- 1School of Stomatology, Hainan Medical University and Hainan Academy of Medical Sciences, Haikou, Hainan, China
- 2Department of Stomatology, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, China
Melatonin (MLT), a naturally occurring hormone produced by the pineal gland, exhibits significant anticancer effects. It has superior antioxidant, inhibit tumor cell proliferation, migration, angiogenesis-inhibiting, and tumor cell apoptosis-inducing functions. Mechanistically, melatonin inhibits tumor development through epigenetic regulation, metabolic reprogramming, immune micro-environment, and regulation of important signaling pathways (PI3K/AKT). In addition, MLT significantly enhances anticancer efficacy in combination with other anticancer drugs, such as cisplatin, 5-fluorouracil, and paclitaxel. However, the shortcomings of melatonin, such as its low bioavailability, rapid metabolism, and significant individual variation in secretion, have limited its clinical application in anticancer therapy. This limitation has been mitigated by targeted delivery and individualized therapy. Therefore, MLT may be a promising candidate for natural hormone therapy in the future.
1 Introduction
Melatonin (MLT) is an indoleamine secreted by the pineal gland and other organs (retina, gastrointestinal tract, lymphocytes, etc.) (Ahmad et al., 2023). Melatonin secreted by the pineal gland is mainly regulated by light exposure, which activates a pathway starting from the retina, transmitting signals to the suprachiasmatic nucleus (SCN) in the hypothalamus, then to the paraventricular nucleus (PVN), brainstem, and spinal cord, and finally to the pineal gland (Vasey et al., 2021). Tryptophan is the precursor for MLT synthesis, entering the pineal gland through the bloodstream and being converted into 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase. 5-HTP is further converted into 5-hydroxytryptamine (5-HT) by aromatic L-amino acid decarboxylase (AADC), which is then converted to N-acetyl-5-hydroxytryptamine (NAS) by arylalkylamine N-acetyltransferase (AANT), and finally to MLT by acetylserotonin O-methyltransferase (ASMT). This MLT enters the cerebrospinal fluid and bloodstream (Figure 1).
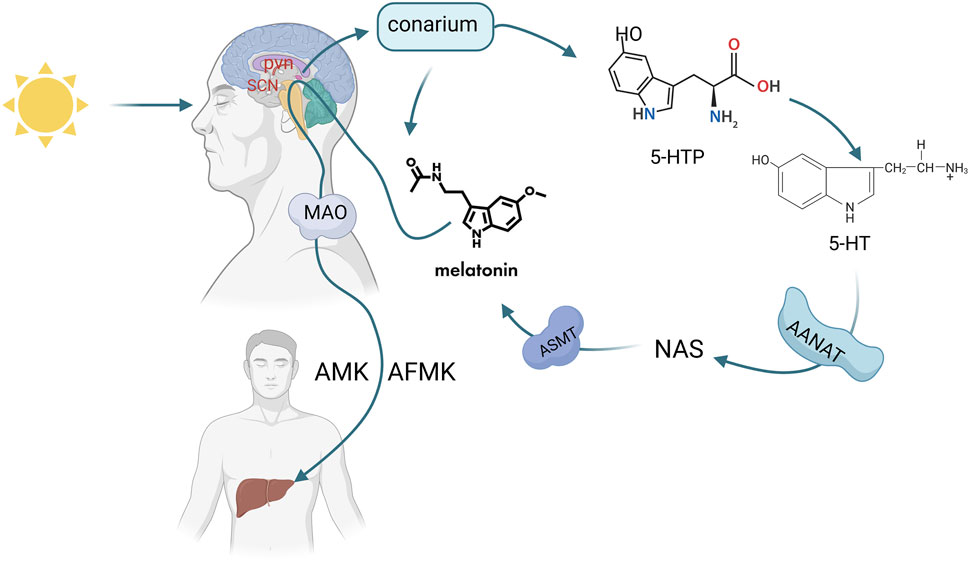
Figure 1. Synthesis and metabolic pathway of melatonin (Melatonin (MLT) synthesis is triggered by light via the retina-SCN-pineal pathway. Tryptophan converts to MLT through enzymatic steps, then enters blood and CSF, metabolizing into AMK/AFMK in the liver). Created in BioRender. cao, y. (2025) https://BioRender.com/p10x701.
Melatonin secreted by the pineal gland is related to the duration of darkness. The main function of melatonin is to transmit darkness signals, which may regulate circadian rhythms and seasonal changes (Claustrat et al., 2005). These circadian rhythms are regulated by the suprachiasmatic nucleus (SCN) of the hypothalamus. The light-dark cycle of the environment plays a key role in the synchronization of the SCN (Pandi-Perumal et al., 2008). MLT synthesis is affected by light, sleep, analgesic drugs, and other factors (Ahmad et al., 2023). Melatonin secretion peaks at night until 3:00 a.m. at the age of 1–3 years, and declines by 80% in adulthood. Seventy% of the melatonin secreted by the pineal gland is metabolized by the liver (Waldhauser et al., 1993). According to reports, in mammals, less than 5% of melatonin is produced by the pineal gland (Reiter et al., 2024). However, most melatonin is secreted outside the pineal gland (in the retina, skin, gastrointestinal tract, immune cells, mitochondria, etc.) and is not affected by circadian rhythms (Acuña-Castroviejo et al., 2014). Due to this characteristic, some researchers have speculated that the local production of melatonin outside the pineal gland plays a more direct and sustained role in the tumor micro-environment, while melatonin secreted by the pineal gland directly and indirectly regulates tumor development during nighttime secretion (Bonmati-Carrion and Tomas-Loba, 2021).
In mammals, melatonin (MLT) activates membrane-bound G protein-coupled receptor (GPCR) receptor binding (MT1, MT2) or by direct action (Jockers et al., 2016). MT1 is a major distribution site in the suprachiasmatic nucleus (SCN), hippocampus, and amygdala (Jockers et al., 2008), and the MTI secreted by the SCN is subject to circadian rhythms. MT2 has a restricted distribution and is mainly confined to the retina. Melatonin has a greater affinity for MTI than MT2 (Liu et al., 2016). It was found that the binding conformation of MTI with 2-iodohydroxytryptamine or ramelteon was more favorable for the binding of high-affinity ligands, and H5.42 and N4.56 of MT2 had weaker affinity due to sequence differences (Wang Q. et al., 2022). These receptors are directly or indirectly linked to a variety of different signaling pathways, thereby inhibiting the response of cancer cells.
2 Antioxidant activity
As a potent redox regulator, melatonin (MLT) exhibits dual antioxidant mechanisms: executing direct ROS/RNS neutralization while concurrently upregulating endogenous antioxidant enzymatic systems through catalytic proficiency modulation, and its main antioxidant effect is the formation of N-acetyl-5-methoxytryptophan (AMK), deformed from the metabolite of melatonin, N1-acetyl-N2-formyl-5-methoxykynurenine (AFMK) (Galano et al., 2013). Melatonin is most concentrated in cell membranes (Venegas et al., 2012),is highly concentrated in mitochondria, and protects proteins, lipids, and DNA from free radical-induced oxidative damage, in addition to preventing mutations and damage to mitochondrial DNA (García et al., 2014).
Mitochondria are the primary sites of ROS production. Within mitochondria, melatonin exerts its antioxidant function by directly scavenging free radicals and also influences the mitochondrial membrane potential to prevent damage from oxidative stress (Chitimus et al., 2020). Melatonin protects the electron transport chain (ETC) by binding to the Fe-S cluster of NADPH dehydrogenase, reducing the generation of superoxide anion radicals (O2•−) caused by electron leakage, blocking the opening of the mitochondrial permeability transition pore (mPTP), and preventing apoptosis caused by cytochrome C leakage (Hardeland, 2017; Tan et al., 2007). Melatonin orchestrates tumor-selective reverse electron transport through mitochondrial Complex I in head and neck squamous cell carcinoma (HNSCC), eliciting site-specific bioenergetic disruption via modulation of NADH/ubiquinone oxidoreductase flux, thereby augmenting ROS-mediated activation of the intrinsic apoptotic cascade through redox-sensitive BAX oligomerization and cytochrome c efflux (Florido et al., 2022a). In addition, the deacetylase sirtuin 3 (Sirt3) increases the content of the pyruvate dehydrogenase complex (PDH) through deacetylation, thereby participating in ATP production. PDH significantly enhances mitochondrial energy metabolism (Ozden et al., 2014). Melatonin enhances superoxide dismutase 2 (SOD2) activity through SIRT3-mediated deacetylation, accelerating the conversion of O2•− to H2O2 (Ning et al., 2022). MLT reverses the Warburg effect and inhibits lung cancer progression in lung cancer cells by stimulating Sirt3 to increase PDH production (Chen X. et al., 2021).
Melatonin exerts its anticancer effects through a multi-level antioxidant mechanism. ROS triggers apoptosis by activating the pro-apoptotic proteins caspase-3/7/9 and cleaved PARP, disrupting mitochondrial function. In pancreatic cancer, melatonin-induced ROS enhances apoptosis through the mitochondrial pathway. ROS inhibits cancer cell invasion and migration by regulating the key JAK2/STAT3 signaling pathway. Additionally, cancer cells combat ROS by relying on their antioxidant system. In hepatocellular carcinoma cells, melatonin increases ROS accumulation and promotes apoptosis by inhibiting GSH levels (Ordoñez et al., 2015). In hepatocellular carcinoma cells, MLT induces hepatocellular carcinoma cell death by increasing ROS production. Concomitant use of melatonin with cisplatin promotes ROS generation and increases cervical cancer cell death (Pariente et al., 2016). Melatonin enhances ROS production in botulinic acid-induced oral squamous cell carcinoma (OSCC) with concomitant activation of DNA repair (Shih et al., 2021).
In addition, melatonin is conditioned to be pro-oxidant. High concentrations of melatonin promote ROS generation. Studies have shown that melatonin promotes ROS production depending on cell type, concentration and duration of action. High concentrations of melatonin are pro-oxidant in cancer cells, but do not increase ROS production in lymphocytes (Büyükavci et al., 2006). The longer a high concentration of melatonin acts, the more ROS it generates (Bejarano et al., 2011). In addition, melatonin promotes ROS production via calmodulin; ROS production is increased when melatonin interacts with calmodulin, and chlorpromazine interrupts ROS production by interrupting the binding of melatonin to calmodulin (Radogna et al., 2009).
3 The anticancer molecular mechanism of melatonin
Extensive research has demonstrated the crucial involvement of melatonin in regulating neoplastic progression. Especially for people who work at night or have low melatonin secretion, the cancer incidence rate increases significantly, implying an inevitable connection between melatonin and various tumors. Second, due to the antioxidant and free radical scavenging activities of melatonin, it has good anticancer activities (Table 1).
3.1 Inhibition of tumor cell proliferation and cycle arrest
Cell proliferation refers to an increase in the number of cells. During tumor growth, abnormal cell proliferation capacity is significantly enhanced. The rapid expansion of cancer cells indicates that the disease is more invasive and spreads faster. Changes in the expression or activity of cell cycle-related proteins are the main markers of proliferation (Jarrett et al., 2018). Numerous studies have shown that MLT can inhibit cell proliferation-related pathways (CDK5 glycosylation, P21, P53, Smad3, etc.) and suppress the cell cycle (G2/M), thereby hindering cell proliferation. For example, Melatonin exerts anti-neoplastic effects in bladder carcinoma through selective suppression of O-GlcNAc post-translational modification on cell cycle-dependent kinase 5 (CDK5), thereby disrupting malignant cell cycle progression (Wu et al., 2021). melatonin demonstrates therapeutic efficacy in gastric malignancies through coordinated downregulation of CDK2/4 oncogenic drivers (Chatterjee et al., 2024). Melatonin inhibits proliferation in the G2/M phase of the hepatocellular carcinoma cell cycle and induces apoptosis by upregulating p21 and p53 (Ammar et al., 2022). Melatonin exerts antitumor efficacy in gastric malignancies through selective downregulation of Smad3-mediated proliferative signaling, effectively disrupting cell cycle progression in neoplastic epithelia (Zhu et al., 2018). In melanoma, melatonin inhibits cell proliferation by interfering with cytoskeleton formation (Alvarez-Artime et al., 2020). Melatonin inhibits proliferation of prostate cancer cells by inhibiting SENP1 protein (Nyamsambuu et al., 2022; Ha et al., 2022). Melatonin demonstrates potent anti-neoplastic activity in cervical carcinoma through dual suppression of NF-κB-mediated inflammatory signaling and COX-2 enzymatic hyperactivity, effectively arresting malignant epithelial proliferation (Minocha et al., 2022). In breast cancer, melatonin promotes breast cancer cell apoptosis through downregulation of Delta-like ligand 4 (Rajabi et al., 2020). Melatonin inhibits gastric cancer proliferation by inhibiting estrogen receptor 1 (ESR1) in bisphenol S-induced gastric cancer production (Wang Y. et al., 2023). In endometrial cancer, melatonin inhibits endometrial cancer proliferation by upregulating GATA-binding protein 2 (Liao et al., 2024). In a mouse model of pancreatic cancer, melatonin supplementation inhibited tumor growth by up to 65%, while blocking endogenous melatonin accelerated tumor growth (Chan et al., 2023).
3.2 Induction of apoptosis and autophagy
In the development and progression of cancer,the anti-apoptosis ability of tumorigenesis is significantly enhanced. Melatonin usually promotes apoptosis by regulating apoptosis-related proteins (caspase family, bax, bcl-2, C-myc, etc.) and inducing endoplasmic reticulum stress. Melatonin orchestrates dual antitumor mechanisms in thyroid carcinoma by simultaneously inducing programmed cell death pathways and suppressing mitogenic signaling cascades, effectively disrupting neoplastic homeostasis (Shih et al., 2021). Melatonin orchestrates dual antitumor mechanisms in cervical carcinoma by initiating endoplasmic reticulum stress-mediated unfolded protein response (UPR) while concurrently activating caspase-dependent apoptotic pathways in neoplastic epithelia (Song and Wang, 2023). ROS trigger apoptosis by activating the pro-apoptotic proteins caspase-3/7/9 and cleaved PARP, disrupting mitochondrial function, and in pancreatic cancer melatonin-induced ROS enhance apoptosis through the mitochondrial pathway (Florido et al., 2022b).
Induction of tumor cell autophagy is a new possibility for studying the potential therapeutic mechanisms of tumors (Liu et al., 2023). Autophagy is a type of programmed cell death and a relatively conserved catabolic process within cells. In cancer, autophagy maintains genomic stability, suppresses the accumulation of oncogenic proteins, and prevents tumorigenesis (Li et al., 2020). Melatonin promotes autophagy in cancer cells by regulating key proteins of autophagy (Beclin-1, LC3-II, ATG7, etc.). During autophagic flux, cytoplasmic LC3-I undergoes lipid conjugation via the ubiquitin-like system (Atg7/Atg3 cascade) to form autophagosome membrane-bound LC3-II. This stoichiometric conversion serves as a quantifiable biomarker for autophagosome biogenesis monitoring, establishing LC3 lipidation as the gold-standard metric in autophagy assessment. Melatonin can induce autophagy in OSCC cells alone or in concert with other drugs (Sung et al., 2020; Wang et al., 2023b), Melatonin membrane receptor induces increased autophagy of TFE3 and induces apoptosis in tongue squamous cell carcinoma (TSCC) (Fan et al., 2018). In gastric cancer, melatonin promotes apoptosis in gastric cancer cells by upregulating HSF1 protein (Li W. et al., 2022). melatonin is also involved in the autophagy process of breast cancer cells, D Wu et al. found that significantly increased anti-apoptotic proteins, LC3- ΙΙ/LC3-Ι ratio of autophagy marker LC3, and the expression of Beclin1 when it was used in the induction of autophagy in breast cancer cells through the combination of MLT and autophagy inhibitor 3-MA. decreased, indicating that autophagy inhibitors can reverse the inhibitory effect of melatonin on breast cancer and that melatonin inhibits breast cancer by inducing autophagy (Wu et al., 2022). Combined treatment with melatonin and Andrographis paniculata in rectal cancer results in an increased LC3-II to LC3-I ratio and promotes autophagy and apoptosis in rectal cancer cells (Zhao et al., 2022).
3.3 Inhibition of metastasis and anti-angiogenesis
The acquisition of epithelial-mesenchymal transition (EMT) characteristics represents a critical indicator of metastatic potential in malignant neoplasms. Among the regulatory network governing this process, Twist and Snail transcription factors have been identified as pivotal molecular regulators that orchestrate both EMT activation and the dissemination of cancerous cells (Gundamaraju et al., 2022),and have been shown to target Twist to inhibit the EMT of lung cancer cells, thereby effectively controlling lung cancer metastasis (Chao et al., 2019). In a seminal study conducted by Karadas et al. Their experimental findings revealed that MLT administration effectively suppressed hepatic and pulmonary metastatic dissemination in murine models of mammary carcinoma. Furthermore, the investigation demonstrated that this indoleamine compound exerted dual inhibitory effects on both angiogenesis and neoplastic proliferation in breast cancer specimens (Karadas et al., 2021). Endothelin-1 (ET-1) inhibits osteoblast differentiation, melatonin inhibits prostate cancer bone metastasis by inhibiting ET-1, making melatonin a promising therapy (Lin et al., 2024). The ECM is a specialized extracellular matrix in the basement membrane that surrounds solid tumors and serves as a structural barrier that anatomically separates the tumor from the surrounding normal tissue (Mond et al., 2020). Matrix metalloproteinases (MMPs) play an important role in explaining the ECM, and melatonin inhibits BC development by downregulating the AKT/MMP9 signaling pathway (Chen et al., 2019). Oncogenic transcription factor (FOSL1) can regulate EMT in a variety of tumors (Sobolev et al., 2022),synergizes with PDL-1 in HNSCC, and significantly inhibits EMT through the ERK1/2/FOSL1 pathway (Luo et al., 2022). In breast cancer, melatonin treatment reduces STAT3 phosphorylation, thereby inhibiting epithelial mesenchymal transformation and metastasis (Das et al., 2024). Melatonin inhibits chondrosarcoma cell proliferation by inhibiting matrix metalloproteinase 7 (MMP7) (Nguyen et al., 2023).
Increased angiogenesis is an important factor in promoting tumorigenesis. Tumor development is usually inhibited by direct or indirect inhibition of angiogenic factors (VEGF, PDGF, HIF1, etc.), and melatonin can achieve this function. Melatonin inhibits the progression of hepatocellular carcinoma in rats by decreasing VEGF levels (Bahaa Eldeen et al., 2023). Melatonin inhibits tumorigenesis by reducing HIF1 levels (Kim et al., 2013; Park et al., 2010). Melatonin enhances the inhibitory effect of netazumab on glioblastoma by inhibiting EGFR, Melatonin significantly reduces microvascular density and vascular endothelial growth factor (VEGF) expression levels in mouse tumors, inhibiting tumor angiogenesis (Wang et al., 2024).
3.4 Metabolic reprogramming
Tumor metabolic reprogramming is the metabolic reprogramming of cancer cells to adapt to hypoxia and nutrient deficiencies, Melatonin has the ability to regulate cancer cell metabolic reprogramming (see Figure 2), in which mitochondrial metabolism is one of the most important factors in cancer development and inhibits the development of HNSCC by regulating mitochondrial metabolism and function (Guerra-Librero et al., 2021). MLT can be combined with vitexoporfin to regulate mitochondrial function and inhibit the growth and stemness of HNSCC (Shin et al., 2022).
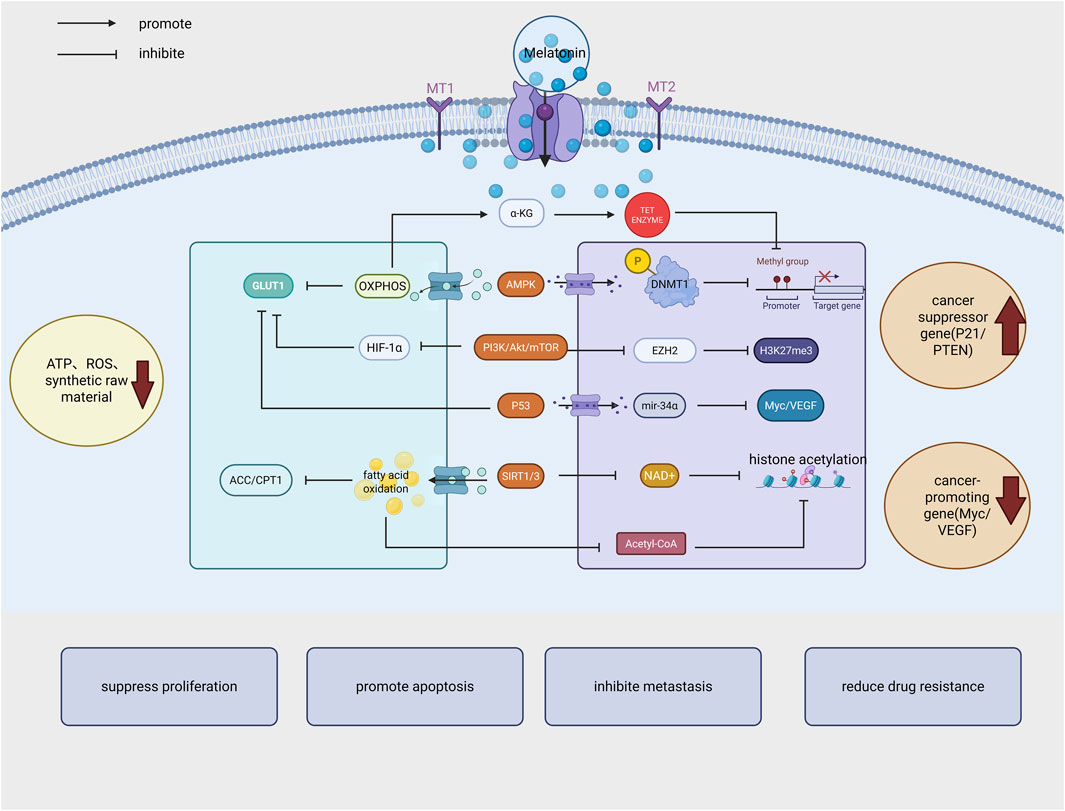
Figure 2. Melatonin exerts its anticancer effects by inhibiting cancer cell proliferation, promoting apoptosis, reducing metastasis and drug resistance through multiple mechanisms, including regulation of energy metabolism, epigenetic modification, signaling pathways, and tumor suppressor gene expression. (GLUT1: Glucose transporter 1; OXPHOS:oxidative phosphorylation; AMPK: AMP-activated protein kinase; HIF-1α: Hypoxia-inducible factor 1α; SIRT1/NAD+: Deacetylase; EZH2 (H3K27me3): Histone methyltransferase; DNMT1: DNA methyltransferase 1; ACC/CPT1: Acetyl-CoA carboxylase and carnitine palmitoyltransferase 1). Created in BioRender. cao, y. (2025) https://BioRender.com/bqm60d5.
In tumor cells, aerobic glycolysis (Warburg effect) leads to tumor promotion through glucose uptake and lactate production for a rapid tumor energy supply. Through its modulatory effects on energy metabolism pathways, melatonin suppresses key oncogenic factors implicated in ovarian carcinoma progression and metastatic potential. This is achieved by downregulating both aerobic glycolytic processes and glutamine degradation mechanisms, thereby fundamentally reshaping the metabolic profile of malignant ovarian cells,In a nude mouse tumor suppression model of ovarian cancer cells, the tumor volume in the melatonin treatment group was reduced by 50% (Silveira et al., 2024). ENO1, best known for catalyzing glycolysis’ ninth enzymatic step, has been identified as a melatonin-regulated protein that mediates downstream metabolic processes, which is mainly involved in the glycolytic process of tumor cells to provide energy support for the survival of tumor cells (Huang et al., 2022). Melatonin induces toxicity of the chemotherapeutic drug gemcitabine in BC cells by silencing the ENO1 upstream factor PPARγ,In a bladder cancer xenograft model, the tumor growth inhibition rate in the melatonin treatment group reached 58%,providing a new perspective for MLT treatment of BC (Shen et al., 2023). Melatonin inhibits smooth muscle sarcoma by suppressing aerobic glycolysis, inhibiting the uptake of linoleic acid (LA) and the release of 13-hydroxy octadecadienoic acid (13-HODE), thereby inhibiting its proliferation and invasion (Mao et al., 2016).
Additionally, the Warburton effect appears to interact with mitochondrial oxidative reactions. Some researchers have speculated that melatonin may act as a glycolytic agent similar to the anticancer drug DCA, targeting the mitochondria of metabolically reprogrammed cancer cells. Melatonin upregulates the pyruvate dehydrogenase complex (PDC), reprogramming pyruvate in mitochondria, promoting the metabolism of pyruvate to acetyl-CoA in mitochondria, and inhibiting the Warburg effect (Reiter et al., 2020).
Fat metabolism is an important part of cancer metabolic reprogramming. Under hypoxic conditions, the rate of fatty acid synthesis increases in cancer cells (Xu et al., 2023). Carboxylesterase 1 (CES1) is an enzyme that inhibits fat accumulation, induces lipid metabolism and increases endoplasmic reticulum stress (Gan et al., 2023),Melatonin can target PCa by upregulating the expression of CES1 to achieve this function (Zhou et al., 2021).
Folic acid drives tumor development by increasing nucleotide synthesis and methylation capacity. In tumor metabolic reprogramming, nucleotide metabolism is linked to glucose metabolism and amino acid metabolism. Methylenetetrahydrofolate dehydrogenase 1-like (MTHFD1L) is a metabolic enzyme that regulates the folate cycle from format production (Agarwal et al., 2019),It is a downstream target of MLT, which was found by Cui et al. to inhibit the development of HNSCC by inhibiting the expression of MTHFD1L mainly through downregulation of CREB1 phosphorylation,In a head and neck squamous cell carcinoma xenograft model, melatonin inhibited tumor growth by more than 60% (Cui et al., 2021).
4 Epigenetic regulation
Epigenetic modifications are primarily categorized into four types: DNA methylation, histone modifications, chromatin remodeling, and non-coding RNA-induced modifications (Xu et al., 2023). DNA methylation maintains dynamic equilibrium within the body to ensure normal physiological functions. In tumor cells, abnormal methylation can lead to the activation of certain proto-oncogenes and the silencing of tumor suppressor genes. The key regulatory enzymes of DNA methylation are DNA methyltransferases (DNMTs), and the key enzymes for active DNA methylation are TET enzymes. In various cancers, the balance between DNA methylation and demethylation is disrupted, leading to impaired expression of DNMT and TET. Melatonin regulates the activity of DNMT and TET, thereby influencing the expression of tumor suppressor genes and oncogenes. Melatonin promotes the expression of DNMT1 and epigenetic suppression of the transcription of the tumor suppressor gene ARHI (Ras homolog 1), thereby reducing the sensitivity of breast cancer to paclitaxel chemotherapy (Xiang et al., 2019). Melatonin reduces the expression of transport proteins and the resistance of brain tumor stem cells to chemotherapy drugs by inducing methylation of the promoter of ABCG2/BCRP, a member of the adenosine triphosphate-binding box (ABC) superfamily (Martín et al., 2013).
Histone modifications influence chromatin structure and gene transcription. The N-terminal regions of histones can undergo post-translational modifications such as methylation, acetylation, lactylation, glycosylation, propionylation, or butyrylation, which alter gene expression. Melatonin exerts its anticancer effects by regulating histone deacetylases (HDACs) and histone acetyltransferases (HATs). Melatonin inhibits the growth of esophageal squamous cell carcinoma by suppressing histone deacetylase 7 (HDAC7) (Ma et al., 2022). HDAC9 knockdown further enhanced the anticancer activity of melatonin treatment in non-small cell lung cancer (Ma et al., 2019). Melatonin inhibits the growth of glioblastoma stem cells by suppressing the NOTCH1 signaling axis induced by histone methyltransferase EZH2 (Zheng et al., 2017). Glycosylation is a post-translationally modified form of the metabolic flux of glucose or other monosaccharides (Pinho and Reis, 2015). Dysregulation of glycosylation triggers tumor development, and O-GlcNAcylation is usually a biomarker of dysregulated glycosylation (Chatham et al., 2021). MLT significantly downregulates O-GlcNAcylation, a dysregulated glycosylation marker, to reduce BC cell proliferation and pro-apoptosis (Wu et al., 2021).
Non-coding RNAs (ncRNAs), consisting of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), have been increasingly recognized as crucial for various biological processes in recent years. Long non-coding RNAs (lncRNAs) represent a class of epigenetically active molecules that orchestrate post-transcriptional gene regulation through competitive sequestration of chromatin modifiers and microRNAs. This RNA-protein interaction paradigm positions lncRNAs as promising therapeutic candidates for targeted oncogenic pathway modulation in precision oncology (McCabe and Rasmussen, 2021). Melatonin coordinates lncRNA to inhibit breast cancer development, and FK506-binding protein (FKBP3) and lnc010561 act as competing endogenous RNAs (ceRNAs) for the tumor suppressor mir-30, which regulates breast cancer development because of the significant downregulation of FKBP3 by melatonin (Liu P. et al., 2020). Melatonin suppresses triple-negative breast cancer (TNBC) oncogenesis through competitive ceRNA-mediated modulation of the lnc049808/miR-101/FUNDC1 mitophagic signaling axis, effectively disrupting mitochondrial homeostasis in malignant epithelia (Yang et al., 2021).
Cyclic RNA is highly conserved and very stable; therefore, it is considered a promising tumor biomarker for precision medicine. Hsa_circ_0017109 Increased expression is a biological process that promotes hyperproliferation and metastatic invasion of lung carcinoma. Wang et al. found that downregulation of Hsa_circ_0017109 expression can effectively inhibit the development of lung cancer, and melatonin plays an exact role (Wang Y. et al., 2022).
Melatonin inhibits cancer cell proliferation to promote apoptosis by up-regulating pro-apoptosis-related miRNAs and down-regulating anti-apoptosis miRNAs. Melatonin also impedes tumor progression through miRNA regulation of pathways related to cancer progression. In addition, melatonin inhibits GC development by suppressing the exosome miR-27b-3p (Zhang et al., 2023). Melatonin inhibits malignant progression of glioblastoma by negatively regulating its downstream target PIM1 through upregulation of mir-16-5p (Yan et al., 2022). Melatonin inhibits human glioblastoma development by regulating HIF1-α/VEGF/MMP9 signaling through the regulation of differentially expressed vascular miRNAs in 6 (Doğanlar et al., 2021). Figure 2 summarizes the interaction mechanisms between melatonin and metabolic reprogramming and epigenetic regulation.
5 Tumor immune microenvironment
The tumor immune microenvironment (TME) is an integral part of cancer progression, influencing metastasis and treatment response. It consists of multiple cell types, extracellular matrix components, and signaling molecules that interact to promote cancer cell growth, invasion, metastasis, and treatment resistance (Bilotta et al., 2022; Jin and Jin, 2020).
Immunosuppressive regulatory T cells (Tregs) are a major mechanism of tumor immune escape (Qin et al., 2024). Targeting Tregs plays an important role in tumor immune escape and has significant antitumor effects. IL-10 and TGF-β are two key cytokines released by Tregs (Sawant et al., 2019). Melatonin reverses immune suppression by reducing the secretion of TGF-β by tumor cells and decreasing the accumulation of myeloid-derived suppressor cells (MDSCs). Melatonin acts on the interactions between Tregs and other cells, thereby eliminating Treg function. Melatonin has also been found to induce the release of inflammatory cytokines such as IFN-γ and TNF-α, which not only promote the proliferation of CD8+ T lymphocytes but also inhibit the proliferation of Tregs (Mu and Najafi, 2021).
Macrophages are divided into two types: classically activated M1 macrophages and selectively activated M2 macrophages (Pan et al., 2020). M1-type macrophages primarily release pro-inflammatory factors, while M2-type macrophages produce anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 within tumors (Murray et al., 2014). Tumor-infiltrating macrophages (TAMs) are the main macrophages in tumors and exhibit M2-type characteristics (Fu et al., 2020). Melatonin can inhibit the release of cytokines such as IL-6, IL-10, and IL-12 by macrophages. After melatonin treatment, the inhibition of the TLR9/ERK1/2 pathway in macrophages plays a key role in preventing the release of pro-inflammatory cytokines (Xu et al., 2018). In addition, melatonin can also inhibit the expression of other inflammatory mediators by macrophages. MLT treatment increased the secretion of TNF-α and CXCL10 by macrophages, thereby inhibiting the growth of gastric cancer cells (Wang K. et al., 2023).
T lymphocytes include various types of cells, such as CD4+ T lymphocytes (i.e., type 1 and type 2 helper T cells), Th17 cells, and cytotoxic CD8+ cells. Th1 cells release inflammatory cytokines, such as IFN-γ, TNF-α, and IL-2. These cytokines activate the immune response of NK cells and CD8+ T lymphocytes and promote the proliferation of CD8+ T lymphocytes. In contrast to Th1 cells, Th2 cells release anti-inflammatory cytokines such as IL-4 and IL-10 (Zhang et al., 2014). In addition, MLT treatment of gastric cancer cells leads to the production of exosomes, which promote the recruitment of CD8+ T cells to the tumor site, thereby inhibiting tumor growth (Wang K. et al., 2023). Melatonin therapy significantly increased the number of CD3+ CD4+ and CD3+ CD8+ T cells, but reduced the infiltration of Ly6G + F4/80- myeloid-derived suppressor cells (MDSCs), significantly inhibiting the growth of non-small cell lung cancer (Chao et al., 2021).
Natural killer (NK) cells are key immune cells in the fight against cancer cells. NK cells kill cancer cells by releasing inflammatory cytokines such as IFN-γ and TNF-α (Vallentin et al., 2015). NK cells may be influenced by molecules released in the tumor microenvironment, thereby promoting angiogenesis and tumor growth (Zhang et al., 2020). Melatonin or its agonists, such as agomelatine and remimegrotin, can promote the release of IL-2, which is a key stimulatory factor for NK cell proliferation (Srinivasan et al., 2011).
Cancer-associated fibroblasts (CAFs) regulate immune responses, alter the composition of the extracellular matrix, and promote angiogenesis to drive tumor progression and metastasis (Chen D. et al., 2021). CAFs can promote endothelial cell proliferation by directly secreting vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) through exosomes. CAFs also secrete chemokine matrix cell-derived factor 1 (SDF-1), which recruits endothelial progenitor cells (EPCs) into peripheral blood and guides their migration to the tumor periphery. Melatonin inhibits the infiltration of triple-negative breast cancer-associated fibroblasts (CAFs) by downregulating the expression of laminin beta-3 (LAMB3) and the C-X-C chemokine ligand 2 (CXCL2) (Lai et al., 2024). IL-8 is primarily expressed in CAFs. Melatonin inhibits IL-8 expression in CAFs by suppressing the NF-κB pathway and AKT pathway, thereby directly or indirectly inhibiting tumor progression (Liao et al., 2023). Figure 3 summarize the role of melatonin in cancer hallmarks.
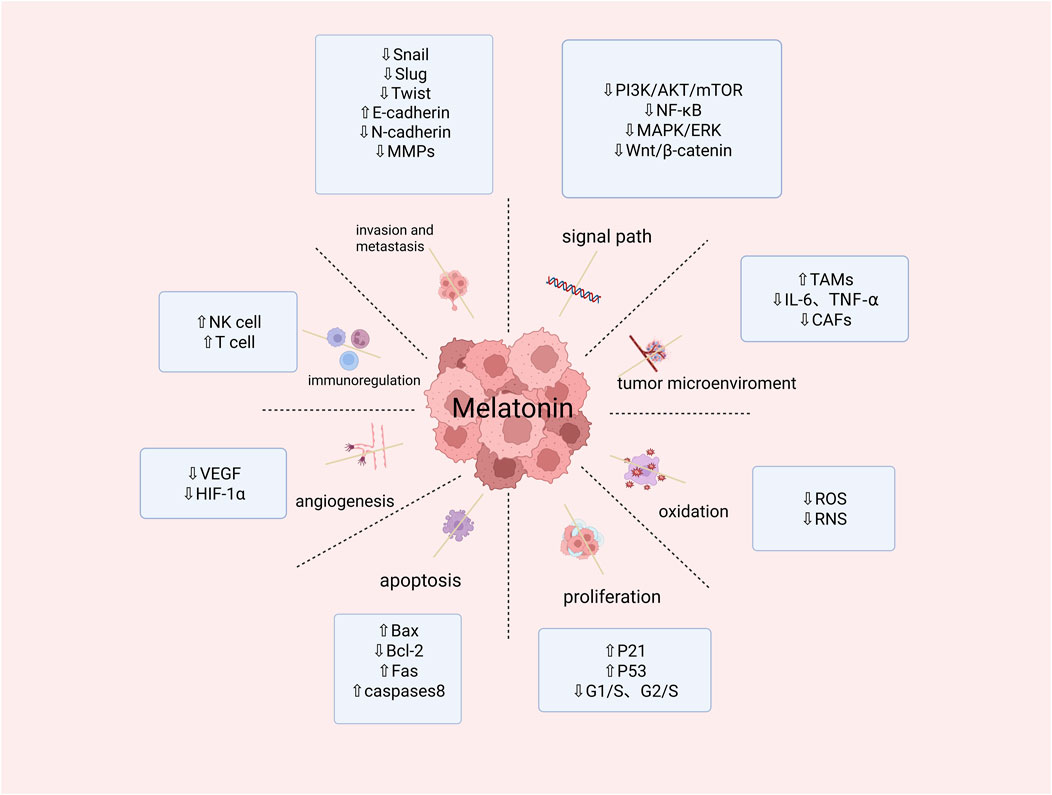
Figure 3. Summary of melatonin activity in restraining cancer hallmarks. (Snail, slug,twist,NF-κB:transcription factor; E-Cadherin,Bax:pro-apoptotic protein; N-Cadherin:inhibitor of apoptoasis protein; MMPs:matrix metalloproteinases; PI3K/AKT/mTOR:phosphatidylinositol-3-kinase/Protenin KinaseB/Mammalian Target of Rapamycin signal channel; MAPK/ERK:signal channel; Wnt/β-catenin:signal channel; TAMs:Tumor-Associated Macrophages; IL-6,TNF-α:proinflammatory cytokine; CAFs:Cancer-Associated Fibroblasts; ROS:Reactive Oxygen Species; RNS:Reactive Nitrogen; P21,P53:cancer suppressor genen; G1/S,G2/S:cell cycle; Bcl-2:anti-apoptosis gene; Fas:tumor necrosis factor; Caspase 8:cysteine protease; VEGF:vascular endothelial growth factor; HIF-1α:the transcription factor hypoxia-inducible factor 1α; NK cell:identify tumor cells; T cell:kill tumor cells). Created in BioRender. cao, y. (2025) https://BioRender.com/u29o844.
6 MLT and signaling pathways
The PI3K/AKT/mTOR (PAM) signaling axis functions as an evolutionarily conserved regulatory network coordinating pro-survival mechanisms, mitogenic processes, and cell cycle regulation through integrated phosphorylation cascades (Glaviano et al., 2023). Through its regulatory effects on the PI3K/AKT signaling cascade, melatonin enhances programmed cell death in ovarian carcinoma cells, thereby suppressing tumor progression and malignant transformation (Baghal-Sadriforoush et al., 2022). MLT inhibits AKT pathway activation by decreasing MMD2, a downstream target of AKT. Inhibition of mTOR induced autophagy in cancer cells through activation of ULK1, leading to Beclin-1 phosphorylation (Pourbarkhordar et al., 2024). MLT plays a major role in inhibiting bladder cancer growth, proliferation and invasion/metastasis by inhibiting Notch/JAG2 signaling through upregulation of PI3K/AKT/mTOR downstream signaling (Chen et al., 2020). Melatonin activates the PI3K/AKT axis, leading to upregulation of ETS and inhibition of apoptosis in hyperoxia-exposed lung cancer cells (He et al., 2023). Figure 4 summarizes the regulatory mechanism of melatonin on the PI3K/AKT signaling pathway.
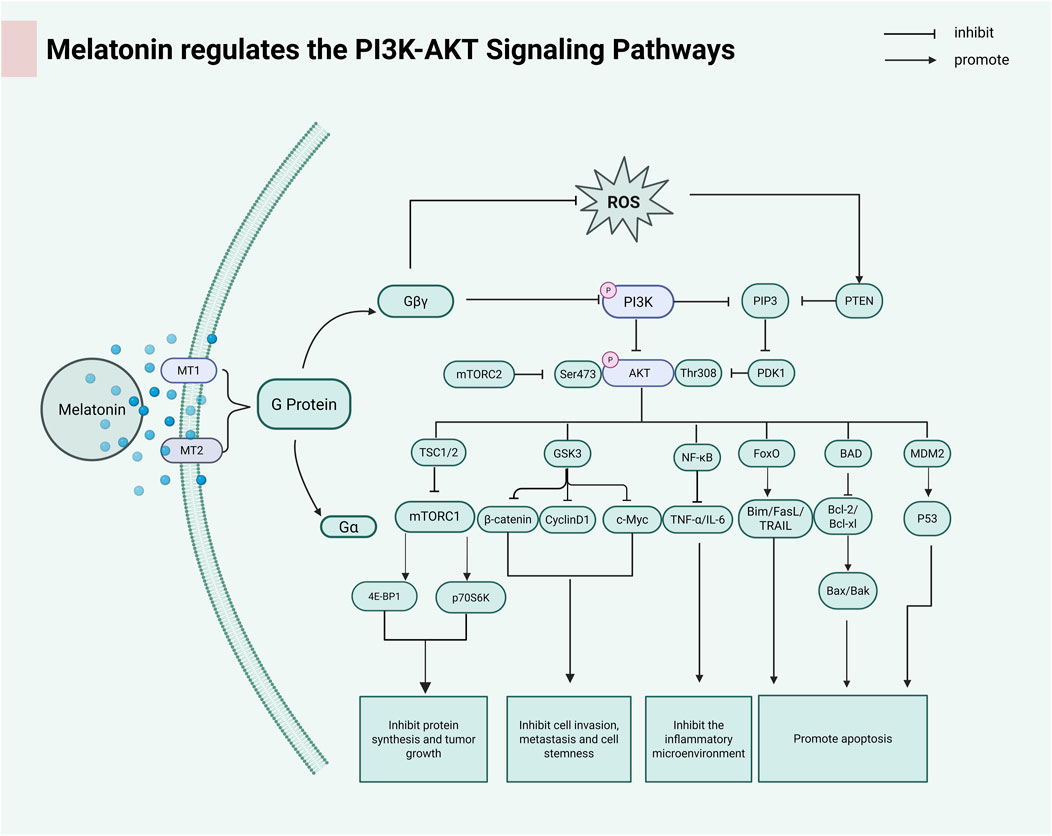
Figure 4. Melatonin activates G proteins by binding to the MT1 and MT2 receptors. The dissociation of the Gβγ subunit further activates the downstream PI3K-AKT signaling pathway. AKT regulates the mTOR complex through phosphorylation, thereby affecting cell growth, metabolism, and survival. (PIP3:Phosphatidylinositol (3,4,5)-trisphosphate; PTEN:anticancer protein; PDK1:3-Phosphoinositide-Dependent Protein Kinase 1; TSC1/2:Tuberous Sclerosis Complex; GSK3:Glycogen Synthase Kinase 3; FoxO:Forkhead box class O; BAD:Pro-apoptotic BCL-2 family; MDM2:p53 tumor suppressor protein; 4EBP1:Translation suppression protein; p70s6k:Serine/threonine protein kinase). Created in BioRender. cao, y. (2025) https://BioRender.com/x11d1oe.
Abnormal activation of Wnt/β-catenin signal transduction is closely related to the occurrence and development of cancer (Yu et al., 2021). Melatonin (MLT) paradoxically enhances metastatic progression in ovarian carcinoma through NE/AKT/β-catenin/SLUG axis potentiation, yet concurrently attenuates chemotherapy-related sequelae (CRS)-driven oncogenesis via SLUG-mediated epithelial-mesenchymal transition (EMT) suppression in preclinical models (Bu et al., 2020). MLT combined with Andrographis paniculata in the treatment of colon cancer, the main mechanism is to induce cell death by inhibiting β-catenin expression and its downregulated signals Cyclin D1 and c-Myc (Sokolov et al., 2022).
Melatonin inhibits cervical cancer cell proliferation by suppressing NF-κB pro-inflammatory transcription factor expression. Melatonin demonstrates oncostatic efficacy in hepatocellular carcinoma (HCC) through dual-pathway modulation: suppressing NF-κB transcriptional activation while attenuating TNF-α-mediated proinflammatory cascades (Ozturk et al., 2023).
7 Synergize with chemotherapeutic agents
In order to further study the synergistic mechanism of melatonin and chemotherapeutic agents, optimize the combined treatment regimen, improve therapeutic efficacy, and reduce side effects, a large number of studies have been conducted. Cisplatin, a platinum-based chemotherapeutic agent, acts on tumorigenesis mainly by inducing DNA damage and apoptosis, to which tumors are prone to develop resistance and its main side effect is that it affects the secretion function of oral salivary glands, resulting in a series of oral-associated diseases (Dasari and Tchounwou, 2014). MLT has superior anti-inflammatory and antioxidant effects, and the combination of melatonin and cisplatin treatment significantly attenuates the destruction of the submandibular gland due to the chemotherapy of cisplatin and reduces the side effects (Badawy et al., 2024). In addition, melatonin enhanced the sensitivity and efficacy of cisplatin for osteosarcoma chemotherapy (Hosseini et al., 2022). MLT attenuated acute kidney injury induced by cisplatin chemotherapy (Kim et al., 2019). Injury to renal tubular epithelial cells is also frequently seen in cisplatin treatment; fatty acid oxidation (FAO) supplies energy to renal tubular epithelial cells, where peroxisome proliferator receptor alpha (PPARα) is a major regulator of FAO (Robbins and Nie, 2012),Melatonin increased PPARα gene and FAO expression and reduced cisplatin-generated acute kidney injury (Li N. et al., 2022).
Melatonin reduces the toxicity of chemotherapeutic drugs while at the same time is significantly anti-decaying and has become a new means of adjuvant chemotherapy for the elderly (Ma et al., 2020). 5-Fluorouracil (5-FU) has become one of the most commonly used chemotherapeutic drugs for cancer treatment, and the use of melatonin in combination with 5-FU reduces the toxicity of the drug and decreases drug resistance (Mafi et al., 2023). Lapatinib is commonly used in the treatment of HER2 positive breast cancer but is prone to recurrence due to drug resistance (Yuan et al., 2023; Yang et al., 2022; Zhang et al., 2024).
Paclitaxel (PTX) is a classic microtubule stabilizer chemotherapy drug that blocks cell mitosis, induces cancer cell apoptosis, and inhibits tumor metastasis (Abbaspour et al., 2025). However, paclitaxel has neurotoxicity and bone marrow suppression issues. In breast cancer, exposure to dim nighttime lighting (dLAN) disrupts the circadian rhythm of melatonin, which drives intrinsic resistance to paclitaxel through epigenetic mechanisms, increases STAT3 expression, and enhances breast tumors’ sensitivity to paclitaxel, inhibiting its growth (Xiang et al., 2019). Melatonin inhibits dryness by activating MT1 to suppress c-Myc, nestin, and histone methylation, thereby promoting the anticancer effect of paclitaxel in brain cancer stem cells (Lee et al., 2018). Table 1 summarizes the anticancer effects of melatonin on different types of cancer and their mechanisms of action.
8 Clinical trial
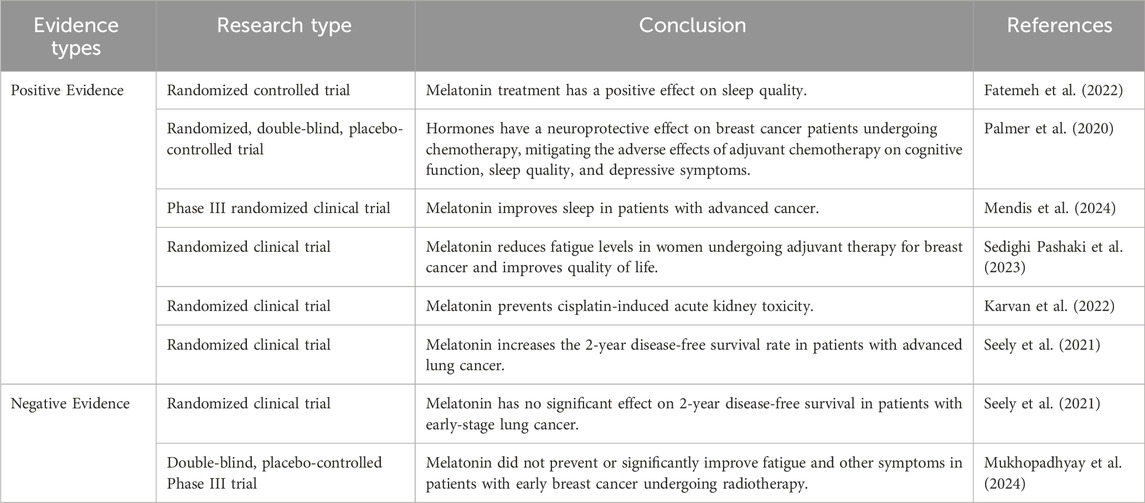
Melatonin has been used in anticancer clinical trials in various types of tumors, and confirmed the beneficial effects of melatonin on various types of cancer. To further promote the use of melatonin as an adjunctive therapy to traditional anticancer treatments, researchers investigated the efficacy of melatonin in clinical studies and patients (Table 2). Most clinical studies used melatonin in combination with chemotherapy or as a protective therapy, including alleviating chemotherapy-induced side effects, reducing the incidence of depressive symptoms, and improving sleep quality in cancer patients (Fatemeh et al., 2022). A prophylactic regimen of 20 mg exogenous melatonin administered 10 days prior to and during initial breast cancer adjuvant chemotherapy (ACBC) demonstrated neuroprotective efficacy, effectively counteracting treatment-induced cognitive impairment, sleep dysregulation, and depressive symptomatology (Palmer et al., 2020). Advanced cancer patients treated with MLT showed significant improvement in sleep disorders in a double-blind clinical trial (Mendis et al., 2024). Among breast cancer patients receiving chemotherapy, showed that melatonin had the ability to significantly ameliorate symptoms such as fatigue after adjuvant therapy for breast cancer (Sedighi Pashaki et al., 2023). Conversely, some clinical trials have also shown conflicting results. Cisplatin, one of the most commonly used cancer chemotherapy drugs, causes significant loss of magnesium and potassium in cancer patients. Melatonin adjunctive therapy improved the incidence of acute kidney injury and the rate of magnesium and potassium loss in urine; however, it did not demonstrate positive results in preventing acute kidney injury (Karvan et al., 2022). A clinical double-blind, phase III randomized controlled trial study indicated that melatonin adjunctive therapy can increase disease-free survival (DFS) in patients with advanced non-small cell lung cancer, but it has no significant effect on postoperative fatigue, depression, and anxiety (Seely et al., 2021). Further research is needed to explore its effectiveness. In addition, recent studies have shown that patients undergoing chemotherapy for breast cancer are prone to fatigue, and the experimental group was administered melatonin 20 mg orally from the night before the start of chemotherapy until 2 weeks after the start of chemotherapy. The results showed that melatonin did not significantly improve the patients’ symptoms of fatigue and sleep disturbance. It is thought-provoking to note that the study did not conduct serologic testing to further validate the (Mukhopadhyay et al., 2024).
Overall, melatonin, as an adjuvant to the main anticancer therapies, can enhance the anticancer effects and significantly improve the quality of life of cancer patients with fatigue, depression and other symptoms associated with chemotherapy. Of course, there are also some conflicting research results, which require well-designed studies with longer follow-up periods and larger sample sizes for verification.
9 Challenges and constraints
Absorption, metabolism, and excretion of melatonin vary from individual to individual, and secondly, the type of drug formulation needs to be considered in order to achieve clinical therapeutic benefit. Ideally, it is recommended that melatonin be administered orally at the usual bedtime time of approximately 45 min to 1 h (Arendt, 1998). Route of administration, age, hepatic function, and potential drug interactions may affect plasma melatonin levels, and melatonin sensitivity and pharmacokinetics vary from person to person; Clinical observations suggest diminished dosing ranges (0.3–0.5 mg) frequently exhibit enhanced therapeutic outcomes compared with elevated dosages across diverse patient populations (Harpsøe et al., 2015). In addition, the collection of melatonin samples in the clinic needs to vary according to the patient’s time of secretion due to differences in the timing of melatonin secretion, which greatly increases the difficulty of sample collection.
10 Challenges and strategies for clinical translation
10.1 Improvement of bioavailability
Melatonin has a short blood half-life, rapid cycling, and high hepatic metabolism. To optimize sustained therapeutic efficacy, developing controlled-release melatonin formulations with prolonged circulation half-life becomes imperative. Pharmacokinetic studies demonstrate that modified-release 2 mg oral tablets achieve peak serum concentration (Tmax) at 6 h post-administration, sustaining bioactive levels above the therapeutic threshold for 3.5 h through first-order elimination kinetics. The sublingual delivery system demonstrated accelerated melatonin absorption kinetics, achieving peak plasma concentration (Cmax) within 30 min - pharmacokinetic behavior analogous to immediate-release (IR) formulations. Comparatively, oral tablet administration exhibited reduced Cmax values but prolonged therapeutic exposure, characterized by an extended elimination half-life (t1/2) and greater area under the curve (AUC) retention (Ait Abdellah et al., 2023). Oniria, an oral extended-release form of melatonin, also significantly increased its bioavailability (Román Martinez et al., 2022).
10.2 Enhanced targeting
When taken orally, melatonin is rapidly metabolized by CYP450 enzymes in the liver into 6-hydroxy melatonin, with a bioavailability of only 3%–15%. Due to its high lipophilicity, melatonin distributes unevenly and tends to accumulate in adipose tissue. Additionally, its short half-life necessitates frequent dosing, which limits its clinical application (Harpsøe et al., 2015). Targeted delivery of melatonin to tumor sites using nanotechnology and nanocoupling to reduce side effects on normal tissues. Melatonin secretion decreases with age, and the use of prostate-specific membrane antigen (PSMA)-targeted nanocarriers loaded with I125 radioactive particles and encapsulated siRNAs targeting APE1 (siAPE1) and melatonin for the treatment of PCa played a good role in tumor-targeted therapy (Liu et al., 2024). Melatonin-containing lactoferrin-chitosan-etoposide nanoparticles show good efficacy in targeting colorectal cancer therapy, increasing bioavailability, and improving drug delivery (Raval et al., 2025). When encapsulated within NIR-responsive chitosan (CS) biopolymers exhibiting superior biocompatibility, melatonin triggers apoptotic cascades in gastric carcinoma through ROS-dependent PI3K/Akt/mTOR axis modulation, leveraging photothermal conversion for spatiotemporal control of therapeutic payload release (Fan et al., 2024). Polylactic acid-hydroxyacetyl copolymer (PLGA) controls degradation rate by regulating the lactic acid/hydroxyacetic acid ratio. Brown algae polysaccharide/chitosan-layered PLGA nanoparticles loaded with melatonin can induce slow release of melatonin, enhance intestinal absorption, and inhibit the progression of triple-negative breast cancer (Yen et al., 2023).
In a mouse model of prostate cancer, the tumor suppression rate of the mitochondrial-targeted nanoparticle Mito-Mel was equivalent to that of 500 mg/kg of free melatonin, representing an approximately 100-fold improvement in efficacy (Chen et al., 2025). Melatonin and gemcitabine were co-delivered in a pancreatic cancer model, achieving a tumor inhibition rate of 68%, significantly higher than the 25% observed in the free group (Ibrahim et al., 2025).
Exosomes mediate intercellular communication. Through exosomes, donor cells can transfer exogenous substances such as proteins, mRNA, microRNA (miRNA), and lipids to recipient cells (Alfonsi et al., 2018). Current research indicates that exosome-mediated drug delivery has low toxicity, low immunogenicity, and high engineering potential (Liang et al., 2021). Recent research has developed engineered M2 macrophage-derived exosomes loaded with melatonin, which can effectively target periodontal inflammation sites and mediate immune reprogramming to promote macrophage repolarization (Cui et al., 2023). Melatonin-pretreated mesenchymal stem cell-derived exosomes (MT-Exo) can suppress inflammation by increasing the ratio of M2 polarization to M1 polarization through activation of the PTEN/AKT signaling pathway, and can promote diabetic wound healing (Liu W. et al., 2020). Endothelial cell-derived primary exosomes mediate melatonin inhibition of vascular calcification and vascular aging in an m6A methylation-dependent manner (Shan et al., 2024). However, current research on the use of exosome-encapsulated melatonin for targeted delivery in cancer is minimal, and we anticipate further studies on melatonin in this area.
10.3 Individualized treatment
Due to the differences in the timing of patients’ melatonin secretion, patients who are sensitive to melatonin are screened for melatonin therapy through genomics, proteomics, and other techniques. The administration time and dose of melatonin are optimized according to the patient’s circadian rhythm, tumor stage, and type.
10.4 Development of novel melatonin analogs
Developing melatonin analogs through chemical modification to enhance their anticancer activity and stability. To design melatonin derivatives with multi-targeted effects that simultaneously act on multiple key pathways in tumors to achieve multiple anti-tumor effects.
11 Conclusion and perspectives
Melatonin, which is a natural hormone with multiple anticancer activities, has made significant progress in cancer prevention research in terms of its role and mechanism. MLT inhibits cancer progression through anti-inflammatory and antioxidant modulation of the immune system, induction of apoptosis, and synergistic chemotherapeutic agents. Targeting MLT is prominent and can effectively reduce side effects and improve bioavailability. Although MLT has been shown to have therapeutic effects on certain cancers in ex vivo and in vivo studies, its molecular mechanism remains unclear, and most of the studies on MLT have focused on the cellular level, with the direct target in tumors still unknown. In addition, due to the time-dependent and concentration-dependent nature of melatonin, although it has been approved by the FDA for the treatment of insomnia and other therapies, no substantial progress has been made in its clinical use in cancer. Currently, there have been related studies using nanosystems to improve the targeting and utilization of melatonin, so melatonin is also a natural anticancer hormone worthy of in-depth study in the future.
Author contributions
YC: Writing – review and editing, Writing – original draft. HZ: Conceptualization, Investigation, Writing – review and editing. XC: Data curation, Writing – review and editing. CL: Writing – review and editing, Supervision. JC: Resources, Funding acquisition, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article was supported by the Hainan Provincial Department of Science and Technology (Grant Numbers: ZDYF2022SHFZ284, ZDYF2021SHFZ115, WSJK2024QN040) and the Hainan Provincial Association for Science and Technology (Grant Number: QCQTXM202215).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaspour, S., Mohamadzadeh, M., and Shojaosadati, S. A. (2025). Protein-based nanocarriers for paclitaxel (PTX) delivery in cancer treatment: a review. Int. J. Biol. Macromol. 310, 143068. doi:10.1016/j.ijbiomac.2025.143068
Acuña-Castroviejo, D., Escames, G., Venegas, C., Díaz-Casado, M. E., Lima-Cabello, E., López, L. C., et al. (2014). Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71, 2997–3025. doi:10.1007/s00018-014-1579-2
Agarwal, S., Behring, M., Hale, K., Al Diffalha, S., Wang, K., Manne, U., et al. (2019). MTHFD1L, A folate cycle enzyme, is involved in progression of colorectal cancer. Transl. Oncol. 12, 1461–1467. doi:10.1016/j.tranon.2019.07.011
Ahmad, S. B., Ali, A., Bilal, M., Rashid, S. M., Wani, A. B., Bhat, R. R., et al. (2023). Melatonin and health: insights of melatonin action, biological functions, and associated disorders. Cell. Mol. Neurobiol. 43, 2437–2458. doi:10.1007/s10571-023-01324-w
Ait Abdellah, S., Raverot, V., Gal, C., Guinobert, I., Bardot, V., Blondeau, C., et al. (2023). Bioavailability of melatonin after administration of an oral prolonged-release tablet and an immediate-release sublingual spray in healthy Male volunteers. Drugs R. D. 23, 257–265. doi:10.1007/s40268-023-00431-9
Alfonsi, R., Grassi, L., Signore, M., and Bonci, D. (2018). The double face of exosome-carried MicroRNAs in cancer immunomodulation. Int. J. Mol. Sci. 19, 1183. doi:10.3390/ijms19041183
Alvarez-Artime, A., Cernuda-Cernuda, R., Francisco Artime, N., Cepas, V., Gonzalez-Menendez, P., Fernadez-Vega, S., et al. (2020). Melatonin-induced cytoskeleton reorganization leads to inhibition of melanoma cancer cell proliferation. Int. J. Mol. Sci. 21, 548. doi:10.3390/ijms21020548
Ammar, O. A., El-Missiry, M. A., Othman, A. I., and Amer, M. E. (2022). Melatonin is a potential oncostatic agent to inhibit HepG2 cell proliferation through multiple pathways. Heliyon 8, e08837. doi:10.1016/j.heliyon.2022.e08837
Arendt, J. (1998). Melatonin and the pineal gland: influence on Mammalian seasonal and circadian physiology. Rev. Reprod. 3, 13–22. doi:10.1530/ror.0.0030013
Badawy, A. M., Ibrahim, M., Taha, M., Helal, A. I., Elmetwally, A. A., El-Shenbaby, I., et al. (2024). Melatonin mitigates cisplatin-induced submandibular gland damage by inhibiting oxidative stress, inflammation, apoptosis, and fibrosis. Cureus 16, e68515. doi:10.7759/cureus.68515
Baghal-Sadriforoush, S., Bagheri, M., Abdi Rad, I., and Sotoodehnejadnematalahi, F. (2022). Melatonin sensitizes OVCAR-3 cells to cisplatin through suppression of PI3K/Akt pathway. Cell Mol. Biol. (Noisy-le-grand) 68, 158–169. doi:10.14715/cmb/2022.68.4.19
Bahaa Eldeen, N. M., Kamel, M. M., Mohamed, A., Kamar, S. S., Rashed, L., and ShamsEldeen, A. M. (2023). Melatonin mitigates the progression of chemically induced hepatocellular carcinoma in rats via targeting Wnt/Β-Catenin pathway, and small noncoding miR-let-7b. Rep. Biochem. Mol. Biol. 12, 403–414. doi:10.61186/rbmb.12.3.403
Bejarano, I., Espino, J., Barriga, C., Reiter, R. J., Pariente, J. A., and Rodríguez, A. B. (2011). Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin. Pharmacol. Toxicol. 108, 14–20. doi:10.1111/j.1742-7843.2010.00619.x
Bilotta, M. T., Antignani, A., and Fitzgerald, D. J. (2022). Managing the TME to improve the efficacy of cancer therapy. Front. Immunol. 13, 954992. doi:10.3389/fimmu.2022.954992
Bonmati-Carrion, M. A., and Tomas-Loba, A. (2021). Melatonin and cancer: a polyhedral network where the source matters. Antioxidants (Basel) 10, 210. doi:10.3390/antiox10020210
Bu, S., Wang, Q., Sun, J., Li, X., Gu, T., and Lai, D. (2020). Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. 11, 644. doi:10.1038/s41419-020-02906-y
Büyükavci, M., Ozdemir, O., Buck, S., Stout, M., Ravindranath, Y., and Savaşan, S. (2006). Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam. Clin. Pharmacol. 20, 73–79. doi:10.1111/j.1472-8206.2005.00389.x
Chan, Y. T., Tan, H. Y., Lu, Y., Zhang, C., Cheng, C. S., Wu, J., et al. (2023). Pancreatic melatonin enhances anti-tumor immunity in pancreatic adenocarcinoma through regulating tumor-associated neutrophils infiltration and NETosis. Acta Pharm. Sin. B 13, 1554–1567. doi:10.1016/j.apsb.2023.01.020
Chao, C. C., Chen, P. C., Chiou, P. C., Hsu, C. J., Liu, P. I., Yang, Y. C., et al. (2019). Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to twist. Clin. Sci. (Lond) 133, 709–722. doi:10.1042/CS20180945
Chao, Y. C., Lee, K. Y., Wu, S. M., Kuo, D. Y., Shueng, P. W., and Lin, C. W. (2021). Melatonin downregulates PD-L1 expression and modulates tumor immunity in KRAS-mutant non-small cell lung cancer. Int. J. Mol. Sci. 22, 5649. doi:10.3390/ijms22115649
Chatham, J. C., Zhang, J., and Wende, A. R. (2021). Role of O-Linked N-Acetylglucosamine protein modification in cellular (patho)Physiology. Physiol. Rev. 101, 427–493. doi:10.1152/physrev.00043.2019
Chatterjee, A., Roy, T., Jyothi, D., Mishra, V. K., Singh, U. P., and Swarnakar, S. (2024). Melatonin inhibits AGS cell proliferation by binding to the ATP binding site of CDK2 under hyperglycemic conditions. Cell Biochem. Biophys. 82, 895–908. doi:10.1007/s12013-024-01241-9
Chen, D., Zhang, X., Li, Z., and Zhu, B. (2021b). Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 11, 1016–1030. doi:10.7150/thno.51777
Chen, X., Hao, B., Li, D., Reiter, R. J., Bai, Y., Abay, B., et al. (2021a). Melatonin inhibits lung cancer development by reversing the warburg effect via stimulating the SIRT3/PDH axis. J. Pineal Res. 71, e12755. doi:10.1111/jpi.12755
Chen, X., Kadier, M., Shi, M., Li, K., Chen, H., Xia, Y., et al. (2025). Targeting melatonin to mitochondria mitigates castration-resistant prostate cancer by inducing pyroptosis. Small 21, e2408996. doi:10.1002/smll.202408996
Chen, Y. T., Huang, C. R., Chang, C. L., Chiang, J. Y., Luo, C. W., Chen, H. H., et al. (2020). Jagged2 progressively increased expression from stage I to III of bladder cancer and Melatonin-mediated downregulation of Notch/Jagged2 suppresses the bladder tumorigenesis via inhibiting PI3K/AKT/mTOR/MMPs signaling. Int. J. Biol. Sci. 16, 2648–2662. doi:10.7150/ijbs.48358
Chen, Y. T., Yang, C. C., Shao, P. L., Huang, C. R., and Yip, H. K. (2019). Melatonin-mediated downregulation of ZNF746 suppresses bladder tumorigenesis mainly through inhibiting the AKT-MMP-9 signaling pathway. J. Pineal Res. 66, e12536. doi:10.1111/jpi.12536
Chitimus, D. M., Popescu, M. R., Voiculescu, S. E., Panaitescu, A. M., Pavel, B., Zagrean, L., et al. (2020). Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10, 1211. doi:10.3390/biom10091211
Claustrat, B., Brun, J., and Chazot, G. (2005). The basic physiology and pathophysiology of melatonin. Sleep. Med. Rev. 9, 11–24. doi:10.1016/j.smrv.2004.08.001
Cucielo, M. S., Freire, P. P., Emílio-Silva, M. T., Romagnoli, G. G., Carvalho, R. F., Kaneno, R., et al. (2023). Melatonin enhances cell death and suppresses the metastatic capacity of ovarian cancer cells by attenuating the signaling of multiple kinases. Pathol. Res. Pract. 248, 154637. doi:10.1016/j.prp.2023.154637
Cui, L., Zhao, X., Jin, Z., Wang, H., Yang, S. F., and Hu, S. (2021). Melatonin modulates metabolic remodeling in HNSCC by suppressing MTHFD1L-formate axis. J. Pineal Res. 71, e12767. doi:10.1111/jpi.12767
Cui, Y., Hong, S., Xia, Y., Li, X., He, X., Hu, X., et al. (2023). Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv. Sci. (Weinh) 10, e2302029. doi:10.1002/advs.202302029
Das, N., Mukherjee, S., Das, A., Gupta, P., Bandyopadhyay, A., and Chattopadhyay, S. (2024). Intra-tumor ROS amplification by melatonin interferes in the apoptosis-autophagy-inflammation-EMT collusion in the breast tumor microenvironment. Heliyon 10, e23870. doi:10.1016/j.heliyon.2023.e23870
Dasari, S., and Tchounwou, P. B. (2014). Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378. doi:10.1016/j.ejphar.2014.07.025
Doğanlar, O., Doğanlar, Z. B., Delen, E., and Doğan, A. (2021). The role of melatonin in angio-mir-associated inhibition of tumorigenesis and invasion in human glioblastoma tumour spheroids. Tissue Cell 73, 101617. doi:10.1016/j.tice.2021.101617
Fan, T., Pi, H., Li, M., Ren, Z., He, Z., Zhu, F., et al. (2018). Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis in tongue squamous cell carcinoma. J. Pineal Res. 64. doi:10.1111/jpi.12457
Fan, Z., Shao, Y., Jiang, X., Zhou, J., Yang, L., Chen, H., et al. (2024). Cytotoxic effects of NIR responsive chitosan-polymersome layer coated melatonin-upconversion nanoparticles on HGC27 and AGS gastric cancer cells: role of the ROS/PI3K/Akt/mTOR signaling pathway. Int. J. Biol. Macromol. 278, 134187. doi:10.1016/j.ijbiomac.2024.134187
Fatemeh, G., Sajjad, M., Niloufar, R., Neda, S., Leila, S., and Khadijeh, M. (2022). Effect of melatonin supplementation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. J. Neurol. 269, 205–216. doi:10.1007/s00415-020-10381-w
Florido, J., Martinez-Ruiz, L., Rodriguez-Santana, C., López-Rodríguez, A., Hidalgo-Gutiérrez, A., Cottet-Rousselle, C., et al. (2022a). Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J. Pineal Res. 73, e12824. doi:10.1111/jpi.12824
Florido, J., Rodriguez-Santana, C., Martinez-Ruiz, L., López-Rodríguez, A., Acuña-Castroviejo, D., Rusanova, I., et al. (2022b). Understanding the mechanism of action of melatonin, which induces ROS production in cancer cells. Antioxidants (Basel) 11, 1621. doi:10.3390/antiox11081621
Fu, L. Q., Du, W. L., Cai, M. H., Yao, J. Y., Zhao, Y. Y., and Mou, X. Z. (2020). The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 353, 104119. doi:10.1016/j.cellimm.2020.104119
Galano, A., Tan, D. X., and Reiter, R. J. (2013). On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 54, 245–257. doi:10.1111/jpi.12010
Gan, C., Wang, J., Martínez-Chávez, A., Hillebrand, M., de Vries, N., Beukers, J., et al. (2023). Carboxylesterase 1 family knockout alters drug disposition and lipid metabolism. Acta Pharm. Sin. B 13, 618–631. doi:10.1016/j.apsb.2022.10.017
García, J. J., López-Pingarrón, L., Almeida-Souza, P., Tres, A., Escudero, P., García-Gil, F. A., et al. (2014). Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J. Pineal Res. 56, 225–237. doi:10.1111/jpi.12128
Glaviano, A., Foo, A. S. C., Lam, H. Y., Yap, K. C. H., Jacot, W., Jones, R. H., et al. (2023). PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22, 138. doi:10.1186/s12943-023-01827-6
Guerra-Librero, A., Fernandez-Gil, B. I., Florido, J., Martinez-Ruiz, L., Rodríguez-Santana, C., Shen, Y. Q., et al. (2021). Melatonin targets metabolism in head and neck cancer cells by regulating mitochondrial structure and function. Antioxidants (Basel) 10, 603. doi:10.3390/antiox10040603
Gundamaraju, R., Lu, W., Paul, M. K., Jha, N. K., Gupta, P. K., Ojha, S., et al. (2022). Autophagy and EMT in cancer and metastasis: who controls whom? Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166431. doi:10.1016/j.bbadis.2022.166431
Hao, L., Dong, Y., Zhang, J. J., He, H. G., Chen, J. G., Zhang, S. Q., et al. (2022). Melatonin decreases androgen-sensitive prostate cancer growth by suppressing SENP1 expression. Transl. Androl. Urol. 11, 91–103. doi:10.21037/tau-21-1110
Hardeland, R. (2017). Melatonin and the electron transport chain. Cell Mol. Life Sci. 74, 3883–3896. doi:10.1007/s00018-017-2615-9
Harpsøe, N. G., Andersen, L. P., Gögenur, I., and Rosenberg, J. (2015). Clinical pharmacokinetics of melatonin: a systematic review. Eur. J. Clin. Pharmacol. 71, 901–909. doi:10.1007/s00228-015-1873-4
He, F., Wang, Q. F., Li, L., Yu, C., Liu, C. Z., Wei, W. C., et al. (2023). Melatonin protects against hyperoxia-induced apoptosis in alveolar epithelial type II cells by activating the MT2/PI3K/AKT/ETS1 signaling pathway. Lung 201, 225–234. doi:10.1007/s00408-023-00610-0
Hosseini, F., Shanehbandi, D., Soleimanpour, J., Yousefi, B., and Alemi, F. (2022). Melatonin increases the sensitivity of osteosarcoma cells to chemotherapy drug cisplatin. Drug Res. (Stuttg) 72, 312–318. doi:10.1055/a-1830-8716
Huang, C. K., Sun, Y., Lv, L., and Ping, Y. (2022). ENO1 and cancer. Mol. Ther. Oncolytics 24, 288–298. doi:10.1016/j.omto.2021.12.026
Ibrahim, S., Yousef, E. H., El-Dessouki, A. M., Raslan, N. A., and Alzokaky, A. A. (2025). Melatonin augments anti-tumor activity and alleviates nephrotoxicity of gemcitabine in a pancreatic cancer xenograft model targeting P62/Keap1 pathway. Naunyn Schmiedeb. Arch. Pharmacol. doi:10.1007/s00210-025-03938-x
Jarrett, A. M., Lima, E., Hormuth, D. A., McKenna, M. T., Feng, X., Ekrut, D. A., et al. (2018). Mathematical models of tumor cell proliferation: a review of the literature. Expert Rev. Anticancer Ther. 18, 1271–1286. doi:10.1080/14737140.2018.1527689
Jin, M. Z., and Jin, W. L. (2020). The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target Ther. 5, 166. doi:10.1038/s41392-020-00280-x
Jockers, R., Delagrange, P., Dubocovich, M. L., Markus, R. P., Renault, N., Tosini, G., et al. (2016). Update on melatonin receptors: IUPHAR review 20. Br. J. Pharmacol. 173, 2702–2725. doi:10.1111/bph.13536
Jockers, R., Maurice, P., Boutin, J. A., and Delagrange, P. (2008). Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br. J. Pharmacol. 154, 1182–1195. doi:10.1038/bjp.2008.184
Kahkesh, M. H., Salehi, Z., Najafi, M., Ghobadi, A., Izad, M., and Shirazi, A. (2020). The inhibitory effect of melatonin on the proliferation of irradiated A549 cell line. J. Cancer Res. Ther. 16, 1500–1505. doi:10.4103/jcrt.JCRT_682_19
Karadas, A. K., Dilmac, S., Aytac, G., and Tanriover, G. (2021). Melatonin decreases metastasis, primary tumor growth and angiogenesis in a mice model of breast cancer. Hum. Exp. Toxicol. 40, 1545–1557. doi:10.1177/09603271211002883
Karvan, S., Sadeghi, A., Farrokhi, P., Nekouee, A., Sharifi, M., and Moghaddas, A. (2022). Melatonin in the prevention of cisplatin-induced acute nephrotoxicity: a randomized, controlled clinical trial. Res. Pharm. Sci. 17, 176–188. doi:10.4103/1735-5362.335176
Kim, J. W., Jo, J., Kim, J. Y., Choe, M., Leem, J., and Park, J. H. (2019). Melatonin attenuates cisplatin-induced acute kidney injury through dual suppression of apoptosis and necroptosis. Biol. (Basel) 8, 64. doi:10.3390/biology8030064
Kim, K. J., Choi, J. S., Kang, I., Kim, K. W., Jeong, C. H., and Jeong, J. W. (2013). Melatonin suppresses tumor progression by reducing angiogenesis stimulated by HIF-1 in a mouse tumor model. J. Pineal Res. 54, 264–270. doi:10.1111/j.1600-079X.2012.01030.x
Lai, Y. W., Liu, Z. W., Lin, M. H., Yang, C. C., Chu, C. Y., Chung, C. H., et al. (2024). Melatonin increases olaparib sensitivity and suppresses cancer-associated fibroblast infiltration via suppressing the LAMB3-CXCL2 axis in TNBC. Pharmacol. Res. 209, 107429. doi:10.1016/j.phrs.2024.107429
Lang, L., Xiong, Y., Prieto-Dominguez, N., Loveless, R., Jensen, C., Shay, C., et al. (2021). FGF19/FGFR4 signaling axis confines and switches the role of melatonin in head and neck cancer metastasis. J. Exp. Clin. Cancer Res. 40, 93. doi:10.1186/s13046-021-01888-9
Lee, H., Lee, H. J., Jung, J. H., Shin, E. A., and Kim, S. H. (2018). Melatonin disturbs SUMOylation-mediated crosstalk between c-Myc and nestin via MT1 activation and promotes the sensitivity of paclitaxel in brain cancer stem cells. J. Pineal Res. 65, e12496. doi:10.1111/jpi.12496
Li, N., Liu, X., Lei, Y., Wang, B., and Li, Z. (2022b). Melatonin ameliorates cisplatin-induced renal tubular epithelial cell damage through PPARα/FAO regulation. Chem. Res. Toxicol. 35, 1503–1511. doi:10.1021/acs.chemrestox.2c00121
Li, W., Hu, C., Zhong, X., Wu, J., and Li, G. (2022a). Melatonin induces AGS gastric cancer cell apoptosis via regulating PERK/eIF2α and HSF1/NF-κB signaling pathway. Ann. Clin. Lab. Sci. 52, 40–47.
Li, X., He, S., and Ma, B. (2020). Autophagy and autophagy-related proteins in cancer. Mol. Cancer 19, 12. doi:10.1186/s12943-020-1138-4
Liang, Y., Duan, L., Lu, J., and Xia, J. (2021). Engineering exosomes for targeted drug delivery. Theranostics 11, 3183–3195. doi:10.7150/thno.52570
Liao, H., Li, H., Dong, J., Song, J., Chen, H., Si, H., et al. (2023). Melatonin blunts the tumor-promoting effect of cancer-associated fibroblasts by reducing IL-8 expression and reversing epithelial-mesenchymal transition. Int. Immunopharmacol. 119, 110194. doi:10.1016/j.intimp.2023.110194
Liao, Y., Li, R., Pei, J., Zhang, J., Chen, B., Dong, H., et al. (2024). Melatonin suppresses tumor proliferation and metastasis by targeting GATA2 in endometrial cancer. J. Pineal Res. 76, e12918. doi:10.1111/jpi.12918
Lin, L. W., Lin, T. H., Swain, S., Fang, J. K., Guo, J. H., Yang, S. F., et al. (2024). Melatonin inhibits ET-1 production to break crosstalk between prostate cancer and bone cells: implication for osteoblastic bone metastasis treatment. J. Pineal Res. 76, e70000. doi:10.1111/jpi.70000
Liu, J., Clough, S. J., Hutchinson, A. J., Adamah-Biassi, E. B., Popovska-Gorevski, M., and Dubocovich, M. L. (2016). MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56, 361–383. doi:10.1146/annurev-pharmtox-010814-124742
Liu, P., Xie, X., Yang, A., Kong, Y., Allen-Gipson, D., Tian, Z., et al. (2020a). Melatonin regulates breast cancer progression by the lnc010561/miR-30/FKBP3 axis. Mol. Ther. Nucleic Acids 19, 765–774. doi:10.1016/j.omtn.2019.12.019
Liu, S., Yao, S., Yang, H., Liu, S., and Wang, Y. (2023). Autophagy: regulator of cell death. Cell Death Dis. 14, 648. doi:10.1038/s41419-023-06154-8
Liu, W., Yu, M., Xie, D., Wang, L., Ye, C., Zhu, Q., et al. (2020b). Melatonin-stimulated MSC-Derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 11, 259. doi:10.1186/s13287-020-01756-x
Liu, Y., Hao, L., Dong, Y., Dong, B. Z., Wang, X. L., Liu, X., et al. (2024). Co-delivery of Siape1 and melatonin by (125)I-loaded PSMA-Targeted nanoparticles for the treatment of prostate cancer. Recent Pat. Anticancer Drug Discov. 19, 503–515. doi:10.2174/1574892818666230419081414
Luo, X., Chen, Y., Tang, H., Wang, H., Jiang, E., Shao, Z., et al. (2022). Melatonin inhibits EMT and PD-L1 expression through the ERK1/2/FOSL1 pathway and regulates anti-tumor immunity in HNSCC. Cancer Sci. 113, 2232–2245. doi:10.1111/cas.15338
Ma, Z., Liu, D., Di, S., Zhang, Z., Li, W., Zhang, J., et al. (2019). Histone deacetylase 9 downregulation decreases tumor growth and promotes apoptosis in non-small cell lung cancer after melatonin treatment. J. Pineal Res. 67, e12587. doi:10.1111/jpi.12587
Ma, Z., Xu, L., Liu, D., Zhang, X., Di, S., Li, W., et al. (2020). Utilizing melatonin to alleviate side effects of chemotherapy: a potentially good partner for treating cancer with ageing. Oxid. Med. Cell Longev. 2020, 6841581. doi:10.1155/2020/6841581
Ma, Z. Q., Feng, Y. T., Guo, K., Liu, D., Shao, C. J., Pan, M. H., et al. (2022). Melatonin inhibits ESCC tumor growth by mitigating the HDAC7/β-catenin/c-Myc positive feedback loop and suppressing the USP10-maintained HDAC7 protein stability. Mil. Med. Res. 9, 54. doi:10.1186/s40779-022-00412-0
Mafi, A., Rezaee, M., Hedayati, N., Hogan, S. D., Reiter, R. J., Aarabi, M. H., et al. (2023). Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun. Signal 21, 33. doi:10.1186/s12964-023-01047-x
Mao, L., Dauchy, R. T., Blask, D. E., Dauchy, E. M., Slakey, L. M., Brimer, S., et al. (2016). Melatonin suppression of aerobic glycolysis (warburg effect), survival signalling and metastasis in human leiomyosarcoma. J. Pineal Res. 60, 167–177. doi:10.1111/jpi.12298
Martín, V., Sanchez-Sanchez, A. M., Herrera, F., Gomez-Manzano, C., Fueyo, J., Alvarez-Vega, M. A., et al. (2013). Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer 108, 2005–2012. doi:10.1038/bjc.2013.188
McCabe, E. M., and Rasmussen, T. P. (2021). lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin. Cancer Biol. 75, 38–48. doi:10.1016/j.semcancer.2020.12.012
Mendis, R., Wong, A., Frenkel, S., Southcott, A. M., Selman, C. J., Martin, J., et al. (2024). Temazepam or melatonin versus placebo for the treatment of insomnia in advanced cancer: a three-arm, double-blind, phase III, multicenter, randomized clinical trial. J. Palliat. Med. 27, 1368–1373. doi:10.1089/jpm.2024.0151
Minocha, T., Das, M., Rai, V., Verma, S. S., Awasthee, N., Gupta, S. C., et al. (2022). Melatonin induces apoptosis and cell cycle arrest in cervical cancer cells via inhibition of NF-κB pathway. Inflammopharmacology 30, 1411–1429. doi:10.1007/s10787-022-00964-6
Mondal, S., Adhikari, N., Banerjee, S., Amin, S. A., and Jha, T. (2020). Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur. J. Med. Chem. 194, 112260. doi:10.1016/j.ejmech.2020.112260
Mu, Q., and Najafi, M. (2021). Modulation of the tumor microenvironment (TME) by melatonin. Eur. J. Pharmacol. 907, 174365. doi:10.1016/j.ejphar.2021.174365
Mukhopadhyay, N. D., Khorasanchi, A., Pandey, S., Nemani, S., Parker, G., Deng, X., et al. (2024). Melatonin supplementation for cancer-related fatigue in patients with early stage breast cancer receiving radiotherapy: a double-blind placebo-controlled trial. Oncologist 29, e206–e212. doi:10.1093/oncolo/oyad250
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. doi:10.1016/j.immuni.2014.06.008
Nguyen, B. T., Lin, C. Y., Chang, T. K., Fong, Y. C., Thadevoos, L. A., Lai, C. Y., et al. (2023). Melatonin inhibits chondrosarcoma cell proliferation and metastasis by enhancing miR-520f-3p production and suppressing MMP7 expression. J. Pineal Res. 75, e12872. doi:10.1111/jpi.12872
Ning, L., Rui, X., Guorui, L., Tinglv, F., Donghang, L., Chenzhen, X., et al. (2022). A novel mechanism for the protection against acute lung injury by melatonin: mitochondrial quality control of lung epithelial cells is preserved through SIRT3-dependent deacetylation of SOD2. Cell Mol. Life Sci. 79, 610. doi:10.1007/s00018-022-04628-0
Nyamsambuu, A., Khan, M. A., Zhou, X., and Chen, H. C. (2022). Molecular mechanism of inhibitory effects of melatonin on prostate cancer cell proliferation, migration and invasion. PLoS One 17, e0261341. doi:10.1371/journal.pone.0261341
Ordoñez, R., Fernández, A., Prieto-Domínguez, N., Martínez, L., García-Ruiz, C., Fernández-Checa, J. C., et al. (2015). Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J. Pineal Res. 59, 178–189. doi:10.1111/jpi.12249
Ozden, O., Park, S. H., Wagner, B. A., Song, H. Y., Zhu, Y., Vassilopoulos, A., et al. (2014). SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic. Biol. Med. 76, 163–172. doi:10.1016/j.freeradbiomed.2014.08.001
Ozturk, I., Elbe, H., Bicer, Y., Karayakali, M., Onal, M. O., and Altinoz, E. (2023). Therapeutic role of melatonin on acrylamide-induced hepatotoxicity in pinealectomized rats: effects on oxidative stress, NF-κB signaling pathway, and hepatocellular proliferation. Food Chem. Toxicol. 174, 113658. doi:10.1016/j.fct.2023.113658
Palmer, A. C. S., Zortea, M., Souza, A., Santos, V., Biazús, J. V., Torres, I. L. S., et al. (2020). Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS One 15, e0231379. doi:10.1371/journal.pone.0231379
Pan, Y., Yu, Y., Wang, X., and Zhang, T. (2020). Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084. doi:10.3389/fimmu.2020.583084
Pandi-Perumal, S. R., Trakht, I., Srinivasan, V., Spence, D. W., Maestroni, G. J., Zisapel, N., et al. (2008). Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85, 335–353. doi:10.1016/j.pneurobio.2008.04.001
Pariente, R., Pariente, J. A., Rodríguez, A. B., and Espino, J. (2016). Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J. Pineal Res. 60, 55–64. doi:10.1111/jpi.12288
Park, S. Y., Jang, W. J., Yi, E. Y., Jang, J. Y., Jung, Y., Jeong, J. W., et al. (2010). Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J. Pineal Res. 48, 178–184. doi:10.1111/j.1600-079x.2009.00742.x
Phiboonchaiyanan, P. P., Puthongking, P., Chawjarean, V., Harikarnpakdee, S., Sukprasansap, M., Chanvorachote, P., et al. (2021). Melatonin and its derivative disrupt cancer stem-like phenotypes of lung cancer cells via AKT downregulation. Clin. Exp. Pharmacol. Physiol. 48, 1712–1723. doi:10.1111/1440-1681.13572
Pinho, S. S., and Reis, C. A. (2015). Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555. doi:10.1038/nrc3982
Pourbarkhordar, V., Rahmani, S., Roohbakhsh, A., Hayes, A. W., and Karimi, G. (2024). Melatonin effect on breast and ovarian cancers by targeting the PI3K/Akt/mTOR pathway. IUBMB Life 76, 1035–1049. doi:10.1002/iub.2900
Qin, D., Zhang, Y., Shu, P., Lei, Y., Li, X., and Wang, Y. (2024). Targeting tumor-infiltrating tregs for improved antitumor responses. Front. Immunol. 15, 1325946. doi:10.3389/fimmu.2024.1325946
Radogna, F., Paternoster, L., De Nicola, M., Cerella, C., Ammendola, S., Bedini, A., et al. (2009). Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol. Appl. Pharmacol. 239, 37–45. doi:10.1016/j.taap.2009.05.012
Rajabi, A., Saber, A., Pourmahdi, M., Emami, A., Ravanbakhsh, R., Khodavirdipour, A., et al. (2020). Anti-cancer effect of melatonin via downregulation of delta-like ligand 4 in estrogen-responsive breast cancer cells. Recent Pat. Anticancer Drug Discov. 15, 329–340. doi:10.2174/1574892815666200929145236
Raval, H., Bhattacharya, S., Bhirud, D., Sangave, P. C., Gupta, G. L., Paraskar, G., et al. (2025). Fabrication of lactoferrin-chitosan-etoposide nanoparticles with melatonin via carbodiimide coupling: in-vitro and in-vivo evaluation for Colon cancer. J. Control Release 377, 810–841. doi:10.1016/j.jconrel.2024.11.077
Reiter, R. J., Sharma, R., Ma, Q., Rorsales-Corral, S., and de Almeida Chuffa, L. G. (2020). Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: a mechanistic hypothesis. Cell Mol. Life Sci. 77, 2527–2542. doi:10.1007/s00018-019-03438-1
Reiter, R. J., Sharma, R., Tan, D. X., Chuffa, L. G. A., da Silva, D. G. H., Slominski, A. T., et al. (2024). Dual sources of melatonin and evidence for different primary functions. Front. Endocrinol. (Lausanne) 15, 1414463. doi:10.3389/fendo.2024.1414463
Robbins, G. T., and Nie, D. (2012). PPAR gamma, bioactive lipids, and cancer progression. Front. Biosci. Landmark Ed. 17, 1816–1834. doi:10.2741/4021
Román Martinez, M., García Aguilar, E., Martin Vílchez, S., González García, J., Luquero-Bueno, S., Camargo-Mamani, P., et al. (2022). Bioavailability of oniria(®), a melatonin prolonged-release formulation, versus immediate-release melatonin in healthy volunteers. Drugs R. D. 22, 235–243. doi:10.1007/s40268-022-00394-3
Sang, X., Li, L., Rui, C., Liu, Y., Liu, Z., Tao, Z., et al. (2021). Induction of EnR stress by melatonin enhances the cytotoxic effect of lapatinib in HER2-positive breast cancer. Cancer Lett. 518, 82–93. doi:10.1016/j.canlet.2021.06.011
Sawant, D. V., Yano, H., Chikina, M., Zhang, Q., Liao, M., Liu, C., et al. (2019). Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735. doi:10.1038/s41590-019-0346-9
Sedighi Pashaki, A., Sheida, F., Moaddab Shoar, L., Hashem, T., Fazilat-Panah, D., Nemati Motehaver, A., et al. (2023). A randomized, controlled, parallel-group, trial on the long-term effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr. Cancer Ther. 22, 15347354231168624. doi:10.1177/15347354231168624
Seely, D., Legacy, M., Auer, R. C., Fazekas, A., Delic, E., Anstee, C., et al. (2021). Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): a randomized placebo controlled clinical trial. EClinicalMedicine 33, 100763. doi:10.1016/j.eclinm.2021.100763
Shan, S. K., Lin, X., Wu, F., Li, C. C., Guo, B., Li, F. X., et al. (2024). Vascular wall microenvironment: endothelial cells original exosomes mediated melatonin-suppressed vascular calcification and vascular ageing in a m6A methylation dependent manner. Bioact. Mater 42, 52–67. doi:10.1016/j.bioactmat.2024.08.021
Shen, D., Deng, Z., Liu, W., Zhou, F., Fang, Y., Shan, D., et al. (2023). Melatonin inhibits bladder tumorigenesis by suppressing PPARγ/ENO1-mediated glycolysis. Cell Death Dis. 14, 246. doi:10.1038/s41419-023-05770-8
Shih, Y. H., Chiu, K. C., Wang, T. H., Lan, W. C., Tsai, B. H., Wu, L. J., et al. (2021). Effects of melatonin to arecoline-induced reactive oxygen species production and DNA damage in oral squamous cell carcinoma. J. Formos. Med. Assoc. 120, 668–678. doi:10.1016/j.jfma.2020.07.037
Shin, Y. Y., Seo, Y., Oh, S. J., Ahn, J. S., Song, M. H., Kang, M. J., et al. (2022). Melatonin and verteporfin synergistically suppress the growth and stemness of head and neck squamous cell carcinoma through the regulation of mitochondrial dynamics. J. Pineal Res. 72, e12779. doi:10.1111/jpi.12779
Silveira, H. S., Cesário, R. C., Vígaro, R. A., Gaiotte, L. B., Cucielo, M. S., Guimarães, F., et al. (2024). Melatonin changes energy metabolism and reduces oncogenic signaling in ovarian cancer cells. Mol. Cell Endocrinol. 592, 112296. doi:10.1016/j.mce.2024.112296
Sobolev, V. V., Khashukoeva, A. Z., Evina, O. E., Geppe, N. A., Chebysheva, S. N., Korsunskaya, I. M., et al. (2022). Role of the transcription factor FOSL1 in organ development and tumorigenesis. Int. J. Mol. Sci. 23, 1521. doi:10.3390/ijms23031521
Sokolov, D., Sharda, N., Giri, B., Hassan, M. S., Singh, D., Tarasiewicz, A., et al. (2022). Melatonin and andrographolide synergize to inhibit the colospheroid phenotype by targeting wnt/Beta-Catenin signaling. J. Pineal Res. 73, e12808. doi:10.1111/jpi.12808
Song, Y., and Wang, S. (2023). Melatonin synergistically enhances docetaxel induced endoplasmic reticulum stress to promote apoptosis by suppressing NF-κB activation in cervical cancer. Med. Oncol. 40, 219. doi:10.1007/s12032-023-02087-6
Srinivasan, V., Pandi-Perumal, S. R., Brzezinski, A., Bhatnagar, K. P., and Cardinali, D. P. (2011). Melatonin, immune function and cancer. Recent Pat. Endocr. Metab. Immune Drug Discov. 5, 109–123. doi:10.2174/187221411799015408
Sung, E. S., Kim, J. Y., Ahn, Y. T., Lee, I. W., Choi, S. W., Jang, H. B., et al. (2020). Melatonin exerts anticancer effects in human tongue squamous cell carcinoma cells by promoting autophagy. Anticancer Res. 40, 6295–6303. doi:10.21873/anticanres.14650
Tan, D. X., Manchester, L. C., Terron, M. P., Flores, L. J., and Reiter, R. J. (2007). One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42, 28–42. doi:10.1111/j.1600-079X.2006.00407.x
Vallentin, B., Barlogis, V., Piperoglou, C., Cypowyj, S., Zucchini, N., Chéné, M., et al. (2015). Innate lymphoid cells in cancer. Cancer Immunol. Res. 3, 1109–1114. doi:10.1158/2326-6066.CIR-15-0222
Vasey, C., McBride, J., and Penta, K. (2021). Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13, 3480. doi:10.3390/nu13103480
Venegas, C., García, J. A., Escames, G., Ortiz, F., López, A., Doerrier, C., et al. (2012). Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52, 217–227. doi:10.1111/j.1600-079X.2011.00931.x
Waldhauser, F., Ehrhart, B., and Förster, E. (1993). Clinical aspects of the melatonin action: impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia 49, 671–681. doi:10.1007/BF01923949
Wang, F., Zhu, Y., Wanggou, S., Lin, D., Su, J., Li, X., et al. (2024). A natural compound melatonin enhances the effects of nimotuzumab via inhibiting EGFR in glioblastoma. Cancer Lett. 592, 216920. doi:10.1016/j.canlet.2024.216920
Wang, K., Cai, R., Fei, S., Chen, X., Feng, S., Zhang, L., et al. (2023c). Melatonin enhances anti-tumor immunity by targeting macrophages PD-L1 via exosomes derived from gastric cancer cells. Mol. Cell Endocrinol. 568-569, 111917. doi:10.1016/j.mce.2023.111917
Wang, L., Su, Y., and Choi, W. S. (2021). Melatonin suppresses oral squamous cell carcinomas migration and invasion through blocking FGF19/FGFR 4 signaling pathway. Int. J. Mol. Sci. 22, 9907. doi:10.3390/ijms22189907
Wang, L., Wang, C., Li, X., Tao, Z., Zhu, W., Su, Y., et al. (2023b). Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS. Cell Mol. Biol. Lett. 28, 36. doi:10.1186/s11658-023-00449-6
Wang, L., Wang, C., Tao, Z., Zhu, W., Su, Y., and Choi, W. S. (2023d). Tumor-associated macrophages facilitate oral squamous cell carcinomas migration and invasion by MIF/NLRP3/IL-1β circuit: a crosstalk interrupted by melatonin. Biochim. Biophys. Acta Mol. Basis Dis. 1869, 166695. doi:10.1016/j.bbadis.2023.166695
Wang, Q., Lu, Q., Guo, Q., Teng, M., Gong, Q., Li, X., et al. (2022a). Structural basis of the ligand binding and signaling mechanism of melatonin receptors. Nat. Commun. 13, 454. doi:10.1038/s41467-022-28111-3
Wang, Y., Song, J., Li, Y., Lin, C., Chen, Y., Zhang, X., et al. (2023a). Melatonin inhibited the progression of gastric cancer induced by bisphenol S via regulating the estrogen receptor 1. Ecotoxicol. Environ. Saf. 259, 115054. doi:10.1016/j.ecoenv.2023.115054
Wang, Y., Tao, B., Li, J., Mao, X., He, W., and Chen, Q. (2020). Melatonin inhibits the progression of oral squamous cell carcinoma via inducing miR-25-5p expression by directly targeting NEDD9. Front. Oncol. 10, 543591. doi:10.3389/fonc.2020.543591
Wang, Y., Wang, Z., Shao, C., Lu, G., Xie, M., Wang, J., et al. (2022b). Melatonin May suppress lung adenocarcinoma progression via regulation of the circular noncoding RNA hsa_circ_0017109/miR-135b-3p/TOX3 axis. J. Pineal Res. 73, e12813. doi:10.1111/jpi.12813
Woo, S. M., Seo, S. U., Min, K. J., and Kwon, T. K. (2022). Melatonin induces apoptotic cell death through bim stabilization by Sp1-mediated OTUD1 upregulation. J. Pineal Res. 72, e12781. doi:10.1111/jpi.12781
Wu, D., Zhang, Y., Tang, H., Yang, J., Li, M., Liu, H., et al. (2022). Melatonin inhibits growth and metastasis of MDA-MB-231 breast cancer cells by activating autophagy. Nan Fang. Yi Ke Da Xue Xue Bao 42, 278–285. doi:10.12122/j.issn.1673-4254.2022.02.16
Wu, J., Tan, Z., Li, H., Lin, M., Jiang, Y., Liang, L., et al. (2021). Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 71, e12765. doi:10.1111/jpi.12765
Xiang, S., Dauchy, R. T., Hoffman, A. E., Pointer, D., Frasch, T., Blask, D. E., et al. (2019). Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. J. Pineal Res. 67, e12586. doi:10.1111/jpi.12586
Xu, X., Peng, Q., Jiang, X., Tan, S., Yang, Y., Yang, W., et al. (2023). Metabolic reprogramming and epigenetic modifications in cancer: from the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 55, 1357–1370. doi:10.1038/s12276-023-01020-1
Xu, X., Wang, G., Ai, L., Shi, J., Zhang, J., and Chen, Y. X. (2018). Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 8, 15579. doi:10.1038/s41598-018-34011-8
Yan, Z., Zhang, X., Hua, L., and Huang, L. (2022). Melatonin inhibits the malignant progression of glioblastoma via regulating miR-16-5p/PIM1. Curr. Neurovasc Res. 19, 92–99. doi:10.2174/1567202619666220406084947
Yang, A., Peng, F., Zhu, L., Li, X., Ou, S., Huang, Z., et al. (2021). Melatonin inhibits triple-negative breast cancer progression through the Lnc049808-FUNDC1 pathway. Cell Death Dis. 12, 712. doi:10.1038/s41419-021-04006-x
Yang, C., Shangguan, C., Lou, G., and Qu, Q. (2022). The efficacy of pyrotinib-based therapy in lapatinib-resistant metastatic HER2-positive breast cancer. Ann. Palliat. Med. 11, 332–338. doi:10.21037/apm-21-3965
Yang, C. C., Chuang, F. C., Chang, C. L., Huang, C. R., Chen, H. H., Yip, H. K., et al. (2023). Melatonin-assisted cisplatin suppresses urinary bladder cancer cell proliferation and growth through inhibiting PrP(C)-Regulated cell stress and cell proliferation signaling. Int. J. Mol. Sci. 24, 3353. doi:10.3390/ijms24043353
Yen, Y. W., Lee, Y. L., Yu, L. Y., Li, C. E., Shueng, P. W., Chiu, H. C., et al. (2023). Fucoidan/Chitosan layered PLGA nanoparticles with melatonin loading for inducing intestinal absorption and addressing triple-negative breast cancer progression. Int. J. Biol. Macromol. 250, 126211. doi:10.1016/j.ijbiomac.2023.126211
Yu, F., Yu, C., Li, F., Zuo, Y., Wang, Y., Yao, L., et al. (2021). Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target Ther. 6, 307. doi:10.1038/s41392-021-00701-5
Yuan, Y., Liu, X., Cai, Y., and Li, W. (2023). Pyrotinib versus lapatinib therapy for HER2 positive metastatic breast cancer patients after first-line treatment failure: a meta-analysis and systematic review. PLoS One 18, e0279775. doi:10.1371/journal.pone.0279775
Zhang, C., Hu, Y., and Shi, C. (2020). Targeting natural killer cells for tumor immunotherapy. Front. Immunol. 11, 60. doi:10.3389/fimmu.2020.00060
Zhang, H., Zhang, L., He, Y., Jiang, D., Sun, J., Luo, Q., et al. (2024). PI3K PROTAC overcomes the lapatinib resistance in PIK3CA-mutant HER2 positive breast cancer. Cancer Lett. 598, 217112. doi:10.1016/j.canlet.2024.217112
Zhang, Y., Zhang, Y., Gu, W., and Sun, B. (2014). TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 841, 15–44. doi:10.1007/978-94-017-9487-9_2
Zhang, Y. Q., Shi, S. S., Li, Y. F., Yang, Y., Bai, P., and Qiao, C. H. (2023). Melatonin inhibits gastric cancer cell proliferation by suppressing exosome miR-27b-3p expression. Anticancer Res. 43, 4413–4424. doi:10.21873/anticanres.16637
Zhao, Y., Wang, C., and Goel, A. (2022). A combined treatment with melatonin and andrographis promotes autophagy and anticancer activity in colorectal cancer. Carcinogenesis 43, 217–230. doi:10.1093/carcin/bgac008
Zheng, X., Pang, B., Gu, G., Gao, T., Zhang, R., Pang, Q., et al. (2017). Melatonin inhibits glioblastoma stem-like cells through suppression of EZH2-NOTCH1 signaling axis. Int. J. Biol. Sci. 13, 245–253. doi:10.7150/ijbs.16818
Zhou, L., Zhang, C., Yang, X., Liu, L., Hu, J., Hou, Y., et al. (2021). Melatonin inhibits lipid accumulation to repress prostate cancer progression by mediating the epigenetic modification of CES1. Clin. Transl. Med. 11, e449. doi:10.1002/ctm2.449
Keywords: melatonin (MLT), anticancer activity, antioxidant activity, chemotherapy synergy, targeted delivery
Citation: Cao Y, Zhang H, Chen X, Li C and Chen J (2025) Melatonin: a natural guardian in cancer treatment. Front. Pharmacol. 16:1617508. doi: 10.3389/fphar.2025.1617508
Received: 24 April 2025; Accepted: 07 July 2025;
Published: 18 July 2025.
Edited by:
Alejandro Romero, Complutense University of Madrid, SpainReviewed by:
Josefa Leon, Fundación para la Investigación Biosanitaria de Andalucía Oriental (FIBAO), SpainZhi-Bin Wang, Central South University, China
Luiz Gustavo Chuffa, Universidade Estadual Paulista, Brazil
Copyright © 2025 Cao, Zhang, Chen, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxin Chen, Y2hqeDIwMDMyMDFAMTYzLmNvbQ==
 Yurou Cao
Yurou Cao Hang Zhang1
Hang Zhang1 Jingxin Chen
Jingxin Chen