- 1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2Department of VIP Region, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: In advanced non-small cell lung cancer with EGFR mutations, third-generation EGFR TKIs (3rd-G TKIs) are currently the preferred first-line treatment. Previous studies have demonstrated that combining first-generation EGFR TKIs with chemotherapy (1st-G TKIs + chemo) also significantly enhances efficacy compared to 1st-G TKIs alone. This study aims to compare the effectiveness of 1st-G TKIs + chemo against 3rd-G TKIs.
Methods: We conducted an indirect meta-analysis of randomized controlled trials comparing 1st-G TKIs + chemo to 3rd-G TKIs. Randomized controlled trials (RCTs) were searched from the PubMed, Embase and Cochrane Library databases. Outcomes included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and grade ≥3 treatment-related adverse events (TRAEs). Data were analyzed using inverse variance and Mantel-Haenszel methods.
Results: Ten RCTs with 3,014 patients met the inclusion criteria. Direct comparisons indicated that 1st-G TKIs + chemo significantly improved PFS (HR 0.54, P < 0.001), OS (HR 0.62, P < 0.001), and ORR (RR 1.21, P < 0.001) compared to 1st-G TKIs alone. Indirect comparisons between 1st-G TKIs + chemo and 3rd-G TKIs revealed no significant differences in PFS (HR 1.17; 95% CI, 0.98 to 1.40; P = 0.075) or OS (HR 0.78; 95% CI, 0.56 to 1.07; P = 0.122). Although 1st-G TKIs + chemo showed a 16% improvement in ORR compared to 3rd-G TKIs (RR 1.16; 95% CI, 1.06 to 1.27; P < 0.001), it was also associated with a notable increase in grade ≥3 TRAEs (RR 2.41; 95% CI, 1.63 to 3.57; P < 0.001).
Conclusion: 1st-G TKIs + chemo demonstrated PFS and OS comparable to 3rd-G TKIs. Moreover, 1st-G TKIs + chemo may be a viable option for patients who prioritize a higher response rate.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023461565 identifer, PROSPERO (CRD42023461565).
1 Introduction
Lung cancer is the predominant cause of cancer death worldwide, with approximately 85% of patients presenting with the histological subtypes of non-small cell lung cancer (NSCLC), the most common of which are lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (Herbst et al., 2018). A substantial proportion of patients are unsuitable for surgical intervention, necessitating palliative internal medicine treatments. Epidermal growth factor receptor (EGFR) mutations occur in 40%–60% of Southeast Asian patients and 10%–20% of Caucasian patients with lung adenocarcinomas and reach occurrences of 50%–60% in non-smoking patients with lung cancer. The most common EGFR mutations include exon 19 deletions and missense mutations in exon 21 (Hsu et al., 2018).
Since 2009, the IPASS study (Mok et al., 2009) has validated the effectiveness of 1st-G TKIs for patients with EGFR-mutant NSCLC. Substantial advancements have been made in the first-line treatment of advanced EGFR-mutant NSCLC, particularly with the development of 3rd-G TKIs, as demonstrated by studies such as FLAURA (Soria et al., 2018), FLAURA China (Cheng et al., 2021), FURLONG (Shi et al., 2022), AENEAS (Lu et al., 2022) and LASER301 (Cho et al., 2023). These advancements have established 3rd-G TKIs as the standard of care. However, the resistance mechanisms to 3rd-G TKIs are complex, and subsequent treatment options remain limited (Fu et al., 2022). Additionally, the adoption of 3rd-G TKIs as first-line therapy has been constrained in certain regions due to lack of inclusion in insurance coverage, rendering it inaccessible for many patients. Moreover, the APPLE trial (Remon et al., 2024) indicated that sequential treatment with 1st-G TKIs followed by 3rd-G TKIs offers comparable overall survival (OS) benefits to upfront 3rd-G TKIs administration. This suggests a continued role for 1st-G TKIs and their combinations in clinical practice, highlighting the potential and necessity for combination therapies based on 1st-G TKIs.
Combination therapy with multiple drugs, particularly the addition of chemotherapy, is a pivotal approach to delaying the onset of drug resistance. Chemotherapy, as a systemic treatment, may eradicate subsets of cancer cells contributing to EGFR-TKIs resistance (Miller and Hanna, 2021). Several clinical studies have demonstrated that 1st-G TKIs + chemo significantly enhances efficacy compared to 1st-G TKIs alone. However, a direct comparison between 1st-G TKIs + chemo and 3rd-G TKIs has not yet been conducted.
To address this gap, we devised an indirect comparison using 1st-G TKIs as a bridge to compare the efficacy and safety of 1st-G TKIs + chemo versus 3rd-G TKIs in treating patients with advanced NSCLC harboring EGFR mutations. This study aims to provide additional comparative data to support informed decision-making in first-line therapy. Additionally, we performed subgroup analyses to identify patient populations that might derive greater benefit from 1st-G TKIs + chemo.
2 Methods
This meta-analysis followed the PRISMA (Page et al., 2021) guidelines and was registered on the PROSPERO website (CRD42023461565).
2.1 Data sources and searches
Relevant studies were comprehensively searched in PubMed, Embase, and Cochrane Library databases prior to 31 December 2023, using the following search terms: “epidermal growth factor receptor,” “non-small cell lung carcinoma,” “Gefitinib,” “Erlotinib,” “Icotinib,” “Chemotherapy,” “Osimertinib,” “Furmonertinib,” “Aumolertinib,” “Lazertinib,” “Olmutinib,” and “randomized clinical trial (RCT).” A detailed description of the retrieval method is provided in Supplementary Material 1. Two investigators (S.P and Z.Y) independently assessed the articles for eligibility, and disagreements between the investigators were resolved through further discussion with a third investigator.
2.2 Study selection
The inclusion criteria for study selection were as follows: 1) patients with advanced, EGFR-mutated NSCLC; 2) interventions: treatment with 1st-G TKIs + chemo versus a 1st-G TKIs as the control arm or treatment with a 3rd-G TKIs versus a 1st-G TKIs as the control arm; 3) Outcome Measures: inclusion of the hazard ratio (HR) and the corresponding 95% confidence interval (CI) for progression-free survival (PFS); and 4) randomized controlled trials.
2.3 Assessment of study quality
The risk of bias for each eligible study was evaluated using the Cochrane Risk of Bias Tool (Higgins et al., 2011).
2.4 Data extraction and outcomes
Two authors (S.P and Z.Y) independently extracted data and reached a consensus. The primary endpoint was PFS, while the secondary endpoints were OS, objective response rate (ORR), and grade ≥3 treatment-related adverse events (TRAEs). PFS and OS were collected as HRs with 95% CIs, while ORR and grade≥3 TRAEs were collected as the dichotomous data.
2.5 Data analyses
In the direct comparison of 1st-G TKIs + chemo or 3rd-G TKIs versus 1st-G TKIs, for continuous variables, which included PFS and OS, HRs with 95% CIs were assessed for each individual study. A meta-analysis of the HRs was performed using the inverse variance technique. Furthermore, for the analysis of ORRs, grade≥3 TRAEs, RRs, and corresponding 95% CIs were calculated for each study by adopting the Mantel-Haenszel method (DerSimonian and Laird, 1986). Heterogeneity was evaluated using Cochran’s Q test; a P value < 0.1 and I2 > 50% indicated statistical heterogeneity, thus requiring the use of a random-effects model; otherwise, a fixed-effects model was used (Cumpston et al., 2019).
When conducting an indirect comparison between 1st-G TKIs + chemo and 3rd-G TKIs, an adjusted indirect comparison was performed on arm A (1st-G TKIs + chemo) versus arm B (3rd-G TKIs), linked by arm C (1st-G TKIs), using the frequentist methods with the following formula (Bucher et al., 1997): log HRAB = log HRAC ˗ log HRBC, and its standard error (SE) for the log HR was SE (log HRAB) =
All statistical analyses were conducted using STATA software (version 17.0). Statistical significance was defined as a two-sided P-value < 0.05.
3 Results
3.1 Characteristics of the eligible studies
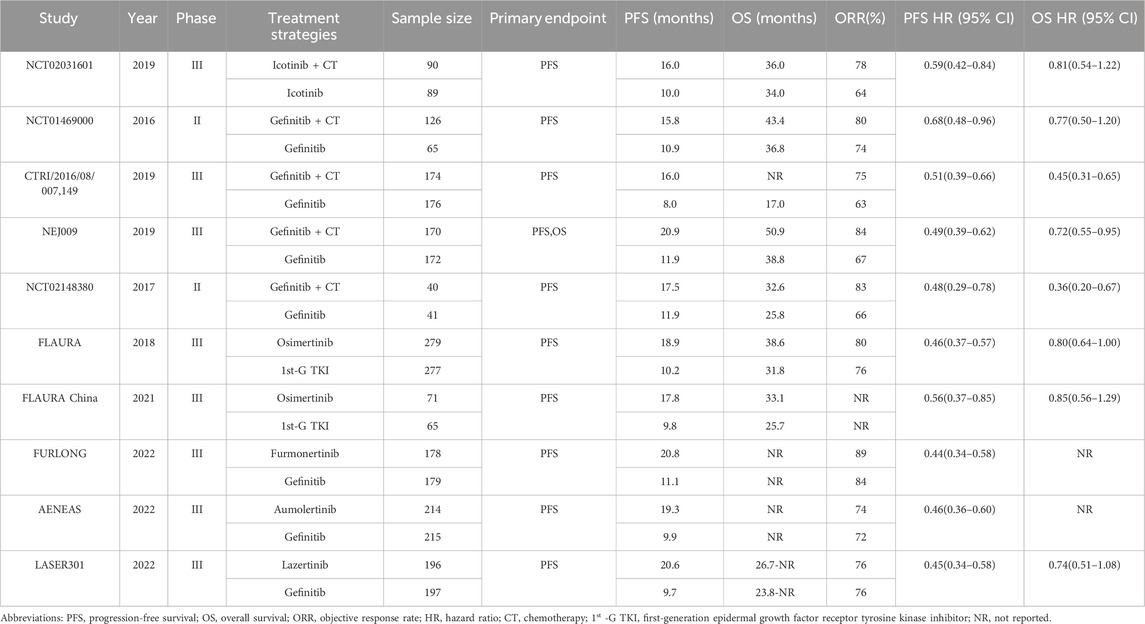
Ten RCTs (Soria et al., 2018; Cheng et al., 2021; Shi et al., 2022; Lu et al., 2022; Cho et al., 2023; Xu et al., 2019; Cheng et al., 2016; Noronha et al., 2020; Hosomi et al., 2020; Han et al., 2017) were deemed eligible for inclusion in the meta-analysis and included a total of 3014 patients. A brief summary of the included studies is presented in Table 1, and the search process is described in Figure 1. Of the included studies, five investigated the efficacy of 1st-G TKIs + chemo (n = 600) versus 1st-G TKIs (n = 543), while the remaining five trials (Soria et al., 2018; Cheng et al., 2021; Shi et al., 2022; Lu et al., 2022; Cho et al., 2023) explored 3rd-G TKIs (n = 938) versus 1st-G TKIs (n = 933). There were two randomized phase II Trials (Cheng et al., 2016; Han et al., 2017) and eight randomized phase III studies. The primary endpoint of all studies was PFS, and the PFS of 1st-G TKIs fluctuated between 8 and 11.9 months. The PFS durations observed for 1st-G TKIs + chemo spanned from 15.8 to 20.9 months, while the PFS profiles for 3rd-G TKIs ranged from 17.8 to 20.8 months.
A summary of the risk of bias evaluation is provided in Supplementary Material 2. The primary source of bias stemmed from the absence of blinding in clinical studies that compared 1st-G TKIs + chemo with 1st-G TKIs. Funnel plots are shown in Supplementary Material 3, with no asymmetry observed in the funnel plots. (PFS Egger test P = 0.107, OS Egger test P = 0.237, ORR Egger test P = 0.105, grade ≥3 TRAEs Egger test P = 0.164).
3.2 Direct comparisons between 1st-G TKIs + chemo or 3rd-G TKIs versus 1st-G TKIs
1st-G TKIs + chemo presented a significant improvement in PFS (HR, 0.54; 95%CI, 0.47 to 0.61, P < 0.001) (Figure 2A), OS (HR, 0.62; 95%CI, 0.47 to 0.81, P < 0.001) (Figure 2B), and ORR (RR, 1.21; 95%CI, 1.12 to 1.30, P < 0.001) (Figure 2C) compared to use of 1st-G TKIs as monotherapy. However, the risk of grade ≥3 TRAEs (RR, 2.24; 95%CI, 1.57 to 3.20, P < 0.001) (Figure 2D) of 1st-G TKIs + chemo was increased compared to 1st-G TKIs monotherapy.
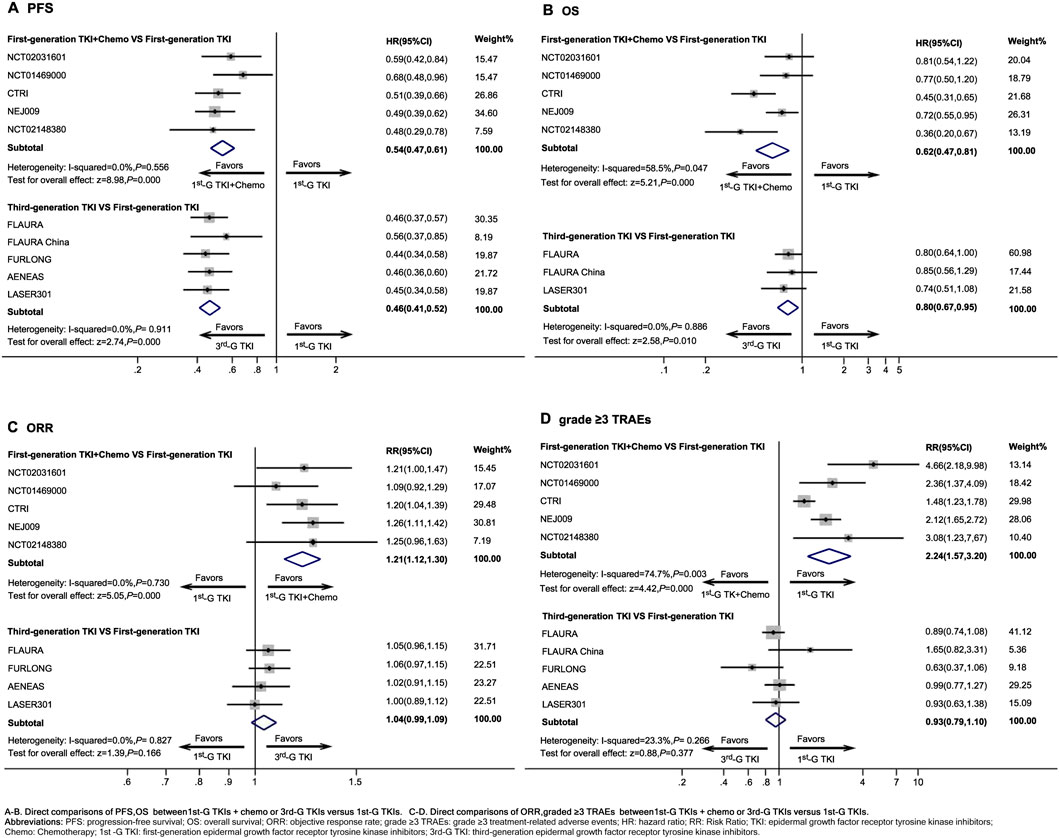
Figure 2. Direct comparisons between first-generation EGFR-TKIs plus chemotherapy or third-generation EGFR-TKIs versus first-generation EGFR-TKIs Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, objective response rate; grade ≥3 TRAEs, grade ≥3 treatment-related adverse events; HR, hazard ratio; RR: Risk Ratio; TKI, epidermal growth factor receptor tyrosine kinase inhibitors; Chemo, Chemotherapy; 1st-G TKI, first-generation epidermal growth factor receptor tyrosine kinase inhibitors; 3rd-G TKI, third-generation epidermal growth factor receptor tyrosine kinase inhibitors.
Compared with 1st-G TKIs, 3rd-G TKIs significantly improved PFS (HR, 0.46; 95%CI, 0.42 to 0.52, P < 0.001) (Figure 2A) and showed a significant improvement in OS (HR, 0.80; 95%CI, 0.67 to 0.95, P = 0.010) (Figure 2B), although there was no statistically significant difference in ORR (RR, 1.04; 95%CI, 0.99 to 1.09, P = 0.166) (Figure 2C). The risk of grade ≥3 TRAEs (RR, 0.93; 95%CI, 0.79 to 1.10, P = 0.377) (Figure 2D) was similar between 1st-G TKIs and 3rd-G TKIs regimens.
3.3 Indirect comparisons between 1st-G TKIs + chemo and 3rd-G TKIs
1st-G TKIs + chemo presented numerically poorer PFS than 3rd-G TKIs regimens (HR, 1.17; 95%CI, 0.98 to 1.40, P = 0.075) (Figure 3A), although this difference lacks statistical significance. Across most subgroups, a shorter PFS was observed in 1st-G TKIs + chemo compared to the 3rd-G TKIs, particularly in patients younger than 65 years of age (HR, 1.40; 95%CI, 1.07 to 1.85, P = 0.02), patients with no history of smoking (HR, 1.32; 95%CI, 1.02 to 1.71, P = 0.04), patients with a PS score of 0 (HR, 1.49; 95%CI, 1.06 to 2.08, P = 0.02), and patients with EGFR exon 19 deletion mutations (HR, 1.32; 95%CI, 1.03 to 1.68, P = 0.03) (Figure 3B). However, in patients with brain metastases (HR, 0.85; 95%CI, 0.55 to 1.32, P = 0.46) (Figure 3B), the efficacy of 1st-G TKIs + chemo was numerically longer than that of 3rd-G TKIs, without statistical significance.
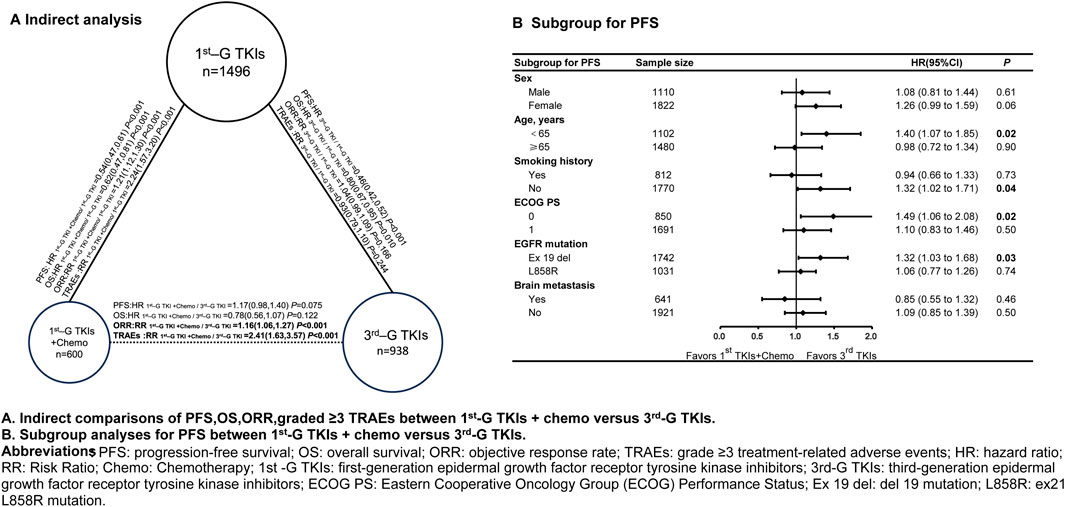
Figure 3. Indirect comparisons and subgroup analyses for PFS between first-generation EGFR-TKIs plus chemotherapy and third-generation EGFR-TKIs Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, objective response rate; TRAEs, grade ≥3 treatment-related adverse events; HR, hazard ratio; RR, Risk Ratio; Chemo, Chemotherapy; 1st -G TKI, first-generation epidermal growth factor receptor tyrosine kinase inhibitors; 3rd-G TKI, third-generation epidermal growth factor receptor tyrosine kinase inhibitors; ECOG PS, Eastern Cooperative Oncology Group (ECOG) Performance Status; Ex 19 del, del 19 mutation; L858R, ex21 L858R mutation.
Conversely, 1st-G TKIs combined with chemotherapy produced a numerically lower risk of death than 3rd-G TKIs (OS: HR 0.78, 95% CI 0.56–1.07; P = 0.122), but this difference did not reach statistical significance (Figure 3A). The combination did, however, yield a significantly higher objective response rate (ORR: RR 1.16, 95% CI 1.06–1.27; P < 0.001) and was associated with a markedly increased incidence of grade ≥3 treatment-related adverse events (RR 2.41, 95% CI 1.63–3.57; P < 0.001) (Figure 3A).
4 Discussion
To the best of our knowledge, this is the first meta-analysis to perform an indirect comparison between 1st-G TKIs + chemo and 3rd-G TKIs alone. Our findings indicate that PFS and OS were comparable between these two regimens in patients with EGFR-mutant NSCLC. However, the 1st-G TKIs + chemo significantly increased the ORR by 16%, at the cost of a marked increase in the risk of grade ≥3 TRAEs (RR, 2.41, P < 0.001).
From the meta-analysis of the direct comparison between 1st-G TKIs + chemo and the 1st-G TKIs, 1st-G TKIs + chemo presented significantly longer PFS and OS than 1st-G TKIs alone, in agreement with the findings of previous meta-analyses (Wu et al., 2021). A possible reason for this is that some patients treated with 1st-G TKIs alone did not receive subsequent chemotherapy after disease progression, for various reasons. Moreover, early combination chemotherapy can delay resistance to EGFR-TKIs and improve the survival rate of patients compared to sequential chemotherapy (Chang et al., 2021). The possible mechanism is that tumors with acquired drug resistance may contain a mixed cell population of drug-sensitive and drug-resistant cells with different growth rates, and early combined chemotherapy can better eliminate this heterogeneity (Moore and Wheatley-Price, 2021).
Furthermore, in the indirect comparison, when the 1st-G TKIs was replaced with the current preferred 3rd-G TKIs, the PFS and OS benefits of 1st-G TKIs + chemo compared to 3rd-G TKIs were comparable, indicating that1st-G TKIs + chemo has potential as a first-line treatment. However, it is important to note that in studies of 1st-G TKIs + chemo, only 23.3% of patients subsequently received 3rd-G TKIs treatment (Hosomi et al., 2020). If more patients were able to receive 3rd-G TKIs after progression, the results of 1st-G TKIs + chemo would likely be even more pronounced.
Despite these potential benefits, 1st-G TKIs + chemo has several notable drawbacks. These include increased adverse reactions and the possibility of rapid disease progression or death in some patients, preventing them from accessing 3rd-G TKIs. Therefore, careful consideration and patient selection are crucial. Identifying subgroups of patients who are more likely to benefit from 1st-G TKIs + chemo is essential to optimize treatment outcomes.
From the results of the primary endpoint, 1st-G TKIs + chemo presented a statistically significant advantage in terms of ORR compared with 3rd-G TKIs. This indicates that the use of 1st-G TKIs + chemo can effectively reduce the tumor burden and is suitable for patients requiring rapid tumor shrinkage. Based on the subgroup analysis, it is noteworthy that, among patients with baseline brain metastases, first-generation EGFR-TKI plus chemotherapy yielded numerically longer progression-free survival (HR 0.85, 95% CI 0.55–1.32; P = 0.46); however, this difference was not statistically significant, and no superiority over third-generation EGFR-TKIs can be claimed. Osimertinib and other third-generation TKIs are known to reach higher cerebrospinal-fluid concentrations than first-generation compounds (Popat et al., 2023), and any apparent advantage of adding chemotherapy remains speculative and requires dedicated pharmacokinetic investigation. Furthermore, our meta-analysis lacked individual-patient data on intracranial progression; future studies should report CNS-specific endpoints such as CNS progression-free survival or time-to-brain-progression to determine the relative efficacy of these strategies in patients with brain metastases.
In other subgroups, including patients younger than 65 years, non-smokers, those with an ECOG PS of 0, and those with EGFR exon 19 deletion mutations, 3rd-G TKIs were the preferred treatment. EGFR mutation type is a crucial biomarker for treating NSCLC with EGFR-TKIs. Compared to 1st-G TKIs + chemo, patients with EGFR exon 19 deletion achieved a superior PFS (HR, 1.32; 95% CI, 1.03–1.68) when treated with 3rd-G TKIs as monotherapy, while patients with exon 21 mutation showed comparable PFS (HR, 1.06; 95% CI, 0.77–1.39) when treated with 1st-G TKIs + chemo and 3rd-G TKIs. This finding could be attributed to the higher incidence of the T790M mutation and the lower incidence of concomitant mutations in patients with EGFR exon 19 deletions than in those with EGFR exon 21 mutations, therefore, the selection of a 3rd-G TKIs could directly overcome the T790M mutation, thereby extending PFS (Hong et al., 2018). Moreover, In patients aged <65 years, never-smokers, and ECOG 0, several biological and clinical factors may underlie the observed superiority of third-generation EGFR-TKIs. First, never-smokers harbour markedly fewer tobacco-related passenger mutations and a lower prevalence of resistance-associated co-mutations such as TP53, STK11 and RB1, which are enriched in smoking-related tumours and accelerate escape from EGFR blockade (Moorthi et al., 2024; Labbe et al., 2017). Second, the absence of heavy mutagenic exposure yields a comparatively low tumour-mutational burden, reinforcing ‘oncogene addiction’ to mutant EGFR and prolonging sensitivity to potent third-generation inhibitors (Rudin et al., 2009). Third, patients with ECOG 0 generally tolerate therapy without dose reduction, preserving full osimertinib exposure; dose-intensity analyses from AURA3 and real-world series confirm that optimal pharmacokinetics translate into deeper and more durable responses (Papadimitrakopoulou et al., 2020; Ramalingam et al., 2020). Although compelling, these subgroup findings remain exploratory and should be validated prospectively, ideally in trials stratified by co-mutation status and performance score.
The recent study FLAURA2 (Planchard et al., 2023) that compared osimertinib combined with chemotherapy to 1st-G TKIs monotherapy revealed that the osimertinib regimen presented a significant improvement in progression-free survival (PFS) (25.5 months vs. 16.7 months) among patients with advanced NSCLC and EGFR mutations. This suggests that combining 3rd-G TKIs and chemotherapy may offer a new treatment option for these patients. However, subsequent OS data must be considered to determine this regimen’s clinical significance. The combination of EGFR-TKI with other drugs will be a focus of future research and may impact the current standard of using 3rd-G TKIs as a first-line therapy.
Based on the results of our current study, considering both efficacy and safety data, 1st-G TKIs + chemo did not present superior performance over 3rd-G TKIs. However, 1st-G TKIs + chemo is a potential choice for patients with brain metastases or those who require high response rates.
Our study has some limitations that should be noted when interpreting our findings. First, the number of included studies was relatively small, and only two phase II studies were present in our dataset, which reduced the level of evidence. Second, no direct comparative study was available. In addition, OS and ORR were not the primary endpoints; thus, the data may be considered immature. Finally, we were unable to further compare 1st-G TKIs + chemo and 3rd-G TKIs + chemo groups.
In this meta-analysis, 1st-G TKIs + chemo regimens presented PFS and OS comparable to those of 3rd-G TKIs, with a statistically significant improvement in ORR. However, the risk of grade ≥3 TRAEs significantly increased. This suggests that in clinical practice, for patients with EGFR mutations, if we intend to use 1st-G TKIs + chemo as a first-line treatment, further population screening is imperative to determine the optimal medication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SP: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review and editing. ZY: Investigation, Methodology, Software, Supervision, Writing – review and editing. HZ: Formal Analysis, Investigation, Validation, Writing – original draft. CL: Methodology, Software, Validation, Writing – review and editing. HQ: Resources, Supervision, Writing – original draft. SH: Conceptualization, Project administration, Visualization, Writing – review and editing. YZ: Funding acquisition, Software, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by grants 2022A1515010386 from Guangdong Basic and Applied Basic Research Foundation, 82272837 from the National Natural Science Funds of China; and 2023A04J2130 from Guangzhou Basic and Applied Basic Research project. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1586332/full#supplementary-material
References
Bucher, H. C., Guyatt, G. H., Griffith, L. E., and Walter, S. D. (1997). The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 50 (6), 683–691. doi:10.1016/s0895-4356(97)00049-8
Chang, Q., Xu, J., Qiang, H., Teng, J., Qian, J., Lv, M., et al. (2021). EGFR tyrosine Kinase Inhibitor (TKI) combined with concurrent or sequential chemotherapy for patients with advanced lung cancer and gradual progression after first-line EGFR-TKI therapy: a randomized controlled Study. Clin. Lung Cancer 22 (3), e395–e404. doi:10.1016/j.cllc.2020.06.005
Cheng, Y., Murakami, H., Yang, P.-C., He, J., Nakagawa, K., Kang, J. H., et al. (2016). Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous Non–Small-Cell lung cancer with activating epidermal growth factor receptor mutations. J. Clin. Oncol. 34 (27), 3258–3266. doi:10.1200/JCO.2016.66.9218
Cheng, Y., He, Y., Li, W., Zhang, H. L., Zhou, Q., Wang, B., et al. (2021). Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-Mutated advanced NSCLC: FLAURA China, A randomized Study. Target. Oncol. 16 (2), 165–176. doi:10.1007/s11523-021-00794-6
Cho, B. C., Ahn, M.-J., Kang, J. H., Soo, R. A., Reungwetwattana, T., Yang, J. C. H., et al. (2023). Lazertinib versus gefitinib as first-line treatment in patients with EGFR-Mutated advanced Non–Small-Cell lung cancer: results from LASER301. J. Clin. Oncol. 41 (26), 4208–4217. doi:10.1200/JCO.23.00515
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane database Syst. Rev. 10 (10), ED000142. doi:10.1002/14651858.ED000142
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control Clin. Trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
Fu, K., Xie, F., Wang, F., and Fu, L. (2022). Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. and Oncol. 15 (1), 173. doi:10.1186/s13045-022-01391-4
Han, B., Jin, B., Chu, T., Niu, Y., Dong, Y., Xu, J., et al. (2017). Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int. J. Cancer 141 (6), 1249–1256. doi:10.1002/ijc.30806
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 553 (7689), 446–454. doi:10.1038/nature25183
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343 (oct18 2), d5928–d. doi:10.1136/bmj.d5928
Hong, S., Gao, F., Fu, S., Wang, Y., Fang, W., Huang, Y., et al. (2018). Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-Mutant advanced non-small cell lung cancer. JAMA Oncol. 4 (5), 739–742. doi:10.1001/jamaoncol.2018.0049
Hosomi, Y., Morita, S., Sugawara, S., Kato, T., Fukuhara, T., Gemma, A., et al. (2020). Gefitinib Alone versus gefitinib plus chemotherapy for Non–Small-Cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J. Clin. Oncol. 38 (2), 115–123. doi:10.1200/JCO.19.01488
Hsu, W. H., Yang, J. C. H., Mok, T. S., and Loong, H. H. (2018). Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 29, i3–i9. doi:10.1093/annonc/mdx702
Labbe, C., Cabanero, M., Korpanty, G. J., Tomasini, P., Doherty, M. K., Mascaux, C., et al. (2017). Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer 111, 23–29. doi:10.1016/j.lungcan.2017.06.014
Lu, S., Dong, X., Jian, H., Chen, J., Chen, G., Sun, Y., et al. (2022). AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or MetastaticNon–Small-Cell lung cancer with EGFR Exon 19 deletion or L858R mutations. J. Clin. Oncol. 40 (27), 3162–3171. doi:10.1200/JCO.21.02641
Miller, M., and Hanna, N. (2021). Advances in systemic therapy for non-small cell lung cancer. Bmj 375, n2363. doi:10.1136/bmj.n2363
Mok, T. S., Wu, Y.-L., Thongprasert, S., Yang, C. H., Chu, D. T., Saijo, N., et al. (2009). Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361 (10), 947–957. doi:10.1056/NEJMoa0810699
Moore, S., and Wheatley-Price, P. (2021). EGFR combination therapy should become the new standard first-line treatment in advanced EGFR-Mutant NSCLC. J. Thorac. Oncol. 16 (11), 1788–1792. doi:10.1016/j.jtho.2021.06.004
Moorthi, S., Paguirigan, A., Itagi, P., Ko, M., Pettinger, M., Hoge, A. C., et al. (2024). The genomic landscape of lung cancer in never-smokers from the Women's Health Initiative. JCI Insight 9 (17), e174643. doi:10.1172/jci.insight.174643
Noronha, V., Patil, V. M., Joshi, A., Menon, N., Chougule, A., Mahajan, A., et al. (2020). Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-Mutated lung cancer. J. Clin. Oncol. 38 (2), 124–136. doi:10.1200/JCO.19.01154
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Papadimitrakopoulou, V. A., Mok, T. S., Han, J. Y., Ahn, M. J., Delmonte, A., Ramalingam, S. S., et al. (2020). Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 31 (11), 1536–1544. doi:10.1016/j.annonc.2020.08.2100
Planchard, D., Janne, P. A., Cheng, Y., Yang, J. C. H., Yanagitani, N., Kim, S. W., et al. (2023). Osimertinib with or without chemotherapy in EGFR-Mutated advanced NSCLC. N. Engl. J. Med. 389 (21), 1935–1948. doi:10.1056/NEJMoa2306434
Popat, S., Ahn, M.-J., Ekman, S., Leighl, N. B., Ramalingam, S. S., Reungwetwattana, T., et al. (2023). Osimertinib for EGFR-Mutant non-small-cell lung cancer central nervous System metastases: current evidence and future perspectives on therapeutic strategies. Target. Oncol. 18 (1), 9–24. doi:10.1007/s11523-022-00941-7
Ramalingam, S. S., Vansteenkiste, J., Planchard, D., Cho, B. C., Gray, J. E., Ohe, Y., et al. (2020). Overall survival with osimertinib in untreated, EGFR-Mutated advanced NSCLC. N. Engl. J. Med. 382 (1), 41–50. doi:10.1056/NEJMoa1913662
Remon, J., Besse, B., Aix, S. P., Callejo, A., Al-Rabi, K., Bernabe, R., et al. (2024). Overall survival from the EORTC LCG-1613 APPLE trial of osimertinib versus gefitinib followed by osimertinib in advanced EGFR-Mutant Non–Small-Cell lung cancer. J. Clin. Oncol. 42 (12), 1350–1356. doi:10.1200/JCO.23.01521
Rudin, C. M., Avila-Tang, E., Harris, C. C., Herman, J. G., Hirsch, F. R., Pao, W., et al. (2009). Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin. Cancer Res. 15 (18), 5646–5661. doi:10.1158/1078-0432.CCR-09-0377
Shi, Y., Chen, G., Wang, X., Liu, Y., Wu, L., Hao, Y., et al. (2022). Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir. Med. 10 (11), 1019–1028. doi:10.1016/S2213-2600(22)00168-0
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in untreated EGFR-Mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
Wu, Q., Luo, W., Li, W., Wang, T., Huang, L., and Xu, F. (2021). First-Generation EGFR-TKI plus chemotherapy versus EGFR-TKI alone as first-line treatment in advanced NSCLC with EGFR activating mutation: a systematic review and meta-analysis of randomized controlled trials. Front. Oncol. 11, 598265. doi:10.3389/fonc.2021.598265
Keywords: EGFR mutant, chemotherapy, targeted therapy, advanced non small cell lung cancer, combined therapy
Citation: Peng S, Yu Z, Zhu H, Liang C, Qiu H, Hong S and Zhou Y (2025) Comparative analysis of first-generation epidermal growth factor receptor inhibitors combined with chemotherapy versus third-generation epidermal growth factor receptor inhibitors in the treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis. Front. Pharmacol. 16:1586332. doi: 10.3389/fphar.2025.1586332
Received: 12 March 2025; Accepted: 31 July 2025;
Published: 14 August 2025.
Edited by:
Jingrui Huang, Central South University, ChinaReviewed by:
Kenji Morimoto, Kyoto Prefectural University of Medicine, JapanHesong Wang, Fourth Hospital of Hebei Medical University, China
Copyright © 2025 Peng, Yu, Zhu, Liang, Qiu, Hong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaodong Hong, aG9uZ3NoZEBzeXN1Y2Mub3JnLmNu; Yixin Zhou, emhvdXl4QHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
 Siyan Peng
Siyan Peng Zhixin Yu1,2†
Zhixin Yu1,2† Chuwen Liang
Chuwen Liang Shaodong Hong
Shaodong Hong Yixin Zhou
Yixin Zhou