- 1Department of Clinical Pharmacy, Xiangtan Central Hospital, Xiangtan, Hunan, China
- 2Hunan Provincial Key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, The “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science), Changsha Medical University, Changsha, Hunan, China
- 3Department of Pharmacy, Xiangtan Central Hospital, Xiangtan, Hunan, China
Diabetic cardiomyopathy (DCM) is a myocardial-specific microvascular disease caused by diabetes that affects the structure and function of the heart and is considered to be the leading cause of morbidity and death in patients with diabetes. Currently, there is no specific treatment or preventive drug for DCM, and there is an urgent need to develop new drugs to treat DCM. Traditional Chinese medicine (TCM) has rich experience in the treatment of DCM, and its characteristics of multi-target, multi-pathway, multi-component, and few side effects can effectively deal with the complexity and long-term nature of DCM. Growing evidence suggests that myocardial fibrosis, inflammation, oxidative stress, apoptosis, cardiac hypertrophy, and advanced glycation end product deposition were the main pathologic mechanisms of DCM. According to the pathological mechanism of DCM, this study revealed the potential of metabolites and prescriptions in TCM against DCM from the perspective of signaling pathways. The results showed that TGF-β/Smad, NF-κB, PI3K/AKT, Nrf2, AMPK, NLRP3, and Wnt/β-catenin signaling pathways were the key signaling pathways for TCM treatment of DCM. The aim of this study was to summarize and update the signaling pathways for TCM treatment of DCM, to screen potential targets for drug candidates against DCM, and to provide new ideas and more experimental evidence for the clinical use of TCM treatment of DCM.
1 Introduction
The incidence of diabetes has increased exponentially over the past few decades as living standards have improved, and the number of people worldwide with the disease is reported to be more than 700 million by 2045. The main feature of diabetes is persistent hyperglycemia, which over time will cause endocrine and metabolic disorders in patients with diabetes and lead to complications of diabetes, which is the final outcome of diabetes and the main cause of great pain and economic loss to patients, and has become one of the most serious global public health problems (Li et al., 2022; Huang et al., 2023; Zhou et al., 2023). Diabetic cardiomyopathy (DCM) refers to a complication caused by diabetes, which cannot be explained by hypertensive heart disease, coronary artery disease, valvular heart disease, and other heart diseases, and is characterized by myocardial-specific microvascular disease. It is the leading cause of morbidity and mortality in people with diabetes (Tan et al., 2020; Zhao et al., 2022; Yan et al., 2023).
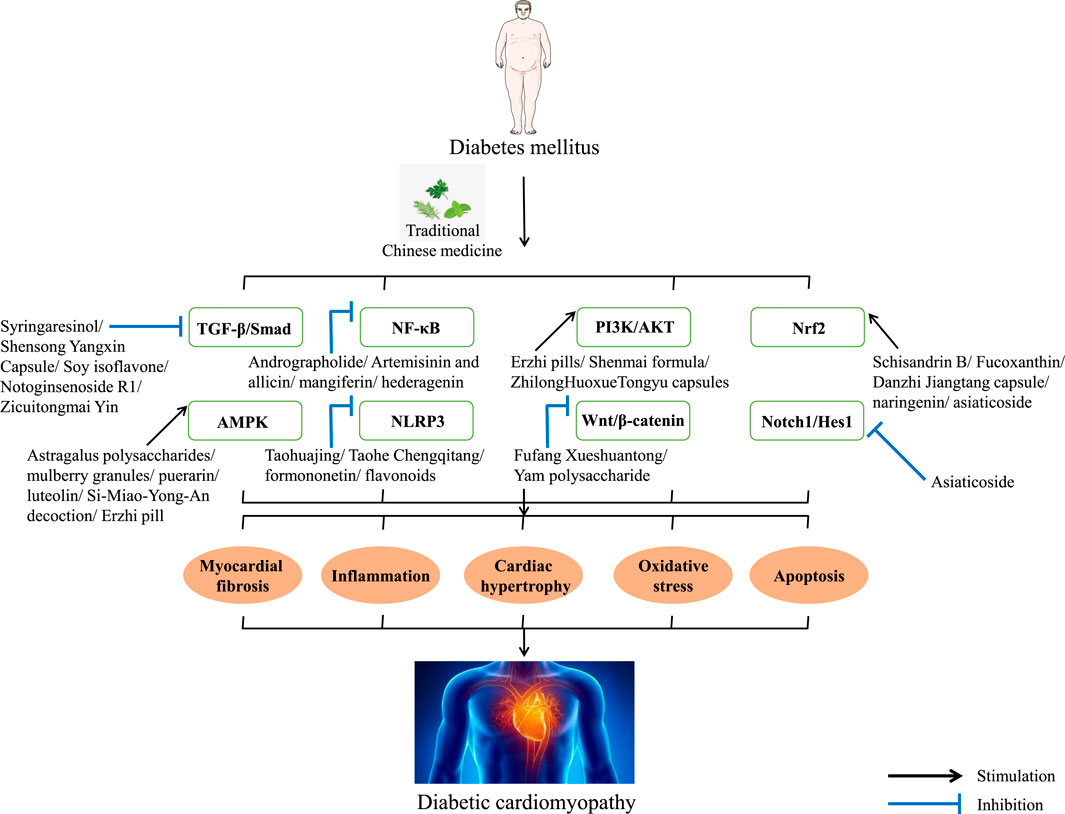
Clinically, DCM can be divided into two stages according to its typical features. The early stage is characterized by diastolic damage and left ventricular hypertrophy without vascular defects, and the late stage is mainly characterized by systolic dysfunction and myocardial fibrosis (Wang et al., 2022; Zhou et al., 2023). Although the pathogenesis of DCM is complex, after long-term exploration, scholars generally believe that the occurrence and development of DCM is mainly related to glucose and lipid metabolism disorders, microvascular endothelial dysfunction, apoptosis, advanced glycation end product (AGE) deposition, myocardial inflammation, and oxidative stress. Under the influence of the crosstalk of the above factors, it will lead to increased myocardial oxygen consumption, myocardial fibrosis, increased metabolic pressure, significantly reduced cardiac efficiency and function, and ultimately DCM (Huo et al., 2021; Wang et al., 2022; Wu et al., 2023; Cai et al., 2023; Zhou et al., 2023). Currently, there is no specific treatment or preventative drug for DCM. Therefore, it is urgent to explore drugs that are new and have fewer side effects or complementary and alternative therapies for DCM treatment (Tan et al., 2020; Nakamura et al., 2022; Zhou et al., 2023). Traditional Chinese medicine (TCM) has its own unique diagnosis and treatment system, which has been used for the prevention and treatment of diseases for more than 2,000 years in Chinese history. TCM is characterized by multiple targets, multiple pathways, fewer side effects, and easy access. It is considered to be a novel therapeutic agent for many diseases, especially for chronic metabolic diseases such as DCM. In order to better understand the efficacy of TCM, a large number of experimental studies have been carried out, trying to uncover the veil from the molecular mechanism (Zheng et al., 2018; Yang et al., 2021; Li X. H. et al., 2022a). Currently, there is a lack of review of TCM against DCM signaling pathways. This article summarizes the related signaling pathways of TCM against DCM in recent years in order to provide ideas for the development of new drugs against DCM (Figure 1).
2 Signaling pathways in TCM intervening the pathological progression of DCM
2.1 TGF-β/Smad signaling pathway
Excessive extracellular matrix deposition in the diabetic heart is associated with overexpression of transforming growth factor-β1 (TGF-β1) and connective tissue growth factors, which can cause cardiac fibrosis and damage the structure and function of the heart. When the body is exposed to persistently hyperglycemia levels, endothelial cells take on the characteristics of fibroblasts, leading to diabetic heart fibrosis. TGF-β is one of the key factors in the emergence of DCM that is expressed in all human cells. TGF-β is mainly divided into TGF-β1, TGF-β2, and TGF-β3 forms, which play an important role in myocardial fibrosis, cardiac repair, and cardiac remodeling, especially myocardial fibrosis. TGF-β also has three receptors, namely, TGFBR1, TGFBR2, and TGFBR3. When one of the TGF-β ligands binds to TGFBR2, it will produce typical TGF-β signal transduction, and then, TGFBR2 will recruit and phosphorylate TGFBR1. Phosphorylated TGFBR1 then phosphorylates downstream Smad2 and Smad3 and recruits Smad4 and translocates to the nucleus to bind to DNA and promote TGF-β transcription. Among them, TGF-β1 has a strong anti-inflammatory function and is involved in almost all early tissue damage and is a key cytokine. In addition, Smad3 is pathogenic in cardiovascular diseases, and its overexpression could lead to myocardial inflammation, myocardial fibrosis, and insulin synthesis and secretion disorders, which is a key mediator in the pathogenesis of DCM. Smad7 has a myocardial protective effect, and its overexpression could reverse Smad3-mediated myocardial fibrosis and nuclear factor kappa B (NF-κB)-driven inflammation (Frangogiannis, 2022; Ghafouri-Fard et al., 2023; Qin et al., 2023; Seksaria et al., 2023; Yang et al., 2023).
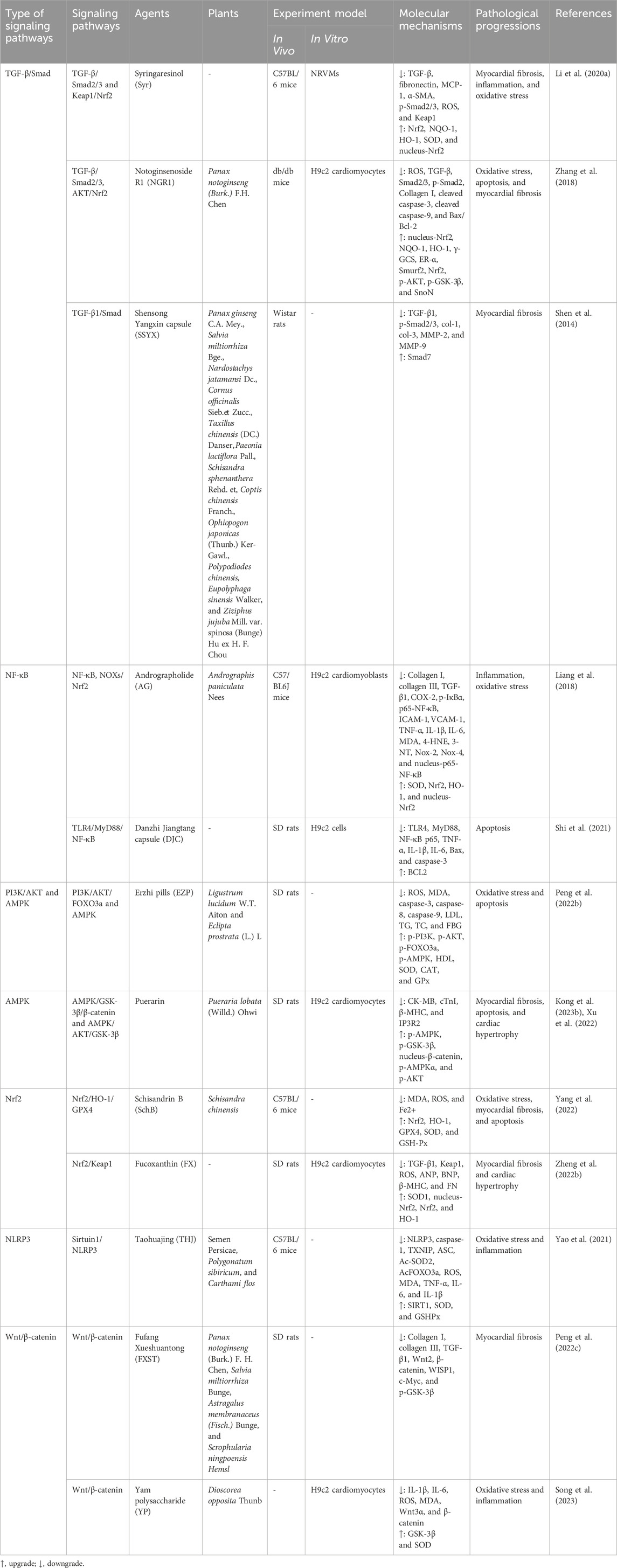
Syringaresinol (Syr) is a polyphenolic metabolite widely found in various TCM formulations, and many pieces of evidence have shown that Syr has anti-inflammatory, antioxidant, and antitumor activities (Li et al., 2023; Wang et al., 2023; Wang et al., 2023). Li et al. (2020) found that Syr significantly reversed the decrease of ejection fraction (EF), fractional shortening (FS), and cardiac output (CO) in streptozotocin (STZ)-induced diabetic mice and reversed the increase in inflammatory factors tumor necrosis factor-α (TNF-α) and interleukin (IL-6) and the increase in IL-1β and MCP-1 in cardiac tissues of diabetic mice, which indicate that Syr could alleviate cardiac damage and inflammatory response in diabetic mice. Masson trichrome staining and Sirius Red staining showed that Syr decreased interstitial collagen accumulation and effectively reversed myocardial fibrosis in diabetic mice, and Syr significantly decreased the high expression of TGF-β, fibronectin, α-SMA, and p-Smad2/3 in cardiac tissues of diabetic mice. In addition, Syr reversed the increase in ROS and the decrease in SOD in the myocardium of diabetic mice; upregulated the expression of Nrf2, NQO-1, and HO-1; and downregulated the expression of Keap1. In further studies, Li et al. established high-glucose (HG)-treated neonatal rat ventricular myocytes (NRVMs) and verified the abovementioned effects of Syr, and the results showed consistency. These results suggested the potential of Syr to attenuate inflammatory response, myocardial fibrosis, and oxidative stress for the treatment of DCM, and its mechanism of action is related to TGF-β/Smad2/3 and Keap1/Nrf2 signaling pathways.
Panax notoginseng (Burk.) F.H. Chen, known as “aspirin” in TCM, has the efficacy of reducing swelling, relieving pain, clearing blood stasis, and stopping bleeding, and has a landmark position in TCM. Notoginsenoside R1 (NGR1) is one of the main bioactive metabolites of P. notoginseng (Burk.) F.H. Chen. It has a wide range of protective and therapeutic effects in anti-diabetes, anticancer, and vascular protection, especially cardiovascular and cerebrovascular protection (Liu et al., 2020; Xie and Wang, 2023; Zhu and Wan, 2023). Zhang et al. (2018) found that NGR1 significantly reduced the apoptosis and mitochondrial damage of AGE-induced H9c2 cells in vitro; downregulated ROS, Smad2/3, p-Smad2, and collagen I; and promoted the expression of estrogen receptor (ER)-α and Smurf2. In vivo, NGR1 decreased LVIDd and LV mass and increased LVVd, LVVs, EF, and FS in diabetic mice. In addition, NGR1 inhibited the increase of LDH, AST, and CK-MB in the serum of diabetic mice. In a further mechanism study, NGR1 upregulated the expression levels of Nrf2, γ-GCS, NQO-1, HO-1, p-AKT, p-GSK-3β, and SnoN and downregulated the expression levels of TGF-β, collagen I, and Smad2/3 in cardiac tissues of diabetic mice. Furthermore, NGR1 promoted the expression levels of ERα and SMurf2 in cardiac tissues of diabetic mice, which was consistent with the results of in vitro experiments. These lines of evidence suggest that NGR1 may improve DCM through TGF-β/Smad2/3 and AKT/Nrf2 signaling pathways. Shensong Yangxin capsule (SSYX) is a TCM prescription widely used to treat arrhythmia and has been used clinically for many years (Cao et al., 2021; Ma et al., 2023). Shen et al. (2014) found that SSYX significantly reduced the heart weight/body weight ratio of fat emulsion + STZ-induced diabetic rats and improved the degree of cardiac dysfunction, myocardial fibrosis, and collagen deposition. In addition, it was found in the mechanism study that SSYX downregulated the expression levels of TGF-β1, p-Smad2/3, col-1, col-3, MMP-2, and MMP-9 and upregulated the expression level of Smad7 in myocardial tissues of diabetic rats.
Moreover, Soy isoflavone (Huang et al., 2023), Zicui Tongmai Yin (Wu et al., 2021), ginkgolide B (Gao and Hou, 2022), and echinacoside (Liao et al., 2022) have shown significant effects in alleviating myocardial fibrosis in diabetes by a mechanism related to the inhibition of the TGF-β/Smad signaling pathway.
2.2 NF-κB signaling pathway
The nuclear factor kappa B (NF-κB) family consists of five members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2). They are involved in a variety of physiological and pathological processes, including cellular immunity, proliferation, inflammation, and oxidative stress. In DCM, persistently high levels of glucose and LDL/VLDL lipoproteins stimulate NF-κB activation in the myocardium. In addition, they can cause circulating and local cells to release vasoactive polypeptides, TGF-β growth factor, and connective tissue growth factor. These molecules stimulate NF-κB activity either directly or through cytokine-mediated expression. There are two major NF-κB activation pathways in the above process, namely, the canonical and non-canonical signaling pathways. The canonical signaling pathway is mainly induced by pro-inflammatory factor tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and lipopolysaccharide (LPS). When cells are stimulated, the IκB kinase (IKK) complex is activated, and signal transduction leads to IκB protein phosphorylation and ubiquitination. IκB is then degraded, releasing the NF-κB dimer from the cytoplasm into the nucleus, where it binds to DNA and promotes its transcription. Among them, IκBα can not only bind to the most common NF-κB heterodimer P65:P50 but also the strongest negative feedback factor in NF-κB activation, ensuring the rapid initiation and termination of the entire process (Lorenzo et al., 2011; Li L. J. et al., 2023; Cheng et al., 2023; Oh et al., 2023).
Andrographis paniculata Nees is distributed all over the world and is widely used, and andrographolide (AG) is a diterpene lactone metabolite, which is the main active metabolite of A. paniculata Nees and can be used to treat hyperlipidemia, obesity, diabetes, and cardiovascular diseases (Li X. H. et al., 2022b; Gou et al., 2023). Liang et al. (2018) found that diabetes could lead to decreased LVEF, FS, E/A, and insulin levels and increased blood glucose in mice, while AG could reverse the above phenomenon. According to the results of Masson’s trichrome and Sirius Red staining, diabetic mice showed increased collagen deposition in the myocardial interstitial area, and collagen I, collagen indigenous, TGF-β1, fibronectin (FN), ANP, and BNP were decreased; the above phenomena could be significantly improved after AG intervention. In a further mechanism study, the expression levels of COX-2, p-IκBα, p65-NF-κB, intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), TNF-α, IL-1β, and IL-6 in myocardial tissues of diabetic mice were increased, while AG could reduce their expression levels. In addition, AG also reduced the expression of MDA, 4-HNE, 3-NT, Nox-2, and Nox-4 and increased the expression of SOD, Nrf2, and HO-1 in myocardial tissues of diabetic mice. In the HG-induced H9c2 cardiomyoblast model, AG reduced the expression of p-IκBα, nucleus-p65-NF-κB, Nox-2, and Nox-4 and promoted the translocation of Nrf2 into the nucleus. These results suggested that AG has the potential to treat DCM by reducing inflammation and oxidative stress, and its mechanism may be related to NF-κB and NOXs/Nrf2 signaling pathways. Artemisinin and allicin (AA) have anticancer, antioxidant, and anti-inflammatory biological activities (Zhou et al., 2022; Jin et al., 2023) and have been proved to be used in clinical anti-myocardial fibrosis (Kong et al., 2023a). Kong et al. (2023a) found that the cardiac function of STZ-induced diabetic rats was damaged, and AA could improve these damages. In addition, the myocardial cells of diabetic rats showed pathological changes, such as increased collagen fibers, a large number of fibrous scars, and increased myocardial cells, and these changes were significantly reduced after AA intervention. In the mechanism study, AA significantly reduced the expression of NF-κB p65 and p-NF-κB p65 in the myocardial tissues of diabetic rats, indicating that the effect of AA on improving myocardial injury and myocardial fibrosis in diabetic rats may be related to the inhibition of the NF-κB signaling pathway. Furthermore, mangiferin (Hou et al., 2016) and hederagenin (Li et al., 2019) play a beneficial role in DCM by inhibiting the NF-κB signaling pathway to reduce the inflammatory response.
2.3 PI3K/AKT signaling pathway
Phosphoinositol-3 kinase (PI3K) is an intracellular lipid kinase, which can be divided into three types according to its molecular structure and substrate selectivity. Class I PI3K is the focus of current research. It is a heterodimer composed of a regulatory domain (p85) and a catalytic domain (p110) that phosphorylate the 3′-hydroxyl group of phosphatidylinositol and phosphoinositides. The PI3K-mediated imbalance of fatty acid metabolism and glucose homeostasis in diabetic hearts has been reported to lead to pathological features of DCM, such as decreased cardiac efficiency, pathological myocardial hypertrophy, fibrosis, and apoptosis. Dysregulation of PI3K-induced metabolic imbalances leads to the development of DCM. AKT is also known as protein kinase B (PKB), a serine/threonine kinase widely expressed in human tissues, divided into AKT1 (PKB α), AKT2 (PKB β), and AKT3 (PKB γ), the first two of which are commonly expressed in the heart, brain, and lungs. Overexpression of AKT3 in the heart can induce myocardial hypertrophy. AKT1 and AKT3 function similarly, and it has also been shown that overexpression of AKT1 induces myocardial hypertrophy, but loss of AKT1 leads to resistance to exercise-induced cardiac growth. AKT2 is a regulator of cardiomyocyte metabolism and cardiomyocyte death in DCM. AKT is the main downstream effector molecule of PI3K, which plays a key role in DCM by regulating cell size, survival, apoptosis, angiogenesis, and inflammatory response (Li et al., 2017; Qin et al., 2021; Ghafouri-Fard et al., 2022).
Erzhi pill (EZP) is a TCM prescription composed of the Ligustrum lucidum W. T. Aiton and Eclipta prostrata (L.) L. in equal proportions. It has the effects of nourishing yin and tonifying the liver and kidney and is commonly used in clinical treatment of diabetic nephropathy and osteoporosis (Li et al., 2020; Peng et al., 2022; Hong et al., 2024). Peng et al. (2022) reported the intervention effect of EZP on diabetic rats induced by high-fat diet combined with STZ. EZP increased the level of high-density lipoprotein (HDL) and decreased the levels of low-density lipoprotein (LDL), triglycerides (TG), total cholesterol (TC), and fasting blood glucose (FBG) in the serum of diabetic rats. In addition, EZP could reverse the decrease in superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) and the increase in caspase-3, caspase-8, caspase-9, reactive oxygen species (ROS), malondialdehyde (MDA), B-cell lymphoma (Bcl)-2, and Bcl-2-associated X protein (Bax) in the serum of diabetic rats. In the study of the mechanism of action, EZP upregulated the expression levels of p-PI3K, p-AKT, p-AMPK, and p-FOXO3a in the heart tissues of diabetic rats. These results suggested that EZP could reduce DCM by inhibiting oxidative stress and apoptosis, and its mechanism is related to PI3K/AKT/FOXO3a and AMPK signaling pathways. The Zhilong Huoxue Tongyu capsule (ZHTC) is a TCM prescription with the effects of tonifying qi, dredging collaterals, promoting blood circulation, and removing blood stasis, which is commonly used in the treatment of ischemic stroke (Wang et al., 2022). Zeng et al. (2023) found that ZHTC could reduce serum GHb, TC, TG, and LDL-C and increase HDL-C in STZ-induced diabetic rats. In addition, the results of HE staining, Masson staining, and TUNEL staining suggested that ZHTC effectively reduced myocardial fibrosis, inflammatory cell infiltration, and apoptosis in diabetic rats. In terms of mechanism, ZHTC upregulated the expression levels of p-PI3K, p-AKT1, and p-FOXO3a in the heart tissues of diabetic rats. These beneficial effects of ZHTC on DCM are related to the regulation of the PI3K/AKT1/FOXO3a signaling pathway.
Moreover, TCM prescriptions Shengjie Tongyu decoction (Wang et al., 2023), Zicui Tongmai decoction (Wu et al., 2023), and Shenmai formula (Li et al., 2023) have shown significant effects in alleviating DCM by a mechanism related to the regulation of the PI3K/AKT signaling pathway.
2.4 Nrf2 signaling pathway
Nuclear factor erythroid 2-related factor 2 (Nrf2), a member of the Cap’n’collar (CNC)-BZIP transcription factor family, is a key transcription factor affecting cellular oxidative stress response. Under normal circumstances, Kelch-like ECH-associated protein 1 (Keap1) is a negative regulator of Nrf2, which can bind to Nrf2 in the cytoplasm and form a complex with cullin3, and mediates the degradation of Nrf2 ubiquitination. Under oxidative stress, with the increase in ROS expression, Nrf2 is dissociated from the complex and translocated into the nucleus to bind to the promoter antioxidant response element (ARE) sequence, promoting the expression of Nrf2-related antioxidant enzymes, such as SOD, CAT, heme oxygenase-1 (HO-1), and GPx (Zhang et al., 2021; Li et al., 2023; Ghareghomi et al., 2023). Related studies have shown that Nrf2 is a key regulator of cardiac resistance to ROS in both normal and diabetic hearts. Primary adult cardiomyocytes from mice with deletion of the Nrf2 gene showed isoproterenol-stimulated loss of contraction. In addition, cardiac Nrf2 expression was significantly downregulated in diabetic animals and patients. Clearly, the downregulation of Nrf2 is one of the important reasons for the occurrence of DCM (Chen et al., 2014).
Schisandrin B (SchB) is the main active metabolite of the fruit of Schisandra chinensis. It has antioxidant biological activity and exhibits protective effects in many tissues in the body, including the heart (Luo et al., 2022; Yang et al., 2022). Yang et al. (2022) reported the intervention effect of SchB on myocardial injury in STZ-induced diabetic mice. SchB could improve heart weight and the cardiac index; reduce FBG, LVEDD, LVESD, LDH, CK-MB, and cTnI; and increase LVEF and LVFS in the serum of diabetic mice. In addition, HE, Masson, and TUNEL staining results showed that SchB reduced the cardiomyocyte apoptosis rate and collagen volume fraction (CVF). SchB reduced MDA, ROS, and Fe2+ and increased SOD and GSH-Px in myocardial tissues of diabetic mice. In terms of mechanism research, SchB upregulated the protein and mRNA expression levels of Nrf2, HO-1, and GPX4 in myocardial tissues of diabetic mice. It is suggested that SchB could improve DCM by exerting antioxidation, anti-fibrosis, and anti-apoptosis effects, and its molecular mechanism may be related to the inhibition of ferroptosis mediated by Nrf2/HO-1/GPX4 signaling pathway activation. Fucoxanthin (FX) is a carotenoid derived from natural marine organisms with antioxidant, anti-diabetic, and anticancer activities (Zheng et al., 2022). Zheng et al. (2022) found that myocardial cells in STZ-induced diabetic rats were hypertrophic and disordered, while FX could improve the above phenomenon. In addition, FX could reduce the surface area of HG-induced H9c2 cells and downregulate the mRNA levels of cell hypertrophy factors ANP, BNP, and β-MHC. These results suggested the ability of FX to reduce cardiomyocyte hypertrophy. In further mechanistic studies, FX reduced the expression levels of ROS, FN, TGF-β1, and Keap1 and upregulated the expression levels of SOD1, nucleus-Nrf2, Nrf2, and HO-1 in heart tissues of diabetic rats. The results suggested that FX has a protective effect on DCM, and its mechanism is related to the Nrf2/Keap1 signaling pathway.
Moreover, naringenin (Li and Lai, 2022), saponins of Aralia taibaiensis (Duan et al., 2015), Danzhi Jiangtang capsule (DJC) (Wang et al., 2023), and asiaticoside (Jin et al., 2020) have shown significant effects in alleviating DCM by a mechanism related to the regulation of the Nrf2 signaling pathway.
2.5 AMPK signaling pathway
Adenosine monophosphate-activated protein kinase (AMPK) is widely present in eukaryotes and various organs, including three unique subunits of α, β, and γ. AMPK is considered an energy sensor that regulates cellular metabolism, and the regulation of AMPK signaling is crucial for the regulation of intracellular dynamic balance, which is also known as the “total metabolic switch” of the human body. In general, AMPK signaling is activated under low-energy stress conditions. Calcium/calmodulin-dependent protein kinase β (CaMKKβ), TGFβ-activated kinase 1, and liver kinase B1 (LKB1), the upstream mediator of AMPK, can induce T172 phosphorylation in AMPK signaling stimulation. Metabolic stress can trigger AMPK signal transduction by regulating AMP and ATP levels. In this process, increased AMP and adenosine diphosphate levels bind to the γ-subunit site and induce T172 phosphorylation, thereby causing AMPK activation. Moreover, the activation of AMPK can be independent of the regulation of AMP levels. Calcium accumulation in cells causes T172 phosphorylation, resulting in CaMKKβ-dependent upregulation of AMPK, in response to increased intracellular calcium and DNA damage caused by glucose starvation. In addition, AMPK can improve DCM by regulating glycolipid metabolism, regulating protein synthesis and degradation, affecting the transcription of proteins related to gluconeogenesis, lipogenesis, and mitochondrial biogenesis, and regulating vascular endothelial dysfunction. Therefore, AMPK is a potential therapeutic target for DCM (Nellaiappan et al., 2019; Peng et al., 2022; Entezari et al., 2022; Liu et al., 2023).
Astragalus polysaccharide (AP) is a key active ingredient extracted from Astragalus membranaceus. Pharmacological studies have shown that AP has anti-inflammatory, antitumor, antioxidation, and anti-fibrosis effects, which is helpful in the management of blood glucose and blood lipids (Zhang and Feng, 2022). Ye et al. (2022) found that LVEDD and LVESD were significantly increased and LVEF and LVFS were significantly decreased in diabetic rats induced by high-sugar and high-fat diets combined with STZ, and these phenomena were significantly reversed after AP intervention. The results of HE staining and Masson staining showed that the myocardial cells of diabetic rats were hypertrophic and disordered, collagen fibers were proliferated, interstitial fibrosis was obvious, and a large number of inflammatory cells were infiltrated. The above conditions were improved after AP intervention. In the mechanism study, AP reduced mTOR and p-mTOR and increased AMPK, p-AMPK protein, and mRNA expression levels in myocardial tissues of diabetic rats. These results indicated that AP could exert a protective effect on DCM by regulating the AMPK/mTOR signaling pathway.
The mulberry granule (MLD), derived from Morus alba L., is a TCM prescription that can be used to treat diabetes (Liu et al., 2017). Liu et al. (2017) reported the intervention effect of MLD on STZ-induced diabetic mice. MLD reduced FBG and fasting blood insulin and blood lipids and increased GSH, SOD, CAT, and GR in cardiac tissues of diabetic mice. In addition, MLD could also improve cardiac function and reduce the area of myocardial infarction in diabetic mice. In further mechanism studies, MLD upregulated the expression levels of p-AMPK and Nrf2 cardiac tissues of diabetic mice. The results suggested that MLD may have a protective effect on DCM, and its mechanism may be related to the AMPK/Nrf2 signaling pathway. Pueraria lobata (Willd.) Ohwi, as a common TCM, has been made into many kinds of anti-diabetic foods. Puerarin is the main active metabolite of P. lobata (Willd.) Ohwi, which has good anti-inflammatory and antioxidant effects (Li et al., 2023). Kong et al. (2023b) found that puerarin significantly reduced the myocardial cell morphology of STZ-induced diabetic rats with irregular and disordered arrangement and a small amount of myocardial cell degeneration and necrosis. The above phenomenon was improved after puerarin intervention. The results of Masson and TUNEL staining showed that puerarin also had a beneficial effect on myocardial fibrosis and apoptosis in diabetic rats. In addition, diabetes resulted in a significant increase in LVESD and LVEDD and a significant decrease in EF and FS in the model rats, which could be reversed by puerarin. Puerarin reduced the levels of FBG, HbA1c, CK-MB, and cTnI in the serum of diabetic rats and upregulated the expression levels of p-AMPK, nucleus-β-catenin, and p-GSK-3β in myocardial tissues. It is suggested that puerarin could improve diabetic myocardial injury by regulating the AMPK/GSK-3β/β-catenin signaling pathway, thus playing a role in protecting DCM. Similarly, Xu et al. (2022) reported that the improvement of puerarin in HG-induced H9c2 cardiomyocyte hypertrophy may be related to the AMPK/AKT/GSK-3β signaling pathway, indicating the importance of AMPK-related signaling pathways in the development of DCM and the great potential of puerarin in the treatment of DCM.
Moreover, luteolin (Huang et al., 2022), Si-Miao-Yong-An decoction (Li et al., 2020), and Erzhi pill (Peng et al., 2022) have shown significant effects in alleviating DCM by a mechanism related to the regulation of the AMPK signaling pathway.
2.6 NLRP3 signaling pathway
Nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome is closely related to metabolic disorders and cell death and is one of the important causes of DCM. One of the important signals during NLRP3 initiation and activation is diabetes-induced hyperglycemia and hyperlipidemia, which can promote ROS overexpression to activate the NF-κB signaling pathway and thus the transcription of NLRP3, pro-IL-1β, and pro-IL-18. Thioredoxin-interacting/inhibiting protein (TXNIP) was another signal during NLRP3 activation that directly combined with NLRP3 to modulate its oligopolization. Hyperglycemia leads to ROS upregulation of TXNIP and thus activation. In addition, hyperglycemia and hyperlipidemia directly exacerbate mitochondrial oxidative stress and proinflammatory cytokine production, thus inducing the formation of NLRP3 inflammasome. Nod-like receptors (NLRs) are a family of pattern recognition receptors (PRRs) used to identify the pathogen-associated molecular pattern (PAMP) and damage-related molecular pattern (DAMP). NLR contains four subfamilies, among which NLRP3 is one of the most representative members. NLRP3 inflammasome consists of NLRP3, pro-caspase-1, and apoptosis-associated speck-like protein containing a CARD domain (ASC). NLRP3 inflammasome activation generally includes two processes. First, when PAMP or DAMP is recognized by the corresponding PRRs, it promotes the transcriptional activation of the NLRP3 inflammasome gene. Then, NLRP3 inflammasome is assembled, caspase-1 is activated, pro-IL-1β and pro-IL-18 shear processing is conducted, and finally, mature forms of IL-1β and IL-18 are produced and secreted extracellularly to trigger the inflammatory response (Luo et al., 2014; Sun and Ding, 2021; Zheng et al., 2022; Jiang et al., 2024).
Taohuajing (THJ) is a TCM prescription consisting of Semen Persicae, Polygonatum sibiricum, and Carthami flos, which has a good clinical effect in the treatment of DCM (Yao et al., 2021). Yao et al. (2021) observed the intervention effect of THJ on DCM by establishing a high-fat diet combined with an STZ-induced diabetic mouse model. THJ reduced the blood glucose and blood lipid of DCM model mice and improved their insulin sensitivity in a dose-dependent manner. In the study of myocardial function, THJ increased LVEF and LVFS and decreased LVEDV and LVESV in DCM model mice, indicating that THJ could improve myocardial function in DCM model mice. Masson and HE staining showed a significant increase in collagen, myocardial cells and mast cells were destroyed, myofibrils were broken and irregular, and mitochondria were swollen in the heart of DCM model mice, while THJ could reverse these diabetes-induced cardiac function damage. In addition, THJ also reduced the inflammatory factors TNF-α, IL-6, and IL-1β in the serum of DCM model mice, reduced the levels of ROS and MDA, and increased the levels of SOD and GSHPx in heart tissues of DCM model mice, reflecting its ability to inhibit oxidative stress and inflammatory response in DCM model mice. In a further mechanism study, THJ reduced the protein and mRNA expression levels of NLRP3, caspase-1, TXNIP, ASC, and IL-1β in the left ventricular tissues of DCM model mice. In addition, THJ downregulated the expression levels of Ac-SOD2 and AcFOXO3a and upregulated the expression level of SIRT1 in heart tissues of DCM model mice. These experimental results indicated that THJ can protect myocardial injury through antioxidative stress and anti-inflammatory effects, and the molecular mechanism of these effects may be related to the Sirtuin1/NLRP3 signaling pathway. Taohe Chengqitang (TCT), a TCM prescription derived from Shanghan Zabing Lun, has the effects of tonifying qi and nourishing yin, promoting blood circulation, and removing blood stasis and could be used clinically to treat diabetic complications (Zhang et al., 2022). Zhang et al. (2022) observed the intervention effect of TCT on diabetic rats. TCT increased EF and FS in STZ-induced diabetic rats and decreased FBG, TC, and TG in the serum of diabetic rats. The inflammatory factors IL-1β and IL-18 in the serum of diabetic rats were significantly increased, and the expression of these inflammatory factors could be significantly reversed after TCT intervention. In the examination of the pathological morphology of myocardial tissues in diabetic rats, it was found that diabetes could lead to hypertrophy of myocardial fibers in rats, disordered arrangement, a small amount of necrotic and apoptotic myocardial fibers, and a small amount of inflammatory cell infiltration, and these symptoms were significantly improved after TCT treatment. In further studies, TCT significantly reduced the expression levels of ASC, caspase-1, NLRP3, and p-NF-κB p65 in myocardial tissues of diabetic rats. It is suggested that TCT could reduce myocardial inflammation and myocardial fibrosis by inhibiting the activation of the NLRP3 inflammasome signaling pathway and improve the cardiac function of DCM rats, thus playing a role in the treatment of DCM.
Moreover, formononetin (Zhang et al., 2023), protocatechualdehyde (Ding et al., 2024), and mulberry leaves flavonoids (Yang and Cao, 2022) have shown significant effects in alleviating DCM by a mechanism related to the inhibition of the NLRP3 signaling pathway.
2.7 Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is an important regulator of cardiac development and growth. Although its activity is low in the normal heart, it is indispensable for maintaining normal cardiac function, and its long-term high activity can lead to cardiac fibrosis and cardiac hypertrophy. In DCM, hyperglycemia causes an overproduction of ROS through the mitochondrial electron transport chain, causing oxidative stress. Oxidative stress can activate the Wnt/β-catenin signaling pathway and nuclear β-catenin/c-Myc axis and aggravate oxidative stress damage under their cascading influence, thus exacerbating DCM. The Wnt signaling pathway is divided into classical and non-classical pathways; the former mainly controls cell proliferation, while the latter regulates cell polarity and migration, and the two main pathways form a mutual regulatory network. In the canonical Wnt pathway, it activates the Wnt/β-catenin signaling pathway by regulating the downstream key transcription factor β-catenin, and then, the Wnt ligand protein Wnt3α binds to frizzled (Fzd), low-density lipoprotein receptor-related protein receptors 5/6 (LRP5/6) to activate Casein kinase 1 and promote the release of GSK3β-related regulatory proteins. PDZ and DvL are recruited to form a protein complex, which inhibits the activation of GSK3β, thereby inhibiting the degradation of β-catenin. β-catenin can stabilize aggregation and transfer to the nucleus to interact with the T-cell factor/lymphoid enhancer factor, initiating Wnt/β-catenin downstream target gene and protein transcription (Liu et al., 2022; Ma et al., 2023; Ni et al., 2023).
FXST is a TCM prescription composed of P. notoginseng (Burk.) F. H. Chen, Salvia miltiorrhiza Bunge, Astragalus membranaceus (Fisch.) Bunge, and Scrophularia ningpoensis Hemsl. It has the function of promoting blood circulation, removing blood stasis, tonifying qi, and nourishing yin and could be used clinically for the treatment of stable angina pectoris and cerebrovascular diseases (Peng et al., 2022; Wang et al., 2022). Peng et al. (2022) observed the intervention effect of FXST on DCM by establishing a STZ-induced diabetic rat model. FXST reduced LVEDP and significantly increased the heart rate, CI, LVSP, dp/dt min, EF, and FS in diabetic rats. Diabetic rats showed myocardial cell disorder, inflammatory cell infiltration, uneven staining of the nucleus and cytoplasm, and a high deposition area of myocardial collagen fibers, which were reversed by FXST intervention. In addition, FXST could reduce the expression levels of collagen I, collagen III, and TGF-β1 in heart tissues of diabetic rats and downregulate the protein expression levels of Wnt2, β-catenin, WISP1, c-Myc, and p-GSK-3β and the mRNA expression levels of β-catenin and c-Myc in myocardial tissues of diabetic rats. These results suggested that FXST could enhance cardiac function and reduce myocardial fibrosis induced by diabetes, thereby improving the role of DCM, which may be related to the downregulation of the Wnt/β-catenin signaling pathway. The work of Song et al. (2023) revealed the intervention of Yam polysaccharide (YP) on HG-induced H9c2 cardiomyocytes. YP decreased the levels of IL-1β, IL-6, ROS, and MDA and increased SOD activity and cell viability in HG-induced H9c2 cardiomyocytes, indicating the anti-inflammatory and antioxidant properties of YP. In terms of mechanism, YP downregulated the expression of Wnt3α and β-catenin and upregulated the expression of GSK3β. These results indicated that YP could improve DCM through anti-inflammatory and antioxidant effects, and its mechanism may be related to the Wnt/β-catenin signaling pathway.
2.8 Other signaling pathways
Centella asiatica (L.) Urban mainly grows in China and Southeast Asia. It is one of the main TCMs to restore the vitality of nerves and brain cells. Asiaticoside (ASI) is its iconic metabolite, which has anti-inflammatory, antioxidant, and cardioprotective effects (Bandopadhyay et al., 2023). Wei et al. (2022) observed the intervention effect of ASI on myocardial injury in DCM mice. According to the results of Masson staining, it was found that the myocardial fibers of DCM mice were significantly broken and CVF increased, while ASI intervention significantly improved these phenomena. ASI could increase the levels of LVEF, FS, LVEDV, and LVESV and decrease the levels of LVAWd, LDH, and CTGF in DCM mice, which indicated that ASI could reduce cardiac injury and improve cardiac function in DCM mice. In addition, ASI increased the ratio of Beclin1, Atg5, and LC3II/I and downregulated the expression levels of Notch1 and Hes1 in myocardial tissues of DCM mice. These results indicated that ASI can improve DCM by reducing cardiomyocyte autophagy, and its mechanism may be related to the Notch1/Hes1 signaling pathway. Similarly, Yang et al. published their study of ASI against DCM (Yang Z. M. et al., 2021). ASI could improve myocardial structural disorder, myocardial fibrosis, and cardiomyocyte apoptosis in DCM mice by regulating the Notch1/Hes1 signaling pathway, thus protecting DCM. These two studies demonstrated the potential of ASI in the treatment of DCM. DJC is a TCM prescription with the effects of nourishing qi and yin and promoting blood circulation. It has a beneficial effect in the treatment of DCM, cardiovascular diseases, and diabetic nephropathy (Shi et al., 2021; Wang et al., 2023). Shi et al. (2021) found that DJC increased EF and FS levels in DCM rats. DCM rats showed cardiomyocyte dissolution, inflammatory cell infiltration, and an increased apoptosis rate, which could be reversed by DJC intervention. DJC reduced the expression levels of inflammatory factors TNF-α, IL-1β, and IL-6 in the serum of DCM rats and downregulated the expression levels of TLR4, MyD88, and NF-κB p65 in myocardial tissues of DCM rats. In addition, in the in vitro study of HG-induced H9c2, DJC could also downregulate the expression levels of TLR4, MyD88, and NF-κB p65, which is consistent with the in vivo results. DJC decreased the expression of Bax and caspase-3 and increased the expression of BCL2, reflecting the anti-apoptotic potential of DJC. These results suggested that DJC could treat DCM by inhibiting cardiomyocyte apoptosis in vitro and in vivo, and its mechanism may be related to the inhibition of the TLR4/MyD88/NF-κB signaling pathway. Furthermore, galangin (Lyu et al., 2022) regulated the IRAK-1/MAPK/NF-κB signaling pathway, gardenoside (Zhang et al., 2021) regulated the VPO1/ERK1/2 signaling pathway, and salvianolic acid B (Zhang and Fu, 2022) regulated the RhoA/ROCK1 signaling pathway to improve DCM.
3 Discussion
TCM is a great crystallization of the wisdom generated by ancient Chinese people through clinical practice over a long history and has made an indelible contribution to the prevention and treatment of human diseases. Although TCM has been treating diseases for thousands of years, its focus has always been on the therapeutic effect of diseases, and the mechanism through which TCM exerts its curative effect has not been paid much attention, which is mainly related to the backward cognition and detection methods of people at that time. Modern medicine and TCM have completely different theoretical systems. With the rapid development of modern medicine and the growing popularity of TCM in the world, researchers have begun to explore the mechanisms of TCM and have completed a large number of mechanism studies of TCM against DCM in the past decade.
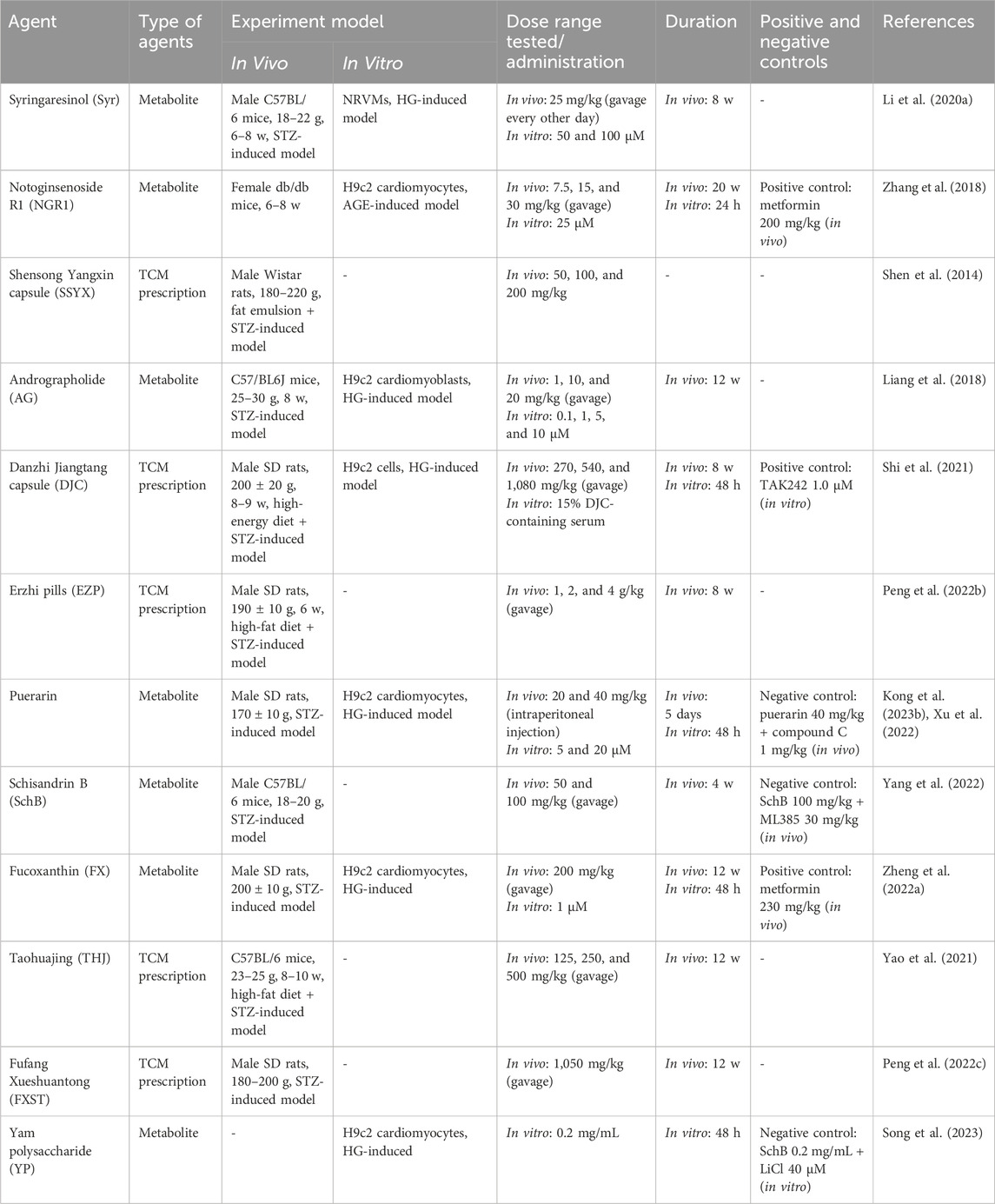
This review describes the mechanism and potential of TCM against DCM from the perspective of signaling pathways (Table 1). It is noteworthy that TGF-β/Smad, NF-κB, PI3K/AKT, Nrf2, AMPK, NLRP3, and Wnt/β-catenin signaling pathways are prominent in the above studies of TCM against DCM. TCM alleviates DCM mainly by improving pathological processes such as myocardial fibrosis, inflammation, oxidative stress, apoptosis, and cardiac hypertrophy. The above conclusions are also consistent with the current state of research. For example, the TGF-β/Smad signaling pathway is a key signaling pathway associated with cardiovascular disease, especially an important factor in the development of myocardial fibrosis. In this study, Syr, NGR1, SSYX, Soy isoflavone, Zicuitongmai Yin, ginkgolide B, and echinacoside could reduce myocardial fibrosis and improve DCM by regulating the TGF-β/Smad signaling pathway, which proves the great prospect of the TGF-β/Smad signaling pathway in TCM against myocardial fibrosis of DCM. It is well-known that inflammation is one of the main causes of DCM in the initial stage. NF-κB and NLRP3 signaling pathways are two of the most classical inflammatory signaling pathways. In this study, AG, mangiferin, and hederagenin inhibited the NF-κB signaling pathway, and THJ, TCT, and formononetin inhibited the NLRP3 signaling pathway to reduce the inflammatory response of DCM. In addition, oxidative stress is another risk factor for the development of DCM and often occurs in tandem with inflammatory reactions, thus accelerating the development of DCM under the influence of the crosstalk between the two. The Nrf2 signaling pathway is one of the classical signaling pathways for antioxidative stress, and it is also prominent in TCM against oxidative stress of DCM. Syr, NGR1, AG, and SchB have been shown to improve the oxidative stress of DCM by regulating the Nrf2 signaling pathway. Compared to the aforementioned predictive processes, there are no signaling pathways with high relevance in improving TCM regulation of DCM by intervening apoptosis and cardiac hypertrophy, which may be related to the difference in the number of references in the current study of different pathological processes of DCM. Due to the popularity of autophagy research in recent years, there has been a gradual emergence of research on TCM regulation of autophagy to improve DCM, but little research has been done on the signaling pathways involved in autophagy. How TCM interferes with autophagy to improve DCM is a promising research direction and may also be one of the focuses of future research work. In addition, many TCMs improve DCM not only by intervening with one signaling pathway but multiple; for example, AG could reduce the inflammatory response of DCM by inhibiting the activation of the NF-κB signaling pathway and could also improve the oxidative stress of DCM by regulating the Nrf2 signaling pathway; Syr could improve myocardial fibrosis, inflammation, and oxidative stress of DCM by regulating TGF-β/Smad2/3 and Keap1/Nrf2 signaling pathways; NGR1 could improve oxidative stress, apoptosis, and myocardial fibrosis of DCM by regulating TGF-β/Smad2/3 and Akt/Nrf2 signaling pathways; and EZP could improve oxidative stress and apoptosis of DCM by regulating PI3K/Akt/FOXO3a and AMPK signaling pathways. There are many such examples in this study, which reflect the multi-target and multi-pathway characteristics of TCM. Considering the multiple-organ damage caused by hyperglycemia and the complexity and long-term nature of DCM pathogenesis, TCM with multi-target and multi-pathway characteristics and few side effects has broad prospects as a drug candidate for the treatment of DCM. Although TCM has shown great potential in the treatment of DCM, there are still some shortcomings. First, the same TCM in different regions may have different components due to differences in climate, which may lead to differences in the efficacy and toxicity of TCM in application. Second, the complexity of TCM components determines its multi-target and multi-pathway characteristics, which is an advantage of TCM but also a disadvantage, making it more difficult to study the specific mechanism of TCM against DCM. Third, there is a lack of large clinical trials of TCM in the treatment of DCM. Fourth, some TCM prescriptions are hospital preparations whose production process standards are not clear and uniform. Finally, it is worth noting that the scientific quality of the references needed to be assessed, and based on the available information, we defined (A) experiments containing animal and cellular experiments with positive or negative control groups; (B) experiments containing animal and cellular experiments without positive or negative control groups; (C) experiments containing only animal experiments; and (D) experiments containing only cellular experiments. Obviously, the evidence of type A not only obtains relevant results from in vivo experiments but can also be further verified by in vitro experiments and positive or negative control groups to confirm the reliability of the results, which is a higher level of experimental evidence. The level of the evidence of type B is only second to that of type A, which also belongs to a higher level of experimental evidence. The evidence of type C and type D belongs to a slightly lower level of evidence relative to A and B, especially type D. Due to the singularity of the cell itself and its growth environment, which is far less complex than that of animals, often evidence that appears in cell experiments is not necessarily validated in animal experiments, and thus, type D belongs to a lower level of evidence among these. In short, all the evidence needs to be viewed dialectically. In addition, dose is a very important event in pharmacological studies, and for in vivo studies, as a general rule, the dose tested should not exceed 1 g/kg and day. However, a small number of TCM prescriptions included in this review have doses exceeding 1 g/kg and day, and the maximum dose even reaches 4 g/kg and day, which is worth considering. If an excessive dose is required to achieve the relevant pharmacological activity, can it be reasonably assumed that it has no activity at lower doses and, thus, that they are not pharmacologically relevant? Even in excessive doses, the toxicity of TCM should be evaluated. Therefore, this review suggests that future pharmacological studies should be evaluated at more relevant dose levels to make the conclusions more convincing. The relevant basic pharmacological data are shown in Table 2.
In summary, in the future, more in-depth studies should be conducted to explore the pathogenesis of DCM and screen potential targets for drug candidates against DCM, thus providing new ideas and more experimental evidence for the clinical use of TCM in the treatment of DCM.
Author contributions
WL: conceptualization, methodology, writing–original draft, and writing–review and editing. XL: writing–original draft, writing–review and editing, and conceptualization. ZL: investigation, methodology, and writing–review and editing. QX: investigation, methodology, and writing–review and editing. RL: investigation, methodology, and writing–review and editing. QW: investigation, methodology, and writing–review and editing. YH: investigation, methodology, and writing–review and editing. JZ: conceptualization, methodology, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Natural Science Foundation of Hunan Province (2023JJ60058, 2024JJ8199, and 2024JJ9560), the Scientific Research Project of Hunan Administration of Traditional Chinese Medicine (B2023056), and the Xiangtan Medical Research Project (2023-xtyx-48).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bandopadhyay, S., Mandal, S., Ghorai, M., Jha, N. K., Kumar, M., Radha, , et al. (2023). Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: a review. J. Cell. Mol. Med. 27 (5), 593–608. doi:10.1111/jcmm.17635
Cai, L., Tan, Y., Watson, S., and Wintergerst, K. (2023). Diabetic cardiomyopathy-Zinc preventive and therapeutic potentials by its anti-oxidative stress and sensitizing insulin signaling pathways. Toxicol. Appl. Pharmacol. 477, 116694. doi:10.1016/j.taap.2023.116694
Cao, X. F., Zhou, M. X., Liu, H. X., Chen, X. F., Li, X., and Jia, S. H. (2021). Clinical efficacy and safety of shensong yangxin capsule-amiodarone combination on heart failure complicated by ventricular arrhythmia: a meta-analysis of randomized controlled trials. Front. Pharmacol. 12, 613922. doi:10.3389/fphar.2021.613922
Chen, J., Zhang, Z. G., and Cai, L. (2014). Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes. Metab. J. 38 (5), 337–345. doi:10.4093/dmj.2014.38.5.337
Cheng, W. J., Cui, C., Liu, G., Ye, C. J., Shao, F., Bagchi, A. K., et al. (2023). NF-κB, A potential therapeutic target in cardiovascular diseases. Cardiovasc. Drugs. Ther. 37 (3), 571–584. doi:10.1007/s10557-022-07362-8
Ding, P., Ye, Z. M., Liu, B., and Zhang, L. Y. (2024). Effect of protocatechualdehyde on streptozotocin induced diabetic cardiomyopathy in mice. Mod. Food Sci. Technol. 40 (3), 1–7. doi:10.13982/j.mfst.1673-9078.2024.3.0400
Duan, J. L., Wei, G., Guo, C., Cui, J., Yan, J. J., Yin, Y., et al. (2015). Aralia taibaiensis protects cardiac myocytes against high glucose-induced oxidative stress and apoptosis. Am. J. Chin. Med. 43 (6), 1159–1175. doi:10.1142/S0192415X15500664
Entezari, M., Hashemi, D., Taheriazam, A., Zabolian, A., Mohammadi, S., Fakhri, F., et al. (2022). AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed. Pharmacother. 146, 112563. doi:10.1016/j.biopha.2021.112563
Frangogiannis, N. G. (2022). Transforming growth factor-β in myocardial disease. Nat. Rev. Cardiol. 19 (7), 435–455. doi:10.1038/s41569-021-00646-w
Gao, Z. H., and Hou, S. P. (2022). Inhibitory effect of ginkgolide B on myocardial fibrosis in diabetes rats. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 20 (8), 1399–1403. doi:10.12102/j.issn.1672-1349.2022.08.010
Ghafouri-Fard, S., Askari, A., Shoorei, H., Seify, M., Koohestanidehaghi, Y., Hussen, B. M., et al. (2023). Antioxidant therapy against TGF-β/SMAD pathway involved in organ fibrosis. J. Cell. Mol. Med. 00, 180522–e18121. doi:10.1111/jcmm.18052
Ghafouri-Fard, S., Khanbabapour, S. A., Hussen, B. M., Shoorei, H., Siddiq, A., Taheri, M., et al. (2022). Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 49 (10), 9767–9781. doi:10.1007/s11033-022-07468-0
Ghareghomi, S., Moosavi-Movahedi, F., Saso, L., Habibi-Rezaei, M., Khatibi, A., Hong, J., et al. (2023). Modulation of Nrf2/HO-1 by natural compounds in lung cancer. Antioxidants (Basel) 12 (3), 735. doi:10.3390/antiox12030735
Gou, T. T., Hu, M. H., Xu, M., Chen, Y. C., Chen, R., Zhou, T., et al. (2023). Novel wine in an old bottle: preventive and therapeutic potentials of andrographolide in atherosclerotic cardiovascular diseases. J. Pharm. Anal. 13 (6), 563–589. doi:10.1016/j.jpha.2023.05.010
Hong, S. W., Wang, Y. F., Chen, Y. J., Zhang, K. Y., Chen, P. Y., Hang, H. X., et al. (2024). Integrative pharmacology reveals the mechanisms of Erzhi pills, A traditional Chinese formulation, stimulating melanogenesis. J. Ethnopharmacol. 324, 117617. doi:10.1016/j.jep.2023.117617
Hou, J., Zheng, D. Z., Fung, G., Deng, H. Y., Chen, L., Liang, J. L., et al. (2016). Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can. J. Physiol. Pharmacol. 94 (3), 332–340. doi:10.1139/cjpp-2015-0073
Huang, H. K., Luo, J. W., and Ran, H. (2023a). Study on the effects of soy isoflavone on cardiac function and fibrosis in rats with diabetic cardiomyopathy and its mechanisms. Chin. J. Diabetes 31 (5), 376–381. doi:10.3969/j.issn.1006-6187.2023.05.012
Huang, T., Tang, J. D., Li, Z. Z., Bai, Y., Liu, H. S., and Xu, Z. G. (2022). Mechanism of luteolin resisting myocardial fibrosis in type 2 diabetic rats. Jilin J. Chin. Med. 42 (9), 1063–1068. doi:10.13463/j.cnki.jlzyy.2022.09.019
Huang, Y. L., Xiang, Q., Zou, J. J., Wu, Y., and Yu, R. (2023b). Zuogui Jiangtang Shuxin formula Ameliorates diabetic cardiomyopathy mice via modulating gut-heart axis. Front. Endocrinol.(Lausanne) 14, 1106812. doi:10.3389/fendo.2023.1106812
Huo, Y., Mijiti, A., Cai, R. N., Gao, Z. H., Aini, M., Mijiti, A., et al. (2021). Scutellarin alleviates type 2 diabetes (HFD/low dose STZ)-induced cardiac injury through modulation of oxidative stress, inflammation, apoptosis and fibrosis in mice. Hum. Exp. Toxicol. 240 (12_Suppl. l), S460–S474. doi:10.1177/09603271211045948
Jiang, X. Y., Zhou, Y. Y., Wang, Q., and Li, Q. (2024). Intervention of traditional Chinese medicine in NLRP3 signaling pathway for prevention and treatment of alzheimer's disease: a review. Chin. J. Exp. Tradit. Med. Formulae. 30 (8), 290–298. doi:10.13422/j.cnki.syfjx.20240302
Jin, Q., Liu, T. T., Chen, D. Q., Yang, L. P., Mao, H. M., Ma, F., et al. (2023). Therapeutic potential of artemisinin and its derivatives in managing kidney diseases. Front. Pharmacol. 14, 1097206. doi:10.3389/fphar.2023.1097206
Jin, Q. Y., Zhu, Q., Li, X. L., Ye, L. F., and Qu, B. M. (2020). Preventive effect of asiaticoside on rat diabetic cardiomyopathy and its mechanisms. J. Electrocardiol. Circ. 39 (4), 326–332+417. doi:10.12124/j.issn.2095-3933.2020.4.2020-3996
Kong, L. J., Ji, X. Q., Liu, Y., and Du, Y. J. (2023a). Effect of artemisinin combined with allicin on improving cardiac function, fibrosis and NF-κB signaling pathway in rats with diabetic cardiomyopathy. Acta. Biochim. Pol. 70 (2), 401–405. doi:10.18388/abp.2020_6692
Kong, L. J., Ji, X. Q., Liu, Y., Liu, H., and Gao, Z. X. (2023b). Puerarin alleviates myocardial injury in diabetic rats by regulating AMPK/GSK-3β/β-catenin signaling pathway. J. Chin. Med. Mat. 46 (2), 474–478. doi:10.13863/j.issn1001-4454.2023.02.035
Li, G. R., Liu, C., Yang, L., Feng, L. F., Zhang, S. Z., An, J. L., et al. (2023a). Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol. Toxicol. 39 (3), 621–639. doi:10.1007/s10565-023-09790-0
Li, G. R., Yang, L., Feng, L. F., Yang, J., Li, Y. F., An, J. L., et al. (2020a). Syringaresinol protects against type 1 diabetic cardiomyopathy by alleviating inflammation responses, cardiac fibrosis, and oxidative stress. Mol. Nutr. Food. Res. 64 (18), e2000231. doi:10.1002/mnfr.202000231
Li, J. F., and Lai, L. H. (2022). Effects of naringenin on apoptosis and Nrf2/ARE signaling pathway of H9c2 cardiomyocytes induced by high glucose. Chin. J. Arterioscler. 30 (12), 1040–1044. doi:10.20039/j.cnki.1007-3949.2022.12.005
Li, L., Chen, X. Y., Su, C. P., Wang, Q., Li, R., Jiao, W. C., et al. (2020b). Si-Miao-Yong-An decoction preserves cardiac function and regulates GLC/AMPK/NF-κB and GLC/PPARα/PGC-1α pathways in diabetic mice. Biomed. Pharmacother. 132, 110817. doi:10.1016/j.biopha.2020.110817
Li, L. J., Liu, J. W., Zhou, K. X., Dai, L. B., and Guan, W. C. (2023b). Study on the Effect of Shenmai formula on improving myocardial injury in diabetes through PI3K/AKT pathway. Lishizhen Med. Mat. Med. Res. 34 (4), 790–792. doi:10.3969/j.issn.1008-0805.2023.04.06
Li, M. C., Murabito, A., Ghigo, A., and Hirsch, E. (2017). PI3Ks in diabetic cardiomyopathy. J. Cardiovasc. Pharmacol. 70 (6), 422–429. doi:10.1097/FJC.0000000000000511
Li, W. C., Li, W., Xing, Q. C., Liu, Z., Hu, Y. X., Liu, X., et al. (2022d). Progress in traditional Chinese medicine on treatment of diabetic retinopathy. Nat. Prod. Commun. 17 (8), 1934578X2211185–10. doi:10.1177/1934578X221118547
Li, W. C., Xing, Q. C., Liu, Z., Liu, R. Z., Hu, Y. X., Yan, Q. Z., et al. (2023c). The signaling pathways of traditional Chinese medicine in treating diabetic retinopathy. Front. Pharmacol. 14, 1165649. doi:10.3389/fphar.2023.1165649
Li, X. H., Yin, F. T., Zhou, X. H., Zhang, A. H., Sun, H., Yan, G. L., et al. (2022a). The signaling pathways and targets of natural compounds from traditional Chinese medicine in treating ischemic stroke. Molecules 27 (10), 3099. doi:10.3390/molecules27103099
Li, X. H., Yuan, W. C., Wu, J. B., Zhen, J. H., Sun, Q. H., and Yu, M. M. (2022b). Andrographolide, a natural anti-inflammatory agent: an Update. Front. Pharmacol. 13, 920435. doi:10.3389/fphar.2022.920435
Li, X. Y., Lu, X. C., Fan, D. P., Li, L., Lu, C., Tan, Y., et al. (2020c). Synergistic effects of Erzhi pill combined with methotrexate on osteoblasts mediated via the wnt1/LRP5/β-catenin signaling pathway in collagen-induced arthritis rats. Front. Pharmacol. 11, 228. doi:10.3389/fphar.2020.00228
Li, Y., Dong, J. L., Shang, Y. H., Zhao, Q. Q., Li, P. C., and Wu, B. (2019). Anti-inflammatory effects of hederagenin on diabetic cardiomyopathy via inhibiting NF-κB and Smads signaling pathways in a type-2 diabetic mice model. RSC Adv. 9 (45), 26238–26247. doi:10.1039/c9ra02043h
Liang, E. S., Liu, X., Du, Z. H., Yang, R. X., and Zhao, Y. X. (2018). Andrographolide ameliorates diabetic cardiomyopathy in mice by blockage of oxidative damage and NF-κB-Mediated inflammation. Oxid. Med. Cell. Longev. 2018, 9086747. doi:10.1155/2018/9086747
Liao, M., Yang, L., Wang, Z., and Hao, Y. R. (2022). Protective effect of echinacoside on myocardium in db/db diabetic mice. Curr. Biotechnol. 12 (1), 129–134. doi:10.19586/j.2095-2341.2021.0061
Liu, C. X., Guo, X. Y., Zhou, Y. B., and Wang, H. (2023). AMPK signalling pathway: a potential strategy for the treatment of heart failure with Chinese medicine. J. Inflamm. Res. 16, 5451–5464. doi:10.2147/JIR.S441597
Liu, H., Yang, J. Q., Yang, W. Q., Hu, S. N., Wu, Y. L., Zhao, B., et al. (2020). Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug. Des. devel. Ther. 14, 551–565. doi:10.2147/DDDT.S240511
Liu, J. Q., Xiao, Q., Xiao, J. N., Niu, C. X., Li, Y. Y., Zhang, X. J., et al. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target. Ther. 7 (1), 3. doi:10.1038/s41392-021-00762-6
Liu, Y., Zhao, Y. B., Wang, S. W., Zhou, Y., Tang, Z. S., and Li, F. (2017). Mulberry granules protect against diabetic cardiomyopathy through the AMPK/Nrf2 pathway. Int. J. Mol. Med. 40 (3), 913–921. doi:10.3892/ijmm.2017.3050
Lorenzo, O., Picatoste, B., Ares-Carrasco, S., Ramírez, E., Egido, J., and Tuñón, J. (2011). Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediat. Inflamm. 2011, 652097. doi:10.1155/2011/652097
Luo, B. B., Li, B., Wang, W. K., Liu, X. J., Xia, Y. F., Zhang, C., et al. (2014). NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 9 (8), e104771. doi:10.1371/journal.pone.0104771
Luo, W., Lin, K., Hua, J., Han, J. Y., Zhang, Q. Y., Chen, L. F., et al. (2022). Schisandrin B attenuates diabetic cardiomyopathy by targeting MyD88 and inhibiting MyD88-dependent inflammation. Adv. Sci. (Weinh). 9 (31), e2202590. doi:10.1002/advs.202202590
Lyu, C. Y., Yang, Y. J., Ao, W., Li, Z. Z., Huang, T., Xu, Z. G., et al. (2022). Impact of galangin on myocardial injury in diabetic rat by inhibiting IRAK-1/MAPK/NF-κB signaling pathway. Tianjin J. Tradit. Chin. Med. 39 (11), 1476–1482. doi:10.11656/j.issn.1672-1519.2022.11.20
Ma, L. Y., Li, L. B., Ma, F. J., Ma, F. Y., and Ma, S. J. (2023a). Efficacy, safety, and economy of shensongyangxin capsules for the treatment of coronary heart disease arrhythmia: a meta-analysis of randomized controlled trials. Ann. Med. Surg. (Lond). 85 (10), 4989–5000. doi:10.1097/MS9.0000000000001244
Ma, Q. M., Yu, J. L., Zhang, X., Wu, X. L., and Deng, G. C. (2023b). Wnt/β-catenin signaling pathway-a versatile player in apoptosis and autophagy. Biochimie 211, 57–67. doi:10.1016/j.biochi.2023.03.001
Nakamura, K., Miyoshi, T., Yoshida, M., Akagi, S., Saito, Y., Ejiri, K., et al. (2022). Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int. J. Mol. Sci. 23 (7), 3587. doi:10.3390/ijms23073587
Nellaiappan, K., Yerra, V. G., and Kumar, A. (2019). Role of AMPK in diabetic cardiovascular complications: an overview. Cardiovasc. Hematol. Disord. Drug. Targets 19 (1), 5–13. doi:10.2174/1871529X18666180508104929
Ni, B. B., Sun, M. J., Zhao, J., Wang, J., and Cao, Z. Q. (2023). The role of β-catenin in cardiac diseases. Front. Pharmacol. 14, 1157043. doi:10.3389/fphar.2023.1157043
Oh, A., Pardo, M., Rodriguez, A., Yu, C. N., Nguyen, L., Liang, O., et al. (2023). NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype. Cell. Commun. Signal. 21 (1), 291. doi:10.1186/s12964-023-01207-z
Peng, M. L., Fu, Y., Wu, C. W., Zhang, Y., Ren, H., and Zhou, S. S. (2022a). Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front. Endocrinol. (Lausanne). 13, 907757. doi:10.3389/fendo.2022.907757
Peng, M. M., Xia, T. Y., Zhong, Y. M., Zhao, M. T., Yue, Y. M., Liang, L. Y., et al. (2022b). Integrative pharmacology reveals the mechanisms of Erzhi Pill, a traditional Chinese formulation, against diabetic cardiomyopathy. J. Ethnopharmacol. 296, 115474. doi:10.1016/j.jep.2022.115474
Peng, M. Z., Liu, H. Y., Ji, Q. X., Ma, P., Niu, Y. T., Ning, S. Q., et al. (2022c). Fufang xueshuantong improves diabetic cardiomyopathy by regulating the wnt/β-catenin pathway. Int. J. Endocrinol. 2022, 3919161. doi:10.1155/2022/3919161
Qin, J. N., Tan, Y., Han, Y., Yu, L. T., Liu, S. L., Zhao, S. M., et al. (2023). Interplay between TGF-β signaling and MicroRNA in diabetic cardiomyopathy. Cardiovasc. Drugs. Ther. doi:10.1007/s10557-023-07532-2
Qin, W. M., Cao, L. H., and Massey, I. Y. (2021). Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell. Biochem. 476 (11), 4045–4059. doi:10.1007/s11010-021-04219-w
Seksaria, S., Mehan, S., Dutta, B. J., Gupta, G. D., Ganti, S. S., and Singh, A. (2023). Oxymatrine and insulin resistance: focusing on mechanistic intricacies involve in diabetes associated cardiomyopathy via SIRT1/AMPK and TGF-β signaling pathway. J. Biochem. Mol. Toxicol. 37 (5), e23330. doi:10.1002/jbt.23330
Shen, N. N., Li, X. G., Zhou, T., Bilal, M. U., Du, N., Hu, Y. Y., et al. (2014). Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. J. Ethnopharmacol. 157, 161–170. doi:10.1016/j.jep.2014.09.035
Shi, H., Zhou, P., Ni, Y. Q., Wang, S. S., Song, R., Shen, A. L., et al. (2021). In vivo and in vitro studies of Danzhi Jiangtang capsules against diabetic cardiomyopathy via TLR4/MyD88/NF-κB signaling pathway. Saudi. Pharm. J. 29 (12), 1432–1440. doi:10.1016/j.jsps.2021.11.004
Song, J. H., Zhang, L., and Chen, H. B. (2023). Based on Wnt/β-Catenin explores the protective effect of yam polysaccharides on high glucose induced myocardial cell damage. Chin. Tradit. Pat. Med. 45 (2), 626–629. doi:10.3969/j.issn.1001-1528.2023.02.051
Sun, Y., and Ding, S. (2021). NLRP3 inflammasome in diabetic cardiomyopathy and exercise intervention. Int. J. Mol. Sci. 22 (24), 13228. doi:10.3390/ijms222413228
Tan, Y., Zhang, Z. G., Zheng, C., Wintergerst, K. A., Keller, B. B., and Cai, L. (2020). Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 17 (9), 585–607. doi:10.1038/s41569-020-0339-2
Wang, J. J., Zou, J. Q., Zhao, C., Yu, H., Teng, J. J., and Dong, L. (2023d). Syringaresinol inhibits cardiorenal fibrosis through HSP90 in a cardiorenal syndrome type 2. Hum. Exp. Toxicol. 42, 9603271231165678. doi:10.1177/09603271231165678
Wang, L., Wang, L. P., Wang, H., and Zhu, T. (2022a). Investigation into the potential mechanism and molecular targets of Fufang Xueshuantong capsule for the treatment of ischemic stroke based on network pharmacology and molecular docking. Front. Pharmacol. 13, 949644. doi:10.3389/fphar.2022.949644
Wang, M. R., Li, Y. S., Li, S., and Lv, J. G. (2022b). Endothelial dysfunction and diabetic cardiomyopathy. Front. Endocrinol. (Lausanne). 13, 851941. doi:10.3389/fendo.2022.851941
Wang, R. Q., Liu, M. N., Ren, G. L., Luo, G., Wang, Z. C., Ge, Z. X., et al. (2022c). Zhilong Huoxue Tongyu Capsules' Effects on ischemic stroke: an assessment using fecal 16S rRNA gene sequencing and untargeted serum metabolomics. Front. Pharmacol. 13, 1052110. doi:10.3389/fphar.2022.1052110
Wang, S. H., Fang, Z. H., Zhao, J. D., Ni, Y. Q., Wang, T. T., Chu, J., et al. (2023a). Danzhi Jiangtang capsule alleviates myocardial fibrosis in diabetic cardiomyopathy rats by up-regulating Nrf2/HO-1 signaling pathway. Chin. J. Gerontol. 43 (10), 2457–2463. doi:10.3969/j.issn.1005-9202.2023.10.044
Wang, S. X., Duan, J., Liao, J. Q., Wang, Y., Xiao, X., Li, L., et al. (2023b). Shengjie Tongyu decoction regulates cardiomyocyte autophagy through modulating ROS-PI3K/Akt/mTOR Axis by LncRNA H19 in diabetic cardiomyopathy. Altern. Ther. Health. Med. 29 (6), 280–287.
Wang, X. F., Wang, D. R., Deng, B. Y., and Yan, L. T. (2023c). Syringaresinol attenuates osteoarthritis via regulating the NF-κB pathway. Int. Immunopharmacol. 118, 109982. doi:10.1016/j.intimp.2023.109982
Wei, D. M., Xu, C. N., Liu, Y., Yang, J., and Yang, Y. (2022). Asiaticoside attenuates myocardial injury in diabetic cardiomyopathy by enhancing autophagy through Notch1/Hes1 signaling. J. Shanxi Med. Univ. 53 (3), 305–311. doi:10.13753/j.issn.1007-6611.2022.03.008
Wu, G. Q., Xiong, C. H., Mao, Y., Wen, X. F., Tan, J., Li, F., et al. (2021). Effect of Zicuitongmai yin on myocardial fibrosis and TGF-β1/smads signaling pathway in diabetic cardiomyopathy rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 32 (1), 29–35. doi:10.19378/j.issn.1003-9783.2021.01.004
Wu, G. Q., Yuan, C. Y., Yuan, S. S., Mao, Y., Chen, Q., Wen, X. F., et al. (2023a). Experimental study on reducing high glucose-induced injury in H9c2 cardiomyocytes by modulating PI3K/Akt/mTOR signaling pathway with Zicui Tongmai decoction. Hebei Med. 29 (1), 31–36. doi:10.3969/j.issn.1006-6233.2023.01.006
Wu, T., Qu, Y. W., Xu, S. J., Wang, Y., Liu, X., and Ma, D. F. (2023b). SIRT6: a potential therapeutic target for diabetic cardiomyopathy. Faseb. J. 37 (8), e23099. doi:10.1096/fj.202301012R
Xie, Y. J., and Wang, C. H. (2023). Herb-drug interactions between Panax notoginseng or its biologically active compounds and therapeutic drugs: a comprehensive pharmacodynamic and pharmacokinetic review. J. Ethnopharmacol. 307, 116156. doi:10.1016/j.jep.2023.116156
Xu, H., Li, L., Pan, G. X., and Li, Y. H. (2022). Study on the effect of puerarin on alleviating high glucose-induced hypertrophy of H9c2 cells via activating AMPK/Akt/GSK-3β signaling pathway. Tianjin J. Tradit. Chin. Med. 39 (10), 1329–1334. doi:10.11656/j.issn.1672-1519.2022.10.18
Yan, M. L., Liu, S. P., Zeng, W. R., Guo, Q. L., Mei, Y., Shao, X. Q., et al. (2023). The Chinese herbal medicine Fufang Zhenzhu Tiaozhi ameliorates diabetic cardiomyopathy by regulating cardiac abnormal lipid metabolism and mitochondrial dynamics in diabetic mice. Biomed. Pharmacother. 164, 114919. doi:10.1016/j.biopha.2023.114919
Yang, P., Liu, T. H., Qin, L. L., Wu, L. L., Zhang, C. F., Peng, C., et al. (2023). Improvement of diabetic cardiomyopathy by Chinese medicine based on TGF-β/smad pathway: a review. Chin. J. Exp. Tradit. Med. Formulae. 29 (1), 215–226. doi:10.13422/j.cnki.syfjx.20221603
Yang, S. Q., He, T. L., Su, J. H., and Wang, W. (2022). Study on the mechanism of schizandrin B regulating Nrf2/HO-1/GPX4 iron death pathway to reduce myocardial injury in diabetes mellitus mice. J. Chin. Med. Mat. 45 (7), 1705–1713. doi:10.13863/j.issn1001-4454.2022.07.033
Yang, W. J., and Cao, J. J. (2022). Effects of mulberry leaves flavonoids inhibiting NLRP3 inflammasome via autophagy on myocardium of rats with diabetes cardiomyopathy. Chin. J. Gerontol. 42 (14), 3570–3573. doi:10.3969/j.issn.1005-9202.2022.14.054
Yang, Y., Wei, D. M., Qiao, R., Liu, Y., and Yang, J. (2021a). Intervention effect of asiaticoside on diabetic cardiomyopathy mice and the mechanism. Shandong Med. J. 61 (32), 10–14. doi:10.3969/j.issn.1002-266X.2021.32.003
Yang, Z. M., Zhang, Q. H., Yu, L. H., Zhu, J. Y., Cao, Y., and Gao, X. F. (2021b). The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 264, 113249. doi:10.1016/j.jep.2020.113249
Yao, R., Cao, Y., Wang, C. M., Xu, L., Zhang, X., Deng, Y. Q., et al. (2021). Taohuajing reduces oxidative stress and inflammation in diabetic cardiomyopathy through the sirtuin 1/nucleotide-binding oligomerization domain-like receptor protein 3 pathway. BMC Complement. Med. Ther. 21 (1), 78. doi:10.1186/s12906-021-03218-0
Ye, T., Ma, G. Q., Wei, M. H., and Chen, J. (2022). Regulation mechanism of Astragalus polysaccharides on diabetic cardiomyopathy rats by AMPK-mTOR pathway. World Chin. Med. 17 (7), 977–982. doi:10.3969/j.issn.1673-7202.2022.07.014
Zeng, Q. H., Liu, M. N., Li, X. L., Bai, X., Yang, S. J., and Luo, G. (2023). Effects of zhilong huoxue Tongyu capsules on diabetic cardiomyopathy rats based on PI3K/AKT1/FoxO3a signaling pathway. J. Chin. Med. Mat. 46 (1), 197–201. doi:10.13863/j.issn1001-4454.2023.01.034
Zhang, B., Zhang, J. Y., Zhang, C. Y., Zhang, X. L., Ye, J. X., Kuang, S. H., et al. (2018). Notoginsenoside R1 protects against diabetic cardiomyopathy through activating estrogen receptor α and its downstream signaling. Front. Pharmacol. 9, 1227. doi:10.3389/fphar.2018.01227
Zhang, H., Zhang, R. X., Liu, J. C., and Ke, H. X. (2023). Formononetin alleviates hyperglycemia-induced cardiomyocyte inflammatory response and pyroptosis by regulating the P2X7R/NLRP3 pathway. Immunol. J. 39 (7), 594–600. doi:10.13431/j.cnki.immunol.j.20230077
Zhang, J., and Feng, Q. S. (2022). Pharmacological effects and molecular protective mechanisms of Astragalus polysaccharides on nonalcoholic fatty liver disease. Front. Pharmacol. 13, 854674. doi:10.3389/fphar.2022.854674
Zhang, Q., Liu, J., Duan, H. X., Li, R. L., Peng, W., and Wu, C. J. (2021a). Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 34, 43–63. doi:10.1016/j.jare.2021.06.023
Zhang, W. P., Jiang, J. J., Chen, X. F., and Xu, S. S. (2021b). Gardenoside alleviates diabetic cardiomyopathy via inhibiting VPO1/ERK1/2 signaling pathway to improve myocardial apoptosis in rats. Chin. J. Clin. Pharmacol. Ther. 26 (2), 129–136. doi:10.12092/j.issn.1009-2501.2021.02.002
Zhang, X., and Fu, L. J. (2022). The study of salvianolic acid B on the improvement of myocardial fibrosis in diabetic rats by RhoA/ROCK1 pathway. Chin. Pharmacol. Bull. 38 (10), 1487–1491. doi:10.12360/CPB202111091
Zhang, Y. N., Ding, Y. J., Xu, H. Z., Li, Z. L., and Si, Q. J. (2022). Effect of modified taohe chengqitang on NLRP3 inflammasomes in rats with diabetic cardiomyopathy. Chin. J. Exp. Tradit. Med. Formulae. 28 (16), 59–65. doi:10.13422/j.cnki.syfjx.20220780
Zhao, X. D., Liu, S. W., Wang, X., Chen, Y. B., Pang, P., Yang, Q. J., et al. (2022). Diabetic cardiomyopathy: clinical phenotype and practice. Front. Endocrinol. (Lausanne) 13, 1032268. doi:10.3389/fendo.2022.1032268
Zheng, D. X., Chen, L. L., Wei, Q. H., Zhu, Z. R., Liu, Z. L., Jin, L., et al. (2022a). Fucoxanthin regulates Nrf2/Keap1 signaling to alleviate myocardial hypertrophy in diabetic rats. J. South. Med. Univ. 42 (5), 752–759. doi:10.12122/j.issn.1673-4254.2022.05.18
Zheng, J., Cheng, J., Zheng, S., Feng, Q. Y., and Xiao, X. H. (2018). Curcumin, A polyphenolic curcuminoid with its protective effects and molecular mechanisms in diabetes and diabetic cardiomyopathy. Front. Pharmacol. 9, 472. doi:10.3389/fphar.2018.00472
Zheng, Y. D., Xu, L., Dong, N. G., and Li, F. (2022b). NLRP3 inflammasome: the rising star in cardiovascular diseases. Front. Cardiovasc. Med. 9, 927061. doi:10.3389/fcvm.2022.927061
Zhou, Y., Li, X. X., Luo, W. Y., Zhu, J. F., Zhao, J. F., Wang, M. Y., et al. (2022). Allicin in digestive system cancer: from biological effects to clinical treatment. Front. Pharmacol. 13, 903259. doi:10.3389/fphar.2022.903259
Zhou, Y. T., Suo, W. D., Zhang, X. A., Liang, J. J., Zhao, W. Z., Wang, Y., et al. (2023). Targeting mitochondrial quality control for diabetic cardiomyopathy: therapeutic potential of hypoglycemic drugs. Biomed. Pharmacother. 168, 115669. doi:10.1016/j.biopha.2023.115669
Keywords: diabetic cardiomyopathy, traditional Chinese medicine, signaling pathway, mechanism, review
Citation: Li W, Liu X, Liu Z, Xing Q, Liu R, Wu Q, Hu Y and Zhang J (2024) The signaling pathways of selected traditional Chinese medicine prescriptions and their metabolites in the treatment of diabetic cardiomyopathy: a review. Front. Pharmacol. 15:1416403. doi: 10.3389/fphar.2024.1416403
Received: 12 April 2024; Accepted: 11 June 2024;
Published: 03 July 2024.
Edited by:
Shen Xiang-chun, Guizhou Medical University, ChinaReviewed by:
Zhang Liang, Nanjing University of Chinese Medicine, ChinaLi Lisheng, Zunyi Medical University, China
Copyright © 2024 Li, Liu, Liu, Xing, Liu, Wu, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wencan Li, bGl3ZW5jYW4xNjhAMTYzLmNvbQ==; Jiani Zhang, amlhbml6aGFuZzMxOEAxNjMuY29t
†These authors have contributed equally to this work
 Wencan Li
Wencan Li Xiang Liu1†
Xiang Liu1† Zheng Liu
Zheng Liu Renzhu Liu
Renzhu Liu