Abstract
The complex etiology and spectrum of kidney diseases necessitate vigilant attention; the focus on early diagnosis and intervention in kidney diseases remains a critical issue in medical research. Recently, with the expanding studies on extracellular vesicles, exosomes have garnered increasing interest as a promising tool for the diagnosis and treatment of kidney diseases. Exosomes are nano-sized extracellular vesicles that transport a diverse array of bioactive substances, which can influence various pathological processes associated with kidney diseases and exhibit detrimental or beneficial effects. Within the kidney, exosomes derived from the glomeruli and renal tubules possess the ability to enter systemic circulation or urine. The biomarkers they carry can reflect alterations in the pathological state of the kidneys, thereby offering novel avenues for early diagnosis. Furthermore, research studies have confirmed that exosomes originating from multiple cell types exhibit therapeutic potential in treating kidney disease; notably, those derived from mesenchymal stem cells (MSCs) have shown significant treatment efficacy. This comprehensive review summarizes the contributions of exosomes from different cell types within the kidneys while exploring their physiological and pathological roles therein. Additionally, we emphasize recent advancements in exosome applications for the diagnosis and treatment of various forms of kidney diseases over the past decades. We not only introduce the urinary and blood biomarkers linked to kidney diseases found within exosomes but also explore their therapeutic effects. Finally, we discuss existing challenges and future directions concerning the clinical applications of exosomes for diagnostic and therapeutic purposes.
1 Introduction
Kidney diseases are varied and prevalent across the globe, impacting the quality of life and health of millions of individuals. These conditions present a significant challenge to healthcare systems worldwide. Severe kidney disease can progress to end-stage renal disease (ESRD); therefore, early recognition and proactive interventions are essential for mitigating the global burden of kidney disease (Eknoyan et al., 2004). Urinalysis and blood tests have been acknowledged as critical indicators for assessing and monitoring renal function, including proteinuria and serum creatinine levels. However, their utility in evaluating renal disease is limited by issues such as specificity and sensitivity as they can be influenced by various physiological and pathological factors, often lagging behind the onset of early kidney damage (Aklilu, 2023). Renal puncture biopsy remains the gold standard for diagnosing most renal diseases; however, it is an invasive procedure that carries risks such as bleeding and infection during puncture, making it unsuitable for routine screening. Consequently, identifying non-invasive and sensitive biomarkers for the diagnosis of renal diseases is imperative (Chen et al., 2018).
Exosomes play a pivotal role in renal physiology and pathology, acting as essential mediators of intercellular communication. Exosomes are present in nearly all cell types and body fluids, including plasma, urine, saliva, breast milk, cerebrospinal fluid, and ascites. Notably, certain components of exosomes, such as microRNAs (miRNAs), have the potential to serve as biomarkers for early-stage renal pathology. The progression of kidney disease can be monitored by assessing alterations in the quantity or composition of exosomes (Grange and Bussolati, 2022). Furthermore, due to their natural properties as vesicular carriers, exosomes can specifically target damaged kidney cells by delivering proteins, RNAs, and therapeutic agents to facilitate cellular repair and regeneration. The unique characteristics of exosomes present promising opportunities for the development of innovative therapeutic strategies aimed at treating kidney diseases.
This review discusses the relevant characteristics of exosomes, summarizes their mechanisms in various renal diseases based on existing studies, and investigates their potential as biomarkers and drug delivery systems for the diagnosis and treatment of renal disorders.
2 Biological characteristics of exosomes
2.1 Biogenesis, secretion, and structure of exosomes
Johnstone et al. (1987) were the pioneers in observing that amniotic reticulocytes can release membrane vesicles during their study of reticulocyte maturation, marking one of the early discoveries related to exocytosis. Initially, extracellular vesicles (EVs) garnered limited interest and were primarily regarded as a mechanism for cells to eliminate waste products. However, with ongoing research, the biological characteristics and significance of exosomes have gradually gained recognition and appreciation. Exosomes are a specific class of extracellular vesicles, ranging from 30 to 200 nm in diameter, produced by various cell types, and widely distributed across biological fluids such as blood, urine, saliva, pleural fluid, cerebrospinal fluid, breast milk, and semen. Exosome biogenesis involves four critical steps, namely, cargo sorting into multivesicular bodies (MVBs), MVB formation and maturation, MVB transport, and the fusion of MVBs with the plasma membrane (Han et al., 2022). Cargo sorting is responsible for targeting specific molecules and proteins within MVBs. The synthesis of exosomes typically begins at the early endosome, which originates from the inward budding of the cell membrane. At this stage, the endocytosed cargo undergoes primary sorting and fate determination. This process generally leads to three potential outcomes: recycled cargoes are localized to the peritubular region of the endosome before being separated and fused with either the Golgi apparatus or the plasma membrane of recycling endosomes. In contrast, the unrecovered cargoes become concentrated in the central region of the early endosome as it follows a maturation pathway toward late endosomes. Within late endosomes, some components of the endocytosed material are further sorted and processed, which ultimately results in MVB formation (Krylova and Feng, 2023). Typically, MVBs face one of the two fates: they may either bind to lysosomes for degradation due to their ubiquitinated contents or fuse with cytoplasmic membranes to release their contents into extracellular space (Doyle and Wang, 2019a).
The structural composition of exosomes is illustrated in Figure 1. Exosomes possess a well-defined membrane structure primarily composed of proteins and phospholipids. They are characterized by various surface proteins, including tetraspanins (CD9, CD63, CD81, and CD82), adhesion proteins, integrins, and glycoproteins. Certain specific surface proteins can be utilized to identify exosomes after isolation, thereby confirming the presence of exosome-like vesicles. Furthermore, the surface of exosomes contains distinct marker molecules associated with their parental cells. These markers can facilitate the identification or selection of particular exosome populations and enable the screening for disease-related biomarkers within these populations or the analysis of their functional roles. The exosomal components are diverse and capable of carrying an array of cargo molecules such as nucleic acids (including DNA, miRNAs, mRNA, siRNA, circular RNA, rRNA, and long non-coding RNA), proteins (e.g., Alix proteins, TSG101, heat shock proteins, and cytoskeletal proteins), and lipids (Liang et al., 2023). The cargoes carried by exosomes are closely linked to those of their parent cells. Consequently, there exists variability in the cargoes derived from different cell sources, which contributes to heterogeneity. Additionally, both the composition and quantity of exosomal cargoes may alter under pathological conditions. Qualitative and quantitative analyses of the contents within exosomes can be performed using techniques such as nucleic acid sequencing, proteomics, and lipidomics (Liu et al., 2024).
FIGURE 1
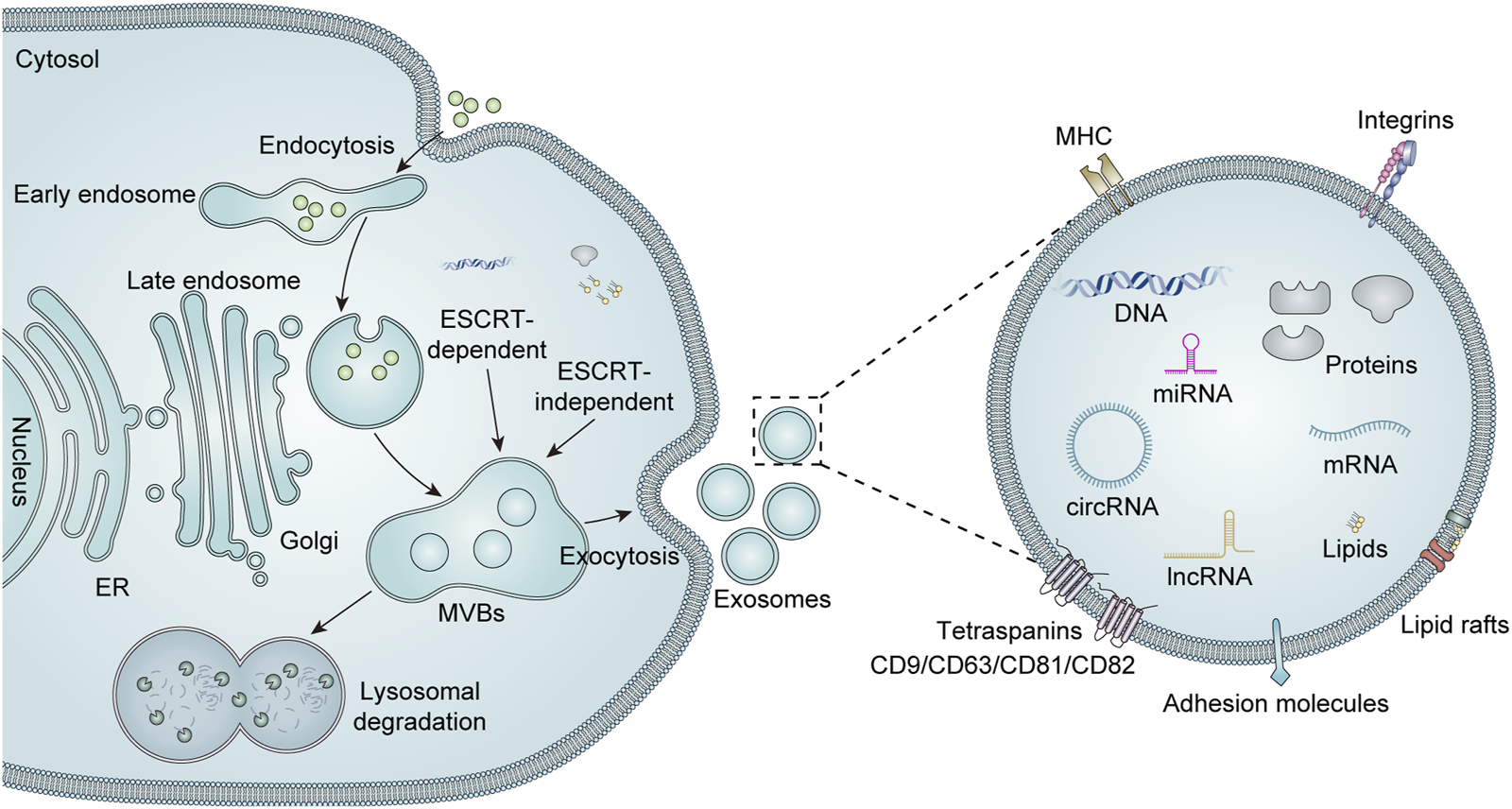
Biogenesis, secretion, and structure of exosomes. Extracellular components can enter the cell via intracellular endocytosis. The biogenesis of exosomes begins with the formation of early endosomes, which are formed by invagination of the cytoplasmic membrane. These early endosomes subsequently mature into late endosomes, which can evolve into MVBs through the accumulation of numerous intraluminal vesicles (ILVs). There are two recognized pathways for regulating MVBs, namely, endosomal sorting complexes required for transport (ESCRT)-dependent and ESCRT-independent mechanisms. Mature MVBs may either fuse with lysosomes for degradation or are transported to the cell membrane to merge with the plasma membrane, thereby releasing their luminal vesicles as exosomes into the extracellular space. Exosomes are mainly enriched with a variety of biologically active substances, including lipids, proteins (e.g., major histocompatibility complex (MHC), integrins, tetra-transmembrane proteins, and heat shock proteins), and nucleic acids (DNA, mRNA, miRNA, lncRNA, and circRNA).
2.2 Characteristics of exosomes
As nanoscale EVs, exosomes have unique and superior properties that render them highly valuable for biomarker screening and clinical diagnosis. First, exosomes feature a membranous structure that ensures stability across various body fluids, thereby protecting their cargoes from enzymatic degradation in the external environment. Second, the contents of exosomes—comprising nucleic acids, proteins, and lipids—exhibit alterations associated with disease states. Moreover, exosomes can be isolated from diverse types of body fluid samples through non-invasive methods, facilitating dynamic monitoring of diseases (Liang et al., 2023). Given the ease of sample acquisition, numerous studies have sought to identify suitable exosomal biomarkers from blood and urine samples for the early diagnosis and therapeutic monitoring of kidney diseases.
Numerous studies have demonstrated that urinary exosomes have great potential for the early diagnosis of a wide range of renal and urological diseases (Harita, 2024). Urinary exosomes possess multiple properties and originate from various cellular sources. They can be produced by different cell types within the genitourinary tract, including glomerular cells, tubular cells, thylakoid cells, and podocytes. These exosomes reflect the physiological and pathological states of the genitourinary system (Grange et al., 2023). The diverse origins of these cell-derived exosomes enable them to carry nucleic acids and proteins from their parent cells, thereby providing insights into the physiological and pathological changes occurring in kidney tissue. Consequently, urine-derived exosomes have emerged as powerful non-invasive diagnostic biomarkers for kidney disease.
Based on these characteristics, exosomes are increasingly recognized as effective drug carriers for targeted drug delivery. Exosomes are small in size, abundant in quantity, low in immunogenicity, and possess significant stability. Additionally, they facilitate the transport of materials and the transfer of information between cells. The drugs carried by exosomes can be directed to specific target cells through the artificial loading of small-molecule drugs or modifications to the exosomal membrane. This targeted approach to drug delivery is expected to enhance medication efficacy for patients, decrease administration frequency, and minimize systemic side effects (Sharma et al., 2024). In summary, exosomes represent a promising next generation of revolutionary drug carriers with considerable potential and numerous advantages as therapeutic agents and delivery systems.
3 Regulation of exosome secretion and its interaction with target cells
As membranous vesicles that play an important role in intercellular communication, the secretion of exosomes is regulated by a variety of factors, including molecular intervention (such as lipopolysaccharides, interferons, cytokines, and growth factors), culture conditions, physicochemical factors, and nanoparticles (Gurunathan et al., 2021b). For example, it has been suggested that the addition of certain small molecule regulators to the medium, such as the treatment of mesenchymal stem cells (MSCs) with a combination of N-methyldopamine and norepinephrine, can promote the production and secretion of exosomes (Wang et al., 2020). Similarly, there is increasing evidence that changes in the culture microenvironment, such as hypoxic preconditioning (Jiang et al., 2022), acidic environment (Logozzi et al., 2018), laser irradiation (Bagheri et al., 2018), mechanical stimulation (Guo et al., 2021), and serum deprivation (Haraszti et al., 2019) , can regulate the secretion of exosomes and change their contents, thereby affecting their biological function. In recent years, it has been reported that various nanoparticles, such as platinum nanoparticles, can enhance the biogenesis and secretion of exosomes (Gurunathan et al., 2021a). The physicochemical properties of micro/nano-textured hierarchical titanium topographies promote the proliferation of bone marrow mesenchymal stem cells (BMSCs), thus promoting the synthesis and secretion of BMSC-derived exosomes (Zhang Z. et al., 2021). In addition, some other biological materials, such as bioglass, can also promote the secretion of exosomes (Wu et al., 2020). In conclusion, exosomes are promising therapeutic tools for the treatment of various diseases, and an in-depth study of the related factors affecting the secretion of exosomes will help better understand their potential applications in disease diagnosis, therapy, and biomarker development.
Exosomes can be secreted by all types of living cells and are widely distributed in body fluids. They can transfer a variety of bioactive molecules, including nucleic acids, proteins, and lipids, to target cells, affect various biological processes of target cells, and ultimately affect the biological activity of target cells. Currently, it is believed that there are three types of interaction mechanisms between exosomes and target cells. (1) Direct membrane fusion: exosomes regulate target cells by directly fusing with the cell membrane and releasing their contents directly into the cell (Mori et al., 2019). (2) Endocytosis: target cells take up exosomes through endocytosis, and their contents are released or reprocessed within the cell, thereby regulating cell function. The endocytosis includes clathrin-independent and clathrin-dependent endocytosis processes. In addition, the clathrin-independent pathways are divided into lipid raft-mediated and caveolin-mediated endocytosis, phagocytosis, and micropinocytosis (Wu et al., 2021). (3) Receptor–ligand interaction: proteins, lipids, or lipoproteins on the membrane of exosomes can bind to specific receptors on the target cell membrane, triggering cell signaling pathways. It includes four transmembrane proteins, integrins, major tissue compatibility complex (MHC), intercellular adhesion molecule-1 (ICAM-1), proteoglycans, and lectins (Mulcahy et al., 2014).
4 Exosome isolation and purification techniques
In order to fully utilize the potential of exosomes in biomedical research, efficient isolation and purification techniques are essential (He et al., 2018). In recent years, with the deepening of exosome research, several methods have been proposed. However, various methods have different advantages and limitations. Several common techniques for exosome isolation and purification are described in Table 1.
TABLE 1
| Method | Advantage | Limitation | Reference |
|---|---|---|---|
| Ultracentrifugation | Low cost, easy to operate, reproducible, and stable | Time-consuming, low yield, and may damage the structure of exosomes | Lai et al. (2022) |
| Polymer precipitation | Simple and fast operation without expensive equipment | Low yield, poor purity, and impact on downstream analyses | Doyle and Wang (2019b) |
| Size exclusion chromatography | High efficiency, high purity, and the preservation of exosome integrity and bioactivity | Time-consuming, low total yield, and suitable for small samples | Liu et al. (2022a) |
| Ultrafiltration | Simple, cost-effective, and efficient | Membrane pore clogging and exosome loss | Yang et al. (2020) |
| Immunoaffinity | High specificity, high purity, and effective in reducing protein interference | High cost of antibodies and small sample size to process | Alzhrani et al. (2021) |
| Microfluidic technology | High efficiency, purity, and integration | Technically complex | Li et al. (2021b) |
Advantages and limitations of different exosome isolation and purification methods.
4.1 Ultracentrifugation
Ultracentrifugation is the most commonly used and classic method, which is regarded as the ‘gold standard’ for exosome extraction. Its basic principle is to take advantage of the difference in settling speeds of different particles under the action of centrifugal force. Ultracentrifugation is widely used for the isolation, purification, and functional study of exosomes, which offers advantages such as low cost, easy operation, good reproducibility, and stability. However, it is time-consuming, has a low yield, and may damage the structure of exosomes (Lai et al., 2022).
4.2 Polymer precipitation
The basic principle of polymer precipitation is to use polymers to interact with the water molecules surrounding the exosomes to form a hydrophobic microenvironment, thereby precipitating the exosomes. Polyethylene glycol (PEG) is the polymer most commonly used to precipitate exosomes. This method of isolation is simple and fast and does not rely on expensive equipment. However, this method is also capable of precipitating non-exosomal proteins, resulting in low yields and poor purity of the isolated exosomes (Doyle and Wang, 2019b).
4.3 Size exclusion chromatography
Size exclusion chromatography (SEC) is based on the size difference in molecules in gel packing. During separation and extraction, large molecules cannot enter the gel pores and are quickly eluted along the gap between the porous gel and the mobile phase, whereas small molecules are retained in the gel pores longer and eluted for a longer period of time. Exosome molecules are larger than normal protein molecules and, therefore, will be eluted first, resulting in the isolation of high-purity exosomes. Compared to centrifugation, SEC has higher separation efficiency and purity, and it can maintain the integrity and biological activity of the isolated exosomes. However, due to its time consuming nature and lower total yield, SEC is only suitable for exosome separation of small samples (Liu W.-Z. et al., 2022).
4.4 Ultrafiltration
Ultrafiltration is a technique in which exosomes are selectively separated by ultrafiltration membranes based on molecular particle size. Based on this principle, many simple and convenient ultrafiltration devices have been developed, such as microfilters configured in series and continuous ultrafiltration. Ultrafiltration is simple, economical, and efficient, but it also has its drawbacks. One of the prominent problems is the clogging of membrane pores, leading to a significant reduction in the service life of ultrafiltration membranes. More efficient forms of ultrafiltration are constantly being optimized to address these problems. For example, tangential flow filtration has become one of the main methods for rapid exosome isolation as it can continuously wash and flush the membrane surface, keeping it clean and functioning efficiently compared to traditional dead-end filtration (Yang et al., 2020).
4.5 Immunoaffinity
Immunoaffinity is a highly specific technique for exosome isolation and purification. It utilizes the affinity between antibodies and specific molecules on the exosome surface (e.g., tetraspanin proteins) to achieve selective capture and isolation of exosomes. Methods of exosome enrichment based on this principle are constantly being developed, such as enzyme-linked immunosorbent assays (ELISAs), immunomagnetic bead assays, and affinity peptides (Li et al., 2017). The immunoaffinity method is superior to ultracentrifugation in terms of sample volume required, purity, and yield. For example, it has been demonstrated that the immunoaffinity method can produce similar results with less sample volume, better morphological integrity of exosomes, and higher specificity than ultracentrifugation. The immunoaffinity method offers advantages such as high specificity, high purity, and the ability to effectively reduce protein interference, but it also has limitations such as high cost of antibodies, small sample size it can process, and its unsuitability for high-throughput separation (Alzhrani et al., 2021).
4.6 Microfluidic technology
Recently, with the continuous development of the field, the traditional exosome separation methods have struggled to meet the current growing demands of scientific research. Emerging exosome separation technology continues to emerge and gradually become the current hot spot of exosome separation research, one of which is based on the microfluidic exosome separation technology. It provides a new solution for the efficient and automated capture and purification of exosomes. The main feature of microfluidics is the precise manipulation of fluids in the micro- and nanoscale space and the use of exosome size, surface charge, and other physicochemical properties for the efficient separation and enrichment of exosomes (Al-Madhagi, 2024). Microfluidics-based assays offer advantages such as high efficiency, purity, and integration. They offer great potential for the discovery of exosomal biomarkers, diagnosis, and prognosis of diseases by integrating purification and enrichment of exosomes into microfluidic platforms (Li S. et al., 2021).
Exosomes have a large amount of genetic information and biomolecules derived from parental cells and play an important role in human physiological or pathological processes. The enrichment of high-purity exosomes from body fluids is a prerequisite for biofunctional and clinical application studies. It has been reported in the literature that combining different isolation methods can improve exosome isolation efficacy. Ultracentrifugation coupled with SEC (UC-SEC) has been identified as the best method for producing high-purity exosomes from the same volume of urine (50 mL) (Gheinani et al., 2018). Meanwhile, more efficient and optimized separation techniques have been successfully developed based on various traditional separation methods. For example, it has been reported that compared to the traditional ultracentrifugation method, the optimized ultrafiltration (OUF) method offers higher purity, yield, and biostability. Moreover, the higher miRNA expression in exosomes isolated by OUF may be suitable for large-scale extraction of clinical urine samples and subsequent experiments (He et al., 2019). In conclusion, with the rapid progress of exosomes in the diagnosis and treatment of clinical diseases, there is an urgent need to develop reproducible and scalable methods for the batch preparation of high-quality exosomes to meet clinical needs.
5 Exosomes from different sources in the kidney
Exosomes are derived from various types of kidney cells, including glomerular endothelial cells (GECs), mesangial cells (MCs), podocytes, and tubular epithelial cells (TECs). These exosomes encapsulate information-containing biomolecules that are crucial for regulating cell–cell interactions within the renal environment. Furthermore, they play a significant role in modulating pathophysiological processes in the kidneys, such as fibrosis, inflammation, and apoptosis (Figure 2).
FIGURE 2
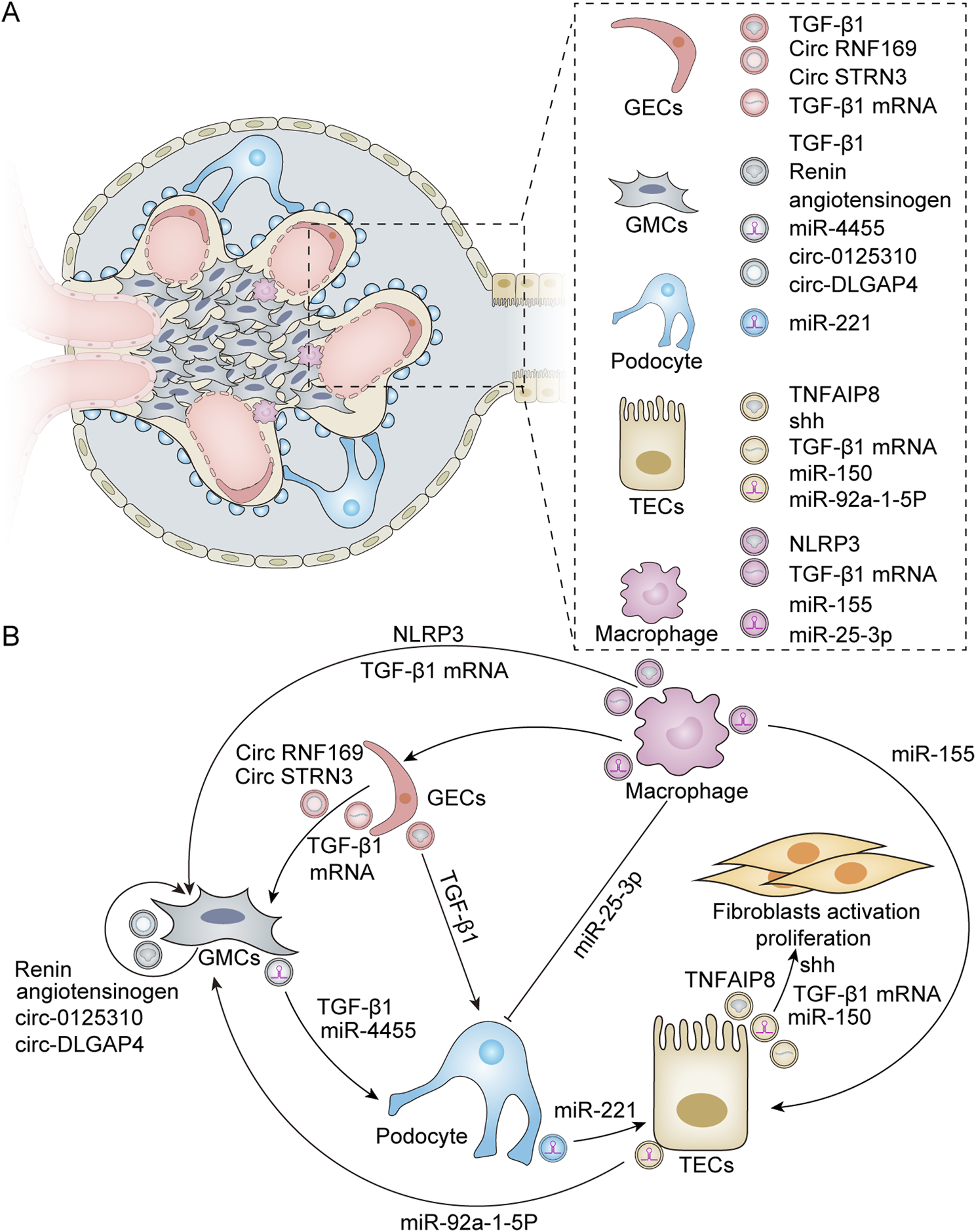
Exosomes derived from various renal resident cells and their crosstalk. (A) Exosomes derived from different renal resident cell types. Exosomes can derive from a variety of cell types within the kidney, including GECs, GMCs, podocytes, TECs, and macrophages. These exosome-derived biomolecules play a crucial role in modulating renal cell–cell interactions. (B) Exosome-mediated intercellular crosstalk among various renal resident cell origins. For GECs, HG treatment leads to the activation of GMCs by secreting exosomes enriched with TGF-β1 mRNA, circRNF169, and circSTRN3 (Wu et al., 2016; Ling et al., 2019). In addition, HG can induce GECs to release exosomes containing TGF-β1 to stimulate podocytes for EMT and dysfunction (Wu et al., 2017). Additionally, GMCs possess self-activation capabilities due to the active substances they produce. Exosomes released from HG-stimulated GMCs exhibit elevated levels of renin and angiotensinogen, which can induce normal GMCs to produce profibrotic and bioactive substances (da Silva Novaes et al., 2019). Furthermore, HG-induced GMCs can also secrete exosomes with high expression of circ_0125,310 and circ_DLGAP4, which in turn promote the proliferation and fibrosis of GMCs (Bai et al., 2020; Zhu et al., 2022). Furthermore, exosomes derived from GMCs can induce podocyte injury through mechanisms involving miR-4455 or TGF-β1 (Wang et al., 2018; Yu M. et al., 2022). For podocytes, exosomes secreted by these cells under HG conditions can induce TEC damage via miR-221 (Su et al., 2020). For TECs, TEC-derived exosomes promote fibroblast activation through Shh, TGF-β1 mRNA, miR-150, and tumor necrosis factor-α-induced protein 8 (TNFAIP8), which inhibits fibroblast apoptosis and stimulates their proliferation (Liu et al., 2020; Borges et al., 2013; Guan et al., 2020; Liu X. et al., 2023). In addition, HG induces TECs to produce exosomes rich in miR-92a-1-5p, which alters endoplasmic reticulum stress and myoblast transdifferentiation in GMCs (Tsai et al., 2023). M2 macrophage-derived exosomal miR-25-3p can ameliorate HG-induced podocyte injury by activating autophagy (Huang et al., 2020). TECs internalize exosomal miR-155 derived from macrophages, leading to an increase in tubular injury (Zhang Z. et al., 2022). Exosomes secreted by HG-treated macrophages also promote the activation of GMCs by carrying TGF-β1 mRNA or NLRP3 (Zhu Q.-J. et al., 2019; Liu Y. et al., 2023). Furthermore, macrophage-derived exosomes can mediate GEC dysfunction in sepsis-associated acute kidney injury (Xiang et al., 2023).
GECs represent the primary barrier of the glomerular capillary wall, playing a crucial role in regulating glomerular filtration and constituting an essential component of the inner filtration barrier. Previous studies have reported that GECs treated with high glucose (HG) can secrete increased amounts of exosomes enriched with transforming growth factor-β1 (TGF-β1) mRNA. These GEC-derived exosomes can enhance α-smooth muscle actin expression, promote proliferation, and lead to excessive extracellular matrix protein production in glomerular mesangial cells (GMCs) via the TGF-β1/Smad3 signaling pathway (Wu et al., 2016). Furthermore, HG-treated GECs also release more exosomes containing circular RNAs (circRNAs), which facilitates the intercellular transfer of circRNAs from GECs to GMCs (Ling et al., 2019). Wu et al. demonstrated that GEC-derived exosomes mediate interactions between GECs and podocytes while contributing to the pathogenesis of diabetic nephropathy (DN). In an HG environment, GECs undergo the endothelium-mesenchymal transition (EMT) and produce a significantly higher number of exosomes. These exosomes can mediate the epithelial–mesenchymal transition and dysfunction of podocytes partly through the transfer of TGF-β1 mRNA (Wu et al., 2017).
The MCs of the kidney are specialized stromal cells within the glomeruli that play an important role in maintaining glomerular homeostasis and mediating the glomerular response to injury. Increasing evidence suggests that MCs not only contribute to tissue architecture but also regulate developmental processes, angiogenesis, and cell fate (Avraham et al., 2021). Current findings indicate that exosomes derived from MCs significantly influence the pathophysiology of DN. Exosomes facilitate communication both among MCs themselves and between MCs and podocytes. Research studies have shown that HG-stimulated human mesangial cells (HMCs) release an increased quantity of exosomes containing elevated levels of renin and angiotensinogen. These exosomes can induce the production of pro-fibrotic and biologically active substances in normally cultured HMCs. These results indicate that cellular communication mechanisms mediated by exosomes may play a pivotal role in transmitting and spreading changes to cells that are not directly affected (da Silva Novaes et al., 2019). This pattern of self-action has also been observed in other mechanisms. Exo-circ_0125310, derived from HG-induced MCs, promotes the proliferation and fibrosis of MCs by sponging miR-422a and targeting the IGF1R/p38 axis (Zhu et al., 2022). Similarly, exo-circ_DLGAP4, derived from HG-treated MCs, significantly enhances the proliferation and fibrosis of MCs through the modulation of the miR-143/ERBB3/NF-κB/MMP-2 axis (Bai et al., 2020). Interestingly, recent studies have demonstrated that MC-derived exosomes can play a role in regulating podocyte injury via crosstalk with neighboring cells. Yu M. et al. (2022) found that exosomal miR-4455 from MCs induces podocyte injury in IgA nephropathy by targeting ULK2. Furthermore, it has been reported that exosomes derived from HG-treated MCs can induce podocyte injury through the TGF-β1-PI3K/AKT pathway. Notably, berberine has been shown to protect podocyte function by inhibiting the transfer of TGF-β1 from the GMCs to the podocytes (Wang et al., 2018).
Podocytes are highly differentiated terminal cells that extend from the foot processes, encircling the glomerular basement membrane and maintaining the homeostasis of the glomerular filtration barrier. Increasing evidence suggests that podocyte injury is a critical event in the pathogenesis of DN. Similarly, podocyte-derived exosomes may also contribute to this pathological process. Su et al. demonstrated that podocyte-derived exosomes serve as key mediators of proximal tubular cell injury in diabetes mellitus. Specifically, miR-221 within these exosomes facilitates cellular damage through Wnt/β-catenin signaling pathways. It has been proposed that miR-221 expression induces β-catenin nuclear accumulation by targeting DKK2, an inhibitor of Wnt signaling, ultimately leading to the dedifferentiation of proximal tubular epithelial cells (Su et al., 2020). A recent study found that a significant increase in podocyte-derived exosomes among patients with DN is associated with dysfunctional sirtuin1-mediated lysosomal acidification (Ding et al., 2023). Beyond their effects on proximal tubule cells, podocyte-derived exosomes are implicated in glomerular inflammation. Huang et al. reported that acid sphingomyelins produced by ceramide can participate in regulating NLRP3 inflammasome activation and inflammatory exosome release in podocytes during obesity via the ceramide signaling pathway, resulting in glomerular inflammation and injury associated with obesity-related glomerulopathy (Huang et al., 2023).
TECs are the primary targets of renal injury. Damaged tubular cells release harmful molecules, mediate intercellular communication pathways, and contribute to the progression of renal diseases. Previous studies have demonstrated that exosomes derived from injured TECs can transfer sonic hedgehog (Shh) protein, TGF-β1 mRNA, and miR-150 into fibroblasts, thereby promoting fibroblast activation, proliferation, and stroma production (Borges et al., 2013; Liu et al., 2020). Furthermore, Liu et al. illustrated that tubule-derived exosomes can block p53 signaling by shuttling tumor necrosis factor-α-induced protein 8 (TNFAIP8), which prevents fibroblast apoptosis and stimulates their proliferation (Liu X. et al., 2023). These findings suggest that TEC-derived exosomes have pro-fibrotic functions in kidney diseases. Exosome-mediated delivery plays a crucial role in renal cell crosstalk and the progression of kidney disease. In addition to interacting with fibroblasts, tubule-derived exosomes also engage with mesangial cells. Proximal tubule-derived exosomes contribute to mesangial cell injury in DN via the transfer of miR-92a-1-5p (Tsai et al., 2023).
Exosomes can also be derived from other kidney cell types, including fibroblasts and macrophages. A recent study indicated that exosomes originating from cancer-associated fibroblasts (CAFs) are capable of being internalized by clear cell renal cell carcinoma (ccRCC) cells, which subsequently enhances the proliferation, migration, and invasion of these cancer cells, thereby promoting the progression of ccRCC (Fu et al., 2022). Fibroblasts have been shown to play an important role in the development of renal fibrosis. In their research, Yu et al. observed that TGF-β1 can stimulate the differentiation of renal fibroblasts into myofibroblasts and promote the release of exosomes from these myofibroblasts. Exosomes derived from myofibroblasts enhance the transition of macrophages into myofibroblasts and contribute to renal fibrosis (Yu et al., 2024). Growing evidence indicates that exosomes derived from macrophages are involved in the occurrence and development of kidney diseases through crosstalk with resident renal cells. Studies have shown that miR-25-3p, originating from M2 macrophage-derived exosomes, ameliorates HG-induced podocyte injury by promoting autophagy via the inhibition of DUSP1 expression (Huang et al., 2020). Conversely, exosomes derived from M1 macrophages are known to inhibit podocyte activity and enhance podocyte apoptosis (Liang et al., 2022b). In an ischemia/reperfusion-induced acute kidney injury model, Yuan et al. investigated the crosstalk between macrophages and tubular cells. They discovered that exosomal miRNA-155 released by activated macrophages can be internalized by tubular cells, leading to increased tubule injury by targeting the suppressor of cytokine signaling-1 (Zhang Z. et al., 2022). In an HG environment, it has been observed that macrophage-derived exosomes contain TGF-β1 mRNA, which can trigger mesangial cell activation, proliferation, fibrosis, and inflammation via the TGF-β1/Smad3 pathway (Zhu Q.-J. et al., 2019). In addition, a recent study has reported that macrophage-derived exosomes can promote the activation of the NLRP3 inflammasome and autophagy deficiency of MCs in DN (Liu Y. et al., 2023). Additionally, Xiang et al. (2023) reported that lipopolysaccharides significantly stimulate the secretion of macrophage-derived exosomes. These exosomes have been implicated in inducing dysfunction in GECs.
6 Mechanisms of exosomes in renal diseases
6.1 Mediating intercellular crosstalk
Exosomes serve as a crucial mechanism for intercellular information transfer, facilitating communication between cells by transporting bioactive molecules such as mRNAs, non-coding RNAs, lipids, and proteins. Their interactions with renal cells are pivotal in the progression of renal diseases. Increasing evidence suggests that exosomes can either exert a local pathogenic effect within nephrons or, through urinary flow, transport pathogenic substances over considerable distances to the distal regions of the nephron, thereby contributing to the pathogenic processes in recipient cells.
In a study utilizing models of LPS-induced acute kidney injury and adriamycin-induced chronic kidney injury, it was observed that the expression of miR-19b-3p was significantly elevated in exosomes derived from TECs compared to control groups. Moreover, this investigation confirmed that TEC-derived exosomes can target the NF-kB/SOCS-1 pathway through a miR-19b-3p-dependent mechanism, leading to the activation of M1 macrophages. This process promotes the progression of tubulointerstitial inflammation and exacerbates renal injury (Lv et al., 2020). Previous research has demonstrated intercellular communication among proximal tubular epithelial cells (PTECs) via exosome transmission in an autocrine pattern. Tsai et al. conducted a study to explore crosstalk within PTECs in DN. They discovered that HG levels stimulate PTECs to upregulate fibronectin-1 (FBLN1), which is subsequently secreted by PTECs through exosomal delivery, thereby inducing the epithelial–mesenchymal transition in these cells (Tsai et al., 2021).
Cells within the glomeruli primarily comprise GECs, podocytes, and mesangial cells. These cell types are capable of releasing exosomes, facilitating crosstalk between different cell populations through these exosomal mediators. This interaction plays a pivotal role in the pathogenesis of glomerular and renal tubular diseases (Hu et al., 2024). Tubulointerstitial fibrosis is a hallmark characteristic of progressive chronic kidney disease. However, its development necessitates the involvement of various cell types—including endothelial cells, fibroblasts, and inflammatory cells—within the tubular interstitium. Following severe injury to renal tubular epithelial cells, exosomes containing diverse active substances can be released. These exosomes function analogously to virus-like seeds, thereby leading to fibroblast activation, inflammatory cell recruitment, and endothelial cells (Guo et al., 2023). Moreover, there exists significant signal crosstalk between tubular epithelial cells and interstitial fibroblasts following renal injury. This interaction contributes to the progression of tubular interstitial damage (Tan et al., 2016). In summary, such crosstalk among different nephron components is exceedingly prevalent in instances of kidney injury (Figure 3).
FIGURE 3
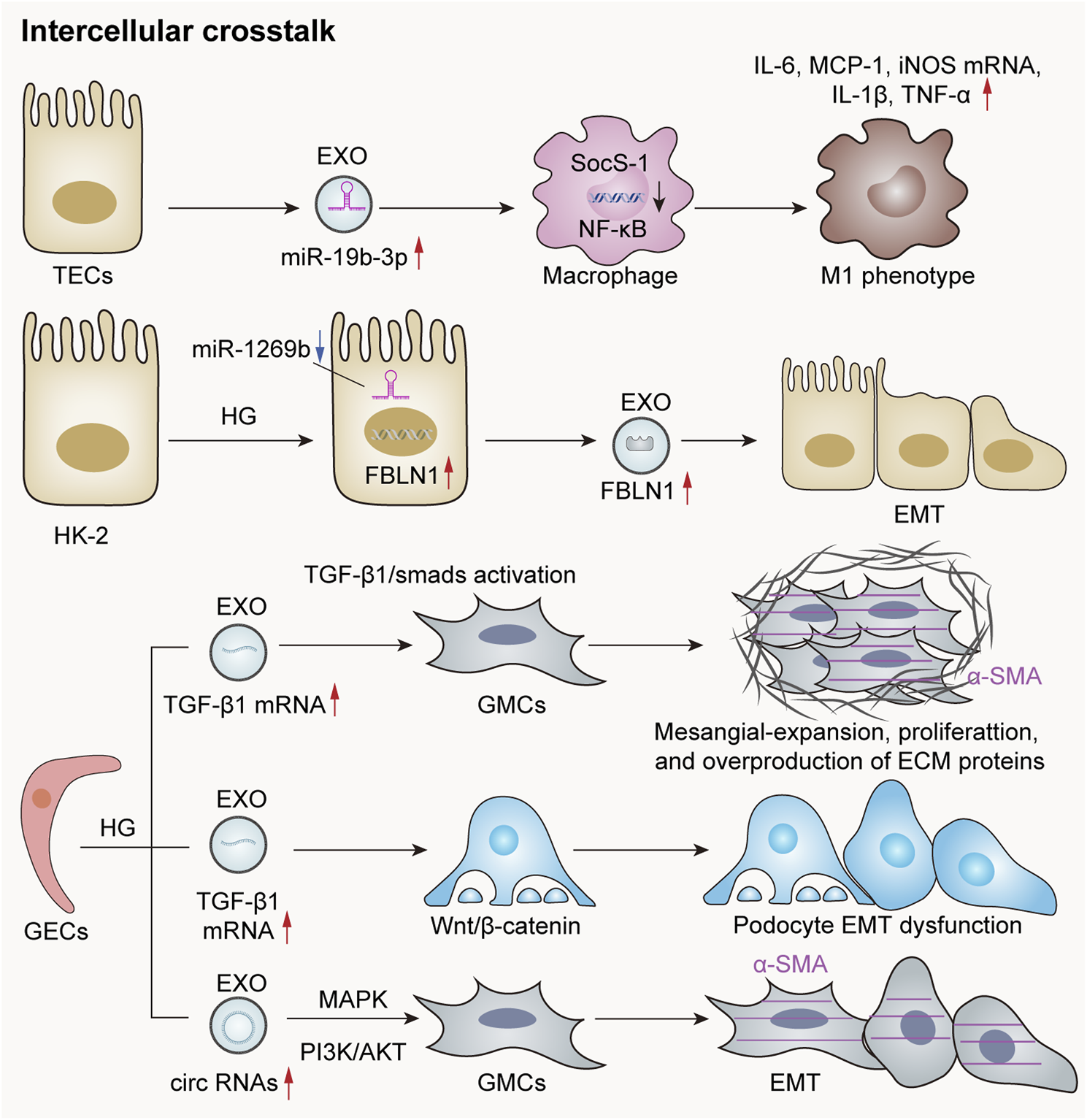
Exosomes mediate crosstalk between kidney cells. The exosomal miR-19b-3p derived from renal TECs is significantly elevated in acute or chronic kidney injury models, leading to M1 phenotypic polarization through internalization by macrophages and targeting the NF-κB/SOCS-1 pathway (Lv et al., 2020). Additionally, the downregulation of miR-1269b in HG-treated HK-2 cells can lead to the increased expression of fibronectin-1 (FBLN1), thereby promoting EMT within these cells (Tsai et al., 2021). HG-induced GECs secrete exosomes rich in TGF-β1 mRNA, which are subsequently internalized by GMCs and mediate GMC activation via the TGF-β1/Smad signaling pathway. Furthermore, exosomes abundant in circRNAs are released to enhance α-smooth muscle actin (α-SMA) expression in GMCs and induce the EMT. Notably, HG-treated GECs also release exosomes rich in TGF-β1 mRNA, which contribute to EMT and podocyte dysfunction through the activation of the Wnt/β-catenin signaling pathway (Hu et al., 2024).
6.2 Inflammation and immune regulation
Exosomes play an important role in inflammation and immune regulation in renal diseases. They can regulate the intensity and course of the immune response by modulating the release of inflammatory factors and the activity of inflammatory cells, thereby impacting both the progression and regression of renal diseases. An increasing number of studies have shown that exosomes can directly or indirectly regulate the immune-inflammatory response through miRNAs, thus participating in kidney injury. In a model of sepsis-induced acute renal injury, Wan et al. observed a significant downregulation in levels of miR-223-3p derived from platelet exosomes. This alteration led to the activation of the NLRP3 inflammasome and pyroptosis in endothelial cells, consequently exacerbating the inflammatory response associated with sepsis-induced acute kidney injury (Wan et al., 2024). Additionally, another study reported that exosomes derived from HK2 cells influenced cisplatin-induced acute kidney injury and HK2 cell pyroptosis via the miR-122/embryonic lethal abnormal vision (ELAVL1) axis (Zhu et al., 2023). A recent study has proposed that activated CD8+ T cells contribute to renal inflammation and tissue injury by releasing exosomes enriched with miR-186-5p. Furthermore, miR-186-5p is suggested to initiate tubular cell apoptosis through the direct activation of the TLR7/8 signaling axis in renal tubules (Xu et al., 2023). Macrophages play a crucial role as inflammatory cells within the kidney, and their functions are influenced by their phenotypic states. Kidney-derived exosomes can modulate the macrophage phenotype via crosstalk, thereby participating in the inflammatory processes associated with kidney disease. Exosomes released from tubular epithelial cells undergoing EMT due to TGF-β treatment have been shown to induce M1 macrophage activation, which may lead to the progression of renal fibrosis (Lu et al., 2023). In conclusion, exosomes are involved in various renal diseases by regulating inflammatory responses and immunological activities (Figure 4).
FIGURE 4
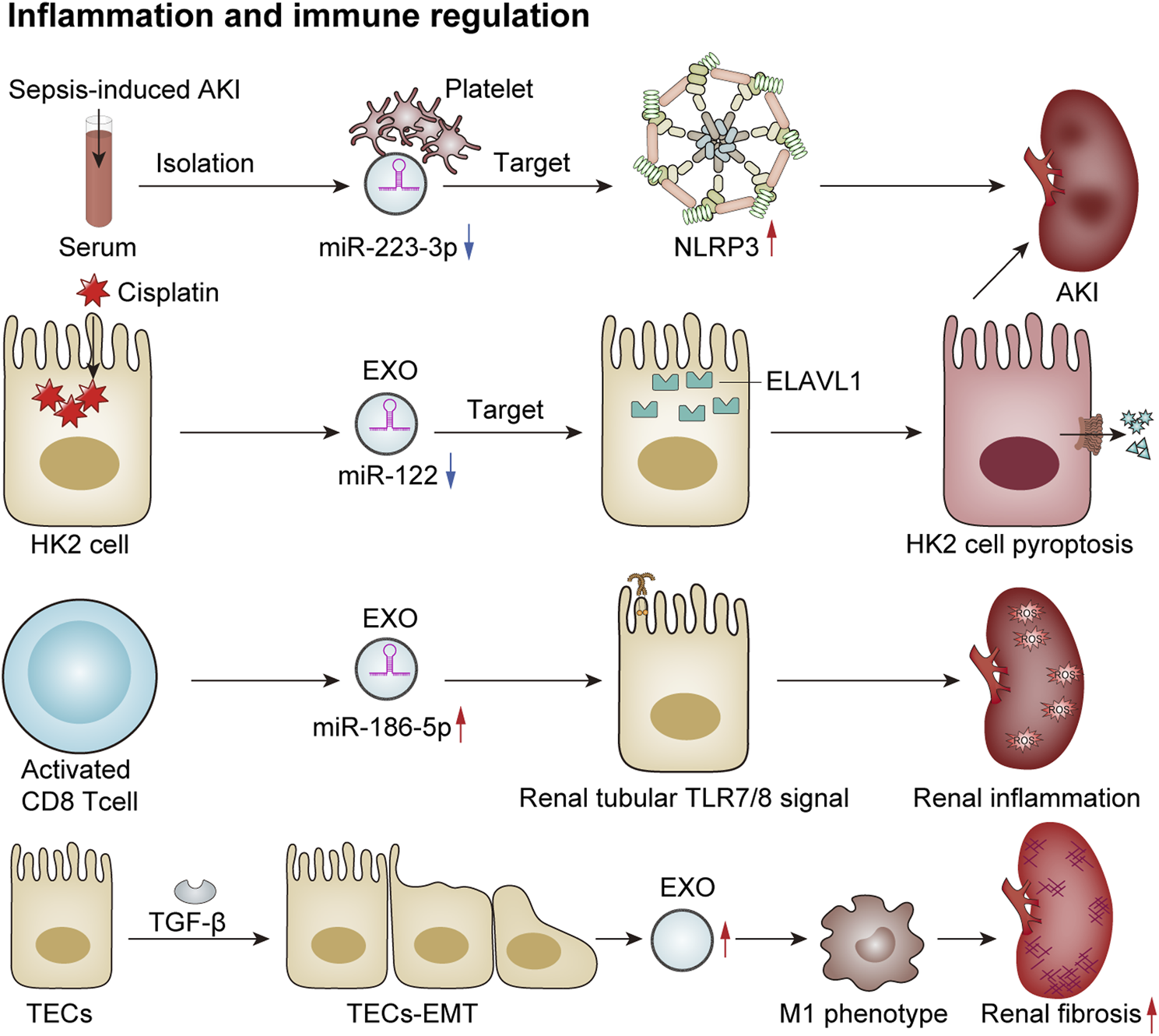
Exosomes mediate immune regulation and renal inflammation. In a sepsis-induced mouse model of AKI, reduced levels of platelet-derived exosomal miR-2233p inhibit endothelial cell pyroptosis, thereby exacerbating AKI by targeting NLRP3 (Wan et al., 2024). Additionally, in cisplatin-treated HK2 cells, the expression of exosomal miR-122 is downregulated, which promotes cellular pyroptosis and contributes to AKI by targeting embryonic lethal abnormal vision (ELAVL1) (Zhu et al., 2023). Activated CD8 T cells release exosomes enriched with elevated levels of miR-186-5p, which activate the renal tubular TLR7/8 signal axis to trigger renal inflammation and tissue damage (Xu et al., 2023). Furthermore, TGF-β induces TECs to undergo the EMT and secrete more exosomes, which induces macrophage M1 polarization and promotes the progression of renal fibrosis (Lu et al., 2023).
6.3 Cell regeneration, repair, and fibrosis
Exosomes have been proposed to play a significant role in promoting repair and regeneration in renal diseases. However, under certain conditions, they may also be involved in the development of fibrosis within the kidney, thereby exacerbating interstitial fibrosis (Figure 5). Renal fibrosis represents a major pathological change observed in the chronic kidney, ultimately progressing to end-stage renal failure. This condition is characterized by the substantial accumulation of the extracellular matrix within the renal interstitium, destruction of renal tissue architecture, and subsequent loss of function. Emerging evidence indicates that following kidney injury, exosomes derived from glomerular endothelial cells, podocytes, renal tubular epithelial cells, and other resident cell types recruit inflammatory cells through various cytokines and pathways. These processes activate myofibroblasts and lead to excessive deposition of the extracellular matrix components, thus promoting the progression of renal fibrosis.
FIGURE 5
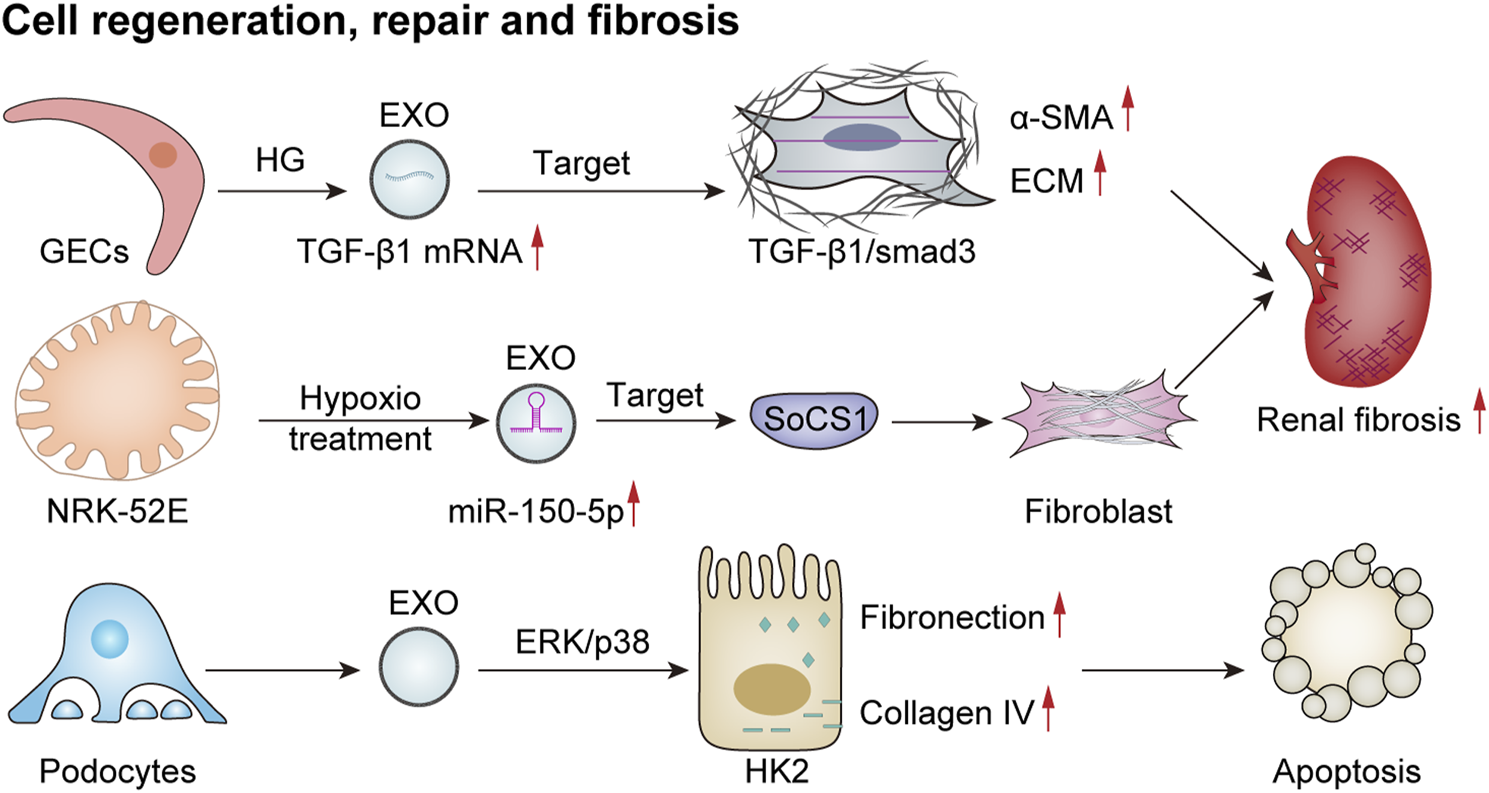
Exosomes mediate cell regeneration, repair, and fibrosis. HG-treated GECs secrete more TGF-β1 mRNA-rich exosomes, which enhance the expression of α-smooth muscle actin (α-SMA), promote proliferation, and lead to excessive production of extracellular matrix proteins (ECMs) in GMCs via the TGF-β1/Smad3 signaling pathway, ultimately resulting in renal fibrosis (Wu et al., 2016). EVs derived from damaged podocytes can activate the ERK and p38 pathways in HK2 cells, thereby increasing fibronectin and collagen IV expression and promoting apoptosis (Jeon et al., 2020). Additionally, hypoxia-treated renal tubular epithelial cells (NRK-52E) release exosomes with increased miR-150-5p expression, thus targeting cytokine signaling 1 (SOCS1) to promote fibroblast activation and exacerbate renal fibrosis (Zhou et al., 2021).
Previous studies have shown that exosomes derived from HG-treated glomerular endothelial cells activate mesangial cells, thereby promoting renal fibrosis (Wu et al., 2016). In addition, a study involving the culture of renal tubular epithelial (HK2) cells with exosomes obtained from either puromycin-treated or healthy human podocytes revealed that exosomes from damaged podocytes significantly increased the expression of fibronectin and collagen IV in renal tubular epithelial cells. This effect was mediated through the activation of ERK and P38 signaling pathways, contributing to tubular epithelial cell injury associated with tubulointerstitial fibrosis in renal disease (Jeon et al., 2020). In a model of acute renal injury induced by ischemia–reperfusion, there was a notable increase in exosomes derived from renal tubular epithelial cells. These exosomes were found to negatively regulate the expression of suppressor of cytokine signaling 1 via miR-150-5p, which subsequently activated fibroblasts and facilitated the progression of renal fibrosis (Zhou et al., 2021). This body of evidence suggests a potential association between exosomes and renal fibrosis. However, further in-depth studies are necessary to elucidate the specific mechanisms underlying this relationship.
6.4 Tumor growth and metastasis
Recently, several studies have indicated that exosomes play a crucial role in tumor growth and metastasis through mechanisms such as intercellular communication, microenvironmental regulation, and immune evasion. Flora et al. observed a loss of function of the von Hippel–Lindau (VHL) oncogene in 70% of clear cell renal cell carcinoma (ccRCC) cases, which is considered an early oncogenic driver of this malignancy. VHL (−) cells can produce significantly higher levels of exosomes. Moreover, exosomes derived from VHL (−) RCC cells can induce epithelial–mesenchymal transition, migration, invasion, and distant metastasis in VHL (+) RCC cells upon uptake (Flora et al., 2023). In addition, Song et al. (2024) reported that exosome-mediated secretion of miR-127-3p can accelerate metastasis in renal cell carcinoma. A recent study investigated the role of tumor-derived exosomes in macrophage phenotypic transformation and tumor development in renal cell carcinoma (RCC). The findings revealed that RCC-derived exosomes carry elevated levels of lncARSR, activate the STAT3 pathway, induce a shift in the macrophage phenotype from M1 to M2, and promote cytokine secretion along with phagocytosis and angiogenesis—thereby significantly enhancing tumor progression (Zhang et al., 2022b). Exosomes influence tumor development through intercellular communication, microenvironmental regulation, and immune system interactions. An extensive exploration of these mechanisms may offer new avenues for future cancer treatments and facilitate the development of therapeutic strategies targeting exosomes to prevent or mitigate tumor growth or proliferation (Figure 6).
FIGURE 6
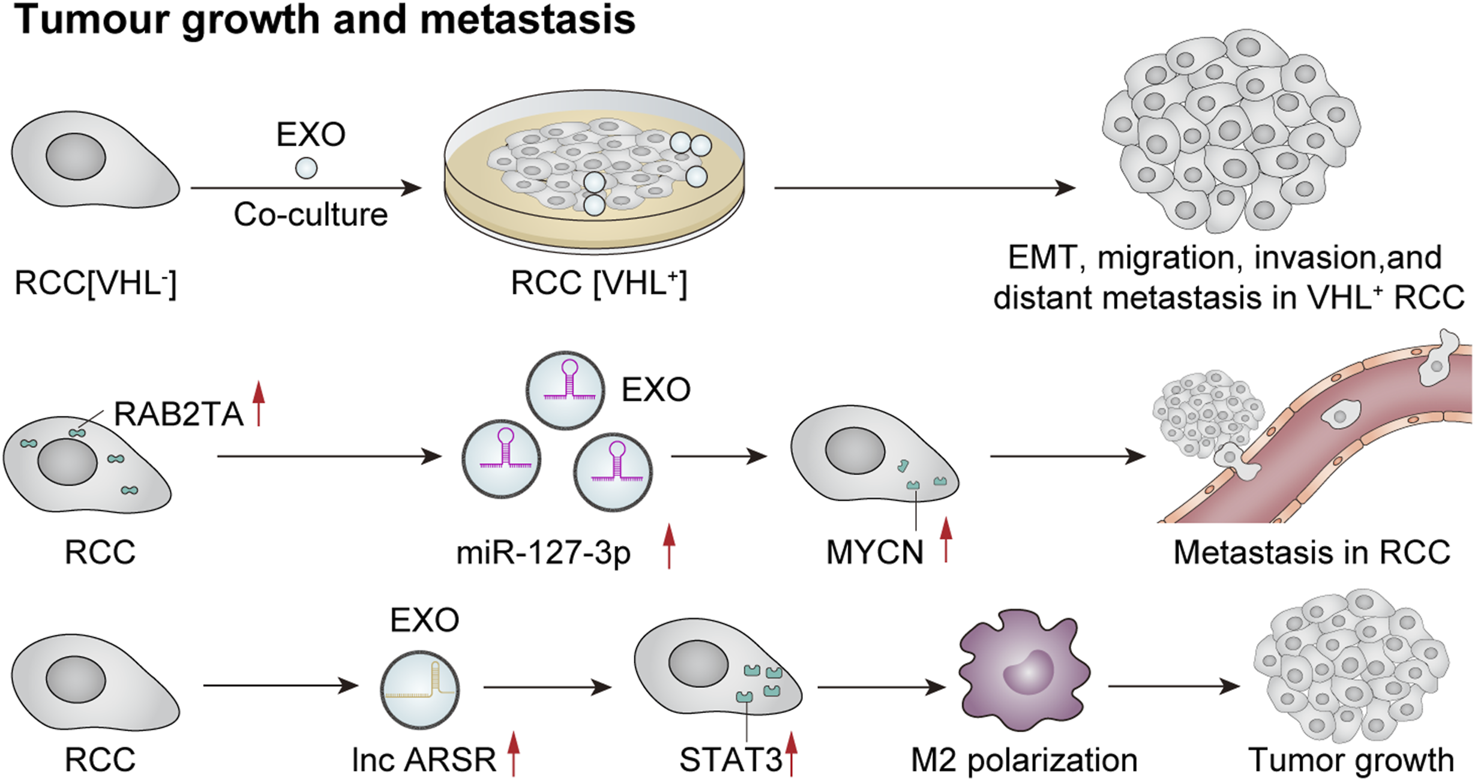
Exosomes mediate tumor growth and metastasis. Co-culture of VHL-renal cell carcinoma (RCC)-derived exosomes with VHL + RCC cells can promote EMT, migration, invasion, and distant metastasis of VHL + cells (Flora et al., 2023). Furthermore, RCC cells that overexpress RAB27A secrete a higher quantity of miR-127-3p-rich exosomes, resulting in elevated MYCN protein levels and augmented metastatic potential of cancer cells (Song et al., 2024). Additionally, RCC-derived exosomes contain significant amounts of lncRNA ARSR, which elevate the expression of signal transducer and activator of transcription 3 (STAT3), promote M2 polarization of macrophages, and contribute to tumor growth (Zhang et al., 2022b).
7 Application of exosomes in kidney diseases
Urine is an ideal material for evaluating kidney disease. Variations in the quantity, origin, or composition of exosomes isolated from urine are closely associated with alterations in the physiological and pathological states of the kidney. Therefore, exosomes can be used as a non-invasive diagnostic tool, showing great potential for application in the early diagnosis, prognostic assessment, and monitoring of kidney diseases (Pisitkun et al., 2004). Moreover, relevant studies indicate that exosomes may function as efficient drug carriers due to their natural substance transport properties, inherent long-term recycling capabilities, and superior biocompatibility. They also hold promise as a gene therapy tool, providing innovative approaches for developing new therapeutic strategies across various diseases (Batrakova and Kim, 2016). Hence, there has been a growing body of studies focused on applying exosomes in diagnosing and treating kidney diseases.
7.1 IgA nephropathy
IgA nephropathy (IgAN) is an autoimmune glomerular disease characterized by a wide spectrum of clinical presentations, ranging from asymptomatic microscopic hematuria to acute progressive nephritis. Therefore, non-invasive biomarkers that reflect histological changes are essential for predicting the progression of IgA nephropathy and determining therapeutic strategies (Feng et al., 2018). Exosomes have emerged as promising novel non-invasive biomarkers for both the diagnosis and treatment of IgAN. Current evidence indicates that the diagnostic biomarkers associated with IgAN primarily originate from urinary exosomal nucleic acids, while other components of exosomes are rarely reported.
Previous studies have explored the differences in urinary exosomal miRNA expression profiles between IgAN patients and healthy controls, revealing significant disparities in these profiles. Among the identified exosomal miRNAs, miR-29c, miR-146a, and miR-205 have emerged as potential novel non-invasive biomarkers for IgAN (Min et al., 2018). Additionally, hsa-miR-451a, hsa-let-7d-3p, and miR-199a-3p may also be used as non-invasive indicators for evaluating IgAN (Li S. et al., 2022). The findings presented by Zhao et al. are particularly compelling. Their results showed that the expression of miR-4639 and miR-210 was markedly upregulated in patients with IgAN. Moreover, increased expression of miR-4639 was associated with more severe histological activity. Importantly, follow-up assessments revealed that exosomal levels of both miR-4639 and miR-210 outperformed proteinuria measurements in predicting renal prognosis. This suggests that these markers may be effective tools for diagnosing IgAN, assessing disease severity, and monitoring progression (Zhao S. et al., 2022). In addition to miRNAs, exosomal lncRNAs and mRNA may also serve as diagnostic biomarkers for IgAN. One study identified that lncRNA-G21551 was significantly downregulated in patients with IgAN when comparing their plasma exosomes to those of their healthy first-degree relatives. The downregulation of lncRNA-G21551 suggests its potential utility as a non-invasive biomarker for IgAN (Guo et al., 2020). In another study, Feng et al. discovered that urinary exosomal chemokine (C-C motif) ligand 2 (CCL2) levels were correlated with the glomerular filtration rate, tubulointerstitial inflammation, and the progression of renal dysfunction. Consequently, urinary exosomal CCL2 mRNA emerges as a promising biomarker for monitoring renal tissue injury and the deterioration of renal function in IgAN (Feng et al., 2018).
Previous reports have highlighted the application of exosomes in the treatment of IgAN. An in vitro study demonstrated that the combination of artemisinin and hydroxychloroquine (AH) enhanced exosome secretion from human renal tubular epithelial cells (HK-2), effectively inhibiting NF-κB/NLRP3 signaling activation in human mesangial cells. This inhibition subsequently reduced pro-inflammatory cytokine production, thereby providing a novel therapeutic strategy for IgAN (Bai et al., 2019). Recent studies have focused on the role of exosomes in the therapeutic response of patients with IgAN. Wu J. et al. (2022) found that plasma exosomal IRAK1 could serve as a potential biomarker for predicting the treatment response to renin–angiotensin system inhibitors among individuals with IgAN. Additionally, another study revealed that urinary exosomal miR-451a may serve as a promising biomarker for diagnosing IgAN and assessing tubulointerstitial damage. Further follow-up studies indicated that the baseline levels of urinary exosomal miR-451a may predict both therapeutic efficacy and disease progression in IgAN patients (Zhang Q. et al., 2024).
7.2 Membranous nephropathy
Membranous nephropathy accounts for approximately 20% of primary nephrotic syndrome in China, typically presenting with an insidious onset. Renal function deterioration generally manifests gradually over a period of 5–10 years following the initial onset of the disease. As the condition progresses, pathological changes intensify, and treatment efficacy diminishes. Therefore, early diagnosis and timely intervention are essential to prevent further decline in renal function. By comparing the expression profiles of exosomes found in the serum and urine of patients with idiopathic membranous nephropathy (IMN) against those of normal controls using gene sequencing technology, Ma et al. (2019) identified significant differences in specific circRNA expressions within the exosomes from IMN patients. Among these, MUC3A can be considered a potential diagnostic biomarker for IMN. In addition, one study revealed that urinary exosomal hsa_circ_0001250 could serve as another promising biomarker for IMN (Li Q. et al., 2022). Subsequently, more urinary exosomal miRNAs specifically expressed in patients have been screened through high-throughput sequencing, and some of them showed increased expression levels, whereas others showed decreased expression levels. Despite their strong association with kidney disease, their potential value as diagnostic biomarkers for IMN remains unclear and warrants further investigation (Zhang J. et al., 2020). Evidence suggests that miR-30b-5p and miR-9-5p may represent novel non-invasive biomarkers for IMN (Guo et al., 2022). In addition to nucleic acids utilized as biomarkers for diagnosing IMN, proteins in urinary exosomes may also have a significant diagnostic value. A recent study revealed that urinary exosomal PLA2R can effectively diagnose PLA2R-associated membranous nephropathy with a sensitivity of 95.4% and specificity of 63.3%. Furthermore, the combined assessment of urinary exosomal PLA2R and the serum anti-PLA2R antibody demonstrates enhanced sensitivity (Wang B. et al., 2024).
7.3 Focal segmental glomerulonephritis
Focal segmental glomerulosclerosis (FSGS) is a prevalent primary glomerular disease, primarily affecting children and serving as a common cause of nephrotic syndrome in this population. FSGS is characterized by lesions involving partial glomerular and/or capillary loops. Earlier studies have shown that urinary exosomal Wilms’ tumor-1 (WT-1) is a promising non-invasive biomarker with significant podocyte specificity, reflecting early podocyte injury in patients with FSGS (Zhou H. et al., 2013). In a recent study, it was found that urinary exosomal miR-193a levels were significantly elevated in patients diagnosed with primary FSGS compared to those with minimal change nephropathy and IgAN. Furthermore, these levels exhibited a positive correlation with the glomerulosclerosis index. These findings suggest that urinary exosomal miR-193a can be used as a reliable non-invasive biomarker for facilitating early diagnosis and prognostic evaluation in individuals suffering from FSGS (Wang et al., 2021). Similar results were corroborated by another study, which reported an area under the ROC curve of 0.85 for urinary exosomal miR-193a in diagnosing primary FSGS (Huang et al., 2017). In addition to miR-193a, one study analyzed the expression profiles of urine and plasma exosomal microRNAs in patients presenting minimal change disease (MCD) versus those with FSGS. The findings revealed that compared to both MCD and control groups, the levels of miR-1915 and miR-663 were downregulated in the urinary exosomes of patients with FSGS, whereas the level of miR-155 was upregulated (Ramezani et al., 2015).
7.4 Diabetic nephropathy
Diabetic nephropathy (DN), also known as diabetic kidney disease (DKD), is one of the most common microvascular complications in diabetes mellitus (DM) patients. Currently, it is the primary cause of chronic kidney disease (CKD) and ESRD (Sagoo and Gnudi, 2020). Microalbuminuria is a widely utilized assessment tool for DKD. Clinically, microalbuminuria, urinary albumin-to-creatinine ratio (ACR), and/or serum creatinine-based estimated glomerular filtration rate (eGFR) are commonly used to assess glomerular injury and renal function. These parameters are also frequently used in the assessment of DN. However, in the early stages of DN, reliance on urinary microalbumin levels and eGFR is less reliable and sensitive for early diagnosis. Kidney biopsy is regarded as the gold standard for diagnosing DN. However, it is an invasive procedure that requires highly experienced technical operators. Furthermore, renal biopsy is not routinely performed in all patients with DN but is recommended primarily when nondiabetic nephropathy is suspected. Therefore, it is important to identify promising biomarkers and develop effective therapeutic agents to prevent, diagnose, and treat DN. Developing biomarkers for the early diagnosis of DN has become a hot topic in nephropathy research. Recent studies have increasingly demonstrated that nucleic acids and proteins found within urinary exosomes can be used as biomarkers for both the diagnosis and prognosis of DN (Table 2).
TABLE 2
| Potential biomarker | Subject | Source of exosomes | Detection method | Main findings | Reference | |
|---|---|---|---|---|---|---|
| miRNAs | ||||||
| miR-451-5p | Diabetic rats | Urinary exosomes | Sequencing technology and qPCR validation | It was significantly increased in DM rats during the progression of diabetes and was better than urinary albumin excretion in predicting kidney damage in DM. | Mohan et al. (2016) | |
| let-7c-5p | T2DM/DN patients and HCs | Urinary exosomes | RT-PCR | The upregulated expression of urinary exosomal let-7c-5p is associated with glomerular filtration rate and disease progression. | Li et al. (2018) | |
| miR-15b, miR-34a, and miR-636 | T2DM patients | Urinary exosomes | Bioinformatic analysis, PCR array analysis, and qPCR validation | There were significant correlations among the three miRNAs, which share common target genes related to glucose homeostasis, angiogenesis, and DKD. | Eissa et al. (2016) | |
| miR-21–5p and miR-30b-5p | T2DM/DKD patients | Urinary exosomes | PCR panels | Increased expression of urinary exosomal miR-21-5p and decreased expression of miR-30b-5p can better distinguish individuals with impaired renal function, but there is no specific association with DKD. | Zang et al. (2019) | |
| miR-4534 | T2DM/DKD patients | Urinary exosomes | miRNA microarray analysis and qRT-PCR validation | It has a characteristic elevated expression in DKD and is strongly correlated with microproteinuria. | Zhao et al. (2020) | |
| miR-145-5p and miR-27a-3p | T2DM/DKD patients and HCs | Urinary exosomes | RT-qPCR and bioinformatics analysis | They are significantly upregulated in DKD and associated with the progression of kidney injury. | Han et al. (2024) | |
| miR-615–3p | T2DM/DKD patients and HCs | Urinary exosomes | RT-qPCR validation | The expression level in DKD patients was significantly elevated. It was positively or negatively correlated with some clinical indicators of DKD. The combination with ACR has a more stable and sensitive diagnostic criteria. | Wang et al. (2023a) | |
| mRNAs | WT1 | DN/MCNS patients and HCs | Urinary exosomes | RT-qPCR | WT1 mRNA levels reflect glomerular damage in diabetic patients and predict a future decline in eGFR. | Abe et al. (2018) |
| CCL21 | DN/DM patients, HCs, and DN rat | Urinary EVs | RT-PCR | The increase in CCL21 mRNA in urine small EVs in DN patients can more effectively distinguish early DN patients from DM patients. | Feng et al. (2021) | |
| WT1 and ACE | DN/DM patients and HCs | Blood EVs | qRT-PCR | The expression of WT1 mRNA in the blood EVs of DN patients was significantly increased, and the accuracy of predicting early DN was 0.63. On the contrary, ACE mRNA expression was significantly decreased, and the accuracy of predicting early DN was 0.62. | Hashemi et al. (2021) | |
| CDH2 and MCP-1 | DN/DM patients and HCs | Blood EVs | qRT-PCR | CDH2 and MCP-1 mRNA expressions in blood EVs were significantly downregulated in DN, and both were inversely correlated with creatinine and the Alb/Cr ratio. | Dehghanbanadaki et al. (2022) | |
| Proteins | WT1 | Type-1 DM patients and HCs | Urinary exosomes | Western blotting | The predominant expression of the WT1 protein in urinary exosomes in type-1 diabetic patients is closely related to increased urinary protein excretion and decreased eGFR. | Kalani et al. (2013) |
| PAK6 and EGFR | DN/T2DM patients and HCs | Urinary exosomes | LC–MS/MS; Western blot and ELISA validation | PAK6 and EGFR were significantly upregulated in DN, negatively correlated with eGFR, and positively correlated with serum creatinine levels. The AUC value of PAK6 diagnostic DN was 0.903, EGFR was 0.842, and the combination of the two proteins was 0.912. | Li et al. (2023b) | |
| CALM1 | DN/T2DM patients and HCs | Urinary exosomes | LC–MS/MS; Western blot and ELISA validation | CALM1 correlated with serum Cr and eGFR levels, and a diagnostic model based on CALM1 and serum ALB levels was established. | Li et al. (2023a) | |
| TF, SERPINA1, AFM, and CTSD | DN/T2DM patients and HCs | Urinary exosomes | LC–MS/MS detection; Western blot validation | A large-scale urinary exosomal proteomic analysis was performed on T2DM patients without or with DKD in different stages, and it was found that TF, SERPINA1, and AFM expressions increased with the progression of DKD, while CTSD decreased. | Du et al. (2023) | |
Potential biomarkers in exosomes of DN.
DN, diabetic nephropathy; qPCR, quantitative real-time polymerase chain reaction; DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; DKD, diabetic kidney disease; HCs, healthy controls; RT-PCR, real-time quantitative polymerase chain reaction; ACR, albumin-to-creatinine ratio; WT1, Wilms tumor 1; eGFR, estimated glomerular filtration rate; EVs, extracellular vesicles; CDH2, cadherin 2; MCP-1, monocyte chemoattractant protein-1; PAK6, serine/threonine-protein kinase PAK6; EGFR, epidermal growth factor receptor; AUC, area under curve; CALM1, calmodulin-1; TF, transferrin; SERPINA1, Serpin α1-antitrypsin; AFM, afamin; CTSD, cathepsin D.
Recently, numerous studies have focused on the therapeutic potential of exosomes in various diseases. Studies have demonstrated that exosomes derived from different stem cell types have significant potential for treating DN. Previous investigations have established that MSCs are multifunctional stem cells located in adipose tissue, bone marrow, umbilical cord, placenta, and other tissues and organs. MSCs display remarkable self-renewal capabilities and differentiation potential. An increasing number of studies have reported that MSC-derived exosomes—particularly those sourced from adipose tissue, bone marrow, and umbilical cord—can exert protective effects on renal function. The underlying mechanisms primarily involve enhancing renal function and mitigating pathological damage to the kidney by reducing inflammation and inhibiting fibrosis and oxidative stress while promoting renal tissue repair (Table 3).
TABLE 3
| Source of exosomes | Cell or animal model | Key factor | Mechanism | Reference |
|---|---|---|---|---|
| ADSCs | Diabetic mice and MPC5 podocytes | miR-486 | The expression of miR-486 was enhanced, and activating the Smad1/mTOR signaling pathway in podocytes was inhibited, leading to increased autophagy and reduced podocyte apoptosis | Jin et al. (2019) |
| MPC5 podocytes | miR-215–5p | By inhibiting ZEB2 transcription, miR-215–5p is mediated to shuttle into podocytes, inhibiting HG-induced EMT progression and podocyte migration | Jin et al. (2020) | |
| Diabetic mice and MP5 podocytes | miR-26a-5p | miR-26a-5p is delivered to podocytes and alleviates pathological symptoms of DN by mediating TLR4/NF-κB/VEGFA signaling pathway inactivation | Duan et al. (2020) | |
| Diabetic mice and MPC5 podocytes | Nrf2/Keap1 pathway and FAM129B | Regulating the Nrf2/Keap1 pathway to alleviate inflammation and oxidative stress in DN by targeting FAM129B | Ren et al. (2023) | |
| GMC and DN rats | miR-125a | Carried miR-125a can be targeted to bind HDAC1 and downregulate ET-1, thereby inhibiting mesangial hyperplasia and renal fibrosis, promoting cell apoptosis, inhibiting DN progression, and relieving symptoms | Hao et al. (2021) | |
| MPC5 cells | miR-15b-5p | miR-15b-5p from ADSC-derived EVs can inhibit VEGF expression by binding to PDK4, thereby reducing MPC5 cell apoptosis and inflammation | Zhao et al. (2022b) | |
| BMSCs | STZ-diabetic mice | Fibrotic markers (collagen I, TGF-β, and α-SMA) and fibrosis-related genes | BMSCs–EVs can ameliorate renal dysfunction, attenuate renal histopathological changes, and reduce fibrosis | Grange et al. (2019) |
| DN rats | Autophagy mTOR pathway | Renal function is improved, and renal tissue histology is restored. LC3 and Beclin-1 are significantly increased, and the expressions of mTOR and fibrosis markers in renal tissue are significantly decreased | Ebrahim et al. (2018) | |
| HKCs | miR-125b | BMSC-derived exosomal miR-125b can induce autophagy and inhibit apoptosis in HG-treated HKCs via the TRAF6/AKT axis | Cai et al. (2021) | |
| HK-2 cells | miR-30e-5p | BMSC-derived exosomal miR-30e-5p can target ELAVL1 and inhibit caspase-1-mediated pyroptosis | Lv et al. (2023) | |
| Diabetic rats | Apoptosis-associated proteins and inflammatory molecules TNF-α and NF-κB | Inhibiting apoptosis and inflammation | Liu et al. (2023a) | |
| USCs | Diabetes rat | Molecules associated with apoptosis, angiogenesis, and cell survival | Inhibiting podocyte apoptosis and promoting angiogenesis and cell survival | Jiang et al. (2016) |
| Diabetic rats and HPDCs | miR-16–5p | miR-16-5p can inhibit VEGFA expression and podocyte apoptosis, thereby ameliorating HG-induced podocyte injury | Duan et al. (2021) | |
| DN rats and macrophage RAW264.7 | circRNA ATG7 | Promoting macrophage M2 polarization by regulating the SOCS1/STAT3 signaling pathway through miR-4500 | Sun et al. (2024) | |
| M2 macrophage | Macrophage RAW264.7 | miR-25-3p | miR-25-3p activates autophagy via inhibiting DUSP1 expression and improves podocyte injury induced by high glucose | Huang et al. (2020) |
| hUC-MSCs | Diabetic mice and podocytes | miR-22-3p | miR-22-3p may reduce the NLRP3 signaling pathway by inhibiting the expression of NLRP3 and alleviating the NLPR3-mediated inflammatory response | Wang et al. (2023c) |
| DN rats and TECs | Hedgehog/SMO signaling molecules | Inhibiting the Hedgehog/SMO pathway reduces tubular epithelial EMT and alleviates renal fibrosis | Zhang et al. (2024a) | |
| DN rats and macrophage RAW264.7 | miR-146a-5p | miR-146a-5p can alleviate renal damage in DN rats by facilitating M2 macrophage polarization by targeting TRAF6 | Zhang et al. (2022c) | |
| Db/db mice and HK2 cells | miR-424-5p | Inhibiting HG-induced apoptosis and EMT through miR-424–5p targeting of YAP1 | Cui et al. (2022) | |
| Skeletal muscle satellite cells | DN patients, db/db mice, and TECs | miR-23a/27a/26a | Ameliorating renal tubular injury and the progression of TIF in DN mice | Ji et al. (2023) |
Potential therapeutic values of exosomes derived from different cell types in DN.
ADSCs, adipose-derived mesenchymal stem cells; DN, diabetic nephropathy; MPC5, mouse podocyte clone 5; HG, high glucose; EMT, endothelium-mesenchymal transition; ZEB2, zinc finger E-box-binding homeobox 2; TLR4, toll-like receptor 4; VEGFA, vascular endothelial growth factor A; BMSCs, bone marrow mesenchymal stem cells (BMSCs); Nrf2, nuclear factor erythroid 2-related factor 2; Keap1; kelch-like ECH-associated protein 1; GMC, glomerular mesangial cell; HDAC1, histone deacetylase 1; ET-1, endothelin-1; HKCs, human embryonic kidney epithelial cells; TRAF6, tumor necrosis factor receptor-associated factor 6; ELAVL1, ELAV, like RNA, binding protein 1; USCs, urine-derived stem cells; HPDCs, human podocytes; ATG7, autophagy-associated gene 7; DUSP1, dual specificity protein phosphatase 1; hUC-MSCs, human umbilical cord mesenchymal stem cells; SMO, smoothened; TECs, tubular epithelial cells; YAP1, Yes-associated protein 1; TIF, tubulointerstitial fibrosis.
7.5 Lupus nephritis
Lupus nephritis (LN) represents a severe manifestation of systemic lupus erythematosus (SLE). The hallmark pathological feature of LN is the deposition of an immune complex formed by the combination of autoantibodies and antigens in the glomeruli. Early diagnosis of renal disease in SLE patients is crucial. Currently, renal biopsy is regarded as the gold standard for diagnosing LN. However, it is invasive. Therefore, searching for a non-invasive diagnostic marker is essential. Recently, numerous studies have demonstrated that specific components of urine- or blood-derived exosomes can be used as biomarkers for the early diagnosis of LN and for monitoring the therapeutic response. Among these components, miRNAs have been extensively studied. Growing evidence from both clinical and experimental models suggests that miRNAs and tRNA-derived small non-coding RNAs (tsRNAs) play pivotal roles in the progression of LN (Table 4).
TABLE 4
| Potential biomarker | Subject | Source of exosomes | Detection method | Main finding | Reference | |
|---|---|---|---|---|---|---|
| miRNA | ||||||
| miR-146a | SLE with or without patients with LN and HCs | Urinary exosomes | RT-PCR | All urinary miRNAs tested were mainly in exosomes in patients with LN. Only miR-146a can discriminate the presence of active LN | Perez-Hernandez et al. (2015) | |
| miR-146a | Patients with SLE, patients with LN, and HCs | Urinary exosomes | RT-qPCR | miR-146a levels are correlated with lupus activity, proteinuria, and histological features | Perez-Hernandez et al. (2021) | |
| miR-29c | Patients with LN and non-lupus chronic kidney diseases and HCs | Urinary exosomes | RT-PCR | miR-29c levels decreased significantly in patients with LN but lacked specificity. It can predict the degree of chronicity and correlate negatively with Smad3 and MMP2 expression | Solé et al. (2015) | |
| miR-21, miR-150, and miR-29c | Patients with LN and HCs; RMCs and RTCs | Urinary exosomes | RT-qPCR | The combined use of three miRNAs provides a non-invasive method for early renal fibrosis and the prediction of disease progression in patients with LN. | Solé et al. (2019) | |
| miR-195-5p | SLE with or without patients with LN | Urinary exosomes | Bioinformatic screen and RT-qPCR | The expression of miR-195-5p is decreased in patients with SLE, and it can distinguish LN from SLE | Cheng et al. (2022) | |
| miR-4796-5p and miR-7974 | SLE with or without patients with LN | Serum exosomes | Small RNA sequencing and RT-qPCR | The levels of Hsa-miR-4796-5p and hsa-miR-7974 in patients with LN are significantly increased, which can be used as better biomarkers to distinguish LN from patients with SLE, with an AUC exceeding 0.8 | Chen et al. (2023a) | |
| tsRNAs | tRF3-Ile-AAT-1 and tiRNA5-Lys-CTT-1 | SLE with or without patients with LN | Urinary exosomes | Sequencing and RT-PCR | Upregulated levels of tRF3-Ile-AAT-1 and tiRNA5-Lys-CTT-1 in the urinary exosomes are measured in LN compared with SLE without patients with LN. These two tsRNAs levels are strongly associated with SLE activity | Chen et al. (2023b) |
Potential biomarkers in exosomes of LN.
LN, lupus nephritis; HCs, healthy controls; RT-PCR, real-time polymerase chain reaction; MMP2, metalloproteinase 2; RMCs, renal mesangial cells; RTCs, renal tubular epithelial cells; SLE, systemic lupus erythematosus; RT-qPCR, real-time quantitative polymerase chain reaction; tsRNA, tRNA-derived small non-coding RNA.
Although numerous studies have confirmed that exosomes are crucial in treating kidney diseases, there are few reports concerning their application in LN patients. Evidence supporting the use of exosomes for predicting clinical responses or assessing disease activity remains limited. A long-term follow-up study was conducted with a small sample size to analyze LN patients during both the active and therapy remission stages. Notably, urinary exosomal let-7a and miR-21 were significantly downregulated in patients experiencing an active phase of LN. However, their expression levels increased following treatment-induced remission. This suggests that these biomarkers may serve as indicators for guiding the clinical stage of LN patients (Tangtanatakul et al., 2019). In another study, qPCR arrays and qRT-PCR were used to screen and identify specific miRNAs from the urinary exosomes in LN patients to predict the clinical therapeutic response. The findings revealed that the expression levels of miR-31, miR-107, and miR-135b-5p were significantly elevated in responders’ urine and kidney tissues during renal flares compared to non-responders. Among these miRNAs, miR-135b exhibited the highest predictive value. Elevated levels of these miRNAs may have contributed positively to recovery from LN (Garcia-Vives et al., 2020). An intriguing observation was reported by Somparn et al., who dynamically monitored the changes in plasma exosomal miRNA-146a levels among juvenile proliferative lupus nephritis (JPLN) patients at various stages—from onset through complete remission. Their results showed that the expression levels of exosomal miRNA-146a remained consistent at diagnosis and early treatment phases but showed a significant increase during complete remission compared to the time of diagnosis. Consequently, miRNA-146a has potential as a non-invasive biomarker for evaluating the therapeutic response in JPLN patients (Somparn et al., 2024).
7.6 Acute kidney injury
Acute kidney injury (AKI) is a clinical syndrome characterized by a sudden decline in renal function. AKI remains a serious global health issue, associated with high morbidity and mortality rates. Many factors can induce AKI, including trauma, major surgical procedures, sepsis, exposure to nephrotoxic agents, and ischemia/reperfusion injury. Notably, AKI is prevalent among critically ill surgical patients. The diagnosis of AKI primarily relies on the assessment of decreased urine volume and elevated serum creatinine levels. However, creatinine measurements are influenced by various confounding factors and may not effectively reflect early renal dysfunction. Furthermore, creatinine has demonstrated poor sensitivity and specificity in evaluating the severity or grade of AKI (Strauß et al., 2024). Therefore, there is an urgent need to identify new and more sensitive biomarkers for the early detection of kidney injury. Such advancements will significantly enhance our ability to monitor the progression of AKI and improve patient outcomes. Several novel indicators have been identified in blood or urine to predict and diagnose AKI (Ferreira et al., 2024). However, fewer biomarkers have been detected in the exosomes (Table 5). Exploring exosomal biomarkers for the early diagnosis of AKI is still in its infancy, and more extensive research is needed.
TABLE 5
| Potential biomarker | Subject | Source of exosomes | Detection method | Main finding | Reference | |
|---|---|---|---|---|---|---|
| Protein | ||||||
| Fetuin-A | AKI rats, ICU patients, and HCs | Urinary exosomes | Gel electrophoresis and mass spectrometry | Fetuin-A levels increase significantly in the early stages of different AKI models | Zhou et al. (2006) | |
| ATF3 | AKI rats, ICU patients with AKI, CKD patients, and HCs | Urinary exosomes | Western blot | ATF3 shows a significant increase trend in the early stage of the AKI model. ATF3 has high specificity in AKI patients | Zhou et al. (2008) | |
| ATF3 | Sepsis-AKI patients, sepsis-non-AKI patients, and HCs | Urinary exosomes | Western blot | ATF3 in urinary exosomes is an interesting sepsis-AKI biomarker with high diagnostic specificity | Panich et al. (2017) | |
| NHE3 | AKI rats, sepsis-associated AKI patients, and HCs | Urinary exosomes | Western blot | NHE3 increases significantly in different AKI rat models and sepsis-associated AKI patients | Yu et al. (2022b) | |
| C3, C4, G3BP, α-2 macroglobulin, and serotransferrin | V-AKI patients and HCs | Urinary exosomes | Proteomic analysis and LC/MS | Inflammatory proteins in urinary exosomes, such as C3, C4, G3BP, alpha-2 macroglobulin, and serotransferrin, are upregulated after nephrotoxic injury in V-AKI. | Awdishu et al. (2021) | |
| miRNAs | miR-181a-5p and miR-23b-3p | Sepsis-induced AKI model | Serum exosomes | RT-qPCR array | miR-181a-5p and miR-23b-3p are upregulated earlier than creatinine in a sepsis-induced AKI rat model | Da-Silva et al. (2022) |
Potential biomarkers in the exosomes of AKI.
AKI, acute kidney injury; HCs, healthy controls; ATF3, activating transcription factor 3; CKD, chronic kidney disease; NHE3, Na+/H+ exchanger isoform 3; ICU, intensive care unit; V-AKI, vancomycin-induced AKI; G3BP, galectin-3-binding protein; LC–MS, liquid chromatography–mass spectrometry; RT-qPCR, real-time quantitative polymerase chain reaction.
Increasing evidence suggests that exosomes derived from various cell sources exhibit therapeutic effects in AKI, becoming a new research hotspot. Among these, exosomes derived from MSCs—including those sourced from bone marrow, adipose tissue, placenta, and umbilical cord—have been shown to have significant therapeutic efficacy (Table 6). In renal ischemia/reperfusion (I/R) injury models or cisplatin-induced AKI, BMSC-Exos have shown anti-apoptotic properties and the ability to reduce DNA damage in renal tubular cells through their encapsulated miRNAs (Zhu G. et al., 2019; Wang S.-Y. et al., 2023). Furthermore, it has been reported that the role of BMSCs-Exos can be genetically modified to improve the effectiveness of treatment. For example, BMSC-Exos can be engineered by overexpressing indoleamine 2, 3-dioxygenase (IDO) to produce IDO-overexpressing BMSC-Exos (BMSC-Exo-IDO) in vitro. The repair ability of BMSC-Exo-IDO is significantly higher than that of BMSC-Exos after renal injury (Xie et al., 2022). Adipose-derived mesenchymal stem cells (ADSCs) are pluripotent stem cells derived from adipose cells. Numerous studies have shown that ADSC-Exos play a crucial role in the treatment of AKI. ADSC-Exos can transport different nucleic acids, such as miR-342–5p (Liu W. et al., 2023) and circVMA21 (He et al., 2023), to interact with specific target molecules and exert protective effects in autophagy, apoptosis, oxidative stress, inflammation, aerobic glycolysis, and other processes. Surprisingly, one study compared the difference in efficacy between ADSC-Exos and BMSC-Exos for LPS-induced AKI. The results of the study showed that ADSC-Exos were significantly more effective than BMSC-Exos in improving kidney function and structure (Zhang et al., 2022a). According to the published literature, hUCMSC-Exos are also involved in the recovery process after kidney injury through various mechanisms. Their protective effects include the inhibition of apoptosis (Zhou Y. et al., 2013), pyroptosis (Wan et al., 2023), inflammation, the promotion of cell proliferation, autophagy (Wang et al., 2017), renal tissue repair and angiogenesis (Huang J. et al., 2022), and the maintenance of mitochondrial homeostasis (Yu et al., 2023). Human amniotic epithelial cells (hAECs) are an ideal source of stem cells. Recently, a research team from the same laboratory reported the therapeutic effects of hAEC-Exos in AKI. In models of AKI induced by different injury factors, hAEC-Exos significantly improved mortality and renal function in mice. The protective mechanisms involve several aspects, including anti-apoptosis, the regulation of angiogenesis and immune function (Ren et al., 2020), anti-inflammatory effects (Kang et al., 2021), and the maintenance of renal endothelial integrity and adhesion junctions (Chi et al., 2022). Human urine-derived stem cells (USCs) exhibit antioxidant, anti-inflammatory, and anti-apoptotic cytoprotective effects. We hypothesize that USC-Exos also exhibit a reno-protective effect on AKI. The current literature suggests that USC-Exos can exert these protective effects by transporting encapsulated nucleic acids, which act on different signaling pathway molecules. These nucleic acids include miR-216a-5p (Zhang Y. et al., 2020), miR-146a-5p (Li et al., 2020), lncRNA taurine-upregulated gene 1 (TUG1) (Sun et al., 2022), and circ DENND4C (Yang et al., 2024) In addition to stem cell-derived exosomes, which can be used as potential therapeutic tools for AKI, exosomes derived from other cell types also exhibit comparable therapeutic benefits. For example, exosomes from apical papilla stem cells (Huang T.-Y. et al., 2022), endothelial progenitor cells (Zhou et al., 2018), and human cord blood endothelial colony-forming cells (Viñas et al., 2016) have shown promising effects. These cell-derived exosomes play a role in kidney protection by alleviating oxidative stress of damaged kidney cells, reducing the expression of inflammatory factors, regulating cell apoptosis, and preventing microvascular dysfunction.
TABLE 6
| Source of exosomes | Cell or animal model | Key factor | Mechanism | Reference |
|---|---|---|---|---|
| BMSCs | I/R rats and I/R HK-2 cells | miR-199a-3p | miR-199a-3p is delivered to renal tubule cells, downregulates Sema3A expression, and activates AKT and ERK pathways, exerting anti-apoptotic effects and alleviating kidney injury induced by I/R | Zhu et al. (2019a) |
| Cisplatin-induced AKI mice and HPTC injury | Let-7b-5p | Let-7b-5p alleviates tubular epithelial cell apoptosis via inhibiting p53, thereby reducing DNA damage and apoptosis pathway activity | Wang et al. (2023b) | |
| IRI-induced AKI mice | Cell proliferation and anti-fibrotic and anti-inflammatory molecules | Accelerating the proliferation of renal tubule cells, inhibiting tubule cell apoptosis and fibrosis, promoting the secretion of anti-inflammatory factors, and thus promoting the self-repair process after kidney injury | Xie et al. (2022) | |
| ADSCs | Sepsis-associated AKI patients and mouse models; LPS-induced HK-2 cells | miR-342–5p | ADSC-derived exosomal miR-342–5p promotes autophagy via the miR-342–5p/TLR9 axis, thereby protecting AKI. | Liu et al. (2023b) |
| LPS-induced mice and HK-2 cells | Circ VMA21 and miR-16–5p | ADSC-derived exosomal circVMA21 inhibits LPS-induced renal tubular cell apoptosis, inflammation, and aerobic glycolysis by targeting miR-16–5p | He et al. (2023) | |
| LPS-induced AKI rat | Inflammatory factors and oxidative stress molecules | ADSCs-Exos inhibit inflammatory factors and oxidative stress levels, improve renal function, and protect against AKI. | Zhang et al. (2022a) | |
| CLP-established AKI mice model | SIRT1 | ADSC-Exos activate the SIRT1 signaling pathway, inhibit kidney inflammation, improve kidney function and tissue morphology, and reduce the mortality of mice | Gao et al. (2020) | |
| hUC-MSCs | Cisplatin-induced AKI rats and NRK-52E cells | Oxidative stress, apoptosis, and proliferation-related molecules | Ameliorating oxidative stress and apoptosis, promoting cell proliferation, and repairing cisplatin-induced renal cell damage | Zhou et al. (2013b) |
| Cisplatin-induced AKI rats and NRK-52E cells | Autophagy-associated proteins | Activating autophagy and inhibiting cisplatin-induced apoptosis and inflammation, thereby reducing the renal toxicity | Wang et al. (2017) | |
| IRI-induced AKI rats and cisplatin-induced NRK-52E cells | Pyroptosis-associated proteins | Improving AKI by inhibiting pyroptosis | Wan et al. (2023) | |
| UUO-induced mice and cisplatin-induced HK-2 cells | miR-874-3p | Regulating necroptosis via miR-874-3p to attenuate renal tubular epithelial cell injury and enhance repair | Yu et al. (2023) | |
| I/R-induced AKI pigs | Apoptosis, inflammation, and angiogenesis molecules | Inhibiting I/R-induced apoptosis and necroptosis promotes renal regeneration, inhibits the expression of pro-inflammatory factors and infiltration of macrophages, and protects against the loss of renal angiogenesis | Huang et al. (2022a) | |
| hAECs | IRI-induced AKI mice H/R-treated HK-2 cells | Anti-apoptotic, pro-angiogenetic, and immunomodulatory molecules | Reducing apoptosis, stimulating cell proliferation, preventing peritubular capillary loss, and increasing M2 macrophage polarization | Ren et al. (2020) |
| CLP-established AKI mice | NF-κB and VCAM-1 | Suppressing systemic inflammation and maintaining the renal endothelial integrity in septic animals | Chi et al. (2022) | |
| Cisplatin-induced AKI mice and HK-2 cells | TNF-α/MAPK and caspase signaling pathways | Alleviating cisplatin-induced kidney inflammation by inhibiting the TNF-α/MAPK signaling pathway and attenuating cisplatin-induced apoptosis in renal tubular epithelial cells | Kang et al. (2021) | |
| USCs | IRI-induced AKI rats and H/R-induced HK-2 cells | miR-216a-5p | miR-216a-5p can target PTEN and regulate apoptosis via the AKT signaling pathway | Zhang et al. (2020b) |
| IRI-induced AKI rats and H/R-induced HK-2 cells | miR-146a-5p | miR-146a-5p can target the 3′UTR of IRAK1 and subsequently inhibit the activation of NF-κB signaling and infiltration of inflammatory cells to protect renal function | Li et al. (2020) | |
| I/R-induced AKI mice and H/R-induced HK-2 cells | LncRNA TUG1 | LncRNA TUG1 regulates the stability of ACSL4 mRNA by interacting with SRSF1 and alleviates IRI-induced AKI by suppressing ACSL4-mediated ferroptosis | Sun et al. (2022) | |
| I/R-induced AKI rats and H/R-induced HK-2 cells | Circ DENND4C | Promoting cell proliferation and inhibiting the activation of NLRP3 via the circ DENND4C/miR-138–5p/FOXO3a pathway, thereby reducing pyroptosis and AKI | Yang et al. (2024) | |
| SCAPs | Cisplatin-treated NRK-52E Cells | Oxidative stress, inflammation, and cell apoptosis molecules | Protecting NRK-52E cells from cisplatin-induced AKI by inhibiting oxidative stress, inflammation, and cell apoptosis | Huang et al. (2022b) |
| EPCs | CLP-established AKI rats | miR-21–5p | EPCs-Exos upregulate the expression of miR-21–5p. When targeting RUNX1, miR-21–5p can improve renal function and pathological kidney tissue damage in CLP rats, inhibit inflammation and oxidative stress response, and reduce the apoptosis of kidney tissue | Zhang et al. (2021a) |
| CLP-induced septic mice | miR-126–3p and 5p | EPCs-Exos prevent microvascular dysfunction and potentially improve sepsis outcomes by delivering miR-126 | Zhou et al. (2018) | |
| ECFCs | I/R-induced AKI mice | miR-486–5p | Delivery of ECFCs-Exos reduces ischemic kidney injury via the transfer of miR-486–5p targeting PTEN. | Viñas et al. (2016) |
Potential therapeutic values of different cell-derived exosomes in AKI.
AKI, acute kidney injury; BMSCs, bone marrow mesenchymal stem cells; I/R, ischemia/reperfusion; Sema3A, semaphorin 3A; AKT, activated the protein kinase B; ERK, extracellular-signal-regulated kinase; HPTCs, human primary renal tubular epithelial cells; IRI; ischemia–reperfusion injury; ADSCs, adipose-derived mesenchymal stem cells; LPS, lipopolysaccharide; TLR9, Toll-like receptor 9; CLP, cecal ligation and puncture; SIRT1, sirtuin 1; hUC-MSCs, human umbilical cord mesenchymal stem cells; UUO, unilateral ureter obstruction; hAECs, human amniotic epithelial cells; H/R, hypoxia-reoxygenation; VCAM-1, vascular cell adhesion molecule-1; NF-κB, nuclear factor kappa B; MAPK, mitogen-activated protein kinase; USCs, urine-derived stem cells; PTEN, phosphatase and tensin homolog; IRAK1, interleukin-1 receptor-associated kinase 1; TUG1, taurine-upregulated gene 1; ACSL4, acyl-CoA synthetase long-chain family member 4; SRSF1, serine/arginine splicing factor 1; circ DENND4C, circDENN, domain-containing 4C; NLRP3, NLR, family pyrin domain containing 3; SCAPs, apical papilla; EPCs, endothelial progenitor cells; RUNX1, runt-related transcription factor 1; ECFCs, endothelial colony-forming cells.
7.7 Polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common single-gene inherited disorders, primarily resulting from mutations in the PKD1 gene (approximately 85%) or the PKD2 gene (approximately 15%). The estimated prevalence of ADPKD ranges from 1/1000 to 1/400. Notably, more than half of affected individuals will progress to end-stage renal disease before the age of 60 years, requiring dialysis or kidney transplantation (Ding et al., 2021). Therefore, it is imperative to identify more sensitive early diagnostic markers and develop effective therapeutic strategies aimed at slowing the progression of ADPKD and enhancing patient prognosis.
Currently, there are limited reports on exosomal biomarkers related to ADPKD. Earlier studies utilizing proteomic analysis revealed that urinary exosomal levels of polycystin-1 (PC1) and polycystin-2 (PC2) were significantly reduced in patients with PKD1 mutations, while the levels of fibrocystic homologous transmembrane protein 2 (TMEM2) were found to be elevated. Subsequent validation through cohort studies indicated that the ratios of PC1/TMEM2 or PC2/TMEM2 could effectively differentiate individuals with PKD1 mutations from healthy controls. This suggests that these urinary exosomal proteins have practical utility in the diagnosis and monitoring of ADPKD (Hogan et al., 2015). Furthermore, the expression of the activator of G-protein signaling 3 (AGS3) is significantly increased in urinary exosomes in both animal models and patients with PKD, suggesting that AGS3 may serve as a novel biomarker for PKD (Keri et al., 2018). Another study identified up to 30 proteins that were significantly elevated in the urinary EVs of patients with ADPKD. Notably, periplakin, envoplakin, villin-1, and complements C3 and C9 are closely associated with the progression of ADPKD (Salih et al., 2016).
The diagnostic value of miRNAs derived from urinary exosomes in renal diseases has been previously demonstrated. Similarly, urinary exosomal miRNAs can be used as diagnostic tools for ADPKD. A previous study showed that miR-192-5p, miR-194-5p, miR-30a-5p, miR-30d-5p, and miR-30e-5p were significantly downregulated in the urinary exosomes of patients, murine PKD1 cystic kidneys, and human PKD1 cystic kidney tissues. This suggests their potential utility as novel biomarkers for disease progression and new therapeutic targets for ADPKD (Magayr et al., 2020). A recent study analyzed small RNAs that are differentially expressed between patients with ADPKD and healthy controls. The findings revealed that miR-320b, miR-320c, miR-146a-5p, miR-199b-3p, miR-671-5p, miR-1246, miR-8485, miR-3656, has_piR_020497, has_piR_020496, and has_piR_016271 were significantly upregulated in urine EVs from ADPKD patients. Conversely, miRNA-29c was significantly downregulated. These specific miRNAs may represent promising biomarkers for ADPKD (Ali et al., 2024). To the best of our knowledge, few studies have investigated the role of exosomes in the treatment of ADPKD. One such study conducted a proteomic analysis of urinary exosomes, which proved useful in distinguishing between rapidly progressing and slowly progressing ADPKD. Additionally, it distinguished between patients who responded favorably to tolvaptan therapy and those who did not respond adequately. The proteomic analysis of urinary exosomes provides a specific and sensitive non-invasive method for identifying disease progression rates and evaluating the efficacy of tolvaptan treatment among individuals with ADPKD—thereby indicating new avenues for personalized prognosis and management strategies related to this condition (Raby et al., 2021).
7.8 Renal cell carcinoma
Renal cell carcinoma (RCC) is one of the most common malignancies in the urinary system. Unfortunately, approximately 30% of patients are already in the metastatic stage at the time of diagnosis. Currently, the diagnosis of RCC primarily relies on imaging techniques and renal biopsy. However, the early clinical symptoms of RCC are non-specific and typically manifest at a later stage in disease progression, thereby missing the best opportunities for timely diagnosis and treatment. Therefore, there is an urgent need to develop non-invasive molecular detection in urine or serum to identify reliable biomarkers, which is crucial for improving the early diagnosis and treatment of RCC (Rui et al., 2023). Numerous studies have highlighted the significant roles of exosomes in several aspects of RCC, including tumorigenesis, metastasis, immune evasion, and drug response. The multiple bioactive molecules encapsulated in exosomes, such as RNA, DNA, proteins, and lipids, provide opportunities for early diagnosis and targeted interventions in RCC (Boussios et al., 2023). Table 7 shows potential exosomal biomarkers for RCC. These exosome-related molecules have also been validated.
TABLE 7
| Potential biomarker | Subject | Source of exosomes | Detection method | Main finding | Reference | |
|---|---|---|---|---|---|---|
| miRNAs | miR-126-3p and different combinations of miRNAs | ccRCC patients and HCs | Urinary exosomes | RT-qPCR | miR-126-3p combined with miR-449a or miR-34b-5p can significantly distinguish ccRCC patients from healthy participants | Butz et al. (2016) |
| miR-30c-5p | ccRCC, prostate cancer, and bladder cancer patients, and HCs | Urinary exosomes | NGS | Sixteen urinary exosomal miRNAs are dissimilarly expressed between ccRCC patients and healthy individuals. Among them, miR-30c-5p is the only miRNA with high specificity in ccRCC. | Song et al. (2019) | |
| miR-204-5p | Xp11 tRCC mouse model, M-1 cells, HK-2 cells, and Xp11 tRCC cells | Urinary exosomes | qPCR array analysis and RT-qPCR | miR-204-5p levels in urinary exosomes from the Xp11 tRCC mouse model significantly increased relative to those in control mice, and these increases occurred at time points before overt tRCC development | Kurahashi et al. (2019) | |
| miR-210 and miR-1233 | ccRCC patients and HCs | Serum exosomes | RT-qPCR | Serum exosomal miR-210 and miR-1233 are upregulated in ccRCC, independently of clinical staging | Zhang et al. (2018) | |
| miR-210 | Renal cell lines, ccRCC patients, and HCs | Serum exosomes | RT-qPCR | Only the serum exosomal miR-210 was significantly upregulated in ccRCC patients. The area under the ROC curve was 0.8779, and the sensitivity and specificity were 82.5% and 80.0%, respectively | Wang et al. (2019) | |
| miR-149–3p, miR-424–3p, and miR-92a-1-5p | RCC patients and HCs | Plasma exosomes | RT-qPCR | miR-149-3p and miR-424-3p are upregulated, and miR-92a-1-5p is significantly downregulated. These three miRNAs may be novel biomarkers for the diagnosis of RCC. | Xiao et al. (2020) | |
| miR-224 | Renal cell lines and ccRCC patients | Serum or cell culture media exosomes | RT-qPCR | Exosomal miR-224 is associated with invasion and metastasis of RCC and can be used as a biomarker reflecting the prognosis of RCC. | Fujii et al. (2017) | |
| miR-let-7i-5p, miR-26a-1-3p, and miR-615–3p | mRCC patients | Plasma exosomes | RT-qPCR | Multiple plasma exosomal miRNAs show an association with survival in mRCC-stage patients | Du et al. (2017) | |
| hsa-miR-320 d | ccRCC patients | Serum EVs | Small RNA deep sequencing and RT-qPCR | Serum EVs-derived hsa-miR-320d can be used as a prognostic biomarker for predicting ccRCC metastasis and recurrence | Xue et al. (2023) | |
| mRNA | CUL9, KMT2D, PBRM1, PREX2, and SETD2 | ccRCC, benign renal masses patients, and HCs | Serum exosomes | RT-qPCR | Establishing and validating novel exosomal mRNA-based signatures for the early detection of ccRCC and differential diagnosis of uncertain renal masses | He et al. (2022) |
| circular RNA | circ_400 068 | RCC patients and RCC cells | Plasma exosomes | circRNA microarray and RT-qPCR | Exosomal circ_400 068 promotes the development of RCC via the miR-210-5p/SOCS1 axis | Xiao and Shi (2020) |
| snoRNAs | SNORD99, SNORD22, SNORD26, and SNORA50C | ccRCC patients and urolithiasis controls | Urine-derived EVs | RNA sequencing and qPCR | Reporting four snoRNAs as biomarkers from urine-derived EVs for the non-invasive detection of ccRCC | Grützmann et al. (2024) |
| Proteins | 10 proteins | RCC patients and HCs | Urinary exosomes | LC–MS/MS | Identifying 261 proteins in HCs and 186 in RCC patients. Significant, reproducible differences exist in the content of some proteins between the two groups | Raimondo et al. (2013) |
Potential biomarkers in the exosomes of RCC.
RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; HCs, healthy controls; RT-qPCR, real-time quantitative polymerase chain reaction; NGS, next-generation sequencing; Xp11 tRCC, Xp11.2 translocation renal cell carcinoma; qPCR, quantitative PCR; mRCC, metastatic renal cell cancer; EVs, extracellular vesicles; SOCS1, suppressor of cytokine signaling 1; snoRNAs, small nucleolar RNAs.
The application of exosomes in tumor therapy has become a new research topic. Exosomes can directly transport bioactive molecules to tumor cells. This characteristic of precise release and targeted therapy makes exosomes a promising treatment for RCC. It has been demonstrated that miR-182, contained within MSC-derived exosomes, can inhibit the expression of vascular endothelial growth factor A (VEGFA) to promote the immune response of T cells, thus slowing RCC progression (Li D. et al., 2021). In 2022, Yoshino et al. found that exosomal miRNA-1 (miR-1) significantly inhibits RCC cell proliferation, migration, and invasion, suggesting that exosomal miR-1 therapy may be an effective therapeutic strategy for RCC (Yoshino et al., 2022).
7.9 Chronic kidney disease
Chronic kidney disease (CKD) is a global public health challenge, affecting approximately 10–15 percent of the adult population. CKD ultimately progresses to kidney failure, also referred to as ESRD, necessitating dialysis or kidney transplantation for patient survival. A critical event in the progression from CKD to ESRD is interstitial fibrosis, which is characterized by a multifactorial pathological process involving oxidative stress and an inflammatory response. Current conventional non-invasive indicators, such as blood creatinine and urea nitrogen levels, exhibit limitations in sensitivity and specificity for the early detection of renal impairment. These markers are influenced by various factors, thereby constraining their clinical application. Recent studies have highlighted that the pathophysiological changes in renal function are closely related to exosomes derived from glomeruli, tubules, interstitial cells, urine, and serum. These exosomes contain proteins and non-coding RNAs that are involved in the onset and progression of renal fibrosis. They play an important role in the early diagnosis and subsequent treatment of CKD (Yeh et al., 2024).
Increasing evidence indicates that exosomal proteins and miRNAs are expressed at abnormal levels in CKD patients and may be regarded as key biomarkers in the progression of CKD. Moreover, it is well known that CKD is characterized by an irreversible loss of renal function and is closely associated with the progression of renal fibrosis. Therefore, CKD biomarkers primarily reflect the extent of renal fibrosis. More than a decade ago, Lv et al. first investigated the value of miRNAs in urinary exosomes as potential biomarkers of renal fibrosis. Their findings revealed that the levels of urinary exosomal miR-29 and miR-200 were significantly reduced in patients with CKD. Notably, both miR-29a and miR-29c have been shown to effectively predict the degree of tubulointerstitial fibrosis, thereby distinguishing mild cases from moderate-to-severe fibrosis (Lv et al., 2013). Subsequently, results from different research teams showed that urinary exosomal miR-200b (Yu et al., 2018), miR-181a (Khurana et al., 2017), and miR-21 (Lange et al., 2019) could serve as promising biomarkers for the early detection of CKD. In addition to exosomal miRNAs, other nucleic acid components, such as non-coding RNAs, may also become a new research direction for identifying biomarkers of renal fibrosis in CKD. CircRNAs are a class of non-coding, linear RNA molecules characterized by their unique circular structure. Recently, a microarray has been used to analyze the differences in urinary exosomal circRNA expression profiles between CKD patients without renal fibrosis and those with renal fibrosis. The results showed that the expression levels of hsa_circ_0036649 correlated with the degree of renal fibrosis, showing high specificity in predicting this condition (Cao et al., 2022b). Additionally, it has also been reported that urinary exosomal hsa_circ_0008925 can act as a non-invasive biomarker for renal fibrosis. The expression level of hsa_circ_0008925 was found to be elevated in urinary exosomes from patients with renal fibrosis and exhibited a significant correlation with tubulointerstitial fibrosis and glomerular sclerosis (Cao et al., 2022a).
Exosomes also have a great therapeutic potential for CKD. Currently, the application of exosomes in CKD treatment primarily focuses on alleviating or preventing the progression of renal fibrosis. Increasing evidence suggests that MSC-derived exosomes from diverse origins have unique advantages in treating renal fibrosis. The potential therapeutic values associated with different cell-derived exosomes in patients with CKD are summarized in Table 8.
TABLE 8
| Source of exosomes | Cell or animal model | key factor | Mechanism | Reference |
|---|---|---|---|---|
| BMSCs | HA-VSMCs and 5/6 SNx rat models | miR-381-3p | BMSCs-Exos play anti-calcification and anti-apoptosis roles in CKD by delivering enclosed miR-381–3p, directly targeting NFAT5 mRNA | Liu et al. (2022c) |
| UUO mice model and TCMK-1 cells | miR-21a-5p | BMSCs-Exos significantly ameliorate UUO-induced renal fibrosis by inhibiting glycolysis in TECs | Xu et al. (2022) | |
| UUO mice model and pericytes | miR-34c-5p | BMSCs-Exos deliver miR-34c-5p into pericytes, fibroblasts, and macrophages, and miR-34c-5p downregulates CF to inhibit multiple signaling pathways, thereby ameliorating multiple cellular activations and RIF | Hu et al. (2022) | |
| UUO mice model and HK-2 cells | miR-374a-5p | Exosomal miR-374a-5p inhibited the progression of renal fibrosis by regulating the MAPK6/MK5/YAP axis | Liang et al. (2022a) | |
| UUO mice model and NRK-52E cells | Let-7i-5p antagomir | Exosomal anti-let-7i-5p from BMSCs exerts anti-fibrotic effects in TGF-β1-induced fibrogenic responses in NRK52E cells in vitro and in the UUO-induced renal fibrosis model in vivo | Jin et al. (2021) | |
| UUO mice model and NRK52E cells | miR-let7c | The anti-fibrotic function of engineered MSCs can selectively transfer miR-let7c to damaged kidney cells and significantly downregulate the expression of fibrosis genes | Wang et al. (2016) | |
| 5/6 SNx rat models | klotho activity | BMSCs-Exos may protect against kidney injury, probably by regulating klotho activity and expression | Wan et al. (2022) | |
| 5/6 SNx rat models and HRPTEpics | Smurf 2/Smad 7 axis | BMSCs-Exos partially inhibit renal fibrosis both in vivo and in vitro by regulating the Smurf 2/Smad 7 axis | Liu et al. (2022b) | |
| 5/6 SNx mouse models | SIRT6 and HMGB1 | BMSCs-Exos inhibit high phosphate-induced aortic calcification and ameliorate renal function via the SIRT6-HMGB1 deacetylation pathway | Wei et al. (2021) | |
| hUC-MSCs | UUO rat model and NRK52E cells | YAP activity | hUC-MSCs-Exos can increase CK1δ and β-TRCP expression and promote YAP ubiquitination and degradation, which inhibits ECM deposition and alleviates renal fibrosis | Ji et al. (2020) |
| UUO mice model and RAW264.7 cells | ARNTL | hUC-MSCs-Exos reduce renal fibrosis in UUO mice by inhibiting MMT and may be associated with regulating ARNTL expression | Guo et al. (2024) | |
| HK-2 cells | miR-335–5p | hUC-MSCs-derived exosomal miR-335–5p attenuates the inflammation and EMT of HK-2 cells by reducing ADAM19 protein levels | Qiu et al. (2022) | |
| ADSCs | CKD mouse model | Molecules associated with inflammation and fibrosis | ADSCs-Exos inhibit renal fibrosis by regulating apoptosis and fibrosis-related cell proliferation, reducing the severity of CKD, and restoring renal function by increasing the levels of aquaporins 2 and 5 | Yea et al. (2021) |
Potential therapeutic values of exosomes derived from different cell types in CKD.
CKD, chronic kidney disease; BMSCs, bone marrow mesenchymal stem cells; SNx, subtotal nephrectomy; HA-VSMCs, human aortic smooth muscle cells; NFAT5, nuclear factor of activated T cells 5; UUO, unilateral ureter obstruction; TECs, tubular epithelial cells; CF, core fucosylation; RIF, renal interstitial fibrosis; TGF-β1, transforming growth factor beta 1; HRPTEpCs, human renal proximal tubular epithelial cells; SIRT6, sirtuin 6; HMGB1, high mobility group box 1; hUC-MSCs, human umbilical cord mesenchymal stem cells; CK1δ, casein kinase 1δ; YAP, yes-associated protein; ECM, extracellular matrix; MMT, macrophage-to-myofibroblast transformation; EMT, endothelium-mesenchymal transition; ADAM19, metalloproteinase domain-containing protein 19; ADSCs, adipose-derived mesenchymal stem cells.
7.10 Kidney transplantation
Kidney transplantation is an effective treatment for end-stage renal disease, significantly improving patients’ quality of life and prolonging survival. Although kidney transplantation is an effective treatment, recipients still often encounter challenges such as rejection, adverse effects from immunosuppressive medications, and delayed recovery of the transplanted kidney’s function. Although renal biopsy is considered the gold standard for the diagnosis of transplant rejection, it is an invasive procedure with a high cost. Consequently, there is a pressing need for non-invasive biomarkers with high sensitivity and specificity to monitor, diagnose, and detect allograft rejection at an early stage (Ramalhete et al., 2024). Exosomes have been identified to play an important role in allograft rejection (Saravanan et al., 2023). Currently, various exosomal biomarkers are used to assess response after kidney transplantation, as detailed in Table 9.
TABLE 9
| Potential biomarker | Subject | Source of exosomes | Detection method | Main finding | Reference | |
|---|---|---|---|---|---|---|
| miRNAs | Hsa-miR-21-5p, hsa-miR-31-5p, and hsa-miR-4532 | AR, STA, and OGI recipients | Urinary exosomes | RT-qPCR | Identifying 29 urinary exosomal microRNAs as the candidate biomarkers of AR. The combination of hsa-miR-21-5p, hsa-miR-31-5p, and hsa-miR-4532 has higher performance for AR identification | Seo et al. (2023) |
| miR-21, miR-210, and miR-4639 | Kidney transplant recipients and HCs | Plasma exosomes | RT-qPCR | miR-21, miR-210, and miR-4639 in plasma exosomes correlate closely with eGFR. The combined diagnostic value of the three miRNAs is better than that of single or double indicators | Chen et al. (2020) | |
| miR-21 | Post-kidney transplantation patients | Plasma exosomes | RT-qPCR | Plasma exosome miR-21 is an interesting non-invasive IF/TA grade II/III biomarker that is better than the current clinical biomarkers of renal function | Saejong et al. (2022) | |
| mRNA | Exosomal mRNA signature | Kidney transplant recipients | Urinary exosomes | RT-PCR | Providing the high-performance characteristics of urinary exosome RNA to discriminate active rejection from no rejection and ABMR from TCMR | El Fekih et al. (2021) |
| Gp130, SH2D1B, TNFα, and CCL4 | Kidney transplant recipients | Plasma exosomes | RT-qPCR | In the plasma exosomes of AMR patients, mRNA levels of several genes are significantly increased. The gene combination of gp130, SH2D1B, TNF-α, and CCL4 is the best | Zhang et al. (2017) | |
| Proteins | 349 exosomal proteins | Kidney transplant patients with and without AR | Urinary exosomes | LC–MS/MS | Three hundred forty-nine proteins are identified in urine exosomes. Among them, 11 proteins are more abundant in urine samples from patients with AR, and three are exclusive to the urine exosome fraction | Sigdel et al. (2014) |
| Tetraspanin-1 and hemopexin | Kidney transplant recipients including STA and TCMR | Urinary exosomes | Nano-UPLC–MS/MS | Seventeen proteins were increased in TCMR patients. Among the five TCMR candidate biomarkers, only tetraspanin-1 and hemopexin perform better | Lim et al. (2018) | |
| SYT17 | CKD patients, kidney transplant patients, and HCs | Urinary exosomes | Western blotting | Urinary exosomal SYT17 levels are significantly elevated in the CAAMR group compared to other histology groups | Takada et al. (2020) | |
| AZGP1 | Kidney transplant recipients including CAMR and LGS | Urinary exosomes | LC–MS analysis and Western blotting | AZGP1, in particular, was found to be a CAMR-specific proteomic biomarker | Jung et al. (2020) | |
| CST3 and LBP | Kidney transplant recipients including ABMR, TCMR, BKVN, and NOMOA | Urinary exosomes | Nano-LC-ESI-MS/MS analysis | Urinary exosomal CST3 and LBP levels are significantly higher in the ABMR group | Kim et al. (2022) | |
Potential biomarkers in the exosomes of kidney transplantation.
AR, acute rejection; STA, stable graft function; OGIs, other graft injuries; RT-qPCR, real-time quantitative polymerase chain reaction; HCs, healthy controls; IF/TA, interstitial fibrosis and tubular atrophy; RT-PCR, real-time polymerase chain reaction; TCMR, T-cell-mediated rejection; ABMR, antibody-mediated rejection; AMR, antibody-mediated rejection; nano-UPLC–MS/MS, nano-ultra performance liquid chromatography–tandem mass spectrometry; SYT17, synaptotagmin-17; CKD, chronic kidney disease; CAAMR, chronic active antibody-mediated rejection; CAMR, chronic active antibody-mediated rejection; AZGP1, zinc-alpha-2-glycoprotein; LGS, long-term graft survival; CST3, cystatin C; LBP, lipopolysaccharide-binding protein; BKVN, BK virus nephropathy; NOMOA, no major abnormality.
Immunosuppressive agents are most commonly used to prevent immune rejection in renal transplantation; however, they cause immune-mediated damage and adverse effects. Therefore, studies on using exosomes for preventing and mitigating immune rejection in renal transplantation have become an area of significant interest. Recent investigations utilizing a mouse kidney transplantation model have focused on the antagonistic non-protein coding RNA (DANCR), a long-chain non-coding RNA (lncRNA) found in bone marrow mesenchymal stem cell-derived exosomes. This lncRNA has been shown to promote immune tolerance in kidney transplantation by downregulating sirtuin-1 (SIRT1) expression and promoting the differentiation of CD4+ T-cells into Treg-cells (Wu X. et al., 2022). Renal ischemia–reperfusion injury (IRI) is a common clinical symptom of renal transplantation. Exosomes derived from umbilical cord mesenchymal stem cells (UC-MSC-Exos) exhibit certain advantages in treating IRI in transplanted kidneys. A recent study conducted by Wu et al. explored how UC-MSC-Exos ameliorate renal transplant-related IRI, identifying miR-19b as a critical factor. miR-19b was found to reduce the expression of the metabolic enzyme PDXK, increase levels of the metabolite pyridoxine, and ultimately reduce apoptosis in I/R-HK2 cells by targeting GSK3β, thereby providing a new therapeutic target for IRI after kidney transplantation (Wu et al., 2024). In summary, there is limited research regarding the role of exosomes in treating and recovering renal function post-kidney transplantation. Further comprehensive studies are warranted to elucidate the therapeutic potential of exosomes more thoroughly.
8 Limitation of exosomes
Although exosome research has made remarkable progress, several challenges still exist. These issues include the following: first, although there are many methods for the production, isolation, and purification of exosomes, most of these procedures are complex and time-consuming, making it difficult to meet the needs of rapid clinical detection. Therefore, more convenient methods must be developed and improved. Moreover, various studies used different methods, complicating the reproducibility of results. Therefore, it is essential to establish more stringent protocols for the isolation and purification of exosomes to achieve unified standardization that allows for comparability and reproducibility across different studies. Second, the study of exosome biomarkers in kidney diseases is mostly a phenomenon description, which is still in the initial stage. The biomarkers used for diagnosing kidney disease primarily stem from exosome-derived miRNAs and proteins. However, many studies have relied on smaller validation sample sizes. Thus, further validation using larger clinical samples is necessary to ascertain the disease specificity of these biomarkers. Additionally, the complexity inherent in various types of kidney diseases must be taken into account. Different types of kidney diseases may exhibit similar trends in biomarker changes, which can increase the difficulty of using exosomes as biomarkers of kidney diseases. Moreover, the coexistence of multiple diseases may further challenge the clinical application of exosomes. Third, current research studies on the role of exosomes in treating kidney diseases have predominantly focused on MSC-derived exosomes. However, the experimental protocols for extracting exosomes from mesenchymal stem cells still lack standardization, and there are no effective methods for the large-scale production and purification of exosomes. Fourth, there are still some other issues to be considered in the clinical treatment of kidney diseases with exosomes. More preclinical studies and pharmacological dynamic monitoring are needed to reveal the optimal dose, mode of administration, time point, and duration for exosome therapy. More importantly, many current in vitro studies, animal experiments, and preclinical studies do not provide a comprehensive analysis of the long-term efficacy and potential side effects of exosomes in the treatment of kidney disease. For instance, while MSC-derived exosomes are known for their immunomodulatory and anti-fibrotic effects, their immunogenicity and pro-tumorigenic risks in high-dose or long-term applications remain unclear. This is especially relevant for chronic kidney disease patients who may require repeated dosing, where issues such as immune tolerance or side effect accumulation could arise. In addition, the immune response evaluations in preclinical models of ADSC-derived exosomes and the exploration of strategies for targeted delivery to reduce systemic side effects require more attention and research. Fifth, the therapeutic effects of exosomes have primarily been demonstrated through experiments utilizing animal and cell models, resulting in a notable absence of clinical research evidence. Nevertheless, it is encouraging to note that research into the roles of exosomes in disease treatment is progressing rapidly. We believe that the application of exosome-based therapies will gradually transition from basic research to clinical practice.
9 Future directions
9.1 Application of engineered exosomes
Engineered exosomes represent a class of modified extracellular vesicles derived from natural exosomes through bioengineering processes. This approach overcomes some of the inherent limitations of natural exosomes, such as unpredictable targeting, short half-lives, and variability in the composition of the functional cargoes. Engineered exosomes exhibit enhanced drug delivery efficiency and specificity, enabling more effective delivery to target cells or tissues. The preparation techniques for engineered exosomes primarily encompass surface modification and content modification. Surface modification involves altering the exosome surface via genetic engineering or chemical methods to improve targeting and stability. For instance, targeting peptides can be conjugated to exosomes to facilitate specific binding to target cells. Content modification integrates therapeutic agents, such as small molecule drugs or nucleic acids, into exosomes for targeted drug delivery. This method increases local therapeutic agent concentration while minimizing side effects. Common methods for content modification include co-incubation, due to its simplicity, and physical methods like ultrasound, electroporation, freeze–thaw cycles, and extrusion, although these may compromise exosomal membrane integrity to varying degrees (Fan et al., 2024). With ongoing technological advancements, the integration of gene editing technologies with exosomes is emerging as a promising direction for disease treatment. The synergistic effect of CRISPR/Cas9 gene editing and exosome therapy offers the potential for highly targeted and personalized therapeutic strategies (Dara et al., 2024). Currently, engineered exosomes show significant promise across various diseases (Tian et al., 2023). However, their application in kidney disease treatment remains nascent and limited. It is reported that Wang L. et al. (2024) demonstrated the renal protective properties of exendin-4-enriched exosomes derived from human umbilical cord mesenchymal stem cells in diabetic nephropathy. Additionally, techniques such as CRISPR/Cas9-mediated gene editing or electroporation-based siRNA loading (e.g., targeting TGF-β1) hold considerable potential for anti-fibrotic treatments in chronic kidney disease.
9.2 Enhancing the transformation of basic research into clinical practice
In recent years, an increasing number of models have been used to investigate the protective role of exosomes in renal diseases. However, to date, the majority of studies have been confined to cellular and animal models. There are several factors limiting clinical trials of exosomes, one of which is the low separation efficiency and purity of exosomes. Additionally, ethical concerns associated with exosome research pose another potential barrier to their clinical translation. There is now an urgent need for clinical trials to assess the efficacy and safety of exosome therapy. In particular, large-scale clinical trials and long-term follow-up studies are essential for elucidating the therapeutic potential of mesenchymal stem cell-derived exosomes in nephropathy treatment. In addition, future studies should pay more attention to the clinical translation of engineered exosomes in the treatment of kidney diseases. Determining the optimal dose, timing, route of administration, and effective measures to avoid side effects of engineered exosome therapy are important issues faced by researchers and essential steps in advancing exosomes to clinical practice.
9.3 Application of combination therapies
Although MSC-derived exosomes have shown significant therapeutic effects in various kidney diseases, these treatments are often single, and their efficacy still needs to be improved. Future research studies should investigate the potential of combining exosome therapy with other therapeutic approaches. For instance, integrating exosome therapy with pharmacological agents or bioactive molecules or utilizing exosome engineering to deliver multifunctional exosomes carrying anti-inflammatory factors (e.g., miR-26a-5p) and anti-fibrotic molecules (e.g., siRNA) can simultaneously achieve dual therapeutic effects against inflammation and fibrosis in diabetic nephropathy and chronic kidney disease.
9.4 Precise classification and extraction of kidney specific exosomes
Exosomes have different cell origins and can be obtained from a variety of cells. The surface proteins and functions of exosomes derived from different cells are different. If the classification of exosomes is not accurate, their clinical application and development can be limited to a certain extent. In kidney disease, urinary exosomes used for diagnosis originate from diverse sources such as glomerular endothelial cells, mesangial cells, podocytes, and renal tubular epithelial cells. The isolation and identification of disease-specific exosomes using specific molecular markers to improve the specificity of disease diagnosis remains an urgent problem.
10 Summary
In conclusion, research on the application of exosomes in renal diseases is currently experiencing rapid advancement. Their potential for diagnosis, treatment, and prognosis monitoring holds great promise. With ongoing technological progress and comprehensive investigations, exosomes are expected to emerge as a crucial tool in the management of renal diseases, thereby significantly enhancing patients’ health outcomes and quality of life.
Statements
Author contributions
HC: writing – original draft and writing – review and editing. ZL: conceptualization and writing – review and editing. JY: writing – review and editing. YL: conceptualization and writing – review and editing. CZ: writing – review and editing. TL: writing – original draft, writing – review and editing, and funding acquisition. YW: writing – original draft, writing – review and editing, funding acquisition, and supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 81570657), the Natural Science Foundation Program of Hubei Province (No. 2024AFB672), and the Free Innovation Pre-research Fund of Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (No. 2023XHYN052).
Acknowledgments
The authors would like to thank all participants for their valuable contributions to this study. In addition, they would also like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abe H. Sakurai A. Ono H. Hayashi S. Yoshimoto S. Ochi A. et al (2018). Urinary exosomal mRNA of WT1 as diagnostic and prognostic biomarker for diabetic nephropathy. J. Med. Invest.65, 208–215. 10.2152/jmi.65.208
2
Aklilu A. M. (2023). Diagnosis of chronic kidney disease and assessing glomerular filtration rate. Med. Clin. North Am.107, 641–658. 10.1016/j.mcna.2023.03.001
3
Ali H. Malik M. Z. Abu-Farha M. Abubaker J. Cherian P. Nizam R. et al (2024). Global analysis of urinary extracellular vesicle small RNAs in autosomal dominant polycystic kidney disease. J. Gene Med.26, e3674. 10.1002/jgm.3674
4
Al-Madhagi H. (2024). The landscape of exosomes biogenesis to clinical applications. Int. J. Nanomedicine19, 3657–3675. 10.2147/IJN.S463296
5
Alzhrani G. N. Alanazi S. T. Alsharif S. Y. Albalawi A. M. Alsharif A. A. Abdel-Maksoud M. S. et al (2021). Exosomes: isolation, characterization, and biomedical applications. Cell Biol. Int.45, 1807–1831. 10.1002/cbin.11620
6
Avraham S. Korin B. Chung J.-J. Oxburgh L. Shaw A. S. (2021). The Mesangial cell - the glomerular stromal cell. Nat. Rev. Nephrol.17, 855–864. 10.1038/s41581-021-00474-8
7
Awdishu L. Le A. Amato J. Jani V. Bal S. Mills R. H. et al (2021). Urinary exosomes identify inflammatory pathways in vancomycin associated acute kidney injury. Int. J. Mol. Sci.22, 2784. 10.3390/ijms22062784
8
Bagheri H. S. Mousavi M. Rezabakhsh A. Rezaie J. Rasta S. H. Nourazarian A. et al (2018). Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med. Sci.33, 1131–1145. 10.1007/s10103-018-2495-8
9
Bai L. Li J. Li H. Song J. Zhou Y. Lu R. et al (2019). Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem. Pharmacol.169, 113619. 10.1016/j.bcp.2019.08.021
10
Bai S. Xiong X. Tang B. Ji T. Li X. Qu X. et al (2020). Exosomal circ_DLGAP4 promotes diabetic kidney disease progression by sponging miR-143 and targeting ERBB3/NF-κB/MMP-2 axis. Cell Death Dis.11, 1008. 10.1038/s41419-020-03169-3
11
Batrakova E. V. Kim M. S. (2016). Development and regulation of exosome-based therapy products. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol8, 744–757. 10.1002/wnan.1395
12
Borges F. T. Melo S. A. Özdemir B. C. Kato N. Revuelta I. Miller C. A. et al (2013). TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol.24, 385–392. 10.1681/ASN.2012101031
13
Boussios S. Devo P. Goodall I. C. A. Sirlantzis K. Ghose A. Shinde S. D. et al (2023). Exosomes in the diagnosis and treatment of renal cell cancer. Int. J. Mol. Sci.24, 14356. 10.3390/ijms241814356
14
Butz H. Nofech-Mozes R. Ding Q. Khella H. W. Z. Szabó P. M. Jewett M. et al (2016). Exosomal MicroRNAs are diagnostic biomarkers and can mediate cell-cell communication in renal cell carcinoma. Eur. Urol. Focus2, 210–218. 10.1016/j.euf.2015.11.006
15
Cai X. Zou F. Xuan R. Lai X.-Y. (2021). Exosomes from mesenchymal stem cells expressing microribonucleic acid-125b inhibit the progression of diabetic nephropathy via the tumour necrosis factor receptor-associated factor 6/Akt axis. Endocr. J.68, 817–828. 10.1507/endocrj.EJ20-0619
16
Cao Y. Shi Y. Wang Y. Yang Y. Guo W. Zhang C. et al (2022a). Exosomal hsa_circ_0008925 from urine is related to chronic renal fibrosis. Dis. Markers2022, 1899282. 10.1155/2022/1899282
17
Cao Y. Shi Y. Yang Y. Wu Z. Peng N. Xiao J. et al (2022b). Urinary exosomes derived circRNAs as biomarkers for chronic renal fibrosis. Ann. Med.54, 1966–1976. 10.1080/07853890.2022.2098374
18
Chen F. Shi B. Liu W. Gong J. Gao J. Sun Y. et al (2023a). Circulating exosomal microRNAs as biomarkers of lupus nephritis. Front. Immunol.14, 1326836. 10.3389/fimmu.2023.1326836
19
Chen L. Su W. Chen H. Chen D.-Q. Wang M. Guo Y. et al (2018). Proteomics for biomarker identification and clinical application in kidney disease. Adv. Clin. Chem.85, 91–113. 10.1016/bs.acc.2018.02.005
20
Chen S. Zhang X. Meng K. Sun Y. Shu R. Han Y. et al (2023b). Urinary exosome tsRNAs as novel markers for diagnosis and prediction of lupus nephritis. Front. Immunol.14, 1077645. 10.3389/fimmu.2023.1077645
21
Chen Y. Han X. Sun Y. He X. Xue D. (2020). A circulating exosomal microRNA panel as a novel biomarker for monitoring post-transplant renal graft function. J. Cell Mol. Med.24, 12154–12163. 10.1111/jcmm.15861
22
Cheng C. Guo F. Yang H. Ma J. Li H. Yin L. et al (2022). Identification and analysis of the predictive urinary exosomal miR-195-5p in lupus nephritis based on renal miRNA-mRNA co-expression network. Lupus31, 1786–1799. 10.1177/09612033221133684
23
Chi D. Chen Y. Xiang C. Yao W. Wang H. Zheng X. et al (2022). Human amnion epithelial cells and their derived exosomes alleviate sepsis-associated acute kidney injury via mitigating endothelial dysfunction. Front. Med. (Lausanne)9, 829606. 10.3389/fmed.2022.829606
24
Cui C. Zang N. Song J. Guo X. He Q. Hu H. et al (2022). Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J.36, e22517. 10.1096/fj.202200488R
25
Dara M. Dianatpour M. Azarpira N. Tanideh N. Tanideh R. (2024). Integrating CRISPR technology with exosomes: revolutionizing gene delivery systems. Biochem. Biophys. Res. Commun.740, 151002. 10.1016/j.bbrc.2024.151002
26
Da-Silva C. C. S. Anauate A. C. Guirao T. P. Novaes A. da S. Maquigussa E. Boim M. A. (2022). Analysis of exosome-derived microRNAs as early biomarkers of lipopolysaccharide-induced acute kidney injury in rats. Front. Physiol.13, 944864. 10.3389/fphys.2022.944864
27
da Silva Novaes A. Borges F. T. Maquigussa E. Varela V. A. Dias M. V. S. Boim M. A. (2019). Influence of high glucose on mesangial cell-derived exosome composition, secretion and cell communication. Sci. Rep.9, 6270. 10.1038/s41598-019-42746-1
28
Dehghanbanadaki H. Forouzanfar K. Kakaei A. Zeidi S. Salehi N. Arjmand B. et al (2022). The role of CDH2 and MCP-1 mRNAs of blood extracellular vesicles in predicting early-stage diabetic nephropathy. PLoS One17, e0265619. 10.1371/journal.pone.0265619
29
Ding H. Li L. X. Harris P. C. Yang J. Li X. (2021). Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat. Commun.12, 4548. 10.1038/s41467-021-24799-x
30
Ding L. Li Z.-L. Zhou Y. Liu N.-C. Liu S.-S. Zhang X.-J. et al (2023). Loss of Sirt1 promotes exosome secretion from podocytes by inhibiting lysosomal acidification in diabetic nephropathy. Mol. Cell Endocrinol.568–569, 111913. 10.1016/j.mce.2023.111913
31
Doyle L. M. Wang M. Z. (2019a). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells8, 727. 10.3390/cells8070727
32
Doyle L. M. Wang M. Z. (2019b). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells8, 727. 10.3390/cells8070727
33
Du M. Giridhar K. V. Tian Y. Tschannen M. R. Zhu J. Huang C.-C. et al (2017). Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget8, 63703–63714. 10.18632/oncotarget.19476
34
Du S. Zhai L. Ye S. Wang L. Liu M. Tan M. (2023). In-depth urinary and exosome proteome profiling analysis identifies novel biomarkers for diabetic kidney disease. Sci. China Life Sci.66, 2587–2603. 10.1007/s11427-022-2348-0
35
Duan Y. Luo Q. Wang Y. Ma Y. Chen F. Zhu X. et al (2020). Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem.295, 12868–12884. 10.1074/jbc.RA120.012522
36
Duan Y.-R. Chen B.-P. Chen F. Yang S.-X. Zhu C.-Y. Ma Y.-L. et al (2021). Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J. Cell Mol. Med.25, 10798–10813. 10.1111/jcmm.14558
37
Ebrahim N. Ahmed I. A. Hussien N. I. Dessouky A. A. Farid A. S. Elshazly A. M. et al (2018). Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells7, 226. 10.3390/cells7120226
38
Eissa S. Matboli M. Aboushahba R. Bekhet M. M. Soliman Y. (2016). Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J. Diabetes Complicat.30, 1585–1592. 10.1016/j.jdiacomp.2016.07.012
39
Eknoyan G. Lameire N. Barsoum R. Eckardt K.-U. Levin A. Levin N. et al (2004). The burden of kidney disease: improving global outcomes. Kidney Int.66, 1310–1314. 10.1111/j.1523-1755.2004.00894.x
40
El Fekih R. Hurley J. Tadigotla V. Alghamdi A. Srivastava A. Coticchia C. et al (2021). Discovery and validation of a urinary exosome mRNA signature for the diagnosis of human kidney transplant rejection. J. Am. Soc. Nephrol.32, 994–1004. 10.1681/ASN.2020060850
41
Fan X. Zhang Y. Liu W. Shao M. Gong Y. Wang T. et al (2024). A comprehensive review of engineered exosomes from the preparation strategy to therapeutic applications. Biomater. Sci.12, 3500–3521. 10.1039/d4bm00558a
42
Feng Y. Lv L.-L. Wu W.-J. Li Z.-L. Chen J. Ni H.-F. et al (2018). Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. Am. J. Pathol.188, 2542–2552. 10.1016/j.ajpath.2018.07.017
43
Feng Y. Zhong X. Ni H.-F. Wang C. Tang T.-T. Wang L.-T. et al (2021). Urinary small extracellular vesicles derived CCL21 mRNA as biomarker linked with pathogenesis for diabetic nephropathy. J. Transl. Med.19, 355. 10.1186/s12967-021-03030-x
44
Ferreira G. S. Frota M. L. Gonzaga M. J. D. Vattimo M. de F. F. Lima C. (2024). The role of biomarkers in diagnosis of sepsis and acute kidney injury. Biomedicines12, 931. 10.3390/biomedicines12050931
45
Flora K. Ishihara M. Zhang Z. Bowen E. S. Wu A. Ayoub T. et al (2023). Exosomes from von hippel-lindau-null cancer cells promote metastasis in renal cell carcinoma. Int. J. Mol. Sci.24, 17307. 10.3390/ijms242417307
46
Fu W. Zhao H. Liu Y. Nie H. Gao B. Yin F. et al (2022). Exosomes derived from cancer-associated fibroblasts regulate cell progression in clear-cell renal-cell carcinoma. Nephron146, 383–392. 10.1159/000520304
47
Fujii N. Hirata H. Ueno K. Mori J. Oka S. Shimizu K. et al (2017). Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget8, 109877–109888. 10.18632/oncotarget.22436
48
Gao F. Zuo B. Wang Y. Li S. Yang J. Sun D. (2020). Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci.255, 117719. 10.1016/j.lfs.2020.117719
49
Garcia-Vives E. Solé C. Moliné T. Vidal M. Agraz I. Ordi-Ros J. et al (2020). The urinary exosomal miRNA expression profile is predictive of clinical response in lupus nephritis. Int. J. Mol. Sci.21, 1372. 10.3390/ijms21041372
50
Gheinani A. H. Vögeli M. Baumgartner U. Vassella E. Draeger A. Burkhard F. C. et al (2018). Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep.8, 3945. 10.1038/s41598-018-22142-x
51
Grange C. Bussolati B. (2022). Extracellular vesicles in kidney disease. Nat. Rev. Nephrol.18, 499–513. 10.1038/s41581-022-00586-9
52
Grange C. Dalmasso A. Cortez J. J. Spokeviciute B. Bussolati B. (2023). Exploring the role of urinary extracellular vesicles in kidney physiology, aging, and disease progression. Am. J. Physiol. Cell Physiol.325, C1439–C1450. 10.1152/ajpcell.00349.2023
53
Grange C. Tritta S. Tapparo M. Cedrino M. Tetta C. Camussi G. et al (2019). Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep.9, 4468. 10.1038/s41598-019-41100-9
54
Grützmann K. Salomo K. Krüger A. Lohse-Fischer A. Erdmann K. Seifert M. et al (2024). Identification of novel snoRNA-based biomarkers for clear cell renal cell carcinoma from urine-derived extracellular vesicles. Biol. Direct19, 38. 10.1186/s13062-024-00467-0
55
Guan H. Peng R. Mao L. Fang F. Xu B. Chen M. (2020). Injured tubular epithelial cells activate fibroblasts to promote kidney fibrosis through miR-150-containing exosomes. Exp. Cell Res.392, 112007. 10.1016/j.yexcr.2020.112007
56
Guo C. Cui Y. Jiao M. Yao J. Zhao J. Tian Y. et al (2023). Crosstalk between proximal tubular epithelial cells and other interstitial cells in tubulointerstitial fibrosis after renal injury. Front. Endocrinol. (Lausanne)14, 1256375. 10.3389/fendo.2023.1256375
57
Guo N. Zhou Q. Huang X. Yu J. Han Q. Nong B. et al (2020). Identification of differentially expressed circulating exosomal lncRNAs in IgA nephropathy patients. BMC Immunol.21, 16. 10.1186/s12865-020-00344-1
58
Guo Q. Li P. Chen M. Yu Y. Wan Y. Zhang Z. et al (2024). Exosomes from human umbilical cord stem cells suppress macrophage-to-myofibroblast transition, alleviating renal fibrosis. Inflammation47, 2094–2107. 10.1007/s10753-024-02027-0
59
Guo S. Debbi L. Zohar B. Samuel R. Arzi R. S. Fried A. I. et al (2021). Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett.21, 2497–2504. 10.1021/acs.nanolett.0c04834
60
Guo S. Hao H. Li S. Zhang L. Li R. (2022). Differential expression of urinary exosomal miRNA in idiopathic membranous nephropathy and evaluation of its diagnostic value. Tohoku J. Exp. Med.256, 327–336. 10.1620/tjem.2022.J002
61
Gurunathan S. Kang M.-H. Jeyaraj M. Kim J.-H. (2021a). Platinum nanoparticles enhance exosome release in human lung epithelial adenocarcinoma cancer cells (A549): oxidative stress and the ceramide pathway are key players. Int. J. Nanomedicine16, 515–538. 10.2147/IJN.S291138
62
Gurunathan S. Kang M.-H. Kim J.-H. (2021b). A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomedicine16, 1281–1312. 10.2147/IJN.S291956
63
Han L.-L. Wang S.-H. Yao M.-Y. Zhou H. (2024). Urinary exosomal microRNA-145-5p and microRNA-27a-3p act as noninvasive diagnostic biomarkers for diabetic kidney disease. World J. Diabetes15, 92–104. 10.4239/wjd.v15.i1.92
64
Han Q.-F. Li W.-J. Hu K.-S. Gao J. Zhai W.-L. Yang J.-H. et al (2022). Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol. Cancer21, 207. 10.1186/s12943-022-01671-0
65
Hao Y. Miao J. Liu W. Cai K. Huang X. Peng L. (2021). Mesenchymal stem cell-derived exosomes carry MicroRNA-125a to protect against diabetic nephropathy by targeting histone deacetylase 1 and downregulating endothelin-1. Diabetes Metab. Syndr. Obes.14, 1405–1418. 10.2147/DMSO.S286191
66
Haraszti R. A. Miller R. Dubuke M. L. Rockwell H. E. Coles A. H. Sapp E. et al (2019). Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. iScience16, 230–241. 10.1016/j.isci.2019.05.029
67
Harita Y. (2024). Urinary extracellular vesicles in childhood kidney diseases. Pediatr. Nephrol.39, 2293–2300. 10.1007/s00467-023-06243-y
68
Hashemi E. Dehghanbanadaki H. Baharanchi A. A. Forouzanfar K. Kakaei A. Mohammadi S. M. et al (2021). WT1 and ACE mRNAs of blood extracellular vesicle as biomarkers of diabetic nephropathy. J. Transl. Med.19, 299. 10.1186/s12967-021-02964-6
69
He C. Zheng S. Luo Y. Wang B. (2018). Exosome theranostics: biology and translational medicine. Theranostics8, 237–255. 10.7150/thno.21945
70
He L. Zhu D. Wang J. Wu X. (2019). A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med.43, 83–90. 10.3892/ijmm.2018.3944
71
He X. Tian F. Guo F. Zhang F. Zhang H. Ji J. et al (2022). Circulating exosomal mRNA signatures for the early diagnosis of clear cell renal cell carcinoma. BMC Med.20, 270. 10.1186/s12916-022-02467-1
72
He Y. Li X. Huang B. Yang Y. Luo N. Song W. et al (2023). Exosomal CIRCVMA21 derived from adipose-derived stem cells alleviates sepsis-induced acute kidney injury by targeting MIR-16-5P. Shock60, 419–426. 10.1097/SHK.0000000000002179
73
Hogan M. C. Bakeberg J. L. Gainullin V. G. Irazabal M. V. Harmon A. J. Lieske J. C. et al (2015). Identification of biomarkers for PKD1 using urinary exosomes. J. Am. Soc. Nephrol.26, 1661–1670. 10.1681/ASN.2014040354
74
Hu S. Hang X. Wei Y. Wang H. Zhang L. Zhao L. (2024). Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review. Cell Commun. Signal22, 136. 10.1186/s12964-024-01502-3
75
Hu X. Shen N. Liu A. Wang W. Zhang L. Sui Z. et al (2022). Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol. Ther.30, 763–781. 10.1016/j.ymthe.2021.10.012
76
Huang D. Kidd J. M. Zou Y. Wu X. Gehr T. W. B. Li P.-L. et al (2023). Regulation of NLRP3 inflammasome activation and inflammatory exosome release in podocytes by acid sphingomyelinase during obesity. Inflammation46, 2037–2054. 10.1007/s10753-023-01861-y
77
Huang H. Liu H. Tang J. Xu W. Gan H. Fan Q. et al (2020). M2 macrophage-derived exosomal miR-25-3p improves high glucose-induced podocytes injury through activation autophagy via inhibiting DUSP1 expression. IUBMB Life72, 2651–2662. 10.1002/iub.2393
78
Huang J. Cao H. Cui B. Ma X. Gao L. Yu C. et al (2022a). Mesenchymal stem cells-derived exosomes ameliorate ischemia/reperfusion induced acute kidney injury in a porcine model. Front. Cell Dev. Biol.10, 899869. 10.3389/fcell.2022.899869
79
Huang T.-Y. Chien M.-S. Su W.-T. (2022b). Therapeutic potential of pretreatment with exosomes derived from stem cells from the apical papilla against cisplatin-induced acute kidney injury. Int. J. Mol. Sci.23, 5721. 10.3390/ijms23105721
80
Huang Z. Zhang Y. Zhou J. Zhang Y. (2017). Urinary exosomal miR-193a can Be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed. Res. Int.2017, 7298160. 10.1155/2017/7298160
81
Jeon J. S. Kim E. Bae Y.-U. Yang W. M. Lee H. Kim H. et al (2020). microRNA in extracellular vesicles released by damaged podocytes promote apoptosis of renal tubular epithelial cells. Cells9, 1409. 10.3390/cells9061409
82
Ji C. Zhang J. Zhu Y. Shi H. Yin S. Sun F. et al (2020). Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis.11, 327. 10.1038/s41419-020-2510-4
83
Ji J.-L. Shi H.-M. Li Z.-L. Jin R. Qu G.-T. Zheng H. et al (2023). Satellite cell-derived exosome-mediated delivery of microRNA-23a/27a/26a cluster ameliorates the renal tubulointerstitial fibrosis in mouse diabetic nephropathy. Acta Pharmacol. Sin.44, 2455–2468. 10.1038/s41401-023-01140-4
84
Jiang H. Zhao H. Zhang M. He Y. Li X. Xu Y. et al (2022). Hypoxia induced changes of exosome cargo and subsequent biological effects. Front. Immunol.13, 824188. 10.3389/fimmu.2022.824188
85
Jiang Z. Liu Y. Niu X. Yin J. Hu B. Guo S. et al (2016). Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther.7, 24. 10.1186/s13287-016-0287-2
86
Jin J. Qian F. Zheng D. He W. Gong J. He Q. (2021). Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA let-7i-5p antagomir. Int. J. Nanomedicine16, 3565–3578. 10.2147/IJN.S299969
87
Jin J. Shi Y. Gong J. Zhao L. Li Y. He Q. et al (2019). Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther.10, 95. 10.1186/s13287-019-1177-1
88
Jin J. Wang Y. Zhao L. Zou W. Tan M. He Q. (2020). Exosomal miRNA-215-5p derived from adipose-derived stem cells attenuates epithelial-mesenchymal transition of podocytes by inhibiting ZEB2. Biomed. Res. Int.2020, 2685305. 10.1155/2020/2685305
89
Johnstone R. M. Adam M. Hammond J. R. Orr L. Turbide C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem.262, 9412–9420. 10.1016/s0021-9258(18)48095-7
90
Jung H.-Y. Lee C.-H. Choi J.-Y. Cho J.-H. Park S.-H. Kim Y.-L. et al (2020). Potential urinary extracellular vesicle protein biomarkers of chronic active antibody-mediated rejection in kidney transplant recipients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1138, 121958. 10.1016/j.jchromb.2019.121958
91
Kalani A. Mohan A. Godbole M. M. Bhatia E. Gupta A. Sharma R. K. et al (2013). Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One8, e60177. 10.1371/journal.pone.0060177
92
Kang X. Chen Y. Xin X. Liu M. Ma Y. Ren Y. et al (2021). Human amniotic epithelial cells and their derived exosomes protect against cisplatin-induced acute kidney injury without compromising its antitumor activity in mice. Front. Cell Dev. Biol.9, 752053. 10.3389/fcell.2021.752053
93
Keri K. C. Regner K. R. Dall A. T. Park F. (2018). Urinary exosomal expression of activator of G protein signaling 3 in polycystic kidney disease. BMC Res. Notes11, 359. 10.1186/s13104-018-3467-6
94
Khurana R. Ranches G. Schafferer S. Lukasser M. Rudnicki M. Mayer G. et al (2017). Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA23, 142–152. 10.1261/rna.058834.116
95
Kim M. J. Lim S. J. Ko Y. Kwon H. E. Jung J. H. Kwon H. et al (2022). Urinary exosomal cystatin C and lipopolysaccharide binding protein as biomarkers for antibody-mediated rejection after kidney transplantation. Biomedicines10, 2346. 10.3390/biomedicines10102346
96
Krylova S. V. Feng D. (2023). The machinery of exosomes: biogenesis, release, and uptake. Int. J. Mol. Sci.24, 1337. 10.3390/ijms24021337
97
Kurahashi R. Kadomatsu T. Baba M. Hara C. Itoh H. Miyata K. et al (2019). MicroRNA-204-5p: a novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci.110, 1897–1908. 10.1111/cas.14026
98
Lai J. J. Chau Z. L. Chen S.-Y. Hill J. J. Korpany K. V. Liang N.-W. et al (2022). Exosome processing and characterization approaches for research and technology development. Adv. Sci. (Weinh)9, e2103222. 10.1002/advs.202103222
99
Lange T. Artelt N. Kindt F. Stracke S. Rettig R. Lendeckel U. et al (2019). MiR-21 is up-regulated in urinary exosomes of chronic kidney disease patients and after glomerular injury. J. Cell Mol. Med.23, 4839–4843. 10.1111/jcmm.14317
100
Li D. Lin F. Li G. Zeng F. (2021a). Exosomes derived from mesenchymal stem cells curbs the progression of clear cell renal cell carcinoma through T-cell immune response. Cytotechnology73, 593–604. 10.1007/s10616-021-00480-5
101
Li P. Kaslan M. Lee S. H. Yao J. Gao Z. (2017). Progress in exosome isolation techniques. Theranostics7, 789–804. 10.7150/thno.18133
102
Li Q. Xu M. Zhang Z. Yin M. Zhang Y. Liu F. (2022a). Urinary exosomal hsa_circ_0001250 as a novel diagnostic biomarker of idiopathic membranous nephropathy. J. Transl. Med.20, 607. 10.1186/s12967-022-03784-y
103
Li S. Hao H. Li R. Guo S. (2022b). Urinary exosomal MicroRNAs as new noninvasive biomarkers of IgA nephropathy. Tohoku J. Exp. Med.256, 215–223. 10.1620/tjem.256.215
104
Li S. Yi M. Dong B. Tan X. Luo S. Wu K. (2021b). The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int. J. Cancer148, 2640–2651. 10.1002/ijc.33386
105
Li T. Ci Liu T. Liu N. Zhang M. (2023a). Changes in urinary exosomal protein CALM1 may serve as an early noninvasive biomarker for diagnosing diabetic kidney disease. Clin. Chim. Acta547, 117466. 10.1016/j.cca.2023.117466
106
Li T. Liu T. C. Liu N. Li M. J. Zhang M. (2023b). Urinary exosome proteins PAK6 and EGFR as noninvasive diagnostic biomarkers of diabetic nephropathy. BMC Nephrol.24, 291. 10.1186/s12882-023-03343-7
107
Li W. Yang S. Qiao R. Zhang J. (2018). Potential value of urinary exosome-derived let-7c-5p in the diagnosis and progression of type II diabetic nephropathy. Clin. Lab.64, 709–718. 10.7754/Clin.Lab.2018.171031
108
Li X. Liao J. Su X. Li W. Bi Z. Wang J. et al (2020). Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics10, 9561–9578. 10.7150/thno.42153
109
Liang M. Zhang D. Zheng D. He W. Jin J. (2022a). Exosomes from miR-374a-5p-modified mesenchymal stem cells inhibit the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis. Bioengineered13, 4517–4527. 10.1080/21655979.2022.2033465
110
Liang M. Zhu X. Zhang D. He W. Zhang J. Yuan S. et al (2022b). Yi-Shen-Hua-Shi granules inhibit diabetic nephropathy by ameliorating podocyte injury induced by macrophage-derived exosomes. Front. Pharmacol.13, 962606. 10.3389/fphar.2022.962606
111
Liang T. Wu Z. Li J. Wu S. Shi W. Wang L. (2023). The emerging double-edged sword role of exosomes in Alzheimer’s disease. Front. Aging Neurosci.15, 1209115. 10.3389/fnagi.2023.1209115
112
Lim J.-H. Lee C.-H. Kim K. Y. Jung H.-Y. Choi J.-Y. Cho J.-H. et al (2018). Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: a cross-sectional study. PLoS One13, e0204204. 10.1371/journal.pone.0204204
113
Ling L. Tan Z. Zhang C. Gui S. Cui Y. Hu Y. et al (2019). CircRNAs in exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells. Am. J. Transl. Res.11, 4667–4682.
114
Liu L. Zhou Y. Zhao X. Yang X. Wan X. An Z. et al (2023a). Bone marrow mesenchymal stem cell-derived exosomes alleviate diabetic kidney disease in rats by inhibiting apoptosis and inflammation. Front. Biosci. Landmark Ed.28, 203. 10.31083/j.fbl2809203
115
Liu M. Wen Z. Zhang T. Zhang L. Liu X. Wang M. (2024). The role of exosomal molecular cargo in exosome biogenesis and disease diagnosis. Front. Immunol.15, 1417758. 10.3389/fimmu.2024.1417758
116
Liu W. Hu C. Zhang B. Li M. Deng F. Zhao S. (2023b). Exosomal microRNA-342-5p secreted from adipose-derived mesenchymal stem cells mitigates acute kidney injury in sepsis mice by inhibiting TLR9. Biol. Proced. Online25, 10. 10.1186/s12575-023-00198-y
117
Liu W.-Z. Ma Z.-J. Kang X.-W. (2022a). Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem.414, 7123–7141. 10.1007/s00216-022-04253-7
118
Liu X. Liu Z. Wang C. Miao J. Zhou S. Ren Q. et al (2023c). Kidney tubular epithelial cells control interstitial fibroblast fate by releasing TNFAIP8-encapsulated exosomes. Cell Death Dis.14, 672. 10.1038/s41419-023-06209-w
119
Liu X. Miao J. Wang C. Zhou S. Chen S. Ren Q. et al (2020). Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int.97, 1181–1195. 10.1016/j.kint.2019.11.026
120
Liu Y. Guo W. Guo Y. Chen X. Liu W. (2022b). Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis via regulating Smurf 2/Smad 7. Front. Biosci. Landmark Ed.27, 17. 10.31083/j.fbl2701017
121
Liu Y. Guo Y. Bao S. Huang H. Liu W. Guo W. (2022c). Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis.13, 278. 10.1038/s41419-022-04703-1
122
Liu Y. Li X. Zhao M. Wu Y. Xu Y. Li X. et al (2023d). Macrophage-derived exosomes promote activation of NLRP3 inflammasome and autophagy deficiency of mesangial cells in diabetic nephropathy. Life Sci.330, 121991. 10.1016/j.lfs.2023.121991
123
Logozzi M. Mizzoni D. Angelini D. F. Di Raimo R. Falchi M. Battistini L. et al (2018). Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers (Basel)10, 370. 10.3390/cancers10100370
124
Lu Y. Zhang R. Gu X. Wang X. Xi P. Chen X. (2023). Exosomes from tubular epithelial cells undergoing epithelial-to-mesenchymal transition promote renal fibrosis by M1 macrophage activation. FASEB Bioadv5, 101–113. 10.1096/fba.2022-00080
125
Lv J. Hao Y.-N. Wang X.-P. Lu W.-H. Xie L.-Y. Niu D. (2023). Bone marrow mesenchymal stem cell-derived exosomal miR-30e-5p ameliorates high-glucose induced renal proximal tubular cell pyroptosis by inhibiting ELAVL1. Ren. Fail45, 2177082. 10.1080/0886022X.2023.2177082
126
Lv L.-L. Cao Y.-H. Ni H.-F. Xu M. Liu D. Liu H. et al (2013). MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Ren. Physiol.305, F1220–F1227. 10.1152/ajprenal.00148.2013
127
Lv L.-L. Feng Y. Wu M. Wang B. Li Z.-L. Zhong X. et al (2020). Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ.27, 210–226. 10.1038/s41418-019-0349-y
128
Ma H. Xu Y. Zhang R. Guo B. Zhang S. Zhang X. (2019). Differential expression study of circular RNAs in exosomes from serum and urine in patients with idiopathic membranous nephropathy. Arch. Med. Sci.15, 738–753. 10.5114/aoms.2019.84690
129
Magayr T. A. Song X. Streets A. J. Vergoz L. Chang L. Valluru M. K. et al (2020). Global microRNA profiling in human urinary exosomes reveals novel disease biomarkers and cellular pathways for autosomal dominant polycystic kidney disease. Kidney Int.98, 420–435. 10.1016/j.kint.2020.02.008
130
Min Q.-H. Chen X.-M. Zou Y.-Q. Zhang J. Li J. Wang Y. et al (2018). Differential expression of urinary exosomal microRNAs in IgA nephropathy. J. Clin. Lab. Anal.32, e22226. 10.1002/jcla.22226
131
Mohan A. Singh R. S. Kumari M. Garg D. Upadhyay A. Ecelbarger C. M. et al (2016). Urinary exosomal microRNA-451-5p is a potential early biomarker of diabetic nephropathy in rats. PLoS One11, e0154055. 10.1371/journal.pone.0154055
132
Mori M. A. Ludwig R. G. Garcia-Martin R. Brandão B. B. Kahn C. R. (2019). Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab.30, 656–673. 10.1016/j.cmet.2019.07.011
133
Mulcahy L. A. Pink R. C. Carter D. R. F. (2014). Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles3. 10.3402/jev.v3.24641
134
Panich T. Chancharoenthana W. Somparn P. Issara-Amphorn J. Hirankarn N. Leelahavanichkul A. (2017). Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol.18, 10. 10.1186/s12882-016-0415-3
135
Perez-Hernandez J. Forner M. J. Pinto C. Chaves F. J. Cortes R. Redon J. (2015). Increased urinary exosomal MicroRNAs in patients with systemic lupus erythematosus. PLoS One10, e0138618. 10.1371/journal.pone.0138618
136
Perez-Hernandez J. Martinez-Arroyo O. Ortega A. Galera M. Solis-Salguero M. A. Chaves F. J. et al (2021). Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol.34, 1157–1167. 10.1007/s40620-020-00832-y
137
Pisitkun T. Shen R.-F. Knepper M. A. (2004). Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U. S. A.101, 13368–13373. 10.1073/pnas.0403453101
138
Qiu Z. Zhong Z. Zhang Y. Tan H. Deng B. Meng G. (2022). Human umbilical cord mesenchymal stem cell-derived exosomal miR-335-5p attenuates the inflammation and tubular epithelial-myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Res. Ther.13, 373. 10.1186/s13287-022-03071-z
139
Raby K. L. Horsely H. McCarthy-Boxer A. Norman J. T. Wilson P. D. (2021). Urinary exosome proteomic profiling defines stage-specific rapid progression of autosomal dominant polycystic kidney disease and tolvaptan efficacy. BBA Adv.1, 100013. 10.1016/j.bbadva.2021.100013
140
Raimondo F. Morosi L. Corbetta S. Chinello C. Brambilla P. Della Mina P. et al (2013). Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Biosyst.9, 1220–1233. 10.1039/c3mb25582d
141
Ramalhete L. Araújo R. Ferreira A. Calado C. R. C. (2024). Exosomes and microvesicles in kidney transplantation: the long road from trash to gold. Pathology56, 1–10. 10.1016/j.pathol.2023.10.004
142
Ramezani A. Devaney J. M. Cohen S. Wing M. R. Scott R. Knoblach S. et al (2015). Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur. J. Clin. Invest.45, 394–404. 10.1111/eci.12420
143
Ren P. Qian F. Fu L. He W. He Q. Jin J. et al (2023). Adipose-derived stem cell exosomes regulate Nrf2/Keap1 in diabetic nephropathy by targeting FAM129B. Diabetol. Metab. Syndr.15, 149. 10.1186/s13098-023-01119-5
144
Ren Y. Chen Y. Zheng X. Wang H. Kang X. Tang J. et al (2020). Human amniotic epithelial cells ameliorate kidney damage in ischemia-reperfusion mouse model of acute kidney injury. Stem Cell Res. Ther.11, 410. 10.1186/s13287-020-01917-y
145
Rui R. Zhou L. He S. (2023). Advances in the research of exosomes in renal cell carcinoma: from mechanisms to applications. Front. Immunol.14, 1271669. 10.3389/fimmu.2023.1271669
146
Saejong S. Townamchai N. Somparn P. Tangtanatakul P. Ondee T. Hirankarn N. et al (2022). MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation. Asian Pac J. Allergy Immunol.40, 94–102. 10.12932/AP-101019-0656
147
Sagoo M. K. Gnudi L. (2020). Diabetic nephropathy: an overview. Methods Mol. Biol.2067, 3–7. 10.1007/978-1-4939-9841-8_1
148
Salih M. Demmers J. A. Bezstarosti K. Leonhard W. N. Losekoot M. van Kooten C. et al (2016). Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J. Am. Soc. Nephrol.27, 3079–3092. 10.1681/ASN.2015090994
149
Saravanan P. B. Kalivarathan J. Khan F. Shah R. Levy M. F. Kanak M. A. (2023). Exosomes in transplantation: role in allograft rejection, diagnostic biomarker, and therapeutic potential. Life Sci.324, 121722. 10.1016/j.lfs.2023.121722
150
Seo J.-W. Lee Y. H. Tae D. H. Kim Y. G. Moon J.-Y. Jung S. W. et al (2023). Development and validation of urinary exosomal microRNA biomarkers for the diagnosis of acute rejection in kidney transplant recipients. Front. Immunol.14, 1190576. 10.3389/fimmu.2023.1190576
151
Sharma A. Yadav A. Nandy A. Ghatak S. (2024). Insight into the functional dynamics and challenges of exosomes in pharmaceutical innovation and precision medicine. Pharmaceutics16, 709. 10.3390/pharmaceutics16060709
152
Sigdel T. K. Ng Y. W. Lee S. Nicora C. D. Qian W.-J. Smith R. D. et al (2014). Perturbations in the urinary exosome in transplant rejection. Front. Med. (Lausanne)1, 57. 10.3389/fmed.2014.00057
153
Solé C. Cortés-Hernández J. Felip M. L. Vidal M. Ordi-Ros J. (2015). miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transpl.30, 1488–1496. 10.1093/ndt/gfv128
154
Solé C. Moliné T. Vidal M. Ordi-Ros J. Cortés-Hernández J. (2019). An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells8, 773. 10.3390/cells8080773
155
Somparn P. Srichaimongkol A. Jungjing S. Wanthong B. Nanthawong S. Asada L. et al (2024). Potential involvement of circulating exosomal miRNA-146a in disease activity and TRAF6 gene expression in juvenile proliferative lupus nephritis. Lupus Sci. Med.11, e001078. 10.1136/lupus-2023-001078
156
Song D. H. Lee J. S. Lee J.-H. Kim D. C. Yang J. W. Kim M. H. et al (2024). Exosome-mediated secretion of miR-127-3p regulated by RAB27A accelerates metastasis in renal cell carcinoma. Cancer Cell Int.24, 153. 10.1186/s12935-024-03334-0
157
Song S. Long M. Yu G. Cheng Y. Yang Q. Liu J. et al (2019). Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J. Cell Mol. Med.23, 6755–6765. 10.1111/jcmm.14553
158
Strauß C. Booke H. Forni L. Zarbock A. (2024). Biomarkers of acute kidney injury: from discovery to the future of clinical practice. J. Clin. Anesth.95, 111458. 10.1016/j.jclinane.2024.111458
159
Su H. Qiao J. Hu J. Li Y. Lin J. Yu Q. et al (2020). Podocyte-derived extracellular vesicles mediate renal proximal tubule cells dedifferentiation via microRNA-221 in diabetic nephropathy. Mol. Cell Endocrinol.518, 111034. 10.1016/j.mce.2020.111034
160
Sun Y. Zhao Y. Lu Y. Li H. Xiang J. Yang D. et al (2024). Urinary stem cell-derived exocrine circRNA ATG7 regulates the SOCS1/STAT3 signaling pathway through miR-4500, inhibits M1 macrophage polarization, and alleviates the progression of diabetes nephropathy. Int. Urol. Nephrol.56, 1449–1463. 10.1007/s11255-023-03819-3
161
Sun Z. Wu J. Bi Q. Wang W. (2022). Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res. Ther.13, 297. 10.1186/s13287-022-02986-x
162
Takada Y. Kamimura D. Jiang J.-J. Higuchi H. Iwami D. Hotta K. et al (2020). Increased urinary exosomal SYT17 levels in chronic active antibody-mediated rejection after kidney transplantation via the IL-6 amplifier. Int. Immunol.32, 653–662. 10.1093/intimm/dxaa032
163
Tan R. J. Zhou D. Liu Y. (2016). Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis. (Basel)2, 136–144. 10.1159/000446336
164
Tangtanatakul P. Klinchanhom S. Sodsai P. Sutichet T. Promjeen C. Avihingsanon Y. et al (2019). Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J. Allergy Immunol.37, 189–197. 10.12932/AP-130318-0280
165
Tian J. Han Z. Song D. Peng Y. Xiong M. Chen Z. et al (2023). Engineered exosome for drug delivery: recent development and clinical applications. Int. J. Nanomedicine18, 7923–7940. 10.2147/IJN.S444582
166
Tsai Y.-C. Hung W.-W. Chang W.-A. Wu P.-H. Wu L.-Y. Lee S.-C. et al (2021). Autocrine exosomal fibulin-1 as a target of MiR-1269b induces epithelial-mesenchymal transition in proximal tubule in diabetic nephropathy. Front. Cell Dev. Biol.9, 789716. 10.3389/fcell.2021.789716
167
Tsai Y.-C. Kuo M.-C. Hung W.-W. Wu P.-H. Chang W.-A. Wu L.-Y. et al (2023). Proximal tubule-derived exosomes contribute to mesangial cell injury in diabetic nephropathy via miR-92a-1-5p transfer. Cell Commun. Signal21, 10. 10.1186/s12964-022-00997-y
168
Viñas J. L. Burger D. Zimpelmann J. Haneef R. Knoll W. Campbell P. et al (2016). Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int.90, 1238–1250. 10.1016/j.kint.2016.07.015
169
Wan F. Yang R.-C. Tang Y.-W. Tang X.-L. Ye T. Zheng J. et al (2022). BMSC-derived exosomes protect against kidney injury through regulating klotho in 5/6 nephrectomy rats. Eur. J. Med. Res.27, 118. 10.1186/s40001-022-00742-8
170
Wan P. Tan X. Sheng M. Xiang Y. Wang P. Yu M. (2024). Platelet exosome-derived miR-223-3p regulates pyroptosis in the cell model of sepsis-induced acute renal injury by targeting mediates NLRP3. Crit. Rev. Immunol.44, 53–65. 10.1615/CritRevImmunol.2023051651
171
Wan Y. Yu Y. Yu C. Luo J. Wen S. Shen L. et al (2023). Human umbilical cord mesenchymal stem cell exosomes alleviate acute kidney injury by inhibiting pyroptosis in rats and NRK-52E cells. Ren. Fail45, 2221138. 10.1080/0886022X.2023.2221138
172
Wang B. Fu Y.-Q. Xie L.-J. Cao J.-Y. Yang M. Li M. et al (2024a). Measurement of urinary exosomal phospholipase A2 receptor is a sensitive method for diagnosis of PLA2R-associated membranous nephropathy. Clin. Kidney J.17, sfad191. 10.1093/ckj/sfad191
173
Wang B. Jia H. Zhang B. Wang J. Ji C. Zhu X. et al (2017). Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther.8, 75. 10.1186/s13287-016-0463-4
174
Wang B. Yao K. Huuskes B. M. Shen H.-H. Zhuang J. Godson C. et al (2016). Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther.24, 1290–1301. 10.1038/mt.2016.90
175
Wang J. Bonacquisti E. E. Brown A. D. Nguyen J. (2020). Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells9, 660. 10.3390/cells9030660
176
Wang J. Tao Y. Zhao F. Liu T. Shen X. Zhou L. (2023a). Expression of urinary exosomal miRNA-615-3p and miRNA-3147 in diabetic kidney disease and their association with inflammation and fibrosis. Ren. Fail45, 2121929. 10.1080/0886022X.2022.2121929
177
Wang L. Liang A. Huang J. (2024b). Exendin-4-enriched exosomes from hUCMSCs alleviate diabetic nephropathy via gut microbiota and immune modulation. Front. Microbiol.15, 1399632. 10.3389/fmicb.2024.1399632
178
Wang L. Wang J. Wang Z. Zhou J. Zhang Y. (2021). Higher urine exosomal miR-193a is associated with a higher probability of primary focal segmental glomerulosclerosis and an increased risk of poor prognosis among children with nephrotic syndrome. Front. Cell Dev. Biol.9, 727370. 10.3389/fcell.2021.727370
179
Wang S.-Y. Xu Y. Hong Q. Chen X.-M. Cai G.-Y. (2023b). Mesenchymal stem cells ameliorate cisplatin-induced acute kidney injury via let-7b-5p. Cell Tissue Res.392, 517–533. 10.1007/s00441-022-03729-3
180
Wang X. Wang T. Chen C. Wu Z. Bai P. Li S. et al (2019). Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell Biochem.120, 1492–1502. 10.1002/jcb.27347
181
Wang Y. Liu J. Wang H. Lv S. Liu Q. Li S. et al (2023c). Mesenchymal stem cell-derived exosomes ameliorate diabetic kidney disease through the NLRP3 signaling pathway. Stem Cells41, 368–383. 10.1093/stmcls/sxad010
182
Wang Y.-Y. Tang L.-Q. Wei W. (2018). Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFβ1-PI3K/AKT pathway. Eur. J. Pharmacol.824, 185–192. 10.1016/j.ejphar.2018.01.034
183
Wei W. Guo X. Gu L. Jia J. Yang M. Yuan W. et al (2021). Bone marrow mesenchymal stem cell exosomes suppress phosphate-induced aortic calcification via SIRT6-HMGB1 deacetylation. Stem Cell Res. Ther.12, 235. 10.1186/s13287-021-02307-8
184
Wu J. Wei X. Li J. Gan Y. Zhang R. Han Q. et al (2022a). Plasma exosomal IRAK1 can be a potential biomarker for predicting the treatment response to renin-angiotensin system inhibitors in patients with IgA nephropathy. Front. Immunol.13, 978315. 10.3389/fimmu.2022.978315
185
Wu P. Zhang B. Ocansey D. K. W. Xu W. Qian H. (2021). Extracellular vesicles: a bright star of nanomedicine. Biomaterials269, 120467. 10.1016/j.biomaterials.2020.120467
186
Wu X. Gao Y. Xu L. Dang W. Yan H. Zou D. et al (2017). Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep.7, 9371. 10.1038/s41598-017-09907-6
187
Wu X. Wang Z. Wang J. Tian X. Cao G. Gu Y. et al (2022b). Exosomes secreted by mesenchymal stem cells induce immune tolerance to mouse kidney transplantation via transporting LncRNA DANCR. Inflammation45, 460–475. 10.1007/s10753-021-01561-5
188
Wu X. Wu X. Wang Z. Tian X. Zhang C. Cao G. et al (2024). Delivery of exogenous miR-19b by Wharton’s Jelly Mesenchymal Stem Cells attenuates transplanted kidney ischemia/reperfusion injury by regulating cellular metabolism. Drug Deliv. Transl. Res.15, 925–938. 10.1007/s13346-024-01645-3
189
Wu X.-M. Gao Y.-B. Cui F.-Q. Zhang N. (2016). Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol. Open5, 484–491. 10.1242/bio.015990
190
Wu Z. He D. Li H. (2020). Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact. Mater6, 823–835. 10.1016/j.bioactmat.2020.09.011
191
Xiang H. Xu Z. Zhang C. Xiong J. (2023). Macrophage-derived exosomes mediate glomerular endothelial cell dysfunction in sepsis-associated acute kidney injury. Cell Biosci.13, 46. 10.1186/s13578-023-00990-z
192
Xiao C.-T. Lai W.-J. Zhu W.-A. Wang H. (2020). MicroRNA derived from circulating exosomes as noninvasive biomarkers for diagnosing renal cell carcinoma. Onco Targets Ther.13, 10765–10774. 10.2147/OTT.S271606
193
Xiao H. Shi J. (2020). Exosomal circular RNA_400068 promotes the development of renal cell carcinoma via the miR-210-5p/SOCS1 axis. Mol. Med. Rep.22, 4810–4820. 10.3892/mmr.2020.11541
194
Xie X. Yang X. Wu J. Tang S. Yang L. Fei X. et al (2022). Exosome from indoleamine 2,3-dioxygenase-overexpressing bone marrow mesenchymal stem cells accelerates repair process of ischemia/reperfusion-induced acute kidney injury by regulating macrophages polarization. Stem Cell Res. Ther.13, 367. 10.1186/s13287-022-03075-9
195
Xu S. Cheuk Y. C. Jia Y. Chen T. Chen J. Luo Y. et al (2022). Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death Dis.13, 876. 10.1038/s41419-022-05305-7
196
Xu X. Qu S. Zhang C. Zhang M. Qin W. Ren G. et al (2023). CD8 T cell-derived exosomal miR-186-5p elicits renal inflammation via activating tubular TLR7/8 signal Axis. Adv. Sci. (Weinh)10, e2301492. 10.1002/advs.202301492
197
Xue Y. Chen T. Hou N. Wu X. Kong W. Huang J. et al (2023). Serum extracellular vesicles derived hsa-miR-320d as an indicator for progression of clear cell renal cell carcinoma. Discov. Oncol.14, 114. 10.1007/s12672-023-00730-2
198
Yang B. Wang J. Qiao J. Zhang Q. Liu Q. Tan Y. et al (2024). Circ DENND4C inhibits pyroptosis and alleviates ischemia-reperfusion acute kidney injury by exosomes secreted from human urine-derived stem cells. Chem. Biol. Interact.391, 110922. 10.1016/j.cbi.2024.110922
199
Yang D. Zhang W. Zhang H. Zhang F. Chen L. Ma L. et al (2020). Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics10, 3684–3707. 10.7150/thno.41580
200
Yea J.-H. Yoon Y. M. Lee J. H. Yun C. W. Lee S. H. (2021). Exosomes isolated from melatonin-stimulated mesenchymal stem cells improve kidney function by regulating inflammation and fibrosis in a chronic kidney disease mouse model. J. Tissue Eng.12, 20417314211059624. 10.1177/20417314211059624
201
Yeh T.-H. Tu K.-C. Wang H.-Y. Chen J.-Y. (2024). From acute to chronic: unraveling the pathophysiological mechanisms of the progression from acute kidney injury to acute kidney disease to chronic kidney disease. Int. J. Mol. Sci.25, 1755. 10.3390/ijms25031755
202
Yoshino H. Tatarano S. Tamai M. Tsuruda M. Iizasa S. Arima J. et al (2022). Exosomal microRNA-1 and MYO15A as a target for therapy and diagnosis in renal cell carcinoma. Biochem. Biophys. Res. Commun.630, 71–76. 10.1016/j.bbrc.2022.09.056
203
Yu M. Shen X. He W. Zheng D. He Q. Jin J. (2022a). Mesangial cell-derived exosomal miR-4455 induces podocyte injury in IgA nephropathy by targeting ULK2. Oxid. Med. Cell Longev.2022, 1740770. 10.1155/2022/1740770
204
Yu W. Song J. Chen S. Nie J. Zhou C. Huang J. et al (2024). Myofibroblast-derived exosomes enhance macrophages to myofibroblasts transition and kidney fibrosis. Ren. Fail46, 2334406. 10.1080/0886022X.2024.2334406
205
Yu Y. Bai F. Qin N. Liu W. Sun Q. Zhou Y. et al (2018). Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron139, 269–282. 10.1159/000487104
206
Yu Y. Chen M. Guo Q. Shen L. Liu X. Pan J. et al (2023). Human umbilical cord mesenchymal stem cell exosome-derived miR-874-3p targeting RIPK1/PGAM5 attenuates kidney tubular epithelial cell damage. Cell Mol. Biol. Lett.28, 12. 10.1186/s11658-023-00425-0
207
Yu Y. Ren Z. Xie A. Jia Y. Xue Y. Wang P. et al (2022b). Assessment of urinary exosomal NHE3 as a biomarker of acute kidney injury. Diagn. (Basel)12, 2634. 10.3390/diagnostics12112634
208
Zang J. Maxwell A. P. Simpson D. A. McKay G. J. (2019). Differential expression of urinary exosomal MicroRNAs miR-21-5p and miR-30b-5p in individuals with diabetic kidney disease. Sci. Rep.9, 10900. 10.1038/s41598-019-47504-x
209
Zhang H. Huang E. Kahwaji J. Nast C. C. Li P. Mirocha J. et al (2017). Plasma exosomes from HLA-sensitized kidney transplant recipients contain mRNA transcripts which predict development of antibody-mediated rejection. Transplantation101, 2419–2428. 10.1097/TP.0000000000001834
210
Zhang J. Zhu Y. Cai R. Jin J. He Q. (2020a). Differential expression of urinary exosomal small RNAs in idiopathic membranous nephropathy. Biomed. Res. Int.2020, 3170927. 10.1155/2020/3170927
211
Zhang K. Zheng S. Wu J. He J. Ouyang Y. Ao C. et al (2024a). Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate renal fibrosis in diabetic nephropathy by targeting Hedgehog/SMO signaling. FASEB J.38, e23599. 10.1096/fj.202302324R
212
Zhang Q. Zhao Y. Luo Y. Guo S. Hou H. Han X. et al (2024b). Urinary exosomal miRNA-451a can be used as a potential noninvasive biomarker for diagnosis, reflecting tubulointerstitial damage and therapeutic response in IgA nephropathy. Ren. Fail46, 2319326. 10.1080/0886022X.2024.2319326
213
Zhang W. Ni M. Su Y. Wang H. Zhu S. Zhao A. et al (2018). MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus4, 412–419. 10.1016/j.euf.2016.09.007
214
Zhang W. Zhang J. Huang H. (2022a). Exosomes from adipose-derived stem cells inhibit inflammation and oxidative stress in LPS-acute kidney injury. Exp. Cell Res.420, 113332. 10.1016/j.yexcr.2022.113332
215
Zhang W. Zheng X. Yu Y. Zheng L. Lan J. Wu Y. et al (2022b). Renal cell carcinoma-derived exosomes deliver lncARSR to induce macrophage polarization and promote tumor progression via STAT3 pathway. Int. J. Biol. Sci.18, 3209–3222. 10.7150/ijbs.70289
216
Zhang Y. Huang H. Liu W. Liu S. Wang X. Y. Diao Z. L. et al (2021a). Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis.12, 335. 10.1038/s41419-021-03578-y
217
Zhang Y. Le X. Zheng S. Zhang K. He J. Liu M. et al (2022c). MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res. Ther.13, 171. 10.1186/s13287-022-02855-7
218
Zhang Y. Wang J. Yang B. Qiao R. Li A. Guo H. et al (2020b). Transfer of MicroRNA-216a-5p from exosomes secreted by human urine-derived stem cells reduces renal ischemia/reperfusion injury. Front. Cell Dev. Biol.8, 610587. 10.3389/fcell.2020.610587
219
Zhang Z. Chen H. Zhou L. Li C. Lu G. Wang L. (2022d). Macrophage-derived exosomal miRNA-155 promotes tubular injury in ischemia-induced acute kidney injury. Int. J. Mol. Med.50, 116. 10.3892/ijmm.2022.5172
220
Zhang Z. Xu R. Yang Y. Liang C. Yu X. Liu Y. et al (2021b). Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnology19, 78. 10.1186/s12951-021-00826-3
221
Zhao S. Sun Y. Mao Q. Zhou C. Chen Y. Xue D. (2022a). Exosomal miR-4639 and miR-210 in plasma and urine as biomarkers in IgA nephropathy. Nephron146, 539–552. 10.1159/000523924
222
Zhao T. Jin Q. Kong L. Zhang D. Teng Y. Lin L. et al (2022b). microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis. J. Bioenerg. Biomembr.54, 17–30. 10.1007/s10863-021-09919-y
223
Zhao Y. Shen A. Guo F. Song Y. Jing N. Ding X. et al (2020). Urinary exosomal MiRNA-4534 as a novel diagnostic biomarker for diabetic kidney disease. Front. Endocrinol. (Lausanne)11, 590. 10.3389/fendo.2020.00590
224
Zhou H. Cheruvanky A. Hu X. Matsumoto T. Hiramatsu N. Cho M. E. et al (2008). Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int.74, 613–621. 10.1038/ki.2008.206
225
Zhou H. Kajiyama H. Tsuji T. Hu X. Leelahavanichkul A. Vento S. et al (2013a). Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Ren. Physiol.305, F553–F559. 10.1152/ajprenal.00056.2013
226
Zhou H. Pisitkun T. Aponte A. Yuen P. S. T. Hoffert J. D. Yasuda H. et al (2006). Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int.70, 1847–1857. 10.1038/sj.ki.5001874
227
Zhou X. Zhao S. Li W. Ruan Y. Yuan R. Ning J. et al (2021). Tubular cell-derived exosomal miR-150-5p contributes to renal fibrosis following unilateral ischemia-reperfusion injury by activating fibroblast in vitro and in vivo. Int. J. Biol. Sci.17, 4021–4033. 10.7150/ijbs.62478
228
Zhou Y. Li P. Goodwin A. J. Cook J. A. Halushka P. V. Chang E. et al (2018). Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther.26, 1375–1384. 10.1016/j.ymthe.2018.02.020
229
Zhou Y. Xu H. Xu W. Wang B. Wu H. Tao Y. et al (2013b). Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther.4, 34. 10.1186/scrt194
230
Zhu B. He J. Ye X. Pei X. Bai Y. Gao F. et al (2023). Role of cisplatin in inducing acute kidney injury and pyroptosis in mice via the exosome miR-122/ELAVL1 regulatory Axis. Physiol. Res.72, 753–765. 10.33549/physiolres.935129
231
Zhu G. Pei L. Lin F. Yin H. Li X. He W. et al (2019a). Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell Physiol.234, 23736–23749. 10.1002/jcp.28941
232
Zhu Q.-J. Zhu M. Xu X.-X. Meng X.-M. Wu Y.-G. (2019b). Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-β1/Smad3 pathway in vivo and in vitro. FASEB J.33, 9279–9290. 10.1096/fj.201802427RRR
233
Zhu Y. Zha F. Tang B. Ji T.-T. Li X.-Y. Feng L. et al (2022). Exosomal hsa_circ_0125310 promotes cell proliferation and fibrosis in diabetic nephropathy via sponging miR-422a and targeting the IGF1R/p38 axis. J. Cell Mol. Med.26, 151–162. 10.1111/jcmm.17065
Summary
Keywords
exosomes, kidney disease, diagnosis, biomarkers, treatment
Citation
Cao H, Li Z, Ye J, Lv Y, Zhang C, Liang T and Wang Y (2025) Emerging roles of exosomes in the diagnosis and treatment of kidney diseases. Front. Pharmacol. 16:1525314. doi: 10.3389/fphar.2025.1525314
Received
09 November 2024
Accepted
20 March 2025
Published
16 April 2025
Volume
16 - 2025
Edited by
Min Chen, Peking University, China
Reviewed by
Dwijendra K. Gupta, Allahabad University, India
Giuseppe Remuzzi, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy
Updates
Copyright
© 2025 Cao, Li, Ye, Lv, Zhang, Liang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Wang, wangyumei75@hust.edu.cn; Tao Liang, lt-liangtao-lt@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.