Abstract
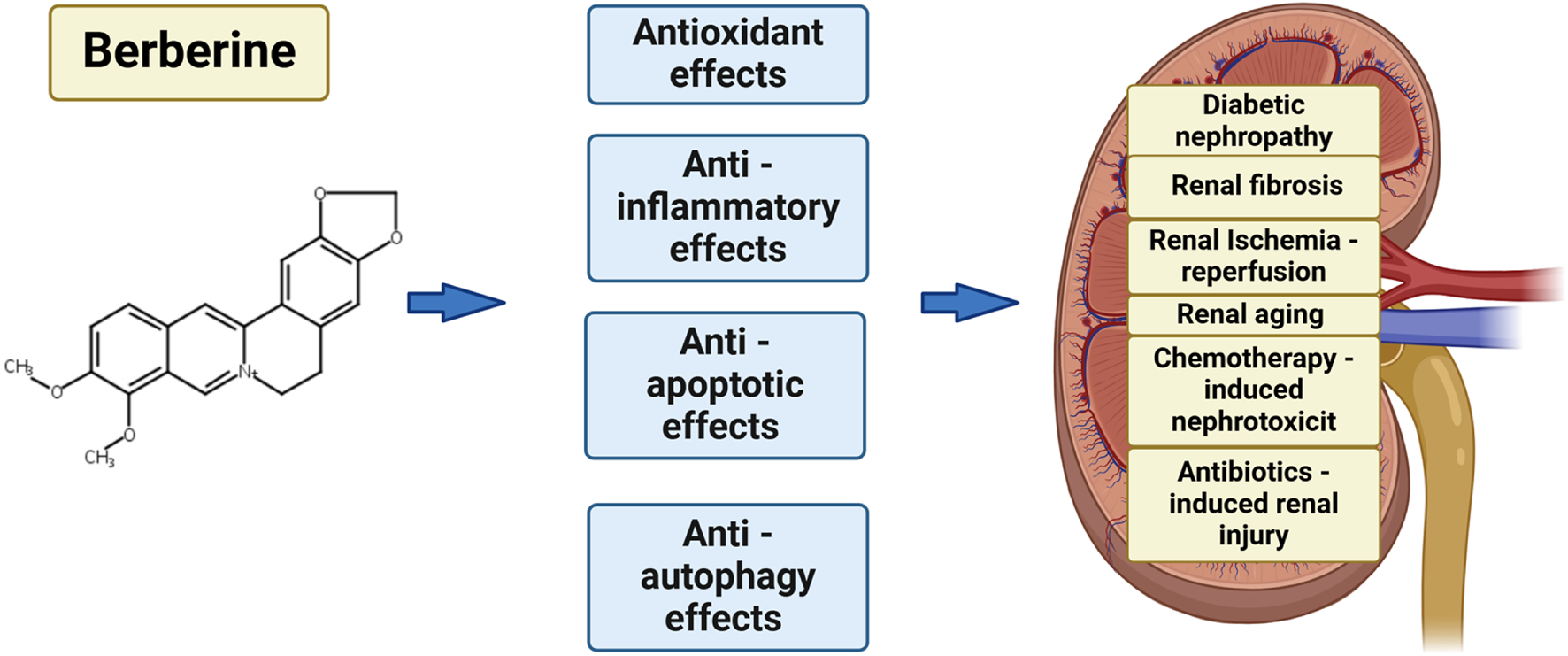
Berberine (BBR) is a pentacyclic benzylisoquinoline alkaloid widely distributed across various medicinal plants. Recent studies have demonstrated that berberine possesses a broad spectrum of pharmacological activities, including not only antioxidant properties but also the ability to lower blood glucose, modulate lipid profiles, and mitigate inflammation. These findings suggest that berberine holds significant potential as a therapeutic agent for renal diseases, highlighting its substantial research value. Moreover, when administered orally, berberine has been shown to exhibit a wide therapeutic safety margin. Several studies have identified berberine’s renoprotective effects across a range of kidney disorders, including diabetic nephropathy, renal fibrosis, renal aging, kidney toxicity induced by chemotherapy and antibiotics. These properties underscore berberine’s evolving therapeutic potential for both acute kidney injury (AKI) and chronic kidney disease (CKD). In summary, the research discussed in this article provides a comprehensive overview of the renoprotective effects of BBR and elucidates the molecular mechanisms underlying its therapeutic potential in the treatment of various renal disease. Furthermore, the article underscores the significance of berberine as a promising therapeutic agent for the treatment of kidney disorders.
Graphical Abstract
1 Introduction
Renal damage is generally categorized into two main forms: acute kidney injury (AKI) and chronic kidney disease (CKD). The etiopathogenesis of intrinsic AKI may be attributed to various factors, including renovascular causes resulting from conditions such as vasculitis or vascular stenosis, glomerular involvement typically linked to immune complex-mediated diseases like systemic lupus erythematosus, and interstitial damage induced by inflammation, ischemia or nephrotoxic agents such as chemotherapeutic agents, nonsteroidal anti-inflammatory drugs (NSAIDs), and aminoglycoside (Radi, 2018; Radi et al., 2018a; Radi et al., 2018b). CKD affects approximately 10%–13% of the global population and is distinguished by its insidious, progressive course, which frequently culminates in an increased susceptibility to cardiovascular complications. In its early stages, CKD typically remains asymptomatic, with patients only exhibiting symptoms related to renal failure in advanced stages (Jager and Fraser, 2017; Levey and Coresh, 2012). Despite the availability of advanced diagnostic and supportive interventions, AKI and CKD continue to exhibit high morbidity and mortality rates, primarily due to the limited efficacy of current therapeutic options. Consequently, there is a critical need to identify and develop more effective therapeutic strategies to both treat and mitigate the progression of renal diseases.
BBR is an alkaloid compound belonging to the isoquinoline class, commonly utilized in traditional Chinese and Ayurvedic medicine. It is derived from a range of plant species within the Berberidaceae, Papaveraceae, and Ranunculaceaefamilies. BBR has garnered significant attention owing to its broad range of pharmacological effects and diverse mechanisms of action. The earliest documented use of Rhizoma coptidis, a source of berberine, for medicinal purposes dates back to the year 200 A.D (Feng et al., 2019). BBR is a bioactive alkaloid with a millennia-long history of therapeutic applications in traditional medicine. It demonstrates a wide array of therapeutic properties, including antioxidative, anti-inflammatory, and anti-apoptotic activities (Lin and Zhang, 2018; Wang et al., 2017). Recent studies have increasingly highlighted berberine’s potential to confer protective effects against ischemia-reperfusion (I/R) injury in multiple organs, including the heart, brain, kidneys, intestines, liver,and testes (Kazaz et al., 2020; Gu et al., 2013). BBR has demonstrated nephroprotective effects across a diverse spectrum of renal disorders, including those associated with ischemia-reperfusion injury (Zhang et al., 2020), kidney fibrosis (Shao et al., 2021), medication or toxin induced injury (Ibrahim Fouad and Ahmed, 2021), kidney stones (Bashir and Gilani, 2011) and kidney aging (El-Horany et al., 2020). This review delves into the molecular pathways underlying the renoprotective properties of berberine in the context of various nephropathies.
2 Berberine
2.1 Chemical and physical properties of BBR
BBR is readily extracted from various traditional medicinal plants, where it is broadly dispersed across the roots, stems, and bark of species with notable pharmacological value. These include Berberis vulgaris, Coptis chinensis and Berberis aristata. The genus Berberis represents the most extensively distributed natural reservoir of BBR, with its concentration in medicinal plants ranging significantly from 0.05 mg/g to 96.10 mg/g. Among documented species, C. chinensis exhibits the highest BBR content, followed by Berberis asiatica and Coptis teeta (Lu et al., 2018). Barberry and other berberine-rich plants have been integral to nearly all traditional medical systems, with their therapeutic use tracing back over 3,000 years in Ayurvedic, Iranian, Chinese and Egyptianand medicine practices (Sarraf et al., 2019).
BBR is a chemically stable quaternary ammonium isoquinoline alkaloid, characterized by the molecular formula C20H18NO4 and the molecular weight is 336.37 g/mol (Feng et al., 2019). Free BBR exhibits limited solubility in water, dissolves readily in hot ethanol, and shows minimal solubility in low-polarity organic solvents for instance chloroform and benzene. Its hydrochloride form has reduced water solubility but dissolves more efficiently in boiling water, while its phosphate derivatives and sulfate are comparatively more water-soluble (Feng et al., 2019).
Despite its extremely low concentration in the bloodstream (Hua et al., 2007), BBR exhibits a bioavailability of less than 1% (Kheir et al., 2010), the pharmacological efficacy of BBR is closely associated with its extensive distribution across tissues. Previous studies have demonstrated that BBR readily crosses the blood–brain barrier, exhibiting rapid accumulation in the hippocampus following intravenous administration, with subsequent slow clearance (Wang et al., 2005). Moreover, research has demonstrated that the levels of BBR and its pharmacologically active metabolites are significantly elevated in various organs compared to their levels in the bloodstream following oral administration. BBR exhibits rapid tissue distribution, with the highest accumulation observed in the liver, followed by the kidneys, lungs heart and pancreas, while minimal distribution occurs in adipose tissue, where levels remain relatively stable for up to 48 h (Tan et al., 2013).
Extensive scientific research indicates that berberine undergoes metabolic transformation primarily through demethylation, glucuronidation, and sulfonation (Ahmed et al., 2015). Notably, the pharmacological properties of its metabolites align closely with those of the parent compound (Wang et al., 2017). The primary active metabolites of BBR can be classified into four major types: berberrubine, thalifendine, demethyleneberberine, and jatrorrhizine (Liu et al., 2009). The oral bioavailability of berberrubine may surpass that of BBR. These findings suggest that investigating the metabolically active derivatives of berberine could offer a promising avenue for enhancing its therapeutic efficacy. Finally, with regard to excretion, BBR is predominantly eliminated via urine and bile in its metabolized forms. In addition, BBR exhibits a robust safety profile, with clinical trials reporting a paucity of adverse events (Cheng et al., 2022).
2.2 Pharmacological and therapeutic effects of BBR
For many years, comprehensive investigations have been undertaken to explore the biological activities of BBR, with clinical studies highlighting its diverse range of pharmacological properties. Numerous studies have emphasized the therapeutic potential of BBR across various domains, including its roles as an anticancer (Nishino et al., 1986), antihyperglycemic (Jiang et al., 2015), antioxidant (Shirwaikar et al., 2006), and anti-inflammatory (Kuo et al., 2004). Additionally, numerous studies have demonstrated that BBR possessescardioprotective, hepatoprotective and neuroprotective properties. Notably, BBR has shown nephroprotective efficacy in various models of AKI and CKD, underscoring its potential as a therapeutic agent for renal disorders (Figure 1).
FIGURE 1
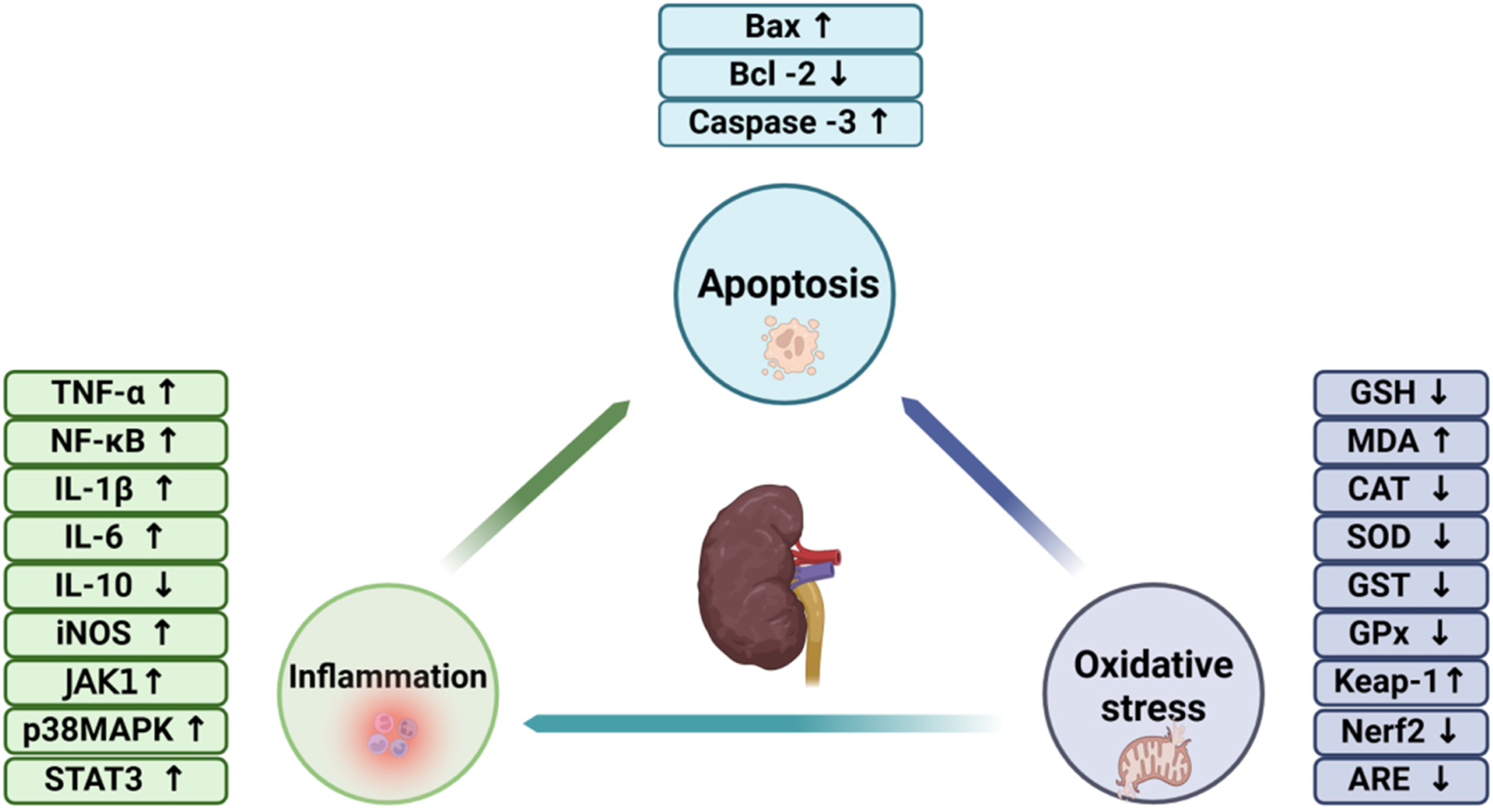
The involvement of oxidative stress, inflammation, and apoptosis in the pathogenesis of renal disorders.
2.2.1 Oxidative stress and renal diseases
At physiological concentrations, reactive oxygen species (ROS) function as key signaling mediators, orchestrating a variety of critical cellular processes (Finkel, 2011). However, excessive ROS production perturbs redox balance, resulting in oxidative damage to key biomolecules, including DNA, lipids and proteins (Liguori et al., 2018). It is broadly estimated that individual human cells experience approximately 105 oxidative assaults daily from ROS, including hydroxyl radicals. Hydroxyl radicals (Valko et al., 2004), capable of interacting with all components of DNA are the principal agents driving DNA damage (Lombardi et al., 1998). Such damage results in genetic alterations, including chromosomal rearrangements and mutations, which can ultimately drive cellular senescence. Renal tubular epithelial cells are closely linked to the progression of renal senescence (Guyton and Kensler, 1993). Elevated levels of tubular epithelial cell senescence have been observed in animal models of AKI induced by ischemia-reperfusion injury (IRI) and CKD caused by unilateral ureteral obstruction (UUO), as well as in patients with CKD (Kim et al., 2021). Senescent cells secrete a range of cytokines, including transforming growth factor-β (TGF-β), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8), collectively termed the senescence-associated secretory phenotype (SASP), which exerts profound effects on the function of adjacent cells and tissues (Takahashi et al., 2018; Freund et al., 2010). The SASP drives inflammatory and pro-fibrotic responses, stimulating the activation of renal interstitial fibroblasts. This activation leads to excessive matrix protein synthesis, disrupting the normal architecture of renal tissues and facilitating fibrotic scar formation (Xu et al., 2020).
2.2.2 Inflammation and renal diseases
Inflammation, a central pathological mechanism underlying both AKI and CKD, is characterized by complex interactions among dendritic cells, macrophages, and circulating leukocytes. This process is closely linked to the activation of nuclear factor-κB (NF-κB) signaling and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasomes, along with the release of pro-inflammatory cytokines, which can result in irreversible tissue damage (Andrade-Oliveira et al., 2019). What’s more, both in vivo and in vitro investigations have elucidated that NF-κB activation in resident glomerular cells plays a pivotal role in driving the progression of renal damage. Numerous stimuli trigger the activation of the canonical NF-κB pathway, which modulates the expression of various cytokines. Members of the TNF superfamily and angiotensin II (Ang II) are prominent activators of NF-κB in renal pathologies (Sanz et al., 2010). In the streptozotocin (STZ)-induced rat model of diabetic nephropathy, Zhu et al. reported that berberine alleviated renal injury by attenuating Toll-like receptor 4 (TLR4)-dependent NF-κB-mediated inflammation (Zhu et al., 2018). The chemokines and pro-inflammatory cytokines produced by tubular epithelial cells serve as key components of the stress response, as well as mechanisms involved in tissue repair and restoration. Nevertheless, persistent renal inflammation can lead to the progression of AKI and CKD. In systemic conditions like sepsis, tubular epithelial cells play a pivotal role in the overproduction and release of inflammatory mediators, which contribute to damage in both the kidneys and remote organs.
2.2.3 Apoptosis and renal disease
Apoptosis, evident through podocyte depletion and tubular cell attrition, exacerbates the decline in renal epithelial cells, a hallmark of both AKI and CKD (Hughes et al., 2004; Lorz et al., 2006). Notably, the kidney appears to be particularly responsive to the protective effects of anti-apoptotic molecules (Lorz et al., 2006). The role of autophagy in AKI has been primarily explored in tubular epithelial cells and podocytes. Initial evidence, derived from a renal I/R injury rat model, revealed upregulation of autophagy-related proteins, including light chain 3 (LC3) and Beclin1, within proximal and distal epithelial cells. Moreover, it has been demonstrated that enhanced expression of B-cell lymphoma-XL (Bcl-XL) in the kidney effectively suppresses both autophagy activation and apoptotic processes (Sureshbabu et al., 2015).
Consistent with this finding, autophagosomes were detected in murine renal tubular cells following I/R injury. It has also been reported that autophagic flux is elevated during the reperfusion phase. Similarly, studies utilizing kidney tubule-specific autophagy-deficient mouse models have provided more conclusive evidence regarding this process. In podocytes, elevated basal autophagic activity is essential for maintaining normal cellular homeostasis (Mizushima et al., 2004). Conditional knockout of autophagy-related genes, including autophagy-related protein 5 (Atg5) or autophagy-related protein 7 (Atg7), in mice led to pronounced vacuolization in both podocytes and tubular cells, ultimately contributing to the development of focal segmental glomerulosclerosis and renal dysfunction (Kawakami et al., 2015).
3 The protective effects of BBR in kidney diseases
3.1 Diabetic nephropathy
Diabetic nephropathy (DN), a major microvascular complication of diabetes mellitus, is the foremost cause of end-stage renal disease (ESRD) worldwide (Yan et al., 2007). It is estimated that approximately one-third of individuals with diabetes, irrespective of whether they have type 1 or type 2 diabetes, are affected by this condition. In the United States, 20%–40% of individuals with diabetes develop DN, which is the leading cause of ESRD (Reutens and Atkins, 2011).
Hyperglycemia impairs renal capillary dilation, induces podocyte depletion, and triggers oxidative stress within the nephron’s tubular system. Additionally, as filtrate albumin levels rise, excessive tubular reabsorption ensues, leading to inflammatory and fibrotic responses that contribute to the progressive decline of renal function (Ibrahim and Hostetter, 1997). The anti-inflammatory and antifibrotic effects of BBR play a role in alleviating diabetic nephropathy.
Initially, the inhibition of the NF-κB signaling pathway is observed. In a STZ-induced DN rat model, BBR alleviated renal damage by reducing fasting blood glucose levels, kidney-to-body weight ratio, 24-h proteinuria, creatinine (Cr) and blood urea nitrogen (BUN) concentrations. BBR mitigated systemic and renal cortical inflammation in STZ-induced DN models and high glucose (HG)-stimulated podocytes by downregulating the TLR4/NF-κB signaling pathway (Zhu et al., 2018). One study demonstrated that, in the context of diabetes, BBR reduces fibronectin (FN) expression in mesangial cells by targeting the sphingosine 1-phosphate (S1P) receptor subtype 2, an effect potentially linked to its suppression of NF-κB activation (Huang et al., 2012). Additionally, recent research highlights BBR’s ability to mitigate type 2 diabetes by attenuating NF-κB-mediated renal inflammation and inhibiting the TGF/Smad3 signaling pathway (Sun et al., 2015).
Secondly, mesangial cell proliferation was effectively attenuated. Excessive proliferation of glomerular mesangial cells represents a key pathological hallmark of DN. Recent research demonstrated that Huang-Gui solid dispersion, an innovative berberine-based formulation, mitigates diabetic nephropathy by inhibiting mesangial matrix expansion in the kidney and enhancing autophagic activity, potentially through the activation of Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) phosphorylation (Zhang et al., 2020).
Thirdly, berberine exhibits potent anti-inflammatory properties. In the kidneys of DN hamster models, the NLRP3–Caspase-1–Gasdermin D (GSDMD) signaling pathway was found to be upregulated. BBR mitigates oxidative stress-induced damage by enhancing the antioxidative activity of nuclear factor erythroid 2-Related Factor 2 (Nrf2), which subsequently modulates the NLRP3–Caspase-1–GSDMD axis. This regulation inhibits pyroptosis and counters inflammation-driven renal injury in DN (Ding et al., 2021). Berberine suppresses HG-induced epithelial-to-mesenchymal transition (EMT) and renal interstitial fibrosis by inhibiting the activation of the NLRP3 inflammasome. These findings suggest the potential of berberine as a novel therapeutic agent for managing tubulointerstitial fibrosis associated with diabetic nephropathy (Ma et al., 2022).
Fourthly, BBR exhibits anti-fibrotic properties. Proliferation of glomerular mesangial cells represents a key pathological feature in DN. In alignment with this, BBR mitigates renal tubular EMT and renal interstitial fibrosis by modulating the Notch/snail signaling pathway (Yang et al., 2017).
Despite these promising effects in DN, the underlying molecular mechanisms and specific pharmacological targets remain poorly elucidated. Consequently, future research should focus on identifying precise therapeutic targets and characterizing the alterations in downstream signaling pathways, enabling berberine to exert a pivotal role in the treatment of DN by targeting these pathways.
3.2 Renal fibrosis
Renal fibrosis (RF) is a prevalent feature of numerous chronic kidney diseases, characterized by enhanced extracellular matrix (ECM) synthesis and impaired degradation, which together facilitate intercellular matrix interactions. Therefore, BBR may confer renal protection by modulating these cellular interactions and their associated molecular pathways (Figure 2).
FIGURE 2
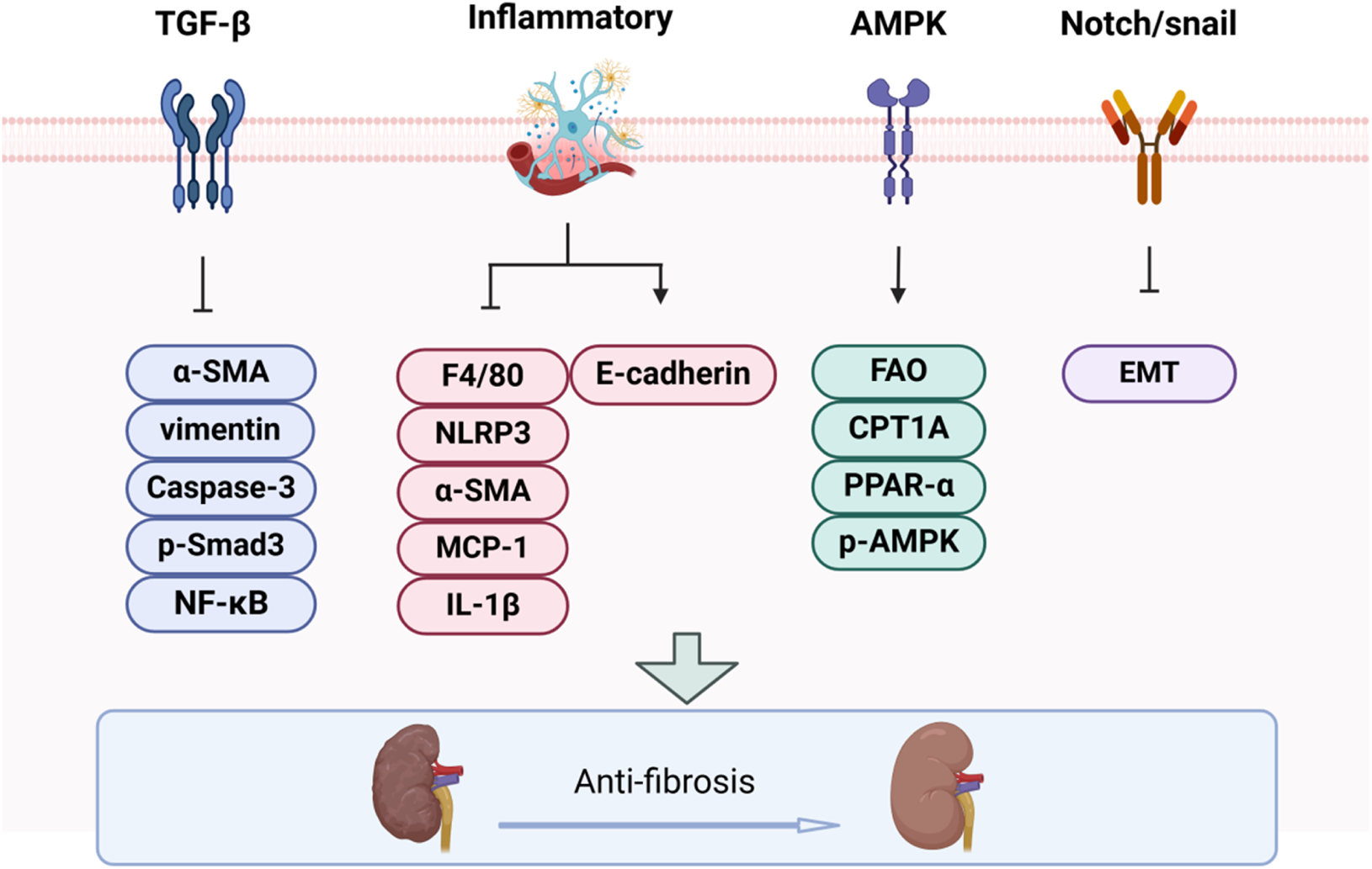
The impact of BBR on renal fibrosis progression.
3.2.1 TGF-β1/Smad signaling pathway
The TGF-β1/Smad signaling axis is pivotal in the progression of renal fibrosis and constitutes a core mechanism in its pathophysiology (Meng et al., 2016). In diabetic rat models, berberine exerts its anti-inflammatory and antifibrotic effects through the modulation of Smad7 expression. What’s more, it inhibits ECM deposition, alleviates glomerular basement membrane thickening, and mitigates renal tubular atrophy, thus attenuating various histopathological injuries associated with diabetes (Sun et al., 2015). In both diabetic rat and unilateral ureteral obstruction models, berberine treatment also attenuated the TGF-β/Smad3 signaling pathway, thereby mitigating renal fibrosis (Sun et al., 2015; Wang et al., 2014). Epiberberine, a potent derivative of berberine, ameliorates renal fibrosis by modulating the Agt-TGF-β/Smad2 signaling pathway (Xiao et al., 2021). Additionally, berberine mitigates tubulointerstitial fibrosis in DN through activation of the Nrf2 pathway and suppression of TGF-β/Smad/EMT signaling (Zhang et al., 2016).
3.2.2 Inflammatory
The pathogenesis of RF primarily involves inflammation-driven injury to tubular epithelial cells (TECs), and modulation of the inflammatory response has the potential to attenuate or reverse the progression of RF (Song et al., 2024; Tan et al., 2023). BBR mitigated interstitial fibrosis and histopathological injury by suppressing NLRP3 inflammasome activation and IL-1β production. This intervention decreased the expression of MCP-1, F4/80, α-SMA, collagen I and collagen IV, while upregulating E-cadherin levels. Additionally, BBR alleviated serum creatinine and urea nitrogen abnormalities in both in vivo and in vitro models (Song et al., 2024; Tan et al., 2023).
3.2.3 AMPK pathway
AMPK is instrumental in the initiation and progression of renal interstitial fibrosis. It regulates mitochondrial fatty acid oxidation (FAO) to compensate for diminished adenosine triphosphate (ATP) levels (Li et al., 2022). BBR treatment restored the phosphorylation of AMPK in the kidney and reversed the downregulation of FAO-associated proteins, including peroxiisome proliferator-activated receptor alpha (PPAR-α) and carnitine palmitoyltransferase 1A (CPT1A), both in vitro and in vivo (Tan et al., 2023). Other drugs may also exert their renal protective effects through AMPK-mediated mechanisms, such as autophagy (Dorweiler et al., 2007) and oxidative stress (Chertow et al., 2005).
3.2.4 Notch/snail pathway
The Notch signaling pathway has been implicated in the regulation of cellular fibrosis, including EMT in DN, and is closely associated with TGF-β1 signaling (Yang et al., 2017). The expression of Snail1 is directly modulated by Notch signaling, and the Notch/Snail pathway plays a critical role in the pathogenesis of renal interstitial fibrosis in DN (Yang et al., 2017). BBR attenuated HG-induced EMT, downregulated Notch and Snail1 expression in renal tubular epithelial cells, and effectively inhibited renal interstitial fibrosis and tubular EMT both in vitro and in vivo (Yang et al., 2017).
3.3 Ischemia-reperfusion induced renal injury
IRI arises when blood supply is reinstated following a period of ischemia, leading to tissue and organ injury. In clinical settings, IRI represents a prevalent pathological mechanism (Jochmans et al., 2017). Renal ischemia is a primary etiology of acute renal failure (ARF), contributing to substantial morbidity and mortality. In addition, AKI has become a significant public health concern, with escalating incidence rates and limited therapeutic options (Neri et al., 2017). In both clinical and experimental studies, IRI precipitates multi-organ dysfunction, contributing to elevated mortality rates (Yu et al., 2013; Xie et al., 2017). Especially, a pioneering study by Yu et al. examined the renoprotective effects of BBR on human renal proximal tubular cells exposed to hypoxia/reoxygenation (H/R) injury. In this study, I/R induced significant damage to HK-2 cells, as demonstrated by reduced cell viability and heightened oxidative stress. Moreover, there was an upregulation of mitochondrial injury-related proteins, accompanied by an increase in both the apoptotic rate and expression of endoplasmic reticulum stress-associated proteins. BBR treatment was shown to significantly mitigate the abnormalities induced by H/R injury (Tait et al., 2015). In another study, Xie et al. demonstrated that berberine nanoparticles (BBR-NP) effectively protect TECs from renal IRI in rats. The research revealed that both BBR and BBR-NP alleviated renal damage, both functionally and morphologically, by reducing ROS, mitigating mitochondrial dysfunction, and attenuating apoptosis in renal cells (Yao et al., 2007).
3.4 Kidney aging
Although global life expectancy continues to rise, this does not necessarily correlate with an improvement in the overall quality of life. The aging process is marked by a gradual yet progressive decline in the functional capacity of multiple organs, impairing their ability to sustain baseline tissue homeostasis and adequately respond to physiological demands under stress (Chválová et al., 2007). Notably, El-Horany et al. explored the protective effects of BBR against kidney aging induced by D-galactose (D-gal) in rats.In this investigation, BBR significantly attenuated urea and serum creatinine (Scr) concentrations, reversed histopathological alterations in the kidneys, and restored redox balance, as evidenced by reduced levels of malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine, alongside the activation of heme oxygenase-1 (HO-1). Moreover, berberine markedly reduced serum levels of pro-inflammatory mediators, downregulated phosphatase and tensin homolog (PTEN) expression, while enhancing Akt hosphorylation and upregulating Bcl-2 protein levels (El-Horany et al., 2020).
3.5 Chemotherapy-induced renal injury
3.5.1 Cisplatin-induced nephrotoxicity
Cisplatin (CP) is a prominent chemotherapeutic agent utilized in the treatment of solid tumors (Fuertes et al., 2003). As an alkylating compound, CP binds to DNA, inducing intrastrand crosslinking and the formation of adducts, which distort the DNA structure and hinder the process of DNA replication (Domitrović et al., 2013). Furthermore, cisplatin-induced cytotoxicity involves mitochondrial dysfunction, reduced ATPase activity, and disruption of cellular transport processes (Malaviya, 2016). In addition to its effects on tumor cells, cisplatin’s cytotoxic activity extends to normal somatic cells, particularly within the kidneys. The kidneys serve as the primary excretory route for cisplatin, with proximal tubular cells being the principal sites of drug accumulation (Yao et al., 2007). Interestingly, berberine has been shown to confer nephroprotection against cisplatin-induced renal injury, as evidenced by Domitrovi et al., through the reduction of oxidative and nitrosative stress, modulation of inflammatory responses, and suppression of apoptotic signaling (Vardi et al., 2013).
3.5.2 Methotrexate-induced renal injury
Methotrexate (MTX), a potent anti-metabolite and folate antagonist, is administered at elevated doses for the treatment of various cancers, while lower doses are utilized for managing non-malignant conditions (Howard et al., 2016). MTX exerts its effects by inhibiting DNA synthesis through the suppression of dihydrofolate reductase (Hassanein et al., 2019). However, the nephrotoxic effects associated with MTX therapy represent a significant clinical challenge, primarily due to the direct tubular toxicity induced by its metabolite, 7-hydroxy-MTX. This nephrotoxicity can lead to compromised renal function, treatment delays, and substantial morbidity (Cheng et al., 2010). Hassanein et al. investigated the pivotal roles of the Bax/Bcl2/caspase-3, Keap1/Nrf2, and P38MAPK/NF-κB signaling pathways in mediating the protective effects of berberine against methotrexate-induced nephropathy (Cardoso et al., 2008).
3.5.3 Doxorubicin-induced renal intoxication
Doxorubicin (DOX), a chemotherapeutic agent classified as an anthracycline, is a highly effective therapeutic used in the management of various hematological cancers and solid tumors (Tangpong et al., 2011). Despite its efficacy in cancer treatment, the clinical use of DOX is significantly constrained by its severe toxicities on off-target organs (Kalender et al., 2005; Bárdi et al., 2007). While the cardiac toxicity of DOX is well-documented, emerging research highlights its detrimental effects on additional organs, including the kidney, liver, and brain (Lopez-Novoa et al., 2011; Adil et al., 2016). Ibrahim Fouad G et al. found that DOX induced nephrotoxicity, characterized by marked elevations in serum urea, creatinine, and kidney injury molecule-1 (KIM-1) levels. Furthermore, DOX induced oxidative stress by elevating renal levels of MDA and hydrogen peroxide (H2O2), while concurrently reducing catalase (CAT) activity in the kidneys. DOX induced renal fibrosis and elevated levels of TGF-β1and increased collagen deposition. Additionally, DOX promoted apoptosis and inflammation in renal tissues, as demonstrated by upregulated expression of caspase-3 and NF-κB, respectively. These pathological alterations were significantly mitigated by concurrent BBR administration. Co-treatment with BBR effectively attenuated DOX-induced inflammatory response, oxidative stress, renal fibrosis and apoptosis (Ibrahim Fouad and Ahmed, 2021).
3.5.4 Antibiotics-induced renal injury
Gentamicin (GM), an aminoglycoside antibiotic, is widely utilized in clinical settings for managing severe Gram-negative bacterial infections. Nonetheless, its clinical application is constrained by its potential to induce significant nephrotoxicity. Renal proximal tubule cells serve as the primary site of accumulation for aminoglycoside antibiotics, where they exert nephrotoxic effects via interaction with specific membrane transporters (Viljoen et al., 2019). Adil et al. explored the impact of BBR on gentamicin-induced nephrotoxicity in a rat model. In this study, GM administration resulted in a significant increase in BUN, Scr, and renal levels of MDA, KIM-1, nitric oxide (NO), NF-κB and neutrophil gelatinase-associated lipocalin (NGAL). Additionally, GM treatment markedly reduced renal superoxide dismutase (SOD) activity and depleted mitochondrial glutathione (GSH), nicotinamide adenine dinucleotide (NADH) dehydrogenase, Bcl-2, and cytochrome-c (Cyt-C) oxidase levels. BBR co-treatment reversed these alterations in a dose-dependent manner, restoring antioxidant defense mechanisms, attenuating inflammation, apoptosis, and markers of AKI, and modulating mitochondrial enzymatic functions (Jha et al., 2013).
3.6 Kidney stone
Kidney stone is a widespread health concern associated with a poor prognosis, often necessitating surgical intervention (Viljoen et al., 2019). Studies have demonstrated that BBR exerts its antiurolithic effects through multiple mechanisms. Similar to hydrochlorothiazide, BBR promotes diuresis, modulates urinary potential of hydrogen, and enhances Na⁺ and K⁺ excretion while reducing Ca2⁺ levels. In a rat model of ethylene glycol-induced calcium oxalate urolithiasis, BBR not only prevented and dissolved tubular crystal deposits but also significantly preserved renal function and mitigated oxidative stress. Additionally, in vivo experiments have confirmed that BBR increases urine output and alkalinity while lowering Ca2⁺ excretion, further supporting its nephroprotective potential (Bashir and Gilani, 2011).
3.7 Hypertensive renal impairment
In many developing countries, CKD is most typically associated with diabetes and/or hypertension (Jha et al., 2013). Wan et al. demonstrated in rats that BBR mitigates chronic renal damage caused by atherosclerotic renovascular disease through the suppression of the NF-κB signaling pathway. BBR significantly reduced blood pressure, low-density lipoprotein cholesterol, urinary albumin, and malondialdehyde levels. Additionally, treatment with BBR in the atherosclerotic renovascular disease model resulted in markedly lower expression levels of TGF-β1 compared to the untreated group, likely through the inhibition of NF-κB-DNA binding activity (Wan et al., 2013). Kishimoto et al. explored the impact of BBR on adipose tissue and renal function using 3T3-L1 cells and spontaneously hypertensive rats. In vitro, BBR supplementation suppressed the expression of CCAAT/enhancer-binding proteins α and β, as well as PPAR-γ, while downregulating PPAR target genes and inhibiting the differentiation of 3T3-L1 fibroblasts into adipocytes. In hypertensive rats, BBR administration led to a significant reduction in adipose tissue mass and alleviated renal injury (Kishimoto et al., 2015).
3.8 Uric acid nephropathy
Uric acid nephropathy, or gouty nephropathy, is a metabolic disorder primarily driven by hyperuricemia. Epidemiological data indicate a rising prevalence of Uric acid nephropathy, closely mirroring the increasing incidence of hyperuricemia, a trend associated with improvements in lifestyle and overall quality of life (Gong et al., 2024). The study demonstrated that BBR exerts an anti-hyperuricemic effect, partially through the modulation of urate transporter expression and the inhibition of the JAK2/STAT3 signaling pathway (Pan et al., 2023). Gong et al. Explored BBR binds to red blood cells, is identified and internalized by monocytes, and subsequently accumulates in damaged renal tissue. Upon entry, it activates AMPK, suppresses NF-κB-mediated inflammatory signaling, inhibits macrophage polarization (Imenshahidi and Hosseinzadeh, 2016).
Recent studies have found that BBR can also relieve renal fibrosis through the gut–kidney axis. Pan et al. identified gut microbiota modulation as a key nephroprotective mechanism of berberine. Their study explored BBR’s therapeutic effects in CKD and demonstrated that it reduces gut-derived uremic toxins while reshaping intestinal microbiota composition. Notably, BBR was found to inhibit the microbial metabolism of tyrosine, thereby suppressing p-cresol production, a process potentially linked to its ability to restrain Clostridium proliferation. These findings provide the first evidence that BBR mitigates CKD through the gut-kidney axis, highlighting its considerable potential as a therapeutic agent for CKD management (Liu et al., 2016).
4 Toxicity of BBR
BBR is generally regarded as safe at standard dosages, exhibiting minimal toxicity and adverse effects (Imenshahidi and Hosseinzadeh, 2019; Chitra et al., 2013). Clinical studies have primarily reported mild gastrointestinal disturbances, such as diarrhea and constipation, as the most common side effects (Germoush and Mahmoud, 2014). On the one hand, BBR has the potential to mitigate the adverse effects and toxicities associated with various chemotherapeutic and analgesic agents, including cyclophosphamide and cisplatin (Vardi et al., 2013; Feng et al., 2018; Holy et al., 2009). On the other hand, under certain conditions, BBR may induce adverse effects. For instance, a dose of 10 mg/kg BBR has been shown to impair immune function in murine models[98–100]. Additionally, interactions between BBR and macrolides or statins can result in cardiac arrhythmias and diminish the therapeutic efficacy of these drugs (Zhi et al., 2015). To date, there have been no documented instances of severe adverse reactions associated with oral BBR administration in clinical settings. In addition, BBR has been deemed safe for the majority of human subjects in both short-term and long-term clinical trials. In conclusion, based on conventional dosages and indications, BBR is considered safe for oral administration.
5 Conclusion
Renal and urological disorders can affect individuals of any age and at any time. In this review, we aim to provide a comprehensive analysis of BBR’s protective effects across a range of renal pathologies. The renoprotective effects of berberine are largely attributed to its ability to mitigate oxidative stress, suppress inflammatory pathways, and inhibit apoptotic processes, as evidenced by the reviewed literature (Table 1). Several studies have demonstrated that BBR exerts substantial renoprotective effects across a range of renal disorders, including diabetic nephropathy, renal fibrosis, renal aging, chemotherapy-induced renal injuryand kidney toxicity induced by chemotherapy and antibiotics. These findings suggest that BBR holds significant and evolving therapeutic potential. Furthermore, extensive research on BBR’s clinical use in humans has established its safety profile, even at higher dosages, in the treatment of various diseases. Consequently, further investigations into the renoprotective properties of berberine in patients with AKI and CKD are warranted. Therefore, future research should prioritize additional clinical trials as well as in vivo and in vitro studies. It is anticipated that the accumulation of robust scientific evidence will significantly enhance the guidance for clinical treatment and prevention. Of course BBR’s potential use in combination therapies with other nephroprotective agents or its role in chronic kidney disease management should also be strengthened. Concurrently, these advancements will strengthen the theoretical foundation for the management of AKI and CKD, ultimately facilitating the standardization of berberine-based therapeutic strategies for various renal disorders.
TABLE 1
| Disease | Animals or cells model | Effects | Pathway | References |
|---|---|---|---|---|
| Diabetic nephropathy | STZ-induced DN rats | Mitigate oxidative stress, suppress inflammatory pathways and inhibit apoptotic processes | TLR4/NF-κB pathway | Zhu et al. (2018) |
| Diabetic nephropathy | STZ-induced DN rats | Suppress the elevation of fibronectin levels triggered by the S1P2 receptor | SphK1/S1P/S1P2 pathway | Huang et al. (2012) |
| Diabetic nephropathy | STZ-induced DN rats | Inhibit renal inflammation blocking the upregulation of pro-inflammatory cytokines (IL-1β, TNFα) and chemokine (MCP-1) | TGF-β/Smad3 pathway | Sun et al. (2015) |
| Diabetic nephropathy | High-fat diet and STZ induced DN rats and Leprdb/db mice | Inhibit glomerular mesangial matrix expansion, activate autophagy and activate of AMPK phosphorylation | Activate AMPK phosphorylation | Zhang et al. (2020) |
| Diabetic nephropathy | High-sugar, high-fat diet and STZ induced DN rats | Regulate NLRP3-Caspase-1-GSDMD signalling pathway, inhibit kidney cell pyroptosis and antagonize DN inflammatory damage | Nrf2-NLRP3-Caspase-1-GSDMD pathway | Ding et al. (2021) |
| Diabetic nephropathy | High-fat diet and STZ induced DN rats and HK2 cell | Suppress the NLRP3 inflammasome | NLRP3 inflammasome | Ma et al. (2022) |
| Diabetic nephropathy | DN model KKAy mice and Mouse renal tubular epithelial cells (mRTECs) | Suppress renal tubular epithelial EMT, reduce renal interstitial fibrosis | Notch/snail pathway | Yang et al. (2017) |
| Renal fibrosis | UUO in rats | Inhibit of oxidative stress, inflammatory responses, and TGF-β1/pSmad3 signalling | TGF-β1/pSmad3 pathway | Wang et al. (2014) |
| Renal fibrosis | db/db mice and high-glucose induced glomerular mesangial cells (GMCs) | Reduce Agt, TGFβ1, and Smad2 expression in vitro and in vivo | Agt-TGFβ/Smad2 pathway | Xiao et al. (2021) |
| Renal fibrosis | STZ-induce diabetic mice and normal rat kidney tubular epithelial (NRK 52E) cells | Activate Nrf2 pathway and inhibit TGF-β/Smad/EMT signaling activity | TGF-β/Smad/EMT pathway | Zhang et al. (2016) |
| Renal fibrosis | UUO in rats and HK-2 cells | Deactivate of the NLRP3 inflammasome and protection of TECs by reversing defective FAO | Activate AMPK | Tan et al. (2023) |
| Renal fibrosis | High-fat diet (HFD)-fed mice | Suppress macrophage migration towards adipocytes by activating SIRT3 | Activate the deacetylase SIRT3 | Li et al. (2022) |
| IRI induced injury | HK-2 cells | Attenuat oxidative stress and apoptosis link to mitochondrial stress | ER stress pathways | Yu et al. (2013) |
| IRI induced injury | IRI in rats | Suppress ROS, mitochondrial dysfunction and apoptosis | ROS and apoptosis | Xie et al. (2017) |
| Kidney aging | D-gal-induced kidney aging in rats | Reduce the serum levels of pro-inflammatory mediators, along with downregulation of PTEN expression, enhanced Akt activity | PTEN/Akt pathway | El-Horany et al. (2020) |
| Chemotherapy induced renal injury | CDDP-induced renal injury in BALB/cN mice | Suppress oxidative, inflammation and apoptosis | Oxidative, inflammation, apoptosis | Domitrović et al. (2013) |
| Chemotherapy induced renal injury | MTX-induced renal injury in rats | Anti-oxidant and anti-inflammatory and anti-apoptotic | Bax/Bcl2/caspase-3, Keap1/Nrf2, and P38MAPK/NF-κB pathway | Hassanein et al. (2019) |
| Chemotherapy induced renal injury | DOX-induced acute toxicity in rats | Suppress oxidative stress | Oxidative stress | Ibrahim Fouad and Ahmed (2021) |
| Antibiotics-induced renal injury | GNT-induced renal intoxication in rats | Attenuat of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction | Oxidative, inflammation, apoptosis | Adil et al. (2016) |
| kidney stone | Ca-oxalate urolithiasis induced by ethylene glycol in rats | Attenuat renal oxidative stress | Oxidative stress | Bashir and Gilani (2011) |
| Hypertensive renal impairment | Renal injury induced by atherosclerotic renovascular disease in rats | Inhibit the NF-κB signalling pathway, attenuat renal oxidative stress | NF-κB signalling pathway, oxidative stress | Borghi et al. (2020) |
| Hypertensive renal impairment | 3T3-L1 cells and spontaneously hypertensive rats | Suppress of PPAR target genes and inhibit of differentiation of 3T3-Ll fibroblast to adipocytes | PPAR signalling pathway | Lin et al. (2021) |
| Uric acid nephropathy | PO/HX induced Uric acid nephropathy in mice | Regulate urate transporter expressions and JAK2/STAT3 signaling pathway | JAK2/STAT3 signaling pathway | Pan et al. (2023) |
| Uric acid nephropathy | THP-1 cells and PO/HX induced Uric acid nephropathy in mice | Activate AMPK, inhibit the activation of inflammatory pathway NF-κB and regulate macrophage polarization | NF-κB signalling pathway | Imenshahidi and Hosseinzadeh (2016) |
An overview of the renoprotective effects of berberine across various renal diseases and the underlying molecular mechanisms.
Statements
Author contributions
ZF: Writing–original draft, Writing–review and editing. XuW: Investigation, Methodology, Writing–review and editing. XZ: Conceptualization, Methodology, Writing–review and editing. KY: Methodology, Project administration, Writing–review and editing. LT: Resources, Software, Writing–review and editing. XiW: Project administration, Writing–review and editing. YD: Funding acquisition, Methodology, Project administration, Writing–review and editing. LY: Funding acquisition, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The investigation protocol was approved by the (Jilin Development and Reform Commission) under Grant (number 2018C052-9); (Department of Science and Technology of Jilin Province) under Grant (number 20190201248JC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- BBR
Berberine
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- I/R
Ischemia-reperfusion
- ROS
Reactive oxygen species
- IRI
Ischemia-reperfusion injury
- UUO
Unilateral ureteral obstruction
- TGF-β
Transforming growth factor-β
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- SASP
Senescence-associated secretory phenotype
- NF-κB
Nuclear factor-κB
- NLRP3
NOD-like receptor thermal protein domain associated protein 3
- Ang II
Angiotensin II
- STZ
Streptozotocin
- TLR4
Toll-like receptor 4
- LC3
Light chain 3
- Bcl-XL
B-cell lymphoma-XL
- Atg5
Autophagy-related protein
- Atg7
Autophagy-related protein 7
- DN
Diabetic nephropathy
- ESRD
End-stage renal disease
- Cr
Creatinine
- BUN
Blood urea nitrogen
- HG
High glucose
- FN
Fibronectin
- S1P
Sphingosine 1-phosphate
- AMPK
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- GSDMD
Gasdermin D
- Nrf2
Nuclear factor erythroid 2-Related Factor 2
- EMT
Epithelial-to-mesenchymal transition
- RF
Renal fibrosis
- ECM
Extracellular matrix
- TECs
Tubular epithelial cells
- FAO
Fatty acid oxidation
- ATP
Adenosine triphosphate
- PPAR-α
Peroxiisome proliferator-activated receptor alpha
- CPT1A
Carnitine palmitoyltransferase 1A
- ARF
Acute renal failure
- H/R
Hypoxia/reoxygenation
- HK-2
Human kidney-2
- BBR-NP
Berberine nanoparticles
- D-gal
D-galactose
- MDA
Malondialdehyde
- HO-1
Heme oxygenase-1
- PTEN
Phosphatase and tensin homolog
- Bcl-2
B-cell lymphoma-2
- CP
Cisplatin
- MTX
Methotrexate
- DOX
Doxorubicin
- KIM-1
Kidney injury molecule-1
- H2O2
Hydrogen peroxide
- CAT
Catalase
- GM
Gentamicin
- NO
Nitric oxide
- NGAL
Neutrophil gelatinase-associated lipocalin
- SOD
Superoxide dismutase
- GSH
Glutathione
- NADH
Nicotinamide adenine dinucleotide
- Cyt-C
Cytochrome-c
References
1
Adil M. Kandhare A. D. Dalvi G. Ghosh P. Venkata S. Raygude K. S. et al (2016). Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail38, 996–1006. 10.3109/0886022X.2016.1165120
2
Ahmed T. Gilani A. U. Abdollahi M. Daglia M. Nabavi S. F. Nabavi S. M. (2015). Berberine and neurodegeneration: a review of literature. Pharmacol. Rep.67, 970–979. 10.1016/j.pharep.2015.03.002
3
Andrade-Oliveira V. Foresto-Neto O. Watanabe I. K. M. Zatz R. Câmara N. O. S. (2019). Inflammation in renal diseases: new and old players. Front. Pharmacol.10, 1192. 10.3389/fphar.2019.01192
4
Bárdi E. Bobok I. A V. O. Kappelmayer J. Kiss C. (2007). Anthracycline antibiotics induce acute renal tubular toxicity in children with cancer. Pathol. Oncol. Res.13, 249–253. 10.1007/BF02893506
5
Bashir S. Gilani A. H. (2011). Antiurolithic effect of berberine is mediated through multiple pathways. Eur. J. Pharmacol.651, 168–175. 10.1016/j.ejphar.2010.10.076
6
Borghi C. Agabiti-Rosei E. Johnson R. J. Kielstein J. T. Lurbe E. Mancia G. et al (2020). Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern Med.80, 1–11. 10.1016/j.ejim.2020.07.006
7
Cardoso S. Santos R. X. Carvalho C. Correia S. Pereira G. C. Pereira S. S. et al (2008). Doxorubicin increases the susceptibility of brain mitochondria to Ca(2+)-induced permeability transition and oxidative damage. Free Radic. Biol. Med.45, 1395–1402. 10.1016/j.freeradbiomed.2008.08.008
8
Cheng C. Xue W. Diao H. Xia S. Zuo L. He A. et al (2010). Antitumor activity and toxicological properties of doxorubicin conjugated to [alpha],[beta]-poly[(2-hydroxyethyl)-L-aspartamide] administered intraperitoneally in mice. Anticancer Drugs21, 362–371. 10.1097/cad.0b013e3283355227
9
Cheng Z. Kang C. Che S. Su J. Sun Q. Ge T. et al (2022). Berberine: a promising treatment for neurodegenerative diseases. Front. Pharmacol.13, 845591. 10.3389/fphar.2022.845591
10
Chertow G. M. Burdick E. Honour M. Bonventre J. V. Bates D. W. (2005). Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol.16, 3365–3370. 10.1681/ASN.2004090740
11
Chitra P. Saiprasad G. Manikandan R. Sudhandiran G. (2013). Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-κB dependant TGF-β activation: a biphasic experimental study. Toxicol. Lett.219, 178–193. 10.1016/j.toxlet.2013.03.009
12
Chválová K. Brabec V. Kaspárková J. (2007). Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res.35, 1812–1821. 10.1093/nar/gkm032
13
Ding B. Geng S. Hou X. Ma X. Xu H. Yang F. et al (2021). Berberine reduces renal cell pyroptosis in golden hamsters with diabetic nephropathy through the nrf2-NLRP3-caspase-1-GSDMD pathway. Evid. Based Complement. Altern. Med.2021, 1–13. 10.1155/2021/5545193
14
Domitrović R. Cvijanović O. Pernjak-Pugel E. Skoda M. Mikelić L. Crnčević-Orlić Z. (2013). Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol.62, 397–406. 10.1016/j.fct.2013.09.003
15
Dorweiler B. Pruefer D. Andrasi T. B. Maksan S. M. Schmiedt W. Neufang A. et al (2007). Ischemia-reperfusion injury: pathophysiology and clinical implications. Eur. J. Trauma Emerg. Surg.33, 600–612. 10.1007/s00068-007-7152-z
16
El-Horany H. E. Gaballah H. H. Helal D. S. (2020). Berberine ameliorates renal injury in a rat model of D-galactose-induced aging through a PTEN/Akt-dependent mechanism. Arch. Physiol. Biochem.126, 157–165. 10.1080/13813455.2018.1499117
17
Feng P. Zhao L. Guo F. Zhang B. Fang L. Zhan G. et al (2018). The enhancement of cardiotoxicity that results from inhibiton of CYP 3A4 activity and hERG channel by berberine in combination with statins. Chem. Biol. Interact.293, 115–123. 10.1016/j.cbi.2018.07.022
18
Feng X. Sureda A. Jafari S. Memariani Z. Tewari D. Annunziata G. et al (2019). Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics9, 1923–1951. 10.7150/thno.30787
19
Finkel T. (2011). Signal transduction by reactive oxygen species. J. Cell Biol.194, 7–15. 10.1083/jcb.201102095
20
Freund A. Orjalo A. V. Desprez P. Y. Campisi J. (2010). Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med.16, 238–246. 10.1016/j.molmed.2010.03.003
21
Fuertes M. A. Alonso C. Pérez J. M. (2003). Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev.103, 645–662. 10.1021/cr020010d
22
Germoush M. O. Mahmoud A. M. (2014). Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol.140, 1103–1109. 10.1007/s00432-014-1665-8
23
Gong S. Chen J. Zheng X. Lu X. Chen M. Li J. et al (2024). Kidney targeting and modulating macrophage polarization through AMPK signaling: therapeutic mechanism of berberine in uric acid nephropathy. Int. Immunopharmacol.138, 112632. 10.1016/j.intimp.2024.112632
24
Gu L. Li N. Yu W. Gong J. Li Q. Zhu W. et al (2013). Berberine reduces rat intestinal tight junction injury induced by ischemia-reperfusion associated with the suppression of inducible nitric oxide synthesis. Am. J. Chin. Med.41, 1297–1312. 10.1142/S0192415X13500870
25
Guyton K. Z. Kensler T. W. (1993). Oxidative mechanisms in carcinogenesis. Br. Med. Bull.49, 523–544. 10.1093/oxfordjournals.bmb.a072628
26
Hassanein E. H. M. Shalkami A. S. Khalaf M. M. Mohamed W. R. Hemeida R. A. M. (2019). The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother.109, 47–56. 10.1016/j.biopha.2018.10.088
27
Holy E. W. Akhmedov A. Lüscher T. F. Tanner F. C. (2009). Berberine, a natural lipid-lowering drug, exerts prothrombotic effects on vascular cells. J. Mol. Cell Cardiol.46, 234–240. 10.1016/j.yjmcc.2008.10.011
28
Howard S. C. McCormick J. Pui C. H. Buddington R. K. Harvey R. D. (2016). Preventing and managing toxicities of high-dose methotrexate. Oncologist21, 1471–1482. 10.1634/theoncologist.2015-0164
29
Hua W. Ding L. Chen Y. Gong B. He J. Xu G. (2007). Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal.44, 931–937. 10.1016/j.jpba.2007.03.022
30
Huang K. Liu W. Lan T. Xie X. Peng J. Huang J. et al (2012). Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One7, e43874. 10.1371/journal.pone.0043874
31
Hughes J. Cailhier J. F. Watson S. Savill J. S. (2004). “Apoptosis in glomerulonephritis,”, 30. Rheum. Dis. Clin. North Am.655–676. 10.1016/j.rdc.2004.04.004
32
Ibrahim H. N. Hostetter T. H. (1997). Diabetic nephropathy. J. Am. Soc. Nephrol.8, 487–493. 10.1681/ASN.V83487
33
Ibrahim Fouad G. Ahmed K. A. (2021). The protective impact of berberine against doxorubicin-induced nephrotoxicity in rats. Tissue Cell73, 101612. 10.1016/j.tice.2021.101612
34
Imenshahidi M. Hosseinzadeh H. (2016). Berberis vulgaris and berberine: an update review. Phytother. Res.30, 1745–1764. 10.1002/ptr.5693
35
Imenshahidi M. Hosseinzadeh H. (2019). Berberine and barberry (Berberis vulgaris): a clinical review. Phytother. Res.33, 504–523. 10.1002/ptr.6252
36
Jager K. J. Fraser S. D. S. (2017). The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol. Dial. Transpl.32, ii121–ii128. 10.1093/ndt/gfw330
37
Jha V. Garcia-Garcia G. Iseki K. Li Z. Naicker S. Plattner B. et al (2013). Chronic kidney disease: global dimension and perspectives. Lancet382, 260–272. 10.1016/S0140-6736(13)60687-X
38
Jiang S. J. Dong H. Li J. B. Xu L. J. Zou X. Wang K. F. et al (2015). Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol.21, 7777–7785. 10.3748/wjg.v21.i25.7777
39
Jochmans I. Meurisse N. Neyrinck A. Verhaegen M. Monbaliu D. Pirenne J. (2017). Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transpl.23, 634–644. 10.1002/lt.24728
40
Kalender Y. Yel M. Kalender S. (2005). Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology209, 39–45. 10.1016/j.tox.2004.12.003
41
Kawakami T. Gomez I. G. Ren S. Hudkins K. Roach A. Alpers C. E. et al (2015). Deficient autophagy results in mitochondrial dysfunction and FSGS. J. Am. Soc. Nephrol.26, 1040–1052. 10.1681/ASN.2013111202
42
Kazaz I. O. Mentese A. Demir S. Kerimoglu G. Colak F. Bodur A. et al (2020). Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am. J. Emerg. Med.38, 33–37. 10.1016/j.ajem.2019.04.001
43
Kheir M. M. Wang Y. Hua L. Hu J. Li L. Lei F. et al (2010). Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol.48, 1105–1110. 10.1016/j.fct.2010.01.033
44
Kim S. R. Puranik A. S. Jiang K. Chen X. Zhu X. Y. Taylor I. et al (2021). Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J. Am. Soc. Nephrol.32, 1987–2004. 10.1681/ASN.2020091373
45
Kishimoto A. Dong S. F. Negishi H. Yasui N. Sun J. N. Ikeda K. (2015). Effects of berberine on adipose tissues and kidney function in 3T3-L1 cells and spontaneously hypertensive rats. Nat. Prod. Commun.10, 1543–1546. 10.1177/1934578x1501000914
46
Kuo C. L. Chi C. W. Liu T. Y. (2004). The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett.203, 127–137. 10.1016/j.canlet.2003.09.002
47
Levey A. S. Coresh J. (2012). Chronic kidney disease. Lancet379, 165–180. 10.1016/S0140-6736(11)60178-5
48
Li D. Yang C. Zhu J. Z. Lopez E. Zhang T. Tong Q. et al (2022). Berberine remodels adipose tissue to attenuate metabolic disorders by activating sirtuin 3. Acta Pharmacol. Sin.43, 1285–1298. 10.1038/s41401-021-00736-y
49
Liguori I. Russo G. Curcio F. Bulli G. Aran L. Della-Morte D. et al (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging13, 757–772. 10.2147/CIA.S158513
50
Lin G. Yu Q. Xu L. Huang Z. Mai L. Jiang L. et al (2021). Berberrubine attenuates potassium oxonate- and hypoxanthine-induced hyperuricemia by regulating urate transporters and JAK2/STAT3 signaling pathway. Eur. J. Pharmacol.912, 174592. 10.1016/j.ejphar.2021.174592
51
Lin X. Zhang N. (2018). Berberine: pathways to protect neurons. Phytother. Res.32, 1501–1510. 10.1002/ptr.6107
52
Liu C. S. Zheng Y. R. Zhang Y. F. Long X. Y. (2016). Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia109, 274–282. 10.1016/j.fitote.2016.02.001
53
Liu Y. Hao H. Xie H. Lv H. Liu C. Wang G. (2009). Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J. Pharm. Sci.98, 4391–4401. 10.1002/jps.21721
54
Lombardi V. Valko L. Stolc S. Valko M. Ondrejicková O. Horáková L. et al (1998). Free radicals in rabbit spinal cord ischemia: electron spin resonance spectroscopy and correlation with SOD activity. Cell Mol. Neurobiol.18, 399–412. 10.1023/a:1022597431593
55
Lopez-Novoa J. M. Quiros Y. Vicente L. Morales A. I. Lopez-Hernandez F. J. (2011). New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int.79, 33–45. 10.1038/ki.2010.337
56
Lorz C. Benito-Martin A. Justo P. Sanz A. B. Sanchez-Niño M. D. Santamaria B. et al (2006). Modulation of renal tubular cell survival: where is the evidence?Curr. Med. Chem.13, 449–454. 10.2174/092986706775527956
57
Lu F. J. Ding L. Q. Cao S. J. Zhang D. Q. Zhang B. L. Qiu F. (2018). Chemistry and biology research on bitter-taste Chinese materia medica with function of regulating glycolipid metabolism. Zhongguo Zhong Yao Za Zhi43, 3834–3840. 10.19540/j.cnki.cjcmm.20180709.006
58
Ma Z. Zhu L. Wang S. Guo X. Sun B. Wang Q. et al (2022). Berberine protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition involving the inactivation of the NLRP3 inflammasome. Ren. Fail44, 923–932. 10.1080/0886022X.2022.2079525
59
Malaviya A. N. (2016). Landmark papers on the discovery of methotrexate for the treatment of rheumatoid arthritis and other systemic inflammatory rheumatic diseases: a fascinating story. Int. J. Rheum. Dis.19, 844–851. 10.1111/1756-185X.12862
60
Meng X. M. Nikolic-Paterson D. J. Lan H. Y. (2016). TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol.12, 325–338. 10.1038/nrneph.2016.48
61
Mizushima N. Yamamoto A. Matsui M. Yoshimori T. Ohsumi Y. (2004). In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell15, 1101–1111. 10.1091/mbc.e03-09-0704
62
Neri M. Riezzo I. Pascale N. Pomara C. Turillazzi E. (2017). Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediat. Inflamm.2017, 7018393. 10.1155/2017/7018393
63
Nishino H. Kitagawa K. Fujiki H. Iwashima A. (1986). Berberine sulfate inhibits tumor-promoting activity of teleocidin in two-stage carcinogenesis on mouse skin. Oncology43, 131–134. 10.1159/000226349
64
Pan L. Yu H. Fu J. Hu J. Xu H. Zhang Z. et al (2023). Berberine ameliorates chronic kidney disease through inhibiting the production of gut-derived uremic toxins in the gut microbiota. Acta Pharm. Sin. B13, 1537–1553. 10.1016/j.apsb.2022.12.010
65
Radi Z. A. (2018). Immunopathogenesis of acute kidney injury. Toxicol. Pathol.46, 930–943. 10.1177/0192623318799976
66
Radi Z. A. Stewart Z. S. O'Neil S. P. (2018a). Accidental and programmed cell death in investigative and toxicologic pathology. Curr. Protoc. Toxicol.76, e51. 10.1002/cptx.51
67
Radi Z. A. Vogel W. M. LaBranche T. Dybowski J. A. Peraza M. A. Portugal S. S. et al (2018b). Renal and hematologic comparative effects of dissociated agonist of the glucocorticoid receptor and prednisone in dogs with and without food restriction. Int. J. Toxicol.37, 223–233. 10.1177/1091581818763804
68
Reutens A. T. Atkins R. C. (2011). Epidemiology of diabetic nephropathy. Contrib. Nephrol.170, 1–7. 10.1159/000324934
69
Sanz A. B. Sanchez-Niño M. D. Ramos A. M. Moreno J. A. Santamaria B. Ruiz-Ortega M. et al (2010). NF-kappaB in renal inflammation. J. Am. Soc. Nephrol.21, 1254–1262. 10.1681/ASN.2010020218
70
Sarraf M. Beig Babaei A. Naji-Tabasi S. (2019). Investigating functional properties of barberry species: an overview. J. Sci. Food Agric.99, 5255–5269. 10.1002/jsfa.9804
71
Shao M. Ye C. Bayliss G. Zhuang S. (2021). New insights into the effects of individual Chinese herbal medicines on chronic kidney disease. Front. Pharmacol.12, 774414. 10.3389/fphar.2021.774414
72
Shirwaikar A. Shirwaikar A. Rajendran K. Punitha I. S. (2006). In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull.29, 1906–1910. 10.1248/bpb.29.1906
73
Song L. Zhang W. Tang S. Y. Luo S. M. Xiong P. Y. Liu J. Y. et al (2024). Natural products in traditional Chinese medicine: molecular mechanisms and therapeutic targets of renal fibrosis and state-of-the-art drug delivery systems. Biomed. Pharmacother.170, 116039. 10.1016/j.biopha.2023.116039
74
Sun S. F. Zhao T. T. Zhang H. J. Huang X. R. Zhang W. K. Zhang L. et al (2015). Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol.42, 662–670. 10.1111/1440-1681.12402
75
Sureshbabu A. Ryter S. W. Choi M. E. (2015). Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol.4, 208–214. 10.1016/j.redox.2015.01.001
76
Tait I. S. Li Y. Lu J. (2015). Effects of PTEN on the longevity of cultured human umbilical vein endothelial cells: the role of antioxidants. Int. J. Mol. Med.35, 277–284. 10.3892/ijmm.2014.1999
77
Takahashi A. Loo T. M. Okada R. Kamachi F. Watanabe Y. Wakita M. et al (2018). Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun.9, 1249. 10.1038/s41467-018-03555-8
78
Tan E. Gao Z. Wang Q. Han B. Shi H. Wang L. et al (2023). Berberine ameliorates renal interstitial inflammation and fibrosis in mice with unilateral ureteral obstruction. Basic Clin. Pharmacol. Toxicol.133, 757–769. 10.1111/bcpt.13947
79
Tan X. S. Ma J. Y. Feng R. Ma C. Chen W. J. Sun Y. P. et al (2013). Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One8, e77969. 10.1371/journal.pone.0077969
80
Tangpong J. Miriyala S. Noel T. Sinthupibulyakit C. Jungsuwadee P. St Clair D. K. (2011). Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neuroscience175, 292–299. 10.1016/j.neuroscience.2010.11.007
81
Valko M. Izakovic M. Mazur M. Rhodes C. J. Telser J. (2004). Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem.266, 37–56. 10.1023/b:mcbi.0000049134.69131.89
82
Vardi N. Parlakpinar H. Ates B. Cetin A. Otlu A. (2013). The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J. Physiol. Biochem.69, 371–381. 10.1007/s13105-012-0219-2
83
Viljoen A. Chaudhry R. Bycroft J. (2019). Renal stones. Ann. Clin. Biochem.56, 15–27. 10.1177/0004563218781672
84
Wan X. Chen X. Liu L. Zhao Y. Huang W. J. Zhang Q. et al (2013). Berberine ameliorates chronic kidney injury caused by atherosclerotic renovascular disease through the suppression of NFκB signaling pathway in rats. PLoS One8, e59794. 10.1371/journal.pone.0059794
85
Wang F. M. Yang Y. J. Ma L. L. Tian X. J. He Y. Q. (2014). Berberine ameliorates renal interstitial fibrosis induced by unilateral ureteral obstruction in rats. Nephrol. Carlt.19, 542–551. 10.1111/nep.12271
86
Wang K. Feng X. Chai L. Cao S. Qiu F. (2017). The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev.49, 139–157. 10.1080/03602532.2017.1306544
87
Wang X. Wang R. Xing D. Su H. Ma C. Ding Y. et al (2005). Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci.77, 3058–3067. 10.1016/j.lfs.2005.02.033
88
Xiao Y. Deng J. Li C. Gong X. Gui Z. Huang J. et al (2021). Epiberberine ameliorated diabetic nephropathy by inactivating the angiotensinogen (Agt) to repress TGFβ/Smad2 pathway. Phytomedicine83, 153488. 10.1016/j.phymed.2021.153488
89
Xie D. Xu Y. Jing W. Juxiang Z. Hailun L. Yu H. et al (2017). Berberine nanoparticles protects tubular epithelial cells from renal ischemia-reperfusion injury. Oncotarget8, 24154–24162. 10.18632/oncotarget.16530
90
Xu J. Zhou L. Liu Y. (2020). Cellular senescence in kidney fibrosis: pathologic significance and therapeutic strategies. Front. Pharmacol.11, 601325. 10.3389/fphar.2020.601325
91
Yan H. D. Li X. Z. Xie J. M. Li M. (2007). Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin. Med. J. Engl.120, 787–793. 10.1097/00029330-200705010-00010
92
Yang G. Zhao Z. Zhang X. Wu A. Huang Y. Miao Y. et al (2017). Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des. Devel Ther.11, 1065–1079. 10.2147/DDDT.S124971
93
Yao X. Panichpisal K. Kurtzman N. Nugent K. (2007). Cisplatin nephrotoxicity: a review. Am. J. Med. Sci.334, 115–124. 10.1097/MAJ.0b013e31812dfe1e
94
Yu W. Sheng M. Xu R. Yu J. Cui K. Tong J. et al (2013). Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med.11, 24. 10.1186/1479-5876-11-24
95
Zhang M. Zhang Y. Xiao D. Zhang J. Wang X. Guan F. et al (2020). Highly bioavailable berberine formulation ameliorates diabetic nephropathy through the inhibition of glomerular mesangial matrix expansion and the activation of autophagy. Eur. J. Pharmacol.873, 172955. 10.1016/j.ejphar.2020.172955
96
Zhang X. He H. Liang D. Jiang Y. Liang W. Chi Z. H. et al (2016). Protective effects of berberine on renal injury in streptozotocin (STZ)-Induced diabetic mice. Int. J. Mol. Sci.17, 1327. 10.3390/ijms17081327
97
Zhi D. Feng P. F. Sun J. L. Guo F. Zhang R. Zhao X. et al (2015). The enhancement of cardiac toxicity by concomitant administration of Berberine and macrolides. Eur. J. Pharm. Sci.76, 149–155. 10.1016/j.ejps.2015.05.009
98
Zhu L. Han J. Yuan R. Xue L. Pang W. (2018). Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol. Res.51, 9. 10.1186/s40659-018-0157-8
Summary
Keywords
berberine, renal diseases, inflammation, oxidative stress, apoptosis
Citation
Fan Z, Wei X, Zhu X, Yang K, Tian L, Wang X, Du Y and Yang L (2025) Unveiling the therapeutic potential of berberine: its therapeutic role and molecular mechanisms in kidney diseases. Front. Pharmacol. 16:1549462. doi: 10.3389/fphar.2025.1549462
Received
21 December 2024
Accepted
05 February 2025
Published
21 February 2025
Volume
16 - 2025
Edited by
Krishna M. Boini, University of Houston, United States
Reviewed by
Ferda Kaleagasioglu, University of Istinye, Türkiye
Sumaya Akter, Noakhali Science and Technology University, Bangladesh
Updates
Copyright
© 2025 Fan, Wei, Zhu, Yang, Tian, Wang, Du and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujun Du, duyj@jlu.edu.cn; Liming Yang, yliming0815@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.