In the published article, there was an error in Figure 4 as published. The displayed structure of compound E4 in the article and on the Enamine site (Catalog ID T5320580) is that of (2-(4-chloro-2,6-dimethylphenoxy)-N-isopropylacetamide). Subsequent research showed that this compound is largely inactive as an inhibitor (Xu et al., 2024), while it was evaluated in the original high throughput screen as a potent inhibitor. An IC50 of 13.7 µM (FLIPR assay) was determined with the ordered compound as shown in Table 2 of the original article and confirmed by radioactive flux assay (IC50 7.7 µM). To resolve the discrepancy, we performed structural analysis and showed that the compound in the HTS collection was in fact (2-(4-chloro-3,5-dimethylphenoxy)-N-isopropylacetamide). The corrected Figure 4 and its corrected caption (‘Properties of second-generation inhibitors of B0AT1. Inhibitors E4, CB3 and E18 were identified by high-throughput screening. The established B0AT1 inhibitor cinromide is shown for comparison (A). Inhibition of B0AT1 activity [red symbols (B–D)] was tested in CHO-BC cells using a FLIPR assay (n = 3, e = 3). The assay allows to test the specificity of the inhibitors against the endogenous LAT1 transporter [green symbols (B–D)].') appear below. The docking experiment presented in Figure 5D remains correct, although it was performed with (2-(4-chloro-2,6-dimethylphienoxy)-N-isopropylacetamide). Subsequent research has, however, shown that the active compound binds to an allosteric site on the transporter (Xu et al., 2024). To avoid confusion, we have since renamed the active compound JX98.
FIGURE 4
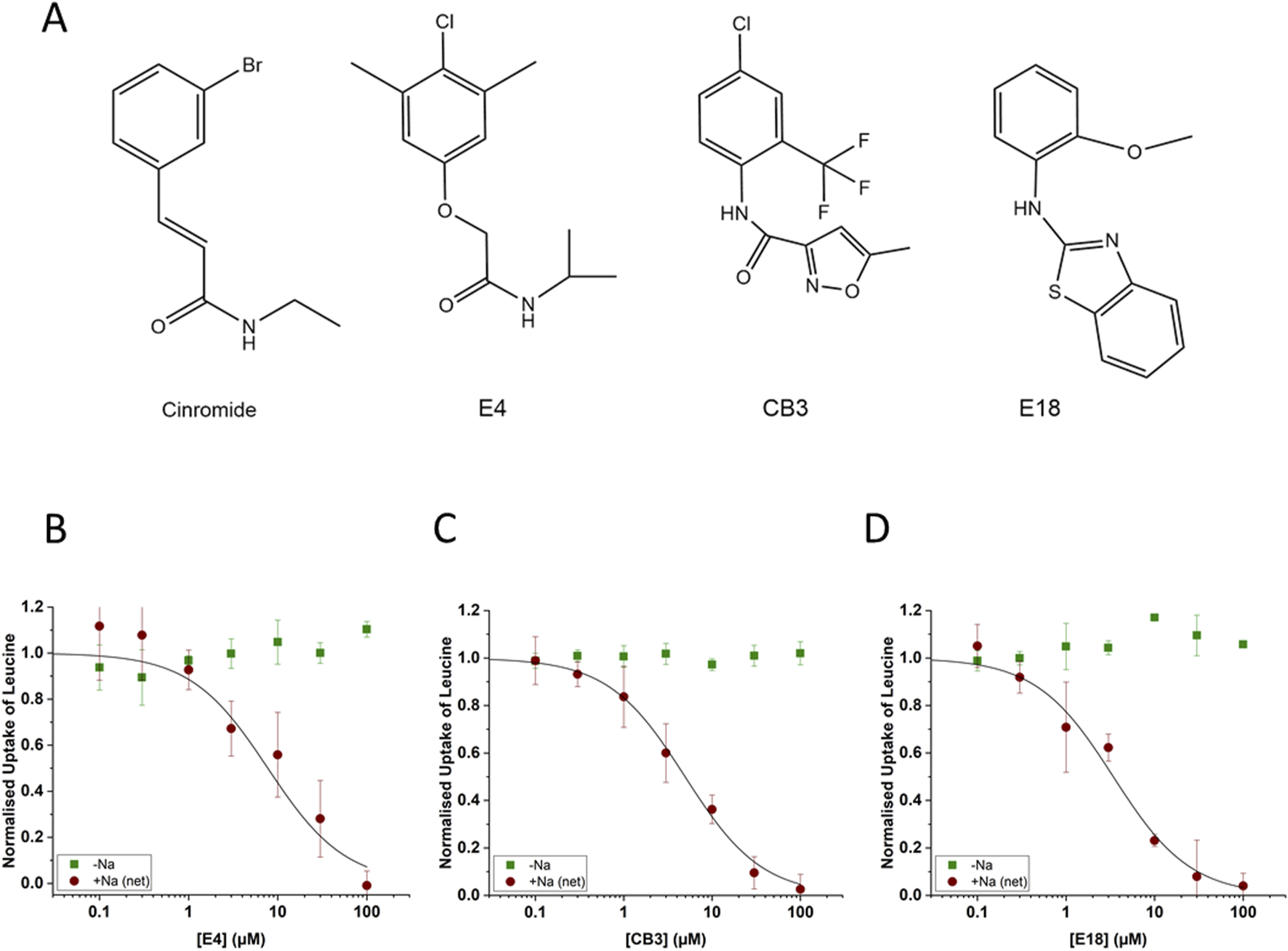
Properties of second-generation inhibitors of B0AT1. Inhibitors E4, CB3 and E18 were identified by high-throughput screening. The established B0AT1 inhibitor cinromide is shown for comparison (A). Inhibition of B0AT1 activity [red symbols (B–D)] was tested in CHO-BC cells using a FLIPR assay (n = 3, e = 3). The assay allows to test the specificity of the inhibitors against the endogenous LAT1 transporter [green symbols (B–D)].
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Statements
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Xu J. Hu Z. Dai L. Yadav A. Jiang Y. Broer A. et al (2024). Molecular basis of inhibition of the amino acid transporter B(0)AT1 (SLC6A19). Nat. Commun.15, 7224.
Summary
Keywords
phenylketonuria, steatohepatitis, non-alcoholic steatohepatitis, solute carrier, high throughput screening, HTS
Citation
Yadav A, Shah N, Tiwari PK, Javed K, Cheng Q, Aidhen IS and Bröer S (2025) Corrigendum: Novel chemical scaffolds to inhibit the neutral amino acid transporter B0AT1 (SLC6A19), a potential target to treat metabolic diseases. Front. Pharmacol. 15:1485054. doi: 10.3389/fphar.2024.1485054
Received
23 August 2024
Accepted
14 October 2024
Published
07 March 2025
Volume
15 - 2024
Edited and reviewed by
Tea Lanisnik Rizner, University of Ljubljana, Slovenia
Updates
Copyright
© 2025 Yadav, Shah, Tiwari, Javed, Cheng, Aidhen and Bröer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Bröer, Stefan.Broeer@anu.edu.au
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.