- Department of Nephrology, The Second Hospital of Zhuzhou City, Zhuzhou, Hunan, China
The endoplasmic reticulum (ER) is the most metabolically active organelle in cells, and recent research has shown that abnormal ER function is involved in the occurrence and development of acute kidney injury (AKI), but the underlying molecular mechanism needs to be further elucidated. Here, we review the biological functions of the ER in cellular metabolism, explore the current research progress on the role of the ER in different triggers of AKI, and summarize the ER stress inhibitors discovered thus far. Finally, we explore the possibility of targeting ER homeostasis as a therapeutic target for AKI.
1 Introduction
As an important excretory and regulatory organ in the human body, the normal maintenance of kidney function is crucial for the stability of the internal environment of the body. Acute kidney injury (AKI) is induced when the kidney is severely affected by the external environment and is a common critical illness in clinical practice (Sun et al., 2025). Its main clinical symptom is a sharp decline in renal function in a short period of time, and patients often need urgent medical intervention (Velluto et al., 2025). Previous studies have suggested that inflammation (Ren et al., 2020), mitochondrial dysfunction (Zhao et al., 2021) and oxidative stress (Zhang et al., 2022) are involved in the occurrence and development of AKI. However, these pathogenesis mechanisms cannot fully explain the occurrence of AKI, and drugs developed on the basis of these mechanisms have little effect on clinical treatment. Therefore, it is necessary to explore the mechanism of AKI from other perspectives. Research has shown that abnormalities in endoplasmic reticulum (ER) homeostasis are involved in the occurrence and development of AKI.
The ER is an important organelle in cells and is responsible for key functions such as protein synthesis, folding, modification, and lipid synthesis (Wu et al., 2025). The maintenance of ER homeostasis is highly important for the normal physiological activities of cells. As a highly active metabolic organ, abnormalities in ER homeostasis in the kidney can induce the occurrence and development of AKI (Gallazzini and Pallet, 2018). In AKI, various pathogenic factors such as ischemia, toxicity, infection, and inflammation, can lead to ER stress in intrinsic renal cells (Yan et al., 2018; Ricciardi and Gnudi, 2020). The occurrence of ER stress activates a series of downstream signaling pathways within cells, such as the unfolded protein response (UPR) (Qian et al., 2024), ER-associated degradation (ERAD) (Christianson et al., 2023), and ER-phagy (Hoyer et al., 2024), in an attempt to restore ER homeostasis. When these protective mechanisms are insufficient to cope with sustained stress, ER stress leads to pathological processes such as cell apoptosis, the inflammatory response, and oxidative stress, further exacerbating kidney damage (Chen and Cubillos-Ruiz, 2021; Chen X. et al., 2023; Oakes and Papa, 2015). Studies have shown that drugs or genes that inhibit ER stress can alleviate the occurrence and development of AKI (Liu et al., 2019; Cao et al., 2024; Deng et al., 2023). These findings suggest that targeting ER homeostasis may be a therapeutic target for AKI.
In-depth research on the relationship between ER homeostasis and AKI not only helps us better understand the pathogenesis of AKI but also may provide a theoretical basis for the development of new treatment strategies. This review systematically elucidates the function of the ER and mechanism of ER homeostasis imbalance in AKI, as well as potential therapeutic targets and intervention measures for ER homeostasis regulation, to provide new ideas and directions for the clinical prevention and treatment of AKI.
2 The functions of the ER
2.1 Ca2+ homeostasis
2.1.1 SERCA pumps: uptake and storage
In the cell, the concentrations of calcium ions in different parts of the cell are significantly different; for example, the concentrations of calcium ions in the cytoplasm and the ER can be up to tens of thousands of times different (de Ridder et al., 2023; Dickson et al., 2016). A stable calcium ion concentration is conducive to a variety of cellular processes, such as cell proliferation, differentiation, metabolism, gene transcription and apoptosis (Terrell et al., 2023; Kito and Ohya, 2021; Nicotera et al., 1994). The ER is the main reservoir of calcium ions in the cell, and homeostasis of the ER is critical for maintaining the balance of the intracellular Ca2+ concentration. Calcium ions in the ER are mediated by the following proteins: Ca2+ pumps, which transport Ca2+ from the cytoplasm upward into the ER lumen; Ca2+-binding proteins, which bind and store Ca2+; and Ca2+ channels, which mediate the release of Ca2+ from the ER into the cytoplasm (Mekahli et al., 2011). The uptake of calcium ions by the ER is mediated mainly by sarco/ER Ca2+ ATPase (SERCA), which belongs to the family of P-type ATPases (Dhureja et al., 2023; Nemirovskaya and Sharlo, 2022). Members of this family also include the plasma membrane Ca2+ ATPase, Na+/K+ ATPase and H+/K+ ATPase (Sweadner and Donnet, 2001). A feature of these P-type ATPase enzymes is the ability to hydrolyze ATP to ADP while transporting metal ions against the gradient of the SR membrane (Xu and Van Remmen, 2021). The SERCA pump is located on the ER and sarcoplasmic reticulum (SR) and can use the energy generated by ATP hydrolysis to transport Ca2+ across membranes. Each SERCA contains two high-affinity Ca2+-binding sites, which can transport two Ca2+ ions for every ATP molecule hydrolyzed (Wu et al., 2023). SERCA is encoded by three different genes, SERCA1, SERCA2 and SERCA3, and a total of seven different subtypes are expressed in different tissues and cells (Periasamy and Kalyanasundaram, 2007). SERCA pump activity is also regulated by a variety of proteins, such as phospholamban, sarcolipin and myoregulin, which can inhibit SERCA pump activity (Hamstra et al., 2020; Chambers et al., 2022; Rathod et al., 2024), whereas the dwarf open reading frame can effectively activate the SERCA pump (Nelson et al., 2016). The presence of the SERCA pump ensures that the concentration of Ca2+ in the ER is much greater than that in the cytoplasm and that high concentrations of Ca2+ in the ER are essential for the regulation of posttranslational modification, folding, and protein transport. In the lumen of the ER/SR, Ca2+ mainly binds to Ca2+ proteins, such as calmodulin in cardiac and skeletal muscle, and calcium-binding proteins such as calnexin or 78-kDa glucose regulatory protein/immunoglobulin heavy chain binding protein (GRP78/BiP), in other tissues (Mekahli et al., 2011).
2.1.2 Ca2+ release channels: IP3Rs and RyRs
Inositol 1,4,5-trisphosphate receptors (IP3Rs) and ryanodine receptor (RyR) channels mediate the release of calcium ions from the ER (Ambudkar, 2014). Both have three mammalian isomers, IP3R1, 2, and 3 and RYR1, 2 and 3, which are distributed in different tissues (Luciani et al., 2009; Raturi et al., 2014). IP3R is expressed in most cells, and when IP3 is present, it can bind to IP3R to promote the release of calcium ions from the ER (Zhang et al., 2020). Compared with IP3Rs, RYRs have greater calcium ion binding activity, and the opening of RYRs depends on the concentration of Ca2+ in the cytoplasm (Berridge, 2016; Gillespie, 2020).
In general, ER-mediated calcium homeostasis is finely regulated, which ensures various metabolic activities within the cell. The high Ca2+ concentration maintained by SERCA is essential for protein folding and posttranslational modifications in the ER lumen.
2.2 Protein synthesis and processing
2.2.1 Chaperone-assisted folding and modifications
In cells, the ER is involved in approximately one-third of protein synthesis, folding, and maturation (Anelli and Sitia, 2008). In particular, some proteins on the plasma membrane and organelles are initially translated at ER-bound ribosomes and subsequently transferred to the ER lumen for further processing (Pehar and Puglielli, 2013). The common feature of these proteins is that they have an ER-targeting sequence at the N-terminus, and this signaling sequence is removed when the polypeptide chain is translated. Polypeptide chains entering the ER lumen are folded into unique three-dimensional shapes and undergo various modifications, such as glycosylation, hydroxylation and acylation, in the presence of high calcium ion concentrations and many chaperone proteins (Pehar and Puglielli, 2013; Johnson et al., 2001; Ogawa et al., 2015). These processes ensure that the polypeptide chain is transformed into an active protein, which is then transported to the next step. In the cell, the processes of the folding, transport and degradation pathways of ER proteins are strictly and finely regulated, a process known as ER quality control, which ensures that the synthesized and processed proteins in the ER are conformationally correct and active (Ferro-Novick et al., 2021). ER quality control is achieved by accelerating protein folding, activating the UPR, and eliminating faulty proteins through ERAD (Wiseman et al., 2022).
2.2.2 UPR pathways
In secretory tissues, cells are often in a continuous high-intensity process of protein secretion. For example, islet beta cells synthesize and secrete up to a million insulin molecules per minute (Seino et al., 2011). In the case of insulin resistance, the demand for insulin is greater, which requires islet beta cells to produce more insulin to meet the body’s needs. High-intensity protein secretion is a great challenge for the synthesis and folding of proteins in the ER. When the number of proteins folded far exceeds the upper limit of the capacity of the ER, ER stress often occurs to self-regulate (Di Conza and Ho, 2020; Hetz, 2012). At this point, the UPR detects whether misfolded proteins in the ER have exceeded a threshold. The UPR can regulate the downstream signaling pathway through three different signaling pathways, namely, inositol-requiring enzyme 1α (IRE1α), pancreatic endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6), thereby reducing the level of ER stress by reducing protein translation and upregulating chaperone expression (Ajoolabady et al., 2023). Unfolded proteins can bind to the lumen domain of Ire1, thereby triggering the self-binding of Ire1 and activating its cytoplasmic effector domain (Credle et al., 2005; Pincus et al., 2010). Proper ER stress effectively regulates the rate of intracellular protein synthesis and maintains cell homeostasis. The persistence of ER stress leads to the continuous activation of the UPR signaling pathway and eventually induces cell death (Marciniak et al., 2022; Rahmati et al., 2018).
2.2.3 ERAD
ERAD is a complex multistep process that involves mainly the recognition, extraction and ubiquitination of ER proteins and ultimately their degradation in the cytoplasmic proteasome (Dreher and Hoppe, 2018). In brief, when the polypeptide chain in the ER cannot be folded, it can be recognized by proteins such as BiP, EDEM, and OS9 in the ER (Sekiya et al., 2017; Seaayfan et al., 2016). The identified substrate is subsequently transported back to the cytoplasm via reverse transcriptional translocation. On the cytoplasmic side of the ER, the substrate is ubiquitinated by ubiquitin ligases and released into the cytoplasm in an ATP-dependent manner, where it is eventually recognized and degraded by the proteasome (Dreher and Hoppe, 2018; Blackwood et al., 2023). ERAD ensures that the unfolded protein is cleared in time, thus maintaining protein homeostasis in the cell.
In addition to protein processing, the ER is also the primary site for lipid metabolism, where synthesized lipids are stored in droplets or transported via membrane contact sites.
2.3 Lipid synthesis and droplet biogenesis
In the cell, the ER is also the key site of lipid metabolism and synthesis and contains many lipid synthetases, such as DGAT1/2 and phosphatidylserine synthase (PSS) (Farese and Walther, 2023; Wang and Benning, 2012). Lipid droplets store neutral fat in the cell, and they are also considered to constitute a single layer of phospholipid membrane organelles (Zadoorian et al., 2023). A previous review adequately described the role of the ER in lipid droplet formation (Walther et al., 2017). In addition, the ER is involved in lipid synthesis along with other organelles. For example, there are mitochondria-associated membranes (MAMs) between the ER and mitochondria, and MAMs are involved in the synthesis of phospholipids and cholesterol (Vance, 2015; Vance, 2014). The part of the ER connected to the Golgi apparatus is rich in tubules and vesicles called the ER-Golgi intermediate compartment (ERGIC), which is involved in the synthesis and redistribution of phospholipids in cells (Schwarz and Blower, 2016).
2.4 ER-phagy: Selective autophagy of the ER
ER-phagy is a newly discovered type of selective autophagy in which the ER can directly bind to LC3 through ER-phagy receptors, thereby mediating ER degradation. ER-phagy maintains the homeostasis of the ER and normal cellular function by clearing damaged, redundant, or dysfunctional components of the ER (Gonzalez et al., 2023). Currently, multiple ER proteins, such as FAM134B, SEC62, reticulon-3 (RTN3), cell cycle progression 1 (CCPG1), atlastin-3 (ATL3), and TEX264, have been shown to mediate the occurrence of ER-phagy, (Gubas and Dikic, 2022; Chino and Mizushima, 2023). When ER homeostasis is abnormal, ER-phagy receptors bind with LC3 to induce dysfunctional ER degradation, thereby blocking secondary cellular dysfunction (Stolz and Grumati, 2019; Chino and Mizushima, 2020).
In cells, the UPR, ERAD and ER-phagy precisely assist each other in jointly maintaining ER homeostasis. When stimulated by the outside world, unfolded and misfolded proteins accumulate in cells. At this time, the early warning system (UPR) in the cell is activated to reduce the number of misfolded proteins through promoting the expression of chaperone proteins and protein-folding enzymes, inhibiting the transcription and translation of proteins, strengthening the degradation tool (ERAD), etc. When the persistent UPR fails to restore the homeostasis of the ER in time, cells activate ER-phagy to degrade the functionally impaired ER. Therefore, the UPR, ERAD, and ER-phagy together form a refined collaborative network that maintains ER homeostasis in cells. They do not operate independently but form a hierarchical defense system through dynamic interactions at the temporal, spatial, and molecular levels, in which they respond to ER stress.
3 ER homeostasis and AKI
With increasing research revealing the importance of the ER in maintaining kidney function, the relationships between abnormal ER homeostasis and the occurrence and development of AKI have also been explored (Figure 1). In the following section, we summarize the current research progress on ER homeostasis abnormalities in AKI induced by different etiologies.
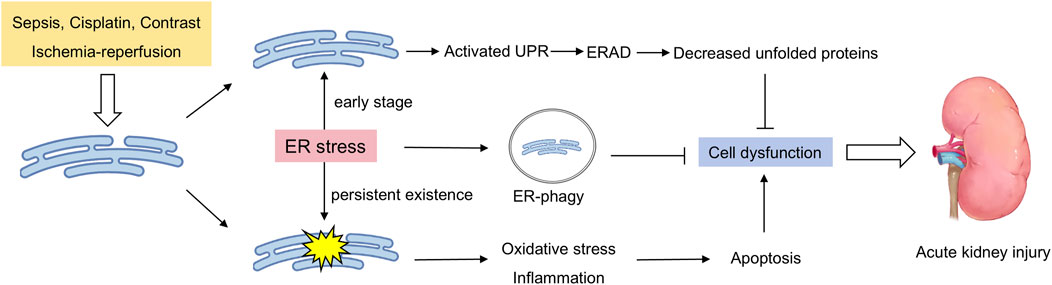
Figure 1. Double-sided endoplasmic reticulum stress in acute renal injury. External stimuli induce ER stress in the kidneys, which activates the unfolded protein response to clear unfolded proteins in a timely manner. Moreover, the occurrence of ER-phagy ensures that the damaged ER is cleared in a timely manner. When these protective mechanisms are insufficient to cope with sustained stress, ER stress leads to pathological processes such as cell apoptosis, the inflammatory response, and oxidative stress, further exacerbating kidney damage.
3.1 Cisplatin-induced AKI
Cisplatin is a very effective chemotherapy drug that is often used to treat solid tumors. Nephrotoxicity is the main side effect, and the risk of nephrotoxicity in cisplatin-treated patients is between 20% and 35%, manifested mainly as severe renal tubule injury and acute renal failure (Fang et al., 2021). In the kidney, cisplatin can be passively absorbed into renal tubule cells by organic cation transporter 2 (OCT2) and then continuously accumulate in the kidney (Eljack et al., 2014). The entry of cisplatin into the urine is mediated by transporters such as multidrug resistance-associated proteins (MRPs) and multiantimicrobial extrusion proteins (MATEs) (Holditch et al., 2019). Severe nephrotoxicity severely limits the use of cisplatin. At present, there is still a lack of effective drugs to prevent cisplatin-induced AKI. There are many molecular mechanisms of cisplatin-induced AKI, such as oxidative stress (Huang et al., 2017) and inflammation (Yu et al., 2023; Chen et al., 2019). However, with the increasing research, the role of ER homeostasis in cisplatin-induced AKI progression has also been revealed.
Multiple studies have shown that ER stress is overactivated in the kidneys of individuals with cisplatin-induced AKI and that the inhibition of ER stress can effectively slow kidney injury (Yang et al., 2024; Liu et al., 2024). NSC228155 is a novel compound with anticancer and antibacterial effects, and it reduces the ER stress level of cisplatin-induced renal tubular cells and HK-2 cells and inhibits apoptosis (Li et al., 2022). Similar results revealed that achyranthes aspera extract and dexmedetomidine attenuate cisplatin-induced kidney damage by inhibiting ER stress (Lin et al., 2024; Chai et al., 2020). Moreover, disturbances in energy metabolism are present in cisplatin-induced AKI and exacerbate the progression of AKI. Deceased fatty acid oxidation (FAO) levels, inhibited ATP production and increased lipid deposition in the kidneys of patients with cisplatin-induced AKI, while increasing the level of FAO effectively protected the kidney function (Xu et al., 2022; Li et al., 2020). The main site of fatty acid oxidation is the mitochondria; however, the MAM domain is located between the ER and mitochondria, and the ER can regulate mitochondrial function through the MAM (Senft and Ronai, 2015; Doghman-Bouguerra and Lalli, 2019). The MAM mediates the flow of calcium ions from the ER into the mitochondria, and increased levels of calcium ions promote mitochondrial ATP synthesis (Sun and Ding, 2020; Li et al., 2019; Barazzuol et al., 2021). In addition, markers of ER-mediated cell death, such as caspase-12 and calpain, are activated in rat kidney tissue (Peyrou et al., 2007). Although a number of studies have revealed that overactivation of ER stress in cisplatin-induced AKI and that ER stress inhibitors can effectively reverse renal injury, how ER homeostasis is disrupted in cisplatin-induced AKI, and its molecular mechanism have yet to be determined. These mechanisms need to be further explored in future studies to better develop drugs that target ER stress.
3.2 Ischemia‒reperfusion injury (IRI)
The kidney is a very sensitive organ to ischemia and hypoxia. Renal ischemia‒reperfusion injury is a common complication after transplantation and heart surgery. Renal blood circulation is restricted during kidney transplantation, which is indispensable for kidney reperfusion after surgery, resulting in kidney damage (Zhao et al., 2020). Kidney cells change from aerobic respiration to anaerobic respiration when the oxygen supply of the kidney decreases, the production of ATP in the kidney also decreases, and the accumulation of lactic acid increases (Nieuwenhuijs-Moeke et al., 2020). Moreover, a decrease in calcium ion excretion leads an increase in its intracellular concentration. When reperfusion occurs, the recovery of the oxygen content leads to the production of many reactive oxygen species, and the concentration of intracellular calcium ions further increases, inducing cell death (Nieuwenhuijs-Moeke et al., 2020). In addition, ER dysfunction plays a key role in the pathophysiological process of renal ischemia-reperfusion injury.
During IRI, renal cells undergo hypoxia, oxidative stress, and disruption of calcium homeostasis, all of which can lead to ER stress. Multiple studies have shown that renal IRI induces ER stress in renal tubular epithelial cells. Tang et al. demonstrated through single-cell RNA sequencing that kidney cells from ischemic AKI patients who differentially expressed genes in renal proximal tubular cells were enriched mainly in ER stress signals (Tang et al., 2023). Similar evidence has been reported in the kidneys of both mice and rats (Zhang et al., 2020; Tang et al., 2020), and persistent ER stress exacerbates kidney damage, whereas drug-mediated inhibition of ER stress can effectively slow kidney damage (Zhang et al., 2024). However, the role of ER stress in IRI still needs further exploration. Although most studies have shown that inhibiting ER stress can alleviate IRI-related renal damage, the role of the ER is different in the early stages of the disease. Chandrika et al. reported that the use of the ER stress inducer tunicamycin to intervene in renal tubular epithelial cells activated ER stress, increased the expression of the ER chaperone protein Grp78, and triggered downstream autophagy pathways, thereby inhibiting the activation of caspase-3 and cell death (Chandrika et al., 2015). This means that when ER stress is in the early to middle stages, autophagy can be induced to promptly clear damaged proteins and organelles, whereas when ER stress is too severe or excessive, the apoptotic pathway may be activated. Therefore, when the ER is targeted as a preventive and therapeutic target for renal IRI, controlling and monitoring ER stress levels is crucial.
3.3 Sepsis
Sepsis is a serious form of organ dysfunction caused mainly by the host’s dysregulated response to infection (Srzic et al., 2022). When faced with severe infections, the body produces excessive inflammatory factors such as interleukins and tumor necrosis factor, and experiences endothelial damage and abnormal secretion of vasoactive substances, which can trigger AKI (He et al., 2022). Bagshaw et al. reported that approximately 64.4% of septic shock patients develop early AKI (Bagshaw et al., 2009). However, even in patients without severe sepsis or shock, AKI is still common: 34% of nonsevere community-acquired pneumonia patients develop AKI (Murugan et al., 2010). Regardless of the species, disease stage, and severity of sepsis, three pathophysiological changes are consistently observed in sepsis patients and animal models: microcirculation dysfunction, inflammation, and the bioenergetic adaptive response to injury (Zarbock et al., 2014). Overall, the occurrence of sepsis AKI significantly increases the risk of in-hospital mortality (2-6 times) and is closely associated with the likelihood of later progression to chronic kidney disease (Hoste et al., 2015; Pais et al., 2024). However, there is still a lack of specific molecular markers and treatment methods for sepsis-induced AKI in clinical practice. Therefore, a deeper understanding of the pathogenesis of sepsis-induced AKI is necessary. Recently, multiple studies have shown that abnormal ER homeostasis is involved in the occurrence and development of sepsis-induced AKI.
Excessive misfolded proteins in the ER can further exacerbate ER stress and lead to apoptosis. Molecular chaperones are particularly important for promoting protein folding in the ER. Porter et al. demonstrated that knocking out the ER-resident protein GRP170 resulted in an acute kidney injury phenotype in mice (Porter et al., 2022). Moreover, in a cell model induced by lipopolysaccharide (LPS), the expression of the key protein GRP78 in ER stress is elevated, accompanied by an increase in apoptosis. The absence of GRP78 can alleviate the LPS-induced immune response and oxidative stress (Teng et al., 2018). Similar results have also been reported, with a significant increase in ER stress marker proteins in septic AKI mouse or rat models (Sun et al., 2024; Luo et al., 2020). Drug or gene knockout-mediated inhibition of ER stress can effectively delay sepsis-induced AKI-related kidney damage. Sun et al. reported that Marins-1 treatment significantly inhibited kidney damage in AKI model mice induced by cecal ligation and puncture, whereas an AMPK inhibitor (Compound C) partially blocked the protective effect of Marins-1 (Sun et al., 2024). Although multiple studies have revealed that maintaining ER homeostasis and inhibiting ER stress can effectively alleviate sepsis-induced AKI, current research has several limitations. At present, the molecular mechanism of ER stress in sepsis-induced AKI is not clear. Currently, only ER stress has been observed during sepsis-induced AKI, but its molecular mechanism still needs to be explored. Therefore, a deeper understanding of the molecular mechanism of ER stress in the occurrence of sepsis AKI is beneficial for targeting ER homeostasis as a therapeutic drug for sepsis AKI in the future.
3.4 Contrast-induced (CI) AKI
CI-AKI refers to the sudden deterioration of renal function caused by intravenous or arterial injection of iodinated contrast agents (Mccullough et al., 2016). Its main manifestation is a sudden and long-term decline in renal function that occurs 48–72 h after injection (Ward and Valentovic, 2019). It was first described by Bartels et al., in 1954 (Bartels et al., 1954). With the development of follow-up imaging, the incidence rate of CI-AKI has gradually increased. Although the incidence of CI-AKI is low in the general population, it is significantly greater in high-risk groups, such as those with renal insufficiency, diabetes, dehydration, heart failure and elderly individuals (Rundback et al., 2011). Currently, there is still a lack of effective prevention and control measures for CI-AKI. Recent studies suggest that ER dysfunction may be involved in the occurrence and development of CI-AKI. The radiocontrast agent meglumine diatrizoate can upregulate the expression of GRP78, ATF4, and CHOP to induce ER stress, leading to the activation of the renin‒angiotensin system and the apoptosis of renal tubular cells in rats. However, pretreatment with valsartan significantly inhibited ER stress levels and renal injury (Sun et al., 2017). Apelin is an endogenous antioxidant and anti-inflammatory physiological regulator (Vinel et al., 2018). Liu et al. reported that exogenous apelin-13 can alleviate cell and renal tissue damage in rats induced by contrast agent intervention by inhibiting ER stress (Liu et al., 2023). These studies have partially revealed the role of ER stress in CI-AKI, and the inhibition of ER stress can alleviate CI-AKI injury. However, the molecular mechanism of ER stress activation in CI-AKI needs to be further explored.
4 ER stress inhibitors and clinical translation
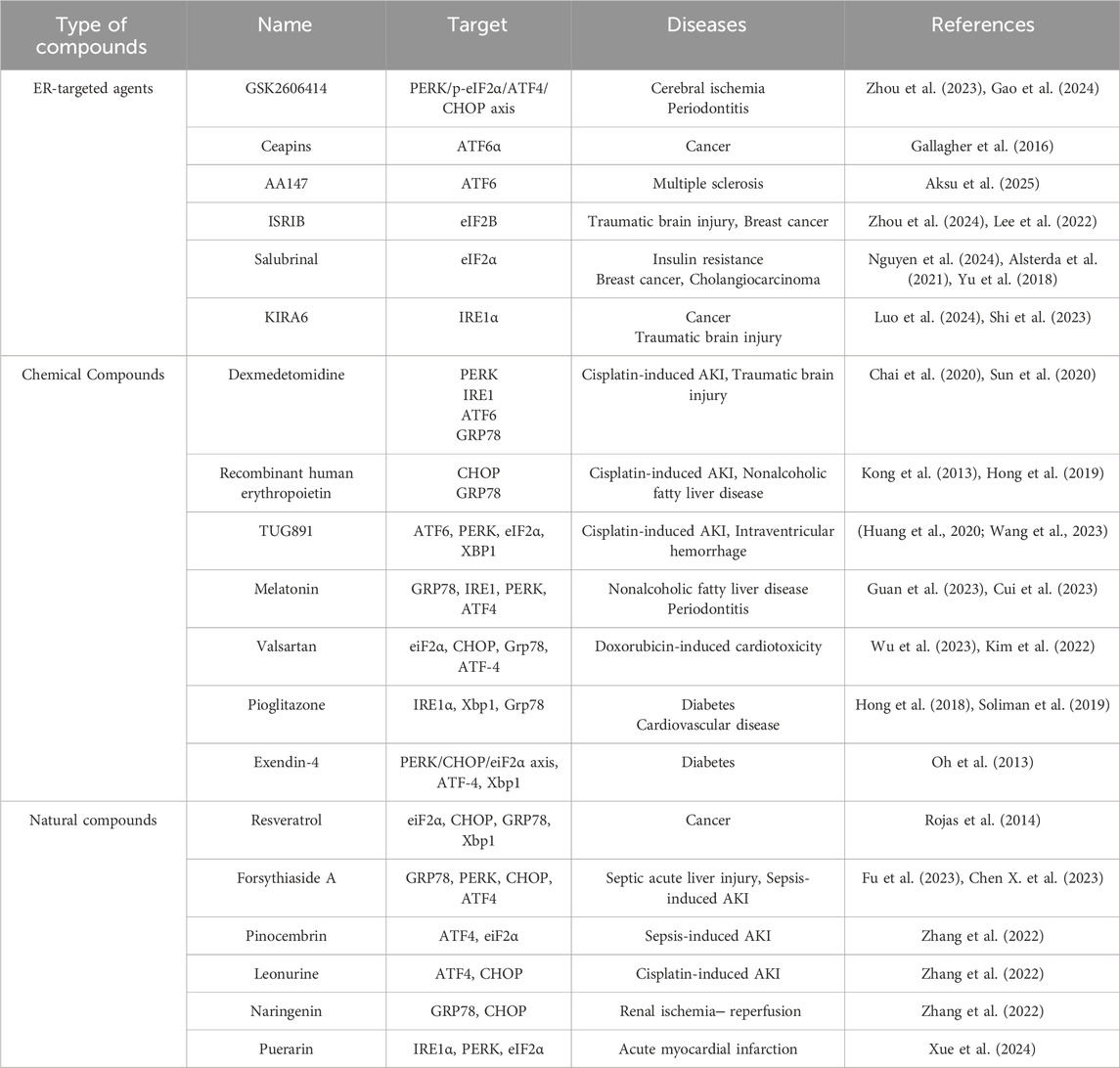
Given the role of ER stress in the occurrence and development of different types of AKI, targeting ER stress is a potential approach for developing drugs for the prevention and treatment of AKI in the future. Currently, multiple studies have reported that some compounds or drugs can alleviate AKI by inhibiting ER stress, and we have summarized these finding here (Table 1).
Drug intervention targeting ER homeostasis is a promising approach for the treatment of AKI. At present, small molecule inhibitors of key proteins in the ER stress pathway and some chemical chaperones, such as 4-PBA, have been found to improve AKI by inhibiting ER stress in cell and animal models, but there are still challenges that need to be further addressed in the use of ER stress as a target for the treatment of AKI in the future. First, many ER stress regulators lack specificity and may have unexpected effects on other cellular processes. For example, some regulators may reduce ER stress while inhibiting the necessary UPR pathways required for cell survival. In addition, ER stress has both protective and harmful effects. Excessive inhibition may hinder necessary adaptive responses, whereas excessive activation may lead to apoptosis and inflammation. Therefore, how to precisely regulate ER stress to achieve the best balance between adaptation and cell death is a considerable challenge. Most of the existing preclinical models use acute injury conditions and cannot fully represent the complexity of human AKI, especially in the case of chronic kidney injury. Furthermore, there may be differences in ER stress responses between animal models and human tissues, making it difficult for preclinical research results to accurately predict clinical outcomes and limiting the transformation of research results into human applications. Finally, at present, relatively few studies on ER stress in samples from AKI patients exist. The very limited clinical research data make it difficult to determine the exact role and therapeutic effect of ER stress in human AKI, and evaluations of the safety and efficacy of related drugs in patients with kidney diseases are lacking.
5 Conclusion
AKI is a common critical disease with high morbidity and mortality. There is an urgent need to investigate its pathogenesis and find effective treatments. The role of ER homeostasis in acute renal injury has been thoroughly investigated. While the findings were surprising, there were several limitations. The double-edged sword effect and spatiotemporal dynamics of ER stress in AKI are worthy of exploration. ER stress is a protective response in the early stage that aims to restore ER homeostasis. However, persistent or excessive ER stress results in proapoptotic and proinflammatory responses. How to precisely define this “turning point” and the exact role (whether it is a driving factor or an accompanying phenomenon) of ER stress at different stages of AKI remain unclear. Moreover, there is complex crosstalk and feedback regulation among the three main pathways of the UPR (PERK, IRE1α, and ATF6). In AKI, how these pathways precisely coordinate to determine cellular outcomes is not fully understood. Currently, research on ER stress and AKI often uses animal models, such as rats and mice. However, there are differences between animal models and humans in terms of physiology, pathology, and immune response, which limits the applicability of research results in humans. In the future, research should focus on the precise regulation of the ER stress signaling pathway and the development of safe and effective treatment strategies. Overall, targeting ER homeostasis is an effective potential therapeutic target for AKI.
Author contributions
LH: Writing – original draft. HC: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajoolabady, A., Kaplowitz, N., Lebeaupin, C., Kroemer, G., Kaufman, R. J., Malhi, H., et al. (2023). Endoplasmic reticulum stress in liver diseases. Hepatology 77 (2), 619–639. doi:10.1002/hep.32562
Aksu, M., Kaschke, K., Podojil, J. R., Chiang, M., Steckler, I., Bruce, K., et al. (2025). AA147 alleviates symptoms in a mouse model of multiple sclerosis by reducing oligodendrocyte loss. Glia 73 (6), 1241–1257. doi:10.1002/glia.70001
Alsterda, A., Asha, K., Powrozek, O., Repak, M., Goswami, S., Dunn, A. M., et al. (2021). Salubrinal exposes anticancer properties in inflammatory breast cancer cells by manipulating the endoplasmic reticulum stress pathway. Front. Oncol. 11, 654940. doi:10.3389/fonc.2021.654940
Ambudkar, I. S. (2014). Ca²⁺ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 55 (6), 297–305. doi:10.1016/j.ceca.2014.02.009
Anelli, T., and Sitia, R. (2008). Protein quality control in the early secretory pathway. EMBO J. 27 (2), 315–327. doi:10.1038/sj.emboj.7601974
Bagshaw, S. M., Lapinsky, S., Dial, S., Arabi, Y., Dodek, P., Wood, G., et al. (2009). Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 35 (5), 871–881. doi:10.1007/s00134-008-1367-2
Barazzuol, L., Giamogante, F., and Cali, T. (2021). Mitochondria associated membranes (MAMs): architecture and physiopathological role. Cell Calcium 94, 102343. doi:10.1016/j.ceca.2020.102343
Bartels, E. D., Brun, G. C., Gammeltoft, A., and Gjorup, P. A. (1954). Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Med. Scand. 150 (4), 297–302. doi:10.1111/j.0954-6820.1954.tb18632.x
Berridge, M. J. (2016). The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 96 (4), 1261–1296. doi:10.1152/physrev.00006.2016
Blackwood, E. A., Macdonnell, L. F., Thuerauf, D. J., Bilal, A. S., Murray, V. B., Bedi, K. J., et al. (2023). Noncanonical form of ERAD regulates cardiac hypertrophy. Circulation 147 (1), 66–82. doi:10.1161/CIRCULATIONAHA.122.061557
Cao, Y., Chen, X., Zhu, Z., Luo, Z., Hao, Y., Yang, X., et al. (2024). STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury. Cell Death Dis. 15 (3), 217. doi:10.1038/s41419-024-06600-1
Chai, Y., Zhu, K., Li, C., Wang, X., Shen, J., Yong, F., et al. (2020). Dexmedetomidine alleviates cisplatin‑induced acute kidney injury by attenuating endoplasmic reticulum stress‑induced apoptosis via the α2AR/PI3K/AKT pathway. Mol. Med. Rep. 21 (3), 1597–1605. doi:10.3892/mmr.2020.10962
Chambers, P. J., Juracic, E. S., Fajardo, V. A., and Tupling, A. R. (2022). Role of SERCA and sarcolipin in adaptive muscle remodeling. Am. J. Physiol. Cell Physiol. 322 (3), C382–C394. doi:10.1152/ajpcell.00198.2021
Chandrika, B. B., Yang, C., Ou, Y., Feng, X., Muhoza, D., Holmes, A. F., et al. (2015). Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS One 10 (10), e0140025. doi:10.1371/journal.pone.0140025
Chen, X., and Cubillos-Ruiz, J. R. (2021). Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer. 21 (2), 71–88. doi:10.1038/s41568-020-00312-2
Chen, X., Shi, C., He, M., Xiong, S., and Xia, X. (2023). Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct. Target Ther. 8 (1), 352. doi:10.1038/s41392-023-01570-w
Chen, X., Wei, W., Li, Y., Huang, J., and Ci, X. (2019). Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 308, 269–278. doi:10.1016/j.cbi.2019.05.040
Chino, H., and Mizushima, N. (2020). ER-phagy: quality control and turnover of endoplasmic reticulum. Trends Cell Biol. 30 (5), 384–398. doi:10.1016/j.tcb.2020.02.001
Chino, H., and Mizushima, N. (2023). ER-phagy: quality and quantity control of the endoplasmic reticulum by autophagy. Cold Spring Harb. Perspect. Biol. 15 (1), a041256. doi:10.1101/cshperspect.a041256
Christianson, J. C., Jarosch, E., and Sommer, T. (2023). Mechanisms of substrate processing during ER-associated protein degradation. Nat. Rev. Mol. Cell Biol. 24 (11), 777–796. doi:10.1038/s41580-023-00633-8
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M., and Walter, P. (2005). On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 102 (52), 18773–18784. doi:10.1073/pnas.0509487102
Cui, Y., Hong, S., Xia, Y., Li, X., He, X., Hu, X., et al. (2023). Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv. Sci. (Weinh) 10 (27), e2302029. doi:10.1002/advs.202302029
Deng, F., Zhang, H., Zhou, W., Ma, S., Kang, Y., Yang, W., et al. (2023). TRPA1 promotes cisplatin-induced acute kidney injury via regulating the endoplasmic reticulum stress-mitochondrial damage. J. Transl. Med. 21 (1), 695. doi:10.1186/s12967-023-04351-9
de Ridder, I., Kerkhofs, M., Lemos, F. O., Loncke, J., Bultynck, G., and Parys, J. B. (2023). The ER-mitochondria interface, where Ca(2+) and cell death meet. Cell Calcium 112, 102743. doi:10.1016/j.ceca.2023.102743
Dhureja, M., Arthur, R., Soni, D., Upadhayay, S., Temgire, P., and Kumar, P. (2023). Calcium channelopathies in neurodegenerative disorder: an untold story of RyR and SERCA. Expert Opin. Ther. Targets 27 (11), 1159–1172. doi:10.1080/14728222.2023.2277863
Dickson, E. J., Jensen, J. B., and Hille, B. (2016). Regulation of calcium and phosphoinositides at endoplasmic reticulum-membrane junctions. Biochem. Soc. Trans. 44 (2), 467–473. doi:10.1042/BST20150262
Di Conza, G., and Ho, P. C. (2020). ER stress responses: an emerging modulator for innate immunity. Cells 9 (3), 695. doi:10.3390/cells9030695
Doghman-Bouguerra, M., and Lalli, E. (2019). ER-mitochondria interactions: both strength and weakness within cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 1866 (4), 650–662. doi:10.1016/j.bbamcr.2019.01.009
Dreher, L. S., and Hoppe, T. (2018). Hepatic ERAD takes control of the organism. EMBO J. 37 (22), e100676. doi:10.15252/embj.2018100676
Eljack, N. D., Ma, H. Y., Drucker, J., Shen, C., Hambley, T. W., New, E. J., et al. (2014). Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics 6 (11), 2126–2133. doi:10.1039/c4mt00238e
Fang, C. Y., Lou, D. Y., Zhou, L. Q., Wang, J. C., Yang, B., He, Q. J., et al. (2021). Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 42 (12), 1951–1969. doi:10.1038/s41401-021-00620-9
Farese, R. J., and Walther, T. C. (2023). Glycerolipid synthesis and lipid droplet formation in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 15 (5), a041246. doi:10.1101/cshperspect.a041246
Ferro-Novick, S., Reggiori, F., and Brodsky, J. L. (2021). ER-phagy, ER homeostasis, and ER quality control: implications for disease. Trends biochem. Sci. 46 (8), 630–639. doi:10.1016/j.tibs.2020.12.013
Fu, J. N., Liu, S. C., Chen, Y., Zhao, J., Lu, N., and Ma, T. (2023). Forsythiaside A alleviates lipopolysaccharide-induced acute liver injury through inhibiting endoplasmic reticulum stress and NLRP3 inflammasome activation. Biol. Pharm. Bull. 46 (7), 979–986. doi:10.1248/bpb.b23-00137
Gallagher, C. M., Garri, C., Cain, E. L., Ang, K. K., Wilson, C. G., Chen, S., et al. (2016). Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. eLife 5, e11878. doi:10.7554/eLife.11878
Gallazzini, M., and Pallet, N. (2018). Endoplasmic reticulum stress and kidney dysfunction. Biol. Cell. 110 (9), 205–216. doi:10.1111/boc.201800019
Gao, C., Shi, Q., Pan, X., Chen, J., Zhang, Y., Lang, J., et al. (2024). Neuromuscular organoids model spinal neuromuscular pathologies in C9orf72 amyotrophic lateral sclerosis. Cell Rep. 43 (3), 113892. doi:10.1016/j.celrep.2024.113892
Gillespie, D. (2020). Recruiting RyRs to open in a Ca(2+) release unit: single-RyR gating properties make RyR group dynamics. Biophys. J. 118 (1), 232–242. doi:10.1016/j.bpj.2019.11.021
Gonzalez, A., Covarrubias-Pinto, A., Bhaskara, R. M., Glogger, M., Kuncha, S. K., Xavier, A., et al. (2023). Ubiquitination regulates ER-phagy and remodelling of endoplasmic reticulum. Nature 618 (7964), 394–401. doi:10.1038/s41586-023-06089-2
Guan, Q., Wang, Z., Hu, K., Cao, J., Dong, Y., and Chen, Y. (2023). Melatonin ameliorates hepatic ferroptosis in NAFLD by inhibiting ER stress via the MT2/cAMP/PKA/IRE1 signaling pathway. Int. J. Biol. Sci. 19 (12), 3937–3950. doi:10.7150/ijbs.85883
Gubas, A., and Dikic, I. (2022). ER remodeling via ER-phagy. Mol. Cell. 82 (8), 1492–1500. doi:10.1016/j.molcel.2022.02.018
Hamstra, S. I., Whitley, K. C., Baranowski, R. W., Kurgan, N., Braun, J. L., Messner, H. N., et al. (2020). The role of phospholamban and GSK3 in regulating rodent cardiac SERCA function. Am. J. Physiol. Cell Physiol. 319 (4), C694–C699. doi:10.1152/ajpcell.00318.2020
He, F. F., Wang, Y. M., Chen, Y. Y., Huang, W., Li, Z. Q., and Zhang, C. (2022). Sepsis-induced AKI: from pathogenesis to therapeutic approaches. Front. Pharmacol. 13, 981578. doi:10.3389/fphar.2022.981578
Hetz, C. (2012). The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13 (2), 89–102. doi:10.1038/nrm3270
Holditch, S. J., Brown, C. N., Lombardi, A. M., Nguyen, K. N., and Edelstein, C. L. (2019). Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 20 (12), 3011. doi:10.3390/ijms20123011
Hong, S. W., Lee, J., Cho, J. H., Kwon, H., Park, S. E., Rhee, E. J., et al. (2018). Pioglitazone attenuates palmitate-induced inflammation and endoplasmic reticulum stress in pancreatic beta-cells. Endocrinol. Metab. Seoul. 33 (1), 105–113. doi:10.3803/EnM.2018.33.1.105
Hong, T., Ge, Z., Zhang, B., Meng, R., Zhu, D., and Bi, Y. (2019). Erythropoietin suppresses hepatic steatosis and obesity by inhibiting endoplasmic reticulum stress and upregulating fibroblast growth factor 21. Int. J. Mol. Med. 44 (2), 469–478. doi:10.3892/ijmm.2019.4210
Hoste, E. A., Bagshaw, S. M., Bellomo, R., Cely, C. M., Colman, R., Cruz, D. N., et al. (2015). Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41 (8), 1411–1423. doi:10.1007/s00134-015-3934-7
Hoyer, M. J., Capitanio, C., Smith, I. R., Paoli, J. C., Bieber, A., Jiang, Y., et al. (2024). Combinatorial selective ER-phagy remodels the ER during neurogenesis. Nat. Cell Biol. 26 (3), 378–392. doi:10.1038/s41556-024-01356-4
Huang, Y. C., Tsai, M. S., Hsieh, P. C., Shih, J. H., Wang, T. S., Wang, Y. C., et al. (2017). Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 329, 128–139. doi:10.1016/j.taap.2017.05.034
Huang, Z., Guo, F., Xia, Z., Liang, Y., Lei, S., Tan, Z., et al. (2020). Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomed. Pharmacother. 126, 110056. doi:10.1016/j.biopha.2020.110056
Johnson, S., Michalak, M., Opas, M., and Eggleton, P. (2001). The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 11 (3), 122–129. doi:10.1016/s0962-8924(01)01926-2
Kim, B. S., Park, I. H., Lee, A. H., Kim, H. J., Lim, Y. H., and Shin, J. H. (2022). Sacubitril/valsartan reduces endoplasmic reticulum stress in a rat model of doxorubicin-induced cardiotoxicity. Arch. Toxicol. 96 (4), 1065–1074. doi:10.1007/s00204-022-03241-1
Kito, H., and Ohya, S. (2021). Role of K(+) and Ca(2+)-permeable channels in osteoblast functions. Int. J. Mol. Sci. 22 (19), 10459. doi:10.3390/ijms221910459
Kong, D., Zhuo, L., Gao, C., Shi, S., Wang, N., Huang, Z., et al. (2013). Erythropoietin protects against cisplatin-induced nephrotoxicity by attenuating endoplasmic reticulum stress-induced apoptosis. J. Nephrol. 26 (1), 219–227. doi:10.5301/jn.5000177
Lee, D. M., Seo, M. J., Lee, H. J., Jin, H. J., and Choi, K. S. (2022). ISRIB plus bortezomib triggers paraptosis in breast cancer cells via enhanced translation and subsequent proteotoxic stress. Biochem. Biophys. Res. Commun. 596, 56–62. doi:10.1016/j.bbrc.2022.01.082
Li, J., Zhang, D., Brundel, B., and Wiersma, M. (2019). Imbalance of ER and mitochondria interactions: prelude to cardiac ageing and disease? Cells 8 (12), 1617. doi:10.3390/cells8121617
Li, M., Li, C. M., Ye, Z. C., Huang, J., Li, Y., Lai, W., et al. (2020). Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell. Mol. Med. 24 (9), 5109–5121. doi:10.1111/jcmm.15148
Li, Y., Jiang, Y., Zhou, W., Wu, Y., Zhang, S., Ding, G., et al. (2022). Maintaining homeostasis of mitochondria and endoplasmic reticulum with NSC228155 alleviates cisplatin-induced acute kidney injury. Free Radic. Biol. Med. 181, 270–287. doi:10.1016/j.freeradbiomed.2022.02.003
Lin, S. Y., Chang, C. L., Liou, K. T., Kao, Y. K., Wang, Y. H., Chang, C. C., et al. (2024). The protective role of Achyranthes aspera extract against cisplatin-induced nephrotoxicity by alleviating oxidative stress, inflammation, and PANoptosis. J. Ethnopharmacol. 319 (Pt 1), 117097. doi:10.1016/j.jep.2023.117097
Liu, H., Wang, L., Weng, X., Chen, H., Du, Y., Diao, C., et al. (2019). Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 24, 101195. doi:10.1016/j.redox.2019.101195
Liu, Q., Duan, S. B., Wang, L., Luo, X. Q., Wang, H. S., Deng, Y. H., et al. (2023). Apelin-13 alleviates contrast-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Ren. Fail. 45 (1), 2179852. doi:10.1080/0886022X.2023.2179852
Liu, Y. T., Zhang, H., Duan, S. B., Wang, J. W., Chen, H., Zhan, M., et al. (2024). Mitofusin2 ameliorated endoplasmic reticulum stress and mitochondrial reactive oxygen species through maintaining mitochondria-associated endoplasmic reticulum membrane integrity in cisplatin-induced acute kidney injury. Antioxid. Redox Signal 40 (1-3), 16–39. doi:10.1089/ars.2022.0178
Luciani, D. S., Gwiazda, K. S., Yang, T. L., Kalynyak, T. B., Bychkivska, Y., Frey, M. H., et al. (2009). Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 58 (2), 422–432. doi:10.2337/db07-1762
Luo, C. J., Luo, F., Bu, Q. D., Jiang, W., Zhang, W., Liu, X. M., et al. (2020). Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 164 (1), 49–56. doi:10.5507/bp.2019.006
Luo, Z., Jiang, M., Cheng, N., Zhao, X., Liu, H., Wang, S., et al. (2024). Remodeling the hepatic immune microenvironment and demolishing T cell traps to enhance immunotherapy efficacy in liver metastasis. J. Control. Release. 373, 890–904. doi:10.1016/j.jconrel.2024.07.057
Marciniak, S. J., Chambers, J. E., and Ron, D. (2022). Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 21 (2), 115–140. doi:10.1038/s41573-021-00320-3
Mccullough, P. A., Choi, J. P., Feghali, G. A., Schussler, J. M., Stoler, R. M., Vallabahn, R. C., et al. (2016). Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 68 (13), 1465–1473. doi:10.1016/j.jacc.2016.05.099
Mekahli, D., Bultynck, G., Parys, J. B., De Smedt, H., and Missiaen, L. (2011). Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 3 (6), a004317. doi:10.1101/cshperspect.a004317
Murugan, R., Karajala-Subramanyam, V., Lee, M., Yende, S., Kong, L., Carter, M., et al. (2010). Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 77 (6), 527–535. doi:10.1038/ki.2009.502
Nelson, B. R., Makarewich, C. A., Anderson, D. M., Winders, B. R., Troupes, C. D., Wu, F., et al. (2016). A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351 (6270), 271–275. doi:10.1126/science.aad4076
Nemirovskaya, T. L., and Sharlo, K. A. (2022). Roles of ATP and SERCA in the regulation of calcium turnover in unloaded skeletal muscles: current view and future directions. Int. J. Mol. Sci. 23 (13), 6937. doi:10.3390/ijms23136937
Nguyen, K., Tang, J., Cho, S., Ying, F., Sung, H. K., Jahng, J. W., et al. (2024). Salubrinal promotes phospho-eIF2α-dependent activation of UPR leading to autophagy-mediated attenuation of iron-induced insulin resistance. Mol. Metab. 83, 101921. doi:10.1016/j.molmet.2024.101921
Nicotera, P., Zhivotovsky, B., and Orrenius, S. (1994). Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium 16 (4), 279–288. doi:10.1016/0143-4160(94)90091-4
Nieuwenhuijs-Moeke, G. J., Pischke, S. E., Berger, S. P., Sanders, J., Pol, R. A., Struys, M., et al. (2020). Ischemia and reperfusion injury in kidney transplantation: relevant mechanisms in injury and repair. J. Clin. Med. 9 (1), 253. doi:10.3390/jcm9010253
Oakes, S. A., and Papa, F. R. (2015). The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 10, 173–194. doi:10.1146/annurev-pathol-012513-104649
Ogawa, M., Sawaguchi, S., Furukawa, K., and Okajima, T. (2015). N-acetylglucosamine modification in the lumen of the endoplasmic reticulum. Biochim. Biophys. Acta 1850 (6), 1319–1324. doi:10.1016/j.bbagen.2015.03.003
Oh, Y. S., Lee, Y. J., Kang, Y., Han, J., Lim, O. K., and Jun, H. S. (2013). Exendin-4 inhibits glucolipotoxic ER stress in pancreatic β cells via regulation of SREBP1c and C/EBPβ transcription factors. J. Endocrinol. 216 (3), 343–352. doi:10.1530/JOE-12-0311
Pais, T., Jorge, S., and Lopes, J. A. (2024). Acute kidney injury in sepsis. Int. J. Mol. Sci. 25 (11), 5924. doi:10.3390/ijms25115924
Pehar, M., and Puglielli, L. (2013). Lysine acetylation in the lumen of the ER: a novel and essential function under the control of the UPR. Biochim. Biophys. Acta 1833 (3), 686–697. doi:10.1016/j.bbamcr.2012.12.004
Periasamy, M., and Kalyanasundaram, A. (2007). SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35 (4), 430–442. doi:10.1002/mus.20745
Peyrou, M., Hanna, P. E., and Cribb, A. E. (2007). Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol. Sci. 99 (1), 346–353. doi:10.1093/toxsci/kfm152
Pincus, D., Chevalier, M. W., Aragon, T., van Anken, E., Vidal, S. E., El-Samad, H., et al. (2010). BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8 (7), e1000415. doi:10.1371/journal.pbio.1000415
Porter, A. W., Nguyen, D. N., Clayton, D. R., Ruiz, W. G., Mutchler, S. M., Ray, E. C., et al. (2022). The molecular chaperone GRP170 protects against ER stress and acute kidney injury in mice. JCI Insight 7 (5), e151869. doi:10.1172/jci.insight.151869
Qian, Q., Li, M., Zhang, Z., Davis, S. W., Rahmouni, K., Norris, A. W., et al. (2024). Obesity disrupts the pituitary-hepatic UPR communication leading to NAFLD progression. Cell Metab. 36 (7), 1550–1565.e9. doi:10.1016/j.cmet.2024.04.014
Rahmati, M., Moosavi, M. A., and Mcdermott, M. F. (2018). ER stress: a therapeutic target in rheumatoid arthritis? Trends Pharmacol. Sci. 39 (7), 610–623. doi:10.1016/j.tips.2018.03.010
Rathod, N., Guerrero-Serna, G., Young, H. S., and Espinoza-Fonseca, L. M. (2024). Replacement of Lys27 by asparagine in the SERCA regulator myoregulin: a Ca(2+) affinity modulator or a catalytic activity switch? Biochim. Biophys. Acta Mol. Cell Res. 1871 (1), 119613. doi:10.1016/j.bbamcr.2023.119613
Raturi, A., Ortiz-Sandoval, C., and Simmen, T. (2014). Redox dependence of endoplasmic reticulum (ER) Ca²⁺ signaling. Histol. Histopathol. 29 (5), 543–552. doi:10.14670/HH-29.10.543
Ren, Q., Guo, F., Tao, S., Huang, R., Ma, L., and Fu, P. (2020). Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 122, 109772. doi:10.1016/j.biopha.2019.109772
Ricciardi, C. A., and Gnudi, L. (2020). The endoplasmic reticulum stress and the unfolded protein response in kidney disease: implications for vascular growth factors. J. Cell. Mol. Med. 24 (22), 12910–12919. doi:10.1111/jcmm.15999
Rojas, C., Pan-Castillo, B., Valls, C., Pujadas, G., Garcia-Vallve, S., Arola, L., et al. (2014). Resveratrol enhances palmitate-induced ER stress and apoptosis in cancer cells. PLoS One 9 (12), e113929. doi:10.1371/journal.pone.0113929
Rundback, J. H., Nahl, D., and Yoo, V. (2011). Contrast-induced nephropathy. J. Vasc. Surg. 54 (2), 575–579. doi:10.1016/j.jvs.2011.04.047
Schwarz, D. S., and Blower, M. D. (2016). The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 73 (1), 79–94. doi:10.1007/s00018-015-2052-6
Seaayfan, E., Defontaine, N., Demaretz, S., Zaarour, N., and Laghmani, K. (2016). OS9 protein interacts with Na-K-2Cl Co-transporter (NKCC2) and targets its immature form for the endoplasmic reticulum-associated degradation pathway. J. Biol. Chem. 291 (9), 4487–4502. doi:10.1074/jbc.M115.702514
Seino, S., Shibasaki, T., and Minami, K. (2011). Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 121 (6), 2118–2125. doi:10.1172/JCI45680
Sekiya, M., Maruko-Otake, A., Hearn, S., Sakakibara, Y., Fujisaki, N., Suzuki, E., et al. (2017). EDEM function in ERAD protects against chronic ER proteinopathy and age-related physiological decline in Drosophila. Dev. Cell. 41 (6), 652–664. doi:10.1016/j.devcel.2017.05.019
Senft, D., and Ronai, Z. A. (2015). UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends biochem. Sci. 40 (3), 141–148. doi:10.1016/j.tibs.2015.01.002
Shi, G., Liu, L., Cao, Y., Ma, G., Zhu, Y., Xu, J., et al. (2023). Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. J. Neuroinflammation 20 (1), 222. doi:10.1186/s12974-023-02903-w
Soliman, E., Behairy, S. F., El-Maraghy, N. N., and Elshazly, S. M. (2019). PPAR-gamma agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats. Life Sci. 239, 117047. doi:10.1016/j.lfs.2019.117047
Srzic, I., Nesek, A. V., and Tunjic, P. D. (2022). Sepsis definition: what's new in the treatment guidelines. Acta Clin. Croat. 61 (Suppl. 1), 67–72. doi:10.20471/acc.2022.61.s1.11
Stolz, A., and Grumati, P. (2019). The various shades of ER-phagy. FEBS J. 286 (23), 4642–4649. doi:10.1111/febs.15031
Sun, D., Wang, J., Liu, X., Fan, Y., Yang, M., and Zhang, J. (2020). Dexmedetomidine attenuates endoplasmic reticulum stress-induced apoptosis and improves neuronal function after traumatic brain injury in mice. Brain Res. 1732, 146682. doi:10.1016/j.brainres.2020.146682
Sun, K., Yao, C., Xu, G., Wang, J., Shou, S., and Jin, H. (2025). Research progress on the pathogenesis of AKI complicated by ECMO. Clin. Exp. Nephrol. 29 (1), 10–20. doi:10.1007/s10157-024-02559-7
Sun, M., Wang, F., Li, H., Li, M., Wang, Y., Wang, C., et al. (2024). Maresin-1 attenuates sepsis-associated acute kidney injury via suppressing inflammation, endoplasmic reticulum stress and pyroptosis by activating the AMPK/SIRT3 pathway. J. Inflamm. Res. 17, 1349–1364. doi:10.2147/JIR.S442729
Sun, Y., and Ding, S. (2020). ER-mitochondria contacts and insulin resistance modulation through exercise intervention. Int. J. Mol. Sci. 21 (24), 9587. doi:10.3390/ijms21249587
Sun, Y., Peng, P. A., Ma, Y., Liu, X. L., Yu, Y., Jia, S., et al. (2017). Valsartan protects against contrast-induced acute kidney injury in rats by inhibiting endoplasmic reticulum stress-induced apoptosis. Curr. Vasc. Pharmacol. 15 (2), 174–183. doi:10.2174/1570161114666161025100656
Sweadner, K. J., and Donnet, C. (2001). Structural similarities of Na,K-ATPase and SERCA, the Ca(2+)-ATPase of the sarcoplasmic reticulum. Biochem. J. 356 (Pt 3), 685–704. doi:10.1042/0264-6021:3560685
Tang, C., Hu, Y., Gao, J., Jiang, J., Shi, S., Wang, J., et al. (2020). Dexmedetomidine pretreatment attenuates myocardial ischemia reperfusion induced acute kidney injury and endoplasmic reticulum stress in human and rat. Life Sci. 257, 118004. doi:10.1016/j.lfs.2020.118004
Tang, R., Jin, P., Shen, C., Lin, W., Yu, L., Hu, X., et al. (2023). Single-cell RNA sequencing reveals the transcriptomic landscape of kidneys in patients with ischemic acute kidney injury. Chin. Med. J. Engl. 136 (10), 1177–1187. doi:10.1097/CM9.0000000000002679
Teng, J., Liu, M., Su, Y., Li, K., Sui, N., Wang, S., et al. (2018). Down-regulation of GRP78 alleviates lipopolysaccharide-induced acute kidney injury. Int. Urol. Nephrol. 50 (11), 2099–2107. doi:10.1007/s11255-018-1911-0
Terrell, K., Choi, S., and Choi, S. (2023). Calcium's role and signaling in aging muscle, cellular senescence, and mineral interactions. Int. J. Mol. Sci. 24 (23), 17034. doi:10.3390/ijms242317034
Vance, J. E. (2014). MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841 (4), 595–609. doi:10.1016/j.bbalip.2013.11.014
Vance, J. E. (2015). Phospholipid synthesis and transport in mammalian cells. Traffic 16 (1), 1–18. doi:10.1111/tra.12230
Velluto, C., Mazzella, G. G., Scaramuzzo, L., Borruto, M. I., Inverso, M., Fulli, L., et al. (2025). Incidence and risk assessment of acute kidney injury (AKI) in spine surgery: a case report and literature review. J. Clin. Med. 14 (4), 1210. doi:10.3390/jcm14041210
Vinel, C., Lukjanenko, L., Batut, A., Deleruyelle, S., Pradere, J. P., Le Gonidec, S., et al. (2018). The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24 (9), 1360–1371. doi:10.1038/s41591-018-0131-6
Walther, T. C., Chung, J., and Farese, R. J. (2017). Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 33, 491–510. doi:10.1146/annurev-cellbio-100616-060608
Wang, H. X., Liu, C., Li, Y. Y., Cao, Y., Zhao, L., Zhao, Y. J., et al. (2023). TUG-891 inhibits neuronal endoplasmic reticulum stress and pyroptosis activation and protects neurons in a mouse model of intraventricular hemorrhage. Neural Regen. Res. 18 (10), 2278–2284. doi:10.4103/1673-5374.369116
Wang, Z., and Benning, C. (2012). Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem. Soc. Trans. 40 (2), 457–463. doi:10.1042/BST20110752
Ward, D. B., and Valentovic, M. A. (2019). Contrast induced acute kidney injury and direct cytotoxicity of iodinated radiocontrast media on renal proximal tubule cells. J. Pharmacol. Exp. Ther. 370 (2), 160–171. doi:10.1124/jpet.119.257337
Wiseman, R. L., Mesgarzadeh, J. S., and Hendershot, L. M. (2022). Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell. 82 (8), 1477–1491. doi:10.1016/j.molcel.2022.03.025
Wu, M., Wu, C., Song, T., Pan, K., Wang, Y., and Liu, Z. (2023). Structure and transport mechanism of the human calcium pump SPCA1. Cell Res. 33 (7), 533–545. doi:10.1038/s41422-023-00827-x
Wu, S. A., Li, Z. J., and Qi, L. (2025). Endoplasmic reticulum (ER) protein degradation by ER-associated degradation and ER-phagy. Trends Cell Biol. doi:10.1016/j.tcb.2025.01.002
Xu, H., and Van Remmen, H. (2021). The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: a potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 11 (1), 25. doi:10.1186/s13395-021-00280-7
Xu, S., Jia, P., Fang, Y., Jin, J., Sun, Z., Zhou, W., et al. (2022). Nuclear farnesoid X receptor attenuates acute kidney injury through fatty acid oxidation. Kidney Int. 101 (5), 987–1002. doi:10.1016/j.kint.2022.01.029
Xue, J., Ren, H., Zhang, Q., Gu, J., Xu, Q., Sun, J., et al. (2024). Puerarin attenuates myocardial ischemic injury and endoplasmic reticulum stress by upregulating the Mzb1 signal pathway. Front. Pharmacol. 15, 1442831. doi:10.3389/fphar.2024.1442831
Yan, M., Shu, S., Guo, C., Tang, C., and Dong, Z. (2018). Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 50 (5), 381–390. doi:10.1080/07853890.2018.1489142
Yang, Y., Xiong, T., Wang, T., Chen, X., Ma, Z., Zuo, B., et al. (2024). Small GTP-binding protein GDP dissociation stimulator influences cisplatin-induced acute kidney injury via PERK-dependent ER stress. Commun. Biol. 7 (1), 1091. doi:10.1038/s42003-024-06792-4
Yu, B., Jin, L., Yao, X., Zhang, Y., Zhang, G., Wang, F., et al. (2023). TRPM2 protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction via modulating autophagy. Theranostics 13 (13), 4356–4375. doi:10.7150/thno.84655
Yu, W., Xiang, Y., Luo, G., Zhao, X., Xiao, B., Cheng, Y., et al. (2018). Salubrinal enhances doxorubicin sensitivity in human cholangiocarcinoma cells through promoting DNA damage. Cancer Biother. Radiopharm. 33 (6), 258–265. doi:10.1089/cbr.2018.2447
Zadoorian, A., Du, X., and Yang, H. (2023). Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 19 (8), 443–459. doi:10.1038/s41574-023-00845-0
Zarbock, A., Gomez, H., and Kellum, J. A. (2014). Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care. 20 (6), 588–595. doi:10.1097/MCC.0000000000000153
Zhang, B., Wan, S., Liu, H., Qiu, Q., Chen, H., Chen, Z., et al. (2022). Naringenin alleviates renal ischemia reperfusion injury by suppressing ER stress-induced pyroptosis and apoptosis through activating Nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2022, 5992436. doi:10.1155/2022/5992436
Zhang, H., Zheng, C., Xu, Y., and Hu, X. (2024). Comprehensive molecular and cellular characterization of endoplasmic reticulum stress-related key genes in renal ischemia/reperfusion injury. Front. Immunol. 15, 1340997. doi:10.3389/fimmu.2024.1340997
Zhang, X., Huang, R., Zhou, Y., Zhou, W., and Zeng, X. (2020). IP3R channels in male reproduction. Int. J. Mol. Sci. 21 (23), 9179. doi:10.3390/ijms21239179
Zhao, H. H., Han, Q. X., Ding, X. N., Yan, J. Y., Li, Q., Zhang, D., et al. (2020). Critical hubs of renal ischemia-reperfusion injury: endoplasmic reticulum-mitochondria tethering complexes. Chin. Med. J. Engl. 133 (21), 2599–2609. doi:10.1097/CM9.0000000000001091
Zhao, M., Wang, Y., Li, L., Liu, S., Wang, C., Yuan, Y., et al. (2021). Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 11 (4), 1845–1863. doi:10.7150/thno.50905
Zhou, W., Liang, Y., Liao, X., Tong, L., Du, W., Fu, W., et al. (2024). ISRIB improves white matter injury following TBI by inhibiting NCOA4-mediated ferritinophagy. Neurochem. Int. 177, 105744. doi:10.1016/j.neuint.2024.105744
Zhou, Y., Liu, J., Xue, P., and Zhang, J. (2023). Collagenase-responsive hydrogel loaded with GSK2606414 nanoparticles for periodontitis treatment through inhibiting inflammation-induced expression of PERK of periodontal ligament stem cells. Pharmaceutics 15 (10), 2503. doi:10.3390/pharmaceutics15102503
Keywords: endoplasmic reticulum (ER), ER stress, kidney, AKI, UPR
Citation: Hu L and Chen H (2025) Research progress on endoplasmic reticulum homeostasis in acute kidney injury. Front. Pharmacol. 16:1595845. doi: 10.3389/fphar.2025.1595845
Received: 18 March 2025; Accepted: 11 June 2025;
Published: 20 June 2025.
Edited by:
Nektarios Barabutis, University of Louisiana at Monroe, United StatesReviewed by:
Yang Yang, First Affiliated Hospital of Zhengzhou University, ChinaAndrew Fribley, Wayne State University, United States
Copyright © 2025 Hu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huike Chen, Y2hlbmh1aWtlNTIwQDE2My5jb20=
 Liling Hu
Liling Hu Huike Chen
Huike Chen