- 1Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 2Department of Clinical Test Laboratory, Hohhot First Hospital, Hohhot, Inner Mongolia, China
Reactive oxygen species (ROS) exhibit a dual regulatory role in cancer biology. While moderate ROS levels promote tumorigenesis via DNA mutagenesis, excessive ROS accumulation induces cancer cell death through oxidative stress. Therefore, ROS homeostasis represents a promising therapeutic target in oncology. Collectively, ROS exhibit context-dependent and multifaceted roles in cancer progression. Emerging evidence highlights the anticancer potential of traditional Chinese medicine (TCM), particularly Paris polyphylla saponin (PPS). PPS modulates oxidative stress through precision targeting of ROS-associated signaling pathways, thereby inducing apoptosis, cell cycle arrest, autophagy, and ferroptosis. These mechanisms collectively suppress tumor growth, metastasis, and angiogenesis, while concurrently mitigating inflammatory responses. Notably, PPS potentiates the efficacy of chemotherapeutic agents by reversing multidrug resistance in refractory cancer cells. The bioactive constituents of PPS, polyphyllin and polyphyllinositol, exhibit potent antitumor activity in preclinical models. This study systematically elucidates the molecular mechanisms underlying PPS-mediated anticancer effects via ROS targeting, offering a robust theoretical framework and translational insights for future oncology research.
1 Introduction
Malignant tumors represent a critical global public health challenge, significantly compromising human health (Cronin et al., 2022; Miller et al., 2022). Cancer incidence and mortality rates are steadily escalating, driven by population aging and cumulative environmental risk exposure (Liao et al., 2019; Qin et al., 2018; Sparg et al., 2004; Torre et al., 2016). Globally, an estimated 19 million incident malignancies and 10 million attributable mortality were encompassed in 2020 (Sung et al., 2021), with projections indicating a surge to over 30 million new cases by 2040 (Murthy et al., 2024). Although conventional therapies (e.g., surgical resection, radiotherapy, and chemotherapy) have markedly improved patient survival, their clinical utility is constrained by dose-limiting toxicities, acquired drug resistance, and limited efficacy against metastatic tumors (Qin et al., 2018; Torre et al., 2016).
Natural medicines are now regarded as promising candidates in oncology research due to their multifaceted therapeutic potential. Multi-target strategies involving bioactive compounds from traditional Chinese medicine (TCM) are gaining prominence for their dual capacity to augment therapeutic outcomes while mitigating toxicity and overcoming chemoresistance (Xiang et al., 2019; Zhang et al., 2021). Among TCM-derived anticancer agents, Paris polyphylla saponins (PPS)—the core bioactive constituents of P. polyphylla—exhibit potent antitumor activity against multiple malignancies, including lung (Kong et al., 2010), gastric, and colorectal carcinomas (Teng et al., 2015). Notably, PPS modulate the redox equilibrium of reactive oxygen species (ROS), triggering tumor cell cycle arrest, apoptotic and autophagic cell death, while concurrently suppressing angiogenesis and metastatic dissemination. This ROS-dependent antitumor mechanism offers a novel paradigm for designing precision-targeted anticancer therapeutics. This review synthesizes recent advances in PPS-mediated ROS signaling modulation, systematically dissecting its molecular underpinnings and evaluating its translational prospects for anticancer drug development.
This research seeks to systematically explore the antitumor mechanisms of PPS through ROS level modulation, with the goal of proposing novel therapeutic strategies for cancer management. By elucidating the molecular mechanisms, signaling networks, and translational potential of PPS-mediated ROS generation, this research seeks to establish a foundational framework for developing precision-targeted antitumor agents and advancing their clinical applicability.
2 Chemical composition and bioactive properties of PPS
The genus Paris (Liliaceae family) comprises 24 species, of which 22 are endemic to China. Paris polyphylla is the most medicinally significant species within this genus, owing to its abundant germplasm diversity and phytochemical richness. To date, over 320 distinct chemical constituents have been identified in P. polyphylla, encompassing steroidal saponins (Zhou et al., 2021), C-21 steroids, phytosterols, ecdysteroids, pentacyclic triterpenoids, and flavonoids (Liu Y. et al., 2023). Of these,the steroidal saponins—collectively termed PPS—represent the predominant bioactive constituent (Ding et al., 2021).
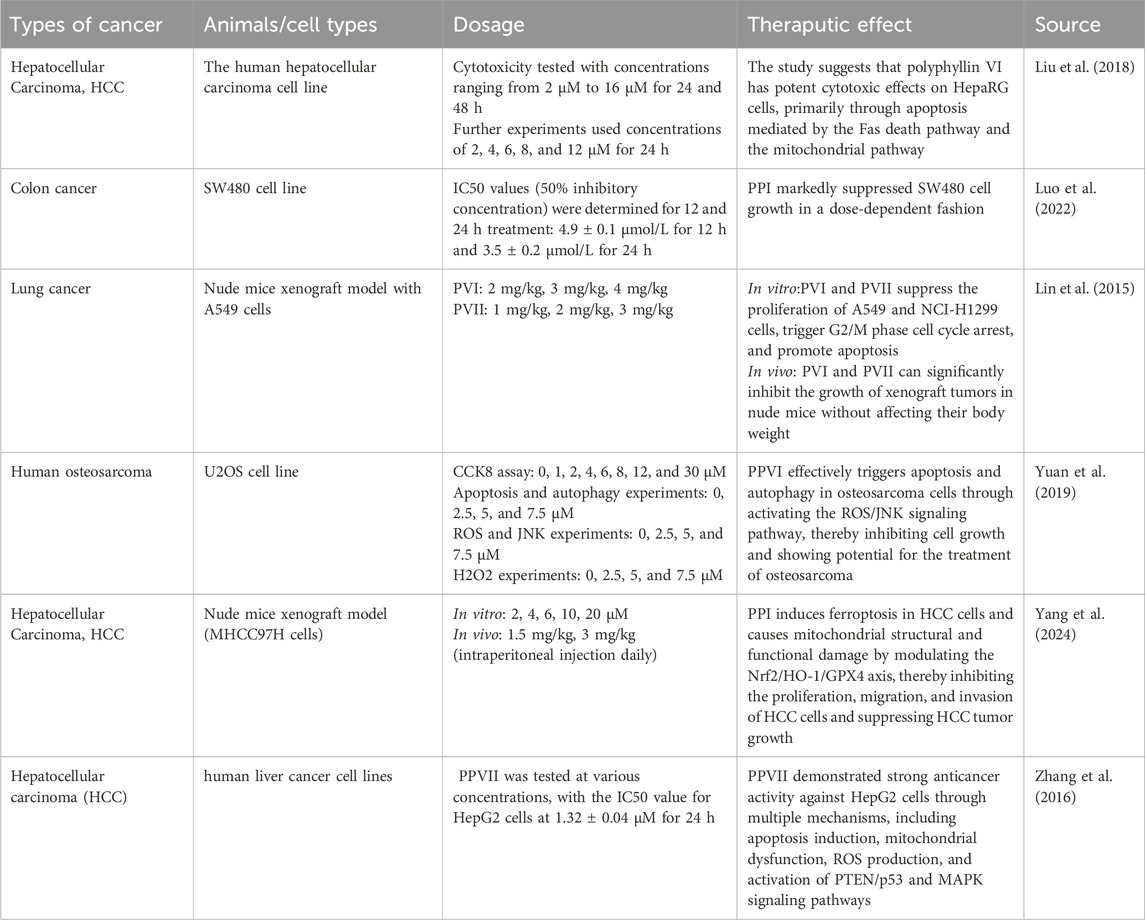
Structurally, saponins are glycosides composed of aglycone moieties (sapogenins) linked to sugar chains. Based on their aglycone frameworks, they are classified into steroidal and triterpenoid subtypes (Sparg et al., 2004). As spirostanol-type steroidal saponins, PPS exhibit a characteristic 27-carbon skeleton with sugar moieties attached at the C-3 and/or C-26 positions (Figure 1). Key structural variants include polyphyllin I, II, III, VI, VII, C, and H, among which polyphyllin I, II, VI, and VII are designated by the Chinese Pharmacopoeia as quality-control markers due to their validated anticancer properties (Qin et al., 2018; He et al., 2019).
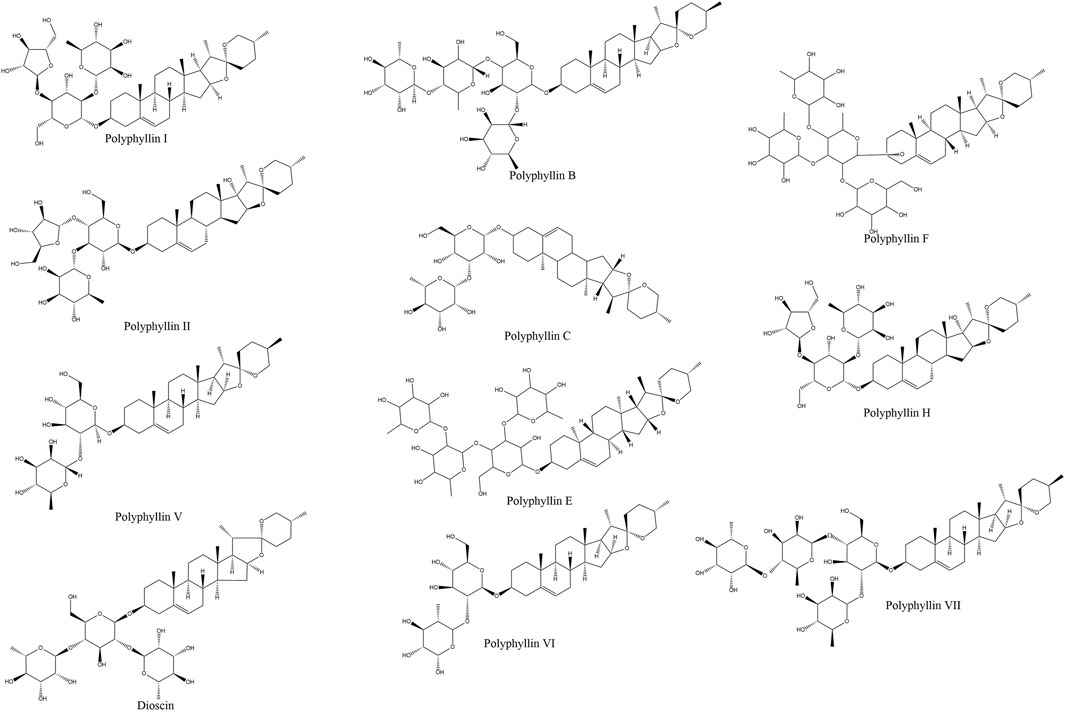
Figure 1. Rhizoma Paridis and its anti-cancer bioactive ingredients Rhizoma Paridis saponins (RPS). Commercially available RPS include polyphyllin I/polyphyllin D, polyphyllin II, Dioscin, polyphyllin V, polyphyllin VI, polyphyllin VII, polyphyllin B, polyphyllin C, polyphyllin E, polyphyllin F, and polyphyllin H.
2.1 Bioactive extracts and pharmacological properties
Extracts of Paris polyphylla rhizomes—prepared using solvents ranging from polar (aqueous, alcoholic) to nonpolar (petroleum ether)—demonstrate broad bioactivity. At the extract level, these preparations exhibit antioxidant, antimicrobial, and antitumor effects, which are primarily attributable to their PPS content (Lepcha et al., 2019). Pharmacological studies have systematically characterized PPS as multifunctional agents with anti-inflammatory, analgesic, immunomodulatory (Yang L. et al., 2021), and antitumor activities, along with hemostatic, antimicrobial (Wei et al., 2014), and detoxifying properties (Guan et al., 2019).
2.2 Structure-activity relationships and mechanistic insights
The bioactivity of PPS is closely linked to their chemical architecture. For instance, the phenolic hydroxyl groups within saponin structures contribute to antioxidant effects by scavenging free radicals and chelating redox-active metal ions (e.g., Fe2+, Cu2+), thereby inhibiting lipid peroxidation and hydroxyl radical generation (Li et al., 2021). Notably, the length of sugar chains and the configuration of glycosidic linkages critically influence antimicrobial potency, as exemplified by the stronger activity of polyphyllin I (PPI) compared to polyphyllin H (PPH) (Qin et al., 2012).
2.3 Specific bioactive compounds
Among characterized PPS monomers, PPI emerges as a multifunctional candidate. It alleviates oxidative stress via activation of the SIRT3/SOD2/ROS signaling axis, while also demonstrating the highest antimicrobial activity against pathogens such as Cutibacterium acnes and Staphylococcus aureus (Firlej et al., 2022). In contrast, PPH exhibits reduced efficacy, highlighting the importance of structural features such as sugar moiety composition for bioactivity (Qin et al., 2012). Furthermore, PPI and related saponins exert antitumor effects through ROS-mediated mechanisms, suppressing proliferation, inducing apoptosis, and reversing multidrug resistance in lung, breast, colorectal, and hepatocellular carcinomas.
3 Role of ROS in tumors
ROS primarily originate from mitochondrial oxidative phosphorylation (Cheung and Vousden, 2022; Forman et al., 2014). While regulating cellular homeostasis, ROS induce cytotoxicity via DNA, lipid, and protein damage (Halliwell, 2022; Ismail et al., 2019). This duality drives ROS-mediated tumor promotion and cell death. Cancer cells exploit ROS by dynamically balancing their production, activating oncogenic pathways (e.g., MAPK/NF-κB), and suppressing antioxidants, collectively enhancing tumor progression (Aggarwal et al., 2019).
3.1 ROS generation and regulation
Mitochondria represent the predominant intracellular ROS source through electron transport chain (ETC.) activity during oxidative phosphorylation (Figure 2). Approximately 1% of molecular oxygen undergoes partial reduction at complexes I and III, generating superoxide (O2−) that partitions into the mitochondrial matrix (Complex I) or intermembrane space (Complex III) (Okoye et al., 2023; Murphy, 2009). These radicals are converted to H2O2 by MnSOD (matrix) and Cu/ZnSOD (cytosol), with cytosolic transfer mediated by mitochondrial permeability transition pores (MPTP).
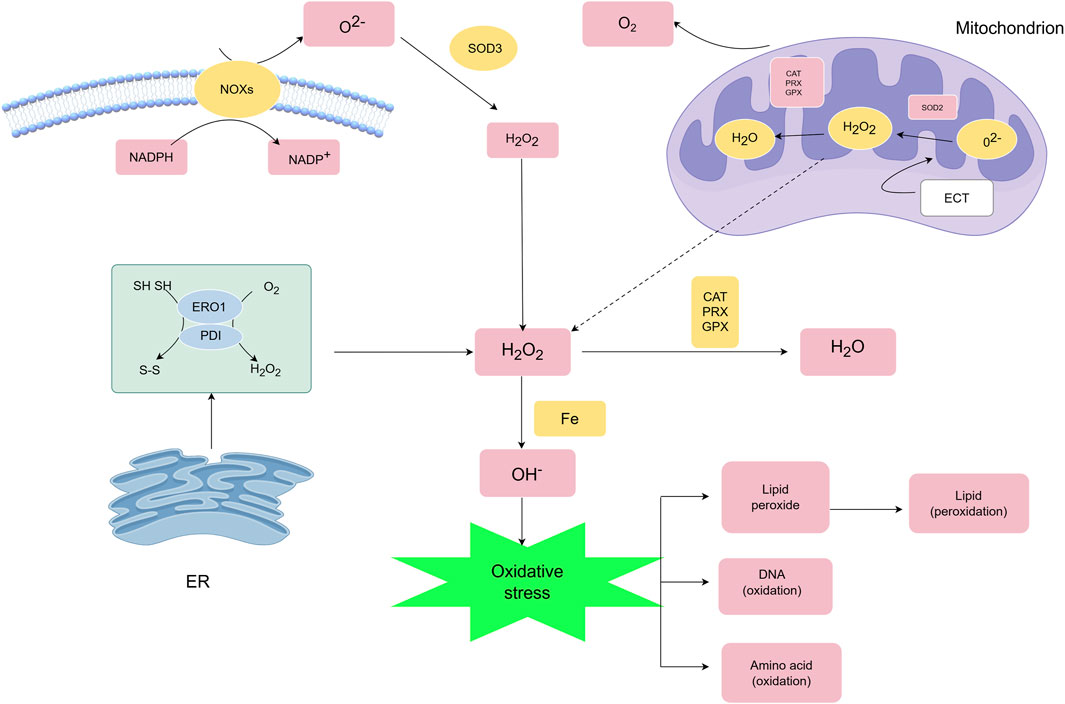
Figure 2. Main generation and modulation of ROS.ROS are primarily generated by mitochondria and cellular membrane NOXs, and their metabolism is regulated by multiple mechanisms. SOD converts O2·- into H2O2. H2O2 has two metabolic fates: ① it can react with Fe2+ through the Fenton reaction to produce hydroxyl radical (OH·), resulting in oxidative injury to cellular macromolecules such as DNA, proteins, and lipids; ② it can be reduced to water by the antioxidant system composed of PRXs, GPXs, and CAT, thereby regulating the intracellular oxidative balance.
NADPH oxidases (NOXs) constitute another major ROS-generating system. NOX isoforms (NOX1-5/DUOX1-2) utilize NADPH (maintained by NADK and regulated by MESH1/Nocturnin phosphatases) to produce O2− or directly generate H2O2 (NOX4) through coordinated action of regulatory subunits (Racphox, p47phox, etc.) (Bedard and Krause, 2007; Ding et al., 2020; Schild et al., 2021; Takac et al., 2011).
ROS signaling exhibits spatiotemporal specificity: Physiological H2O2 diffuses via aquaporins (AQP3/8) to activate redox-sensitive targets, while pathological overproduction disrupts compartmentalization, causing oxidative damage and cell death (Edmondson and Binda, 2018; Pak et al., 2020). Beyond mitochondrial and NOX-derived ROS, secondary sources include endoplasmic reticulum (ER) stress (protein misfolding) (Tu and Weissman, 2002; Haynes et al., 2004), inflammatory cytokines (TNF-α/IL-1β), and hypoxia signaling (Cheung and Vousden, 2022). This dynamic network integrates diverse stimuli to regulate redox homeostasis in health and disease.
3.2 The double-edged role of ROS
ROS exhibit concentration-dependent duality in cancer—promoting tumorigenesis at physiological levels through oncogene activation and metabolic reprogramming (Szatrowski and Nathan, 1991), while triggering apoptosis/ferroptosis when exceeding cellular antioxidant thresholds (Wang et al., 2021) This spatiotemporal dynamic positions ROS as both oncogenic drivers and therapeutic targets. Figure 3 delineates their dual roles in homeostasis and tumor progression (Figure 3).
Figure 3. Effects of ROS on cells: physiological function, cancer development, and cell demise.In normal cells, ROS production, antioxidant responses, and cellular repair processes are tightly balanced, maintaining ROS at optimal levels to restrict excessive cell persistence and multiplication. Elevated ROS concentration can induce cellular harm; however, tumor cells often exhibit increased antioxidant capacity and adapt through metabolic reprogramming and hypoxia-induced signaling pathways, supporting tumor-promoting effects. Nevertheless, when ROS surpass a critical threshold, oxidative stress causes irreversible cellular damage, overwhelms adaptive mechanisms, and ultimately triggers tumor cell death.
3.2.1 Tumor-promoting effects of ROS
ROS exhibit multifaceted roles in tumorigenesis and cancer progression (Cheung and Vousden, 2022). Chronic oxidative stress induces DNA damage and genomic instability, hallmarks of carcinogenesis (Huang et al., 2022). ROS modulate redox-sensitive signaling pathways in cancer cells through post-translational modifications of cysteine residues in regulatory proteins. These redox-regulated proteins orchestrate pro-tumorigenic processes, including proliferation, metabolic adaptation, invasion, and metastatic dissemination—key drivers of cancer aggressiveness.
3.2.1.1 ROS promotes cell proliferation
ROS play a crucial role in driving cellular survival and proliferation, acting as signaling intermediates in growth factor-mediated pathways, particularly through PI3K/Akt/mTOR and MAPK/ERK pathways, which are central to proliferation and survival (Moloney and Cotter, 2018a). ROS further modulate the nuclear factor kappa B (NF-κB) pathway (Lingappan, 2018) and induce oxidative modifications of transcription factors (e.g., Nrf2, p53), altering their stability and activity in cancer-related processes (Humpton et al., 2022). Collectively, ROS are master regulators of oncogenic signaling networks.
The Akt pathway promotes cell survival by inhibiting pro-apoptotic proteins (e.g., Bad, Bax) and Foxo transcription factors (Essers et al., 2004). ROS-mediated activation of the PI3K/Akt survival pathway represents an early driver of oncogenesis in multiple malignancies. Negative modulators of the PI3K/Akt pathway, including PTEN and PTP1B, contain redox-sensitive cysteine residues in their catalytic sites. Oxidative modification of these residues by H2O2 inactivates phosphatase activity, resulting in constitutive pathway activation and enhanced tumor cell survival (Hu et al., 2019). This mechanism is observed in various cancer types (Guo et al., 2020).
The MAPK pathway, activated through a three-tiered kinase cascade, regulates proliferation, growth, differentiation, apoptosis, and tumorigenesis. The ERK1/2 branch drives proliferation via growth factor receptors and K-Ras (Khavari and Rinn, 2007). ROS activate ASK1(a member of three-tiered kinase cascade), by oxidizing thioredoxin (TRX), leading to its dissociation from ASK1 (Liu et al., 2000). Beyond upstream regulation, ROS sustain MAPK activation by oxidizing JNK’s cysteine residue to sulfenic acid, preventing dephosphorylation (Son et al., 2011).
ROS can drive drug resistance in tumors. ROS enhance tumor survival via NF-κB/Nrf2 activation, upregulating antioxidant (SOD) and anti-apoptotic (Bcl-2) mediators, directly driving chemoresistance.
3.2.1.2 ROS-driven metastatic progression
ROS drive the process of tumor metastasis: (1) stimulating angiogenesis through vascular endothelial growth factor(VEGF) upregulation, and (2) enhancing tumor cell invasion and metastasis via mitochondrial membrane potential (MMP) activation.
ROS drive pathological angiogenesis in cancer through hypoxia-inducible factor-1α (HIF-1α) stabilization, inducing pseudohypoxic HIF complex formation. This activates angiogenic pathways via VEGF upregulation (Catrina and Zheng, 2021; Arbiser et al., 2002; Chatterjee and Chatterjee, 2020; Gerald et al., 2004; Zhao et al., 2023),promoting tumor neovascularization. Beyond angiogenesis, ROS also promote metastasis through integrin/FAK (focal adhesion kinase)-mediated tumor-endothelial adhesion (ten Kate et al., 2006). Paradoxically, targeted suppression of melanoma cells in the microvasculature may elevate endothelial ROS to cytotoxic thresholds, triggering tumor cell apoptosis—a potential antimetastatic strategy (Wang et al., 2000).
ROS activate matrix metalloproteinases (MMPs) (Nelson and Melendez, 2004)to degrade extracellular matrix (ECM) (Mori et al., 2019), enabling tumor invasion. Aggressive epithelial cancers undergo epithelial-mesenchymal transition (EMT) (Brabletz, 2012), imparting migratory and invasive capacities.ROS-driven EMT enables metastatic colonization. (Wu et al., 2014; Lambert and Weinberg, 2021; Pastushenko et al., 2021).Figure 4.
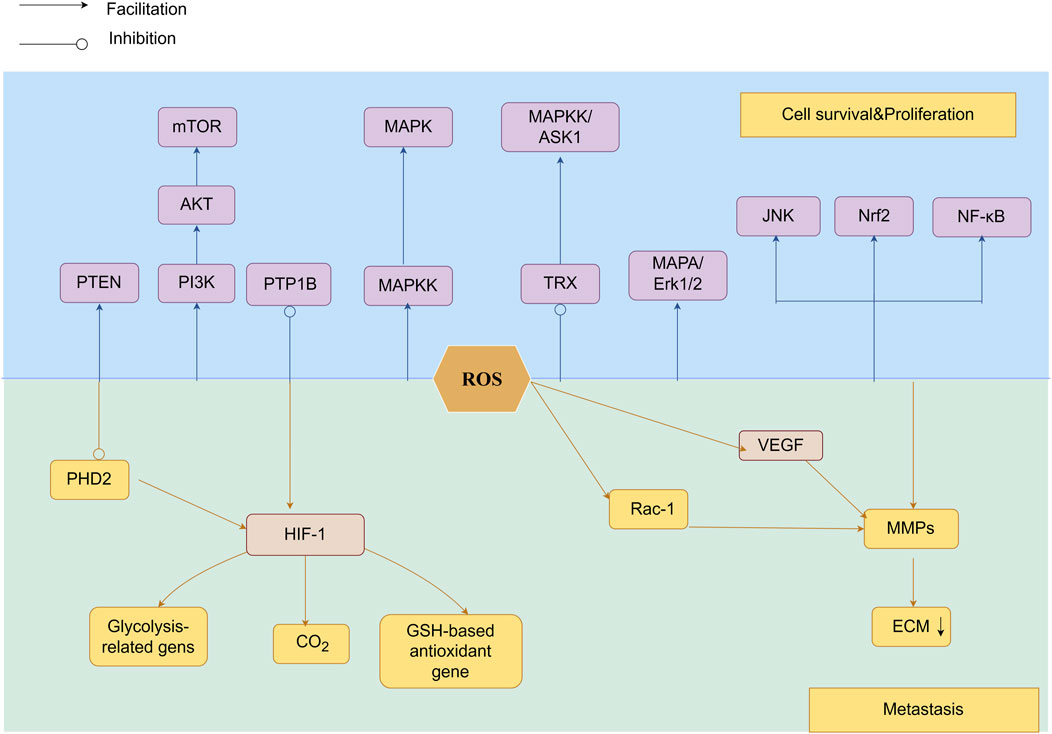
Figure 4. As signaling molecules, ROS participate in the PI3K/Akt/mTOR and MAPK/ERK pathways, modulate NF-κB activity, and are associated with Nrf2 mutations. By inhibiting PHD2 and stabilizing HIF-1α, ROS facilitate tumor cell motility and invasiveness.HIF-1α activation induces the expression of lactate dehydrogenase and pyruvate dehydrogenase kinase 1, suppresses antioxidant genes involved in GSH metabolism, and reduces mitochondrial ROS production, thereby promoting extracellular matrix degradation and invasive behavior. Furthermore, HIF-1α-driven signaling facilitates VEGF-mediated angiogenesis, while ROS accelerate tumor metastasis through MMP-mediated breakdown of ECM proteins, enhancing both vascularization and metastatic spread.
3.2.2 Carcinogenic effects of ROS
ROS accumulation exhibits dual cytotoxic/mutagenic effects, mediating tumorigenesis and cell death. This causes oxidative macromolecular damage (DNA/proteins/lipids) (Bekeschus, 2023), while activating tumor-suppressive programmed cell death (PCD) pathways, including apoptosis, ferroptosis, etc., as intrinsic tumor suppression (Hengartner, 2000). Therapeutically elevating ROS induces tumor-specific PCD (Figure 5) (Liu et al., 2022; Loke et al., 2023).
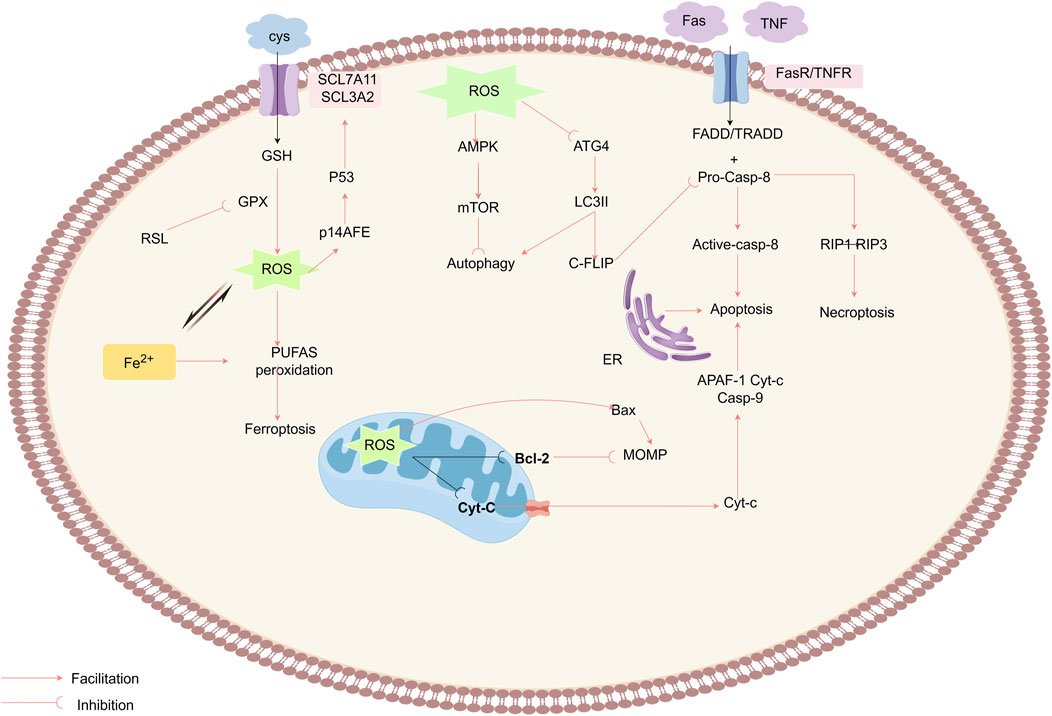
Figure 5. ROS mediate antitumor effects by triggering RCD pathways, including apoptosis, autophagy, necroptosis, and ferroptosis. ROS act on the MPTP, reducing the mitochondrial membrane potential (MMP), which prompts the release of Cyt-c into the cytoplasm, where it binds to APAF-1 and procaspase-9, initiating the caspase-9 cascade reaction and triggering apoptosis.The ROS also enhance the extrinsic apoptosis pathway by degrading c-FLIP and activate RIP1 and RIP3 to induce necroptosis. ROS inactivate the autophagy-related gene Atg4, increase LC3-associated autophagosomes, and promote autophagy, while the inhibition of mTORC1 and the activation of AMPK negatively regulate autophagy. Ferroptosis is an ROS-driven, iron-dependent form of programmed cell death characterized by lipid peroxidation. The Fenton reaction enhances the activity of lipoxygenase and the production of ROS. Erastin disrupts the XC− system, damaging the GPX antioxidant mechanism.Elevated ROS levels disrupt the integrity of the outer mitochondrial membrane, while RSL3 induces ferroptosis by suppressing GPX activity.
3.2.2.1 ROS promote apoptosis via extrinsic pathway regulation
Apoptosis (type I PCD) proceeds via extrinsic (death receptor) and intrinsic (mitochondrial) pathways. Extrinsic apoptosis initiates through death receptor (TNFR1/Fas) ligation, recruiting adaptor proteins (e.g., FADD, TRADD) and procaspase-8 to form the death-inducing signaling complex (DISC) (Pan et al., 2022). Procaspase-8 undergoes autocatalytic activation within the DISC, initiating a caspase cascade that executes apoptosis (Wang et al., 2008), counteracted by cellular FLICE inhibitory protein (c-FLIP) through competitive DISC binding. DISC contains death domains (DD/DED), adaptor proteins (FADD/TRADD), and procaspase-8 (Medina et al., 2020). ROS promote this pathway via c-FLIP degradation, enhancing caspase-8 activation (Wilkie-Granth et al., 2013).
The mitochondrial pathway represents the primary ROS-mediated apoptotic route, regulated through MPTPs. ROS oxidize within MPTP components—voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and cyclophilin D (CypD)—increasing membrane permeability (Madesh and Hajnóczky, 2001). This leads to ΔΨm collapse and cytochrome c release (Halliwell, 2022), triggering apoptosome-mediated caspase activation (Bock and Tait, 2020; Kagan et al., 2005; Moloney and Cotter, 2018b; Zuo et al., 2009; Stennicke et al., 1999).
Intrinsic apoptosis is regulated by Bcl-2 family proteins. ROS induce Bcl-2 degradation via ubiquitination-proteasome pathways and upregulate Bax/Bad (Kalkavan et al., 2022; Li et al., 2004). Additionally, ROS activate JNK/p38/MAPK pathways to promote apoptosis (Cadenas, 2004; Liou and Storz, 2010), a mechanism exploited by targeted anticancer therapies (Dimitrov and Marks, 2009). For instance, imatinib triggers ROS-dependent apoptosis in glioblastoma through JNK activation and mitochondrial membrane potential (ΔΨm) collapse (Liu et al., 2019). Similarly, rituximab elevates ROS levels, suppresses Bcl-2, and inhibits p38 MAPK to induce apoptosis in B-cell lymphoma (Alas et al., 2002).
Beyond the extrinsic and intrinsic pathways, ROS modulate apoptosis via the ER stress pathway, which is mediated by cysteine-dependent proteases such as caspase-12. ER stress induced by ROS activates caspase-12 through unfolded protein response (UPR), triggering a caspase cascade that ultimately executes apoptotic cell death (Oppenheim et al., 2001).
3.2.2.2 ROS modulate oncogenesis through autophagy regulation
ROS exhibit dual roles in autophagy, either suppressing or promoting tumorigenesis based on stress intensity (Scherz-Shouval and Elazar, 2011). Autophagy (type II programmed cell death) acts as a cytoprotective mechanism under mild stress but triggers cell death under persistent damage (Debnath et al., 2023; He et al., 2009). Three canonical autophagy forms are recognized: macroautophagy, selective autophagy (e.g., mitophagy), and the microautophagy (Rake et al., 2022). Among its subtypes, selective autophagy (e.g., mitophagy) recruits receptors like p62/SQSTM1 to degrade DAMPs(damage-associated molecular patterns)-marked organelles (Kuchitsu and Taguchi, 2024; Wang et al., 2023). ROS enhance autophagosome accumulation by inhibiting ATG4B-mediated LC3-II delipidation (Perillo et al., 2020). ROS induce autosis through synergism with agents such as 2-mercaptoethanol (2-ME) (Chen et al., 2008). Mechanistically,ROS activate AMPK to suppress mTORC1 (43), thereby initiating ULK1/2-dependent autophagosome formation (Alexander et al., 2010; Poillet-Perez et al., 2015). For instance, H2O2 combined with the polycyclic ammonium ion sanguinarine amplifies mitochondrial ROS via NOX to induce glioma cell autophagic death (Li et al., 2012). Furthermore, co-targeting mTORC1 (e.g., rapamycin) and HSP90 exacerbates mitochondrial dysfunction, augmenting ROS-driven autophagy against RAS-driven tumors (De Raedt et al., 2011).
3.2.2.3 ROS modulate oncogenesis through ferroptosis regulation
Ferroptosis, an iron-dependent regulated cell death driven by ROS-induced lipid peroxidation (Dixon et al., 2012). This process selectively oxidizes membrane polyunsaturated fatty acids (PUFAs). Its progression depends on iron overload and glutathione (GSH) depletion (Jiang et al., 2015; Jiang et al., 2021; Tang et al., 2021). PUFA oxidation compromises membrane integrity through bilayer destabilization (Dixon and Stockwell, 2019). Cysteine/glutamate anti-transporter - system Xc- and GPX4 - glutathione peroxidase four constitute the major ferrocytic defence axis (Kuang et al., 2020; Liao et al., 2024; Liu K. et al., 2023; Wang et al., 2024).System Xc− imports cystine for GSH synthesis, while GPX4 detoxifies lipid peroxides. Pharmacological inhibitors (e.g., erastin, RSL3) targeting this axis induce ferroptosis (Jang et al., 2021; Kim et al., 2020).Parallel ferroptosis-suppressing pathways include:1)FSP1-CoQH2 axis: FSP1(ferroptosis suppressor protein 1)regenerates reduced coenzyme Q10 (CoQH2), mitigating lipid radical accumulation. 2)DHODH-CoQH2 system: Mitochondrial dihydroorotate dehydrogenase (DHODH) sustains CoQH2 pools independent of GPX4. 3)GCH1-BH4 pathway: GTP cyclohydrolase 1 (GCH1) synthesizes tetrahydrobiopterin (BH4), protecting phospholipids from peroxidation (Mao et al., 2021; Mishima et al., 2022; Soula et al., 2020).
Iron serves as a catalytic redox center in ferroptosis by driving lipid peroxidation (Galy et al., 2024). Labile iron pools amplify ROS via Fenton reactions and lipoxygenase (LOX) activation, sustaining a pro-ferroptotic cycle (Conrad and Pratt, 2020; Mishchenko et al., 2021; Yan et al., 2021). Ferroptosis-inducing agents (FIAs), such as erastin, selectively target tumors with iron dysregulation by inhibiting system Xc−, depleting GSH, and elevating mitochondrial ROS (Dolma et al., 2003; Yagoda et al., 2007). While ROS-mediated ferroptosis represents a promising anticancer strategy, emerging evidence cautions its context-dependent effects—e.g., ferroptosis induction combined with macrophage infiltration may paradoxically accelerate pancreatic cancer progression (Dai et al., 2020).
4 Anti-tumor mechanism of PPS through regulation of ROS
4.1 PPS-induced apoptosis via ROS modulation
Apoptosis, a tightly regulated form of programmed cell death, serves as an important mechanism for maintaining tissue stationary and eliminating malignant cells. PPS demonstrate potent pro-apoptotic activity across multiple cancer cell lineages, thereby validating their therapeutic potential as natural anticancer agents. PPS orchestrate apoptotic signaling via dual regulatory mechanisms: modulation of intracellular ROS homeostasis through both intrinsic (mitochondrial) and extrinsic (death receptor-mediated) pathways.
4.1.1 Mitochondrial activation pathway
The mitochondrial pathway is a primary mechanism through which PPS induce apoptosis. Polyphyllin II (PPII), a major bioactive component of PPS, activates the mitochondrial apoptotic pathway by elevating ROS levels, triggering cytochrome c discharge, and initiating caspase cascade initiation. For instance, PPII suppresses proliferation and induces apoptosis in human gastric cancer MGC-830 cells via caspase-3 activation, mediated by ROS-dependent cytochrome c upregulation. Experimental evidence indicates that polyphyllin VII (PPVII) treatment in bladder cancer cells markedly elevates intracellular and mitochondrial ROS levels, directly linking oxidative stress to mitochondrial apoptosis (Guo et al., 2018).
Increased ROS accumulation reduce mitochondrial membrane potential (ΔΨm), thereby promoting cytochrome c release from mitochondria into the cytosol. This cytosolic cytochrome c activates caspase-9, which subsequently cleaves and activates executioner caspase-3, culminating in apoptosis (Kshetrimayum et al., 2023). Furthermore, PPS activates ROS-generating signaling pathways, including the MAPK and PTEN/p53 axes. These pathways drive ROS accumulation beyond cellular antioxidant capacity, initiating apoptosis through oxidative stress overload. In a cellular model of human hepatocellular carcinoma (HepG2 cells), Zhang et al. systematically evaluated the anti-tumor effects of PPS. After PPVII treatment, JC-1 staining revealed a dose-dependent increase in green/red fluorescence ratio, indicating that PPVII increased ΔΨm collapse and mitochondrial permeability transition pore opening. Concurrently, DCFH-DA fluorescence intensity surged, confirming PPVII-induced ROS overproduction. Western blot analysis demonstrated significant phosphorylation enhancement of MAPK pathway components—JNK, ERK, and p38. These findings mechanistically link PPVII-induced mitochondrial dysfunction and ROS overgeneration to MAPK/PTEN-p53 pathway activation, establishing a cascading pro-apoptotic signaling network (Zhang et al., 2016).
4.1.2 Activation of death receptor pathways
PPS additionally modulate intracellular ROS to activate extrinsic apoptotic pathways in cancer cells. PPS upregulates death receptor expression (e.g., Fas/CD95, TNFR) on tumor cell surfaces, initiating extrinsic apoptosis (Hengartner, 2001). Liu and his teams demonstrated that Paris polyphylla saponin VI (PPVI) suppresses HepaRG cells viability through Fas-dependent apoptosis. Western blot analysis revealed PPVI-induced upregulation of Fas, caspases-3/8/9, and Poly (ADP-ribose) polymerase (PARP) cleavage, confirming activation of the extrinsic pathway. PPVI treatment elevated intracellular ROS levels, inducing oxidative stress that triggered Fas overexpression and caspase-8 activation. Caspase-8 subsequently activated executioner caspase-3, culminating in apoptosis.These findings indicate that PPVI triggers apoptosis in HepaRG cells via the Fas-dependent extrinsic pathway (Liu et al., 2018). Similarly, PPI dose and time-dependently elevated ROS levels, disrupted mitochondrial membrane potential (ΔΨm), and promoted cytochrome c release in HepG2 cells. Concurrently, PPI upregulated Fas, p53, and p21 expression, increased Bax/Bcl-2 ratios, and activated caspases-3/8/9, leading to PARP cleavage and apoptosis (Zeng et al., 2020). Lin et al. have provided additional evidence that resorcinolic acid saponins activate the extrinsic apoptotic pathway to induce cancer cell death in an A549 xenograft model. Treatment with saponin fractions PPVI and PPVII elevated intracellular ROS concentrations, thereby initiating oxidative stress and upregulating death receptor expression. Western blot analysis of both cultured cells and xenograft tumor tissues revealed that PPVI/PPVII treatment upregulated pro-apoptotic regulators (p53, p21WAF1/CIP1), death receptors (DR3, DR5, Fas), and apoptosis execution markers (cleaved PARP, caspase-3), while downregulating cell cycle promoters cyclin B1 and decoy receptor DcR3. These death receptors (DR3, DR5, Fas) initiate proteolytic cascades that activate executioner caspases (-3, -8, -9), mechanistically linking TRAIL- and Fas-mediated apoptosis pathways (Lin et al., 2015).Furthermore, Ke et al. validated the pro-apoptotic effects of PPS in tongue squamous cell carcinoma, demonstrating ROS-dependent activation of the caspase-8/caspase-3 axis as the principal mechanism. As the initiator caspase in extrinsic apoptosis, caspase-8 activation represents a critical regulatory node governing programmed cell death. Through formation of the death-inducing signaling complex (DISC), caspase-8 catalyzes proteolytic activation of downstream effectors, committing cells to apoptosis (Ke et al., 2016).
4.1.3 Endoplasmic reticulum stress and Other pathways
ER stress-induced apoptosis is a key mechanism by which PPS exert their effects on oxidative stress regulation. Previous studies have shown that polyphylla saponins induce apoptosis in lung cancer cells through modulation of ER stress, resulting in significantly increased intracellular oxidative stress and ROS accumulation. Researchers conducting cDNA microarray analysis on polyphylla saponin-treated lung cancer cells observed significant upregulation of multiple ER stress-associated genes. Western blot analysis confirmed the elevated expression of ER stress markers including BiP/GRP78, PDI, and HSP70, demonstrating ER stress activation and its association with apoptosis induction (Siu et al., 2008). In a seminal study, Tan’s team established the inhibitory effects of polyphylla saponin on nasopharyngeal carcinoma cells using in vitro models. Quantitative Western blot analysis revealed both enhanced PERK phosphorylation and upregulated expression of ER stress mediators including CHOP, BiP, PDI, ERO1α, and IRE1α, thereby confirming ER stress activation. Consistent with the established pro-apoptotic role of ER stress, morphological analysis revealed characteristic apoptotic features in CNE-2 cells post-treatment, including nuclear fragmentation, chromatin condensation, and cytoplasmic shrinkage. Flow cytometry with Annexin V-FITC/PI staining quantitatively demonstrated increased apoptotic cell populations, thereby providing multimodal evidence for ER stress-mediated apoptosis (Tan et al., 2019).
4.2 PPS promotes cancer cell autophagy by regulating ROS
Autophagy is a conserved intracellular degradation process that maintains cellular homeostasis through lysosomal recycling of superfluous or damaged cytoplasmic components (Miller and Thorburn, 2021). The dual role of autophagy in tumorigenesis—acting as both a pro-survival and tumor-suppressive mechanism—is well characterized. While autophagy may sustain cancer cell survival under metabolic stress by replenishing energy and nutrients, it can also trigger autophagic cell death or suppress tumor progression under specific contexts (Gozuacik and Kimchi, 2004). Emerging evidence highlights ROS as critical regulators of autophagy (Chen et al., 2009). ROS accumulation activates AMPK, a master energy sensor that induces autophagy under low energy conditions (e.g., ATP depletion), thereby restoring metabolic homeostasis in cancer cells (Carling, 2017).
Luo et al. demonstrated that PPI induces autophagy in colon cancer cells at specific therapeutic concentrations. This autophagic activity was evidenced by an elevated LC3-II/LC3-I ratio, a hallmark of autophagosome formation. Concurrently, PPI treatment in SW480 cells induced ROS accumulation and suppressed AKT phosphorylation, effects reversible by N-acetylcysteine (NAC) cotreatment. These findings indicate ROS-dependent AKT/mTORC1 pathway inhibition as the mechanistic basis for PPI-mediated autophagy induction (Luo et al., 2022).Similarly, PPVII activates autophagic flux in glioma models through LC3-II upregulation coupled with AKT inactivation and SQSTM1/p62 degradation (Pang et al., 2020). Notably, PPI exhibits dual pro-death effects in temozolomide-resistant gliomas, coordinately activating p38-JNK MAPK signaling to drive ROS-mediated apoptosis and autophagy (Feng et al., 2024).Yuan and his team further confirmed that PPVI triggers autophagy in U2OS osteosarcoma cells through ROS modulation. Mechanistically, PPVI elevates intracellular ROS via H2O2 generation, activating the JNK signaling pathway. This cascade promotes autophagy as evidenced by LC3-II accumulation, with JNK activation showing direct correlation to autophagic marker upregulation, confirming its pivotal role in PPVI-mediated autophagy (Yuan et al., 2019).Figure 6.
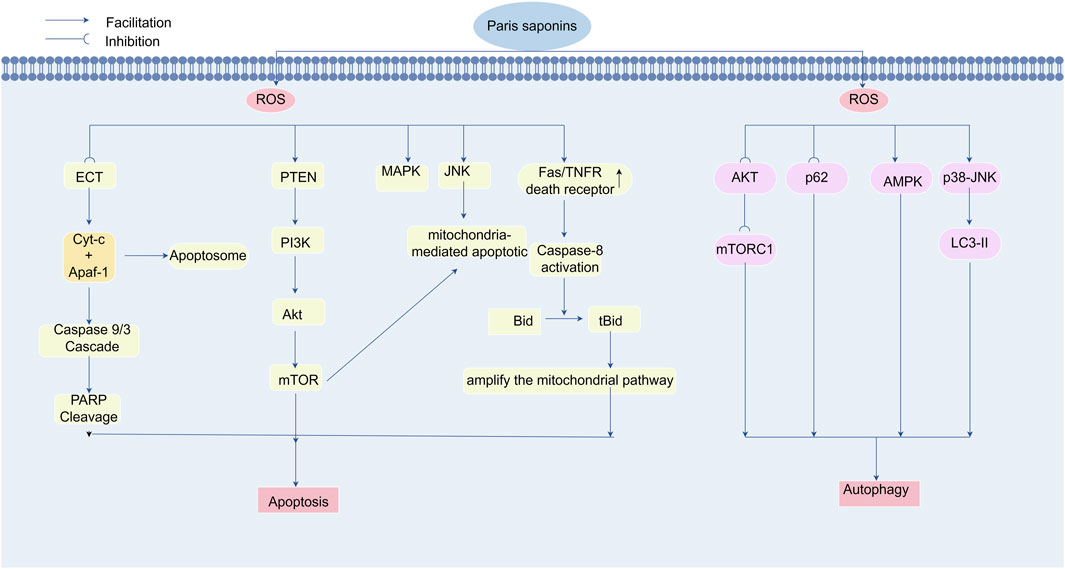
Figure 6. Polyphyllin triggers apoptosis and autophagy for cancer treatment by regulating reactive oxygen species levels. Paradisaponin increases intracellular ROS levels, triggering oxidative stress, which in turn activates endogenous and exogenous apoptosis pathways, leading to the death of cancer cells. It can elevate ROS levels in the mitochondria and cytoplasm, resulting in a decrease in mitochondrial membrane potential, the release of cytochrome c, and the subsequent activation of the Caspase cascade system, leading to PARP cleavage and DNA fragmentation, ultimately triggering apoptosis. Paradisaponin, by increasing ROS levels, inhibits the AKT/mTOR pathway, activates the AMPK and MAPK pathways to promote LC3-II expression, and induces autophagy in cancer cells.
4.3 ROS-mediated cell cycle arrest by PPS
Effective suppression of tumorigenesis and progression is contingent upon arresting cancer cell proliferation. Malignant cells evade growth-suppressive checkpoints through oncogenic reprogramming, acquiring limitless replicative potential. Such dysregulated proliferation imposes systemic pathophysiological stress and disrupts tissue homeostasis.The tumor suppressor p53 orchestrates cellular homeostasis through pleiotropic regulation of growth arrest, DNA repair, and apoptotic pathways. As a key p53 effector, p21 (CDKN1A) mediates cell cycle arrest by inhibiting CDK2-cyclin complexes and modulating PCNA functionality. p21 overexpression induces G1 phase arrest, effectively suppressing neoplastic growth across experimental models (Gartel et al., 1996). PPS exert antitumor effects through pharmacologically-induced cell cycle arrest (Tian et al., 2020).
Elevated ROS levels modulate cell cycle progression by targeting key regulatory proteins. ROS overproduction upregulates p21 expression and its downstream effector cyclin B1, a critical driver of G2/M phase transition (Nagesh et al., 2017). Yu et al. demonstrated that PPI induces concentration-dependent G2/M phase arrest, an effect reversible by NAC pretreatment. Notably, PPI selectively modulates cell cycle regulators: downregulating cyclin B1 while upregulating p21 in a concentration-dependent manner, without altering CDC2, p27, CDC25C, or GAPDH expression. These findings delineate a ROS-dependent mechanism underlying PPI-induced G2/M arrest (Yu et al., 2018). Complementary studies in HepaRG models revealed that PPII triggers ROS accumulation and mitochondrial depolarization, concomitant with S-phase arrest. Antioxidant pretreatment with NAC attenuates these effects, restoring membrane potential and reducing apoptotic commitment (Wang et al., 2019) Figure 7.
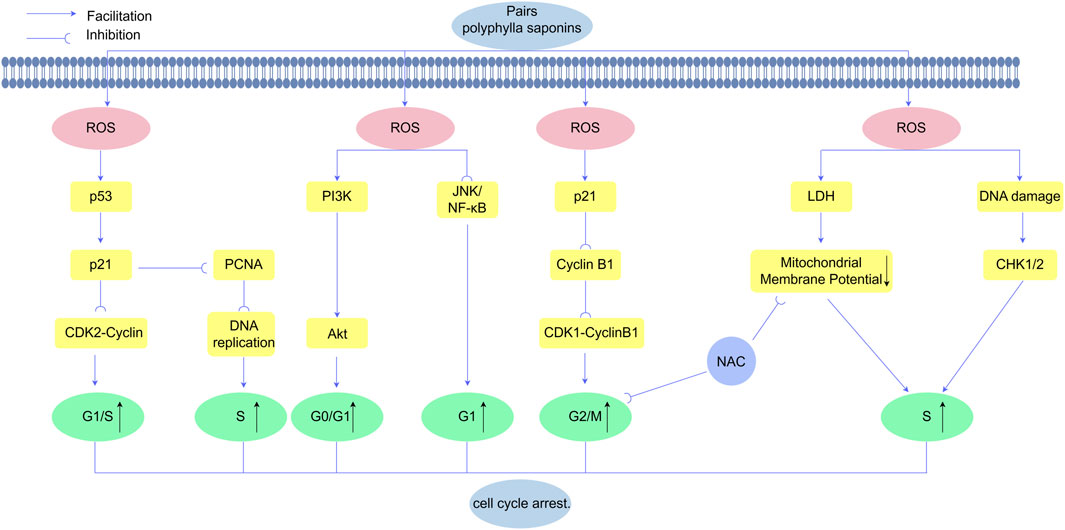
Figure 7. Polyphyllin exerts anticancer effects by inducing ROS-mediated cell cycle arrest in cancer cells. Polyphyllin increases ROS levels to activate the PI3K/Akt signaling cascade and JNK/NF-κB, leading to cell cycle arrest at the G0/G1 and G1 phases The upregulation of p53 promotes the expression of p21, which inhibits CDK2-cyclin complexes and proliferating cell nuclear antigen (PCNA), thereby blocking G1/S phase transition and inducing S phase arrest. Additionally, Polyphyllinsuppresses cyclin B1 expression, disrupting the CDK1-cyclin B1 complex and resulting in G2/M phase arrest.
4.4 PPS promote ferroptosis in cancer cells by modulating ROS
Ferroptosis is a regulated cell death mechanism that relies on iron and is marked by lipid peroxidation and excessive intracellular iron accumulation. Excessive iron accumulation and GSH depletion synergistically drive oxidative stress, resulting in lethal peroxidation of PUFAs. PPS induce ferroptosis in cancer cells through ROS-mediated mechanisms. For instance, PPI suppresses acute myeloid leukemia progression by activating the PI3K/SREBP-1/SCD1 axis to promote lipid oxidative damage and ferroptosis (Zhou et al., 2024).Yang and his team demonstrated that PPI induces ferroptosis in hepatocellular carcinoma (HCC) cells with elevating intracellular Fe2+ and ROS levels, depleting GSH, increasing malondialdehyde (MDA), and downregulating xCT and GPX4 expression. In a nude mouse xenograft model, PPI significantly suppressed tumor growth and inhibited the Nrf2/HO-1 antioxidant axis. This suppression reduced GPX4 activity, further amplifying ROS accumulation and iron overload, ultimately driving HCC cells into ferroptosis (Yang et al., 2024). Feng et al. demonstrated that PPI suppresses the Nrf2/HO-1 antioxidant pathway in glioblastoma, thereby elevating ROS levels and inducing ferroptosis. These results emphasize the therapeutic potential of PPI for drug-resistant gliomas (Feng et al., 2024). Furthermore, Zou et al. revealed that PPI modulates the ERK/DNMT1/ACSL4 axis in castration-resistant prostate cancer (CRPC) cells, driving ferroptosis through elevated MDA, Fe2+, and ROS, alongside reduced GSH and GPX4 levels—a hallmark of ferroptosis (Zou et al., 2024).
4.5 PPS in combination with drugs reverses resistance and improves therapeutic efficacy
Clinical applications of ROS-modulating monotherapies frequently demonstrate limited efficacy in tumor growth suppression. However, combinatorial regimens integrating these compounds with conventional chemotherapeutic agents not only potentiate natural product efficacy but also reverse chemoresistance via synergistic ROS-mediated mechanisms. Pang et al. demonstrated that PPVII modulates the Bcl-2/Bax apoptotic rheostat, thereby initiating mitochondrial pathway apoptosis. Mechanistically, PPVII administration significantly suppressed phospho-AKT/mTORC1 signaling axis activity. N-acetylcysteine (NAC) pretreatment abolished PPVII-mediated cytotoxicity, autophagic flux, and AKT/mTORC1 suppression, establishing ROS overproduction as the central mechanistic driver of these phenomena. The PPVII-TMZ combination exhibited marked synergism in glioma models, particularly in overcoming O6-methylguanine-DNA methyltransferase (MGMT)-mediated chemoresistance. This sensitization correlated with PPVII-induced epigenetic silencing of MGMT via promoter hypermethylation (Pang et al., 2019). Chen’s group elucidated that PPVII-paclitaxel coadministration provokes profound ROS burst, culminating in mitochondrial depolarization, ferroptosis induction, and complete reversal of prostate cancer chemoresistance (Chen et al., 2025). Zhang et al. pioneered the discovery that Polyphyllin D (PPD) dose- and time-dependently induces mitochondrial transmembrane potential (ΔΨm) collapse, H2O2 accumulation, and ROS-mediated apoptosis in chemoresistant HepG2 cells, thereby establishing PPD as a first-in-class mitochondrial disruptor capable of bypassing hepatocellular carcinoma chemoresistance (Cheung et al., 2005). Notably, PPS exhibits broad-spectrum chemosensitization potential, effectively reversing resistance to erlotinib (NSCLC), gefitinib (LUAD), and cisplatin (TNBC) while preventing acquired resistance in these malignancies (Lou et al., 2017; Zhang et al., 2024; Al Sawah et al., 2015). Collectively, these findings position PPS as a multimodal chemopotentiator that enhances conventional chemotherapy (paclitaxel, temozolomide, cisplatin) through three-pronged mechanisms: (1) ROS amplification, (2) p53-PUMA-Bax apoptotic axis activation, and (3) mitochondrial death pathway engagement.
4.6 Interaction of PPS with other signaling pathways by regulating ROS
Signaling pathways are pivotal in cellular function, serving as the primary mechanisms through which cells perceive external stimuli and coordinate physiological responses. These pathways orchestrate diverse cellular processes, including differentiation, survival, apoptosis, migration, invasion, metabolism, stress adaptation, and cell cycle progression. Through intricate crosstalk within a signaling network, these pathways collectively enable cellular adaptation to environmental perturbations while preserving homeostasis. Beyond the aforementioned pathways, PPS exhibit anti-tumor effects by modulating ROS to target additional signaling cascades (Zhao et al., 2021) Table.1.
5 Other bioactivities of PPS
The NF-κB signaling pathway is a central transcriptional regulator of inflammatory responses. Macrophages activate NF-κB through Toll-like receptors (TLRs), triggering the secretion of pro-inflammatory factors, including NO, prostaglandin E2 (PGE2), COX-2, MMPs, TNF-α, IL-1β, and IL-6. These mediators attract immune cells to sites of infectious sites or injured tissue (Mussbacher et al., 2019; Moncada, 1999). In canonical NF-κB activation, stimuli like lipopolysaccharide (LPS), interferon-γ (IFN-γ), and TNF-α induce IκB kinase (IKK) complex activation. IKK phosphorylates specific serine residues (e.g., Ser32/36) on IκBα, marking it for proteasomal degradation. IκBα degradation liberates NF-κB, enabling its nuclear translocation and subsequent transcription of pro-inflammatory genes (e.g., TNF-α, IL-1β, IL-6, iNOS) (Fearon et al., 2016).
PPS exert multi-faceted anti-inflammatory effects by targeting key nodes in this pathway.PPS exhibit dose-dependent anti-inflammatory activity by suppressing key pro-inflammatory mediators, including TNF-α (Zhu et al., 2019), IL-1β (Yang S. et al., 2021), NO, IL-1α, IL-6, and IL-8. In collagen-induced arthritis (CIA) murine models, PPI alleviates joint inflammation by inhibiting NF-κB-dependent inflammatory cytokine production in macrophages. These findings highlight the therapeutic potential of PPI for rheumatoid arthritis (RA) management (Wang et al., 2018). PPVII suppresses LPS-triggered macrophage activation primarily through NF-κB pathway inhibition. LPS stimulation induces IκB-α phosphorylation and degradation, enabling NF-κB p65 nuclear translocation and subsequent transcription of pro-inflammatory genes. PPVII attenuates this cascade by stabilizing IκB-α and blocking NF-κB p65 nuclear translocation, thereby reducing pro-inflammatory mediator synthesis. Furthermore, PPVII inhibits LPS-activated JNK, ERK, and p38 MAPK signaling, suggesting an additional mechanism to suppress IκB-α degradation (Zhang et al., 2019).
In addition to the above inflammation-related diseases, PPS can also play an anti-tumor role by regulating inflammatory factors, Chen et al. elucidated that PPII suppresses NF-κB activation via blockade of IKKβ/p65 nuclear translocation, thereby suppressing colorectal cancer progression (Chen et al., 2019). Studies have demonstrated PPI concentration-dependently enhances HepG2 cell chemosensitivity to cisplatin.Mechanistically, PPI dose-dependently attenuates basal phosphorylation of the NF-κB p65 subunit and downregulates downstream oncogenic targets (Bcl-2, c-Myc, VEGF), priming HepG2 cells for chemotherapy response (Han et al., 2015). In translational models, PPI potently suppressed CRPC growth and induced G1/S phase arrest in both xenograft models and primary cell cultures.PPI concomitantly reduced p65 phosphorylation, mucin 1 (MUC1) oncoprotein levels, and long non-coding RNA HOTAIR expression.These findings establish PPI as multimodal inhibitors that disrupt NF-κB/p65-MUC1 signaling crosstalk in stroma-rich prostate tumors (Xiang et al., 2018).
In summary, inflammation and cancer exhibit bidirectional crosstalk, forming a self-amplifying pathological cycle. Tumor cells perpetuate inflammation through pro-inflammatory cytokine secretion, whereas chronic inflammatory microenvironments promote oncogenesis, tumor progression, and therapeutic resistance. As a dual anti-inflammatory and anti-neoplastic agent, PPS suppress NF-κB signaling and attenuate inflammatory cytokine activity, thereby disrupting the inflammation-cancer axis and suppressing tumor progression.
6 Conclusion and outlook
In conclusion, preclinical studies of PPS in cellular and animal models demonstrate promising antitumor efficacy; however, clinical translation remains limited by critical challenges: 1) Low bioavailability: The poor aqueous solubility of PPS limits gastrointestinal absorption, resulting in suboptimal systemic exposure and heterogeneous tissue distribution. 2) Rapid hepatic metabolism: PPS undergoes extensive first-pass metabolism, generating inactive or potentially toxic metabolites that compromise therapeutic efficacy. 3) Drug-drug interactions: PPS may interfere with cytochrome P450 enzymes, altering the pharmacokinetics of co-administered therapeutics. 4) Dose-limiting toxicity: While PPS exhibits potent in vitro antitumor activity, in vivo studies report hepatotoxicity, nephrotoxicity, and myelosuppression at elevated doses, necessitating rigorous safety profiling. 5) Insufficient translational data: Gaps in pharmacokinetic-pharmacodynamic (PK/PD) modeling and early-phase clinical trials hinder rational dose optimization and risk-benefit assessment.
In future research, the following strategies should be prioritized to address these limitations: 1) Pharmacokinetic optimization: Advanced nano-delivery platforms (e.g., liposomes, exosomes) require systematic development to overcome the poor aqueous solubility and limited bioavailability of PPS. 2) Toxicity mitigation: Structural engineering approaches, such as site-specific glycosylation or prodrug design, should be investigated to alleviate dose-dependent hepatotoxicity while preserving bioactivity. 3) Clinical translation: Phase I/II clinical trials must be initiated to establish safety profiles, determine optimal dosing regimens, and validate therapeutic efficacy in human populations, building upon current preclinical evidence. 4) Combinatorial therapy exploration: Synergistic regimens integrating PPS with immune checkpoint inhibitors or targeted therapies could enhance therapeutic indices while minimizing systemic toxicity. 5) Biomarker-driven stratification: Identification of predictive biomarkers (e.g., Nrf2 expression, ROS levels) may enable personalized dosing and patient selection.
Author contributions
JLu: Conceptualization, Writing – review and editing, Visualization, Writing – original draft, Methodology. YM: Supervision, Formal analysis, Funding acquisition, Validation, Writing – original draft, Writing – review and editing.. KQ: Writing – review and editing, Formal Analysis. JyL: Formal Analysis, Writing – review and editing. YH: Writing – review and editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received financial support from the Inner Mongolia Autonomous Region Natural Science Foundation (No. 2024MS08045) and Inner Mongolia Autonomous Region Health Commission, 2023 Capital Region Public Hospital High-Level Clinical Specialty Construction Science and Technology Project (No. 2023SGGZ074). Inner Mongolia Health and Wellness Commission Project, the Key Science Foundation (No. 202203158). Additionally, it was supported by the Tumor Molecular Diagnosis Laboratory Precision Medicine Improvement Construction of Inner Mongolia Medical University (No. YKD2003XK011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, V., Tuli, H. S., Varol, A., Thakral, F., Yerer, M. B., Sak, K., et al. (2019). Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules 9 (11), 735. doi:10.3390/biom9110735
Alas, S., Ng, C. P., and Bonavida, B. (2002). Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-hodgkin's lymphoma. Clin. Cancer Res. 8 (3), 836–845.
Alexander, A., Cai, S. L., Kim, J., Nanez, A., Sahin, M., MacLean, K. H., et al. (2010). ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. U. S. A. 107 (9), 4153–4158. doi:10.1073/pnas.0913860107
Al Sawah, E., Marchion, D. C., Xiong, Y., Ramirez, I. J., Abbasi, F., Boac, B. M., et al. (2015). The Chinese herb polyphyllin D sensitizes ovarian cancer cells to cisplatin-induced growth arrest. J. Cancer Res. Clin. Oncol. 141 (2), 237–242. doi:10.1007/s00432-014-1797-x
Arbiser, J. L., Petros, J., Klafter, R., Govindajaran, B., McLaughlin, E. R., Brown, L. F., et al. (2002). Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. U. S. A. 99 (2), 715–720. doi:10.1073/pnas.022630199
Bedard, K., and Krause, K. H. (2007). The NOX family of ROS-Generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87 (1), 245–313. doi:10.1152/physrev.00044.2005
Bekeschus, S. (2023). Medical gas plasma technology: roadmap on cancer treatment and immunotherapy. Redox Biol. 65, 102798. doi:10.1016/j.redox.2023.102798
Bock, F. J., and Tait, S. W. G. (2020). Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21 (2), 85–100. doi:10.1038/s41580-019-0173-8
Brabletz, T. (2012). To differentiate or not-routes towards metastasis. Nat. Rev. Cancer 12 (6), 425–436. doi:10.1038/nrc3265
Cadenas, E. (2004). Mitochondrial free radical production and cell signaling. Mol. Aspects Med. 25 (1), 17–26. doi:10.1016/j.mam.2004.02.005
Carling, D. (2017). AMPK signalling in health and disease. Curr. Opin. Cell Biol. 45, 31–37. doi:10.1016/j.ceb.2017.01.005
Catrina, S.-B., and Zheng, X. (2021). Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 64 (4), 709–716. doi:10.1007/s00125-021-05380-z
Chatterjee, R., and Chatterjee, J. (2020). ROS and oncogenesis with special reference to EMT and stemness. Eur. J. Cell Biol. 99 (2), 151073. doi:10.1016/j.ejcb.2020.151073
Chen, M., Ye, K., Zhang, B., Xin, Q., Li, P., Kong, A. N., et al. (2019). Paris saponin II inhibits colorectal carcinogenesis by regulating mitochondrial fission and NF-κB pathway. Pharmacol. Res. 139, 273–285. doi:10.1016/j.phrs.2018.11.029
Chen, Y., Azad, M. B., and Gibson, S. B. (2009). Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 16 (7), 1040–1052. doi:10.1038/cdd.2009.49
Chen, Y., McMillan-Ward, E., Kong, J., Israels, S. J., and Gibson, S. B. (2008). Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 15 (1), 171–182. doi:10.1038/sj.cdd.4402233
Chen, Y. Y., Hua, W. X., Huang, Y. H., and Ding, X. (2025). Polyphyllin VII enhances the sensitivity of prostate cancer cells to docetaxel by promoting mitochondrial dysfunction and inducing ferroptosis. Chem. Biol. Drug Des. 105 (2), e70053. doi:10.1111/cbdd.70053
Cheung, E. C., and Vousden, K. H. (2022). The role of ROS in tumour development and progression. Nat. Rev. Cancer 22 (5), 280–297. doi:10.1038/s41568-021-00435-0
Cheung, J. Y., Ong, R. C., Suen, Y. K., Ooi, V., Wong, H. N., Mak, T. C., et al. (2005). Polyphyllin D is a potent apoptosis inducer in drug-resistant HepG2 cells. Cancer Lett. 217 (2), 203–211. doi:10.1016/j.canlet.2004.06.042
Conrad, M., and Pratt, D. A. (2020). Publisher correction: the chemical basis of ferroptosis. Nat. Chem. Biol. 16 (2), 223–224. doi:10.1038/s41589-019-0434-z
Cronin, K. A., Scott, S., Firth, A. U., Sung, H., Henley, S. J., Sherman, R. L., et al. (2022). Annual report to the nation on the status of cancer, part 1: national cancer statistics. Cancer 128 (24), 4251–4284. doi:10.1002/cncr.34479
Dai, E., Han, L., Liu, J., Xie, Y., Zeh, H. J., Kang, R., et al. (2020). Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 11 (1), 6339. doi:10.1038/s41467-020-20154-8
Debnath, J., Gammoh, N., and Ryan, K. M. (2023). Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 24 (8), 560–575. doi:10.1038/s41580-023-00585-z
De Raedt, T., Walton, Z., Yecies Jessica, L., Li, D., Chen, Y., Malone Clare, F., et al. (2011). Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell 20 (3), 400–413. doi:10.1016/j.ccr.2011.08.014
Dimitrov, D. S., and Marks, J. D. (2009). Therapeutic antibodies: current state and future trends-is a paradigm change coming soon? Methods Mol. Biol. 525, 1–27, xiii. doi:10.1007/978-1-59745-554-1_1
Ding, C. C., Rose, J., Sun, T., Wu, J., Chen, P. H., Lin, C. C., et al. (2020). MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat. Metab. 2 (3), 270–277. doi:10.1038/s42255-020-0181-1
Ding, Y. G., Zhao, Y. L., Zhang, J., Zuo, Z. T., Zhang, Q. Z., and Wang, Y. Z. (2021). The traditional uses, phytochemistry, and pharmacological properties of paris L. (liliaceae): a review. J. Ethnopharmacol. 278, 114293. doi:10.1016/j.jep.2021.114293
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., and Stockwell, B. R. (2019). “The hallmarks of ferroptosis,” in Annual review of cancer biology.
Dolma, S., Lessnick, S. L., Hahn, W. C., and Stockwell, B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3 (3), 285–296. doi:10.1016/s1535-6108(03)00050-3
Edmondson, D. E., and Binda, C. (2018). Monoamine oxidases. Subcell. Biochem. 87, 117–139. doi:10.1007/978-981-10-7757-9_5
Essers, M. A., Weijzen, S., de Vries-Smits, A. M., Saarloos, I., de Ruiter, N. D., Bos, J. L., et al. (2004). FOXO transcription factor activation by oxidative stress mediated by the small GTPase ral and JNK. Embo J. 23 (24), 4802–4812. doi:10.1038/sj.emboj.7600476
Fearon, U., Canavan, M., Biniecka, M., and Veale, D. J. (2016). Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 12 (7), 385–397. doi:10.1038/nrrheum.2016.69
Feng, F., Sun, C., Wang, X., Zhang, H., and Cheng, P. (2024). Polyphyllin I induces apoptosis and autophagy in temozolomide-resistant glioma via modulation of NRF2 and MAPK-Signaling activation. Biotechnol. Genet. Eng. Rev. 40 (3), 2409–2428. doi:10.1080/02648725.2023.2199553
Firlej, E., Kowalska, W., Szymaszek, K., Roliński, J., and Bartosińska, J. (2022). The role of skin immune system in acne. J. Clin. Med. 11 (6), 1579. doi:10.3390/jcm11061579
Forman, H. J., Ursini, F., and Maiorino, M. (2014). An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 73, 2–9. doi:10.1016/j.yjmcc.2014.01.018
Galy, B., Conrad, M., and Muckenthaler, M. (2024). Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25 (2), 133–155. doi:10.1038/s41580-023-00648-1
Gartel, A. L., Serfas, M. S., and Tyner, A. L. (1996). p21-negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 213 (2), 138–149. doi:10.3181/00379727-213-44046
Gerald, D., Berra, E., Frapart, Y. M., Chan, D. A., Giaccia, A. J., Mansuy, D., et al. (2004). JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118 (6), 781–794. doi:10.1016/j.cell.2004.08.025
Gozuacik, D., and Kimchi, A. (2004). Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23 (16), 2891–2906. doi:10.1038/sj.onc.1207521
Guan, X., Ruo-shi, L. I., Duan, B.-z., Wang, Y., Fan, M., Wang, S., et al. (2019). Advances in research on chemical constituents and pharmacological effects of Paris genus and prediction and analysis of quality markers. Chi. Herb. Med. 50 (19), 4838–4852. doi:10.7501/j.issn.0253-2670.2019.19.034
Guo, A., Li, K., and Xiao, Q. (2020). Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochem. Biophysical Res. Commun. 526 (4), 1069–1076. doi:10.1016/j.bbrc.2020.04.002
Guo, Y., Liu, Z., Li, K., Cao, G., Sun, C., Cheng, G., et al. (2018). Paris polyphylla-derived saponins inhibit growth of bladder cancer cells by inducing mutant P53 degradation while Up-Regulating CDKN1A expression. Curr. Urol. 11 (3), 131–138. doi:10.1159/000447207
Halliwell, B. (2022). Reactive oxygen species (ROS), oxygen radicals and antioxidants: where are we now, where is the field going and where should we go? Biochem. Biophysical Res. Commun. 633, 17–19. doi:10.1016/j.bbrc.2022.08.098
Han, W., Hou, G., Liu, L., and Polyphyllin, I. (2015). Polyphyllin I (PPI) increased the sensitivity of hepatocellular carcinoma HepG2 cells to chemotherapy. Int. J. Clin. Exp. Med. 8 (11), 20664–20669.
Haynes, C. M., Titus, E. A., and Cooper, A. A. (2004). Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15 (5), 767–776. doi:10.1016/j.molcel.2004.08.025
He, C., and Klionsky, D. J. (2009). Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93. doi:10.1146/annurev-genet-102808-114910
He, J., Yu, S., Guo, C., Tan, L., Song, X., Wang, M., et al. (2019). Polyphyllin I induces autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal and downregulating cyclin B1 in human gastric carcinoma HGC-27 cells. Biomed. Pharmacother. 117, 109189. doi:10.1016/j.biopha.2019.109189
Hengartner, M. O. (2000). The biochemistry of apoptosis. Nature 407 (6805), 770–776. doi:10.1038/35037710
Hengartner, M. O. (2001). Apoptosis: corralling the corpses. Cell 104 (3), 325–328. doi:10.1016/s0092-8674(01)00219-7
Hu, M., Zhu, S., Xiong, S., Xue, X., and Zhou, X. (2019). MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (review). Oncol. Rep. 41 (3), 1439–1454. doi:10.3892/or.2019.6962
Huang, Z., Ding, Y., Luo, Y., Chen, M., Zeng, Z., Zhang, T., et al. (2022). ROS-Triggered cycle amplification effect: a prodrug activation nanoamplifier for tumor-specific therapy. Acta Biomater. 152, 367–379. doi:10.1016/j.actbio.2022.08.072
Humpton, T. J., Hall, H., Kiourtis, C., Nixon, C., Clark, W., Hedley, A., et al. (2022). p53-mediated redox control promotes liver regeneration and maintains liver function in response to CCl4. Cell Death and Differ. 29 (3), 514–526. doi:10.1038/s41418-021-00871-3
Ismail, T., Kim, Y., Lee, H., Lee, D.-S., and Lee, H.-S. (2019). Interplay between mitochondrial peroxiredoxins and ROS in cancer development and progression. Int. J. Mol. Sci. 20 (18), 4407. doi:10.3390/ijms20184407
Jang, S., Chapa-Dubocq, X. R., Tyurina, Y. Y., St Croix, C. M., Kapralov, A. A., Tyurin, V. A., et al. (2021). Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 45, 102021. doi:10.1016/j.redox.2021.102021
Jiang, L., Kon, N., Li, T., Wang, S. J., Su, T., Hibshoosh, H., et al. (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520 (7545), 57–62. doi:10.1038/nature14344
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22 (4), 266–282. doi:10.1038/s41580-020-00324-8
Kagan, V. E., Tyurin, V. A., Jiang, J., Tyurina, Y. Y., Ritov, V. B., Amoscato, A. A., et al. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1 (4), 223–232. doi:10.1038/nchembio727
Kalkavan, H., Chen, M. J., Crawford, J. C., Quarato, G., Fitzgerald, P., Tait, S. W. G., et al. (2022). Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 185 (18), 3356–74.e22. doi:10.1016/j.cell.2022.07.025
Ke, J. Y., Zhang, W., Gong, R. S., Cen, W. J., Huang, H. Q., Li, Y. R., et al. (2016). A monomer purified from paris polyphylla (PP-22) triggers S and G2/M phase arrest and apoptosis in human tongue squamous cell carcinoma SCC-15 by activating the p38/cdc25/cdc2 and caspase 8/caspase 3 pathways. Tumour Biol. 37 (11), 14863–14872. doi:10.1007/s13277-016-5376-4
Khavari, T. A., and Rinn, J. L. (2007). Ras/erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle 6 (23), 2928–2931. doi:10.4161/cc.6.23.4998
Kim, D. H., Kim, W. D., Kim, S. K., Moon, D. H., and Lee, S. J. (2020). TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death and Dis. 11 (5), 406. doi:10.1038/s41419-020-2618-6
Kong, M., Fan, J., Dong, A., Cheng, H., and Xu, R. (2010). Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. Acta Biochim. Biophys. Sin. (Shanghai) 42 (11), 827–833. doi:10.1093/abbs/gmq091
Kshetrimayum, V., Heisnam, R., Keithellakpam, O. S., Radhakrishnanand, P., Akula, S. J., Mukherjee, P. K., et al. (2023). Paris polyphylla sm. Induces reactive oxygen species and caspase 3-Mediated apoptosis in colorectal cancer cells in vitro and potentiates the therapeutic significance of fluorouracil and cisplatin. Plants (Basel) 12 (7), 1446. doi:10.3390/plants12071446
Kuang, F., Liu, J., Tang, D., and Kang, R. (2020). Oxidative damage and antioxidant defense in ferroptosis. Front. Cell Dev. Biol. 8, 586578. doi:10.3389/fcell.2020.586578
Kuchitsu, Y., and Taguchi, T. (2024). Lysosomal microautophagy: an emerging dimension in Mammalian autophagy. Trends Cell Biol. 34 (7), 606–616. doi:10.1016/j.tcb.2023.11.005
Lambert, A. W., and Weinberg, R. A. (2021). Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 21 (5), 325–338. doi:10.1038/s41568-021-00332-6
Lepcha, D., Chhetri, A., and Chhetri, D. (2019). Antioxidant and cytotoxic attributes of Paris polyphylla smith from Sikkim himalaya. Pharmacogn. J. 11, 705–711. doi:10.5530/pj.2019.11.112
Li, D., Ueta, E., Kimura, T., Yamamoto, T., and Osaki, T. (2004). Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 95 (8), 644–650. doi:10.1111/j.1349-7006.2004.tb03323.x
Li, L., Ishdorj, G., and Gibson, S. B. (2012). Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic. Biol. Med. 53 (7), 1399–1410. doi:10.1016/j.freeradbiomed.2012.07.011
Li, Y.-M., Guan, L.-J., Chen, L.-M., Zhao, M., Ding, L.-S., Meng, C.-X.-N., et al. (2021). Qualitative and quantitative analysis of Paris polyphylla Var. chinensis by UPLC-Q-TOF-MS/MS and HPLC. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia medica 46, 2900–2911. doi:10.19540/j.cnki.cjcmm.20210205.301
Liao, Z., Tan, Z. W., Zhu, P., and Tan, N. S. (2019). Cancer-associated fibroblasts in tumor microenvironment - accomplices in tumor malignancy. Cell Immunol. 343, 103729. doi:10.1016/j.cellimm.2017.12.003
Liao, Z., Wen, E., and Feng, Y. (2024). GSH-Responsive degradable nanodrug for glucose metabolism intervention and induction of ferroptosis to enhance magnetothermal anti-tumor therapy. J. Nanobiotechnology 22 (1), 147. doi:10.1186/s12951-024-02425-4
Lin, Z., Liu, Y., Li, F., Wu, J., Zhang, G., Wang, Y., et al. (2015). Anti-lung cancer effects of polyphyllin VI and VII potentially correlate with apoptosis in vitro and in vivo. Phytother. Res. 29 (10), 1568–1576. doi:10.1002/ptr.5430
Lingappan, K. (2018). NF-κB in oxidative stress. Curr. Opin. Toxicol. 7, 81–86. doi:10.1016/j.cotox.2017.11.002
Liou, G.-Y., and Storz, P. (2010). Reactive oxygen species in cancer. Free Radic. Res. 44 (5), 479–496. doi:10.3109/10715761003667554
Liu, F., He, T., Gong, S., Shen, M., Ma, S., Huang, X., et al. (2022). A tumor pH-responsive autocatalytic nanoreactor as a H2O2 and O2 self-supplying depot for enhanced ROS-Based chemo/photodynamic therapy. Acta Biomater. 154, 510–522. doi:10.1016/j.actbio.2022.10.002
Liu, H., Nishitoh, H., Ichijo, H., and Kyriakis, J. M. (2000). Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell Biol. 20 (6), 2198–2208. doi:10.1128/MCB.20.6.2198-2208.2000
Liu, K., Huang, L., Qi, S., Liu, S., Xie, W., Du, L., et al. (2023b). Ferroptosis: the entanglement between traditional drugs and nanodrugs in tumor therapy. Adv. Healthc. Mater 12 (12), e2203085. doi:10.1002/adhm.202203085
Liu, X., Zhao, P., Wang, X., Wang, L., Zhu, Y., Song, Y., et al. (2019). Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. and Clin. Cancer Res. 38 (1), 184. doi:10.1186/s13046-019-1173-4
Liu, Y., Dong, X., Wang, W., You, L., Yin, X., Yang, C., et al. (2018). Molecular mechanisms of apoptosis in HepaRG cell line induced by polyphyllin VI via the fas death pathway and mitochondrial-dependent pathway. Toxins (Basel). 10 (5), 201. doi:10.3390/toxins10050201
Liu, Y., Liu, M.-Y., Bi, L.-L., Tian, Y.-Y., Qiu, P.-C., Qian, X.-Y., et al. (2023a). Cytotoxic steroidal glycosides from the rhizomes of Paris polyphylla Var. yunnanensis. Phytochemistry 207, 113577. doi:10.1016/j.phytochem.2022.113577
Loke, Y. L., Beishenaliev, A., Wang, P.-W., Lin, C.-Y., Chang, C.-Y., Foo, Y. Y., et al. (2023). ROS-Generating alginate-coated gold nanorods as biocompatible nanosonosensitisers for effective sonodynamic therapy of cancer. Ultrason. Sonochemistry 96, 106437. doi:10.1016/j.ultsonch.2023.106437
Lou, W., Chen, Y., Zhu, K. Y., Deng, H., Wu, T., and Wang, J. (2017). Polyphyllin I overcomes EMT-associated resistance to erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition. Biol. Pharm. Bull. 40 (8), 1306–1313. doi:10.1248/bpb.b17-00271
Luo, Q., Jia, L., Huang, C., Qi, Q., Jahangir, A., Xia, Y., et al. (2022). Polyphyllin I promotes autophagic cell death and apoptosis of Colon cancer cells via the ROS-inhibited AKT/mTOR pathway. Int. J. Mol. Sci. 23 (16), 9368. doi:10.3390/ijms23169368
Madesh, M., and Hajnóczky, G. (2001). VDAC-Dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 155 (6), 1003–1015. doi:10.1083/jcb.200105057
Mao, C., Liu, X., Zhang, Y., Lei, G., Yan, Y., Lee, H., et al. (2021). DHODH-Mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593 (7860), 586–590. doi:10.1038/s41586-021-03539-7
Medina, C. B., Mehrotra, P., Arandjelovic, S., Perry, J. S. A., Guo, Y., Morioka, S., et al. (2020). Metabolites released from apoptotic cells act as tissue messengers. Nature 580 (7801), 130–135. doi:10.1038/s41586-020-2121-3
Miller, D. R., and Thorburn, A. (2021). Autophagy and organelle homeostasis in cancer. Dev. Cell 56 (7), 906–918. doi:10.1016/j.devcel.2021.02.010
Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R., Jemal, A., et al. (2022). Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72 (5), 409–436. doi:10.3322/caac.21731
Mishchenko, T. A., Balalaeva, I. V., Vedunova, M. V., and Krysko, D. V. (2021). Ferroptosis and photodynamic therapy synergism: enhancing anticancer treatment. Trends Cancer 7 (6), 484–487. doi:10.1016/j.trecan.2021.01.013
Mishima, E., Ito, J., Wu, Z., Nakamura, T., Wahida, A., Doll, S., et al. (2022). A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608 (7924), 778–783. doi:10.1038/s41586-022-05022-3
Moloney, J. N., and Cotter, T. G. (2018a). ROS signalling in the biology of cancer. Seminars Cell and Dev. Biol. 80, 50–64. doi:10.1016/j.semcdb.2017.5.023
Moloney, J. N., and Cotter, T. G. (2018b). ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 80, 50–64. doi:10.1016/j.semcdb.2017.05.023
Moncada, S. (1999). Nitric oxide: discovery and impact on clinical medicine. J. R. Soc. Med. 92 (4), 164–169. doi:10.1177/014107689909200402
Mori, K., Uchida, T., Yoshie, T., Mizote, Y., Ishikawa, F., Katsuyama, M., et al. (2019). A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-Activated cancer cells. Febs J. 286 (3), 459–478. doi:10.1111/febs.14671
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417 (1), 1–13. doi:10.1042/BJ20081386
Murthy, S. S., Trapani, D., Cao, B., Bray, F., Murthy, S., Kingham, T. P., et al. (2024). Premature mortality trends in 183 countries by cancer type, sex, WHO region, and world bank income level in 2000-19: a retrospective, cross-sectional, population-based study. Lancet Oncol. 25 (8), 969–978. doi:10.1016/S1470-2045(24)00274-2
Mussbacher, M., Salzmann, M., Brostjan, C., Hoesel, B., Schoergenhofer, C., Datler, H., et al. (2019). Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 10, 85. doi:10.3389/fimmu.2019.00085
Nagesh, R., Kiran Kumar, K. M., Naveen Kumar, M., Patil, R. H., Kavya, K., Babu, R. L., et al. (2017). Aqueous areca nut extract induces oxidative stress in human lung epithelial A549 cells: probable role of p21 in inducing cell death. Gene Rep. 6, 103–111. doi:10.1016/j.genrep.2016.12.008
Nelson, K. K., and Melendez, J. A. (2004). Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 37 (6), 768–784. doi:10.1016/j.freeradbiomed.2004.06.008
Okoye, C. N., Koren, S. A., and Wojtovich, A. P. (2023). Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 67, 102926. doi:10.1016/j.redox.2023.102926
Oppenheim, R. W., Flavell, R. A., Vinsant, S., Prevette, D., Kuan, C. Y., and Rakic, P. (2001). Programmed cell death of developing Mammalian neurons after genetic deletion of caspases. J. Neurosci. 21 (13), 4752–4760. doi:10.1523/JNEUROSCI.21-13-04752.2001
Pak, V. V., Ezeriņa, D., Lyublinskaya, O. G., Pedre, B., Tyurin-Kuzmin, P. A., Mishina, N. M., et al. (2020). Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 31 (3), 642–53.e6. doi:10.1016/j.cmet.2020.02.003
Pan, L., Chou, J. J., and Fu, T. (2022). Editorial: targeting TNF/TNFR signaling pathways. Front. Pharmacol. 13, 1120954. doi:10.3389/fphar.2022.1120954
Pang, D., Li, C., Yang, C., Zou, Y., Feng, B., Li, L., et al. (2019). Polyphyllin VII promotes apoptosis and autophagic cell death via ROS-inhibited AKT activity, and sensitizes glioma cells to temozolomide. Oxid. Med. Cell Longev. 2019, 1805635. doi:10.1155/2019/1805635
Pang, D., Yang, C., Li, C., Zou, Y., Feng, B., Li, L., et al. (2020). Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biol. Open 9 (2), bio046854. doi:10.1242/bio.046854
Pastushenko, I., Mauri, F., Song, Y., de Cock, F., Meeusen, B., Swedlund, B., et al. (2021). Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589 (7842), 448–455. doi:10.1038/s41586-020-03046-1
Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., et al. (2020). ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 52 (2), 192–203. doi:10.1038/s12276-020-0384-2
Poillet-Perez, L., Despouy, G., Delage-Mourroux, R., and Boyer-Guittaut, M. (2015). Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 4, 184–192. doi:10.1016/j.redox.2014.12.003
Qin, X.-J., Ni, W., Chen, C.-X., and Liu, H.-Y. (2018). Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla Var. yunnanensis. J. Ethnopharmacol. 224, 134–139. doi:10.1016/j.jep.2018.05.028
Qin, X.-J., Sun, D.-J., Ni, W., Chen, C.-X., Hua, Y., He, L., et al. (2012). Steroidal saponins with antimicrobial activity from stems and leaves of Paris polyphylla Var. yunnanensis. Steroids 77 (12), 1242–1248. doi:10.1016/j.steroids.2012.07.007
Rakesh, R., PriyaDharshini, L. C., Sakthivel, K. M., and Rasmi, R. R. (2022). Role and regulation of autophagy in cancer. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1868 (7), 166400. doi:10.1016/j.bbadis.2022.166400
Scherz-Shouval, R., and Elazar, Z. (2011). Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36 (1), 30–38. doi:10.1016/j.tibs.2010.07.007
Schild, T., McReynolds, M. R., Shea, C., Low, V., Schaffer, B. E., Asara, J. M., et al. (2021). NADK is activated by oncogenic signaling to sustain pancreatic ductal adenocarcinoma. Cell Rep. 35 (11), 109238. doi:10.1016/j.celrep.2021.109238
Siu, F. M., Ma, D. L., Cheung, Y. W., Lok, C. N., Yan, K., Yang, Z., et al. (2008). Proteomic and transcriptomic study on the action of a cytotoxic saponin (polyphyllin D): induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics 8 (15), 3105–3117. doi:10.1002/pmic.200700829
Son, Y., Cheong, Y. K., Kim, N. H., Chung, H. T., Kang, D. G., and Pae, H. O. (2011). Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct. 2011, 792639. doi:10.1155/2011/792639
Soula, M., Weber, R. A., Zilka, O., Alwaseem, H., La, K., Yen, F., et al. (2020). Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16 (12), 1351–1360. doi:10.1038/s41589-020-0613-y
Sparg, S. G., Light, M. E., and van Staden, J. (2004). Biological activities and distribution of plant saponins. J. Ethnopharmacol. 94 (2), 219–243. doi:10.1016/j.jep.2004.05.016
Stennicke, H. R., Deveraux, Q. L., Humke, E. W., Reed, J. C., Dixit, V. M., and Salvesen, G. S. (1999). Caspase-9 can be activated without proteolytic processing. J. Biol. Chem. 274 (13), 8359–8362. doi:10.1074/jbc.274.13.8359
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Szatrowski, T. P., and Nathan, C. F. (1991). Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51 (3), 794–798.
Takac, I., Schröder, K., Zhang, L., Lardy, B., Anilkumar, N., Lambeth, J. D., et al. (2011). The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 286 (15), 13304–13313. doi:10.1074/jbc.M110.192138
Tan, G. X., Wang, X. N., Tang, Y. Y., Cen, W. J., Li, Z. H., Wang, G. C., et al. (2019). PP-22 promotes autophagy and apoptosis in the nasopharyngeal carcinoma cell line CNE-2 by inducing endoplasmic reticulum stress, downregulating STAT3 signaling, and modulating the MAPK pathway. J. Cell Physiol. 234 (3), 2618–2630. doi:10.1002/jcp.27076
Tang, D., Chen, X., Kang, R., and Kroemer, G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31 (2), 107–125. doi:10.1038/s41422-020-00441-1
Teng, W. J., Chen, P., Zhu, F. Y., Di, K., Zhou, C., Zhuang, J., et al. (2015). Effect of rhizoma paridis total saponins on apoptosis of colorectal cancer cells and imbalance of the JAK/STAT3 molecular pathway induced by IL-6 suppression. Genet. Mol. Res. 14 (2), 5793–5803. doi:10.4238/2015.May.29.11
ten Kate, M., van der Wal, J. B. C., Sluiter, W., Hofland, L. J., Jeekel, J., Sonneveld, P., et al. (2006). The role of superoxide anions in the development of distant tumour recurrence. Br. J. Cancer 95 (11), 1497–1503. doi:10.1038/sj.bjc.6603436
Tian, Y., Gong, G.-Y., Ma, L.-L., Wang, Z.-Q., Song, D., and Fang, M.-Y. (2020). Anti-cancer effects of polyphyllin I: an update in 5 years. Chemico-Biological Interact. 316, 108936. doi:10.1016/j.cbi.2019.108936
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global cancer incidence and mortality rates and Trends-An update. Cancer Epidemiol. Biomarkers Prev. 25 (1), 16–27. doi:10.1158/1055-9965.EPI-15-0578
Tu, B. P., and Weissman, J. S. (2002). The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 10 (5), 983–994. doi:10.1016/s1097-2765(02)00696-2
Wang, H. H., McIntosh, A. R., Hasinoff, B. B., Rector, E. S., Ahmed, N., Nance, D. M., et al. (2000). B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 60 (20), 5862–5869.
Wang, L., Azad, N., Kongkaneramit, L., Chen, F., Lu, Y., Jiang, B. H., et al. (2008). The fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J. Immunol. 180 (5), 3072–3080. doi:10.4049/jimmunol.180.5.3072
Wang, L., Klionsky, D. J., and Shen, H.-M. (2023). The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 24 (3), 186–203. doi:10.1038/s41580-022-00529-z
Wang, Q., Zhou, X., Zhao, Y., Xiao, J., Lu, Y., Shi, Q., et al. (2018). Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front. Immunol. 9, 2091. doi:10.3389/fimmu.2018.02091
Wang, R., Song, W., Zhu, J., Shao, X., Yang, C., Xiong, W., et al. (2024). Biomimetic nano-chelate diethyldithiocarbamate Cu/Fe for enhanced metalloimmunity and ferroptosis activation in glioma therapy. J. Control. Release 368, 84–96. doi:10.1016/j.jconrel.2024.02.004
Wang, W., Dong, X., You, L., Sai, N., Leng, X., Yang, C., et al. (2019). Apoptosis in HepaRG and HL-7702 cells inducted by polyphyllin II through caspases activation and cell-cycle arrest. J. Cell Physiol. 234 (5), 7078–7089. doi:10.1002/jcp.27462
Wang, Y., Qi, H., Liu, Y., Duan, C., Liu, X., Xia, T., et al. (2021). The double-edged roles of ROS in cancer prevention and therapy. Theranostics 11 (10), 4839–4857. doi:10.7150/thno.56747
Wei, J. C., Gao, W. Y., Yan, X. D., Wang, Y., Jing, S. S., and Xiao, P. G. (2014). Chemical constituents of plants from the genus paris. Chem. Biodivers. 11 (9), 1277–1297. doi:10.1002/cbdv.201300083
Wilkie-Grantham, R. P., Matsuzawa, S., and Reed, J. C. (2013). Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. J. Biol. Chem. 288 (18), 12777–12790. doi:10.1074/jbc.M112.431320
Wu, J. B., Shao, C., Li, X., Li, Q., Hu, P., Shi, C., et al. (2014). Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J. Clin. Invest 124 (7), 2891–2908. doi:10.1172/JCI70982
Xiang, S., Zou, P., Wu, J., Zheng, F., Tang, Q., Zhou, J., et al. (2018). Crosstalk of NF-κB/P65 and LncRNA HOTAIR-mediated repression of MUC1 expression contribute to synergistic inhibition of castration-resistant prostate cancer by polyphyllin 1-Enzalutamide combination treatment. Cell Physiol. Biochem. 47 (2), 759–773. doi:10.1159/000490028
Xiang, Y., Guo, Z., Zhu, P., Chen, J., and Huang, Y. (2019). Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 8 (5), 1958–1975. doi:10.1002/cam4.2108
Yagoda, N., von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447 (7146), 864–868. doi:10.1038/nature05859
Yan, B., Ai, Y., Sun, Q., Ma, Y., Cao, Y., Wang, J., et al. (2021). Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell 81 (2), 355–69.e10. doi:10.1016/j.molcel.2020.11.024
Yang, L., Shou, Y. H., Yang, Y. S., and Xu, J. H. (2021a). Elucidating the immune infiltration in acne and its comparison with rosacea by integrated bioinformatics analysis. PLoS One 16 (3), e0248650. doi:10.1371/journal.pone.0248650
Yang, R., Gao, W., Wang, Z., Jian, H., Peng, L., Yu, X., et al. (2024). Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine 122, 155135. doi:10.1016/j.phymed.2023.155135
Yang, S., Jiang, Y., Yu, X., Zhu, L., Wang, L., Mao, J., et al. (2021b). Polyphyllin I inhibits propionibacterium acnes-induced IL-8 secretion in HaCaT cells by downregulating the CD36/NOX1/ROS/NLRP3/IL-1β pathway. Evid. Based Complement. Altern. Med. 2021, 1821220. doi:10.1155/2021/1821220
Yu, S., Wang, L., Cao, Z., Gong, D., Liang, Q., Chen, H., et al. (2018). Anticancer effect of polyphyllin Ι in colorectal cancer cells through ROS-Dependent autophagy and G2/M arrest mechanisms. Nat. Prod. Res. 32 (12), 1489–1492. doi:10.1080/14786419.2017.1353512
Yuan, Y. L., Jiang, N., Li, Z. Y., Song, Z. Z., Yang, Z. H., Xue, W. H., et al. (2019). Polyphyllin VI induces apoptosis and autophagy in human osteosarcoma cells by modulation of ROS/JNK activation. Drug Des. Devel Ther. 13, 3091–3103. doi:10.2147/DDDT.S194961
Zeng, Y., Zhang, Z., Wang, W., You, L., Dong, X., Yin, X., et al. (2020). Underlying mechanisms of apoptosis in HepG2 cells induced by polyphyllin I through fas death and mitochondrial pathways. Toxicol. Mech. Methods 30 (6), 397–406. doi:10.1080/15376516.2020.1747125
Zhang, C., Jia, X., Bao, J., Chen, S., Wang, K., Zhang, Y., et al. (2016). Polyphyllin VII induces apoptosis in HepG2 cells through ROS-Mediated mitochondrial dysfunction and MAPK pathways. BMC Complement. Altern. Med. 16, 58. doi:10.1186/s12906-016-1036-x
Zhang, C., Li, C., Jia, X., Wang, K., Tu, Y., Wang, R., et al. (2019). In vitro and in vivo anti-inflammatory effects of polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules 24 (5), 875. doi:10.3390/molecules24050875
Zhang, D., Tian, X., Wang, Y., Liu, F., Zhang, J., Wang, H., et al. (2024). Polyphyllin I ameliorates gefitinib resistance and inhibits the VEGF/VEGFR2/p38 pathway by targeting HIF-1a in lung adenocarcinoma. Phytomedicine 129, 155690. doi:10.1016/j.phymed.2024.155690
Zhang, X., Qiu, H., Li, C., Cai, P., and Qi, F. (2021). The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 15 (5), 283–298. doi:10.5582/bst.2021.01318
Zhao, L., Liu, Z., Deng, X., Wang, J., Sun, L., Fan, L., et al. (2021). Polyphyllin VII induces mitochondrial apoptosis by regulating the PP2A/AKT/DRP1 signaling axis in human ovarian cancer. Oncol. Rep. 45 (2), 513–522. doi:10.3892/or.2020.7879
Zhao, Y., Xiong, W., Li, C., Zhao, R., Lu, H., Song, S., et al. (2023). Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 8 (1), 431. doi:10.1038/s41392-023-01652-9
Zhou, N., Xu, L., Park, S.-M., Ma, M.-G., Choi, S.-E., and Si, C. (2021). Genetic diversity, chemical components, and property of biomass Paris polyphylla Var. yunnanensis. Front. Bioeng. Biotechnol. 9, 713860. doi:10.3389/fbioe.2021.713860
Zhou, X., Zhang, D., Lei, J., Ren, J., Yang, B., Cao, Z., et al. (2024). Polyphyllin I induces rapid ferroptosis in acute myeloid leukemia through simultaneous targeting PI3K/SREBP-1/SCD1 axis and triggering of lipid peroxidation. J. Nat. Med. 78 (3), 618–632. doi:10.1007/s11418-024-01811-4
Zhu, T., Wu, W., Yang, S., Li, D., Sun, D., and He, L. (2019). Polyphyllin I inhibits propionibacterium acnes-induced inflammation in vitro. Inflammation 42 (1), 35–44. doi:10.1007/s10753-018-0870-z
Zou, P., Chen, Z., He, Q., and Zhuo, Y. (2024). Polyphyllin I induces ferroptosis in castration-resistant prostate cancer cells through the ERK/DNMT1/ACSL4 axis. Prostate 84 (1), 64–73. doi:10.1002/pros.24626
Keywords: Paris polyphylla, reactive oxygen species, anti-cancer, mechanism, saponins
Citation: Liu J, Mu Y, Qi K, Li J and Hu Y (2025) Regulation of anti-tumour effects of Paris polyphylla saponins via ROS: molecular mechanisms and therapeutic potentials. Front. Pharmacol. 16:1611911. doi: 10.3389/fphar.2025.1611911
Received: 15 April 2025; Accepted: 16 June 2025;
Published: 02 July 2025.
Edited by:
Huanling Lai, Guangzhou National Laboratory, ChinaReviewed by:
Wenrui Xia, Chengdu University of Traditional Chinese Medicine, ChinaWenjun Wang, Hong Kong University of Science and Technology, Hong Kong SAR, China
Copyright © 2025 Liu, Mu, Qi, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongping Mu, eXBtdTA0MEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jia Liu
Jia Liu Yongping Mu
Yongping Mu Ke Qi
Ke Qi Jiayi Li1
Jiayi Li1