Abstract
As global population and lifestyles change, osteoarthritis (OA) is becoming a major healthcare challenge world. OA, a chronic condition characterized by inflammatory and degeneration, often present with joint pain and can lead to irreversible disability. While there is currently no cure for OA, it is commonly managed using nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and glucosamine. Although these treatments can alleviate symptoms, it is difficult to effectively deliver and sustain therapeutic agents within joints. The emergence of nanotechnology, particularly in form of smart nanomedicine, has introduced innovative therapeutic approaches for OA treatment. Nanotherapeutic strategies offer promising advantages, including more precise targeting of affected areas, prolonged therapeutic effects, enhanced bioavailability, and reduced systemic toxicity compared to traditional treatments. While nanoparticles show potential as a viable delivery system for OA therapies based on encouraging lab-based and clinical trials results, there remails a considerable gap between current research and clinical application. This review highlights recent advances in nanotherapy for OA and explore future pathways to refine and optimize OA treatments strategies.
1 Introduction
Osteoarthritis (OA) is a prevalent condition, affecting over 500 million people worldwide, which is equates to approximately 7% of the world’s population (Duan et al., 2023). From 1990 to 2019, the prevalence of OA increased by 48% (Hunter et al., 2020), underscoring its growing impact. Research increasingly indicates that factors such as obesity, aging, poor dietary habits, and hypertension significantly contribute to the progression of OA (Colletti and Cicero, 2021). The primary objectives in managing OA are pain relief, reduce of joint inflammation, enhancing joint function, and minimizing overall disability (Filardo et al., 2015; De Faro Silva et al., 2022). Standard treatment typically involves intra-articular drug injections and surgical interventions (Ebert et al., 2013); however, these approaches often fall short in efficacy. Surgical treatments, in particular, are complex and require prolonged recovery periods (Conaghan et al., 2019). While commonly used clinical treatments like NSAIDs, glucocorticoids, and glycosaminoglycan can provide symptomatic relief, but do little to halt the progression of OA (Katz et al., 2021), highlighting the urgent need for more effective treatments.
In recent decades, significant progress has been made in the field of nanomedicine for treating various diseases (Janowicz et al., 2022; Zhang Z. et al., 2022; Han and Huang, 2023). In the context of OA, researchers are working on developing nanocarrier systems to utilize nanoparticles to extend the duration of drug efficacy, allowing for a gradual release of the therapeutic agents and improving their penetration into chondrocytes or synovial cells (Mitchell et al., 2021; Dilliard et al., 2021; Boehnke et al., 2022). Despite these promising advancements, no nanotherapeutic drugs for OA have yet received clinical approval. This review aims to explore recent developments in nanomedicine for OA treatment, providing a comprehensive overview of its properties, potential benefits, and the challenges that must be addressed for clinical application.
2 Pathogenesis of osteoarthritis
Osteoarthritis (OA) is characterized by a complex interplay of pathological changes within affected joints, including the deterioration of articular cartilage (Krishnan and Grodzinsky, 2018), inflammation of the synovial membrane (Hu et al., 2019), remodeling of the subchondral bone (Zhu et al., 2021), and the development of osteophytes (McCulloch et al., 2017). The widely accepted theory is that OA arises from an imbalance between degradation and repair of cartilage (Guilak et al., 2018). Articular cartilage, which is crucial for smooth joint movement, consists primarily of chondrocytes embedded in an extracellular matrix (ECM) rich in type II collagen and proteoglycans (Zou et al., 2021). Under normal conditions, chondrocytes maintain cartilage integrity by synthesizing type II collagen and proteoglycans while also regulating the activity of enzymes such as matrix metalloproteinases (MMPs). These enzymes are responsible for maintaining a balance between the breakdown (catabolism) and synthesis (anabolism) of cartilage components (Guo et al., 2018; Li T. et al., 2022).
Various factors, including mechanical stress, metabolic changes, aging, and inflammation, contribute to the progression of OA. Mechanical stress, such as that caused by joint overuse or injury, can lead to the release of damage-associated molecular patterns (DAMPs) from damaged tissues, which activate pattern recognition receptors (PRRs) on immune cells line macrophages (Li and Wu, 2021). This, in turn, stimulates the production of pro-inflammatory cytokines, including Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1β (IL-1β) (Molnar et al., 2021). Obesity exacerbates this process through chronic low-grade inflammation, known as “metaflammation”, where expanded adipose tissue expansion leads to increased macrophage infiltration pro-inflammatory M1 phenotype, characterized by high levels of TNF-α and IL-1β production (Li et al., 2023). Aging further contributes to OA through “inflammaging,” a state of chronic, low-grade inflammation driven by the accumulation of senescent cells, oxidative stress, and altered immune function, all of which contribute to increased production of inflammatory (Chow and Chin, 2020; Wojdasiewicz et al., 2014; Liu et al., 2022a).
Inflammatory cytokines have a profound effect on chondrocytes, leading to phenotypic changes that disrupt cartilage homeostasis. These cytokines suppress the production of type II collagen and proteoglycans in chondrocytes by activating pathways like Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor-kappa B (NF-κB), particularly through the influence of mediator like prostaglandin E2 (PGE-2), Nitric Oxide (NO), and cyclooxygenase-2 (COX-2) (Ma et al., 2016; Teng et al., 2023; Chen et al., 2018; Yao et al., 2022; Lu R. et al., 2023). This shift increases the production of specific MMPs (e.g., MMP1, MMP3, MMP9, MMP13) while reducing the synthesis of collagen and proteoglycans (Ling et al., 2021; Tian et al., 2021), which collectively contribute to the breakdown of the ECM.
As ECM breaks down, it releases various molecular fragments that act as DAMPs, further activating PRRs on macrophages and chondrocytes (Son et al., 2020; Lambert et al., 2021; Foell et al., 2007; Rahmati et al., 2016; Hügle et al., 2022). This perpetuates the inflammatory cycle, enhancing the activation of NF-κB and MAPK pathways, and accelerating cartilage degradation (Moon et al., 2018; Liao et al., 2020; Xu et al., 2022; Boehme and Rolauffs, 2018). Additionally, hypoxia within the joint microenvironment exacerbates inflammation, activating the NLRP3 inflammasome in macrophages and leading to the release of pro-inflammatory cytokines such as IL-8, MCP-1, CXCL12, CCL22, and MIP-1α (Quero et al., 2020; Raghu et al., 2017; Kuang et al., 2020; Ren et al., 2021; Hou et al., 2020; Zhao et al., 2020). These cytokines contribute to the recruitment and activation of additional immune cells, further perpetuating the inflammatory cycle (Nelson et al., 2011) (Figure 1).
FIGURE 1
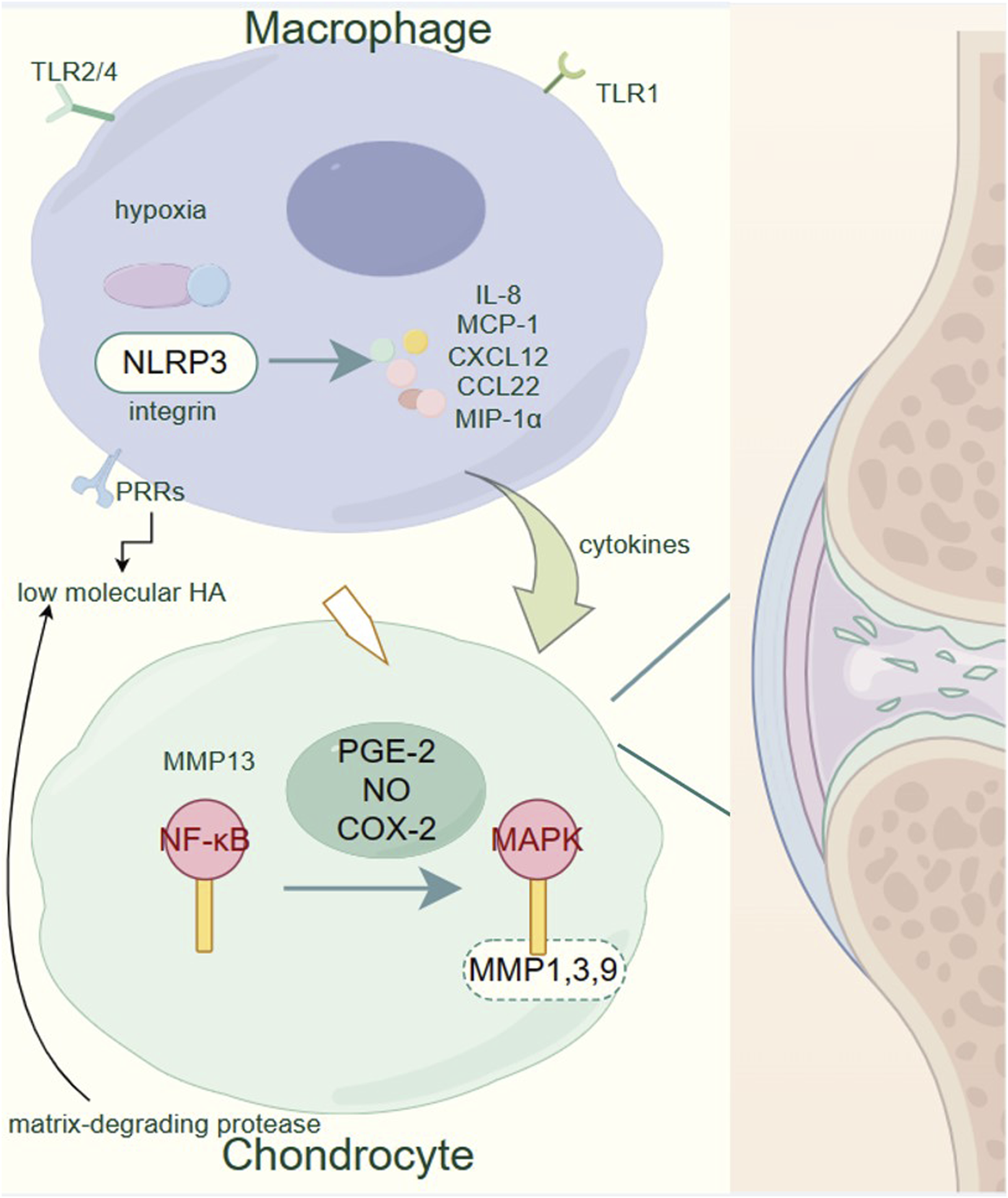
Pathogenesis of osteoarthritis. Within the OA-affected joints, macrophages play a critical role by secreting pro-inflammatory cytokines and chemokines such as IL-8, MCP-1, CXCL12, CCL22, and MIP-1α in response to various stimuli including hypoxia and low molecular HA. These inflammatory mediators contribute to the activation of chondrocytes. Activated chondrocytes express elevated levels of matrix-degrading proteases, particularly MMP1, 3, 9, and 13, via the NF-κB and MAPK signaling pathways, induced by pro-inflammatory factors like PGE-2, NO, and COX-2. This upregulation of proteases leads to the degradation of the extracellular matrix, a hallmark of OA progression. The figure underscores the importance of the NF-κB and MAPK pathways in the catabolic processes of cartilage degradation, highlighting potential targets for therapeutic intervention to mitigate the progression of OA.
As OA progresses, cartilage degeneration extends into the calcified layer, promoting pathological changes in the subchondral bone, including the formation of osteophytes around the joint periphery (Hu et al., 2021; Roelofs et al., 2020). Addressing these inflammatory pathways and restoring the balance between cartilage degradation and synthesis are critical strategies in preventing and treating OA.
3 Nanomedicines to reduce inflammation of synovial and articular cartilage
Current clinical approaches to OA treatment are categorized into three main types: non-pharmacologic management, pharmacologic management, and surgical interventions (Emami et al., 2018; Evans et al., 2014). Non-pharmacologic methods, such as exercise and physical therapy, are often employed to alleviate symptoms. However, there strategies primarily address the symptoms rather than the underlying pathology of OA, and in some cases, improper application may exacerbate the condition (Lin et al., 2023; Furtado et al., 2022). Surgical interventions, such as arthroscopic surgery or joint replacement, provide more direct solution but are associated with high costs, invasiveness, and substantial risks, particularly in elderly patients or those with comorbidities (Skou et al., 2015).
Pharmacologic treatments, although less invasive than surgery, also present significant limitations. Commonly used drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), glycosaminoglycans (GAGs), opioid analgesics, steroids, and hyaluronic acid (HA), can be administered via various routes, including oral, intravenous, intra-articular, and transdermal methods (McGuckin et al., 2022; Oo et al., 2021). Despite their widespread use, these pharmacologic approaches are hindered by issues such as limited local drug concentration in the joints, rapid drug clearance from synovial fluid, and systemic side effects, including gastrointestinal, renal, and cardiovascular complications, particularly with long-term use (Evans et al., 2014; Togo et al., 2022; Cooper et al., 2019).
Nanotechnology has emerged as a promising avenue for overcoming the limitations of traditional OA therapies. Nanoparticles, when utilized as drug carriers, have the unique ability to selectively accumulate in affected joints, minimizing systemic exposure and maximizing local therapeutic effects (Huang et al., 2022; Li X. et al., 2021). These carriers can also stabilize encapsulated drugs, enabling controlled and sustained release, which prolongs drug retention time and reduces site-specific toxicity (Huang et al., 2022). Moreover, nanomaterials can be engineered as stimulus–responsive drug delivery systems, triggered by external stimuli such as temperature, magnetic fields, and electric fields (Said et al., 2019). This targeted and on-demand drug delivery system enhances therapeutic efficacy while mitigating the risk of side effects.
Nanomedicine in OA treatment involves the delivery of various therapeutic agents, including small-molecule drugs, nucleic acids, and peptides/proteins, via nanomaterials designed to inhibit OA progression. By offering more precise, sustained, and responsive drug delivery, nanomedicine holds significant promise for improving OA management, addressing both symptoms and underlying pathologies, and overcoming the limitations of traditional therapies (Dou et al., 2020; Kumar et al., 2019; Xu et al., 2023).
3.1 Combination of nanotechnology with anti-inflammatory drugs
OA is a multifactorial disease characterized by the progressive degradation of joint cartilage and the inflammation of synovial membranes (Scanzello and Goldring, 2012). Inflammation is a key driver of OA progression, where inflammatory cytokines, such as TNF-α and IL-1β, play a central role. Traditional pharmacological interventions like NSAIDs, glucocorticoids (GCs), steroids, and Glycosaminoglycans (GAGs), aim to alleviate symptoms by suppressing inflammation (Strokotova and Grigorieva, 2022; Magni et al., 2021; Nunes et al., 2021). However, these treatments often have limited efficacy and are associated with significant systemic side effects. The development of nanomedicines offers a promising alternative. Some nanoparticles are designed to target the inflamed synovium and cartilage directly. By encapsulating anti-inflammatory agents within these nanovesicles, these nanomedicines can achieve sustained drug release and higher local drug concentrations within the joints (Wang Y. et al., 2022; Wen et al., 2023). This targeted approach not only enhances the therapeutic potential of existing drugs, but also minimized the adverse effects. Another strategy explored the use of metallic nanoparticles. Some metallic particles can directly act on biological molecules, they can either act as nanoenzyme to reduce oxidative stress, or act through inhibition of the NF-κB signaling pathway or activation of NLRP3 inflammasome (Li R. et al., 2022; Luo et al., 2021; Ma, 2023) (Figure 2). In the following sections, we will explore the application of nanomedicines for reducing inflammation in OA, focusing on the mechanisms by which these advanced therapies can improve clinical outcomes by targeting the synovial and articular cartilage.
FIGURE 2
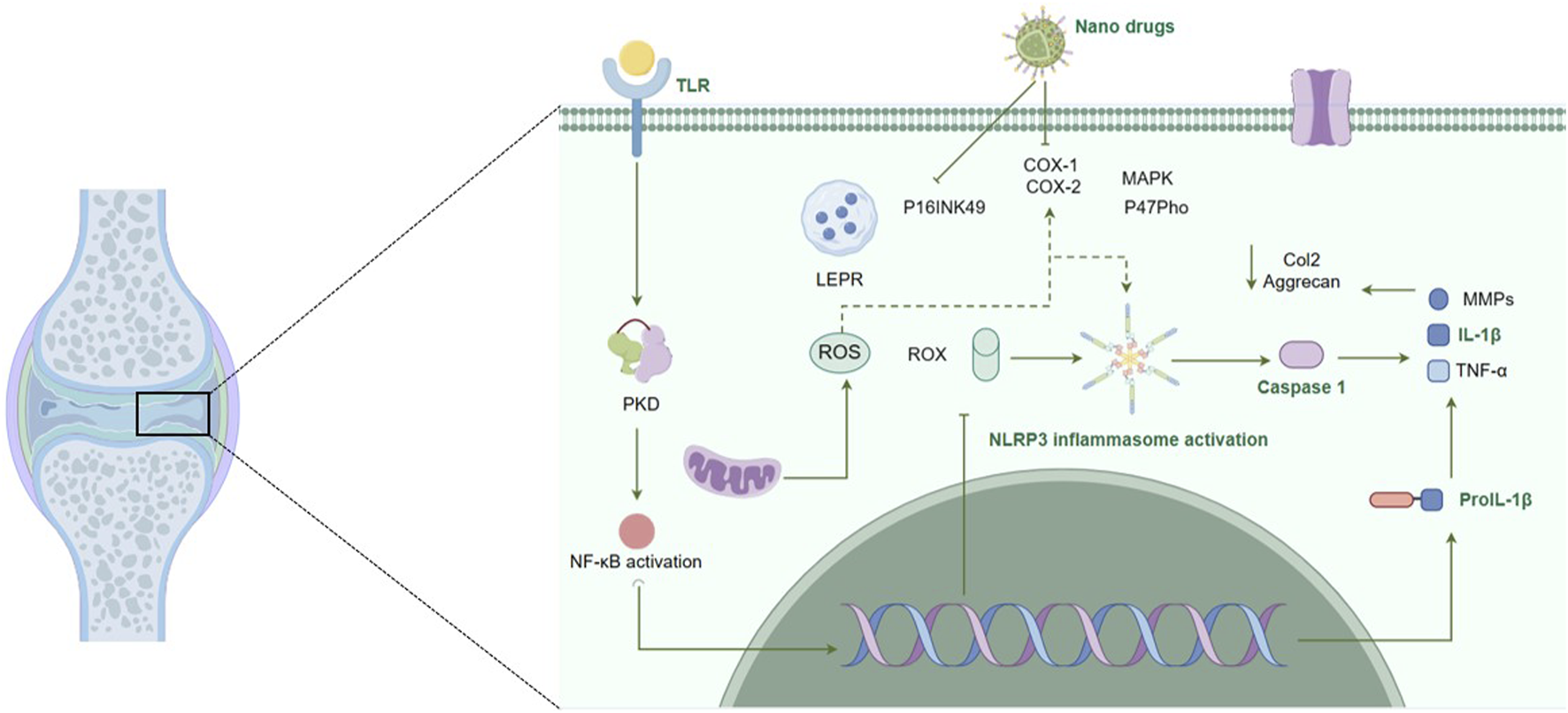
Nanomedicines and their immune regulatory role in osteoarthritis. This figure depicts the multifaceted mechanisms of action of nanomedicines in the regulation of immune responses within the pathophysiological context of osteoarthritis. It illustrates the targeted delivery and sustained release of nanoformulated drugs, highlighting their interactions with cellular and molecular components are integral to the inflammatory process and consequent cartilage degradation in osteoarthritis. The figure underscores the potential of nanomedicines to reduce inflammation by inhibiting these pathways and to enhance the bioavailability and efficacy of therapeutic agents, offering a strategic approach in osteoarthritis treatment.
3.1.1 Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs such as ibuprofen, flurbiprofen, diclofenac, celecoxib, indomethacin, meloxicam, piroxicam, and naproxen, are among the most widely prescribed medications for alleviating symptoms in OA patients. Their primary mode of action involves robust inhibition of cyclooxygenase (COX) enzymes, particularly COX-1 and COX-2 at sites of inflammation, which in turn suppresses the biosynthesis of prostaglandins (PGs) (Ahmadi et al., 2022; Eisenstein et al., 2022; Stiller and Hjemdahl, 2022; Busa et al., 2022). PGs play a key role in sensitizing pain pathways by interacting with TRPV1, Nav1.8 and other ion channels to reduce their threshold, leading to heightened pain perception, amplifying pain perception (Magni et al., 2021; Zhu et al., 2020).
Besides their primary COX-inhibitory action, NSAIDs also modulate various other inflammatory pathways in OA, they reduce the production of pro-inflammatory cytokines and leukotrienes, which are central to joint inflammation and tissue degradation (Alvarez-Soria et al., 2006; Maślanka and Jaroszewski, 2013; Hassan and Ghobara, 2016; Li et al., 2018; Nagy et al., 2017). Furthermore, NSAIDs exert immunomodulatory effects by inhibiting the activation and migration of immune cells (Molnar et al., 2021; Li Z. et al., 2021). This broad spectrum, of anti-inflammatory actions make NSAIDs effective in managing the symptoms of OA. However, long-term use of NSAIDs is associated with several adverse effects, including gastrointestinal (GI) distress, peptic and duodenal ulcers, small bowl erosion, colitis, acute renal failure, hypertension, chronic kidney disease, heart failure, myocardial infarction, stroke, seizures, and delayed wound healing (Sohail et al., 2023; Chevalier et al., 2009). These risks have driven research towards improving NSAID delivery methods to enhance their therapeutic index while minimizing systemic side effects.
Nanotechnology has significantly advanced the field of drug delivery, offering innovative solutions to overcome the limitation associated with traditional drug delivery. Encapsulating NSAIDs within nanoscale carriers, provides several advantages, including protection from premature degradation, targeted drug delivery, and controlled release (Ashfaq et al., 2023). These nanocarriers typically range from 10–200 nm in size, typical nanocarriers that are used to encapsulating NSAIDs are liposomes, polymeric nanoparticles, and solid lipid nanoparticles (SLNs) (Badri et al., 2016; Pontes et al., 2022).
Liposomes are composed of lipid bilayers, which can encapsulate both hydrophilic and hydrophobic NSAIDs (Lee, 2020). Liposomes can be engineered to release the drug in response to specific stimuli, such as changes in pH or temperature, making them effective for localized delivery in inflamed joints (Liu P. et al., 2022). This stimulus-responsive release mechanism is particular effective for localized drug delivery with inflamed joints thereby reducing systemic exposure and associated side effects.
Polymeric nanoparticles are made from biocompatible and biodegradable polymers, these nanoparticles can encapsulate NSAIDs, ensuring a sustained release over time (Baek et al., 2017). This controlled release minimizes the need for frequent dosing and reduces systemic side effects.
SLNs are composed of solid lipids, which remain solid at body temperature. The surface of SLNs can be further coated with enteric polymers that are resistant to acidic environments, by using the combination of solid lipids and enteric coatings, SLNs are designed to bypass the stomach without releasing their contents (Pandey et al., 2021). The SLNs then release the NSAID once they reach the more neutral pH of the intestines or are absorbed into the bloodstream (Mancini et al., 2021). Once the SLNs reach the joint environment, their stability is influenced by the specific modifications made to the nanoparticle (Hsu et al., 2023). For example, SLNs can be designed to degrade in response to enzymes that are overexpressed in the inflamed joint, such as matrix metalloproteinases (MMPs). When these enzymes come into contact with the SLNs, they break down the solid lipid matrix, releasing the encapsulated NSAID directly into the inflamed tissue (Chuang et al., 2018). This coating dissolves only in the more neutral pH of the small intestine, allowing the SLNs to remain intact until they are absorbed into the bloodstream. SLNs offer a stable platform for NSAID delivery, with enhanced bioavailability and reduced gastrointestinal side effects due to localized drug release (Singh et al., 2019).
In comparison to SLNs or liposomes, inorganic nanoparticles, such as silica (SiO2), gold (AuNPs), iron oxide (Fe2O3 or Fe3O4), or cerium oxide (CeO2), provide a unique advantage in delivering NSAIDs for OA treatment because of their structural robustness, precise targeting capabilities, and high stability in various physiological environments (Corsi et al., 2023; Pourmadadi et al., 2022; He et al., 2021; Kalashnikova et al., 2020). Inorganic nanoparticles are inherently stable under a wide range of pH conditions, including the acidic environment of the stomach, making them highly effective for drug delivery, including NSAID encapsulation. Their robust structure prevents the encapsulated NSAID from being exposed to gastric acid, thereby protecting it from premature degradation (Hsu et al., 2023). The surface of inorganic nanoparticles can be modified with targeting ligands, such as antibodies, peptides, or small molecules that recognize and bind to specific receptors overexpressed in inflamed joints. For example,: folate receptors, which are often overexpressed in inflamed tissues, can be targeted by modifying the surface of inorganic nanoparticles with folate molecules. This targeting mechanism can lead to increased drug accumulation in the inflamed joint, improving therapeutic efficacy while reducing systemic side effects (Yu et al., 2010; Sanità et al., 2020; Huang et al., 2024). In the context of OA treatment, inorganic nanoparticles can enhance the bioavailability and therapeutic action of NSAIDs by providing protection against premature degradation, enabling controlled release, and improving tissue targeting (Yi et al., 2024).
3.1.2 Glycosaminoglycans (GAGs)
Glycosaminoglycans (GAGs), such as hyaluronic acid (HA), chondroitin sulfate, and keratan sulfate, are essential components of the extracellular matrix in cartilage. They help maintain the structural integrity and mechanical function of cartilage by providing lubrication and shock absorption in joints. HA is the most commonly used GAG in the treatment of OA (Altman et al., 2015; Testa et al., 2021; Jargin, 2012). HA products vary in molecular weight and cross-linking, which influences their viscosity and duration of action within the joint. These formulations are either derived from bacterial fermentation or extracted from rooster combs and must undergo purification to remove impurities (Serra et al., 2023).
HA is administered via intra-articular injection to restore the viscoelastic properties of synovial fluid, reducing pain and improving joint mobility (Legré-Boyer, 2015; Chavda et al., 2022). Beyond its lubricating function, HA also modulates the inflammatory response by inhibiting the activity of pro-inflammatory cytokines and enzymes, such as matrix metalloproteinases (MMPs), which degrade cartilage (Marinho et al., 2021). Furthermore, HA can inhibit the activation of the NF-κB pathway, thereby reducing the expression of inflammatory mediators like COX-2 and MMPs (Marinho et al., 2021). It may also influence the MAPK signaling pathway, affecting cell survival and inflammation (Chen et al., 2019).
Since HA is typically applied directly into the affected joint, nanoparticle technologies for HA-based treatments aim to overcome different challenges than those associated with NSAIDs. While NSAIDs nanoparticles formulations target tissue-specific delivery to inflamed joints, HA-based treatments focus on extending the therapeutic effects, enhancing stability, and improving cartilage penetration. One key limitation of HA is its rapid degradation due to enzymatic activity and its large molecular size, which restricts penetration into deeper cartilage layers (Gan et al., 2024). To address these issues, various nanoparticle technologies have been explored. For instance, poly (lactic-co-glycolic acid) (PLGA) nanoparticles can encapsulate HA, protecting it from enzymatic degradation and enabling sustained release over time. Additionally, PLGA nanoparticles can also be modified with polyethylene glycol (PEG) to increase circulation time and reduce immune clearance (Pontes et al., 2022; Zerrillo et al., 2022; Householder et al., 2023). Natural polymers such as chitosan, alginate, and cellulose derivatives show therapeutic potential for intra-articular drug delivery. Chitosan is another promising natural polymer for nanoparticle formation with HA. Its mucoadhesive properties help retain the nanoparticles in the joint, and its positive charge facilitates interaction with the negatively charged cartilage, improving penetration (Pontes et al., 2022). Other nanoparticle systems, such as hollow mesoporous silica nanoparticles (HMSNs) and liposomes can also be used to encapsulate HA. HMSNs offer a porous surface for control release, while liposomes protect HA from enzymatic degradation and allow for sustained delivery (Wen et al., 2023; Teixeira et al., 2022).
Overall, the application of nanoparticle technologies in HA-based OA treatments focuses on prolonging the therapeutic effects of HA, enhancing its stability, and improving its penetration into cartilage tissue, rather than focusing on tissue-specific delivery as is the case with NSAIDs.
3.1.3 Natural products from medicinal plants
Natural plant-derived medicines have gained significant attention in the treatment of OA due to their potential to offer anti-inflammatory, analgesic, and cartilage-protective effects with fewer side effects compared to conventional pharmaceuticals (Fang et al., 2024; Akhileshwar Jha et al., 2024; Mu et al., 2022). The significance of plant-based therapies in OA treatment is seen from multiple perspectives, including their historical use in traditional medicine, increasing scientific validation, and the growing interest in integrative approaches to managing chronic diseases like OA (Kuang et al., 2023). However, many plant-derived compounds face challenges such as poor bioavailability, rapid metabolism, and insufficient tissue targeting. Nanoparticle technologies present a promising solution by enhancing the delivery, bioavailability, and therapeutic efficacy of these compounds, improving their potential in OA treatment. While clinical studies on nanoparticles for OA are still in early stages, preclinical data suggest that multiple natural products-loaded nanoparticles could represent a novel and effective approach for mitigating OA symptoms and slowing disease progression (Fang et al., 2024; Cao et al., 2022).
Cannabidiol (CBD), a non-psychoactive component of Cannabis sativa, has been recognized for its anti-inflammatory and analgesic properties (Lowin et al., 2020). It interacts primarily with the endocannabinoid system (ECS), specifically CB1 and CB2 receptors, which play roles in modulating pain, inflammation, and immune responses (Fine and Rosenfeld, 2013; Vučković et al., 2018). CBD reduces pro-inflammatory cytokine production, inhibits immune cell, and mitigates oxidative stress, all contributing to joint protection in OA. However, due to its lipophilic nature and poor bioavailability, traditional oral administration of CBD can be inefficient (Bryk and Starowicz, 2021). Nanotechnology can enhance CBD’s delivery by encapsulating it in lipid-based nanoparticles similar as encapsulating NSAIDs. Liposomes, polymeric nanoparticles, or SLNs, have been employed to improve CBD’s solubility, bioavailability, and stability (Assadpour et al., 2023; Alcantara et al., 2024). Nanoparticles, especially those functionalized with targeting ligands, can direct CBD to inflamed joints, thereby enhancing its therapeutic potential while minimizing systemic side effects. For instance, CBD-loaded PLGA nanoparticles have been shown to enhance bioavailability, allowing for more effective delivery to target tissues, by reducing inflammation and improving the therapeutic outcomes associated with OA (Jin et al., 2023).
3.1.3.1 Curcumin (Curcuma domestica)
Curcumin, a polyphenol derived from turmeric (Curcuma longa), is well-recognized for its anti-inflammatory properties (Salehi et al., 2019), particularly its ability to inhibit the NF-κB pathway, which is central to inflammation in OA (Buhrmann et al., 2021). Additionally, curcumin scavenges free radicals, reducing oxidative stress in affected joints. However, curcumin’s low solubility in water and rapid metabolism limits its clinical use (Crivelli et al., 2019; Guan et al., 2022). SLNs can enhance the oral bioavailability of curcumin by protecting it from the acidic environment of the stomach and enabling sustained release (Ban et al., 2020). It is also reported that the curcumin can be dissolved with mPEG (5kD)-PCL(2kD) polymer to produce the curcumin-loaded polymeric micelles, which has a 74.8 ± 8.68 days nm (Gupta et al., 2020; Kang et al., 2020). This approach can improve curcumin’s solubility and enhance its systemic circulation time, allowing for more effective delivery to the joints (Kang et al., 2020).
3.1.3.2 Boswellia serrata (boswellic acid)
Boswellic acid, from Boswellia serrata (Indian frankincense), inhibits the 5-lipoxygenase enzyme, reducing leukotriene synthesis, which is involved in inflammation and pain (Börner et al., 2021; Gomaa et al., 2021). It also protects cartilage by reducing the degradation caused by MMPs (Shin et al., 2022). Due to its hydrophobic nature and poor gastrointestinal absorption, nanoparticle systems such as nanoemulsion and cyclodextrin inclusion complex have been developed to improve the bioavailability of boswellic acid (Ting et al., 2018). Different from nanocapsules where the drug is confined to a cavity surrounded by a polymeric membrane and are typically 10–500 nm in size, nanoemulsions are colloidal dispersions where two immiscible liquids, typically oil and water, are stabilized by surfactants. The droplets in nanoemulsions are usually in the range of 20–200 nm, with the drug is dissolved in the dispersed phase (Borrajo et al., 2024). Instead of protecting the drug from the harsh acidic environment of the stomach and the enzymatic activity in the intestine, nanoemulsions are designed to enhance the digestion of encapsulated lipophilic compounds by allowing them to be more easily absorbed through the intestinal lining. They can enhance solubility and bioactivity of boswellic acids in the gastrointestinal tract (Choi and McClements, 2020; Han et al., 2022). The small droplet size and large surface area of nanoemulsions enable better absorption and distribution of the boswellic acids, enhancing their therapeutic effects. Cyclodextrins improve the solubility and stability of hydrophobic drugs by forming inclusion complexes where the drug is hosted inside the hydrophobic cavity of the cyclodextrin molecule. Cyclodextrin complexes can help the boswellic acids dissolve more easily in the aqueous environment of the GI tract, improving its bioavailability (Sarabia-Vallejo et al., 2023). Similarly, cyclodextrin complexations do not protect boswellic acids from the acidic environment like nanocapsules but rather enhance the drug’s solubility and absorption in the intestines (Tambe et al., 2018).
3.1.3.3 Quercetin
Quercetin, a flavonoid found in various fruits and vegetables, exhibits anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines and oxidative stress, as well as stabilization lysosomal membranes and protecting chondrocytes (Aldrich et al., 2023). Quercetin’s protective role in cartilage involves inhibiting MMPs and promoting autophagy, which helps maintain cartilage healthy (Li W. et al., 2021; Wang L. et al., 2022). However, like many natural compounds, quercetin has low bioavailability due to poor absorption and rapid metabolism (Carrillo-Martinez et al., 2024). Nanoparticle approaches, including quercetin-loaded liposomes, polymeric nanoparticles, solid lipid nanoparticles (SLNs), as while as nanostructured lipid carriers (NLCs) that are lipid-based delivery systems that combine both solid and liquid lipid, have been developed to enhance quercetin’s bioavailability, stability, and targeted delivery to inflamed joints, making it a more viable treatment option for OA (Carrillo-Martinez et al., 2024). Quercetin-loaded nanoparticles, when administered intra-articularly, showed prolonged retention in joint tissues, providing sustained release of quercetin and improving its anti-inflammatory effects in OA models (Akhileshwar Jha et al., 2024; Jennings et al., 2016). Besides the lipid nanoparticles, gold nanoparticles have been explored as carriers for various bioactive compounds, including quercetin. Due to their small size, AuNPs can efficiently deliver quercetin to targeted sites, enhancing cellular uptake and therapeutic efficacy (Sadalage et al., 2021).
3.1.3.4 Baicalin (Scutellaria baicalensis)
Baicalin, a flavonoid derived from Scutellaria baicalensis, is known for its potent anti-inflammatory, antioxidant, and chondroprotective effects, making it an attractive candidate for the treatment of OA (Hu et al., 2022). However, like many other natural compounds, baicalin suffers from poor water solubility, low bioavailability, and rapid systemic clearance, which limit its therapeutic potential in clinical applications (Huang et al., 2019). To address these limitations, various nanoparticle-based delivery systems have emerged as a promising strategy to enhance effectiveness of baicalin in OA management.
Lipid-based nanoparticles, including liposome, SLNs and NLCs, have been explored for improving baicalin’s bioavailability and targeted delivery to inflamed joints (Zhang et al., 2016; Shi et al., 2016). There nanoparticles protect baicalin from rapid degradation, facilitate sustained release, and improve its solubility in biological fluids. For instance, baicalin-loaded SLNs have demonstrated enhanced anti-inflammatory activity and greater cartilage protection in OA models, compared to baicalin in its free form (Gao et al., 2022). Another innovative approach involves the use of polymeric nanoparticles, particularly those made from biodegradable materials like PLGA. These nanoparticles allow for the controlled release of Baicalin, ensuring prolonged therapeutic effects at the site of inflammation. Functionalization of these nanoparticles with targeting ligands, such as hyaluronic acid, further enhances their ability to accumulate in osteoarthritic joints by targeting CD44 receptors expressed on synovial cells, which are implicated in OA pathology (Gaio et al., 2020; Salathia et al., 2023; Bigaj-Józefowska and Grześkowiak, 2022).
Nanoparticles provide sustained release and enhanced targeting of inflamed tissues. Additionally, lipid-based nanoparticles, such as SLNs and nanoemulsions, can further improve baicilin’s solubility and systemic circulation, allowing for more efficient delivery to OA-affected joints (Ashfaq et al., 2023; Ghasemiyeh and Mohammadi-Samani, 2018). Moreover, nanofibers and hydrogels have been employed as localized delivery platforms for baicalin (Wang Z.-Z. et al., 2022; Liu et al., 2022c). These nanostructures can be injected directly into the joint space, providing a sustained release of baicalin over an extended period. This localized administration minimizes systemic side effects while maintaining high concentrations of the active compound in the affected joints (Bai et al., 2021).
3.1.3.5 Andrographolide (AG)
Andrographolide (AG), extracted from Andrographis panicula, is a potent anti-inflammatory compound known for its ability to modulate immune responses and inhibit pro-inflammatory cytokine production (Xiong et al., 2021). Its application in OA treatment, however, is limited due to poor water solubility and rapid systemic clearance (Zhao et al., 2019). Recent advancements in nanotechnology have enabled the development of AG-loaded nanoparticles that can overcome these limitations. For instance, AG encapsulated in poly (acrylic acid)-modified mesoporous silica nanoparticles has been shown to provide a pH-responsive platform for sustained release, allowing the drug to be released more effectively in the acidic environments of inflamed OA joints (He et al., 2021). This targeted delivery not only improves AG’s therapeutic efficacy but also minimizes systemic side effects (Cheng et al., 2023). Another innovative approach involves the use of AG-loaded liposomes, which enhance the stability and bioavailability of AG, while allowing for controlled release in the affected joint tissues, thereby reducing inflammation and protecting cartilage from degradation. Although clinical applications of AG nanoparticles in OA remain in their early stages, preclinical studies suggest promising therapeutic outcomes (He et al., 2021; Hodgkinson et al., 2022).
3.1.3.6 Diacerein (DIA)
Diacerein (DIA) is a widely studied anti-inflammatory drug used to slow the progression of OA (Bernetti et al., 2019). It inhibits the synthesis of pro-inflammatory cytokines like IL-1β, reducing cartilage degradation. However, DIA is associated with gastrointestinal side effects, which limit its long-term use (Panova and Jones, 2015). Nanoparticle-based delivery systems, such as PLGA nanoparticles, have been employed to enhance the bioavailability and minimize the adverse effects of DIA (Jung et al., 2020). DIA-loaded PLGA nanoparticles provide a sustained release profile, ensuring that therapeutic levels of the drug are maintained for extended periods within the joint space. This not only reduces the frequency of administration but also improves patient compliance (Jung et al., 2020). Another advanced approach involves the development of pH-responsive DIA-loaded nanoparticles, which allow for drug release specifically in inflamed environments, minimizing side effects while maximizing efficacy in OA treatment. Although human clinical trials are still ongoing, these innovations represent a significant step forward in enhancing the therapeutic utility of DIA for OA (Hu et al., 2020).
3.1.3.7 Naringin nanoparticles
Naringin, a flavonoid derived from citrus fruits, has gained attention for its ability to promote cartilage regeneration and inhibit inflammatory pathways, making it a valuable candidate for OA therapy (Gan et al., 2023; Peng et al., 2024). However, like many other natural compounds, its clinical use is hindered by poor bioavailability and rapid clearance. Nanotechnology has provided solutions to these challenges through the development of advanced delivery systems. For example, polycaprolactone/polyethylene glycol-naringin (PCL/PEG-Nar) nanofiber membranes have been developed as a pH-responsive system for the sustained release of Naringin in OA-affected joints (Lan et al., 2020). This innovative approach ensures a steady release of the compound over time, reducing the severity of OA symptoms and promoting cartilage repair. Another promising technique involves the use of Naringin-loaded SLNs, which enhance the compound’s stability, bioavailability, and controlled release within joint tissues. SLNs have the added advantage of being biocompatible and biodegradable, making them an ideal platform for long-term OA treatment (Munir et al., 2021). While clinical applications of Naringin nanoparticles are still under investigation, preclinical studies have shown promising results, particularly in reducing inflammation and promoting cartilage regeneration in OA models (Ravetti et al., 2023).
3.2 Metallic nanoparticles in the treatment of OA
Oxidative stress, an imbalance between reactive oxygen species (ROS) and antioxidant defenses, plays a crucial role in the pathophysiology of OA. Excessive ROS disrupts redox signaling and damages key macromolecules such as proteins, lipids, and DNA, accelerating cartilage degradation and exacerbating joint inflammation (Ansari et al., 2020). In additionally, OA is also characterized by the activation of pro-inflammatory pathways, notably the NF-κB pathway, and the NLRP3 inflammasome, both of which contribute to the production of pro-inflammatory cytokines and enzymes responsible for cartilage breakdown (Ramirez-Perez et al., 2022; Xiao and Zhang, 2023; Yang et al., 2022).
Recent advances in nanotechnology have highlighted metallic nanoparticles (MNPs) as potential therapeutic agents for OA, not only as carriers for drugs and natural compounds but also as a promising therapeutic approach to directly counteract these destructive processes (Akhileshwar Jha et al., 2024). Metallic nanoparticles such as gold (AuNPs), silver (AgNPs), cerium oxide (CeO2NPs), and zinc oxide (ZnONPs) have shown potent antioxidant properties by scavenging ROS and reducing oxidative damage. Furthermore, these nanoparticles can directly modulate inflammatory processes and inhibit cartilage degradation, targeting key pathways like NF-κB and NLRP3 inflammasome activation, thus offering new possibility for OA treatment (Nayal et al., 2024).
3.2.1 Gold nanoparticles
Gold nanoparticles (AuNPs) are extensively studied for their anti-inflammatory and antioxidative effects. AuNPs counteract oxidative stress, a major driver of OA, by scavenging ROS and restoring redox balance. Their inhibition of the NF-κB pathway reduces the production of pro-inflammatory cytokines like IL-1β and TNF-α, thus mitigating cartilage degradation and synovial inflammation (Wen et al., 2023; Abdel-Aziz et al., 2021). Functionalized AuNPs can neutralize ROS through interaction with protein thiol groups, further preventing cartilage damage. AuNPs also have favorable biocompatibility and low toxicity, though their tendency to accumulate raises concerns about long-term safety. Moreover, the high cost of gold limits large-scale production (Kus-Liśkiewicz et al., 2021).
3.2.2 Silver nanoparticles
Silver nanoparticles (AgNPs) exhibit strong anti-inflammatory and antimicrobial properties, offering dual protection by reducing inflammation and preventing infections that may exacerbate OA (Ferdous and Nemmar, 2020; Gherasim et al., 2020). AgNPs effectively scavenge ROS and inhibit the NF-κB pathway, thereby lowering the production of matrix metalloproteinases (MMPs) and other cartilage-degrading enzymes (He et al., 2024; Akter et al., 2018). Furthermore, AgNPs can be incorporated into hydrogels or nanocomposites to provide sustained anti-inflammatory effects. However, their relatively higher cytotoxicity poses limitations for long-term use (Pangli et al., 2021; Nandhini et al., 2024).
3.2.3 Cerium oxide nanoparticles
Cerium oxide nanoparticles (CeO2NPs), or nanoceria, stand out due to their redox-active properties, mimicking natural antioxidant enzymes like superoxide dismutase (SOD) and catalase (Dhall and Self, 2018). These nanoparticles continuously scavenge ROS through their ability to switch between Ce3+ and Ce4+ oxidation states, reducing oxidative stress in OA joints (Corsi et al., 2023; Xiong et al., 2023). CeO2NPs also inhibit NLRP3 inflammasome activation, which helps lower pro-inflammatory cytokines like IL-1β and IL-18 (Li et al., 2024a). Preclinical studies have shown that CeO2NPs preserve cartilage integrity and improve joint function, with low cytotoxicity making them suitable for long-term use (Xiong et al., 2023). However, the technical challenges in producing precise CeO2NPs and the need for further investigation into their long-term effects remain.
3.2.4 Zinc oxide nanoparticles
Zinc oxide nanoparticles (ZnONPs) present a promising approach to OA treatment due to their antioxidant and anti-inflammatory effects (Lopez-Miranda et al., 2023). They modulate the NF-κB pathway, decreasing the production of pro-inflammatory mediators such as MMPs and cytokines, while directly scavenging ROS to prevent oxidative damage in cartilage and synovial tissues (Azeez et al., 2024). ZnONPs also enhance chondrocyte survival and promote cartilage regeneration by upregulating anabolic factors and increasing antioxidant enzyme production (Li et al., 2020; Mirza et al., 2015). Zinc’s affordability and the non-toxic byproducts of ZnONP degradation make them an appealing option for large-scale therapeutic applications, though more research is needed to ensure their long-term safety in clinical settings (Eker et al., 2024).
3.3 Molecular OA therapies enhanced by nanotechnologies
Nanotechnologies, when combined with gene-specific inhibitors like siRNA and shRNA, represent a promising frontier in OA therapy (Rai et al., 2019). Nanoparticles enhance drug delivery by increasing stability, targeting capabilities, and sustaining therapeutic release. This results in more precise modulation of key OA drivers, such as inflammation, oxidative stress, and cartilage degradation. Below, we explore the technical applications of various molecular therapies, such as Protein Kinase D (PKD) inhibitors, p47phox, p66shc, and the peptide KAFAK inhibitors in combination with the nanoparticle systems (Kumari et al., 2023; Li et al., 2024b; Yan et al., 2019; Jeong et al., 2020).
3.3.1 Protein kinase D (PKD) inhibitors in nanoparticle systems
PKD is critical in regulating extracellular matrix (ECM) destruction and driving OA progression by activating the NF-κB pathway, which intensifies inflammation and matrix degradation (Baker et al., 2018). Nanoparticles, particularly PLGA, have been shown to deliver PKD inhibitors effectively, offering controlled and sustained release. This system enhances the inhibition of NF-κB activation and cytokine production (e.g., IL-1β), minimizing ECM degradation more effectively than free PKD inhibitors (Cho et al., 2019). Moreover, biomimetic nanoparticles, such as M2 macrophage-coated particles, exhibit enhanced targeting capabilities by mimicking immune responses, allowing for high concentrations of PKD inhibitors directly in inflamed joints, thereby reducing local inflammation. Biomimetic nanoparticles offer enhanced targeting of inflamed tissues, making them ideal for localized OA therapy (Cho et al., 2019; Rao and Shi, 2022). The choice of nanoparticle system should depend on the specific characteristics of the PKD inhibitor (e.g., hydrophobicity) and more clinical trials are needed for ideal therapeutic outcomes (e.g., sustained release, targeted delivery, or combination therapy) (Kumar et al., 2024; Tenchov et al., 2022; Yetisgin et al., 2020).
3.3.2 Peptide KAFAK inhibitors
KAFAK is a peptide known for suppressing pro-inflammatory cytokines, including IL-1β and IL-6, key factors in OA pathology (Bartlett et al., 2013). Nanoparticles coated with M2 macrophage membranes, incorporating KAFAK, have demonstrated precision in targeting inflamed joints (Zhou et al., 2023). By modifying these nanoparticles with iRGD peptides and hyaluronic acid, sustained release is achieved, leading to reduced inflammation and protection of cartilage (Zhou et al., 2023; Zhang S. et al., 2022). The use of macrophage membrane coatings enhances precise delivery to inflamed joints and immune evasion, increasing the therapeutic efficacy of KAFAK inhibitors. However, the potential immune response to foreign coatings may necessitate further optimization through complex clinical trials (Zheng et al., 2023).
3.3.3 shRNA-LEPR encapsulation in nanoparticle systems
The leptin receptor (LEPR) contributes to inflammation and cartilage breakdown in OA. Targeting LEPR with shRNA has proven effective in reducing its expression and mitigating inflammation. Biomimetic nanoparticles incorporating shRNA-LEPR, along with polyethylenimine (PEI) for gene delivery, offer sustained intra-articular release (Zhou et al., 2023; Li S. et al., 2024). Hyaluronic acid further enhances targeting to joint tissues, while M2 macrophage coatings improve localization in inflamed areas, efficient gene silencing on top of sustained release, leading to effective reduction in cartilage degradation (Li S. et al., 2024). However, long-term application maybe limited by the potential off-target effects, which could complicate the regulation of gene expression (Zha et al., 2021).
3.3.4 siRNA-p47phox in PLGA nanoparticles
The p47phox subunit of NADPH oxidase is involved in reactive oxygen species (ROS) production, contributing to OA-related oxidative stress and cartilage damage. Encapsulating siRNA-p47phox in PLGA nanoparticles provides sustained release, reducing ROS production and inflammation (Shin et al., 2020a). This system not only alleviates oxidative damage but also preserves cartilage integrity for a prolonged therapeutic effect. The limited stability of siRNA in biological environments has hindered its clinical application in OA treatment (Kumari et al., 2023).
3.3.5 siRNA-p66shc in nanoparticles
Overexpression of p66shc contributes to mitochondrial dysfunction and ROS overproduction in OA. Nanoparticles encapsulating siRNA-p66shc have shown efficacy in reducing ROS levels and inflammatory markers (e.g., IL-1β, TNF-α). By suppressing p66shc expression, these nanoparticles can modulate oxidative stress and inflammation, providing a targeted approach to slow OA progression (Shin et al., 2020b). Future studies should focus on optimizing the nanoparticle delivery efficiency and improving formulations stability (Liu et al., 2023a).
3.3.6 P16INK4a and synovial inflammation
P16INK4a, a cell cycle regulator, is upregulated in fibroblast-like synoviocytes (FLS) during OA, contributing to joint damage (Damerau et al., 2024). siRNA targeting P16INK4a, encapsulated in PLGA nanoparticles, accumulates in synovial tissues and reduces inflammation by lowering IL-1β levels in FLS, providing a localized therapeutic option for specific reduction of synovial inflammation (Park et al., 2022). However, challenges such as off-target effects and delivery efficiency to specific joint compartments remain obstacles for clinical applications (Kumari et al., 2023; Li et al., 2024b).
3.3.7 Black phosphorus nanosheets for cartilage and bone repair
Black phosphorus nanosheets represent a novel platform for OA treatment due to their pH-responsive behavior and ability to scavenge ROS (Zhang X. et al., 2022). These nanosheets promote cartilage regeneration and subchondral bone repair by protecting tissues from oxidative stress and modulating the joint’s inflammatory environment (Lu H. et al., 2023) (Table 1). However, research on black phosphorus nanosheet for OA treatment is still in the early-stages. Comprehensive clinical data are needed, particularly regarding sustained release, targeted delivery, combination therapies, and potential toxicity concerns (Zhuang et al., 2024).
TABLE 1
| Composition | Cell | Animal | Dose | Outcome | Refernces |
|---|---|---|---|---|---|
| MLX-Ca (AC)2Lipo | ATDC5 | Rats |
In vitro: 20 μM In vivo:4 mM |
Degenerated cartilage area↓ | Hu et al., (2020) |
| CBD-PLGA-NPs | Rats’ primary chondrocytes | — |
In vitro: 20 μg/mL In vivo:/ |
IL-1β, IL-6, TNF-α, MMP13↓ | Gherasim et al., (2020) |
| SFNs-CXB | Human primary chondrocytes | — |
In vitro: 800 μg/mL In vivo:/ |
ROS, IL-6↓ | Pangli et al., (2021) |
| CUR-PLGA NPs | — | Rats |
In vitro:/ In vivo: 200 mg/kg |
NK-κB, Cleaved caspase3↓ | Dhall and Self, (2018) |
| ACP | RAW264.7 | Mice |
In vitro: 10,25 μg/mL In vivo: 2.5, 5 mg/kg |
TNF-α; IL-1β; ROS↓ Aggrecan↑ Degenerated cartilage area↓ |
Xiong et al., (2023) |
| PEG-FMN NPs | Rats’ primary chondrocytes | Rats |
In vitro: 1.25 μg/mL, 14 μg/mL In vivo: 1.25 ug/mL |
MMP13↓ Degenerated cartilage area↓ |
Azeez et al., (2024) |
| AG@MSNs-PAA | Rats’ primary chondrocytes | Rats |
In vitro: 8 μM In vivo: 8 μM |
MMP13↓ Aggrecan; COL2↑ |
Eker et al., (2024) |
| DIA-PLGA NPs | Rats’ primary synoviocytes | Rats |
In vitro: 10 μg/mL In vivo: 90.61 μg/rat, 470.20 μg/mL |
IL-1; IL-6; MMP3; COX-2; TNF-α↓ IL-4; IL-10↑ |
Li et al., (2024b) |
| MRC-PPL-PSO | Mice Primary chondrocytes | Mice |
In vitro: 15 μM In vivo:15 μM |
IL-1β; MMP3, TNF-α; MMP13; NF-κB, p-P38, p-AKT↓ COL2↑ |
Yan et al., (2019) |
| Ta-NH2 NPs | Rats’ primary chondrocytes | Rats |
In vitro: 100 μg/mL In vivo: 10 μg |
iNOS↓ Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ |
Bartlett et al., (2013) |
| AuNPs | — | Rats |
In vitro:/ In vivo: AuNPs: 30 μg/kg |
serum estrogen↓ IL-6, IL-β, TNF-α, COX-1, COX-2↓ |
Zhang et al., (2022b) |
| E@Au-Ag NPs | Rats’ primary chondrocytes | Rats |
In vitro: 12 μg/mL In vivo:/ |
ROS↓ Apoptosis↓ Degenerated cartilage area↓ |
Zheng et al., (2023) |
| Au@PDA-WL NPs | ATDC5 | Mice |
In vitro: 15, 30, 60, 120, 240, 360 p.m. In vivo: 25 μL |
Collage II↑ ROS↓ Degenerated cartilage area↓ |
Zha et al., (2021) |
| H-MnO2 NPs | — | Mice |
In vitro:/ In vivo: 6 μg |
IL-6; IL-1β; TNF-α↓ Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ |
Zhuang et al., (2024) |
| Mn3O4@CS | SW1353 | Mice |
In vitro: 8 μg/mL In vivo: 0.4 μg |
iNOS, COX2, MMP13↓ SOD, CAT, COL2↑ |
Knights et al., (2023) |
| Mil-88a nano-enzyme | Mice Primary chondrocytes | Mice |
In vitro:1–10 μg/mL In vivo:/ |
MMP13↓ SOD, Col2↑ Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ |
Van Osch et al., (2009) |
| PLGA NP AB | C28/I2 | Mice |
In vitro:10–60 μg/mL In vivo: 20 μg |
Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ | Jeyaraman et al., (2024) |
| PLGA-HA | RAW264.7 | Mice |
In vitro:3.9, 7.8, 15.6, 31.25, 62.5, 125 μg/mL In vivo: 10 mg/mL |
NO↓ | Roseti et al., (2019) |
| HA-NP | Mice primary chondrocytes | Mice |
In vitro: 80 μg/mL In vivo: 0.2 mg/mL |
NK-κB, MMP3, MMP13,COX-2, PGE2↓ Degenerated cartilage area↓ |
Liang et al., (2023) |
| PEG-PLGA-HA | C28/I2 | Mice | In vivo: 2.3 mg/mL | Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ | Wang et al., (2024) |
| PLEL@PL-NPs | ATDC5; primary human articular chondrocytes | Rats |
In vitro: 0–2,500 μg/mL In vivo: 50 μL |
IL-6, TNF-α, iNOS, COX-2,CD68↓ COL2↑ Degenerated cartilage area↓ |
Wu et al., (2024) |
| PDKi-NPs | Pigs’ primary chondrocytes | — |
In vitro: 10 μM In vivo:/ |
Caspase3, p-Akt, NO, PGE2, NF-κB, MMP14, P53↓ ACAN, COL2, SOX9↑ |
Chen et al., (2020) |
| macrophage membrane-coated KAFAK-shRNA-LEPR-PEI-NPs | RAW264.7 | Rats |
In vitro: 1 μg/mL In vivo:/ |
TNF-α; IL-2β, CD86↓ IL-10, COL2, CD2↑ |
Atwal et al., (2023) |
| P47phox siRNA-PLGA-NPs | — | Rats |
In vitro:/ In vivo: 0.2 μM |
ROS↓ Degenerated cartilage area↓ |
Carton and Malatesta, (2024) |
| P16INK4a siRNA-PLGA-NPs | Primary cultured human articular chondrocytes and fibroblast-like synoviocytes | Mice |
In vitro: 50, 100, 200,600, 1,000 μg/mL In vivo: 200 μg |
TNF-α, IL-1β, IL-6, MMP13↓ | Li et al., (2024d) |
| BPNSs | Rats’ primary chondrocytes | Rats |
In vitro: 10 μg/mL, 20 μg/mL In vivo: 10 μg/mL |
ADAMTS5, ADAMTS1↓ COL2, Aggrecan, RUNX2, BMP2↑ |
Jiang et al., (2024) |
Nanomedicines to reduce inflammation of synovial and articular cartilage.
SFNs: silk fibroin nanoparticles; CXB: celecoxib; ACP: acid-activatable curcumin polymer; PEG: poly (ethylene glycol); FMN: formononetin; AG: andrographolide; MSNs: mesoporous silica nanoparticles; PAA: pH-responsive polyacrylic acid; DIA: diacerein; PLGA: poly (d,l-lactide-co-glycolide); NPs: nanoparticles; MRC-PPL: cartilage-targeting and OA-specific theranostic nanoplatforms; PSO: psoralen; Au: Gold; DIA: diacerein; Ta-NH2-NPs: tantalum nanoparticles; E@Au-Ag NPs: EGCG (Epigallocatechin gallate) decorated Au-Ag nano-jars; PDA: polydopamine; WL: WYRGRL; H-MnO2: Hollow- MnO2; HA: hyaluronic acid; PLEL: poly (d, L-lactide)-poly (ethylene glycol)-poly (d, L-lactide); PL: platelet lysate; PDKi: protein kinase D inhibitor. BPNSs: Black phosphorus nanosheets; CS: chondroitin sulfate.
In summary, nanoparticle-based delivery systems for molecular OA treatments offer numerous advantages, including enhanced targeting, sustained release, and reduced side effects. However, challenges such as off-target effects, nanoparticle stability, and scaling up for clinical applications remain. Future studies should prioritize optimizing nanoparticle formulations, enhancing bioavailability, and conducing long-term safety evaluations.
4 Nanomedicines for cartilage regeneration in OA
While inflammation plays a significant role in the progression of OA, regenerating damaged cartilage is key to achieving long-term disease modification (Knights et al., 2023). The unique challenges posed by cartilage, such as its avascularity and limited cellular repair mechanisms, make effective regeneration difficult (Van Osch et al., 2009; Jeyaraman et al., 2024; Roseti et al., 2019). Nanotechnology offers a promising avenue for overcoming these obstacles, providing innovative strategies that focus on cartilage repair and regeneration. In this chapter, we explore advanced nanomedicine approaches that aim to restore cartilage integrity, utilizing nanoparticles, scaffolds, and biologically active molecules for sustained, localized, and effective treatment (Eftekhari et al., 2020; Liang et al., 2023).
4.1 Key nanomedicines that promote cartilage regeneration
While inflammation is a significant contributor to OA progression, the ability to regenerate damaged cartilage remains a critical challenge in achieving meaningful disease modification (Roseti et al., 2019). Current pharmacological and surgical interventions often fail to fully restore articular cartilage due to the tissue’s limited self-repair capabilities. The avascular nature of cartilage, combined with its low density of chondrocytes, restricts its ability to recover from injury, leading to the gradual deterioration of joint function (Wang et al., 2024).
Traditional regenerative approaches, such as cell-based therapies and growth factor injections, have shown potential but are hampered by issues such as poor cell survival, lack of integration with native tissues, and the inability to control the precise delivery of therapeutic agents over time (Tsujii et al., 2024). These limitations highlight the need for advanced strategies that can enhance cartilage repair while ensuring sustained, localized effects (Wang et al., 2024).
Nanotechnology offers transformative solutions to these challenges by enabling the controlled and targeted delivery of bioactive molecules directly to sites of cartilage damage (Qiao et al., 2022). Nanomedicines can be engineered to deliver a variety of therapeutic agents—including growth factors, cytokines, and gene therapies—in a sustained manner, maximizing their efficacy (Liu et al., 2023b). Nanofibrous scaffolds, designed to mimic the extracellular matrix (ECM) of cartilage, provide structural support for cell attachment and proliferation. These scaffolds can be functionalized with growth factors or cells to further promote cartilage regeneration (Huang et al., 2024).
Key growth factors, such as transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs), play pivotal roles in cartilage repair (Zhang X. et al., 2022; Wu et al., 2024). However, delivering these proteins effectively is challenging due to their short half-life and rapid degradation in the joint environment (Thielen et al., 2019). Nanoparticle carriers, such as PLGA nanoparticles combined with nanofibrous scaffolds, have demonstrated success in encapsulating these growth factors, providing sustained release while protecting them from degradation. These integrated nanotechnologies have been shown to stimulate chondrocyte activity more effectively, thereby enhancing cartilage regeneration (Chen et al., 2020; Qin et al., 2023).
Additionally, injectable hydrogels containing nanoparticles and cartilage-promoting factors can fill cartilage defects, offering both mechanical support and bioactivity. These hydrogels are often modified with materials like hyaluronic acid or collagen to better mimic the native cartilage environment. As they promote the growth of new cartilage tissue, they also integrate with existing tissues, with their biodegradability ensuring gradual replacement by natural tissue (Atwal et al., 2023; Carton and Malatesta, 2024).
4.2 Exosome-mimicking nanoparticles
Exosome-mimicking nanoparticles represent an innovative therapeutic strategy for OA by replicating the biological functions of natural exosomes, which are small cell-derived vesicles involved in intercellular communication and tissue repair (Chavda et al., 2023). These engineered nanoparticles hold promise in drug delivery, gene therapy, and tissue regeneration, offering targeted solutions for modulating the inflammatory and degenerative processes associated with OA (Li L. et al., 2024).
Natural exosomes, typically 30–150 nm in diameter, are secreted by various cell types such as mesenchymal stem cells (MSCs) and chondrocytes. They facilitate the transfer of bioactive molecules, including proteins, lipids, and RNA, which play critical roles in inflammation modulation and cartilage repair (Jiang et al., 2024). MSC-derived exosomes can promote chondrocyte migration and proliferation via the Mir-106b-5P/TIMP2 signaling pathway or activating YAP through the Wnt pathway (Tao et al., 2017; Tan et al., 2020). They can also reduce MMP13 and ADAMTS5 levels in chondrocytes, reverse mitochondrial membrane potential changes, and alleviate OA (Cosenza et al., 2017), potentially by inhibiting phosphorylation of p38 and ERK and promoting protein kinase B phosphorylation (Zhai et al., 2022). However, their clinical application faces challenges, such as low yield, heterogeneity, and instability during storage. Exosome-mimicking nanoparticles address these limitations by offering greater stability, scalability, and the ability to customize properties for specific therapeutic needs (Wang X. et al., 2022; Tan et al., 2024).
These nanoparticles are engineered to emulate the structural and functional characteristics of natural exosomes. Constructed from biodegradable materials like liposomes, polymeric nanoparticles, or silica nanoparticles, they are functionalized with surface proteins, ligands, or targeting molecules like hyaluronic acid or MSC-derived membrane proteins. These surface modifications enable the delivery of bioactive molecules, such as miRNAs or proteins, to stimulate cartilage regeneration without the complexity of direct stem cell therapies (Li Y.-J. et al., 2021). Several studies have shown the potential of exosome-mimicking nanoparticles in OA treatment. For instance, nanoparticles mimicking exosomes derived from bone marrow mesenchymal stem cells (BM-MSCs), loaded with growth factors and RNA molecules, have been shown to reduce cartilage degradation, improve joint function, and lower pro-inflammatory cytokine levels in OA models (Cosenza et al., 2017). Additionally, HA-coated exosome-mimicking nanoparticles have demonstrated effective delivery of siRNA targeting MMP13—an enzyme involved in cartilage breakdown—leading to enhanced cartilage preservation in animal studies (Kumari et al., 2023; Qiu et al., 2013) (Table 2).
TABLE 2
| Composition | Cell | Animal | Dose | Outcome | References |
|---|---|---|---|---|---|
| KGN-PLGA-PEG-PLGA-BMSCs | — | Rabbits |
In vitro:/ In vivo: 50 mg/kg |
Degenerated cartilage area↓ | Zhou et al. (2015) |
| CD90@ NPs | — | Rabbits |
In vitro:/ In vivo:12 mg/rat |
CD68, iNOS↓ IL-10, IGF1, CYCLIN B↑ Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ |
Cosenza et al. (2017) |
| hASC-EVs | Human chondrocytes-osteoarthritis | Rats |
In vitro: 1×108, 2×108 particles/mL In vivo: 1×108 particles/rat |
MMP1, MMP3, MMP13, ADAMTS5, IL-1β, CD86↓ | Zhai et al. (2022) |
| PPD-MSC-sEVs | Human chondrocytes-osteoarthritis | Mice |
In vitro:/ In vivo: 1×107 particles/mice |
ADAMTS5, MMP13, IL-1β, TNF-α↓ Aggrecan, COL1↑ |
Li et al. (2021a) |
| MMP13 siRNA NPs | ATDC5 | Mice |
In vitro:/ In vivo: 1,875 nmol |
MMP13↓ Degenerated cartilage area, degenerated surface cartilage width, total osteophyte volume ↓ |
Kalashnikova et al. (2020) |
Nanomedicine that promotes cartilage regeneration and repair.
KGN: kartogenin; BMSCs: bone marrow MSCs; mPEG-Hz-b-PCL: methoxy poly (ethylene oxide)-hydrazone-poly (ε-caprolactone) copolymers; CD90@ NPs: CD90+ MCS-derived micro-vesicle-coated nanoparticle; hASC-EVs: human adipose-derived stem cells extracellular vesicles; PPD: ε-polylysine-polyethylene-distearyl phosphatidylethanolamine; MMP: matrix metalloproteinase.
4.3 Mesenchymal stem cells in nanoparticle therapy
Nanoparticle-encapsulated mesenchymal stem cell (MSC) therapy is a promising new approach for OA treatment, combining the regenerative capabilities of MSCs with the advantages of nanoparticles, such as targeted delivery and enhanced stability (Ding et al., 2022; Bunnell, 2021; Mizuno et al., 2022; Hoang et al., 2022). This method addresses key limitations of traditional MSC-based therapies, such as low cell survival, poor retention in joint tissues, and insufficient targeting (Zou et al., 2023). By using nanoparticles, the therapeutic potential of MSCs in cartilage repair, inflammation modulation, and slowing OA progression is significantly enhanced (Cai et al., 2023).
MSCs are multipotent cells that can differentiate into chondrocytes, the cells responsible for maintaining cartilage integrity. This chondrogenic potential is linked to the upregulation of SOX9, a key marker for chondrocyte progenitors (Zhao et al., 2017). MSCs also secrete bioactive molecules like TGF-β and BMP, which promote bone formation and increase extracellular matrix (ECM) production by inducing SOX9 expression (Cai et al., 2023; Du et al., 2023). Studies have shown that MSC injections can restore chondrocyte proliferation, inhibit apoptosis, regulate inflammation, and help ki67 expression in damaged cartilage (Zhang et al., 2021) (Figure 3). However, despite these benefits, MSCs often exhibit poor retention and rapid degradation after intra-articular injection, limiting their therapeutic efficacy.
FIGURE 3
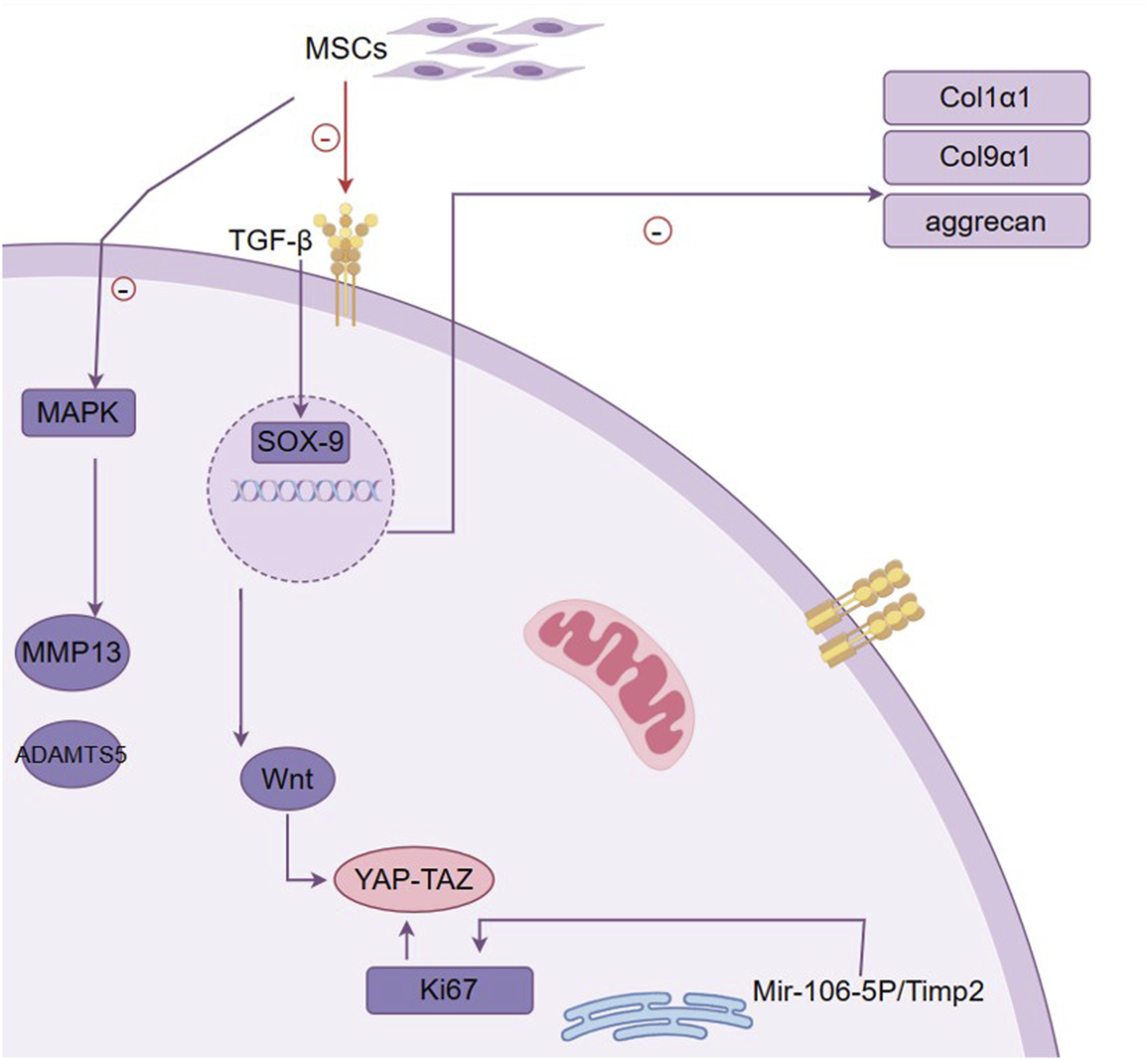
Mechanism of MSC in promoting cartilage repair. This figure outlines the pivotal role of mesenchymal stem cells (MSCs) in cartilage repair and the molecular mechanisms involved. It demonstrates how MSCs contribute to the restoration and maintenance of cartilage through their differentiation into chondrocytes, influenced by signaling molecules such as TGF-β. The figure highlights the activation of the transcription factor SOX-9, which is essential for chondrogenic differentiation and is upregulated by TGF-β. SOX-9 then promotes the expression of key extracellular matrix (ECM) components, including type II collagen (Col2a1) and aggrecan, which are crucial for cartilage structure and function. Additionally, the figure indicates the involvement of the MAPK pathway, which can lead to increased expression of MMP13 and ADAMTS5, enzymes associated with cartilage degradation. The interaction between the Wnt pathway and YAP-TAZ signaling is also depicted, illustrating their role in cell proliferation and tissue regeneration. The figure encapsulates the complex network of interactions that underlie MSC-induced chondrogenesis, highlighting the therapeutic potential of MSCs in treating osteoarthritis by enhancing cartilage repair and reducing inflammation.
To overcome these challenges, various types of nanoparticles are being investigated to encapsulate MSCs or their secreted products. These include polymeric nanoparticles, liposomes, and hydrogels. For example, PLGA nanoparticles have been used to encapsulate MSCs or MSC-derived exosomes, promoting chondrocyte proliferation and enhancing cartilage matrix production. In preclinical models, this approach has shown potential in reducing cartilage degradation (Fan et al., 2006). Nanoparticles can also deliver anti-inflammatory cytokines, such as IL-10 and TGF-β, in a controlled manner, leading to reduced synovitis and cartilage destruction. While most research in nanoparticle-encapsulated MSC therapy remains at the preclinical stage, early results in animal models are promising (Gonzalez-Fernandez et al., 2022). These studies demonstrate improved cartilage regeneration, reduced inflammation, and better joint function. However, further investigation is required to optimize nanoparticle formulations, ensure long-term safety, and assess the clinical efficacy of these therapies in human OA patients (Seo et al., 2022).
5 Conclusion: Recent advances in nanotechnologies for OA treatment
Recent advancements in nanotechnologies offer promising avenues for overcoming the limitations of traditional OA therapies. Nanoparticles have been utilized as vectors to improve the delivery, bioavailability, and stability of existing OA treatments, such as NSAIDs, by enabling targeted, sustained release directly into the inflamed joints. Metal-based nanoparticles, particularly those leveraging silver and copper, have shown potential in reducing oxidative stress, though concerns about toxicity and cost remain.
The combination of nanoparticles with molecular agents, including peptides, siRNA, and shRNA, further enhances the therapeutic potential by allowing for precise targeting of inflammation, oxidative stress, and cartilage degradation at the molecular level. Additionally, nanomedicines designed to promote cartilage regeneration, such as black phosphorus nanosheets, represent a breakthrough in the tissue repair process. Another emerging area is the encapsulation of mesenchymal stem cells (MSCs) in nanoparticles, which improves the efficacy of stem cell therapies in repairing joint tissues and reducing inflammation.
While these nanotechnologies demonstrate significant promise, their clinical applications are still in early stages, with most studies focusing on animal models. Continued research is essential to address challenges related to nanoparticle safety, long-term effects, and scaling up for clinical use. Future directions will likely involve refining nanoparticle formulations for better bioavailability, stability, and minimizing off-target effects, ensuring that nanomedicine becomes a transformative tool in OA management.
Statements
Author contributions
JL: Writing–original draft. QG: Writing–original draft. ZL: Writing–original draft. HW: Writing–original draft. XY: Writing–original draft. RY: Writing–original draft. XZ: Writing–original draft. SS: Writing–review and editing, Conceptualization. LW: Writing–review and editing, Conceptualization. YW: Funding acquisition, Project administration, Software, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (81802504), and a grant from the Sichuan Science and Technology Bureau (2023YFH0010). This study is also supported by the Chengdu Science and Technology Program (2024-YF05-01315-SN).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdel-Aziz M. A. Ahmed H. M. S. El-Nekeety A. A. Sharaf H. A. Abdel-Aziem S. H. Abdel-Wahhab M. A. (2021). Biosynthesis of gold nanoparticles for the treatment of osteoarthritis alone or in combination with Diacerein® in a rat model. Inflammopharmacology29, 705–719. 10.1007/s10787-021-00833-8
2
Ahmadi M. Bekeschus S. Weltmann K.-D. Von Woedtke T. Wende K. (2022). Non-steroidal anti-inflammatory drugs: recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem.13, 471–496. 10.1039/d1md00280e
3
Akhileshwar Jha L. Imran M. Shrestha J. Prasad Devkota H. Bhattacharya K. Alsayari A. et al (2024). Effectiveness of phytoconstituents and potential of phyto-nanomedicines combination to treat osteoarthritis. Eur. Polym. J.215, 113243. 10.1016/j.eurpolymj.2024.113243
4
Akter M. Sikder M. T. Rahman M. M. Ullah A. K. M. A. Hossain K. F. B. Banik S. et al (2018). A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res.9, 1–16. 10.1016/j.jare.2017.10.008
5
Alcantara K. P. Malabanan J. W. T. Nalinratana N. Thitikornpong W. Rojsitthisak P. Rojsitthisak P. (2024). Cannabidiol-loaded solid lipid nanoparticles ameliorate the inhibition of proinflammatory cytokines and free radicals in an in vitro inflammation-induced cell model. Int. J. Mol. Sci.25, 4744. 10.3390/ijms25094744
6
Aldrich J. L. Panicker A. Ovalle R. Sharma B. (2023). Drug delivery strategies and nanozyme technologies to overcome limitations for targeting oxidative stress in osteoarthritis. Pharmaceuticals16, 1044. 10.3390/ph16071044
7
Altman R. Manjoo A. Fierlinger A. Niazi F. Nicholls M. (2015). The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet. Disord.16, 321. 10.1186/s12891-015-0775-z
8
Alvarez-Soria M. A. Largo R. Santillana J. Sánchez-Pernaute O. Calvo E. Hernández M. et al (2006). Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann. Rheum. Dis.65, 998–1005. 10.1136/ard.2005.046920
9
Ansari M. Y. Ahmad N. Haqqi T. M. (2020). Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed. Pharmacother.129, 110452. 10.1016/j.biopha.2020.110452
10
Ashfaq R. Rasul A. Asghar S. Kovács A. Berkó S. Budai-Szűcs M. (2023). Lipid nanoparticles: an effective tool to improve the bioavailability of nutraceuticals. Int. J. Mol. Sci.24, 15764. 10.3390/ijms242115764
11
Assadpour E. Rezaei A. Das S. S. Krishna Rao B. V. Singh S. K. Kharazmi M. S. et al (2023). Cannabidiol-loaded nanocarriers and their therapeutic applications. Pharmaceuticals16, 487. 10.3390/ph16040487
12
Atwal A. Dale T. P. Snow M. Forsyth N. R. Davoodi P. (2023). Injectable hydrogels: an emerging therapeutic strategy for cartilage regeneration. Adv. Colloid Interface Sci.321, 103030. 10.1016/j.cis.2023.103030
13
Azeez S. Fatima M. Gul O. Rehman H. Shad M. A. Nawaz H. (2024). Zinc oxide nanoparticles-doped curcumin-assisted recovery of rheumatoid arthritis and antioxidant status in experimental rabbits. BioMedicine14, 49–59. 10.37796/2211-8039.1446
14
Badri W. Miladi K. Nazari Q. A. Greige-Gerges H. Fessi H. Elaissari A. (2016). Encapsulation of NSAIDs for inflammation management: overview, progress, challenges and prospects. Int. J. Pharm.515, 757–773. 10.1016/j.ijpharm.2016.11.002
15
Baek J.-S. Yeo E. W. Lee Y. H. Tan N. S. Loo S. C. (2017). Controlled-release nanoencapsulating microcapsules to combat inflammatory diseases. Drug Des. devel. Ther.11, 1707–1717. 10.2147/DDDT.S133344
16
Bai H. Yuan R. Zhang Z. Liu L. Wang X. Song X. et al (2021). Intra‐articular injection of baicalein inhibits cartilage catabolism and NLRP3 inflammasome signaling in a posttraumatic OA model. Oxid. Med. Cell. Longev.2021, 6116890. 10.1155/2021/6116890
17
Baker J. Falconer A. M. D. Wilkinson D. J. Europe-Finner G. N. Litherland G. J. Rowan A. D. (2018). Protein kinase D3 modulates MMP1 and MMP13 expression in human chondrocytes. PLOS ONE13, e0195864. 10.1371/journal.pone.0195864
18
Ban C. Jo M. Park Y. H. Kim J. H. Han J. Y. Lee K. W. et al (2020). Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem.302, 125328. 10.1016/j.foodchem.2019.125328
19
Bartlett R. L. Sharma S. Panitch A. (2013). Cell-penetrating peptides released from thermosensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomedicine Nanotechnol. Biol. Med.9, 419–427. 10.1016/j.nano.2012.09.003
20
Bernetti A. Mangone M. Villani C. Alviti F. Valeo M. Grassi M. C. et al (2019). Appropriateness of clinical criteria for the use of SYmptomatic slow-acting drug for OsteoArthritis (sysadoa). A delphi method consensus initiative among experts in Italy. Eur. J. Phys. Rehabil. Med.55, 658–664. 10.23736/S1973-9087.19.05633-8
21
Bigaj-Józefowska M. J. Grześkowiak B. F. (2022). Polymeric nanoparticles wrapped in biological membranes for targeted anticancer treatment. Eur. Polym. J.176, 111427. 10.1016/j.eurpolymj.2022.111427
22
Boehme K. A. Rolauffs B. (2018). Onset and progression of human osteoarthritis—can growth factors, inflammatory cytokines, or differential miRNA expression concomitantly induce proliferation, ECM degradation, and inflammation in articular cartilage?Int. J. Mol. Sci.19, 2282. 10.3390/ijms19082282
23
Boehnke N. Straehla J. P. Safford H. C. Kocak M. Rees M. G. Ronan M. et al (2022). Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science377, eabm5551. 10.1126/science.abm5551
24
Börner F. Werner M. Ertelt J. Meins J. Abdel-Tawab M. Werz O. (2021). Analysis of boswellic acid contents and related pharmacological activities of frankincense-based remedies that modulate inflammation. Pharmaceuticals14, 660. 10.3390/ph14070660
25
Borrajo M. L. Lou G. Anthiya S. Lapuhs P. Álvarez D. M. Tobío A. et al (2024). Nanoemulsions and nanocapsules as carriers for the development of intranasal mRNA vaccines. Drug Deliv. Transl. Res.14, 2046–2061. 10.1007/s13346-024-01635-5
26
Bryk M. Starowicz K. (2021). Cannabinoid-based therapy as a future for joint degeneration. Focus on the role of CB2 receptor in the arthritis progression and pain: an updated review. Pharmacol. Rep.73, 681–699. 10.1007/s43440-021-00270-y
27
Buhrmann C. Brockmueller A. Mueller A.-L. Shayan P. Shakibaei M. (2021). Curcumin attenuates environment-derived osteoarthritis by Sox9/NF-kB signaling Axis. Int. J. Mol. Sci.22, 7645. 10.3390/ijms22147645
28
Bunnell B. A. (2021). Adipose tissue-derived mesenchymal stem cells. Cells10, 3433. 10.3390/cells10123433
29
Busa P. Lee S.-O. Huang N. Kuthati Y. Wong C.-S. (2022). Carnosine alleviates knee osteoarthritis and promotes synoviocyte protection via activating the Nrf2/HO-1 signaling pathway: an in-vivo and in-vitro study. Antioxidants11, 1209. 10.3390/antiox11061209
30
Cai Y. Wu C. Ou Q. Zeng M. Xue S. Chen J. et al (2023). Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-β1. Bioact. Mater.19, 444–457. 10.1016/j.bioactmat.2022.04.021
31
Cao F. Gui S. Y. Gao X. Zhang W. Fu Z. Y. Tao L. M. et al (2022). Research progress of natural product-based nanomaterials for the treatment of inflammation-related diseases. Mater. Des.218, 110686. 10.1016/j.matdes.2022.110686
32
Carrillo-Martinez E. J. Flores-Hernández F. Y. Salazar-Montes A. M. Nario-Chaidez H. F. Quercetin L. D. (2024). Quercetin, a flavonoid with great pharmacological capacity. Molecules29, 1000. 10.3390/molecules29051000
33
Carton F. Malatesta M. (2024). Nanotechnological research for regenerative medicine: the role of hyaluronic acid. Int. J. Mol. Sci.25, 3975. 10.3390/ijms25073975
34
Chavda S. Rabbani S. A. Wadhwa T. (2022). Role and effectiveness of intra-articular injection of hyaluronic acid in the treatment of knee osteoarthritis: a systematic review. Cureus14, e24503. 10.7759/cureus.24503
35
Chavda V. P. Pandya A. Kumar L. Raval N. Vora L. K. Pulakkat S. et al (2023). Exosome nanovesicles: a potential carrier for therapeutic delivery. Nano Today49, 101771. 10.1016/j.nantod.2023.101771
36
Chen L. Liu J. Guan M. Zhou T. Duan X. Xiang Z. (2020). Growth factor and its polymer scaffold-based delivery system for cartilage tissue engineering. Int. J. Nanomedicine15, 6097–6111. 10.2147/IJN.S249829
37
Chen M. Li L. Wang Z. Li P. Feng F. Zheng X. (2019). High molecular weight hyaluronic acid regulates P. gingivalis–induced inflammation and migration in human gingival fibroblasts via MAPK and NF-κB signaling pathway. Arch. Oral Biol.98, 75–80. 10.1016/j.archoralbio.2018.10.027
38
Chen W. Jin G. J. Xiong Y. Hu P. F. Bao J. P. Wu L. D. (2018). Rosmarinic acid down‐regulates NO and PGE 2 expression via MAPK pathway in rat chondrocytes. J. Cell. Mol. Med.22, 346–353. 10.1111/jcmm.13322
39
Cheng X. Xie Q. Sun Y. (2023). Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol.11, 1177151. 10.3389/fbioe.2023.1177151
40
Chevalier X. Goupille P. Beaulieu A. D. Burch F. X. Bensen W. G. Conrozier T. et al (2009). Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis Care Res.61, 344–352. 10.1002/art.24096
41
Cho H. Bhatti F.-U.-R. Hasty K. A. Yi A.-K. (2019). Nanosome-Mediated delivery of protein kinase D inhibitor protects chondrocytes from interleukin-1β-induced stress and apoptotic death. Int. J. Nanomedicine14, 8835–8846. 10.2147/IJN.S218901
42
Choi S. J. McClements D. J. (2020). Nanoemulsions as delivery systems for lipophilic nutraceuticals: strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol.29, 149–168. 10.1007/s10068-019-00731-4
43
Chow Y. Y. Chin K.-Y. (2020). The role of inflammation in the pathogenesis of osteoarthritis. Mediat. Inflamm.2020, 8293921. 10.1155/2020/8293921
44
Chuang S.-Y. Lin C.-H. Huang T.-H. Fang J.-Y. (2018). Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials8, 42. 10.3390/nano8010042
45
Colletti A. Cicero A. F. G. (2021). Nutraceutical approach to chronic osteoarthritis: from molecular research to clinical evidence. Int. J. Mol. Sci.22, 12920. 10.3390/ijms222312920
46
Conaghan P. G. Cook A. D. Hamilton J. A. Tak P. P. (2019). Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol.15, 355–363. 10.1038/s41584-019-0221-y
47
Cooper C. Chapurlat R. Al-Daghri N. Herrero-Beaumont G. Bruyère O. Rannou F. et al (2019). Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say?Drugs Aging36, 15–24. 10.1007/s40266-019-00660-1
48
Corsi F. Deidda Tarquini G. Urbani M. Bejarano I. Traversa E. Ghibelli L. (2023). The impressive anti-inflammatory activity of cerium oxide nanoparticles: more than redox?Nanomaterials13, 2803. 10.3390/nano13202803
49
Cosenza S. Ruiz M. Toupet K. Jorgensen C. Noël D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep.7, 16214. 10.1038/s41598-017-15376-8
50
Crivelli B. Bari E. Perteghella S. Catenacci L. Sorrenti M. Mocchi M. et al (2019). Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm.137, 37–45. 10.1016/j.ejpb.2019.02.008
51
Damerau A. Rosenow E. Alkhoury D. Buttgereit F. Gaber T. (2024). Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis. Front. Immunol.15, 1385006. 10.3389/fimmu.2024.1385006
52
De Faro Silva R. Barreto A. S. Trindade G. d. G. G. Lima C. M. Araújo A. A. d. S. Menezes I. R. A. et al (2022). Enhancement of the functionality of women with knee osteoarthritis by a gel formulation with Caryocar coriaceum Wittm (“Pequi”) nanoencapsulated pulp fixed oil. Biomed. Pharmacother.150, 112938. 10.1016/j.biopha.2022.112938
53
Dhall A. Self W. (2018). Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants7, 97. 10.3390/antiox7080097
54
Dilliard S. A. Cheng Q. Siegwart D. J. (2021). On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci.118, e2109256118. 10.1073/pnas.2109256118
55
Ding P. Gao C. Gao Y. Liu D. Li H. Xu J. et al (2022). Osteocytes regulate senescence of bone and bone marrow. eLife11, e81480. 10.7554/eLife.81480
56
Dou Y. Li C. Li L. Guo J. Zhang J. (2020). Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release327, 641–666. 10.1016/j.jconrel.2020.09.008
57
Du X. Cai L. Xie J. Zhou X. (2023). The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res.11, 2. 10.1038/s41413-022-00239-4
58
Duan W.-L. Zhang L. N. Bohara R. Martin-Saldaña S. Yang F. Zhao Y. Y. et al (2023). Adhesive hydrogels in osteoarthritis: from design to application. Mil. Med. Res.10, 4. 10.1186/s40779-022-00439-3
59
Ebert J. R. Joss B. Jardine B. Wood D. J. (2013). Randomized trial investigating the efficacy of manual lymphatic drainage to improve early outcome after total knee arthroplasty. Arch. Phys. Med. Rehabil.94, 2103–2111. 10.1016/j.apmr.2013.06.009
60
Eftekhari A. Maleki Dizaj S. Sharifi S. Salatin S. Rahbar Saadat Y. Zununi Vahed S. et al (2020). The use of nanomaterials in tissue engineering for cartilage regeneration; current approaches and future perspectives. Int. J. Mol. Sci.21, 536. 10.3390/ijms21020536
61
Eisenstein A. Hilliard B. K. Pope S. D. Zhang C. Taskar P. Waizman D. A. et al (2022). Activation of the transcription factor NRF2 mediates the anti-inflammatory properties of a subset of over-the-counter and prescription NSAIDs. Immunity55, 1082–1095.e5. 10.1016/j.immuni.2022.04.015
62
Eker F. Duman H. Akdaşçi E. Bolat E. Sarıtaş S. Karav S. et al (2024). A comprehensive review of nanoparticles: from classification to application and toxicity. Molecules29, 3482. 10.3390/molecules29153482
63
Emami A. Tepper J. Short B. Yaksh T. L. Bendele A. M. Ramani T. et al (2018). Toxicology evaluation of drugs administered via uncommon routes: intranasal, intraocular, intrathecal/intraspinal, and intra-articular. Int. J. Toxicol.37, 4–27. 10.1177/1091581817741840
64
Evans C. H. Kraus V. B. Setton L. A. (2014). Progress in intra-articular therapy. Nat. Rev. Rheumatol.10, 11–22. 10.1038/nrrheum.2013.159
65
Fan H. Hu Y. Zhang C. Li X. Lv R. Qin L. et al (2006). Cartilage regeneration using mesenchymal stem cells and a PLGA–gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials27, 4573–4580. 10.1016/j.biomaterials.2006.04.013
66
Fang S. Zhang B. Xiang W. Zheng L. Wang X. Li S. et al (2024). Natural products in osteoarthritis treatment: bridging basic research to clinical applications. Chin. Med.19, 25. 10.1186/s13020-024-00899-w
67
Ferdous Z. Nemmar A. (2020). Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci.21, 2375. 10.3390/ijms21072375
68
Filardo G. Di Matteo B. Di Martino A. Merli M. L. Cenacchi A. Fornasari P. et al (2015). Platelet-rich plasma intra-articular knee injections show No superiority versus viscosupplementation: a randomized controlled trial. Am. J. Sports Med.43, 1575–1582. 10.1177/0363546515582027
69
Fine P. G. Rosenfeld M. J. (2013). The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J.4, e0022. 10.5041/RMMJ.10129
70
Foell D. Wittkowski H. Roth J. (2007). Mechanisms of Disease: a ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol.3, 382–390. 10.1038/ncprheum0531
71
Furtado M. Chen L. Chen Z. Chen A. Cui W. (2022). Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen.3, 217–231. 10.1016/j.engreg.2022.05.002
72
Gaio E. Conte C. Esposito D. Reddi E. Quaglia F. Moret F. (2020). CD44 targeting mediated by polymeric nanoparticles and combination of chlorine TPCS2a-PDT and docetaxel-chemotherapy for efficient killing of breast differentiated and stem cancer cells in vitro. Cancers12, 278. 10.3390/cancers12020278
73
Gan J. Deng X. Le Y. Lai J. Liao X. (2023). The development of naringin for use against bone and cartilage disorders. Molecules28, 3716. 10.3390/molecules28093716
74
Gan X. Wang X. Huang Y. Li G. Kang H. (2024). Applications of hydrogels in osteoarthritis treatment. Biomedicines12, 923. 10.3390/biomedicines12040923
75
Gao Z.-R. Feng Y. Z. Zhao Y. Q. Zhao J. Zhou Y. H. Ye Q. et al (2022). Traditional Chinese medicine promotes bone regeneration in bone tissue engineering. Chin. Med.17, 86. 10.1186/s13020-022-00640-5
76
Ghasemiyeh P. Mohammadi-Samani S. (2018). Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci.13, 288–303. 10.4103/1735-5362.235156
77
Gherasim O. Puiu R. A. Bîrcă A. C. Burdușel A.-C. Grumezescu A. M. (2020). An updated review on silver nanoparticles in biomedicine. Nanomaterials10, 2318. 10.3390/nano10112318
78
Gomaa A. A. Mohamed H. S. Abd-ellatief R. B. Gomaa M. A. (2021). Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology29, 1033–1048. 10.1007/s10787-021-00841-8
79
Gonzalez-Fernandez P. Rodríguez-Nogales C. Jordan O. Allémann E. (2022). Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm.172, 41–52. 10.1016/j.ejpb.2022.01.003
80
Guan T. Ding L. G. Lu B. Y. Guo J. Y. Wu M. Y. Tan Z. Q. et al (2022). Combined administration of curcumin and chondroitin sulfate alleviates cartilage injury and inflammation via NF-κB pathway in knee osteoarthritis rats. Front. Pharmacol.13, 882304. 10.3389/fphar.2022.882304
81
Guilak F. Nims R. J. Dicks A. Wu C.-L. Meulenbelt I. (2018). Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol.71–72, 40–50. 10.1016/j.matbio.2018.05.008
82
Guo X. Wang L. Xu M. Bai J. Shen J. Yu B. et al (2018). Shikimic acid prevents cartilage matrix destruction in human chondrocytes. Int. Immunopharmacol.63, 155–160. 10.1016/j.intimp.2018.07.021
83
Gupta A. Costa A. P. Xu X. Lee S. L. Cruz C. N. Bao Q. et al (2020). Formulation and characterization of curcumin loaded polymeric micelles produced via continuous processing. Int. J. Pharm.583, 119340. 10.1016/j.ijpharm.2020.119340
84
Han H. S. Koo S. Y. Choi K. Y. (2022). Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact. Mater.14, 182–205. 10.1016/j.bioactmat.2021.11.027
85
Han Y. Huang S. (2023). Nanomedicine is more than a supporting role in rheumatoid arthritis therapy. J. Control. Release356, 142–161. 10.1016/j.jconrel.2023.02.035
86
Hassan M. H. Ghobara M. M. (2016). Antifibrotic effect of meloxicam in rat liver: role of nuclear factor kappa B, proinflammatory cytokines, and oxidative stress. Naunyn. Schmiedeb. Arch. Pharmacol.389, 971–983. 10.1007/s00210-016-1263-1
87
He J. Ma Y. Niu X. Pei J. Yan R. Xu F. et al (2024). Silver nanoparticles induce endothelial cytotoxicity through ROS-mediated mitochondria-lysosome damage and autophagy perturbation: the protective role of N-acetylcysteine. Toxicology502, 153734. 10.1016/j.tox.2024.153734
88
He M. Qin Z. Liang X. He X. Zhu B. Lu Z. et al (2021). A pH-responsive mesoporous silica nanoparticles-based drug delivery system with controlled release of andrographolide for OA treatment. Regen. Biomater.8, rbab020. 10.1093/rb/rbab020
89
Hoang D. M. Pham P. T. Bach T. Q. Ngo A. T. L. Nguyen Q. T. Phan T. T. K. et al (2022). Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther.7, 272. 10.1038/s41392-022-01134-4
90
Hodgkinson T. Kelly D. C. Curtin C. M. O’Brien F. J. (2022). Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol.18, 67–84. 10.1038/s41584-021-00724-w
91
Hou S.-M. Chen P. C. Lin C. M. Fang M. L. Chi M. C. Liu J. F. (2020). CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther.22, 251. 10.1186/s13075-020-02331-8
92
Householder N. A. Raghuram A. Agyare K. Thipaphay S. Zumwalt M. (2023). A review of recent innovations in cartilage regeneration strategies for the treatment of primary osteoarthritis of the knee: intra-articular injections. Orthop. J. Sports Med.11, 23259671231155950. 10.1177/23259671231155950
93
Hsu C.-Y. Rheima A. M. Kadhim M. M. Ahmed N. N. Mohammed S. H. Abbas F. H. et al (2023). An overview of nanoparticles in drug delivery: properties and applications. South Afr. J. Chem. Eng.46, 233–270. 10.1016/j.sajce.2023.08.009
94
Hu B. Ga F. Li C. Zhang B. An M. Lu M. et al (2020). Rhein laden pH-responsive polymeric nanoparticles for treatment of osteoarthritis. Amb. Express10, 158. 10.1186/s13568-020-01095-3
95
Hu W. Chen Y. Dou C. Dong S. (2021). Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis.80, 413–422. 10.1136/annrheumdis-2020-218089
96
Hu Y. Gui Z. Zhou Y. Xia L. Lin K. Xu Y. (2019). Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med.145, 146–160. 10.1016/j.freeradbiomed.2019.09.024
97
Hu Z. Guan Y. Hu W. Xu Z. Ishfaq M. (2022). An overview of pharmacological activities of baicalin and its aglycone baicalein: new insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci.25, 14–26. 10.22038/ijbms.2022.60380.13381
98
Huang H. Lou Z. Zheng S. Wu J. Yao Q. Chen R. et al (2022). Intra-articular drug delivery systems for osteoarthritis therapy: shifting from sustained release to enhancing penetration into cartilage. Drug Deliv.29, 767–791. 10.1080/10717544.2022.2048130
99
Huang T. Liu Y. Zhang C. (2019). Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur. J. Drug Metab. Pharmacokinet.44, 159–168. 10.1007/s13318-018-0509-3
100
Huang Y. Guo X. Wu Y. Chen X. Feng L. Xie N. et al (2024). Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment. Signal Transduct. Target. Ther.9, 34. 10.1038/s41392-024-01745-z
101
Hügle T. Nasi S. Ehirchiou D. Omoumi P. Busso N. (2022). Fibrin deposition associates with cartilage degeneration in arthritis. eBioMedicine81, 104081. 10.1016/j.ebiom.2022.104081
102
Hunter D. J. March L. Chew M. (2020). Osteoarthritis in 2020 and beyond: a lancet commission. Lancet396, 1711–1712. 10.1016/S0140-6736(20)32230-3
103
Janowicz P. W. Houston Z. H. Bunt J. Fletcher N. L. Bell C. A. Cowin G. et al (2022). Understanding nanomedicine treatment in an aggressive spontaneous brain cancer model at the stage of early blood brain barrier disruption. Biomaterials283, 121416. 10.1016/j.biomaterials.2022.121416
104
Jargin S. V. (2012). Supplementation of glycosaminoglycans and their precursors in osteoarthritis versus diet modification. Int. J. Rheum. Dis.15, e45–e46. 10.1111/j.1756-185X.2012.01707.x
105
Jennings C. L. Dziubla T. D. Puleo D. A. (2016). Combined effects of drugs and plasticizers on the properties of drug delivery films. J. Bioact. Compat. Polym.31, 323–333. 10.1177/0883911515627178
106
Jeong S. Kang M. Park J. Im G. (2020). Dual functional nanoparticles containing SOX duo and ANGPT4 shRNA for osteoarthritis treatment. J. Biomed. Mater. Res. B Appl. Biomater.108, 234–242. 10.1002/jbm.b.34383
107
Jeyaraman M. Jeyaraman N. Nallakumarasamy A. Ramasubramanian S. Yadav S. (2024). Critical challenges and frontiers in cartilage tissue engineering. Cureus16, e53095. 10.7759/cureus.53095
108
Jiang Y. Lv H. Shen F. Fan L. Zhang H. Huang Y. et al (2024). Strategies in product engineering of mesenchymal stem cell-derived exosomes: unveiling the mechanisms underpinning the promotive effects of mesenchymal stem cell-derived exosomes. Front. Bioeng. Biotechnol.12, 1363780. 10.3389/fbioe.2024.1363780
109
Jin Z. Zhan Y. Zheng L. Wei Q. Xu S. Qin Z. (2023). Cannabidiol‐loaded poly lactic‐Co‐glycolic acid nanoparticles with improved bioavailability as a potential for osteoarthritis therapeutic. ChemBioChem24, e202200698. 10.1002/cbic.202200698
110
Jung J. H. Kim S. E. Kim H. J. Park K. Song G. G. Choi S. J. (2020). A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int. J. Pharm.581, 119249. 10.1016/j.ijpharm.2020.119249
111
Kalashnikova I. Chung S. J. Nafiujjaman M. Hill M. L. Siziba M. E. Contag C. H. et al (2020). Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics10, 11863–11880. 10.7150/thno.49069
112
Kang C. Jung E. Hyeon H. Seon S. Lee D. (2020). Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomedicine Nanotechnol. Biol. Med.23, 102104. 10.1016/j.nano.2019.102104
113
Katz J. N. Arant K. R. Loeser R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA325, 568–578. 10.1001/jama.2020.22171
114
Knights A. J. Redding S. J. Maerz T. (2023). Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr. Opin. Rheumatol.35, 128–134. 10.1097/BOR.0000000000000923
115
Krishnan Y. Grodzinsky A. J. (2018). Cartilage diseases. Matrix Biol.71–72, 51–69. 10.1016/j.matbio.2018.05.005
116
Kuang L. Wu J. Su N. Qi H. Chen H. Zhou S. et al (2020). FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann. Rheum. Dis.79, 112–122. 10.1136/annrheumdis-2019-215696
117
Kuang S. Liu L. Hu Z. Luo M. Fu X. Lin C. et al (2023). A review focusing on the benefits of plant-derived polysaccharides for osteoarthritis. Int. J. Biol. Macromol.228, 582–593. 10.1016/j.ijbiomac.2022.12.153
118
Kumar M. A. Baba S. K. Sadida H. Q. Marzooqi S. A. Jerobin J. Altemani F. H. et al (2024). Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther.9, 27. 10.1038/s41392-024-01735-1
119
Kumar S. Adjei I. M. Brown S. B. Liseth O. Sharma B. (2019). Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials224, 119467. 10.1016/j.biomaterials.2019.119467
120
Kumari A. Kaur A. Aggarwal G. (2023). The emerging potential of siRNA nanotherapeutics in treatment of arthritis. Asian J. Pharm. Sci.18, 100845. 10.1016/j.ajps.2023.100845
121
Kus-Liśkiewicz M. Fickers P. Ben Tahar I. (2021). Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int. J. Mol. Sci.22, 10952. 10.3390/ijms222010952
122
Lambert C. Zappia J. Sanchez C. Florin A. Dubuc J. E. Henrotin Y. (2021). The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front. Med.7, 607186. 10.3389/fmed.2020.607186
123
Lan Q. Lu R. Chen H. Pang Y. Xiong F. Shen C. et al (2020). MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J. Nanobiotechnology18, 117. 10.1186/s12951-020-00666-7
124
Lee M.-K. (2020). Liposomes for enhanced bioavailability of water-insoluble drugs: in vivo evidence and recent approaches. Pharmaceutics12, 264. 10.3390/pharmaceutics12030264
125
Legré-Boyer V. (2015). Viscosupplementation: techniques, indications, results. Orthop. Traumatol. Surg. Res.101, S101–S108. 10.1016/j.otsr.2014.07.027
126
Li D. Wu M. (2021). Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther.6, 291. 10.1038/s41392-021-00687-0
127
Li H. Luo Y. Xu Y. Yang L. Hu C. Chen Q. et al (2018). Meloxicam improves cognitive impairment of diabetic rats through COX2-PGE2-EPs-cAMP/pPKA pathway. Mol. Pharm.15, 4121–4131. 10.1021/acs.molpharmaceut.8b00532
128
Li L. Wang F. Zhu D. Hu S. Cheng K. Li Z. (2024d). Engineering exosomes and exosome-like nanovesicles for improving tissue targeting and retention. Fundam. Res.S2667325824001481. 10.1016/j.fmre.2024.03.025
129
Li R. Hou X. Li L. Guo J. Jiang W. Shang W. (2022b). Application of metal-based nanozymes in inflammatory disease: a review. Front. Bioeng. Biotechnol.10, 920213. 10.3389/fbioe.2022.920213
130
Li S. Xiong Y. Zhu H. Ma T. Sun X. Xiao J. et al (2024c). Microenvironment-responsive nanosystems for osteoarthritis therapy. Eng. Regen.5, 92–110. 10.1016/j.engreg.2023.12.002
131
Li T. Peng J. Li Q. Shu Y. Zhu P. Hao L. (2022a). The mechanism and role of ADAMTS protein family in osteoarthritis. Biomolecules12, 959. 10.3390/biom12070959
132
Li W. Wang Y. Tang Y. Lu H. Qi Y. Li G. et al (2021c). Quercetin alleviates osteoarthritis progression in rats by suppressing inflammation and apoptosis via inhibition of IRAK1/NLRP3 signaling. J. Inflamm. Res.14, 3393–3403. 10.2147/JIR.S311924
133
Li X. Dai B. Guo J. Zheng L. Guo Q. Peng J. et al (2021a). Nanoparticle–cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nano-Micro Lett.13, 149. 10.1007/s40820-021-00670-y
134
Li X. Ren Y. Chang K. Wu W. Griffiths H. R. Lu S. et al (2023). Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol.14, 1153915. 10.3389/fimmu.2023.1153915
135
Li Y. Xia X. Niu Z. Wang K. Liu J. Li X. (2024a). hCeO2@ Cu5.4O nanoparticle alleviates inflammatory responses by regulating the CTSB–NLRP3 signaling pathway. Front. Immunol.15, 1344098. 10.3389/fimmu.2024.1344098
136
Li Y. Yang Y. Qing Y. Li R. Tang X. Guo D. et al (2020). Enhancing ZnO-NP antibacterial and osteogenesis properties in orthopedic applications: a review. Int. J. Nanomedicine15, 6247–6262. 10.2147/IJN.S262876
137
Li Y. Zhao J. Guo S. He D. (2024b). siRNA therapy in osteoarthritis: targeting cellular pathways for advanced treatment approaches. Front. Immunol.15, 1382689. 10.3389/fimmu.2024.1382689
138
Li Y.-J. Wu J. Y. Liu J. Xu W. Qiu X. Huang S. et al (2021d). Artificial exosomes for translational nanomedicine. J. Nanobiotechnology19, 242. 10.1186/s12951-021-00986-2
139
Li Z. Wang J. Ma Y. (2021b). Montelukast attenuates interleukin IL-1β-induced oxidative stress and apoptosis in chondrocytes by inhibiting CYSLTR1 (Cysteinyl Leukotriene Receptor 1) and activating KLF2 (Kruppel like Factor 2). Bioengineered12, 8476–8484. 10.1080/21655979.2021.1984003
140
Liang J. Liu P. Yang X. Liu L. Zhang Y. Wang Q. et al (2023). Biomaterial-based scaffolds in promotion of cartilage regeneration: recent advances and emerging applications. J. Orthop. Transl.41, 54–62. 10.1016/j.jot.2023.08.006
141
Liao C.-R. Wang S. N. Zhu S. Y. Wang Y. Q. Li Z. Z. Liu Z. Y. et al (2020). Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol.28, 101306. 10.1016/j.redox.2019.101306
142
Lin F. Li Y. Cui W. (2023). Injectable hydrogel microspheres in cartilage repair. Biomed. Technol.1, 18–29. 10.1016/j.bmt.2022.11.002
143
Ling H. Zeng Q. Ge Q. Chen J. Yuan W. Xu R. et al (2021). Osteoking decelerates cartilage degeneration in DMM-induced osteoarthritic mice model through TGF-β/smad-dependent manner. Front. Pharmacol.12, 678810. 10.3389/fphar.2021.678810
144
Liu L. Tang H. Wang Y. (2023a). Polymeric biomaterials: advanced drug delivery systems in osteoarthritis treatment. Heliyon9, e21544. 10.1016/j.heliyon.2023.e21544
145
Liu L. Tang H. Wang Y. (2023b). Nanotechnology-boosted biomaterials for osteoarthritis treatment: current status and future perspectives. Int. J. Nanomedicine18, 4969–4983. 10.2147/IJN.S423737
146
Liu P. Chen G. Zhang J. (2022b). A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules27, 1372. 10.3390/molecules27041372
147
Liu S. Deng Z. Chen K. Jian S. Zhou F. Yang Y. et al (2022a). Cartilage tissue engineering: from proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep.25, 99. 10.3892/mmr.2022.12615
148
Liu S. Gao X. Wang Y. Wang J. Qi X. Dong K. et al (2022c). Baicalein-loaded silk fibroin peptide nanofibers protect against cisplatin-induced acute kidney injury: fabrication, characterization and mechanism. Int. J. Pharm.626, 122161. 10.1016/j.ijpharm.2022.122161
149
Lopez-Miranda J. L. Molina G. A. González-Reyna M. A. España-Sánchez B. L. Esparza R. Silva R. et al (2023). Antibacterial and anti-inflammatory properties of ZnO nanoparticles synthesized by a green method using sargassum extracts. Int. J. Mol. Sci.24, 1474. 10.3390/ijms24021474
150
Lowin T. Tingting R. Zurmahr J. Classen T. Schneider M. Pongratz G. (2020). Cannabidiol (CBD): a killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis.11, 714. 10.1038/s41419-020-02892-1
151
Lu H. Wei J. Liu K. Li Z. Xu T. Yang D. et al (2023b). Radical-scavenging and subchondral bone-regenerating nanomedicine for osteoarthritis treatment. ACS Nano17, 6131–6146. 10.1021/acsnano.3c01789
152
Lu R. Wang Y. G. Qu Y. Wang S. X. Peng C. You H. et al (2023a). Dihydrocaffeic acid improves IL-1β-induced inflammation and cartilage degradation via inhibiting NF-κB and MAPK signalling pathways. Bone Jt. Res.12, 259–273. 10.1302/2046-3758.124.BJR-2022-0384.R1
153
Luo J. Zhang Y. Zhu S. Tong Y. Ji L. Zhang W. et al (2021). The application prospect of metal/metal oxide nanoparticles in the treatment of osteoarthritis. Naunyn. Schmiedeb. Arch. Pharmacol.394, 1991–2002. 10.1007/s00210-021-02131-0
154
Ma Q. (2023). Pharmacological inhibition of the NLRP3 inflammasome: structure, molecular activation, and inhibitor-NLRP3 interaction. Pharmacol. Rev.75, 487–520. 10.1124/pharmrev.122.000629
155
Ma Z. Wang Y. Piao T. Liu J. (2016). echinocytic acid inhibits IL-1β-induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation39, 543–549. 10.1007/s10753-015-0278-y
156
Magni A. Agostoni P. Bonezzi C. Massazza G. Menè P. Savarino V. et al (2021). Management of osteoarthritis: expert opinion on NSAIDs. Pain Ther.10, 783–808. 10.1007/s40122-021-00260-1
157
Mancini G. Gonçalves L. M. D. Marto J. Carvalho F. A. Simões S. Ribeiro H. M. et al (2021). Increased therapeutic efficacy of SLN containing etofenamate and ibuprofen in topical treatment of inflammation. Pharmaceutics13, 328. 10.3390/pharmaceutics13030328
158
Marinho A. Nunes C. Reis S. (2021). Hyaluronic acid: a key ingredient in the therapy of inflammation. Biomolecules11, 1518. 10.3390/biom11101518
159
Maślanka T. Jaroszewski J. J. (2013). In vitro effects of meloxicam on the number, Foxp3 expression, production of selected cytokines, and apoptosis of bovine CD25+CD4+ and CD25-CD4+ cells. J. Vet. Sci.14, 125–134. 10.4142/jvs.2013.14.2.125
160
McCulloch K. Litherland G. J. Rai T. S. (2017). Cellular senescence in osteoarthritis pathology. Aging Cell16, 210–218. 10.1111/acel.12562
161
McGuckin M. B. Wang J. Ghanma R. Qin N. Palma S. D. Donnelly R. F. et al (2022). Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J. Control. Release345, 334–353. 10.1016/j.jconrel.2022.03.012
162
Mirza E. H. Pan‐Pan C. Wan Ibrahim W. M. A. B. Djordjevic I. Pingguan‐Murphy B. (2015). Chondroprotective effect of zinc oxide nanoparticles in conjunction with hypoxia on bovine cartilage‐matrix synthesis. J. Biomed. Mater. Res. A103, 3554–3563. 10.1002/jbm.a.35495
163
Mitchell M. J. Billingsley M. M. Haley R. M. Wechsler M. E. Peppas N. A. Langer R. (2021). Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov.20, 101–124. 10.1038/s41573-020-0090-8
164
Mizuno M. Matsuzaki T. Ozeki N. Katano H. Koga H. Takebe T. et al (2022). Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res. Ther.13, 177. 10.1186/s13287-022-02850-y
165
Molnar V. Matišić V. Kodvanj I. Bjelica R. Jeleč Ž. Hudetz D. et al (2021). Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci.22, 9208. 10.3390/ijms22179208
166
Moon S.-M. Lee S. A. Han S. H. Park B. R. Choi M. S. Kim J. S. et al (2018). Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharmacother.97, 264–270. 10.1016/j.biopha.2017.10.130
167
Mu P. Feng J. Hu Y. Xiong F. Ma X. Tian L. (2022). Botanical drug extracts combined with biomaterial carriers for osteoarthritis cartilage degeneration treatment: a review of 10 Years of research. Front. Pharmacol.12, 789311. 10.3389/fphar.2021.789311
168
Munir A. Muhammad F. Zaheer Y. Ali M. A. Iqbal M. Rehman M. et al (2021). Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol.66, 102854. 10.1016/j.jddst.2021.102854
169
Nagy E. Vajda E. Vari C. Sipka S. Fárr A. M. Horváth E. (2017). Meloxicam ameliorates the cartilage and subchondral bone deterioration in monoiodoacetate-induced rat osteoarthritis. PeerJ5, e3185. 10.7717/peerj.3185
170
Nandhini J. Karthikeyan E. Elizabeth Rani E. Karthikha V. Sakthi Sanjana D. Jeevitha H. et al (2024). Advancing engineered approaches for sustainable wound regeneration and repair: harnessing the potential of green synthesized silver nanoparticles. Eng. Regen.5, 306–325. 10.1016/j.engreg.2024.06.004
171
Nayal R. Mejjo D. Abajy M. Y. (2024). Anti inflammatory properties and safety of green synthesized metal and metal oxide nanoparticles: a review article. Eur. J. Med. Chem. Rep.11, 100169. 10.1016/j.ejmcr.2024.100169
172
Nelson A. E. Renner J. B. Schwartz T. A. Kraus V. B. Helmick C. G. Jordan J. M. (2011). Differences in multijoint radiographic osteoarthritis phenotypes among african Americans and caucasians: the johnston county osteoarthritis Project. Arthritis Rheum.63, 3843–3852. 10.1002/art.30610
173
Nunes R. D. M. Girão V. C. C. Cunha P. L. R. Feitosa J. P. A. Pinto A. C. M. D. Rocha F. A. C. (2021). Decreased sulfate content and zeta potential distinguish glycosaminoglycans of the extracellular matrix of osteoarthritis cartilage. Front. Med.8, 612370. 10.3389/fmed.2021.612370
174
Oo W. M. Little C. Duong V. Hunter D. J. (2021). The development of disease-modifying therapies for osteoarthritis (DMOADs): the evidence to date. Drug Des. devel. Ther.15, 2921–2945. 10.2147/DDDT.S295224
175
Pandey S. Shaikh F. Gupta A. Tripathi P. Yadav J. S. (2021). A recent update: solid lipid nanoparticles for effective drug delivery. Adv. Pharm. Bull.1, 17–33. 10.34172/apb.2022.007
176
Pangli H. Vatanpour S. Hortamani S. Jalili R. Ghahary A. (2021). Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. J. Burn Care Res.42, 785–793. 10.1093/jbcr/iraa205
177
Panova E. Jones G. (2015). Benefit–risk assessment of diacerein in the treatment of osteoarthritis. Drug Saf.38, 245–252. 10.1007/s40264-015-0266-z
178
Park H. Lee H. R. Shin H. J. Park J. A. Joo Y. Kim S. M. et al (2022). p16INK4a-siRNA nanoparticles attenuate cartilage degeneration in osteoarthritis by inhibiting inflammation in fibroblast-like synoviocytes. Biomater. Sci.10, 3223–3235. 10.1039/d1bm01941d
179
Peng Y. Qu R. Xu S. Bi H. Guo D. (2024). Regulatory mechanism and therapeutic potentials of naringin against inflammatory disorders. Heliyon10, e24619. 10.1016/j.heliyon.2024.e24619
180
Pontes A. P. Welting T. J. M. Rip J. Creemers L. B. (2022). Polymeric nanoparticles for drug delivery in osteoarthritis. Pharmaceutics14, 2639. 10.3390/pharmaceutics14122639
181
Pourmadadi M. Rahmani E. Shamsabadipour A. Mahtabian S. Ahmadi M. Rahdar A. et al (2022). Role of iron oxide (Fe2O3) nanocomposites in advanced biomedical applications: a state-of-the-art review. Nanomaterials12, 3873. 10.3390/nano12213873
182
Qiao K. Xu L. Tang J. Wang Q. Lim K. S. Hooper G. et al (2022). The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnology20, 141. 10.1186/s12951-022-01342-8
183
Qin S. Zhu J. Zhang G. Sui Q. Niu Y. Ye W. et al (2023). Research progress of functional motifs based on growth factors in cartilage tissue engineering: a review. Front. Bioeng. Biotechnol.11, 1127949. 10.3389/fbioe.2023.1127949
184
Qiu L.-Y. Yan L. Zhang L. Jin Y.-M. Zhao Q.-H. (2013). Folate-modified poly(2-ethyl-2-oxazoline) as hydrophilic corona in polymeric micelles for enhanced intracellular doxorubicin delivery. Int. J. Pharm.456, 315–324. 10.1016/j.ijpharm.2013.08.071
185
Quero L. Tiaden A. N. Hanser E. Roux J. Laski A. Hall J. et al (2020). miR-221-3p drives the shift of M2-macrophages to a pro-inflammatory function by suppressing JAK3/STAT3 activation. Front. Immunol.10, 3087. 10.3389/fimmu.2019.03087
186
Raghu H. Lepus C. M. Wang Q. Wong H. H. Lingampalli N. Oliviero F. et al (2017). CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis.76, 914–922. 10.1136/annrheumdis-2016-210426
187
Rahmati M. Mobasheri A. Mozafari M. (2016). Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone85, 81–90. 10.1016/j.bone.2016.01.019
188
Rai M. F. Pan H. Yan H. Sandell L. J. Pham C. T. N. Wickline S. A. (2019). Applications of RNA interference in the treatment of arthritis. Transl. Res.214, 1–16. 10.1016/j.trsl.2019.07.002
189
Ramirez-Perez S. Reyes-Perez I. V. Martinez-Fernandez D. E. Hernandez-Palma L. A. Bhattaram P. (2022). Targeting inflammasome-dependent mechanisms as an emerging pharmacological approach for osteoarthritis therapy. iScience25, 105548. 10.1016/j.isci.2022.105548
190
Rao C. Shi S. (2022). Development of nanomaterials to target articular cartilage for osteoarthritis therapy. Front. Mol. Biosci.9, 900344. 10.3389/fmolb.2022.900344
191
Ravetti S. Garro A. G. Gaitán A. Murature M. Galiano M. Brignone S. G. et al (2023). Naringin: nanotechnological strategies for potential pharmaceutical applications. Pharmaceutics15, 863. 10.3390/pharmaceutics15030863
192
Ren G. Al-Jezani N. Railton P. Powell J. N. Krawetz R. J. (2021). CCL22 induces pro-inflammatory changes in fibroblast-like synoviocytes. iScience24, 101943. 10.1016/j.isci.2020.101943
193
Roelofs A. J. Kania K. Rafipay A. J. Sambale M. Kuwahara S. T. Collins F. L. et al (2020). Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann. Rheum. Dis.79, 1625–1634. 10.1136/annrheumdis-2020-218350
194
Roseti L. Desando G. Cavallo C. Petretta M. Grigolo B. (2019). Articular cartilage regeneration in osteoarthritis. Cells8, 1305. 10.3390/cells8111305
195
Sadalage P. S. Patil R. V. Havaldar D. V. Gavade S. S. Santos A. C. Pawar K. D. (2021). Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnology19, 84. 10.1186/s12951-021-00836-1
196
Said S. S. Campbell S. Hoare T. (2019). Externally addressable smart drug delivery vehicles: current technologies and future directions. Chem. Mater.31, 4971–4989. 10.1021/acs.chemmater.9b01798
197
Salathia S. Gigliobianco M. R. Casadidio C. Di Martino P. Censi R. (2023). Hyaluronic acid-based nanosystems for CD44 mediated anti-inflammatory and antinociceptive activity. Int. J. Mol. Sci.24, 7286. 10.3390/ijms24087286
198
Salehi B. Stojanović-Radić Z. Matejić J. Sharifi-Rad M. Anil Kumar N. V. Martins N. et al (2019). The therapeutic potential of curcumin: a review of clinical trials. Eur. J. Med. Chem.163, 527–545. 10.1016/j.ejmech.2018.12.016
199
Sanità G. Carrese B. Lamberti A. (2020). Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front. Mol. Biosci.7, 587012. 10.3389/fmolb.2020.587012
200
Sarabia-Vallejo Á. Caja M. D. M. Olives A. I. Martín M. A. Menéndez J. C. (2023). Cyclodextrin inclusion complexes for improved drug bioavailability and activity: synthetic and analytical aspects. Pharmaceutics15, 2345. 10.3390/pharmaceutics15092345
201
Scanzello C. R. Goldring S. R. (2012). The role of synovitis in osteoarthritis pathogenesis. Bone51, 249–257. 10.1016/j.bone.2012.02.012
202
Seo B.-B. Kwon Y. Kim J. Hong K. H. Kim S. E. Song H. R. et al (2022). Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater.7, 14–25. 10.1016/j.bioactmat.2021.05.028
203
Serra M. Casas A. Toubarro D. Barros A. N. Teixeira J. A. (2023). Microbial hyaluronic acid production: a review. Molecules28, 2084. 10.3390/molecules28052084
204
Shi F. Wei Z. Zhao Y. Xu X. (2016). Nanostructured lipid carriers loaded with baicalin: an efficient carrier for enhanced antidiabetic effects. Pharmacogn. Mag.12, 198–202. 10.4103/0973-1296.186347
205
Shin H. J. Park H. Shin N. Kwon H. H. Yin Y. Hwang J. A. et al (2020a). p47phox siRNA-loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers12, 443. 10.3390/polym12020443
206
Shin H. J. Park H. Shin N. Shin J. Gwon D. H. Kwon H. H. et al (2020b). p66shc siRNA nanoparticles ameliorate chondrocytic mitochondrial dysfunction in osteoarthritis. Int. J. Nanomedicine15, 2379–2390. 10.2147/IJN.S234198
207
Shin M.-R. Kim H. Y. Choi H. Y. Park K. S. Choi H. J. Roh S. S. (2022). Boswellia serrata extract, 5-loxin®, prevents joint pain and cartilage degeneration in a rat model of osteoarthritis through inhibition of inflammatory responses and restoration of matrix homeostasis. Evid. Based Complement. Altern. Med.2022, 1–11. 10.1155/2022/3067526
208
Singh A. P. Biswas A. Shukla A. Maiti P. (2019). Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther.4, 33. 10.1038/s41392-019-0068-3
209
Skou S. T. Roos E. M. Laursen M. B. Rathleff M. S. Arendt-Nielsen L. Simonsen O. et al (2015). A randomized, controlled trial of total knee replacement. N. Engl. J. Med.373, 1597–1606. 10.1056/NEJMoa1505467
210
Sohail R. Mathew M. Patel K. K. Reddy S. A. Haider Z. Naria M. et al (2023). Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: a narrative review. Cureus15, e37080. 10.7759/cureus.37080
211
Son K. M. Hong J. I. Kim D. H. Jang D. G. Crema M. D. Kim H. A. (2020). Absence of pain in subjects with advanced radiographic knee osteoarthritis. BMC Musculoskelet. Disord.21, 640. 10.1186/s12891-020-03647-x
212
Stiller C. Hjemdahl P. (2022). Lessons from 20 years with COX‐2 inhibitors: importance of dose–response considerations and fair play in comparative trials. J. Intern. Med.292, 557–574. 10.1111/joim.13505
213
Strokotova A. V. Grigorieva E. V. (2022). Glucocorticoid effects on proteoglycans and glycosaminoglycans. Int. J. Mol. Sci.23, 15678. 10.3390/ijms232415678
214
Tambe A. Pandita N. Kharkar P. Sahu N. (2018). Encapsulation of boswellic acid with β- and hydroxypropyl-β-cyclodextrin: synthesis, characterization, in vitro drug release and molecular modelling studies. J. Mol. Struct.1154, 504–510. 10.1016/j.molstruc.2017.10.061
215
Tan F. Li X. Wang Z. Li J. Shahzad K. Zheng J. (2024). Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther.9, 17. 10.1038/s41392-023-01704-0
216
Tan F. Wang D. Yuan Z. (2020). The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 Axis. Inflammation43, 1498–1509. 10.1007/s10753-020-01227-8
217
Tao S.-C. Yuan T. Zhang Y. L. Yin W. J. Guo S. C. Zhang C. Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics7, 180–195. 10.7150/thno.17133
218
Teixeira S. Carvalho M. A. Castanheira E. M. S. (2022). Functionalized liposome and albumin-based systems as carriers for poorly water-soluble anticancer drugs: an updated review. Biomedicines10, 486. 10.3390/biomedicines10020486
219
Tenchov R. Sasso J. M. Wang X. Liaw W. S. Chen C. A. Zhou Q. A. (2022). Exosomes─Nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano16, 17802–17846. 10.1021/acsnano.2c08774
220
Teng L. Shen Y. Qu Y. Yang L. Yang Y. Jian X. et al (2023). Cyasterone inhibits IL-1β-mediated apoptosis and inflammation via the NF-κB and MAPK signaling pathways in rat chondrocytes and ameliorates osteoarthritis vivo. Chin. J. Nat. Med.21, 99–112. 10.1016/S1875-5364(23)60388-7
221
Testa G. Giardina S. M. C. Culmone A. Vescio A. Turchetta M. Cannavò S. et al (2021). Intra-articular injections in knee osteoarthritis: a review of literature. J. Funct. Morphol. Kinesiol.6, 15. 10.3390/jfmk6010015
222
Thielen N. Van Der Kraan P. Van Caam A. (2019). TGFβ/BMP signaling pathway in cartilage homeostasis. Cells8, 969. 10.3390/cells8090969
223
Tian Y. Gou J. Zhang H. Lu J. Jin Z. Jia S. et al (2021). The anti-inflammatory effects of 15-HETE on osteoarthritis during treadmill exercise. Life Sci.273, 119260. 10.1016/j.lfs.2021.119260
224
Ting Y. Jiang Y. Zhao S. Nibber T. Huang Q. (2018). Self-nanoemulsifying system (SNES) enhanced oral bioavailability of boswellic acids. J. Funct. Foods40, 520–526. 10.1016/j.jff.2017.11.043
225
Togo K. Ebata N. Yonemoto N. Abraham L. (2022). Safety risk associated with use of nonsteroidal anti‐inflammatory drugs in Japanese elderly compared with younger patients with osteoarthritis and/or chronic low back pain: a retrospective database study. Pain Pract.22, 200–209. 10.1111/papr.13079
226
Tsujii A. Ohori T. Hanai H. Nakamura N. (2024). Next generation approaches for cartilage repair and joint preservation. J. Cartil. Jt. Preserv.4, 100177. 10.1016/j.jcjp.2024.100177
227
Van Osch G. J. V. M. Brittberg M. Dennis J. E. Bastiaansen-Jenniskens Y. M. Erben R. G. Konttinen Y. T. et al (2009). Cartilage repair: past and future – lessons for regenerative medicine. J. Cell. Mol. Med.13, 792–810. 10.1111/j.1582-4934.2009.00789.x
228
Vučković S. Srebro D. Vujović K. S. Vučetić Č. Prostran M. (2018). Cannabinoids and pain: new insights from old molecules. Front. Pharmacol.9, 1259. 10.3389/fphar.2018.01259
229
Wang L. Xu B. Li S. (2022b). Quercetin activates autophagy suppresses apoptosis, inflammation and cartilage matrix degradation in LPS-induced chondrocytes through targeting AMPK/mTOR/ULK1 signaling pathway. Prepr. A. T. 10.21203/rs.3.rs-1541634/v1
230
Wang M. Wu Y. Li G. Lin Q. Zhang W. Liu H. et al (2024). Articular cartilage repair biomaterials: strategies and applications. Mater. Today Bio24, 100948. 10.1016/j.mtbio.2024.100948
231
Wang X. Zhao X. Zhong Y. Shen J. An W. (2022d). Biomimetic exosomes: a new generation of drug delivery system. Front. Bioeng. Biotechnol.10, 865682. 10.3389/fbioe.2022.865682
232
Wang Y. Liu L. Le Z. Tay A. (2022a). Analysis of nanomedicine efficacy for osteoarthritis. Adv. NanoBiomed Res.2, 2200085. 10.1002/anbr.202200085
233
Wang Z.-Z. Jia Y. Wang G. He H. Cao L. Shi Y. et al (2022c). Dynamic covalent hydrogel of natural product baicalin with antibacterial activities. RSC Adv.12, 8737–8742. 10.1039/d1ra07553e
234
Wen J. Li H. Dai H. Hua S. Long X. Li H. et al (2023). Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio19, 100597. 10.1016/j.mtbio.2023.100597
235
Wojdasiewicz P. Poniatowski Ł. A. Szukiewicz D. (2014). The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm.2014, 1–19. 10.1155/2014/561459
236
Wu M. Wu S. Chen W. Li Y.-P. (2024). The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res.34, 101–123. 10.1038/s41422-023-00918-9
237
Xiao Y. Zhang L. (2023). Mechanistic and therapeutic insights into the function of NLRP3 inflammasome in sterile arthritis. Front. Immunol.14, 1273174. 10.3389/fimmu.2023.1273174
238
Xiong L. Bao H. Li S. Gu D. Li Y. Yin Q. et al (2023). Cerium oxide nanoparticles protect against chondrocytes and cartilage explants from oxidative stress via Nrf2/HO-1 pathway in temporomandibular joint osteoarthritis. Front. Bioeng. Biotechnol.11, 1076240. 10.3389/fbioe.2023.1076240
239
Xiong W. Lan Q. Liang X. Zhao J. Huang H. Zhan Y. et al (2021). Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J. Nanobiotechnology19, 197. 10.1186/s12951-021-00945-x
240
Xu C. Jiang Y. Wang H. Zhang Y. Ye Y. Qin H. et al (2023). Arthritic microenvironment actuated nanomotors for active rheumatoid arthritis therapy. Adv. Sci.10, 2204881. 10.1002/advs.202204881
241
Xu F. Zhao L. J. Liao T. Li Z. C. Wang L. L. Lin P. Y. et al (2022). Ononin ameliorates inflammation and cartilage degradation in rat chondrocytes with IL-1β-induced osteoarthritis by downregulating the MAPK and NF-κB pathways. BMC Complement. Med. Ther.22, 25. 10.1186/s12906-022-03504-5
242
Yan H. Duan X. Pan H. Akk A. Sandell L. J. Wickline S. A. et al (2019). Development of a peptide-siRNA nanocomplex targeting NF- κB for efficient cartilage delivery. Sci. Rep.9, 442. 10.1038/s41598-018-37018-3
243
Yang G. Kang H. C. Cho Y.-Y. Lee H. S. Lee J. Y. (2022). Inflammasomes and their roles in arthritic disease pathogenesis. Front. Mol. Biosci.9, 1027917. 10.3389/fmolb.2022.1027917
244
Yao M. Zhang C. Ni L. Ji X. Hong J. Chen Y. et al (2022). Cepharanthine ameliorates chondrocytic inflammation and osteoarthritis via regulating the MAPK/NF-κB-Autophagy pathway. Front. Pharmacol.13, 854239. 10.3389/fphar.2022.854239
245
Yetisgin A. A. Cetinel S. Zuvin M. Kosar A. Kutlu O. (2020). Therapeutic nanoparticles and their targeted delivery applications. Molecules25, 2193. 10.3390/molecules25092193
246
Yi X. Leng P. Wang S. Liu L. Xie B. (2024). Functional nanomaterials for the treatment of osteoarthritis. Int. J. Nanomedicine19, 6731–6756. 10.2147/IJN.S465243
247
Yu B. Tai H. C. Xue W. Lee L. J. Lee R. J. (2010). Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol.27, 286–298. 10.3109/09687688.2010.521200
248
Zerrillo L. Gigliobianco M. R. D'Atri D. Garcia J. P. Baldazzi F. Ridwan Y. et al (2022). PLGA nanoparticles grafted with hyaluronic acid to improve site-specificity and drug dose delivery in osteoarthritis nanotherapy. Nanomaterials12, 2248. 10.3390/nano12132248
249
Zha K. Li X. Yang Z. Tian G. Sun Z. Sui X. et al (2021). Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. Npj Regen. Med.6, 14. 10.1038/s41536-021-00122-6
250
Zhai Q. Chen X. Fei D. Guo X. He X. Zhao W. et al (2022). Nanorepairers rescue inflammation‐induced mitochondrial dysfunction in mesenchymal stem cells. Adv. Sci.9, 2103839. 10.1002/advs.202103839
251
Zhang Q. Xiang E. Rao W. Zhang Y. Xiao C. Li C. et al (2021). Intra-articular injection of human umbilical cord mesenchymal stem cells ameliorates monosodium iodoacetate-induced osteoarthritis in rats by inhibiting cartilage degradation and inflammation. Bone Jt. Res.10, 226–236. 10.1302/2046-3758.103.BJR-2020-0206.R2
252
Zhang S. Wang J. Pan J. (2016). Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv.23, 3696–3703. 10.1080/10717544.2016.1223218
253
Zhang S. Zhang M. Li X. Li G. Yang B. Lu X. et al (2022b). Nano-based Co-delivery system for treatment of rheumatoid arthritis. Molecules27, 5973. 10.3390/molecules27185973
254
Zhang X. You Y. Sun Y. Guo X. Lin H. Zong M. et al (2022c). Catalytic anti-oxidative stress for osteoarthritis treatment by few-layered phosphorene. Mater. Today Bio17, 100462. 10.1016/j.mtbio.2022.100462
255
Zhang Z. Dalan R. Hu Z. Wang J. W. Chew N. W. Poh K. K. et al (2022a). Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Adv. Mater.34, 2202169. 10.1002/adma.202202169
256
Zhao C. Jiang W. Zhou N. Liao J. Yang M. Hu N. et al (2017). Sox9 augments BMP2-induced chondrogenic differentiation by downregulating Smad7 in mesenchymal stem cells (MSCs). Genes Dis.4, 229–239. 10.1016/j.gendis.2017.10.004
257
Zhao G. Zeng Q. Zhang S. Zhong Y. Wang C. Chen Y. et al (2019). Effect of carrier lipophilicity and preparation method on the properties of andrographolide–solid dispersion. Pharmaceutics11, 74. 10.3390/pharmaceutics11020074
258
Zhao X. Gu M. Xu X. Wen X. Yang G. Li L. et al (2020). CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil.28, 613–625. 10.1016/j.joca.2020.01.009
259
Zheng K. Bai J. Yang H. Xu Y. Pan G. Wang H. et al (2023). Nanomaterial-assisted theranosis of bone diseases. Bioact. Mater.24, 263–312. 10.1016/j.bioactmat.2022.12.014
260
Zhou K. Yang C. Shi K. Liu Y. Hu D. He X. et al (2023). Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage. Biomaterials295, 122036. 10.1016/j.biomaterials.2023.122036
261
Zhou X. Tao Y. Liang C. Zhang Y. Li H. Chen Q. et al (2015). BMP3 alone and together with TGF-β promote the differentiation of human mesenchymal stem cells into a nucleus pulposus-like phenotype. Int. J. Mol. Sci.16 (9), 20344–20359. 10.3390/ijms160920344
262
Zhu J. Zhen G. An S. Wang X. Wan M. Li Y. et al (2020). Aberrant subchondral osteoblastic metabolism modifies NaV1.8 for osteoarthritis. eLife9, e57656. 10.7554/eLife.57656
263
Zhu X. Chan Y. T. Yung P. S. H. Tuan R. S. Jiang Y. (2021). Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front. Cell Dev. Biol.8, 607764. 10.3389/fcell.2020.607764
264
Zhuang C. Sun R. Zhang Y. Zou Q. Zhou J. Dong N. et al (2024). Treatment of rheumatoid arthritis based on the inherent bioactivity of black phosphorus nanosheets. Aging Dis., 0. 10.14336/AD.2024.0319
265
Zou J. Yang W. Cui W. Li C. Ma C. Ji X. et al (2023). Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing. J. Nanobiotechnology21, 14. 10.1186/s12951-023-01778-6
266
Zou Z. Sun M. Yin W. Yang L. Kong L. (2021). Avicularin suppresses cartilage extracellular matrix degradation and inflammation via TRAF6/MAPK activation. Phytomedicine91, 153657. 10.1016/j.phymed.2021.153657
Summary
Keywords
osteoarthritis, nanodrug, immunological responses, mesenchymal stem cells, signaling pathway
Citation
Liao J, Gu Q, Liu Z, Wang H, Yang X, Yan R, Zhang X, Song S, Wen L and Wang Y (2024) Edge advances in nanodrug therapies for osteoarthritis treatment. Front. Pharmacol. 15:1402825. doi: 10.3389/fphar.2024.1402825
Received
18 March 2024
Accepted
25 September 2024
Published
30 October 2024
Volume
15 - 2024
Edited by
Chris A. Bashur, Florida Institute of Technology, United States
Reviewed by
Amilcare Barca, University of Salento, Italy
Sadiq Umar, University of Illinois Chicago, United States
Updates
Copyright
© 2024 Liao, Gu, Liu, Wang, Yang, Yan, Zhang, Song, Wen and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Wang, w_yi2022@163.com; Lebin Wen, 58619763@qq.com; Siyuan Song, si-yuan.song@bcm.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.