Abstract
Background:
Insulin glargine is a long-acting drug and the first synthetic insulin to mimic human metabolism. The safety of insulin glargine in the real world remains to be further investigated. This study aims to analyze insulin glargine-related adverse events (ADEs) to guide its safe clinical use.
Methods:
This study collected ADE reports from the FDA Adverse Event Reporting System (FAERS) between the first quarter of 2004 and the third quarter of 2024, where insulin glargine was identified as the primary suspect drug. Four disproportionate analytical methods were employed to analyze positive signals for drug-related ADEs, including the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). The study also describes the time to onset of ADEs and uses the Weibull distribution to analyze the temporal trend of ADEs occurrence over time.
Results:
This study included 97,350 ADE reports, containing 228,258 ADEs, and identified 130 ADEs with positive signal. The study confirmed several known ADEs, such as hypoglycemia, injection site pain and acquired lipodystrophy. Additionally, several unexpected ADEs were identified, including pancreatic neoplasm, medullary thyroid cancer, and bone marrow tumor cell infiltration. 28.13% of ADEs occurred within the first month. The Weibull distribution indicated that the occurrence of ADEs decreased over time.
Conclusion:
This study explored the real-world safety of insulin glargine and revealed several unexpected ADEs. These findings provide new insights into the safety profile of insulin glargine for clinicians.”
1 Introduction
Diabetes mellitus is a metabolic disorder characterized by persistent hyperglycemia resulting from impaired insulin secretion, insulin function, or a combination of both (Darenskaya et al., 2021). Researchers estimated that in 2021, the global prevalence of diabetes among individuals aged 20–79 was approximately 10.5% (536.6 million individuals), and it is projected to increase to 12.2% (783.2 million individuals) by 2045 (Sun et al., 2022).
Insulin glargine is a long-acting insulin analog designed to mimic human metabolism (Zhou et al., 2019). It was approved by the U.S. Food and Drug Administration (FDA) in April 2000 for the treatment of diabetes mellitus (Goykhman et al., 2009). Multiple randomized controlled trials (RCTs) have demonstrated that insulin glargine exerts stable and sustained glucose-lowering effects by binding to insulin receptors, promoting glucose uptake and utilization in peripheral tissues, and inhibiting hepatic glucose output. Despite its significant efficacy in glycemic management, the use of insulin glargine is also associated with some adverse events (ADEs). Common ADEs related to insulin glargine include hypoglycemia, weight gain, and injection site reactions (Kişioğlu et al., 2021; Saboo et al., 2024). Furthermore, with the widespread use of this drug in the market, increasing concerns have emerged regarding its real-world safety profile. One study suggested that insulin glargine may be associated with an increased risk of breast cancer (Suissa et al., 2011). A case report from Taiwan described the occurrence of stiff-person syndrome following subcutaneous insulin injection (Lee and Ahn, 2020). Additionally, studies from both the United States and Taiwan have reported insulin-induced amyloidosis (Carll and Antic, 2020; Chen and Lee, 2020). However, most of these studies were systematic reviews, case reports, or RCTs, which are limited by small sample sizes, short follow-up periods, and stringent inclusion and exclusion criteria. Given the extensive global use of insulin glargine since its approval, understanding its real-world safety profile is crucial for assisting clinicians in ensuring the safe use of this medication.
The FDA Adverse Event Reporting System (FAERS) is a public database that collects ADE reports spontaneously submitted by physicians, pharmacists, paramedics, and patients, playing a crucial role in post-marketing drug safety monitoring (Chen S. et al., 2024; Zou et al., 2024). Due to its publicly available large volume of data and real-world data characteristics, an increasing number of researchers have explored the real-world safety of drugs through the FAERS database (Zhao et al., 2024; He et al., 2025). This study employs four disproportionality analysis methods to analyze reports related to insulin glargine in the FAERS database, aiming to provide real-world safety information of insulin glargine for clinicians and regulatory agencies.
2 Methods
2.1 Data source and process
The data for this study was sourced from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). The FAERS database is updated quarterly and contains seven datasets: demographic and administrative information (DEMO), drug information (DRUG), ADEs information (REAC), patient outcome information (OUCT), reporting source information (RPSR), therapy information (THER), and indications for drug administration (INDI). The relationship between drugs and ADE reports is categorized into primary suspected (PS), secondary suspected (SS), concomitant (C), and interaction (I). We collected ADE reports from Q1 2004 to Q3 2024 where “insulin glargine” was listed as the PS. Given that duplicate reports may exist in the FAERS database, we followed the FDA’s deduplication principles (Sakaeda et al., 2013). Specifically, if the case identification (CASEID) values were the same, the report with the largest FDA date (FDA_DT) value was retained. If both CASEID and FDA_DT values were identical, the report with the largest primary identification (PRIMARYID) value was retained. We then standardized the ADEs in the reports using the Medical Dictionary for Regulatory Activities (MedDRA, Version 27.0), primarily mapping the data to the preferred term (PT) and system organ class (SOC) levels. The detailed study process is shown in Figure 1.
FIGURE 1
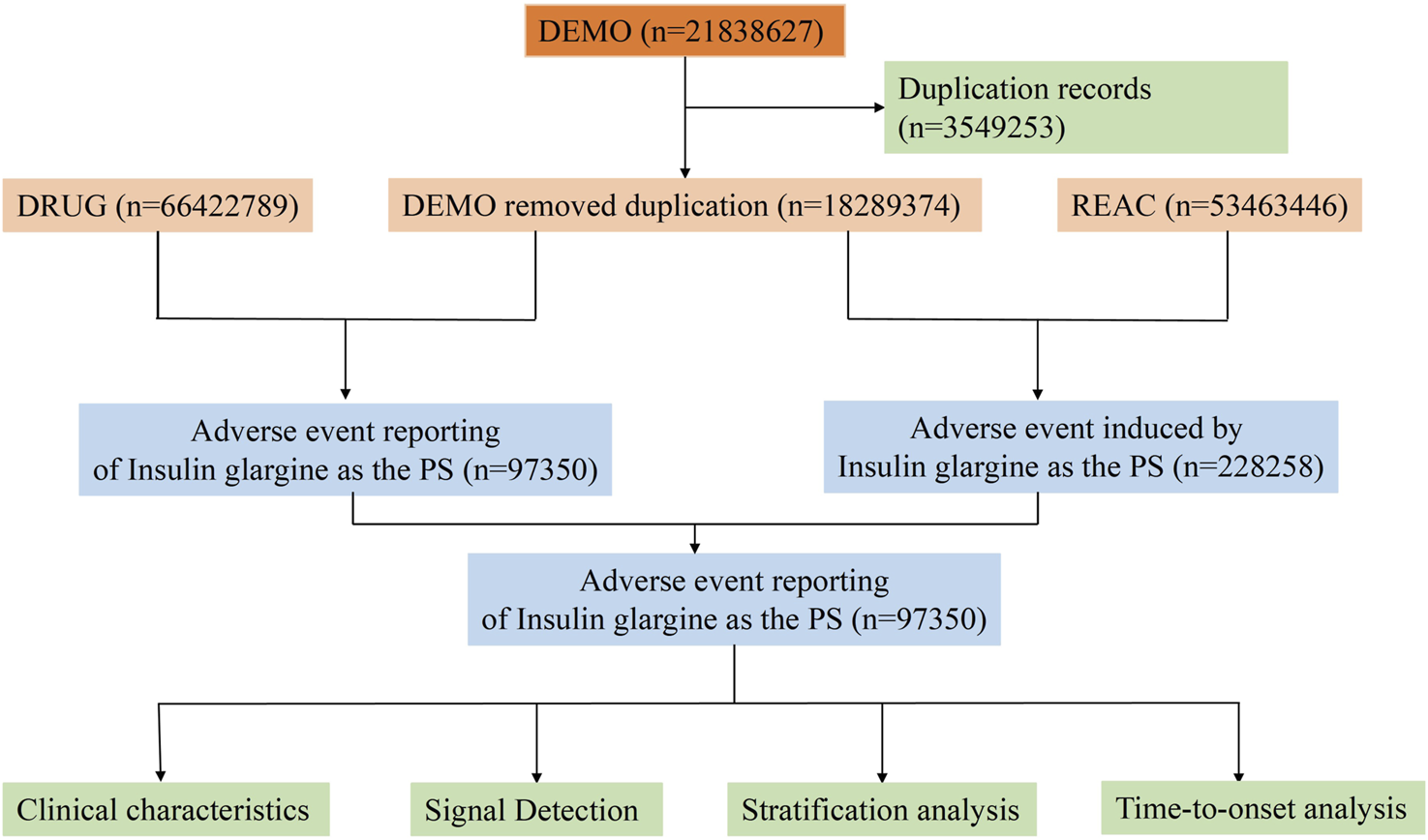
Flowchart of the whole study.
2.2 Classification criteria for serious adverse events (SADEs)
The severity of ADEs was classified according to Council for International Organizations of Medical Sciences (CIOMS) criteria. Serious adverse events (SADEs) were categorized as follows: DE (Death), LT (Life-Threatening), HO (Hospitalization - Initial or Prolonged), DS (Disability), CA (Congenital Anomaly), RI (Required Intervention to Prevent Permanent Impairment/Damage), and OT (Other Serious - Important Medical Event).
2.3 Statistical analysis
2.3.1 Disproportionality analysis
This study employed four disproportionality analysis methods to identify positive signals, including the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (MCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS). The ROR and PRR are the most classical disproportionality analysis methods, widely applied in drug safety surveillance due to their broad applicability and ease of implementation. However, these methods are prone to reporting bias and confounding factors, with limited capability in detecting rare ADEs, potentially resulting in false-positive outcomes. The MCPNN and MGPS are more suitable for large-scale data analysis, as they account for multiple variables such as patient age, gender, and concomitant medication use. This improves the accuracy of signal detection and reduces the occurrence of false positives. In this study, an ADE was defined as a positive signal only when it met the criteria of all four disproportionality analysis methods. This stringent criterion significantly improved detection accuracy, reduced false-positive rates, and enhanced the reliability of the results. The fundamental calculation principles of these four methods are presented in Table 1, while their specific computational rules and threshold criteria are detailed in Table 2.
TABLE 1
| Target ADEs | Other ADEs | Total | |
|---|---|---|---|
| Insulin glargine | a | b | A+ b |
| Other drugs | c | d | c+d |
| Total | a+c | b + d | a+b+c+d |
Two-by-two contingency table for disproportionality analyses.
Abbreviation: ADEs, adverse events; a, number of reports containing both the target drug and target adverse drug reaction; b, number of reports containing other adverse drug reaction of the target drug; c, number of reports containing the target adverse drug reaction of other drugs; d, number of reports containing other drugs and other adverse drug reactions.
TABLE 2
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR = ad/b/c | lower limit of 95% CI > 1, N ≥ 3 |
| 95%CI = eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| PRR | PRR = a (c+d)/c/(a+b) | PRR≥2, χ2 ≥ 4, N ≥ 3 |
| χ2 = [(ad-bc)^2](a+b+c+d)/[(a+b) (c+d) (a+c) (b+d)] | ||
| BCPNN | IC = log2a (a+b+c+d) (a+c) (a+b) | IC025 > 0 |
| 95%CI = E (IC) ± 2V(IC)^0.5 | ||
| MGPS | EBGM = a (a+b+c+d)/(a+c)/(a+b) | EBGM05 > 2 |
| 95%CI = eln(EBGM)±1.96(1/a+1/b+1/c+1/d)^0.5 |
Four major algorithms used for signal detection.
Abbreviation: a, number of reports containing both the target drug and target adverse drug reaction; b, number of reports containing other adverse drug reaction of the target drug; c, number of reports containing the target adverse drug reaction of other drugs; d, number of reports containing other drugs and other adverse drug reactions. 95%CI, 95% confidence interval; N, the number of reports; χ2, chi-squared; IC, information component; IC025, the lower limit of 95% CI, of the IC; E (IC), the IC, expectations; V(IC), the variance of IC; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95% CI, of EBGM.
2.3.2 Time to onset (TTO) and weibull distribution analysis
The TTO of ADEs related to insulin glargine was defined as the time from treatment initiation to the occurrence of the ADEs. The TTO was described using the median and interquartile range (IQR). Additionally, Weibull distribution analysis was conducted to assess the time trend of ADEs. The shape parameter (β) was used to interpret the dynamic changes in failure rates over time. When the β value is <1 and the 95% confidence interval (CI) is also <1, it indicates an early failure trend, where ADEs frequency initially increases but decreases over time. If β = 1 and the 95% CI includes 1, it suggests a constant failure rate, meaning the risk of ADEs remains stable throughout the treatment period. Conversely, when the β value is >1 and the 95% CI excludes 1, it reflects a wear-out failure pattern, indicating that the risk of ADEs increases significantly with prolonged treatment duration.
2.3.3 Sensitivity analysis
To rigorously evaluate the independent safety profile of insulin glargine in real-world settings, this study excluded reports involving the three most frequently co-administered medications: glimepiride, metformin, and sitagliptin. A subsequent disproportionality analysis was performed to minimize potential confounding effects and reduce the impact of drug interactions, thereby enhancing the robustness and reliability of the findings.
3 Results
3.1 Basic characteristics of insulin glargine ADE reports
This study included 97,350 ADE reports. Among these reports, 51.90% involved female patients, while 38.40% involved male patients. The majority of reports were submitted by individuals aged 65–85 years, accounting for 31.38%. Reports from the United States comprised 85.7% of the total. Regarding ADE outcomes, 13.0% were classified as “Hospitalization - Initial or Prolonged.” Detailed information on other ADE reports is provided in Table 3.
TABLE 3
| Characteristics | Case numbers | Case proportion (%) |
|---|---|---|
| Number of events | 97,350 | 100.00% |
| Gender | ||
| Male | 37,409 | 38.40% |
| Female | 50,567 | 51.90% |
| Miss | 9,374 | 9.60% |
| Age | ||
| <18 | 930 | 1.00% |
| 18–65 | 25,512 | 26.20% |
| 65–85 | 30,536 | 31.38.00% |
| >85 | 2,318 | 2.40% |
| Miss | 38,054 | 39.10% |
| Top 5 Reported Countries | ||
| United States | 83,453 | 85.70% |
| Brazil | 1999 | 2.10% |
| Egypt | 965 | 1.00% |
| Japan | 913 | 0.90% |
| Spain | 861 | 0.90% |
| Reporter | ||
| Consumer | 79,424 | 81.60% |
| Nurse | 4,092 | 4.20% |
| Lawyer | 22 | 0.00% |
| Physician | 4,539 | 4.70% |
| Other health professional | 2,964 | 3.00% |
| Pharmacist | 4,634 | 4.80% |
| Missing | 1,674 | 1.70% |
| Reporting year | ||
| 2024 | 7,510 | 7.71% |
| 2023 | 7,650 | 7.86% |
| 2022 | 8,342 | 8.57% |
| 2021 | 8,548 | 8.78% |
| 2020 | 8,179 | 8.40% |
| 2019 | 9,849 | 10.12% |
| 2018 | 12,139 | 12.47% |
| 2017 | 5,173 | 5.31% |
| 2016 | 4,165 | 4.28% |
| 2015 | 6,323 | 6.50% |
| 2014 | 6,021 | 6.18% |
| 2013 | 3,733 | 3.83% |
| 2012 | 1,446 | 1.49% |
| 2011 | 1862 | 1.91% |
| 2010 | 2,343 | 2.41% |
| 2009 | 1,220 | 1.25% |
| 2008 | 730 | 0.75% |
| 2007 | 792 | 0.81% |
| 2006 | 512 | 0.53% |
| 2005 | 485 | 0.50% |
| 2004 | 328 | 0.34% |
| Serious Outcomes | ||
| Death | 3,024 | 3.10% |
| Disability | 1,374 | 1.40% |
| Hospitalization - Initial or Prolonged | 12,654 | 13.00% |
| Life-Threatening | 1,039 | 1.00% |
| Congenital Anomaly | 84 | 0.1% < |
| Damage | 41 | 0.1% < |
| Other Serious | 19,694 | 20.2% |
| Missing | 59,440 | 61.0% |
Clinical characteristics of insulin glargine ADE reports from the FAERS database (Q1 2004 – Q3 2024).
3.2 Disproportionality analysis of ADEs based at SOC level
At the SOC level, the ADEs are distributed across 24 SOC, with the specific signal values for these ADEs shown in Table 4. The five SOC with the highest number of reports were general disorders and administration site conditions, nervous system disorders, eye disorders, metabolism and nutrition disorders, and gastrointestinal disorders. The distribution of ADEs at the SOC level is shown in Figure 2. Among all the SOC, positive signals were observed for eye disorders and metabolism and nutrition disorders.
TABLE 4
| SOC | Numbers | ROR (95%CI) | PRR (χ2) | EBGM (EBGM0) | IC (IC025) |
|---|---|---|---|---|---|
| Injury, Poisoning And Procedural Complications | 44,965 | 2.37 (2.35–2.4) | 2.1 (28,375.07) | 2.09 (2.07) | 1.06 (1.05) |
| General Disorders And Administration Site Conditions | 30,540 | 0.72 (0.72–0.73) | 0.76 (2760.02) | 0.76 (0.75) | −0.39 (−0.41) |
| Nervous System Disorders | 17,523 | 0.89 (0.87–0.9) | 0.9 (226.53) | 0.9 (0.89) | −0.16 (−0.18) |
| Eye Disorders | 14,521 | 3.32 (3.27–3.38) | 3.18 (21,798.48) | 3.15 (3.1) | 1.65 (1.63) |
| Metabolism And Nutrition Disorders | 11,395 | 2.39 (2.34–2.43) | 2.32 (8636.82) | 2.3 (2.27) | 1.2 (1.18) |
| Gastrointestinal Disorders | 7,274 | 0.35 (0.34–0.36) | 0.37 (8589.14) | 0.37 (0.36) | −1.44 (−1.47) |
| Infections And Infestations | 6,277 | 0.5 (0.49–0.52) | 0.52 (2989.31) | 0.52 (0.51) | −0.95 (−0.99) |
| Musculoskeletal And Connective Tissue Disorders | 6,271 | 0.5 (0.49–0.51) | 0.51 (3020.8) | 0.52 (0.51) | −0.95 (−0.99) |
| Skin And Subcutaneous Tissue Disorders | 5,616 | 0.44 (0.43–0.45) | 0.45 (3912.25) | 0.45 (0.44) | −1.14 (−1.18) |
| Psychiatric Disorders | 5,413 | 0.4 (0.39–0.41) | 0.41 (4833.16) | 0.41 (0.4) | −1.28 (−1.32) |
| Respiratory, Thoracic And Mediastinal Disorders | 5,327 | 0.47 (0.46–0.48) | 0.48 (3076.93) | 0.48 (0.47) | −1.04 (−1.08) |
| Surgical And Medical Procedures | 4,876 | 1.58 (1.54–1.63) | 1.57 (1020.42) | 1.57 (1.53) | 0.65 (0.61) |
| Cardiac Disorders | 4,759 | 0.77 (0.75–0.8) | 0.78 (308.31) | 0.78 (0.76) | −0.36 (−0.4) |
| Renal And Urinary Disorders | 2,938 | 0.69 (0.66–0.71) | 0.69 (409.75) | 0.69 (0.67) | −0.53 (−0.58) |
| Neoplasms Benign, Malignant And Unspecified (Incl Cysts And Polyps) | 2,826 | 0.45 (0.44–0.47) | 0.46 (1821.04) | 0.46 (0.45) | −1.11 (−1.17) |
| Vascular Disorders | 2,444 | 0.49 (0.47–0.5) | 0.49 (1318.41) | 0.49 (0.48) | −1.02 (−1.08) |
| Ear And Labyrinth Disorders | 1882 | 1.91 (1.83–2) | 1.9 (803.08) | 1.9 (1.82) | 0.92 (0.86) |
| Immune System Disorders | 1,017 | 0.39 (0.37–0.42) | 0.4 (940.09) | 0.4 (0.38) | −1.33 (−1.42) |
| Hepatobiliary Disorders | 895 | 0.42 (0.4–0.45) | 0.42 (703.74) | 0.43 (0.4) | −1.23 (−1.33) |
| Pregnancy, Puerperium And Perinatal Conditions | 514 | 0.52 (0.47–0.56) | 0.52 (231.4) | 0.52 (0.48) | −0.95 (−1.07) |
| Blood And Lymphatic System Disorders | 443 | 0.11 (0.1–0.12) | 0.11 (3153.03) | 0.11 (0.1) | −3.15 (−3.28) |
| Reproductive System And Breast Disorders | 355 | 0.19 (0.17–0.21) | 0.19 (1266.09) | 0.19 (0.17) | −2.42 (−2.57) |
| Endocrine Disorders | 295 | 0.5 (0.45–0.56) | 0.5 (144.39) | 0.5 (0.46) | −0.99 (−1.15) |
| Congenital, Familial And Genetic Disorders | 240 | 0.34 (0.3–0.39) | 0.34 (306.47) | 0.34 (0.31) | −1.55 (−1.73) |
Signal strength of insulin glargine ADEs across system organ classes (SOC) in the FAERS database.
Abbreviation: ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of the 95% CI, of EBGM; IC, information component; IC025, the lower limit of the 95% CI, of the IC; CI, confidence interval; ADEs, adverse events.
FIGURE 2
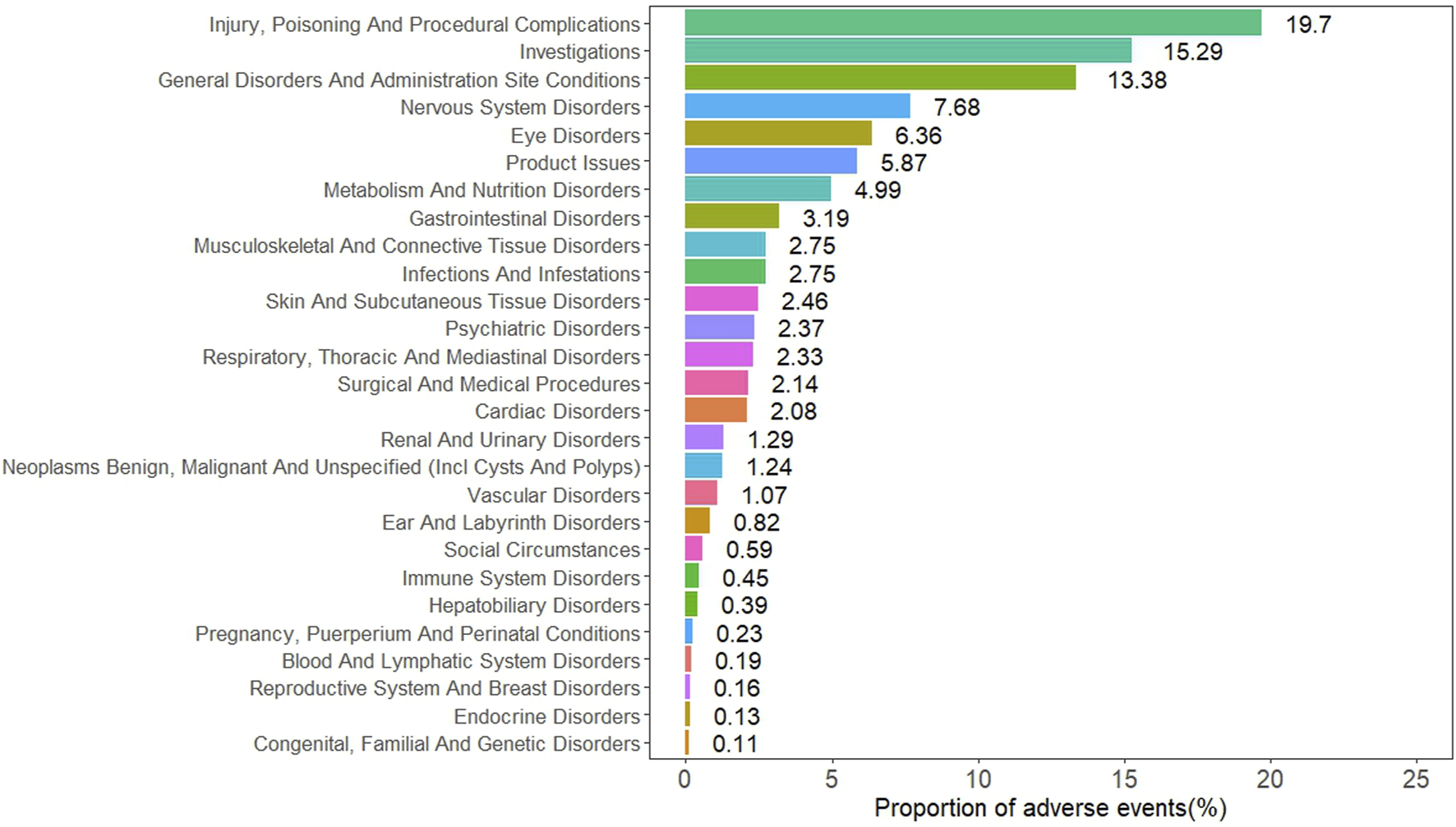
Distribution of ADEs based at SOC level.
3.3 Disproportionality analysis of ADEs based at PT level
This study identified a total of 130 positive ADEs, with their frequencies and corresponding signal strengths presented in Table 5. The most common positive ADEs include visual impairment, hypoglycemia, injection site pain, hyperglycemia, and cerebrovascular accident. The study confirmed several known ADEs, such as hypoglycemia, injection site pain, injection site hemorrhage, and acquired lipodystrophy. In addition, the study identified some drug-related ADEs not mentioned in the product label, such as pancreatic neoplasm, medullary thyroid cancer, visual impairment, malignant neoplasm of the eye, and bone marrow tumor cell infiltration.
TABLE 5
| PT | Case reports | ROR (95%CI) | PRR (χ2) | EBGM (EBGM05) | IC (IC025) |
|---|---|---|---|---|---|
| Visual Impairment* | 5,850 | 13.92 (13.55–14.3) | 13.59 (64,579.48) | 12.89 (12.61) | 3.69 (3.65) |
| Hypoglycaemia | 3,718 | 21.9 (21.17–22.65) | 21.56 (66,754.7) | 19.81 (19.26) | 4.31 (4.26) |
| Injection Site Pain | 3,613 | 3.4 (3.29–3.52) | 3.36 (5944.83) | 3.33 (3.24) | 1.74 (1.69) |
| Hyperglycaemia | 2,474 | 19.25 (18.47–20.06) | 19.05 (39,132.06) | 17.68 (17.08) | 4.14 (4.08) |
| Cerebrovascular Accident | 2026 | 3.09 (2.96–3.23) | 3.07 (2800.74) | 3.04 (2.93) | 1.61 (1.54) |
| Diabetes Mellitus Inadequate Control | 1,596 | 26.29 (24.96–27.69) | 26.11 (34,656.87) | 23.57 (22.57) | 4.56 (4.48) |
| Cataract | 1,531 | 7.21 (6.85–7.59) | 7.17 (7890.44) | 6.98 (6.69) | 2.8 (2.73) |
| Hypoacusis | 1,328 | 8.57 (8.11–9.05) | 8.52 (8512.84) | 8.26 (7.89) | 3.05 (2.96) |
| Injection Site Haemorrhage | 1,271 | 4.4 (4.16–4.65) | 4.38 (3258.03) | 4.32 (4.12) | 2.11 (2.03) |
| Memory Impairment | 1,135 | 2.16 (2.04–2.29) | 2.15 (697.34) | 2.14 (2.04) | 1.1 (1.01) |
| Hyperhidrosis | 1,075 | 2.17 (2.04–2.3) | 2.16 (665.95) | 2.15 (2.04) | 1.1 (1.02) |
| Injection Site Bruising | 1,003 | 3.53 (3.32–3.76) | 3.52 (1785.39) | 3.48 (3.31) | 1.8 (1.71) |
| Blindness | 986 | 6.68 (6.27–7.12) | 6.66 (4609.6) | 6.5 (6.16) | 2.7 (2.61) |
| Injury Associated With Device | 839 | 12.19 (11.37–13.07) | 12.15 (8161.71) | 11.6 (10.94) | 3.54 (3.43) |
| Cardiac Disorder | 812 | 2.24 (2.09–2.4) | 2.24 (552.62) | 2.23 (2.1) | 1.16 (1.05) |
| Visual Acuity Reduced | 790 | 5.95 (5.54–6.39) | 5.93 (3161.74) | 5.81 (5.48) | 2.54 (2.43) |
| Eye Disorder | 746 | 6.21 (5.77–6.68) | 6.19 (3163.8) | 6.06 (5.7) | 2.6 (2.49) |
| Renal Disorder | 643 | 3.65 (3.37–3.94) | 3.64 (1212.25) | 3.6 (3.37) | 1.85 (1.73) |
| Diabetic Ketoacidosis | 625 | 7 (6.47–7.59) | 6.99 (3114.34) | 6.81 (6.37) | 2.77 (2.65) |
| Dementia | 448 | 4.5 (4.1–4.94) | 4.49 (1193.06) | 4.42 (4.09) | 2.15 (2.01) |
| Glaucoma | 391 | 5.42 (4.9–5.99) | 5.41 (1375.6) | 5.31 (4.89) | 2.41 (2.26) |
| Eye Haemorrhage | 369 | 7.29 (6.58–8.09) | 7.28 (1940.06) | 7.09 (6.5) | 2.83 (2.67) |
| Macular Degeneration | 360 | 8.42 (7.58–9.36) | 8.41 (2268.55) | 8.15 (7.46) | 3.03 (2.87) |
| Injection Site Mass | 338 | 2.49 (2.24–2.77) | 2.49 (297.7) | 2.47 (2.26) | 1.31 (1.15) |
| Blindness Unilateral | 321 | 6.18 (5.54–6.91) | 6.18 (1357.1) | 6.04 (5.51) | 2.6 (2.43) |
| Diabetic Retinopathy | 296 | 25.15 (22.31–28.36) | 25.12 (6185.58) | 22.76 (20.59) | 4.51 (4.33) |
| Ketoacidosis | 266 | 9.61 (8.5–10.86) | 9.6 (1968.04) | 9.26 (8.35) | 3.21 (3.03) |
| Localised Infection | 231 | 2.51 (2.21–2.86) | 2.51 (208.34) | 2.5 (2.24) | 1.32 (1.13) |
| Hypoglycaemic Coma | 230 | 22.53 (19.67–25.79) | 22.5 (4308.59) | 20.6 (18.4) | 4.36 (4.17) |
| Back Disorder | 205 | 3.31 (2.88–3.8) | 3.31 (325.37) | 3.27 (2.92) | 1.71 (1.51) |
| Hypoglycaemic Unconsciousness | 201 | 27.81 (24.02–32.19) | 27.78 (4635.08) | 24.92 (22.05) | 4.64 (4.43) |
| Dementia Alzheimer'S Type | 194 | 5.57 (4.83–6.43) | 5.57 (710.27) | 5.46 (4.85) | 2.45 (2.24) |
| Injection Site Discolouration | 184 | 4.05 (3.5–4.69) | 4.05 (414.96) | 3.99 (3.54) | 2 (1.78) |
| Retinopathy | 183 | 13.26 (11.43–15.39) | 13.25 (1961.02) | 12.59 (11.11) | 3.65 (3.44) |
| Diabetic Neuropathy | 161 | 8.45 (7.22–9.89) | 8.44 (1019.58) | 8.18 (7.17) | 3.03 (2.8) |
| Cold Sweat | 159 | 2.36 (2.02–2.76) | 2.36 (123.67) | 2.35 (2.06) | 1.23 (1) |
| Hunger | 158 | 3.72 (3.18–4.35) | 3.71 (308.55) | 3.67 (3.22) | 1.88 (1.65) |
| Injection Site Injury | 151 | 9.04 (7.68–10.63) | 9.03 (1037.92) | 8.73 (7.62) | 3.13 (2.89) |
| Diabetic Coma | 144 | 13.22 (11.18–15.64) | 13.21 (1538.32) | 12.56 (10.91) | 3.65 (3.4) |
| Retinal Detachment | 139 | 4.09 (3.46–4.84) | 4.09 (319.22) | 4.04 (3.51) | 2.01 (1.77) |
| Pancreatic Disorder | 138 | 7.99 (6.74–9.46) | 7.98 (814.97) | 7.75 (6.72) | 2.95 (2.71) |
| Diabetic Foot | 132 | 11.33 (9.51–13.5) | 11.33 (1184.85) | 10.84 (9.37) | 3.44 (3.18) |
| Injection Site Extravasation | 130 | 2.56 (2.15–3.04) | 2.56 (122.18) | 2.54 (2.2) | 1.35 (1.09) |
| Frustration Tolerance Decreased | 123 | 4.24 (3.55–5.07) | 4.24 (299.29) | 4.18 (3.6) | 2.06 (1.8) |
| Diabetic Metabolic Decompensation | 120 | 25.08 (20.77–30.28) | 25.07 (2502.65) | 22.72 (19.41) | 4.51 (4.23) |
| Hypoglycaemic Seizure | 118 | 38.16 (31.41–46.37) | 38.14 (3665.51) | 32.9 (27.95) | 5.04 (4.76) |
| Retinal Haemorrhage | 113 | 4.23 (3.51–5.1) | 4.23 (273.6) | 4.17 (3.57) | 2.06 (1.79) |
| Diabetic Complication | 110 | 11.1 (9.17–13.45) | 11.1 (964.78) | 10.64 (9.06) | 3.41 (3.13) |
| Injection Site Discomfort | 95 | 2.67 (2.18–3.27) | 2.67 (98.08) | 2.65 (2.24) | 1.41 (1.11) |
| Insulin Resistance | 86 | 11.7 (9.42–14.53) | 11.69 (800.61) | 11.18 (9.33) | 3.48 (3.17) |
| Gangrene | 84 | 3.55 (2.86–4.4) | 3.55 (151.49) | 3.51 (2.93) | 1.81 (1.5) |
| Infarction | 71 | 2.56 (2.02–3.23) | 2.56 (66.52) | 2.54 (2.09) | 1.34 (1) |
| Retinal Disorder | 67 | 6.85 (5.38–8.74) | 6.85 (325.34) | 6.69 (5.46) | 2.74 (2.39) |
| Injection Site Scar | 59 | 5.8 (4.48–7.51) | 5.8 (228.65) | 5.68 (4.58) | 2.51 (2.13) |
| Shock Hypoglycaemic | 50 | 29.46 (21.95–39.54) | 29.45 (1219.56) | 26.25 (20.52) | 4.71 (4.29) |
| Diabetic Hyperglycaemic Coma | 47 | 43.47 (31.83–59.36) | 43.46 (1642.13) | 36.76 (28.33) | 5.2 (4.75) |
| Diabetic Nephropathy | 47 | 5.42 (4.06–7.24) | 5.42 (165.62) | 5.32 (4.18) | 2.41 (1.99) |
| Injection Site Atrophy | 43 | 6.79 (5.01–9.19) | 6.78 (206.04) | 6.62 (5.14) | 2.73 (2.29) |
| Throat Clearing | 43 | 3.27 (2.42–4.42) | 3.27 (66.86) | 3.24 (2.52) | 1.7 (1.26) |
| Hypoglycaemia Neonatal | 39 | 6.42 (4.67–8.83) | 6.42 (173.8) | 6.28 (4.81) | 2.65 (2.19) |
| Lipodystrophy Acquired | 38 | 4.55 (3.3–6.27) | 4.55 (103.17) | 4.48 (3.42) | 2.16 (1.7) |
| Reading Disorder | 36 | 4.11 (2.95–5.71) | 4.11 (83.11) | 4.05 (3.08) | 2.02 (1.54) |
| Diabetic Eye Disease | 36 | 25.25 (17.9–35.61) | 25.24 (755.92) | 22.86 (17.15) | 4.51 (4.02) |
| Hypoglycaemia Unawareness | 34 | 25.29 (17.75–36.04) | 25.29 (715.3) | 22.9 (17.03) | 4.52 (4.01) |
| Lipohypertrophy | 31 | 13.55 (9.43–19.47) | 13.55 (340.44) | 12.86 (9.5) | 3.68 (3.16) |
| Ketosis | 29 | 8.82 (6.09–12.78) | 8.82 (193.74) | 8.53 (6.26) | 3.09 (2.56) |
| Injection Site Hypersensitivity | 28 | 2.9 (2–4.21) | 2.9 (34.42) | 2.88 (2.11) | 1.52 (0.98) |
| Myopia | 27 | 2.98 (2.04–4.35) | 2.98 (35) | 2.95 (2.15) | 1.56 (1.01) |
| Hypoglycaemic Encephalopathy | 27 | 19.83 (13.39–29.38) | 19.83 (444.77) | 18.35 (13.21) | 4.2 (3.63) |
| Dyslexia | 26 | 6.23 (4.22–9.19) | 6.23 (111.1) | 6.09 (4.4) | 2.61 (2.04) |
| Neuropathic Arthropathy | 24 | 9.72 (6.46–14.63) | 9.72 (180.22) | 9.37 (6.66) | 3.23 (2.64) |
| Vascular Occlusion | 24 | 2.95 (1.97–4.42) | 2.95 (30.59) | 2.93 (2.09) | 1.55 (0.97) |
| Pancreatic Neoplasm* | 23 | 5.06 (3.34–7.64) | 5.05 (73.22) | 4.97 (3.52) | 2.31 (1.72) |
| Fat Tissue Increased | 22 | 4.09 (2.68–6.23) | 4.09 (50.43) | 4.03 (2.84) | 2.01 (1.41) |
| Injection Site Hypertrophy | 22 | 22.11 (14.27–34.23) | 22.1 (404.72) | 20.27 (14.06) | 4.34 (3.71) |
| Diabetic Foot Infection | 20 | 3.46 (2.22–5.38) | 3.46 (34.4) | 3.42 (2.36) | 1.77 (1.14) |
| Hypermetropia | 19 | 5.08 (3.22–8) | 5.08 (60.93) | 4.99 (3.41) | 2.32 (1.67) |
| Colour Blindness | 17 | 4.84 (2.99–7.83) | 4.84 (50.75) | 4.76 (3.19) | 2.25 (1.56) |
| Dawn Phenomenon | 17 | 50.59 (29.94–85.48) | 50.58 (678.39) | 41.71 (26.89) | 5.38 (4.64) |
| Hyperinsulinaemic Hypoglycaemia | 17 | 26.84 (16.25–44.35) | 26.84 (379.07) | 24.16 (15.87) | 4.59 (3.88) |
| Ketonuria | 15 | 4.8 (2.88–8.01) | 4.8 (44.24) | 4.72 (3.08) | 2.24 (1.51) |
| Diabetic Retinal Oedema | 14 | 7.32 (4.3–12.46) | 7.32 (74.03) | 7.12 (4.56) | 2.83 (2.08) |
| Starvation | 14 | 5.41 (3.18–9.18) | 5.41 (49.13) | 5.31 (3.41) | 2.41 (1.65) |
| Pulmonary Vasculitis | 13 | 8.62 (4.96–15) | 8.62 (84.44) | 8.35 (5.25) | 3.06 (2.28) |
| Ocular Vascular Disorder | 12 | 3.58 (2.02–6.33) | 3.58 (21.97) | 3.54 (2.2) | 1.82 (1.02) |
| Cutaneous Amyloidosis | 12 | 44.92 (24.21–83.35) | 44.92 (431.74) | 37.8 (22.53) | 5.24 (4.37) |
| Hyperglycaemic Unconsciousness | 11 | 23.64 (12.71–43.96) | 23.64 (216.44) | 21.55 (12.82) | 4.43 (3.56) |
| Injection Site Laceration | 11 | 4.49 (2.47–8.15) | 4.49 (29.24) | 4.42 (2.68) | 2.14 (1.3) |
| Diabetic Blindness | 10 | 32.24 (16.64–62.46) | 32.23 (265.74) | 28.42 (16.34) | 4.83 (3.9) |
| Stomach Mass | 10 | 3.42 (1.83–6.39) | 3.42 (16.9) | 3.39 (2.01) | 1.76 (0.88) |
| Retinopathy Proliferative | 10 | 13.73 (7.26–25.99) | 13.73 (111.46) | 13.02 (7.64) | 3.7 (2.81) |
| Brain Stem Stroke | 9 | 4.77 (2.46–9.23) | 4.77 (26.27) | 4.69 (2.7) | 2.23 (1.31) |
| Acetonaemia | 8 | 9.06 (4.47–18.36) | 9.06 (55.19) | 8.75 (4.85) | 3.13 (2.15) |
| Retinal Vascular Disorder | 8 | 3.74 (1.86–7.53) | 3.74 (15.83) | 3.7 (2.06) | 1.89 (0.92) |
| Insulin Autoimmune Syndrome | 8 | 7.74 (3.82–15.65) | 7.74 (45.41) | 7.52 (4.17) | 2.91 (1.93) |
| Diabetic Ketosis | 8 | 7.49 (3.7–15.14) | 7.49 (43.56) | 7.28 (4.04) | 2.86 (1.89) |
| Diabetic Gastroparesis | 8 | 5.4 (2.68–10.88) | 5.4 (28.01) | 5.3 (2.95) | 2.41 (1.43) |
| Pancreas Infection | 7 | 5.16 (2.44–10.91) | 5.16 (22.95) | 5.07 (2.71) | 2.34 (1.31) |
| Diabetic Ketoacidotic Hyperglycaemic Coma | 7 | 9.85 (4.62–20.98) | 9.85 (53.37) | 9.49 (5.04) | 3.25 (2.2) |
| Cataract Diabetic | 7 | 35.32 (15.95–78.23) | 35.32 (202.59) | 30.78 (15.83) | 4.94 (3.85) |
| Drug Effect Faster Than Expected | 7 | 6.6 (3.12–14) | 6.6 (32.37) | 6.45 (3.44) | 2.69 (1.65) |
| Lens Disorder | 6 | 5.36 (2.38–12.03) | 5.36 (20.78) | 5.26 (2.67) | 2.39 (1.29) |
| Malignant Neoplasm Of Eye* | 6 | 5.83 (2.59–13.1) | 5.83 (23.4) | 5.71 (2.9) | 2.51 (1.41) |
| Kidney Malformation | 6 | 4.43 (1.98–9.95) | 4.43 (15.66) | 4.37 (2.22) | 2.13 (1.03) |
| Decreased Insulin Requirement | 6 | 27.85 (11.94–64.95) | 27.85 (138.68) | 24.97 (12.3) | 4.64 (3.49) |
| Diabetic Glaucoma | 6 | 58.02 (23.72–141.95) | 58.02 (268.98) | 46.62 (22.05) | 5.54 (4.34) |
| Medullary Thyroid Cancer* | 5 | 4.62 (1.91–11.2) | 4.62 (13.92) | 4.55 (2.17) | 2.19 (0.99) |
| Benign Pancreatic Neoplasm | 5 | 7.08 (2.91–17.23) | 7.08 (25.31) | 6.9 (3.28) | 2.79 (1.59) |
| Acanthosis Nigricans | 5 | 7.08 (2.91–17.23) | 7.08 (25.31) | 6.9 (3.28) | 2.79 (1.59) |
| Postprandial Hypoglycaemia | 5 | 8.6 (3.52–20.99) | 8.6 (32.36) | 8.32 (3.94) | 3.06 (1.85) |
| Increased Insulin Requirement | 5 | 5.77 (2.38–14.02) | 5.77 (19.25) | 5.66 (2.69) | 2.5 (1.31) |
| Diabetic Wound | 5 | 19.02 (7.64–47.34) | 19.02 (78.91) | 17.66 (8.23) | 4.14 (2.91) |
| Sodium Retention | 5 | 6.27 (2.58–15.25) | 6.27 (21.58) | 6.13 (2.92) | 2.62 (1.42) |
| Eye Degenerative Disorder | 4 | 6.45 (2.39–17.41) | 6.45 (17.91) | 6.3 (2.74) | 2.66 (1.34) |
| Insulinoma | 4 | 5.99 (2.22–16.16) | 5.99 (16.21) | 5.86 (2.56) | 2.55 (1.24) |
| Cerebrovascular Stenosis | 4 | 6.83 (2.53–18.45) | 6.83 (19.32) | 6.66 (2.9) | 2.74 (1.42) |
| Diabetic Foetopathy | 4 | 15.47 (5.62–42.57) | 15.47 (50.76) | 14.57 (6.25) | 3.86 (2.53) |
| Erythema Induratum | 4 | 8 (2.95–21.68) | 8 (23.69) | 7.77 (3.37) | 2.96 (1.64) |
| Diabetic Hepatopathy | 4 | 92.84 (29.12–296.01) | 92.83 (259.56) | 66.6 (25.24) | 6.06 (4.57) |
| Kussmaul Respiration | 4 | 4.79 (1.78–12.88) | 4.79 (11.73) | 4.71 (2.06) | 2.24 (0.93) |
| Somogyi Phenomenon | 3 | 46.42 (13.44–160.34) | 46.42 (111.1) | 38.85 (13.77) | 5.28 (3.69) |
| Splenic Neoplasm Malignancy Unspecified | 3 | 5.9 (1.88–18.56) | 5.9 (11.91) | 5.78 (2.22) | 2.53 (1.06) |
| Bone Marrow Tumour Cell Infiltration* | 3 | 6.05 (1.92–19.05) | 6.05 (12.34) | 5.93 (2.27) | 2.57 (1.1) |
| Fumbling | 3 | 6.22 (1.97–19.57) | 6.22 (12.79) | 6.08 (2.33) | 2.6 (1.14) |
| Injection Site Fibrosis | 3 | 6.51 (2.07–20.5) | 6.51 (13.6) | 6.36 (2.43) | 2.67 (1.2) |
| Congenital Bladder Anomaly | 3 | 5.36 (1.71–16.82) | 5.36 (10.39) | 5.26 (2.02) | 2.39 (0.93) |
| Abdominal Fat Apron | 3 | 8.6 (2.72–27.21) | 8.6 (19.42) | 8.32 (3.17) | 3.06 (1.58) |
| Non-Proliferative Retinopathy | 3 | 87.03 (23.09–328.07) | 87.03 (185.55) | 63.57 (20.94) | 5.99 (4.32) |
| Vessel Puncture Site Injury | 3 | 29.01 (8.74–96.34) | 29.01 (72.12) | 25.9 (9.49) | 4.69 (3.15) |
| Abnormal Labour | 3 | 5.8 (1.85–18.25) | 5.8 (11.63) | 5.68 (2.18) | 2.51 (1.04) |
All positive ADEs at the PT Level in the FAERS database.
Abbreviation:ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of the 95% CI, of EBGM; IC, information component; IC025, the lower limit of the 95% CI, of the IC; CI, confidence interval; PT, preferred term; * The asterisks indicate noteworthy and unexpected adverse events that are not listed in the drug’s label.
3.4 ADEs characteristics across different genders and age groups
The top 50 ADEs associated with insulin glargine in men and women were analyzed at the PT level, with the detailed distribution presented in Supplementary Tables S1, S2. In men, the most frequently reported ADEs were visual impairment, hypoglycemia, and cataract. In women, the most frequently reported ADEs were visual impairment, injection site pain, and hypoglycemia. Additionally, we described the most common ADEs across different age groups. Supplementary Tables S3–S5 present the insulin glargine-related ADEs in each age group. In individuals under 18 years old, hypoglycemia was the most frequently reported PT, while in those aged 18–64 years and ≥65 years, visual impairment was the most commonly reported PT.
3.5 ADEs characteristics across different countries
This study further analyzed the top 20 most frequently reported ADEs in the three countries with the highest number of reports, namely, the United States, Brazil, and Egypt. In the United States, the most frequently reported ADEs included visual impairment, injection site pain, hypoglycemia, cerebrovascular accident, cataract, etc. In Brazil, the most commonly reported ADEs were hyperglycemia, hypoglycemia, visual impairment, cerebrovascular accident, injection site pain, etc. In Egypt, the most frequently reported ADEs included death, hyperglycemia, disease progression, cerebrovascular accident, liver disorders, etc. The top 20 most frequently reported ADEs in these three countries are presented in Supplementary Table S6.
3.6 ADEs characteristics at different drug dosages
In this study, we analyzed the top 20 most frequently reported ADEs associated with insulin glargine at different doses (10 IU, 20 IU, 30 IU, and 40 IU). The specific distribution of the most common ADEs in each dosage group is shown in Supplementary Table S7. Injection site pain, hypoglycemia, and hyperglycemia were the most frequently reported ADEs across all dosage groups. As the dose increased, both the variety and severity of ADEs exhibited an upward trend. In the 30 IU and 40 IU dosage groups, more severe ADEs, such as myocardial infarction, memory impairment, and cerebrovascular accident, were reported.
3.7 Analysis of TTO and Weibull distribution
We analyzed the TTO of 6199 ADE reports, with the detailed distribution presented in Figure 3. A total of 28.13% of ADEs occurred within the first month, followed by a gradual decline in numbers. Additionally, we employed the Weibull distribution to predict the temporal pattern of ADEs, and the model exhibited an early failure pattern. Further details regarding the parameters are provided in Table 6.
FIGURE 3
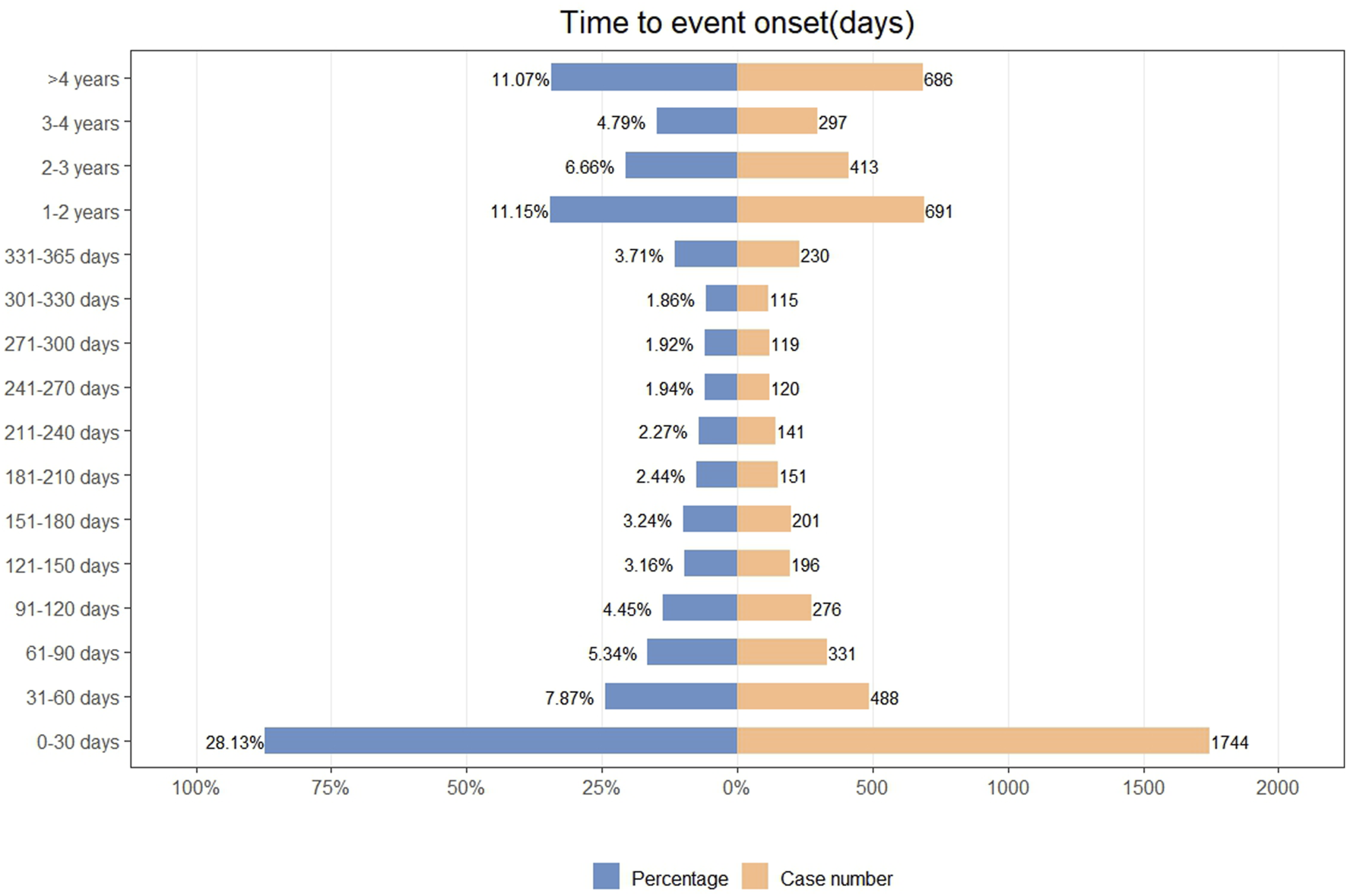
Time to onset of insulin glargine associated ADEs.
TABLE 6
| Drug | TTO (days) | Weibull distribution | |||
|---|---|---|---|---|---|
| Insulin glargine | Case reports | Median(d) (IQR) | Scale parameter: α(95%CI) | Shape parameter: β(95%CI) | Type |
| 6,199 | 158 (24–644) | 309.48 (294.31–324.65) | 0.54 (0.53–0.55) | Early failure | |
Time to onset of insulin glargine associated ADEs and Weibull distribution analysis.
Abbreviation: TTO, time to onset; CI, confidence interval; IQR, interquartile range.
3.8 Sensitivity analysis
Insulin glargine is commonly used in combination with glimepiride, metformin, and sitagliptin. This study further excluded reports involving these concomitant medications and conducted a disproportionality analysis again. A total of 91,698 reports were included, with 209,705 ADEs. The ADEs that continued to show positive signals included hypoglycemia, injection site pain, injection site hemorrhage, acquired lipodystrophy, pancreatic neoplasm, medullary thyroid cancer, visual impairment, malignant neoplasm of the eye, and bone marrow tumor cell infiltration. These findings were largely consistent with the results of the previous overall analysis, with specific signal strengths and frequencies presented in Supplementary Table S8.
4 Discussion
This study analyzed the real-world safety of insulin glargine using the FAERS database. The study confirmed several known ADEs, such as hypoglycemia, injection site reactions, and lipodystrophy. Additionally, the study identified some unexpected ADEs, including pancreatic neoplasm, medullary thyroid cancer, malignant neoplasm of the eye, and bone marrow tumor cell infiltration. These findings provide new safety insights for healthcare professionals regarding insulin glargine in real-world settings.
Hypoglycemia typically presents with symptoms such as dizziness, sweating, nausea, and hunger (Tripyla et al., 2023). Several clinical studies and reviews have indicated that hypoglycemia is a common ADE associated with the use of insulin glargine (Sebastian et al., 2023; Chen L. et al., 2024; Hong et al., 2024; Home, 2025). This finding is consistent with the results of the present study. Mild hypoglycemia may only cause symptoms like dizziness, palpitations, and sweating (Tripyla et al., 2023). However, the dangers of hypoglycemia should not be underestimated, as severe hypoglycemia can directly lead to coma, cardiac arrest, or seizures, all of which are potentially fatal (Husain et al., 2023). Furthermore, recurrent hypoglycemia over time may lead to psychological issues such as anxiety and depression, and even increase the risk of cardiovascular ADEs (Li et al., 2014; Hinnen and Kruger, 2019). Fortunately, hypoglycemia, as a common ADE associated with insulin glargine use, has attracted widespread attention from clinicians, and severe ADEs due to hypoglycemia are rare.
Injection site reactions are common ADEs associated with insulin glargine. The injection site reactions that showed positive signals in this study included injection site pain, injection site hemorrhage, injection site bruising, and injection site mass. A multicenter clinical trial demonstrated that injection site reactions are common ADEs with insulin glargine use (Rosenstock et al., 2015). Another RCT study also confirmed that injection site reactions are frequent ADEs (Yan et al., 2022). The findings of this study are consistent with these researches. Fortunately, injection site reactions are typically mild and do not affect patient adherence to treatment. However, it is worth noting that this study also found that local infections showed positive signals, and clinicians should advise patients to ensure proper disinfection before injection to prevent infection.
In addition, this study also identified several unexpected ADEs, including pancreatic neoplasm, medullary thyroid cancer, visual impairment, malignant neoplasm of eye, and bone marrow tumour cell infiltration. Pancreatic neoplasm, particularly pancreatic cancer, can affect both the endocrine and exocrine functions of the pancreas, leading to symptoms such as indigestion, jaundice, and hyperglycemia (Hussain et al., 2022). The progression and metastasis of pancreatic cancer can lead to complications such as ascites, thrombosis, and respiratory distress, and it is often diagnosed at an advanced stage, with an overall 5-year survival rate of approximately 10% (Jiang et al., 2023). A cohort study investigating cancer risks in insulin users across five countries found that, except among Norwegians, pancreatic cancer was reported as the most common cancer (But et al., 2017). A systematic review and meta-analysis also indicated that insulin use is associated with an increased risk of pancreatic cancer (Karlstad et al., 2013). Another systematic review and meta-analysis of observational studies found that new use of insulin glargine is associated with an increased risk of pancreatic cancer (Colmers et al., 2012). These findings are consistent with the results of this study and support the notion that insulin use may be linked to pancreatic cancer. Given the significant impact of pancreatic cancer on patient health, we recommend that pancreatic tumor monitoring should be closely observed during insulin therapy for diabetes to ensure timely detection and management.
Visual impairment is another unexpected ADE. Based on multiple FAERS studies and clinical practice experience (Xiang et al., 2023; Zhang et al., 2024), we believe that the use of insulin glargine in diabetic patients complicates the differentiation between whether visual impairment is an ADE induced by insulin glargine. This is because visual impairment itself may be related to the clinical manifestations of diabetes, such as cataracts or glaucoma, which can lead to visual impairment or even blindness (Grauslund, 2022). However, considering the significant impact of visual impairment on patients’ daily life, work, and education, it can also affect medication adherence (Paudel et al., 2022). Our study recommends that, when using insulin glargine to treat diabetes, close monitoring of patients’ clinical manifestations is essential to prevent further deterioration of vision.
Moreover, medullary thyroid cancer (MTC) is another unexpected ADE that warrants attention. MTC presents clinically with symptoms such as neck masses, hypercalcemia, dysphagia, and hoarseness (Khan et al., 2022). The relationship between insulin glargine and thyroid cancer remains unclear. A retrospective study indicated no significant association between insulin glargine and pancreatic cancer (Abi Zeid Daou et al., 2025). However, animal studies have shown that insulin glargine can promote thyroid cell proliferation through mitogenic signaling pathways (Sheng et al., 2019). Furthermore, cell experiments suggest that insulin glargine may facilitate thyroid cell proliferation and migration through the insulin-like growth factor-1 receptor and the downstream Akt-signaling pathway (Zhang et al., 2019). Our study found a statistical association between insulin glargine and medullary thyroid cancer, though further prospective studies are needed to validate these findings.
Subgroup analysis suggests that in male patients, attention should be given to the occurrence of cataracts, while in female patients, the occurrence of injection site pain should be closely monitored and promptly managed, which may be related to women’s increased sensitivity to pain (Bulls et al., 2015). In patients under 18 years of age, the occurrence of hypoglycemia should be a primary concern. In patients aged 18 and above, visual impairment should be closely monitored, which is more likely associated with long-term diabetic complications rather than the use of insulin glargine.
After analyzing the top 20 ADEs in the United States, Brazil, and Egypt, we found that hypoglycemia and injection site reactions occurred in all three countries, suggesting that these ADEs are not significantly influenced by genetic variation, environmental factors, or healthcare conditions. Additionally, vision-related disorders (e.g., visual impairment, cataract) were more prevalent in the United States and Brazil, while severe metabolic events (e.g., diabetic ketoacidosis, death) were more frequently reported in Egypt. These differences may be influenced by genetic factors, healthcare infrastructure, and public awareness of diabetes management. The differences in ADEs among different countries warrant further investigation.
This study reveals the distribution of ADEs associated with insulin glargine at different doses. Compared to lower doses (e.g., 10 IU), higher doses (e.g., 40 IU) showed significantly different numbers and types of reported ADEs. Common ADEs at lower doses included milder metabolic reactions such as hypoglycemia and hyperglycemia, while with increasing doses, a greater number of metabolic and neurological ADEs were reported, such as myocardial infarction, memory impairment, and cerebrovascular accidents. However, these may not be directly related to the drug dosage itself, but rather due to the fact that patients with higher dosages often have more severe diabetes and may also have comorbid conditions (Russell and Cooper, 2015), making them more likely to experience severe ADEs. Future prospective studies should further explore the ADEs and clinical impacts of insulin glargine at different doses to better guide individualized treatment.
The TTO analysis indicates that the majority of insulin glargine-related ADEs occur within the first month, with a gradual reduction in ADEs thereafter. The Weibull distribution aligns with the early failure model, suggesting that the occurrence of ADEs decreases over time, consistent with the TTO analysis. This study emphasizes the importance of heightened vigilance in monitoring insulin glargine-related ADEs during the first month of use, with continuous monitoring throughout the treatment period to ensure timely management of ADEs and guarantee patient medication safety.
Sensitivity analysis showed that after excluding reports of insulin glargine in combination with glimepiride, metformin, and sitagliptin, a disproportionality analysis was re-conducted. ADEs such as hypoglycemia, injection site pain, injection site hemorrhage, acquired lipodystrophy, pancreatic neoplasm, medullary thyroid cancer, visual impairment, malignant neoplasm of the eye, and bone marrow tumor cell infiltration still exhibited positive signals, reinforcing the robustness of the study’s findings.
This study has several limitations. First, ADE reports primarily come from spontaneous reports by physicians, pharmacists, paramedics, and patients, which may introduce reporting bias (Zhao et al., 2024). Furthermore, as spontaneous reports are prone to underreporting, selective reporting, and misreporting, these reports often lack critical information, such as detailed treatment regimens, drug dosages, duration of use, and patients’ comorbidities, which may affect the accuracy of the results. Therefore, the findings of this study should be interpreted with caution. Additionally, 85.7% of the reports in this study came from the United States, which may affect the external validity of the results. Future research should consider including reports from other countries to conduct broader analyses. Finally, disproportionality analysis methods only reveal statistical associations between drugs and ADEs, but cannot establish causality (Sakaeda et al., 2013). Future large-scale prospective studies are needed to validate the findings of this study.
5 Conclusion
This study analyzed ADE reports related to insulin glargine in the FAERS database using four disproportionate analysis methods. The study confirmed several known ADEs, such as hypoglycemia and injection site reactions. The study also identified some unexpected ADEs, such as pancreatic neoplasm and medullary thyroid cancer. These findings provide new insights into the real-world safety of insulin glargine for clinicians and regulatory agencies. Future prospective studies are needed to validate the findings of this study.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
TW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. GH: Supervision, Writing – original draft, Writing – review and editing. XW: Resources, Validation, Writing – original draft, Writing – review and editing. JH: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Province (2024JJ8202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1563238/full#supplementary-material
References
1
Abi Zeid Daou C. Aboul Hosn O. Ghzayel L. Mourad M. (2025). Exploring connections between weight-loss medications and thyroid cancer: a look at the FDA adverse event reporting system database. Endocrinol. Diabetes Metab.8 (2), e70038. 10.1002/edm2.70038
2
Bulls H. Freeman E. Anderson A. Robbins M. Ness T. Goodin B. (2015). Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. J. pain Res.8, 311–320. 10.2147/jpr.S84607
3
But A. De Bruin M. L. Bazelier M. T. Hjellvik V. Andersen M. Auvinen A. et al (2017). Cancer risk among insulin users: comparing analogues with human insulin in the CARING five-country cohort study. Diabetologia60 (9), 1691–1703. 10.1007/s00125-017-4312-5
4
Carll T. Antic T. (2020). An abdominal wall mass of exogenous insulin amyloidosis in setting of metastatic sarcoma. J. Cutan. Pathol.47 (4), 406–408. 10.1111/cup.13613
5
Chen L. Wen B. Liu H. Wu H. Duan B. Shu H. et al (2024a). Efficacy and safety of insulin glargine 300 U/mL in people with type 2 diabetes in China: the INITIATION study. Diabetes Obes. Metab.26 (10), 4571–4582. 10.1111/dom.15814
6
Chen M. C. Lee D. D. (2020). Atypical presentation of localized insulin-derived amyloidosis as protruding brownish skin tumours. Clin. Exp. Dermatol45 (3), 353–355. 10.1111/ced.14097
7
Chen S. Fang W. Zhao L. Xu H. (2024b). Safety assessment of cenobamate: real-world adverse event analysis from the FAERS database. Front. Pharmacol.15, 1369384. 10.3389/fphar.2024.1369384
8
Colmers I. N. Bowker S. L. Tjosvold L. A. Johnson J. A. (2012). Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab.38 (6), 485–506. 10.1016/j.diabet.2012.08.011
9
Darenskaya M. A. Kolesnikova L. I. Kolesnikov S. I. (2021). Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med.171 (5). 10.1007/s10517-021-05191-7
10
Grauslund J. (2022). Diabetic retinopathy screening in the emerging era of artificial intelligence. Diabetologia. 10.1007/s00125-022-05727-0
11
Goykhman S. Drincic A. Desmangles J. C. Rendell M. (2009). Insulin Glargine: a review 8 years after its introduction. Expert Opin Pharmacother. 10 (4), 705–18. 10.1517/14656560902775677
12
He K. Zhao K. Yin T. Liu M. Liu J. Du W. et al (2025). A real-world Pharmacovigilance study of brodalumab based on the FDA adverse event reporting system. Sci. Rep.15 (1), 2346. 10.1038/s41598-025-86976-y
13
Hinnen D. Kruger D. F. (2019). Cardiovascular risks in type 2 diabetes and the interpretation of cardiovascular outcome trials. Diabetes, Metabolic Syndrome Obes. Targets Ther.12, 447–455. 10.2147/DMSO.S188705
14
Home P. D. (2025). An overview of insulin therapy for the non-specialist. Diabetes Obes. Metab. 10.1111/dom.16280
15
Hong E. G. Min K. W. Lim J. S. Ahn K. J. Ahn C. W. Yu J. M. et al (2024). Real-world outcomes of individualized targeted therapy with insulin glargine 300 Units/mL in insulin-naïve Korean people with type 2 diabetes: TOBE study. Adv. Ther.41 (5), 1967–1982. 10.1007/s12325-024-02830-z
16
Husain K. H. Sarhan S. F. AlKhalifa H. Buhasan A. Moin A. S. M. Butler A. E. (2023). Dementia in diabetes: the role of hypoglycemia. Int. J. Mol. Sci.24 (12). 10.3390/ijms24129846
17
Hussain A. Weimer D. S. Mani N. (2022). Diagnosing pancreatic adenocarcinoma with contrast-enhanced ultrasonography: a literature review of research in europe and asia. Cureus14 (2), e22080. 10.7759/cureus.22080
18
Jiang W. Xiang C. Du Y. Li X. Li X. Zhou W. (2023). Time trend of pancreatic cancer mortality in the Western Pacific Region: age-period-cohort analysis from 1990 to 2019 and forecasting for 2044. BMC Cancer23 (1), 876. 10.1186/s12885-023-11369-1
19
Karlstad O. Starup-Linde J. Vestergaard P. Hjellvik V. Bazelier M. T. Schmidt M. K. et al (2013). Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr. Drug Saf.8 (5), 333–348. 10.2174/15680266113136660067
20
Khan S. A. Aziz A. Esbhani U. A. Masood M. Q. (2022). Medullary thyroid cancer: an experience from a tertiary care hospital of a developing country. Indian J. Endocrinol. Metab.26 (1), 68–72. 10.4103/ijem.ijem_474_21
21
Kişioğlu S. Demir A. Tufekci D. Gunay Y. Coskun H. Ucuncu O. et al (2021). Clinical research of insulin glargine U300 in type 1 diabetes mellitus patients with frequent hypoglycaemia: real-world experience. Archives Med. Sci. Atheroscler. Dis.6, e102–e108. 10.5114/amsad.2021.105562
22
Lee I. H. Ahn D. J. (2020). Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes mellitus: a case report. Med. Baltim.99 (21), e20228. 10.1097/md.0000000000020228
23
Li S. Zhai X. Rong P. McCabe M. F. Wang X. Zhao J. et al (2014). Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One9 (11), e112066. 10.1371/journal.pone.0112066
24
Paudel G. Vandelanotte C. Dahal P. K. Biswas T. Yadav U. N. Sugishita T. et al (2022). Self-care behaviours among people with type 2 diabetes mellitus in South Asia: a systematic review and meta-analysis. J. Glob. Health12, 04056. 10.7189/jogh.12.04056
25
Rosenstock J. Hollander P. Bhargava A. Ilag L. L. Pollom R. K. Zielonka J. S. et al (2015). Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes. Metab.17 (8), 734–741. 10.1111/dom.12482
26
Russell N. D. Cooper M. E. (2015). 50 years forward: mechanisms of hyperglycaemia-driven diabetic complications. Diabetologia58 (8), 1708–1714. 10.1007/s00125-015-3600-1
27
Saboo B. Chandalia H. Ghosh S. Kesavadev J. Kochar I. Prasannakumar K. et al (2024). Insulin glargine in type 1 diabetes mellitus: a review of clinical trials and real-world evidence across two decades. Curr. diabetes Rev.20 (1), e100323214554. 10.2174/1573399819666230310150905
28
Sakaeda T. Tamon A. Kadoyama K. Okuno Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci.10 (7), 796–803. 10.7150/ijms.6048
29
Sebastian S. A. Co E. L. Mehendale M. Hameed M. (2023). Insulin analogs in the treatment of type II diabetes and future perspectives. Dis. Mon.69 (3), 101417. 10.1016/j.disamonth.2022.101417
30
Sheng X. Yao K. Shao A. Tu S. Zhang X. Chen T. et al (2019). The role of insulin glargine and human insulin in the regulation of thyroid proliferation through mitogenic signaling. Front. Endocrinol. (Lausanne)10, 594. 10.3389/fendo.2019.00594
31
Suissa S. Azoulay L. Dell'Aniello S. Evans M. Vora J. Pollak M. (2011). Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia54 (9), 2254–2262. 10.1007/s00125-011-2190-9
32
Sun H. Saeedi P. Karuranga S. Pinkepank M. Ogurtsova K. Duncan B. B. et al (2022). IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119. 10.1016/j.diabres.2021.109119
33
Tripyla A. Herzig D. Reverter-Branchat G. Pavan J. Schiavon M. Eugster P. J. et al (2023). Counter-regulatory responses to postprandial hypoglycaemia in patients with post-bariatric hypoglycaemia vs surgical and non-surgical control individuals. Diabetologia66 (4), 741–753. 10.1007/s00125-022-05861-9
34
Xiang D. C. Chen W. Fu Z. W. Wu X. H. Gao P. Wu Y. (2023). Adverse events of guselkumab in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Expert Opin. Drug Saf.22 (10), 943–955. 10.1080/14740338.2023.2223956
35
Yan X. Feng C. Lou Y. Zhou Z. (2022). Efficacy and safety of LY2963016 insulin glargine in Chinese patients with type 1 diabetes previously treated with insulin glargine (Lantus(®)): a post hoc analysis of a randomized, open-label, phase 3 trial. Diabetes Ther.13 (6), 1161–1174. 10.1007/s13300-022-01262-8
36
Zhang X. Sheng X. Miao T. Yao K. Yao D. (2019). Effect of insulin on thyroid cell proliferation, tumor cell migration, and potentially related mechanisms. Endocr. Res.44 (1-2), 55–70. 10.1080/07435800.2018.1522641
37
Zhang Z. Yao Y. Zhu L. (2024). Assessing real-world safety of plecanatide: a pharmacovigilance study based on the FDA adverse event reporting system. Front. Pharmacol.15, 1500810. 10.3389/fphar.2024.1500810
38
Zhao K. Zhao Y. Xiao S. Tu C. (2024). Assessing the real-world safety of tralokinumab for atopic dermatitis: insights from a comprehensive analysis of FAERS data. Front. Pharmacol.15, 1458438. 10.3389/fphar.2024.1458438
39
Zhou W. Tao J. Zhou X. Chen H. (2019). Insulin degludec, a novel ultra-long-acting basal insulin versus insulin glargine for the management of type 2 diabetes: a systematic review and meta-analysis. Diabetes Ther.10 (3), 835–852. 10.1007/s13300-019-0624-4
40
Zou F. Cui Z. Lou S. Ou Y. Zhu C. Shu C. et al (2024). Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database. Front. Pharmacol.15, 1338902. 10.3389/fphar.2024.1338902
Summary
Keywords
insulin glargine, adverse drug event, FAERS, pharmacovigilance, diabetes mellitus
Citation
Wang T, He G, Xiong W and Huang J (2025) Adverse drug events associated with insulin glargine: a real-world pharmacovigilance study based on the FAERS database. Front. Pharmacol. 16:1563238. doi: 10.3389/fphar.2025.1563238
Received
19 January 2025
Accepted
18 April 2025
Published
28 April 2025
Volume
16 - 2025
Edited by
Anil K. Giri, University of Helsinki, Finland
Reviewed by
Abad Khan, University of Swabi, Pakistan
Kimberly Crosby, University of Oklahoma, United States
Updates
Copyright
© 2025 Wang, He, Xiong and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juanjuan Huang, 85529726@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.