Abstract
Background:
Cardiovascular diseases are predominant health conditions across the world due to their rising prevalence and association with several disorders. Inclisiran, a small interfering RNA (siRNA) therapy, lowers low density lipoproteins cholesterol (LDL-C) by targeting proprotein convertase subtilisin/kexin type 9 (PCSK9). Its exact role in cardiovascular diseases is not fully understood.
Aim:
This review examines current usage of Inclisran for cardiovascular diseases.
Method:
A detailed search of Clinicaltrials.gov was conducted to identify relevant studies that investigated heart diseases using Inclisran. Data on study design, sample size, intervention details, and outcomes related to Inclisran were extracted and analyzed.
Results:
As of 30 December 2024, there were 92 clinical trials on involving inclisiran found at clinicaltrials.gov. The investigation focused on studies that used inclisiran for cardiovascular diseases and found that limited clinical trials were identified with limited interventional measures. The final number of analyzed trials was 11. The follow-up duration ranged from 270 to 1,695 days with a total of 214,176 participants with a favorable safety profile and twice-yearly dosing after initial loading dose. The collective findings from these trials demonstrated effective LDL-C and PCSK9 lowering compared to baseline measurements. Most studies focused on LDL-C lowering rather than measuring cardiovascular outcomes.
Conclusion:
Although the studies showed inclisiran to lower LDL-C effectively, the evidence is still limited with regards to cardiovascular outcomes data. There is a need for real world studies addressing long-term safety, adherence and cost-effectiveness and therapeutic outcomes of combination therapy.
Introduction
Cardiovascular disease (CVD) is a health concern globally contributing significantly to illness and death on a scale, including in the Middle East (Lippi and Sanchis-Gomar, 2020; Diez and Butler, 2023). Each year CVD is responsible for an estimated 17.9 million deaths (World Health Organization, 2023). It is linked to conditions such as heart disease, heart attacks, strokes and ischemic heart disease (Nabel, 2003; Rehman et al., 2021). Particularly noteworthy is that heart disease and strokes account for over 80% of all CVD related deaths with a troubling one-third occurring in individuals under 70 years old (Tsao et al., 2022).
Multiple behavioral risk factors play a substantial role in the development of heart disease. Excessive dietary consumption of saturated fat, transaturated fat, salt and sugar can increase risk of obesity, increased blood pressure and high levels of cholesterol. The increased risk of these are considered important causes of cardiovascular disease (Anand et al., 2015; Kazemi et al., 2022). Sedentary lifestyles, which involve minimal physical exercise and lengthy periods of sitting, can impair the heart and circulatory system over time (Leon-Latre et al., 2014; Same et al., 2016). Behavioral risk factors such as smoking and chronic stress can trigger inflammation, alter normal physiology of blood vessels and raise blood pressure (Ambrose and Barua, 2004; Duncan et al., 2019; Franklin et al., 2021). In order to prevent and treat heart disease, it is essential to address these risk factors through behavioral treatments such as stress management, regular exercise, good diet, and quitting smoking.
Recent years have seen the exploration of treatments to combat diseases through innovative therapies such, as monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) and strategies aimed at reducing low density lipoprotein cholesterol (LDL-C) levels effectively (Wong and Shapiro, 2019; Lippi and Sanchis-Gomar, 2020; Diez and Butler, 2023). Lowering LDL-C levels has been shown to bring benefits by decreasing the risk of heart attacks, enhancing function, reducing vessel wall inflammation and stabilizing atherosclerotic plaques (Douglas and Channon, 2014). PCSK9 is an enzyme that acts as a regulator of cholesterol levels by binding to LDL receptors on the surface of hepatocytes (Lagace, 2014; Hajar, 2019; Rimbert et al., 2021). These LDL receptors play an important role in the uptake of LDL-C from the ciruculation. However, the binding of PCSK9 to LDL receptors causes the LDL receptors to degrade and thus less LDL is cleared from the circulation (Lagace, 2014). Consequently, more LDL is accumulated in blood which significantly increases the risk of CVD (Yang et al., 2020). For this reason, PCSK9 is a considered a promising target for lowering LDL-C (Shapiro et al., 2015). A novel strategy for targeting PCSK9 with RNA based therapies has emerged in the past several years (Ickenstein and Garidel, 2019; Gareri et al., 2022). In this scenario inclisiran, a first-in-class small interfering RNA (siRNA) designed to specifically break down messenger RNA (mRNA) responsible, for PCSK9 has shown great potential in treating high cholesterol in patients with a high risk of CV events (Fitzgerald et al., 2014). Reduced PCSK9 levels allows LDL receptors to remain active on hepatocyte surfaces, hence promoting LDL-C removal from the circulation (Wang and Tall, 2017).
Aims
To review and summarize the clinical trials pertaining to the use and effectiveness of inclisiran in cardiovascular diseases and to discuss its potential for long-term cardiovascular risk reduction.
Methodological framework and research design
Search strategy and inclusion criteria
On 30 December 2024, the ClinicalTrials.gov database was searched for clinical trials involving inclisiran to and including 30th December 2024. The keywords for condition/disease were; Cardiovascular diseases, Heart diseases, Coronary artery disease, Major adverse cardiovascular events, MACE, Cardiovascular outcomes, Atherosclerotic Cardiovascular Disease and atherosclerotic plaque. Inclisiran was fixed in the “other terms” search engine. To qualify, Completed and any phase clinical trials that included inclisiran as an intervention.
Data extraction
From the results reported in the ClinicalTrials.gov registry, data were manually retrieved, and analyzed, according to the following elements. Study type: interventional/observational. Conditions: Trial design: All phases. Location: Open to any location. Study status: Completed and no longer looking for participants. A summary of the characteristics of the trials was obtained from the database, including the study title, NCT number, acronym, study status, conditions, interventions, primary outcome measure, study design, masking, phase, duration and number of patients enrolled are presented in Table 1.
TABLE 1
| Serial No | NCT number | Study title | Acronym/study status | Brief summary | Conditions | Interventions |
|---|---|---|---|---|---|---|
| 1 | NCT02597127 | Trial to Evaluate the Effect of ALN-PCSSC treatment on low density lipoprotein cholesterol (LDL-C) | ORION-1/completed with results | This study is a Phase II, placebo-controlled, double-blind, randomized trial in 480 participants with atherosclerotic cardiovascular disease (ASCVD) or ASCVD-risk equivalents and elevated LDL-C despite maximum tolerated dose of LDL-C lowering therapies to evaluate the efficacy, safety, and tolerability of inclisiran injection(s) | Atherosclerotic cardiovascular disease| familial hypercholesterolemia| Diabetes | DRUG: ALN-PCSSC | DRUG: Normal Saline |
| 2 | NCT03060577 | An extension trial of inclisiran in participants with cardiovascular disease and high cholesterol | ORION-3/completed with results | This clinical study was designed to assess the efficacy, safety, and tolerability of long-term dosing of inclisiran and evolocumab given as subcutaneous injections in participants with high cardiovascular risk and elevated low-density lipoprotein cholesterol (LDL-C) | Atherosclerotic cardiovascular Disease| Symptomatic Atherosclerosis| Type2 Diabetes| Familial Hypercholesterolemia | DRUG: inclisiran |DRUG: evolocumab |
| 3 | NCT03814187 | Trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C | ORION-8/Completed with results | The purpose of this extension study was to evaluate the efficacy, safety, and tolerability of long-term dosing of Inclisiran. The study was a global multicenter study | ASCVD| Elevated Cholesterol| Heterozygous Familial Hypercholesterolemia Homozygous Familial Hypercholesterolemia | DRUG: Inclisiran Sodium |
| 4 | NCT03399370 | Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-density Lipoprotein Cholesterol | ORION-10/Completed with results | This is a Phase III, placebo-controlled, double-blind, randomized study in participants with ASCVD and elevated LDL-C despite maximum tolerated dose of LDL-C lowering therapies to evaluate the efficacy, safety, and tolerability of subcutaneous (SC) inclisiran injection(s). The study will be a multicenter study in the United States | ASCVD| Elevated Cholesterol | DRUG: Inclisiran Sodium| DRUG: Placebo |
| 5 | NCT03400800 | Inclisiran for Subjects With ASCVD or ASCVD-Risk Equivalents and Elevated Low-density Lipoprotein Cholesterol | ORION-11/Completed with results | This is a Phase III, placebo-controlled, double-blind, randomized study in participants with ASCVD or ASCVD-Risk equivalents and elevated LDL-C despite maximum tolerated dose of LDL-C lowering therapies to evaluate the efficacy, safety, and tolerability of subcutaneous (SC) inclisiran injection(s). The study will be an international multicenter study (non-United States) | ASCVD| Risk Factor, Cardiovascular| Elevated Cholesterol | DRUG: Inclisiran Sodium| DRUG: Placebo |
| 6 | NCT04666298 | Study of efficacy and safety of inclisiran in japanese participants with high cardiovascular risk and elevated LDL-C | ORION-15/completed with results | This was a placebo-controlled, double-blind, randomized trial in Japanese participants with history of coronary artery disease (CAD) or participants categorized in ‘high risk’ by JAS 2017 guideline, or Japanese participants with heterozygous familial hypercholesterolemia (HeFH) and elevated Low-density lipoprotein cholesterol (LDL-C) despite maximum tolerated dose of statin(s) to evaluate the efficacy, safety, tolerability, and PK of subcutaneous inclisiran injection(s) | Hypercholesterolemia Heterozygous Familial Hypercholesterolemia | DRUG: Inclisiran sodium| DRUG: Placebo |
| 7 | NCT04929249 | A Randomized Study to Evaluate the Effect of an “Inclisiran First” Implementation Strategy Compared to Usual Care in Patients With Atherosclerotic Cardiovascular Disease and Elevated LDL-C Despite Receiving Maximally Tolerated Statin Therapy (VICTORION-INITIATE) | V-INITIATE/Completed with results | The purpose of this study was to assess the effectiveness of an “inclisiran first” implementation strategy (addition of inclisiran to maximally tolerated statin therapy immediately upon failure to achieve acceptable LDL-C with maximally tolerated statin therapy alone) compared to usual care in an atherosclerotic cardiovascular disease (ASCVD) population | Atherosclerotic Cardiovascular Disease | DRUG: Inclisiran |
| 8 | NCT04873934 | Management of LDL-cholesterol With Inclisiran + Usual Care Compared to Usual Care Alone in Participants With a Recent Acute Coronary Syndrome | V-INCEPTION/Completed | The purpose of this study is to study the effectiveness of implementation of a systematic LDL-C management pathway including treatment with inclisiran in participants who have experienced a recent acute coronary syndrome (ACS) and have an increased LDL-cholesterol (>70 mg/dL) despite being treated with a statin drug | Acute Coronary Syndrome | DRUG: Inclisiran |
| 9 | NCT06507852 | A Real-world Study of Inclisiran Adherence, Treatment Patterns, Patient Characteristics, and Effectiveness in ASCVD Patients With Hypercholesterolemia, ASCVD-risk Equivalent Patients With Hypercholesterolemia and Familial Hypercholesterolemia | NA/Completed | This was a descriptive, non-interventional, retrospective cohort study among patients with atherosclerotic cardiovascular disease (ASCVD) and hypercholesterolemia, ASCVD-risk equivalent (ASCVD-RE) or familial hypercholesterolemia (FH) administered inclisiran in a real-world setting in Austria | Atherosclerotic Cardiovascular Disease| Hypercholesterolemia | DRUG: Inclisiran |
| 10 | NCT04807400 | Study in Primary Care Evaluating Inclisiran Delivery Implementation + Enhanced Support | SPIRIT/Completed with results | The purpose of this study was to evaluate the implementation of inclisiran in a regional primary care setting in the UK | Atherosclerotic Cardiovascular Disease| Atherosclerotic Cardiovascular Disease Risk Equivelents| Elevated Low Density Lipoprotein Cholesterol | DRUG: Inclisiran| BEHAVIORAL: Behavioral Support| DRUG: Background lipid lowering therapy |
| 11 | NCT05974345 | In Silico Study Assessing the Impact of Inclisiran on Major Adverse Cardiovascular Events in Patients With Established Cardiovascular Disease | SIRIUS/Completed | Study CKJX839B1FR01 in an In silico trial to predict the efficacy of Inclisiran therapy on major adverse cardiovascular events (MACE) and cardiovascular (CV) death in virtual patients with atherosclerotic cardiovascular disease (ASCVD) and elevated LDL-C | Atherosclerotic Cardiovascular Disease | DRUG: Inclisiran sodium| DRUG: Placebo| DRUG: Ezetimibe| DRUG: Evolocumab |
| Phase | Follow-up duration | Enrollment | Study type | Study design | Locations |
|---|---|---|---|---|---|
| PHASE 2 | 523 days | 501 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: DOUBLE | Primary Purpose: TREATMENT | Multiple, International |
| PHASE2 | 1,695 days | 382 | INTERVENTIONAL | Allocation: NONRANDOMIZED Intervention Model: PARALLEL| Masking: NONE| Primary Purpose: TREATMENT | Multiple, International |
| PHASE3 | 1,399 days | 3,275 | INTERVENTIONAL | Allocation: NA |Intervention Model: SINGLE GROUP| Masking: NONE| Primary Purpose: TREATMENT | Multiple, International |
| PHASE3 | 628 days | 1,561 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: DOUBLE | Primary Purpose: TREATMENT | United States |
| PHASE3 | 637 days | 1,617 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: DOUBLE/Primary Purpose: TREATMENT | Multiple, International |
| PHASE2 | 444 days | 312 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: QUADRUPLE | Primary Purpose: TREATMENT | Japan |
| PHASE3 | 812 days | 450 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: NONE| Primary Purpose: TREATMENT | United States |
| PHASE3 | 330 days | 400 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL |Masking: NONE| Primary Purpose: TREATMENT | United States |
| NA | NA | 95 | OBSERVATIONAL | Observational Model: |Time Perspective: p | Austria |
| PHASE3 | 270 days | 892 | INTERVENTIONAL | Allocation: RANDOMIZED| Intervention Model: PARALLEL| Masking: NONE| Primary Purpose: HEALTH_SERVICES_RESEARCH | United Kingdom |
| NA | NA | 204,691 | OBSERVATIONAL | Observational Model: |Time Perspective: p | France |
Summary of extracted clinical trials characteristics.
ASCVD, atherosclerotic cardiovascular disease; NA, low density lipoprotein-cholesterol; ALN-PCSSC/CKJX839B1FR01, Investigational designation of inclisiran
Results
Number of studies returned by the search
The preliminary search of the ClinicalTrials.gov database for the “condition/disease” keywords and inclisiran the “other terms” field yielded 92 trials. These trials were extraced and manually examined. After screening the 92 trials, 70 studies were discarded that had a study status of active not recruting, not yet recruting and recruting were excluded. The remaining 41 trials were further examined and 22 completed trials were found but 11 duplicates were removed. Finally, this process yielded 11 completed studies that were included in this review. A synopsis of the study selection process is presented in Figure 1.
FIGURE 1
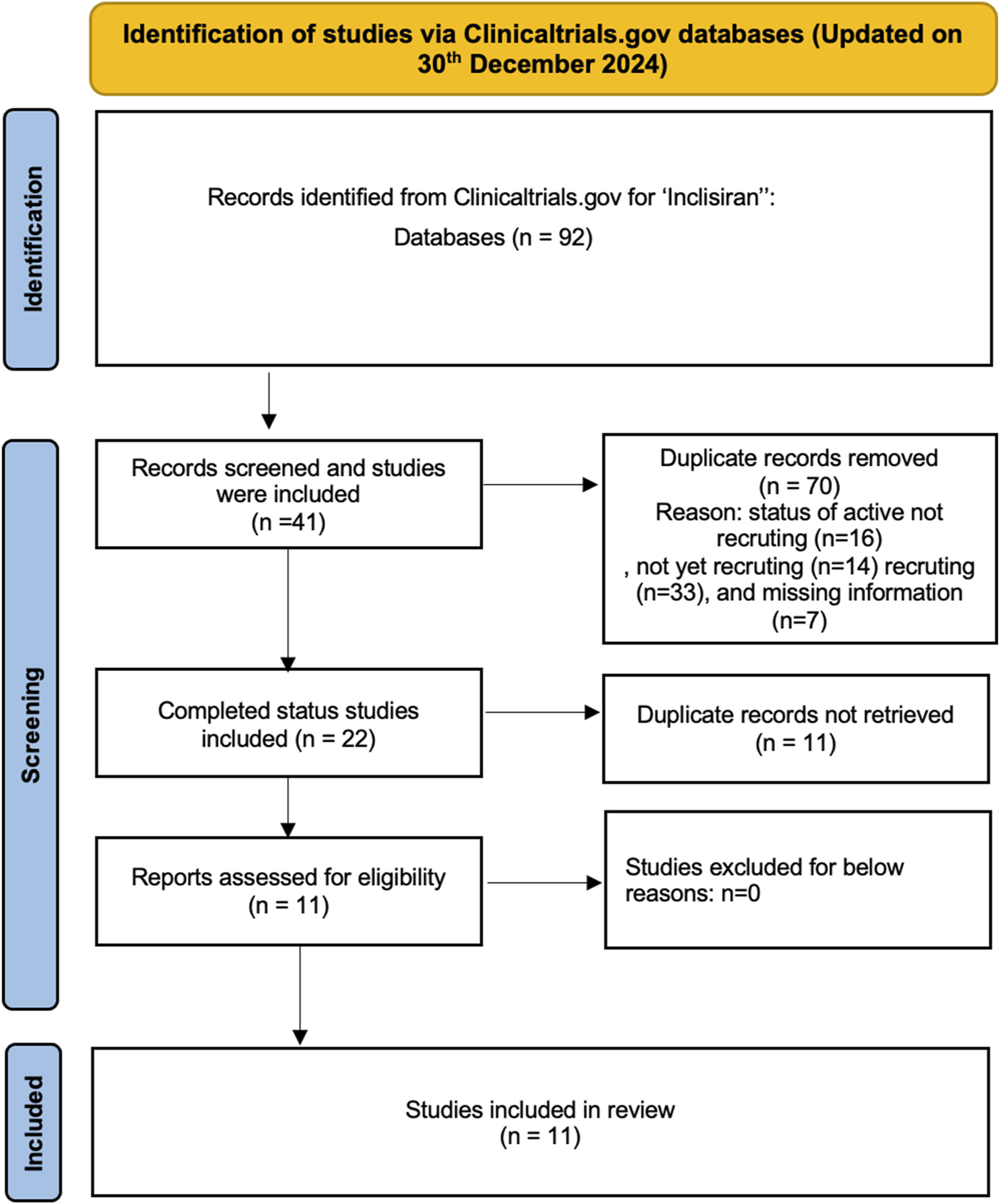
Identification of studies via Clinicaltrials.gov databases.
Characteristics of included studies
The included clinical trials mainly examined the effect of inclisiran in patients with cardiovascular diseases. They included a total of 214,176 participants. Most of the studies included were randomized (n = 8). In terms of masking, four of the studies involved either double or quadruple masking, and the remaining studies did not involve any masking due to the study design. Geographically, four of the studies involved multiple western countries with one study conducted Japan. The full details of the included trials are shown in Table 1.
Discussion
Inclisiran, a first-in-class siRNA-based therapy, represents a novel approach to lipid management by targeting PCSK9 mRNA, enhancing LDL receptor recycling, and providing sustained LDL-C reduction (Do et al., 2013; Fitzgerald et al., 2014). Across pivotal trials, including ORION-9, ORION-10, and ORION-11, inclisiran demonstrated LDL-C reductions of approximately 50%, with effects maintained over extended periods (Ray et al., 2023). These findings highlight its role in addressing unmet needs for hypercholesterolemia management in diverse patient populations, particularly those with atherosclerotic cardiovascular disease (ASCVD) and familial hypercholesterolemia.
Regulatory status and global adoption
Inclisiran has achieved regulatory approval in multiple countries, reflecting its growing acceptance as a novel lipid-lowering therapy. Initially approved by the European Medicines Agency (EMA) in December 2020, inclisiran is indicated for the treatment of hypercholesterolemia and mixed dyslipidemia in adults who require additional LDL-C lowering despite maximally tolerated statin therapy (Leqvio, 2024). Shortly thereafter, the U.S. Food and Drug Administration (FDA) approved inclisiran in December 2021 as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or ASCVD who require additional lowering of LDL-C (Administration, 2021). Regulatory approvals have also been granted in other regions, including Japan, Canada, and Australia, further expanding its global reach.
Practical advantages, safety, and adherence
Inclisiran offers significant practical advantages, due in large part to its unique biannual dosing regimen. Administered subcutaneously by healthcare professionals twice yearly following an initial loading dose, inclisiran reduces the treatment burden associated with daily or biweekly dosing (Ray et al., 2020). This dosing regimen aligns with routine clinical visits, thus facilitating regular dosing and significantly improving adherence compared with self-administered therapies such as PCSK9 monoclonal antibodies (Soffer et al., 2022). The SPIRIT trial provided evidence that the integration of inclisiran in primary care improves patient adherence and eases treatment for both patients and healthcare providers (Wilson et al., 2023). Additionally, the targeted mechanism of action of inclisiran limits systemic exposure, thereby improving its safety profile (Wright et al., 2023). Clinical trials consistently show that the most frequent adverse events reported are injection site reactions, which are generally mild and transient (Wright et al., 2021). Longer trials, such as ORION-3 and ORION-8, have confirmed this good safety profile without any significant increase in serious adverse events (Author Anonymous, 2019; Ray et al., 2023). These characteristics make inclisiran especially useful in high-risk populations, such as those with challenges to adherence or who have limited access to health care services (Wright et al., 2023). However, the extended effect may result in persistent unpleasant reactions that are difficult to reverse, as the drug’s activity remains in the absence of early intervention. This feature demands cautious consideration while providing inclisiran, particularly in patients who may be at increased risk of side effects (Mercep et al., 2022).
Combination use with statins for statin-intolerant patients
Inclisiran complements the existing lipid-lowering therapies, particularly when used in combination with statins. Statins remain a cornerstone in the management of LDL-C levels, with an established history of effectiveness in reducing cardiovascular events. However, not all patients with statin treatment achieve target levels of LDL-C; some are also intolerant, with adverse effects such as muscle symptoms (Serban et al., 2017). Inclisiran closes these gaps effectively, offering a further reduction in LDL-C by almost 50% when used in combination with statins or other treatments as ezetimibe (Ray et al., 2020). Inclisiran is also a potential option for those who cannot tolerate statins, either as monotherapy or in combination with ezetimibe, to achieve substantial reductions in LDL-C levels without the systemic side effects generally associated with statin therapy (Writing et al., 2022; Wright et al., 2023). Real-world settings have confirmed that inclisiran, given biannually, provides consistent LDL-C lowering and adherence, including in high-risk populations (Writing et al., 2022; Naoum et al., 2024). This is of special value for those patients who have trouble following the dosing regimens of other therapies that are taken more frequently. Ongoing studies assessing the combination of inclisiran with various lipid-lowering agents will further define its place in the management of hypercholesterolemia in a variety of patient populations (Wright et al., 2023).
Comparison with Anti-PCSK9 monoclonal antibodies
Both the PCSK9 monoclonal antibodies (e.g., evolocumab and alirocumab), and inclisiran target the same PCSK9 pathway but but differ significantly in their mechanisms of action, administration schedules, and clinical implications. Inclisiran is an RNA interference technology that silences PCSK9 mRNA (Khvorova, 2017). This mechanism allows twice yearly dosing following an initial loading dose, in contrast to the biweekly or monthly dosing required for monoclonal antibodies directly binding circulating PCSK9 proteins (Chan et al., 2009; Scicchitano et al., 2021). When combined with maximally tolerated statins, self-administered biweekly or monthly anti-PCSK9 monoclonal antibodies reduce LDL-C by approximately 60%, while twice-yearly inclisiran (administered by a healthcare professional) reduces LDL-C by ≈ 50% (Sabatine, 2019; Raal et al., 2020; Ray et al., 2020). Long-term data from ORION-3 confirmed LDL-C reductions were sustained over 4 years (Sabatine, 2019; Raal et al., 2020; Ray et al., 2020; Ray et al., 2023). Both classes show a minor reduction in lipoprotein(a) and triglycerides, but inclisiran shows a modest increase in high-density lipoprotein cholesterol (HDL) (Sabatine, 2019; Raal et al., 2020; Ray et al., 2020). Monoclonal antibodies will be more optimal in cases where a fast reduction of LDL-C levels is required because the effect of them begins immediately after administration. Both drugs are usually well tolerated, but inclisiran is delivered directly, so the possibility of systemic side effects is reduced. All these differences make inclisiran a potential additional or alternative treatment option, especially for patients with indications for long-term, easy control of cholesterol levels.
Recent studies and real-world evidence
Clinical trials, including the Phase 3 program and related exploratory analyses, have shown compelling evidence supporting inclisiran’s efficacy and safety. However, crucial concerns remain unsolved, notably about the long-term effects on atherosclerosis and cardiovascular outcomes (Wilkinson et al., 2024). Furthermore, the controlled design of these trials does not fully simulate the complexity encountered in daily clinical practice. Notably, the majority of trial participants were White males, indicating a lack of diversity in the study group (Wright et al., 2021). More research is needed to acquire a better knowledge of inclisiran’s long-term effects, cardiovascular benefits, and safety in varied racial, ethnic, and socioeconomic groups.
Several completed and ongoing studies are evaluating the efficacy, safety, and real-world application of inclisiran across various patient populations. ORION-13 and ORION-16 are phase 3 trials assessing inclisiran in adolescents with homozygous familial hypercholesterolemia (HoFH) and HeFH, respectively (Author Anonymous, 2020b; Author Anonymous, 2020a). The VICTORION-PLAQUE trial is studying inclisiran’s impact on atherosclerotic plaque progression (Author Anonymous, 2022). Real-world implementation trials, including VICTORION-INITIATE and VICTORION-INCEPTION, aim to assess inclisiran’s integration earlier in treatment pathways compared to usual care in patients with ASCVD who had not achieved LDL-C levels below 70 mg/dL despite maximally tolerated statin therapy and patients at high risk for a recurrent cardiovascular event in the first year following acute coronary syndrome (ACS), respectively (Knowlton, 2024; Koren et al., 2024). Early real-world studies from the UK and US indicate significant LDL-C reductions (∼49–56%) and favorable tolerability, mirroring clinical trial results, though further observational data are anticipated to confirm these findings in broader clinical settings (Padam et al., 2022; Chiou et al., 2023).
Cardiovascular benefits
Cardiovascular outcomes trials in patients with established ASCVD have reported a 15%–20% lower risk of major adverse cardiovascular events (MACE) for anti-PCSK9 monoclonal antibodies (Sabatine, 2019). In an exploratory analysis of 3,655 patients (ORION-9, ORION-10, and ORION-11), the addition of inclisiran was linked with a 26% decreased probability of MACE and a lower risk of fatal and nonfatal myocardial infarction compared to placebo. However, these trials were not designed to directly assess MACE as primary endpoints. Additionally, the SIRIUS trial uses computational models to extrapolate the observed LDL-C reductions achieved with inclisiran to predict its potential impact on MACE, offering insights into its long-term cardiovascular benefits in high-risk populations (Angoulvant et al., 2024). Ongoing studies, such as ORION-4, VICTORION-2 PREVENT, and VICTORION-1 PREVENT, are addressing this gap by evaluating inclisiran’s long-term effects on cardiovascular outcomes, including myocardial infarction, stroke, and cardiovascular mortality, in high-risk populations (Novartis, 2018; Author Anonymous, 2021). Despite these promising projections, real-world data and longer-term follow-up are essential to validate these findings and provide conclusive evidence. These studies will also help identify patient subgroups that might derive the greatest benefit. By bridging this evidence gap, ongoing and future trials will solidify inclisiran’s role not just as a lipid-lowering agent but as a critical component of comprehensive cardiovascular risk reduction strategies.
Cost-effectiveness and economic considerations
The cost-effectiveness of inclisiran remains a critical consideration in its broader adoption across diverse healthcare systems. Its high initial cost presents issues, especially in resource-constrained contexts where affordability and accessibility are crucial (Stoekenbroek et al., 2018). In recent years, there have been various cost-effectiveness studies conducted on inclisiran. The current state of its cost-effectiveness varies based on the geographic region and the price criteria. In the US market it may be considered cost-effective at certain price points, while in China, significant price reductions would be necessary to achieve cost-effectiveness (Desai et al., 2022; Zhou et al., 2024). European healthcare systems have indeed demonstrated positive outcomes for the addition of inclisiran to standard care in the secondary prevention of ASCVD increases life expectancy by 0.199 years and provides an additional 0.159 QALYs (Galactionova et al., 2022; Deaney et al., 2024). As more real-world data becomes available and pricing strategies evolve, the cost-effectiveness profile of inclisiran may continue to change.
Implications for special populations
Inclisiran has a number of advantages in several special populations due to its efficacy, safety, and very convenient dosing schedule. No dose adjustment is required for patients with mild, moderate, or severe renal impairment, as was shown in the ORION-7 study (Wright et al., 2020). Similarly, no dose adjustment is necessary for the geriatric population or patients with mild to moderate hepatic impairment, though it has not been evaluated in patients with severe hepatic impairment (Dec et al., 2023). Inclisiran reduces LDL cholesterol in patients with HeFH and appears to be promising in rare HoFH, as seen in ORION-2 (Hovingh et al., 2020). In addition, the drug is being evaluated for use in adolescents with hypercholesterolemia; trials are currently ongoing in studies ORION-13 and ORION-16 (Author Anonymous, 2020b; Author Anonymous, 2020a). It has also shown impressive LDL-C reductions in both diabetic and nondiabetic patients (Leiter et al., 2019). Because of the long-acting effect of the drug, administered every 6 months, inclisiran is of particular benefit in such patients who need long-term management of LDL-C with no treatment burden (Ray et al., 2023). These features are advantages and present the promising versatile option of treating hypercholesterolemia across various populations.
Study limitations and future directions
Despite its promising profile, inclisiran’s current evidence base has limitations. The lack of direct cardiovascular outcome data necessitates reliance on surrogate endpoints such as LDL-C reduction. Real-world studies addressing long-term safety, adherence, and cost-effectiveness in diverse populations are crucial to validate its broad applicability. Additionally, exploring combination therapy with other lipid-lowering agents could optimize cardiovascular outcomes (Raal et al., 2020). Future research should also focus on expanding evidence in underserved populations, including those in low-resource settings, to ensure inclisiran’s benefits are accessible to all. These efforts will refine inclisiran’s clinical utility and solidify its role in the global management of cardiovascular disease.
Conclusion
The results and outcomes from the clinical trials justify its inclusion into clinical guidelines. The sustained LDL-C lowering and favorable safety profile set inclisiran to become a valuable addition to patient groups with high cardiovascular risk. Its unique mechanism of action and dosing frequency has the potential to change hypercholesterolemia therapy, especially for patients who are statin intolerant or have difficulty achieving LDL-C reduction with current therapy. Its implementation might result in a shift in the approach to controlling cardiovascular risk, particularly in high-risk groups. The potential public health effect of inclisiran is significant. Inclisiran can contribute to lowering the total burden of cardiovascular disease by offering a dependable and patient-friendly choice for LDL-C reduction. This can lead to fewer cardiovascular events and hospitalizations, hence improving public health outcomes and lowering healthcare expenditures.
Statements
Author contributions
MH: Conceptualization, Methodology, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Administration (2021). LEQVIO® (inclisiran) injection, for subcutaneous use. U.S. Food and Drug Administration. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214012lbl.pdf (Accessed January 09, 2025).
2
Ambrose J. A. Barua R. S. (2004). The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol.43 (10), 1731–1737. 10.1016/j.jacc.2003.12.047
3
Author Anonymous (2022). A multi-center, randomized, double-blind, placebo-controlled, parallel-group phase IIIb study evaluating the effect of inclisiran on atherosclerotic plaque progression assessed by coronary computed tomography angiography (CCTA) in participants with a diagnosis of non-obstructive coronary artery disease without previous cardiovascular events. VICTORION-PLAQUE. Available at: https://clinicaltrials.gov/study/NCT05360446 (Accessed December 15, 2024).
4
Author Anonymous (2023). A double-blind, randomized, placebo- and active-comparator controlled study to evaluate the efficacy of inclisiran as monotherapy in patients with primary hypercholesterolemia not receiving lipid-lowering therapy VictORION-Mono. Available at: https://clinicaltrials.gov/study/NCT05763875 (Accessed December 15, 2024).
5
Anand S. S. Hawkes C. de Souza R. J. Mente A. Dehghan M. Nugent R. et al (2015). Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the world heart federation. J. Am. Coll. Cardiol.66 (14), 1590–1614. 10.1016/j.jacc.2015.07.050
6
Angoulvant D. Granjeon-Noriot S. Amarenco P. Bastien A. Bechet E. Boccara F. et al (2024). In-silico trial emulation to predict the cardiovascular protection of new lipid-lowering drugs: an illustration through the design of the SIRIUS programme. Eur. J. Prev. Cardiol.31 (15), 1820–1830. 10.1093/eurjpc/zwae254
7
Author Anonymous (2019). An open-label extension trial of the phase III lipid-lowering trials to assess the effect of long term dosing of inclisiran given as subcutaneous injections in subjects with high cardiovascular risk and elevated LDL-C. Available at: https://clinicaltrials.gov/study/NCT03814187 (Accessed December 15, 2024).
8
Author Anonymous (2020a). “Two part (Double-blind inclisiran versus placebo [year 1] followed by open-label inclisiran [year 2]) randomized multicenter study to evaluate safety, tolerability, and efficacy of inclisiran in adolescents (12 to less than 18 Years) with heterozygous familial hypercholesterolemia and elevated LDL-cholesterol (ORION-16)”. Available at: https://clinicaltrials.gov/study/NCT04652726 (Accessed December 15, 2024).
9
Author Anonymous (2020b). “Two part (Double-blind inclisiran versus placebo [year 1] followed by open-label inclisiran [year 2]) randomized multicenter study to evaluate safety, tolerability, and efficacy of inclisiran in adolescents (12 to less than 18 Years) with homozygous familial hypercholesterolemia and elevated LDL-cholesterol (ORION-13)”. Available at: https://clinicaltrials.gov/study/NCT04659863 (Accessed December 16, 2024).
10
Author Anonymous (2021). A randomized, double-blind, placebo-controlled, multicenter trial, assessing the impact of inclisiran on major adverse cardiovascular events in participants with established cardiovascular disease. VICTORION-2 PREVENT. Available at: https://clinicaltrials.gov/study/NCT05030428 (Accessed December 15, 2024).
11
Chan J. C. Piper D. E. Cao Q. Liu D. King C. Wang W. et al (2009). A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. U. S. A.106 (24), 9820–9825. 10.1073/pnas.0903849106
12
Chiou T. T. Tomasi K. Taub P. R. Wilkinson M. J. (2023). One year experience with inclisiran in an academic lipid clinic. Am. J. Prev. Cardiol.15, 100567. 10.1016/j.ajpc.2023.100567
13
Deaney C. N. Donaldson M. P. Reesby D. M. Scott V. L. Ellis V. Cole G. E. et al (2024). Retrospective evaluation of LDL-C levels following first treatment with inclisiran as part of secondary prevention ASCVD risk reduction in a real-world primary care setting. J. Prim. Care and Community Health15, 21501319241236339. 10.1177/21501319241236339
14
Dec A. Niemiec A. Wojciechowska E. Maliglowka M. Buldak L. Boldys A. et al (2023). Inclisiran-A revolutionary addition to a cholesterol-lowering therapy. Int. J. Mol. Sci.24 (7), 6858. 10.3390/ijms24076858
15
Desai N. R. Campbell C. Electricwala B. Petrou M. Trueman D. Woodcock F. et al (2022). Cost effectiveness of inclisiran in atherosclerotic cardiovascular patients with elevated low-density lipoprotein cholesterol despite statin use: a threshold analysis. Am. J. Cardiovasc Drugs22 (5), 545–556. 10.1007/s40256-022-00534-9
16
Diez J. Butler J. (2023). Growing heart failure burden of hypertensive heart disease: a call to action. Hypertension80 (1), 13–21. 10.1161/HYPERTENSIONAHA.122.19373
17
Do R. Q. Vogel R. A. Schwartz G. G. (2013). PCSK9 Inhibitors: potential in cardiovascular therapeutics. Curr. Cardiol. Rep.15 (3), 345. 10.1007/s11886-012-0345-z
18
Douglas G. Channon K. M. (2014). The pathogenesis of atherosclerosis. Medicine42 (9), 480–484. 10.1016/j.mpmed.2014.06.011
19
Duncan M. S. Freiberg M. S. Greevy R. A. Jr. Kundu S. Vasan R. S. Tindle H. A. (2019). Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA322 (7), 642–650. 10.1001/jama.2019.10298
20
Fitzgerald K. Frank-Kamenetsky M. Shulga-Morskaya S. Liebow A. Bettencourt B. R. Sutherland J. E. et al (2014). Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet383 (9911), 60–68. 10.1016/S0140-6736(13)61914-5
21
Franklin B. A. Rusia A. Haskin-Popp C. Tawney A. (2021). Chronic stress, exercise and cardiovascular disease: placing the benefits and risks of physical activity into perspective. Int. J. Environ. Res. Public Health18 (18), 9922. 10.3390/ijerph18189922
22
Galactionova K. Salari P. Mattli R. Rachamin Y. Meier R. Schwenkglenks M. (2022). Cost-effectiveness, burden of disease and budget impact of inclisiran: dynamic cohort modelling of a real-world population with cardiovascular disease. Pharmacoeconomics40 (8), 791–806. 10.1007/s40273-022-01152-8
23
Gareri C. Polimeni A. Giordano S. Tamme L. Curcio A. Indolfi C. (2022). Antisense oligonucleotides and small interfering RNA for the treatment of dyslipidemias. J. Clin. Med.11 (13), 3884. 10.3390/jcm11133884
24
Hajar R. (2019). PCSK 9 inhibitors: a short history and a new era of lipid-lowering therapy. Heart Views20 (2), 74–75. 10.4103/HEARTVIEWS.HEARTVIEWS_59_19
25
Hovingh G. K. Lepor N. E. Kallend D. Stoekenbroek R. M. Wijngaard P. L. J. Raal F. J. (2020). Inclisiran durably lowers low-density lipoprotein cholesterol and proprotein convertase subtilisin/kexin type 9 expression in homozygous familial hypercholesterolemia: the ORION-2 pilot study. Circulation141 (22), 1829–1831. 10.1161/CIRCULATIONAHA.119.044431
26
Ickenstein L. M. Garidel P. (2019). Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv.16 (11), 1205–1226. 10.1080/17425247.2019.1669558
27
Kazemi A. Sasani N. Mokhtari Z. Keshtkar A. Babajafari S. Poustchi H. et al (2022). Comparing the risk of cardiovascular diseases and all-cause mortality in four lifestyles with a combination of high/low physical activity and healthy/unhealthy diet: a prospective cohort study. Int. J. Behav. Nutr. Phys. Act.19 (1), 138. 10.1186/s12966-022-01374-1
28
Khvorova A. (2017). Oligonucleotide therapeutics - a new class of cholesterol-lowering drugs. N. Engl. J. Med.376 (1), 4–7. 10.1056/NEJMp1614154
29
Knowlton K. U. (2024). Baseline characteristics of participants enrolled in victorion-inception: a randomized study of inclisiran vs. Usual care in patients with recent hospitalization for an acute coronary syndrome. Am. J. Prev. Cardiol.19, 100756. 10.1016/j.ajpc.2024.100756
30
Koren M. J. Rodriguez F. East C. Toth P. P. Watwe V. Abbas C. A. et al (2024). An “inclisiran first” strategy vs usual care in patients with atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol.83 (20), 1939–1952. 10.1016/j.jacc.2024.03.382
31
Lagace T. A. (2014). PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol.25 (5), 387–393. 10.1097/MOL.0000000000000114
32
Leiter L. A. Teoh H. Kallend D. Wright R. S. Landmesser U. Wijngaard P. L. J. et al (2019). Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial. Diabetes Care42 (1), 173–176. 10.2337/dc18-1491
33
Leon-Latre M. Moreno-Franco B. Andres-Esteban E. M. Ledesma M. Laclaustra M. Alcalde V. et al (2014). Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Rev. Esp. Cardiol. Engl. Ed.67 (6), 449–455. 10.1016/j.rec.2013.10.015
34
Leqvio (2024). European Medicines agency (EMA). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/leqvio (Accessed December 28, 2024).
35
Lippi G. Sanchis-Gomar F. (2020). Global epidemiology and future trends of heart failure. AME Med. J.5, 15. 10.21037/amj.2020.03.03
36
Mercep I. Friscic N. Strikic D. Reiner Z. (2022). Advantages and disadvantages of inclisiran: a small interfering ribonucleic acid molecule targeting PCSK9-A narrative review. Cardiovasc Ther.2022, 8129513. 10.1155/2022/8129513
37
Nabel E. G. (2003). Cardiovascular disease. N. Engl. J. Med.349 (1), 60–72. 10.1056/NEJMra035098
38
Naoum I. Saliba W. Aker A. Zafrir B. (2024). Lipid-lowering therapy with inclisiran in the real-world setting: initial data from a national health care service. J. Clin. Lipidol.18 (5), e809–e816. 10.1016/j.jacl.2024.05.003
39
Novartis P. (2018). HPS-4/TIMI 65/ORION-4: a double-blind randomized placebo-controlled trial assessing the effects of inclisiran on clinical outcomes among people with atherosclerotic cardiovascular disease. Available at: https://clinicaltrials.gov/study/NCT03705234 (Accessed December 30, 2024).
40
Padam P. Barton L. Wilson S. David A. Walji S. de Lorenzo F. et al (2022). Lipid lowering with inclisiran: a real-world single-centre experience. Open Heart9 (2), e002184. 10.1136/openhrt-2022-002184
41
Raal F. J. Kallend D. Ray K. K. Turner T. Koenig W. Wright R. S. et al (2020). Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med.382 (16), 1520–1530. 10.1056/NEJMoa1913805
42
Ray K. K. Troquay R. P. T. Visseren F. L. J. Leiter L. A. Scott Wright R. Vikarunnessa S. et al (2023). Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol.11 (2), 109–119. 10.1016/S2213-8587(22)00353-9
43
Ray K. K. Wright R. S. Kallend D. Koenig W. Leiter L. A. Raal F. J. et al (2020). Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med.382 (16), 1507–1519. 10.1056/NEJMoa1912387
44
Rehman S. Rehman E. Ikram M. Jianglin Z. (2021). Cardiovascular disease (CVD): assessment, prediction and policy implications. BMC Public Health21 (1), 1299. 10.1186/s12889-021-11334-2
45
Rimbert A. Smati S. Dijk W. Le May C. Cariou B. (2021). Genetic inhibition of PCSK9 and liver function. JAMA Cardiol.6 (3), 353–354. 10.1001/jamacardio.2020.5341
46
Sabatine M. S. (2019). PCSK9 inhibitors: clinical evidence and implementation. Nat. Rev. Cardiol.16 (3), 155–165. 10.1038/s41569-018-0107-8
47
Same R. V. Feldman D. I. Shah N. Martin S. S. Al Rifai M. Blaha M. J. et al (2016). Relationship between sedentary behavior and cardiovascular risk. Curr. Cardiol. Rep.18 (1), 6. 10.1007/s11886-015-0678-5
48
Scicchitano P. Milo M. Mallamaci R. De Palo M. Caldarola P. Massari F. et al (2021). Inclisiran in lipid management: a Literature overview and future perspectives. Biomed. Pharmacother.143, 112227. 10.1016/j.biopha.2021.112227
49
Serban M. C. Colantonio L. D. Manthripragada A. D. Monda K. L. Bittner V. A. Banach M. et al (2017). Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J. Am. Coll. Cardiol.69 (11), 1386–1395. 10.1016/j.jacc.2016.12.036
50
Shapiro M. D. Fazio S. Tavori H. (2015). Targeting PCSK9 for therapeutic gains. Curr. Atheroscler. Rep.17 (4), 499. 10.1007/s11883-015-0499-4
51
Soffer D. Stoekenbroek R. Plakogiannis R. (2022). Small interfering ribonucleic acid for cholesterol lowering - inclisiran: Inclisiran for cholesterol lowering. J. Clin. Lipidol.16 (5), 574–582. 10.1016/j.jacl.2022.06.009
52
Stoekenbroek R. M. Kallend D. Wijngaard P. L. Kastelein J. J. (2018). Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol.14 (6), 433–442. 10.2217/fca-2018-0067
53
Tsao C. W. Aday A. W. Almarzooq Z. I. Alonso A. Beaton A. Z. Bittencourt M. S. et al (2022). Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation145 (8), e153–e639. 10.1161/CIR.0000000000001052
54
Wang N. Tall A. R. (2017). A new approach to PCSK9 therapeutics. Circ. Res.120 (7), 1063–1065. 10.1161/CIRCRESAHA.117.310610
55
Wilkinson M. J. Bajaj A. Brousseau M. E. Taub P. R. (2024). Harnessing RNA interference for cholesterol lowering: the bench-to-bedside story of inclisiran. J. Am. Heart Assoc.13 (6), e032031. 10.1161/JAHA.123.032031
56
Wilson P. M. Dixon S. Vell T. Bower P. Rootkin L. Williams C. et al (2023). Implementation of inclisiran in United Kingdom primary care for patients with atherosclerotic cardiovascular disease or its-risk equivalents: rationale and design of Victorion-spirit, a pragmatic phase IIIb, randomised controlled study. Int. J. Clin. Trials10 (2), 156–165. 10.18203/2349-3259.ijct20231106
57
Wong N. D. Shapiro M. D. (2019). Interpreting the findings from the recent PCSK9 monoclonal antibody cardiovascular outcomes trials. Front. Cardiovasc Med.6, 14. 10.3389/fcvm.2019.00014
58
World Health Organization (2023). Cardiovascular diseases. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (Accessed December 15, 2024).
59
Wright R. S. Collins M. G. Stoekenbroek R. M. Robson R. Wijngaard P. L. J. Landmesser U. et al (2020). Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin. Proc.95 (1), 77–89. 10.1016/j.mayocp.2019.08.021
60
Wright R. S. Koenig W. Landmesser U. Leiter L. A. Raal F. J. Schwartz G. G. et al (2023). Safety and tolerability of inclisiran for treatment of hypercholesterolemia in 7 clinical trials. J. Am. Coll. Cardiol.82 (24), 2251–2261. 10.1016/j.jacc.2023.10.007
61
Wright R. S. Ray K. K. Raal F. J. Kallend D. G. Jaros M. Koenig W. et al (2021). Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J. Am. Coll. Cardiol.77 (9), 1182–1193. 10.1016/j.jacc.2020.12.058
62
Writing C. Lloyd-Jones D. M. Morris P. B. Ballantyne C. M. Birtcher K. K. Covington A. M. et al (2022). 2022 acc expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology solution set oversight committee. J. Am. Coll. Cardiol.80 (14), 1366–1418. 10.1016/j.jacc.2022.07.006
63
Yang H. X. Zhang M. Long S. Y. Tuo Q. H. Tian Y. Chen J. X. et al (2020). Cholesterol in LDL receptor recycling and degradation. Clin. Chim. Acta500, 81–86. 10.1016/j.cca.2019.09.022
64
Zhou W. Liang Z. Lou X. Wang N. Liu X. Li R. et al (2024). The combination use of inclisiran and statins versus statins alone in the treatment of dyslipidemia in mainland China: a cost-effectiveness analysis. Front. Pharmacol.15, 1283922. 10.3389/fphar.2024.1283922
Summary
Keywords
cardiovascular diseases, low density lipoprotein, inclisiran, clinical trials, pcsk9, atherosclerotic cardiovascular disease, coronary artery disease
Citation
Harbi MH (2025) Current usage of inclisiran for cardiovascular diseases: overview of current clinical trials. Front. Pharmacol. 16:1449712. doi: 10.3389/fphar.2025.1449712
Received
15 June 2024
Accepted
20 January 2025
Published
14 February 2025
Volume
16 - 2025
Edited by
Dawn Katrina Coletta, University of Arizona, United States
Reviewed by
Valentina Vellecco, University of Naples Federico II, Italy
Željko Reiner, University Hospital Centre Zagreb, Croatia
Updates
Copyright
© 2025 Harbi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maan H. Harbi, mhiharbi@uqu.edu.sa
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.