- Department of Pharmaceutical Sciences, Tshwane University of Technology, Pretoria, South Africa
Background: The genus Adenia has a rich history of traditional medicinal use across various cultures, particularly in Africa and parts of Asia. The present review summarizes key features of the genus Adenia, focusing on its occurrence, distribution, isolation, bioactivities, and toxicities.
Material and methods: A thorough literature review was conducted using databases such as Google Scholar, PubMed, ScienceDirect, JSTOR, and Web of Science. The search utilized the keyword “Adenia” in combination with relevant terms like “distribution,” “traditional use,” “phytochemicals,” “chemical compounds,” “pharmacology,” “bioactivity,” and “toxicity.”
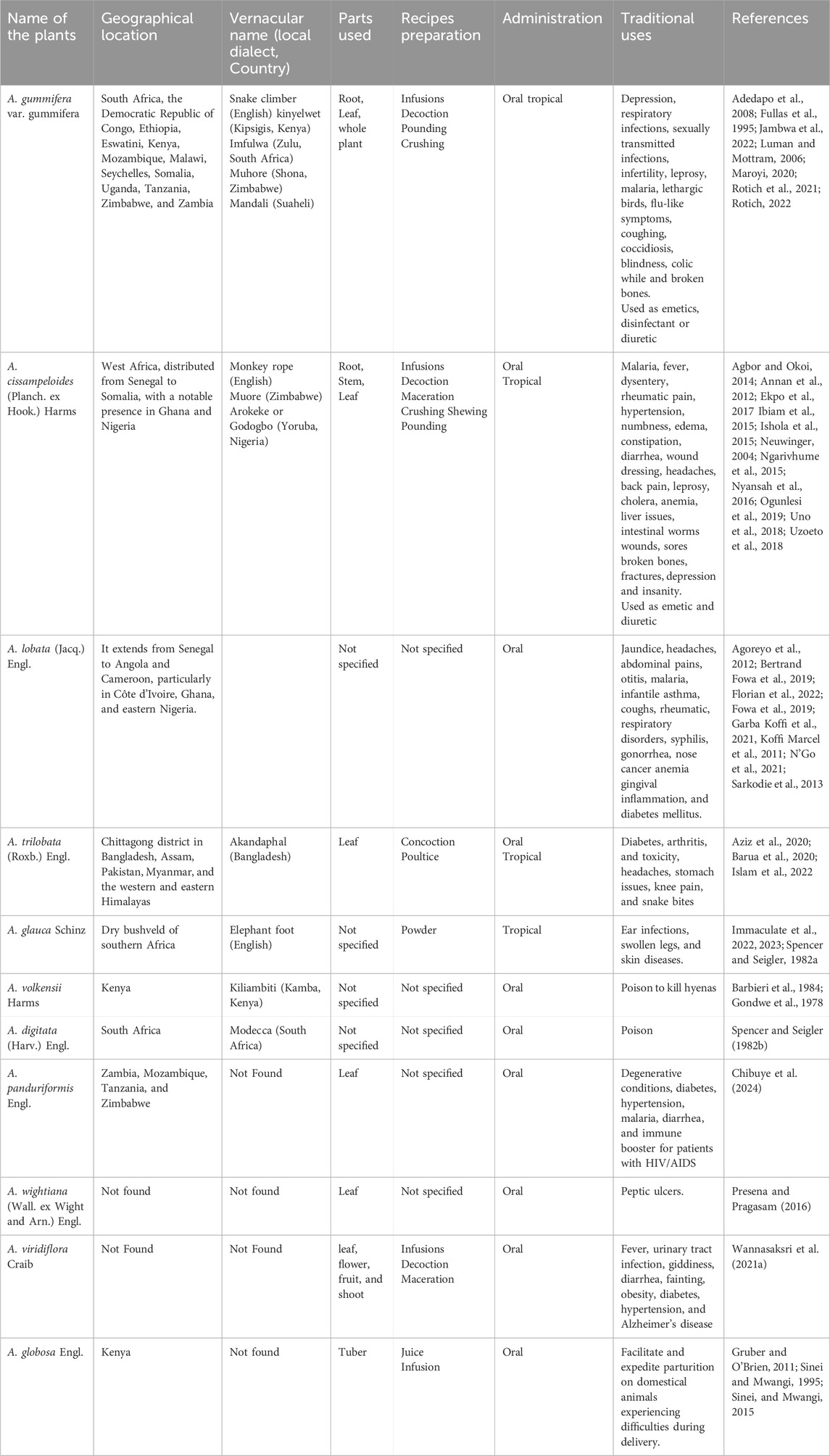
Results: Our search yielded 3,724 records, with 88 studies from 1935 to 2024 meeting our criteria. The findings indicate that the genus Adenia comprises over 106 species of climbing plants, commonly found in tropical and subtropical regions. In traditional medicine, several Adenia species have been employed in various cultures for medicinal purposes, treating ailments such as wounds, leprosy, malaria, infections, infertility, colic, dysentery, hypertension, rheumatism, headaches, abdominal pain, and cancer. Chemical investigations have identified 27 secondary metabolites including alkaloids, glycosides, flavonoids, and ribosome-inactivating proteins (RIPs), particularly type 2, which are associated with cytotoxic and toxic effects. Pharmacological studies of chemical constituents and extracts from Adenia species have revealed a broad spectrum of biological activities, including cytotoxicity, antimicrobial, antioxidant, anti-inflammatory, analgesic, anticholinesterase, neuropharmacological, antidepressant, antihyperglycaemic, anti-anemic, anticoagulant, antithrombotic, thrombolytic, and anesthetic activities. Despite their therapeutic benefits, concerns regarding safety and toxicity are significant, necessitating comprehensive evaluations and standardized methodologies for assessing their efficacy. Thus, future research should focus on validating the traditional uses of Adenia species through rigorous scientific methods to ensure their safety and efficacy in modern medicine.
1 Introduction
The genus Adenia Forssk. (Passifloraceae) comprises over 106 species of climbing plants, predominantly found in tropical and subtropical regions of Asia, Africa, and the Pacific Islands. This genus is notable for its extensive morphological diversity and ecological versatility, allowing species to thrive in various environments, from arid landscapes to lush rainforests (Hearn, 2007; Sinei and Mwangi, 2015; Rotich, 2022). Traditionally, Adenia species are utilized across numerous cultures for medicinal purposes, with different parts of the plant—such as leaves, roots, stems, fruits, and seeds—being employed to treat various ailments, including wounds, fever, gastrointestinal issues, pain, and inflammation (Sarkodie et al., 2013; Sinei and Mwangi, 2015; Barua et al., 2020; Maroyi, 2020; Rotich, 2022; Chibuye et al., 2024). The presence of bioactive compounds further underscores the therapeutic potential of these species.
Ethnobotanical studies have consistently emphasized the importance of Adenia species in traditional medicine, particularly in Africa, where species like A. gummifera, A. cissampeloides, and A. lobata are utilized for various medicinal applications (Ibiam et al., 2015; Ogunlesi et al., 2019; Garba Koffi et al., 2021; N’Go et al., 2021; Rotich et al., 2021; Florian et al., 2022). The pharmacological relevance of these plants is enhanced by their chemical diversity, featuring numerous secondary metabolites including alkaloids, glycosides, flavonoids, steroids, and terpenoids (Ogunlesi et al., 2019; Garba Koffi et al., 2021; Immaculate et al., 2022). These compounds are believed to contribute to the pharmacological effects seen in Adenia species, including antimicrobial, antioxidant, anticancer, anti-inflammatory, and analgesic activities (Fowa et al., 2019; Barua et al., 2020; Maroyi, 2020; Bortolotti et al., 2021; N’Go et al., 2021; Rotich, 2022). Notably, ribosome-inactivating proteins (RIPs), particularly type 2, may play a significant role in the cytotoxic and toxic properties of certain Adenia species, with several demonstrating selective toxicity against cancer cell lines, making them promising candidates for anticancer drug development (Tosi et al., 2009; Battelli et al., 2010; Bortolotti et al., 2021; Maiello et al., 2021; Rotich, 2022).
Despite their potential therapeutic benefits, concerns about the safety and toxicity of Adenia species are growing, especially given their extensive use in traditional medicine. Limited toxicological data indicate that some species may exhibit adverse effects at specific dosages (Ekpo et al., 2017; Uno et al., 2018; Barua et al., 2020; Rage et al., 2024). This highlights the necessity for thorough safety evaluations before recommending Adenia products for therapeutic purposes. Furthermore, the lack of clinical data regarding the safety, efficacy, and pharmacokinetics of Adenia compounds necessitates more rigorous research to establish clear guidelines for their medicinal use. This review aims to synthesize the existing literature on the ethnobotany, phytochemistry, pharmacology, and toxicity of the genus Adenia. By exploring traditional uses, chemical constituents, pharmacological properties, and potential risks, this review underscores the therapeutic potential and the need for further scientific investigation into these remarkable plants.
2 Methodology
A thorough literature review was carried out using databases like Google Scholar, ScienceDirect, PubMed, JSTOR, and Web of Science, covering articles published from 1935 to 2024. These databases were selected for their relevance to medicinal plant research and their recognition by peer-reviewed literature. The search utilized the keyword “Adenia” combined with terms like “distribution,” “traditional use,” “phytochemicals,” “chemical compounds,” “pharmacology,” “bioactivity,” and “toxicity.” For botanical descriptions, the Plant List was accessed via WFO plant list, to compile an exhaustive list of all published Adenia species. Articles were evaluated based on their titles and abstracts, with the following inclusion criteria: (1) only full-text articles or reports that were published in or translated into English; (2) original peer-reviewed studies or reports focusing on the ethnobotany, pharmacology, phytochemistry, and toxicity of Adenia species. Exclusion criteria included papers that (1) did not study Adenia species; (2) were not full-text articles; and (3) were narrative or systematic reviews, or reports lacking original data (such as editorials, expert opinions, and perspective papers). To avoid duplicate results, all downloaded articles were imported into EndNote® X9 (Thomson Reuters, Philadelphia, PA, USA), where duplicate reports were removed.
3 Results and discussion
Our electronic database search generated 3,724 records. After deduplication, screening titles and abstracts, and applying our inclusion and exclusion criteria, 88 studies from 1935 to 2024 were selected for full-text review. The evidence from these included studies is summarized narratively below and listed in Table 1. The review begins with an overview of the botanical description and distribution of Adenia species, followed by sections on their ethnobotany and medicinal uses, phytochemistry, pharmacology, and toxicity studies.
3.1 Botanical description and distribution
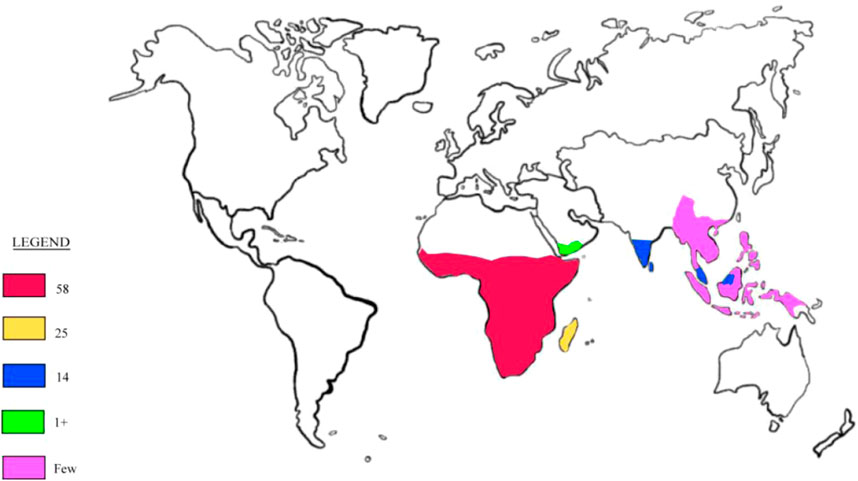
The genus Adenia, a group of flowering plants in the family Passifloraceae, is characterized by its climbing or sprawling habit, with stems emerging from above-ground tubers that can reach up to 2.5 m wide and feature fleshy, lobed leaves (Liebenberg, 1935; Sinei and Mwangi, 2015). It comprises around 106 species and is considered the second-largest genus in the family after Passiflora with over 500 species (Rotich, 2022). The genus Adenia is primarily found in the Old World tropics, where it has undergone significant evolutionary diversification. Its plants are adapted to diverse environments, including dry and moist forests, savanna woodlands, and bushlands (Sinei and Mwangi, 2015). It shows a notable diversity in growth forms, ranging from succulent trees, shrubs, and vines to geophytes, lianas, and other unique growth habits. It is believed that the ancestral Adenia species was likely a liana with storage roots (Hearn, 2006). Several species within Adenia have evolved distinct traits, such as stem succulence, which is thought to have evolved independently at least three times, and the development of storage roots, with multiple gains and losses of these features across different species (Hearn, 2006; Hearn, 2009). Regarding morphological variation, Adenia species can range from small herbaceous climbers to larger, woodier plants (De Wilde, 1971). Many species are dioecious, meaning that male and female flowers are found on separate plants, though some species exhibit monoecy. The flowers, generally glabrous, have variable shapes, with the male flowers being smaller than the female flowers. The fruits of Adenia are typically 1-celled capsules that dehisce loculicidally, leaving seeds attached to the middle of the valves. Fruit shape can range from fusiform to globular, with colors varying from greenish to yellow or bright red when ripe (De Wilde, 1971).
The distribution of Adenia (Figure 1) is predominantly paleotropical, with a significant concentration in Africa, where 58 species are found, alongside 25 species in Madagascar and 14 species in South India, Ceylon, and Indo-Malesia (De Wilde, 1971; Hearn, 2007). The genus extends as far north as the savanna belt south of the Sahara, passing through Sudan and Ethiopia, and into Yemen, where A. Venenata, the first species described, is found (Liebenberg, 1935; De Wilde, 1971). To the south, Adenia species, such as A. repanda and A. gummifera, are found as far as South Africa, reaching as far south as approximately 30° and 33° latitude, respectively (De Wilde, 1971). In Madagascar, Adenia species are found in arid and wet regions, from the lowlands to the central highlands. The genus also thrives in various habitats, ranging from the driest deserts of coastal Namibia to the humid rainforests of Southeast Asia (De Wilde, 1971; Hearn, 2006). In the Flora of Southern Africa, which includes South Africa, Namibia, Botswana, Lesotho, and Swaziland, the genus Adenia is represented by ten species and 13 taxa (Crouch et al., 2016). Among these, Adenia gummifera is notable as one of the two infraspecific varieties of A. gummifera, native to a range from southwest Ethiopia to South Africa and the Seychelles. This species is a tall climber with somewhat woody stems that grow in altitudes of 50 m. The leaves of A. gummifera are typically entire or deeply lobed (3-5 lobes), ovate or orbicular in shape, featuring a truncated base (Rotich, 2022).
3.2 Ethnobotany and medicinal uses
While many species of Adenia are toxic and have been used in traditional practices for poisoning or hunting, some are utilized in conventional medicine across various regions for ailments ranging from common illnesses to more severe conditions (Sarkodie et al., 2013; Sinei, K. A. and Mwangi, 2015; Barua et al., 2020; Maroyi, 2020; Maroyi, 2020; Rotich, 2022; Chibuye et al., 2024). For instance, A. gummifera is particularly recognized for its traditional medicinal uses throughout tropical Africa, occurring naturally in countries such as South Africa, the Democratic Republic of Congo, Ethiopia, Eswatini, Kenya, Mozambique, Malawi, Seychelles, Somalia, Uganda, Tanzania, Zimbabwe, and Zambia. It typically grows on rocky slopes, termite mounds, and ravines, adapting to various ecological niches (Maroyi, 2020). It is well-known as Snake climber (English) and also called kinyelwet in the Kipsigis community of Kenya; imfulwa in the Zulu community in South Africa; Muhore in the Shona community of Zimbabwe; and Mandali or Ngole in Suaheli or Sukuma communities respectively in Eastern Africa (Luman and Mottram, 2006; Adedapo et al., 2008; Rotich et al., 2021; Jambwa et al., 2022; Rotich, 2022). The infusions of this plant are used as emetics and for treating depression (Adedapo et al., 2008). The roots are part of an herbal concoction called CareVid, serves as an immune booster and health tonic for treating and managing HIV/AIDS opportunistic infections in Kenya (Maroyi, 2020; Rotich, 2022). These roots are also employed as a disinfectant or diuretic and traditional medicine for ailments like respiratory infections, filariasis, Oral candidiasis, sexually transmitted infections, gastrointestinal infections, and infertility (Fullas et al., 1995; Maroyi, 2020; Rotich et al., 2021). In nearby Mozambique, a root infusion is prepared by boiling a six-inch section of the root in three to four pints of hot water (100°C) for consumption by the patient to treat malaria and leprosy, often inducing vomiting and perspiration (Luman and Mottram, 2006). The entire plant can be pounded and dissolved in drinking water to treat lethargic birds, coughing, flu-like symptoms, Newcastle disease, coccidiosis, and blindness (Jambwa et al., 2022). In Tanzania, the juice derived from the leaves of this plant is used to alleviate colic, while crushed leaves are used topically to help heal broken bones (Fullas et al., 1995). However, hepatic toxicity has been associated with the chronic traditional use of A. leaves among the Zulu of South Africa (Fullas et al., 1995; Luman and Mottram, 2006). The leaves are also cooked as a vegetable in tropical Africa (Maroyi, 2020). The stems and roots of the plant are sold as traditional remedies in informal herbal medicine markets in Mozambique, Botswana, Gauteng, and KwaZulu-Natal provinces in South Africa (Maroyi, 2020). The stems of A. gummifera are combined with other plants to create a mixture for protective charms (Luman and Mottram, 2006).
Another most used plant is A. cissampeloides, which is commonly known as snake climber or monkey rope, and locally called Muore in Zimbabwe, Arokeke or Godogbo among the Yoruba in Nigeria (Ishola et al., 2015; Ngarivhume et al., 2015). It thrives in humid areas of West Africa, distributed from Senegal to Somalia, with a notable presence in Ghana and Nigeria (Agbor and Okoi, 2014; Uno et al., 2018; Uzoeto et al., 2018). This plant has a wide range of traditional medicinal applications, including cold or hot infusions of the root or bark for treating malaria and fever (Ngarivhume et al., 2015). It is also widely used as a fishing poison by various tribes from Senegal to Tanzania; in Nigeria, fresh leaves are roasted, pounded, and thrown into rivers, resulting in small fish floating dead within 15 minutes and larger fish within an hour (Neuwinger, 2004; Agbor, R.P. and Okoi, 2014; Ibiam et al., 2015; Uno et al., 2018). The stem is used in Nigeria as fish poison when macerated in water and as emetic for biliousness (Ibiam et al., 2015). In Ghana, it treats malaria, dysentery, rheumatic pain, hypertension, and numbness (Annan et al., 2012), while its leaves are used as a vegetable, and the sap serves as a cosmetic (Ibiam et al., 2015; Ogunlesi et al., 2019). In Ivory Coast, the leaf extract is applied to the breast after childbirth to encourage lactation (Ibiam et al., 2015). Decoctions and infusions of the leaves, stem, or root are employed for gastrointestinal disorders like constipation and diarrhea, as well as rheumatic pain and wound dressing (Nyansah et al., 2016; Ekpo et al., 2017). These decoctions or infusions are also used to address various inflammatory conditions, including edema and rheumatism, as well as to provide pain relief, particularly for headaches and back pain. Moreover, pounded roots are commonly used to dress sores and wounds (Ekpo et al., 2017). The decoction made from the leaves or roots is consumed as a diuretic and to treat malaria and fever (Ekpo et al., 2017). For leprosy, a decoction made from the leaves is applied directly to the sores, while an oral intake of root decoction is combined with a steam bath using the leaves. To treat scabies, ashes from the root or bark are combined with castor oil. A root decoction is consumed to manage cholera and is taken with milk to address anemia in eastern Africa. An extract from the roots and stems is used orally for intestinal worms, and a decoction from leaves is consumed for liver issues. A paste made from leaves is used on fractures and broken bones in Tanzania (Ekpo et al., 2017). Additionally, leaf infusions serve as stimulants for depression and insanity in Zimbabwe, with the inner bark used by the Mano people of Liberia to induce amnesia (Ishola et al., 2015). The plant is noted for its antiplasmodial and antimicrobial properties (Agbor and Okoi, 2014; Nyansah et al., 2016). While it possesses toxic properties, particularly due to the gum resin containing modeccin, A. cissampeloides remains economically important for its diverse medicinal uses and fishing practices (Ibiam et al., 2015).
Additionally, A. lobata is a species widely utilized in traditional medicine across African regions. It extends from Senegal to Angola and Cameroon, and is particularly used in Côte d'Ivoire, Ghana, and eastern Nigeria. It is a liana that is often employed for the treatment of various ailments, including jaundice, malaria, headaches, abdominal pains, otitis, coughs, infantile asthma, rheumatic, respiratory disorders, syphilis, gonorrhea, and even nose cancer (Koffi Marcel et al., 2011; Agoreyo et al., 2012; Bertrand Fowa et al., 2019; Fowa et al., 2019; Garba Koffi et al., 2021). In Ivorian traditional pharmacopeia, A. lobata is also used to alleviate anemia in children and pregnant women, relieve gingival inflammation, facilitate labor, and treat malaria fever (N’Go et al., 2021; Florian et al., 2022). In Ghana, it is used to treat diabetes mellitus (Sarkodie et al., 2013), while in some eastern communities of Nigeria, the plant extracts are used for rheumatic pain, headaches, abdominal pain, and cancer (N’Go et al., 2021). Moreover, the plant is recognized for its antihyperglycemic and antioxidant properties (Bertrand Fowa et al., 2019; Fowa et al., 2019).
A. trilobata, locally referred to as akandaphal in Bangladesh, is distributed in the Chittagong district, as well as in Assam, Pakistan, Myanmar, and the western and eastern Himalayas (Barua et al., 2020). Traditionally, it is used in Bangladesh (South Asia) for various herbal treatments, particularly for diabetes, arthritis, toxicity, and other age-related diseases (Islam et al., 2022). The poultice made from its leaves is applied to alleviate headaches, stomach issues, knee pain, and snake bites (Aziz et al., 2020; Barua et al., 2020).
A. glauca, commonly referred to as “Elephant foot,” is a climbing, tuberous, perennial shrub native to the dry bushveld of southern Africa (Immaculate et al., 2022; 2023). This plant is primarily found in rocky areas and has been noted to be poisonous to cattle; however, children in Africa have been observed to consume the fruit without adverse effects (Spencer K. C. and Seigler D. S., 1982). The leaves at the plant’s base are cyanogenic, while the tuber is non-cyanogenic and considered non-poisonous (Spencer K. C. and Seigler D. S., 1982). Traditionally, the powdered form of A. glauca has been utilized to treat various medical conditions, including ear infections, swollen legs, and skin diseases. The preparation typically involves grinding the plant material into a powder, which is then applied as a topical treatment (Immaculate et al., 2023). Furthermore, the plant is known to contain galactose-binding lectins and extracts that are useful for protein synthesis and hemagglutinating activity, highlighting its potential as a therapeutic resource (Immaculate et al., 2022).
A. volkensii is a perennial shrub found growing wild in several regions of Kenya. The plant is particularly noted for its high toxicity and is traditionally used to kill hyenas, which is reflected in its local Kamba name “kiliambiti,” meaning “eater of hyenas” (Gondwe et al., 1978; Barbieri et al., 1984). Documented cases of poisoning have been reported in Kenya, where individuals became ill after consuming a local gruel that was reportedly contaminated with an extract of A. volkensii (Gondwe et al., 1978).
A. digitata, commonly known as Modecca, is a tuberous, perennial shrub that thrives in dry regions of South Africa. Both the tuber and fruit are known to be highly toxic to humans, with cases of death reported following accidental, suicidal, or homicidal ingestion (Spencer K. C. and Seigler D. S., 1982).
A. panduriformis is a woody climber plant primarily found in Zambia, Mozambique, Tanzania, and Zimbabwe. It could thrive in similar climatic conditions elsewhere in the world.
The plant is a wild vegetable that is commonly consumed as part of the daily diet in various Zambia communities. It is also utilized in traditional medicine to treat and manage a range of diseases, such as degenerative conditions. Traditional healers often prescribe A. panduriformis to patients with weakened immune systems, including those living with HIV/AIDS, as it is believed to be an excellent immune booster. Additionally, this leafy vegetable is claimed to have healing properties for several ailments, including diabetes, hypertension, malaria, and diarrhea (Chibuye et al., 2024).
A. wightiana is a tuberous, perennial liana characterized by axillary, branched tendrils. It is widely recognized in folk medicine for its medicinal properties, particularly in treating ailments like peptic ulcers. The leaves of this plant are also consumed as a vegetable by the tribes residing in the Anamalai Hills of the Western Ghats (Presena and Pragasam, 2016).
A. viridiflora is one of the medicinal plants of the Adenia genus used traditionally to treat fever, urinary tract infection, diarrhea, giddiness, and fainting. Its extracts show potential in managing conditions such as obesity, diabetes, hypertension, and Alzheimer’s disease. The edible parts of A. viridiflora include the leaves, flowers, young fruits, and shoots which are typically fermented or blanched using washing rice water and served with a spicy sauce as a vegetable accompaniment (Wannasaksri et al., 2021a).
A. globosa Engl. is also used traditionally in Kenya, where freshly prepared juice from the tuber is administered to cattle, cows, and goats to facilitate and expedite parturition, particularly for those experiencing difficulties during delivery (Sinei and Mwangi, 1995; Sinei, and Mwangi, 2015). Additionally, an infusion made from this tuber has been shown to induce dose-dependent contractions of the rat uterus, further supporting its traditional use in promoting faster parturition in domestic animals (Gruber and OʼBrien, 2011).
3.3 Phytochemistry
Adenia species are also known for their rich phytochemical profile. These plants contain a diverse array of secondary metabolites that have been explored for their medicinal properties. Phytochemical studies on different species of Adenia such as A. cissampeloides, A. gummifera, A. lobate, A. panduriformis, A. trilobata, and A. volkensii have revealed a variety of compound classes. These include alkaloids, flavonoids, tannins, phenols, steroids, terpenoids, polyphenols, saponins, carbohydrates, proanthocyanins, glycosides, and even potentially toxic proteins like type 2 ribosome-inactivating proteins (RIPs) (Gondwe et al., 1978; Severino et al., 2009; Okem et al., 2014; Bertrand Fowa et al., 2019; Ogunlesi et al., 2019; Barua et al., 2020; Maroyi, 2020; Rotich, 2022; Chibuye et al., 2024). The investigation of some of these species led to the isolation of 27 compounds belonging to different classes. These include six cyanohydrin glycosides, one alkaloid, one ceramide, six flavonoids, three steroids, one triterpene, two fatty acids, one polyacetylene, and six type 2 RIPs. In addition, 17 compounds were found in the essential oils obtained from A. cissampeloides leaves. Furthermore, some heavy metals including aluminum, chromium, cadmium, copper, iron, manganese, lead, nickel, mercury, zinc, and tin were also found in an Adenia species (A. gummifera) (Rotich, 2022).
3.3.1 Cyanohydrin glycosides, alkaloid and ceramide
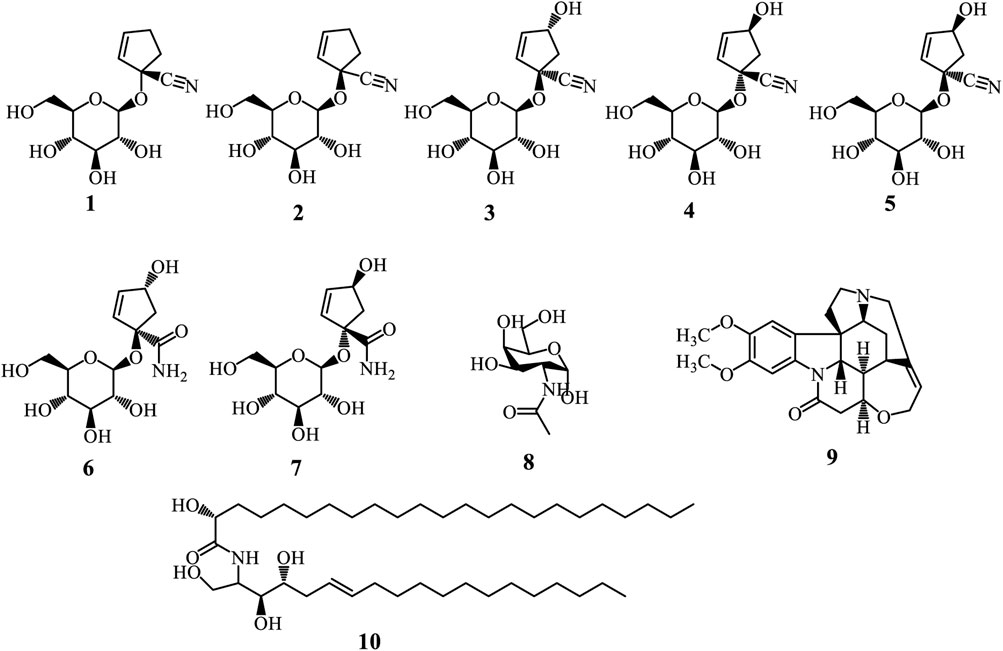
Cyanohydrin glycosides are unique plant compounds defined by a cyanohydrin group attached to a sugar moiety, forming a glycosylated structure. These compounds originate from amino acids and are crucial for plant defense, particularly against herbivores, as they can release toxic hydrogen cyanide when hydrolysed enzymatically (Jørgensen et al., 2011; Gleadow and Møller, 2014). The biosynthesis of cyanohydrin glycosides involves several enzymatic steps, primarily catalysed by cytochrome P450 enzymes. The process typically begins with the conversion of amino acids into oximes, which are then transformed into cyanohydrins through the action of specific enzymes (Jørgensen et al., 2011; Gleadow and Møller, 2014). Alkaloids are a diverse group of naturally occurring organic compounds that primarily contain basic nitrogen atoms. Six cyanohydrin glycosides were isolated from the Adenia genus (Figure 2). These include tetraphyllin A (1) and deidaclin (2) isolated from A. globosa (Sinei and Mwangi, 1995), volkenin (3) from A. volkensii roots (Jaroszewski et al., 1987), tetraphyllin B (4) and epi-tetraphyllin B (5) from the fresh tubers of A. digitate, A. volkensii, and A. glauca (Gondwe et al., 1978; Spencer K. C. and Seigler D. S., 1982; Jaroszewski et al., 1987). Compound (4) was also isolated from the roots of A. cissampeloides (Ogunlesi et al., 2019). Importantly, (Jaroszewski et al. (1987) demonstrated compounds (3) and (3) can be converted into (1R,4R)- (7) and (1S,4S)-1-(β-D-glucopyranosyloxy)-4-hydroxy-2-cyclopentene-1-carboxamides (7) respectively, through a series of reactions, including alkaline hydrolysis and spontaneous lactonization in methanol (Jaroszewski et al., 1987). Another cyanohydrin glycoside is N-Acetyl galactosamine (8) isolated from A. hondala (Sharma et al., 2018). However, brucine (9), isolated from the leaves of A. lobata, is the only alkaloid reported from the genus (Agoreyo et al., 2012). At the same time, adeniamide (10) was the only ceramide isolated from the stem bark of A. lobata (Garba Koffi et al., 2021).
3.3.2 Flavonoids
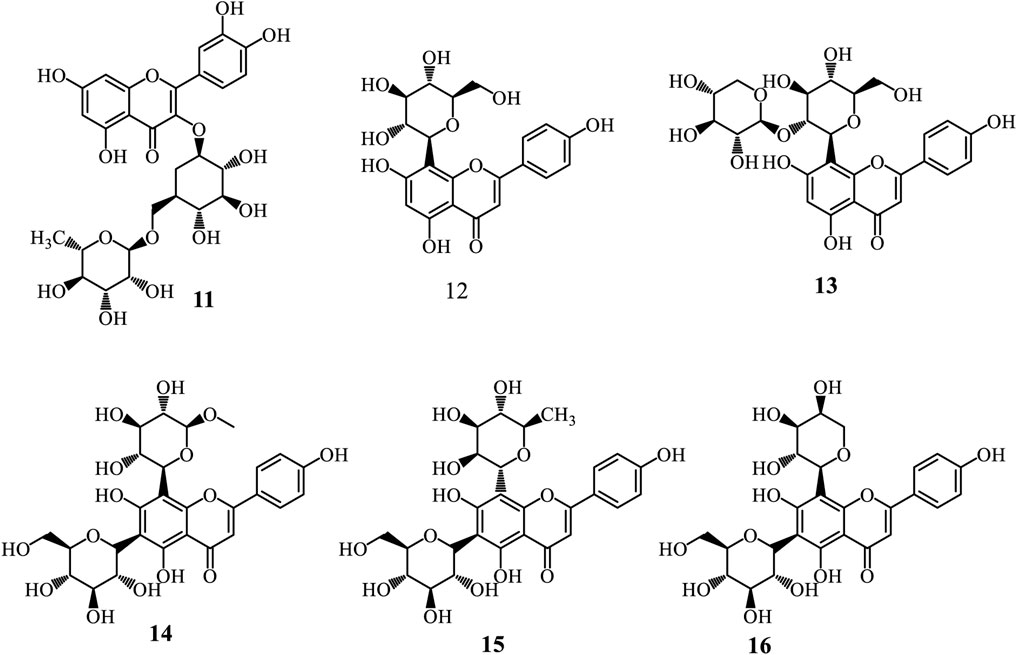
Flavonoids represent a diverse group of natural polyphenolic compounds with a basic structure of 2-phenyl-benzopyran, consisting of two aromatic rings (A and B) linked by a heterocyclic pyran ring (C) containing an oxygen atom (Falcone Ferreyra et al., 2012; Yang et al., 2018; Liga et al., 2023). Glycosyl flavonoids are bonded to sugar molecules, enhancing their solubility and bioavailability (Liga et al., 2023). The flavonoid biosynthesis occurs through the phenylpropanoid pathway, starting with phenylalanine. Key enzymatic steps include the transformation of phenylalanine into trans-cinnamic acid by phenylalanine ammonia-lyase (PAL), followed by its transformation into 4-coumaric acid and then into 4-coumaroyl-CoA. Chalcone synthase catalyses the condensation of 4-coumaroyl-CoA with malonyl-CoA to produce chalcone, which can be further modified into various flavonoid subclasses (Falcone Ferreyra et al., 2012; Yang et al., 2018; Liga et al., 2023). Glycosylation occurs via glycosyltransferases, attaching sugar moieties to the flavonoid backbone, resulting in glycosyl flavonoids with distinct biological activities (Falcone Ferreyra et al., 2012). Only six glycosyl flavonoids have been isolated from the genus Adenia (Figure 3). These include Rutin (11) isolated from A. glauca roots (Immaculate et al., 2022), vitexin (12), 2″-xylosylvitexin (13), and a mixture of vicenin-2 (14), violanthin (15), and schaftoside (16) isolated from the leaves of A. mannii (Ulubelen and Oksuz, 1983).
3.3.3 Steroids and triterpenes
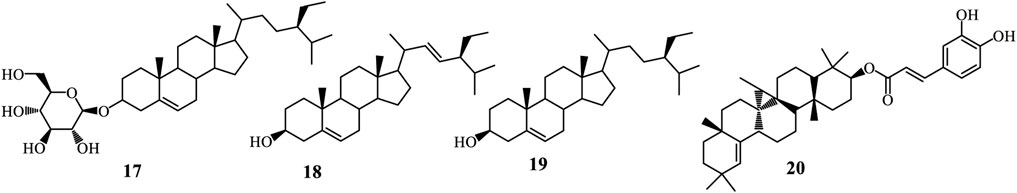
Steroids are organic compounds defined by a core structure of four fused carbon rings, known as the steroid nucleus. Their biosynthesis primarily derives from cholesterol through the mevalonate pathway, which begins with acetyl-CoA converting to mevalonic acid via HMG-CoA reductase. Mevalonic acid is then transformed into isoprenoid units and assembled into squalene, which undergoes cyclization to form lanosterol. This precursor is further modified to produce various steroid hormones (Harrison, 1985). Triterpenes, composed of six isoprene units and resulting in a 30-carbon skeleton, are synthesized via the same mevalonate pathway. The process starts with the formation of isopentenyl pyrophosphate (IPP) from acetyl-CoA, followed by the condensation of IPP units into squalene. Specific enzymes, such as oxidosqualene cyclases, then catalyze the cyclization of squalene, yielding diverse triterpenoid structures (Harrison, 1985; Lu et al., 2024). These compound classes are extensively found in plants and are recognised for their various biological activities. From Adenia species, three steroids have been isolated (Figure 4). These include β-sitosterol-3-O-β-D-glucopyranoside (17) and a mixture of stigmasterol (18) and β-sitosterol (19) isolated from A. lobata stem bark. Whereas, only one triterpene, germanicol caffeoyl ester (20), has been isolated from A. lobata stem bark (Garba Koffi et al., 2021).
3.3.4 Fatty acid and polyacetylene
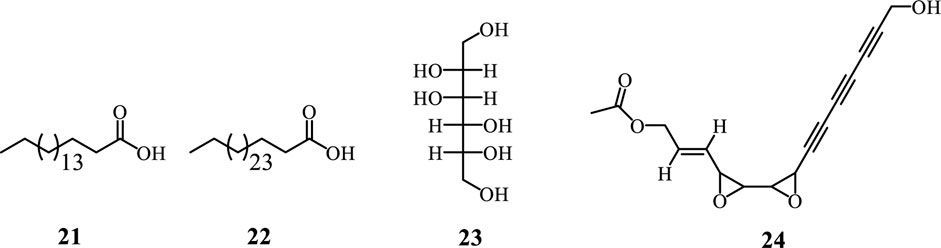
Fatty acids are long-chain carboxylic acids, either saturated or unsaturated, crucial for energy storage, cellular structure, and signaling in organisms. Their synthesis mainly occurs via the fatty acid synthase (FAS) complex, beginning with acetyl-CoA, which is converted to malonyl-CoA and then condensed into a 3-ketoacyl-ACP intermediate. This intermediate undergoes reduction and dehydration, producing saturated fatty acids, with elongation processes resulting in common fatty acids like palmitic and stearic acid (Minto and Blacklock, 2008; Scott et al., 2022; Salomon, 2024). Polyacetylenes, known for their carbon-carbon triple bonds, are derived from fatty acid precursors such as crepenynic and stearolic acids. Their biosynthesis includes desaturation of fatty acids and the formation of acetylenic bonds through oxidative dehydrogenation or decarboxylative enol elimination, leading to diverse polyacetylene derivatives (Minto and Blacklock, 2008; Scott et al., 2022; Salomon, 2024). From Adenia species only two fatty acids are reported, including octacosanoic acid (21) and D-mannitol (22) isolated from A. lobata stem bark (Figure 5) (Garba Koffi et al., 2021). Whereas gummiferol (23) is the only polyacetylene isolated from A. gummifera leaves (Fullas et al., 1995; Luman, F.M., and Mottram, 2006).
3.3.5 Essential oils
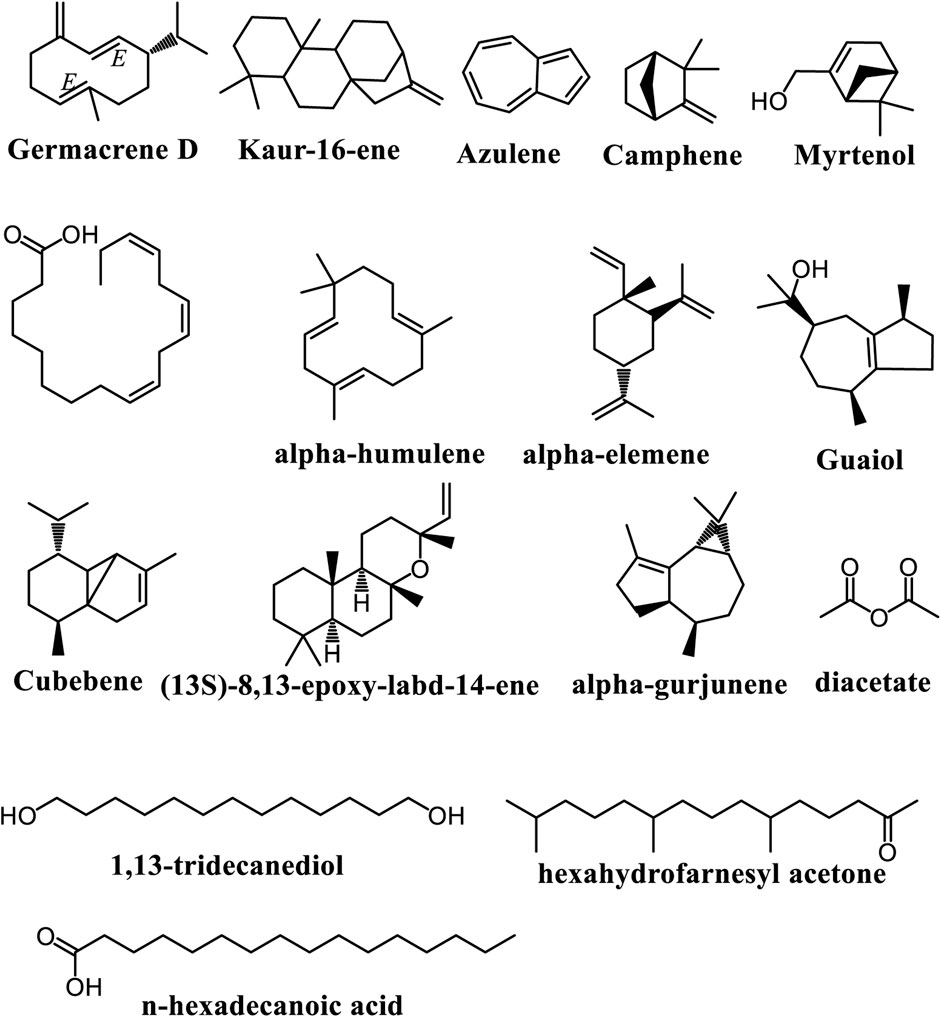
Essential oils (EOs) are concentrated, hydrophobic liquids derived from plants, known for their strong aromas, and composed mainly of monoterpenes and sesquiterpenes. Typically, EOs contain 20 to 60 components, with two or three major constituents making up 20%–70% of the mixture (Rashidinejad and Jafari, 2020; Santos et al., 2022). Their chemical composition can vary significantly due to factors such as season, climate, water stress, geographic location, soil conditions, and other abiotic influences. In 2019, Ogunlesi and collaborators analysed the EOs from A. cissampeloidesdried leaves. They found a total of 17 constituents from five essential derived from the species. These include phytol, germacrene D, n-hexadecanoic acid, α-linolenic acid, 1,13-tridecanediol, diacetate, hexahydrofarnesyl acetone, kaur-16-ene, (13S)-8,13-epoxy-labd-14-ene, guaiol, α-elemene, α-gurjunene, α-humulene, myrtenol, camphene, azulene, and cubebene (Figure 6). The major compound in the EO samples was phytol, with other notable constituents including germacrene D, diacetate, and 1,13-tridecanediol which were predominant in the first and second hours of collection, respectively (Ogunlesi et al., 2019).
3.3.6 Type 2 ribosome-inactivating proteins (RIPs)
RIPs are a class of enzymes primarily derived from plants that can inhibit protein synthesis by targeting eukaryotic ribosomes. They are categorized into three main types: type I RIPs, which are single-chain proteins; type II RIPs, which consist of an A-chain and a B-chain; and type III RIPs, which are less common and have additional domains (Stirpe et al., 2007; Bortolotti et al., 2021; 2024; Maiello et al., 2021). Type 2 RIPs, mostly isolated from the Adenia genus, are responsible for binding to cell surface receptors and then highly cytotoxic, making them of interest for both therapeutic and research applications. These proteins are purified by using Affinity Chromatography and Gel Filtration techniques and characterized by using SDS/PAGE and ESI-MS techniques (Barbieri et al., 1980; Chambery et al., 2004; Severino et al., 2009; Tosi et al., 2009; Mercatelli et al., 2020; Bortolotti et al., 2021; Bortolotti et al., 2024). Type 2 RIPs from Adenia species include modeccin, isolated from A. digitata and A. volkensii roots. This toxin is a protein with a molecular weight of 57,000, which can dissociated into two subunits weighing 25,000 and 32,000 (Gasperi-campani et al., 1978; Barbieri et al., 1980; Bortolotti et al., 2021; Bortolotti et al., 2024). Another type 2 RIP from Adenia species, is volkensin, also isolated from the roots of A. volkensii, with a molecular weight of 60,000. The A-chain has a molecular weight of 29,000, while the B-chain displays double molecular weights of 37,400 and 36,000 (Chambery et al., 2004; Severino et al., 2009). Volkensin has additionally been extracted from A. digitate roots (Bortolotti et al., 2024) and from the caudex of A. kirkii (Bortolotti et al., 2021). Stenodactylin is isolated from the caudex of A. stenodactyla, consisting of two chains connected by a disulfide bond. The A-chain acts on 28S rRNA, while the B-chain binds to glycan structures. Its molecular weight is 53,800, reduced into two bands of 24,800 and 30,000 for the A and B chains respectively (Stirpe et al., 2007; Tosi et al., 2009; Mercatelli et al., 2020; Bortolotti et al., 2024). This protein was characterized through crystallization and X-ray diffraction, resulting in crystals that diffract at high resolution, reaching up to 2.15 Å (Tosi et al., 2009). Lanceolin, isolated from the caudex of A. lanceolata, has a molecular weight of 63,000, with A and B chains ranging from 27,000 to 33,400 (Stirpe et al., 2007; Bortolotti et al., 2021; 2024). Kirkiin, derived from the caudex of A. kirkii, has an approximate molecular weight of 58.5 kDa, which reduces to bands of 27.1 kDa and 35.3 kDa (Bortolotti et al., 2021; 2024). Lastly, heterophyllin from the caudex of A. heterophylla corresponds to 60 kDa under non-reducing conditions and reduces to bands of about 25 kDa and 33 kDa (Bortolotti et al., 2024).
3.4 Pharmacological properties of Adenia
The pharmacological properties of Adenia species highlight their potential for therapeutic applications, including antimicrobial, antioxidant, anti-inflammatory, analgesic, anticholinesterase, neuropharmacological, antidepressant, antihyperglycaemic, anti-anemic, anticoagulant, antithrombotic, thrombolytic, and anesthetic, uterine contraction, and cytotoxicity activities (Table 2).
3.4.1 Antimicrobial activitiesaaaaa
Adenia species exhibit notable antimicrobial properties, supported by multiple studies. A. gummifera demonstrates broad-spectrum antibacterial activity against Gram-negative pathogens like Escherichia coli and Klebsiella pneumoniae using acetone extracts (5.0 mg/mL) (Rotich, 2022), while its ethanol and aqueous root bark extracts show strong antifungal effects against Candida albicans (37.0 mm inhibition zones) (Maroyi, 2020; Rotich, 2022). These activities are linked to flavonoids, alkaloids, and ribosome-inactivating proteins (RIPs) that disrupt microbial membranes, DNA synthesis, and protein synthesis (Tosi et al., 2009; Battelli et al., 2010). Although A. gummifera exhibits moderate antiplasmodial activity against chloroquine-sensitive Plasmodium falciparum (IC50 = 50 μg/mL), it lacks efficacy against resistant strains (Kraft et al., 2003). In contrast, A. lobata’s hydroethanolic leaf extract clears Salmonella typhi infections in rats within 8–12 days (Fowa et al., 2019) and inhibits TEM-1 β-lactamase by 90%, suggesting potential to counter antibiotic resistance (Gangoué-Piéboji et al., 2007). Its ethyl acetate stem bark fraction and a purified compound (20) (IC50 = 6.03 μg/mL) show potent antileishmanial activity against Leishmania donovani, outperforming less effective methanol extracts (Garba Koffi et al., 2021; Okpekon et al., 2004). Structure-activity relationships highlight hydroxyl/acetyl groups and nitrogen-containing alkaloids as key drivers of efficacy (Gangoué-Piéboji et al., 2007; Garba Koffi et al., 2021). In addition, A. trilobata stands out for its gastrointestinal benefits: methanol leaf extracts (200–400 mg/kg) significantly reduced diarrhea episodes and intestinal motility in mice, with the 400 mg/kg dose outperforming the standard drug loperamide in motility inhibition—a promising finding for natural antidiarrheal agents (Barua et al., 2020). Meanwhile, A. cissampeloides shows dual antimicrobial potential. Its petroleum ether extract effectively combats fish pathogens like Aeromonas veronii (8.2 mm inhibition zones), while methanol extracts target Raoutella ornithinolytica (7 mm zones), offering potential solutions for aquaculture-related bacterial infections (Uzoeto et al., 2018). Notably, the same species exhibits moderate antiplasmodial activity against drug-resistant P. falciparum, achieving 73.95% parasite growth inhibition at low concentrations (1.562 μg/mL), though its IC50 (8.521 μg/mL) remains far higher than artesunate’s 0.03 μg/mL benchmark (Annan et al., 2012). Together, these findings position Adenia as a multi-target medicinal resource—from gut health to malaria management—with efficacy often rivalling synthetic drugs, though potency gaps for specific applications warrant further research into compound optimization. They underscore Adenia’s potential as a source of novel antimicrobial agents, particularly for drug-resistant infections.
3.4.2 Antioxidant, anti-inflammatory, and nutritional properties
Adenia species offer a compelling array of antioxidant benefits, as evidenced by extensive research. A. lobata stands out, with studies detailing its ability to modulate oxidative stress in vivo. For example, a hydroethanolic leaf extract administered to S. typhi-infected rats significantly reduced malondialdehyde levels while boosting crucial antioxidant enzymes like superoxide dismutase, glutathione, catalase, and nitric oxide (Fowa et al., 2019). In vitro analyses of A. lobata stem extracts revealed that ethanol extracts outperform petroleum ether in multiple antioxidant assays, displaying a total phenolic content of 0.623 mg/g, a total antioxidant capacity of 0.687 mg/g, and a reducing power of 0.645 mg/g (Sarkodie et al., 2013). The ethanol extract also exhibited lower IC50 values in DPPH scavenging (0.5210 mg/mL) and lipid autoxidation inhibition (0.5985 mg/mL) assays compared to the petroleum ether extract (Sarkodie et al., 2013). Furthermore, an ethyl acetate fraction from the cordate leaf variety of A. lobata showed exceptional antioxidant activity (63.87%), suggesting its potential in cancer therapy (Agoreyo et al., 2012). A. lobata’s antioxidant potential extends to neuroprotection, reducing pain and anxiety in mice, effects linked to decreased levels of nitric oxide and malondialdehyde and increased levels of non-protein thiols (N’Go et al., 2021). Other Adenia species also demonstrate impressive antioxidant capabilities. A. gummifera stem methanol extracts exhibited a reducing ability of 159.12 mm Fe (II)/g and acted as potent ABTS radical scavengers, with activity comparable to the synthetic antioxidant BHT (94.2% inhibition for A. gummifera vs. 96.8% for BHT at 0.08 mg/mL) (Adedapo et al., 2008). A. trilobata n-hexane leaf fractions displayed the highest scavenging activity (89.19% at 500 μg/mL, IC50 = 139.65 μg/mL) (Barua et al., 2020), while A. panduriformis leaf methanol extracts even surpassed ascorbic acid in DPPH free radical quenching (IC50 = 53.48 μg/mL vs. 74.47 μg/mL) (Chibuye et al., 2024). In A. viridiflora, older leaves exhibited higher DPPH scavenging (0.96–1.44 μmol TE/100 g DW) and FRAP activities (27.08–42.90 μmol TE/g DW) compared to younger shoots (Wannasaksri et al., 2021b), suggesting an age-related accumulation of protective compounds. Finally, a compound isolated from A. glauca demonstrated notable free radical scavenging, with an IC50 value of 51.56 μg/mL in the DPPH assay (Immaculate et al., 2022). These antioxidant activities are primarily attributed to flavonoids and phenolic compounds, which neutralize free radicals and boost antioxidant enzymes like SOD and glutathione peroxidase (Sarkodie et al., 2013). Variations in activity among extracts from different species such as A. trilobata, A. gummifera, A. panduriformis, and A. viridiflora are linked to the specific antioxidants extracted by different solvents (n-hexane vs. methanol) and the accumulation of compounds like flavonoids and tannins as leaves mature (Adedapo et al., 2008; Barua et al., 2020; Chibuye et al., 2024; Wannasaksri et al., 2021b). These antioxidant properties contribute to antinociceptive and anti-anxiety effects by mitigating oxidative stress and inflammation in neuronal tissues, as demonstrated in studies with A. lobata (N’Go et al., 2021).These collective findings underscore the significant potential of Adenia species as a source of natural antioxidants for various therapeutic applications, offering a promising avenue for further investigation and development.
Furthermore, the nutritional compositions of old leaves and young shoots from A. viridiflora were studied using samples collected from four regions of Thailand during various periods (March-April, May-June, and July-August). The young shoots provided 371.45–387.69 kcal of energy and contained 18.15–21.94 g of protein, while old leaves offered 373.22–386.97 kcal and 16.53–22.68 g of protein, generally showing higher protein levels in young shoots, particularly during May-June. In terms of fat content, young shoots ranged from 0.62 to 4.10 g, compared to 1.66–3.28 g in old leaves. Both parts had similar carbohydrate contents (67.21–74.57 g in young shoots and 67.12–73.53 g in old leaves), but young shoots contained significantly more total dietary fiber (32.89–65.26 g) than old leaves (28.37–51.46 g), especially in samples from July-August. Young shoots had total sugar levels of 8.45–16.16 g, while old leaves contained 11.83–18.22 g, with old leaves generally exhibiting higher sugar content. Vitamin C levels were notable, with young shoots ranging from 573.93 to 1696.46 mg and old leaves from 862.64 to 2240.65 mg, the latter providing 1.1–1.9 times more. Lutein was the predominant carotenoid in both parts, with old leaves having significantly higher levels of both β-carotene (1.7–3.6 times) and lutein (1.4–2.6 times). In terms of minerals, potassium was most abundant, with young shoots containing 1740.09–2549.33 mg and old leaves 1357.16–2144.11 mg, while old leaves showed slightly higher iron content (4.90–8.57 mg versus 4.27–7.08 mg in young shoots). Overall, young shoots offered higher energy, protein, and fat, while old leaves provided greater antioxidant activities, phenolic content, and specific vitamins and minerals, highlighting the unique nutritional benefits of both plant parts (Wannasaksri et al., 2021b). A. viridiflora offers nutritional benefits, with young shoots rich in protein and fiber and old leaves packed with vitamin C and carotenoids like lutein and β-carotene for antioxidant protection. These nutritional differences arise from the plant’s growth stages, with young shoots focusing on building new tissues and older leaves accumulating protective compounds against environmental stressors (Wannasaksri et al., 2021b). The presence of these vitamins, carotenoids, and essential minerals contributes to overall human health by supporting immune function, eye health, and cellular balance (Wannasaksri et al., 2021b).
3.4.3 Analgesic, anticholinesterase, neuropharmacological, and antidepressant activities
Adenia species showcase significant neuropharmacological potential, offering hope for novel treatments in pain management, anxiety, and depression. A. trilobata extracts have demonstrated analgesic effects, with a methanol leaf extract reducing writhing by 57.85% in acetic acid-induced writhing tests and significantly inhibiting formalin-induced licking in mice (Barua et al., 2020). These effects are potentially mediated by flavonoids and terpenoids, which are known for their anti-inflammatory and pain-relieving properties. In addition to pain relief, A. trilobata exhibits anxiolytic and antidepressant-like activities. Studies using elevated plus mazes, hole-board tests, forced swimming tests, and tail suspension tests in mice revealed that different fractions of A. trilobata extracts could alter behavior, suggesting a modulation of neurotransmitter systems (Aziz et al., 2020). Specifically, open arm entries in the elevated plus maze increased by up to 41.84% with certain extracts, indicating a reduction in anxiety-like behavior (Aziz et al., 2020). In addition, A. cissampeloides has demonstrated promising antidepressant and anxiolytic effects. A hydroethanolic extract of A. cissampeloides leaves increased swimming activity by 75.20% and climbing activity by 190.00% in the forced swimming test, comparable to the effects of imipramine, a standard antidepressant (Ishola et al., 2015). The extract also showed anxiolytic effects by increasing head dips by 41.67% in the hole-board test and enhancing open-arm entries in the elevated plus maze (Ishola et al., 2015). These effects appear to be linked to the modulation of dopaminergic, adrenergic, and serotonergic pathways, as the antidepressant effects were blocked by sulpiride (a dopamine D2 receptor antagonist) and yohimbine (an α2-adrenoceptor antagonist), while the anxiolytic effects were reversed by cyproheptadine (a 5-HT2 receptor antagonist) (Ishola et al., 2015). Finally, A. gummifera has shown anticholinesterase activity, with an IC50 value of 0.02 mg/mL, suggesting potential benefits for cognitive function (Adewusi and Steenkamp, 2011). Additionally, steam distillates from A. gummifera exhibited anesthetic effects on bees (Apis mellifera), likely due to volatile terpenoids interacting with neuronal receptors (Ngarivhume et al., 2008). These diverse neuropharmacological effects suggest that Adenia species hold considerable promise for the development of new treatments for pain, anxiety, depression, and cognitive disorders, and warrant further investigation to isolate and characterize the active compounds responsible for these effects.
3.4.4 Antihyperglycaemic and anti-anemic activities
Adenia species showcase potential in managing diabetes and anemia and demonstrate diverse antioxidant activity. A. lobata stem extracts significantly reduced blood glucose levels in diabetic rats, with the 70% ethanol extract showing up to 82.8% reduction, likely due to flavonoids and phenolics that enhance insulin sensitivity (Sarkodie et al., 2013). Flavonoids’ structure allows them to donate electrons, reducing oxidative stress and promoting iron bioavailability for red blood cell production. Also, A. lobata leaf extracts normalized hematological parameters in anemic rats, with the aqueous extract showing a superior recovery rate of 79.66% at 250 mg/kg and increased hemoglobin levels (Florian et al., 2022). Beyond these effects, A. lobata demonstrates significant antioxidant properties, reducing malondialdehyde while increasing antioxidant enzyme levels in rats (Fowa et al., 2019) and showing potent DPPH scavenging activity, with the ethyl acetate fraction of the cordate leaf variety exhibiting 63.87% activity (Agoreyo et al., 2012). In addition, A. gummifera demonstrated comparable ABTS radical scavenging activity to BHT (Adedapo et al., 2008), A. trilobata n-hexane leaf fraction displayed 89.19% scavenging activity (Barua et al., 2020), A. panduriformis surpassed ascorbic acid in DPPH quenching (Chibuye et al., 2024), and A. glauca compound showed an IC50 of 51.56 μg/mL (Immaculate et al., 2022), reinforcing Adenia’s potential as a source of therapeutic agents.
3.4.5 Anticoagulant, antithrombotic, and thrombolytic activities
Adenia species exhibit promising anticoagulant and antithrombotic properties with insights from a mix of compounds that promote these effects. An A. cissampeloides and Pseudocedrela kotschyi mixture prolonged aPTT and bleeding time in rats, which are both indicators of anticoagulant activity, with no observed adverse effects below 2000 mg/kg (Nyansah et al., 2016). The same extract also reduced tail infarction and microthrombi in a carrageenan-induced thrombosis model (Nyansah et al., 2021). A. trilobata methanol stem extract showed thrombolytic activity, lysing clots by 25.58%, though less effectively than streptokinase, which is a known pharmaceutical agent used to treat blood clots (Barua et al., 2020). Together, the data suggest compounds present in Adenia interact with coagulation factors which ultimately disrupt normal blood coagulation processes leading to an increase in bleeding time and reduced clot formation. Furthermore, this body of research highlights several Adenia species are demonstrating diverse neuropharmacological activities, particularly in pain management, anxiety, and depression. This is important as it suggests that compounds isolated from the Adenia genus may have a therapeutic effect across multiple targets. For example, A. trilobata showed analgesic effects, reducing writhing by up to 57.85% and significantly inhibiting formalin-induced licking, effects that are attributed to flavonoids and terpenoids (Barua et al., 2020). This same extract had demonstrated anxiolytic and antidepressant-like activities, as evidenced by increased open arm entries in animal models of elevated plus maze experiments (up to 41.84%) and altered behavior in forced swimming and tail suspension tests, suggesting neurotransmitter modulation (Aziz et al., 2020). Similarly, A. cissampeloides has displayed antidepressant-like activity, increasing swimming activity and climbing activity and anxiolytic effects, increasing head dips and open-arm entries, an effect that is linked to dopaminergic, adrenergic, and serotonergic pathways. Finally, A. gummifera displayed anticholinesterase activity and anesthetic effects on bees, a reaction that can be attributed to alkaloids and volatile terpenoids (Adewusi and Steenkamp, 2011; Ngarivhume et al., 2008). The culmination of these results highlights an expansive potential of Adenia species to effectively manage pain, anxiety, and depression, which has created a basis for further investigation into these applications.
3.4.6 Anesthetic and uterine contraction properties
The anesthetic properties of steam distillates from the stem of A. gummifera were assessed using a diffusion method on A. mellifera. Live, direct, and fractional distillates (61°C–80°C) exhibited stronger anesthetic effects, while vacuum distillates were milder. The aqueous stem extract also showed dose-dependent anesthetic effect on African bees, with effectiveness increasing up to a 6.0% concentration, beyond which it plateaued, and excessive exposure proved lethal. Factors such as bee-distillate distance (F2,6 = 5.14; Fcal = 31.0) and container volume (F3,8 = 4.07; Fcal = 66.4) significantly influenced anesthetic activity, indicating that the rate of diffusion of the active components was critical. These components likely include amines and halogenated alkanes. Additionally, the leaf extracts of A. gummifera contained gummiferol, a polyacetylene known to be toxic to certain human cancer cell lines (Ngarivhume et al., 2008). The cytotoxicity activity of gummiferol may contribute to the anesthetic effect by disrupting cellular functions in nerve tissues. The SAR of this compound involves its ability to bind to and modulate the activity of neuronal receptors, leading to the observed anesthetic effects. In 2015, Sinei and Mwangi investigated the effects of the aqueous extract of the A. globosa tuber on isolated rat uterus preparations and its interaction with prostaglandin F2α and ergometrine, both known uterine stimulants. The study found that the plant extract induced a dose-dependent contraction of the rat uterus. Notably, small doses of prostaglandin F2α (2.5 μg/mL) and ergometrine (0.125 μg/mL) significantly enhanced the contractile effects of the extract, with the presence of prostaglandin F2α increasing the effect (p < 0.02 and p < 0.01). Alone, this dose of prostaglandin F2α resulted in approximately 15% contraction, while ergometrine resulted in approximately 5% contraction on its own (p < 0.05, p < 0.02, and p < 0.01). These findings indicate a synergistic relationship between the extract and these uterine stimulants (Sinei and Mwangi, 2015). The uterine contraction properties of A. globosa are likely mediated by the presence of oxytocic compounds, which can stimulate uterine smooth muscle contraction. The synergistic relationship with prostaglandin F2α and ergometrine suggests that the extract may enhance the sensitivity of uterine receptors to these stimulants or act through a similar mechanism (Sinei et al., 1994).
3.4.7 Cytotoxicity activities
The genus Adenia has garnered attention in pharmacological research due to its bioactive compounds with significant cytotoxic properties. These include ribosome-inactivating proteins (RIPs), which exhibit promising activities against cancer cells and neurological targets. These properties could be attributed to their strong cell binding, resistance to proteolysis, and efficient endocytosis. These properties enable their retrograde transport along the central nervous system and peripheral nerves, suggesting potential applications in neurophysiology and as components of immunotoxins for targeted cancer therapy and other treatments (Bortolotti et al., 2021). The cytotoxic activity of RIPs is primarily due to their ability to inhibit protein synthesis by depurinating a specific adenine residue in ribosomal RNA, thereby disrupting ribosome function. The structure-activity relationship (SAR) of RIPs is characterized by two key domains: an N-glycosidase domain responsible for the enzymatic activity and a lectin domain that facilitates binding to cell surface glycoproteins or glycolipids, enabling cellular entry (Bortolotti et al., 2021). The specific amino acid sequence and glycosylation patterns of these domains influence their binding affinity and enzymatic activity, ultimately determining their cytotoxicity. For instance, Monti et al. (2007) examined the impacts of lanceolin and stenodactylin, two type 2 RIPs from A. lanceolata and A. stenodactyla respectively, on neural cells and their neurotoxicity in vivo. The concentrations inhibiting 50% protein synthesis were measured at 10−11 M and 10−12 M for cerebellar granule neurons, 10−12 M and 10−13 M for astrocytes, and 10−13 M for microglia. Both RIPs were toxic to glial cells, with microglia being the most sensitive, showing 50% cell death at around 10−14 M. Stenodactylin demonstrated high neurotoxicity when injected intracerebrally and leading to the loss of cholinergic neurons in the medial septal nucleus following stereotaxic injection of 1.3 ng. The retrograde transport of these RIPs along neurons enables targeted lesioning experiments and offers potential for immunolesioning specific neuronal populations (Monti et al., 2007). The differential cytotoxicity of lanceolin and stenodactylin towards different neural cell types highlights the importance of specific cell surface receptors and endocytic pathways in mediating RIP entry and toxicity. In another study, Stirpe et al. (2007) demonstrated that both RIPs demonstrated the ability to agglutinate red blood cells, inhibit protein synthesis in cell-free systems and whole cells, and depurinate ribosomes and DNA, while not affecting tRNA or poly(A). They were found to be highly toxic, inducing apoptosis in cells and exhibiting LD50 values of 8.16 mg/kg for lanceolin and 2.76 mg/kg for stenodactylin in mice after 48 h (Stirpe et al., 2007). The ability of lanceolin and stenodactylin to agglutinate red blood cells and inhibit protein synthesis underscores their potent cytotoxic activity. The lower LD50 value of stenodactylin compared to lanceolin indicates that it is more toxic, possibly due to differences in their amino acid sequences, glycosylation patterns, or cellular trafficking. In addition, stenodactylin have been proven to be a promising candidate for anticancer therapy due to its ability to induce apoptosis and necroptosis in treated cells through reactive oxygen species production. In studies involving Raji and Ramos (human Burkitt’s lymphoma) and MOLM-13 (acute myeloid leukemia) cells, stenodactylin demonstrated significant effects on protein synthesis and cell viability. After 48 h of exposure to 10−9 M stenodactylin, protein synthesis was nearly completely inhibited across all cell lines, although MOLM-13 cells were the most sensitive, exhibiting an IC50 of 3.75 × 10−12 M, compared to 1.95 × 10−11 M for Raji and 3.49 × 10−11 M for Ramos. Cell viability assays indicated that all cell lines showed similar sensitivity to stenodactylin, with EC50 values of 2.09 × 10−10 M (Raji), 3.43 × 10−10 M (Ramos), and 1.06 × 10−10 M (MOLM-13). Time-course studies revealed a dose-dependent reduction in MOLM-13 cell viability, with significant effects observed after 12 h and complete loss by 24 h (Mercatelli et al., 2020). Moreover, stenodactylin-induced rapid activation of apoptotic pathways was confirmed through flow cytometry and microscopy, with an IC50 of 4 × 10−14 M and an EC50 of 2.5 × 10−14 M noted after 48 h (Polito et al., 2016). The induction of apoptosis and necroptosis by stenodactylin in cancer cells, coupled with its ability to generate reactive oxygen species (ROS), highlights its potential as an anticancer agent. The variations in IC50 and EC50 values across different cell lines suggest that stenodactylin’s cytotoxicity is influenced by cellular factors such as the expression of specific receptors or the activity of antioxidant defense mechanisms. The rapid activation of apoptotic pathways further supports its potent cytotoxic activity. Furthermore, in 2010, Battelli et al. evaluated the concentration-response curves for protein synthesis and cell viability after 24 h of exposure to RIPs lanceolin, volkensin, and stenodactylin. They found that lanceolin and volkensin exhibited similar cytotoxicities, while stenodactylin was two orders of magnitude more cytotoxic. The IC50 values for each RIP closely coincided with the LC50 values, although a low percentage of cell viability remained even after complete inhibition of protein synthesis, which occurred at 10−12 M for stenodactylin and 10−10 M for lanceolin and volkensin. Viability tests indicated that 20% of metabolizing cells persisted after 24 h of treatment at these concentrations. Complete cell death (100%) was observed only after 72 h for both lanceolin and stenodactylin. HeLa cell morphology was assessed by optical microscopy, revealing significant alterations after exposure to 10−12 M lanceolin, stenodactylin, or volkensin compared to control cultures (Battelli et al., 2010). The higher cytotoxicity of stenodactylin compared to lanceolin and volkensin reinforces the notion that subtle structural differences among RIPs can significantly impact their biological activity. The persistence of metabolizing cells even after complete inhibition of protein synthesis suggests that cells may activate alternative survival mechanisms, underscoring the complexity of cellular responses to RIP-induced stress. Volkensin, purified from the roots of A. volkensii, inhibits protein synthesis in rabbit reticulocyte lysates and HeLa cells. It is highly toxic to mice, with an LD50 of 1.38 μg/kg body weight (Barbieri et al., 1984). The potent toxicity of volkensin, as evidenced by its low LD50 value, underscores its potential as a cytotoxic agent. Its ability to inhibit protein synthesis in both cell-free systems and whole cells highlights its broad-spectrum activity. Whereas Gummiferol, a type 2 RIP isolated from the leaves of A. gummifera, exhibits significant cytotoxic activity against the human epidermoid carcinoma (KB) cell line. The organic portion of a 20% MeOH/CHCl3/H2O partition from a 50% MeOH/CHCl3 extract of the leaves demonstrated an ED50 value of 1.1 μg/mL in the kB cell line assay (Fullas et al., 1995; Adedapo et al., 2008). The cytotoxic activity of gummiferol against the kB cell line suggests that it may have potential as an anticancer agent. Its isolation from the organic portion of the extract indicates that it is a relatively non-polar compound, consistent with the properties of many RIPs.
In addition, the cytotoxic effects and the pathways of cell death induced by heterophyllin, a type 2 RIP isolated from A. heterophylla, were assessed in three human-derived cell lines: MCF7, NB100, and T24, and compared to ricin (a well-studied type 2 RIP). Heterophyllin was found to entirely eliminate cell viability at nanomolar concentrations, inducing strong apoptosis without necrosis, alongside oxidative stress and necroptosis. Cell viability was assessed after 72 h of incubation using concentration-response experiments. Ricin significantly reduced cell viability starting at 10−13 M, whereas heterophyllin began to show significant effects at 10−12 M (T24 and MCF7) and 10−11 M (NB100). The EC50 for heterophyllin was approximately 10−11 M across all cell lines. Complete cell killing occurred at 10−9 M for T24 and NB100 cells and 10−10 M for MCF7 cells. In contrast, ricin demonstrated higher toxicity with an EC50 of 10−13 M and complete cell killing at 10−11 M. Cytofluorimetric analysis using Annexin V-EGFP/Propidium iodide (PI) staining revealed that after 24 h of exposure to 10−9 M heterophyllin, over 95% of treated cells were in early or late apoptosis, with minimal necrosis observed. The cytotoxicity of heterophyllin (10−9 M) and ricin (10−11 M) was evaluated after 24 h, showing that both scavengers and cell death inhibitors significantly increased cell survival (p < 0.0001) in all tested cell lines. In NB100 cells, pre-treatment with scavengers/inhibitors offered greater protection against heterophyllin than ricin. Conversely, in T24 and MCF7 cells, pre-treatment was more effective for ricin than for heterophyllin, particularly in MCF7 cells (Bortolotti et al., 2024). The distinct effects of heterophyllin and ricin on cell viability and the varying degrees of protection conferred by scavengers and cell death inhibitors across different cell lines highlight the complexity of RIP-induced cytotoxicity. The induction of apoptosis without necrosis by heterophyllin suggests a specific mechanism of action distinct from ricin, possibly involving different intracellular targets or signaling pathways. The higher sensitivity of NB100 cells to heterophyllin compared to ricin may be related to differences in cell surface receptors or endocytic pathways, while the opposite trend in T24 and MCF7 cells suggests that these cell lines are more susceptible to ricin’s mechanism of action.
Furthermore, the effects of crude extracts from A. racemosa, A. fruticosa, A. venenata, A. keramanthus, A. glauca, and A. lanceolata on protein synthesis were assessed using rabbit reticulocyte lysates, HeLa, and Raji cell lines. Hemagglutinating activity was also assessed with normal human erythrocytes (group O, Rh+). The results revealed that, the extracts from A. racemosa, A. fruticosa, and A. venenata exhibited low inhibitory activity, with IC50 values ranging from 1.5 to 36 mg/mL. While, extracts from A. goetzii, A. lanceolata, and A. stenodactyla were highly potent, with IC50 values below 1 ng/mL to less than 10 ng/mL. Most extracts, except those from A. keramanthus, A. glauca, and A. lanceolata, demonstrated hemagglutinating activity, particularly strong in A. goetzii, A. ellenbeckii, and A. racemosa. Agglutination was inhibited by 0.2 M galactose in all cases (Pelosi et al., 2005). The varying potencies of crude extracts from different Adenia species in inhibiting protein synthesis and inducing hemagglutination reflect differences in their RIP content and lectin profiles. The high inhibitory activity of extracts from A. goetzii, A. lanceolata, and A. stenodactyla suggests that these species are rich in potent RIPs. The hemagglutinating activity of most extracts, particularly those from A. goetzii, A. ellenbeckii, and A. racemosa, indicates the presence of lectins capable of binding to erythrocytes. The inhibition of agglutination by galactose suggests that these lectins recognize galactose-containing glycans on the erythrocyte surface. AHL, a single lectin B chain isolated from A. hondala roots, is consider as a human blood group non-specific lectin that agglutinates rabbit erythrocytes. Glycan array analysis revealed that AHL has the greatest affinity for terminal lactosamine or polylactosamine of N-glycans, which are overexpressed in colon cancer and hepatocellular carcinoma. AHL displayed strong binding to human hepatocellular carcinoma HepG2 cells, with a mean fluorescence intensity (MFI) of 59.1, which was effectively blocked by 93.1% by asialofetuin. It also exhibited dose- and time-dependent growth inhibitory effects on HepG2 cells, with an IC50 of 4.8 μg/mL at 72 h. Specifically, AHL (at 10 μg/mL) inhibited HepG2 cell growth by 43% (n = 6, P < 0.01) at 48 h and 76% (n = 6, P < 0.001) at 72 h (Sharma et al., 2018). Additionally, in 2021 Inamdar et al. assessed the interaction of AHL with human colon cancer epithelial HT-29 cells and colon cancer tissues. For that, the cell viability was assessed using the MTT assay, while cell surface binding, apoptosis, and ROS production were analysed by flow cytometry with the Annexin-V-PI and DCFDA kits. Immunohistochemistry utilised biotinylated AHL, and protein purification was conducted via affinity chromatography with asialofetuin-coupled Sepharose-4B. Their results showed that, AHL demonstrated strong binding to HT-29 cells, with a Mean Fluorescence Intensity of 12.4, which was inhibited by asialofetuin. It reduced HT-29 cell growth in a dose- and time-dependent manner, yielding an IC50 of 2.5 μg/mL, and showed differential binding to normal and cancerous human tissues. AHL induced apoptosis, with early apoptotic populations increasing to 25.1% and 36% at 24 h and 48 h, respectively, and necrotic populations at 1.5% and 4.6%. Moreover, AHL triggered the release of Reactive Oxygen Species in a dose-dependent manner (Inamdar et al., 2021). The preferential binding of AHL to lactosamine or polylactosamine of N-glycans, which are overexpressed in colon cancer and hepatocellular carcinoma, suggests that it may have potential as a targeted therapeutic agent for these cancers. The dose- and time-dependent growth inhibitory effects of AHL on HepG2 and HT-29 cells, coupled with its ability to induce apoptosis and ROS production, further support its potential as an anticancer agent. The differential binding of AHL to normal and cancerous human tissues suggests that it can selectively target cancer cells while sparing normal cells, reducing the risk of toxicity.
3.5 Toxicity and safety
The toxicity of A. cissampeloides has been extensively studied, particularly regarding its effects on catfish larvae and juveniles. The methanol extract of the stem was tested under static bioassay conditions for 96 h, revealing various pathological changes in fish larvae, including erratic swimming, moribund behavior, depigmentation, and mortality at concentrations of 25, 50, 75, and 100 mg/L. The lethal concentration for 50% mortality (LC50) was determined to be 50 mg/L within 72 h, indicating high toxicity to catfish juveniles (Agbor and Okoi, 2014). In a separate study, Ibiam et al. (2015) evaluated the acute toxicity of stem-bark aqueous extract of A. cissampeloides in Clarias batrachus juveniles. The study involved 144 fish randomized into groups exposed to varying concentrations (0.0, 1.25, 2.50, 5.0, 10.0, and 20.0 g/L) for 72 h. The calculated LC50 values were 5.0 g/L at 24 h and 2.5 g/L at both 48 and 72 h, demonstrating a time and concentration-dependent toxic effect. Significant (P > 0.05) increases in aspartate aminotransferase (AST) activity and other biochemical parameters were noted, highlighting the acute toxicity of the extract (Ibiam et al., 2015). Further research by Ekpo et al. (2017) assessed the acute toxicity of the leaf 70% ethanol extract of A. cissampeloides on early developmental stages of farmed African catfish, with 160 fingerlings exposed to concentrations of 25, 50, and 100 mg/L for 24–96 h. The study found a significant increase in mortality that was dependent on the dose, with an LC50 of 42.38 mg/L for 96 h, indicating acute toxic effects on the fish (Ekpo et al., 2017). Another study involving 370 catfish (Clarias gariepinus) examined the effects of varying concentrations (0, 25, 50, 75, 100 mg/L) of the leaf extract of A. cissampeloides over different durations (24, 48, 72, and 96 h). Results showed that both concentration and exposure duration significantly (p < 0.05) affected mortality rates, with the highest mortality observed at the highest concentration and longest exposure time (Uno et al., 2018). The toxicity of A. cissampeloides in fish is likely due to the presence of toxic compounds such as saponins, alkaloids, and cyanogenic glycosides. Saponins can disrupt cell membranes, leading to hemolysis and tissue damage, while alkaloids can interfere with neuronal function and cause paralysis. Cyanogenic glycosides release cyanide upon hydrolysis, inhibiting cellular respiration and causing asphyxiation. The SAR of these compounds involves their ability to interact with cellular targets, disrupting normal physiological processes and leading to toxicity. The concentration- and time-dependent effects observed in these studies underscore the importance of dose and duration of exposure in determining the severity of toxicity. In contrast, a study evaluated the therapeutic and potential toxic effects of an aqueous extract of A. cissampeloides in seven female hypertensive subjects undergoing treatment and seven matched controls who had not yet started the treatment. Diastolic and systolic blood pressures were measured, and serum levels of various enzymes, including creatine kinase (CK), α-hydroxybutyrate dehydrogenase (HBDH), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST), were analysed to assess the extract’s effectiveness as an antihypertensive agent. The results showed that systolic blood pressure was significantly reduced in subjects taking A. cissampeloides compared to controls (p < 0.01). Although CK and HBDH levels were reduced in the treatment group, these differences did not reach statistical significance. Additionally, conjugated and total bilirubin levels were significantly reduced in the subjects on A. cissampeloides (p < 0.05). The findings suggest that the plant preparation does not exhibit hepatotoxic or nephrotoxic effects (Nyarko and Addy, 1990). The lack of hepatotoxic or nephrotoxic effects observed in hypertensive subjects treated with A. cissampeloides aqueous extract suggests that the toxic compounds present in the plant may be poorly absorbed or rapidly metabolized, or that the concentration of these compounds in the aqueous extract is below the threshold for toxicity. The reduction in systolic blood pressure may be due to the presence of compounds that act as vasodilators or ACE inhibitors. However, it is important to note that this study involved a small sample size and further research is needed to confirm these findings and to assess the long-term safety of A. cissampeloides in humans. In 2020, Barua et al. investigated the lethality of the A. trilobata stems and leaves methanol extracts, as well as their n-hexane fractions, using the brine shrimp (Artemia salina) bioassay. They found that none of the extracts demonstrated toxicity when compared to vincristine sulfate (2.16 μg/mL), the positive control. The results indicated that the methanol stem extract had a moderately toxic effect, with an LC50 of 328 μg/mL, while the n-hexane leaf fraction exhibited weak toxicity, with an LC50 of 616.85 μg/mL (Barua et al., 2020). The moderate toxicity of the methanol stem extract and the weak toxicity of the n-hexane leaf fraction from A. trilobata in the brine shrimp bioassay suggest that these extracts contain relatively low concentrations of toxic compounds. The differences in toxicity between the methanol and n-hexane extracts may be due to the presence of different classes of compounds with varying degrees of toxicity.
Volkensin, a type 2 RIP derived from the roots of A. volkensii, is a highly toxic protein with a LD50 for rats reported to be between 50 and 60 ng/kg. It is closely related to other well-known toxins such as abrin and ricin, which have been recognized since the late 20th century (Stirpe et al., 1985; Chambery et al., 2004; Chambery et al., 2007; Severino et al., 2009). In experimental settings, the toxicity of volkensin has been evaluated in male Swiss mice, where it was administered intraperitoneally at varying doses (0.1–10 µg per 100 g body weight) dissolved in saline. This method of administration allows for the assessment of its lethal effects in a controlled environment (Barbieri et al., 1984). In addition, modeccin, another toxin purified from the roots of A. digitata, has been studied for its effects on protein synthesis and toxicity in animal models. Two isoforms, modeccin 4B and modeccin 6B, were isolated, with modeccin 6B exhibiting slightly less toxicity than modeccin 4B. Notably, modeccin 6B does not agglutinate erythrocytes and is a more effective inhibitor of protein synthesis in rabbit reticulocyte lysates, achieving 50% inhibition at a concentration of 0.31 μg/mL. While the LD50 of modeccin 6B was not explicitly determined, experiments showed that all rats administered 1 µg of the toxin per 100 g body weight died, whereas those receiving 0.1 µg survived, indicating its intermediate toxicity compared to modeccin 4B and its reduced form treated with 2-mercaptoethanol (Barbieri et al., 1980). Another study demonstrated that modeccin inhibits protein synthesis both in vitro in rabbit reticulocyte lysates and Ehrlich ascites cells, with the inhibitory effect diminished in the presence of lactose. Interestingly, dissociating modeccin into subunits reduces its toxicity in vivo, as seen in rats that survived doses of 5 µg of dissociated modeccin per 100 g body weight. Furthermore, studies revealed that modeccin is generally more toxic to rats than to mice, causing pathological changes somewhat similar to those observed in ricin poisoning (Gasperi-campani et al., 1978). Furthermore, studies have demonstrated that stenodactylin, a type 2 RIP derived from the A. stenodactyla caudex, exhibits high toxicity in mice, resulting in 100% mortality among those injected with 1.21 μg/kg within 7 days (Tosi et al., 2009). The high toxicity of volkensin, modeccin, and stenodactylin is due to their ability to inhibit protein synthesis, a critical cellular function.
Importantly, a case was reported involving four siblings who unintentionally ingested the A. ellenbeckii fruits in the rural Togdheer region of northern Somaliland. Following consumption, they experienced severe illness, including sweating, fever, frequent vomiting, and itching. One sibling progressed to acute kidney injury, resulting in a fatal outcome (Rage et al., 2024). In South Africa, the genus has garnered attention primarily due to poisoning incidents associated with A. digitata. In 1922, a poisoning event resulted in the death of an adult who mistakenly chewed the tubers of A. digitata, believing them to be from a cucurbitaceous plant. Previous reports indicated cases of poisoning among children, including a death attributed to this species in 1928. Investigations at the Veterinary Research Laboratory in Onderstepoort identified two toxic principles: hydrocyanic acid and modeccin. Hydrocyanic acid was found in the fresh leaves of both A. digitata and A. glauca, although the latter’s root was deemed edible. Additionally, observations were made of children consuming the fruits of A. glauca, which they described as pleasant. Confusion arises regarding the consumption of the sap from the “tuber” of A. midtiflora, likely due to its overlap with the non-toxic and edible A. glauca. Furthermore, A. venenata and A. palmata are recognized as having poisonous tubers (Liebenberg, 1935). These case reports and historical accounts of poisoning incidents highlight the potential dangers associated with the ingestion of certain Adenia species. The presence of hydrocyanic acid (cyanide) in A. digitata and A. glauca can lead to cyanide poisoning, which inhibits cellular respiration and causes rapid death. Modeccin, another toxic compound found in A. digitata, inhibits protein synthesis and can cause organ damage. The confusion between toxic and non-toxic species underscores the importance of accurate identification and caution when using Adenia plants for medicinal or food purposes. The symptoms reported in the siblings who ingested A. ellenbeckii fruits, including acute kidney injury, suggest that the toxic compounds present in this species can cause severe organ damage.
4 Conclusion
In conclusion, the genus Adenia represents a significant area of study at the intersection of traditional medicine and modern pharmacology. While many species within this genus are recognized for their ethnobotanical uses, particularly in treating conditions like hypertension and infections, the presence of toxic compounds—especially in species like A. digitata and A. volkensii—raises important safety concerns. Phytochemical analyses have identified various bioactive compounds, such as ribosome-inactivating proteins, alkaloids, flavonoids, and glycosides, which contribute to their therapeutic effects, including anticancer, antimicrobial, antioxidant, and analgesic properties. However, the variability in toxicity among different species and the influence of factors such as geographical location and preparation methods necessitate careful evaluation. Future research should prioritize standardizing methodologies for assessing both the safety and efficacy of Adenia species, alongside comprehensive toxicological studies to elucidate their mechanisms of action. By addressing these safety concerns, the therapeutic potential of the genus Adenia can be more effectively harnessed in modern medical practices.
Author contributions
IM: Conceptualization, Methodology, Writing – original draft. DK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1581659/full#supplementary-material
References
Adedapo, A. A., Jimoh, F. O., Afolayan, A. J., and Masika, P. J. (2008). Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med. 8, 54–57. doi:10.1186/1472-6882-8-54
Adewusi, E. A., and Steenkamp, V. (2011). In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian pac. J. Trop. Med. 4, 829–835. doi:10.1016/S1995-7645(11)60203-4
Agbor, R. P., and Okoi, E. P. (2014). The early life toxicity of Godogbo (Adenia cissampeloides) stem against catfish juveniles. 6, 17–19.
Agoreyo, B. O., Okoro, N. C., and Choudhary, M. I. (2012). Preliminary phytochemical analyses of two varieties of adenia lobata (jacq) and the antioxidant activity of their various solvent fractions. Bayero J. Pure Appl. Sci. 5, 182–186. doi:10.4314/bajopas.v5i1.32
Annan, K., Sarpong, K., Asare, C., Dickson, R., Amponsah, K., Gyan, B., et al. (2012). In vitro anti-plasmodial activity of three herbal remedies for malaria in Ghana: Adenia cissampeloides (Planch.) Harms., Termina liaivorensis A. Chev, and Elaeis guineensis Jacq. Pharmacogn. Res. 4, 225–229. doi:10.4103/0974-8490.102270
Aziz, M. A. I., Tareq, A. M., Sohel, M., Uddin, M., Mahmud, M. H., Hoque, M., et al. (2020). Possible neuropharmacological effects of Adenia trilobata (Roxb.) in the Swiss albino mice model. Futur. J. Pharm. Sci. 6, 2–8. doi:10.1186/s43094-020-00102-5
Barbieri, L., Falasca, A. I., and Stirpe, F. (1984). Volkensin, the toxin of Adenia volkensii (kilyambiti plant). FEBS Lett. 171, 277–279. doi:10.1016/0014-5793(84)80503-7
Barbieri, L., Zamboni, M., Montanaro, L., Sperti, S., and Stirpe, F. (1980). Purification and properties of different forms of modeccin, the toxin of Adenia digitata: separation of subunits with inhibitory and lectin activity. Biochem. J. 185, 203–210. doi:10.1042/bj1850203
Barua, N., Ibn Aziz, M. A., Tareq, A. M., Sayeed, M. A., Alam, N., Alam, N. ul, et al. (2020). In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 23, 100772. doi:10.1016/j.bbrep.2020.100772
Battelli, M. G., Scicchitano, V., Polito, L., Farini, V., Barbieri, L., and Bolognesi, A. (2010). Binding and intracellular routing of the plant-toxic lectins, lanceolin and stenodactylin. Biochim. Biophys. Acta - Gen. Subj. 1800, 1276–1282. doi:10.1016/j.bbagen.2010.09.006
Bertrand Fowa, A., Pierre Chegaing Fodouop, S., Ntungwen Fokunang, C., Gaelle Djoueudam, F., Ndel Famen, L.-C., Sylvie Ongbayokolak, N., et al. (2019). Antityphoid and antioxidant activities of hydroethanolic leaf extract of Adenia lobata Jacq. (Passifloraceae) on Salmonella typhi infected wistar rats. ∼ 13 ∼ J. Med. Plants Stud. 7, 13–22.
Bortolotti, M., Biscotti, F., Zanello, A., Polito, L., and Bolognesi, A. (2024). Heterophyllin: a new adenia toxic lectin with peculiar biological properties. Toxins (Basel) 16, 1–13. doi:10.3390/toxins16010001
Bortolotti, M., Maiello, S., Ferreras, J. M., Iglesias, R., Polito, L., and Bolognesi, A. (2021). Kirkiin: a new toxic type 2 ribosome-inactivating protein from the caudex of Adenia kirkii. Toxins (Basel). 13, 81–18. doi:10.3390/toxins13020081
Chambery, A., Di Maro, A., Monti, M. M., Stirpe, F., and Parente, A. (2004). Volkensin from Adenia volkensii Harms (kilyambiti plant), a type 2 ribosome-inactivating protein: gene cloning, expression and characterization of its A-chain. Eur. J. Biochem. 271, 108–117. doi:10.1046/j.1432-1033.2003.03909.x
Chambery, A., Severino, V., Stirpe, F., and Parente, A. (2007). Cloning and expression of the B chain of volkensin, type 2 ribosome inactivating protein from Adenia volkensii harms: Co-folding with the A chain for heterodimer reconstitution. Protein Expr. Purif. 51, 209–215. doi:10.1016/j.pep.2006.08.004
Chibuye, B., Singh, I. S., Chimuka, L., and Maseka, K. K. (2024). Phytochemical profiling and bioactivity study of Adenia panduriformis in Zambia using UHPLC-MS/MS-MZmine3, GNPS, and METLIN Gen2. Sci. Afr. 24, e02151. doi:10.1016/j.sciaf.2024.e02151
Crouch, N. R., Smith, G. F., Figueiredo, E., and Styles, D. G. A. (2016). Rediscovery of adenia natalensis W.J.De Wilde (passifloraceae) after 150 years. Haselt. 2016-Decem 22, 73–80. doi:10.2985/026.022.0101
De Wilde, W. J. J. O. (1971). A monograph of the genus Adenia FORSK (Passifloraceae). Meded. Landbouwhogesch. Wagening. 71, 1–278. doi:10.5962/p.337637
Ekpo, P. B., Uno, U. U., Okolo, C. M., Agu, R. B., and Onwudike, C. F. (2017). Acute toxicity of adenia cissampeloides in farmed African catfish (Clarias gariepinus). Annu. Res. Rev. Biol. 20, 1–5. doi:10.9734/ARRB/2017/38114
Falcone Ferreyra, M. L., Rius, S. P., and Casati, P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3, 222–315. doi:10.3389/fpls.2012.00222
Florian, Y. B., David, T. J. N., and Marius, D. K. J. (2022). Phytochemical screening, acute toxicity and anti-anaemic activity of aqueous and hydroethanolic extracts of Adenia lobata leaves. J. Pharmacogn. Phytochem. 11, 309–314.
Fowa, A. B., Djoueudam, F. G., Njateng, G. S. S., Tsafack, D. N., Sokoudjou, J. B., Kodjio, N., et al. (2019). “In vitro antisalmonellal activities of leaf extracts of Adenia lobata Jacq,” in Passifloraceae and mechanism of action of the most active extract on Salmonella Typhi ATCC6539. In Vitro 4.
Fullas, F., Brown, D. M., Wani, M. C., Wall, M. E., Chagwedera, T. E., Farnsworth, N. R., et al. (1995). Gummiferol, a cytotoxic polyacetylene from the leaves OF Adenia gummifera. October 58, 1625–1628. doi:10.1021/np50124a027
Gangoué-Piéboji, J., Baurin, S. J. M. F., Ngassam, P., Ngameni, B., Azebaze, A., et al. (2007). Screening of some medicinal plants from Cameroon for β-lactamase inhibitory activity. Phyther. Res. 21, 284–287. doi:10.1002/ptr.2001
Garba Koffi, J., Keumoe, R., Ngansop, C. A. N., Kagho, D. U. K., Tchegnitegni, B. T., Fotsing, Y. S. F., et al. (2021). Constituents of endodesmia calophylloides benth. And adenia lobata (jacq.) engl. With antileihsmanial activities. Chem. Data Collect. 35, 100751. doi:10.1016/j.cdc.2021.100751
Gasperi-campani, B. A., Barbieri, L., Lorenzoni, E., Montanaro, L., Sperti, S., Bonetti, E., et al. (1978). Modeccin, the toxin of Adenia digitata. Purification, toxicity and inhibition of protein synthesis in vitro. Biochem. J. 174, 491–496. doi:10.1042/bj1740491
Gleadow, R. M., and Møller, B. L. (2014). Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 65, 155–185. doi:10.1146/annurev-arplant-050213-040027
Gondwe, A. T. D., Seigler, D. S., and Dunn, J. E. (1978). Two cyanogenic glucosides, tetraphyllin B and epi-tetraphyllin B, from Adenia volkensii. Phytochemistry 17, 271–274. doi:10.1016/S0031-9422(00)94162-1
Gruber, C. W., and OʼBrien, M. (2011). Uterotonic plants and their bioactive constituents. Planta Med. 77, 207–220. doi:10.1055/s-0030-1250317
Harrison, D. M. (1985). The biosynthesis of triterpenoids, steroids, and carotenoids. Nat. Prod. Rep. 6, 459–484. doi:10.1039/np9900700459
Hearn, D. J. (2006). Adenia (Passifloraceae) and its adaptive radiation: phylogeny and growth form diversification. Syst. Bot. 31, 805–821. doi:10.1600/036364406779695933
Hearn, D. J. (2007). Novelties in Adenia (Passifloraceae): four new species, a new combination, a vegetative key, and diagnostic characters for known Madagascan species. Brittonia 59, 308–327. doi:10.1663/0007-196x(2007)59[308:niapfn]2.0.co;2
Hearn, D. J. (2009). Developmental patterns in anatomy are shared among separate evolutionary origins of stem succulent and storage root-bearing growth habits in Adenia (Passifloraceae). Am. J. Bot. 96, 1941–1956. doi:10.3732/ajb.0800203
Ibiam, U. A., Egwu, C. O., Ezeani, N., Edwin, N., C, O. U. P., and Alum, E. (2015). Acute toxicity and biochemical response of Clarias batrachus exposed to adenia cissampeloides stem extract. 2, 6–12.
Immaculate, A. A., Vimala, J. R., and Takuwa, D. T. (2022). Isolation and characterisation of flavonoids (Rutin) from the roots of cadaba aphylla (thunb) and adenia glauca of potential in anti-oxidant activity. Orient. J. Chem. 38, 1404–1413. doi:10.13005/ojc/380610
Immaculate, A. A., Vimala, J. R., and Takuwa, D. T. (2023). Phytochemical analysis in roots and tubers of Botswana medicinal plants-solanum aculeastrum dunal, elephantorrhiza elephentina, cadaba aphylla (Thunb) and Adenia glauca. Rasayan J. Chem. 16, 01–08. doi:10.31788/RJC.2023.1618097
Inamdar, S. R., Jagadeesh, N., Hiremath, K. Y., Belur, S., and Sharma, M. (2021). A polylactosamine specific lectin from adenia hondala induces apoptosis and necrosis in human epithelial colon cancer HT-29 cells. Protein pept. Lett 28, 1108–1114. doi:10.2174/0929866528666210616100140
Ishola, I. O., Olayemi, S. O., Yemitan, O. K., and Akinseye, K. (2015). Role for monoaminergic systems in the antidepressant and anxiolytic properties of the hydroethanolic leaf extract from Adenia cissampeloides. J. Basic Clin. Physiol. Pharmacol. 26, 301–312. doi:10.1515/jbcpp-2014-0015
Islam, M. J., Kamaruzzaman, Hossain, M. F., Awal, M. A., Rahman, M. R., and Alam, M. M. (2022). Green synthesis of silver nanoparticles (AgNPs) incorporated with Adenia trilobata leaf extracts and its anti-bacterial application. AIP Adv. 12. doi:10.1063/5.0116781
Jambwa, P., Katsande, S., Matope, G., and McGaw, L. J. (2022). Ethnoveterinary remedies used in avian complementary medicine in selected communal areas in Zimbabwe. Planta Med. 88, 313–323. doi:10.1055/a-1529-8618
Jaroszewski, J. W., Olafsdottir, E. S., Cornett, C., and Schaumburg, K. (1987). Cyanogenesis of adenia volkensii harms and tetrapathae tetrandra cheeseman (passifloraceae) revisited: tetraphyllin B volkenin. Optical rotary power of cyclopentenoid cyanohydrin glucosides. Acta Chem. Scand. 41, 410–421.
Jørgensen, K., Morant, A. V., Morant, M., Jensen, N. B., Olsen, C. E., Kannangara, R., et al. (2011). Biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in cassava: Isolation, biochemical characterization, and expression pattern of cyp71e7, the oxime-metabolizing cytochrome P450 enzyme. Plant Physiol. 155, 282–292. doi:10.1104/pp.110.164053
Koffi Marcel, K., Janat Akhanovna, M.-B., Yves-Alain, B., Bi Marc Gabin, D., and Bi Tra Jérôme, Z. (2011). In vitro antioxidant activities of total flavonoids extracts from leaves and stems of Adenia lobata (Jacq.) Engl.(Passifloraceae). Acad. Org. 3, 8–12. Available online at: https://academicjournals.org/journal/JPP/article-full-text-pdf/E0C3F774959.
Kraft, C., Jenett-Siems, K., Siems, K., Jakupovic, J., Mavi, S., Bienzle, U., et al. (2003). In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phyther. Res. 17, 123–128. doi:10.1002/ptr.1066
Liebenberg, L. C. C. (1935). A revision of the South African species of Juncus. J. Linn. Soc. Lond. Bot. 50, 513–570. doi:10.1111/j.1095-8339.1935.tb01500.x
Liga, S., Paul, C., and Péter, F. (2023). Flavonoids: overview of biosynthesis, biological activity, and current extraction techniques. Plants 12, 2732–2825. doi:10.3390/plants12142732
Lu, J., Yan, S., and Xue, Z. (2024). Biosynthesis and functions of triterpenoids in cereals. J. Adv. Res. doi:10.1016/j.jare.2024.05.021
Luman, F. M., and Mottram, R. (2006). Pharmacognosy, phytochemistry, chemurgy and ethnobotany of succulents author (s): frank M luman and roy Mottram. British Cactus and Succulent Society Stable. Available online at: https://www.jstor.org/stable/42795091.24, 31–34.
Maiello, S., Iglesias, R., Polito, L., Citores, L., Bortolotti, M., Ferreras, J. M., et al. (2021). Sequence, structure, and binding site analysis of kirkiin in comparison with ricin and other type 2 rips. Toxins (Basel). 13, 862. doi:10.3390/toxins13120862
Maroyi, A. (2020). Evaluation of medicinal uses, phytochemistry and biological activities of adenia gummifera (harv.) harms. J. Pharm. Nutr. Sci. 10, 280–286. doi:10.29169/1927-5951.2020.10.05.14
Mercatelli, D., Bortolotti, M., Andresen, V., Sulen, A., Polito, L., Gjertsen, B. T., et al. (2020). Early response to the plant toxin stenodactylin in acute myeloid leukemia cells involves inflammatory and apoptotic signaling. Front. Pharmacol. 11, 630–717. doi:10.3389/fphar.2020.00630
Minto, R. E., and Blacklock, B. J. (2008). Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 47, 233–306. doi:10.1016/j.plipres.2008.02.002
Monti, B., D’Alessandro, C., Farini, V., Bolognesi, A., Polazzi, E., Contestabile, A., et al. (2007). In vitro and in vivo toxicity of type 2 ribosome-inactivating proteins lanceolin and stenodactylin on glial and neuronal cells. Neurotoxicology 28, 637–644. doi:10.1016/j.neuro.2007.01.008
Neuwinger, H. D. (2004). Plants used for poison fishing in tropical Africa. Toxicon 44, 417–430. doi:10.1016/j.toxicon.2004.05.014
Ngarivhume, T., Dzomba, P., Gwizangwe, I., Zendera, C. H., Katsvanga, C. A. T., Jimu, L., et al. (2008). Anaesthetic effects of Adenia gummifera distillates on Apis mellifera (Honeybee). Nat. Prod. Res. 22, 1370–1378. doi:10.1080/14786410701782213
Ngarivhume, T., Van’T Klooster, C. I. E. A., De Jong, J. T. V. M., and Van Der Westhuizen, J. H. (2015). Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J. Ethnopharmacol. 159, 224–237. doi:10.1016/j.jep.2014.11.011
N’Go, P. K., Tehoua, L., Bolou, E.-K. G., Touré, A. S., and Tako, A. N. (2021). Effects of hydroethanolic extract of adenia lobata (jacq.) engl. (Passifloraceae) on nociception and subsequent anxiety-like behavior: both anti-inflammatory and antioxidant approaches. Eur. J. Med. Plants 32, 10–22. doi:10.9734/ejmp/2021/v32i830407
Nyansah, W., Koffuor, G., Ben, I., Gyanfosu, L., and Ehigiator, B. (2021). Antithrombotic property of an aqueous extract from Pseudocedrela kotschyi and Adenia cissampeloides. Res. Pharm. Sci. 16, 436–446. doi:10.4103/1735-5362.319581
Nyansah, W. B., Koffuor, G. A., Asare, F., and Gyanfosu, L. (2016). Anticoagulant effect and safety assessment of an aqueous extract of Pseudocedrela kotschyi (Schweinf.) harms and Adenia cissampeloides (Planch. ex Hook.) harms. J. Intercult. Ethnopharmacol. 5, 153–161. doi:10.5455/jice.20160324054355
Nyarko, A. A., and Addy, M. E. (1990). Effect of aqueous extract of Adenia cissampeloides on blood pressure and serum analytes of hypertensive patients. Phyther. Res. 4, 25–28. doi:10.1002/ptr.2650040107
Ogunlesi, M., Okiei, W., Ofor, E., and Eniola, A. (2019). Medicinal and pesticidal potentials of the constituents of the essential oil from Adenia cissampeloides leaves. Kem. u Ind. 68, 7–21. doi:10.15255/kui.2018.027
Okem, A., Southway, C., Stirk, W. A., Street, R. A., Finnie, J. F., and Van Staden, J. (2014). Heavy metal contamination in South African medicinal plants: a cause for concern. South Afr. J. Bot. 93, 125–130. doi:10.1016/j.sajb.2014.04.001
Okpekon, T., Yolou, S., Gleye, C., Roblot, F., Loiseau, P., Bories, C., et al. (2004). Antiparasitic activities of medicinal plants used in Ivory Coast. J. Ethnopharmacol. 90, 91–97. doi:10.1016/j.jep.2003.09.029
Pelosi, E., Lubelli, C., Polito, L., Barbieri, L., Bolognesi, A., and Stirpe, F. (2005). Ribosome-inactivating proteins and other lectins from Adenia (Passifloraceae). Toxicon 46, 658–663. doi:10.1016/j.toxicon.2005.07.008
Polito, L., Bortolotti, M., Pedrazzi, M., Mercatelli, D., Battelli, M. G., and Bolognesi, A. (2016). Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 23, 32–41. doi:10.1016/j.phymed.2015.11.006
Presena, J., and Pragasam, A. (2016). Evaluation of anatomical features of adenia wightiana (wall. ex wight and arn.) engl. of passifloraceae for proper identification of the species. Int. J. Plant, Anim. Environ. Sci. 6, 132–139.
Rage, H., Jibril, E., Mohamoud, S., Xia, C. T., and Jha, P. K. (2024). Wcn24-967 acute kidney injury due to adenia ellenbeckii poisoning. A case report. Kidney Int. Rep. 9, S26–S27. doi:10.1016/j.ekir.2024.02.041
Rashidinejad, A., and Jafari, S. M. (2020). Nanoencapsulation of bioactive food ingredients. INC, 279–344. doi:10.1016/B978-0-12-815866-1.00008-X
Rotich, W. (2022). Botanical aspects, chemical overview, and pharmacological activities of 14 plants used to formulate a Kenyan Multi-Herbal Composition (CareVidTM). Sci. Afr. 17, e01287. doi:10.1016/j.sciaf.2022.e01287
Rotich, W., Sadgrove, N. J., Mas-Claret, E., Padilla-González, G. F., Guantai, A., and Langat, M. K. (2021). Hiv-1 reverse transcriptase inhibition by major compounds in a kenyan multi-herbal composition (Care-vidTM): in vitro and in silico contrast. Pharmaceuticals 14, 1009. doi:10.3390/ph14101009
Salomon, R. G. (2024). Complex molecular synthesis book; chapter 3: fatty acid and prostaglandins. Libr. Chem. Available online at: https://chem.libretexts.org/@go/page/285428.
Santos, A. C., Nogueira, M. L., Oliveira, F. P.De, Costa, E. V., and Bezerra, D. P. (2022). Essential oils of duguetia species A. St. Hill (annonaceae): chemical diversity and pharmacological potential. Biomolecules 12, 615. doi:10.3390/biom12050615
Sarkodie, J. A., Fleischer, T. C., Edoh, D. A., Dickson, R. A., Mensah, M. L. K., Annan, K., et al. (2013). Antihyperglycaemic activity of ethanolic extract of the stem of adenia lobata engl (passifloraceae). Int. J. Pharm. Sci. Res. 4, 1370–1377.
Scott, S., Cahoon, E. B., and Busta, L. (2022). Variation on a theme: the structures and biosynthesis of specialized fatty acid natural products in plants. 111, 954–965. doi:10.1111/tpj.15878
Severino, V., Paiardini, A., Pascarella, S., Parente, A., and Chambery, A. (2009). Structural analysis of toxic volkensin, a type 2 ribosome inactivating protein from Adenia volkensii Harm (kilyambiti plant): molecular modeling and surface analysis by computational methods and limited proteolysis. Int. J. Biol. Macromol. 45, 407–413. doi:10.1016/j.ijbiomac.2009.06.015
Sharma, M., Hegde, P., Hiremath, K., Reddy H, V., Kamalanathan, A. S., Swamy, B. M., et al. (2018). Purification, characterization and fine sugar specificity of a N-Acetylgalactosamine specific lectin from Adenia hondala. Glycoconj. J. 35, 511–523. doi:10.1007/s10719-018-9843-6
Sinei, K. A., and Mwangi, J. W. (1995). Effect of the tuber of adenia globosa on isolated rat uterus preparation. Pharm. Biol. 33, 346–347. doi:10.3109/13880209509065391
Sinei, K. A., and Mwangi, J. W. (2015). An in vitro study on the oxytocic action of adenia globosa engl. East Cent. Afr. J. Pharm. Sci. 18, 96–99.
Sinei, K. A., Mwangi, J. W., Achola, K. J., Munenge, R. W., and Mwaura, A. M. (1994). Potentiation of uterine stimulatory action of Adenia globosa Engl. by oxytocin in vitro. Afr. J. Health Sci. 1, 191–193. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/12153348.
Spencer, K. C., and Seigler, D. S. (1982a). Tetraphyllin B and epi-tetraphyllin B from adenia glauca schinz. Onderstepoort J. Vet. Res. 49, 137–138.
Spencer, K. C., and Seigler, D. S. (1982b). B from Adenia digitata occurring in the olefinic region of the spectrum. 6. 21, 653–655.
Stirpe, F., Barbieri, L., Abbondanza, A., Falasca, A. I., Brown, A. N., Sandvig, K., et al. (1985). Properties of volkensin, a toxic lectin from Adenia volkensii. J. Biol. Chem. 260, 14589–14595. doi:10.1016/s0021-9258(17)38608-8
Stirpe, F., Bolognesi, A., Bortolotti, M., Farini, V., Lubelli, C., Pelosi, E., et al. (2007). Characterization of highly toxic type 2 ribosome-inactivating proteins from Adenia lanceolata and Adenia stenodactyla (Passifloraceae). Toxicon 50, 94–105. doi:10.1016/j.toxicon.2007.02.020
Tosi, G., Fermani, S., Falini, G., Polito, L., Bortolotti, M., and Bolognesi, A. (2009). Crystallization and preliminary X-ray diffraction data analysis of stenodactylin, a highly toxic type 2 ribosome-inactivating protein from Adenia stenodactyla. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 66, 51–53. doi:10.1107/S1744309109047654
Ulubelen, A., Oksuz, S., Mabry, T. J., Dellamonica, G., and Chopin, J. (1983). C-glycosylflavonoids from Passiflora pittieri, P. alata, P. ambigua and Adenia mannii. Phytochemistry 22, 783. doi:10.1021/np50024a030
Uno, U., Ekpo, P., Onwudiwe, C., and Agu, R. (2018). Comparative acute toxicity of ichthyotoxic plants (Tephrosia vogelii, adenia cissampeloides and asystasia vogeliana) on farmed african catfish (Clarias gariepinus). Asian J. Biol. 5, 1–7. doi:10.9734/ajob/2018/41275
Uzoeto, H. O., Ayogu, T. E., and Nwakaeze, E. A. (2018). In vitro antibacterial activity of adenia cissampeloides plant extracts on bacteria isolated from fishes recovered from uwana river, ebonyi state, Nigeria. J. Middle East North Afr. Sci. 4, 1–7. doi:10.12816/0044057
Wannasaksri, W., On-Nom, N., Chupeerach, C., Temviriyanukul, P., Charoenkiatkul, S., and Suttisansanee, U. (2021a). In vitro phytotherapeutic properties of aqueous extracted adenia viridiflora craib towards civilization diseases. Molecules 26, 1082–1115. doi:10.3390/molecules26041082
Wannasaksri, W., Temviriyanukul, P., Aursalung, A., Sahasakul, Y., Thangsiri, S., Inthachat, W., et al. (2021b). Influence of plant origins and seasonal variations on nutritive values, phenolics and antioxidant activities of adenia viridiflora craib., an endangered species from Thailand. Foods 10, 2799. doi:10.3390/foods10112799
Keywords: Adenia, ethnobotany, bioactive compounds, pharmacology, ribosome-inactivating proteins, toxicity, traditional medicine
Citation: Mouafon IL and Katerere DR (2025) Review of the ethnobotany, phytochemistry, pharmacology, and toxicity studies of the genus Adenia. Front. Pharmacol. 16:1581659. doi: 10.3389/fphar.2025.1581659
Received: 22 February 2025; Accepted: 23 April 2025;
Published: 21 May 2025.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoCopyright © 2025 Mouafon and Katerere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iliassou L. Mouafon, bW91YWZvbi1sYWhpQHR1dC5hYy56YQ==, bGFoaWxpYXNzQHlhaG9vLmZy
 Iliassou L. Mouafon
Iliassou L. Mouafon David R. Katerere
David R. Katerere