- 1The Second Department of Critical Care Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 2Laboratory of Cardiopulmonary Resuscitation and Critical Care, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 3National Clinical Research Center for TCM Cardiology, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 4Key Laboratory of Disease and Syndrome Integration Prevention and Treatment of Vascular Aging, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
Therapy for acute myocardial infarction often causes myocardial ischemia-reperfusion injury (MIRI), which is characterized by oxidative stress, inflammation, and apoptosis. Traditional therapies have shown poor effectiveness because of their low absorption and inappropriate targeting. Recently, nanotechnology has emerged as a promising treatment option for MIRI. Nanocarriers, such as liposomes, polymers, inorganic nanoparticles, and hybrid nanoparticles, make therapies more effective by making drugs more stable, improving targeting accuracy and lowering side effects. Plant-derived secondary metabolites and nanoparticles, specifically those containing Panax notoginseng saponins and flavonoids, have been shown to work together as a therapeutic approach. These nanoparticles have antioxidant, anti-inflammatory, and anti-apoptotic properties that significantly reduce myocardial injury after reperfusion. Targeting specificity and safety limit clinical translation, even with significant technological developments in these areas. Herein, we review current studies on nanocarriers and plant-derived secondary metabolite nanoparticles for MIRI treatment, as well as potential future clinical applications and limitations.
1 Introduction
The incidence of ischemic heart disease, a leading cause of death and disability worldwide, has been rapidly increasing as people age and adopt unhealthy lifestyles. Public health services have been substantially affected by this trend (Safiri et al., 2022). Major symptoms of ischemic heart disease, acute myocardial infarction, are often treated with reperfusion treatments, such as percutaneous coronary intervention and coronary artery bypass grafting, which attempt to restore blood flow to the ischemic myocardium. Although reperfusion significantly reduces myocardial necrosis, it may also aggravate myocardial ischemia-reperfusion injury (MIRI), thereby compromising the patient’s prognosis (Schäfer et al., 2022). Often leading to arrhythmia and heart failure, MIRI is characterized by a range of pathogenic mechanisms, including oxidative stress, inflammation, and apoptosis (Welt et al., 2024).
Current clinical intervention strategies for MIRI face multidimensional technical challenges (Liu et al., 2023). Existing therapeutic approaches primarily include pharmacological treatments, ischemic conditioning, and physical interventions; however, their clinical efficacy remains significantly constrained. In the pharmacological domain, conventional agents such as antioxidants and calcium channel blockers suffer from limitations in targeted delivery efficiency and suboptimal pharmacokinetics, characterized by rapid systemic clearance, insufficient myocardial tissue accumulation, and adverse effects (e.g., immunosuppression associated with cyclosporine A) (Upadhaya et al., 2017). For non-pharmacological strategies, ischemic conditioning demonstrates procedural simplicity but exhibits substantial heterogeneity in clinical outcomes across randomized controlled trials (Pei et al., 2014; Donato et al., 2017) Hypothermia therapy, as a physical intervention, faces critical technical challenges in translational medicine, particularly in precise temperature control and rewarming management (Voronkov et al., 2024). Notably, although certain botanical drugs show therapeutic potential, their clinical reproducibility is hindered by compositional complexity, undefined molecular targets, and non-standardized preparation protocols, leading to inconsistent pharmacokinetic profiles (Xu et al., 2021).
Recent advances in nanodelivery systems based on plant-derived secondary metabolites (PDSMs) offer innovative avenues to overcome MIRI treatment limitations. Through rational structural design and functional modification of nanocarriers, these systems effectively address the drawbacks of conventional drug delivery, significantly enhancing targeting efficiency and biostability (Wei et al., 2022). Research priorities focus on bioactive secondary metabolites with well-characterized pharmacological properties, including baicalein (flavonoid from Scutellaria baicalensis Georgi), notoginsenoside R1 (triterpenoid saponin from Panax notoginseng (Burk.) F.H. Chen), and curcumin (polyphenol from Curcuma longa L.). While these compounds exhibit anti-inflammatory and antioxidant activities, their inherent low solubility and nonspecific biodistribution limit therapeutic efficacy. Current studies demonstrate that polydopamine-modified nanocarriers encapsulating baicalein activate the Nrf2-ARE pathway, markedly suppressing reactive oxygen species (ROS) accumulation in myocardial tissues (Chen et al., 2024). Mesoporous silica nanoparticles conjugated with CD11b antibody (MSN-NGR1-CD11b) enhance cardiac repair by suppressing reactive oxygen species (ROS) accumulation through activation of AKT/MAPK signaling pathways in myocardial infarction (Li H. et al., 2022). Similarly, curcumin nanoparticles significantly reduce oxidative stress markers and elevate antioxidant capacity in diabetic rats with acute myocardial injury, further supporting the efficacy of nanotechnological approaches in mitigating ROS-mediated myocardial damage (Boarescu et al., 2019b).
This work reviews recent advancements in the treatment of MIRI using PDSM nanoparticles. By focusing on their potential to enhance therapeutic efficacy, improve targeting precision, and reduce side effects, we evaluate current research and explore the clinical feasibility of PDSM nanoparticles. Additionally, we propose novel approaches for cardiovascular disease treatment.
2 Methods
A systematic literature search was conducted across multiple electronic databases (PubMed, Web of Science, Scopus, ScienceDirect, Embase) to identify studies published up to November 2024. Inclusion criteria encompassed in vitro and in vivo investigations evaluating the therapeutic effects of botanical drugs/PDSMs combined with nanotechnology for MIRI. The search strategy employed the following Boolean operators and keywords in titles/abstracts/keywords (nano OR nanoparticle* OR nanocarrier OR “drug delivery” OR nanophytochemical* OR nanophytomedicine* OR liposome* OR polymer* OR inorganic*) AND (“traditional Chinese medicine” OR TCM OR “Chinese herbal medicine” OR plant* OR “phytochemical extract” OR “herbal drug*” OR “botanical drug*” OR “medicinal plants” OR “plant-derived secondary metabolites”) AND (“myocardial ischemia-reperfusion injury” OR MIRI OR “cardiac ischemia-reperfusion injury” OR “acute myocardial infarction”)*. Non-English publications and studies lacking experimental validation were excluded to ensure methodological rigor.
3 Targeting strategies of nanoparticles
Nanotechnology demonstrates enhanced drug delivery efficiency and therapeutic efficacy in MIRI through multimodal targeting approaches. Passive targeting capitalizes on the enhanced permeability and retention (EPR) effect to promote nanoparticle accumulation in ischemic myocardium, thereby reducing systemic adverse effects (Yao et al., 2015; Li et al., 2020; Lan et al., 2022). Active targeting strategies utilize antibody or peptide-modified nanoparticles to improve cardiomyocyte-specific binding affinity and drug internalization efficiency (Wei et al., 2024). Magnetic guidance systems enable precise spatiotemporal delivery through external magnetic field manipulation (Wei et al., 2024). Stimuli-responsive platforms integrated with biomimetic designs achieve pathology-triggered drug release mechanisms, such as oxidative stress-responsive payload deployment, while maintaining enhanced biocompatibility and targeting specificity (Bae et al., 2016; Wang J. et al., 2023; Wang et al., 2023 Y.; Zhou et al., 2023). Emerging hybrid systems combining passive-active targeting synergies with multidrug co-delivery capabilities present a transformative paradigm for optimizing MIRI intervention (Sun et al., 2012; Yang et al., 2023).
4 Application of nanocarriers in MIRI
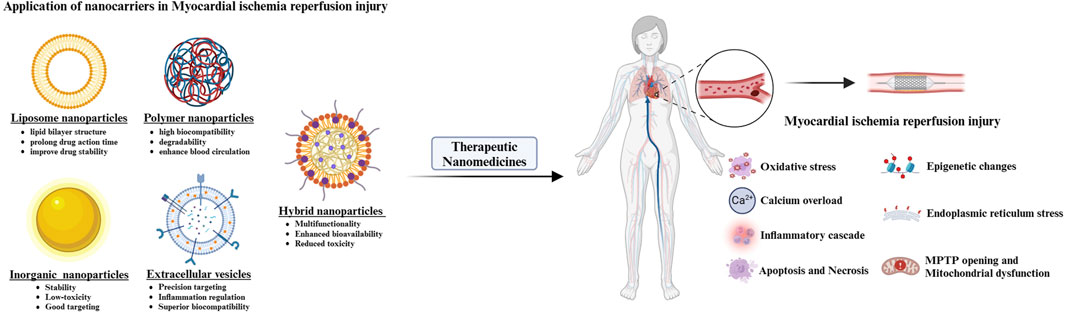
Nanotechnology offers innovative therapeutic strategies for MIRI by enabling precision drug delivery, enhancing pharmaceutical stability, and improving bioavailability. The integration of PDSMs’ inherent bioactivities with functionalized nanocarrier designs synergistically modulates oxidative stress, inflammatory responses, and apoptotic pathways, thereby overcoming the limitations of conventional treatment modalities. The following sections review several common nanocarriers applied in MIRI intervention, detailing their structural configurations and therapeutic mechanisms (Table 1; Figure 1).
Synthetic nanocarriers enable efficient drug loading and controlled release through material engineering design, primarily including polymer-based nanocarriers (e.g., poly (lactic-co-glycolic acid), PLGA), inorganic nanocarriers, and lipid-based systems. Du et al. engineered PLGA nanoparticles incorporating dihydromyricetin (DMY, a flavonoid metabolite derived from Ampelopsis grossedentata (Hand.-Mazz.) W.T. Wang), which demonstrated enhanced oral bioavailability. These DMY-PLGA nanoparticles attenuated oxidative damage by activating the PGC1α/PPARα pathway (Du et al., 2024). Jing et al. formulated nanoparticles (OVQ-NPs) containing bioactive flavonoids (hyperoside, vitexin, quercetin) from Polygonum orientale L., which regulated oxidative stress and inflammatory pathways through sustained-release properties, effectively reducing myocardial enzyme levels and suppressing apoptosis (Jing et al., 2024). Yu et al. designed magnesium-doped mesoporous bioactive glass nanoparticles (MgNPs/GA) loaded with gallic acid (GA, a polyphenolic metabolite from Rhus chinensis Mill.), achieving dual-phase myocardial protection through early-phase ROS scavenging and late-phase Mg2+-mediated angiogenesis promotion with macrophage M2 polarization (Yu et al., 2024). Carbon dots derived from carbonized Curcuma longa L. rhizomes (CRC-CDs) directly enhanced myocardial antioxidant capacity and inhibited apoptosis, demonstrating the innovative potential of nanoscale botanical drug formulations (Dong et al., 2024). Additionally, PEGylated liposomes (P-CLP-A/R) loaded with apigenin (a flavonoid from Apium graveolens L.) synergistically modulated the RAGE/NF-κB pathway through targeted delivery, significantly ameliorating ischemic myocardial injury (Liu et al., 2024).
Extracellular vesicles represent biomimetic nanocarriers that enhance drug targeting and biocompatibility by mimicking biological components. Zhang et al. isolated exosome-like nanoparticles from Salvia miltiorrhiza Bunge, which promoted endothelial cell migration and myocardial neovascularization through inherent pro-angiogenic activity, offering a non-invasive therapeutic strategy for reperfusion injury (Zhang et al., 2024). These exosomes inherit bioactivity from parent cells while exhibiting cost-effectiveness and high yield, highlighting clinical translation potential. For composite nanocarriers, Li et al. engineered neutrophil membrane-camouflaged mesoporous silica nanoparticles (AL@MSNs@NM) that leveraged natural neutrophil interactions with inflamed myocardial microvascular endothelial cells. This system enabled precision delivery of allicin (a sulfur-containing compound from Allium sativum L.), inhibiting ferroptosis and upregulating PECAM-1 expression to improve cardiac function and reduce infarct size (Li et al., 2024). Similarly, Han et al. developed neutrophil degranulosomes (NDs) loaded with 18β-glycyrrhetinic acid (GA, a triterpenoid saponin from Glycyrrhiza uralensis Fisch.), which achieved synchronized mitigation of oxidative stress and inflammation via H2O2-responsive release, effectively attenuating myocardial fibrosis and remodeling (Han et al., 2024). These hybrid systems overcome limitations of single-component carriers by integrating biological membrane functionality with synthetic material-controlled release properties.
5 Plant-derived secondary metabolite nanoparticles in MIRI
The increasing recognition of PDSMs in the treatment of MIRI has highlighted the significant therapeutic potential of botanical drug extracts, which possess strong anti-inflammatory, antioxidant, anti-apoptotic, and cardiovascular-protective properties (Dong et al., 2023). Thus, PDSMs are promising therapeutic approaches for the treatment of MIRI. Concurrently, the rapid advancement of nanotechnology has opened new avenues for research and clinical applications of PDSMs, particularly in two key areas.
Processing drugs into nanoscale suspensions or co-crystals markedly increases their surface areas. This approach enhances the solubility of these drugs and improves their chemical stability. For example, studies have demonstrated that converting curcumin and quercetin into nanodispersions significantly boosts their bioavailability and pharmacological efficacy. This nanoprocessing technology effectively addresses the inadequate absorption of PDSMs by the body (Gao et al., 2010; Li H. et al., 2021).
Nanoparticles are often used for the targeted delivery of PDSMs. Unlike synthetic drugs, PDSMs often non-selectively affect multiple organ systems. However, these natural chemicals present significant therapeutic challenges, including low absorption rates, poor chemical stability, limited permeability, and risk of liver and kidney damage. Nanotechnology has distinct benefits in addressing these difficulties, as nanoparticle-based delivery methods may significantly enhance the bioavailability and therapeutic efficiency of PDSMs while reducing side effects. This precise delivery approach addresses long-standing issues in PDSMs treatment and offers new possibilities for the therapeutic use of potent botanical drug medications (Xie et al., 2024).
Nanotechnology has provided new prospects for expanding the study of PDSMs while offering technical support for their clinical application. The integration of several PDSMs with nanotechnology has been successfully implemented in therapeutic investigations targeting MIRI, with outstanding results in the targeted distribution of critical components. These include ginsenoside, puerarin, tanshinone IIA, baicalin, triptolide, and ligustrazine (Kim et al., 2018; Yan et al., 2019; Xu et al., 2020; Mi et al., 2021; Yalikong et al., 2021; Zhu et al., 2021). Nanoparticle delivery technologies significantly increase the targeting precision and therapeutic effectiveness of these drugs while effectively mitigating adverse effects.
The following section introduces several representative PDSMs with nanocarriers that are currently under investigation, along with examples of their applications and mechanisms in the treatment of MIRI (Table 2; Figures 2, 3). The successful implementation of these nanoparticles not only validates the potential of nanotechnology in enhancing the efficacy of botanical drugs but also provides valuable insights into the modernization of botanical drugs and its integration with precision medicine.
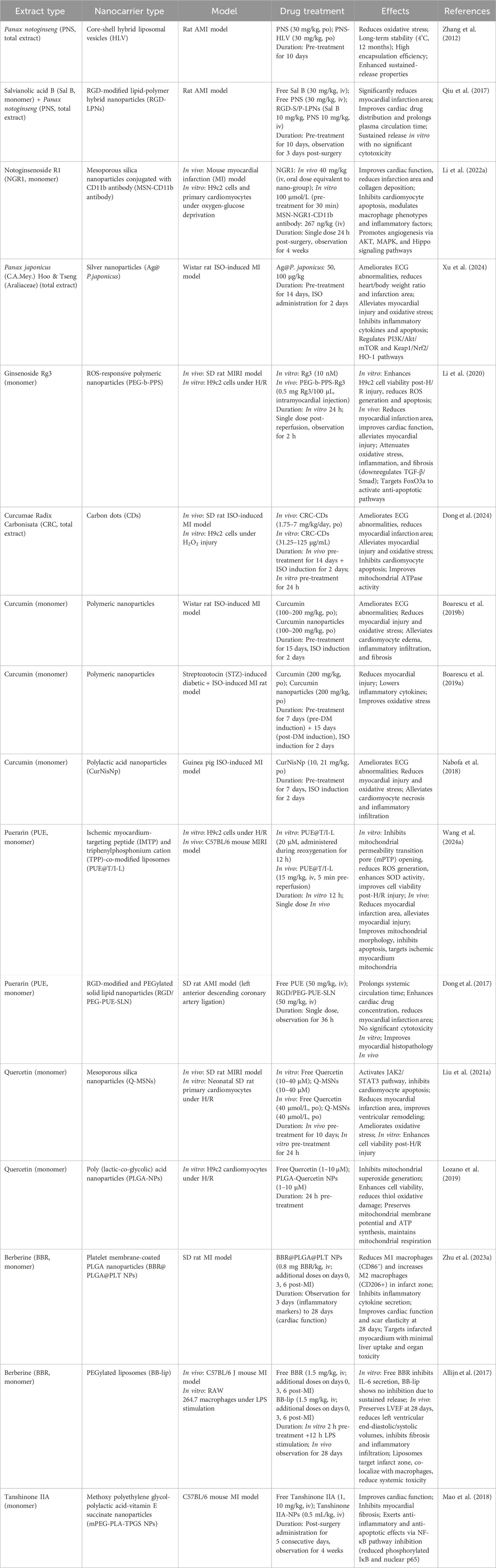
Table 2. Mechanisms and therapeutic advantages of nanocarriers delivering plant-derived secondary metabolites in myocardial ischemia-reperfusion injury.
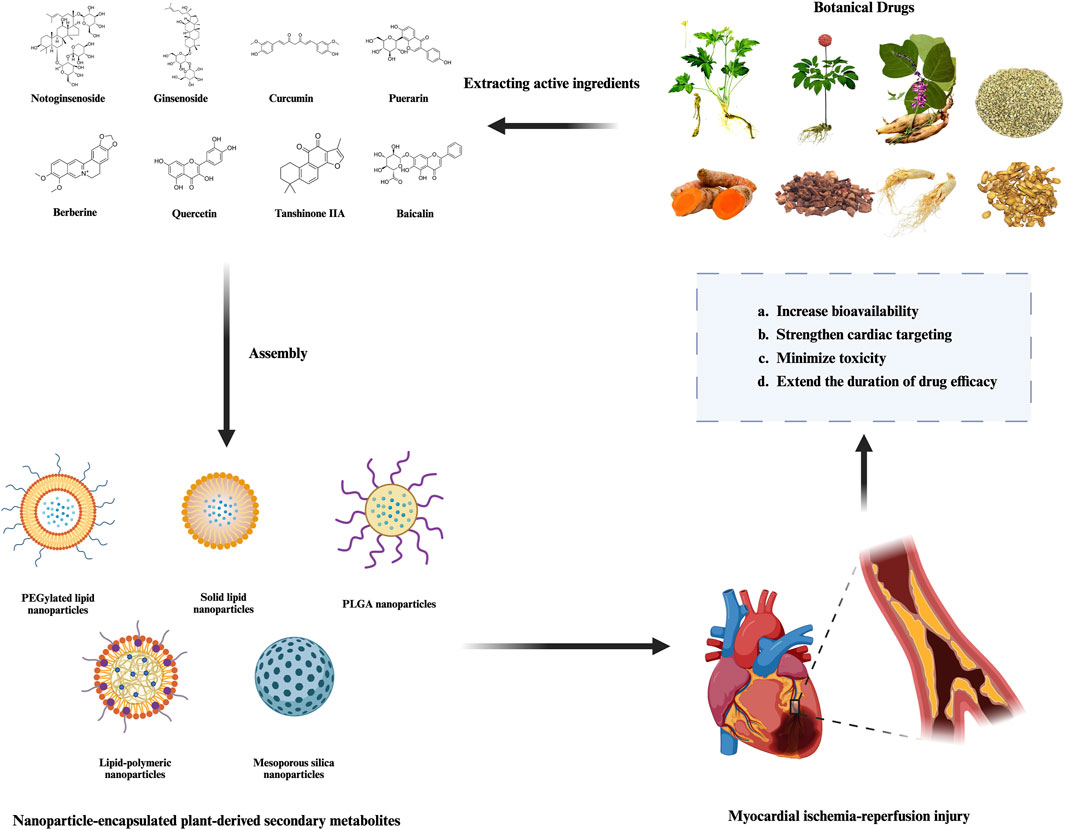
Figure 2. Therapeutic advantages of nanoparticle-encapsulated plant-derived secondary metabolites in MIRI.
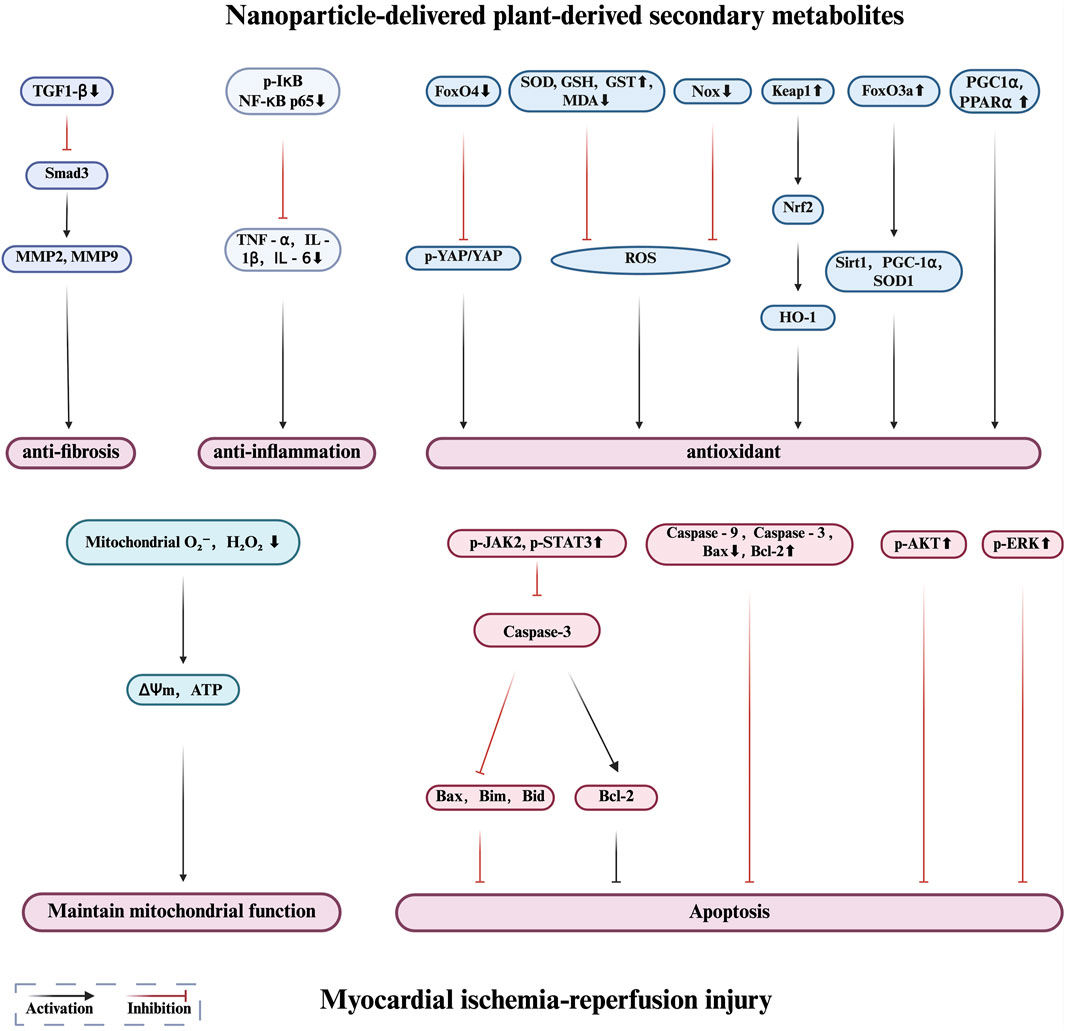
Figure 3. Protective mechanisms of nanoparticle-delivered plant-derived secondary metabolites in myocardial ischemia reperfusion injury.
5.1 Notoginsenoside
Notoginsenoside R1 (NGR1), a triterpenoid saponin primarily isolated from the dried roots and rhizomes of Panax notoginseng (Burk.) F.H.Chen (Araliaceae family, Panax genus), demonstrates potent cardioprotective effects against MIRI (Zhu and Wan, 2023). NGR1 suppresses the transforming growth factor-β-activated kinase 1-JNK/p38 signaling pathway, reducing inflammatory cytokine production, considerably reducing infarct size, and enhancing heart function (Zeng et al., 2023). NGR1 reduces apoptosis by regulating critical signaling pathways, including AKT and JAK2, thereby enhancing its cardioprotective effects (Lei et al., 2022; Xu et al., 2022).
Owing to its anti-inflammatory, antioxidant, and anti-apoptotic properties, NGR1 has been extensively investigated. MIRI-related damage is primarily caused by oxidative stress and myocardial cell death. Superoxide dismutase, which lowers ROS generation and shields cardiac cells from oxidative stress (Tong et al., 2019), is an antioxidant enzyme that NGR1 promotes. Moreover, NGR1 helps stabilize mitochondrial membrane potential, thereby lowering mortality and shielding against heart injury (Yan et al., 2021).
Although notoginsenoside has enormous therapeutic potential, its low bioavailability, poor water solubility, and sensitivity to metabolic breakdown restrict its therapeutic uses (Li et al., 2007). To overcome these limitations, researchers have devised mesoporous silica nanoparticle-based delivery systems, including NGR1. This method, together with CD11b antibodies, focuses on injured regions after myocardial infarction, thereby increasing the local concentration of NGR1 in the myocardium. This method reduces myocardial reperfusion damage, enhances heart performance, and increases the antioxidant, anti-inflammatory, and anti-apoptotic actions of NGR1. Moreover, the treatment controls energy metabolism and induces angiogenesis (Li H. et al., 2022). However, the long-term biosafety profile of Notoginsenoside R1-loaded mesoporous silica nanoparticles conjugated with CD11b antibody and their potential off-target effects on non-infarcted tissues expressing CD11b remain uncharacterized, necessitating further preclinical validation.
NGR1 was used in a core-shell hybrid liposome nanoparticle system to increase the bioavailability of notoginsenoside. After oral delivery, this method greatly enhanced the pharmacological effects of notoginsenosides, thereby lowering the severity of myocardial infarction, controlling oxidative stress, and increasing myocardial cell survival rates (Zhang et al., 2012). This study proposes a novel approach to enhance the delivery of notoginsenoside. Notably, the current findings are limited by the lack of pharmacokinetic evaluations to delineate the absorption mechanisms of panax notoginsenoside-encapsulated core-shell hybrid liposomal delivery systems and insufficient toxicological monitoring over extended durations, which necessitates further investigation prior to human trials.
Researchers incorporated notoginsenoside and salvianolic acid B into an RGD-modified dual-drug delivery system. This technique greatly improved myocardial targeting, lowered infarction size, and improved heart function (Qiu et al., 2017). With significant consequences for future cardiovascular disease treatments, this dual-drug delivery system epitomizes the benefits of multi-target therapeutic approaches. The limitations of this study include that the experimental findings were primarily derived from a rat model of acute myocardial ischemia, which may not fully replicate the pathophysiological complexity of human cardiovascular disease, potentially restricting the direct clinical translatability of the results.
5.2 Ginsenoside
Ginsenosides, a class of dammarane-type and oleanane-type triterpenoid saponins characteristic of Panax genus (Araliaceae) plants, are secondary metabolites predominantly distributed in the roots and rhizomes of Panax ginseng C.A.Mey., Panax notoginseng (Burk.) F.H.Chen, and Panax quinquefolius L. These bioactive compounds exhibit multi-pharmacological properties including anti-inflammatory, antioxidant, antitumor, and neuroprotective activities. Common ginsenosides, such as Rg1, Rg3, Rb1, and Rc, affect many physiological processes by regulating cellular signaling pathways (Chen, 2020). In addition to their potential in treating neurodegenerative diseases and cancer, ginsenosides have attracted attention for their protective effects on the cardiovascular system (Sarhene et al., 2021).
Qin et al. have demonstrated that ginsenoside Rb1 significantly improved MIRI by reducing myocardial autophagy through the modulation of the PI3K/Akt/mTOR signaling pathway (Qin et al., 2021). Similarly, Wang et al. have reported that ginsenoside Rd alleviates MIRI by reducing inflammation and apoptosis via inhibition of the PI3K/AKT signaling pathway (Wang et al., 2024c). Ye et al. have found that ginsenoside Re protects cardiomyocytes by inhibiting ferroptosis by regulating the miR-144–3p/SLC7A11 signaling pathway (Ye et al., 2023). Furthermore, Xue et al. have found that ginsenoside Rc mitigates MIRI by activating the sirtuin 1 (SIRT1 pathway), thereby reducing mitochondrial oxidative stress and apoptosis (Xue et al., 2023). Li et al. have demonstrated that ginsenoside Rg2 significantly improves MIRI by inhibiting necrosis by modulating the transforming growth factor-β-activated kinase one signaling pathway (Li Y. et al., 2022). Huang et al. have demonstrated that the ginsenoside Rb2 reduces MIRI by inhibiting SF3A2 acetylation and regulating Fscn1 selective splicing (Huang et al., 2023). Another study by Xue et al. has revealed that ginsenoside Rb2 improves cardiac function by reducing oxidative stress through SIRT1 activation (Xue et al., 2020). Collectively, these studies highlight the diverse cardioprotective mechanisms of ginsenosides against MIRI.
Despite their promising therapeutic effects, the clinical application of ginsenosides is limited owing to their low water solubility and poor bioavailability (Pan et al., 2018). Therefore, improving their bioavailability and pharmacological efficacy has become a major focus of current research. Recently, nanotechnology has been used to enhance the stability, targeting, and circulation time of ginsenoside-based drugs, thereby boosting their clinical efficacy. Nanoparticles not only improve the solubility of hydrophobic ginsenosides, such as Rg3, but also concentrate drug effects at specific disease sites through targeted delivery, thereby minimizing side effects. For example, Xu et al. developed Ag@P. japonicus nanoparticles by integrating silver nanoparticles with Panax japonicus extract. These nanoparticles reduced myocardial apoptosis and inflammation, enhanced antioxidant enzyme activity (e.g., superoxide dismutase and glutathione peroxidase), decreased infarct size, and improved cardiac function in an isoproterenol-induced myocardial infarction model. This study underscored the potential of nanotechnology in enhancing the efficacy and stability of ginsenosides (Xu et al., 2024). Li et al. investigated a ROS-responsive nanoparticle delivery system for ginsenoside Rg3. By targeting the FoxO3a pathway, Rg3 inhibited oxidative stress, inflammation, and fibrosis, resulting in improved cardiac function after MIRI. Nanotechnology significantly enhanced the bioavailability of Rg3, demonstrating its potential as a delivery strategy for other natural products (Li et al., 2020). Li et al. demonstrated limitations in their study by failing to comprehensively evaluate the long-term biosafety and potential immunogenicity of reactive oxygen species (ROS)-responsive nanoparticles, while the lack of validation in preclinical large animal models further constrained their clinical translatability (Li et al., 2020). Xu et al. did not address the In vivo pharmacokinetics or long-term bioaccumulation risks of silver nanoparticles, nor did they systematically analyze dose-dependent toxicity or stability in complex pathological microenvironments, thereby compromising a thorough assessment of therapeutic safety (Xu et al., 2024).
5.3 Curcumin
Curcumin, a diarylheptanoid compound (chemical formula C21H20O6, molecular weight 368.38 Da), is primarily isolated from the dried rhizomes of C. longa L (Zingiberaceae). Secondary botanical sources include rhizomes of the congeneric species Curcuma aromatica Salisb. And Curcuma zedoaria (Berg.) Rosc., albeit with lower extraction yields. In MIRI, curcumin mitigates oxidative stress by scavenging ROS and enhancing antioxidant enzyme activity. It also inhibits the production of pro-inflammatory cytokines such as TNF-α and IL-6, thereby reducing inflammation while suppressing myocardial cell apoptosis by regulating apoptosis-related proteins. Numerous studies have investigated multiple protective mechanisms of curcumin in MIRI, consistently revealing its significant cardioprotective effects.
For example, Wu et al. have demonstrated that curcumin exerts protective effects against myocardial damage by activating the PI3K/Akt/mTOR signaling pathway, providing key insights into its role in cellular signal transduction (Wu et al., 2021). Cui et al. have revealed that curcumin stimulates vascular endothelial cells to secrete fibroblast growth factor 2, thereby reducing hypoxia/reoxygenation damage to myocardial cells and reinforcing the role of curcumin in promoting myocardial repair (Cui J.-K. et al., 2024). Moreover, Cui et al. have revealed that curcumin alleviates myocardial injury by increasing endogenous H2S levels and regulating m6A RNA modifications, offering deeper insights into its molecular regulatory mechanisms (Cui J. et al., 2024). Zhang et al. have shown that curcumin regulates mitochondrial metabolism and inhibits apoptosis, significantly improving the outcomes of cardiopulmonary resuscitation following cardiac arrest (Zhang et al., 2022).
Nanotechnology has been widely used to enhance the bioavailability and stability of curcumin. Nabofa et al. investigated curcumin-nisin-based polylactic acid nanoparticles (CurNisNp) and found that they effectively prevented isoproterenol-induced myocardial injury by reducing oxidative stress and inflammation (Nabofa et al., 2018). Boarescu et al. used nano-curcumin particles with a PLGA carrier, which significantly improved the absorption rate of curcumin and demonstrated stronger antioxidant and anti-inflammatory effects, particularly by enhancing electrocardiogram readings and protecting cardiac function (Boarescu et al., 2019b). In another study, Boarescu et al. validated the superior performance of nano-curcumin in a diabetic MIRI model, demonstrating that it not only significantly reduced oxidative stress and inflammation but also exhibited excellent cardioprotective effects (Boarescu et al., 2019a). Additionally, Dong et al. studied carbon quantum dot nanoparticles (CRC-CDs) extracted from turmeric, which further reduced cardiac damage in MIRI by enhancing antioxidant enzyme activity and inhibiting myocardial cell apoptosis (Dong et al., 2024).
While existing studies provide valuable insights into nanomaterial-based cardioprotective strategies, several critical limitations persist. Dong et al. focused exclusively on short-term rodent models, neglecting evaluations of chronic toxicity and sustained therapeutic efficacy of carbon dots in clinically relevant myocardial ischemia paradigms, thereby limiting translational validity (Dong et al., 2024). Boarescu et al. omitted pharmacokinetic profiling of nanocurcumin, particularly its long-term bioavailability and metabolic fate, and employed a restricted experimental dose range that may inadequately characterize dose-response relationships (Boarescu et al., 2019b); their subsequent study further constrained generalizability by excluding non-diabetic myocardial infarction models and failing to address nanoparticle stability and tissue distribution dynamics (Boarescu et al., 2019a). Additionally, Nabofa et al. relied solely on guinea pig models, which exhibit marked cardiovascular physiological divergence from humans, while overlooking immunogenicity assessments and chronic safety risks of their composite nanoparticles (Nabofa et al., 2018). Collectively, these limitations highlight gaps in interspecies physiological relevance, comprehensive chronic exposure evaluations, and rigorous pharmacokinetic characterization, necessitating further research to bridge preclinical findings and clinical applicability.
5.4 Puerarin
Puerarin, an isoflavone C-glycoside (chemical formula C21H20O9, molecular weight 416.38 Da), is primarily isolated from the roots of Pueraria lobata (Willd.) Ohwi and Pueraria thomsonii Benth (Fabaceae family, Pueraria genus). This phytochemical demonstrates significant cardioprotective effects within the cardiovascular system, underpinning its widespread clinical application in managing hypertension and coronary artery disease (Zhou et al., 2014). Consequently, it is widely used in the treatment of cardiovascular diseases such as hypertension and coronary artery disease. Numerous studies have demonstrated the beneficial effects of puerarin on MIRI with its protective mechanisms operating at multiple levels (Zhou et al., 2021).
Puerarin regulates miR-21, reducing apoptosis and inhibiting oxidative stress, which enhances antioxidant capacity and significantly improves the survival rate of myocardial cells during MIRI (Xu et al., 2019). It further protects cardiac cells by increasing the long non-coding RNA ANRIL and blocking autophagy (Han et al., 2021). Puerarin inhibits the SIRT1/NF-κB pathway and prevents NLRP3 inflammasome activation, thereby reducing MIRI-induced inflammation (Wang et al., 2020). Puerarin also prevents ferroptosis, a unique type of cell death that reduces cardiac cell damage and provides overall cardioprotection (Ding et al., 2023).
Despite its promising therapeutic potential, its low water solubility and short half-life limit its clinical use (Wang et al., 2022). Nanoparticle carrier technologies have been developed to improve the bioavailability and targeting efficiency of PUR. For example, triphenylphosphonium cation and ischemic myocardium-targeting peptide-modified liposomes (PUE@T/I-L) were designed to target mitochondria, increasing puerarin localization in the myocardium, reducing oxidative stress, protecting myocardial cells from ischemia-reperfusion injury, and decreasing infarct size (Wang et al., 2024a). Moreover, RGD-modified and PEGylated solid lipid nanoparticles significantly prolonged the retention time of puerarin in the body, augmenting its concentration and efficacy in the heart while offering substantial protection against acute myocardial ischemia [148]. These nanoparticle carriers not only promote medication stability and controlled release but also maximize targeted distribution, highlighting the promise of puerarin in the precision treatment of cardiovascular disorders (Dong et al., 2017).
However, the following methodological limitations may constrain their translational relevance: Wang et al. did not validate the long-term biodistribution or organotoxicity of their dual-targeted liposomes in large mammals or advanced preclinical models, potentially undermining clinical translatability (Wang et al., 2024b); Dong et al. omitted systematic evaluation of RGD-modified nanoparticles’ targeting stability under dynamic pathological conditions (e.g., fibrosis or chronic inflammation), limiting generalizability of their therapeutic strategy (Dong et al., 2017).
5.5 Quercetin
Quercetin, a flavonol ubiquitously distributed in the plant kingdom (chemical formula C15H10O7, molecular weight 302.24 Da), predominantly occurs in nature as glycosidic forms such as rutin, liberating free quercetin upon acid hydrolysis or enzymatic conversion. Its principal botanical sources include the flower buds (Huaimi) of Sophora japonica L (Fabaceae), epidermal tissues of Allium cepa L (Amaryllidaceae), and fruit peel of Malus domestica Borkh (Rosaceae). Quercetin have demonstrated notable cardioprotective effects in MIRI therapy owing to their strong antioxidant and anti-inflammatory properties (Zhang et al., 2020). Quercetin regulates the main pathways involved in MIRI. To prevent cell death, it scavenges ROS, reduces oxidative stress, quiesces inflammatory responses, and preserves mitochondrial activity. Moreover, it influences signaling channels, including ATP-sensitive potassium channels and the nitric oxide system, which cooperate to minimize cardiac cell damage and necrosis. Many studies have demonstrated the effectiveness of quercetin in reducing cardiac damage caused by MIRI via various molecular pathways. Liu Y et al. have discovered that quercetin greatly reduced myocardial cell death and provided cardiac protection by inducing the NO system and mitochondrial ATP-sensitive potassium channels (Liu Y. et al., 2021). Chang et al. have shown that via a DNA-PKcs-SIRT5-regulated mitochondrial quality control mechanism, quercetin reduces necroptosis and, therefore, enhances heart function (Chang et al., 2024). Furthermore, Li et al. have discovered that quercetin significantly reduced oxidative stress and mitochondrial-mediated death by modulating the extracellular signal-regulated protein kinases one and 2/DRP1 signaling pathway, thereby increasing the resistance of the heart to damage. These results demonstrate the enormous therapeutic potential of quercetin in MIRI (Li F. et al., 2021).
Despite the potential therapeutic effects of quercetin, its clinical use is limited because of its physicochemical properties. Quercetin has poor water solubility and is rapidly metabolized in the body, resulting in low bioavailability and difficulty in maintaining a sufficient drug concentration and duration of action, which restricts its therapeutic effectiveness (Alizadeh and Ebrahimzadeh, 2022). To overcome these challenges, nanotechnology has been used to enhance the drug delivery performance of quercetin.
Nanotechnology has significantly improved the therapeutic efficacy of quercetin. Liu et al. have demonstrated that loading quercetin into mesoporous silica nanoparticles effectively reduced myocardial infarction size and improved cardiac physiological and biochemical functions, particularly in protecting against MIRI by activating the JAK2/STAT3 signaling pathway and inhibiting apoptosis and oxidative stress (Liu C.-J. et al., 2021). Similarly, Lozano et al. encapsulated quercetin in PLGA nanoparticles and found that this nanodelivery system exhibited stronger cardioprotective effects against oxidative stress compared with free quercetin, especially by reducing mitochondrial ROS production, maintaining mitochondrial function and preserving ATP synthesis (Lozano et al., 2019).
Although both studies provide critical evidence for the therapeutic potential of nanoparticle-mediated quercetin delivery, their methodological frameworks exhibit significant limitations. Liu et al. exclusively employed a 10-day prophylactic pretreatment regimen prior to ischemia-reperfusion (IR), failing to evaluate acute therapeutic interventions or clinically relevant post-ischemic treatment windows (e.g., intra-reperfusion or emergency post-infarction administration), thereby limiting the clinical translatability of their efficacy assessments (Liu C.-J. et al., 2021). Similarly, Lozano et al. relied solely on in vitro hypoxia-reoxygenation models using the H9c2 rat cardiomyoblast cell line, omitting validation in primary cardiomyocytes or pathophysiologically complex conditions (e.g., hypertension or metabolic comorbidities), which may overestimate the applicability of their findings to human cardiac pathophysiology (Lozano et al., 2019).
5.6 Berberine
Berberine, an isoquinoline alkaloid (chemical formula C20H18NO4+, molecular weight 336.36 Da), is predominantly isolated from the rhizomes of Coptis chinensis Franch (Ranunculaceae), root bark of Berberis julianae Schneid (Berberidaceae), and bark of Phellodendron amurense Rupr (Rutaceae). This phytochemical has garnered significant pharmacological attention due to its broad-spectrum bioactivities. These include anti-inflammatory, antioxidant, antibacterial, and metabolic regulatory effects (Song et al., 2020). Berberine’s potential in cardiovascular illnesses has recently received considerable attention for its preventive function against MIRI (Feng et al., 2019). Jia et al. showed that berberine greatly reduces myocardial damage by decreasing inflammation and oxidative stress, predominantly via the miR-26b-5p-mediated prostaglandin-endoperoxide synthase 2/MAPK signaling pathway (Jia et al., 2022). Long et al. have discovered that berberine improves cardioprotection by upregulating miR-340-5p and inhibiting high mobility group box 1 (HMGB1)-mediated inflammatory response (Long et al., 2023). Abdulredha et al. have demonstrated the ability of berberine to prevent MIRI by interfering with oxidative stress and inflammatory pathways (Abdulredha et al., 2021). Hu et al. have discovered that berberine decreased myocardial damage by blocking excessive autophagy via the RhoE/adenosine monophosphate-activated protein kinase pathway (Hu et al., 2024). Yang et al. have clarified this process and found that berberine suppressed cardiomyocyte ferroptosis, providing further protection (Yang et al., 2022). Overall, these findings show that berberine has a broad cardioprotective effect against MIRI by modulating inflammation, oxidative stress, autophagy, and ferroptosis.
Although berberine has significant pharmacological properties, its low water solubility and rapid metabolism restrict its clinical use and reduce its bioavailability, making it difficult to maintain therapeutic concentrations in target tissues (Wang et al., 2017). To overcome these limitations, researchers have used nanotechnology to add berberine to nanoparticle delivery methods. This approach enhances solubility, stability, and focused distribution, thereby increasing therapeutic effectiveness.
To restore heart function after myocardial infarction, Allijn et al. synthesized berberine in liposomes. Their results revealed that long-circulating liposomes enhanced the stability and bioavailability of berberine. By preventing ventricular remodeling, preserving the ejection fraction, and reducing the risk of heart failure, berberine liposomes provided significant cardioprotection after myocardial infarction. This demonstrates how liposomal technology can improve the medicinal and cardioprotective properties of berberine (Allijn et al., 2017). Zhu et al. investigated the use of platelet membrane-coated nanoparticles to treat MIRI with berberine. Their results revealed that extended drug release after reperfusion was made possible by berberine encapsulated in PLGA nanoparticles coated with a platelet membrane that was preferentially localized at the location of myocardial infarction. Platelet membrane-coated nanoparticles accumulated more in the heart tissue than standard PLGA nanoparticles, resulting in significantly fewer systemic side effects. Significant post-reperfusion cardiac function improvements resulted from this new delivery strategy, changing macrophage polarization, lowering inflammation and death, accelerating myocardial regeneration, and reducing fibrosis (Zhu K. et al., 2023).
While Allijn et al. demonstrated improved cardiac function preservation through liposomal berberine encapsulation, their study did not investigate the long-term stability and potential off-target effects beyond 28 days post-myocardial infarction, limiting comprehensive safety assessments (Allijn et al., 2017). Notably, Zhu et al. addressed nanoparticle delivery but did not quantitatively validate the In vivo drug release kinetics within the infarcted myocardium, leaving uncertainties regarding sustained therapeutic efficacy and dose optimization under physiological conditions (Zhu K. et al., 2023).
5.7 Tanshinone IIA
Tanshinone IIA, a lipophilic diterpenoid quinone (chemical formula C19H18O3, molecular weight 294.35 Da), is unambiguously identified as a secondary metabolite derived from the dried roots and rhizomes of S. miltiorrhiza Bunge (Lamiaceae). This compound exerts core cardiovascular protective effects, including anti-atherosclerotic activity, myocardial ischemic protection, and platelet aggregation inhibition, through mechanisms involving suppression of the NF-κB inflammatory pathway and modulation of lipid metabolism (Gao et al., 2012). Tanshinone IIA exerts its cardioprotective effects in MIRI through various mechanisms, including scavenging ROS, inhibiting the NF-κB signaling pathway to reduce inflammation, regulating apoptosis-related proteins to prevent myocardial cell death, protecting mitochondrial function to mitigate oxidative stress, and preventing myocardial fibrosis to prevent additional damage to cardiac function (Zhu P.-C. et al., 2023). Through these multi-pathway, multi-target actions, tanshinone IIA’s strong cardioprotective ability efficiently reduced cardiac damage induced by MIRI.
However, the poor solubility and low bioavailability of tanshinone IIA limit its clinical application (Huang et al., 2022). To address these challenges, researchers have employed nanocarrier technologies to enhance the pharmacological properties of carriers. One study developed a lipid-polymer nanocarrier system loaded with tanshinone IIA, modified with triphenylphosphine and D-α-tocopherol polyethylene glycol succinate, for targeted mitochondrial therapy in myocardial infarction. This system demonstrated significantly improved compatibility and therapeutic efficacy compared with free drugs and other similar nanocarriers in both in vitro and in vivo experiments. Pharmacokinetic and biodistribution studies confirmed the superior therapeutic effects of this nanocarrier system (Zhang et al., 2018).
Focusing on their effects on left ventricular remodeling after myocardial infarction, researchers in another study created monomethoxy polyethylene glycol-polylactic acid-D-α-tocopherol polyelsky nanoparticles containing tanshinone IIA. Within 4 weeks following myocardial infarction, tanshinone IIA nanoparticle treatment significantly recovered cardiac function, decreased infarct size, and effectively averted left ventricular dilatation in a mouse model. By lowering inhibitor of nuclear factor kappa B phosphorylation and the NF-κB signaling system, this drug clearly lowered cardiac inflammation, death, and fibrosis. Following myocardial infarction, tanshinone IIA nanoparticles lowered inhibitor of nuclear factor kappa B phosphorylation and NF-κB activity, hence improving heart remodeling (Mao et al., 2018). While the study demonstrates promising cardioprotective efficacy, its acute-phase dosing protocol (5-day post-MI administration) may be insufficient to mitigate persistent inflammatory and fibrotic cascades underlying chronic cardiac remodeling (Mao et al., 2018).
6 Discussion
Significant progress has been made in the treatment of MIRI using PDSM nanoparticles (Li et al., 2020; Li et al., 2022). The combination of modern nanotechnology with botanical drugs in this new strategy provides synergistic benefits for several targets and pathways (Chen et al., 2024). These nanoparticles overcome the main limitations of botanical drug treatments, such as restricted absorption and targeting (Liu C.-J. et al., 2021), thus improving therapeutic efficacy. Although initial results showed promise, considerable obstacles still exist in the therapeutic use of PDSM nanoparticles.
A key advantage of PDSM nanoparticles is their ability to exert synergistic effects across several targets and processes. Although many botanical drugs function through various pathways, PDSMs combined with nanotechnology provide significant benefits by simultaneously targeting many avenues. This technique enables the complete regulation of pathogenic processes, such as antioxidation, anti-inflammation, apoptosis suppression, and mitochondrial function modification. In MIRI models, quercetin-loaded nanoparticles demonstrated strong antioxidant effects, reducing oxidative stress, myocardial apoptosis, and inflammation while promoting angiogenesis and cardiac repair via the JAK2/STAT3 signaling pathway (Liu C.-J. et al., 2021). Panax notoginseng saponin-loaded nanoparticles decrease inflammation by blocking TNF-α and IL-6, activating the AKT and MAPK pathways, and reducing oxidative stress. As a result, post-reperfusion heart function has greatly improved (Li H. et al., 2022). Ginsenoside nanoparticles provide further cardioprotection by inhibiting apoptosis via the FoxO3a signaling pathway, as well as antioxidative and anti-inflammatory effects. This multifaceted approach makes PDSM nanoparticles a more effective therapeutic option for MIRI than traditional medicines.
The incorporation of nanotechnology has markedly enhanced the efficacy of the targeted delivery of PDSMs for MIRI treatment. Nanoparticles can precisely deliver drugs to damaged cardiac tissues while minimizing adverse effects in healthy areas by integrating passive and active targeting mechanisms. For instance, when vascular permeability increases during MIRI, nanoparticles using the EPR effect effectively aggregate in the injured regions (Lan et al., 2022). Drug localization can also be enhanced by active targeting strategies using nanoparticles covered with antibodies or peptides. Using external magnetic fields, magnetic targeting drives nanoparticles to the location of the injury, thereby increasing their therapeutic effects (Wei et al., 2024). By lowering the medication levels in non-targeted organs and reducing oxidative stress, mitochondria-targeted nanoparticles protect the mitochondria. This concentrated approach lowers adverse effects and increases medical efficacy (Gao et al., 2022).
Because they target many MIRI pathogen routes, multifunctional nanocarriers have synergistic therapeutic potential. By delivering antioxidants and anti-inflammatory medicines, these nanocarriers can effectively address several aspects of disease (Yang et al., 2023). For instance, while reducing inflammation and cardiomyocyte injury, nanoparticle solutions combining the EPR effect with tailored modifications can enhance drug delivery efficacy (Sun et al., 2012). Particularly pH- or ROS-responsive systems, liposomes, and polymeric nanoparticles provide precise control over drug release, thereby enhancing therapeutic effectiveness and reducing adverse effects (Li et al., 2020). Using these multifunctional nanocarriers in MIRI treatment showed their ability to cooperate across various systems, including anti-inflammation, anti-oxidation, and anti-apoptosis systems, thus producing considerably superior myocardial damage interventions.
Although PDSMs nanoparticles have shown potential therapeutic advantages in animal studies, various obstacles limit their clinical use. The long-term safety of nanocarriers requires further confirmation through clinical research, mostly in terms of their metabolism, excretion, and possible effects on other organs (Piscatelli et al., 2021). The complexity and variety of botanical drug constituents can impact the stability and efficacy of nanoparticles in clinical applications (Wei et al., 2022). Moreover, improving the pharmaceutical distribution and release techniques is a major challenge. Addressing the critical challenges in the clinical translation of botanical nanomedicines requires strategic advancements across material innovation, pharmacological optimization, and translational validation. In biodegradable carrier development, biocompatible polymers such as PLGA and natural polysaccharides are modified through surface engineering techniques (e.g., PEGylation) to reduce immunogenicity (Chatterjee and Chanda, 2022), with parallel long-term toxicological evaluations to assess organ-specific biodistribution profiles. Exploration of plant-derived natural vesicles, including exosomes and polysaccharide-based carriers, demonstrates potential for minimizing xenobiotic toxicity compared to synthetic alternatives (Basyoni et al., 2025; Chai et al., 2025). Following HPLC-MS-guided phytochemical screening and metabolomics-optimized drug loading (Plumb et al., 2023), nanocarriers are functionalized with immunomodulatory agents (e.g., siRNA targeting inflammatory mediators) to activate therapeutic pathways such as cGAS-STING signaling (Nagarajan et al., 2023). Translational validation employs 3D human organoid models and large-animal disease prototypes to quantify targeting specificity and biosafety parameters, integrated with standardized good manufacturing practice (GMP) protocols. Advanced pathology-responsive release systems, including oxidative stress-activated formulations, enable localized drug enrichment while maintaining systemic exposure below toxicity thresholds (Zheng et al., 2022).
Future studies should focus on integrating biomimetic nanotechnology with PDSMs to enhance biocompatibility and targeted efficacy. Encapsulating nanoparticles with myocardial or immune cell membranes may facilitate immune clearance and improve drug retention in the heart tissue (Liu et al., 2020; Zhou et al., 2023). This biomimetic approach improves the precise distribution of PDSM nanoparticles, enhancing their therapeutic efficacy in MIRI treatment. Moreover, the development of multifunctional hybrid nanoparticles has enhanced their potential for multitarget treatment. Future research should investigate how nanoparticles can concurrently carry genes, proteins, and other therapeutic compounds to address the complex disease processes of MIRI (Wang et al., 2021). For example, nanoparticle platforms integrating miRNA delivery methods have shown considerable promise in supporting myocardial regeneration and repair, indicating a possible breakthrough in the future treatment of cardiovascular disorders (Tan et al., 2021).
7 Conclusion
The clinical prospects of PDSM nanoparticles in the treatment of MIRI are highly promising. Nanotechnology not only boosts the bioavailability of bioactive phytochemicals but also enhances therapeutic outcomes through multi-target synergistic effects. However, successful translation to clinical practice requires further human trials to confirm long-term safety and efficacy. With advances in nanotechnology, more PDSM nanoparticles are expected to enter clinical trials, offering diverse and precise treatment options for MIRI.
Author contributions
WS: Visualization, Writing – original draft. YX: Data curation, Visualization, Writing – review and editing. JW: Data curation, Visualization, Writing – review and editing. XZ: Data curation, Visualization, Writing – review and editing. SZ: Data curation, Visualization, Writing – review and editing. HG: Validation, Writing – review and editing. QH: Validation, Writing – review and editing. CQ: Validation, Writing – review and editing. TH: Conceptualization, Project administration, Supervision, Writing – review and editing. YL: Conceptualization, Project administration, Supervision, Writing – review and editing. MY: Conceptualization, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [no. 8207213], Research Fund of Anhui Institute of Translational Medicine [no. 2023zhyx-C64 and 2022zhyx-C76]; the Basic and Clinical Enhancement Project of Anhui Medical University [no. 2019xkjT028 and 2023xkjT042]; the Postgraduate Innovation Research and Practice Program of Anhui Medical University [no. YJS20230133]; Anhui Province Key Research and Development Plan High-tech Special Project [no. 202304a05020071]; Anhui University Excellent Young Talents Support Plan [no. gxyqZD2018026].
Acknowledgments
The authors would like to thank Editage for the English language editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MIRI, Myocardial ischemia-reperfusion injury; PDSMs, Plant-derived secondary metabolites; EPR, Enhanced Permeability and Retention; ROS, Reactive oxygen species; PEG, Polyethylene glycol; RAGE, Receptor of Advanced Glycation Endproducts; TPP, Triphenylphosphonium; PLGA, Polylactic acid/glycolic acid; NGR1, Notoginsenoside R1.
References
Abdulredha, A., Abosaooda, M., Al-Amran, F., and Hadi, N. R. (2021). Berberine protests the heart from ischemic reperfusion injury via interference with oxidative and inflammatory pathways. Med. Arch. 75, 174–179. doi:10.5455/medarh.2021.75.174-179
Alizadeh, S. R., and Ebrahimzadeh, M. A. (2022). Quercetin derivatives: drug design, development, and biological activities, a review. Eur. J. Med. Chem. 229, 114068. doi:10.1016/j.ejmech.2021.114068
Allijn, I. E., Czarny, B. M. S., Wang, X., Chong, S. Y., Weiler, M., da Silva, A. E., et al. (2017). Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J. Control Release 247, 127–133. doi:10.1016/j.jconrel.2016.12.042
Bae, S., Park, M., Kang, C., Dilmen, S., Kang, T. H., Kang, D. G., et al. (2016). Hydrogen peroxide-responsive nanoparticle reduces myocardial ischemia/reperfusion injury. J. Am. Heart Assoc. 5, e003697. doi:10.1161/JAHA.116.003697
Basyoni, A. E., Atta, A., Salem, M. M., and Mohamed, T. M. (2025). Harnessing exosomes for targeted drug delivery systems to combat brain cancer. Cancer Cell. Int. 25, 150. doi:10.1186/s12935-025-03731-z
Boarescu, P.-M., Boarescu, I., Bocşan, I. C., Gheban, D., Bulboacă, A. E., Nicula, C., et al. (2019a). Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants (Basel) 8, 504. doi:10.3390/antiox8100504
Boarescu, P.-M., Boarescu, I., Bocşan, I. C., Pop, R. M., Gheban, D., Bulboacă, A. E., et al. (2019b). Curcumin nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules 24, 2802. doi:10.3390/molecules24152802
Chai, M., Gao, B., Wang, S., Zhang, L., Pei, X., Yue, B., et al. (2025). Leveraging plant-derived nanovesicles for advanced nucleic acid-based gene therapy. Theranostics 15, 324–339. doi:10.7150/thno.104507
Chang, X., Zhang, Q., Huang, Y., Liu, J., Wang, Y., Guan, X., et al. (2024). Quercetin inhibits necroptosis in cardiomyocytes after ischemia-reperfusion via DNA-PKcs-SIRT5-orchestrated mitochondrial quality control. Phytother. Res. 38, 2496–2517. doi:10.1002/ptr.8177
Chatterjee, M., and Chanda, N. (2022). Formulation of PLGA nano-carriers: specialized modification for cancer therapeutic applications. Mat. Adv. 3, 837–858. doi:10.1039/D1MA00600B
Chen, C., Liu, W., Gu, X., Zhang, L., Mao, X., Chen, Z., et al. (2024). Baicalin-loaded Polydopamine modified ZIF-8 NPs inhibits myocardial ischemia/reperfusion injury in rats. J. Biomater. Sci. Polym. Ed. 35, 1863–1878. doi:10.1080/09205063.2024.2358640
Cui, J., Wang, X., Dong, L., and Wang, Q. (2024a). Curcumin reduces myocardial ischemia-reperfusion injury, by increasing endogenous H2S levels and further modulating m6A. Mol. Biol. Rep. 51, 558. doi:10.1007/s11033-024-09478-6
Cui, J.-K., Fan, M., and Wang, Q. (2024b). Curcumin reduces hypoxia/reperfusion injury of cardiomyocytes byStimulating vascular endothelial cells to secrete FGF2. Comb. Chem. High. Throughput Screen 27, 2101–2109. doi:10.2174/0113862073239166231103102648
Ding, Y., Li, W., Peng, S., Zhou, G., Chen, S., Wei, Y., et al. (2023). Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting ferroptosis. Biol. Pharm. Bull. 46, 524–532. doi:10.1248/bpb.b22-00174
Donato, M., Evelson, P., and Gelpi, R. J. (2017). Protecting the heart from ischemia/reperfusion injury: an update on remote ischemic preconditioning and postconditioning. Curr. Opin. Cardiol. 32, 784–790. doi:10.1097/HCO.0000000000000447
Dong, L., Shen, Z., Chi, H., Wang, Y., Shi, Z., Fang, H., et al. (2023). Research progress of Chinese medicine in the treatment of myocardial ischemia-reperfusion injury. Am. J. Chin. Med. 51, 1–17. doi:10.1142/S0192415X23500015
Dong, L., Zhao, Y., Luo, J., Li, X., Wang, S., Li, M., et al. (2024). Carbon dots derived from curcumae radix and their heartprotective effect. IJN 19, 3315–3332. doi:10.2147/IJN.S444125
Dong, Z., Guo, J., Xing, X., Zhang, X., Du, Y., and Lu, Q. (2017). RGD modified and PEGylated lipid nanoparticles loaded with puerarin: formulation, characterization and protective effects on acute myocardial ischemia model. Biomed. Pharmacother. 89, 297–304. doi:10.1016/j.biopha.2017.02.029
Du, L., Lu, H., Xiao, Y., Guo, Z., and Li, Y. (2024). Protective effect and pharmacokinetics of dihydromyricetin nanoparticles on oxidative damage of myocardium. PLoS One 19, e0301036. doi:10.1371/journal.pone.0301036
Feng, X., Sureda, A., Jafari, S., Memariani, Z., Tewari, D., Annunziata, G., et al. (2019). Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics 9, 1923–1951. doi:10.7150/thno.30787
Gao, F., Zhao, Y., Zhang, B., Xiao, C., Sun, Z., Gao, Y., et al. (2022). Mitochondrial targeted astaxanthin liposomes for myocardial ischemia-reperfusion injury based on oxidative stress. J. Biomater. Appl. 37, 303–314. doi:10.1177/08853282221087102
Gao, S., Liu, Z., Li, H., Little, P. J., Liu, P., and Xu, S. (2012). Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 220, 3–10. doi:10.1016/j.atherosclerosis.2011.06.041
Gao, Y., Li, Z., Sun, M., Li, H., Guo, C., Cui, J., et al. (2010). Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev. Ind. Pharm. 36, 1225–1234. doi:10.3109/03639041003695139
Han, D., Wang, F., Jiang, Q., Qiao, Z., Zhuang, Y., An, Q., et al. (2024). Enhancing cardioprotection through neutrophil-mediated delivery of 18β-glycyrrhetinic acid in myocardial ischemia/reperfusion injury. Adv. Sci. (Weinh) 11, e2406124. doi:10.1002/advs.202406124
Han, Y., Wang, H., Wang, Y., Dong, P., Jia, J., and Yang, S. (2021). Puerarin protects cardiomyocytes from ischemia-reperfusion injury by upregulating LncRNA ANRIL and inhibiting autophagy. Cell. Tissue Res. 385, 739–751. doi:10.1007/s00441-021-03463-2
Hu, F., Hu, T., Qiao, Y., Huang, H., Zhang, Z., Huang, W., et al. (2024). Berberine inhibits excessive autophagy and protects myocardium against ischemia/reperfusion injury via the RhoE/AMPK pathway. Int. J. Mol. Med. 53, 49. doi:10.3892/ijmm.2024.5373
Huang, Q., Yao, Y., Wang, Y., Li, J., Chen, J., Wu, M., et al. (2023). Ginsenoside Rb2 inhibits p300-mediated SF3A2 acetylation at lysine 10 to promote Fscn1 alternative splicing against myocardial ischemic/reperfusion injury. J. Adv. Res. S2090-1232 (23), 365–379. doi:10.1016/j.jare.2023.12.012
Huang, X., Deng, H., Shen, Q.-K., and Quan, Z.-S. (2022). Tanshinone IIA: pharmacology, total synthesis, and progress in structure-modifications. Curr. Med. Chem. 29, 1959–1989. doi:10.2174/0929867328666211108110025
Jia, X., Shao, W., and Tian, S. (2022). Berberine alleviates myocardial ischemia-reperfusion injury by inhibiting inflammatory response and oxidative stress: the key function of miR-26b-5p-mediated PTGS2/MAPK signal transduction. Pharm. Biol. 60, 652–663. doi:10.1080/13880209.2022.2048029
Jing, J., Fang, S., Li, Y., Liu, W., Wang, C., Lan, Y., et al. (2024). An enhanced cardio-protective effect of nanoparticles loaded with active components from Polygonum orientale L. against isoproterenol-induced myocardial ischemia in rats. Int. J. Pharm. 655, 124047. doi:10.1016/j.ijpharm.2024.124047
Kim, H., Lee, J. H., Kim, J. E., Kim, Y. S., Ryu, C. H., Lee, H. J., et al. (2018). Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 42, 361–369. doi:10.1016/j.jgr.2017.12.003
Lan, M., Hou, M., Yan, J., Deng, Q., Zhao, Z., Lv, S., et al. (2022). Cardiomyocyte-targeted anti-inflammatory nanotherapeutics against myocardial ischemia reperfusion (IR) injury. Nano Res. 15, 9125–9134. doi:10.1007/s12274-022-4553-6
Lei, W., Yan, Y., Ma, Y., Jiang, M., Zhang, B., Zhang, H., et al. (2022). Notoginsenoside R1 regulates ischemic myocardial lipid metabolism by activating the AKT/mTOR signaling pathway. Front. Pharmacol. 13, 905092. doi:10.3389/fphar.2022.905092
Li, F., Li, D., Tang, S., Liu, J., Yan, J., Chen, H., et al. (2021a). Quercetin protects H9c2 cardiomyocytes against oxygen-glucose deprivation/reoxygenation-induced oxidative stress and mitochondrial apoptosis by regulating the ERK1/2/DRP1 signaling pathway. Evid. Based Complement. Altern. Med. 2021, 7522175. doi:10.1155/2021/7522175
Li, H., Li, M., Fu, J., Ao, H., Wang, W., and Wang, X. (2021b). Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 28, 1226–1236. doi:10.1080/10717544.2021.1927244
Li, H., Zhu, J., Xu, Y.-W., Mou, F.-F., Shan, X.-L., Wang, Q.-L., et al. (2022a). Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 54, 102384. doi:10.1016/j.redox.2022.102384
Li, L., Wang, Y., Guo, R., Li, S., Ni, J., Gao, S., et al. (2020). Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J. Control Release 317, 259–272. doi:10.1016/j.jconrel.2019.11.032
Li, M., Wu, J., Yang, T., Zhao, Y., Ren, P., Chang, L., et al. (2024). Engineered biomimetic nanoparticles-mediated targeting delivery of allicin against myocardial ischemia-reperfusion injury by inhibiting ferroptosis. Int. J. Nanomedicine 19, 11275–11292. doi:10.2147/IJN.S478276
Li, X., Wang, G., Sun, J., Hao, H., Xiong, Y., Yan, B., et al. (2007). Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional Chinese medicine (TCM) in rats. Biol. Pharm. Bull. 30, 847–851. doi:10.1248/bpb.30.847
Li, Y., Hao, H., Yu, H., Yu, L., Ma, H., and Zhang, H. (2022b). Ginsenoside Rg2 ameliorates myocardial ischemia/reperfusion injury by regulating TAK1 to inhibit necroptosis. Front. Cardiovasc Med. 9, 824657. doi:10.3389/fcvm.2022.824657
Liu, C., Zhang, X., Yang, H., Zhao, M., Liu, Y., Zhao, R., et al. (2024). PEG-modified nano liposomes co-deliver Apigenin and RAGE-siRNA to protect myocardial ischemia injury. Int. J. Pharm. 649, 123673. doi:10.1016/j.ijpharm.2023.123673
Liu, C.-J., Yao, L., Hu, Y.-M., and Zhao, B.-T. (2021a). Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism. IJN 16, 741–752. doi:10.2147/IJN.S277377
Liu, Y., Li, L., Wang, Z., Zhang, J., and Zhou, Z. (2023). Myocardial ischemia-reperfusion injury; Molecular mechanisms and prevention. Microvasc. Res. 149, 104565. doi:10.1016/j.mvr.2023.104565
Liu, Y., Song, Y., Li, S., and Mo, L. (2021b). Cardioprotective effect of quercetin against ischemia/reperfusion injury is mediated through NO system and mitochondrial K-ATP channels. Cell. J. 23, 184–190. doi:10.22074/cellj.2021.7183
Liu, Y., Xie, X., Chen, H., Hou, X., He, Y., Shen, J., et al. (2020). Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomedicine 29, 102237. doi:10.1016/j.nano.2020.102237
Long, T., Pan, W., Li, F., Sheikh, S. A., Xie, Q., and Zhang, C. (2023). Berberine up-regulates miR-340-5p to protect myocardial ischaemia/reperfusion from HMGB1-mediated inflammatory injury. Esc. Heart Fail 10, 931–942. doi:10.1002/ehf2.14235
Lozano, O., Lázaro-Alfaro, A., Silva-Platas, C., Oropeza-Almazán, Y., Torres-Quintanilla, A., Bernal-Ramírez, J., et al. (2019). Nanoencapsulated quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxid. Med. Cell. Longev. 2019, 7683051. doi:10.1155/2019/7683051
Mao, S., Wang, L., Chen, P., Lan, Y., Guo, R., and Zhang, M. (2018). Nanoparticle-mediated delivery of Tanshinone IIA reduces adverse cardiac remodeling following myocardial infarctions in a mice model: role of NF-κB pathway. Artif. Cells Nanomed Biotechnol. 46, S707–S716. doi:10.1080/21691401.2018.1508028
Mi, X., Hu, M., Dong, M., Yang, Z., Zhan, X., Chang, X., et al. (2021). Folic acid decorated zeolitic imidazolate framework (ZIF-8) loaded with baicalin as a nano-drug delivery system for breast cancer therapy. Int. J. Nanomedicine 16, 8337–8352. doi:10.2147/IJN.S340764
Nabofa, W. E. E., Alashe, O. O., Oyeyemi, O. T., Attah, A. F., Oyagbemi, A. A., Omobowale, T. O., et al. (2018). Cardioprotective effects of curcumin-nisin based poly lactic acid nanoparticle on myocardial infarction in Guinea pigs. Sci. Rep. 8, 16649. doi:10.1038/s41598-018-35145-5
Nagarajan, A. G., Neeley, N., Doroodian, P., Olazabel, A., and Kupfer, G. (2023). Genome wide siRNA screen identifies CGAS-sting pathway as a pharmacological target that promotes survival of hematopoietic stem cells deficient in fanconi genes. Blood 142, 1083. doi:10.1182/blood-2023-187180
Pan, W., Xue, B., Yang, C., Miao, L., Zhou, L., Chen, Q., et al. (2018). Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 129, 272–282. doi:10.1016/j.fitote.2018.06.001
Pei, H., Wu, Y., Wei, Y., Yang, Y., Teng, S., and Zhang, H. (2014). Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta-analysis of 11 randomized trials. PLoS One 9, e115500. doi:10.1371/journal.pone.0115500
Piscatelli, J. A., Ban, J., Lucas, A. T., and Zamboni, W. C. (2021). Complex factors and challenges that affect the pharmacology, safety and efficacy of nanocarrier drug delivery systems. Pharmaceutics 13, 114. doi:10.3390/pharmaceutics13010114
Plumb, R. S., Gethings, L. A., Rainville, P. D., Isaac, G., Trengove, R., King, A. M., et al. (2023). Advances in high throughput LC/MS based metabolomics: a review. TrAC Trends Anal. Chem. 160, 116954. doi:10.1016/j.trac.2023.116954
Qin, G.-W., Lu, P., Peng, L., and Jiang, W. (2021). Ginsenoside Rb1 inhibits cardiomyocyte autophagy via PI3K/Akt/mTOR signaling pathway and reduces myocardial ischemia/reperfusion injury. Am. J. Chin. Med. 49, 1913–1927. doi:10.1142/S0192415X21500907
Qiu, J., Cai, G., Liu, X., and Ma, D. (2017). αvβ3 integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed. Pharmacother. 96, 1418–1426. doi:10.1016/j.biopha.2017.10.086
Safiri, S., Karamzad, N., Singh, K., Carson-Chahhoud, K., Adams, C., Nejadghaderi, S. A., et al. (2022). Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990-2019. Eur. J. Prev. Cardiol. 29, 420–431. doi:10.1093/eurjpc/zwab213
Sarhene, M., Ni, J. Y., Duncan, E. S., Liu, Z., Li, S., Zhang, J., et al. (2021). Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol. Res. 166, 105481. doi:10.1016/j.phrs.2021.105481
Schäfer, A., König, T., Bauersachs, J., and Akin, M. (2022). Novel therapeutic strategies to reduce reperfusion injury after acute myocardial infarction. Curr. Probl. Cardiol. 47, 101398. doi:10.1016/j.cpcardiol.2022.101398
Song, D., Hao, J., and Fan, D. (2020). Biological properties and clinical applications of berberine. Front. Med. 14, 564–582. doi:10.1007/s11684-019-0724-6
Sun, G., Lin, X., Hong, Y., Feng, Y., Ruan, K., and Xu, D. (2012). PEGylation for drug delivery to ischemic myocardium: pharmacokinetics and cardiac distribution of poly(ethylene glycol)s in mice with normal and ischemic myocardium. Eur. J. Pharm. Sci. 46, 545–552. doi:10.1016/j.ejps.2012.04.010
Tan, H., Song, Y., Chen, J., Zhang, N., Wang, Q., Li, Q., et al. (2021). Platelet-like fusogenic liposome-mediated targeting delivery of miR-21 improves myocardial remodeling by reprogramming macrophages post myocardial ischemia-reperfusion injury. Adv. Sci. (Weinh) 8, e2100787. doi:10.1002/advs.202100787
Tong, Q., Zhu, P.-C., Zhuang, Z., Deng, L.-H., Wang, Z.-H., Zeng, H., et al. (2019). Notoginsenoside R1 for organs ischemia/reperfusion injury: a preclinical systematic review. Front. Pharmacol. 10, 1204. doi:10.3389/fphar.2019.01204
Upadhaya, S., Madala, S., Baniya, R., Subedi, S. K., Saginala, K., and Bachuwa, G. (2017). Impact of cyclosporine A use in the prevention of reperfusion injury in acute myocardial infarction: a meta-analysis. Cardiol. J. 24, 43–50. doi:10.5603/CJ.a2016.0091
Voronkov, N. S., Popov, S. V., Naryzhnaya, N. V., Prasad, N. R., Petrov, I. M., Kolpakov, V. V., et al. (2024). Effect of cold adaptation on the state of cardiovascular system and cardiac tolerance to ischemia/reperfusion injury. Iran. Biomed. J. 28, 59–70. doi:10.61186/ibj.3872
Wang, D., Bu, T., Li, Y., He, Y., Yang, F., and Zou, L. (2022). Pharmacological activity, pharmacokinetics, and clinical research progress of puerarin. Antioxidants (Basel) 11, 2121. doi:10.3390/antiox11112121
Wang, J., Liu, Y., Liu, Y., Huang, H., Roy, S., Song, Z., et al. (2023a). Recent advances in nanomedicines for imaging and therapy of myocardial ischemia-reperfusion injury. J. Control Release 353, 563–590. doi:10.1016/j.jconrel.2022.11.057
Wang, K., Feng, X., Chai, L., Cao, S., and Qiu, F. (2017). The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 49, 139–157. doi:10.1080/03602532.2017.1306544
Wang, Q., Song, Y., Chen, J., Li, Q., Gao, J., Tan, H., et al. (2021). Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 276, 121028. doi:10.1016/j.biomaterials.2021.121028
Wang, Y., Li, F., Wei, S., Li, W., Wu, J., Li, S., et al. (2024a). Puerarin-loaded liposomes Co-modified by ischemic myocardium-targeting peptide and triphenylphosphonium cations ameliorate myocardial ischemia-reperfusion injury. Int. J. Nanomedicine 19, 7997–8014. doi:10.2147/IJN.S468394
Wang, Y., Li, S., Li, W., Wu, J., Hu, X., Tang, T., et al. (2024b). Cardiac-targeted and ROS-responsive liposomes containing puerarin for attenuating myocardial ischemia-reperfusion injury. Nanomedicine (Lond) 19, 2335–2355. doi:10.1080/17435889.2024.2402678
Wang, Y., Wang, Q., Wang, X., Yao, P., Dai, Q., Qi, X., et al. (2023b). Docetaxel-loaded pH/ROS dual-responsive nanoparticles with self-supplied ROS for inhibiting metastasis and enhancing immunotherapy of breast cancer. J. Nanobiotechnology 21, 286. doi:10.1186/s12951-023-02013-y
Wang, Y., Wang, Y., Wang, X., and Hu, P. (2018). Tilianin-loaded reactive oxygen species-scavenging nano-micelles protect H9c2 cardiomyocyte against hypoxia/reoxygenation-induced injury. J. Cardiovasc Pharmacol. 72, 32–39. doi:10.1097/FJC.0000000000000587
Wang, Y., Zheng, J., Xiao, X., Feng, C., Li, Y., Su, H., et al. (2024c). Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury by inhibiting inflammation and apoptosis through PI3K/akt signaling pathway. Am. J. Chin. Med. 52, 433–451. doi:10.1142/S0192415X24500186
Wang, Z.-K., Chen, R.-R., Li, J.-H., Chen, J.-Y., Li, W., Niu, X.-L., et al. (2020). Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: the role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 89, 107086. doi:10.1016/j.intimp.2020.107086
Wei, D., Yang, H., Zhang, Y., Zhang, X., Wang, J., Wu, X., et al. (2022). Nano-traditional Chinese medicine: a promising strategy and its recent advances. J. Mater Chem. B 10, 2973–2994. doi:10.1039/D2TB00225F
Wei, Q., Xiao, Y., Du, L., and Li, Y. (2024). Advances in nanoparticles in the prevention and treatment of myocardial infarction. Molecules 29, 2415. doi:10.3390/molecules29112415
Welt, F. G. P., Batchelor, W., Spears, J. R., Penna, C., Pagliaro, P., Ibanez, B., et al. (2024). Reperfusion injury in patients with acute myocardial infarction: JACC scientific statement. J. Am. Coll. Cardiol. 83, 2196–2213. doi:10.1016/j.jacc.2024.02.056
Wu, H.-J., Zhang, K., Ma, J.-J., Wang, L., and Zhuang, Y. (2021). Mechanism of curcumin against myocardial ischaemia-reperfusion injury based on the P13K/Akt/mTOR signalling pathway. Eur. Rev. Med. Pharmacol. Sci. 25, 5490–5499. doi:10.26355/eurrev_202109_26658
Xie, Y., Shen, X., Xu, F., and Liang, X. (2024). Research progress of nano-delivery systems for the active ingredients from traditional Chinese medicine. Phytochem. Anal. doi:10.1002/pca.3381
Xu, H., Hu, M., Liu, M., An, S., Guan, K., Wang, M., et al. (2020). Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 235, 119769. doi:10.1016/j.biomaterials.2020.119769
Xu, H., Zhang, X., Shi, Y., Yu, K., and Jiang, Y. (2022). Notoginsenoside R1 relieves the myocardial infarction via activating the JAK2/STAT3 signaling pathway in vivo and in vitro. Bioengineered 13, 5653–5662. doi:10.1080/21655979.2022.2037366
Xu, H.-X., Pan, W., Qian, J.-F., Liu, F., Dong, H.-Q., and Liu, Q.-J. (2019). MicroRNA-21 contributes to the puerarin-induced cardioprotection via suppression of apoptosis and oxidative stress in a cell model of ischemia/reperfusion injury. Mol. Med. Rep. 20, 719–727. doi:10.3892/mmr.2019.10266
Xu, S., Wu, B., Zhong, B., Lin, L., Ding, Y., Jin, X., et al. (2021). Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 12, 10924–10934. doi:10.1080/21655979.2021.1995994
Xu, X., Diao, Z., Zhao, B., Xu, H., Yan, S., and Chen, H. (2024). Protective activities of silver nanoparticles containing Panax japonicus on apoptotic, inflammatory, and oxidative alterations in isoproterenol-induced cardiotoxicity. Open Chem. 22, 20240006. doi:10.1515/chem-2024-0006
Xue, Y., Fu, W., Liu, Y., Yu, P., Sun, M., Li, X., et al. (2020). Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J. Food Sci. 85, 4039–4049. doi:10.1111/1750-3841.15505
Xue, Y., Fu, W., Yu, P., Li, Y., Yu, X., Xu, H., et al. (2023). Ginsenoside Rc alleviates myocardial ischemia-reperfusion injury by reducing mitochondrial oxidative stress and apoptosis: role of SIRT1 activation. J. Agric. Food Chem. 71, 1547–1561. doi:10.1021/acs.jafc.2c06926
Yalikong, A., Li, X.-Q., Zhou, P.-H., Qi, Z.-P., Li, B., Cai, S.-L., et al. (2021). A triptolide loaded HER2-targeted nano-drug delivery system significantly suppressed the proliferation of HER2-positive and BRAF mutant colon cancer. Int. J. Nanomedicine 16, 2323–2335. doi:10.2147/IJN.S287732
Yan, L., Pan, C.-S., Liu, Y.-Y., Cui, Y.-C., Hu, B.-H., Chang, X., et al. (2021). The composite of 3, 4-dihydroxyl-phenyl lactic acid and notoginsenoside R1 attenuates myocardial ischemia and reperfusion injury through regulating mitochondrial respiratory chain. Front. Physiol. 12, 538962. doi:10.3389/fphys.2021.538962
Yan, S., Yue, Y., Zeng, L., Jiang, C., Li, W., Li, H., et al. (2019). Ligustrazine nanoparticles nano spray’s activation on Nrf2/ARE pathway in oxidative stress injury in rats with postoperative abdominal adhesion. Ann. Transl. Med. 7, 379. doi:10.21037/atm.2019.07.72
Yang, C., Yang, S., Fang, S., Li, L., Jing, J., Liu, W., et al. (2023). PLGA nanoparticles enhanced cardio-protection of scutellarin and paeoniflorin against isoproterenol-induced myocardial ischemia in rats. Int. J. Pharm. 648, 123567. doi:10.1016/j.ijpharm.2023.123567
Yang, K.-T., Chao, T.-H., Wang, I.-C., Luo, Y.-P., Ting, P.-C., Lin, J.-H., et al. (2022). Berberine protects cardiac cells against ferroptosis. Tzu Chi Med. J. 34, 310–317. doi:10.4103/tcmj.tcmj_236_21
Yao, C., Shi, X., Lin, X., Shen, L., Xu, D., and Feng, Y. (2015). Increased cardiac distribution of mono-PEGylated Radix Ophiopogonis polysaccharide in both myocardial infarction and ischemia/reperfusion rats. Int. J. Nanomedicine 10, 409–418. doi:10.2147/IJN.S73462
Ye, J., Lyu, T.-J., Li, L.-Y., Liu, Y., Zhang, H., Wang, X., et al. (2023). Ginsenoside re attenuates myocardial ischemia/reperfusion induced ferroptosis via miR-144-3p/SLC7A11. Phytomedicine 113, 154681. doi:10.1016/j.phymed.2023.154681
Yu, W., Ding, J., Chen, J., Jiang, Y., Zhao, J., Liu, J., et al. (2024). Magnesium ion-doped mesoporous bioactive glasses loaded with gallic acid against myocardial ischemia/reperfusion injury by affecting the biological functions of multiple cells. Int. J. Nanomedicine 19, 347–366. doi:10.2147/IJN.S444751
Zeng, J.-J., Shi, H.-Q., Ren, F.-F., Zhao, X.-S., Chen, Q.-Y., Wang, D.-J., et al. (2023). Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol. Sin. 44, 1366–1379. doi:10.1038/s41401-023-01057-y
Zhang, J., Han, X., Li, X., Luo, Y., Zhao, H., Yang, M., et al. (2012). Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int. J. Nanomedicine 7, 4299–4310. doi:10.2147/IJN.S32385
Zhang, J., Liu, S., Jiang, L., Hou, J., and Yang, Z. (2022). Curcumin improves cardiopulmonary resuscitation outcomes by modulating mitochondrial metabolism and apoptosis in a rat model of cardiac arrest. Front. Cardiovasc Med. 9, 908755. doi:10.3389/fcvm.2022.908755
Zhang, S., Li, J., Hu, S., Wu, F., and Zhang, X. (2018). Triphenylphosphonium and D-α-tocopheryl polyethylene glycol 1000 succinate-modified, tanshinone IIA-loaded lipid-polymeric nanocarriers for the targeted therapy of myocardial infarction. Int. J. Nanomedicine 13, 4045–4057. doi:10.2147/IJN.S165590
Zhang, S., Xia, J., Zhu, Y., Dong, M., and Wang, J. (2024). Establishing Salvia miltiorrhiza-derived exosome-like nanoparticles and elucidating their role in angiogenesis. Molecules 29, 1599. doi:10.3390/molecules29071599
Zhang, Y.-M., Zhang, Z.-Y., and Wang, R.-X. (2020). Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 11, 956. doi:10.3389/fphys.2020.00956
Zheng, Y., Wang, Y., Xia, M., Gao, Y., Zhang, L., Song, Y., et al. (2022). The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: review on nanotechnology in TCM-based drug delivery systems. Drug Deliv. Transl. Res. 12, 1306–1325. doi:10.1007/s13346-021-01029-x
Zhou, Y., Liang, Q., Wu, X., Duan, S., Ge, C., Ye, H., et al. (2023). siRNA delivery against myocardial ischemia reperfusion injury mediated by reversibly camouflaged biomimetic nanocomplexes. Adv. Mater 35, e2210691. doi:10.1002/adma.202210691
Zhou, Y.-X., Zhang, H., and Peng, C. (2014). Puerarin: a review of pharmacological effects. Phytother. Res. 28, 961–975. doi:10.1002/ptr.5083
Zhou, Y.-X., Zhang, H., and Peng, C. (2021). Effects of puerarin on the prevention and treatment of cardiovascular diseases. Front. Pharmacol. 12, 771793. doi:10.3389/fphar.2021.771793
Zhu, K., Yao, Y., Wang, K., Shao, F., Zhu, Z., Song, Y., et al. (2023a). Berberin sustained-release nanoparticles were enriched in infarcted rat myocardium and resolved inflammation. J. Nanobiotechnol 21, 33. doi:10.1186/s12951-023-01790-w
Zhu, P.-C., Shen, J., Qian, R.-Y., Xu, J., Liu, C., Hu, W.-M., et al. (2023b). Effect of tanshinone IIA for myocardial ischemia/reperfusion injury in animal model: preclinical evidence and possible mechanisms. Front. Pharmacol. 14, 1165212. doi:10.3389/fphar.2023.1165212
Zhu, T., and Wan, Q. (2023). Pharmacological properties and mechanisms of Notoginsenoside R1 in ischemia-reperfusion injury. Chin. J. Traumatol. 26, 20–26. doi:10.1016/j.cjtee.2022.06.008
Keywords: nanoparticles, myocardial ischemia-reperfusion injury, plant-derived secondary metabolites, nanocarriers, targeting strategies
Citation: Shi W, Xu Y, Wei J, Zhang X, Zhu S, Guo H, Huang Q, Qi C, Hua T, Liu Y and Yang M (2025) Plant-derived secondary metabolites and nanotechnology: innovative strategies and emerging challenges in myocardial ischemia-reperfusion injury therapy. Front. Pharmacol. 16:1529478. doi: 10.3389/fphar.2025.1529478
Received: 17 November 2024; Accepted: 19 May 2025;
Published: 29 May 2025.
Edited by:
Chen Huei Leo, National University of Singapore, SingaporeReviewed by:
Guang Chen, The University of Hong Kong, Hong Kong, SAR ChinaYuxiang Fei, China Pharmaceutical University, China
Copyright © 2025 Shi, Xu, Wei, Zhang, Zhu, Guo, Huang, Qi, Hua, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Yang, eWFuZ21pbkBhaG11LmVkdS5jbg==; Yue Liu, bGl1eXVlaGVhcnRAaG90bWFpbC5jb20=
 Wei Shi
Wei Shi Yang Xu1,2
Yang Xu1,2 Xiaoyu Zhang
Xiaoyu Zhang Shuaijie Zhu
Shuaijie Zhu Yue Liu
Yue Liu Min Yang
Min Yang