention of orally absorbed prodrug, 437 (Dabigatran etexilate). The prodrug 437 reached clinical trials and its active form 438 (Dabigatran) was discovered with excellent thrombin inhibitory potency and tolerability.
Recently, Ren et al. (Ren et al., 2016) designed a series of benzimidazole derivatives and tested them for thrombin inhibitory effects. Compound 439 with IC50 value of 3.11 ± 0.21 nM appeared to be a potent thrombin inhibitor exhibiting better activity than the standard argatroban (IC50 9.88 ± 2.26 nM). A series of 1,2,5-trisubstituted benzimidazole fluorinated derivatives (440–442) were also evaluated for in vitro inhibitory activity against thrombin (Yang et al., 2016). Compounds 440–442 with IC50 values of 2.26 ± 0.38, 1.54 ± 0.09 and 3.35 ± 0.87 nmol/L, respectively showed improved result compared to argatroban (IC50 9.88 ± 2.26 nmol/L), thus showing their potential as thrombin inhibitors. A library of benzimidazole derived 1,3,4-oxadiazole derivatives (443–445) were assessed for ex vivo anticoagulant activity by determining the effect of compounds in increasing prothrombin time (PT) and activated partial thromboplastin time (aPTT) (Vishwanathan and Gurupadayya, 2015). Compounds 443–445 displayed significant increase in PT (32 ± 0.7, 36 ± 0.5 and 41 ± 0.4 s, respectively) compared to standard drug acenocoumarol (48 ± 0.5 s). The compounds, however, caused a slight increase in aPTT in comparison with the reference drug, unfractionated heparin (500 IU/kg).
Factor IXa (fIXa), an important coagulation factor, is a useful target for developing potent and selective antithrombotic agents. A research involving pharmacophore modelling of a new series of benzimidazole analogues presented the chemical features necessary for designing fIXa inhibitors and showed that benzimidazole derivatives have the potential to be developed into effective antithrombotic agents (Kumbhar et al., 2017). Compound 446 was found to be the most active compound from the series, indicated by fIXa binding affinity (Ki) value of 0.016 µM.
Recently, Zhang et al. (Zhang et al., 2020) designed and synthesized ten novel dabigatran derivatives (447–456) with high docking score. The study uncovered that all the compounds showed more than 50% in vitro thrombin inhibitory property at 1 mg/ml concentration, where the IC50 values of compounds 447, 450 and 456 were 1.92, 2.17 and 1.54 nM, respectively, comparable to the IC50 value of positive control dabigatran (1.20 nM). The derivatives 425 and 428, previously reported in this review for antidiabetic property, exerted anticoagulant activity by augmenting the clotting duration. However, only compound 425 exhibited excellent inhibition (93.4%) of epinephrine-induced platelet aggregation (Bharadwaj et al., 2018).
Anticonvulsant Activity
Epilepsy is one of the most prevalent and serious neurological disorders, and recurrent seizures or convulsions are its characteristic syndrome. Around one-third of patients in the world show poor response to currently available antiepileptic drugs (Keri et al., 2015). In search of novel clinically effective anticonvulsant medications, benzimidazole nucleus has recently been explored by scientists with promising results. The benzimidazole derivatives with anticonvulsant property are shown in Figure 19.
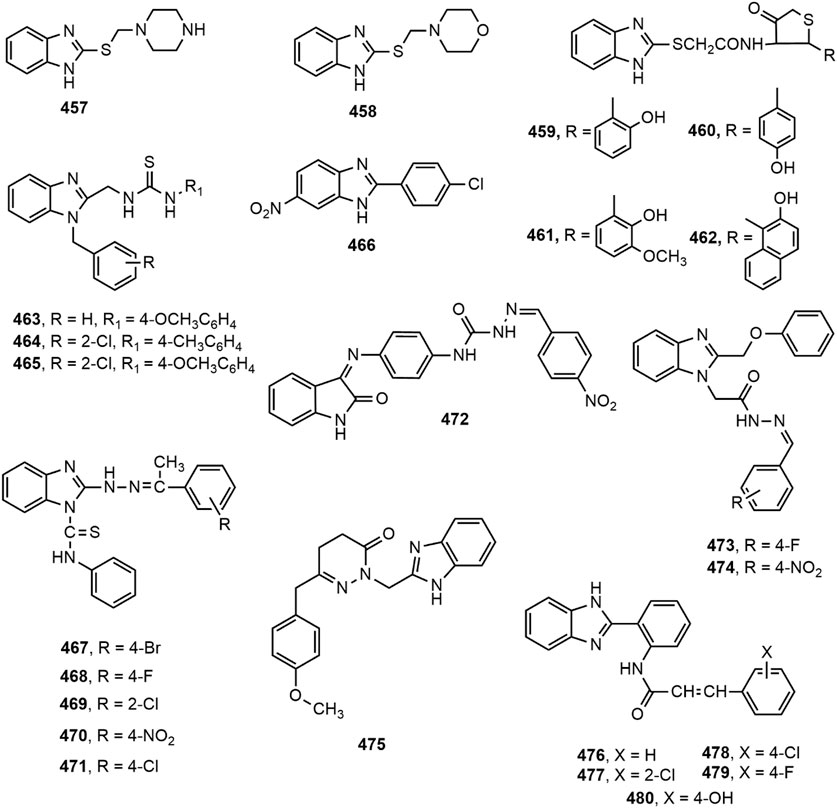
A library of 2-mercaptobenzimidazole derivatives were evaluated for anticonvulsant activity using maximal electroshock seizure (MES) model. The synthesized compounds displayed anticonvulsant property at a dose of 20 mg/kg (i.p.) compared to standard phenytoin and compounds 457–458 appeared to be the most potent of all (Anandarajagopal et al., 2010). A series of 4-thiazolidinones and 1,3,4-oxadiazoles bearing 2-mercaptobenzimidazole moiety were assessed for in vivo anticonvulsant activity (Shingalapur et al., 2010). Compounds 459–462 emerged as the most promising anticonvulsants in MES model.
Siddiqui and co-workers described the synthesis of several 1-{(1-(2-substituted benzyl)-1H-benzo[d]imidazol-2-yl)methyl}-3-arylthioureas which have been mentioned earlier for their analgesic potential. The research group in a previous study reported about the anticonvulsant and cytotoxic effects of the same series of compounds. Compounds 463–465 were found to possess potent anticonvulsant property in comparison with standard drug phenytoin (Siddiqui and Alam, 2010). A series of nitro-benzimidazole derivatives were synthesized by Jain et al. and screened for anticonvulsant activity using MES and subcutaneous pentylenetetrazole (scPTZ) models. Compound 466 displayed the most promising result in inhibiting convulsion induced in mice by both methods (Jain et al., 2010). Bhrigu et al. synthesized a library of 2-[(1-substituted phenylethylidine) hydrazine]-N-phenyl-1H-benzo[d]imidazole-1-carbothioamides (467–471) from the reaction of 2-mercaptobenzimidazole with hydrazine hydrate, substituted acetophenones and phenylisothiocyanate. Compounds 467–471 were found to be active compounds in MES and scPTZ models, and devoid of neurotoxicity (Bhrigu et al., 2012).
A series of benzimidazole substituted semicarbazones were synthesized and tested for anticonvulsant activity using MES model. Compound 472 at a dose of 50 mg/kg (i.p.) appeared to be the most potent among the derivatives (Rajak, 2015). In another study, some oxadiazole bearing benzimidazoles and several derivatives of 2-[2-(phenoxymethyl)-1H-benzimidazol-1-yl]-N0-[(Z)-phenylmethylidene] acetohydrazide (473–474) were evaluated employing MES and scPTZ methods. Compounds 473–474 were found to be effective anticonvulsant agents (Shaharyar et al., 2016). A new series of hybrid benzimidazole containing pyridazinones were developed to assess anticonvulsant property. Compound 475 emerged as an effective and safe anticonvulsant in both MES and scPTZ models. The compound also exhibited notable increase in GABA level (1.7-fold) compared to control which was attributed to its good binding property with the GABAA receptor (Partap et al., 2017). Recently, Sahoo et al. (Sahoo et al., 2019) synthesized several benzimidazole derivatives and disclosed that compounds 476–480 exhibited notable anticonvulsant potency (around 70–80%) in comparison to standard drug phenytoin.
Benzimidazole as Neuro-protective Agent
Neurological disorders comprise the disorders of central and peripheral nervous system. Alzheimer disease (AD), dementia, stroke, Parkinson’s disease, multiple sclerosis, brain tumor etc. are the most common diseases affecting a large number of people. AD is a chronic neurodegenerative disorder and the most common form of dementia manifested by loss of memory, language, cognitive functions, behavior and emotion. The factors which mostly contribute in the development of AD include the levels of acetylcholine (ACh) and deposits of neurotoxic amyloid-β-peptide (Aβ) (Akhtar et al., 2017). A number of substituted benzimidazole derivatives have been developed for the management and treatment of neurological diseases. The benzimidazole derivatives explored recently as neuro-protective agents are shown in Figure 20.
Khan et al. synthesized a series of 6-nitrobenzimidazole derivatives and screened their phosphodiesterase inhibitory property. Among them, compounds 481 (IC50 = 1.5 ± 0.043 µM) and 482 (IC50 = 2.4 ± 0.049 µM) were found to possess better activity than the standard EDTA (IC50 = 274 ± 0.007 µM). The presence of 2,3-dihydroxyphenyl (481) and 4-hydroxyphenyl (482) moiety might have contributed for their notable property (Khan et al., 2012). Hu et al. designed and synthesized a series of keto-benzimidazole derivatives (483–484) as potent and selective inhibitors of Phosphodiesterase 10A (PDE10A). Compound 484 showed 55% receptor occupancy (RO) of PDE10A at 30 mg/kg p.o. in rat brain. Further research led to the identification of compound 484, with improvements in in vivo efficacy (57% RO in rats at 10 mg/kg p.o.), rat clearance and oral bioavailability (Hu et al., 2013). Hamaguchi et al. (Hamaguchi et al., 2013) prepared a series of benzimidazole derivatives as PDE10A inhibitors with reduced CYP1A2 inhibition among which the compound 485 appeared to be the most prominent.
Tamura and co-workers synthesized a series of benzimidazole derivatives for neuropeptide Y (NPY) receptor antagonistic activity which is an important pharmacological target for the treatment of different neurodegenerative disorders. Compound 486 appeared to be the most promising agent (Tamura et al., 2012a). In another study by the same research group (Tamura et al., 2012b), compound 487 was reported to show high NPY Y5 receptor binding affinity along with good absorption, distribution, metabolism and elimination (ADME) profile resulting in notable in vivo efficacy as NPY Y5 receptor antagonist.
Kim et al. evaluated a series of 1-heteroaryl-2-aryl-1H-benzimidazole derivatives as inhibitors of c-Jun N-terminal kinases (JNK3), a group of degenerative signal transducers and potential target of neurodegenerative diseases, e.g. Alzheimer’s and Parkinson’s diseases. Majority of the compounds exhibited high affinity (Kd = 10 µM–46 nM) to JNK3 when investigated through SPR, JNK3 kinase assay and cell-viability of human neuroblastoma cells. The most potent compound 488 showed notable cell protective effect (IC50 = 1.09 µM) against toxicity induced by anisomycin (Kim et al., 2013).
Human Presequence Protease (hPreP) is a mitochondrial metalloprotease capable of degrading amyloid-β peptide and increasing its proteolysis in human neuronal cells. Identification of potential agonists of hPreP is of great importance for Alzheimer’s drug design. Compounds 489–490 showed marked enhancement of hPreP-mediated proteolysis of Aβ, pF1β and fluorogenic-substrate V and thus presented great potential in the treatment of Alzheimer’s disease (Vangavaragu et al., 2014).
Among the therapeutic agents used for the treatment of traumatic brain injury (TBI), positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGluR5) are important. He et al. (He et al., 2015) designed and synthesized a series of acyl-2-aminobenzimidazole derivatives based on the chemical structure of a well-known mGluR5 PAM called 3,3’-difluorobenzaldazine (DFB). The compounds were tested for binding affinity to transmembrane domain of mGluR5 using nitric oxide (NO) production assay, among which compound 491 (IC50 = 6.4 µM) was found to be around 20 times more potent than DFB (IC50 = 136 µM) (He et al., 2015).
Zhu et al. synthesized a series of 2-aminobenzimidazole derivatives (492–494) under microwave irradiation and assessed their acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitory activities. Compounds 492–494 displayed more than 25-fold more selectivity towards BuChE than AChE (Zhu et al., 2013). In another study, the compounds (495–496) showed selective inhibition on BChE and appeared to be the most potent BuChE inhibitors (IC50 = 5.18 and 5.22 µM, respectively) (Ozadali-Sari et al., 2017). Besides, compound 497 was found to be the most potent AChE inhibitor (IC50 = 0.93 ± 0.04 µM) with marked selectivity ratio (13.68) (Alpan et al., 2017). Similarly, compound 498 carrying a methyl group at 5-position of benzimidazole ring and 2-fluorobenzyl group connected to 1,2,3-triazole system was found to be the most potent inhibitor of AChE displaying 84% inhibition at 100 μM concentration (Faraji et al., 2017). The nitrophenyl piperazine substituted derivative 499 showed 57.25 and 77.92% inhibition of Rho-associated protein kinase II (ROCK II) enzyme at a concentration of 0.5 and 1 mM, respectively and exerted prominent IOP lowering effect (51.56%) compared to the standard fasudil (Abbhi et al., 2017).
Recently, compounds 500–501 were reported as promising AChE and BuChE inhibitors (IC50 = 0.14 and 0.22 μM, respectively), highly neuroprotective against hydrogen peroxide mediated toxicity, metal chelators, and free radical scavengers in the treatment of Alzheimer’s disease (Sarıkaya et al., 2018). Similarly, compounds 502–503 demonstrated duel effects as anti-Alzheimer agents by beta amyloid cleavage enzyme-1 (BACE1) inhibition and neuroprotection (Gurjar et al., 2020). In a 6-hydroxydopamine (6-OHDA)-induced oxidative stress in vitro model assay, compound 504 exerted neuroprotective action compared to melatonin, and reduced superoxide anion radical and hypochlorite level in a luminol-dependent chemiluminescent assay (Anastassova et al., 2020). In another study, compound 505 displayed significant anti-neuroinflammatory property (IC50 = 5.07 mM to prevent nitric oxide generation) and 65.7% hBACE1 inhibition. Besides, the compound (505) increased glutathione (GSH) level, reduced ROS production, and subsequently opened a door for further development to be established as a promising treatment option against Alzheimer’s disease (Fang et al., 2019). Finally, compound 506 exhibited promising neuroprotective role on SH-SY5Y cells by preserving the synaptosomal viability and minimizing GST level compared to the standards melatonin and rasagiline (Anastassova et al., 2021).
Miscellaneous Activities
Several other classes of benzimidazole derivatives have been prepared by different scientists during the last few years. Some of these novel benzimidazole based compounds are shown in Figure 21.
Saify et al. (Saify et al., 2014) synthesized a series of 2-(2’-pyridyl) benzimidazole derivatives and evaluated their urease inhibitory property. Compound 507 displayed significant urease inhibition compared to the standard thiourea (IC50 = 19.22 ± 0.49 µM and 21.00 ± 0.01 µM, respectively) and twofold more activity than another standard acetohydroxamic acid (IC50 = 42.00 ± 1.26 µM). Several 1-alkylbenzimidazoles and 1,3-dialkyl benzimidazolium salts were evaluated for their tyrosinase inhibitory activity among which compound 508 emerged as the most potent derivative (IC50 = 0.31 mM) (Karatas et al., 2014).
A series of 2-pyridylbenzimidazole derivatives were designed and synthesized as Cannabinoid 1 (CB1) receptor agonists amongst which compound 509 appeared to be the most potent with a binding affinity value (KiCB1) of 0.53 nM (Mella-Raipán et al., 2013). The same research group synthesized a series of N-acyl-2,5-dimethoxyphenyl-1H-benzimidazole derivatives and assessed them for human CB1 receptor binding affinity using competitive binding assays (Espinosa-Bustos et al., 2015). Compound 510, carrying 3-nitrophenyl group in the aroyl region of benzimidazole moiety was found to be the most promising agent (Ki = 1.2 nM). More recently, they synthesized a new series of benzimidazole derivatives which displayed more selectivity towards human Cannabinoid 2 (CB2) receptor than that towards CB1 receptor (Romero-Parra et al., 2016). Compound 511 demonstrated the best binding affinity (Ki = 0.08 µM), high selectivity index (KiCB1/KiCB2 >125) and low toxicity.
Lim et al. synthesized a series of 2-heteroaryl substituted benzimidazole derivatives bearing piperidinylphenyl acetamide group at 1-position and screened them for their effect on melanin-concentrating hormone (MCH), an attractive target for developing anti-obesity agents. Compound 512 displayed prominent MCH receptor 1(MCH-R1) binding affinity (IC50 = 1 nM), as well as low human ether-a-go-go-related gene (hERG) binding affinity thereby ensuring low risks of cardiovascular diseases, metabolic stability and preferable pharmacokinetic profile (Lim et al., 2013). Igawa et al. synthesized several 1-(1H-benzimidazol-6-yl) pyridin-2(1H)-one compounds among which compound 513 showed most prominent antagonistic property against MCH-R1 (Igawa et al., 2016).
Mochizuki and co-workers (Mochizuki et al., 2016) assessed several benzimidazole analogues for in vivo corticotropin releasing factor type 1 (CRF1) receptor antagonistic property. Compound 514 with an electron withdrawing cyano group at the 4-position of benzimidazole nucleus emerged as the most promising among the series. In continuation with their research to develop CRF1 receptor antagonists, compound 515 was reported as a potent CRF1 binding inhibitor (IC50 = 4.1 nM) and in vitro CRF1 antagonist (IC50 = 44 nM) (Mochizuki et al., 2017). More recently they designed a series of 1,2,3,4-tetrahydropyrimido[1,2-a]benzimidazole analogues as novel CRF1 receptor antagonists. Compound 516 displayed very prominent CRF1 receptor binding activity (IC50 = 58 nM) and oral bioavailability (F = 68% in rat model) indicating its potential in developing clinically effective CRF1 receptor antagonist in the future (Kojima et al., 2018).
A library of small molecules were tested for their property as human gonadotropin releasing hormone (GnRH) receptor antagonists. The derivative 517 (Ki = 13.8 nM) exhibited highest affinity towards human GnRH receptor and emerged as the most prominent GnRH receptor antagonist (Fjellaksel et al., 2017). Furthermore, a recent study reported several benzimidazole analogs (518–521) as intraocular pressure (IOP) reducer in the treatment of dexamethasone-induced ocular hypertension. Among these, compound 521 exerted the best anti-glaucoma action by the maximum IOP reduction of 22.32% from baseline following single drop administration (0.1%) (Marcus et al., 2019).
Maltsev et al. (Maltsev et al., 2020) evaluated a series of Diazepino[1,2-a]benzimidazole derivatives for their possible psychotropic properties. Compound 522 at a dose of 2.34 mg/kg showed prominent anxiolytic, antidepressant and anticonvulsant activities in both rat and mice models. Vasil’ev et al. (Vasil’ev et al., 2017) investigated a series of imidazo[1,2-a]benzimidazole derivatives using corazole-induced seizure model. The compounds 523–526 displayed notable anticonvulsive activity in comparison with standard valproic acid. The prominent activity is attributed to the presence of a 4-fluoro substituent in the 2-position and a dialkylaminoalkyl or cycloalkylaminoalkyl substituent in the 9-position of the fused ring.
Change et al. (Chang et al., 2017) investigated the antiplatelet activity of some novel saccharide-based benzimidazole derivatives. Compound 527 exhibited concentration-dependent inhibitory property against thrombin (0.01 U/ml) and collagen (1 µg/ml)-induced human platelet aggregation in an in vitro model. Its inhibitory effect might be attributed to the presence of 1-imidazolyl moiety at one end carrying a long chain of three sugar moieties. A series of benzimidazole derivatives bearing a sterically hindered phenolic group in their structures were evaluated for in vitro antiplatelet activity using the Adenosine diphosphate (ADP)-induced platelet aggregation model of rabbit’s plasma (Spasov et al., 2020). Compound 528 showed notable antiplatelet activity by exceeding the standard acetylsalicylic acid by 21.8 times. In the in vivo study of inhibition of intravascular platelet aggregation, the same compound displayed 1.5 times superior activity than acetylsalicylic acid and slightly inferior activity than another standard clopidogrel.
Idris and his co-workers (Idris et al., 2019) reported that benzimidazole derivatives 529 and 530 decreased repeated morphine administration-induced thermal hyperalgesia and tactile allodynia as well as the expression of spinal Tumor Necrosis Factor-alpha (TNF-α) in Balb-c mice. The compounds displayed PPARγ agonist activity, indicating their potential in the management of morphine-induced paradoxical pain. Akhtar et al. (Akhtar et al., 2021) evaluated the role of benzimidazole derivative 531 in managing nalbuphine-induced tolerance in cisplatin-induced neuropathic pain in mice. Cisplatin, a platinum-based anticancer medication, is often coupled with Nalbuphine, an opioid analgesic used for treating acute and chronic pain. The compound 531 attenuated the tolerance to the analgesic activity of nalbuphine developed due to its long-term use, as well as TNF-α expression in the spinal cord of mice.
Moreover, Raka et al. (Raka et al., 2021) reported a series of substituted benzimidazole derivatives and found a notable inhibitory property of compound 532 against the acetylcholinesterase enzyme with an IC50 value of 29.64 μg/ml compared to the standard drug donepezil (IC50 = 9.54 μg/ml).
Expert Opinion
Heterocyclic compounds possess versatile applications, such as antibacterial, antiviral, antitubercular, anticancer, anti-inflammatory, analgesics, herbicidal, fungicidal, insecticidal, antidiabetic, antihypertensive, and so on (Pathan et al., 2020). Some of these heterocyclic compounds, having several photochromic, solvatochromic, and biochemical luminescence characteristics, are widely distributed in natural sources as in plant alkaloids, hemoglobin, anthocyanins, flavones, etc., and play a vital role as drugs, vitamins, chlorophyll pigment, dyes, amino acids, and enzymes in human life (Pathan et al., 2020).
Among the heterocyclic compounds, benzimidazole derivatives have been playing the most pulsing and eye-catching pioneer role in synthetic pharmaceutical and agrochemical sectors for the last couple of decades (Pardeshi et al., 2021). As having the similarity of the benzimidazole nucleus with many naturally occurring nucleotides and its presence in several natural compounds, the benzimidazole derivatives can easily interact with various biomacromolecules or target proteins. Thus, the compounds derived from the benzimidazole ring system exert broad-spectrum efficacy against various human disorders like cancer, hypertension, diabetes, bacterial or viral infection, inflammation, gastritis, neurodegenerative disorders, and so on (Wang et al., 2015).
Moreover, the first effective therapy developed through benzimidazole moiety against the Ebola virus was an innovative breakthrough (De Clercq, 2019). Categorically, several approved drugs derived from benzimidazole are currently available in the market and they can be grouped as 1) proton pump inhibitors (PPIs) exemplified by omeprazole, esomeprazole, lansoprazole, pantoprazole, etc., 2) anthelmintic drugs demonstrated by mebendazole, albendazole, oxibendazole, etc., 3) non-sedative H1-receptor blockers or antihistamine presented by astemizole, norastemizole, emedastine, mizolastine, etc., 4) angiotensin II receptor blocker illustrated by telmisartan, candesartan, azilsartan, and 5) calcium sensitized cardiac agents instanced by isomazole (Tahlan et al., 2019).
As stated in the comprehensive review, there are diverse classes of benzimidazole derivatives synthesized by several groups, which showed prominent bioactivities even sometimes better than the existing drugs. Although several structure-activity relationship studies were accomplished by several groups in a particular area of bioactivities, it is challenging to draw any conclusion on the structural features needed for a specific activity. In many cases, further studies on specific benzimidazole derivatives leading to the development of potential drug candidates were not continued. The synthesis of a new library of benzimidazole structures is expanding day by day, with a unique spectrum of bioactivity being reported regularly. Despite having extensive biological and diagnostic applications with the lustrous futuristic potentiality of benzimidazole scaffolds, barriers and challenges are still viable in the clinical development process. Drug resistance is the major drawback of the antiviral, antifungal, antibacterial, antimalarial, anti-cancer, anti-hepatitis, and anti-HIV agents (Wang et al., 2015). Another notable concern is that several benzimidazole derivatives such as PPIs are showing in vitro and in vivo drug-drug interactions with several classes of therapeutics such as anti-cancer (Bezabeh et al., 2012) and antidiabetics (Hossain et al., 2020; Hossain et al., 2021).
Benzimidazole derivatives containing pyrrolidine side-chain exhibited a beacon of hope in malignancy treatment where several available drugs (for example, sorafenib) have become resistant due to long-term exposure against several cancers, such as hepatocellular carcinoma (Suk et al., 2019). It is a crucial fact that benzimidazole derivatives have been synthesized and screened against many disease conditions; among them, very few enter into clinical trials. For example, selumetinib (NCT01933932) and galeterone (NCT04098081) are two promising agents undergoing several clinical trials to be established as effective cancer agents. Besides, several ongoing clinical trials (For instance, NCT01611974) of various drugs (maribavir) bearing benzimidazole moiety showed promising effects against resistant cytomegalovirus (Maertens et al., 2019; Papanicolaou et al., 2019). Dexlansoprazole completed the phase IV trial (NCT01093755) and showed extensive effect against esophageal inflammation (Sharma et al., 2020). However, these reported trials are still very negligible compared to the preclinical status of the ample amount of benzimidazole drug candidates.
It is crystal clear that an exciting number of benzimidazole pharmacophore derivatives have become potential drug candidates to treat several disease conditions in ongoing clinical trials. However, this promising moiety is copious to be further investigated for designing a number of different target molecules, which might reveal some encouraging outcomes in medicinal research. It can easily be assumed at the current stage that several new strategies might be designed and developed for multi-target agents with the highest affinity, specificity, and enhanced bioactivities. In the future, the benzimidazole scaffolds might be fused with another heterocyclic ring system to be explored several novel chemically stable and potent pharmacological agents against several rare or critical diseases or with better pharmacokinetic and pharmacodynamic profiles. This might be an outrageous and worldwide revolutionary achievement in the next century. Toxicity, drug resistance, and poor bioavailability are the significant concerns during drug development or the underlying reasons behind the compounds be untouched in the human trial. Structural modifications of the existing synthesized molecules might be an excellent technique to be brought about improved or novel activities with a better safety profile. Besides, the prodrug strategy can also be applied to ameliorate the bioavailability of the present derivatives. Furthermore, combinations of the benzimidazole derivatives with other existing drugs might be explored for finding synergistic effects or exciting pharmacological activities against multidrug-resistant microorganisms.
Moreover, several benzimidazole-containing clinically used medications, e.g., omeprazole, lansoprazole, pantoprazole, and rabeprazole, are actually in the form of prodrugs for which the onset of action is slower. Thus, there is an increased demand for producing the active form of drugs directly from benzimidazoles, and for this, researchers are paying attention to novel benzimidazole-derived therapeutics with lesser toxicity and better pharmacodynamic profile. In this case, PPIs containing benzimidazole ring might be modified, or the benzimidazole pharmacophore might be replaced by imidazole ring with the improved biological profile. The non-approved benzimidazole scaffolds for cancer therapy having eminent pharmacokinetics and safety profile might be repurposed to treat several life-threatening cancers with a lack of safe and effective treatment. Some anthelmintic drugs containing benzimidazole nuclei, such as flubendazole, albendazole, and mebendazole, could be considered to apply against various types of cancer in future investigations. Besides, stereochemistry could be regarded as to trace and explore more potent isomers of the existing approved benzimidazole derivatives because one enantiomer may exert distinct biochemical property than the other enantiomer.
Conclusion
Benzimidazole, an essential nitrogen-containing heterocyclic moiety, can be found in various therapeutically used compounds playing a vital role in treating many diseases. Many efforts have been given so far on the development of target-based benzimidazole derivatives, and the interest to produce new therapeutically active agents to treat different diseased conditions has grown noticeably during the last few years. However, this field has several challenges, especially to bring the numerous synthesized compounds into the clinical trial, which showed valuable pharmacological properties in different studies, and later to ensure their availability in the market and clinical practice. The present review has focused on the current status of benzimidazole moiety, emphasizing the SAR of different benzimidazole-based compounds explored by scientists around the world. So far this is the most inclusive and informative account about biological and therapeutic potential of benzimidazole derivatives. With a compilation of information from more than 250 latest pieces of literature, we aimed to aid the researchers, medicinal chemists, and drug designers with valuable and comprehensive knowledge and provide them the rationale to develop target-oriented and clinically useful benzimidazole-based molecules.
Author Contributions
SMAR and SRB developed the idea of the article, SRB and MJH performed the literature search and data analysis. SRB and MJH wrote the original draft of the manuscript. MJH, MUK, MRIF, HO, and SMAR critically revised the work. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the Research Universiti Grant, Universiti Kebangsaan Malaysia, Geran Universiti Penyelidikan (GUP), code: 2021-074.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbhi, V., Saini, L., Mishra, S., Sethi, G., Kumar, A. P., and Piplani, P. (2017). Design and Synthesis of Benzimidazole-Based Rho Kinase Inhibitors for the Treatment of Glaucoma. Bioorg. Med. Chem. 25, 6071–6085. doi:10.1016/J.BMC.2017.09.045
Abd El-All, A. S., Magd-El-Din, A. A., Ragab, F. A., ElHefnawi, M., Abdalla, M. M., Galal, S. A., et al. (2015). New Benzimidazoles and Their Antitumor Effects with Aurora A Kinase and KSP Inhibitory Activities. Arch. Pharm. (Weinheim) 348, 475–486. doi:10.1002/ardp.201400441
Abdel-Motaal, M., Almohawes, K., and Tantawy, M. A. (2020). Antimicrobial Evaluation and Docking Study of Some New Substituted Benzimidazole-2yl Derivatives. Bioorg. Chem. 101, 103972. doi:10.1016/j.bioorg.2020.103972
Abdelgawad, M. A., Bakr, R. B., Ahmad, W., Al-Sanea, M. M., and Elshemy, H. A. H. (2019). New Pyrimidine-Benzoxazole/benzimidazole Hybrids: Synthesis, Antioxidant, Cytotoxic Activity, In Vitro Cyclooxygenase and Phospholipase A2-V Inhibition. Bioorg. Chem. 92, 103218. doi:10.1016/j.bioorg.2019.103218
Abou-Seri, S. M., Abouzid, K., and Abou El Ella, D. A. (2011). Molecular Modeling Study and Synthesis of Quinazolinone-Arylpiperazine Derivatives as α1-adrenoreceptor Antagonists. Eur. J. Med. Chem. 46, 647–658. doi:10.1016/J.EJMECH.2010.11.045
Abu-Bakr, S. M., Bassyouni, F. A., and Rehim, M. A. (2012). Pharmacological Evaluation of Benzimidazole Derivatives with Potential Antiviral and Antitumor Activity. Res. Chem. Intermed. 38, 2523–2545. doi:10.1007/s11164-012-0569-y
Acar Çevik, U., Kaya Çavuşoğlu, B., Sağlık, B. N., Osmaniye, D., Levent, S., Ilgın, S., et al. (2020). Synthesis, Docking Studies and Biological Activity of New Benzimidazole- Triazolothiadiazine Derivatives as Aromatase Inhibitor. Molecules 25, 1642. doi:10.3390/molecules25071642
Acar Çevik, U., Sağlık, B. N., Korkut, B., Özkay, Y., and Ilgın, S. (2018). Antiproliferative, Cytotoxic, and Apoptotic Effects of New Benzimidazole Derivatives Bearing Hydrazone Moiety. J. Heterocyclic Chem. 55, 138–148. doi:10.1002/jhet.3016
Adak, V. S., Awate, P. B., Bhagat, V. C., Bodake, V. S., Kardile, D. P., Kilaje, S. V., et al. (2018). Process of Synthesis of Benzimidazole Derivatives against M.Tb. AU2020104192A4 https://patents.google.com/patent/AU2020104192A4/en?oq=AU2020104192A4.
Akhtar, S., Abbas, M., Naeem, K., Faheem, M., Nadeem, H., and Mehmood, A. (2021). Benzimidazole Derivative Ameliorates Opioid-Mediated Tolerance During Anticancer- Induced Neuropathic Pain in Mice. Anticancer. Agents Med. Chem. 21, 365–371. doi:10.2174/1871520620999200818155031
Akhtar, W., Khan, M. F., Verma, G., Shaquiquzzaman, M., Rizvi, M. A., Mehdi, S. H., et al. (2017). Therapeutic Evolution of Benzimidazole Derivatives in the Last Quinquennial Period. Eur. J. Med. Chem. 126, 705–753. doi:10.1016/J.EJMECH.2016.12.010
Akkoç, S., Tüzün, B., İlhan, İ. Ö., and Akkurt, M. (2020). Investigation of Structural, Spectral, Electronic, and Biological Properties of 1,3-disubstituted Benzimidazole Derivatives. J. Mol. Struct. 1219, 128582. doi:10.1016/j.molstruc.2020.128582
AlAjmi, M. F., Hussain, A., Alsalme, A., and Khan, R. A. (2016). In Vivo assessment of Newly Synthesized Achiral Copper(ii) and Zinc(ii) Complexes of a Benzimidazole Derived Scaffold as a Potential Analgesic, Antipyretic and Anti-inflammatory. RSC Adv. 6, 19475–19481. doi:10.1039/C5RA25071D
Alaqeel, S. I. (2017). Synthetic Approaches to Benzimidazoles from O-Phenylenediamine: A Literature Review. J. Saudi Chem. Soc. 21, 229–237. doi:10.1016/J.JSCS.2016.08.001
Alp, M., Göker, H., Brun, R., and Yildiz, S. (2009). Synthesis and Antiparasitic and Antifungal Evaluation of 2'-arylsubstituted-1H,1'H-[2,5']bisbenzimidazolyl-5-carboxamidines. Eur. J. Med. Chem. 44, 2002–2008. doi:10.1016/j.ejmech.2008.10.003
Alpan, A. S., Sarıkaya, G., Çoban, G., Parlar, S., Armagan, G., and Alptüzün, V. (2017). Mannich-Benzimidazole Derivatives as Antioxidant and Anticholinesterase Inhibitors: Synthesis, Biological Evaluations, and Molecular Docking Study. Arch. Pharm. (Weinheim) 350, e1600351. doi:10.1002/ardp.201600351
Amine Khodja, I., Boulebd, H., Bensouici, C., and Belfaitah, A. (2020). Design, Synthesis, Biological Evaluation, Molecular Docking, DFT Calculations and In Silico ADME Analysis of (Benz)imidazole-hydrazone Derivatives as Promising Antioxidant, Antifungal, and Anti-acetylcholinesterase Agents. J. Mol. Struct. 1218, 128527. doi:10.1016/j.molstruc.2020.128527
Anandarajagopal, K., Tiwari, R. N., Bothara, K. G., Sunilson, J. A. J., Dineshkumar, C., and Promwichit, P. (2010). 2-Mercaptobenzimidazole Derivatives: Synthesis and Anticonvulsant Activity. Adv. Appl. Sci. Res. 1, 132–138.
Anastassova, N., Aluani, D., Kostadinov, A., Rangelov, M., Todorova, N., Hristova-Avakumova, N., et al. (2021). Evaluation of the Combined Activity of Benzimidazole Arylhydrazones as New Anti-parkinsonian Agents: Monoamine Oxidase-B Inhibition, Neuroprotection and Oxidative Stress Modulation. Neural Regen. Res. 16, 2299–2309. doi:10.4103/1673-5374.309843
Anastassova, N., Yancheva, D., Hristova-Avakumova, N., Hadjimitova, V., Traykov, T., Aluani, D., et al. (2020). New Benzimidazole-Aldehyde Hybrids as Neuroprotectors with Hypochlorite and Superoxide Radical-Scavenging Activity. Pharmacol. Rep. 72, 846–856. doi:10.1007/s43440-020-00077-3
Anastassova, N. O., Mavrova, A. T., Yancheva, D. Y., Kondeva-Burdina, M. S., Tzankova, V. I., Stoyanov, S. S., et al. (2018a). Hepatotoxicity and Antioxidant Activity of Some New N,N′-disubstituted Benzimidazole-2-Thiones, Radical Scavenging Mechanism and Structure-Activity Relationship. Arabian J. Chem. 11, 353–369. doi:10.1016/J.ARABJC.2016.12.003
Anastassova, N. O., Yancheva, D. Y., Mavrova, A. T., Kondeva-Burdina, M. S., Tzankova, V. I., Hristova-Avakumova, N. G., et al. (2018b). Design, Synthesis, Antioxidant Properties and Mechanism of Action of New N,N′-disubstituted Benzimidazole-2-Thione Hydrazone Derivatives. J. Mol. Struct. 1165, 162–176. doi:10.1016/J.MOLSTRUC.2018.03.119
Anguru, M. R., Taduri, A. K., Bhoomireddy, R. D., Jojula, M., and Gunda, S. K. (2017). Novel Drug Targets for Mycobacterium tuberculosis: 2-heterostyrylbenzimidazoles as Inhibitors of Cell Wall Protein Synthesis. Chem. Cent. J. 11, 68. doi:10.1186/s13065-017-0295-z
Apgar, J. M., Biftu, T., Chen, P., Feng, D., Hicks, J. D., Kekec, A., et al. (2015). Novel Benzimidazole Tetrahydrofuran Derivatives. US20150218149A1 https://patents.google.com/patent/US20150218149A1/en?oq=US+20150218149+A1.
Arora, R. K., Kaur, N., Bansal, Y., and Bansal, G. (2014). Novel Coumarin-Benzimidazole Derivatives as Antioxidants and Safer Anti-inflammatory Agents. Acta Pharm. Sin. B 4, 368–375. doi:10.1016/J.APSB.2014.07.001
Ashok, D., Gundu, S., Aamate, V. K., and Devulapally, M. G. (2018). Conventional and Microwave-Assisted Synthesis of New Indole-Tethered Benzimidazole-Based 1,2,3-triazoles and Evaluation of Their Antimycobacterial, Antioxidant and Antimicrobial Activities. Mol. Divers. 22, 769–778. doi:10.1007/s11030-018-9828-1
Atkinson, S. J., Barker, M. D., Campbell, M., Diallo, H., Douault, C., Garton, N. S., et al. (2015). 2-(azaindol-2-yl)benzimidazoles as Pad4 Inhibitors. US20150175600A1 https://patents.google.com/patent/US20150175600A1/en?oq=US+20150175600+A1.
Atmaca, H., İlhan, S., Batır, M. B., Pulat, Ç. Ç., Güner, A., and Bektaş, H. (2020). Novel Benzimidazole Derivatives: Synthesis, In Vitro Cytotoxicity, Apoptosis and Cell Cycle Studies. Chem. Biol. Interact. 327, 109163. doi:10.1016/j.cbi.2020.109163
Babkov, D. A., Zhukowskaya, O. N., Borisov, A. V., Babkova, V. A., Sokolova, E. V., Brigadirova, A. A., et al. (2019). Towards Multi-Target Antidiabetic Agents: Discovery of Biphenyl-Benzimidazole Conjugates as AMPK Activators. Bioorg. Med. Chem. Lett. 29, 2443–2447. doi:10.1016/j.bmcl.2019.07.035
Baig, M. F., Nayak, V. L., Budaganaboyina, P., Mullagiri, K., Sunkari, S., Gour, J., et al. (2018). Synthesis and Biological Evaluation of Imidazo[2,1-B]thiazole-Benzimidazole Conjugates as Microtubule-Targeting Agents. Bioorg. Chem. 77, 515–526. doi:10.1016/J.BIOORG.2018.02.005
Baldisserotto, A., Demurtas, M., Lampronti, I., Tacchini, M., Moi, D., Balboni, G., et al. (2020). Synthesis and Evaluation of Antioxidant and Antiproliferative Activity of 2-arylbenzimidazoles. Bioorg. Chem. 94, 103396. doi:10.1016/j.bioorg.2019.103396
Bansal, Y., and Silakari, O. (2012). The Therapeutic Journey of Benzimidazoles: A Review. Bioorg. Med. Chem. 20, 6208–6236. doi:10.1016/J.BMC.2012.09.013
Bao, X.-L., Zhu, W.-B., Shan, T.-L., Wu, Z., Zhang, R.-J., Liao, P.-Y., et al. (2017). Design, Synthesis and Evaluation of Novel Angiotensin II Receptor 1 Antagonists with Antihypertensive Activities. RSC Adv. 7, 26401–26410. doi:10.1039/C7RA03915H
Bariwal, J. B., Shah, A. K., Kathiravan, M. K., Somani, R. S., Jagtap, J. R., and Jain, K. S. (2008). Synthesis and Antiulcer Activity of Novel Pyrimidylthiomethyl-And Pyrimidylsulfinylmethyl Benzimidazoles as Potential Reversible Proton Pump Inhibitors. Indian J. Pharm. Educ. Res. 42, 225–231.
Barot, K. P., Nikolova, S., Ivanov, I., and Ghate, M. D. (2013). Novel Research Strategies of Benzimidazole Derivatives: A Review. Mini Rev. Med. Chem. 13, 1421–1447. doi:10.2174/13895575113139990072
Bartberger, M. D., Chakka, N., Gao, H., Guzman-Perez, A., Horne, D. B., Hua, Z., and Cirandur, S. R. (2019). Benzimidazole Derivatives and Their Uses. CA3079081A1 https://patents.google.com/patent/CA3079081A1/en?oq=CA3079081A1.
Bellam, M., Gundluru, M., Sarva, S., Chadive, S., Tartte, V., et al. (2017). Synthesis and Antioxidant Activity of Some New N-Alkylated Pyrazole-Containing Benzimidazoles. Chem. Heterocycl Comp. 53, 173–178. doi:10.1007/s10593-017-2036-6
Berrebi-Bertrand, I., Billot, X., Calmels, T., Capet, M., Krief, S., Labeeuw, O., et al. (2021). Benzimidazole Derivatives as Dual Ligands of the Histamine H1 Receptor and the Histamine H4 Receptor. ES2807191T3 https://patents.google.com/patent/ES2807191T3/en?oq=ES2807191T3.
Bezabeh, S., Mackey, A. C., Kluetz, P., Jappar, D., and Korvick, J. (2012). Accumulating Evidence for a Drug-Drug Interaction between Methotrexate and Proton Pump Inhibitors. Oncologist 17, 550–554. doi:10.1634/theoncologist.2011-0431
Bhambra, A. S., Edgar, M., Elsegood, M. R. J., Horsburgh, L., Kryštof, V., Lucas, P. D., et al. (2016). Novel Fluorinated Benzimidazole-Based Scaffolds and Their Anticancer Activity In Vitro. J. Fluorine Chem. 188, 99–109. doi:10.1016/J.JFLUCHEM.2016.06.009
Bharadwaj, S. S., Poojary, B., Nandish, S. K. M., Kengaiah, J., Kirana, M. P., Shankar, M. K., et al. (2018). Efficient Synthesis and In Silico Studies of the Benzimidazole Hybrid Scaffold with the Quinolinyloxadiazole Skeleton with Potential α-Glucosidase Inhibitory, Anticoagulant, and Antiplatelet Activities for Type-II Diabetes Mellitus Management and Treating Thrombotic Disorders. ACS Omega 3, 12562–12574. doi:10.1021/acsomega.8b01476
Bhrigu, B., Siddiqui, N., Pathak, D., Alam, M. S., Ali, R., and Azad, B. (2012). Anticonvulsant Evaluation of Some Newer Benzimidazole Derivatives: Design and Synthesis. Acta Pol. Pharm. 69, 53–62.
Birajdar, S. S., Hatnapure, G. D., Keche, A. P., and Kamble, V. M. (2013). Synthesis and Biological Evaluation of Amino Alcohol Derivatives of 2-methylbenzimidazole as Antitubercular and Antibacterial Agents. J. Chem. Pharm. Res. 5, 583–589.
Birch, A. M., Buckett, L. K., and Turnbull, A. V. (2010). DGAT1 Inhibitors as Anti-obesity and Anti-diabetic Agents. Curr. Opin. Drug Discov. Devel. 13, 489–496.
Bourque, E. M. J., and Skerlj, R. (2019). Cxcr4 Inhibitors and Uses Thereof. US20190322671A1 https://patents.google.com/patent/US20190322671A1/en?oq=US20190322671A1.
Bramhananda Reddy, N., Burra, V. R., Ravindranath, L. K., Naresh Kumar, V., Sreenivasulu, R., and Sadanandam, P. (2016). Synthesis and Biological Evaluation of Benzimidazole Fused Ellipticine Derivatives as Anticancer Agents. Monatsh Chem. 147, 599–604. doi:10.1007/s00706-016-1684-z
Brink, N. G., and Folkers, K. (1949). Vitamin B12. Vi. 5,6-Dimethylbenzimidazole, A Degradation Product of Vitamin B12. J. Am. Chem. Soc. 71, 2951. doi:10.1021/ja01176a532
Brishty, S. R., Saha, P., Mahmud, Z. A., and Rahman, S. A. (2020). Synthesis and Evaluation of Analgesic and Antioxidant Activities of Substituted Benzimidazole Derivatives. Dhaka Univ. J. Pharm. Sci. 19, 37–46. doi:10.3329/dujps.v19i1.47817
Brown, S. D., and Matthews, D. J. (2015). (alpha-substituted Aralkylamino and Heteroarylalkylamino) Pyrimidinyl and 1,3,5-triazinyl Benzimidazoles, Pharmaceutical Compositions Thereof, and Their Use in Treating Proliferative Diseases. US20150265625A1 https://patents.google.com/patent/US20150265625A1/en?oq=US+20150265625+A1.
Bukhari, S. N., Lauro, G., Jantan, I., Fei Chee, C., Amjad, M. W., Bifulco, G., et al. (2016). Anti-inflammatory Trends of New Benzimidazole Derivatives. Future Med. Chem. 8, 1953–1967. doi:10.4155/fmc-2016-0062
Błaszczak-Świątkiewicz, K., Olszewska, P., and Mikiciuk-Olasik, E. (2014). Biological Approach of Anticancer Activity of New Benzimidazole Derivatives. Pharmacol. Rep. 66, 100–106. doi:10.1016/J.PHAREP.2014.01.001
Camacho, J., Barazarte, A., Gamboa, N., Rodrigues, J., Rojas, R., Vaisberg, A., et al. (2011). Synthesis and Biological Evaluation of Benzimidazole-5-Carbohydrazide Derivatives as Antimalarial, Cytotoxic and Antitubercular Agents. Bioorg. Med. Chem. 19, 2023–2029. doi:10.1016/j.bmc.2011.01.050
Caymaz, B., Yıldız, U., Akkoç, S., Gerçek, Z., Şengül, A., and Coban, B. (2020). Synthesis, Characterization, and Antiproliferative Activity Studies of Novel Benzimidazole‐Imidazopyridine Hybrids as DNA Groove Binders. ChemistrySelect 5, 8465–8474. doi:10.1002/slct.202001580
Chandrasekhar, J., Perreault, S., Patel, L., Phillips, G., Till, N. A., and Treiberg, J. A. (2018). Benzimidazole Derivatives and Their Use as Phosphatidylinositol 3-kinase Inhibitors. WO2018057810A1 https://patents.google.com/patent/WO2018057810A1/en?oq=WO2018057810A1.
Chandrika, N. T., Shrestha, S. K., Ngo, H. X., and Garneau-Tsodikova, S. (2016). Synthesis and Investigation of Novel Benzimidazole Derivatives as Antifungal Agents. Bioorg. Med. Chem. 24, 3680–3686. doi:10.1016/j.bmc.2016.06.010
Chang, C. S., Liu, J. F., Lin, H. J., Lin, C. D., Tang, C. H., Lu, D. Y., et al. (2012). Synthesis and Bioevaluation of Novel 3,4,5-trimethoxybenzylbenzimidazole Derivatives that Inhibit Helicobacter Pylori-Induced Pathogenesis in Human Gastric Epithelial Cells. Eur. J. Med. Chem. 48, 244–254. doi:10.1016/j.ejmech.2011.12.021
Chang, Y., Hsu, W. H., Yang, W. B., Jayakumar, T., Lee, T. Y., Sheu, J. R., et al. (2017). Structure-activity Relationship of Three Synthesized Benzimidazole-Based Oligosaccharides in Human Platelet Activation. Int. J. Mol. Med. 40, 1520–1528. doi:10.3892/IJMM.2017.3133
Chappie, T. A., Verhoest, P. R., Patel, N. C., and Hayward, M. M. (2015). Azabenzimidazole Compounds. US20150322065A1 https://patents.google.com/patent/US20150322065A1/en?oq=US+20150322065+A1.
Chen, X., Yang, X., Mao, F., Wei, J., Xu, Y., Li, B., et al. (2021). Development of Novel Benzimidazole-Derived Neddylation Inhibitors for Suppressing Tumor Growth Invitro and Invivo. Eur. J. Med. Chem. 210, 112964. doi:10.1016/j.ejmech.2020.112964
Cheong, J. E., Zaffagni, M., Chung, I., Xu, Y., Wang, Y., Jernigan, F. E., et al. (2018). Synthesis and Anticancer Activity of Novel Water Soluble Benzimidazole Carbamates. Eur. J. Med. Chem. 144, 372–385. doi:10.1016/J.EJMECH.2017.11.037
Chikkula, K. V., and Sundararajan, R. (2017). Analgesic, Anti-inflammatory, and Antimicrobial Activities of Novel Isoxazole/pyrimidine/pyrazole Substituted Benzimidazole Analogs. Med. Chem. Res. 26, 3026–3037. doi:10.1007/s00044-017-2000-0
Chojnacki, K., Wińska, P., Skierka, K., Wielechowska, M., and Bretner, M. (2017). Synthesis, In Vitro Antiproliferative Activity and Kinase Profile of New Benzimidazole and Benzotriazole Derivatives. Bioorg. Chem. 72, 1–10. doi:10.1016/J.BIOORG.2017.02.017
Crew, A. P., Crews, C. M., Dong, H., Hornberger, K. R., Jaime-Figueroa, S., Qian, Y., et al. (2021). Compounds and Methods for the Targeted Degradation of Rapidly Accelerated Fibrosarcoma Polypeptides. AU2017382436A1 https://patents.google.com/patent/AU2017382436A1/en?oq=AU2017382436A1.
Czardybon, W., Brzózka, K., Galezowski, M., Windak, R., Milik, M., Zawadzka, M., et al. (2015). Novel Benzimidazole Derivatives as Kinase Inhibitors. US20150336967A1 https://patents.google.com/patent/US20150336967A1/en?oq=US+20150336967+A1.
Dai, D., Burgeson, J. R., Gharaibeh, D. N., Moore, A. L., Larson, R. A., Cerruti, N. R., et al. (2013). Discovery and Optimization of Potent Broad-Spectrum Arenavirus Inhibitors Derived from Benzimidazole. Bioorg. Med. Chem. Lett. 23, 744–749. doi:10.1016/J.BMCL.2012.11.095
Datani, R. H., Kini, S. G., and Mubeen, M. (2012). Design, Synthesis and Vasorelaxant Activity of 5-Nitro Benzimidazole Derivatives. J. Comput. Methods Mol. Des. 2, 149–157.
Datar, P. A., and Limaye, S. A. (2015). Design and Synthesis of Mannich Bases as Benzimidazole Derivatives as Analgesic Agents. Antiinflamm. Antiallergy. Agents Med. Chem. 14, 35–46. doi:10.2174/1871523014666150312164625
De Clercq, E. (2019). New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem. Asian J. 14, 3962–3968. doi:10.1002/asia.201900841
De Luca, L., Ferro, S., Buemi, M. R., Monforte, A. M., Gitto, R., Schirmeister, T., et al. (2018). Discovery of Benzimidazole-Based Leishmania Mexicana Cysteine Protease CPB2.8ΔCTE Inhibitors as Potential Therapeutics for Leishmaniasis. Chem. Biol. Drug Des. 92 (3), 1585–1596. doi:10.1111/cbdd.13326
Desai, N. C., Pandya, D., and Vaja, D. (2018). Synthesis and Antimicrobial Activity of Some Heterocyclic Compounds Bearing Benzimidazole and Pyrazoline Motifs. Med. Chem. Res. 27, 52–60. doi:10.1007/s00044-017-2040-5
Desai, N. C., Shihory, N. R., and Kotadiya, G. M. (2014). Facile Synthesis of Benzimidazole Bearing 2-pyridone Derivatives as Potential Antimicrobial Agents. Chin. Chem. Lett. 25, 305–307. doi:10.1016/J.CCLET.2013.11.026
Dhanamjayulu, P., Boga, R. B., and Mehta, A. (2019). Inhibition of Aflatoxin B1 Biosynthesis and Down Regulation of aflR and aflB Genes in Presence of Benzimidazole Derivatives without Impairing the Growth of Aspergillus flavus. Toxicon 170, 60–67. doi:10.1016/j.toxicon.2019.09.018
Dittmer, A., Woskobojnik, I., Adfeldt, R., Drach, J. C., Townsend, L. B., Voigt, S., et al. (2017). Tetrahalogenated Benzimidazole D-Ribonucleosides Are Active against Rat Cytomegalovirus. Antivir. Res 137, 102–107. doi:10.1016/J.ANTIVIRAL.2016.11.012
Djemoui, A., Naouri, A., Ouahrani, M. R., Djemoui, D., Lahcene, S., Lahrech, M. B., et al. (2020). A Step-by-step Synthesis of Triazole-Benzimidazole-Chalcone Hybrids: Anticancer Activity in Human Cells+. J. Mol. Struct. 1204, 127487. doi:10.1016/j.molstruc.2019.127487
Dokla, E. M. E., Abutaleb, N. S., Milik, S. N., Li, D., El-Baz, K., Shalaby, M. W., et al. (2020). Development of Benzimidazole-Based Derivatives as Antimicrobial Agents and Their Synergistic Effect with Colistin against Gram-Negative Bacteria. Eur. J. Med. Chem. 186, 111850. doi:10.1016/j.ejmech.2019.111850
El-Gohary, N. S., and Shaaban, M. I. (2017). Synthesis and Biological Evaluation of a New Series of Benzimidazole Derivatives as Antimicrobial, Antiquorum-Sensing and Antitumor Agents. Eur. J. Med. Chem. 131, 255–262. doi:10.1016/j.ejmech.2017.03.018
El-Meguid, E. A. A., El-Deen, E. M. M., Nael, M. A., and Anwar, M. M. (2020). Novel Benzimidazole Derivatives as Anti-cervical Cancer Agents of Potential Multi-Targeting Kinase Inhibitory Activity. Arabian J. Chem. 13, 9179–9195. doi:10.1016/j.arabjc.2020.10.041
Emerson, G., Brink, N. G., Holly, F. W., Koniuszy, F., Heyl, D., and Folkers, K. (1950). Vitamin B12. VIII. Vitamin B12-like Activity of 5,6-Dimethylbenzimidazole and Tests on Related Compounds. J. Am. Chem. Soc. 72, 3084–3085. doi:10.1021/ja01163a078
Espinosa-Bustos, C., Lagos, C. F., Romero-Parra, J., Zárate, A. M., Mella-Raipán, J., Pessoa-Mahana, H., et al. (2015). Design, Synthesis, Biological Evaluation and Binding Mode Modeling of Benzimidazole Derivatives Targeting the Cannabinoid Receptor Type 1. Arch. Pharm. (Weinheim) 348, 81–88. doi:10.1002/ardp.201400201
Eswayah, A., Khaliel, S., Saad, S., Shebani, N., Fhid, O., Belaid, A., et al. (2017). Synthesis and Analgesic Activity Evaluation of Some New Benzimidazole Derivatives. Am. J. Chem. Appl. 4, 30–35.
Fang, Y., Zhou, H., Gu, Q., and Xu, J. (2019). Synthesis and Evaluation of Tetrahydroisoquinoline-Benzimidazole Hybrids as Multifunctional Agents for the Treatment of Alzheimer's Disease. Eur. J. Med. Chem. 167, 133–145. doi:10.1016/j.ejmech.2019.02.008
Farahat, A. A., Ismail, M. A., Kumar, A., Wenzler, T., Brun, R., Paul, A., et al. (2018). Indole and Benzimidazole Bichalcophenes: Synthesis, DNA Binding and Antiparasitic Activity. Eur. J. Med. Chem. 143, 1590–1596. doi:10.1016/J.EJMECH.2017.10.056
Faraji, L., Shahkarami, S., Nadri, H., Moradi, A., Saeedi, M., Foroumadi, A., et al. (2017). Synthesis of Novel Benzimidazole and Benzothiazole Derivatives Bearing a 1,2,3-triazole Ring System and Their Acetylcholinesterase Inhibitory Activity. J. Chem. Res. 41, 30–35. doi:10.3184/174751917X14836231670980
Fei, F., and Zhou, Z. (2013). New Substituted Benzimidazole Derivatives: a Patent Review (2010 - 2012). Expert Opin. Ther. Pat. 23, 1157–1179. doi:10.1517/13543776.2013.800857
Ferro, S., Buemi, M. R., De Luca, L., Agharbaoui, F. E., Pannecouque, C., and Monforte, A. M. (2017). Searching for Novel N1-Substituted Benzimidazol-2-Ones as Non-nucleoside HIV-1 RT Inhibitors. Bioorg. Med. Chem. 25, 3861–3870. doi:10.1016/J.BMC.2017.05.040
Fjellaksel, R., Boomgaren, M., Sundset, R., Haraldsen, I. H., Hansen, J. H., and Riss, P. J. (2017). Small Molecule Piperazinyl-Benzimidazole Antagonists of the Gonadotropin-Releasing Hormone (GnRH) Receptor. Medchemcomm 8, 1965–1969. doi:10.1039/C7MD00320J
Flores-Carrillo, P., Velázquez-López, J. M., Aguayo-Ortiz, R., Hernández-Campos, A., Trejo-Soto, P. J., Yépez-Mulia, L., et al. (2017). Synthesis, Antiprotozoal Activity, and Chemoinformatic Analysis of 2-(methylthio)-1h-Benzimidazole-5-Carboxamide Derivatives: Identification of New Selective Giardicidal and Trichomonicidal Compounds. Eur. J. Med. Chem. 137, 211–220. doi:10.1016/J.EJMECH.2017.05.058
Gaba, M., and Mohan, C. (2015). Design, Synthesis and Biological Evaluation of Novel 1, 2, 5-Substituted Benzimidazole Derivatives as Gastroprotective Anti-inflammatory and Analgesic Agents. Med. Chem. 5, 58–63. doi:10.4172/2161-0444.1000243
Gobis, K., Foks, H., Suchan, K., Augustynowicz-Kopeć, E., Napiórkowska, A., and Bojanowski, K. (2015). Novel 2-(2-Phenalkyl)-1h-Benzo[d]imidazoles as Antitubercular Agents. Synthesis, Biological Evaluation and Structure-Activity Relationship. Bioorg. Med. Chem. 23 (9), 2112–2120. doi:10.1016/j.bmc.2015.03.008
Gurjar, A. S., Solanki, V. S., Meshram, A. R., and Vishwakarma, S. S. (2020). Exploring Beta Amyloid Cleavage Enzyme‐1 Inhibition and Neuroprotective Role of Benzimidazole Analogues as Anti‐alzheimer Agents. J. Chin. Chem. Soc. 67, 864–873. doi:10.1002/jccs.201900200
Hamaguchi, W., Masuda, N., Isomura, M., Miyamoto, S., Kikuchi, S., Amano, Y., et al. (2013). Design and Synthesis of Novel Benzimidazole Derivatives as Phosphodiesterase 10A Inhibitors with Reduced CYP1A2 Inhibition. Bioorg. Med. Chem. 21, 7612–7623. doi:10.1016/j.bmc.2013.10.035
Hameed, P. S., Chinnapattu, M., Shanbag, G., Manjrekar, P., Koushik, K., Raichurkar, A., et al. (2014). Aminoazabenzimidazoles, a Novel Class of Orally Active Antimalarial Agents. J. Med. Chem. 57, 5702–5713. doi:10.1021/jm500535j
Hao, L.-P., Xue, W.-Z., Han, X.-F., He, X., Zhang, J., and Zhou, Z.-M. (2015). Design, Synthesis and Biological Activity of 4′-[(benzimidazol-1-Yl)methyl]biphenyl-2-Sulphonamides as Dual Angiotensin II and Endothelin A Receptor Antagonists. Med. Chem. Commun. 6, 715–718. doi:10.1039/C4MD00499J
Harika, M. S., Bhargavi, S., Renukadevi, V., Karishma, S., Abbinaya, L., Ramya, L., et al. (2017). Synthesis and Pharmacological Screening of New Benzimidazole Derivatives. Indian J. Heterocycl. Chem. 27, 217–221.
Hauel, N. H., Nar, H., Priepke, H., Ries, U., Stassen, J. M., and Wienen, W. (2002). Structure-Based Design of Novel Potent Nonpeptide Thrombin Inhibitors. J. Med. Chem. 45, 1757–1766. doi:10.1021/jm0109513
He, X., Lakkaraju, S. K., Hanscom, M., Zhao, Z., Wu, J., Stoica, B., et al. (2015). Acyl-2-aminobenzimidazoles: A Novel Class of Neuroprotective Agents Targeting mGluR5. Bioorg. Med. Chem. 23, 2211–2220. doi:10.1016/J.BMC.2015.02.054
Henderson, J. A., Bilimoria, D., Bubenik, M., Cadilhac, C., Cottrell, K. M., Dietrich, E., et al. (2015). Benzimidazole-containing HCV NS5A Inhibitors: Effect of 4-substituted Pyrrolidines in Balancing Genotype 1a and 1b Potency. Bioorg. Med. Chem. Lett. 25, 944–947. doi:10.1016/J.BMCL.2014.12.045
Hernández-Luis, F., Hernández-Campos, A., Castillo, R., Navarrete-Vázquez, G., Soria-Arteche, O., Hernández-Hernández, M., et al. (2010). Synthesis and Biological Activity of 2-(trifluoromethyl)-1h-Benzimidazole Derivatives against Some Protozoa and Trichinella spiralis. Eur. J. Med. Chem. 45, 3135–3141. doi:10.1016/j.ejmech.2010.03.050
Hernández-Núñez, E., Tlahuext, H., Moo-Puc, R., Moreno, D., González-Díaz, M. O., Vázquez, G. N., et al. (2017). Design, Synthesis and Biological Evaluation of 2-(2-Amino-5(6)-nitro-1H-benzimidazol-1-yl)-N-arylacetamides as Antiprotozoal Agents. Molecules 22, 579. doi:10.3390/molecules22040579
Hossain, M. J., Sultan, M. Z., Rashid, M. A., and Kuddus, M. R. (2020). Does Rabeprazole Sodium Alleviate the Anti-diabetic Activity of Linagliptin? Drug-Drug Interaction Analysis by In Vitro and In Vivo Methods. Drug Res. (Stuttg) 70, 519–527. doi:10.1055/a-1233-3371
Hossain, M. J., Sultan, M. Z., Rashid, M. A., and Kuddus, M. R. (2021). Interactions of Linagliptin, Rabeprazole Sodium, and Their Formed Complex with Bovine Serum Albumin: Computational Docking and Fluorescence Spectroscopic Methods. Anal. Sci. Adv. 7, 202000153. doi:10.1002/ansa.202000153
Hsieh, C. Y., Ko, P. W., Chang, Y. J., Kapoor, M., Liang, Y. C., Lin, H. H., et al. (2019). Design and Synthesis of Benzimidazole-Chalcone Derivatives as Potential Anticancer Agents. Molecules 24, 3259. doi:10.3390/molecules24183259
Hu, E., Kunz, R. K., Chen, N., Rumfelt, S., Siegmund, A., Andrews, K., et al. (2013). Design, Optimization, and Biological Evaluation of Novel Keto-Benzimidazoles as Potent and Selective Inhibitors of Phosphodiesterase 10A (PDE10A). J. Med. Chem. 56, 8781–8792. doi:10.1021/jm401234w
Ibrahim, H. A., Awadallah, F. M., Refaat, H. M., and Amin, K. M. (2018). Molecular Docking Simulation, Synthesis and 3D Pharmacophore Studies of Novel 2-substituted-5-nitro-benzimidazole Derivatives as Anticancer Agents Targeting VEGFR-2 and C-Met. Bioorg. Chem. 77, 457–470. doi:10.1016/J.BIOORG.2018.01.014
Idris, Z., Abbas, M., Nadeem, H., and Khan, A. U. (2019). The Benzimidazole Derivatives, B1 (N-[(1H-Benzimidazol-2-yl)Methyl]-4-Methoxyaniline) and B8 (N-{4-[(1H-Benzimidazol-2-yl)Methoxy]Phenyl}Acetamide) Attenuate Morphine-Induced Paradoxical Pain in Mice. Front. Neurosci. 13, 101. doi:10.3389/FNINS.2019.00101
Igawa, H., Takahashi, M., Shirasaki, M., Kakegawa, K., Kina, A., Ikoma, M., et al. (2016). Amine-free Melanin-Concentrating Hormone Receptor 1 Antagonists: Novel 1-(1h-Benzimidazol-6-Yl)pyridin-2(1h)-One Derivatives and Design to Avoid CYP3A4 Time-dependent Inhibition. Bioorg. Med. Chem. 24, 2486–2503. doi:10.1016/j.bmc.2016.04.011
Ishikawa, M., Nonoshita, K., Ogino, Y., Nagae, Y., Tsukahara, D., Hosaka, H., et al. (2009). Discovery of Novel 2-(pyridine-2-Yl)-1h-Benzimidazole Derivatives as Potent Glucokinase Activators. Bioorg. Med. Chem. Lett. 19, 4450–4454. doi:10.1016/j.bmcl.2009.05.038
İbişoğlu, H., Erdemir, E., Atilla, D., Şahin Ün, Ş., Topçu, S., and Gül Şeker, M. (2020). Synthesis, Characterization and Antimicrobial Properties of Cyclotriphosphazenes Bearing Benzimidazolyl Rings. Inorg. Chim. Acta 509, 119679. doi:10.1016/j.ica.2020.119679
Jain, P., Sharma, P. K., Rajak, H., Pawar, R. S., Patil, U. K., and Singour, P. K. (2010). Design, Synthesis and Biological Evaluation of Some Novel Benzimidazole Derivatives for Their Potential Anticonvulsant Activity. Arch. Pharm. Res. 33, 971–980. doi:10.1007/s12272-010-0701-8
Kacar, S., Unver, H., and Sahinturk, V. (2020). A Mononuclear Copper(II) Complex Containing Benzimidazole and Pyridyl Ligands: Synthesis, Characterization, and Antiproliferative Activity against Human Cancer Cells. Arabian J. Chem. 13, 4310–4323. doi:10.1016/j.arabjc.2019.08.002
Kahveci, B., Yılmaz, F., Menteşe, E., Özil, M., and Karaoğlu, Ş. A. (2014). Microwave-Assisted Synthesis of Some Novel Benzimidazole Derivatives Containing Imine Function and Evaluation of Their Antimicrobial Activity. J. Heterocyclic Chem. 51, 982–990. doi:10.1002/jhet.1593
Kalalbandi, V. K. A., and Seetharamappa, J. (2015). 1-[(2E)-3-Phenylprop-2-enoyl]-1H-benzimidazoles as Anticancer Agents: Synthesis, Crystal Structure Analysis and Binding Studies of the Most Potent Anticancer Molecule with Serum Albumin. Med. Chem. Commun. 6, 1942–1953. doi:10.1039/C5MD00293A
Kanwal, A., Ullah, S., Ahmad, M., Pelletier, J., Aslam, S., Sultan, S., et al. (2020). Synthesis and Nucleotide Pyrophosphatase/Phosphodiesterase Inhibition Studies of Carbohydrazides Based on Benzimidazole‐Benzothiazine Skeleton. ChemistrySelect 5, 14399–14407. doi:10.1002/slct.202003479
Kapil, S., Singh, P. K., Kashyap, A., and Silakari, O. (2019). Structure Based Designing of Benzimidazole/benzoxazole Derivatives as Anti-leishmanial Agents. SAR QSAR Environ. Res. 30, 919–933. doi:10.1080/1062936X.2019.1684357
Karaali, N., Baltaş, N., and Menteşe, E. (2018). Synthesis and Antioxidant, Antiurease and Anti-xanthine Oxidase Activities of Some New Benzimidazoles Bearing Triazole, Oxadiazole, Thiadiazole and Imin Function. Indian J. Chem. - Sect. B Org. Med. Chem. 57, 374–384.
Karatas, M. O., Alici, B., Çetinkaya, E., Bilen, C., Gençer, N., and Arslan, O. (2014). Synthesis, Characterization, and Tyrosinase Inhibitory Properties of Benzimidazole Derivatives. Bioorg. Khim 40, 497–502. doi:10.1134/S1068162014040049
Kathrotiya, H. G., and Patel, M. P. (2013). An Efficient Synthesis of 3′-indolyl Substituted Pyrido[1,2-A]benzimidazoles as Potential Antimicrobial and Antioxidant Agents. J. Chem. Sci. 125, 993–1001. doi:10.1007/s12039-013-0468-9
Keri, R. S., Hiremathad, A., Budagumpi, S., and Nagaraja, B. M. (2015). Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 86, 19–65. doi:10.1111/cbdd.12462
Keurulainen, L., Siiskonen, A., Nasereddin, A., Kopelyanskiy, D., Sacerdoti-Sierra, N., Leino, T. O., et al. (2015). Synthesis and Biological Evaluation of 2-arylbenzimidazoles Targeting Leishmania Donovani. Bioorg. Med. Chem. Lett. 25, 1933–1937. doi:10.1016/j.bmcl.2015.03.027
Khan, K. M., Shah, Z., Ahmad, V. U., Ambreen, N., Khan, M., Taha, M., et al. (2012). 6-Nitrobenzimidazole Derivatives: Potential Phosphodiesterase Inhibitors: Synthesis and Structure-Activity Relationship. Bioorg. Med. Chem. 20, 1521–1526. doi:10.1016/J.BMC.2011.12.041
Khan, M. T., Razi, M. T., Jan, S. U., Mukhtiar, M., Gul, R., IzharUllah, S., et al. (2018). Synthesis, Characterization and Antihypertensive Activity of 2-phenyl Substituted Benzimidazoles. Pak. J. Pharm. Sci. 31, 1067–1074.
Kharitonova, M. I., Denisova, A. O., Andronova, V. L., Kayushin, A. L., Konstantinova, I. D., Kotovskaya, S. K., et al. (2017). New Modified 2-aminobenzimidazole Nucleosides: Synthesis and Evaluation of Their Activity against Herpes Simplex Virus Type 1. Bioorg. Med. Chem. Lett. 27, 2484–2487. doi:10.1016/J.BMCL.2017.03.100
Kharitonova, M., Antonov, K., Fateev, I., Berzina, M., Kaushin, A., Paramonov, A., et al. (2016). Chemoenzymatic Synthesis of Modified 2′-Deoxy-2′-Fluoro-β-D-Arabinofuranosyl Benzimidazoles and Evaluation of Their Activity against Herpes Simplex Virus Type 1. Synthesis 49, 1043–1052. doi:10.1055/s-0036-1588625
Kim, M. H., Lee, J., Jung, K., Kim, M., Park, Y. J., Ahn, H., et al. (2013). Syntheses and Biological Evaluation of 1-Heteroaryl-2-Aryl-1h-Benzimidazole Derivatives as C-Jun N-Terminal Kinase Inhibitors with Neuroprotective Effects. Bioorg. Med. Chem. 21, 2271–2285. doi:10.1016/J.BMC.2013.02.021
Kojima, T., Mochizuki, M., Takai, T., Hoashi, Y., Morimoto, S., Seto, M., et al. (2018). Discovery of 1,2,3,4-Tetrahydropyrimido[1,2-A]benzimidazoles as Novel Class of Corticotropin Releasing Factor 1 Receptor Antagonists. Bioorg. Med. Chem. 26, 2229–2250. doi:10.1016/J.BMC.2018.01.020
Kolaczkowski, L. (2013). 2-{(R)-2-methylpyrrolidin-2-yl)-1H-benzimidazole-4-carboxamide Crystalline Form 1. US8372987B2 https://patents.google.com/patent/US8372987B2/en?oq=US8372987+B2.
Kuduk, S. D., McComas, C. C., and Reger, T. S. (2015). Cyclobutyl Benzimidazoles as Pde 10 Inhibitors. US20150307479A1 https://patents.google.com/patent/US20150307479A1/en?oq=US+20150307479+A1.
Kumar, A., Banerjee, S., Roy, P., Sondhi, S. M., and Sharma, A. (2018). Solvent-free Synthesis and Anticancer Activity Evaluation of Benzimidazole and Perimidine Derivatives. Mol. Divers. 22, 113–127. doi:10.1007/s11030-017-9790-3
Kumar, N., Sharma, C. S., Ranawat, M. S., Singh, H. P., Chauhan, L. S., and Dashora, N. (2015). Synthesis, Analgesic and Anti-inflammatory Activities of Novel Mannich Bases of Benzimidazoles. J. Pharm. Invest. 45, 65–71. doi:10.1007/s40005-014-0145-0
Kumbhar, S. S., Choudhari, P. B., and Bhatia, M. S. (2017). 3D QSAR and Pharmacophore Modelling of Selected Benzimidazole Derivatives as Factor IXa Inhibitors. pharmaceutical-sciences 79, 813–819. doi:10.4172/pharmaceutical-sciences.1000295
Kusumoto, K., Igata, H., Ojima, M., Tsuboi, A., Imanishi, M., Yamaguchi, F., et al. (2011). Antihypertensive, Insulin-Sensitising and Renoprotective Effects of a Novel, Potent and Long-Acting Angiotensin II Type 1 Receptor Blocker, Azilsartan Medoxomil, in Rat and Dog Models. Eur. J. Pharmacol. 669, 84–93. doi:10.1016/J.EJPHAR.2011.07.014
Kwak, H. J., Pyun, Y. M., Kim, J. Y., Pagire, H. S., Kim, K. Y., Kim, K. R., et al. (2013). Synthesis and Biological Evaluation of Aminobenzimidazole Derivatives with a Phenylcyclohexyl Acetic Acid Group as Anti-obesity and Anti-diabetic Agents. Bioorg. Med. Chem. Lett. 23, 4713–4718. doi:10.1016/J.BMCL.2013.05.081
Lamotte, Y., Faucher, N., Sançon, J., Pineau, O., Sautet, S., Fouchet, M. H., et al. (2014). Discovery of Novel Indazole Derivatives as Dual Angiotensin II Antagonists and Partial PPARγ Agonists. Bioorg. Med. Chem. Lett. 24, 1098–1103. doi:10.1016/J.BMCL.2014.01.004
Leban, J., and Zaja, M. (2015). Bifluorodioxalane-amino-benzimidazole Kinase Inhibitors for the Treatment of Cancer, Autoimmuneinflammation and Cns Disorders. US20150158878A1 https://patents.google.com/patent/US20150158878A1/en?oq=US+20150158878+A1.
Li, Q., Hu, Q., Wang, X., Zong, Y., Zhao, L., Xing, J., et al. (2015). Discovery of Novel 2-(piperidin-4-Yl)-1h-Benzo[d]imidazole Derivatives as Potential Anti-inflammatory Agents. Chem. Biol. Drug Des. 86, 509–516. doi:10.1111/cbdd.12513
Lim, C. J., Kim, J. Y., Lee, B. H., Oh, K.-S., and Yi, K. Y. (2013). 2-Heteroaryl Benzimidazole Derivatives as Melanin Concentrating Hormone Receptor 1 (MCH-R1) Antagonists. Bull. Korean Chem. Soc. 34, 2305–2310. doi:10.5012/bkcs.2013.34.8.2305
Liu, H., Wang, Y., Sharma, A., Mao, R., Jiang, N., Dun, B., et al. (2015). Derivatives Containing Both Coumarin and Benzimidazole Potently Induce Caspase-dependent Apoptosis of Cancer Cells through Inhibition of PI3K-AKT-mTOR Signaling. Anticancer. Drugs 26, 667–677. doi:10.1097/CAD.0000000000000232
Liu, H. B., Gao, W. W., Tangadanchu, V. K. R., Zhou, C. H., and Geng, R. X. (2018). Novel Aminopyrimidinyl Benzimidazoles as Potentially Antimicrobial Agents: Design, Synthesis and Biological Evaluation. Eur. J. Med. Chem. 143, 66–84. doi:10.1016/J.EJMECH.2017.11.027
Liu, X., Han, Y., and Yang, L. (2020). Benzimidazole Compound and Preparation Method Thereof. US10787420B2 https://patents.google.com/patent/US10787420B2/en?oq=US10787420B2.
Luo, Y.-L., Baathulaa, K., Kannekanti, V. K., Zhou, C.-H., and Cai, G.-X. (2015). Novel Benzimidazole Derived Naphthalimide Triazoles: Synthesis, Antimicrobial Activity and Interactions with Calf Thymus DNA. Sci. China Chem. 58, 483–494. doi:10.1007/s11426-014-5296-3
Maertens, J., Cordonnier, C., Jaksch, P., Poiré, X., Uknis, M., Wu, J., et al. (2019). Maribavir for Preemptive Treatment of Cytomegalovirus Reactivation. N. Engl. J. Med. 381, 1136–1147. doi:10.1056/nejmoa1714656
Mahmoud, M. A., Ibrahim, S. K., and Rdaiaan, M. A. (2020). Antibacterial Evaluation of Some New Benzimidazole Derivatives. Ijpr 12, 282–287. doi:10.31838/ijpr/2020.12.01.050
Malasala, S., Ahmad, M. N., Akunuri, R., Shukla, M., Kaul, G., Dasgupta, A., et al. (2021). Synthesis and Evaluation of New Quinazoline-Benzimidazole Hybrids as Potent Anti-microbial Agents against Multidrug Resistant Staphylococcus aureus and Mycobacterium tuberculosis. Eur. J. Med. Chem. 212, 112996. doi:10.1016/j.ejmech.2020.112996
Maltsev, D. V., Spasov, A. A., Miroshnikov, M. V., Skripka, M. O., and Divaeva, L. N. (2020). Influence of Diazepino[1,2-A]benzimidazole Derivative (DAB-19) on Behavioral Aspects of Animals. Rrp 6, 9–14. doi:10.3897/RRPHARMACOLOGY.6.55142
Mantu, D., Antoci, V., Moldoveanu, C., Zbancioc, G., and Mangalagiu, I. I. (2016). Hybrid Imidazole (Benzimidazole)/pyridine (Quinoline) Derivatives and Evaluation of Their Anticancer and Antimycobacterial Activity. J. Enzyme Inhib. Med. Chem. 31, 96–103. doi:10.1080/14756366.2016.1190711
Marcus, A. J., Iezhitsa, I., Agarwal, R., Vassiliev, P., Spasov, A., Zhukovskaya, O., et al. (2019). Intraocular Pressure-Lowering Effects of Imidazo[1,2-A]- and Pyrimido[1,2-A]benzimidazole Compounds in Rats with Dexamethasone-Induced Ocular Hypertension. Eur. J. Pharmacol. 850, 75–87. doi:10.1016/j.ejphar.2019.01.059
Mariappan, G., Hazarika, R., Alam, F., Karki, R., Patangia, U., and Nath, S. (2015). Synthesis and Biological Evaluation of 2-substituted Benzimidazole Derivatives. Arabian J. Chem. 8, 715–719. doi:10.1016/J.ARABJC.2011.11.008
Maske, P. P., Lokapure, S. G., Nimbalkar, D., and Disouza, J. I. (2012). Synthesis and Antiprotozoal Activity of Nitro and Halogeno Substituted Some Novel Mercaptobenzimidazole Derivatives. Der Pharma Chem. 4, 1283–1287.
Matadamas-Martínez, F., Castillo, R., Hernández-Campos, A., Méndez-Cuesta, C., de Souza, W., Gadelha, A. P., et al. (2016). Proteomic and Ultrastructural Analysis of the Effect of a New Nitazoxanide-N-Methyl-1h-Benzimidazole Hybrid against Giardia Intestinalis. Res. Vet. Sci. 105, 171–179. doi:10.1016/J.RVSC.2016.02.006
Mathew, B., Suresh, J., and Anbazhagan, S. (2013). Synthesis and PASS-Assisted In Silico Approach of Some Novel 2-substituted Benzimidazole Bearing a Pyrimidine-2, 4, 6(trione) System as Mucomembranous Protector. J. Pharm. Bioallied Sci. 5, 39–43. doi:10.4103/0975-7406.106563
Mella-Raipán, J. A., Lagos, C. F., Recabarren-Gajardo, G., Espinosa-Bustos, C., Romero-Parra, J., Pessoa-Mahana, H., et al. (2013). Design, Synthesis, Binding and Docking-Based 3D-QSAR Studies of 2-Pyridylbenzimidazoles-Aa New Family of High Affinity CB1 Cannabinoid Ligands. Molecules 18, 3972–4001. doi:10.3390/molecules18043972
Menet, C. J. M., Mammoliti, O., Blanc, J., Orsulic, M., and Roscic, M. (2015). Novel Compounds and Pharmaceutical Compositions Thereof for the Treatment of Inflammatory Disorders. US20150203455A1 https://patents.google.com/patent/US20150203455A1/en?oq=US+20150203455+A1.
Menteşe, E., Yılmaz, F., Baltaş, N., Bekircan, O., and Kahveci, B. (2015). Synthesis and Antioxidant Activities of Some New Triheterocyclic Compounds Containing Benzimidazole, Thiophene, and 1,2,4-triazole Rings. J. Enzyme Inhib. Med. Chem. 30, 435–441. doi:10.3109/14756366.2014.943203
Mishra, V. R., Ghanavatkar, C. W., Mali, S. N., Qureshi, S. I., Chaudhari, H. K., and Sekar, N. (2019). Design, Synthesis, Antimicrobial Activity and Computational Studies of Novel Azo Linked Substituted Benzimidazole, Benzoxazole and Benzothiazole Derivatives. Comput. Biol. Chem. 78, 330–337. doi:10.1016/j.compbiolchem.2019.01.003
Mobinikhaledi, A., Asghari, B., and Jabbarpour, M. (2015). Design and Synthesis of New Benzimidazole and Pyrimidine Derivatives as α-glucosidase Inhibitor. Iran J. Pharm. Res. 14, 723–731.
Mochizuki, M., Kojima, T., Kobayashi, K., Kotani, E., Ishichi, Y., Kanzaki, N., et al. (2017). Discovery of 4-Chloro-2-(2,4-Dichloro-6-Methylphenoxy)-1-Methyl-7-(pentan-3-Yl)-1h-Benzimidazole, a Novel CRF1 Receptor Antagonist. Bioorg. Med. Chem. 25, 1556–1570. doi:10.1016/j.bmc.2016.11.011
Mochizuki, M., Kori, M., Kobayashi, K., Yano, T., Sako, Y., Tanaka, M., et al. (2016). Design and Synthesis of Benzimidazoles as Novel Corticotropin-Releasing Factor 1 Receptor Antagonists. J. Med. Chem. 59, 2551–2566. doi:10.1021/acs.jmedchem.5b01715
Moneer, A. A., Mohammed, K. O., and El-Nassan, H. B. (2016). Synthesis of Novel Substituted Thiourea and Benzimidazole Derivatives Containing a Pyrazolone Ring as Anti-inflammatory Agents. Chem. Biol. Drug Des. 87, 784–793. doi:10.1111/cbdd.12712
Monforte, A. M., De Luca, L., Buemi, M. R., Agharbaoui, F. E., Pannecouque, C., and Ferro, S. (2018). Structural Optimization of N1-Aryl-Benzimidazoles for the Discovery of New Non-nucleoside Reverse Transcriptase Inhibitors Active against Wild-type and Mutant HIV-1 Strains. Bioorg. Med. Chem. 26, 661–674. doi:10.1016/J.BMC.2017.12.033
Morais, G. R., Palma, E., Marques, F., Gano, L., Oliveira, M. C., Abrunhosa, A., et al. (2017). Synthesis and Biological Evaluation of Novel 2-Aryl Benzimidazoles as Chemotherapeutic Agents. J. Heterocyclic Chem. 54, 255–267. doi:10.1002/jhet.2575
Morcoss, M. M., Abdelhafez, E. S. M. N., Ibrahem, R. A., Abdel-Rahman, H. M., Abdel-Aziz, M., and Abou El-Ella, D. A. (2020). Design, Synthesis, Mechanistic Studies and In Silico ADME Predictions of Benzimidazole Derivatives as Novel Antifungal Agents. Bioorg. Chem. 101, 103956. doi:10.1016/j.bioorg.2020.103956
Mueller, R., Reddy, V., Nchinda, A. T., Mebrahtu, F., Taylor, D., Lawrence, N., et al. (2020). Lerisetron Analogues with Antimalarial Properties: Synthesis, Structure-Activity Relationship Studies, and Biological Assessment. ACS Omega 5, 6967–6982. doi:10.1021/acsomega.0c00327
Nair, S. M., Beevi, J., Nj, M., Emmanuel, B. D., Dharan, S. S., and Cr, R. (2016). Insilico Design, Synthesis and In Vitro Antidiabetic and Anti-inflammatory Activities of 1,3,4-Thiadiazole Substituted 2-Methyl Benzimidazole Derivatives. J. Pharm. Res. Clin. Pract. 6, 27–36. doi:10.21817/ijpsr/2020/v11i6/201106011
Nandha, B., Nargund, L. G., Nargund, S. L., and Bhat, K. (2017). Design and Synthesis of Some Novel Fluorobenzimidazoles Substituted with Structural Motifs Present in Physiologically Active Natural Products for Antitubercular Activity. Iran J. Pharm. Res. 16, 929–942.
Nashaat, S., Henen, M. A., El-Messery, S. M., and Eisa, H. (2020). Synthesis, State-Of-The-Art NMR-Binding and Molecular Modeling Study of New Benzimidazole Core Derivatives as Pin1 Inhibitors: Targeting Breast Cancer. Bioorg. Med. Chem. 28, 115495. doi:10.1016/j.bmc.2020.115495
Nieto-Meneses, R., Castillo, R., Hernández-Campos, A., Maldonado-Rangel, A., Matius-Ruiz, J. B., Trejo-Soto, P. J., et al. (2018). In Vitro Activity of New N-Benzyl-1h-Benzimidazol-2-Amine Derivatives Against Cutaneous, Mucocutaneous and Visceral Leishmania Species. Exp. Parasitol. 184, 82–89. doi:10.1016/J.EXPPARA.2017.11.009
Nofal, Z. M., Soliman, E. A., Abd El-Karim, S. S., El-Zahar, M. I., Srour, A. M., Sethumadhavan, S., et al. (2014). Synthesis of Some New Benzimidazole-Thiazole Derivatives as Anticancer Agents. J. Hetercyclic Chem. 51, 1797–1806. doi:10.1002/jhet.1886
Noolvi, M., Agrawal, S., Patel, H., Badiger, A., Gaba, M., and Zambre, A. (2014). Synthesis, Antimicrobial and Cytotoxic Activity of Novel Azetidine-2-One Derivatives of 1H-Benzimidazole. Arabian J. Chem. 7, 219–226. doi:10.1016/J.ARABJC.2011.02.011
Noor, A., Qazi, N. G., Nadeem, H., Khan, A. U., Paracha, R. Z., Ali, F., et al. (2017). Synthesis, Characterization, Anti-ulcer Action and Molecular Docking Evaluation of Novel Benzimidazole-Pyrazole Hybrids. Chem. Cent. J. 11, 85. doi:10.1186/s13065-017-0314-0
Ozadali-Sari, K., Tüylü Küçükkılınç, T., Ayazgok, B., Balkan, A., and Unsal-Tan, O. (2017). Novel Multi-Targeted Agents for Alzheimer's Disease: Synthesis, Biological Evaluation, and Molecular Modeling of Novel 2-[4-(4-Substitutedpiperazin-1-Yl)phenyl]benzimidazoles. Bioorg. Chem. 72, 208–214. doi:10.1016/J.BIOORG.2017.04.018
Özil, M., Emirik, M., Beldüz, A., and Ülker, S. (2016). Molecular Docking Studies and Synthesis of Novel Bisbenzimidazole Derivatives as Inhibitors of α-glucosidase. Bioorg. Med. Chem. 24, 5103–5114. doi:10.1016/J.BMC.2016.08.024
Özil, M., Parlak, C., and Baltaş, N. (2018). A Simple and Efficient Synthesis of Benzimidazoles Containing Piperazine or Morpholine Skeleton at C-6 Position as Glucosidase Inhibitors with Antioxidant Activity. Bioorg. Chem. 76, 468–477. doi:10.1016/j.bioorg.2017.12.019
Özkay, Y., Tunalı, Y., Karaca, H., and Işıkdağ, I. (2011). Antimicrobial Activity of a New Series of Benzimidazole Derivatives. Arch. Pharm. Res. 34, 1427–1435. doi:10.1007/s12272-011-0903-8
Padalkar, V. S., Borse, B. N., Gupta, V. D., Phatangare, K. R., Patil, V. S., Umape, P. G., et al. (2016). Synthesis and Antimicrobial Activity of Novel 2-substituted Benzimidazole, Benzoxazole and Benzothiazole Derivatives. Arabian J. Chem. 9, S1125–S1130. doi:10.1016/J.ARABJC.2011.12.006
Pajouhesh, H., Holland, R., Zhang, L., Pajouhesh, H., Lamontagne, J., and Whelan, B. (2015). Benzimidazole Inhibitors of the Sodium Channel. US20150361032A1 https://patents.google.com/patent/US20150361032A1/en?oq=US20150361032A1.
Papanicolaou, G. A., Silveira, F. P., Langston, A. A., Pereira, M. R., Avery, R. K., Uknis, M., et al. (2019). Maribavir for Refractory or Resistant Cytomegalovirus Infections in Hematopoietic-Cell or Solid-Organ Transplant Recipients: A Randomized, Dose-Ranging, Double-Blind, Phase 2 Study. Clin. Infect. Dis. 68, 1255–1264. doi:10.1093/cid/ciy706
Pardeshi, V. A. S., Chundawat, N. S., Pathan, S. I., Sukhwal, P., Chundawat, T. P. S., and Singh, G. P. (2021). A Review on Synthetic Approaches of Benzimidazoles. Synth. Commun. 51, 485–513. doi:10.1080/00397911.2020.1841239
Partap, S., Yar, M. S., Hassan, M. Z., Akhtar, M. J., and Siddiqui, A. A. (2017). Design, Synthesis, and Pharmacological Screening of Pyridazinone Hybrids as Anticonvulsant Agents. Arch. Pharm. (Weinheim) 350, 1700135. doi:10.1002/ardp.201700135
Pathan, S. I., Chundawat, N. S., Chauhan, N. P. S., and Singh, G. P. (2020). A Review on Synthetic Approaches of Heterocycles via Insertion-Cyclization Reaction. Synth. Commun. 50, 1251–1285. doi:10.1080/00397911.2020.1712609
Patil, A., Ganguly, S., Hundiwale, J., and Tayade, S. (2012). Synthesis and Study of Some Novel Benzimidazole Analogs as Potential Antiulcer Agents. Int. J. Pharm. Chem. 2, 89–92. doi:10.7439/ijpc.v2i3.679
Patil, A., Ganguly, S., and Surana, S. (2010). Synthesis and Antiulcer Activity of 2-[5-Substituted-1-H-Benzo(d) Imidazol-2-Yl Sulfinyl]methyl-3-Substituted quinazoline-4-(3H) Ones. J. Chem. Sci. 122, 443–450. doi:10.1007/s12039-010-0052-5
Pevzner, V., and Moses-Heller, S. (2020). Stable Orally Disintegrating Pharmaceutical Compositionsns. US10835488B2 https://patents.google.com/patent/US10835488B2/en?oq=US10835488B2.
Poddar, S. K., Saqueeb, N., and Rahman, S. A. (2016). Synthesis and Biological Evaluation of 2-methyl-1Hbenzimidazole and 1H-Benzimidazol-2-Yl-Methanol. Dhaka Univ. J. Pharm. Sci. 15, 83–87. doi:10.3329/dujps.v15i1.29201
Popov, A. B., Krstulović, L., Koštrun, S., Jelić, D., Bokulić, A., Stojković, M. R., et al. (2020). Design, Synthesis, Antitrypanosomal Activity, DNA/RNA Binding and In Vitro ADME Profiling of Novel Imidazoline-Substituted 2-arylbenzimidazoles. Eur. J. Med. Chem. 207, 112802. doi:10.1016/j.ejmech.2020.112802
Prajapat, P., and Talesara, G. L. (2016). Synthesis and Anti-inflammatory Screening of Some Mono and Bis-Alkoxyphthalimide Linked Benzimidazole and Their Quinazoline and Pyrimidine Derivatives. J. Heterocyclic Chem. 53, 1603–1610. doi:10.1002/jhet.2471
Prasad, P. M. K., and Sundararajan, R. (2017). Design, Synthesis, Antitubercular and Antimicrobial Activities of Novel Thiazole Substituted Benzimidazole Derivatives. Der Pharm. Lett. 9, 270–284.
Rajak, H. (2015). Synthesis and Evaluation of Some Novel Semicarbazones Based Benzimidazole Derivatives as Anticonvulsant Agent. Ijcea 6, 142–145. doi:10.7763/IJCEA.2015.V6.469
Rajesh, R., Manikandan, A., Sivakumar, A., Ramasubbu, C., and Nagaraju, N. (2017). Substituted Methoxybenzyl-Sulfonyl-1h-Benzo[d]imidazoles Evaluated as Effective H+/K+-ATPase Inhibitors and Anti-ulcer Therapeutics. Eur. J. Med. Chem. 139, 454–460. doi:10.1016/J.EJMECH.2017.08.001
Raka, S. C., Rahman, A., Hussain, F., and Rahman, S. M. A. (2021). Synthesis, Characterization and In Vitro, In Vivo, In Silico Biological Evaluations of Substituted Benzimidazole Derivatives. Saudi J. Biol. Sci. 9, 2. doi:10.1016/j.sjbs.2021.08.082
Ramos Rodríguez, O. A., Magaña Vergara, N. E., Mojica Sánchez, J. P., Sumaya Martínez, M. T., Gómez Sandoval, Z., Cruz, A., et al. (2020). Synthesis, crystal Structure, Antioxidant Activity and Dft Study of 2-Aryl-2,3-Dihydro-4h-[1,3]thiazino[3,2-A]benzimidazol-4-One. J. Mol. Struct. 1199, 127036. doi:10.1016/j.molstruc.2019.127036
Rathore, A., Sudhakar, R., Ahsan, M. J., Ali, A., Subbarao, N., Jadav, S. S., et al. (2017). In Vivo Anti-inflammatory Activity and Docking Study of Newly Synthesized Benzimidazole Derivatives Bearing Oxadiazole and Morpholine Rings. Bioorg. Chem. 70, 107–117. doi:10.1016/J.BIOORG.2016.11.014
Ren, W., Ren, Y., Dong, M., and Gao, Y. (2016). Design, Synthesis, and Thrombin Inhibitory Activity Evaluation of Some Novel Benzimidazole Derivatives. Helv. Chim. Acta 99, 325–332. doi:10.1002/hlca.201500527
Romero-Parra, J., Mella-Raipán, J., Palmieri, V., Allarà, M., Torres, M. J., Pessoa-Mahana, H., et al. (2016). Synthesis, Binding Assays, Cytotoxic Activity and Docking Studies of Benzimidazole and Benzothiophene Derivatives with Selective Affinity for the CB2 Cannabinoid Receptor. Eur. J. Med. Chem. 124, 17–35. doi:10.1016/J.EJMECH.2016.08.005
Saha, P., Brishty, S. R., and Rahman, S. A. (2021). Pharmacological Screening of Substituted Benzimidazole Derivatives. Dhaka Univ. J. Pharm. Sci. 20, 95–102. doi:10.3329/dujps.v20i1.54037
Saha, P., Brishty, S. R., and Rahman, S. M. A. (2020). Synthesis and Evaluation of Disubstituted Benzimidazole Derivatives as Potential Analgesic and Antidiarrheal Agents. pharmaceutical-sciences 82, 222–229. doi:10.36468/pharmaceutical-sciences.642
Sahoo, B. M., Banik, B. K., Mazaharunnisa, N. S., and Rao, B. (2019). Microwave Assisted Green Synthesis of Benzimidazole Derivatives and Evaluation of Their Anticonvulsant Activity. Cmic 6, 23–29. doi:10.2174/2213335606666190429124745
Saify, Z. S., Kamil, A., Akhtar, S., Taha, M., Khan, A., Khan, K. M., et al. (2014). 2-(2′-Pyridyl) Benzimidazole Derivatives and Their Urease Inhibitory Activity. Med. Chem. Res. 23, 4447–4454. doi:10.1007/s00044-014-1015-z
Sarıkaya, G., Çoban, G., Parlar, S., Tarikogullari, A. H., Armagan, G., Erdoğan, M. A., et al. (2018). Multifunctional Cholinesterase Inhibitors for Alzheimer's Disease: Synthesis, Biological Evaluations, and Docking Studies of O/p -propoxyphenylsubstituted-1H -benzimidazole Derivatives. Arch. Pharm. Chem. Life Sci. 351, 1800076. doi:10.1002/ardp.201800076
Schade, T., Skubsch, K., Brinkmann, S., Bleckmann, A., and Schlenker, D. (2015). Octocrylene-free Sunscreen Composition with Low Stickiness. US20150209259A1 https://patents.google.com/patent/US20150209259A1/en?oq=US+20150209259+A1.
Sethi, P., Bansal, Y., and Bansal, G. (2018). Synthesis and PASS-Assisted Evaluation of Coumarin-Benzimidazole Derivatives as Potential Anti-inflammatory and Anthelmintic Agents. Med. Chem. Res. 27, 61–71. doi:10.1007/s00044-017-2036-1
Sethi, R., Arora, S., Saini, D., Singh, T. G., and Jain, S. (2017). Design and Synthesis of N-(benzimidazol-1-yl Methyl)-Benzamide Derivatives Anti-inflammatory and Analgesic Agents. ACTA Pol. Pharm. 74, 1413–1425.
Shaharyar, M., Mazumder, A., Salahuddin, R., and Garg, R. D. (2016). Synthesis, Characterization and Pharmacological Screening of Novel Benzimidazole Derivatives. Arabian J. Chem. 9, S342–S347. doi:10.1016/J.ARABJC.2011.04.013
Shaikh, I. N., Hosamani, K. M., and Kurjogi, M. M. (2018). Design, Synthesis, and Evaluation of New α-aminonitrile-based Benzimidazole Biomolecules as Potent Antimicrobial and Antitubercular Agents. Arch. Pharm. (Weinheim) 351, 1700205. doi:10.1002/ardp.201700205
Shaikh, I. N., Hosamani, K. M., Seetharamareddy, H. R., and Hugar, M. H. (2012). Synthesis and In-Vivo Evaluation of Carbonyl-Amide Linkage Based New Benzimidazole Derivatives. Arch. Pharm. (Weinheim) 345, 65–72. doi:10.1002/ardp.201100068
Shaker, Y. M., Omar, M. A., Mahmoud, K., Elhallouty, S. M., El-Senousy, W. M., Ali, M. M., et al. (2015). Synthesis, In Vitro and In Vivo Antitumor and Antiviral Activity of Novel 1-substituted Benzimidazole Derivatives. J. Enzyme Inhib. Med. Chem. 30, 826–845. doi:10.3109/14756366.2014.979344
Shankar, B., Jalapathi, P., Valeru, A., Kishor Kumar, A., Saikrishna, B., and Kudle, K. r. (2017). Synthesis and Biological Evaluation of New 2-(6-Alkyl-Pyrazin-2-Yl)-1h-Benz[d]imidazoles as Potent Anti-inflammatory and Antioxidant Agents. Med. Chem. Res. 26, 1835–1846. doi:10.1007/s00044-017-1897-7
Sharma, K., Shrivastava, A., Mehra, R. N., Deora, G. S., Alam, M. M., Zaman, M. S., et al. (2018). Synthesis of Novel Benzimidazole Acrylonitriles for Inhibition of Plasmodium Falciparum Growth by Dual Target Inhibition. Arch. Pharm. (Weinheim) 351, 1700251. doi:10.1002/ardp.201700251
Sharma, M. C., Kohli, D. V., and Sharma, S. (2010). Benzimidazoles Derivatives with (2-{6-Chloro-5-nitro-1-[2-(1H-tetrazol-5-yl) Biphenyl-4-Ylmethyl] 1H-Benzoimidazol-2-Yl}-Phenyl)-(substituted-Benzylidene)-Amine with Potential Angiotensin II Receptor Antagonists as Antihypertensive Activity. Int. J Drug Delivery 2, 228–237. doi:10.5138/ijdd.2010.0975.0215.02033
Sharma, P., Reddy, T. S., Kumar, N. P., Senwar, K. R., Bhargava, S. K., and Shankaraiah, N. (2017a). Conventional and Microwave-Assisted Synthesis of New 1H-Benzimidazole-Thiazolidinedione Derivatives: A Potential Anticancer Scaffold. Eur. J. Med. Chem. 138, 234–245. doi:10.1016/J.EJMECH.2017.06.035
Sharma, R., Bali, A., and Chaudhari, B. B. (2017b). Synthesis of Methanesulphonamido-Benzimidazole Derivatives as Gastro-Sparing Antiinflammatory Agents with Antioxidant Effect. Bioorg. Med. Chem. Lett. 27, 3007–3013. doi:10.1016/J.BMCL.2017.05.017
Sharma, S., Kumar, D., Singh, G., Monga, V., and Kumar, B. (2020). Recent Advancements in the Development of Heterocyclic Anti-inflammatory Agents. Eur. J. Med. Chem. 200, 112438. doi:10.1016/j.ejmech.2020.112438
Shingalapur, R. V., Hosamani, K. M., Keri, R. S., and Hugar, M. H. (2010). Derivatives of Benzimidazole Pharmacophore: Synthesis, Anticonvulsant, Antidiabetic and DNA Cleavage Studies. Eur. J. Med. Chem. 45, 1753–1759. doi:10.1016/J.EJMECH.2010.01.007
Shintre, S. A., Ramjugernath, D., Singh, P., Mocktar, C., and Koorbanally, N. A. (2017). Microwave Synthesis, Biological Evaluation and Docking Studies of 2-substituted Methyl 1-(4-Fluorophenyl)-1h-Benzimidazole-5-Carboxylates. Med. Chem. Res. 26, 484–498. doi:10.1007/s00044-016-1763-z
Siddiqui, N., and Alam, M. S. (2010). Anticonvulsant and Toxicity Evaluation of Newer 1-{(1-(2-substituted Benzyl)-1h-Benzo [d] Imidazol-2-Yl) Methyl}-3-Arylthioureas. Der Pharma Chem. 2, 163–171.
Siddiqui, N., Alam, M. S., Sahu, M., Yar, M. S., Alam, O., and Siddiqui, M. J. A. (2016). Antidepressant, Analgesic Activity and SAR Studies of Substituted Benzimidazoles. Asian Jour. Pharmac. Rese. 6, 170–174. doi:10.5958/2231-5691.2016.00024.1
Singh, A., Yadav, D., Yadav, M., Dhamanage, A., Kulkarni, S., and Singh, R. K. (2015). Molecular Modeling, Synthesis and Biological Evaluation of N-Heteroaryl Compounds as Reverse Transcriptase Inhibitors against HIV-1. Chem. Biol. Drug Des. 85, 336–347. doi:10.1111/cbdd.12397
Singh, G., Singh, A., Verma, R. K., Mall, R., and Azeem, U. (2018). Synthesis, Biological Evaluation and Molecular Docking Studies of Novel Benzimidazole Derivatives. Comput. Biol. Chem. 72, 45–52. doi:10.1016/J.COMPBIOLCHEM.2017.12.010
Singh, K., Okombo, J., Brunschwig, C., Ndubi, F., Barnard, L., Wilkinson, C., et al. (2017a). Antimalarial Pyrido[1,2-A]benzimidazoles: Lead Optimization, Parasite Life Cycle Stage Profile, Mechanistic Evaluation, Killing Kinetics, and In Vivo Oral Efficacy in a Mouse Model. J. Med. Chem. 60, 1432–1448. doi:10.1021/acs.jmedchem.6b01641
Singh, L. R., Avula, S. R., Raj, S., Srivastava, A., Palnati, G. R., Tripathi, C. K. M., et al. (2017b). Coumarin-benzimidazole Hybrids as a Potent Antimicrobial Agent: Synthesis and Biological Elevation. J. Antibiot. (Tokyo) 70, 954–961. doi:10.1038/ja.2017.70
Singh, N., Pandurangan, A., Rana, K., Anand, P., Ahamad, A., and Tiwari, A. K. (2012). Benzimidazole: A Short Review of Their Antimicrobial Activities. Int. Curr. Pharm. J. 1, 110–118. doi:10.3329/icpj.v1i5.10284
Singhal, S., Khanna, P., and Khanna, L. (2019). Synthesis, DFT Studies, Molecular Docking, Antimicrobial Screening and UV Fluorescence Studies on Ct-DNA for Novel Schiff Bases of 2-(1-aminobenzyl) Benzimidazole. Heliyon 5, e02596. doi:10.1016/j.heliyon.2019.e02596
Singla, P., Luxami, V., and Paul, K. (2015). Triazine-benzimidazole Hybrids: Anticancer Activity, DNA Interaction and Dihydrofolate Reductase Inhibitors. Bioorg. Med. Chem. 23, 1691–1700. doi:10.1016/J.BMC.2015.03.012
Sireesha, R., Sreenivasulu, R., Chandrasekhar, C., Jadav, S. S., Pavani, Y., Rao, M. V. B., et al. (2021). Design, Synthesis, Anti-cancer Evaluation and Binding Mode Studies of Benzimidazole/benzoxazole Linked β-carboline Derivatives. J. Mol. Struct. 1226, 129351. doi:10.1016/j.molstruc.2020.129351
Sirim, M. M., Krishna, V. S., Sriram, D., and Unsal Tan, O. (2020). Novel Benzimidazole-Acrylonitrile Hybrids and Their Derivatives: Design, Synthesis and Antimycobacterial Activity. Eur. J. Med. Chem. 188, 112010. doi:10.1016/j.ejmech.2019.112010
Song, J., Gao, Q. L., Wu, B. W., Li, D., Shi, L., Zhu, T., et al. (2019). Novel Tertiary Sulfonamide Derivatives Containing Benzimidazole Moiety as Potent Anti-gastric Cancer Agents: Design, Synthesis and SAR Studies. Eur. J. Med. Chem. 183, 111731. doi:10.1016/j.ejmech.2019.111731
Sontakke, V. A., Kate, A. N., Ghosh, S., More, P., Gonnade, R., Kumbhar, N. M., et al. (2015). Synthesis, DNA Interaction and Anticancer Activity of 2-anthryl Substituted Benzimidazole Derivatives. New J. Chem. 39, 4882–4890. doi:10.1039/C4NJ02415J
Spasov, A. A., Kucheryavenko, A. F., Gaidukova, K. A., Kosolapov, V. A., and Zhukovskaya, O. N. (2020). Antiplatelet Activity of New Derivatives of Benzimidazole Containing Sterically Hindered Phenolic Group in Their Structure. Rrp 6, 1–9. doi:10.3897/RRPHARMACOLOGY.6.50373
Sridhar Goud, N., Pooladanda, V., Muni Chandra, K., Lakshmi Soukya, P. S., Alvala, R., Kumar, P., et al. (2020). Novel Benzimidazole-Triazole Hybrids as Apoptosis Inducing Agents in Lung Cancer: Design, Synthesis, 18F-Radiolabeling & Galectin-1 Inhibition Studies. Bioorg. Chem. 102, 104125. doi:10.1016/j.bioorg.2020.104125
Srinivas Reddy, M., Nath Anisetti, R., Durga Prasad, K., Sannigrahi, S., and Arvinda Reddy, P. (2011). Synthesis, Characterization and Biological Evaluation of Some Novel 2-substituted Mercaptobenzimidazole Derivatives. Pharm. Chem. J. 44, 642–645. doi:10.1007/s11094-011-0537-7
Srivastava, R., Gupta, S. K., Naaz, F., Sen Gupta, P. S., Yadav, M., Singh, V. K., et al. (2020). Alkylated Benzimidazoles: Design, Synthesis, Docking, DFT Analysis, ADMET Property, Molecular Dynamics and Activity Against HIV and YFV. Comput. Biol. Chem. 89, 107400. doi:10.1016/j.compbiolchem.2020.107400
Srour, A. M., Ahmed, N. S., Abd El-Karim, S. S., Anwar, M. M., and El-Hallouty, S. M. (2020). Design, Synthesis, Biological Evaluation, QSAR Analysis and Molecular Modelling of New Thiazol-Benzimidazoles as EGFR Inhibitors. Bioorg. Med. Chem. 28, 115657. doi:10.1016/j.bmc.2020.115657
Su, W.-Y., Pan, R.-K., Song, J.-L., Li, G.-B., and Liu, S.-G. (2019). Synthesis, Crystal Structures and Cytotoxic Activity of Two Zinc(II) Complexes Derived from Benzimidazole Derivatives. Polyhedron 161, 268–275. doi:10.1016/j.poly.2019.01.012
Suk, F. M., Liu, C. L., Hsu, M. H., Chuang, Y. T., Wang, J. P., and Liao, Y. J. (2019). Treatment with a New Benzimidazole Derivative Bearing a Pyrrolidine Side Chain Overcomes Sorafenib Resistance in Hepatocellular Carcinoma. Sci. Rep. 9, 17259. doi:10.1038/s41598-019-53863-2
Taha, M., Mosaddik, A., Rahim, F., Ali, S., Ibrahim, M., and Almandil, N. B. (2020). Synthesis, Antiglycation and Antioxidant Potentials of Benzimidazole Derivatives. J. King Saud Univ. - Sci. 32, 191–194. doi:10.1016/j.jksus.2018.04.003
Tahlan, S., Kumar, S., and Narasimhan, B. (2019). Pharmacological Significance of Heterocyclic 1H-Benzimidazole Scaffolds: A Review. BMC Chem. 13, 101. doi:10.1186/s13065-019-0625-4
Tahri, A., Jonckers, T. H. M., Raboisson, P. J.-M. B., Vendeville, S. M. H., and Hu, L. (2015). Novel 4-substituted 1,3-Dihydro-2h-Benzimidazol-2-One Derivatives Substituted with Benzimidazoles as Respiratory Syncytial Virus Antiviral Agents. US20150175608A1 https://patents.google.com/patent/US20150175608A1/en?oq=US+20150175608+A1.
Tamura, Y., Omori, N., Kouyama, N., Nishiura, Y., Hayashi, K., Watanabe, K., et al. (2012a). Design, Synthesis and Identification of Novel Benzimidazole Derivatives as Highly Potent NPY Y5 Receptor Antagonists with Attractive In Vitro ADME Profiles. Bioorg. Med. Chem. Lett. 22, 5498–5502. doi:10.1016/J.BMCL.2012.07.020
Tamura, Y., Omori, N., Kouyama, N., Nishiura, Y., Hayashi, K., Watanabe, K., et al. (2012b). Identification of a Novel and Orally Available Benzimidazole Derivative as an NPY Y5 Receptor Antagonist with In Vivo Efficacy. Bioorg. Med. Chem. Lett. 22, 6554–6558. doi:10.1016/J.BMCL.2012.09.025
Tanaka, J., Iida, H., Abe, M., Yuda, Y., Inoue, S., and Okabe, S. (2011). Gastric Antisecretory and Anti-ulcer Effect of ME3407, A New Benzimidazole Derivative, in Rats. Arzneimittelforschung 54, 221–229. doi:10.1055/s-0031-1296963
Tomovic, K., Ilic, B. S., Smelcerovic, Z., Miljkovic, M., Yancheva, D., Kojic, M., et al. (2020). Benzimidazole-based Dual Dipeptidyl Peptidase-4 and Xanthine Oxidase Inhibitors. Chem. Biol. Interact. 315, 108873. doi:10.1016/j.cbi.2019.108873
Tonelli, M., Gabriele, E., Piazza, F., Basilico, N., Parapini, S., Tasso, B., et al. (2018). Benzimidazole Derivatives Endowed with Potent Antileishmanial Activity. J. Enzyme Inhib. Med. Chem. 33, 210–226. doi:10.1080/14756366.2017.1410480
Torres-Gómez, H., Hernández-Núñez, E., León-Rivera, I., Guerrero-Alvarez, J., Cedillo-Rivera, R., Moo-Puc, R., et al. (2008). Design, Synthesis and In Vitro Antiprotozoal Activity of Benzimidazole-Pentamidine Hybrids. Bioorg. Med. Chem. Lett. 18, 3147–3151. doi:10.1016/j.bmcl.2008.05.009
Tsay, S. C., Hwu, J. R., Singha, R., Huang, W. C., Chang, Y. H., Hsu, M. H., et al. (2013). Coumarins Hinged Directly on Benzimidazoles and Their Ribofuranosides to Inhibit Hepatitis C Virus. Eur. J. Med. Chem. 63, 290–298. doi:10.1016/J.EJMECH.2013.02.008
Ushiroda, K., Maruta, K., Takazawa, T., Nagano, T., Taiji, M., Kohno, T., et al. (2011). Synthesis and Pharmacological Evaluation of Novel Benzoylazole-Based PPAR α/γ Activators. Bioorg. Med. Chem. Lett. 21, 1978–1982. doi:10.1016/j.bmcl.2011.02.032
Vangavaragu, J. R., Valasani, K. R., Gan, X., and Yan, S. S. (2014). Identification of Human Presequence Protease (hPreP) Agonists for the Treatment of Alzheimer's Disease. Eur. J. Med. Chem. 76, 506–516. doi:10.1016/J.EJMECH.2014.02.046
Varshney, H., Ahmad, A., Rauf, A., Sherwani, A., and Owais, M. (2015). Multistep Synthesis of 1-[{(5-Alkenyl/hydroxyalkenylsubstituted)-1,3,4-Oxadiazol-2-Yl}-Methyl]-2-Methyl-1h-Benzimidazole Series and In Vitro Anticancer Screening, SAR Studies. Med. Chem. Res. 24, 944–953. doi:10.1007/s00044-014-1162-2
Vasantha, K., Basavarajaswamy, G., Vaishali Rai, M., Boja, P., Pai, V. R., Shruthi, N., et al. (2015). Rapid 'one-Pot' Synthesis of a Novel Benzimidazole-5-Carboxylate and its Hydrazone Derivatives as Potential Anti-inflammatory and Antimicrobial Agents. Bioorg. Med. Chem. Lett. 25, 1420–1426. doi:10.1016/J.BMCL.2015.02.043
Vasil’ev, P. M., Kalitin, K. Y., Spasov, A. A., Grechko, O. Y., Poroikov, V. V., Filimonov, D. A., et al. (2017). Prediction and Study of Anticonvulsant Properties of Benzimidazole Derivatives. Pharm. Chem. J. 50, 775–780. doi:10.1007/S11094-017-1530-6
Veerasamy, R., Roy, A., Karunakaran, R., and Rajak, H. (2021). Structure-Activity Relationship Analysis of Benzimidazoles as Emerging Anti-inflammatory Agents: An Overview. Pharmaceuticals 14 (7), 663. doi:10.3390/ph14070663
Vishwanathan, B., and Gurupadayya, B. (2015). Anticoagulant Evaluation of 1,3,4-oxadiazole Derivatives Derived from Benzimidazole. World J. Pharm. Sci. 3, 154–157.
Vitale, G., Corona, P., Loriga, M., Carta, A., Paglietti, G., Giliberti, G., et al. (2012). 5-Acetyl-2-arylbenzimidazoles as Antiviral Agents. Part 4. Eur. J. Med. Chem. 53, 83–97. doi:10.1016/J.EJMECH.2012.03.038
Wang, M., Han, X., and Zhou, Z. (2015). New Substituted Benzimidazole Derivatives: A Patent Review (2013 - 2014). Expert Opin. Ther. Pat. 25, 595–612. doi:10.1517/13543776.2015.1015987
Wang, Y. J., Yang, L., and Zuo, J. P. (2016). Recent Developments in Antivirals against Hepatitis B Virus. Virus. Res. 213, 205–213. doi:10.1016/J.VIRUSRES.2015.12.014
Wang, Y. N., Bheemanaboina, R. R. Y., Cai, G. X., and Zhou, C. H. (2018). Novel Purine Benzimidazoles as Antimicrobial Agents by Regulating ROS Generation and Targeting Clinically Resistant Staphylococcus aureus DNA Groove. Bioorg. Med. Chem. Lett. 28, 1621–1628. doi:10.1016/J.BMCL.2018.03.046
Wang, Z., Deng, X., Xiong, S., Xiong, R., Liu, J., Zou, L., et al. (2017). Design, Synthesis and Biological Evaluation of Chrysin Benzimidazole Derivatives as Potential Anticancer Agents. Nat. Product. Res. 32, 2900–2909. doi:10.1080/14786419.2017.1389940
Warekar, P. P., Patil, P. T., Patil, K. T., Jamale, D. K., Kolekar, G. B., and Anbhule, P. V. (2016). Ecofriendly Synthesis and Biological Evaluation of 4-(4-nitro-phenyl)-2-phenyl-1,4-dihydro-benzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylic Acid Ethyl Ester Derivatives as an Antitubercular Agents. Synth. Commun. 46, 2022–2030. doi:10.1080/00397911.2016.1244273
Woolley, D. W. (1944). Some Biological Effects Produced by Benzimidazole and Their Reversal by Purines. J. Biol. Chem. 152, 225–232. doi:10.1016/s0021-9258(18)72045-0
Wu, Z., Xia, M. B., Bertsetseg, D., Wang, Y. H., Bao, X. L., Zhu, W. B., et al. (2020). Design, Synthesis and Biological Evaluation of Novel Fluoro-Substituted Benzimidazole Derivatives with Anti-hypertension Activities. Bioorg. Chem. 101, 104042. doi:10.1016/j.bioorg.2020.104042
Wubulikasimu, R., Yang, Y., Xue, F., Luo, X., Shao, D., Li, Y., et al. (2013). Synthesis and Biological Evaluation of Novel Benzimidazole Derivatives Bearing a Heterocyclic Ring at 4/5 Position. Bull. Korean Chem. Soc. 34, 2297–2304. doi:10.5012/bkcs.2013.34.8.2297
Xu, Y. B., Yang, L., Wang, G. F., Tong, X. K., Wang, Y. J., Yu, Y., et al. (2014). Benzimidazole Derivative, BM601, a Novel Inhibitor of Hepatitis B Virus and HBsAg Secretion. Antivir. Res 107, 6–15. doi:10.1016/J.ANTIVIRAL.2014.04.002
Xue, F., Luo, X., Ye, C., Ye, W., and Wang, Y. (2011). Inhibitory Properties of 2-Substituent-1h-Benzimidazole-4-Carboxamide Derivatives Against Enteroviruses. Bioorg. Med. Chem. 19, 2641–2649. doi:10.1016/J.BMC.2011.03.007
Yadav, S., Narasimhan, B., Lim, S. M., Ramasamy, K., Vasudevan, M., Shah, S. A. A., et al. (2018). Synthesis and Evaluation of Antimicrobial, Antitubercular and Anticancer Activities of Benzimidazole Derivatives. Egypt. J. Basic Appl. Sci. 5, 100–109. doi:10.1016/J.EJBAS.2017.11.001
Yamani, A., Zdżalik-Bielecka, D., Lipner, J., Stańczak, A., Piórkowska, N., Stańczak, P. S., et al. (2021). Discovery and Optimization of Novel Pyrazole-Benzimidazole CPL304110, as a Potent and Selective Inhibitor of Fibroblast Growth Factor Receptors FGFR (1-3). Eur. J. Med. Chem. 210, 112990. doi:10.1016/j.ejmech.2020.112990
Yan, Y., Liu, Z., Zhang, J., Xu, R., Hu, X., and Liu, G. (2011). A Reverse Method for Diversity Introduction of Benzimidazole to Synthesize H(+)/K(+)-ATP Enzyme Inhibitors. Bioorg. Med. Chem. Lett. 21, 4189–4192. doi:10.1016/J.BMCL.2011.05.080
Yang, C., Balsells, J., Chu, H. D., Cox, J. M., Crespo, A., Ma, X., et al. (2015). Discovery of Benzimidazole Oxazolidinediones as Novel and Selective Nonsteroidal Mineralocorticoid Receptor Antagonists. ACS Med. Chem. Lett. 6, 461–465. doi:10.1021/acsmedchemlett.5b00010
Yang, H., Ren, Y., Gao, X., and Gao, Y. (2016). Synthesis and Anticoagulant Bioactivity Evaluation of 1,2,5-trisubstituted Benzimidazole Fluorinated Derivatives. Chem. Res. Chin. Univ. 32, 973–978. doi:10.1007/s40242-016-6205-4
Yang, Y., Zhang, S., Zhou, Q., Zhang, C., Gao, Y., Wang, H., et al. (2020). Discovery of Highly Selective and Orally Available Benzimidazole-Based Phosphodiesterase 10 Inhibitors with Improved Solubility and Pharmacokinetic Properties for Treatment of Pulmonary Arterial Hypertension. Acta Pharm. Sin. B 10, 2339–2347. doi:10.1016/j.apsb.2020.04.003
Yeong, K. Y., Nor Azizi, M. I. H., Berdigaliyev, N., Chen, W. N., Lee, W. L., Shirazi, A. N., et al. (2019). Sirtuin Inhibition and Anti-cancer Activities of Ethyl 2-Benzimidazole-5-Carboxylate Derivatives. Medchemcomm 10, 2140–2145. doi:10.1039/c9md00323a
Yeong, K. Y., Ang, C. W., Ali, M. A., Osman, H., and Tan, S. C. (2017). Antituberculosis Agents Bearing the 1,2-disubstituted Benzimidazole Scaffold. Med. Chem. Res. 26, 770–778. doi:10.1007/s00044-017-1784-2
Youssif, B. G., Mohamed, Y. A., Salim, M. T., Inagaki, F., Mukai, C., and Abdu-Allah, H. H. (2016). Synthesis of Some Benzimidazole Derivatives Endowed with 1,2,3-triazole as Potential Inhibitors of Hepatitis C Virus. Acta Pharm. 66, 219–231. doi:10.1515/acph-2016-0014
Yuan, X., Yang, Q., Liu, T., Li, K., Liu, Y., Zhu, C., et al. (2019). Design, Synthesis and In Vitro Evaluation of 6-Amide-2-Aryl Benzoxazole/benzimidazole Derivatives Against Tumor Cells by Inhibiting VEGFR-2 Kinase. Eur. J. Med. Chem. 179, 147–165. doi:10.1016/j.ejmech.2019.06.054
Yılmaz, Ü., Tekin, S., Buğday, N., Yavuz, K., Küçükbay, H., and Sandal, S. (2019). Synthesis and Evaluation of Anticancer Properties of Novel Benzimidazole Ligand and Their Cobalt(II) and Zinc(II) Complexes Against Cancer Cell Lines A-2780 and DU-145. Inorg. Chim. Acta 495, 118977. doi:10.1016/j.ica.2019.118977
Zawawi, N. K., Taha, M., Ahmat, N., Ismail, N. H., Wadood, A., and Rahim, F. (2017). Synthesis, Molecular Docking Studies of Hybrid Benzimidazole as α-glucosidase Inhibitor. Bioorg. Chem. 70, 184–191. doi:10.1016/J.BIOORG.2016.12.009
Zhang, T., Liu, Q., and Ren, Y. (2020). Design, Synthesis and Biological Activity Evaluation of Novel Methyl Substituted Benzimidazole Derivatives. Tetrahedron 76, 131027. doi:10.1016/j.tet.2020.131027
Zhang, Y., Xu, J., Li, Y., Yao, H., and Wu, X. (2015). Design, Synthesis and Pharmacological Evaluation of Novel NO-Releasing Benzimidazole Hybrids as Potential Antihypertensive Candidate. Chem. Biol. Drug Des. 85, 541–548. doi:10.1111/cbdd.12442
Zhu, J., Wu, C. F., Li, X., Wu, G. S., Xie, S., Hu, Q. N., et al. (2013). Synthesis, Biological Evaluation and Molecular Modeling of Substituted 2-aminobenzimidazoles as Novel Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Bioorg. Med. Chem. 21, 4218–4224. doi:10.1016/J.BMC.2013.05.001
Keywords: nitrogenous heterocyclic compounds, diverse pharmacological activities, structure-activity relationship (SAR), anti-infectious, anti-proliferative, cardiovascular agents
Citation: Brishty SR, Hossain MJ, Khandaker MU, Faruque MRI, Osman H and Rahman SMA (2021) A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 12:762807. doi: 10.3389/fphar.2021.762807
Received: 22 August 2021; Accepted: 01 October 2021;
Published: 03 November 2021.
Edited by:
Andres Trostchansky, Universidad de la República, UruguayReviewed by:
Alexander Spasov, Volgograd State Medical University, RussiaNatalia Rios, Universidad de la República, Uruguay
Copyright © 2021 Brishty, Hossain, Khandaker, Faruque, Osman and Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. M. Abdur Rahman, c21hcmFobWFuQGR1LmFjLmJk
†ORCID: Shejuti Rahman Brishty, orcid.org/0000-0001-7794-6775; Md. Jamal Hossain, orcid.org/0000-0001-9706-207X; S. M. Abdur Rahman, orcid.org/0000-0002-9963-8885
 Shejuti Rahman Brishty
Shejuti Rahman Brishty Md. Jamal Hossain
Md. Jamal Hossain Mayeen Uddin Khandaker3
Mayeen Uddin Khandaker3 Mohammad Rashed Iqbal Faruque
Mohammad Rashed Iqbal Faruque Hamid Osman
Hamid Osman S. M. Abdur Rahman
S. M. Abdur Rahman