- 1Pharmaceutical Department, The Second People’s Hospital of Yibin-Yibin Hospital of West China Hospital of Sichuan University, Yibin, China
- 2Clinical Pharmacy Department, Third Affiliated Hospital of Chengdu Medical College-Chengdu Pidu District People’s Hospital, Chengdu, China
- 3Department of Oncology, The Second People’s Hospital of Yibin-Yibin Hospital of West China Hospital of Sichuan University, Yibin, China
Coronary Heart Disease (CHD) stands as a predominant cardiovascular ailment globally, posing a severe menace to human health. Central to both hemostasis and the pathogenesis of coronary atherosclerotic thrombosis are platelets. In recent years, their significance has expanded beyond mere involvement in clot formation; they have been implicated in heightened immune responses, contributing to tissue inflammation (evident in myocardial ischemia) and vascular inflammation (crucial in vulnerable plaque formation). While contemporary antiplatelet therapies have markedly enhanced clinical outcomes for patients with coronary artery disease, they inadvertently escalate the hazard of bleeding complications. This review delves into the intricate mechanisms by which platelets influence the progression of coronary artery disease and meticulously examines the prospective utility of herbal antiplatelet interventions. Our objective is twofold: firstly, to furnish clinicians with scientifically grounded and valuable therapeutic alternatives for managing coronary artery disease, and secondly, to stimulate research and development endeavors aimed at creating novel, more efficacious antiplatelet medications that strike a balance between efficacy and safety.
1 Introduction
Coronary heart disease (CHD), also known as coronary atherosclerotic heart disease, is a cardiovascular disorder characterized by the gradual narrowing of coronary arteries due to plaque accumulation or atherosclerosis (Baaten et al., 2024). Its hallmark feature is the substantial obstruction within the vascular lumen, which not only results in myocardial ischaemia and hypoxiabut can also precipitate cardiomyocyte necrosis. These pathological alterations ultimately culminate in severe outcomes such as heart failure, arrhythmias, and even sudden cardiac death (Dong et al., 2019). Currently, CHD ranks among the leading causes of mortality and morbidity worldwide (Bi et al., 2015).
Platelets, multifunctional nucleate cells with a diameter of 2–4 μm, are present at concentrations of 150–350 × 109/L in healthy individuals. Despite lacking genomic DNA, they contain megakaryocyte-derived messenger RNAs (mRNAs) and translational machinery essentialfor protein synthesis (Davizon-Castillo et al., 2020). Platelets play a critical role in maintaining vascular wall integrity, preventing excessive blood loss due to tissue damage (de Gaetano and Cerletti, 2002; Brewer, 2006), and are central to processes such as thrombosis and haemostasis (Davì and Patrono, 2007). Consequently, platelets are increasingly recognised as key players in many other pathophysiological processes, including inflammation, atherosclerosis, obstructive and non-obstructive coronary artery disease (CAD), and a wide range of serious diseases such as ischemic stroke (Sabetta et al., 2022). Antiplatelet therapy is therefore considered one of the most important tools for the prevention of cardiovascular diseases such as ischemic heart disease (IHD) (Wiviott et al., 2007; Wallentin et al., 2009), stroke (Amarenco et al., 2020), and peripheral arterial disease (PAD) (Bonaca et al., 2013).
In traditional medicine, CHD is classified under the conditions of chest paralysis and heart pain. Its etiology primarily encompasses factors such as qi depression, phlegm, blood stasis, fire-heat, cold condensation and zhengqi deficiency. The pathological mechanisms mainly involve processes like coagulation, inflammation and immune response (Lan et al., 2024). As a traditional medical resource mainly derived from natural herbs, Traditional Chinese Medicine (TCM) boasts advantages such as multi-targeting, multi-pathway, low adverse effects and a wealth of clinical practice, has long been employed to maintain human health. At present, a multitude of published randomised controlled trials (RCTs), systematic evaluations and meta-analyses have affirmed that TCM offers certain benefits in the comprehensive prevention and control of coronary artery lesions (Teng et al., 2024). It can not only effectively impede early critical lesions but also delay the onset of heart failure complications following advanced myocardial infarction (Cheang et al., 2024; Chen K. et al., 2023). Furthermore, TCM improves symptoms associated with CHD like chest tightness and chest pain (Zhang et al., 2025). Hence, it is crucial to investigate the pharmacological and material basis of anti-CHD treatments and their related mechanism of action within the framework of TCM.
2 Mechanism of platelets in the development of CHD
CHD is characterized by myocardial ischemia resulting from coronary artery atherosclerosis. It frequently manifests with symptoms such as angina pectoris and can lead to severe complications, including myocardial infarction. Given the critical nature of this condition, it becomes imperative to understand the role of platelets in its pathogenesis. What, then, is the contribution of platelets to the development and progression of CHD?
2.1 Platelets and thrombosis
As is well known, during normal blood circulation, the vascular wall remains intact and platelets remain in a non-activated state, maintaining the integrity of the vascular endothelium (Knowles and Warner, 2019). However, when platelets are activated, they will further adhere and aggregate, forming thromboembolic bodies, leading to the occurrence of diseases related to arterial thromboembolism such as ischemic stroke and coronary heart disease (Chen K. et al., 2023). For example, clinical study in the context of acute coronary syndrome (ACS), there is a notable alteration in the platelet lipidome, which not only stimulates platelet activation but also accelerates the progression of ACS by promoting thrombotic events (Harm et al., 2022).
When the vascular wall is damaged by external factors or pathological stimuli, the activated thrombin or exposed subendothelial matrix produced after endothelial injury can trigger platelet activation and aggregation, leading to changes in platelet biochemistry and structure and stimulating platelet activation. Subsequently, activated platelets adhere and aggregate through a series of reactions, forming an embolic body. For example, research has demonstrated that the platelet transmembrane chemokine SR-PSOX/CXCL16-CXCR6 synergistically accelerates the release of platelet degranulation and platelet aggregation to promote thrombosis in CAD (Guan et al., 2022). In turn, dense granules and α-granules released by activated platelets further amplify platelet activation and promote thrombosis via the release of adenosine triphosphate (ATP) or adenosine diphosphate (ADP) and complement factors (Stenberg et al., 1984). Concurrently, activated platelets also lead to the exposure of negatively charged phospholipids on the surface, which stimulates the binding and activation of coagulation and tissue factors, further promoting thrombosis (Gremmel et al., 2024); and its derivative proprotein convertase subtilisin/kexin type 9 (PCSK9) also induces a platelet-dependent thrombotic inflammatory response and promotes atherosclerotic thrombosis (Petersen-Uribe et al., 2021). Furthermore, inhibition of cystic fibrosis transmembrane conductance regulator protein (CFTR) in platelets also enhances platelet activity and accelerates arterial thrombosis by increasing intracellular Cl− concentration and promoting the recombinant serum/glucocorticoid regulated kinase 1 (SGK1) signaling pathway (Yang et al., 2022). In general, activated platelets promote thrombus formation through the following mechanisms: (1) activation of integrin αⅡbβ3 (signal transduction from the inside out), binding to fibrinogen, fibronectin, or VWF, and aggregating and adhering at the damaged site (Grover et al., 2018); (2) Promote thrombin activation, initiate coagulation cascade reaction, and form stable and irreversible thrombus (Furie and Furie, 2008); (3) By increasing intracellular Ca2+concentration, bioactive molecules such as adenosine diphosphate (ADP), P-selectin, thromboxane A2 (TXA2), and serotonin (5-HT) are released (Jurk and Kehrel, 2005), further enhancing platelet aggregation and forming firm embolic bodies (Kubes, 2016), thereby promoting the progression of cardiovascular disease.
Therefore, platelet activation plays a crucial role in occurrence, development and prognosis of CHD by facilitating thrombosis.
2.2 Platelets and vascular endothelial function
The vascular endothelium comprises extremely thin vascular endothelial cells that serve as a mechanical barrier between the vessel wall and the bloodstream. These cells selectively regulate the passage of substances of various sizes through the vessel wall, as well as modulate vascular smooth muscle contraction, platelet aggregation, vascular smooth muscle cell proliferation, leukocyte adhesion, and thrombus formation. Consequently, damage to the vascular endothelium and subsequent inflammatory responses are significant contributors to the development of CHD (Roth et al., 2020; GBD 2019 Diseases and Injuries Collaborators, 2020). In general, endothelial cells (ECs) covering the vascular lumen protect vascular integrity and homeostasis by sensing and responding to physical, chemical, and biological stimuli (Feaver et al., 2013; Giannotta et al., 2013). When exposed to external stimuli such as high/low shear forces, these cells undergo major phenotypic changes, leading to increased endothelial cell permeability, cytokine release, and leukocyte adhesion (Ni et al., 2010; Gimbrone, 2010), ultimately resulting in endothelial cell damage. In addition, the imbalance between the generation and accumulation of ROS/free radicals and the ability of antioxidants (such as superoxide dismutase, glutathione peroxidase, catalase, vitamin E) to clear them can lead to oxidative stress, resulting in cell damage (Pizzino et al., 2017), thus leading to the onset and progression of atherosclerotic cardiovascular disease.
Studies have shown that platelets rapidly recognize and respond to damage in the vascular system during injury, further activating it and affecting endothelial cell function by releasing a variety of cytokines. For instance, platelets stimulate the synthesis and release of interleukin-1α (IL-1α) (Thornton et al., 2010), interleukin-1β (IL-1β) (Lindemann et al., 2001) and chemokines such as monocyte chemotactic protein 1 (MCP-1) (Blair and Flaumenhaft, 2009; Pennings et al., 2022). They also promote interactions between platelets and leukocytes, neutrophils, and endothelial cells (Gawaz et al., 2005; Merhi et al., 1995), which exacerbating plaque rupture and thrombotic inflammatory responses (Bakogiannis et al., 2019; Steg et al., 2011). Concurrently, activated platelets can also directly adhere to endothelial cells following thrombin stimulation (Kaplan et al., 1989); or bind to Willebrand factor (vWF) via glycoprotein (GP)Iβα on their surface before adhering to endothelial cells (Auid-Orcid Zifkos et al., 2024), ultimately intensifying inflammatory injury. The stable binding of platelets to vWF further stimulates collagen interacts with GPVI and α2β1 integrin. This interaction triggers platelet activation, leading to inside-out signaling, which then activates Glycoprotein IIb/IIIa (GPIIb/IIIa), allowing it to bind fibrinogen and von vWF for platelet aggregation (Denis and Wagner, 2007). This, in turn, promotes the cytosolic action of dense granules such as ADP, ATP, Ca2+ and 5-hydroxytryptamine (5-HT), thereby enhancing platelet aggregation and activation at sites of vascular injury (Fraer and Kilic, 2015). Additionally, platelet-expressed toll-like receptor 2 (TLR2) boost platelet activity and interactions with vascular endothelial cells, contributing to the development of thrombo-inflammatory disease (Parra-Izquierdo et al., 2021).
These findings suggest that platelet activation and vascular endothelial cell adhesion can induce a vascular inflammatory response, which in turn accelerates the progression of cardiovascular disease (Badimon et al., 2012).
2.3 Platelets and inflammatory response
The immune system plays a central role in atherosclerotic thrombosis (Verdoia et al., 2015). In contrast, the inflammatory response is a catalyst for CHD disease progression.
In acute myocardial infarction, activated platelets release transforming growth factor B-inducible (TGFBI) protein, which stimulates platelet activation, adhesion, migration, and vascular inflammation, thereby contributing to the disease progression of myocardial infarction (MI) (Kraemer et al., 2022). At the same time, these activated platelets also release 5-HT or serotonin (Mauler et al., 2019), inducing neutrophil degranulation and the release of myeloperoxidase, hydrogen peroxide (H2O2), and the membrane-bound leukocyte adhesion molecule recombinant integrin alpha M (CD11b). This ultimately leads to enhanced inflammation in the infarcted area and reduced myocardial salvage. Furthermore, platelet-neutrophil aggregates formed by activated platelets and neutrophils can contribute to the development of various thrombotic-inflammatory disorders (Zarbock and Ley, 2009; Schmitt et al., 2015). Additionally, platelet microvesicles (PMV), ultramicrofilm vesicle released by platelets during activation, can also exacerbate the vicious cycle of inflammation and thrombosis during ACS (Gkaliagkousi et al., 2021). In ApoE−/−mice, platelet-released P-selectin facilitates the delivery of platelet-derived pro-inflammatory factors to monocytes/leukocytes and the vessel wall, thereby inducing an inflammatory response and plaque enlargement (Huo et al., 2003). Additionally, platelet surface CD154 can also accelerate atherosclerosis by inhibiting regulatory T cells and reducing plaque stability (Lievens et al., 2010).
However, it has also been shown that soluble guanylate cyclase (sGC) in platelets can inhibit atherosclerotic plaque formation by stimulating the release of angiopoietin-1 from platelets and reducing leukocyte recruitment in vitro (Mauersberger et al., 2022). Platelet-derived miRNAs can ameliorate myocardial inflammation following myocardial ischemia-reperfusion by inhibiting myocardial inflammation and cardiac fibrosis (Schütte et al., 2023). Additionally, platelet microparticles containing miR-4306 can also improve CAD by inhibiting human monocyte-derived macrophage migration via the VEGFA/ERK1/2/NF-κB signaling pathway (Yang et al., 2019). In addition, miR-34c-5p derived from extracellular vesicles of activated platelets (PLT-EVs) also attenuates inflammatory responses in HCAEC through inhibition of the podocalyxin (PODXL) and P38 MAPK signaling pathways, thereby preventing the development of CAD (Bai et al., 2022).
Collectively, these studies indicate that platelets possess the dual capacity to both enhance and suppress inflammatory responses via a multitude of pathways, as illustrated in Figure 1. Consequently, they play a significant role in influencing the progression and outcome of atherosclerotic disease.
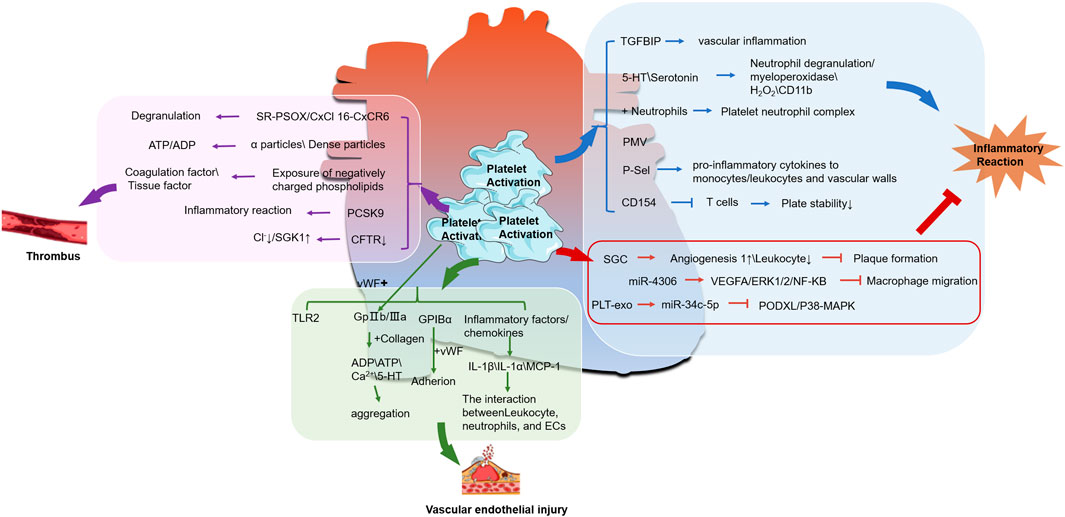
Figure 1. The relationship between activated platelets and CHD. (The molecular mechanism between platelet activation and thrombus formation is shown in the pink box; The green box represents the relationship between platelet activation and endothelial injury, while the blue box represents the molecular mechanism between platelet activation and inflammatory response. ↑ is upregulation, ↓ is downregulation,


3 Progress in the study of antiplatelet effects in TCM
At present, representative drugs widely used in clinical practice for the treatment and prevention of thrombotic diseases, such as commonly used antiplatelet drugs (aspirin, clopidogrel, and GPIIbIIIa inhibitors) (Mega and Simon, 2015), often come with side effects such as drug resistance (Rozalski et al., 2005), intolerance, allergies, “aspirin resistance”, and gastrointestinal bleeding (Angiolillo, 2012). TCM is considered by scholars as a new alternative therapy (Li et al., 2019). TCM, primarily derived from natural herbs, has a long history in treating thrombotic diseases. Modern pharmacological studies have demonstrated that TCM are effective in antithrombotic therapy, particularly in inhibiting platelet activity (Qiu et al., 2019; Zhou et al., 2019), such as plant extracts ketones, alkaloids, and alcohol glycosides, all have antiplatelet activity, promote fibrinolysis, and anticoagulant activity (Wei et al., 2020).
3.1 Inhibition of platelet adhesion
Platelet adhesion refers to the adhesion of platelets to the surface of subendothelial tissues or other substances in blood vessels. It is the initial reaction of normal hemostasis after vascular damage. Studies have shown that the release of P-selectin from stimulated endothelial cells mediates the initial loose contact between platelets and endothelial cells (Frenette et al., 1998; Frenette et al., 1995), which in turn affects the progression of CHD.
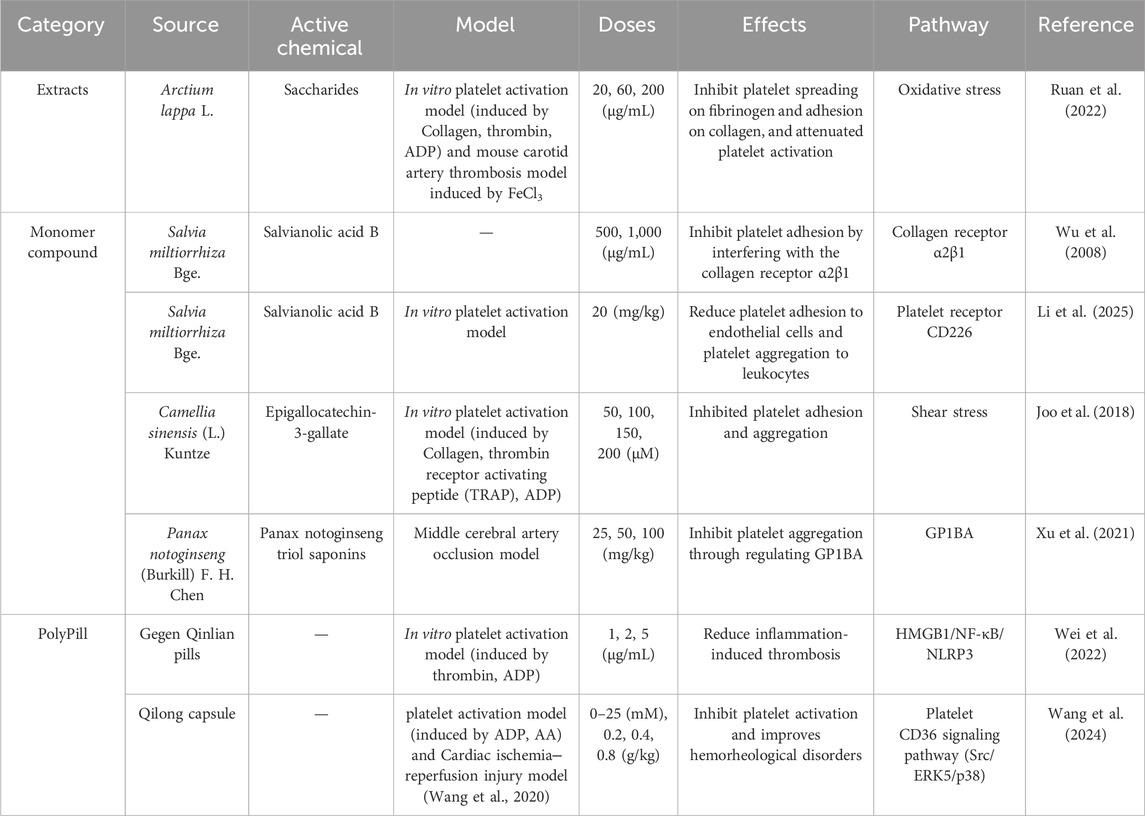
In the study of herbal extracts, Saccharides from Arctium lappa L. root, inhibits platelet spreading on fibrinogen and adhesion on collagen under shear (Ruan et al., 2022).
Regarding the single components of TCM, research has demonstrated that the combination of total saponins from the stem and leaves of Panax quinquefolius L. and total saponins from PNF attenuates injured endothelium-induced platelet adhesion by up-regulating the PI3K/PKB pathway in the endothelial cells (Ming-ming et al., 2016). Panax notoginseng saponin inhibits low shear stress-induced platelet adhesion and activation on dysfunctional endothelial cells (Liu et al., 2024). Panax notoginseng triol saponin reduces vascular VWF-mediated platelet adhesion to damaged vascular endothelium, enhancing the potential for antiplatelet aggregation and antithrombosis under pathological conditions (Xu et al., 2021). Salvianolic acid B from Salvia miltiorrhiza Bge, inhibits platelet adhesion to immobilized collagen by interfering with the collagen receptor α2β1 (Wu et al., 2008). It also reduced platelet adhesion to endothelial cells and platelet aggregation to leukocytes (Li et al., 2025). Epigallocatechin-3-gallate, found in high levels in dried green tea leaves, has been shown to inhibit platelet adhesion and aggregation (Joo et al., 2018).
In addition, the Chinese medicine compound preparation QiLong capsule reduces platelet adhesion by interfering with platelet CD36 expression and affecting the level of β-thromboglobulin (β-TG), PF-4, P-selectin and platelet activating factor (PAF) (Wang et al., 2024). Gegen Qinlian pills also inhibit platelet aggregation and adhesion by regulating HMGB1/NF-κB/NLRP3 signaling pathway and attenuating keratine-induced thrombosis in mice (Wei et al., 2022).
In summary, TCM exhibit significant inhibitory effects on platelet activity. These effects can be effective in both the prevention and treatment of cardiovascular diseases through mechanisms such as anti-platelet aggregation, adhesion, and inhibition of platelet content release, as illustrated in Table 1.
3.2 Inhibition of platelet aggregation
In recent years, numerous pharmacological studies have demonstrated the beneficial effects of TCM in inhibiting platelet aggregation.
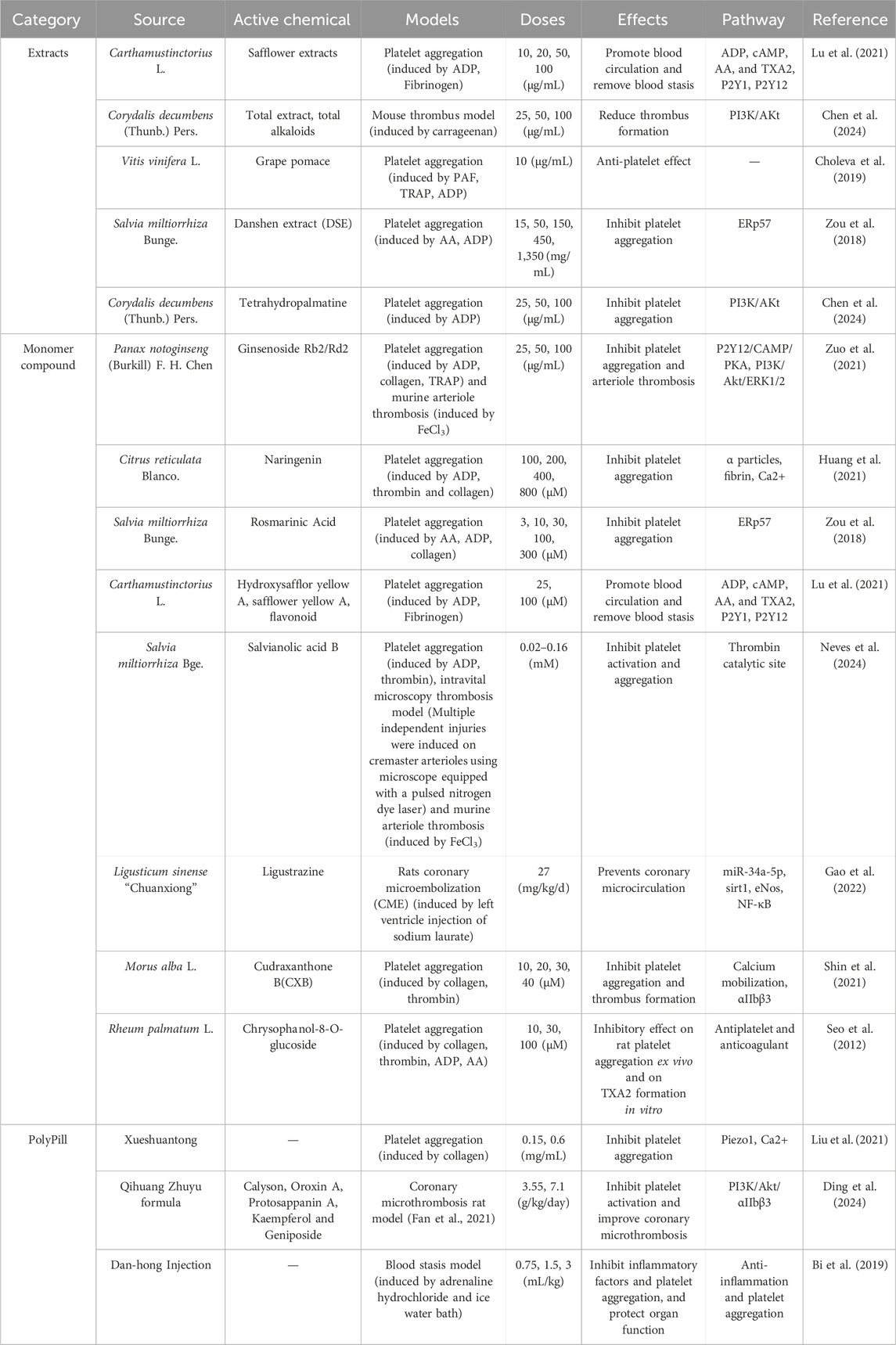
In the study of herbal extracts, the safflower extract compounds, such as hydroxysafflor yellow A, safflower yellow A, and luteolin, extracted from Rheum palmatum L., has been shown to inhibit platelet aggregation by affecting the activation of ADP receptor downstream signaling pathways, such as calcium and cAMP production, and modulating the expression of activated glycoproteins on platelet membranes (Lu et al., 2021). The extract of Corydalis decumbens (Thunb.) Pers. inhibits thrombosis by reducing platelet aggregation through the modulation of the PI3K-Akt pathway (Chen et al., 2024). Grape pomace extract significantly inhibits platelet aggregation (Choleva et al., 2019).
Regarding the single components of TCM, studies have shown that ginsenosides Rb2 and Rd2 in Panax notoginseng (Burkill) F. H. Chen ex C. H. Chow flowers play an important preventive role in thrombotic diseases by attenuating platelet activation through P2Y12-mediated cAMP/PKA and PI3K/Akt/Erk1/2 signaling (Zuo et al., 2021). Salvianolic acid B (SAB), an active ingredient in Salvia miltiorrhiza Bunge (SMB), inhibit platelet activation and aggregation induced by thrombin, adenosine diphosphate and collagen (Neves et al., 2024). Danshen extract (DSE), and rosmarinic acid (another active ingredient in SMB), all exert antiplatelet aggregation effects by inhibiting the enzymatic activity of endoplasmic reticulum resident protein 57 (ERp57) (Zou et al., 2018). Tetramethylpyrazine, an active ingredient in Ligusticum sinense “Chuanxiong,” promotes the expression of Sirtuin1 (Sirt1) and endothelial nitric oxide synthase (eNOS) by inhibiting miR-34a-5p expression and nuclear factor-κB (NF-κB) activation, thereby attenuating endothelial dysfunction and inhibiting platelet aggregation and inflammatory responses (Gao et al., 2022). Cudraxanthone B (CXB), isolated from Morus alba L., suppressed collagen-induced human platelet aggregation, Ca2+ ([Ca2+]i) mobilization, fibrinogen binding, fibronectin adhesion (Shin et al., 2021). While naringenin from citrus fruits suppresses platelet aggregation and arterial thrombosis by inhibiting platelet α-granule secretion, fibrinogen binding, intracellular calcium mobilization, and platelet adhesion to collagen-coated surfaces (Huang et al., 2021). Other plant extracts such as coumarins, flavonoids, alkaloids, flavonoids and anthraquinones may also exhibit antiplatelet and fibrinolytic activities (Saluk-Juszczak et al., 2010; Yoo et al., 2014; Seo et al., 2012).
In the study of Chinese herbal compound preparations (a kind of Chinese traditional medicine product that takes Chinese traditional medicine as raw material and is processed into a certain dosage form according to the prescribed prescription and preparation process under the guidance of Chinese traditional medicine theory), Xueshuantong Capsules inhibit shear-induced platelet aggregation by targeting the Piezo1 channel-mediated Ca2+ signaling pathway (Liu et al., 2021). The Qihuang Zhuyu formula improves coronary artery micro-thrombosis by inhibiting PI3K/Akt/αIIbβ3-mediated platelet activation and inflammatory responses (Ding et al., 2024). Danhong Injection (DHI) inhibits inflammation and platelet aggregation, reduces immune responses and peroxidation, and protects vascular endothelial and organ function, thereby preventing and treating cardiovascular disease (Bi et al., 2019). In clinical study, several researchers have found that DHI is beneficial to patients with ACS after percutaneous coronary intervention (Jing et al., 2014; Wei-hua et al., 2015; Zhuhua et al., 2016); after treating ischemic stroke patients with Qishen Quyu Formula, the platelet aggregation function indicators ADP and arachidonic acid (AA) decreased compared to before treatment (Jian-feng et al., 2023), as shown in Table 2.
3.3 Inhibition of platelet contents release
Platelet release reaction refers to the process in which particles or substances stored in the lysosome of platelets are stimulated and released from the open pipeline system of platelets to the outside. These substances include P-selectin, 5-HT, ADP, Ca2+, B-thromboglobulin (B-TG), platelet factor 4 (PF4), etc. In addition to inhibiting platelet aggregation, TCM also play an important role in inhibiting the release of platelet contents.
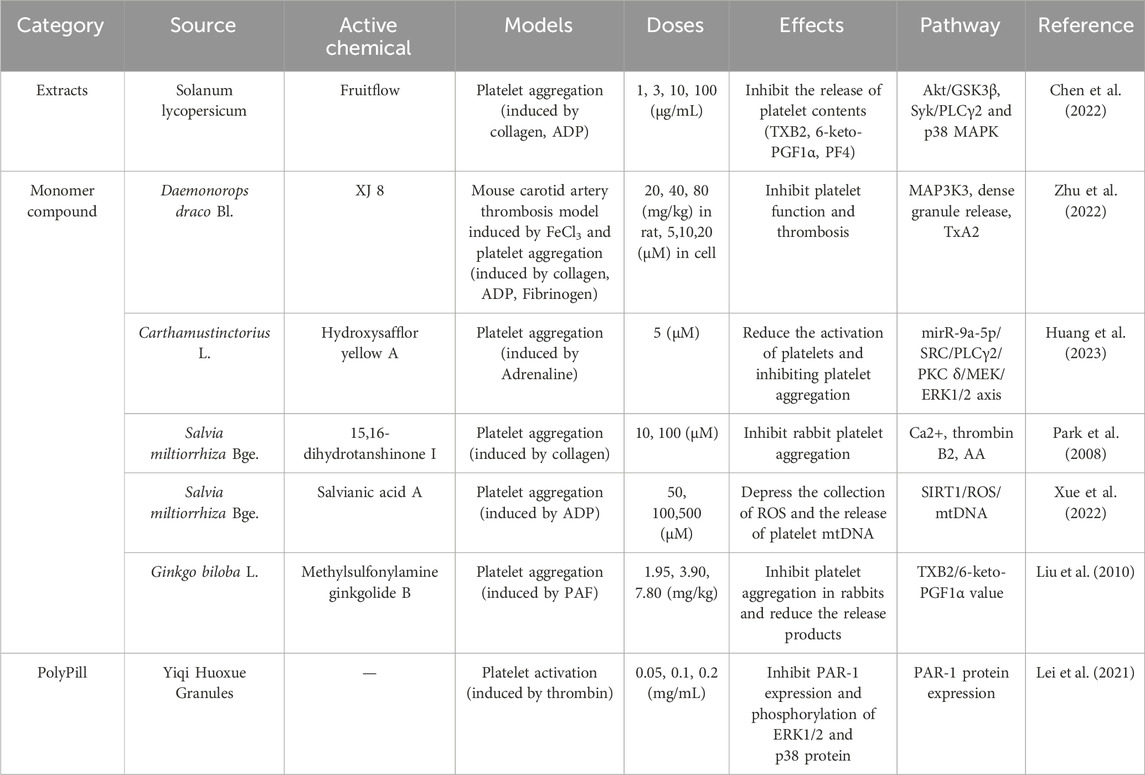
In the study of herbal extracts, Fruitflow, a water-soluble tomato extract, also interferes with collagen-stimulated platelet phosphorylation of Akt/GSK3β, Syk/PLCγ2, and p38 MAPK. This is achieved by inhibiting the level of platelet TXB2, 6-keto-PGF1α and platelet factor (PF4), thereby preventing platelet activation (Chen et al., 2022).
Regarding the single components of TCM, Salvianic acid, an active ingredient in SMB, can effectively inhibits platelet activation by blocking the aggregation of reactive oxygen species (ROS) and the release of mitochondrial DNA (mtDNA) from platelets (Xue et al., 2022). Another major constituent of SMB, 15,16-dihydrotanshinone I, also significantly inhibits intracellular Ca2+ mobilisation, AA release, and prothrombin B2 production, demonstrating potent antiplatelet activity (Park et al., 2008). Hydroxysafflor yellow A inhibits platelet surface glycoprotein IIb/IIIa (GPIIb/IIIa) and thromboxane A2 (TXA2) expression by modulating the miR-9a-5p/SRC axis, thereby reducing platelet Ca2+ accumulation and subsequent platelet overactivation in rats (Huang et al., 2023). XJ-8, a flavonoid separated from Sanguis draxonis extract (extracted from Daemonorops draco Bl.), inhibit platelet function and thrombosis by targeting MAP3K3, which suppresses platelet dense granule release, TxA2 synthesis, and aggregation (Zhu et al., 2022). Methylsulfonylamine ginkgolide B, can significantly inhibit platelet activating factor induced Ca2+ release from rabbit platelets (Liu et al., 2010).
In the study of Chinese herbal compound preparations, Yiqi Huoxue Granules may inhibit thrombin-induced platelet activation by suppressing protease-activated receptor 1 (PAR-1) expression in platelets (Lei et al., 2021). Buyang Huanwu Tang can also inhibit the state of excessive platelet release, significantly reducing the release of TXB2 and β-TG (Jingqi et al., 2020). DHI also has a significant inhibitory effect on platelet aggregation. It can inhibit the synthesis process of TXA2 and effectively reduce the levels of TXB2 and plasma platelet activating factor, thereby reducing the formation of large platelet aggregation and ultimately reducing the occurrence of thrombosis (Cui et al., 2021), relevant research reports illustrated in Table 3.
4 Clinical application of TCM in cardiovascular diseases
TCM have a long history of use in treating cardiovascular diseases. Among them, blood-activating and blood-stasis-eliminating Chinese medicines such as Salvia divinorum, safflower, Panax ginseng and Chuanxiong rhizome are major groups of commonly used medicines with multiple advantages. Compared to single-component chemical drugs, TCM exists in the form of multiple components, offering the benefits of multi-targeting, multi-pathway actions, low adverse effects, and rich clinical practice. Modern pharmacological studies have also shown that TCM can improve thrombotic diseases by regulating platelet activation, aggregation, adhesion and granule release. Secondly, TCM treats diseases holistically, including maintaining or alleviating the disease, improving overall health, and enhancing immunity, etc. In addition to eliminating cardiac lesions, TCM can also alleviate the symptoms, slow down the progression, and effectively prevent disease recurrence. Therefore, Chinese medicine is practical and promising in the treatment of CHD. In clinical case studies, TCM extracts have also demonstrated unique advantages, such as the combination of Xueshuantong injection and Danhong injection in the clinical treatment of coronary heart disease, which can effectively improve patients’ plasma specific viscosity and whole blood viscosity, and has high safety (Li, 2023); Xuefu Zhuyu Tang can basically restore chest pain symptoms in patients with coronary heart disease, and also alleviate other accompanying symptoms (Jia and Wang, 2022); Modified Danggui Sini Tang can reduce the symptom score and Seattle Angina Questionnaire score of angina pectoris, improve hemorheological indicators, and thereby improve unstable angina pectoris in coronary heart disease (Du et al., 2022); Xuefu Zhuyu Tang can significantly reduce platelet activity units (PRU), increase coagulation formation time, coagulation reaction time, platelet inhibition rate, etc. in patients with primary stable angina pectoris (Changfu et al., 2021); Zhishi Xiebai Guizhi Decoction is effective and safe in treating CHD (Zhou, 2023). It can be seen that traditional Chinese medicine has significant therapeutic effects in improving myocardial ischemia, alleviating angina symptoms, and regulating cardiac function.
5 Prospects and challenges
At present, there are about 11.4 million patients with CHD in China, and its morbidity and mortality rate are rising annually, imposing an extremely high medical burden to society and the public (Lan et al., 2024). Platelets play a crucial role in the development of CHD, affecting the blood supply to the coronary arteries and promoting the disease process through various biological mechanisms. These include promoting thrombus formation, regulating inflammatory responses, inducing vasoconstriction, and altering endothelial function.
Currently, most of the commonly used antiplatelet drugs in the clinic are G protein coupled receptors inhibitors, such as the P2Y12 receptor inhibitors ticagrelor and clopidogrel, and the TXA2 cyclooxygenase inhibitor aspirin. These drugs reduce the occurrence and progression of cardiovascular diseases by inhibiting thrombus formation. However, long-term administration of these drugs can lead to adverse effects. A meta-analysis showed that the prevalence of Aspirin resistance was 52.1% and the prevalence of Clopidogrel resistance was 20.5% (Parsa-Kondelaji and Mansouritorghabeh, 2023). Additionally, the incidence of in-hospital 30 day bleeding in acute coronary syndromes ranges from 3.0% to 8.3%(Moscucci et al., 2003). Consequently, an increasing number of patients are opting for TCM treatment.
Although some progress has been made in the study of platelet activity inhibition by TCM, but the Bioavailability, the pharmacology, pharmacokinetics, adverse effects, and mechanism of action has not been thoroughly investigated due to the complexity of TCM composition. This lack of comprehensive understanding has long hindered clarity on the mechanisms through which TCM combats cardiovascular diseases. As a result, the transferability of many research results is relatively low, and the efficacy of many TCM monomers/compounds is limited to laboratory validation and has not entered clinical use. Therefore, due to the complex composition of TCM, in subsequent research, we can first study the main ingredients and targets that exert pharmacological effects from single Chinese medicinal herbs or individual components in classic formulas. Secondly, we can also explore multiple pathways simultaneously to identify more therapeutic targets. Thirdly, we can further explore the therapeutic targets of diseases by combining the characteristics of disease course and the dialectical treatment of TCM. In addition, we need to pay more attention to the combination of traditional Chinese medicine and modern medical treatment methods, and use modern pharmacological research technologies such as chips, organoids, multi omics, network pharmacology, targeted screening, molecular biology, and information science to strengthen the experimental and clinical research of TCM in various platelet related diseases. This approach will enable a more comprehensively exploration of the antiplatelet effects of Chinese medicines, as well as the material basis and mechanisms underlying their anti-cardiovascular properties.
6 Limitation
In addition, the sample size analyzed in this review was relatively small, we only reviewed the TCM that improves CHD through anti-platelet adhesion, anti-platelet aggregation, and anti-platelet content release. Actually, the multi-target of TCM is also reflected in its ability to resist myocardial/endothelial cell damage, inhibit inflammatory reactions, suppress oxidative stress reactions, and improve symptoms of CHD, but this is not listed in this article. We will present this part in the next article.
7 Conclusion
There is a close interaction between platelets and CHD, and antiplatelet therapy plays a significant role in the prevention and treatment of cardiovascular diseases. TCM exhibits notable antiplatelet activity and can regulate platelet function through specific targets and pathways, thereby intervening in the progression of CHD. Therefore, exploring new therapies against CHD from the perspective of TCM holds high application prospects.
Author contributions
LL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. XL: Investigation, Supervision, Writing – review and editing. HX: Formal Analysis, Investigation, Writing – original draft. JT: Software, Writing – review and editing. BL: Visualization, Writing – review and editing. YT: Validation, Visualization, Writing – review and editing. FX: Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Sichuan Province Science and Technology Plan Project (NO. 2024NSFSC1843), the Sichuan Provincial Medical Youth Innovation Research Project (NO. Q20027), and the Sichuan Hospital Association Young Pharmacist Research Special Fund Project (NO. 22047).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1641780.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amarenco, P., Denison, H., Evans, S. R., Himmelmann, A., James, S., Knutsson, M., et al. (2020). Ticagrelor added to aspirin in acute nonsevere ischemic stroke or transient ischemic attack of atherosclerotic origin. Stroke 51 (12), 3504–3513. doi:10.1161/STROKEAHA.120.032239
Angiolillo, D. J. (2012). The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 72 (16), 2087–2116. doi:10.2165/11640880-000000000-00000
Auid-Orcid Zifkos, K., Auid-Orcid Bochenek, M. I., Auid-Orcid Gogiraju, R., Robert, S., Auid-Orcid Pedrosa, D., Auid-Orcid Kiouptsi, K., et al. (2024). Endothelial PTP1B Deletion promotes VWF exocytosis and venous thromboinflammation. Circ. Res 134 (10), e93–e111. doi:10.1161/CIRCRESAHA.124.324214
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396 (10258), 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Baaten, C. C. F. M. J., Nagy, M., Bergmeier, W., Spronk, H. M. H., and van der Meijden, P. E. J. (2024). Platelet biology and function: plaque erosion vs. Rupture. Eur. Heart J. 45 (1), 18–31. doi:10.1093/eurheartj/ehad720
Badimon, L., Padró, T., and Vilahur, G. (2012). Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Acute Cardiovasc. Care 1 (1), 60–74. doi:10.1177/2048872612441582
Bai, X., Zhang, H., Li, Z., Chen, O., He, H., Jia, X., et al. (2022). Platelet-derived extracellular vesicles encapsulate microrna-34c-5p to ameliorate inflammatory response of coronary artery endothelial cells via podxl-mediated P38 mapk signaling pathway. Nutr. metabolism & Cardiovasc. Dis. 32 (10), 2424–2438. doi:10.1016/j.numecd.2022.06.013
Bakogiannis, C., Sachse, M., Stamatelopoulos, K., and Stellos, K. (2019). Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 122, 154157. doi:10.1016/j.cyto.2017.09.013
Bi, C., Li, P. L., Liao, Y., Rao, H. Y., Yi, J., Yi, J., et al. (2019). Pharmacodynamic effects of dan-hong injection in rats with blood stasis syndrome. Biomédecine & pharmacothérapie 118, 109187. doi:10.1016/j.biopha.2019.109187
Bi, Y., Jiang, Y., He, J., Xu, Y., Wang, L., Xu, M., et al. (2015). Status of cardiovascular health in Chinese adults. J. Am. Coll. Cardiol. 65 (10), 1013–1025. doi:10.1016/j.jacc.2014.12.044
Blair, P., and Flaumenhaft, R. (2009). Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 23 (4), 177–189. doi:10.1016/j.blre.2009.04.001
Bonaca, M. P., Scirica, B. M., Creager, M. A., Olin, J., Bounameaux, H., Dellborg, M., et al. (2013). Vorapaxar in patients with peripheral artery disease: results from Tra2{Degrees}P-timi 50. Circulation 127 (14), 1522–1529. doi:10.1161/CIRCULATIONAHA.112.000679
Brewer, D. B. (2006). Max schultze (1865), G. Bizzozero (1882) and the discovery of the platelet. Br. J. Haematol. 133 (3), 251–258. doi:10.1111/j.1365-2141.2006.06036.x
Changfu, X. U., Shen, S., Lin, X. U., Jianhua, Y. E., and Tong, H. (2021). The effect of Xuefu Zhuyu decoction on primary stable microvascular angina in patients with different tcm syndromes. J. Electrocardiol. Circulation 40 (03), 282–286+95. doi:10.12124/j.issn.2095-3933.2021.3.2021-4267
Cheang, I., Yao, W., Zhou, Y., Zhu, X., Lu, X., Ni, G., et al. (2024). The traditional Chinese medicine qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial. Nat. Med. 30 (8), 2295–2302. doi:10.1038/s41591-024-03169-2
Chen, H., Zhang, S., Wang, H., Bao, L., Wu, W., and Qi, R. (2022). Fruitflow inhibits platelet function by suppressing akt/gsk3β, syk/plcγ2 and P38 mapk phosphorylation in collagen-stimulated platelets. BMC complementary Med. Ther. 22 (1), 75. doi:10.1186/s12906-022-03558-5
Chen, J., Wei, X., Zhang, Q., Wu, Y., Xia, G., Xia, H., et al. (2023). The traditional Chinese medicines treat chronic heart failure and their main bioactive constituents and mechanisms. Acta Pharm. Sin. B 13 (5), 1919–1955. doi:10.1016/j.apsb.2023.02.005
Chen, K., Zhang, R., Chen, S., Fan, X., Shen, L., and Yuan, H. (2023). Clinical value of platelet indices in premature coronary artery disease. Int Heart J. 64 (3), 336–343. doi:10.1536/ihj.22-442
Chen, S., Tian, C.-B., Bai, L.-Y., He, X. C., Lu, Q. Y., Zhao, Y. L., et al. (2024). Thrombosis inhibited by Corydalis decumbens through regulating pi3k-akt pathway. J. Ethnopharmacol. 329, 118177. doi:10.1016/j.jep.2024.118177
Choleva, M., Boulougouri, V., Panara, A., Panagopoulou, E., Chiou, A., Thomaidis, N. S., et al. (2019). Evaluation of anti-platelet activity of grape pomace extracts. Food & Funct. 10 (12), 8069–8080. doi:10.1039/c9fo02138h
Cui, L., Yi, S., Xu, M., Yang, K., Wang, Y., and Lv, F. (2021). Research progress on traditional Chinese medicine treatment of lower limb deep vein thrombosis in icu patients. J. Emerg. Traditional Chin. Med. 30 (07), 1308–1310. doi:10.3969/j.issn.1004-745X.2021.07.050
Davì, G., and Patrono, C. (2007). Platelet activation and Atherothrombosis. N. Engl. J. Med. 357 (24), 2482–2494. doi:10.1056/NEJMra071014
Davizon-Castillo, P., Rowley, J. W., and Rondina, M. T. (2020). Megakaryocyte and platelet transcriptomics for discoveries in human health and disease. Arteriosclerosis, Thrombosis, Vasc. Biol. 40 (6), 1432–1440. doi:10.1161/ATVBAHA.119.313280
de Gaetano, G., and Cerletti, C. (2002). Platelet adhesion and aggregation and fibrin formation in flowing blood: a historical contribution by giulio bizzozero. Platelets 13 (2), 85–89. doi:10.1080/09537100220122457
Denis, C. V., and Wagner, D. D. (2007). Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arteriosclerosis, Thrombosis, Vasc. Biol. 27 (4), 728–739. doi:10.1161/01.ATV.0000259359.52265.62
Ding, Y., Xiang, Q., Zhu, P., Fan, M., Tong, H., Wang, M., et al. (2024). Qihuang Zhuyu formula alleviates coronary microthrombosis by inhibiting pi3k/akt/αiibβ3-mediated platelet activation. Phytomedicine Int. J. Phytotherapy Phytopharm. 125, 155276. doi:10.1016/j.phymed.2023.155276
Dong, Y., Chen, H., Gao, J., Liu, Y., Li, J., and Wang, J. (2019). Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 136, 27–41. doi:10.1016/j.yjmcc.2019.09.001
Du, H., Lei, R., Zhang, H., Mou, H., and Yuanyuan, Y. (2022). Clinical observation of modified Danggui Sini decoction in the treatment of unstable angina pectoris (yin cold stagnation syndrome) in coronary heart disease. J. Emerg. Traditional Chin. Med. 31 (05), 875–877. doi:10.3969/j.issn.1004-745X.2022.05.033
Fan, M. L., Tong, H. Q., Sun, T., Zhang, H. W., Han, J., Cheng, S. Y., et al. (2021). Animal model of coronary microembolization under transthoracic echocardiographic guidance in rats. Biochem. Biophys. Res. Commun. 568, 174–179. doi:10.1016/j.bbrc.2021.05.045
Feaver, R. E., Fau - Blackman, B. R. G.Bd, and Blackman, B. R. (2013). Human haemodynamic frequency harmonics regulate the inflammatory phenotype of vascular endothelial cells. Nat. Commun. 4, 1525. doi:10.1038/ncomms2530
Fraer, M., and Kilic, F. (2015). Serotonin: a different player in hypertension-associated thrombosis. Hypertension 65 (5), 942–948. doi:10.1161/HYPERTENSIONAHA.114.05061
Frenette, P. S., Johnson, R. C., Hynes, R. O., and Wagner, D. D. (1995). Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc. Natl. Acad. Sci. U. S. A. 92 (16), 7450–7454. doi:10.1073/pnas.92.16.7450
Frenette, P. S., Moyna, C., Hartwell, D. W., Lowe, J. B., Hynes, R. O., and Wagner, D. D. (1998). Platelet-endothelial interactions in inflamed mesenteric venules. Blood 91 (4), 1318–1324. doi:10.1182/blood.v91.4.1318
Furie, B., and Furie, B. C. (2008). Mechanisms of thrombus formation. N. Engl. J. Med. 359 (9), 938–949. doi:10.1056/NEJMra0801082
Gao, J., Ren, J., Ma, X., Zhang, Y., Song, L., Liu, J., et al. (2022). Ligustrazine prevents coronary microcirculation dysfunction in rats via suppression of mir-34a-5p and promotion of Sirt1. Eur. J. Pharmacol. 929, 175150. doi:10.1016/j.ejphar.2022.175150
Gawaz, M., Fau - May, A. E. L. H., and May, A. E. (2005). Platelets in inflammation and atherogenesis. J. Clin. Investigation 115 (12), 3378–3384. doi:10.1172/JCI27196
Giannotta, M., Fau - Dejana, E. T. M., and Dejana, E. (2013). Ve-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26 (5), 441–454. doi:10.1016/j.devcel.2013.08.020
Gimbrone, M. A. (2010). The gordon wilson lecture. Understanding vascular endothelium: a pilgrim's progress. Endothelial dysfunction, biomechanical forces and the pathobiology of atherosclerosis. Trans. Am. Clin. Climatol. Assoc. 121, 115–127.
Gkaliagkousi, E., Gavriilaki, E., Yiannaki, E., Vasileiadis, I., Nikolaidou, B., Lazaridis, A., et al. (2021). Platelet microvesicles are associated with the severity of coronary artery disease: comparison between peripheral and coronary circulation. J. thrombosis thrombolysis 51 (4), 1138–1143. doi:10.1007/s11239-020-02302-5
Gremmel, T., Frelinger, A. L., and Michelson, A. D. (2024). Platelet physiology. Semin. Thromb. Hemost. 50 (8), 1173–1186. doi:10.1055/s-0044-1786387
Grover, S. P., Bergmeier, W., and Mackman, N. (2018). Platelet signaling pathways and new inhibitors. Arteriosclerosis, Thrombosis, Vasc. Biol. 38 (4), e28–e35. doi:10.1161/ATVBAHA.118.310224
Guan, T., Emschermann, F., Schories, C., Groga-Bada, P., Martus, P., Borst, O., et al. (2022). Platelet Sr-Psox/Cxcl16-Cxcr6 Axis influences thrombotic propensity and prognosis in coronary artery disease. Int. J. Mol. Sci. 23 (19), 11066. doi:10.3390/ijms231911066
Harm, T., Bild, A., Dittrich, K., Goldschmied, A., Nestele, J., Chatterjee, M., et al. (2022). Acute coronary syndrome is associated with a substantial change in the platelet lipidome. Cardiovasc. Res. 118 (8), 1904–1916. doi:10.1093/cvr/cvab238
Huang, M., Deng, M., Nie, W., Zou, D., Wu, H., and Xu, D. (2021). Naringenin inhibits platelet activation and arterial thrombosis through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Front. Pharmacol. 12, 722257. doi:10.3389/fphar.2021.722257
Huang, W., Yao, W., Weng, Y., Xie, X., Jiang, J., Zhang, S., et al. (2023). Hydroxysafflor yellow a inhibits the hyperactivation of rat platelets by regulating the mir-9a-5p/src Axis. Archives Biochem. biophysics 747, 109767. doi:10.1016/j.abb.2023.109767
Huo, Y., Fau - Forlow, S. B. S. A., Fau - Smith, D. F. F.Sb, Smith, D. F., Hyman, M. C., Jung, S., et al. (2003). Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9 (1), 61–67. doi:10.1038/nm810
Jia, L., and Wang, H. (2022). Professor Wang hai's clinical case study on the treatment of coronary heart disease with modified Xuefu Zhuyu tang. Chin. J. Ethn. Med. 28 (09), 21–23. doi:10.16041/j.cnki.cn15-1175.2022.09.010
Jian-feng, ZHOU, Ke-qing, ZHANG, Zhi-yang, YANG, Jin-zhong, KANG, and Guo-ni, ZHANG (2023). Clinical efficacy of qishen Quyu formula in treating patients with ischemic stroke of qi deficiency and blood stasis syndrome and its influence on serum nerve cytokines and platelet aggregation function. World J. Integr. Traditional West. Med. 18 (12), 2493–2497. doi:10.13935/j.cnki.sjzx.231229
Jing, D., Chen, Y., Liu, H., Jinsong, C., Ying, Z., Jing, W., et al. (2014). Effects of Danhong injection on endothelial function of perioperative unstable angina patients with blood stasis syndrome undergoing percutaneous coronary intervention. J. Traditional Chin. Med. 55 (13), 1109–1112. doi:10.13288/j.11-2166/r.2014.13.009
Jingqi, B., Niu, W., Yuenan, F., Zhang, Y., and Xiao, H. (2020). Effect of buyang huanwu decoction on platelet activation in qi deficiency and blood stasis model 22 (02), 43–46. doi:10.13194/j.issn.1673-842x.2020.02.013
Joo, H. J., Park, J.-Y., Jun Hong, S., Kim, K. A., Lee, S. H., Cho, J. Y., et al. (2018). Anti-platelet effects of epigallocatechin-3-gallate in addition to the concomitant aspirin, clopidogrel or ticagrelor treatment. Korean J. Intern. Med. 33 (3), 522–531. doi:10.3904/kjim.2016.228
Jurk, K., and Kehrel, B. E. (2005). Platelets: physiology and biochemistry. Semin. Thromb. Hemost. 31 (4), 381–392. doi:10.1055/s-2005-916671
Kaplan, J. E., Moon Dg Fau - Weston, L. K., Weston Lk Fau - Minnear, F. L., Minnear, F. L., Del Vecchio, P. J., Shepard, J. M., et al. (1989). Platelets adhere to thrombin-treated endothelial cells in vitro. Am. J. Physiol. 257 (2 Pt 2), H423–H433. doi:10.1152/ajpheart.1989.257.2.H423
Knowles, R. B., and Warner, T. D. (2019). Anti-platelet drugs and their necessary interaction with endothelial mediators and platelet cyclic nucleotides for therapeutic efficacy. Pharmacol. Ther. 193, 83–90. doi:10.1016/j.pharmthera.2018.08.004
Kraemer, B, Martinovic, I., and Lindemann, S. (2022). Human platelets release tgfbip in acute myocardial infarction. Heart Vessels 37 (11), 1962–1970. doi:10.1007/s00380-022-02086-z
Kubes, P. (2016). The versatile platelet contributes to inflammation, infection, hemostasis, coagulation and cancer. Seminars Immunol. 28 (6), 535. doi:10.1016/j.smim.2016.11.002
Lan, Y., Fu-Kun, L., Yue, Y., Wang, X.-Y., Wang, P.-Q., and Xiong, X.-J. (2024). Coronary heart disease: innovative understanding from traditional Chinese medicine and treatment by classic formulas. China J. Chin. Materia Medica 49 (13), 3684–3692. doi:10.19540/j.cnki.cjcmm.20240326.501
Lei, Z., Gao, S., Wang, X., Gao, H., Han, Y., Wang, Z., et al. (2021). Effect of Yiqi Huoxue granules on platelet activation induced by thrombin. Evidence-based complementary Altern. Med. 2021, 6622848. doi:10.1155/2021/6622848
Li, Da (2023). Clinical effect of Xueshuantong injection combined with Danhong injection in the treatment of coronary heart disease. Chin. J. Clin. Ration. Drug Use 16 (11), 1–3+7. doi:10.15887/j.cnki.13-1389/r.2023.11.001
Li, J., Liang, Q., and Sun, G. (2019). Interaction between traditional Chinese medicine and anticoagulant/antiplatelet drugs. Curr. drug Metab. 20 (9), 701–713. doi:10.2174/1389200220666190827160212
Li, X., Liu, S., Xie, J., Liu, L., Duan, C., Yang, L., et al. (2025). Salvianolic acid B improves the microcirculation in a mouse model of sepsis through a mechanism involving the platelet receptor Cd226. Br. J. Pharmacol. 182 (4), 988–1004. doi:10.1111/bph.17371
Lievens, D., Fau - Seijkens, T. Z. A., Oliver, S. T. F.-S., Soehnlein, O., Beckers, L., Munnix, I. C. A., et al. (2010). Platelet Cd40l mediates thrombotic and inflammatory processes in atherosclerosis. Blood 116 (20), 4317–4327. doi:10.1182/blood-2010-01-261206
Lindemann, S., Tolley, N. D., Dixon, D. A., McIntyre, T. M., Prescott, S. M., Zimmerman, G. A., et al. (2001). Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. cell Biol. 154 (3), 485–490. doi:10.1083/jcb.200105058
Liu, H., Pan, S., Liu, C., Wei, M., and Xu, H. (2010). The role of dimethylaminoethyl ginkgolide B mesylate on inhibiting platelet Ag- gregation and release function. Chin. Tradit. Pat. Med. 32 (06), 914–918. doi:10.3969/j.issn.1001-1528.2010.06.008
Liu, L., Jia, L., Wang, Y., Gong, P., Feng, J., Xiao, S., et al. (2024). Effects of Panax notoginseng saponins on alleviating low shear induced endothelial inflammation and thrombosis via Piezo1 signalling. J. Ethnopharmacol. 335, 118639. doi:10.1016/j.jep.2024.118639
Liu, L., Zhang, Q., Xiao, S., Sun, Z., Ding, S., Chen, Y., et al. (2021). Inhibition of shear-induced platelet aggregation by Xueshuantong via targeting Piezo1 channel-mediated Ca(2+) signaling pathway. Front. Pharmacol. 12, 606245. doi:10.3389/fphar.2021.606245
Lu, P.-H., Kuo, C.-Y., Chan, C.-C., Wang, L. K., Chen, M. L., Tzeng, I. S., et al. (2021). Safflower extract inhibits adp-induced human platelet aggregation. Plants (Basel, Switz.) 10 (6), 1192. doi:10.3390/plants10061192
Mauersberger, C., Sager, H., Wobst, J., Dang, T. A., Lambrecht, L., Koplev, S., et al. (2022). Loss of soluble guanylyl cyclase in platelets contributes to atherosclerotic plaque formation and vascular inflammation. Nat. Cardiovasc. Res. 1 (12), 1174–1186. doi:10.1038/s44161-022-00175-w
Mauler, M., Herr, N., Schoenichen, C., Witsch, T., Marchini, T., Härdtner, C., et al. (2019). Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation 139 (7), 918–931. doi:10.1161/CIRCULATIONAHA.118.033942
Mega, J. L., and Simon, T. (2015). Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet 386 (9990), 281–291. doi:10.1016/S0140-6736(15)60243-4
Merhi, Y., Guidoin R Fau - Provost, P., Provost, T. K., Fau - Leung, P., Leung Tk Fau - Lam, J. Y., and Lam, J. Y. (1995). Increase of neutrophil adhesion and vasoconstriction with platelet deposition after deep arterial injury by angioplasty. Am. heart J. 129 (3), 445–451. doi:10.1016/0002-8703(95)90266-x
Ming-ming, W., Xue, M., and Yang, L. (2016). Chinese herbal compounds for supplementing qi and activating blood circulation combined with dual anTiplatelet drugs alleviated human umbilical vein endothelial cell injury and platelet adhesion via up-regulartion of pi3k/akt pathway. Chin. J. Integr. Med. 36 (07), 842–848. doi:10.7661/CJIM.2016.07.0842
Moscucci, M., Fox Ka Fau - Cannon, C. P., Fau - Klein, W. C.C., Klein, W., López-Sendón, J., Montalescot, G., et al. (2003). Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (grace). Eur. Heart J. 24 (20), 1815–1823. doi:10.1016/s0195-668x(03)00485-8
Neves, M. A. D., Ni, T. T., Mackeigan, D. T., Shoara, A. A., Lei, X., Slavkovic, S., et al. (2024). Salvianolic acid B inhibits thrombosis and directly blocks the thrombin catalytic site. Res. Pract. Thrombosis Haemostasis 8 (4), 102443. doi:10.1016/j.rpth.2024.102443
Ni, C. W., Fau - Rezvan, A. Q. H., Fau - Kwon, K. R. A., Fau - Nam, D. K., Fau - Son, D. J. N., Dj Fau - Visvader, J. E. S., et al. (2010). Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116 (15), e66–e73. doi:10.1182/blood-2010-04-278192
Park, J. W., Lee, S. H, Yang, M. K., Lee, J. J., Song, M. J., Ryu, S. Y., et al. (2008). 15,16-Dihydrotanshinone I, a major component from Salvia miltiorrhiza Bunge (dansham), inhibits rabbit platelet aggregation by suppressing intracellular calcium mobilization. Arch. Pharm. Res. 31 (1), 47–53. doi:10.1007/s12272-008-1119-4
Parra-Izquierdo, I., Lakshmanan, H. H. S., Melrose, A. R., Pang, J., Zheng, T. J., Jordan, K. R., et al. (2021). The toll-like receptor 2 ligand Pam2csk4 activates platelet nuclear factor-κb and bruton’s tyrosine kinase signaling to promote platelet-endothelial cell interactions. Front. Immunol. 12, 729951. doi:10.3389/fimmu.2021.729951
Parsa-Kondelaji, M., and Mansouritorghabeh, H. (2023). Aspirin and clopidogrel resistance; a neglected gap in stroke and cardiovascular practice in Iran: a systematic review and meta-analysis. Thrombosis J. 21 (1), 79. doi:10.1186/s12959-023-00522-2
Pennings, G. J., Reddel, C. J., Traini, M., Lam, M., Kockx, M., Chen, V. M., et al. (2022). Rapid release of interleukin-1β from human platelets is independent of Nlrp3 and caspase. Thrombosis Haemostasis 122 (4), 517–528. doi:10.1055/s-0041-1731288
Petersen-Uribe, Á., Kremser, M., Rohlfing, A., Castor, T., Kolb, K., Dicenta, V., et al. (2021). Platelet-derived Pcsk9 is associated with ldl metabolism and modulates atherothrombotic mechanisms in coronary artery disease. Int. J. Mol. Sci. 22 (20), 11179. doi:10.3390/ijms222011179
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 8416763. doi:10.1155/2017/8416763
Qiu, J., Lingna, W., Jinghong, H., and Yongqing, Z. (2019). Oral administration of leeches (shuizhi): a review of the mechanisms of action on antiplatelet aggregation. J. Ethnopharmacol. 232, 103–109. doi:10.1016/j.jep.2018.12.010
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the gbd 2019 study. J. Am. Coll. Cardiol. 76 (25), 2982–3021. doi:10.1016/j.jacc.2020.11.010
Rozalski, M., Fau - Luzak, B. B. M., Fau - Watala, C. L. B., and Watala, C. (2005). Genetic factors underlying differential blood platelet sensitivity to inhibitors. Pharmacol. Rep. 57 (1), 1–13.
Ruan, Y., Ding, Y., Li, X., Zhang, C., Wang, M., Liu, M., et al. (2022). Saccharides from Arctium lappa L. Root reduce platelet activation and thrombus formation in a laser injury thrombosis mouse model. Exp. Ther. Med. 23 (5), 344. doi:10.3892/etm.2022.11274
Sabetta, A., Lombardi, L., and Stefanini, L. X. (2022). Sex differences at the platelet-vascular interface. Intern. Emerg. Med. 17 (5), 1267–1276. doi:10.1007/s11739-022-02994-y
Saluk-Juszczak, J., Fau - Olas, B. P. I., Fau - Kołodziejczyk, J. O. B., Kołodziejczyk, J., Ponczek, M., Nowak, P., et al. (2010). The effect of polyphenolic-polysaccharide conjugates from selected medicinal plants of asteraceae family on the peroxynitrite-induced changes in blood platelet proteins. Int. J. Biol. Macromol. 47 (5), 700–705. doi:10.1016/j.ijbiomac.2010.09.007
Schmitt, C., Fau - Ciorciaro, C. A. M., Fau - Kling, D. C. C., Fau - Jamois, C. K. D., Fau - Schick, E. J. C., Fau - Solier, C. S. E., et al. (2015). First-in-Man study with inclacumab, a human monoclonal antibody against P-selectin. J. Cardiovasc. Pharmacol. 65 (6), 1533–4023. doi:10.1097/FJC.0000000000000233
Schütte, J. P., Manke, M., Hemmen, K., Münzer, P., Schörg, B. F., Ramos, G. C., et al. (2023). Platelet-derived micrornas regulate cardiac remodeling after myocardial ischemia. Circulation Res. 132 (7), e96–e113. doi:10.1161/CIRCRESAHA.122.322459
Seo, E.Ji, Tran, M. N., Lee, S.-M., Kim, Y. S., and Jung, Y.-S. (2012). Chrysophanol-8-O-Glucoside, an anthraquinone derivative in rhubarb, has antiplatelet and anticoagulant activities. J. Pharmacol. Sci. 118 (2), 245–254. doi:10.1254/jphs.11123fp
Shin, J.-H., Irfan, M., Rhee, M. H., and Kwon, H.-W. (2021). Antiplatelet effect of cudraxanthone B is related to inhibition of calcium mobilization, Αiibβ3 activation, and clot retraction. Appl. Biol. Chem. 64 (1), 4. doi:10.1186/s13765-020-00575-1
Steg, P. G., Dorman, S., and Amarenco, P. (2011). Atherothrombosis and the role of antiplatelet therapy. J. Thrombosis Haemostasis JTH 9 (Suppl. 1), 325–332. doi:10.1111/j.1538-7836.2011.04277.x
Stenberg, P. E., Shuman, M. A., Levine, S. P., and Bainton, D. F. (1984). Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J. cell Biol. 98 (2), 748–760. doi:10.1083/jcb.98.2.748
Teng, Y., Yang, L., Wang, L., Wang, B., Su, S., Chen, J., et al. (2024). Effectiveness and pharmacological mechanisms of Chinese herbal medicine for coronary heart disease complicated with heart failure. J. Ethnopharmacol. 322, 117605. doi:10.1016/j.jep.2023.117605
Thornton, P., McColl, B. W., Greenhalgh, A., Denes, A., Allan, S. M., and Rothwell, N. J. (2010). Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 115 (17), 3632–3639. doi:10.1182/blood-2009-11-252643
Verdoia, M., Schaffer, A., Barbieri, L., Aimaretti, G., Marino, P., Sinigaglia, F., et al. (2015). Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes metabolism 41 (4), 304–311. doi:10.1016/j.diabet.2015.01.001
Wallentin, L., Fau - Budaj, A. B., Fau - Cannon, C. P. B. A., Fau - Emanuelsson, H. C. C., Fau - Held, C. E. H., Fau - Horrow, J. H. C., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. Mass. Med. Soc. 361 (11), 1533–4406. doi:10.1056/NEJMoa0904327
Wang, M., Li, L., Tang, S., Liu, J., Liu, S., Ye, J., et al. (2024). Qilong capsule prevents myocardial ischemia/reperfusion injury by inhibiting platelet activation via the platelet Cd36 signaling pathway. J. Ethnopharmacol. 330, 118211. doi:10.1016/j.jep.2024.118211
Wang, R., Wang, M., Zhou, J., Dai, Z., Sun, G., and Sun, X. (2020). Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J. Adv. Res. 34, 173–186. doi:10.1016/j.jare.2020.10.005
Wei, G., Xu, X., Tong, H., Wang, X., Chen, Y., Ding, Y., et al. (2020). Salidroside inhibits platelet function and thrombus formation through akt/gsk3β signaling pathway. Aging 12 (9), 8151–8166. doi:10.18632/aging.103131
Wei, X., Zhang, B., Wei, F., Ding, M., Luo, Z., Han, X., et al. (2022). Gegen Qinlian pills alleviate carrageenan-induced thrombosis in mice model by regulating the hmgb1/nf-κb/nlrp3 signaling. Phytomedicine Int. J. Phytotherapy Phytopharm. 100, 154083. doi:10.1016/j.phymed.2022.154083
Wei-hua, ZHENG, Xue-zhou, W. U., and Ai-peng, ZHANG (2015). Influence of Danhong injection on il-17 expression after pci in stemi patients. Chin. J. Cardiovasc. Rehabilitation 24 (01), 84–87. doi:10.3969/j.issn.1008-0074.2015.01.25
Wiviott, S. D., Fau - McCabe, C. H. B. E., Fau - Montalescot, G. M. C.Ch, Fau - Ruzyllo, W. M. G., Fau - Gottlieb, S. R. W., Fau - Neumann, F. J. G., et al. (2007). Prasugrel versus clopidogrel in patients with acute coronary syndromes. Mass. Med. Soc. 357 (20), 1533–4406. doi:10.1056/NEJMoa0706482
Wu, Ya P., Xiao, M. Z., Pan, S. D., Guo, D. A., Wei, R., Han, J. J., et al. (2008). Salvianolic acid B inhibits platelet adhesion under conditions of flow by a mechanism involving the collagen receptor Alpha2beta1. Thrombosis Res. 123 (2), 298–305. doi:10.1016/j.thromres.2008.05.020
Xu, Z.-Y., Xu, Y., Xie, X.-F., Tian, Y., Sui, J. H., Sun, Y., et al. (2021). Anti-platelet aggregation of Panax notoginseng triol saponins by regulating Gp1ba for ischemic stroke therapy. Chin. Med. 16 (1), 12. doi:10.1186/s13020-021-00424-3
Xue, Y., Zhang, L., Zhang, L., Sun, W., Fang, Z., Leng, Y., et al. (2022). Danshensu prevents thrombosis by inhibiting platelet activation via sirt1/ros/mtdna pathways without increasing bleeding risk. Phytomedicine Int. J. Phytotherapy Phytopharm. 104, 154271. doi:10.1016/j.phymed.2022.154271
Yang, H. Y., Zhang, C., Hu, L., Liu, C., Pan, N., Li, M., et al. (2022). Platelet cftr inhibition enhances arterial thrombosis via increasing intracellular Cl(-) concentration and activation of Sgk1 signaling pathway. Acta Pharmacol. Sin. 43 (10), 2596–2608. doi:10.1038/s41401-022-00868-9
Yang, Y., Luo, H., Liu, S., Zhang, R., Zhu, X., Liu, M., et al. (2019). Platelet microparticles-containing mir-4306 inhibits human monocyte-derived macrophages migration through vegfa/erk1/2/Nf-Κb signaling pathways. Clin. Exp. Hypertens. 41 (5), 481–491. doi:10.1080/10641963.2018.1510941
Yoo, H., Ku, S.-K., Lee, W., Kwak, S., Baek, Y. D., Min, B. W., et al. (2014). Antiplatelet, anticoagulant, and Profibrinolytic activities of cudratricusxanthone A. Archives pharmacal Res. 37 (8), 1069–1078. doi:10.1007/s12272-013-0290-4
Zarbock, A., and Ley, K. (2009). The role of platelets in acute lung injury (Ali). Front. Biosci. (Landmark Ed.) 14 (1), 150–158. doi:10.2741/3236
Zhang, A., Liu, X., Li, X., Duan, N., and Huang, B. (2025). A Narrative review on the role of traditional Chinese medicine (tcm) in treating coronary artery disease. JPMA 75 (3), 462–468. doi:10.47391/JPMA.11610
Zhou, H. (2023). Clinical observation on the treatment of coronary heart disease angina pectoris with phlegm turbidity and internal resistance type with modified Zhishi Xiebai Guizhi decoction. J. Pract. Traditional Chin. Med. 39 (11), 2143–2145. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=bEegF8awJvzJtSau0C2tt_sXNTubeSALqdRItyri-aBZ5ckQUg0gCmWsMT2jV10NEM_3USggRDSoj8tmdDMIn1iOmb5gkZMiX3kV5P6cdozlCay1lD0DRSig3GCFwh0sXNRhyJjm4RhMohknSIl7CkVJlhFPzkJghmB5XLheTLLmw9sPIqInf_2ihEdtOkT-SsF8h0PmLjA=&uniplatform=NZKPT&language=CHS.
Zhou, J., Zhai, J., Zheng, W., Han, N., Liu, Z., Lv, G., et al. (2019). The antithrombotic activity of the active fractions from the fruits of Celastrus orbiculatus thunb through the anti-coagulation, anti-platelet activation and anti-fibrinolysis pathways. J. Ethnopharmacol. 241, 111974. doi:10.1016/j.jep.2019.111974
Zhu, Z. X., Wang, L., Guo, R., Pang, D., Wang, W., Wu, Y., et al. (2022). Xj-8, a natural compound isolated from Sanguis draxonis, inhibits platelet function and thrombosis by targeting Map3k3. J. Thrombosis Haemostasis 20 (3), 605–618. doi:10.1111/jth.15593
Zhuhua, Z., Liu, H., Zhang, D., Yundai, C., Juju, S., Mei, D., et al. (2016). The impact of Danhong injection on unstable angina patients after percutaneous coronary intervention reflected upon velocity vector imaging. World J. Traditional Chin. Med. 11 (03), 388–391. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=HgkNOCd8VPioVtTQenfvATaDk97P-qYco8JN8cd1f61j_COgcf90A5kLHuxtiFvYbYhDL5T8XYixYmR8NU8Yd7AR098nLtHAhaPUnN7Hp1q0epRsdSfTYFkxnsgekQdbcajBL8jagZ1F6D6YP4G_6yat8ZVcDkUtGkROFopJ_WzxxmErwuWSoJC-48L5zihJ6fVbGpIQ1iw=&uniplatform=NZKPT&language=CHS.
Zou, J., Chen, Y., Hoi, M., Li, J., Wang, T., Zhang, Y., et al. (2018). Discovery of a novel Erp57 inhibitor as antiplatelet agent from danshen (Salvia miltiorrhiza). Evidence-based complementary Altern. Med. 2018, 9387568. doi:10.1155/2018/9387568
Zuo, X., Li, Q., Fuli, Y., Ma, L. J., Tian, Z., Zhao, M., et al. (2021). Ginsenosides Rb2 and Rd2 isolated from Panax notoginseng flowers attenuate platelet function through P2y12-mediated camp/Pka and Pi3k/Akt/Erk1/2 signaling. Food & Funct. 12 (13), 5793–5805. doi:10.1039/d1fo00531f
Glossary
CHD Coronary heart disease
IHD ischemic heart disease
PAD peripheral arterial disease
TCM Traditional Chinese Medicine
RCTs randomised controlled trials
ACS acute coronary syndrome
CFTR cystic fibrosis transmembrane conductance regulator protein
SGK1 Serum/Glucocorticoid Regulated Kinase 1
ATP Adenosine Triphosphate
ADP Adenosine Diphosphate
PCSK9 Proprotein Convertase Subtilisin/Kexin Type 9
IL-1α Interleukin-1α
IL-1β Interleukin-1β
GPIIb/IIIa Glycoprotein IIb/IIIa
5-HT 5-hydroxytryptamine
TLR2 Toll-Like Receptor 2
CD11b Recombinant Integrin Alpha M
MCP-1 monocyte chemotactic protein 1
ECs endothelial cells
vWF Willebrand factor
PLT-exo platelet exosomes
TGFBI transforming growth factor B-inducible
PF4 platlet factor 4
β-TG β-thromboglobulin
P-Sel P-selectin
PAF P-selectin and Platelet Activating Factor
PAR-1 protease-activated receptor 1
MI myocardial infarction
H2O2 hydrogen peroxide
PMV platelet microvesicles
sGC soluble guanylate cyclase
Sirt1 Sirtuin1
eNOS endothelial nitric oxide synthase
NF-Kβ nuclear factor-kβ
mtDNA mitochondrial DNA
TXA2 thromboxane A2
CAD coronary artery disease
PODXL Podocalyxin-Like Protein 1
VEGFA vascular endothelial growth factor A
ERK extracellular regulated protein kinases
AA arachidonic acid
P2Y12 Purinergic Receptor P2Y, G Protein Coupled 12
TRAP thrombin receptor activating peptide
Keywords: coronary heart disease, platelets, thrombus, inflammation, traditional Chinese medicine
Citation: Liao L, Li X, Xu H, Tang J, Li B, Tang Y and Xie F (2025) Systematic analysis of the interaction mechanism between platelets and coronary heart disease: from molecular pathways to new strategies for plant based antiplatelet therapy. Front. Pharmacol. 16:1586265. doi: 10.3389/fphar.2025.1586265
Received: 02 March 2025; Accepted: 28 April 2025;
Published: 03 June 2025; Corrected: 28 July 2025.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Changle Shao, Shanghai University of Traditional Chinese Medicine, ChinaIoannis Koutsaliaris, University of Ioannina, Greece
Asma Haffouz, Centre of Biotechnology of Sfax, Tunisia
Muttia Amalia, Jakarta Veterans National Development University, Indonesia
Copyright © 2025 Liao, Li, Xu, Tang, Li, Tang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Liao, NTE5ODU1NDM2QHFxLmNvbQ==; Yan Tang, MTIwMDY0MjAxQHFxLmNvbQ==; Fang Xie, cGVubnl4aWVmYW5nQGZveG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Li Liao
Li Liao Xiaoxuan Li1†
Xiaoxuan Li1† Hong Xu
Hong Xu Bo Li
Bo Li