Abstract
Background:
The use of herbal medicines (HMs) for the treatment of hypertension (HTN) is increasing globally, but research on the potential adverse effects and safety of HMs in HTN patients is limited. Therefore, this systematic review and meta-analysis aim to determine the global prevalence of HM usage among HTN patients and assess the safety of identified herbs based on current scientific evidence.
Methods:
The PubMed/MEDLINE, EMBASE (Ovid), and Cumulated Index to Nursing and Allied Health Literature (CINAHL) databases were searched for cross-sectional studies on the use of HM among HTN patients. Our review includes studies published in English up to the year 2023. After extracting and appraising the data from the studies, a meta-analysis was conducted using the Stata version 16.0 to estimate the pooled prevalence of HM use in patients with HTN (PROSPERO: CRD42023405537). The safety classification of the identified HM was done based on the existing scientific literature.
Results:
This study analyzed 37 cross-sectional studies from 21 countries and found that 37.8% of HTN patients used HM to manage their health. The prevalence of HM use varied significantly based on publication year and geographical region. Among the 71 identified herbs, Allium sativum L., Hibiscus sabdariffa L., and Olea europaea L. were the most commonly used. However, four herbs were identified as contraindicated, 50 herbs required caution, and only 11 herbs were considered safe for use.
Conclusion:
The study highlights the potential risks of toxicities and adverse effects associated with HM use in the treatment of HTN. Ensuring patient safety involves using safe HMs in appropriate doses and avoiding contraindicated HMs. Future research should focus on identifying commonly used herbs, especially in resource-limited countries with poor HTN management, and additional clinical research is required to assess the toxicity and safety of commonly used HMs.
1 Introduction
High blood pressure is a considerable global health concern (Kearney et al., 2005; Mills et al., 2016), presenting a significant risk factor for cardiovascular disease and premature death (Mills et al., 2020; Roth et al., 2020; Koya et al., 2023). Despite the availability of effective treatment options, over half of the diagnosed patients continue to struggle with managing hypertension (HTN) (Mohsen Ibrahim, 2018; Anand et al., 2019; Burnier and Egan, 2019; Mills et al., 2020; Schutte et al., 2021; Zhou et al., 2021), primarily due to poor adherence to antihypertensive medication, adding to the global disease burden (Kearney et al., 2005; Mills et al., 2020; Mohammed Nawi et al., 2021; Schutte et al., 2021; Hamrahian et al., 2022). Several behavioral risk factors are associated with non-adherence to medication (Burnier and Egan, 2019); complementary and alternative medicine (CAM) use is believed to be one of the contributing factors (Krousel-Wood et al., 2004; Krousel-Wood et al., 2010; Dhar et al., 2017). Among different types of CAM, herbal medicine (HM) is the most popular treatment used by HTN patients (Ali-Shtayeh et al., 2013; James et al., 2018b; Kifle et al., 2021; Palileo-Villanueva et al., 2022). HM has gained popularity as a treatment option for HTN, driven by personal beliefs, preference for natural remedies, cultural traditions, and barriers to accessing conventional care (Liwa et al., 2014; Rahmawati and Bajorek, 2017; Azizah et al., 2021).
However, concerns have been raised about the adulteration or contamination of HM with toxic substances such as heavy metals, as well as the risk of adverse effects when HM is taken with conventional medications due to the pharmacological properties of herbs that may interact with antihypertensive drugs (Pathak and Kothiyal, 2013; Posadzki et al., 2013; Ekor, 2014; Anwar et al., 2016; Azizah et al., 2021; Luo et al., 2021; Im et al., 2023). For instance, concurrent consumption of Azadirachta indica A. Juss, Aloe vera (L.) Burm. f., and Hibiscus sabdariffa L., along with antihypertensive drugs, can compromise the clinical effectiveness of conventional medication by reducing drug absorption (Rahmawati and Bajorek, 2017; Azizah et al., 2021). Furthermore, concurrent use increases the risk of adverse drug reactions, including headaches, gastrointestinal disorders, diarrhea, skin reactions, and frequent urination (Amira and Okubadejo, 2007; Olisa and Oyelola, 2009). Despite the lack of scientific evidence supporting the safety and clinical efficacy of HM (Shafiq et al., 2003; Amira and Okubadejo, 2007; Osamor and Owumi, 2010; Tabassum and Ahmad, 2011; Liwa et al., 2014; Asfaw Erku and Basazn Mekuria, 2016), its usage remains high among HTN patients, with as many as 70% using medicinal herbs due to accessibility and affordability, especially among vulnerable groups (McMahon et al., 1973; Clement et al., 2007; Cuzzolin and Benoni, 2009; Nuwaha and Musinguzi, 2013; Owusu et al., 2020).
These issues have raised concerns about the inappropriate use of HM among HTN patients, highlighting the importance of identifying potential toxicity and adverse effects associated with HM use and developing evidence-based clinical guidelines (Burnier and Egan, 2019). However, limited evidence is available to examine the use of HM among HTN patients and to evaluate the safety of commonly used HMs (Xiong et al., 2015a; Azizah et al., 2021). Therefore, this systematic review and meta-analysis aimed to investigate the pooled prevalence of HM use among HTN patients globally and assess the safety of identified herbs based on current scientific evidence.
2 Materials and methods
This systematic review was reported to comply with the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) checklist (Moher et al., 2009; Page et al., 2021; Supplementary Table S1). The rationale and methods of the study protocol were registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number: CRD42023405537).
2.1 Search strategy
An electronic database search was conducted on 19 June 2023 and included the systematic investigation of the PubMed/MEDLINE, EMBASE (Ovid), and Cumulated Index to Nursing and Allied Health Literature (CINAHL) databases. The initial version was developed using keywords suggested by the literature based on previous studies, and a comprehensive search strategy including specific words, phrases, and controlled vocabulary was then developed by an information specialist in collaboration with two cardiologists and three public health specialists. The search strategy and results are provided in Supplementary Table S2. Medical subject heading (MeSH) terms, EMBASE subject headings (Emtree), and keywords from related articles were explored to guide the selection of relevant search terms. The search terms were further refined by referring to related literature reviews. Finally, variations in three major terms (hypertension, herbal medicine, and cross-sectional study) were used for the search.
2.2 Eligibility criteria
This review includes studies that (i) were published from inception to 2023, (ii) were published in English, (iii) report cross-sectional data of HM use among HTN patients, and (iv) report the name of each herb used and the corresponding number of users. Additionally, studies with mixed study populations (i.e., studies on chronic disease patients that include more than one disease group) were only included if the findings related to the HTN population were presented independently.
Studies were excluded if they met one or more of the following criteria: (i) lack of full English text; (ii) non-use of cross-sectional study designs; (iii) inclusion of non-hypertensive study samples or failure to separate data on hypertensive subjects from other study populations; (iv) failure to report the type of HM used by HTN patients and provide information on the number of users per HM type; (v) incorrect publication types, such as posters, letters, conference abstracts, review articles, or case reports (Mills and Bone, 2004).
2.3 Safety classification of identified herbal medicines
The safety of identified herbs was classified into four categories: potentially harmful to use, use with caution, safety evidence not available, and safe to use (Table 1), and these categories were determined based on the previous literature (Kennedy et al., 2016; Ahmed et al., 2017; Kim H.-L. et al., 2023; Im et al., 2023). The safety classification of identified HMs was determined by reviewing the existing scientific literature and reference material, including the latest published literature, websites, and textbooks related to safety (Ulbricht and Basch, 2005; Johnston, 2006; Gruenwald et al., 2007; Ulbricht, 2010; Gardner and McGuffin, 2013), and the quality of the evidence was assessed based on the hierarchy of evidence (Concato et al., 2000). Evidence from clinical studies on HTN patients was considered first, followed by human studies and animal studies.
TABLE 1
| Category | Classification | Description |
|---|---|---|
| ✕ | Contraindicated for use | The available evidence has shown adverse impacts on hypertension, following the use of the herb |
| △ | Should be used with caution | Caution must be taken when using this herb due to the lack of sufficient human evidence or limited research available. Therefore, it is advisable to use this herb under the guidance and supervision of a qualified healthcare practitioner |
| ᅳ | Safety evidence not available | No reference was found regarding the use of the herb for hypertension |
| ○ | Safe to use | Available human evidence suggests that the herb can be safely used by hypertensive patients |
Safety classification of identified herbal medicines used by HTN patients.
If safety information for an HM was not identified in the above reference sources, we conducted an additional search on PubMed, EMBASE, and Google Scholar. When inconsistencies were found among the reviewed sources, we prioritized the most recently published study on the safety classification of an HM as the primary reference source (Ahmed et al., 2017; Im et al., 2023). Lastly, because the current study is primarily concerned with the safety classification of the identified herbs, the efficacy of each herb was simply categorized based solely on whether there was evidence of blood pressure (BP) lowering from either animal or clinical studies.
2.4 Data extraction and study quality assessment
Based on the eligibility criteria, three researchers (DC, HI, and SJ) conducted a full-text review using a review template that was developed to examine study characteristics (i.e., publication year, country, study design, setting, and research subjects) and the inclusion of primary study outcomes (i.e., the prevalence of HM use, as well as the type of HM used to treat HTN). The data extracted from each study were compared, and any discrepancies between the three reviewers were resolved through consultation with the senior researcher (DW).
The quality of the included studies was independently evaluated by three reviewers using a validated tool to assess the risk of bias in prevalence studies (Hoy et al., 2012). The tool consists of 10 questions that address four items of external validity of the study and six domains of internal validity issues. A score of 0 (no) or 1 (yes) was given for each item, and scores were summed across items to calculate an overall score that ranged from 0 to 10. Studies were then classified as having a low (0–3), moderate (4–6), or high (7–9) risk of bias.
2.5 Data synthesis and statistical analysis
A meta-analysis was conducted to estimate the pooled prevalence of HM use among HTN patients and the corresponding 95% confidence intervals (CI). Articles that did not report the prevalence of HM use (i.e., studies reporting the prevalence of CAM, biologically based therapies, or home remedy use) were excluded from the meta-analysis. The prevalence of HM use from each study was initially recorded in the Microsoft Excel spreadsheet. If a study only reported the number or percent values of HTN patients or HM use, the researchers re-calculated the value of events based on the given percentages. The recorded values were then imported into the Stata version 16.0 tool for further analysis. Due to high heterogeneity among the studies (I2 = 99.61%), a random-effect meta-analysis was conducted. The results were displayed using a forest plot.
The presence of publication bias was evaluated using the funnel plot and the Egger test (p = 0.013; Supplementary Figure S3). A subgroup meta-analysis was conducted to investigate potential differences in the use of HM by geographical region (i.e., individual countries and continents) and the publication year (i.e., studies published before 2011 versus after 2011).
3 Results
3.1 Selection of studies
The PRISMA flow diagram of the study selection process is shown in Figure 1. A total of 2,469 studies were identified from databases and other sources. After removing duplicate records, 2,335 articles were eligible for title and abstract review. During the title and abstract screening, 2,096 records were excluded, leaving 239 articles that were selected for the full-text review. During the full-text review, 201 articles were excluded for the following reasons: unavailability of full-text or English text, ineligible study design or publication type (i.e., reviews, conference abstracts, the letter to the editor, posters, case reports, and animal studies), unrelated to HTN patients, and insufficient reporting of the prevalence of HM use by HTN patients.
FIGURE 1
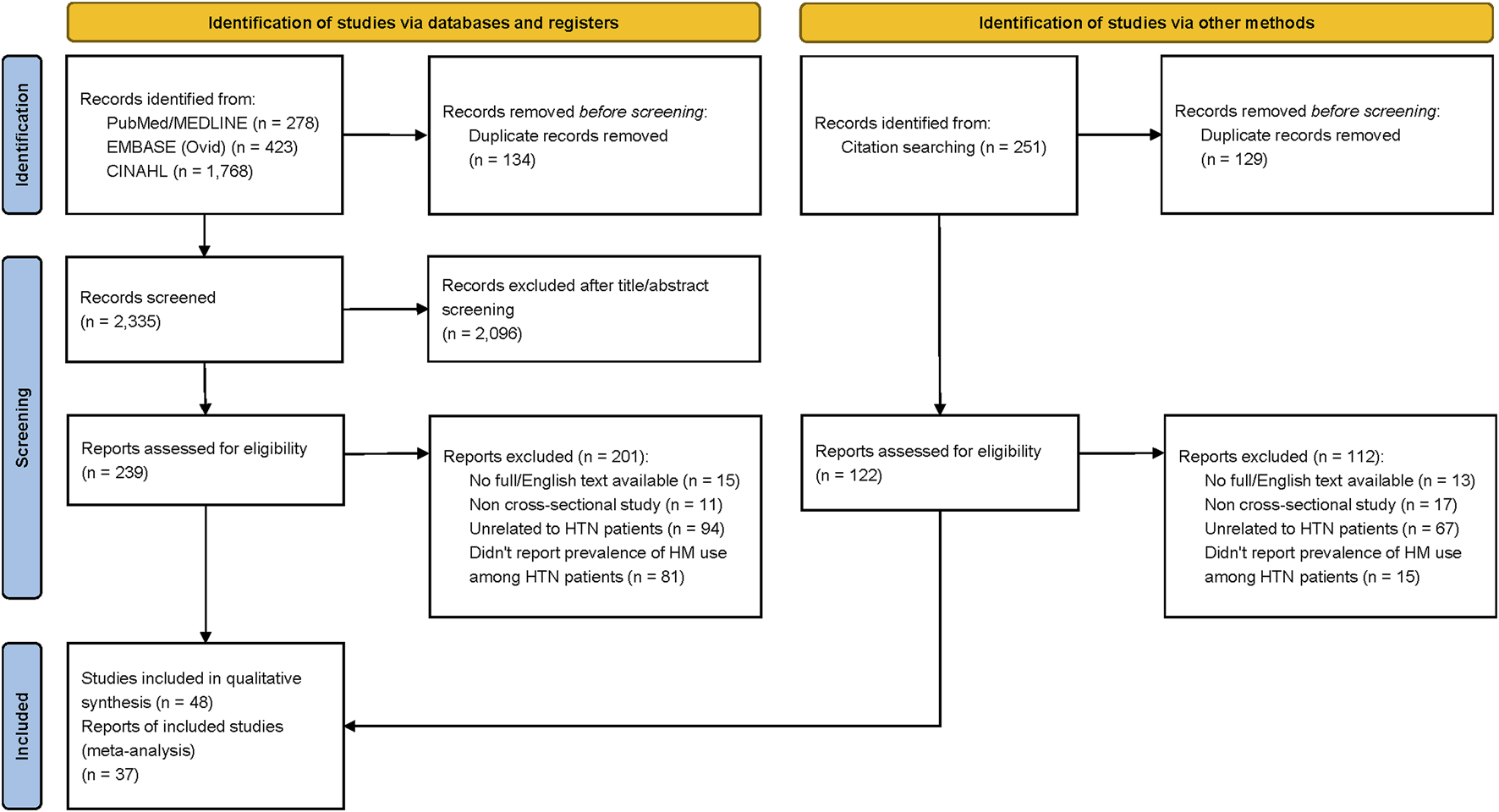
Flow diagram of the search strategy.
A total of 251 additional records were identified by reviewing the reference lists of the 38 included studies. After removing duplicates, 122 additional studies were included for further review. However, during the full-text appraisal, 112 studies were excluded as they did not meet the inclusion criteria (i.e., unrelated to HTN patients, non-cross-sectional study). As a result, 48 articles were eligible for the risk of bias assessment.
3.2 Study quality and risk of bias assessment
The external validity of the reviewed studies showed a high risk of bias for the target population (item 1), sampling frame (item 2), and random sample selection (item 3) because most studies included in this review were conducted at a single hospital and used the convenience sampling method. In addition, as for the internal validity, most items exhibited a low risk of bias, except for item 9. The prevalence period (item 9) was considered to have a high risk of bias if a study did not report or examine the respondent’s HM use beyond the past 12 months. As a result, out of the 48 studies examined, 11 showed a high risk of bias, 7 exhibited a low risk of bias, and 30 displayed a moderate risk. Therefore, excluding 11 studies with a high risk of bias, 37 studies were included in the final review (Supplementary Table S4).
3.3 Characteristics of cross-sectional studies of HM use among HTN patients
In the review, a total of 37 cross-sectional studies conducted in 21 countries were examined. Among these, 27 studies were published after 2010, while ten were conducted between 2000 and 2010. The characteristics of the studies included in the review are illustrated in Table 2. The highest number of studies were carried out in Asia (48.6%), followed by Africa (35.1%), North America (13.5%), and Europe (2.7%). The sample sizes varied from a minimum of 19 participants to a maximum of 2,436 participants (Mahfudz and Chan, 2005; Ali-Shtayeh et al., 2013). Fifteen studies provided detailed information regarding the specific types of HM used and the number of users for each herb (Amira and Okubadejo, 2007; Clement et al., 2007; Gohar et al., 2008; Olisa and Oyelola, 2009; Ali-Shtayeh et al., 2013; Bahar et al., 2013; Kretchy et al., 2014; Tajadini et al., 2015; Baran et al., 2017; Liwa et al., 2017; James et al., 2018a; Al-Hadid et al., 2020; Adeniyi et al., 2021; Joachimdass et al., 2021; Kifle et al., 2021).
TABLE 2
| No. | Study (author, year) | Country | Setting | Population | Sample sizea (Response rate) | HM users N (%) | No. of herbs identifiede |
|---|---|---|---|---|---|---|---|
| Total | 23,947* | 6,316 | 166d | ||||
| 1 | Shafiq et al. (2003) | India | HTN clinic of a teaching hospital | HTN patients visiting the HTN clinic | 333 (63.9%) | 48 (14.4%) | NR |
| 2 | Mahfudz and Chan (2005) | Malaysia | Public primary care center | HTN patients attending the outpatient HTN department | 124 (NR) | 19 (15.3%) | 6 |
| 3 | Yeh et al. (2006) | United States | National household survey (National Health Interview Survey) | Adults ≥18 years of age | Total: 31,044 (NR) | 65 (0.8%) | NR |
| HTN: 8,055* | |||||||
| 4 | Amira and Okubadejo (2007) | Nigeria | HTN clinic of the university teaching hospital | HTN patients attending the HTN clinic for at least 6 months | 225 (NR) | 88 (39.1)b | 5 |
| 5 | Clement et al. (2007) | Trinidad and Tobago | 16 primary healthcare facilities | Patients ≥16 years of age who confirmed their use of herbal remedies | Total: 265 (NR)c | 53 (71.6%) | 15 |
| HTN: 74* (28.0%) | |||||||
| 6 | Gohar et al. (2008) | United Kingdom | Secondary teaching hospital | HTN patients attending the outpatient HTN clinic | 153 (78.1%) | 24 (15.7%)c | 4 |
| 7 | Olisa and Oyelola (2009) | Nigeria | Secondary hospital (state level) | Ambulatory HTN patients attending the HTN clinic | 480 (96.0%) | 120 (25.0%) | 24 |
| 8 | Al-Hamdan et al. (2010) | Saudi Arabia | Community-based survey in 20 primary health centers | All Saudi population aged 15–64 years | Total: 4,719 (99.2%) | 45 (8.3%) | NR |
| HTN: 542* | |||||||
| 9 | Delgoda et al. (2010) | Jamaica | Eighteen pharmacies | Patients or parents/carers of children visiting the study pharmacy | 365 (91.5%) | 103 (79.8%) | NR |
| 10 | Nur (2010) | Turkey | Community-based survey at a semi-rural province | Adults ≥18 years of age | 3,876 (96.3%) | 273 (48.8%) | NR |
| HTN: 559* | |||||||
| 11 | Ali-Shtayeh et al. (2013) | Palestine | HTN outpatient departments at governmental hospitals, military medical clinics, and refugee camp clinics in eight towns | HTN patients who had been diagnosed with HTN and attending the HTN outpatient clinic | 4,575 (NR) | 2,436 (53.25%)b | 83 |
| 12 | Bahar et al. (2013) | Turkey | Three primary care centers located within the same district | Patients who had been diagnosed with HTN by a physician, receiving HTN treatment, and admitted to a primary care center | 193 (NR) | 99 (51.3%) | 8 |
| 13 | Wazaify et al. (2013) | Jordan | University teaching hospital | Patients with CKD, dyslipidemia, and HTN cases attending the outpatient departments | Total: 700 (91.3%) | 44 (6.9%)c | 9 |
| HTN: 636* | |||||||
| 14 | Nuwaha and Musinguzi (2013) | Uganda | Community-based survey at two rural districts | HTN patients ≥15 years of age | 258 (91.8%) | 73 (28.3%) | NR |
| 15 | Hu et al. (2018) | China | Survey conducted within a local community of a metropolitan city | HTN patients ≥35 years of age who have had HTN for a minimum of 12 months | 318 (81.4%) | 59 (18.6%) | NR |
| 16 | Mollaoğlu et al. (2013) | Turkey | Outpatient clinics of a general hospital | Adults ≥21 years of age and treated for one or more of six chronic diseases, including HTN, within the past year in Turkey | 252 (NR) | 51 (61.4%) | NR |
| HTN: 83* | |||||||
| 17 | Açıkgöz et al. (2014) | Turkey | Tertiary care education hospital | All patients admitted to outpatient cardiology clinics with prior prescription of at least one cardiovascular drug | 390 (84.5%) | 79 (29.7%) | NR |
| HTN: 266* | |||||||
| 18 | Kretchy et al. (2014) | Ghana | Two tertiary teaching Hospitals | HTN patients ≥18 years of age attending the outpatient departments | 400 (100.0%) | 51 (12.8%) | 13 |
| 19 | Boima et al. (2015) | Ghana and Nigeria | Three tertiary teaching hospitals and one general hospital | HTN patients ≥18 years of age who had been diagnosed with HTN and placed on medication for at least 12 months | 357 (NR) | 62 (17.4%) | NR |
| 20 | Li et al. (2015) | China | Community-based survey at the two rural counties | HTN patients ≥30 years of age in two counties | 665 (NR) | 93 (14.0%) | NR |
| 21 | Tajadini et al. (2015) | Iran | Telephone interview | HTN patients participated in the KERCADER project in the Beast subspecialty clinic | 612 (94.2%) | 180 (29.4%) | 3 |
| 22 | Asfaw Erku and Basazn Mekuria (2016) | Ethiopia | University teaching hospital | HTN patients ≥18 years of age who started taking medication for reduction of BP and visited the outpatient clinic | 412 (97.4%) | 189 (45.9%) | NR |
| 23 | Lulebo et al. (2017) | Congo | Fifteen primary healthcare facilities | HTN patients >18 years of age attending Kinshasa Primary Healthcare (KPHC) facilities | 280 (NR) | 119 (42.5%) | NR |
| 24 | Baran et al. (2017) | Turkey | Tertiary hospital | HTN patients ≥18 years attending the Family Health Center | 465 (80.4%) | 259 (55.7%)b | 2 |
| 25 | Liwa et al. (2017) | Tanzania | Tertiary teaching hospital | Patients >18 years of age admitted with HTN-related diagnoses | 213 (92.6%) | 52 (24.4%) | 15 |
| 26 | Adidja et al. (2018) | Cameroon | Community-based survey at a single district | HTN patients >21 years of age who were on hypertensive medication(s) for at least 1 month | 183 (NR) | 38 (20.8%) | NR |
| 27 | James et al. (2018a) | Sierra Leone | Four public and two private health facilities | HTN patients ≥18 years of age attending the outpatient departments | 260 (NR) | 148 (56.9%) | 14 |
| 28 | Peltzer& Pengpid (2019) | Thailand | Seven district hospitals | Outpatients ≥21 years of age and had a chronic disease | 1,396 (98.6%) | 272 (32.5%) | NR |
| HTN: 838* | |||||||
| 29 | Sabery et al. (2019) | Iran | Community-based survey at Kashan city | Adults >60 years of age | 770 (100.0%) | 235 (68.5%) | NR |
| HTN: 343* | |||||||
| 30 | Al-Hadid et al. (2020) | Jordan | A health center | HTN patients ≥16 years of age who had been managed at a selected health center for at least 6 months | 208 (100.0%) | 107 (51.4%) | 4 |
| 31 | Alshabi (2020) | Saudi Arabia | University hospital | Adults >18 years of age | 1,000 (NR) | 51 (43.6%) | NR |
| HTN: 117* | |||||||
| 32 | El-Dahiyat et al. (2020) | Jordan | Two universities | University students, staff, and their family members | 378 (75.6%) | 32 (88.9%) | NR |
| HTN: 36* | |||||||
| 33 | Owusu et al. (2020) | Jamaica | Any of the seven chronic disease clinics in one of the four parishes under the WRHA | Adults ≥18 years of age who had been diagnosed with HTN and/or T2DM and were attending a chronic disease clinic | Total: 362 (95.3%) | 224 (72.1%)a | 6 |
| HTN: 311* (90.1%) | |||||||
| 34 | Adeniyi et al. (2021) | Jamaica | Clinics for HTN and T2DM in the four parishes | Adults ≥18 years of age who had been diagnosed with HTN and/or T2DM and attended health clinics in one of the four WRHA parishes | 60 (NR) | 48 (96.0%) | 6 |
| HTN: 50* | |||||||
| 35 | Joachimdass et al. (2021) | Malaysia | Primary healthcare clinic in the suburban district | HTN patients ≥18 years of age who had attended the clinic for at least three prior appointments for HTN | 294 (96.1%) | 90 (30.6%) | 52 |
| 36 | Kifle et al. (2021) | Ethiopia | General hospital located in a town | HTN patients ≥18 years of age who received medical care at the adult hypertensive care services | 450 (94.7%) | 167 (37.1%) | 9 |
| 37 | Thangsuk et al. (2021) | Thailand | Primary care clinic in university hospital | Patients ≥35 years of age who had been diagnosed with essential HTN and taking at least one antihypertensive drug | 450 (80.6%) | 80 (17.8%) | NR |
Characteristics of included studies.
HTN, hypertension; T2DM, Type 2 diabetes mellitus; CKD, chronic kidney disease; BP, blood pressure; HL, hyperlipidemia; HM, herbal medicine; CAM, complementary and alternative medicine; NR, not reported.
The original study examined the use of HM among both HTN patients and non-HTN adults; thus, only the data on HTN patients were extracted and included in this review.
The researchers manually calculated the exact number of respondents as the prevalence was only reported as percentages in the published article.
This number indicates the number of CAM users as the original study encompasses the use of CAM, and only the number of individual HM users is reported (the overall number of HM users is not provided).
The original study encompasses the use of complementary medicine; thus, only the data on HM use were extracted and included in this review.
The total number of HMs indicated in this table excludes duplicate records (e.g., if an HM mortality was reported in more than one study, it is counted once).
The total number of herbal medicines with their full names mentioned in each study.
3.4 Prevalence of HM use among HTN patients
The prevalence of HM use among HTN patients ranged from 0.8% to 96.0% (Yeh et al., 2006; Adeniyi et al., 2021), the pooled prevalence was 37.8% (95% CI: 27.4%–48.9%; Table 3; Figure 2). Among the 21 countries included in this review, HM use was the lowest in the United States and the highest in Jamaica (Wazaify et al., 2013; Owusu et al., 2020). The prevalence of HM utilization varied significantly by publication year and geographic region, with higher utilization rates observed in studies published after 2011 (41.0%, 95% CI: 33.9%–48.3%) than those published before 2011 (28.6%, 95% CI: 11.3%–50.1%; p < 0.001). The highest use was reported in North America (62.5%, 95% CI: 9.0%–99.9%), followed by Asia (36.7%, 95% CI: 28.2%–45.7%) and Africa (30.9%, 95% CI: 22.8%–39.6%; Table 3; Figure 3).
TABLE 3
| Characteristic | Included studiesa | Sample size | Meanb (%) | 95% CI | p-value |
|---|---|---|---|---|---|
| Overall | 37 | 23,947 | 37.8 | 27.4–48.9 | |
| Publication year | |||||
| Before 2011 | 10 | 10,674 | 28.6 | 11.3–50.1 | <0.001 |
| After 2011 | 27 | 13,273 | 41.0 | 33.9–48.3 | |
| Geographical region | |||||
| Africa | 11 | 3,518 | 30.9 | 22.8–39.6 | <0.001 |
| Asia | 20 | 11,657 | 36.7 | 28.2–45.7 | |
| Europe | 1 | 153 | 15.7 | 10.3–22.4 | |
| North America | 5 | 8,619 | 62.5 | 9.0–99.9 | |
Pooled prevalence of HM use by study characteristics.
*Estimated using a random-effects model.
aNumber of studies included in each subgroup.
bPooled estimate.
FIGURE 2
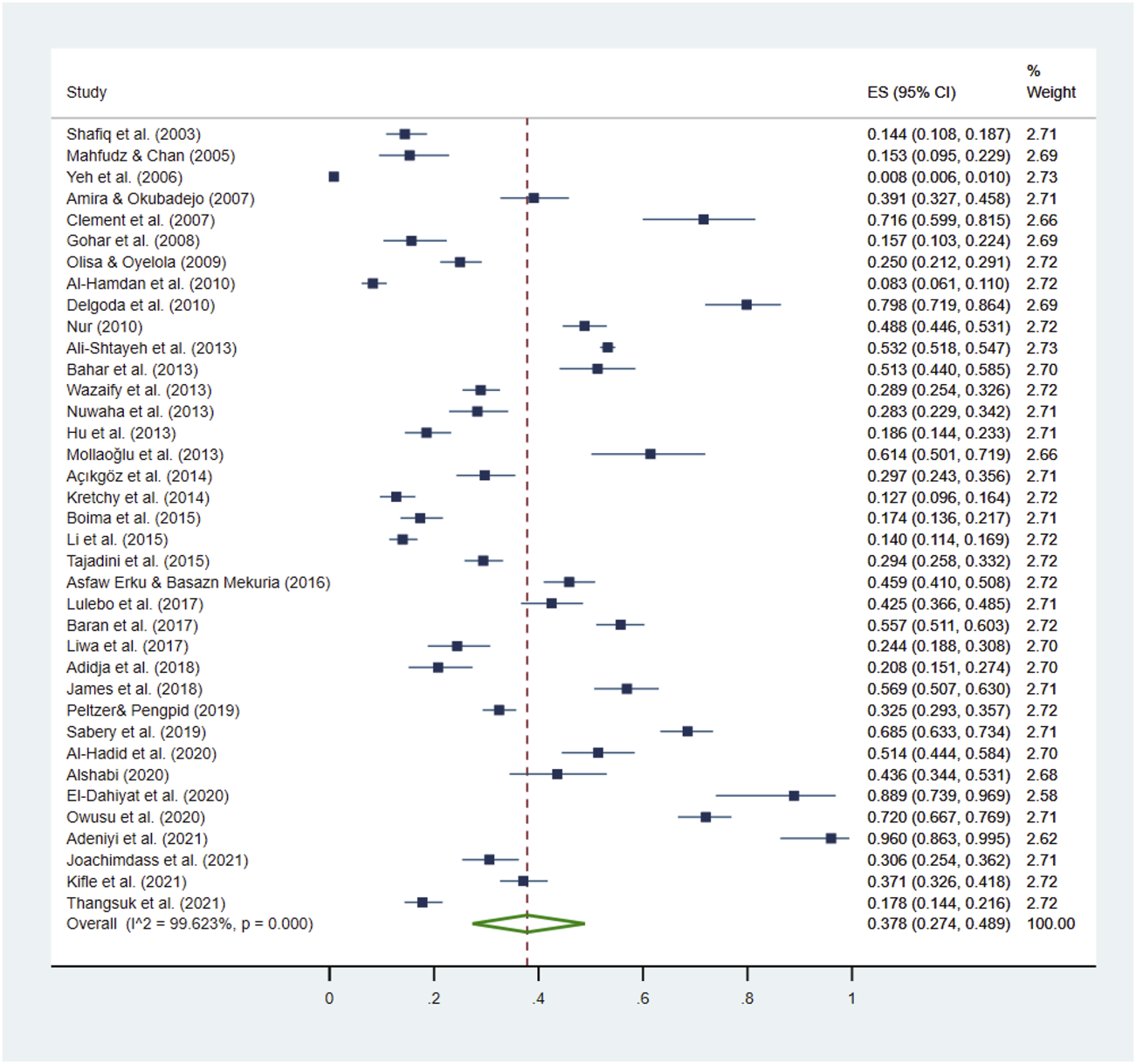
Pooled prevalence of HM use by HTN patients. This figure presents a forest plot of a random-effect meta-analysis. Thirty-seven studies on HTN patients reported the HM use rate and were included in the pooled estimation of HM use. The square blue dots and the dashed line passing through represent the effect size and corresponding 95% confidence intervals (CIs) reported in individual studies, and the green diamond on the bottom and the size of its lateral tips denote the pooled effect size and its 95% CI.
FIGURE 3
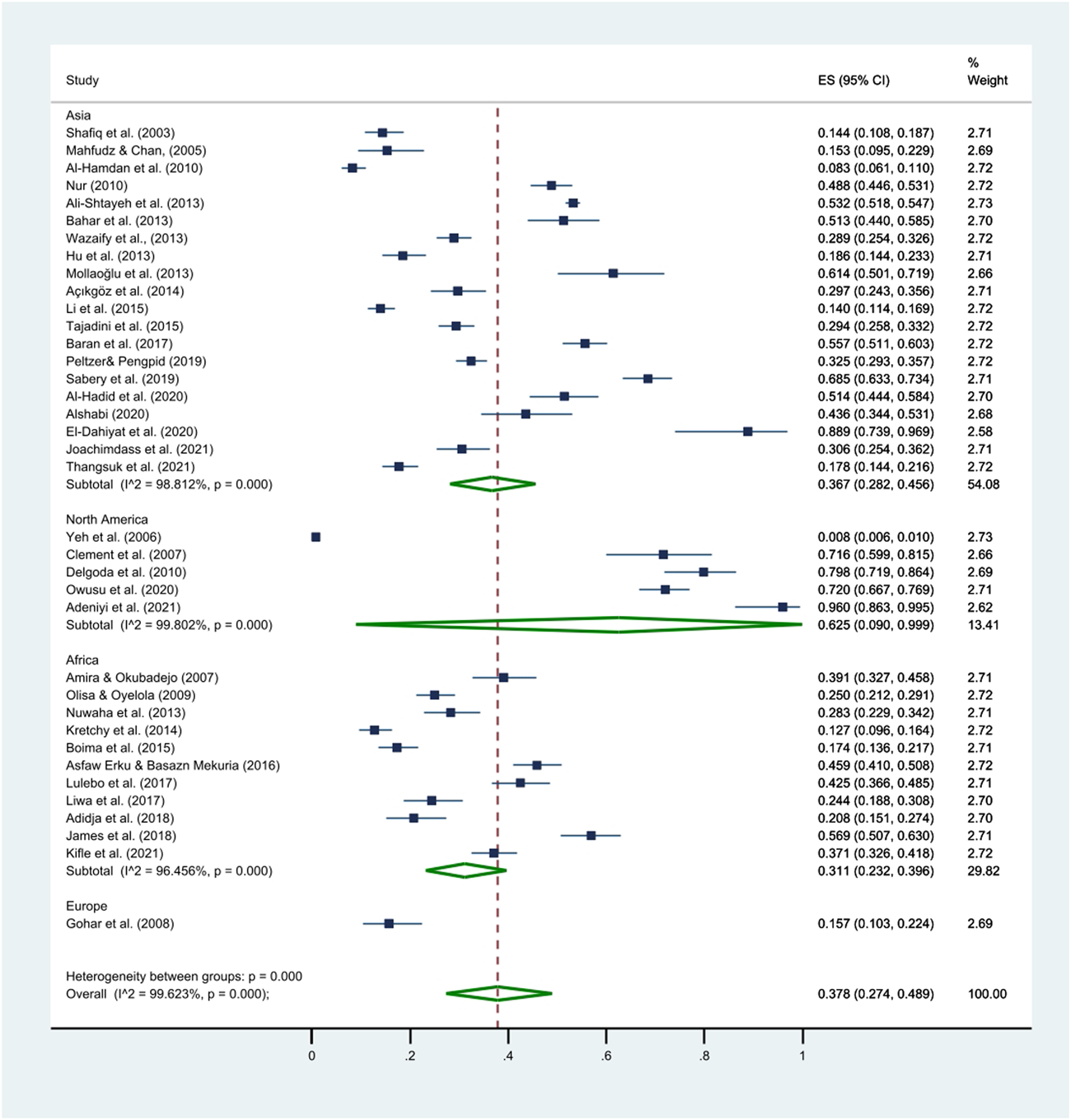
Differences in the pooled prevalence of HM use by continents. This figure presents a forest plot of a random-effect meta-analysis. Subgroup meta-analysis was conducted to investigate potential differences in the prevalence of HM use by geographical region (i.e., Asia, North America, Africa, and Europe). The square blue dots and dashed line passing through represent the effect size and corresponding 95% CI reported in individual studies, and the green diamond on the bottom and the size of its lateral tips denote the pooled effect size and its 95% CI for each subgroup.
3.5 The most commonly used HM and reported indications for HM use
The use of 165 herbs was observed in the reviewed articles, but only the modalities used by ten or more study subjects were included in the safety evaluation. As a result, the use of 71 different HMs (individual herbs or mixture as preparation) was identified from 15 studies (Table 4). The most frequently used herbal medicines included Allium sativum L. (31.3%), H. sabdariffa L. (12.3%), Olea europaea L. (10.4%), and Crataegus oxyacantha L (8.0%).
TABLE 4
| No. | Herbal medicinesb | No. of usersa (Total= 3,922) N (%) | Route | Reported indication for HM use | |
|---|---|---|---|---|---|
| English name | Scientific name | ||||
| 1 | Garlica–o | Allium sativum L. | 1,229 (31.3) | Oral, topical, inhalation | Control or treat HTN, DM, or cancerreducing the side effects of prescription drugs; carminative; improve overall health; malaria; weight reduction; dyslipidemia; arthritis; hyperlipidemia; and meningitis |
| 2 | Rosellef,m | Hibiscus sabdariffa L. | 484 (12.3) | Oral, topical, and inhalation | Control or treat HTN or DM |
| 3 | Olivef | Olea europaea L. | 407 (10.4) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer |
| 4 | Hawthornf | Crataegus oxyacantha L. | 314 (8.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer |
| 5 | Lemonf,g,j,k,o | Citrus limon L. | 288 (7.3) | Oral | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 6 | Green teaf,g,i | Camellia sinensis (L.) Kuntze | 248 (6.3) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer |
| 7 | Gingerb,e,f,k,n | Zingiber officinale Rosc. | 242 (6.2) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 8 | Anisef | Pimpinella anisum L. | 229 (5.8) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 9 | Chamomilef,l | Matricaria chamomilla L. | 211 (5.4) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs, and malaria |
| 10 | Common sagef | Salvia officinalis L. | 181 (4.6) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 11 | Rosemaryf | Salvia rosmarinus L. | 142 (3.6) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 12 | Cinnamonf,g,m | Cinnamomum verum J. Presl. | 121 (3.1) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 13 | Fenugreekf,o,p | Trigonella foenum-graecum L. | 118 (3.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 14 | Moringae,h,k,l,n,o | Moringa oleifera L. | 118 (3.0) | Oral | Control or treat HTN or DM, weight reduction, arthritis, hyperlipidemia, meningitis, and tuberculosis |
| 15 | Peppermintf,p | Mentha piperita L. | 117 (3.0) | Oral, topical, and inhalation | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 16 | Syrian oreganof | Majorana syriaca (L.) Rafin. | 117 (3.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 17 | Parsleyf | Petroselinum crispum (Mill.) Nyman | 109 (2.8) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 18 | African moringap | Moringa stenoptela (Baker f.) Cufod. | 105 (2.7) | Oral | Control or treat HTN |
| 19 | Black cuminf | Nigella sativa L. | 91 (2.3) | Oral, topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 20 | Honeyk,l | Honey | 91 (2.3) | Oral | Control or treat HTN or DM, weight reduction, arthritis, asthma, and meningitis |
| 21 | Damakasep | Ocimum lamiifolium Hochst. ex Benth. | 81 (2.1) | Oral | Control or treat HTN |
| 22 | Cat thymef | Teucrium polium L. | 79 (2.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 23 | Chickweedf | Stellaria media L. | 75 (1.9) | Oral and topical | Control or treat HTN, reducing the side effects of prescription drugs |
| 24 | Marshmallowi | Althaea officinalis L. | 63 (1.6) | Oral | Control or treat HTN and DM |
| 25 | Wild laburnump | Calpurnia aurea (Ait.) Benth. | 62 (1.6) | Inhalation (nasal) | Control or treat HTN |
| 26 | Pomegranatef | Punica granatum L. | 57 (1.5) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 27 | Guavaf,o | Psidium guajava L. | 53 (1.4) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 28 | Bananaf,o | Musa paradisiaca L. | 48 (1.2) | Oral and topical | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 29 | Barleyf | Hordeum vulgare L. | 45 (1.1) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 30 | Nepal dockp | Rumex nepalensis Spreng. | 44 (1.1) | Oral | Control or treat HTN |
| 31 | Fennelf | Foeniculum vulgare Mill. | 40 (1.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 32 | Onionf,k | Allium cepa L. | 40 (1.0) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 33 | Applef,g,o | Malus domestica Borkh. | 30 (0.8) | Oral | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 34 | Sweet-marjoramf | Origanum majorana L. | 30 (0.8) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 35 | Indian pennyworto | Centella asiatica L. | 28 (0.7) | Oral | Control or treat HTN |
| 36 | Almondf | Prunus dulcis L. | 25 (0.6) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 37 | Lupinef | Lupinus albus L. | 25 (0.6) | Oral, topical, and inhalation | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 38 | African redwoodp | Hagenia abyssinica J.F. Gmel. | 24 (0.6) | Oral | Control or treat HTN |
| 39 | Carrotf,k,o | Daucus carota L. | 24 (0.6) | Oral | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 40 | Papayae,k,o | Carica papaya L. | 24 (0.6) | Oral | Control or treat HTN |
| 41 | Sweet peppersf | Capsicum annuum L. | 24 (0.6) | Oral | Control or treat HTN or DM, reducing the side effects of prescription drugs |
| 42 | Bitter gourdo | Momordica charantia L. | 23 (0.6) | Oral | Control or treat HTN |
| 43 | Black mulberryf | Morus nigra L. | 23 (0.6) | Oral, topical | Control or treat HTN or DM, reducing the side effects of prescription drugs |
| 44 | Cucumberf,o | Cucumis sativus L. | 22 (0.6) | Oral | Control or treat HTN |
| 45 | Saffloweri | Carthamus tinctorius L. | 22 (0.6) | Oral | Control or treat HTN and DM |
| 46 | Bitter leafb,h,l,o | Vernonia amygdalina Delile | 21 (0.5) | Oral | Control or treat HTN, improve overall health, weight reduction, arthritis, malaria, typhoid, abdominal pain, and tuberculosis |
| 47 | White wormwoodf | Artemisia herba-alba Asso. | 21 (0.5) | Oral | Control or treat HTN or DM, reducing the side effects of prescription drugs |
| 48 | Palestine arumf | Arum palaestinum Boiss. | 20 (0.5) | Oral and topical | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 49 | Cabbagef,o | Brassica oleracea L. | 19 (0.5) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 50 | Tosign (Dry thyme)p | Thymus schimperi R. | 19 (0.5) | Oral | Control or treat HTN |
| 51 | Licoricef | Glycyrrhiza glabra L. | 16 (0.4) | Oral, topical, and inhalation | Control or treat HTN, reducing the side effects of prescription drugs |
| 52 | Pink flaxf | Linum pubescens Willd. ex Schult. | 16 (0.4) | Oral and topical | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 53 | Sugar beetf | Beta vulgaris L. | 16 (0.4) | Oral and topical | Control or treat HTN, reducing the side effects of prescription drugs |
| 54 | Mekmekof | Rumex abyssinicus Jacq. | 15 (0.4) | Oral | Control or treat HTN |
| 55 | Wild mustardf | Sinapis arvensis L. | 15 (0.4) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 56 | Kiwif,o | Actinidia deliciosa A. Chev | 14 (0.4) | Oral | Control or treat HTN, reducing the side effects of prescription drugs |
| 57 | Pearf,h | Pyrus communis L. | 14 (0.4) | Oral and topical | Control or treat HTN, reducing the side effects of prescription drugs |
| 58 | Aloeb,e,k | Aloe vera (L.) Burm.f. | 13 (0.3) | Oral | Control or treat HTN or malaria |
| 59 | Flax seedg,m | Linum usitatissimum L. | 13 (0.3) | Oral | Control or treat HTN |
| 60 | Limen,o | Citrus aurantiifolia (Christm.) Swingle | 13 (0.3) | Oral | Control or treat HTN |
| 61 | Neeme | Azadirachta indica A. Juss. | 13 (0.3) | Oral | Control or treat HTN or malaria |
| 62 | Roman nettlef | Urtica pilulifera L. | 13 (0.3) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 63 | Coffeef | Coffea arabica L. | 12 (0.3) | Inhalation and topical | Control or treat HTN, reducing the side effects of prescription drugs |
| 64 | Guinea henweedn | Petiveria alliacea L. | 12 (0.3) | Oral | Control or treat HTN |
| 65 | Grapef,o | Vitis vinifera L. | 11 (0.3) | Oral | Control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 66 | Persimmonf | Diospyros kaki L. | 11 (0.3) | Oral | Control or treat HTN, reducing the side effects of prescription drugs |
| 67 | Petaio | Parkia speciosa Hassk. | 10 (0.3) | Oral | Control or treat HTN |
| 68 | Prickly pearf | Opuntia ficus-indica (L.) Mill. | 10 (0.3) | Oral and topical | control or treat HTN or cancer, reducing the side effects of prescription drugs |
| 69 | Sesamef | Sesamum indicum L. | 10 (0.3) | Oral and topical | Control or treat HTN, DM, or cancer, reducing the side effects of prescription drugs |
| 70 | Tamarinde,o | Tamarindus indica L. | 10 (0.3) | Oral | Control or treat HTN |
| 71 | White micromeriaf | Micromeria fruticosa (L.) Druce | 10 (0.3) | Oral and topical | Control or treat HTN |
Most commonly used HMs and reported indications for HM use among HTN patients.
HTN, hypertension; T2DM, Type 2 diabetes mellitus; HM, herbal medicine.
Table 4 includes studies that provide the specific names of the HMs used by HTN patients, along with the corresponding number of users exceeding 10.
Superscript numbers from 1 to 15 on every herbal modality indicate the study that reported use of that modality: Amira et al.
Clement et al.
Gohar et al.
Olisa et al.
Ali-Shtayeh et al.
Bahar et al.
Kretchy et al.
Tajadini et al.
Baran et al.
Liwa et al.
James et al.
Al-Hadid et al.
Adeniyi et al.
Joachimdass et al.
pKifle et al.
The most commonly reported indications for HM use were to control or treat HTN and diabetes mellitus-related symptoms, followed by weight reduction, arthritis, meningitis, hyperlipidemia, malaria, and tuberculosis (Table 4). Oral administration of the HM was the most common route of administration.
3.6 Safety classification of commonly used HMs
Supplementary Table S5 provides a detailed analysis of the safety classification of commonly used HMs among patients with HTN. In this review, 71 herbs were identified, and four of them were classified as contraindicated based on four safety criteria. Notably, clinical evidence involving human subjects was available for two of these herbs (Glycyrrhiza glabra L. and A. indica A. Juss.), while the safety classification for Arum palaestinum Boiss. and Micromeria fruticosa (L.) Druce relied solely on animal studies. Furthermore, 50 herbs were categorized to be used with caution, while 11 herbs were classified as safe and suitable for HTN patients. Lastly, the safety of the remaining six herbs could not be determined due to insufficient evidence in the current literature for HTN patients (Figure 4).
FIGURE 4
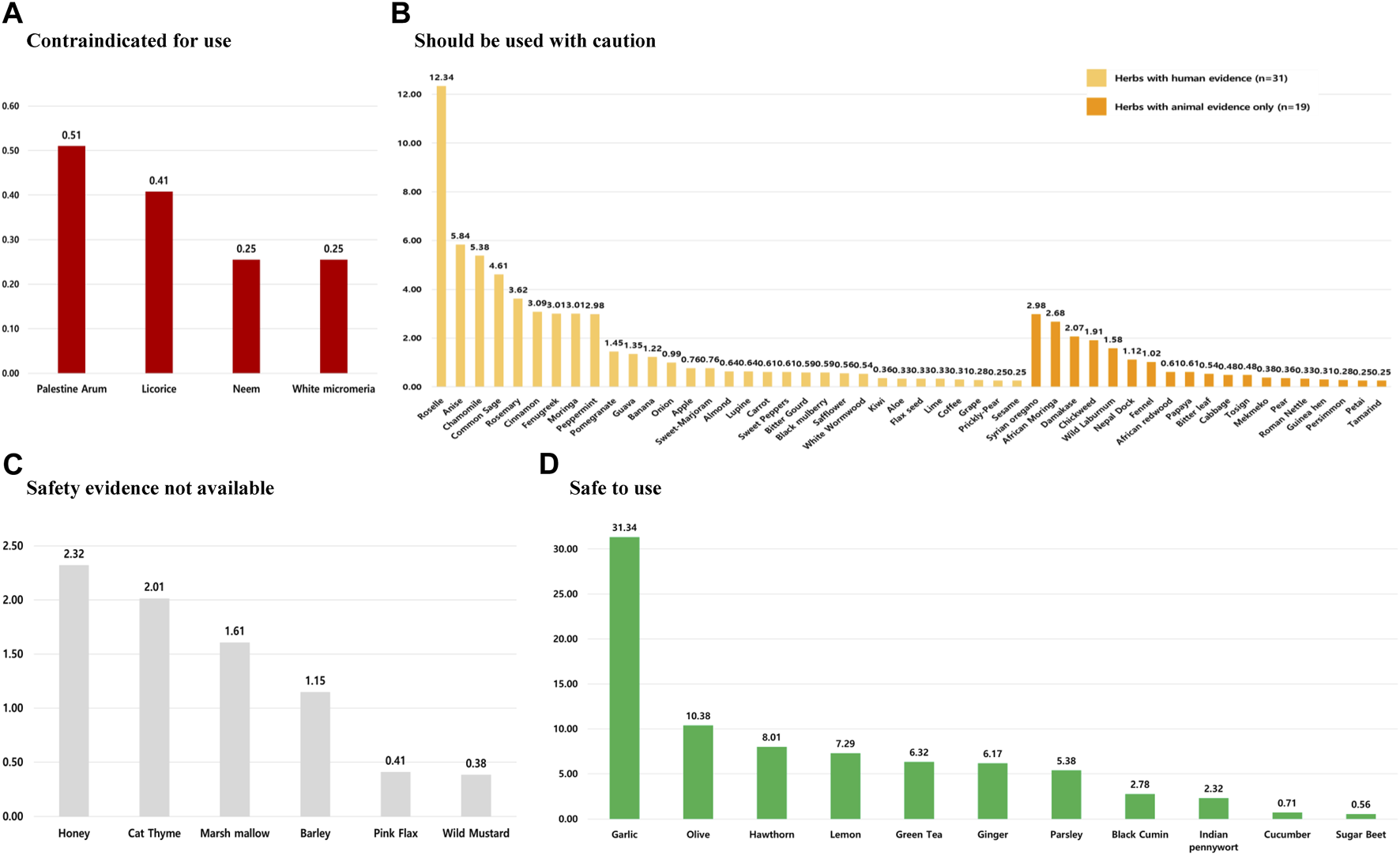
Prevalence of HMs used among hypertensive patients by safety classification. HMs in these sections are classified as follows: (A) can be potentially harmful to use, (B) should not be used without the supervision of a qualified healthcare practitioner, (C) safety evidence for herbal medicines not available, and (D) safe to use among hypertensive patients. *Note: The prevalence of each herb’s use was calculated by dividing the number of users for a particular herb by the total number of herbal medicine users.
4 Discussion
The present study presents the first systematic review and meta-analysis of HM use among HTN patients, identifying herbs commonly and globally used and assessing their safety based on current evidence. In total, 37 cross-sectional studies were included, with a combined sample size of 23,947 HTN patients. Of these, 37.8% reported using one or more types of HM during their treatment. The prevalence of HM utilization varied significantly by publication year and continent. Higher utilization rates were observed in studies published after 2011 (41.0%) than before 2011 (28.6%). Additionally, there were significant regional variations, with the highest use reported in North America (62.5%) and the lowest in Europe (15.7%). These regional differences may be attributed to various factors, including geographical characteristics, social and cultural influences, sociodemographic characteristics of study participants, and the quality of conventional therapy (Liwa et al., 2014; Peltzer and Pengpid, 2018; Azizah et al., 2021). Consequently, further research is warranted to explore the potential associations among these factors.
Patients with HTN commonly turn to HM to manage a range of physical conditions. The most frequently reported reasons for using HM include controlling blood pressure (BP), managing common comorbidities associated with HTN, and alleviating side effects from prescription drugs, such as frequent urination, headaches, and fatigue (Joshi et al., 2010; Olowofela and Isah, 2017). Additionally, some patients use HM to enhance their overall health status and wellbeing. The decision to use HM among HTN patients is often based on the belief that HM is safer and has fewer adverse effects than conventional antihypertensive drugs. Some patients opt for non-conventional treatment modalities due to concerns about the potential toxicity or dissatisfaction with conventional medicine in meeting their healthcare needs (Ahmed et al., 2017).
Despite the widespread use of HM among HTN patients, previous reviews on HM use among HTN patients have overlooked the evaluation of the safety of commonly used herbs. Thus, this study evaluated the safety profiles of the 71 most common HMs used by HTN patients, as identified in the included studies. The results revealed that four herbs were contraindicated for use, while 11 herbs were considered safe for use. Of the four herbs classified as contraindicated, two had no evidence of efficacy in lowering BP, and the other two herbs, G. glabra and A. indica, were supported only by a limited number of animal studies and had no clinical evidence of efficacy (Khoshnam and Bahaoddini, 2013; Shah et al., 2014). While these animal studies demonstrated potential benefits in controlling BP and managing common comorbidities associated with hypertension, numerous adverse effects were observed. For example, the use of G. glabra was associated with increased BP, hypokalemic-induced secondary disorders, rhabdomyolysis, acute renal failure, metabolic alkalosis, acute tubular necrosis, uremic, and paralysis in cases of chronic use (Van Uum, 2005; Sontia et al., 2008; Nazari et al., 2017; Penninkilampi et al., 2017; Kwon et al., 2020). Similarly, the misuse of A. indica was linked to severe stomatitis, marked oliguria, sanguineous vomiting, and even death (Wajdy et al., 2021). As a result, the consumption of these herbs or supplements containing their extracts should be avoided for HTN patients. These results stress the importance of safety in using HM for HTN management and highlight the need for evidence-based recommendations to enhance healthcare practices for HTN patients.
Amidst the potential risks, several herbs have been identified to provide clinical benefits while also being safe for use. A. sativum, used by 31.3% of HTN patients worldwide, is a popular HM used for managing HTN. Patients turn to A. sativum not only for HTN control but also for managing diabetes mellitus, alleviating prescription drug side effects, and improving overall health. Numerous studies and reviews have reported the antihypertensive effects of A. sativum, with a mean reduction in systolic/diastolic BP of 8.3/5.5 mmHg observed after administration of various A. sativum preparations and doses ranging from 600 mg/day to 1,200 mg/day over a median follow-up of 12 weeks (Shouk et al., 2014; Chrysant and Chrysant, 2017; Ried, 2020; EKİCİ et al., 2023). Its use has not been linked to any harmful events among HTN patients, although caution is advised with higher doses to prevent minor gastrointestinal disturbances (Matsutomo, 2020).
Similarly, O. europaea and C. oxyacantha are HMs that are commonly used by HTN patients and are known for their safety and efficacy in managing HTN at recommended doses, with no significant side effects reported (Susalit et al., 2011; Cloud et al., 2020; Venkatakrishnan et al., 2020; Ismail et al., 2021; Sun Y. et al., 2022; Johnson-Moore et al., 2023). The mechanism of action of O. europaea involves ACE inhibition, Ca2+ channel blockade, vasodilation, and antioxidant effects of flavonoids such as quercetin and rutin. Clinical trials administering 500 mg/day of O. europaea leaf extract versus placebo or no treatment resulted in a significant reduction in systolic/diastolic BP of 11.5/4.8 mmHg over 8 weeks (EKİCİ et al., 2023; Álvares et al., 2024). The beneficial effects of 900 mg/day C. oxyacantha on HTN have been consistently reported, where significant reductions in both SBP and DBP of approximately 17.2 mmHg and 9.2 mmHg, respectively, have been observed, especially when used for at least 12 weeks. These effects are primarily attributed to its flavonoids and oligomeric proanthocyanidins. Specifically, quercetin, the major polyphenolic flavonoid in C. oxyacantha, has shown efficacy in reducing BP through its antioxidant, anti-inflammatory, and vasorelaxant properties (Al-Gareeb, 2012; Cloud et al., 2020). Compounds found in C. limon (hesperidin and naringin) and C. sinensis (catechin) also act as vasodilators with antioxidant, anti-inflammatory, and antihypertensive properties, contributing to the use of HM in HTN patients (Peng et al., 2014; Rawat et al., 2016; David, 2017; Shilpa and Souza, 2020).
Fifty herbs have been identified for use with caution, as some of them have been associated with serious adverse effects, while others lack sufficient human evidence to determine their safety, particularly in HTN patients. H. sabdariffa, the second most commonly used HM among HTN patients, is frequently used to manage mild ailments and lower BP, and clinical literature reported no harmful effects in HTN patients (McKay et al., 2010; Serban et al., 2015). However, caution is still advised when using H. sabdariffa, as some studies reported diuretic effects and hepatotoxicity associated with high doses (Hopkins et al., 2013; Diallo et al., 2019). Also, P. anisum L. and Salvia officinalis L. are generally considered safe, but high doses or frequent intake of P. anisum seeds and its oil may cause nausea, vomiting, and pulmonary edema (Singletary, 2022), and excessive use of S. officinalis with high thujone content can lead to allergic reactions in some individuals (Mills and Bone, 2004; Hamidpour et al., 2014). In addition, although the consumption of Stellaria media L. tea and Foeniculum vulgare Mill. has not shown any toxicity or adverse events in animal studies, their safety and efficacy in HTN patients have yet to be investigated. As a result, it is recommended to use these herbs under the supervision of a qualified healthcare practitioner.
Finally, the safety of six HMs, such as honey, Teucrium polium L., and Althaea officinalis L., could not be established due to the lack of scientific research on their potential toxicity in humans (Reinelt and Melzig, 2017; Kianitalaei et al., 2019; Akhbari et al., 2021; Gholami et al., 2022). Therefore, it is recommended to avoid using these HMs until clinical evidence is available to ensure safety. In addition, certain substances that are commonly used in foods, such as honey, A. officinalis, and Hordeum vulgare L., should only be consumed in moderation and in amounts typical of culinary practices because excessive consumption of these HM may result in potential adverse effects. These findings emphasize the importance of conducting clinical studies to establish the toxicity and safety of commonly used herbs.
Before interpreting the findings of this systematic review, it is important to consider the following limitations. First, the reviewed studies were selected from three databases and restricted to articles published in the English language, which many potentially limit the generalizability of findings regarding the utilization of HM among HTN patients. Second, significant variations in sample size and study settings were observed among the reviewed studies, with the sample size ranging from 74 to 4,575 participants and the study settings varying from primary healthcare centers to tertiary teaching hospitals. The number of hospitals surveyed varied from a single institution to as many as 16 facilities (Mahfudz and Chan, 2005; Clement et al., 2007; Ali-Shtayeh et al., 2013; Boima et al., 2015).
The extent of HM use investigated also varied among the studies, with four studies excluded from the final analysis as they only investigated whether patients used HM or not and did not report the number of users for individual HM modalities (Mahfudz and Chan, 2005; Wazaify et al., 2013; Boima et al., 2015; Owusu et al., 2020). Lastly, due to variations in study quality, numerous studies were omitted from the final analysis during the risk of bias assessment phase. Initially, 48 studies from 25 countries were considered for the review, but after the quality assessment of the studies, 37 cross-sectional studies from 21 countries remained for the final analysis. Hence, it is essential to consider the discrepancies when interpreting the results of this review, and additional studies with a focus on methodological rigor are needed to achieve a more comprehensive understanding of HM use among HTN patients globally. Despite these limitations, our findings offer valuable insights into the safety profile of HMs commonly used by HTN patients, facilitating the development of evidence-based guidance for policymakers and healthcare providers involved in HTN management.
5 Conclusion
The use of HM among HTN patients is widespread globally. Our study emphasizes the significance of recognizing the risks of toxicities and adverse effects associated with HM use for HTN treatment. To prioritize patient safety, it is crucial to take only HM classified as safe in normal doses and avoid contraindicated HM. The safety classification in this review can raise awareness among physicians and healthcare providers regarding potential adverse effects in HTN patients. Future research should prioritize identifying commonly used herbs among HTN patients, particularly in resource-limited countries with poor HTN management. Conducting additional clinical studies is imperative to thoroughly evaluate the toxicity and safety of these medicinal plants.
Statements
Data availability statement
All data generated and analyzed, including study protocol, search strategy, list of included and excluded studies, extracted data, analysis plans, quality assessment, and assessment of publication bias, will be made available by the authors upon reasonable request. Requests to access these datasets should be directed to the corresponding author.
Author contributions
DC: conceptualization, data curation, formal analysis, methodology, writing–original draft, writing- review and editing, visualization, and software. HI: conceptualization, data curation, formal analysis, investigation, writing–review and editing, and software. SC: formal analysis, investigation, writing- review and editing. DH: conceptualization, supervision, writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to all the reviewers for their valuable comments and feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1321523/full#supplementary-material
Abbreviations
BP, blood pressure; CAM, complementary and alternative medicine; CI, confidence interval; DM, diabetes mellitus; HM, herbal medicine; HTN, hypertension; RCT, randomized controlled trial.
References
1
Abo-elmatty D. M. Essawy S. S. Badr J. M. Sterner O. (2013). Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type2 diabetic rats. J. Ethnopharmacol.145 (1), 269–277. 10.1016/j.jep.2012.11.002
2
Açıkgöz S. K. Açıkgöz E. Topal S. Okuyan H. Yaman B. Er O. et al (2014). Effect of herbal medicine use on medication adherence of cardiology patients. Complement. Ther. Med.22 (4), 648–654. 10.1016/j.ctim.2014.05.013
3
Adane F. Asres K. Ergete W. Woldekidan S. Abebe A. Lengiso B. et al (2021). Composition of the essential oil Thymus schimperi and evaluation of its acute and subacute toxicity in wistar albino rats: in silico toxicity studies. Evid. Based Complement. Altern. Med.2021, 5521302. 10.1155/2021/5521302
4
Adane F. Assefa W. Alem M. B. Dessalegn M. (2023). Sub-chronic toxicity of the aqueous leaf extract of Ocimum lamiifolium Hochst. ex Benth on biochemical parameters and histopathology of liver and kidney in rats: in vivo and in-silico toxicity studies. BMC Complement. Med. Ther.23 (1), 30. 10.1186/s12906-023-03863-7
5
Adeniyi O. Washington L. Glenn C. J. Franklin S. G. Scott A. Aung M. et al (2021). The use of complementary and alternative medicine among hypertensive and type 2 diabetic patients in Western Jamaica: a mixed methods study. PloS one16 (2), e0245163. 10.1371/journal.pone.0245163
6
Adidja N. M. Agbor V. N. Aminde J. A. Ngwasiri C. A. Ngu K. B. Aminde L. N. (2018). Non-adherence to antihypertensive pharmacotherapy in Buea, Cameroon: a cross-sectional community-based study. BMC Cardiovasc. Disord.18, 1–9. 10.1186/s12872-018-0888-z
7
Adokoh C. K. Asante D.-B. Acheampong D. O. Kotsuchibashi Y. Armah F. A. Sirikyi I. H. et al (2019). Chemical profile and in vivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicol. Rep.6, 692–702. 10.1016/j.toxrep.2019.06.020
8
Aekthammarat D. Pannangpetch P. Tangsucharit P. (2019). Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine54, 9–16. 10.1016/j.phymed.2018.10.023
9
Ahmad N. I. Rahman S. A. Leong Y.-H. Azizul N. H. (2019). A review on the phytochemicals of Parkia Speciosa, stinky beans as potential Phytomedicine. J. Food Sci. Nutr. Res.2 (3), 151–173. 10.26502/jfsnr.2642-11000017
10
Ahmed M. Hwang J. H. Choi S. Han D. (2017). Safety classification of herbal medicines used among pregnant women in Asian countries: a systematic review. BMC Complement. Altern. Med.17 (1), 489. 10.1186/s12906-017-1995-6
11
Ahmed-Farid O. Abdelrazek A. M. Elwakel H. Mohamed M. M. (2023). Hordeum vulgare ethanolic extract mitigates high salt-induced cerebellum damage via attenuation of oxidative stress, neuroinflammation, and neurochemical alterations in hypertensive rats. Metab. Brain Dis.38 (7), 2427–2442. 10.1007/s11011-023-01277-5
12
Ajebli M. Eddouks M. (2019). Antihypertensive activity of Petroselinum crispum through inhibition of vascular calcium channels in rats. J. Ethnopharmacol.242, 112039. 10.1016/j.jep.2019.112039
13
Ajijolakewu K. A. Ayoola A. S. Agbabiaka T. O. Zakariyah F. R. Ahmed N. R. Oyedele O. J. et al (2021). A review of the ethnomedicinal, antimicrobial, and phytochemical properties of Musa paradisiaca (plantain). Bull. Natl. Res. Cent.45 (1), 86. 10.1186/s42269-021-00549-3
14
Akaberi M. Hosseinzadeh H. (2016). Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother. Res.30 (4), 540–556. 10.1002/ptr.5570
15
Akhbari M. Jabbari M. Ayati M. H. Namazi N. (2021). The effects of oral consumption of honey on key metabolic profiles in adult patients with type 2 diabetes mellitus and nondiabetic individuals: a systematic review of clinical trials. Evid. Based Complement. Altern. Med.2021, 6666832. 10.1155/2021/6666832
16
Akhtar S. Rauf A. Imran M. Qamar M. Riaz M. Mubarak M. S. (2017). Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: a review. T Trends Food Sci. Technol.66, 36–47. 10.1016/j.tifs.2017.05.004
17
Al Batran R. Al-Bayaty F. Jamil Al-Obaidi M. M. Abdualkader A. M. Hadi H. A. Ali H. M. et al (2013). In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PloS one8 (5), e64751. 10.1371/journal.pone.0064751
18
Al Disi S. S. Anwar M. A. Eid A. H. (2016). Anti-hypertensive herbs and their mechanisms of action: part I. Front. Pharmacol.6, 323. 10.3389/fphar.2015.00323
19
Al-Gareeb A. I. A. (2012). Effect of hawthorn extract on blood pressure and lipid profile in patients with stage I hypertension: a placebo-controlled, double-blind randomized trial. Mustansiriya Med. J.11 (1), 52–57.
20
Al-Hadid D. Musa R. J. Al-Talhuni A. Alkrad J. A. (2020). Prevalence of traditional herbs and supplements use among hypertensive patients in om elamad health center. Pharmacogn. J.12 (6s), 1612–1622. 10.5530/pj.2020.12.221
21
Al-Hamdan N. Saeed A. Kutbi A. Choudhry A. J. Nooh R. (2011). Characteristics, risk factors, and treatment practices of known adult hypertensive patients in Saudi Arabia. Int. J. Hypertens.2010. 10.4061/2010/168739
22
Alshabi A. M. (2020). Knowledge and attitudes toward use of herbal medicine among Saudi Arabian Patients. Curr. Top. Nutraceutical Res.18 (4). 10.37290/ctnr2641-452X.18:303-309
23
Ali-Shtayeh M. S. Jamous R. M. Jamous R. M. Salameh N. M. (2013). Complementary and alternative medicine (CAM) use among hypertensive patients in Palestine. Complement. Ther. Clin. Pract.19 (4), 256–263. 10.1016/j.ctcp.2013.09.001
24
Aliwaini S. Lubbad A. M. (2016). Anti carcinogenic effect of roman nettle against chemical induced colon cancer in sprague–dawley rats. IUG J. Nat. Stud.24 (2).
25
Al-Qudah M. (2016). Histological and biochemical studies on liver of female rats treated with different concentrations of ethanolic extract of Arum palaestinum. J. Appl. Environ. Biol. Sci.6 (7), 1.
26
Al-Qura’n S. (2005). Ethnobotanical survey of folk toxic plants in southern part of Jordan. Toxicon46 (2), 119–129. 10.1016/j.toxicon.2005.04.010
27
Aluko E. O. Olubobokun T. H. Enobong I. B. Atang D. E. (2013). Comparative study of effect of honey on blood pressure and heart rate in healthy male and female subjects. Br. J. Med. Med. Res.3 (4), 2214–2221. 10.9734/bjmmr/2013/4152
28
Álvares A. A. Garcêz A. Silva L. T. Averbuch N. Garavaglia J. (2024). Olive leaf extract effect on cardiometabolic risk factors: a systematic review and meta-analysis of randomized clinical trials. Nutr. Rev., nuad164. 10.1093/nutrit/nuad164
29
Al-Waili N. (1986). Treatment of diabetes mellitus by Artemisia herba-alba extract: preliminary study. Clin. Exp. Pharmacol. Physiol.13 (7), 569–573. 10.1111/j.1440-1681.1986.tb00940.x
30
Amira O. C. Okubadejo N. U. (2007). Frequency of complementary and alternative medicine utilization in hypertensive patients attending an urban tertiary care centre in Nigeria. BMC Complement. Altern. Med.7, 30–35. 10.1186/1472-6882-7-30
31
Anand T. Joseph L. M. Geetha A. Prabhakaran D. Jeemon P. (2019). Task sharing with non-physician health-care workers for management of blood pressure in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob. Health7 (6), e761–e771. 10.1016/s2214-109x(19)30077-4
32
Andargie Y. Sisay W. Molla M. Norahun A. Singh P. (2022). Evaluation of the antiulcer activity of methanolic extract and solvent fractions of the leaves of Calpurnia aurea (Ait.) Benth.(Fabaceae) in rats. Evid. Based Complement. Altern. Med.2022, 4199284. 10.1155/2022/4199284
33
Anh N. H. Kim S. J. Long N. P. Min J. E. Yoon Y. C. Lee E. G. et al (2020). Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients12 (1), 157. 10.3390/nu12010157
34
Anwar M. A. Al Disi S. S. Eid A. H. (2016). Anti-hypertensive herbs and their mechanisms of action: part II. Front. Pharmacol.7 (50), 50–25. 10.3389/fphar.2016.00050
35
Arma M. R. Sumarni S. (2020). Effectiveness of beetroot (beta vulgaris L) extracts on blood pressure level among postpartum mothers with hypertension. Str. Health Sci. J.9 (2), 678–685. 10.30994/sjik.v9i2.356
36
Asfaw Erku D. Basazn Mekuria A. (2016). Prevalence and correlates of complementary and alternative medicine use among hypertensive patients in Gondar town, Ethiopia. Evid. Based Complement. Altern. Med.2016, 6987636. 10.1155/2016/6987636
37
Asiwe J. N. Kolawole T. A. Ben-Azu B. Ajayi A. M. Ojetola A. A. Moke E. G. et al (2022). Up-regulation of B-cell lymphoma factor-2 expression, inhibition of oxidative stress and down-regulation of pro-inflammatory cytokines are involved in the protective effect of cabbage (Brassica oleracea) juice in lead-induced endothelial dysfunction in rats. J. Trace Elem. Med. Biol.73, 127014. 10.1016/j.jtemb.2022.127014
38
Assad T. Khan R. A. Feroz Z. (2014). Evaluation of hypoglycemic and hypolipidemic activity of methanol extract of Brassica oleracea. Chin. J. Nat. Med.12 (9), 648–653. 10.1016/s1875-5364(14)60099-6
39
Assefa B. Glatzel G. Buchmann C. (2010). Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel. among rural communities of Ethiopia. J. Ethnobiol. Ethnomedicine6, 20–10. 10.1186/1746-4269-6-20
40
Astutik F. E. F. Zuhroh D. F. Ramadhan M. R. L. (2021). The effect of gotu kola (Centella asiatica L.) tea on blood pressure of hypertension. Enferm. Clin.31, S195–S198. 10.1016/j.enfcli.2020.12.021
41
Avello M. Jofre P. Pastene E. Fernandez P. (2014). Use of citrus limon, L.(lemon) in treating blood pressure sudden rises. Int. J. Pharmacogn. Phytochem. Res.6 (3), 606–611.
42
Awaad A. A. El‐Meligy R. M. Zain G. M. Safhi A. A. Al Qurain N. A. Almoqren S. S. et al (2018). Experimental and clinical antihypertensive activity of Matricaria chamomilla extracts and their angiotensin‐converting enzyme inhibitory activity. Phytother. Res.32 (8), 1564–1573. 10.1002/ptr.6086
43
Ayal G. Belay A. Kahaliw W. (2019). Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med.25 (1), 100151. 10.1016/j.wndm.2019.100151
44
Ayşe K. Ertuğrul K. (2020). Medical and cosmetic applications of persimmon (Diospyros Kaki): toxicity assessment-A review. Int. J. Tradit. Complement. Med. Res.1 (3), 162–176.
45
Azemi A. K. Nordin M. L. Hambali K. A. Noralidin N. A. Mokhtar S. S. Rasool A. H. G. (2022). Phytochemical contents and pharmacological potential of Parkia speciosa hassk. For diabetic vasculopathy: a review. Antioxidants11 (2), 431. 10.3390/antiox11020431
46
Azizah N. Halimah E. Puspitasari I. M. Hasanah A. N. (2021). Simultaneous use of herbal medicines and antihypertensive drugs among hypertensive patients in the community: a review. J. Multidiscip. Healthc.14, 259–270. 10.2147/jmdh.s289156
47
Bahadoran Z. Mirmiran P. Kabir A. Azizi F. Ghasemi A. (2017). The nitrate-independent blood pressure–lowering effect of beetroot juice: a systematic review and meta-analysis. Adv. Nutr.8 (6), 830–838. 10.3945/an.117.016717
48
Bahar Z. Kizilci S. Beser A. Besen D. B. Gördes N. Ersin F. et al (2013). Herbal therapies used by hypertensive patients in Turkey. Afr. J. Tradit. Complement. Altern. Med.10 (2), 292–298. 10.4314/ajtcam.v10i2.14
49
Bahmani M. Shirzad H. Mirhosseini M. Mesripour A. Rafieian-Kopaei M. (2016). A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L). J. Evid. Based Complement. Altern. Med.21 (1), 53–62. 10.1177/2156587215583405
50
Bakour M. Al-Waili N. El-Haskoury R. El-Menyiy N. Al-Waili T. Ali A. et al (2017). Comparison of hypotensive, diuretic and renal effects between cladodes of Opuntia ficus-indica and furosemide. Asian pac. J. Trop. Med.10 (9), 900–906. 10.1016/j.apjtm.2017.08.016
51
Baran A. K. Demirci H. Budak E. Candar A. Akpınar Y. (2017). What do people with hypertension use to reduce blood pressure in addition to conventional medication–Is this related to adherence?Eur. J. Integr. Med.13, 49–53. 10.1016/j.eujim.2017.07.004
52
Bardai S. E. Lyoussi B. Wibo M. Morel N. (2001). Pharmacological evidence of hypotensive activity of Marrubium vulgare and Foeniculum vulgare in spontaneously hypertensive rat. Clin. Exp. Hypertens.23 (4), 329–343. 10.1081/ceh-100102671
53
Basch E. Gabardi S. Ulbricht C. (2003). Bitter melon (Momordica charantia): a review of efficacy and safety. Am. J. Health-Syst. Pharm.60 (4), 356–359. 10.1093/ajhp/60.4.356
54
Basch E. Mphil S. B. Collins J. Dacey C. Harrison M. Szapary P. et al (2007). Flax and flaxseed oil (Linum usitatissimum): a review by. J. Soc. Integr. Oncol.5 (3), 92–105. 10.2310/7200.2007.005
55
Belsty T. Ekanem P. E. Gebremedhin G. Gebreselassie H. Kebede H. (2019). Evaluation of Rumex nepalensis Spreng. root extract on biochemical and histopathologic parameters of mice liver. J. Anat. Soc. India68 (3), 205–210. 10.4103/jasi.jasi_48_19
56
Benjamim C. J. R. Porto A. A. Valenti V. E. Sobrinho A. C. d.S. Garner D. M. Gualano B. et al (2022). Nitrate derived from beetroot juice lowers blood pressure in patients with arterial hypertension: a systematic review and meta-analysis. Front. Nutr.9, 823039. 10.3389/fnut.2022.823039
57
Benkhaira N. Ech-Chibani N. Fikri-Benbrahim K. (2021). Ethnobotanical survey on the medicinal usage of two common medicinal plants in Taounate Region: Artemisia herba-alba Asso and Ormenis mixta (L.) Dumort. Ethnobot. Res. Appl.22, 1–19. 10.32859/era.22.48.1-19
58
Birhanu Z. Wuhab M. Abula T. (2015). Antimalarial activity of Calpurnia aurea hydroalcoholic leaf extract in mice infected with Plasmodium berghei. PharmacologyOnLine2, 73–79.
59
Boima V. Ademola A. D. Odusola A. O. Agyekum F. Nwafor C. E. Cole H. et al (2015). Factors associated with medication nonadherence among hypertensives in Ghana and Nigeria. Int. J. Hypertens.2015, 205716. 10.1155/2015/205716
60
Bonilla Ocampo D. A. Paipilla A. F. Marín E. Vargas-Molina S. Petro J. L. Pérez-Idárraga A. (2018). Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules8 (4), 134. 10.3390/biom8040134
61
Bouyahya A. Chamkhi I. Benali T. Guaouguaou F.-E. Balahbib A. El Omari N. et al (2021). Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol.265, 113318. 10.1016/j.jep.2020.113318
62
Brasil G. A. Ronchi S. N. do Nascimento A. M. de Lima E. M. Romão W. da Costa H. B. et al (2014). Antihypertensive effect of Carica papaya via a reduction in ACE activity and improved baroreflex. Planta Med.80 (17), 1580–1587. 10.1055/s-0034-1383122
63
Bunaim M. K. Kamisah Y. Mohd Mustazil M. N. Fadhlullah Zuhair J. S. Juliana A. H. Muhammad N. (2021). Centella asiatica (L.) Urb. prevents hypertension and protects the heart in chronic nitric oxide deficiency rat model. Front. Pharmacol.12, 742562. 10.3389/fphar.2021.742562
64
Bunbupha S. Pakdeechote P. Maneesai P. Prachaney P. Boonprom P. (2019). Carthamus Tinctorius L. extract attenuates cardiac remodeling in L-NAME-induced hypertensive rats by inhibiting the NADPH oxidase-mediated TGF-β1 and MMP-9 pathway. Ann. Anat.222, 120–128. 10.1016/j.aanat.2018.12.006
65
Burnier M. Egan B. M. (2019). Adherence in hypertension: a review of prevalence, risk factors, impact, and management. Circ. Res.124 (7), 1124–1140. 10.1161/circresaha.118.313220
66
Cardoso C. A. Oliveira G. M. M. d. Gouveia L. d.A. V. Moreira A. S. B. Rosa G. (2018). The effect of dietary intake of sesame (Sesamumindicum L.) derivatives related to the lipid profile and blood pressure: a systematic review. Crit. Rev. Food Sci. Nutr.58 (1), 116–125. 10.1080/10408398.2015.1137858
67
Cesarone M. Belcaro G. De Sanctis M. Incandela L. Cacchio M. Bavera P. et al (2001). Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology52 (2_Suppl. l), S15–S18. 10.1177/000331970105202s04
68
Chakraborty A. J. Uddin T. M. Zidan B. R. M. Mitra S. Das R. Nainu F. et al (2022). Allium cepa: a treasure of bioactive phytochemicals with prospective health benefits. Evid. Based Complement. Altern. Med.2022, 4586318. 10.1155/2022/4586318
69
Chen S. Li J. Gao M. Li D. Shen R. Lyu L. et al (2022). Association of caffeine intake with all-cause and cardiovascular mortality in elderly patients with hypertension. Front. Nutr.9, 1023345. 10.3389/fnut.2022.1023345
70
Chrysant S. G. Chrysant G. S. (2017). Herbs used for the treatment of hypertension and their mechanism of action. Curr. Hypertens. Rep.19, 77–10. 10.1007/s11906-017-0775-5
71
Clement Y. N. Morton-Gittens J. Basdeo L. Blades A. Francis M.-J. Gomes N. et al (2007). Perceived efficacy of herbal remedies by users accessing primary healthcare in Trinidad. BMC Complement. Altern. Med.7 (1), 4–9. 10.1186/1472-6882-7-4
72
Cloud A. Vilcins D. McEwen B. (2020). The effect of hawthorn (Crataegus spp.) on blood pressure: a systematic review. Adv. Integr. Med.7 (3), 167–175. 10.1016/j.aimed.2019.09.002
73
Concato J. Shah N. Horwitz R. I. (2000). Randomized, controlled trials, observational studies, and the hierarchy of research designs. N. Engl. J. Med.342 (25), 1887–1892. 10.1056/nejm200006223422507
74
Costa E. S. França C. N. Fonseca F. A. Kato J. T. Bianco H. T. Freitas T. T. et al (2019). Beneficial effects of green banana biomass consumption in patients with pre-diabetes and type 2 diabetes: a randomised controlled trial. Br. J. Nutr.121 (12), 1365–1375. 10.1017/s0007114519000576
75
Cui W. Luo K. Xiao Q. Sun Z. Wang Y. Cui C. et al (2023). Effect of mulberry leaf or mulberry leaf extract on glycemic traits: a systematic review and meta-analysis. Food Funct.14 (3), 1277–1289. 10.1039/d2fo02645g
76
Cuzzolin L. Benoni G. (2009). Safety issues of phytomedicines in pregnancy and paediatrics. Herb. drugs ethnomedicine Mod. Med., 381–396. 10.1007/978-3-540-79116-4_21
77
Dai Y.-L. Li Y. Wang Q. Niu F.-J. Li K.-W. Wang Y.-Y. et al (2022). Chamomile: a review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules28 (1), 133. 10.3390/molecules28010133
78
Damtie D. Mekonnen Y. Eyado A. (2017). Acute oral toxicity study of Thymus serrulatus and Thymus schimperi from Ethiopia. Ethiop. J. Sci. Technol.10 (3), 181–192. 10.4314/ejst.v10i3.3
79
da Paixà T. P. Silva J. P. Oliveira F. R. Silva N. Santos P. C. Baetas A. C. et al (2016). In vitro and in vivo assessment of genotoxic activity of Petiveria alliacea. Afr. J. Pharm. Pharmacol.10 (34), 718–727. 10.5897/AJPP2016.4581
80
Da Silva M. V. B. dos Santos Barbosa G. da Rocha A. C. da Rocha D. da Silva T. A. da Silva J. A. et al (2022). Therapeutic potential of flavonoid-rich plants in the treatment of arterial hypertension and diabetes mellitus: focus on antioxidant role. Res. Soc. Dev.11 (8), e52911831364. 10.33448/rsd-v11i8.31364
81
David R. (2017). Effectiveness of lemon juice in reduction of blood pressure among people with essential hypertension in peelamedu, coimbator. Community Public Health Nurs.2 (2), 27–41. 10.21088/cphn.2455.8621.2217.4
82
Delgoda R. Younger N. Barrett C. Braithwaite J. Davis D. (2010). The prevalence of herbs use in conjunction with conventional medicines in Jamaica. Complement. Ther. Med.18 (1), 13–20. 10.1016/j.ctim.2010.01.002
83
Demján V. Sója A. Kiss T. Fejes A. Gausz F. D. Szűcs G. et al (2022). Stellaria media tea protects against diabetes-induced cardiac dysfunction in rats without affecting glucose tolerance. J. Tradit. Complement. Med.12 (3), 250–259. 10.1016/j.jtcme.2021.08.003
84
Dhar L. Earnest J. Ali M. (2017). A systematic review of factors influencing medication adherence to hypertension treatment in developing countries. Open J. Epidemiol.7, 211–250. 10.4236/ojepi.2017.73018
85
Diallo M. Traore M. Balde M. Camara A. Baldé E. Traore S. et al (2019). Prevalence, management and ethnobotanical investigation of hypertension in two Guinean urban districts. J. Ethnopharmacol.231, 73–79. 10.1016/j.jep.2018.07.028
86
Djordjević S. Nikolić N. Ć. (2021). Hawthorn (Crataegus spp.) from botanical source to phytopreparations. Lek. sirovine41, 63–71. 10.5937/leksir2141063d
87
Dosoky N. S. Setzer W. N. (2018). Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci.19 (7), 1966. 10.3390/ijms19071966
88
Duttaroy A. K. Jørgensen A. (2004). Effects of kiwi fruit consumption on platelet aggregation and plasma lipids in healthy human volunteers. Platelets15 (5), 287–292. 10.1080/09537100410001710290
89
Ekici E. Tuncay H. O. Akalin E. Bucak A. Y. Üresin U. Y. (2023). Evaluation of the efficacy, safety, and mechanism of action of plants traditionally used in the treatment of hypertension in Turkey. J. Herb. Med.43, 100835. 10.1016/j.hermed.2023.100835
90
Ekor M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.4, 177. 10.3389/fphar.2013.00177
91
El-Dahiyat F. Rashrash M. Abuhamdah S. Abu Farha R. Babar Z. U. D. (2020). Herbal medicines: a cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharm. Policy Pract.13 (1), 2. 10.1186/s40545-019-0200-3
92
Enejoh O. S. Ogunyemi I. O. Bala M. S. Oruene I. S. Suleiman M. M. Ambali S. F. (2015). Ethnomedical importance of Citrus aurantifolia (christm) swingle. Pharma Innov.4 (8), 1.
93
Eno A. Owo O. Itam E. Konya R. (2000). Blood pressure depression by the fruit juice of Carica papaya (L.) in renal and DOCA‐induced hypertension in the rat. Phytother. Res.14 (4), 235–239. 10.1002/1099-1573(200006)14:4<235::aid-ptr574>3.0.co;2-g
94
Erejuwa O. O. Sulaiman S. A. Wahab M. S. A. Sirajudeen K. N. Salleh M. S. M. Gurtu S. (2011). Differential responses to blood pressure and oxidative stress in streptozotocin-induced diabetic wistar-kyoto rats and spontaneously hypertensive rats: effects of antioxidant (Honey) treatment. Int. J. Mol. Sci.12 (3), 1888–1907. 10.3390/ijms12031888
95
Eslampour E. Asbaghi O. Hadi A. Abedi S. Ghaedi E. Lazaridi A.-V. et al (2020). The effect of almond intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med.50, 102399. 10.1016/j.ctim.2020.102399
96
Evania D. Punjastuti B. Yunitasari P. Maryati S. (2022). The impact of cucumber (cucumissativus) juice on blood pressure in elderly with hypertension. KnE Life Sci., 481–487. 10.18502/kls.v7i2.10346
97
Foshati S. Nouripour F. Sadeghi E. Amani R. (2022). The effect of grape (Vitis vinifera) seed extract supplementation on flow-mediated dilation, blood pressure, and heart rate: a systematic review and meta-analysis of controlled trials with duration-and dose-response analysis. Pharmacol. Res.175, 105905. 10.1016/j.phrs.2021.105905
98
García-Pérez M.-E. Alfonso-Castillo A. Lores O. F. Batista-Duharte A. Lemus-Rodríguez Z. (2018). Toxicological evaluation of an aqueous suspension from leaves and stems of Petiveria alliacea L.(Phytolaccaceae). J. Ethnopharmacol.211, 29–37. 10.1016/j.jep.2017.09.022
99
Gardner Z. McGuffin M. (2013). American Herbal Products Association’s botanical safety handbook. Boca Raton, FL: CRC Press.
100
Gayer B. A. Avendano E. E. Edelson E. Nirmala N. Johnson E. J. Raman G. (2019). Effects of intake of apples, pears, or their products on cardiometabolic risk factors and clinical outcomes: a systematic review and meta-Analysis. Curr. Dev. Nutr.3 (10), nzz109. 10.1093/cdn/nzz109
101
Gebrekidan A. Desta A. A. (2019). Assessment on the levels of selected essential and non-essential metals in sesame seeds (Sesamum indicum L.) collected from Sheraro town, Northwest Tigray, Ethiopia. Bull. Chem. Soc. Ethiop.33 (2), 191–202. 10.4314/bcse.v33i2.1
102
Geleta B. Makonnen E. Debella A. Tadele A. (2016). In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front. Pharmacol.7, 97. 10.3389/fphar.2016.00097
103
Getiye Y. Tolessa T. Engidawork E. (2016). Antihypertensive activity of 80% methanol seed extract of Calpurnia aurea (Ait.) Benth. subsp. aurea (Fabaceae) is mediated through calcium antagonism induced vasodilation. J. Ethnopharmacol.189, 99–106. 10.1016/j.jep.2016.04.056
104
Gholami Z. Sohrabi Z. Zare M. Pourrajab B. Nasimi N. (2022). The effect of honey on lipid profiles: a systematic review and meta-analysis of controlled clinical trials. Br. J. Nutr.127 (10), 1482–1496. 10.1017/s0007114521002506
105
Gohar F. Greenfield S. M. Beevers D. G. Lip G. Y. Jolly K. (2008). Self-care and adherence to medication: a survey in the hypertension outpatient clinic. BMC Complement. Altern. Med.8, 4. 10.1186/1472-6882-8-4
106
Gonfa Y. H. Beshah F. Tadesse M. G. Bachheti A. Bachheti R. K. (2021). Phytochemical investigation and potential pharmacologically active compounds of Rumex nepalensis: an appraisal. Beni-Suef Univ. J. Basic Appl. Sci.10 (1), 18–11. 10.1186/s43088-021-00110-1
107
Gruenwald J. Brendler T. Jaenicke C. (2007). PDR for Herbal Medicines. 4th Edition. Montvale, NJ: Thomson, Reuters.
108
Gu D.-T. Tung T.-H. Jiesisibieke Z. L. Chien C.-W. Liu W.-Y. (2022). Safety of cinnamon: an umbrella review of meta-analyses and systematic reviews of randomized clinical trials. Front. Pharmacol.12, 790901. 10.3389/fphar.2021.790901
109
Gunde M. C. Amnerkar N. D. (2016). Nutritional, medicinal and pharmacological properties of papaya (Carica papaya linn.): a review. J. Innov. Pharm. Biol. Sci.3 (1), 162–169.
110
Hadis M. Gebreyohannes Y. Gemeda N. (2020). Potential therapeutic uses of Moringa stenopetala: a scoping review. J. Glob. Health Sci.2 (2). 10.35500/jghs.2020.2.e26
111
Haj‐Husein I. Tukan S. Alkazaleh F. (2016). The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J. Hum. Nutr. Diet.29 (1), 105–111. 10.1111/jhn.12290
112
Hamidpour M. Hamidpour R. Hamidpour S. Shahlari M. (2014). Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med.4 (2), 82–88. 10.4103/2225-4110.130373
113
Hamrahian S. M. Maarouf O. H. Fülöp T. (2022). A critical review of medication adherence in hypertension: barriers and Facilitators Clinicians should consider. Patient prefer. Adherence16, 2749–2757. 10.2147/ppa.s368784
114
Han E. H. Lim M. K. Lee S. H. Rahman M. M. Lim Y.-H. (2019). An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var. saboten. BMC Complement. Altern. Med.19, 31–10. 10.1186/s12906-019-2442-7
115
Hariyanti R. Hadisaputro S. Sumarni S. Widyastuti E. (2020). The effectiveness of cucumber suri juice (cucumis sativus) on blood pressure in menopausal hypertension. Str. J. Ilm. Kesehat.9 (2), 1771–1778. 10.30994/sjik.v9i2.532
116
Hasani H. Arab A. Hadi A. Pourmasoumi M. Ghavami A. Miraghajani M. (2019). Does ginger supplementation lower blood pressure? A systematic review and meta‐analysis of clinical trials. Phytother. Res.33 (6), 1639–1647. 10.1002/ptr.6362
117
Hasimun D. P. Sulaeman A. Maharani I. D. P. (2022). Supplementation of Carica papaya leaves (Carica papaya L.) in nori preparation reduced blood pressure and arterial stiffness on hypertensive animal model. J. Young Pharm.12 (1), 63–66. 10.5530/jyp.2020.12.12
118
Hassani F. V. Shirani K. Hosseinzadeh H. (2016). Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn-Schmiedeb. Arch. Pharmacol.389, 931–949. 10.1007/s00210-016-1256-0
119
Hatmal M. Abderrahman S. Alsholi D. (2017). Determining the anti-tumor effects of differnt extracting methods of Arum palaestinum on different cancer cell lines by in vitro assay. Pharmacologyonline1, 28–45.
120
Hong S. J. Yoon S. Jo S. M. Jeong H. Youn M. Y. Kim Y. J. et al (2022). Olfactory stimulation by fennel (Foeniculum vulgare Mill.) essential oil improves lipid metabolism and metabolic disorders in high fat-induced obese rats. Nutrients14 (4), 741. 10.3390/nu14040741
121
Hopkins A. L. Lamm M. G. Funk J. L. Ritenbaugh C. (2013). Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia85, 84–94. 10.1016/j.fitote.2013.01.003
122
Hosseini A. Razavi B. M. Hosseinzadeh H. (2022). Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: a comprehensive review. Phytomedicine109, 154581. 10.1016/j.phymed.2022.154581
123
Hoy D. Brooks P. Woolf A. Blyth F. March L. Bain C. et al (2012). Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol.65 (9), 934–939. 10.1016/j.jclinepi.2011.11.014
124
Hu J. Webster D. Cao J. Shao A. (2018). The safety of green tea and green tea extract consumption in adults–results of a systematic review. Regul. Toxicol. Pharmacol.95, 412–433. 10.1016/j.yrtph.2018.03.019
125
Huang H. Zhou G. Pu R. Cui Y. Liao D. (2022). Clinical evidence of dietary supplementation with sesame on cardiovascular risk factors: an updated meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr.62 (20), 5592–5602. 10.1080/10408398.2021.1888689
126
Husein A. I. Ali-Shtayeh M. S. Jondi W. J. Zatar N.A.-A. Abu-Reidah I. M. Jamous R. M. (2014). In vitro antioxidant and antitumor activities of six selected plants used in the Traditional Arabic Palestinian herbal medicine. Pharm. Biol.52 (10), 1249–1255. 10.3109/13880209.2014.886274
127
Hutachok N. Koonyosying P. Pankasemsuk T. Angkasith P. Chumpun C. Fucharoen S. et al (2021). Chemical analysis, toxicity study, and free-radical scavenging and iron-binding assays involving coffee (Coffea arabica) extracts. Molecules26 (14), 4169. 10.3390/molecules26144169
128
Im H. B. Ghelman R. Portella C. F. S. Choi D. Han D. Kunwor S. K. et al (2023). Assessing the safety and use of medicinal herbs during pregnancy: a cross-sectional study in São Paulo, Brazil. Front. Pharmacol.14, 1268185. 10.3389/fphar.2023.1268185
129
Ishaq A. R. El-Nashar H. A. Younis T. Mangat M. A. Shahzadi M. Ul Haq A. S. et al (2022). Genus Lupinus (Fabaceae): a review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol.74 (12), 1700–1717. 10.1093/jpp/rgac058
130
Islas J. F. Acosta E. Zuca G. Delgado-Gallegos J. L. Moreno-Treviño M. G. Escalante B. et al (2020). An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods74, 104171. 10.1016/j.jff.2020.104171
131
Ismail M. A. Norhayati M. N. Mohamad N. (2021). Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: a systematic review and meta-analysis. PeerJ9, e11173. 10.7717/peerj.11173
132
James P. B. Kamara H. Bah A. J. Steel A. Wardle J. (2018a). Herbal medicine use among hypertensive patients attending public and private health facilities in Freetown Sierra Leone. Complement. Ther. Clin. Pract.31, 7–15. 10.1016/j.ctcp.2018.01.001
133
James P. B. Wardle J. Steel A. Adams J. (2018b). Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Glob. Health3 (5), e000895. 10.1136/bmjgh-2018-000895
134
James-Martin G. Williams G. Stonehouse W. O’Callaghan N. Noakes M. (2015). Health and nutritional properties of pears (Pyrus): a literature review. Adelaide: CSIRO.
135
Jang H.-H. Lee J. Lee S.-H. Lee Y.-M. (2020). Effects of Capsicum annuum supplementation on the components of metabolic syndrome: a systematic review and meta-analysis. Sci. Rep.10 (1), 20912. 10.1038/s41598-020-77983-2
136
Joachimdass R. J. Subramaniam K. Sit N. W. Lim Y. M. Teo C. H. Ng C. J. et al (2021). Self-management using crude herbs and the health-related quality of life among adult patients with hypertension living in a suburban setting of Malaysia. Plos one16 (9), e0257336. 10.1371/journal.pone.0257336
137
Johnston D. (2006). Natural standard herb and supplement reference: evidence based clinical reviews. Ulst. Med. J.75, 166.
138
Johnson S. A. Navaei N. Pourafshar S. Akhavan N. S. Elam M. L. Foley E. et al (2016). Fresh pear (Pyrus communis) consumption may improve blood pressure in middle‐aged men and women with metabolic syndrome. FASEB J.30, 1175.1112. 10.1096/fasebj.30.1_supplement.1175.12
139
Johnson-Moore T. Pooler D. Hooker H. Crentsil N. Kas-Osoka U. Fullas F. et al (2023). A review of the uses of five dietary supplements in the management of hypertension and a composite survey. Int. J. Sci. Res. Arch. (IJSRA)8 (02), 074–084. 10.30574/ijsra.2023.8.2.0207
140
Joshi V. D. Dahake A. P. Suthar A. P. (2010). Adverse effects associated with the use of antihypertensive drugs: an overview. Int. J. Pharm. Tech. Res.2, 10–13.
141
Kamath J. Rahul N. Kumar C. A. Lakshmi S. M. (2008). Psidium guajava L: a review. Int. J. Green Pharm.2 (1), 9. 10.4103/0973-8258.39155
142
Kamyab R. Namdar H. Torbati M. Ghojazadeh M. Araj-Khodaei M. Fazljou S. M. B. (2021). Medicinal plants in the treatment of hypertension: a review. Adv. Pharm. Bull.11 (4), 601–617. 10.34172/apb.2021.090
143
Karimi Z. Firouzi M. Dadmehr M. Javad‐Mousavi S. A. Bagheriani N. Sadeghpour O. (2021). Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: a narrative review. Phytother. Res.35 (6), 2997–3012. 10.1002/ptr.7006
144
Karimzadeh L. Behrouz V. Sohrab G. Hedayati M. Emami G. (2022). A randomized clinical trial of beetroot juice consumption on inflammatory markers and oxidative stress in patients with type 2 diabetes. J. Food Sci.87 (12), 5430–5441. 10.1111/1750-3841.16365
145
Kaur L. (2019). A review on antihypertensive properties of Raphanus sativus, Daucus carota and Tribulus terrestris. J. Crit. Rev.6 (6), 2019.
146
Kawakami K. Yamada K. Takeshita H. Yamada T. Nomura M. (2021). Antihypertensive effect of lemon juice squeezed residue on spontaneously hypertensive rats. F. Food Sci. Technol. Res.27 (3), 521–527. 10.3136/fstr.27.521
147
Kearney P. M. Whelton M. Reynolds K. Muntner P. Whelton P. K. He J. (2005). Global burden of hypertension: analysis of worldwide data. Lancet365 (9455), 217–223. 10.1016/S0140-6736(05)17741-1
148
Kennedy D. Lupattelli A. Koren G. Nordeng H. (2016). Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement. Altern. Med.16, 102–109. 10.1186/s12906-016-1079-z
149
Khoshnam S. E. Bahaoddini A. (2013). The effect of hydro-alcoholic extract of Glycyrrhiza glabra on the cardiovascular system of male rats with normal blood pressure and its interaction with cholinergic and adrenergic systems. Physiol. Pharmacol.17 (3), 349–358.
150
Kianitalaei A. Feyzabadi Z. Hamedi S. Qaraaty M. (2019). Althaea officinalis in traditional medicine and modern phytotherapy. J. Adv. Pharm. Educ. Res.9 (S2), 154–161.
151
Kifle Z. D. Belayneh Y. M. (2020). Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of Hagenia abyssinica (Rosaceae) leaves in streptozotocin-induced diabetic mice. Diabetes Metab. Syndr. Obes.13, 4085–4094. 10.2147/dmso.s279475
152
Kifle Z. D. Yesuf J. S. Atnafie S. A. (2020). Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of Hagenia abyssinica (rosaceae). J. Exp. Pharmacol.12, 151–167. 10.2147/jep.s249964
153
Kifle Z. D. Yimenu D. K. Kidanu B. B. (2021). Complementary and alternative medicine use and its associated factors among hypertensive patients in Debre Tabor General Hospital, Ethiopia. Metabol. Open12, 100132. 10.1016/j.metop.2021.100132
154
Kim B. Lee H. S. Kim H.-J. Lee H. Lee I.-y. Ock S. et al (2023a). Momordica charantia (bitter melon) efficacy and safety on glucose metabolism in Korean prediabetes participants: a 12-week, randomized clinical study. Food Sci. Biotechnol.32 (5), 697–704. 10.1007/s10068-022-01214-9
155
Kim H.-L. Lee E. M. Ahn S. Y. Kim K.-i. Kim H. C. Kim J. H. et al (2023b). The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin. Hypertens.29 (1), 11. 10.1186/s40885-023-00234-9
156
Kim S. J. Anh N. H. Jung C. W. Long N. P. Park S. Cho Y. H. et al (2022). Metabolic and cardiovascular benefits of apple and apple-derived products: a systematic review and meta-analysis of randomized controlled trials. Front. Nutr.9, 766155. 10.3389/fnut.2022.766155
157
Kim S. K. Jung J. Jung J. H. Yoon N. Kang S. S. Roh G. S. et al (2020). Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement. Ther. Med.52, 102524. 10.1016/j.ctim.2020.102524
158
Kmail A. Jaradat N. Mansour B. Abu-Labdeh R. Zakarneh S. Abu-Farha S. et al (2022). Phytochemical analysis, cytostatic, cytotoxic, and anti-inflammatory effects of Arum palaestinum, Ocimum basilicum, and Trigonella foenum-graecum in human monocytic cell line (THP-1)-derived macrophages. Eur. J. Integr. Med.54, 102159. 10.1016/j.eujim.2022.102159
159
Komakech R. Kim Y.-g. Matsabisa G. M. Kang Y. (2019). Anti-inflammatory and analgesic potential of Tamarindus indica Linn.(Fabaceae): a narrative review. Integr. Med. Res.8 (3), 181–186. 10.1016/j.imr.2019.07.002
160
Koya S. F. Pilakkadavath Z. Chandran P. Wilson T. Kuriakose S. Akbar S. K. et al (2023). Hypertension control rate in India: systematic review and meta-analysis of population-level non-interventional studies, 2001–2022. Lancet Reg. Health Southeast Asia9, 100113. 10.1016/j.lansea.2022.100113
161
Kretchy I. A. Owusu-Daaku F. Danquah S. (2014). Patterns and determinants of the use of complementary and alternative medicine: a cross-sectional study of hypertensive patients in Ghana. BMC Complement. Altern. Med.14 (1), 44–47. 10.1186/1472-6882-14-44
162
Krishna R. N. Anitha R. Ezhilarasan D. (2020). Aqueous extract of Tamarindus indica fruit pulp exhibits antihyperglycaemic activity. Avicenna J. Phytomed.10 (5), 440–447.
163
Krousel-Wood M. Thomas S. Muntner P. Morisky D. (2004). Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr. Opin. Cardiol.19 (4), 357–362. 10.1097/01.hco.0000126978.03828.9e
164
Krousel‐Wood M. A. Muntner P. Joyce C. J. Islam T. Stanley E. Holt E. W. et al (2010). Adverse effects of complementary and alternative medicine on antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J. Am. Geriatr. Soc.58 (1), 54–61. 10.1111/j.1532-5415.2009.02639.x
165
Kuru P. (2014). Tamarindus indica and its health related effects. Asian pac. J. Trop. Biomed.4 (9), 676–681. 10.12980/apjtb.4.2014apjtb-2014-0173
166
Kwon Y.-J. Son D.-H. Chung T.-H. Lee Y.-J. (2020). A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J. Med. Food23 (1), 12–20. 10.1089/jmf.2019.4459
167
Laurindo L. F. Barbalho S. M. Marquess A. R. Grecco A. I. d.S. Goulart R. d.A. Tofano R. J. et al (2022). Pomegranate (Punica granatum L.) and metabolic syndrome risk factors and outcomes: a systematic review of clinical studies. Nutrients14 (8), 1665. 10.3390/nu14081665
168
Leong X.-F. Rais Mustafa M. Jaarin K. (2013). Nigella sativa and its protective role in oxidative stress and hypertension. Evid. Based Complement. Altern. Med.2013, 120732. 10.1155/2013/120732
169
Li X. Peng M. Li Y. Kang Z. Hao Y. Sun H. et al (2015). Chinese herbal therapy and western drug use, belief and adherence for hypertension management in the rural areas of Heilongjiang province, China. PLoS One10, e0123508. 10.1371/journal.pone.0123508
170
Lim S. H. Choi C.-I. (2019). Pharmacological properties of Morus nigra L.(black mulberry) as a promising nutraceutical resource. Nutrients11 (2), 437. 10.3390/nu11020437
171
Liu C. Kurakane S. Takita J. Itano R. Soga T. Oikawa A. et al (2012). Antihypertensive effects of unripe persimmon (Diospyros kaki L. cv. Hiratanenashi) fruit and its component in spontaneously hypertensive rats. Food Sci. Technol. Res.18 (3), 391–398. 10.3136/fstr.18.391
172
Liwa A. Roediger R. Jaka H. Bougaila A. Smart L. Langwick S. et al (2017). Herbal and alternative medicine use in Tanzanian adults admitted with hypertension-related diseases: a mixed-methods study. Int. J. Hypertens.2017, 5692572. 10.1155/2017/5692572
173
Liwa A. C. Smart L. R. Frumkin A. Epstein H.-A. B. Fitzgerald D. W. Peck R. N. (2014). Traditional herbal medicine use among hypertensive patients in sub-Saharan Africa: a systematic review. Curr. Hypertens. Rep.16, 437–439. 10.1007/s11906-014-0437-9
174
Lucius K. (2022). Integrative medicine in the management of hypertension. Integr. Complement. Ther.28 (5), 240–250. 10.1089/ict.2022.29043.klu
175
Lulebo A. M. Mapatano M. A. Mutombo P. B. Mafuta E. M. Samba G. Coppieters Y. (2017). Prevalence and determinants of use of complementary and alternative medicine by hypertensive patients attending primary health care facilities in Kinshasa, Democratic Republic of the Congo: a cross-sectional study. BMC Complement. Altern. Med.17, 1–9. 10.1186/s12906-017-1722-3
176
Luo B. Mohammad W. T. Jalil A. T. Saleh M. M. Al-Taee M. M. Alshahrani M. Y. et al (2023). Effects of almond intake on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Complement. Ther. Med.73, 102935. 10.1016/j.ctim.2023.102935
177
Luo L. Wang B. Jiang J. Fitzgerald M. Huang Q. Wei J. et al (2021). Heavy metal contaminations in herbal medicines: determination, comprehensive risk assessments, and solutions. Front. Pharmacol.11, 595335. 10.3389/fphar.2020.595335
178
Luz D. A. Pinheiro A. M. Silva M. L. Monteiro M. C. Prediger R. D. Maia C. S. F. et al (2016). Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L.(Phytolaccaceae): a review. J. Ethnopharmacol.185, 182–201. 10.1016/j.jep.2016.02.053
179
Mahdavi-Roshan M. Salari A. Ghorbani Z. Ashouri A. (2020). The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: a systematic review and meta-analysis. Complement. Ther. Med.51, 102430. 10.1016/j.ctim.2020.102430
180
Mahendran G. Rahman L. U. (2020). Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha× piperita L.)—a review. Phytother. Res.34 (9), 2088–2139. 10.1002/ptr.6664
181
Mahfudz A. Chan S. (2005). Use of complementary medicine amongst hypertensive patients in a public primary care clinic in Ipoh. Med. J. Malays.60 (4), 454–459.
182
Majeed U. Shafi A. Majeed H. Akram K. Liu X. Ye J. et al (2022). Grape (Vitis vinifera L.) phytochemicals and their biochemical protective mechanisms against leading pathologies. Food Chem.134762, 134762. 10.1016/j.foodchem.2022.134762
183
Malekmohammad K. Rafieian-Kopaei M. Sardari S. Sewell R. D. (2021). Toxicological effects of Mentha x piperita (peppermint): a review. Toxin Rev.40 (4), 445–459. 10.1080/15569543.2019.1647545
184
Martins M. Ribeiro M. H. Miranda A. Lopes S. Franco R. Paiva J. et al (2023). New foods with history: nutritional and toxic profile of prickly pear. J. Food Meas. Charact.17 (1), 956–972. 10.1007/s11694-022-01680-z
185
Matsutomo T. (2020). Potential benefits of garlic and other dietary supplements for the management of hypertension. Exp. Ther. Med.19 (2), 1479–1484. 10.3892/etm.2019.8375
186
McKay D. L. Chen C. O. Saltzman E. Blumberg J. B. (2010). Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr.140 (2), 298–303. 10.3945/jn.109.115097
187
McMahon F. G. Cole P. A. Ryan J. R. (1973). A study of hypertension in the inner city. A student hypertension survey. Am. Heart J.85 (1), 65–71. 10.1016/0002-8703(73)90526-7
188
Meher B. Dash D. K. Roy A. (2014). A review on: phytochemistry, pharmacology and traditional uses of Tamarindus indica L. World J. Pharm. Pharm. Sci.3 (10), 229–240.
189
Mekonnen T. Urga K. Engidawork E. (2010). Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J. Ethnopharmacol.127 (2), 433–439. 10.1016/j.jep.2009.10.020
190
Melesie Taye G. Bule M. Alemayehu Gadisa D. Teka F. Abula T. (2020). In vivo antidiabetic activity evaluation of aqueous and 80% methanolic extracts of leaves of Thymus schimperi (Lamiaceae) in alloxan-induced diabetic mice. Diabetes Metab. Syndr. Obes.13, 3205–3212. 10.2147/dmso.s268689
191
Mengistu M. Abebe Y. Mekonnen Y. Tolessa T. (2012). In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. Afr. Health Sci.12 (4), 545–551. 10.4314/ahs.v12i4.23
192
Mequanint W. Makonnen E. Urga K. (2011). In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J. Ethnopharmacol.134 (1), 32–36. 10.1016/j.jep.2010.11.051
193
Mesmar J. Abdallah R. Badran A. Maresca M. Baydoun E. (2022). Origanum syriacum phytochemistry and pharmacological properties: a comprehensive review. Molecules27 (13), 4272. 10.3390/molecules27134272
194
Mills K. T. Bundy J. D. Kelly T. N. Reed J. E. Kearney P. M. Reynolds K. et al (2016). Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation134 (6), 441–450. 10.1161/circulationaha.115.018912
195
Mills K. T. Stefanescu A. He J. (2020). The global epidemiology of hypertension. Nat. Rev. Nephrol.16 (4), 223–237. 10.1038/s41581-019-0244-2
196
Mills S. Y. Bone K. (2004). The essential guide to herbal safety. Elsevier Health Sciences.
197
Mirmiran P. Houshialsadat Z. Gaeini Z. Bahadoran Z. Azizi F. (2020). Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab.17, 3–15. 10.1186/s12986-019-0421-0
198
Mohajeri G. Safaee M. Sanei M. H. (2014). Effects of topical Kiwifruit on healing of neuropathic diabetic foot ulcer. J. Res. Med. Sci.19 (6), 520–524.
199
Mohammed Nawi A. Mohammad Z. Jetly K. Abd Razak M. A. Ramli N. S. Wan Ibadullah W. A. H. et al (2021). The prevalence and risk factors of hypertension among the urban population in southeast asian countries: a systematic review and meta-analysis. Int. J. Hypertens.2021, 6657003–6657014. 10.1155/2021/6657003
200
Moher D. Liberati A. Tetzlaff J. Altman D. G. PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med.151 (4), 264–W64. 10.7326/0003-4819-151-4-200908180-00135
201
Mohsen Ibrahim M. (2018). Hypertension in developing countries: a major challenge for the future. Curr. Hypertens. Rep.20, 38–10. 10.1007/s11906-018-0839-1
202
Mollaoğlu M. Aciyurt A. (2013). Use of complementary and alternative medicine among patients with chronic diseases. Acta Clin. Croat. 52 (2), 181–188.
203
Mondal A. Banerjee S. Bose S. Das P. P. Sandberg E. N. Atanasov A. G. et al (2021). Cancer preventive and therapeutic potential of banana and its bioactive constituents: a systematic, comprehensive, and mechanistic review. Front. Oncol.11, 697143. 10.3389/fonc.2021.697143
204
Musharraf H. M. Arman M. S. I. (2018). Prophetic medicine is the cheapest, safest and the best remedy in the prevention and treatment of hypertension (high blood pressure)–a mini review. Int. J. Mol. Biol. Open Access3 (6), 245–250. 10.15406/ijmboa.2018.03.00084
205
Nassiri‐Asl M. Hosseinzadeh H. (2009). Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytotherapy Res.23 (9), 1197–1204. 10.1002/ptr.2761
206
Nassiri‐Asl M. Hosseinzadeh H. (2016). Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: an update. Phytother. Res.30 (9), 1392–1403. 10.1002/ptr.5644
207
Navaei N. Pourafshar S. Akhavan N. S. Litwin N. S. Foley E. M. George K. S. et al (2019). Influence of daily fresh pear consumption on biomarkers of cardiometabolic health in middle-aged/older adults with metabolic syndrome: a randomized controlled trial. Food Funct.10 (2), 1062–1072. 10.1039/c8fo01890a
208
Nazari S. Rameshrad M. Hosseinzadeh H. (2017). Toxicological effects of Glycyrrhiza glabra (licorice): a review. Phytother. Res.31 (11), 1635–1650. 10.1002/ptr.5893
209
Niazmand S. Esparham M. Hassannia T. Derakhshan M. (2011). Cardiovascular effects of Teucrium polium L. extract in rabbit. Pharmacogn. Mag.7 (27), 260–264. 10.4103/0973-1296.84244
210
Nikkhah Bodagh M. Maleki I. Hekmatdoost A. (2019). Ginger in gastrointestinal disorders: a systematic review of clinical trials. Food Sci. Nutr.7 (1), 96–108. 10.1002/fsn3.807
211
Nur N. (2010). Knowledge and behaviours related to herbal remedies: a cross-sectional epidemiological study in adults in Middle Anatolia, Turkey. Health Soc. Care Community18 (4), 389–395. 10.1111/j.1365-2524.2010.00911.x
212
Nuwaha F. Musinguzi G. (2013). Use of alternative medicine for hypertension in Buikwe and Mukono districts of Uganda: a cross sectional study. BMC Complement. Altern. Med.13, 301–306. 10.1186/1472-6882-13-301
213
Olaiya C. Choudhary M. Ogunyemi O. Nwauzoma A. (2013). Nutraceuticals from bitter leaf (Vernonia amygdalina Del.) protects against cadmium chloride induced hypertension in albino rats. Nat. Sci.11 (6), 136–145.
214
Olisa N. S. Oyelola F. T. (2009). Evaluation of use of herbal medicines among ambulatory hypertensive patients attending a secondary health care facility in Nigeria. Int. J. Pharm. Pract.17 (2), 101–105. 10.1211/ijpp.17.02.0005
215
Olowofela A. O. Isah A. O. (2017). A profile of adverse effects of antihypertensive medicines in a tertiary care clinic in Nigeria. Ann. Afr. Med.16 (3), 114–119. 10.4103/aam.aam_6_17
216
Onakpoya I. J. O'Sullivan J. Heneghan C. J. (2015). The effect of cactus pear (Opuntia ficus-indica) on body weight and cardiovascular risk factors: a systematic review and meta-analysis of randomized clinical trials. Nutrition31 (5), 640–646. 10.1016/j.nut.2014.11.015
217
Onyema-iloh O. Meludu S. Iloh E. Dioka C. Obi-Ezeani C. (2018). Effects of methanolic extract of Vernonia amygdalina on electrolytes and renal biomarkers in NaCl–induced hypertensive male wistar rats. J. Pharm. Res. Int.23 (1), 1–7. 10.9734/jpri/2018/41908
218
Orgah J. O. He S. Wang Y. Jiang M. Wang Y. Orgah E. A. et al (2020). Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res.153, 104654. 10.1016/j.phrs.2020.104654
219
Osamor P. E. Owumi B. E. (2010). Complementary and alternative medicine in the management of hypertension in an urban Nigerian community. BMC Complement. Altern. Med.10, 36–39. 10.1186/1472-6882-10-36
220
Osuna-Martínez U. Reyes-Esparza J. Rodríguez-Fragoso L. (2014). Cactus (Opuntia ficus-indica): a review on its antioxidants properties and potential pharmacological use in chronic diseases. Nat. Prod. Chem. Res.2, 153. 10.4172/2329-6836.1000153
221
Owusu S. Gaye Y.-E. Hall S. Junkins A. Sohail M. Franklin S. et al (2020). Factors associated with the use of complementary and alternative therapies among patients with hypertension and type 2 diabetes mellitus in Western Jamaica: a cross-sectional study. BMC Complement. Med. Ther.20 (1), 314. 10.1186/s12906-020-03109-w
222
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg.88, 105906. 10.1016/j.ijsu.2021.105906
223
Palatini P. Fania C. Mos L. Garavelli G. Mazzer A. Cozzio S. et al (2016). Coffee consumption and risk of cardiovascular events in hypertensive patients. Results from the HARVEST. Int. J. Cardiol.212, 131–137. 10.1016/j.ijcard.2016.03.006
224
Palileo-Villanueva L. M. Palafox B. Amit A. M. L. Pepito V. C. F. Ab-Majid F. Ariffin F. et al (2022). Prevalence, determinants and outcomes of traditional, complementary and alternative medicine use for hypertension among low-income households in Malaysia and the Philippines. BMC Complement. Med. Ther.22 (1), 252. 10.1186/s12906-022-03730-x
225
Park S. W. Shin K. C. Yoou S.-K. Park H. J. Eun S. H. Bae Y. M. et al (2019). Effects of an ethanolic extract of mulberry fruit on blood pressure and vascular remodeling in spontaneous hypertensive rats. Clin. Exp. Hypertens.41 (3), 280–286. 10.1080/10641963.2018.1469645
226
Pathak L. Kothiyal P. (2013). Antihypertensive drugs interaction with herbal medicine—review. Int. J. Pharm. Phytopharm. Res.3 (2), 139–143.
227
Patil R. A. Lokwani P. S. Amrutkar S. V. (2022). Phytochemical evaluation and antihypertensive activity of Malus domestica peel in experimental animals. Pharmacophore13 (3), 1–7. 10.51847/ectmxxm8iw
228
Peltzer K. Pengpid S. (2018). Prevalence and determinants of traditional, complementary and alternative medicine provider use among adults from 32 countries. Chin. J. Integr. Med.24, 584–590. 10.1007/s11655-016-2748-y
229
Peng X. Zhou R. Wang B. Yu X. Yang X. Liu K. et al (2014). Effect of green tea consumption on blood pressure: a meta-analysis of 13 randomized controlled trials. Sci. Rep.4 (1), 6251. 10.1038/srep06251
230
Penninkilampi R. Eslick E. Eslick G. (2017). The association between consistent licorice ingestion, hypertension and hypokalaemia: a systematic review and meta-analysis. J. Hum. Hypertens.31 (11), 699–707. 10.1038/jhh.2017.45
231
Popoola J. O. Aworunse O. S. Oyesola O. L. Akinnola O. O. Obembe O. O. (2020). <i/>A systematic review of pharmacological activities and safety ofMoringa oleifera. J. Herbmed Pharmacol.9 (3), 174–190. 10.34172/jhp.2020.24
232
Posadzki P. Watson L. Ernst E. (2013). Herb–drug interactions: an overview of systematic reviews. Br. J. Clin. Pharmacol.75 (3), 603–618. 10.1111/j.1365-2125.2012.04350.x
233
Prasad S. Hima R. Trainee P. S. (2020). Efficacy of supplementation of kiwi fruit juice on selected hypertensive adult patients of Suchindrum in Kanniyakumari District. Int. J. Adv. Res. Eng. Technol.7 (5), 2394–3785.
234
Punoševac M. Radović J. Leković A. Kundaković-Vasović T. (2021). A review of botanical characteristics, chemical composition, pharmacological activity and use of parsley. Arh. Farm.71 (Notebook 3), 177–196. 10.5937/arhfarm71-30800
235
Putri A. A. Mukaromah I. Pratiwi N. D. Alivian G. N. (2022). Utilization of African Leaves (Vernonia amygdalina) to lower blood pressure in patients with hypertension: a systematic review. Int. J. Biomed. Nurs. Rev.1 (1), 52–56. 10.20884/1.ijbnr.2022.1.1.6534
236
Radha M. H. Laxmipriya N. P. (2015). Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J. Tradit. Complement. Med.5 (1), 21–26. 10.1016/j.jtcme.2014.10.006
237
Rahimi R. Ardekani M. R. S. (2013). Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin. J. Integr. Med.19, 73–79. 10.1007/s11655-013-1327-0
238
Rahmawati R. Bajorek B. V. (2017). Self-medication among people living with hypertension: a review. Fam. Pract.34 (2), 147–153. 10.1093/fampra/cmw137
239
Rambod M. Rakhshan M. Tohidinik S. Nikoo M. H. (2020). The effect of lemon inhalation aromatherapy on blood pressure, electrocardiogram changes, and anxiety in acute myocardial infarction patients: a clinical, multi-centered, assessor-blinded trial design. Complement. Ther. Clin. Pract.39, 101155. 10.1016/j.ctcp.2020.101155
240
Rana R. Mehmood M. H. Shaukat B. Shahid S. Malik A. Murtaza B. (2022). Evaluation of the cardiovascular effects of Coriandrum sativum and Citrus limon to treat arsenic-Induced endothelial damage and hypertension in rats. Life12 (11), 1842. 10.3390/life12111842
241
Rawat P. Singh P. K. Kumar V. (2016). Anti-hypertensive medicinal plants and their mode of action. J. Herb. Med.6 (3), 107–118. 10.1016/j.hermed.2016.06.001
242
Reinelt N. Melzig M. (2017). Marshmallow (Althaea officinalis L.). Z. fur Phytother.38, 91–96. 10.1055/s-0043-103256
243
Richardson D. P. Ansell J. Drummond L. N. (2018). The nutritional and health attributes of kiwifruit: a review. Eur. J. Nutr.57, 2659–2676. 10.1007/s00394-018-1627-z
244
Ried K. (2020). Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: a review and meta-analysis. Exp. Ther. Med.19 (2), 1472–1478. 10.3892/etm.2019.8374
245
Rodríguez-Artalejo F. López-García E. (2017). Coffee consumption and cardiovascular disease: a condensed review of epidemiological evidence and mechanisms. J. Agric. Food Chem.66 (21), 5257–5263. 10.1021/acs.jafc.7b04506
246
Roth G. A. Mensah G. A. Johnson C. O. Addolorato G. Ammirati E. Baddour L. M. et al (2020). Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol.76 (25), 2982–3021. 10.1016/j.jacc.2020.11.010
247
Rouhi-Boroujeni H. Heidarian E. Rouhi-Boroujeni H. Deris F. Rafieian-Kopaei M. (2017). Medicinal plants with multiple effects on cardiovascular diseases: a systematic review. Curr. Pharm. Des.23 (7), 999–1015. 10.2174/1381612822666161021160524
248
Ruyvaran M. Zamani A. Mohamadian A. Zarshenas M. M. Eftekhari M. H. Pourahmad S. et al (2022). Safflower (Carthamus tinctorius L.) oil could improve abdominal obesity, blood pressure, and insulin resistance in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol.282, 114590. 10.1016/j.jep.2021.114590
249
Sabbaghzadegan S. Golsorkhi H. Soltani M. H. Kamalinejad M. Bahrami M. Kabir A. et al (2021). Potential protective effects of Aloe vera gel on cardiovascular diseases: a mini‐review. Phytother. Res.35 (11), 6101–6113. 10.1002/ptr.7219
250
Sabery M. Adib-Hajbaghery M. Rafiee S. (2019). Satisfaction with and factors related to medicinal herb consumption in older Iranian adults. Eur. J. Integr. Med.25, 100–105. 10.1016/j.eujim.2018.12.005
251
Sah A. Naseef P. P. Kuruniyan M. S. Jain G. K. Zakir F. Aggarwal G. (2022). A comprehensive study of therapeutic applications of Chamomile. Pharmaceuticals15, 1284. 10.3390/ph15101284
252
Sanati S. Razavi B. M. Hosseinzadeh H. (2018). A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iran. J. Basic Med. Sci.21 (5), 439–448. 10.22038/IJBMS.2018.25200.6238
253
Sane R. Dawkhar S. Ambulkar P. Mandole R. (2018). The effect of a polyherbal oral formulation in the management of essential hypertension: an open label, pilot clinical study. Int. J. Basic Clin. Pharmacol.7 (7), 1427. 10.18203/2319-2003.ijbcp20182694
254
Santana L. F. Inada A. C. Espirito Santo B. L. S. D. Filiú W. F. Pott A. Alves F. M. et al (2019). Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients11 (7), 1608. 10.3390/nu11071608
255
Schutte A. E. Srinivasapura Venkateshmurthy N. Mohan S. Prabhakaran D. (2021). Hypertension in low-and middle-income countries. Circ. Res.128 (7), 808–826. 10.1161/circresaha.120.318729
256
Seifu E. (2015). Actual and potential applications of Moringa stenopetala, underutilized indigenous vegetable of Southern Ethiopia: a review. Int. J. Agric. Food Res.3 (4). 10.24102/ijafr.v3i4.381
257
Serban C. Sahebkar A. Ursoniu S. Andrica F. Banach M. (2015). Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J. Hypertens.33, 1119–1127. 10.1097/hjh.0000000000000585
258
Shafiq N. Gupta M. Kumari S. Pandhi P. (2003). Prevalence and pattern of use of complementary and alternative medicine (CAM) in hypertensive patients of a tertiary care center in India. Int. J. Clin. Pharmacol. Ther.41 (7), 294–298. 10.5414/cpp41294
259
Shah A. J. Gilani A.-H. Hanif H. M. Ahmad S. Khalid S. Bukhari I. (2014). Neem (Azadirachta indica) lowers blood pressure through a combination of Ca++ channel blocking and endothelium-dependent muscarinic receptors activation. Int. J. Pharmacol.10 (8), 418–428. 10.3923/ijp.2014.418.428
260
Shah S. Cho I.-J. Lee W. Pyun W. B. Ha E. (2023). Coffee intake and hypertension in Korean adults: results from KNHANES 2012–2016. Clin. Hypertens.29 (1), 20–28. 10.1186/s40885-023-00239-4
261
Shehab N. G. Abu-Gharbieh E. (2012). Constituents and biological activity of the essential oil and the aqueous extract of Micromeria fruticosa (L.) Druce subsp. serpyllifolia. Pak. J. Pharm. Sci.25 (3), 687–692.
262
Shilpa T. Souza J. D. (2020). Effectiveness of Lemon juice on reduction of blood pressure among hypertensive clients in selected rural community, Mangaluru. Asian J. Nurs. Educ. Res.10 (1), 31–37. 10.5958/2349-2996.2020.00008.7
263
Shirani F. Foshati S. Tavassoly M. Clark C. C. Rouhani M. H. (2021). The effect of red pepper/capsaicin on blood pressure and heart rate: a systematic review and meta‐analysis of clinical trials. Phytother. Res.35 (11), 6080–6088. 10.1002/ptr.7217
264
Shoaei‐Hagh P. Kamelan Kafi F. Najafi S. Zamanzadeh M. Heidari Bakavoli A. Ramezani J. et al (2021). A randomized, double‐blind, placebo‐controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother. Res.35 (8), 4388–4400. 10.1002/ptr.7140
265
Shoara R. Hashempur M. H. Ashraf A. Salehi A. Dehshahri S. Habibagahi Z. (2015). Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: a randomized controlled clinical trial. Complement. Ther. Clin. Pract.21 (3), 181–187. 10.1016/j.ctcp.2015.06.003
266
Shouk R. Abdou A. Shetty K. Sarkar D. Eid A. H. (2014). Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr. Res.34 (2), 106–115. 10.1016/j.nutres.2013.12.005
267
Singh R. Chaudhary M. Chauhan E. S. (2022). Stellaria media Linn.: a comprehensive review highlights the nutritional, phytochemistry, and pharmacological activities. J. HerbMed Pharmacol.11 (3), 330–338. 10.34172/jhp.2022.38
268
Singletary K. W. (2022). Anise. Potential health benefits. Nutr. Today57 (2), 96–109. 10.1097/nt.0000000000000534
269
Sinha A. Meena A. Panda P. Srivastava B. Gupta M. Padhi M. (2012). Phytochemical, pharmacological and therapeutic potential of Hordeum vulgare Linn.-a review. Asian J. Res. Chem.5 (10), 1303–1308.
270
Sochorova L. Prusova B. Cebova M. Jurikova T. Mlcek J. Adamkova A. et al (2020). Health effects of grape seed and skin extracts and their influence on biochemical markers. Molecules25 (22), 5311. 10.3390/molecules25225311
271
Sontia B. Mooney J. Gaudet L. Touyz R. M. (2008). Pseudohyperaldosteronism, liquorice, and hypertension. J. Clin. Hypertens.10 (2), 153–157. 10.1111/j.1751-7176.2008.07470.x
272
Srinivasan K. (2016). Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit. Rev. Food Sci. Nutr.56 (9), 1488–1500. 10.1080/10408398.2013.772090
273
Stohs S. J. Hartman M. J. (2015). Review of the safety and efficacy of Moringa oleifera. Phytother. Res.29 (6), 796–804. 10.1002/ptr.5325
274
Sun L. Chi B. Xia M. Ma Z. Zhang H. Jiang H. et al (2022a). LC–MS-based lipidomic analysis of liver tissue sample from spontaneously hypertensive rats treated with extract hawthorn fruits. Front. Pharmacol.13, 963280. 10.3389/fphar.2022.963280
275
Sun Y. Liu J. Xin L. Wen J. Zhou Q. Chen X. et al (2022b). Traditional Chinese medicine is associated with reduced risk of readmission in rheumatoid arthritis patients with anemia: a retrospective cohort study. Evid. Based Complement. Altern. Med.2022, 4553985. 10.1155/2022/4553985
276
Surma S. Oparil S. (2021). Coffee and arterial hypertension. Curr. Hypertens. Rep.23, 38. 10.1007/s11906-021-01156-3
277
Susalit E. Agus N. Effendi I. Tjandrawinata R. R. Nofiarny D. Perrinjaquet-Moccetti T. et al (2011). Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine18 (4), 251–258. 10.1016/j.phymed.2010.08.016
278
Svendsen M. Tonstad S. Heggen E. Pedersen T. R. Seljeflot I. Bøhn S. K. et al (2015). The effect of kiwifruit consumption on blood pressure in subjects with moderately elevated blood pressure: a randomized, controlled study. Blood Press24 (1), 48–54. 10.3109/08037051.2014.976979
279
Tabassum N. Ahmad F. (2011). Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev.5 (9), 30–40. 10.4103/0973-7847.79097
280
Taiwo I. A. Odeigah P. G. C. Jaja S. Mojiminiyi F. (2010). Cardiovascular effects of Vernonia amygdalina in rats and the implications for treatment of hypertension in diabetes. Researcher2 (1), 76–79.
281
Tajadini H. Divsalar K. Mehrabani M. Haghdoost A. A. Esmaili Z. Shadkam M. et al (2015). The frequency of using herbal medicines among patients with hypertension in Kerman, Iran, 2012-2013. J. Evid. Based Complement. Altern. Med.20 (3), 199–202. 10.1177/2156587215573141
282
Takeda L. N. Laurindo L. F. Guiguer E. L. Bishayee A. Araújo A. C. Ubeda L. C. C. et al (2022). Psidium guajava L.: a systematic review of the multifaceted health benefits and economic importance. Food Rev. Int.39, 4333–4363. 10.1080/87559129.2021.2023819
283
Thaipitakwong T. Supasyndh O. Rasmi Y. Aramwit P. (2020). A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med.49, 102292. 10.1016/j.ctim.2019.102292
284
Thangsuk P. Pinyopornpanish K. Jiraporncharoen W. Buawangpong N. Angkurawaranon C. (2021). Is the association between herbal use and blood-pressure control mediated by medication adherence? A cross-sectional study in primary care. Int. J. Environ. Res. Public Health18 (24), 12916. 10.3390/ijerph182412916
285
Toulabi T. Yarahmadi M. Goudarzi F. Ebrahimzadeh F. Momenizadeh A. Yarahmadi S. (2022). Effects of flaxseed on blood pressure, body mass index, and total cholesterol in hypertensive patients: a randomized clinical trial. Explore18 (4), 438–445. 10.1016/j.explore.2021.05.003
286
Toungos M. D. (2019). Tamarind (Tamarindus indicus L) fruit of potential value but underutilized in Nigeria. Int. J. Innov. Food Nutr. Sustain. Agric.7 (1), 1–10.
287
Tripathy B. Satyanarayana S. Khan K. A. Raja K. (2017). An updated review on traditional uses, taxonomy, phytochemistry, pharmacology and toxicology of Origanum majorana. Int. J. Pharm. Res. Health Sci.5 (4), 1717–1723. 10.21276/ijprhs.2017.04.01
288
Tyagi S. Nanher A. Sahay S. Kumar V. Bhamini K. Nishad S. K. et al (2015). Kiwifruit: health benefits and medicinal importance. Rashtriya Krishi10 (2), 98–100.
289
Ugbogu E. A. Ude V. C. Elekwa I. Arunsi U. O. Uche-Ikonne C. Nwakanma C. (2018). Toxicological profile of the aqueous-fermented extract of Musa paradisiaca in rats. Avicenna J. Phytomed.8 (6), 478–487.
290
Ulbricht C. Basch E. Burke D. Cheung L. Ernst E. Giese N. et al (2008). Fenugreek (Trigonella foenum-graecum L. Leguminosae): an evidence-based systematic review by the natural standard research collaboration. J. Herb. Pharmacother.7 (3-4), 143–177. 10.1080/15228940802142852
291
Ulbricht C. E. (2010). Natural standard herb and supplement guide: an evidence-based reference. Missouri, USA: Elsevier/Mosby.
292
Ulbricht C. E. Basch E. M. (2005). Natural standard herb and supplement reference: evidence-based clinical reviews. St. Louis, MO: Mosby.
293
Umer S. Tekewe A. Kebede N. (2013). Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement. Altern. Med.13, 21–25. 10.1186/1472-6882-13-21
294
Ursoniu S. Sahebkar A. Andrica F. Serban C. Banach M. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group et al (2016). Effects of flaxseed supplements on blood pressure: a systematic review and meta-analysis of controlled clinical trial. Clin. Nutr.35 (3), 615–625. 10.1016/j.clnu.2015.05.012
295
Uuh‐Narvaez J. J. Segura‐Campos M. R. (2021). Cabbage (Brassica oleracea var. capitata): a food with functional properties aimed to type 2 diabetes prevention and management. J. Food Sci.86 (11), 4775–4798. 10.1111/1750-3841.15939
296
Valenzuela R. Das U. N. Videla L. A. Llorente C. G. (2018). Nutrients and diet: a relationship between oxidative stress, aging, obesity, and related noncommunicable diseases. Oxid. Med. Cell. Longev.2018, 7460453. 10.1155/2018/7460453
297
Van Uum S. (2005). Liquorice and hypertension. Neth J. Med.63 (4), 119–120.
298
Varshney R. Budoff M. J. (2016). Garlic and heart disease. J. Nutr.146, 416S–421S. 10.3945/jn.114.202333
299
Velmurugan C. Bhargava A. (2013). Anti-diabetic and hypolipidemic activity of fruits of Pyrus communis L. in hyperglycemic rats. Asian J. Pharm. Clin. Res.6 (5), 108–111.
300
Venkatakrishnan K. Chiu H.-F. Wang C.-K. (2020). Impact of functional foods and nutraceuticals on high blood pressure with a special focus on meta-analysis: review from a public health perspective. Food Funct.11 (4), 2792–2804. 10.1039/d0fo00357c
301
Verma T. Sinha M. Bansal N. Yadav S. R. Shah K. Chauhan N. S. (2021). Plants used as antihypertensive. Nat. Prod. bioprospecting11, 155–184. 10.1007/s13659-020-00281-x
302
Wahdi A. Astuti P. Puspitosari D. R. Maisaroh S. Pratiwi T. F. (2020). The Effectiveness of giving papaya fruit (Carica papaya) toward blood pressure on elderly hypertension patients. IOP Conf. Ser. Earth Environ. Sci.519, 012007. 10.1088/1755-1315/519/1/012007
303
Wajdy J. Tayseer A. Dheyaa K. (2021). Constituents, pharmacological and toxicological effects of Meliaazadirachta-A review. Sapporo Med. J.55.
304
Wazaify M. Alawwa I. Yasein N. Al-Saleh A. Afifi F. U. (2013). Complementary and alternative medicine (CAM) use among Jordanian patients with chronic diseases. Complement. Ther. Clin. Pract.19 (3), 153–157. 10.1016/j.ctcp.2013.03.001
305
Wei P. Zhao F. Wang Z. Wang Q. Chai X. Hou G. et al (2022). Sesame (Sesamum indicum L.): a comprehensive review of nutritional value, phytochemical composition, health benefits, development of food, and industrial applications. Nutrients14 (19), 4079. 10.3390/nu14194079
306
Wibowo M. A. Anita D. C. (2021). Cucumber juice treatment to the decrease of systolic and diastolic blood pressure. Pak. J. Med. Health Sci.15 (3), 1137–1140.
307
Xie C. Xie Z. Xu X. Yang D. (2015). Persimmon (Diospyros kaki L.) leaves: a review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol.163, 229–240. 10.1016/j.jep.2015.01.007
308
Xiong X. Li X. Zhang Y. Wang J. (2015a). Chinese herbal medicine for resistant hypertension: a systematic review. BMJ Open5 (1), e005355. 10.1136/bmjopen-2014-005355
309
Xiong X. Wang P. Li S. Li X. Zhang Y. Wang J. (2015b). Garlic for hypertension: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine22 (3), 352–361. 10.1016/j.phymed.2014.12.013
310
Xu R. Yang K. Ding J. Chen G. (2020). Effect of green tea supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Medicine99 (6), e19047. 10.1097/md.0000000000019047
311
Yamaji T. Harada T. Hashimoto Y. Nakano Y. Kajikawa M. Yoshimura K. et al (2022). Relationship of daily coffee intake with vascular function in patients with hypertension. Nutrients14 (13), 2719. 10.3390/nu14132719
312
Yaqoob N. Fatimah M. Naqvi F. Sarfraz J. Mushtaq S. Chiragh S. (2021). Comparative evaluation of Aloe vera and Diclofenac on body weight, blood pressure and renal function of hypertensive rats. Proc. S.Z.M.C.35, 39–44. 10.47489/p000s351z7821-6mc
313
Yeh G. Y. Davis R. B. Phillips R. S. (2006). Use of complementary therapies in patients with cardiovascular disease. Am. J. Cardiol.98 (5), 673–680. 10.1016/j.amjcard.2006.03.051
314
Yu G. Luo Z. Zhou Y. Zhang L. Wu Y. Ding L. et al (2019). Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother.117, 109094. 10.1016/j.biopha.2019.109094
315
Zeggwagh N. Farid O. Michel J. Eddouks M. (2008). Cardiovascular effect of Artemisia herba alba aqueous extract in spontaneously hypertensive rats. Methods Find. Exp. Clin. Pharmacol.30 (5), 375–381. 10.1358/mf.2008.30.5.1186081
316
Zhang R. Zhang Q. Zhu S. Liu B. Liu F. Xu Y. (2022). Mulberry leaf (Morus alba L.): a review of its potential influences in mechanisms of action on metabolic diseases. Pharmacol. Res.175, 106029. 10.1016/j.phrs.2021.106029
317
Zhao C.-N. Meng X. Li Y. Li S. Liu Q. Tang G.-Y. et al (2017). Fruits for prevention and treatment of cardiovascular diseases. Nutrients9 (6), 598. 10.3390/nu9060598
318
Zhao X.-X. Lin F.-J. Li H. Li H.-B. Wu D.-T. Geng F. et al (2021). Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr.8, 669805. 10.3389/fnut.2021.669805
319
Zhou B. Perel P. Mensah G. A. Ezzati M. (2021). Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol.18 (11), 785–802. 10.1038/s41569-021-00559-8
320
Zhou Q. Lei X. Fu S. Li Z. Chen Y. Long C. et al (2022). Efficacy of cinnamon supplementation on glycolipid metabolism in T2DM diabetes: a meta-analysis and systematic review. Front. Physiol.13, 960580. 10.3389/fphys.2022.960580
321
Zhou X. Tang L. Xu Y. Zhou G. Wang Z. (2014). Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol.151 (1), 27–43. 10.1016/j.jep.2013.10.050
322
Ziaei S. Heidari M. Amin G. Kochmeshki A. Heidari M. (2009). Inhibitory effects of germinal angiotensin converting enzyme by medicinal plants used in Iranian traditional medicine as antihypertensive. J. Kerman Univ. Med. Sci.16 (4), 134–143.
Summary
Keywords
hypertension, herbal medicine, safety, adverse effects, systematic review, meta-analysis
Citation
Choi D, Im HB, Choi SJ and Han D (2024) Safety classification of herbal medicine use among hypertensive patients: a systematic review and meta-analysis. Front. Pharmacol. 15:1321523. doi: 10.3389/fphar.2024.1321523
Received
14 October 2023
Accepted
27 March 2024
Published
31 May 2024
Volume
15 - 2024
Edited by
Anthony Booker, University of Westminster, United Kingdom
Reviewed by
Gianfranco Pintus, University of Sharjah, United Arab Emirates
Omar Estrada, Instituto Venezolano de Investigaciones Científicas (IVIC), Venezuela
Updates
Copyright
© 2024 Choi, Im, Choi and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongwoon Han, dwhan@hanyang.ac.kr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.