Abstract
Background:
Currently, there remains substantial controversy in research regarding whether the concomitant use of colchicine and statins increases the occurrence of rhabdomyolysis, warranting further substantiation.
Objective:
This study aimed to identify the likelihood drug-drug interactions (DDIs) for the co-administration of colchicine and statins resulting in rhabdomyolysis.
Methods:
A disproportionality analysis was conducted by using data sourced from the US Food and Drug Administration Adverse Event Reporting System (FAERS) to detect rhabdomyolysis signals associated with the combined use of colchicine and statins. The association between (colchicine/statins/colchicine and statins) and rhabdomyolysis were evaluated using information component (IC). DDI signals were calculated based on the Ω shrinkage measure and Bayesian confidence propagation neural network (BCPNN) method. Furthermore, stratification was performed based on colchicine and individual statins agents.
Results:
In total, 11,119 reports of rhabdomyolysis were identified in the FAERS database, 255 (2.29%) involved both colchicine and statins. Our analysis showed potential DDI signals of rhabdomyolysis (Ω025 = 1.17) among individuals concurrent use of colchicine and statins. Moreover, further drug-specific analysis suggests DDI signals in the colchicine-atorvastatin pair (Ω025 = 1.12), and colchicine-rosuvastatin pair (Ω025 = 1.05), along with a higher proportion of rhabdomyolysis (IC025 = 5.20) and (IC025 = 4.26), respectively.
Conclusion:
The findings suggest that concomitant use of colchicine and statins may increase the risk of rhabdomyolysis, particularly when combined with atorvastatin or rosuvastatin. Therefore, healthcare professionals should pay special attention to life-threatening AE such as rhabdomyolysis, when co-prescribing colchicine statins.
1 Introduction
Statins, also known as 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, are used to reduce cholesterol and triglycerides by blocking the formation of cholesterol in the liver (Fiolet et al., 2021). Statins have been a cornerstone class of medications in cardiovascular therapeutics for decades, owing to their lipid-lowering and anti-inflammatory benefits as well as their role in the primary and secondary prevention of cardiovascular disease (Grundy et al., 2004).
Colchicine, a traditional anti-inflammatory medication, has been used to treat and prevent gout flares for millennia. In the last decade, large placebo-controlled trials in nearly 12,000 patients have confirmed that colchicine significantly reduces the risk of myocardial infarction, ischemic stroke, and the need for unscheduled revascularization by 25%–30% based on anti-platelet and lipid-lowering therapy (Turner and Pirmohamed, 2019; Siak et al., 2021). Thus, the US Food and Drug Administration (FDA) approved colchicine (Sadiq et al., 2024) in 2023 for patients with atherosclerotic disease or multiple cardiovascular disease risk factors to reduce the risk of myocardial infarction, stroke, coronary revascularization, and cardiovascular death (Aimo et al., 2021). Evidence suggests that gout is independently linked to an increased risk of cardiovascular diseases (Choi and Curhan, 2007; Mouradjian et al., 2020). As the high and direct correlation between gout and cardiovascular disease, colchicine is extensively used in those patients (Tardif et al., 2019; Kaul et al., 2021). Accordingly, colchicine and statins are frequently co-prescribed for prevention and treatment of cardiovascular diseases, auto-inflammatory diseases, and gout, which is high and clinically significant.
Despite the benefits, both statins and colchicine are known to be associated with an increased risk of myotoxicity, ranging from mild myalgia to rhabdomyolysis, are major side effects and a leading cause of statin intolerance. While the precise incidence of myotoxicity induced by colchicine remains uncertain, statins-associated myotoxicity occurs in 5%–10% of cases, with rhabdomyolysis occurring in 0.01%–0.1% (Ramachandran and Wierzbicki, 2017; Newman et al., 2019). Rhabdomyolysis is the most serious side effect of statins, which leads to a high mortality rate of approximately 10% (Zutt et al., 2014; Toth et al., 2018; Fracchiolla et al., 2009). Therefore, there is a growing concern about the association between the combined use of colchicine and statins and the occurrence of myotoxicity, but this remains controversial. Several clinical studies have confirmed that long use of colchicine based on statins therapy does not increase the risk of myotoxicity and rhabdomyolysis (Wiggins et al., 2016; Kwon et al., 2017; Vrachatis et al., 2021). Conversely, other studies show an association between colchicine use and the occurrence of statins-related myotoxicity (Wiggins et al., 2016; Sen et al., 2021; Schwier et al., 2022).
The inconsistent results surrounding this issue remain controversial, thus, necessitating further clarification regarding whether the concurrent administration of colchicine and statins enhances the likelihood of myotoxicity, particularly rhabdomyolysis. Clinical trials and foundational research play a pivotal role in elucidating the mechanisms of action and the resultant effects of medications (Massari et al., 2020). On the other hand, real-world research holds significance in post-marketing drug safety monitoring and guiding clinical practice (Meng et al., 2022). Importantly, analyses combined with pharmacovigilance data could provide valuable evidential support. To enhance these endeavors, the FDA launched the US FDA Adverse Event Reporting System (FAERS) database to meticulously monitor the safety profile of approved pharmaceutical products. Data mining from the FAERS dataset can be employed for quantitative assessment in detecting drug-drug interactions (DDIs).
This study aimed to clarify whether concomitant use of statins and colchicine increases the incidence of rhabdomyolysis, thereby effectively addressing the limitations of previous studies.
2 Methods
2.1 Data acquisition and preprocessing
FAERS is a publicly available database that facilitates the surveillance of post-marketing safety for approved medications and biologics (Fukazawa et al., 2018). As an open-access platform, it proactively encourages adverse events (AEs) reporting via the MedWatch program, including consumers, healthcare professionals, pharmaceutical companies, and the general public (Meng et al., 2022). In our study, publicly available FAERS data from quarter 1 of 2004 to quarter 4 of 2023 were downloaded as raw data. The exclusion criteria were outlined in the study schematic (Figure 1): reports officially removed by FDA authorities, duplicates, those lacking case ID or date, and those containing inaccurate gender or age data were excluded. Ethical approval was not required as only anonymous data was used.
FIGURE 1
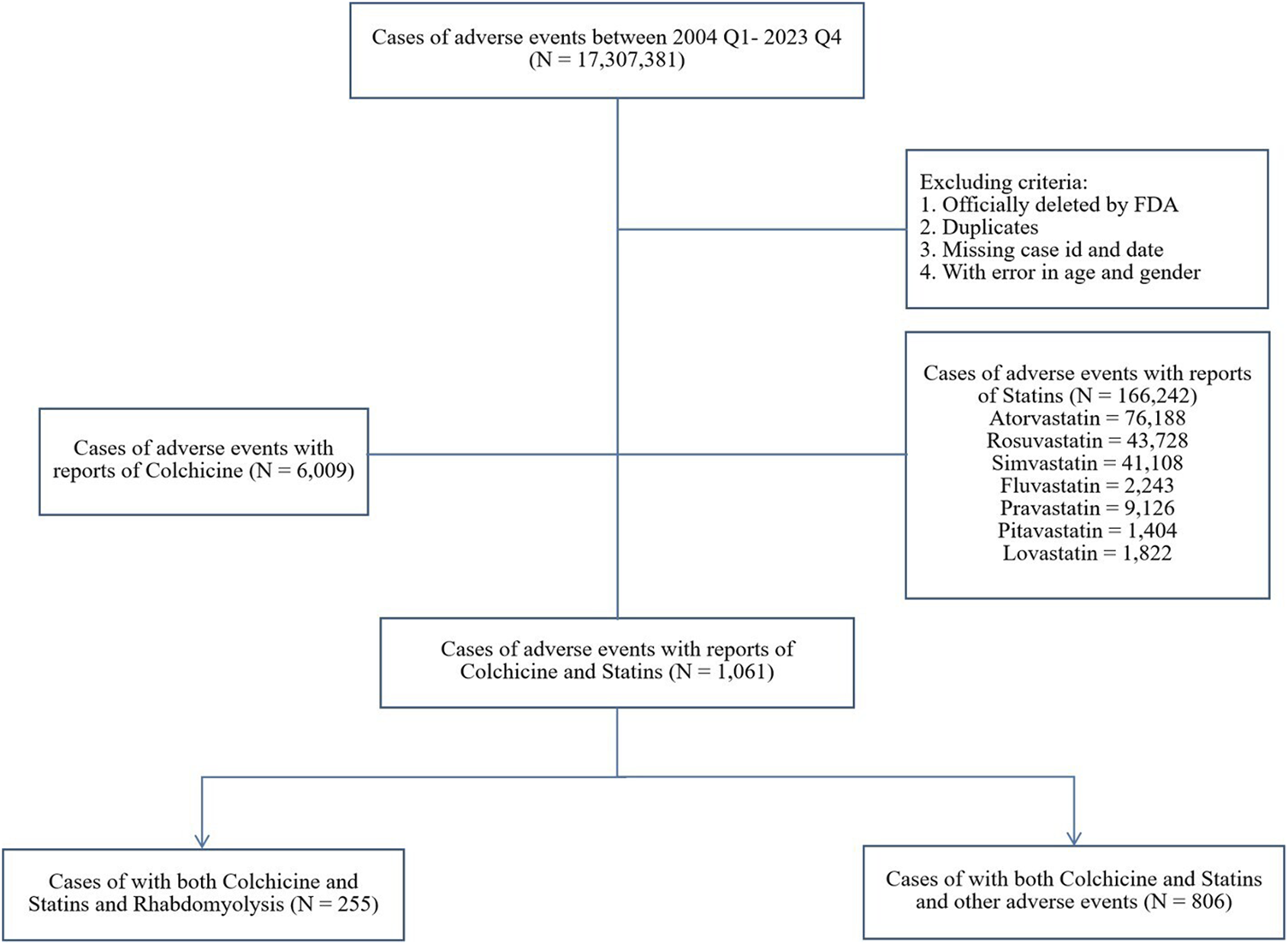
Data flow chart.
2.2 Identification of adverse events
In the FAERS database, AEs were coded using Preferred Terms from the Medical Dictionary for Regulatory Activities (MedDRA, version 26). AEs of myotoxicity were identified using the preferred term “rhabdomyolysis (Code10039020)” in MedDRA. Statins were defined as the following drugs: atorvastatin, rosuvastatin, fluvastatin, simvastatin, pravastatin, pitavastatin, and lovastatin, which are all listed in the FAERS. All reports referring to the trade name and generic name of the interested drugs were extracted for further analysis according to the scheme of study, and cleaned by the including or excluding criteria.
2.3 Descriptive analysis
Descriptive analysis on the demographic profiles was determined based on patients’ demographic. Categorical variables are presented with case number (N) and frequencies (N %), while continuous variables are presented using mean and medians with quartile ranges.
2.4 Disproportionality analysis
A sequential methodology was adopted to mitigate significant confounding variables and biases, adhering to the principles of Good Signal Detection Practices in Pharmacovigilance (Khaleel et al., 2022).
1) The disproportionality analysis based on differences in reporting portion of AEs. If there is no causal relationship between target drug and interested AE, the reporting rate will be similar to the average reporting rate for all other drugs, otherwise the associated drug-AE combination will demonstrate high reporting rate than random drug-AE pairs. Herein, we did not apply restrictions to the role code of each drug as either primary suspect or secondary suspect, as our aim was to analyze DDIs. As a result, all drugs were included in the study. Three mutually exclusive datasets were categorized: reports of colchicine alone, reports of statins alone, as well as reports with both colchicine and statins (colchicine + statins). In addition, we further refined the colchicine and statins dataset to focus specifically on colchicine in combination with individual statins. A disproportionality analysis was performed to compare these three datasets with all other drugs reported in the FAERS database by using the Bayesian confidence propagation neural network (BCPNN) method. This approach is more accurate than the Reporting Odds Ratio (ROR), particularly in situations with a limited number of cases (Noren et al., 2013). A reporting signal was defined as the lower limit of the 95% confidence interval (CI) of information component (IC025) > 0. The higher the IC025, the stronger signal between the drug and AE. The calculation procedure for the IC can be referenced in Supplementary Table S1.
2) To investigate the potential impact on the disproportionate reporting of rhabdomyolysis associated with co-administration of statins and colchicine by gender and age, we conducted a subgroup analysis by stratifying patients based on gender and age.
2.5 Drug-drug interaction analysis
A DDI analysis were conducted between colchicine and statins class level and specific-statins level by using BCPNN method and Ω shrinkage measure. We compared the IC025 values of colchicine and statins administered alone versus in combination, when the IC025 for the colchicine and statins combination exceeds that of other non-combination groups, it indicates evidence of interaction (IC025 of combination drugs greater than IC025 of individual drugs used). Additionally, to test the consistency of the findings, we further measured the DDI signals using the Ω shrinkage recommended by the World Health Organization Uppsala Monitoring Center, as an earlier study (Noguchi et al., 2020) showed it to be the most conservative of the multiple algorithms. Norén et al. (Noguchi et al., 2021) proposed the Ω shrinkage measure to calculate the observed-to-expected ratio as a measure of disproportionality to explore signals of DDIs. The detection criterion is the lower limit of the 95% CI of the Ω (Ω025) > 0. Consequently, the DDI signals detection criterion in our study is the lower limit of the 95% CI of the Ω (Ω025) > 0. The calculation methodology for Ω were elucidated in Supplementary Table S2. Therefore, the DDI signals detection criteria in this study require simultaneous meeting of the standards of both detection methods mentioned above.
2.6 Sensitivity analyses
To test the robustness of the DDI signals, we performed a sensitivity analysis using concomitant use of atorvastatin and clarithromycin as the positive control, in which we replicated our primary analysis using an established cytochrome p450-mediated DDIs between statins and clarithromycin to evaluate our ability to detect interaction-positive signals (Alkabbani et al., 2022; Chuma et al., 2022). In addition, we repeated our analysis with concomitant use of colchicine and aspirin as a negative control group, because the package-inserts of aspirin and previous studies (Wiggins et al., 2016) showed that there is no DDI between statins and aspirin. All Data processing and analysis were conducted using Microsoft Office Excel (2010) and SPSS (version 22), while R (4.12) was applied for graphics.
3 Results
3.1 Descriptive analysis
From quarter 1 of 2004 to quarter 4 of 2023, a total of 17,307,381 reports were retrieved through FAERS after data cleaning, among which 11,119 reports were associated with rhabdomyolysis (Figure 1). Of these, 255 (2.29%) involved both colchicine and statins, and the most common statin was atorvastatin (N = 140, 54.90%). The distribution of reported cases shows a notable upward trend over the years, primarily submitted by healthcare professionals. Specifically, reports involving colchicine alone, statins alone, or a combination of both were reported by healthcare professionals at rates of 88.76%, 89.81%, and 94.51%, respectively. For the combination therapy, the average age was 66.32 ± 13.31, with 75.29% being male patients, about three times as many as female. A significant portion of AE reports related to rhabdomyolysis were severe, leading to hospitalization (85.49%), life-threatening conditions (12.55%), or even death (11.37%) (Table 1).
TABLE 1
| Characteristic | Rhabdomyolysis | ||
|---|---|---|---|
| Colchicine alone, N (%) N = 267 |
Statin alone, N (%) N = 10,597 |
Colchicine and statin, N (%) N = 255 |
|
| Age in years, mean (SD) | 55.58 ± 18.62 | 66.64 ± 13.30 | 66.32 ± 13.31 |
| Age in years, median(IQR) | 59.00 (43.00,70.00) | 68.00 (58.00,76.00) | 69.00 (61.00, 75.00) |
| Gender | |||
| Male | 202 (75.66) | 6,617 (62.44) | 192 (75.29) |
| Female | 65 (24.34) | 3,977 (37.53) | 63 (24.71) |
| Other | NA | 3 (0.03) | NA |
| Colchicine n (%) | 267 (100.00) | NA | 255 (100.00) |
| Statins, n (%) | NA | 10,597 (100.00) | 255 (100.00) |
| Atorvastatin | NA | 3,498 (33.01) | 140 (54.90) |
| Rosuvastatin | NA | 2,263 (21.36) | 51 (20.00) |
| Simvastatin | NA | 4,266 (40.26) | 62 (24.31) |
| Fluvastatin | NA | 173 (1.63) | 9 (3.53) |
| Pravastatin | NA | 363 (3.43) | 12 (4.71) |
| Lovastatin | NA | 155 (1.46) | 2 (0.78) |
| Year | |||
| 2004 | 7 (2.62) | 961 (9.07) | 10 (3.92) |
| 2005 | 7 (2.62) | 962 (9.08) | 6 (2.35) |
| 2006 | 3 (1.12) | 699 (6.60) | 8 (3.14) |
| 2007 | 3 (1.12) | 585 (5.52) | 9 (3.53) |
| 2008 | 7 (2.62) | 625 (5.90) | 10 (3.92) |
| 2009 | 15 (5.62) | 716 (6.76) | 6 (2.35) |
| 2010 | 16 (5.99) | 903 (8.52) | 21 (8.24) |
| 2011 | 28 (10.49) | 985 (9.30) | 19 (7.45) |
| 2012 | 11 (4.12) | 675 (6.37) | 13 (5.10) |
| 2013 | 13 (4.87) | 538 (5.08) | 8 (3.14) |
| 2014 | 10 (3.75) | 457 (4.31) | 15 (5.88) |
| 2015 | 7 (2.62) | 445 (4.20) | 1 (0.39) |
| 2016 | 23 (8.61) | 401 (3.78) | 27 (10.59) |
| 2017 | 16 (5.99) | 409 (3.86) | 13 (5.10) |
| 2018 | 11 (4.12) | 866 (8.17) | 17 (6.67) |
| 2019 | 18 (6.74) | 844 (7.96) | 28 (10.98) |
| 2020 | 17 (6.37) | 873 (8.24) | 31 (12.16) |
| 2021 | 39 (14.61) | 695 (6.56) | 29 (11.37) |
| 2022 | 30 (11.24) | 545 (5.14) | 17 (6.67) |
| 2023 | 20 (7.49) | 609 (5.75) | 10 (3.92) |
| Time to onset | |||
| instant | 11 (4.12) | 255 (2.41) | 2 (0.78) |
| 1–2 days | 3 (1.12) | 47 (0.44) | NA |
| 2–3 days | 2 (0.75) | 35 (0.33) | NA |
| 3 days–1 week | 6 (2.25) | 113 (1.07) | NA |
| 1–2 weeks | 6 (2.25) | 165 (1.56) | NA |
| 0.5–1 month | 19 (7.12) | 372 (3.51) | 7 (2.75) |
| 1–3 months | 6 (2.25) | 723 (6.82) | 12 (4.71) |
| 3–6 months | 2 (0.75) | 372 (3.51) | 3 (1.18) |
| 0.5–1 year | 3 (1.12) | 363 (3.43) | 5 (1.96) |
| 1–2 years | NA | 388 (3.66) | 6 (2.35) |
| 2–3 years | NA | 236 (2.23) | 1 (0.39) |
| 3–4 years | 1 (0.37) | 166 (1.57) | NA |
| 4–5 years | NA | 111 (1.05) | 3 (1.18) |
| 5–10 years | 4 (1.50) | 248 (2.34) | 6 (2.35) |
| TTO mean (SD) (day) | 205.46 ± 622.41 | 560.72 ± 1062.25 | 578.42 ± 882.95 |
| TTO median(IQR) (day) | 21.00 (2.50, 28.00) | 119.00 (27.00,647.00) | 129.00 (40.00,555.00) |
| Reporter | |||
| Health professional | 237 (88.76) | 9,517 (89.81) | 241 (94.51) |
| Nonhealth professional | 11 (4.12) | 736 (6.94) | 5 (1.96) |
| Unknown or missing | 19 (7.12) | 344 (3.25) | 9 (3.53) |
| Outcomes | |||
| Hospitalization/hospitalization prolonged | 216 (80.90) | 8,109 (76.52) | 218 (85.49) |
| Other serious illness | 136 (50.94) | 4,921 (46.42) | 113 (44.31) |
| Death | 57 (21.35) | 1,043 (9.84) | 29 (11.37) |
| Life-threatening | 26 (9.74) | 1995 (18.83) | 32 (12.55) |
| Requair medical intervention | 7 (2.62) | 389 (3.67) | 8 (3.14) |
| Disability | 3 (1.12) | 864 (8.15) | 11 (4.31) |
| Other | NA | 92 (0.82) | NA |
| Occurred countries | |||
| United States | 49 (18.35) | 1,058 (9.98) | 47 (18.43) |
| France | 47 (17.60) | 845 (7.97) | 64 (25.09) |
| Italy | 9 (3.37) | 521 (4.92) | 20 (7.84) |
| Canada | 11 (4.12) | 287 (2.71) | 7 (2.75) |
| Portugal | 7 (2.62) | 82 (0.77) | 7 (2.75) |
| China | 7 (2.62) | 142 (1.34) | 1 (0.39) |
| Turk | 7 (2.62) | 10 (0.09) | 1 (0.39) |
| Missing | 130 (48.7) | 7,652 (72.2) | 108 (42.4) |
Demographic characteristics of cases of rhabdomyolysis.
SD, standard deviation; IQR, interquartile range; NA, not applicable; TTO, Time-to-onset. The onset time was calculated as (onset date of AE)—(administration start date).
3.2 Disproportionality analysis
A disproportionality analysis was conducted by comparing the three datasets with all other drugs reported in the FAERS database. Colchicine (IC025 = 3.68) or statins (IC025 = 4.43) alone both showed a signal of rhabdomyolysis. Among the included statins, including atorvastatin (IC025 = 3.94), simvastatin (IC025 = 5.11), rosuvastatin (IC025 = 4.09), and pravastatin (IC025 = 3.58), fluvastatin (IC025 = 4.29), and lovastatin (IC025 = 4.37), presented a disproportionate signal of rhabdomyolysis. Similarly, the concomitant use of colchicine and statins suggested a high-intensity rhabdomyolysis signal (IC025 = 5.73). Regarding colchicine-specific statins, including colchicine-atorvastatin, colchicine-rosuvastatin, colchicine-simvastatin, and colchicine-pravastatin, there are indications of rhabdomyolysis signals with signal strengths of IC025 = 5.20, 4.29, 4.26, and 1.63, respectively (Table 2). On performing gender and age stratification, we did not detect significant differences between males and females on the safety signals for the combination of colchicine and statins leading to rhabdomyolysis, either at the class level (IC025 = −0.28) or for specific drugs (Figure 2). Likewise, we did not find a significant difference in rhabdomyolysis signals between individuals more than 65 years and those under 65 years when colchicine was used in combination with statins (IC025 = −0.15) (Figure 3).
TABLE 2
| Drug1 | Drug2 | N of rhabdomyolysis with drug1+drug2/N of all AEs with drug1+drug2 | Drug1+Drug2 IC(IC025-IC075) | Ω(Ω025-Ω075) | N of rhabdomyolysis with drug1/N of all AEs with drug1 | Drug1 IC(IC025-IC075) | N of rhabdomyolysis with drug2/N of all AEs with drug2 | Drug2 IC(IC025-IC075) |
|---|---|---|---|---|---|---|---|---|
| Colchicine | Statins | 255/1,061 | 5.98 (5.73–6.22) | 1.24 (1.17–1.32) | 267/6,009 | 3.87 (3.68–4.06) | 10,597/166,242 | 4.46 (4.43–4.49) |
| Colchicine | Atorvastatin | 140/708 | 5.53 (5.20–5.87) | 1.22 (1.12–1.32) | 267/6,009 | 3.87 (3.68–4.06) | 3,498/76,188 | 3.99 (3.94–4.04) |
| Colchicine | Simvastatin | 62/426 | 4.82 (4.29–5.35) | 0.08 (-0.08–0.23) | 267/6,009 | 3.87 (3.68–4.06) | 4,266/41,108 | 5.16 (5.11–5.21) |
| Colchicine | Rosuvastatin | 51/242 | 4.93 (4.26–5.61) | 1.21 (1.05–1.38) | 267/6,009 | 3.87 (3.68–4.06) | 2,263/43,728 | 4.16 (4.09–4.22) |
| Colchicine | Pravastatin | 12/108 | 3.30 (1.63–4.98) | 0.48 (0.13–0.83) | 267/6,009 | 3.87 (3.68–4.06) | 363/9,126 | 3.74 (3.58–3.90) |
| Colchicine | Fluvastatin | 9/13 | 3.26 (-1.37–7.89) | 2.27 (1.87–2.67) | 267/6,009 | 3.87 (3.68–4.06) | 173/2,243 | 4.54 (4.29–4.79) |
IC025 and Ω025 of Colchicine and statins.
When IC025 > 0, a significant signal was detected between target drug and rhabdomyolysis; When Ω025 > 0,and (drug1+drug2 IC025)> (drug1C025) and (drug2C025), a significant drug–drug interaction signal was detected.; IC, information component; AEs, Adverse events.
FIGURE 2
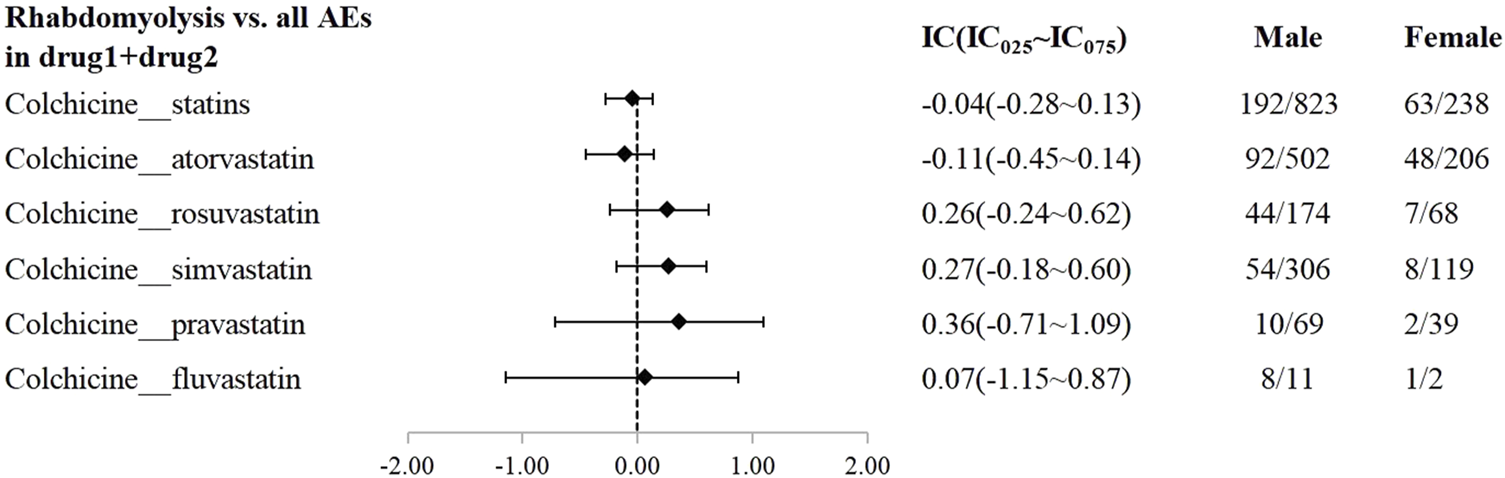
The Effect of gender on rhabdomyolysis reports with colchicine and statins. When IC025 > 0, a significant signal of rhabdomyolysis associated with combination therapy was detected between genders, with a higher risk in males compared to females.
FIGURE 3
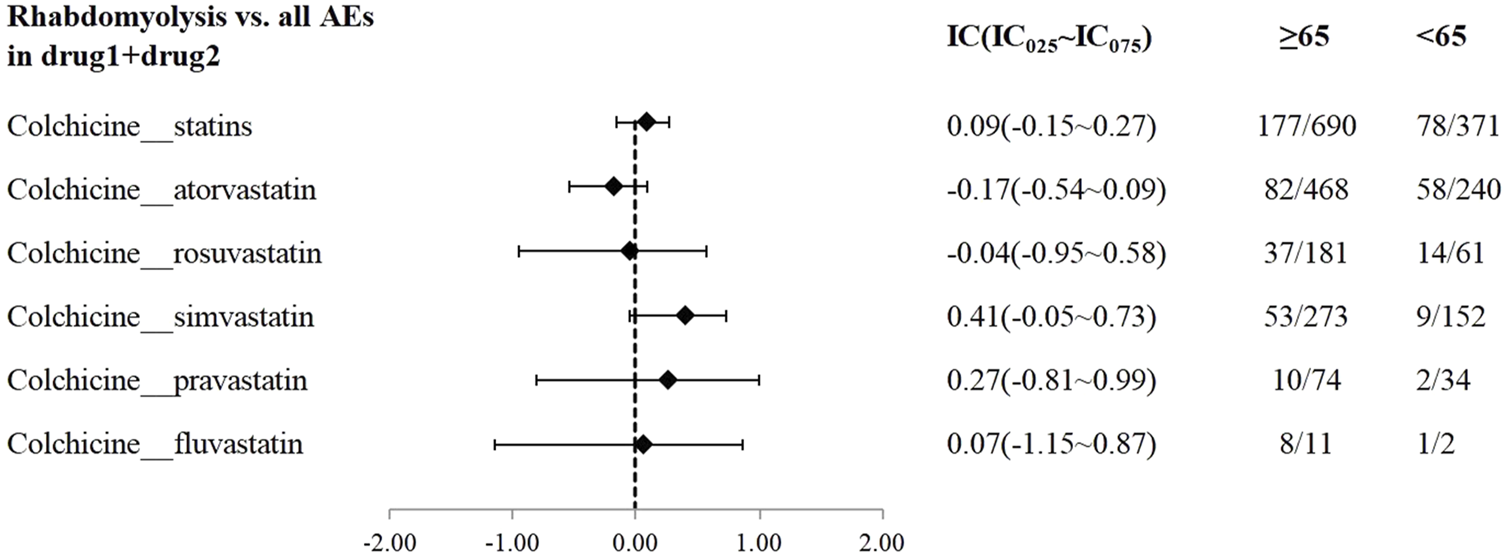
The Effect of age on rhabdomyolysis reports with colchicine and statins. When IC025 > 0, a significant association between age and the risk of rhabdomyolysis related to combination therapy was observed, with a higher risk in individuals aged ≥65 years compared to those <65 years.
3.3 Drug-drug interactions
A DDI analysis were conducted between colchicine and statins associated with rhabdomyolysis. We observed a heightened disproportionality in the reporting of rhabdomyolysis with concomitant administration of colchicine and statins, evidenced by the IC025 value (IC025 = 5.73) for the combination exceeding that of the individual drugs (IC025 = 3.68 and IC025 = 4.43). Meanwhile, the Ω shrinkage model (Ω025 = 1.17) identified a DDI signal when colchicine was co-treated with statins, indicating an elevated risk of rhabdomyolysis (Table 2).
In addition, we carried out this possible DDI on a drug-specific level between colchicine and statins. Concerning drug-specific results, colchicine-atorvastatin pair and colchicine-rosuvastatin pair demonstrated DDI signals of rhabdomyolysis in both the Ω shrinkage model (Ω025 = 1.12 and Ω025 = 1.05) and BCPNN method (IC025 = 5.20 and IC025 = 4.26). However, significant DDI signals of rhabdomyolysis were observed for colchicine-pravastatin pair and colchicine-fluvastatin pair in the Ω shrinkage model (Ω025 = 0.13 and Ω025 = 1.87), but they were absent in the BCPNN method. And the combined treatment cases of colchicine and fluvastatin are limited, comprising only 13 cases, including 9 cases and 4 non-cases. Furthermore, regarding colchicine and simvastatin, there was no evidence of a significant interaction from the Ω-shrinkage model, but it was at the edge of the threshold (Ω025 = −0.08). (Table 2).
3.4 Sensitivity analyses
Two pre-specified sensitivity analyses were conducted, encompassing positive control analysis and negative control analysis. In the positive control analysis establishing DDI signals between clarithromycin and simvastatin, both estimations including the Ω-shrinkage model (Ω025 = 0.84) and BCPNN method (IC025 = 5.61, greater than IC025 of individual drugs used) indicated significant DDI signals. Conversely, in the negative control analysis assessing the combined use of atorvastatin and aspirin, all estimations suggested the absence of a DDI (Ω025 = -0.39) and BCPNN method (IC025 = 3.44, less than IC025 of individual drugs used) (Table 3).
TABLE 3
| Drug1 | Drug2 | N of rhabdomyolysis with drug1+drug2/N of all AEs with drug1+drug2 | Drug1+Drug2 IC(IC025-IC075) | Ω(Ω025-Ω075) | N of rhabdomyolysis with drug1/N of all AEs with drug1 | Drug1 IC(IC025-IC075) | N of rhabdomyolysis with drug2/N of all AEs with drug2 | Drug2 IC(IC025-IC075) |
|---|---|---|---|---|---|---|---|---|
| Clarithromycin | Simvastatin | 239/1,084 | 5.86 (5.61–6.11) | 0.91 (0.84–0.99) | 343/18,506 | 2.66 (2.50–2.82) | 4,266/41,108 | 5.16 (5.11–5.21) |
| Acetylsalicylic | Atorvastatin | 244/6,465 | 3.64 (3.44–3.84) | −0.32 (-0.39-0.24) | 95/25,539 | 0.36 (0.07–0.66) | 3,498/76,188 | 3.99 (3.94–4.04) |
IC025 and Ω025 of sensitivity analyses.
When IC025 > 0, a significant signal was detected between target drug and rhabdomyolysis; When Ω025 > 0,and (drug1+drug2 IC025)> (drug1C025) and (drug2C025), a significant drug–drug interaction signal was detected. IC, information component; AEs, Adverse events.
4 Discussion
Current evidence from clinical research and case series studies does not conclude whether colchicine combined with statins therapy increases rhabdomyolysis (Aimo et al., 2021; Hansten et al., 2023). In this study, we performed a disproportionation analysis in tandem with BCPNN based on the large publicly available FAERS database to investigate possible DDI signals generated from combination therapy of colchicine and statins. We have four main findings: first, we confirmed signals of disproportionate reporting for rhabdomyolysis with colchicine or statins; second, we found gender did not exert a significant influence on the safety signal associated with the combination of colchicine and statins leading to rhabdomyolysis; third, we detected potential DDI signals between colchicine and statins that co-administration patterns increase the incidence of rhabdomyolysis; finally, we further identified potential DDI signals of rhabdomyolysis at a drug-specific level between colchicine and atorvastatin or rosuvastatin.
We carried out a disproportionality analysis of the three datasets, including colchicine, statins, and both colchicine and statins, against all other drugs in the FAERS database, and we found that colchicine or statins use alone had a clear potential signal for rhabdomyolysis. The findings regarding colchicine and statins align with existing literatures and labeling information, affirming the reliability of our study’s methodology and data analysis. Prior studies indicate a heightened risk of myotoxicity, notably rhabdomyolysis, among individuals using either colchicine or statins (Turner and Pirmohamed, 2019; Newman et al., 2019; Zutt et al., 2014). Furthermore, drug inserts underscore the importance of vigilance regarding the occurrence of myotoxic AEs during administration.
Statins are the cornerstone of treating cardiovascular diseases as well as metabolic disorders, more and more patients are being treated with statins in clinical practice (Wiggins et al., 2016). Given the growing interest in targeting inflammation to mitigate major cardiovascular risk and the significant anti-inflammatory effects of colchicine, along with its potential to reduce major cardiovascular events, related research is receiving increasing attention (Xie et al., 2024). This is further compounded by the FDA’s 2023 approval of colchicine for patients with atherosclerotic vascular disease or multiple cardiovascular risk factors, as extensive clinical studies over the past decade have validated its contribution in reducing cardiovascular events (Aimo et al., 2021; Mouradjian et al., 2020; Tardif et al., 2019). Therefore, the potential co-medication of colchicine and statins is high. We performed a co-administration analysis of colchicine and statins to investigate potential DDIs by BCPNN method and Ω shrinkage analysis. The results showed that there are potential DDI signals between colchicine and statins which increased the reported frequency of rhabdomyolysis. Our findings are consistent with a recent review reported by Schwier et al. (2022) which suggests that the statins-colchicine drug interaction may be related to potentially life-threatening myotoxicity. Upon performing gender stratification, we found that gender did not significantly influence the safety signal associated with the combination of colchicine and statins leading to rhabdomyolysis. Previous studies have highlighted gender as a significant risk factor for myopathy or rhabdomyolysis, with females exhibiting a higher frequency of reported myotoxicity compared to males (Ward et al., 2019; Mancini et al., 2016). Our results can be interpreted as the incidence of cardiovascular diseases being higher in males than females (Virani et al., 2023),as well as the number of individuals co-administering colchicine and statin being significantly greater in males. Specifically, in our study, the number of males combined with colchicine and statins was three times greater than that of females.
In the age-stratified analysis, this study found that the number of patients over 65 years using both colchicine and statins is twice that of those under 65 years old. This finding reflects the increasing use of both colchicine and statins among the elderly, consistent with longer life expectancy and rising rates of cardiovascular disease. Studies have shown that the elderly may be more susceptible to statins-induced myotoxicity, but specific age ranges have not been clearly defined (Adhyaru and Jacobson, 2018). However, this study found no significant difference in rhabdomyolysis signals between individuals over and under 65 years old when colchicine was combined with statins. This result aligns with Camerino et al. (2017) which indicated that in most subjects, modifications in skeletal muscle biomarkers due to statins therapy were independent of age. It is important to note that our result does not suggest that advanced age is not a critical factor. Statins-associated muscle toxicity is influenced by several factors, including high doses, advanced age, female sex, hypothyroidism, reduced muscle mass, and increased physical activity (Adhyaru and Jacobson, 2018). We hypothesize that the risk of myotoxicity may increase only when multiple complex adverse factors are present. Addressing one or more of these factors could reduce or eliminate the risk of muscle toxicity. Importantly, clinicians should carefully evaluate each patient’s condition and address factors contributing to statins-induced muscle toxicity to optimize the safety and efficacy of statins therapy. Besides, positive and negative controls were employed to assess both the internal validity of the database and the robustness of the DDI signals. The clarithromycin-simvastatin pair was utilized as the positive control, while the atorvastatin-aspirin pair served as the negative control. The outcomes pertaining to these pre-defined control drugs aligned with our expectations, thus reinforcing the reliability and validity of this study concerning both methodology and data analysis.
We conducted further analyses to explore potential DDIs specifically between colchicine and certain statins. Our drug-specific analysis revealed potential signals of DDIs leading to increase of drug-induced rhabdomyolysis due to co-administration of colchicine and atorvastatin. For many years, it has been posited that lipophilic statins, such as atorvastatin and simvastatin, are more prone to inducing muscle toxicity compared to hydrophilic statins like rosuvastatin and pravastatin (Sabanis et al., 2021). This is attributed to the fact that lipophilic statins are predominantly metabolized by the hepatic cytochrome P450 3A4 (CYP3A4) enzyme system, whereas hydrophilic statins are less reliant on CYP3A4 for their metabolism. Similarly, colchicine is lipophilicis and metabolized primarily by CYP3A4 and is eliminated via the P-glycoprotein pump, akin to lipophilic statins (Dahan et al., 2009; Finkelstein et al., 2010). The competition for the same metabolic enzymes and efflux pumps between colchicine and lipophilic statins may result in elevated plasma levels of these drugs (Davis and Wason, 2014). This is probably the most significant reason for the increase in adverse effects due to the combination of colchicine and statins. In addition, previous meta-analysis (Schwier et al., 2022) showed that among patients experiencing AEs due to the combination therapy of statins and colchicine, over 70% were attributed to atorvastatin or simvastatin, and simvastatin has the highest propensity for DDIs, especially in terms of pharmacokinetics (Siriangkhawut et al., 2017). Our study did not uncover a significant DDI signal between colchicine and simvastatin, but the results approached the threshold of significance. This suggested the necessity for additional research to delve deeper and corroborate these findings. However, it is important to emphasize that the absence of positive results does not necessarily imply the absence of rhabdomyolysis when colchicine and simvastatin are combined. Our research still indicated a strong signal for rhabdomyolysis with the concurrent use of these two drugs.
Our study also revealed potential DDI signals of rhabdomyolysis between colchicine and rosuvastatin, despite that rosuvastatin neither underwent metabolism via the CYP3A4 enzyme system nor being transported via the P-glycoprotein pathway. Another potential mechanism underlying the interaction between statins and colchicine may involve combined myotoxic effects, potentially additive or synergistic, given that each medication class has distinct mechanisms associated with causing myopathies (Ward et al., 2019; Fernandez-Cuadros et al., 2019; Wilbur and Makowsky, 2004; Camerino et al., 2021). Encouragingly, our findings were consistent with previous studies (Wiggins et al., 2016; Hansten et al., 2023), and indicate that the concomitant use of pravastatin and colchicine minimal increase in the risk of rhabdomyolysis. Considering the benefits of co-administration and potential DDIs that may result in severe adverse outcomes, we should select statins with fewer DDI in combination with colchicine to maximize effectiveness and minimize side effects. While our findings contribute evidence towards enhancing safety warnings for these medications, further preclinical and large-scale clinical studies are remained warranted.
Our study has some certain limitations. The FAERS database is a spontaneous and anonymous reporting system, making underreporting, overreporting, or missing information inevitable (Gravel et al., 2023); Second, reporting sources in the FAERS database are diverse, encompassing healthcare professionals and consumers. This heterogeneity can impact the data quality and result in the omission of crucial details regarding treatment-related myotoxicity, such as significant comorbidities and comorbid medications; Third, spontaneous reporting data suffer from inherent limitations including the inability to establish a causal relationship between myotoxicity and the combined colchicine and statin, as well as the failure to calculate the incidence rate of myotoxicity (Meng et al., 2022).
5 Conclusion
Our large-scale pharmacovigilance study indicates that concomitant use of colchicine and statins may increase the risk of rhabdomyolysis. Moreover, this interaction primarily pertains to colchicine and specific statins, including atorvastatin and rosuvastatin. Therefore, healthcare professionals should pay special attention to life-threatening AEs such as rhabdomyolysis, when co-prescribing colchicine and statins.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Author contributions
SZ: Data curation, Writing–original draft. M-MY: Data curation, Writing–original draft. HZ: Data curation, Writing–review and editing. X-YQ: Conceptualization, Supervision, Writing–review and editing. DZ: Conceptualization, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shanghai Municipal Health Commission [2018] No.8.
Acknowledgments
We express our gratitude to the US FDA for generously providing the dataset used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1445324/full#supplementary-material
References
1
Adhyaru B. B. Jacobson T. A. (2018). Safety and efficacy of statin therapy. Nat. Rev. Cardiol.15 (12), 757–769. 10.1038/s41569-018-0098-5
2
Aimo A. Pascual-Figal D. A. Barison A. Cediel G. Vicente A. H. Saccaro L. F. et al (2021). Colchicine for the treatment of coronary artery disease. Trends Cardiovasc Med.31 (8), 497–504. 10.1016/j.tcm.2020.10.007
3
Alkabbani W. Pelletier R. Beazely M. A. Labib Y. Quan B. Gamble J. M. (2022). Drug-drug interaction of the sodium glucose co-transporter 2 inhibitors with statins and myopathy: a disproportionality analysis using adverse events reporting data. Drug Saf.45 (3), 287–295. 10.1007/s40264-022-01166-3
4
Camerino G. M. Musumeci O. Conte E. Musaraj K. Fonzino A. Barca E. et al (2017). Risk of myopathy in patients in therapy with statins: identification of biological markers in a pilot study. Front. Pharmacol.8, 500. 10.3389/fphar.2017.00500
5
Camerino G. M. Tarantino N. Canfora I. De Bellis M. Musumeci O. Pierno S. (2021). Statin-induced myopathy: translational studies from preclinical to clinical evidence. Int. J. Mol. Sci.22 (4), 2070. 10.3390/ijms22042070
6
Choi H. K. Curhan G. (2007). Independent impact of gout on mortality and risk for coronary heart disease. Circulation116 (8), 894–900. 10.1161/CIRCULATIONAHA.107.703389
7
Chuma M. Nakamoto A. Bando T. Niimura T. Kondo Y. Hamano H. et al (2022). Association between statin use and daptomycin-related musculoskeletal adverse events: a mixed approach combining a meta-analysis and a disproportionality analysis. Clin. Infect. Dis.75 (8), 1416–1422. 10.1093/cid/ciac128
8
Dahan A. Sabit H. Amidon G. L. (2009). Multiple efflux pumps are involved in the transepithelial transport of colchicine: combined effect of p-glycoprotein and multidrug resistance-associated protein 2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metab. Dispos.37 (10), 2028–2036. 10.1124/dmd.109.028282
9
Davis M. W. Wason S. (2014). Effect of steady-state atorvastatin on the pharmacokinetics of a single dose of colchicine in healthy adults under fasted conditions. Clin. Drug Investig.34 (4), 259–267. 10.1007/s40261-013-0168-8
10
Fernandez-Cuadros M. E. Goizueta-San-Martin G. Varas-de-Dios B. Casique-Bocanegra L. O. Manrique-de-Lara-Cadinanos P. Albaladejo-Florin M. J. et al (2019). Colchicine-induced rhabdomyolysis: clinical, biochemical, and neurophysiological features and review of the literature. Clin. Med. Insights Arthritis Musculoskelet. Disord.12, 1179544119849883. 10.1177/1179544119849883
11
Finkelstein Y. Aks S. E. Hutson J. R. Juurlink D. N. Nguyen P. Dubnov-Raz G. et al (2010). Colchicine poisoning: the dark side of an ancient drug. Clin. Toxicol. (Phila)48 (5), 407–414. 10.3109/15563650.2010.495348
12
Fiolet A. Opstal T. Mosterd A. Eikelboom J. W. Jolly S. S. Keech A. C. et al (2021). Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur. Heart J.42 (28), 2765–2775. 10.1093/eurheartj/ehab115
13
Fracchiolla G. Laghezza A. Piemontese L. Tortorella P. Mazza F. Montanari R. et al (2009). New 2-aryloxy-3-phenyl-propanoic acids as peroxisome proliferator-activated receptors alpha/gamma dual agonists with improved potency and reduced adverse effects on skeletal muscle function. J. Med. Chem.52 (20), 6382–6393. 10.1021/jm900941b
14
Fukazawa C. Hinomura Y. Kaneko M. Narukawa M. (2018). Significance of data mining in routine signal detection: analysis based on the safety signals identified by the fda. Pharmacoepidemiol Drug Saf.27 (12), 1402–1408. 10.1002/pds.4672
15
Gravel C. A. Krewski D. Mattison D. R. Momoli F. Douros A. (2023). Concomitant use of statins and sodium-glucose co-transporter 2 inhibitors and the risk of myotoxicity reporting: a disproportionality analysis. Br. J. Clin. Pharmacol.89 (8), 2430–2445. 10.1111/bcp.15711
16
Grundy S. M. Cleeman J. I. Merz C. N. Brewer H. J. Clark L. T. Hunninghake D. B. et al (2004). Implications of recent clinical trials for the national cholesterol education program adult treatment panel iii guidelines. Circulation110 (2), 227–239. 10.1161/01.CIR.0000133317.49796.0E
17
Hansten P. D. Tan M. S. Horn J. R. Gomez-Lumbreras A. Villa-Zapata L. Boyce R. D. et al (2023). Colchicine drug interaction errors and misunderstandings: recommendations for improved evidence-based management. Drug Saf.46 (3), 223–242. 10.1007/s40264-022-01265-1
18
Kaul S. Gupta M. Bandyopadhyay D. Hajra A. Deedwania P. Roddy E. et al (2021). Gout pharmacotherapy in cardiovascular diseases: a review of utility and outcomes. Am. J. Cardiovasc Drugs21 (5), 499–512. 10.1007/s40256-020-00459-1
19
Khaleel M. A. Khan A. H. Ghadzi S. Adnan A. S. Abdallah Q. M. (2022). A standardized dataset of a spontaneous adverse event reporting system. Healthc. (Basel)10 (3), 420. 10.3390/healthcare10030420
20
Kwon O. C. Hong S. Ghang B. Kim Y. G. Lee C. K. Yoo B. (2017). Risk of colchicine-associated myopathy in gout: influence of concomitant use of statin. Am. J. Med.130 (5), 583–587. 10.1016/j.amjmed.2016.12.006
21
Mancini G. B. Baker S. Bergeron J. Fitchett D. Frohlich J. Genest J. et al (2016). Diagnosis, prevention, and management of statin adverse effects and intolerance: canadian consensus working group update (2016). Can. J. Cardiol.32 (7 Suppl. l), S35–S65. 10.1016/j.cjca.2016.01.003
22
Massari F. Mollica V. Rizzo A. Cosmai L. Rizzo M. Porta C. (2020). Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: a systematic review and meta-analysis. Expert Opin. Drug Saf.19 (10), 1329–1338. 10.1080/14740338.2020.1811226
23
Meng L. Huang J. Qiu F. Shan X. Chen L. Sun S. et al (2022). Peripheral neuropathy during concomitant administration of proteasome inhibitors and factor xa inhibitors: identifying the likelihood of drug-drug interactions. Front. Pharmacol.13, 757415. 10.3389/fphar.2022.757415
24
Mouradjian M. T. Plazak M. E. Gale S. E. Noel Z. R. Watson K. Devabhakthuni S. (2020). Pharmacologic management of gout in patients with cardiovascular disease and heart failure. Am. J. Cardiovasc Drugs20 (5), 431–445. 10.1007/s40256-020-00400-6
25
Newman C. B. Preiss D. Tobert J. A. Jacobson T. A. Page R. N. Goldstein L. B. et al (2019). Statin safety and associated adverse events: a scientific statement from the american heart association. Arterioscler. Thromb. Vasc. Biol.39 (2), e38–e81. 10.1161/ATV.0000000000000073
26
Noguchi Y. Tachi T. Teramachi H. (2020). Comparison of signal detection algorithms based on frequency statistical model for drug-drug interaction using spontaneous reporting systems. Pharm. Res.37 (5), 86. 10.1007/s11095-020-02801-3
27
Noguchi Y. Tachi T. Teramachi H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform22 (6), bbab347. 10.1093/bib/bbab347
28
Noren G. N. Hopstadius J. Bate A. (2013). Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res.22 (1), 57–69. 10.1177/0962280211403604
29
Ramachandran R. Wierzbicki A. S. (2017). Statins, muscle disease and mitochondria. J. Clin. Med.6 (8), 75. 10.3390/jcm6080075
30
Sabanis N. Paschou E. Drylli A. Papanikolaou P. Zagkotsis G. (2021). Rosuvastatin and colchicine combined myotoxicity: lessons to be learnt. Cen. Case Rep.10 (4), 570–575. 10.1007/s13730-021-00598-7
31
Sadiq N. M. Robinson K. J. Terrell J. M. (2023). Colchicine. In:StatPearls. StatPearls Publishing.
32
Schwier N. C. Cornelio C. K. Boylan P. M. (2022). A systematic review of the drug-drug interaction between statins and colchicine: patient characteristics, etiologies, and clinical management strategies. Pharmacotherapy42 (4), 320–333. 10.1002/phar.2674
33
Sen S. Karahan E. Buyukulas C. Polat Y. O. Uresin A. Y. (2021). Colchicine for cardiovascular therapy: a drug interaction perspective and a safety meta-analysis. Anatol. J. Cardiol.25 (11), 753–761. 10.5152/AnatolJCardiol.2021.707
34
Siak J. Flint N. Shmueli H. G. Siegel R. J. Rader F. (2021). The use of colchicine in cardiovascular diseases: a systematic review. Am. J. Med.134 (6), 735–744.e1. 10.1016/j.amjmed.2021.01.019
35
Siriangkhawut M. Tansakul P. Uchaipichat V. (2017). Prevalence of potential drug interactions in Thai patients receiving simvastatin: the causality assessment of musculoskeletal adverse events induced by statin interaction. Saudi Pharm. J.25 (6), 823–829. 10.1016/j.jsps.2016.12.006
36
Tardif J. C. Kouz S. Waters D. D. Bertrand O. F. Diaz R. Maggioni A. P. et al (2019). Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med.381 (26), 2497–2505. 10.1056/NEJMoa1912388
37
Toth P. P. Patti A. M. Giglio R. V. Nikolic D. Castellino G. Rizzo M. et al (2018). Management of statin intolerance in 2018: still more questions than answers. Am. J. Cardiovasc Drugs18 (3), 157–173. 10.1007/s40256-017-0259-7
38
Turner R. M. Pirmohamed M. (2019). Statin-related myotoxicity: a comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J. Clin. Med.9 (1), 22. 10.3390/jcm9010022
39
Virani S. S. Newby L. K. Arnold S. V. Bittner V. Brewer L. C. Demeter S. H. et al (2023). 2023 aha/acc/accp/aspc/nla/pcna guideline for the management of patients with chronic coronary disease: a report of the american heart association/american college of cardiology joint committee on clinical practice guidelines. Circulation148 (9), e9–e119. 10.1161/CIR.0000000000001168
40
Vrachatis D. A. Papathanasiou K. A. Giotaki S. G. Iliodromitis K. E. Papaioannou T. G. Stefanini G. G. et al (2021). Repurposing colchicine's journey in view of drug-to-drug interactions. A review. Toxicol. Rep.8, 1389–1393. 10.1016/j.toxrep.2021.07.009
41
Ward N. C. Watts G. F. Eckel R. H. (2019). Statin toxicity. Circ. Res.124 (2), 328–350. 10.1161/CIRCRESAHA.118.312782
42
Wiggins B. S. Saseen J. J. Page R. L. Reed B. N. Sneed K. Kostis J. B. et al (2016). Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the american heart association. Circulation134 (21), e468–e495. 10.1161/CIR.0000000000000456
43
Wilbur K. Makowsky M. (2004). Colchicine myotoxicity: case reports and literature review. Pharmacotherapy24 (12), 1784–1792. 10.1592/phco.24.17.1784.52334
44
Xie S. Galimberti F. Olmastroni E. Catapano A. L. Casula M. (2024). Effect on c-reactive protein levels of the addition of ezetimibe, bempedoic acid, or colchicine to statin treatment: a network meta-analysis. J. Intern Med.296, 302–305. 10.1111/joim.13824
45
Zutt R. van der Kooi A. J. Linthorst G. E. Wanders R. J. de Visser M. (2014). Rhabdomyolysis: review of the literature. Neuromuscul. Disord.24 (8), 651–659. 10.1016/j.nmd.2014.05.005
Summary
Keywords
colchicine, statins, rhabdomyolysis, drug-drug interactions, pharmacovigilance
Citation
Zhang S, Yan M-M, Zhao H, Qiu X-Y and Zhu D (2024) Rhabdomyolysis associated with concomitant use of colchicine and statins in the real world: identifying the likelihood of drug–drug interactions through the FDA adverse event reporting system. Front. Pharmacol. 15:1445324. doi: 10.3389/fphar.2024.1445324
Received
07 June 2024
Accepted
30 August 2024
Published
16 September 2024
Volume
15 - 2024
Edited by
George Nikov Chaldakov, Medical University of Varna, Bulgaria
Reviewed by
Sabata Pierno, University of Bari Aldo Moro, Italy
Chi-Chuan Wang, National Taiwan University, Taiwan
Updates
Copyright
© 2024 Zhang, Yan, Zhao, Qiu and Zhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deqiu Zhu, zdq_0726@163.com; Xiao-Yan Qiu, xyqiu@fudan.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.