Abstract
Fagopyri Dibotryis Rhizoma (FDR) is an effective Chinese herbal medicine with a long history of use in China. FDR is effective in heat clearing and detoxifying, promotion of blood circulation, relieving carbuncles, dispelling wind, and removing dampness. Its seeds also have high nutritional value, are rich in protein, and contain a variety of mineral elements and vitamins. Therefore, FDR is considered a natural product with medical and economic benefits, and its chemical composition and pharmacological activity are of interest to scientists. The current review provides an overview of the available scientific information on FDR, particularly its botany, chemical constituents, and pharmacological activities. Various sources of valid and comprehensive relevant information were consulted, including the China National Knowledge Infrastructure, Web of Science, and PubMed. Among the keywords used were “Fagopyri Dibotryis Rhizoma”, “botanical features”, “chemical composition”, and “pharmacological activity” in combination. Various ailments are treated with FDR, such as diabetes, tumor, sore throat, headache, indigestion, abdominal distension, dysentery, boils, carbuncles, and rheumatism. FDR is rich in organic acids, tannins, flavonoids, steroids, and triterpenoids. Experiments performed in vitro and in vivo showed that FDR extracts or fractions had a wide range of pharmacological activities, including antitumor, anti-inflammatory, immunomodulatory, antioxidant, antimicrobial, and antidiabetic. The current review provides an integrative perspective on the botany, phytochemistry and pharmacological activities of FDR. FDR may be used as a medicine and food. Based on its chemical composition and pharmacological effects, the main active ingredients of FDR are organic acids, tannins, and flavonoids, and it has obvious antitumor pharmacological activity against a variety of malignant tumors. Therefore, FDR is worthy of further study and application as a potential antitumor drug.
1 Introduction
Chinese herbal medicine plays a crucial role in the prevention and treatment of diseases as a drug resource for the traditional medical system and as an important raw material for chemical drugs, international botanicals, and the food industry. A significant amount of evidence suggests that medicinal plants may be used to treat a variety of diseases and for the discovery of novel pharmacologically active molecules. The phytochemicals identified from medicinal plants have provided promising lead compounds for effective new drugs (Ríos and Recio, 2005; Batiha et al., 2019a; Batiha et al., 2020; El-Saber et al., 2020). Medicinal plants have gained wider acceptance in recent years due to the perception that they are natural products and less likely to induce side effects than their synthetic counterparts (Abushouk et al., 2017a; Abushouk et al., 2017b). Various medicinal plants possess anti-inflammatory, antibacterial, antitumor, antiviral, and other activities (Bakkali et al., 2008). Herbal extracts and pharmacologically active molecules extracted from different plant species that were previously used in traditional medicine have received much attention (Essawi and Srour, 2000; Batiha et al., 2019b; Beshbishy et al., 2019).
Fagopyrum dibotrys (D. Don) Hara is a perennial herb of the genus Fagopyrum in the family Polygonaceae, and it is widely distributed in the Sichuan Basin, the hills of Guangdong and Guangxi, and the Yunnan-Guizhou Plateau in China and Thailand, Nepal, India and other countries (Peng et al., 1996). The dried rhizome is often used as medicine and food because it effectively clears heat, removes toxins, drains pus, removes blood stasis and invigorates the spleen to strengthen the stomach. For several thousand years in China, Fagopyri Dibotryis Rhizoma (FDR) has been widely used as a folk medicine to cure forms of chronic bronchitis, lung cancer, sore throat, rheumatic disease, dysentery, and enteritis (Chan, 2003a; Jing et al., 2016; Zhao et al., 2018). The medicinal properties of FDR are attracting the attention of an increasing number of academics due to its tremendous medicinal value. FDR components have been extensively examined, and an increasing number of compounds have been identified and isolated. A variety of components have been identified in FDR, including organic acids, tannins, flavonoids, steroids, and triterpenoids (Shao et al., 2005; Cao et al., 2019). FDR also has a broad spectrum of pharmacological effects, including antitumor, antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects (Chan, 2003b; Shen, 2013; Wang et al., 2017).
FDR has a variety of chemical components and diverse pharmacological activities, and it is a highly valuable medicinal resource plant for development. Many studies recently investigated the botany, phytochemistry and pharmacology of FDR and found that organic acids, tannins, and flavonoids were the most important active components underlying the broad-spectrum antitumor, anti-inflammatory, and other effects (Li et al., 2019). However, comprehensive and up-to-date information on FDR is lacking. Therefore, the current review summarizes recent progress on the phytoconstituents, chemical components and pharmacological activity of FDR, especially the organic acids, tannins and flavonoids that inhibit tumors and the specific mechanisms of these effects, and adds its botanical characteristics and clinical applications. Various published data of valid and comprehensive relevant information were consulted, including the China National Knowledge Infrastructure, Web of Science, and PubMed. Among the keywords used were “Fagopyri Dibotryis Rhizoma”, “botanical features”, “chemical composition”, and “pharmacological activity” in combination. This review provides references for the further development and use of FDR in traditional Chinese medicine.
2 Botanical characterization and application
2.1 Botanical characterization
Fagopyrum dibotrys (D. Don) Hara is a perennial herb that is native to eastern, central and southwestern China, India, Nepal, Vietnam, Thailand, and other countries. The habitat of Fagopyrum dibotrys (D. Don) Hara is 250–3,200 m above sea level in valley wetlands and hillside forests. The rhizomes are black‒brown, stout, and ligneous, and the stems are long and erect, green, or brownish, 40–100 cm high, branched, striate, and glabrous. The petiole is 2–10 cm, and the leaf blade is triangular at 4-12 × 3–11 cm. Both surfaces are papillate, the base is nearly hastate, the leaf margin is entire, and the apex is acuminate. The ocrea is brown, 5–10 mm, membranous, and oblique, and the apex is truncate, not ciliate. Plants have terminal, axillary or corymbose inflorescence. Bracts are ovate-lanceolate, ca. 3 mm, with membranous margins, and an acute apex, each 4-flowered and rarely 6-flowered. Pedicels are in equaling bracts that articulate at the middle. Perianth are white, and tepals are narrowly elliptical, ca. 2.5 mm. Stamens are included. The styles are free, and stigmas are much longer than the persistent perianth, capitate, and opaque. During April-August, the chenes are blackish brown, dull, broadly ovoid, 6–8 mm long, trigonous, sometimes narrowly winged, with smooth to repandous angles, and an acute apex (Editorial Committee of Chinese Flora, 1998).
2.2 Application
The anti-inflammatory and antiseptic effects of FDR may be used to treat a variety of respiratory diseases. FDR tablets combined with tiotropium bromide powder nebulizer exhibited clinical efficacy and high safety, and it effectively improved the acute exacerbation of COPD patients with clinical symptoms and blood gas analysis indicators and reduced the inflammatory response (Li et al., 2022). FDR capsules combined with salmeterol ticapone inhalation powder nebulizer for the treatment of bronchial asthma in children had good results, and it effectively relieved clinical symptoms, improved lung and immune functions, regulated serum inflammatory factor levels, and had a good safety profile (Wei et al., 2022). FDR capsules significantly reduced the acute exacerbation of asthma patients’ serum EOS and IgE levels, reduced the respiratory inflammatory response, improved the patient’s lung ventilation function and the clinical symptoms of patients, which are worthy of clinical promotion (Feng et al., 2021).
The anti-inflammatory, analgesic and antibacterial pharmacological effects of FDR significantly improved the symptoms of infectious diseases of the intestinal tract. FDR tablets combined with cefdinir dispersible tablets effectively improved the symptoms of acute bacterial dysentery patients with diarrhea, purulent stools and other symptoms and reduced the level of serum inflammatory indicators (Zhang and Li, 2019).
3 Phytochemistry
Various parts of FDR have yielded more than 100 compounds, including organic acids, tannins, flavonoids, steroids, and triterpenoids (Lin et al., 2016), which support its potential use as a medicinal and food plant. These compounds likely explain the differentiated pharmacological effects based on the characteristics of these chemical components. A list of phytochemical constituents is presented in Table 1.
TABLE 1
| No. | Chemical component | Plant Part | Chemistry | Chemical Formula | Chemical Structures | Biological activity |
|---|---|---|---|---|---|---|
| 1 | (-)-Epicatechin-3-O-gallate acid ester | Rhizome | Organic acids | C22H18O10 |

|
Anti-inflammatory Antioxidant |
| 2 | Gallic acid | Rhizome | Organic acids | C₇H₆O₅ |

|
Antitumor |
| Antimicrobial | ||||||
| Antioxidant | ||||||
| 3 | Protocatechuic acid | Rhizome | Organic acids | C7H6O4 |

|
Anti-inflammatory |
| Antimicrobial | ||||||
| 4 | 3,4-Dihydroxy benzamide | Rhizome | Organic acids | C7H7O3 |

|
Anti-inflammatory |
| Antimicrobial | ||||||
| 5 | Monopalmitin | Rhizome | Organic acids | C19H38O4 |

|
Immunomodulatory |
| 6 | Protocatechuic acid methyl ester | Rhizome | Organic acids | C8H8O4 |

|
Antioxidant |
| 7 | Tans-p-hy-droxy cinnamic methyl ester | Rhizome | Organic acids | C10H10O3 |

|
Antitumor |
| Antimicrobial | ||||||
| 8 | 3,5-Dimethoxy benzene carbonic acid-4-O-glucoside | Rhizome | Organic acids | C12H18O3 |

|
Anti-inflammatory |
| 9 | Ferulic acid | Rhizome | Organic acids | C10H10O4 |

|
Antioxidant |
| Antimicrobial | ||||||
| Anti-viral | ||||||
| 10 | Syringic acid | Rhizome | Organic acids | C9H10O5 |

|
Antimicrobial |
| 11 | p-Hydroxyl-benzaldehyde | Rhizome | Organic acids | C7H6O2 |

|
Anti-inflammatory |
| Antimicrobial | ||||||
| 12 | Succinic acid | Rhizome | Organic acids | C4H6O4 |

|
Antimicrobial |
| Immunomodulatory | ||||||
| 13 | Luteolin | Rhizome | Flavonoids | C15H10O6 |

|
Anti-inflammatory |
| 14 | (-)-Epicatechin | Rhizome | Flavonoids | C15H14O6 |

|
Antitumor |
| Anti-inflammatory | ||||||
| 15 | 3-Galloyl (-) epicatechin | Rhizome | Flavonoids | C22H18O10 |

|
Antitumor |
| Antioxidant | ||||||
| Anti-inflammatory | ||||||
| 16 | Dimeric procyanidin | Rhizome | Flavonoids | C45H38O18 |

|
Anti-inflammatory |
| Antimicrobial | ||||||
| Immunomodulatory | ||||||
| 17 | (+)-Catechin | Rhizome | Flavonoids | C15H14O6 |

|
Antitumor |
| Antidiabetic | ||||||
| Anti-inflammatory | ||||||
| 18 | Eriodictyol | Roots | Flavonoids | C15H12O6 |

|
Anti-inflammatory |
| Antioxidant | ||||||
| Antidiabetic | ||||||
| 19 | Quercetin | Seeds, Stems, Roots, Leaves | Flavonoids | C15H10O7 |

|
Anti-inflammatory |
| Antioxidant | ||||||
| Antimicrobial | ||||||
| Immunomodulatory | ||||||
| 20 | Rutin | Flowers, Seeds, Leaves | Flavonoids | C27H30O16 |

|
Anti-inflammatory |
| Antioxidant | ||||||
| Anti-viral | ||||||
| 21 | Genkwanin | Rhizome | Flavonoids | C16H12O5 |

|
Antitumor |
| Anti-viral | ||||||
| 22 | Chrysoeriol | Rhizome | Flavonoids | C16H12O6 |

|
Anti-inflammatory |
| 23 | Pratol | Rhizome | Flavonoids | C16H12O4 |

|
Anti-inflammatory |
| 24 | Luteolin-7,4′-dime-thylether | Rhizome | Flavonoids | C17H14O6 |

|
Anti-inflammatory |
| 25 | Rhamnetin | Rhizome | Flavonoids | C16H12O7 |

|
Antitumor |
| Anti-inflammatory | ||||||
| 26 | 3,6,3′,4′-Tetrahydroxy-7-methoxyflavon | Rhizome | Flavonoids | C16H12O7 |

|
Anti-inflammatory |
| 27 | Procyanidin B2 | Rhizome | Tannins | C30H26O12 |

|
Anti-inflammatory Antimicrobial |
| 28 | Procyanidin C1 | Rhizome | Tannins | C45H38O18 |

|
Antitumor |
| Anti-diabetic | ||||||
| 29 | Procyanidin B4 | Rhizome | Tannins | C30H26O12 |

|
Antitumor |
| Antioxidant | ||||||
| 30 | 3,3'-Digalloyl procyanidin B2 | Rhizome | Tannins | C44H34O20 |

|
Antitumor |
| Antioxidant | ||||||
| 31 | -Sitosterol | Rhizome | Steroids | C30H52O |

|
Antitumor |
| 32 | -Daucosterol | Rhizome | Steroids | C35H60O6 |

|
Antitumor |
| 33 | Hecogenin | Rhizome | Steroids | C27H42O4 |

|
Anti-inflammatory |
| 34 | Glutinone | Rhizome | Terpenoids | C19H18O3 |

|
Antimicrobial |
| 35 | Glutinol | Rhizome | Terpenoids | C30H50O1 |

|
Antimicrobial |
| 36 | N-Butyl--D-fructopy-ronoside | Rhizome | Others | C10H20O6 |

|
Antitumor |
| 37 | Methyl-3,4-dihydroxybenzoatem | Rhizome | Others | C8H8O4 |

|
Anti-inflammatory |
| Antioxidant | ||||||
| 38 | Gglycerol monop-almitate | Rhizome | Others | C19H38O4 |

|
Anti-inflammatory |
| 39 | p-Hydroxyl-benzaidehyde | Roots | Others | C7H6O2 |

|
Anti-inflammatory |
| Antimicrobial | ||||||
| 40 | N-Trans-coumaroyl tyramine | Rhizome | Others | C17H17NO3 |

|
Anti-inflammatory |
| 41 | Emodin | Rhizome | Others | C15H10O5 |

|
Antimicrobial |
| 42 | Diboside A | Rhizome | Others | C49H48O20 |

|
Anti-inflammatory |
| 43 | 3-Methyl-gossypetin 8-O-d-glucopyranoside | Rhizome | Others | C22H22O13 |

|
Anti-inflammatory |
| 44 | 5,5-Di-α-furaldehyde dimethylether | Rhizome | Others | C7H10O3 |

|
Immunomodulatory |
Phytochemical constituents of FDR.
3.1 Organic acids
Organic acids are compounds that contain -COOH, -SO3H, RSOOH, and RCOSH in their molecular structure, and leaves, roots, and Chinese herbs are abundant in these molecular structures. Twelve organic acids have been identified in FDR, including gallic acid, protocatechuic acid, (-)-epicatechin (Li et al., 2020; Huang et al., 2022), (-)-epicatechin-3-O-gallate acid ester, tans-p-hy-droxy cinnamic methyl ester, 3,4-dihydroxy benzamide, monopalmitin, protocatechuic acid methyl ester (Shao et al., 2004), 3,5-dimethoxy benzene carbonic acid-4-O-glucoside, syringic acid, ferulic acid, p-hydroxyl-benzaldehyde, and succinic acid (Zhao et al., 2011).
3.2 Flavonoids
Flavonoids are widely present in naturally growing plants and refer to a class of compounds with two benzene rings connected by three carbon atoms that create the C6-C3-C6 structure (Cook and Samman, 1996). Quercetin, rutin (Tang et al., 2014), luteolin (Shao et al., 2005), genkwanin, chrysoeriol (Yan, 2006), pratol, luteolin-7,4′-dime-thylether, rhamnetin, iorhamnetin, 3,6,3′,4′-tetrahydroxy-7-methoxyflavon (Zhang et al., 2016), eriodictyol (Zhao et al., 2011), dimeric procyanidin (Liu et al., 1983), 3-galloyl (+) catechin, 3-galloyl (-) epicatechin (Liu et al., 1998), (+)-catechin, (-) epicatechin (Zhang et al., 1994) and other flavonoids were isolated from FDR using column chromatography and high-performance liquid chromatography (HPLC).
3.3 Tannins
Tannins are phenolic compounds with complex structures that are widely distributed in plants. Procyanidin b2, procyanidin c1 (Huang et al., 2022), procyanidin b4 (Peng et al., 1996), and 3,3′-digalloyl procyanidin b2 were isolated from FDR.
3.4 Steroids
Steroids are a class of natural chemical components that exist widely in nature and have the steroid parent nucleus of cyclopentane-polyhydrophenanthrene in their structure. Chromatography on silica and Sephadex LH-20 columns isolated β-sitosterol and β-daucosterol from FDR (Wu et al., 2008). Liu et al. obtained hecogenin from FDR (Liu et al., 1983).
3.5 Terpenoids
Terpenoid is a general term that summarizes all polymers of isoprene and their derivatives, which are commonly found in plants. Terpenoids have important physiological activities and are an important resource for the study of natural products and the development of new drugs. Silica gel column chromatography, Sephadex LH-20 column chromatography and recrystallization were used to separate the ethyl acetate extract as glutinone and glutinol (Shao et al., 2005).
3.6 Other components
Emodin (Wu et al., 2008), glycerol monop-almitate, n-butyl-β-D-fructopy-ronoside, methyl-3,4-dihydroxybenzoate (Shao et al., 2005), diboside A, 3-methyl-gossypetin 8-O-d-glucopyranoside (Wang et al., 2005), 5,5-di-α-furaldehyde dimethylether (Tian et al., 1997), n-trans-coumaroyl tyramine, and p-hydroxy-benzaidehyde (Zhao et al., 2011) were also isolated from FDR.
4 Pharmacological activities
FDR is widely used in Chinese herbal medicine for its antitumor, anti-inflammatory, antimicrobial, antioxidant, and immunomodulatory properties in recent years (Figure 1). A variety of extracts and their chemical constituents showed various and significant biological and pharmacological activities in previous studies (Yang et al., 2019). Extracts and constituents of FDR were tested, and the results support their renowned applications in the treatment of a variety of ailments. Detailed pharmacological studies are discussed in the following sections.
FIGURE 1
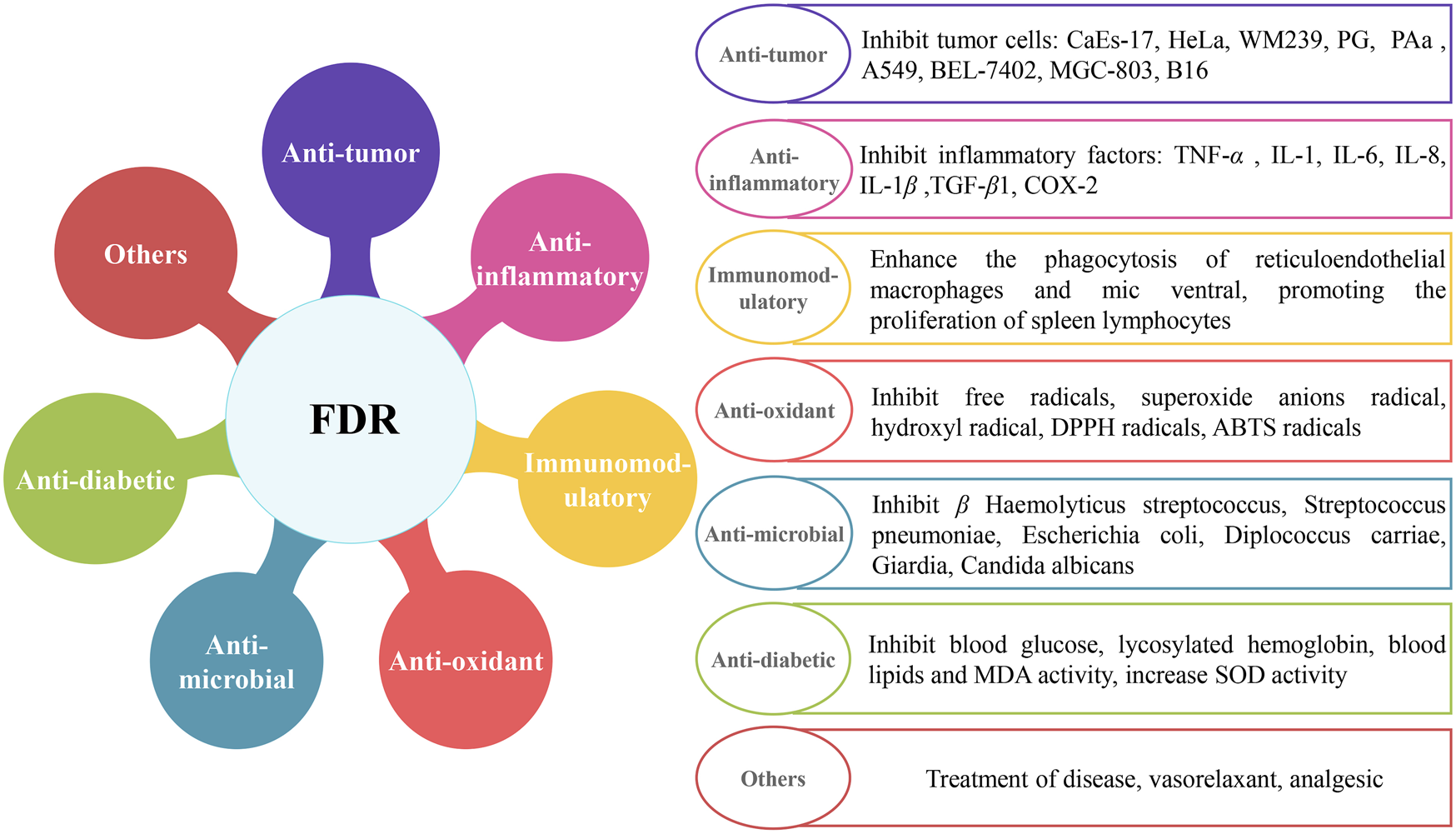
Molecular pharmacological activity mechanisms of FDR.
4.1 Antitumor activity
The antitumor activity of FDR has drawn increasing attention over the past decades. FDR components had beneficial effects in the treatment of a variety of cancers in several studies. As shown in Figure 2, Wang et al. (Wang and Bao, 2020) found that gallic acid prevented non-small cell lung cancer progression via inhibition of epidermal growth factor receptor activation and impairment of the binding of coactivator-associated arginine methyltransferase 1 to proline, glutamic acid, and leucine-rich protein 1. Vergara et al. (Pereyra-Vergara et al., 2020) showed that reactive oxygen species (ROS) mediated (-)-epicatechin-induced apoptosis in human breast cancer cells. Apoptosis and autophagy were induced by procyanidin b2 in colorectal cancer cells (CRC) in a dose-dependent manner via downregulation of the expression of phosphorylated-phosphatidylinositol 3-kinase (p-PI3K), phosphorylated-protein kinase B (p-Akt) and phosphorylated-mammalian target of rapamycin (p-mTOR) of the PI3K/Akt pathway (Zhang et al., 2019). Procyanidin b2 prevented the binding of nuclear factor kappa B (NF-κB) to DNA in the H-RS cell line and inhibited NF-κB-driven genes, including anti-apoptotic proteins (Mackenzie et al., 2008). Another study revealed that β-sitosterol regulated the treatment response in CRC by mediating the p53/NF-κB/BCRP signal transduction axis (Wang et al., 2020). Melanoma cell growth inhibition by procyanidin c1 was attributed to activation of the 67LR/PKA/PP2A/CPI17/MRLC pathways (Bae et al., 2020). Genkwanin increased host immunity and decreased the levels of inflammatory cytokines, which may make it an effective chemotherapeutic agent for the treatment of CRC (Wang X et al., 2015). Rhamnetin inhibited the expression of the pregnant x receptor (PXR) by increasing miR-148a levels, which decreased the expression of its downstream genes. Therefore, sorafenib was more effective against hepatocellular carcinoma (Li Y et al., 2021).
FIGURE 2
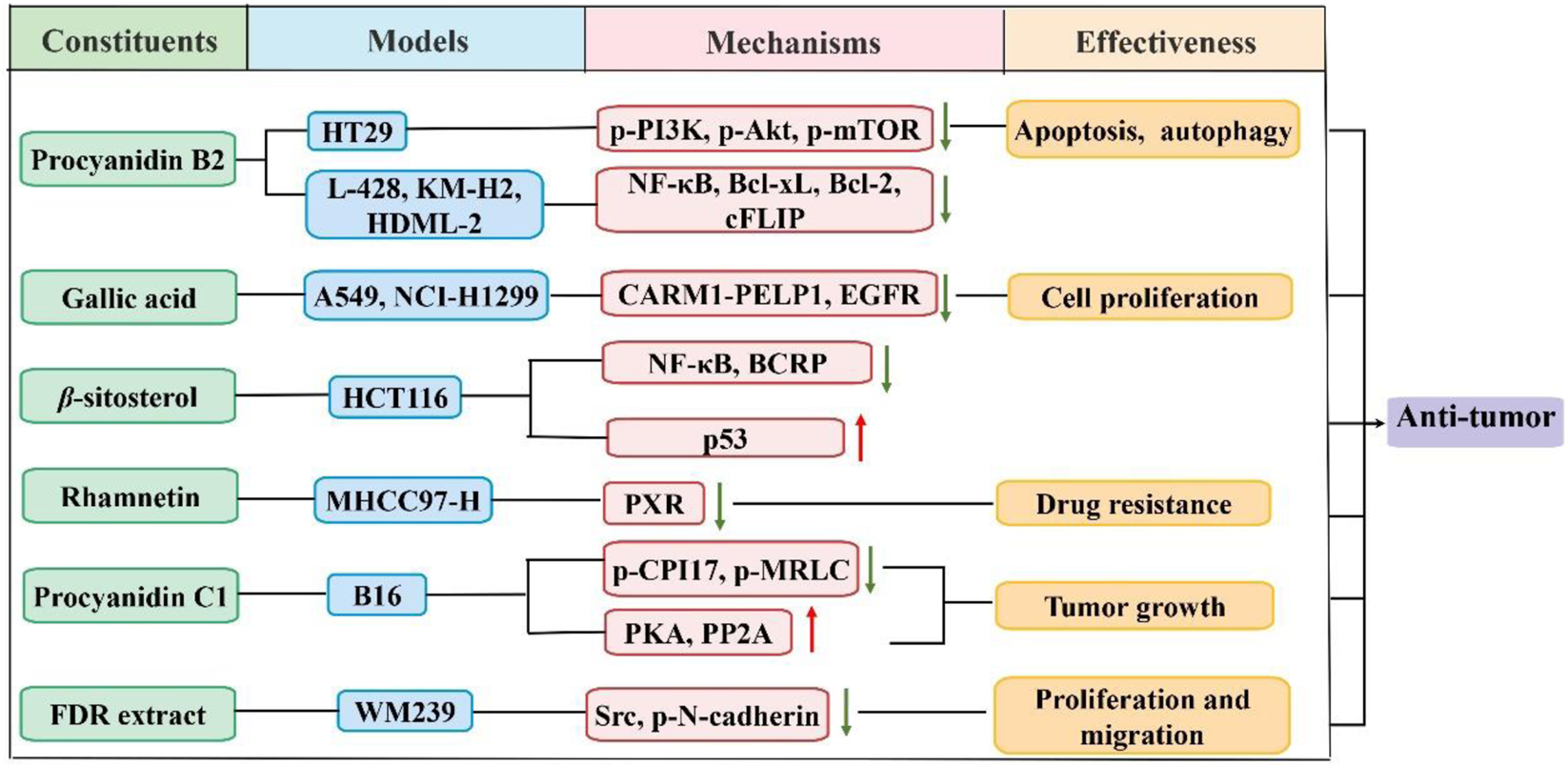
Schematic diagram of the antitumor mechanisms of FDR and its constituents.
The treatment of cancer cells with FDR extract inhibited their growth. FDR extract showed significant proliferation inhibitory activity on HeLa cells, which was primarily associated with modulation of the expression of the apoptotic inducible factor Bcl2-associated X (Bax) and inhibition of the anti-apoptotic factor B-cell lymphoma-2 (Bcl2). The extract of FDR can also activated caspase-8, caspase-9, and caspase-3 and released mitochondrial cytochrome C (Pan et al., 2018). Some extracts from FDR possessed potential antitumor activity. For example, an extract from the FDR rhizome had antiproliferative and proapoptotic effects on the human esophageal cancer cell line CaEs-17 (Zhang et al., 2010). Chen et al. (Chen et al., 2012) revealed that FDR prevented Bowes melanoma cell WM239 proliferation and migration, which was accomplished via reduced activation of Src protein, decreased levels of N-cadherin intracellular segment phosphorylation and dissociation of N-cadherin from β-catenin. Fr4 is a polyphenolic substance extracted from FDR. Fr4 reduced tumor weight, increased tumor suppression, and showed good antitumor activity in a mouse Lewis lung cancer model (Chen et al., 2005). Fr4 promoted a dose-dependent increase in the inhibition of HL-60 proliferation in leukemic cells and induced apoptosis (Chen et al., 2006). The FDR extract Fr4 also had an antitumor effect on kidney cancer. Fr4 inhibited the proliferation and induced apoptosis of kidney cancer cells via a mechanism related to the upregulation of DNA damage-induced transcript 4 protein expression (Song et al., 2020).
Wei Mai Ning capsules are the main raw material extracts from FDR, which inhibit tumor growth, invasion, and blood flow metastasis, and it has been approved for clinical cancer therapy (Lou et al., 2004a). Wei Mai Ning had effects on the lung cancer cell lines PG, PAa and A549 and inhibited the liver cancer cell line BEL-7402, gastric cancer cell line MGC-803 and melanoma cell line B16 to varying degrees (Lou et al., 2004b). Wei Mai Ning inhibited the adhesion between PG and HUVECs in vitro via the dual action of PG cells and HUVECs, which inhibited tumor cell metastasis in the blood channel (Lou et al., 2007).
4.2 Anti-inflammatory activity
Various in vitro and in vivo experiments investigated the anti-inflammatory effects of FDR extracts (Figure 3). The effects of (-)-epicatechin on lipopolysaccharide (LPS)-induced inflammation in RAW264.7 cells were demonstrated, and its anti-inflammatory effect may be related to a reduction in inflammatory cytokines, such as nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), interleukin-1 and interleukin-6 (IL-6) and inhibition of the expression of nitric oxide synthase, phosphorylation of p38 mitogen-activated protein kinase (p-p38MAPK), extracellular signal regulated kinases 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) (Ruan and Mu, 2017). Different (-)-epicatechin metabolites have anti-inflammatory properties that boost vascular health partially by reprogramming epigenetic signaling in endothelial-immune cells and reversing low-grade systemic inflammation (Milenkovic et al., 2020). Protocatechuic acid inhibited BV2 microglia and keratinocytes by reducing the activation of toll-like receptor 4 (TLR4)-dependent Akt, mTOR, and NF-κB transcription factors and activating JNK and p38 MAPK (Wang H. Y et al., 2015; Amini et al., 2018; Nam and Lee, 2018). Rhamnetin treatment inhibited the inflammatory and proatherosclerosis pathways in ApoE−/− mice, and aortic tissue from ApoE−/−mice exhibited amelioration of TLR4 mRNA and components of the TLR4 pathway after treatment with rhamnetin (Wang et al., 2021).
FIGURE 3
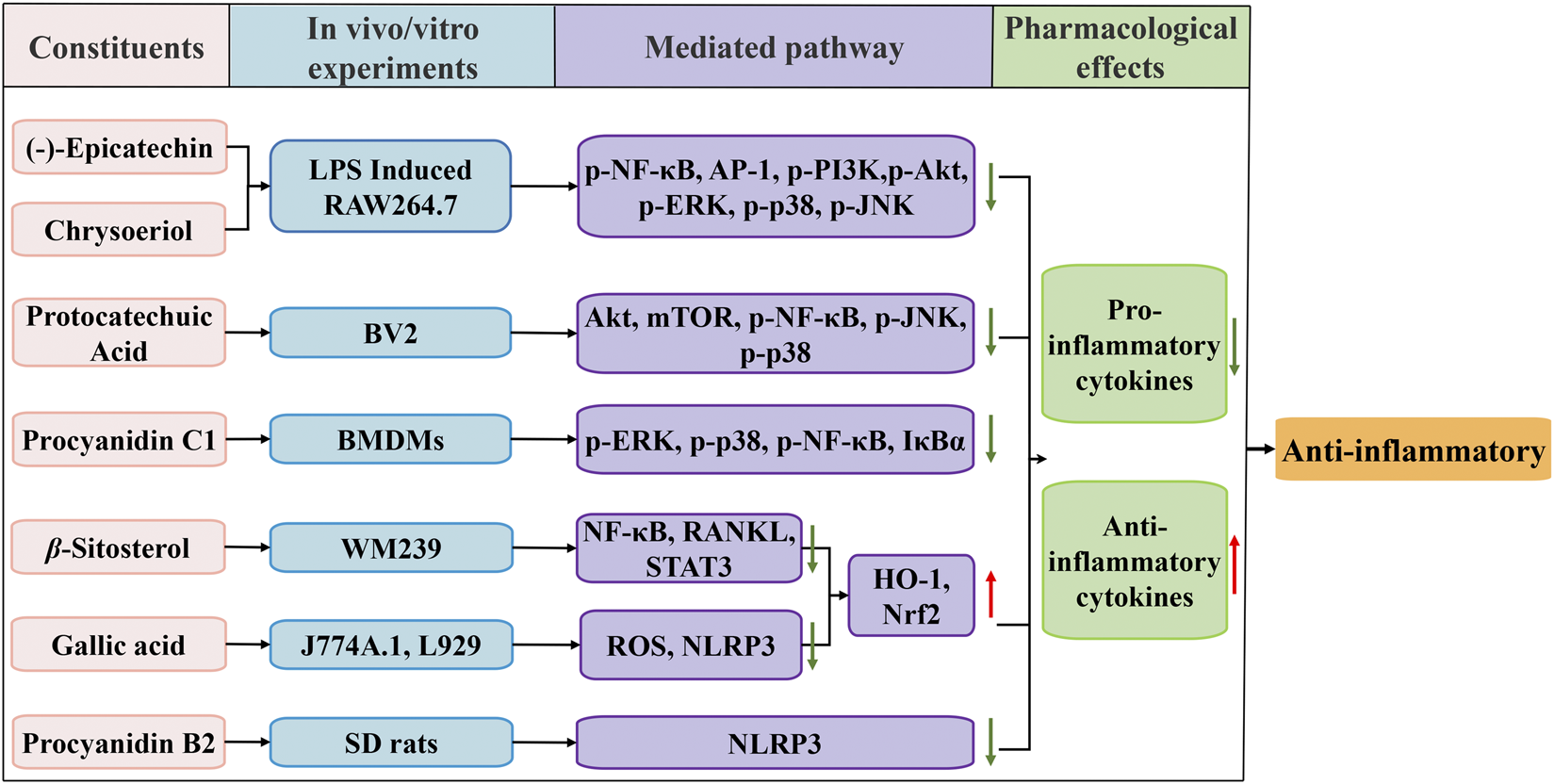
Schematic diagram of the anti-inflammatory mechanisms of FDR and its constituents.
By inhibiting the TLR4-mediated activation of NF-κB and activator protein 1 and suppressing the phosphorylation of PI3K/Akt and MAPK, chrysoeriol inhibited the inflammatory response of LPS-stimulated RAW 264.7 cells (Yoon and Park, 2021). Gallic acid is a promising treatment for gouty arthritis. These effects are induced by suppression of ROS generation, which limits NOD-like receptor protein 3 (NLRP3) inflammasome activation and pyroptosis dependent on nuclear factor erythroid 2-related factor 2 (Nrf2) signaling (Lin et al., 2020). The anti-inflammatory properties of procyanidin b2 are attributed to suppression of NLRP3 inflammasome activation (Jiang et al., 2018). Byun et al. (Byun et al., 2013) indicated that procyanidin c1 inhibited LPS-induced activation of MAPK and NF-κB signaling via TLR4 in macrophages. Zhang et al. (Zhang et al., 2020) demonstrated that β-sitosterol suppressed NF-κB and activated heme oxygenase-1 (HO-1)/Nrf-2 pathways to inhibit arthritis.
FDR extract inhibits the transcription factor NF-κB and the induced production of TNF-α, interleukin-8, IL-6, transforming growth factor-β1 and precollagen peptide III activity in chronic obstructive pulmonary disease rats, which improves lung tissue inflammation (Tang et al., 2014; Tang et al., 2016). The FDR extract prevented lung tissue injury in rats with pneumonia by downregulating TLR2/4, myeloid differentiation primary response 88 mRNA and NF-κB inhibitor alpha protein expression (Dong et al., 2011). FDR tablets attenuated inflammatory symptoms and inflammatory damage in colorectal tissues of mice with a dextran sulfate sodium-induced inflammatory bowel disease model by downregulating TNF-α, IL-6 and interleukin-1β factor expression (Shen et al., 2019; Tan et al., 2020).
Clinical studies proposed combination therapy with Chinese medicines as an effective treatment strategy. FDR tablets combined with salazosulfapyridine (SASP) were more effective than SASP alone in ulcerative colitis (UC), and the mechanism may be the anti-inflammatory and immunomodulatory effects of intervening in UC via the TLR4/NLRP3 signaling pathway (Ge et al., 2021). FDR tablets were combined with compound kangfuxin solution and showed good efficacy in the treatment of UC (Hua and Yin, 2016). The effectiveness of FDR in controlling lung disease has been demonstrated in several clinical studies, including the treatment of adult and childhood bronchial asthma, and FDR capsules combined with salmeterol xinafoate and fluticasone propionate powder were effective (Li and Wu, 2018; Feng et al., 2021). Some studies also revealed that FDR tablets combined with cefoperazone and gubenkechuan tablets had a significant effect in chronic bronchial patients (Li, 2010; Han, 2020).
4.3 Immunomodulatory activity
Pharmacological studies confirmed that the extract from FDR showed an anti-rheumatoid arthritis effect, which may be due to its anti-inflammatory and immune activities (Shen, 2013). The polysaccharide content of FDR repairs the immune function of the thymus and spleen, enhances nonspecific immune function, improves specific humoral immunity and cellular immune function, and ultimately enhances the body’s immune function via multiple pathways, links, and targets (Gu et al., 2015). An extract of FDR reduced the expression of caspase-1, caspase-3, caspase-9, and matrix metallopeptidase-1 (MMP-1) in articular cartilage of a rabbit knee osteoarthritis model, which reduced cartilage damage and had an osteoprotective effect (Pan et al., 2019). FDR enhanced the phagocytosis of ventral and reticuloendothelial macrophages, which showed that it enhanced the immune function of mice (Yang et al., 1992; Zhang and Lin, 1999). Ethanol extract from FDR roots had an immunomodulatory role by promoting the proliferation of chicken spleen lymphocytes and the secretion of interleukin-2 and interferon-γ by peripheral blood T lymphocytes (Qiao et al., 2010).
4.4 Antioxidant activity
Organic acids, flavonoids, and tannins found in FDR demonstrate scavenging properties against free radicals and superoxide anions. Figure 4 shows the antioxidant effect of FDR via some pathways. Flavonoids remarkably reduced superoxide anion radicals and hydroxyl radicals in a concentration-dependent manner (Wang et al., 2017). Protoconuic acid is a naturally occurring organic acid that is widely distributed. Han et al. (Han et al., 2018; Han et al., 2019) found that the antioxidant properties of protocatechuic acid were beneficial for reducing the oxidative damage caused by palmitic acid in induced human umbilical vein endothelial cells (HUVECs) or high fat-induced oxidative damage in mice via downregulation of the CD36/AMPK-dependent pathway. PA had a beneficial effect on oxidative damage to the gastrointestinal mucosa by upregulating the DJ-1/PI3K pathways, increasing Nrf2 and mTOR expression, reducing ROS levels and lipid peroxidation, downregulating proapoptotic and inflammatory factors, and enhancing antioxidant enzyme activity and cell viability (Farombi et al., 2016; Cheng et al., 2019). (-)-Epicatechin in FDR extract exhibited stronger antioxidant activity and reduced superoxide anion radicals and hydroxyl radicals (Huang R et al., 2014). Procyanidin b2 prevented oxidative injury in aged mice via citrate cycle regulation, fatty acid regulation, and bile acid regulation, and procyanidin b2 suppressed intracellular ROS generation by activating Nrf2 expression to prevent oxidative damage (Xiao et al., 2018; Li B et al., 2021). Procyanidin c1 plays an important role in antioxidant activity by mediating the nuclear translocation of Nrf2 and increasing the expression levels of HO-1. Procyanidin c1 also blocks glutamate-induced phosphorylation of MAPKs, including ERK1/2 and p38, but not JNK (Song et al., 2019). Kim et al. (Kim et al., 2021) confirmed that chrysoeriol treatment prevented HO-induced oxidative stress in RPE cells, which significantly decreased the mitochondrial dysfunction caused by HO-induced oxidative stress. A reduction in MMP and an increase in mitochondrial-associated genes and proteins were also observed. Chrysoeriol also markedly induced the transcription factors Nrf2 and NAD(P)H:quinone oxidoreductase 1, which are related to antioxidants.
FIGURE 4
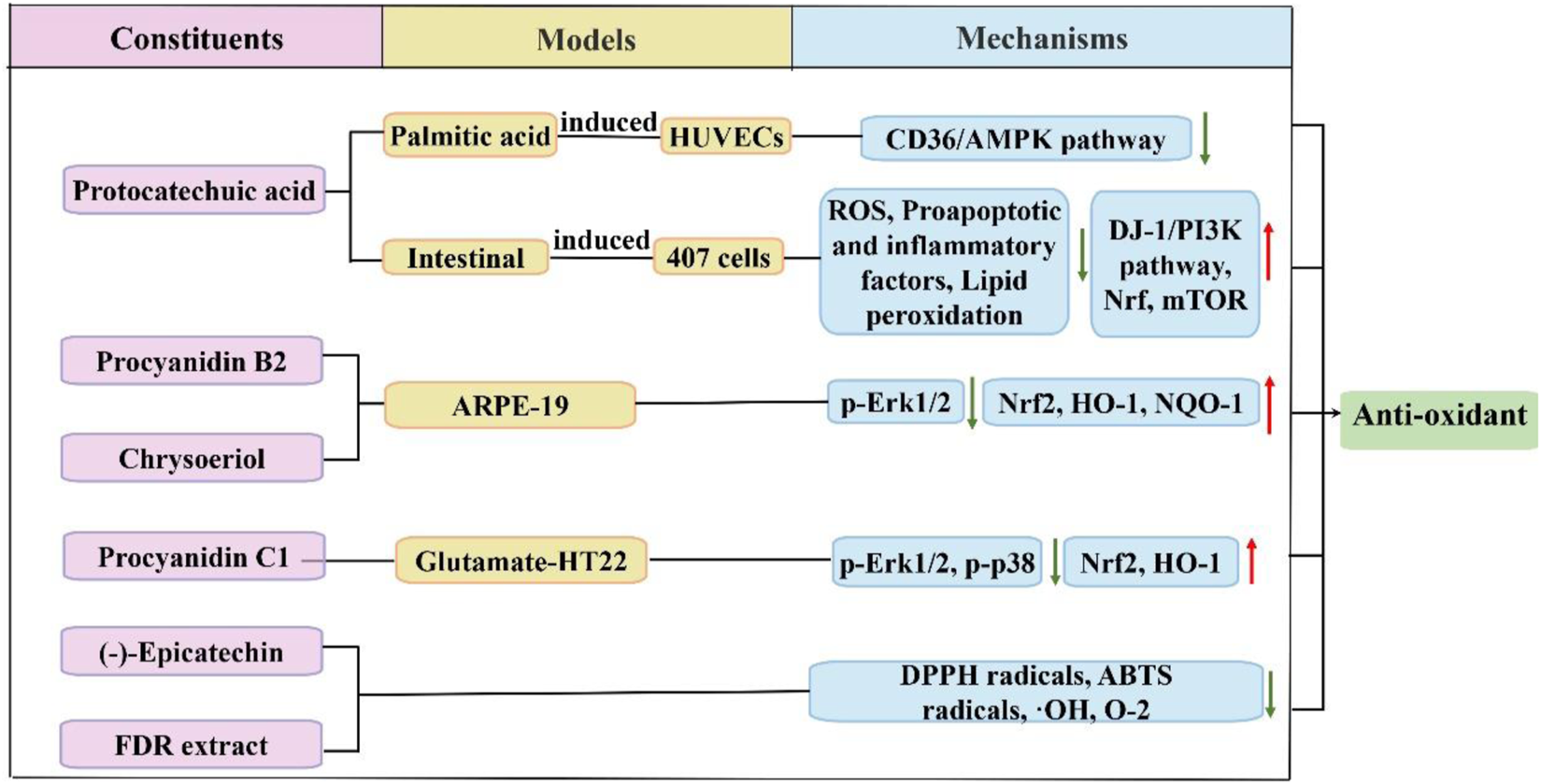
Schematic diagram of the antioxidant mechanisms of FDR and its constituents.
FDR leaf tea has significant antioxidant scavenging ability against DPPH radicals, ABTS radicals and hydroxyl radicals (Huang et al., 2016). FDR extract reduces cartilage damage by reducing malondialdehyde (MDA) and lipid peroxide content and enhancing superoxide dismutase (SOD) activity, which reduce oxygen free radicals and provide osteoprotective effects (Pan et al., 2020).
4.5 Antimicrobial activity
Ethanol extracts of FDR showed antimicrobial effects (Ai et al., 2002; Zhou et al., 2009). The ethanol extract of FDR showed inhibition of β Hemolyticus Streptococcus and Streptococcus pneumoniae in vitro and inhibited infections caused by strains of Streptococcus pneumoniae in mice in vivo (Yan et al., 2006). Fang et al. (Feng et al., 2006) evaluated the inhibitory activities of FDR against bacteria and fungi, and their results indicated that FDR exhibited obvious antibacterial effects on Staphylococcus aureus, Escherichia coli and Diplococcus carriae It also exhibited antifungal activities against Giardia and Candida albicans. FDR also demonstrated antibacterial activity by scavenging Staphylococcus aureus, Bacillus subtilis and Saccharomyces cerevisiae (Huang and Yi, 2015). KQH-01, KQH-02 and JQY-1 isolated from FDR showed strong antibiotic activity against some indicator microorganisms, such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis and Pythium aphanidermatum (Zhang et al., 2011). FDR powder protected mice from Salmonella infection and suggested a dose-activity relationship (Wang et al., 2013).
FDR tablets combined with ceftriaxone had important antimicrobial activities in acute bacillary dysentery, and one of the mechanisms of action was the promotion of inflammatory absorption (Li, 2012). When FDR tablets combined with levofloxacin showed significant antimicrobial activity and may be used for the treatment of acute bacillary dysentery (Bi et al., 2012; Yu, 2014).
4.6 Antidiabetic activity
FDR flavonoids improved objective indices in streptozotocin-induced diabetes mellitus type 2 (T2DM) mice and regulated lipid metabolism and oxidative stress levels in model animals (Ruan et al., 2017). FDR leaf tea reduced blood glucose, blood lipids and MDA activity, increased SOD activity and improved pancreatic and liver lesions in mice with T2DM (Huang X et al., 2014). The FDR mixture significantly improved the clinical symptoms of diabetic nephropathy and significantly reduced the patient’s blood glucose, glycosylated hemoglobin and blood lipids (Huang et al., 2009).
4.7 Others
FDR also performs other functions in the above-described pharmacological activities. For example, FDR extract has an obvious antiviral effect in vitro, and its active ingredient is the flavonoid of FDR, which is concentration dependent (Zhao et al., 2019). Lianhua Qingwen associated with FDR tablets was more effective, faster and safer than oseltamivir alone in the treatment of patients with influenza A (Guo, 2015). Procyanidin b1 may be an effective treatment for hepatitis C virus, which may be an HCV RNA polymerase inhibitor (Li et al., 2010).
The tannic compound procyanidin b2 has analgesic effects, primarily via anti-inflammatory antioxidant free radicals to protect nerve cell membranes and prevent the production and release of the neurotransmitter 5-HT. It also antagonizes the ligand-type receptor 5-HT3A expression or promotes the expression of the G protein-coupled superfamily 5-HT1A receptor via the upstream signaling pathway to improve irritable bowel syndrome (IBS). Downregulation of transient receptor potential vanilloid 1 (TRPV1) expression also had a therapeutic effect on hyperalgesia in IBS rats (Liu et al., 2012a; Liu et al., 2012b; Liu et al., 2016). The hot plate test and the acetic acid twist test showed that FDR medicinal liquid had analgesic effects, and it increased the pain threshold and reduced the number of twists in mice (Pan and Wan, 2015). Jia et al. used dysmenorrhea models in mice to evaluate the analgesic effect of FDR extract and found that it showed potential analgesic activity (Jia et al., 2010).
Othman et al. suggested that the vasorelaxant effect of ethyl cinnamate was mediated via multiple pathways, and the inhibition of Ca2+ influx into vascular cells and release of NO and prostacyclin from endothelial cells were involved (Othman et al., 2002).
5 Conclusions and perspectives
The current review systematically discussed the ethnobotany, phytochemistry, and pharmacology of FDR. Various ailments have traditionally been treated with FDR, including chronic bronchitis, tumor, sore throat, rheumatic disease, dysentery, and enteritis. The predominant natural compounds in FDR are organic acids, tannins, and flavonoids, but over 100 compounds have been identified. FDR exerts antitumor, anti-inflammatory, immunomodulatory, antioxidant, antimicrobial, antidiabetic, and other pharmacological activities. In addition to the phytochemical and pharmacological studies mentioned above, FDR has also received considerable attention because it contains a variety of essential nutrients, and the chemical composition of human health has received widespread attention. Therefore, a better understanding of the phytochemistry and pharmacology of FDR will undoubtedly promote a more rational development and utilization of FDR.
FDR has rich nutritional value and healthcare functions. It is a medicinal resource plant with high developmental value. It contains organic acids, tannins, flavonoids, and other antitumor active ingredients, and gallic acid, procyanidin B2, (-)-epicatechin and genkwanin show significant antitumor activity. However, whether the antitumor effects of FDR are the result of the joint action of various components and the specific antitumor mechanism are not clear. Therefore, there is a need for in-depth research on the following aspects. According to pharmacodynamic studies, the effective site of the antitumor effect of FDR must be clarified via separation and purification to improve its antitumor potency. From the molecular or genetic level, more in-depth research is needed to reveal the antitumor effect of FDR. Because FDR has certain anti-invasive and metastatic effects, it is necessary to perform further research to understand the value and significance of its intervention in tumor cell invasion and metastasis. FDR tablets have also been clinically proven to increase the efficacy of pneumonia treatment, pulmonary abscess treatment, and rheumatic disease treatment. However, as a “Chinese Pharmacopoeia” collection, its mechanism of action of “removing heat and toxins and removing pus and stasis” must be further clarified. This pharmacological effect of FDR may be due to the extract or active ingredients as clearing and detoxifying lung agents in drinks and beverages to promote the full use of FDR resources. The antitumor and anti-inflammatory effects and mechanisms of FDR have been widely studied. However, the mechanism of antimicrobial, antidiabetic and immunomodulatory activity of FDR still needs to be further explored. The research on the mechanism of action of FDR should be continuously strengthened, and the potential medicinal function of it should be expanded to promote the development and utilization of its medicinal resources.
Statements
Author contributions
QG Designing the review, Writing original draft, Writing—review and editing. BL Designing the review, Revising the pharmacology part, Writing—review and editing. ZC designed the review and revised the botanical characterization and phytochemistry section. LiL designed the review and revised the pharmacological part. PL designed the review and revised the manuscript. LinL preparation table and schematic diagram. LY Preparation schematic diagram and polishing the language and grammar. CL was involved in conception, supervision, manuscript reviewing and editing. Submission of the final version was approved by all authors.
Funding
This study was supported by the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Sciences (CI2021B003), the National Key R&D Program of China (2020YFE0205100) and the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abushouk A. I. Ismail A. Salem A. Afifi A. M. Abdel-Daim M. M. (2017a). Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. = Biomedecine Pharmacother.90, 935–946. 10.1016/j.biopha.2017.04.033
2
Abushouk A. I. Negida A. Ahmed H. Abdel-Daim M. M. (2017b). Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: Future applications in Parkinson's disease. Biomed. Pharmacother. = Biomedecine Pharmacother.85, 635–645. 10.1016/j.biopha.2016.11.074
3
Ai Q. Wang B. Wang G. (2002). Sterilization function of the abstracting products of rhizom of fagopyrum cymosum maaisn. Heilongjiang Med. J.666. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7lwLRIsgSA99HxaEcueJ8s_vYdvP1Fwx4fKWzAnkmibK6J7lgRI35sNS7wuNA6hCh&uniplatform=NZKPT
4
Amini A. M. Spencer J. Yaqoob P. (2018). Effects of pelargonidin-3-O-glucoside and its metabolites on lipopolysaccharide-stimulated cytokine production by THP-1 monocytes and macrophages. Cytokine103, 29–33. 10.1016/j.cyto.2017.12.031
5
Bae J. Kumazoe M. Murata K. Fujimura Y. Tachibana H. (2020). Procyanidin C1 inhibits melanoma cell growth by activating 67-kDa laminin receptor signaling. Mol. Nutr. Food Res.64 (7), e1900986. 10.1002/mnfr.201900986
6
Bakkali F. Averbeck S. Averbeck D. Idaomar M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol.46 (2), 446–475. 10.1016/j.fct.2007.09.106
7
Batiha G. E. Beshbishy A. M. Alkazmi L. Adeyemi O. S. Nadwa E. Rashwan E. et al (2020). Gas chromatography-mass spectrometry analysis, phytochemical screening and antiprotozoal effects of the methanolic Viola tricolor and acetonic Laurus nobilis extracts. Ther20 (1), 87. 10.1186/s12906-020-2848-2
8
Batiha G. E. Beshbishy A. M. Tayebwa D. S. Adeyemi O. S. Shaheen H. Yokoyama N. et al (2019a). The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. Plos Negl. Trop. Dis.13, e0007030. 10.1371/journal.pntd.0007030
9
Batiha G. E. Beshbishy A. M. Tayebwa D. S. Shaheen H. M. Yokoyama N. Igarashi I. (2019b). Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick-Borne Dis.10 (5), 949–958. 10.1016/j.ttbdis.2019.04.016
10
Beshbishy A. M. Batiha G. E. Yokoyama N. Igarashi I. (2019). Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasites Vectors12 (1), 269. 10.1186/s13071-019-3520-x
11
Bi C. Gao X. Zhang Q. (2012). Therapeutic efficacy of fagopyrum cymosum tabletcombined with levofloxacin on acute bacillary dysentery. Infect. Dis. Inf.25 (1), 31–33.
12
Byun E. B. Sung N. Y. Byun E. H. Song D. S. Kim J. K. Park J. H. et al (2013). The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Immunopharmacol15 (2), 450–456. 10.1016/j.intimp.2012.11.021
13
Cao T. Zhao L. Luo H. Shi J. Jiang Y. (2019). Study on the extraction and purification technology for active ingredients of JinQiaoMai by taking epicatechin as the marker. West. J. Traditional Chin. Med.32 (03), 36–40. https://kns.cnki.net/kcms2/article/abstract?v=M5zCgC8QIcrbD1bgB8dzzWR2b3IZcBSNp-89zD9F4u7iLCgaMNvaiUS-49gqyL94w3B1MqpUjf9SYmG8Ynn-_AN1WcrGj4gUuw8wlkQ31wg4OuUj56s0zg==&uniplatform=NZKPT&language=CHS
14
Editorial Committee Of Chinese Flora (1998). “Editorial committee of Chinese Flora,” in Flora of China. (Beijing: Science Press). http://www.cn-flora.ac.cn/
15
Chan P. (2003b). Inhibition of tumor growth in vitro by the extract of Fagopyrum cymosum. J. Chin. Integr. Med.1 (2), 128–131. 10.3736/jcim20030213
16
Chan P. (2003a). Inhibition of tumor growth in vitro by the extract of fagopyrum cymosum (fago-c). Life Sci.72 (16), 1851–1858. 10.1016/s0024-3205(03)00013-4
17
Chen H. Zou Z. Xu X. Zhang J. Zhang X. Meng D. et al (2012). Inhibitory effect of Fagopyri Dibotryis Rhizoma extract on migration of WM239 and phosphorylation of N-cadherin in co-culture system of WM239 and Huvec. Chin. J. Cell. Mol. Immunol.28 (1), 37–39. 10.13423/j.cnki.cjcmi.006300
18
Chen X. Gu Z. Yang H. Liang Z. (2005). The effect of Fr4 on expression of matrix metalloproteinase-9 and tissue inhibitors of metallproteinase-1 in mouse Lewis lung cancer tissue, 25. China: Suzhou University Journal of Medical Science, 383–386. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SYXU200503011&DbName=CJFQ2005
19
Chen X. Gu Z. Yang H. Liang Z. Zhu M. Chen B. (2006). Effect of Fagopyri Dibotryis Rhizoma Fr4 on apoptosis and telomerase activity of HL-60 cells induced. Chin. Pharmacol. ical Bull.22 (7), 836–840. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7eWoVfj7plMzGtzV4keeauUu5P4TXrh2R22DCg6Mt70DPAe5itXyMOOMlDzJoEbVF&uniplatform=NZKPT
20
Cheng Y. T. Lin J. A. Jhang J. J. Yen G. C. (2019). Protocatechuic acid-mediated DJ-1/PARK7 activation followed by PI3K/mTOR signaling pathway activation as a novel mechanism for protection against ketoprofen-induced oxidative damage in the gastrointestinal mucosa. Free Radic. Biol. Med.130, 35–47. 10.1016/j.freeradbiomed.2018.10.415
21
Cook N. C. Samman S. J. (1996). Flavonoids Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem.7 (2), 66–76. 10.1016/0955-2863(95)00168-9
22
Dong L. Wang C. Wu C. Jiang Q. Zhang Z. (2011). [Effect of jinqiaomai on expression of TLR2/4, MyD88 mRNA and IkappaB-alpha in lung tissue of rats with Klebsiella pneumonia]. China J. Chin. Materia Medica36 (2), 200–204. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7tvjWANqNvp86QERf2Ty5clht06VmARYrSkr8coldZTKr1AdVj66cbPy0UKAbAa7U&uniplatform=NZKPT
23
El-Saber B. G. Magdy B. A. Stephen A. O. Nadwa E. Rashwan E. Yokoyama N. et al (2020). Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS One15, e0228996. 10.1371/journal.pone.022899
24
Essawi T. Srour M. (2000). Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol.70 (3), 343–349. 10.1016/s0378-8741(99)00187-7
25
Farombi E. O. Adedara I. A. Awoyemi O. V. Njoku C. R. Micah G. O. Esogwa C. U. et al (2016). Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct.7 (2), 913–921. 10.1039/c5fo01228g
26
Feng B. Huang X. Pang X. Long Q. (2021). Clinical efficacy of jinqiaomai capsules combined with salmeterol xinafoate and fluticason propionate powder for inhalation in the treatment of children with bronchial asthma. China Pharm.30 (7), 46–48. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=YYGZ202107012&DbName=CJFQ2021
27
Feng L. Chen F. Bai J. (2006). Study of antimicrobial in vitro from fagopyrum dibotrys extracts. J. Wuhan Botanical Res.24 (3), 240–244. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7eWoVfj7plMwamtZ5XYOWUW_egjbGHE7JlQO6DfGdNRAMmO4umUjbu3e5dR7Nz3yO&uniplatform=NZKPT
28
Ge F. Liu L. Yan J. Kang A. Zhu S. Tian Z. et al (2021). Influence of jinqiaomai pill combined with SASP on treating UC with dampness-heat in large intestine, 37. Nanjing, China: Journal of Nanjing University of Traditional Chinese Medicine, 16 (1)–20. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iy_Rpms2pqwbFRRUtoUImHdXb0YLgRehclWyfiCiFAKvqIeaItVyGYM9i1fkEfrsN&uniplatform=NZKPT
29
Gu F. Huang R. Liu Y. (2015). Effect of Fagopyri Dibotryis polysaccharide on immune function of immunocompromised mice induced by cyclophosphamide. J. Chin. Med. Mater.38 (2), 370–372. 10.13863/j.issn1001-4454.2015.02.040
30
Guo W. (2015). Clinical observation of lianhua-qingwen capsule joint with jingqiaomai tablet in treatment of influeza A (H1N1). J. Chengdu Med. Coll.10 (3), 357–359. 10.3969/j.issn.1674-2257.2015.03.027
31
Han J. (2020). Effect of Gubenkechuan tablet combined with Fagopyri Dibotryis Rhizoma tablet on symptom score and lung function in patients with chronic bronchitis. Contemp. Med.26 (27), 170–171. 10.3969/j.issn.1009-4393.2020.27.073
32
Han L. Yang Q. Li J. Cheng F. Zhang Y. Li Y. et al (2019). Protocatechuic acid-ameliorated endothelial oxidative stress through regulating acetylation level via CD36/AMPK pathway. J. Agric. Food Chem.67 (25), 7060–7072. 10.1021/acs.jafc.9b02647
33
Han L. Yang Q. Ma W. Li J. Qu L. Wang M. (2018). Protocatechuic acid ameliorated palmitic-acid-induced oxidative damage in endothelial cells through activating endogenous antioxidant enzymes via an adenosine-monophosphate-activated-protein-kinase-dependent pathway. J. Agric. Food Chem.66 (40), 10400–10409. 10.1021/acs.jafc.8b03414
34
Hua M. Yin Z. (2016). Clinical observation on the treatment of mild and moderate ulcerative colitis by oral administration of Fagopyri Dibotryis Rhizoma tablets combined with rectal drip of compound Kangfuxin solution. J. Med. Theory Pract.29 (24), 3354–3356. 10.19381/j.issn.1001-7585.2016.24.025
35
Huang G. Huang M. Chen W. Huang Y. Yang Z. You Y. (2009). Clinical study on the treatment of early diabetic nephropathy with Fagopyri dibotryis mixture. J. Chin. Med. Mater.32 (12), 1932–1935. 10.13863/j.issn1001-4454.2009.12.011
36
Huang J. Wang L. Tang B. Ren R. Shi T. Zhu L. et al (2022). Integrated transcriptomics and wide targeted metabolomics analyses provide insights into flavonoid biosynthesis in the rhizomes of golden buckwheat (fagopyrum cymosum). Front. Plant Sci.20, 803472. 10.3389/fpls.2022.803472
37
Huang R. Yi F. (2015). Bacteriostasis of epicatechin active substances from Buckwheat (-) in vitro. Jiangsu Agric. Sci.43 (01), 308–310. 10.15889/j.issn.1002-1302.2015.01.103
38
Huang R. Yi F. He H. Wu S. Fang J. Yang M. (2014). Antioxidant activity of (-)-Epicatechin from the root tubers of fagopyrum dibotrys. Food Sci.35 (15), 118–121. 10.7506/spkx1002-6630-201415024
39
Huang S. Wang J. Chen Q. Huang X. (2016). Study on the antioxidant effect of the active components of Fagopyri Dibotryis Rhizoma leaf tea. Cereals & Oils29 (2), 30–32. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7ijP0rjQD-AVm8oHBO0FTadjrjgFLq8hawP0ZrsKTTc6ko7kCFohVL7vTz86B1tgYI&uniplatform=NZKPT
40
Huang X. Wang J. Chen Q. (2014). Golden buckwheat (Fagopyrum cymosum) leaf tea function and mechanism of resistance to type II diabetes. Lishizhen Med. Materia Medica Res.25 (6), 1334–1337. 10.3969/j.issn.1008-0805.2014.06.020
41
Jia W. Lu J. Li X. Li C. (2010). Effects of rhizoma fagopyrum dibotryis extraction on dysmenorrhea models in mouse. J. LIAONING Univ. TRADITIONAL Chin. Med.12 (2), 198–199. 10.13194/j.jlunivtcm.2010.02.200.jiaw.069
42
Jiang Y. Yang W. Gui S. (2018). Procyanidin B2 protects rats from paraquat-induced acute lung injury by inhibiting NLRP3 inflammasome activation. Immunobiology223 (10), 555–561. 10.1016/j.imbio.2018.07.001
43
Jing R. Li H. Q. Hu C. L. Jiang Y. P. Qin L. P. Zheng C. J. (2016). Phytochemical and pharmacological profiles of three fagopyrum buckwheats. Int. J. Mol. Sci.17, 589. 10.3390/ijms17040589
44
Kim M. H. Kwon S. Y. Woo S. Y. Seo W. D. Kim D. Y. (2021). Antioxidative effects of chrysoeriol via activation of the Nrf2 signaling pathway and modulation of mitochondrial function. Molecules26, 313. 10.3390/molecules26020313
45
Li H. Wen D. Zhou M. Wang H. Peng X. Gao L. (2019). Advances in extraction and mechanism of antitumor active components from Fagopyrum dibotrys. Chin. J. Clin. Pharmacol. Ther.24 (7), 833–840. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iLik5jEcCI09uHa3oBxtWoDKPjZvajtk0U5H0I2RWOF3DfyjnCdAmn4dUKqJentv6&uniplatform=NZKPT
46
Li J. Hossain M. S. Ma H. Yang Q. Gong X. Yang P. et al (2020). Comparative metabolomics reveals differences in flavonoid metabolites among different coloured buckwheat flowers. J. Food Compos. Anal.85, 103335. 10.1016/j.jfca.2019.103335
47
Li L. (2012). Treatment of 32 cases of acute bacillary dysentery with Fagopyri Dibotryis Rhizoma tablets combined with ceftriaxone sodium. Guid. J. Traditional Chin. Med. Pharm.18 (3), 90. 10.13862/j.cnki.cn43-1446/r.2012.03.061
48
Li L. Wu X. (2018). Clinical observation of jinqiaomai capsules combined with salmeterol xinafoate and fluticasone propionate powder for inhalation in treatment of bronchial asthma. Drugs & Clin.33 (12), 3234–3237. 10.7501/j.issn.1674-5515.2018.12.034
49
Li S. (2010). Observation on the curative effect of gold buckwheat tablet combined with cefoperazone in treating acute attack of chronic bronchitis. J. Clin. Pulm. Med.15 (4), 466–467. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7_IFawAif0mxIaKhd5F2gn6ZFPc9juEyia0jox23Zn4iBpAhSuhp4ar1a_vqOp23F&uniplatform=NZKPT
50
Li S. Kodama E. N. Inoue Y. Tani H. Matsuura Y. Zhang J. et al (2010). Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication. Antivir. Chem. Chemother.20 (6), 239–248. 10.3851/IMP1597
51
Li X. Wu J. Liu Z. Zhu H. Zhang Z. (2022). Clinical study on the treatment of acute exacerbation of chronic ObstructivePulmonary disease with jinqiaomai tablets combined with tiotropium BromidePowder for inhalation. Chin. J. Ration. Drug Use19 (04), 59–64. 10.3969/j.issn.2096-3327.2022.04.011
52
Li Y. Cheng Z. Wang K. Zhu X. Ali Y. Shu W. et al (2021). Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (RPE) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp. Eye Res.207, 108586. 10.1016/j.exer.2021.108586
53
Li, B B. Feng F. Jia H. Jiang Q. Cao S. Wei L. et al (2021). Rhamnetin decelerates the elimination and enhances the antitumor effect of the molecular-targeting agent sorafenib in hepatocellular carcinoma cells via the miR-148a/PXR axis. Food Funct.12 (6), 2404–2417. 10.1039/d0fo02270e
54
Lin J. Zhao L. Guo J. Liu L. Kuang Y. Yang S. (2016). Chemical constituents from aerial parts of cagopyrum dibotrys. Chin. Traditional Herb. Drugs46 (11), 1841–1844. 10.7501/j.issn.0253-2670.2016.11.005
55
Lin Y. Luo T. Weng A. Huang X. Yao Y. Fu Z. et al (2020). Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Immunol11, 580593. 10.3389/fimmu.2020.580593
56
Liu L. Sun Z. Zhang X. Zhou L. Tian C. Chen L. et al (2012a). Analgesic mechanisms of Fagopyrum cymosum extracts in rats with irritable bowel syndrome. World Chin. J. Dig.20 (15), 1290–1295. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7fm4X_1ttJAmcliuAXHuQoTp9KWtxk6uq-JqpQKMnByXyHvO9qN9HXmCaMwsp_uWK&uniplatform=NZKPT
57
Liu L. Yan J. Lu W. Chen G. Lu Y. Sun Z. (2016). Effects of Fagopyrum cymosum extracts on visceral hyperalgesia of IBS rats and themechanism. West China J. Pharm. Sci.31 (2), 135–138. 10.13375/j.cnki.wcjps.2016.02.008
58
Liu L. Zhou L. Tian C. Chen L. Guo H. Sun Z. (2012b). Improvement of IBS rats’hyperalgesia by total flavones of Fagopyrum cymosum via down - regulation of NR2B expression. Chin. Pharmacol. Bull.28 (9), 1289–1293. 10.3969/j.issn.1001-1978.2012.09.024
59
Liu S. Tian L. Chen L. (1998). Research progress of buckwheat aureus. Prim. J. Chin. Mater Med.3, 46–47. 10.13728/j.1673-6427.1998.03.035
60
Liu Y. Fang Q. Zhang X. Feng X. Zhang L. He X. (1983). Study on active components of Fagopyri dibotryis rhizoma. Acta Pharm. Sin.18 (7), 545–547. 10.16438/j.0513-4870.1983.07.013
61
Lou J. Lin H. Qiu Q. Pei Y. Qi X. He X. (2004b). Experimental study on the antitumor effect of Weimining in vitro. Chin. Archives Traditional Chin. Med.22 (5), 810–811. 10.13193/j.archtcm.2004.05.39.loujl.025
62
Lou J. Lin H. Qiu Q. Pei Y. Qi X. He X. (2004a). The molecular mechanism of inhibition of murine Lewis lung carcinoma metastasis by weimaining in vivo. Chin. J. Pathophysiol.20 (4), 627–631. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7eeyE9jLkqq9HoZSVvnrpXjWT2L1a8vL_JroqhLyR_wAc82w9iKit0cGTyG5xf3mW&uniplatform=NZKPT
63
Lou J. Qiu Q. Lin H. Pei Y. Qi X. Wu X. et al (2007). Effect of Weimaining on adhesion between PG cell and human umbilical vein endothelial cells, 30. Beijing, China: Journal of Beijing University of Traditional Chinese Medicine, 29–31.1. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=JZYB200701009&DbName=CJFQ2007
64
Mackenzie G. G. Adamo A. M. Decker N. P. Oteiza P. I. (2008). Dimeric procyanidin B2 inhibits constitutively active NF-kappaB in Hodgkin's lymphoma cells independently of the presence of IkappaB mutations. Biochem. Pharmacol.75 (7), 1461–1471. 10.1016/j.bcp.2007.12.013
65
Milenkovic D. Declerck K. Guttman Y. Kerem Z. Claude S. Weseler A. R. et al (2020). (-)-Epicatechin metabolites promote vascular health through epigenetic reprogramming of endothelial-immune cell signaling and reversing systemic low-grade inflammation. Biochem. Pharmacol.173, 113699. 10.1016/j.bcp.2019.113699
66
Nam Y. J. Lee C. S. (2018). Protocatechuic acid inhibits Toll-like receptor-4-dependent activation of NF-κB by suppressing activation of the Akt, mTOR, JNK and p38-MAPK. Int. Immunopharmacol.55, 272–281. 10.1016/j.intimp.2017.12.024
67
Othman R. Ibrahim H. Mohd M. A. Awang K. Gilani A. U. Mustafa M. R. (2002). Vasorelaxant effects of ethyl cinnamate isolated from Kaempferia galanga on smooth muscles of the rat aorta. Planta Med.68 (7), 655–657. 10.1055/s-2002-32900
68
Pan C. Wan J. (2015). Study on analgesic and anti - inflammatory effects of Fagopyri Dibotryis Rhizoma medicinal liquor, 22. Hezhou, China: Journal of Ezhou University, 110–112. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=EZDX201509034&DbName=CJFQ2015
69
Pan C. Wang W. Qi X. Yang J. Zhou X. (2018). Anti-proliferative and apoptosis-inducing activity of rhizoma Fagopyri cymosi extract in cervical cancer hela cells. Pharmacol. Clin. Chin. Materia Medica34 (5), 96–100. 10.13412/j.cnki.zyyl.2018.05.023
70
Pan C. Wang W. Wan J. Ji D. Qing L. (2019). Effects of Fagopyri Dibotryis Rhizoma extract on caspase-1 39 and MMP-1 of articular cartilage in rabbits with knee osteoarthritis. Chin. J. Gerontology39 (20), 5066–5069. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZLXZ201920059&DbName=CJFQ2019
71
Pan C. Yang J. Ji D. Wan J. (2020). Effect of Fagopyri Dibotryis Rhizoma extract on oxygen free radical in rat model of knee osteoarthritis, 27. Hezhou, China: Journal of Ezhou University, 106–108.5. 10.16732/j.cnki.jeu.2020.05.033
72
Peng Y. Sun Z. Xiao P. (1996). Research and development of fagopyrum dibotrys (D.don) Hara. Chin. Traditional Herb. Drugs27 (10), 629–631. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9NkZNmQNo4kSVpbdjw7UNKfQ-1kzAb7Yq9-LI70Ck2x-j41l-1716-RaQuMuOwJRw0r&uniplatform=NZKPT
73
Pereyra-Vergara F. Olivares-Corichi I. M. Perez-Ruiz A. G. Luna-Arias J. P. García-Sánchez J. R. (2020). Apoptosis induced by (-)-Epicatechin in human breast cancer cells is mediated by reactive oxygen species. Molecules. 25. 1020. 10.3390/molecules25051020
74
Qiao H. Li C. Wang G. (2010). Serum pharmacological study on the effect of Rhizoma Fagopyri dibotryis root extract on th1-like lymphofactor mRNA expression in chicken. Heilongjiang Animal Sci. Veterinary Med.22, 130–132. 10.13881/j.cnki.hljxmsy.2010.09.056
75
Ríos J. L. Recio M. C. (2005). Medicinal plants and antimicrobial activity. J. Ethnopharmacol.100 (1-2), 80–84. 10.1016/j.jep.2005.04.025
76
Ruan H. Ji T. Ji W. Ma S. Zhang Z. (2017). Effects of flavonoids from Fagopyri dibotryis rhizoma on the glycolipidmetabolism and antioxidation in type 2 diabetic rats. Pharmacol. Clin. Chin. Materia Medica33 (5), 73–76. 10.13412/j.cnki.zyyl.2017.05.020
77
Ruan H. Mu J. (2017). Effect of epicatechin on inflammatory cytokines in LPS-induced RAW264.7 cell. Chin. J. Exp. Traditional Med. Formulae32, 159–163. 10.13422/j.cnki.syfjx.2017040159
78
Shao M. Yang Y. Gao H. Wu B. Wang L. Wu L. (2004). Phenolic acid derivatives from the rhizome of Fagopyrum cymosum. China J. Chin. Materia Medica30 (20), 1591–1593. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7F1IFNsBV5UtgR4AbwGdxZneHv9jhpZXpwlxY5V9dwWzUOIEEAGjbNxYbpvhbVpSv&uniplatform=NZKPT
79
Shao M. Yang Y. Gao H. Wu B. Wang L. Wu L. (2005). Study on chemical constituents of Fagopyri Dibotryis rhizoma, 22. Shenyang, China: Journal of Shenyang Pharmaceutical University, 1006–2858.2. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7F1IFNsBV5Uu2KAJBLaRynTuLXOBKO2uWV2l-Png5drI8NNUUNdDGqNb1fp3Tyg3A&uniplatform=NZKPT
80
Shen L. (2013). The anti-rheumatoid arthritis activity of Fagopyrum cymosum and its mechanism study. Wuhan, China: Huazhong University Of Science And Technology. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1014025348.nh&DbName=CDFD2014
81
Shen Y. Bian Z. Shao J. (2019). Effect of fagopyrum cymosum tablets on the level of inflammatory factor in DSS-induced inflammation model mice. Acta Chin. Med.34 (9), 1916–1920. 10.16368/j.issn.1674-8999.2019.09.448
82
Song H. Li L. Zhang J. (2020). Effect of Fagopyri Dibotryis Rhizoma extract on apoptosis of renal carcinoma cells regulated by DDIT4. Chin. J. Integr. Traditional West. Nephrol.21 (8), 708–711. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=JXSB202008020&DbName=CJFQ2020
83
Song J. H. Lee H. J. Kang K. S. (2019). Procyanidin C1 activates the Nrf2/HO-1 signaling pathway to prevent glutamate-induced apoptotic HT22 cell death. Int. J. Mol. Sci.20, 142. 10.3390/ijms20010142
84
Tan Y. Gan M. Fan Y. Wang J. Zhang X. Huang B. et al (2020). Protective effects of Fagopyri dibotryis on lipopolysaccharide - induced intestinal inflammation in mice. China Animal Husb. Veterinary Med.47 (2), 597–604. 10.16431/j.cnki.1671-7236.2020.02.032
85
Tang Y. Gao X. Jiang F. Guo S. You J. Zhu J. et al (2014). Effect of extract of fagopyrum dibotrys on serum cytokines and pulmonary histopathological changes in chronic obstructive pulmonary disease rats. Traditional Chin. Drug Res. Clin. Pharmacol.25 (6), 679–683. 10.3969/j.issn.1003-9783.2014.06.008
86
Tang Y. Gao X. Jiang F. Guo S. Su C. Hou H. (2016). Effect of the Wild Buckwheat Rhizome Extract on the serum inflammatory cytokines and the expression of NF - κB in chronic obstructive pulmonary disease model of rats. Mod. J. Integr. Traditional Chin. West. Med.25 (15), 1600–1603. 10.3969/j.issn.1008-8849.2016.15.002
87
Tang Y. Jia H. Sun J. Zhong Z. Shao J. (2014). Study on active components and content change of Fagopyri Dibotryis Rhizoma. Hubei Agric. Sci.53 (3), 672–675. 10.14088/j.cnki.issn0439-8114.2014.03.010
88
Tian L. Xu L. Shilin Y. (1997). Study on Chemical constituents of the aboveground part of Buckwheat. China J. Chin. Materia Medica22 (12), 100–102.
89
Wang D. Bao B. (2020). Gallic acid impedes non-small cell lung cancer progression via suppression of EGFR-dependent CARM1-PELP1 complex. Drug Des. Devel Ther.14, 1583–1592. 10.2147/DDDT.S228123
90
Wang H. Tang C. Yue H. (2013). Studies on antibacterial function of superfine powder fagopyrum dibotryis in vivo in mice. Prog. Veterinary Med.34 (10), 130–132. 10.16437/j.cnki.1007-5038.2013.10.029
91
Wang H. Y. Wang H. Wang J. H. Wang Q. Ma Q. F. Chen Y. Y. (2015). Protocatechuic acid inhibits inflammatory responses in LPS-stimulated BV2 microglia via NF-κB and MAPKs signaling pathways. Neurochem. Res.40 (8), 1655–1660. 10.1007/s11064-015-1646-6
92
Wang K. J. Zhang Y. J. Yang C. R. (2005). Antioxidant phenolic constituents from Fagopyrum dibotrys. J. Ethnopharmacol.99 (2), 259–264. 10.1016/j.jep.2005.02.029
93
Wang M. Wu Y. Li W. (2021). Rhamnetin ameliorates macrophage-mediated inflammation and pro-atherosclerosis pathways in apolipoprotein E-deficient mice. J. Physiol. Pharmacol.72, 10. 10.26402/jpp.2021.2.10
94
Wang P. Wang Y. Fang Y. (2017). Study on anti-oxidantion activity of the total flavone extract from fagopyrum dibotrys(D.Don)Hara. Anhui Agric. Sci. Bull.23 (8), 23–24. 10.16377/j.cnki.issn1007-7731.2017.08.011
95
Wang Z. Zhan Y. Xu J. Wang Y. Sun M. Chen J. et al (2020). β-Sitosterol reverses multidrug resistance via BCRP suppression by inhibiting the p53-MDM2 interaction in colorectal cancer. J. Agric. Food Chem.68 (12), 3850–3858. 10.1021/acs.jafc.0c00107
96
Wang, X X. Song Z. J. He X. Zhang R. Q. Zhang C. F. Li F. et al (2015). Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APC(Min/+) mice. Int. Immunopharmacol.29 (2), 701–707. 10.1016/j.intimp.2015.09.006
97
Wei W. Sun J. Shuai X. (2022). Effect of Jinqiaomai Capsules on the pulmonary ventilation function and the EOSlgE levels in the patients with mild to moderate asthma during its acute attack. Jilin J. Traditional Chin. Med.41 (06), 751–754. 10.13463/j.cnki.jlzyy.2021.06.015
98
Wu H. Zhou J. Pan H. (2008). Study on chemical constituents of fagopyrumdibotrys (D.don) Hara. Chin. J. Hosp. Pharm.28 (21), 1829–1831. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7VSL-HJEdEx3onS7wCSmyabHM2q82d4k-b7YwTR-EwQ9tBXiWUQc2MXh-MfrFedt2&uniplatform=NZKPT
99
Xiao Y. Dong J. Yin Z. Wu Q. Zhou Y. Zhou X. (2018). Procyanidin B2 protects against d-galactose-induced mimetic aging in mice: Metabolites and microbiome analysis. Food Chem. Toxicol.119, 141–149. 10.1016/j.fct.2018.05.017
100
Yan J. (2006). Study on Chemical constituents & HPLC fingerprint of buckwheat. Shenyang, China: Shenyang Pharmaceutical University. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAk6X_k1IQGNCLwAgnuJ-hC0_lcpntt3h-DHUcgEmqBCCkz8iejljMj5jLDULva8NQD&uniplatform=NZKPT
101
Yan J. Wang L. Li W. Gao H. Wu L. (2006). Study on bacteriostasis of fagopyrum dibotrys (D.don) Hara. Mod. Chin. Med.8 (6), 21–23. 10.13313/j.issn.1673-4890.2006.06.008
102
Yang T. Rong Z. Wu Y. (1992). Effect of Fagopyri Dibotryis Rhizoma E on phagocytosis of reticuloendothelial system in mice. Sichuan J. Physiological Sci.22, 9–12. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9NkyGkCpOZCCaeYi10YmvBDhlGCMGoEFfVYjcBPb-oTRtrumQOj7cHoS2uvLlFeYB_6&uniplatform=NZKPT
103
Yang X. Zhang Y. Li L. (2019). Advances in studies on medicinal plant of fagopyrum dibotrys. Mod. Chin. Med.21 (6), 837–846. 10.13313/j.issn.1673-4890.20180709003
104
Yin D. Lin S. (1999). Effect of Fagopyri Dibotryis Rhizoma on phagocytic function of mouse peritoneal macrophages. Cap. Med.6 (12), 28–29. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9Nkm5tS6uBYqSGHbvWnFosSPHb_Qf7ezhOT0DOH1YfuehruJzt_RqjoCUwV1AidIvYZ&uniplatform=NZKPT
105
Yoon H. S. Park C. M. (2021). Chrysoeriol ameliorates COX-2 expression through NF-κB, AP-1 and MAPK regulation via the TLR4/MyD88 signaling pathway in LPS-stimulated murine macrophages. Exp. Ther. Med.22 (1), 718. 10.3892/etm.2021.10150
106
Yu J. (2014). Effect of Fagopyri Dibotryis Rhizoma tablet combined with levofloxacin on acute bacillary dysentery. Chin. Foreign Med. Res.12 (10), 49–50. 10.14033/j.cnki.cfmr.2014.10.039
107
Zhang C. Chen R. Yin Y. Wang Z. (2011). Isolation and identification of endophytic fungi with antimicrobial activity from Fagopyrum dibotrys. Microbiol. China38 (1), 70–77. 10.13344/j.microbiol.china.2011.01.016
108
Zhang F. Liu Z. He X. Li Z. Shi B. Cai F. (2020). β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete freund's adjuvant-induced arthritis in rats: Involvement of NF-кB and HO-1/Nrf-2 pathway. Drug Deliv.27 (1), 1329–1341. 10.1080/10717544.2020.1818883
109
Zhang H. Li L. (2019). Clinical study on Jinqiaomai Tablets combined with cefdinir in treatment of acute bacterial dysentery. Drugs & Clin.34 (02), 499–503. 10.7501/j.issn.1674-5515.2019.02.050
110
Zhang H. Li S. Cui J. Zhao T. (2010). Effects of extract from rosa roxburghir tratt or extract of fagopyrum cymosum meissn on cell prolife. J. Oncol.16 (01), 35–39. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7_IFawAif0mz-Ilz_uHmWlNMTZhzl0zx7_vOJm9bBco2au0067CUXs1uK2se5uYba&uniplatform=NZKPT
111
Zhang J. Kuang Y. Liu L. Yang S. Zhao C. (2016). Chemical constituents from root tubers of Fagopyrum dibotrys. Chin. Traditional Herb. Drugs47 (5), 722–725. 10.7501/j.issn.0253-2670.2016.05.005
112
Zhang R. Yu Q. Lu W. Shen J. Zhou D. Wang Y. et al (2019). Grape seed procyanidin B2 promotes the autophagy and apoptosis in colorectal cancer cells via regulating PI3K/Akt signaling pathway. OncoTargets Ther.12, 4109–4118. 10.2147/OTT.S195615
113
Zhang W. Li X. Liu Y. Yao R. Nonaka G. Yang C. (1994). Phenolic constituents from fagopyrum dibotrys. Acta Bot. Yunnanica16 (4), 354–356. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9NkGsvn6cq9Bo0GTEcJPCg6sx7krNckKfvPcY49x34luPbmxOqhjwEttn-fvNXzSoTZ&uniplatform=NZKPT
114
Zhao J. Jiang L. Tang X. Peng L. Li X. Zhao G. et al (2018). Chemical composition, antimicrobial and antioxidant activities of the flower volatile oils of fagopyrum esculentum. Fagopyrum tataricum Fagopyrum Cymosum. Mol.23, 182. 10.3390/molecules23010182
115
Zhao L. Zhang X. Zhang C. (2011). Fractionation and identification of ethyl ecetate extract from rhizoma Fagopyri cymosi. Food Sci.32 (19), 16–22. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SPKX201119005&DbName=CJFQ2011
116
Zhao Y. Liu Y. Xie S. Wu Q. (2019). Study on the anti-influenza virus of extracts from fagopyrum dibotrys(D. Don) Hara in vitro. Chin. J. Mod. Appl. Pharm.36 (21), 2648–2651. 10.16368/j.issn.1674-8999.2019.09.448
117
Zhou Y. Qiao H. Li C. Wang G. (2009). Study on antibacterial activity of Fagopyri dibotryis rhizoma extract in vitro. J. Traditional Chin. Veterinary Med.28 (5), 44–46. 10.13823/j.cnki.jtcvm.2009.05.045
Summary
Keywords
Fagopyri Dibotryis Rhizoma, ethnobotany, application, phytochemistry, pharmacology
Citation
Geng Q, Liu B, Cao Z, Li L, Lu P, Lin L, Yan L and Lu C (2023) Ethnobotany, phytochemistry and pharmacological properties of Fagopyri Dibotryis Rhizoma: A review. Front. Pharmacol. 14:1095554. doi: 10.3389/fphar.2023.1095554
Received
11 November 2022
Accepted
20 February 2023
Published
06 March 2023
Volume
14 - 2023
Edited by
Somasundaram Arumugam, National Institute of Pharmaceutical Education and Research, Kolkata, India
Reviewed by
Carlos L. Cespedes-Acuña, University of Bío-Bío, Chile
Laiba Arshad, Forman Christian College, Pakistan
Updates
Copyright
© 2023 Geng, Liu, Cao, Li, Lu, Lin, Yan and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Lu, lv_cheng0816@163.com
†These authors have contributed equally to this work
This article was submitted to Ethnopharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.