- Department of Environmental Physiology, Faculty of Medicine, Shimane University, Izumo, Shimane, Japan
Vasospasm is a sustained abnormal contraction of vascular smooth muscle (VSM), which is commonly observed in the coronary and cerebral arteries. This abnormal VSM contraction leads to reduced blood flow to tissues or organs, ultimately causing severe diseases such as myocardial infarction and cerebral infarction. Studies have demonstrated that oxidative stress and sphingosylphosphorylcholine (SPC)-induced Rho-kinase signaling pathways are related to this abnormal contraction. Flavonoids, a class of natural compounds, are found in various plants, fruits, vegetables, and traditional Chinese medicines. They have anti-inflammatory, antioxidative, and anticarcinogenic properties. Recent studies have shown that some flavonoids strongly inhibit the abnormal contraction of VSM. This review explores the potential of flavonoids as candidate drugs for the treatment and prevention of vasospasm through oxidative stress and the SPC-induced Rho-kinase signaling pathway. Nevertheless, more extensive studies are required to fully elucidate the mechanism by which flavonoids exert their anti-vasospastic effects and explore their potential benefits as adjunctive therapy for critical cardiovascular and cerebrovascular diseases.
1 Introduction
Vasospasm, such as coronary vasospasm and cerebral vasospasm, is the abnormal contraction of vascular smooth muscle (VSM) leading to blood vessel narrowing. This narrowing reduces blood flow, resulting in serious cardiovascular and cerebrovascular diseases, such as myocardial infarction, angina, and cerebral infarction (Hung et al., 2014; Slavich and Patel, 2016; Maruhashi and Higashi, 2021; Sinha et al., 2022). The underlying pathophysiology of vasospasm involves multiple factors, including endothelial dysfunction, autonomic nervous system imbalances, vascular smooth muscle hypercontractility, and activated signaling pathway of vasoactive mediators (Kolias et al., 2009; Matta et al., 2020). Although the pathogenesis of vasospasm has not been fully elucidated, endothelial dysfunction and increased VSM contractility are considered to be the main underlying mechanisms (Kusama et al., 2011; Franczyk et al., 2022). Oxidative stress plays an important role in vasospasm by promoting endothelial dysfunction, activating vasoconstrictors such as endothelin-1 (ET-1), and impairing the nitric oxide (NO) vasodilator system (Ruef et al., 2001; Higashi et al., 2009; Higashi, 2022). Sphingosylphosphorylcholine (SPC) is an active sphingolipid that induces the Rho-kinase signaling pathway involved in abnormal contraction of VSM (Todoroki-Ikeda et al., 2000; Shirao et al., 2002). So far, there is no effective treatment for vasospasm. Therefore, understanding the underlying mechanism of vasospasm and finding new therapeutic strategies are crucial for treating vasospasm-associated diseases.
Recent studies have found that natural compounds with antioxidant properties can reduce oxidative stress and improve vascular function (Mukherjee et al., 2024). Among them, flavonoids, widely distributed in plants and traditional Chinese medicine herbs, have shown promise in protecting against oxidative damage, restoring vascular homeostasis, and preventing vasospasm (Xu et al., 2022; Li R. L. et al., 2023). Some evidence suggests that flavonoids reduce the risk of cardiovascular diseases and have cardiovascular protective effects (Erdman et al., 2007; Horakova, 2011). Quercetin, kaempferol, and catechins can relax the smooth muscle of the coronary artery, thereby reducing the incidence of coronary vasospasm (Xu et al., 2015; Mangels and Mohler, 2017; Dagher et al., 2021). Flavonoids have also been found to improve endothelial function and reduce mortality of cardiovascular diseases (Perez-Vizcaino et al., 2006; Yamagata and Yamori, 2020). In addition, they contribute to regulate cholesterol levels, lower blood pressure, and reduce the risk of thrombosis (Ciumarnean et al., 2020; Kozlowska and Szostak-Wegierek, 2022). A recent study showed that flavonoids effectively prevent brain damage caused by intracerebral hemorrhage and subarachnoid hemorrhage (SAH) by inhibiting inflammation and oxidative stress (Dong et al., 2024). Furthermore, hesperetin and tangeretin significantly inhibit SPC-induced abnormal contraction (Li et al., 2022; Lu et al., 2022).
In recent years, there has been an increasing interest in how oxidative stress affects vascular function. At the same time, antioxidants have been widely used as adjuvant therapy for a variety of clinical diseases. A deeper understanding of the mechanisms regulating abnormal vascular contraction is essential for further revealing the pathophysiology of cardiovascular and cerebrovascular diseases. This review comprehensively explores the mechanism of action of oxidative stress and SPC in vasospasm and summarizes the research progress on the regulation of vascular tension by natural flavonoids. This article aims to promote in-depth research in this field and provide effective intervention strategies for improving and preventing coronary artery spasm and cerebral vasospasm.
2 Roles of oxidative stress and SPC in vasospasm
2.1 Role of oxidative stress in vasospasm
Oxidative stress is a state of imbalance between the generation and accumulation of reactive oxygen species (ROS) and the clearance of these ROS in cells and tissues (Vona et al., 2021). Under pathological conditions, such as brain injury and SAH, oxidative stress impairs endothelial function and increases the production of ROS during hemoglobin degradation, mitochondrial dysfunction, and disrupted antioxidant systems (Ayer and Zhang, 2008; Hao et al., 2022). These processes result in vasoconstriction, increased vascular resistance, and a propensity for vasospasm. Studies have demonstrated that oxidative stress contributes to cerebral vasospasm after SAH (Macdonald and Weir, 1994; Kim et al., 2002; Wu F. et al., 2021; Hao et al., 2022). Increased levels of superoxide anions in the cerebrospinal fluid following SAH have been reported to be associated with cerebral vasospasm (Fumoto et al., 2019). In animal models of SAH, accumulating evidence has shown that free radical scavengers, such as iron chelators, ebselen, U74006F, and inhibitors of free radical-generating enzymes attenuate cerebral vasospasm (Matsui and Asano, 1994; Watanabe et al., 1997; Horky et al., 1998; Handa et al., 2000; Zheng et al., 2005). Maeda Y et al. demonstrated that oxidative stress significantly impairs bradykinin-induced, endothelium-dependent relaxation in bovine middle cerebral arteries. (Maeda et al., 2004). In addition, oxidative stress contributes to smooth muscle cell proliferation and hypertrophy, as well as endothelial cell apoptosis (Satoh et al., 2010). Research is ongoing to further elucidate how oxidative stress affects cerebral vasoconstrictor responses and contributes to the development of vasospasm.
2.2 Oxidative stress-related signaling pathways in vasospasm
Oxidative stress activates multiple molecular pathways that regulate vascular tone, playing a crucial role in the development of vasospasm. The production of ROS during oxidative stress damages endothelial cells, leading to endothelial dysfunction and impairing the endothelium’s ability to produce vasodilators such as NO (Shaito et al., 2022), which contributes to vasoconstriction and the development of vasospasm. Additionally, ROS enhance the expression of ET-1, and elevated ET-1 levels are closely associated with vasospasm (Saitoh et al., 2009). ROS also activate NF-κB, which triggers the expression of pro-inflammatory cytokines and adhesion molecules, further exacerbating endothelial dysfunction and vascular constriction (Scioli et al., 2020). Increased oxidative stress can also activate the RhoA/Rho-kinase signaling pathway (de Souza et al., 2016), a key factor in the pathophysiology of cerebral vasospasm after SAH (Naraoka et al., 2013). This activation, along with mechanical stress and hypotonic conditions, influences paxillin phosphorylation and RhoA translocation (Lopez-Colome et al., 2017). Accumulating studies have emphasized Fyn expression is upregulated and activated by oxidative stress (Gao et al., 2009; Giannoni and Chiarugi, 2014; Matsushima et al., 2016). Additionally, ROS activate the mitogen-activated protein kinase (MAPK) pathway, leading to the phosphorylation of proteins that promote vasoconstriction and inflammation (Higashi, 2022). These findings demonstrate that oxidative stress triggers various molecular pathways and activates vasoconstriction-related proteins, which play an important role in the onset and progression of vasospasm (Figure 1).
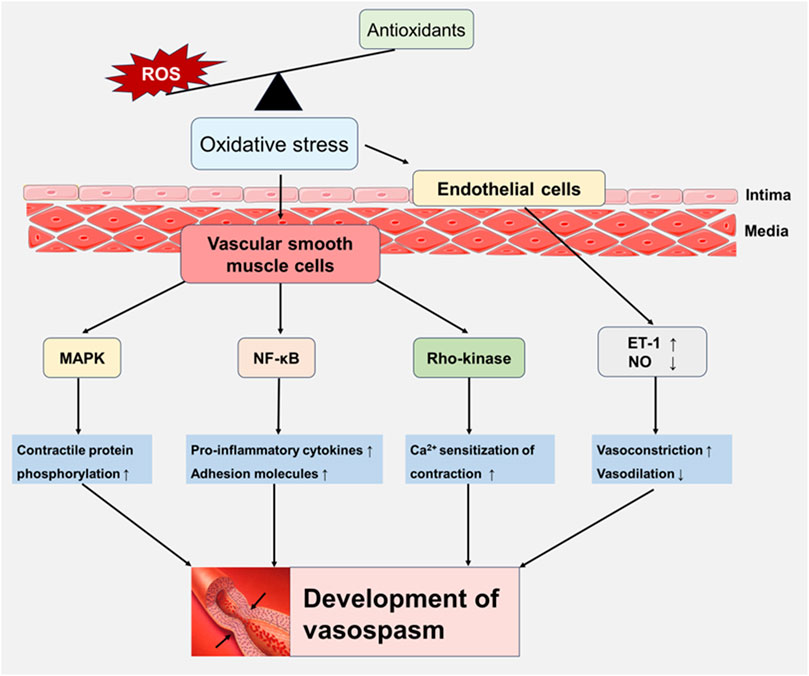
Figure 1. Oxidative stress-induced signaling pathways involved in vasospasm. Oxidative stress damages endothelial cell function and increases the expression of endothelin-1 (ET-1) by increasing the generation of reactive oxygen species (ROS), while inhibiting the vasodilator factor NO, increasing vasoconstriction and weakening vasodilation. In addition, ROS activate key signaling pathways of vascular smooth muscle cells, including NF-κB, Rho-kinase, and MAPK, leading to inflammation and vascular dysfunction, playing a key role in development of vasospasm.
Additionally, increasing evidence indicates that mitochondrial dysfunction–induced oxidative stress may play a critical role in the development of vasospasm (Jacobsen et al., 2014; Zhang et al., 2022). Mitochondria are essential for cellular energy metabolism and redox homeostasis, and mitochondrial dysfunction can lead to oxidative stress, impaired bioenergetics, and vascular dysregulation (Zong et al., 2024). Mitochondria are the main source of ROS, mainly produced in complexes I and III of the electron transport chain (Angelova and Abramov, 2018; Zhao R. Z. et al., 2019). Under pathological conditions, impaired mitochondrial dynamics lead to elevated mitochondrial ROS, which in turn reduces the bioavailability of NO by reacting with it to form peroxynitrite (Prajapat et al., 2024), ultimately causing impaired endothelium-dependent vasodilation and contributing to the occurrence of vasospasm. Increased mitochondrial ROS and calcium overload in cerebral arteries of SAH patients suggest that they may aggravate delayed cerebral vasospasm (Zhang et al., 2022). In addition, oxidative stress induced by mitochondrial dysfunction leads to reduced ATP synthesis (Bhatti et al., 2017), which in turn affects the sarcoplasmic or endoplasmic reticulum Ca2+-ATPase (SERCA) pump’s ability to actively transport Ca2+ from the cytosol into the lumen of the sarcoplasmic reticulum and endoplasmic reticulum (Wu et al., 2001), a process that is critical for maintaining low cytoplasmic calcium levels and promoting muscle relaxation, thereby impairing vascular tone regulation and possibly inducing vasospasm.
2.3 The role of SPC in vasospasm
Sphingosylphosphorylcholine (SPC) is a naturally occurring bioactive sphingolipid in blood plasma that has emerged as an important modulator of cardiovascular functions (Ge et al., 2018). Under physical conditions, circulating SPC levels are about 50 nM in plasma and 130 nM in serum (Liliom et al., 2001). SPC acts both as an extracellular first messenger via G protein-coupled receptors (such as S1P1–3 and GPR12) (Meyer zu Heringdorf and Jakobs, 2007; Ge et al., 2018) and as an intracellular second messenger by directly regulating the ryanodine receptor in cardiomyocytes (Uehara et al., 1999; Yasukochi et al., 2003). In the heart, SPC regulates Na+ and Ca2+ currents (Yasui and Palade, 1996) and protects cardiomyocytes against ischemia/reperfusion-induced apoptosis and inflammation through autophagy mediated by the lipid raft/PTEN/Akt/mTOR pathway (Yue et al., 2015). In vascular endothelial cells, SPC at low concentrations (≤10 μM) exhibits anti-apoptotic and anti-inflammatory effects (Ge et al., 2011), whereas at higher concentrations (≥10 μM), it may induce oxidative stress and inflammation (Jeon et al., 2007). In vascular smooth muscle cells, SPC promotes cell migration (Boguslawski et al., 2002; Zhang et al., 2021) and Ca2+ sensitization through the Src/Rho-kinase pathway (Nakao et al., 2002), contributing to vascular remodeling and vasospasm. Furthermore, SPC induces the differentiation of mesenchymal stem cells into smooth muscle-like cells (Jeon et al., 2006), suggesting a potential role in vascular repair. In summary, SPC exerts multifaceted effects on the cardiovascular system. In this review, we focus on the role of SPC in the abnormal contraction of vascular smooth muscle.
Under normal physiological conditions, VSM contraction plays a crucial role in maintaining blood pressure, blood flow, and vascular tone. This contraction is dependent on Ca2+ (Somlyo and Somlyo, 1994) and can be triggered by mechanical, electrical, or chemical stimuli through the Ca2+/calmodulin (CaM)-myosin light chain kinase (MLCK) signaling pathway (Kamm and Stull, 1985). When vascular smooth muscle cells are stimulated, the intracellular Ca2+ concentration increases, binding to CaM to form a Ca2+/CaM complex. This complex induces a conformational change in MLCK, thereby activating it (Kemp and Pearson, 1991). Activated MLCK then phosphorylates myosin light chain (MLC), promoting the formation of actin-myosin crossbridges, leading to muscle contraction (Kamm and Stull, 1985). However, the Ca2+-independent mechanism has also been reported to contribute to VSM contraction. The pathways involved including RhoA-Rho-kinase (Kureishi et al., 1997; Mizuno et al., 2008), protein kinase C (Walsh et al., 1996; Dimopoulos et al., 2007), MAPK signaling (Cain et al., 2002), and ROS (Jin et al., 2004). These signals ultimately phosphorylate myosin phosphatase targeting subunit 1 (MYPT1), a subunit of myosin light chain phosphatase (MLCP), reducing MLCP activity and preventing the dephosphorylation of MLC (Alvarez-Santos et al., 2020). As a result, MLC remains in a sustaining phosphorylated state, promoting actin-myosin crossbridge formation and muscle contraction, independent of calcium ion (Webb, 2003; Hirano, 2007). Vasospasm is considered a pathological condition characterized by sustained vascular hyperresponsiveness or Ca2+-sensitization of VSM contraction (Tanaka et al., 1998; Dimopoulos et al., 2007). SPC has been identified as a key bioactive lipid mediator that activates Rho-kinase and plays a central role in vasospasm. A study by Kurokawa T et al. showed that SPC concentration is significantly elevated in the cerebrospinal fluid of patients with cerebral vasospasm (Kurokawa et al., 2009). Additionally, injecting SPC into the cisterna magna of the cerebellum and medulla oblongata induces significant and prolonged vasospasm in the canine basilar artery (Shirao et al., 2008). Furthermore, SPC has been shown to induce smooth muscle contraction in various vascular tissues, including the cerebral and coronary arteries, as well as human coronary artery smooth muscle cells (Shirao et al., 2002; Zhang et al., 2017; Li et al., 2022; Lu et al., 2022; Zhang et al., 2024).
2.4 The SPC-induced signaling pathway involved in vasospasm
Early studies have demonstrated that the Rho-kinase pathway is significantly activated in cerebral arterial smooth muscle during cerebral vasospasm, a severe complication following SAH (Sato M. et al., 2000; Wang et al., 2014; Hu et al., 2023). This finding suggests that Rho-kinase plays a crucial role in the pathogenesis of vasospasm, contributing to sustained smooth muscle contraction. Shirao S et al. reported that SPC activates Rho-kinase, leading to Ca2+-independent contraction in bovine cerebral artery VSM strips (Shirao et al., 2002). A specific Rho-kinase inhibitor, Y27632, along with a dominant-negative Rho-kinase construct, effectively abolished the abnormal contraction of VSM strips induced by SPC (Shirao et al., 2002). Furthermore, SPC stimulation also triggers the translocation of Rho-kinase from the cytoplasm to the cell membrane in VSM cells (Zhang et al., 2017; Li et al., 2022; Lu et al., 2022). These results suggest that Rho-kinase is a downstream molecule in the SPC-induced signaling pathway, which is involved in abnormal contraction.
Fyn is a member of the Src family of non-receptor tyrosine kinases, which plays an important role in cellular signaling, including cell growth, differentiation, and motility (Peng and Fu, 2023). Fyn is also involved in several pathways that regulate smooth muscle contraction, and recent studies have highlighted its role in mediating SPC-induced abnormal contraction in vascular smooth muscle cells (VSMCs). Inhibition of Fyn activity using specific Fyn inhibitors decreased the contraction of VSM strips exposed to SPC, highlighting the importance of Fyn in mediating SPC-induced VSM contraction (Nakao et al., 2002; Lu et al., 2022). Further study found that specific Fyn inhibitors such as PP1 and EPA blocked the activation and translocation of Rho-kinase (Nakao et al., 2002). These findings collectively suggest that Fyn and Rho-kinase play a critical role in mediating abnormal contraction of VSM induced by SPC.
Paxillin, a scaffolding protein located at focal adhesion, recruits various signaling molecules including Fyn and FAK, playing an important role in cytoskeletal reorganization (Singh et al., 2019; Zhang et al., 2021; Zhang et al., 2023). Several studies have shown that paxillin regulates the Ca2+-dependent contraction in tracheal smooth muscle and cardiac contractility (Hirth et al., 2016; Zhang W. et al., 2016). Recent study reported that paxillin is a binding molecule of the active Fyn (Zhang et al., 2021). Paxillin deletion attenuates the SPC-induced contraction of VSM, suggesting that paxillin is involved in the SPC-induced abnormal contraction of VSM (Zhang et al., 2024). In addition, the activity of Rho-kinase but not Fyn is inhibited in paxillin deleted cells and tissues (Zhang et al., 2024), indicating that paxillin involves in the SPC-induced abnormal contraction as a signaling molecule between Fyn and Rho-kinase.
In addition, SPC increases ROS generation by activating PLC, PKC1, and Src-dependent NADPH oxidase 1 (NOX1), thereby enhancing Ca2+ entry through L-type channels and strongly enhancing vascular reactivity (Shaifta et al., 2015). Jin L et al. demonstrated that ROS-induced vascular contraction is mediated through the activation of the Rho/Rho-kinase pathway, as evidenced by the increased translocation of Rho to the membrane and phosphorylation of MYPT1, both of which were inhibited by the Rho-kinase inhibitor Y-27632 (Jin et al., 2004). SPC induces apoptosis of endothelial cells through ROS-mediated activation of ERK, causing endothelial dysfunction (Jeon et al., 2007). These studies suggest that SPC could impact vascular contraction activity through ROS-mediated pathways. The signaling pathways induced by SPC are summarized as shown in Figure 2.
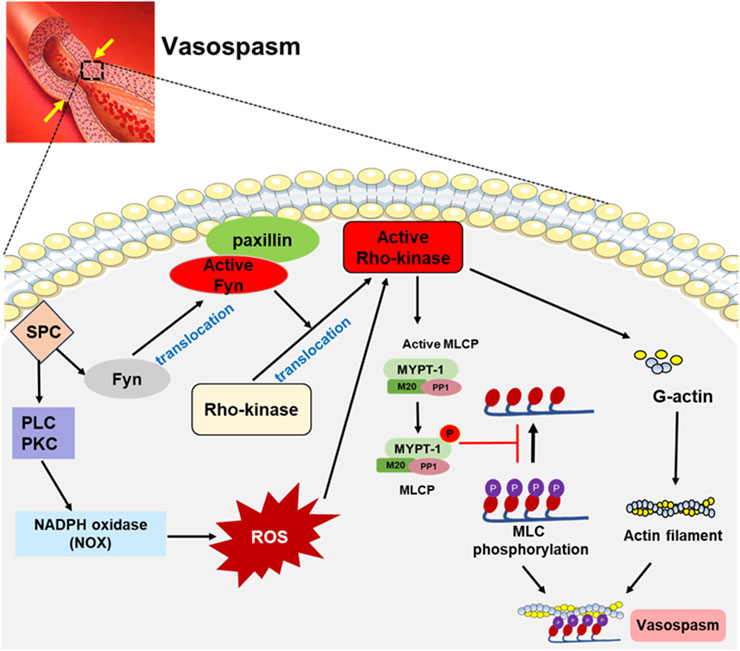
Figure 2. SPC-induced signaling pathways involved in vasospasm. SPC-mediated signaling pathway involves Fyn, a Src family kinase, which promotes Rho-kinase activation by interacting with paxillin. In addition, SPC can activate NADPH oxidase (NOX) through PLC and PKC, increasing ROS generation, which activates the Rho-kinase. The activated Rho-kinase phosphorylates myosin light chain phosphatase (MLCP) to inactivate it, and myosin light chain (MLC) remains in a highly phosphorylated state, leading to vasospasm.
3 Anti-vasospasm effect of flavonoids
Flavonoids are compounds extracted from many fruits, vegetables, and traditional Chinese herbal medicines. The basic backbone, classification, and chemical structures of representative flavonoids are shown in Figure 3. These compounds exhibit a variety of beneficial biological activities for human health, specially anti-inflammatory and antioxidant effects (Maleki et al., 2019; Cho et al., 2020), as well as antimicrobial and antiviral properties (Badshah et al., 2021; Cascaes et al., 2021). They have also the potential to reduce the risk of chronic diseases such as cancer (Busch et al., 2015), cardiovascular diseases (Ponzo et al., 2015; Ciumarnean et al., 2020), and neurodegenerative diseases (Devi et al., 2021). Here, we will discuss the potential anti-vasospasm effects of flavonoids through virous mechanisms.
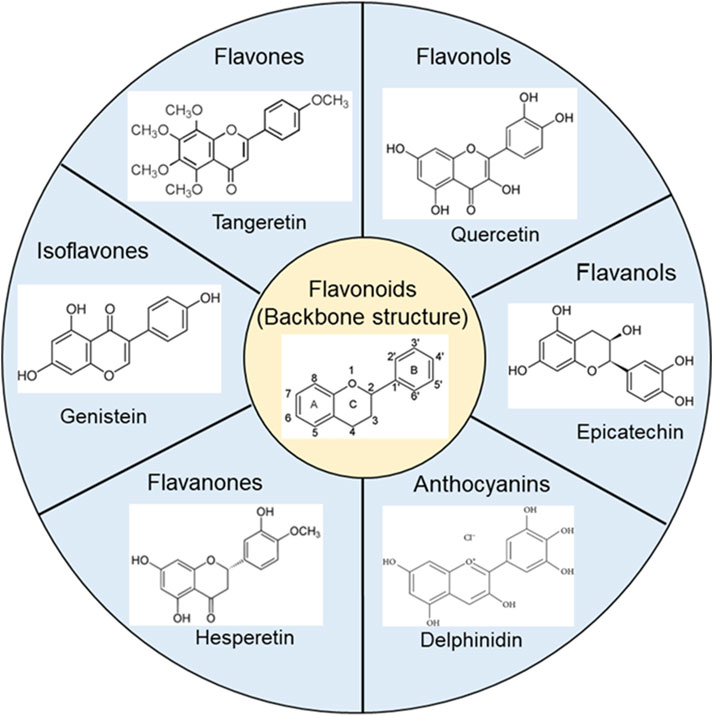
Figure 3. The basic backbone structure of flavonoids and representative compounds of their category.
3.1 Anti-vasospasm effect of flavonoids by inhibiting the SPC-induced pathway
In recent years, researchers have shown growing interest in the potential of flavonoids to inhibit abnormal contraction of VSM. To date, hesperetin and tangeretin have been found to exert significant inhibitory effects on abnormal contraction induced by SPC (Li et al., 2022; Lu et al., 2022). In addition, we discuss the anti-vasospastic effects of several other flavonoids, potentially through the attenuation of oxidative stress.
3.1.1 Hesperetin
Hesperetin (the structure is shown in Figure 3), a metabolite of hesperidin, has shown promising potential in improving cardiovascular health. Animal studies have demonstrated that hesperetin can improve endothelial function (Liu et al., 2008), reduce inflammation (Wu J. et al., 2021), and exert antioxidant effects (Li et al., 2021). Furthermore, hesperetin has been found to reduce the hepatic triacylglycerol (TG) accumulation induced by 1% orotic acid (Cha et al., 2001). These findings suggest that hesperetin exerts a protective effect against cardiovascular diseases by reducing risk factors such as hypertension and dyslipidemia.
In our recent study, we observed that hesperetin effectively inhibits the abnormal contraction induced by SPC in coronary artery smooth muscle (Lu et al., 2022). Hesperetin (30 μM) markedly suppressed SPC-induced contraction, resulting in a 79.4% ± 7.4% inhibition, with an IC50 value of 13.94 μM (Lu et al., 2022). Furthermore, pretreatment with hesperetin exhibited a significant protective effect, with an inhibition rate of 80.3% ± 6.6% in response to SPC-induced contraction (Lu et al., 2022). Additionally, hesperetin was found to inhibit the translocation of Fyn and Rho-kinase from the cytoplasm to the membrane, and to attenuate SPC-induced phosphorylation of both Fyn and MLC (Lu et al., 2022). Collectively, these findings suggest that hesperetin has promising potential as a therapeutic agent for the prevention and treatment of vasospasm.
3.1.2 Tangeretin
Tangeretin (the structure is shown in Figure 3), a natural compound found in citrus plants, shows promising therapeutic effects in cardiovascular diseases. Studies indicate that tangeretin exhibits anti-inflammatory (Funaro et al., 2016; Lee et al., 2016; Li et al., 2019), antioxidant (Lee et al., 2016; Li et al., 2019), and endothelial protective effects (Wu et al., 2019), which are crucial for preventing and treating cardiovascular diseases. Tangeretin has also been found to inhibit platelet activation, aggregation, and preventing thrombosis (Vaiyapuri et al., 2013). Additionally, tangeretin has been found to have lipid-lowering effects, reduce serum cholesterol levels and inhibit the expression of genes involved in lipid metabolism (Chen et al., 2021). Shiroorkar PN et al. found that tangeretin is a novel cardioprotective therapeutic agent for the treatment of sepsis-induced myocardial dysfunction (Shiroorkar et al., 2020).
A recent study by Li et al. showed that both pretreatment and posttreatment at the optimal concentration, tangeretin exhibited a remarkable inhibitory effect on the SPC-induced contraction, indicating its protective potential against cardiovascular diseases (Li et al., 2022). At the concentration of 2.5 μM, tangeretin effectively inhibited the SPC-induced contraction by 85.4% ± 8.23%, while only showing a slightly inhibitory effect (1.53% ± 2.57% inhibition) on the high K+-induced Ca2+-dependent contraction (Li et al., 2022). In addition, pretreatment with tangeretin exhibited a marked inhibitory effect on the SPC-induced abnormal contraction, with an inhibition rate of 72.51% ± 10.04%, while showing an inhibition rate of 31.89% ± 9.74% for 40 mM K+-induced Ca2+-dependent contraction (Li et al., 2022). These results suggest that tangeretin may be a potential compound for the treatment and/or prevention of vasospasm. The underlying mechanism of tangeretin’s action appears to be similar to that of hesperetin. In cultured VSM cells, tangeretin reduced MLC phosphorylation by inhibiting SPC-induced activation of Fyn and Rho-kinase, as well as their translocation from the cytoplasm to the membrane, thereby inhibiting SPC-induced abnormal contraction (Li et al., 2022).
Although inhibitory effects of hesperetin and tangeretin on SPC-induced abnormal contraction have been demonstrated in isolated tissues and smooth muscle cells, they have not yet been investigated in an animal model of vasospasm. Future in-depth studies exploring these flavonoids in this context will be crucial for advancing preclinical trials.
3.1.3 Genistein
Genistein (the structure is shown in Figure 3), a natural isoflavone primarily found in soy products, has been extensively studied for its cardiovascular protective effects (Fukutake et al., 1996; Jafari et al., 2023). With its vasodilatory (Walker et al., 2001), anti-inflammatory (Goh et al., 2022), and antioxidant (Kerry and Abbey, 1998) properties, genistein has emerged as a promising candidate for the prevention and treatment of vasospasm, attracting growing interest in recent years.
As a well-known protein tyrosine kinase inhibitor, genistein significantly inhibits smooth muscle contraction, suggesting that protein tyrosine kinase plays a crucial role in regulating Ca2+ sensitivity of smooth muscle (Steusloff et al., 1995). Src family protein tyrosine kinases have been implicated in SPC-induced abnormal vasoconstriction (Nakao et al., 2002). It is reasonable to hypothesize that genistein may mitigate SPC-induced abnormal vascular contraction by inhibiting the activity of Src family protein tyrosine kinases. Genistein decreases RhoA activation, inhibits vascular contraction induced by U46619 and KCl, and reduces phosphorylation of MLC and MYPT1 (Thr855), suggesting that genistein may contribute to vascular relaxation and blood pressure regulation by targeting the RhoA/Rho-kinase pathway (Seok et al., 2008). Additionally, genistein’s key mechanisms of action is the enhancement of endothelial nitric oxide synthase (eNOS) activity, leading to increased NO production (Liu et al., 2004; Si et al., 2012). This, in turn, may help alleviate vasospasm and improve endothelial function.
Preclinical studies have demonstrated genistein’s vasodilatory effects in animal vascular tissues (Sato A. et al., 2000; Sun et al., 2015). Moreover, epidemiological research suggests that individuals with a diet rich in genistein-containing foods have a lower incidence of vasospastic disorders (Squadrito et al., 2003; Cruz et al., 2008). However, clinical trials specifically evaluating genistein’s efficacy in vasospasm management remain limited, underscoring the need for further investigation.
3.1.4 Delphinidin
Delphinidin (the structure is shown in Figure 3) is the major anthocyanidin found in various berries and other colored fruits and possesses potent antioxidant properties (Yun et al., 2009). Studies have shown that SPC-induced NADPH oxidase (NOX) enzyme-mediated ROS generation (Shaifta et al., 2015) followed by ROS-promoted Rho-kinase activation (Jin et al., 2004; MacKay et al., 2017) may play a key role in SPC-induced vasospasm. The NOX enzyme family is a major source of ROS in various cell types (Lambeth, 2007; Zhang J. et al., 2016; Cipriano et al., 2023). A study by Lim TG et al. identified that NOX as the molecular target of delphinidin in suppressing UVB-induced MMP-1 expression in human dermal fibroblasts (Lim et al., 2013). They further found that delphinidin inhibits NOX activity, reduces ROS production, and prevents p47 (phox) translocation, thereby downregulating MKK4-JNK1/2, MKK3/6-p38, and MEK-ERK1/2 signaling pathways (Lim et al., 2013), indicating that delphinidin effectively inhibits NOX-dependent ROS generation, making it a promising candidate for therapeutic intervention in vasospasm. Delphinidin has been demonstrated to reduce ROS generation through the AMPK/NOX/MAPK signaling pathway (Chen et al., 2020). In addition, delphinidin has been demonstrated to inhibit the PDGFAB-induced release of VEGF in vascular smooth muscle cells by scavenging ROS and blocking p38MAPK and JNK pathway activation (Oak et al., 2006). These studies make delphinidin a promising candidate for therapeutic intervention in vasospasm.
3.2 Anti-vasospasm effect of flavonoids by inhibiting oxidative stress
Studies have shown that in order to counteract the harmful effects of oxidative stress caused by ROS, certain antioxidant enzymes play a key role in the scavenging of ROS, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Jomova et al., 2024). SOD not only scavenges superoxide anion radicals, thereby preventing the formation of more damaging peroxynitrite, but also maintains the physiologically required level of NO (Fukai and Ushio-Fukai, 2011). Flavonoids have significant antioxidant activity, which can not only effectively scavenge ROS, but also inhibit the activity of NADPH oxidase (Luo et al., 2019), thereby reducing the production of ROS. In addition, flavonoids can also induce the activation of nuclear factor 2-related factor 2 (Nrf2) (Suraweera et al., 2020) and promote mitochondrial biogenesis (Omidian et al., 2020), thereby improving oxidative stress caused by mitochondrial dysfunction. Here, we discuss the antioxidant effects of several representative flavonoids.
3.2.1 Tangeretin
Tangeretin, a polymethoxylated flavone, exhibits antioxidant biological activities. Wu et al. investigated the protective effects of tangeretin against oxygen-glucose deprivation (OGD)-induced injury in human brain microvascular endothelial cells. The results showed that tangeretin increased the SOD activity while decreasing ROS and malondialdehyde (MDA) levels (Wu et al., 2019). These effects were mediated through the suppression of the neuroinflammatory JNK signaling pathway (Wu et al., 2019). In another recent study, researchers induced brain neurotoxicity in BALB/c mice using cisplatin and investigated the potential protective effects of tangeretin. They found that ROS and MDA levels were significantly increased in cisplatin-treated brain tissue, while treatment with tangeretin reduced these levels (Cicek et al., 2024), suggesting that tangeretin has a beneficial antioxidant effect. Additionally, tangeretin activates the Nrf2 pathway (Lv et al., 2023; Peng et al., 2024), thereby enhancing the body’s endogenous antioxidant defenses and alleviating oxidative stress. Tangeretin is also reported to enhance mitochondrial biogenesis via activating the AMPK-PGC1-α pathway (Kou et al., 2018).
3.2.2 Quercetin
Quercetin (the structure is shown in Figure 3), a flavonoid found in onions, apples, berries, and tea, has been extensively studied for its antioxidant and vasodilatory properties. It has been shown to effectively reduce oxidative stress by scavenging ROS and inhibiting NADPH oxidase (Zhang et al., 2020), a key enzyme responsible for ROS production in VSMCs (Drummond et al., 2011). Several studies have shown that activation of NADPH oxidase is involved in the development of coronary artery spasm and cerebral vasospasm (Kim et al., 2002; Murase et al., 2004; Saitoh et al., 2015). Quercetin mitigates vascular endothelial dysfunction in atherosclerotic mice by inhibiting the activity of myeloperoxidase and NADPH oxidase (Li J. X. et al., 2023). Furthermore, dietary quercetin has been shown to enhance NO levels and reduce ET-1 concentrations, potentially improving endothelial function (Loke et al., 2008). Quercetin can prevent ET-1-induced upregulation of NADPH oxidase and uncoupling of eNOS, thereby improving endothelial dysfunction (Romero et al., 2009). Several studies have also shown that quercetin can reduce oxidative stress response and alleviate brain damage following experimental subarachnoid hemorrhage (Dong et al., 2014; Gul et al., 2020; Jiao et al., 2023). Additionally, quercetin can also modulate mitochondrial biogenesis in various cell types (Rayamajhi et al., 2013; Li et al., 2016; Koshinaka et al., 2020). These reports suggest that quercetin has the potential to prevent cerebral vasospasm after subarachnoid hemorrhage.
3.2.3 Kaempferol
Kaempferol, another flavonoid found in a variety of fruits and vegetables, has similar properties to quercetin in modulating oxidative stress and improving endothelial function (Alrumaihi et al., 2024). Recent studies highlight the link between vascular pathology, oxidative stress, and inflammation (Siti et al., 2015; Steven et al., 2019; Higashi, 2022). Kaempferol acts as a direct scavenger of ROS, reducing oxidative stress, showing great potential in the treatment of many diseases (Yao et al., 2020; Hussain et al., 2024; Yao et al., 2024). Yao et al. found that in a mouse model of vascular injury, kaempferol inhibited TNF-α and IL-6 expression and activated the Nrf2/HO-1 pathway, thereby reducing oxidative stress and inflammation and providing a protective effect on the vascular endothelium (Yao et al., 2020). A recent review summarized that kaempferol prevented neurological dysfunction in experimental models of ischemia-reperfusion and 3-nitropropionic acid-induced brain injury by inhibiting mitochondrial dysfunction, suggesting its potential use in both the prevention and post-injury treatment of brain injury (Lopez-Sanchez et al., 2024). Studies in isolated arteries have shown that kaempferol induces vasorelaxation (Xu et al., 2006; Yoon et al., 2024), which can counteract the effects of vasospasm, particularly in conditions like coronary artery spasm. Kaempferol is also reported to alleviate mitochondrial damage by reducing mitochondrial ROS production (Han et al., 2021; Lee et al., 2023).
3.2.4 Apigenin
Apigenin, a flavone found in parsley, chamomile, and celery, has been reported to possess various cardioprotective effects, including modulating oxidative stress pathways involved in vasospasm (Allemailem et al., 2024). Apigenin reduces ROS levels and inhibits the activation of pro-inflammatory pathways, which are often triggered by oxidative stress in vascular tissues (Clayton et al., 2021). Apigenin induces vasodilation by promoting NO production and improving eNOS activity (Jin et al., 2009; Chen et al., 2010). Apigenin supplementation restores endothelial-dependent dilation, increases NO availability, reduces oxidative stress, and improves antioxidant enzyme expression in aged mice (Clayton et al., 2021). Zhang et al. investigated the effects of apigenin on early brain injury following SAH in rats. Apigenin administration significantly reduces brain edema, blood-brain barrier disruption, neurological deficits, and cell apoptosis by inhibiting the TLR4/NF-κB signaling pathway and upregulating tight junction proteins, suggesting its potential as a therapeutic for SAH-induced brain injury (Zhang et al., 2015). Another study by Han et al. demonstrated that apigenin treatment alleviated neurological deficits, brain edema, blood-brain barrier permeability, and cell apoptosis by reducing oxidative stress markers (ROS, MDA, myeloperoxidase (MPO)) and enhancing antioxidant activity (SOD, glutathione (GSH)), suggesting its potential as a therapeutic for SAH through its anti-oxidative effects (Han et al., 2017). By reducing oxidative stress and inflammation, apigenin can help maintain vascular homeostasis and prevent the excessive constriction of blood vessels associated with vasospasm. Furthermore, in a rat SAH model, apigenin decreased brain swelling, cell death, and neurological damage by blocking the TLR4/NF-κB pathway, which drives inflammation (Zhang et al., 2015), supporting its potential as a therapeutic agent for preventing cerebral vasospasm.
Figure 4 shows the effects of flavonoids on oxidative stress and SPC-induced signaling pathways and their effects on vasospasm. In summary, flavonoids have shown great potential in preventing and treating vasospasm by alleviating oxidative stress, improving endothelial function, and inhibiting vascular smooth muscle contraction. Although existing studies have provided valuable experimental evidence for the use of flavonoids in vasospasm, their effects need to be further verified in preclinical studies. So far, there are few clinical trials that directly evaluate the effects of flavonoids on vasospasm. Although flavonoids are known for their powerful antioxidant and anti-inflammatory properties, their therapeutic effects are limited by low bioavailability, including low solubility, instability, rapid metabolism, and poor intestinal absorption (Thilakarathna and Rupasinghe, 2013). To overcome these challenges, several strategies have been explored, including nanocarriers, structural modifications, and advanced drug delivery systems (Nagula and Wairkar, 2019; Zhao J. et al., 2019). The pharmacokinetic properties, dose dependence, and long-term safety of flavonoids need to be further studied. Therefore, future studies should focus on exploring the effects of flavonoids in animal models and using drug delivery systems to improve their bioavailability. It is expected that clinical trials will be conducted in the near future to evaluate the effects of flavonoids on vasospasm. In addition, the synergistic effects of flavonoids combined with other drugs are also worthy of further study.
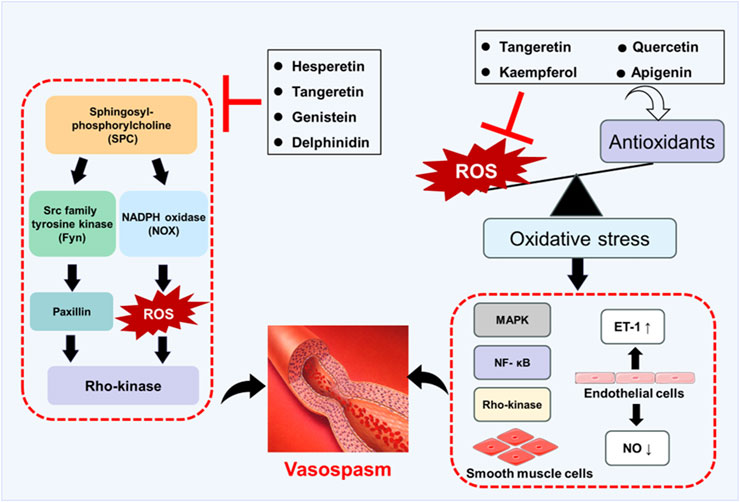
Figure 4. Schematic diagram of flavonoids preventing and treating vasospasm by regulating oxidative stress and SPC-induced signaling pathways.
4 Conclusions and future perspectives
Vasospasm is an abnormal contraction of VSM, commonly occurring in coronary and cerebral arteries, which reduces blood flow and can lead to serious conditions such as myocardial and cerebral infarctions. Studies have suggested that oxidative stress and the SPC-induced signaling pathway are involved in this contraction. Flavonoids, natural compounds found in plants and traditional Chinese medicines, have shown efficacy in reducing oxidative stress and inhibiting SPC-induced pathways, indicating that they have great potential in the treatment of cardiovascular and cerebrovascular diseases associated with vasospasm. Despite the promising results of preclinical studies on these flavonoids have been demonstrated in vitro, additional research is required to examine their effects in vivo. Clinical studies are needed to validate and develop these active flavonoids for the treatment of vasospasm-associated diseases.
Author contributions
YZ: Writing – original draft, Visualization, Conceptualization, Writing – review and editing. SM: Writing – review and editing. KM: Writing – review and editing. HK: Conceptualization, Writing – review and editing, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was partly supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant Numbers: 24K10026 for HK).
Acknowledgments
We would like to express our sincere gratitude to all those who provided assistance during the writing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allemailem, K. S., Almatroudi, A., Alharbi, H. O. A., Alsuhaymi, N., Alsugoor, M. H., Aldakheel, F. M., et al. (2024). Apigenin: a bioflavonoid with a promising role in disease prevention and treatment. Biomedicines 12, 1353. doi:10.3390/biomedicines12061353
Alrumaihi, F., Almatroodi, S. A., Alharbi, H. O. A., Alwanian, W. M., Alharbi, F. A., Almatroudi, A., et al. (2024). Pharmacological potential of kaempferol, a flavonoid in the management of pathogenesis via modulation of inflammation and other biological activities. Molecules 29, 2007. doi:10.3390/molecules29092007
Alvarez-Santos, M. D., Alvarez-Gonzalez, M., Estrada-Soto, S., and Bazan-Perkins, B. (2020). Regulation of myosin light-chain phosphatase activity to generate airway smooth muscle hypercontractility. Front. Physiol. 11, 701. doi:10.3389/fphys.2020.00701
Angelova, P. R., and Abramov, A. Y. (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 592, 692–702. doi:10.1002/1873-3468.12964
Ayer, R. E., and Zhang, J. H. (2008). Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir. Suppl. 104, 33–41. doi:10.1007/978-3-211-75718-5_7
Badshah, S. L., Faisal, S., Muhammad, A., Poulson, B. G., Emwas, A. H., and Jaremko, M. (2021). Antiviral activities of flavonoids. Biomed. Pharmacother. 140, 111596. doi:10.1016/j.biopha.2021.111596
Bhatti, J. S., Bhatti, G. K., and Reddy, P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1066–1077. doi:10.1016/j.bbadis.2016.11.010
Boguslawski, G., Grogg, J. R., Welch, Z., Ciechanowicz, S., Sliva, D., Kovala, A. T., et al. (2002). Migration of vascular smooth muscle cells induced by sphingosine 1-phosphate and related lipids: potential role in the angiogenic response. Exp. Cell Res. 274, 264–274. doi:10.1006/excr.2002.5472
Busch, C., Burkard, M., Leischner, C., Lauer, U. M., Frank, J., and Venturelli, S. (2015). Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 7, 64. doi:10.1186/s13148-015-0095-z
Cain, A. E., Tanner, D. M., and Khalil, R. A. (2002). Endothelin-1--induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension 39, 543–549. doi:10.1161/hy0202.103129
Cascaes, M. M., Guilhon, G., Zoghbi, M. D. G., Andrade, E. H. A., Santos, L. S., Kelly, R. D. S. J., et al. (2021). Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae). Nat. Prod. Res. 35, 1717–1721. doi:10.1080/14786419.2019.1629912
Cha, J. Y., Cho, Y. S., Kim, I., Anno, T., Rahman, S. M., and Yanagita, T. (2001). Effect of hesperetin, a citrus flavonoid, on the liver triacylglycerol content and phosphatidate phosphohydrolase activity in orotic acid-fed rats. Plant Foods Hum. Nutr. 56, 349–358. doi:10.1023/a:1011884200848
Chen, C. C., Ke, W. H., Ceng, L. H., Hsieh, C. W., and Wung, B. S. (2010). Calcium- and phosphatidylinositol 3-kinase/Akt-dependent activation of endothelial nitric oxide synthase by apigenin. Life Sci. 87, 743–749. doi:10.1016/j.lfs.2010.10.014
Chen, P. Y., Chao, T. Y., Hsu, H. J., Wang, C. Y., Lin, C. Y., Gao, W. Y., et al. (2021). The lipid-modulating effect of tangeretin on the inhibition of angiopoietin-like 3 (ANGPTL3) gene expression through regulation of LXRα activation in hepatic cells. Int. J. Mol. Sci. 22, 9853. doi:10.3390/ijms22189853
Chen, Y., Ge, Z., Huang, S., Zhou, L., Zhai, C., Chen, Y., et al. (2020). Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway. Aging (Albany NY) 12, 5362–5383. doi:10.18632/aging.102956
Cho, S. Y., Kim, H. W., Lee, M. K., Kim, H. J., Kim, J. B., Choe, J. S., et al. (2020). Antioxidant and anti-inflammatory activities in relation to the flavonoids composition of pepper (capsicum annuum L.). Antioxidants (Basel) 9, 986. doi:10.3390/antiox9100986
Cicek, B., Danisman, B., Bolat, I., Kiliclioglu, M., Kuzucu, M., Suleyman, H., et al. (2024). Effect of tangeretin on cisplatin-induced oxido-inflammatory brain damage in rats. J. Cell Mol. Med. 28, e18565. doi:10.1111/jcmm.18565
Cipriano, A., Viviano, M., Feoli, A., Milite, C., Sarno, G., Castellano, S., et al. (2023). NADPH oxidases: from molecular mechanisms to current inhibitors. J. Med. Chem. 66, 11632–11655. doi:10.1021/acs.jmedchem.3c00770
Ciumarnean, L., Milaciu, M. V., Runcan, O., Vesa, S. C., Rachisan, A. L., Negrean, V., et al. (2020). The effects of flavonoids in cardiovascular diseases. Molecules 25, 4320. doi:10.3390/molecules25184320
Clayton, Z. S., Hutton, D. A., Brunt, V. E., Vandongen, N. S., Ziemba, B. P., Casso, A. G., et al. (2021). Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am. J. Physiol. Heart Circ. Physiol. 321, H185–H196. doi:10.1152/ajpheart.00118.2021
Cruz, M. N., Agewall, S., Schenck-Gustafsson, K., and Kublickiene, K. (2008). Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis 196, 49–58. doi:10.1016/j.atherosclerosis.2007.01.038
Dagher, O., Mury, P., Thorin-Trescases, N., Noly, P. E., Thorin, E., and Carrier, M. (2021). Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front. Cardiovasc Med. 8, 658400. doi:10.3389/fcvm.2021.658400
De Souza, P., Guarido, K. L., Scheschowitsch, K., Da Silva, L. M., Werner, M. F., Assreuy, J., et al. (2016). Impaired vascular function in sepsis-surviving rats mediated by oxidative stress and Rho-Kinase pathway. Redox Biol. 10, 140–147. doi:10.1016/j.redox.2016.09.016
Devi, S., Kumar, V., Singh, S. K., Dubey, A. K., and Kim, J. J. (2021). Flavonoids: potential candidates for the treatment of neurodegenerative disorders. Biomedicines 9, 99. doi:10.3390/biomedicines9020099
Dimopoulos, G. J., Semba, S., Kitazawa, K., Eto, M., and Kitazawa, T. (2007). Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ. Res. 100, 121–129. doi:10.1161/01.RES.0000253902.90489.df
Dong, H., Gao, X., Li, H., Gao, J., and Zhang, L. (2024). Protective effects of flavonoids against intracerebral and subarachnoid hemorrhage (Review). Exp. Ther. Med. 28, 350. doi:10.3892/etm.2024.12639
Dong, Y. S., Wang, J. L., Feng, D. Y., Qin, H. Z., Wen, H., Yin, Z. M., et al. (2014). Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 11, 282–290. doi:10.7150/ijms.7634
Drummond, G. R., Selemidis, S., Griendling, K. K., and Sobey, C. G. (2011). Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 10, 453–471. doi:10.1038/nrd3403
Erdman, J. W., Balentine, D., Arab, L., Beecher, G., Dwyer, J. T., Folts, J., et al. (2007). Flavonoids and heart health: proceedings of the ILSI north America flavonoids workshop, may 31-june 1, 2005, Washington, DC. J Nutr 137, 718S–737S. doi:10.1093/jn/137.3.718S
Franczyk, B., Dybiec, J., Frak, W., Krzeminska, J., Kucmierz, J., Mlynarska, E., et al. (2022). Cellular mechanisms of coronary artery spasm. Biomedicines 10, 2349. doi:10.3390/biomedicines10102349
Fukai, T., and Ushio-Fukai, M. (2011). Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal 15, 1583–1606. doi:10.1089/ars.2011.3999
Fukutake, M., Takahashi, M., Ishida, K., Kawamura, H., Sugimura, T., and Wakabayashi, K. (1996). Quantification of genistein and genistin in soybeans and soybean products. Food Chem. Toxicol. 34, 457–461. doi:10.1016/0278-6915(96)87355-8
Fumoto, T., Naraoka, M., Katagai, T., Li, Y., Shimamura, N., and Ohkuma, H. (2019). The role of oxidative stress in microvascular disturbances after experimental subarachnoid hemorrhage. Transl. Stroke Res. 10, 684–694. doi:10.1007/s12975-018-0685-0
Funaro, A., Wu, X., Song, M., Zheng, J., Guo, S., Rakariyatham, K., et al. (2016). Enhanced anti-inflammatory activities by the combination of luteolin and tangeretin. J. Food Sci. 81, H1320–H1327. doi:10.1111/1750-3841.13300
Gao, Y., Howard, A., Ban, K., and Chandra, J. (2009). Oxidative stress promotes transcriptional up-regulation of Fyn in BCR-ABL1-expressing cells. J. Biol. Chem. 284, 7114–7125. doi:10.1074/jbc.M804801200
Ge, D., Jing, Q., Meng, N., Su, L., Zhang, Y., Zhang, S., et al. (2011). Regulation of apoptosis and autophagy by sphingosylphosphorylcholine in vascular endothelial cells. J. Cell Physiol. 226, 2827–2833. doi:10.1002/jcp.22632
Ge, D., Yue, H. W., Liu, H. H., and Zhao, J. (2018). Emerging roles of sphingosylphosphorylcholine in modulating cardiovascular functions and diseases. Acta Pharmacol. Sin. 39, 1830–1836. doi:10.1038/s41401-018-0036-4
Giannoni, E., and Chiarugi, P. (2014). Redox circuitries driving Src regulation. Antioxid. Redox Signal 20, 2011–2025. doi:10.1089/ars.2013.5525
Goh, Y. X., Jalil, J., Lam, K. W., Husain, K., and Premakumar, C. M. (2022). Genistein: a review on its anti-inflammatory properties. Front. Pharmacol. 13, 820969. doi:10.3389/fphar.2022.820969
Gul, S., Aydogmus, E., Bahadir, B., Buyukuysal, M. C., and Guven, B. (2020). Neuroprotective effects of quercetin on cerebral vasospasm following experimental subarachnoid haemorrhage in rats. Turk J. Med. Sci. 50, 1106–1110. doi:10.3906/sag-1904-207
Han, X., Zhao, S., Song, H., Xu, T., Fang, Q., Hu, G., et al. (2021). Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: implications in Parkinson's disease. Redox Biol. 41, 101911. doi:10.1016/j.redox.2021.101911
Han, Y., Zhang, T., Su, J., Zhao, Y., Chenchen, W., Li, X., et al. (2017). Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 40, 157–162. doi:10.1016/j.jocn.2017.03.003
Handa, Y., Kaneko, M., Takeuchi, H., Tsuchida, A., Kobayashi, H., and Kubota, T. (2000). Effect of an antioxidant, ebselen, on development of chronic cerebral vasospasm after subarachnoid hemorrhage in primates. Surg. Neurol. 53, 323–329. doi:10.1016/s0090-3019(00)00168-3
Hao, G., Eser, P., and Mo, J. (2022). Oxidative stress and intracranial hypertension after aneurysmal subarachnoid hemorrhage. Antioxidants (Basel) 11, 2423. doi:10.3390/antiox11122423
Higashi, Y. (2022). Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxidants (Basel) 11, 1958. doi:10.3390/antiox11101958
Higashi, Y., Noma, K., Yoshizumi, M., and Kihara, Y. (2009). Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 73, 411–418. doi:10.1253/circj.cj-08-1102
Hirano, K. (2007). Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J. Pharmacol. Sci. 104, 109–115. doi:10.1254/jphs.cp0070027
Hirth, S., Buhler, A., Buhrdel, J. B., Rudeck, S., Dahme, T., Rottbauer, W., et al. (2016). Paxillin and focal adhesion kinase (FAK) regulate cardiac contractility in the zebrafish heart. PLoS One 11, e0150323. doi:10.1371/journal.pone.0150323
Horakova, L. (2011). Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 4, 114–124. doi:10.2478/v10102-011-0019-5
Horky, L. L., Pluta, R. M., Boock, R. J., and Oldfield, E. H. (1998). Role of ferrous iron chelator 2,2'-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J. Neurosurg. 88, 298–303. doi:10.3171/jns.1998.88.2.0298
Hu, Z., Deng, X., Zhou, S., Zhou, C., Shen, M., Gao, X., et al. (2023). Pathogenic mechanisms and therapeutic implications of extracellular matrix remodelling in cerebral vasospasm. Fluids Barriers CNS 20, 81. doi:10.1186/s12987-023-00483-8
Hung, M. J., Hu, P., and Hung, M. Y. (2014). Coronary artery spasm: review and update. Int. J. Med. Sci. 11, 1161–1171. doi:10.7150/ijms.9623
Hussain, M. S., Altamimi, A. S. A., Afzal, M., Almalki, W. H., Kazmi, I., Alzarea, S. I., et al. (2024). Kaempferol: paving the path for advanced treatments in aging-related diseases. Exp. Gerontol. 188, 112389. doi:10.1016/j.exger.2024.112389
Jacobsen, A., Nielsen, T. H., Nilsson, O., Schalen, W., and Nordstrom, C. H. (2014). Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 130, 156–163. doi:10.1111/ane.12258
Jafari, S., Shoghi, M., and Khazdair, M. R. (2023). Pharmacological effects of genistein on cardiovascular diseases. Evid. Based Complement. Altern. Med. 2023, 8250219. doi:10.1155/2023/8250219
Jeon, E. S., Lee, M. J., Sung, S. M., and Kim, J. H. (2007). Sphingosylphosphorylcholine induces apoptosis of endothelial cells through reactive oxygen species-mediated activation of ERK. J. Cell Biochem. 100, 1536–1547. doi:10.1002/jcb.21141
Jeon, E. S., Moon, H. J., Lee, M. J., Song, H. Y., Kim, Y. M., Bae, Y. C., et al. (2006). Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J. Cell Sci. 119, 4994–5005. doi:10.1242/jcs.03281
Jiao, D., Xu, J., Lou, C., Luo, Y., Ni, C., Shen, G., et al. (2023). Quercetin alleviates subarachnoid hemorrhage-induced early brain injury via inhibiting ferroptosis in the rat model. Anat. Rec. Hob. 306, 638–650. doi:10.1002/ar.25130
Jin, B. H., Qian, L. B., Chen, S., Li, J., Wang, H. P., Bruce, I. C., et al. (2009). Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 616, 200–205. doi:10.1016/j.ejphar.2009.06.020
Jin, L., Ying, Z., and Webb, R. C. (2004). Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am. J. Physiol. Heart Circ. Physiol. 287, H1495–H1500. doi:10.1152/ajpheart.01006.2003
Jomova, K., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., and Valko, M. (2024). Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 98, 1323–1367. doi:10.1007/s00204-024-03696-4
Kamm, K. E., and Stull, J. T. (1985). The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 25, 593–620. doi:10.1146/annurev.pa.25.040185.003113
Kemp, B. E., and Pearson, R. B. (1991). Intrasteric regulation of protein kinases and phosphatases. Biochim. Biophys. Acta 1094, 67–76. doi:10.1016/0167-4889(91)90027-u
Kerry, N., and Abbey, M. (1998). The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro. Atherosclerosis 140, 341–347. doi:10.1016/s0021-9150(98)00138-5
Kim, D. E., Suh, Y. S., Lee, M. S., Kim, K. Y., Lee, J. H., Lee, H. S., et al. (2002). Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 33, 2687–2691. doi:10.1161/01.str.0000033071.99143.9e
Kolias, A. G., Sen, J., and Belli, A. (2009). Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J. Neurosci. Res. 87, 1–11. doi:10.1002/jnr.21823
Koshinaka, K., Honda, A., Masuda, H., and Sato, A. (2020). Effect of quercetin treatment on mitochondrial biogenesis and exercise-induced AMP-activated protein kinase activation in rat skeletal muscle. Nutrients 12, 729. doi:10.3390/nu12030729
Kou, G., Li, Z., Wu, C., Liu, Y., Hu, Y., Guo, L., et al. (2018). Citrus tangeretin improves skeletal muscle mitochondrial biogenesis via activating the AMPK-PGC1-alpha pathway in vitro and in vivo: a possible mechanism for its beneficial effect on physical performance. J. Agric. Food Chem. 66, 11917–11925. doi:10.1021/acs.jafc.8b04124
Kozlowska, A., and Szostak-Wegierek, D. (2022). Targeting cardiovascular diseases by flavonols: an update. Nutrients 14, 1439. doi:10.3390/nu14071439
Kureishi, Y., Kobayashi, S., Amano, M., Kimura, K., Kanaide, H., Nakano, T., et al. (1997). Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 272, 12257–12260. doi:10.1074/jbc.272.19.12257
Kurokawa, T., Yumiya, Y., Fujisawa, H., Shirao, S., Kashiwagi, S., Sato, M., et al. (2009). Elevated concentrations of sphingosylphosphorylcholine in cerebrospinal fluid after subarachnoid hemorrhage: a possible role as a spasmogen. J. Clin. Neurosci. 16, 1064–1068. doi:10.1016/j.jocn.2009.01.010
Kusama, Y., Kodani, E., Nakagomi, A., Otsuka, T., Atarashi, H., Kishida, H., et al. (2011). Variant angina and coronary artery spasm: the clinical spectrum, pathophysiology, and management. J. Nippon. Med. Sch. 78, 4–12. doi:10.1272/jnms.78.4
Lambeth, J. D. (2007). Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 43, 332–347. doi:10.1016/j.freeradbiomed.2007.03.027
Lee, M. J., Cho, Y., Hwang, Y., Jo, Y., Kim, Y. G., Lee, S. H., et al. (2023). Kaempferol alleviates mitochondrial damage by reducing mitochondrial reactive oxygen species production in lipopolysaccharide-induced prostate organoids. Foods 12, 3836. doi:10.3390/foods12203836
Lee, Y. Y., Lee, E. J., Park, J. S., Jang, S. E., Kim, D. H., and Kim, H. S. (2016). Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J. Neuroimmune Pharmacol. 11, 294–305. doi:10.1007/s11481-016-9657-x
Li, J. X., Tian, R., and Lu, N. (2023). Quercetin attenuates vascular endothelial dysfunction in atherosclerotic mice by inhibiting myeloperoxidase and NADPH oxidase function. Chem. Res. Toxicol. 36, 260–269. doi:10.1021/acs.chemrestox.2c00334
Li, N., Zhang, Y., Morita, T., Kishi, H., and Kobayashi, S. (2022). Inhibitory mechanism of tangeretin, a citrus flavone on the sphingosylphosphorylcholine (SPC)-induced vascular smooth muscle contraction. J. Pharmacol. Sci. 149, 189–197. doi:10.1016/j.jphs.2022.05.002
Li, R. L., Wang, L. Y., Duan, H. X., Qian, D., Zhang, Q., He, L. S., et al. (2023). Natural flavonoids derived from herbal medicines are potential anti-atherogenic agents by inhibiting oxidative stress in endothelial cells. Front. Pharmacol. 14, 1141180. doi:10.3389/fphar.2023.1141180
Li, S., Shao, L., Fang, J., Zhang, J., Chen, Y., Yeo, A. J., et al. (2021). Hesperetin attenuates silica-induced lung injury by reducing oxidative damage and inflammatory response. Exp. Ther. Med. 21, 297. doi:10.3892/etm.2021.9728
Li, X., Wang, H., Gao, Y., Li, L., Tang, C., Wen, G., et al. (2016). Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1α signaling pathway. Am. J. Transl. Res. 8, 3558–3566.
Li, X., Xie, P., Hou, Y., Chen, S., He, P., Xiao, Z., et al. (2019). Tangeretin inhibits oxidative stress and inflammation via upregulating nrf-2 signaling pathway in collagen-induced arthritic rats. Pharmacology 104, 187–195. doi:10.1159/000501163
Liliom, K., Sun, G., Bunemann, M., Virag, T., Nusser, N., Baker, D. L., et al. (2001). Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 355, 189–197. doi:10.1042/0264-6021:3550189
Lim, T. G., Jung, S. K., Kim, J. E., Kim, Y., Lee, H. J., Jang, T. S., et al. (2013). NADPH oxidase is a novel target of delphinidin for the inhibition of UVB-induced MMP-1 expression in human dermal fibroblasts. Exp. Dermatol 22, 428–430. doi:10.1111/exd.12157
Liu, D., Homan, L. L., and Dillon, J. S. (2004). Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5'-monophosphate-dependent mechanism. Endocrinology 145, 5532–5539. doi:10.1210/en.2004-0102
Liu, L., Xu, D. M., and Cheng, Y. Y. (2008). Distinct effects of naringenin and hesperetin on nitric oxide production from endothelial cells. J. Agric. Food Chem. 56, 824–829. doi:10.1021/jf0723007
Loke, W. M., Hodgson, J. M., Proudfoot, J. M., Mckinley, A. J., Puddey, I. B., and Croft, K. D. (2008). Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 88, 1018–1025. doi:10.1093/ajcn/88.4.1018
Lopez-Colome, A. M., Lee-Rivera, I., Benavides-Hidalgo, R., and Lopez, E. (2017). Paxillin: a crossroad in pathological cell migration. J. Hematol. Oncol. 10, 50. doi:10.1186/s13045-017-0418-y
Lopez-Sanchez, C., Lagoa, R., Poejo, J., Garcia-Lopez, V., Garcia-Martinez, V., and Gutierrez-Merino, C. (2024). An update of kaempferol protection against brain damage induced by ischemia-reperfusion and by 3-nitropropionic acid. Molecules 29, 776. doi:10.3390/molecules29040776
Lu, Q., Kishi, H., Zhang, Y., Morita, T., and Kobayashi, S. (2022). Hesperetin inhibits sphingosylphosphorylcholine-induced vascular smooth muscle contraction by regulating the fyn/rho-kinase pathway. J. Cardiovasc Pharmacol. 79, 456–466. doi:10.1097/FJC.0000000000001210
Luo, M., Tian, R., Yang, Z., Peng, Y. Y., and Lu, N. (2019). Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Arch. Biochem. Biophys. 671, 69–76. doi:10.1016/j.abb.2019.06.007
Lv, C., Li, Y., Liang, R., Huang, W., Xiao, Y., Ma, X., et al. (2023). Characterization of tangeretin as an activator of nuclear factor erythroid 2-related factor 2/antioxidant response element pathway in HEK293T cells. Curr. Res. Food Sci. 6, 100459. doi:10.1016/j.crfs.2023.100459
Macdonald, R. L., and Weir, B. K. (1994). Cerebral vasospasm and free radicals. Free Radic. Biol. Med. 16, 633–643. doi:10.1016/0891-5849(94)90064-7
Mackay, C. E., Shaifta, Y., Snetkov, V. V., Francois, A. A., Ward, J. P. T., and Knock, G. A. (2017). ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: role of Src-family kinases and ARHGEF1. Free Radic. Biol. Med. 110, 316–331. doi:10.1016/j.freeradbiomed.2017.06.022
Maeda, Y., Hirano, K., Nishimura, J., Sasaki, T., and Kanaide, H. (2004). Endothelial dysfunction and altered bradykinin response due to oxidative stress induced by serum deprivation in the bovine cerebral artery. Eur. J. Pharmacol. 491, 53–60. doi:10.1016/j.ejphar.2004.03.019
Maleki, S. J., Crespo, J. F., and Cabanillas, B. (2019). Anti-inflammatory effects of flavonoids. Food Chem. 299, 125124. doi:10.1016/j.foodchem.2019.125124
Mangels, D. R., and Mohler, E. R. (2017). Catechins as potential mediators of cardiovascular health. Arterioscler. Thromb. Vasc. Biol. 37, 757–763. doi:10.1161/ATVBAHA.117.309048
Maruhashi, T., and Higashi, Y. (2021). An overview of pharmacotherapy for cerebral vasospasm and delayed cerebral ischemia after subarachnoid hemorrhage. Expert Opin. Pharmacother. 22, 1601–1614. doi:10.1080/14656566.2021.1912013
Matsui, T., and Asano, T. (1994). Effects of new 21-aminosteroid tirilazad mesylate (U74006F) on chronic cerebral vasospasm in a “two-hemorrhage” model of beagle dogs. Neurosurgery 34, 1035–1039. doi:10.1227/00006123-199406000-00012
Matsushima, S., Kuroda, J., Zhai, P., Liu, T., Ikeda, S., Nagarajan, N., et al. (2016). Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J. Clin. Invest 126, 3403–3416. doi:10.1172/JCI85624
Matta, A., Bouisset, F., Lhermusier, T., Campelo-Parada, F., Elbaz, M., Carrie, D., et al. (2020). Coronary artery spasm: new insights. J. Interv. Cardiol. 2020, 5894586. doi:10.1155/2020/5894586
Meyer Zu Heringdorf, D., and Jakobs, K. H. (2007). Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta 1768, 923–940. doi:10.1016/j.bbamem.2006.09.026
Mizuno, Y., Isotani, E., Huang, J., Ding, H., Stull, J. T., and Kamm, K. E. (2008). Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am. J. Physiol. Cell Physiol. 295, C358–C364. doi:10.1152/ajpcell.90645.2007
Mukherjee, S., Chopra, H., Goyal, R., Jin, S., Dong, Z., Das, T., et al. (2024). Therapeutic effect of targeted antioxidant natural products. Discov. Nano 19, 144. doi:10.1186/s11671-024-04100-x
Murase, Y., Yamada, Y., Hirashiki, A., Ichihara, S., Kanda, H., Watarai, M., et al. (2004). Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur. Heart J. 25, 970–977. doi:10.1016/j.ehj.2004.02.020
Nagula, R. L., and Wairkar, S. (2019). Recent advances in topical delivery of flavonoids: a review. J. Control Release 296, 190–201. doi:10.1016/j.jconrel.2019.01.029
Nakao, F., Kobayashi, S., Mogami, K., Mizukami, Y., Shirao, S., Miwa, S., et al. (2002). Involvement of Src family protein tyrosine kinases in Ca(2+) sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ. Res. 91, 953–960. doi:10.1161/01.res.0000042702.04920.bf
Naraoka, M., Munakata, A., Matsuda, N., Shimamura, N., and Ohkuma, H. (2013). Suppression of the Rho/Rho-kinase pathway and prevention of cerebral vasospasm by combination treatment with statin and fasudil after subarachnoid hemorrhage in rabbit. Transl. Stroke Res. 4, 368–374. doi:10.1007/s12975-012-0247-9
Oak, M. H., Bedoui, J. E., Madeira, S. V., Chalupsky, K., and Schini-Kerth, V. B. (2006). Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br. J. Pharmacol. 149, 283–290. doi:10.1038/sj.bjp.0706843
Omidian, K., Rafiei, H., and Bandy, B. (2020). Increased mitochondrial content and function by resveratrol and select flavonoids protects against benzo[a]pyrene-induced bioenergetic dysfunction and ROS generation in a cell model of neoplastic transformation. Free Radic. Biol. Med. 152, 767–775. doi:10.1016/j.freeradbiomed.2020.01.021
Peng, B., Hu, J., Sun, Y., Huang, Y., Peng, Q., Zhao, W., et al. (2024). Tangeretin alleviates inflammation and oxidative response induced by spinal cord injury by activating the Sesn2/Keap1/Nrf2 pathway. Phytother. Res. 38, 4555–4569. doi:10.1002/ptr.8294
Peng, S., and Fu, Y. (2023). FYN: emerging biological roles and potential therapeutic targets in cancer. J. Transl. Med. 21, 84. doi:10.1186/s12967-023-03930-0
Perez-Vizcaino, F., Duarte, J., and Andriantsitohaina, R. (2006). Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radic. Res. 40, 1054–1065. doi:10.1080/10715760600823128
Ponzo, V., Goitre, I., Fadda, M., Gambino, R., De Francesco, A., Soldati, L., et al. (2015). Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J. Transl. Med. 13, 218. doi:10.1186/s12967-015-0573-2
Prajapat, S. K., Maharana, K. C., and Singh, S. (2024). Mitochondrial dysfunction in the pathogenesis of endothelial dysfunction. Mol. Cell Biochem. 479, 1999–2016. doi:10.1007/s11010-023-04835-8
Rayamajhi, N., Kim, S. K., Go, H., Joe, Y., Callaway, Z., Kang, J. G., et al. (2013). Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxid. Med. Cell Longev. 2013, 154279. doi:10.1155/2013/154279
Romero, M., Jimenez, R., Sanchez, M., Lopez-Sepulveda, R., Zarzuelo, M. J., O'valle, F., et al. (2009). Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 202, 58–67. doi:10.1016/j.atherosclerosis.2008.03.007
Ruef, J., Moser, M., Kubler, W., and Bode, C. (2001). Induction of endothelin-1 expression by oxidative stress in vascular smooth muscle cells. Cardiovasc Pathol. 10, 311–315. doi:10.1016/s1054-8807(01)00095-3
Saitoh, S., Matsumoto, K., Kamioka, M., Ohkawara, H., Kaneshiro, T., Ishibashi, T., et al. (2009). Novel pathway of endothelin-1 and reactive oxygen species in coronary vasospasm with endothelial dysfunction. Coron. Artery Dis. 20, 400–408. doi:10.1097/MCA.0b013e32832e5c8c
Saitoh, S., Takeishi, Y., and Maruyama, Y. (2015). Mechanistic insights of coronary vasospasm and new therapeutic approaches. Fukushima J. Med. Sci. 61, 1–12. doi:10.5387/fms.2015-2
Sato, A., Hattori, Y., and Kanno, M. (2000). Effects of genistein and daidzein on enhanced vascular contractile reactivity and Ca2+ sensitivity in cardiomyopathic hamsters. Methods Find. Exp. Clin. Pharmacol. 22, 25–30. doi:10.1358/mf.2000.22.1.795816
Sato, M., Tani, E., Fujikawa, H., and Kaibuchi, K. (2000). Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ. Res. 87, 195–200. doi:10.1161/01.res.87.3.195
Satoh, K., Nigro, P., and Berk, B. C. (2010). Oxidative stress and vascular smooth muscle cell growth: a mechanistic linkage by cyclophilin A. Antioxid. Redox Signal 12, 675–682. doi:10.1089/ars.2009.2875
Scioli, M. G., Storti, G., D'amico, F., Rodriguez Guzman, R., Centofanti, F., Doldo, E., et al. (2020). Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: potential diagnostic biomarkers and therapeutic targets. J. Clin. Med. 9, 1995. doi:10.3390/jcm9061995
Seok, Y. M., Baek, I., Kim, Y. H., Jeong, Y. S., Lee, I. J., Shin, D. H., et al. (2008). Isoflavone attenuates vascular contraction through inhibition of the RhoA/Rho-kinase signaling pathway. J. Pharmacol. Exp. Ther. 326, 991–998. doi:10.1124/jpet.108.138529
Shaifta, Y., Snetkov, V. A., Prieto-Lloret, J., Knock, G. A., Smirnov, S. V., Aaronson, P. I., et al. (2015). Sphingosylphosphorylcholine potentiates vasoreactivity and voltage-gated Ca2+ entry via NOX1 and reactive oxygen species. Cardiovasc Res. 106, 121–130. doi:10.1093/cvr/cvv029
Shaito, A., Aramouni, K., Assaf, R., Parenti, A., Orekhov, A., Yazbi, A. E., et al. (2022). Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front. Biosci. (Landmark Ed.) 27, 105. doi:10.31083/j.fbl2703105
Shirao, S., Fujisawa, H., Kudo, A., Kurokawa, T., Yoneda, H., Kunitsugu, I., et al. (2008). Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc. Dis. 26, 30–37. doi:10.1159/000135650
Shirao, S., Kashiwagi, S., Sato, M., Miwa, S., Nakao, F., Kurokawa, T., et al. (2002). Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ. Res. 91, 112–119. doi:10.1161/01.res.0000026057.13161.42
Shiroorkar, P. N., Afzal, O., Kazmi, I., Al-Abbasi, F. A., Altamimi, A. S. A., Gubbiyappa, K. S., et al. (2020). Cardioprotective effect of tangeretin by inhibiting PTEN/AKT/mTOR Axis in experimental sepsis-induced myocardial dysfunction. Molecules 25, 5622. doi:10.3390/molecules25235622
Si, H., Yu, J., Jiang, H., Lum, H., and Liu, D. (2012). Phytoestrogen genistein up-regulates endothelial nitric oxide synthase expression via activation of cAMP response element-binding protein in human aortic endothelial cells. Endocrinology 153, 3190–3198. doi:10.1210/en.2012-1076
Singh, J., Sharma, K., Frost, E. E., and Pillai, P. P. (2019). Role of PDGF-A-activated ERK signaling mediated FAK-paxillin interaction in oligodendrocyte progenitor cell migration. J. Mol. Neurosci. 67, 564–573. doi:10.1007/s12031-019-1260-1
Sinha, A., Rahman, H., and Perera, D. (2022). Vasospastic angina: a contemporary review of its pathophysiology, diagnosis and management. Heart Int. 16, 99–104. doi:10.17925/HI.2022.16.2.99
Siti, H. N., Kamisah, Y., and Kamsiah, J. (2015). The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 71, 40–56. doi:10.1016/j.vph.2015.03.005
Slavich, M., and Patel, R. S. (2016). Coronary artery spasm: current knowledge and residual uncertainties. Int. J. Cardiol. Heart Vasc. 10, 47–53. doi:10.1016/j.ijcha.2016.01.003
Somlyo, A. P., and Somlyo, A. V. (1994). Signal transduction and regulation in smooth muscle. Nature 372, 231–236. doi:10.1038/372231a0
Squadrito, F., Altavilla, D., Crisafulli, A., Saitta, A., Cucinotta, D., Morabito, N., et al. (2003). Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am. J. Med. 114, 470–476. doi:10.1016/s0002-9343(03)00059-7
Steusloff, A., Paul, E., Semenchuk, L. A., Di Salvo, J., and Pfitzer, G. (1995). Modulation of Ca2+ sensitivity in smooth muscle by genistein and protein tyrosine phosphorylation. Arch. Biochem. Biophys. 320, 236–242. doi:10.1016/0003-9861(95)90005-5
Steven, S., Frenis, K., Oelze, M., Kalinovic, S., Kuntic, M., Bayo Jimenez, M. T., et al. (2019). Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell Longev. 2019, 7092151. doi:10.1155/2019/7092151
Sun, L., Hou, Y., Zhao, T., Zhou, S., Wang, X., Zhang, L., et al. (2015). A combination of genistein and magnesium enhances the vasodilatory effect via an eNOS pathway and BK(Ca) current amplification. Can. J. Physiol. Pharmacol. 93, 215–221. doi:10.1139/cjpp-2014-0306
Suraweera, T. L., Rupasinghe, H. P. V., Dellaire, G., and Xu, Z. (2020). Regulation of Nrf2/ARE pathway by dietary flavonoids: a friend or foe for cancer management? Antioxidants (Basel) 9, 973. doi:10.3390/antiox9100973
Tanaka, Y., Masuzawa, T., Saito, M., Yamada, T., and Fujimoto, K. (1998). Change in Ca2+ sensitivity of cerebrovascular smooth muscle in experimental chronic cerebral vasospasm. Neurol. Med. Chir. (Tokyo) 38, 459–463. doi:10.2176/nmc.38.459
Thilakarathna, S. H., and Rupasinghe, H. P. (2013). Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5, 3367–3387. doi:10.3390/nu5093367
Todoroki-Ikeda, N., Mizukami, Y., Mogami, K., Kusuda, T., Yamamoto, K., Miyake, T., et al. (2000). Sphingosylphosphorylcholine induces Ca(2+)-sensitization of vascular smooth muscle contraction: possible involvement of rho-kinase. FEBS Lett. 482, 85–90. doi:10.1016/s0014-5793(00)02046-9
Uehara, A., Yasukochi, M., Imanaga, I., and Berlin, J. R. (1999). Effect of sphingosylphosphorylcholine on the single channel gating properties of the cardiac ryanodine receptor. FEBS Lett. 460, 467–471. doi:10.1016/s0014-5793(99)01385-x
Vaiyapuri, S., Ali, M. S., Moraes, L. A., Sage, T., Lewis, K. R., Jones, C. I., et al. (2013). Tangeretin regulates platelet function through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Arterioscler. Thromb. Vasc. Biol. 33, 2740–2749. doi:10.1161/ATVBAHA.113.301988
Vona, R., Pallotta, L., Cappelletti, M., Severi, C., Matarrese, P., and Pietraforte, D. (2021). The impact of oxidative stress in human pathology: focus on gastrointestinal disorders. Antioxidants (Basel) 10, 201. doi:10.3390/antiox10020201
Walker, H. A., Dean, T. S., Sanders, T. A., Jackson, G., Ritter, J. M., and Chowienczyk, P. J. (2001). The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation 103, 258–262. doi:10.1161/01.cir.103.2.258
Walsh, M. P., Horowitz, A., Clement-Chomienne, O., Andrea, J. E., Allen, B. G., and Morgan, K. G. (1996). Protein kinase C mediation of Ca(2+)-independent contractions of vascular smooth muscle. Biochem. Cell Biol. 74, 485–502. doi:10.1139/o96-053
Wang, C. J., Lee, P. Y., Wu, B. N., Wu, S. C., Loh, J. K., Tsai, H. P., et al. (2014). Alteration of basilar artery rho-kinase and soluble guanylyl cyclase protein expression in a rat model of cerebral vasospasm following subarachnoid hemorrhage. Biomed. Res. Int. 2014, 531508. doi:10.1155/2014/531508
Watanabe, T., Nishiyama, M., Hori, T., Asano, T., Shimizu, T., and Masayasu, H. (1997). Ebselen (DR3305) ameliorates delayed cerebral vasospasm in a canine two-hemorrhage model. Neurol. Res. 19, 563–565. doi:10.1080/01616412.1997.11740859
Webb, R. C. (2003). Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 27, 201–206. doi:10.1152/advan.00025.2003
Wu, C., Zhao, J., Chen, Y., Li, T., Zhu, R., Zhu, B., et al. (2019). Tangeretin protects human brain microvascular endothelial cells against oxygen-glucose deprivation-induced injury. J. Cell Biochem. 120, 4883–4891. doi:10.1002/jcb.27762
Wu, F., Liu, Z., Li, G., Zhou, L., Huang, K., Wu, Z., et al. (2021). Inflammation and oxidative stress: potential targets for improving prognosis after subarachnoid hemorrhage. Front. Cell Neurosci. 15, 739506. doi:10.3389/fncel.2021.739506
Wu, J., Qian, Y., Chen, C., Feng, F., Pan, L., Yang, L., et al. (2021). Hesperetin exhibits anti-inflammatory effects on chondrocytes via the AMPK pathway to attenuate anterior cruciate ligament transection-induced osteoarthritis. Front. Pharmacol. 12, 735087. doi:10.3389/fphar.2021.735087
Wu, K. D., Bungard, D., and Lytton, J. (2001). Regulation of SERCA Ca2+ pump expression by cytoplasmic Ca2+ in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 280, C843–C851. doi:10.1152/ajpcell.2001.280.4.C843
Xu, W., Lu, H., Yuan, Y., Deng, Z., Zheng, L., and Li, H. (2022). The antioxidant and anti-inflammatory effects of flavonoids from propolis via Nrf2 and NF-κB pathways. Foods 11, 2439. doi:10.3390/foods11162439
Xu, Y. C., Leung, S. W., Leung, G. P., and Man, R. Y. (2015). Kaempferol enhances endothelium-dependent relaxation in the porcine coronary artery through activation of large-conductance Ca(2+) -activated K(+) channels. Br. J. Pharmacol. 172, 3003–3014. doi:10.1111/bph.13108
Xu, Y. C., Yeung, D. K., Man, R. Y., and Leung, S. W. (2006). Kaempferol enhances endothelium-independent and dependent relaxation in the porcine coronary artery. Mol. Cell Biochem. 287, 61–67. doi:10.1007/s11010-005-9061-y
Yamagata, K., and Yamori, Y. (2020). Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J. Cardiovasc Pharmacol. 75, 1–9. doi:10.1097/FJC.0000000000000757
Yao, H., Sun, J., Wei, J., Zhang, X., Chen, B., and Lin, Y. (2020). Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Front. Pharmacol. 11, 1118. doi:10.3389/fphar.2020.01118
Yao, Y. X., Yu, Y. J., Dai, S., Zhang, C. Y., Xue, X. Y., Zhou, M. L., et al. (2024). Kaempferol efficacy in metabolic diseases: molecular mechanisms of action in diabetes mellitus, obesity, non-alcoholic fatty liver disease, steatohepatitis, and atherosclerosis. Biomed. Pharmacother. 175, 116694. doi:10.1016/j.biopha.2024.116694
Yasui, K., and Palade, P. (1996). Sphingolipid actions on sodium and calcium currents of rat ventricular myocytes. Am. J. Physiol. 270, C645–C649. doi:10.1152/ajpcell.1996.270.2.C645
Yasukochi, M., Uehara, A., Kobayashi, S., and Berlin, J. R. (2003). Ca2+ and voltage dependence of cardiac ryanodine receptor channel block by sphingosylphosphorylcholine. Pflugers Arch. 445, 665–673. doi:10.1007/s00424-002-0945-3
Yoon, H. J., Moon, H. W., Min, Y. S., Jin, F., Bang, J. S., Sohn, U. D., et al. (2024). Effect of kaempferol on modulation of vascular contractility mainly through PKC and CPI-17 inactivation. Biomol. Ther. Seoul. 32, 361–367. doi:10.4062/biomolther.2023.186
Yue, H. W., Liu, J., Liu, P. P., Li, W. J., Chang, F., Miao, J. Y., et al. (2015). Sphingosylphosphorylcholine protects cardiomyocytes against ischemic apoptosis via lipid raft/PTEN/Akt1/mTOR mediated autophagy. Biochim. Biophys. Acta 1851, 1186–1193. doi:10.1016/j.bbalip.2015.04.001
Yun, J. M., Afaq, F., Khan, N., and Mukhtar, H. (2009). Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. 48, 260–270. doi:10.1002/mc.20477
Zhang, J., Wang, X., Vikash, V., Ye, Q., Wu, D., Liu, Y., et al. (2016). ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016, 4350965. doi:10.1155/2016/4350965
Zhang, T., Su, J., Guo, B., Wang, K., Li, X., and Liang, G. (2015). Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int. Immunopharmacol. 28, 79–87. doi:10.1016/j.intimp.2015.05.024
Zhang, W., Huang, Y., and Gunst, S. J. (2016). p21-Activated kinase (Pak) regulates airway smooth muscle contraction by regulating paxillin complexes that mediate actin polymerization. J. Physiol. 594, 4879–4900. doi:10.1113/JP272132
Zhang, Y., Kishi, H., and Kobayashi, S. (2023). Direct active Fyn-paxillin interaction regulates vascular smooth muscle cell migration. J. Smooth Muscle Res. 59, 58–66. doi:10.1540/jsmr.59.58
Zhang, Y., Kishi, H., Morita, T., and Kobayashi, S. (2021). Paxillin controls actin stress fiber formation and migration of vascular smooth muscle cells by directly binding to the active Fyn. FASEB J. 35, e22012. doi:10.1096/fj.202101035RR
Zhang, Y., Li, N., and Kobayashi, S. (2024). Paxillin participates in the sphingosylphosphorylcholine-induced abnormal contraction of vascular smooth muscle by regulating Rho-kinase activation. Cell Commun. Signal 22, 58. doi:10.1186/s12964-023-01404-w
Zhang, Y., Zhang, M., Lyu, B., Kishi, H., and Kobayashi, S. (2017). Omega-3 and omega-6 DPA equally inhibit the sphingosylphosphorylcholine-induced Ca(2+)-sensitization of vascular smooth muscle contraction via inhibiting Rho-kinase activation and translocation. Sci. Rep. 7, 36368. doi:10.1038/srep36368
Zhang, Y. M., Zhang, Z. Y., and Wang, R. X. (2020). Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 11, 956. doi:10.3389/fphys.2020.00956
Zhang, Z., Zhang, A., Liu, Y., Hu, X., Fang, Y., Wang, X., et al. (2022). New mechanisms and targets of subarachnoid hemorrhage: a focus on mitochondria. Curr. Neuropharmacol. 20, 1278–1296. doi:10.2174/1570159X19666211101103646
Zhao, J., Yang, J., and Xie, Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int. J. Pharm. 570, 118642. doi:10.1016/j.ijpharm.2019.118642
Zhao, R. Z., Jiang, S., Zhang, L., and Yu, Z. B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 44, 3–15. doi:10.3892/ijmm.2019.4188
Zheng, J. S., Zhan, R. Y., Zheng, S. S., Zhou, Y. Q., Tong, Y., and Wan, S. (2005). Inhibition of NADPH oxidase attenuates vasospasm after experimental subarachnoid hemorrhage in rats. Stroke 36, 1059–1064. doi:10.1161/01.STR.0000163102.49888.b7
Keywords: flavonoids, vasospasm, oxidative stress, SPC, ROS
Citation: Zhang Y, Maejima S, Matsuzaki K and Kishi H (2025) Potential of functional flavonoids in targeting vasospasm through modulation of oxidative stress and SPC-induced signaling pathways. Front. Pharmacol. 16:1594060. doi: 10.3389/fphar.2025.1594060
Received: 15 March 2025; Accepted: 29 May 2025;
Published: 11 June 2025.
Edited by:
Chris A. Bashur, Florida Institute of Technology, United StatesReviewed by:
Christopher Gerner, University of Vienna, AustriaKarla Hernández-Fonseca, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), Mexico
Copyright © 2025 Zhang, Maejima, Matsuzaki and Kishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroko Kishi, aGlya2lzaGlAbWVkLnNoaW1hbmUtdS5hYy5qcA==
 Ying Zhang
Ying Zhang Sho Maejima
Sho Maejima Kentaro Matsuzaki
Kentaro Matsuzaki Hiroko Kishi
Hiroko Kishi