Abstract
Aim: To know whether metformin use has different influence on cardiovascular risks in patients with type 2 diabetes mellitus (T2DM) and chronic obstructive pulmonary disease (COPD) as compared with metformin no-use.
Methods: This study employed a retrospective cohort study design. Using propensity score matching, we recruited 55 ,224 pairs of metformin users and nonusers from Taiwan’s National Health Insurance Research Database between 1 January 2000, and 31 December 2017. Cox proportional-hazards models with robust standard error estimates were used to compare the risks of cardiovascular outcomes.
Results: The mean study period of metformin users and nonusers was 11.04 (5.46) and 12.30 (4.85) years, respectively. Compared with the nonuse of metformin, the adjusted hazard ratios (95% CI) of metformin use for composited cardiovascular events, stroke, coronary artery disease, and heart failure were 0.51 (0.48–0.53), 0.62 (0.59–0.64), 0.48 (0.46–0.50), and 0.61 (0.57–0.65), respectively. The longer cumulative duration of metformin use had even lower adjusted hazard ratios compared with metformin nonuse.
Conclusion: In patients with coexisting T2DM and COPD, metformin use was associated with significantly lower risks of CVD; moreover, longer duration of metformin use was associated with a lower risk of CVD. A well-designed prospective study is required to verify the results.
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by smoking or air pollution and progressively limits the airflow. Furthermore, it can cause chronic inflammation of the respiratory tract and whole body and may exacerbate intermittently (Bhatt and Dransfield, 2013). Approximately 5%–10% of the population of the United States has COPD (Bhatt and Dransfield, 2013). Currently, COPD affects approximately 380 million individuals worldwide (Lopez et al., 2006). It is sixth among diseases in terms of global mortality and caused approximately 3.3 million deaths in 2019 (GBD 2019 Diseases and Injuries Collaborators, 2020). Type 2 diabetes mellitus (T2DM) is a hyperglycemic disease caused by insulin resistance and insufficient insulin secretion, which may be caused by obesity and reduced physical activity (International Diabetes Federation (IDF), 2019). The global prevalence rate of T2DM is approximately 9.3%. Currently, there are approximately 463 million patients with diabetes worldwide, and this figure is expected to increase to 578 million by 2030 (International Diabetes Federation (IDF), 2019). In 2019, it ranked eighth among diseases in terms of mortality (GBD 2019 Diseases and Injuries Collaborators, 2020).
T2DM, possibly through hyperglycemia-induced oxidative stress and inflammation, can cause pulmonary microvascular complications, diminish lung function, exacerbate COPD, and increase mortality (Gläser et al., 2015). Approximately 10% of people with T2DM also have COPD. Moreover, T2DM aggravates the progression and mortality risk of patients with COPD (Gläser et al., 2015). COPD, possibly through chronic inflammation and high-dose corticosteroid use, can lead to the development or deterioration of T2DM (Gläser et al., 2015). Therefore, COPD coexisting with T2DM is not uncommon, and it represents a relatively serious chronic disease that requires careful management (Gläser et al., 2015). Cardiovascular disease (CVD) shares many of the same causes with T2DM and COPD, such as old age, smoking, metabolic syndrome, and systemic inflammation (Bhatt and Dransfield, 2013). Therefore, CVD often occurs in patients with T2DM and COPD and is the leading cause of death among them (Bhatt and Dransfield, 2013; Low Wang et al., 2016). When using medications to treat patients with T2DM and COPD, it may be crucial to consider whether those medications have any effect on CVD risk.
Metformin has long been the world’s first-line medication for managing T2DM (American Diabetes Association, 2021). In the UK Prospective Diabetes Study (UKPDS), metformin use in overweight patients with T2DM was demonstrated to reduce the risk of myocardial infarction (UK Prospective Diabetes Study (UKPDS) Group, 1998). Its use in patients with COPD can cause lactic acidosis because they are prone to hypoxia; however, a later study confirmed that the incidence is very low (approximately 0.03 cases per 1000 person-years) (Bailey and Turner, 1996). Our previous study disclosed that metformin use in patients with COPD was associated with a reduced risk of mortality compared with metformin nonuse (Yen et al., 2018). Metformin exhibits antioxidant, anti-inflammatory, and possibly cardioprotective actions despite its glucose-lowering effect (Nesti and Natali, 2017). Therefore, we conducted this retrospective cohort study to investigate whether the influence of metformin use on CVD in patients with comorbid T2DM and COPD are different compared with metformin nonuse.
Materials and methods
Study population
Taiwan established its National Health Insurance (NHI) program in 1995. People are only required to pay a small premium, while their employers and the government pay the largest proportion (Cheng, 2003). By 2000, approximately 99% of Taiwan’s people had joined the NHI program. The NHI Research Database (NHIRD) contains data on the insured’s age, sex, place of residence, salary, medications, clinical procedures, and diagnosis according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM. The NHI Bureau regularly evaluates the diagnostic codes and medical management of clinical units to ensure correct diagnoses as well as the proper disposal of the NHI system. Additionally, to govern the usage of the NHIRD, Taiwan’s Ministry of Health and Welfare constructed the Health and Welfare Data Center (HWDC) in 2016 to standardize data management for all available health care data. All methods employed by this study were performed in accordance with the Declaration of Helsinki. The identifiable information of patients and caregivers was deidentified before release; therefore, the need for informed consent was waived. This study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH109-109-REC2-031).
Study design
We consecutively selected patients who had received the diagnoses of T2DM and COPD between 1 January 2000, and 31 December 2017; they were followed up to 31 December 2018. The diagnosis of T2DM and COPD was considered using the ICD-9-CM code or ICD-10-CM code (Supplementary Table S1) for at least two outpatient visits or one hospitalization record. Validation using ICD codes to define T2DM, COPD, and CVD has been performed by previous studies in Taiwan (Lin et al., 2005; Cheng et al., 2014; Lin et al., 2017; Su et al., 2019). We have further evaluated the severity of COPD. Mild-to-moderate COPD exacerbation was defined by the medication of antibiotics or systemic corticosteroids within 1 year, and which was managed in the outpatient setting. Severe COPD exacerbation was considered in the event of an emergency room (ER) visit or hospitalization within 1 year (Rodriguez-Roisin, 2000). Patients were excluded (Figure 1) if they (1) were < 40 or > 100 years; (2) did not use antidiabetic drugs; (3) had missing data of age or sex; (4) were diagnosed as having type 1 DM, hepatic failure, or were undergoing dialysis; (5) were followed up for less than 180 days after the index date to exclude latent diseases; or (6) had been diagnosed as having COPD or T2DM before January 1, 2000, to exclude prevalent diseases.
FIGURE 1
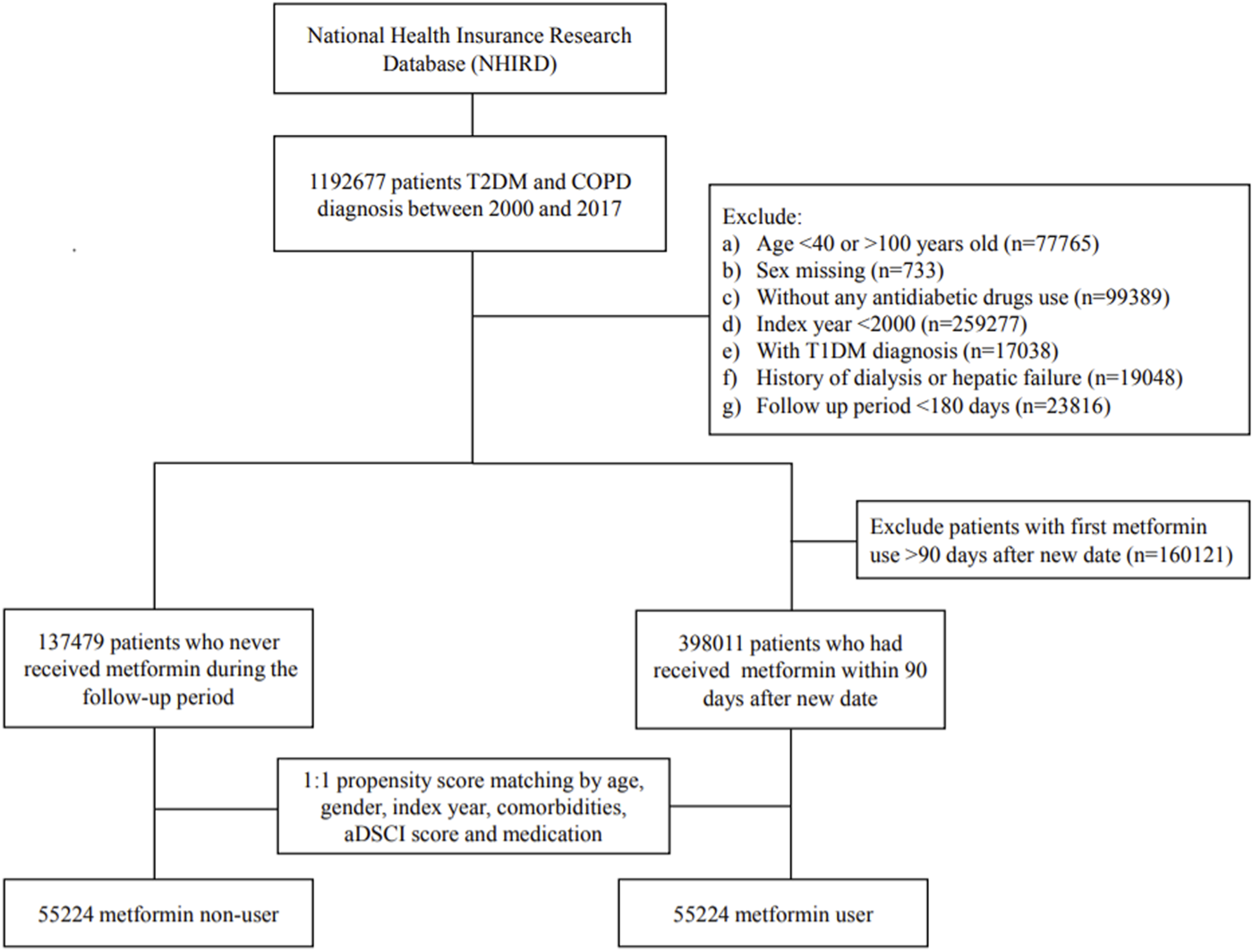
Flow diagram of the recruitment process.
Procedures
Comorbidity date was defined as the date of concurrent diagnosis of COPD and T2DM (Figure 2). Within 90 days following said date, patients who received metformin for at least 30 days were defined as metformin users, whereas those who never received metformin during the study period were defined as metformin nonusers. We defined the first date of metformin use after the comorbidity date as the index date. The index date of the control group was defined as the same period from the comorbidity date to the index date of the study group. Some related variables were assessed and matched between the metformin user and nonuser, including age, sex, comorbidity, Diabetes Complication Severity Index (DCSI) score and medication. Preexisting comorbidities including overweight; obesity; severe obesity; smoking status; the comorbidities of hypertension, dyslipidemia, chronic kidney disease (CKD), coronary artery disease (CAD), stroke, heart failure, peripheral arterial occlusive disease (PAOD), and atrial fibrillation diagnosed within 1 year before the index date; and medications use including respiratory drugs, antidiabetic drugs, antihypertensive drugs, statins, and aspirin during the study period. Glucagon-like peptide one receptor agonists were marketed in Taiwan since 2011, sodium-glucose cotransporter-2 inhibitors were marketed in Taiwan since 2015. Because the number of patients receiving these two antidiabetic medications was so small that they didn’t be contained in the baseline co-medication. To accurately reflect the characteristics of our patients with COPD and T2DM, we used the DCSI score (Young et al., 2008) to evaluate T2DM severity and the number of moderate or severe exacerbations of COPD to investigate the stability of COPD.
FIGURE 2
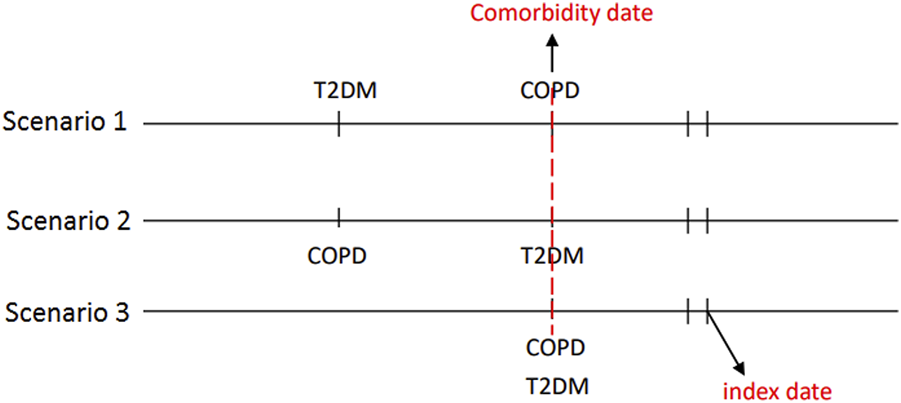
The concurrent diagnosis of T2DM and COPD was defined as the comorbidity date. The first date of metformin use after the comorbidity date was defined as the index date.
Main outcomes
Hospitalized stroke, CAD, heart failure, and composite cardiovascular events were the main outcomes of this study. We calculated the events and incidence rates of composite cardiovascular events, stroke, CAD, and heart failure during the study period. The cumulative incidences of stroke, CAD, and heart failure were also compared between metformin users and nonusers.
Statistical analyses
Propensity-score matching was used to match the related covariates between metformin users and nonusers (D’Agostino, 1998). The propensity score was estimated for each patient using nonparsimonious multivariable logistic regression, with metformin use as the dependent variable. We included 32 clinically relevant covariates as independent variables (Table 1). The nearest-neighbor algorithm was used to construct matched pairs, assuming the standardized mean difference of ≤ 0.05 to be a negligible difference between the case and comparison cohorts.
TABLE 1
| Characteristics | Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin nonusers | Metformin users | SMD | Metformin nonusers | Metformin users | SMD | |||||
| N = 1,37 ,479 | N = 3,98 ,011 | N = 55 ,224 | N = 55 ,224 | |||||||
| n | % | n | % | n | % | n | % | |||
| Age, y | ||||||||||
| <55 | 28 ,030 | 20.39 | 131 642 | 33.07 | 0.28 | 14 ,252 | 29.91 | 12 ,833 | 26.93 | 0.04 |
| 55–65 | 35 ,742 | 26.00 | 132 119 | 33.19 | 0.15 | 15 ,566 | 32.66 | 16 ,929 | 35.52 | 0.04 |
| >65 | 73 ,707 | 53.61 | 134 250 | 33.73 | 0.4 | 17 ,838 | 37.43 | 17 ,894 | 37.55 | 0.002 |
| Mean ± SD | 65.88 ± 12.02 | 60.46 ± 10.93 | 0.47 | 61.47 ± 11.02 | 61.78 ± 10.42 | 0.01 | ||||
| Sex | ||||||||||
| Female | 66 ,328 | 48.25 | 172 661 | 43.38 | 0.1 | 24 ,970 | 52.40 | 24 ,628 | 51.68 | 0.009 |
| Male | 71 ,151 | 51.75 | 2,25 ,350 | 56.62 | 0.1 | 22 ,686 | 47.60 | 23 ,028 | 48.32 | 0.009 |
| Comorbidity | ||||||||||
| Obesity | ||||||||||
| Overweight | 147 | 0.11 | 556 | 0.14 | 0.009 | 70 | 0.15 | 64 | 0.13 | 0.003 |
| Obesity | 384 | 0.28 | 2048 | 0.51 | 0.03 | 225 | 0.47 | 200 | 0.42 | 0.007 |
| Severe obesity | 58 | 0.04 | 249 | 0.06 | 0.008 | 32 | 0.07 | 30 | 0.06 | 0.001 |
| Smoking | 1698 | 1.24 | 6491 | 1.63 | 0.03 | 8821 | 1.72 | 777 | 1.63 | 0.007 |
| Chronic kidney disease | 19 ,146 | 13.93 | 28 ,733 | 7.22 | 0.21 | 5107 | 10.72 | 5226 | 10.97 | 0.008 |
| PAOD | 4160 | 3.03 | 8033 | 2.02 | 0.06 | 1313 | 2.76 | 1341 | 2.81 | 0.003 |
| Atrial fibrillation | 2245 | 1.63 | 3780 | 0.95 | 0.06 | 517 | 1.08 | 511 | 1.07 | 0.001 |
| Previous stroke | 31 ,329 | 22.79 | 52 ,919 | 13.30 | 0.24 | 8319 | 17.46 | 8413 | 17.65 | 0.005 |
| Previous CAD | 45 ,317 | 32.96 | 94 ,474 | 23.74 | 0.21 | 14 ,770 | 30.99 | 14 ,751 | 30.95 | 0.009 |
| Previous HF | 6854 | 4.99 | 11 ,599 | 2.91 | 0.11 | 1719 | 3.61 | 1804 | 3.79 | 0.01 |
| Exacerbation of COPD | ||||||||||
| Mild | 51 ,230 | 37.26 | 1,59 ,713 | 40.13 | 0.06 | 18 ,508 | 38.83 | 18 ,554 | 38.93 | 0.004 |
| Moderate | 74 ,310 | 54.05 | 2,20 ,326 | 55.36 | 0.03 | 26 ,758 | 56.15 | 26 ,654 | 55.93 | 0.002 |
| Severe | 11 ,939 | 8.68 | 17 ,972 | 4.52 | 0.17 | 2390 | 5.02 | 2448 | 5.14 | 0.006 |
| DCSI score | ||||||||||
| 0 | 37 ,518 | 27.29 | 144 371 | 36.27 | 0.19 | 15 ,851 | 33.26 | 15 ,865 | 33.29 | 0.001 |
| 1 | 17 ,762 | 12.92 | 55 ,773 | 14.01 | 0.03 | 7421 | 15.57 | 7253 | 15.22 | 0.009 |
| 2 | 82 ,199 | 59.79 | 197 867 | 49.71 | 0.2 | 24 ,384 | 51.17 | 24 ,538 | 51.49 | 0.006 |
| Medication | ||||||||||
| Respiratory drugs | ||||||||||
| β2 bronchodilator inhalants | 59 ,154 | 43.03 | 149 300 | 37.51 | 0.11 | 14 ,227 | 29.85 | 14 ,439 | 30.30 | 0.01 |
| Anticholinergic inhalants | 41 ,564 | 30.23 | 97 ,761 | 24.56 | 0.13 | 9007 | 18.90 | 9078 | 19.05 | 0.004 |
| Corticosteroid inhalants | 91 ,110 | 66.27 | 251 303 | 63.14 | 0.07 | 30 ,674 | 64.37 | 30 ,701 | 64.42 | 0.001 |
| Oral systemic corticosteroid | 6954 | 5.07 | 16 ,320 | 4.10 | 0.05 | 1593 | 3.34 | 1561 | 3.28 | 0.003 |
| Methylxanthine | 42 ,011 | 30.56 | 1,00 ,381 | 25.22 | 0.11 | 9317 | 19.55 | 9456 | 19.84 | 0.007 |
| Antidiabetic drugs | ||||||||||
| Sulphonylurea | 24 ,443 | 17.78 | 183 854 | 46.19 | 0.95 | 17 ,821 | 32.27 | 17 ,881 | 32.38 | 0.002 |
| TZD | 16 ,153 | 11.75 | 1,00 ,740 | 25.31 | 0.73 | 12 ,928 | 23.41 | 13 ,170 | 23.85 | 0.02 |
| DPP4 inhibitor | 21 ,460 | 15.61 | 148 219 | 37.24 | 1.07 | 11 ,128 | 21.09 | 11 ,641 | 21.08 | 0.003 |
| AGI | 21 ,776 | 15.84 | 1,25 ,181 | 31.45 | 0.69 | 16 ,225 | 20.15 | 11 ,381 | 20.61 | 0.01 |
| Insulin | 59 ,734 | 43.45 | 59 ,246 | 14.89 | 0.44 | 14 ,000 | 29.38 | 15 ,644 | 28.33 | 0.02 |
| Antihypertensive drugs | ||||||||||
| ACEI/ARB | 40 ,428 | 29.41 | 1,03 ,747 | 26.07 | 0.07 | 14 ,837 | 31.13 | 14 ,754 | 30.96 | 0.003 |
| β-blockers | 46 ,534 | 33.85 | 109 940 | 27.62 | 0.14 | 17 ,143 | 35.97 | 16 ,894 | 35.45 | 0.01 |
| Calcium-channel blockers | 31 ,461 | 22.88 | 74 ,171 | 18.64 | 0.1 | 11 ,325 | 23.76 | 11 ,234 | 23.57 | 0.004 |
| Diuretics | 32 ,546 | 23.67 | 82 ,521 | 20.73 | 0.07 | 12 ,426 | 26.07 | 12 ,270 | 25.75 | 0.007 |
| Other drugs | ||||||||||
| Statin | 52 ,748 | 77.75 | 247 547 | 85.11 | 0.19 | 37 ,402 | 78.48 | 37 ,545 | 78.78 | 0.007 |
| Aspirin | 70 ,016 | 50.93 | 228 406 | 57.39 | 0.13 | 24 ,984 | 52.43 | 25 ,030 | 52.52 | 0.002 |
Characteristics and medication use between metformin users and nonusers among patients with T2DM and COPD.
Data shown as n (%) or mean ± SD.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AGI, alpha-glucosidase inhibitors; ARB, angiotensin receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DCSI, diabetes complications severity index; DM, diabetes; DPP4 inhibitor, dipeptidyl peptidase-4, inhibitor; HF, heart failure; PSM, propensity-score matching; SMD, standardized mean difference; TZD, thiazolidinedione. A standardized mean difference of 0.05 or less indicates a negligible difference.
Crude and multivariable-adjusted Cox proportional-hazards models were utilized to compare outcomes between metformin users and nonusers. Results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs) of metformin users compared with nonusers. To calculate the investigated CVD risks, we censored patients until the date of death, date of respective outcomes, or at the end of the follow-up on 31 December 2018, whichever occurred first. The Kaplan-Meier method and log-rank tests were used to compare the cumulative incidence of stroke, CAD, and heart failure during the follow-up period between metformin users and nonusers. We also assessed the cumulative duration of metformin use for the risk of stroke, CAD, and heart failure compared with metformin nonuse.
SAS (version 9.4; SAS Institute, Cary, NC, United States) was used for the statistical analyses; a 2-tailed p value < 0.05 was considered significant.
Results
Participants
From 1 January 2000, to 31 December 2017, 1 192 677 patients were diagnosed as having COPD and T2DM. After certain ineligible cases were excluded, we identified 398 ,011 metformin users and 137 ,479 nonusers during the study period. Figure 1 presents a flow diagram of the recruitment process.
Before propensity-score matching, we observed that the distribution of demographics, comorbidities and medications were significantly different between metformin users and nonusers (Table 1). After matching, 55 ,224 paired patients with COPD and t2DM were selected. In the matched cohorts, the mean (SD) age was 61.63 (10.72) years, and the mean T2DM duration was 2.02 (3.35) years. The mean study period of metformin users and nonusers was 11.04 (5.46) and 12.30 (4.85) years, respectively.
Main outcomes
In the matched cohorts (Table 2), 9236 (16.72%) metformin users and 14 ,806 (26.81%) nonusers developed composite cardiovascular events during the follow-up period (incidence rate: 15.75 vs. 28.12 per 1000 person-years). In multivariable model, the metformin user had a significantly lower incidence of composite cardiovascular events compared with nonusers (aHR = 0.51, 95%CI = 0.48–0.53).
TABLE 2
| Outcomes | Metformin nonusers (n = 55 224) | Metformin users(n = 55 224) | Crude HR (95% CI) | p value | Adjusted HR (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | PY | IR | Events | PY | IR | |||||
| Composited cardiovascular outcomes | 14 ,806 | 526 554 | 28.12 | 9236 | 586 ,432 | 15.75 | 0.55 (0.54–0.56) | <0.0001 | 0.51 (0.48–0.53) | <0.0001 |
| Stroke | 6702 | 579 ,875 | 11.56 | 4614 | 605 619 | 7.62 | 0.66 (0.63–0.68) | <0.0001 | 0.62 (0.59–0.64) | <0.0001 |
| CAD | 9728 | 559 ,808 | 17.38 | 5251 | 603 ,868 | 8.70 | 0.50 (0.48–0.52) | <0.0001 | 0.48 (0.46–0.50) | <0.0001 |
| Heart failure | 2220 | 612 ,224 | 3.63 | 1368 | 620 ,659 | 2.20 | 0.60 (0.57–0.64) | <0.0001 | 0.61 (0.57–0.65) | <0.0001 |
Hazard ratios and 95% confidence intervals of hospitalization cardiovascular outcomes associated with metformin use.
Abbreviations: CI, confidence interval; HR, hazard ratio; IR, Incidence rate = per 1000 person-years; PY, Person-years. aHR, adjusted for age, sex, comorbidities, DSCI, score, and medication listed in Table 1.
As presented in Table 2, compared with nonusers, metformin users were associated with significantly lower risks of hospitalized stroke (aHR 0.62, 95% CI 0.59–0.64), hospitalized CAD (aHR 0.48, 95% CI 0.46–0.50), and heart failure (aHR 0.61, 95% CI 0.57–0.65), respectively. The Kaplan−Meier analysis illustrated that the cumulative incidences of stroke, CAD, and heart failure were significantly lower in metformin users than in nonusers (Figure 3).
FIGURE 3
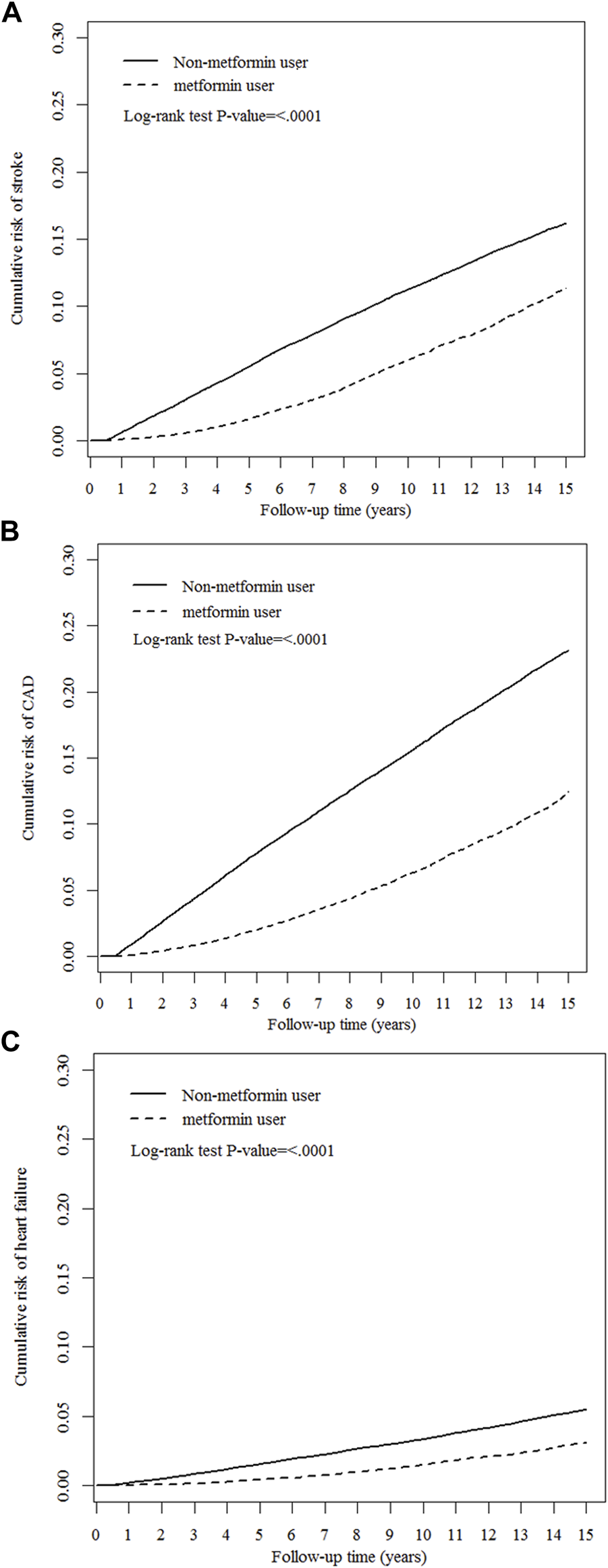
Cumulative risks of cardiovascular events between metformin users and nonusers. (A) stroke, (B) coronary artery disease (CAD), and (C) heart failure.
Cumulative duration of metformin use
The association between the cumulative duration of metformin use and the risk of stroke, CAD and heart failure were further analyzed and presented in Table 3. We found that longer cumulative durations of metformin use were associated with lower risks of stroke, CAD, and heart failure; the p values for the trend of cumulative duration of metformin use in stroke, CAD and heart failure risk were less than 0.0001.
TABLE 3
| Event | PY | IR | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| Cumulative duration of metformin use | |||||
| a) Stroke (n = 11 ,316) | |||||
| Nonuse | 6702 | 579 ,875 | 11.56 | 1 (Reference) | 1 (Reference) |
| <180 days | 1962 | 186 ,538 | 10.52 | 0.91 (0.87–0.96)*** | 0.81 (0.77–0.86)*** |
| 180–360 days | 761 | 97 ,788 | 7.78 | 0.67 (0.63–0.73)*** | 0.64 (0.60–0.69)*** |
| 361–720 days | 846 | 129 ,633 | 6.53 | 0.56 (0.52–0.61)*** | 0.57 (0.53–0.61)*** |
| >720 days | 1045 | 191 659 | 5.45 | 0.46 (0.43–0.49)*** | 0.42 (0.40–0.45)*** |
| P for trend | <0.0001 | <0.0001 | |||
| Cumulative duration of metformin use | |||||
| b) CAD (n = 14 ,979) | |||||
| Nonuse | 9728 | 559 ,808 | 17.38 | 1 (Reference) | 1 (Reference) |
| <180 days | 2194 | 187 484 | 11.70 | 0.67 (0.64–0.71)*** | 0.64 (0.61–0.67)*** |
| 180–360 days | 918 | 97 ,787 | 9.39 | 0.54 (0.51–0.58)*** | 0.53 (0.50–0.57)*** |
| 361–720 days | 986 | 129 ,600 | 7.61 | 0.44 (0.41–0.46)*** | 0.43 (0.40–0.46)*** |
| >720 days | 1153 | 188 996 | 6.10 | 0.34 (0.32–0.36)*** | 0.31 (0.30–0.32)*** |
| P for trend | <0.0001 | <0.0001 | |||
| Cumulative duration of metformin use | |||||
| c) Heart failure (n = 3588) | |||||
| Nonuse | 2220 | 612 ,224 | 3.63 | 1 (Reference) | 1 (Reference) |
| <180 days | 516 | 184 674 | 2.79 | 0.78 (0.71–0.86)*** | 0.76 (0.69–0.84)*** |
| 180–360 days | 216 | 98 ,218 | 2.20 | 0.61 (0.53–0.70)*** | 0.64 (0.56–0.74)*** |
| 361–720 days | 276 | 133 018 | 2.07 | 0.57 (0.51–0.65)*** | 0.63 (0.56–0.71)*** |
| >720 days | 360 | 204 748 | 1.76 | 0.48 (0.43–0.53)*** | 0.44 (0.40–0.50)*** |
| P for trend | <0.0001 | <0.0001 | |||
Hazard ratios of cardiovascular outcomes associated with the cumulative duration of metformin use.
*** p < 0.001.
Abbreviations: CI, confidence interval; HR, hazard ratio; IR, Incidence rate = per 1000 person-years; PY, Person-years. aHR, adjusted for age, sex, comorbidities, DSCI, score, and medication listed in Table 1.
Discussion
This study demonstrated that metformin use in patients with T2DM and COPD was associated with significantly lower risks of composite cardiovascular events, CAD, stroke, and heart failure. In addition, a longer cumulative duration of metformin use was associated with an even lower risk of cardiovascular events.
Cardiovascular disease is the most critical complication of T2DM (Low Wang et al., 2016), and patients with T2DM for a longer duration are at higher risk of CVD (Sattar, 2013). Patients with COPD, possibly through the common pathogenic factors of aging, smoking, and chronic inflammation, are also prone to complications from CVD (Bhatt and Dransfield, 2013; Rabe et al., 2018). CVDs are the most common and lethal comorbidities in patients with T2DM and COPD (Bhatt and Dransfield, 2013; Rabe et al., 2018). Metformin has been reported to reduce the mortality risk in patients with COPD or chronic lower respiratory diseases (Yen et al., 2018; Ho et al., 2019; Mendy et al., 2019). The classic UKPDS also demonstrated that metformin use in overweight patients with T2DM had a significantly lower risk of mortality and myocardial infarction (MI), and this result persisted in the posttrial observational data (Holman et al., 2008). A randomized trial of insulin-treated patients with T2DM revealed that metformin use was associated with a lower risk of a composite cardiovascular endpoint compared with a placebo (Kooy et al., 2009). A meta-analysis of observational studies confirmed that metformin use was related to reduced MI risk compared with sulphonylureas use (Pladevall et al., 2016). Furthermore, metformin use in patients with T2DM was reported to be associated with a lower risk of hospitalization for heart failure in Taiwan (Tseng, 2019). However, a meta-analysis of randomized clinical trials of metformin use in patients with T2DM disclosed a neutral effect in reducing CVD risk (Griffin et al., 2017). This may be because of the small size of some studies as well as their short follow-up periods with low event rates. In brief, these studies have suggested that metformin may have beneficial cardiovascular effects, especially in certain populations such as overweight or insulin-treated patients (Nesti and Natali, 2017). Our study also disclosed that metformin use in patients with T2DM and COPD was associated with a prominently lower risk of CVD compared with metformin nonuse; furthermore, a longer duration of metformin use was associated with a lower risk of CVD.
Metformin, through inhibiting mitochondrial respiratory-chain complex 1, can activate the liver kinase B1 (LKB1)/adenosine 5′-monophosphate-activated protein kinase (AMPK) pathway, inhibit hepatic gluconeogenesis, reduce advanced glycation end products (AGEs), diminish oxidative stress and chronic systemic inflammation (Nesti and Natali, 2017). Metformin can also improve insulin resistance by activating tyrosine kinase, which can attenuate the sympathetic tone, decrease vasoconstriction, and reduce blood pressure (Nesti and Natali, 2017). Clinical and animal studies have demonstrated that metformin could reduce cardiac endothelial dysfunction (Sardu et al., 2019), attenuate cardiac ischemia reperfusion injury, and positively affect cardiac performance during acute heart stress (Bhatt and Dransfield, 2013; Nesti and Natali, 2017). Moreover, metformin can attenuate remodeling of the heart after cardiac injury as well as reduce cardiac hypertrophy (Bhatt and Dransfield, 2013). A clinical study disclosed that metformin can increase nitro-oxide production and vasodilatation as well as improve pulmonary hypertension (Agard et al., 2009). Metformin has also been reported to be associated with some improvement in lipoprotein levels (Bhatt and Dransfield, 2013; Nesti and Natali, 2017). Taken together, metformin may reduce CVD risk by reducing insulin resistance, systemic inflammation, endothelial dysfunction, cardiac injury and remodeling.
Our study has some strengths. First, this study started from 2000 to 2018, which could be sufficiently long, and the large number of patients to observe the effect of a drug or intervention on cardiovascular events. Second, the population was recruited from nationwide consecutive cases, and therefore, the selection bias may be minimal.
This study also has some limitations. First, the dataset lacked hemoglobin A1C, renal function, and other biochemical tests; moreover, it did not contain pulmonary function or cardioechogram results, precluding the precise calculation of diabetes and COPD severity scores. Instead, we used clinical records of the number of moderate and severe exacerbations to evaluate COPD severity, and the DCSI to evaluate the severity of T2DM. Second, the NHIRD is lack of complete information on dietary patterns, family history, and physical activity; accurate population data for alcohol consumption and smoking status are also difficult to obtain. The lack of such data may have influenced the results of metformin use and outcome assessment. Furthermore, we cannot completely rule out the possibility of confounding by indication; that is, metformin users may have a lower disease burden than nonusers who take insulin or other oral antidiabetic drugs. However, we performed propensity-score matching to optimally balance many relevant variables between the study and control groups to minimize bias from possible confounding factors. Third, we are never sure about the effective intake of metformin, because the blood metformin levels are not available. Fourth, this study was Chinese population–based research, and therefore, its result may not be applicable to other ethnicities. Finally, cohort studies inevitably face some unknown confounding factors, and thus, randomized controlled studies are required to verify our results.
COPD and T2DM are both common chronic diseases with a usually indolent evolution and systemic complications. CVDs are critical complications of T2DM and COPD, and usually underdiagnosed and under-treated in these patients (Holman et al., 2008). CVD can also worsen the prognosis of patients with T2DM and COPD. Our study demonstrated that metformin use was associated with a significantly lower risk of CVD compared with metformin nonuse. If metformin can truly reduce the risk of CVD in this high risk group of patients, it shall have the potential to modify the natural history of these diseases. A well-designed prospective study is warranted to confirm our results.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Data of this study are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Administration. The data utilized in this study cannot be made available in the paper, the supplemental files, or in a public repository due to the “Personal Information Protection Act” executed by Taiwan government starting from 2012. Requests for data can be sent as a formal proposal to the NHIRD Office (https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html) or by email to stsung@mohw.gov.tw.
Ethics statement
The studies involving human participants were reviewed and approved by this study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH109-109-REC2-031). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, F-SY, C-CH, and C-MH. Methodology, F-SY and C-CH. Software, C-MH. Validation, L-TC and C-CH. Formal analysis, L-TC. Investigation, L-TC. Resources, JC-CW. Data curation, C.M.H. Writing—original draft preparation, F-SY. Writing—review and editing, JC-CW and C-CH. Visualization, C-MH. Supervision, C-CH and C-MH. Project administration, JC-CW and L-TC. Funding acquisition, C-MH. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by Taiwan’s Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), the MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-110-222), and Tseng-Lien Lin Foundation, Taichung, Taiwan. This work was also supported by grants from Taipei Veterans General Hospital (V105C-204, V110C-175, V109C-189, V108C-172, and VN107-07). This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), China Medical University Hospital (DMR-111-105). This work was also supported by grants from the Taipei Veterans General Hospital (V105C-204, V110C-175) and the Ministry of Science and Technology, R.O.C (MOST 110-2314-B-075-027-MY3).
Acknowledgments
We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.919881/full#supplementary-material
References
1
Agard C. Rolli-Derkinderen M. Dumas-de-La-Roque E. Rio M. Sagan C. Savineau J. P. et al (2009). Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br. J. Pharmacol.158, 1285–1294. 10.1111/j.1476-5381.2009.00445.x
2
American Diabetes Association (2021). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care44, S111–S124. 10.2337/dc21-S009
3
Bailey C. J. Turner R. C. (1996). Metformin. N. Engl. J. Med.334, 574–579. 10.1056/NEJM199602293340906
4
Bhatt S. P. Dransfield M. T. (2013). Chronic obstructive pulmonary disease and cardiovascular disease. Transl. Res.162, 237–251. 10.1016/j.trsl.2013.05.001
5
Cheng C. L. Lee C. H. Chen P. S. Li Y. H. Lin S. J. Yang Y. H. (2014). Validation of acute myocardial infarction cases in the national health insurance research Database in taiwan. J. Epidemiol.24, 500–507. 10.2188/jea.JE20140076
6
Cheng T. M. (2003). Taiwan’s new national health insurance program: Genesis and experience so far. Health Aff.22, 61–76. 10.1377/hlthaff.22.3.61
7
D’Agostino R. B. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med.17, 2265–2281. 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
8
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet396, 1204–1222. 10.1016/S0140-6736(20)30925-9
9
Gläser S. Krüger S. Merkel M. Bramlage P. Herth F. J. (2015). Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration.89, 253–264. 10.1159/000369863
10
Griffin S. J. Leaver J. K. Irving G. J. (2017). Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia60, 1620–1629. 10.1007/s00125-017-4337-9
11
Ho T. W. Huang C. T. Tsai Y. J. Lien A. S. Lai F. Yu C. J. (2019). Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir. Res.20, 69. 10.1186/s12931-019-1035-9
12
Holman R. R. Paul S. K. Bethel M. A. Matthews D. R. Neil H. A. (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med.359, 1577–1589. 10.1056/NEJMoa0806470
13
International Diabetes Federation (IDF) (2019).Diabetes atlas, 9th ed. Available: https://www.diabetesatlas.org/en/sections/demographic-and-geographic-outline.html (Accessed October 08, 2021).
14
Kooy A. de Jager J. Lehert P. Wulffele M. G. Bets D. Wulffelé M. G. et al (2009). Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med.169, 616–625. 10.1001/archinternmed.2009.20
15
Lin C. C. Lai M. S. Syu C. Y. Chang S. C. Tseng F. Y. (2005). Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc.104, 157–163.
16
Lin Y. S. Chen T. H. Chi C. C. Lin M. S. Tung T. H. Liu C. H. et al (2017). Different implications of heart failure, ischemic stroke, and mortality between non-valvular atrial fibrillation and atrial flutter-a view from a national cohort study. J. Am. Heart Assoc.6, e006406. 10.1161/JAHA.117.006406
17
Lopez A. D. Shibuya K. Rao C. Mathers C. D. Hansell A. L. Held L. S. et al (2006). Chronic obstructive pulmonary disease: Current burden and future projections. Eur. Respir. J.27, 397–412. 10.1183/09031936.06.00025805
18
Low Wang C. C. Hess C. N. Hiatt W. R. Goldfine A. B. (2016). Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation133, 2459–2502. 10.1161/CIRCULATIONAHA.116.022194
19
Mendy A. Gopal R. Alcorn J. F. Forno E. (2019). Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology24, 646–651. 10.1111/resp.13486
20
Nesti L. Natali A. (2017). Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis.27, 657–669. 10.1016/j.numecd.2017.04.009
21
Pladevall M. Riera-Guardia N. Margulis A. V. Varas-Lorenzo C. Calingaert B. Perez-Gutthann S. (2016). Cardiovascular risk associated with the use of glitazones, metformin and sufonylureas: meta-analysis of published observational studies. BMC Cardiovasc. Disord.16, 14. 10.1186/s12872-016-0187-5
22
Rabe K. F. Hurst J. R. Suissa S. (2018). Cardiovascular disease and COPD: Dangerous liaisons?Eur. Respir. Rev.27, 180057. 10.1183/16000617.0057-2018
23
Rodriguez-Roisin R. (2000). Toward a consensus definition for COPD exacerbations. Chest117, 398S–401S. 10.1378/chest.117.5_suppl_2.398S
24
Sardu C. Paolisso P. Sacra C. Mauro C. Minicucci F. Portoghese M. et al (2019). Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: The codyce multicenter prospective study. Diabetes Care42, 1946–1955. 10.2337/dc18-2356
25
Sattar N. (2013). Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia56, 686–695. 10.1007/s00125-012-2817-5
26
Su V. Y. Yang Y. H. Perng D. W. Tsai Y. H. Chou K. T. Su K. C. et al (2019). Real-world effectiveness of medications on survival in patients with COPD-heart failure overlap. Aging11, 3650–3667. 10.18632/aging.102004
27
Tseng C. H. (2019). Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Am. Heart Assoc.8, e011640. 10.1161/JAHA.118.011640
28
UK Prospective Diabetes Study (UKPDS) Group (1998). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet352, 854–865. 10.1016/S0140-6736(98)07037-8
29
Yen F. S. Chen W. Wei J. C. Hsu C. C. Hwu C. M. (2018). Effects of metformin use on total mortality in patients with type 2 diabetes and chronic obstructive pulmonary disease: A matched-subject design. PLoS One13, e0204859. 10.1371/journal.pone.0204859
30
Young B. A. Lin E. Von Korff M. Simon G. Ciechanowski P. Ludman E. J. et al (2008). Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am. J. Manag. Care14, 15–23.
Summary
Keywords
coronary artery disease, heart failure, metformin, stroke, cardiovascular events
Citation
Yen F-S, Wei JC-C, Chiu L-T, Hsu C-C and Hwu C-M (2022) Cardiovascular outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Front. Pharmacol. 13:919881. doi: 10.3389/fphar.2022.919881
Received
14 April 2022
Accepted
01 August 2022
Published
22 August 2022
Volume
13 - 2022
Edited by
Leonello Fuso, Catholic University of the Sacred Heart, Italy
Reviewed by
Jean-Daniel Lalau, University Hospital Center (CHU) of Amiens, France
Emiel Wouters, Maastricht University, Netherlands
Updates
Copyright
© 2022 Yen, Wei, Chiu, Hsu and Hwu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Cheng Hsu, cch@nhri.edu.tw; Chii-Min Hwu, chhwu@vghtpe.gov.tw
This article was submitted to Pharmacoepidemiology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.