Abstract
Atopic dermatitis (AD) is a common chronic, recurrent, inflammatory skin disease characterized by pruritus, lichen-like changes and dry skin. Due to the complex pathogenesis of AD, its mechanism is primarily associated with genetic, skin barrier dysfunction, environmental, and immune factors. AD has been routinely treated with glucocorticoids, antihistamines, local immunomodulators, biological agents, and small molecules; however, the side effects are significant, and the treatment efficacy is limited. In recent years, traditional Chinese medicine (TCM) has gradually been widely used in the treatment of AD. Many studies have shown that TCM mainly regulates inflammatory cytokines, gut microbiota and the immune system. Therefore, it plays a crucial role in the treatment of AD. The treatment of atopic dermatitis using TCM is characterized by targeting multiple pathways and multiple targets, and it demonstrates significant therapeutic effects. This paper reviews the pathogenesis of AD and reports the efficacy of TCM on AD (including TCM prescription, single TCM, treatment of TCM metabolites), which provides a theoretical basis for TCM treatment of AD. TCM has certain therapeutic effects on AD. It can alleviate and treat AD in various ways. We should base our differentiation on syndrome differentiation and treatment differentiation. With the help of modern medicine, the clinical efficacy of TCM in treating AD can be improved.
1 Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease with a genetic predisposition related to the environment, skin barrier, immune system, and bacterial infection. AD is a global health problem, and although its pathogenesis involves multiple factors such as genetic predisposition and environmental triggers, it is not yet fully understood (Zhang et al., 2022a). The prevalence of AD is increasing worldwide, affecting approximately 20% of children and 3% of adults (Zeng et al., 2019). It imposes a significant psychosocial burden on patients because it creates an unsightly appearance and functional limitations. It also increases the likelihood of developing diseases, including asthma, arthritis, allergic rhinitis, food allergies and other inflammatory diseases, including mental disorders (Hemrajani et al., 2022). The purpose of treatment is to alleviate symptoms, prevent recurrent attacks, and reduce risks. Clinical medications for AD include glucocorticoids, calcineurin inhibitors, antibiotics, antihistamines, targeted biological agents, small molecules, and immunosuppressants (Figure 1). Many studies have shown a lack of appropriate, safe and effective AD treatments (Liu W. et al., 2022). Over recent years, a growing number of people are paying attention to the more effective and safe use of traditional Chinese medicine (TCM) to treat AD.
FIGURE 1
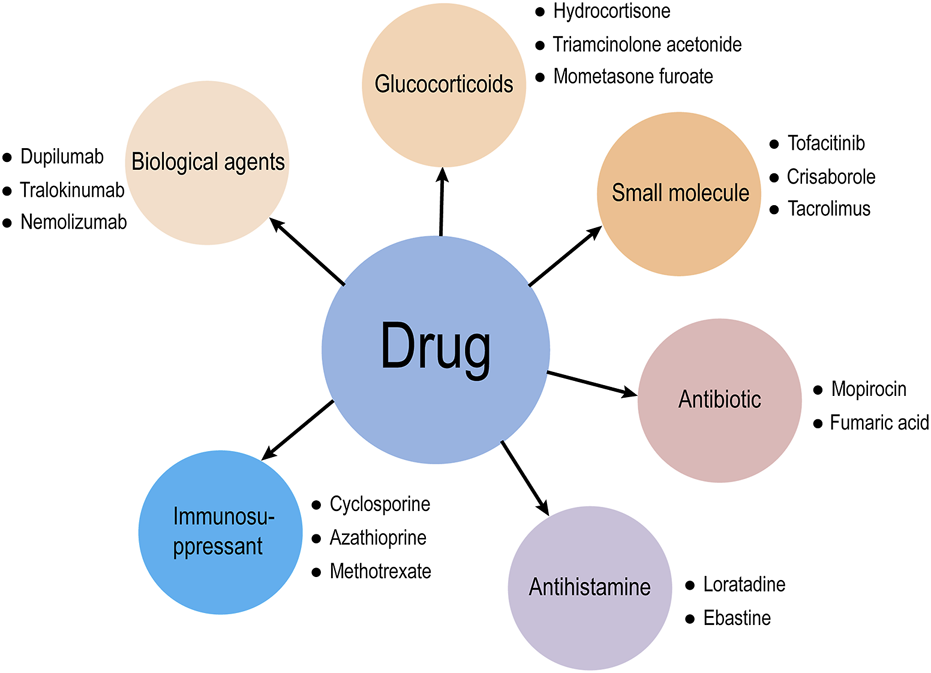
Drugs for clinical treatment of AD.
2 Pathogenesis of AD
The etiology and pathogenesis of the disease are not yet fully understood (Jia, 2020), and it may have an inseparable relationship with genetics, skin barrier dysfunction (Chong et al., 2022), environmental factors (Gu et al., 2023), and also involves immunology, neuropsychiatric factors, and gut microbiota (Liu Y. et al., 2022).
2.1 Genetic factors and skin barrier damage
AD often occurs in individuals with a family history of allergies, including asthma and allergic rhinitis (Williamson et al., 2020). Genetic factors play a significant role in AD pathogenesis, as evidenced by twin studies showing high heritability (72%–86% in monozygotic twins vs. 21%–23% in dizygotic twins) (Thomsen et al., 2007). Recent studies have identified multiple genes potentially associated with AD susceptibility across diverse populations (Chen and Chen, 2022), with at least 34 genetic loci and 46 genes linked to AD risk globally (Schmidt and de Guzman Strong, 2021). The most well-characterized genetic association involves the filaggrin gene (FLG). The protein encoded by this gene is crucial for maintaining the integrity of the epidermal barrier (Naeem et al., 2017). FLG mutations impair skin barrier function, and emerging evidence highlights the significance of epigenetic mechanisms regulating FLG expression in disease pathogenesis (Yang et al., 2020), FLG levels are generally reduced in AD patients, which destroys the structure of the stratum corneum, increases the permeability of the cell membrane, and causes a large loss of epidermal water, which may be related to the mutation of FLG gene (Drislane and Irvine, 2020), therefore, FLG gene mutations, which lead to dysfunction, are among the most significant pathogenic factors in AD (Smieszek et al., 2020). The skin’s primary function resides in establishing an efficient barrier between an organism’s internal milieu and external environment. Consequently, skin serves as a critical interface providing both protective and structural support for the organism it encapsulates (Miura et al., 2025). Barrier dysfunction initiates a pathogenic cascade wherein environmental stimuli activate keratinocytes to release alarmins (such as thymic stromal lymphopoietin), triggering type 2 immune responses through interleukin production. This mechanism exacerbates barrier defects and potentiates allergic inflammation (van den Bogaard et al., 2023).
2.2 Environmental factors
The influence of environment is related to the pathogenesis of AD, and many studies have shown that environmental factors can exacerbate the symptoms of AD patients (Kantor and Silverberg, 2017). The onset of AD may change the skin penetration through the stimulation of various substances in the environment to the skin, resulting in the impairment of skin barrier function, making it easier for external stimuli to enter the skin and trigger inflammatory reactions (Thibault Greugny et al., 2023). This may be related to the abnormal synthesis and secretion of skin barrier proteins such as keratin and intercellular lipids. Exposure to dynamic environmental changes may induce or cause disease in susceptible populations. There are complex interactions between different environmental factors, including personal use of personal care products and exposure to climate, pollution, food, and other extrinsic factors (Narla and Silverberg, 2020; Lai et al., 2023; Mailepessov et al., 2024).
2.3 Diseases of the immune system
Immune dysfunction is the central link in the pathogenesis of AD (Figure 2). Keratinocytes (KCs) are the regulatory cells of the innate immune response. When the expression of KCs is abnormal, AD-related symptoms are triggered on the surface of the skin, and there are also many abnormalities in immune factors (De Bruyn Carlier et al., 2021), such as KCs, dendritic cells (DCs), mast cells (MCs) and Th cells. These are the sources of the pathogenesis of AD. Previous studies on AD have been focused predominantly on Th2-mediated immune responses. The imbalance of Th1/Th2 immunity caused by cytokines such as IL-4 causes skin inflammation and damages skin barrier function (Oh et al., 2021; Sroka-Tomaszewska and Trzeciak, 2021). According to recent studies, ad is obviously Th2/Th22 centered with the overexpression of Th1 and Th17 cell related cytokines (Li H. et al., 2021), and the additional activation pathways of Th22 and Th17 cytokines through the release of IL-17, IL-19, and IL-22, as well as the role of regulating lymphocytes as another mechanism of AD have been widely discussed (Brunner et al., 2017; Gatmaitan and Lee, 2023).
FIGURE 2
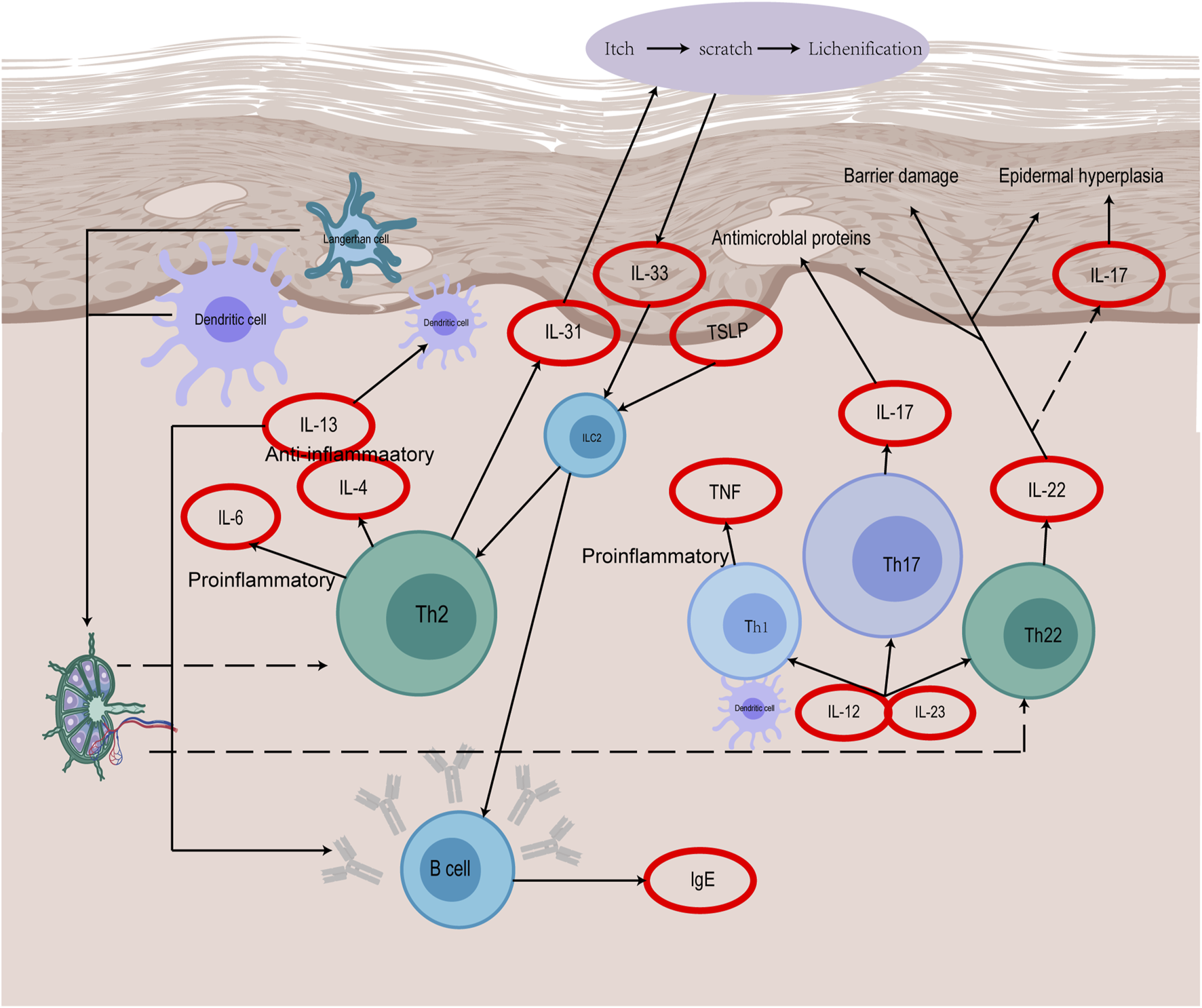
The immune mechanism of AD.
2.4 Neural and psychiatric factors
The core symptom of AD is pruritus, which is primarily mediated by neurogenic signaling pathways. Th2 cytokines can directly activate sensory neurons and mediate itch signals (Kwatra et al., 2022). Transient receptor potential channels play a key role in inflammation and nerve sensitization, leading to a decrease in itch threshold (Aldossary et al., 2024). Sensory nerve endings in the skin release neuropeptides, trigger “neurogenic inflammation,” promote mast cell degranulation, and release inflammatory mediators such as histamine and TNF-α (Xu et al., 2020), activate keratinocytes, secrete cytokines such as IL-33 and TSLP, and amplify Th2 immune response (Ikai et al., 2023). At the same time, inflammatory factors further affect the central nervous system through the “brain skin axis” (Chitnis and Weiner, 2017). The density of nerve fibers increases in the skin of AD patients, especially itch related nerves, resulting in enhanced itch perception. Overexpression of nerve growth factor promotes nerve fiber proliferation and sensitization (Tominaga et al., 2009). Nerve and mental factors play a key role in the occurrence and chronicity of AD through complex neuroimmune interactions, itch signaling, and psychophysiological feedback loops. Future research needs to further analyze the molecular mechanism of “brain skin axis” to provide basis for individualized treatment.
2.5 Intestinal floras
Studies have shown that early life is a critical period for appropriate immune responses to microorganisms, which may be a critical period for initiating microbiome interventions (Koh et al., 2022). In recent years, with the concept of the “gut skin” axis being proposed (Zhang et al., 2024), the imbalance of gut microbiota abundance is considered to be another important factor in the occurrence of AD. The status of gut microbiota greatly affects the differentiation of naive T cells into other types of Th cells, regulating the immune system of the body and skin (Markowiak-Kopeć and Śliżewska, 2020). Further studies on the interaction between human microbiota and AD will provide new targets for the treatment of AD in the future.
3 TCM for AD treatment
The concept of AD does not have a complete counterpart in TCM, ancient physicians, who formerly referred to AD as eczema, wet sores, wet tinea, blood wind sores, or four-curved wind sores, believed that the onset of AD was related to the environment, diet, and daily life. It was caused by internal factors such as Heart Fire and Spleen Dampness, and external factors such as Wind Evil.
3.1 Five elements and five Yin/Yang in TCM
TCM interprets disease pathogenesis through a holistic lens that integrates the Five Elements (Wu Xing), Yin-Yang organ relationships, and exogenous/endogenous pathogenic factors. These theories collectively explain systemic imbalances and guide therapeutic strategies, such as those for AD. While modern dermatology focuses on molecular pathways, TCM emphasizes the interplay between organ networks and environmental influences. Table 1 is a structured summary of these foundational concepts and their clinical relevance.
TABLE 1
| Theory | Key components | Clinical correlation |
|---|---|---|
| Five Elements | Wood: Liver/Gallbladde; Governs Qi flow, emotional regulation Fire: Heart/Small Intestine; Regulates blood circulation Earth: Spleen/Stomach; Controls digestion and dampness Metal: Lung/Large Intestine; Manages skin and immunity Water: Kidney/Bladder; Governs growth and hydration |
Chronic stress (Wood/Liver dysfunction) → Qi stagnation → eczema flares Heart Fire exacerbates erythema and itching Spleen deficiency → dampness accumulation → oozing lesions Lung Qi deficiency → dry, flaky skin (impaired barrier function) Kidney Yin deficiency leads to chronic dryness and lichenification |
| Yin-Yang Organ Pairs | Yin (Zang): Solid, nutrient-storing organs (Liver, Spleen) Yang (Fu): Hollow, transport-focused organs (Stomach, Gallbladder) |
Yin deficiency (Spleen) → poor skin hydration Yang excess (Stomach Heat) → inflammatory erythema |
| Pathogenic Factors | External: Wind (itching), Damp (exudation), Heat (redness), Dryness (scaling) Internal: Emotional stress (Liver Qi stagnation), Blood deficiency |
Acute AD: Wind-Heat-Damp invasion → pruritic, erythematous plaques Chronic AD: Blood deficiency → xerosis; Qi stagnation → lichenification |
Five elements, Yin-Yang organ Pairs, and pathogenic factors.
3.2 Research progress in the treatment of AD with TCM
Although AD is not accurately and in detail recorded in TCM, the discussions in ancient Chinese medicine books have important reference value and guiding significance for today’s understanding and treatment of AD (Figure 3). It is recorded in Shen Shi Fang of the Northern and Southern Dynasties that the decoction of black plum can Dry Damp ringworm Zhang proposed in Treatise on Febrile Diseases in the Eastern Han Dynasty that Ephedra, Forsythia and Chixiaodou Decoction could clear away Heat Toxin, Dispel Dampness and Streng Spleen. During the Southern Song Dynasty, there was a record in the General Prescription of Pediatric Healt that Diyu treated facial ulcers, redness, swelling, and pain. During the Yuan Dynasty, Zhu proposed the use of Ermiao Powder in the Danxi Heart Method to treat inflammation, redness, swelling, and exudation caused by dampness and heat. During the Ming Dynasty, Chen proposed the use of Xiaofeng Powder for the treatment of eczema in his Surgical Authentic. During the Qing Dynasty, Wang recorded in his Essentials of Matea Medica that Kushen was used to Dry Dampness and remove heat. Nowadays, TCM prescription is the most common TCM treatment method, which allows the easy adjustment of dosage and drug composition according to the conditions (Figure 4). In recent years, some external creams and moisturizing agents based on active substances found in TCM have been commonly used in the treatment of AD and have achieved satisfactory results. TCM ointment is a proprietary medicine with a long history of treating diseases. It is a semiliquid thick paste that is created by preparing a large decoction through the addition of certain excipients after concentration. Umbilical compression treatment and acupuncture treatment have produced unexpected effects on many chronic medical diseases, both of which belong to the classical treatment methods of TCM. TCM baths are a classic TCM method for treating skin diseases and are also an effective method of treating intractable skin diseases. The use of medicinal baths makes the treatment method more scientific, safe, environmentally friendly, and humanized.
FIGURE 3
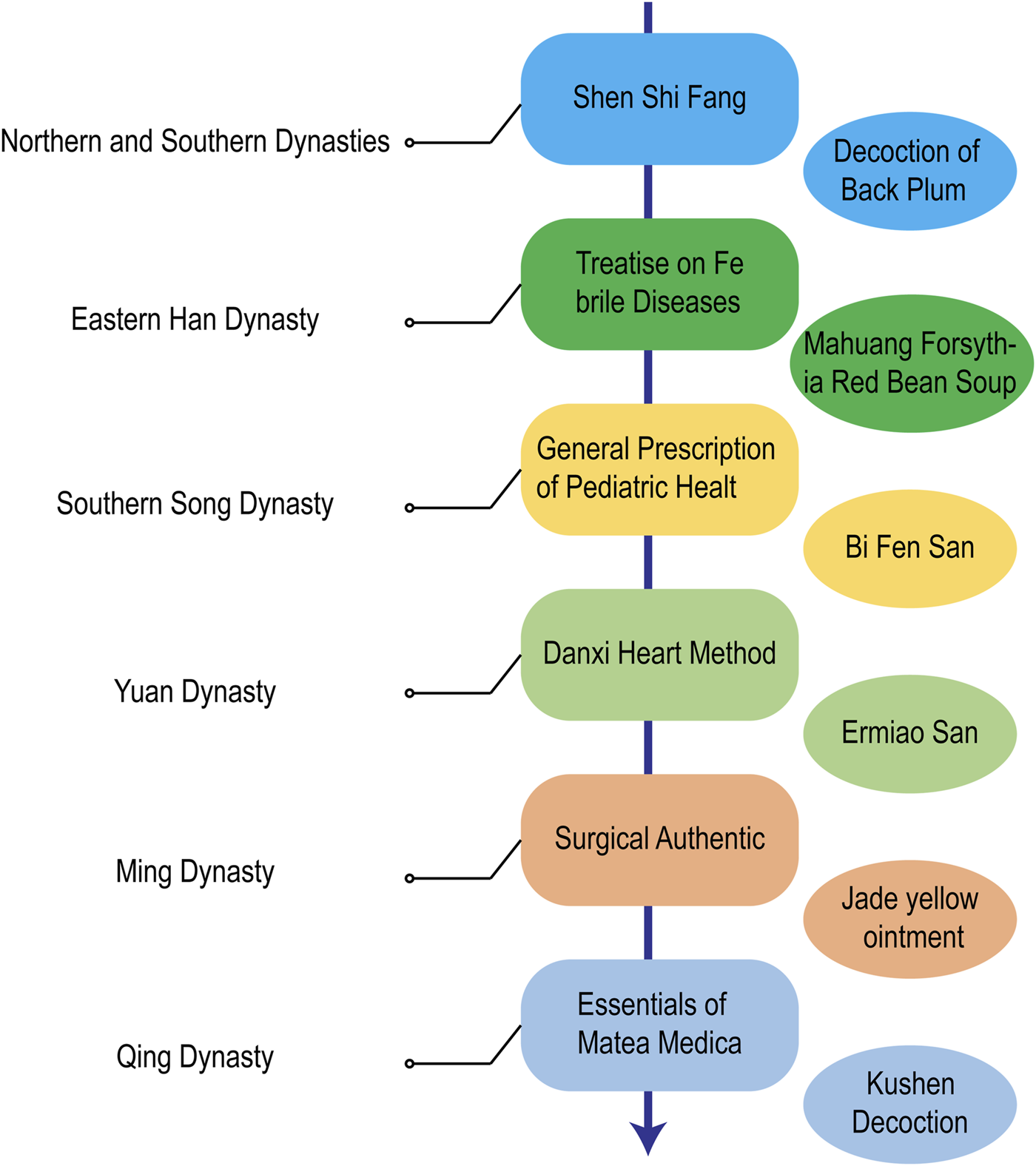
Historical evolution of AD treatment with TCM in China.
FIGURE 4
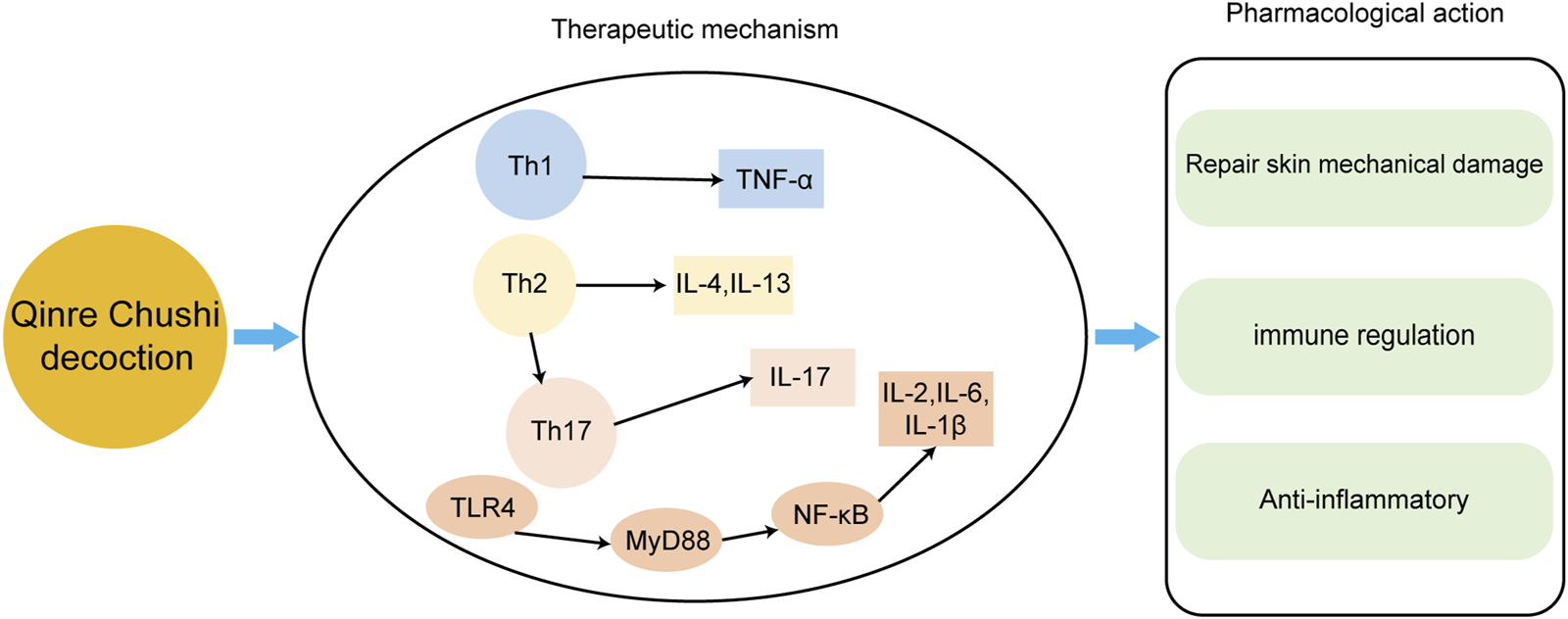
The main mechanisms of Qingre Chushi decoction against AD.
3.3 Therapeutic effect of TCM on AD
3.3.1 TCM prescription
TCM preparations have unique advantages in the treatment of AD. Experimental studies have found that TCM preparations have multiple targets for the treatment of AD, and most of them have been applied in clinical practice with demonstrated favorable outcomes. Among them is the classic botanical drug formula Angelica Yinzi (AYZ) comes from the Yan’s Jisheng Formula during the Southern Song Dynasty (Yan’s Jisheng Formula volume VI). The efficacy of AYZ has been scientifically and clinically proven and there have been no serious adverse reactions. The AYZ formula contains 11 different botanical drug: Dang Gui (Angelica sinensis (Oliv.) Diels [Apiaceae]), Shao Yao (Paeonia lactiflora Pall. [Paeoniaceae]), Chuan Xiong (Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae]), Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Orobanchaceae]), Ji Li (Tribulus cistoides L. [Zygophyllaceae]), Fang Feng (Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk.), Jing Jie (Nepeta tenuifolia Benth. [Lamiaceae]), He Shou Wu (Reynoutria multiflora (Thunb.) Moldenke [Polygonaceae]), Huang QI (Astragalus mongholicus Bunge [Fabaceae]), Gan Cao (Glycyrrhiza glabra L. [Fabaceae]), ShengJiang (Zingiber officinale Roscoe [Zingiberaceae]). Wang et al. (2021) used network pharmacology methods to suggest AYZ may be able to intervene in AD by regulating the MAPK/NF-κB signalling pathways. Liu et al. (see Table 2) treated DNCB-induced AD-like mice with AYZ and found that AYZ decreased the serum IgE level, decreased the epidermal thickness of AD-like lesion skin, and AYZ can downregulate inflammatory factors TNF-α and IL-1β by inhibiting the MAPK/NF-κB signalling pathways. The treatment downregulates the expression of NLRP3, which significantly decreases the activation of inflammatory bodies in NLRP3, improves the symptoms of AD (Liu W. et al., 2022).
TABLE 2
| No. | Chinese name | Family name | Latin name | Source species |
|---|---|---|---|---|
| 1 | Dang Gui | Apiaceae | Angelicae Sinensis Radix | Angelica sinensis (Oliv.) Diels [Apiaceae] |
| 2 | Shao Yao | Paeoniaceae | Paeonia lactiflora Pall | Paeonia lactiflora Pall. [Paeoniaceae] |
| 3 | Chuan Xiong | Apiaceae | Ligusticum chuanxiong Hort. | Conioselinum anthriscoides ‘Chuanxiong’ [Apiaceae] |
| 4 | Di Huang | Ledangae | Rehmanniae Radix Praeparata | Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Orobanchaceae] |
| 5 | Ji Li | Tribulus | Tribulus terrestris Linnaeus | Tribulus terrestris L. [Zygophyllaceae] |
| 6 | Fang Feng | Apiaceae | Saposhnikoviae Rqadix | Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. [Apiaceae] |
| 7 | Jing Jie | Labiatae | Schizonepetae Herba | Nepeta tenuifolia Benth. [Lamiaceae] |
| 8 | He Shou Wu | Polygonaceae | Polygoni Multiflori Radix | Reynoutria multiflora (Thunb.) Moldenke [Polygonaceae] |
| 9 | Huang Qi | Leguminosae | Astragali Radix | Astragalus mongholicus Bunge [Fabaceae] |
| 10 | Gan cao | Leguminosae | Glycyrrhizae Radix et Rhizoma | Glycyrrhiza glabra L. [Fabaceae] |
| 11 | ShengJiang | Curcumaceae | Zingiber officinale Roscoe | Zingiber officinale Roscoe [Zingiberaceae] |
| 12 | Ma Huang | Ephedraceae | Ephedra Herba | Ephedra sinica Stapf [Ephedraceae] |
| 13 | Lian Qiao | Oleaceae | Forsythia suspensa | Forsythia suspensa (Thunb.) Vahl [Oleaceae] |
| 14 | Xing Ren | Rosaceae | Prunus armeniaca | Prunus amygdalus Batsch [Rosaceae] |
| 15 | Chi Dou | Leguminosae | Vigna umbellata | Vigna umbellata (Thunb.) Ohwi & H.Ohashi [Fabaceae] |
| 16 | Da Zao | Rhamnaceae | Jujubae Fructus | Ziziphus jujuba Mill. [Rhamnaceae] |
| 17 | Sang Bai Pi | Moriaceae | Morus alba L. | Morus indica L. [Moraceae] |
| 18 | Zi Hua Di Ding | Violaceae | Viola yedoensis Makino | Viola hamiltoniana D.Don [Violaceae] |
| 19 | Ku Shen | Leguminosae | Sophorae Flavescentis Radix | Sophora flavescens Aiton [Fabaceae] |
| 20 | Bai Xian | Rutaceae | Dictamnus albus | Dictamnus dasycarpus Turcz. [Rutaceae] |
| 21 | Jin Yin Hua | Lonicera | Lonicera japonica Thunb | Lonicera japonica Thunb. [Caprifoliaceae] |
| 22 | Huang Bai | Rutaceae | Cortex Phellodendri | Phellodendron amurense Rupr. [Rutaceae] |
| 23 | Pu Gong Yin | Asteraceae | Taraxacum mongolicum | Taraxacum mongolicum Hand.-Mazz. [Asteraceae] |
| 24 | Da Hang | Polygonaceae | Radix et Rhizoma Rhei | Rheum palmatum L. [Polygonaceae] |
| 25 | Ku Di Ding | Papaveridae | Corydalis Bungeanae Herba | Corydalis bungeana Turcz. [Papaveraceae] |
| 26 | Huang Qin | Labiatae | Scutellariae Radix | Scutellaria baicalensis Georgi [Lamiaceae] |
| 27 | Di Yu | Rosaceae | Radix Sanguisorbae | Sanguisorba officinalis L. [Rosaceae] |
| 28 | Ma Chi Xian | Portulaceae | Portulacae Herba | Portulaca oleracea L. [Portulacaceae] |
| 29 | Cang Shu | Asteraceae | Atractylodes | Atractylodes lancea (Thunb.) DC. [Asteraceae] |
| 30 | Yin Yang Huo | Berberidaceae | Epimedium chlorandrum Stearn | Epimedium brevicornu Maxim. [Berberidaceae] |
| 31 | San Qi | Araliaceae | Notoginseng Radix | Panax notoginseng (Burkill) F.H.Chen [Araliaceae] |
| 32 | Tu Fu Ling | Smilacaceae | Smilacis Glabrae Rhixoma | Smilax glabra Roxb. [Smilacaceae] |
| 33 | Che Qian Cao | Plantaginaceae | Plantaginis Herba | Plantago asiatica L. [Plantaginaceae] |
| 34 | Ban Lan Gen | Brassicaceae | Isatidis Radix | Isatis tinctoria L. [Brassicaceae] |
| 35 | Huang Shan Yao | Dioscoreaceae | Dioscorea panthaiccae rhizoma | Dioscorea panthaica Prain & Burkill [Dioscoreaceae] |
| 36 | Ze Xie | Alismaceae | Alisma Orientale | Alisma plantago-aquatica subsp. orientale (Sam.) Sam. [Alismataceae] |
| 37 | Mu Dan Pi | Ranunculaceae | Paeoniae Radix Moutan | Paeonia × suffruticosa Andrews [Paeoniaceae] |
| 38 | Bo He | Lamiaceae | Menthae Herba | Mentha canadensis L. [Lamiaceae] |
| 39 | Bai Shu | Asteraceae | Atractylodes Macrocephala Koidz. | Atractylodes macrocephala Koidz. [Asteraceae] |
| 40 | BaiShao | Paeoniaceae | Paeoniae Radix Alba | Paeonia lactiflora Pall. [Paeoniaceae] |
| 41 | Bai zhi | Apiaceae | Angelica dahurica | Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. [Apiaceae] |
| 42 | Zi Ding Xiang | Oleaceae | Syringa oblata | Syringa oblata Lindl. [Oleaceae] |
| 43 | Ji Xue Teng | Fabaceae | Spatholobus Suberectus Dunn | Spatholobus suberectus Dunn [Fabaceae] |
| 44 | She Chuang Zi | Apiaceae | Cnidii Fructus | Cnidium monnieri (L.) Cusson [Apiaceae] |
| 45 | Lu Hui | Asphodelaceae | Aloe | Aloe vera (L.) Burm.f. [Asphodelaceae] |
| 46 | Yi Ren | Poaceae | Coicis Semen | Coix lacryma-jobi L. [Poaceae] |
| 47 | Hai Tong Pi | Leguminosae | Cortex Erythrinae | Erythrina variegata L. [Fabaceae] |
| 48 | Zi Cao | Boraginaceae | Lithospermum Erythrorhizon | Lithospermum erythrorhizon Siebold & Zucc. [Boraginaceae] |
| 49 | Ya Zui Hua | Acanthaceae | Justicia adhatoda L. | Justicia adhatoda L. [Acanthaceae] |
| 50 | Hu Zhang | Polygonaceae | Polygoni Cuspidati Rhizoma Et Radix | Reynoutria japonica Houtt. [Polygonaceae] |
| 51 | Deng Zhan Hua | Asteraceae | Erigeron breviscapus | Erigeron breviscapus (Vaniot) Hand.-Mazz. [Asteraceae] |
| 52 | Mu Ju | Asteraceae | Matricaria recutita | Matricaria chamomilla L. [Asteraceae] |
| 53 | Huang Lian | Ranunculaceae | Coptidis Rhizoma | Coptis trifolia (L.) Salisb. [Ranunculaceae] |
The Source species of TCM involved in this paper.
In addition, the Mahuang Lianqiao Chixiaodou Decoction (MLCD) incorporated in “Treatise on Cold Injury and Miscellaneous Diseases” is a classic TCM formula that is believed to resolve external (i.e., skin surface) and internal (i.e., visceral dysfunction) symptoms and signs by Expelling Wind, Moisture, and Heat Pathogens to Strengthen Spleen, reduce fever, and Expel Moisture. Recently, Yuan et al. showed that modified MLCD exhibits promising results in the treatment of AD, and significantly improves the pathological state of AD patients, alleviates itching, and reduces the recurrence rate. The mechanism may be related to regulating Th1/Th2 immune imbalance (Yuan et al., 2022).
There are also some classic prescriptions, such as the Viola yedoensis Makino anti-itching metabolite, the Huangbai liniment and Qingxue jiedu formulation, which can be used to treat AD (Table 3). They come from ancient books and modern clinical treatment cases, and are worth learning and further development and utilization.
TABLE 3
| Name | Botanical drugs | Evaluation model | The effect mechanism | The literature |
|---|---|---|---|---|
| Angelica Yinzi Decoction | Dang Gui (Angelica sinensis (Oliv.) Diels), Shao Yao (Paeonia lactiflora Pall.), Chuan Xiong (Conioselinum anthriscoides ‘Chuanxiong’), Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC.), Ji Li (Tribulus terrestris L.), Fang Feng (Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk.), Jing Jie (Nepeta tenuifolia Benth.), He Shou Wu (Reynoutria multiflora (Thunb.) Moldenke), Huang Qi (Astragalus mongholicus Bunge), Gan Cao (Glycyrrhiza glabra L.), Sheng Jiang (Zingiber officinale Roscoe) | DNCB - induced AD in mice | Inhibiting the activation of the NLRP3 inflammasome and the MAPKs/NF-κB signaling | Liu et al. (2022a) |
| Qingre Chushi decoction | Long Dan Cao (Gentiana scabra Bunge), Bai Mao Gen (Imperata cylindrica (L.) Raeusch.), Sheng Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC.), Da Qing Ye (Isatis tinctoria subsp. tinctoria), Che Qian Cao (Plantago asiatica L.), Sheng Shi Gao (Gypsum Fibrosum),Huang Qin (Scutellaria baicalensis Georgi),Hua Shi (Talcum), Gan Cao (Glycyrrhiza glabra L.). | DNFB - induced AD in mice | Alleviate inflammatory reactions, reduce the number of mast cells, and regulate Th1/Th2 balance | Meng (2021) |
| Mahuang Lianqiao Chixiaodou decoction | Ma Huang (Ephedra sinica Stapf), Lian Qiao (Forsythia suspensa (Thunb.) Vahl), Xing Ren (Prunus armeniaca L.), Chi Xiao Dou (Vigna umbellata (Thunb.) Ohwi & H.Ohashi), Da Zao (Ziziphus jujuba Mill.), Sang Bai Pi (Morus indica L.), Sheng Jiang (Zingiber officinale Roscoe), Gan Cao (Glycyrrhiza glabra L.). | DNFB - induced AD in mice | Significantly reduced therelative expression of IL-4 and TSLP mRNA in the lesion tissues,as well as the serum level of IL-4 | Yuan et al. (2022) |
| Shen Chan decoction | Dang Shen (Codonopsis pilosula (Franch.) Nannf.), Bai Zhu (Atractylodes Macrocephala Koidz.), Fu Ling (Poria Cocos (Schw.) Wolf.), Shan Yao (Dioscorea oppositifolia L.), Yi Yi Ren (Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf), Huang Qi (Astragalus mongholicus Bunge.), Da Zao (Ziziphus jujuba Mill.), Gan Cao (Glycyrrhiza glabra L.). | DNCB - induced AD in mice | Balance Th1/Th2 cytokines and inhibit H1R/PAR-2/TRPV1 Itch signal transduction; adjust microorganisms in the gut |
Zhang et al. (2024) |
| Viola yedoensisMakino anti-itching metabolite | Zi Hua Di Ding (Viola hamiltoniana D.Don), Ku Shen (Sophora flavescens Aiton), Bai Xian (Dictamnus dasycarpus Turcz.) | DNCB - induced AD in mice | Inhibiting the inflammatory mediator productions and blocking mast cell degranulationviasuppressing Syk mediated NF-κB pathway reduces the levels of inflammatory factors by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization | Zeng et al. (2021) |
| Huangbai Liniment | Lian Qiao (Forsythia suspensa (Thunb.) Vahl), Jin Yin Hua (Lonicera japonica Thunb.), Huang Bai (Phellodendron amurense Rupr.), Pu Gong Yin (Taraxacum sect. Taraxacum F.H.Wigg.), Wu Gong (centipede) | DNCB - induced AD in mice | Reduced the expression ofproinflammatory cytokines, including IL-1β, IL-4, IFN-γ, IL-13,and IL-17, and increased the expression of anti-inflammatorycytokine IL-10 | Zheng et al. (2021) |
| Qingxue jiedu formulation | Da Hang (Rheum palmatum L), Jing Jie (Nepeta tenuifolia Benth.), Pu Gong Ying (Taraxacum sect. Taraxacum F.H.Wigg.), Fang Feng (Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk.), Ku Di Ding (Corydalis bungeana Turcz.), Huang Qin (Scutellaria baicalensis Georgi), Lian Qiao (Forsythia suspensa (Thunb.) Vahl), Gan Cao (Glycyrrhiza glabra L.), Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC.) | DNFB - induced AD in mice | Inhibited the activations of STAT3, MAPK and NF-κB signaling pathways and possessed a significant therapeutic effect on AD | Xiong et al. (2020) |
| Ta-Xi-San | Ku Shen (Sophora flavescens Aiton), Huang Bai (Phellodendron amurense Rupr.), Di Yu (Sanguisorba officinalis L.), Ma Chi Xian (Portulaca oleracea L.), Cang Shu (Atractylodes lancea (Thunb.) DC.), Bai Fan (Alumen) | DNCB - induced AD in mice | Via PI3K-Akt signaling pathway, MAPK signaling pathway, and TLR signaling pathway with the regulation of inflammatory response and transcription | Zhi et al. (2022) |
| BuShenYiQi Granule | Yin Yang Huo (Epimedium brevicornum Maxim), Huang Qi (Astragalus mongholicus Bunge), Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC.) | OVA - induced AD in mice | Presented anti-inflam-matory and anti-allergic potential by systemically elevated levels of endogenous glucocorticoids and locally normalized function of skin HPA axis-like system | Kong et al. (2015) |
| Jiu-Wei-Yong-An Formula | Tu Fu Ling (Smilax glabra Roxb.), Di Huang (Rehmannia glutinosa (Gaertn.) Libosch. ex DC.), Che Qian Cao (Plantago asiatica L.), Ban Lan Gen (Isatis tinctoria L.), Lian Qiao (Forsythia suspensa (Thunb.) Vahl), Dang Gui (Angelica sinensis (Oliv.) Diels), Shan Yao (Dioscorea oppositifolia L.), Huang Shan Yao (Dioscorea panthaica Prain & Burkill), Ze Xie (Alisma lanceolatum With.) | DNCB - induced AD in mice | Attributed to blocking the JAK1/STAT3 and MAPK signaling pathways | Qinwufeng et al. (2022) |
| Pentaherbs formulaz | Jin Yin Hua (Lonicera japonica Thunb.), Bo He (Mentha canadensis L.), Huang Bai (Phellodendron amurense Rupr.), Mu Dan Pi (Paeonia × suffruticosa Andrews), Cang Shu (Atractylodes lancea (Thunb.) DC.) | OX - induced AD in mice | Suppress the releaseof pro-inflammatory cytokine IL-6 and chemokine CCL7 and CXCL8 | Chu et al. (2020) |
The effects of TCM formulas on AD.
AD, atopic dermatitis; DNCB, 1-Chloro-2,4-dinitrobenzene; DNFB, 2,4-dinitrofluorobenzene; OVA, ovalbumin; OX, oxazolone; IL, interleukin.
3.3.2 Single TCM botanical drug
In addition to the above preparations, various individual botanical drug can also treat AD (Table 4). Lycopus lucidus Turcz has been shown to alleviate DNCB-induced AD in BALB/c mice. Scutellaria baicalensis Georgi (Huangqin) is commonly used to treat AD and has broad anti-inflammatory, immunosuppressive, and antibacterial functions (Wang L. et al., 2022; Lee and Im, 2023) (Figure 5).
TABLE 4
| Single botanical drugs | Evaluation model | The effect mechanism | The literature |
|---|---|---|---|
| He Zi (Terminalia chebula Retz.) | Dfe - induced AD in mice | Regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-κB signaling in vitro | Kim et al. (2022) |
| Bai Shao (Paeonia lactiflora Pall.) | DNCB - induced AD in mice | Increased the diversity of the gut microbiota and changed the microbial composition suppressing inflammatory cytokine production, inducing Foxp3 expression | Lee et al. (2022b) |
| Bai Xian (Dictamnus dasycarpus Turcz.) | DNFB - induced AD in mice | Inhibited AD-induced chronic itch, inflammation symptoms, epidermal thickening, in-flammatory cell infiltration, and downregulated the expression of MrgprA3 and TRPA1 | Yang et al. (2022) |
| Bai zhi (Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav.) | MC903 - induced AD in mice | Decreased TRPV1 activity and protein expression in mice with inflammatory diseases | Zhu et al. (2022) |
| Zi Ding Xiang (Syringa oblata Lindl.) | DNCB - induced AD in mice | Down-regulatedthe expression of iNOS and COX-2, inhibiting the T cell-mediated allergic immune response | Fan et al. (2021) |
| Ji Xue Teng (Spatholobus suberectus Dunn.) | Dfe - induced AD in mice | Inhibits IFN-γ/TNF-α–Induced MAPK/STAT1/NF-κB | Song et al. (2022a) |
| She Chuang Zi (Cnidium monnieri (L.) Cusson) | OX - induced AD in mice | Inhibition of upregulation of p-Akt protein expression and downregulation of ZO-3 protein expression in the skin of AD model mice | Hu (2020) |
| Lu Hui (Aloe vera (L.) Burm.f.) | DNFB- induced AD in mice | Suppresses Th17 cell immune response, reduces IgE levels, and inhibits NF- κB signaling pathway | Chen et al. (2019) |
| Ku Shen (Sophora flavescens Aiton) | OVA- induced AD in mice | Regulate the expression of PAR-2 and downstream receptor protein TrK-A in skin lesions, and reduce the content of PGE2, LTB4, SP, and CGRP in serum | Song et al. (2022b) |
| Yi Yi Ren (Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf) | DNCB - induced AD in mice | Reduce the levels of IgE and IL-4 in serum, and increase IFN- γ Level, regulating the expression of AQP3, TLR2, and TLR4 in the skin | Wang (2018) |
| Hai Tong Pi (Erythrina variegata L.) | DNCB - induced AD in mice | Regulate p38/NF- κ B signaling pathways | Chen et al. (2023) |
| Zi Cao (Lithospermum erythrorhizon Siebold & Zucc.) | OX - induced AD in mice | Reduce the level of inhibitor protein IκBα, thereby inhibiting the expression of COX-2 and iNOS | Kim et al. (2008) |
The effects of single TCM botanical drugs on AD.
Dfe, Dermatophagoides farinae extract; AD, atopic dermatitis; DNCB, 1-Chloro-2,4-dinitrobenzene; DNFB, 2,4-dinitrofluorobenzene; OVA, ovalbumin; OX, oxazolone; MC903, Calcipotriol; IL, interleukin.
FIGURE 5
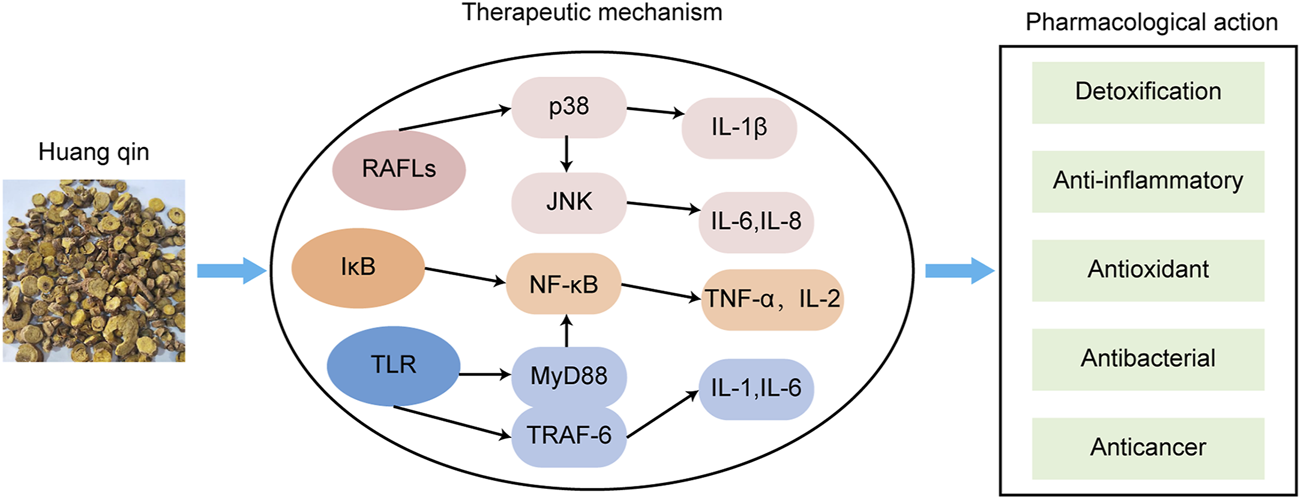
The main mechanisms of Huangqin against AD.
Research has shown that in addition to the aforementioned preparations, various individual TCM extracts also treat AD.
Terminalia chebula Retz. [Combretaceae] (HeZi, TC) is a botanical drug that belongs to a tree of the genus Elephantia in the family Meneraceae, and it is a dried and mature fruit. TC is commonly used as a TCM. (Li H. et al., 2022). The chemical metabolites isolated from TC are mainly divided into three types according to their structure: phenolic acids, tannins, and triterpenes. Most of the metabolites in TC have anti-inflammatory and antioxidant effects. As a result, TC single TCM may become a therapeutic drug for treating AD. Hye Jin Kim et al. used AD in induced by Dermatophagoides farinae extract (Dfe) in NC/Nga mice. The results showed that after treatment with TC reduced lesions caused by AD. TC treatment reduced the serum levels of histamine, IgE, and other chemokines, and can inhibit STAT1/3 and NF-κB signaling pathway by regulating inflammatory factors (Kim et al., 2022).
Paeonia lactiflora Pall. [Paeoniaceae] (BaiShao, PL) is a traditional botanical drug. Recently, several reports have shown that PL is effective for the treatment of various diseases (Ou et al., 2011; Zhang and Dai, 2012). Paeoniflorin and total paeoniflorin are the main metabolites of PL. Paeoniflorin has been shown to induce lymphocyte apoptosis (Tsuboi et al., 2004; Wu et al., 2019). The results of Lee et al. indicate that PL attenuates pathological changes in antibiotic-induced AD model mice (Lee S. Y. et al., 2022). Many studies have shown that white peony has anti-inflammatory and immunomodulatory activities (He and Dai, 2011). Shen et al. constructed 1-chloro-2,4-dinitrobenzene (DNCB) -induced mouse AD model and administered total peony glucosides (200 mg/kg) by gavage. Under the effect of total peony glucosides, the thickness of the ear, the score of the skin lesion degree, and the number of scratches were significantly reduced, and the damage to the skin structure compared with the model group, there was a significant decrease. Moreover, the infiltration of inflammatory cells and mast cells was reduced, and reduced the levels of IgE and IL-6, indicating that total peony glucosides can reduce the inflammatory reaction in AD model mice and improve the pathological symptoms of AD (Shen et al., 2021). Liu et al. treated AD model mice established with DNCB with total peony glycosides and found that total peony glycosides had an inhibitory effect on the symptoms of AD model mice. PL may be could inducing the expression of Foxp3, increasing the integrity of the intestinal barrier, and altering the composition of intestinal microbiota. TGP can significantly inhibit the activation of NLRP3, increase Th1-secreted IFN-γ levels, and inhibit Th2-secreted IL-4, thereby inhibiting the synthesis and expression of IgE and regulating the balance of Thl/Th2, producing a certain therapeutic effect on AD (Liu et al., 2017).
Dictamnus dasycarpus Turcz. [Rutaceae] (Bai Xian) also known as White Fresh Skin, Eight Stranded Cow, and Mountain Peony, is a perennial botanical drug in the Rutaceae family, belonging to the genus Lysimachia. The stem of Bai Xian is rigid and woody and grows to a height of up to 100 cm. In TCM theory, its effects are defined as reducing fever, detoxification, dispelling wind, and relieving itching. It can also treat skin itching, eczema, jaundice, rheumatism, and other diseases (Qin et al., 2021). It also has antitumour (Park et al., 2015), antioxidation (Lin et al., 2021), anti-inflammatory (Yang N. et al., 2023), antibacterial, and antiallergic effects (Qin et al., 2021). It can be taken orally in the form of a decocted liquid or used externally by grinding it into a powder. It can not only treat various diseases but also be used as a pollution-free pesticide (Du et al., 2023). Yang et al. used DNCB-induced AD mouse model to observe scratching behaviour and inflammatory behaviour and to evaluate the expression of MrgprA3 and TRPA1 in the skin and DRG. The data showed that dichloraz effectively inhibited AD-induced chronic itching, inflammatory symptoms, epidermal thickening, and inflammatory cell infiltration and downregulated the expression of MrgprA3 and TRPA1. Molecular docking also shows that fresh amine has a better binding affinity with MrgprA3. Dichloraz may inhibit chronic itching caused by AD through the histamine-independent itching pathway mediated by MrgprA3-TRPA1, indicating that dichloraz may have potential efficacy in the treatment of AD (Yang et al., 2022).
Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. [Apiaceae](Bai Zhi) belongs to the Umbrellaceae genus and is a famous Chinese botanical drug. The roots of Angelica dahurica are often used to treat headaches, toothache, abscesses, boils and acne. They have functions such as alleviating disease symptoms, removing dampness, expelling pus, generating muscle, promoting blood circulation and relieving pain. Chen et al. used a gel with xanthopterin, an extract of Angelica dahurica, to treat mice with MC-903-induced AD-like skin. The experimental results showed that xanthopterin gel significantly improved the symptoms of AD in mice and significantly reduced the thickness of the mouse epidermal scales and serum IgE levels (Chen et al., 2022). The results of Zhu et al. showed that Bai Zhi also reduced activity and protein expression of TRPV1 (Zhu et al., 2022). Wang et al. used Angelica dahurica aloe gel to treat 16 patients with radiation dermatitis for 4 weeks. The final results showed that the clinical effect of Angelica dahurica aloe gel was equivalent to that of steroid drugs, which could help patients effectively improve skin pain and itching, and no adverse reactions occurred (Wang and Lei, 2018).
Cnidium monnieri (L.) Cusson [Apiaceae] (She Chuang Zi) is the mature and dried fruit of the Apiaceae plant Cnidii. Osthol (OS) in Cnidii is a natural coumarin with a long medicinal history and can be used for a variety of diseases, both internally and externally, with excellent external treatment and multiple effects, such as anti-inflammatory, antiviral and antiallergic effects. Kordulewska et al. used osthol to treat 3D skin models of AD stimulated by LPS and histamine in vitro, and the results showed that osthol significantly improved the integrity of the 3D skin models and reduced the secretion of pro-inflammatory cytokines, chemokines, and proteins (Kordulewska et al., 2021). Chen et al. intraperitoneally injected mice with osthol, and the skin lesions of the mice were significantly improved. The expression of connexin mRNA was abnormally downregulated, while the expression of p-Akt was downregulated in mice after treatment with osthol. The expression of connexin mRNA was abnormally downregulated, with ZO-3 being the most significantly downregulated. Moreover, the use of osthol downregulated the expression of p-Akt, indicating that osthol can regulate the expression of tight junction proteins in the skin of AD model mice through the PI3K/Akt pathway, which can improve skin barrier damage (Chen J. et al., 2021). Du et al. pointed out that osthol has strong active oxygen scavenging and anti-inflammatory effects, and its anti-inflammatory activity is mediated through a variety of mechanisms, including inhibition of various transcription factors, and downregulation of a variety of pro-inflammatory factors and chemokines (Du et al., 2020).
3.3.3 TCM metabolites
In recent years, with the advancement of science, many researchers have conducted detailed research on the active metabolites of TCM and identified many plant metabolites.
Piceatannol is a natural polyphenol metabolite found in a variety of edible plants (blueberries, grapes, passionfruit, etc.). It has a variety of health promotive functions, such as anti-diabetes, neuroprotection, anti-allergy, and anti-ageing activities (Yang W. et al., 2023). Lee et al. successfully reduced the symptoms of AD, by using a treatment drug supplemented with piceatannol in an AD mouse model induced by dust mite extract (Lee C. H. et al., 2022). Yang used network pharmacology and cytology experiments to demonstrate that piceatannol has an inhibitory effect on the expression of NF-κB and that NF-κB is an effective target for the treatment of AD with piceatannol, providing a new possibility for the treatment of AD. Piceatannol inhibited the infiltration of immune cells into the skin of DFE-induced AD model mice, reducing TNF α/IFN-γ levels, and induced phosphorylation of JAK STAT protein in HaCaT cell lines. The results of Macromolecular docking study showed that there was a strong interaction between piceatannol and JAK1 (Yang, 2022).
Vasicine is a pyrrolo [2,1-b] quinazoline alkaloid isolated from the flower of duckbill. Duckbill alkaloids have many active sites and exhibit a wide range of biological activities after binding to receptors, and have anti-inflammatory, antiasthmatic, antioxidant, antitussive and low toxicity characteristics (Kong et al., 2022). Zhang et al. evaluated the anti-AD effect of duckbill alkaloids induced by DNCB in mice. The potential anti-allergic effect of duckbill alkaloids was evaluated using a passive skin anaphylaxis (PCA) test. They found that oral administration of duckbill alkaloids could reduce histopathological changes and restore epidermal thickness, improving the severity of AD-like lesions in the skin. Duckbill alkaloids can significantly inhibit mast cell infiltration and effectively reduce serum IgE and Th2 cytokine levels, demonstrating their therapeutic potential for AD. In a PCA mouse model, duckbill alkaloids reduced serum IgE levels (Zhang et al., 2022a). This result suggests duckbill alkaloids may benefit allergic or atopic diseases associated with elevated IgE levels (Table 5).
TABLE 5
| Name | Botanical drug | Evaluationmodel | Mechanism of action | The literature |
|---|---|---|---|---|
| Piceatannol | Reynoutria japonica Houtt. | Dfe - induced AD in mice | Downregulation of inflammatory markers, including serum and skin TARC and MDC. Piceatannol decreased phosphorylation of JAK-STAT protein | Lee et al. (2022a) |
| Vasicine | Justicia adhatoda L. | DNCB - induced AD in mice | Inhibited the infiltration of mast cells in the skin and reduced the levels of pro-Th2 and Th2 cytokines as well as immunoglobulin E in the serum. Finally, vasicine inhibited the expression of pro-Th2 and Th2 cytokines in skin tissues | Zhang et al. (2022a) |
| Myricetin | NA | MC903 - induced AD in mice | Blocking the NF-κB and STAT1 signal pathway | Hou et al. (2022a) |
| Quercetin | NA | MC903 - induced AD in mice | Reduce the expression of CCL17, CCL22, IL-4, IL-6, IFN-γ and TNF-α | Hou et al. (2019) |
| Scutellarein | Erigeron breviscapus (Vaniot) Hand.-Mazz. | DNFB - induced AD in mice | Inhibiting TRPV3 | Wang et al. (2022b) |
| (-) -α-Bisabolol | Matricaria chamomilla L. | DNCB - induced AD in mice | Inhibiting MAPK and NF-κB Signaling in MC | Li et al. (2022a) |
| Phellopterin | Glehnia littoralis (J.G.Cooper) F.Schmidt ex Miq. | MC903 - induced AD in mice | Phellopterin suppressed phosphorylation of signal transducer and STAT3 at Tyr705, and the expression of TSLP and IL-33 in epidermal keratinocytes of AD-like lesions | Chen et al. (2022) |
| Notoginsenoside R1 | Panax notoginseng (Burkill) F.H.Chen | LPS - establish the in vitro cell | Inhibiting inflammation through suppressing the NF-κB signaling pathway and NLRP3 inflammasome activation | Wang and Ma (2019) |
| 7-Methoxyisoflavone | NA | OX - induced AD in mice | Regulating Th1/Th2 balance | Dong et al. (2022) |
| Pseudoephedrine | Ephedra sinica Stapf | DNCB - induced AD in mice | Suppressed the activation of MAPKs and NF-κB signaling pathways in vivo and in vitro | Chen et al. (2020) |
| Daphnetin | Daphne kamtschatica var. kamtschatica | DNCB - induced AD in mice | Decreased the expression levels of histamine, IL-4, IL-6, IL-13, MIP-1α and TNF-α, and reduced the protein expression levels of phosphorylated MAPKs, P-Lyn and P-syk in the RBL-2H3 cells | Zhang et al. (2022b) |
| Lonicera japonica polysaccharide | Lonicera japonica Thunb. | DNCB - induced AD in mice | By promoting Nrf2 activation and NLRP3 inflammasome degradation via p62 | Bai et al. (2023) |
| 2,4-dimethoxy-6-methylbenzene-1,3-diol | Antrodia camphorata | OVA - induced AD in mice | Stopped the upregulation of chemokines (CCL5 and CCL17) and increased the expression of differentiation proteins (filaggrin, involucrin, and integrin β-1) | Yang et al. (2018) |
| Paeonol | Paeonia × suffruticosa Andrews | DNCB - induced AD in mice | Reduced the protein expression levels of p-p38 and p-ERK | Meng et al. (2019) |
| Bisdemethoxycurcumin | Zingiber officinale Roscoe | DNCB - induced AD in mice | Inhibit the mRNA expression levels of chemokines and inflammatory cytokines and the activation of the MAPK and NF-κB signaling pathways | Wang et al. (2022c) |
| Crude polysaccharide | White wax scale | DNCB - induced AD in mice | Through modulating T cell-elicited immune responses and CD4+T cell polarization | Lin et al. (2017) |
| Glycyrrhizic acid | Glycyrrhiza glabra L. | MC903 - induced AD in mice | Suppressed the Th1/Th2/Th17-immune responses in the dLNs, inhibited the migration of LCs in dLNs | Hou et al. (2022b) |
| Rutaecarpine | Tetradium ruticarpum (A.Juss.) T.G.Hartley | DNFB- induced AD in mice | Regulation of IL-4/STAT6 signaling pathway | Dun et al. (2022) |
| Isoglycyrrhizin | Glycyrrhiza glabra L. | DNCB- induced AD in mice | Reduce serum IL-4 and TNF- α And IgE levels, inhibiting the JAK-STAT pathway to mediate | Li et al. (2021b) |
| Berberine tannate | Coptis chinensis Franch. | DNCB- induced AD in mice | Suppress participation in PPAR-γ Upregulated HMGB1, RAGE, NF-κB, COX-2 signaling pathway and inflammatory cytokines such as IL-1β, TNF-α, IL-4 and IFN-γ | Ma et al. (2023) |
The effect of TCM metabolites on AD.
NA, not applicable; Dfe, Dermatophagoides farinae extract; AD, atopic dermatitis; DNCB, 1-Chloro-2,4-dinitrobenzene; DNFB, 2,4-dinitrofluorobenzene; OVA, ovalbumin; OX, oxazolone; MC903, Calcipotriol; LPS, lipopolysaccharide; IL, interleukin.
3.4 Clinical evidence for the use of TCM in AD
Currently, many TCM treatment methods have been used for the clinical treatment of AD, and many TCMs for AD treatment have entered clinical research (reference website:https://www.yaozh.com, Table 6). Controlled studies of AD are often compared to glucocorticoids, calcineurin inhibitors, antibiotics and antihistamines.
TABLE 6
| Stage | Drug name | Metabolites | Method | Intervention measures | Indication | |
|---|---|---|---|---|---|---|
| Experimental group | Control group | |||||
| Phase II | Peitu Qingxin Granules | Atractylodes macrocephala Koidz., Forsythia suspensa (Thunb.) Vahl, Hydrastis canadensis L., Pseudostellaria heterophylla (Miq.) Pax, Coix lacryma-jobi L., Dioscorea oppositifolia L., Concha Margaritifera, Dictamnus dasycarpus Turcz., Glycyrrhiza glabra L. | Random double-blind | PeiTu QingXin Granules | PeiTu QingXin Granules | AD |
| Exploratory study/pre- test | Zicao Ointment | Arnebia euchroma (Royle ex Benth.) I.M.Johnst., Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav., Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Angelica sinensis (Oliv.) Diels | Randomized parallel control | Zicao Ointment | Mometasone furoate | AD |
| Exploratory study/pre- test | Taxisan | Rhus chinensis Mill., Zanthoxylum armatum DC., Cnidium monnieri (L.) Cusson, Sophora flavescens Aiton, Alumen, Allium cepa L. | Randomized parallel control | Taxisan | Moisturizer (Vaseline, glycerin and paraffin oil) | AD |
| NA | Anshenjianpizhiyang granule | NA | Randomized parallel | Anshenjianpizhiyang granule | placebo | Mild to moderate AD |
| Exploratory study/pre- test | Jiuwei Yongan Tang Granules | Alumen, Dioscorea oppositifolia L., Forsythia suspensa (Thunb.) Vahl, Isatis tinctoria subsp. tinctoria, Dioscorea collettii var. hypoglauca (Palib.) S.J.Pei & C.T.Ting, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Angelica sinensis (Oliv.) Diels, Alisma plantago-aquatica subsp. orientale (Sam.) Sam., Plantago ovata Forssk. | Randomized parallel | Jiuwei Yongan Tang Granules | Xianteming | AD |
| Exploratory study/pre- test | Qinzhu Liangxue granule | Scutellaria baicalensis Georgi, Margaritifera Concha, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Scrophularia ningpoensis Hemsl., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Lithospermum erythrorhizon Siebold & Zucc., Glycyrrhiza glabra L., Vincetoxicum mukdenense Kitag., Paeonia lactiflora Pall., Paeonia × suffruticosa Andrews, Lonicera japonica Thunb. | Randomized parallel | Qinzhu Liangxue granule | Mizolastine Sustained Release Tablets | AD |
| NA | Chushi Zhiyang Mixture | Lonicera japonica Thunb, Scutellaria baicalensis Georgi, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Paeonia lactiflora Pall., Bassia scoparia (L.) Beck, Poria Cocos (Schw.) Wolf. | Randomized parallel | Chushi Zhiyang Mixture + Hydrocortisone butyrate cream | Cetirizine + Hydrocortisone butyrate cream | Mild to moderate AD |
| Phase IV | Bu Shen Yi Qi Fang | Astragalus mongholicus Bunge, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Epimedium brevicornum Maxim, etc | Randomized parallel | Bu Shen Yi Qi Fang + Desloratadine tablet | Imitation of Bu Shen Yi Qi Fang + Desloratadine tablet | Severe AD (Patients with syndrome of kidney deficiency and qi deficiency) |
| Exploratory study/pre- test | Chuankezhi | Epimedium sagittatum (Siebold & Zucc.) Maxim., Gynochthodes officinalis (F.C.How) Razafim. & B.Bremer | Randomized parallel | Basic treatment + Chuankezhi | Basic treatment | AD (Yin deficiency syndrome) |
Clinical trials of TCM on AD.
NA, not applicable; AD, atopic dermatitis.
At the same time, the clinical efficacy of some TCM was also noted (Table 7). For example, Geng and Zhang (2023) conducted a clinical study on the efficacy of orally administered Danggui Zhiyang Formula (prescription composition: Angelica sinensis, Ligusticum chuanxiong, Radix Rehmanniae, Stir-fried white peony, S. divaricata (Turcz. ex Ledeb.) Schischk., Stir-fried tribulus, bergamot, Zhetong bark, astragalus, raw liquorice) on AD. The effective rate was 91.89%, which was significantly larger than the control group (61.76%).
TABLE 7
| Drug name | Control group | Group prescription | Number ofcases | Effective rate | Control group | Reference |
|---|---|---|---|---|---|---|
| Experience group | Experience group | |||||
| Maidong Dendrobium lotion + Desloratadine tablets | Desloratadine tablets | Dendrobium officinale Kimura & Migo, Ophiopogon intermedius D.Don, Bletilla striata (Thunb.) Rchb.f., Portulaca oleracea L., Phellodendron amurense Rupr., Phaseolus vulgaris L., Angelica sinensis (Oliv.) Diels, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Glycyrrhiza glabra L., Linum usitatissimum L., Prunus persica (L.) Batsch, Carthamus tinctorius L. | 40 | 79.95% | 45.74% | Liu and Sun (2023) |
| Danggui Zhiyang Formula | Runzao Zhiyang Capsule | Angelica sinensis (Oliv.) Diels, Conioselinum anthriscoides ‘Chuanxiong’, Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Plumbago zeylanica L., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Tribulus terrestris L., Citrus × limon (L.) Osbeck, Euphorbia pekinensis Rupr., Astragalus mongholicus Bunge, Glycyrrhiza glabra L. | 70 | 91.89% | 61.76% | Geng and Zhang (2023) |
| Addition and subtraction of Sijunzi Decoction and Daochi Powder | Loratadine Syrup + Hydrocortisone Butyrate Ointment | Pseudostellaria heterophylla (Miq.) Pax, Atractylodes macrocephala Koidz., Poria Cocos (Schw.) Wolf., ehmannia glutinosa (Gaertn.) Libosch. ex DC., Lophatherum gracile Brongn., Portulaca oleracea L., Scutellaria baicalensis Georgi, Bauhinia × blakeana Dunn, Bassia scoparia (L.) Beck, Glycyrrhiza glabra L. | 80 | 95.24% | 88.10% | Ceng et al. (2022) |
| Lingshu Qushi Decoction Combined with Dry Branch Ear Position + Tacrolimus ointment | Loratadine Tablets + Tacrolimus ointment | Poria Cocos (Schw.) Wolf., Atractylodes macrocephala Koidz., Codonopsis pilosula (Franch.) Nannf., Lophatherum gracile Brongn., Platycodon grandiflorus (Jacq.) A.DC., Phragmites australis (Cav.) Trin. ex Steud., Citrus reticulata Blanco, Magnolia officinalis Rehder & E.H.Wilson, Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen, Bassia scoparia (L.) Beck, Coix lacryma-jobi L.,Atractylodes macrocephala Koidz., Glycyrrhiza glabra L. | 56 | 92.31% | 69.23% | Gao (2022) |
| Fu Ling Tai Bai Zhi Yang Decoction + Mucopolysaccharide Polysulfate Cream | Mucopolysaccharide Polysulfate Cream | Atractylodes macrocephala Koidz., Poria Cocos (Schw.) Wolf., Pseudostellaria heterophylla (Miq.) Pax, Dioscorea oppositifolia L., Bassia scoparia (L.) Beck, Perilla frutescens (L.) Britton, Alisma plantago-aquatica subsp. orientale (Sam.) Sam., Angelica sinensis (Oliv.) Diels, Platycodon grandiflorus (Jacq.) A.DC., Glycyrrhiza uralensis Fisch. ex DC. | 70 | 80% | 34.3% | Shen (2022) |
| Huaiqi Yellow Granules + Fluticasone propionate cream,+desloratadine tablets | Fluticasone propionate cream + desloratadine tablets | Vicia faba L., Lycium barbarum L., Polygonatum odoratum (Mill.) Druce | 60 | 96.67% | 73.3% | Liu (2022) |
| Long Mu Jia Wei Decoction | Loratadine Tablets | Os Draconis, Concha Ostreae Calcinationis, Forsythia suspensa (Thunb.) Vahl, Pastinaca sativa L., Ephedra sinica Stapf, Poria Cocos (Schw.) Wolf. | 60 | 90.3% | 76.67% | Chi et al. (2021) |
| Yangxue Qufeng Decoction | Bastine tablets | Paeonia lactiflora Pall., Reynoutria japonica Houtt., Sesamum indicum L., Tribulus terrestris L., Angelica sinensis (Oliv.) Diels, Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. ,Bombyx mori Linnaeus, Conioselinum anthriscoides ‘Chuanxiong’, Cicadae Periostracum | 95 | 95.83% | 80.85% | Xiao and Zhao (2021) |
| Yupingfeng Granules + Baiji Polysaccharide + Fu’an Xiaojiao | Yupingfeng Granules + Baiji Polysaccharide + Zinc Oxide | Fu’an Xiaojiao Lotion: Tarpaulin, Chrysanthemum indicum L., Poria Cocos (Schw.) Wolf., Alumen, and Cnidium monnieri (L.) Cusson. Yupingfeng Granules: Astragalus mongholicus Bunge, Atractylodes macrocephala Koidz., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. |
156 | 100% | 84.6% | Chen et al. (2021a) |
| Qiwei Baizhu Powder | Cetirizine hydrochloride drops | Pseudostellaria heterophylla (Miq.) Pax, Dolomiaea costus (Falc.) Kasana & A.K.Pandey, Poria Cocos (Schw.) Wolf., Atractylodes macrocephala Koidz., Agastache rugosa (Fisch. & C.A.Mey.) Kuntze, Pueraria lobata, Glycyrrhiza glabra L. | 80 | 86.84% | 67.57% | Hong (2021) |
| Shenling Baizhu Powder Modified Granules | Mometasone Furoate Cream | Codonopsis pilosula (Franch.) Nannf., Poria Cocos (Schw.) Wolf., Atractylodes macrocephala Koidz.,Vicia lens (L.) Coss. & Germ., Citrus reticulata Blanco, Dioscorea oppositifolia L., Glycyrrhiza glabra L., Nelumbo nucifera Gaertn., Coix lacryma-jobi L., Platycodon grandiflorus (Jacq.) A.DC., Ziziphus jujuba Mill. | 60 | 76.7% | 46.7% | Liu (2021) |
Clinical study of TCM on AD in literature.
3.5 Advantages and disadvantages of TCM in the treatment of AD
AD, a multifactorial inflammatory skin disorder, necessitates therapeutic strategies that address both symptomatic relief and long-term disease modulation. While modern medical treatments, such as topical corticosteroids and biologics, prioritize rapid suppression of inflammation, TCM emphasizes holistic regulation of immune dysregulation, skin barrier repair, and systemic balance. To elucidate their distinct profiles, a comparative evaluation of TCM and modern medicine is essential, focusing on efficacy, safety, mechanisms, and practicality. The Table 8 summarizes their respective advantages and limitations in AD management.
TABLE 8
| Aspect | TCM | Modern medical |
|---|---|---|
| Speed of efficacy | Gradual (weeks to months) | Rapid (days to weeks) |
| Mechanistic focus | Multi-target (anti-inflammatory, barrier repair, immune modulation) | Targeted (IL-4/IL-13 inhibition, JAK-STAT blockade) |
| Anti-inflammatory action | Broad, multi-target (cytokines, barrier repair) | Targeted (IL-4/IL-13 inhibitors) |
| Side effects | Generally mild; risk of botanical drug interactions or contaminants | Common (skin atrophy, immunosuppression, rebound flares) |
| Prevention of Relapse | Emphasizes systemic balance (Root Treatment) | Limited to symptom control; recurrence common post-discontinuation |
| Personalization | Tailored to individual constitution and syndrome differentiation | Standardized protocols with limited customization |
| Cost-effectiveness | Lower long-term costs (botanical drug formulations) | High |
| Evidence base | Growing preclinical data; limited large-scale RCTs | Robust RCTs and regulatory validation |
Comparison between TCM and modern medicine.
TCM, traditional Chinese medicine; RCT, randomized controlled trial.
4 Conclusion and prospects
TCM has shown promising potential in the treatment of AD. This article confirms the important therapeutic effect of TCM on AD symptoms from both research and clinical studies.
The pathogenesis of AD involves the complex interaction of multiple cell types and genetic and environmental factors and is not fully understood. Although topical glucocorticoid therapy has a certain clinical effect on AD, it has a high recurrence rate, adverse reactions are difficult to avoid, and the long-term treatment effect is not ideal. In recent years, as research on TCM and its clinical applications has progressed, it has been found that TCM plays an increasingly important role in the treatment of AD, with significant curative effects, few side effects and a low recurrence rate. TCM and certain preparations can play a therapeutic role through corresponding mechanisms regardless of dosage and route of administration. The treatment of atopic dermatitis using TCM is characterized by targeting multiple pathways and multiple targets, and it demonstrates significant therapeutic effects.
Although there are still some issues in existing research, the clinical efficacy of TCM in treating AD is evident. To enhance the effectiveness of TCM in treating AD, we should delve deeper into the correlation between various mechanisms and indicators. The mechanism of action of a drug refers to the reasons why the tested drug exerts relevant effects on the body. This index represents the response of the subject after administration. By observing this index, we analyzed the internal causes that led to changes in this index, determined the internal relationships between these indicators, and explored their mechanisms of action. Integrating the advantages of TCM and Western medicine, along with the principles of TCM syndrome differentiation, may enhance efficacy and reduce adverse reactions. This integrated approach leverages the strengths of both medical traditions, potentially leading to more effective and safer treatment options for AD patients.
Statements
Author contributions
LZ: Writing – original draft. HL: Writing – review and editing. NC: Writing – review and editing. SZ: Supervision, Writing – review and editing. YH: Methodology, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Ningbo key research and development project (No. 2023Z207, No. 2022Z148), Zhejiang key research and development project (No. 2024C03107), Ningbo key laboratory project, China (20221CXJD030002), Ningbo major research and development plan project (No. 2024Z193).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aldossary S. A. Alsalem M. Grubb B. D. (2024). Role of bradykinin and prostaglandin EP4 receptors in regulating TRPV1 channel sensitization in rat dorsal root ganglion neurons. Basic Clin. Pharmacol. Toxicol.134, 345–360. 10.1111/bcpt.13967
2
Bai X. Rao X. Wang Y. Shen H. Jin X. (2023). A homogeneous Lonicera japonica polysaccharide alleviates atopic dermatitis by promoting Nrf2 activation and NLRP3 inflammasome degradation via p62. J. Ethnopharmacol.309, 116344. 10.1016/j.jep.2023.116344
3
Brunner P. M. Guttman-Yassky E. Leung D. Y. M. (2017). The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol.139, S65–S76. 10.1016/j.jaci.2017.01.011
4
Ceng Z. Lai B. Liu S. (2022). Clinical observation on sijunzi tang wan and daochi powder in treating childhood atopic dermatitis. Sichuan Tradit. Chin. Med.40, 178–181.
5
Chen F. Chen L. Chen J. Chang M. (2021a). Clinical observation on the treatment of 78 cases of atopic dermatitis in children with triple therapy of traditional Chinese medicine. J. Tradit. Chin. Med. Pediatr.17, 46–50. 10.16840/j.issn1673-4297.2021.04.13
6
Chen J. Hong X. Duan Y. Zhang Y. Han Y. (2021b). The effect of osthol on skin barrier and chronic itching in mice with specific dermatitis. Chin. Pat. Drug43, 3489–3492. 10.3969/j.issn.1001-1528.2021.12.045
7
Chen S. Wang J. Zhou X. Chen L. Teng J. (2023). Based on p38/NF- κ exploring the mechanism of B signal pathway in the treatment of atopic dermatitis with haitong skin. J. Shandong Univ. Tradit. Chin. Med.47, 194–201. 10.16294/j.cnki.1007-659x.2023.02.013
8
Chen X. Lin J. Liang Q. Chen X. Wu Z. (2020). Pseudoephedrine alleviates atopic dermatitis-like inflammatory responses in vivo and in vitro. Life Sci.258, 118139. 10.1016/j.lfs.2020.118139
9
Chen X. Zhang Y. Pei J. Zeng X. Yang Y. Zhang Y. et al (2022). Phellopterin alleviates atopic dermatitis-like inflammation and suppresses IL-4-induced STAT3 activation in keratinocytes. Int. Immunopharmacol.112, 109270. 10.1016/j.intimp.2022.109270
10
Chen Y. Chen W. (2022). Genome-wide integration of genetic and genomic studies of atopic dermatitis: insights into genetic architecture and pathogenesis. J. Invest. Dermatol.142, 2958–2967.e8. 10.1016/j.jid.2022.04.021
11
Chen Z. Li X. Han L. Zhai W. (2019). Effect of aloe vera extract on IgE, IL-17, and NF in a mouse model of atopic dermatitis- κ the impact of B pathway. Chin. J. Dermatovenereology33, 644–650. 10.13735/j.cjdv.1001-7089.201809103
12
Chi H. Mao C. Wang T. Ma Y. Huang Y. Yao C. et al (2021). Clinical observation on the treatment of 31 cases of moderate to severe atopic dermatitis with rheumatic heat accumulation syndrome with Long Mu Jia Wei Tang. J. Tradit. Chin. Med.62, 1617–1621. 10.13288/j.11-2166/r.2021.18.011
13
Chitnis T. Weiner H. L. (2017). CNS inflammation and neurodegeneration. J. Clin. Invest.127, 3577–3587. 10.1172/JCI90609
14
Chong A. C. Visitsunthorn K. Ong P. Y. (2022). Genetic/environmental contributions and immune dysregulation in children with atopic dermatitis. J. Asthma Allergy.15, 1681–1700. 10.2147/jaa.s293900
15
Chu M. Tsang M. S.-M. He R. Lam C. W.-K. Quan Z. B. Wong C. K. (2020). The active compounds and therapeutic mechanisms of pentaherbs formula for oral and topical treatment of atopic dermatitis based on network pharmacology. Plants9, 1166. 10.3390/plants9091166
16
De Bruyn Carlier T. Badloe F. M. S. Ring J. Gutermuth J. Kortekaas Krohn I. (2021). Autoreactive T cells and their role in atopic dermatitis. J. Autoimmun.120, 102634. 10.1016/j.jaut.2021.102634
17
Dong H. Feng C. Cai X. Hao Y. Gu X. Cai L. et al (2022). 7-Methoxyisoflavone ameliorates atopic dermatitis symptoms by regulating multiple signaling pathways and reducing chemokine production. Sci. Rep.12, 8760. 10.1038/s41598-022-12695-3
18
Drislane C. Irvine A. D. (2020). The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol.124, 36–43. 10.1016/j.anai.2019.10.008
19
Du J. Nan G. Li X. Wang W. Piao S. (2023). Research progress on medicinal plant Bai Xian. China Feed, 09, 148–154. 10.15906/j.cnki.cn11-2975/s.2022040007-01
20
Du M. Xiang R. Fan Y. Li Q. Xu B. (2020). Research progress on the pharmacological effects and anti-inflammatory mechanism of osthol. J. Yunnan Coll. Tradit. Chin. Med.43, 92–98. 10.19288/j.cnki.issn.1000-2723.2020.06.015
21
Dun G. Qi X. Wu D. Zang F. Wang Z. (2022). Study on the improvement of Tetradium ruticarpum hypobase on IL-4/STAT6 pathway in mice with atopic dermatitis. J. Tradit. Chin. Med.37, 2643–2648. 10.16368/j.issn.1674-8999.2022.12.471
22
Fan P. Yang Y. Liu T. Lu X. Huang H. Chen L. et al (2021). Anti-atopic effect of Viola yedoensis ethanol extract against 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin dysfunction. J. Ethnopharmacol.280, 114474. 10.1016/j.jep.2021.114474
23
Gao X. (2022). Clinical observation on the treatment of atopic dermatitis of spleen deficiency and dampness accumulation type with Lingshu Qushi Yin combined with dry branch ear position. Shandong University of Traditional Chinese Medicine. 10.27282/d.cnki.gsdzu.2022.000688
24
Gatmaitan J. G. Lee J. H. (2023). Challenges and future trends in atopic dermatitis. Int. J. Mol. Sci.24, 11380. 10.3390/ijms241411380
25
Geng M. Zhang H. (2023). Clinical observation on the therapeutic effect of Xia’s Danggui Zhiyang formula on adult atopic dermatitis of blood deficiency and wind dryness type. Liaoning J. Tradit. Chin. Med., 1–11. 10.13192/j.issn.1000-1719.2024.02.023
26
Gu X. Jing D. Xiao Y. Zhou G. Yang S. Liu H. et al (2023). Association of air pollution and genetic risks with incidence of elderly-onset atopic dermatitis: a prospective cohort study. Ecotoxicol. Environ. Saf.253, 114683. 10.1016/j.ecoenv.2023.114683
27
He D.-Y. Dai S.-M. (2011). Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional Chinese herbal medicine. Front. Pharmacol.2, 10. 10.3389/fphar.2011.00010
28
Hemrajani C. Negi P. Parashar A. Gupta G. Jha N. K. Singh S. K. et al (2022). Overcoming drug delivery barriers and challenges in topical therapy of atopic dermatitis: a nanotechnological perspective. Biomed. Pharmacother.147, 112633. 10.1016/j.biopha.2022.112633
29
Hong P. (2021). Clinical observation on the treatment of atopic dermatitis of spleen deficiency type in children with Qiwei Baizhu powder (attachment: investigation on the influential factors of atopic dermatitis in preschool children). Hunan Univ. Chin. Med.10.27138/d.cnki.ghuzc.2021.000307
30
Hou D.-D. Gu Y.-J. Wang D.-C. Niu Y. Xu Z.-R. Jin Z.-Q. et al (2022a). Therapeutic effects of myricetin on atopic dermatitis in vivo and in vitro. Phytomedicine102, 154200. 10.1016/j.phymed.2022.154200
31
Hou D.-D. Wang X.-X. Li S.-J. Wang D.-C. Niu Y. Xu Z.-R. et al (2022b). Glycyrrhizic acid suppresses atopic dermatitis-like symptoms by regulating the immune balance. J. Cosmet. Dermatol.21, 7090–7099. 10.1111/jocd.15383
32
Hou D.-D. Zhang W. Gao Y.-L. Sun Y. Wang H.-X. Qi R.-Q. et al (2019). Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol.74, 105676. 10.1016/j.intimp.2019.105676
33
Hu X. (2020). Study on the mechanism of osthoprim regulating akt/ZO-3 signal pathway to repair skin barrier. Nanjing University of Chinese Medicine. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkyRJRH-nhEQBuKg4okgcHYqndK74Ho33odx2Birh6eldF7dOjE9qSQqb-nJG-_S6A&uniplatform=NZKPT (Accessed June 21, 2023).
34
Ikai M. Murakami M. Kanei T. Asahina R. Iwata M. Kamishina H. et al (2023). Phosphorylation of Janus kinase 1 and signal transducer and activator of transcription 3 and 6 in keratinocytes of canine atopic dermatitis. Vet. Dermatol34, 318–326. 10.1111/vde.13156
35
Jia J. (2020). Progress in traditional Chinese medicine research on atopic dermatitis. Mod. Chin. Med.40, 92–96. 10.13424/j.cnki.mtcm.2020.06.022
36
Kantor R. Silverberg J. I. (2017). Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol.13, 15–26. 10.1080/1744666X.2016.1212660
37
Kim H. J. Song H.-K. Park P. Jang S. Park K.-S. Song K. H. et al (2022). Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-ĸB signaling in vitro. Phytomedicine104, 154318. 10.1016/j.phymed.2022.154318
38
Kim J. Kim Y. Seo D. Kim S. Lee S. Cho Y. (2008). Oral supplementation of gromwell (Lithospermum erythrorhizon) extract prevents the development of atopic dermatitis with reducing ceramide degradation in the epidermis of NC/Nga mice. Faseb J.22. 1108.8–1108.8. 10.1096/fasebj.22.1_supplement.1108.8
39
Koh L. F. Ong R. Y. Common J. E. (2022). Skin microbiome of atopic dermatitis. Allergol. Int.71, 31–39. 10.1016/j.alit.2021.11.001
40
Kong L. Li M. Zou Q. Sun Y. Mao Z. Huang F. (2022). Synthesis and anti-inflammatory activity evaluation of novel derivatives of duckbill alkaloids. Synth. Chem.30, 269–273. 10.15952/j.cnki.cjsc.1005-1511.21153
41
Kong L. Wu J. Wu J. Lin Y. Wang G. Wang J. et al (2015). BuShenYiQi granule inhibits atopic dermatitis via improving central and skin hypothalamic-Pituitary-Adrenal axis function. PLoS One10, e0116427. 10.1371/journal.pone.0116427
42
Kwatra S. G. Misery L. Clibborn C. Steinhoff M. (2022). Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin. Transl. Immunol.11, e1390. 10.1002/cti2.1390
43
Lai A. Owens K. Patel S. Nicholas M. (2023). The impact of air pollution on atopic dermatitis. Curr. Allergy Asthma Rep.23, 435–442. 10.1007/s11882-023-01095-w
44
Lee C. H. Yang H. Park J. H. Y. Kim J.-E. Lee K. W. (2022a). Piceatannol, a metabolite of resveratrol, attenuates atopic dermatitis by targeting Janus kinase 1. Phytomedicine99, 153981. 10.1016/j.phymed.2022.153981
45
Lee S. Y. Hong S. H. Kim H. I. Ku J. M. Choi Y.-J. Kim M.-J. et al (2022b). Paeonia lactiflora Pallas extract alleviates antibiotics and DNCB-induced atopic dermatitis symptoms by suppressing inflammation and changing the gut microbiota composition in mice. Biomed. Pharmacother.154, 113574. 10.1016/j.biopha.2022.113574
46
Lee Y.-J. Im D.-S. (2023). Inhibitory effect of oroxylin A in a mouse model of atopic dermatitis. Inflammation46, 679–687. 10.1007/s10753-022-01764-4
47
Li G. Wu H. Sun L. Cheng K. Lv Z. Chen K. et al (2022a). (-) -α-Bisabolol alleviates atopic dermatitis by inhibiting MAPK and NF-κB signaling in mast cell. Molecules27, 3985. 10.3390/molecules27133985
48
Li H. Liu Y. Yang H. Liu Y. (2022b). Research progress on chemical components, pharmacological mechanisms, quality control, and processing of Terminalia chebula. Nat. Prod. Res. Dev.34, 2130–2141. 10.16333/j.1001-6880.2022.12.017
49
Li H. Zhang Z. Zhang H. Guo Y. Yao Z. (2021a). Update on the pathogenesis and therapy of atopic dermatitis. Clin. Rev. Allergy Immunol.61, 324–338. 10.1007/s12016-021-08880-3
50
Li N. Pan X. Chi L. (2021b). Mechanism study on the treatment of allergic dermatitis in mice with isoglycyrrhizin through the JAK/STAT pathway. Hebei Med.27, 710–715. 10.3969/j.issn.1006-6233.2021.05.02
51
Lin L. Zhou Y. Li H. Pang D. Zhang L. Lu X. et al (2017). Polysaccharide extracted from Chinese white wax scale ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like symptoms in BALB/c mice. Saudi Pharm. J.25, 625–632. 10.1016/j.jsps.2017.04.035
52
Lin Q. Guan H. Ma C. Chen L. Cao L. Liu H. et al (2021). Biotransformation patterns of dictamnine in vitro/in vivo and its relative molecular mechanism of dictamnine-induced acute liver injury in mice. Environ. Toxicol. Pharmacol.85, 103628. 10.1016/j.etap.2021.103628
53
Liu B. Li J. Hu H. Zhu L. (2017). Protective effect of total glycosides of peony on Nc/Nga mouse atopic dermatitis. China J. Lepr. Skin. Dis.33, 31–33.
54
Liu L. (2022). Clinical efficacy of Huaiqi Huang granules in the treatment of atopic dermatitis and its effect on serum Th1/Th2 cytokines. Chengde Medical College. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkaWjBDt8_rTOnKA7PWSN5MLEDQU0fRsaA-P8zHIWgiMy_FAxaSJdupNquIzGSLTSG&uniplatform=NZKPT (Accessed June 21, 2023). 10.27691/d.cnki.gcdyx.2022.000030
55
Liu M. (2021). The clinical effect of Shenling Baizhu powder on atopic dermatitis of spleen deficiency type and its influence on gut microbiota. Jiangxi Univ. Traditional Chin. Med.10.27180/d.cnki.gjxzc.2021.000117
56
Liu W. Song W. Luo Y. Dan H. Li L. Zhang Z. et al (2022a). Angelica Yinzi alleviates 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis by inhibiting activation of NLRP3 inflammasome and down-regulating the MAPKs/NF-kB signaling pathway. Saudi Pharm. J.30, 1426–1434. 10.1016/j.jsps.2022.07.003
57
Liu Y. Du X. Zhai S. Tang X. Liu C. Li W. (2022b). Gut microbiota and atopic dermatitis in children: a scoping review. BMC Pediatr.22, 323. 10.1186/s12887-022-03390-3
58
Liu Y. Sun S. (2023). Clinical observation on the treatment of atopic dermatitis of blood deficiency and wind dryness type with maidong dendrobium lotion. Shanxi Tradit. Chin. Med.39, 47–48. 10.20002/j.issn.1000-7156.2023.03.019
59
Ma Y. Men Y. Chu M. Wang Z. Huang G. Jiang L. et al (2023). Study on the therapeutic effect of tannic acid Berberine on atopic dermatitis. J. Liaoning Univ. Tradit. Chin. Med.25, 21–24+221. 10.13194/j.issn.1673-842x.2023.04.006
60
Mailepessov D. Ong J. Nasir M. Z. M. Aik J. Woo M. Zhao X. et al (2024). Association between exposure to ambient air pollution, meteorological factors and atopic dermatitis consultations in Singapore—a stratified nationwide time-series analysis. Sci. Rep.14, 10320. 10.1038/s41598-024-60712-4
61
Markowiak-Kopeć P. Śliżewska K. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients12, 1107. 10.3390/nu12041107
62
Meng Y. (2021). The regulatory effect and mechanism of Qingre Chushi decoction and its active components on atopic dermatitis mice and mast cell. Beijing University of Chinese Medicine. 10.26973/d.cnki.gbjzu.2021.000111
63
Meng Y. Liu Z. Zhai C. Di T. Zhang L. Zhang L. et al (2019). Paeonol inhibits the development of 1-chloro-2,4-dinitrobenzene-induced atopic dermatitis via mast and T cells in BALB/c mice. Mol. Med. Rep.19, 3217–3229. 10.3892/mmr.2019.9985
64
Miura A. Kitayama T. Ouchi Y. Saga K. Shimbo T. Tamai K. (2025). Evaluation of the digestion protocol of mouse neonatal epidermis for single-cell RNA sequencing. Biochem. Biophys. Res. Commun.743, 151159. 10.1016/j.bbrc.2024.151159
65
Naeem A. S. Tommasi C. Cole C. Brown S. J. Zhu Y. Way B. et al (2017). A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls filaggrin expression and processing in skin, a novel mechanism for skin barrier disruption in patients with atopic dermatitis. J. Allergy Clin. Immunol.139, 1228–1241. 10.1016/j.jaci.2016.09.052
66
Narla S. Silverberg J. I. (2020). The role of environmental exposures in atopic dermatitis. Curr. Allergy Asthma Rep.20, 74. 10.1007/s11882-020-00971-z
67
Oh J.-S. Seong G.-S. Kim Y.-D. Choung S.-Y. (2021). Deacetylasperulosidic acid ameliorates pruritus, immune imbalance, and skin barrier dysfunction in 2,4-dinitrochlorobenzene-induced atopic dermatitis NC/Nga mice. Int. J. Mol. Sci.23, 226. 10.3390/ijms23010226
68
Ou T.-T. Wu C.-H. Hsu J.-D. Chyau C.-C. Lee H.-J. Wang C.-J. (2011). Paeonia lactiflora Pall inhibits bladder cancer growth involving phosphorylation of Chk2 in vitro and in vivo. J. Ethnopharmacol.135, 162–172. 10.1016/j.jep.2011.03.011
69
Park H. Hong N. Ahn T. Kim H. Jung M. Kim B. (2015). Apoptosis of AGS human gastric adenocarcinoma cells by methanolic extract of Dictamnus. Pharmacogn. Mag.11, s329–S336. 10.4103/0973-1296.165994
70
Qin Y. Quan H.-F. Zhou X.-R. Chen S.-J. Xia W.-X. Li H. et al (2021). The traditional uses, phytochemistry, pharmacology and toxicology of Dictamnus dasycarpus: a review. J. Pharm. Pharmacol.73, 1571–1591. 10.1093/jpp/rgab141
71
Qinwufeng G. Jiacheng L. Xiaoling L. Tingru C. Yunyang W. Yanlong Y. (2022). Jiu-Wei-Yong-An Formula suppresses JAK1/STAT3 and MAPK signaling alleviates atopic dermatitis-like skin lesions. J. Ethnopharmacol.295, 115428. 10.1016/j.jep.2022.115428
72
Schmidt A. D. de Guzman Strong C. (2021). Current understanding of epigenetics in atopic dermatitis. Exp. Dermatol.30, 1150–1155. 10.1111/exd.14392
73
Shen Y. (2022). Clinical study on fuling taibai Zhiyang tang in treating atopic dermatitis of spleen deficiency and dampness accumulation type. Chengde Med. Coll.10.27691/d.cnki.gcdyx.2022.000305
74
Shen Y. Zhu L. Lin F. Yang H. (2021). The mechanism of action of total glycosides of peony in the treatment of atopic dermatitis in mice. Chin. J. Clin. Pharmacol.37, 2842–2846. 10.13699/j.cnki.1001-6821.2021.20.031
75
Smieszek S. P. Welsh S. Xiao C. Wang J. Polymeropoulos C. Birznieks G. et al (2020). Correlation of age-of-onset of atopic dermatitis with Filaggrin loss-of-function variant status. Sci. Rep.10, 2721. 10.1038/s41598-020-59627-7
76
Song H.-K. Park S. H. Kim H. J. Jang S. Kim T. (2022a). Spatholobus suberectus dunn water extract ameliorates atopic dermatitis–like symptoms by suppressing proinflammatory chemokine production in vivo and in vitro. Front. Pharmacol.13, 919230. 10.3389/fphar.2022.919230
77
Song X. Wang C. Yuan J. Zhang X. Wang G. Gao S. et al (2022b). The effect of Sophora flavescens on the expression of PAR-2 and TrK-A proteins in skin lesions of atopic dermatitis model mice. J. Tradit. Chin. Med.63, 869–874. 10.13288/j.11-2166/r.2022.09.014
78
Sroka-Tomaszewska J. Trzeciak M. (2021). Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci.22, 4130. 10.3390/ijms22084130
79
Thibault Greugny E. Bensaci J. Fages F. Stamatas G. N. (2023). Computational modelling predicts impaired barrier function and higher sensitivity to skin inflammation following pH elevation. Exp. Dermatol.32, 177–185. 10.1111/exd.14698
80
Thomsen S. F. Ulrik C. S. Kyvik K. O. Hjelmborg J. V. B. Skadhauge L. R. Steffensen I. et al (2007). Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc.28, 535–539. 10.2500/aap2007.28.3041
81
Tominaga M. Tengara S. Kamo A. Ogawa H. Takamori K. (2009). Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J. Dermatol. Sci.55, 40–46. 10.1016/j.jdermsci.2009.03.007
82
Tsuboi H. Hossain K. Akhand A. A. Takeda K. Du J. Rifa’i M. et al (2004). Paeoniflorin induces apoptosis of lymphocytes through a redox-linked mechanism. J. Cell. Biochem.93, 162–172. 10.1002/jcb.20134
83
van den Bogaard E. H. Elias P. M. Goleva E. Berdyshev E. Smits J. P. H. Danby S. G. et al (2023). Targeting skin barrier function in atopic dermatitis. J. Allergy Clin. Immunol. Pract.11, 1335–1346. 10.1016/j.jaip.2023.02.005
84
Wang J. (2018). Observation on the therapeutic effect of coix seed extract on BALB/c atopic dermatitis model mice. Tianjin Medical University. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkEcTGK3Qt5VuzQzk0e7M1z9uc3VnZQ0IJcbazyix_TTYzaougW3JBBq8qksPJcjAb&uniplatform=NZKPT (Accessed June 21, 2023).
85
Wang J. Lei Z. (2018). Study on the therapeutic effect of angelica dahurica and aloe gel on acute radiation Dermatitis. J. Hubei Univ. Sci. Technol. Med.32, 325–326. 10.16751/j.cnki.2095-4646.2018.04.0325
86
Wang L. Xian Y.-F. Loo S. K. F. Ip S. P. Yang W. Chan W. Y. et al (2022a). Baicalin ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway. Bioorg. Chem.119, 105538. 10.1016/j.bioorg.2021.105538
87
Wang M. Ma J. (2019). Effect of NGR1 on the atopic dermatitis model and its mechanisms. Open Med.14, 847–853. 10.1515/med-2019-0099
88
Wang Y. Tan L. Jiao K. Xue C. Tang Q. Jiang S. et al (2022b). Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol.179, 4792–4808. 10.1111/bph.15913
89
Wang Y. Zhang P. Zhang J. Hong T. (2022c). Inhibitory effect of bisdemethoxycurcumin on DNCB-induced atopic dermatitis in mice. Molecules28, 293. 10.3390/molecules28010293
90
Wang Z. Xu M. Wang H. Liu X. (2021). Study on the mechanism of angelica decoction in treating atopic dermatitis based on network pharmacology and Macromolecular docking. J. Zhengzhou Univ. Med.56, 313–319. 10.13705/j.issn.1671-6825.2020.08.152
91
Williamson S. Merritt J. De Benedetto A. (2020). Atopic dermatitis in the elderly. Br. J. Dermatol.182, e21. 10.1111/bjd.18652
92
Wu X.-X. Huang X.-L. Chen R.-R. Li T. Ye H.-J. Xie W. et al (2019). Paeoniflorin prevents intestinal barrier disruption and inhibits Lipopolysaccharide (LPS) -induced inflammation in caco-2 cell monolayers. Inflammation42, 2215–2225. 10.1007/s10753-019-01085-z
93
Xiao J. Zhao X. (2021). Clinical efficacy of Yangxue Qufeng Tang combined with ear acupoint pressing beans in the treatment of atopic dermatitis of blood deficiency and wind dryness type. J. Xinjiang Med. Univ.44, 963–967. 10.3639/j.issn.1009-5551.2021.08.019
94
Xiong X. Huang C. Wang F. Dong J. Zhang D. Jiang J. et al (2020). Qingxue jiedu formulation ameliorated DNFB-induced atopic dermatitis by inhibiting STAT3/MAPK/NF-κB signaling pathways. J. Ethnopharmacol.270, 113773. 10.1016/j.jep.2020.113773
95
Xu H. Shi X. Li X. Zou J. Zhou C. Liu W. et al (2020). Neurotransmitter and neuropeptide regulation of mast cell function: a systematic review. J. Neuroinflammation17, 356. 10.1186/s12974-020-02029-3
96
Yang G. Seok J. K. Kang H. C. Cho Y.-Y. Lee H. S. Lee J. Y. (2020). Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci.21, 2867. 10.3390/ijms21082867
97
Yang M. (2022). To explore the therapeutic effect of Piceatannol on rheumatoid arthritis through network pharmacology and experimental verification. Yan’an University. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAke-wuWrktdE-tSIT2YIbQ2CfxSbu6UuF6KxqMVlZylwqqNrTWNiMTw6eUxjL3Kjbc&uniplatform=NZKPT (Accessed June 21, 2023).
98
Yang N. Shao H. Deng J. Liu Y. (2023a). Network pharmacology-based analysis to explore the therapeutic mechanism of Cortex Dictamni on atopic dermatitis. J. Ethnopharmacol.304, 116023. 10.1016/j.jep.2022.116023
99
Yang N. Shao H. Deng J. Yang Y. Tang Z. Wu G. et al (2022). Dictamnine ameliorates chronic itch in DNFB-induced atopic dermatitis mice via inhibiting MrgprA3. Biochem. Pharmacol.208, 115368. 10.1016/j.bcp.2022.115368
100
Yang S.-C. Huang T.-H. Chiu C.-H. Chou W.-L. Alalaiwe A. Yeh Y.-C. et al (2018). The atopic dermatitis-like lesion and the associated MRSA infection and barrier dysfunction can be alleviated by 2,4-dimethoxy-6-methylbenzene-1,3-diol from Antrodia camphorata. J. Dermatol. Sci.92, 188–196. 10.1016/j.jdermsci.2018.09.002
101
Yang W. Wang Z. Wang Z. Liu J. Zhang M. Wang J. (2023b). Effect of piceatanno on cognitive function of Alzheimer’s disease mice. J. Food Sci. Technol.41, 126–134. 10.12301/spxb202200175
102
Yuan H. Sun Y. Tang Y. Zhang Y. Liu S. Liu J. et al (2022). Intervention of the Mahuang Lianqiao Chixiaodou decoction on immune imbalance in atopic dermatitis-like model mice. J. Tradit. Chin. Med. Sci.9, 392–399. 10.1016/j.jtcms.2022.09.001
103
Zeng H. R. Zhao B. Rui X. Jia G. H. Wu Y. Zhang D. et al (2021). A TCM formula VYAC ameliorates DNCB-induced atopic dermatitis via blocking mast cell degranulation and suppressing NF-κB pathway. J. Ethnopharmacol.280, 114454. 10.1016/j.jep.2021.114454
104
Zeng L. Huang Y. Sun G. (2019). Research progress on animal models, immunological mechanisms, and traditional Chinese medicine treatment of atopic dermatitis. Zhongnan Pharm.17, 696–700. 10.7539/j.issn.1672-2981.2019.05.012
105
Zhang L. Chen N. Liao Y. Kong Y. Yang X. Zhan M. et al (2024). Efficacy and action mechanisms of compound Shen Chan decoction on experimental models of atopic dermatitis. Int. Immunopharmacol.137, 112479. 10.1016/j.intimp.2024.112479
106
Zhang W. Dai S.-M. (2012). Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int. Immunopharmacol.14, 27–31. 10.1016/j.intimp.2012.06.001
107
Zhang Y. Du W. Zhu D. Li M. Qu L. Rao G. et al (2022a). Vasicine alleviates 2,4-dinitrochlorobenzene-induced atopic dermatitis and passive cutaneous anaphylaxis in BALB/c mice. Clin. Immunol.244, 109102. 10.1016/j.clim.2022.109102
108
Zhang Y. Qu L. Sun Y. Lin Y. Zeng J. He L. et al (2022b). Daphnetin contributes to allergen-induced Th2 cytokine expression and type 2-immune responses in atopic dermatitis and asthma. Food Funct.13, 12383–12399. 10.1039/d2fo02518c
109
Zheng T. Fan M. Wei Y. Feng J. Zhou P. Sun X. et al (2021). Huangbai liniment ameliorates skin inflammation in atopic dermatitis. Front. Pharmacol.12, 726035. 10.3389/fphar.2021.726035
110
Zhi W. Li C. Zhang H. Zhao Y. Zong S. Liu Q. et al (2022). Ta-Xi-San suppresses atopic dermatitis involved in multitarget mechanism using experimental and network pharmacology analysis. Evid. Based Complement. Altern. Med.2022, 8441938. 10.1155/2022/8441938
111
Zhu C. Wang M. Guo J. Su S. L. Yu G. Yang Y. et al (2022). Angelica dahurica extracts attenuate CFA-induced inflammatory pain via TRPV1 in mice. Evid. Based Complement. Altern. Med.2022, 4684830. 10.1155/2022/4684830
Summary
Keywords
atopic dermatitis, traditional Chinese medicine, therapeutic drugs, action mechanism, review
Citation
Zhang L, Lin H, Chen N, Zhu S and Hu Y (2025) Selected traditional Chinese herbal medicines for the treatment of atopic dermatitis - research progress on the effect and mechanism of actions. Front. Pharmacol. 16:1553251. doi: 10.3389/fphar.2025.1553251
Received
30 December 2024
Accepted
12 March 2025
Published
26 March 2025
Volume
16 - 2025
Edited by
Ruiwen Zhang, University of Houston, United States
Reviewed by
Anna Zalewska-Janowska, Medical University of Lodz, Poland
Diandong Hou, Huzhou University, China
Updates
Copyright
© 2025 Zhang, Lin, Chen, Zhu and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Hu, pharmhawk@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.