Abstract
Background: The effectiveness of the novel oral antiviral agents, nirmatrelvir plus ritonavir and molnupiravir, in treating COVID-19 in patients with nonalcoholic fatty liver disease is unclear.
Objective: To assess the effectiveness of novel oral antiviral agents against COVID-19 among patients with nonalcoholic fatty liver diseases.
Methods: This retrospective cohort study used the TriNetX Research Network to identify non-hospitalized patients with COVID-19 and nonalcoholic fatty liver disease between 1 January 2022, and 30 June 2023. Propensity score matching was used to form two matched cohorts treated with or without nirmatrelvir-ritonavir or molnupiravir.
Results: In the two matched cohorts of 6,358 patients each, the use of novel oral antiviral agents was associated with a significantly lower risk of all-cause emergency department visits, hospitalization, or mortality (6.59% versus 8.24%; hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.70–0.91). The novel antiviral group had a significantly lower risk of all-cause emergency department visits (HR, 0.85; 95% CI, 0.74–0.99). Additionally, the incidence of hospitalization was significantly lower in the oral antiviral group than in the control group (HR, 0.71; 95% CI, 0.55–0.90). There were no deaths in the oral antiviral group but 12 deaths in the control group.
Conclusion: Novel oral antiviral agents are beneficial for treating COVID-19 in patients with nonalcoholic fatty liver disease.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a prevalent chronic liver disease worldwide. According to the Global Burden of Diseases, Injuries, and Risk Factors Study, it affects approximately 15,023 individuals per 100,000 population, with an annual increase of 0.83% from 2000 to 2019 (Younossi et al., 2011; Marjot et al., 2021; Chew et al., 2023). The US National Health and Nutrition Examination Surveys have shown a gradual increase in prevalence in the US, from 20.0% in 1988–1994, to 28.3% in 1999–2004, 33.2% in 2009–2012, and 31.9% in 2013–2016 (Younossi et al., 2020). A meta-analysis showed the highest prevalence in the Middle East (37.19%) and South America (30.45%), followed by Asia (27.17%), North America (24.13%), Europe (23.17%), and Africa (13.48%) (Younossi et al., 2016), suggesting that the burden of NAFLD is high worldwide.
Recently, several studies have shown that NAFLD and other types of chronic liver disease, including alcohol-related liver disease, autoimmune hepatitis, and cirrhosis, may increase the risk of severe coronavirus disease (COVID-19) (Singh and Khan, 2020; Marjot et al., 2021; Middleton et al., 2021; Vardavas et al., 2022). A retrospective study conducted in China found that patients with NAFLD had a higher risk of COVID-19 progression (odds ratio [OR], 6.4; 95% confidence interval [CI], 1.5–31.2) and longer viral shedding time (17.5 ± 5.2 days vs. 12.1 ± 4.4 days, p < 0.0001) compared with patients without NAFLD (Ji et al., 2020). Another study found that patients with NAFLD and SARS-CoV-2 infection were more likely to present with severe illness on admission and used more hospital resources (Younossi et al., 2022). Moreover, Rui et al. (Huang et al., 2020) found that patients with NAFLD had an increased risk of developing liver injury if they developed COVID-19 (OR, 2.956; 95% CI, 1.526–5.726; p = 0.001). Lastly, a systematic review and meta-analysis conducted in 2021 revealed that patients with NAFLD had an increased risk of severe COVID-19 (adjusted odds ratio [aOR], 2.60; 95% CI, 2.24–3.06; p < 0.001) and intensive care unit (ICU) admission (aOR, 2.60; 95% CI, 1.26–2.60; p < 0.001) (Singh et al., 2021). Because NAFLD is associated with severe COVID-19, effective management of COVID-19 in this high-risk population is important. Several oral antiviral agents such as nirmatrelvir plus ritonavir (NMV-r, Paxlovid™) and molnupiravir (MOV) are recommended for non-hospitalized high-risk patients with COVID-19 (Bhimraj et al., 2022). Paxlovid, consists of nirmatrelvir, which is boosted by the addition of ritonavir. The active ingredient nirmatrelvir works by reducing the ability of SARS-CoV-2 to multiply within the body through a mechanism of inhibition of some viral proteases necessary for the virus to replicate. Ritonavir, on the other hand, inhibits the metabolism operated by cytochrome P450 prolonging the action of nirmatrelvir, allowing to remain within the body in quantities that allow it to interfere with the replication of the virus for a longer period of time than would happen if the nirmatrelvir was administered alone. However, there is a paucity of evidence regarding the effectiveness of these drugs in patients with NAFLD. Therefore, we conducted a multicenter retrospective study to investigate the short-term clinical effectiveness of these oral antiviral agents in patients with NAFLD and COVID-19.
Materials and methods
Data source
Patient data were retrieved from the TriNetX Research Network, a global health collaborative clinical research platform containing deidentified records of more than 100 million patients. This research network includes de-identified electronic medical records from multiple healthcare organizations worldwide. The records contain ICD-10 diagnoses, procedures, medications, laboratory data, and genomic information. This study was approved by the Institutional Review Board of Chi Medical Center (No. 11202-002) and the requirement for informed consent was waived because of its retrospective design.
Patient selection
This retrospective study analyzed data of adult patients (aged ≥18 years), with more than two visits to a healthcare provider, who had preexisting NAFLD diagnosed at least 3 months prior, and either tested positive for SARS-CoV-2 infection or were diagnosed with COVID-19 between 1 January 2022, and 30 June 2023. Patients with COVID-19 were identified using ICD-10 diagnosis codes (U07.1, J12.81, and J12.82), positive SARS-CoV-2 RNA tests (LOINC 94309-2, 94,500-6, 95,406-5, 94,502-2, 94,565-9, 95,608-6, 94,759-8, and 94,845-5), and SARS-CoV-2 antigen immunoassays (LOINC 94558-4 and 96,119-3). Patients with NAFLD were identified using the ICD-10 codes K76.0 (fatty liver, not elsewhere classified) and K75.81 (nonalcoholic steatohepatitis).
As the TriNetX database does not provide information on the severity of COVID-19, and considering that oral antivirals were specifically recommended for patients with mild-to-moderate cases, we focused our study exclusively on non-hospitalized patients to ensure both the study and control groups fell within this category. Patients were excluded from the analysis if they were admitted to hospital from 3 days before to 1 day after the COVID-19 diagnosis; received convalescent plasma or monoclonal antibody treatment (tixagevimab, cilgavimab, bebtelovimab, and bamlanivimab); died on the day of COVID-19 diagnosis; had been diagnosed with chronic liver disease not related to NAFLD; had a history of excessive alcohol use/abuse or alcohol-related disorders; or had both NAFLD and other coexisting liver diseases. Supplementary Table S1 provides additional information on the inclusion and exclusion criteria.
The patients were further divided into those who received oral antivirals (NMV-r or MOV) within 5 days of the COVID-19 diagnosis (oral antiviral group) and untreated patients (control group).
Covariates
Propensity score matching was performed in a 1:1 ratio using the TriNetX built-in platform. Demographic characteristics (age, sex, and race) and comorbidities (asthma [45], diabetes mellitus [ICD10 code E08-E13], hypertension [ICD10 code E10], ischemic heart disease [I20–I25], respiratory diseases [J00–J99], chronic kidney disease [N18], chronic lower respiratory diseases [J40–J47], and neoplasms [C00–D49]) were used to eliminate the confounding factors and selection bias. A standard difference of <0.1 was considered an acceptable balanced match.
Outcomes
Primary outcome was defined as the composite outcome of all-cause emergency department (ED) visits, hospitalization, or mortality during the 30-day follow-up period. Second outcomes were the individual outcomes of all-cause ED visits, hospitalization, and mortality during the follow-up.
Statistical analyses
Statistical analyses were performed using the TriNetX platform, and baseline characteristics were reported as means and standard deviations, or frequencies and proportions. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression. The cumulative probability was determined using Kaplan–Meier curves, with statistical significance set at p < 0.05. Subgroup analyses were conducted based on patient age, sex, and hypertension, diabetes, dyslipidemia, obesity, vaccination status and type of oral antivirals.
Results
Based on the initial database search, a total of 49,737 patients with NAFLD and COVID-19 were identified. Of these, 6,394 patients belonged to the oral antiviral group, while 43,343 patients were in the control group (Figure 1). Table 1 displays the baseline characteristics of the patients included in the analysis before and after propensity score matching. Before matching, the oral antiviral group was characterized by older age and a higher proportion of patients classified as white race compared to the control group. Additionally, the oral antiviral group exhibited a higher prevalence of systemic disorders, including endocrine, nutritional, and metabolic diseases, diseases of the circulatory and nervous systems, as well as skin and subcutaneous diseases, in comparison to the control group. Specifically, the oral antiviral group had a greater prevalence of essential hypertension, hyperlipidemia, and neoplasms than the control group. After propensity score matching, two matched cohorts, each consisting of 6,358 patients, were identified, resulting in a total of 12,716 patients included in the analysis. The absolute standardized mean differences between the study and control groups were all <0.1, indicating a successful match between the two groups.
FIGURE 1

Flowchart of patient selection and the cohort selection process.
TABLE 1
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Oral antiviral group (n = 6,358) | Control group (n = 43,215) | Std diff | Oral antiviral group (n = 6,358) | Control group (n = 6,358) | Std diff | |
| Age at index, years | 58.2 ± 13.7 | 55.5 ± 15.0 | 0.185 | 58.2 ± 13.7 | 58.3 ± 14.2 | 0.011 |
| Sex | ||||||
| Male | 2,365 (37.2%) | 15,957 (36.9%) | 0.006 | 2,365 (37.2%) | 2,362 (37.2%) | 0.001 |
| Female | 3,639 (57.2%) | 26,360 (61.0%) | 0.077 | 3,639 (57.2%) | 3,668 (57.7%) | 0.009 |
| Race | ||||||
| White | 4,930 (77.5%) | 30,598 (70.8%) | 0.154 | 4,930 (77.5%) | 4,954 (77.9%) | 0.009 |
| Black or African American | 359 (5.6%) | 3,879 (9.0%) | 0.128 | 359 (5.6%) | 358 (5.6%) | 0.001 |
| Asian | 220 (3.5%) | 2,062 (4.8%) | 0.066 | 220 (3.5%) | 223 (3.5%) | 0.003 |
| Unknown Race | 808 (12.7%) | 6,242 (14.4%) | 0.051 | 808 (12.7%) | 776 (12.2%) | 0.015 |
| Organ system dysfunction | ||||||
| Endocrine, nutritional, and metabolic diseases | 5,849 (92.0%) | 38,008 (88.0%) | 0.135 | 5,849 (92.0%) | 5,856 (92.1%) | 0.004 |
| Diseases of the circulatory system | 4,883 (76.8%) | 31,992 (74.0%) | 0.064 | 4,883 (76.8%) | 4,949 (77.8%) | 0.025 |
| Diseases of the nervous system | 4,581 (72.1%) | 28,904 (66.9%) | 0.112 | 4,581 (72.1%) | 4,432 (69.7%) | 0.052 |
| Diseases of the respiratory system | 4,253 (66.9%) | 28,695 (66.4%) | 0.010 | 4,253 (66.9%) | 4,227 (66.5%) | 0.009 |
| Diseases of the genitourinary system | 4,098 (64.5%) | 27,864 (64.5%) | <0.001 | 4,098 (64.5%) | 4,126 (64.9%) | 0.009 |
| Diseases of the skin and subcutaneous tissue | 3,369 (53.0%) | 20,441 (47.3%) | 0.114 | 3,369 (53.0%) | 3,442 (54.1%) | 0.023 |
| Underlying diseases | ||||||
| Essential hypertension | 4,196 (66.0%) | 27,152 (62.8%) | 0.066 | 4,196 (66.0%) | 4,282 (67.3%) | 0.029 |
| Hyperlipidemia | 2,967 (46.7%) | 19,397 (44.9%) | 0.036 | 2,967 (46.7%) | 3,026 (47.6%) | 0.019 |
| Neoplasms | 2,628 (41.3%) | 16,022 (37.1%) | 0.087 | 2,628 (41.3%) | 2,658 (41.8%) | 0.010 |
| Diabetes mellitus | 2,481 (39.0%) | 16,937 (39.2%) | 0.003 | 2,481 (39.0%) | 2,470 (38.8%) | 0.004 |
| Obesity | 2,153 (33.9%) | 15,005 (34.7%) | 0.018 | 2,153 (33.9%) | 2,139 (33.6%) | 0.005 |
| Chronic lower respiratory diseases | 1,938 (30.5%) | 13,066 (30.2%) | 0.005 | 1,938 (30.5%) | 1,899 (29.9%) | 0.013 |
| Asthma | 1,324 (20.8%) | 8,826 (20.4%) | 0.010 | 1,324 (20.8) | 1,298 (20.4%) | 0.010 |
| Ischemic heart diseases | 1,019 (16.0%) | 8,181 (18.9%) | 0.077 | 1,019 (16.0%) | 1,060 (16.7%) | 0.017 |
| Chronic kidney disease | 622 (9.8%) | 5,202 (12.0%) | 0.072 | 622 (9.8%) | 627 (9.9%) | 0.003 |
Baseline characteristics of antiviral and non-antiviral group in patients with nonalcoholic fatty liver disease and COVID-19 before and after propensity score mathcing matching.
Standardized difference (Std diff) < 0.1 is considered an insignificant difference.
Primary outcome
During the 30-day follow-up period, the oral antiviral group had a significantly lower incidence of all-cause ED visits, hospitalization, or mortality than the control group, with 419 (6.59%) patients in the oral antiviral group and 524 (8.24%) patients in the control group experiencing the composite outcome of all-cause ED visits, hospitalization, or mortality (HR, 0.80; 95% CI, 0.70–0.91) (Table 2). The Kaplan–Meier curves of the probability of time-to-event, showed that the risk of all-cause ED visits, hospitalization, or mortality was significantly lower in the oral antiviral group than control group (p < 0.001, log-rank test) (Figure 2).
TABLE 2
| Outcomes | Patients with outcome | Hazard ratio (95%CI) | p-value | |
|---|---|---|---|---|
| Antiviral group (n = 6,358) | Control group (n = 6,358) | |||
| Primary outcome | ||||
| All-cause ED visits, hospitalization, or death | 419 | 524 | 0.80 (0.70–0.91) | <0.001 |
| Secondary outcome | ||||
| All-cause ED visits | 329 | 386 | 0.85 (0.74–0.99) | 0.03 |
| All-cause hospitalization | 112 | 159 | 0.71 (0.55–0.90) | 0.05 |
| All-cause death | 0 | 12 | - | - |
The hazard ratio for comparing matched nirmatrelvir-ritonavir or molnupiravir between control cohorts for the outcomes.
FIGURE 2
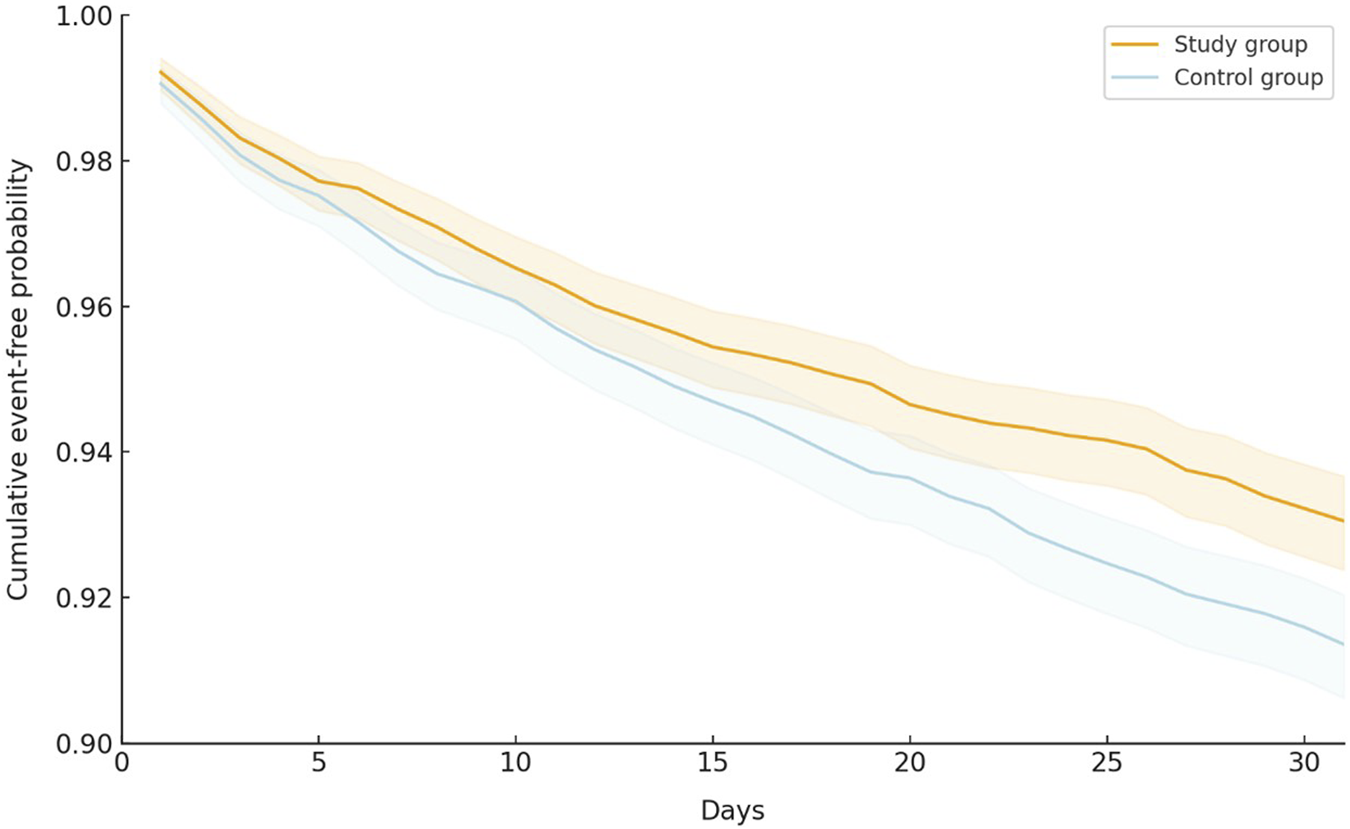
Kaplan–Meier curves of the primary outcome (composite of all-cause emergency department visits, hospitalization, or mortality).
Secondary outcomes
Regarding individual outcomes, the oral antiviral group had a significantly lower risk of all-cause ED visits than the control group (5.17% vs. 6.07%; HR, 0.85; 95% CI, 0.74–0.99). The incidence of all-cause hospitalization was also lower in the oral antiviral group than that in the control group (HR, 0.71; 95% CI, 0.55–0.90). There were no deaths in the oral antiviral group but 12 deaths in the control group during the follow-up period (Table 2).
Subgroup analyses
Figure 3 displays the results of the subgroup analyses. The significantly lower risk of all-cause ED visits, hospitalization, or mortality in the oral antiviral group compared to the control group was consistent across most of subgroups, including patients aged 18–64 years (HR, 0.77; 95% CI, 0.66–0.89) and those aged ≥65 years (HR, 0.80; 95% CI, 0.66–0.98), patients with dyslipidemia (HR, 0.77; 95% CI, 0.64–0.93), hypertension (HR, 0.87; 95% CI, 0.75–0.99), obesity (HR, 0.75; 95% CI, 0.63–0.90), patients receiving NMV-r (HR, 0.86; 95% CI, 0.76–0.97), and unvaccinated patients (HR, 0.74; 95% CI, 0.61–0.88). Additionally, there was a non-significant trend towards a lower risk of the primary outcome in subgroups comprising males, females, individuals with diabetes, those receiving molnupiravir, and those who had received more than 2 vaccinations (Figure 3).
FIGURE 3
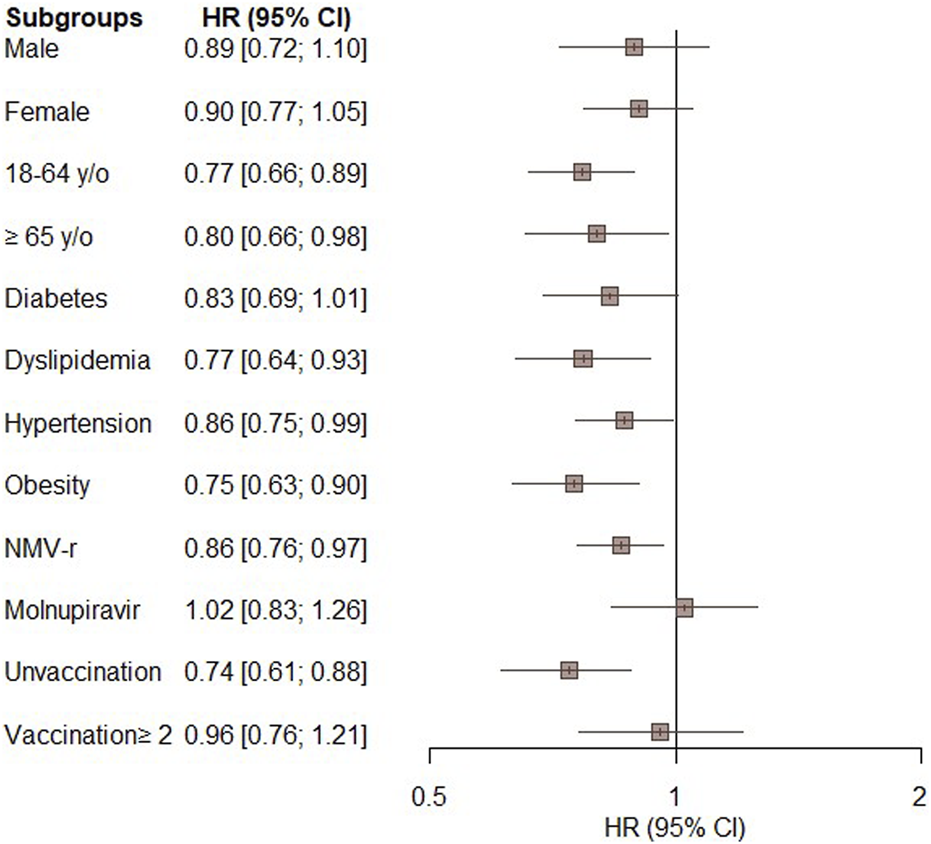
Subgroup analysis of the primary outcome (composite of all-cause emergency department visits, hospitalization, or mortality) in patients treated with novel oral antiviral agents compared with untreated patients.
Discussion
To our knowledge, this retrospective study of 12,716 patients is the first to evaluate the short-term effects of novel oral antiviral agents for the treatment of COVID-19 in patients with NAFLD. Our findings suggest that treatment with oral antiviral agents can improve short-term outcomes in this patient population, as evidenced by the significantly lower risk of all-cause ED visits, hospitalization, and mortality in patients receiving oral antiviral agents than in the untreated control group. This lower risk of primary outcomes was consistent across most of subgroups based on age, sex, and underlying comorbidities and the type of antiviral agents despite some differences did not reach statistical significances. The oral antiviral group also had a significantly lower risk of all-cause ED visits and hospitalization than the control group. Lastly, there were no deaths in the oral antiviral group compared with 12 deaths in the control group. These findings suggest that novel oral antiviral agents have a beneficial effect in the treatment of COVID-19 in patients with NAFLD and support their use in this vulnerable population.
Similar to previous studies (Li et al., 2021; Li et al., 2022; Ahmed et al., 2023), we found that patients with NAFLD had a high prevalence of cardio-metabolic dysfunction and associated diseases (Table 1). Diabetes mellitus has been described as an additional risk factor for severe COVID-19 (Guo et al., 2020). Independent of diabetes, the presence of adiposity (i.e., excess body fat that impairs health, including overweight, obesity, and metabolic syndrome) appears to be a risk factor in infectious viral diseases due to impaired immunity. Obese individuals with COVID-19 are at higher risk of hospitalization, ICU admission, and mortality (Popkin et al., 2020). Based on the subgroup analysis, the clinical benefit of novel oral antiviral agents remained in the specific subgroup of patients with dyslipidemia, hypertension, obesity or diabetes mellitus, which is consistent with the present COVID-19 treatment guidelines (Bhimraj et al., 2022).
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new term proposed in 2020 that includes hepatic steatosis along with the presence of at least one of the following conditions: obesity, type 2 diabetes, or evidence of multiple metabolic risk abnormalities (Eslam and George, 2019). Unlike NAFLD, MAFLD is more closely associated with the main risk factors of atherosclerosis and cardiovascular disease (Boccatonda et al., 2023). The MAFLD definition has higher sensitivity for detecting significant fibrosis (Yamamura et al., 2020). In a US population-based study, MAFLD was associated with an increased risk of all-cause mortality, whereas NAFLD was not associated with all-cause mortality after adjusting for metabolic risk factors (Kim et al., 2021). Furthermore, many studies (Zhou et al., 2020a; Zhou et al., 2020b; Gao et al., 2021) and a meta-analysis of 16 studies with a total of 11,484 patients found that MAFLD was associated with an increased risk of severe COVID-19 (OR, 3.07; 95% CI, 2.30–4.09), and ICU admission (OR, 1.46; 95% CI, 1.12–1.91) (Hayat et al., 2022). In this study, the subgroup analysis showed that the administration of novel oral antiviral agents might provide potential clinical benefits for patients with NAFLD and comorbidities such as obesity, dyslipidemia, and hypertension, which are considered to be associated with MAFLD. Although we may not have captured all patients with MAFLD based on the TriNetX platform, we were able to identify a subset of patients with NAFLD and coexisting major metabolic risk abnormalities similar to MAFLD, who may potentially have similar outcomes in the context of COVID-19. Accordingly, our findings suggest the use of novel oral antiviral agents is effective for treating COVID-19 in patients with MAFLD.
The choice of antiviral agent should consider various factors, including patient characteristics, comorbidities, drug interactions, and local availability. Previous studies have suggested that NMV-r may be more effective than MOV in treating patients with COVID-19 (Lai et al., 2022). Similarly, this study found that patients receiving NMV-r had better outcomes than untreated patients, but the beneficial effect of MOV was not statistically significant. This finding aligns with current guidelines, which recommend using MOV in situations where other antiviral agents are not available, feasible, or clinically appropriate (National Institutes of Health, 2023).
This study had several strengths. First, although chronic liver disease is known to be an indication for the use of an intensive antiviral agent, to our knowledge, this is the first study to focus on the effectiveness of novel antiviral agents for treating COVID-19 in patients with NAFLD. Second, we included robust controls and adjusted for baseline characteristics and confounders using a large sample in the propensity-matched analyses, resulting in narrow CIs. Third, we performed a subgroup analysis including hypertension, dyslipidemia, diabetes, and obesity, which helps to assess the effectiveness of antiviral agents for treating COVID-19 in patients with MAFLD. Finally, our results remained consistent across sensitivity analyses of risk factors for mortality.
This study also has some limitations. The patient data, derived from an electronic health record-based database, were susceptible to errors in diagnostic coding and data entry. In addition, coding errors may have been present. Because NAFLD is an exclusive diagnosis, we considered all diagnostic codes for liver disease that should be excluded according to the NAFLD diagnostic criteria in as much detail as possible. However, there may have been errors in diagnosis. Second, despite our efforts to adjust for many confounding factors, there is still a possibility of residual confounding. Our data did not include imaging modalities, such as conventional imaging techniques and elastography, to confirm the diagnosis of NAFLD. We could not evaluate the severity and progressive forms of NAFLD, such as nonalcoholic steatohepatitis and cirrhosis, owing to the unavailability of common surrogate serum marker-based fibrosis scoring systems, including the NAFLD fibrosis score, Fibrosis-4 Index, and ALT/AST ratio. Finally, our inclusion criteria were not based on a histological diagnosis, which may have increased the risk of misdiagnosis. Further studies are warranted to confirm these findings.
In conclusion, this retrospective study found that treatment with novel oral antiviral agents within 5 days of COVID-19 diagnosis was associated with a lower risk of all-cause ED visits, hospitalization, and mortality in patients with preexisting NAFLD. Therefore, early treatment with novel oral antiviral agents may be beneficial in patients with NAFLD and COVID-19. In subgroup analysis, patients with NAFLD and major metabolic disorders still had significantly better outcomes with the use of oral antiviral agents. These findings suggest the potential role of the antiviral agents in patients with MAFLD. Further studies are needed to confirm these findings and investigate the long-term outcomes of this treatment approach.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
C-CY: Conceptualization, Data curation, Investigation, Writing–original draft. Y-WT: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. SW: Data curation, Investigation, Methodology, Writing–original draft. J-YW: Data curation, Writing–original draft. T-HL: Data curation, Formal Analysis, Investigation, Software, Writing–original draft. W-HH: Data curation, Methodology, Writing–original draft. P-YH: Data curation, Methodology, Writing–original draft. M-HC: Data curation, Formal Analysis, Investigation, Software, Writing–original draft. MS: Supervision, Writing–review and editing. C-CL: Conceptualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1321155/full#supplementary-material
References
1
Ahmed H. S. Wang N. Carr J. J. Ding J. Terry J. G. Vanwagner L. B. et al (2023). The association between hepatic steatosis and incident cardiovascular disease, cancer, and all-cause mortality in a US multi-cohort study. Hepatology77, 2063–2072. 10.1097/HEP.0000000000000286
2
Bhimraj A. Morgan R. L. Shumaker A. H. Baden L. Cheng V. C. C. Edwards K. M. et al (2022). Infectious diseases society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19). Clin. Infect. Dis., ciaa478. 10.1093/cid/ciaa478
3
Boccatonda A. Andreetto L. D'ardes D. Cocco G. Rossi I. Vicari S. et al (2023). From NAFLD to MAFLD: definition, pathophysiological basis and cardiovascular implications. Biomedicines11, 883. 10.3390/biomedicines11030883
4
Chew N. W. S. Ng C. H. Tan D. J. H. Kong G. Lin C. Chin Y. H. et al (2023). The global burden of metabolic disease: data from 2000 to 2019. Cell Metab.35, 414–428.e3. 10.1016/j.cmet.2023.02.003
5
Eslam M. George J. (2019). Refining the role of epicardial adipose tissue in non-alcoholic fatty liver disease. Hepatol. Int.13, 662–664. 10.1007/s12072-019-09990-z
6
Gao F. Zheng K. I. Wang X. B. Yan H. D. Sun Q. F. Pan K. H. et al (2021). Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J. Gastroenterol. Hepatol.36, 204–207. 10.1111/jgh.15112
7
Guo W. Li M. Dong Y. Zhou H. Zhang Z. Tian C. et al (2020). Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev.36, e3319. 10.1002/dmrr.3319
8
Hayat U. Ashfaq M. Z. Johnson L. Ford R. Wuthnow C. Kadado K. et al (2022). The association of metabolic-associated fatty liver disease with clinical outcomes of COVID-19: a systematic review and meta-analysis. Kans J. Med.15, 241–246. 10.17161/kjm.vol15.16522
9
Huang R. Zhu L. Wang J. Xue L. Liu L. Yan X. et al (2020). Clinical features of patients with COVID-19 with nonalcoholic fatty liver disease. Hepatol. Commun.4, 1758–1768. 10.1002/hep4.1592
10
Ji D. Qin E. Xu J. Zhang D. Cheng G. Wang Y. et al (2020). Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J. Hepatol.73, 451–453. 10.1016/j.jhep.2020.03.044
11
Kim D. Konyn P. Sandhu K. K. Dennis B. B. Cheung A. C. Ahmed A. (2021). Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J. Hepatol.75, 1284–1291. 10.1016/j.jhep.2021.07.035
12
Lai C. C. Wang Y. H. Chen K. H. Chen C. H. Wang C. Y. (2022). The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Viruses14, 1706. 10.3390/v14081706
13
Li M. Zhao Z. Qin G. Chen L. Lu J. Huo Y. et al (2021). Non-alcoholic fatty liver disease, metabolic goal achievement with incident cardiovascular disease and eGFR-based chronic kidney disease in patients with prediabetes and diabetes. Metabolism124, 154874. 10.1016/j.metabol.2021.154874
14
Li W. Wen W. Xie D. Qiu M. Cai X. Zheng S. et al (2022). Association between non-alcoholic fatty liver disease and risk of incident heart failure: a meta-analysis of observational studies. Ther. Adv. Chronic Dis.13, 20406223221119626. 10.1177/20406223221119626
15
Marjot T. Webb G. J. Barritt A. S. T. Moon A. M. Stamataki Z. Wong V. W. et al (2021). COVID-19 and liver disease: mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol.18, 348–364. 10.1038/s41575-021-00426-4
16
Middleton P. Hsu C. Lythgoe M. P. (2021). Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol.8, e000739. 10.1136/bmjgast-2021-000739
17
National Institutes of Health (2023). Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ (Accessed on March 29, 2023).
18
Popkin B. M. Du S. Green W. D. Beck M. A. Algaith T. Herbst C. H. et al (2020). Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes. Rev.21, e13128. 10.1111/obr.13128
19
Singh A. Hussain S. Antony B. (2021). Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: a comprehensive systematic review and meta-analysis. Diabetes Metab. Syndr.15, 813–822. 10.1016/j.dsx.2021.03.019
20
Singh S. Khan A. (2020). Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology159, 768–771. 10.1053/j.gastro.2020.04.064
21
Vardavas C. I. Mathioudakis A. G. Nikitara K. Stamatelopoulos K. Georgiopoulos G. Phalkey R. et al (2022). Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: a systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev.31, 220098. 10.1183/16000617.0098-2022
22
Yamamura S. Eslam M. Kawaguchi T. Tsutsumi T. Nakano D. Yoshinaga S. et al (2020). MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int.40, 3018–3030. 10.1111/liv.14675
23
Younossi Z. M. Koenig A. B. Abdelatif D. Fazel Y. Henry L. Wymer M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology64, 73–84. 10.1002/hep.28431
24
Younossi Z. M. Stepanova M. Afendy M. Fang Y. Younossi Y. Mir H. et al (2011). Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol.9, 524–530. e521; quiz e560. 10.1016/j.cgh.2011.03.020
25
Younossi Z. M. Stepanova M. Lam B. Cable R. Felix S. Jeffers T. et al (2022). Independent predictors of mortality among patients with NAFLD hospitalized with COVID-19 infection. Hepatol. Commun.6, 3062–3072. 10.1002/hep4.1802
26
Younossi Z. M. Stepanova M. Younossi Y. Golabi P. Mishra A. Rafiq N. et al (2020). Epidemiology of chronic liver diseases in the USA in the past three decades. Gut69, 564–568. 10.1136/gutjnl-2019-318813
27
Zhou Y. J. Zheng K. I. Wang X. B. Sun Q. F. Pan K. H. Wang T. Y. et al (2020a). Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int.40, 2160–2163. 10.1111/liv.14575
28
Zhou Y. J. Zheng K. I. Wang X. B. Yan H. D. Sun Q. F. Pan K. H. et al (2020b). Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J. Hepatol.73, 719–721. 10.1016/j.jhep.2020.04.027
Summary
Keywords
COVID-19, molnupiravir, nirmatrelvir plus ritonavir, nonalcoholic fatty liver disease, outcome
Citation
Yang C-C, Tsai Y-W, Wang S-H, Wu J-Y, Liu T-H, Hsu W-H, Huang P-Y, Chuang M-H, Sheu M-J and Lai C-C (2024) The effectiveness of oral anti-SARS-CoV-2 agents in non-hospitalized COVID-19 patients with nonalcoholic fatty liver disease: a retrospective study. Front. Pharmacol. 15:1321155. doi: 10.3389/fphar.2024.1321155
Received
13 October 2023
Accepted
01 February 2024
Published
15 February 2024
Volume
15 - 2024
Edited by
Amedeo De Nicolò, University of Turin, Italy
Reviewed by
Dafeng Liu, Public Health and Clinical Center of Chengdu, China
Alice Palermiti, University of Turin, Italy
Updates
Copyright
© 2024 Yang, Tsai, Wang, Wu, Liu, Hsu, Huang, Chuang, Sheu and Lai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Jen Sheu, hmj@mail.chimei.org.tw; Chih-Cheng Lai, dtmed141@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.