- 1Department of Pharmacy, Jilin Medical University, Jilin, China
- 2Department of Medical Examination, Jilin Medical University, Jilin, China
- 3Department of Cardiovascular Surgery, Gaozhou People’s Hospital, Gaozhou, China
Photothermal therapy (PTT) offers revolutionary breakthroughs in tumor treatment due to its minimally invasive nature, high selectivity and efficiency. Photothermal therapy is a method of using laser irradiation (near-infrared light) to convert light energy into heat, reaching a relatively high temperature to kill tumor cells. Efficient and stable photothermal conversion materials are the key factors in PTT. There are inorganic and organic nanomaterials used in photothermal therapy. Through chemical modification of them, the functions of targeted drug delivery and combination therapy can be achieved. This work generalizes the features, excellent performance and therapeutic effects of photothermal conversion nanomaterials such as polydopamine (PDA), semiconductor nanoparticles (SNPs), Au nanomaterials, palladium nanosheets (PdNs), and carbon nanomaterials. Their functions and the advantages in photothermal therapy, tumor targeting and inactivation, and the mechanisms of nanophotothermal therapy are summarized. By continuously improving the performance and treatment methods of nanomaterials, more efficient, safe and minimally invasive solutions for tumor treatment are expected.
1 Introduction
Many preparations and treatment methods are used to overcome cancer (Liu et al., 2024; Yue et al., 2024). Nanophotothermal agents in photothermal therapy (PTT) convert near-infrared (NIR) light into thermal energy (Liu et al., 2025), directly destroy tumor tissues and efficiently kill them (Ma et al., 2023). Compared with traditional therapies, PTT offers higher localized treatment efficiency (Cui et al., 2022a), targeting tumors more precisely (Shi et al., 2024a). By targeting modification, nanophotothermal agents can actively or passively accumulate in tumor sites (Shi et al., 2024b; Xiong et al., 2024; Ren et al., 2024), enhancing treatment specificity. This specificity allows PTT to eradicate tumors while minimizing damage to normal cells (Komedchikova et al., 2024). Moreover, PTT is a non-invasive or minimally invasive method that avoids surgical incisions or large-scale irradiation (Zhou et al., 2025), reducing discomfort and recovery time of patients (Zheng et al., 2024). So compared to chemotherapy and radiotherapy, PTT has less side effects (Cui et al., 2022b). Additionally, PTT promotes tumor vascular normalization, increasing the accumulation and deep penetration of nanomedicines in tumor tissues (Feng et al., 2024), allowing for synergistic effects when combined with chemotherapy, radiotherapy, or immunotherapy (Xu et al., 2024). Furthermore, PTT induces the local release of tumor-associated antigens (TAAs), which activates the host immune response and reduces the risk of tumor recurrence and metastasis. So combining PTT with immunotherapy create the synergistic immune effect (Fang et al., 2024; Liu et al., 2024). When tumor cells undergo ICD (induced immunogenic cell death) under PTT, they release damage-associated molecular patterns (DAMPs) acting as “danger signals” to recruit dendritic cells (DCs), enhancing tumor antigen presentation, and activating cytotoxic T lymphocytes (CTLs). Furthermore, the localized inflammatory microenvironment generated by PTT can reverse immunosuppressive tumor niches, thereby sensitizing tumors to immune checkpoint inhibitors. This multimodal synergy not only amplifies the direct tumor-killing effects of PTT but also establishes systemic antitumor immunity to suppress distant metastases (Shi et al., 2024). Additionally, by surface modification, nanophotothermal agents can be equipped with imaging function, facilitating tumor diagnosis (Zhang et al., 2024). In summary, nanophotothermal tumor therapy exhibits remarkable advantages such as efficiency, specificity, minimal invasiveness, low adverse reactions, multifunctionality and synergy (Lu et al., 2023; Lian et al., 2021; Wang et al., 2024). Nonetheless, clinical application still faces critical issues to be addressed. Approved experimental PTT nanosystem gold nanoshell (AuroShell) NCT02648035 has demonstrated the precision of local thermal ablation in clinical trials of head and neck cancer, but the recurrence rate after a single treatment (−30%) suggests the need for combination chemotherapy or immunotherapy to improve long-term efficacy (Rastinehad et al., 2019). Carbon based materials, such as graphene oxide, exhibit low systemic toxicity in the treatment of melanoma (NCT04323020), but are limited by the depth of light penetration (<2 cm), resulting in insufficient efficacy for deep tumors (Zhao et al., 2023). The major clinical application bottlenecks include (a) biosafety: some metal based nanomaterials (such as CuS, Fe3O4) have not yet undergone long-term toxicological evaluation by FDA/EMA due to their potential toxicity caused by long-term retention; (b) lack of standardized parameters: the clinical protocol for laser power density (0.3–2 W/cm2) and irradiation time (1–10 min) lacks a unified standard, resulting in significant fluctuations in therapeutic efficacy. It is inferred that precise delivery, controllable release and multimodal combination therapy are the keys for technological optimization and future direction of nano PTT.
2 Nanomaterials for photothermal therapy
The nanomaterials in PTT are mainly divided into two categories: inorganic and organic nanomaterials. Inorganic nanomaterials were studied earlier, mainly including noble metal nanoparticles, metal chalcogenide nanomaterials, carbon based nanomaterials, magnetic nanoparticles and quantum dots. Precious metal nanoparticles such as gold, silver, platinum and palladium, are commonly used in tumor PTT. Other kind of inorganic nanomaterials such as tungsten nitride (WN) nanoparticles and carbon nanospheres have also been used. Compared to inorganic nanomaterials, the research on organic nanomaterials is relatively limited. Organic nanomaterials typically have good biocompatibility and degradability, but may not be as efficient and stable in photothermal conversion as inorganic nanomaterials. The followings provide detailed introductions to photothermal conversion nanomaterials of polydopamine (PDA), semiconductor nanoparticles (SPNPs), AuNPs, palladium nanosheets and carbon nanomaterials. The performance comparisons of different nanomaterials are listed in Tables 1, 2 shows the applications of the nanomaterials discussed in this review.
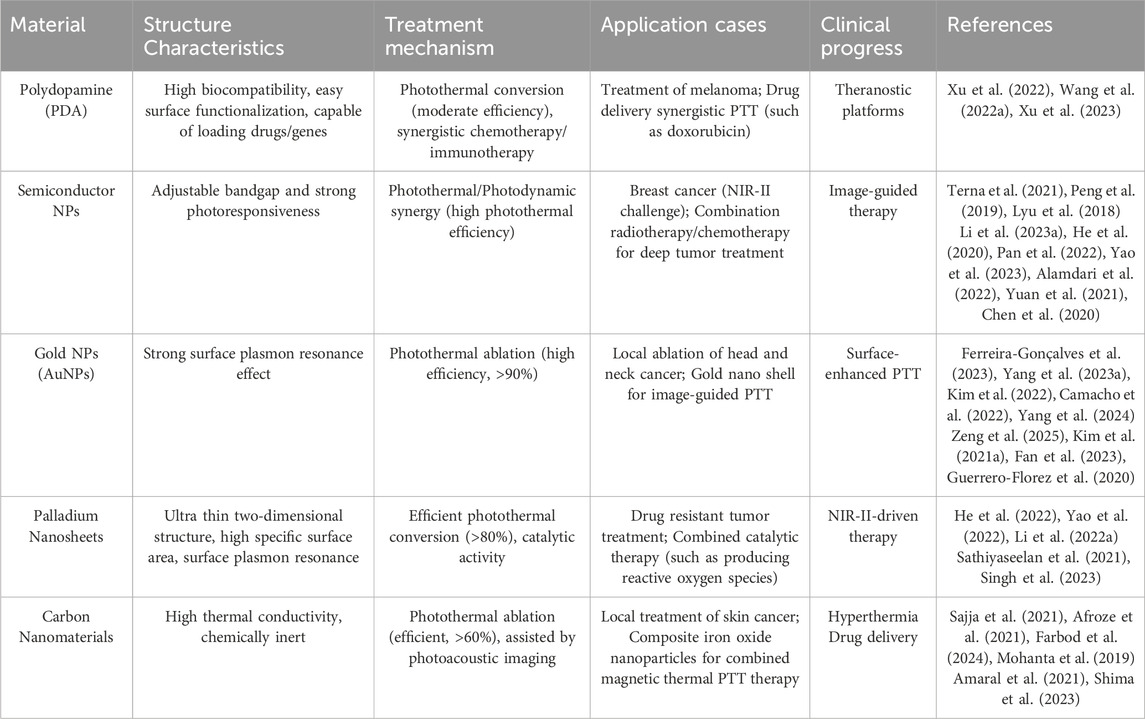
Table 2. The structure characteristics, treatment mechanism and clinical applications of PDA, SNPs, Au nanomaterials, PdNs and carbon nanomaterials.
2.1 Polydopamine (PDA)
PDA possess a unique core-shell structure, and the surfaces are enriched with active functional groups such as phenolic hydroxyl and amino groups (Lu et al., 2021). These groups confer excellent modification and multifunctionality to PDA (Balavigneswa et al., 2024). PDA exhibits strong adhesion, similar to the foot threads of natural mussels (Feng et al., 2024), allowing it to firmly adhere to almost any material surface, forming a conformal layer (Jie et al., 2023). In vivo, PDA demonstrates good biocompatibility and limited biological toxicity, which makes it suitable for biomedical applications (Xu et al., 2022). The photothermal effect of PDA originates from the broadband optical absorption (300–900 nm) of its polyphenol-quinone conjugated structure. Under NIR excitation, electrons within the π-π stacking layers convert photon energy into lattice vibrations (phonons) via non-radiative relaxation, releasing thermal energy (Wang et al., 2022). The hydrogen-bonding network further enhances photothermal conversion efficiency through intermolecular vibrational coupling. Surface amino modification (e.g. RGD peptides) enables targeted binding to integrin receptors (e.g., αvβ3) overexpressed on tumor cells, facilitating clathrin-mediated endocytosis. Following internalization, PDA accumulates in lysosomes (pH 4.5–5.0), where its alkaline groups induce lysosomal membrane permeabilization (LMP), releasing heat shock protein 70 (HSP70) and activating apoptosis pathways. PDA degradation products (e.g., dopamine monomers) suppress M2 polarization of tumor-associated macrophages (TAMs), synergistically enhancing antitumor immune responses (Xu et al., 2023). The schematic diagram of the process of using DPA based biomimetic nanomaterials for PTT and killing colon cancer is shown in Figure 1 (Gong et al., 2022), which has a pH-responsive property and can be degraded in a weakly acidic tumor microenvironment (TME), leading to loaded drug release, and the released Apoptin (AP) can work as a radiosensitizer to improve the RT and destroy tumor cells by promoting apoptosis directly. Researchers have constructed many PDA drug delivery systems that simultaneously encapsulate photosensitizers and antitumor drugs within, enabling targeted delivery (Zhang et al., 2022). Zeng addressed the issue of tumor hypoxia by using MnO2 nanoparticles loaded with chlorin E6 (Ce6) and coated with folic acid-functionalized PDA layer (MCPFNP) (Zeng et al., 2020). Due to the active targeting mediated by folic acid and the passive transport of enhanced permeability and retention (EPR) effect, MCPFNP significantly accumulated in the tumor sites of mice. In the acidic tumor microenvironment, PDA disassembled and released the photosensitizer Ce6, completing the precise tumor targeting. Simultaneously, under 808 nm laser irradiation, the system generated high temperatures and burned tumor cells, which reduced MCP-7 cell viability to 11.74%. MCPFNP also exhibited excellent biodegradability and low long-term toxicity. Zhang and Wang synthesized a PDA nanomaterial (PTTPB) with PDA and tributyl tetradecylphosphonium bromide (TTPB) (Li et al., 2022), which similarly got precise tumor treatment. PTTPB can recognize sialic acid (SA), which is underexpressed in normal cells and overexpressed on the surface of tumor cells such as B16 F10, thus it targets tumor cells. Upon irradiation, PTTPB elevated the temperature through photothermal effect and ablate the tumor tissue. Additionally, the donor-acceptor (D-A) electronic structure of PTTPB generated singlet oxygen and other reactive oxygen species (ROS) under light, further killing tumor cells, achieving combined photothermal-photodynamic therapy, and significantly improving therapeutic efficiency. Biocompatibility tests showed that PTTPB exhibited low cytotoxicity and good biosafety. Activating the interferon gene stimulating factor (STING) pathway is a highly promising approach for tumor treatment, but its clinical application is limited by the inability to administer systemically and the immunosuppressive tumor microenvironment (TME). Zeng constructed a PDA multifunctional platform loaded with the STING agonist methylated adenylate-2 (MAS-2) and chelated Mn2+ with mesoporous PDA (Zeng et al., 2023). This system accumulated in the tumor region through the EPR effect and performed PTT on tumor under NIR irradiation, inducing apoptosis of tumor. During the process, tumor cells released TAAs and pro-inflammatory factors, alleviating the immunosuppressive TME. The platform released MAS-2 and Mn2+, activating the STING pathway, ultimately triggered a strong immune response and showed high anticancer effects. In summary, the innovative design of PDA materials in PTT can significantly enhance their functionality. The latest technologies include: Ⅰ. Multifunctional composite carriers, such as gold nanorods or carbon dots wrapped in PDA, to enhance photothermal conversion efficiency (up to 60% or more) and integrate drug delivery/imaging functions; Ⅱ. Surface engineering involves modifying targeted ligands (such as folate) with amino or carboxyl groups to enhance tumor selectivity; Ⅲ. Responsive release system utilizes the pH/ROS sensitivity of PDA to achieve controlled drug release. The challenges lie in long-term biosafety, large-scale synthesis stability, and limitations on deep tissue penetration. Future development will focus on synergistic therapies (such as PTT/chemotherapy/immunotherapy), development of biodegradable PDA derivatives, and AI assisted material design to promote clinical translation, optimize performance balance, and address metabolic mechanism issues.
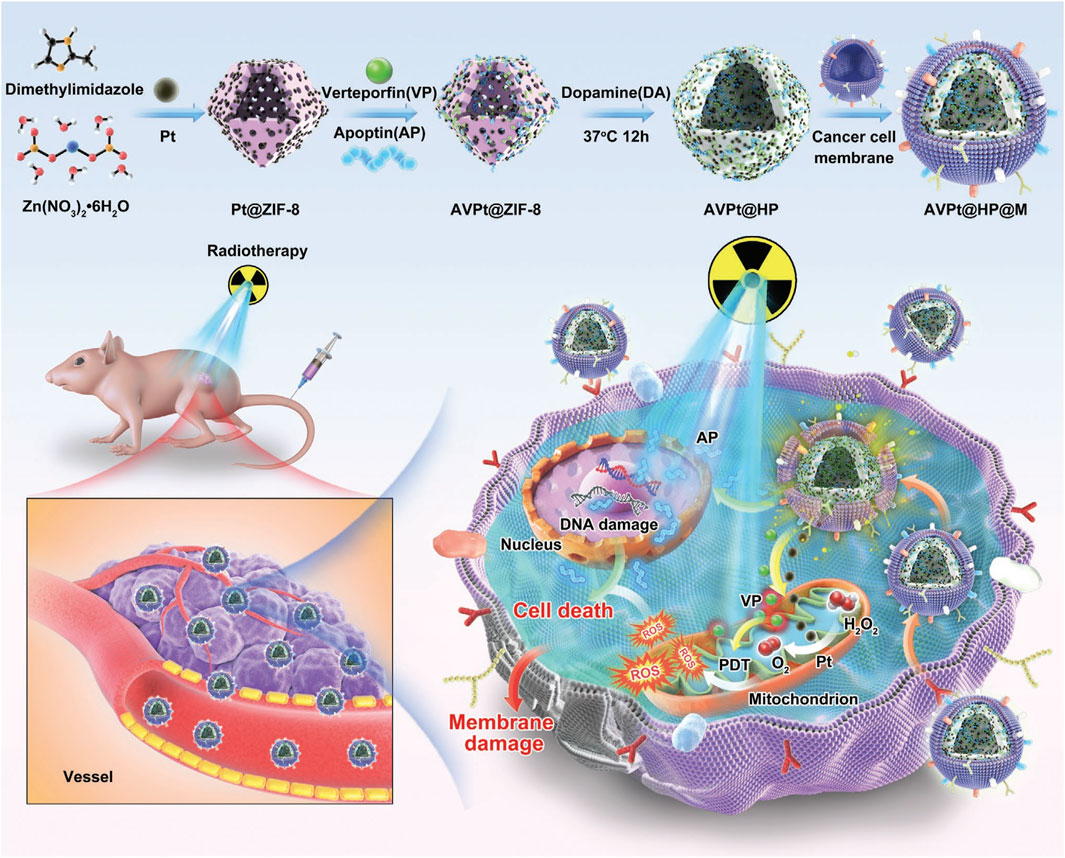
Figure 1. Schematic illustration of biomimetic nanoplatform AVPt@HP@M for colon cancer radiotherapy sensitization through hypoxia relief (Pt-doped ZIF-8), apoptin-enhanced apoptosis (AP), and VP-mediated X-PDT, with cancer cell membrane camouflage for targeted delivery (reprinted with permission from Gong et al., 2022. Copyright 2022 Wiley - VCH GmbH).
2.2 Semiconductor nanoparticles (SNPs)
The core structure of SNPs determines their fundamental properties (Terna et al., 2021), while the shell serves to protect the core, enhance optical properties, and improve stability (Peng et al., 2019). Unlike precious metal materials, the ion release properties of SNPs endow them with the potential for photothermal chemodynamic combined therapy (Zhang et al., 2024). The photothermal effect of SNPs comes from bandgap modulation and free carrier oscillation. Narrow bandgap design allows NIR light to excite electrons from the valence band to the conduction band, and then convert energy into heat through “electron phonon scattering” (Li et al., 2023). Sulfur vacancies or oxygen doping (such as MoS2-xOx) can form intermediate energy levels, enhancing non radiative recombination pathways (carrier lifetime<1ns). SNPs enter cells through caveolae mediated endocytosis, and particles smaller than 50 nm in size can penetrate the nuclear membrane. The local high temperature (ΔT>10°C) generated by photothermal stimulation triggers the release of metal ions from SNPs through Fenton reaction to generate hydroxyl radicals (·OH), leading to mitochondrial DNA damage (Li et al., 2021). Figure 2 shows a schematic diagram of hollow structured CuS NPs composite with carbon dots (CuSCDs) enhancing PTT through ubiquitin dependent proteasome degradation pathway (Yu et al., 2020). SNPs remain stable in biological systems, resist to aggregation or degradation, and exhibit low biotoxicity, ensuring effective PTT with minimal side effects (Zhao et al., 2022). The surface of SNPs have unsaturated coordination, resulting in numerous surface defects and active sites which are modifiable to achieve multifunctionality, such as targeted delivery and biocompatibility (Peng et al., 2019). For instance, Yan Lyu developed a water-soluble SNP (SPNV) through vinylene bonds through simple chemical reactions and physical cross-linking processes (Lyu et al., 2018). SPNV owns the mass absorption coefficient (1.3-fold) and PCE (2.4-fold). The study revealed that the vinylene bonds enhance the biodegradability and optical activity of SNPs, while also improving their imaging and therapeutic capabilities. Thus, appropriate chemical modifications can further expand the biomedical applications of SNPs (Kshatriya et al., 2024), including drug delivery and PTT (Alghamri et al., 2022). In tumor vascular disruption therapy, the off-target effects and repeated dose toxicity of vascular disrupting agents (VDAs) limit overall therapeutic efficacy. To solve this problem, Li constructed biomimetic SNPs containing surface-modified platelet membranes (Li et al., 2023). The SNPs are capable of precise tumor vascular disruption via two-stage light manipulation. During the first irradiation, the nanoparticles generated mild heat to induce tumor vascular bleeding, activating the coagulation cascade and recruiting more nanoparticles to the damaged vessels. During the second irradiation, enhanced targeting of tumor vasculature by the photothermal agents led to intense hyperthermia effectively, destroying the tumor vasculature and completely eradicating the tumor, while also inhibited metastasis. He reported iron-chelated SPFeN composed of ferroptosis inducers (Fe3+) and amphiphilic semiconductor copolymers (SPC) (He et al., 2020). Upon NIR irradiation, localized heating occurred and these particles accelerated the Fenton reaction to generate free radicals, assisting tumor suppression and achieved combined PTT-photodynamic therapy. In the acidic tumor microenvironment, SPFeN generated hydroxyl radicals, leading to ferroptosis. Compared to previous studies, SPFeN-mediated ferroptosis PTT can minimize iron dosage and effectively inhibit tumor growth in vivo. Additionally, Pan synthesized similar nanoparticles, gadolinium-containing SPN-Gd which exhibited significant inhibitory effects on oral squamous cell carcinoma (OSCC) (Pan et al., 2022). In vivo, SPN-Gd as an MRI contrast agent and optical imaging agent, showed a prolonged retention time and significantly inhibited OSCC tumors in mouse models through PTT. The latest technologies for enhancing the therapeutic effect of SNPs include: Ⅰ. Band engineering, which enhances NIR absorption by adjusting the bandgap (such as doping or heterostructure design); Ⅱ. Multi modal collaboration, combined with PDT or chemodynamic therapy (CDT), such as MoS2 loaded Fe2+ to achieve PTT/CDT (Zhang et al., 2020); Ⅲ. Surface functionalization, such as polyethylene glycol (PEG) modification to enhance biocompatibility, or coupling antibodies to enhance targeting. The challenges of SNPs lie in long-term toxicity, photothermal stability and deep tissue penetration efficiency. Developing new narrow bandgap semiconductors (such as organic-inorganic hybrid perovskites), intelligent responsive nanosystems (such as photo/thermal dual controlled release drugs) and clinical translational research can promote precise PTT tumor treatment.
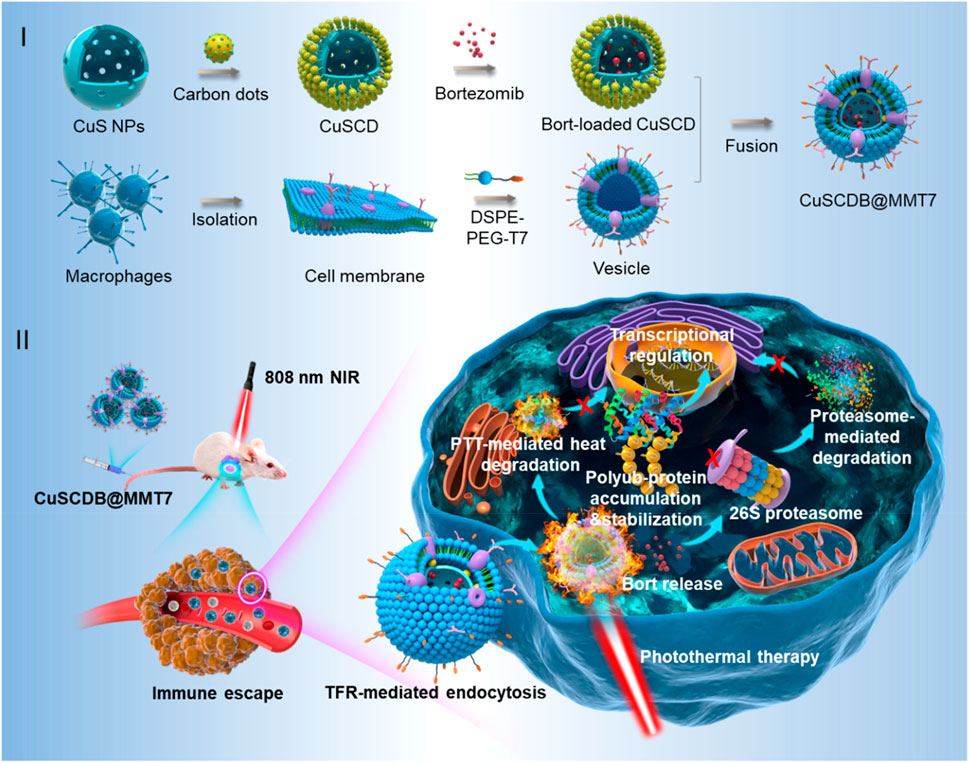
Figure 2. Schematic illustration of the generation of proteasome inhibitor-encapsulated CuS/carbondots nanocomposites for enhanced photothermal therapy viaheat-stabilization of varioussubstrates in the ubiquitin-dependent proteasomal degradation pathway (reprinted with permission from Yu et al., 2020. Copyright 2020 American Chemical Society).
2.3 Au nanoparticles and nanorods
Au nanomaterials exhibit strong light absorption ability, and their photothermal effect originates from localized surface plasmon resonance (LSPR). When the frequency of incident light matches the oscillation frequency of free electrons on the material surface, a strong electromagnetic field is generated, and energy is converted into hot electrons through Landau damping, followed by the release of thermal energy through electron phonon scattering. Due to the influence of the specific surface area, size and shape of Au nanomaterials on their PTT performance, different shapes of Au nanomaterials have been developed, such as Au nanoparticles (AuNPs) and Au nanorods (AuNRs) (Zhao et al., 2022). The longitudinal LSPR of AuNRs can be adjusted to the NIR-II region (1,000–1,350 nm), and the larger the aspect ratio (AR), the deeper the penetration depth. The surface charge of Au nanomaterials (such as CTAB modified positive charges) can disrupt the lipid bilayer structure of tumor cell membranes, leading to increased membrane permeability and calcium ion influx. Accumulation of gold nanomaterials in lysosomes inhibit the mTOR pathway, promote autophagosome formation, and antagonize photothermal induced apoptosis. It is necessary to combine autophagy inhibitors (such as chloroquine) to enhance therapeutic efficacy (Wei et al., 2022).
2.3.1 Au nanoparticles (AuNPs)
AuNPs demonstrate good PCE (Ferreira-Gonçalves et al., 2023), localized surface plasmon resonance (LSPR) absorption (Yang et al., 2023), high transport efficiency and supramolecular recognition ability (Kim et al., 2022), enabling them to precisely adsorb proteins and target macromolecules within cells or on cell surfaces (Camacho et al., 2022). However, for the reason that working temperature during photothermal therapy is often high (>50°C), surrounding tissues near the tumor are frequently burned, limiting the further application of chemotherapy-photothermal combination therapies (Yang et al., 2024). For that, Yu designed a mild AuNPs photothermal agent (ADHM), composed of dopamine (DA) and hyaluronic acid (HA)-coated AuNPs, assembled with metformin (MET) via electrostatic interactions (Figure 3) (Yang et al., 2023). As shown in Figure 3, ADHM selectively accumulates in tumors via HA-mediated active targeting and the EPR effect, followed by pH-responsive dispersion. The 808 nm NIR-induced mild hyperthermia then synergizes with MET therapy for enhanced antitumor efficacy. This system achieved 94.6% inhibition rate for chemotherapy-photothermal combination therapy of 4T1 tumors in mice at a mild temperature (43°C) while effectively preventing tumor metastasis. The chemical interactions within ADHM exhibit a high degree of synergy, bringing the system excellent targeting ability and biocompatibility. ADHM provides a potential candidate for mild chemotherapy-photothermal combination therapy of tumors. To extend drug accumulation and retention within tumor cells, and enhance the therapeutic efficacy of tumor photothermal therapy, the modifications of surface ligand types, charge, chemical polarity, chemical reactivity, and hydrophobicity of AuNPs photothermal agents (PTAs) are usually used. Wang prepared a mixed-charge zwitterionic surface Au nanoparticle (Au-MUA-TMA) with chemical modification and altered the shape and size of the AuNP to shift the light absorption to the near-infrared (NIR) region (Kong et al., 2023). Under 808 nm laser irradiation, Au-MUA5-TMA5 targeted and accumulated at the U87MG tumor site in mice, causing a significant increase in local temperature and leading to complete tumor disappearance within 14 days of treatment.
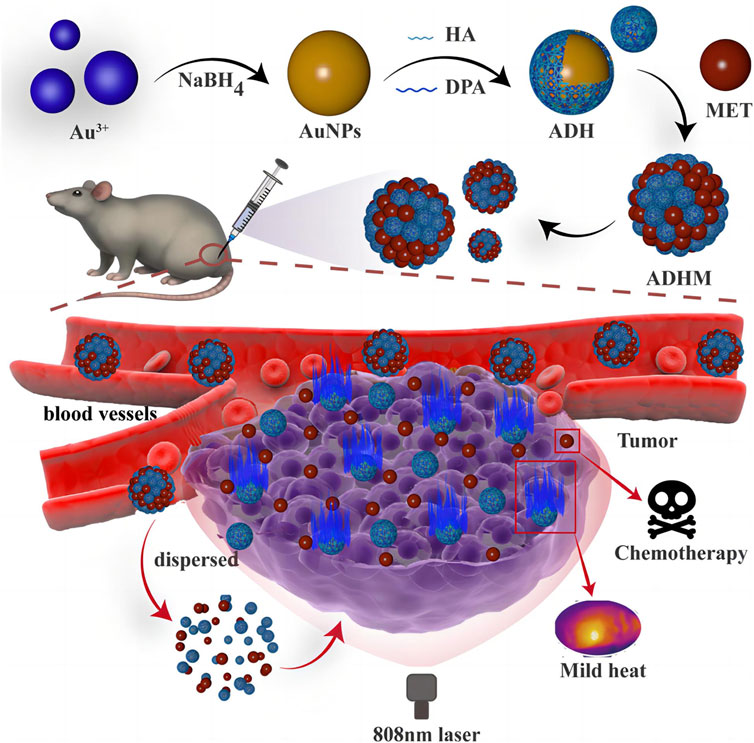
Figure 3. Scheme of mild chemo-photothermal synergistic therapy based on gold nanopaticles coupled with metformin (reprinted with permission from Yang et al., 2023. Copyright 2023 American Chemical Society).
2.3.2 Au nanorods (AuNRs)
In contrast to the spherical structure of AuNPs, AuNRs are elongated and rod-like, which makes AuNRs more prone to absorb and scatter light in the long-wavelength region (Roach et al., 2021). Similar to AuNPs, AuNRs also exhibit excellent optical absorption and scattering cross-sections, PCE, LSPR and their surface is easy to be functionalized (Dong et al., 2024). Compared to zero- and one-dimensional nanomaterials, two-dimensional nanomaterials offer significant advantages in PTT-based tumor therapies, such as ultra-thin structures, high specific surface areas and unique optical properties (Wu et al., 2022). For example, Kong combined 2D peptide nanosheets (PNS) with AuNRs to form PNS-AuNRs (Cheong et al., 2021). Upon irradiation, MTT assays on human breast cancer cells and MCF-7 cells showed more than 75% inhibition rates. PNS-AuNRs were also tested in vivo for PTT on mice bearing MCF-7 tumors. Within 10 min of irradiation, the tumor cells underwent apoptosis. After 14 days, tumor disappeared completely. Meanwhile, the mice exhibited good health, which demonstrates the low toxicity and general anticancer effects of AuNRs. AuNRs show two characteristic light absorption peaks due to the SPR effects on transverse and longitudinal surfaces. Cheong adjusted the aspect ratio of AuNRs to shift the light absorption peak to NIR region and achieved effective PTT (Figures 4A–C) (Zhao et al., 2024a). The anti-angiogenic effects of BCP50-2-AuNRs were evaluated in Tg (fli1:EGFP) zebrafish. BCP50-2-AuNRs suppressed angiogenesis in a dose-dependent manner (14.6% at 5 μg/mL, 21.5% at 10 μg/mL, and 31.2% at 20 μg/mL) (Figures 4D,E). Some nanomaterials are unstable in physiological environments and tend to aggregate and form precipitates. To improve the stability and antitumor activity, Zhao extracted a homogeneous polysaccharide (BCP50-2) from Belamcanda (a plant) and conjugated it with AuNRs to produce BCP50-2-AuNRs (Kong et al., 2024). BCP50-2-AuNRs exhibited good stability and PCE. Under NIR irradiation, the local temperature of HepG2 tumors increased significantly with BCP50-2-AuNRs and the tumor cell growth were inhibited efficiently. Moreover, the biological models showed good growth without much side effect.
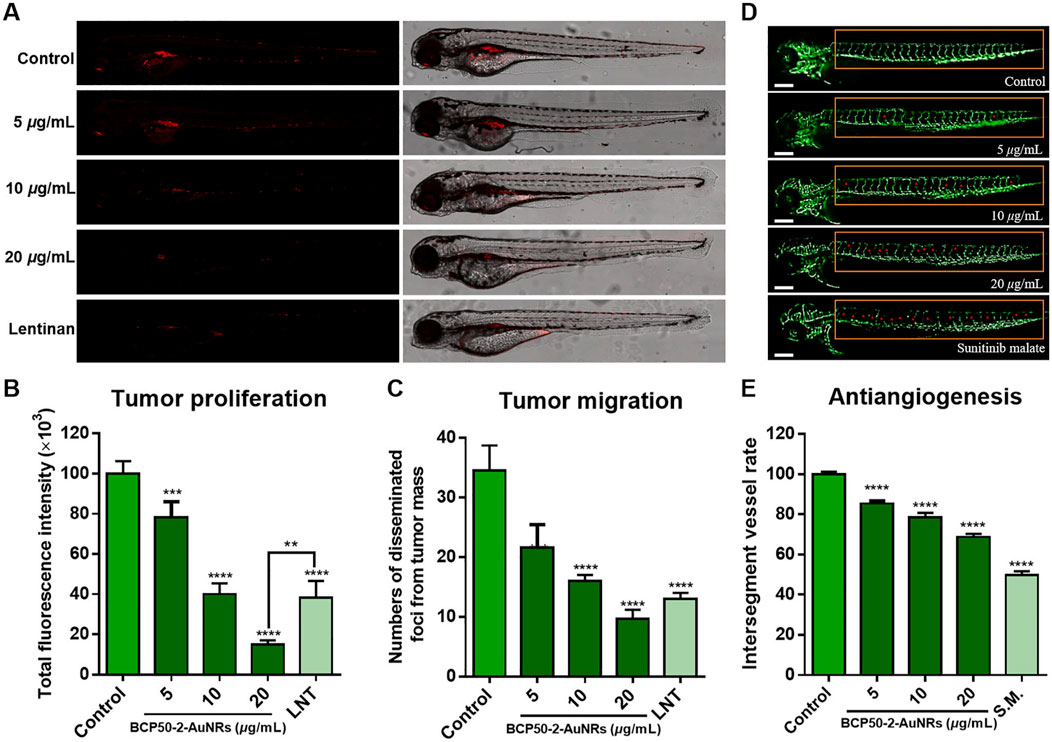
Figure 4. In vivo antitumor activity of BCP50-2-AuNRs. (A) Confocal images of HepG2-xenografted zebrafish treated with BCP50-2-AuNRs (vs. Lentinan, 400 μg/mL). (B,C) Quantification of tumor fluorescence intensity and foci (n = 20 in each group). (D) Intersegmental vessels development in transgenic zebrafish (vs. sunitinib malate, 1 μg/mL). (E) Angiogenesis inhibition rate (n = 20 in each group) (reprinted with permission from Zhao et al., 2024a. Copyright 2024 Elsevier B.V.).
In summary, innovative strategies to enhance the efficacy of AuNPs and AuNRs include the following: Ⅰ. Morphology optimization: adjusting the aspect ratio of Au NRs (such as 50–70 nm length) to match the near-infrared (NIR) window and improve photothermal conversion efficiency (>90%); Ⅱ. Surface plasmon coupling: constructing core-shell structures (such as Au@SiO2) or polymer enhanced local thermal field; Ⅲ. Multi functional integration: combining drug loading (such as doxorubicin), immune regulation (PD-L1 inhibitors), or photoacoustic imaging to achieve integrated diagnosis and treatment. The latest technologies currently include intelligent responsive Au NRs (such as pH/GSH triggered drug release) and improved tumor penetration and renal clearance through ultra small Au NPs (<5 nm). The future challenges also face long-term retention toxicity, uniformity in large-scale preparation, and limitations in tissue penetration depth. The key points for future development lie in accelerating clinical translation (such as combination immunotherapy), developing biodegradable Au based materials and optimizing photothermal performance with AI assisted morphology design.
2.4 Palladium nanosheets (PdNSs)
PdNSs are thin and possess large specific surface area and planar size (Li et al., 2024). The planar surface allows for better absorption of light, as a unique advantage in PTT (He et al., 2022). The NIR-II response characteristics of Pd NSs are derived from their two-dimensional electronic confinement effect. The ultra-thin structure (thickness<2 nm) causes electrons to move freely in the plane, forming a wideband LSPR (1,000–1,350 nm), with a significantly higher photothermal efficiency (∼60%) than bulk Pd (<10%). The hot electrons generated by photoexcitation transition from d-band to sp band, and then release energy through electron phonon coupling. Unlike spherical NPs, the planar structure of Pd NSs endows them with unique membrane interaction modes (Li et al., 2022). The sharp edges of Pd NSs can physically damage the cell membrane and enter the cytoplasm directly through non endocytic pathways, avoiding lysosomal degradation. Pd NSs can inhibit key enzymes involved in tumor cell glycolysis, such as HK2 and LDHA, reduce ATP production, and enhance hyperthermia sensitivity (Chen et al., 2017). Figure 5 shows a schematic diagram of a bimetallic palladium nanocapsule (Pd Ncap) targetting the breast cancer cell line SK-BR-3 (Singh et al., 2023). Pd Ncap owns a rattle like morphology because of a solid gold bead as a core located inside a porous thin Pd shell. These nanostructures possess broad absorbance in the NIR biological windows (600–1,300 nm), thus enhancing their applicability in PPTT. PdNSs remain stable in vivo without aggregation or degradation, and they exhibit low biotoxicity (Yao et al., 2022). Additionally, PdNSs can be used in combination with other therapies, such as chemotherapy and immunotherapy (Li et al., 2022). For example, Jiang developed PdNSs Pd (5)-CpG (PS), which significantly enhanced the absorption of cytosine polyguanine (CpG) by immune cells and boosts the immune-stimulatory activity of CpG (Ming et al., 2020). Combined with Pd (5)-CpG (PS)-mediated PTT and immunotherapy, using safe NIR radiation (808 nm laser, 0.15 W cm−2), highly effective tumor inhibition and a significant increase in the survival rate of tumor-bearing mice were achieved. By surface modification, PdNSs can target tumor tissues (Yang et al., 2021). They cause local high temperature which disrupts the structure of diseased cells and interferes with their metabolism. Singh reported a bimetallic palladium nanocapsule (Pd Ncap) with a solid gold core and a thin, perforated palladium shell that demonstrated excellent photothermal stability (Singh et al., 2023). At a very low laser power density of 0.5 W cm−2, the PCE in 1,064 nm region reached 49%. The nanocapsules were further functionalized with Herceptin (Pd Ncap-Her) to target the breast cancer cell line SK-BR-3. In vitro PTT applications with NIR light, at a concentration of 50 μg/mL and a laser power density of 0.5 W cm−2 with an output power of only 100 mW, more than 98% of the cells were killed. Innovative designs and strategies for developing Pd NSs include: Ⅰ. Ultra thin structure optimization: regulating thickness (2–5 nm) and lateral size (50–200 nm) to enhance surface plasmon resonance (SPR) and improve photothermal conversion efficiency (>80%). Ⅱ. Multi functional composite: such as drug loaded composite catalytic function, utilizing the catalytic ability of Pd to decompose H2O2 and enhance CDT. Ⅲ. Heterogeneous structures (such as Pd@Au) improve photothermal stability and biocompatibility. Ⅳ. Surface modification: PEGylation or targeted molecule (such as RGD peptide) modification enhances tumor enrichment ability. The latest developed technologies currently include dual-mode therapy combining PTT/CDT or photothermal immunotherapy, and responsive drug release systems that utilize the tumor microenvironment (such as low pH/high GSH) to trigger drug release. The challenges of the material development still lie in the lack of long-term biosafety data and the control of size uniformity in large-scale synthesis. Future development can be achieved through the study of ultra-thin degradable Pd based nanosheets, combined with AI prediction of optimal morphology parameters to promote clinical translation.
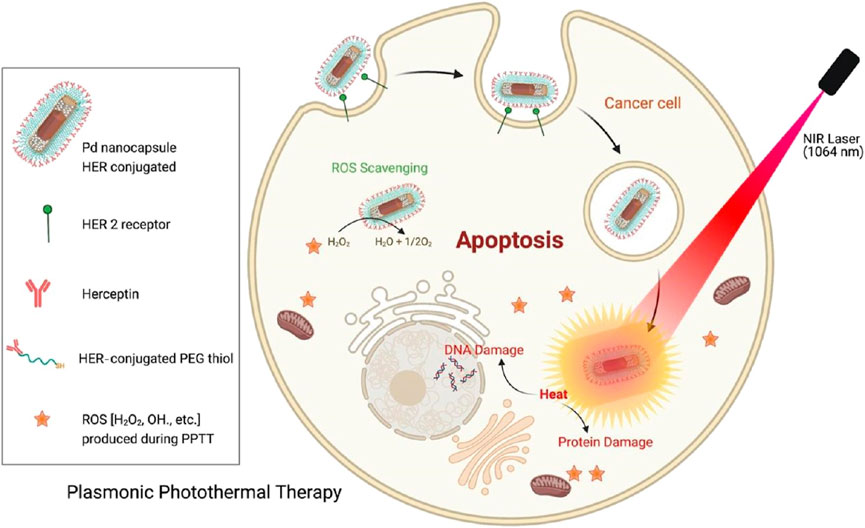
Figure 5. Schematic representation of the targeted plasmonic photothermal therapy of breast cancer cells using bimetallic herceptin-conjugated palladium nanocapsules (reprinted with permission from Singh et al., 2023. Copyright 2023 American Chemical Society).
2.5 Carbon nanomaterials
Carbon nanotubes (CNTs), carbon dots (CDs) and quantum dots (QDs) (Figure 6A) (Hong et al., 2015) achieve photothermal conversion through different molecular mechanisms. CNTs are plasmonic resonances excited by conjugated π electrons in NIR light, generating heat through electron phonon coupling (Liu et al., 2015). Their efficiency is regulated by diameter, chirality, and surface modification. CDs generate heat mainly through non radiative transitions between surface defect states and functional groups (such as amino groups), and doping can extend absorption to the NIR-II region (1000–1350 nm). QDs are controlled by quantum confinement effects to regulate their bandgap, electron hole pairs generate heat through Auger relaxation, and carbon based QDs combine with surface state effects to reduce toxicity (Sun et al., 2019). CNTs rely on clathrin endocytosis to disrupt lysosomes. CDs/QDs regulate endocytosis efficiency through size and charge regulation, while utilizing targeted modifications (such as folate) to target mitochondria, enhancing tumor specificity. CNTs/CDs/QDs can interfere with DNA repair in tumor cells and induce protein denaturation, ROS burst, and apoptosis/necrosis through local temperature rise (42°C–48°C). The PTT performance of carbon materials is highly dependent on their defect density - moderate oxidation treatment (C/O ratio≈8:1) can optimize absorption spectra while maintaining stability. Figures 6B,C shows the typical schematic diagrams of using CDs and QDs for PTT (Li J. et al., 2021; Cao et al., 2024).
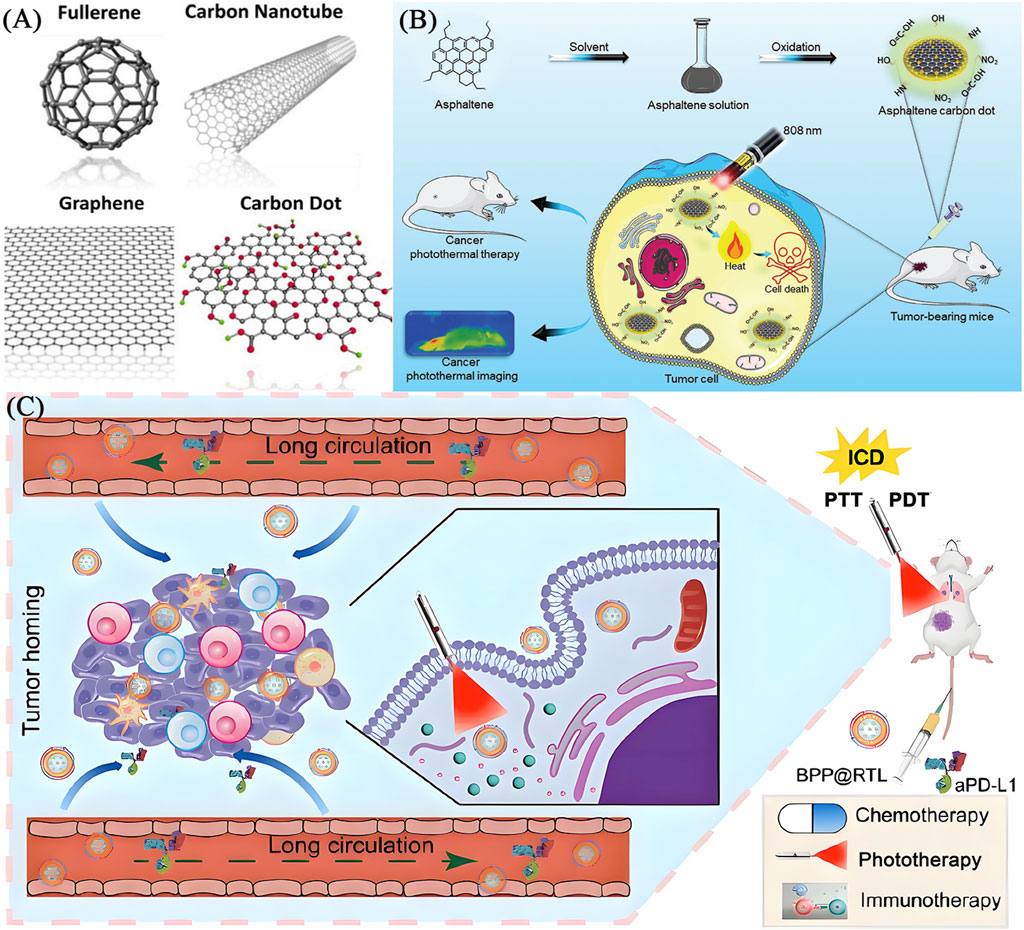
Figure 6. (A) The structures of carbon nanomaterials including carbon nanotube, graphene, fullerene and carbon dot (reprinted with permission from Hong et al., 2015. Copyright 2015 American Chemical Society). (B) Schematic illustration of ACDs synthesis from asphaltenes precursor and subsequent application in cancer photothermal therapy (reprinted with permission from Akakuru et al., 2025, licensed under CC BY). (C) Schematic image of fabrication and application of a QD-based nano-transformer (GQDNT) for diagnosis and treatment of cancer (reprinted with permission from Cao et al., 2024. Copyright 2024 American Chemical Society).
2.5.1 Carbon nanotubes (CNTs)
Depending on the number of graphene layers, CNTs are classified into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) (Sajja et al., 2021). SWCNTs consist of sp-hybridized carbon atoms arranged in a hexagonal honeycomb lattice, forming a hollow tubular structure (Afroze et al., 2021), while MWCNTs are composed of multiple concentric tubes nested within each other. CNTs exhibit strong absorption in the near-infrared (NIR) spectrum and PCE (Farbod et al., 2024). In addition, functionalization of the CNT surface with targeting ligands can reduce the toxicity and immunogenicity (Mohanta et al., 2019), which makes CNTs highly suitable for PTT and PDT. The high aspect ratio and specific surface area enable CNTs to adsorb a variety of drug molecules (Zhao et al., 2022). Their needle-like structure facilitates internalization into target cells, which could be an advantage for drug delivery (Naief et al., 2024). The PTT with CNTs has primarily focused on relatively small primary tumors, but the approach has limitations in preventing tumor metastasis. To overcome this, Mc Kernan developed SWCNT-ANXA5 by modifying SWCNTs with annexin A5 (ANXA5) (McKernan et al., 2021). Under NIR irradiation, this bioconjugate selectively ablated primary orthotopic EMT6 breast tumors in mice, synergistically enhancing the T lymphocyte-associated protein-dependent abscopal response, thus inhibiting tumor metastasis. As a result, the survival rate of mice treated with PTT was significantly extended, with survival persisting up to 100 days after tumor inoculation. Guo prepared an optically and thermally stable multi-walled CNT-hyaluronic acid composite (MWCNT-HA) (Guo et al., 2023). MWCNT-HA upregulates the expression of the apoptotic factor Caspase-3, which in turn affects the expression of the downstream anti-apoptotic factor Bcl-2, leading to apoptosis in CNE-1 cells, thereby inhibiting tumor cell proliferation and promoting apoptosis. Additionally, this material significantly raises the surrounding temperature under laser irradiation. When applied MWCNT-HA to tumor cells in mice, assisted by NIR laser irradiation, cell viability decreased by over 80% within 3 days. MWCNT-HA also greatly enhances the biosafety and compatibility of the drug, improving the therapeutic efficacy. Lee synthesized MWCNTs conjugated with thyroid-stimulating hormone receptor antibodies (TSHR), which selectively accumulate in tumor sites and remain for extended time (Figures 7C,D) (Lee et al., 2023). In BCPAP xenograft mice, αTSHR-Cy5.5-MWCNT (1 mg/kg, i.v.) showed predominant tumor accumulation at 24 h, with significantly enhanced targeting (p = 0.038 vs. IgG-Cy5.5-MWCNT) (Figures 7A,B). This tumor-specific distribution guided subsequent laser treatment timing. Upon laser irradiation, the conjugates induced tumor cell ablation, minimizing non-specific damage, which demonstrates a strong cytotoxic effect on thyroid tumor cells, inhibiting their regeneration and delaying tumor recurrence.
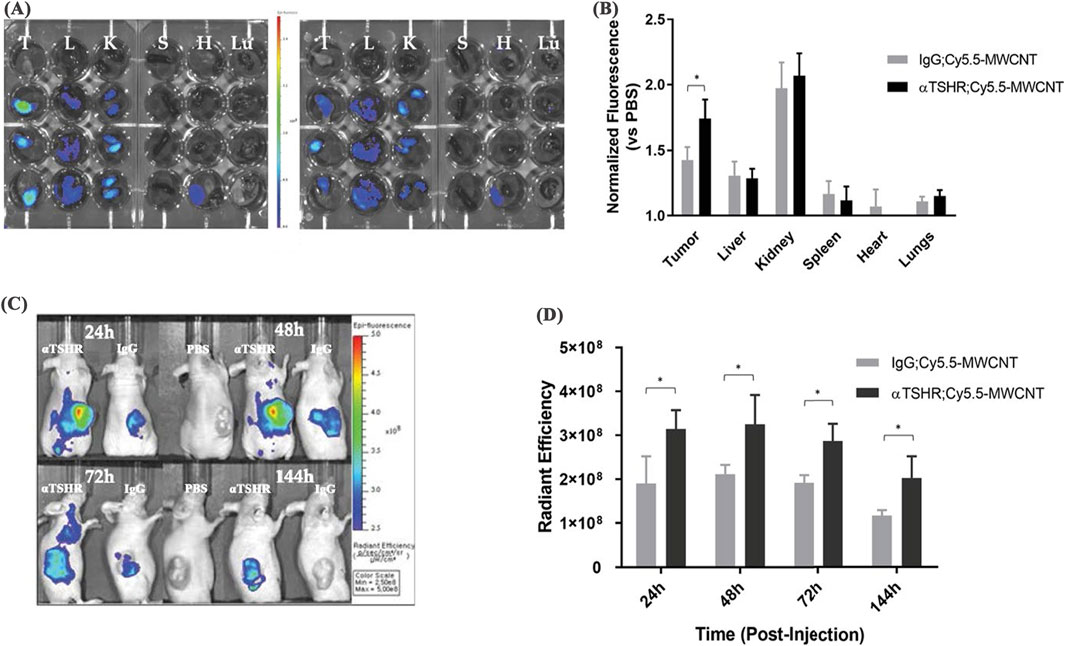
Figure 7. In vivo biodistribution of αTSHR-Cy5.5-MWCNT. (A) IVIS images of major organs (T = tumor, L = liver, K = kidney, S = spleen, H = heart, Lu = lung) 24 h post-injection. (B) Tumor accumulation showed 1.22-fold increase for αTSHR-Cy5.5-MWCNT vs. IgG-Cy5.5-MWCNT (p = 0.0376), with no significant changes in other organs. (C) Weekly tumor accumulation profile. (D) Time-course showing enhanced tumor targeting by αTSHR-Cy5.5-MWCNT (p = 0.046, 0.049, 0.019, and 0.043 at 24, 48, 72, and 144 h, respectively) (reprinted with permission from Lee et al., 2023, licensed under CC BY 4.0).
2.5.2 Carbon dots (CDs)
CDs possess a large π-conjugated system with a core composed primarily of graphitized sp2 carbon (Huo et al., 2021). Their shell contains abundant functional groups, such as carboxyl, hydroxyl and amine groups, which bring excellent water solubility and facilitate the combination of CDs with therapeutic agents (Lagos et al., 2023). Wang synthesized copper-doped Cu-CDs using urea and ethylene glycol as carbon sources and copper sulfate as an active dopant, employing one-step hydrothermal method (Wang et al., 2024). Cu-CDs effectively inhibited the proliferation of breast cancer cells (MDA-MB-231) by disrupting their malignant behavior. CDs can convert NIR photons into heat and promote the production of reactive oxygen species (ROS) in the surrounding environment, leading to cell apoptosis and achieving tumor treatment. Kim synthesized sulfur-doped CDs (S-CDs) with strong NIR absorption, achieving a photothermal conversion efficiency of 55.4% (Kim et al., 2021). When injected at a low dose (45 μg/mL) and combined with moderate laser irradiation (808 nm, 1.1 W cm−2), S-CDs could completely ablate cancer cells with minimal side effects. CDs possess tunable size and structural units, small volume, modifiability, good biocompatibility and low toxicity. For example, Bao designed PEG-modified Cu-CD cross-linked nanosheets, which effectively killed MCF-7 cells without exhibiting toxicity towards healthy cells (Bao et al., 2018). Additionally, Phuong developed a highly selective and sensitive PTT using CDs for mitochondrial-targeted cellular imaging (Phuong et al., 2020). The CDs were combined with TiO2 (C-CD/TiO2) to create a pH-responsive system that precisely targeted the cell membranes and nuclei of acidic cancer cells, effectively ablating the cancer cells and upregulating pro-apoptotic markers within them. This system demonstrated excellent targeting abilities for both bio-imaging and therapy. These advantages enable functional CDs to serve as drug carriers.
2.5.3 Quantum dots (QDs)
QDs are constrained in three spatial directions. QDs exhibit excellent optical stability and biocompatibility (Tade and More, 2025). The surface of QDs contains oxygen-rich functional groups such as hydroxyl and carboxyl, which are soluble in water and facilitate the application of QDs in biological systems. QDs also possess NIR absorption properties, allowing them to generate photothermal effect (Zhan et al., 2022). During localized hyperthermia, QDs promote the generation of ROS, so QDs are suitable for synergistic PTT and PDT. Tian synthesized DOX-ZIF-8/GQD, which showed significant temperature increases under NIR radiation (Iannazzo et al., 2017). The nanoparticles deeply penetrate biological tissues and be absorbed by tumor tissues, where they generate localized hyperthermia to kill tumor cells. QDs have a high surface area-to-volume ratio, providing more active interactions with the surrounding environment, which is also crucial for their adsorption ability (Yang et al., 2023). By linking specific ligands or antibodies to the surface of QDs, they can bind specific receptors to tumor cells, achieving active targeting. For instance, Zhong developed highly water-soluble (104 μg mL−1 at 25°C), highly luminescent (quantum yields of 77%) graphene QD with folate receptor targeting using folic acid as a precursor (Khodadadei et al., 2017). These QDs demonstrated excellent fluorescence labeling and targeted binding to breast and ovarian cancer cells expressing different levels of folate receptors, without exhibiting cytotoxicity. Interestingly, although the targeting mechanism is mediated by folate receptor endocytosis, the QDs do not bind through the pterin-folate receptor interaction. Instead, after thermal decomposition of the pterin ring on the folate molecules, residues remain on the surface of the QDs, interacting with organic functional groups and promoting specific binding to folate receptors on tumor cells, achieving targeted selectivity. Additionally, the structure of QDs allows them to own higher drug loads, making them more efficient in delivering chemotherapeutic agents. The π-orbitals in the sp2-hybridized QD lattice can also stack with aromatic rings in chemotherapeutic drugs and enhance drug delivery without covalent conjugation (Zhao et al., 2024b).
The above indicates that the functional efficiency of carbon nanomaterials is usually improved through structural optimization, doping with heteroatoms and multifunctional composites. Such as reducing graphene oxide (GO) to regulate the sp2/s3 carbon ratio and enhance the photothermal conversion efficiency (>40%), hollow carbon spheres or mesoporous carbon enhance drug loading capacity, nitrogen/sulfur doped carbon dots (N-CDs) enhance NIR absorption and catalytic activity, achieving PTT/CDT synergistic therapy, carbon based carrier loaded metal nanoparticles (such as Au@C) enhance photothermal effect, surface modify targeted molecules (such as folate) to improve tumor selectivity and so on. The latest emerging technologies include carbon nano intelligent response systems, such as pH/photothermal dual controlled drug release and ultra small carbon based quantum dots (<10 nm) to improve tumor penetration. The development bottleneck is the unclear long-term metabolic mechanism in the body and the batch stability of large-scale preparation of carbon nanomaterials. Developing biodegradable carbon based materials in the future and combining them with AI to optimize the band structure will promote clinical translation.
3 Low-temperature-driven multidimensional antitumor paradigm
The key to low-temperature PTT lies in how it is carried out at relatively low temperatures (usually less than 42°C). Its advantage lies in the ability to inhibit the synthesis of heat shock proteins, reduce the heat resistance of tumor cells, thereby improving the effectiveness of PTT and reducing the temperature required for treatment. This has important value for the future clinical translation of cancer PTT (Yan et al., 2024). Mild thermal stress (42°C–45°C) can selectively lead ICD in tumor cells, release DAMPs (such as CRT, HMGB1, ATP), promote DCs maturation and initiate CTLs responses. Taking PDA as an example, its low-temperature photothermal effect (ΔT ≈ 8°C–10°C) not only avoids upregulation of HSP70, but also activates the cGAS STING pathway through LMP, driving type I interferon secretion and transforming “immune cold tumors” into T cell enriched phenotypes. This “heat immune synergy” effect breaks through the local limitations of traditional PTT and can significantly prolong survival when combined with immune checkpoint inhibitors such as anti-PD-1. Abnormal metabolism of tumor cells, such as the Warburg effect, leads to microenvironmental acidification and immune suppression, and low-temperature PTT can break this vicious cycle through metabolic intervention. For example, under NIR-II irradiation, Pd NSs block the glycolytic pathway by inhibiting hexokinase 2 (HK2), reduce lactate production, reverse M2 polarization of TAMs, and reduce the expression of heat tolerant proteins, thereby increasing the sensitivity of tumor cells to sublethal temperature rise by 3–5 times. This dual targeted strategy of “metabolism hyperthermia” provides a new approach to overcome tumor heterogeneity (Uson et al., 2022).
To achieve precise low-temperature PTT, a new generation of intelligent materials (such as pH/ROS dual responsive semiconductor nanoparticles) are activated specifically by the lesion microenvironment, strictly limiting thermal effects to the tumor area (spatial accuracy<200 μm) to avoid off target damage. For example, CuS@MnO The core-shell structure generates heat in situ under acidic and high H2O2 conditions, resulting in a temperature gradient of 6°C–8°C between tumor and normal tissue (Jin et al., 2024). However, clinical application still faces the following challenges: lack of material biodegradability and long-term toxicity assessment system; The dose-response relationship between the low-temperature effect and immune response is unclear; The establishment of scientific standards for large-scale preparation processes and supervision is urgently needed. This review calls for the establishment of three-level evaluation indicators to replace traditional tumor inhibition rates: (1) immunogenicity index (such as CD8+T cell infiltration rate); (2) Depth of metabolic regulation (such as changes in lactate/ATP ratio); (3) Induction rate of remote effect. In addition, exploring the cross fusion of low-temperature PTT with epigenetic regulation (such as HDAC inhibitors) or microbial therapy may open up a new dimension of “photothermal microbiome immune” multidimensional therapy. This paradigm shift not only drives PTT from “rough thermal ablation” to “precise immune metabolism regulation,” but also offers a multi-scale mode for personalized tumor treatment.
4 Loading strategy and application of photothermal agents on nanocarriers
Various integrated nanoplatforms have been developed by loading photothermal agents onto multifunctional nanocarriers or combining them with other therapeutic molecules, effectively overcoming the limitations of single PTT (such as insufficient penetration depth, drug resistance, off target damage). This section focuses on the strategies and application progress of PDA, SNPs, AuNPs, palladium nanosheets and carbon materials as nanocarriers for loading photothermal agents or constructing composite systems. Based on the physical and chemical properties such as surface functional groups, porosity and plasma resonance effects, different nanocarriers can adopt the following strategies to achieve efficient loading and functional synergy of photothermal agents: Ⅰ. Physical adsorption: Utilizing π - π stacking, hydrophobic interactions, or electrostatic adsorption to load small molecule photothermal agents (such as indocyanine green ICG) or drugs. For example, PDA nanoparticles load doxorubicin (DOX) through π-π interactions, achieving photothermal triggered drug release (pH/NIR dual response) (Wang et al., 2022). Ⅱ. Chemical coupling: Fixing targeted ligands or photosensitizers on the surface of a carrier through covalent bonds (such as thiol gold bonds, amino carboxyl condensation). For instance, surface modification of AuNPs with HA targets tumor cells overexpressing CD44, and covalently Ce6 to achieve photothermal/photodynamic synergistic therapy (Liu et al., 2022). Ⅲ. Encapsulation: Co-loading of photothermal agents and drugs is achieved through mesoporous structure, core-shell design or liposome encapsulation. Such as mesoporous CuS@SiO NPs coated with DOX utilize NIR-II to trigger drug release and enhance deep tumor killing (Li et al., 2022). Ⅳ. Composite hybridization: Combining with other nanomaterials such as metal organic frameworks (MOFs) and graphene to enhance photothermal conversion efficiency or introduce catalytic functions. Table 3 summarizes the loading strategies, synergistic therapeutic mechanisms and application cases of five types of nanomaterials. Although loaded nanocarriers have shown great potential, some key issues still need to be addressed, such as loading efficiency and stability, penetration depth limitations, metabolism and toxicity, and clinical translation bottlenecks. In the future, intelligent responsive carriers will be developed to achieve precise drug release. Build an integrated PTT immunotherapy platform by combining mRNA vaccines or CAR-T cell therapy. Using AI assisted design of nanocarrier structures to optimize photothermal conversion efficiency and biological distribution.
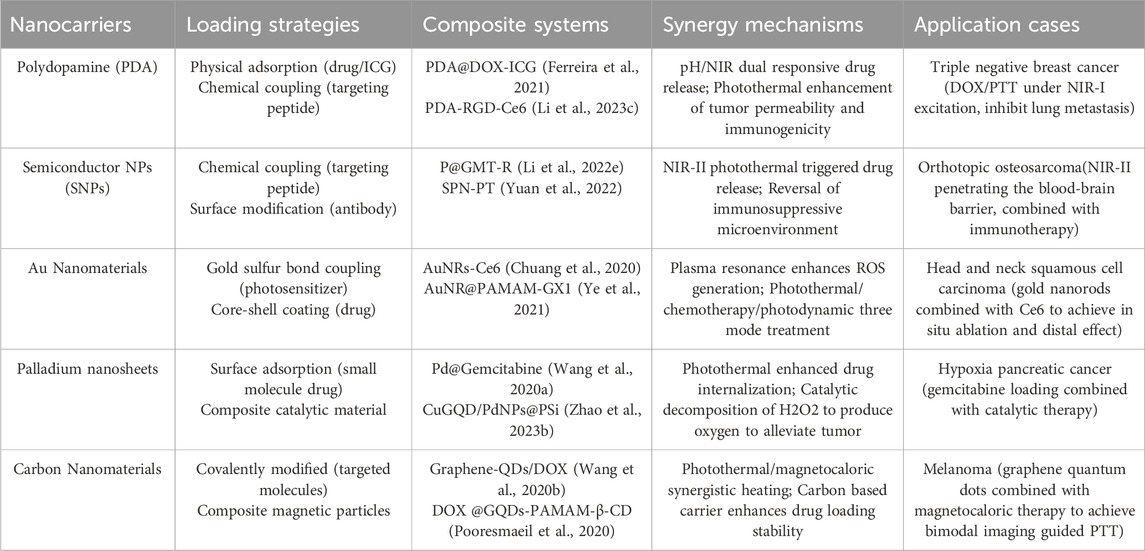
Table 3. The loading strategies, synergistic therapeutic mechanisms and application cases of five types of nanomaterials.
5 Conclusion and outlook
In recent years, functional nanomaterials have made progress in the field of tumor PTT, providing strategies to break through the limitations of traditional cancer treatment. By combining efficient photothermal materials with functional modification, researchers have successfully developed nanodelivery system with targeted delivery, multimodal imaging, synergistic therapy and so on. The materials can achieve local high-temperature killing tumor cells under NIR light excitation, while significantly improving tumor enrichment efficiency and reducing damage to normal tissues through surface functionalization (such as antibody and peptide modification). In addition, the synergistic application of nanomaterials with chemotherapy, radiotherapy and immunotherapy further amplifies the anti-tumor effect, such as activating the systemic anti-tumor immune response through photothermal induced immunogenic cell death (ICD), providing the possibility to inhibit metastasis and recurrence. Preclinical studies have confirmed that some nanosystems exhibit significant advantages in penetration depth, photothermal stability and biosafety, laying the foundation for translational medicine. However, the clinical translation of functional nanomaterials still faces multiple challenges. Firstly, the long-term biocompatibility and metabolic pathways of nanomaterials are not yet clear, and some metal based materials have potential toxicity risks, which urgently require the development of degradable or biologically inert carriers. Secondly, tumor heterogeneity and complex microenvironments (such as hypoxia and acidic pH) may weaken PTT therapy, requiring the design of intelligent responsive nanomaterials to achieve on-demand release of energy or drugs. Again, existing research has mostly focused on small animal models, and the depth of light penetration, thermal diffusion effect and large-scale preparation process of human tissues still need to be systematically optimized. In addition, the targeting efficiency of nanomaterials is limited by the accuracy of tumor vascular permeability and surface modification, and real-time treatment monitoring needs to be achieved by combining molecular imaging technology. Future research directions include the followings: Ⅰ. Material innovation, developing ultra efficient photothermal agents with NIR-II response and utilizing theoretical calculation to accelerate material design; Ⅱ. System integration, building an integrated diagnosis and treatment platform, integrating photoacoustic imaging, photothermal/photodynamic/immunotherapy synergistic therapy functions; Ⅲ. Clinical adaptation, evaluating individualized treatment plans through organoid or patient derived xenograft (PDX) models; Ⅳ. Deepen the mechanism and analyze the molecular correlation between tumor cell death and immune microenvironment remodeling under photothermal stress. With the integration of interdisciplinary technologies and the improvement of translational medicine systems, functional nanomaterials are expected to promote tumor PTT from the laboratory to clinical practice, opening up new paths for precision oncology.
Author contributions
XH: Writing – original draft. GF: Writing – original draft. XL: Writing – original draft. SM: Writing – original draft. CX: Writing – original draft. JY: Writing – original draft. LY: Writing – original draft. RZ: Writing – original draft. YJ: Writing – original draft. XX: Writing – original draft, Writing – review and editing. LD: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Jilin science and technology department, science and technology development project (Nos 20240101147JC, 20220508069RC and 20210203189SF), Jilin Province Development and Reform Commission, Industrial Technology Research and Development Project (No. 2024C019-9), College Student Innovation and Entrepreneurship Training Program (Nos JYSC24004, 2023CXXL030, and S202213706008).
Acknowledgments
The authors are grateful to all the reviewers of this article for their comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afroze, J. D., Tong, L., Abden, M. J., Yuan, Z., and Chen, Y. (2021). Hierarchical honeycomb graphene aerogels reinforced by carbon nanotubes with multifunctional mechanical and electrical properties. Carbon 175, 312–321. doi:10.1016/j.carbon.2021.01.002
Akakuru, O. U., Xing, J., Huang, S., Iqbal, Z. M., Bryant, S., Wu, A., et al. (2025). Leveraging non-radiative transitions in asphaltenes-derived carbon dots for cancer photothermal therapy. Small. 21, 2404591. doi:10.1002/smll.202404591
Alamdari, S. G., Amini, M., Jalilzadeh, N., Baradaran, B., Mohammadzadeh, R., Mokhtarzadeh, A., et al. (2022). Recent advances in nanoparticle-based photothermal therapy for breast cancer. JCR, 349: 269–303. doi:10.1016/j.jconrel.2022.06.050
Alghamri, M. S., Banerjee, K., Mujeeb, A. A., Mauser, A., Taher, A., Thalla, R., et al. (2022). Systemic delivery of an adjuvant CXCR4-CXCL12 signaling inhibitor encapsulated in synthetic protein nanoparticles for glioma immunotherapy. Acs Nano 16, 8729–8750. doi:10.1021/acsnano.1c07492
Amaral, S. I., Costa-Almeida, R., Goncalves, I. C., Magalhães, F. D., and Pinto, A. M. (2021). Carbon nanomaterials for phototherapy of cancer and microbial infections. Carbon 190, 194–244. doi:10.1016/j.carbon.2021.12.084
Balavigneswaran, C. K., Jaiswal, V., Venkatesan, R., Karuppiah, P. S., Sundaram, M. K., Vasudha, T. K., et al. (2024). Mussel-inspired adhesive hydrogels based on laponite-confined dopamine polymerization as a transdermal patch. Biomolecules 24, 724–738. doi:10.1021/acs.biomac.2c01168
Bao, Y. W., Hua, X. W., Li, Y. H., Jia, H. R., and Wu, F. G. (2018). Hyperthemia-promoted cytosolic and nuclear delivery of copper/carbon quantum dot-crosslinked nanosheets: multimodal imaging-guided photothermal cancer therapy. ACS Appl. Mater. Interfaces. 10, 1544–1555. doi:10.1021/acsami.7b15332
Camacho, S. A., Kobal, M. B., Moreira, L. G., Bistaffa, M. J., Roque, T. C., Pazin, W. M., et al. (2022). The efficiency of photothermal action of gold shell-isolated nanoparticles against tumor cells depends on membrane interactions. Colloids Surf. B 211, 112301. doi:10.1016/j.colsurfb.2021.112301
Cao, Y., Tang, L., Fu, C., Yin, Y., Liu, H., Feng, J., et al. (2024). Black phosphorus quantum dot loaded bioinspired nanoplatform synergized with aPD-L1 for multimode cancer immunotherapy. Nano Lett. 24, 6767–6777. doi:10.1021/acs.nanolett.4c01511
Chen, X., Shi, S., Wei, J., Chen, M., and Zheng, N. (2017). Two-dimensional Pd-based nanomaterials for bioapplications. Sci. Bull. 62, 579–588. doi:10.1016/j.scib.2017.02.012
Chen, X., Zou, J., Zhang, K., Zhu, J., Zhang, Y., Zhu, Z., et al. (2020). Photothermal/Matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Sci. Direct 11, 271–282. doi:10.1016/j.apsb.2020.08.009
Cheong, J. K. K., Popov, V., Alchera, E., Locatelli, I., Alfano, M., Menichetti, L., et al. (2021). A numerical study to investigate the effects of tumour position on the treatment of bladder cancer in mice using gold nanorods assisted photothermal ablation. Comput. Biol. Med. 138, 104881. doi:10.1016/j.compbiomed.2021.104881
Chuang, C. C., Chen, Y. N., Wang, Y. Y., Huang, Y. C., Lin, S. Y., Huang, R. Y., et al. (2020). Stem cell-based delivery of gold/chlorin e6 nanocomplexes for combined photothermal and photodynamic therapy. ACS Appl. Mater. Interfaces. 12, 30021–30030. doi:10.1021/acsami.0c03446
Cui, G., Dong, S., Sui, C., Kakuchi, T., Duan, Q., and Feng, B. (2022a). Fabrication of composite Fe3O4 nanoparticles coupled by thermo-responsive and fluorescent Eu complex on surface. IJPOM. Po. 71, 109–115. doi:10.1080/00914037.2020.1809404
Cui, G., Wang, H., Long, S., Zhang, T., Guo, X., and Chen, S. (2022b). Thermo and light-responsive polymer-coated magnetic nanoparticles as potential drug carriers. FBB 10, 931830. doi:10.3389/fbioe.2022.931830
Dong, S., Huang, Y., Yan, H., Tan, H., Fan, L., Chao, M., et al. (2024). Ternary heterostructure-driven photoinduced electron-hole separation enhanced oxidative stress for triple-negative breast cancer therapy. J. Nanobiotechnol 22, 240. doi:10.1186/s12951-024-02530-4
Fan, R., Chen, C., Hu, J., Mu, M., Chuan, D., Chen, Z., et al. (2023). Multifunctional gold nanorods in low-temperature photothermal interactions for combined tumor starvation and RNA interference therapy. Sci. Direct. 159, 324–337. doi:10.1016/j.actbio.2023.01.036
Fang, J. Y., Lin, L., Cao, Y., Tan, J., Liang, Y., Xiao, X., et al. (2024). Targeting the CD24-Siglec10 axis: a potential strategy for cancer immunotherapy. BIO Integr. 5, 1–11. doi:10.15212/bioi-2023-0022
Farbod, M., Sharif, L., and Rezatofighi, S. E. (2024). Photothermal performance of carbon nanotubes in the visible and near-infrared regions: applications in water evaporation and destroying cancer cells. Colloid Polym. Sci. 303, 25–31. doi:10.1007/s00396-024-05327-x
Feng, Y., Lu, S., Zhao, Q., Sun, T., Shi, Y., Gao, G., et al. (2024a). Controlled phase transition of the onion-like carbon nanostructure for photothermal cancer therapy. ACS Appl. Nano Mater. 7, 7008–7017. doi:10.1021/acsanm.3c06107
Feng, Z., Lin, X., and Wu, Z. (2024b). Recent development and applications of polydopamine in tissue repair and regeneration. Biomater. Int. J. Nanomed. 19, 859–881. doi:10.2147/IJN.S437854
Fernandes, N., Rodrigues, C. F., Moreira, A. F., and Correia, I. J. (2020). Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci-uk 8, 2990–3020. doi:10.1039/d0bm00222d
Ferreira, L. P., Gaspar, V. M., Monteiro, M. V., Freitas, B., Silva, N. J. O., and Mano, J. F. (2021). Screening of dual chemo-photothermal cellular nanotherapies in organotypic breast cancer 3D spheroids. J.Control. Release. 331, 85–102. doi:10.1016/j.jconrel.2020.12.054
Ferreira-Gonçalves, T., Nunes, D., Fortunato, E., Martins, R., de Almeida, A. P., Carvalho, L., et al. (2023). Rational approach to design gold nanoparticles for photothermal therapy: the effect of gold salt on physicochemical, optical and biological properties. Int. J. Pharm. 650, 123659. doi:10.1016/j.ijpharm.2023.123659
Gong, L., Zhang, Y., Zhao, J., Zhang, Y., Tu, K., Jiao, L., et al. (2022). All-in-one biomimetic nanoplatform based on hollow polydopamine nanoparticles for synergistically enhanced radiotherapy of colon cancer. SMALL 4, 2107656. doi:10.1002/smll.202107656
Guerrero-Florez, V., Mendez-Sanche, S. C., Patrón-Soberano, O. A., Rodríguez-González, V., Blach, D., and Martínez O, F. (2020). Gold nanoparticle-mediated generation of reactive oxygen species during plasmonic photothermal therapy: a comparative study for different particle sizes, shapes, and surface conjugations. J. Mater. Chem. B 8, 2862–2875. doi:10.1039/d0tb00240b
Guo, Z., Liu, X., Li, Y., Sang, Z., and Chen, D. (2023). Hyaluronic acid modified carbon nanotubes using for photothermal therapy by promoting apoptosis of nasopharyngeal carcinoma cells. Front. Bioeng. Biotechnol. 11, 1229852. doi:10.3389/fbioe.2023.1229852
He, J. F., Wang, J., Gao, S. B., Cui, Y., Ji, X., Zhang, X., et al. (2022). Biomineralized synthesis of palladium nanoflowers for photothermal treatment of cancer and wound healing. Int. J. Pharm. 615, 121489. doi:10.1016/j.ijpharm.2022.121489
He, S. S., Jiang, Y. Y., Li, J. C., and Pu, K. (2020). Semiconducting polycomplex nanoparticles for photothermal ferrotherapy of cancer. Angew. Chem. Int. Ed. 10633 - 10638, 10633–10638. doi:10.1002/anie.202003004
Hong, G., Diao, S., Antaris, A. L., and Dai, H. (2015). Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 115, 10816–10906. doi:10.1021/acs.chemrev.5b00008
Huo, F., Li, W., Liu, Y., Liu, X., Lee, C. Y., and Zhang, W. (2021). Review of long wavelength luminescent carbon-based nanomaterials: preparation, biomedical application and future challenges. J. Mater.Sci. 56, 2814–2837. doi:10.1007/s10853-020-05435-3
Iannazzo, D., Pistone, A., Salamo, M., Galvagno, S., Romeo, R., Giofré, S. V., et al. (2017). Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 518, 185–192. doi:10.1016/j.ijpharm.2016.12.060
Jie, Q., Chen, H., Yuan, G., and Jiang, X. (2023). Fast coating of polydopamine enables Scratch/Moisture resistant flexible electronics. ACS Appl. Mater. Interfaces. 15, 25393–25402. doi:10.1021/acsami.3c03412
Jin, Z., Wang, Y., Han, M., Wang, L., Lin, F., Jia, Q., et al. (2024). Tumor microenvironment-responsive size-changeable and biodegradable HA-CuS/MnO2 nanosheets for MR imaging and synergistic chemodynamic therapy/phototherapy. Colloid Surf. B 238, 113921. doi:10.1016/j.colsurfb.2024.113921
Khodadadei, F., Safarian, S., and Ghanbari, N. (2017). Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mat. Sci. Eng. C-MATER 79, 280–285. doi:10.1016/j.msec.2017.05.049
Kim, D., Jo, G., Chae, Y., Subramani, S., Lee, B. Y., Kim, E. J., et al. (2021b). Bioinspired camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy. Nanoscale 13, 14426–14434. doi:10.1039/D1NR03999G
Kim, H. S., Lee, S. J., and Lee, D. Y. (2021a). Milk protein-shelled gold nanoparticles with gastrointestinally active absorption for aurotherapy to brain tumor. Sci. Direct. 8, 35–48. doi:10.1016/j.bioactmat.2021.06.026
Kim, H. S., Seo, M., Park, T. E., and Lee, D. Y. (2022). A novel therapeutic strategy of multimodal nanoconjugates for state-of-the-art brain tumor phototherapy. J. Nanobiotechnology 20, 14. doi:10.1186/s12951-021-01220-9
Komedchikova, E. N., Kolesnikova, O. A., Syuy, A. V., Volkov, V. S., Deyev, S. M., Nikitin, M. P., et al. (2024). Targosomes: Anti-HER2 PLGA nanocarriers for bioimaging, chemotherapy and local photothermal treatment of tumors and remote metastases. JCR 365, 317–330. doi:10.1016/j.jconrel.2023.11.036
Kong, H., Han, J., Yang, M., Lai, L., Sun, Y., Luan, X., et al. (2023). Two-dimensional peptide nanosheets functionalized with gold nanorods for photothermal therapy of tumors. J. Mater. Chem. B 11, 3445–3452. doi:10.1039/d3tb00074e
Kong, X., Li, M., Xiao, W., Li, Y., Luo, Z., Shen, J. W., et al. (2024). Ω-Shaped fiber optic LSPR coated with hybridized nanolayers for tumor cell sensing and photothermal treatment. Talanta 278, 126381. doi:10.1016/j.talanta.2024.126381
Kshatriya, V. V., Kumbhare, M. R., Jadhav, S. V., Thorat, P. J., and Bhambarge, R. G. (2024). Updated review on recent advances in silver nanoclusters in bioanalytical and biomedical applications. BIO Integr. 5, 1–13. doi:10.15212/bioi-2024-0003
Lagos, K. J., García, D., Cuadrado, C. F., de Souza, L. M., Mezzacappo, N. F., da Silva, A. P., et al. (2023). Carbon dots: types, preparation, and their boosted antibacterial activity by photoactivation. Current status and future perspectives. Curr. status future Perspect. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnoiogy. 15, 1887. doi:10.1002/wnan.1887
Lee, S. S., Oudjedi, F., Kirk, A. G., Paliouras, M., and Trifiro, M. A. (2023). Photothermal therapy of papillary thyroid cancer tumor xenografts with targeted thyroid stimulating hormone receptor antibody functionalized multiwalled carbon nanotubes. Cancer nano. 14, 31. doi:10.1186/s12645-023-00184-9
Li, D., Zhang, C., Tai, X., Xu, D., Xu, J., Sun, P., et al. (2022e). 1064 nm activatable semiconducting polymer-based nanoplatform for NIR-II fluorescence/NIR-II photoacoustic imaging guided photothermal therapy of orthotopic osteosarcoma. Chem. Eng. J. 445, 136836. doi:10.1016/j.cej.2022.136836
Li, H. Z., Zhou, S. S., Wu, M., Qu, R., Wang, X., Chen, W., et al. (2023a). Light-driven self-recruitment of biomimetic semiconducting polymer nanoparticles for precise tumor vascular disruption. AM 221, e2210920–e2210920. doi:10.1002/adma.202210920
Li, J., Chen, L., Qin, Q., Wang, D., Zhao, J., Gao, H., et al. (2022c). Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson's disease. Neurobiol. Dis. 163, 105605. doi:10.1016/j.nbd.2021.105605
Li, J., Zhang, W., Ji, W., Wang, J., Wang, N., Wu, W., et al. (2021b). Near infrared photothermal conversion materials: mechanism, preparation, and photothermal cancer therapy applications. J.Mater. Chem. B 9, 7909–7926. doi:10.1039/d1tb01310f
Li, L., Li, J., Hu, R., Zhang, X., Ding, L., Ren, G., et al. (2023c). Tumor cell targeting and responsive nanoplatform for multimodal-imaging guided chemodynamic/photodynamic/photothermal therapy toward triple negative breast cancer. ACS Appl. Mater. Interfaces. 15, 27706–27718. doi:10.1021/acsami.3c04709
Li, S., Xu, B., Lu, M., Sun, M., Yang, H., Liu, S., et al. (2022a). Tensile-strained palladium nanosheets for synthetic catalytic therapy and phototherapy. AM. 34, e2202609. doi:10.1002/adma.202202609
Li, S. X., Chen, Y., He, P., Ma, Y., Cai, Y., Hou, X., et al. (2022b). Aggregation-induced emission (AIE) photosensitizer combined polydopamine nanomaterials for organelle-targeting photodynamic and photothermal therapy by the recognition of sialic acid. Adv. Healthc. MATER 11, 2200242. doi:10.1002/adhm.202200242
Li, X., Chen, J., Liu, S., Wang, N., Li, L., Shi, X., et al. (2023b). A therapeutic platform based on hollow CuSe nanostructures for multi-mode antitumor therapy. ACS Appl. Nano Mater. 6, 17728–17739. doi:10.1021/acsanm.3c03000
Li, Y., Du, L., Li, F., Deng, Z., and Zeng, S. (2022d). Intelligent nanotransducer for deep-tumor hypoxia modulation and enhanced dual-photosensitizer photodynamic therapy. ACS Appl. Mater. Interfaces. 14, 14944–14952. doi:10.1021/acsami.1c24172
Li, Y., Yu, J., Zhang, W., Shan, J., Chen, H., Ma, Y., et al. (2024). Copper selenide nanosheets with photothermal therapy-related properties and multienzyme activity for highly effective eradication of drug resistance. J. Colloid. Interf. Sci. 666, 434–446. doi:10.1016/j.jcis.2024.03.176
Li, Z., Mu, Y., Peng, C., Lavin, M. F., Shao, H., and Du, Z. (2021a). Understanding the mechanisms of silica nanoparticles for nanomedicine. WIRES Nanomed. Nanobiotechnol. 13, 1658. doi:10.1002/wnan.1658
Lian, H., Guan, P., Tan, H., Zhang, X., and Meng, Z. (2021). Near-infrared light triggered multi-hit therapeutic nanosystem for tumor specific photothermal effect amplified signal pathway regulation and ferroptosis. Biomater. Sci. 21, 63–76. doi:10.1016/j.bioactmat.2021.07.014
Liu, J., Wang, C., Wang, X., Cheng, L., Li, Y., Liu, Z., et al. (2015). Mesoporous silica coated single-walled carbon nanotubes as a multifunctional light-responsive platform for cancer combination therapy. Adv. Funct. Mater. 25, 384–392. doi:10.1002/adfm.201403079
Liu, P., Hao, L., Hsu, J. C., Zhou, M., Luo, Z., Peng, Y., et al. (2025). Biomineralized nanocomposite-integrated microneedle patch for combined brachytherapy and photothermal therapy in postoperative melanoma recurrence and infectious wound healing. Adv. Sci. 12, 2414468. doi:10.1002/advs.202414468
Liu, S., Tian, L., Mu, M., Liu, Z., Dong, M., Gong, Y., et al. (2024c). Platinum nanoparticles-enhanced Ferritin–Mn2+ interaction for magnetic resonance contrast enhancement and efficient tumor photothermal therapy. Adv. Healthc. Mater. 13, 2303939. doi:10.1002/adhm.202303939
Liu, T., Guo, S., Zhang, D., Xie, D., Zhang, X., Chen, L., et al. (2024b). Rhythmic mild photothermal therapy enhancing anti-PD-L1 response for oral squamous cell carcinoma immunotherapy, Chem. Eng. J., Rhythm. mild photothermal Ther. enhancing anti-PD-L1 response oral squamous Cell carcinoma Immunother. 488, 150908. doi:10.1016/j.cej.2024.150908
Liu, W., Wang, Y., Wang, Y., Li, X., Qi, K., Wang, J., et al. (2022a). Black silver nanocubes@amino acid-encoded highly branched gold shells with efficient photothermal conversion for tumor therapy. ACS 15, 236–248. doi:10.1021/acsami.2c14436
Liu, X., Sun, T., Sun, Y., Manshina, A., and Wang, L. (2024). Polyoxometalate-based peroxidase-like nanozymes. Adv. Mater. 7, 24–48. doi:10.1016/j.nanoms.2024.03.002
Lu, J., Cai, L., Dai, Y., Liu, Y., Zuo, F., Liu, Y., et al. (2021). Polydopamine-based nanoparticles for photothermal therapy/chemotherapy and their synergistic therapy with autophagy inhibitor to promote antitumor treatment. Chem. Rec. 21, 781–794. doi:10.1002/tcr.202000170
Lu, Y., Wang, Y., Liu, W., Ma, H., Yang, B., Shao, K., et al. (2023). Photothermal “nano-dot” reactivate “immune-hot” for tumor treatment via reprogramming cancer cells metabolism. Biomaterials 296, 122089–122093. doi:10.1016/j.biomaterials.2023.122089
Lyu, Y., Zeng, Z. F., Jiang, Y. Y., Zhen, X., Wang, T., Qiu, S., et al. (2018). Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Nano 12, 1801–1810. doi:10.1021/acsnano.7b08616
Ma, J., Li, N., Wang, J., Liu, Z., Han, Y., and Zeng, Y. (2023). In vivo synergistic tumor therapies based on copper sulfide photothermal therapeutic nanoplatforms. Exploration 3, 20220161. doi:10.1002/EXP.20220161
McKernan, P., Virani, N. A., Faria, G. N. F., Karch, C. G., Prada Silvy, R., Resasco, D. E., et al. (2021). Targeted single-walled carbon nanotubes for photothermal therapy combined with immune checkpoint inhibition for the treatment of metastatic breast cancer. Nanoscale Res. Lett. 16, 9. doi:10.1186/s11671-020-03459-x
Ming, J., Zhang, J., Shi, Y., Yang, W., Li, J., Sun, D., et al. (2020). A trustworthy CpG nanoplatform for highly safe and efficient cancer photothermal combined immunotherapy. Nanoscale 12, 3916–3930. doi:10.1039/c9nr09402d
Mohanta, D., Patnaik, S., Sood, S., and Das, N. (2019). Carbon nanotubes: evaluation of toxicity at biointerfaces. J. Pharm. Anal. 9, 293–300. doi:10.1016/j.jpha.2019.04.003
Moonshi, S. S., Vazquez-Prada, K. X., Tang, J., Westra van Holthe, N. J., Cowin, G., Wu, Y., et al. (2023). Spiky silver-iron oxide nanohybrid for effective dual-imaging and synergistic thermo-chemotherapy. ACS 15, 42153–42169. doi:10.1021/acsami.3c04696
Naief, M. F., Mohammed, S. N., and Mohammed, A. M. (2024). Carbon nanotubes: a review on synthesis and drug delivery for cancer treatment. Inorg. Chem. Commun. 159, 111694. doi:10.1016/j.inoche.2023.111694
Pan, X., Gao, A. T., Hu, Y. N., Hu, Z., Xie, C., and Lin, Z. (2022). Gadolinium-containing semiconducting polymer nanoparticles for magnetic resonance/fluorescence dual-modal imaging and photothermal therapy of oral squamous cell carcinoma. NANO Res. 16, 2808–2820. doi:10.1007/s12274-022-4947-5
Peng, Y., Liu, Q. M., and Chen, S. (2019). Structural engineering of semiconductor nanoparticles by conjugated interfacial bonds. TCR 20, 41–50. doi:10.1002/tcr.201900010
Phuong, P. T. M., Won, H. J., Robby, A. I., Kim, S. G., Im, G. B., Bhang, S. H., et al. (2020). NIR-vis-induced pH-sensitive TiO2 immobilized carbon dot for controllable membrane-nuclei targeting and photothermal therapy of cancer cells. ACS Appl. Mater. Interfaces 12, 37929–37942. doi:10.1021/acsami.0c11979
Pooresmaeil, M., Namazi, H., and Salehi, R. (2020). Synthesis of photoluminescent glycodendrimer with terminal β-cyclodextrin molecules as a biocompatible pH-sensitive carrier for doxorubicin delivery. Carbohydr. Polym. 246, 116658. doi:10.1016/j.carbpol.2020.116658
Rastinehad, A. R., Anastos, H., Wajswol, E., Winoker, J. S., Sfakianos, J. P., Doppalapudi, S. K., et al. (2019). Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. U. S. A. 116, 18590–18596. doi:10.1073/pnas.1906929116
Ren, Z., Wan, Q., Zhu, Y., Li, L., Wang, K., Zhao, F., et al. (2024). Atypical artificial cells: novel biomimetic materials for combating cancer. IM 3, 658–714. doi:10.1002/idm2.12199
Roach, L., Booth, M. E., Ingram, N., Paterson, D. A., Batchelor, D. V. B., Moorcroft, S. C. T., et al. (2021). Evaluating phospholipid-functionalized gold nanorods for in vivo applications. SMALL 17, 2006797. doi:10.1002/smll.202006797
Runzan, Z., Xianya, Q., Junyu, L., Xu, H., Zhao, S., Li, X., et al. (2023). Chemodynamic/Photothermal synergistic cancer immunotherapy based on yeast microcapsule-derived Au/Pt nanoparticles. ACS 15, 24134–24148. doi:10.1021/acsami.3c02646
Sajjadi, M., Nasrollahzadeh, M., Jaleh, B., Soufi, G. J., and Iravani, S. (2021). Carbon-based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects. J. Drug Target 29 (7), 716–741. doi:10.1080/1061186X.2021.1886301
Sathiyaseelan, A., Saravanakumar, K., Manivasagan, P., Jeong, M. S., Jang, E. S., and Wang, M. H. (2021). Folic acid conjugated chitosan encapsulated palladium nanoclusters for NIR triggered photothermal breast cancer treatment. Carbohydr. Polym. 280, 119021. doi:10.1016/j.carbpol.2021.119021
Shi, B., Li, D., Yao, W., Wang, W., Jiang, J., Wang, R., et al. (2024c). Multifunctional theranostic nanoparticles for multi-modal imaging-guided CAR-T immunotherapy and chemo-photothermal combinational therapy of non-hodgkin's lymphoma. Biomater. Sci. 10, 2577–2589. doi:10.1039/d1bm01982a
Shi, M., Fu, Z., Pan, W., Wang, K., Liu, X., Li, N., et al. (2024a). A golgi apparatus-targeted photothermal agent with protein anchoring for enhanced cancer photothermal therapy. AHM 13, 2303749. doi:10.1002/adhm.202303749
Shi, M., Li, Y., Pan, W., Fu, Z., Wang, K., Liu, X., et al. (2024b). A BRD4-Targeting photothermal agent for controlled protein degradation. ANGEW 63, e202403258–e202407851. doi:10.1002/anie.202403258
Shima, M. A., Guerrero, E. D., Bugarini, G., Cayme, J., De Avila, N., Garcia, J., et al. (2023). Theranostic applications of multifunctional carbon nanomaterials. VIEW 4, 20220056. doi:10.1002/viw.20220056
Singh, P., Haloi, P., Singh, K., Roy, S., Sarkar, A., B, S. L., et al. (2023). Palladium nanocapsules for photothermal therapy in the near-infrared II biological window. ACS Appl. Mater. & Interfaces 15, 39081–39098. doi:10.1021/acsami.3c06186
Sun, W., Zhang, X., Jia, H. R., Zhu, Y. X., Guo, Y., Gao, G., et al. (2019). Water-dispersible candle soot-derived carbon nano-onion clusters for imaging-guided photothermal cancer therapy. SMALL 15, 1804575. doi:10.1002/smll.201804575
Tade, R. S., and More, M. P. (2025). Emerging application of graphene quantum dots in photodynamic/photothermal and hyperthermia therapies for cancer treatment. Nano Biomed. Eng. 17, 111–128. doi:10.26599/NBE.2024.9290083
Terna, A. D., Elemike, E. E., Mbonu, J. I., Osafile, O. E., and Ezeani, R. O. (2021). The future of semiconductors nanoparticles:Synthesis, properties and applications. MSEB 11, 115363–63. doi:10.1016/j.mseb.2021.115363
Uson, L., Yus, C., Mendoza, G., Leroy, E., Irusta, S., Alejo, T., et al. (2022). Nanoengineering palladium plasmonic nanosheets inside polymer nanospheres for photothermal therapy and targeted drug delivery. Adv. Funct. Mater. 32, 2106932. doi:10.1002/adfm.202106932
Wang, C., Chen, Y., Xu, Z., Chen, B., Zhang, Y., Yi, X., et al. (2020b). Fabrication and characterization of novel cRGD modified graphene quantum dots for chemo-photothermal combination therapy. Sens. Actuators B Chem. 309, 127732. doi:10.1016/j.snb.2020.127732
Wang, H., Gao, J., Xu, C., Jiang, Y., Liu, M., Qin, H., et al. (2024a). Light-driven biomimetic nanomotors for enhanced photothermal therapy. Small 20, e2306208. doi:10.1002/smll.202306208
Wang, M., Lan, S., Zhang, W., Jin, Q., Du, H., Sun, X., et al. (2024b). Anti-cancer potency of copper-doped carbon quantum dots against breast cancer progression. IJN 19, 1985–2004. doi:10.2147/IJN.S449887
Wang, Q., Zhu, X., Wu, Z., Sun, T., Huang, W., Wang, Z., et al. (2020a). Theranostic nanoparticles enabling the release of phosphorylated gemcitabine for advanced pancreatic cancer therapy. J. Mater. Chem. B 8, 2410–2417. doi:10.1039/d0tb00017e
Wang, W., Li, M., Huang, X., Fang, J., Peng, F., and Huang, H. (2022a). Structural evolution mechanisms of Polydopamine/CdS and photothermal effect boosted photocatalytic H2 production activity. APPLSURFSCI 601, 154114. doi:10.1016/j.apsusc.2022.154114
Wang, Y., Wang, W., Wang, X., Wu, H., Zhao, W., and Zhao, C. (2022b). Immune-stealth carboxymethyl chitosan-based nanomaterials for magnetic resonance imaging-guided photothermal therapy. Carbohydr. Polym. 288, 119382. doi:10.1016/j.carbpol.2022.119382
Wei, Y., Wang, Z., Yang, J., Xu, R., Deng, H., Ma, S., et al. (2022). Reactive oxygen species/photothermal therapy dual-triggered biomimetic gold nanocages nanoplatform for combination cancer therapy via ferroptosis and tumor-associated macrophage repolarization mechanism. J. Colloid Interface Sci. 606, 1950–1965. doi:10.1016/j.jcis.2021.09.160
Wu, B., Shao, Y., Zhao, W., Zheng, Y., Wang, Y., and Sun, D. (2022). Dual functions of epigallocatechin gallate surface-modified Au nanorods@selenium composites for near-infrared-II light-responsive synergistic antibacterial therapy. J. Biomater. Appl. 36, 1812–1825. doi:10.1177/08853282211048570
Xiong, Y., Li, M., and Qing, G. (2024). Biomolecule-responsive polymers and their bio-applications. 3, 865–896. doi:10.1002/idm2.12210
Xu, H., Zhang, Y., Zhang, H., Zhang, Y., Xu, Q., Lu, J., et al. (2023). Smart polydopamine-based nanoplatforms for biomedical applications: state-of-art and further perspectives. CCR 488, 215153. doi:10.1016/j.ccr.2023.215153
Xu, L., Wu, R., Ni, J., Jin, L., Xu, K., Zhu, Y., et al. (2024). Black TiO2-based nanoparticles as toll-like receptor stimulator delivery system for enhanced photothermal-immunotherapy of pancreatic cancer. Cancer nano. 15, 27. doi:10.1186/s12645-024-00266-2
Xu, Q., Zhao, S., Deng, L., Ouyang, J., Wen, M., Zeng, K., et al. (2019). A NIR-II light responsive hydrogel based on 2D engineered tungsten nitride nanosheets for multimode chemo/photothermal therapy. Chem. Commun. 64, 9471–9474. doi:10.1039/c9cc04132j
Xu, Z., Wang, T., and Liu, J. (2022). Recent development of polydopamine anti-bacterial nanomaterials. Int. J. Mol. Sci. 23, 7278. doi:10.3390/ijms23137278
Yan, J., Yu, H., Liu, C., Li, B., Wei, D., He, B., et al. (2024). Low-temperature photothermal-chemotherapy enhancing tumor immunotherapy by tetrahedral framework nucleic acids nanogels based drug delivery system. Chem. Eng. J. 481, 148616. doi:10.1016/j.cej.2024.148616
Yang, H. L., Bai, L. F., Geng, Z. R., Chen, H., Xu, L. T., Xie, Y. C., et al. (2023b). Carbon quantum dots: preparation, optical properties, and biomedical applications. Mater. Today. Adv. 18, 100376. doi:10.1016/j.mtadv.2023.100376
Yang, M., Pang, M., Chen, J., Gao, F., Li, H., and Guo, P. (2021). Surfactant-assisted synthesis of palladium nanosheets and nanochains for the electrooxidation of ethanol. ACS Appl. 13, 9830–9837. doi:10.1021/acsami.0c20146
Yang, T., Wang, H., Zhou, Q., Huang, W., Zhang, J., Yu, Y., et al. (2023a). Mild chemo-photothermal synergistic therapy for tumors based on gold-nanoparticles coupled with metformin. ACS Appl. Nano Mater. 6, 5729–5736. doi:10.1021/acsanm.3c00140
Yang, Z., Zhang, Y., Tang, L., Yang, X., Song, L., Shen, C., et al. (2024). “All in one” nanoprobe Au-TTF-1 for target FL/CT bioimaging, machine learning technology and imaging-guided photothermal therapy against lung adenocarcinoma. J. Nanobiotechnol 22, 22. doi:10.1186/s12951-023-02280-9
Yao, L. J., Cao, W. Q., Cui, Y. J., and Qian, G. (2022). An adenosine triphosphate-responsive metal-organic framework decorated with palladium nanosheets for synergistic tri-modal therapy. CrystEngComm. 24, 2558–2566. doi:10.1039/d2ce00015f
Yao, X., Rao, Y., Hu, J., Luo, Z., and Chen, C. (2023). Nanoparticle-based photothermal therapy for breast cancer noninvasive treatment. Adv. Mater. 1–28. doi:10.1002/adma.202305140
Ye, L., Chen, Y., Mao, J., Lei, X., Yang, Q., and Cui, C. (2021). Dendrimer-modified gold nanorods as a platform for combinational gene therapy and photothermal therapy of tumors. J. Exp. Clin. Cancer Res. 40, 303–316. doi:10.1186/s13046-021-02105-3
Ying, Y., Su, Y., Wang, A., Li, T., Zhuang, H., Li, S., et al. (2025). Highly efficient NIR-II photothermal therapy amplified ROS oxide breast tumor therapy by mesoporous gallium-enriched platinum nanomedicine. MATER TODAY BIO. 32, 101869. doi:10.1016/j.mtbio.2025.101869
Yu, Y., Song, M., Chen, C., Du, Y., Li, C., Han, Y., et al. (2020). Bortezomib-encapsulated CuS/carbon dot nanocomposites for enhanced photothermal therapy via stabilization of polyubiquitinated substrates in the proteasomal degradation pathway. ACS Nano 14, 10688–10703. doi:10.1021/acsnano.0c05332
Yuan, X., Cen, J., Chen, X., Jia, Z., Zhu, X., Huang, Y., et al. (2021). Iridium oxide nanoparticles mediated enhanced photodynamic therapy combined with photothermal therapy in the treatment of breast cancer. Sci. Direct. 605, 851–862. doi:10.1016/j.jcis.2021.07.136
Yuan, Y., Diao, S., Ni, X., Zhang, D., Yi, W., Jian, C., et al. (2022). Peptide-based semiconducting polymer nanoparticles for osteosarcoma-targeted NIR-II fluorescence/NIR-I photoacoustic dual-model imaging and photothermal/photodynamic therapies. J. Nanobiotechnol 20, 44. doi:10.1186/s12951-022-01249-4
Yue, Z., Li, J., Tang, M., Sun, T., Chen, C., and Wu, Z. (2024). Nanozyme-based clusterphene for enhanced electrically catalytic cancer therapy. Adv. Healthc. Mater. 13, 2303222. doi:10.1002/adhm.202303222
Zeng, W., Li, Z., Huang, Q., Ding, C., Yang, L., Wang, W., et al. (2023). Multifunctional mesoporous polydopamine-based systematic delivery of STING agonist for enhanced synergistic photothermal-immunotherapy. Adv. Funct. Mater. 34, 2307241. doi:10.1002/adfm.202307241
Zeng, W., Zhang, H., Deng, Y., Jiang, A., Bao, X., Guo, M., et al. (2020). Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. J. Chem. Eng. Chin. Univ. 389, 124494. doi:10.1016/j.cej.2020.124494
Zeng, X., Zhang, W., Xue, C., Hong, X., and Xiao, Y. (2025). Shape and size effects of gold nanoparticles for tumor photoacoustic imaging and photothermal therapy within the NIR-I and NIR-II biowindows. SMALL. 2412296 21, 1–11. doi:10.1002/smll.202412296
Zhang, H., Abdiryim, T., Jamal, R., Liu, X., Niyaz, M., Xie, S., et al. (2022). Coal-based carbon quantum dots-sensitized TiO2 NRs/PTTh heterostructure for self-powered UV detection. Appl. Surf. Sci. 605, 154797. doi:10.1016/j.apsusc.2022.154797
Zhang, J., Zhang, K., Hao, Y., Yang, H., Wang, J., Zhang, Y., et al. (2022). Polydopamine nanomotors loaded indocyanine green and ferricion for photothermal and photodynamic synergistic therapy of tumor. J. Colloid Interface Sci. 633, 679–690. doi:10.1016/j.jcis.2022.11.099
Zhang, R., Wang, L., Wang, X., Jia, Q., Chen, Z., Yang, Z., et al. (2020). Acid-induced in vivo assembly of gold nanoparticles for enhanced photoacoustic imaging-guided photothermal therapy of tumors. Adv. Healthc. Mater 9, e2000394. doi:10.1002/adhm.202000394
Zhang, S., Jin, L., Liu, J., Liu, Y., Zhang, T., Zhao, Y., et al. (2020). Boosting Chemodynamic Therapy by the Synergistic Effect of Co-Catalyze and Photothermal Effect Triggered by the Second Near-Infrared Light. Nano-Micro Letters, Nano-Micro Lett 12, 180. doi:10.1007/s40820-020-00516-z
Zhang, S., Yu, S., Sun, J., Huang, T., Lin, H., Li, Z., et al. (2024a). Au@CuS nanoshells for surface-enhanced raman scattering image-guided tumor photothermal therapy with accelerated hepatobiliary excretion. Pharmaceutics 16, 1089. doi:10.3390/pharmaceutics16081089
Zhang, Y., Mou, H., Huang, Q., Jian, C., Li, X., Chen, S., et al. (2024b). Engineering of phytic acid-coated prussian nanoparticles for combined nitric oxide and low-temperature photothermal therapy of osteosarcoma. Chem. Eng. J. 491, 151730. doi:10.1016/j.cej.2024.151730
Zhao, J., Duan, W., Liu, X., Xi, F., and Wu, J. (2023b). Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 33, 2308183. doi:10.1002/adfm.202308183
Zhao, J., Zhang, Q., Liu, W., Shan, G., and Wang, X. (2022). Biocompatible BSA-Ag2S nanoparticles for photothermal therapy of cancer. Colloids Surf. B 211, 112295–112303. doi:10.1016/j.colsurfb.2021.112295
Zhao, X., Zhang, K., Chen, L., Wang, Z. Y., and Yan, X. P. (2023a). Multifunctionalized tumor-triggered targeting theranostic nanoparticles as a precision NIR imaging-guided nanoplatform for photothermal/photodynamic therapy. ACS Appl. Nano Mater. 6, 10501–10510. doi:10.1021/acsanm.3c01473
Zhao, Y., Sarhan, R. M., Eljarrat, A., Kochovski, Z., Koch, C., Schmidt, B., et al. (2022c). Surface-functionalized Au-Pd nanorods with enhanced photothermal conversion and catalytic performance. ACS Appl. Mater. Interfaces 14, 17259–17272. doi:10.1021/acsami.2c00221
Zhao, Y., Tang, T., Yang, J., Liu, F., Guo, J., Cui, R., et al. (2024b). Bioorthogonal NIR-IIb quantum dots for imaging-guided photothermal therapy. ACS Appl. Nano Mater. 7, 14354–14362. doi:10.1021/acsanm.4c01794
Zhao, Y., Wang, X., Li, Y., Liu, Y., Hou, J., and Guo, Y. (2024a). Preparation and photothermal therapy of gold nanorods modified by belamcanda chinensis (L.) DC polysaccharide. Int. J. Biol. Macromol. 255, 127854. doi:10.1016/j.ijbiomac.2023.127854
Zheng, C. ., Wang, Z. ., Xu, H. ., Huang, H., Tao, X., Hu, Y., et al. (2024). Redox-activatable magnetic nanoarchitectonics for self-enhanced tumor imaging and synergistic photothermal-chemodynamic therapy. SMALL METHODS 8, e2301099–13. doi:10.1002/smtd.202301099
Keywords: nanomaterial, polymer nanoparticles, nanophotothermal agent, photothermal therapy, tumor therapy
Citation: Han X, Feng G, Li X, Mo S, Xu C, Yan J, Yu L, Zhang R, Jin Y, Xiao X and Deng L (2025) Functional nanomaterials for enhanced tumor photothermal therapy - the mechanisms and applications. Front. Pharmacol. 16:1604965. doi: 10.3389/fphar.2025.1604965
Received: 02 April 2025; Accepted: 04 June 2025;
Published: 14 July 2025.
Edited by:
Wu Junyong, Central South University, ChinaReviewed by:
Tiedong Sun, Northeast Forestry University, ChinaVeena Vijayan, University of Rhode Island, United States
Copyright © 2025 Han, Feng, Li, Mo, Xu, Yan, Yu, Zhang, Jin, Xiao and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Xiao, eGlhb3gxNDVAbmVudS5lZHUuY24=; Li Deng, ZGVuZ2xpXzE5ODExOEAxNjMuY29t
 Xiaoling Han1
Xiaoling Han1 Xiao Xiao
Xiao Xiao Li Deng
Li Deng