Abstract
Diabetic nephropathy (DN), a prevalent microvascular complication of diabetes mellitus, is the primary contributor to end-stage renal disease in developed countries. Existing clinical interventions for DN encompass lifestyle modifications, blood glucose regulation, blood pressure reduction, lipid management, and avoidance of nephrotoxic medications. Despite these measures, a significant number of patients progress to end-stage renal disease, underscoring the need for additional therapeutic strategies. The endoplasmic reticulum (ER) stress response, a cellular defense mechanism in eukaryotic cells, has been implicated in DN pathogenesis. Moderate ER stress can enhance cell survival, whereas severe or prolonged ER stress may trigger apoptosis. As such, the role of ER stress in DN presents a potential avenue for therapeutic modulation. Chinese herbal medicine, a staple in Chinese healthcare, has emerged as a promising intervention for DN. Existing research suggests that some herbal remedies may confer renoprotective benefits through the modulation of ER stress. This review explores the involvement of ER stress in the pathogenesis of DN and the advancements in Chinese herbal medicine for ER stress regulation, aiming to inspire new clinical strategies for the prevention and management of DN.
1 Introduction
Diabetes mellitus (DM) constitutes an escalating global public health concern. As reported by the International Diabetes Federation, the prevalence of DM surged to 536.6 million individuals globally in 2021, with projections indicating a rise to 783.2 million by 2045 (Sun et al., 2022). A significant proportion, ranging from 20% to 40% of these individuals, concurrently live with diabetic kidney disease (de Boer et al., 2011; Afkarian et al., 2016). Diabetic nephropathy (DN), a form of renal damage precipitated by chronic hyperglycemia, can affect the entire kidney structure, including the glomerulus, renal tubules, renal interstitium, and renal vessels. Clinically, DN is characterized by persistent albuminuria and/or a progressive decrease in glomerular filtration rate, which can eventually progress to end-stage renal disease. Given the complex pathogenesis of DN, no curative treatment has been established to date. Existing therapeutic regimens primarily focus on glycemic control, blood pressure management, cardiovascular risk reduction, and inhibition of the renin-angiotensin system (Umanath and Lewis, 2018). In recent years, novel hypoglycemic agents, namely, sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists, have garnered significant attention for their renoprotective effects (Alicic et al., 2019; Kristensen et al., 2019). Despite these efforts, one-third of patients ultimately progress to end-stage renal disease necessitating renal replacement therapy (Collins et al., 2012). Therefore, there is currently an urgent need to identify novel therapeutic strategies that can impede the progression of DN.
Recent advancements in research have unveiled the potential significance of endoplasmic reticulum (ER) stress in the pathogenesis and progression of DN. Elevations in ER stress markers, namely, glucose-regulated protein 78 (GRP78) and the C/EBP homologous protein (CHOP), have been observed in the renal tissue of DN patients. These molecular alterations are often associated with histopathological aberrations such as glomerulosclerosis, tubular atrophy, and interstitial fibrosis (Pang et al., 2016). Notably, several DN-associated factors, including proteinuria, hyperglycemia, free fatty acids, and advanced glycation end products (AGEs), have been reported to trigger ER stress, thereby contributing to renal intrinsic cell damage (Lindenmeyer et al., 2008; Park et al., 2017; Jeong and Lee, 2021). Recent studies have underscored the renoprotective effects of ER stress inhibition, thereby highlighting its therapeutic potential (Yuan et al., 2018; Xiong et al., 2020). In this context, the strategy of normalizing ER stress through pharmacological interventions has been postulated as an effective approach to curtail DN progression (Chen H. Y. et al., 2019). Moreover, an increasing body of evidence suggests the potential of Chinese herbal medicine in attenuating DM-induced renal damage by modulating ER stress. This review article explores the critical role of ER stress in the pathogenesis of DN and the pertinent advancements in the field of Chinese herbal medicine for the prevention and treatment of DN via ER stress regulation.
2 ER stress and the unfolded protein response
The ER is an intracellular organelle found in eukaryotic cells, critical for various functions including protein synthesis and folding, lipid biosynthesis, calcium storage, and detoxification processes (Schwarz and Blower, 2016; Huang et al., 2022). The ER is highly sensitive to environmental alterations, and several physiological or pathological conditions can adversely affect its function. Factors such as nutritional deprivation, oxidative stress, tissue hypoxia, lipid overload, calcium imbalance, and low pH can precipitate the accumulation of unfolded or misfolded proteins within the ER, thereby impairing its function and precipitating what is known as ER stress. The manifestation of ER stress can be categorized into three primary responses: the unfolded protein response (UPR), the ER overload response, and the sterol regulatory element-binding protein response. The first two responses arise from disturbances in protein processing, whereas the latter response is a consequence of cholesterol depletion synthesized at the endoplasmic reticulum’s surface. The UPR, the most extensively studied of these responses, plays a pivotal role in mitigating damage induced by ER stress. In response to ER stress, cells initiate the UPR to alleviate damage, including inhibition of protein translation, enhancement of protein folding capabilities, and degradation of misfolded proteins via ER-associated degradation (Engin and Hotamisligil, 2010). This response mechanism assists in restoring ER homeostasis and promotes cell survival. However, in cases where ER stress is either too severe or sustained for extended periods, the UPR may paradoxically activate programmed cell death (Engin and Hotamisligil, 2010; So, 2018) (Figure 1).
FIGURE 1
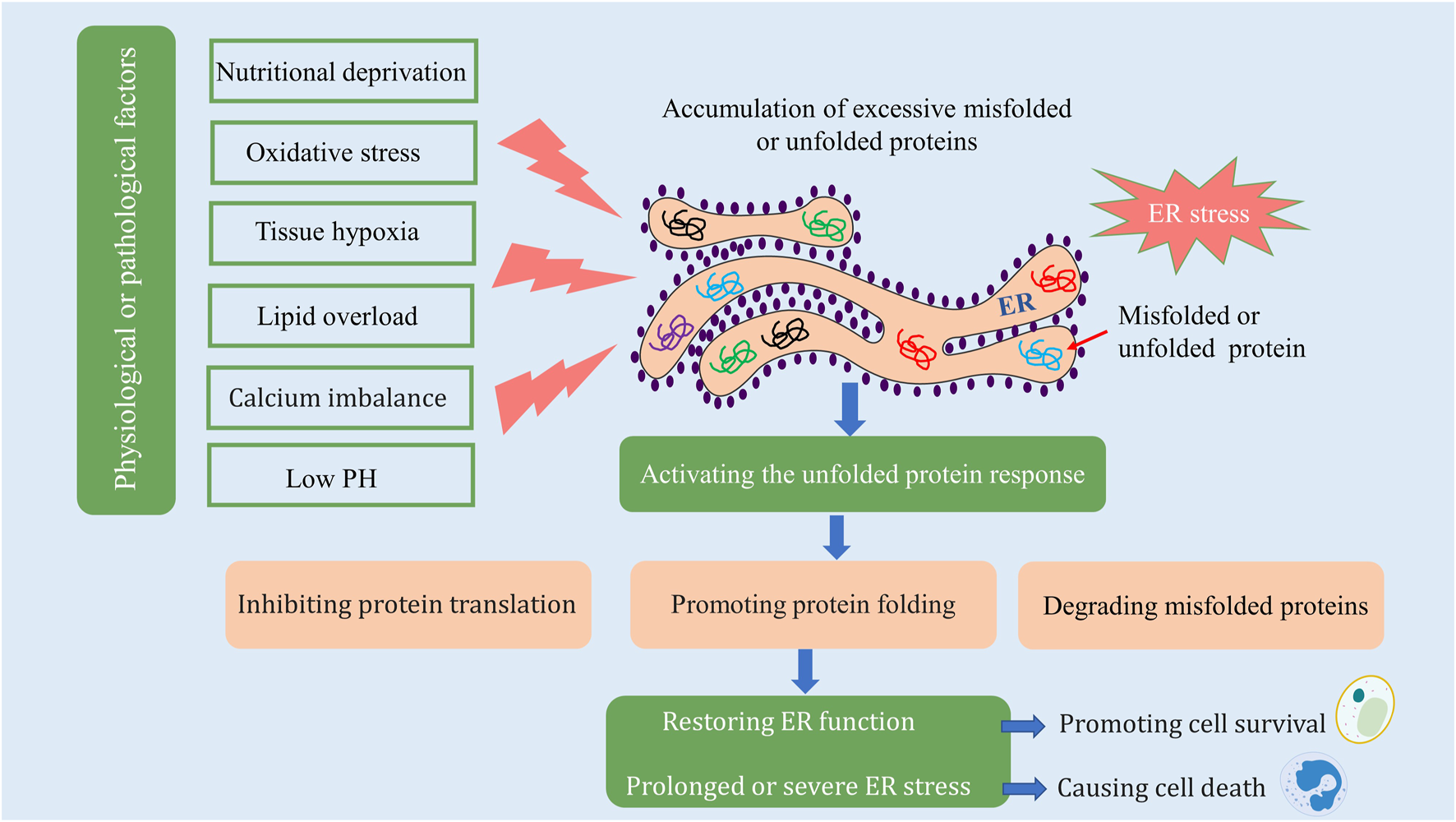
ER stress and the unfolded protein response. Materials provided by Servier Medical Art (smart.servier.com).
3 Signaling pathways for the unfolded protein response
UPR is an adaptive cellular mechanism governed by three ER transmembrane proteins: protein kinase R (PRK)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). Under standard physiological conditions, these transmembrane proteins bind to GRP78, thereby maintaining an inactive state. However, when ER stress is induced, an accumulation of unfolded or misfolded proteins results in the binding to GRP78, causing the disassociation of GRP78 from PERK, IRE1, and ATF6. This disassociation instigates the subsequent induction of downstream signaling pathways (Figure 2).
FIGURE 2
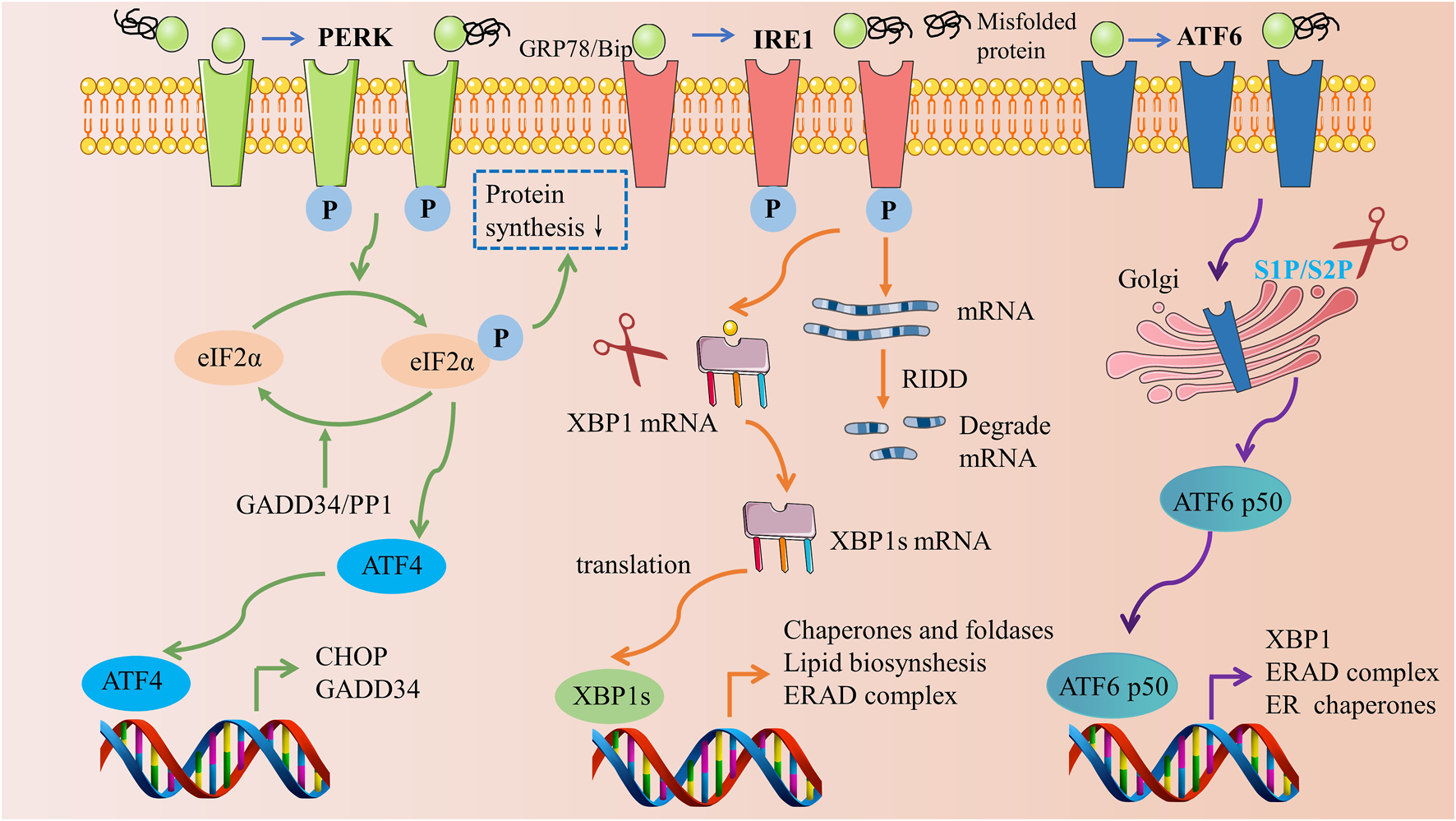
Three signaling pathways of the unfolded protein response. Materials provided by Servier Medical Art (smart.servier.com).
Among these proteins, PERK plays a pivotal role in the modulation of ER stress. Upon liberation from GRP78, oligomerization and autophosphorylation activate PERK (Wang et al., 2018). Once activated, PERK proceeds to phosphorylate the eukaryotic translation initiation factor (eIF2α), thereby mitigating protein synthesis in the ER (Guo et al., 2021). Subsequently, phosphorylated eIF2α selectively enables the translation of activating transcription factor 4 (ATF4) mRNA, concomitantly inhibiting other protein translations, thus fostering the expression of ATF4 (Mukherjee et al., 2020). ATF4 then prompts the generation of two crucial target genes: CHOP and growth arrest and DNA damage-inducible protein (GADD34). While CHOP, a transcription factor, modulates apoptosis-related genes, GADD34 exerts a negative regulation on the PERK pathway by catalyzing the dephosphorylation of eIF2α (Oyadomari and Mori, 2004; Tsaytler et al., 2011).
IRE1 is a type I transmembrane protein located within the ER membrane, exhibiting dual enzymatic activities of serine/threonine protein kinase and endonuclease. Upon the initiation of ER stress, IRE1 undergoes dissociation from GRP78, facilitating a signal transduction across the membrane to the cytoplasmic domain, which subsequently undergoes oligomerization and autophosphorylation. The activated state of IRE1 exhibits endonuclease activity capable of cleaving the mRNA of X-box binding protein 1 (XBP1), thus enabling its translation into the active transcription factor XBP1s. This activated factor translocates into the nucleus, instigating an upregulation in the expression of genes associated with protein folding, thereby mitigating ER stress and reinstating intracellular homeostasis (Yoshida et al., 2001; Bashir et al., 2021). Moreover, IRE1 promotes degradation of numerous ER-targeted mRNAs through the regulated IRE1α-dependent decay (RIDD) cleavage mechanism, impeding their translation and alleviating the protein folding burden on the ER (Hetz et al., 2020).
ATF6, a type II transmembrane protein residing within the ER membrane, possesses a unique mode of action compared to IRE1 and PERK. Upon release, ATF6 is transported to the Golgi apparatus where it is cleaved by site 1 protease (S1P) and site 2 protease (S2P), resulting in the formation of the active form, p50 ATF6 (Haze et al., 1999). Subsequently, p50 ATF6 migrates into the nucleus to bind with the endoplasmic reticulum stress response element (ERSE) located on the promoter, thereby triggering the transcription of XBP1, an ER chaperone protein, and the endoplasmic reticulum-associated degradation (ERAD) complex, all of which contribute to the alleviation of ER stress (Yamamoto et al., 2007).
4 The role of ER stress in diabetic nephropathy
4.1 Existence of ER stress in diabetic nephropathy
Renal intrinsic cells possess a substantial ER system, thereby establishing the conditions and foundation for the occurrence of ER stress. The renal tissues of patients with progressive, proteinuric DN have reported markedly elevated mRNA levels of HSPA5, HYOU1, and XBP1—principal genes implicated in the UPR (Lindenmeyer et al., 2008). This indicates an activation of ER stress in the kidneys of DN patients. In parallel, Guo et al. (2016) noted the activation of ER transmembrane sensory proteins—ATF6, PERK, and IRE1α—and their downstream targets eIF2α, ATF4, and XBP1 in both in vivo and in vitro DN models. These findings corroborate that all three branches of the UPR signaling pathway are activated during the course of DN.
A growing body of evidence implicates various factors such as hyperglycemia, proteinuria, free fatty acids, and AGEs in triggering ER stress in renal cells. For instance, Bai et al. (2018) identified that the expression of LINC01619 was downregulated in high glucose-cultured podocytes. This reduction in LINC01619 led to the suppression of FOXO1 expression via acting as a miR-27a “sponge,” thereby initiating ER stress and causing podocyte damage in DN. Sirtuin-1, a nicotinamide adenine dinucleotide-dependent deacetylase, has also been implicated in these processes. A study by Kang et al. (2018) illustrated that sirtuin-1 deacetylated PERK, mitigating the PERK/eIF2α/CHOP ER stress pathway. Furthermore, LncRNA TUG1, downregulated in a high-glucose environment, was found to inhibit sirtuin-1 expression through a sponge-like interaction with miR-29c-3p, which in turn exacerbated ER stress-mediated injury in HK-2 cells (Wang S. et al., 2021). Notably, proteinuria serves not merely as an indicator of glomerular damage but also as a significant risk factor for kidney disease progression. It has been demonstrated that podocytes and renal tubular epithelial cells exposed to high protein loads experience ER stress (Ohse et al., 2006; Gonçalves et al., 2018; Jia et al., 2019). In this context, Jia et al. (2019) discovered that albumin stimulated the expression of miR-4756 in HK-2 cells, which directly suppressed sestrin2 expression by targeting its 3'-untranslated region, consequently inducing epithelial-mesenchymal transition and ER stress in these cells. Another study revealed that simultaneous exposure of HK-2 cells to albumin and high glucose notably enhanced ER stress-related gene expression as compared to exposure to albumin alone (Lindenmeyer et al., 2008).
Palmitic acid, the most abundant free fatty acid in human plasma, has demonstrated profound effects on podocytes, cells particularly sensitive to this fatty acid. Studies by Xu et al. (2015). Highlighted that palmitate stimulates ER Ca2+ depletion in mouse podocytes, initiating ER stress. However, this effect was mitigated by silencing the CHOP gene, which attenuated palmitic acid-induced podocyte death (Sieber et al., 2010). In patients with diabetes, AGEs, harmful protein byproducts, are notably elevated and have a propensity to accumulate within all renal structures. Chiang et al. (2016) observed that AGEs trigger ER stress signaling in a time- and dose-dependent fashion. Importantly, the inhibition of ER stress via 4-phenylbutyric acid (4-PBA) successfully reversed AGE-induced apoptosis in mesangial cells. Subsequently, Liu et al. (2015) determined that the ATF4/p16 pathway, regulated by ER stress, contributes to AGE-induced premature senescence of renal tubular epithelial cells. This effect was significantly attenuated by both 4-PBA and ATF4 gene silencing. Moreover; Chen et al. (2008) discovered that AGEs induce GRP78 expression and podocyte apoptosis in a dose- and time-dependent manner, while also triggering a rapid increase in intracellular calcium through the release of ER stores and the influx of extracellular calcium. These effects were substantially diminished following treatment with tauroursodeoxycholic acid; Liang et al. (2016) further demonstrated that salubrinal, a selective inhibitor of eIF2α dephosphorylation, blocked the conversion of human glomerular endothelial cells to mesenchymal cells, instigated by advanced oxidation protein products. Several in vivo studies also revealed that ER stress inhibitors substantially reduced proteinuria, ameliorated renal function, and attenuated renal histopathological damage in animal models of DN (Cao A. L. et al., 2016; Fan et al., 2017). Collectively, these findings underscore the presence of ER stress in DN and its critical role in the disease’s onset and progression.
4.2 Underlying mechanisms of ER stress involved in the progression of diabetic nephropathy
4.2.1 ER stress and oxidative stress
Oxidative stress is a physiological state characterized by an overproduction of reactive oxygen and reactive nitrogen radicals, surpassing the body’s capacity for oxide removal. This state is typically induced by various harmful stimuli and results in an imbalance between oxidative and antioxidant systems, subsequently leading to tissue or cellular dysfunction (Daenen et al., 2019). Extended periods of ER stress generate a hyperoxic environment within the ER lumen, thereby releasing H2O2 into the cytoplasm and directly forming cytotoxic intracellular reactive oxygen species (ROS) within the cytoplasm. One empirical human study confirmed that a significant interplay exists between ER stress and oxidative stress, contributing notably to the progression of DN (Victor et al., 2021). In this study, ER oxidase 1α (ERO1α) levels were found to be markedly elevated in the peripheral blood mononuclear cells of DN patients and positively correlated with PERK and p22pHox (Victor et al., 2021). ERO1α, a key intermediary linking ER stress and ROS, also serves as a target of CHOP. A CHOP knockdown mitigated the expression of ERO1α, consequently reducing ROS production (Rao et al., 2015). Moreover, either selective PERK blockade (via GSK2606414) or PERK silencing (shPERK) successfully curtailed the elevated cytoplasmic Ca2+ and intracellular ROS levels (Zhang Y. et al., 2019; Ko et al., 2021). Multiple studies have demonstrated the antioxidant capabilities of ER stress inhibitors. Cao A. et al. (2016) suggested that ursodeoxycholic acid (UDCA) could potentially enhance renal pathology and function by attenuating high glucose-induced oxidative stress. Additionally, Luo et al. (2010) found that 4-PBA effectively inhibited NADPH oxidase activity, reduced malondialdehyde (MDA) levels, and enhanced superoxide dismutase (SOD) activity in DN rats.
4.2.2 ER stress and inflammation
Inflammation serves as a critical mechanism underlying the onset and progression of DN. Recent evidence has implicated ER stress in fostering kidney inflammation under diabetic conditions. Specifically, Zhu et al. (2017) observed that ER stress could incite the expression of CXCL10 and CCL2 via the activation of nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) pathways. Notably, these effects were counteracted by PERK knockdown. In the context of the db/db mouse model of diabetes, ER stress was found to stimulate the expression of monocyte chemoattractant protein-1 (MCP-1) via the SET7/9-mediated induction of histone methylation. Furthermore, the silencing of the XBP1s gene using siRNA markedly diminished the expression of both SET7/9 and MCP-1 (Chen J. et al., 2014). The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is known to orchestrate the secretion, maturation, and release of various inflammatory mediators, such as interleukin 18 (IL-18) and IL-1β, that further exacerbate glomerular and tubular damage in DN (Williams et al., 2022). In β-cells, ER stress has been found to upregulate the expression of thioredoxin-interacting protein (TXNIP) through the PERK and IRE1 pathways, thereby activating the NLRP3 inflammasome (Oslowski et al., 2012). Consistent with these findings, Yang et al. (2022) demonstrated that chronic ER stress augments the levels of renal pro-inflammatory cytokines, such as TNF-α, MCP-1, IL-1β, and IL-18, by excessively activating the NLRP3 inflammasome. Significantly, the ER stress inhibitor, UDCA, was found to mitigate the bovine serum albumin-induced activation of the NLRP3 inflammasome in renal tubular epithelial cells (Fang et al., 2013).
4.2.3 ER stress and apoptosis
Analogous to the endogenous (mitochondrial) pathway and the exogenous (death receptor) pathway, endoplasmic reticulum stress represents a crucial avenue for apoptosis induction (Hu et al., 2018). Prior research has confirmed that ER stress can steer apoptosis through three pathways: CHOP/GADD153, IRE1/ASK1/JNK, and caspase-12 (Oyadomari and Mori, 2004; Hetz, 2012). Notably, activation of ER-associated apoptotic proteins CHOP, JNK, and caspase-12 in DN rat kidneys was documented as early as 2008 (Liu et al., 2008). CHOP, a pro-apoptotic transcription factor in the ER stress process, resides downstream of PERK. Interventions involving PERK knockdown or CHOP depletion have exhibited protective effects against podocyte apoptosis (Fan et al., 2017; Tian N. et al., 2018). In parallel, HRD1, an E3 ubiquitin ligase, is known to advance eIF2α ubiquitination and degradation. Huang et al. (2017) observed that HRD1 expression was diminished and apoptosis was enhanced in palmitic acid- or high glucose-induced HKC-8 cells. Conversely, overexpression of HRD1 led to decreased p-eIF2α and eIF2α expression, thereby mitigating HKC-8 cell apoptosis. Protein arginine methyltransferase-1 (PRMT1), a key enzyme catalyzing the process of protein arginine methylation, showed marked elevation in DN models. Importantly, PRMT1 knockdown was found to alleviate high glucose- or palmitic acid-induced ER stress and renal intrinsic cell apoptosis through the inactivation of PERK and ATF6 (Park et al., 2017; Chen Y. Y. et al., 2019). A recent investigation demonstrated that KIRA6, a type II IRE1α inhibitor, reversed high-glucose-induced apoptosis in HK-2 cells by attenuating ER stress via the inhibition of IRE1α expression (Xie et al., 2022). In contrast, Xie et al. (2021) reported that podocyte-specific disruption of IRE1α amplified renal cell apoptosis, proteinuria, and renal fibrosis in diabetic mice through the suppression of ADH1 expression. The reasons behind these discrepancies in the role of IRE1α in DN remain elusive, with potential influencers being the differences in cell types and experimental conditions.
4.2.4 ER stress and autophagy
Autophagy represents a highly conserved lysosomal degradation pathway, crucial for maintaining intracellular homeostasis through the degradation of cytoplasmic metabolites and damaged organelles. It has been demonstrated in earlier research that impaired autophagy of renal cells under hyperglycemic conditions plays a pivotal role in the pathogenesis of DN (Koch et al., 2020). More recent investigations have drawn a close connection between ER stress and deficiencies in autophagy. In their study, Cao A. L. et al. (2016) observed that both UDCA and 4-PBA significantly boosted the expression of LC3 A/B II and Beclin-1, thereby mitigating high glucose-induced apoptosis in podocytes. ATF4 serves as a principal regulator of ER stress. Liang et al. (2021) demonstrated that ATF4 knockout resulted in improved urinary albumin levels, renal function, and renal fibrosis in DN mice. Mechanistically, the silencing of the ATF4 gene curtailed p62 and Col-IV protein expression, elevated LC3-II protein expression, and reinstated autophagosomes and autophagic lysosomes in NRK-52E cells cultured under high glucose conditions. In contrast to (Liang et al.) 's findings, Yuan et al. (2021) reported that ATF4 silencing diminished podocyte autophagy triggered by DN mouse serum and heightened podocyte apoptosis. The evidence thus suggests that the influence of ATF4 on the autophagic activity of renal cells during DN pathology may exhibit cell-specific variations. Therefore, future studies could provide further elucidation to reconcile this apparent inconsistency.
4.2.5 ER stress and pyroptosis
Pyroptosis represents a recently identified mode of programmed cell death. It involves the activation of caspases 1, 4, 5, and 11, mediated by inflammasomes, which initiate the cleavage and polymerization of several Gasdermin family members, thereby triggering the formation of cell membrane pores and the subsequent release of inflammatory factors. TXNIP serves as an important molecular link between ER stress and pyroptosis, with TXNIP upregulation being dependent on the activation of PERK and IRE1α (Fu et al., 2021). In DN models, Ke et al. (2020) observed the activation of ER stress, where IRE1α specifically degraded miR-200a. This degradation in turn elevated the TXNIP/NLRP3 inflammasome, which induced pyroptosis in renal tubular epithelial cells and exacerbated renal injury. In line with this, another study corroborated that ER stress-induced pyroptosis via activation of the NF-κB/NLRP3 pathway constitutes a pivotal mechanism of high glucose-induced renal injury (Li et al., 2023). Moreover, the silencing of XBP1 in cadmium-induced HK-2 cells resulted in inhibited NLRP3 inflammasome activation and pyroptosis (Chou et al., 2019). The interrelationships between ER stress and oxidative stress, inflammation, apoptosis, autophagy, and pyroptosis in DN are summarized in Figure 3.
FIGURE 3
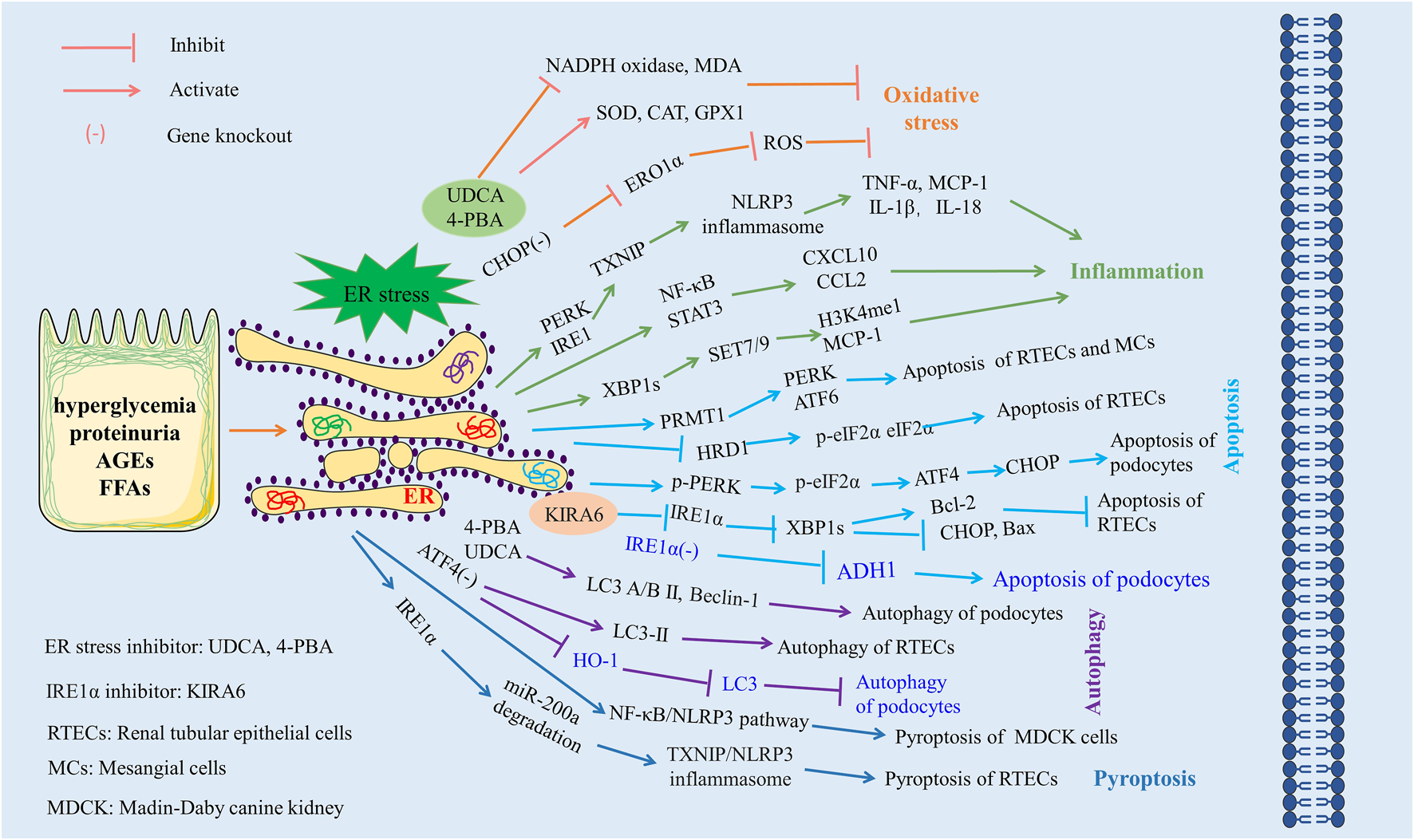
The role of ER stress in diabetic nephropathy. Excessive ER stress exacerbates kidney injury by aggravating oxidative stress, inflammation, apoptosis, and pyroptosis and inhibiting autophagy. Materials provided by Servier Medical Art (smart.servier.com).
ER stress, a complex biological process, plays a dual role in DN. While the majority of studies to date have reported on the pathogenic implications of ER stress in DN, with excessive ER stress exacerbating pathological processes such as oxidative stress, inflammatory responses, apoptosis, autophagy, and pyroptosis, a few studies intriguingly suggest a potentially protective role for ER stress in DN. Therefore, the question of how to maintain ER stress within a moderate range presents an important direction for future research.
5 Modulatory role of Chinese herbal medicine in ER stress in diabetic nephropathy
5.1 Chinese herbal formulas
Chinese herbal formulas, comprised of two or more herbs, are a cornerstone of clinical treatments for diabetic kidney disease in China. Recent literature has substantiated the effectiveness of these formulas in improving renal function and reducing proteinuria in patients with diabetic kidney disease (Zhang L. et al., 2019; Liu et al., 2022). Recent investigations have also highlighted a potential link between the amelioration of DN and the regulation of ER stress by certain Chinese herbal formulas (Table 1).
TABLE 1
| Chinese herbal formula | Experimental models | Effects | Mechanisms | References |
|---|---|---|---|---|
| Tongluo Yishen formula | Glomerular mesangial cells cultured with high glucose | Reduced apoptosis | JNK, CHOP, and Caspase-12↓, the increase of GRP78↓ | Li et al. (2016a); Li et al. (2016b) |
| Inhibited ER stress | Li et al. (2016c) | |||
| Tongluo Baoshen formula | SD rats injected with 50 mg/kg STZ | Reduced apoptosis | GRP78↓, p-IRE1α↓, p-JNK↓ | Zhang et al. (2017a) |
| Inhibited ER stress | Zhang et al. (2017b) | |||
| Zuogui Jiangtang Yishen decoction | MKR mice treated with high-fat diet and unilateral nephrectomy | Lowered blood glucose urine | GRP78↑, CHOP↓ | Tang et al. (2015) |
| Reduced urine protein | ||||
| Inhibited ER stress | ||||
| Danggui Buxue decoction | SD rats treated with high sugar, high-fat diet and 60mg/kg STZ Glomerular mesangial cells cultured with high glucose | Lowered blood glucose | GRP78↓, PERK↓, eIF2α↓, p-IRE1α↓, ATF6↓, CHOP↓, Caspase-3↓, Caspase-12↓, p-JNK↓ | Zhang et al. (2015) |
| Reduced urine protein | Zhang et al. (2018) | |||
| Improved renal function | Shuai et al. (2018) | |||
| Reduced apoptosis | Shen et al. (2018) | |||
| Inhibited ER stress | ||||
| Modified Shenqi Dihuang decoction | Patients with diabetic kidney disease | Lowered blood glucose | GRP78, ATF6, CHOP, and Caspase-12 ↓ | Zhang et al. (2022) |
| Alleviated insulin resistance | ||||
| Reduced urine protein | ||||
| Improved lipid profiles | ||||
| Improved renal function | ||||
| Inhibited ER stress |
The role of Chinese herbal formula in the regulation of ER stress in DN.
Glomerular mesangial cell apoptosis is implicated in the worsening of proteinuria and renal function in DN. Prior studies suggest that high glucose can trigger an endogenous pro-apoptotic signaling pathway, leading to mesangial cell apoptosis and subsequent DN progression (Mishra et al., 2005; Lu et al., 2018). In vitro studies have shown that the serum containing the Tongluo Yishen formula hindered the surge of GRP78 mRNA and downregulated the expression of c-Jun N-terminal kinase (JNK), CHOP, and caspase-12 mRNA, thereby impeding high glucose-induced apoptosis of glomerular mesangial cells (Li et al., 2016a; Li et al., 2016b; Li et al., 2016c). In the streptozotocin (STZ)-induced diabetic rat model, the Tongluo Baoshen formula treatment led to a considerable reduction in the protein expression of p-IRE1-α, GRP78, and p-JNK in the kidneys, suggesting a renal protective role mediated through ER stress inhibition (Zhang et al., 2017a; Zhang et al., 2017b). Tang et al. (2015) found that the Zuogui Jiangtang Yishen decoction elevated GRP78 expression while lowering CHOP expression in DN mouse podocytes. This implied a possible mechanism of DN retardation through the amelioration of ER stress-mediated podocyte injury. Consistent with this finding, numerous studies have noted that the Danggui Buxue decoction substantially downregulated the expression of ER stress and apoptosis-related proteins in DN rat kidney tissue, including GRP78, PERK, p-IRE1α, ATF6, CHOP, caspase-12, p-JNK, among others (Zhang et al., 2015; Shen et al., 2018; Shuai et al., 2018; Zhang et al., 2018). Further corroborating these observations, Zhang et al. (2022) demonstrated that the modified Shenqi Dihuang decoction not only significantly improved glucolipid metabolism and renal function in DN patients but also exerted a considerable inhibitory effect on serum ER stress and apoptosis markers, such as GRP78, ATF6, CHOP, and caspase-12 mRNA expression. In Supplementary Table S1, we have listed the composition of the above Chinese herbal formulas in detail.
5.2 Chinese patent medicine
Chinese patent medicine, an integral component of traditional Chinese medicine, exhibits stable efficacy and offers the advantage of convenient administration. It is extensively utilized in treating diabetic kidney disease to enhance renal function and augment patient clinical outcomes (Sheng et al., 2020; Yu et al., 2022). Certain Chinese patent medicines used in DN treatment, like Chinese herbal formulas, also exert regulatory effects on ER stress (Table 2).
TABLE 2
| Chinese patent medicine | Experimental models | Effects | Mechanisms | References |
|---|---|---|---|---|
| Qiwei granule | KK-Ay mice treated with high-fat diet | Lowered blood glucose | GRP78↓, p-IRE1↓, XBP1↓, p-PERK↓, Caspase-12↓ | Han and Gao (2019) |
| Reduced urine protein | Tian et al. (2019) | |||
| Mouse podocytes cultured with high glucose | Improved renal function | Tian et al. (2018b) | ||
| Reduced apoptosis | ||||
| Inhibited ER stress | ||||
| Huangkui capsule | SD rats treated with unilateral nephrectomy, high-fat diet, and 35 mg/kg STZ | Lowered blood glucose | TNF-α↓, IL-6↓, IL-1β↓, IL-2↓,ATF6α↓, p-PERK↓, p-JNK↓ | Ge et al. (2016) |
| Improved renal function | ||||
| Reduced urine protein | ||||
| Improved lipid profiles | ||||
| Alleviated glomerular injury | ||||
| Suppressed renal inflammation | ||||
| Inhibited ER stress | ||||
| Shenyan Kangfu tablet | SD rats treated with 40 mg/kg STZ | Reduced urine protein | GRP78↓, p-JNK↓, CHOP↓ | Chang et al. (2017) |
| Improved renal function | ||||
| Reduced apoptosis | ||||
| Inhibited ER stress | ||||
| Shenshao oral liquid | Wistar rats treated with high-fat diet and 25 mg/kg STZ | Lowered blood glucose | GRP78↓, PERK↓, CHOP↓ | Yuan et al. (2017) |
| Reduced urine protein | ||||
| Improved renal function | ||||
| Reduced apoptosis | ||||
| Inhibited ER stress | ||||
| Shenkang injection | SD rats treated with unilateral nephrectomy and 3 5mg/kg STZ NRK-52E cells cultured with high glucose | Reduced urine protein | E-cadherin↑, α-SMA↓, Vimentin↓, Collagen I↓, Bax↓, Bcl-2↑, Caspase-12↓, Bax/Bcl-2↓, GRP78↓, p-PERK/PERK↓, p-eIF2α/eIF2α↓, ATF4↓, CHOP↓ | Wang et al. (2021b) |
| Improved renal function | ||||
| Alleviated renal tubular injury | ||||
| Suppressed epithelial-mesenchymal transition | ||||
| Reduced apoptosis | ||||
| Inhibited ER stress |
The role of Chinese patent medicine in the regulation of ER stress in DN.
It has been reported that Qiwei granules can diminish the expression of ER stress-related factors such as GRP78, p-IRE1, XBP1, and p-PERK in the renal tissue of KK-Ay mice. This suggests that Qiwei granules safeguard renal function and alleviate renal pathological damage by inhibiting the activation of IRE1 and PERK pathways (Han and Gao, 2019; Tian et al., 2019). Further in vitro studies have revealed that the drug-containing serum of Qiwei granules suppresses the ER stress-mediated caspase-12 apoptotic pathway, thereby reducing podocyte apoptosis (Tian N. X. et al., 2018). Another Chinese patent medicine, Huangkui capsule, an extract from Abelmoschus manihot (L.) Medic, has demonstrated efficacy in reducing proteinuria, improving renal function, and delaying DN progression (Xu et al., 2018). Ge et al. (2016) noted that the Huangkui capsule alleviated renal inflammation and glomerular injury in DN rats in a dose-dependent manner. Mechanistically, Huangkui capsule modulates ER stress by downregulating the expression of ATF6, p-PERK, p-JNK, and JNK proteins. Shenyan Kangfu tablet, commonly used in chronic kidney disease treatment, has been shown to reduce the expression of GRP78, p-JNK, and CHOP in the kidneys of DN rats, suggesting its role in delaying renal function deterioration via inhibiting ER stress-induced apoptosis of renal intrinsic cells (Chang et al., 2017). Yuan et al. (2017) similarly observed that Shenshao oral liquid inhibited the expression of GRP78, PERK, and CHOP proteins in the kidneys of DN rats. Additionally, a separate study found that Shenkang injection effectively inhibited the activity of the PERK-eIF2α-ATF4-CHOP signaling pathway both in vitro and in vivo. This inhibition serves to mitigate diabetic tubulopathy by restraining renal tubular epithelial-mesenchymal transition and ER stress-induced apoptosis (Wang W. W. et al., 2021). In Supplementary Table S2, we have listed the composition of the above Chinese patent medicine in detail.
5.3 Extractive compounds in Chinese herbal medicine
Bioactive compounds derived from Chinese herbal medicine are instrumental in the therapeutic effects of these remedies. They have been identified as significant contributors in the treatment of DN. The present study suggests that the renoprotective effects of these bioactive compounds could be attributed to their ability to modulate ER stress (Table 3).
TABLE 3
| Category | Extractive compounds of Chinese herbal medicine | Experimental models | Effects | Mechanisms | References |
|---|---|---|---|---|---|
| Polyphenols | Curcumins | SD rats treated with 50 mg/kg STZ | Lowered blood glucose | GRP78↓, p-eIF2α/eIF2α↓, ATF4↓, CHOP↓, Caspase-3↓, Bax/Bcl-2↓, JNK↓, Notch2↓, hes1↓ | Yu et al. (2020) |
| Improved renal function | |||||
| Mouse podocytes induced by angiotensin II | Reduced apoptosis | Deng et al. (2020) | |||
| Inhibited ER stress | |||||
| Epigallocatechin-3-gallate | Mouse podocytes cultured with high glucose | Reduced apoptosis | GRP78↓, p-PERK↓, Caspase-12↓ | Xiang et al. (2017) | |
| Inhibited ER stress | |||||
| Resveratrol | db/db diabetic mice NRK-52E cells cultured with high glucose | Reduced urine protein | GRP78↓, CHOP↓, Cleaved Caspase-12↓ | Zhang et al. (2020a) | |
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Chlorogenic acid | SD rats treated with 60 mg/kg STZ | Lowered blood glucose | CAT↑, SOD↑, GSH-Px↑, MDA↓, CHOP↓, ATF6↓, p-PERK/PERK↓, p-eIF2α/eIF2α↓ | Zhu et al. (2019) | |
| Reduced urine protein | |||||
| Improved renal function | |||||
| Attenuated oxidative stress | |||||
| Inhibited ER stress | |||||
| Category | Extractive compounds of Chinese herbal medicine | Experimental models | Effects | Mechanisms | References |
| Flavonoids | Chrysin | db/db diabetic mice | Reduced urine protein | p-PERK↓, p-eIF2α↓, ATF4↓, CHOP↓, Bax↓, Bcl-2↑, Podocin↑, Nephrin↑, Apaf-1↓ |
Kang et al. (2017) |
| Mouse podocytes cultured with high glucose | Attenuated glomerular and podocyte damage | ||||
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Naringenin | Wistar rats treated with 120 mg/kg nicotinamide and 60 mg/kg STZ | Lowered blood glucose | ROS↓, GSH↑, SOD↑, CAT↑, XBP-1s↓, p-PERK/PERK↓,p-eIF2α/eIF2α↓, ATF4↓, CHOP↓, Bax/Bcl-2↓, Cleaved caspase-3↓ | Khan et al. (2022) | |
| Improved glucose tolerance | |||||
| Mitigated hyperinsulinemia | |||||
| NRK-52E cells cultured with high glucose | Improved renal function | ||||
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Attenuated oxidative stress | |||||
| Total flavones of Abelmoschus manihot | SD rats treated with unilateral nephrectomy and 35 mg/kg STZ HK-2 cells cultured with AGEs | Lowered blood glucose | TNF-α↓, IL-6↓, MCP-1↓,TACE↓, p-iRhom2↓, GRP94↓, XBP1s↓ | Liu et al. (2017) | |
| Reduced urine protein | |||||
| Improved renal function | |||||
| Alleviated glomerulosclerosis fibrosis | |||||
| Inhibited ER stress | |||||
| Suppressed renal inflammation | |||||
| Quinones | Emodin | KK-Ay mice treated with high fat diet | Reduced urine protein | GRP78↓, p-PERK↓, p-eIF2α↓, ATF4↓, CHOP↓, Bax↓, Bcl-2↑ | Tian et al. (2018a) |
| Improved renal function | |||||
| Mouse podocytes cultured with high glucose | Reduced apoptosis | ||||
| Inhibited ER stress | |||||
| Tanshinone IIA | SD rats treated with 60 mg/kg STZ | Lowered blood glucose | GRP78↓, CHOP↓, p-PERK↓, p-eIF2α↓, ATF4 ↓ | Xu et al. (2020) | |
| Improved renal function | |||||
| Inhibited ER stress | |||||
| Alkaloids | Berberine | Mouse podocytes induced by palmitic acid | Reduced apoptosis | Cleaved Caspase-3↓, BIP↓, PERK↓, ATF4↓, CHOP↓, ATF6↓, IRE1α↓, Caspase-12↓,ROS↓ | Xiang et al. (2021) |
| Inhibited ER stress | |||||
| Attenuated oxidative stress | |||||
| Terpenoids | Astragaloside IV(a) | SD rats treated with high fat diet and 35 mg/kg STZ | Lowered blood glucose | Bax/Bcl-2↓, Cleaved Caspase-3↓,GRP78↓, p-PERK/PERK↓, ATF4↓, CHOP↓ | Ju et al. (2019) |
| Reduced urine protein | |||||
| Improved renal function | |||||
| Improved lipid profiles | |||||
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Astragaloside IV(b) | SD rats treated with 65 mg/kg STZ | Reduced urine protein | Bax↓, Bcl-2↑, GRP78↓, p-PERK/PERK↓, p-eIF2α/eIF2α↓, ATF4↓, CHOP↓, TRB3↓ | Chen et al. (2014b) | |
| Reduced apoptosis | |||||
| Mouse podocytes cultured with high glucose | Inhibited ER stress | ||||
| Astragaloside IV(c) | C57BL/6J mice treated with 100 mg/kg STZ | Reduced urine protein | GRP78↓, Cleaved ATF6↓, p-PERK↓, p-eIF2α↓, CHOP↓, p-IRE1α↓, Spliced XBP1↓, TRAF2↓, p-JNK↓, SERCA2↑, Cleaved caspase-12↓, LC3 II↑, Beclin↑, Atg12↑, p62↓ | Guo et al. (2017) | |
| Improved renal function | |||||
| Mouse podocytes cultured with high glucose | Reduced apoptosis | ||||
| Inhibited ER stress | |||||
| Induced autophagy | |||||
| Astragaloside IV(d) | db/db diabetic mice | Reduced urine protein | MCP-1↓, TNF-α↓, SERCA2↑, GRP78↓, Cleaved ATF6↓, p-PERK↓, p-eIF2α↓, ATF4↓, CHOP↓, p-IRE1α↓, Spliced XBP1↓, ASK1↓, TRAF2↓, p-JNK↓, Cleaved caspase-12↓, Cleaved caspase-9↓, Cleaved caspase-3↓, Bcl-2↑, Bax↓ |
Guo et al. (2016) | |
| Mouse podocytes induced by palmitic acid | Improved renal function | ||||
| Lowered systolic blood pressure | |||||
| Improved glucose tolerance | |||||
| Increased insulin sensitivity | |||||
| Suppressed renal inflammation | |||||
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Restored Ca2+ homeostasis | |||||
| Astragaloside IV(e) | SD rats treated with 40 mg/kg STZ | Reduced urine protein | p-PERK/PERK↓, p-JNK/JNK↓, p-eIF2α/eIF2α↓, GRP78↓, ORP150↓,CHOP↓, Cleaved caspase-3↓ | Wang et al. (2015) | |
| Improved renal function | |||||
| Human podocytes induced by tunicamycin | Reduced apoptosis | ||||
| Inhibited ER stress | |||||
| Lignans | Arctigenin | db/db diabetic mice | Lowered blood glucose | GRP78↓, CHOP↓, Caspase-12↓ | Zhang et al. (2019a) |
| HK-2 cells cultured with high glucose | Reduced urine protein | ||||
| Reduced apoptosis | |||||
| Inhibited ER stress | |||||
| Additional agents | Ginkgo biloba extract EGB761 | C57BL/6 mice treated with high fat diet and 50 mg/kg STZ | Lowered blood glucose | a-SMA↓, E-cadherin↑, collagen IV↓, fibronectin↓, GRP78↓, ATF6↓ | Han et al. (2021) |
| Reduced urine protein | |||||
| Improved renal function | |||||
| HK-2 cells cultured with high glucose | Alleviated renal tubular injury | ||||
| Suppressed epithelial-mesenchymal transition | |||||
| Reduced extracellular matrix accumulation | |||||
| Inhibited ER stress | |||||
| Terpene glycoside component of Moutan Cortex | SD rats treated with high sugar, high fat diet, and 30 mg/kg STZ | Lowered blood glucose | GRP78↓, XBP-1s↓, p-IRE1α↓, IL-6↓, MCP-1↓, ICAM-1↓, p-NF-κB p65↓ | Chen et al. (2016) | |
| Reduced urine protein | |||||
| Improved renal function | |||||
| Rat glomerular mesangial cell line HBZY-1 induced by AGEs | Alleviated glomerular injury | ||||
| Inhibited ER stress | |||||
| Suppressed renal inflammation | |||||
| Total glucosides of peony | Wistar rats treated with 65 mg/kg STZ | Reduced urine protein | GRP78↓, p-PERK↓, p-eIF2α↓, CHOP↓, TXNIP↓ | Shao et al. (2017) | |
| Inhibited ER stress |
The role of Extractive compounds of Chinese herbal medicine in the regulation of ER stress in DN.
5.3.1 Polyphenols
Curcumin, a lipophilic polyphenol derived from Curcumae Longae Rhizoma, exhibits antioxidant, anti-inflammatory, anti-apoptotic, renin-angiotensin-aldosterone system regulatory, and anti-fibrotic properties (Yaribeygi et al., 2021). Recent research indicates that curcumin mitigates angiotensin II-induced podocyte damage and apoptosis, partly through ER stress inhibition (Yu et al., 2020). In vivo studies confirm that curcumin mediates nephroprotective effects in DN rats by suppressing the activation of the ER stress-mediated apoptotic signaling pathways JNK and Notch2/hes1 (Deng et al., 2020). Epigallocatechin-3-gallate (EGCG), the primary bioactive compound in catechins, has demonstrated significant hypotensive, hypolipidemic, anti-diabetic, and nephroprotective activities in previous studies (Bazyar et al., 2020; Mohan et al., 2020; Zhu et al., 2022). Xiang et al. (2017) reported that EGCG downregulated the protein expression of GRP78, p-PERK, and caspase-12, thereby protecting podocytes against high glucose-induced apoptosis. Resveratrol (RSV), a natural polyphenolic compound, is predominantly found in grapes, peanuts, Polygoni Cuspidati Rhizoma et Radix, among other plants. A recent clinical trial suggested that RSV could effectively supplement angiotensin II receptor antagonists, significantly reducing urinary albumin excretion in DN patients (Sattarinezhad et al., 2019). Additional investigations have shown that the mechanism through which RSV improves DN is associated with the suppression of ER stress-induced apoptosis in renal tubular epithelial cells. Specifically, RSV reduces the expression of GRP78, CHOP, and caspase-12 in the DN model (Zhang et al., 2020a). Chlorogenic acid, a widespread dietary polyphenol, exhibits ER stress-inhibiting and antioxidant properties. Zhu et al. (2019) found that chlorogenic acid attenuated the protein expression of renal tissue p-PERK, p-eIF2α, ATF6, and CHOP, augmented the activity of SOD, catalase, and glutathione peroxidase, and reduced MDA levels in a dose-dependent manner. The cumulative evidence suggests that polyphenolic compounds, including curcumin, EGCG, RSV, and chlorogenic acid, could have potential therapeutic applications in DN, largely due to their ability to inhibit ER stress.
5.3.2 Flavonoids
Chrysin, a naturally occurring flavonoid compound, is primarily derived from propolis, Scutellaria baicalensis, and Oroxylum indicum. Contemporary pharmacological research has demonstrated that chrysin possesses an array of pharmacological properties, including anti-cancer, anti-diabetic, antioxidant, anti-inflammatory, and hepatoprotective characteristics (Naz et al., 2019). Kang et al. (2017) found that chrysin mitigated ER stress via inhibition of the PERK-eIF2α-ATF4-CHOP pathway, thereby improving high glucose-induced podocyte injury and preventing the loss of slit diaphragm proteins. Naringenin, a common dihydroflavonoid, is predominantly found in citrus fruits and Chinese herbs such as Aurantii Fructus Immaturus and Aurantii Fructus. Khan et al. (2022) reported that naringenin augmented the antioxidant capacity of renal cells during hyperglycemic renal toxicity while also demonstrating significant anti-ER stress and anti-apoptotic effects. Naringenin was found to prevent renal tubular epithelial cell apoptosis by diminishing the expression of ER stress-related proteins (p-PERK, p-eIF2α, XBP1s, ATF4, and CHOP) and mitigating disruption to the ER ultrastructure within renal cells. Total flavones of Abelmoschus manihot (TFA) represent a total flavonoid component extracted from the flowers of A. manihot (L.) Medic. Liu et al. (2017) reported that TFA alleviated renal inflammation and glomerular injury in DN rats by attenuating ER stress and suppressing the activation of iRhom2/TACE signaling.
5.3.3 Quinones
Emodin, a naturally occurring anthraquinone derivative, is prevalent in various Chinese herbal medicines such as Radix Rhei et Rhizome, Polygoni Cuspidati Rhizoma et Radix, and Fallopia multiflora (Thunb.) Harald, among others. This compound exhibits a broad spectrum of pharmacological properties including, but not limited to, anti-inflammatory, antimicrobial, antioxidant, anti-diabetic, anti-fibrotic, immunosuppressive, and hepatoprotective activities (Semwal et al., 2021). Tian N. et al. (2018) reported that emodin treatment ameliorated both renal function and histopathological damage in a DN mouse model. The researchers further established that emodin’s effects were comparable to those of PERK knockdown, with the compound mitigating high glucose-induced podocyte apoptosis by inhibiting the PERK-eIF2α signaling pathway. Tanshinone IIA (Tan IIA), a phenanthraquinone derived from Salvia miltiorrhiza, also exhibits a variety of pharmacological activities, such as anti-inflammatory, antioxidant, anti-tumor, and blood circulation-improving effects (Guo et al., 2020). In a STZ-induced DN rat model, Tan IIA showcased hypoglycemic, renal protective, and anti-fibrotic activities. These effects are linked to its ability to inhibit the PERK pathway, thereby mitigating ER stress (Xu et al., 2020).
5.3.4 Alkaloids
Berberine is an isoquinoline alkaloid primarily extracted from the Chinese herbs Berberis aristata and Coptis chinensis. Extensive research has corroborated its multifaceted biological activities, including lipid-lowering, anti-diabetic, anti-obesity, and anti-tumor effects (Och et al., 2020). A recent clinical investigation confirmed that combining berberine with valsartan treatment significantly outperformed valsartan monotherapy in enhancing renal and vascular endothelial function in patients with diabetic kidney disease (Fang et al., 2017). In another study, berberine was shown to counteract palmitic acid-induced podocyte apoptosis by curbing ER stress and ROS production (Xiang et al., 2021).
5.3.5 Terpenoids
Astragaloside IV (AS-IV), a key bioactive constituent of the traditional Chinese herbal medicine Astragalus membranaceus, exhibits an array of pharmacological properties including anti-tumor, anti-diabetic, hepatoprotective, and neuroprotective effects, as evidenced by modern pharmacological studies (Zhang et al., 2020b). Numerous investigations have attributed the nephroprotective capability of AS-IV in DN rats to its inhibition of ER stress (Chen Y. et al., 2014; Wang et al., 2015; Ju et al., 2019). Specifically, AS-IV mitigates apoptosis in diabetic rat renal tubular epithelial cells and podocytes by suppressing the PERK-ATF4-CHOP signaling pathway (Chen Y. et al., 2014; Ju et al., 2019). Recent reports suggest that AS-IV’s capacity to impede podocyte apoptosis is calcium-dependent (Zang et al., 2021). The Sarco/ER Ca2+-ATPase (SERCA) plays a pivotal role in maintaining ER Ca2+ homeostasis by facilitating the transportation of cytoplasmic Ca2+ into the ER. Guo et al. (2017) (Guo et al., 2016); demonstrated that AS-IV’s inhibitory effect on ER stress-mediated podocyte apoptosis correlated with upregulated SERCA2 expression. Notably, the knockdown of SERCA2 markedly dampened the anti-ER stress and anti-apoptotic effects of AS-IV.
5.3.6 Lignans
Arctigenin, a lignan compound derived from Fructus arctii, boasts anti-inflammatory, anti-cancer, antioxidant, and immunoregulation properties (Wu et al., 2022). Zhang J. et al. (2019) found that arctigenin significantly reduced blood glucose and urinary protein levels, while mitigating renal pathological damage in db/db mice. At the molecular level, arctigenin inhibited ER stress by downregulating the expression of GRP78, CHOP, and caspase-12 proteins, thereby mitigating high glucose-induced apoptosis in HK-2 cells.
5.3.7 Additional agents
EGB761, a standardized extract of Ginkgo biloba produced by the German Schwabe Company, consists of flavonoids and terpenoids as its primary active components. In a murine model of DN, Han et al. (2021) demonstrated that EGB761 improved renal function and mitigated renal tubular extracellular matrix accumulation and epithelial-mesenchymal transition through ER stress inhibition. The principal ingredient of the Chinese herbal medicine Moutan Cortex is terpene glycoside. Chen et al. (2016) reported considerable nephroprotective and cytoprotective impacts of the terpene glycoside component of Moutan Cortex (MC-TG) in DN models. Mechanistically, MC-TG alleviated ER reticulum stress-associated inflammation by blocking the activation of the IRE1/NF-κB pathway. The total glucosides of paeony (TGP), an active ingredient derived from the root of Paeonia alba, possess anti-inflammatory, anti-apoptotic, antioxidant, and immunomodulatory pharmacological properties (Jin and Zhang, 2022). Shao et al. (2017) indicated that TGP significantly diminished urinary protein in diabetic rats, an effect linked to its inhibition of ER stress-related markers and TXNIP expression.
The aforementioned results imply that Chinese herbal formulas, patent medicines, and extracts—including polyphenols, flavonoids, quinones, alkaloids, terpenoids, among others—may ameliorate DN by inhibiting ER stress (Figure 4). The PERK pathway is the predominant signaling pathway leveraged in Chinese herbal medicine for ER stress inhibition. Attenuating renal intrinsic cell apoptosis is a crucial mechanism of action in providing renal protection after ER stress inhibition by Chinese herbal medicine. In summary, the inhibition of ER stress and associated signaling pathways could potentially represent a significant therapeutic strategy by Chinese herbal medicine to improve DN.
FIGURE 4
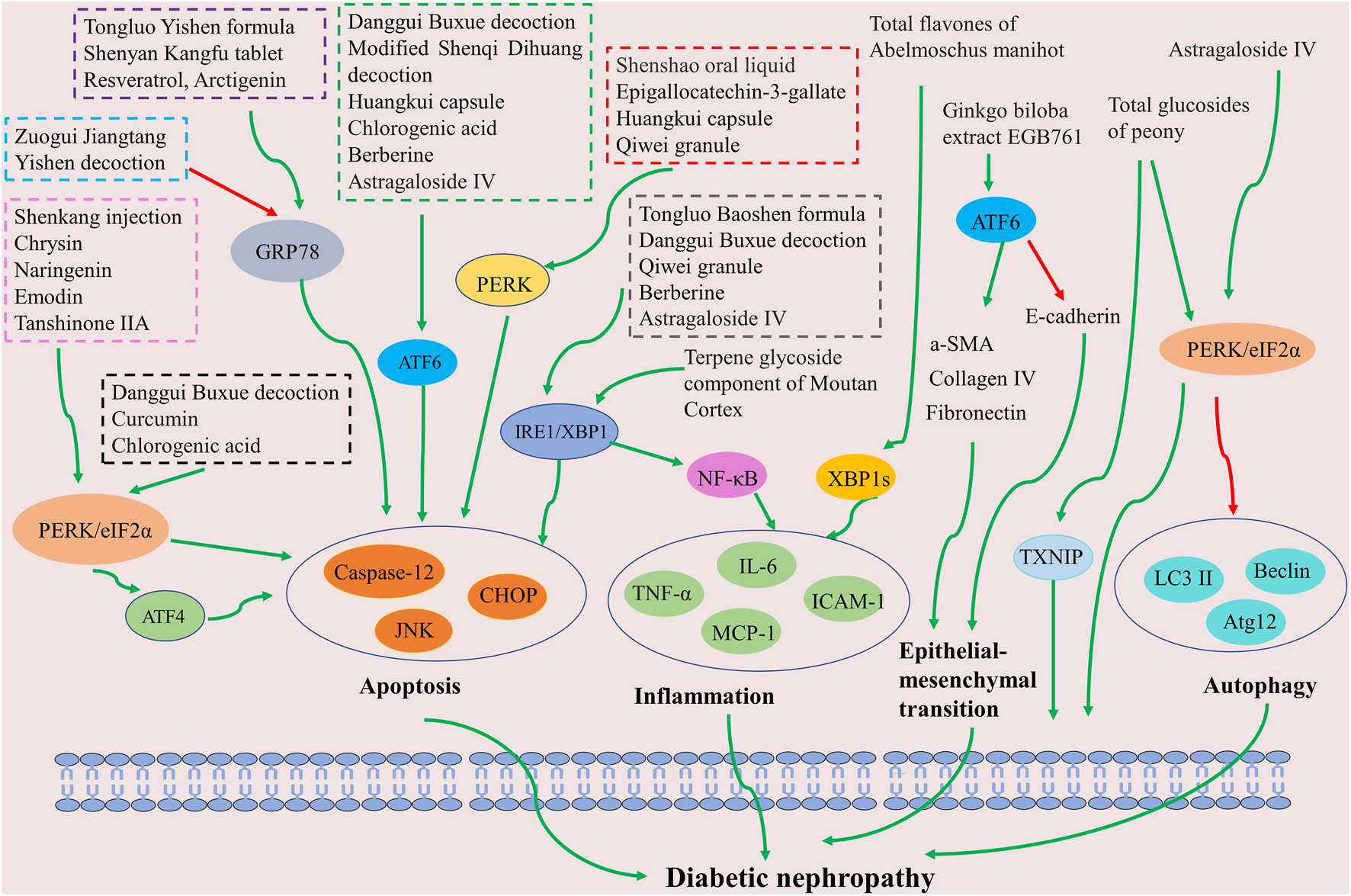
Schematic of Chinese herbal medicine against diabetic nephropathy by inhibiting ER stress. The inhibition of ER stress signaling pathways (including PERK, ATF6, and XBP1) can improve apoptosis, inflammation, autophagy, and epithelial-mesenchymal transition, which benefits diabetic nephropathy treatment.
6 Conclusion and future directions
As the global incidence of DM escalates, there is a corresponding rise in the number of patients with end-stage renal disease instigated by DM. This trend increases the risk of premature mortality in these patients, amplifying both economic and societal burdens. The etiology of DN is multifaceted, intertwined with a plethora of pathological factors including oxidative stress, inflammation, apoptosis, autophagy, and pyroptosis. The body’s response to detrimental stimuli, embodied by ER stress, exhibits a close association with these pathological processes, collectively promoting the onset and progression of DN.
Empirical clinical data have demonstrated the beneficial effects of certain Chinese herbal medicines, including symptom amelioration, reduction of urinary albumin levels, and renal function preservation in diabetic kidney disease patients. Foundational research corroborates these findings, confirming that ER stress modulation by Chinese herbal medicine can contribute to mitigating renal structural and functional damage, thereby delaying the progression of DN to a certain degree. However, the existing research is not without limitations. The role of Chinese herbal medicine in regulating ER stress has been primarily investigated through the detection of ER stress marker proteins. Whether these herbal medicines directly interact with these proteins or modulate them by influencing upstream signaling pathways warrants further exploration. Moreover, the focus has been predominantly on extracted compounds from Chinese herbal medicine, some of which exhibit disadvantages such as low water solubility, inadequate gastrointestinal absorption, and suboptimal bioavailability. Additionally, the majority of these studies are currently at the preclinical phase, lacking substantial clinical validation. Therefore, future research endeavors should seek to further elucidate the regulatory effects of Chinese herbal medicine on ER stress, overcome challenges related to poor water solubility and low bioavailability of extracted compounds, and initiate high-quality clinical trials. These steps will contribute significantly toward expanding the body of clinical and experimental data supporting the preventive and therapeutic potential of Chinese herbal medicine in the context of DN.
Statements
Author contributions
MW designed the study and completed the first draft, so she is the first author. XL and ML revised English grammar. XT and MF contributed in the scientific writing of the manuscript. BP and ZF examined the literature. MW and JW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Beijing Municipal Natural Science Foundation (No. 7202172).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1174415/full#supplementary-material
References
1
Afkarian M. Zelnick L. R. Hall Y. N. Heagerty P. J. Tuttle K. Weiss N. S. et al (2016). Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA316 (6), 602–610. 10.1001/jama.2016.10924
2
Alicic R. Z. Neumiller J. J. Johnson E. J. Dieter B. Tuttle K. R. (2019). Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes68 (2), 248–257. 10.2337/dbi18-0007
3
Bai X. Geng J. Li X. Wan J. Liu J. Zhou Z. et al (2018). Long noncoding RNA LINC01619 regulates microRNA-27a/forkhead box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxid. Redox Signal29 (4), 355–376. 10.1089/ars.2017.7278
4
Bashir S. Banday M. Qadri O. Bashir A. Hilal N. Nida I. F. et al (2021). The molecular mechanism and functional diversity of UPR signaling sensor IRE1. Life Sci.265, 118740. 10.1016/j.lfs.2020.118740
5
Bazyar H. Hosseini S. A. Saradar S. Mombaini D. Allivand M. Labibzadeh M. et al (2020). Effects of epigallocatechin-3-gallate of camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med.18 (2), 405–411. 10.1515/jcim-2020-0090
6
Cao A. L. Wang L. Chen X. Wang Y. M. Guo H. J. Chu S. et al (2016b). Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab. Invest.96 (6), 610–622. 10.1038/labinvest.2016.44
7
Cao A. Wang L. Chen X. Guo H. Chu S. Zhang X. et al (2016a). Ursodeoxycholic acid ameliorated diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress. Biol. Pharm. Bull.39 (8), 1300–1308. 10.1248/bpb.b16-0009410.1248/bpb.b16-00094
8
Chang X. D. Yang Y. Q. Xue H. Gan H. (2017). The effect of Shenyankangfu tablets on endoplasmic reticulum stress in diabetic nephropathy rats. J. North Sichuan Med. Coll.32 (3), 422–425. 10.3969/j.issn.1005-3697.2017.03.030
9
Chen H. Y. Pan H. C. Chen Y. C. Chen Y. C. Lin Y. H. Yang S. H. et al (2019a). Traditional Chinese medicine use is associated with lower end-stage renal disease and mortality rates among patients with diabetic nephropathy: A population-based cohort study. BMC Complement. Altern. Med.19 (1), 81. 10.1186/s12906-019-2491-y
10
Chen J. Guo Y. Zeng W. Huang L. Pang Q. Nie L. et al (2014a). ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Ren. Physiol.306 (8), F916–F925. 10.1152/ajprenal.00697.2012
11
Chen J. Hou X. F. Wang G. Zhong Q. X. Liu Y. Qiu H. H. et al (2016). Terpene glycoside component from Moutan Cortex ameliorates diabetic nephropathy by regulating endoplasmic reticulum stress-related inflammatory responses. J. Ethnopharmacol.193, 433–444. 10.1016/j.jep.2016.09.043
12
Chen Y. Gui D. Chen J. He D. Luo Y. Wang N. (2014b). Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol. Biochem.33 (6), 1975–1987. 10.1159/000362974
13
Chen Y. Liu C. P. Xu K. F. Mao X. D. Lu Y. B. Fang L. et al (2008). Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am. J. Nephrol.28 (6), 1014–1022. 10.1159/000148209
14
Chen Y. Y. Peng X. F. Liu G. Y. Liu J. S. Sun L. Liu H. et al (2019b). Protein arginine methyltranferase-1 induces ER stress and epithelial-mesenchymal transition in renal tubular epithelial cells and contributes to diabetic nephropathy. Biochim. Biophys. Acta Mol. Basis Dis.1865 (10), 2563–2575. 10.1016/j.bbadis.2019.06.001
15
Chiang C. K. Wang C. C. Lu T. F. Huang K. H. Sheu M. L. Liu S. H. et al (2016). Involvement of endoplasmic reticulum stress, autophagy, and apoptosis in advanced glycation end products-induced glomerular mesangial cell injury. Sci. Rep.6, 34167. 10.1038/srep34167
16
Chou X. Ding F. Zhang X. Ding X. Gao H. Wu Q. (2019). Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch. Toxicol.93 (4), 965–986. 10.1007/s00204-019-02415-8
17
Collins A. J. Foley R. N. Chavers B. Gilbertson D. Herzog C. Johansen K. et al (2012). 'United States renal data system 2011 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am. J. Kidney Dis.59, e1–e420. 10.1053/j.ajkd.2011.11.015
18
Daenen K. Andries A. Mekahli D. Van Schepdael A. Jouret F. Bammens B. (2019). Oxidative stress in chronic kidney disease. Pediatr. Nephrol.34 (6), 975–991. 10.1007/s00467-018-4005-4
19
de Boer I. H. Rue T. C. Hall Y. N. Heagerty P. J. Weiss N. S. Himmelfarb J. (2011). Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA305 (24), 2532–2539. 10.1001/jama.2011.861
20
Deng W. R. Shi W. Q. Li H. Q. Zhou Z. L. (2020). Effects of curcumin on renal function and JNK and Notch 2/hes1 signaling pathway in renal tissues of diabetic nephropathy rats. Shandong Med. J.60 (14), 5–8. 10.3969/j.issn.1002-266X.2020.14.002
21
Engin F. Hotamisligil G. S. (2010). Restoring endoplasmic reticulum function by chemical chaperones: An emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab.12, 108–115. 10.1111/j.1463-1326.2010.01282.x
22
Fan Y. Zhang J. Xiao W. Lee K. Li Z. Wen J. et al (2017). Rtn1a-mediated endoplasmic reticulum stress in podocyte injury and diabetic nephropathy. Sci. Rep.7 (1), 323. 10.1038/s41598-017-00305-6
23
Fang L. Xie D. Wu X. Cao H. Su W. Yang J. (2013). Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLoS One8 (8), e72344. 10.1371/journal.pone.0072344
24
Fang X. L. Han X. J. Su R. T. (2017). Impacts of berberine on vascular endothelial function in the patients of early diabetic nephropathy. World J. Integr. Traditional West. Med.12 (12), 1707–1710+1714. 10.13935/j.cnki.sjzx.171219
25
Fu Y. Shen J. Li Y. Liu F. Ning B. Zheng Y. et al (2021). Inhibition of the PERK/TXNIP/NLRP3 axis by baicalin reduces NLRP3 inflammasome-mediated pyroptosis in macrophages infected with mycobacterium tuberculosis. Mediat. Inflamm.2021, 1805147. 10.1155/2021/1805147
26
Ge J. Miao J. J. Sun X. Y. Yu J. Y. (2016). Huangkui capsule, an extract from Abelmoschus manihot (L) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol.189, 238–249. 10.1016/j.jep.2016.05.033
27
Gonçalves G. L. Costa-Pessoa J. M. Thieme K. Lins B. B. Oliveira-Souza M. (2018). Intracellular albumin overload elicits endoplasmic reticulum stress and PKC-delta/p38 MAPK pathway activation to induce podocyte apoptosis. Sci. Rep.8 (1), 18012. 10.1038/s41598-018-36933-9
28
Guo H. Cao A. Chu S. Wang Y. Zang Y. Mao X. et al (2016). Astragaloside IV attenuates podocyte apoptosis mediated by endoplasmic reticulum stress through upregulating sarco/endoplasmic reticulum Ca(2+)-ATPase 2 expression in diabetic nephropathy. Front. Pharmacol.7, 500. 10.3389/fphar.2016.00500
29
Guo H. Wang Y. Zhang X. Zang Y. Zhang Y. Wang L. et al (2017). Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci. Rep.7 (1), 6852. 10.1038/s41598-017-07061-7
30
Guo J. Ren R. Sun K. He J. Shao J. (2021). PERK signaling pathway in bone metabolism: Friend or foe?Cell Prolif.54 (4), e13011. 10.1111/cpr.13011
31
Guo R. Li L. Su J. Li S. Duncan S. E. Liu Z. et al (2020). Pharmacological activity and mechanism of Tanshinone IIA in related diseases. Drug Des. Devel Ther.14, 4735–4748. 10.2147/dddt.S266911
32
Han J. Pang X. Shi X. Zhang Y. Peng Z. Xing Y. (2021). Ginkgo biloba extract EGB761 ameliorates the extracellular matrix accumulation and mesenchymal transformation of renal tubules in diabetic kidney disease by Inhibiting endoplasmic reticulum stress. Biomed. Res. Int.2021, 6657206. 10.1155/2021/6657206
33
Han Z. J. Gao Y. B. (2019). Effects of Qiwei granule on process of endoplasmic reticulum stress through IRE1 pathway in type 2 diabetic nephropathy KK-Ay mice. Glob. Tradit. Chin. Med.12 (2), 166–170. 10.3969/j.issn.1674-1749.2019.02.003
34
Haze K. Yoshida H. Yanagi H. Yura T. Mori K. (1999). Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell10 (11), 3787–3799. 10.1091/mbc.10.11.3787
35
Hetz C. (2012). The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol.13 (2), 89–102. 10.1038/nrm3270
36
Hetz C. Zhang K. Kaufman R. J. (2020). Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol.21 (8), 421–438. 10.1038/s41580-020-0250-z
37
Hu H. Tian M. Ding C. Yu S. (2018). The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol.9, 3083. 10.3389/fimmu.2018.03083
38
Huang Q. Chen Y. Zhang Z. Xue Z. Hua Z. Luo X. et al (2022). The endoplasmic reticulum participated in drug metabolic toxicity. Cell Biol. Toxicol.38 (6), 945–961. 10.1007/s10565-021-09689-8
39
Huang Y. Sun Y. Cao Y. Sun H. Li M. You H. et al (2017). HRD1 prevents apoptosis in renal tubular epithelial cells by mediating eIF2α ubiquitylation and degradation. Cell Death Dis.8 (12), 3202. 10.1038/s41419-017-0002-y
40
Jeong S. R. Lee K. W. (2021). Methylglyoxal-derived advanced glycation end product (AGE4)-induced apoptosis leads to mitochondrial dysfunction and endoplasmic reticulum stress through the RAGE/JNK pathway in kidney cells. Int. J. Mol. Sci.22 (12), 6530. 10.3390/ijms22126530
41
Jia Y. Zheng Z. Yang Y. Zou M. Li J. Wang L. et al (2019). MiR-4756 promotes albumin-induced renal tubular epithelial cell epithelial-to-mesenchymal transition and endoplasmic reticulum stress via targeting Sestrin2. J. Cell Physiol.234 (3), 2905–2915. 10.1002/jcp.27107
42
Jin Y. Zhang A. (2022). Total glucosides of paeony ameliorates oxidative stress, apoptosis and inflammatory response by regulating the Smad7-TGF-β pathway in allergic rhinitis. Mol. Med. Rep.25 (3), 83. 10.3892/mmr.2022.12599
43
Ju Y. Su Y. Chen Q. Ma K. Ji T. Wang Z. et al (2019). Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother.109, 84–92. 10.1016/j.biopha.2018.10.041
44
Kang M. K. Park S. H. Kim Y. H. Lee E. J. Antika L. D. Kim D. Y. et al (2017). Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacol. Sin.38 (8), 1129–1140. 10.1038/aps.2017.30
45
Kang X. Yang W. Wang R. Xie T. Li H. Feng D. et al (2018). Sirtuin-1 (SIRT1) stimulates growth-plate chondrogenesis by attenuating the PERK-eIF-2α-CHOP pathway in the unfolded protein response. J. Biol. Chem.293 (22), 8614–8625. 10.1074/jbc.M117.809822
46
Ke R. Wang Y. Hong S. Xiao L. (2020). Endoplasmic reticulum stress related factor IRE1α regulates TXNIP/NLRP3-mediated pyroptosis in diabetic nephropathy. Exp. Cell Res.396 (2), 112293. 10.1016/j.yexcr.2020.112293
47
Khan M. F. Mathur A. Pandey V. K. Kakkar P. (2022). Naringenin alleviates hyperglycemia-induced renal toxicity by regulating activating transcription factor 4-C/EBP homologous protein mediated apoptosis. J. Cell Commun. Signal16 (2), 271–291. 10.1007/s12079-021-00644-0
48
Ko J. Kim J. Y. Kyoung Chae M. Jig Lee E. Sook Yoon J. (2021). PERK mediates oxidative stress and adipogenesis in Graves' orbitopathy pathogenesis. J. Mol. Endocrinol.66 (4), 313–323. 10.1530/jme-21-0057
49
Koch E. A. T. Nakhoul R. Nakhoul F. Nakhoul N. (2020). Autophagy in diabetic nephropathy: A review. Int. Urol. Nephrol.52 (9), 1705–1712. 10.1007/s11255-020-02545-4
50
Kristensen S. L. Rørth R. Jhund P. S. Docherty K. F. Sattar N. Preiss D. et al (2019). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol.7 (10), 776–785. 10.1016/s2213-8587(19)30249-9
51
Li Q. Zhang K. Hou L. Liao J. Zhang H. Han Q. et al (2023). Endoplasmic reticulum stress contributes to pyroptosis through NF-κB/NLRP3 pathway in diabetic nephropathy. Life Sci.322, 121656. 10.1016/j.lfs.2023.121656
52
Li X. H. Cheng X. P. Zhao Y. K. Li Y. J. Lei G. P. Xu J. J. (2016a). Effects of Tongluo Yishen decoction containing serum to GRP78,CHOP expression of high glucose induced glomerular mesangial cells. J. Shanxi Univ. Chin. Med.39 (5), 89–92. 10.13424/j.cnki.jsctcm.2016.05.033
53
Li X. H. Li Y. J. Zhao Y. K. Lei G. P. Xu J. J. (2016b). Effect of Tongluoyishen decoction on the expression of GRP78 and Caspase - 12 in the mesangial cells Induced by high glucose. Chin. J. Integr. Traditional West. Nephrol.17 (2), 102–105.
54
Li X. H. Zhao Y. K. Li Y. J. Lei G. P. Xu J. J. (2016c). Effect of Tongluo Yishen formula on expression of GRP78 mRNA and JNK mRNA in high glucose induced glomerular mesangial cells. J. Traditional Chin. Med.57 (7), 605–609. 10.13288/j.11-2166/r.2016.07.017
55
Liang Q. Liu T. Guo T. Tao W. Chen X. Chen W. et al (2021). ATF4 promotes renal tubulointerstitial fibrosis by suppressing autophagy in diabetic nephropathy. Life Sci.264, 118686. 10.1016/j.lfs.2020.118686
56
Liang X. Duan N. Wang Y. Shu S. Xiang X. Guo T. et al (2016). Advanced oxidation protein products induce endothelial-to-mesenchymal transition in human renal glomerular endothelial cells through induction of endoplasmic reticulum stress. J. Diabetes Complicat.30 (4), 573–579. 10.1016/j.jdiacomp.2016.01.009
57
Lindenmeyer M. T. Rastaldi M. P. Ikehata M. Neusser M. A. Kretzler M. Cohen C. D. et al (2008). Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol.19 (11), 2225–2236. 10.1681/asn.2007121313
58
Liu G. Sun Y. Li Z. Song T. Wang H. Zhang Y. et al (2008). Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem. Biophys. Res. Commun.370 (4), 651–656. 10.1016/j.bbrc.2008.04.031
59
Liu J. Gao L. D. Fu B. Yang H. T. Zhang L. Che S. Q. et al (2022). Efficacy and safety of zicuiyin decoction on diabetic kidney disease: A multicenter, randomized controlled trial. Phytomedicine100, 154079. 10.1016/j.phymed.2022.154079
60
Liu J. Yang J. R. Chen X. M. Cai G. Y. Lin L. R. He Y. N. (2015). Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am. J. Physiol. Cell Physiol.308 (8), C621–C630. 10.1152/ajpcell.00096.2014
61
Liu S. Ye L. Tao J. Ge C. Huang L. Yu J. (2017). Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm. Biol.56 (1), 1–11. 10.1080/13880209.2017.1412467
62
Lu Q. Zhou Y. Hao M. Li C. Wang J. Shu F. et al (2018). The mTOR promotes oxidative stress-induced apoptosis of mesangial cells in diabetic nephropathy. Mol. Cell Endocrinol.473, 31–43. 10.1016/j.mce.2017.12.012
63
Luo Z. F. Feng B. Mu J. Qi W. Zeng W. Guo Y. H. et al (2010). Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: Regulation of endoplasmic reticulum stress-oxidative activation. Toxicol. Appl. Pharmacol.246 (1-2), 49–57. 10.1016/j.taap.2010.04.005
64
Mishra R. Emancipator S. N. Kern T. Simonson M. S. (2005). High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int.67 (1), 82–93. 10.1111/j.1523-1755.2005.00058.x
65
Mohan T. Narasimhan K. K. S. Ravi D. B. Velusamy P. Chandrasekar N. Chakrapani L. N. et al (2020). Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med.160, 227–238. 10.1016/j.freeradbiomed.2020.07.037
66
Mukherjee D. Bercz L. S. Torok M. A. Mace T. A. (2020). Regulation of cellular immunity by activating transcription factor 4. Immunol. Lett.228, 24–34. 10.1016/j.imlet.2020.09.006
67
Naz S. Imran M. Rauf A. Orhan I. E. Shariati M. A. Iahtisham Ul H. et al (2019). Chrysin: Pharmacological and therapeutic properties. Life Sci.235, 116797. 10.1016/j.lfs.2019.116797
68
Och A. Podgórski R. Nowak R. (2020). Biological activity of berberine-A summary update. Toxins (Basel).12 (11), 713. 10.3390/toxins12110713
69
Ohse T. Inagi R. Tanaka T. Ota T. Miyata T. Kojima I. et al (2006). Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int.70 (8), 1447–1455. 10.1038/sj.ki.5001704
70
Oslowski C. M. Hara T. O'Sullivan-Murphy B. Kanekura K. Lu S. Hara M. et al (2012). Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab.16 (2), 265–273. 10.1016/j.cmet.2012.07.005
71
Oyadomari S. Mori M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.11 (4), 381–389. 10.1038/sj.cdd.4401373
72
Pang X. X. Bai Q. Wu F. Chen G. J. Zhang A. H. Tang C. S. (2016). Urotensin II induces ER stress and EMT and increase extracellular matrix production in renal tubular epithelial cell in early diabetic mice. Kidney Blood Press Res.41 (4), 434–449. 10.1159/000443445
73
Park M. J. Han H. J. Kim D. I. (2017). Lipotoxicity-induced PRMT1 exacerbates mesangial cell apoptosis via endoplasmic reticulum stress. Int. J. Mol. Sci.18 (7), 1421. 10.3390/ijms18071421
74
Rao J. Zhang C. Wang P. Lu L. Qian X. Qin J. et al (2015). C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1α signalling in acute liver failure. Biochem. J.466 (2), 369–378. 10.1042/bj20140412
75
Sattarinezhad A. Roozbeh J. Shirazi Yeganeh B. Omrani G. R. Shams M. (2019). Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab.45 (1), 53–59. 10.1016/j.diabet.2018.05.010
76
Schwarz D. S. Blower M. D. (2016). The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci.73 (1), 79–94. 10.1007/s00018-015-2052-6
77
Semwal R. B. Semwal D. K. Combrinck S. Viljoen A. (2021). Emodin - a natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry190, 112854. 10.1016/j.phytochem.2021.112854
78
Shao Y. Qi X. Xu X. Wang K. Wu Y. Xia L. (2017). TGP attenuates endoplasmic reticulum stress and regulates the expression of thioredoxin-interacting protein in the kidneys of diabetic rats. Biosci. Trends10 (6), 489–495. 10.5582/bst.2016.01188
79
Shen X. Zhang S. Q. Zhang Y. W. Ke H. L. Shuai Y. (2018). Effect of Danggui Buxue decoction on PERK pathway in rats with diabetic nephropathy. J. Tianjin Univ. Traditional Chin. Med.37 (2), 131–136. 10.11656/j.issn.1673-9043.2018.02.11
80
Sheng X. Dong Y. Cheng D. Wang N. Guo Y. (2020). Efficacy and safety of bailing capsules in the treatment of type 2 diabetic nephropathy: A meta-analysis. Ann. Palliat. Med.9 (6), 3885–3898. 10.21037/apm-20-1799
81
Shuai Y. Zhang S. Q. Shen X. Zhang Y. W. (2018). Effect of Astragals and Angelica mixture on expression of IRE1α-JNK pathway in diabetic nephropathy rats. Chin. Archives Traditional Chin. Med.36 (6), 1372–1375. 10.13193/j.issn.1673-7717.2018.06.022
82
Sieber J. Lindenmeyer M. T. Kampe K. Campbell K. N. Cohen C. D. Hopfer H. et al (2010). Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Ren. Physiol.299 (4), F821–F829. 10.1152/ajprenal.00196.2010
83
So J. S. (2018). Roles of endoplasmic reticulum stress in immune responses. Mol. Cells41 (8), 705–716. 10.14348/molcells.2018.0241
84
Sun H. Saeedi P. Karuranga S. Pinkepank M. Ogurtsova K. Duncan B. B. et al (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119. 10.1016/j.diabres.2021.109119
85
Tang Y. Yu R. Luo W. J. Chen C. Wu Y. J. Zeng J. et al (2015). Effects of Zuogui Jiangtang Yishen decoction on expressions of glucose regulated protein 78 and C/EBP homology protein in podocyte of MKR mice witn diabetic nephropathy. Chin. J. Inf. Traditional Chin. Med.22 (3), 65–68. 10.3969/j.issn.1005-5304.2015.03.017
86
Tian N. Gao Y. Wang X. Wu X. Zou D. Zhu Z. et al (2018a). Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des. Devel Ther.12, 2195–2211. 10.2147/dddt.S167405
87
Tian N. X. Wang X. L. Gao Y. B. (2019). Effect and the mechanism of Qiwei Granules on renal function in KK-Ay mice with spontaneous type 2 diabetes. Beijing J. Traditional Chin. Med.38 (1), 21–25. 10.16025/j.1674-1307.2019.01.005
88
Tian N. X. Wang X. L. Wang T. Shi Y. M. Gao Y. B. (2018b). Research on the mechanism of drug-containing serum of Qiwei Granules on the relief of nephritic podocyte injury in high-glucose environment through caspase12 pathway. China J. Traditional Chin. Med. Pharm.33 (5), 1858–1862.
89
Tsaytler P. Harding H. P. Ron D. Bertolotti A. (2011). Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science332 (6025), 91–94. 10.1126/science.1201396
90
Umanath K. Lewis J. B. (2018). Update on diabetic nephropathy: Core curriculum 2018. Am. J. Kidney Dis.71 (6), 884–895. 10.1053/j.ajkd.2017.10.026
91
Victor P. Umapathy D. George L. Juttada U. Ganesh G. V. Amin K. N. et al (2021). Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones26 (2), 311–321. 10.1007/s12192-020-01176-z
92
Wang P. Li J. Tao J. Sha B. (2018). The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J. Biol. Chem.293 (11), 4110–4121. 10.1074/jbc.RA117.001294
93
Wang S. Yi P. Wang N. Song M. Li W. Zheng Y. (2021a). LncRNA TUG1/miR-29c-3p/SIRT1 axis regulates endoplasmic reticulum stress-mediated renal epithelial cells injury in diabetic nephropathy model in vitro. PLoS One16 (6), e0252761. 10.1371/journal.pone.0252761
94
Wang W. W. Liu Y. L. Wang M. Z. Li H. Liu B. H. Tu Y. et al (2021b). Inhibition of renal tubular epithelial mesenchymal transition and endoplasmic reticulum stress-induced apoptosis with Shenkang injection attenuates diabetic tubulopathy. Front. Pharmacol.12, 662706. 10.3389/fphar.2021.662706
95
Wang Z. S. Xiong F. Xie X. H. Chen D. Pan J. H. Cheng L. (2015). Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol.16, 44. 10.1186/s12882-015-0031-7
96
Williams B. M. Cliff C. L. Lee K. Squires P. E. Hills C. E. (2022). The role of the NLRP3 inflammasome in mediating glomerular and tubular injury in diabetic nephropathy. Front. Physiol.13, 907504. 10.3389/fphys.2022.907504
97
Wu D. Jin L. Huang X. Deng H. Shen Q. K. Quan Z. S. et al (2022). Arctigenin: Pharmacology, total synthesis, and progress in structure modification. J. Enzyme Inhib. Med. Chem.37 (1), 2452–2477. 10.1080/14756366.2022.2115035
98
Xiang C. Xiao X. Jiang B. Zhou M. Zhang Y. Li H. et al (2017). Epigallocatechin-3-gallate protects from high glucose induced podocyte apoptosis via suppressing endoplasmic reticulum stress. Mol. Med. Rep.16 (5), 6142–6147. 10.3892/mmr.2017.7388
99
Xiang X. Y. Liu T. Wu Y. Jiang X. S. He J. L. Chen X. M. et al (2021). Berberine alleviates palmitic acid-induced podocyte apoptosis by reducing reactive oxygen species-mediated endoplasmic reticulum stress. Mol. Med. Rep.23 (1), 3. 10.3892/mmr.2020.11641
100
Xie H. Shi Y. Zhou Y. Liu H. (2022). TMBIM6 promotes diabetic tubular epithelial cell survival and albumin endocytosis by inhibiting the endoplasmic reticulum stress sensor, IRE1α. Mol. Biol. Rep.49 (10), 9181–9194. 10.1007/s11033-022-07744-z
101
Xie L. Guo K. Lu S. Wang N. Wang Y. Chen H. et al (2021). Diabetic nephropathy in mice is aggravated by the absence of podocyte IRE1 and is correlated with reduced kidney ADH1 expression. Ann. Transl. Med.9 (8), 636. 10.21037/atm-20-6356
102
Xiong S. Han Y. Gao P. Zhao H. Jiang N. Sun L. (2020). AdipoRon protects against tubular injury in diabetic nephropathy by inhibiting endoplasmic reticulum stress. Oxid. Med. Cell Longev.2020, 6104375. 10.1155/2020/6104375
103
Xu G. H. Yuan L. Chen Y. H. Li Y. Xie P. Zhao J. Y. et al (2018). Effects of Huangkui Capsule on oxidative stress and endothelial function in diabetes nephropathy. Chin. J. Integr. Traditional West. Nephrol.19 (2), 137–139.
104
Xu S. He L. Ding K. Zhang L. Xu X. Wang S. et al (2020). Tanshinone IIA ameliorates streptozotocin-induced diabetic nephropathy, partly by attenuating PERK pathway-induced fibrosis. Drug Des. Devel Ther.14, 5773–5782. 10.2147/dddt.S257734
105
Xu S. Nam S. M. Kim J. H. Das R. Choi S. K. Nguyen T. T. et al (2015). Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis.6 (11), e1976. 10.1038/cddis.2015.331
106
Yamamoto K. Sato T. Matsui T. Sato M. Okada T. Yoshida H. et al (2007). Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell13 (3), 365–376. 10.1016/j.devcel.2007.07.018
107
Yang R. Chen J. Jia Q. Yang X. Mehmood S. (2022). Epigallocatechin-3-gallate ameliorates renal endoplasmic reticulum stress-mediated inflammation in type 2 diabetic rats. Exp. Biol. Med. (Maywood)247 (16), 1410–1419. 10.1177/15353702221106479
108
Yaribeygi H. Maleki M. Majeed M. Jamialahmadi T. Sahebkar A. (2021). Renoprotective roles of curcumin. Adv. Exp. Med. Biol.1328, 531–544. 10.1007/978-3-030-73234-9_38
109
Yoshida H. Matsui T. Yamamoto A. Okada T. Mori K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell107 (7), 881–891. 10.1016/s0092-8674(01)00611-0
110
Yu N. Yang L. Ling L. Liu Y. Yu Y. Wu Q. et al (2020). Curcumin attenuates angiotensin II-induced podocyte injury and apoptosis by inhibiting endoplasmic reticulum stress. FEBS Open Bio10 (10), 1957–1966. 10.1002/2211-5463.12946
111
Yu X. Yan D. Lan Q. Fang J. Ding Z. Guan Y. et al (2022). Efficacy and safety of jinshuibao capsule in diabetic nephropathy: A systematic review and meta-analysis of randomized controlled trials. Comput. Math. Methods Med.2022, 9671768. 10.1155/2022/9671768
112
Yuan D. Dong Y. P. Zeng Z. J. Huang A. J. Zhang C. L. Zhou H. X. (2017). Effect of Shenshao oral liquid on expression of GRP78, PERK and CHOP in rat models of diabetic nephropathy. China J. Mod. Med.27 (27), 22–26.
113
Yuan D. Liu X. M. Fang Z. Du L. L. Chang J. Lin S. H. (2018). Protective effect of resveratrol on kidney in rats with diabetic nephropathy and its effect on endoplasmic reticulum stress. Eur. Rev. Med. Pharmacol. Sci.22 (5), 1485–1493. 10.26355/eurrev_201803_14497
114
Yuan S. Liang X. He W. Liang M. Jin J. He Q. (2021). ATF4-dependent heme-oxygenase-1 attenuates diabetic nephropathy by inducing autophagy and inhibiting apoptosis in podocyte. Ren. Fail43 (1), 968–979. 10.1080/0886022x.2021.1936040
115
Zang Y. Liu S. Cao A. Shan X. Deng W. Li Z. et al (2021). Astragaloside IV inhibits palmitic acid-induced apoptosis through regulation of calcium homeostasis in mice podocytes. Mol. Biol. Rep.48 (2), 1453–1464. 10.1007/s11033-021-06204-4
116
Zhang J. Cao P. Gui J. Wang X. Han J. Wang Y. et al (2019a). Arctigenin ameliorates renal impairment and inhibits endoplasmic reticulum stress in diabetic db/db mice. Life Sci.223, 194–201. 10.1016/j.lfs.2019.03.037
117
Zhang J. Dong X. J. Ding M. R. You C. Y. Lin X. Wang Y. et al (2020a). Resveratrol decreases high glucose-induced apoptosis in renal tubular cells via suppressing endoplasmic reticulum stress. Mol. Med. Rep.22 (5), 4367–4375. 10.3892/mmr.2020.11511
118
Zhang J. Li J. M. Rong X. L. Liu X. P. Zhou G. M. Han X. et al (2017a). Influence of Tongluo Baoshen Fang on indexes of renal endoplasmic reticulum stress-p-IRE1α and GRP78 in rats with diabetes. J. Beijing Univ. Traditional Chin. Med.40 (3), 219–225.
119
Zhang J. Rong X. L. Li J. M. Liu X. P. Zhou G. M. Huang C. X. et al (2017b). Effects of Tongluo Baoshen formula on apoptosis signaling pathways JNK, Caspase-12 mediated by endoplasmic reticulum stress. China J. Traditional Chin. Med. Pharm.32 (11), 5109–5112.
120
Zhang J. Wu C. Gao L. Du G. Qin X. (2020b). Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol.87, 89–112. 10.1016/bs.apha.2019.08.002
121
Zhang L. Wang D. Jin Y. H. Zhao D. P. Li X. D. Han Y. S. (2022). Efficacy of Modified Shenqi Dihuang decoction as an adjuvant therapy for diabetic nephropathy via modulating endoplasmic reticulum stress and its effect on renal function and glucose and lipid metabolism. China J. Mod. Med.32 (10), 47–52. 10.3969/j.issn.1005-8982.2022.10.009
122
Zhang L. Wei W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther.207, 107452. 10.1016/j.pharmthera.2019.107452
123
Zhang L. Yang L. Shergis J. Zhang L. Zhang A. L. Guo X. et al (2019b). Chinese herbal medicine for diabetic kidney disease: A systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open9 (4), e025653. 10.1136/bmjopen-2018-025653
124
Zhang S. Q. Shuai Y. Zhang Y. W. Shen X. (2018). Effect of Danggui Buxue decoction on endoplasmic reticulum stress-specific Caspase-12 apoptosis way. J. Liaoning Univ. Traditional Chin. Med.20 (3), 20–24. 10.13194/j.issn.1673-842x.2018.03.006
125
Zhang S. Q. Zhang Y. W. Shuai Y. Shen X. (2015). Effects of Danggui Buxue decoction on expressions of ATF6, CHOP and Caspase-3 in renal tissue of diabetic nephropathy rats. Shanghai J. Traditional Chin. Med.52 (4), 91–95. 10.16305/j.1007-1334.2018.04.025
126
Zhang Y. Sun R. Geng S. Shan Y. Li X. Fang W. (2019c). Porcine circovirus type 2 induces ORF3-independent mitochondrial apoptosis via PERK activation and elevation of cytosolic calcium. J. Virol.93 (7), e01784–18. 10.1128/jvi.01784-18
127
Zhu C. Xu J. Wang X. Q. Chen M. (2019). Improvement of chlorogenic acid for oxidative renal injury in diabetic rats through inhibiting endoplasmic reticulum stress. China Pharm.22 (10), 1824–1828.
128
Zhu S. Liu H. Sha H. Qi L. Gao D. S. Zhang W. (2017). PERK and XBP1 differentially regulate CXCL10 and CCL2 production. Exp. Eye Res.155, 1–14. 10.1016/j.exer.2017.01.002
129
Zhu T. Li M. Zhu M. Liu X. Huang K. Li W. et al (2022). Epigallocatechin-3-gallate alleviates type 2 diabetes mellitus via β-cell function improvement and insulin resistance reduction. Iran. J. Basic Med. Sci.25 (4), 483–488. 10.22038/ijbms.2022.58591.13016
Summary
Keywords
Chinese herbal medicine, diabetic nephropathy, endoplasmic reticulum stress, mechanism, renal protection
Citation
Wei M, Liu X, Li M, Tian X, Feng M, Pang B, Fang Z and Wei J (2023) The role of Chinese herbal medicine in the treatment of diabetic nephropathy by regulating endoplasmic reticulum stress. Front. Pharmacol. 14:1174415. doi: 10.3389/fphar.2023.1174415
Received
26 February 2023
Accepted
15 June 2023
Published
26 June 2023
Volume
14 - 2023
Edited by
Arifullah Mohammed, Universiti Malaysia Kelantan, Malaysia
Reviewed by
Wenyan Gong, Hangzhou Normal University, China
Honglian Wang, Southwest Medical University, China
Updates
Copyright
© 2023 Wei, Liu, Li, Tian, Feng, Pang, Fang and Wei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junping Wei, weijunping@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.