Abstract
Central nervous system (CNS) disorders and diseases are expected to rise sharply in the coming years, partly because of the world’s aging population. Medicines for the treatment of the CNS have not been successfully made. Inadequate knowledge about the brain, pharmacokinetic and dynamic errors in preclinical studies, challenges with clinical trial design, complexity and variety of human brain illnesses, and variations in species are some potential scenarios. Neurodegenerative diseases (NDDs) are multifaceted and lack identifiable etiological components, and the drugs developed to treat them did not meet the requirements of those who anticipated treatments. Therefore, there is a great demand for safe and effective natural therapeutic adjuvants. For the treatment of NDDs and other memory-related problems, many herbal and natural items have been used in the Ayurvedic medical system. Anxiety, depression, Parkinson’s, and Alzheimer’s diseases (AD), as well as a plethora of other neuropsychiatric disorders, may benefit from the use of plant and food-derived chemicals that have antidepressant or antiepileptic properties. We have summarized the present level of knowledge about natural products based on topological evidence, bioinformatics analysis, and translational research in this review. We have also highlighted some clinical research or investigation that will help us select natural products for the treatment of neurological conditions. In the present review, we have explored the potential efficacy of phytoconstituents against neurological diseases. Various evidence-based studies and extensive recent investigations have been included, which will help pharmacologists reduce the progression of neuronal disease.
1 Introduction
Information is sent across the body via a specialized network of neurons. Neurons use chemical and electrical signals to support the coordination of all fundamental aspects of life. When a neuron releases an electrical or chemical signal, it travels down its axon (a specialized projection) to the neighboring cell. These signals can be retained by root-like dendrites. There are around 86 billion neurons in the human brain. Hence, a growing fetus generates approximately 250,000 neurons each minute (Fields et al., 2020; Heiney et al., 2021). An enormous communication network is created because each neuron is connected to a thousand others. Neurons are the cells that make up the nervous system. Neurons are the cells in the brain responsible for transmitting and receiving signals. Despite their similarities to other types of cells, neurons are characterized by distinct physical and functional properties. Similar to the hundreds of kinds of animals and plants on Earth, thousands of distinct types of neurons exist. Neurons are not all the same in terms of structure, function, or genetics (Duan et al., 2020a; Yang et al., 2020). Neurons are further divided into three categories: sensory (carrying signals from the senses to the CNS), motor (carrying signals from the CNS to muscles), and interneurons (carrying signals from one place to another within the CNS) (Hor et al., 2018; Wan et al., 2018; Smolilo et al., 2019; Duan et al., 2020b). However, neurons come in five distinct varieties. Each exhibits a unique variation on the standard neuron shape.
Brain elements, including cognitive and motor neuron function, can be lost rapidly due to neurodegenerative illnesses, posing a significant problem for the elderly. Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) are neurodegenerative illnesses (Angelucci et al., 2019; Jensen et al., 2020). Despite their various clinical manifestations, neurodegenerative symptoms share common traits and mechanisms. Regional cytosolic or nuclear protein aggregation is one of these characteristics (Xu et al., 2021). In AD, extracellular amyloid-beta (Aβ) plaques and intracellular hyperphosphorylated microtubule-binding tau inclusions form (Katsumoto et al., 2019; Roda et al., 2022). Some of the distinguishing features of these diseases are the accumulation of polyglutamine protein aggregates in HD and other repeat CAG-polyglutamine diseases, the intracellular storage of Aβ-synuclein in PD, and the inclusion of TAR DNA-binding protein (TDP)-43 transactive response in ALS, frontotemporal dementia, and other related disorders (Arnold et al., 2013; Toyoshima and Takahashi, 2014). Although a few genetic origins have been found, the primary factor is a complex mixture of genetic and environmental predisposition factors (a balance of hereditary and “sporadic” types in every major neurodegenerative condition). AD is a neurological condition that is the leading cause of dementia among the elderly (Pan et al., 2021a). The amyloid cascade hypothesis proposes that the accumulation of amyloid peptides as fibrils in the human brain is causally related to AD development (Ibrahim and Gabr, 2019). The binding of amyloid-β aggregates to neuronal and non-neuronal plasma membranes causes synaptic and neural network disruption, which is associated with cognitive abnormalities in patients with AD (Hampel et al., 2021). Symptoms include a progressive loss of memory and other cognitive skills as a result of the damage of specific forms of neurons and synapses, which leads to neuronal death (Angelucci et al., 2019). PD is a progressive neurological condition that leads to mortality. It affects 3% of the worldwide population over the age of 60 (Ball et al., 2019). There are two types of PD: familial (inherited in an autosomal dominant or recessive way) and sporadic (idiopathic), which is caused primarily by gene–environment interactions (Halperin and Healey, 2011; Verstraeten et al., 2015; Lill, 2016). Alpha-synuclein (SNCA), glucocerebrosidase (GBA), leucine-rich repeat kinase 2 (LRRK2), vacuolar protein sorting-associated protein 35 (VPS35), parkin RBR E3 ubiquitin protein ligase (PARK2), and phosphatase and tensin homolog-induced kinase 1 (PTHIK1) are the seven genes linked to familial (PARK7) (Kruse et al., 2012; Ma et al., 2013; Ankireddy and Kim, 2015; Kalinderi et al., 2016; Mursaleen et al., 2017; Zhao et al., 2018). These genes, as well as particular metabolites and PD-related biomarkers, have been utilized to investigate prospective early detection strategies for PD. The fundamental etiology of idiopathic PD is considered to be gene–environment interactions. Individuals exposed to the same environmental cause are impacted differently, resulting in various illness manifestations (Ball et al., 2019).
2 Common targets of neurological disorders
The various targets found in neurological conditions (Figure 1) that further can be explored for the drug treatment are mentioned as follows.
FIGURE 1
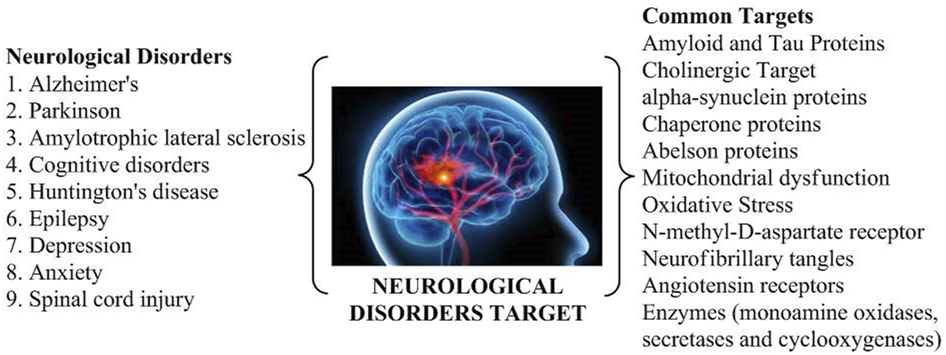
Common targets of various neurological disorders.
2.1 Amyloid and tau proteins
The tau and amyloid receptors have been tremendously researched as AD targets (Kent et al., 2020). The main aim is to lower amyloid levels and inhibit amyloid or tau accumulation. Various neuro-proteins, including APOE, APP, BACE (Aβ cleaving enzyme), PS1/2, secretase, and tau, play a key role in the pathogenesis of AD (Chen et al., 2017). Hence, studies are based on the development of novel compounds restricting the aforementioned process for the management of AD.
2.2 Cholinergic target
Various research findings have resulted in a facile grasp of the cholinesterase role inside the brain, along with the implementation of cholinesterase inhibitors in the treatment of AD (Stanciu et al., 2020). The further process of the newer generation of acetyl and butyryl cholinesterase inhibitors is being deliberated and scrutinized clinically for AD, resulting in the discovery of antioxidants, hormonal delivery, hypolipidemic compounds, anti-inflammatory drugs, and vaccinations (Santos et al., 2018).
The current study analyzes the common pharmacological targets and biological prospects for current and futuristic natural drugs. Multi-targeted techniques in oxidative stress and neuroinflammatory pathways, along with other target approaches and the extensive role of different phytoconstituents in neurodegenerative diseases (NDDs), are highlighted (Martins et al., 2020).
2.3 α-Synuclein protein
A 140-residue protein, presynaptic in the brain and called α-syn, is essential for the movement and synaptic vesicle fusion and controls dopamine (DA) release at presynaptic terminals. In the typical human brain and cerebral spinal fluid (CSF) fluid, α-syn has a physiological concentration of 1 μM and 70 pM, respectively (Domenighetti et al., 2022). When it binds to lipid vesicles, it transforms from its natural state of an unfolded monomer to α-helical conformations (secondary structure). This results in the misfolding and accumulation of α-syn upon destabilization in neurons. The monomeric protein α-syn is inherently disordered and exists in several conformational states. It is important for several vital metabolic pathways and increasing misfolding-related illnesses, most notably neurodegenerative disorders (Fields et al., 2019).
2.4 Chaperone proteins
Pharmacological chaperoning is emerging as a viable therapeutic strategy for the management of several disorders linked to single gene mutations. Small molecules known as chaperones attach to proteins, stabilize them against proteolytic breakdown, or guard them against heat denaturation. Additionally, they function similarly to molecular chaperones in aiding or hindering certain protein–protein complexes (Gouda et al., 2022). Several animal models of neurodegeneration have shown that distinct chaperone proteins are neuroprotective. Targeting the cytoplasmic chaperone Hsp90 and, by extension, enhancing the cellular response to stress may constitute a feasible therapeutic strategy for NDDs, although this hypothesis has to be proven and new drugs have to be developed (Lindberg et al., 2015).
2.5 Abelson (c-Abl) proteins
Cellular and oxidative stresses activate the protein Abelson (c-Abl), a member of the tyrosine kinase family. It is made up of the SH3, SH2, and catalytic domains. The function of c-Abl depends on where it is located within the cell. c-Abl promotes cellular adhesion with a survival mechanism inside the cytoplasm, but it also induces cell death inside the mitochondria and nucleus (Lindberg et al., 2015). Recent studies revealed that c-Abl is activated in response to amyloid beta fibrils and oxidative stress in AD and PD, as well as in animal models and neuronal cultures (Haron et al., 2021).
2.6 Mitochondrial region
It has been discovered that mitochondrial dysfunction is a universal trait of all neurological diseases. It is a major contributor to the onset and advancement of NDDs. Mitochondria play a pivotal role in health and disease by participating in various cellular processes, including maintaining a healthy intracellular Ca2+ balance, producing reactive oxygen species (ROS), initiating the intrinsic apoptotic pathway, and synthesizing heme and steroids. Mitochondria also play an important role in neural activity and plasticity and the formation and differentiation of brain cells (Werner and Olanow, 2022). Unusually formed and differentiated neurons emerge from defects in these pathways. Altered signaling of the apoptotic pathway has been linked to neurodegenerative disorders, such as HD, PD, ALS, epilepsy, schizophrenia, multiple sclerosis, neuropathic pain, and AD (Ikawa et al., 2021). Although the relationship between mitochondrial dysfunction and neurodegenerative disease onset and development is still not clearly understood, researchers are exploring treatments that control mitochondrial functioning to reduce neuronal damage and mutant protein aggregation (Jamwal et al., 2021).
2.7 Oxidative stress
Oxidative stress is still considered the primary treatment target in NDDs. It is important to investigate the several mechanisms that might considerably restore damage caused by ROS and thus slow or stop the progression of NDDs. The enzyme nicotinamide adenine dinucleotide phosphate oxidase is essential for oxidative stress and is a potential therapeutic target for the treatment of NDDs (Murphy and Hartley, 2018).
2.8 NMDA receptors
Neurodegenerative disorders, such as AD and PD, have attracted much attention regarding N-methyl-D-aspartate (NMDA) receptors and their functions in these conditions. Overactivation of NMDA receptors (NMDARs) mediates various elements of synaptic dysfunction in numerous central nervous system (CNS) disorders, prompting a great deal of focus on the development of drugs that can inhibit NMDAR activity (Marí and Colell, 2021; Rahman et al., 2022).
2.9 MAO enzyme
As an enzyme, monoamine oxidase (MAO) deaminates monoamines and other proteins. Nervous system diseases, such as AD, PD, ALS, HD, and depression-like disorders, are associated with the large formation of ROS caused by MAO hyperactivation. Although synthetic MAO inhibitors are currently used in clinical practice, they are linked to adverse events such as hepatotoxicity, cheese response, and hypertensive crisis. This has prompted the search for natural MAO inhibitors with a much more excellent safety profile (Gonzalez et al., 2015). The most prevalent neurodegenerative disorders are AD and PD. Based on current research into PD, type B MAO inhibitors, such as selegiline and rasagiline, show highly promising results as neuroprotective medicines. In cellular and animal models, neuronal cells are protected against death by these inhibitors. Stabilizing mitochondria, blocking the death signaling cascade, and activating the pro-survival anti-apoptotic Bcl-2 protein family and neurotrophic factors are all responsible for the neuroprotective actions (Naoi and Maruyama, 2010).
2.10 Neurofibrillary tangles
In neurofibrillary tangles, tau, a microtubule-associated protein, has become hyperphosphorylated. An imbalance between the activity of protein kinases and phosphatases acting on tau may occur even before neurofibrillary tangles form because phosphorylated tau proteins accumulate in neurons even before tangles form. To date, no in vivo development of neurofibrillary tangles has been observed in experimental models, and the molecular linkage between neurofibrillary tangle and senile plaque formation is poorly known (Mannan et al., 2022).
2.11 Angiotensin receptors
The rennin angiotensin system is made up of several different parts, including angiotensinogen, the (pro)renin receptor (PRR), angiotensin-converting enzyme 1 (ACE1), ACE2, angiotensin I (ATI), angiotensin II (ATII), ATII receptor 1 (AT11R), ATII receptor 2 (AT22R), and the Mas receptor (MasR). The rennin angiotensin system plays a crucial role in systemic and cellular pathways to maintain normal blood pressure, fluid balance, and cellular homeostasis. An ACE1/ATII/AT11R axis regulates oxidative stress and neuroinflammation pathways, whereas an ATII/AT22R and/or ACE2/Ang(1–7)/MasR axis enhances neuroprotection pathways. ATII is the primary effector of the RAS, and it exerts its impact by binding to AT11R and AT22R through two competitive arms (Srinivasan et al., 2022a).
2.12 COX enzyme
Several research studies have revealed the association between different pro-inflammatory cytokines and PD, and their findings suggest that immunological responses may explain a portion of PD etiology. Evidence supports the hypothesis that cyclooxygenase-2 (COX-2) is over-expressed in mouse models with PD. However, the same research showed that blocking COX-2 reduced the risk of PD by inhibiting the production of potentially harmful DA-quinones (Chinraj and Raman, 2022). Another research revealed that the neuronal cells of PD are severely damaged due to an invasion of T lymphocytes (Brochard et al., 2009).
Memory is a cognitive process in the brain that encodes, stores, and recalls information that has been received. Memory is crucial for learning and communicating with the surroundings (Fuloria et al., 2022). Subjective memory impairment is a frequent finding in adults, although the underlying condition is not detected in most of these patients. Memory impairment (MI) has various etiologies in the absence of physical or psychological disease, including being stressed, feeling ill, feeling melancholy, being exposed to air and noise pollution, adverse effects of certain medicines and substance addiction, and lifestyle factors, such as tobacco use, heavy alcohol consumption, poor physical exercise, and high-fat diet. Memory problems, often known as MI, are important markers for detecting syndromes and their underlying causes. AD, PD, HD, Korsakoff’s syndrome, and Creutzfeldt–Jakob disease are only a few examples (Sarris et al., 2014; Zlotnik and Vansintjan, 2019; Gao et al., 2022). With amnesia and dementia, MI mostly impairs declarative memory; however, this is not necessarily the case with dementia, defined as a decrease in two or more domains of cognition. In other words, dementia not only damages declarative memory but also affects other aspects of memory. Dementia has direct and secondary effects on memory (Vidyanti et al., 2022). Primary memory impairment can involve a deficit in declarative memory, which is one of the cognitive regions affected by AD. Memory capacity is harmed in a secondary case when there are cognitive abnormalities that might limit memory performance, such as attentional deficit (Callahan et al., 2022; Guo et al., 2022). There is currently no proven medication that can completely prevent MI from occurring. In contrast, memory enhancement treatments are critical for preserving a patient’s cognitive function to counteract MI risk factors (Gold and Budson, 2008; Wichansawakun et al., 2022).
3 Traditional holistic approach for the management of neurological disorders
Traditional medicines could be an alternative option to cure various neurodegenerative disorders because allopathic treatments are limited and have severe adverse effects. Indian ayurvedic medicine offers several plant-derived compounds that may be useful in future research, especially on neurological disorders. The ayurvedic system provides a holistic approach to managing different polyherbal formulations that act as antioxidants and reduce amyloid deposits and neuroprotective, anti-inflammatory, and immunomodulating compounds that alter neuroendocrine-immune activities, enhance memory, activate neurofunctions, and enhance the quality of life. A balanced lifestyle, good eating habits, socio-psychological support, Rasayanas, and psychotherapies as defined in Ayurveda have been recognized as effective approaches to prevent and treat AD and other neurodegenerative disorders (Rastogi, 2010; Ravikumar and Aittokallio, 2018; Sharma et al., 2018; Rastogi, 2019; Sharma et al., 2022).
Natural products, secondary metabolites, and bioactive molecules derived from plants, animals, and microorganisms are key sources of bioactive molecules that have been turned into disease remedies in many circumstances (Zucchella et al., 2018; Miranda et al., 2019; Ratcliffe et al., 2020). On land and at sea, nature has bestowed surplus resources (natural products) on humans. Natural products play an important role in disease prevention and health promotion for people and animals (Cragg and Newman, 2002; Mantovani et al., 2008; Cragg et al., 2009; Villa and Gerwick, 2010). These natural compounds have been shown to have various biological qualities, including antioxidant, anti-inflammatory, and anti-apoptotic capabilities (Villoslada et al., 2008). Natural products used in numerous preclinical models of neurodegenerative conditions have been further confirmed by in vitro and in vivo investigations. Phytoconstituents, such as polyphenolic antioxidants, are present in herbs, fruits, nuts, and vegetables, as well as marine and freshwater flora (Aboulwafa et al., 2019; Rehman et al., 2019). These phytoconstituents may help prevent neurodegeneration and improve brain memory and cognitive abilities. They are also thought to play a key role in preventing and treating neurodegenerative illnesses, including AD, epilepsy, and PD (Ratcliffe et al., 2020; Sharifi-Rad et al., 2020; Mendonça-Junior et al., 2021). The plants that show and prove their therapeutic action against neurological diseases are discussed in Table 1.
TABLE 1
| Plant name/species | Family | Source | Ingredient with biologically significant activity | Action | References |
|---|---|---|---|---|---|
| Ginkgo biloba | Ginkgoaceae | Leaves | Quercetin, kaempferol, and isorhamnetin | Boosts circulation to the brain | Mashayekh et al. (2021) |
| Panax ginseng C.A. Meyer | Araliaceae | Root and aerial parts | Aglycones, protopanaxadiol, and protopanaxatriol | Neurons survive longer by increasing their supply of survival compounds known as neurotrophic factors | Miranda et al. (2019) |
| Scutellaria baicalensis Georgi | Lamiaceae | Root and aerial parts | Baicalein, baicalin, and wogonin | Protect neurons from oxidative damage | Yoon et al. (2017) |
| Curcuma longa | Zingiberaceae | Rhizome | Curcumin | Inhibition of cytokine production and microglia activation | Yu et al. (2018) |
| Vitis vinifera | Vitaceae | Fruits and seeds | Resveratrol, quercetin, and catechin | Neuroprotective effects | Tabeshpour et al. (2018) |
| Salvia officinalis L. | Lamiaceae | Leaves and flowers | 1,8-Cineole, camphor, borneol, caryophyllene, and linalool | Anticholinesterase activity | Kennedy et al. (2006) |
| Coffea | Rubiaceae | Seeds | Caffeine | Acts on adenosine receptors | López-Cruz et al. (2018) |
| Camellia sinensis Kuntze | Theaceae | Leaves | Epigallocatechin, epigallocatechin-3-gallate, myricetin, quercetin, kaempferol, and epicatechin | Antioxidants, protects from oxidative stress, reduces amyloid proteins | Bazyar et al. (2021) |
| Bacopa monniera | Plantaginaceae | Whole plant | Herpestine, d-mannitol, hersaponin, and monnierin | Enhancing neuronal synthesis, kinase activity, restoring synaptic activity, and nerve impulse transmission | Mathur et al. (2016) |
| Centella asiatica | Apiaceae | Leaves | Asiaticoside, brahmoside, brahminoside, asiatic acid, madecassic acid, brahmic acid, isobrahmic acid, and betulic acid | Antioxidant action, acetylcholine esterase inhibitor activity | Hafiz et al. (2020) |
| Picrorhiza scrophulariiflora | Plantaginaceae | Roots | Glycosides, terpenoids, phenylethanoids, glycosides, and phenolic glycosides | Neuritogenic activity | Kumar et al. (2015) |
Different types of plants along with their biological effects.
Neuroinformatics is the study of the neurological system via the development of databases and tools that aims to design and manage web-accessible databases of experimental and computational data and novel software tools that are necessary for understanding the nervous system in diseased and healthy states (Pu and Li, 2018; Usman et al., 2022). Brain imaging using positron emission tomography (Kaswan et al., 2021; Ruiz-Olazar et al., 2021), functional magnetic resonance imaging (Stefanovski et al., 2021; Li et al., 2022), electroencephalography (Wojcik et al., 2018; Shirbandi et al., 2021), magnetoencephalography (Gorina-Careta et al., 2021), and other methods; several electrophysiological recording methods; and clinical neurological data are examples of neuroinformatics (Sharma et al., 2019). In an interesting study, 679 flavonoid-based compounds and their 481 relative targets were screened, and their bioinformatic analysis exhibited multiple pharmacological pathways, especially for neuronal diseases. Flavone-based targets were remarkably augmented in mitogen-activated protein kinase (MAPK) signaling and neurotrophin signaling pathways, suggesting that natural flavone compounds possess biological effects on neuronal diseases (Qiu et al., 2018; Ravikumar and Aittokallio, 2018). Based on the pattern of substitution of phenyl rings and oxidation and saturation of pyran rings, different modified flavonoid-based compounds can be synthesized, thus exhibiting potent physico-chemical properties and biological activities acceptable for the effective management of neurological-related diseases (Figure 2) (Ayaz et al., 2019).
FIGURE 2
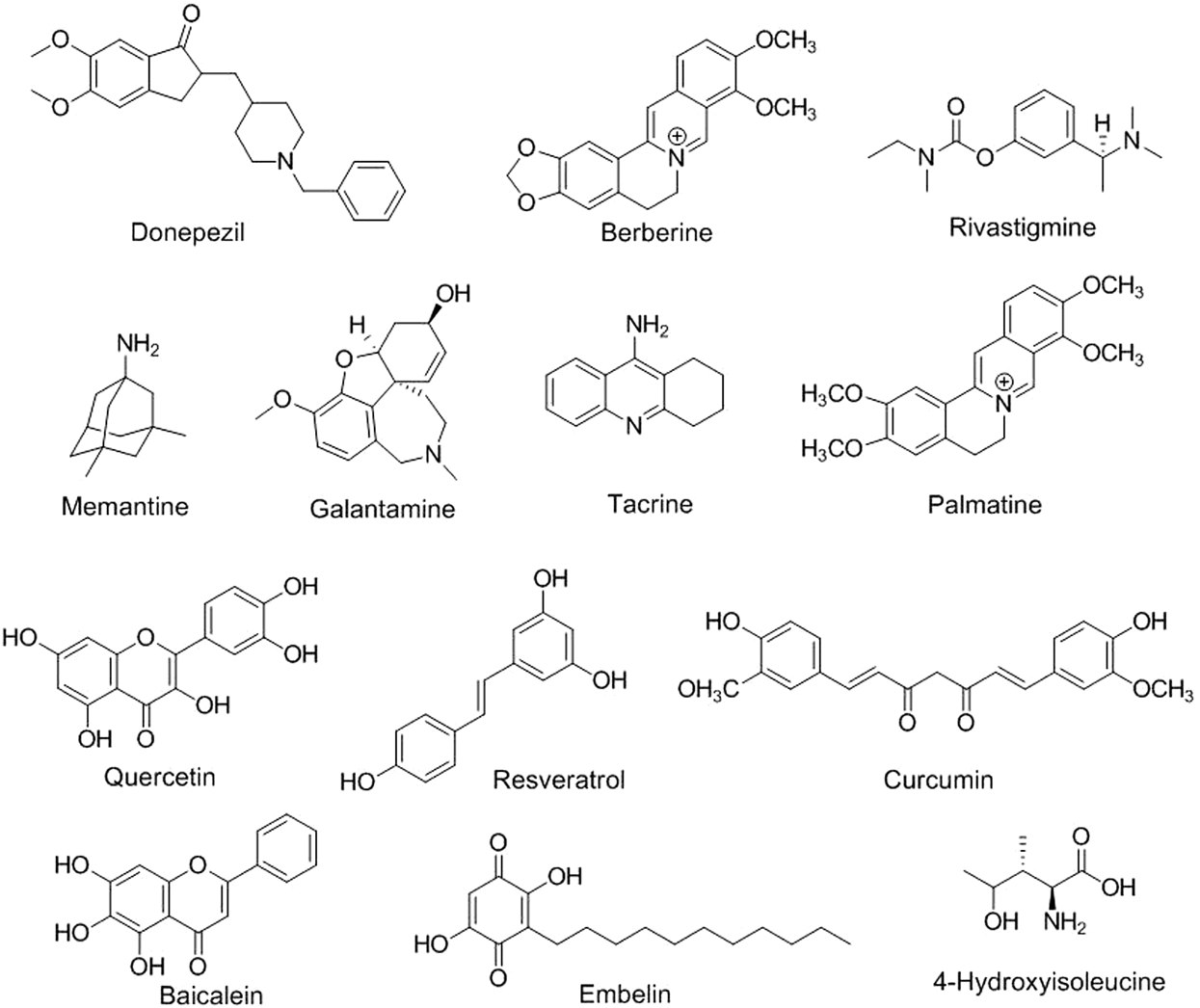
Chemical structures of different phytoconstituents in neurological disorders.
The concept of medications interacting with many targets has long been seen as undesirable, as it is inevitably related to negative side effects but theoretically can be safer compared to a single-hit target molecule (Hampel et al., 2021). Target-driven approaches often find a poor association between in vitro medication effects and in vivo effectiveness, thus finding a pivotal research scope. While understanding the underlying pathomechanisms of neurological and psychiatric disorders, searching for new biomarkers, and developing innovative therapies, translational research is one of the most important yet difficult fields for pharmacologists (Wan et al., 2018; Angelucci et al., 2019). Significant progress has been achieved in our understanding of the polygenic, complex, and heterogeneous disease pathways due to the advancement of disease models in vivo and in vitro (Xu et al., 2021). Diseases that can be studied through translational research include neurodegenerative disorders, such as AD, PD, multiple sclerosis, HD, and ALS, and psychiatric disorders, such as major depressive disorder, bipolar disorder, substance abuse disorder, post-traumatic stress disorder, anxiety disorder, schizophrenia, somatic symptom disorder, autism spectrum disorder, and hyperactive ataxia (Kaswan et al., 2021). There are clinician guides for using neuroscience to guide case framework, understand psychotherapeutic techniques, aid in treatment personalization and outcome prediction, and develop novel mechanistically targeted treatments for disorders (Shirbandi et al., 2021). We extensively added recent and updated key findings and additionally showed the applicability of natural products to improve their appropriate usage in neurological disorders, followed by the incorporation of various clinical studies and patents on phytoconstituents for neuronal diseases. This study focused on assessing various research studies related to the prevention and treatment of NDDs and provided evidence for the efficacy of natural products. It also sparked interest in the development of novel medications for neurological disorders derived from plant sources.
4 Phytoconstituents in different neurological disorders
4.1 Alzheimer’s disease
AD and dementia are diseases of the elderly society and have become one of the major concerns in health management because of the unattainability of medicinal treatment in this area (Liu et al., 2022). Pathophysiologically, AD is an accelerating neuro-degenerative disease, resulting in the change of behavioral patterns and cognitive defects, and is the recurring source of dementia in approximately 80% of the diseased population, expected to increase three times by 2050 (Zhang et al., 2021a). Various target receptors are responsible for this condition, including the scarcity of important neurotransmitter acetylcholine (ACh), accumulation of β-amyloid proteins, largely phosphorylated tau plaques, and variation in glutamate pathways, neuro-inflammation, and different pathways, which participate in the pathological mechanism of the particular diseased condition (Thomford et al., 2018). In fact, the following are the natural phytoconstituent-based drugs that have been accepted clinically in AD, such as cholinesterase inhibitors (tacrine, galantamine, donepezil, and rivastigmine) and glutamatergic system modulators (memantine). However, they have shown lesser symptomatic effect and hepatotoxicity with tacrine (Joshi et al., 2022).
The important pathological attributes observed in the brains of patients with AD are as follows (
Husain et al., 2021):
1) Neuritic plaques containing polymorphous deposits of Aβ, a peptide constructed through the deterioration of Aβ initiators;
2) Neuro-fibrillary tangles, along with the dense irregular bundles inside cytoplasm based in the neuronal system consisting of the modified form of the microtubular-assisted proteins.
The present pharmacological treatment depicts lesser symptomatic positive outcomes. Due to the multi-factorial causes, the advancement of novel molecules is aimed at multi-targeting therapy such as cholinesterase inhibition, anti-amyloid effects, β-secretase and MAO blockage, nitric oxide delivering ability and interactivity with cannabinoid, and NMDA or histamine receptors, contributing to an effective approach in AD. Interestingly, the clinically approved treatment for AD is based on natural phytoconstituents, and its recent developments are described in the following (Jankowska-Kieltyka et al., 2021).
By considering the “single-molecule multiple-target regimen” for the discovery of newer drugs in AD, natural molecules have found dominant interest. Regardless of the less-acknowledged success of synthetic compounds in AD, pharmacokinetics and pharmacodynamics (safety issues) are their crucial restricting steps (Stanciu et al., 2020). Contrarily, natural molecules extracted from herbal, nutritional, or marine origins have shown effectiveness in research studies based on a multi-targeting approach (Ciccone et al., 2021). Among many phytoconstituents, curcumin mitigates cognitive impairment symptoms by modulating inflammatory mechanisms in the brain, decreases free radical burden and metal ion chelation, and blocks Aβ aggregation. Furthermore, has proved to be a favorable candidate for AD and PD. Various flavonoids such as apigenin, luteolin, catechins, gossypetin, and myricetin have also been shown to inhibit Aβ accumulation in AD (Wang et al., 2018). Apigenin can modulate matrix metalloproteinases (MMP)-2 and 9, thus playing a neurodegenerative and neuroinflammatory role, especially in AD. Structure–activity relationship (SAR) research data on flavonoids observed that a catechol ring contributes to an important pharmacophoric moiety in multi-pharmacological activity, including AD. Other products, including alkaloids (huperzine A) and resveratrol, have different biological effects and can interact simultaneously with more than one target of this neurological disorder, showing better effectiveness (Patil et al., 2020; Fantacuzzi et al., 2022).
4.1.1 Berberine
Berberine is a natural compound in which quaternary ammonium salt of isoquinoline alkaloids extracted from different plant species such as Berberis aquifolium, B. vulgaris, B. aristata, Hydrastis canadensis, and Tinospora cordifolia (Neag et al., 2018). Several pharmacological actions of this compound are mentioned in the literature, such as antioxidant, cholinesterase inhibition, MAO inhibition, and hypocholesterolemic effect, along with fewer gastrointestinal side effects (Akbar et al., 2021). In a recent study, berberine (260 mg/kg, oral) has been reported to reduce Aβ42 aggregation and tau hyperphosphorylation through remarkably mitigating endoplasmic reticulum (ER) stress (Wu et al., 2021). Similarly, Liang et al. and group discovered the effect of berberine in 3xTg AD (triple-transgenic AD) mice and observed that protein kinase RNA-like ER kinase/eukaryotic translation initiation factor 2α signal pathway was diminished, further declining Aβ growth and thus improving neuronal functions by mitigating ER and oxidative stress (Liang et al., 2021). In another study, berberine was found to lower MI effects as assessed in a triple-transgenic (3xTg) AD mouse model-based assay. Berberine (100 mg/kg, oral) could simultaneously target autophagic clearance and hyperphosphorylation of tau by regulating the Akt-glycogen synthase pathway (Chen et al., 2020).
4.1.2 Resveratrol
Resveratrol is a polyphenolic compound categorized as stilbenes extracted from plants after exposure to stress, injury, infection (fungal), or UV radiation (Perrone et al., 2017). This phytoconstituent has been reported to have antitumor, anti-inflammatory, cardiovascular, hypoglycemic, and neuro-protective effects with no adverse effects (Zhang et al., 2021b). Resveratrol is readily absorbed in the gastrointestinal lumen, simultaneously exhibiting lesser bioavailability because of its fast metabolism and elimination. Resveratrol plays a significant role in boosting non-amyloidogenic cleavage of the amyloid precursor protein, resulting in advancing the clearance of Aβ peptides and decreasing the degradation of neurons (Sergides et al., 2016). Resveratrol (15, 45, and 135 mg/kg) has been reported to block the cholinesterase effect in AD-based animal assays (Jia et al., 2017). A combination study of melatonin (80 mg/kg) with resveratrol (40 mg/kg) showed that melatonin augmented memory deficit effects in novel object recognition task (NORT) and passive avoidance task (PAT) assays of AD-based mouse models. In contrast, resveratrol enhanced only PAT response in respective animal studies (Jabir et al., 2018). Mehringer et al. explored phosphorylated resveratrol (Figure 3) for their AD-based neuronal properties and observed that these analogs could diminish the accumulation of proteins along with the fibrillation of Aβ42 and insulin based on in vitro studies. The in vivo drosophila fly model also showed prominent effects with decreased Aβ42 accumulation and enhanced neuroprotective locomotor action (Labban et al., 2021; Mehringer et al., 2022).
FIGURE 3
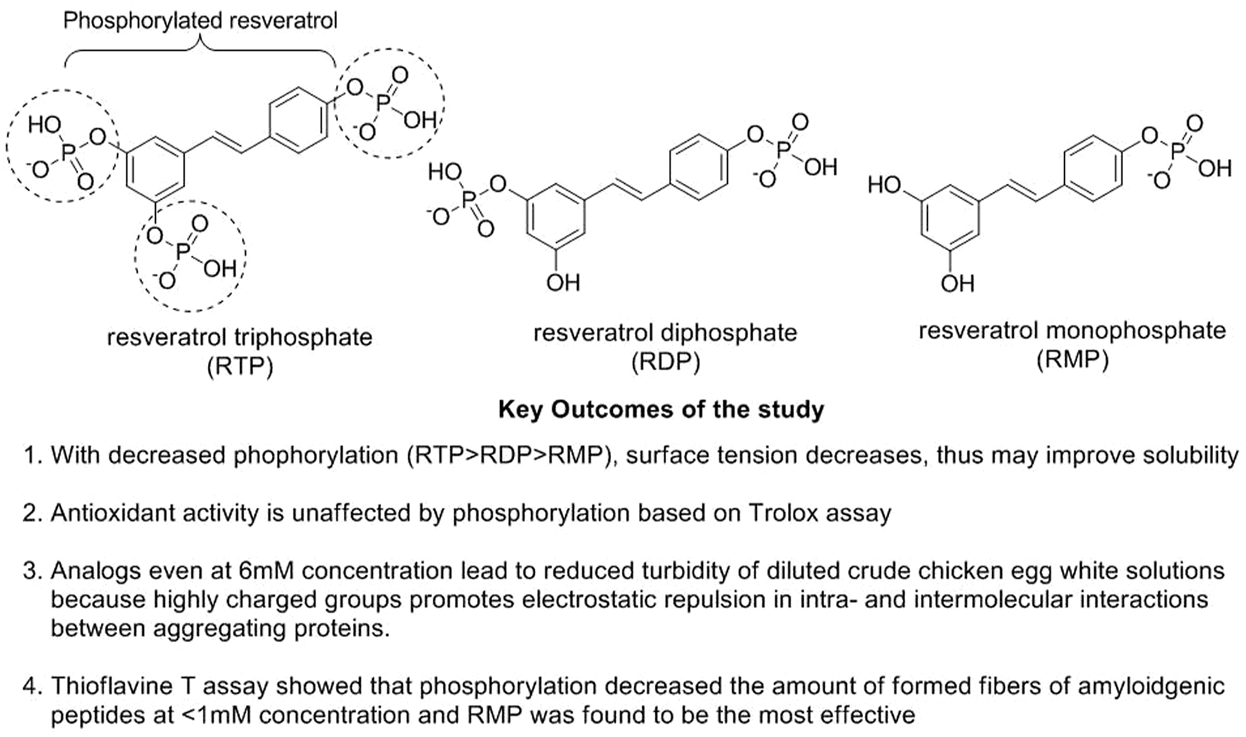
Mechanistic study of phosphorylated resveratrol in AD.
4.1.3 Curcumin
Curcumin is the most pivotal turmeric-based curcuminoid and is a popular yellow-colored Indian spice obtained from the rhizome part of Curcuma longa and corresponds to the ginger family (Hewlings and Kalman, 2017). Preclinical research has suggested curcumin to prevent or treat many disorders, such as colorectal cancer, cystic fibrosis, and inflammatory and neurological diseases (Jyotirmayee and Mahalik, 2022). Based on phase I clinical data, an oral curcumin dosage of 8,000 mg/day has not resulted in any major adverse effects besides mild nausea and diarrhea. However, excessive usage of this natural compound can harm the gut microbiome, thus obstructing the normal physiological and immunological processes (Gupta et al., 2013). The oral bioavailability of curcumin is relatively low, and many of its metabolites have been detected in plasma after oral intake (Lopresti, 2018). Many recent reviews have assessed the extraordinary role of curcumin in developing tau-focused therapeutics in AD, mainly due to the failure of most of the Aβ-based AD drugs in clinical trials (Sivanantharajah and Mudher, 2022). Current advances have revealed that the phenolic hydroxyl group of curcumin can contribute to the anti-amyloidogenic effect. Phenyl-substituted methoxy groups can show suppression of Aβ42 and APP (amyloid precursor protein), and hydrophobic interactions have also played an amplifying role. Furthermore, the elongation of phenyl rings can have decreased effect in patients with AD (Chainoglou and Hadjipavlou-Litina, 2020). Another systemic analysis carried out the correlation of the 74 target genes of curcumin with AD and experimented through Gene Ontology (GO) mechanism enrichment analysis and Kyoto Encyclopaedia of Genes and Genomes (KEGG). Five important genes were identified using the network pharmacological approach: RARA, APP, PRARG, STAT3, and MAPK1. Computational studies were also carried out to observe that curcumin has a prospective to attach with big active sites of PPARγ, observing better binding scores compared to other protein targets (Vijh et al., 2022). Another molecular docking study showed the molecular modeling studies of curcumin displaying a remarkable binding affinity toward mTOR, TrkB, LXR-β, TLR-2, ER-β, GluN2B, β-secretase, and GSK-3β, which are the critical modifiers of molecular and cellular pathways related to AD (Hannan et al., 2020). Recently, Utomo et al. verified curcumin-based compounds 1 and 2 (Figure 4) in Alzheimer’s Drosophila model and observed disassembled Aβ fibrils. The study further showed very low toxicity at 1 µM concentration in N2a cells (neuroblastoma) and prominently recovered its locomotor activity in AD model flies (Utomo et al., 2022).
FIGURE 4
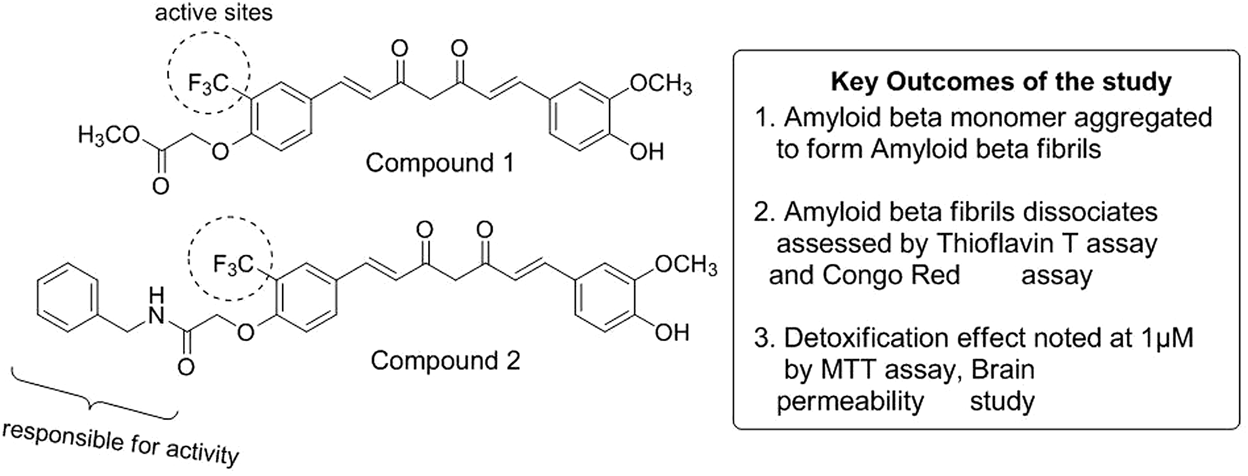
Mechanistic study of curcumin in AD.
4.2 Parkinson’s disease
The brain motor system is most primarily affected by PD, which causes inflammation and depletion of dopaminergic neurons inside the substantia nigra. A series of factors, including mitochondrial dysfunction, oxidative stress, protein misfolding during synthesis, excitotoxicity caused by different biochemical pathways (such as the glutamate pathway), lysosome impairment, chaperone-mediated autophagy, and the development of Lewy bodies as a result of protein misfolding, contribute to the onset of the disease (Amro et al., 2018). Associated protein (neurofilament) and protein targeting, such as ubiquitinated α-synuclein, are the components of cellular bodies. According to Braak’s staging, Lewy bodies are often located in the olfactory area and the lower part of the brain stem. However, as the illness advances, Lewy bodies also appear in the midbrain (substantia nigra) and forebrain, as well as the neocortex in an advanced stage. The most prevalent classes of phytochemicals with known antiparkinsonian actions include terpenes and numerous subtypes of polyphenols. Alkaloids, carbohydrates, acids (amino and fatty acids), and amides are a few more phytochemical groups containing representatives that have beneficial effects on PD (Lill and Klein, 2017; Zoey et al., 2021). Proinflammatory cytokines, such as prostaglandin E2, interleukin-6 and 1β, and nuclear factor of kappa cells, are reduced in expression, as nuclear and cellular inflammatory signaling, and phytochemicals suppress apoptosis (by reducing either caspases orα-synuclein aggregation), lower dopaminergic neuronal damage, and alleviate DA exhaustion. In order to increase the effectiveness and lower the biological side effects of PD, herbal compounds might be thought of as prospective pharmaceutical medications or as adjuvant therapy, along with traditional therapeutic procedures (Aliakbari et al., 2018).
The striatum has two primary output pathways. 1) The indirect route, which is carried out by inhibiting D2 DA receptors by DA in which the striatum sends GABA-mediated signals toward the neuronal cells in the lateral GP (GPe) and the GPe then sends signals to the STN, which sends glutamate-based excitatory signals to segment (internal) of GP (GPi), as well as SN pars reticulata (SNr). Rigidity and bradykinesia are clinical manifestations of the thalamocortical-spinal route suppressed by GPi and SNr. 2) Simultaneously, the unobstructed path is regulated by DA’s excitatory impact-bearing striatal receptors, and the lack of this neurotransmitter lessens the striatum’s ability to inhibit GPi and SNr (Merzougui et al., 2021).
Although the precise etiology and PD process are still unclear, there has been great progress in understanding the illness’s fundamental mechanisms. This was accomplished by research on genetics, experimental forms of PD, pathological and pharmacological abnormalities of PD, and novel findings on the structural characteristics and physiology of basal ganglia. In this study, we cover the pathophysiology of PD and the natural use of several herbal medicines, as well as their modes of action (Jankovic and Sherer, 2014).
Numerous studies on the use of various herbal remedies and natural items in the treatment of PD have been conducted over the past few years and have been explained below (Ion et al., 2021).
4.2.1 Curcuma longa
In India, Curcuma longa is frequently used as a medication for various health issues. It has been established that this plant has anti-inflammatory, antioxidant, chemotherapeutic, anti-proliferative, wound-healing, and antiparasitic properties. Curcumin, the plant’s active polyphenolic component, is assumed to be responsible for these properties (Nebrisi, 2021). Using fibroblasts from patients with PD, who have LRRK2 mutation, as well as healthy controls, curcumin is an effective treatment to address mitochondrial dysfunction in the condition. While post-curcumin treatment showed little impact, pre-curcumin treatment enhanced maximum and ATP-associated respiration. These findings are significant for the therapeutic use of curcumin because they suggest that it would be the most advantageous pre-treatment to toxin exposure. PD fibroblasts with the LRRK2 mutation and healthy control fibroblasts may benefit from pre-treatment with curcumin to prevent mitochondrial damage (Abrahams et al., 2021). Nerve regeneration and anti-apoptotic effects are considerably aided by phosphatidylinositol-3-kinase (PI3k)/protein kinase B (Akt) signaling mechanism and abrineurin pathway. According to recent studies, curcumin regulates the above-mentioned signaling pathways in neurodegenerative disease, positively affecting neuroprotection (Jin et al., 2022).
4.2.2 Resveratrol
Resveratrol, a natural polyphenol, is present in different plant species of grapes and berries. In PD etiology, altered PGC-1 activity and transcriptional dysregulation of its target genes were demonstrated by a recent study, suggesting that PGC-1 may represent a new target for therapeutic intervention. Resveratrol has been reported to increase mitochondrial action by activating multiple metabolic sensors, which in turn activates PGC-α. In addition, the resveratrol administration led to an uptick in the complex I and citrate synthase activity, a reduction in lactate content, an increase in baseline oxygen consumption, and the synthesis of mitochondrial ATP (Katila et al., 2022). These changes supported the transition from glycolytic to oxidative metabolism. Additionally, resveratrol administration increased macro-autophagic flux by activating a mechanism unrelated to LC3. The findings on PD fibroblasts from patients with early onset implied that resveratrol may have potential clinical use in some PD patients. In a different study, Su et al. investigated transgenic and chemically generated mouse PD models, including those caused by MPTP, rotenone, 6-OHDA, paraquat, and maneb (Su et al., 2021). Resveratrol’s neuroprotective effects were mostly focused on reducing oxidative stress and inflammation and improving mitochondrial dysfunction and motor function. Resveratrol also inhibits the production of the enlargement of mitochondria along with the compaction of chromatin and prevents the enlargement of mitochondria and condensation of chromatin (George et al., 2019).
4.2.3 Quercetin
Quercetin, a flavonol-type flavonoid, is present in several fruits and vegetables and is identified as a complementary treatment for PD. The neuroprotective action of quercetin is directly linked with its antioxidant activity, besides stimulating cellular defense against oxidative stress. Additional associated pathways are activating sirtuins (SIRT1) and stimulating autophagy, besides the induction of Nrf2-ARE and paraoxonase 2 (PON2) (Grewal et al., 2021). In another investigation by Josiah et al., the animal studies observed the promising efficacy of quercetin on NF-κB and IκKB gene expressions compared to the rotenone group only. Different research data have exhibited the potential of quercetin for PD by relieving oxidative stress, observing dopaminergic breakdown, and altering neuroinflammation, along with apoptosis (Josiah et al., 2022).
4.2.4 Walnut
The water extract of walnut (Juglandis semen) has exhibited pivotal neuroprotective action in various research studies. This extract was found to deplete ROS and NO (nitric oxide) growth, further blocking the loss of DA, thus showing exceptional recovery in patients with PD (Esselun et al., 2022). In another investigation by Yang et al., the walnut-derived polypeptide (TW-7) observed antioxidant action simultaneously initiating autophagy. They further investigated that TW-7 restricted the mitochondrial apoptosis through downregulation of the cytoplasmic cytochrome C, caspase-9, and cleaved-caspase-3 expression (Yang et al., 2022).
4.2.5 Olive leaves extract
Derivatives are isolated from olive leaves, including phenolic compounds, such as hydroxytyrosol, and flavonoids, such as luteolin, apigenin, and apigenin-7-O-glucoside, and their wide range of pharmacological activities, including several properties, such as neuroprotective, antioxidative, antibacterial, antiviral, anti-obese, and anti-inflammatory. The phenolic compounds isolated from olive lowered the syndrome (metabolic) associated with PD (Hadrich et al., 2022).
4.2.6 Myricitrin
Myricitrin, a naturally originated phenolic compound with antioxidant and anti-inflammatory properties, is also known as myricetin-3-O-rhamnoside. Myricitrin’s therapeutic potential was examined in a mouse brain model by Banerjee et al. In the mouse brain, myricitrin reduced MAO activity and increased DA levels. In the PD mouse model, myricitrin could lessen motor incoordination and elevate the DA levels in the striatum (Banerjee et al., 2022).
4.2.7 Baicalein
Baicalein is an active constituent in which Scutellaria baicalensis is its natural source. The alcohol extract of Scutellaria baicalensis has been reported to decrease nitric oxide (NO) and COX-2 levels (Jeong et al., 2011). This compound also restricts the accumulation of ROS, ATP degradation, apoptosis, and mitochondrial disruption based on rotenone-generated neuronal toxicity (PC12 cells) (Li et al., 2012). Zhao et al. showed that baicalein-treated mice exhibited lower depression-based symptoms after a monthly treatment, and its repeated usage induced α-synuclein dissociation, neuroinflammation blockage, and regulating the homeostasis of neurotransmitters (Zhao et al., 2021). In another study, Song et al. investigated that baicalein can also inhibit the MAO enzyme, and its blocking action on oxidative stress is governed by ERK inhibition in PD (Song et al., 2021; Xu et al., 2022).
4.2.8 Glycyrrhizin
The primary active component of licorice roots and rhizomes (Glycyrrhiza glabra L.) is glycyrrhizin, which is typically used to treat inflammatory illnesses or even as a tonifying herbal remedy. Ren et al. reported inhibition of the degeneration of DA neurons, reduction of the count of apoptotic cells in the zebrafish brain, prevention of the loss of their vasculature as well as disordered vasculature, and suppression of the locomotor impairment to exert an anti-PD effect on MPTP-induced PD in zebrafish (Ren et al., 2022).
4.2.9 Chicoric acid
A polyphenolic acid called chicoric acid (CA), which is derived from the purple coneflower (Echinacea purpurea) and chicory, has been promoted as a nutraceutical to fight infections, inflammation, and obesity. Wang et al. showed that oral pretreatments of CA significantly prevented the motor dysregulation and death of nigrostriatal dopaminergic neurons exacerbated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), including the inhibition of glial hyperactivation and the increase in striatal neurotrophins. It may be inferred that CA showed neuroprotective effects on mice with MPTP-induced PD. These benefits may have been caused by altering the gut microbiota and reducing inflammation along the brain–gut axis (Wang et al., 2021).
4.3 Amyotrophic lateral sclerosis
There are broadly two types of ALS: sporadic and familial types. The family variety (5%–10%) has a genetic component but is genetically inherited, whereas the irregular type, which is prevalent (90%–95%), is not inherited. Various neurological conditions, including ALS, are characterized by the degeneration of both motor neurons (upper and lower). Intraneuronal protein aggregates, including protein TAR DNA-binding, superoxide dismutase, and fused in sarcoma, may interrupt normal protein homeostasis and cause ALS and cellular stress (Chandran et al., 2022). These proteins have been thoroughly discovered in ALS animal models and pathological examinations of individuals. Muscle twitching, cramping, soreness, and weakness are static analyses of ALS. Patients eventually develop dysphagia (difficulty swallowing), dysarthria (difficulty speaking), and dyspnea (difficulty breathing) in the advanced stage of the disease. Diet and environmental toxins have also been researched for their links to ALS. For ALS treatment, multidisciplinary methods are reported to be beneficial (Kim and Taylor, 2017; Anakor et al., 2022).
4.3.1 Mecasin
Mecasin, traditional medicine that originated in India, has been shown to have various biological effects in vivo and in vitro. It also possesses anti-inflammatory properties based on previous investigations and has been discovered for ALS by Kim et al. Mecasin was found to lessen symptom development without causing significant side effects, and the long-term effects of the drug are currently being studied in a phase IIb clinically (Kim et al., 2022).
4.3.2 Morin
It is possible to isolate the yellow chemical component known as morin from the leaves of Psidium guajava, Maclura pomifera, and Maclura tinctoria. Srinivasan et al. studied the effectiveness of flavonoids against amyloids, such as morin, myricetin, and epigallocatechin gallate. Additionally, it was determined that morin has a significant therapeutic potential for developing extremely effective inhibitors for reducing deadly and incurable ALS (Srinivasan et al., 2022b).
4.3.3 4-Hydroxyisoleucine
The insulin sensitivity of rodents is improved by the bioactive amino acid (4-hydroxyisoleucine, HI) extracted from Trigonella foenum-graecum. This study focused on brain IGF1/GLP-1 activation, and a study evaluating adult Wistar rats with ALS-like signs found that 4-HI had neuroprotective properties that had been treated with methyl mercury (MeHg+). Additionally, evidence points to the neuroprotective advantages of 4-HI in minimizing MeHg+-induced behavioral changes, chemical alteration in neurons, and histological impairments in ALS in rats exposed to methylmercury (Shandilya et al., 2022).
4.4 Huntington’s disease
The neurological abnormality known as HD is inherited in an autosomal dominant manner and is monogenic. Patients and their families find the illness state traumatizing due to its inheritance pattern (autosomal dominant), progressive nature, and mix of physical, cognitive, and behavioral deficits (Lum et al., 2021). HD is a pathological condition caused by an enlarged CAG trinucleotide repeat in the gene (HTT5) on the chromosome (Yang et al., 2020), which codes for aberrant huntingtin, a potentially pathogenic protein with several functions. The enlarged CAG repeat seen in the mutant protein’s unique polyglutamine pattern is recognized to be hazardous and causes the death or malfunction of neuronal cells (Träger et al., 2015). The striatum neurons are vulnerable to this mutant protein, although HD has been shown to affect the whole brain and body. Exon 1 of the mutant huntingtin protein directly affects transport (axonal), homeostasis (protein), and mitochondrial functioning. The mutant protein’s propensity to aggregate also directly affects these processes. Abnormal huntingtin protein causes neuronal death through several methods. The alternative theory links HD’s neuronal damage to neurotrophic factor losses, glutamate excitotoxicity, and toxic consequences of repetitive associated non-ATG translation mechanisms (Kay et al., 2015).
Memory loss and motor loss of coordination caused by 3-nitropropionic (3-NP) acid were greatly reduced by natural precursors. Reduced lipid peroxidation, enhanced endogenous antioxidants enzymatically, decreased activity (acetylcholinesterase), and increased mitochondrial generation have significantly reduced biochemical changes. Interestingly, 3-NP-induced damage to the striatum was lessened after therapy with certain natural ingredients, as seen by histology. Overall, antioxidant and anti-inflammatory characteristics, maintenance of mitochondrial function, suppression of apoptosis, and activation of autophagy in natural products provided varied levels of neuroprotection throughout preclinical trials of HD (Lum et al., 2021).
4.4.1 Embelin
Embelin’s ability to fortify neurons against 3-NP-induced exploratory HD in rats was examined by Dhadde et al. in which vehicle/embelin was pretreated in adult Wistar rats (doses of 10 and 20 mg/kg p.o.) for a week. Furthermore, embelin significantly reversed behavioral changes, improved antioxidant status, and repaired striatal neuronal damage brought on by 3-nitropropionic acid (Kundap et al., 2017). In an interesting study, embelin and levodopa were analyzed for PD and HD animal studies, which were shown to mitigate oxidative and neuroinflammatory stress. Tyrosine hydroxylase and Nurr1 protein levels were significantly recovered. In silico computational studies between embelin and α-syn fibrils were also demonstrated, which validated the strong affinity of embelin approaching α-syn with the help of hydrogen bonding with Lys45(D) and His50(D) residues of α-syn (Figure 5) (Ramachandra et al., 2022).
FIGURE 5
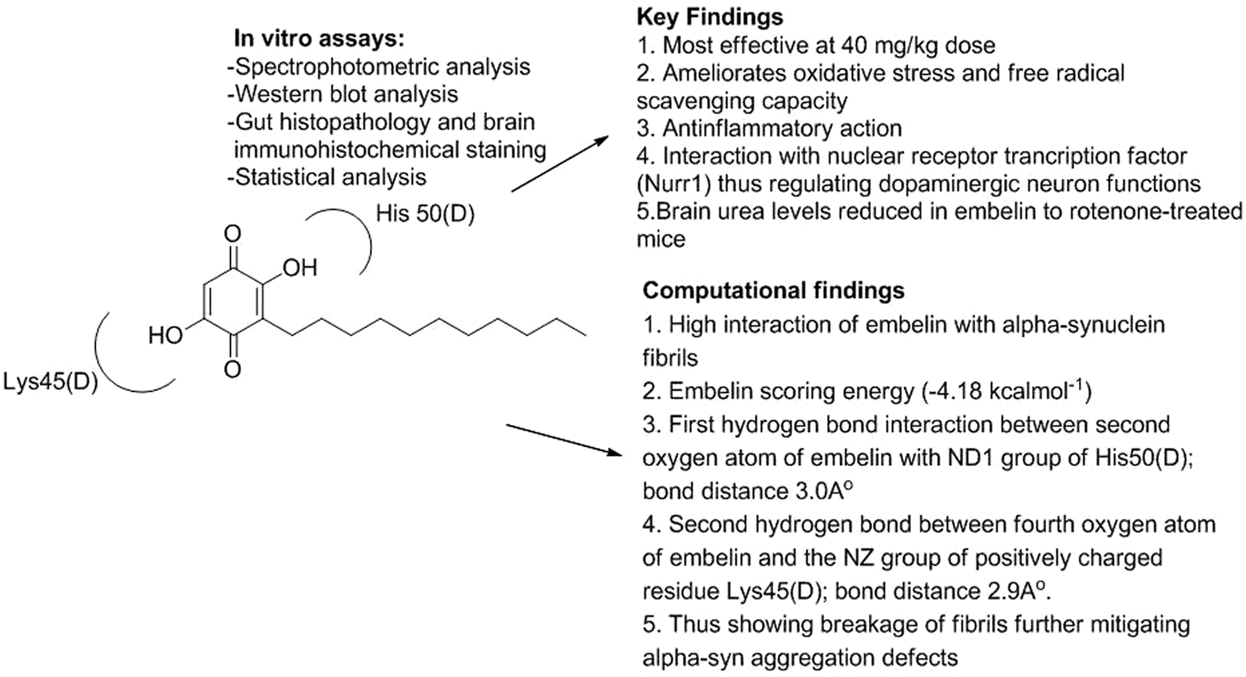
Mechanistic study of embelin in PD and Huntington’s disease.
4.4.2 Curcumin
In India, Curcuma longa is frequently used as a medication for several health issues. It has been established that this plant has several properties and is a potential candidate for antioxidant, anti-inflammatory, wound healing, chemical, therapeutic, anti-proliferative, and antiparasitic properties (Mohammadi et al., 2022). Curcumin, the plant’s active polyphenolic component, is assumed to be responsible for these properties. Curcumin’s effectiveness was examined by Aditi et al. in a Drosophila model of HD. The injection of curcumin was observed to increase locomotor performance and enhance lifespan in HD flies with advanced illness stages and reduce high reactive oxygen species levels in adult adipose tissue of sick flies (Aditi et al., 2022). The effectiveness of melatonin and curcumin in avoiding the motor deficit and disordered eclosion behavior in the Drosophila model of HD was examined by Khyati et al. It can also be deduced that melatonin (100 μg) and curcumin dramatically enhanced the abilities of HD flies to move around and behave in an enclosing manner, restoring the 24 h rhythm of mRNA expression of period and timeless to normal (control) levels (KhyatiMalik et al., 2021).
4.4.3 Lactuca sativa
Malik et al. produced extracts (ethanolic) of the leaves of three different Lactuca sativa (LS) cultivars and assimilated them using HPLC according to their quercetin concentration. The extract with the highest activity level was progressively separated in increasing polarity employing organic solvents (hexane, ethyl acetate, and n-butanol) and an aqueous solvent. It was further concluded that improved behavioral and biochemical indicators demonstrated the greatest reduction of 3-NP-induced HD-like symptoms (Malik et al., 2022).
4.4.4 Baicalein
The neuropharmacological efficiency of baicalein against QA-induced hypertension was assessed in recent research. In the striatum of HD-induced rats, naturally found baicalein, technically known as 5,6,7 trihydroxy flavone, including Scutellaria baicalensis and Oroxylum indicum (edible plants), has a stronger neuroprotective effect when administered intraperitoneally in doses of 10 and 30 mg/kg. Further analysis reveals that the neuroprotective effectiveness of baicalein exhibits the advancement of psychological and cognitive alterations spurred on by QA (Purushothaman and Sumathi, 2022).
4.4.5 Ugni molinae berries
Arancibia et al. discovered that extracts (phenolic rich) from murtilla berries of the 19-1 genotype significantly decreased peptide (polyglutamine) accumulation amounts, corresponding with the regulation in the expression patterns of proteins, which are related to autophagy and thus promising in HD therapy. Berries were extracted by exhaustive maceration with increasing polarity solvents (Pérez-Arancibia et al., 2021).
4.5 Epilepsy
Epilepsy is a neurological disease identified with attacks of altered brain responses, resulting in convulsions and seizures, and has affected around 50 million people worldwide (Pearson-Smith et al., 2017). A series of pharmacological events include cognitive impedance and oxidative stress, further contributing to epilepsy-linked recurrent seizures (Mao et al., 2019). In addition, Mao et al. explored the pharmacological mechanism at the molecular level via different redox-related neurological cell death modalities in onset seizures. The group also analyzed ferroptosis, a newly discovered lipid ROS-dependent regulatory cell death, which is likely to be a critical mechanism for unfolding epileptic phenotype (Rho and Boison, 2022).
Epilepsy has been classified broadly into four main components (
Goldenberg, 2010):
1) Seizure: partial, generalized, and unknown onset;
2) Epilepsies: partial, generalized, combined generalized, and partial unknown;
3) Epilepsy syndrome: juvenile myoclonic epilepsy and Lennox–Gastaut syndrome;
4) Etiology: structural, genetic, metabolic, infectious, immune, unknown.
Epileptic seizures also arise due to the imbalance in the excitation/inhibition response of decreased GABA receptors and the rise in glutamatergic transmission (Karim et al., 2021). Thus, phytoconstituents maintaining this balance [in between the GABA (brain neurotransmitter) and glutamate and blocking of glutamate receptors] will have an efficacious antiepileptic response compared to allopathic antiepileptic drugs showing major side effects among which impairment (cognitive) is undesirable (Kaur et al., 2021). Natural products have exhibited experimentally encouraging results in animal models based on epilepsy. An interesting study discovered the modifications of GABA, GABAA, and GABAB targets in the cerebral cortex of epileptic rats, along with the pharmacological application of Bacopa monnieri. This plant variety and bacoside-A reversed epilepsy-associated symptoms exhibiting the diminishing role of GABA receptors in epilepsy recurrence (Mathew et al., 2012).
4.5.1 Cannabidiol
Cannabidiol (a phytocannabinoid) is a natural constituent in the Cannabis sativa, also known as cannabis or hemp, comprising 80 different forms. One of the cannabidiol forms was approved as an anti-seizure drug in the United States in 2018 (Ryan, 2020). Cannabidiol has been proved via recent studies to exhibit anti-epileptic and anticonvulsant activities in acute animal models of seizures. However, their detailed pharmacological pathways remain under investigation (Devinsky et al., 2014). Gray et al. proposed three different pharmacological targets for cannabidiol, including transient receptor potential vanilloid-1, G protein-coupled receptor-55, and equilibrated nucleoside transporter 1, as this phytoconstituent has an attraction for more than one target resulting in neurological excitation applicable in epilepsy (Gray and Whalley, 2020). Concomitantly, cannabidiol was investigated along with other anticonvulsant drugs for its safety, pharmacokinetics, and drug–drug interaction with the help of double-blinded placebo-controlled trials in the recurrent epilepsies in pediatric patients, not just in the epileptic encephalopathy. Cannabidiol administration was observed to be safe and well-tolerated, and new levothyroxine–cannabidiol interaction was reported (Raucci et al., 2020; Cáceres Guido et al., 2021). The structural modification of cannabidiol phytoconstituent majorly comprises its alkyl side chain and the incorporation of phenolic hydroxyl groups on the propenylcyclohexene moiety. The SAR-based studies on cannabidiol, especially on neurodegenerative disorders, are well-reviewed by various groups. Thus, this phytoconstituent has shown great potential in neuropharmacological action (Morales et al., 2017; Prandi et al., 2018; Yousaf et al., 2022).
4.5.2 Apigenin
Apigenin is a flavonoid with several anti-inflammatory, antioxidant, and neurological effects (Salehi et al., 2019). Apigenin and its derivatives are obtained from several plants, such as fruits, vegetables, nuts, citrus, tea, chamomile, thyme, celery, and celeriac, in their glycosidic form (Ginwala et al., 2019). Shao et al. discovered that apigenin could alleviate myeloperoxidase-related oxidative stress and block the ferroptosis of neurological cells. The study developed a multifunctional brain-imaging fluorescence tool and explicated the role of HClO (endogenous hypochlorite) generation by myeloperoxidase in the physiology of epileptic seizures, thus inventing new antiepileptic agents for the prevention and treatment of epilepsy (Shao et al., 2020). The cognitive deficit, a common symptom in epilepsy, was treated with apigenin. Hashemi et al. concluded the biological role of this phytoconstituent in restoring memory deficiency (apigenin significantly increased the number of living neurons in the hilus), thus showing potent anticonvulsant and neuroprotective action (Hashemi et al., 2019).
4.6 Depression
Depression is a neurological condition that affects people of all ages worldwide. It is distinguished by emotional, behavioral, health, cognitive capabilities, and behavioral and sleep patterns (Wang et al., 2007). The family and medical history of the patient, early childhood traumas, brain anatomy, and drug consumption are all key contributing variables. Depression is the main cause of disability and a substantial contribution to illness, according to a new World Health Organization report. Multiple complicated biological processes are involved in the pathophysiology of depression (Duman and Voleti, 2012; Zhang et al., 2019). MAPK and cyclic adenosine phosphate signaling are globally accepted to be connected with depression progression, which has sparked much interest in antidepressant research (Pandey et al., 2013; Ekor, 2014). The traditional medical system, which is based on natural ingredients from numerous sources, provides a framework for several commercial depression treatments (Pan et al., 2021b; Álvarez et al., 2022). Metabolic extracts and metabolites derived from many medicinal plants have been shown to have antidepressant effects. In addition to leaves, flowers, and fruits (powdered or unripe), the metabolic extracts are generated from many plant components, such as stem bark, bulb (powdered), the whole plant (seed), petal (stigma), and rhizome (hypocotyl) (Singh et al., 2003; Fakhri et al., 2021; Ranjbar et al., 2022). Collectively, some researchers carried out antidepressant action or neuroprotective benefits by several methods that target the neurological signaling pathways or molecules responsible for depressive illnesses (Lu et al., 2022; Zarneshan et al., 2022). Natural compounds produced from various parts of the plants with a common mode of action are addressed in Table 2. This mechanism includes MAO (MAO-A and MAO-B) inhibitory activity and interactions with dopaminergic (D2), serotonergic, GABA (gamma-aminobutyric acid), adrenergic (α1), and noradrenergic receptor system interactions (Ekor, 2014).
TABLE 2
Different types of plants used for depression.
4.7 Anxiety
Anxiety disorders are common, incapacitating, frequently chronic, and very co-morbid conditions (Saha et al., 2022). Plant-based medications may provide an extra safe and useful option in addition to traditional pharmacotherapies and psychological therapy, which are the front-line techniques. The term “anxiolytics” refers to phytotherapeutic treatments that may be helpful for anxiety disorders. These treatments typically have effects on the GABA system (Sarris, 2007; Sarris and Kavanagh, 2009), either affecting ionic channel transmission through voltage-gated blocking, altering membrane architecture (Greenfield, 2022), or, less frequently, binding to benzodiazepine receptor sites (such as GABA-a) (Awad et al., 2007), inhibiting GABA transaminase or glutamic acid decarboxylase (Rastogi et al., 2016). Preclinical research in this field has been widely explored, especially by nations such as China, India, Brazil, the United States, Spain, and Germany. Over the past several decades, clinical studies have been undertaken on various plant-based medications for different anxiety and mood disorders. Preclinical research is essential because it frequently expands on existing knowledge of the traditional uses of plant medicines and informs possible human applications. Table 3 discusses medicinal plants used in clinical trials for anxiolytic effects.
TABLE 3
| Botanical name | Family | Active constituents | Neurochemical pathways | References |
|---|---|---|---|---|
| Achillea millefolium | Asteraceae | Flavonoids, sesquiterpene lactones, and dicaffeoylquinic acids | — | Nemeth and Bernath (2008), Baretta et al. (2012) |
| Aloysia polystachya | Verbenaceae | Thujone carvone | GABA | Mora et al. (2005), Hellion-Ibarrola et al. (2006) |
| Abies pindrow | Pinaceae | Terpenoids, flavonoids, and glycosides | — | Assad et al. (2021) |
| Albizia julibrissin | Fabaceae | Flavonoids and triterpenoid saponins | Serotonin, 5-HT1A | Kim et al. (2004), Jung et al. (2005) |
| Bacopa monnieri (Brahmi) | Plantaginaceae | Bacoside A | ACh, DA, NA, 5-HT | Stough et al. (2001), Calabrese et al. (2008), Charles et al. (2011), Pase et al. (2012) |
| Cannabis sativa/indica (marijuana) | Cannabaceae | Cannabidiol | Cannabinoid | Campos and Guimarães (2008), Resstel et al. (2009), Bergamaschi et al. (2011) |
| Citrus aurantium (bitter orange) | Rutaceae | Volatile oils and flavonoids | GABA | Akhlaghi et al. (2011), Saiyudthong and Marsden (2011) |
| Galphimia glauca | Malpighiaceae | Nor-seco-triterpene (galphimine B) | 5-HT | Herrera-Ruiz et al. (2006a), Herrera-Ruiz et al. (2006b), Herrera-Arellano et al. (2007), Jiménez-Ferrer et al. (2011), Herrera-Arellano et al. (2012) |
| Apocynum venetum | Apocynaceae | Flavonoids | GABA and 5-HT | Grundmann et al. (2007), Xie et al. (2007) |
| Crocus sativus | Iridaceae | Safranal, crocin, and picrocrocin | 5-HT, NE, DA, GLU, and GABA | Hosseinzadeh and Sadeghnia (2007), Schmidt et al. (2007), Pitsikas et al. (2008), Hosseinzadeh and Noraei (2009), Ghadrdoost et al. (2011) |
| Eschscholzia californica | Papaveraceae | Benzophenanthridine alkaloids | GABA | Rolland et al. (1991), Rolland et al. (2001), Klvana et al. (2006) |
| Euphorbia hirta | Euphorbiaceae | Alkaloids and phenolics | GABA | Lanhers et al. (1990), Anuradha et al. (2008) |
| Justicia spp. | Acanthaceae | Elenoside | GABA | Navarro et al. (2004), Venâncio et al. (2011) |
| Leea indica | Vitaceae | Triterpenoid glycosides, hydrocarbons, and ursolic acid | Srinivasan et al. (2008), Raihan et al. (2011) | |
| Panax ginseng | Araliaceae | Triterpenoid saponins (ginsenosides | Monoamines, HPA-axis, and BDNF | Dang et al. (2009), Jiang et al. (2021) |
| Ginkgo biloba | Ginkgoaceae | Ginkgolides | Dopamine, noradrenaline (norepinephrine) | Kuribara et al. (2003), Woelk et al. (2007), Fehske et al. (2009), Yoshitake et al. (2010) |
| Passiflora incarnata (passion flower) | Passifloraceae | Amino acids, chrysin, b-carboline alkaloids, and flavonoids | GABA | Akhondzadeh et al. (2001), Movafegh et al. (2008), Aslanargun et al. (2012) |
| Withania somnifera (ashwagandha) | Solanaceae | Glycowithanolides | GABA | Andrade et al. (2000) |
| Valeriana spp. (valerian) | Caprifoliaceae | Valerenic acid and valepotriates | Adenosine and GABA | Andreatini and Leite (1994), Andreatini et al. (2002), Benke et al. (2009), Nunes and Sousa (2011), Javan Gholiloo et al. (2019) |
| Turnera diffusa | Turneraceae | Flavonoids (apigenin) and essential oils | GABA | Kumar and Sharma (2005), Kumar et al. (2008) |
Various plant species used for anxiety.
4.8 Spinal cord injury
Mechanisms such as multiple cellular and molecular are activated by acute spinal cord injury (SCI). Su et al. inquired how effectively the Jisuikang (JSK), a traditional drug, works as a treatment in a rat model with established SCI. High-performance liquid chromatography in conjunction with photodiode array detection, electrospray ionization-mass spectrometry, and phytochemical fingerprinting of JSK was used. Additionally, JSK seems to target several pathways (biochemical and cellular) to promote functional recovery and enhance the results of SCI (Su et al., 2013; Islam et al., 2022). To evaluate the therapeutic effects of ethanolic extract of Mucuna pruriens (MP) in treating SCI, Chandran et al. used the widely researched standardized Multicenter Animal Spinal Cord Injury Study animal model of the contusive spinal cord. Additionally, MP, at equivalent dosages, was found to be very beneficial in reducing inflammation and/or oxidative stress in various disease circumstances (Rastogi, 2014).
5 Role of natural products as biomarkers in neuronal diseases
Using biomarkers of neurodegeneration and neuronal dysfunction can enhance the precision of diagnosis, the ability to track disease progression, prognosis, and the efficacy of therapeutic interventions. Neurological biomarkers are present in the CSF but rarely or at undetectable levels in the blood. Different proteins presented in the CSF, such as neurofilament proteins, tau, and tar DNA-binding protein (TDP-43), have been considerably applied markers to monitor the CNS activity (Viswambharan et al., 2017).
Natural substances have rarely been used as biomarkers in neurodegenerative disorders. However, many biomarkers have been utilized to disclose the molecular pathways of plant extracts for the therapy of NDDs. For example, plasma Aβ40 levels were used to detect the effect of curcumin on AD (Hardy and Selkoe, 2002; Baum et al., 2008). Aβ40 belongs to the βAPP gene, the first AD susceptibility gene found, which encodes a glycosylated transmembrane protein of 770 amino acids in its longest isoform. The amyloid cascade theory postulates that an increase in the production of the proteins would result from a mutation in the βAPP gene, with more of the protein eventually broken down to produce the poisonous β-amyloid peptides (Aβ) (Huang et al., 2014). Aβ was also used in a Huperzia serrata (Chinese herb) study in the treatment of AD. Cholinesterase inhibitor isolated from Huperzia serrata was reported to decrease levels of soluble and insoluble β-amyloid and amyloid plaques in AD mice (Ghodsi et al., 2022a; Mitra et al., 2022).
In the case of PD, α-synuclein aggregation has been used as a biomarker in various in vivo studies. Basically, α-synuclein gene is most commonly expressed on elongated arm of chromosome 4 and is a characteristic of PD and also leads to faster progression of the disease. They occur in most forms, including the rare early-onset familial form of PD. A study reported that curcumin extract prevented α-synuclein aggregation and fibrillation in animal models of PD (Bakhtiari et al., 2017).
6 Role of bioinformatic studies of plant metabolites in neuronal diseases
Several plants have been used in medicine for neuronal diseases since historical times, and some natural extracts have been developed to commercial medical products. The conventional method of the discovery of plant-based pharmaceuticals is frequently time-consuming and costly. The fast development of high-throughput technology has made it difficult for these labor-intensive methods to stay up. Bioinformatics is vital in the era of high-volume, high-throughput data creation in biosciences. In the realm of drug design and discovery, this has typically been the case. However, the potential use of bioinformatics techniques that can harness plant-based knowledge has received little attention so far. Bioinformatics research has benefited medicinal plant research. In medicinal plant research, the application of bioinformatics techniques leads to faster and potentially more cost-effective discoveries of plant-based treatments.
Most bioinformatic studies of plant metabolites in neuronal diseases have focused on flavonoids. Flavonoids are a family of phenolic substances. This group of phenolic substances has been reported to affect neuroprotection in AD (Mohebali et al., 2018; Sharma et al., 2021). Different side chains may considerably impact the biological activities of flavonoid subclasses, according to systematic correlations between fragments of the chemical structure and biological effects. Flavonoids might considerably enhance the pathways of HD and AD compared to other natural plant products. In addition, systemic examination of targets for various flavonoid subclasses revealed that targets such as MAPT, APEX1, and ALDH1A1, which are strongly associated with the nervous system, were considerably enriched in nearly all flavonoid subclasses. In this situation, the flavonoid multimodal therapeutic potential suggests their value in nervous system medication discovery (Qiu et al., 2018).
7 Limitations
Therapeutic efficacy in human patients remains uncertain and limited, although natural products or plant extracts with antioxidant activity have shown excellent efficacy in in vitro and in vivo animal models. This might be attributed in part to the fact that most clinical studies focus on single compounds. In contrast, plant extracts containing a range of secondary metabolites are more commonly investigated in studies preceding clinical trials. The combination of several active components in extracts can have additive or synergistic effects, resulting in enhanced antioxidant or disease-modifying activities. In addition, clinical trials examine a wide range of subjects with various environmental and genetic origins, as well as various illness symptoms and, in some cases, disease stages. It can be interesting to look at specific people or small groups who show substantial improvement rather than the overall importance of the entire participant population to see why some respond to the treatment and others do not. Furthermore, most clinical studies on natural antioxidants (i.e., natural products or plant extracts) have focused on behavioral or cognitive improvements in patients. In contrast, relatively few trials have properly examined molecular signs of sickness or oxidative stress (Pohl and Kong Thoo Lin, 2018).
8 Patent overview
Varied medicinal plant species have been explored in neuronal disorders in the conventional system of natural medicines, and interestingly, unknown species are yet to be scientifically explored. The emphasis on research in the field of herbal compounds in neurological disorders expanded after phytoconstituents were used as a basis for the human treatment of several neurological disorders (Table 4). Ravid et al. formulated a combination of Uncaria rhynchophylla herb and an antidepressant or anxiolytic drug therapy for treating or preventing anxiety, stress, depression, and/or symptoms. The combinations, therefore, elicit fast on-set responses in patients (Ravid, 2022). Ichim et al. formulated a nutraceutical of green tea extract and/or Nigella sativa, pterostilbene, and/or sulforaphane to overcome treatment resistance of the currently used antidepressants (Thomas et al., 2022). Thamaraikanet et al. prepared a phytochemical extract containing indole alkaloids. Camalexin in aldehyde dehydrogenases mediated benomyl-induced PD. The formulation provides a suitable multi-targeted molecule with antioxidant, neuroprotective, and minimal side-effect properties that can be used as an anti-PD drug (Manasa et al., 2022). Sudhakara Sastry et al. formulated a therapeutically effective nano-polyherbal composition comprising herbal extracts, such as Allium sativum, Bacopa monniera, Citrus lemon, Citrus sinensis, Curcuma longa, Cyperus rotundus, Lycopersicon esculentum L., Mucuna pruriens, Nardostachys jatamansi, Nigella sativa, Prunus dulcis, Psidium guajava, Sesame indicum, Vicia faba, Vitis vinifera, Withania somnifera, and Zingiber officinale using the phytonanoceutics method, thereby enhancing high bio-efficacy fortified in quality. The composition provides an alternative treatment option for subjects suffering from neurological disorders, anxiety, and/or management of related complications without any side effects (AmanchiBala et al., 2021). Mohanty et al. isolated an anticonvulsant drug from Cucurbita maxima and tested it in a convulsion-based animal assay. The pre-treatment with this water–alcohol extract was given biweekly and later exposed to induced electroshock seizures at optimized conditions, and it proved to be effective for electroshock-induced convulsions in rats (Kumar and Nagnath, 2021). Kodimule formulated a composition containing chlorogenic acid and sunflower seed extract in AD (Kodimule, 2021). Palkar and Prasad formulated a synergistic mixture of celery-based extract and various pharmaceutical excipients in brain stroke in different ratios (1:0.1 to 1:5) (Palkar and Prasad, 2021). Vaijanath et al. formulated a Wedelolactone Nasal Formulation. This formulation is made for the nasal drug delivery system to achieve its brain bioavailability for treating or preventing seizures or epilepsy (Vaijnath and Suraj, 2019). Chaudhary et al. formulated a water-soluble extract of Alpinia galanga for improving mental alertness and sustaining attention in humans (Chaudhary et al., 2021).
TABLE 4
| Patent no. | Invention | Applicant | Date of publication | References |
|---|---|---|---|---|
| WO/2022/123572 | “A combination therapy comprising uncaria for treating anxiety and depression” | The Open University | 16.06.2022 | Ravid (2022) |
| US20220175701 | “Treatment of major depressive disorder and suicidal ideations through stimulation of hippocampal neurogenesis utilizing plant-based approaches” | Therapeutic Solutions International, Inc. | 09.06.2022 | Thomas et al. (2022) |
| IN202141020016 | “Phytochemical extract containing indole alkaloid camalexin for management of benomyl-induced Parkinson’s disease” | Dr. Tamilanban Thamaraikani | 11.03.2022 | Manasa et al. (2022) |
| IN201941028495 | “A synergistic nanopolyherbal formulation for Parkinson’s disease” | Srimaharshi Research Institute of Vedic Technology | 22.01.2021 | AmanchiBala et al. (2021) |
| IN202121057739 | “Isolation and identification of suitable anticonvulsant drug from Curcurbita maxima” | Dr. Pradeep Kumar Mohanty Nagnath Ramrao Kadam | 24.12.2021 | Kumar and Nagnath (2021) |
| US20210330627 | “Method of using a chlorogenic acid composition for supporting cognitive function” | Vidya Herbs, Inc. | 28.10.2021 | Kodimule (2021) |
| WO/2021/084559 | “Synergistic nutritional compositions for treating cerebrovascular diseases” | Celagenex Research (India) Pvt. Ltd. | 06.05.2021 | Palkar and Prasad (2021) |
| IN201921009898 | “Development and evaluation of wedelolactone nasal formulation for antiepileptic activity” | Sathaye Sadhana Vaijanath | 18.09.2020 | Vaijnath and Suraj (2019) |
| US20210205400A1 | “Formulation containing an extract of Alpinia galanga, a process for the preparation thereof, and uses thereof” | Enovate Biolife Pvt. Ltd. | 16.03.2021 | Chaudhary et al. (2021) |
List of different patents on different phytoconstituents for neurological disorders.
9 Clinical research
Recently, clinical trial reports manifested that mild-to-moderate dementia patients have been cured by employing naturally originated therapeutics (Yiannopoulou and Papageorgiou, 2013). Both studies including clinical trials for test scores and randomized trial for 30 weeks placebo study, were restricted due to resulting hepatotoxicity (Alfirevic et al., 2007). Berberine, another phytoconstituent, displayed symptoms including constipation, diarrhea, bloating, and stomach pain in human subjects with type 2 diabetes (Yin et al., 2008). In a short-term study based on resveratrol, its repeated dose revealed no major adverse effects, but nearly 13% of the individuals had a frontal headache as a side effect (Shaito et al., 2020). In another phase III trial, cholinesterase inhibitors, including galantamine, donepezil, and rivastigmine, were observed to have a lesser memory-enhancing effect, and side effects, including vomiting, nausea, diarrhea, sleeplessness, muscular spasm, loss of fatigue, and loss of hunger, were observed in severe AD subjects (https://clinicaltrials.gov/ct2/show/NCT02035982). In recent report findings, the investigated anti-AD drugs have been excluded based on approximately 200 clinical trials because of inefficacy and toxicity (Mo et al., 2018). Amyloid blockers have not been marketed yet, although they undergo clinical testing (Huang et al., 2020). Toxicity has been reported, Commercialization of such drugs is constrained by concerns of toxicity, but scientists are discovering a novel pharmacological entity with natural existence (Cummings et al., 2021). Indeed, the multitargeting approach by natural agents observes enhanced safety and potentially cognitive modulating abilities, thus contributing to remarkable efficacious compounds (Sartori and Singewald, 2019). Many clinical observations are available in the form of case reports or preliminary clinical trials, which provide essential clinical leads for the initiation of any serious clinical trial in the related area on the background of experimental studies. Interestingly, Ghodsi et al. designed a randomized, triple-blind, placebo-controlled study and evaluated curcumin in 30 idiopathic PD patients and 30 placebo groups as an add-on therapy at 80 mg/kg dose for 9 months. The movement disorder society revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part-III was p = 0.04, exhibiting a significant difference in patient groups, and nausea and vomiting with p = 0.25 and gastroesophageal reflux with p = 0.42 were side effects (Ghodsi et al., 2022b). Recently, Wang et al. examined a systematic and meta-analysis overview of the accessible preclinical data and plausible mechanisms of baicalein based on in vivo PD studies. Twenty different studies were implied, and the data analysis observed that baicalein can enhance neuroprotective action such as instant motor activity (n = 5), pole (n = 2), rotarod (n = 9), apomorphine-induced rotations (n = 4), grid (n = 2), and tremor (n = 2) tests in comparison to control. The study reported multi-signaling pathways, including neurotransmitter modulation, modifying enzyme activity, relieving oxidative stress, blocking protein aggregation, and further restricting apoptosis (Wang et al., 2020). In another retrospective trial, the pharmacological effect of the artisanal oil formulation of cannabidiol was investigated for epilepsy among 108 pediatric populations. The study observed that 39% of patients showed a major decrease in seizures (more than 50%), and 10% showed no seizures. In contrast, 44% patients exhibited a 50% reduction compared to the 33% with only cannabidiol in the group that was observed receiving combination therapy with cannabidiol and clobazam. The overall results exhibited better alertness and enhanced verbal communication in cannabidiol patients in comparison to the cannabidiol and clobazam patient group, which also showed sedation as its side effect (non-statistically significant difference) (Porcari et al., 2018).
There are few and conflicting pharmacological and clinical studies on the effectiveness of traditional Chinese systems and herbal mixtures in AD. Concerns with irreproducibility may result from this incapacity to deal with uncertainty. Consequently, due to their natural occurrence, promising drug delivery to the brain, and lower adverse effects, the complexes of nanoparticles and herbal plants or their constituents called nano-phytomedicine have currently become essential in the progression of novel neuro-therapeutics. Nanotheranostics is a strategy attracting much interest worldwide for the management of neurodegenerative disorders. Nanoformulations are used in management and diagnosis at the same time. Researchers have created a revolutionary nanotheranostic system that reflects the utilization of nanoparticles and expands the potential applications in this field (Bhattacharya et al., 2022). Toward this direction, Noor et al. established curcumin-based intracerebroventricular injection at a sub-diabetogenic dose of streptozotocin for AD. Curcumin ameliorated the behavioral, immunohistochemical, and most of the neurochemical alterations induced by streptozotocin in the hippocampus and cortex portion, thus showing prospects for brain drug delivery. Thuraisingam et al. formulated nanoemulsions containing Centella asiatica crude extract to penetrate the blood–brain barrier using the low-energy emulsification method, showing promising results against epilepsy. Junior et al. compared nanoemulsions of curcumin with free curcumin through an experimental model for PD. The study concluded that curcumin-loaded nanoemulsions and free curcumin enhanced motor impairment decreased lipoperoxidation, modified antioxidant protection, and inhibited the formation of complex I (Ramires Júnior et al., 2021; Noor et al., 2022; Thuraisingam et al., 2022).
10 Conclusion
In summary, medicinal plants constitute a significant reservoir of various bioactive ingredients. The implementation of effective multi-targeted drugs for the treatment and prevention of various diseases, including neurological disorders, may result from ethnopharmacology-focused studies that provide a scientific basis for the effective dose and promising toxicological effects on the local community. The key insight is that natural products may hold enormous therapeutic potential for varied neurological diseases as conventional treatments, including synthetic medications, only aim to relieve symptoms and are completely inadequate because they cannot arrest the evolution of the diseased condition. However, the uncertainties regarding the effectiveness and efficacy of several natural products present a challenge. A lot still needs to be studied, described, and discovered. The chemical modification of natural phytoconstituents and molecular docking of those compounds may improve the potency and efficacy of natural products. Thus, to improve patient safety and ethical treatment, clinicians must frequently investigate the employability of all products, such as conventional, complementary, and alternative. Furthermore, experts should deliberately begin to increase scientific understanding of the efficacy and safety of natural products, underlining the need for fundamental research to enhance scientific understanding of the fundamental biological mechanisms. The best sources of novel therapeutics and active frameworks are still natural products. When synthetic and biological chemists collaborate on these case studies, novel structures with the potential to treat a range of human diseases can be investigated.
11 Future prospectives
Pain associated with neurodevelopmental disorders and neurodegenerative diseases are common, as are conditions which includes Parkinson’s disease (PD), dementia, epilepsy, and neuro infections caused by malnutrition. The pharmacological properties of medicinal plants have been effective in treating various neurological conditions. Although many different types of plants are available globally, only a few have been researched for neurological problems. Therefore, there are several chances for more exploration of botanicals and their bioactive compounds in this field. In recent years, there has been an increase in interest in natural alternative treatments that encourage fast recovery and avoid side effects. The use of natural compounds in alternative and complementary therapies may result in the identification of novel drug lead compounds. The use of natural compounds to treat neurodegenerative illnesses has gradually become a growing industry. In addition to providing a scientific foundation for the ideal dose and potential toxicological effects on the local community, pharmacological studies can aid in the development of even more effective therapeutically multi-targeted natural compounds for the treatment of various neurological disorders.
Statements
Author contributions
Conceptualization: VP and DD; writing—original draft preparation: all authors; writing—review and editing: VP, DD, and TS; funding acquisition: TS. All authors read and agreed to the published version of the manuscript.
Acknowledgments
The authors are thankful to Ashok Chitkara, Chancellor, Chitkara University, Himachal Pradesh (HP), India; Madhu Chitkara, Pro-Chancellor, Chitkara University, HP, India; Varinder Kanwar, Vice-Chanceller, Chitkara University, HP, India, and Nitin Verma, Principal, School of Pharmacy, Chitkara University, HP, India, for constant encouragement and providing necessary facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AboulwafaM. M.YoussefF. S.GadH. A.AltyarA. E.Al-AziziM. M.AshourM. L. (2019). A comprehensive insight on the health benefits and phytoconstituents of Camellia sinensis and recent approaches for its quality control. Antioxidants8, 455. 10.3390/antiox8100455
2
AbrahamsS.MillerH. C.LombardC.van der WesthuizenF. H.BardienS. (2021). Curcumin pre-treatment may protect against mitochondrial damage in LRRK2-mutant Parkinson’s disease and healthy control fibroblasts. Biochem. Biophys. Rep.27, 101035. 10.1016/j.bbrep.2021.101035
3
AbreuT. M.CorpeF. P.TelesF. B.da ConceiçãoRivanorR. L.de SousaC. N.da Silva MedeirosI.et al (2022). Lectin isolated from the red marine alga Solieria filiformis (kützing) PW gabrielson: Secondary structure and antidepressant-like effect in mice submitted to the lipopolysaccharide-induced inflammatory model of depression. Algal Res.65, 102715. 10.1016/j.algal.2022.102715
4
Abu-IzneidT.RaufA.KhalilA. A.OlatundeA.KhalidA.AlhumaydhiF. A.et al (2022). Nutritional and health beneficial properties of saffron (Crocus sativus L.): A comprehensive review. Crit. Rev. Food Sci. Nutr.62, 2683–2706. 10.1080/10408398.2020.1857682
5
AditiK.SinghA.ShakaradM. N.AgrawalN. (2022). Management of altered metabolic activity in Drosophila model of Huntington’s disease by curcumin. Exp. Biol. Med.247, 152–164. 10.1177/15353702211046927
6
AkbarM.ShabbirA.RehmanK.AkashM. S. H.ShahM. A. (2021). Neuroprotective potential of berberine in modulating Alzheimer’s disease via multiple signaling pathways. J. Food Biochem.45, e13936. 10.1111/jfbc.13936
7
AkhlaghiM.ShabanianG.Rafieian-KopaeiM.ParvinN.SaadatM.AkhlaghiM. (2011). Citrus aurantium blossom and preoperative anxiety. Rev. Bras. Anestesiol.61, 702–712. 10.1016/S0034-7094(11)70079-4
8
AkhondzadehS.NaghaviH. R.VazirianM.ShayeganpourA.RashidiH.KhaniM. (2001). Passionflower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J. Clin. Pharm. Ther.26, 363–367. 10.1046/j.1365-2710.2001.00367.x
9
AlfirevicA.MillsT.CarrD.BarrattB. J.JawaidA.SherwoodJ.et al (2007). Tacrine-induced liver damage: An analysis of 19 candidate genes. Pharmacogenet. Genomics17, 1091–1100. 10.1097/FPC.0b013e3282f1f12b
10
AliB. H.BashirA. K.TaniraM. O.MedvedevA. E.JarrettN.SandlerM.et al (1998). Effect of extract of Rhazya stricta, a traditional medicinal plant, on rat brain tribulin. Pharmacol. Biochem. Behav.59, 671–675. 10.1016/S0091-3057(97)00464-4
11
AliB. H.BashirA. K.TaniraM. O. (1998). The effect of Rhazya stricta Decne, a traditional medicinal plant, on the forced swimming test in rats. Pharmacol. Biochem. Behav.59, 547–550. 10.1016/S0091-3057(97)00470-X
12
AliakbariF.Mohammad-BeigiH.Rezaei-GhalehN.BeckerS.EsmatabadF. D.SeyediH. A. E.et al (2018). The potential of zwitterionic nanoliposomes against neurotoxic alpha-synuclein aggregates in Parkinson’s disease. Nanoscale10, 9174–9185. 10.1039/C8NR00632F
13
ÁlvarezS. A.Rocha-GuzmánN. E.González-LaredoR. F.Gallegos-InfanteJ. A.Moreno-JiménezM. R.Bravo-MuñozM. (2022). Ancestral food sources rich in polyphenols, their metabolism, and the potential influence of gut microbiota in the management of depression and anxiety. J. Agric. Food Chem.70, 944–956. 10.1021/acs.jafc.1c06151
14
AmanchiBalaS. S.UpadhyayalaN.Ganga ModN. V. (2021). “A synergistic nano polyherbal formulation for Parkinson disorder,”. IN201941028495.
15
AmroM. S.TeohS. L.NorzanaA. G.SrijitD. (2018). The potential role of herbal products in the treatment of Parkinson’s disease. Clin. Ter.169, 23–33. 10.7417/T.2018.2050
16
AnakorE.MillaV.ConnollyO.MartinatC.PradatP. F.DumonceauxJ.et al (2022). The neurotoxicity of vesicles secreted by ALS patient myotubes is specific to exosome-like and not larger subtypes. Cells11, 845. 10.3390/cells11050845
17
AndradeC.AswathA.ChaturvediS. K.SrinivasaM.RaguramR. (2000). A double-blind, placebo-controlled evaluation of the anxiolytic efficacy ff an ethanolic extract of Withania somnifera. Indian J. Psychiatry42, 295–301.
18
AndreatiniR.LeiteJ. (1994). Effect of valepotriates on the behavior of rats in the elevated plus-maze during diazepam withdrawal. Eur. J. Pharmacol.260, 233–235. 10.1016/0014-2999(94)90342-5
19
AndreatiniR.SartoriV. A.SeabraM. L.LeiteJ. R. (2002). Effect of valepotriates (valerian extract) in generalized anxiety disorder: A randomized placebo-controlled pilot study. Phytother. Res.16, 650–654. 10.1002/ptr.1027
20
AngelucciF.CechovaK.ValisM.KucaK.ZhangB.HortJ. (2019). MicroRNAs in alzheimer’s disease: Diagnostic markers or therapeutic agents?Front. Pharmacol.10, 665. 10.3389/fphar.2019.00665
21
AnkireddyS. R.KimJ. (2015). Selective detection of dopamine in the presence of ascorbic acid via fluorescence quenching of InP/ZnS quantum dots. Int. J. Nanomedicine10, 113–119. 10.2147/IJN.S88388
22
AnuradhaH.SrikumarB. N.RaoS.LakshmanaM. (2008). Euphorbia hirta reverses chronic stress-induced anxiety and mediates its action through the GABAA receptor benzodiazepine receptor-Cl− channel complex. J. Neural Transm.115, 35–42. 10.1007/s00702-007-0821-6
23
AraújoJ. R.de MeloJ. D.DamascenoM. D.SantosS. A.Vieira-NetoA. E.LoboM. D.et al (2018). Neuropharmacological characterization of frutalin in mice: Evidence of an antidepressant-like effect mediated by the NMDA receptor/NO/cGMP pathway. Int. J. Biol. Macromol.112, 548–554. 10.1016/j.ijbiomac.2018.01.180
24
ArnoldS., J.DuggerB. N.BeachT. G. (2013). TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: Correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol.126, 51–57. 10.1007/s00401-013-1110-0
25
AslanargunP.CuvasO.DikmenB.AslanE.YukselM. U. (2012). Passiflora incarnata Linneaus as an anxiolytic before spinal anesthesia. J. Anesth.26, 39–44. 10.1007/s00540-011-1265-6
26
AssadR.ReshiZ. A.MirS. H.RashidI.ShoucheY.DhotreD. (2021). Bioprospecting appraisal of Himalayan pindrow fir for pharmacological applications. Phytomedicine Academic Press, 461–482. 10.1016/B978-0-12-824109-7.00003-0
27
AwadR.LevacD.CybulskaP.MeraliZ.TrudeauV. L.ArnasonJ. T. (2007). Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol.85, 933–942. 10.1139/Y07-083
28
AyazM.SadiqA.JunaidM.UllahF.OvaisM.UllahI.et al (2019). Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci.11, 155. 10.3389/fnagi.2019.00155
29
BakhtiariM.PanahiY.AmeliJ.DarvishiB. (2017). Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed. Pharmacother.93, 218–229. 10.1016/j.biopha.2017.06.010
30
BallN.TeoW. P.ChandraS.ChapmanJ. (2019). Parkinson's disease and the environment. Front. Neurol.218, 218. 10.3389/fneur.2019.00218
31
BanerjeeC.NandyS.ChakrabortyJ.KumarD. (2022). Myricitrin–a flavonoid isolated from the Indian olive tree (Elaeocarpus floribundus)–inhibits monoamine oxidase in the brain and elevates striatal dopamine levels: Therapeutic implications against Parkinson's disease. Food Funct.13, 6545–6559. 10.1039/D2FO00734G
32
BarettaI. P.FelizardoR. A.BimbatoV. F.dos SantosM. G.KassuyaC. A.JuniorA. G.et al (2012). Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J. Ethnopharmacol.140, 46–54. 10.1016/j.jep.2011.11.047
33
BaumL.LamC. W. K.CheungS. K.-K.KwokT.LuiV.TsohJ.et al (2008). Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol.28, 110–113. 10.1097/jcp.0b013e318160862c
34
BazyarH.HosseiniS. A.SaradarS.MombainiD.AllivandM.LabibzadehM.et al (2021). Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med.18, 405–411. 10.1515/jcim-2020-0090
35
BenkeD.BarberisA.KoppS.AltmannK. H.SchubigerM.VogtK. E.et al (2009). GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology56, 174–181. 10.1016/j.neuropharm.2008.06.013
36
BergamaschiM. M.QueirozR. H.ChagasM. H.De OliveiraD. C.De MartinisB. S.KapczinskiF.et al (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology36, 1219–1226. 10.1038/npp.2011.6
37
BhattacharyaT.SoaresG. A.ChopraH.RahmanM. M.HasanZ.SwainS. S.et al (2022). Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Materials15, 804. 10.3390/ma15030804
38
BonokwaneM. B.LekhooaM.StruwigM.AremuA. O. (2022). Antidepressant effects of south african plants: An appraisal of ethnobotanical surveys, ethnopharmacological and phytochemical studies. Front. Pharmacol.13, 895286. 10.3389/fphar.2022.895286
39
BrochardV.CombadiereB.PrigentA.LaouarY.PerrinA.Beray-BerthatV.et al (2009). Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest.119, 182–192. 10.1172/JCI36470
40
ButterweckV.NishibeS.SasakiT.UchidaM. (2001). Antidepressant effects of Apocynum venetum leaves in a forced swimming test. Biol. Pharm. Bull.24, 848–851. 10.1248/bpb.24.848
41
Cáceres GuidoP.RivaN.CaraballoR.ReyesG.HuamanM.GutierrezR.et al (2021). Pharmacokinetics of cannabidiol in children with refractory epileptic encephalopathy. Epilepsia62, e7–e12. 10.1111/epi.16781
42
CalabreseC.GregoryW. L.LeoM.KraemerD.BoneK.OkenB. (2008). Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: A randomized, double-blind, placebo-controlled trial. J. Altern. Complement. Med.14, 707–713. 10.1089/acm.2008.0018
43
CallahanB. L.RamakrishnanN.ShammiP.BierstoneD.TaylorR.OzzoudeM.et al (2022). Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J. Atten. Disord.26, 1118–1129. 10.1177/10870547211060546
44
CamposA. C.GuimarãesF. S. (2008). Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology199, 223–230. 10.1007/s00213-008-1168-x
45
ChainoglouE.Hadjipavlou-LitinaD. (2020). Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci.21, 1975. 10.3390/ijms21061975
46
ChandranP.ChandramohanK.IyerK.MichaelF. M.SeppanP.VenkatachalamS. (2022). Beneficial effects of ethanolic extract of the medicinal herb Mucuna pruriens against oxidative stress and inflammation might be limited in contusive spinal cord injury. Biomed. Pharmacol. J.15, 235–248. 10.13005/bpj/2359
47
CharlesP. D.AmbigapathyG.GeraldineP.AkbarshaM. A.RajanK. E. (2011). Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: Implications in memory formation. J. Ethnopharmacol.134, 55–61. 10.1016/j.jep.2010.11.045
48
ChaudharyJ.ChaudharyL.DigheS.SrivastvaS. (2021). “Formulation containing an extract of Alpinia galanga, a process for the preparation thereof, and uses thereof,”. US20210205400A1.
49
ChenG. F.XuT. H.YanY.ZhouY. R.JiangY.MelcherK.et al (2017). Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin.38, 1205–1235. 10.1038/aps.2017.28
50
ChenY.ChenY.LiangY.ChenH.JiX.HuangM. (2020). Berberine mitigates cognitive decline in an Alzheimer’s disease mouse model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother.121, 109670. 10.1016/j.biopha.2019.109670
51
ChengM. C.LiC. Y.KoH. C.KoF. N.LinY. L.WuT. S. (2006). Antidepressant principles of the roots of Polygala tenuifolia. J. Nat. Prod.69, 1305–1309. 10.1021/np060207r
52
ChinrajV.RamanS. (2022). Neuroprotection by resveratrol: A review on brain delivery strategies for alzheimer’s and Parkinson’s disease. J. Appl. Pharm. Sci.12, 001–017. 10.7324/JAPS.2022.120701
53
CicconeL.VandoorenJ.NencettiS.OrlandiniE. (2021). Natural marine and terrestrial compounds as modulators of matrix metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer's disease. Pharm. (Basel, Switz.14, 86. 10.3390/ph14020086
54
CraggG. M.GrothausP. G.NewmanD. J. (2009). Impact of natural products on developing new anti-cancer agents. Chem. Rev.109, 3012–3043. 10.1021/cr900019j
55
CraggG. M.NewmanD. J. (2002). Chemical diversity: A function of biodiversity. Trends Pharmacol. Sci.23, 404–405. 10.1016/s0165-6147(02)02099-0
56
CuiC.YangM.YaoZ.CaoB.LuoZ.XuY.et al (1995). [Antidepressant active constituents in the roots of Morinda officinalis How]. China J. Chin. Mater. Medica.20, 36–63.
57
CummingsJ.LeeG.ZhongK.FonsecaJ.TaghvaK. (2021). Alzheimer's disease drug development pipeline: 2021. Alzheimers Dement.7, e12179. 10.1002/trc2.12179
58
DangH.ChenY.LiuX.WangQ.WangL.JiaW.et al (2009). Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry33, 1417–1424. 10.1016/j.pnpbp.2009.07.020
59
DevinskyO.CilioM. R.CrossH.Fernandez-RuizJ.FrenchJ.HillC.et al (2014). Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia55, 791–802. 10.1111/epi.12631
60
DhingraD.GoyalP. K. (2008). Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of Tinospora cordifolia in mice. Indian J. Pharm. Sci.70, 761–767. 10.4103/0250-474X.49118
61
DhingraD.JoshiP. (2012). Antidepressant-like activity of Benincasa hispida fruits in mice: Possible involvement of monoaminergic and GABAergic systems. J. Pharmacol. Pharmacother.3, 60–62. 10.4103/0976-500X.92521
62
DhingraD.JoshiP.GuptaA.ChhillarR. (2012). Possible involvement of monoaminergic neurotransmission in antidepressant-like activity of Emblica officinalis fruits in mice. CNS Neurosci. Ther.18, 419–425. 10.1111/j.1755-5949.2011.00256.x
63
DhingraD.KumarV. (2007). Pharmacological evaluation for antidepressant-like activity of Asparagus racemosus Wild. in mice. Pharmacologyonline3, 133–152. 10.1007/s40495-022-00300-0
64
DhingraD.SharmaA. (2006). Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry30, 449–454. 10.1016/j.pnpbp.2005.11.019
65
DomenighettiC.DouillardV.SugierP. E.SreelathaA. A.SchulteC.GroverS.et al (2022). The interaction between HLA-DRB1 and smoking in Parkinson's disease revisited. Mov. Disord.37, 1929–1937. 10.1002/mds.29133
66
DuanQ.JingZ.ZouX.WangY.YangK.ZhangT.et al (2020). Spiking neurons with spatiotemporal dynamics and gain modulation for monolithically integrated memristive neural networks. Nat. Commun.11, 1–13. 10.1038/s41467-020-17215-3
67
DuanZ.LiA.GongH.LiX. (2020). A whole-brain map of long-range inputs to GABAergic interneurons in the mouse caudal forelimb area. Neurosci. Bull.36, 493–505. 10.1007/s12264-019-00458-6
68
DumanR. S.VoletiB. (2012). Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci.35, 47–56. 10.1016/j.tins.2011.11.004
69
EkorM. (2014). The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.4, 177. 10.3389/fphar.2013.00177
70
EsselunC.DieterF.SusN.FrankJ.EckertG. P. (2022). Walnut oil reduces Aβ levels and increases neurite length in a cellular model of early alzheimer disease. Nutrients14, 1694. 10.3390/nu14091694
71
EttehadiH.MojabiS. N.RanjbaranM.ShamsJ.SahraeiH.HedayatiM.et al (2013). Aqueous extract of saffron (Crocus sativus) increases brain dopamine and glutamate concentrations in rats. J. Behav. Brain Sci.3, 315–319. 10.4236/jbbs.2013.33031
72
FakhriS.IranpanahA.GravandiM. M.MoradiS. Z.RanjbariM.MajnooniM. B.et al (2021). Natural products attenuate PI3K/Akt/mTOR signaling pathway: A promising strategy in regulating neurodegeneration. Phytomedicine.91, 153664. 10.1016/j.phymed.2021.153664
73
FanZ. Z.ZhaoW. H.GuoJ.ChengR. F.ZhaoJ. Y.YangW. D.et al (2012). Antidepressant activities of flavonoids from Glycyrrhiza uralensis and its neurogenesis protective effect in rats. Yao xuexue bao=Acta Pharm.Sin. B47, 1612–1617. 23460966.
74
FantacuzziM.AmorosoR.CarradoriS.De FilippisB. (2022). Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur. J. Med. Chem.233, 114242. 10.1016/j.ejmech.2022.114242
75
FehskeC. J.LeunerK.MüllerW. E. (2009). Ginkgo biloba extract (EGb761®) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol. Res.60, 68–73. 10.1016/j.phrs.2009.02.012
76
FieldsC.BischofJ.LevinM. (2020). Morphological coordination: A common ancestral function unifying neural and non-neural signaling. Physiology35, 16–30. 10.1152/physiol.00027.2019
77
FieldsC. R.Bengoa-VergnioryN.Wade-MartinsR. (2019). Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front. Mol. Neurosci.12, 299. 10.3389/fnmol.2019.00299
78
FuloriaS.YusriM. A.SekarM.GanS. H.RaniN. N.LumP. T.et al (2022). Genistein: A potential natural lead molecule for new drug design and development for treating memory impairment. Molecules27, 265. 10.3390/molecules27010265
79
GadagaL. L.TagwireyiD.DzangareJ.NhachiC. F. (2011). Acute oral toxicity and neurobehavioural toxicological effects of hydroethanolic extract of Boophone disticha in rats. Hum. Exp. Toxicol.30, 972–980. 10.1177/0960327110384524
80
GaoH.LeiX.YeS.YeT.HuaR.WangG.et al (2022). Genistein attenuates memory impairment in Alzheimer's disease via ERS-mediated apoptotic pathway in vivo and in vitro. J. Nutr. Biochem.109, 109118. 10.1016/j.jnutbio.2022.109118
81
GaurV.BodhankarS. L.MohanV.ThakurdesaiP. (2012). Antidepressant-like effect of 4-hydroxyisoleucine from Trigonella foenum graecum L. seeds in mice. Biomed. Aging Pathology2, 121–125. 10.1016/j.biomag.2012.07.002
82
GeorgeJ.NihalM.SinghC. K.AhmadN. (2019). 4′-Bromo-resveratrol, a dual Sirtuin-1 and Sirtuin-3 inhibitor, inhibits melanoma cell growth through mitochondrial metabolic reprogramming. Mol. Carcinog.58, 1876–1885. 10.1002/mc.23080
83
GhadrdoostB.VafaeiA. A.Rashidy-PourA.HajisoltaniR.BandegiA. R.Motamedi FhaghighiS.et al (2011). Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol.667, 222–229. 10.1016/j.ejphar.2011.05.012
84
GhodsiH.RahimiH. R.AghiliS. M.SaberiA.ShoeibiA. (2022). Evaluation of curcumin as add-on therapy in patients with Parkinson's disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin. Neurol. Neurosurg.218, 107300. 10.1016/j.clineuro.2022.107300
85
GhodsiH.RahimiH. R.AghiliS. M.SaberiA.ShoeibiA. (2022). Evaluation of curcumin as add-on therapy in patients with Parkinson's disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin. Neurol. Neurosurg.18, 107300. 10.1016/j.clineuro.2022.107300
86
GinwalaR.BhavsarR.ChigbuD. G. I.JainP.KhanZ. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants8, 35. 10.3390/antiox8020035
87
GirishC.RajV.AryaJ.BalakrishnanS. (2012). Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol.682, 118–125. 10.1016/j.ejphar.2012.02.034
88
GoldC. A.BudsonA. E. (2008). Memory loss in alzheimer’s disease: Implications for development of therapeutics. Expert Rev. Neurother.8, 1879–1891. 10.1586/14737175.8.12.1879
89
GoldenbergM. M. (2010). Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. P Trans.35, 392–415. 20689626.
90
GonzalezJ.Jurado-CoronelJ. C.AvilaM. F.SabogalA.CapaniF.BarretoG. E. (2015). NMDARs in neurological diseases: A potential therapeutic target. Int. J. Neurosci.125, 315–327. 10.3109/00207454.2014.940941
91
Gorina-CaretaN.KurkelaJ. L.HämäläinenJ.AstikainenP.EsceraC. (2021). Neural generators of the frequency-following response elicited to stimuli of low and high frequency: A magnetoencephalographic (MEG) study. Neuroimage231, 117866. 10.1016/j.neuroimage.2021.117866
92
GoudaN. A.ElkamhawyA.ChoJ. (2022). Emerging therapeutic strategies for Parkinson’s disease and future prospects: A 2021 update. Biomedicines10, 371. 10.3390/biomedicines10020371
93
GrayR. A.WhalleyB. J. (2020). The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord.22, 10–15. 10.1684/epd.2020.1135
94
GreenfieldD. P. (2022). “Basic principles of pharmacology, psychopharmacology, and psychopharmacotherapy,” in Psychopharmacology for NonpsychiatristsSpringer (Cham, 9–25. 10.1007/978-3-030-82507-2_2
95
GrewalA. K.SinghT. G.SharmaD.SharmaV.SinghM.RahmanM. H.et al (2021). Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother.140, 111729. 10.1016/j.biopha.2021.111729
96
GrundmannO.NakajimaJ. I.SeoS.ButterweckV. (2007). Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J. Ethnopharmacol.110, 406–411. 10.1016/j.jep.2006.09.035
97
GuoP.BenitoBallesteros A.YeungS. P.LiuR.SahaA.CurtisL.et al (2022). Covcog 2: Cognitive and memory deficits in long COVID: A second publication from the Covid and cognition study. Front. Aging Neurosci.14, 804937. 10.3389/fnagi.2022.804937
98
GuptaS. C.PatchvaS.AggarwalB. B. (2013). Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J.15, 195–218. 10.1208/s12248-012-9432-8
99
HadrichF.ChamkhaM.SayadiS. (2022). Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for alzheimer and Parkinson diseases modulation. Food Chem. Toxicol.159, 112752. 10.1016/j.fct.2021.112752
100
HafizZ. Z.AminM. A.Johari JamesR. M.TehL. K.SallehM. Z.AdenanM. I. (2020). Inhibitory effects of raw-extract Centella asiatica (RECA) on acetylcholinesterase, inflammations, and oxidative stress activities via in vitro and in vivo. Molecules25, 892. 10.3390/molecules25040892
101
HalperinJ. M.HealeyD. M. (2011). The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: Can we alter the developmental trajectory of ADHD?Neurosci. Biobehav. Rev.35, 621–634. 10.1016/j.neubiorev.2010.07.006
102
HampelH.HardyJ.BlennowK.ChenC.PerryG.KimS. H.et al (2021). The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry26, 5481–5503. 10.1038/s41380-021-01249-0
103
HannanM. A.DashR.HabibM.AlA.SohagA.MoonI.-S. (2020). Mechanistic insights into the curcumin-mediated neuroprotection in Alzheimer’s disease: An integrated system pharmacology and molecular simulation study . 10.20944/preprints202001.0109.v1Preprints2020010109
104
HardyJ.SelkoeD. J. (2002). The amyloid hypothesis of alzheimer's disease: Progress and problems on the road to therapeutics. Science297, 353–356. 10.1126/science.1072994
105
HaronS.KilmisterE. J.DavisP. F.StylliS. S.MantamadiotisT.KayeA. H.et al (2021). The renin-angiotensin system in central nervous system tumors and degenerative diseases. Front. Biosci.26, 628–642. 10.52586/4972
106
HashemiP.Fahanik BabaeiJ.VazifekhahS.NikbakhtF. (2019). Evaluation of the neuroprotective, anticonvulsant, and cognition-improvement effects of apigenin in temporal lobe epilepsy: Involvement of the mitochondrial apoptotic pathway. Iran. J. Basic Med. Sci.22, 752–758. 10.22038/ijbms.2019.33892.8064
107
HeineyS. A.WojaczynskiG. J.MedinaJ. F. (2021). Action-based organization of a cerebellar module specialized for predictive control of multiple body parts. Neuron109, 2981–2994.e5. 10.1016/j.neuron.2021.08.017
108
Hellion-IbarrolaM. C.IbarrolaD. A.MontalbettiY.KennedyM. L.HeinichenO.CampuzanoM.et al (2006). The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. J. Ethnopharmacol.105, 400–408. 10.1016/j.jep.2005.11.013
109
Herrera-ArellanoA.Jiménez-FerrerE.ZamilpaA.Morales-ValdézM.García-ValenciaC. E.TortorielloJ. (2007). Efficacy and tolerability of a standardized herbal product from Galphimia glauca on generalized anxiety disorder. A randomized, double-blind clinical trial controlled with lorazepam. Planta Med.73, 713–717. 10.1055/s-2007-981539
110
Herrera-ArellanoA.Jiménez-FerrerJ. E.ZamilpaA.García-AlonsoG.Herrera-AlvarezS.TortorielloJ. (2012). Therapeutic effectiveness of Galphimia glauca vs. lorazepam in generalized anxiety disorder. A controlled 15-week clinical trial. Planta Med.78, 1529–1535. 10.1055/s-0032-1315110
111
Herrera-RuizM.González-CortazarM.Jiménez-FerrerE.ZamilpaA.AlvarezL.RamírezG.et al (2006). Anxiolytic effect of natural galphimines from Galphimia glauca and their chemical derivatives. J. Nat. Prod.69, 59–61. 10.1021/np050305x
112
Herrera-RuizM.Jiménez-FerrerJ. E.De LimaT. C.Avilés-MontesD.Pérez-GarcíaD.González-CortazarM.et al (2006). Anxiolytic and antidepressant-like activity of a standardized extract from Galphimia glauca. Phytomedicine13, 23–28. 10.1016/j.phymed.2005.03.003
113
HewlingsS. J.KalmanD. S. (2017). Curcumin: A review of its effects on human health. Foods (Basel, Switz.6, 92. 10.3390/foods6100092
114
HorJ. H.SohE. S.TanL. Y.LimV. J.SantosaM. M.HoB. X.et al (2018). Cell cycle inhibitors protect motor neurons in an organoid model of spinal muscular atrophy. Cell Death Dis.9, 1100–1102. 10.1038/s41419-018-1081-0
115
HosseinzadehH.KarimiG.NiapoorM. (2003). Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic.650, 435–445. 10.17660/ActaHortic.2004.650.54
116
HosseinzadehH.NoraeiN. B. (2009). Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res.23, 768–774. 10.1002/ptr.2597
117
HosseinzadehH.SadeghniaH. R. (2007). Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine14, 256–262. 10.1016/j.phymed.2006.03.007
118
HuY.LiuP.GuoD. H.RahmanK.WangD. X.XieT. T. (2010). Antidepressant effects of the extract YZ-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharm. Biol.48, 794–800. 10.3109/13880200903280034
119
HuangL.-K.ChaoS.-P.HuC.-J. (2020). Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci.27 (1), 18. 10.1186/s12929-019-0609-7
120
HuangX.-T.QianZ.-M.HeX.GongQ.WuK.-C.JiangL.-R.et al (2014). Reducing iron in the brain: A novel pharmacologic mechanism of huperzine A in the treatment of alzheimer's disease. Neurobiol. Aging35, 1045–1054. 10.1016/j.neurobiolaging.2013.11.004
121
HusainM. A.LaurentB.PlourdeM. (2021). APOE and alzheimer’s disease: From lipid transport to physiopathology and therapeutics. Front. Neurosci.15, 630502. 10.3389/fnins.2021.630502
122
IbrahimM. M.GabrM. T. (2019). Multitarget therapeutic strategies for alzheimer’s disease. Neural Regen. Res.14, 437–440. 10.4103/1673-5374.245463
123
IkawaM.OkazawaH.YonedaM. (2021). Molecular imaging for mitochondrial metabolism and oxidative stress in mitochondrial diseases and neurodegenerative disorders. Biochim. Biophys. Acta. Gen. Subj.1865, 129832. 10.1016/j.bbagen.2020.129832
124
IonD.NiculescuA. G.PăduraruD. N.AndronicO.MușatF.GrumezescuA. M.et al (2021). An up-to-date review of natural nanoparticles for cancer management. Pharmaceutics14, 18. 10.3390/pharmaceutics14010018
125
IslamF.BeparyS.NafadyM. H.IslamM.EmranT. B.SultanaS.et al (2022). Polyphenols targeting oxidative stress in spinal cord injury: Current status and future vision. Oxid. Med. Cell. Longev.2022, 8741787. 10.1155/2022/8741787
126
ItoN.YabeT.GamoY.NagaiT.OikawaT.YamadaH.et al (2008). Rosmarinic acid from PerillaeHerba produces an antidepressant-like effect in mice through cell proliferation in the hippocampus. Biol. Pharm. Bull.31, 1376–1380. 10.1248/bpb.31.1376
127
JabirN. R.KhanF. R.TabrezS. (2018). Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of alzheimer’s disease. CNS Neurosci. Ther.24, 753–762. 10.1111/cns.12971
128
JamwalS.BlackburnJ. K.ElsworthJ. D. (2021). PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther.219, 107705. 10.1016/j.pharmthera.2020.107705
129
JankovicJ.ShererT. (2014). The future of research in Parkinson disease. JAMA Neurol.71, 1351–1352. https://doi.org/10.1001/jamaneurol.2014.1717.
130
Jankowska-KieltykaM.RomanA.NalepaI. (2021). The air we breathe: Air pollution as a prevalent proinflammatory stimulus contributing to neurodegeneration. Front. Cell. Neurosci.15, 647643. 10.3389/fncel.2021.647643
131
Javan GholilooM.YarniaM.GhorttapehA. H.FarahvashF.DaneshianA. M. (2019). Evaluating effects of drought stress and bio-fertilizer on quantitative and qualitative traits of valerian (Valeriana officinalis L.)J. Plant Nutr.42, 1417–1429. 10.1080/01904167.2019.1628972
132
JensenN. J.WodschowH. Z.NilssonM.RungbyJ. (2020). Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci.21, 8767. 10.3390/ijms21228767
133
JeongK.ShinY. C.ParkS.ParkJ. S.KimN.UmJ. Y.et al (2011). Ethanol extract of Scutellaria baicalensis Georgi prevents oxidative damage and neuroinflammation and memorial impairments in artificial senescense mice. J. Biomed. Sci.18, 14. 10.1186/1423-0127-18-14
134
JiaY.WangN.LiuX. (2017). Resveratrol and amyloid-beta: Mechanistic insights. Nutrients9, 1122. 10.3390/nu9101122
135
JiangN.WangH.LiC.ZengG.LvJ.WangQ.et al (2021). The antidepressant-like effects of the water extract of Panax ginseng and Polygala tenuifolia are mediated via the BDNF-TrkB signaling pathway and neurogenesis in the hippocampus. J. Ethnopharmacol.267, 113625. 10.1016/j.jep.2020.113625
136
Jiménez-FerrerE.Herrera-RuizM.Ramírez-GarcíaR.Herrera-ArellanoA.TortorielloJ. (2011). Interaction of the natural anxiolytic Galphimine-B with serotonergic drugs on dorsal hippocampus in rats. J. Ethnopharmacol.137, 724–729. 10.1016/j.jep.2011.06.029
137
JinT.ZhangY.BensonO. A.ZhangJ.FanR.ZhangY.et al (2022). Curcumin can improve Parkinson's disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol.113091, 113091. 10.1016/j.fct.2022.113091
138
JinZ. L.GaoN.ZhouD.ChiM. G.YangX. M.XuJ. P. (2012). The extracts of fructus akebiae, a preparation containing 90% of the active ingredient hederagenin: Serotonin, norepinephrine and dopamine reuptake inhibitor. Pharmacol. Biochem. Behav.100, 431–439. 10.1016/j.pbb.2011.10.001
139
JoshiP.BishtA.JoshiS.SemwalD.NemaN. K.DwivediJ.et al (2022). Ameliorating potential of curcumin and its analogue in central nervous system disorders and related conditions: A review of molecular pathways. Phytother. Res.36, 3143–3180. 10.1002/ptr.7522
140
JosiahS. S.FamusiwaC. D.CrownO. O.LawalA. O.OlaleyeM. T.AkindahunsiA. A.et al (2022). Neuroprotective effects of catechin and quercetin in experimental Parkinsonism through modulation of dopamine metabolism and expression of IL-1β, TNF-α, NF-κB, IκKB, and p53 genes in male Wistar rats. Neurotoxicology90, 158–171. 10.1016/j.neuro.2022.03.004
141
JungJ. W.ChoJ. H.AhnN. Y.OhH. R.KimS. Y.JangC. G.et al (2005). Effect of chronic Albizzia julibrissin treatment on 5-hydroxytryptamine1A receptors in rat brain. Pharmacol. Biochem. Behav.81, 205–210. 10.1016/j.pbb.2005.03.014
142
JyotirmayeeB.MahalikG. (2022). A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop.25, 1377–1398. https://doi.org/10.1080/10942912.2022.2082464.
143
KalinderiK.BostantjopoulouS.FidaniL. (2016). The genetic background of Parkinson's disease: Current progress and future prospects. Acta Neurol. Scand.134, 314–326. 10.1111/ane.12563
144
KarimN.KhanI.AbdelhalimA.HalimS. A.KhanA.Al-HarrasiA. (2021). Stigmasterol can be new steroidal drug for neurological disorders: Evidence of the GABAergic mechanism via receptor modulation. Phytomedicine.90, 153646. 10.1016/j.phymed.2021.153646
145
KarkadaG.ShenoyK. B.HalahalliH.KaranthK. (2012). Nardostachys jatamansi extract prevents chronic restraint stress-induced learning and memory deficits in a radial arm maze task. J. Nat. Sci. Biol. Med.3, 125–132. 10.4103/0976-9668.101879
146
KaswanK. S.GaurL.DhatterwalJ. S.KumarR. (2021). Advanced AI techniques and applications in bioinformatics. Boca Raton, FL: CRC Press, 41–86. 10.1201/9781003126164AI-based natural language processing for the generation of meaningful information electronic health record (EHR) data
147
KatilaN.DuwaR.BhurtelS.KhanalS.MaharjanS.JeongJ. H.et al (2022). Enhancement of blood–brain barrier penetration and the neuroprotective effect of resveratrol. J. Control. Release346, 1–19. 10.1016/j.jconrel.2022.04.003
148
KatsumotoA.TakeuchiH.TanakaF. (2019). Tau pathology in chronic traumatic encephalopathy and alzheimer's disease: Similarities and differences. Front. Neurol.10, 980. 10.3389/fneur.2019.00980
149
KaurJ.FamtaP.FamtaM.MehtaM.SatijaS.SharmaN.et al (2021). Potential anti-epileptic phytoconstituents: An updated review. J. Ethnopharmacol.268, 113565. 10.1016/j.jep.2020.113565
150
KayC.CollinsJ. A.SkotteN. H.SouthwellA. L.WarbyS. C.CaronN. S.et al (2015). Huntingtin haplotypes provide prioritized target panels for allele-specific silencing in Huntington disease patients of European ancestry. Mol. Ther.23, 1759–1771. 10.1038/mt.2015.128
151
KennedyD. O.PaceS.HaskellC.OkelloE. J.MilneA.ScholeyA. B. (2006). Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stress or battery. Neuropsychopharmacology31, 845–852. 10.1038/sj.npp.1300907
152
KentS. A.-O.Spires-JonesT. A.-O.DurrantC. A.-O. (2020). The physiological roles of tau and Aβ: Implications for alzheimer's disease pathology and therapeutics. Acta Neuropathol.417, 417–447. 10.1007/s00401-020-02196-w
153
KhanH.PervizS.SuredaA.NabaviS. M.TejadaS. (2018). Current standing of plant derived flavonoids as an antidepressant. Food Chem. Toxicol.119, 176–188. 10.1016/j.fct.2018.04.052
154
Khyati, MalikI.AgrawalN.KumarV. (2021). Melatonin and curcumin reestablish disturbed circadian gene expressions and restore locomotion ability and eclosion behavior in Drosophila model of Huntington’s disease. Chronobiol. Int.38, 61–78. 10.1080/07420528.2020.1842752
155
KimH. J.TaylorJ. P. (2017). Lost in transportation: Nucleocytoplasmic transport defects in ALS and other neurodegenerative diseases. Neuron96, 285–297. 10.1016/j.neuron.2017.07.029
156
KimS.YangM.KuB.ChaE.SeoW.SonI.et al (2022). Efficacy of mecasin for treatment of amyotrophic lateral sclerosis: A phase IIa multicenter randomized double-blinded placebo-controlled trial, KCT0001984. 10.2139/ssrn.4086326
157
KimW. K.JungJ. W.AhnN. Y.OhH. R.LeeB. K.OhJ. K.et al (2004). Anxiolytic-like effects of extracts from Albizzia julibrissin bark in the elevated plus-maze in rats. Life Sci.75, 2787–2795. 10.1016/j.lfs.2004.05.024
158
KimuraY.SumiyoshiM. (2011). Effects of various flavonoids isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. J. Pharm. Pharmacol.63, 1613–1623. 10.1111/j.2042-7158.2011.01365.x
159
KlvanaM.ChenJ.LépineF.LegrosR.JolicoeurM. (2006). Analysis of secondary metabolites from Eschscholtzia californica by high-performance liquid chromatography. Phytochem. Anal.17, 236–242. 10.1002/pca.913
160
KodimuleP. S. (2021). “Method of using a chlorogenic acid composition for supporting cognitive function,”. US20210330627.
161
KruseN.Schulz-SchaefferW. J.SchlossmacherM. G.MollenhauerB. (2012). Development of electrochemiluminescence-based singleplex and multiplex assays for the quantification of α-synuclein and other proteins in cerebrospinal fluid. Methods56, 514–518. 10.1016/j.ymeth.2012.03.016
162
KumarG. P.AnilakumarK. R.NaveenS. (2015). Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Phcog. J.7, 1–17. 10.5530/pj.2015.7.1
163
KumarM. P.NagnathK. R. (2021). “Isolation and identification of suitable anticonvulsant drug from Cucurbita maxima,”. IN202121057739.
164
KumarS.MadaanR.SharmaA. (2008). Estimation of apigenin, an anxiolytic constituent, in Turnera aphrodisiaca. Indian J. Pharm. Sci.70, 847–851. 10.4103/0250-474X.49143
165
KumarS.SharmaA. (2005). Anti-anxiety activity studies of various extracts of Turnera aphrodisiaca Ward. J. Herb. Pharmacother.5, 13–21. 10.1080/J157v05n04_02
166
KundapU. P.BhuvanendranS.KumariY.OthmanI.ShaikhM. F. (2017). Plant derived phytocompound, embelin in CNS disorders: A systematic review. Front. Pharmacol.8, 76. 10.3389/fphar.2017.00076
167
KuribaraH.WeintraubS. T.YoshihamaT.MaruyamaY. (2003). An anxiolytic-like effect of Ginkgo biloba extract and its constituent, ginkgolide-A, in mice. J. Nat. Prod.66, 1333–1337. 10.1021/np030122f
168
LabbanS.AlghamdiB. S.AlshehriF. S.KurdiM. (2021). Effects of melatonin and resveratrol on recognition memory and passive avoidance performance in a mouse model of Alzheimer’s disease. Behav. Brain Res.402, 113100. 10.1016/j.bbr.2020.113100
169
LanhersM. C.FleurentinJ.CabalionP.RollandA.DorfmanP.MisslinR.et al (1990). Behavioral effects of Euphorbia hirta L.: Sedative and anxiolytic properties. J. Ethnopharmacol.29, 189–198. 10.1016/0378-8741(90)90055-X
170
LeeS. A.HongS. S.HanX. H.HwangJ. S.OhG. J.LeeK. S.et al (2005). Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull.53, 832–835. 10.1248/cpb.53.832
171
LeeS. A.HwangJ. S.HanX. H.LeeC.LeeM. H.ChoeS. G.et al (2008). Methylpiperate derivatives from Piper longum and their inhibition of monoamine oxidase. Arch. Pharm. Res.31, 679–683. 10.1007/s12272-001-1212-7
172
LiR.WangZ. M.WangY.DongX.ZhangL. H.WangT.et al (2021). Antidepressant activities and regulative effects on serotonin transporter of Nardostachys jatamansi DC. J. Ethnopharmacol.268, 113601. 10.1016/j.jep.2020.113601
173
LiW.ZhaoJ.ShenC.ZhangJ.HuJ.XiaoM.et al (2022). Regional brain fusion: Graph convolutional network for alzheimer's disease prediction and analysis. Front. Neuroinform.16, 886365. 10.3389/fninf.2022.886365
174
LiX. X.HeG. R.MuX.XuB.TianS.YuX.et al (2012). Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur. J. Pharmacol.674, 227–233. 10.1016/j.ejphar.2011.09.181
175
LiangY.YeC.ChenY.ChenY.DiaoS.HuangM. (2021). Berberine improves behavioral and cognitive deficits in a mouse model of Alzheimer’s disease via regulation of β-amyloid production and endoplasmic reticulum stress. ACS Chem. Neurosci.12, 1894–1904. 10.1021/acschemneuro.0c00808
176
LillC. M. (2016). Genetics of Parkinson's disease. Mol. Cell. Probes30, 386–396. 10.1016/j.mcp.2016.11.001
177
LillC. M.KleinC. (2017). Epidemiology and causes of Parkinson’s disease. Nervenarzt88, 345–355. 10.1007/s00115-017-0288-0
178
LimaZ. P.dos SantosR. D.TorresT. U.SannomiyaM.RodriguesC. M.dos SantosL. C.et al (2008). Byrsonima fagifolia: An integrative study to validate the gastroprotective, healing, antidiarrheal, antimicrobial and mutagenic action. J. Ethnopharmacol.120, 149–160. 10.1016/j.jep.2008.07.047
179
LindbergI.ShorterJ.WisemanR. L.ChitiF.DickeyC. A.McLeanP. J. (2015). Chaperones in neurodegeneration. J. Neurosci.35, 13853–13859. 10.1523/jneurosci.2600-15.2015
180
LiuW.WuL.LiuW.TianL.ChenH.WuZ.et al (2022). Design, synthesis and biological evaluation of novel coumarin derivatives as multifunctional ligands for the treatment of Alzheimer's disease. Eur. J. Med. Chem.242, 114689. 10.1016/j.ejmech.2022.114689
181
LopesI. S.OliveiraI. C.CapibaribeV. C.ValentimJ. T.da SilvaD. M.de SouzaA. G.et al (2018). Riparin II ameliorates corticosterone-induced depressive-like behavior in mice: Role of antioxidant and neurotrophic mechanisms. Neurochem. Int.120, 33–42. 10.1016/j.neuint.2018.07.007
182
López-CruzL.SalamoneJ. D.CorreaM. (2018). Caffeine and selective adenosine receptor antagonists as new therapeutic tools for the motivational symptoms of depression. Front. Pharmacol.9, 526. 10.3389/fphar.2018.00526
183
LoprestiA. L. (2018). The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects?Adv. Nutr.9, 41–50. 10.1093/advances/nmx011
184
LuJ.WangX.WuA.CaoY.DaiX.LiangY.et al (2022). Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytother. Res.36, 1523–1544. 10.1002/ptr.7395
185
LumP. T.SekarM.GanS. H.BonamS. R.ShaikhM. F. (2021). Protective effect of natural products against Huntington’s disease: An overview of scientific evidence and understanding their mechanism of action. ACS Chem. Neurosci.12, 391–418. 10.1021/acschemneuro.0c00824
186
MaW.KuangH.WangL.XuL.ChangW. S.ZhangH.et al (2013). Chiral plasmonics of self-assembled nanorod dimers. Sci. Rep.3, 1934–1936. 10.1038/srep01934
187
MachadoD. G.CunhaM. P.NeisV. B.BalenG. O.CollaA.BettioL. E.et al (2013). Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem.136, 999–1005. 10.1016/j.foodchem.2012.09.028
188
MachadoD. G.NeisV. B.BalenG. O.CollaA.CunhaM. P.DalmarcoJ. B.et al (2012). Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. In mice: Evidence for the involvement of the dopaminergic system. Pharmacol. Biochem. Behav.103, 204–211. 10.1016/j.pbb.2012.08.016
189
MahantiN. K.UpendarK.ChakrabortyS. K. (2022). Comparison of artificial neural network and linear regression model for the leaf morphology of fenugreek (Trigonella foenum graecum) grown under different nitrogen fertilizer doses. Smart Agric. Technol.2, 100058. 10.1016/j.atech.2022.100058
190
MalikJ.KaurS.KaranM.ChoudharyS. (2022). Neuroprotective effect of standardized extracts of three Lactuca sativa Linn. varieties against 3-NP induced Huntington’s disease like symptoms in rats. Nutr. Neurosci.25, 1173–1187. 10.1080/1028415X.2020.1841500
191
ManasaK.VellapandianC.VasanthanM. M.JaisankarN. (2022). Phytochemical extract containing indole alkaloid camalexin for management of benomyl induced Parkinson’s disease. 21. IN202141020016.
192
MannanA.SinghT. G.SinghV.GargN.KaurA.SinghM. (2022). Insights into the mechanism of the therapeutic potential of herbal monoamine oxidase inhibitors in neurological diseases. Curr. Drug Targets23, 286–310. 10.2174/1389450122666210707120256
193
MannucciC.NavarraM.CalzavaraE.CaputiA. P.CalapaiG. (2012). Serotonin involvement in Rhodiola rosea attenuation of nicotine withdrawal signs in rats. Phytomedicine19, 1117–1124. 10.1016/j.phymed.2012.07.001
194
MantovaniA.AllavenaP.SicaA.BalkwillF. (2008). Cancer-related inflammation. Nature454, 436–444. 10.1038/nature07205
195
MaoQ.HuangZ.IpS.CheC. (2008). Antidepressant-like effect of ethanol extract from Paeonia lactiflora in mice. Phytother. Res.22, 1496–1499. 10.1002/ptr.2519
196
MaoQ. Q.IpS. P.TsaiS. H.CheC. T. (2008). Antidepressant-like effect of peony glycosides in mice. J. Ethnopharmacol.119, 272–275. 10.1016/j.jep.2008.07.008
197
MaoX.-Y.ZhouH.-H.JinW.-L. (2019). Redox-related neuronal death and crosstalk as drug targets: Focus on epilepsy. Front. Neurosci.13, 512. 10.3389/fnins.2019.00512
198
MaríM.ColellA. (2021). Mitochondrial oxidative and nitrosative stress as a therapeutic target in diseases. Antioxidants10, 314. 10.3390/antiox10020314
199
MartinsJ.BrijeshS. (2018). Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother.104, 343–365. 10.1016/j.biopha.2018.05.044
200
MartinsM.SilvaR.PintoM. M. M.SousaE. (2020). Marine natural products, multitarget therapy and repurposed agents in Alzheimer's disease. Pharm. (Basel)13, 242. 10.3390/ph13090242
201
MashayekhA.PhamD. L.YousemD. M.DizonM.BarkerP. B.LinD. D. (2021). Effects of Ginkgo biloba on cerebral blood flow assessed by quantitative MR perfusion imaging: A pilot study. Neuroradiology53, 185–191. 10.1007/s00234-010-0790-6
202
MathewJ.BalakrishnanS.AntonyS.AbrahamP. M.PauloseC. S. (2012). Decreased GABA receptor in the cerebral cortex of epileptic rats: Effect of Bacopa monnieri and bacoside-A. J. Biomed. Sci.19, 25. 10.1186/1423-0127-19-25
203
MathurD.GoyalK.KoulV.AnandA. (2016). The molecular links of re-emerging therapy: A review of evidence of brahmi (Bacopa monniera). Front. Pharmacol.7, 44. 10.3389/fphar.2016.00044
204
McCarthyB.O’NeillG.Abu-GhannamN. (2022). Potential psychoactive effects of microalgal bioactive compounds for the case of sleep and mood regulation: Opportunities and challenges. Mar. Drugs20, 493. 10.3390/md20080493
205
MehringerJ.NavarroJ. A.TouraudD.SchneuwlyS.KunzW. (2022). Phosphorylated resveratrol as a protein aggregation suppressor in vitro and in vivo. RSC Chem. Biol.3, 2250–2260. https://doi.org/10.1039/D1CB00220A.
206
Mendonça-JuniorF. J.ScottiM. T.MuratovE. N.ScottiL.NayarisseriA. (2021). Natural bioactive products with antioxidant properties useful in neurodegenerative diseases 2020. Oxid. Med. Cell. Longev.2021, 6262316. 10.1155/2021/6262316
207
MerzouguiW. H.MyersM. A.HallS.ElmansouriA.ParkerR.RobsonA. D.et al (2021). Multiple-choice versus open-ended questions in advanced clinical neuroanatomy: Using a national neuroanatomy assessment to investigate variability in performance using different question types. Anat. Sci. Educ.14, 296–305. 10.1002/ase.2053
208
MirandaM.MoriciJ. F.ZanoniM. B.BekinschteinP. (2019). Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci.13, 363. 10.3389/fncel.2019.00363
209
MitraS.MuniM.ShawonN. J.DasR.EmranT. B.SharmaR.et al (2022). Tacrine derivatives in neurological disorders: Focus on molecular mechanisms and neurotherapeutic potential. Oxid. Med. Cell. Longev.2022, 7252882. 10.1155/2022/7252882
210
MoY.XuE.WeiR.LeB.SongL.LiD.et al (2018). Bushen-yizhi formula alleviates neuroinflammation via inhibiting NLRP3 inflammasome activation in a mouse model of Parkinson’s disease. Evid. Based. Complement. Altern. Med.2018. 10.1155/2018/35716043571604
211
MohammadiA.ColagarA. H.KhorshidianA.AminiS. M. (2022). The functional roles of curcumin on astrocytes in neurodegenerative diseases. Neuroimmunomodulation29, 4–14. 10.1159/000517901
212
MohebaliN.ShahzadehFazeliS. A.GhafooriH.FarahmandZ.MohammadKhaniE.VakhshitehF.et al (2018). Effect of flavonoids rich extract of Capparis spinosa on inflammatory involved genes in amyloid-beta peptide injected rat model of Alzheimer's disease. Nutr. Neurosci.21, 143–150. 10.1080/1028415X.2016.1238026
213
MoraS.Díaz-VélizG.MillánR.LungenstrassH.QuirósS.Coto-MoralesT.et al (2005). Anxiolytic and antidepressant-like effects of the hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol. Biochem. Behav.82, 373–378. 10.1016/j.pbb.2005.09.007
214
MoralesP.ReggioP. H.JagerovicN. (2017). An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol.8, 422. 10.3389/fphar.2017.00422
215
MovafeghA.AlizadehR.HajimohamadiF.EsfehaniF.NejatfarM. (2008). Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Anesth. Analg.106, 1728–1732. 10.1213/ane.0b013e318172c3f9
216
MurphyM. P.HartleyR. C. (2018). Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov.17, 865–886. 10.1038/nrd.2018.174
217
MursaleenL. R.StamfordJ. A.JonesD. A.WindleR.IsaacsT. (2017). Attitudes towards data collection, ownership and sharing among patients with Parkinson's disease. J. Park. Dis.7, 523–531. 10.3233/JPD-161045
218
MuzaffarK.SofiS. A.MakrooH. A.MajidD.DarB. N. (2022). Insight about the biochemical composition, postharvest processing, therapeutic potential of Indian gooseberry (amla), and its utilization in development of functional foods—A comprehensive review. J. Food Biochem.1. e14132. 10.1111/jfbc.14132
219
NakazawaT.YasudaT.UedaJ.OhsawaK. (2003). Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull.26, 474–480. 10.1248/bpb.26.474
220
NaoiM.MaruyamaW. (2010). Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharm. Des.16, 2799–2817. 10.2174/138161210793176527
221
NavarroE.AlonsoS. J.TrujilloJ.JorgeE.PérezC. (2004). Central nervous activity of elenoside. Phytomedicine11, 498–503. 10.1016/j.phymed.2003.06.003
222
NeagM. A.MocanA.EcheverríaJ.PopR. M.BocsanC. I.CrişanG.et al (2018). Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol.9, 557. 10.3389/fphar.2018.00557
223
NebrisiE. E. (2021). Neuroprotective activities of curcumin in Parkinson’s disease: A review of the literature. Int. J. Mol. Sci.22, 11248. https://doi.org/10.3390/ijms222011248.
224
NemethE.BernathJ. (2008). Biological activities of yarrow species (Achillea spp.)Curr. Pharm. Des.14, 3151–3167. 10.2174/138161208786404281
225
NoorN. A.HosnyE. N.KhadrawyY. A.MouradI. M.OthmanA. I.Aboul EzzH. S.et al (2022). Effect of curcumin nanoparticles on streptozotocin-induced male wistar rat model of Alzheimer’s disease. Metab. Brain Dis.37, 343–357. 10.1007/s11011-021-00897-z
226
NunesA.SousaM. (2011). Use of valerian in anxiety and sleep disorders: What is the best evidence?Acta Med. Port.24, 961–966.
227
PalkarJ.PrasadR. (2021). “Synergistic nutritional compositions for treating cerebrovascular diseases,”. WO/2021/084559.
228
PanG.KingA.WuF.Simpson-YapS.WoodhouseA.PhippsA.et al (2021). The potential roles of genetic factors in predicting ageing-related cognitive change and Alzheimer’s disease. Ageing Res. Rev.70, 101402. 10.1016/j.arr.2021.101402
229
PanY.ZhaoX.WangY.TanJ.ChenD. X. (2021). Metabolomics integrated with transcriptomics reveals the distribution of iridoid and crocin metabolic flux in Gardenia jasminoides Ellis. Plos One16, e0256802. 10.1371/journal.pone.0256802
230
PandeyM. M.RastogiS.RawatA. K. (2013). Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid. Based. Complement. Altern. Med.2013. 10.1155/2013/376327376327
231
PaseM. P.KeanJ.SarrisJ.NealeC.ScholeyA. B.StoughC. (2012). The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J. Altern. Complement. Med.18, 647–652. 10.1089/acm.2011.0367
232
PatilP.ThakurA.SharmaA.FloraS. J. S. (2020). Natural products and their derivatives as multifunctional ligands against alzheimer's disease. Drug Dev. Res.81, 165–183. 10.1002/ddr.21587
233
PatilR. A.HirayY. A.KastureS. B. (2012). Reversal of reserpine-induced orofacial dyskinesia and catalepsy by Nardostachys jatamansi. Indian J. Pharmacol.44, 340–344. 10.4103/0253-7613.96307
234
Pearson-SmithJ. N.LiangL. P.RowleyS. D.DayB. J.PatelM. (2017). Oxidative stress contributes to status epilepticus associated mortality. Neurochem. Res.42, 2024–2032. 10.1007/s11064-017-2273-1
235
Pérez-ArancibiaR.OrdoñezJ. L.RivasA.PihánP.SagredoA.AhumadaU.et al (2021). A phenolic-rich extract from Ugnimolinae berries reduces abnormal protein aggregation in a cellular model of Huntington’s disease. PloS One16, 0254834. 10.1371/journal.pone.0254834
236
PerroneD.FuggettaM. P.ArditoF.CottarelliA.De FilippisA.RavagnanG.et al (2017). Resveratrol (3, 5, 4’-trihydroxystilbene) and its properties in oral diseases. Exp. Ther. Med.14, 3–9. 10.3892/etm.2017.4472
237
PiatoÂ. L.RizonL. P.MartinsB. S.NunesD. S.ElisabetskyE. (2009). Antidepressant profile of Ptychopetalum olacoides bentham (marapuama) in mice. Phytother. Res.23, 519–524. 10.1002/ptr.2664
238
PitsikasN.BoultadakisA.GeorgiadouG.TarantilisP. A.SakellaridisN. (2008). Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine15, 1135–1139. 10.1016/j.phymed.2008.06.005
239
PohlF.Kong Thoo LinP. (2018). The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules23, 3283. 10.3390/molecules23123283
240
PorcariG. S.FuC.DollE. D.CarterE. G.CarsonR. P. (2018). Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: Practical experiences in a tertiary medical center. Epilepsy Behav.80, 240–246. 10.1016/j.yebeh.2018.01.026
241
PrandiC.BlangettiM.NamdarD.KoltaiH. (2018). Structure-activity relationship of cannabis derived compounds for the treatment of neuronal activity-related diseases. Molecules23, 1526. 10.3390/molecules23071526
242
PuJ.LiX. N. D. D. N. (2018). Nddn: A cloud-based neuroinformation database for developing neuronal networks. J. Healthc. Eng.2018. 3839094. 10.1155/2018/3839094
243
PurushothamanB.SumathiT. (2022). 5, 6, 7-Trihydroxy flavone armoured neurodegeneration caused by quinolinic acid induced Huntington’s like disease in rat striatum-reinstating the level of brain neurotrophins with special reference to cognitive-socio behaviour, biochemical and histopathological aspects. Neurosci. Res.174, 25–35. 10.1016/j.neures.2021.08.003
244
QiuF.ZhongX.MaoQ.HuangZ. (2013). The antidepressant-like effects of paeoniflorin in mouse models. Exp. Ther. Med.5, 1113–1116. 10.3892/etm.2013.925
245
QiuT.WuD.YangL.YeH.WangQ.CaoZ.et al (2018). Exploring the mechanism of flavonoids through systematic bioinformatics analysis. Front. Pharmacol.9, 918. 10.3389/fphar.2018.00918
246
RahmanM.IslamM. R.MimM.AkashS.Noor AlamM.NepovimovaE.et al (2022). Exploring the role of nano-medicines for the therapeutic approach of central nervous system dysfunction: At a glance. Front. Cell Dev. Biol.1780, 989471. 10.3389/fcell.2022.989471
247
RaihanM. O.HabibM. R.BrishtiA.RahmanM. M.SaleheenM. M.MannaM. (2011). Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. leaf. Drug Discov. Ther.5, 185–189. 10.5582/ddt.2011.v5.4.185
248
RamachandraV. H.SivanesanS.KoppalA.AnandakumarS.HowellM. D.SukumarE.et al (2022). Embelin and levodopa combination therapy for improved Parkinson’s disease treatment. Transl. Neurosci.13, 145–162. 10.1515/tnsci-2022-0224
249
Ramires JúniorO. V.AlvesB. D.BarrosP. A.RodriguesJ. L.FerreiraS. P.MonteiroL. K.et al (2021). Nanoemulsion improves the neuroprotective effects of curcumin in an experimental model of Parkinson’s disease. Neurotox. Res.39, 787–799. 10.1007/s12640-021-00362-w
250
RanjbarM.MazaheriM.AnsaripourM.BabaeianM.JalaliA.ZarshenasM. M. (2022). Herbal medications to manage insomnia: An overview of clinical trials using herbal treatment for insomnia. Trad. Integr. Med.1. 254–265. 10.18502/tim.v7i2.9928
251
RapakaD.BitraV. R.UmmidiR.AkulaA. (2021). Benincasa hispida alleviates amyloid pathology by inhibition of keap1/nrf2-axis: Emphasis on oxidative and inflammatory stress involved in alzheimer's disease model. Neuropeptides88, 102151. 10.1016/j.npep.2021.102151
252
RastogiS.BaiswarA.NischalA.SrivastavaP. S.NischalA. (2016). Effects of shirodhara in generalized anxiety disorder. Cell Med.6, 27. 10.5667/tang.2016.0016
253
RastogiS. (2010). Building bridges between ayurveda and modern science. Int. J. Ayurveda Res.1, 41–46. 10.4103/0974-7788.59943
254
RastogiS. (2019). Coma with glasgow coma scale score 3 at admission following acute head injury: Experiencing the complete recovery supported through ayurveda–a case report. Complement. Med. Res.26, 353–360. 10.1159/000498912
255
RastogiS. (2014). Rehabilitative potential of ayurveda for neurological deficits caused by traumatic spinal cord injury. J. Ayurveda Integr. Med.5, 56–59. 10.4103/0975-9476.128868
256
RatcliffeC.WandschneiderB.BaxendaleS.ThompsonP.KoeppM. J.CaciagliL. (2020). Cognitive function in genetic generalized epilepsies: Insights from neuropsychology and neuroimaging. Front. Neurol.11, 144. 10.3389/fneur.2020.00144
257
RaucciU.PietrafusaN.PaolinoM. C.Di NardoG.VillaM. P.PavoneP.et al (2020). Cannabidiol treatment for refractory epilepsies in pediatrics. Front. Pharmacol.11, 586110. 10.3389/fphar.2020.586110
258
RavidD. (2022). A combination therapy comprising uncaria for treating anxiety and depression. WO/2022/123572.
259
RavikumarB.AittokallioT. (2018). Improving the efficacy-safety balance of polypharmacology in multi-target drug discovery. Expert Opin. Drug Discov.13, 179–192. 10.1080/17460441.2018.1413089
260
RehmanM. U.WaliA. F.AhmadA.ShakeelS.RasoolS.AliR.et al (2019). Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol.17, 247–267. 10.2174/1570159X16666180911124605
261
RenL. X.LuoY. F.LiX.ZuoD. Y.WuY. L. (2006). Antidepressant-like effects of sarsasapogenin from Anemarrhena asphodeloides B UNGE (liliaceae). Biol. Pharm. Bull.29, 2304–2306. 10.1248/bpb.29.2304
262
RenQ.JiangX.PaudelY. N.GaoX.GaoD.ZhangP.et al (2022). Co-treatment with natural HMGB1 inhibitor Glycyrrhizin exerts neuroprotection and reverses Parkinson’s disease like pathology in zebrafish. J. Ethnopharmacol.292, 115234. 10.1016/j.jep.2022.115234
263
ResstelL. B.TavaresR. F.LisboaS. F.JocaS. R.CorrêaF. M.GuimarãesF. S. (2009). 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol.156, 181–188. 10.1111/j.1476-5381.2008.00046.x
264
RhoJ. M.BoisonD. (2022). The metabolic basis of epilepsy. Nat. Rev. Neurol.18, 333–347. 10.1038/s41582-022-00651-8
265
RodaA. R.Serra-MirG.Montoliu-GayaL.TiesslerL.VillegasS. (2022). Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res.17, 1666–1674. 10.4103/1673-5374.332127
266
RodriguesA. L.da SilvaG. L.MateussiA. S.FernandesE. S.MiguelO. G.YunesR. A.et al (2002). Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci.70, 1347–1358. 10.1016/S0024-3205(01)01498-9
267
RollandA.FleurentinJ.LanhersM. C.MisslinR.MortierF. (2001). Neurophysiological effects of an extract of Eschscholzia californica cham.(papaveraceae). Phytother. Res.15, 377–381. 10.1002/ptr.884
268
RollandA.FleurentinJ.LanhersM. C.YounosC.MisslinR.MortierF.et al (1991). Behavioural effects of the American traditional plant Eschscholzia californica: Sedative and anxiolytic properties. Planta Med.57, 212–216. 10.1055/s-2006-960076
269
Ruiz-OlazarM.RochaE. S.VargasC. D.BraghettoK. R. (2021). The neuroscience experiments system (nes)-a software tool to manage experimental data and its provenance. Front. Neuroinform.77, 768615. 10.3389/fninf.2021.768615
270
RyanM. (2020). Cannabidiol in epilepsy: The indications and beyond. Ment. Health Clin.10, 317–325. 10.9740/mhc.2020.11.317
271
SahS. P.MathelaC. S.ChopraK. (2011). Antidepressant effect of Valerianawallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J. Ethnopharmacol.135, 197–200. 10.1016/j.jep.2011.02.018
272
SahS. P.MathelaC. S.ChopraK. (2011). Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valerianawallichii Patchouli alcohol chemotype. Phytomedicine18, 1269–1275. 10.1016/j.phymed.2011.06.009
273
SahaT.SahaS.KarmakarA.ChatterjeeM.MaitraS.SinhaS.et al (2022). Differential effect of folate metabolic system genetic variants on attention deficit hyperactivity disorder severity. Hum. Gene34, 201096. 10.1016/j.humgen.2022.201096
274
SairamK.DorababuM.GoelR. K.BhattacharyaS. K. (2002). Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine9, 207–211. 10.1078/0944-7113-00116
275
SaiyudthongS.MarsdenC. A. (2011). Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother. Res.25, 858–862. 10.1002/ptr.3325
276
SakakibaraH.YoshinoS.KawaiY.TeraoJ. (2008). Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Biosci. Biotechnol. Biochem.72, 94–100. 10.1271/bbb.70454
277
SalehiB.VendittiA.Sharifi-RadM.KręgielD.Sharifi-RadJ.DurazzoA.et al (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci.20, 1305. 10.3390/ijms20061305
278
SantosT. Cd.GomesT. M.PintoB. A. S.CamaraA. L.PaesA. MdA. (2018). Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer's disease therapy. Front. Pharmacol.9, 1192. 10.3389/fphar.2018.01192
279
SarrisJ. (2007). Herbal medicines in the treatment of psychiatric disorders: A systematic review. Phytother. Res.21, 703–716. 10.1002/ptr.2187
280
SarrisJ.KavanaghD. J. (2009). Kava and st. John's wort: Current evidence for use in mood and anxiety disorders. J. Altern. Complement. Med.15, 827–836. 10.1089/acm.2009.0066
281
SarrisJ.O’NeilA.CoulsonC. E.SchweitzerI.BerkM. (2014). Lifestyle medicine for depression. BMC Psychiatry14, 1–13. 10.1186/1471-244X-14-107
282
SartoriS. B.SingewaldN. (2019). Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther.204, 107402. 10.1016/j.pharmthera.2019.107402
283
SchmidtM.BettiG.HenselA. (2007). Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr.157, 315–319. 10.1007/s10354-007-0428-4
284
SergidesC.ChirilăM.SilvestroL.PittaD.PittasA. (2016). Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med.11, 164–170. 10.3892/etm.2015.2895
285
ShaitoA.PosadinoA. M.YounesN.HasanH.HalabiS.AlhababiD.et al (2020). Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci.21, 2084. 10.3390/ijms21062084
286
ShandilyaA.MehanS.KumarS.SethiP.NarulaA. S.AlshammariA.et al (2022). Activation of IGF-1/GLP-1 signalling via 4-hydroxyisoleucine prevents motor neuron impairments in experimental ALS-rats exposed to methylmercury-induced neurotoxicity. Molecules27, 3878. 10.3390/molecules27123878
287
ShaoC.YuanJ.LiuY.QinY.WangX.GuJ.et al (2020). Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad. Sci. U. S. A.117, 10155–10164. 10.1073/pnas.1917946117
288
Sharifi-RadM.LankatillakeC.DiasD. A.DoceaA. O.MahomoodallyM. F.LobineD.et al (2020). Impact of natural compounds on neurodegenerative disorders: From preclinical to pharmacotherapeutics. J. Clin. Med.9, 1061. 10.3390/jcm9041061
289
SharmaR.GargN.VermaD.RathiP.SharmaV.KucaK.et al (2021). “Indian medicinal plants as drug leads in neurodegenerative disorders,” in Nutraceuticals in brain health and beyond (Academic Press), 1–45. 10.1016/B978-0-12-820593-8.00004-5
290
SharmaR.KabraA.RaoM. M.PrajapatiP. K. (2018). Herbal and holistic solutions for neurodegenerative and depressive disorders: Leads from ayurveda. Curr. Pharm. Des.24, 2597–2608. 10.2174/1381612824666180821165741
291
SharmaR.KucaK.NepovimovaE.KabraA.RaoM. M.PrajapatiP. K. (2019). Traditional ayurvedic and herbal remedies for alzheimer’s disease: From bench to bedside. Expert Rev. Neurother.19, 359–374. 10.1080/14737175.2019.1596803
292
SharmaR.SinglaR. K.BanerjeeS.SinhaB.ShenB.SharmaR. (2022). Role of shankhpushpi (Convolvulus pluricaulis) in neurological disorders: An umbrella review covering evidence from ethnopharmacology to clinical studies. Neurosci. Biobehav. Rev.140, 104795. 10.1016/j.neubiorev.2022.104795
293
ShirbandiK.KhalafiM.Mirza-Aghazadeh-AttariM.TahmasbiM.ShahvandiH. K.JavanmardiP.et al (2021). Accuracy of deep learning model-assisted amyloid positron emission tomography scan in predicting alzheimer's disease: A systematic review and meta-analysis. Inf. Med. Unlocked25, 100710. 10.1016/j.imu.2021.100710
294
SibiP. I.MeeraP. (2013). In silico docking analysis of constituents of Zingiber officinale as antidepressant. J. Pharmacogn. Phytother.5, 101–105. 10.5897/JPP2013.0280
295
SinghB.BhatT. K.SinghB. (2003). Potential therapeutic applications of some antinutritional plant secondary metabolites. J. Agric. Food Chem.51, 5579–5597. 10.1021/jf021150r
296
SinghG. K.GarabaduD.MuruganandamA. V.JoshiV. K.KrishnamurthyS. (2009). Antidepressant activity of Asparagus racemosus in rodent models. Pharmacol. Biochem. Behav.91, 283–290. 10.1016/j.pbb.2008.07.010
297
SinghR. P.JainR.MishraR.TiwariP. (2012). Antidepressant activity of hydroalcoholic extract of Zingiber officinale. Int. Res. J. Pharm.3, 149–151. 10.2174/1871527321666220128091408
298
SinghR.RamakrishnaR.BhateriaM.BhattaR. S. (2014). In vitro evaluation of Bacopa monniera extract and individual constituents on human recombinant monoamine oxidase enzymes. Phytother. Res.28, 1419–1422. 10.1002/ptr.5116
299
SinglaR. K.JoonS.ShenL.ShenB. (2021). Translational informatics for natural products as antidepressant agents. Front. Cell Dev. Biol.9, 738838. 10.3389/fcell.2021.738838
300
SinglaR. K.ScottiL.DubeyA. K. (2017). In silico studies revealed multiple neurological targets for the antidepressant molecule ursolic acid. Curr. Neuropharmacol.15, 1100–1106. 10.2174/1570159X14666161229115508
301
SiqueiraI. R.CimarostiH.FochesattoC.NunesD. S.SalbegoC.ElisabetskyE.et al (2004). Neuroprotective effects of Ptychopetalum olacoides Bentham (Olacaceae) on oxygen and glucose deprivation induced damage in rat hippocampal slices. Life Sci.75, 1897–1906. 10.1016/j.lfs.2004.06.001
302
SivanantharajahL.MudherA. (2022). Curcumin as a holistic treatment for tau pathology. Front. Pharmacol.13, 903119. 10.3389/fphar.2022.903119
303
SmoliloD. J.CostaM.HibberdT. J.BrookesS. J.WattchowD. A.SpencerN., J. (2019). Distribution, projections, and association with calbindin baskets of motor neurons, interneurons, and sensory neurons in Guinea-pig distal colon. J. Comp. Neurol.527, 1140–1158. 10.1002/cne.24594
304
SongQ.PengS.ZhuX. (2021). Baicalein protects against MPP+/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson’s disease. Neurotoxicology87, 188–194. 10.1016/j.neuro.2021.10.003
305
SpectorP. E.FoxS.PenneyL. M.BruursemaK.GohA.KesslerS. (2006). The dimensionality of counterproductivity: Are all counterproductive behaviors created equal?J. Vocat. Behav.68, 446–460. 10.1016/j.jvb.2005.10.005
306
SpeersA. B.CabeyK. A.SoumyanathA.WrightK. M. (2021). Effects of Withania somnifera (ashwagandha) on stress and the stress-related neuropsychiatric disorders anxiety, depression, and insomnia. Curr. Neuropharmacol.19, 1468–1495. 10.2174/1570159X19666210712151556
307
SrinivasanE.ChandrasekharG.RajasekaranR. (2022). Probing the polyphenolic flavonoid, morin as a highly efficacious inhibitor against amyloid (A4V) mutant SOD1 in fatal Amyotrophic Lateral Sclerosis. Arch. Biochem. Biophys.727, 109318. 10.1016/j.abb.2022.109318
308
SrinivasanG.RanjithC.VijayanK. (2008). Identification of chemical compounds from the leaves of Leea indica. Acta Pharm.58, 207–214. 10.2478/v10007-008-0002-7
309
SrinivasanS.GalJ.BachstetterA.NelsonP. T. (2022). Alpha adaptins show isoformspecific association with neurofibrillary tangles in Alzheimer's disease. Neuropathol. Appl. Neurobiol.48, 12776. 10.1111/nan.12776
310
StanciuG. D.LucaA.RusuR. N.BildV.BescheaChiriac S. I.SolcanC.et al (2020). Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules10, 40. 10.3390/biom10010040
311
StefanovskiL.MeierJ. M.PaiR. K.TriebkornP.LettT.MartinL.et al (2021). Bridging scales in alzheimer's disease: Biological framework for brain simulation with the virtual brain. Front. Neuroinform.15, 630172. 10.3389/fninf.2021.630172
312
StoughC.LloydJ.ClarkeJ.DowneyL.HutchisonC.RodgersT.et al (2001). Nathan, P. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology156, 481–484. 10.1007/s002130100815
313
SuC. F.JiangL.ZhangX. W.IyaswamyA.LiM. (2021). Resveratrol in rodent models of Parkinson’s disease: A systematic review of experimental studies. Front. Pharmacol.12, 644219. 10.3389/fphar.2021.644219
314
SuC.ZhangD.TruongJ.JiangC.LeeS.JaroucheM.et al (2013). Effects of a novel herbal formulation JSK on acute spinal cord injury in rats. Restor. Neurol. Neurosci.31, 597–617. 10.3233/RNN-120303
315
SubarnasA.OshimaY.OhizumiY. (1992). An antidepressant principle of Lobelia inflata L. (Campanulaceae). J. Pharm. Sci.81, 620–621. 10.1002/jps.2600810705
316
SubarnasA.TadanoT.NakahataN.AraiY.KinemuchiH.OshimaY.et al (1993). A possible mechanism of antidepresant activity of beta-amyrin palmitate isolated from Lobelia inflata leaves in the forced swimming test. Life Sci.52, 289–296. 10.1016/0024-3205(93)90220-W
317
SubhanF.KarimN.GilaniA. H.SewellR. D. (2010). Terpenoid content of Valerianawallichii extracts and antidepressant-like response profiles. Phytother. Res.24, 686–691. 10.1002/ptr.2980
318
TabeshpourJ.MehriS.Shaebani BehbahaniF.HosseinzadehH. (2018). Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: A comprehensive review. Phytother. Res.32, 2164–2190. 10.1002/ptr.6168
319
TakemotoY.KishiC.EhiraH.MatsuiN.YamaguchiT.YoshiokaY.et al (2022). Inhaled turmerones can be incorporated in the organs via pathways different from oral administration and can affect weight-gain of mice. Sci. Rep.12, 1–8. 10.1038/s41598-022-15168-9
320
ThomasI.TimothyD. G.VeltmeyerJ.O'ConnorK. (2022). “Treatment of major depressive disorder and suicidal ideations through stimulation of hippocampal neurogenesis utilizing plant-based approaches,”. US20220175701.
321
ThomfordN. E.SenthebaneD. A.RoweA.MunroD.SeeleP.MaroyiA.et al (2018). Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci.19, 1578. 10.3390/ijms19061578
322
ThuraisingamS.SalimN.AzmiI. D.KartineeN. (2022). Development of nanoemulsion containing Centella asiatica crude extract as a promising drug delivery system for epilepsy treatment. Biointerface Res. Appl. Chem.13, 17. 10.33263/BRIAC131.017
323
ToyoshimaY.TakahashiH. (2014). TDP-43 pathology in polyglutamine diseases: With reference to amyotrphic lateral sclerosis. Neuropathology34, 77–82. 10.1111/neup.12053
324
TrägerU.AndreR.Magnusson-LindA.MillerJ. R.ConnollyC.WeissA.et al (2015). Characterisation of immune cell function in fragment and full-length Huntington's disease mouse models. Neurobiol. Dis.73, 388–398. 10.1016/j.nbd.2014.10.012
325
TungmunnithumD.DrouetS.HanoC. (2022). Phytochemical diversity and antioxidant potential of natural populations of Nelumbo nucifera Gaertn. throughout the floristic regions in Thailand. Molecules27, 681. 10.3390/molecules27030681
326
UsmanM. B.OjhaS.JhaS. K.ChellappanD. K.GuptaG.SinghS. K.et al (2022). Biological databases and tools for neurological disorders. J. Integr. Neurosci.21, 41. 10.31083/j.jin2101041
327
UtomoR. Y.SugieA.OkadaS.MiuraK.NakamuraH. (2022). Detoxification of amyloid β fibrils by curcumin derivatives and their verification in a Drosophila Alzheimer’s model. Chem. Commun.58, 2576–2579. 10.1039/D1CC07000B
328
VaijnathS. S.SurajA. (2019). Development and evaluation of wedelolactone nasal formulation for antiepileptic activity, IN201921009898.
329
Van DiermenD.MarstonA.BravoJ.ReistM.CarruptP. A.HostettmannK. (2009). Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol.122, 397–401. 10.1016/j.jep.2009.01.007
330
VenâncioE. T.RochaN. F.RiosE. R.FeitosaM. L.LinharesM. I.MeloF. H.et al (2011). Anxiolytic-like effects of standardized extract of Justicia pectoralis (SEJP) in mice: Involvement of GABA/benzodiazepine in receptor. Phytother. Res.25, 444–450. 10.1002/ptr.3274
331
VerstraetenA.TheunsJ.VanBroeckhovenC. (2015). Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet.31, 140–149. 10.1016/j.tig.2015.01.004
332
VianaA.do RegoJ. C.von PoserG.FerrazA.HecklerA. P.CostentinJ.et al (2005). The antidepressant-like effect of Hypericum caprifoliatum Cham &Schlecht (Guttiferae) on forced swimming test results from an inhibition of neuronal monoamine uptake. Neuropharmacology49, 1042–1052. 10.1016/j.neuropharm.2005.06.002
333
VidyantiA. N.AwaliyahM. T.FauziA. R.HarahapI. S.MulyaD. P. (2022). Dementia in a patient with autoimmune disease and hypercoagulable state worsened by COVID-19 vaccination: A case report. Ann. Med. Surg.78, 103886. 10.1016/j.amsu.2022.103886
334
VijhD.ImamM.HaqueM.DasS.IslamA.MalikM. (2022). Network pharmacology and bioinformatics approach reveals the therapeutic mechanism of action of curcumin in Alzheimer disease. Res. Sq. Prepr. version. 1. 1.
335
VillaF. A.GerwickL. (2010). Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol. Immunotoxicol.32, 228–237. 10.3109/08923970903296136
336
VillosladaP.MorenoB.MeleroI.PablosJ. L.MartinoG.UccelliA.et al (2008). Immunotherapy for neurological diseases. Clin. Immunol.128, 294–305. 10.1016/j.clim.2008.04.003
337
ViswambharanV.ThanseemI.VasuM. M.PoovathinalS. A.AnithaA. (2017). miRNAs as biomarkers of neurodegenerative disorders. Biomark. Med.11, 151–167. 10.2217/bmm-2016-0242
338
WanC.ChenG.FuY.WangM.MatsuhisaN.PanS.et al (2018). An artificial sensory neuron with tactile perceptual learning. Adv. Mat.30, 1801291. 10.1002/adma.201801291
339
WangN.PanX. Y.ZhuH. K.GuoY. H.QianH. (2021). Chicoric acid prevents neurodegeneration via microbiota-gut-brain axis in a mouse Parkinson’s disease model. bioRxiv. 1. 1. 10.1101/2021.05.03.442390
340
WangP. S.Aguilar-GaxiolaS.AlonsoJ.AngermeyerM. C.BorgesG.BrometE. J.et al (2007). Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet370, 841–850. 10.1016/S0140-6736(07)61414-7
341
WangT.-Y.LiQ.BiK.-S. (2018). Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci.13, 12–23. 10.1016/j.ajps.2017.08.004
342
WangW.HuX.ZhaoZ.LiuP.HuY.ZhouJ.et al (2008). Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry32, 1179–1184. 10.1016/j.pnpbp.2007.12.021
343
WangY.WeiN.LiX. (2020). Preclinical evidence and possible mechanisms of baicalein for rats and mice with Parkinson's disease: A systematic review and meta-analysis. Front. Aging Neurosci.12, 277. 10.3389/fnagi.2020.00277
344
WernerM. H.OlanowC. W. (2022). Parkinson's disease modification through abl kinase inhibition: An opportunity. Mov. Disord.37, 6–15. 10.1002/mds.28858
345
WichansawakunS.ChupisanyaroteK.WongpipathpongW.KaurG.ButtarH. S. (2022). Functional foods and nutraceuticals in metabolic and non-communicable diseases. Academic Press, 533–549. 10.1016/B978-0-12-819815-5.00028-8Antioxidant diets and functional foods attenuate dementia and cognition in elderly subjects
346
WoelkH.ArnoldtK. H.KieserM.HoerrR. (2007). Ginkgo biloba special extract EGb 761® in generalized anxiety disorder and adjustment disorder with anxious mood: A randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res.41, 472–480. 10.1016/j.jpsychires.2006.05.004
347
WojcikG. M.MasiakJ.KawiakA.SchneiderP.KwasniewiczL.PolakN.et al (2018). New protocol for quantitative analysis of brain cortex electroencephalographic activity in patients with psychiatric disorders. Front. Neuroinform.12, 27. 10.3389/fninf.2018.00027
348
WuY.ChenQ.WenB.WuN.HeB.ChenJ. (2021). Berberine reduces Aβ42 deposition and tau hyperphosphorylation via ameliorating endoplasmic reticulum stress. Front. Pharmacol.12, 640758. 10.3389/fphar.2021.640758
349
XieW.ZhangX.WangT.HuJ. (2007). Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L.(Luobuma): A review. J. Ethnopharmacol.141, 1–8. 10.1016/j.jep.2012.02.003
350
XuB.WangX.XuZ.LiQ.QuanJ. (2022). N-cystaminylbiguanide MC001 prevents neuron cell death and alleviates motor deficits in the MPTP-model of Parkinson’s disease. Neurosci. Lett.784, 136751. 10.1016/j.neulet.2022.136751
351
XuK.LiY.AllenE. G.JinP. (2021). Therapeutic development for CGG repeat expansion-associated neurodegeneration. Front. Cell. Neurosci.15, 655568. 10.3389/fncel.2021.655568
352
XuQ.PanY.YiL. T.LiY. C.MoS. F.JiangF. X.et al (2008). Antidepressant-like effects of psoralen isolated from the seeds of Psoralea corylifolia in the mouse forced swimming test. Biol. Pharm. Bull.31, 1109–1114. 10.1248/bpb.31.1109
353
YangJ.FangL.LuH.LiuC.WangJ.WuD.et al (2022). Walnut-derived peptide enhances mitophagy via JNK-mediated PINK1 activation to reduce oxidative stress in HT-22 cells. J. Agric. Food Chem.70, 2630–2642. 10.1021/acs.jafc.2c00005
354
YangJ. Q.WangR.RenY.MaoJ. Y.WangZ. P.ZhouY.et al (2020). Neuromorphic engineering: From biological to spike-based hardware nervous systems. Adv. Mat.32, 2003610. 10.1002/adma.202003610
355
YiL. T.LiJ.GengD.LiuB. B.FuY.TuJ. Q.et al (2013). Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in mice. J. Ethnopharmacol.147, 245–253. 10.1016/j.jep.2013.03.015
356
YiL. T.LiY. C.PanY.LiJ. M.XuQ.MoS. F.et al (2008). Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea corylifolia in the forced swimming test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry32, 510–519. 10.1016/j.pnpbp.2007.10.005
357
YiannopoulouK. G.PapageorgiouS. G. (2013). Current and future treatments for Alzheimer's disease. Ther. Adv. Neurol. Disord.6, 19–33. 10.1177/1756285612461679
358
YinJ.XingH.YeJ. (2008). Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism.57, 712–717. 10.1016/j.metabol.2008.01.013
359
YoonJ. J.JeongJ. W.ChoiE. O.KimM. J.Hwang-BoH.KimH. J.et al (2017). Protective effects of Scutellaria baicalensis Georgi against hydrogen peroxide-induced DNA damage and apoptosis in HaCaT human skin keratinocytes. EXCLI J.16, 426–438. 10.17179/excli2016-817
360
YoshitakeT.YoshitakeS.KehrJ. (2010). The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol.159, 659–668. 10.1111/j.1476-5381.2009.00580.x
361
YousafM.ChangD.LiuY.LiuT.ZhouX. (2022). Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules27, 4961. 10.3390/molecules27154961
362
YuY.ShenQ.LaiY.ParkS. Y.OuX.LinD.et al (2018). Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol.9, 386. 10.3389/fphar.2018.00386
363
YuZ. F.KongL. D.ChenY. (2022). Antidepressant activity of aqueous extracts of Curcuma longa in mice. J. Ethnopharmacol.83, 161–165. 10.1016/S0378-8741(02)00211-8
364
ZaazaaA. M.DaoudN. N.El-GendyO. A.Al-ShafeiA. I. (2022). Neuroprotective role of Bacopa monnieri extract in modulating depression in an experimental rat model. J. Affect. Disord.308, 229–235. 10.1016/j.jad.2022.04.021
365
ZarneshanS. N.FakhriS.KhanH. (2022). Targeting akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res.177, 106099. 10.1016/j.phrs.2022.106099
366
ZhangB.ZhaoJ.WangZ.GuoP.LiuA.DuG. (2021). Identification of multi-target anti-ad chemical constituents from traditional Chinese medicine formulae by integrating virtual screening and in vitro validation. Front. Pharmacol.12, 709607. 10.3389/fphar.2021.709607
367
ZhangL.-X.LiC.-X.KakarM. U.KhanM. S.WuP.-F.AmirR. M.et al (2021). Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother.143, 112164. 10.1016/j.biopha.2021.112164
368
ZhangS.GongT. T.LiuF. H.JiangY. T.SunH.MaX. X.et al (2019). Global, regional, and national burden of endometrial cancer, 1990–2017: Results from the global burden of disease study. Front. Oncol.9, 1440. 10.3389/fonc.2019.01440
369
ZhangZ. Q.YuanL.YangM.LuoZ. P.ZhaoY. M. (2002). The effect of Morinda officinalis How, a Chinese traditional medicinal plant, on the DRL 72-s schedule in rats and the forced swimming test in mice. Pharmacol. Biochem. Behav.72, 39–43. 10.1016/S0091-3057(01)00730-4
370
ZhaoH.WangC.ZhaoN.LiW.YangZ.LiuX.et al (2018). Potential biomarkers of Parkinson's disease revealed by plasma metabolic profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1081, 101–108. 10.1016/j.jchromb.2018.01.025
371
ZhaoX.KongD.ZhouQ.WeiG.SongJ.LiangY.et al (2021). Baicalein alleviates depression-like behavior in rotenone-induced Parkinson's disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother.140, 111556. 10.1016/j.biopha.2021.111556
372
ZhaoZ.WangW.GuoH.ZhouD. (2008). Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res.194, 108–113. 10.1016/j.bbr.2008.06.030
373
ZhengM.FanY.ShiD.LiuC. (2013). Antidepressant-like effect of flavonoids extracted from Apocynum venetum leaves on brain monoamine levels and dopaminergic system. J. Ethnopharmacol.147, 108–113. 10.1016/j.jep.2013.02.015
374
ZhouD.JinH.LinH. B.YangX. M.ChengY. F.DengF. J.et al (2010). Antidepressant effect of the extracts from Fructus akebiae. Pharmacol. Biochem. Behav.94, 488–495. 10.1016/j.pbb.2009.11.003
375
ZhouN.GuX.ZhuangT.XuY.YangL.ZhouM. (2020). Gut microbiota: A pivotal hub for polyphenols as antidepressants. J. Agric. Food Chem.68, 6007–6020. 10.1021/acs.jafc.0c01461
376
ZlotnikG.VansintjanA. (2019). Memory: An extended definition. Front. Psychol.10, 2523. 10.3389/fpsyg.2019.02523
377
ZoeyF. L.PalanivelM.PadmanabhanP.GulyásB. (2021). Parkinson’s disease: A nanotheranostic approach targeting alpha-synuclein aggregation. Front. Cell Dev. Biol.9. 10.3389/fcell.2021.707441707441
378
ZucchellaC.SinforianiE.TamburinS.FedericoA.MantovaniE.BerniniS.et al (2018). The multidisciplinary approach to Alzheimer's disease and dementia. A narrative review of non-pharmacological treatment. Front. Neurol.9, 1058. 10.3389/fneur.2018.01058
Summary
Keywords
natural products, neurological disorders, clinical research, bioinformatic tools, translational research
Citation
Puri V, Kanojia N, Sharma A, Huanbutta K, Dheer D and Sangnim T (2022) Natural product-based pharmacological studies for neurological disorders. Front. Pharmacol. 13:1011740. doi: 10.3389/fphar.2022.1011740
Received
04 August 2022
Accepted
18 October 2022
Published
07 November 2022
Volume
13 - 2022
Edited by
Suresh Kumar, Punjabi University, India
Reviewed by
Sanjeev Rastogi, University of Lucknow, India
Pradeep Kumar Prajapati, University of Delhi, India
Updates
Copyright
© 2022 Puri, Kanojia, Sharma, Huanbutta, Dheer and Sangnim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Divya Dheer, divya.dheer@chitkarauniversity.edu.in; Tanikan Sangnim, tanikan@go.buu.ac.th
This article was submitted to Neuropharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.