Abstract
Bone infection remains a challenging condition to fully eradicate due to its intricate nature. Traditional treatment strategies, involving long-term and high-dose systemic antibiotic administration, often encounter difficulties in achieving therapeutic drug concentrations locally and may lead to antibiotic resistance. Bone cement, serving as a local drug delivery matrix, has emerged as an effective anti-infective approach validated in clinical settings. Calcium phosphate cements (CPCs) have garnered widespread attention and application in the local management of bone infections due to their injectable properties, biocompatibility, and degradability. The interconnected porous structure of calcium phosphate particles, not only promotes osteoconductivity and osteoinductivity, but also serves as an ideal carrier for antibacterial agents. Various antimicrobial agents, including polymeric compounds, antibiotics, antimicrobial peptides, therapeutic inorganic ions (TIIs) (and their nanoparticles), graphene, and iodine, have been integrated into CPC matrices in numerous studies aimed at treating bone infections in diverse applications such as defect filling, preparation of metal implant surface coatings, and coating of implant surfaces. Additionally, for bone defects and nonunions resulting from chronic bone infections, the utilization of calcium phosphate-calcium sulfate composite multifunctional cement loaded with antibacterial agents serves to efficiently deal with infection, stimulate new bone formation, and attain an optimal degradation rate of the bone cement matrix. This review briefly delves into various antibacterial strategies based on calcium phosphate cement for the prevention and treatment of bone infections, while also discussing the application of calcium phosphate-calcium sulfate composites in the development of multifunctional bone cement against bone infections.
1 Introduction
Bone infection, an inflammatory disease resulting from pyogenic bacterial infection, leads to osteolysis and necrosis. Its primary causes include trauma, orthopedic surgeries, joint replacements, and the dissemination of diabetic foot infection (DFI) lesions. Hematogenous diseases have also been implicated as a source of bone infection (Zhong et al., 2023; Kavanagh et al., 2018; Senneville et al., 2020). This condition is associated with high recurrence and disability rates, prolonged hospital stays, and a significant economic burden for patients (Kavanagh et al., 2018; Vallet-Regí et al., 2020; Schade et al., 2021). Staphylococcus aureus (S. aureus) infection is particularly prevalent, with approximately 30% of cases involving Methicillin-resistant S. aureus (MRSA) (Zhong et al., 2023; Nandi et al., 2016). The treatment of bone infection poses a significant challenge for both patients and orthopedic surgeons (Zhong et al., 2023; Geurts et al., 2021; McNally et al., 2020).
1.1 Analysis of the challenges associated with the treatment of bone infections
S. aureus stands as the foremost etiological agent responsible for bone infections, exhibiting the highest pathogenicity among microbial pathogens. The genesis of S. aureus infection is attributed to the emergence of drug-resistant bacterial strains and their evasion of host immune surveillance. The recalcitrant nature of osteomyelitis instigated by S. aureus primarily arises from intracellular infection within the host, invasion of osteocyte lacuno-canicular network (OLCN), biofilm formation, and the development of staphylococcal abscesses (Masters et al., 2022). (Figure 1) Concurrently, diabetic foot osteomyelitis (DFO) and soft tissue infection, recognized as significant complications of diabetes, pose formidable treatment challenges and impart a considerable burden on both public health systems and individual patients (Leone et al., 2020; Senneville et al., 2024; Rubitschung et al., 2021).
FIGURE 1
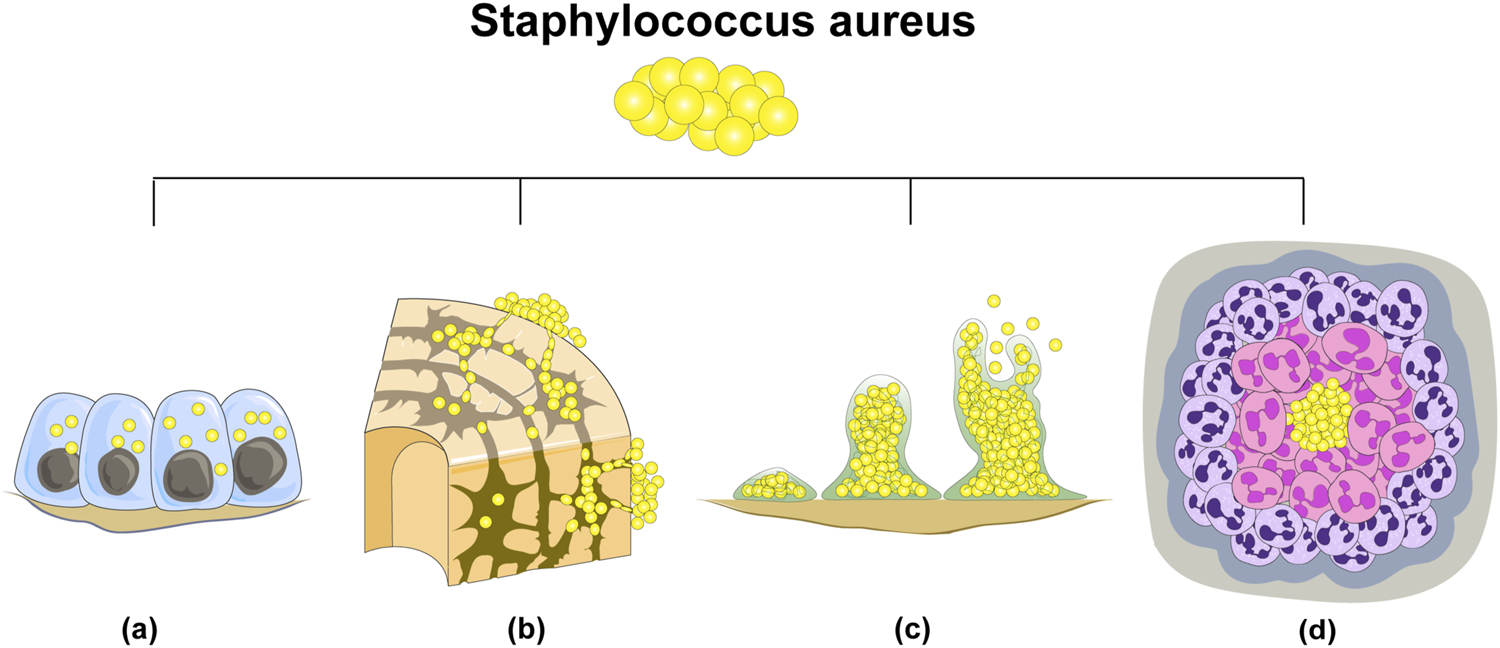
There are four principal reasons underlying the difficulty in eradicating S. aureus in bone infections: (A) intracellular bacterial colonization; (B) invasion of osteocyte lacuno-canicular network (OLCN); (C) biofilm formation; (D) abscess formation.
1.1.1 Intracellular bacterial colonization
Extensive research has demonstrated that S. aureus is capable of persisting within a diverse range of cellular environments, including macrophages, osteoblasts, osteoclasts, osteocytes, and fibroblasts, for prolonged durations (Masters et al., 2022). Macrophages harboring intracellular bacterial invasion are referred to as “Trojan horse” macrophages, facilitating the accumulation of small bacterial colony variants (Garzoni and Kelley, 2011). This intracellular residency of S. aureus accelerates osteoblast apoptosis and further augments osteoclast activity. Additionally, when pathogenic bacteria colonize bone cells, they trigger the secretion of inflammatory factors that induce osteoclasts, ultimately leading to pathological bone loss (Tong et al., 2022; Yoshimoto et al., 2022). Furthermore, S. aureus colonization within osteoblasts promotes their differentiation and maturation into osteocytes, serving as a vehicle for long-term immune evasion within the host (Alder et al., 2020; Watkins and Unnikrishnan, 2020).
1.1.2 OLCN invasion
The invasion of OLCN by S. aureus represents a novel mechanism of bacterial persistence and immune evasion in chronic osteomyelitis (Masters et al., 2021). Traditionally, S. aureus was considered a non-motile bacterium. However, recent advancements in research have established that S. aureus is capable of invading and residing within OLCN for extended durations (Yu et al., 2020). Notably, it has been demonstrated that the bacterium can successfully traverse the narrow spaces within the tubules by altering its shape (Jensen et al., 2023). In vitro investigations have revealed that the cell wall transpeptidase-penicillin-binding protein 4 (PBP4) and surface adhesin-S. aureus surface protein C (SasC) play pivotal roles in S. aureus’s ability to deform and replicate during its traversal through the tubules (Masters et al., 2021).
1.1.3 Biofilm formation
The intricate mechanism underlying biofilm formation during osteomyelitis has garnered significant attention in recent research. Biofilms, particularly those formed by S. aureus play a vital role. in persistent infections. Firstly, the biofilm matrix, composed of extracellular polymeric substances (EPS) including polysaccharides, proteins, and extracellular DNA secreted by bacteria during growth, serves as a protective barrier. This matrix not only hinders the diffusion of antibiotics to bacteria within the biofilm but also inhibits the penetration of immune cells, thereby contributing to bacterial persistence (Masters et al., 2022; Schilcher and Horswill, 2020). Secondly, the interaction between EPS and bacterial aggregates confers cohesion and viscoelasticity to the biofilm, enabling bacteria to adhere firmly to both biotic and abiotic surfaces and resist external mechanical forces (Peng et al., 2022). Thirdly, S. aureus exhibits altered metabolic phenotypes within biofilms, rendering it more resilient against the effects of antibiotics (Muthukrishnan et al., 2019). Additionally, the accessory gene regulator (Agr) quorum sensing system serves as a crucial regulator of S. aureus biofilm formation, which is intricately linked to imbalances in bone homeostasis and disease progression (Masters et al., 2022; Butrico and Cassat, 2020).
1.1.4 Staphylococcal abscess formation
Abscess formation serves as an additional mechanism for the long-term survival and immune evasion of S. aureus. Initially, S. aureus overcomes the host’s innate immune defense mechanisms by expressing microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). As S. aureus cells accumulate and establish colonies, a significant influx of neutrophils occurs in the affected area. These neutrophils undergo necrosis due to the effects of S. aureus, inadvertently creating a “protective barrier” that hinders the ability of newly recruited host immune cells to eliminate the bacteria within the abscess. Eventually, as the abscess ruptures, the bacteria disseminate to new anatomic locations, perpetuating the infection process (Masters et al., 2022; Muthukrishnan et al., 2019).
Furthermore, as inflammation ensues, the delicate balance between osteoblasts and osteoclasts within the local bone tissue is disrupted. Osteoclasts, the sole cell type responsible for bone resorption, exhibit excessive differentiation and proliferation, which are intricately linked to bone loss and ultimately give rise to infectious bone defects. S. aureus can directly bind to osteoclasts and promote bone resorption through its own virulence factors, such as Staphylococcal protein A (SpA), peptidoglycan, and lipoproteins. Additionally, it can indirectly stimulate osteoclasts to increase bone resorption by upregulating the synthesis of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) in osteoblasts. Notably, the activated immune system generates a copious amount of proinflammatory cytokines, which further promote osteoclast differentiation and exacerbate bone resorption (Tong et al., 2022; Mendoza et al., 2016; Kassem and Lindholm, 2016; Udagawa et al., 2021).
1.1.5 DFI
The involvement of bone as a secondary complication of DFIs is particularly severe, especially in cases of the most advanced soft tissue DFIs. Therefore, a high degree of vigilance is essential in the occurrence of DFO (Senneville et al., 2020).
DFIs are defined as infections involving soft tissue or bone located anywhere below the ankle in diabetic patients. These infections frequently originate from diabetic foot ulcers (DFUs) and are closely correlated with the eventual risk of lower limb amputation in affected patients (Noor et al., 2017; Mohseni et al., 2019). Multiple pathological factors elevate the risk of foot infections in individuals with diabetes, including neuropathy, vascular insufficiency, immune dysfunction, and alterations in foot biomechanics, all of which are contributing elements to the development of DFIs (Rubitschung et al., 2021; Noor et al., 2017). Neurological complications in diabetic patients encompass motor, sensory, and autonomic dysfunction. Motor neuropathy results in atrophy of foot muscles and associated deformities, leading to traumatic injuries. Impaired sensory function often leads to the body overlooking damage to the lower limbs, fostering a vicious cycle of injury. Autonomic neuropathy disrupts normal blood flow to the soles of the feet. When accompanied by a loss of sweat and sebaceous gland function, the skin becomes dry and keratinized, rendering it more susceptible to rupture and serving as a portal for initial infection (Pitocco et al., 2019). Concurrently, the presence of peripheral artery disease exacerbates tissue ischemia, impairs wound healing processes, and fosters an environment conducive to infection (Armstrong et al., 2023). Hyperglycemia-induced decreases in host defense capacity encompass deficiencies in neutrophil function, alterations in macrophage morphology, elevations in proinflammatory cytokines, and impairments in diabetic polymorphonuclear cell functions, including chemotaxis, phagocytosis, and bactericidal activity. Additionally, locally elevated blood glucose levels serve as an optimal medium for enhancing the virulence of pathogenic bacteria (Baroni and Russo, 2014).
Given that DFIs are the culmination of multiple factors, their treatment necessitates a multidisciplinary and coordinated comprehensive approach. Effective antibacterial interventions and necessary surgical procedures are pivotal. Local antimicrobial therapy and strategies to combat local drug resistance, encompassing antibiotic-impregnated biomaterials, novel antimicrobial peptides, nanomedicine, and photodynamic therapy, represent key considerations in the management of DFIs (Maity et al., 2024). Clinicians are continually focused on addressing how to facilitate the healing of locally damaged tissue, which involves revascularization, while rigorously managing blood glucose levels. Local growth factors, shockwave therapy, negative pressure wound therapy, and stem cell therapy have emerged as promising avenues for accelerating wound healing and reducing the incidence of DFUs as well as the amputation rate (Raja et al., 2023).
1.2 Recent advances in local treatment of bone infection
Conventional treatment strategies for bone infection have historically encompassed surgical debridement and long-term, high-dose systemic antibiotic therapy. However, these approaches exhibit notable limitations. Firstly, the attainment of effective antibiotic concentrations at the lesion site is challenging due to bone loss, sequestrum formation, and soft tissue insufficiency (Zhong et al., 2023). Secondly, bone loss at the osteomyelitis lesion and surgical debridement-induced bone defects pose significant challenges for natural healing, particularly when exceeding a certain threshold, significantly compromising patient recovery and quality of life (Yoshimoto et al., 2022; Migliorini et al., 2021). Furthermore, since the introduction of antibiotics in osteomyelitis treatment in the 1940s, the emergence of bacterial resistance has garnered increasing attention from orthopedic surgeons, posing a significant obstacle in the therapeutic process (Zhong et al., 2023; Wang X. et al., 2023).
As an effective and feasible alternative strategy to solve the above shortcomings, local treatment has been successfully used in clinical practice (Zhong et al., 2023). The utilization of multifunctional antibacterial materials in clinical practice has successfully demonstrated their therapeutic potential in achieving slow-release of high-dose local antibacterial agents, effectively eradicating bacteria, and promoting angiogenesis and bone formation (Wang X. et al., 2023; Cobb and McCabe, 2020). Notably, the integration of antibacterial drugs with bone cement in a local drug delivery system has proven effective in both preventing and treating bone infections, while simultaneously enhancing osseointegration efficiency of bone cement implants (Alegrete et al., 2023; Boyle et al., 2019; Wu et al., 2021). Currently, three types of bone cement are commonly employed in clinical settings for the local treatment of bone infections: poly (methyl methacrylate)(PMMA) cements, CPCs, and calcium sulfate cements (CSCs). Each of these cements exhibits distinct characteristics that contribute to their respective therapeutic outcomes (Quan et al., 2023).
2 Essential characteristics of CPCs for medical applications
CPCs are defined as a combination of one or multiple calcium phosphate powders or particles. Upon mixing with the corresponding liquid phase, they transform into a paste capable of solidifying and hardening within the bone defect site, ultimately forming a scaffold (Ginebra et al., 2012). The common CPCs encompass tricalcium phosphate (TCP), tetracalcium phosphate (TTCP), octacalcium phosphate (OCP), hydroxyapatite (HA), and calcium-deficient hydroxyapatite (CDHA), among others. Unlike acrylic bone cements, which are hardened by polymerization reactions, CPCs are the result of a dissolution and precipitation process and fall into two main categories: precipitated apatite cement and brushite cement. In the context of apatite cement formation, CDHA is formed when a single-composition calcium phosphate compound undergoes hydrolysis without altering the Ca/P ratio. Conversely, precipitated HA results from an acid-base reaction yielding multiple calcium phosphate compounds. Brushite cement is primarily obtained through the acid-base reaction involving more than one type of calcium phosphate (Ginebra et al., 2012; Vezenkova and Locs, 2022). Under physiological conditions, brushite cement exhibits more rapid dissolution rates in comparison to apatite cement (Ginebra et al., 2012; Apelt et al., 2004). CPCs possess injectable properties and their composition closely resembles the chemical structure of bone mineral. HA, a bioactive inorganic ceramic with a chemical and crystal structure resembling that of natural bone apatite [Ca10(PO4)6(OH)2], exhibits excellent biological properties (Fang et al., 2006). When compared to PMMA cements, CPCs exhibit superior biocompatibility and bioabsorbability. Their degradation products provide essential calcium and phosphate ions at the implantation site, vital for local mineralization. Furthermore, CPCs demonstrate greater osteoconductivity and osteoinductivity than CSCs, thereby facilitating new bone formation (Ginebra et al., 2012; Vezenkova and Locs, 2022).
The inherent interconnected macropores (pore size> 100 μm) and micropores (pore size <100 μm) of CPCs not only surpass CSCs in osteoconductivity and osteoinduction, thereby promoting new bone formation (Ginebra et al., 2012; Vezenkova and Locs, 2022), but also expand their specific surface area for the adsorption of bioactive substances. These substances, including drugs, bioactive molecules, and metal or non-metal ions, further enhance the functionality of CPCs (Ginebra et al., 2012; Vezenkova and Locs, 2022; Grosfeld et al., 2016; Kost et al., 2023) (Figure 2)
FIGURE 2
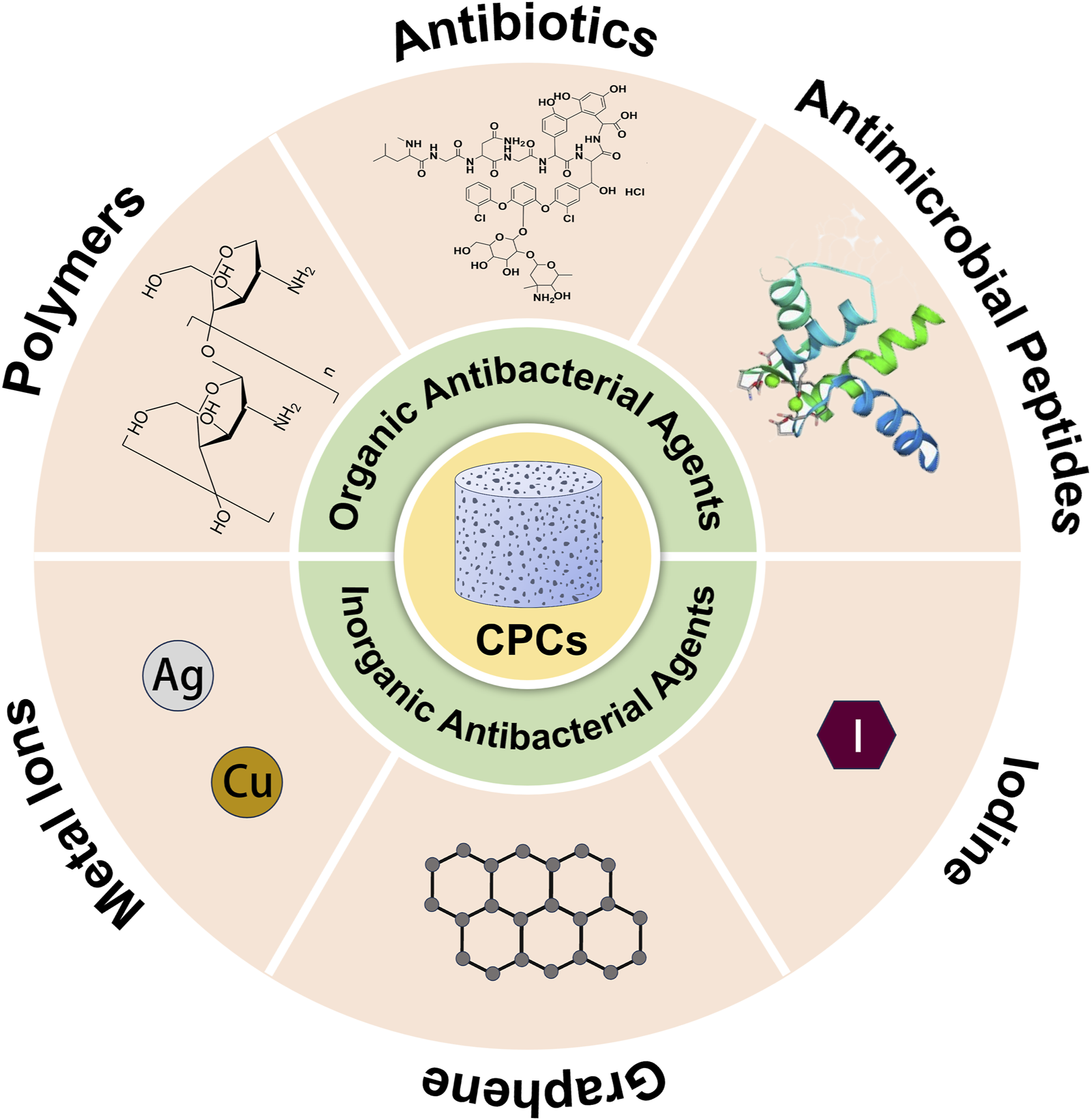
Antibacterial agents loaded in calcium phosphate bone cement.
Furthermore, CPCs possess the ability to harden at normal or room temperature, permitting bioactive substances to penetrate their inherent pores without compromising their activity (Ginebra et al., 2012; Vezenkova and Locs, 2022; Rh Owen et al., 2018). Consequently, CPCs exhibit exceptional biocompatibility in vivo and serves as an optimal drug delivery system for the locally controlled release of antibacterial agents.
3 CPC-based bone filler for the treatment of bone infection
From a clinical perspective, selecting appropriate CPC application strategies tailored to patients’ individual conditions is pivotal for disease treatment. The appropriate form of CPC implantation is closely associated with various patient factors, including whether the bone defect is located in a weight-bearing area, its size, and whether it is a complex defect. Furthermore, certain intrinsic properties of CPC also influence the choice of its final application form, encompassing porosity, mechanical strength, injectability, solidification reaction and time, cohesion, biodegradability, and the inclusion of additives. These factors are interdependent and collectively determine the ultimate characteristics of CPC in practical applications (Ginebra et al., 2012; Vezenkova and Locs, 2022; Mucalo, 2019). In cases where bone infection leads to significant defects, the Masquelet technique is initially employed for membrane induction, followed by autologous bone transplantation. To address the defects, larger porous particles of CPC, which have been pre-solidified and sterilized prior to surgery, can be utilized as a replacement (Andrzejowski et al., 2020; Gupta et al., 2019). It is noteworthy that CPC particles exhibit brittleness and possess limited mechanical strength, with pores playing a crucial role in this context (Baudín et al., 2019). This limits application to weight-bearing sites. Additional interventions, such as internal fixation, are necessary to augment the mechanical strength of the affected region. Ideally, the mechanical properties of the implant should closely approximate those of the surrounding bone tissue (Vezenkova and Locs, 2022). On the other hand, in cases where the bone defect is small or irregularly shaped, to prevent cavity residuals, the use of paste-form CPC with high injectability is recommended for cavity filling (Demir-Oğuz et al., 2023). The injectability of the material is intimately associated with the ratio of solid to liquid components, as well as the particle size distribution of the material (Vezenkova and Locs, 2022). Luo et al. (2018) developed a pre-mixed acid cement formulated from monocalcium phosphate monohydrate (MCPM) paste and β-TCP paste, specifically tailored for the rapid and minimally invasive filling of bone defects. This approach is effective in reducing procedure time and minimizing the risk of contamination.
For the drug loading of CPCs, two primary methods can be employed (Parent et al., 2017): direct loading and indirect loading. Direct loading involves the mixing of solid or liquid antibacterial drugs directly with the bone cement prior to administration (Yu et al., 2010). During surgical intervention, when CPC powder is intended for mixing with the corresponding curing liquid, the therapeutic agent is generally incorporated directly within the bone cement compound. While this approach enables CPCs hardening at low temperatures, thereby preventing drug denaturation or inactivation that might occur at higher temperatures as compared to PMMA (Ginebra et al., 2012; Vezenkova and Locs, 2022; Rh Owen et al., 2018), it is not without its limitations. These include a lack of standardization in the production process, inhomogeneous drug distribution within the bone cement matrix, restricted drug quantities to maintain mechanical strength, and challenges in achieving an optimal in vitro release profile for macromolecular drugs (Mouriño and Boccaccini, 2010; Chen L. et al., 2024). Indirect loading, on the other hand, involves impregnating a calcium phosphate carrier in a drug solution or suspension, followed by separation of the impregnated carrier from the liquid phase and evaporation of the solvent to obtain a dry drug-loaded carrier (Wang et al., 2022). This method is particularly suitable when the micro three-dimensional structure of CPCs is crucial for exerting its therapeutic effects and when the fragility of the material is a concern (Parent et al., 2017). When selecting pre-mixed bone cement prior to surgery, antibiotics can be incorporated via dipping to enhance their antibacterial efficacy. However, the limitation of a shortened shelf life associated with this method cannot be overlooked (Luo et al., 2018).
The regulation of release kinetics from a drug carrier is pivotal in maintaining the therapeutic window, defined as the concentration range between the minimal inhibitory concentration for bacteria and the minimal toxic concentration for humans, for the administered drug (McInnes et al., 2016; Wu et al., 2024). A research team has demonstrated the efficacy of utilizing calcium phosphate-loaded antibiotics for the localized treatment of bone infections. Notably, the serum concentration of the antibiotic in patients remains low, staying below the manufacturer-recommended safety limits (Sasaki et al., 2005). For a constant quantity of a given drug, when blended directly with CPC matrix components, drug release is primarily governed by matrix diffusion, with the concentration and duration of release exhibiting a positive correlation with the drug dosage. Conversely, when the drug is adsorbed onto the substrate surface through incubation, the release mechanism becomes anomalous, displaying an increase in release as the drug loading augments (Fosca et al., 2022).
The rate of drug release from the carrier is influenced by various factors, encompassing not only the method of drug loading but also characteristics of the bone cement matrix, drug type, drug loading quantity or concentration, and the utilization of additives (Parent et al., 2017; Fosca et al., 2022). Specifically, the porosity, pore size distribution, specific surface area, and crystallinity of the bone cement matrix all play a significant role. Furthermore, the addition of polymer additives to the CPC matrix represents a relatively straightforward approach to regulate drug release kinetics.
3.1 Organic antibacterial substances loaded with CPCs
3.1.1 Polymer-doped CPCs
By incorporating biodegradable polymers, such as chitosan (CS), mannitol, hyaluronic acid, alginate, gelatin, collagen, hydroxypropyl methyl cellulose (HPMC), and poly (lactic-co-glycolic acid) (PLGA), into the drug-loaded matrix under specific conditions, it is possible to mitigate the initial burst release of drugs from the carriers. This modulation results in a more sustained and controlled drug release profile from the cement matrix (Vezenkova and Locs, 2022; Fosca et al., 2022; Mistry et al., 2016; Xu et al., 2017; Dorozhkin, 2019), thereby effectively fulfilling the objective of preventing and treating bone infection.
The influence of additives on the drug release rate within CPC manifests firstly through alterations in the drug’s affinity for the matrix, where a negative correlation exists between affinity and release rate. Secondly, the intrinsic properties of the additives themselves can modulate the rate of drug release. When polymers are employed as additives, they induce a spatial effect by swelling and filling the pores of the matrix, thereby inhibiting drug release. Subsequently, as the polymer undergoes degradation, the release rate augments due to the facilitated diffusion of drug molecules (Vezenkova and Locs, 2022; Fosca et al., 2022; Li et al., 2007; David Chen et al., 2011). When the local concentrations of antibiotics released by CPC remain subtherapeutic for an extended period following the initial burst release, the potential for bacterial resistance may arise (Thomes et al., 2002; Kendall et al., 1996). The incorporation of a polymer as an additive in the CPC matrix results in a reduction of the initial burst release of the drug and promotes a more sustained release profile of the drug over time (David Chen et al., 2011; Jin, 2015). This characteristic of the polymer is advantageous for modulating drug release within the therapeutic window in CPC, thereby mitigating the adverse effects of bacterial resistance and ensuring the antibacterial efficacy of the treated area within a safe concentration range. Furthermore, the rate of drug release in the CPC matrix can be controlled by adjusting the concentration of the polymer in the reaction solution (Tiğli et al., 2009).
Normal human bone comprises a complex combination of inorganic and organic materials. By incorporating polymeric organic polymers into CPC, we can better mimic the natural state of bone tissue, thereby enhancing the biocompatibility of CPC in vivo. Additionally, the inclusion of these polymers is advantageous in improving the injectability and mechanical strength of the bone cement matrix. Furthermore, it has been demonstrated that the antimicrobial properties of drug-loaded cement are also enhanced through the addition of polymers (Wu et al., 2021; Wu et al., 2020a; Bose et al., 2023).
CS is a polysaccharide compound derived from chitin through deacetylation (Bakshi et al., 2020). Its molecular structure harbors multiple functional groups, facilitating modifications and chemical reactions (Zhong et al., 2023). Apart from its biodegradability and biocompatibility, CS exhibits a strong affinity towards negatively charged bacterial membranes due to its cationic nature, thereby displaying moderate antibacterial activity (Tao et al., 2020; Negm et al., 2020). Yang et al. (2018) successfully fabricated a PLGA-HA scaffold grafted with CS using 3D printing technology. Both in vitro and animal experiments confirmed that the CS-PLGA-HA scaffold demonstrated remarkable antibacterial properties against S. aureus, along with bone conductivity, offering a novel approach to enhance the local therapeutic efficacy of CPCs in the treatment of infectious bone defects.
3.1.2 Antibiotic-loaded CPCs
Antibiotics have long been extensively studied as a primary approach for preventing and treating bone infections. Surgical procedures such as wound contamination, fracture repair with or without internal fixation, joint prosthesis implantation, and spinal surgery can often lead to severe bone infections. Therefore, the successful osseointegration of CPCs during surgical procedures critically depends on the loading of antibacterial drugs, which effectively prevents bone infection at the surgical site (Ginebra et al., 2006). To prevent bone infection, it is essential that the antibiotics released from the bone cement carrier rapidly reach the minimum inhibitory concentration (MIC) of the corresponding pathogenic bacteria, while avoiding prolonged exposure to sub-inhibitory concentrations that may promote bacterial resistance. For the treatment of bone infection, it is necessary to achieve local long-term antibiotic release (Ginebra et al., 2012).
Currently, the antibiotics loaded into CPCs for the treatment of bone infections are primarily derived from a diverse range of antimicrobial agents. These include, but are not limited to, aminoglycosides such as gentamicin sulfate, tobramycin, and amikacin; β-lactams like meropenem and hydroxy benzylpenicillin; glycopeptides such as vancomycin and teicoplanin; quinolones like moxifloxacin and ciprofloxacin; and tetracyclines, such as doxycycline. These antibiotics, used singly or in combination, have demonstrated effectiveness in the prevention and treatment of bone infections (Ginebra et al., 2012; Mistry et al., 2016; Ribeiro et al., 2022; Nasser et al., 2022; Mabroum et al., 2023; Radwan et al., 2021; Liu et al., 2023; Mistry et al., 2022; Pradeep et al., 2023; Bhanwala and Kasaragod, 2022; Kumar Dewangan et al., 2023) (Table 1)
TABLE 1
| Antibiotic type | Antibiotic(s) | CPC Ingredient(s) | Antibacterial efficiency | References |
|---|---|---|---|---|
| Aminoglycosides | Gentamicin | On the basis of the commercially available CPC (Neocement®), CS and HPMC were added to enhance its performance and ensure sustained release of gentamicin | The formula containing 42% Liquid phase + HPMC + 1.87% wt gentamicin was identified as the optimal formulation, exhibiting desirable coagulation and mechanical properties, with an injectability of approximately 87% (compared to the original Neocement at 31%). This formulation ensures local release of gentamicin for 14 days at concentrations above antibacterial levels, demonstrating excellent antibacterial activity against S. epidermidis and moderate activity against S. aureus | Ribeiro et al. (2022) |
| β-lactams | Amoxicillin (AMX) | Citrate-modified mesoporous hydroxyapatite nanocarrier (Ctr-mpHANCs) | In vitro: AMX @ Ctr-mpHANs significantly reduced the growth of S. aureus, E. coli, and P. aeruginosa compared to Ctr-mpHANs | Nasser et al. (2022) |
| Quinolones | Ciprofloxacin | A reactive mixture containing 25% wt of bioactive glass (46S6) powder, along with an equimolar blend of calcium carbonate and dicalcium phosphate dihydrate, was prepared. Subsequently, these reactive powders were combined with a gel consisting of sodium alginate dissolved in a 0.25 M disodium hydrogen phosphate solution, maintaining an Liquid/Powder ratio of 0.7 | In vitro: the cement composite loaded with ciprofloxacin exhibited excellent antibacterial activity against S. aureus and E. coli | Mabroum et al. (2023) |
| Moxifloxacin | Biodegradable composite scaffolds of poly-lactide-co-ε-caprolactone/calcium phosphate (calcium phosphate: commercial-β-TCP, commercial-HA, commercial-dicalcium hydrogen phosphate) | The composite calcium phosphate scaffold effectively reduces bacterial load, inflammation, and sequestrum formation in the local lesions of an animal model of chronic osteomyelitis caused by S. aureus. As a result, it represents a promising candidate material for further clinical trials in the treatment of chronic osteomyelitis | Radwan et al. (2021) | |
| Tetracycline class | Doxycycline | α-TCP (CAM Bioceramics B.V.) powders contained: 40 wt% PLGA, 1.5 wt% carboxymethyl cellulose (CMC), or 39.4 wt% PLGA plus 1.5 wt% CMC | In vitro: doxycycline released from the bone cement retained its antibacterial activity against S. aureus. Animal implantation models: a rapid reduction in the number of S. aureus bacteria on the surface of CPC and in surrounding tissues following implantation | Liu et al. (2023) |
| Glycopeptides (with other antibiotics) | Vancomycin Tobramycin |
50 wt% HA+50 wt% β-TCP+α-CSH. After drug loading, BCP particles were encapsulated with PLGA for sustained release | Utilizing a rabbit tibial osteomyelitis model (MRSA), the superiority of composite bone cement in controlling infection rates and promoting bone healing was demonstrated compared to PMMA bone cement and parenteral therapy alone | Mistry et al. (2016) |
| In the treatment of patients with chronic osteomyelitis (MRSA), composite bone cement exhibits superior performance in terms of cellular compatibility, hemostatic activity, infection control effectiveness, and bone regeneration compared to PMMA bone cement and parenteral therapy | Mistry et al. (2022) | |||
| Vancomycin Amikacin |
TTCP + Phosphoserine (PS) (The mass ratio: 10:6) | In vitro: antibacterial bone cement exerted inhibitory effects on the formation of biofilms by E. coli, K. pneumoniae, and S. aureus, and also suppressed the growth of these bacteria when cocultured. In vivo: the application of bone cement to rats with sternal infections caused by S. aureus or E. coli significantly inhibited bacterial activity | Pradeep et al. (2023) | |
| Vancomycin Meropenem |
HA | A case report about Single step treatment of frontal sinus osteomyelitis using bone cement. During the follow-up period, no intracranial, nasal, or intraorbital complications were observed. Additionally, no recurrence or residual disease was detected at the conclusion of the 6-month period | Bhanwala and Kasaragod (2022) | |
| Vancomycin Gentamicin Meropenem Rifampicin |
CDHA | In vitro experiments have demonstrated that eggshell-derived apatite bone cement is a more suitable injectable bone substitute for anterior use compared to injection-molded synthetic bone cement, effectively avoiding postoperative implant-related and other types of bone infections | Kumar Dewangan et al. (2023) | |
| Vancomycin | α-TCP based CPC modification with vancomycin loaded poly (lactic acid) (PLA) microcapsules | CPC modification with vancomycin loaded PLA microcapsules decreased the initial burst release of drug down to 7.7% ± 0.6%, while only 30.4% ± 1.3% of drug was transferred into the dissolution medium within 43days, compared to pure vancomycin loaded CPC, where 100% drug release was observed already after 12days | Loca et al. (2015) | |
| Teicoplanin | An injectable drug delivery system based on poloxamer 407 hydrogel containing undoped Mg, Zn-doped β-TCP, and teicoplanin | The encapsulated teicoplanin showed a sustained release over the evaluated period, enough to trigger antibacterial properties against Gram-positive bacteria. Besides, the formulations were biocompatible and showed bone healing ability and osteogenic properties. Finally, in vivo studies confirmed that the proposed locally injected formulations yielded osteomyelitis treatment with superior outcomes than parenteral administration while promoting bone regeneration | Kai et al. (2024) | |
| Teicoplanin | CPC powder containing 3% teicoplanin | To assess the effectiveness of calcium phosphate as a delivery system of teicoplanin, MRSA osteomyelitis was induced in 36 rabbits. And calcium phosphate cement with 3% teicoplanin was implanted. Bacterial eradication signified a considerable decrease of the total histologic scores of osteomyelitis compared with controls, accompanied with newly growing host bone | Lazarettos et al. (2004) | |
| Teicoplanin | CPC (Biopex; Pentax, Tokyo, Japan) | A 71-year-old man developed skin ulceration with cranial osteomyelitis after bypass surgery for diffuse cerebral infarction and internal carotid artery obstruction. MRSA was detected on wound culture test. Cranioplasty with a combination of calcium phosphate bone cement impregnated with teicoplanin, and a titaniummesh sheet and scalp reconstruction were performed. As of 6 months after surgery, no infection has relapsed | Ogino et al. (2020) |
Antibiotics loaded into calcium phosphate bone cement.
S. epidermidis, Staphylococcus epidermidis; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; MRSA, Methicillin-resistant Staphylococcus aureus; K. pneumoniae, Klebsiella pneumoniae.
However, the emergence of antibiotic-resistant bacteria, including those that are refractory to almost all antibiotics and the challenges associated with the removal of biofilms, has garnered increasing attention. Consequently, the development of effective non-antibiotic alternatives has become a significant research focus in recent years (Aslam et al., 2018; Nir-Paz et al., 2019; Kalelkar et al., 2022). Additionally, the inclusion of antibiotics in bone cement matrices has been shown to compromise their mechanical stability, further emphasizing the need for alternative antimicrobial strategies (Arciola et al., 2018).
3.1.3 Antimicrobial peptide-loaded CPCs
In the context of the escalating problem of antibiotic resistance in pathogenic bacteria, particularly caused by multidrug-resistant pathogens and biofilm formation (Peng et al., 2022; Muthukrishnan et al., 2019; van Staden and Heunis, 2011), the urgent need to identify reliable alternatives to antibiotics has become paramount. This is due to the difficulties in discovering new antibiotics that can effectively address this challenge in the near future (Volejníková et al., 2019; Sinha and Shukla, 2019; Costa et al., 2021).
Antimicrobial peptides (AMPs) represent a class of basic active peptides that exhibit broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. These peptides demonstrate high antimicrobial efficacy even at low concentrations, possess anti-biofilm activity, and exert immunomodulatory effects (Costa et al., 2021; Melicherčík et al., 2018; Yazici et al., 2019; Luo and Song, 2021). Given that AMPs target multiple components within the plasma membrane and cytosol of bacteria, the likelihood of them inducing pathogen resistance is extremely low (Costa et al., 2021; Van Staden and Dicks, 2012). Currently, several AMPs have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe bacterial infections, underscoring their potential as viable alternatives to traditional antibiotics (Dijksteel et al., 2021).
Notably, the application of drug-eluting coatings loaded with antimicrobial peptides onto porous calcium phosphate substrates represents a promising strategy for the prevention of orthopedic implant-related infections and the formation of biofilms on their surfaces:
Kazemzadeh-Narbat et al. (2010) selected the broad-spectrum AMP Tet213 and applied it to a titanium surface, creating coatings composed of microporous OCP as a drug carrier. These coatings, with a thickness of 7 μm and an AMP loading of 9 μg/cm2, exhibited antimicrobial activity in vitro against both S. aureus (Gram-positive) and P. aeruginosa (Gram-negative). These results indicate that calcium phosphate-Tet213 coatings could potentially serve as an effective solution for the prevention of infections associated with orthopedic implants. Furthermore, the team demonstrated significantly lower in vitro cytotoxicity (200 μg/mL) of AMP HHC36 compared to AMP Tet213 (50 μg/mL) in calcium phosphate-HHC36 coatings applied to a titanium surface. Additionally, these coatings exhibited antimicrobial activity against both S. aureus and P. aeruginosa (Kazemzadeh-Narbat et al., 2012). Additionally, they also validated the ideal cytotoxicity and antibacterial efficiency of AMP HHC36 in vitro through antibacterial experiments utilizing titanium surface nanotubes coated with a palmitoyl oleoyl phosphatidyl-choline (POPC) film after loading AMP HHC36 into a supersaturated calcium phosphate coating matrix for sustained drug release (Kazemzadeh-Narbat et al., 2013).
Yazici et al. (2019) aimed to enhance the delivery efficiency of AMPs to localized regions and consequently developed bifunctional peptides by fabricating titanium surface nanotubes through HA deposition. The dual functionality of these peptides was achieved by conjugating hydroxyapatite binding peptide-1 (HABP1) with AMP using a flexible linker. The resulting HABP1-AMP conjugate demonstrated favorable affinity for HA and exhibited high antibacterial activity against S. mutans (Gram-positive) and E. coli (Gram-negative), thus presenting a promising approach for targeted delivery of AMPs to specific areas.
3.2 Inorganic antibacterial substances loaded with CPCs
3.2.1 TII-loaded CPCs
In the realm of biomaterial engineering stents, the integration of drug delivery functionality with therapeutic efficacy is a common strategy employed to augment stent performance. From a pharmaceutical standpoint, the manufacturing methodologies of stents must ensure compatibility with drug stability and sustained release profiles. In comparison to organic macromolecular drug molecules, TIIs exhibit several notable advantages, including reduced cost, superior stability, enhanced safety, and fewer constraints on the fabrication process of tissue-engineered scaffolds (Mouriño et al., 2012; Hoppe et al., 2013). Currently, in the ongoing research endeavors aimed at utilizing CPC as scaffolds for bone tissue engineering, researchers have selected specific metal ions as TIIs. These TIIs have been successfully validated for their notable efficacy in combating bacterial infections and enhancing the material matrix’s capacity to induce new bone formation (Gritsch et al., 2019; Feng et al., 2021).
Metals have been utilized as antibacterial agents and as materials for various biomedical research and applications (Zhong et al., 2023; Samuel et al., 2020; Makvandi et al., 2020). When CPC is employed as a drug delivery device for bone infections, a small quantity of ions within the calcium phosphate lattice can be substituted by other ions. Several studies have corroborated that the doping of metal ions, such as silver, copper, and zinc, into the bone cement matrix enables calcium phosphate to exhibit antibacterial properties (Kamphof et al., 2023).
Silver (Ag) is among the most extensively studied metal ions incorporated into calcium phosphate due to its remarkable antibacterial properties (Kamphof et al., 2023). Notably, silver nanoparticles (AgNPs) exhibit potent antibacterial activity by releasing silver ions that attach to the bacterial cell membrane, altering the lipid bilayer or surface charge state. This process generates reactive oxygen species (ROS) and free radicals, which damage organelles and biomolecules and regulate associated signal transduction pathways, ultimately leading to antibacterial efficacy (Zheng et al., 2018; Lee and Jun, 2019).
Choi et al. (2023) conducted a study evaluating the antibacterial effect of TTCP-dicalcium phosphate dihydrate (DCPD) cement impregnated with AgNPs. In a rat tibial infection model, local treatment with TTCP-DCPD cement containing varying concentrations of AgNPs was administered for either 3 or 12 weeks. The findings revealed a decrease in bacterial colony counts in the 12-week treatment group compared to the 3-week group. Furthermore, a trend towards a lower bacterial colony count was observed in groups treated with higher doses of AgNPs compared to those without AgNP impregnation. Separately, Köse et al. (2021) established a model of methicillin-induced osteomyelitis in the proximal tibia of New Zealand white rabbits. The animals were divided into three groups: the control group received vancomycin-impregnated PMMA bone cement beads, the experimental group received silver ion-doped calcium phosphate beads, and the negative control group received pure calcium phosphate beads. After 10 weeks, radiographic assessments demonstrated significant improvement in osteomyelitis in the experimental group compared to the control group. These results suggest that silver ion-doped calcium phosphate beads have the potential to stimulate bone tissue growth, resist infection, and ultimately treat experimental chronic osteomyelitis in animal models. However, it is crucial to acknowledge that the cytotoxicity of silver towards normal tissues and cells cannot be overlooked, posing a limitation to its widespread application in the treatment of bone infections (Mijnendonckx et al., 2013; Rizzello, 2014).
Copper (Cu), an essential trace element in the human body, possesses diverse biological functions, including broad-spectrum antibacterial effects, bone regeneration capabilities, and angiogenesis properties (Shen et al., 2021; Marziya et al., 2023; Holloway, 2019).
Foroutan et al. (2019) conducted a study to investigate the influence of Cu2+ release on the properties and underlying mechanisms of CPCs. They synthesized CPC containing varying concentrations of Cu2+ (0, 2, 4, and 6 mol%) through a room temperature precipitation reaction. In vitro experiments demonstrated that as the Cu content increased, the release of phosphorus and calcium decreased, while the release of Cu2+ escalated. Furthermore, an analysis of the antibacterial activity against S. aureus revealed that the antimicrobial potential of the bone cement enhanced with increasing Cu2+ concentration. Additionally, when human osteoblast-like osteosarcoma cells (Saos-2, HTB85) were inoculated onto the bone cement particles for a defined period, a significant increase in cell count was observed, thereby confirming its biocompatibility. This study underscores the dual benefits of copper-doped CPCs, which not only exhibit antimicrobial activity but also possess bone regeneration properties. Näf et al. (2024) developed bilayer nanocomposites consisting of PLGA and amorphous calcium phosphate, incorporating various copper nanoparticles, specifically copper oxide (CuONPs) and copper-doped tricalcium phosphate (CuTCPNPs). To assess the antimicrobial properties of these copper-containing materials, clinically isolated S. aureus and S. epidermidis were employed. Furthermore, the angiogenic potential of these nanocomposites was evaluated using the chick embryo chorioallantoic membrane (CAM) model. The findings revealed that both CuONPs and CuTCPNPs possess antimicrobial activities and serve as effective components for stimulating angiogenesis.
Moreover, numerous in vitro and in vivo experiments have demonstrated the antimicrobial properties of metals and their alloys, including magnesium, strontium, and zinc, when incorporated into CPCs against bone infections. Additionally, these metals have been shown to enhance the antimicrobial effectiveness of existing therapeutic agents (Daskalova et al., 2023; Sikder et al., 2020; Wang Y. et al., 2023; Dapporto et al., 2022; Shu et al., 2024; Wolf-Brandstetter et al., 2020). However, it is crucial to emphasize that the precise correlation between the antimicrobial activity and cytotoxicity of metal-doped CPCs remains to be elucidated in future investigations (Kamphof et al., 2023).
3.2.2 Graphene-loaded CPCs
Graphene, an allotropic isomer of carbon, has been shown to enhance the mechanical properties of CPC matrices and improve their biocompatibility for bone repair and regeneration (Seonwoo et al., 2022; Ozder et al., 2023). Furthermore, studies have confirmed the antimicrobial efficacy of graphene against a broad range of bacteria, including both Gram-positive and Gram-negative strains (Akhavan et al., 2011; Gurunathan et al., 2012; He et al., 2015; Veerapandian et al., 2013).
Wu et al. (2020b) pioneered the preparation of an injectable CPC-CS-GO slurry through the doping of graphene oxide (GO) into CPC. This novel slurry exhibited a robust inhibitory effect against S. aureus, demonstrating an inhibition zone of 55.2 ± 2.5 mm, significantly surpassing that of the control CPC-CS (30.1 ± 2.0 mm) (p < 0.05). Furthermore, CPC-CS-GO displayed superior antibacterial activity against S. aureus biofilm in vitro (p > 0.05). Khosalim et al. (2022) synthesized biomaterials composed of HA, agarose, and GO. Incorporating 1.0wt% GO into these biomaterials resulted in a notable reduction of S. aureus in vitro colony-forming unit tests. Additionally, the excellent biocompatibility of these materials was confirmed through tests using mouse embryo osteoblast precursor cells (MC3T3-E1).
3.2.3 Iodine-loaded CPCs
Iodine possesses a broad antimicrobial spectrum, demonstrating effectiveness against viruses, Mycobacterium tuberculosis, fungi, and bacteria (Tsuchiya et al., 2012). In a groundbreaking study, Morinaga et al. (2023) impregnated CPC with various concentrations of iodine to assess its in vitro antibacterial activity and in vivo biocompatibility. Their findings revealed that CPC containing 5% iodine retained a higher iodine content compared to other CPCs after 1 week of release. Furthermore, this CPC formulation exhibited antimicrobial efficacy against S. aureus and E. coli for up to 8 weeks, while maintaining a similar number of fibroblast colony formations as the control sample.
However, there are limited reports on the application of doping graphene or iodine into CPCs for the treatment of bone infections, and further exploration of these possibilities is warranted.
4 Multifunctional calcium phosphate-calcium sulfate complex bone cement for the treatment of bone infections
As a bone cement filler for the treatment of bone infections, it aims to promote functional recovery of the affected limb or area while ensuring the desired preventative and therapeutic outcomes. Consequently, orthopedic surgeons face stringent requirements in selecting an appropriate bone cement matrix. A crucial aspect of this selection process is ensuring that the degradation rate of the cement matrix aligns with the rate of new bone formation.
Although CPC possesses ideal biocompatibility and recapitulates the inorganic phase of human bone, exhibiting porosity that renders it a suitable carrier for the slow release of osteoconductive drugs (Ginebra et al., 2012; Vezenkova and Locs, 2022; Fang et al., 2006), it is recognized that its degradation rate is rather sluggish. Studies have demonstrated that the degradation and absorption of CPC in vivo requires over 20 weeks to complete (Russell et al., 2008; Bohner, 2000). Conversely, CSC, a frequently utilized bone defect filler in clinical practice, is associated with limitations such as its limited osteogenic potential, rapid drug elution rate (Mistry et al., 2016), and swift degradation rate in vivo (<12 weeks) (Zhao et al., 2020).
To harmonize the resorption rate of the bone cement matrix with the rate of new bone formation, a multifunctional calcium phosphate-calcium sulfate complex bone cement was developed, exhibiting antimicrobial, osseointegrative, and degradable properties. Building upon the established potential of this cement as an effective antibiotic delivery system, as demonstrated by Mistry et al. (2016) through animal experiments, Zhao et al. (2020) reported the first clinical application of calcium phosphate (dicalcium phosphate (DCP) + β-TCP)-calcium sulfate complex bone cement impregnated with vancomycin in the treatment of chronic osteomyelitis caused by S. aureus infection. When compared to vancomycin-impregnated CSC used as a control, the composite bone cement exhibited superior new bone growth and a lower rate of infection recurrence (Table 2; Figure 3).
TABLE 2
| Group A (CS/CP) |
Group B (CS) |
P value | |
|---|---|---|---|
| Hospital stay (days), mean ± SD | 35.05 ± 14.83 | 29.30 ± 7.12 | 0.156 |
| Follow-up (weeks), mean ± SD | 61.29 ± 33.75 | 61.29 ± 33.75 | 0.041* |
| Systemic antibiotic treatment duration (days), mean ± SD | 36.33 ± 18,45 | 45.80 ± 8.92 | 0.064 |
| Post-op ESR (mm/h), mean ± SD | 9.10 ± 8.69 | 27.50 ± 26.24 | 0.056 |
| Post-op serum hs-CRP (mg/L), mean ± SD | 4.91 ± 7.78 | 15.65 ± 18.09 | 0.100 |
| Post-op WBC (◊109), mean ± SD | 6.65 ± 2.59 | 6.64 ± 1.48 | 0.984 |
| Filling dose, mean ± SD | 3.95 ± 1.56 | 4.49 ± 1.36 | 0.353 |
| Defect length, mean ± SD | 5.28 ± 0.98 | 5.66 ± 0.74 | 0.280 |
| Defect width, mean ± SD | 2.31 ± 0.50 | 2.28 ± 0.54 | 0.883 |
| Microorganisms isolated (Staph, E. cloacae, Bacillus subtilis, negative) | 14/1/1/5 | 5/1/0/4 | 0.643 |
| Internal fixation | 4/17 | 2/8 | 1.000 |
| Complications | 1/20 | 3/7 | 0.087 |
| Recurrence | 0/20 | 2/8 | 0.034* |
Postoperative data of the two groups ( ±s).
*, P < 0.05. ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; WBC, white blood cell count; CS, calcium sulfate; CP, calcium phosphate (Zhao et al., 2020). Reprinted with permission from. Copyright 2020 Annals of Palliative Medicine.
FIGURE 3
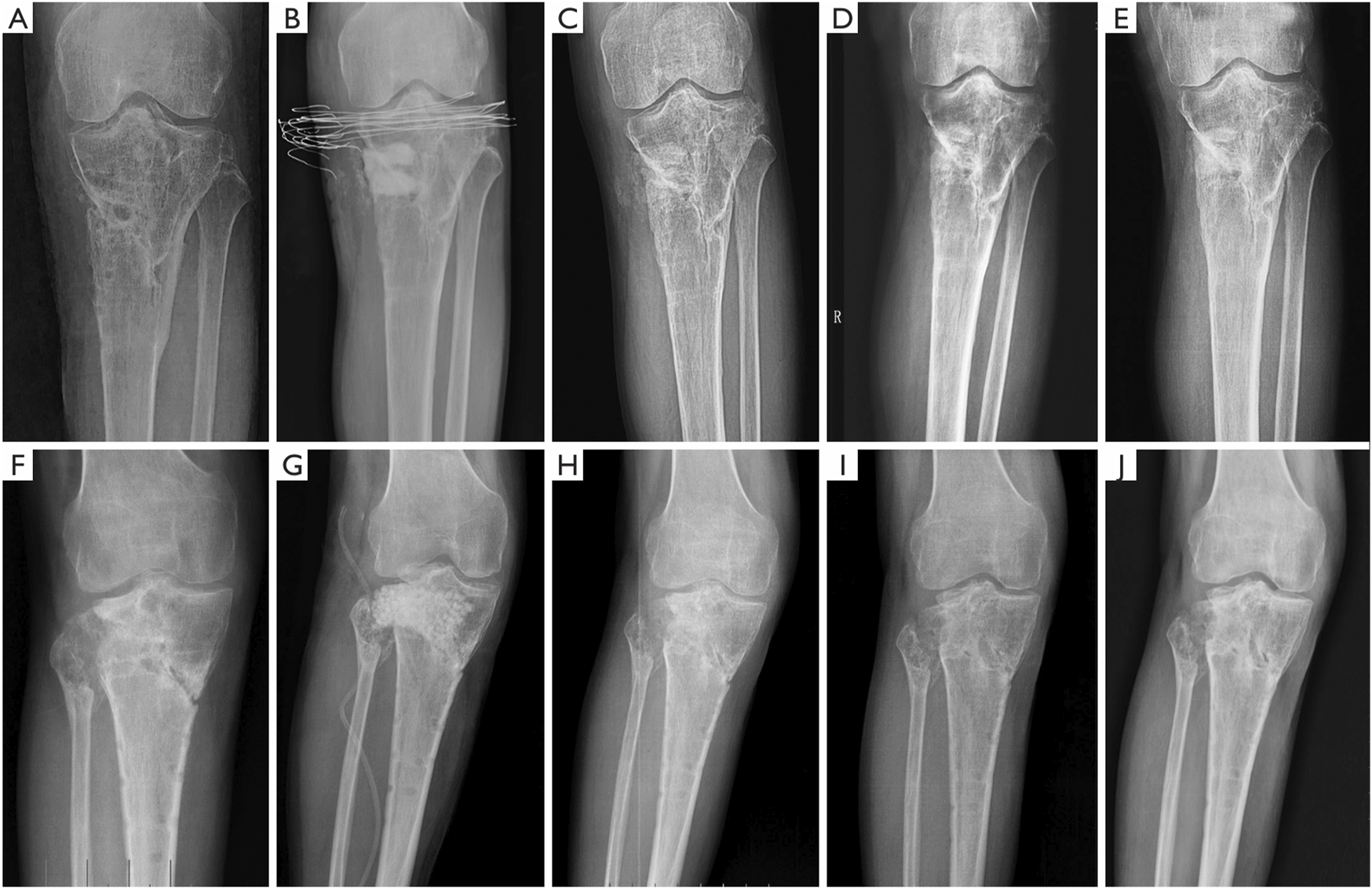
Typical radiographs of chronic osteomyelitis treated with CS/CP preoperatively (A), immediately (B), at 3 months (C), at 6 months (D) and at 1 year (E) postoperatively. With the formation of new bone, the bone substitute composite was gradually absorbed and no new defects formed until the formation of new bone completed (E). Typical radiographs of chronic osteomyelitis treated with CS preoperatively (F), immediately (G), at 3 months (H), at 6 months (I) and at 1 year (J) postoperatively. Three months after the surgery, CS had been greatly absorbed. However, the new bone formation was slow and bone defect cavities still existed (H, I, J). CS, calcium sulfate; CP, calcium phosphate (Zhao et al., 2020). Reprinted with permission from. Copyright 2020 Annals of Palliative Medicine.
On the other hand, Mistry et al. (2022) employed biphasic calcium phosphate (BCP), consisting of HA blended with β-TCP and processed through foaming and high-temperature sintering, in the development of a calcium sulfate complex bone cement loaded with antibiotics (vancomycin + tobramycin). This formulation had been previously validated in animal experiments. It was utilized in the treatment of chronic osteomyelitis caused by MRSA infection, with gentamicin sulfate-loaded PMMA bone cement and intravenous vancomycin drip serving as control groups. Over time, clinical and radiological assessments demonstrated the superiority of the composite bone cement compared to the other two treatment modalities, evident in its antimicrobial efficacy, rapidity in sepsis control, and enhancement of new bone production.
Orthopaedic surgeons continue to grapple with the challenges of fracture-related infections (FRI) and periprosthetic infections (PJI). To address these issues, Freischmidt et al. (2020) introduced a novel, individualized surgical technique utilizing HA-calcium sulfate complexes impregnated with antibiotics (vancomycin or gentamicin sulphate). These composites were applied to intramedullary nails and plates, aiming to prevent biofilm formation and subsequent recurrence of bone or joint infections. The antimicrobial and osteoconductive properties of the composite bone cement coating the internal fixation devices yielded promising results in three reported cases of bone infection.
Recently, magnesium phosphate cement (MPC) has garnered significant attention as a novel bone repair material. Besides possessing advantages such as mechanical strength, biocompatibility, injectability, and modifiability, MPC exhibits superior bone conductivity and absorbability compared to CPC. These properties are particularly beneficial for bone tissue regeneration and repair. Furthermore, MPC offers a simpler operation and a degradation rate that is more in harmony with new bone formation (Tian et al., 2024; Ostrowski and Roy, 2016; Kaiser et al., 2022; Goldberg et al., 2020). Therefore, in comparison to calcium phosphate-calcium sulfate complex bone cement, MPC may exhibit comparable or even superior performance in exerting multiple effects simultaneously. However, this hypothesis necessitates further verification in subsequent studies.
5 Conclusion
Extensive research efforts have been directed towards addressing bone infections and the associated bone defects, focusing on osteointegration through the incorporation of antimicrobial drugs into CPCs or enhancing the physicochemical properties of CPCs. This review aims to present a comprehensive overview of various CPC-based antimicrobial agents, aimed at augmenting the antimicrobial efficacy and osteointegrative capabilities of bone cement fillers or coatings. While antibiotics remain the most extensively studied antimicrobial agents loaded into CPCs, the emergence of drug-resistant pathogens has garnered increasing attention. Additionally, several research teams have validated alternative antimicrobial agents, such as antimicrobial peptides and metal ions, through in vitro and animal experiments. The physicochemical properties of CPC play a pivotal role in both the antimicrobial activity of drugs loaded within the cement matrix and the stimulation of new bone formation. Notably, the interconnected pores inherent to calcium phosphate particles serve as reservoirs for antimicrobial agents. By encapsulating these pores with polymers, the localized and sustained release of these drugs can be modulated, thereby facilitating the expression of CPC’s osteoconductive and osteoinductive properties. Utilizing advanced techniques, antimicrobial drug-loaded calcium phosphates can be formulated as surface coatings on metallic endoprostheses (such as titanium alloys) or as slurries applied to endoprosthetic surfaces. This approach offers not only robust mechanical support but also localized antimicrobial and osseointegrative effects, thereby enhancing the overall therapeutic outcome.
However, a significant drawback of developing multifunctional CPC is the loading of diverse antimicrobial drugs. This loading process can compromise the physicochemical properties of the CPC matrix, thereby hindering the maximizaion of the antimicrobial efficacy of these drugs. Furthermore, the intricate nature of the antimicrobial CPCs fabrication process, coupled with the stringent regulations imposed by national legislatures and regulatory agencies regarding the approval of in vivo implants in the healthcare industry, has restricted the commercial viability of CPCs in the treatment of bone infections to a certain extent.
6 Future perspectives
In the future, multifunctional CPCs are poised to play a pivotal role in the prevention and treatment of bone infections. However, the sole reliance on calcium phosphate-loaded antimicrobial agents has proven insufficient in clinical practice. Therefore, it is imperative to integrate local drug delivery technology with antimicrobials to develop CPCs that possess optimal physicochemical properties, including antimicrobial activity, osseointegration promotion, and self-degradability. Additionally, it is crucial to mitigate cytotoxicity through the judicious combination of antimicrobial agents, such as bioactive metallic silver. Nonetheless, the pursuit of multifunctional modifications in bone cements often necessitates the utilization of multiple techniques, thereby increasing the overall cost and time required for the preparation of antimicrobial bone cements.
The development of anti-infective CPCs capable of specifically targeting pathogenic bacteria colonized within host cells, coupled with advanced targeting technologies, remains a pressing challenge for researchers. To address this challenge, future research should focus on modifying antimicrobial drugs while maintaining the biocompatibility and osseointegrative properties of CPCs, aiming to effectively treat chronic bone infections that persist due to intracellular bacterial colonization (Watkins and Unnikrishnan, 2020). Silver-copper-boron (AgCuB) nanoparticles (NPs), as prepared by Qadri et al. (2019), have demonstrated no harmful effects on host cells in vitro at concentrations ranging from 1 to 5 μg/g of CPC. In vitro experiments have shown that AgCuB NPs at concentrations of 1–5 μg/mL significantly reduce the internalization of S. aureus infection in osteoblasts in a dose-dependent manner, with a single treatment dose, without causing harm to host cells. To enhance the anti-cellular properties of AgCuB NPs, Abdulrehman et al. (2020) conjugated the cadherin-11 antibody (OBAb) to the original nanoparticles in vitro, resulting in AgCuB-OBAb NPs that could specifically target osteoblasts and subsequently exhibited remarkable antibacterial activity against intracellular S. aureus. Additionally, the use of liposomes for delivering antimicrobial drugs to host cells has been reported to effectively kill intracellular pathogens (Ferreira et al., 2021). The antimicrobial efficacy of liposomes can be further validated by utilizing targeting technology to identify specific markers on host cells and subsequently delivering the drugs to these cells efficiently.
The employment of bacteriophages (phages) in the combat against bone infections, notably those orchestrated by S. aureus, has garnered substantial attention. Phage therapy, in contrast to conventional antibiotic regimens, showcases remarkable benefits including pronounced heterogeneity and a diminished proclivity for eliciting bacterial resistance (Plumet et al., 2022; Kim et al., 2021). By efficiently lysing pathogenic bacteria and mitigating biofilm accumulation, phages assume an optimal stance in the prophylaxis and management of bone infections (Young et al., 2024). Notably, the therapeutic efficacy is further bolstered when phages are synergistically administered with antibiotics, heralding a novel, non-antibiotic approach to counteract drug-refractory bacterial pathogens implicated in bone infections (Moghadam et al., 2024; Bouchart et al., 2020). Innovatively, researchers have harnessed 3D-printed calcium phosphate bioceramics as targeted phage delivery vehicles, enabling direct infusion of phages to the infected site. This strategy not only mitigates systemic adverse effects but also augments therapeutic outcomes (Bouchart et al., 2020). Nevertheless, the clinical translation of phage-based interventions for bone infection prevention and treatment faces undeniable challenges, encompassing the safety profile, stability of the phages themselves, as well as regulatory hurdles that necessitate careful consideration and ongoing investigation.
Numerous studies have explored the application of photodynamic therapy (PDT) in the treatment of malignant tumors. Calcium phosphate, as a biomaterial with broad application potential in the biomedical field, has demonstrated anti-tumor effects when combined with PDT through the construction of bionic systems. This integration can involve strategies such as the combination of chemotherapy with PDT, pH responsiveness, and other mechanisms (Zhong et al., 2021; Nomoto et al., 2016; Liu et al., 2019). Recently, several studies have confirmed the beneficial effects of combining calcium phosphate with PDT in combating bacterial infections (Tan et al., 2024; Chen X. et al., 2024; Hu et al., 2022). In the pathological progression of infected bone defects, the advancement of infection serves as the primary obstacle to successful bone regeneration, thereby complicating treatment strategies. Consequently, the management of infectious bone defects necessitates a coordinated approach that addresses both anti-infection measures and the promotion of new bone formation. Tan et al. (2024) successfully incorporated 2D Ti3C2 MXene and berberine (BBR) into a 3D-printed calcium phosphate scaffold. Through both in vitro and in vivo experiments, the Ti3C2-BBR functionalized calcium phosphate scaffold exhibited remarkable antibacterial and osteogenic properties. The persistence of intracellular bacterial colonization in bone infections has posed a formidable challenge for an extended period. Traditional antibiotics targeting intracellular bacteria often struggle to achieve satisfactory anti-infective outcomes and may carry the risk of inducing bacterial resistance. Chen X. et al. (2024) introduced a novel photodynamic/photothermal calcium phosphate nanoparticle coated with mannose-based lipids (MAN-LCaP@Indocyanine green (ICG)) for the eradication of intracellular MRSA. Both in vitro and in vivo experiments demonstrated that MAN-LCaP, serving as a drug delivery vehicle, exhibited preferential uptake by macrophages and facilitated the transport of ICG to intracellular pathogens. MAN-LCaP@ICG offers a promising avenue for the clinical application in the treatment of anti-intracellular infections.
Furthermore, the immune response is frequently overlooked in traditional therapeutic approaches for bone infections. When CPC is implanted as an inlay material, it is recognized as a foreign substance by the immune system. Bone immunomodulation introduces a novel concept for antimicrobial bone cement, aiming to mitigate bacterial colonization (Chen et al., 2016). Additionally, the integration of immunomodulatory effects into biomaterials can mitigate the body’s excessive immune response locally, fostering the establishment of favorable osseointegration between the implanted material and surrounding bone tissues. Olechnowicz et al. (2018) demonstrated that zinc can reduce local tissue damage during repair by activating antioxidant enzymes, scavenging reactive oxygen species, and mitigating oxidative stress, thus promoting the progression of localized damage towards repair. Macrophages have emerged as crucial players in enhancing the osseointegration of CPCs (Li et al., 2020; Wu et al., 2022). It is crucial to highlight that, while pursuing the desired immunomodulatory effects, further research is imperative to elucidate strategies that safeguard the antimicrobial efficiency of CPCs and their physicochemical properties, vital for the formation of new bone, from being compromised.
Statements
Author contributions
XL: Conceptualization, Writing–original draft. CW: Conceptualization, Supervision, Writing–review and editing. HW: Conceptualization, Writing–review and editing. GW: Conceptualization, Writing–review and editing. YoZ: Conceptualization, Writing–review and editing. YuZ: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research received the financial support from the incubation project from the Military Medical Science and Technology Youth Training Program of the Air Force Medical University of the Chinese People’s Liberation Army (No. 18QNP022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- CPC(s)
Calcium phosphate cement(s)
- TII(s)
Therapeutic inorganic ion(s)
- DFI(s)
Diabetic foot infection(s)
- S. aureus
Staphylococcus aureus
- MRSA
Methicillin-resistant Staphylococcus aureus
- OLCN
Osteocyte lacuno-canicular network
- DFO
Diabetic foot osteomyelitis
- PBP4
Penicillin-binding protein 4
- SasC
Surface adhesin-S. aureussurface protein C
- EPS
Extracellular polymeric substances
- Agr
Accessory gene regulator
- MSCRAMMs
Microbial surface components recognizing adhesive matrix molecules
- SpA
Staphylococcal protein A
- M-CSF
Macrophage colony-stimulating factor in osteoblasts
- RANKL
Receptor activator of nuclear factor-κB ligand
- DFU(s)
Diabetic foot ulcer(s)
- PMMA
Poly(methyl methacrylate)
- CSC(s)
Calcium sulfate cement(s)
- TCP
Tricalcium phosphate
- TTCP
Tetracalcium phosphate
- OCP
Octacalcium phosphate
- HA
Hydroxyapatite
- CDHA
Calcium-deficient hydroxyapatite
- MCPM
Monocalcium phosphate monohydrate
- CS
Chitosan
- HPMC
Hydroxypropyl methyl cellulose
- PLGA
Poly (lactic-co-glycolic acid)
- MIC
Minimum inhibitory concentration
- AMX
Amoxicillin
- PLA
Poly (lactic acid)
- Ctr-mpHANCs
Citrate-modified mesoporous hydroxyapatite nanocarrier
- CMC
Carboxymethyl cellulose
- PS
Phosphoserine
- S. epidermidis
Staphylococcus epidermidis
- E. coli
Escherichia coli
- P. aeruginosa
Pseudomonas aeruginosa
- K. pneumoniae
Klebsiella pneumoniae
- AMP(s)
Antimicrobial peptide(s)
- FDA
The U.S. Food and Drug Administration
- POPC
Palmitoyl oleoyl phosphatidyl-choline
- HABP1
Hydroxyapatite binding peptide-1
- Ag
Silver
- AgNP(s)
Silver nanoparticle(s)
- ROS
Reactive oxygen species
- DCPD
Dicalcium phosphate dihydrate
- Saos-2, HTB85
Human osteoblast-like osteosarcoma cells
- CuONP(s)
Copper oxide nanoparticle(s)
- CuTCPNP(s)
Copper-doped tricalcium phosphate
- CAM
Chorioallantoic membrane
- GO
Graphene oxide
- MC3T3-E1
Mouse embryo osteoblast precursor cell(s)
- DCP
Dicalcium phosphate
- ESR
Erythrocyte sedimentation rate
- hs-CRP
High-sensitivity C-reactive protein
- WBC
White blood cell count
- CS
Calcium sulfate
- CP
Calcium phosphate
- BCP
Biphasic calcium phosphate
- FRI
Fracture-related infection(s)
- PJI
Periprosthetic infection(s)
- MPC(s)
Magnesium phosphate cement(s)
- AgCuB
Silver-copper-boron
- NP(s)
Nanoparticle(s)
- OBAb
Cadherin-11 antibody
- PDT
Photodynamic therapy
- BBR
Berberine
- MAN-LCaP
Calcium phosphate nanoparticle coated with mannose-based lipids
- ICG
Indocyanine green
References
1
Abdulrehman T. Qadri S. Skariah S. Sultan A. Mansour S. Azzi J. et al (2020). Boron doped silver-copper alloy nanoparticle targeting intracellular S. aureus in bone cells. PLoS One15, e0231276. 10.1371/journal.pone.0231276
2
Akhavan O. Ghaderi E. Esfandiar A. (2011). Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B115, 6279–6288. 10.1021/jp200686k
3
Alder K. D. Lee I. Munger A. M. Kwon H.-K. Morris M. T. Cahill S. V. et al (2020). Intracellular Staphylococcus aureus in bone and joint infections: a mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone, 141. 10.1016/j.bone.2020.115568
4
Alegrete N. Sousa S. R. Peleteiro B. Monteiro F. J. Gutierres M. (2023). Local antibiotic delivery ceramic bone substitutes for the treatment of infected bone cavities and bone regeneration: a systematic review on what we have learned from animal models. Mater. (Basel)16, 2387. 10.3390/ma16062387
5
Andrzejowski P. Masquelet A. Giannoudis P. V. (2020). Induced membrane technique (masquelet) for bone defects in the distal tibia, foot, and ankle: systematic review, case presentations, tips, and techniques. Foot Ankle Clin.25, 537–586. 10.1016/j.fcl.2020.08.013
6
Apelt D. Theiss F. El-Warrak A. O. Zlinszky K. Bettschart-Wolfisberger R. Bohner M. et al (2004). In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials25, 1439–1451. 10.1016/j.biomaterials.2003.08.073
7
Arciola C. R. Campoccia D. Montanaro L. (2018). Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol.16, 397–409. 10.1038/s41579-018-0019-y
8
Armstrong D. G. Tan T. W. Boulton A. J. M. Bus S. A. (2023). Diabetic foot ulcers: a review. Jama330, 62–75. 10.1001/jama.2023.10578
9
Aslam B. Wang W. Arshad M. I. Khurshid M. Muzammil S. Rasool M. H. et al (2018). Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist11, 1645–1658. 10.2147/IDR.S173867
10
Bakshi P. S. Selvakumar D. Kadirvelu K. (2020). Chitosan as an environment friendly biomaterial - a review on recent modifications and applications. Int. J. Biol. Macromol.150, 1072–1083. 10.1016/j.ijbiomac.2019.10.113
11
Baroni A. Russo T. (2014). Relationship between local neuroimmune impairment and diabetic foot: the immunocompromised district theory. Int. J. Dermatol53, 263–266. 10.1111/ijd.12328
12
Baudín C. Benet T. Pena P. (2019). Effect of graphene on setting and mechanical behaviour of tricalcium phosphate bioactive cements. J. Mech. Behav. Biomed. Mater89, 33–47. 10.1016/j.jmbbm.2018.09.002
13
Bhanwala S. Kasaragod S. K. (2022). Single step treatment of frontal sinus osteomyelitis using bone cement: a case report. Indian J. Otolaryngology Head and Neck Surg.74, 1523–1526. 10.1007/s12070-021-02667-w
14
Bohner M. (2000). Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury31 (Suppl. 4), 37–47. 10.1016/s0020-1383(00)80022-4
15
Bose S. Sarkar N. Majumdar U. (2023). Micelle encapsulated curcumin and piperine-laden 3D printed calcium phosphate scaffolds enhance in vitro biological properties. Colloids Surfaces B Biointerfaces, 231. 10.1016/j.colsurfb.2023.113563
16
Bouchart F. Vidal O. Lacroix J. M. Spriet C. Chamary S. Brutel A. et al (2020). 3D printed bioceramic for phage therapy against bone nosocomial infections. Mater Sci. Eng. C Mater Biol. Appl.111, 110840. 10.1016/j.msec.2020.110840
17
Boyle K. K. Sosa B. Osagie L. Turajane K. Bostrom M. P. G. Yang X. (2019). Vancomycin-laden calcium phosphate-calcium sulfate composite allows bone formation in a rat infection model. PLoS One14, e0222034. 10.1371/journal.pone.0222034
18
Butrico C. E. Cassat J. E. (2020). Quorum sensing and toxin production in Staphylococcus aureus osteomyelitis: pathogenesis and paradox. Toxins (Basel), 12. 10.3390/toxins12080516
19
Chen L. Lin X. Wei M. Zhang B. Sun Y. Chen X. et al (2024a). Hierarchical antibiotic delivery system based on calcium phosphate cement/montmorillonite-gentamicin sulfate with drug release pathways. Colloids Surf. B Biointerfaces238, 113925. 10.1016/j.colsurfb.2024.113925
20
Chen X. Shi X. Liu X. Zhai X. Li W. Hong W. (2024b). Eliminating intracellular MRSA via mannosylated lipid-coated calcium phosphate nanoparticles. Mol. Pharm.21, 5772–5783. 10.1021/acs.molpharmaceut.4c00779
21
Chen Z. Klein T. Murray R. Z. Crawford R. Chang J. Wu C. et al (2016). “Osteoimmunomodulation for the development of advanced bone biomaterials,” in Materials today. Amsterdam: Elsevier Science B.V.304–321.
22
Choi Y. S. Kim Y. H. An H. M. Bae S. K. Lee Y. K. (2023). Efficacy of silver nanoparticles-loaded bone cement against an MRSA induced-osteomyelitis in a rat model. Med. Kaunas.59, 811. 10.3390/medicina59040811
23
Cobb L. H. McCabe E. M. (2020). Therapeutics and delivery vehicles for local treatment of osteomyelitis. J. Orthop. Res.38, 2091–2103. 10.1002/jor.24689
24
Costa B. Martínez-de-Tejada G. Gomes P. A. C. Mc L. M. Costa F. (2021). Antimicrobial peptides in the battle against orthopedic implant-related infections: a review. Pharmaceutics13, 1918. 10.3390/pharmaceutics13111918
25
Dapporto M. Tavoni M. Restivo E. Carella F. Bruni G. Mercatali L. et al (2022). Strontium-doped apatitic bone cements with tunable antibacterial and antibiofilm ability. Front. Bioeng. Biotechnol.10, 969641. 10.3389/fbioe.2022.969641
26
Daskalova A. Sezanova K. Angelova L. Paunova-Krasteva T. Gergulova R. Kovacheva D. et al (2023). Ultra-short laser-assisted micro-structure formations on Mg/Zn double-doped calcium phosphate ceramics for enhanced antimicrobial activity. Mater. (Basel)16, 6626. 10.3390/ma16206626
27
David Chen C.-H. Chen C.-C. Shie M.-Y. Huang C.-H. Ding S.-J. (2011). Controlled release of gentamicin from calcium phosphate/alginate bone cement. Mater. Sci. and Eng. C31, 334–341. 10.1016/j.msec.2010.10.002
28
Demir-Oğuz Ö. Boccaccini A. R. Loca D. (2023). Injectable bone cements: what benefits the combination of calcium phosphates and bioactive glasses could bring?Bioact. Mater19, 217–236. 10.1016/j.bioactmat.2022.04.007
29
Dijksteel G. S. Ulrich M. M. W. Middelkoop E. Boekema B. (2021). Review: lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol.12, 616979. 10.3389/fmicb.2021.616979
30
Dorozhkin S. V. (2019). Functionalized calcium orthophosphates (CaPO(4)) and their biomedical applications. J. Mater Chem. B7, 7471–7489. 10.1039/c9tb01976f
31
Fang L. Leng Y. Gao P. (2006). Processing and mechanical properties of HA/UHMWPE nanocomposites. Biomaterials27, 3701–3707. 10.1016/j.biomaterials.2006.02.023
32
Feng S. He F. Fang X. Deng X. Lu T. Ye J. (2021). Enhancement of mechanical strength and osteogenesis, and inhibition of osteoclastic activity for β-tricalcium phosphate bioceramics by incorporating calcium silicate and magnesium-strontium phosphate. Ceram. Int.47, 24191–24197. 10.1016/j.ceramint.2021.05.130
33
Ferreira M. Aguiar S. Bettencourt A. Gaspar M. M. (2021). Lipid-based nanosystems for targeting bone implant-associated infections: current approaches and future endeavors. Drug Deliv. Transl. Res.11, 72–85. 10.1007/s13346-020-00791-8
34
Foroutan F. McGuire J. Gupta P. Nikolaou A. Kyffin B. A. Kelly N. L. et al (2019). Antibacterial copper-doped calcium phosphate glasses for bone tissue regeneration. ACS Biomater. Sci. Eng.5, 6054–6062. 10.1021/acsbiomaterials.9b01291
35
Fosca M. Rau J. V. Uskoković V. (2022). Factors influencing the drug release from calcium phosphate cements. Bioact. Mater7, 341–363. 10.1016/j.bioactmat.2021.05.032
36
Freischmidt H. Armbruster J. Reiter G. Grützner P. A. Helbig L. Guehring T. (2020). Individualized techniques of implant coating with an antibiotic-loaded, hydroxyapatite/calcium sulphate bone graft substitute. Ther. Clin. Risk Manag.16, 689–694. 10.2147/TCRM.S242088
37
Garzoni C. Kelley W. L. (2011). Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol. Med.3, 115–117. 10.1002/emmm.201100123
38
Geurts J. A. P. van Vugt T. A. G. Arts J. J. C. (2021). Use of contemporary biomaterials in chronic osteomyelitis treatment: clinical lessons learned and literature review. J. Orthop. Res.39, 258–264. 10.1002/jor.24896
39
Ginebra M. P. Canal C. Espanol M. Pastorino D. Montufar E. B. (2012). Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev.64, 1090–1110. 10.1016/j.addr.2012.01.008
40
Ginebra M. P. Traykova T. Planell J. A. (2006). Calcium phosphate cements as bone drug delivery systems: a review. J. Control Release113, 102–110. 10.1016/j.jconrel.2006.04.007
41
Goldberg M. A. Krohicheva P. A. Fomin A. S. Khairutdinova D. R. Antonova O. S. Baikin A. S. et al (2020). Insitu magnesium calcium phosphate cements formation: from one pot powders precursors synthesis to in vitro investigations. Bioact. Mater5, 644–658. 10.1016/j.bioactmat.2020.03.011
42
Gritsch L. Maqbool M. Mouriño V. Ciraldo F. E. Cresswell M. Jackson P. R. et al (2019). Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium. J. Mater Chem. B7, 6109–6124. 10.1039/c9tb00897g
43
Grosfeld E. C. Hoekstra J. W. Herber R. P. Ulrich D. J. Jansen J. A. van den Beucken J. J. J. P. (2016). Long-term biological performance of injectable and degradable calcium phosphate cement. Biomed. Mater12, 015009. 10.1088/1748-605X/12/1/015009
44
Gupta S. Malhotra A. Jindal R. Garg S. K. Kansay R. Mittal N. (2019). Role of beta tri-calcium phosphate-based composite ceramic as bone-graft expander in masquelet's-induced membrane technique. Indian J. Orthop.53, 63–69. 10.4103/ortho.IJOrtho_240_17
45
Gurunathan S. Han J. W. Dayem A. A. Eppakayala V. Kim J. H. (2012). Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomedicine7, 5901–5914. 10.2147/IJN.S37397
46
He J. Zhu X. Qi Z. Wang C. Mao X. Zhu C. et al (2015). Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater Interfaces7, 5605–5611. 10.1021/acsami.5b01069
47
Holloway J. L. (2019). One step solution for fighting bacteria and growing bone. Sci. Transl. Med.11 (479). 10.1126/scitranslmed.aaw5326
48
Hoppe A. Mouriño V. Boccaccini A. R. (2013). Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci.1, 254–256. 10.1039/c2bm00116k
49
Hu J. Ding Y. Tao B. Yuan Z. Yang Y. Xu K. et al (2022). Surface modification of titanium substrate via combining photothermal therapy and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection. Bioact. Mater18, 228–241. 10.1016/j.bioactmat.2022.03.011
50
Jensen L. K. Birch J. M. Jensen H. E. Kirketerp‐Møller K. Gottlieb H. (2023). “Bacterial invasion of the submicron osteocyte lacuna–canaliculi network (OLCN): a part of osteomyelitis disease biology,” Apmis. Gt., 131: 325–332. 10.1111/apm.13312
51
Jin N (2015). WBPIPWX-FLIH-LYX-SZHU Sustained release of Semaphorin 3A from a-tricalcium phosphate based cement composite contributes to osteoblastic differentiation of MC3T3-E1 cells. Front. Mater. Sci. China9, 282–292. 10.1007/s11706-015-0293-9
52
Kai K. C. Borges R. Pedroni A. C. F. Pelosine A. M. da Cunha M. R. Marques M. M. et al (2024). Tricalcium phosphate-loaded injectable hydrogel as a promising osteogenic and bactericidal teicoplanin-delivery system for osteomyelitis treatment: an in vitro and in vivo investigation. Biomater. Adv.164, 213966. 10.1016/j.bioadv.2024.213966
53
Kaiser F. Schröter L. Stein S. Krüger B. Weichhold J. Stahlhut P. et al (2022). Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater.145, 358–371. 10.1016/j.actbio.2022.04.019
54
Kalelkar P. P. Riddick M. García A. J. (2022). Biomaterial-based delivery of antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater.7, 39–54. 10.1038/s41578-021-00362-4
55
Kamphof R. Lima R. N. O. Schoones J. W. Arts J. J. Nelissen R. Cama G. et al (2023). Antimicrobial activity of ion-substituted calcium phosphates: a systematic review. Heliyon9, e16568. 10.1016/j.heliyon.2023.e16568
56
Kassem A. Lindholm C. (2016). Toll-like receptor 2 stimulation of osteoblasts mediates Staphylococcus aureus induced bone resorption and osteoclastogenesis through enhanced RANKL. PLoS One11, e0156708. 10.1371/journal.pone.0156708
57
Kavanagh N. Ryan E. J. Widaa A. Sexton G. Fennell J. O'Rourke S. et al (2018). Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev.31. 10.1128/CMR.00084-17
58
Kazemzadeh-Narbat M. Kindrachuk J. Duan K. Jenssen H. Hancock R. E. Wang R. (2010). Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials31, 9519–9526. 10.1016/j.biomaterials.2010.08.035
59
Kazemzadeh-Narbat M. Lai B. F. Ding C. Kizhakkedathu J. N. Hancock R. E. Wang R. (2013). Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials34, 5969–5977. 10.1016/j.biomaterials.2013.04.036
60
Kazemzadeh-Narbat M. Noordin S. Masri B. A. Garbuz D. S. Duncan C. P. Hancock R. E. W. et al (2012). Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater Res. B Appl. Biomater.100, 1344–1352. 10.1002/jbm.b.32701
61
Kendall R. W. Duncan C. P. Smith J. A. (1996). Persistence of bacteria on antibiotic loaded acrylic depots. A reason for caution. Clin. Orthop. Relat. Res.329, 273–280. 10.1097/00003086-199608000-00034
62
Khosalim I. P. Zhang Y. Y. Yiu C. K. Y. Wong H. M. (2022). Synthesis of a graphene oxide/agarose/hydroxyapatite biomaterial with the evaluation of antibacterial activity and initial cell attachment. Sci. Rep.12, 1971. 10.1038/s41598-022-06020-1
63
Kim H. Y. Chang R. Y. K. Morales S. Chan H. K. (2021). Bacteriophage-delivering hydrogels: current progress in combating antibiotic resistant bacterial infection. Antibiot. (Basel)10, 130. 10.3390/antibiotics10020130
64
Köse N. Asfuroğlu Z. M. Köse A. Şahintürk V. Gürbüz M. Doğan A. (2021). Silver ion-doped calcium phosphate-based bone-graft substitute eliminates chronic osteomyelitis: an experimental study in animals. J. Orthop. Res.39, 1390–1401. 10.1002/jor.24946
65
Kost J. Huwyler J. Puchkov M. (2023). Calcium phosphate microcapsules as multifunctional drug delivery devices. Adv. Funct. Mater.33, 1–11. 10.1002/adfm.202303333
66
Kumar Dewangan V. Sampath Kumar T. S. Doble M. Daniel Varghese V. (2023). Fabrication of injectable antibiotic-loaded apatitic bone cements with prolonged drug delivery for treating post-surgery infections. J. Biomed. Mater. Res. Part A111, 1750–1767. 10.1002/jbm.a.37584
67
Lazarettos J. Efstathopoulos N. Papagelopoulos P. J. Savvidou O. D. Kanellakopoulou K. Giamarellou H. et al (2004). A bioresorbable calcium phosphate delivery system with teicoplanin for treating MRSA osteomyelitis. Clin. Orthop. Relat. Res.423, 253–258. 10.1097/01.blo.0000127422.06956.35
68
Lee S. H. Jun B. H. (2019). Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci.20, 865. 10.3390/ijms20040865
69
Leone A. Vitiello C. Gullì C. Sikora A. K. Macagnino S. Colosimo C. (2020). Bone and soft tissue infections in patients with diabetic foot. Radiol. Med.125, 177–187. 10.1007/s11547-019-01096-8
70
Li D. X. Fan H. S. Zhu X. D. Tan Y. F. Xiao W. Q. Lu J. et al (2007). Controllable release of salmon-calcitonin in injectable calcium phosphate cement modified by chitosan oligosaccharide and collagen polypeptide. J. Mater Sci. Mater Med.18, 2225–2231. 10.1007/s10856-007-3084-8
71
Li M. Guo X. Qi W. Wu Z. de Bruijn J. D. Xiao Y. et al (2020). Macrophage polarization plays roles in bone formation instructed by calcium phosphate ceramics. J. Mater Chem. B8, 1863–1877. 10.1039/c9tb02932j
72
Liu Q. Lodoso-Torrecilla I. Gunnewiek R. K. Harhangi H. R. Mikos A. G. van Niftrik L. et al (2023). Tunable calcium phosphate cement formulations for predictable local release of doxycycline. Materialia28, 101769. 10.1016/j.mtla.2023.101769
73
Liu S. Li W. Dong S. Gai S. Dong Y. Yang D. et al (2019). Degradable calcium phosphate-coated upconversion nanoparticles for highly efficient chemo-photodynamic therapy. ACS Appl. Mater Interfaces11, 47659–47670. 10.1021/acsami.9b11973
74
Loca D. Sokolova M. Locs J. Smirnova A. Irbe Z. (2015). Calcium phosphate bone cements for local vancomycin delivery. Mater Sci. Eng. C Mater Biol. Appl.49, 106–113. 10.1016/j.msec.2014.12.075
75
Luo J. Engqvist H. Persson C. (2018). A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater.81, 304–314. 10.1016/j.actbio.2018.10.001
76
Luo Y. Song Y. (2021). Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci.22, 11401. 10.3390/ijms222111401
77
Mabroum H. Elbaza H. Ben Youcef H. Oudadesse H. Noukrati H. Barroug A. (2023). Design of antibacterial apatitic composite cement loaded with Ciprofloxacin: investigations on the physicochemical Properties, release Kinetics, and antibacterial activity. Int. J. Pharm.637, 122861. 10.1016/j.ijpharm.2023.122861
78
Maity S. Leton N. Nayak N. Jha A. Anand N. Thompson K. et al (2024). A systematic review of diabetic foot infections: pathogenesis, diagnosis, and management strategies. Front. Clin. Diabetes Healthc.5, 1393309. 10.3389/fcdhc.2024.1393309
79
Makvandi P. Wang C. Zare E. Borzacchiello A. Niu L. Tay F. R. et al (2020). “Metal‐based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects,” in Advanced functional materials. Germany: John Wiley and Sons, Ltd.
80
Marziya A. Yingao M. Liang C. Henigul O. Liu M. Jiang T. et al (2023). Electrospinning fibers modified with near infrared light‐excited copper nanoparticles for antibacterial and bone regeneration. Adv. Mater. Interfaces10. 10.1002/admi.202300113
81
Masters E. A. Muthukrishnan G. Ho L. Gill A. L. de Mesy Bentley K. L. Galloway C. A. et al (2021). Staphylococcus aureus cell wall biosynthesis modulates bone invasion and osteomyelitis pathogenesis. Front. Microbiol.12, 723498. 10.3389/fmicb.2021.723498
82
Masters E. A. Ricciardi B. F. Bentley K. L. M. Moriarty T. F. Schwarz E. M. Muthukrishnan G. (2022). Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol.20, 385–400. 10.1038/s41579-022-00686-0
83
McInnes S. J. Michl T. D. Delalat B. Al-Bataineh S. A. Coad B. R. Vasilev K. et al (2016). Thunderstruck: plasma-polymer-coated porous silicon microparticles as a controlled drug delivery system. ACS Appl. Mater Interfaces8, 4467–4476. 10.1021/acsami.5b12433
84
McNally M. Govaert G. Dudareva M. Morgenstern M. Metsemakers W. J. (2020). Definition and diagnosis of fracture-related infection. EFORT Open Rev.5, 614–619. 10.1302/2058-5241.5.190072
85
Melicherčík P. Nešuta O. Čeřovský V. (2018). Antimicrobial peptides for topical treatment of osteomyelitis and implant-related infections: study in the spongy bone. Pharm. (Basel)11, 20. 10.3390/ph11010020
86
Mendoza B. A. Delpino M. V. Lattar S. Giai C. Llana M. N. Sanjuan N. et al (2016). Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochim. Biophys. Acta1862, 1975–1983. 10.1016/j.bbadis.2016.07.016
87
Migliorini F. La Padula G. Torsiello E. Spiezia F. Oliva F. Maffulli N. (2021). Strategies for large bone defect reconstruction after trauma, infections or tumour excision: a comprehensive review of the literature. Eur. J. Med. Res.26, 118. 10.1186/s40001-021-00593-9
88
Mijnendonckx K. Leys N. Mahillon J. Silver S. (2013). Antimicrobial silver: uses, toxicity and potential for resistance. Biometals26, 609–621. 10.1007/s10534-013-9645-z
89
Mistry S. Roy R. Jha A. K. Pandit N. Das S. Burman S. et al (2022). Treatment of long bone infection by a biodegradable bone cement releasing antibiotics in human. J. Control Release346, 180–192. 10.1016/j.jconrel.2022.04.018
90
Mistry S. Roy S. Maitra N. J. Kundu B. Chanda A. Datta S. et al (2016). A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model. J. Control Release239, 169–181. 10.1016/j.jconrel.2016.08.014
91
Moghadam M. T. Mojtahedi A. Salamy S. Shahbazi R. Satarzadeh N. Delavar M. et al (2024). Phage therapy as a glimmer of hope in the fight against the recurrence or emergence of surgical site bacterial infections. Infection52, 385–402. 10.1007/s15010-024-02178-0
92
Mohseni S. Aalaa M. Atlasi R. Mohajeri Tehrani M. R. Sanjari M. Amini M. R. (2019). The effectiveness of negative pressure wound therapy as a novel management of diabetic foot ulcers: an overview of systematic reviews. J. Diabetes Metab. Disord.18, 625–641. 10.1007/s40200-019-00447-6
93
Morinaga S. Yamamoto N. Tokoro M. Hayashi K. Takeuchi A. Miwa S. et al (2023). Antibacterial effect and biological reaction of calcium phosphate cement impregnated with iodine for use in bone defects. J. biomaterials Appl.37, 1716–1723. 10.1177/08853282231164827
94
Mouriño V. Boccaccini A. R. (2010). Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface7, 209–227. 10.1098/rsif.2009.0379
95
Mouriño V. Cattalini J. P. Boccaccini A. R. (2012). Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J. R. Soc. Interface9, 401–419. 10.1098/rsif.2011.0611
96
Mucalo M. R. (2019). Special issue: novel advances and approaches in biomedical materials based on calcium phosphates. Mater. (Basel)12, 405. 10.3390/ma12030405
97
Muthukrishnan G. Masters E. A. Daiss J. L. Schwarz E. M. (2019). Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr. Osteoporos. Rep.17, 395–404. 10.1007/s11914-019-00548-4
98
Näf L. Miescher I. Pfuderer L. Schweizer T. A. Brunner D. Dürig J. et al (2024). Pro-angiogenic and antibacterial copper containing nanoparticles in PLGA/amorphous calcium phosphate bone nanocomposites. Heliyon10, e27267. 10.1016/j.heliyon.2024.e27267
99
Nandi S. K. Bandyopadhyay S. Das P. Samanta I. Mukherjee P. Roy S. et al (2016). Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv.34, 1305–1317. 10.1016/j.biotechadv.2016.09.005
100
Nasser H. A. Muhammad Usman M. Nabil K. A. Khalid Saad A. Ayesha I. Almurshedi A. S. et al (2022). Synthesis and characterization of antibiotic–loaded biodegradable citrate functionalized mesoporous hydroxyapatite nanocarriers as an alternative treatment for bone infections. Pharmaceutics14, 975. 10.3390/pharmaceutics14050975
101
Negm N. A. Hefni H. H. H. Abd-Elaal A. A. A. Badr E. A. (2020). Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol.152, 681–702. 10.1016/j.ijbiomac.2020.02.196
102
Nir-Paz R. Gelman D. Khouri A. Sisson B. M. Fackler J. Alkalay-Oren S. et al (2019). Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin. Infect. Dis.69, 2015–2018. 10.1093/cid/ciz222
103
Nomoto T. Fukushima S. Kumagai M. Miyazaki K. Inoue A. Mi P. et al (2016). Calcium phosphate-based organic-inorganic hybrid nanocarriers with pH-responsive on/off switch for photodynamic therapy. Biomater. Sci.4, 826–838. 10.1039/c6bm00011h
104
Noor S. Khan R. U. Ahmad J. (2017). Understanding diabetic foot infection and its management. Diabetes Metab. Syndr.11, 149–156. 10.1016/j.dsx.2016.06.023
105
Ogino A. Nakamichi M. Takeda K. Onishi K. (2020). Cranial reconstruction using antibiotic-impregnated calcium phosphate bone cement with a titanium mesh sheet. J. Craniofac Surg.31, 1452–1454. 10.1097/SCS.0000000000006427
106
Olechnowicz J. Tinkov A. Skalny A. Suliburska J. (2018). Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci.68, 19–31. 10.1007/s12576-017-0571-7
107
Ostrowski N. Roy A. (2016). Magnesium phosphate cement systems for hard tissue applications: a review. ACS Biomater. Sci. Eng.2, 1067–1083. 10.1021/acsbiomaterials.6b00056
108
Ozder M. N. Ciftci F. Rencuzogullari O. Arisan E. D. Ustündag C. B. (2023). In situ synthesis and cell line studies of nano-hydroxyapatite/graphene oxide composite materials for bone support applications. Ceram. Int.49, 14791–14803. 10.1016/j.ceramint.2023.01.075
109
Parent M. Baradari H. Champion E. Damia C. Viana-Trecant M. (2017). Design of calcium phosphate ceramics for drug delivery applications in bone diseases: a review of the parameters affecting the loading and release of the therapeutic substance. J. Control Release252, 1–17. 10.1016/j.jconrel.2017.02.012
110
Peng Q. Tang X. Dong W. Sun N. Yuan W. A. (2022). A review of biofilm formation of Staphylococcus aureus and its regulation mechanism. Antibiot. (Basel)12, 12. 10.3390/antibiotics12010012
111
Pitocco D. Spanu T. Di Leo M. Vitiello R. Rizzi A. Tartaglione L. et al (2019). Diabetic foot infections: a comprehensive overview. Eur. Rev. Med. Pharmacol. Sci.23, 26–37. 10.26355/eurrev_201904_17471
112
Plumet L. Ahmad-Mansour N. Dunyach-Remy C. Kissa K. Sotto A. Lavigne J. P. et al (2022). Bacteriophage therapy for Staphylococcus aureus infections: a review of animal models, treatments, and clinical trials. Front. Cell. Infect. Microbiol.12, 907314. 10.3389/fcimb.2022.907314
113
Pradeep A. Varma P. K. Arumugam T. Vijayan A. M. Vasudevan A. K. Rangasamy J. (2023). Antibacterial bone adhesive cement for preventing sternal infections after cardiac surgery. Ceram. Int.49, 16110–16122. 10.1016/j.ceramint.2023.01.209
114
Qadri S. Abdulrehman T. Azzi J. Mansour S. Haik Y. (2019). AgCuB nanoparticle eradicates intracellular S. aureus infection in bone cells: in vitro. Emergent Mater.2, 219–231. 10.1007/s42247-019-00035-7
115
Quan Q. Gongping X. Ruisi N. Shiwen L. (2023). New research progress of modified bone cement applied to vertebroplasty. World Neurosurg.176, 10–18. 10.1016/j.wneu.2023.04.048
116
Radwan N. H. Nasr M. Ishak R. A. H. (2021). Moxifloxacin-loaded in situ synthesized Bioceramic/Poly(L-lactide-co-ε-caprolactone) composite scaffolds for treatment of osteomyelitis and orthopedic regeneration. Int. J. Pharm.602, 120662. 10.1016/j.ijpharm.2021.120662
117
Raja J. M. Maturana M. A. Kayali S. Khouzam A. Efeovbokhan N. (2023). Diabetic foot ulcer: a comprehensive review of pathophysiology and management modalities. World J. Clin. Cases11, 1684–1693. 10.12998/wjcc.v11.i8.1684
118
Rh Owen G. Dard M. Larjava H. (2018). Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater Res. B Appl. Biomater.106, 2493–2512. 10.1002/jbm.b.34049
119
Ribeiro N. Reis M. Figueiredo L. Pimenta A. Santos L. F. Branco A. et al (2022). Improvement of a commercial calcium phosphate bone cement by means of drug delivery and increased injectability. Ceram. Int.48, 33361–33372. 10.1016/j.ceramint.2022.07.279
120
Rizzello L. (2014). Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev.43, 1501–1518. 10.1039/c3cs60218d
121
Rubitschung K. Sherwood A. Crisologo A. P. Bhavan K. Haley R. W. Wukich D. K. et al (2021). Pathophysiology and molecular imaging of diabetic foot infections. Int. J. Mol. Sci.22, 11552. 10.3390/ijms222111552
122
Russell T. A. Leighton R. K. Alpha-BSM Tibial Plateau Fracture Study Group (2008). Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J. Bone Jt. Surg. Am.90, 2057–2061. 10.2106/JBJS.G.01191
123
Samuel C. Andrew J. C. Rashad K. Daniel C. Torben D. Crawford R. J. et al (2020). Antimicrobial metal nanomaterials: from passive to stimuli‐activated applications. Adv. Sci.7, 1902913. 10.1002/advs.201902913
124
Sasaki T. Ishibashi Y. Katano H. Nagumo A. Toh S. (2005). In vitro elution of vancomycin from calcium phosphate cement. J. Arthroplasty20, 1055–1059. 10.1016/j.arth.2005.03.035
125
Schade A. T. Khatri C. Nwankwo H. Carlos W. Harrison W. J. Metcalfe A. J. (2021). The economic burden of open tibia fractures: a systematic review. Injury52, 1251–1259. 10.1016/j.injury.2021.02.022
126
Schilcher K. Horswill A. R. (2020). Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev.84. 10.1128/MMBR.00026-19
127
Senneville É. Albalawi Z. van Asten S. A. Abbas Z. G. Allison G. Aragón-Sánchez J. et al (2024). Diagnosis of infection in the foot of patients with diabetes: a systematic review. Diabetes Metab. Res. Rev.40, e3723. 10.1002/dmrr.3723
128
Senneville E. M. Lipsky B. A. van Asten S. A. V. Peters E. J. (2020). Diagnosing diabetic foot osteomyelitis. Diabetes Metab. Res. Rev.36 (Suppl. 1), e3250. 10.1002/dmrr.3250
129
Seonwoo H. Choung H.-W. Park S. Choi K. S. Jang K.-J. Kim J. et al (2022). Reduced graphene oxide-incorporated calcium phosphate cements with pulsed electromagnetic fields for bone regeneration. RSC Adv.12, 5557–5570. 10.1039/d1ra05717k
130
Shen Q. Qi Y. Kong Y. Bao H. Wang Y. Dong A. et al (2021). Advances in copper-based biomaterials with antibacterial and osteogenic properties for bone tissue engineering. Front. Bioeng. Biotechnol.9, 795425. 10.3389/fbioe.2021.795425
131
Shu X. Liao J. Wang Q. Wang L. Shi Q. Xie X. (2024). Enhanced osteogenic and bactericidal performance of premixed calcium phosphate cement with photocrosslinked alginate thin film. J. Biomed. Mater Res. A112, 1057–1069. 10.1002/jbm.a.37688
132
Sikder P. Coomar P. P. Mewborn J. M. (2020). Antibacterial calcium phosphate composite cements reinforced with silver-doped magnesium phosphate (newberyite) micro-platelets. J. Mech. Behav. Biomed. Mater110, 103934. 10.1016/j.jmbbm.2020.103934
133
Sinha R. Shukla P. (2019). Antimicrobial peptides: recent insights on biotechnological interventions and future perspectives. Protein Pept. Lett.26, 79–87. 10.2174/0929866525666181026160852
134
Tan Y. Sun H. Lan Y. Khan H. M. Zhang H. Zhang L. et al (2024). Study on 3D printed MXene-berberine-integrated scaffold for photo-activated antibacterial activity and bone regeneration. J. Mater Chem. B12, 2158–2179. 10.1039/d3tb02306k
135
Tao F. Cheng Y. Shi X. Zheng H. Du Y. Xiang W. et al (2020). Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym.230, 115658. 10.1016/j.carbpol.2019.115658
136
Thomes B. Murray P. Bouchier-Hayes D. (2002). Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J. Bone Jt. Surg. Br.84, 758–760. 10.1302/0301-620x.84b5.11907
137
Tian Y. Sun R. Li Y. Liu P. Fan B. Xue Y. (2024). Research progress on the application of magnesium phosphate bone cement in bone defect repair: a review. Biomed. Mater Eng.35, 265–278. 10.3233/BME-230164
138
Tiğli R. S. Akman A. C. Gümüşderelioğlu M. Nohutçu R. M. (2009). In vitro release of dexamethasone or bFGF from chitosan/hydroxyapatite scaffolds. J. Biomater. Sci. Polym. Ed.20, 1899–1914. 10.1163/156856208X399945
139
Tong Z. Chen Z. Li Z. Xie Z. Zhang H. (2022). Mechanisms of promoting the differentiation and bone resorption function of osteoclasts by Staphylococcus aureus infection. Int. J. Med. Microbiol.312, 151568. 10.1016/j.ijmm.2022.151568
140
Tsuchiya H. Shirai T. Nishida H. Murakami H. Kabata T. Yamamoto N. et al (2012). Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci.17, 595–604. 10.1007/s00776-012-0247-3
141
Udagawa N. Koide M. Nakamura M. Nakamichi Y. Yamashita T. Uehara S. et al (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab.39, 19–26. 10.1007/s00774-020-01162-6
142
Vallet-Regí M. Lozano D. González B. Izquierdo-Barba I. (2020). Biomaterials against bone infection. Adv. Healthc. Mater9, e2000310. 10.1002/adhm.202000310
143
Van Staden A. D. Dicks L. M. (2012). Calcium orthophosphate-based bone cements (CPCs): applications, antibiotic release and alternatives to antibiotics. J. Appl. Biomater. Funct. Mater10, 2–11. 10.5301/JABFM.2012.9279
144
van Staden A. D. Heunis T. D. J. (2011). Release of Enterococcus mundtii bacteriocin ST4SA from self-setting brushite bone cement. Probiotics Antimicrob. Proteins3, 119–124. 10.1007/s12602-011-9074-7
145
Veerapandian M. Zhang L. Krishnamoorthy K. Yun K. (2013). Surface activation of graphene oxide nanosheets by ultraviolet irradiation for highly efficient anti-bacterials. Nanotechnology24, 395706. 10.1088/0957-4484/24/39/395706
146
Vezenkova A. Locs J. (2022). Sudoku of porous, injectable calcium phosphate cements - path to osteoinductivity. Bioact. Mater17, 109–124. 10.1016/j.bioactmat.2022.01.001
147
Volejníková A. Melicherčík P. Nešuta O. Vaňková E. Bednárová L. Rybáček J. et al (2019). Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol.68, 961–972. 10.1099/jmm.0.001000
148
Wang S. Y. Yao R. B. Yang K. S. Liang H. C. Su C. Y. Fang H. W. et al (2022). The efficacy of vancomycin-loaded biphasic calcium phosphate bone substitute in the promotion of new bone growth and the prevention of postoperative infection. Front. Bioeng. Biotechnol.10, 988436. 10.3389/fbioe.2022.988436
149
Wang X. Zhang M. Zhu T. Wei Q. Liu G. Ding J. (2023a). Flourishing antibacterial strategies for osteomyelitis therapy. Adv. Sci. (Weinh)10, e2206154. 10.1002/advs.202206154
150
Wang Y. Gou Z. Ma S. Jin Z. Chen S. Ye J. et al (2023b). Remote eradication of delayed infection on orthopedic implants via magnesium-based total morphosynthesis of biomimetic mineralization strategy. Mater. and Des.233, 112233. 10.1016/j.matdes.2023.112233
151
Watkins K. E. Unnikrishnan M. (2020). Evasion of host defenses by intracellular Staphylococcus aureus. Adv. Appl. Microbiol.112, 105–141. 10.1016/bs.aambs.2020.05.001
152
Wolf-Brandstetter C. Beutner R. Hess R. Bierbaum S. Wagner K. Scharnweber D. et al (2020). Multifunctional calcium phosphate based coatings on titanium implants with integrated trace elements. Biomed. Mater15, 025006. 10.1088/1748-605X/ab5d7b
153
Wu J. Feng C. Wang M. Wu H. Zhu X. Li X. et al (2022). Whisker of biphasic calcium phosphate ceramics: osteo-immunomodulatory behaviors. Nano Res.15, 9169–9182. 10.1007/s12274-022-4591-0
154
Wu S. Lei L. Bao C. Liu J. Weir M. D. Ren K. et al (2021). An injectable and antibacterial calcium phosphate scaffold inhibiting Staphylococcus aureus and supporting stem cells for bone regeneration. Mater Sci. Eng. C Mater Biol. Appl.120, 111688. 10.1016/j.msec.2020.111688
155
Wu S. Lei L. Zhang H. Liu J. Weir M. D. Schneider A. et al (2020a). Nanographene oxide-calcium phosphate to inhibit Staphylococcus aureus infection and support stem cells for bone tissue engineering. J. tissue Eng. Regen. Med.14, 1779–1791. 10.1002/term.3139
156
Wu S. Lei L. Zhang H. Liu J. Weir M. D. Schneider A. et al (2020b). Nanographene oxide-calcium phosphate to inhibit Staphylococcus aureus infection and support stem cells for bone tissue engineering. J. Tissue Eng. Regen. Med.14, 1779–1791. 10.1002/term.3139
157
Wu Y. Garren M. R. Estes Bright L. M. Maffe P. Brooks M. Brisbois E. J. et al (2024). Enhanced antibacterial efficacy against antibiotic-resistant bacteria via nitric oxide-releasing ampicillin polymer substrates. J. Colloid Interface Sci.653, 1763–1774. 10.1016/j.jcis.2023.09.188
158
Xu H. H. Wang P. Wang L. Bao C. Chen Q. Weir M. D. et al (2017). Calcium phosphate cements for bone engineering and their biological properties. Bone Res.5, 17056. 10.1038/boneres.2017.56
159
Yang Y. Chu L. Yang S. Zhang H. Qin L. Guillaume O. et al (2018). Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater.79, 265–275. 10.1016/j.actbio.2018.08.015
160
Yazici H. Habib G. Boone K. Urgen M. Utku F. S. Tamerler C. (2019). Self-assembling antimicrobial peptides on nanotubular titanium surfaces coated with calcium phosphate for local therapy. Mater Sci. Eng. C Mater Biol. Appl.94, 333–343. 10.1016/j.msec.2018.09.030
161
Yoshimoto T. Kittaka M. Doan A. A. P. Urata R. Prideaux M. Rojas R. E. et al (2022). Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection. Nat. Commun.13, 6648. 10.1038/s41467-022-34352-z
162
Young J. Lee S. W. Shariyate M. J. Cronin A. Wixted J. J. Nazarian A. et al (2024). Bacteriophage therapy and current delivery strategies for orthopedic infections: a SCOPING review. J. Infect.88, 106125. 10.1016/j.jinf.2024.106125
163
Yu B. Pacureanu A. Olivier C. Cloetens P. Peyrin F. (2020). Assessment of the human bone lacuno-canalicular network at the nanoscale and impact of spatial resolution. Sci. Rep.10, 4567. 10.1038/s41598-020-61269-8
164
Yu T. Ye J. Gao C. Yu L. Wang Y. (2010). “Synthesis and drug delivery property of calcium phosphate cement with special crystal morphology,” Journal- Am. Ceram. Soc., 93, 1241–1244. 10.1111/j.1551-2916.2009.03537.x
165
Zhao Z. Wang G. Zhang Y. Luo W. Liu S. Liu Y. et al (2020). The effect of calcium sulfate/calcium phosphate composite for the treatment of chronic osteomyelitis compared with calcium sulfate. Ann. Palliat. Med.9, 1821–1833. 10.21037/apm.2020.03.23
166
Zheng K. Setyawati M. I. Leong D. T. (2018). Antimicrobial silver nanomaterials. Coord. Chem. Rev.357, 1–17. 10.1016/j.ccr.2017.11.019
167
Zhong C. Wu Y. Lin H. Liu R. (2023). Advances in the antimicrobial treatment of osteomyelitis. Compos. Part B, 249. 10.1016/j.compositesb.2022.110428
168
Zhong D. Li W. Hua S. Qi Y. Xie T. Qiao Y. et al (2021). Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy. Theranostics11, 3580–3594. 10.7150/thno.55441
Summary
Keywords
calcium phosphate cement, resistance to antibiotics, bone infection, antibacterial agents, multifunctional bone cement
Citation
Liu X, Wang C, Wang H, Wang G, Zhang Y and Zhang Y (2025) Calcium phosphate-based anti-infective bone cements: recent trends and future perspectives. Front. Pharmacol. 16:1522225. doi: 10.3389/fphar.2025.1522225
Received
04 November 2024
Accepted
07 February 2025
Published
26 February 2025
Volume
16 - 2025
Edited by
Anutthaman Parthasarathy, University of Bradford, United Kingdom
Reviewed by
Roger Borges, Federal University of ABC, Brazil
Francesca Ridi, University of Florence, Italy
Updates
Copyright
© 2025 Liu, Wang, Wang, Wang, Zhang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfei Zhang, tdbone@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.